Abstract
Transient global ischemia induces selective, delayed neuronal death of pyramidal neurons in the hippocampal CA1. Whereas long term treatment of middle-aged female rats with estradiol at physiological doses ameliorates neuronal death, the signaling pathways that mediate the neuroprotection are, as yet, unknown. Protein kinase B (Akt) and downstream transcription factors, the cAMP response element binding protein (CREB) and signal transducer and activator of transcription (STAT3) are critical players in cellular survival following injury. The present study was undertaken to determine whether long term estradiol alters the phosphorylation status and activity of Akt, STAT3 and CREB in ovariohysterectomized, middle-aged and young female rats subjected to global ischemia. Irrespective of either hormone or ischemic condition, middle-aged females exhibited lower levels of p-CREB and higher levels of Akt and STAT3 in CA1 than did young females, as assessed by Western blot. In middle-aged animals, ischemia increased the phosphorylation status/activity of Akt and STAT3, and decreased the phosphorylation status/activity of CREB in the hippocampal CA1. Whereas estradiol did not detectably alter the phosphorylation status/activity of Akt or STAT3, it prevented the ischemia-induced decrease in nuclear p-CREB. Similar results were observed for young females. Collectively, these data demonstrate that CREB, STAT3, and Akt are involved in the molecular response to global ischemia and that age influences the status of CREB, STAT3 and Akt activity in CA1 under physiological as well as pathological conditions, further emphasizing the importance of including older rodents in neuroprotection studies.
Keywords: estradiol, global ischemia, neuroprotection, Signal transducer and activator of transcription-3, cAMP response element binding protein, Akt, hippocampus, ovariectomy
1. INTRODUCTION
Stroke and cardiac arrest occur more frequently in older individuals, and in women, the risk increases after menopause (Towfighi et al., 2007). Postmenopausal women are more likely to suffer greater disability and mortality following a stroke than men (Niewada et al., 2005; Roquer et al., 2003), highlighting the importance of using older female animals in studies assessing potential treatments for global ischemia. To be more clinically relevant, neuroprotection studies should examine whether a particular treatment is also effective in older animals as outcomes of ischemia differ between young and older rodents. For instance, older rodents exhibit age-related changes in the brain that affect not only their vulnerability to ischemic injury, but also their responsiveness to pharmacological agents (Buga et al., 2008; Chen et al., 2010; Sutherland et al., 1996).
Transient global ischemia induced experimentally in animals causes selective, delayed death of hippocampal CA1 pyramidal neurons and, in many cases, cognitive deficits. Neuronal death in CA1 is not histologically detectable until 2–3 d after ischemia in rats (Kirino, 1982; Kirino et al., 1984). The significant delay between injury and onset of cell death is consistent with an important role for transcription in global ischemia-induced neuronal death. Global ischemia promotes phosphorylation of protein kinase B (Akt) and its downstream target signal transducer and activator of transcription (STAT3) and dephosphorylation of cAMP response element binding protein (CREB) in the hippocampal CA1 within the first few hours following reperfusion (Choi et al., 2003; Endo et al., 2006; Jover-Mengual et al., 2007; Jover-Mengual et al., 2010; Miyawaki et al., 2009). These findings are consistent with the possibility that Akt and transcription factors such as CREB and STAT3 play a role in the signaling response to global ischemia.
The serine-threonine kinase Akt and downstream transcription factors such as CREB and STAT3 play a pivotal role in neuronal survival and protection, and their activation can protect against cellular stress and injury (Chong et al., 2005; Dziennis & Alkayed, 2008; Tanaka et al., 2000). Expression of Akt, a downstream effector of the phosphoinositide-3-kinase pathway, is activated during cellular injury (Chong et al., 2005). Once activated, Akt inhibits the pro-apoptotic proteins Bad and caspase-9. Additionally, Akt activation promotes cellular survival by phosphorylation and activation of CREB at Ser133, resulting in the up-regulation of pro-survival CREB target genes such as the anti-apoptotic protein, Bcl-2 (Walton & Dragunow, 2000). Following inflammation and cellular oxidative stress, levels of various cytokines increase, leading to the activation of the JAK/STAT pathway (Planas et al., 2006). Once activated, STAT3 promotes neuroprotection by inducing transcription of pro-survival genes such as Bcl-2 and Bcl-xL (Dziennis & Alkayed, 2008).
To date, there are remarkably few safe and effective treatments for the neuronal death or neurological sequellae associated with global ischemia. It is now well established that endogenous and exogenous estrogens exert profound neuroprotective effects in animal models of global ischemia (Etgen et al., 2011; Brown et al., 2009; Lebesgue et al., 2009; Suzuki et al., 2009). Recently, we reported that estradiol retains its neuroprotective actions after global ischemia in middle-aged female rats, even after long-term hormone withdrawal (De Butte-Smith et al., 2009; Lebesgue et al., 2010; Traub et al., 2009). To date, the molecular mechanisms underlying estradiol protection against global ischemia-induced neuronal death in middle-aged rats are unclear. The present study was undertaken to determine whether estradiol pretreatment intervenes at similar or different signaling pathways in middle-aged compared to young female rats subjected to global ischemia. Because vulnerability to ischemic injury and responsiveness to pharmacological agents and hormones such as estradiol may change with age, we compared the effects of estradiol on the phosphorylation status and abundance of Akt, CREB and STAT3 in the selectively vulnerable hippocampal CA1 in ovariohysterectomized (OVX) young and middle-aged female rats.
2. RESULTS
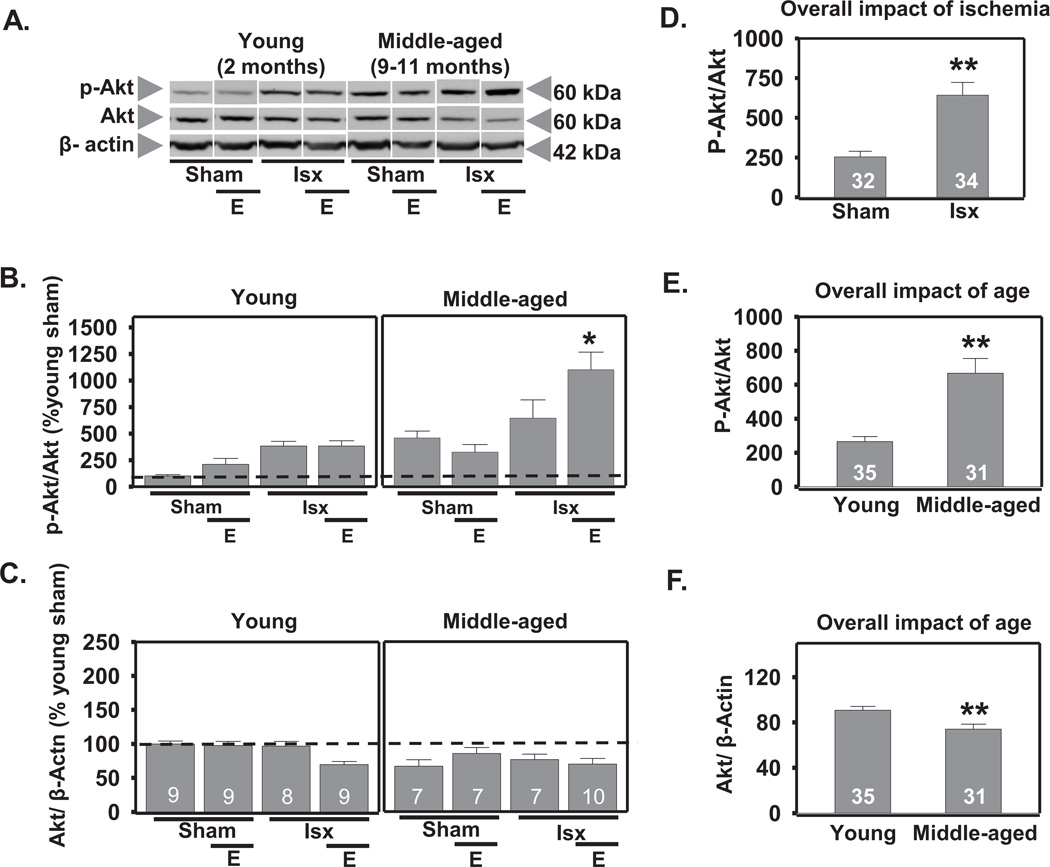
2.1. Global ischemia and age increase p-Akt in the CA1 3 h following reperfusion
Activation of Akt occurs following cellular stress or injury, suggesting Akt may play a critical role in neuronal survival (Chong et al., 2005; Choi et al., 2005; Jover-Mengual et al., 2010; Li et al., 2003; Miyawaki et al., 2009). We used 3-way ANOVA to determine the impact of age, ischemia, and estradiol on the phosphorylation status of Akt 3 h following reperfusion. This time-point was chosen as it has been reported that p-Akt (Ser 473) increases within the first few hours following ischemia (Zhao et al., 2006). There was a main effect of age (F1,65=25.3, P<.0001) with middle-aged female rats exhibiting higher overall levels of p-Akt than young rats across all conditions (increase to 251% of control; Scheffe, P < .0001; Figs. 1A, B, E). In contrast, total Akt was slightly lower in middle-aged rats than in young rats, indicated by a main effect of age (F1,65=10.6, P < .002; decrease by 19%; Scheffe, P < 0.003; Figs. 1A, F). There was also a main effect of ischemia (F1,65=25.4, P<.0001) in that global ischemia increased p-Akt in the CA1 of all females at 3 h following reperfusion (increase to 253% of control; Scheffe, P < 0.0001; all ischemic rats vs all sham rats; Figs. 1A, B, D). In addition, there was a significant estradiol × ischemia × age interaction (F1, 65=6.19, P<.02). Estradiol further increased p-Akt in CA1 of middle-aged ischemic females but not in young females subjected to ischemia (Fig. 1A, B). There were no interactions between age and ischemia, age and estradiol, or estradiol and ischemia on the abundance of p-Akt or total Akt.
Figure 1.
Age and global ischemia increase Akt phosphorylation at 3 h after reperfusion. Representative Western blots (A) and relative abundance of p-Akt and Akt (B and C) in the cytosolic fraction of CA1 from placebo and estradiol (E) treated rats subjected to sham surgery or ischemia (isx). Data are expressed relative to controls (young sham) (B and C). Irrespective of age and E, ischemia increased phosphorylation of Akt (D; P < 0.01, all ischemic groups vs all sham groups). Middle-aged rats had higher p-Akt than young rats regardless of ischemia or E (E; P < 0.01 all middle-aged groups vs all young groups), but total Akt was lower in middle-aged than in young rats (F; p< 0.01 all young groups vs all middle-aged groups).
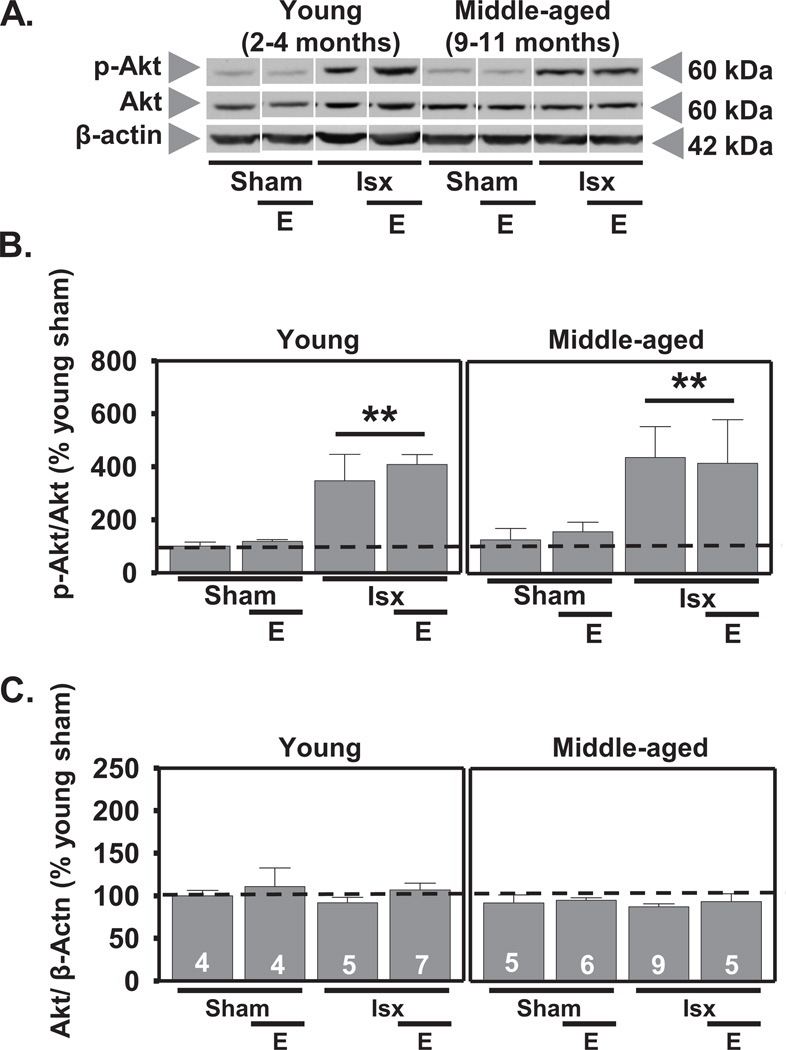
We also determined the effects of age, estradiol, and ischemia on Akt abundance and phosphorylation in whole cell lysates at 12 h after reperfusion. A main effect of ischemia (F1,44=16.0, P<.0001) revealed that global ischemia significantly increased p-Akt in CA1 of all rats with no significant change in total Akt (Scheffe, P < 0.002 vs. sham; Figs. 2A, B, C). Estradiol did not alter total or p-Akt in sham or ischemic animals at this time, nor was there a main effect of age on p-Akt or total Akt. There were no significant interactions.
Figure 2.
Global ischemia increases p-Akt at 12 h after reperfusion. Representative Western blots (A) and relative abundance of p-Akt and Akt (B and C) in CA1 whole-cell lysates from placebo and estradiol (E)-treated rats subjected to sham surgery or ischemia (isx). Data are expressed relative to controls (young sham) (B and C). Ischemia increased the phosphorylation of Akt in both young and middle-aged OVX rats (B; P < 0.01 all ischemic groups vs all sham groups). There were no differences in total Akt at 12 h post-reperfusion (C). ** P < 0.01.
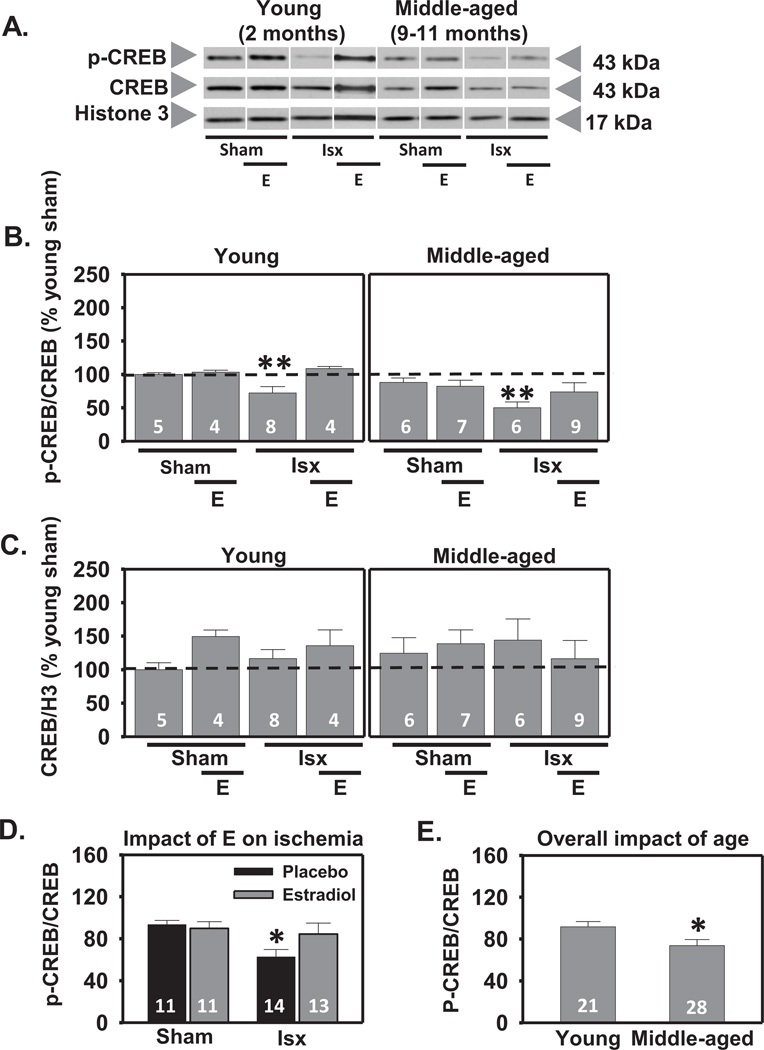
2.2. Estradiol prevents ischemia-induced dephosphorylation of CREB in young and middle-aged female rats
We then focused on CREB, a transcription factor downstream of Akt implicated in the transcriptional control of genes involved in neuronal survival, synaptic plasticity and memory in the brain (Sakamoto et al., 2011; Sweatt, 2004). OVX middle-aged female rats were treated with estradiol or placebo, subjected to global ischemia or sham surgery, and examined for nuclear CREB and p-CREB abundance in the CA1 at 3 h following reperfusion. This time-point was chosen as ischemia has been shown to promote the inactivation of CREB in the first few hours following reperfusion (Jover-Mengual et al., 2007). Interestingly, there was a main effect of age (F1,48=9.41, P<.004); p-CREB abundance in the CA1 of middle-aged female rats was modestly lower than in young rats (decrease by 20%; Scheffe, P < 0.02; Figs. 3A, B, E). Total CREB did not detectably change with age. As expected, there was a significant main effect of ischemia (F1, 48=5.41, P<.03) to reduce p-CREB (decrease by 28% in young and 50% in middle-aged, Scheffe, P < 0.01 vs. sham-operated rats; Figs. 3A, B, C), but there was no significant change in total CREB abundance in the CA1. There was also a significant ischemia × estradiol interaction in that estradiol prevented the ischemia-induced decrease in p-CREB in OVX rats (Scheffe P < 0.04 vs. placebo-treated ischemic rats; Figs. 3A, B, D), but did not influence total CREB abundance (Figs. 3A, C). These findings demonstrate that estradiol retains its ability to prevent ischemia-induced dephosphorylation of CREB in middle-aged females subjected to long-term ovarian hormone deprivation and that age modestly but significantly lowers p-CREB levels in the hippocampal CA1.
Figure 3.
Estradiol prevents ischemia-induced dephosphorylation of CREB in CA1 cell nuclei 3 h after global ischemia in OVX rats. Representative Western blots (A) and relative abundance (B and C) of p-CREB and CREB in the nuclear fraction of CA1 of placebo and estradiol (E) treated rats subjected to sham surgery or ischemia (isx) at 3 h after reperfusion. Data are expressed relative to controls (young sham) (B and C). Irrespective of age and E treatment, ischemia induced dephosphorylation of CREB (B) (P < 0.01 vs sham). Estradiol prevented the decrease in nuclear p-CREB in young and middle-aged ischemic rats (D; P < 0.05 vs all ischemic rats). Overall, middle-aged rats had lower p-CREB in the CA1 than young rats (E; P < 0.05 all young groups vs all middle-aged groups). No differences were found in total CREB (C). *, ** P < 0.05, P < 0.01, respectively.
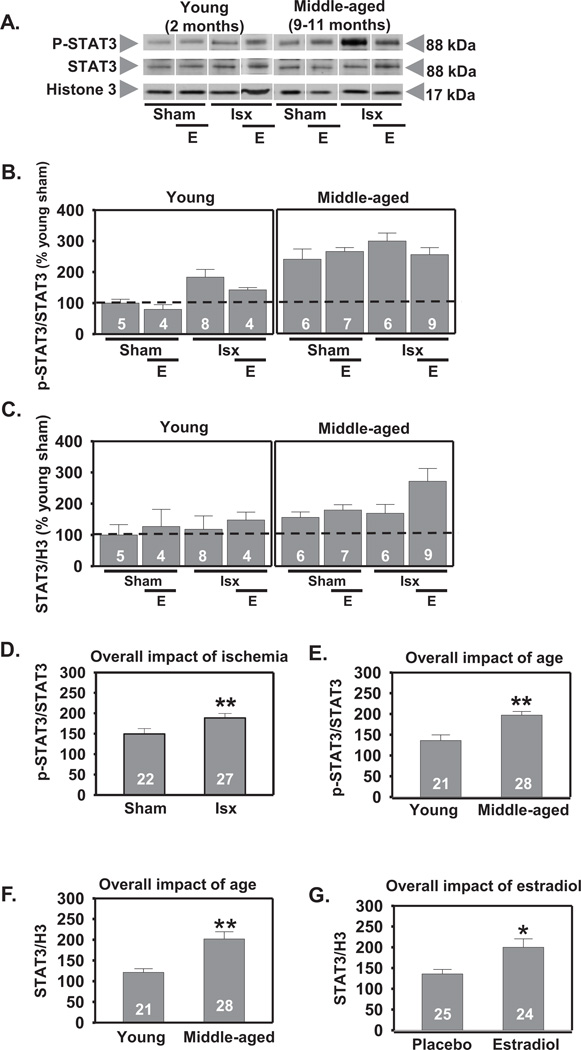
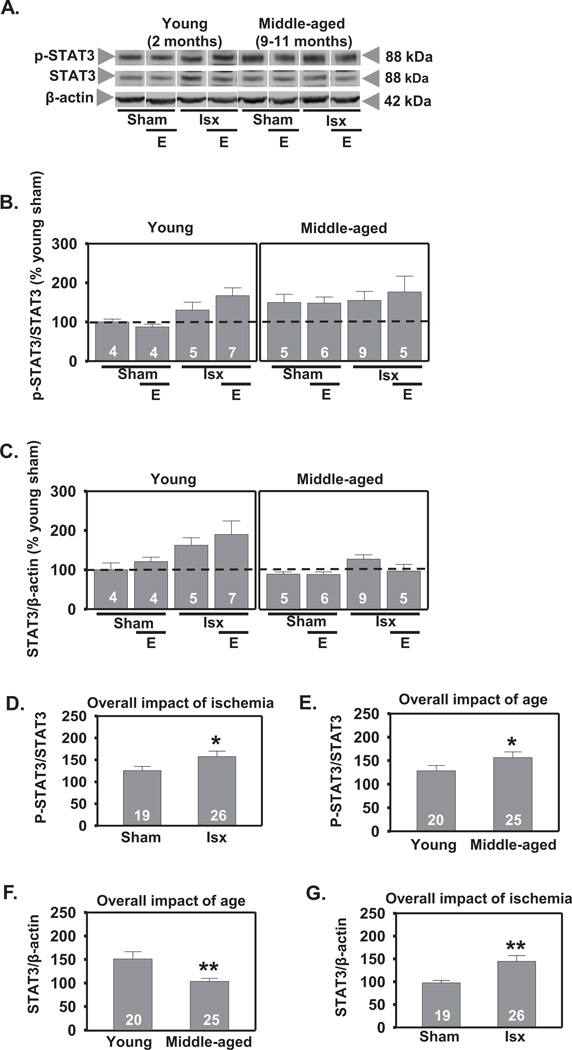
2.3. Ischemia, but not estradiol, increases phosphorylation of STAT3 in the CA1
Both global (Choi et al., 2003) and focal (Justicia et al., 2000; Suzuki et al., 2001) ischemia can activate STAT3 in the brain. We first measured p-STAT3 and total STAT3 in cell nuclei of CA1 at 3 h following global ischemia. There was a significant effect of age with middle-aged females exhibiting higher overall levels of p-STAT3 (F1,48=25.0, P<.0001) and total STAT3 (F1,48=12.1, P<.001) than young rats (increase to 145% and 167% respectively, Scheffe, P < 0.001; Figs. 4A, B, E, F), suggesting an overall elevation of STAT3 signaling in CA1 of middle-aged females. There was also a significant main effect of global ischemia to increase p-STAT3 (F1,48=10.2, P<.003; increase to 127%, Scheffe, P < 0.001 vs sham; Figs. 4A, B, D). Ischemia did not alter total STAT3 (Figs. 4A, C). Estradiol pretreatment did not alter p-STAT3 in sham-operated or ischemic females but did increase total STAT3 in the CA1 3 h following reperfusion (F1,48=4.98, P<.05; increase to 147%, Scheffe, P < .02 vs placebo; Figs. 4A, C, G). There were no significant interactions for either p-STAT3 or total STAT3.
Figure 4.
Effect of age and ischemia on phosphorylation of STAT3 in CA1 cell nuclei 3 h following reperfusion. Representative Western blots (A) and relative abundance of p-STAT3 and STAT3 (B and C) in the nuclear fraction of CA1 of placebo and estradiol (E) treated rats subjected to sham surgery or ischemia (isx). Data are expressed relative to controls (young sham) (B and C). Ischemia increased p-STAT3 in CA1 in both young and middle-aged rats (D; P < 0.01 all sham rats vs all ischemic rats). Both sham-operated and ischemic middle-aged rats had elevated p-STAT3 and total STAT3 compared to young rats (E and F; P < 0.01 all young groups vs all middle-aged groups). Estradiol increased total STAT3 in both young and middle-aged rats (G; P < 0.05 all placebo groups vs all estradiol groups). *, ** P < 0.05 and P < 0.01, respectively.
We also determined the impact of age, ischemia, and estradiol, on p-STAT3 and total STAT3 in whole cell lysates of CA1 at 12 h following reperfusion. There was a significant main effect of age on p-STAT3 with middle-aged females demonstrating elevated p-STAT3 in CA1 (F1,44=4.48, P<.04; increase to 121%, Scheffe, P < 0.04; Fig 5A, B, E). In contrast, total STAT3 abundance was lower in CA1 of middle-aged than of young rats at 12 h (F1,44=9.44, P<.004; decrease by 33%, Scheffe, P < 0.001; Fig 5A, C, F). Ischemia increased the phosphorylation of STAT3 in CA1 in all rats (F1,44=4.50, P<.04; increase to 126%, Scheffe, P < 0.04 vs sham-operated animals; Fig 5A, B, D). There were no significant interactions. There was also a significant increase in total STAT3 abundance in CA1 of all ischemic rats (F1,44=9.99, P< .003, increase to 149%, Scheffe, P < 0.001 vs sham; Fig 5A, C, G). There were no significant interactions. Estradiol did not alter either p-STAT3 or total STAT3 abundance at 12 h.
Figure 5.
Effect of age and ischemia on STAT3 in CA1 12 h following global ischemia. Representative Western blots (A) and relative abundance of p-STAT3 and STAT3 (B and C) in CA1 whole-cell lysates from placebo and estradiol (E) treated rats subjected to sham surgery or ischemia (isx). Data are expressed relative to controls (young sham) (B and C). Ischemia increased p-STAT3 and total STAT3 in CA1 of both young and middle-aged rats (D and G; P < 0.05 all ischemic groups vs all sham groups). Middle-aged rats showed higher levels of p-STAT3 than young rats (E; P < 0.05 all middle-aged groups vs all young groups). Estradiol did not alter levels of either p-STAT3 or total STAT3 at 12 h following ischemia (B and C; P > 0.05). Total STAT3 levels were lower in middle-aged than in young rats (F; P < 0.01 all middle-aged groups vs all young groups). *, ** P < 0.05 and P < 0.01, respectively.
3. DISCUSSION
It is well established that pretreatment with physiological levels of estradiol affords neuroprotection in young and middle-aged female rodents against both focal (Alkayed, 2000; Brown et al., 2009) and global ischemia (De Butte-Smith et al., 2009; Lebesgue et al., 2009). In apparent contrast with these findings, the Women’s Health Initiative Memory Study demonstrated that hormone treatment beginning many years post-menopause does not confer protection against dementia or cognitive decline in elderly women (Espeland et al., 2004; Rapp et al., 2003) . One explanation for these unexpected results is that there is a critical time window after cessation of ovarian function during which estradiol retains its beneficial effects and that these actions may decline following a chronic period of hormone deprivation (Daniel et al., 2006; MacLennan et al., 2006; Selvamani & Sohrabji, 2010; Sherwin, 2009; Suzuki et al., 2007).
Support for the critical period hypothesis comes from both human studies and research in middle-age rodents demonstrating that the timing of hormone treatment determines the effects of estradiol on the brain. For instance, Daniel and colleagues (2006) found that estradiol ameliorated age-related impairments in working memory in middle-aged female rats when initiated immediately following OVX but not when initiated 5 months after OVX. Estradiol also confers protection from middle-cerebral artery occlusion (MCAO)-induced injury when treatment is instigated immediately but not 10 weeks after hormone deprivation (Suzuki et al., 2007). Another recent study (Selvamani & Sohrabji, 2010) showed that estradiol reduced cortical infarct volume in young adult females subjected to MCAO but worsened injury in middle-aged, reproductively senescent females.
In contrast, we recently demonstrated that estradiol pretreatment is neuroprotective in middle-aged females that are OVX for 8 weeks prior to insult (De Butte-Smith et al., 2009; Traub et al., 2009). Hence, the current study investigated the effects of estradiol on the phosphorylation status of several key molecular targets involved in neuronal survival, such as Akt, CREB, and STAT3, in middle-aged female rats subjected to global ischemia 8 weeks after OVX. Importantly, we sought to determine whether estradiol had the same or different effects on these molecular targets in middle-aged and young OVX rats subjected to global ischemia. The results suggest several potential molecular mechanisms underlying the ability of estradiol to ameliorate hippocampal cell death induced by global ischemia in middle-aged females subjected to prolonged hormone deprivation. The present study demonstrates that (1) global ischemia promotes phosphorylation of Akt and STAT3 and dephosphorylation of CREB 3 h after insult in the hippocampal CA1 of middle-aged females. Similar results were found for young animals; (2) estradiol treatment for 14 days prior to induction of ischemia and during the postischemic period prevents ischemia-induced early dephosphorylation of CREB and increases total nuclear STAT3 3 h following reperfusion. Similar results were observed in young animals; (3) middle-aged OVX rats show modestly lower p-CREB and markedly higher p-STAT3 and p-Akt levels in the CA1 than young OVX rats regardless of ischemia or estradiol treatment.
3.1. Effects of age, ischemia, and estradiol on CREB activity in middle-aged female rats
We recently reported that estradiol retains its neuroprotective actions after global ischemia in middle-aged female rats even after 8 weeks of ovarian hormone deprivation (De Butte-Smith et al., 2009; Traub et al., 2009). Both acute (Raval et al., 2009) and chronic (Jover-Mengual et al., 2007) pretreatment with estradiol elevates p-CREB in young female rats subjected to global ischemia. The present study extends the earlier studies in that here we report the novel observation that estradiol retains its ability to maintain nuclear CREB phosphorylation in CA1 of middle-aged females that were OVX for 8 weeks. This provides evidence that maintenance of nuclear CREB signaling may be key for neuronal survival after ischemic insults.
Interestingly, irrespective of hormone or ischemic condition, the levels of p-CREB in CA1 of middle-aged OVX rats were lower than in young OVX rats. This observation is consistent with other findings that phosphorylation of CREB decreases significantly in the dentate gyrus, CA1 and CA3 regions of the hippocampus of middle-aged male rats (Hattiangady et al., 2005). Also consistent with previous reports (Jover-Mengual et al., 2007; Walton et al., 1996), global ischemia promoted dephosphorylation and presumably inactivation of nuclear CREB in the early hours following reperfusion in middle-aged females deprived of estradiol for 8 weeks. CREB activity promotes neuronal survival (Sakamoto et al., 2011); hence, its dephosphorylation in injured neurons may be detrimental for their survival. This is consistent with the finding byWalton et al. (1996) that p-CREB decreases in the highly vulnerable CA1 region of the hippocampus but not in resistant cortical and dentate areas following ischemia in young animals. Similarly, neuronal death is associated with a decrease in CREB phosphorylation following traumatic brain injury (O'Dell et al., 2000). Although not addressed by the present study, global ischemia also decreases expression of the prosurvival CREB target genes brain-derived neurotrophic factor and Bcl-2 in the vulnerable CA1 in young animals (Ferrer et al., 1998; Jover-Mengual et al., 2007). In young female rats, estradiol prevents the ischemia-induced suppression of p-CREB and the post-ischemic decline in Bcl-2 (Jover-Mengual et al., 2007). Thus, maintenance of CREB activity may be causally related to the neuroprotective actions of estradiol in the hippocampus.
Extensive evidence suggests activation of CREB is necessary for the cellular events underlying long-term memory (Bourtchuladze et al., 1994; Mizuno et al., 2002; Yin & Tully, 1996) and that changes in p-CREB in the hippocampus of older rodents is associated with memory impairment (Monti et al., 2005). We recently showed that pretreatment with estradiol enhanced CA1 neuron survival but did not ameliorate ischemia-induced cognitive deficits in middle-aged female rats (De Butte-Smith et al., 2009). Our present results offer a plausible molecular explanation as to why estradiol did not confer cognitive benefit in middle-aged rats. Although estradiol pretreatment prevented the early (3 h) ischemia-induced decrease in p-CREB in CA1 of middle-aged females, overall levels of p-CREB were significantly lower than in young females. Thus, p-CREB levels may have been sufficient for neuronal survival but not high enough to attenuate cognitive deficits induced by global ischemia.
3.2. Effects of ischemia, estradiol, and age on Akt and STAT3 activity
Akt and STAT3 are important modulators of cellular injury, and expression of both is up-regulated in response to brain ischemia (Chong et al., 2005; Planas et al., 2006). Consistent with previous reports (Choi et al., 2003; Endo et al., 2006; Jover-Mengual et al., 2010), our data demonstrate that global ischemia increases the phosphorylation of Akt and STAT3 at both 3 and 12 h after injury in young and middle-aged female rats. Estradiol further increased p-Akt in middle-aged ischemic rats at 3 h post-reperfusion. This finding is consistent with studies showing that estradiol increases phosphorylation of Akt (Endo et al., 2006; Janelidze et al., 2001; Jover-Mengual et al., 2010; Li et al., 2003; Noshita et al., 2001) following cerebral ischemia. Neuroprotective treatments such as estradiol (Jover-Mengual et al., 2010; Wilson et al., 2002) and ischemic preconditioning (Zhan et al., 2010) promote phosphorylation of Akt and subsequent phosphorylation and inactivation of downstream targets implicated in cell death. The ability to markedly elevate p-Akt at 3 h after ischemia in older females may contribute to the hormone’s efficacy in reducing CA1 cell death after global ischemia (De Butte-Smith et al., 2009; Traub et al., 2009).
In contrast with its effects on p-CREB and p-Akt, estradiol did not detectably alter STAT3 phosphorylation in the CA1 of either young or middle-aged animals; however, it did increase total nuclear STAT3 at 3 h. This increase was most apparent in the middle-aged ischemic females. To date, only one study has examined the effects of estradiol on STAT3 signaling in female rats subjected to ischemic brain injury (Dziennis et al., 2007). Unlike our findings, these authors found that estradiol increased STAT3 phosphorylation without affecting total STAT3 in the cerebral cortex following 2 h of middle cerebral artery occlusion. This discrepancy may reflect differences between global and focal ischemia or brain region-specific effects of estradiol on STAT3.
Our study also documents the novel finding that middle-aged OVX rats exhibited higher p-STAT3 and p-Akt levels compared to young OVX animals at 3 h post reperfusion. At 12 h, only p-STAT3 levels were increased, suggesting the elevation of p-Akt in middle-aged rats was a transient effect. Interestingly, this elevation was also observed in sham-operated rats (irrespective of hormone condition), suggesting baseline levels of these signaling molecules may be altered. Whether this elevation is due to age per se or differences in the effect of OVX between young and middle-aged rats is not known. Although OVX duration also differed between the middle-aged and young animals, preliminary data in our laboratory shows similar results using middle-aged rats OVS for 1 week (data not illustrated), suggesting OVX duration cannot account for these findings.
Additionally, we found differences in total STAT3 and Akt signaling between middle-aged and young OVX rats. Specifically, middle-aged OVX females exhibited higher total STAT3 at 3 h compared to young OVX rats while lower levels were detected in middle-aged rats at 12 h. This reduction does not seem to be due to age per se as total STAT3 levels in the different middle-aged groups seem to be comparable to those of the young control. Rather, this difference may be apparent because of a slight elevation in STAT3 in the young ischemic rats at 12 h. Whereas middle-aged rats exhibited an increase in STAT3 a few hours post-reperfusion, young ischemic animals did not exhibit an elevation until 12 h after ischemia. This may reflect a truncated response in the middle-aged animals. In regards to total Akt, middle-aged rats did show an overall reduction in total Akt that was apparent at 3 h but not at 12 h. As with our finding for p-Akt, whether this reduction is due to age per se or differences in the effect of OVX between young and middle-aged rats is not known.
3.3. Conclusion
The current study demonstrates that the baseline activation of several important signaling molecules involved in neuronal survival is altered in the hippocampal CA1 of middle-aged female rats. Specifically, whereas p-Akt and p-STAT3 are higher in the CA1 of middle-aged than young animals, p-CREB is lower. In agreement with published work on neuronal survival, these molecules show similar responses to ischemia and estradiol pretreatment in both age groups. Most notably, estradiol prevents the early ischemia-induced reduction in p-CREB (and presumably CREB activity) in all females. These findings may have important implications for the development of therapeutic treatments for older individuals. This study further demonstrates the importance of including older animals in studies assessing potential neuroprotective treatments for global ischemia.
4. EXPERIMENTAL PROCEDURES
4.1. Animals
This study was conducted in accordance with the National Institutes of Health Guidelines for Care and Use of Animals in Research, and the protocol was approved by the Institutional Animal Care and Use Committee of the Albert Einstein College of Medicine. Female Sprague-Dawley rats [young (2–3 mo) and middle-aged (retired breeders, 9–11 mo)] were obtained from Charles River (Wilmington, DE). Rats were housed 3 per cage, maintained in a temperature and light-controlled environment with a 14 h light:10 h dark cycle, and given food and water ad libitum. As in our past studies (e.g., DeButte-Smith et al., 2009), we used retired breeders for the middle-aged group rather than virgin females, because the majority of middle-aged women have had at least one pregnancy. Therefore, the use of retired breeders is more clinically relevant.
A total of 151 rats entered the study. Of these, 17 died during surgery, and 5 were excluded because they failed to show neurological signs of ischemia, either awakening (n=4) or failing to show both complete loss of righting reflex and dilation of the pupils from 1 min after occlusion was initiated until the end of occlusion (n=1). Rats that died during surgery or failed to show neurological signs of ischemia were not disproportionately represented in any of the age or hormone groups.
4.2. Ovariohysterectomy and estradiol treatment
All rats were OVX under isoflurane (4% induction, 2% maintenance in 70% N2O and 30% O2) anesthesia. Animals were ovariohysterectomized to minimize surgical stress and to allow for comparisons to previous reports (De Butte-Smith et al., 2009; Jover-Mengual et al., 2007; Jover-Mengual et al., 2010). Intact (sham-OVX) animals were not included in the present study because we previously reported no differences in either behavioral or histological outcomes between sham-operated intact and OVX, middle-aged rats (De Butte-Smith et al., 2009). Secondly, the hormonal fluctuations associated with estrous cycle stage make it impossible to control the hormonal status of intact animals at the time of ischemia surgery. Young rats received subcutaneous implants of either 17β-estradiol pellets (0.05 mg, 21d sustained release; Innovative Research of America, Inc., Sarasota, FL) or no pellet (sham surgery) one week following OVX. Middle-aged rats underwent pellet implantation (0.1 mg, 60d sustained release; Innovative Research of America) or sham surgery 8 weeks after OVX. In recent reports (De Butte-Smith et al., 2009; Traub et al., 2009) we found that estradiol was effective in rescuing CA1 pyramidal neurons from ischemia-induced cell death in middle-aged rats that were OVX for 8 weeks before experimentation. Hence, we used the 8 week OVX duration as these animals would be similar to women who are 5–10 years post-menopause. The pellets were selected so that we could compare the results to other reports from our laboratory (De Butte-Smith et al., 2009; Jover-Mengual et al., 2007; Miller et al., 2005), and they yield serum estradiol levels in the physiological range (34–48 pg/ml) (De Butte-Smith et al., 2009).
4.3 Global ischemia
Two weeks following pellet implantation or sham surgery, rats were subjected to global ischemia by four-vessel occlusion as previously described (Jover-Mengual et al., 2007; Jover-Mengual et al., 2010; Miyawaki et al., 2008; Miyawaki et al., 2009). Briefly, rats were deeply anesthetized with isoflurane (4% induction, 2% maintenance in 70% N2O: 30% O2), and the vertebral arteries were irreversibly occluded by electrocoagulation to prevent collateral blood flow to the forebrain during subsequent occlusion of the common carotid arteries. Twenty-four hours later, animals were anesthetized again, the wound was reopened and both carotid arteries were manipulated (sham) or occluded with microarterial clamps for 10 min. During clamping, animals were awake and spontaneously ventilating. Following clamping, arteries were visually inspected to ensure adequate reflow. During both surgeries, rectal temperature was monitored and maintained at 36.5–37.5°C. Ischemic animals that failed to show complete loss of righting reflex and dilation of the pupils from 1 min after occlusion was initiated until the end of occlusion were excluded from the study. Sham-operated rats were subjected to the identical coagulation and carotid artery manipulations, except the carotid arteries were not occluded.
4.4 Western blot analysis
For quantification of protein abundance in the hippocampal CA1, western blot analysis was performed as previously described (Jover-Mengual et al., 2007). Briefly, animals were deeply anesthetized and euthanized at 3 or 12h after global ischemia or sham surgery. Hippocampi were rapidly dissected and cut into 1-mm transverse slices on a McIllwain tissue chopper (Vibratome Co., St. Louis, MO). CA1 from both right and left hippocampus was rapidly separated by microdissection, placed in ice-cold saline containing protease inhibitor cocktail (1%, Sigma-Aldrich, St. Louis, MO) and phosphatase inhibitor cocktail (1%, Sigma-Aldrich), and homogenized in lysis buffer containing HEPES (5 mM), MgCl2 (1 mM), EGTA (2 mM), dithiothreitol (1 mM), sucrose (10%), protease inhibitor cocktail (1%) and phosphatase inhibitor cocktail 1 (1%). prepared by differential centrifugation at the 3 h time-point. At 12 h (p-Akt, Akt, p-STAT3, STAT3) following reperfusion, whole cell lysates were prepared.
Protein concentration was determined using the BCA protein assay (Pierce, Rockford, IL). Protein samples (40 µg) were dissolved in Laemmli sample buffer, loaded onto 4–12% Bis-Tris Midi gels (Invitrogen, Carlsbad, CA), separated by SDS-PAGE and transferred to nitrocellulose membranes for immunoblotting with antibodies as described below. Following incubation with primary and appropriate secondary antibodies, membranes were treated with enhanced chemiluminescence reagents (ECL, GE Healthcare Life Sciences, Piscataway, NJ) and apposed to x-ray film (Eastman Kodak Co., Rochester, NY).
Quantitation of protein abundance was performed as previously described (Jover-Mengual et al., 2010). Briefly, a Scan Jet 4-C computing densitometer (Hewlett-Packard Co., Palo Alto, CA) was used with NIH image 1.61 software. Band densities for p-CREB, p-STAT3, and p-Akt were normalized to the corresponding band densities for total CREB, total STAT3, and total Akt, respectively; normalized means were expressed as a percentage of the corresponding value for control (young OVX, sham-operated) animals. Membranes were stripped and reprobed with anti-β-actin (whole cell and cytosolic fractions) or anti-histone H3 (nuclear fractions) as loading controls. Because it was not possible to run samples from the entire experiment on a single gel, band densities for all samples on a given gel were normalized to the band density of samples from young sham placebo (“control”) animals. Each gel included 2–3 samples from control animals to permit comparisons of data across different cohorts of animals, and different control animals were prepared for each experiment. In addition, all gels had 2–3 samples from each treatment group.
4.5 Antibodies
The following antibodies were used: 1) anti-p-CREB rabbit polyclonal antibody, which recognizes CREB phosphorylated at serine 133 (1:3000; Millipore, Billerica, MA); 2) anti-CREB rabbit polyclonal antibody, which recognizes total CREB (1:3000; Millipore); 3) p-STAT3 rabbit polyclonal antibody which recognizes STAT3 phosphorylated at Tyr 705 (1:3000; Santa Cruz Biotechnology, Santa Cruz, CA); anti-STAT3 rabbit polyclonal, which recognizes total STAT3 (1:1000; Santa Cruz Biotechnology); 4) anti-pAkt rabbit polyclonal antibody, which recognizes Akt phosphorylated at Ser473 (1:1000; Cell Signaling Technology, Danvers, MA); 5) anti-Akt rabbit polyclonal antibody, which recognizes total Akt (1:3000; Cell Signaling Technology); 6) anti-β-actin mouse monoclonal antibody, which recognizes an epitope in the N-terminal domain of the β- isoform of actin (1: 20,000; Sigma-Aldrich); and 7) anti-histone H3 rabbit polyclonal antibody (1:1000; Cell Signaling Technology). Secondary antibodies were horseradish peroxidase-conjugated donkey anti-rabbit IgG (1:5000; GE Life Sciences) for polyclonal antibodies or sheep anti-mouse IgG (1:2500; GE Life Sciences) for monoclonal antibodies.
4.6. Statistical Analysis
Data were normalized as described above and are presented as mean ± standard error of the mean (SEM). Data analysis was performed using Statview®. Statistical comparisons were determined using 3-way (age × hormone × ischemia) ANOVA. When significant main effects and interactions were detected, Scheffe’s post-hoc analyses were used to identify specific between-group differences. Level of significance was set at P < 0.05.
Highlights.
Examined effect of E2 and ischemia on CREB, Akt, and STAT3 in OVX female rats
Ischemia increased phosphorylation of Akt and STAT3 and decreased phosphorylation of CREB
E2 prevented dephosphorylation of CREB and increased total STAT3
Older rats showed lower p-CREB and increased p-Akt and p-STAT3 compared to young
Acknowledgments
The authors gratefully acknowledge support by DHHS grant R01 AG027702 to A.M.E., D.P. Purpura Department of Neuroscience, Albert Einstein College of Medicine and the F.M. Kirby Program in Neural Repair and Protection. The authors would also like to thank Mr. Fabrizio Pontarelli and Ms. Adrianna Latuszek for their technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alkayed NJ, Murphy SJ, Traystman RJ, Hurn PD, Miller VM. Neuroprotective effects of female gonadal steroids in reproductively senescent female rats. Stroke. 2000;31(1):161–168. doi: 10.1161/01.str.31.1.161. [DOI] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79(1):59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Brown CM, Suzuki S, Jelks KA, Wise PM. Estradiol is a potent protective, restorative, and trophic factor after brain injury. Semin Reprod Med. 2009;27(3):240–249. doi: 10.1055/s-0029-1216277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buga AM, Dunoiu C, Balseanu A, Popa-Wagner A. Cellular and molecular mechanisms underlying neurorehabilitation after stroke in aged subjects. Rom J Morphol Embryol. 2008;49(3):279–302. [PubMed] [Google Scholar]
- Chen RL, Balami JS, Esiri MM, Chen LK, Buchan AM. Ischemic stroke in the elderly: an overview of evidence. Nat Rev Neurol. 2010;6(5):256–265. doi: 10.1038/nrneurol.2010.36. [DOI] [PubMed] [Google Scholar]
- Choi JS, Kim SY, Cha JH, Choi YS, Sung KW, Oh ST, et al. Upregulation of gp130 and STAT3 activation in the rat hippocampus following transient forebrain ischemia. Glia. 2003;41(3):237–246. doi: 10.1002/glia.10186. [DOI] [PubMed] [Google Scholar]
- Choi JS, Park HJ, Kim HY, Kim SY, Lee JE, Choi YS, et al. Phosphorylation of PTEN and Akt in astrocytes of the rat hippocampus following transient forebrain ischemia. Cell Tissue Res. 2005;319(3):359–366. doi: 10.1007/s00441-004-1033-0. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Li F, Maiese K. Activating Akt and the brain's resources to drive cellular survival and prevent inflammatory injury. Histol Histopathol. 2005;20(1):299–315. doi: 10.14670/hh-20.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel JM, Hulst JL, Berbling JL. Estradiol replacement enhances working memory in middle-aged rats when initiated immediately after ovariectomy but not after a long-term period of ovarian hormone deprivation. Endocrinology. 2006;147(1):607–614. doi: 10.1210/en.2005-0998. [DOI] [PubMed] [Google Scholar]
- De Butte-Smith M, Gulinello M, Zukin RS, Etgen AM. Chronic estradiol treatment increases CA1 cell survival but does not improve visual or spatial recognition memory after global ischemia in middle-aged female rats. Horm Behav. 2009;55(3):442–453. doi: 10.1016/j.yhbeh.2008.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziennis S, Alkayed NJ. Role of signal transducer and activator of transcription 3 in neuronal survival and regeneration. Rev Neurosci. 2008;19(4–5):341–361. doi: 10.1515/revneuro.2008.19.4-5.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziennis S, Jia T, Ronnekleiv OK, Hurn PD, Alkayed NJ. Role of signal transducer and activator of transcription-3 in estradiol-mediated neuroprotection. J Neurosci. 2007;27(27):7268–7274. doi: 10.1523/JNEUROSCI.1558-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo H, Nito C, Kamada H, Nishi T, Chan PH. Activation of the Akt/GSK3beta signaling pathway mediates survival of vulnerable hippocampal neurons after transient global cerebral ischemia in rats. J Cereb Blood Flow Metab. 2006;26(12):1479–1489. doi: 10.1038/sj.jcbfm.9600303. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Rapp SR, Shumaker SA, Brunner R, Manson JE, Sherwin BB, et al. Conjugated equine estrogens and global cognitive function in postmenopausal women: Women's Health Initiative Memory Study. Jama. 2004;291(24):2959–2968. doi: 10.1001/jama.291.24.2959. [DOI] [PubMed] [Google Scholar]
- Etgen AM, Jover-Mengual T, Suzanne Zukin R. Neuroprotective actions of estradiol and novel estrogen analogs in ischemia: Translational implications. Front Neuroendocrinol. 2011;32(3):336–352. doi: 10.1016/j.yfrne.2010.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Lopez E, Pozas E, Ballabriga J, Marti E. Multiple neurotrophic signals converge in surviving CA1 neurons of the gerbil hippocampus following transient forebrain ischemia. J Comp Neurol. 1998;394(4):416–430. [PubMed] [Google Scholar]
- Hattiangady B, Rao MS, Shetty GA, Shetty AK. Brain-derived neurotrophic factor, phosphorylated cyclic AMP response element binding protein and neuropeptide Y decline as early as middle age in the dentate gyrus and CA1 and CA3 subfields of the hippocampus. Exp Neurol. 2005;195(2):353–371. doi: 10.1016/j.expneurol.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Janelidze S, Hu BR, Siesjo P, Siesjo BK. Alterations of Akt1 (PKBalpha) and p70(S6K) in transient focal ischemia. Neurobiol Dis. 2001;8(1):147–154. doi: 10.1006/nbdi.2000.0325. [DOI] [PubMed] [Google Scholar]
- Jover-Mengual T, Miyawaki T, Latuszek A, Alborch E, Zukin RS, Etgen AM. Acute estradiol protects CA1 neurons from ischemia-induced apoptotic cell death via the PI3K/Akt pathway. Brain Res. 2010;1321:1–12. doi: 10.1016/j.brainres.2010.01.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jover-Mengual T, Zukin RS, Etgen AM. MAPK signaling is critical to estradiol protection of CA1 neurons in global ischemia. Endocrinology. 2007;148(3):1131–1143. doi: 10.1210/en.2006-1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justicia C, Gabriel C, Planas AM. Activation of the JAK/STAT pathway following transient focal cerebral ischemia: signaling through Jak1 and Stat3 in astrocytes. Glia. 2000;30(3):253–270. doi: 10.1002/(sici)1098-1136(200005)30:3<253::aid-glia5>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982;239(1):57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- Kirino T, Tamura A, Sano K. Delayed neuronal death in the rat hippocampus following transient forebrain ischemia. Acta Neuropathol. 1984;64(2):139–147. doi: 10.1007/BF00695577. [DOI] [PubMed] [Google Scholar]
- Lebesgue D, Chevaleyre V, Zukin RS, Etgen AM. Estradiol rescues neurons from global ischemia-induced cell death: multiple cellular pathways of neuroprotection. Steroids. 2009;74(7):555–561. doi: 10.1016/j.steroids.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebesgue D, Traub M, De Butte-Smith M, Chen C, Zukin RS, Kelly MJ, et al. Acute administration of non-classical estrogen receptor agonists attenuates ischemia-induced hippocampal neuron loss in middle-aged female rats. PLoS One. 2010;5(1):e8642. doi: 10.1371/journal.pone.0008642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li F, Omori N, Jin G, Wang SJ, Sato K, Nagano I, et al. Cooperative expression of survival p-ERK and p-Akt signals in rat brain neurons after transient MCAO. Brain Res. 2003;962(1–2):21–26. doi: 10.1016/s0006-8993(02)03774-5. [DOI] [PubMed] [Google Scholar]
- MacLennan AH, Henderson VW, Paine BJ, Mathias J, Ramsay EN, Ryan P, et al. Hormone therapy, timing of initiation, and cognition in women aged older than 60 years: the REMEMBER pilot study. Menopause. 2006;13(1):28–36. doi: 10.1097/01.gme.0000191204.38664.61. [DOI] [PubMed] [Google Scholar]
- Miller NR, Jover T, Cohen HW, Zukin RS, Etgen AM. Estrogen can act via estrogen receptor alpha and beta to protect hippocampal neurons against global ischemia-induced cell death. Endocrinology. 2005;146(7):3070–3079. doi: 10.1210/en.2004-1515. [DOI] [PubMed] [Google Scholar]
- Miyawaki T, Mashiko T, Ofengeim D, Flannery RJ, Noh KM, Fujisawa S, et al. Ischemic preconditioning blocks BAD translocation, Bcl-xL cleavage, and large channel activity in mitochondria of postischemic hippocampal neurons. Proc Natl Acad Sci U S A. 2008;105(12):4892–4897. doi: 10.1073/pnas.0800628105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyawaki T, Ofengeim D, Noh KM, Latuszek-Barrantes A, Hemmings BA, Follenzi A, et al. The endogenous inhibitor of Akt, CTMP, is critical to ischemia-induced neuronal death. Nat Neurosci. 2009;12(5):618–626. doi: 10.1038/nn.2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno M, Yamada K, Maekawa N, Saito K, Seishima M, Nabeshima T. CREB phosphorylation as a molecular marker of memory processing in the hippocampus for spatial learning. Behav Brain Res. 2002;133(2):135–141. doi: 10.1016/s0166-4328(01)00470-3. [DOI] [PubMed] [Google Scholar]
- Monti B, Berteotti C, Contestabile A. Dysregulation of memory-related proteins in the hippocampus of aged rats and their relation with cognitive impairment. Hippocampus. 2005;15(8):1041–1049. doi: 10.1002/hipo.20099. [DOI] [PubMed] [Google Scholar]
- Niewada M, Kobayashi A, Sandercock PA, Kaminski B, Czlonkowska A. Influence of gender on baseline features and clinical outcomes among 17,370 patients with confirmed ischaemic stroke in the international stroke trial. Neuroepidemiology. 2005;24(3):123–128. doi: 10.1159/000082999. [DOI] [PubMed] [Google Scholar]
- Noshita N, Lewen A, Sugawara T, Chan PH. Evidence of phosphorylation of Akt and neuronal survival after transient focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2001;21(12):1442–1450. doi: 10.1097/00004647-200112000-00009. [DOI] [PubMed] [Google Scholar]
- O'Dell DM, Raghupathi R, Crino PB, Eberwine JH, McIntosh TK. Traumatic brain injury alters the molecular fingerprint of TUNEL-positive cortical neurons In vivo: A single-cell analysis. J Neurosci. 2000;20(13):4821–4828. doi: 10.1523/JNEUROSCI.20-13-04821.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planas AM, Gorina R, Chamorro A. Signalling pathways mediating inflammatory responses in brain ischaemia. Biochem Soc Trans. 2006;34(Pt 6):1267–1270. doi: 10.1042/BST0341267. [DOI] [PubMed] [Google Scholar]
- Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner RL, Manson JE, et al. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women's Health Initiative Memory Study: a randomized controlled trial. Jama. 2003;289(20):2663–2672. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- Raval AP, Saul I, Dave KR, DeFazio RA, Perez-Pinzon MA, Bramlett H. Pretreatment with a single estradiol-17beta bolus activates cyclic-AMP response element binding protein and protects CA1 neurons against global cerebral ischemia. Neuroscience. 2009;160(2):307–318. doi: 10.1016/j.neuroscience.2009.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roquer J, Campello AR, Gomis M. Sex differences in first-ever acute stroke. Stroke. 2003;34(7):1581–1585. doi: 10.1161/01.STR.0000078562.82918.F6. [DOI] [PubMed] [Google Scholar]
- Sakamoto K, Karelina K, Obrietan K. CREB: a multifaceted regulator of neuronal plasticity and protection. J Neurochem. 2011;116(1):1–9. doi: 10.1111/j.1471-4159.2010.07080.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvamani A, Sohrabji F. Reproductive age modulates the impact of focal ischemia on the forebrain as well as the effects of estrogen treatment in female rats. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherwin BB. Estrogen therapy: is time of initiation critical for neuroprotection? Nat Rev Endocrinol. 2009;5(11):620–627. doi: 10.1038/nrendo.2009.193. [DOI] [PubMed] [Google Scholar]
- Sutherland GR, Dix GA, Auer RN. Effect of age in rodent models of focal and forebrain ischemia. Stroke. 1996;27(9):1663–1667. doi: 10.1161/01.str.27.9.1663. discussion 1668. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Brown CM, Dela Cruz CD, Yang E, Bridwell DA, Wise PM. Timing of estrogen therapy after ovariectomy dictates the efficacy of its neuroprotective and antiinflammatory actions. Proc Natl Acad Sci U S A. 2007;104(14):6013–6018. doi: 10.1073/pnas.0610394104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Brown CM, Wise PM. Neuroprotective effects of estrogens following ischemic stroke. Front Neuroendocrinol. 2009;30(2):201–211. doi: 10.1016/j.yfrne.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S, Tanaka K, Nogawa S, Dembo T, Kosakai A, Fukuuchi Y. Phosphorylation of signal transducer and activator of transcription-3 (Stat3) after focal cerebral ischemia in rats. Exp Neurol. 2001;170(1):63–71. doi: 10.1006/exnr.2001.7701. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr Opin Neurobiol. 2004;14(3):311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Nogawa S, Nagata E, Ito D, Suzuki S, Dembo T, et al. Persistent CREB phosphorylation with protection of hippocampal CA1 pyramidal neurons following temporary occlusion of the middle cerebral artery in the rat. Exp Neurol. 2000;161(2):462–471. doi: 10.1006/exnr.1999.7313. [DOI] [PubMed] [Google Scholar]
- Towfighi A, Saver JL, Engelhardt R, Ovbiagele B. A midlife stroke surge among women in the United States. Neurology. 2007;69(20):1898–1904. doi: 10.1212/01.wnl.0000268491.89956.c2. [DOI] [PubMed] [Google Scholar]
- Traub ML, De Butte-Smith M, Zukin RS, Etgen AM. Oestradiol and insulin-like growth factor-1 reduce cell loss after global ischaemia in middle-aged female rats. J Neuroendocrinol. 2009;21(12):1038–1044. doi: 10.1111/j.1365-2826.2009.01927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton M, Dragunow I. Is CREB a key to neuronal survival? Trends Neurosci. 2000;23(2):48–53. doi: 10.1016/s0166-2236(99)01500-3. [DOI] [PubMed] [Google Scholar]
- Walton M, Sirimanne E, Williams C, Gluckman P, Dragunow M. The role of the cyclic AMP-responsive element binding protein (CREB) in hypoxic-ischemic brain damage and repair. Brain Res Mol Brain Res. 1996;43(1–2):21–29. doi: 10.1016/s0169-328x(96)00144-1. [DOI] [PubMed] [Google Scholar]
- Wilson ME, Liu Y, Wise PM. Estradiol enhances Akt activation in cortical explant cultures following neuronal injury. Brain Res Mol Brain Res. 2002;102(1–2):48–54. doi: 10.1016/s0169-328x(02)00181-x. [DOI] [PubMed] [Google Scholar]
- Yin JC, Tully T. CREB and the formation of long-term memory. Curr Opin Neurobiol. 1996;6(2):264–268. doi: 10.1016/s0959-4388(96)80082-1. [DOI] [PubMed] [Google Scholar]
- Zhan L, Wang T, Li W, Xu ZC, Sun W, Xu E. Activation of Akt/FoxO signaling pathway contributes to induction of neuroprotection against transient global cerebral ischemia by hypoxic pre-conditioning in adult rats. J Neurochem. 2010;114(3):897–908. doi: 10.1111/j.1471-4159.2010.06816.x. [DOI] [PubMed] [Google Scholar]
- Zhao H, Sapolsky RM, Steinberg GK. Phosphoinositide-3-kinase/akt survival signal pathways are implicated in neuronal survival after stroke. Mol Neurobiol. 2006;34(3):249–270. doi: 10.1385/MN:34:3:249. [DOI] [PubMed] [Google Scholar]