Abstract
Developing cell-based diabetes therapies requires examining transcriptional mechanisms underlying human β cell development. However, increased knowledge is hampered by low availability of fetal pancreatic tissue and gene targeting strategies. Rodent models have elucidated transcription factor roles during islet organogenesis and maturation, but differences between mouse and human islets have been identified. The past 5 years have seen strides toward generating human β cell lines, the examination of human transcription factor expression, and studies utilizing induced pluripotent stem cells (iPS cells) and human embryonic stem (hES) cells to generate β-like cells. Nevertheless, much remains to be resolved. We present current knowledge of developing human β cell transcription factor expression, as compared to rodents. We also discuss recent studies employing transcription factor or epigenetic modulation to generate β cells.
Keywords: diabetes mellitus, transcription factor, human, organogenesis, epigenetics
Introduction
Diabetes mellitus affects over 300 million people worldwide, based on US Centers for Disease Control and Prevention (CDC) estimates. In the USA diabetes treatments cost an estimated $245 billion in 2012 (American Diabetes Association, ADA), and projections suggest that one in three adults in the USA will develop diabetes by 2050 at current trends (ADA). Central to glucose homeostasis, pancreatic β cells secrete insulin in response to increased blood glucose levels, promoting its uptake in peripheral tissues. β cells work in concert with α cells, which secrete glucagon to promote glucose release from stores in response to hypoglycemia. Diabetic hyperglycemia results from the inability of β cells to secrete insulin properly, or from a lack of insulin action at target tissues, leading to increased mortality from complications. Diabetes mellitus is divided into two classes. Type 1 (T1DM, see Glossary) is characterized by autoimmune-mediated destruction of β cells, whereas type 2 (T2DM) is associated with insulin resistance, and β and α cell dysfunction [1].
To reverse diabetes, transplantation of β cells is a promising replacement therapy, but limitations that include tissue rejection and low donor availability pose a challenge to widespread application [1]. With the need for innovative therapies to better treat the growing numbers of patients with diabetes, research has focused on understanding the molecular mechanisms promoting β cell formation and identity. Transcription factors are gene regulatory proteins that play an integral role in islet cell development, directing cell fates by regulating the transcription of genes involved in specification and ultimately mature function. Much of what is known of transcription factors in β cell development has been revealed in rodent model systems such as genetically manipulated mouse models and cell lines (reviewed in [2]). Although these models remain our best tools for study, there are notable distinctions between mouse and human pancreas, with implications in development and function.
We focus our discussion on human β cell transcription factors, and provide an overview of what is known about their expression and function during development and how this parallels the expression profile in rodents (Figure 1). Human loss-of-function mutations that have revealed similar roles for many transcription factors in β cells will be discussed. We will also address how knowledge of human β cell transcriptional regulation is being applied toward generating therapeutic β cells.
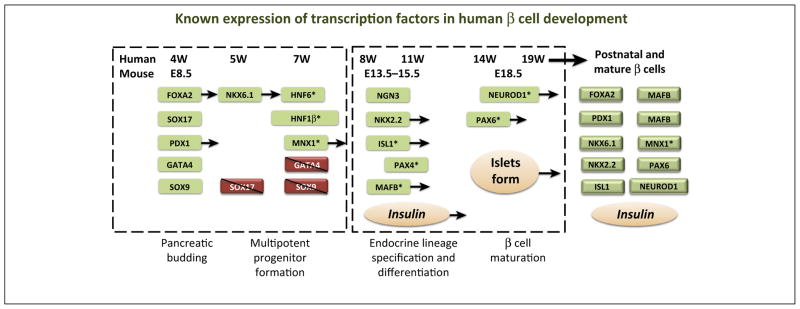
Figure 1.
Approximate timeline of transcription factor expression during human β cell development. A relative course of human and mouse β cell development is depicted. Transcription factors (in shaded boxes) are listed under each time-point where their expression is first observed, or lost, according to the literature. For comparison, approximate mouse developmental points are included under the human timeline. Early [i.e., pancreatic budding and multipotent pancreatic progenitor cell (MPC) formation] and late (i.e., lineage specification and β cell maturation) stages of pancreas formation are approximated by labels and dashed boxes. Some transcription factors are marked with an *, denoting that whole pancreas mRNA was used to characterize expression, thus cell type-specificity was not determined. Transcription factors with arrows extending denote expression that persists into postnatal and/or mature β cells. Abbreviations: E, embryonic day; W, weeks gestation.
Transcriptional regulation of human β cell development
Human and rodent pancreatic islets are composed of five hormone-secreting endocrine cell types. These include the aforementioned α and β cells, plus somatostatin-secreting δ cells, pancreatic polypeptide (PP) cells, and the ghrelin-producing ε cells. During human and rodent development, early endodermal tissue becomes specified toward a pancreatic fate before evagination of dorsal and ventral pancreatic buds [3]. These buds are populated with multipotent pancreatic progenitor cells (MPCs). Signaling and transcriptional events then promote the MPCs towards acinar, endocrine, or ductal fates. Once specified, endocrine progenitors undergo further differentiation with lineage-specific transcription factors promoting final maturation steps.
Importantly, several key differences have been observed between species. Human islets comprise a lower proportion of β cells, with more α and δ cells than mouse islets [4]. Islet architecture differs, with human β cells being dispersed among α and δ cells, whereas mouse islets maintain a β cell core surrounded by the four other endocrine cell types [5]. Moreover, two genes encode insulin in rodents (Ins1, Ins2), whereas only one is present in humans (INS) [6]. Human islets were found to secrete more insulin at baseline glucose levels, but less in response to a stimulatory glucose challenge, compared to mouse islets [7]. As we will discuss further, human and rodent β cells share many transcription factors during β cell development and adult function, but their expression pattern, timing, and overall activities may differ (Figure 1).
In addition to fetal studies describing human transcription factor expression, functional roles for many transcription factors have been revealed in genome-wide analyses of various forms of diabetes. For example, maturity-onset diabetes of the young (MODY) genes are responsible for rare forms of diabetes that are caused by single gene mutations [8]. Although MODY accounts for only 1–2% of diagnosed cases, their monogenic nature links these factors to roles in β cell identity, having implications for T2DM and T1DM. Neonatal diabetes mellitus is a more rare monogenic form of non-autoimmune diabetes that can be permanent or transient after presenting in the first 6 months of life. Several recent reviews have focused on genes studied in these contexts [8]; therefore we will briefly note the transcription factors that are most commonly involved and which appear to act by affecting human β cell development and/or function.
Foregut development and budding
About 4 weeks into human gestation, the dorsal pancreatic bud emerges, followed by the ventral bud. This early phase of pancreatic specification is marked by several transcription factors that are also linked to early mouse development [9]. Many of these factors are expressed throughout organogenesis, and have unique roles in distinct temporal windows and populations. Early human pancreas studies are particularly limited, thus many additional factors are likely involved in this early process.
Transcription factors involved in early stages of development Forkhead box A2 (FOXA2)
During early pancreatic development, the FOXA2 transcription factor is consistently expressed from week 4 forward, as revealed by recent studies on human fetal pancreas [10–12]. This expression profile is similar to broad mouse FoxA2 expression throughout pancreatic development, acting as a pioneer factor to regulate Pdx1 (pancreatic and duodenal homeo-box 1) expression, a relationship that has not been determined in humans [3,13]. FOXA2 persists in all mature pancreatic cell types of both mice and humans [2,3].
SOX17 [SRY (sex determining region Y)-box 17]
In contrast to FOXA2, expression of the HMG (high mobility group) box transcription factor SOX17 is observed immediately before 4 weeks in humans and is then excluded from pancreatic cells about 1 week later, similar to the down-regulation of Sox17 during mouse pancreatic development [9,10]. Studies in mice have indicated that although early Sox17 expression is necessary for endoderm formation, it later represses the pancreatic fate [14].
Hepatocyte nuclear factor (HNF6)
mRNA analysis of human pancreas aged 7–21 weeks demonstrated that HNF6 is consistently expressed [11,12]. This parallels mouse Hnf6 expression at embryonic day (E) 8.5 with broad expression throughout development, directing endocrine allocation until just before birth when it becomes restricted to α and acinar cells [15].
Hepatocyte nuclear factor 1 homeobox β (HNF1β)
A high level of HNF1β expression begins as early as 7 weeks in humans, and persists throughout pancreatic development [12]. Interestingly, heterozygous loss-of-function HNF1β mutations (termed MODY5) result in diabetes in humans but only homozygous mutations produced diabetes in mice [16]. This could be due to a potentiated single wave of human endocrine differentiation versus the two phases observed in rodents, rendering these human cells more sensitive to HNF1β dosage [2]. Hnf1β-deficient mice exhibit pancreatic agenesis by E13.5, suggesting that the role of HNF1β in pancreatic development is evolutionarily conserved [17].
PDX1
Also known as insulin promoter factor 1 (IPF1), PDX1 has been studied for its role throughout all phases of pancreatic development. PDX1 is broadly expressed at around 4 weeks with a high level of expression being restricted later to adult human β cells [10,11]. PDX1 high expression is specific to rodent β cells by E15.5, where it regulates the expression of Ins1, MafA (musculoaponeurotic fibrosarcoma oncogene family, protein A), and Pdx1, whereas only low-level expression is observed in the exocrine pancreas [3]. Based on the staging of the surrounding tissue morphology, PDX1 appears slightly later in human development than in mice. Expression is only evident after the notochord and aorta are separate from the dorsal foregut in humans [10,12]. Although PDX1 is most studied for its role in β cells, low-level PDX1 expression has also been reported in mouse acinar tissue. By contrast, PDX1 expression is also observed in human adult duct cells, thus it remains unclear if human PDX1 is regulated in a similar manner [18,19]. Otherwise, PDX1 spatial expression compares with mouse Pdx1 first seen in the pre-pancreatic endoderm around E8.5 [20]. Mouse lineage-tracing studies demonstrated that Pdx1+ cells mark progenitors of all the mature pancreatic cell types including endocrine, acinar, and ductal cells [21]. Similar to mice with targeted disruption of Pdx1, homozygous inactivating mutations of PDX1/IPF1 result in pancreatic agenesis (termed MODY4) [22,23]. Autosomal recessive mutations in the PDX1 locus have also been reported to cause permanent neonatal diabetes, comparable to the Pdx1+/− mice that likewise exhibit a diabetic phenotype [24]. PDX1 levels were compromised in rodent models of T2DM and human T2DM islets, suggesting conservation in adult islet β cells [25].
Pancreas transcription factor 1A (PTF1A)
PTF1A expression is barely detectable by quantitative RT-PCR until midgestation in whole human fetal pancreas, presumably due to its enriched expression at that timepoint in acinar cells. It is better characterized in mice, with broad expression at E9 in dorsal and ventral pancreatic buds that is later restricted to acinar cells [12,26]. Mutations in the PTF1A locus result in autosomal recessive cases of permanent neonatal diabetes that require insulin for survival [27]. This is similar to Ptf1a−/− mice, which die postnatally with impaired pancreatic development [28]. A recent study identified mutations in the human PTF1A enhancer resulting in pancreatic agenesis [29]. These human and mouse mutant phenotypes support an evolutionarily conserved role during early pancreatic development.
GATA binding protein 4 (GATA4)
This transcription factor is expressed during early human pancreatic budding between 4 and 5 weeks of age, but then becomes drastically reduced in pancreatic progenitors, remaining only in mature acinar cells [10]. This pattern is comparable to mice [30]. Although human and mouse GATA4/Gata4 mutations have been associated with congenital heart defects, a pancreatic phenotype has only been documented in the mouse model [2,31]. This suggests compensation in the human pancreas by another GATA transcription factor [2,31].
SOX9
SOX9 is found in PDX1+ cells in early human and mouse pancreas by about 4 weeks and E9, respectively, and is then excluded from mature endocrine cells [2,3,10,32]. Sox9 is necessary for the maintenance of multipotent progenitor populations in mice [3,10,32,33]. The mouse Sox9+/− model phenocopies SOX9 haploinsufficiency in humans, with islet hypoplasia from failed maintenance of endocrine progenitors [33–35].
Homeobox protein NK-6 homolog (NKX6.1)
Human NKX6.1 is expressed in early multipotent pancreatic progenitors after 4 weeks, once SOX17 is excluded from the pancreatic buds [10]. Its expression then becomes restricted to β cells by 14–16 weeks [10,24]. Similarly, early rodent Nkx6.1 expression is broad, then gradually becomes β cell specific [10,34,36]. Nkx6.1 null mice exhibit a severe reduction in β cells, and conditional Nkx6.1 mutants reveal its requirement for specifying endocrine precursors toward a β cell lineage [36–38]. In adult pancreas, NKX6.1 is a key β cell identity factor with severely reduced expression in diabetic and obese db/db mice and human T2DM islets [7,25].
Motor neuron and pancreas homeobox 1 (MNX1)
MNX1, also known as homeobox HB9 (HLXB9), is expressed as early as 7 weeks in the developing pancreas, then its expression is reduced to lower levels by 14–16 weeks into gestation [11,12]. MNX1 transcripts have been identified in the adult human pancreas, although the cell type distribution has yet to be characterized [3,12]. It is unknown whether MNX1 becomes progressively restricted to β cells, as found in mice. Detailed expression analysis found mouse Mnx1 expression in the E9.5 endoderm, and expression was then gradually restricted to the Pax6+ (paired box 6) endocrine population by E15.5, and finally only in adult β cells [2,39]. Recently, a patient presenting with permanent neonatal diabetes was found to harbor a homozygous mutation within the DNA-binding homeodomain of MNX1 [40,41]. Similar to the null mouse model, these patients had no obvious exocrine deficits but exhibited reduced β cell numbers and likely dorsal pancreatic lobe agenesis [2,39,40].
MPCs
Continued expression of the transcription factors FOXA2, PDX1, SOX9, NKX6.1, and GATA4 in developing human pancreatic cells likely demarcates the MPCs that will be further restricted to ductal, endocrine, and exocrine compartments [2]. The MPC population also controls ultimate pancreas size [42]. Most of the factors described above also have distinct functional roles in later differentiated acinar, islet, and ductal populations.
GATA6
Another MPC-expressed transcription factor, GATA6, appears more important during human pancreatic development than in mice, with Gata6+/− mice exhibiting no obvious phenotype [43]. However, de novo heterozygous human GATA6 mutations, often in the DNA-binding domain, cause pancreatic agenesis [44,45]. Also important for rodent β cell generation, Gata6 is expressed in multipotent pancreatic progenitors and Gata6−/− embryos have fewer Pdx1+ cells compared to heterozygous controls [46]. Strikingly, Gata6/Gata4 compound mouse mutants present with a similar pancreatic agenesis to that seen in GATA6 patients [30]. The human fetal temporal GATA6 expression pattern has yet to be determined [2,10].
Endocrine cell specification
After MPCs commit to a pancreatic acinar, ductal, or endocrine fate, a host of transcription factors are required in mice for production of islet endocrine cell lineages, discussed below. There is evidence that these factors are also important in human endocrine commitment.
Transcription factors involved in the production of islet endocrine cell lineages Neurogenin 3 (NGN3)
Coincident with SOX9 loss, endocrine commitment in pancreatic epithelial cells initiates with NGN3 expression, a factor also required for endocrine cell specification in mouse [10,11,47]. NGN3 is seen as early as 8 weeks and becomes more highly expressed at around 11 weeks, then expression subsequently declines to only low levels at 19 weeks [10,12,47,48]. Later induction of human transcription factors including ISL1, NEU-ROD1 (neurogenic differentiation 1), MAFB, NKX2.2, and PAX6 near week 15 indicates that NGN3 expression precedes the expression of these factors that are implicated in late endocrine cell differentiation [12]. Similarly, these islet-enriched factors act downstream of NGN3 in mice [2,47]. A rare NGN3 null mutation resulted in permanent neonatal diabetes with no histologically detectable islets, although the patient maintained low C-peptide levels [49]. Similarly, Ngn3−/− mice develop diabetes and die a few days after birth with a complete lack of endocrine cells [47].
Regulatory factor X 6 (RFX6)
By quantitative real-time PCR, pancreatic RFX6 expression is limited to adult human islet cells, and autosomal recessive mutations at this locus result in neonatal diabetes, with absence of insulin+, glucagon+, and somatostatin+ cells [50,51]. Similarly, Rfx6-deficient mice exhibit impaired formation of all endocrine cell types except for pancreatic polypeptide [50]. Although Rfx6 is expressed more broadly and earlier than Ngn3 during mouse development, Rfx6 expression is not detected in Ngn3−/− mice. Interestingly, human NGN3 mutations result in milder diabetes cases than RFX6 mutations [51]. Thus, RFX6 could act either upstream or downstream of NGN3 in coordinating the production of a subset of islet cell types [50,51].
Paired box gene 4 (PAX4)
Human PAX4 expression is evident by 9 weeks in whole fetal pancreatic mRNA analysis [12]. Although its spatial pattern has yet to be reported in humans, PAX4 is found in mouse endocrine progenitors and later in β cell precursors, as a regulator of β cell commitment [34]. Further, PAX4 (termed MODY9) mutations result in diabetic symptoms resembling those of Pax4+/− mice [52]. Mutant mice also exhibit a severe reduction in β cells and abnormal α cell clustering, similar to Pdx1+/− mice [24,34].
GLIS family zinc finger 3 (GLIS3)
Patients with GLIS3 mutations present with autosomal recessive diabetes and impaired islet cell development [53]. The Glis3-deficient mouse pancreatic phenotype is similar [54]. This is further supported by a study indicating that Glis3 may interact with Hnf6 to regulate Ngn3 expression [55]. The expression pattern has yet to be determined in humans.
MAFB
Unlike in mice, where β cell MafB expression diminishes postnatally, MAFB increases from 7 to 21 weeks then remains in mature α and β cells, in humans [7,12,37]. Sustained MAFB expression may have functional implications in β cell development and identity. Indeed, severe reductions in MAFB levels were found in human T2DM islet α and β cells, suggesting a role in their functional maintenance [25].
Endocrine cell differentiation and maturation
Pancreatic hormone expression first occurs about 8 weeks into human gestation, with the appearance of insulin+ cells, which become more abundant by week 9 when glucagon+ cells also appear [12,56]. In rodents, two waves of endocrine development have been observed. A first wave from about E9.5–12.5 is characterized by insulin and glucagon coexpressing cells, whereas the second wave, from about E12.5 to birth, produces endocrine cells that will populate mature islets [57]. By contrast, human development lacks two waves of endocrine cell formation [2,10]. Several islet-enriched transcription factors have been implicated in mouse and human β cell differentiation. Here we will discuss these factors in their relative order of expression during human development.
Transcription factors involved in β cell differentiation NKX2.2
Another key difference between mice and humans is seen with NKX2.2 expression [10]. Although its expression is only observed later in human α and β cells, first appearing at 8 weeks with later increased expression by 14–16 weeks, Nkx2.2 expression is observed earlier in rodent development around E9.5 [10,11,58]. Only later in development does NKX2.2/Nkx2.2 expression overlap, with rodent Nkx2.2 being restricted to β cells and a subset of α and PP cells [10,11,58]. This implies a more limited role for NKX2.2 in human β cell differentiation.
Insulin gene enhancer protein ISL-1 (ISL1)
ISL1, also called ISLET1, appears to be required for pancreatic development in humans and mice [59]. Isl1 is a pan-endocrine cell marker, with endocrine Isl1 mouse mutants becoming diabetic, exhibiting impaired islet cell maturation and reduced postnatal islet mass expansion [60,61]. A nonsense mutation in a Japanese T2DM patient indicated a role for ISL1 in the maturation of functional human β cells [59]. In humans, its expression has been observed in the fetal pancreas at age 8–10 weeks, and expression then gradually increases from mid-gestation [11,12]. This is similar to the situation in mice, where Isl1 is first expressed broadly in the pancreatic mesenchyme at E9 and is then maintained in the mature hormone+ endocrine cells [60].
NEUROD1
NEUROD1 is expressed at week 15 and is then found in all endocrine cell types of adult islets [10–12]. However, NeuroD1 expression occurs relatively earlier in mouse development – by E10.5 – but is similarly restricted to the endocrine compartment [62,63]. Although rare cases of heterozygous NEUROD1 (termed MODY6) mutations have been reported, the phenotype of homozygous NEU-ROD1 mutations appear similar to the mutant mouse phenotype, causing autosomal recessive neonatal diabetes [64,65]. β cell-specific mutants revealed that NeuroD1 is required for β cell maturation because the β cells formed are immature, with increased glycolytic gene expression, neuropeptide Y overexpression (a hormone whose expression in islets normally decreases after birth), and elevated basal insulin secretion [62].
PAX6
PAX6 is induced by 14–16 weeks in the human pancreas and is then maintained in all adult islet cells [11]. This is similar to the known Pax6 expression pattern in mice [66]. Pax6 null mice die at birth from brain abnormalities, but embryos have reduced islet cell numbers, impaired hormone synthesis, and defective islet morphogenesis, indicating a role in endocrine cell allocation and differentiation [66]. The only study linking human PAX6 to β cell function identified a common single-nucleotide polymorphism that resulted in reduced PAX6 mRNA associated with reduced insulin response and sensitivity [67].
MAFA
Mouse MafA is expressed relatively late in development and is found only in second wave insulin+ cells that will become mature β cells [37]. In adults, MafA is known as a maturation marker, crucial for glucose-responsive β cells through regulation of insulin and Glut2 (also known as Slc2a2, solute carrier family 2 facilitated glucose transporter, member 2) [37]. Similar to the late onset of expression in developing rodent β cells, MAFA is nearly undetectable in embryonic human samples from seven to 21 weeks [12,25,37]. Later, in mice and humans, MAFA is specifically expressed in mature adult β cells [7]. Recently, reductions of MAFA levels were found in db/db mice and in human T2DM islets, potentially a signature of dysfunctional β cells [25].
Applying knowledge of transcriptional control to promote human β cell fates
A collective goal of islet biologists is to apply what has been learned of transcription factor expression and function toward creating renewable and transplantable therapeutic β cells. Several strategies have been developed, including in vitro human embryonic stem cell (ES cell) directed differentiation [68,69] and modulation of existing β cell proliferation [70]. Transcription factors important during β cell development and function can be used in approaches to convert non-β cells into β (or β-like) cells. Several examples of mouse cell conversion have been described, which can guide studies in human cells. Zhou et al. converted mouse acinar cells to β-like cells using adenovirus-delivered Mafa, Pdx1, and Ngn3 [71], whereas α-to-β cell conversion was observed after forced Pdx1 [72] and Pax4 [73] overexpression, or Arx (aristaless related homeobox) inactivation [74] in the developing pancreas. Further, conversion of non-pancreatic cells has been promising, including converting liver cells to insulin+ cells [75–77]. However, it is possible that the cells produced still retain non-β cell phenotypes, an obvious impediment for use in human studies.
Recent studies have demonstrated that the epigenetic landscapes of β and non-β cells are unique, suggesting that transcription factor-mediated cell type conversion is impacted by chromatin modifications. Dhawan and colleagues reported that mouse β cell loss of the Dnmt1 DNA methyltransferase induces derepression of the Arx transcription factor gene and subsequent conversion into α cells [78]. This lends evidence to the plasticity of these cells and the potential for interconversion of endocrine cells for the generation of β cells. Histone acetylation/deacetylation also influences cell type specification. Haumaitre and colleagues treated cultured rat embryonic pancreas rudiments with histone deacetylase inhibitors, finding an increase in the Ngn3 endocrine pool and enhanced β and δ cell lineage allocation [79]. The above studies suggest that plasticity exists between the various endoderm-derived cell populations. Mouse studies appear to serve as proof-of-principle that cells can undergo functional β cell conversion. However, given differences between mouse and human islet development, architecture, and glucose-sensing properties, a next step is to employ available human samples for conversion experiments.
Several recent reports utilized human tissue and cell lines to demonstrate cellular plasticity similar to that observed in mice. Reprogramming non-pancreatic cell types into insulin+ cells was achieved using ectopic PDX1 expression in human liver cells [80,81] and keratinocytes [82]. Pennarossa et al. utilized a DNA methyl-transferase inhibitor to convert skin fibroblasts into cells that expressed pancreatic transcription factors and insulin C-peptide, and could reverse streptozotocin (STZ)-induced diabetes in severe combined immunodeficiency (SCID) mice [83]. These studies indicate that altering the transcription factor expression profile and chromatin modification state of a human cell may enhance conversion to insulin+ cells. However, much like the mouse studies described above, it remains unclear how closely the trans-differentiated cells resemble true β cells.
To this end, many are focusing on examining the level of plasticity between closely related human pancreatic cell types. Recently, human exocrine tissue was directly converted into a ductal phenotype, with lineage-traced exocrine cells expressing ductal markers CK-19, HNF1β, and SOX9 [84]. Similarly, human ductal cells are also able to convert because Ngn3 alone or added with MafA, Pdx1, and Pax6 was able to activate an endocrine program and produce insulin+ cells, although these cells are only β-like and are likely to lack the glucose-sensing and insulin secretion properties of endogenous β cells [85–87]. Perhaps more tantalizing is direct conversion between human α and β phenotypes without addition of exogenous transcription factors. As proof-of-principle for human cell conversion, Spijker et al. used dispersed human donor islets and lineage-tracing methods to illustrate the conversion of β cells to α-like cells [88]. This was shown to be mediated by ARX, an essential regulator of α cell development [88]. Recently, Bramswig et al. established that human α cells possess an epigenetic profile more amenable to conversion than other pancreatic cell types, including β cells [89]. α cells retain bivalently marked histones (i.e., activating H3K4me3 and repressing H3K27me3), particularly at β cell signature genes such as MAFA and PDX1, suggesting the human α cell epigenetic landscape is primed for cellular conversion [89]. Treatment with a histone methyltransferase inhibitor impacted H3K27me3 levels at β cell genes within α cells, allowing partial α-to-β conversion [89]. Consideration of epigenetics during reprogramming or in hES cell differentiation was suggested in another recent study. The authors utilized the Novocell protocol to direct hES cells while monitoring epigenetic and gene expression profiles [68,69,90]. The key findings included the dysfunctional in vitro-produced polyhormonal cells having inappropriately remodeled chromatin, as compared to primary human islets. This dysfunction was linked to a failure to eliminate polycomb group-mediated repression of endocrine-specific genes. These studies collectively suggest that careful modification of transcription factors and epigenetic profiles may allow conversion of pancreatic and non-pancreatic cells into bona fide β cells.
Concluding remarks and future perspectives
Future studies of human β cell transcription factors will not only yield insight into β cell conversion protocols, but will also provide a foundation for development of novel in vitro tools for studying β cell development [2]. Although transcription factor profile manipulation will certainly facilitate efforts toward protocols that convert non-β cells to β cells, it is important to note that insulin expression alone does not yield a functional β cell with the appropriate secretory machinery to maintain glucose homeostasis. Regardless, there is a need for innovative genetic systems that enable deeper analysis of human β cells, as compared to mouse. For example, iPS cells derived from MODY patients have the potential to elucidate protein interactions and expression cascades dysregulated in patients [91]. Functionally immortalized human β cell lines have recently become available, and these will allow numerous in vitro studies of human transcription factor function [92,93]. Although rodent models have been essential to understanding transcription factor roles in pancreatic organogenesis, key differences in human islet expression and function place limits on what can be learned without consideration of the human context (Box 1) [8]. Greater comprehension of the transcriptional regulation that defines a human β cell will benefit from more sensitive genetic tools, better markers for purifying human islet cells, and increased availability of human samples and cell lines [2,94].
Box 1. Outstanding questions.
How similar/dissimilar are mouse and human pancreatic specification, endocrine cell differentiation, and β cell maturation?
Do transcription factors expressed during both mouse and human β cell development have the same activities and target genes?
How closely do converted cells approximate the function of endogenous β cells?
Can tools be developed to better study human transcription factor control over β cell development and function?
What are the causes of the noted differences between mouse and human islet architectures and function?
Acknowledgments
C.S.H. was supported by National Institutes of Health (NIH) grant DK094842; R.S. was supported by NIH grants DK090570, DK050203, and DK089572; E.C. was supported by NIH grant T32GM008554.
Glossary
- Chromatin
the packaged complex of DNA and histone proteins located in the nucleus of a cell
- Endocrine
the system of cells, glands, and tissues that secretes hormones into the bloodstream to regulate physiological activities
- Epigenetics
heritable changes in gene activity not resulting from alterations in DNA sequence; can occur as DNA modifications (e.g., DNA methylation) impacting expression patterns
- Glucagon
a peptide hormone secreted by pancreatic α cells to promote glucose release from glycogen stores in the liver
- Histone acetylation/deacetylation
a modification of histone proteins within chromatin in which acetyl groups are added or removed from the N-terminal tails of lysine residues to alter underlying DNA accessibility by regulatory factors
- Histone bivalency
when histones at gene regulatory domains are marked with both activating (e.g., H3K4me3) and repressive (e.g., H3K27me3) modifications, leading to a poised state
- Insulin
a peptide hormone secreted by pancreatic β cells into the bloodstream that promotes glucose uptake in liver, skeletal muscle, and adipose tissue
- Islets of Langerhans
clusters of endocrine cells within in the pancreas composed of α, β, δ, pancreatic polypeptide (PP), and ε cells
- Maturity onset diabetes of the young (MODY)
a heritable form of diabetes caused by autosomal dominant gene mutations; also known as monogenic diabetes
- Methyltransferase (DNA or histone)
an enzyme that transfers a methyl group onto nucleic acids or amino acids to alter transcriptional activity
- Multipotent pancreatic progenitor cells (MPCs)
embryonic cells with the capacity to develop into the three lineages (endocrine, acinar, and ductal) that comprise the mature pancreas
- Neonatal diabetes
similar to PNDM but this condition is transient, disappearing during infancy
- Pancreatic agenesis
a condition in which the pancreas, or part of the pancreas, fails to develop
- Permanent neonatal diabetes (PNDM)
diabetes that usually appears within the first 6 months of life, and persists throughout the lifespan; can be caused by several gene mutations
- Transcription factor
a protein that recognizes and binds to specific regulatory DNA sequences to activate or repress the transcription of target genes
- Type 1 diabetes (T1DM)
a disease caused by autoimmune destruction of pancreatic β cells, resulting in hyperglycemia and insulin dependence
- Type 2 diabetes (T2DM)
a multifactorial disease characterized by hyperglycemia resulting from insulin resistance. Failed compensation by β cells ultimately results in dysfunction
Footnotes
Note added in proof
During publication of this review Shaw-Smith et al. reported on several patients with GATA4 mutations [95], thus revealing functional roles for GATA4 in human pancreas development. These new observations alter our interpretation of human GATA4 function in the text above.
References
- 1.Muoio DM, Newgard CB. Mechanisms of disease: molecular and metabolic mechanisms of insulin resistance and beta-cell failure in type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:193–205. doi: 10.1038/nrm2327. [DOI] [PubMed] [Google Scholar]
- 2.Cano DA, et al. Transcriptional control of mammalian pancreas organogenesis. Cell Mol Life Sci. 2013 doi: 10.1007/s00018-013-1510-2. http://dx.doi.org/10.1007/s00018-013-1510-2. [DOI] [PMC free article] [PubMed]
- 3.Pan FC, Wright C. Pancreas organogenesis: from bud to plexus to gland. Dev Dyn. 2011;240:530–565. doi: 10.1002/dvdy.22584. [DOI] [PubMed] [Google Scholar]
- 4.Brissova M. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem. 2005;53:1087–1097. doi: 10.1369/jhc.5C6684.2005. [DOI] [PubMed] [Google Scholar]
- 5.Cabrera O, et al. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci USA. 2006;103:2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Melloul D, et al. Regulation of insulin gene transcription. Diabetologia. 2002;45:309–326. doi: 10.1007/s00125-001-0728-y. [DOI] [PubMed] [Google Scholar]
- 7.Dai C, et al. Islet-enriched gene expression and glucose-induced insulin secretion in human and mouse islets. Diabetologia. 2011;55:707–718. doi: 10.1007/s00125-011-2369-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashcroft FM, Rorsman P. Diabetes mellitus and the β cell: the last ten years. Cell. 2012;148:1160–1171. doi: 10.1016/j.cell.2012.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Piper K, et al. Beta cell differentiation during early human pancreas development. J Endocrinol. 2004;181:11–23. doi: 10.1677/joe.0.1810011. [DOI] [PubMed] [Google Scholar]
- 10.Jennings RE, et al. Development of the human pancreas from foregut to endocrine commitment. Diabetes. 2013;62:3514–3522. doi: 10.2337/db12-1479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyttle BM, et al. Transcription factor expression in the developing human fetal endocrine pancreas. Diabetologia. 2008;51:1169–1180. doi: 10.1007/s00125-008-1006-z. [DOI] [PubMed] [Google Scholar]
- 12.Jeon J, et al. Endocrine cell clustering during human pancreas development. J Histochem Cytochem. 2009;57:811–824. doi: 10.1369/jhc.2009.953307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gao N, et al. Dynamic regulation of Pdx1 enhancers by Foxa1 and Foxa2 is essential for pancreas development. Genes Dev. 2008;22:3435–3448. doi: 10.1101/gad.1752608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spence JR, et al. Sox17 regulates organ lineage segregation of ventral foregut progenitor cells. Dev Cell. 2009;17:62–74. doi: 10.1016/j.devcel.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang H, et al. Multiple, temporal-specific roles for HNF6 in pancreatic endocrine and ductal differentiation. Mech Dev. 2009;126:958–973. doi: 10.1016/j.mod.2009.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horikawa Y, et al. Mutation in hepatocyte nuclear factor-1 beta gene (TCF2) associated with MODY. Nat Genet. 1997;17:384–385. doi: 10.1038/ng1297-384. [DOI] [PubMed] [Google Scholar]
- 17.Haumaitre C, et al. Lack of TCF2/vHNF1 in mice leads to pancreas agenesis. Proc Natl Acad Sci USA. 2005;102:1490–1495. doi: 10.1073/pnas.0405776102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heimberg H, et al. Adult human pancreatic duct and islet cells exhibit similarities in expression and differences in phosphorylation and complex formation of the homeodomain protein Ipf-1. Diabetes. 2000;49:571–579. doi: 10.2337/diabetes.49.4.571. [DOI] [PubMed] [Google Scholar]
- 19.Castaing M, et al. Ex vivo analysis of acinar and endocrine cell development in the human embryonic pancreas. Dev Dyn. 2005;234:339–345. doi: 10.1002/dvdy.20547. [DOI] [PubMed] [Google Scholar]
- 20.Ahlgren U, et al. The morphogenesis of the pancreatic mesenchyme is uncoupled from that of the pancreatic epithelium in IPF1/PDX1-deficient mice. Development. 1996;122:1409–1416. doi: 10.1242/dev.122.5.1409. [DOI] [PubMed] [Google Scholar]
- 21.Gu G, et al. Direct evidence for the pancreatic lineage: NGN3+ cells are islet progenitors and are distinct from duct progenitors. Development. 2002;129:2447–2457. doi: 10.1242/dev.129.10.2447. [DOI] [PubMed] [Google Scholar]
- 22.Stoffers DA, et al. Early-onset type-II diabetes mellitus (MODY4) linked to IPF1. Nat Genet. 1997;17:138–139. doi: 10.1038/ng1097-138. [DOI] [PubMed] [Google Scholar]
- 23.Stoffers DA, et al. Pancreatic agenesis attributable to a single nucleotide deletion in the human IPF1 gene coding sequence. Nat Genet. 1997;15:106–110. doi: 10.1038/ng0197-106. [DOI] [PubMed] [Google Scholar]
- 24.Brissova M. Reduced PDX-1 expression impairs islet response to insulin resistance and worsens glucose homeostasis. Am J Physiol Endocrinol Metab. 2004;288:E707–E714. doi: 10.1152/ajpendo.00252.2004. [DOI] [PubMed] [Google Scholar]
- 25.Guo S, et al. Inactivation of specific β cell transcription factors in type 2 diabetes. J Clin Invest. 2013;123:3305–3316. doi: 10.1172/JCI65390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Obata J, et al. p48 subunit of mouse PTF1 binds to RBP-Jkappa/CBF-1, the intracellular mediator of Notch signalling, and is expressed in the neural tube of early stage embryos. Genes Cells. 2001;6:345–360. doi: 10.1046/j.1365-2443.2001.00422.x. [DOI] [PubMed] [Google Scholar]
- 27.Sellick GS, et al. Mutations in PTF1A cause pancreatic and cerebellar agenesis. Nat Genet. 2004;36:1301–1305. doi: 10.1038/ng1475. [DOI] [PubMed] [Google Scholar]
- 28.Krapp A, et al. The bHLH protein PTF1-p48 is essential for the formation of the exocrine and the correct spatial organization of the endocrine pancreas. Genes Dev. 1998;12:3752–3763. doi: 10.1101/gad.12.23.3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weedon MN, et al. Recessive mutations in a distal PTF1A enhancer cause isolated pancreatic agenesis. Nat Genet. 2014;46:61–64. doi: 10.1038/ng.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xuan S, et al. Pancreas-specific deletion of mouse Gata4 and Gata6 causes pancreatic agenesis. J Clin Invest. 2012;122:3516–3528. doi: 10.1172/JCI63352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garg V, et al. GATA4 mutations cause human congenital heart defects and reveal an interaction with TBX5. Nature. 2003;424:438–443. doi: 10.1038/nature01827. [DOI] [PubMed] [Google Scholar]
- 32.McDonald E, et al. SOX9 regulates endocrine cell differentiation during human fetal pancreas development. Int J Biochem Cell Biol. 2012;44:72–83. doi: 10.1016/j.biocel.2011.09.008. [DOI] [PubMed] [Google Scholar]
- 33.Piper K, et al. Novel SOX9 expression during human pancreas development correlates to abnormalities in Campomelic dysplasia. Mech Dev. 2002;116:223–226. doi: 10.1016/s0925-4773(02)00145-4. [DOI] [PubMed] [Google Scholar]
- 34.Sosa-Pineda B, et al. The Pax4 gene is essential for differentiation of insulin-producing beta cells in the mammalian pancreas. Nature. 1997;386:399–402. doi: 10.1038/386399a0. [DOI] [PubMed] [Google Scholar]
- 35.Seymour PA, et al. A dosage-dependent requirement for Sox9 in pancreatic endocrine cell formation. Dev Biol. 2008;323:19–30. doi: 10.1016/j.ydbio.2008.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sander M, et al. Homeobox gene Nkx6.1 lies downstream of Nkx2.2 in the major pathway of beta-cell formation in the pancreas. Development. 2000;127:5533–5540. doi: 10.1242/dev.127.24.5533. [DOI] [PubMed] [Google Scholar]
- 37.Hang Y, Stein R. MafA and MafB activity in pancreatic β cells. Trends Endocrinol Metab. 2011;22:364–373. doi: 10.1016/j.tem.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schaffer AE, et al. Nkx6.1 controls a gene regulatory network required for establishing and maintaining pancreatic beta cell identity. PLoS Genet. 2013;9:e1003274. doi: 10.1371/journal.pgen.1003274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li H, et al. Selective agenesis of the dorsal pancreas in mice lacking homeobox gene Hlxb9. Nat Genet. 1999;23:67–70. doi: 10.1038/12669. [DOI] [PubMed] [Google Scholar]
- 40.Flanagan SE, et al. Analysis of transcription factors key for mouse pancreatic development establishes NKX2-2 and MNX1 mutations as causes of neonatal diabetes in man. Cell Metab. 2014;19:146–154. doi: 10.1016/j.cmet.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bonnefond A, et al. Transcription factor gene MNX1 is a novel cause of permanent neonatal diabetes in a consanguineous family. Diabetes Metab. 2013;39:276–280. doi: 10.1016/j.diabet.2013.02.007. [DOI] [PubMed] [Google Scholar]
- 42.Stanger BZ, et al. Organ size is limited by the number of embryonic progenitor cells in the pancreas but not the liver. Nature. 2007;445:886–891. doi: 10.1038/nature05537. [DOI] [PubMed] [Google Scholar]
- 43.De Franco E, et al. GATA6 mutations cause a broad phenotypic spectrum of diabetes from pancreatic agenesis to adult-onset diabetes without exocrine insufficiency. Diabetes. 2013;62:993–997. doi: 10.2337/db12-0885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bonnefond A, et al. GATA6 inactivating mutations are associated with heart defects and, inconsistently, with pancreatic agenesis and diabetes. Diabetologia. 2012;55:2845–2847. doi: 10.1007/s00125-012-2645-7. [DOI] [PubMed] [Google Scholar]
- 45.Allen HL, et al. GATA6 haploinsufficiency causes pancreatic agenesis in humans. Nat Genet. 2012;44:20–22. doi: 10.1038/ng.1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Watt AJ, et al. Development of the mammalian liver and ventral pancreas is dependent on GATA4. BMC Dev Biol. 2007;7:37. doi: 10.1186/1471-213X-7-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gradwohl G, et al. neurogenin3 is required for the development of the four endocrine cell lineages of the pancreas. Proc Natl Acad Sci USA. 2000;97:1607–1611. doi: 10.1073/pnas.97.4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Capito C, et al. Mouse muscle as an ectopic permissive site for human pancreatic development. Diabetes. 2013;62:3479–3487. doi: 10.2337/db13-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rubio-Cabezas O, et al. Permanent neonatal diabetes and enteric anendocrinosis associated with biallelic mutations in NEUROG3. Diabetes. 2011;60:1349–1353. doi: 10.2337/db10-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Smith SB, et al. Rfx6 directs islet formation and insulin production in mice and humans. Nature. 2010;463:775–780. doi: 10.1038/nature08748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taleb N, Polychronakos C. RFX6 is needed for the development and maintenance of the β-cell phenotype. Islets. 2011;3:291–293. doi: 10.4161/isl.3.5.15944. [DOI] [PubMed] [Google Scholar]
- 52.Plengvidhya N, et al. PAX4 mutations in Thais with maturity onset diabetes of the young. J Clin Endocrinol Metab. 2007;92:2821–2826. doi: 10.1210/jc.2006-1927. [DOI] [PubMed] [Google Scholar]
- 53.Senée V, et al. Mutations in GLIS3 are responsible for a rare syndrome with neonatal diabetes mellitus and congenital hypothyroidism. Nat Genet. 2006;38:682–687. doi: 10.1038/ng1802. [DOI] [PubMed] [Google Scholar]
- 54.Watanabe N, et al. A murine model of neonatal diabetes mellitus in Glis3-deficient mice. FEBS Lett. 2009;583:2108–2113. doi: 10.1016/j.febslet.2009.05.039. [DOI] [PubMed] [Google Scholar]
- 55.Kim YS, et al. Glis3 regulates neurogenin 3 expression in pancreatic beta-cells and interacts with its activator, Hnf6. Mol Cells. 2012;34:193–200. doi: 10.1007/s10059-012-0109-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Polak M, et al. Early pattern of differentiation in the human pancreas. Diabetes. 2000;49:225–232. doi: 10.2337/diabetes.49.2.225. [DOI] [PubMed] [Google Scholar]
- 57.Herrera PL. Adult insulin- and glucagon-producing cells differentiate from two independent cell lineages. Development. 2000;127:2317–2322. doi: 10.1242/dev.127.11.2317. [DOI] [PubMed] [Google Scholar]
- 58.Sussel L, et al. Mice lacking the homeodomain transcription factor Nkx2.2 have diabetes due to arrested differentiation of pancreatic beta cells. Development. 1998;125:2213–2221. doi: 10.1242/dev.125.12.2213. [DOI] [PubMed] [Google Scholar]
- 59.Shimomura H, et al. Nonsense mutation of islet-1 gene (Q310X) found in a type 2 diabetic patient with a strong family history. Diabetes. 2000;49:1597–1600. doi: 10.2337/diabetes.49.9.1597. [DOI] [PubMed] [Google Scholar]
- 60.Ahlgren U, et al. Independent requirement for ISL1 in formation of pancreatic mesenchyme and islet cells. Nature. 1997;385:257–260. doi: 10.1038/385257a0. [DOI] [PubMed] [Google Scholar]
- 61.Du A, et al. Islet-1 is required for the maturation, proliferation, and survival of the endocrine pancreas. Diabetes. 2009;58:2059–2069. doi: 10.2337/db08-0987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gu C, et al. Pancreatic beta cells require NeuroD to achieve and maintain functional maturity. Cell Metab. 2010;11:298–310. doi: 10.1016/j.cmet.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Naya FJ, et al. Tissue-specific regulation of the insulin gene by a novel basic helix-loop-helix transcription factor. Genes Dev. 1995;9:1009–1019. doi: 10.1101/gad.9.8.1009. [DOI] [PubMed] [Google Scholar]
- 64.Malecki MT, et al. Mutations in NEUROD1 are associated with the development of type 2 diabetes mellitus. Nat Genet. 1999;23:323–328. doi: 10.1038/15500. [DOI] [PubMed] [Google Scholar]
- 65.Rubio-Cabezas O, et al. Homozygous mutations in NEUROD1 are responsible for a novel syndrome of permanent neonatal diabetes and neurological abnormalities. Diabetes. 2010;59:2326–2331. doi: 10.2337/db10-0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sander M, et al. Genetic analysis reveals that PAX6 is required for normal transcription of pancreatic hormone genes and islet development. Genes Dev. 1997;11:1662–1673. doi: 10.1101/gad.11.13.1662. [DOI] [PubMed] [Google Scholar]
- 67.Ahlqvist E, et al. A common variant upstream of the PAX6 gene influences islet function in man. Diabetologia. 2011;55:94–104. doi: 10.1007/s00125-011-2300-8. [DOI] [PubMed] [Google Scholar]
- 68.D’Amour KA, et al. Production of pancreatic hormone-expressing endocrine cells from human embryonic stem cells. Nat Biotechnol. 2006;24:1392–1401. doi: 10.1038/nbt1259. [DOI] [PubMed] [Google Scholar]
- 69.Kroon E, et al. Pancreatic endoderm derived from human embryonic stem cells generates glucose-responsive insulin-secreting cells in vivo. Nat Biotechnol. 2008;26:443–452. doi: 10.1038/nbt1393. [DOI] [PubMed] [Google Scholar]
- 70.Ackermann AM, Gannon M. Molecular regulation of pancreatic beta-cell mass development, maintenance, and expansion. J Mol Endocrinol. 2007;38:193–206. doi: 10.1677/JME-06-0053. [DOI] [PubMed] [Google Scholar]
- 71.Zhou Q, et al. In vivo reprogramming of adult pancreatic exocrine cells to β-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang YP, et al. Context-specific α-to-β-cell reprogramming by forced Pdx1 expression. Genes Dev. 2011;25:1680–1685. doi: 10.1101/gad.16875711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Collombat P, et al. The ectopic expression of Pax4 in the mouse pancreas converts progenitor cells into α and subsequently β cells. Cell. 2009;138:449–462. doi: 10.1016/j.cell.2009.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Courtney M, et al. The inactivation of Arx in pancreatic α-cells triggers their neogenesis and conversion into functional β-like Cells. PLoS Genet. 2013;9:e1003934. doi: 10.1371/journal.pgen.1003934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kojima H, et al. NeuroD-betacellulin gene therapy induces islet neogenesis in the liver and reverses diabetes in mice. Nat Med. 2003;9:596–603. doi: 10.1038/nm867. [DOI] [PubMed] [Google Scholar]
- 76.Ber I. Functional, persistent, and extended liver to pancreas transdifferentiation. J Biol Chem. 2003;278:31950–31957. doi: 10.1074/jbc.M303127200. [DOI] [PubMed] [Google Scholar]
- 77.Banga A, et al. In vivo reprogramming of Sox9+ cells in the liver to insulin-secreting ducts. Proc Natl Acad Sci USA. 2012;109:15336–15341. doi: 10.1073/pnas.1201701109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dhawan S, et al. Pancreatic β cell identity is maintained by DNA methylation-mediated repression of Arx. Dev Cell. 2011;20:419–429. doi: 10.1016/j.devcel.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haumaitre C, et al. Histone deacetylase inhibitors modify pancreatic cell fate determination and amplify endocrine progenitors. Mol Cell Biol. 2008;28:6373–6383. doi: 10.1128/MCB.00413-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zalzman M, et al. Reversal of hyperglycemia in mice by using human expandable insulin-producing cells differentiated from fetal liver progenitor cells. Proc Natl Acad Sci USA. 2003;100:7253–7258. doi: 10.1073/pnas.1136854100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zalzman M, et al. Differentiation of human liver-derived, insulin-producing cells toward the beta-cell phenotype. Diabetes. 2005;54:2568–2575. doi: 10.2337/diabetes.54.9.2568. [DOI] [PubMed] [Google Scholar]
- 82.Mauda-Havakuk M, et al. Ectopic PDX-1 expression directly reprograms human keratinocytes along pancreatic insulin-producing cells fate. PLoS ONE. 2011;6:e26298. doi: 10.1371/journal.pone.0026298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pennarossa G, et al. Brief demethylation step allows the conversion of adult human skin fibroblasts into insulin-secreting cells. Proc Natl Acad Sci USA. 2013;110:8948–8953. doi: 10.1073/pnas.1220637110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Houbracken I, et al. Lineage tracing evidence for transdifferentiation of acinar to duct cells and plasticity of human pancreas. Gastroenterology. 2011;141:731–741. doi: 10.1053/j.gastro.2011.04.050. [DOI] [PubMed] [Google Scholar]
- 85.Heremans Y. Recapitulation of embryonic neuroendocrine differentiation in adult human pancreatic duct cells expressing neurogenin 3. J Cell Biol. 2002;159:303–312. doi: 10.1083/jcb.200203074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Swales N, et al. Plasticity of adult human pancreatic duct cells by neurogenin3-mediated reprogramming. PLoS ONE. 2012;7:e37055. doi: 10.1371/journal.pone.0037055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lee J, et al. Expansion and conversion of human pancreatic ductal cells into insulin-secreting endocrine cells. Elife. 2013;2:e00940. doi: 10.7554/eLife.00940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Spijker HS, et al. Conversion of mature human β-cells into glucagon-producing α-cells. Diabetes. 2013;62:2471–2480. doi: 10.2337/db12-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bramswig NC, et al. Epigenomic plasticity enables human pancreatic α to β cell reprogramming. J Clin Invest. 2013;123:1275–1284. doi: 10.1172/JCI66514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xie R, et al. Dynamic chromatin remodeling mediated by polycomb proteins orchestrates pancreatic differentiation of human embryonic stem cells. Cell Stem Cell. 2013;12:224–237. doi: 10.1016/j.stem.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Teo AKK, et al. Derivation of human Induced pluripotent stem cells from patients with maturity onset diabetes of the young. J Biol Chem. 2013;288:5353–5356. doi: 10.1074/jbc.C112.428979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.McCluskey JT, et al. Development and functional characterization of insulin-releasing human pancreatic beta cell lines produced by electrofusion. J Biol Chem. 2011;286:21982–21992. doi: 10.1074/jbc.M111.226795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ravassard P, et al. A genetically engineered human pancreatic β cell line exhibiting glucose-inducible insulin secretion. J Clin Invest. 2011;121:3589–3597. doi: 10.1172/JCI58447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pagliuca FW, Melton DA. How to make a functional β-cell. Development. 2013;140:2472–2483. doi: 10.1242/dev.093187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shaw-Smith C, et al. GATA4 mutations are a cause of neonatal and childhood-onset diabetes. Diabetes. 2014 doi: 10.2337/db14-0061. published ahead of print April 2, 2014. http://dx.doi.org/10.2337/db14-0061. [DOI] [PMC free article] [PubMed]