Abstract
Reduced-intensity conditioning (RIC) improves the outcomes of hematopoietic cell transplantation (HCT) in patients with hemophagocytic lymphohistiocytosis (HLH). Proximal (ie, close to graft infusion) dosing of alemtuzumab is associated with a high incidence of mixed chimerism, whereas distal (ie, distant from graft infusion) dosing is associated with less mixed chimerism but more acute graft-versus-host disease (GVHD). The alemtuzumab dose per kilogram of body weight also influences these outcomes. We hypothesized that an intermediate alemtuzumab dosing schedule would reduce mixed chimerism and maintain a low incidence of acute GVHD. In this study, 24 consecutive HCTs were performed in patients with HLH or a related disorder using a novel intermediate alemtuzumab schedule of 1 mg/kg starting on day -14. The cumulative incidences (CIs) of mixed chimerism, upfront acute GVHD grades II-IV, and receipt of additional hematopoietic cell products after HCT were compared in patients treated with a distal alemtuzumab schedule (n = 15) and those treated with a proximal alemtuzumab schedule (n = 33). All patients received fludarabine and melphalan. The CI of mixed chimerism was 31% in the intermediate group, 72% in the proximal group (P < .01), and 75% in the distal group patients who received ≥2 mg/kg alemtuzumab (P = .03). The CI of acute GVHD grades II-IV before the development of mixed chimerism was 4% in the intermediate group, 0% in the proximal group, and 13% in the distal group (P = .04, proximal versus distal). The 1-year CI of administration of additional hematopoietic cell products for mixed chimerism (donor lymphocyte infusion ± hematopoietic stem cell boost ± repeat HCT) was 14% in the intermediate group, 53% in the proximal group (P = .01), and 38% in the distal ≥2 mg/kg alemtuzumab group (P = .02). Our findings indicate that intermediate RIC reduces the incidence of mixed chimerism, is associated with a low incidence of upfront acute GVHD, and decreases the need for additional hematopoietic cell products after HCT.
Keywords: Hemophagocytic, lymphohistiocytosis, Familial hemophagocytic, lymphohistiocytosis, Bone marrow transplantation, Hematopoietic cell, transplantation, XIAP deficiency, SAP deficiency, X-linked lymphoproliferative, disease, Reduced-intensity conditioning, Alemtuzumab
Introduction
The use of reduced-intensity conditioning (RIC) regimens for allogeneic hematopoietic cell transplantation (HCT) in patients with nonmalignant disease has increased in recent years. Specifically, RIC regimens containing alemtuzumab, fludarabine, and melphalan have shown good results and are commonly used inthe setting of primary immune deficiencies [1-3]. RIC has especially been shown to improve outcomes in patients with hemophagocytic lymphohistiocytosis (HLH), who may suffer significant early transplantation-related toxicity and mortality in association with myeloablative regimens. In these patients, survival after RIC is approximately 80% to 90%, compared with 45% to 70% after myeloablative chemotherapeautic regimens [4-12].
Despite the improved survival of patients with HLH who undergo allogeneic HCT with RIC, transplantation is often complicated by the high incidence of mixed donor and recipient chimerism, as high as 80% [11]. This is troublesome because HLH disease can recur when the donor contribution to hematopoiesis declines to less than approximately 10% to 20% [8,11]. To reduce the risk of disease recurrence or graft loss, physicians may pursue the administration of additional stem cell or T cell products to stabilize or increase the donor contribution to hematopoiesis. Second transplantations are also required in some cases. Clearly, improvements in RIC regimens are needed to decrease the incidence of mixe donor and recipient chimerism.
We previously reported that the timing (with relation graft infusion) and dose of alemtuzumab affect the development of both mixed chimerism and acute graft-versu host disease (GVHD) after allogeneic HCT in patients with HLH [11]. Patients treated with proximal alemtuzumab schedules (close to the time of graft infusion) develop more mixed chimerism, and less acute GVHD, compared with patients treated with distal (more distant from the time of graft infusion) schedules. The total dose of alemtuzumab per patient kilogram weight also influences the outcomes.
Alemtuzumab (Campath-1H) is a recombinant monoclonal antibody that is directed against the CD52 cell surface glycoprotein present on lymphocytes. Because alemtuzumab can persist at lympholytic concentrations for 1 to 2 months after HCT [13,14], our observations may be the result of varying degrees of in vivo lymphocyte depletion of donor grafts, which is dependent on the presence of residual alemtuzumab at the time of graft infusion. In fact, alemtuzumab dose reductions in adults have been reported to reduce the incidence of mixed chimerism, with significant reductions sometimes occurring at a cost of an increase in acute GVHD [15-17].
Based on these observations, we hypothesized that an intermediate alemtuzumab schedule would reduce the incidence of mixed chimerism compared with the proximal schedule, yet maintain a low incidence of acute GVHD compared with the increase observed in the distal schedule. We designed a RIC regimen with an intermediate alemtuzumab schedule, starting on day -14 with a set dose of 1 mg/kg, with maintenance of historical fludarabine and melphalan dosing schedules. Here we compare the results of this novel intermediate RIC protocol to the outcomes of historical cohorts of patients with HLH and related disorders treated with proximal and distal RIC regimens.
Patients and methods
Patients
Institutional Review Board permission was granted for this study. Between 2006 and March 4, 2012, we performed 79 consecutive HCTs with RIC regimens consisting of alemtuzumab, fludarabine, and melphalan in patients with HLH and related disorders. Seven patients were excluded from this analysis because of changes in the alemtuzumab dosing schedule from the protocol, owing to concomitant treatment of HLH with alemtuzumab (n = 2), temporary cessation of the preparative regimen owing to reactivation of HLH (n = 1) or another illness (n = 2), reduction of melphalan dose by 50% owing to concerns about toxicity in infants (n = 1), or receipt of an alemtuzumab dose below that specified in the dosing protocol (n = 1). In the remaining patients, HLH and related disorders were due to defects in XIAP/BIRC4 (n = 6), SH2D1 A (n = 12), PRF1 (n = 7), UNC13D (n = 8), STXBP2 (n = 9), RAB27 A (n = 1), or HLH related to an unknown genetic defect (n = 23), or the patients was found to be heterozygous for single UNC13D mutations (n = 2) or for single STXBP2 mutations (n = 2), or to have single heterozygous mutations in both UNC13D and STXBP2 (n = 1) (Table 1). Of the patients with unknown genetic defects, 1 was also diagnosed with mitochondrial disease, 2 were also diagnosed with juvenile idiopathic arthritis, and 1 patient had been previously diagnosed with Langerhans cell histiocytosis.
Table 1. Patient Characteristics.
| Proximal RIC (n = 33) | Intermediate RIC (n = 24 HCT in 23 Patients) | Distal RIC (n = 15) | P Value | |
|---|---|---|---|---|
| Age at HCT, yr, median (range) | 3.9 (0.25-25.5) | 4 (0.41-24.3) | 6.1 (0.43-10.8) | .79 |
| Underlying genetic diagnosis | ||||
| PRF1 | 3 (9) | 2 (9) | 2 (13) | |
| UNC13D | 2 (6) | 4 (17) | 2 (13) | |
| STXBP2 | 6 (18) | 2 (9) | 1 (7) | |
| STX11 | 0 | 0 | 0 | |
| XIAP/BIRC4 | 4 (12) | 1 (4) | 1 (7) | |
| SH2D1A | 7 (21) | 5 (22) | 0 | |
| RAB27A | 0 | 0 | 1 (7) | |
| Unknown | 9 (27) | 7 (30) | 7 (47) | |
| Heterozygous for UNC13D and/or STXBP2 | 2 (6) | 2 (9) | 1 (7) | |
| Donor match | .12 | |||
| 8/8 HLA-A, -B, -C, -DR | 21 (64) | 21 (88) | 12 (80) | |
| 1 or 2 allele mismatch | 12 (36) | 3 (13) | 3 (20) | |
| Donor relation | 1.00 | |||
| Sibling | 8 (24) | 6 (25) | 3 (20) | |
| Unrelated | 25 (76) | 18 (75) | 12 (80) | |
| Stem cell source | .44 | |||
| Bone marrow | 31 (94) | 24 (100) | 14 (93) | |
| Peripheral blood stem cells | 2 (6) | 0 | 1 (7) | |
| Total nucleated cell dose, × 108/kg, median (range) | 7 (1.4-23.3) | 7.4 (2.3-15) | 7 (3.2-10) | .73 |
| CD3+ cell dose, × 107/kg, median (range) | 6.8 (1.8-58.8) | 5.9 (2.6-16.2) | 6.2 (2.1-15.7) | .69 |
Transplantation Procedures
Twenty-four consecutive HCTs were performed in 23 patients diagnosed with HLH or X-linked lymphoproliferative disease (XLP) using a novel intermediate RIC protocol with an alemtuzumab dosing schedule of1 mg/kg divided over days -14 to -10 (Figure 1). Alemtuzumab was administered s.c., and the first dose was limited to 3 mg. The cumulative incidences of mixed chimerism and acute GVHD were compared in 2 historical cohorts of patients with HLH and XLP treated with distal (n = 15) or proximal (n = 33) RIC protocols modeled after the protocols published by Shenoy et al. [1] and Cooper et al. [18]. The patients treated with distal RIC had a dose escalation schedule of 3 mg/10 mg/15 mg/20 mg alemtuzumab given over days -22 to -19. Patients weighing <10 kg received 3 mg/10 mg/10 mg/10 mg. The patients treated with proximal RIC received either this dose escalation schedule from day -12 to day -9 or from day -11 to day -8 (n = 12) or a cumulative 1 mg/kg dose divided over days -9 to -5 or over days -8 to -4 (n = 19). Two other patients received proximal alemtuzumab dose escalation schedules on alternative days, either from day -13 to day -10 or from day -9 to day -6. All patients received fludarabine 150 mg/m2 (1 mg/kg in those weighing <10 kg) divided over days -8 to-4 orover days-7 to-3, along with melphalan 140 mg/m2 (4.7 mg/kg in those weighing <10 kg) on day -3 or day -2. Graft characteristics are summarized in Table 1.
Figure 1.
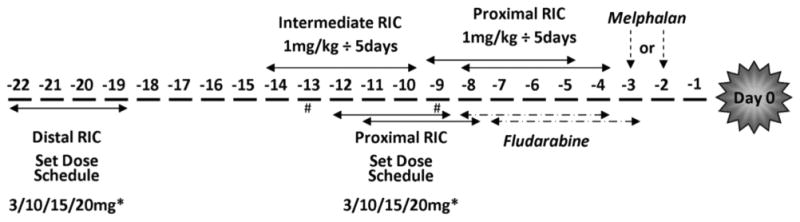
Schematic of alemtuzumab schedules. *Patients weighing <10 kg treated with set dosage schedules received 3 mg/10 mg/10 mg/10 mg of alemtuzumab. #In the proximal RIC group, alemtuzumab was started on day -13 in 1 patient and on day -9 in 1 patient.
Acute GVHD prophylaxis consisted of methylprednisolone 1 to 2 mg/kg and cyclosporine or tacrolimus (dosed to maintain a goal level of 250 to 350 ng/mL or 5 to 10 ng/mL, respectively) in all but 2 patients, who received methylprednisolone and mycophenolate mofetil (MMF). Two patients also received methotrexate on days +1, +3, and +6. Engraftment studies were performed using either fluorescence in situ hybridization (FISH) with X and Y chromosome probes in cases of a sex-mismatched donor or variable number tandem repeat (VNTR) analysis in cases of a same-sex donor. Whole-blood chimerism studies were performed at the discretion of the primary physician and as clinically indicated, generally twice weekly, weekly, or biweekly. All whole-blood chimerism studies were performed in the clinical genetics laboratory at Cincinnati Children's Hospital.
Post-Transplantation Outcomes
The time to mixed chimerism, acute GVHD, administration of donor lymphocyte infusion (DLI), hematopoietic stem cell boost, retransplantation, and time of death or last follow-up were obtained from laboratory and medical records. Mixed chimerism was defined as detection of <95% donorderived cells inperipheral whole-blood samplesonmore than 2 consecutive occasions. Acute GVHD was graded based on standard criteria [19].
Statistical Analyses
We compared the patient characteristics listed in Table 1 among the 3 study groups using either the Kruskal-Wallis test or Fisher exact test [20,21]. For patient outcomes, we analyzed times to cause-specific events by estimating cumulative incidence curves and performing comparisons using the log-rank test [22,23]. We analyzed the time to the development of mixed chimerism, time to the first of any cell product intervention for mixed chimerism (DLI and/or hematopoietic stem cell boost and/or repeat allogeneic HCT), and time to development of acute GVHD grade II-IV and chronic GVHD occurring after graft infusion but before the development of mixed chimerism (ie, upfront acute and chronic GVHD). Patients who underwent repeat allogeneic HCT or died were not at risk for any of these events, and repeat allogeneic HCT and death were considered competing events. These event times are called “cause-specific” event times to distinguish the events of interest from the competing events. For these analyses, patients were censored at the time of last follow-up, repeat allogeneic HCT, or death if the events had not developed by the time of last follow-up, repeat HCT, or death.
Overall survival was defined as time to death or last follow-up if death did not occur. We used the Kaplan-Meier estimator [24] to estimate survival curves by groups, and used the log-rank test [22,23] to compare these curves.
Multivariate analysis was performed with Cox proportional hazard regression [25]. The regression modeled the hazard for overall survival when the outcome of interest was time to death or modeled the cause-specific hazards when the outcomes were times to cause- specific events. We considered donor match (8/8 HLA-A, -B -C, -DR match versus less than 8/ 8 HLA match) and total nucleated cell (TNC) dose (dichotomized around the median cell dose of 7/kg) as covariates. For overall survival analysis, we also considered age (dichotomized as 1 to 6 years or 7 + years). A P value ≤.05 was considered to indicate statistical significance. Computations were done using SAS (SAS Institute, Cary, NC) or XLSTAT (Addinsoft, New York, NY) software.
Results
Patients and Preliminary Analyses
In 23 patients with HLH or a related disorder, we performed 24 consecutive HCTs using an intermediate RIC regimen consisting of fludarabine and melphalan coupled with a novel intermediate alemtuzumab dosing schedule of 1 mg/kg s.c. divided over 5 days starting on day -14. We compared outcomes in 2 retrospective cohorts of patients with HLH and related disorders treated with distal (n = 15) or proximal (n = 33) RIC regimens (Figure 1).
First, because patients treated with proximal alemtuzumab received either a dose escalation schedule (n = 14) or a 1 mg/kg total dose (n = 19), with timing as detailed in Figure 1, we performed preliminary subanalyses to compare the incidence of whole- blood mixed chimerism between these proximal subgroups. The cumulative incidence of mixed chimerism was 86% in the proximal group treated with a dose escalation schedule and 61% in the proximal group treated with 1 mg/kg alemtuzumab (P = .32).
Second, we performed additional preliminary subanalyses to identify any significant differences in the incidence of mixed chimerism based on alemtuzumab dose per kilogram within the proximal or distal groups. In the proximal group, there was no significant difference based on receipt of a total alemtuzumab dose equal to or less than the median dose of 1 mg/kg (65% versus 83%; P = .77). However, in the distal RIC group, the incidence of mixed chimerism was significantly lower in the 7 patients who received an alemtuzumab dose below the median dose of 2 mg/kg compared with the 8 patients who received ≥2 mg/kg (0% versus 75%; P < .01). Given the significant difference in the distal group, we performed all comparisons of whole-blood mixed chimerism among the proximal, intermediate, and distal RIC groups both with and without subdividing the distal RIC group.
Incidence of Whole-Blood Mixed Chimerism
We next compared the cumulative incidence of whole-blood mixed chimerism among the intermediate, proximal, and distal RIC schedule groups (Figure 2A). The incidence of mixed chimerism was lower in the intermediate group compared with the proximal group (31% versus 72%; P < .01). This difference was also significant with regard to each proximal subgroup's incidence of 61% to 86% (data not shown; P < .01). There was a suggestion of less mixed chimerism in the intermediate group compared with the distal group's cumulative incidence of 40%, but clearly less mixed chimerism compared with the distal subgroup patients who received ≥2 mg/kg alemtuzumab (31% versus 75%; P = .03) (Figure 2B). No patients developed mixed chimerism in the distal subgroup that received <2 mg/kg alemtuzumab, compared with an incidence of 31% in the intermediate RIC group, but the difference was not statistically significant (P = .12), perhaps because of the small sample size. In all groups, onset of mixed chimerism generally occurred before 100 days post-HCT (Figure 2C).
Figure 2.
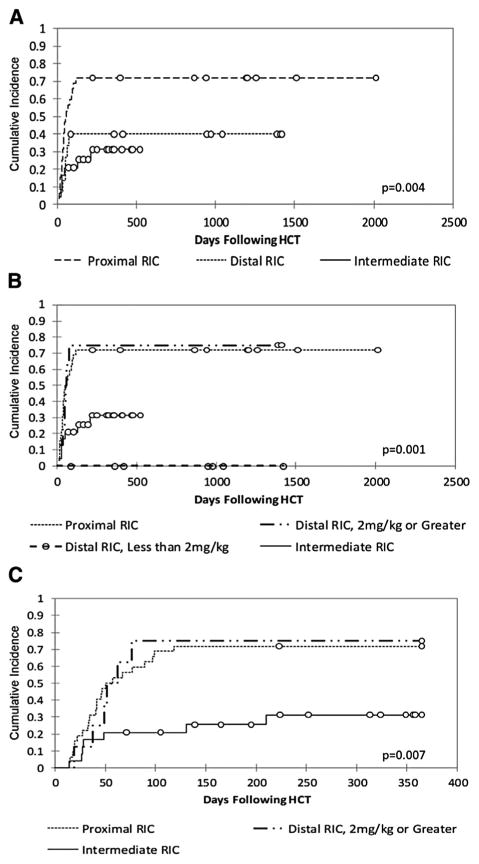
(A) Cumulative incidence of whole-blood mixed chimerism in the intermediate (n = 24 HCTs in 23 patients), proximal (n = 33), and distal (n = 15) groups to last follow-up. (B) Cumulative incidence of whole-blood mixed chimerism in the intermediate, proximal, and 2 distal subgroups (7 patients receiving <2 mg/kg alemtuzumab and 8 patients receiving ≥2 mg/kg) to last follow-up. (C) Cumulative incidence of whole-blood mixed chimerism in the intermediate, proximal, and distal ≥2 mg/kg alemtuzumab groups to 1 year.
We performed multivariate cause-specific hazard modeling to control for any effect of donor match or TNC dose. As shown in Table 2, when controlling for these effects, mixed chimerism was significantly increased in both the proximal RIC group (HR, 3.11; 95% confidence interval [CI], 1.30 to 7.40; P < .01) and the distal ≥2 mg/kg alemtuzumab group (HR, 3.33; 95% CI, 1.10 to 10.08; P = .04) compared with the intermediate group. Because no patients in the distal subgroup who received <2 mg/kg alemtuzumab developed mixed chimerism, we were not able to compute a HR for this group, but this group's lack of mixed chimerism was significant as well (P = .04).
Table 2. Multivariate Cause-specific Hazard Modeling of Whole-Blood Mixed Chimerism.
| Mixed Chimerism | HR (95% CI) | P Value* |
|---|---|---|
| Intermediate RIC (n = 24; HCT in 23 patients) | 1.00 | |
| Proximal RIC (n = 33) | 3.11 (1.30-7.40) | .006 |
| Distal RIC <2 mg/kg alemtuzumab (n = 7) | † | .0406 |
| Distal RIC ≥ 2 mg/kg alemtuzumab (n = 8) | 3.33 (1.10-10.08) | .0407 |
Donor match and total nucleated cell dose were covariates, but were not significant and thus are not included in the table.
P values resulted from the likelihood ratio test. The standard test relies on HR estimates to test for significance. This is not applicable here because the distal subgroup received <2 mg/kg alemtuzumab. The likelihood ratio test is an alternative test that does not rely on the HR estimates [26].
No patients developed mixed chimerism in the distal subgroup receiving <2 mg/kg alemtuzumab. This suggests zero observed hazard for the group, and so the HR estimate is not computable.
Levels of Whole-Blood Donor Chimerism
We then examined the levels of donor chimerism. Whole-blood donor chimerism declined to <50% in 17% of the intermediate RIC group, compared with 50% of both the proximal and distal ≥2 mg/kg alemtuzumab RIC groups (Figure 3). Whole-blood donor chimerism further declined to <20% in 13% of the intermediate RIC group, compared with 25% of both the proximal and distal ≥2 mg/kg alemtuzumab RIC groups (Figure 3).
Figure 3.
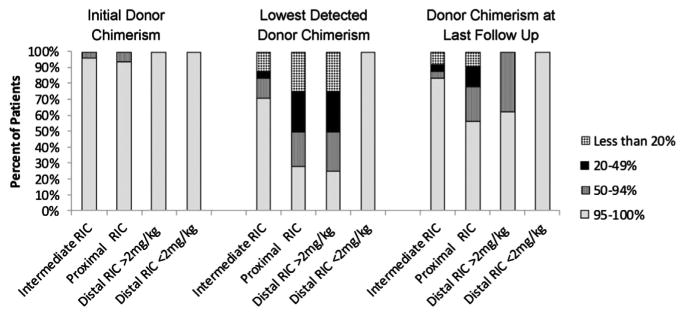
Percentages of patients in each of the groups with whole-blood donor chimerism of >95%, 50-94%, 20-49%, and <20% at initial engraftment, at lowest observation, and last follow-up. One patient in the proximal group died at day +9 without an engraftment study and is not included.
Cell Product Interventions for Whole-Blood Mixed Chimerism
Patients who developed mixed donor and recipient chimerism were initially treated with withdrawal of immune suppression. In addition, at the discretion of the treating physicians, 3 patients in the intermediate RIC group (13%), 15 patients in the proximal group (45%), and 3 patients in the distal ≥2 mg/kg alemtuzumab group (38%) were treated with 1 or more DLIs and/or hematopoietic stem cell boosts within 1 year after HCT (Table 3). The first or sole DLI was administered at a median of 130 days (range, 46 to 230 days) post-HCT in the proximal group, 62 days (range, 39 to 177 days) post-HCT in the intermediate group, and 106 days (range, 44 to 121 days) post-HCT in the distal ≥2 mg/kg group (Table 3). Many patients received additional DLIs (Table 3), including 4% of the intermediate group, 27% of the proximal group, and 25% of the distal ≥2 mg/kg dose group. After these interventions, donor contribution to hemato-poiesis stabilized or increased in many patients, such that >75% of patients in each group demonstrated a >50% donor contribution to hematopoiesis at the time of last follow-up (Figure 3). One patient in the intermediate group and 3 patients in the proximal group required a second allogeneic HCT. The 1-year cumulative incidence of administration of any of these cell products for mixed chimerism (DLI ± boost ± repeat HCT) was 14% in the intermediate group, 53% in the proximal group (P = .01), and 38% in the distal ≥2 mg/kg dose group (P = .02) (Figure 4).
Table 3. Additional Hematopoietic Cell Products Given after HCT.
| Proximal RIC (n = 33) | IntermediateRIC (n = 24HCT in 23 Patients) | Distal RIC <2 mg/kg (n = 7) | Distal RIC ≥2 mg/kg (n = 8) | |
|---|---|---|---|---|
| Patients with mixed chimerism, n (%) | 23 (70) | 7 (29) | 0 | 6 (75) |
| Patients with resolution after withdrawal of immune suppression, n | 2 | 2 | NA | 1 |
| Patients receiving first or only DLI for mixed chimerism, n (%) | 15 (45) | 3 (13) | NA | 3 (38) |
| Day of first DLI after HCT, median (range) | 130 (46-230) | 62 (39-177) | NA | 106 (44-121) |
| Whole-blood donor chimerism just before DLI, %, median (range) | 41 (9-71) | 23 (5-25) | NA | 41 (29-48) |
| Dose of first or only DLI, CD3+ × 106/kg, median (range) | 1 (0.02-11) | 2 (1-5) | NA | 2 (0.4-10) |
| Patients receiving second DLI, n (%) | 9 (27) | 1 (4) | NA | 2 (25) |
| Dose of second DLI, CD3+ × 106/kg, median (range) | 3 (0.2-12.3) | 2 | NA | 13 (6-20) |
| Patients receiving third DLI, n (%) | 8 (24) | 1 (4) | NA | 1 (13) |
| Dose of third DLI, CD3+ × 106/kg, median (range) | 10 (0.5-40) | 4 | NA | 10 |
| Patients receiving fourth DLI, n (%) | 8 (24) | 1 (4) | NA | 1 (13) |
| Dose of fourth DLI, CD3+ × 106/kg, median (range) | 25 (0.5-125.6) | 13.1 | NA | 32 |
| Patients receiving further DLI, n (%) | 3 (9) | 1 (4) | NA | 1 (13) |
| Patients developing acute GVHD grade II-IV after withdrawal of immune suppression and/or DLI, n | 5 | 2 | NA | 2 |
| Patients receiving CD34+-selected boost for mixed chimerism ± DLI, n | 4 | 0 | NA | 1 |
| Patients with resolution of mixed chimerism after DLI ± boost, n | 6 | 1 | NA | 2 |
| Patients who underwent second allogeneic HCT, n | 3 | 1 | NA | 0 |
NA indicates not applicable.
Figure 4.
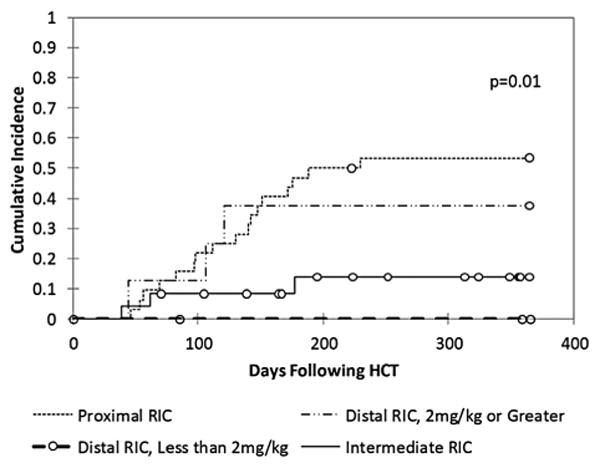
One-year cumulative incidence of cell product intervention for mixed chimerism (DLI ± boost ± repeat HCT).
Upfront Acute GVHD Grades II-IV
Before the development of mixed chimerism or administration of interventions for mixed chimerism, we found a very low incidence of acute GVHD grade II-IV in the proximal and intermediate RIC groups (0% and 4%, respectively; P = .248). We observed an increased incidence of upfront acute GVHD in the distal RIC group (13%; P = .04, proximal versus distal), with 1 patient in each distal subgroup developing upfront acute GVHD.
Unfortunately, we were not able to perform multivariate analyses to confirm these findings, owing to the very low incidence of acute GVHD grade II-IV. However, it is notable that all patients who developed acute GVHD before mixed chimerism received grafts from 8/8 HLA-matched donors, which makes a confounding effect of match unlikely.
Upfront Chronic GVHD
Few patients developed upfront chronic GHVD in the proximal RIC group (3%), intermediate RIC group (8%), and distal RIC subgroup that received ≥2 mg/kg alemtuzumab (0%). However, 2 of 7 patients in the distal RIC subgroup who received <2 mg/kg alemtuzumab developed chronic GHVD (29%). Both of these patients had previous grade I acute GVHD. The cumulative incidence of chronic GVHD in the distal <2 mg/kg RIC group was significantly higher (33%) compared with all of the other groups combined (6%) (P = .01).
Postintervention Acute GVHD Grade II-IV
We next analyzed the incidences of acute GVHD grades II-IV after interventions for mixed chimerism. The incidence of acute GVHD grades II-IV after withdrawal of immune suppression with or without subsequent DLI was 8% in the intermediate group, 14% in the distal group, and 16% in the proximal group (P = .741).
Survival
Finally, we sought to determine if there was any significant effect of alemtuzumab dosing schedule on overall survival. Kaplan-Meier analysis demonstrated similar overall survival in all groups, ranging from 80% to 91% at 1 year (P = .49) and from 80% to 82% at last follow-up (P = .56) (Figure 5). Multivariate analysis also failed to detect any significant differences among the alemtuzumab schedule groups (data not shown). Causes of death were related to infectious complications in all groups, except for 1 patient who died from complications of a multivisceral transplantation at 2 years after HCT. Sepsis, pneumonia or pneumonitis with respiratory failure, acute respiratory distress syndrome, and multiorgan failure were associated with various organisms, including Klebsiella pneumoniae, Enterococcus faecium, Pseudomonas aeruginosa, Acinetobacter baumannii Pneumocystis jiroveci, Scedosporium apiospermum, Paecilomyces species, Candida albicans, adenovirus, and human metapneumovirus.
Figure 5.
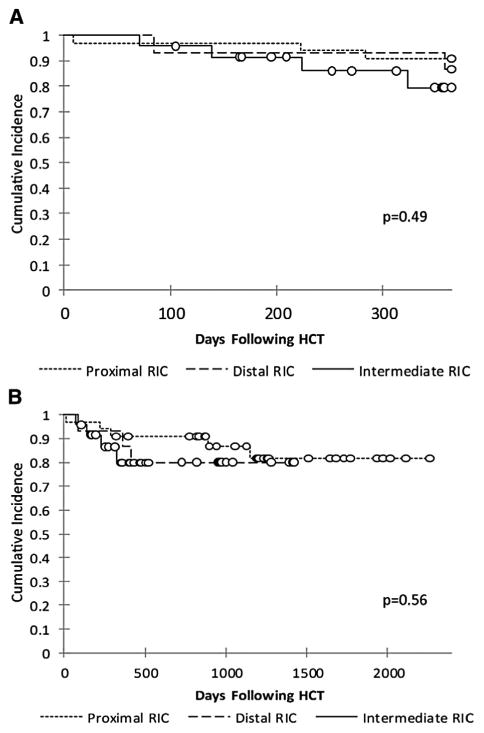
Overall survival at 1 year (A) and long term (B).
Discussion
Our results demonstrate that intermediate RIC regimens significantly reduce the incidence of mixed chimerism after allogeneic HCT in patients with HLH and related disorders. Although this study is limited by our small number of patients, both univariate and multivariate analyses support this conclusion. This is in comparison to both proximal alemtuzumab schedules as well the distal schedule with an alemtuzumab dose ≥2 mg/kg. A distal dose of <2 mg/kg also conveyed protection against mixed chimerism. However, we found an increased incidence of upfront acute GVHD in the distal RIC group and an increased incidence of chronic GVHD in the distal subgroup with an alemtuzumab dose of <2 mg/ kg. Thus, the intermediate RIC protocol appears to better balance the risks of mixed chimerism and upfront GVHD compared with the other regimens.
In addition to decreasing the incidence of mixed chimerism, the intermediate alemtuzumab regimen appeared to decrease the subsequent use of cell product interventions for mixed chimerism compared with the proximal and distal regimens. The ability to avoid DLI or a second HCT offers obvious benefits to patients in terms of decreased risk of associated morbidity and mortality and reduced psychosocial and financial burdens related to the receipt of additional hematopoietic cell products.
Based on our results, we recommend that the 14-day intermediate RIC regimen described herein be considered for use in patients with HLH and related disorders. This intermediate RIC regimen decreases the risk of mixed chimerism, carries a minimal risk of upfront acute GVHD, and reduces the need for additional hematopoietic cell products after HCT. Future studies that prospectively correlate recipient blood levels of alemtuzumab with the risks of mixed chimerism and acute GVHD may promote continued improvements to RIC HCT. Once an optimal therapeutic range of alemtuzumab is determined, personalized dosing protocols that target the therapeutic range can then be developed to further optimize the outcomes of RIC HCT for patients with HLH and related disorders.
Acknowledgments
The authors thank the physicians, nurses, care managers, transplantation coordinators, and other care providers and staff at Cincinnati Children's Hospital, and especially the patients and their families. They also thank Angie Bonavita, Christine Sper, Linda Carl, Mat Goodridge, and Amy Lawton for their help with data collection, along with the clinical genetics laboratory at Cincinnati Children's Hospital.
Footnotes
Conflict of interest statement: There are no conflicts of interest to report.
Authorship statement: R.M. designed the study, collected and analyzed data, performed statistical analyses, created figures, and wrote the manuscript. M.K. and C.L. designed and performed statistical analyses. D.B. and L.H. collected data. M.G., A.K., S.J., K.M., S.C., T.L., P.M., and J.B. contributed patients and edited the manuscript. S.M. directed the design of statistical analyses and edited the manuscript. M.J. and A.H.F. designed the study and edited the manuscript.
Financial disclosure: The authors declare that there are no financial interests to declare.
References
- 1.Shenoy S, Grossman WJ, DiPersio J, et al. A novel reduced-intensity stem cell transplant regimen for nonmalignant disorders. Bone Marrow Transplant. 2005;35:345–352. doi: 10.1038/sj.bmt.1704795. [DOI] [PubMed] [Google Scholar]
- 2.Rao K, Amrolia PJ, Jones A, et al. Improved survival after unrelated donor bone marrow transplantation in children with primary immunodeficiency using a reduced-intensity conditioning regimen. Blood. 2005;105:879–885. doi: 10.1182/blood-2004-03-0960. [DOI] [PubMed] [Google Scholar]
- 3.Satwani P, Cooper N, Rao K, et al. Reduced-intensity conditioning and allogeneic stem cell transplantation in childhood malignant and nonmalignant diseases. Bone Marrow Transplant. 2008;41:173–182. doi: 10.1038/sj.bmt.1705923. [DOI] [PubMed] [Google Scholar]
- 4.Ohga S, Kudo K, Ishii E, et al. Hematopoietic stem cell transplantation for familial hemophagocytic lymphohistiocytosis and Epstein-Barr virus-associated hemophagocytic lymphohistiocytosis in Japan. Pediatr Blood Cancer. 2010;54:299–306. doi: 10.1002/pbc.22310. [DOI] [PubMed] [Google Scholar]
- 5.Yoon HS, Im HJ, Moon HN, et al. The outcome of hematopoietic stem cell transplantation in Korean children with hemophagocytic lymphohistiocytosis. Pediatr Transplant. 2010;14:735–740. doi: 10.1111/j.1399-3046.2009.01284.x. [DOI] [PubMed] [Google Scholar]
- 6.Cesaro S, Locatelli F, Lanino E, et al. Hematopoietic stem cell transplantation for hemophagocytic lymphohistiocytosis: a retrospective analysis of data from the Italian Association of Pediatric Hematology Oncology (AIEOP) Haematologica. 2008;93:1694–1701. doi: 10.3324/haematol.13142. [DOI] [PubMed] [Google Scholar]
- 7.Baker KS, Filipovich AH, Gross TG, et al. Unrelated donor hematopoietic cell transplantation for hemophagocytic lymphohistiocytosis. Bone Marrow Transplant. 2008;42:175–180. doi: 10.1038/bmt.2008.133. [DOI] [PubMed] [Google Scholar]
- 8.Ouachee-Chardin M, Elie C, de Saint Basile G, et al. Hematopoietic stem cell transplantation in hemophagocytic lymphohistiocytosis: a single-center report of 48 patients. Pediatrics. 2006;117:e743–e750. doi: 10.1542/peds.2005-1789. [DOI] [PubMed] [Google Scholar]
- 9.Horne A, Janka G, Maarten Egeler R, et al. Haematopoietic stem cell transplantation in haemophagocytic lymphohistiocytosis. Br J Haema-tol. 2005;129:622–630. doi: 10.1111/j.1365-2141.2005.05501.x. [DOI] [PubMed] [Google Scholar]
- 10.Cooper N, Rao K, Goulden N, et al. The use of reduced-intensity stem cell transplantation in haemophagocytic lymphohistiocytosis and Langerhans cell histiocytosis. Bone Marrow Transplant. 2008;42(Suppl 2):S47–S50. doi: 10.1038/bmt.2008.283. [DOI] [PubMed] [Google Scholar]
- 11.Marsh RA, Vaughn G, Kim MO, et al. Reduced-intensity conditioning significantly improves survival of patients with hemophagocytic lymphohistiocytosis undergoing allogeneic hematopoietic cell transplantation. Blood. 2010;116:5824–5831. doi: 10.1182/blood-2010-04-282392. [DOI] [PubMed] [Google Scholar]
- 12.Henter JI, Samuelsson-Horne A, Arico M, et al. Treatment of hemophagocytic lymphohistiocytosis with HLH-94 immunochemotherapy and bone marrow transplantation. Blood. 2002;100:2367–2373. doi: 10.1182/blood-2002-01-0172. [DOI] [PubMed] [Google Scholar]
- 13.Morris EC, Rebello P, Thomson KJ, et al. Pharmacokinetics of alemtuzumab used for in vivo and in vitro T-cell depletion in allogeneic transplantations: relevance for early adoptive immunotherapy and infectious complications. Blood. 2003;102:404–406. doi: 10.1182/blood-2002-09-2687. [DOI] [PubMed] [Google Scholar]
- 14.Rebello P, Cwynarski K, Varughese M, et al. Pharmacokinetics of CAMPATH-1H in BMT patients. Cytotherapy. 2001;3:261–267. doi: 10.1080/146532401317070899. [DOI] [PubMed] [Google Scholar]
- 15.Shore T, Harpel J, Schuster MW, et al. A study of a reduced-intensity conditioning regimen followed by allogeneic stem cell transplantation for patients with hematologic malignancies using Campath-1H as part of a graft-versus-host disease strategy. Biol Blood Marrow Transplant. 2006;12:868–875. doi: 10.1016/j.bbmt.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 16.Dodero A, Carrabba M, Milani R, et al. Reduced-intensity conditioning containing low-dose alemtuzumab before allogeneic peripheral blood stem cell transplantation: graft-versus-host disease is decreased but T-cell reconstitution is delayed. Exp Hematol. 2005;33:920–927. doi: 10.1016/j.exphem.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Chakraverty R, Orti G, Roughton M, et al. Impact of in vivo alemtuzumab dose before reduced-intensity conditioning and HLA-identical sibling stem cell transplantation: pharmacokinetics, GVHD, and immune reconstitution. Blood. 2010;116:3080–3088. doi: 10.1182/blood-2010-05-286856. [DOI] [PubMed] [Google Scholar]
- 18.Cooper N, Rao K, Gilmour K, et al. Stem cell transplantation with reduced-intensity conditioning for hemophagocytic lymphohistiocy-tosis. Blood. 2006;107:1233–1236. doi: 10.1182/blood-2005-05-1819. [DOI] [PubMed] [Google Scholar]
- 19.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–828. [PubMed] [Google Scholar]
- 20.Kruskal WH, Wallis WA. Use of ranks in one-criterion variance analysis. J Am Stat Assoc. 1952;47:583–621. [Google Scholar]
- 21.Fisher RA. Statistical methods for research workers. Edinburgh, UK: Oliver & Boyd; 1954. [Google Scholar]
- 22.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 23.Peto R, Peto J. Asymptotically efficient rank invariant test procedures. J R Stat Soc A. 1972;135:185–207. [Google Scholar]
- 24.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 25.Cox D. Regression models and life tables (with discussion) J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 26.Klein JP, Moeschberger ML. Survival analysis: techniques for censored and truncated data. New York: Springer-Verlag; 2010. [Google Scholar]


