A fundamental tenet of any screening program is that it should “reduce individual and societal burden from specific disorders.”1 Screening is predicated on the assumption that early detection and subsequent treatment will improve the clinical course for individuals without symptoms. Conversely, when an individual presents with complaints of memory problems, the physician engages in case-finding. 1 Screening for some conditions in some populations has resulted in improvements in individual and societal burdens of diseases (e.g., colon and breast cancer), but screening can also cause individual and societal harm (e.g., prostate-specific antigen testing). According to the 2003 report of the U.S. Preventive Services Task Force, there is insufficient evidence to recommend for or against routine screening for dementia in primary care.2
Data were reviewed from multiple studies,2–7 conducted by Indiana University Center for Aging Research, investigating the benefits and harms of dementia screening in the primary care setting, and used to answer three questions:
Can current primary care practices implement dementia screening without leading to negative, unintended consequences?
Are there potential harms affecting asymptomatic individuals undergoing dementia screening in primary care?
Is there evidence supporting the benefit of early implementation of best-practice dementia care in screening-detected cases of dementia?
Can current primary care practices implement dementia screening without leading to negative, unintended consequences?
The typical primary care physician has a panel of approximately 2,000 patients, including 300 aged 65 and older, and provides care for only approximately 18 patients with recognized or unrecognized dementia.6,7 If a typical primary care physician screened these 300 older adults for dementia, 39 would have a positive screen, but only 20 would accept a formal diagnostic evaluation, and no more than nine would have a confirmed dementia diagnosis, costing the system approximately $39,000 (as a result of the cost of the screening process and the diagnostic assessment) without having an effective intervention that will stop the progression of dementia.6 A large number of individuals with and without dementia seek out their primary care physician to manage their complex biopsychosocial health needs, such as multiple chronic conditions, physical and psychological symptoms, and acute illnesses.7 This typical primary care provider needs an estimated 17 hours per working day to comply fully with evidence-based practice regarding management of chronic conditions and providing preventive services.6–8 Organizational and procedural changes in this care environment, such as introducing routine dementia screening, are not only difficult, but also may have unintended consequences, such as high use of psychotropic medications and longer length of hospital stay. 9–11
Are there potential harms affecting asymptomatic individuals undergoing dementia screening in primary care?
Over the past decade, the refusal rates of undergoing a cognitive screening for dementia in primary care vary from 7% to 23%. 3–5 To understand attitudes toward dementia screening, a survey instrument called Perceptions Regarding Investigational Screening for Memory in Primary Care was developed and completed on more than 1,000 individuals aged 65 and older attending primary care clinics in Indianapolis. The majority of subjects were concerned about potential harms of dementia screening.3–5 The biggest concerns involved loss of independence; 78% of older adults in primary care feared losing their driver’s license as a consequence of undergoing dementia screening, 34% feared being put into a nursing home, and 20% feared losing their own home. Furthermore, 40% of subjects feared that the results of dementia screening would affect their long-term care insurance, and 37% worried that they might lose their job. In addition, study participants were concerned about the effect of dementia screening on their family and their own emotional well-being; 75% feared their family would suffer emotionally, and approximately 40% feared that they would feel anxious and depressed. Furthermore, it was found that the experience of being an informal dementia caregiver might influence one’s own attitude about undergoing dementia screening.5 After adjusting for age, race, sex, and education, caregiver s were less likely to accept dementia screening and more likely to perceive emotional suffering as a result of dementia screening than individuals with no caregiving experience (NCG). The top three barriers to screening that both groups identified were emotional suffering of the family (86% of caregivers, 75% of noncaregivers), loss of driving privileges (75% of caregivers, 78% of noncaregivers), and becoming depressed (64% of caregivers, 43% of noncaregivers).
Is there evidence supporting the benefit of early implementation of best-practice dementia care in screening-detected cases of dementia?
In 2006, the results of a randomized controlled trial that tested the efficacy of a collaborative care model that delivered best practice dementia care were reported.9 Over 12 months, intervention participants received care management from an interdisciplinary team led by a nurse practitioner serving as a care coordinator who worked with individuals’ family caregivers and integrated within primary care. The team used standard protocols to identify, monitor, and treat behavioral and psychological symptoms of dementia (BPSDs). At 12 and 18 months, intervention participants had fewer BPSDs, and intervention caregivers reported improvements in distress and depression,9 but there are no data to demonstrate that providing the collaborative dementia care model to screening-detected individuals with dementia leads to better outcomes than providing this model to individuals with recognized dementia in primary care.
In 2009, with support from the National Institutes of Health (NIH), an interdisciplinary implementation team from Indiana University Center for Aging Research and the urban safety net healthcare system in Indianapolis implemented the collaborative dementia care model.10 The team used the lens of complex adaptive system theory and the tools of the reflective adaptive process to translate the collaborative models into a locally sensitive program, called the Aging Brain Care (ABC) Program. The ABC Program includes a clinical team that has an advanced practice nurse as the care coordinator, a medical assistant as a care coordinator assistant, and a social worker. A medical director supervises these providers, and care coordination support software is used. Serving individuals with recognized dementia, the ABC Program improved access for participants and informal caregivers, improved participant satisfaction with care, enhanced quality of dementia care, and reduced use of acute care.10
Although the efficacy of the collaborative dementia care model has been demonstrated, and it was possible to implement it within the safety net healthcare system, the generalizability and sustainability of this model in other healthcare systems has not been tested, and the benefit of this model in screening-detected individuals with dementia has not been demonstrated. Thus, the value, including harms and benefits, of screening populations of asymptomatic older adults for dementia in an attempt to identify the disease at its earliest manifestations remains controversial.
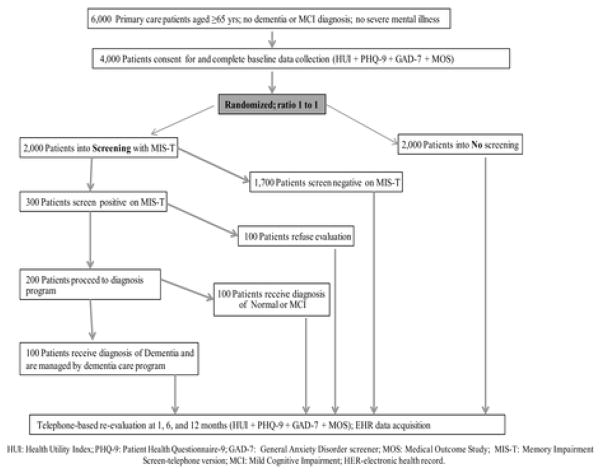
To fill this gap in the literature, funding was received from the NIH to conduct a randomized controlled dementia screening trial to obtain new knowledge to judge the benefits and potential harms of early exposure to the collaborative dementia care model through screening. The trial will randomize 4,000 adults aged 65 and older into a no screening group or a dementia screening, diagnosis, and management group. To fully explore the potential benefits and harms of dementia screening, the trial will provide subjects randomized into the screening arm with the resources of the ABC Program, which will conduct a diagnostic assessment of individuals with positive screening results, deliver the collaborative care model for those with a confirmed dementia diagnosis, and counsel and follow-up those with positive screening results but without evidence of dementia. The primary aims of the trial are to test the effect of dementia screening on the health-related quality of life, mood, and anxiety of the individual at 1, 6, and 12 months. The trial will also estimate the cost effectiveness of dementia screening (Figure 1).
Figure 1.
CONCLUSION
Building a population screening program for dementia in primary care requires a solid foundation of strong data favoring the benefit of such a program over its potential harms. Until that foundation has been built, screening should wait, and limited resources should be focused on implementing the collaborative dementia care model as a best practice dementia care for recognized dementia cases within the primary care system. A wide range of experts have called for routine screening for dementia in primary care, and a growing interest in dementia prevention and early treatment has already begun to push primary care clinicians toward earlier recognition and treatment, yet the benefits and harms of dementia screening are unknown.
Footnotes
Author Contributions: Dr. Boustani participated in the study concept and design, acquisition of subjects and/or data, analysis and interpretation of data, and preparation of manuscript.
Sponsor’s Role: The sponsor had no role in the study design, evaluation, or manuscript development.
Conflict of Interest: Dr. Boustani is funded by the IU CHOICE R01 (R01AG040220–01A1). This work was supported by grants R01AG040220 and R01AG029884 from the National Institute on Aging.
References
- 1.Brayne C, Fox C, Boustani M. Dementia screening in primary care: Is it time? JAMA. 2007;298:2409–2411. doi: 10.1001/jama.298.20.2409. [DOI] [PubMed] [Google Scholar]
- 2.U S. Preventive Services Task Force. Screening for dementia: Recommendations and rationale. Ann Intern Med. 2003;138:925–926. doi: 10.7326/0003-4819-138-11-200306030-00014. [DOI] [PubMed] [Google Scholar]
- 3.Boustani M, Perkins AJ, Monahan P, et al. Measuring primary care patients’ attitudes about dementia screening. Int J Geriatr Psychiatry. 2008;23:812–820. doi: 10.1002/gps.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fowler NR, Boustani MA, Frame A, et al. Effect of patients’ perceptions on dementia screening in primary care. J Am Geriatr Soc. 2012;60:1037–1043. doi: 10.1111/j.1532-5415.2012.03991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boustani MA, Justiss MD, Frame A, et al. Caregivers and non-caregivers attitudes toward dementia screening. J Am Geriatr Soc. 2011;59:681–686. doi: 10.1111/j.1532-5415.2011.03327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boustani M, Callahan CM, Unverzagt FW, et al. Implementing a screening and diagnosis program for dementia in primary care. J Gen Intern Med. 2005;20:572–577. doi: 10.1111/j.1525-1497.2005.0126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boustani M, Sachs G, Callahan CM. Can primary care meet the biopsychosocial needs of older adults with dementia? J Gen Intern Med. 2007;22:1625–1627. doi: 10.1007/s11606-007-0386-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ostbye T, Yarnall KS, Krause KM, et al. Is there time for management of patients with chronic diseases in primary care? Ann Fam Med. 2005;3:209–214. doi: 10.1370/afm.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Callahan CM, Boustani M, Unverzagt FW, et al. Effectiveness of guideline-level care for older adults with Alzheimer’s disease in primary care: A clinical trial. JAMA. 2006;295:2148–2157. doi: 10.1001/jama.295.18.2148. [DOI] [PubMed] [Google Scholar]
- 10.Boustani MA, Sachs GA, Alder CA, et al. Implementing innovative models of dementia care: The Healthy Aging Brain Center. J Aging Mental Health. 2011;15:13–22. doi: 10.1080/13607863.2010.496445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boustani M, Campbell N, Khan B, et al. Enhancing care for hospitalized older adults with cognitive impairment. A randomized controlled trial. J Gen Intern Med. 2012;27:561–567. doi: 10.1007/s11606-012-1994-8. [DOI] [PMC free article] [PubMed] [Google Scholar]