Abstract
Purpose
To assess the clinical activity and safety of gemcitabine (G) plus capecitabine (X) in metastatic renal cell cancer (mRCC) patients previously treated with immunotherapy.
Methods
In this phase II trial, patients received G 1000 mg/m2 intravenously on days 1, 8 and 15, plus X 830 mg/m2 orally twice daily on days 1-21 of 28-day cycles. The primary endpoint was progression-free survival (PFS) time. Secondary endpoints included overall survival (OS) time, objective response rate (ORR), and toxicity.
Results
Of 84 patients enrolled, 83 were evaluable for response and toxicity. Sixty-five patients had intermediate- or poor-risk prognosis. Median PFS and OS times were 4.6 months (95% CI, 3.7-7.3) and 17.9 months (95% CI, 13.2-23.6), respectively. There were one complete response and six partial responses [ORR 8.4% (95% CI, 3.5-16.6)]. Two patients remain in unmaintained remission close to 3 years from initiation of GX. By multivariate analyses, >3 disease sites was significantly associated with shorter PFS time, and patients with thrombocytosis, >3 disease sites or anemia had a significantly increased risk of death. Adverse events, occurring at least once in >5% of patients, included grade ≥3 neutropenia (83%), grade ≥2 hand-foot syndrome (13%), grade ≥3 thrombocytopenia (12%), grade ≥3 thromboembolic events (8%), grade ≥3 fatigue (8%), and grade ≥2 mucositis (6%).
Conclusions
At the doses and schedule tested, GX demonstrated a modest clinical activity in mRCC after cytokine failure and produced significant neutropenia. A modified GX regimen may be evaluated in patients with mRCC after failure of approved targeted therapies.
Keywords: chemotherapy, metastatic renal cell carcinoma, targeted therapies, angiogenesis inhibition
Introduction
Until recently, there was no standard approach for the management of metastatic renal cell cancer (mRCC) patients who developed progressive disease after cytokine therapy. Patients with disease progression during first-line immunotherapy did not benefit from additional cytokine treatment. Therefore, the salvage setting after cytokine failure provided an area of investigation for new therapies in mRCC.
Novel agents, targeting the vascular endothelial growth factor (VEGF) pathway, have shown clinical benefit in patients previously treated with immunotherapy 1-3. Two multi-tyrosine kinase inhibitors (TKI), sorafenib and sunitinib, received regulatory approval based on improved progression-free survival (PFS) in the second-line setting 2, 3. While targeted agents have significantly influenced the management of mRCC, their long-term impact on overall survival (OS) is not known. Furthermore, many patients do not respond initially to these agents or develop progressive disease after tumor stabilization or shrinkage. Therefore, there continues to be a need to investigate alternative therapies for the management of mRCC.
A frequently studied chemotherapeutic agent in mRCC, 5-fluorouracil (5-FU), produced a 5% response rate 4. Gemcitabine yielded 6% and 8.1% response rates in two phase II trials 5, 6. A phase II trial of gemcitabine plus infusional 5-FU produced a 17% partial response (PR) rate in previously treated patients 7. However, this regimen requires an in-dwelling intravenous catheter and an infusion pump and carries the risks of catheter-related complications.
Capecitabine offers the advantage of being an oral prodrug of 5-FU and is selectively activated within the tumor, mainly through the action of thymidine phosphorylase. Capecitabine produced an 8.7% PR rate in mRCC previously treated with immunotherapy 8. Preclinical synergy provided the rationale for combining gemcitabine plus capecitabine in mRCC. Two phase II trials of this regimen in previously treated mRCC have been published. Waters et al administered gemcitabine at 1200 mg/ m2 on days 1 and 8 and capecitabine 1300 mg/m2 twice daily on days 1-14 of 21-day cycles 9. Among 19 patients treated, three had a PR. Median time to progression (TTP) and OS time were 7.6 months and 14.2 months, respectively. Stadler et al administered gemcitabine at 1000 mg/m2 on days 1, 8 and 15 and capecitabine at 830 mg /m2 twice daily on days 1-21 of 28-day cycles 10. Among 56 patients treated, six had a PR, with median TTP and OS time 5.6 months and 14.5 months, respectively.
Herein, we report our experience with gemcitabine plus capecitabine (GX) in a larger number of mRCC patients previously treated with immunotherapy.
Materials and Methods
Patients were registered to this study if they had mRCC with progressive disease (PD) after or during IL-2 and/or IFN-α therapy, measurable disease, life expectancy > 12 weeks, Eastern Cooperative Oncology Group (ECOG) performance status ≤ 2, adequate organ and marrow function, and signed an informed consent. Patients with creatinine clearance < 30 ml/min, brain metastasis, > 3 prior therapies, or taking warfarin therapeutically were excluded.
A chest X-ray, CT scans of the chest/abdomen/pelvis, MRI of the brain and an electrocardiogram were obtained within 4 weeks from enrollment. CBC, differential, platelet count, routine chemistries, and prothrombin time were obtained within 2 weeks from enrollment. During therapy, patients had weekly blood counts and chemistries every 4 weeks. CT scans were repeated every 8 weeks. Response was assessed by RECIST.
The treatment schema followed the published phase I trial 11. We administered gemcitabine (G) at 1000 mg/m2 intravenously over 60 minutes on days 1, 8, and 15 and capecitabine (X) at 830 mg/m2 orally twice daily on days 1-21, during 28-day cycles. For patients with creatinine clearance 30-50 ml/min at baseline or during therapy, X was given at 622 mg/m2 twice daily for 21 days.
Management of Toxicity and Dose Modifications
All toxicities were assessed using the National Cancer Institute Common Toxicity Criteria, version 2.0. A cycle interrupted for toxicity was not completed; however, a new cycle would begin, as long as one but not > 3 weeks had elapsed from interruption of therapy. Adverse events (AEs) had to resolve or improve to grade 1 or baseline prior to commencing a new cycle. The protocol initially allowed 2 dose reductions per drug (25%, 50%) and later an additional reduction of 20% for each drug. During any given cycle, therapy with both agents was withheld for platelet count < 75,000/μl or ANC < 1000/μl on days 8 or 15. For subsequent cycles, the doses of both drugs were decreased by 1 dose level. For hepatotoxicity grade 2, dermatitis grade 3, or nausea/vomiting grade ≥3 despite adequate prophylaxis, both drugs were withheld; for subsequent cycles, the doses of G and X were decreased by 1 dose level (2 dose levels for grade 3 hepatotoxicity). For AEs grade ≥2 felt to be secondary to X such as mucositis, diarrhea or hand-foot syndrome, both drugs were withheld; for subsequent cycles, the dose of G was resumed at 100% but the dose of X was decreased by 1 dose level for grade 2 and 2 dose levels for grade >2. For pneumonitis grade ≥2, hepatotoxicity/dermatitis grade 4, or recurrent hepatotoxicity/mucositis/diarrhea grade ≥2, protocol treatment was discontinued.
Statistical Considerations
Patients were accrued up to a planned maximum sample size of 84. The primary endpoint of this single-arm phase II trial was PFS time. Secondary endpoints were objective response rate (ORR), OS time, and toxicity. A combined futility-safety monitoring rule was constructed using the Bayesian method of Thall et al 12. The rule was defined in terms of the “time to treatment failure” ,TF, defined as the more general event including disease progression or death, and also any of these events: (1) toxicity or intercurrent illness precluding further administration of GX, (2) patient drop-out, (3) physician decision to change therapy. The monitoring rule was constructed and applied as follows: At any given point in the trial, the data from each patient consisted of TF or, if the patient had not had a failure event, the time since the start of the patient's therapy, that is, the administrative censoring time in the latter case. Denote the historical mean of TF obtained with 5-FU plus gemcitabine (FG) by mFG and the mean of TF with GX by mGX. FG was chosen as the basis for comparison in the early stopping rule because at that time, it had shown beneficial clinical effect. Based on historical experience with FG, including published PFS time results7 and unpublished data on patients treated with FG at our institution, it was assumed that mFG followed an inverse gamma (IG) prior distribution with mean 7.7 months and variance 12.2. These values were based on PFS time rather than failure time, since the more general failure time data with FG were not available. It was assumed that mGX followed an IG prior distribution with the same mean of 7.7 months but with variance 1000, to reflect little prior knowledge about mGX. The trial would have been stopped early if, based on the current data, Pr(mFG < mGX | data) < .14. That is, the trial would have been stopped early if, based on the current data, it was unlikely that the mean failure time with GX was at least the mean failure time with FG. The probability cutoff .14 was chosen to obtain a .05 false negative (early stopping) probability if the true median time-to-failure were 7 months. The rule was applied each time a new patient was eligible for enrollment in the trial, with the probability criterion recomputed based on the most recent data available at that time. Because the mean of mFG was set equal to the historical mean PFS time of 7.7 months, and this was a single-arm of GX only and no additional data on FG were obtained, the stopping rule was conservative in that it was more likely to stop the trial than if a smaller prior mean value for mFG that might be considered to correspond to mean failure time had been used. However, this additional measure of safety was considered desirable. The maximum sample size of 84 was chosen to ensure a maximum trial duration of 20 months, based on an anticipated accrual rate of 6 patients per month and final follow up of 6 months, and also to ensure reasonably reliable final estimators of median PFS time and median failure time. For example, if 70 patients progressed or died with a “total time on test” (the sum of all times to progression or death, or last follow up) of 539 months, corresponding to an empirical mean PFS time of 539/70 = 7.7 months, then a posterior 95% credible interval (ci) for mean PFS time would be 6.15-9.63. Since median = ln(2) x mean under the exponential distribution, the corresponding 95% ci for median PFS time would be 4.26-6.68 with these data. Unadjusted PFS and OS time distributions were estimated using the Kaplan-Meier method. The Cox regression model was used to account for the effects of patient prognostic covariates on PFS and OS times with goodness-of-fit assessed using the Grambsch-Therneau test and martingale residual plots 13.
Results
Patient Characteristics
Between June 26th, 2003 and January 6th 2005, 84 patients were enrolled. One patient withdrew consent 9 days after registration and received no further therapy. Characteristics of the 83 evaluable patients are summarized in Table 1. More than 75% of the patients had intermediate-risk or poor-risk prognosis, as defined by Motzer et al 14.
Table 1. Patient Characteristics (N=83).
| Variable | N | |
|---|---|---|
| Age | ||
| Median (range) : 61 (36 - 78) | ||
| Gender | ||
| Men | 65 | |
| Women | 18 | |
| ECOG performance status | ||
| 0 | 22 | |
| 1 | 48 | |
| 2 | 13 | |
| Histology | ||
| Conventional | 76 | |
| Papillary | 4 | |
| Sarcomatoid | 3 | |
| Prior nephrectomy | ||
| Yes | 77 | |
| No | 6 | |
| Number of disease sites | ||
| 1 | 15 | |
| 2 | 38 | |
| ≥3 | 30 | |
| Disease sites | ||
| Lung | 65 | |
| Lymph Nodes | 44 | |
| Liver | 24 | |
| Bone | 23 | |
| Others | 49 | |
| MSKCC* prognostic groups | ||
| Favorable | 18 | |
| Intermediate | 45 | |
| Poor | 20 | |
| Number of prior regimens | ||
| 1 | 64 | |
| ≥2 | 19 | |
| Prior systemic therapies** | ||
| IFN-α | 52 | |
| High-dose bolus IL-2 | 15 | |
| Thalidomide combinations | 14 | |
| Bevacizumab + erlotinib | 3 | |
| Sorafenib | 2 |
MSKCC: Memorial Sloan-Kettering Cancer Center
One patient each received erlotinib, temsirolimus, temsirolimus plus IFN-α, heat shock protein peptide complex autologous tumor vaccine, continuous IL-2 infusion, IFN-α plus 13-cis-retinoic acid, ABT-751.
Treatment Outcomes
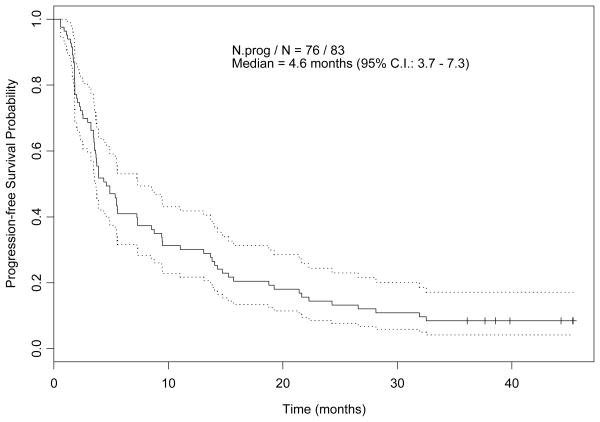
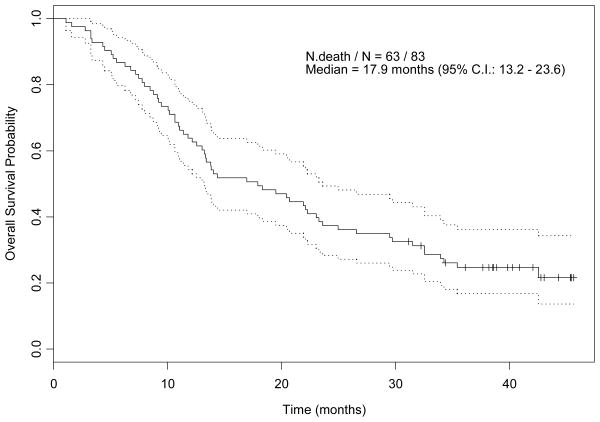
The median follow up time for all 83 patients, as of July 30, 2007, was 40.3 months (range, 31.1- 45.6). Kaplan-Meier curves for PFS and OS are shown in Figures 1A and 1B respectively, with median PFS and OS times 4.6 months (95% CI, 3.7-7.3), and 17.9 months (95% CI, 13.2-23.6), respectively. For 19 patients who had received thalidomide, bevacizumab, sorafenib, or temsirolimus, in addition to immunotherapy, median PFS and OS times were 7.3 months (95% CI, 4.3-18.8) and 14 months (95% CI, 7.9-35.4), respectively. All 83 patients experienced treatment failure, with median failure time 3.9 months (95% CI, 3.5-5.5). Univariate and multivariate Cox proportional hazards regression models for PFS are shown in Tables 2A and 2B, respectively. By multivariate analysis, number of disease sites >3 was significantly associated with a shorter PFS time. Univariate and multivariate Cox proportional hazards regression models for OS are shown in Tables 3A and 3B, respectively. By multivariate analysis, patients with a higher platelet count, >3 disease sites or anemia had a significantly increased risk of death.
Figure 1.
A: Kaplan-Meier Estimate of Progression-free Survival Probability
B: Kaplan-Meier Estimate of Overall Survival Probability
Table 2A. Univariate Cox Proportional Hazards Models for Progression-free Survival.
| Variable | Relative Risk | 95% Confidence Intervals | P-value |
|---|---|---|---|
| Number of disease sites >=4 (versus <4) | 2.43 | 1.39 – 4.23 | 0.002 |
| Prior nephrectomy = yes (versus no) | 0.98 | 0.39 - 2.43 | 0.96 |
| Number of prior therapy >1 (versus 1) | 0.65 | 0.37 - 1.13 | 0.13 |
| MSKCC risk group = good (versus intermediate) | 0.87 | 0.49 - 1.54 | 0.64 |
| MSKCC risk group = poor (versus intermediate) | 1.42 | 0.82 - 2.47 | 0.21 |
| WBC | 0.99 | 0.91 - 1.07 | 0.81 |
| Log(platelet count) | 1.41 | 0.83 - 2.39 | 0.20 |
| Log(albumin) | 0.98 | 0.23 - 4.27 | 0.98 |
| Corrected serum calcium | 1.35 | 1.04 - 1.75 | 0.02 |
| Log(alkaline phosphatase) | 1.03 | 0.69 - 1.54 | 0.89 |
| Log(LDH) | 1.42 | 0.86 - 2.34 | 0.17 |
| Hemoglobin | 0.87 | 0.77 – 0.98 | 0.02 |
| PS = 2 (versus 0, 1) | 1.45 | 0.78 – 2.71 | 0.24 |
Table 2B. Multivariate Cox Proportional Hazards Model for Progression-free Survival.
| Variable | Relative Risk | 95% Confidence Intervals | P-value |
|---|---|---|---|
| Number of disease sites >=4 (versus <4) | 2.14 | 1.21 – 3.78 | 0.009 |
| Hemoglobin | 0.90 | 0.80 – 1.01 | 0.08 |
Table 3A. Univariate Cox Proportional Hazards Models for Overall Survival.
| Variable | Relative Risk | 95% Confidence Intervals | P-value |
|---|---|---|---|
| Prior nephrectomy = yes (versus no) | 0.75 | 0.30 - 1.88 | 0.54 |
| Number of prior therapy >1 (versus 1) | 1.27 | 0.72 - 2.25 | 0.40 |
| MSKCC risk group = good (versus intermediate) | 0.45 | 0.21 - 0.93 | 0.03 |
| MSKCC risk group = poor (versus intermediate) | 1.85 | 1.06 - 3.25 | 0.03 |
| WBC | 1.01 | 0.93 - 1.10 | 0.76 |
| Log(platelet count) | 3.36 | 1.82 - 6.18 | 0.0001 |
| Log(albumin) | 0.12 | 0.02 - 0.76 | 0.02 |
| Corrected serum calcium | 1.61 | 1.22 - 2.14 | 0.0009 |
| Log(alkaline phosphatase) | 1.7 | 1.12 - 2.58 | 0.01 |
| Log(LDH) | 1.71 | 0.92 - 3.19 | 0.09 |
| Hemoglobin | 0.76 | 0.67 – 0.86 | < 0.0001 |
| PS = 2 (versus 0, 1) | 2.64 | 1.39 – 4.99 | 0.003 |
Table 3B. Multivariable Cox Proportional Hazards Model for Overall Survival.
| Variable | Relative Risk | 95% Confidence Intervals | P-value |
|---|---|---|---|
| Log(platelet count) | 2.77 | 1.37 – 5.61 | 0.005 |
| Number of disease sites>=4 (versus <4) | 3.03 | 1.67 – 5.50 | 0.0003 |
| Hemoglobin | 0.83 | 0.72 – 0.95 | 0.006 |
There were one complete response and six PRs for ORR of 8.4% (95% CI, 3.5-16.6). Fifty-six patients had stable disease as their best response and 20 patients had PD. The characteristics of the responding patients are shown in Table 4. It is noteworthy that two responders had received prior sorafenib. It is also noteworthy that two patients remain in unmaintained remission for close to three years, from initiation of GX.
Table 4. Characteristics of Responders.
| Acc # | 41 | 17 | 27 | 22 | 14 | 63 | 80 |
| Age | 55 | 56 | 59 | 62 | 46 | 48 | 45 |
| Gender | M | M | F | M | F | M | M |
| Performance status | 1 | 2 | 1 | 0 | 2 | 1 | 1 |
| Prognostic group | Intermediate | Poor | Favorable | Intermediate | Intermediate | Favorable | Favorable |
| Prior therapy | IFN | IL-2; Bay43-9006 | IL-2 + Thalidomide + GMSCF | IFN; High-dose IL-2 | High-dose IL-2 | IFN + Thalidomide; High-dose IL-2; Bay 43-9006 | IFN |
| Prior nephrectomy | No | Yes | Yes | Yes | Yes | Yes | Yes |
| Pathology | Conventional | Conventional | Conventional | Conventional | Conventional | Conventional | Conventional |
| Metastatic site(s) | Lymph nodes; adrenal glands | Liver; bone; pleura | Lungs | Lungs | Lungs | Lungs; lymph nodes | Lungs |
| Treatment duration (wks) | 19 | 99 | 63 | 57 | 30 | 29 | 52 |
| Response duration (wks) | 16+ | 195+ | 56 | 183+ | 27 | 25+ | 92 |
The median number of cycles administered was 5 (range, 1-26). Only 24 patients were able to receive their first cycle at full doses of both agents without interruption, and only 19 patients were able to receive all their cycles without dose-limiting toxicity (DLT). There were no therapy-related deaths. Thirteen patients were taken off protocol treatment, three due to intercurrent illnesses and ten because of DLT (Table 5). Seventy-seven patients (93%) had grade 2 AEs, 62 patients (75%) had grade 3 AEs and 18 patients (22%) had grade 4 AEs, at least once. Summarized by maximum grade of any AE, 18 patients (22%) had grade 2 AEs, 45 patients (54%) had grade 3 AEs, and 18 patients (22%) had grade 4 AEs. Dose-limiting neutropenia, hand-foot syndrome, thrombocytopenia, thromboembolic events, fatigue and mucositis occurred in 83%, 13%, 12%, 8%, 8%, and 6% of patients, respectively (Table 6).
Table 5. Reasons for Protocol Discontinuation.
| Intercurrent Illness (n = 3 patients) | |
| Small bowel obstruction due to adhesions requiring surgery | 1 |
| Myocardial infarction/congestive heart failure/renal insufficiency | 1 |
| Deep vein thrombosis/heparin-induced thrombocytopenia | 1 |
| Dose Limiting Toxicity (n = 10 patients) | |
| Gemcitabine-related pneumonitis | 2 |
| Renal insufficiency | 2 |
| Recurrent thrombocytopenia | 1 |
| Recurrent neutropenia | 1 |
| Fatigue | 1 |
| Fatigue/diarrhea | 1 |
| Renal insufficiency/thrombocytopenia | 1 |
| Thrombocytopenia/fatigue | 1 |
Table 6. Toxicity.
Shown here are AEs with Frequency >1%
| Adverse Events | Grade 2 | Grade 3 | Grade 4 | |||
|---|---|---|---|---|---|---|
|
| ||||||
| N* | n** | N* | n** | N* | n** | |
| Hematologic | ||||||
| Neutropenia | 101 | 11 | 83 | 59 | 10 | 10 |
| Leukopenia | 69 | 7 | 18 | 11 | 1 | 1 |
| Thrombocytopenia | 43 | 7 | 11 | 9 | 1 | 1 |
| Anemia | 36 | 6 | 7 | 3 | 1 | 1 |
| Gastrointestinal/Hepatic | ||||||
| Diarrhea | 10 | 1 | 4 | 3 | 0 | 0 |
| Nausea | 12 | 1 | 3 | 3 | 0 | 0 |
| Vomiting | 10 | 1 | 3 | 3 | 0 | 0 |
| Mucositis | 23 | 2 | 3 | 3 | 0 | 0 |
| Elevated ALT | 3 | 1 | 2 | 0 | 1 | 1 |
| Cutaneous | ||||||
| Rash/desquamation | 10 | 2 | 0 | 0 | 0 | 0 |
| Hand-foot skin reaction | 35 | 6 | 5 | 5 | 0 | 0 |
| Constitutional/Infectious | ||||||
| Fatigue | 18 | 1 | 11 | 7 | 1 | 0 |
| Fever*** | 14 | 2 | 0 | 0 | 0 | 0 |
| Infection without neutropenia | 5 | 0 | 5 | 4 | 0 | 0 |
| Cardiovascular | ||||||
| Thromboembolism | 0 | 0 | 6 | 5 | 2 | 2 |
| Cardiac-ischemia/infarction | 0 | 0 | 0 | 1 | 1 | 1 |
N represents number of AEs in each category and grade with frequency ≥1%
n represents number of patients having at least one episode of the AE at grade ≥2
Includes one episode of neutropenic fever
Discussion
This large phase II trial of GX in mRCC patients previously treated with immunotherapy confirms the results of two smaller phase II trials with the same regimen in similar patient populations. Notwithstanding the pitfalls of comparisons across non-randomized trials, particularly in a heterogeneous disease such as mRCC, the observed 8.4% ORR (95% CI, 3.5-16.6) and the 4.6 months median PFS time (95% CI, 3.7-7.3) are comparable to those reported by Waters et al 9 and Stadler et al 10. In our study, number of disease sites was predictive of PFS and OS, confirming Stadler et al's finding 15. Furthermore, and consistent with previous reports, anemia 14 and thrombocytosis 16 were independent prognostic factors for OS. It is not possible to estimate the individual contributions of gemcitabine or capecitabine to the modest clinical efficacy of this regimen, but the ORR with the combination was in the range observed with either agent alone. Although it is reasonable to assume that the antitumor activity of these agents is related to their direct cytotoxicity, the exact mechanism underlying tumor regression is not known.
The antitumor activity of GX is comparable to that of bevacizumab, sorafenib, and temsirolimus in the salvage setting 1, 3, 17, and many adverse events observed in this trial are similar to those reported with TKI, although the incidence and grade of myelosuppression were higher in our study. Approximately 25% of the patients in our study had more than one prior regimen, unlike the patients reported by Yang et al 1 and Escudier et al 3 who all had only one prior therapy. Although OS depends on therapy administered during a trial and subsequent therapies, the 17.9 months median OS time observed in our study is comparable to that reported by Escudier et al 3. The durability of response in two patients with clear-cell mRCC who had received prior sorafenib suggests there is a subset of patients with clear-cell mRCC who would benefit from this regimen, even after TKI failure. It is noteworthy that the median PFS for the subgroup of patients who had received thalidomide, bevacizumab, sorafenib or temsirolimus, in addition to immunotherapy, was 7.3 months. However, our data, together with Waters et al's 9 and Stadler et al's 10, suggest this regimen has no demonstrable activity in papillary mRCC. In this trial, neutropenia was substantial; therefore, if GX is to be further developed in mRCC, we recommend modification of the dosage and/or schedule.
Since the newly approved agents, sorafenib, sunitinib, and temsirolimus are not curative 3, 18, 19, it is reasonable to evaluate the GX regimen in clear-cell mRCC after failure of targeted therapies. Future research should focus on identifying the molecular features associated with the clinical benefit of this regimen in responding patients. A rationale for combining chemotherapy with anti-VEGF agents exists and is based on preclinical data that anti-VEGF therapy normalizes tumor vasculature and leads to the improved delivery of chemotherapeutic agents to tumor cells 20. Encouraged by the results of our trial, we have initiated a phase II protocol to evaluate the activity of gemcitabine plus capecitabine plus bevacizumab in advanced RCC.
In summary, this trial confirms the modest clinical activity of GX in clear-cell mRCC. Despite the toxicity observed, a modified GX regimen warrants further evaluation in patients with clear-cell mRCC, after failure of TKI and mammalian target of rapamycin (mTOR) inhibitors.
Acknowledgments
The authors wish to thank Ms. Charla McMichael for help with the enrollment of patients, Ms. Loretta Patterson for data collection, and Ms. Gloria Curtis for help in the manuscript preparation.
Supported in part by Roche and Eli Lilly and Company
Abbreviations
- mRCC
metastatic renal cell cancer
- IL-2
interleukin-2
- IFN-α
interferon alfa
- VEGF
vascular endothelial growth factor
- PFS
progression-free survival
- OS
overall survival
- 5-FU
5-fluorouracil
- PR
partial response
- TTP
time to progression
- PD
progressive disease
- ORR
objective response rate
Footnotes
Presented in part at the 41st Annual Meeting of the American Society of Clinical Oncology, Orlando, FL, May 13-17, 2005.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, et al. A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med. 2003;349:427–434. doi: 10.1056/NEJMoa021491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Motzer RJ, Rini BI, Bukowski RM, Curti BD, George DJ, Hudes GR, et al. Sunitinib in patients with metastatic renal cell carcinoma. JAMA. 2006;295:2516–2524. doi: 10.1001/jama.295.21.2516. [DOI] [PubMed] [Google Scholar]
- 3.Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, et al. Sorafenib in advanced clear-cell renal cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- 4.Kish JA, Wolf M, Crawford ED, Leimert JT, Bueschen A, Neefe JR, et al. Evaluation of low-dose continuous infusion 5-Fluorouracil in patients with advanced and recurrent renal cell carcinoma. A Southwest Oncology Group Study. Cancer. 1994;74:916–919. doi: 10.1002/1097-0142(19940801)74:3<916::aid-cncr2820740319>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 5.Mertens WC, Eisenhauer EA, Moore M, Venner P, Stewart D, Muldal A, et al. Gemcitabine in advanced renal cell carcinoma. A phase II study of the National Cancer Institute of Canada Clinical Trials Group. Ann Oncol. 1993;4:331–332. doi: 10.1093/oxfordjournals.annonc.a058494. [DOI] [PubMed] [Google Scholar]
- 6.de Mulder PH, Weissbach L, Jakse G, Osieka R, Blatter J. Gemcitabine: a phase II study in patients with advanced renal cancer. Cancer Chemother Pharmacol. 1996;37(5):491–495. doi: 10.1007/s002800050417. [DOI] [PubMed] [Google Scholar]
- 7.Rini BI, Vogelzang NJ, Dumas MC, Wade JL, 3rd, Taber DA, Stadler WM. Phase II trial of weekly intravenous gemcitabine with continuous infusion fluorouracil in patients with metastatic renal cell cancer. J Clin Oncol. 2000;18:2419–2426. doi: 10.1200/JCO.2000.18.12.2419. [DOI] [PubMed] [Google Scholar]
- 8.Wenzel CC, Schmidinger MP, Locker GJ, Mader RM, Kramer G, Margerger M, et al. Capecitabine in the treatment of metastatic renal cell carcinoma failing immunotherapy – The Vienna experience. Proc Am Soc Clin Oncol. 2001;20:196a. doi: 10.1053/ajkd.2002.29879. abstr 782. [DOI] [PubMed] [Google Scholar]
- 9.Waters JS, Moss C, Pyle L, James M, Hackett S, A'hern R, et al. Phase II clinical trial of capecitabine and gemcitabine chemotherapy in patients with renal carcinoma. Br J Cancer. 2004;91:1763–1768. doi: 10.1038/sj.bjc.6602209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stadler WM, Halabi S, Rini B, Ernstoff MS, Davila E, Picus J, et al. A phase II study of gemcitabine and capecitabine in metastatic renal cancer. Cancer. 2006;107(6):1273–1279. doi: 10.1002/cncr.22117. [DOI] [PubMed] [Google Scholar]
- 11.Schilsky RL, Bertucci D, Vogelzang NJ, Kindler HL, Ratain MJ. Dose-escalating study of capecitabine plus gemcitabine combination therapy in patients with advanced cancer. J Clin Oncol. 2002;20:582–587. doi: 10.1200/JCO.2002.20.2.582. [DOI] [PubMed] [Google Scholar]
- 12.Thall PF, Wooten LH, Tannir NM. Monitoring event times in early phase clinical trials: some practical issues. Clin Trials. 2005;2(6):467–478. doi: 10.1191/1740774505cn121oa. [DOI] [PubMed] [Google Scholar]
- 13.Therneau TM, Grambsch PM. Modeling Survival Data: Extending the Cox Model New York. Springer; 2000. [Google Scholar]
- 14.Motzer RJ, Bacik J, Schwartz LH, Reuter V, Russo P, Marion S, et al. Prognostic factors for survival in previously treated patients with metastatic renal cell carcinoma. J Clin Oncol. 2004;22:454–463. doi: 10.1200/JCO.2004.06.132. [DOI] [PubMed] [Google Scholar]
- 15.Stadler WM, Huo D, George C, Yang X, Ryan CW, Karrison T, et al. Prognostic factors for survival with gemcitabine plus 5-fluorouracil based regimens for metastatic renal cancer. J Urology. 2003;170:1141–1145. doi: 10.1097/01.ju.0000086829.74971.4a. [DOI] [PubMed] [Google Scholar]
- 16.Suppiah R, Shaheen PE, Elson P, Misbah SA, Wood L, Motzer RJ, et al. Thrombocytosis as a prognostic factor for survival in patients with metastatic renal cell carcinoma. Cancer. 2006;107(8):1793–1800. doi: 10.1002/cncr.22237. [DOI] [PubMed] [Google Scholar]
- 17.Atkins MB, Hidalgo M, Stadler WM, Logan TF, Dutcher JP, Hudes GR, et al. Randomized phase II study of multiple dose levels of CI-779, a novel mammalian target of rapamycin kinase inhibitor, in patients with advanced refractory renal cell carcinoma. J Clin Oncol. 2004;22:909–918. doi: 10.1200/JCO.2004.08.185. [DOI] [PubMed] [Google Scholar]
- 18.Motzer RJ, Hutson TE, Tomczak P, Michaelson MD, Bukowski RM, Rixe O, et al. Sunitinib versus IFN-α in metastatic renal cell carcinoma. N Engl J Med. 2007;356:115–124. doi: 10.1056/NEJMoa065044. [DOI] [PubMed] [Google Scholar]
- 19.Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–2281. doi: 10.1056/NEJMoa066838. [DOI] [PubMed] [Google Scholar]
- 20.Jain RK. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science. 2005;307:58–62. doi: 10.1126/science.1104819. [DOI] [PubMed] [Google Scholar]