Abstract
The brain, in particular the hypothalamus, plays a role in regulating glucose homeostasis; however, it remains unclear if the brain is causally involved in diabetic development. Here, we identified that hypothalamic TGF-β is excessive under conditions of not only obesity but aging, which are two general etiological factors of diabetes. Pharmacological and genetic approaches consistently revealed that brain TGF-β excess caused hyperglycemia and glucose intolerance in a body weight-independent manner. Cell-specific genetic models demonstrated that astrocytes are responsible for brain TGF-β excess, and POMC neurons are crucial for the pro-diabetic effect of TGF-β excess. Mechanistically, TGF-β excess induced hypothalamic RNA stress response to accelerate IκBα mRNA decay, leading to an atypical, mRNA metabolism-driven hypothalamic NF-κB activation which links obesity as well as aging to hypothalamic inflammation. In conclusion, brain TGF-β excess and induction of RNA stress response and hypothalamic inflammation are important for the pro-diabetic effects of obesity or aging.
Keywords: Transforming growth factor, brain, hypothalamus, RNA stress granule, IκBα, NF-κB, inflammation, glucose tolerance, insulin resistance, diabetes, obesity, aging
Introduction
Type-2 diabetes (T2D), one of most prevalent chronic diseases in developed societies, is initiated by the induction of glucose intolerance, a pre-diabetic state of hyperglycemia that is frequently caused by insulin resistance in peripheral tissues such as liver and muscles. Over decades, many research activities have been focusing on peripheral tissues in order to depict the mechanisms of glucose intolerance and insulin resistance, and indeed, multiple models of molecular mechanisms were elucidated1-4. Despite these important progresses, there is still a critical lack of successful solutions for stopping T2D epidemic, perhaps implicating that additional mechanisms remain to be unveiled. Interestingly, recent research advance in neuroendocrinology has increasingly suggested that the central nervous system (CNS), in particular the comprised hypothalamus, has explicit impacts on glucose homeostasis5-9, and these effects can be dissociable from the role of the hypothalamus in regulating body weight – which has been extensively studied over the past decades10,11. However, it remains unexplored if the brain could casually translate certain pro-diabetic etiology, such as obesity and aging, into the development of T2D. Of note, hypothalamic inflammation was recently demonstrated to occur in not only obesity12-22 but also aging23-25. In general, hypothalamic inflammation in obesity or aging is attributed to an atypical format of pro-inflammatory NF-κB activation12-14,18-20,23-27; yet, the causes and characteristics of this atypical inflammation were poorly defined. Here in this work, we found that the brain can directly induce pre-diabetic glucose disorder through the local, excessive effect of transforming growth factor-β (TGF-β), a cytokine which is often overproduced during inflammation and has mixed biological functions28. Mechanistically, brain TGF-β excess induces hypothalamic RNA stress granules to enhance IκBα mRNA decay which activates hypothalamic NF-κB atypically, and thus mediates a hypothalamic inflammatory basis in co-linking obesity and aging to T2D development.
Results
Hypothalamic TGF-β excess in pro-T2D etiological conditions
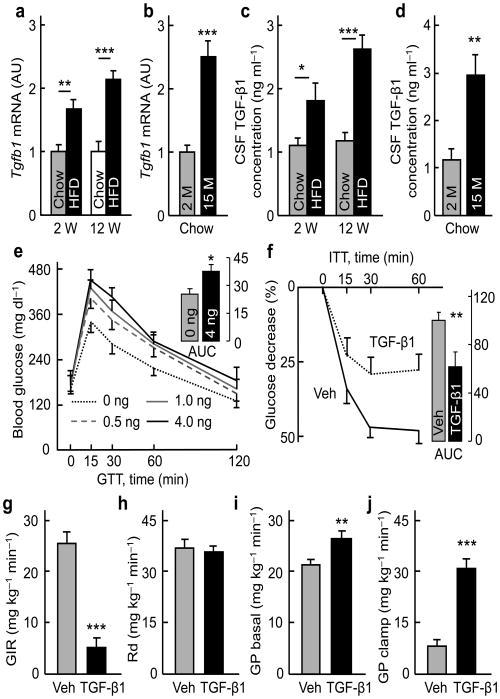
In etiology, obesity and aging are known as two important physiological conditions that lead to T2D development. In recent research12-27, it has been demonstrated that obesity and aging are both associated with hypothalamic inflammation, which is induced by a mild, chronic activation of inflammatory NF-κB signaling. In this study, we comparatively analyzed HFD-fed vs. chow-fed mice, middle-aged vs. young mice, and caloric restriction (CR) vs. ad libitum-fed mice for the hypothalamic expression levels of genes which are closely associated with inflammation but are not strongly pro-inflammatory. With interest, we observed that hypothalamic TGF-β1 levels were changed across these conditions. As shown in supplementary Table 1, Tgfb1 mRNA levels increased in HFD-fed mice as well as aged mice compared to their controls, and these increases were prevented by short-term CR, an approach which exerts effects in counteracting against not only obesity but aging. We further examined additional time-course points of HFD feeding or aging, and found that short-term HFD feeding or early-stage aging was both sufficient to increase hypothalamic Tgfb1 mRNA levels (Fig. 1a, b). These mRNA changes indeed led to increased protein levels, as TGF-β contents in the cerebrospinal fluid of HFD-fed mice and aged mice were both higher than matched controls (Fig. 1c, d). In addition to TGF-β1, which is the predominant and most important TGF-β isoform29, we analyzed TGF-β2 and 3, and found that the hypothalamic mRNA levels of these two isoforms also increased in HFD-fed mice or aged mice (supplementary Fig. 1a – d). Suggested by this information, we decided to study if brain TGF-β excess might have effects on metabolic physiology in mouse models.
Figure 1. Brain TGF-β1 excess induces systemic glucose disorder.
Male C57BL/6 mice fed on a HFD vs. chow for indicated weeks (W) (a, c), and chow-fed C57BL/6 mice at the ages of indicated months (M) (b, d) were analyzed for Tgfb1 mRNA in the hypothalamus (a, b) or TGF-β1 concentrations in the CSF (c, d). C57BL/6 mice were injected with vehicle (Veh) vs. TGF-β1 at the indicated doses (e) or 4 ng (f – j) and examined with GTT (e), ITT (f) or insulin clamp (g – j). Inserted bars (e, f) show the area under curve (AUC) of GTT (unit: mg dl−1 ×120 min, ×103) and ITT (% of control). Glucose infusion rate (GIR) (g), rate of glucose disposal (Rd) (h), and hepatic glucose production (GP) (i – j) in the clamp experiment were determined. * P < 0.05, ** P < 0.01, *** P < 0.001; n = 4 (a – d), 7 – 9 (e, f), and 5 (g – j) mice per group. Error bars reflect mean ± SEM.
Brain TGF-β excess impairs glucose tolerance
To study the metabolic effects of brain TGF-β excess, we first used a pharmacological approach by which TGF-β was delivered into the hypothalamic third ventricle of normal C57BL/6 mice via pre-implanted cannula. To optimize the dosage, we injected different doses of TGF-β1 (0, 0.5, 1.0, and 4.0 ng), and measured TGF-β1 concentrations in the CSF at various time points post-injection. Data showed that the peak increase of CSF TGF-β1 concentration was seen at 15 min post-injection in a dose-dependent manner, and at 60 min post-injection, TGF-β1 in the CSF declined to the concentrations which were roughly 2-fold higher than the basal concentration (supplementary Fig. 2a). None of these doses significantly increased the blood TGF-β1 concentration (supplementary Fig. 2b). Based on this condition, we gave overnight-fasted mice intra-third ventricle injections of TGF-β1 in these doses, one injection at night after food was removed, and the second injection in the following morning at 4 hours prior to glucose tolerance test (GTT). Data revealed that TGF-β1 treatment at each dose led to glucose intolerance, and the effect from 4 ng TGF-β was slightly strongest (Fig. 1e). Following this observation, we further studied if impaired glucose tolerance in these mice was a result of insulin resistance, and through insulin tolerance test (ITT), we obtained data showing that the sensitivity of insulin in lowing blood glucose was significantly dampened in TGF-β1-injected mice (Fig. 1f). In contrast to these effects on blood glucose, the same protocol of TGF-β1 treatment did not affect food intake or body weight for 24 hours after injection (supplementary Fig. 2c, d). In sum, a pharmacological induction of brain TGF-β excess can acutely cause pre-diabetic changes including glucose intolerance and insulin resistance.
Brain TGF-β excess increases hepatic glucose production
In physiology, blood glucose levels are balanced by glucose uptake in metabolic tissues (such as muscles) and glucose production in the liver. We employed hyperinsulinemic-euglycemic clamp to analyze if any of these metabolic processes was relevant to the pro-diabetic effect of brain TGF-β excess. In the experiment, normal C57BL/6 mice were pre-implanted with a catheter into the jugular vein and also a cannula into the hypothalamic third ventricle. Following surgical recovery, overnight-fasted mice were injected with TGF-β1 (4 ng) using the same protocol as described in Fig. 1e, and were subjected to the clamp procedure. During the clamp period, blood glucose concentrations were maintained at approximate 120 – 130 mg/dl, with steady insulin infusion (4 mU kg−1 min−1) together with various rates of 20% glucose infusion to maintain euglycemia (supplementary Fig. 3a). Glucose infusion rates in TGF-β1-injected mice were significantly lower than vehicle-injected mice since the first 20 min of clamp (supplementary Fig. 3a). While the basal blood insulin concentrations in TGF-β-injected mice were higher which indicated insulin resistance, constant insulin infusion in the clamp elevated the blood insulin concentrations of these mice and controls to similar levels (supplementary Fig. 3b). The calculated data revealed that as a response to similarly increased insulin concentrations, glucose infusion rate in TGF-β1-injected mice was on average 80% lower than vehicle-injected mice (Fig. 1g). This change was consistent with the data in GTT, both showing that these mice were glucose intolerant (Fig. 1e). The rates of glucose disposal were, however, similar between TGF-β1 and vehicle-injected mice (Fig. 1h), suggesting that tissue glucose uptake was not significantly altered by brain TGF-β. In contrast, even under the basal condition, hepatic glucose production in TGF-β1-treated mice was higher compared to control mice (Fig. 1i), and during clamp, while insulin infusion suppressed hepatic glucose production in control mice by ∼75%, it failed to do so in TGF-β1-treated mice (Fig. 1j). Blood glucagon concentrations were similar between TGF-β1-treated mice and controls (supplementary Fig. 3c). Therefore, the brain-liver axis mediates the pro-diabetic effect of brain TGF-β excess.
Genetic model of astrocytic TGF-β excess is pro-diabetic
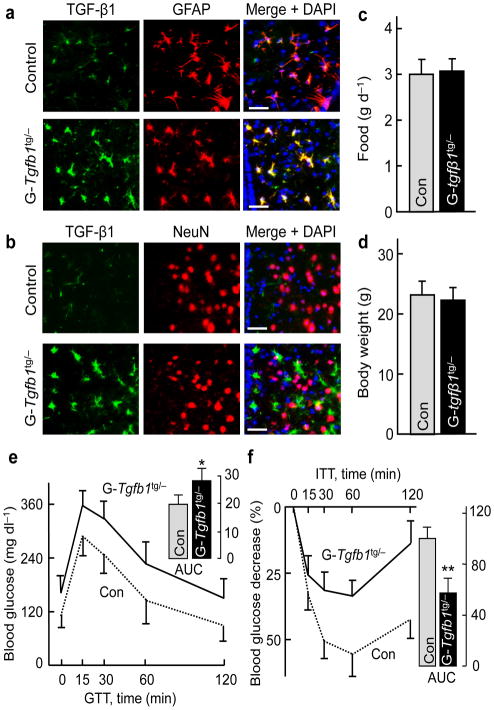
Following our pharmacological studies, we developed a genetic mouse model with brain TGF-β1 excess, using transgenic mouse line containing CMV-flox-stop-flox-Tgfb1 in the genome30. As established30, the floxed fragment in this transgenic line prevents expression of transgenic TGF-β1, while introduction of Cre leads to the removal of the floxed fragment and therefore TGF-β1 overexpression in Cre-positive cells. Because we detected TGF-β1 mostly in astrocytes (rather than in neurons) in C57BL/6 mice, we generated astrocyte-specific TGF-β1 transgenic mice by breeding CMV-flox-stop-flox-Tgfb1 with astrocyte-specific (GFAP-Cre) mice, and obtained the compound mice termed GFAP-Tgfb1tg/− mice and littermate control Tgfb1tg/− mice. Hypothalamic immunostaining images demonstrated that while TGF-β1 was detected in the astrocytes of control mice, the expression levels were specifically increased in the astrocytes of GFAP-Tgfb1tg/− mice (Fig. 2a, b). We maintained GFAP-Tgfb1tg/− and littermate control mice under chow feeding, and confirmed that they had normal development as well as normal food intake and body weight (Fig. 2c, d). In contrast, compared to the normal glucose profile in control mice, chow-fed GFAP-Tgfb1tg/− mice were glucose intolerant (Fig. 2e), and even fasting blood glucose levels of these mice tended to be higher. ITT revealed that GFAP-Tgfb1tg/− mice were severely insulin intolerant (Fig. 2f). Taken together, GFAP-Tgfb1tg/− mice developed glucose intolerance and insulin resistance independently of body weight change.
Figure 2. Astrocyte-specific TGF-β1 transgenic expression leads to glucose disorder.
Co-immunostaining of TGF-β1 with GFAP (a) or NeuN (b) of hypothalamic sections generated from male GFAP-Tgfb1tg/− mice (G-Tgfb1tg/−) and littermate controls (Con). Images show a sub-area in the MBH, and nuclear staining by DAPI revealed cells in sections. Scale bar = 50 μm. Food intake (c), body weight (d), GTT (e) and ITT (f) were determined in chow-fed G-Tgfb1tg/− and littermate Con. Inserted bar graphs show the area under curve (AUC) values of GTT (unit: mg dl−1 ×120 min, × 103) and ITT (% of Con). * P < 0.05, ** P < 0.01; n = 7 – 8 mice per group. Error bars reflect mean ± SEM.
Genetic inhibition of astrocytic TGF-β is anti-diabetic
In parallel with the TGF-β1 gain-of-function model, we studied how an inhibition of brain TGF-β could affect blood glucose levels in physiology or disease. Because astrocytes are important for brain TGF-β excess and the consequent pro-diabetic outcome (Fig. 2), we continued to target astrocytes, and to do so, we bred Tgfb1lox/lox mice with GFAP-Cre mice, resulting in astrocyte-specific Tgfb1 knockout (GFAP-Tgfb1lox/lox) mice and littermate control Tgfb1lox/lox mice. When maintained under chow feeding, we found that GFAP-Tgfb1lox/lox mice and control mice were comparable in terms of development, food intake, body weight, glucose tolerance and insulin tolerance (supplementary Fig. 4a – d). In the meanwhile, we employed HFD feeding to induce glucose and insulin intolerance and test how these disorders were affected by astrocytic TGF-β inhibition. To better appreciate a primary, obesity-dissociable pro-diabetic mechanism of brain TGF-β, we used HFD feeding for a relative short duration (3 weeks), because while this dietary treatment is sufficient to induce glucose and insulin intolerance, it helpfully addressed if a pro-diabetic brain mechanism could occur in early-stage rather than late-stage obesity development in which complex peripheral mechanisms are pronounced. Following 3-week HFD feeding, we confirmed that control mice developed impairments in glucose and insulin resistance; in contrast, GFAP-Tgfb1lox/lox mice were found resistant to both of these changes (Fig. 3a, b). Under this 3-week HFD regime, food intake and body weight levels in GFAP- Tgfb1lox/lox mice and control mice were, however, still comparable (supplementary Fig. 4e, f), although we did not study if obesity development via long-term HFD feeding could be affected in this genetic model. Hence, supported by astrocyte-specific TGF-β1 loss-of-function as well as gain-of-function models, astrocytes are conclusively important for the pro-diabetic effect of brain TGF-β excess.
Figure 3. Cell-specific TGF-β1 inhibition reduces diet-induced glucose disorder.
Adult male GFAP-Tgfb1lox/lox mice (G-Tgfb1l/l; a, b), POMC-Tgfbr2lox/lox mice (P-Tgfbr2l/l; c, d) and matched littermate controls (Con) were fed on a HFD for 3 weeks and examined for GTT (a, c), ITT (b, d). * P < 0.05, n = 6 – 8 mice per group. Error bars reflect mean ± SEM.
POMC neurons direct the pro-T2D effect of TGF-β excess
We subsequently studied if hypothalamic neurons are critical for the pro-diabetic effects of brain TGF-β excess. Indeed, research has revealed that TGF-β signaling increased in the hypothalamus of aged mice, and TGF-β was further shown to inhibit pro-opiomelanorcortin (POMC) peptide in the hypothalamus32,33. We also found that Pomc mRNA levels in the hypothalamus of TGF-β1-injected mice were lower compared to the controls (supplementary Fig. 5a). In this background, we studied if POMC neurons could be crucial for the pro-diabetic effect of brain TGF-β excess. To do so, we targeted TGF-β receptor-2 (TGFβR2), given that TGFβR2 is required for TGF-β signaling, and experimentally TGFβR2 knockout has been shown to inhibit TGF-β signaling32. To carry out this study, we generated a genetic mouse model with Tgfbr2 knockout specifically in POMC neurons by crossing Tgfbr2lox/lox mice with POMC-Cre mice. As a result, we obtained compound offspring POMC-Tgfbr2lox/lox mice and littermate control Tgfbr2lox/lox mice. These mice were maintained on normal chow feeding or 3-week HFD feeding. Data showed that chow-fed POMC-Tgfbr2lox/lox mice and controls demonstrated normal levels in food intake, body weight and glucose and insulin tolerance (supplementary Fig. 5b – e). On the other hand, under the condition of 3-week HFD feeding, while food intake and body weight of these mice were still comparable (supplementary Fig. 5f, g), HFD-induced glucose and insulin intolerance were both markedly lessened in POMC-Tgfbr2lox/lox mice (Fig. 3c, d). Taken together, the action of TGF-β in POMC neurons is important for the pro-diabetic effect of brain TGF-β excess. We also point out that POMC-Cre can target other types of neurons during the developmental stage34, thus, the pro-diabetic mechanism of TGF-β may involve additional hypothalamic or brain neurons.
Induction of hypothalamic NF-κB activation by TGF-β
TGF-β is often appreciated for anti-inflammatory feature in immune response, but, depending on physiological context and particularly in pathological conditions, TGF-β can be inflammatory28 – although it is atypical and many details are still unclear. Here, because NF-κB has been known to atypically mediate hypothalamic inflammation in obesity or aging12-14,18-20,23-27, we analyzed if NF-κB signaling components were different in the hypothalamus of mice with third-ventricle injection of TGF-β vs. vehicle. Data revealed that while many of these components had similar protein levels between two groups, TGF-β1 treatment led to a significant reduction in IκBα protein levels (Fig. 4a). Because IκBα is the canonical and specific inhibitor of NF-κB, this result suggested that hypothalamic NF-κB was activated in TGF-β1-treated mice, and our subsequent experiments confirmed this perdition. First, since IκBα loss is the specific step that immediately liberates cytoplasmic NF-κB for nuclear translocation, and NF-κB subunit RelA in the nucleus undergoes phosphorylation, we measured RelA phosphorylation in mice injected with TGF-β1 or vehicle. The results revealed that TGF-β1 injection significantly increased hypothalamic RelA phosphorylation (Fig. 4a). Second, we evaluated if TGF-β1-triggered NF-κB activation could be involved in HFD-induced hypothalamic inflammation. By employing heterogeneous Tgfb1 knockout (Tgfb1+/−) mice, we examined if haplodeficiency of Tgfb1 in this mouse model could affect the induction of hypothalamic inflammation by HFD feeding. To do so, we subjected adult Tgfb1+/− mice and littermate WT controls to HFD feeding for three weeks, and chow feeding was included to provide as dietary control. Indeed, hypothalamic Tgfb1 mRNA in chow-fed Tgfb1+/− mice dropped by ∼50% compared to chow-fed WT (Fig. 4b), which was consistent with the literature35. Under 3-week HFD feeding, hypothalamic Tgfb1 mRNA increased significantly in WT mice, but to a much lesser extent in HFD-fed Tgfb1+/− mice (Fig. 4b). Using these hypothalamic samples, we examined mRNA levels of a list of inflammation-related molecules including TNFα, IL-6, SOCS3, TLR4, PTP1B, PKCλ and PKCι. Results demonstrated that 3-week HFD feeding increased hypothalamic mRNA levels of these genes in WT mice but barely in Tgfb1+/− knockout mice (Fig. 4b). Therefore, brain TGF-β excess plays a role in inducing diet-induced hypothalamic inflammation.
Figure 4. Effect of TGF-β1 excess on hypothalamic inflammation.
(a) Male C57BL/6 mice were injected with TGF-β1 vs. vehicle (Veh), and hypothalami were collected for Western blots. Western blot data represent 4 mice per group. (b) Hypothalami of male Tgfb1+/− and littermate WT mice were collected and analyzed for mRNA levels of indicated genes. (c, d) Male Tlr4−/− mice and littermate WT were injected with TGF-β1 vs. vehicle, and subjected to GTT (e) or ITT (f). * P < 0.05, ** P < 0.01, ns, non-significant; n = 4 (b) and 8 – 10 (c – f) mice per group. Error bars reflect mean ± SEM.
Kinase-independent, hypothalamic NF-κB activation by TGF-β
While TGF-β1 clearly led to activation of hypothalamic NF-κB, we noted that it did not change the phosphorylated levels of IκBα when normalized by IκBα protein levels (Fig. 4a). Thus, while kinase (e.g., IKK)-induced IκBα phosphorylation is a crucial, rapid signaling reaction in classical inflammation, this process is not primarily critical in TGF-β1-induced hypothalamic inflammation. We also examined TAK1, a kinase which can mediate TGF-β-induced NF-κB activation in some immune cells, but data revealed that TGF-β1 did not lead to hypothalamic TAK1 phosphorylation (Fig. 4a). All these observations suggest that TGF-β1 modulates IκBα levels in a manner which is independent of upstream kinase signaling, leading to atypical activation of hypothalamic NF-κB. To further assess this point, we asked if the effects of brain TGF-β excess could be impaired by ablation of a signaling component, such as toll-like receptor-4 (TLR4) or myeloid differentiation primary response gene 88 (MyD88), because they employ kinase signaling to induce IκBα phosphorylation and thus NF-κB activation. Using a genetic model, Tlr4 knockout (Tlr4-/-) mice, we delivered TGF-β1 or vehicle into the hypothalamic third ventricle of these mice and littermate WT mice via pre-implanted cannula. Vehicle-injected Tlr4-/- mice and WT mice were both normal in glucose and insulin tolerance tests (Fig. 4c, d). Of note, TGF-β1 treatment led to similar extents of glucose intolerance and insulin resistance in Tlr4-/- mice and WT mice (Fig. 4c, d). Also, using Myd88 knockout mice, we observed that the lack of Myd88 did not lead to a significant reduction in glucose or insulin intolerance following hypothalamic third ventricle TGF-β1 delivery (data not shown). Altogether, brain TGF-β excess may use a mechanism that directly targets IκBα rather than upstream kinase signaling to activate hypothalamic NF-κB.
Induction of hypothalamic RNA SGs/PBs by TGF-β or HFD
In exploring how TGF-β could activate hypothalamic NF-κB without requiring pro-inflammatory kinase signaling, our attention was directed to a possible role from mRNA regulation. In coping with inflammatory stress, eukaryotic cells can develop a process known as RNA stress response which is characterized by RNA stress granules (SGs) and processing bodies (PBs)36-38. In this reaction, messenger ribonuclear protein (mRNP) by products exit from polysomes and form RNA SGs at discrete cytoplasmic foci. RNA SGs primarily consist of poly(A)+ mRNAs-containing 48S pre-initiation complexes, small ribosomal subunits, mRNA decay factor tristetraprolin (TTP), translation initiation factors such as eukaryotic translation initiation factor-4E (eIF4E), eIF4G, eIF4A, eIF4B, poly(A)-binding protein (PABP), and RNA helicases36-38. RNA stress response can lead to the export of mRNPs into the PBs, a complex which harbors an array of mRNA decay machineries that act to dispose mRNAs from SGs or polysomes36-38. PBs contain nontranslating mRNAs, translation repressors, mRNA decay machineries (including 5′–3′ mRNA decay system, nonsense-mediated decay pathway, and RNA-induced silencing complex), and mRNA decay factors such as TTP, eIF4E, DEAD box RNA helicase family member p54/RCK, cAMP response element-binding transcription factor (CPEB), B-related factor 1/RNA polymerase III transcription initiatin factor IIIB subunit (BRF1), eukaryotic translation initiation factor 4E transporter (4-ET), and RNA-binding protein Smaug36-38. In this context, we analyzed the expression levels of these RNA SGs/PBs genes in our mouse models, and found that many of them were increased in the hypothalamus of C57BL/6 mice with 3-month HFD feeding (supplementary Fig. 6a). Notably, hypothalamic increases of these genes were similarly induced by an injection of TGF-β1 into the third ventricle of normal mice (Fig. 5a). In line with these observations, we examined if the morphology of RNA SGs could be detected in the hypothalamus of these mice. We performed immunostaining of HuR, a molecular component of RNA SGs, and found that HuR-containing aggregates were present in the perinuclear regions of hypothalamic cells in TGF-β1-injected mice but barely in control mice (Fig. 5b). Consistently, these morphological changes were found in the hypothalamus of HFD-fed mice but almost not chow-fed mice (supplementary Fig. 6b). These data suggest that RNA stress response could be causally important for the induction of obesity-associated hypothalamic inflammation.
Figure 5. Effects of TGF-β1 on hypothalamic RNA SGs and IκBα mRNA decay.
(a, b) Male C57BL/6 mice were injected with TGF-β1 (4 ng) vs. vehicle (Veh), and hypothalami were harvested for measuring mRNA levels of SGs/PBs components (a) or HuR immunostaining (b). Nuclear staining by DAPI revealed cells in sections. Images show a representative sub-area of the MBH. Scale bar = 10 μm. (c, d) GT1-7 cells were treated with TGF-β1 (10 ng/ml) for the indicated durations and were harvested for measuring IκBα mRNA levels. (e) Male C57BL/6 mice were injected with TGF-β1 (4 ng) vs. vehicle (Veh), and hypothalami were harvested for measuring mRNA levels of IκBα. (f – g) Male C57BL/6 mice received MBH injection of lentiviral dominant-negative IκBα vs. control (Con), and were injected with TGF-β1 vs. vehicle. Mice were killed for Western blots (f), or examined with ITT (g). Bar graph shows the area under curve (AUC) values of ITT. * P < 0.05, ** P < 0.01, *** P < 0.001, n = 4 mice per group (a, e), and n = 4 samples per group (c, d), and n = 5 – 8 mice per group (g). Error bars reflect mean ± SEM. AU: arbitrary unit.
TGF-β degrades IκBα mRNA to atypically activate NF-κB
RNA SGs/PBs have the function to degrade mRNAs by targeting the AU-rich element (AREs) at the 3′ untranslated region (UTR)36-38. As shown in supplementary Fig. 7, our analysis of gene sequences showed that AUE is conserved in IκBα mRNA across species. This information led us to suspect that TGF-β could work to degrade IκBα mRNA. Using both hypothalamic GT1-7 cells (Fig. 5c) and HEK 293 cells (data not shown), our experiments revealed that IκBα mRNA levels in these cells notably decreased following TGF-β1 treatment. When these cells were added with transcription inhibitor actinomycin D, IκBα mRNA decay further accelerated (Fig. 5d). These results indicated that the turnover of IκBα mRNA is fast, and TGF-β has a strong effect in promoting IκBα mRNA decay. Consistent with in vitro data, an intra-third ventricle injection of TGF-β1 was sufficient to decrease hypothalamic IκBα mRNA levels (Fig. 5e), and we predicted that this mRNA change led to hypothalamic IκBα protein loss in TGF-β1-injected mice (Fig. 4a). To test if TGF-β induced IκBα mRNA decay is accountable for the glucose metabolism disorder, we designed an experiment to study if the pro-diabetic effect of brain TGF-β excess could be reversed through directly increasing hypothalamic IκBα mRNA. Using an approach which we established previously23, we employed a lentiviral system to deliver exogenous Iκbα mRNA into the mediobasal hypothalamus of chow-fed C57BL/6 mice, and indeed, this lentivirus-delivered Iκbα mRNA prevented TGF-β1 injection from activating hypothalamic NF-κB (Fig. 5f). Our metabolic analysis confirmed that this treatment attenuated the effect of TGF-β1 from impairing insulin-dependent glucose control (Fig. 5g). Altogether, our findings suggest that a hypothalamic process consisting of TGF-β excess, RNA stress response and IκBα mRNA decay mediates the pro-diabetic mechanism of the brain in the condition of dietary obesity.
Hypothalamic TGF-β and RNA SGs/PBs link aging to glucose intolerance
Finally, we studied if the pro-diabetic role of hypothalamic TGF-β-directed RNA SGs/PBs is also important for aging-related glucose and insulin disorders. In supplementary Table 1, we showed that hypothalamic TGF-β1 mRNA levels increased in aged mice but were decreased by CR. In this context, we analyzed expression levels of RNA SBs/PBs components, and found that many of these molecules were upregulated in the hypothalamus of aged mice compared to young mice (Fig. 6a). Using immunostaining, we confirmed that RNA SGs were present in the hypothalamus of old mice but barely in young mice (Fig. 6b). These aging-associated changes significantly overlap with those induced by HFD feeding, implicating that dietary obesity and aging have a common abnormality dictated by RNA stress response-triggered inflammation. In this context, we extended to use Tgfb1+/− mice to study if the partial inhibition of TGF-β1 in this model could protect against aging-induced glucose and insulin intolerance. In this experiment, Tgfb1+/− mice and WT controls were maintained under chow feeding and studied for metabolic physiology in young vs. middle-aged conditions. Our follow-up showed that chow-fed Tgfb1+/− mice and WT controls had similar food intake and body weight (Fig. 6c, d). At young ages, Tgfb1+/− mice and WT had similar glucose levels in GTT and ITT (Fig. 6e, f). Glucose and insulin tolerance were both impaired in middle-aged WT mice compared to young WT mice; in contrast, middle-aged Tgfb+/− mice showed significant improvements of glucose tolerance in GTT and insulin tolerance in ITT (Fig. 6e, f). Taken together, TGF-β excess and inflammatory RNA metabolism represent two critical factors located in the crossroad of translating not only obesity but also aging into pro-diabetic complications.
Figure 6. Hypothalamic TGF-β and RNA SGs/PBs link aging to glucose disorders.
Male C57BL/6 mice (a, b) and Tgfb1+/− vs. WT mice (Con) (c – f) were analyzed at young vs. middle-aged age (2 vs. ∼15 months old). Hypothalamic mRNA levels of SGs/PBs components (a) and HuR immunostaining (b), food intake (c), body weight (d), and blood glucose in GTT (e) and ITT (f) were analyzed. Scale bar = 10 μm (b). * P < 0.05, ** P < 0.01, *** P < 0.001; n = 4 mice per group (a, b), and n = 5 – 8 mice per group (c – f). Error bars reflect mean ± SEM.
Discussion
Glucose intolerance by TGF-β: potentially adaptive but chronically pro-diabetic
Based on epidemiological and clinical evidences, hyperglycemia and glucose intolerance are frequently found in brain diseases such as Alzheimer's disease39-41. Recently, manipulations of the CNS or the hypothalamus were found to change hepatic glucose production in experimental models5-9, but it remains unexplored whether the brain could mediate diabetic development. Here, we found that obesity and aging are both associated with overproduction of TGF-β in the brain, and our pharmacological and genetic models consistently revealed that excess of TGF-β in the brain leads to glucose and insulin intolerance in a manner which is dissociable from obesity or aging. The brain-liver axis was found critical for the pro-diabetic effect of brain TGF-β excess, agreeing with the knowledge demonstrating that the hypothalamus has a regulatory role in hepatic glucose production5-8. Conceptually, our finding was in line with the literature showing that TGF-β excess was not only seen in many brain diseases but also implicated in their pathogenesis42-46. On the other hand, despite this disease relevance of TGF-β excess, we want to point out that TGF-β has biological functions in cell growth, differentiation and transformation, and complete absence of TGF-β is developmentally lethal47,48. With regards to the CNS, lack of brain TGF-β signaling can affect neurological development or synapse function32,49,50, indicating that a normal level of brain TGF-β is biologically required and therefore neuroprotective. In this background, we speculate that increase of TGF-β in brain diseases may represent an adaptive response, and by inducing glucose intolerance, it can increase glucose availability for the brain, since glucose is almost the exclusive fuel for the brain, and an increase in glucose flux can help the brain to cope with stress and damages. However, when such induction of glucose intolerance is chronic, it lowers the threshold of developing diabetes, leading to the T2D-prone condition. Consistent with this idea, systemic TGF-β neutralization in db/db mice or ob/ob mice was shown to have effects in reducing glucose or renal disorders in the context of body weight reduction51,52. Herein, our findings demonstrate that brain TGF-β excess is pro-diabetic which is independent of body weight, and therefore, appropriate TGF-β suppression can represent a therapeutic basis for diabetic patients with or without obesity. Nevertheless, it needs to be further studied if TGF-β itself or the downstream mechanism represents a viable target for treating human diabetes.
A mediator of atypical hypothalamic inflammation: RNA stress response
Classical NF-κB activation is induced by membrane receptor-dependent kinases such as IKK and TAK153. Activation of these kinases rapidly leads to IκBα phosphorylation, ubiquitination and degradation, and subsequently, NF-κB is liberated from binding to IκBα, enters the nucleus and induce gene transcription53. This paradigm of classical NF-κB activation requires extracellular stimuli, such as pathogens or related molecules which activate TLRs. As a result, activated NF-κB induces gene expression of inflammatory cytokines (e.g., TNF-α and interleukins), which are released to induce subsequent NF-κB activation through cytokine receptor signaling. Recently, obesity and aging were both revealed to be associated with hypothalamic NF-κB activation12-14,18-20,23-27; however, how hypothalamic NF-κB activation is triggered in these conditions was unclear. Here, we demonstrated that hypothalamic RNA stress response induces IκBα mRNA decay to initiate NF-κB activation, an intracellular RNA metabolism-driven event which does not rely on receptor signaling. In the literature, it has been documented that the biological function of RNA stress response is to provide an early intracellular defense during which RNA SGs/PBs are formed to degrade ARE-containing mRNAs36-38. In this study, we demonstrated that IκBα mRNA has a fast turnover rate and is sensitively subjected to RNA SGs/PBs-mediated mRNA decay. TGF-β excess can trigger this process, despite that it remains to be studied if there are other contributing factor(s). Furthermore, we found that induction of NF-κB-dependent inflammatory genes by short-term HFD feeding was suppressed by TGF-β1 inhibition, suggesting that TGF-β excess is involved in initiating obesity-related hypothalamic inflammation. In physiology, since hypothalamic NF-κB mediates the pro-diabetic effect of brain TGF-β excess, it provides a strong support to an integrated model (supplementary Fig. 8) that the brain mechanism of T2D involves the activation of hypothalamic NF-κB by many other factors, such as endoplasmic reticulum stress, cytokines and other inflammatory signaling molecules (e.g., JNK). While the predicted pro-diabetic effects of these factors are intertwined with obesity development, here we dissected out an obesity-independent mechanism to support the conclusion that hypothalamic inflammation is primarily involved in diabetic development.
Inflammatory milieu: key for disease relevance of inflammation-related cytokines
The overall findings in this study further elucidated the notion that the content of hypothalamic inflammation in obesity or aging has unique features, and the involvement of TGF-β reflects this point. TGF-β has often been studied for its anti-inflammatory functions28, but here we revealed that excess brain TGF-β activates proinflam¬matory NF-κB. In agreement with our finding, it has been recently noticed that TGF-β can support proinflammatory functions in the context of other inflammation-related cytokines28,54,55. Hence, in the context of the recent literature that has increasingly uncovered the role of hypothalamic inflammation in the development of phenotypes associated with the metabolic syndrome12–25,56–60, the findings in this work suggest that the hypothalamic inflammation–related mechanism of these phenotypes involves molecules whose proinflammatory actions are relatively moderate. Also, given our findings here and those in the previous studies, it seems the cellular mechanism of this form of hypothalamic inflammation is driven by a cross-talk between astrocytes and neurons. Notably, recent studies have demonstrated that HFD feeding can rapidly induce hypothalamic gliosis and increase of microglia17,22. Our results here show that TGF-β is produced and released predominantly from astrocytes and acts on hypothalamic neurons such as POMC neurons to induce metabolic changes. This finding further indicates that hypothalamic glia-neuron interaction is important in the regulation of metabolic physiology and pathophysiology.
Online Methods Section
Animals
Tgfb1lox/lox mice, Tgfbr2lox/lox mice, Tgfb1+/− mice, GFAP-Cre mice, and Tlr4−/− mice on C57BL/6 were obtained from Jackson32,61-64 and continued to be maintained on C57BL/6. CMV-lox-stop-lox-Tgfb1 mice30 obtained from Jackson were backcrossed into C57BL/6. POMC-Cre mice maintained on C57BL/6 were used in our previous research20,65. All mice were housed in standard, pathogen-free animal facility with 12h/12h light and darkness cycles, and adult male mice were used in experiments of this work. Mice were maintained on normal chow since weaning, and for some experiments involving HFD feeding, a HFD (45% kcal fat, Research Diets, Inc.) was used when mice were two to three months old. Food intake and body weight of mice were measured using a laboratory scale. GTT was performed in mice through intraperitoneal (i.p.) injection of glucose at 2g/kg body weight. ITT was performed in mice through i.p. injection of human recombinant insulin (Nova Nordisk) at the dose of 0.7U/kg body weight. Blood glucose levels during GTT and ITT were measured with LifeScan® blood glucose monitoring system. All procedures were approved by the Institutional Animal Care and Use Committee of Albert Einstein College of Medicine.
Brain injection
As we previously described14,66, using an ultra-precise (10 μm resolution) small animal stereotactic apparatus (David Kopf Instruments), a 26 gauge guide cannula (Plastics One, Inc.) was implanted into third ventricle of anesthetized mice at the midline coordinates of 1.8 mm posterior to bregma and 5.0 mm below the surface of skull. Intra-third ventricular injection was carried out with a 33-gauge internal cannula (Plastics One) connected to a 5-μl Hamilton Syringe. TGF-β1 (Sigma) was dissolved in 1 μl artificial cerebrospinal fluid (aCSF) for injection. Injection of aCSF was used as vehicle control. Pharmacological treatment: Mice were fasted for an overnight period, and received two injections of TGF-β1 vs. vehicle via pre-implanted cannula, one injection at night after food was removed, and the second injection in the following morning at 4 hours prior to a metabolic test such as GTT and ITT. Bilateral intra-MBH viral injections were directed by an ultra-precise stereotactic apparatus at coordinates of 1.5 mm posterior to bregma, 5.8 mm below the surface of skull, and 0.3 mm lateral to midline, as previously described14. Purified lentiviruses suspended in 0.2 μl aCSF was injected over 10-min period via a 26-gauge guide cannula and a 33-gauge internal injector (Plastics One) connected to a 5-μl Hamilton Syringe and infusion pump (WPI Instruments).
Hyperinsulinemic-euglycemic clamp
Mice that were pre-implanted with cannula in the third ventricle were anesthetized, and a catheter was inserted into the right jugular vein and crossed over from the underneath and out the back of the neck. Following surgical recovery, overnight-fasted mice were injected with TGF-β1 (4 ng) vs. vehicle, once at the beginning of fasting and the other in the following morning at four hours prior to clamp. Conscious mice were then subjected to euglycemic clamp, with the blood glucose concentrations maintained at 120 – 130 mg/dl for four hours, followed by steady-state human insulin infusion (4 mU kg−1 min−1) together with infusion of 20% glucose at variable rates to maintain euglycemia. During the final 1 hour of the clamp, [3-3H] glucose and 2-deoxy-D-[1-14C] glucose (PerkinElmer) infusions were used. Blood samples were collected from tail vein. At the end of the procedure, mice were euthanized, and various tissues were removed and quickly frozen in liquid nitrogen. Plasma insulin and glucagon concentratios were analyzed with ELISA kits (Crystal Chem. and R&D Systems).
Lentiviruses and histology
Lentiviral IκBα was cloned by inserting cDNA of dominant-negative IκBα in synapsin promoter-driven lentiviral vector as previously described14, and replacement of IκBα by GFP cDNA was used as the matched control. Lentiviruses were generated in HEK293T cells and then purified as previously described14. Brain histology was analyzed using brain sections and immunostaining. Mice under anesthesia were transcardially perfused with 4% PFA and brains were removed, post-fixed in 4% PFA for four hours, and infiltrated with 20% – 30% sucrose. 20 μm-thick brain sections were blocked with serum of appropriate species, penetrated with 0.2% Triton-X 100, treated with primary antibodies including mouse anti-GFAP (Millipore, MAB3402, 1:1000), mouse anti-NeuN (Millipore, MAB377, 1:1000), rabbit anti-TGF-β (Abcam, ab53169, 1:200), and mouse anti-HuR (Santa Cruz, sc5261, 1:500), and subsequently reacted with fluorescent secondary antibodies (Invitrogen, 1:1000). Naïve IgGs of appropriate species were used as negative controls. DAPI staining was used to reveal all cells in the section. Images were taken using a confocal microscope.
Protein, mRNA and peptide analyses
Hypothalami were isolated as previously described14. Tissue lysis, SDS/PAGE and Western blotting were performed as previously described19. Primary antibodies in Western blots included anti-IκBα (Santa Cruz, #SC847, 1:500), anti-p-TAK1 (Cell Signaling, #4531, 1:1000), anti-p-IκBα (Cell Signaling, #2859, 1:1000), anti-RelA (Cell Signaling, #3039, 1:1000), anti-p-RelA (Cell Signaling, #4764, 1:1000), and anti-β-actin (Cell Signaling, #4967S, 1:1000), and secondary antibodies were HRP-conjugated anti-rabbit or goat antibody (Pierce, 1:2000). Quantification of Western blots was processed with Image J. Total RNA was extracted from hypothalamic tissue using TRIzol® (Invitrogen), and cDNA was synthesized using M-MLV RT System (Promega). Using SYBR® Green PCR Master Mix (Applied Biosystems), expression levels of target genes were analyzed via PCR amplification and quantification. GAPDH or TBP mRNA levels were used for normalization. CSF collection and TGFβ measurement: An anesthetized mouse was placed onto the stereotactic apparatus with the head forming an angle of about 135° with the body, and then a sagittal incision in the neck skin was made inferior to the occiput, followed by penetrating a capillary tube through the dura mater into the citerna magna to draw the CSF. Serum and CSF TGF-β content were measured using TGF-β ELISA kit (R&D Systems).
Cell culture
GT1-7 cells were cultured as described previously14. Briefly, GT1-7 cells were maintained in Dulbecco's Modified Eagle's Medium (Invitrogen) with 10% fetal bovine serum (Hyclone) and penicillin/streptomycin (Invitrogen), at 37°C in a humidified atmosphere containing 5% CO2. Cells were fasted in serum-free medium for an overnight period, and then were subjected to TGF-β1 treatment at the indicated dose and time course.
Statistics
Two-tailed Student's t test was used for comparisons between two groups, and ANOVA and appropriate post hoc analyses were used for comparisons among more than two groups. Data presented met normal distribution, and statistical tests for each figure were justified appropriate. Sample sizes were chosen with adequate power based on the literature. Data were presented as mean ± SEM. P < 0.05 was considered statistically significant.
Supplementary Material
Acknowledgments
The authors sincerely thank Cai's laboratory members for providing technical assistance. This study was supported by NIH R01 DK078750, R01 AG031774, R01 HL113180, and R01 DK 099136 (all to D. Cai).
Footnotes
Author contributions: DC conceived and designed the study. JY, HZ, YY, JL, and YT performed experiments and data analysis with experimental assistance from SP and LL. DC and JY wrote the paper.
Competing Interests Statement: The authors have no competing financial interests.
Reference List
- 1.Samuel VT, Shulman GI. Mechanisms for insulin resistance: common threads and missing links. Cell. 2012;148:852–871. doi: 10.1016/j.cell.2012.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. doi: 10.1146/annurev-immunol-031210-101322. [DOI] [PubMed] [Google Scholar]
- 3.Muoio DM, Newgard CB. Obesity-related derangements in metabolic regulation. Annu Rev Biochem. 2006;75:367–401. doi: 10.1146/annurev.biochem.75.103004.142512. [DOI] [PubMed] [Google Scholar]
- 4.Glass CK, Olefsky JM. Inflammation and lipid signaling in the etiology of insulin resistance. Cell Metab. 2012;15:635–645. doi: 10.1016/j.cmet.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mighiu PI, et al. Hypothalamic glucagon signaling inhibits hepatic glucose production. Nat Med. 2013;19:766–772. doi: 10.1038/nm.3115. [DOI] [PubMed] [Google Scholar]
- 6.Berglund ED, et al. Direct leptin action on POMC neurons regulates glucose homeostasis and hepatic insulin sensitivity in mice. J Clin Invest. 2012;122:1000–1009. doi: 10.1172/JCI59816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Obici S, Zhang BB, Karkanias G, Rossetti L. Hypothalamic insulin signaling is required for inhibition of glucose production. Nat Med. 2002;8:1376–1382. doi: 10.1038/nm1202-798. [DOI] [PubMed] [Google Scholar]
- 8.Lam TK, et al. Hypothalamic sensing of circulating fatty acids is required for glucose homeostasis. Nat Med. 2005;11:320–327. doi: 10.1038/nm1201. [DOI] [PubMed] [Google Scholar]
- 9.Joly-Amado A, et al. Hypothalamic AgRP-neurons control peripheral substrate utilization and nutrient partitioning. EMBO J. 2012;31:4276–4288. doi: 10.1038/emboj.2012.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wisse BE, Kim F, Schwartz MW. Physiology. An integrative view of obesity. Science. 2007;318:928–929. doi: 10.1126/science.1148032. [DOI] [PubMed] [Google Scholar]
- 11.Cone RD. Anatomy and regulation of the central melanocortin system. Nat Neurosci. 2005;8:571–578. doi: 10.1038/nn1455. [DOI] [PubMed] [Google Scholar]
- 12.Cai D. Neuroinflammation and neurodegeneration in overnutrition-induced diseases 2250. Trends Endocrinol Metab. 2013;24:40–47. doi: 10.1016/j.tem.2012.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cai D, Liu T. Hypothalamic inflammation: a double-edged sword to nutritional diseases. Ann N Y Acad Sci. 2011;1243:E1–39. doi: 10.1111/j.1749-6632.2011.06388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang X, et al. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Souza CT, et al. Consumption of a fat-rich diet activates a proinflammatory response and induces insulin resistance in the hypothalamus. Endocrinology. 2005;146:4192–4199. doi: 10.1210/en.2004-1520. [DOI] [PubMed] [Google Scholar]
- 16.Kleinridders A, et al. MyD88 signaling in the CNS is required for development of fatty acid-induced leptin resistance and diet-induced obesity. Cell Metab. 2009;10:249–259. doi: 10.1016/j.cmet.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horvath TL, et al. Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proc Natl Acad Sci U S A. 2010;107:14875–14880. doi: 10.1073/pnas.1004282107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meng Q, Cai D. Defective hypothalamic autophagy directs the central pathogenesis of obesity via the IkappaB kinase beta (IKKbeta)/NF-kappaB pathway. J Biol Chem. 2011;286:32324–32332. doi: 10.1074/jbc.M111.254417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Purkayastha S, et al. Neural dysregulation of peripheral insulin action and blood pressure by brain endoplasmic reticulum stress. Proc Natl Acad Sci U S A. 2011;108:2939–2944. doi: 10.1073/pnas.1006875108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Purkayastha S, Zhang G, Cai D. Uncoupling the mechanisms of obesity and hypertension by targeting hypothalamic IKK-beta and NF-kappaB. Nat Med. 2011;17:883–887. doi: 10.1038/nm.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milanski M, et al. Inhibition of hypothalamic inflammation reverses diet-induced insulin resistance in the liver. Diabetes. 2012;61:1455–1462. doi: 10.2337/db11-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thaler JP, et al. Obesity is associated with hypothalamic injury in rodents and humans. J Clin Invest. 2012;122:153–162. doi: 10.1172/JCI59660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang G, et al. Hypothalamic programming of systemic ageing involving IKK-beta, NF-kappaB and GnRH. Nature. 2013;497:211–216. doi: 10.1038/nature12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Purkayastha S, Cai D. Neuroinflammatory basis of metabolic syndrome. Mol Metab. 2013;2:356–363. doi: 10.1016/j.molmet.2013.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tang Y, Cai D. Hypothalamic inflammation and GnRH in aging development. Cell Cycle. 2013;12:2711–2712. doi: 10.4161/cc.26054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Tang Y, Cai D. IKKbeta/NF-kappaB disrupts adult hypothalamic neural stem cells to mediate a neurodegenerative mechanism of dietary obesity and pre-diabetes. Nat Cell Biol. 2012;14:999–1012. doi: 10.1038/ncb2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li J, et al. Control of obesity and glucose intolerance via building neural stem cells in the hypothalamus. Mol Metab. 2014;3:313–324. doi: 10.1016/j.molmet.2014.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wan YY, Flavell RA. ‘Yin-Yang’ functions of transforming growth factor-beta and T regulatory cells in immune regulation. Immunol Rev. 2007;220:199–213. doi: 10.1111/j.1600-065X.2007.00565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li MO, et al. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 30.Hall BE, et al. Conditional overexpression of TGF-beta1 disrupts mouse salivary gland development and function. Lab Invest. 2010;90:543–555. doi: 10.1038/labinvest.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouret S, et al. Evidence that TGF beta may directly modulate POMC mRNA expression in the female rat arcuate nucleus. Endocrinology. 2001;142:4055–4065. doi: 10.1210/endo.142.9.8361. [DOI] [PubMed] [Google Scholar]
- 32.Falk S, et al. Brain area-specific effect of TGF-beta signaling on Wnt-dependent neural stem cell expansion. Cell Stem Cell. 2008;2:472–483. doi: 10.1016/j.stem.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 33.Beynon AL, Thome J, Coogan AN. Age and time of day influences on the expression of transforming growth factor-beta and phosphorylated SMAD3 in the mouse suprachiasmatic and paraventricular nuclei. Neuroimmunomodulation. 2009;16:392–399. doi: 10.1159/000228914. [DOI] [PubMed] [Google Scholar]
- 34.Padilla SL, Carmody JS, Zeltser LM. Pomc-expressing progenitors give rise to antagonistic neuronal populations in hypothalamic feeding circuits. Nat Med. 2010;16:403–405. doi: 10.1038/nm.2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scherf W, Burdach S, Hansen G. Reduced expression of transforming growth factor beta 1 exacerbates pathology in an experimental asthma model. Eur J Immunol. 2005;35:198–206. doi: 10.1002/eji.200425209. [DOI] [PubMed] [Google Scholar]
- 36.Thomas MG, Loschi M, Desbats MA, Boccaccio GL. RNA granules: the good, the bad and the ugly. Cell Signal. 2011;23:324–334. doi: 10.1016/j.cellsig.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Buchan JR, Parker R. Eukaryotic stress granules: the ins and outs of translation. Mol Cell. 2009;36:932–941. doi: 10.1016/j.molcel.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson P, Kedersha N. Stress granules: the Tao of RNA triage. Trends Biochem Sci. 2008;33:141–150. doi: 10.1016/j.tibs.2007.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Dai Y, Kamal MA. Fighting Alzheimer's Disease and Type 2 Diabetes: Pathological Links and Treatment Strategies. CNS Neurol Disord Drug Targets. 2013 doi: 10.2174/18715273113126660134. [DOI] [PubMed] [Google Scholar]
- 40.McCrimmon RJ, Ryan CM, Frier BM. Diabetes and cognitive dysfunction. Lancet. 2012;379:2291–2299. doi: 10.1016/S0140-6736(12)60360-2. [DOI] [PubMed] [Google Scholar]
- 41.de la Monte SM, Wands JR. Alzheimer's disease is type 3 diabetes-evidence reviewed. J Diabetes Sci Technol. 2008;2:1101–1113. doi: 10.1177/193229680800200619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Luo J, et al. Glia-dependent TGF-beta signaling, acting independently of the TH17 pathway, is critical for initiation of murine autoimmune encephalomyelitis. J Clin Invest. 2007;117:3306–3315. doi: 10.1172/JCI31763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ilzecka J, Stelmasiak Z, Dobosz B. Transforming growth factor-Beta 1 (tgf-Beta 1) in patients with amyotrophic lateral sclerosis. Cytokine. 2002;20:239–243. doi: 10.1006/cyto.2002.2005. [DOI] [PubMed] [Google Scholar]
- 44.Tarkowski E, et al. Increased intrathecal levels of the angiogenic factors VEGF and TGF-beta in Alzheimer's disease and vascular dementia. Neurobiol Aging. 2002;23:237–243. doi: 10.1016/s0197-4580(01)00285-8. [DOI] [PubMed] [Google Scholar]
- 45.Wyss-Coray T, Borrow P, Brooker MJ, Mucke L. Astroglial overproduction of TGF-beta 1 enhances inflammatory central nervous system disease in transgenic mice. J Neuroimmunol. 1997;77:45–50. doi: 10.1016/s0165-5728(97)00049-0. [DOI] [PubMed] [Google Scholar]
- 46.Grammas P, Ovase R. Cerebrovascular transforming growth factor-beta contributes to inflammation in the Alzheimer's disease brain. Am J Pathol. 2002;160:1583–1587. doi: 10.1016/s0002-9440(10)61105-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bottinger EP, Letterio JJ, Roberts AB. Biology of TGF-beta in knockout and transgenic mouse models. Kidney Int. 1997;51:1355–1360. doi: 10.1038/ki.1997.185. [DOI] [PubMed] [Google Scholar]
- 48.Dunker N, Krieglstein K. Tgfbeta2 -/- Tgfbeta3 -/- double knockout mice display severe midline fusion defects and early embryonic lethality. Anat Embryol (Berl) 2002;206:73–83. doi: 10.1007/s00429-002-0273-6. [DOI] [PubMed] [Google Scholar]
- 49.Heupel K, et al. Loss of transforming growth factor-beta 2 leads to impairment of central synapse function. Neural Dev. 2008;3:25. doi: 10.1186/1749-8104-3-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vogel T, Ahrens S, Buttner N, Krieglstein K. Transforming growth factor beta promotes neuronal cell fate of mouse cortical and hippocampal progenitors in vitro and in vivo: identification of Nedd9 as an essential signaling component. Cereb Cortex. 2010;20:661–671. doi: 10.1093/cercor/bhp134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ziyadeh FN, et al. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proc Natl Acad Sci U S A. 2000;97:8015–8020. doi: 10.1073/pnas.120055097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yadav H, et al. Protection from obesity and diabetes by blockade of TGF-beta/Smad3 signaling. Cell Metab. 2011;14:67–79. doi: 10.1016/j.cmet.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 54.Veldhoen M, et al. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 55.Mangan PR, et al. Transforming growth factor-beta induces development of the T(H)17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 56.Calegari VC, et al. Inflammation of the hypothalamus leads to defective pancreatic islet function. J Biol Chem. 2011;286:12870–12880. doi: 10.1074/jbc.M110.173021. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 57.Milanski M, et al. Saturated fatty acids produce an inflammatory response predominantly through the activation of TLR4 signaling in hypothalamus: implications for the pathogenesis of obesity. J Neurosci. 2009;29:359–370. doi: 10.1523/JNEUROSCI.2760-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ropelle ER, et al. IL-6 and IL-10 anti-inflammatory activity links exercise to hypothalamic insulin and leptin sensitivity through IKKβ and ER stress inhibition. PLoS Biol. 2010;8:e1000465. doi: 10.1371/journal.pbio.1000465. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59.Gao Y, et al. Hormones and diet, but not body weight, control hypothalamic microglial activity. Glia. 2014;62:17–25. doi: 10.1002/glia.22580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ito Y, et al. GABA type B receptor signaling in proopiomelanocortin neurons protects against obesity, insulin resistance, and hypothalamic inflammation in male mice on a high-fat diet. J Neurosci. 2013;33:17166–17173. doi: 10.1523/JNEUROSCI.0897-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Garcia AD, et al. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- 62.Azhar M, et al. Generation of mice with a conditional allele for transforming growth factor β1 gene. Genesis. 2009;47:423–431. doi: 10.1002/dvg.20516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shull MM, et al. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature. 1992;359:693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yoshinaga K, et al. Perturbation of transforming growth factor (TGF)- β1 association with latent TGF-β binding protein yields inflammation and tumors. Proc Natl Acad Sci USA. 2008;105:18758–18763. doi: 10.1073/pnas.0805411105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang H, et al. Hypoxia-inducible factor directs POMC gene to mediate hypothalamic glucose sensing and energy balance regulation. PLoS Biol. 2011;9:e1001112. doi: 10.1371/journal.pbio.1001112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhang G, et al. Neuropeptide exocytosis involving synaptotagmin-4 and oxytocin in hypothalamic programming of body weight and energy balance. Neuron. 2011;69:523–535. doi: 10.1016/j.neuron.2010.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.