Abstract
Quorum sensing (QS) is a process mediated via small molecules termed autoinducers (AI) that allow bacteria to respond and adjust according to the cell population density by altering the expression of multitudinous genes. Since QS governs numerous bioprocesses in bacteria, including virulence, its inhibition promises to be an ideal target for the development of novel therapeutics. We found that the aqueous leaf extract of Psidium guajava (GLE) exhibited anti-QS properties as evidenced by inhibition of violacein production in Chromobacterium violaceum and swarming motility of Pseudomonas aeruginosa. The gram-negative bacterium, C. violaceum is a rare pathogen with high mortality rate. In this study, perhaps for the first time, we identified the target genes of GLE in C. violaceum MTCC 2656 by whole transcriptome analysis on Ion Torrent. Our data revealed that GLE significantly down-regulated 816 genes at least three fold, with p value≤0.01, which comprises 19% of the C. violaceum MTCC 2656 genome. These genes were distributed throughout the genome and were associated with virulence, motility and other cellular processes, many of which have been described as quorum regulated in C. violaceum and other gram negative bacteria. Interestingly, GLE did not affect the growth of the bacteria. However, consistent with the gene expression pattern, GLE treated C. violaceum cells were restrained from causing lysis of human hepatoma cell line, HepG2, indicating a positive relationship between the QS-regulated genes and pathogenicity. Overall, our study proposes GLE as a QS inhibitor (QSI) with the ability to attenuate virulence without affecting growth. To the best of our knowledge, this is the first report which provides with a plausible set of candidate genes regulated by the QS system in the neglected pathogen C. violaceum.
Introduction
With the increase in the number of multi-drug-resistant pathogenic bacteria worldwide, there is a dire need for developing strategies to fight bacterial infections. The indiscriminate use of novel antibiotics that interfere with the metabolism of bacteria have only added to this number. Since Quorum Sensing (QS) regulates many virulence determinants of various pathogens, it has emerged as an attractive target to control their pathogenicity [1], [2], [3]. QS is a cell-to-cell communication mechanism, regulated by small diffusible signalling molecules termed autoinducers (AI), which allows bacteria to respond and adjust their needs in a population density-dependent manner by altering the expression of multitudinous genes [4], [5], [6]. The AIs used by Gram-negative bacteria are known as N-acyl homoserine lactones (AHLs), while Gram-positive bacteria utilize post-translationally modified oligopeptides as signaling molecules [7], [8]. In most Gram-negative bacteria, QS systems are based on LuxI/LuxR homologues. The LuxI homologues encode an AHL synthetase involved in the synthesis of signal molecules, and the LuxR homologues encode the transcription regulatory protein which, upon binding of the cognate signal molecules, activates the transcription of the QS target genes [9].
Chromobacterium violaceum, an opportunistic pathogen, is a free-living, gram-negative, facultative anaerobic β-proteobacterium commonly found in water and soil in the tropical and subtropical regions [10]. In human, C. violaceum infection is rare, but this may be attributed to under-reporting of such cases in areas where the risks of exposure are high and diagnostic facilities are scarce [11]. In spite of this, more than 150 cases of infection were reported in tropical and subtropical regions, including India, where C. violaceum is normally found [12]. This rare infection is associated with a high mortality rate, between 60% and 80%, if not diagnosed at an early stage or treated correctly [11]. A recent case of a man from South India with septicemic C. violaceum infection and septic arthritis, who had a fatal outcome, was reported [13]. A likely explanation for the high mortality rate could be the resistance of C. violaceum to a wide range of antibiotics and to other mechanisms that pump out the cytotoxic drugs [14]. Thus, appropriate therapy is absolutely essential to control this neglected, potentially fatal infection. The strategy of controlling pathogens by interrupting its QS phenomenon was the prime focus of research in the recent years. The importance of quorum sensing in C. violaceum pathogenesis was demonstrated by the fact that QS-antagonist molecules protect the nematode Caenorhabditis elegans from C. violaceum-mediated killing [15]. The C. violaceum quorum-sensing system consists of the LuxI/LuxR homologues CviI/CviR, which controls virulence and the production of a variety of phenotypic characteristics that includes the production of the purple pigment, violacein, cyanide, chitinase and the antibiotic phenazine. The complete genomic sequence of C. violaceum ATCC 12472 has also revealed the presence of these QS-associated genes [16].
Inhibition of QS by some chemically synthesized compounds was identified but most of the QS inhibitors were isolated and characterized from plant sources [17], [18]. Crude extracts of many plant parts were shown to possess anti-QS activity using C. violaceum as a model bacterium [19], [20], [21]. Crude plant extracts are often found to be more effective than isolated constituents at an equivalent dose perhaps owing to positive interactions between components of whole plant extracts. This synergy may involve prevention of the active component from degradation by enzymes or facilitate transport across cell barriers that result in higher efficacy of the crude drug when compared with purified components [22], [23]. Hence, it is lately realized that crude extracts may possibly be the right strategy to treat multi-drug resistant pathogens as compared to the purified compound isolated from the same extract. In fact, the use of traditional herbal medicines is sometimes considered more effective than conventional drugs for the treatment of disease such as malaria [24]. It is proposed that the new generation of phytopharmaceuticals may enable successful use of herbal drug combinations to treat diseases in comparison to single active component [25].
Psidium guajava L. (Guava), widely distributed throughout India, belongs to the family Myrtaceae and is a well known traditional medicinal plant widely used in folk medicine [26], [27]. The leaf extracts of this plant were shown to possess anti-microbial [28], anti-inflammatory, antidiarrhoea [29], anti-oxidant [30], antimutagenic [31], anti- cancer [32], anti-diabetic [33] and anti-plaque [34] activities. However, no molecular mechanism of antimicrobial property of guava-leaf extract was explored.
To the best of our knowledge, this is the first attempt to reveal gene expression profile of C. violaceum with the aid of whole transcriptome analyses on Ion-Torrent in presence of guava-leaf extract (GLE). GLE inhibited QS-controlled genes and QS-regulated phenotypes without affecting the bacterial growth up to 24 h suggesting these effects to be unrelated to bacteriostatic or bactericidal effects. Furthermore, the down-regulation of the wide array of genes, including those encoding virulence factors, affect pathogenicity as revealed by the ability of GLE to arrest C. violaceum induced cell lysis of human hepatoma cells.
Materials and Methods
Bacterial strains
The Chromobacterium violaceum wild type strain MTCC 2656 and Pseudomonas aeruginosa MTCC 2297 were obtained from the Microbial Type Culture Collection Center (MTCC), IMTECH, Chandigarh, India. MTCC 2656 and MTCC 2297 cells were routinely cultured on Nutrient broth (NB, Hi-Media- M002) agar and Luria-Broth (LB, Hi-Media-M575) agar respectively and maintained at 37°C.
Extraction of guava leaves
Leaves of Psidium guajava L. (Guava) were collected from the Centre of Floriculture and Agro Buisness Management (COFAM), University of North Bengal and extracted following standard method [35]. The leaves were washed thoroughly with sterile distilled water and rinsed with 70% (v/v) ethanol. Washed leaves were dried under sun initially and finally in the oven at 50°C for 1 hour. The dried leaves were crushed to fine powder, passed through an 80 mesh sieve and stored in a sealed plastic bag. To 50 gm of powder, 500 ml of sterile distilled water was added and the mixture was heated at 70°C for 1 hour and incubated at 30°C for 72 hours with shaking at regular intervals. The extract was filtered through a Whatman No. 1 filter paper (pore size 11 µm) and centrifuged at 10,000 rpm for 10 min. The resultant supernatant was freeze-dried using a lyophilizer (IIC Industrial Corporation, India). The dried sample was reconstituted with water, filter-sterilized (0.45 µm pore size, Sartorius Stedim, Germany) and tested for its ability to modulate quorum sensing in Chromobacterium violaceum strain MTCC 2656.
Demonstration of Inhibitory effect of Guava leaf extract (GLE) on Quorum Sensing (QS) activity of C. violaceum MTCC 2656
The inhibition of QS-mediated violacein production in C. violaceum, by GLE, was studied by the agar well assay [36]. Sterile molten Nutrient agar (Hi-Media, India) was pour-plated with the cells of C. violaceum MTCC 2656. After solidification of the plates, wells were made in which 100 µl of the aqueous extract was placed, and incubated at 37°C for 24 h. In this assay system, inhibition of bacterial growth (if any) results in producing a clear circular zone around the well, while a positive result of quorum sensing inhibition is demonstrated by a colourless circular translucent zone, signifying growth of cells with no production of pigment, around the well, circumscribed by purple pigmented bacterial growth in the remaining part of the plate.
Demonstration of anti-QS property of GLE using Pseudomonas aeruginosa MTCC 2297
To further confirm and or validate the anti-QS property of the GLE, motility assays of Pseudomonas aeruginosa MTCC 2297 were undertaken in petri dishes [37]. For this, LB media supplemented with 5% glucose and 0.5% agar containing either water (control) or 400 µg ml− 1 of GLE was poured in petri dishes. An inoculum of 10 µl of overnight grown MTCC 2297 was inoculated at the center of the plates. The inoculated plates were incubated at 37°C for 20h and motility across the agar surface was visualized.
Determination of QS-inhibitory concentration of GLE with reference to violacein production
Log phase cells of MTCC 2656 (2.5×106 CFU ml−1) were inoculated (1.0% inocula) into 10 ml volumes of sterile nutrient broth in flasks containing different concentrations of the GLE (100 µg ml−1to 1000 µg ml−1) and incubated at 37°C for 24 h with shaking. The control set was devoid of GLE. The violacein pigment formation in the flask was quantified following the method of Choo et al. [38]. Briefly, 1 ml culture from each flask was centrifuged at 13,000 rpm for 10 min. The culture supernatant was discarded and 1 ml of DMSO was added to the pellet. The solution was vortexed vigorously to completely solubilize violacein and centrifuged at 10,000 rpm for 10 min to remove the cells. The absorbance of the supernatant was read at a wavelength of 585 nm in a digital spectrophotometer (ThermoSpectronic UV 1). The maximum O.D.585 value observed in case of GLE-untreated cells was considered as 100% production of violacein. The percentage (%) inhibition in violacein production was calculated as follows: % inhibition in violacein production = [(O.D.585 value observed in the absence of GLE – O.D.585 oberved in presence of a defined quantity of GLE)÷O.D.585 value observed in absence of GLE]×100.
Determination of growth and violacein production of C. violaceum MTCC 2656 in presence and absence of GLE
Growth of MTCC 2656 was quantified in terms of viable cell number present in the culture (dilution plating followed by counting CFUs) at different time interval [39]. Briefly, an overnight culture of MTCC 2656 (in NB medium), that had been inoculated with a freshly grown single colony, was diluted 100-fold into 10 ml NB medium and allowed to grow for 4 h to obtain log phase cells. The culture was then inoculated into 800 ml NB medium and divided into two portions, to one of which water (control) and to the other, GLE at a final concentration of 400 µg ml−1, was added. These were finally distributed in 10 ml aliquots per pre-sterilized 100 ml Erlenmeyer flask, and incubated at 37°C with shaking. At different time intervals, cultures were withdrawn from time-defined flasks (both control and test) for dilution plating onto NB agar plates as well as for the estimation of violacein by the method described above.
Whole genome transcriptome analysis
The detail of the methodology is provided in File S1. In brief, RNA was isolated from control (cells grown without GLE) and experiment (cells grown with GLE) samples and cDNA Library was prepared using the Ion Total RNA-Seq Kit v2 (Catalog Number 4475936). Template preparation and enrichment was performed as per Ion OneTouch 200 Template Kit (Cat no. 4471263) and 200 base-read sequencing was performed using the Ion PGM 200 Sequencing Kit (Cat no 4474004) on ION TORRENT.
Infection and morphological assessment of HepG2 cells by phase-contrast inverted microscope
HepG2 cells (Human hepatocellular liver carcinoma cell line), obtained from Cell Culture Collection, NCCS, Pune, were grown in 100 mm polyvinyl coated plates, in DMEM (Dulbeco’s Modified Eagle’s Medium) media with 10% FCS at 37°C in a humidified atmosphere containing 5% CO2. Cells (1×106) were seeded in 60 mm plates in DMEM medium supplemented with 10 U/ml penicillinG and 100 µg ml−1 streptomycin. After 24 h, the media was removed and cells were washed thrice with phosphate buffer (PBS, pH 7.2) and grown in DMEM media without antibiotics until C. violaceum infection. Bacteria (MTCC 2656) were grown overnight at 37°C in NB either without or with 400 µg ml−1GLE. The following day, the bacterial cells were grown further in fresh media under the same conditions for 3 h. Finally, the untreated or GLE treated C. violaceum cells were diluted in DMEM with 10% FCS supplemented either with water or GLE, and added to HepG2 cells at the infectivity ratio of 10∶1. The plates were incubated at 37°C in an atmosphere of 5% CO2 and observed under inverted microscope (Olympus, ck40-slp) at 0 and 4 h of infection at 200X magnification and photographed. HepG2 cells incubated in DMEM containing GLE served as control.
LDH cytotoxicity assay
Cytotoxicity induced in HepG2 cells was quantitated by measuring the release of the cytosolic enzyme lactate dehydrogenase (LDH) in the culture medium. For this, HepG2 cells were grown and exposed to C. violaceum as described above. After 4 h of incubation, supernatants were collected and evaluated for the presence of LDH using the LDH Cytotoxicity Assay Kit (Item No. 10008882, Cayman Chemical Company). The LDH activity (µU) was determined from the standard curve and the total LDH activity (µU ml−1) was calculated as value from LDH activity assay (µU)/sample volume assayed (ml).
Statistical Analysis
All experiments were performed in triplicate. Results are expressed as mean value ± standard deviations (S.D) of three replicates and analyzed by using the software SPSS 15.0 for windows (SPSS Inc. Chicago, IL, USA). The statistical treatment of “Whole Transcriptome Analysis” data is elucidated in File S1.
Results and Discussion
Inhibition of violacein production in the presence of aqueous extracts of guava leaves
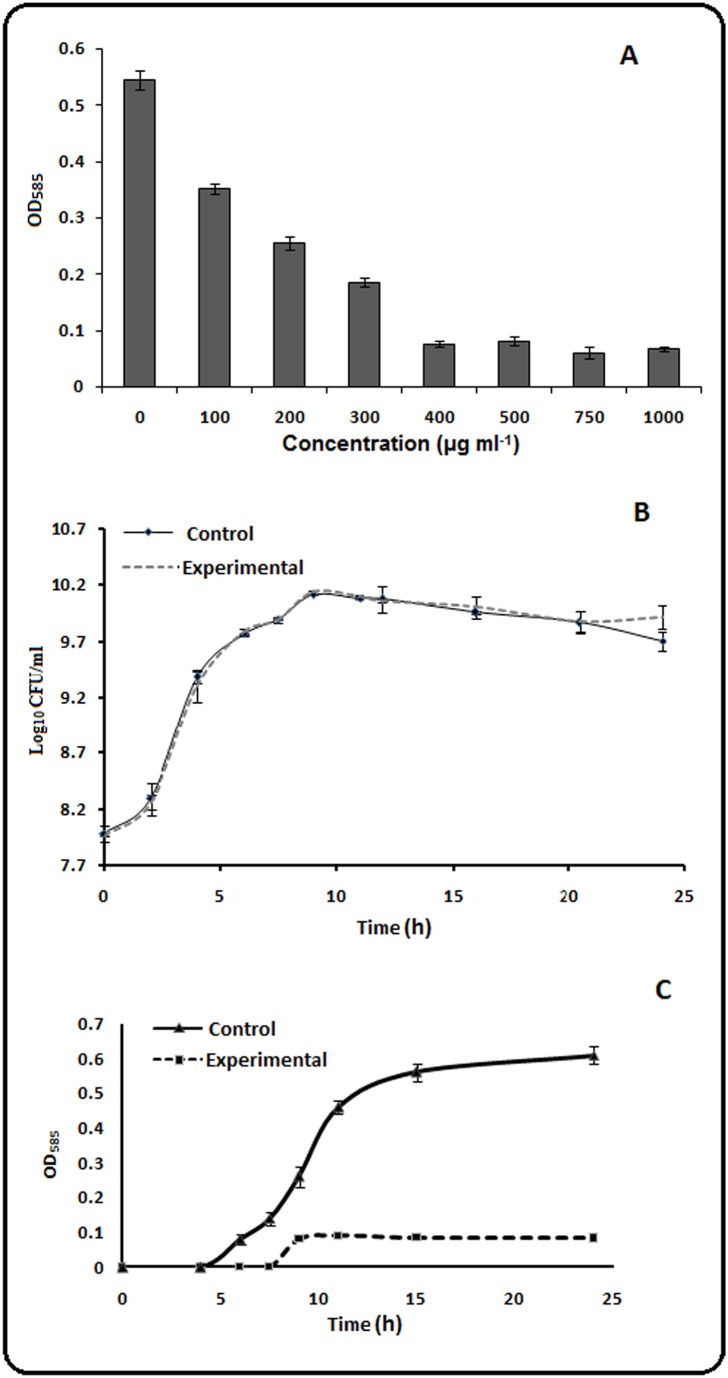
The best studied trait controlled by QS in C. violaceum is the production of the purple pigment, violacein [40]. To investigate QS inhibitory activity of aqueous extract of Psidium guajava L. leaves (GLE), its effect on violacein synthesis by C. violaceum MTCC 2656 was examined. A colourless transluscent zone around the zone of diffusion of GLE from the agar cup indicated growth of C. violaceum failing to produce violacein (purple pigment, Figure S1A). The production of violacein pigment at 24 h in the presence of varied concentration of GLE was quantified spectrophotometrically (Figure 1A). Violacein production was inhibited by 85% in presence of 400 µg ml−1 of GLE and the extent of inhibition remained similar at concentrations up to 1000 µg ml−1. Thus, the concentration of GLE to be used for further studies was selected as 400 µg ml−1. It has been documented in the Literature that nearly all parts of Psidium guajava L. tree, including fruits, leaves, bark, and roots, were used traditionally as a medicinal plant throughout the world for varied ailments [27]. The present study revealed that the guava-leaf extract was capable of inhibiting the quorum-dependent violacein production in C. violaceum.
Figure 1. Effect of GLE on violacein production and growth of wild-type C. violaceum MTCC 2656 cells.
A. Violacein production in the presence of different concentrations of GLE. B. Viable cell number in batch culture grown without (Control) or with (Experimental) supplementation of 400 µg ml−1GLE. C. Quantitation of violacein in batch culture grown without (Control) or with (Experimental) supplementation of 400 µg ml−1 GLE.
The growth of C. violaceum is unaffected in presence of GLE
The number of viable cell enumerated from growth studies in presence and absence of the GLE (400 µg/ml) showed no significant difference (Figure 1B). On the contrary, the violacein production was significantly inhibited under similar growth conditions (Figure 1C). It has been noted by earlier authors that the toxicity of putative quorum sensing inhibitors towards bacterial cells may be assessed by addressing three principal issues; that the QS inhibitory effects occur below the minimum inhibitory concentration (if the inhibitor exhibits antimicrobial property), the quorum-sensing inhibitory concentration used in the study does not affect the final cell density after a certain period of incubation, and the QS inhibitor does not grossly affect the kinetics of growth [41]. Our results show that the growth of C. violaceum remained unaffected but the quorum-dependent production of violacein was significantly reduced in the presence of GLE. These observations led us to conclude that the decrease in violacein production was not due to any form of growth inhibition of the cells in the culture medium at least up to 24 h of incubation. Similar results with respect to concentration-dependent decrease in violacein production without affecting bacterial growth were reported with extracts of various medicinal plants [42], [43]. Assuming that the quorum-inhibiting property of GLE should also be yielding in other bacteria that demonstrate quorum-dependent diverse phenotypes, we tested GLE for its activity using another test organism, Pseudomonas aeruginosa. The gram-negative bacterium Pseudomonas aeruginosa exhibits swarming motility which requires the bacteria to effectively work together via the process of quorum-sensing [37]. The cells of P. aeruginosa, MTCC 2297, in absence of GLE, formed tendrils migrating outwards from the point of bacterial inoculation, with continued branching as the bacteria moved farther from the center, while in presence of GLE, cells grew to form a localized colony in the center with no signs of swarming (Figure S1B). The ability of natural products to disrupt the quorum regulated swarming motility in P. aeruginosa was demonstrated earlier [44]. Thus, the manifestation of anti-QS property in the form of inhibition of swarming motility of P. aeruginosa, further provides evidence for the proposed quorum quenching property of GLE.
Impact of GLE on the genome-wide gene expression of C. violaceum
The quorum-regulated gene expression has been extensively studied in gram negative pathogenic bacteria such as P. aeruginosa [45] and Escherichia coli [46]. However, C. violaceum transcriptomic studies have not yet attracted the similar attention. The increasing reports of C. violaceum cases [47] prompted us to unravel the status of gene expression in the presence or absence of GLE with a view to establish GLE as a QS inhibitory candidate for controlling the bacterial pathogenicity. Schuster et al. have observed that the timing affects the quorum-controlled gene expression in P. aeruginosa and most of the transcripts under QS-regulation were induced maximally at late log or stationary phase [48]. Thus, to get an insight into the effect of GLE on global gene expression of C. violaceum, we used 24 h grown cultures either in the absence (Control) or presence (Experimental) of 400 µg ml−1GLE.
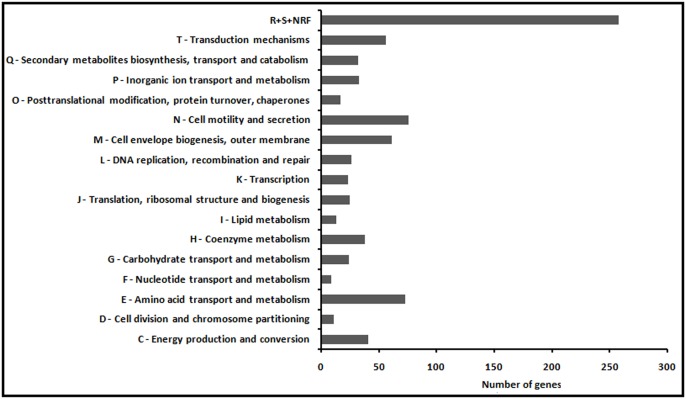
A total of 33,54,744 and 30,09,475 high quality Ion Torrent reads for Control and Experiment sample respectively were mapped on the reference genome of Chromobacterium violaceum ATCC 12472 with genome size of 4.75 Mb. There are 4,529 genes present in C. violaceum ATCC 12472 GFF file. The number of genes expressed in (i) both control and experiment; (ii) exclusively in control; (iii) exclusively in experiment; (iv) not expressed in both control and experiment were determined to be 3025, 1229, 32, and 243 respectively, as illustrated in Figure 2. Raw sequences were deposited at the NCBI Sequence Read Archive, under Bioproject accession number PRJNA243990 and SRA accession code SRP041018 (http://www.ncbi.nlm.nih.gov/bioproject/?term=PRJNA243990). The FPKM value for each gene was calculated for the combination of samples Control and Experiment. These FPKM values (FPKM_control and FPKM_experiment) were further used to calculate the log fold change as log2 (FPKM_experiment/FPKM_control). Moreover, uncorrected p-value of the test statistic for each gene was also computed. Using a stringent log2 threefold cut-off with p-value≤0.01, we observed that 816 genes, scattered throughout the C. violaceum MTCC 2656 genome, comprising about 19% of the total genes expressed, were significantly down regulated in the presence of GLE (Table S1). The 816 differentially expressed genes were categorised into 17 groups (Figure 3) based on the C. violaceum ATCC12472 database from the Brazilian Genome Virtual Institute of Genomic Research (BRGene) (http://www.brgene.lncc.br). The category representing the largest percentage, ∼32% of the total 816 genes, encoded for the group of proteins assigned unclassified and unknown functions. The categories representing>6.5% belonged to the functional classes of proteins namely cell motility and secretion (9.31%), cell envelope biogenesis, outer membrane (7.47%), transduction mechanisms (6.86%) and amino acid transport and metabolism (8.94%). Among these, the first three groups of proteins are related to virulence in many pathogens since these processes mediate interaction of bacteria with their immediate environment and genes encoding virulence factors. GLE down-regulated genes like lyases (argH, aspA), dehydrogenase and hydratase (usg, sdaA1) and transporters (sdaC, argT), that encode products involved in metabolism and transport of amino acids. In addition, GLE down-regulated, about 4%, the genes involved in energy production and conversion, secondary metabolites biosynthesis, inorganic ion transport and metabolism, and coenzyme metabolism. The possibility of interference of GLE with one or several global regulators in C. violaceum resulting in differential expression of multiple genes cannot be ruled out. However, since GLE targeted virulent genes which are often QS controlled [49] and QS activated genes involved in uptake, synthesis and degradation of amino acids [50]; inhibited QS controlled phenotype, violacein production, without hampering growth of C. violaceum upto 24 h, pointed to the possible anti-QS property of GLE. Repression of genes related to cellular processes with no effect on growth was revealed by microarray analysis of P. aeruginosa gene expression in the presence of QS inhibitor furanone C-30 [51]. Earlier microarray studies with E. coli and P. aeruginosa have revealed that varied cellular functions are largely affected by the QS system [45], [46]. Both the gram negative bacteria have more than one QS regulatory system. The cross-talk between different quorum signal pathways lead to either synergestic or antagonistic effects. It is thus tempting to assume that C. violaceum may also possess multiple QS systems and any interference would thus lead to a global differential expression of genes as observed with GLE. Previous transcriptomic studies have shown that as much as>10% of the total genes in the genome of P. aeruginosa are QS regulated [45], however, we observed a higher percentage (19%) of differential gene expression. This could be explained either due to the presence of multiple components of GLE or if we assume that these genes are QS-regulated, the higher number of genes revealed in this study may be due to the fact that earlier groups detected QS regulons using specific mutants but our studies involves revelation of QS regulons using a putative QS inhibitor on wild type cells. Thus, although the data obtained does not reflect a definitive analysis of gene regulation by quorum sensing, perhaps for the first time, our results provide with a set of probable candidate genes under QS regulation in C. violaceum for further investigation.
Figure 2. Profile of genome-wide gene expression in C. violaceum MTCC 2656 cells.
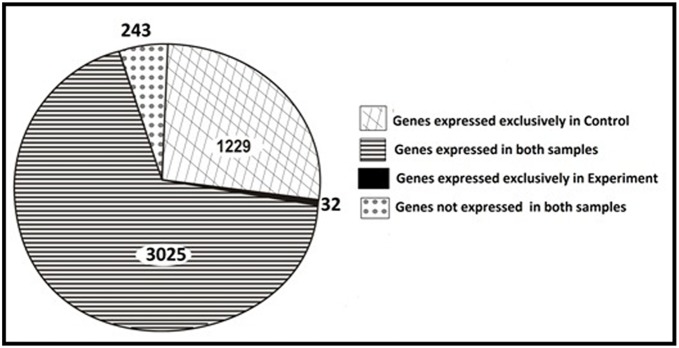
Cells were grown in presence (experimental) and absence of 400 µg ml−1 GLE (control).
Figure 3. Differential expression of genes in C. violaceum in presence of GLE.
The classification was based on Clusters of Orthologous Groups (COG) functional classification (R, General function prediction only; S, Function unknown; and NRF, No results found).
Differential expression of genes predicted to be under QS-regulation in C. violaceum
Cell to cell signaling in C. violaceum is based on the CviI/CviR circuit. At high cell density, the cytoplasmic quorum-sensing receptor, CviR, binds to the AHL autoinducer. The CviR-AHL complex then binds to DNA and activates transcription of a number of genes. The fact that GLE has the ability to induce break in the CviI/CviR circuitry is reflected phenotypically by the abolition of violacein production by C. violaceum in the growth medium. The gene responsible for violacein production, vioA, was log2>threefold repressed. Since the level of expression of vioA gene (FPKM_control value) in absence of GLE was low (perhaps owing to the experimental conditions), the comparison with FPKM_experiment value (in presence of GLE) was concluded as statistically non-significant. Our data revealed that GLE down-regulated the expression of major virulence factors (Table 1) such as, lasA (CV_2571), lasB (CV_0057), hcnABC operon (CV_1682 to CV_1684) and chitinase (CV_4240). The quorum regulated proteases LasA and LasB are capable of inactivating a wide range of biological tissues and immunological agents to allow the bacteria to invade and colonize host tissues [52], [53]. The hcnABC operon responsible for biosynthesis of hydrogen cyanide (HCN), a secondary metabolite and a potent inhibitor of cytochrome oxidase and several other metalloenzymes, is known to be under the control of quorum-sensing mechanism in C. violaceum [54]. The enzyme, chitinase, is hypothesized to be involved in blocking the growth of fungi with chitinous exoskeleton, allowing a competitive advantage to C. violaceum during colonization [55]. In addition to these secreted factors, transcription of gene encoding acyl carrier protein (ACP) synthase, fabF (CV_3412) and other ACP-encoding genes like fabZ (CV_2207) and CV_3413, were significantly repressed by GLE. These gene products are proposed to be involved in synthesis of AHLs [56]. Similar down-regulation of these genes in P. aeruginosa was observed in the presence of the QS inhibitor, furanone C-30 [51]. Moreover, C-30 also targets P. aeruginosa virulence but it does not affect the expression of gene clusters encoding the components of QS system. On the contrary, GLE not only affected virulence but significantly repressed the expression of both cviI (CV_4091) and cviR (CV_4090) genes, the basic constituents of C. violaceum QS system, suggesting direct transcriptional interference of GLE. A putative cviR binding site was detected in the cviI promoter region and so its expression was expected to be down-regulated. In addition, a number of other cviR -regulated genes based on the presence of an ideal CviR binding site coupled with genome scanning were predicted [57]. GLE down-regulated a subset of these genes, which included CV_1328 (methyl-accepting chemotaxis protein), CV_2321 (recN, DNA repair protein) and CV_3062 (enoyl-CoA hydratase). The downregulation of QS-controlled genes with no effect on bacterial cell growth led us to hypothesize the anti-QS property of GLE to be responsible for the observed differential gene expression.
Table 1. Significantly downregulated C. violaceum genes associated with quorum-sensing and pathogenicity, in presence of GLE.
| ORF no. | Genename | Description | Control1 | Expt2 | log2(foldchange) | p value |
| Constituent of Quorum sensing circuit | ||||||
| CV_4091 | cviI | N-acyl homoserine synthase;autoinducer synthase,quorum sensing controlled system | 171.85 | 19.44 | −3.14 | 5.39E-005 |
| CV_4090 | cviR | transcriptional activator,LuxR/UhpA family of regulators | 194.51 | 14.75 | −3.72 | 3.04E-006 |
| Genes predicted to have cviR binding sites | ||||||
| CV_1311 | hypothetical | 46.41 | 1.6 | −4.86 | 0 | |
| CV_1329 | sbcB | exonuclease I | 15.82 | 0.66 | −4.59 | 0 |
| CV_1328 | methyl-accepting chemotaxis protein | 39.91 | 2.86 | −3.8 | 3.86E-006 | |
| CV_1408 | sdaA2 | serine dehydratase | 23.25 | 2.67 | −3.12 | 0 |
| CV_1444 | hypothetical | 14.81 | 0.94 | −3.98 | 0.01 | |
| CV_2091 | putative tetR-family transcriptional regulator | 23.69 | 2.89 | −3.03 | 0.01 | |
| CV_2321 | recN | DNA repair protein | 19.88 | 0.54 | −5.19 | 0 |
| CV_2434 | hypothetical | 56.1 | 1.93 | −4.86 | 0 | |
| CV_2656 | cytochrome P450 hydroxylase | 96.94 | 8.63 | −3.49 | 7.18E-006 | |
| CV_2716 | hypothetical | 52.45 | 5.47 | −3.26 | 4.07E-005 | |
| CV_3062 | enoyl-CoA hydratase | 32.72 | 1.31 | −4.64 | 0 | |
| CV_3300 | treB | protein phosphohistidine-sugar phosphotransferase | 8.82 | 0.55 | −4 | 0.01 |
| CV_4091 | cviI | autoinducer synthase | 171.85 | 19.44 | −3.14 | 5.39E-005 |
| CV_4092 | aldehyde dehydrogenase | 20.7 | 1.39 | −3.9 | 0 | |
| CV_4142 | hoxX | HoxX-like protein | 7.99 | 0.53 | −3.9 | 0.01 |
| CV_4240 | putative chitinase | 42.51 | 2.82 | −3.92 | 2.08E-006 | |
| CV_4379 | hypothetical inside biotin synthesis operon | 16.18 | 0.61 | −4.74 | 1.65E-005 | |
| CV_4382 | comF | competence protein F | 26.97 | 1.71 | −3.98 | 0.01 |
| Quorum Sensing controlled genes | ||||||
| hcn operon | ||||||
| CV_1682 | hcnC | hydrogen cyanide synthase HcnC | 187.54 | 6.8 | −4.78 | 7.43E-009 |
| CV_1683 | hcnB | hydrogen cyanide synthase HcnB | 600.99 | 4.65 | −7.01 | 0 |
| CV_1684 | hcnA | hydrogen cyanide synthase HcnA | 600.99 | 4.65 | −7.01 | 0 |
| Protease | ||||||
| CV_2571 | LasA | LasA protease precursor | 175.34 | 13.11 | −3.74 | 1.13E-006 |
| CV_0057 | LasB | class 4 metalloprotease | 211.37 | 20.93 | −3.34 | 1.09E-006 |
| Cellulose biosynthesis | ||||||
| CV_2675 | bscC | cellulose synthase, subunit C | 9.3 | 0.22 | −5.39 | 0 |
| CV_2676 | bscZ | endo-1,4-D-glucanase | 9.3 | 0.22 | −5.39 | 0 |
| CV_2677 | bcsB | cellulose synthase, subunit B | 9.3 | 0.22 | −5.39 | 0 |
| Other genes related to virulence and pathogenicity | ||||||
| CV_3824 | conserved hypothetical protein | 64.28 | 5.74 | −3.49 | 6.81E-005 | |
| CV_3828 | pilB | type 4 fimbrial biogenesis protein | 63.95 | 5.32 | −3.59 | 5.99E-006 |
| CV_0829 | pilQ | type 4 fimbrial biogenesis protein PilQ | 96.39 | 2.11 | −5.51 | 1.01E-013 |
| CV_0830 | pilP | type 4 fimbrial biogenesis protein PilP | 96.39 | 2.11 | −5.51 | 1.01E-013 |
| CV_0832 | pilN | type 4 fimbrial biogenesis protein PilN | 85.03 | 6.41 | −3.73 | 0 |
| CV_0833 | pilM | type 4 fimbrial biogenesis protein PilM | 130.37 | 15.8 | −3.04 | 5.79E-005 |
| CV_0179 | pilT | twitching motility protein PilT | 53.97 | 6.7 | −3.01 | 0 |
| CV_0180 | pilU2 | twitching mobility protein transport | 32.64 | 2.57 | −3.67 | 0 |
| CV_1458 | pilU1 | twitching motility protein | 41.43 | 5.17 | −3 | 0 |
| CV_3112 | pilV | type-4 fimbrial biogenesis PilV transmembrane protein | 79.2 | 2.24 | −5.14 | 0 |
| CV_2618 | sipC | cell invasion protein | 17.62 | 0.92 | −4.25 | 0.01 |
| CV_2619 | sipB | cell invasion protein | 116.65 | 3.16 | −5.21 | 6.23E-005 |
| CV_2620 | spaT | surface presentation of antigens; secretory proteins | 116.65 | 3.16 | −5.21 | 6.23E-005 |
| CV_2198 | pyrH | uridylate kinase, Pyrimidine metabolism | 40.96 | 4.63 | −3.15 | 0 |
| CV_2205 | ompH | hypothetical protein, outer membrane protein | 46.62 | 5.62 | −3.05 | 0.01 |
| CV_2206 | lpxD | Lipopolysaccharide biosynthesis | 41.56 | 4.12 | −3.33 | 0.01 |
| CV_2207 | fabZ | 3-hydroxyacyl-[acyl-carrier-protein]dehydratase, Fatty acid biosynthesis | 41.56 | 4.12 | −3.33 | 0.01 |
| CV_2209 | lpxB | lipid-A-disaccharide synthase, Lipopolysaccharide biosynthesis | 29.44 | 3.69 | −3 | 0 |
| CV_2210 | rnhB | ribonuclease HII | 29.44 | 3.69 | −3 | 0 |
| CV_2212 | hypothetical protein | 561.06 | 41.05 | −3.77 | 0 | |
| CV_4338 | ftsZ | cell division protein | 61.92 | 7.3 | −3.08 | 9.58E-005 |
| CV_4340 | ftsQ | cell division transmembrane protein | 41.07 | 1.13 | −5.19 | 0 |
| CV_4341 | ddlB | D-alanine-D-alanine ligase, Peptidoglycan biosynthesis | 41.07 | 1.13 | −5.19 | 0 |
| CV_4344 | ftsW | cell division protein | 25.93 | 3.05 | −3.09 | 0 |
| CV_4345 | murD | Peptidoglycan biosynthesis | 25.93 | 3.05 | −3.09 | 0 |
| CV_4346 | mraY | Peptidoglycan biosynthesis | 19.51 | 1.81 | −3.43 | 0 |
| CV_4349 | ftsI | cell division protein FtsI | 157.11 | 0.52 | −8.25 | 3.40E-008 |
| CV_4350 | cell division protein | 157.11 | 0.52 | −8.25 | 3.40E-008 | |
| CV_4351 | mraW | 16 S rRNA (cytosine1402-N4)-methyltransferase | 157.11 | 0.52 | −8.25 | 3.40E-008 |
| CV_4352 | conserved hypothetical protein | 157.11 | 0.52 | −8.25 | 3.40E-008 | |
| CV_0516 | Ca binding hemolysin | 7.43 | 0.71 | −3.39 | 3.36E-005 | |
| CV_0360 | thermolabile hemolysin | 25.86 | 2.28 | −3.5 | 0 | |
| CV_1989 | porin | 257.04 | 24.41 | −3.4 | 6.76E-006 | |
| CV_3104 | porin | 1207.93 | 95.97 | −3.65 | 7.68E-006 | |
| CV_3829 | porin | 813.08 | 69.81 | −3.54 | 6.04E-006 | |
| CV_3342 | hemolysin III | 274 | 26.88 | −3.35 | 1.39E-005 | |
gene expression in cells grown for 24 h.
gene expression in cells grown for 24 h in presence of 400 µg ml−1 of GLE.
Status of expression of genes plausibly networked with quorum sensing mechanism
The pathogenicity of C. violaceum is still poorly understood. Cause and effect relationship between QS system and pathogenicity of C. violaceum is yet an enigma. On the basis of whole genome sequence of C. violaceum, a catalogue of genes encoding probable virulence factors was prepared [58]. These included the genes encoding pil proteins and genes involved in lipopolysaccharide (LPS) and peptidoglycan biosynthesis. The type IV pilus gene cluster encodes Type IV pili which are appendages emanating from the surfaces of several gram-negative bacteria that are associated with pathogenicity [59], [60]. The expression of a majority of the pil genes found in C. violaceum, namely pil B, T, U2, U1, V and pilQPNM, were inhibited by GLE. Transcription of lpxB (CV_2209) and lpxD (CV_ 2206) genes, responsible for LPS biosynthesis and murA (CV_0440) and murD (CV_4345), genes required for peptidoglycan biosynthesis, were significantly down-regulated in the presence of GLE. As found in other gram negative bacteria, the LPS and peptidoglycan of C. violaceum are associated with activation of immune response in the host, which induces secretion of inflammatory cytokines resulting in septic shock [58]. Very recently, genes encoding potential secreted virulence factors of C. violaceum were identified [61]. Among these, GLE targeted the hemolysins (CV_0516, CV_0360 and CV_3342) and porins (CV_1989, CV_3104, and CV_3829). Increased expression of hemolysin and porins in presence of quorum signal autoinducer 2 (AI-2), mediator of second signaling pathway, has previously been documented in E.coli [46]. While unravelling the virulence determinant for C. violaceum induced cell lysis, Miki et al. observed that the formation of pore structures on the host cell membrane results in cell death in hepatocytes [62]. The pore formation involved CipB (sipB, CV_2619), a translocator of the Chromobacterium pathogenicity islands 1 and 1a (Cpi-1/-1a). GLE significantly down-regulated the expression of the sipB gene and two other linked genes, spaT (CV_2620) and sipC (CV_2618), cell invasion protein. Transcription of a vast number of genes involved in flagellar biosynthesis (flhB1, flhF, fliL, fliE, fliM, fliN, fliC2, fliC3, flgK1, flaG, flil1, fleN and fliA1, RNA polymerase sigma factor for flagellar operon) and chemotaxis (cheV1, cheR3, cheR1, cheA, cheA2, cheZ, trg, nahY, motA1, motB1 and motB2) were repressed by GLE. Regulation of flagellar genes by QS has been previously reported in other bacteria such as Yersinia pseudotuberculosis and Vibrio harvei [63], [64]. A decreased expression of motA was observed in QS-mutant strains of E. coli [49]. The results indicate that down-regulation of the expression of the varied genes, responsible for virulence and cell motility, by GLE, may alter C. violaceum pathogenicity.
C. violaceum genome consists of four genes involved in cellulose production: bcsA, bcsB, bcsZ and bcsC [65]. Interestingly, in the presence of GLE, expression of bsc CZ and bcsB (CV_2675-CV_2677) were down-regulated indicative of a low level of cellulose production. Bacterial biofilms consist of bacteria colonies embedded in their own extracellular matrix composed of exopolysaccharides (EPS). Cellulose has been identified as an exopolysaccharide (EPS) in the extracellular matrix produced by several bacterial species. Decreased cellulose production in the presence of GLE will thus lead to inhibition in the formation of biofilms. This emphasises the proposed QS inhibitory property of GLE in light of the finding that QS has been implicated as an important step in biofilm formation [5], [66].
Earlier studies on anti-QS activities of crude plant extracts in bacteria have focussed mainly on demonstrating inhibition of expression of well-established specific QS-induced gene(s) [67]. In C. violaceum, quantitative real-time -PCR assay for expression of specific genes, namely, AI synthetase gene (cviI) and one of the violacein biosynthetic genes (vioB), was performed in order to establish that sub-lethal concentration of antibiotics can inhibit QS in C. violaceum [68]. Validation of the claims of remedies resulting from consumption of crude plant extracts (e.g crude extracts of Ginkgo biloba) by transcriptome profiling of the affected organs (e.g liver of rats on a high-fat diet) have provided with the molecular basis of herbal therapy [69]. The outcome of the present transcriptomic data improvises quorum inhibitory property of crude extract of guava leaves, GLE, and provides with a list of probable quorum regulated genes in C. violaceum giving insights into the features of this versatile bacterium. The attenuation of virulence by GLE, as revealed by the differential gene expression data, compelled us to test the phenotypic manifestation of C. violaceum induced cytolysis of human hepatoma cell line in presence of GLE.
GLE inhibits C. violaecum-induced cytotoxicity in HepG2 cells
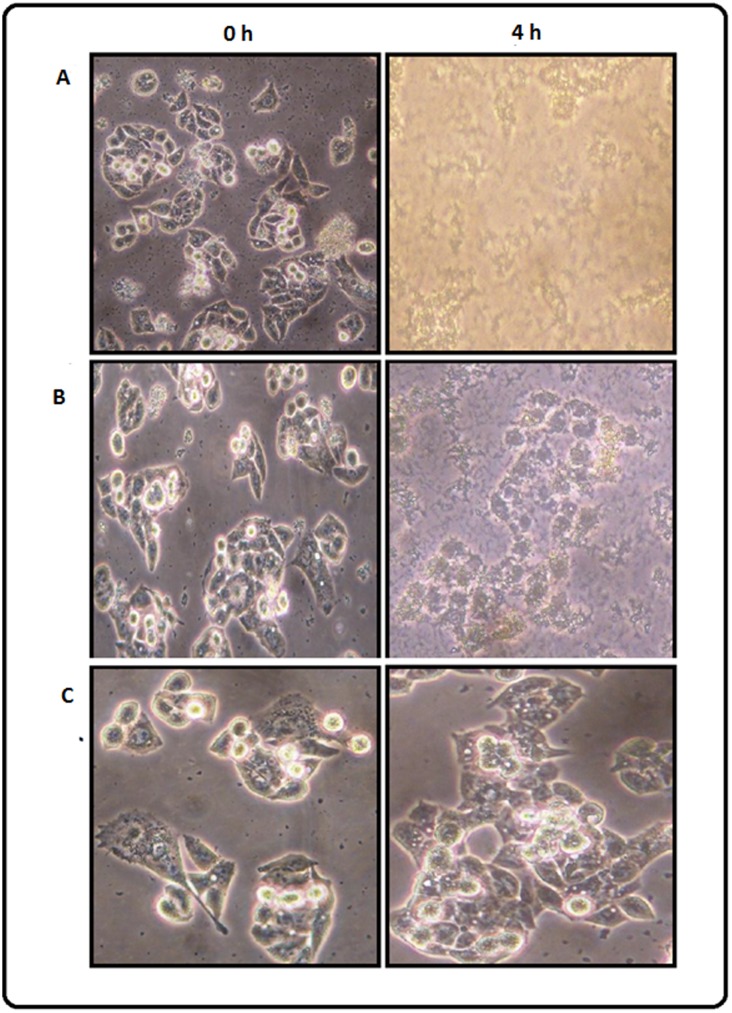
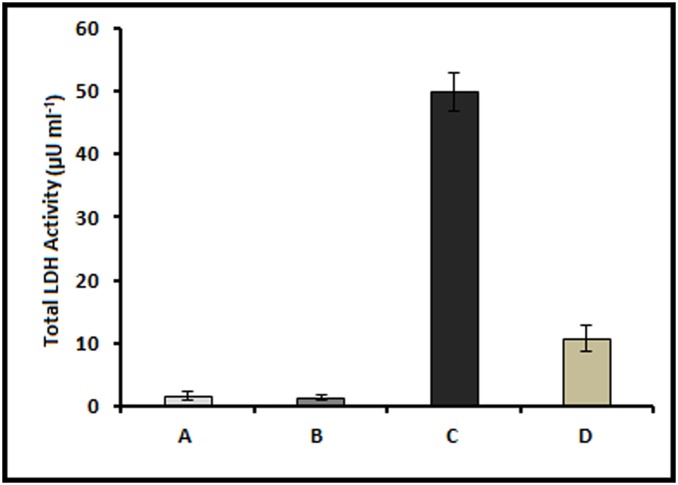
To analyze the effect of GLE on cell death induced by C. violaecum, human hepatocellular liver carcinoma cell line HepG2 was infected with the bacteria either in the absence or presence of GLE. The course of infection was monitored by phase contrast microscopy at different time points. It was observed that C. violaecum was able to induce cytotoxicity in HepG2 cells, characterized by shrinkage of the cells and reduction in cell density, with time (Figure. 4A). Whereas, no morphological changes were observed in the cells when infection was carried out in presence of GLE indicating that the extract could protect the HepG2 cells from C. violaecum infection (Figure 4B). Notably, GLE alone had no inhibitory effect on the growth of HepG2 cells (Figure 4C). Furthermore, C. violaceum-induced cytotoxicity was quantified by assaying the release of the cytosolic enzyme lactate dehydrogenase (LDH). The cell death observed after C. violaceum infection in hepatocytes is characterized by membrane rupture with subsequent release of LDH [62]. In agreement, after 4 h following C. violaceum infection of HepG2 cells, a significant increase in extracellular LDH activity was observed as compared to the non-infected cells (Fig. 5). Interestingly, the amount of LDH release from C. violaceum-infected HepG2 cells decreased by ∼80% in the presence of GLE indicating the ability of GLE to hinder cell death instigated by C. violaceum. A similar decrease in cytotoxicity in different cell lines was demonstrated using mutant strains of C. violaceum [62]. Moreover, GLE itself had no effect on human hepatoma HepG2 cells showing its non-toxicity towards the host cell (Figure 5). The guava leaf paste was highly likely to be non-toxic since it is used immensely in folklore practices, including as toothpaste for the cure of dental caries [70]. This result shows that GLE regulates the pathogenicity of C. violaceum by preventing the initiation of the cascade of gene expression required for successful infection and establishment in the host which, in turn, may provide time to the immune system of the host to eliminate the pathogen. This effect of GLE may be attributed to its ability to either directly or indirectly inhibit the QS system.
Figure 4. GLE inhibits C. violaceum induced cell-lysis of HepG2 cells.
Phase contrast micrographs at 200X magnification showing (A) lysis of HepG2 cells after 4 h of infection with C. violaceum MTCC 2656 and (B) inhibition of lysis in presence of 400 µg ml−1 GLE. (C) growth of HepG2 cells in presence of GLE alone (to nullify any effect of GLE on HepG2 growth).
Figure 5. LDH activity in the culture medium of HepG2 cells.
LDH assay was performed after 4 h treatment with: (A) only water; (B) only 400 µg ml−1 of GLE; (C) only C. violaceum; and (D) C. violaceum along with 400 µg ml−1 GLE.
Conclusions
Identification of QS inhibitors (QSIs) from natural products as an alternative to antibiotics is currently an area of intense interest. However, establishment of a real QSI and the phenomenon of resistance to QSIs are debatable aspects in this field of research [71]. The present study proposes guava leaf aqueous extract (GLE) as QSI since it inhibits quorum regulated phenotypes such as violacein production and swarming in the pathogenic bacteria C. violaceum and P. aeruginosa respectively and induces a global differential gene expression in C. violaceum, a pathogen with high mortality rate, without affecting its growth. The complexity of GLE prevents us from directly linking it with the QS system. However, reduction in QS gene expression was correlated to the attenuation of bacterial virulence resulting in prevention of C. violaceum induced lysis of host (HepG2) cells in vitro. More studies are required to establish the exact nature of the metabolic cross-talks in presence of GLE but this study provides with the platform to think on using the crude extract of plants to combat pathogenic bacteria. Overall, our results provide insights into the candidature of GLE as QSI and the identification of a set of probable quorum regulated genes in C. violaceum.
Supporting Information
Inhibition of QS-regulated phenotypes by GLE. A. Formation of a colourless translucent zone around the well containing GLE indicating absence of violacein production by C. violaceum cells. B. Inhibition of swarming motility of P. aeruginosa cells grown in presence of GLE.
(TIF)
Significantly down-regulated genes of C. violaceum when grown in presence of GLE.
(DOCX)
Method: Whole genome transcriptome analysis.
(DOCX)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All sequence files are available from the SRA database (accession number(s) SRR1221300. All other relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was funded by the Department of Biotechnology, Government of India (BT/Bio-CARe/06/141/2010-11) [Project Investigator- RG; Junior Research Fellow- BKT]. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Rasmussen TB, Givskov M (2006) Quorum-sensing inhibitors as anti-pathogenic drugs. Intl J Med Microbiol 296: 149–161. [DOI] [PubMed] [Google Scholar]
- 2. Clatworthy AE, Pierson E, Hung DT (2007) Targeting virulence: a new paradigm for antimicrobial therapy. Nat Chem Biol 3: 541–548. [DOI] [PubMed] [Google Scholar]
- 3. Kalia VC, Purohit HJ (2011) Quenching the quorum sensing system: potential antibacterial drug targets. Crit Rev Microbiol 37: 121–140. [DOI] [PubMed] [Google Scholar]
- 4. Williams P (2007) Quorum sensing, communication and cross-kingdom signalling in the bacterial world. Microbiology 153: 3923–3938. [DOI] [PubMed] [Google Scholar]
- 5. Sarkar S, Chakraborty R (2008) Quorum sensing in metal tolerance of Acinetobacter junii BB1A is associated with biofilm production. FEMS Microbiol. Lett 282: 160–165. [DOI] [PubMed] [Google Scholar]
- 6. Dandekar AA, Chugani S, Greenberg EP (2012) Bacterial quorum sensing and metabolic incentives to cooperate. Science 338: 264–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Waters CM, Bassler BL (2005) Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol 21: 319–346. [DOI] [PubMed] [Google Scholar]
- 8. Schuster M, Sexton DJ, Diggle SP, Greenberg EP (2013) Acyl-homoserine lactone quorum sensing: From evolution to application. Annu Rev Microbiol 67: 43–63. [DOI] [PubMed] [Google Scholar]
- 9. Rutherford ST, Bassler BL (2012) Bacterial quorum sensing: Its role in virulence and possibilities for its control. Cold Spring Harb Perspect Med 2: a01242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Koburger JA, May SO (1982) Isolation of Chromobacterium spp. from foods, soil, and water. Appl Environ Microbiol 44: 1463–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Orsetti AG, Markiewicz P, Epstein MG, Conceiçao OJG, D’Ippolito G, et al. (2013) Liver abscesses by Chromobacterium violaceum: a case report of a rare disease. OA Case Reports 28: 19–23. [Google Scholar]
- 12. Teoh AYB, Hui M, Ngo KY, Wong J, Lee KF, et al. (2006) Fatal septicaemia from Chromobacterium violaceum: Case reports and review of the literature. Hong Kong Med J 12: 228–231. [PubMed] [Google Scholar]
- 13. Karthik R, Pancharatnam P, Balaji V (2012) Case Report Fatal Chromobacterium violaceum septicemia in a South Indian adult. J Infect Dev Ctries 6: 751–755. [DOI] [PubMed] [Google Scholar]
- 14. Fantinatti-Garboggini F, Almeida R, Portillo VA, Barbosa TA, Trevilato PB, et al. (2004) Drug resistance in Chromobacterium violaceum . Genet Mol Res 3: 134–147. [PubMed] [Google Scholar]
- 15. Swem LR, Swem DL, O'Loughlin CT, Gatmaitan R, Zhao B, et al. (2009) A quorum-sensing antagonist targets both membrane-bound and cytoplasmic receptors and controls bacterial pathogenicity. Mol Cell 35: 143–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Vasconcelos ATR, Almeida de DF, Hungria M, Guimarães CT, Antonio RV, et al. (2003) The complete genome sequence of Chromobacterium violaceum reveals remarkable and exploitable bacterial adaptability. Proc Natl Acad Sci USA 100: 11660–11665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martinelli D, Grossmann G, Sequin U, Brandl H, Bachofen R (2004) Effects of natural and chemically synthesized furanones on quorum sensing in Chromobacterium violaceum . BMC Microbiol 4: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kalia VC (2013) Quorum sensing inhibitors: An overview. Biotechnol Adv 31: 224–245. [DOI] [PubMed] [Google Scholar]
- 19. Koh KH, Tham FY (2011) Screening of traditional Chinese medicinal plants for quorum-sensing inhibitors activity. J Microbiol Immunol Infect 44: 144–148. [DOI] [PubMed] [Google Scholar]
- 20. Vasavi HS, Arun AB, Rekha PD (2013) Inhibition of quorum sensing in Chromobacterium violaceum by Syzygium cumini L. and Pimenta dioica L. Asian Pac J Trop Biomed. 3: 954–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Priya K, Yin W-F, Chan K-GF (2013) Anti-Quorum Sensing Activity of the Traditional Chinese Herb, Phyllanthus amarus . Sensors 13: 14558–14569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gilbert B, Alves LF (2003) Synergy in Plant Medicines. Curr Med Chem 10: 13–20. [DOI] [PubMed] [Google Scholar]
- 23.Rasoanaivo P, Wright CW, Willcox ML, Gilbert B (2011) Whole plant extracts versus single compounds for the treatment of malaria: synergy and positive interactions. Malar J (Suppl 1): S4. [DOI] [PMC free article] [PubMed]
- 24.Ginsburg H, Deharo E (2011) A call for using natural compounds in the development of new antimalarial treatments– an introduction. Malar J (Suppl 1): S1. [DOI] [PMC free article] [PubMed]
- 25. Wagner H, Ulrich-Merzenich G (2009) Synergy research: approaching a new generation of phytopharmaceuticals. Phytomedicine 16: 97–110. [DOI] [PubMed] [Google Scholar]
- 26. Gutierrez RM, Mitchell S, Solis RV (2008) Psidium guajava: a review of its traditional uses, phytochemistry and pharmacology. J Ethnopharmacol 117: 1–27. [DOI] [PubMed] [Google Scholar]
- 27. Barbalho SM, Farinazzi-Machado FMV, de Alvares GR, Brunnati ACS, Otoboni AM, et al. (2012) Psidium Guajava (Guava): A plant of multipurpose medicinal applications. Med Aromat Plants 1: 104–109. [Google Scholar]
- 28. Metwally AM, Omar AA, Harraz FM, El Sohafy SM (2010) Phytochemical investigation and antimicrobial activity of Psidium guajava L leaves. Pharmacogn Mag 6: 212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Birdi T, Daswani P, Brijesh S, Tetali P, Natu A, et al. (2010) Newer insights into the mechanism of action of Psidium guajava L. leaves in infectious diarrhoea. BMC Complement Altern Med 10: 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Chen HY, Yen GC (2007) Antioxidant activity and free radical-scavenging capacity of extracts from guava (Psidium guajava L) leaves. Food Chem 101: 686–694. [Google Scholar]
- 31. Matsuo T, Hanamure N, Shimoi K, Nakamura Y, Tomita I (1994) Identification of (+)-gallocatechin as a bio-antimutagenic compound in Psidium guava leaves. Phytochemistry 36: 1027–1029. [DOI] [PubMed] [Google Scholar]
- 32. Ryu NH, Park KR, Kim SM, Yun HM, Nam D, et al. (2012) A Hexane Fraction of Guava Leaves (Psidium guajava L.) Induces Anticancer Activity by Suppressing AKT/Mammalian Target of Rapamycin/Ribosomal p70 S6 Kinase in Human Prostate Cancer Cells. J Med Food 15: 231–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Deguchi Y, Miyazaki K (2010) Anti-hyperglycemic and anti-hyperlipidemic effects of guava leaf extract. Nutr Metab (Lond) 7: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Prabu GR, Gnanamani A, Sadulla S (2006) Guaijaverin - a plant flavonoid as potential antiplaque agent against Streptococcus mutans . J Appl Microbiol 101: 487–495. [DOI] [PubMed] [Google Scholar]
- 35. Quave CL, Plano LRW, Pantuso T, Bennett BC (2008) Effects of extracts from Italian medicinal plants on planktonic growth, biofilm formation and adherence of methicillin-resistant Staphylococcus aureus . J Ethnopharmacol 118: 418–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Al-Hussaini R, Mahasneh AM (2009) Microbial growth and quorum sensing antagonist activities of herbal plants extracts. Molecules 14: 3425–3435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tremblay J, Richardson AP, Lepine F, Deziel E (2007) Self-produced extracellular stimuli modulate the Pseudomonas aeruginosa swarming motility behaviour. Environ Microbiol 9: 2622–2630. [DOI] [PubMed] [Google Scholar]
- 38. Choo JH, Rukayadi Y, Hwang J-K (2006) Inhibition of bacterial quorum sensing by vanilla extract. Lett Appl Microbiol 42: 637–641. [DOI] [PubMed] [Google Scholar]
- 39.Miller JH (1972) Determination of viable cell counts: bacterial growth curves. Experiments in Molecular Genetics. Cold Spring Harbor Press: New York, pp 31–36.
- 40. McLean RJ, Pierson LS III, Fuqua C (2004) A simple screening protocol for the identification of quorum signal antagonists. J Microbiol Methods 58: 351–360. [DOI] [PubMed] [Google Scholar]
- 41. Defoirdt T, Brackman G, Coenye T (2013) Quorum sensing inhibitors: how strong is the evidence? Trends Microbiol 21: 619–624. [DOI] [PubMed] [Google Scholar]
- 42. Adonizio AL, Downum K, Bennett BC, Mathee K (2006) Anti-quorum sensing activity of medicinal plants in southern Florida. J Ethnopharmacol 105: 427–435. [DOI] [PubMed] [Google Scholar]
- 43. Koh KH, Tham FY (2011) Screening of traditional Chinese medicinal plants for quorum-sensing inhibitors activity. J Microbiol Immunol Infect 44: 144–148. [DOI] [PubMed] [Google Scholar]
- 44. May CO, Tufenkji N (2011) The swarming motility of P. aeruginosa is blocked by cranberry proanthocyanidins and other tannin containing materials. Appl Environ Microbiol 77: 3061–3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wagner VE, Bushnell D, Passador L, Brooks AI, Iglewski BH (2003) Microarray analysis of Pseudomonas aeruginosa quorum-sensing regulons: effects of growth phase and environment. J Bacteriol 185: 2080–2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. DeLisa MP, Wu CF, Wang L, Valdes JJ, Bentley WE (2001) DNA microarray-based identification of genes controlled by autoinducer 2-stimulated quorum sensing in Escherichia coli . J Bacteriol 183: 5239–5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Yang CH, Li YH (2011) Chromobacterium violaceum infection: a clinical review of an important but neglected infection. J Chin Med Assoc 74: 435–441. [DOI] [PubMed] [Google Scholar]
- 48. Schuster M, Lostroh CP, Ogi T, Greenberg EP (2003) Identification, timing, and signal specificity of Pseudomonas aeruginosa quorum-controlled genes: a transcriptome analysis. J Bacteriol 185: 2066–2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Passador LJ, Cook M, Gambello MJ, Rust L, Iglewski BH (1993) Expression of Pseudomonas aeruginosa virulence genes requires cell-to-cell communication. Science 260: 1127–1130. [DOI] [PubMed] [Google Scholar]
- 50. Baca-DeLancey RR, South MM, Ding X, Rather PN (1999) Escherichia coli genes regulated by cell-to-cell signaling. Proc Natl Acad Sci USA 96: 4610–4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hentzer M, Wu H, Andersen JB, Riedel K, Rasmussen TB, et al. (2003) Attenuation of Pseudomonas aeruginosa virulence by quorum sensing inhibitors. EMBO J 22: 3803–3815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kessler E (1995) Beta-lytic endopeptidases. Methods Enzymol 248: 740–756. [DOI] [PubMed] [Google Scholar]
- 53. Preston M, Seed P, Toder D, Iglewski B, Ohman D, et al. (1997) Contribution of proteases and LasR to the virulence of Pseudomonas aeruginosa during corneal infections. Infect Immun 65: 3086–3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Duran N, Menck CF (2001) Chromobacterium violaceum: a review of pharmacological and industiral perspectives. Crit Rev Microbiol 27: 201–222. [DOI] [PubMed] [Google Scholar]
- 55. Chernin LS, Winson MK, Thompson JM, Haran S, Bycroft BW, et al. (1998) Chitinolytic activity in Chromobacterium violaceum: substrate analysis and regulation by quorum sensing. J Bacteriol 180: 4435–4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schaefer AL, Val DL, Hanzelka BL, Cronan JE, Greenberg EP (1996) Generation of cell-to-cell signals in quorum sensing: acyl homoserine lactone synthase activity of a purified Vibrio fischeri LuxI protein. Proc Natl Acad Sci USA 93: 9505–9509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stauff DL, Bassler BL (2011) Quorum Sensing in Chromobacterium violaceum: DNA Recognition and Gene Regulation by the CviR Receptor. J Bact 193: 3871–3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Brito CFAD, Carvalho CMB, Santos FR, Gazzinelli RT, Oliveira SC, et al. (2004) Chromobacterium violaceum genome: molecular mechanisms associated with pathogenicity. Genet Mol Res 3: 148–161. [PubMed] [Google Scholar]
- 59. Bieber D, Ramer SW, Wu CY, Murray WJ, Tobe T, et al. (1998) Type IV pili, transient bacterial aggregates, and virulence of enteropathogenic Escherichia coli . Science 280: 2114–2118. [DOI] [PubMed] [Google Scholar]
- 60. Kennan RM, Dhungyel OP, Whittington RJ, Egerton JR, Rood JI (2001) The type IV fimbrial subunit gene (fimA) of Dichelobacter nodosus is essential for virulence, protease secretion, and natural competence. J Bacteriol 183: 4451–4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Castro-Gomes T, Cardoso MS, DaRocha WD, Laibida LA, Nascimento AM, et al. (2014) Identification of secreted virulence factors of Chromobacterium violaceum . J Microbiol 52: 350–353. [DOI] [PubMed] [Google Scholar]
- 62. Miki T, Iguchi M, Akiba K, Hosono M, Sobue T, et al. (2010) Chromobacterium pathogenicity island 1 type III secretion system is a major virulence determinant for Chromobacterium violaceum-induced cell death in hepatocytes. Mol Microbiol 77: 855–872. [DOI] [PubMed] [Google Scholar]
- 63. Atkinson S, Throup JP, Stewart GSAB, Williams P (1999) A hierarchical quorum-sensing system in Yersinia pseudotuberculosis is involved in the regulation of motility and clumping. Mol Microbiol 33: 1267–1277. [DOI] [PubMed] [Google Scholar]
- 64.Yang Q, Defoirdt T (2014) Quorum sensing positively regulates flagellar motility in pathogenic Vibrio harveyi. Env Microbiol doi:10.1111/1462–2920.12420 (in Press). [DOI] [PubMed]
- 65. Recouvreux DO, Carminatti CA, Pitlovanciv AK, Rambo CR, Porto LM, et al. (2008) Cellulose biosynthesis by the beta-proteobacterium, Chromobacterium violaceum . Curr Microbiol 57: 469–476. [DOI] [PubMed] [Google Scholar]
- 66. Wood TK (2009) Insights on Escherichia coli biofilm formation and inhibition from whole-transcriptome profiling. Environ Microbiol 11: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lee J-HJ, Kim Y-GY, Cho MHM, Wood TKT, Lee JJ (2011) Transcriptomic analysis for genetic mechanisms of the factors related to biofilm formation in Escherichia coli O157: H7. Curr Microbiol 62: 1321–1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Liu Z, Wang W, Zhu Y, Gong Q, Yu W, et al. (2013) Antibiotics at subinhibitory concentrations improve the quorum sensing behavior of Chromobacterium violaceum . FEMS Microbiol Lett 341: 37–44. [DOI] [PubMed] [Google Scholar]
- 69. Gu X, Xie Z, Wang Q, Liiu G, Qu Y, et al. (2009) Transcriptome profiling analysis reveals multiple modulatory effects of Ginkgo biloba extract in the liver of rats on a high-fat diet. Febs J 276: 1450–1458. [DOI] [PubMed] [Google Scholar]
- 70. Fathilah AR, Rahim ZHA (2003) The anti-adherence effect of Piper betle and Psidium guajava extracts on the adhesion of early settlers in dental plaque to saliva-coated glass surfaces. J Oral Sci 45: 201–206. [DOI] [PubMed] [Google Scholar]
- 71. Defoirdt T, Boon N, Bossier P (2010) Can Bacteria Evolve Resistance to Quorum Sensing Disruption, PLoS Pathog. 6: e1000989. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Inhibition of QS-regulated phenotypes by GLE. A. Formation of a colourless translucent zone around the well containing GLE indicating absence of violacein production by C. violaceum cells. B. Inhibition of swarming motility of P. aeruginosa cells grown in presence of GLE.
(TIF)
Significantly down-regulated genes of C. violaceum when grown in presence of GLE.
(DOCX)
Method: Whole genome transcriptome analysis.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All sequence files are available from the SRA database (accession number(s) SRR1221300. All other relevant data are within the paper and its Supporting Information files.