Abstract
Background
In many countries of the industrialised world second-generation (“atypical”) antipsychotics (SGAs) have become the first line drug treatment for people with schizophrenia. The question as to whether and if so how much the effects of the various SGAs differ is a matter of debate. In this review we examined how the efficacy and tolerability of risperidone differs from that of other SGAs.
Objectives
To evaluate the effects of risperidone compared with other atypical antipsychotics for people with schizophrenia and schizophrenia-like psychosis.
Search methods
1. Electronic searching
We searched the Cochrane Schizophrenia Group Trials Register (April 2007) which is based on regular searches of BIOSIS, CENTRAL, CINAHL, EMBASE, MEDLINE and PsycINFO.
2. Reference searching
We inspected the references of all identified studies for more trials.
3. Personal contact
We contacted the first author of each included study for missing information.
4. Drug companies
We contacted the manufacturers of all atypical antipsychotics included for additional data.
Selection criteria
We included all randomised, blinded trials comparing oral risperidone with oral forms of amisulpride, aripiprazole, clozapine, olanzapine, quetiapine, sertindole, ziprasidone or zotepine in people with schizophrenia or schizophrenia-like psychosis.
Data collection and analysis
We extracted data independently. For dichotomous data we calculated risk ratio (RR) and their 95% confidence intervals (CI) on an intention-to-treat basis based on a random-effects model. We calculated numbers needed to treat/harm (NNT/NNH) where appropriate. For continuous data, we calculated mean differences (MD), again based on a random-effects model.
Main results
The review currently includes 45 blinded RCTs with 7760 participants. The number of RCTs available for each comparison varied: four studies compared risperidone with amisulpride, two with aripiprazole, 11 with clozapine, 23 with olanzapine, eleven with quetiapine, two with sertindole, three with ziprasidone and none with zotepine. Attrition from these studies was high (46.9%), leaving the interpretation of results problematic. Furthermore, 60% were industry sponsored, which can be a source of bias.
There were few significant differences in overall acceptability of treatment as measured by leaving the studies early. Risperidone was slightly less acceptable than olanzapine, and slightly more acceptable than ziprasidone in this regard.
Risperidone improved the general mental state (PANSS total score) slightly less than olanzapine (15 RCTs, n = 2390, MD 1.94 CI 0.58 to 3.31), but slightly more than quetiapine (9 RCTs, n = 1953, MD −3.09 CI −5.16 to −1.01) and ziprasidone (3 RCTs, n = 1016, MD −3.91 CI −7.55 to −0.27). The comparisons with the other SGA drugs were equivocal. Risperidone was also less efficacious than olanzapine and clozapine in terms of leaving the studies early due to inefficacy, but more efficacious than ziprasidone in the same outcome.
Risperidone produced somewhat more extrapyramidal side effects than a number of other SGAs (use of antiparkinson medication versus clozapine 6 RCTs, n = 304, RR 2.57 CI 1.47 to 4.48, NNH 6 CI 33 to 3; versus olanzapine 13 RCTs, n = 2599, RR 1.28 CI 1.06 to 1.55, NNH 17 CI 9 to 100; versus quetiapine 6 RCTs, n = 1715, RR 1.98 CI 1.16 to 3.39, NNH 20 CI 10 to 100; versus ziprasidone 2 RCTs, n = 822, RR 1.42 CI 1.03 to 1.96, NNH not estimable; parkinsonism versus sertindole 1 RCT, n = 321, RR 4.11 CI 1.44 to 11.73, NNH 14 CI 100 to 8). Risperidone also increased prolactin levels clearly more than all comparators, except for amisulpride and sertindole for which no data were available.
Other adverse events were less consistently reported, but risperidone may well produce more weight gain and/or associated metabolic problems than amisulpride (weight gain: 3 RCTs, n = 585, MD 0.99 CI 0.37 to 1.61), aripiprazole (cholesterol increase: 1 RCT, n = 83, MD 22.30 CI 4.91 to 39.69) and ziprasidone (cholesterol increase 2 RCTs, n = 767, MD 8.58 CI 1.11 to 16.04) but less than clozapine (weight gain 3 RCTs n = 373, MD −3.30 CI −5.65 to −0.95), olanzapine (weight gain 13 RCTs, n = 2116, MD −2.61 CI −3.74 to −1.48), quetiapine (cholesterol increase: 5 RCTs, n = 1433, MD −8.49 CI −12. 23 to −4.75) and sertindole (weight gain: 2 RCTs, n = 328, MD −0.99 CI −1.86 to −0.12). It may be less sedating than clozapine and quetiapine, lengthen the QTc interval less than sertindole (QTc change: 2 RCTs, n = 495, MD −18.60 CI −22.37 to 14.83), produce fewer seizures than clozapine (2 RCTs, n = 354, RR 0.22 CI 0.07 to 0.70, NNT 14 CI 8 to 33) and less sexual dysfunction in men than sertindole (2 RCTs, n = 437, RR 0.34 CI 0.16 to 0.76, NNT 13 CI 8 to 33).
Authors’ conclusions
Risperidone seems to produce somewhat more extrapyramidal side effects and clearly more prolactin increase than most other SGAs. It may also differ from other compounds in efficacy and in the occurrence of other adverse effects such as weight gain, metabolic problems, cardiac effects, sedation and seizures. Nevertheless, the large proportion of participants leaving studies early and incomplete reporting of outcomes makes it difficult to draw firm conclusions. Further large trials, especially comparing risperidone with those other new drugs for which only a few RCTs are available, are needed.
Medical Subject Headings (MeSH): Antipsychotic Agents [adverse effects; * therapeutic use], Benzodiazepines [therapeutic use], Clozapine [therapeutic use], Dibenzothiazepines [therapeutic use], Imidazoles [therapeutic use], Indoles [therapeutic use], Piperazines [therapeutic use], Quinolones [therapeutic use], Randomized Controlled Trials as Topic, Risperidone [adverse effects; * therapeutic use], Schizophrenia [* drug therapy], Sulpiride [analogs & derivatives; therapeutic use], Thiazoles [therapeutic use]
MeSH check words: Humans
BACKGROUND
Description of the condition
Schizophrenia is usually a chronic and disabling psychiatric disorder which afflicts approximately one per cent of the population world-wide with little gender differences. The annual incidence of schizophrenia averages 15 per 100,000, the point prevalence averages approximately 4.5 per population of 1000, and the risk of developing the illness over one’s lifetime averages 0.7%. (Tandon 2008). Its typical manifestations are positive symptoms such as fixed, false beliefs (delusions) and perceptions without cause (hallucinations), negative symptoms such as apathy and lack of drive, disorganisation of behaviour and thought, and catatonic symptoms such as mannerisms and bizarre posturing (Carpenter 1994). The degree of suffering and disability is considerable, with 80%-90% not working (Marvaha 2004) and up to 10% dying (Tsuang 1978). In the age group of 15-44 years, schizophrenia is among the top 10 leading causes of disease-related disability in the world (WHO 2001).
Description of the intervention
Conventional antipsychotic drugs such as chlorpromazine and haloperidol have traditionally been used as first-line antipsychotics for people with schizophrenia (Kane 1993). The reintroduction of clozapine in the United States of America and a finding that clozapine was more efficacious and associated with fewer movement disorders than chlorpromazine (Kane 1988) has boosted the development of so-called “atypical” or new (second) generation antipsychotics (SGA). There is no good definition of what an “atypical” or SGA is, but they were initially said to differ from typical antipsychotics in that they do not cause movement disorders (catalepsy) in rats at clinically effective doses (Arnt 1998). The terms “new” or “second generation” antipsychotics are not much better, because clozapine is a very old drug. According to treatment guidelines (APA 2004; Gaebel 2006) SGAs include drugs such as amisulpride, aripiprazole, clozapine, olanzapine, quetiapine, risperidone sertindole, ziprasidone and zotepine, although it is unclear whether some old and cheap compounds such as sulpiride or perazine have similar properties (Möller 2000). The SGAs raised major hopes of superior effects in a number of areas such as compliance, cognitive functioning, negative symptoms, movement disorders, quality of life and the treatment of refractory people with schizophrenia.
How the intervention might work
Risperidone has high affinity to 5-HT2 and D2 receptors; it also binds to a1 receptors and with lower affinity to H1 and a2 receptors. It was developed following the observation that a selective serotonin receptor blocker (ritanserin) produced a beneficial effect when combined with conventional neuroleptics (Gupta 1994; Curtis 1995). Risperidone is described to have no affinity to cholinergic receptors. Although being a potential D2 antagonist it causes less motor retardation and cataleptic symptoms than typical antipsychotics (Janssen-Cilag 2005).
Why it is important to do this review
The debate as to how far the SGA improve these outcomes compared to conventional antipsychotics continues (Duggan 2005;El-Sayeh 2006) and the results from recent studies are sobering (Jones 2006; Lieberman 2005). Nevertheless, in some parts of the world, especially in the highly industrialised countries, SGA have become the mainstay of treatment. The SGAs also differ in terms of their costs: while amisulpride and risperidone are already generic in many countries in 2009, aripiprazole, olanzapine, quetiapine, sertindole and ziprasidone are still not. Therefore the question as to whether they differ from each other in their clinical effects becomes increasingly important. In this review we aim to summarise evidence from randomised controlled trials that compared risperidone with other SGAs.
OBJECTIVES
To review the effects of risperidone compared with other atypical antipsychotics for people with schizophrenia and schizophrenialike psychosis.
METHODS
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) which were at least single-blind (blind raters). Where a trial was described as ‘double-blind’, but it was only implied that the study was randomised, we included these trials in a sensitivity analysis. If there was no substantive difference within primary outcomes (see types of outcome measures) when these ‘implied randomisation’ studies were added, then we included these in the final analysis. If there was a substantive difference, we only used clearly randomised trials and described the results of the sensitivity analysis in the text. We excluded quasi-randomised studies, such as those allocating by using alternate days of the week. In crossover studies, we only included the first treatment phase prior to crossover.
We included randomised crossover studies, but only data up to the point of first crossover because of the instability of the problem behaviours and the likely carry-over effects of all treatments.
Types of participants
We included people with schizophrenia and other types of schizophrenia-like psychosis (e.g. schizophreniform and schizoaffective disorders), irrespective of the diagnostic criteria used. There is no clear evidence that the schizophrenia-like psychoses are caused by fundamentally different disease processes or require different treatment approaches (Carpenter 1994).
Types of interventions
Risperidone: any oral form of application, any dose
Other “atypical” antipsychotic drugs: amisulpride, aripiprazole, clozapine, olanzapine, quetiapine, sertindole, ziprasidone, zotepine: any oral form of application, any dose.
Types of outcome measures
We grouped outcomes into the short term (up to 12 weeks), medium term (13-26 weeks) and long term (over 26 weeks).
Primary outcomes
Global state: no clinically important response as defined by the individual studies (e.g. global impression less than much improved or less than 50% reduction on a rating scale).
Secondary outcomes
-
1.
Leaving the studies early (any reason, adverse events, inefficacy of treatment)
-
2.
Global state
-
2.1
No clinically important change in global state (as defined by individual studies)
-
2.2
Relapse (as defined by the individual studies)
-
3.
Mental state (with particular reference to the ‘positive’ and ‘negative’ symptoms of schizophrenia)
-
3.1
No clinically important change in general mental state score
-
3.2
Average endpoint general mental state score
-
3.3
Average change in general mental state score
-
3.4
No clinically important change in specific symptoms (positive symptoms of schizophrenia, negative symptoms of schizophrenia)
-
3.5
Average endpoint specific symptom score
-
3.6
Average change in specific symptom score
-
4.
General functioning
-
4.1
No clinically important change in general functioning
-
4.2
Average endpoint general functioning score
-
4.3
Average change in general functioning score
-
5.
Quality of life/satisfaction with treatment
-
5.1
No clinically important change in general quality of life
-
5.2
Average endpoint general quality of life score
-
5.3
Average change in general quality of life score
-
6.
Cognitive functioning
-
6.1
No clinically important change in overall cognitive functioning
-
6.2
Average endpoint of overall cognitive functioning score
-
6.3
Average change of overall cognitive functioning score
-
7.
Service use
-
7.1
Number of people hospitalised
-
8.
Adverse effects
-
8.1
Number of participants with at least one adverse effect
-
8.2
Clinically important specific adverse effects (cardiac effects, death, movement disorders, prolactin increase and associated effects, sedation, seizures, weight gain, effects on white blood cell count)
-
8.3
Average endpoint in specific adverse effects
-
8.4
Average change in specific adverse effects
Search methods for identification of studies
We applied no language restriction within the limitations of the search tools.
Electronic searches
We searched the Cochrane Schizophrenia Group’s Specialised Register (April 2007) using the phrase:
[((ziprasidon* AND (amisulprid* OR aripiprazol* OR clozapin* OR olanzapin* OR quetiapin* OR sertindol* OR risperidon* OR zotepin*)) in title, abstract or index terms of REFERENCE) or ((ziprasidon* AND (amisulprid* OR aripiprazol* OR clozapin* OR olanzapin* OR quetiapin* OR sertindol* OR risperidon * OR zotepin*)) in interventions of STUDY)]
This register is compiled by systematic searches of major databases, hand searches and conference proceedings (see Group Module). The Cochrane Schizophrenia Group Trials Register is maintained on Meerkat 1.5. This version of Meerkat stores references as studies. When an individual reference is selected through a search, all references which have been identified as the same study are also selected.
Searching other resources
1. Reference searching
We inspected the reference lists of all studies identified in the search for more trials.
2. Personal contact
We contacted the first author of each included study for missing information.
3. Drug companies
We contacted the manufacturers of all atypical antipsychotics included for additional data.
Data collection and analysis
Selection of studies
KK, CRK and SL independently inspected all reports. We resolved any disagreement by discussion, and where there was still doubt, we acquired the full article for further inspection. Once we obtained the full articles, we independently decided whether the studies met the review criteria. If disagreement could not be resolved by discussion, we sought further information and added these trials to the list of those awaiting assessment.
Data extraction and management
1. Data extraction
KK, CRK and SL independently extracted data from selected trials. When disputes arose we attempted to resolve these by discussion. When this was not possible and further information was necessary to resolve the dilemma, we did not enter data and added the trial to the list of those awaiting assessment.
2. Management
KK, CRK, SS, HH, FS and SL extracted the data onto standard simple forms. Where possible, we entered data in such a way that the area to the left of the line of no effect indicated a favourable outcome for risperidone.
3. Rating scales
A wide range of instruments are available to measure outcomes in mental health studies. These instruments vary in quality and many are not validated, or are even ad hoc. It is accepted generally that measuring instruments should have the properties of reliability (the extent to which a test effectively measures anything at all) and validity (the extent to which a test measures that which it is supposed to measure) (Rust 1989). Unpublished scales are known to be subject to bias in trials of treatments for schizophrenia (Marshall 2000). Therefore we included continuous data from rating scales only if the measuring instrument had been described in a peer-reviewed journal.
Assessment of risk of bias in included studies
Again working independently, KK and SL assessed risk of bias using the tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). This tool encourages consideration of how the sequence was generated, how allocation was concealed, the integrity of blinding at outcome, the completeness of outcome data, selective reporting and other biases.
We assessed the risk of bias in each domain and overall and categorised them into:
Low risk of bias: plausible bias unlikely to seriously alter the results (categorised as ‘Yes’ in Risk of Bias table)
High risk of bias: plausible bias that seriously weakens confidence in the results (categorised as ‘No’ in Risk of Bias table)
Unclear risk of bias: plausible bias that raises some doubt about the results (categorised as ‘Unclear’ in Risk of Bias table)
We categorised trials with high risk of bias (defined as at least four out of seven domains) as ‘No’. Where allocation was clearly not concealed, we did not include these trials in the review. If the initial raters disagreed, we made the final rating by consensus with the involvement of another member of the review group. Where inadequate details of randomisation and other characteristics of trials were provided, we contacted authors of the studies in order to obtain further information. We reported non-concurrence in quality assessment.
Measures of treatment effect
1. Data types
We assessed outcomes using continuous (for example changes on a behaviour scale), categorical (for example, one of three categories on a behaviour scale, such as “little change”, “moderate change” or “much change”) or dichotomous (for example, either “no important changes or ”important change“ in a person’s behaviour) measures. Currently The Cochrane Collaboration’s Review Manager (RevMan) software (RevMan 2008) does not support categorical data so we were unable to analyse this.
1.1 Dichotomous- yes/no- data
We carried out an intention-to-treat analysis. We counted every-one allocated to the intervention, whether they completed the follow-up or not. We assumed that those who dropped out had no change of their outcome. This rule is conservative concerning response to treatment, because it assumes that those discontinuing the studies would not have responded. It is not conservative concerning side effects, because it assumes that those discontinuing the studies would not have developed the side effect if they had remained in the study, but we felt that assuming that all drop-outs would have developed side effects would overestimate the risk. Where possible, we made efforts to convert outcome measures to dichotomous data. This can be done by identifying cut-off points on rating scales and dividing participants accordingly into “clinically improved” or “not clinically improved”. It was generally assumed that if there had been a 50% reduction in a scale-derived score such as the Brief Psychiatric Rating Scale (BPRS, Overall 1962) or the Positive and Negative Syndrome Scale (PANSS, Kay 1986); this could be considered as a clinically significant response (Leucht 2005a; Leucht 2005b). If data based on these thresholds were not available, we used the primary cut-off presented by the original authors.
We calculated the risk ratio (RR) and its 95% confidence interval (CI) based on the random-effects model, as this takes into account any differences between studies even if there is no statistically significant heterogeneity. It has been shown that RR is more intuitive (Boissel 1999) than odds ratios (OR) and that OR tend to be interpreted as RR by clinicians (Deeks 2000). This misinterpretation then leads to an overestimate of the impression of the effect. When the overall results were significant we calculated the number needed to treat (NNT) and the number- needed- to- harm (NNH) as the inverse of the risk difference.
1.2 Continuous data
1.2.1 Normal distribution of the data
The meta-analytic formulae applied by RevMan Analyses (the statistical programme included in RevMan) require a normal distribution of data (RevMan 2008). The software is robust towards some skew, but to which degree of skewness meta-analytic calculations can still be reliably carried out is unclear. Conversely, excluding all studies on the basis of estimates of the normal distribution of the data also leads to a bias, because a considerable amount of data may be lost leading to a selection bias. Therefore, we included all studies in the primary analysis. In a sensitivity analysis we excluded potentially skewed data applying the following rules.
When a scale started from the finite number zero the standard deviation, when multiplied by two, was less than the mean (as otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution, Altman 1996).
If a scale started from a positive value (such as PANSS which can have values from 30 to 210), we modified the calculation described above to take the scale starting point into account. In these cases skew is present if 2SD>(S-Smin), where S is the mean score and Smin is the minimum score.
In large studies (as a cut-off we used 200 participants) skewed data pose less of a problem. In these cases we entered the data in a synthesis.
The rules explained in a) and b) do not apply to change data.
The reason is that when continuous data are presented on a scale which includes a possibility of negative values, it is difficult to tell whether data are non-normally distributed (skewed) or not. This is also the case for change data (endpoint minus baseline). In the absence of individual patient data it is impossible to know if data are skewed, though this is likely. After consulting the ALL-STAT electronic statistics mailing list, we presented change data in RevMan Analyses in order to summarise available information. In doing this, it was assumed either that data were not skewed or that the analysis could cope with the unknown degree of skew. Without individual patient data it is impossible to test this assumption. We therefore included change data and did not apply a sensitivity analysis.
2. Data synthesis
For continuous outcomes we estimated a mean difference (MD) between groups. MDs were again based on the random-effects model, as this takes into account any differences between studies even if there is no statistically significant heterogeneity. We combined both endpoint data and change data in the analysis, because there is no principal statistical reason why endpoint and change data should measure different effects (Higgins 2008). When standard errors instead of standard deviations (SD) were presented, we converted the former to standard deviations. If both were missing we estimated SDs from P values or used the average SD of the other studies (Furukawa 2006).
Unit of analysis issues
1. Cluster trials
Studies increasingly employ ‘cluster randomisation’ (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intraclass correlation in clustered studies, leading to a ‘unit of analysis’ error (Divine 1992) whereby P values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This causes type 1 errors (Bland 1997; Gulliford 1999).
Where clustering was not accounted for in primary studies, we presented the data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review we will seek to contact first authors of studies to obtain intraclass correlation coefficients of their clustered data and to adjust for this using accepted methods (Gulliford 1999). Where clustering has been incorporated into the analysis of primary studies, we will also present these data as if from a non-cluster randomised study, but adjusted for the clustering effect.
We have sought statistical advice and have been advised that the binary data as presented in a report should be divided by a ‘design effect’. This is calculated using the mean number of participants per cluster (m) and the intraclass correlation coefficient (ICC) [Design effect = 1+(m-1)*ICC] (Donner 2002). If the ICC was not reported we assumed it to be 0.1 (Ukoumunne 1999).
If cluster studies had been appropriately analysed taking into account ICC and relevant data documented in the report, we synthesised these with other studies using the generic inverse variance technique.
2. Crossover trials
A major concern of crossover trials is the carry-over effect. It occurs if an effect (e.g. pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence, on entry to the second phase the participants can differ systematically from their initial state despite a washout phase. For the same reason crossover trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in schizophrenia, we will only use data of the first phase of crossover studies.
3. Studies with multiple treatment groups
Where a study involved more than two treatment groups, if relevant, we presented the additional treatment groups in additional relevant comparisons. We have not double counted data. Where the additional treatment groups were not relevant, we have not reproduced these data.
Dealing with missing data
At some degree of loss of follow-up data must lose credibility (Xia 2007). Although high rates of premature discontinuation are a major problem in this field, we feel that it is unclear which degree of attrition leads to a high degree of bias. We, therefore, did not exclude trials on the basis of the percentage of participants completing them. However we addressed the drop-out problem in all parts of the review, including the abstract. For this purpose we calculated, presented and commented on frequency statistics (overall rates of leaving the studies early in all studies and comparators pooled and their ranges).
Assessment of heterogeneity
1. Clinical heterogeneity
We considered all the included studies within any comparison to judge for clinical heterogeneity.
2. Statistical
2.1 Visual inspection
We visually inspected graphs to investigate the possibility of statistical heterogeneity.
2.2 Employing the I2 statistic
We supplemented visual inspection using, primarily, the I2statistic. This provides an estimate of the percentage of variability due to heterogeneity rather than chance alone. Where the I2 estimate was greater than or equal to 50% we interpreted this as indicating the presence of considerable levels of heterogeneity (Higgins 2003).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). These are described in section 10.1 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). We are aware that funnel plots may be useful in investigating small-study effects but are of limited power to detect such effects when there are few studies. We entered data from all identified and selected trials into a funnel graph (trial effect versus trial size) in an attempt to investigate the likelihood of overt publication bias. We did not undertake a formal test for funnel-plot asymmetry.
Data synthesis
Where possible for both dichotomous and continuous data, we used the random-effects model for data synthesis as this takes into account any differences between studies even if there is no statistically significant heterogeneity. We understand that there is no closed argument for preference for use of fixed or random-effects models. The random-effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This does seem true to us, however, random-effects does put added weight onto the smaller of the studies - those trials that are most vulnerable to bias.
Subgroup analysis and investigation of heterogeneity
If data are clearly heterogeneous we checked that data are correctly extracted and entered and that we had made no unit of analysis errors. If inconsistency was high and clear reasons explaining the heterogeneity were found, we presented the data separately. If not, we commented on the heterogeneity of the data.
Sensitivity analysis
In sensitivity analyses we excluded studies with potentially skewed data. A recent report showed that some of the comparisons of atypical antipsychotics may have been biased by using inappropriate comparator doses (Heres 2006). We, therefore, also analysed whether the exclusion of studies with inappropriate comparator doses changed the results of the primary outcome and the general mental state.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
For substantive description of studies please see Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
The search strategy yielded 3620 reports. We closely inspected 330 reports. We excluded 241 studies, included 45 and designated seven as ongoing (Eli Lilly 2003; Eli Lilly 2006; Gafoor 2005;Lieberman 2001; Ratna 2003; Reveley 2000; Sireling 2003). No study is awaiting assessment. For further descriptions please see below and the included, excluded and ongoing studies tables.
The 45 included studies provided data on seven comparisons: four studies compared risperidone with amisulpride, two with aripiprazole, 11 with clozapine, 23 with olanzapine, 11 with quetiapine, two with sertindole and three with ziprasidone. We identified no RCTs comparing risperidone with zotepine.
Included studies
The 45 studies randomised approximately 7700 people with schizophrenia and schizophrenia-like disorders. All but six included studies were double blind; the remaining six were single blind (blind raters). Eight studies were sponsored by pharmaceutical companies producing risperidone; 19 studies were sponsored by pharmaceutical companies producing the comparator substances and 14 studies had a neutral sponsor. The sponsoring of four studies remained unclear despite written requests.
1. Length of studies
Thirty-one studies presented short-term (up to 12 weeks) data. Six studies fell into the medium-term category (13-26 weeks) and eight trials fell in the long-term category (more than 26 weeks).
2. Setting
Eighteen trials took place in both inpatient and outpatient settings; 12 used an inpatient design and three studies an outpatient setting. The setting of 10 studies was not reported.
3. Participants
All studies used operationalised diagnostic criteria, most frequently the Diagnostic and Statistical Manual (DSM, APA 1994), usually version IV, only Addington 2004, Daniel 1996 and Bondolfi 1998 used DSM-III). Svestka 2003 applied the International Classification of Diseases and Related Health Problems (ICD-10). Riedel 2005 diagnosed according to DSM-IV and ICD-10. Sikich 2004 applied DSM-IV as well as the Schedule for Affective Disorders and Schizophrenia (K-SADS-P). Ren 2002, a study from China, used the Chinese Classification of Mental Disorders Version 3 (CCDM-3) and Zhou 2000 used Version 2 (CCDM-2) of the same classification.
All studies included people with schizophrenia; 21 studies additionally included those with schizoaffective disorder (Addington 2004; Chan 2007; Conley 2001; Daniel 1996; Gureje 2003;Heinrich 1994; Jeste 2003; Keefe 2006; McEvoy 2007; Möller 2005; McGurk 2005; Potkin 2003; Potkin 2006; Robinson 2005; Sikich 2004; Svestka 2003; Tran 1997; Van Nimwegen 2006; Volavka 2002; Wang 2006; Wynn 2007) and seven studies also included people with schizophreniform disorder (Gureje 2003; McEvoy 2007; Möller 2005; Robinson 2005; Sikich 2004;Tran 1997; Van Nimwegen 2006). Nevertheless, people with schizophrenia clearly dominated the trials.
Five studies randomised people with acute exacerbations (Addington 2004; Chan 2007; Heinrich 1994; Potkin 2003;Potkin 2006). McEvoy 2007, Robinson 2005 and Svestka 2003 examined those with a first episode of schizophrenia, and Purdon 2000 included only participants in the early phase of the illness. Five studies restricted randomisation to chronic schizophrenia (Breier 1999; Daniel 1996; Lieberman 2005; Sechter 2002; Stroup 2006). Insufficient response or resistance to previous treatment was an inclusion criterion in nine studies (Azorin 2001; Azorin 2006; Bondolfi 1998; Conley 2005; Kane 2005; McEvoy 2006;McGurk 2005; Volavka 2002; Wahlbeck 2000), but the criteria varied widely. One study examined people with postpsychotic depression (Dollfus 2005) and another one participants with pre-dominant negative symptoms (Riedel 2005).
In most studies participants were 18 years old or older. Two studies randomised only patients aged 60 or older (Jeste 2003; Möller 2005). Another study examined childhood schizophrenia and included only children and adolescents with ages between 8 and 19 years (Sikich 2004).
In Van Nimwegen 2006 all participants had a recent history of cannabis use in addition to schizophrenia.
4.Study size
Lieberman 2005 was the largest study (1460 participants) whereas Daniel 1996 and Wahlbeck 2000 included the smallest samples with 20 participants each. Twelve studies had fewer than 50 participants, 13 had 50 to 100 participants, 16 had 100 to 400 participants and two randomised more than 400 people. Two studies did not indicate the total number of randomised participants.
5 Interventions
5.1 Risperidone
The trialists gave risperidone in a wide range of doses from 0.5 mg/day to 12 mg/day (McGurk 2005 up to 16 mg/per day). Three studies used a fixed dose design. Seven studies did not report the dose range.
5.2 Comparators
Seven other SGA drugs were used as comparators with the following dose ranges: amisulpride (100 mg/day to 1000 mg/day), aripiprazole (15 mg/day to 30 mg/day), clozapine (25 mg/day to 900 mg/day), quetiapine (50 mg/day to 800 mg/day), olanzapine (2.5 mg/day to 40 mg/day), sertindole (12 mg/day to 24 mg/day), and zisprasidone (40 mg/day to 160 mg/day). Eight studies included more than one comparator arms of interest therefore number of comparisons (56) is higher than number of included studies (45). Some studies also included additional arms with the typical antipsychotic drugs haloperidol, perospirone or perphenazine as comparators. These results were not considered in the current review.
6. Outcomes
6.1 Leaving the study early
We evaluated the number of participants leaving the studies early due to any reason, due to adverse events and due to lack of efficacy.
6.2 Response to treatment
The studies rarely reported the response cut off at least 50% reduction (Addington 2004; Dollfus 2005; Gureje 2003; Sechter 2002;Tran 1997) of a scale’s baseline value that we considered clinically meaningful. In contrast, Breier 1999 used at least 20% BPRS total score reduction from baseline, Conley 2001 and Wahlbeck 2000 at least 20% PANSS total score reduction, and Potkin 2006 and Zhong 2006a at least 30% PANSS total score reduction from baseline.
Heinrich 1994 based its assessment of response on the Clinical Global Impression Scale (CGI) and defined ‘at least much improved compared to baseline’ as a cutoff.
Other studies applied combined criteria: Chan 2007 and Potkin 2003 used at least much improved on the CGI or at least 30% PANSS total score reduction from baseline; Conley 2005 and Sikich 2004 at least minimally improved on the CGI and at least 20% BPRS total reduction from baseline; McEvoy 2007 all PANSS items mild or better and a CGI-severity scale of mildly ill or better; McGurk 2005 at least 20% improvement on the BPRS psychosis cluster and no psychotic symptom rated worse than mild and Robinson 2005 certain SADS-C+PD items mild or better and at least much improved on the CGI.
6.3 Relapse
Only three studies (Dollfus 2005; Lieberman 2005; Stroup 2006) provided data for relapse and used different definitions.
6.4 Service use
A few studies reported the number of participants who had to be readmitted to the hospital.
6.5 Outcome scales
We have provided details below of scales that provided usable data. We have provided reasons for exclusion of data from other instruments under ‘Outcomes’ in the ‘Included studies’ section.
6.5.1 Global state scales
6.5.1.1 Clinical Global Impression Scale - CGI Scale (Guy 1976)
This is used to assess both severity of illness and clinical improvement, by comparing the conditions of the person standardised against other people with the same diagnosis. A seven-point scoring system is usually used, with low scores showing decreased severity and/or overall improvement.
6.5.2 Mental state scales
6.5.2.1 Brief Psychiatric Rating Scale - BPRS (Overall 1962)
This is used to assess the severity of abnormal mental state. The original scale has 16 items, but a revised 18-item scale is commonly used. Each item is defined on a seven-point scale varying from ‘not present’ to ‘extremely severe’, scoring from 0-6 or 1-7. Scores can range from 0-126, with high scores indicating more severe symptoms.
6.5.2.2 Positive and Negative Syndrome Scale - PANSS (Kay 1986)
This schizophrenia scale has 30 items, each of which can be defined on a seven-point scoring system varying from 1 - absent to 7 - extreme. It can be divided into three sub-scales for measuring the severity of general psychopathology, positive symptoms (PANSS-P), and negative symptoms (PANSS-N). A low score indicates less severity.
6.5.2.3 Scale for the Assessment of Negative Symptoms - SANS (Andreasen 1983)
This six-point scale gives a global rating of the following negative symptoms: alogia, affective blunting, avolition-apathy, anhedonia-asociality and attention impairment. Higher scores indicate more symptoms.
6.5.2.4 Scale for the Assessment of Positive Symptoms - SAPS (Andreasen 1984)
This four-point scale gives a global rating of the following positive symptoms: hallucinations, paranoia, disorganised behaviour and disorganised thinking. Higher scores indicate more symptoms.
6.5.3 General functioning scales
6.5.3.1 Social and Occupational Functioning Assessment Scale - SOFAS (Goldman 1992)
The SOFAS focuses on the different levels of social and occupational functioning. Higher scores indicate a higher level of functioning.
6.5.3.2 Global Assessment of Functioning - GAF (APA 1994)
This is a rating scale for a patients overall capacity of psychosocial functioning scoring from 1-100. Higher scores indicate a higher level of functioning.
6.5.4 Quality of life scales
6.5.4.1 Quality of Life Scale - QLS (Carpenter 1984)
This semi-structured interview is administered and rated by trained clinicians. It contains 21 items rated on a seven-point scale based on the interviewers judgement of patient functioning. A total QLS and four subscale scores are calculated, with higher scores indicating less impairment.
6.5.4.2 Subjective Well-being under Neuroleptics Scale - SWN (De Haan 2002)
The SWN is an instrument to measure the subtle subjective changes, such as restrictions in emotionality, the clarity of thinking and spontaneity, that are often referred as ‘pharmacogenic depression’ or the ‘neuroleptic induced deficit syndrome’.
6.5.5 Cognitive functioning scales
6.5.5.1 Global Neurocognitive Score (Volavka 2002)
This score consists of 15 tests that assess the domains’ general ability, learning and memory, attention, executive functions, and motor skills. Sixteen variables were selected from 12 tests. For each test variable, z-scores were computed. This global score was then computed by averaging the z-scores of contributing variables. All z-scores were computed in a way that positive scores indicate better performance.
6.5.5.2 Neurocognitive Composite Score (Keefe 2006)
The Neurocognitive Composite Score comprises individual cognitive domains (executive function, learning and memory, processing speed, attention/vigilance, verbal working memory, verbal fluency, motor function, and visuospatial ability) measured by various tests that were transformed into a composite score.
6.5.5.3 PANSS cognitive subscore
This score has been derived from the Positive and Negative Syndrome Scale - PANSS (Kay 1986).
6.5.6 Adverse effects scales
6.5.6.1 Abnormal Involuntary Movement Scale - AIMS (Guy 1976)
This has been used to assess tardive dyskinesia, a long-term, drug-induced movement disorder and short-term movement disorders such as tremor.
6.5.6.2 Barnes Akathisia Scale - BAS (Barnes 1989)
The scale comprises items rating the observable, restless movements that characterise akathisia, a subjective awareness of restlessness, and any distress associated with the condition. These items are rated from 0 - normal to 3 - severe. In addition, there is an item for rating global severity (from 0 - absent to 5 - severe). A low score indicates low levels of akathisia.
6.5.6.3 Extrapyramidal Symptom Rating Scale - ESRS (Chouinard 1980)
This consists of a questionnaire relating to parkinsonian symptoms (nine items), a physician’s examination for parkinsonism and dyskinetic movements (eight items), and a clinical global impression of tardive dyskinesia. High scores indicate severe levels of movement disorder.
6.5.6.4 Hillside Akathisia Scale - HAS (Fleischhacker 1989)
The Hillside Akathisia Scale (HAS) is another rating scale to examine akathisia, a subjective awareness of restlessness, and any distress associated with the condition. It has two subjective and three objective items for which anchored rating points are provided.
6.5.6.5 Simpson Angus Scale - SAS (Simpson 1970)
This 10-item scale, with a scoring system of 0-4 for each item, measures drug-induced parkinsonism, a short-term drug-induced movement disorder. A low score indicates low levels of parkinsonism.
Excluded studies
We excluded 241 studies for the following reasons: 22 studies because they were not randomised; 186 because they were open-label trials without any effort to blind medication; 17 because they were pooled-analyses of several trials; 11 because they did not use appropriate interventions; and five because they did not present any usable data.
Awaiting assessment
No studies are awaiting assessment.
Ongoing studies
We identified six RCTs comparing risperidone with other atypical antipsychotics which appear to be ongoing (Astra Zeneca 2002;Eli Lilly 2006; Eli Lilly 2003; Lieberman 2001; Lundbeck 2002;Reveley 2000). We have presented further details in the ongoing studies table.
Risk of bias in included studies
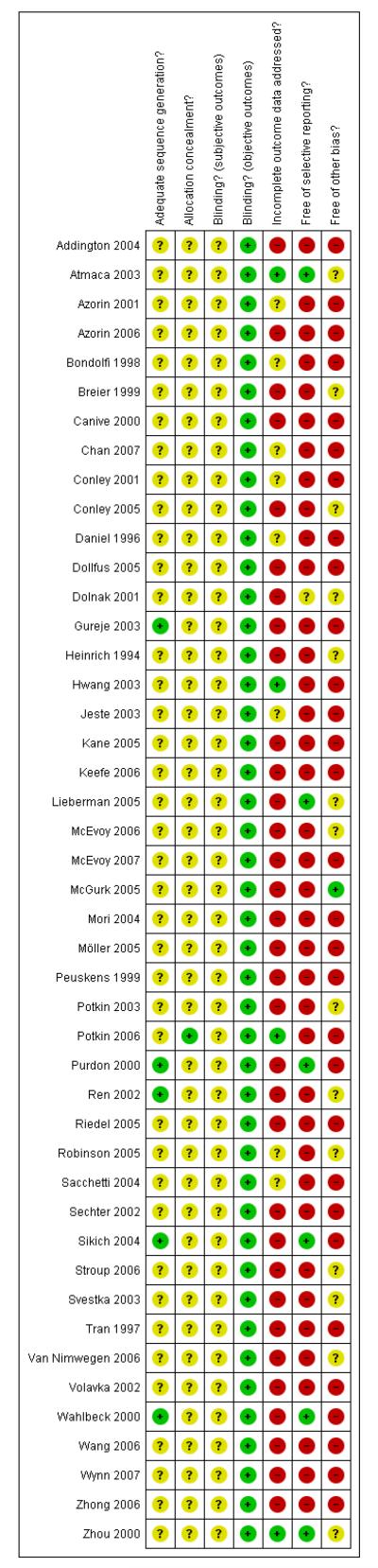
For details of risk of bias, please refer to risk of bias table (Figure 1, Figure 2)
Figure 1. Methodological quality summary: review authors’ judgements about each methodological quality item for each included study.
Figure 2. Methodological quality graph: review authors’ judgements about each methodological quality item presented as percentages across all included studies.
Allocation
All included studies were randomised, but only eight trials provided some details about the randomisation process (Chan 2007;Gureje 2003; Purdon 2000; Ren 2002; Sikich 2004; Stroup 2006;Wahlbeck 2000; Wynn 2007). Four studies (Gureje 2003; Purdon 2000; Sikich 2004; Wahlbeck 2000) used a computer-generated randomisation procedure, and Ren 2002 described a process to make participants draw a ball out of a box. For all the other studies the information was so little that it remained unclear whether there was a risk of bias. Details on allocation concealment were not provided for any of the studies.
Blinding
Of the included studies, 38 were double-blind. The remaining seven trials were single-blind (Atmaca 2003; Conley 2005; Daniel 1996; Robinson 2005; Sacchetti 2004; Wahlbeck 2000; Zhou 2000). Another 16 studies described using identical capsules for blinding. No study examined whether blinding was effective. We found that the side-effect profiles of the examined compounds are quite different, which may have made blinding difficult. We therefore conclude that the risk of bias for objective outcomes (e.g. death or laboratory values) was low, but there was a risk of bias for subjective outcomes.
Incomplete outcome data
The overall number of participants leaving the studies early was high (46.9%), although the percentages in the different comparisons varied. Eight studies did not report on leaving the study early (Breier 1999; Mori 2004; Möller 2005; Ren 2002; Svestka 2003; Van Nimwegen 2006; Wang 2006; Wynn 2007). Nearly all the studies analysed their data on an intention-to-treat basis using the last observation carried forward. This method of accounting for missing data is imperfect, because it assumes that participants would not have experienced a change of their condition if they had stayed in the trial. This assumption can obviously be wrong. Conversely it is a positive aspect of the evidence base that most studies indicated the specific reasons for leaving early.
Selective reporting
In most studies the reporting of secondary or even primary outcome data was incomplete (Addington 2004; Bondolfi 1998;Conley 2005; Daniel 1996; Dollfus 2005; Heinrich 1994; Hwang 2003; McGurk 2005; Mori 2004; Möller 2005; Peuskens 1999;Potkin 2003; Potkin 2006; Purdon 2000; Ren 2002; Riedel 2005;Robinson 2005; Sacchetti 2004; Stroup 2006; Svestka 2003; Van Nimwegen 2006; Volavka 2002; Wang 2006; Wynn 2007; Zhou 2000). Often only those adverse events that occurred in at least 5% or 10% of the participants, or that were of moderate or worse severity, or that were significantly different between groups were reported (Azorin 2006; Bondolfi 1998; Chan 2007; Conley 2001;Gureje 2003; Hwang 2003; Jeste 2003; Kane 2005; Keefe 2006;McEvoy 2007; Peuskens 1999; Potkin 2003; Potkin 2006; Sechter 2002; Tran 1997; Zhong 2006). This procedure is problematic, because rare, but potentially serious side effects may be missed. Only five studies appeared to have a low risk of bias (Atmaca 2003;Lieberman 2005; Purdon 2000; Sikich 2004; Wahlbeck 2000).
Other potential sources of bias
One study was free of other bias (McGurk 2005), but for all others we felt that there was at least a possibility of risk of bias. The main reason was sponsoring of the pharmaceutical industry. Of the included studies, 60% were sponsored by pharmaceutical companies, 18% by the manufacturers of risperidone and 42% by the manufacturers of the comparator compounds. Pharmaceutical companies have an inevitable conflict of interest which may well lead to bias (Heres 2006; Leucht 2008). Other potential sources of bias were missing wash-out phases (Lieberman 2005; McEvoy 2006; Mori 2004), low numbers of participants (Conley 2001;Sikich 2004; Wahlbeck 2000), baseline imbalance in duration of illness (Conley 2005) and missing data on allowed dose ranges (Ren 2002).
Effects of interventions
1. Comparison 1. Risperidone versus amisulpride
Four included studies fell in this category.
1.1 Global state
1.1.1 No clinically significant response - as defined by the original studies
The results of three studies were slightly heterogeneous (I2 54%). The combined analysis did not indicate a difference (3 RCTs, n = 586, RR 1.12 CI 0.83 to 1.50), but when the outlier study (Hwang 2003) was excluded there was a significant superiority of amisulpride (2 RCTs, n = 538, RR 1.25 CI1.05 to 1.50, NNH 9 CI 5 to 50).
1.1.2 Global state: no clinically important change
Overall there was no significant difference (3 RCTs, n = 586, RR 1.12 CI 0.82 to 1.53), but some heterogeneity (I2 54%). Again, when the outlier study (Hwang 2003) was excluded there was a significant superiority of amisulpride (2 RCTs, n = 586, RR 1.28 CI 1.04 to 1.56, NNH 10 CI 6 to 50) in the short (Peuskens 1999) (1 RCT, n=228, RR 0.96 CI 0.50 to 1.85) and medium term (Sechter 2002) (1 RCT, n = 310, RR 1.27 CI 0.99 to 1.64).
1.1.3 Global state: relapse - medium term
Only one study provided data for this outcome and did not reveal a significant difference between amisulpride and risperidone (1 RCT, n = 173, RR 1.50 CI 0.94 to 2.39).
1.2 Leaving the study early
There was no significant difference between groups. Of the participants, 34% in the amisulpride group and 32% in the risperidone group left the studies early due to any reason (3 RCTs, n = 586, RR 1.02 CI 0.68 to 1.53); 11% in the amisulpride group and 12% in the risperidone group left the studies early due to adverse events (4 RCTs, n = 622, RR 0.93 CI 0.61 to 1.42); and 10 % in the amisulpride group compared to 7% in the risperidone group left the studies early due to lack of efficacy of treatment (3 RCTs, n = 586, RR 1.45 CI 0.83 to 2.53).
1.3 Mental state
1.3.1 General - no clinically important change - medium term (less than 50% PANSS total score reduction)
Sechter 2002 showed a tendency in favour of amisulpride which did not reach a conventional level of statistical significance (1 RCT, n = 310, RR 1.24 CI 1.00 to 1.53).
1.3.2 General - no clinically important change - short term (less than 20% PANSS total score reduction)
In Hwang 2003 there was no significant difference between groups (1 RCT, n = 48, RR 0.69 CI 0.28 to 1.69).
1.3.3 General - no clinically important change - medium term (less than 50% BPRS total score reduction)
A single study (Sechter 2002) found a significant superiority of amisulpride (1 RCT, n = 310, RR 1.29 CI 1.02 to 1.62, NNH 8 CI 4 to 100).
1.3.4 General - no clinically important change - short term (less than 40% BPRS total score reduction)
Peuskens 1999 found no significant difference (1 RCT, n = 228, RR 1.29 CI 0.92 to 1.80).
1.3.5 General - average score at endpoint - PANSS total
There was no significant difference (2 RCTs, n = 291, MD −0.38 CI −5.33 to 4.57) in either the short (1 RCT, n = 47, MD −4.30 (-14.59 to 5.99) or the medium term (1 RCT, n = 244, MD 0.80 CI −4.5 to 6.45).
1.3.6 General - average score at endpoint - BPRS total
There was no significant difference (3 RCTs, n = 519, MD 0.68 CI −1.79 to 3.14) in either the short (2 RCTs, n = 275, MD 0.50 CI −4.54 to 5.53) or medium term (1 RCT, n = 244, MD 0.20 CI −3.28 to 3.68).
1.3.7 Positive symptoms - average score at endpoint - PANSS positive
There was no significant difference (3 RCTs, n = 519, MD 0.03 CI −1.24 to 1.29) in either the short (2 RCTs, n = 275, MD 0.15 CI −2.17 to 2.47) or medium term (1 RCT, n = 244, MD −0.30 CI −2.11 to 1.51).
1.3.8 Positive symptoms - average score at endpoint - BPRS positive
There was no significant difference (1 RCT, n = 228, MD 0.50 CI −0.89 to 1.89).
1.3.9 Negative symptoms - average score at endpoint - PANSS negative
There was no significant difference (3 RCTs, n = 519, MD 1.00 CI −0.11 to 2.11) in either the short (2 RCTs, n = 275, MD 0.60 CI −1.72 to 2.92) or the medium term (1 RCT, n = 244, MD 1.20 CI −0.21 to 2.61).
1.3.10 Negative symptoms - average score at endpoint - SANS total
There was no significant difference (1 RCT, n = 224, MD 2.70 CI −2.33 to 7.73).
1.4 General functioning
1.4.1 General functioning - no clinically important change - medium term (less than 50 % SOFAS total score reduction)
There was no significant difference (1 RCT, n = 310, MD 1.11 CI 0.98 to 1.25).
1.4.2 General functioning - average score at endpoint - SOFAS total score
There was no significant difference (2 RCTs, n = 291, MD 2.31 CI −1.28 to 5.90) in either the short (1 RCT, n = 47, MD 1.10 CI −7.03 to 9.23) or medium term (1 RCT, n = 244, MD 2.60 CI −1.40 to 6.60).
1.5 Adverse effects
1.5.1 General - at least one adverse effect
There was no significant difference (4 RCTs, n = 622, RR 1.00 CI 0.90 to 1.10).
1.5.2 Death
Only Sechter 2002 reported on death and did not find a significant difference in terms of natural causes (1 RCT, n = 310, RR 0.32 CI 0.01 to 7.81) or suicide (1 RCT, n = 310, RR 0.48 CI 0.04 to 5.25).
1.5.3 Cardiac effects - number of participants with QTc prolongation
No participant had a prolongation of the QTc interval (2 RCTs, n = 276, RR not estimable).
1.5.4 Central nervous system - sedation
There was no significant difference (1 RCT, n = 310, RR 1.44 CI 0.61 to 3.43).
1.5.5 Central nervous system - seizures
Only Sechter 2002 reported on this outcome. No participant had a seizure (1 RCT, n = 310, RR not estimable).
1.5.6 Extrapyramidal effects
There was no significant difference between risperidone and amisulpride in the frequency of akathisia (3 RCTs, n = 586, RR 1.25 CI 0.90 to 1.74), extrapyramidal symptoms (1 RCT, n = 228, RR 0.88 CI 0.60 to 1.30), hyperkinesia (2 RCTs, n = 538, RR 0.75 CI 0.49 to 1.14), parkinsonism (2 RCTs, n = 538, RR 1.13 CI 0.66 to 1.95), rigor (2 RCTs, n = 276, RR 1.08 CI 0.27 to 4.36), tremor (3 RCTs, n = 586, RR 1.46 CI 0.66 to 3.21) or use of antiparkinson medication (3 RCTs, n = 586, RR 1.07 CI 0.72 to 1.57).
1.5.7 Extrapyramidal effects - scale measured
There was no significant difference in dyskinesia (AIMS: 2 RCTs, n = 538, MD −0.08 CI −0.72 to 0.55) or extrapyramidal side effects in general (SAS: 2 RCTs, n = 538, MD 0.03 CI −0.06 to 0.13).
1.5.8 Prolactin associated side effects
There was no significant difference in the frequency of amenorrhea (1 RCT, n = 310, RR 0.96 CI 0.06 to 15.24) and galactorrhea (2 RCTs, n = 538, RR 0.74 CI 0.17 to 3.27). In men sexual dysfunction occurred more frequently in the risperidone group than in the amisulpride group (2 RCTs, n = 538, RR 9.52 CI 1.22 to 74.44, NNH 33 CI 100 to 17).
1.5.9 Metabolic - weight gain
More participants in the risperidone group than in the amisulpride group had a clinically important weight gain (2 RCTs, n = 538, RR 1.75 CI 1.07 to 2.87, NNH not estimable).
1.5.10 Metabolic - weight gain - mean change from baseline in kg
Risperidone was associated with more weight gain than amisulpride (3 RCTs, n = 585, MD 0.99 CI 0.37 to 1.61).
1.6 Publication bias
Due to small number of included studies (< 10 studies), we did not perform a funnel plot analysis.
1.7 Sensitivity analyses
The reasons for the preplanned sensitivity analyses did not apply and therefore we have not performed these.
2. Comparison 2. Risperidone versus aripiprazole - all data short term
Two included studies fell in this category.
2.1 Global state
2.1.1 Global state - no clinically significant response - as defined by the original studies
There was no significant difference (2 RCTs, n = 384, RR 0.88 CI 0.62 to 1.24).
2.1.2 Global state - no clinically important change
There was no significant difference (2 RCTs, n = 384, RR 0.88 CI 0.62 to 1.24).
2.2 Leaving the study early
There was no significant difference between groups. 35% of the participants in the risperidone group and 34% of the participants in the aripiprazole group left the studies early due to any reason (2 RCTs, n = 384, RR 1.06 CI 0.79 to 1.41). There was also no significant difference in leaving the studies early due to adverse events (8% versus 10%; 2 RCTs, n = 384, RR 0.79 CI 0.39 to 1.61), or in leaving the studies early due to inefficacy of treatment (6% versus 8%; 2 RCTs, n = 384, RR 0.89 CI 0.41 to 1.93).
2.3 Mental state
2.3.1 General - average endpoint score - PANSS total
There was no significant difference (2 RCTs, n = 372, MD −1.50 CI −5.96 to 2.96).
2.3.2 Positive symptoms - average endpoint score - PANSS positive
There was no significant difference (2 RCTs, n = 372, MD −1.24 CI −2.74 to 0.26).
2.3.3 Negative symptoms - average endpoint score - PANSS negative
There was no significant difference (2 RCTs, n = 372, MD 0.45 CI −0.87 to 1.78).
2.4 Adverse effects
2.4.1 General - at least one adverse effect
There was no significant difference (2 RCTs, n = 384, RR 1.02 CI 0.95 to 1.09).
2.4.2 Cardiac effects - number of participants with QTc prolongation
There was no significant difference (1 RCT, n = 301, RR 14.21 CI 0.74 to 272.45).
2.4.3 Cardiac effects - mean change of QTc interval from baseline in ms
There was no significant difference (2 RCTs, n = 383, MD 7.19 CI 2.19 to 12.19).
2.4.4 Extrapyramidal effects
There was no significant difference in akathisia (2 RCTs, n = 384, RR 1.56 CI 0.21 to 11.45). The results of the two included studies were heterogeneous, but neither one found a significant difference between groups (Potkin 2003: n = 301, RR 0.71 CI 0.41 to 1.25 and Chan 2007: n = 83, RR 5.76 CI 0.67 to 49.35). There was also no significant difference in extrapyramidal symptoms (2 RCTs, n = 384, RR 1.18 CI 0.68 to 2.06), parkinsonism (1 RCT, n = 301, RR 0.14 CI 0.01 to 2.35) and use of antiparkinson medication (1 RCT, n = 83, RR 1.68 CI 0.89 to 3.17). More participants in the risperidone group than in the amisulpride group had a dystonia (1 RCT, n = 301, RR 7.14 CI 2.41 to 21.13, NNH 8 CI 20 to 5), while in the same study more participants in the aripiprazole group suffered from tremor (1 RCT, n = 301, RR 0.21 CI 0.05 to 0.90, NNH 14 CI 8 to 50).
2.4.5 Extrapyramidal symptoms - scale measured
There was no significant difference in dyskinesia (AIMS: 2 RCTs, n = 383, MD 0.25 CI −0.75 to 1.24), akathisia (BAS: 2 RCTs, n = 388, MD 0.11 CI −0.11 to 0.49) or extrapyramidal side effects in general (SAS: 2 RCTs, n = 388, MD 0.70 CI −0.82 to 2.22).
2.4.6 Prolactin associated side effects
In one study many more participants in the risperidone group than in the aripiprazole group had elevated prolactin levels (1 RCT, n = 301, RR 26.23 CI 12.64 to 54.46, NNH not estimable). There was no significant difference in the frequency of dysmenorrhoea (1 RCT, n = 91, RR 0.32 CI 0.02 to 5.91).
2.4.7 Prolactin - mean change from baseline in ng/ml
Risperidone was associated with clearly more prolactin increase than aripiprazole (2 RCTs, n = 383, MD 54.71 CI 49.36 to 60.06).
2.4.8 Metabolic - cholesterol - mean change from baseline in mg/dl
Risperidone was associated with significantly more cholesterol increase than aripiprazole (1 RCT, n = 83, MD 22.30 CI 4.91 to 39.69).
2.4.9 Metabolic - glucose - mean change from baseline in mg/dl
There was no significant difference (1 RCT, n = 83, MD −6.80 CI −19.70 to 6.10).
2.4.10 Metabolic - weight gain of 7% or more of total body weight
There was no significant difference (2 RCTs, n = 284, RR 1.30 CI 0.55 to 3.07).
2.4.11 Metabolic - weight gain - mean change from baseline in kg
There was no significant difference (2 RCTs, n = 383, MD 0.54 CI −0.15 to 1.24).
2.5 Publication bias
Due to the small number of included studies, we have not performed a funnel plot analysis.
2.6 Sensitivity analysis
The reason for the preplanned sensitivity analyses did not apply and we have therefore not performed these.
3. Comparison 3. Risperidone versus clozapine
Eleven included studies fell in this category.
3.1 Global state
3.1.1 Global state - no clinically significant response - as defined by the original studies
There was no significant difference (6 RCTs, n = 575, RR 1.07 CI 0.98 to 1.16).
3.1.2 Global state - no clinically important change - short term - as defined by the original studies
There was no significant difference (2 RCTs, n = 333, RR 1.07 CI 0.88 to 1.30).
3.2 Leaving the study early
A similar number of participants in the risperidone group (35%) and the clozapine group (31%) left the studies early due to any reason (8 RCTs, n = 675, RR 1.10 CI 0.86 to 1.41). Nevertheless, fewer participants in the risperidone group (7%) than in the clozapine group (12%) left the studies early due to adverse events (7 RCTs, n = 647, RR 0.55 CI 0.31 to 0.98, NNH not estimable). In contrast, more participants in the risperidone group (14%) than in the clozapine group (5%) left the studies early due to inefficacy of treatment (7 RCTs, n = 647, RR 2.51 CI 1.43 to 4.40, NNH not estimable).
3.3 Mental state
3.3.1 General - no clinically important change - short term - less than 20% PANSS total score reduction
There was no significant difference (2 RCTs, n = 106, RR 0.84 CI 0.50 to 1.42).
3.3.2 General - no clinically important change - short term
(Kane 1988 criteria)
There was no significant difference (1 RCT, n = 273, RR 1.05 CI 0.85 to 1.29).
3.3.3 General - no clinically important change - long term - less than 20% BPRS total score reduction
There was no significant difference (1 RCT, n = 29, RR 1.24 CI 0.78 to 1.98).
3.3.4 General - no clinically important change - short term - less than 40% BPRS total score reduction
There was no significant difference (1 RCT, n = 107, RR 1.05 CI 0.76 to 1.45).
3.3.5 General - average score at endpoint - PANSS total
There was no significant difference, in the short term (4 RCTs, n = 387, MD 0.75 CI −5.35 to 6.85) in the medium term (1 RCT, n = 81, MD 3.60 CI −6.12 to 13.32), or overall (5 RCTs, n = 468, RR 1.49 CI −3.44 to 6.42). The results were heterogeneous due to the study by Azorin 2001 which showed a pronounced superiority of clozapine. Excluding this study did not change the results.
3.3.6 General - average score at endpoint - BPRS total
There was a trend in favour of clozapine which did not reach the conventional 5% level of statistical significance (4 RCTs, n = 364, MD 3.07 CI −0.01 to 6.16). This was in contrast to long-term (1 RCT, n = 52, MD −0.20 CI −4.12 to 3.72) and short-term data (3 RCTs, n = 345, MD 4.90 CI 2.17 to 7.64).
3.3.7 Positive symptoms - average score at endpoint - PANSS positive
Data on this outcome showed a superiority of clozapine (6 RCTs, n = 591, 1.26 CI 0.18 to 2.35), but when two studies with potentially skewed data were excluded (Breier 1999; Ren 2002) the difference was no longer statistically significant (4 RCTs, n = 442, MD 0.60, CI −1.34 to 2.53).
3.3.8 Positive symptoms - average score at endpoint - short term - BPRS positive
There was no significant difference (1 RCT, n = 29, RR 2.10 CI −0.56 to 4.76).
3.3.9 Negative symptoms - average score at endpoint - PANSS negative
There was no significant difference, in the short (4 RCTs, n = 481, MD −0.55 CI −2.80 to 1.71) or medium term (1 RCT, n = 81, MD 1.40 CI −1.42 to 4.22), or overall (5 RCTs, n = 562, MD −0.13 CI −1.96 to 1.71).
3.3.10 Negative symptoms - average score at endpoint - short term - SANS total
There was no significant difference (2 RCTs, n = 69, MD −0.62 CI −3.74 to 2.51).
3.4 General functioning
3.4.1 General functioning - average score at endpoint - GAF
There was no significant difference (1 RCT, n = 19, MD 9.00 CI −0.44 to 18.44).
3.4.2 General functioning - average endpoint - short term - social functioning scale
There was no significant difference (1 RCT, n = 19, MD 47.00 CI 0.45 to 93.55, P = 0.05).
3.5 Cognitive functioning
3.5.1 Cognitive functioning - medium term - no clinically important change in global neurocognitive score (less than 1/2 SD)
There was no significant difference (1 RCT, n = 81, RR 0.79 CI 0.60 to 1.05).
3.5.2 Cognitive functioning - average endpoint - medium term - global neurocognitive score
There was no significant difference (1 RCT, n = 50, RR 0.33 CI −0.06 to 0.72).
3.6 Adverse effects
3.6.1 General - at least one adverse effect
There was no significant difference (2 RCTs, n = 333, RR 0.85 CI 0.51 to 1.42), but the results of two studies were heterogeneous (Heinrich 1994: n = 60, RR 0.63 CI 0.42 to 0.96; Azorin 2001: n = 273, RR 1.06 CI 0.94 to 1.19). We did not identify obvious reasons for the heterogeneity.
3.6.2 Death
One study reported on death due to any reason and showed no significant difference between groups (1 RCT, n = 273, RR 1.02 CI 0.06 to 16.18).
3.6.3 Cardiac effects
Cardiac effects were reported as ‘preterminally negative t-wave’ (1 RCT, n = 60, RR 1.54 CI 0.07 to 36.11) and ‘any significant cardiac effect’ (1 RCT, n = 86, RR not estimable), but there was not significant difference.
3.6.4 Central nervous system - sedation
There was a significant difference in favour of risperidone (5 RCTs, n = 479, RR 0.53 CI 0.32 to 0.86, NNT 5 CI not estimable).
3.6.5 Central nervous system - seizures
There was a significant difference in favour of risperidone (2 RCTs, n = 354, RR 0.22 CI 0.07 to 0.70, NNT 14 CI 8 to 33).
3.6.6 Extrapyramidal effects
There was no significant difference in akathisia (1 RCT, n = 40, RR 0.11 CI 0.01 to 1.94), akinesia (1 RCT, n = 86, RR 1.00 CI 0.38 to 2.61), dystonia (1 RCT, n = 86, RR 2.00 CI 0.19 to 21.24), extrapyramidal symptoms (2 RCTs, n = 333, RR 1.30 CI 0.44 to 3.85) and tremor (1 RCT, n = 40, RR 0.50 CI 0.18 to 1.40).
However, a single small study found more parkinsonism (1 RCT, n = 86, RR 0.63 CI 0.41 to 0.97, NNH 4 CI 2 to 33) in the clozapine group. In contrast, the combination of six RCTs revealed a consistently more frequent use of antiparkinson medication in the risperidone group (6 RCTs, n = 304, RR 2.57 CI 1.47 to 4.48, NNH 6 CI 33 to 3).
3.6.7 Extrapyramidal effects - scale measured
There was no difference in extrapyramidal side effects according to the Simpson-Angus Scale (2 RCTs, n = 69, MD 0.81 CI −0.10 to 1.73) nor the ESRS total score (1 RCT, n = 81, MD −0.30 CI −1.91 to 1.31).
3.6.8 Haematological - white blood cells - low white blood cell count
There was no significant difference (5 RCTs, n = 567, RR 1.69 CI 0.51 to 5.58).
3.6.9 Prolactin associated side effects
There was no significant difference in the frequency of sexual dysfunction (1 RCT, n = 86, RR 2.00 CI 0.39 to 10.35).
3.6.10 Prolactin - change from baseline in ng/ml
Risperidone was associated with a clearly higher increase of prolactin levels than clozapine (men and women: 1 RCT, n = 27, MD 38.50 CI 23.30 to 53.70; men only: 1 RCT, n = 28, MD 20.00 CI 8.19 to 31.81).
3.6.11 Metabolic - cholesterol - change from baseline in mg/dl
There was no significant difference .(1 RCT, n = 31, MD −7.10 CI −34.01 to 19.81).
3.6.12 Metabolic - glucose - change from baseline in mg/dl
There was no significant difference (1 RCT, n = 31, MD −1.70 CI −12.04 to 8.64).
3.6.13 Metabolic - weight gain
There was no significant difference in ‘weight gain of 10% or more of total body weight’ (1 RCT, n = 81, RR 0.56 CI 0.18 to 1.76) or in ‘weight gain reported as an adverse event’ (1 RCT, n = 86, RR 0.63 CI 0.32 to 1.22).
3.6.14 Metabolic - weight gain - mean change from baseline in kg
Clozapine was associated with significantly more weight gain than risperidone (3 RCTs, n = 373, MD −3.30 CI −5.65 to −0.95). Although all three studies showed a consistent trend in favour of risperidone, the results were heterogeneous, with Atmaca 2003 revealing a particularly high difference (Azorin 2001: n = 270, MD −2.20 CI −3.50 to −0.90; Volavka 2002: n = 77, MD −1.90 CI −3.63 to −0.17; Atmaca 2003: n = 26, MD −5.98 CI −7.87 to −4.09).
3.7 Publication bias
Due to the small number of included studies, we did not perform a funnel plot analysis.
3.8 Investigation for heterogeneity and sensitivity analysis
Excluding Breier 1999 (skewed data) from the outcome BPRS total score did not change the overall result. Excluding Ren 2002 (skewed data) and Breier 1999 (skewed data) from the outcome PANSS positive subscore, the advantage for clozapine was no longer significant (4 RCTs, n = 441, MD 0.60 CI −1.34 to 2.53). The exclusion of Ren 2002 (skewed data) from the outcome PANSS negative subscore did not influence the result to any important extent.
4. Comparison 4. Risperidone versus olanzapine
Twenty-three studies fell into this category.
4.1 Global state
4.1.1 Global state - no clinically significant response - as defined by the original studies
There was no significant difference (7 RCTs, n = 1376, RR 1.06 CI 0.99 to 1.13).
4.1.2 Global state: no clinically important change (as defined by the original studies)
There was no significant difference (5 RCTs, n = 975, RR 0.98 CI 0.88 to 1.09) in short-term (3 RCTs, n = 589, RR 1.00 CI 0.87 to 1.16), medium-term (1 RCT, n = 120, RR 0.83 CI 0.60 to 1.16) or long-term data (1 RCT, n = 266, 0.98 CI 0.71 to 1.35).
4.1.3 Global state: relapse (as defined by the original studies)
There was no significant difference (2 RCTs, n = 211, RR 1.25 CI 0.57 to 2.71) in either short-term (1 RCT, n = 76, RR 0.75 CI 0.25 to 2.25) or long-term data (1 RCT, n = 135, RR 1.70 CI 0.79 to 3.67).
4.2 Leaving the study early
Significantly more participants in the risperidone group (56%) than in the olanzapine group (48%) left the studies early due to any reason (15 RCTs, n = 2662, RR 1.14 CI 1.07 to 1.21, NNH 13 CI 9 to 25).
Leaving the studies early due to adverse events did not differ between groups (11% versus 12%, 13 RCTs, n = 2519, RR 0.96 CI 0.71 to 1.30). There was a trend that more participants in the risperidone group (15%) than in the olanzapine group (11%) left the studies early due to inefficacy of treatment, but the difference did not reach a conventional level of significance (14 RCTs, n = 2668, RR 1.28 CI 1.02 to 1.60).
4.3 Mental state
4.3.1 General - no clinically important change - (less than 50% PANSS total score reduction)
There was a significant difference in favour of olanzapine (3 RCTs, n = 472, RR 1.09 CI 1.00 to 1.18, NNH not estimable) in shortterm (1 RCT, n = 71, RR 0.43 CI 0.04 to 4.57) and long-term outcomes (2 RCTs, n = 401, RR 1.09 CI 1.00 to1.18).
4.3.2 General - no clinically important change - short term (less than 20% PANSS total score reduction)
There was no significant difference (2 RCTs, n = 553, RR 1.02 CI 0.88 to 1.19).
4.3.3 General - average endpoint score - PANSS total
There was a significant difference in favour of olanzapine (15 RCTs, n = 2390, MD 1.94 CI 0.58 to 3.31), which was most prominent in long-term (5 RCTs, n = 1431, MD 2.59 CI 0.20 to 4.98), short-term (7 RCTs, n = 728, MD 0.97 CI −1.10 to 3.05) and medium-term outcomes (4 RCTs, n = 231, MD 4.11 CI −0.71 to 8.93).
4.3.4 General - average endpoint score - BPRS total
There was no significant difference (3 RCTs, n = 428, MD 4.16 CI 0.03 to 8.29) in either short-term (1 RCT, n = 35, MD 5.00 CI −5.74 to 15.74) or long-term outcomes (2 RCTs, n = 493, MD 4.28 CI −1.34 to 9.91).
4.3.5 Positive symptoms - no clinically important change - short term - less than 50% PANSS positive subscore reduction
There was no significant difference (1 RCT, n = 377, RR 0.98 CI 0.93 to 1.04).
4.3.6 Positive symptoms - average endpoint score - PANSS positive
There was no significant difference (13 RCTs, n = 1702, MD 0.46 CI −0.09 to 1.02), but medium-(3 RCTs, n = 231, MD 1.58 CI −0.03 to 3.20) and long-term data (5 RCTs, n = 810, MD 0.68 CI −0.04 to 1.40) almost indicated a benefit for the control group, compared to short-term (5 RCTs, n = 661, MD −0.48 CI −1.53 to 0.57).
4.3.7 Negative symptoms - average endpoint score - PANSS negative
There was no significant difference (13 RCTs, n = 1702, MD 0.46 CI −0.09 to 1.02). Long-term data (5 RCTs, n = 810, MD 0.81 CI 0.07 to 1.54) indicated a benefit for the control group, whereas short-term (5 RCTs, n = 661, MD 0.19 CI −0.85 to 1.22) and medium-term data did not show a significant difference (3 RCTs, n = 231, MD 0.00 CI −1.58 to 1.59).
4.3.8 Negative symptoms - average endpoint score - long term - SANS total
There was a significant superiority in favour of olanzapine (1 RCT, n = 308, MD 1.40 CI 0.37 to 2.43).
4.4 Quality of life - average endpoint score - long term - QLS total
In two trials the quality of life of the participants in the olanzapine group was significantly better than that of those in the risperidone group (2 RCTs, n = 296, MD 5.10 CI 1.09 to 9.1).
4.5 Cognitive functioning
4.5.1 Cognitive functioning - no clinically important change - medium term (less than half a standard deviation improvement in a global neurocognitive score)
There was no significant difference (1 RCT, n = 80, RR 1.30 CI 0.88 to 1.94).
4.5.2 Cognitive functioning - average score at endpoint - medium term - mean global neurocognitive score
There was no significant difference (1 RCT, n = 52, MD 0.04 CI −0.31 to 0.39).
4.5.3 Cognitive functioning - average score at endpoint - long term - neurocognitive composite score
There was no significant difference (1 RCT, n = 263, MD 0.01 CI −0.11 to 0.13).
4.6 Service use - number of patients rehospitalised
There was no significant difference overall (3 RCTs, n = 965, RR 1.34 CI 0.96 to 1.86) or in the short (1 RCT, n = 76, RR 1.35 CI 0.41 to 4.40), medium (1 RCT, n = 212, RR 1.38 CI 0.69 to 2.78) or long term (1 RCT, n = 677, RR 1.32 CI 0.89 to 1.96).
4.7 Adverse effects
4.7.1 At least one adverse effect
There was no significant difference (11 RCTs, n = 2576, RR 0.96 CI 0.88 to 1.03).
4.7.2 Death
Tran 1997 reported on death due to any reason and found no difference between groups (1 RCT, n = 339, RR 3.09 CI 0.13 to 75.30).
Two studies examined death due to natural causes. Overall there was no significant difference, but there was only one death which occurred in the olanzapine group (2 RCTs, n = 252, RR 0.34 CI 0.01 to 8.26).
Completed suicides and suicide attempts were reported by four and five studies, respectively. There was no significant difference in either outcome (suicide: 4 RCTs, n = 730, RR 3.11 CI 0.13 to 75.59; suicide attempts: 5 RCTs, n = 1724, RR 1.15 CI 0.37 to 3.54).
4.7.3 Cardiac effects
Cardiac effects were reported as ‘ECG abnormalities’ (2 RCTs, n = 415, RR 0.42 CI 0.08 to 2.30) and ‘QTc prolongation’ (2 RCTs, n = 853, RR 2.69 CI 0.12 to 60.00), without significant difference between groups.
The data on QTc prolongation were heterogeneous, possibly due to a difference in age groups, because Jeste 2003 included only elderly participants with schizophrenia. Nevertheless, neither one of the two individual studies found a significant difference between groups (Jeste 2003: n = 176, RR 0.77 CI 0.18 to 3.33; Lieberman 2005: 1 RCT, n = 677, RR 14.78 CI 0.85 to 257.77).
4.7.4 Cardiac effects - mean change of QTc interval from baseline in ms
There was no significant difference (6 RCTs, n = 1518, MD 0.96 CI −2.74 to 4.67).
4.7.5 Central nervous system - sedation
There was no significant difference (11 RCTs, n = 2576, RR 0.93 CI 0.84 to 1.04).
4.7.6 Central nervous system - seizures
There was no significant difference (4 RCTs, n = 671, RR 0.26 CI 0.03 to 2.35).
4.7.7 Extrapyramidal effects
There was no significant difference in the number of participants with akinesia (3 RCTs, n = 681, RR 1.21 CI 0.82 to 1.79), dyskinesia (3 RCTs, n = 580, RR 1.02 CI 0.36 to 2.90), dystonia (3 RCTs, n = 591, RR 1.79 CI 0.37 to 8.77), rigor (2 RCTs, n = 141, RR 0.41 CI 0.06 to 2.70), tremor (5 RCTs, n = 973, RR 0.87 CI 0.48 to 1.57) or extrapyramidal symptoms (4 RCTs, n = 1104, RR 1.33 CI 0.83 to 2.13). The results of the latter outcome were heterogeneous, possibly due to a single study in elderly participants which showed an opposite trend compared to the other studies (Jeste 2003). Excluding Jeste 2003 there was a significant superiority of olanzapine (RR 1.54, CI 1.05 to 2.28, NNH 13 CI 8 to 33).
Risperidone produced more akathisia (8 RCTs, n = 1988, RR 1.30 CI 1.02 to 1.66, NNH not estimable) and parkinsonism (1 RCT, n = 776, RR 1.65 CI 1.08 to 2.51, NNH not estimable), and it was associated with more frequent use of antiparkinson medication (13 RCTs, n = 2599, RR 1.28 CI 1.06 to 1.55, NNH 17 CI 9 to 100).
4.7.8 Extrapyramidal symptoms - scale measured
There was no significant difference in akathisia (BAS: 2 RCTs, n = 353, MD 0.72 CI −0.36 to 1.81; ESRS akathisia subscore: 1 RCT, n = 359, MD 0.00 CI −0.27 to 0.27), dyskinesia (AIMS: 11 RCTs, n = 302, MD 0.03 CI −0.72 to 0.78; ESRS dyskinesia subscore: 3 RCTs, n = 572, MD −0.08 CI −0.76 to 0.60), dystonia (ESRS dystonia subscore: 1 RCT, n = 42, MD −0.09 CI −0.91 to 0.73), overall general extrapyramidal symptoms (ESRS total score: 4 RCTs, n = 682, MD 0.30 CI −0.35 to 0.94; SAS: 5 RCTs, n = 522, MD 0.62 CI −0.08 to 1.33) or parkinsonism (ESRS parkinsonism subscore: 3 RCTs, n = 572, MD 0.24 CI −1.09 to 1.57. It should be noted that some of these results were heterogeneous, but no clear reason for the heterogeneity could be identified.
4.7.9 Haematological - white blood cells - number of participants with low white blood cell count
Overall there was no significant difference (3 RCTs, n = 484, RR 1.00 CI 0.09 to 10.59). The results of the three studies were heterogeneous, but the single trials did not show significant differences between risperidone and olanzapine either (Tran 1997: n = 339, RR 0.15 CI 0.02 to 1.18; Volavka 2002: n = 80, RR 4.76 CI 0.24 to 96.16; and Gureje 2003: n = 65, RR 2.91 CI 0.12 to 68.95).
4.7.10 Prolactin associated side effects
Significantly more participants in the risperidone group suffered from amenorrhea (7 RCTs, n = 565, RR 1.50 CI 1.02 to 2.22, NNH not estimable) and ‘abnormal ejaculation’ (3 RCTs, n = 531, RR 4.34 CI 1.49 to 12.69, NNH not estimable). More participants in the risperidone group had abnormally high prolactin levels, but this result did not reach conventional levels of statistical significance (3 RCTs, n = 477, RR 3.02 CI 0.99 to 9.23).
There were no significant differences in decreased libido (3 RCTs, n = 781, RR 2.48 CI 0.77 to 8.00), galactorrhea (7 RCTs, n = 547, RR 1.64 CI 0.79 to 3.39), gynaecomastia (5 RCTs, n = 1083, RR 1.39 CI 0.70 to 2.77), impotence (3 RCTs, n = 531, RR 2.00 CI 0.68 to 5.89), orgasmic dysfunction (1 RCT, n = 377, RR 5.03 CI 0.24 to 104.00) and sexual dysfunction (7 RCTs, n = 1715, RR 1.07 CI 0.90 to 1.28).
4.7.11 Prolactin - mean change from baseline in ng/ml
Risperidone was associated with significantly more prolactin increase than olanzapine (men and women combined: 6 RCTs, n = 1291, MD 22.84 CI 17.69 to 27.98; men only: 2 RCTs, n = 70, MD 19.91 CI 13.64 to 26.18; women only: 1 RCT, n = 71, MD 41.40 CI 29.64 to 53.16). There was some heterogeneity in the degree of the difference, but all studies consistently favoured olanzapine.
4.7.12 Metabolic - cholesterol - number of participants with a cholesterol increase
There was no significant difference (1 RCT, n = 266, RR 0.78 CI 0.44 to 1.38).
4.7.13 Metabolic - cholesterol - mean change from baseline in mg/dl
There was a significant difference favouring risperidone (7 RCTs, n = 1391, MD −10.36 CI −14.43 to −6.28).
4.7.14 Metabolic - glucose - abnormally high fasting glucose value
There was no significant difference (3 RCTs, n = 670, RR 0.50 CI 0.22 to 1.16).
4.7.15 Metabolic - glucose - change from baseline in mg/dl
There was a significant difference in favour of risperidone (7 RCTs, n = 1201, MD −7.58 CI −11.23 to −3.93).
4.7.16 Metabolic - weight gain - number of participants with weight gain
Significantly fewer participants in the risperidone group than in the olanzapine group suffered from weight gain (11 RCTs, n = 2594, RR 0.55 CI 0.43 to 0.72, NNT 9 CI 7 to 14). There was some heterogeneity due to a first episode study (McEvoy 2007) which showed only a small difference between groups, but the overall trend was very consistent in favour of risperidone.
4.7.17 Metabolic - weight gain - mean change from baseline in kg
Risperidone was associated with significantly less weight gain than olanzapine (3 RCTs, n = 2116, MD −2.61 CI −3.74 to −1.48). The results were heterogeneous, because Atmaca 2003 showed an extreme superiority of risperidone. Excluding this study resolved the heterogeneity and risperidone’s superiority prevailed (12 RCTs, n = 2090, MD −2.06 CI −2.74 to −1.37).
4.8 Publication bias
A funnel plot of the outcome PANSS total score (> 10 included studies) did not suggest a significant publication bias.
4.9 Investigation for heterogeneity and sensitivity analysis
Excluding Mori 2004 (skewed data) from the outcome ‘PANSS positive score’ did not change the result. The exclusion of Sikich 2004 (skewed data) from the analysis of the BPRS total score did not have an important impact on this outcome.
5. Comparison 5. Risperidone versus quetiapine
Eleven included studies fell in this category.
5.1 Global state
5.1.1 Global state - no clinically significant response - as defined by the original studies
Overall there was no significant difference. As the results were heterogeneous we present the single studies separately. Potkin 2006 reported a significant difference in favour of risperidone (1 RCT, n = 177, RR 0.79 CI 0.65 to 0.96), while Zhong 2006a (1 RCT, n = 495, RR 1.00 CI 0.92 to 1.10), McEvoy 2007 (1 RCT, n = 103, RR 0.85 CI 0.62 to 1.15) and Conley 2005 found no significant difference between groups (1 RCT, n = 25, RR not estimable).
5.1.2 Global state - no clinically important change (as defined by the original studies)
Short-term studies (3 RCTs, n = 1007, RR 0.86 CI 0.70 to 1.07) and long-term studies (1 RCT, n = 267, RR 0.85 CI 0.62 to 1.15) tended to favour risperidone, but the difference was not statistically significant.
5.2 Leaving the study early
There was no significant difference in the number of participants leaving the studies early due to any reason (risperidone 54%, quetiapine 57%, 10 RCTs, n = 2278, RR 0.94 CI 0.87 to 1.02) or due to adverse events (9% versus 11%, 7 RCTs, n = 1851, RR 0.84 CI 0.56 to 1.27). Leaving early due to inefficacy showed an almost significant superiority of risperidone (24% versus 19%, 7 RCTs, n = 1851, RR 0.79 CI 0.62 to 1.01).
5.3 Mental state
5.3.1 General - no clinically important change - short term - less than 30% PANSS total score reduction from baseline
There was no significant difference (2 RCTs, n = 982, RR 0.90 CI 0.71 to 1.15), but the results were heterogeneous. Potkin 2006 found a superiority of risperidone (n = 177, RR 0.79 CI 0.65 to 0.96), whereas Zhong 2006a found no difference between groups (n = 495, RR 1.00 CI 0.92 to 1.10).
5.3.2 General - no clinically important change - short term - less than 20% BPRS total score reduction from baseline
There was no significant difference (1 RCT, n = 25, RR 1.03 CI 0.66 to 1.60).
5.3.3 General - average score at endpoint - PANSS total
There was a significant difference in favour of risperidone (9 RCTs, n = 1953, MD −3.09 CI −5.16 to −1.01), which was seen in long-term data (2 RCTs, n = 743, MD −3.11 CI −5.82 to −0.40), but not in medium-term (2 RCTs, n = 146, MD −6.27 CI −16.48 to 3.94) or short-term (5 RCTs, n = 1064, MD −2.44 CI −5.69 to 0.81) data.
5.3.4 General - average score at endpoint - short term - BPRS total
There was no significant difference (1 RCT, n = 25, MD −1.68 CI −11.69 to 8.33).
5.3.5 Positive symptoms - short term - less than 40% PANSS positive subscore reduction from baseline
There was no significant difference (1 RCT, n = 673, RR 1.00 CI 0.9 to 1.12).
5.3.6 Positive symptoms - average endpoint score - PANSS positive
There was a significant difference in favour of risperidone (7 RCTs, n = 1264, MD −1.82 CI −2.48 to −1.16), which was most prominent in short-term data (4 RCTs, n = 1037, MD −2.10 CI −3.19 to −1.00) as well as medium-term (2 RCTs, n = 146, MD −2.15 CI −4.31 to 0.01) and long-term (1 RCT, n = 81, MD −1.30 CI −2.73 to 0.13) data.
5.3.7 Positive symptoms - average endpoint score - short term - BPRS positive
There was a significant difference in favour of risperidone (1 RCT, n = 25, MD −1.10 CI −2.02 to −0.18).
5.3.8 Negative symptoms - average endpoint score - short term - less than 40% PANSS negative subscore reduction from baseline
There was no significant difference (1 RCT, n = 673, RR 1.02 CI 0.96 to 1.08).
5.3.9 Negative symptoms - average endpoint score - PANSS negative
There was no significant difference in the short (4 RCTs, n = 956, MD −1.46 CI −4.11 to 1.19), medium (2 RCTs, n = 146, MD 1.3 CI −3.35 to 0.75) or long term (1 RCT, n = 81, MD 0.8 CI −2.27 to 0.61).
5.3.10 Negative symptoms - average endpoint score - short term - BPRS negative
There was a significant difference favouring risperidone (1 RCT, n = 25, MD −0.57 CI −0.97 to −0.17).
5.4 Quality of life: average endpoint score - short term - QLS total
There was no significant difference (1 RCT, n = 22, MD 0.5 CI −12.87 to 13.87).
5.5 Service use: number of participants rehospitalised
There was a benefit in favour of risperidone in the overall analysis (2 RCTs, n = 877, RR 0.75 CI 0.56 to 1.00), as well as in the medium (1 RCT, n = 199, RR 0.77 CI 0.42 1.41) and long term (1 RCT, n = 681, RR 0.7 CI 0.53 1.03).
5.6 Adverse effects
5.6.1 General - at least one adverse effect
There was no significant difference (8 RCTs, n = 2226, RR 0.96 CI 0.85 to 1.08).
5.6.2 Death
There was no significant difference in the number of completed suicides (3 RCTs, n = 1139, RR 0.71 CI 0.05 to 9.16) or in the number of suicide attempts (2 RCTs, n = 945, RR 2.30 CI 0.34 to 15.65).
5.6.3 Cardiac effects - number of participants with QTc prolongation
There was no significant difference between groups (2 RCTs, n = 1351, RR 1.15 CI 0.39 to 3.40).
5.6.4 Cardiac effects - mean change of QTc interval from baseline in ms
There was no significant difference (3 RCTs, n = 940, MD −2.21 CI −9.48 to 5.05).
5.6.5 Central nervous system - sedation
There was a significant difference in favour of risperidone (8 RCTs, n = 2226, RR 0.82 CI 0.69 to 0.97, NNT 20 CI 11 to 50).
5.6.6 Central nervous system - somnolence
There was a significant difference in favour of risperidone (1 RCT, n = 309, RR 0.25 CI 0.09 to 0.75, NNT 13 CI 8 to 50).
5.6.7 Extrapyramidal effects - number of participants with extrapyramidal side effects
There was no significant difference in akinesia (1 RCT, n = 267, RR 1.10 CI 0.73 to 1.65) and rigor (1 RCT, n = 309, RR 2.24 CI 0.80 to 6.30). Nevertheless, risperidone produced more dystonias (1 RCT, n = 673, RR 18.16 CI 2.44 to 135.27, NNH 20 CI 33 to 13) and more extrapyramidal symptoms (2 RCTs, n = 872, RR 1.69 CI 1.23 to 2.34, NNH 14 CI 33 to 8). More participants in the risperidone group used antiparkinson medication at least once (6 RCTs, n = 1715, RR 1.98 CI 1.16 to 3.39, NNH 20 CI 10 to 100).
5.6.8. Extrapyramidal symptoms - scale measured
Risperidone induced more extrapyramidal side effects than quetiapine according to the Simpson-Angus Scale (5 RCTs, n = 1077, MD 0.59 CI 0.02 to 1.16). There was no significant difference in dyskinesia (AIMS, 2 RCTs, n = 958, MD 0.34 CI −0.08 to 0.75) and akathisia (BAS, 2 RCTs, n = 700, MD 0.73 CI −0.54 to 2.0).
5.6.9 Haematological - white blood cell count - number of participants with low white blood cell count
There was no significant difference (1 RCT, n = 673, RR 0.34 CI 0.01 to 8.23)
5.6.10 Prolactin associated side effects
Amenorrhea (4 RCTs, n = 359, RR 2.14 CI 1.26 to 3.64, NNH not estimable) and galactorrhea (5 RCTs, n = 478, RR 2.65 CI 1.19 to 5.91, NNH 25 CI 100 to 13) were more frequent in the risperidone group, while gynaecomastia (1 RCT, n = 78, RR 4.33 CI 1.34 to 14.02, NNH 4 CI 11 to 2) occurred more frequently in the quetiapine group. There was no significant difference in the frequency of sexual dysfunction (6 RCTs, n = 2157, RR 1.24 CI 0.99 to 1.54) or dysmenorrhoea (1 RCT, n = 163, RR 2.23 CI 0.42 to 11.86).
5.6.11 Prolactin - change from baseline in ng/ml
There was a significant and consistent difference in favour of quetiapine (6 RCTs, n = 1731, MD 35.28 CI 26.19 to 44.36). Only the amount of the difference varied, leading to significant heterogeneity (Lieberman 2005: n = 678, MD 24.70 CI −20.68 to 28.72;McEvoy 2006: n = 24, MD 28.6 CI 14.18 to 43.02; Potkin 2006: n = 309, MD 50.4 CI −40.56 to 60.24; Stroup 2006: n = 199, MD 30.3 CI 23.50 to 37.10; Zhong 2006a: n = 440, MD 47.0 CI 41.03 to 52.97; McEvoy 2007: n = 81, MD 30.8 CI 23.50 to 38.10).
5.6.12 Metabolic - cholesterol - number of participants with increased cholesterol
There was no significant difference (2 RCTs, n = 940, RR 0.79 CI 0.45 to 1.39).
5.6.13 Metabolic - cholesterol - mean change from baseline in mg/dl
Risperidone was associated with less cholesterol increase than quetiapine (5 RCTs, n = 1433, MD −8.49 CI −12.23 to 4.75).
5.6.14 Metabolic - glucose - abnormally high fasting glucose value
There was no significant difference (2 RCTs, n = 940, RR 0.72 CI 0.29 to 1.79).
5.6.15 Metabolic - glucose - mean change from baseline in mg/dl
There was no significant difference (5 RCTs, n = 1436, MD 0.04 CI −2.83 to 2.92).
5.7.16 Metabolic - weight gain - number of participants with 7% or more gain of total body weight
There was no significant difference (7 RCTs, n = 1942, RR 1.03 CI 0.88 to 1.22).
5.7.17 Metabolic - weight gain - mean change from baseline in kg
Overall there was no significant difference (7 RCTs, n = 1446, MD −0.71 CI −2.47 to 1.04), but the data were highly heterogeneous presumably due to one small outlier study (Atmaca 2003) that showed a dramatic advantage of risperidone. Nevertheless, excluding this study did not change the overall result.
5.8 Publication bias
The funnel plot of the outcome PANSS total score (> 10 included studies) did not suggest a significant publication bias.
5.9 Investigation for heterogeneity and sensitivity analysis
Excluding Mori 2004 (skewed data) from the analysis of the PANSS positive subscore did not change the overall result. When Riedel 2005 was excluded from the analysis of the SAS score, quetiapine was no longer significantly superior in terms of general extrapyramidal side effects.
6. Comparison 6. Risperidone versus sertindole - all data short term
Two included studies fell in this category.
6.1 Global state
6.1.1 Global state - no clinically significant response - as defined by the original studies
There was no significant difference (1 RCT, n = 187, RR 1.14 CI 0.92 to 1.40).
6.1.2 Global state - no clinically important change
There was no significant difference (1 RCT, n = 187, RR 1.24 CI 0.91 to 1.68).
6.2 Leaving the study early
There were no significant differences between groups. 30% of the participants in the risperidone group and 36% of those in the sertindole group left the study early due to any reason (2 RCTs, n = 508, RR 0.81 CI 0.63 to 1.06). 7% of the participants in the treatment group and 9% of those in the sertindole group left the studies due to adverse events (2 RCTs, n = 508, RR 0.73 CI 0.39 to 1.35). 10% and 14% of the participants in the risperidone and sertindole groups, respectively, left the studies early due to lack of efficacy (2 RCTs, n = 508, RR 0.76 CI 0.46 to 1.25).
6.3 Mental state
6.3.1 General - no clinically important change (less than 50% PANSS total score reduction)
There was no significant difference (1 RCT, n = 187, RR 1.14 CI 0.92 to 1.40).
6.3.2 General - average endpoint score - PANSS total
There was no significant difference (2 RCTs, n = 493, MD −1.98 CI −12.20 to 8.24), but the results were heterogeneous. One study in treatment refractory participants showed a significant superiority of risperidone (Kane 2005: n = 321, MD −6.94 CI −12.14 to −1.74), whereas another study without this criterion revealed no significant difference (Azorin 2006: n = 172, MD 3.50 CI −3.42 to 10.42).
6.3.3 Positive symptoms - average endpoint score - PANSS positive
There was no significant difference (1 RCT, n = 172, MD 0.80 CI −1.35 to 2.95).
6.3.4 Negative symptoms - average endpoint score - PANSS negative
There was no significant difference (1 RCT, n = 172, MD 1.30 CI −0.53 to 3.13).
6.3.5 General functioning - average endpoint score - GAF total
There was no significant difference (1 RCT, n = 114, RR 2.90 CI −2.61 to 8.41).
6.4 Adverse effects
6.4.1 General - at least one adverse effect
There was no significant difference (2 RCTs, n = 508, RR 0.97 CI 0.90 to 1.05).
6.4.2 Death
There was no significant difference (1 RCT, n = 187, RR 3.30 CI 0.14 to 79.98).
6.4.3 Cardiac effects - number of participants with QTc prolongation
Fewer participants in the risperidone group had a prolongation of the QTc interval (2 RCTs, n = 508, RR 0.21 CI 0.08 to 0.51, NNH not estimable).
6.4.4 Cardiac effects - mean change of QTc interval from baseline in ms
Risperidone was associated with less QTc change than sertindole (2 RCTs, n = 495, MD −18.60 CI −22.37 to −14.83).
6.4.5 Central nervous system - sedation
There was no significant difference (2 RCTs, n = 508, RR 1.15 CI 0.69 to 1.92).
6.4.6 Extrapyramidal effects
Risperidone produced more akathisia (1 RCT, n = 321, RR 2.24 CI 1.02 to 4.92, NNH not estimable) and more parkinsonism (1 RCT, n = 321, RR 4.11 CI 1.44 to 11.73, NNH 14 CI 100 to 8) than sertindole. There was no significant difference in the frequency extrapyramidal symptoms (1 RCT, n = 187, RR 1.53 CI 0.90 to 2.61) or tremor (1 RCT, n = 187, RR 0.66 CI 0.16 to 2.69).
6.4.7 Extrapyramidal effects - scale measured
There was no significant difference in dyskinesia (AIMS: 2 RCTs, n = 477, MD 0.31 CI −0.25 to 0.86) and general EPS (SAS: 2 RCTs, n = 500, MD 0.37 CI −0.61 to 1.35), but there was a significant superiority of sertindole in akathisia (BAS: 2 RCTs, n = 500, MD 0.22 CI 0.03 to 0.41).
6.4.8 Prolactin associated effects - sexual dysfunction
Overall, risperidone produced less sexual dysfunction than sertindole (2 RCT, n = 437, RR 0.34 CI 0.16 to 0.76, NNT 13 CI 8 to 33).
6.4.9 Metabolic - cholesterol - change from baseline in mg/dl
There was no significant difference (1 RCT, n = 176, MD 4.90 CI −3.73 to 13.53).
6.4.10 Metabolic - glucose - mean change from baseline in mg/dl
There was no significant difference (1 RCT, n = 176, MD 2.00 CI −5.85 to 9.85).
6.4.11 Metabolic - weight gain - number of participants with weight gain
There was no significant difference (1 RCT, n = 187, RR 0.77 CI 0.41 to 1.43).
6.4.12 Metabolic - weight gain - mean change from baseline in kg
Risperidone was associated with less weight gain than sertindole (2 RCTs, n = 328, MD −0.99 CI −1.86 to −0.12).
6.5 Publication bias
Due to the small number of included studies, a funnel plot analysis was not meaningful.
6.6 Investigation for heterogeneity and sensitivity analysis
The reason for the preplanned sensitivity analysis did not apply and we therefore have not performed this.
7. Comparison 7. Risperidone versus ziprasidone
Three included studies fell in this category.
7.1 Global state
7.1.1 Global state - no clinically significant response - as defined by the original studies
There was no significant difference (1 RCT, n = 296, RR 1.02 CI 0.93 to 1.12).
7.1.2 Global state - no clinically important change - short term - as defined by the original studies
There was no significant difference (1 RCT, n=296, RR 0.81 CI 0.63 to 1.04).
7.2 Leaving the study early
There were no significant differences. 58% of the participants in the risperidone group and 65% of those in the ziprasidone group left the studies early due to any reason (3 RCTs, n = 1036, RR 0.90 CI 0.83 to 0.98). 9% of the risperidone group and 11% of the ziprasidone group left the studies early due to adverse events (3 RCTs, n = 1036, RR 0.82 CI 0.50 to 1.32). 20% of the risperidone group and 23% of the ziprasidone group left the studies early due to lack of efficacy of treatment (3 RCTs, n = 1036, RR 0.88 CI 0.60 to 1.27).
7.3 Mental state
7.3.1 General - no clinically important change - short term - less than 50% PANSS total reduction
There was no significant difference (1 RCT, n = 296, RR 1.02 CI 0.93 to 1.12).
7.3.2 General - average endpoint score - PANSS total
There was a significant difference in favour of risperidone (3 RCTs, n = 1016, MD −3.91 CI −7.55 to −0.27), but the results were heterogeneous. The long-term study by Lieberman 2005 revealed a significant difference (1 RCT, n = 516, MD −6.01 CI −10.03 to −1.99), whereas a short-term study (Addington 2004: 1 RCT, n = 296, MD −1.50 CI −3.16 to 0.16), and a medium-term study (Stroup 2006: 1 RCT, n = 204, MD −6.30 CI −12.77 to 0.17) revealed only a trend in favour of risperidone.
7.3.3 General - average endpoint score - short term - BPRS total
There was no significant difference (1 RCT, n = 296, MD −0.70 CI −4.33 to 2.93).
7.3.4 Positive symptoms - average endpoint score - medium term - PANSS positive
There was a significant difference in favour of risperidone (1 RCT, n = 204, MD −2.50 CI −4.62 to −0.38).
7.4.5 Positive symptoms - average endpoint score - medium term - BPRS positive
There was no significant difference (1 RCT, n = 296, MD-0.50 CI −1.15 to 0.15).
7.4.6 Negative symptoms - average endpoint score - PANSS negative
There was no significant difference overall (2 RCTs, n = 500, MD −0.04 CI −1.20 to 1.12), or in the short (1 RCT, n = 296, MD 0.00 CI −1.48 to 1.48) or medium term (1 RCT, n = 204, MD −0.10 CI −1.98 to 1.78).
7.5 Service use - number of participants rehospitalised
There was no significant difference (2 RCTs, n = 777, RR 0.87 CI 0.63 to 1.22).
7.6 Adverse effects
7.6.1 At least one adverse effect
There was no significant difference (3 RCTs, n = 1063, RR 1.07 CI 0.98 to 1.17).
7.6.2 Death
There was no significant difference in the number of suicide attempts (1 RCT, n = 526, RR 1.09 CI 0.10 to 11.89) or in the number of completed suicides (1 RCT, n = 241, RR 0.66 CI 0.06 to 7.17)
7.6.3 Cardiac effects - number of participants with QTc prolongation
There was no significant difference (2 RCTs, n = 822, RR 1.90 CI 0.40 to 9.05).
7.6.4 Cardiac effects - mean change of QTc interval from baseline in ms
There was no significant difference (3 RCTs, n = 793, RR −2.24 CI −6.39 to 1.92).
7.6.5 Central nervous system - sedation
There was no significant difference (3 RCTs, n = 1063, RR 1.15 CI 0.83 to 1.59).
7.6.6 Extrapyramidal effects
There was no significant difference in the frequency of akathisia (2 RCTs, n = 1063, RR 1.02 CI 0.55 to 1.89) and tremor (1 RCT, n = 296, RR 0.95 CI 0.47 to 1.89), but risperidone produced more extrapyramidal symptoms (1 RCT, n = 241, RR 3.16 CI 1.15 to 8.69, NNH 13 CI 100 to 7) and more participants in the risperidone group used antiparkinson medication at least once (2 RCTs, n = 822, RR 1.42 CI 1.03 to 1.96, NNH not estimable).
7.6.7 Extrapyramidal effects - scale measured
There were no significant differences in dykinesia (AIMS: 1 RCT, n = 296, MD 0.21 CI 0.17 to 0.25) and akathisia (BAS: 1 RCT, n = 296, MD 0.56 CI 0.51 to 0.61), but risperidone produced more overall EPS than ziprasidone (SAS: 1 RCT, n = 296, MD 0.34 CI 0.26 to 0.42).
7.6.8 Prolactin-associated side effects
There was no significant difference in the frequency of abnormal ejaculation (1 RCT, n = 215, RR 2.10 CI 0.65 to 6.75), amenorrhea (1 RCT, n = 81, RR 0.93 CI 0.20 to 4.33), decreased libido (1 RCT, n = 296, RR 1.65 CI 0.70 to 3.86), erectile dysfunction (1 RCT, n = 215, RR 1.05 CI 0.38 to 2.88) and galactorrhea (2 RCTs, n = 159, RR 5.07 CI 0.61 to 42.45). Only orgastic dysfunction occurred more frequently in the risperidone group than in the ziprasidone group (2 RCTs, n = 516, RR 1.44 CI 1.03 to 2.02).
7.6.9 Prolactin - mean change from baseline in ng/ml
Risperidone was associated with more prolactin increase than ziprasidone (2 RCTs, n = 767, MD 21.97 CI 16.60 to 27.34).
7.6.10 Metabolic - cholesterol - change from baseline in mg/dl
There was a significant difference in favour of ziprasidone (2 RCTs, n = 767, MD 8.58 CI 1.11 to 16.04).
7.6.11 Metabolic - glucose - mean change from baseline in mg/dl
There was no significant difference (2 RCTs, n = 767, RR 4.94 CI −1.91 to 11.80).
7.6.12 Metabolic - weight gain of 7% or more of total body weight
Three studies showed a significant difference in favour of ziprasidone (3 RCTs, n = 1063, RR 2.03 CI 1.35 to 3.06, NNH 14 CI 33 to 10).
7.6.13 Metabolic - weight gain - change from baseline in kg
Only one study reported on this outcome. It found no significant difference (1 RCT, n = 461, MD 1.10 CI −0.15 to 2.35).
7.7 Publication bias
Due to the small number of included studies, a funnel plot analysis was not meaningful.
7.8 Sensitivity analyses
The reasons for the preplanned sensitivity analysis did not apply and we have therefore not performed this.
DISCUSSION
Summary of main results
1. General
In the last years the number of randomised risperidone trials has dramatically increased. A previous Cochrane review comparing risperidone with other SGA drugs included only nine RCTs (Gilbody 2000). The current review includes 45 RCTs, although we had more stringent inclusion criteria and excluded open RCTs. Nevertheless, many problems that were identified by the previous review have not been solved:
The number of participants leaving schizophrenia trials prematurely remain high (Wahlbeck 2001). The overall attrition of 47% in the included studies is a threat to the validity of the findings. Adverse events were often only reported if they had a frequency of 5%/10% or greater. This procedure results in underreporting of rare but important adverse effects. We suggest to abandon the > 5%/10% frequency rule for reporting of adverse effects and suggest that all adverse events should be reported instead, for example as online supplements that are nowadays made available by most journals.
Most trials provided data on leaving the studies early and overall efficacy. Outcomes that are possibly more important for daily life such as general functioning or satisfaction with treatment are rarely presented. Authors keep using different criteria for ‘response to treatment’ making comparisons difficult, although validated suggestions for the presentation of response to treatment are available (Leucht 2005a; Leucht 2005b; Van Os 2006).
More than half of the 45 included trials were categorised as ‘short-term’ studies and only eight were ‘long-term’ studies with a length of more than 26 weeks. Schizophrenia is a chronic, often life-long disorder, making more long-term studies necessary.
60% of the studies were sponsored by pharmaceutical companies producing either risperidone or its comparator drugs, whereas only 31% of the studies had a neutral sponsor (the sponsor of the remaining RCTs remained unclear). Due to the inevitable conflict of interest, industry sponsorship is a concern (Heres 2006).
Finally, most studies compared risperidone with clozapine, olanzapine and quetiapine. Fewer RCTs comparing risperidone with amisulpride, aripiprazole, sertindole and ziprasidone are available, and comparisons with zotepine are completely missing.
2. Comparison 1. Risperidone versus amisulpride
2.1 Leaving the studies early
There were no significant differences in the number of participants leaving the studies early due to any reason, due to adverse events or due to inefficacy of treatment. These results suggest a similar overall acceptability, tolerability and efficacy of risperidone and amisulpride. Nevertheless, four studies with 622 participants do not provide a firm basis for such a conclusion. Furthermore, although the overall rate of participants leaving the studies early of 33.2% was lower than that of some other comparisons, it was still considerable.
2.2 Efficacy outcomes (global state, overall and specific mental state)
There was no clear efficacy difference between risperidone and amisulpride. There was a significant superiority of amisulpride in terms of 50% BPRS reduction. However, this result was based on only one trial and may have well occurred by chance alone, given the high number of statistical tests applied (Sechter 2002). Furthermore, the same trial did not find any difference in the mean values at endpoint of the BPRS.
2.3 General functioning
A single study using the SOFAS scale reported on social functioning and found no difference between risperidone and amisulpride. Pragmatic studies that are conducted in situations that are more similar to routine care are needed to address this important outcome.
2.4 Adverse effects
There were some data on extrapyramidal side effects, cardiac effects, prolactin associated side effects, sedation, seizures and death. Besides the reporting on sexual dysfunction which indicated a benefit for amisulpride, the only adverse event showing a significant difference was weight gain which was 1kg more in the risperidone group. This difference was found in short- to medium-term studies. It may well be that the difference would be more pronounced in the long term, but longer studies are needed to verify this assumption. Nevertheless, if metabolic issues are a concern, amisulpride may be a better choice than risperidone.
3. Comparison 2. Risperidone versus aripiprazole
3.1 Leaving the studies early
Again, the number of participants leaving the two studies early was considerable (34.4%). There was no significant difference between both compounds, suggesting that their acceptability is similar, but two included studies - both sponsored by the manufacturers of aripiprazole - are no firm basis for any conclusion.
3.2 Efficacy outcomes (global state, overall and specific mental state)
There were no statistically significant differences in global state, general mental state, positive and negative symptoms. The currently available small evidence base does thus not suggest a difference in efficacy between both compounds. Nevertheless, “no evidence of effect does not mean evidence of no effect” (Tarnow-Mordi 1999).
3.3 Adverse effects
Limited data were available on extrapyramidal side effects, cardiac effects, cholesterol, glucose, prolactin increase, prolactin associated side effects and weight gain.
There was a significant benefit for aripiprazole in terms of dystonia, QTc abnormalities, prolactin increase and cholesterol levels, whereas tremor was less frequent in the risperidone group. Overall risperidoné s tolerability profile may be somewhat worse than that of aripiprazole, but these results are based on very limited data. Any conclusion would be premature.
4. Comparison 3. Risperidone versus clozapine
4.1 Leaving the studies early
The 11 included studies showed a considerable overall attrition of 33.4%. Although this attrition was not as high as in some other comparisons of this review, it nevertheless limits the interpretation of all results beyond the outcome of leaving the studies early.
A similar number of participants in the risperidone and the clozapine groups left the studies early due to any reason, suggesting a comparable overall acceptability of both compounds. Nevertheless, there were significant differences in the reasons why participants left the studies.
Adverse events were a greater problem in the clozapine group. Clozapine is associated with a number of serious and partly dangerous adverse effects such agranulocytosis, seizures, sedation or weight gain (see 4.4 below), which may explain risperidone’s superiority in this regard.
Inefficacy of treatment led more frequently to leaving the studies early in the risperidone group. This suggests a certain efficacy superiority of clozapine. Indeed, clozapine was associated with a somewhat more pronounced reduction of positive symptoms than risperidone, although the difference was not robust (see 4.2 below).
4.2 Efficacy outcomes (global state, overall and specific mental state)
The only significant difference between risperidone and clozapine was a superiority of the latter in terms of positive symptoms. Even this difference was not robust, because when two studies with possibly skewed data were excluded it was no longer statistically significant.
This failure to find clozapine superior was surprising, because clozapine is generally considered to be the most efficacious antipsychotic drug available. This superiority has recently been confirmed by the industry independent studies CATIE II (McEvoy 2006) and CUtLASS (Lewis 2006) which could not be included here. The clozapine group of CATIE II was a non-blinded study arm and CUtLASS compared clozapine with a number of second generation antipsychotics as a group.
One reason for the failure to find a consistent superiority of clozapine may be that efficacy was addressed in different ways (e.g. different scales or different definitions of response to treatment), making a summation difficult. Indeed at most six out of 11 studies could be combined in a meta-analysis, and frequently the results were even based on only one RCT.
Another possible explanation may be relatively low clozapine doses. The mean doses in two pivotal studies demonstrating clozapine’s superiority to first-generation antipsychotic drugs were 600mg/day (Kane 1988) and 523mg/day (Rosenheck 1997). A randomised, blinded dose finding study found that a clozapine dose of 600mg/day was more efficacious than lower doses (Simpson 1999). In contrast, of the 11 trials included in this review only two studies had mean clozapine doses higher than 500mg/day (Volavka 2002: 526mg/day; Azorin 2001: 642 mg/day), and indeed the latter study found a superiority of clozapine. Several trials limited the upper clozapine dose range to 400mg/day.
Nevertheless, a definitive, industry independent trial with sufficient clozapine doses is necessary to establish the relative efficacy of clozapine and risperidone.
4.3 General functioning
Only a very small study reported on general and social functioning, but found no significant difference between groups. From a more global public health perspective, but also for people with schizophrenia, it may be more important to know whether a drug improves functioning in the community than whether it reduces symptoms. It is therefore disappointing that so few data on these important outcomes were available.
4.4 Adverse effects
Very few data on extrapyramidal side effects, cardiac effects, change in cholesterol level, death, prolactin increase and associated side effects, sedation, seizures, and low white blood cell count were available. At most five (sedation, white blood cell count) to six (use of antiparkinson medication) out of 11 included studies could be combined in a meta-analysis. This may well reflect selective reporting and limits the conclusions.
Nevertheless, risperidone was associated with clearly more use of antiparkinson medication than clozapine. Use of antiparkinson medication is a useful proxy measure of movement disorders. One out of six people treated with risperidone instead of clozapine suffered from these very unpleasant adverse events which are well visible and can therefore contribute to the stigma associated with schizophrenia.
Risperidone also produced more prolactin increase than clozapine. This result was based on only two RCTs, but prolactin increase is a well-known adverse event of risperidone. The long-term consequences can be osteoporosis and sexual side effects, although the latter could not be demonstrated in this review, at least partly because the individual studies presented so few data.
Conversely clozapine was associated with more seizures, sedation and weight gain. One out of 14 participants treated with clozapine instead of risperidone had a seizure. This is a considerable and clinically important difference, because seizures are dangerous adverse events. Clozapine is well known for its sedating effects. Many people taking antipsychotic drugs do not like the sedation associated in varying degrees with these compounds, but sometimes sedation is only transient. The weight gain produced by clozapine is a major concern, because in the long term it can lead to diabetes and cardiovascular diseases such as myocardial infarction or stroke. It is reassuring that - despite the limited data available - the review was able to document some of the expected differences in tolerability between risperidone and clozapine. Clinicians and people with schizophrenia may use the results in their choice of drug.
5. Comparison 4. Risperidone versus olanzapine
5.1 Leaving the studies early
Risperidone and olanzapine have been compared in a relatively large number of 23 blinded RCTs and 3207 participants. Nevertheless, the high overall rate of participants leaving the studies early (52%) is a source of concern. The field must urgently find ways to decrease the amount of attrition in schizophrenia trials, because the typically high discontinuation rates make the validity of the results questionable.
Risperidone may be a somewhat less acceptable treatment than olanzapine for people with schizophrenia, because more participants in the risperidone group left the studies early due to any reason. In addition, more risperidone treated participants left the studies early due to inefficacy of treatment. This may reflect a somewhat better efficacy of olanzapine which is also supported by a stronger improvement of the participants’ general mental state (see below). Leaving the studies early due to adverse events showed no difference between groups suggesting a similar overall tolerability of risperidone and olanzapine.
5.2 Efficacy outcomes (global state, overall and specific mental state)
Most data were available for the general mental state (PANSS total score, 15 RCTs) and positive and negative symptoms of schizophrenia (PANSS positive and negative subscore, 13 RCTs). Olanzapine was slightly superior in the improvement of the general mental state, but not superior for specific symptoms of schizophrenia. The difference was numerically very small (two points difference on the PANSS total score) and of questionable clinical importance. Only three studies reported on responder rates defined as ‘at least 50% reduction of the PANSS total score’ and found a marginal but statistically significant difference in favour of olanzapine (RR 1.09). A number needed to harm could not be calculated, because the risk difference was not significant. Most other efficacy-related outcomes were based on very small numbers and showed equivocal results.
5.3 Quality of life
The results suggested a better quality of life of participants treated with olanzapine compared to risperidone. Since only two studies provided data on this outcome, any recommendation for practice would be premature.
5.4 Cognitive functioning
Only two studies compared the cognitive effects of risperidone and olanzapine and found no significant difference between groups.
5.5 Service use
In three trials a similar number of participants in the risperidone and olanzapine groups had to be rehospitalised. This lack of a difference suggests a similar efficacy of both compounds. Or possible efficacy differences are so small that they do not translate in more global outcomes such as rehospitalisation.
5.6 Adverse effects
The adverse effects that occurred in a statistically significantly different frequency can be grouped in three categories.
Olanzapine was associated with more weight gain and associated metabolic problems such as cholesterol and glucose increase. Therefore, risperidone might be a more appropriate treatment for people at risk to develop a metabolic syndrome, overweight people, individuals suffering from diabetes or those with high cholesterol levels.
Risperidone produced some extrapyramidal side effects more frequently than olanzapine. Namely, the participants in the risperidone group used more antiparkinson medication and suffered more frequently from akathisia and parkinsonism. Although the number needed to treat for use of antiparkinson medication was relatively high (NNT 17), movement disorders are very unpleasant side effects and should be avoided.
Risperidone was also associated with clearly more prolactin increase and related sexual dysfunctions such as abnormal ejaculation in men and amenorrhea in women. Clinicians and people with schizophrenia may consider these different tolerability profiles of both compounds in their drug choice.
6. Comparison 5. Risperidone versus quetiapine
6.1 Leaving the studies early
We included 11 studies with 3770 participants in this comparison. This could be a reasonable basis for the examination of the relative effects of risperidone and quetiapine, but the overall discontinuation rate was high (56.7%). Such high attrition limits the interpretation of any other results beyond the outcome leaving the studies early. If more than 50% of the data must be estimated by statistical modelling, the validity of the findings is called into question. Nevertheless, there was no clear difference in the number of participants leaving the studies early due to any reason or due to adverse events, suggesting a similar overall acceptability and tolerability of risperidone and quetiapine. Only the outcome leaving the studies early due to inefficacy tended to favour risperidone, which is consistent with a certain efficacy superiority of risperidone (see 6.2 below).
6.2 Efficacy outcomes (global state, overall and specific mental state)
The only statistically significant differences in efficacy were found for the general mental state and positive symptoms. Risperidone was more efficacious than quetiapine in these aspects of psychopathology. Nevertheless, the differences were small (e.g. only 3 points on the PANSS total score). The clinical relevance of this difference is difficult to interpret. Unfortunately, dichotomous data on response to treatment, which can be interpreted more intuitively, were rarely indicated. Only four studies (less than half of those available for the PANSS total score) showed a trend in favour of risperidone in terms of ‘no important improvement of the participants’ global state’ (P = 0.06) which did not reach the conventional 5% level of statistical significance.
6.3 Adverse effects
Adverse effects were available for at least one adverse effect, cardiac effects, cholesterol increase, changes in serum glucose, increase of prolactin level and associated side effects, death, extrapyramidal side-effects, sedation, weight gain and white blood cell count.
Among these, risperidone was worse than quetiapine in various measures of extrapyramidal side effects and prolactin associated effects. Although the differences were not very large (e.g. among 20 participants treated with risperidone instead of quetiapine one needed antiparkinson medication) extrapyramidal side effects are very unpleasant adverse events which should be avoided. Prolactin increase can lead to sexual side effects and indeed the frequency of amenorrhea and galactorrhea was approximately two times higher in the risperidone group.
Conversely quetiapine was associated with more sedation and cholesterol increase than risperidone. The long-term consequences of the latter adverse event can be cardiovascular problems such as myocardial infarction or stroke.
These differences in the side effect profile and the slightly better efficacy of risperidone may be weighed in drug choice.
7. Comparison 6. Risperidone versus sertindole
Again only two studies could be included in this comparison and one of the studies (Kane 2005) has to date only been published as a conference poster presenting limited information. This evidence base is too limited to draw firm conclusions.
7.1 Leaving the studies early
Although the number of participants leaving the studies early due to any reason was considerable (33.7%), there was no significant difference between sertindole and risperidone, suggesting a similar overall acceptability of treatment. Specific reasons for leaving the studies early (adverse events, inefficacy of treatment) did not show a difference between both compounds either.
7.2 Efficacy outcomes (global state, overall and specific mental state)
There was no clear difference in efficacy based on CGI, PANSS totals score, PANSS positive and negative subscore. Nevertheless, the results on the general mental state as measured by the PANSS total score were heterogeneous. One study suggested that in people with treatment-resistant schizophrenia, risperidone may be somewhat more efficacious (Kane 2005), while in the other study without this criterion, no difference was found (Azorin 2006). Any interpretation is certainly limited by the small number of included trials and participants. Replications are needed.
7.3 General functioning
Only one short-term study provided data on general functioning and showed no difference between groups. We believe that longterm, real world, pragmatic studies are needed to examine this important outcome.
7.4 Adverse effects
There were some data on extrapyramidal symptoms, cardiac effects, cholesterol, glucose, sedation, sexual dysfunction, suicide and weight gain.
These limited data suggest that akathisia and parkinsonism may occur more frequently under treatment with risperidone than with sertindole. However, relatively high risperidone dose ranges of 4-10 mg/day and 4-12 mg/day in the two included studies must be taken into account. It is well known that the EPS risk of risperidone is dose related and lower doses (e.g. 2-8mg/day) may produce similar efficacy, but are better tolerated (Marder 1994).
Conversely cardiac effects (QTc prolongation), male sexual dysfunction and weight change indicated a benefit for risperidone. These differences in tolerability may be considered in drug choice. Due to the known effects of sertindole on the QTc interval, the drug cannot be recommended for people with schizophrenia and cardiac problems. The long-term consequences of weight gain such as diabetes and cardiovascular disease can be dramatic.
8. Comparison 7. Risperidone versus ziprasidone
Data based on three studies were available for this comparison. Similar to the comparisons with olanzapine and quetiapine, a very high overall attrition of 63.1% clearly limits the interpretation of any findings beyond the outcome leaving the study early.
8.1 Leaving the studies early
As fewer participants in the risperidone group than in the ziprasidone group left the studies prematurely, risperidone may be more acceptable for people with schizophrenia. As there were no significant differences in specific reasons (adverse events of inefficacy of treatment) for study discontinuation, the reason for this better acceptability is unclear.
8.2 Efficacy outcomes (global state, overall and specific mental state)
A statistically significant, but numerically small benefit (4 points difference on the PANSS total score) for risperidone was present in general mental state and in positive symptoms. Although the small number of included trials and the high drop-out rates limit the validity of this finding, risperidone maybe somewhat more efficacious for the positive symptoms of schizophrenia than ziprasidone. Whether the numerically small difference is clinically important is again difficult to say. Unfortunately dichotomous data on response to treatment which can be more intuitively understood were only reported by a single study which did not find a significant difference. It cannot be excluded that selective reporting played a role.
8.3 Adverse effects
Ziprasidone was more tolerable than risperidone concerning a number of adverse events: certain extrapyramidal side effects, glucose levels, cholesterol increase, prolactin increase, and weight gain.
In treatment decisions this better tolerability profile of ziprasidone needs to be weighted with the somewhat lower efficacy, always keeping in mind the small amount of available data.
9. Summary
The review currently includes 45 at least single blind studies and 7760 participants. The number of RCTs available for each comparison varied: four studies compared risperidone with amisulpride, two with aripiprazole, 11 with clozapine, 23 with olanzapine, 11 with quetiapine, two with sertindole, three with ziprasidone and none with zotepine. Attrition from these studies was high (46.9%). This high attrition makes the interpretation of the results problematic, because half of the results must be estimated by statistical modelling. Furthermore, 60% were industry sponsored, which can be a source of bias.
Risperidone was slightly less acceptable than olanzapine, and slightly more acceptable than ziprasidone in terms of leaving the studies early due to any reason. The results of all other comparisons were equivocal. There were also only few differences in efficacy-related outcome; risperidone may be somewhat more efficacious than quetiapine and ziprasidone, but slightly less efficacious than olanzapine and clozapine. Whether the differences are clinically meaningful is difficult to say, because most studies reported the mean scores of rating scales, whereas only a few reported more intuitive data on response to treatment and used different definitions for this.
It was the best documented tolerability difference of the review that risperidone produced somewhat more extrapyramidal side effects than a number of other SGA drugs (all except for amisulpride and aripiprazole compared to which only a few RCTs were available). Risperidone also increased prolactin levels clearly more than all comparators, except for amisulpride and sertindole for which no data were available.
Other differences in adverse effects were less well documented, but risperidone may well produce more weight gain and/or associated metabolic problems than amisulpride, aripiprazole and ziprasidone, but less than clozapine, olanzapine, quetiapine and sertindole. It may be less sedating than clozapine and quetiapine, lengthen the QTc interval less than sertindole, produce fewer seizures than clozapine and less sexual dysfunction in men than sertindole.
Overall completeness and applicability of evidence
The amount of RCTs comparing risperidone with the other SGA drugs varied substantially. A high number of studies compared risperidone with olanzapine with risperidone (N = 23). A reasonable amount of trials comparing olanzapine with clozapine (N = 11) and quetiapine (N = 11) was available. In contrast, few trials compared olanzapine with amisulpride (N = 4), aripiprazole (N = 2), sertindole (N = 2) and ziprasidone (N = 3). We did not identify any RCT comparing risperidone with zotepine. Therefore the evidence is incomplete.
Furthermore, it is also obvious that most of the studies reported on leaving the studies early due to any reason and overall symptoms of schizophrenia. All other outcomes were usually based on much smaller numbers. Very little information is available on general functioning, satisfaction with care or cognition. These outcomes may be more important for people suffering from schizophrenia than the improvement of symptoms. Only three included studies reported on service use, although such data would be crucial for policy makers.
Most of the included studies had tight inclusion criteria limiting external validity. Further effectiveness studies are needed.
Quality of the evidence
A major threat for the quality of the evidence is the high overall attrition of 47% in the studies. It is questionable whether even a sophisticated statistical method can account for such a high percentage of participants leaving the studies before their end. Most studies used the last observation carried forward method which is based on the assumption that a participant leaving a study early would not have changed if he had stayed in the study. This assumption can obviously be wrong.
All included studies were stated to be randomised and all but seven studies were double-blind. The remaining seven trials described blinded raters. Nevertheless, the randomisation and blinding methods were rarely described. The study authors did also not make attempts to verify whether blinding was successful.
The majority of the trials fell in the short-term category, which is problematic in a chronic disease such as schizophrenia. All these factors limit the overall quality of the evidence.
Potential biases in the review process
We are not aware of obvious flaws in our review process. Nevertheless, we admit that we present only a selection of outcomes. Although these outcomes were defined a priori in the protocols and although we think that we made a meaningful selection, other people may have different opinions and differences in other outcomes may have been missed.
Agreements and disagreements with other studies or reviews
Many new RCTs have compared risperidone with other SGA drugs since the publication of a previous Cochrane review on the same topic (Gilbody 2000). Gilbody 2000 included only nine RCTs which compared risperidone with clozapine, olanzapine and amisulpride. Due to this large difference in sample size the reviews are hardly comparable. Nevertheless, as in our review risperidone was overall as acceptable as clozapine for people with schizophrenia (leaving the study early due to any reason), but slightly less acceptable than olanzapine. Efficacy data tended to favour clozapine and olanzapine, but statistically significant effects were rare which is not surprising, because even in the current much larger review the differences were small. Risperidone also produced more extrapyramidal side effects but less weight gain than olanzapine, while very few side effect data compared to clozapine were available. As in the current review there were no major differences between risperidone and amisulpride and the evidence base has grown only slightly (from one to four RCTs).
Another more recent review specifically compared risperidone with olanzapine (Jayaram 2006). Again, more participants in the risperidone group left the studies early due to any reason, but there were no clear differences in the efficacy of both compounds. Risperidone produced more extrapyramidal side effects, but less weight gain and associated metabolic effects than olanzapine. Overall, we believe that many findings of the previous reviews are compatible with the current report. Therefore, the evidence has become more robust, although still insufficient in quality, over time.
AUTHORS’ CONCLUSIONS
Implications for practice
1. For people with schizophrenia
People with schizophrenia need to know that risperidone differs in adverse effects from other SGA drugs. It seems to produce somewhat more movement disorders and clearly more prolactin increase than most other SGA drugs. Its risk for weight gain and associated metabolic problems was intermediate - less than clozapine, olanzapine, quetiapine and sertindole, but more than amisulpride, aripiprazole and ziprasidone. Differences in efficacy were less clear, but risperidone may be overall slightly less efficacious than olanzapine and clozapine, but slightly more efficacious than quetiapine and ziprasidone.
2. For clinicians
Attrition was very high (overall 47%). Almost half of the results had to be estimated by statistical modelling which calls the validity of the findings in question. It is also important to know that risperidone has been compared in many RCTs with olanzapine and in a reasonable amount of trials with clozapine and quetiapine, but few comparisons with the other SGA drugs are available. Differences in side effects should be considered in choice of drug. Risperidone seems to produce somewhat more extrapyramidal side effects, clearly more prolactin increase and intermediate weight gain compared to other SGA drugs.
3. For managers/policy makers
There is insufficient information to guide the decisions of managers and policy makers. Service use was reported by only three of 45 included studies and the results were equivocal. The evidence base is too limited for making any recommendation. Nevertheless, in some countries risperidone is now available as a generic and could therefore be cheaper than other SGA drugs.
Implications for research
1. General
There is room for improvement in the conduct and reporting of RCTs for schizophrenia. Rating scale derived efficacy outcomes dominate the trials and even in this regard authors keep using different definitions for response to treatment, making a comparison of the results difficult. Potentially important outcomes such as satisfaction with care, functioning in the community or service use are rarely examined. Simple descriptions of the randomisation or blinding methods are usually not presented. Strict adherence to the CONSORT statement (Moher 2001) would improve the reporting and conduct of future trials.
2. Specific
Comparisons of risperidone with most SGA drugs are currently based on small numbers and no RCT which compares risperidone to zotepine is available. These gaps need to be filled by future trials (Table 1).
Table 1. Suggested design of future study.
| Methods | Allocation: randomised - clearly described generation of sequence and concealment of allocation. Blindness.: double - described and tested. Duration: 6 months minimum. |
| Participants | Diagnosis: schizophrenia (operational criteria). N=2700.* Age: any. Sex: both. History: any. |
| Interventions |
|
| Outcomes | Leaving study early (any reason, adverse events, inefficacy). Service outcomes: hospitalised, time in hospital, attending out patient clinics. Global impression: CGI**, relapse. Mental state: PANSS. Adverse events: UKU. Employment, family satisfaction, patient satisfaction. |
power calculation suggested 300/group would allow good chance of showing a 10% difference between groups for primary outcome.
Primary outcome
PLAIN LANGUAGE SUMMARY.
Risperidone versus other atypical antipsychotics for schizophrenia
This review examines the effects of risperidone compared to other second-generation antipsychotic (SGA) drugs for schizophrenia. We identified 45 relevant studies with 7760 participants comparing risperidone with amisulpride, aripiprazole, clozapine, olanzapine, quetiapine, sertindole and ziprasidone. Comparisons of risperidone with zotepine are currently not available. Risperidone was somewhat more successful than quetiapine and ziprasidone, but somewhat less successful than clozapine and olanzapine. The main disadvantage of risperidone were more frequent movement disorders and more prolactin increase compared to most other SGA drugs.
ACKNOWLEDGEMENTS
We would like to thank all members of the Cochrane Schizophrenia Group for their editorial assistance. We also thank Simon Gilbody, Anne-Marie Bagnall, Lorna Duggan and Arja Tuunainen for their work on a previous version of this review, and additionally the following authors and pharmaceutical companies for providing additional information on their studies: D. Addington, R. Conley, D. Daniel, S. Dollfus, H. Heinrich, T. Hwang, D. Jeste, J. Kane, R. Keefe, S. McGurk, K. Mori, Q. Ren, M. Riedel, N. Schooler, D. Sechter, J. Svestka, J. Volavka, K. Zhong, Astra Zeneca, Eli Lilly, Lundbeck, Pfizer and Sanofi Aventis.
SOURCES OF SUPPORT
Internal sources
Psychiatrische Klinik, Klinikum rechts der Isar, TU München, Friestaat Bayern, Germany.
External sources
Bundesministerium für Bildung und Forschung, Nr FKZ: 01 KG 0606, GZ:GF-GFKG01100506, Germany.
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Allocation: random, no further details. Blindness: double, no further details. Duration: 8 weeks. Design: parallel. Location: multicentre. |
|
| Participants | Diagnosis: (DSM-III-R) schizophrenia (n = 260) or schizoaffective disorder (n = 36), acute exacerbation, PANSS total score of 60 or more. N = 296. Age: 18-64 years. Sex: 215 M, 81 F. History: duration not reported, age at onset mean risperidone = 24.6 years, mean ziprasidone = 25.2 years. Setting: not reported. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Global State: CGI. Mental State: PANSS total score, PANSS derived BPRS, BPRS positive subscore, PANSS negative subscore, depression MADRS. General functioning: GAF. Adverse effects: open interviews, EPS (akathisia, tremor, use of antiparkinson medication, AIMS, BAS, SAS), cardiac effects (ECG), prolactin-associated side effects, sedation, weight gain, laboratory (urine, blood chemistry) Unable to use: GAF total score (no usable data). QTc abnormalities in ms (no usable data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, no further details. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
High risk | The overall attrition was rather high (33.3%). The LOCF method was used to account for people leaving the study early. It assumes that a participant who discontinued the study would not have had a change of his condition if he had remained in the study. This assumption can obviously be wrong. Data on study completers were also available. It is unclear whether this led to bias |
| Free of selective reporting? | High risk | Reporting on secondary outcomes was incomplete. |
| Free of other bias? | High risk | The study was sponsored by the manufacturer of ziprasidone. |
| Methods | Allocation: random, no further details. Blindness: single, rater-blinded. Duration: 6 weeks. Design: parallel. Location: single centre. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia. N=56. Sex: 24 M, 29 F (3 not reported) Age: 19-46 years (mean clozapine = 31.3 years, mean olanzapine = 29.6 years, mean quetiapine = 30.1 years, mean risperidone = 27.9 years, mean control group = 32.1 years). History: duration ill mean clozapine = 6.6 years, mean olanzapine = 6.3 years, mean quetiapine = 5.9 years, mean risperidone = 5.6, age at onset: not reported |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason. Mental State: PANSS total score. Adverse effects: EPS (use of antiparkinson medication), weight gain (BMI), laboratory (serum leptin, triglyceride levels) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Single, rater-blind. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
Low risk | Three subjects in the control groups left the study early (5.4%). Reason for dropout were not assessed, only completer data were presented. But due to the very low rate we do not think that there was a risk of bias |
| Free of selective reporting? | Low risk | Probably free of bias. The study focused on serum leptin and triglyceride levels which were adequately described |
| Free of other bias? | Unclear risk | Data on the allowed dose range have not been presented. Furthermore, the pre-study treatment was quite heterogeneous as 19 participants had never taken any psy-chotropic drugs while most other participants had a long history of previous treatment |
| Methods | Allocation: random, no further details. Blindness: double, no further details. Duration: 12 weeks. Design: parallel. Location: multicenter. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia disorganised (n = 46), catatonic (n = 4), paranoid (n = 140), residual (n = 15) or undifferentiated (n = 51) of intent to treat population, poor previous treatment response, CGI of 4 or more, BPRS of 45 or more. N = 273. Sex: 182 M, 74 F (of intent-to-treat population, n = 256). Age: 18-65 years (mean clozapine = 37.8, mean risperidone = 39.5) (of intent-to-treat population). History: duration ill mean clozapine = 13.0 years, mean risperidone = 15.5 years (of intent-to-treat population), age at onset: not reported. Setting: in- and outpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Global State: CGI. Mental State: PANSS total score, BPRS total score, PANSS positive subscore, PANSS negative subscore, Calgary depression scale, psychotic depression scale, psychotic anxiety scale. Adverse effects: open interviews, death, EPS, sedation, seizures, constipation, hypotension, sialorrhoea, tachycardia, agitation, anxiety, insomnia, nausea, headache, fatigue, fever, weight gain, laboratory (white blood cell count) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, no further details. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
Unclear risk | The overall attrition was moderate (26.7%) . The LOCF method was used to account for people leaving the study early. It assumes that a participant who discontinued the study would not have had a change of his condition if he had remained in the study. This assumption can obviously be wrong. It is unclear whether this led to bias |
| Free of selective reporting? | High risk | Only those adverse events that occurred in at least 5% of the participants were reported. This procedure can miss rare, but important adverse events |
| Free of other bias? | High risk | The study was sponsored by the manufacturer of clozapine. |
| Methods | Allocation: random, no further details. Blindness: double, identical capsules. Duration: 12 weeks. Design: parallel. Location: multicentre. |
|
| Participants | Diagnosis: schizophrenia (DSM-IV). N = 187. Sex: 113 M, 73 F (intent-to-treat group). Age: 18-65 years (mean ~ 36 years). History: duration ill unclear, age at onset unclear, at least moderately ill on CGI-S. Setting: inpatient and outpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving study early: any reason, adverse effects, inefficacy. Global State: CGI. Mental State: PANSS (total, positive, negative sub-score). Functioning: GAF. Attitude: Drug attitude inventory. Adverse effect/event: at least one adverse effect, QTc prolongation, death, extrapyramidal side effects (tremor, AIMS, BAS, SAS), prolactin associated effects (amenorrhoea, sexual dysfunction), sedation, weight change |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, identical capsules. Whether blinding was successful has not been examined, but both compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by lack of blinding |
| Incomplete outcome data addressed? All outcomes |
High risk | Data on reasons for leaving the study early were available, but total number was considerably high (35.8%). The LOCF method was used to account for people leaving the study early. It assumes that a participant who discontinued the study would not have had a change of his condition if he had remained in the study. This assumption can obviously be wrong |
| Free of selective reporting? | High risk | Only those adverse events that occurred in at least 5% of the participants were reported. This procedure can miss rare, but important adverse events |
| Free of other bias? | High risk | The study was industry sponsored by the manufacturer of sertindole |
| Methods | Allocation: random, no further details. Blindness: double, no further details. Duration: 8 weeks. Design: parallel. Location: multicenter. |
|
| Participants | Diagnosis: (DSM-III-R) chronic schizophrenia, non-responders or intolerance, PANSS between 60 and 120. N = 86. Sex: 61 M, 25 F. Age: 18-65 years (mean = 37.3). History: age at first hospitalisation mean clozapine = 25.0, mean risperidone = 26.0, age at onset mean clozapine = 23.5, mean risperidone = 22.4. Setting: inpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Mental State: PANSS total score, PANSS positive subscore, PANSS negative subscore). Adverse effects: cardiac effects, EPS (akinesia, dystonia, parkinsonism, use ofantiparkin-son medication), prolactin associated side effects (sexual dysfunction), sedation, failing memory, concentration difficulties, nausea, weight gain, laboratory (hematology) Unable to use: Change of weight in kg (no usable data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, no further details. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
Unclear risk | The overall attrition was moderate (20.9%) . The LOCF method was used to account for people leaving the study early. It assumes that a participant who discontinued the study would not have had a change of his condition if he had remained in the study. This assumption can obviously be wrong |
| Free of selective reporting? | High risk | Secondary outcomes were not fully reported. For treatment emergent adverse events there was an incidence ofat lest 5% occurrence for being reported |
| Free of other bias? | High risk | The study was sponsored by the manufacturer of risperidone. |
| Methods | Allocation: random, no further details. Blindness: double, no further details. Duration: 6 weeks. Design: parallel. Location: not reported. |
|
| Participants | Diagnosis: (DSM-IV) chronic schizophrenia, BPRS (positive subscale) of 8 or more, SANS of 20 or more. N = 29. Sex: 19 M, 10 F. Age: 18-55 years (mean clozapine = 37.7, mean risperidone = 32.4). History: duration ill mean clozapine = 13.9 years, mean risperidone =11.1 years, age at onset mean clozapine = 23.7, mean risperidone = 21.3. Setting: not reported. |
|
| Interventions |
|
|
| Outcomes | Mental State: BPRS total score, BPRS positive subscore, SANS. Adverse effects: EPS (use of antiparkinson medication, Simpson Angus Scale), laboratory (prolactin) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, no further details. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
High risk | Data on subjects leaving the study early were not available. |
| Free of selective reporting? | High risk | Exclusion of 5 participants after randomisation, incomplete information |
| Free of other bias? | Unclear risk | One of the authors is an employee of a pharmaceutical company producing olanzapine |
| Methods | Allocation: random, no further details. Blindness: double, no further details. Duration: 16 weeks (first 8 weeks observed). Design: crossover. Location: not reported. |
|
| Participants | Diagnosis: schizophrenia. N = 8. Sex: not reported. Age: not reported. History: duration ill not reported, age at onset not reported. Setting: in- and outpatient. |
|
| Interventions |
|
|
| Outcomes | Global State: CGI. Mental State: PANSS total score, PANSS positive subscore, PANSS negative subscore depression Calgary depression scale Unable to use: CGI (no usable data). Mental State (no usable data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, no further details. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
High risk | Data on leaving the study early were not available. |
| Free of selective reporting? | High risk | Data were only presented as a poster, data on primary outcomes were missing |
| Free of other bias? | High risk | The study was sponsored by the manufacturer of olanzapine. |
| Methods | Allocation: random, permuted block randomisation stratified by centre. Blindness: double, identical capsules. Duration: 4 weeks. Design: parallel. Location: multicentre. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia (n = 80) or schizoaffective disorder (n = 3), acute relapse. PANSS total score of 60 or more. N = 83. Age: 18-65 years (mean aripiprazole = 35.2 years, mean risperidone = 35.1 years). Sex: 45 M, 38 F. History: duration illness not reported, age at onset not reported. Setting: inpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Global State: CGI. Mental State: PANSS total score, PANSS positive subscore, PANSS negative subscore. Adverse effects: at least one adverse effect, cardiac effects (QTc), extrapyramidal side effects (use of antiparkinson medication, extrapyramidal symptoms, AIMS, BAS, SAS), cholesterol increase, glucose elevation, prolactin increase, weight |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, permuted block randomisation stratified by centre. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, identical capsules. Whether blinding was successful has not been examined, but both compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
Unclear risk | Total number of participants leaving the study early was possibly acceptable (25%). The LOCF method was used to account for people leaving the study early. It assumes that a participant who discontinued the study would not have had a change of his condition if he had remained in the study. This assumption can obviously be wrong. It is unclear whether this led to bias |
| Free of selective reporting? | High risk | Only adverse events with an incidence of at least 5% in any treatment group were reported, therefore important side effects may have been missed by this procedure |
| Free of other bias? | High risk | The study was industry sponsored by the manufacturer of aripiprazole |
| Methods | Allocation: random, stratified by site. Blindness: double, no further details. Duration: 8 weeks. Design: parallel. Location: multicentre. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia (n = 325) paranoid (n = 213) or schizoaffective disorder (n = 52), PANSS between 60 and 120. N = 377. Sex: 274 M, 103 F. Age: 18-64 years (mean = 40.0 years). History: duration ill mean olanzapine =15.4 years, mean risperidone = 16.5 years, age at onset mean olanzapine = 23.6 years, mean risperidone = 24.5 years. Setting: in- and outpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Global State: CGI. Mental State: PANSS total score, PANSS positive subscore, PANSS negative subscore. Adverse effects: open interviews, cardiac effects (ECG), death (suicide attempt), EPS (use of antiparkinson medication, ESRS), prolactin associated side effects (abnormal ejaculation, amenorrhea, decreased libido, galactorrhea, gynecomastia, impotence, orgastic dysfunction, sexual dysfunction) depression, insomnia, dry mouth, agitation, rhinitis, dizziness, anxiety, vision abnormalities, sedation, weight gain, laboratory (liver enzymes, lipids) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, no further details. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
Unclear risk | The overall attrition was moderate (25.5 %). The LOCF method was used to account for people leaving the study early. It assumes that a participant who discontinued the study would not have had a change of his condition if he had remained in the study. This assumption can obviously be wrong. It is unclear whether this led to bias in a study with moderate attrition |
| Free of selective reporting? | High risk | Only those adverse events that occurred in at least 10% of the participants were reported. This procedure can miss rare, but important adverse events |
| Free of other bias? | High risk | The study was sponsored by the manufacturer of risperidone. The total number of participants was very low (n = 13), which may limit the validity of results |
| Methods | Allocation: random, no further details. Blindness: double, no further details. Duration: 12 weeks. Design: parallel. Location: not reported. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia, treatment resistance, persistent positive psychotic symptoms, BPRS total score of 35 or more plus CGI score of 4 or more. N = 38. Sex: 30 M, 8 F. Age: 18-65 years (mean fluphenazine = 44.2 years, mean quetiapine = 43.7 years, mean risperidone = 46.3 years). History: duration ill not reported, age at onset not reported. Setting: inpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Global State: CGI. Mental State: BPRS total score, BPRS positive subscore, BPRS negative subscore. Cognitive functioning: Neuropsychological testing. Quality of life: QLS. Adverse effects: open interviews, EPS (use of antiparkinson medication, SAS), prolactin increase, sexual dysfunction, sedation, weight gain, laboratory (thyroidal hormones) Unable to use: Prolactin increase: no useable data. Sexual dysfunction: no useable data. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, no further details. Whether blinding was successful has not been examined, but both compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
High risk | The drop-out rate was considerable (36%) . The analysis was based on the intention-to-treat sample and a mixed effects model. It is unclear whether any statistical method can account for higher drop-out rates |
| Free of selective reporting? | High risk | Not all of the predefined adverse effects were assessed. |
| Free of other bias? | Unclear risk | There was a slight baseline imbalance in terms of mean age and previous numbers of hospitalizations (14 in the risperidone and 9.7 in the quetiapine group) |
| Methods | Allocation: random, no further details. Blindness: single, rater-blinded. Duration: 12 weeks (6 weeks observed). Design: crossover. Location: not reported. |
|
| Participants | Diagnosis: (DSM-III-R) chronic schizophrenia (n = 16) or schizoaffective disorder (n = 4). N = 20. Sex: 7 M, 13 F. Age: 22-51 years (mean = 33.8 years). History: duration ill n.i., age at onset mean = 22.7 years. Setting: outpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Global State: CGI-S. Cognitive functioning: Wechsler memory scale. Adverse effects: insomnia, malaise, nausea, vomiting, diarrhoea, anorexia, depressed mood, agitation, weight gain Unable to use: Weight gain: no useable data. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Single, rater-blind. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
Unclear risk | The overall attrition was moderate (15%). Further details were not provided |
| Free of selective reporting? | High risk | Outcomes were reported incompletely. Six weeks’ interim data before crossover were hardly reported. The study was only published as an abstract |
| Free of other bias? | High risk | The study was sponsored by the manufacturer of risperidone. |
| Methods | Allocation: random, no further details. Blindness: double, no further details. Duration: 8 weeks. Design: parallel. Location: multicentre. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia with post-psychotic depression, PANSS positive subscore of 28 or less and MADRS score of 16 or more. N = 76. Sex: 53 M, 23 F. Age: 18-65 years (mean olanzapine = 39 years, mean risperidone = 39.6 years). History: duration ill mean olanzapine = 13.7 years, mean risperidone = 13.1 years, age at onset not reported. Setting: not reported. |
|
| Interventions |
|
|
| Outcomes | Global state: relapse. Mental State: PANSS total score, PANSS positive subscore, PANSS negative subscore, depression MADRS. Service use: number of participants rehospitalised. Adverse effects: open interviews, cardiac effects (ECG), death (natural causes, suicide) , EPS (akathisia, akinesia, dystonia, parkinsonism, rigor, tremor, use of antiparkinson medication, continuous: ESRS total score), prolactin associated side effects (abnormally high prolactin value, amenorrhea, sexual dysfunction), sedation, seizures, weight gain. Weight (change from baseline in kg). Unable to use: White blood cell count (no usable data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, no further details. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
High risk | Data on leaving the study early were not published separately for each group, the overall attrition was moderate (25%). (Data on both treatment attrition were provided from contact of the author) |
| Free of selective reporting? | High risk | Data on efficacy outcomes were incompletely reported. |
| Free of other bias? | High risk | The study was sponsored by the manufacturer of olanzapine. |
| Methods | Allocation: random, no further details. Blindness: double, no further details. Duration: 8 weeks. Design: parallel. Location: not reported. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia. N = 40. Sex: not reported. Age: 18-65 years. History: duration ill not reported, age at onset not reported. Setting: not reported. |
|
| Interventions |
|
|
| Outcomes | General functioning: scale of functioning. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, no further details. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
High risk | No data on leaving the study early available. |
| Free of selective reporting? | Unclear risk | Insufficient data. |
| Free of other bias? | Unclear risk | Insufficient data. |
| Methods | Allocation: random, computer-generated randomisation. Blindness: double, double-dummy design. Duration: 30 weeks. Design: parallel. Location: multicentre. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia, schizoaffective disorder or schizophreniform disorder, BPRS total score of 36 or more. N = 65. Sex: 38 M, 27 F. Age: 18 years or more (mean olanzapine = 35.6 years, mean risperidone = 34.8 years). History: duration ill not reported, age at onset not reported. Setting: in- and outpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, inefficacy. Global State: CGI-S. Mental State: PANSS total score, BPRS total score, PANSS positive subscore, PANSS negative subscore. Quality of life: QLS, SF-36. Adverse effects: open interviews, death (suicide attempt), cardiac effects (ECG), EPS (akathisia, dyskinesia, parkinsonism, rigor, tremor, use of antiparkinson medication), prolactin associated side effects (abnormal ejaculation, decreased libido, gynaecomastia, impotence), sedation, weight change, laboratory (glucose, leukopenia). Unable to use: Cardiac effects (no data). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random, computer-generated randomisation. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, double-dummy design. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
High risk | The overall attrition was high (55.4%). The LOCF method was used to account for people leaving the study early. It assumes that a participant who discontinued the study would not have had a change of his condition if he had remained in the study. This assumption can obviously be wrong |
| Free of selective reporting? | High risk | Only those adverse events that occurred in at least 10% of the participants were reported. This procedure can miss rare, but important adverse events |
| Free of other bias? | High risk | The study was sponsored by the manufacturer of olanzapine. |
| Methods | Allocation: random, no further details. Blindness: double, no further details. Duration: 4 weeks. Design: parallel. Location: single-centre. |
|
| Participants | Diagnosis: acute schizophrenia, disorganised (n = 1), catatonic (n = 1), paranoid (n = 47), unspecified (n = 2) type. Schizoaffective psychosis schizodominant type (n = 8). N = 59. Sex: 31 M, 28 F. Age: 19-65 years. History: duration ill not reported, age at onset not reported. Setting: not reported. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Global State: CGI. General Mental State: BPRS total score. Adverse effects: open interviews, cardiac effects (preterminal negative T-wave), EEG, EPS (extrapyramidal symptoms, use of antiparkinson medication, Simpson-Angus Scale) , sedation, vital signs, laboratory (agranulocytosis) Unable to use: White blood cell count: agranulocytosis (no data). |
|
| Notes | BPRS subscores available. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, no further details. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
High risk | The overall attrition was high (48.3%). The LOCF method was used to account for people leaving the study early. It assumes that a participant who discontinued the study would not have had a change of his condition if he had remained in the study. This assumption can obviously be wrong |
| Free of selective reporting? | High risk | Acutely ill subjects were included but data on the reduction of positive symptoms were not available. Secondary outcome data in terms of adverse effects were reported incompletely |
| Free of other bias? | Unclear risk | Fixed doses of 4mg/day and 8mg/day of risperidone were compared with a fixed dose of 400mg of clozapine, for a fixed dose regimen it might be difficult to decide which doses are appropriate |
| Methods | Allocation: random, no further details. Blindness: double, identical capsules. Duration: 6 weeks. Design: parallel. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia disorganised (n = 9), paranoid (n = 22) or undifferentiated (n = 16) (of intent-to-treat population). N = 48. Age: 18-65 years (mean amisulpride = 36.3 years, mean risperidone = 34.1 years). Sex: 20 M, 27 F (of intent-to-treat population). Location: multicentre. Setting: not described. History: duration ill mean amisulpride = 13.3 years, mean risperidone = 13.4 years, age at onset not reported |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Global State: CGI. Mental State: PANSS total, BPRS total, PANSS positive subscore, PANSS negative subscore. General functioning: SOFAS total score. Adverse effects: open interviews, cardiac effects (QTc interval of > 500 ms), EPS (akathisia, rigor, tremor, use of antiparkinson medication), weight, laboratory (hematology, blood chemistry, urinanalysis) Unable to use: ESRS: no data. Leukopenia: no data. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, identical capsules. Whether blinding was successful has not been examined, but both compounds differ in side effects. This may be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
Low risk | The rate of participants leaving the study early was below 10% and data on reasons for drop-out were available. The data were analysed on an intent-to-treat basis with the LOCF method. This method is not perfect, but due to the very low attrition the risk of bias was low |
| Free of selective reporting? | High risk | Data on the extrapyramidal symptom scale have not been presented. Only those adverse events that occurred in at least 5% of the participants were reported. This procedure can miss rare, but important adverse events |
| Free of other bias? | High risk | The study was sponsored by the manufacturer of amisulpride. |
| Methods | Allocation: random, no further details. Blindness: double, no further details. Duration: 8 weeks. Design: parallel. Location: multicentre. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia (n = 149) or schizoaffective disorder (n = 26), PANSS between 50 and 120. N = 176. Sex: 62 M, 113 F (of intent-to-treat population). Age: 60 years or more (mean olanzapine = 71.4 years, mean risperidone = 70.9 years) (of intent-to-treat population). History: duration ill mean = 36.5 years, age at onset mean olanzapine = 33.4 years, mean risperidone = 36.0 years (of intent-to-treat population). Setting: in- and outpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Global State: CGI. Mental State: PANSS total score, PANSS positive subscore, PANSS negative subscore. Adverse effects: open interviews, cardiac effects (ECG), death (natural causes, suicide) EPS (akinesia, dystonia, extrapyramidal symptoms, parkinsonism, tremor, use of antiparkinson medication, ESRS), sedation, seizures, weight change, laboratory (cholesterol, glucose, prolactin) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, no further details. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
Unclear risk | Number of participants leaving the study early was moderate (23.9%). The LOCF method was used to account for people leaving the study early. It assumes that a participant who discontinued the study would not have had a change of his condition if he had remained in the study. This assumption can obviously be wrong. It is unclear whether this led to bias |
| Free of selective reporting? | High risk | Only those adverse events that occurred in at least 10% of the participants were reported. This procedure can miss rare, but important adverse events |
| Free of other bias? | High risk | The study was sponsored by the manufacturer of risperidone. The mean age of included subjects was about 71 years. Probably due to this reason the upper dose range limit of risperidone was rather low (3mg/day), compared to that of olanzapine (20mg/day) |
| Methods | Allocation: random, no further details. Blindness: double, no further details. Duration: 12 weeks. Design: parallel. Location: multicentre. Setting: not specified. |
|
| Participants | Diagnosis: schizophrenia (DMS-IV). N = 321. Sex: 250 M, 71 F. Age: 18-55 years (mean ~ 39). History: duration ill not specified, mean age at onset - 22 years, treatment resistance, PANSS total score ≧ 60 |
|
| Interventions |
|
|
| Outcomes | Leaving study early: any reason, adverse events, inefficacy. Mental State: PANSS total score. Adverse effects/event: at least one adverse effect, QTc prolongation, cholesterol, extrapyramidal effects (akathisia, parkinsonism, AIMS, BAS, SAS), glucose, sexual dysfunction, sedation, weight |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, no further details. Whether blinding was successful has not been examined. Both compounds differ substantially in side effects which can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
High risk | Data on reason for drop-out were available but total numbers was considerably high (32.4%). The LOCF method was used to account for people leaving the study early. It assumes that a participant who discontinued the study would not have had a change of his condition if he had remained in the study. This assumption can obviously be wrong |
| Free of selective reporting? | High risk | Only those adverse events that occurred in at least 10% of the participants were reported. This procedure can miss rare, but important adverse events |
| Free of other bias? | High risk | The study was sponsored by the manufacturer of sertindole. |
| Methods | Allocation: random, no further details. Blindness: double, no further details. Duration: 52 weeks. Design: parallel. Location: multicentre. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia or schizoaffective disorder. N = 414. Sex: 282 M, 132 F. Age: 18-55 years (mean = 39 years). History: duration ill not reported, age at onset not reported. Setting: in- and outpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Global State: relapse. Mental State: PANSS total score, PANSS positive subscore, PANSS negative subscore, depression (MADRS), anxiety (Hamilton anxiety scale). Cognitive Functioning: Neurocognitive Composite Score. Adverse effects: open interviews, EPS (akathisia, tremor, use of antiparkinson medication, AIMS, BAS, SAS), sedation, weight change, laboratory (cholesterol, prolactin, urinanalysis) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, no further details. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
High risk | The overall attrition was high (62.8%). The LOCF method was used to account for people leaving the study early. It assumes that a participant who discontinued the study would not have had a change of his condition if he had remained in the study. This assumption can obviously be wrong |
| Free of selective reporting? | High risk | Only those adverse events that occurred in at least 10% of the participants were reported. This procedure can miss rare, but important adverse events |
| Free of other bias? | High risk | The study was sponsored by the manufacturer of olanzapine. |
| Methods | Allocation: random, no further details. Blindness: double, identical capsules. Duration: 78 weeks. Design: parallel. Location: multicentre. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia, previously more than one schizophrenic episode, responder. N = 1493. Age: 18-65 years (mean = 40.6 years). Sex: 1080 M, 380 F. History: duration not reported, age at onset not reported. Setting: in- and outpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Global State: CGI-S. Mental State: PANSS total score. Service use: number of patients re-hospitalised. Death: suicide attempt. Adverse effects: open interviews, EPS (use of antiparkinson medication, akathisia), cardiac effects (ECG), prolactin-associated side effects, sedation, weight gain, laboratory (prolactin, lipids, glucose) Unable to use: Withdrawal due to “extrapyramidal effects” (no usable data). |
|
| Notes | Note: 33 patients were excluded before analysis. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, identical capsules. Whether blinding was successful has not been examined, but both compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
High risk | The overall attrition was high (75%), and it is unclear whether any statistical method can account for such a high drop-out rate. Efficacy outcomes were evaluated based on mixed effects model analysis |
| Free of selective reporting? | Low risk | There was no evidence for selective reporting. |
| Free of other bias? | Unclear risk | Dose ranges were quite different, the upper dose range of olanzapine was 30 mg whereas risperidone could only be titrated up to 6mg /day. There was no wash-out period. An overlap in the administration of formerly given antipsychotics was permitted for the first four weeks after randomisation. Allocation to ziprasidone treatment was not possible from the start of the study due to the later availability of ziprasidone |
| Methods | Allocation: random, no further details. Blindness: double, identical capsules. Duration: 52 weeks (26 weeks observed, because of small group sizes). Design: parallel. Location: multicentre. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia, inadequate efficacy in previous study, clozapine treatment (n = 49) was open-label. N = 99 (observed N = 50). Sex: 80 M, 19 F. Age: 18-65 years (mean = 39.7 years). History: duration ill, age at onset: not reported. Setting: in- and outpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Global State: CGI. Mental State: PANSS total score, PANSS positive subscore, PANSS negative subscore. Adverse effects: open interviews, amenorrhoea, galactorrhoea, sexual dysfunction, sedation, laboratory (lipids, glucose, prolactin, haemoglobin A1C level), weight gain Unable to use: Global state CGI: no data. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, identical capsules. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
High risk | The overall attrition was high (74%). It is doubtful that the validity was not affected by this high rate |
| Free of selective reporting? | High risk | Due to small numbers and the very high attrition only data on 26 weeks treatment (rather than 52 weeks) were presented |
| Free of other bias? | Unclear risk | Dose ranges were quite different, the upper dose range of olanzapine was 30 mg whereas risperidone could only be titrated up to 6mg /day. Patients had a history of former inefficacy to one of the medications. It was excluded that the same medication could be given again but still this might implicate a risk of bias due to baseline imbalance in terms of former treatment. There was no wash-out period |
| Methods | Allocation: random, no further details. Blindness: double, no further details. Duration: 52 weeks. Design: parallel. Location: multicentre. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia (n = 231), schizophreniform disorder (n = 115) or schizoaffective disorder (n = 54), first episode, psychotic symptoms for 1 month to 5 years, PANSS psychosis and CGI-S score of 4 or more. N = 400. Sex: 292 M, 108 F. Age: 16-40 years (mean = 24.5 years). History: duration ill mean = 1.08 years, age at onset 23.5 years. Setting: in- and outpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Global State: CGI. Mental State: PANSS total, PANSS positive subscore, PANSS negative subscore, depression Calgary depression scale. Adverse effects: open interviews, death (suicide attempt, suicide, EPS (akathisia, akinesia, use of antiparkinson medication, laboratory (cholesterol, fasting glucose, prolactin) , prolactin associated side effects (amenorrhoea, galactorrhoea, gynaecomastia, sexual dysfunction), sedation, insomnia, dry mouth, orthostatic faintness, constipation, sialorrhoea, skin rash, gynaecomastia, urinary hesitancy, incontinence, weight gain (BMI, waist circumference) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, no further details. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
High risk | The attrition was high (70.3.%). The primary analysis was based on a mixed effect model, secondary approaches used the last-observation-carried forward approach or included only study completers. Nevertheless, it is unclear whether any statistical method can account for such a high attrition |
| Free of selective reporting? | High risk | Adverse events were presented only in case of moderate or worse severity |
| Free of other bias? | High risk | The study was sponsored by the manufacturer of quetiapine. |
| Methods | Allocation: random, no further details. Blindness: double, double-dummy protocol. Duration: 29 weeks. Design: parallel. Location: multicentre. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia (n = 93) or schizoaffective disorder (n = 14), treatment resistance, moderate severity score on BPRS or SANS. N = 107. Sex: 84 M, 23 F. Age: 18-60 years (mean = 42 years). History: duration ill n.i., age at onset mean clozapine = 23 years, mean risperidone = 22 years. Setting: in- and outpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Global State: CGI. General Mental State: BPRS total score, SANS. Cognitive functioning: Spatial working memory performance, social skill and problem solving assessment. Adverse effects: TESS, laboratory (hematology). Unable to use: SANS (modified version). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, double-dummy protocol. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
High risk | The overall attrition was high 63.9%. The analysis was based on mixed effect models, but it is unclear whether any statistical method can account for such a high dropout rate |
| Free of selective reporting? | High risk | Outcome reporting was incomplete. Quote: “…adverse events data have been reported elsewhere (unpublished study of N.R. Schooler et al.) |
| Free of other bias? | Low risk | Review authors did not find other sources of bias. Probably yes |
| Methods | Allocation: random, no further details. Blindness: double, no further details. Duration: 8 weeks (last 4 weeks observed). Design: parallel. Location: single centre. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia disorganised (n = 23), paranoid (n = 10), undifferentiated (n = 34). N = 77. Sex: 39 M, 38 F. Age: 28-84 years (mean = 59.9 years). History: duration ill mean = 34.51 years, age at onset: not reported. Setting: inpatient. |
|
| Interventions |
|
|
| Outcomes | Mental State: PANSS total score, PANSS positive subscore, PANSS negative subscore. Cognitive functioning: digit span distractibility test. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, no further details. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
High risk | There was no data on attrition available. |
| Free of selective reporting? | High risk | Adverse events were not reported. Numbers on use of antiparkinson medication have not been presented |
| Free of other bias? | High risk | There was no wash-out period. The previous antipsychotic treatment was gradually tapered over 4 weeks. Thus, during a period of 4 weeks the participants were on two drugs |
| Methods | Allocation: random, no further details. Blindness: double, no further details. Duration: 6 weeks. Design: parallel. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia, schizophreniform disorder, schizoaffective disorder, delusional disorder or shared psychotic disorder. N = 36. Age: 65 years or more. Sex: not described. Location: not described. Setting: not described. History: duration ill, age at onset: not described. Excluded: conditions such as: not described. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: adverse events. Cognitive functioning: MMSE. Adverse effects: open interviews. Unable to use: Mental state, PANSS total score, BPRS total score: no usable data. Cardiac effects (QTc), laboratory tests: no data. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, no further details. Whether blinding was successful has not been examined, but both compounds differ in side effects. This may be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
High risk | Numbers of participants leaving the study early were only reported for the category “due to adverse effects”. The statistical method used to account for incomplete data was not described |
| Free of selective reporting? | High risk | The study is only available as an abstract. The data for primary outcomes were either not available or only available in percentage change without a standard deviation |
| Free of other bias? | High risk | The study was sponsored by the manufacturer of amisulpride. Whether there was a baseline imbalance could not be judged due to insufficient data |
| Methods | Allocation: random, no further details. Blindness: double, identical capsules. Duration: 8 weeks. Design: parallel. Location: multicentre. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia disorganised, paranoid or undifferentiated, BPRS of 36 or more. N = 228. Age: 18-65 years (mean amisulpride = 36 years, mean risperidone = 37 years). Sex: 137 M, 91 F. Setting: in- and outpatient. History: duration ill mean amisulpride = 7.9 years, mean risperidone = 10.2 years, age at onset n.i |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Global State: CGI. Mental State: BPRS total score, PANSS positive subscore, BPRS positive subscore, PANSS negative subscore. General functioning: SOFAS. Adverse effects: open interviews, cardiac effects (arrhythmia, ECG, QTc interval of > 500 ms), EPS (akathisia, extrapyramidal symptoms, hyperkinesia, parkinsonism, rigor, tremor, use of antiparkinson medication, AIMS, SAS), prolactin associated side effects, weight Unable to use: General Functioning - SOFAS: no data. |
|
| Notes | Individual BPRS factors are also available. | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, identical capsules. Whether blinding was successful has not been examined, but both compounds in side effects. This may be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
High risk | The rate of leaving the study early was moderate (30.3%). The statistical analysis was based on a last-observation-carried-for-ward method to account for people leaving the study early. It assumes that a participant who discontinued the study would not have had a change of his condition if he had remained in the study. This assumption can obviously be wrong |
| Free of selective reporting? | High risk | Data on general functioning (secondary outcome) were not available. Only those adverse events that occurred in at least 5% of the participants were reported. This procedure can miss rare, but important adverse events |
| Free of other bias? | High risk | The study was sponsored by the manufacturer of amisulpride. |
| Methods | Allocation: random, no further details. Blindness: double, identical capsules. Duration: 4 weeks. Design: parallel. Location: multicentre. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia (n = 289) or schizoaffective disorder (n = 115), hospitalised due to an acute relapse, response to previous antipsychotic treatment other than clozapine, PANSS of 60 or more. N = 404. Age: 18-65 years (mean = 38.9 years). Sex: 283 M, 121 F. History: duration illness not reported, age at onset not reported. Setting: inpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Global State: CGI. Mental State: PANSS total score, PANSS positive subscore, PANSS negative subscore. Adverse effects: At least one adverse effect, cardiac effects (ECG, QTc prolongation, QTc abnormalities in ms), extrapyramidal side effects (akathisia, dystonia, parkinsonism, rigor, tremor, AIMS, BAS, SAS), prolactin-associated side effects (dysmenorrhoea, increase of prolactin level above 23 ng/ml, change from baseline in ng/ml), sedation, weight gain |
|
| Notes | Note: There is a placebo group (n = 103), which is not relevant for this review | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, identical capsules. Whether blinding was successful has not been examined, but both compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by lack of blinding |
| Incomplete outcome data addressed? All outcomes |
High risk | The overall drop-out rate was high (36. 9%). The LOCF method was used to account for people leaving the study early. It assumes that a participant who discontinued the study would not have had a change of his condition if he had remained in the study. This assumption can obviously be wrong |
| Free of selective reporting? | High risk | For efficacy outcomes standard deviations or standard errors had not been indicated which had to be back calculated from P values. Only adverse events with an incidence of more than 5% were reported. This procedure may have missed important adverse events |
| Free of other bias? | Unclear risk | The study was sponsored by the manufacturer of aripiprazole. The study used a fixed-dose regimen. It is difficult to say whether the chosen doses were appropriate |
| Methods | Allocation:random, no further details. Blindness: double, identical capsules. Duration: 6 weeks (2 weeks observed). Design: parallel. Location: multicentre. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia (n = 341) disorganised, paranoid or undifferentiated or schizoaffective disorder (n = 30) plus (n = 11), CGI-S of 5 or more, recent exacerbation. N = 382. Sex: 251 M, 131 F. Age: 18-65 years (mean = 34.8 years). History: duration ill, age at onset: not reported. Setting: inpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason. Global State: CGI. Mental State: PANSS total score, PANSS positive subscore, PANSS negative subscore, Depression Hamilton Rating Scale for Depression, Readiness for Discharge Questionnaire. Satisfaction of treatment: Study Medication Satisfaction. Adverse effects: open interviews, death (natural cause), cardiac effects (ECG), EPS (akathisia, rigor, AIMS, BAS, SAS), prolactin associated side effects (amenorrhoea, decreased libido), sedation, headache , insomnia, constipation, laboratory (prolactin) Unable to use: BAS: no data. Cardiac effects - QTc-prolongation: no data. |
|
| Notes | There was a placebo group (n = 73). | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Low risk | Quote: were assigned using a centralised interactive voice response system. Probably done |
| Blinding? subjective outcomes |
Unclear risk | Double, identical capsules. Whether blinding was successful has not been examined, but both compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
Low risk | The overall attrition was rather low (12%). The LOCF method was used to account for people leaving the study early. It assumes that a participant who discontinued the study would not have had a change of his condition if he had remained in the study. This assumption can obviously be wrong. Nevertheless, due to the overall low attrition it is unlikely that the results have been affected |
| Free of selective reporting? | High risk | Data on some adverse effects were not available. Only those adverse events that occurred in at least 10% of the participants were reported. This procedure can miss rare, but important adverse events |
| Free of other bias? | High risk | The study was sponsored by the manufacturer of risperidone. |
| Methods | Allocation: random, computer-generated randomisation. Blindness: double, no further details. Duration: 54 weeks. Design: parallel. Location: multicentre. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia, in early phase. N = 65. Sex: 46 M, 19 F. Age: 18-65 years (mean haloperidol = 28.83 years, mean olanzapine = 26.01 years, mean risperidone = 31.77 years). History: duration ill mean haloperidol = 2.45 years, mean olanzapine = 2.79 years, mean risperidone = 2.67 years, age at onset mean haloperidol = 24.25 years, mean olanzapine = 23.37 years, mean risperidone = 28.86 years. Setting: outpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Mental State: PANSS positive subscore, PANSS negative subscore. Cognitive functioning: Cognitive test battery (finger tapping, digit span, Peabody picture vocabulary test, trailmaking test). Adverse effects: EPS (use of antiparkinson medication, ESRS) Unable to use: Cognitive Functioning (no overall score). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random, computer-generated randomisation. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, no further details. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
High risk | The overall attrition was high (54.8%). The LOCF method was used to account for people leaving the study early. It assumes that a participant who discontinued the study would not have had a change of his condition if he had remained in the study. This assumption can obviously be wrong |
| Free of selective reporting? | Low risk | The study focused on neuropsychological changes, but data for efficacy and some side effects were also presented. Probably no bias |
| Free of other bias? | High risk | The study was sponsored by the manufacturer of olanzapine. |
| Methods | Allocation:random, ball drawing out of box. Blindness: double. Duration: 12 weeks. Design: parallel. Location: single centre. |
|
| Participants | Diagnosis: schizophrenia (CCMD-3). N:120. Sex: not reported. Age mean: 29.45 History duration ill: clozapine = 6.2 years, risperidone = 6.4 years. Setting: outpatient. |
|
| Interventions |
|
|
| Outcomes | Mental State: PANSS positive subscore, PANSS negative subscore). Quality of life: General quality of life inventory. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Allocation: random, ball drawing out of a covered box. Probably yes |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, no further details. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
High risk | Data on leaving the study early were not provided. |
| Free of selective reporting? | High risk | Secondary outcomes were poorly reported. |
| Free of other bias? | Unclear risk | The allowed dose ranges were not indicated. |
| Methods | Allocation: random, no further details. Blindness: double, identical capsules. Duration: 12 weeks. Design: parallel. Location: not reported. |
|
| Participants | Diagnosis: (DSM-IV or ICD-10) schizophrenia, predominant negative symptoms, CGI of 4 or more, PANSS negative subscore of 21 or more. N = 44. Sex: 27 M, 17 F. Age: mean quetiapine = 30.6 years, mean risperidone = 39.3 years. History: duration ill mean quetiapine = 5.4 years, mean risperidone = 2.5 years, age at onset mean quetiapine = 25.3 years, mean risperidone = 36.9 years. Setting: partially in- and outpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Mental State: PANSS total score, PANSS positive subscore, PANSS negative subscore, SANS total score. Cognitive functioning: auditory verbal memory test, Trail Making Test, Wechsler visual memory scale. Adverse effects: open interviews, cardiac effects (ECG), EPS (akathisia, parkinsonism, use of antiparkinson medication, SAS), sedation, headache, nausea, insomnia, dizziness, weight gain, laboratory (prolactin) Unable to use: SANS total score: no data. Prolactin change from baseline in ng/ml: no data. Cardiac effects: no data. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, identical capsules. Whether blinding was successful has not been examined, but both compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
High risk | The overall attrition was high (45.2%). The data were analysed using both a LOCF method and a study completer approach. Nevertheless, it is questionable whether any statistical method can account for such a high attrition |
| Free of selective reporting? | High risk | Data on negative symptoms (SANS) and some adverse effects were not available |
| Free of other bias? | High risk | The study was sponsored by the manufacturer of quetiapine. |
| Methods | Allocation: random, no further details. Blindness: single, rater-blinded. Duration: 16 weeks. Design: parallel. Location: multicentre. |
|
| Participants | Diagnosis: (DSM-IV) first episode schizophrenia (n = 84), schizophreniform disorder (n = 19) or schizoaffective disorder (n = 9) (of intent-to-treat population). N = 120. Sex: 78 M, 34 F (of intent-to-treat population). Age: 16-40 years (mean = 23.3 years) (of intent-to-treat population). History: duration ill mean = 2.2 years (of intent-to-treat population), age at onset mean = 20.7 years (of intent-to-treat population). Setting: not reported. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: inefficacy. Global State. Adverse effects: EPS (parkinsonism, use of antiparkinson medication, Simpson-Angus) , weight gain Unable to use: Leaving the study early: incomplete data. Weight gain: no data. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Single-blind, rater-blinded. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
Unclear risk | The overall attrition was possibly acceptable (28%), but data on leaving the study early were incompletely reported. Eight patients were excluded from the analysis for various reasons. The statistical analysis was based on mixed model approach |
| Free of selective reporting? | High risk | Data on the general mental state (PANSS total score) were not presented. The data available for adverse effects were incomplete. Data on weight gain were missing |
| Free of other bias? | Unclear risk | Quote: “.. the study was designed to detect differences in our primary analysis at alpha = 0.05 with 80% power based upon 130 subjects, the stability analysis included only 47 subjects and therefore might lack adequate power.” |
| Methods | Allocation: random, no further details. Blindness: single (rater-blinded). Duration: 16 weeks (8 weeks observed). Design: parallel. Location: multicentre. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia, PANSS total score of 70 or more, PANSS positive subscore of 4 or more on at least 2 items. N = 75. Sex: M, F: not reported. Age: 18-65 years. History: duration ill not reported, age at onset not reported. Setting: inpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason. Mental State: PANSS total score, BPRS hostility cluster score, PANSS positive subscore, PANSS negative subscore). Adverse effects: EPS (BAS, SAS), weight gain. Unable to use: Mental State - PANSS total score, PANSS positive subscore, PANSS negative subscore: no usable data |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Single: rater-blind. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
Unclear risk | The overall attrition was moderate (18.6%) . The LOCF method was used to account for people leaving the study early. It assumes that a participant who discontinued the study would not have had a change of his condition if he had remained in the study. This assumption can obviously be wrong |
| Free of selective reporting? | High risk | Efficacy data (PANSS) were only presented as percentage change without standard deviations, standard errors, P values or ranges. Only interim data after recruitment of half the patients have been presented |
| Free of other bias? | High risk | The study was sponsored by the manufacturer of quetiapine. |
| Methods | Allocation: random, no further details. Blindness: double, no further details. Duration: 26 weeks. Design: parallel. Location: multicentre. |
|
| Participants | Diagnosis: (DSM-IV) chronic schizophrenia disorganised (n = 37), paranoid (n = 227), residual (n = 19) or undifferentiated (n = 27), PANSS between 60 and 120, recent worsening of symptoms. N = 310. Age: 18-65 years (mean = 38.4 years). Sex: 170 M, 140 F. Setting: in- and outpatient. History: duration ill mean = 11.8 years, age at onset not described |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Global State: CGI, relapse. Mental State: PANSS total score, BPRS total score, PANSS positive subscore, PANSS negative subscore, SANS total score. General functioning: SOFAS total score. Adverse effects: open interviews, death (natural causes, suicide), EPS (akathisia, hyperkinesia, parkinsonism, tremor, use of antiparkinson medication, AIMS, SAS), prolactin associated side effects (amenorrhea, galactorrhea, sexual dysfunction), sedation, seizures, weight |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, no further details. Whether blinding was successful has not been examined, but both compounds differ in side effects. This may be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
High risk | The rate of participants leaving the study early was high (40%). The LOCF method was used to account for people leaving the study early. It assumes that a participant who discontinued the study would not have had a change of his condition if he had remained in the study. This assumption can obviously be wrong |
| Free of selective reporting? | High risk | Only those adverse events that occurred in at least 5% of the participants were reported. This procedure can miss rare, but important adverse events |
| Free of other bias? | High risk | The study was sponsored by the manufacturer of amisulpride. |
| Methods | Allocation: random, computer-generated randomisation. Blindness: double, no further details. Duration: 8 weeks. Design: parallel. Location: multicentre. |
|
| Participants | Diagnosis: children and adolescents with (K-SADS-P or DSM-IV) schizophrenia, schizoaffective disorder, schizophreniform disorder, delusional disorder, major depression with psychotic features or bipolar affective disorder with psychotic features, schizophrenia spectrum (n = 26), affective disorders (n = 24) subjects selected because of prominent positive psychotic symptoms (of intent-to-treat population). N= 51. Sex: 30 M, 21 F. Age: 8-19 years (mean =14.8 years). History: duration ill not reported, age at onset mean = 12.4 years. Setting: in- and outpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Global State: CGI. Mental State: BPRS-C total score, CPRS. Adverse effects: open interviews, cardiac effects (QTc, vital signs), EPS (akathisia, use of antiparkinson medication, Simpson-Angus), prolactin associated side effects (amenorrhea, galactorrhea, gynaecomastia), sedation, gastrointestinal malfunction, weight (BMI) , laboratory (glucose, prolactin) |
|
| Notes | There is a Haloperidol Group (N = 15). | |
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random, computer-generated randomisation. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, no further details. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
High risk | The overall attrition was high (33.3%). The LOCF method was used to account for people leaving the study early. It assumes that a participant who discontinued the study would not have had a change of his condition if he had remained in the study. This assumption can obviously be wrong |
| Free of selective reporting? | Low risk | No evidence for selective reporting. |
| Free of other bias? | High risk | Quote: “…this study has a number of limitations including limited sample size, differences in the diagnosis of participants, use of concomitant medication, variations in age and pupertal status” |
| Methods | Allocation: random, 2 steps of randomisation before and after availability of ziprasidone, subjects received other medication than in previous phase 1 treatment. Re-randomised. Blindness: double, identical capsules. Duration: 26 weeks. Design: parallel. Location: not reported. |
|
| Participants | Diagnosis: (DSM-IV) chronic schizophrenia. N = 444. Sex: 308 M, 136 F. Age: 18-65 years (mean olanzapine = 40.0 years, mean quetiapine = 40.1 years, mean risperidone = 41.8 years, mean ziprasidone = 41.3 years). History: duration ill not reported, age at onset not reported. Setting: in- and outpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Global State: CGI. Mental State: PANSS total score. Adverse effects: open interviews, death (suicide), EPS (akathisia), cardiac effects (ECG), prolactin-associated side effects, weight gain, laboratory (prolactin, glucose, cholesterol) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, 2 steps of randomisation before and after availability of ziprasidone, subjects received other medication than in previous phase 1 treatment. Re-randomised |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, identical capsules. Whether blinding was successful has not been examined, but both compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
High risk | The overall attrition was high (72.5%). The main statistical analysis used a mixed effects model approach. It is unclear whether any statistical method can account for such high rates of leaving the study early |
| Free of selective reporting? | High risk | Use of antiparkinson medication was permitted but data on this outcome have not been presented |
| Free of other bias? | Unclear risk | Patients had a history of former intolerance to atypical antipsychotic treatment but baseline data on this was not provided |
| Methods | Allocation: random, no further details. Blindness: double, no further details. Duration: 6 weeks. Design: parallel. Location: not reported. |
|
| Participants | Diagnosis: schizophrenia or schizoaffective disorder, first episode. N = 42. Sex: not reported. Age: not reported. History: duration ill not reported, age at onset not reported. Setting: inpatient. |
|
| Interventions |
|
|
| Outcomes | Mental State: PANSS total score. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, no further details. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
High risk | Data on subjects leaving the study early were not available. |
| Free of selective reporting? | High risk | Allowed study medication dose ranges were not indicated. A publication was not available |
| Free of other bias? | Unclear risk | Insufficient information. |
| Methods | Allocation: random, no further details. Blindness: double, no further details. Duration: 28 weeks. Design: parallel. Location: multicentre. Setting: in- and outpatient. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia (n = 277), schizophreniform disorder or schizoaffective disorder, BPRS score of 42 or more. N = 339. Sex: 220 M, 119 F. Age: 18-65 years (mean = 36.21 years). History: duration ill not reported, age at onset mean = 23.7 years |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Mental State: PANSS total score, BPRS total score, PANSS positive subscore, PANSS negative subscore, SANS total score. Quality of life: QLS total score. Adverse effects: open interviews, cardiac effects (ECG), death (any reason, suicide attempt), EPS (akathisia, akinesia, dyskinesia, dystonia, extrapyramidal symptoms, parkinsonism, tremor, use of antiparkinson medication), Prolactin associated side effects (abnormal ejaculation, abnormally high prolactin value, amenorrhea, decreased libido, galactorrhea, gynaecomastia, impotence), sedation, backache, blurred vision, breathing difficulties, early wakening, nightmares, seizures, weight gain, laboratory (glucose, white blood cell count) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, no further details. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
High risk | The overall attrition was high 47.5 %. The LOCF method was used to account for people leaving the study early. It assumes that a participant who discontinued the study would not have had a change of his condition if he had remained in the study. This assumption can obviously be wrong |
| Free of selective reporting? | High risk | Adverse effects were only reported in the case of a significant difference between groups. Important side effects may have been missed by this procedure |
| Free of other bias? | High risk | The study was sponsored by the manufacturer of olanzapine. |
| Methods | Allocation: random, no further details. Blindness: double, no further details. Duration: 6 weeks. Design: parallel. Location: not reported. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia, schizophreniform disorder or schizoaffective disorder, cannabis positive last month olanzapine (n = 20), risperidone (n = 23). N = 131. Sex: 106 M, 25 F. Age: mean olanzapine = 24.4 years, mean risperidone = 25.1 years. History: duration ill not reported, age at onset not reported. Setting: not reported. |
|
| Interventions |
|
|
| Outcomes | Quality of life: Subject well being. Adverse effects: EPS (BAS). Cannabis use. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, no further details. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
High risk | Data on leaving the study early were not provided. |
| Free of selective reporting? | High risk | Outcome reporting was incomplete, standard deviations were not published |
| Free of other bias? | Unclear risk | Additional usage of cannabis. |
| Methods | Allocation: random, no further details. Blindness: double, identical capsules. Duration: 14 weeks. Design: parallel. Location: multicentre. |
|
| Participants | Diagnosis: (DSM-IV) chronic schizophrenia (n = 135) or schizoaffective disorder (n = 22), suboptimal response to previous treatment, PANSS of 60 or more. N = 167. Sex: 133 M, 24 F (of intent-to-treat population). Age: 18-60 years (mean = 40.8 years) (of intent-to-treat population). History: duration ill mean = 19.5 years (of intent-to-treat population), age at onset not reported. Setting: inpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early. any reason, adverse events, inefficacy. Mental State: PANSS total score, PANSS positive subscore, PANSS negative subscore. Quality of life: Quality of life scale, Nurses’observation scale for inpatient evaluation. Cognitive Functioning: Global Neurocognitive Score. Adverse effects: EPS (Use of antiparkinson medication, ESRS), seizures, weight gain, laboratory (cholesterol, glucose, prolactin, white blood cell count) Unable to use: Quality of life scale: no data. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, identical capsules. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
High risk | The overall attrition was high (41.7%). The LOCF method was used to account for people leaving the study early. It assumes that a participant who discontinued the study would not have had a change of his condition if he had remained in the study. This assumption can obviously be wrong |
| Free of selective reporting? | High risk | Some outcomes were reported on subgroup from the entire sample. Quality of life data were not presented |
| Free of other bias? | High risk | Quote: “The olanzapine arm was added in November 1997 and required a modified randomization procedure”…It entails the potential for a bias that could be manifested as a cohort effect.” |
| Methods | Allocation: random, computer-generated randomisation. Blindness: single, rater-blinded. Duration: 10 weeks. Design: parallel. Location: multicentre. Setting: in- and outpatient (initially inpatient). |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia, resistance to previous treatment. N = 20. Sex: 10 M, 9 F (of intent-to-treat population). Age: 24-55 years (mean clozapine = 35.7 years, mean risperidone = 36.8 years) (of intent-to-treat population). History: duration ill mean clozapine = 12.6 years, mean risperidone = 13.1 years (of intent-to-treat population), age at onset not reported. Setting: in- and outpatient (initially inpatient). |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Mental State: PANSS total score, PANSS positive subscore, PANSS negative subscore. General functioning: GAF, social functioning scale, patient global impression scale. Satisfaction with treatment: Drug attitude inventory. Adverse effects: death (natural causes, suicide), EPS (use of antiparkinson medication), sedation, laboratory (white blood cell count) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random, computer-generated randomisation. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Single, rater-blind. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
High risk | The overall attrition was high (35%). The LOCF method was used to account for people leaving the study early. It assumes that a participant who discontinued the study would not have had a change of his condition if he had remained in the study. This assumption can obviously be wrong |
| Free of selective reporting? | Low risk | No evidence for selective reporting. |
| Free of other bias? | High risk | The number of participants included was rather low. Quote: “…readers have to bear in mind, that the statistical power of this study may hide some clinically relevant differences in drug efficacy.” |
| Methods | Allocation: random, no further details. Blindness: double, identical capsules. Duration: 22 weeks (last 12 weeks observed). Design: parallel. Location: multicentre. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia (n = 24) or schizoaffective disorder (n = 12). N = 36. Sex: 17 M, 19 F. Age: mean = 47.0 years. History: duration ill not reported, age at onset not reported. Setting: outpatient. |
|
| Interventions |
|
|
| Outcomes | Mental State: PANSS total score, PANSS positive subscore, PANSS negative subscore. Adverse effects: EPS (SAS), weight gain. Unable to use: Leaving the study early: no data. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, identical capsules. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
High risk | Data on leaving the study early were not available. |
| Free of selective reporting? | High risk | Standard deviations for the primary outcome were not available |
| Free of other bias? | High risk | Dose ranges were not indicated. The study was sponsored by the manufacturer of risperidone |
| Methods | Allocation: random, 33 participants were assigned to a three-arm randomisation (1:1:1, blocks of 15) and 18 participants with a history of adverse experiences with haloperidol were assigned to a two-arm randomisation (1:1) for risperidone and olanzapine only. Blindness: double, no further details. Duration: 8 weeks. Design: parallel. Location: multicentre. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia or schizoaffective disorder. N = 51. Sex: 43 M, 8 F. Age: 18-60 years (mean = 48.8 years). History: duration ill not reported, age at onset not reported. Setting: not reported. |
|
| Interventions |
|
|
| Outcomes | Neurological functioning: Prepulse inhibition, EMG. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, 33 participants were assigned to a three-arm randomisation (1:1:1, blocks of 15) and 18 participants with a history of adverse experiences with haloperidol were assigned to a two-arm randomisation (1:1) for risperidone and olanzapine only |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, no further details. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
High risk | Data on leaving the study early were not available. |
| Free of selective reporting? | High risk | Efficay outcomes such as the general mental state (PANSS total score) were not presented |
| Free of other bias? | High risk | The study was sponsored by the manufacturer of risperidone. |
| Methods | Allocation: random, no further details. Blindness: double, no further details. Duration: 8 weeks. Design: parallel. Location: multicentre. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia, PANSS of 60 or more, CGI-S of 4 or more. N = 673. Sex: 510 M, 163 F. Age: 18-65 years (mean quetiapine = 40.2 years, mean risperidone = 39.6 years). History: duration ill, age at onset: not reported. Setting: in- and outpatient, initially inpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Global State: CGI. Mental State: PANSS total score, PANSS positive subscore, PANSS negative subscore. Adverse effects: open interviews, cardiac effects (QTc), death (natural causes, suicide), EPS (akathisia, dystonia, parkinsonism, use of antiparkinson medication, AIMS, BAS, SAS), sedation, prolactin associated side effects (dysmenorrhoea, galactorrhea, sexual dysfunction) weight gain, laboratory (cholesterol, glucose, prolactin, white blood cell count) |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Double, no further details. Whether blinding was successful has not been examined, but both compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
High risk | The overall attrition was high (52.1%). The LOCF method was used to account for people leaving the study early. It assumes that a participant who discontinued the study would not have had a change of his condition if he had remained in the study. This assumption can obviously be wrong. Data on study completers were also available. Nevertheless it is unclear whether any statistical method can account for such a degree of attrition |
| Free of selective reporting? | High risk | Only those adverse events that occurred in at least 5% of the participants were reported. This procedure can miss rare, but important adverse events |
| Free of other bias? | High risk | The study was sponsored by the manufacturer of quetiapine. |
| Methods | Allocation: random, no further details. Blindness: single, rater-blinded. Duration: 8 weeks. Design: parallel. Location: single centre. |
|
| Participants | Diagnosis: (CCMD-2) schizophrenia. N = 40. Sex: 23 M, 17 F. Age: mean clozapine = 27.6 years, mean risperidone = 29 years. History: duration ill mean clozapine = 2.7 years, mean risperidone = 3.2 years, age at onset: not reported. Setting: inpatient. |
|
| Interventions |
|
|
| Outcomes | Mental State: SANS. Advers effects: EPS (akathisia, tremor, treatment emergent symptom scale), vital signs, dry mouth hypersalivation, weight gain, sedation | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors’ judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | Random, no further details. |
| Allocation concealment? | Unclear risk | No further details. |
| Blinding? subjective outcomes |
Unclear risk | Single, rater-blind. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? objective outcomes |
Low risk | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
Low risk | No participant left the study early. |
| Free of selective reporting? | Low risk | No clear evidence for selective reporting. |
| Free of other bias? | Unclear risk | The description of blinding differed between the abstract (double-blind) and the method section (single-blind) |
Diagnostic tool
DSM III-R and DSM-IV - Diagnostic Statistical Manual version 3 Revised and version 4.
ICD 10 - The International Statistical Classification of Diseases and Related Health Problems.
BMI - Body Mass Index.
Rating Scales:
Global rating scales:
CGI - Clinical Global Impressions.
CGI-S - Clinical Global Impression-Severity.
CGI-I - Clinical Global Impression-Improvement.
Mental state:
BPRS - Brief Psychiatric Rating Scale.
MADRS - Montgomery-Asberg Depression Rating Scale.
MMSE - Wiing Mini Mental State Examination.
PANSS - Positive and Negative Syndrome Scale.
SANS - Scale for the Assessment of Negative Symptoms.
Side effects:
AIMS - Abnormal Involuntary Movement Scale.
BAS - Barnes Akathisia Scale.
BMI - Body mass index.
ESRS - Extrapyramidal Syndrome Rating Scale.
HAS - Hillside Akathisia Scale.
SAS - Simpson-Angus Index - for neurological side effects.
TESS - Treatment Emergent Symptom Scale.
UKU - Udvalg for kliniske ndersogelser Side Effect Rating Scale -side effect rating scale.
Quality of Life:
QoL - Quality of Life Scale.
SWN -Subjective Well-being List.
CCMD-2:
ECG:
EEG:
EPS:
GAF:
LOCF: last observation carried forward
QLS:
QTc:
SOFAS:
TESS:
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akdede 2006 |
|
| Almond 1999 |
|
| Alvarez 2006 |
|
| Alvarez-Jimenez 2006 |
|
| Antonova 2005 |
|
| Apiquian 2003 |
|
| Aquila 2000 |
|
| Ascher-Svanum 2006 | 1. Allocation: not randomised, cohort study. |
| Bai 2003 |
|
| Baloescu 2006 | 1. Allocation: not randomised, controlled trial. |
| Basson 2001 |
|
| Beasley 2001 |
|
| Beasley 2003 |
|
| Bera 2001 |
|
| Beuzen 2005 |
|
| Bitter 2004 | 1. Allocation: not randomised, cohort study. |
| Boylan 2004 |
|
| Briken 2002 | 1. Blindness: open-label. |
| Byerly 2006 |
|
| Cai 2000 | 1. Blindness: open-label. |
| Canas 2006 | 1. Allocation: not randomised. |
| Cao 2001 | 1. Allocation: inadequate randomisation. 2: Blindness: not mentioned. |
| Cao 2003 | 1. Blindness: open-label. |
| Cao 2005 | 1. Blindness: open-label. |
| Cha 2002 | 1. Blindness: open-label. |
| Chaudhry 2006 |
|
| Chen 1999 |
|
| Chen 2002 |
|
| Chen 2003 |
|
| Chen 2004 |
|
| Chen 2004a |
|
| Chen 2005 |
|
| Chen 2005a |
|
| Chen 2005b |
|
| Cheng 2002 |
|
| Chou 1999 |
|
| Chowdhury 1999 |
|
| Citrome 2004 |
|
| Ciudad 2004 |
|
| Conley 1999 |
|
| Cornblatt 2002 |
|
| Crespo-Facorro 2006 |
|
| Cui 2002 |
|
| Czekalla 2001 |
|
| David 1999 |
|
| David 2000 |
|
| De Haan 2002 |
|
| Ding 2004 |
|
| Ding 2005 |
|
| Dossenbach 2005 |
|
| Du 2003 |
|
| Du 2004 |
|
| Du 2004a |
|
| Du 2005 |
|
| Earnst 1999 | 1. Allocation: not randomized. |
| Ertugrul 2006 | 1. Allocation: not randomized, controlled trial. |
| Estrella 1996 |
|
| Fan 2003 |
|
| Feng 2004 |
|
| Gan 1999 |
|
| García 2006 |
|
| Ge 2004 |
|
| Goldberg 2000 |
|
| Guan 2005 |
|
| Guo 2001 |
|
| Guo 2003 |
|
| Hagger 1997 |
|
| Han 2000 |
|
| Han 2005 |
|
| Harrigan 2004 |
|
| He 2005 |
|
| Heresco-Levy 2005 |
|
| Hou 2001 |
|
| Hrdlicka 2001 | 1. Allocation: not randomized, cohort study. |
| Hu 2000 |
|
| Hu 2005 |
|
| Huang 2000 |
|
| Huang 2001 |
|
| Huber 2004 |
|
| Janssen-Ortho 2006 |
|
| Jones 2006 |
|
| Karow 2002 |
|
| Keks 2006 |
|
| Kelemen 2006 |
|
| Kim 2004 | 1. Allocation: not randomized, controlled trial. |
| Knegtering 2004 |
|
| Koenigsberg 2000 |
|
| Kolff 2000 |
|
| Kong 2001 |
|
| Kores 2003 |
|
| Lee 2005 |
|
| Lee 2006 |
|
| Lei 2002 |
|
| Lewis 2004 |
|
| Li 2000 |
|
| Li 2001 |
|
| Li 2003 |
|
| Li 2003a |
|
| Li 2004 |
|
| Li 2005 |
|
| Liang 2005 |
|
| Liao 2004 |
|
| Lin 2005 | 1. Allocation: not randomized (inadequate randomization). |
| Lipkovich 2005 |
|
| Littrell 1999 |
|
| Liu 1999 |
|
| Liu 2001 |
|
| Liu 2003 |
|
| Liu 2003a |
|
| Liu 2004 |
|
| Liu 2004a |
|
| Liu 2004b |
|
| Liu 2004c |
|
| Liu 2005 |
|
| Liu 2005a |
|
| Liu 2005c |
|
| Loza 2005 |
|
| Lu 2002 |
|
| Lu 2004 |
|
| Lu 2005 |
|
| Ma 1999 |
|
| Mai 2005 |
|
| Malla 2004 | 1. Allocation: not randomized, controlled trial. |
| Malyarov 1999 | 1. Allocation: not mentioned. |
| Malykhin 2003 |
|
| Mao 2000 |
|
| Mazurek 2003 |
|
| Mei 2001 |
|
| Meltzer 2002 |
|
| Meltzer 2004 |
|
| Mintzer 2004 |
|
| Mohr 2000 |
|
| Mu 2002 |
|
| Mullen 2001 |
|
| Musil 2006 |
|
| Naber 2001 | 1. Allocation: not randomized, pooled analysis. |
| Naber 2002 |
|
| Nan 2001 |
|
| Ni 2001 |
|
| Nicholls 2003 |
|
| Opjordsmoen 2000 |
|
| Ortega-Soto 1997 |
|
| Pan 2004 | 1.Allocation: not randomized (inadequate randomization). |
| Pan 2004a |
|
| Peng 2001 |
|
| Peng 2004 |
|
| Perro 1999 |
|
| Peuskens 2004 |
|
| Qi 2004a |
|
| Qian 2004 |
|
| Qin 2005 |
|
| Rabinowitz 2005 |
|
| Ren 2000 |
|
| Ren 2004 |
|
| Roerig 2004 |
|
| Ryu 2006 |
|
| Sajatovic 2002 |
|
| Shao 1999 |
|
| Sheng 2003 |
|
| Sheng 2005 |
|
| Shi 2000 |
|
| Shi 2001 |
|
| Shi 2004 |
|
| Simpson 2004 |
|
| Sowell 2002 |
|
| Su 2004 |
|
| Su 2005 | 1. Allocation: not randomized. |
| Sun 2000 | 1. Allocation: not randomized. |
| Sun 2001 |
|
| Swanson 2006 |
|
| Tandon 2006 |
|
| Tang 2002 |
|
| Tang 2002a | 1. Allocation: inadequate randomisation. |
| Tian 2005 |
|
| Tong 2005 |
|
| Tunis 2006 |
|
| Van Bruggen 2003 |
|
| Vaughan 2000 |
|
| Wang 2000a |
|
| Wang 2001 |
|
| Wang 2002 |
|
| Wang 2002a |
|
| Wang 2003 |
|
| Wang 2003a |
|
| Wang 2005 |
|
| Wang 2005a |
|
| Wang 2005b |
|
| Weickert 2003 |
|
| Weng 1998 |
|
| Wolf 2002 |
|
| Wolf 2005 |
|
| Wu 2001 |
|
| Wu 2002 |
|
| Wu 2004 |
|
| Wu 2006 |
|
| Wyszogrodzka 2006 |
|
| Xiao 2003 |
|
| Xie 2005 |
|
| Xin 2001 |
|
| Xu 2001 |
|
| Xu 2005 |
|
| Yagdiran 2000 |
|
| Yamashita 2005 |
|
| Yan 2002 |
|
| Yang 1998 |
|
| Yang 2003 |
|
| Yang 2004 |
|
| Yang 2004a |
|
| Ye 2005 |
|
| Ye 2005a |
|
| Yin 2002 |
|
| Yin 2004 |
|
| Yu 1999 |
|
| Yu 2002 |
|
| Yu 2005 |
|
| Yue 2004 |
|
| Zelaschi 2006 | 1. Allocation: not randomized, cohort study. |
| Zeng 2002 |
|
| Zhan 2002 |
|
| Zhang 1999 | 1. Allocation: not randomized- inadequate randomization. |
| Zhang 2000 | 1.Allocation: inadequate randomization. |
| Zhang 2002 |
|
| Zhang 2002a |
|
| Zhang 2003 |
|
| Zhang 2005 |
|
| Zhao 2004 |
|
| Zhao 2005 |
|
| Zheng 2001 |
|
| Zheng 2003 |
|
| Zhi 2005 |
|
| Zhong 2003 |
|
| Zhong 2006a |
|
| Zhou 2005 |
|
| Zhu 1999 |
|
| Zhu 2002 |
|
| Zhu 2003 |
|
| Zhu 2003a |
|
| Zoccali 2003 |
|
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Trial 5296 F1D-MC-S014 |
| Methods | Allocation: random, no further details. Blindness.: double, no further details. Duration: 12 weeks. Design: parallel. Location: not reported. Setting: not reported. |
| Participants | Diagnosis: schizophrenia or schizoaffective disorder. N=not reported. Sex: not reported. M, not reported. F. Age: 18-65 years. History: duration ill not reported., age at onset not reported |
| Interventions |
|
| Outcomes | Global state: CGI-S. Mental State: BPRS. Adverse effects: EPS (AIMS, BAS, SAS), Eating Behavior Assessment Scale, Insuline sensitivity index, weight, BMI, waist circumference, visceral fat area, subcutaneous fat area, ratio of visceral fat area to subcutaneous fat area |
| Starting date | October 2003 |
| Contact information | Eli Lilly and Company. |
| Notes |
| Trial name or title | Trial 10769 F1D-US-HGMN |
| Methods | Allocation: random, no further details. Blindness.: double, no further details. Duration: 12 weeks. Design: parallel. Location: not reported. |
| Participants | Diagnosis: schizophrenia or schizoaffective disorder or schizophreniform disorder. N=not reported. Sex: not reported. M, not reported. F. Age: 18-65 years. History: duration ill not reported., age at onset not reported. Setting: not reported. |
| Interventions |
|
| Outcomes | Global state: Response, remission. Mental State: PANSS. Service use: Psychiatric hospitalisations. Adverse effects. |
| Starting date | June 2006 |
| Contact information | not reported. |
| Notes |
| Trial name or title | A comparative study of quetiapine and risperidone in patients with first episode psychosis |
| Methods | Allocation: random, no further details. Blindness.: rater-blinded. |
| Participants | Diagnosis: first episode of schizophreniform psychosis (ICD-10 criteria) |
| Interventions |
|
| Outcomes | Global state: CGI. Mental State: PANSS positive subscale, PANSS negative subscale, Calgary Depression Scale for Schizophrenia, Calgary Anxiety Scale Schizophrenia. General functioning: GAF. |
| Starting date | Not reported. |
| Contact information | |
| Notes |
| Trial name or title | Risperidone and clozapine in chronic schizophrenia. |
| Methods | Allocation: random, no further details. Blindness.: double, no further details. |
| Participants | Severely ill treatment resistant patients. |
| Interventions |
|
| Outcomes | Global state: CGI. Mental State: PANSS, Overt Agression Scale. General functioning: Social functioning and activities of daily living. Quality of life interview. Cognitive functioning: Cognitive test battery. Adverse effects: EPS (ESRS). |
| Starting date | Not reported. |
| Contact information | |
| Notes |
| Trial name or title | Improved response in Schizophrenia -IRIS. |
| Methods | Allocation: random, no further details. Blindness.: double, no further details. |
| Participants | Diagnosis: schizophrenia. |
| Interventions |
|
| Outcomes | Global state: CGI-S. Mental State: PANSS, GAS, HAM-D. Quality of life - SQLS and care giving inventory scores. Adverse effects: EPS (AIMS, SAS, BAS). Health Economics. |
| Starting date | 1 October 2002. |
| Contact information | Dr Lawrence Ratna Barnet Hospital Wellhouse Lane Barnet EN5 3DJ UK Telephone: 020 8216 4617 Fax: 020 8216 4595 |
| Notes |
| Trial name or title | RIS-INT-45 |
| Methods | Allocation: random, using a central randomisation procedure. Blindness.: double, no further details. Duration: 8 weeks. Design: parallel. Location: multicentre. |
| Participants | Diagnosis: (DSM-IV) schizophrenia, PANSS between 60 and 120. N=not reported. Sex: not reported. Age: 18-65 years. History: duration ill not reported., age at onset not reported. Setting: in- and outpatient. |
| Interventions |
|
| Outcomes | Efficacy. Cognitive functioning. Adverse effects: Sleepiness, weight gain, safety. |
| Starting date | 1 April 1997 |
| Contact information | Professor Michael Reveley Department of Psychiatry Clinical Sciences Building University of Leicester Leicester Royal Infirmary PO BOX 65 LE2 7LX United Kingdom Telephone: 0116 252 3242 |
| Notes |
| Trial name or title | Sertindole versus risperidone safety outcome study. |
| Methods | Allocation: random, no further details. Blindness.: partially blinded. |
| Participants | Diagnosis: not reported. |
| Interventions | Sertindole versus risperidone. |
| Outcomes | Not reported. |
| Starting date | November 2002. |
| Contact information | Lester.sireling@beh-mht.nhs.uk |
| Notes |
DATA AND ANALYSES
Comparison 1. RISPERIDONE versus AMISULPRIDE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1a. No clinically significant response (as defined by the original studies) | 3 | 586 | Risk Ratio (M-H, Random, 95% CI) | 1.12 [0.83, 1.50] |
| 2 Global state: 1b. No clinically important change (as defined by the original studies) | 3 | 586 | Risk Ratio (M-H, Random, 95% CI) | 1.12 [0.82, 1.53] |
| 2.1 short term | 2 | 276 | Risk Ratio (M-H, Random, 95% CI) | 0.96 [0.50, 1.85] |
| 2.2 medium term | 1 | 310 | Risk Ratio (M-H, Random, 95% CI) | 1.27 [0.99, 1.64] |
| 3 Global state: 1c. Relapse - medium term (as defined by the original studies) | 1 | 173 | Risk Ratio (M-H, Random, 95% CI) | 1.50 [0.94, 2.39] |
| 4 Leaving the study early | 4 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 4.1 due to any reason | 3 | 586 | Risk Ratio (M-H, Random, 95% CI) | 1.02 [0.68, 1.53] |
| 4.2 due to adverse events | 4 | 622 | Risk Ratio (M-H, Random, 95% CI) | 0.93 [0.61, 1.42] |
| 4.3 due to inefficacy | 3 | 586 | Risk Ratio (M-H, Random, 95% CI) | 1.45 [0.83, 2.53] |
| 5 Mental state: 1a. General - no clinically important change - medium term (less than 50% PANSS total reduction) | 1 | 310 | Risk Ratio (M-H, Random, 95% CI) | 1.24 [1.00, 1.53] |
| 6 Mental state: 1b. General - no clinically important change - short term (less than 20% PANSS total reduction) | 1 | 48 | Risk Ratio (M-H, Random, 95% CI) | 0.69 [0.28, 1.69] |
| 7 Mental state: 1c. General - no clinically important change - medium term (less than 50% BPRS total reduction) | 1 | 310 | Risk Ratio (M-H, Random, 95% CI) | 1.29 [1.02, 1.62] |
| 8 Mental state: 1d. General - no clinically important change - short term (less than 40% BPRS total reduction) | 1 | 228 | Risk Ratio (M-H, Random, 95% CI) | 1.29 [0.92, 1.80] |
| 9 Mental state: 1e. General - average endpoint score - (PANSS total, high = poor) | 2 | 291 | Mean Difference (IV, Random, 95% CI) | −0.38 [−5.33, 4.57] |
| 9.1 short term | 1 | 47 | Mean Difference (IV, Random, 95% CI) | −4.30 [−14.59, 5.99] |
| 9.2 medium term | 1 | 244 | Mean Difference (IV, Random, 95% CI) | 0.80 [−4.85, 6.45] |
| 10 Mental state: 1f. General - average endpoint score - BPRS total score (high = poor) | 3 | 519 | Mean Difference (IV, Random, 95% CI) | 0.68 [−1.79, 3.14] |
| 10.1 short term | 2 | 275 | Mean Difference (IV, Random, 95% CI) | 0.50 [−4.54, 5.53] |
| 10.2 medium term | 1 | 244 | Mean Difference (IV, Random, 95% CI) | 0.20 [−3.28, 3.68] |
| 11 Mental state: 2a. Positive symptoms - average endpoint score - (PANSS positive, high = poor) | 3 | 519 | Mean Difference (IV, Random, 95% CI) | 0.03 [−1.24, 1.29] |
| 11.1 short term | 2 | 275 | Mean Difference (IV, Random, 95% CI) | 0.15 [−2.17, 2.47] |
| 11.2 medium term | 1 | 244 | Mean Difference (IV, Random, 95% CI) | −0.30 [−2.11, 1.51] |
| 12 Mental state: 2b. Positive symptoms - average endpoint score - short term (BPRS positive, high = poor) | 1 | 228 | Mean Difference (IV, Random, 95% CI) | 0.5 [−0.89, 1.89] |
| 13 Mental state: 3a. Negative symptoms - average endpoint score (PANSS negative, high = poor) | 3 | 519 | Mean Difference (IV, Random, 95% CI) | 1.00 [−0.11, 2.11] |
| 13.1 short term | 2 | 275 | Mean Difference (IV, Random, 95% CI) | 0.60 [−1.72, 2.92] |
| 13.2 medium term | 1 | 244 | Mean Difference (IV, Random, 95% CI) | 1.20 [−0.21, 2.61] |
| 14 Mental state: 3b. Negative symptoms - average endpoint scale (SANS total, high = poor) | 1 | 244 | Mean Difference (IV, Random, 95% CI) | 2.70 [−2.33, 7.73] |
| 14.1 medium term | 1 | 244 | Mean Difference (IV, Random, 95% CI) | 2.70 [−2.33, 7.73] |
| 15 General functioning: General 1a. No clinically important change - medium term (less than 50% SOFAS total score reduction) | 1 | 310 | Risk Ratio (M-H, Random, 95% CI) | 1.11 [0.98, 1.25] |
| 16 General functioning: General 1b. average endpoint score - (SOFAS total score, high = poor) | 2 | 291 | Mean Difference (IV, Random, 95% CI) | 2.31 [−1.28, 5.90] |
| 16.1 short term | 1 | 47 | Mean Difference (IV, Random, 95% CI) | 1.10 [−7.03, 9.23] |
| 16.2 medium term | 1 | 244 | Mean Difference (IV, Random, 95% CI) | 2.60 [−1.40, 6.60] |
| 17 Adverse effects: 1. General - at least one adverse effect | 4 | 622 | Risk Ratio (M-H, Random, 95% CI) | 1.00 [0.90, 1.10] |
| 18 Adverse effects: 2. Death | 1 | 620 | Risk Ratio (M-H, Random, 95% CI) | 0.42 [0.06, 2.82] |
| 18.1 natural causes | 1 | 310 | Risk Ratio (M-H, Random, 95% CI) | 0.32 [0.01, 7.81] |
| 18.2 suicide | 1 | 310 | Risk Ratio (M-H, Random, 95% CI) | 0.48 [0.04, 5.25] |
| 19 Adverse effects: 3a. Cardiac effects - QTc prolongation | 2 | 276 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Adverse effects: 4a. Central nervous system - sedation | 1 | 310 | Risk Ratio (M-H, Random, 95% CI) | 1.44 [0.61, 3.43] |
| 21 Adverse effects: 4b. Central nervous system - seizures | 1 | 310 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 22 Adverse effects: 5a. Extrapyramidal effects | 3 | 3338 | Risk Ratio (M-H, Random, 95% CI) | 1.04 [0.90, 1.21] |
| 22.1 akathisia (Barnes) | 3 | 586 | Risk Ratio (M-H, Random, 95% CI) | 1.25 [0.90, 1.74] |
| 22.2 extrapyramidal symptoms | 1 | 228 | Risk Ratio (M-H, Random, 95% CI) | 0.88 [0.60, 1.30] |
| 22.3 hyperkinesia | 2 | 538 | Risk Ratio (M-H, Random, 95% CI) | 0.75 [0.49, 1.14] |
| 22.4 parkinsonism | 2 | 538 | Risk Ratio (M-H, Random, 95% CI) | 1.13 [0.66, 1.95] |
| 22.5 rigor | 2 | 276 | Risk Ratio (M-H, Random, 95% CI) | 1.08 [0.27, 4.36] |
| 22.6 tremor | 3 | 586 | Risk Ratio (M-H, Random, 95% CI) | 1.46 [0.66, 3.21] |
| 22.7 use of antiparkinson medication | 3 | 586 | Risk Ratio (M-H, Random, 95% CI) | 1.07 [0.72, 1.57] |
| 23 Adverse effects: 5b Extrapyramidal effects - scale measured | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 23.1 abnormal involuntary movement: AIMS (high = poor) | 2 | 538 | Mean Difference (IV, Random, 95% CI) | −0.08 [−0.72, 0.55] |
| 23.2 extrapyramidal symptoms: Simpson-Angus Scale (high = poor) | 2 | 538 | Mean Difference (IV, Random, 95% CI) | 0.03 [−0.06, 0.13] |
| 24 Adverse effects: 6. Prolactin associated side effects | 2 | 678 | Risk Ratio (M-H, Random, 95% CI) | 2.34 [0.50, 10.92] |
| 24.1 amenorrhea | 1 | 140 | Risk Ratio (M-H, Random, 95% CI) | 0.97 [0.06, 15.23] |
| 24.2 galactorrhea | 2 | 231 | Risk Ratio (M-H, Random, 95% CI) | 0.69 [0.16, 3.03] |
| 24.3 sexual dysfunction (men) | 2 | 307 | Risk Ratio (M-H, Random, 95% CI) | 13.17 [1.74, 99.42] |
| 25 Adverse effects: 8a. Metabolic effects - weight gain | 2 | 538 | Risk Ratio (M-H, Random, 95% CI) | 1.75 [1.07, 2.87] |
| 25.1 weight gain of 7 % or more of total body weight | 1 | 310 | Risk Ratio (M-H, Random, 95% CI) | 1.71 [1.00, 2.91] |
| 25.2 as “weight gain” reported adverse event | 1 | 228 | Risk Ratio (M-H, Random, 95% CI) | 2.04 [0.52, 7.94] |
| 26 Adverse effects: 8b Metabolic effects - weight gain - change from baseline in kg | 3 | 585 | Mean Difference (IV, Random, 95% CI) | 0.99 [0.37, 1.61] |
Comparison 2. RISPERIDONE versus ARIPIPRAZOLE - all data short term.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1a. No clinically significant response (as defined by the original studies) | 2 | 384 | Risk Ratio (M-H, Random, 95% CI) | 0.88 [0.62, 1.24] |
| 2 Global state: 1b. No clinically important change (as defined by the original studies) | 2 | 384 | Risk Ratio (M-H, Random, 95% CI) | 0.88 [0.62, 1.24] |
| 3 Leaving the study early | 2 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 due to any reason | 2 | 384 | Risk Ratio (M-H, Random, 95% CI) | 1.06 [0.79, 1.41] |
| 3.2 due to adverse events | 2 | 384 | Risk Ratio (M-H, Random, 95% CI) | 0.79 [0.39, 1.61] |
| 3.3 due to inefficacy | 2 | 384 | Risk Ratio (M-H, Random, 95% CI) | 0.89 [0.41, 1.93] |
| 4 Mental state: 1a. General - average endpoint score (PANSS total, high = poor) | 2 | 372 | Mean Difference (IV, Random, 95% CI) | −1.5 [−5.96, 2.96] |
| 5 Mental state: 2. Positive symptoms average endpoint score (PANSS positive, high = poor) | 2 | 372 | Mean Difference (IV, Random, 95% CI) | −1.24 [−2.74, 0.26] |
| 6 Mental state: 3. Negative symptoms - average endpoint score - (PANSS negative, high = poor) | 2 | 372 | Mean Difference (IV, Random, 95% CI) | 0.45 [−0.87, 1.78] |
| 7 Adverse effects: 1. General - at least one adverse effect | 2 | 384 | Risk Ratio (M-H, Random, 95% CI) | 1.02 [0.95, 1.09] |
| 8 Adverse effects: 2a. Cardiac effects - QTc prolongation | 1 | 301 | Risk Ratio (M-H, Random, 95% CI) | 14.21 [0.74, 272.45] |
| 9 Adverse effects: 2b. Cardiac effects - QTc abnormalities - change from baseline in ms | 2 | 383 | Mean Difference (IV, Random, 95% CI) | 7.19 [2.19, 12.19] |
| 10 Adverse effects: 3a. Extrapyramidal effects | 2 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 10.1 akathisia | 2 | 384 | Risk Ratio (M-H, Random, 95% CI) | 1.56 [0.21, 11.45] |
| 10.2 dystonia | 1 | 301 | Risk Ratio (M-H, Random, 95% CI) | 7.14 [2.41, 21.13] |
| 10.3 extrapyramidal symptoms | 2 | 384 | Risk Ratio (M-H, Random, 95% CI) | 1.18 [0.68, 2.06] |
| 10.4 parkinsonism | 1 | 301 | Risk Ratio (M-H, Random, 95% CI) | 0.14 [0.01, 2.35] |
| 10.5 tremor | 1 | 301 | Risk Ratio (M-H, Random, 95% CI) | 0.21 [0.05, 0.90] |
| 10.6 use of antiparkinson medication | 1 | 83 | Risk Ratio (M-H, Random, 95% CI) | 1.68 [0.89, 3.17] |
| 11 Adverse effects: 3b. Extrapyramidal effects - scale measured | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 11.1 abnormal involuntary movement: AIMS (high = poor) | 2 | 383 | Mean Difference (IV, Random, 95% CI) | 0.25 [−0.75, 1.24] |
| 11.2 akathisia: Barnes Akathisia Scale (high = poor) | 2 | 383 | Mean Difference (IV, Random, 95% CI) | 0.11 [−0.27, 0.49] |
| 11.3 extrapyramidal symptoms: Simpson-Angus Scale (high = poor) | 2 | 383 | Mean Difference (IV, Random, 95% CI) | 0.70 [−0.82, 2.22] |
| 12 Adverse effects: 4a. Prolactin associated side effects | 1 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 12.1 abnormally high prolactin value | 1 | 301 | Risk Ratio (M-H, Random, 95% CI) | 26.23 [12.64, 54.46] |
| 12.2 dysmenorrhea | 1 | 91 | Risk Ratio (M-H, Random, 95% CI) | 0.32 [0.02, 5.91] |
| 13 Adverse effects: 4b. Prolactin - change from baseline in ng/ml | 2 | 383 | Mean Difference (IV, Random, 95% CI) | 54.71 [49.36, 60.06] |
| 14 Adverse effects: 5a. Metabolic effects - cholesterol - change from baseline in mg/dl | 1 | 83 | Mean Difference (IV, Random, 95% CI) | 22.3 [4.91, 39.69] |
| 15 Adverse effects: 5b. Metabolic effects - glucose - change from baseline in mg/dl | 1 | 83 | Mean Difference (IV, Random, 95% CI) | −6.8 [−19.70, 6.10] |
| 16 Adverse effects: 5c. Metabolic effects - weight gain of 7% or more of total body weight | 2 | 384 | Risk Ratio (M-H, Random, 95% CI) | 1.30 [0.55, 3.07] |
| 17 Adverse effects: 5d. Metabolic effects - weight gain - change from baseline in kg | 2 | 383 | Mean Difference (IV, Random, 95% CI) | 0.54 [−0.15, 1.24] |
Comparison 3. RISPERIDONE versus CLOZAPINE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1a. No clinically significant response (as defined by the original studies) | 6 | 575 | Risk Ratio (M-H, Random, 95% CI) | 1.07 [0.98, 1.16] |
| 2 Global state: 1b. No clinically important change - short term (as defined by the original studies) | 2 | 333 | Risk Ratio (M-H, Random, 95% CI) | 1.07 [0.88, 1.30] |
| 3 Leaving the study early | 8 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 due to any reason | 8 | 675 | Risk Ratio (M-H, Random, 95% CI) | 1.10 [0.86, 1.41] |
| 3.2 due to adverse events | 7 | 647 | Risk Ratio (M-H, Random, 95% CI) | 0.55 [0.31, 0.98] |
| 3.3 due to inefficacy | 7 | 647 | Risk Ratio (M-H, Random, 95% CI) | 2.51 [1.43, 4.40] |
| 4 Mental state: 1a.General - no clinically important change - short term (less than 20 % PANSS total reduction) | 2 | 106 | Risk Ratio (M-H, Random, 95% CI) | 0.84 [0.50, 1.42] |
| 5 Mental state: 1b. General - no clinically important change - short term (as defined by the original studies) | 1 | 273 | Risk Ratio (M-H, Random, 95% CI) | 1.05 [0.85, 1.29] |
| 6 Mental State: 1c. General - no clinically important change - long term (less than 40% BPRS reduction) | 1 | 107 | Risk Ratio (M-H, Random, 95% CI) | 1.05 [0.76, 1.45] |
| 7 Mental State: 1d. General - no clinically important change - short term (less than 20% BPRS reduction) | 1 | 29 | Risk Ratio (M-H, Random, 95% CI) | 1.24 [0.78, 1.98] |
| 8 Mental state: 1e. General - average endpoint score (PANSS total, high = poor) | 5 | 468 | Mean Difference (IV, Random, 95% CI) | 1.49 [−3.44, 6.42] |
| 8.1 short term | 4 | 387 | Mean Difference (IV, Random, 95% CI) | 0.75 [−5.35, 6.85] |
| 8.2 medium term | 1 | 81 | Mean Difference (IV, Random, 95% CI) | 3.60 [−6.12, 13.32] |
| 9 Mental state: 1f. General - average endpoint score (BPRS total, high = poor) | 4 | 397 | Mean Difference (IV, Random, 95% CI) | 3.07 [−0.01, 6.16] |
| 9.1 short term | 3 | 345 | Mean Difference (IV, Random, 95% CI) | 4.90 [2.17, 7.64] |
| 9.2 long term | 1 | 52 | Mean Difference (IV, Random, 95% CI) | −0.20 [−4.12, 3.72] |
| 10 Mental state: 2a. Positive symptoms - average endpoint score (PANSS positive, high = poor) | 6 | 591 | Mean Difference (IV, Random, 95% CI) | 1.26 [0.18, 2.35] |
| 10.1 short term | 5 | 510 | Mean Difference (IV, Random, 95% CI) | 1.28 [−0.01, 2.57] |
| 10.2 medium term | 1 | 81 | Mean Difference (IV, Random, 95% CI) | 0.40 [−2.78, 3.58] |
| 11 Mental state: 2b. Positive symptoms - average endpoint score - short term (BPRS positive, high = poor) | 1 | 29 | Mean Difference (IV, Random, 95% CI) | 2.10 [−0.56, 4.76] |
| 12 Mental state: 3a. Negative symptoms - average endpoint score (PANSS negative, high = poor) | 5 | 562 | Mean Difference (IV, Random, 95% CI) | −0.13 [−1.96, 1.71] |
| 12.1 short term | 4 | 481 | Mean Difference (IV, Random, 95% CI) | −0.55 [−2.80, 1.71] |
| 12.2 medium term | 1 | 81 | Mean Difference (IV, Random, 95% CI) | 1.40 [−1.42, 4.22] |
| 13 Mental state: 3b. Negative symptoms - average endpoint score - short term (SANS total, high = poor) | 2 | 69 | Mean Difference (IV, Random, 95% CI) | −0.62 [−3.74, 2.51] |
| 14 General functioning: 1a. General - average endpoint score - short term (GAF total, high = poor) | 1 | 19 | Mean Difference (IV, Random, 95% CI) | 9.0 [−0.44, 18.44] |
| 15 General functioning: 1b. Social functioning - average endpoint score - short term (SFS, high = poor) | 1 | 19 | Mean Difference (IV, Random, 95% CI) | 47.0 [0.45, 93.55] |
| 16 Cognitive functioning: 1a. Global - no clinically important change in global neurocognitive score - medium term (less than 1/2 SD) | 1 | 81 | Risk Ratio (M-H, Random, 95% CI) | 0.79 [0.60, 1.05] |
| 17 Cognitive functioning: 1b. Global - average endpoint score - medium term - (global neurocognitive score, high = poor) | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 0.33 [−0.06, 0.72] |
| 18 Adverse effects: 1. General - at least one adverse effect | 2 | 333 | Risk Ratio (M-H, Random, 95% CI) | 0.85 [0.51, 1.42] |
| 19 Adverse effects: 2. Death | 2 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 19.1 any reason | 1 | 273 | Risk Ratio (M-H, Random, 95% CI) | 1.02 [0.06, 16.18] |
| 19.2 natural causes | 1 | 20 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.3 suicide | 1 | 20 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Adverse effects: 3. Cardiac effects | 2 | 146 | Risk Ratio (M-H, Random, 95% CI) | 1.54 [0.07, 36.11] |
| 20.1 preterminal negative T-wave | 1 | 60 | Risk Ratio (M-H, Random, 95% CI) | 1.54 [0.07, 36.11] |
| 20.2 any significant cardiac effect | 1 | 86 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 21 Adverse effects: 4a. Central nervous system - sedation | 5 | 479 | Risk Ratio (M-H, Random, 95% CI) | 0.58 [0.41, 0.81] |
| 22 Adverse effects: 4b. Central nervous system - seizures | 2 | 354 | Risk Ratio (M-H, Random, 95% CI) | 0.22 [0.07, 0.70] |
| 23 Adverse effects: 5a. Extrapyramidal effects | 8 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 23.1 akathisia | 1 | 40 | Risk Ratio (M-H, Random, 95% CI) | 0.11 [0.01, 1.94] |
| 23.2 akinesia | 1 | 86 | Risk Ratio (M-H, Random, 95% CI) | 1.0 [0.38, 2.61] |
| 23.3 dystonia | 1 | 86 | Risk Ratio (M-H, Random, 95% CI) | 2.0 [0.19, 21.24] |
| 23.4 extrapyramidal symptoms | 2 | 333 | Risk Ratio (M-H, Random, 95% CI) | 1.30 [0.44, 3.85] |
| 23.5 parkinsonism | 1 | 86 | Risk Ratio (M-H, Random, 95% CI) | 0.63 [0.41, 0.97] |
| 23.6 tremor | 1 | 40 | Risk Ratio (M-H, Random, 95% CI) | 0.5 [0.18, 1.40] |
| 23.7 use of antiparkinson medication | 6 | 304 | Risk Ratio (M-H, Random, 95% CI) | 2.57 [1.47, 4.48] |
| 24 Adverse effects: 5b. Extrapyramidal symptoms - scale measured | 3 | 150 | Mean Difference (IV, Random, 95% CI) | 0.54 [−0.25, 1.34] |
| 24.1 extrapyramidal symptoms: Simpson-Angus Scale (high = poor) | 2 | 69 | Mean Difference (IV, Random, 95% CI) | 0.81 [−0.10, 1.73] |
| 24.2 extrapyramidal symptoms: ESRS total score (high = poor) | 1 | 81 | Mean Difference (IV, Random, 95% CI) | −0.30 [−1.91, 1.31] |
| 25 Adverse effects: 6. Haematological - white blood cells - significant low white blood cell count (as def. by the original studies) | 5 | 567 | Risk Ratio (M-H, Random, 95% CI) | 1.69 [0.51, 5.58] |
| 26 Adverse effects: 7a. Prolactin associated side effects | 1 | 86 | Risk Ratio (M-H, Random, 95% CI) | 2.0 [0.39, 10.35] |
| 26.1 sexual dysfunction | 1 | 86 | Risk Ratio (M-H, Random, 95% CI) | 2.0 [0.39, 10.35] |
| 27 Adverse effects: 7b. Prolactin associated side effects - change from baseline in ng/ml | 2 | 55 | Mean Difference (IV, Random, 95% CI) | 28.61 [10.52, 46.69] |
| 27.1 change from baseline in ng/ml | 1 | 27 | Mean Difference (IV, Random, 95% CI) | 38.5 [23.30, 53.70] |
| 27.2 change fom baseline in mg/ml - of men only | 1 | 28 | Mean Difference (IV, Random, 95% CI) | 20.0 [8.19, 31.81] |
| 28 Adverse effects: 3a. Metabolic - cholesterol - change from baseline in mg/dl | 1 | 31 | Mean Difference (IV, Random, 95% CI) | −7.10 [−34.01, 19. 81] |
| 29 Adverse effects: 3b. Metabolic - glucose - change from baseline in mg/dl | 1 | 31 | Mean Difference (IV, Random, 95% CI) | −1.70 [−12.04, 8.64] |
| 30 Adverse effects: 3c. Metabolic - weight gain | 2 | 167 | Risk Ratio (M-H, Random, 95% CI) | 0.61 [0.34, 1.08] |
| 30.1 10% of total body weight or more | 1 | 81 | Risk Ratio (M-H, Random, 95% CI) | 0.56 [0.18, 1.76] |
| 30.2 as “weight gain” reported adverse event | 1 | 86 | Risk Ratio (M-H, Random, 95% CI) | 0.63 [0.32, 1.22] |
| 31 Adverse effects: 3d. Metabolic - weight gain - change from baseline in kg | 1 | 373 | Mean Difference (IV, Random, 95% CI) | −3.30 [−5.65, −0.95] |
Comparison 4. RISPERIDONE versus OLANZAPINE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1a. No clinically significant response (as defined by the original studies) | 7 | 1376 | Risk Ratio (M-H, Random, 95% CI) | 1.06 [0.99, 1.13] |
| 2 Global state: 1b. No clinically important change (as defined by the original studies) | 5 | 975 | Risk Ratio (M-H, Random, 95% CI) | 0.98 [0.88, 1.09] |
| 2.1 short term | 3 | 589 | Risk Ratio (M-H, Random, 95% CI) | 1.00 [0.87, 1.16] |
| 2.2 medium term | 1 | 120 | Risk Ratio (M-H, Random, 95% CI) | 0.83 [0.60, 1.16] |
| 2.3 long term | 1 | 266 | Risk Ratio (M-H, Random, 95% CI) | 0.98 [0.71, 1.35] |
| 3 Global state: 1c. Relapse (as defined by the original studies) | 2 | 211 | Risk Ratio (M-H, Random, 95% CI) | 1.25 [0.57, 2.71] |
| 3.1 short term | 1 | 76 | Risk Ratio (M-H, Random, 95% CI) | 0.75 [0.25, 2.25] |
| 3.2 long term | 1 | 135 | Risk Ratio (M-H, Random, 95% CI) | 1.7 [0.79, 3.67] |
| 4 Leaving the study early | 17 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 4.1 due to any reason | 16 | 2738 | Risk Ratio (M-H, Random, 95% CI) | 1.14 [1.07, 1.21] |
| 4.2 due to adverse events | 13 | 2595 | Risk Ratio (M-H, Random, 95% CI) | 0.96 [0.71, 1.30] |
| 4.3 due to inefficacy | 14 | 2744 | Risk Ratio (M-H, Random, 95% CI) | 1.28 [1.02, 1.60] |
| 5 Mental state: 1a. General - no clinically important change (less than 50% PANSS total score reduction) | 3 | 472 | Risk Ratio (M-H, Random, 95% CI) | 1.09 [1.00, 1.18] |
| 5.1 short term | 1 | 71 | Risk Ratio (M-H, Random, 95% CI) | 0.43 [0.04, 4.57] |
| 5.2 long term | 2 | 401 | Risk Ratio (M-H, Random, 95% CI) | 1.09 [1.00, 1.18] |
| 6 Mental state: 1b. General - no clinically important change - short term (less than 20% PANSS total score reduction) | 2 | 553 | Risk Ratio (M-H, Random, 95% CI) | 1.02 [0.88, 1.19] |
| 7 Mental state: 1c. General - average endpoint score (PANSS total, high = poor) | 15 | 2390 | Mean Difference (IV, Random, 95% CI) | 1.94 [0.58, 3.31] |
| 7.1 short term | 7 | 728 | Mean Difference (IV, Random, 95% CI) | 0.97 [−1.10, 3.05] |
| 7.2 medium term | 3 | 231 | Mean Difference (IV, Random, 95% CI) | 4.11 [−0.71, 8.93] |
| 7.3 long term | 5 | 1431 | Mean Difference (IV, Random, 95% CI) | 2.59 [0.20, 4.98] |
| 8 Mental state: 1d. General - average endpoint score (BPRS total score, high = poor) | 3 | 428 | Mean Difference (IV, Random, 95% CI) | 4.16 [0.03, 8.29] |
| 8.1 short term | 1 | 35 | Mean Difference (IV, Random, 95% CI) | 5.0 [−5.74, 15.74] |
| 8.2 long term | 2 | 393 | Mean Difference (IV, Random, 95% CI) | 4.28 [−1.34, 9.91] |
| 9 Mental state: 2a. Positive symptoms - no clinically important change - short term (less than 50% PANSS positive subscore reduction) | 1 | 377 | Risk Ratio (M-H, Random, 95% CI) | 0.98 [0.93, 1.04] |
| 10 Mental state: 2b. Positive symptoms - average endpoint score (PANSS positive, high = poor) | 13 | 1702 | Mean Difference (IV, Random, 95% CI) | 0.46 [−0.09, 1.02] |
| 10.1 short term | 5 | 661 | Mean Difference (IV, Random, 95% CI) | −0.48 [−1.53, 0.57] |
| 10.2 medium term | 3 | 231 | Mean Difference (IV, Random, 95% CI) | 1.58 [−0.03, 3.20] |
| 10.3 long term | 5 | 810 | Mean Difference (IV, Random, 95% CI) | 0.68 [−0.04, 1.40] |
| 11 Mental state: 3a. Negative symptoms - average endpoint score (PANSS negative, high = poor) | 13 | 1702 | Mean Difference (IV, Random, 95% CI) | 0.44 [−0.08, 0.96] |
| 11.1 short term | 5 | 661 | Mean Difference (IV, Random, 95% CI) | 0.19 [−0.85, 1.22] |
| 11.2 medium term | 3 | 231 | Mean Difference (IV, Random, 95% CI) | 0.00 [−1.58, 1.59] |
| 11.3 long term | 5 | 810 | Mean Difference (IV, Random, 95% CI) | 0.81 [0.07, 1.54] |
| 12 Mental state: 3b. Negative symptoms - average endpoint score - long term (SANS total, high = poor) | 1 | 308 | Mean Difference (IV, Random, 95% CI) | 1.4 [0.37, 2.43] |
| 13 Quality of life: General - average endpoint score - long term (QLS total score, high = poor) | 2 | 296 | Mean Difference (IV, Random, 95% CI) | 5.10 [1.09, 9.10] |
| 14 Cognitive functioning: 1a.General - no clinically important change - medium term (less than V SD in Global Neurocognitive Score improved) | 1 | 80 | Risk Ratio (M-H, Random, 95% CI) | 1.30 [0.88, 1.94] |
| 15 Cognitive functioning: 1b. General - average endpoint score - medium term (global neurocognitive score, high = poor) | 1 | 52 | Mean Difference (IV, Random, 95% CI) | 0.04 [−0.31, 0.39] |
| 16 Cognitive functioning: 1c. General - average endpoint score - long term (neurocognitive composite score, high = poor) | 1 | 263 | Mean Difference (IV, Random, 95% CI) | 0.01 [−0.11, 0.13] |
| 17 Service use - number of patients rehospitalised | 3 | 965 | Risk Ratio (M-H, Random, 95% CI) | 1.34 [0.96, 1.86] |
| 17.1 short term | 1 | 76 | Risk Ratio (M-H, Random, 95% CI) | 1.35 [0.41, 4.40] |
| 17.2 medium term | 1 | 212 | Risk Ratio (M-H, Random, 95% CI) | 1.38 [0.69, 2.78] |
| 17.3 long term | 1 | 677 | Risk Ratio (M-H, Random, 95% CI) | 1.32 [0.89, 1.96] |
| 18 Adverse effects: 1. General - at least one adverse effect | 11 | 2576 | Risk Ratio (M-H, Random, 95% CI) | 0.96 [0.88, 1.03] |
| 19 Adverse effects: 2. Death | 8 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 19.1 any reason | 1 | 339 | Risk Ratio (M-H, Random, 95% CI) | 3.09 [0.13, 75.30] |
| 19.2 natural causes | 2 | 252 | Risk Ratio (M-H, Random, 95% CI) | 0.34 [0.01, 8.26] |
| 19.3 suicide attempt | 5 | 1724 | Risk Ratio (M-H, Random, 95% CI) | 1.15 [0.37, 3.54] |
| 19.4 suicide | 4 | 730 | Risk Ratio (M-H, Random, 95% CI) | 3.11 [0.13, 75.59] |
| 20 Adverse effects: 3a. Cardiac effects | 4 | 1268 | Risk Ratio (M-H, Random, 95% CI) | 1.03 [0.23, 4.64] |
| 20.1 abnormal ECG | 2 | 415 | Risk Ratio (M-H, Random, 95% CI) | 0.42 [0.08, 2.30] |
| 20.2 QTc prolongation | 2 | 853 | Risk Ratio (M-H, Random, 95% CI) | 2.69 [0.12, 60.00] |
| 21 Adverse effects: 3b. Cardiac effects - QTc abnormalities - change from baseline in ms | 6 | 1518 | Mean Difference (IV, Random, 95% CI) | 0.96 [−2.74, 4.67] |
| 22 Adverse effects: 4a. Central nervous system - sedation | 11 | 2576 | Risk Ratio (M-H, Random, 95% CI) | 0.93 [0.84, 1.04] |
| 23 Adverse effects: 4b. Central nervous system - seizures | 4 | 671 | Risk Ratio (M-H, Random, 95% CI) | 0.26 [0.03, 2.35] |
| 24 Adverse effects: 5a. Extrapyramidal effects | 14 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 24.1 akathisia | 8 | 1988 | Risk Ratio (M-H, Random, 95% CI) | 1.30 [1.02, 1.66] |
| 24.2 akinesia | 3 | 681 | Risk Ratio (M-H, Random, 95% CI) | 1.21 [0.82, 1.79] |
| 24.3 dyskinesia | 3 | 580 | Risk Ratio (M-H, Random, 95% CI) | 1.02 [0.36, 2.90] |
| 24.4 dystonia | 3 | 591 | Risk Ratio (M-H, Random, 95% CI) | 1.79 [0.37, 8.77] |
| 24.5 extrapyramidal symptoms | 4 | 1104 | Risk Ratio (M-H, Random, 95% CI) | 1.33 [0.83, 2.13] |
| 24.6 parkinsonism | 5 | 776 | Risk Ratio (M-H, Random, 95% CI) | 1.65 [1.08, 2.51] |
| 24.7 rigor | 2 | 141 | Risk Ratio (M-H, Random, 95% CI) | 0.41 [0.06, 2.70] |
| 24.8 tremor | 5 | 973 | Risk Ratio (M-H, Random, 95% CI) | 0.87 [0.48, 1.57] |
| 24.9 use of antiparkinson medication | 13 | 2599 | Risk Ratio (M-H, Random, 95% CI) | 1.28 [1.06, 1.55] |
| 25 Adverse effects: 5b. Extrapyramidal effects - scale measured | 10 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 25.1 abnormal involuntary movement: AIMS (high = poor) | 1 | 302 | Mean Difference (IV, Random, 95% CI) | 0.03 [−0.72, 0.78] |
| 25.2 akathisia: Barnes Akathisia Scale (high = poor) | 2 | 353 | Mean Difference (IV, Random, 95% CI) | 0.72 [−0.36, 1.81] |
| 25.3 akathisia: ESRS subscore for akathisia (high = poor) | 1 | 359 | Mean Difference (IV, Random, 95% CI) | 0.0 [−0.27, 0.27] |
| 25.4 dyskinesia: ESRS subscore for dyskinesia (high = poor) | 3 | 572 | Mean Difference (IV, Random, 95% CI) | −0.08 [−0.76, 0.60] |
| 25.5 dystonia: ESRS subscore for dystonia (high = poor) | 1 | 42 | Mean Difference (IV, Random, 95% CI) | −0.09 [−0.91, 0.73] |
| 25.6 extrapyramidal symptoms: ESRS total score (high = poor) | 4 | 682 | Mean Difference (IV, Random, 95% CI) | 0.30 [−0.35, 0.94] |
| 25.7 extrapyramidal symptoms: Simpson-Angus Scale (high = poor) | 5 | 522 | Mean Difference (IV, Random, 95% CI) | 0.62 [−0.08, 1.33] |
| 25.8 parkinsonism: ESRS subscore for parkinsonism (high = poor) | 3 | 572 | Mean Difference (IV, Random, 95% CI) | 0.24 [−1.09, 1.57] |
| 26 Adverse effects: 6. Haematological: white blood cells - significant low white blood cell count (as def. by the original studies) | 3 | 484 | Risk Ratio (M-H, Random, 95% CI) | 1.00 [0.09, 10.59] |
| 27 Adverse effects: 7a. Prolactin associated side effects | 10 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 27.1 abnormal ejaculation | 3 | 531 | Risk Ratio (M-H, Random, 95% CI) | 4.34 [1.49, 12.69] |
| 27.2 abnormally high prolactin concentration | 3 | 477 | Risk Ratio (M-H, Random, 95% CI) | 3.02 [0.99, 9.23] |
| 27.3 amenorrhea | 7 | 565 | Risk Ratio (M-H, Random, 95% CI) | 1.50 [1.02, 2.22] |
| 27.4 decreased libido | 3 | 781 | Risk Ratio (M-H, Random, 95% CI) | 2.48 [0.77, 8.00] |
| 27.5 galactorrhea | 7 | 1044 | Risk Ratio (M-H, Random, 95% CI) | 1.59 [0.77, 3.28] |
| 27.6 gynecomastia | 5 | 1083 | Risk Ratio (M-H, Random, 95% CI) | 1.39 [0.70, 2.77] |
| 27.7 impotence | 3 | 531 | Risk Ratio (M-H, Random, 95% CI) | 2.00 [0.68, 5.89] |
| 27.8 orgastic dysfunction | 1 | 377 | Risk Ratio (M-H, Random, 95% CI) | 5.03 [0.24, 104.00] |
| 27.9 sexual dysfunction | 7 | 1715 | Risk Ratio (M-H, Random, 95% CI) | 1.07 [0.90, 1.28] |
| 28 Adverse effects: 7b. Prolactin - change from baseline in ng/ml | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 28.1 change from baseline in ng/ml | 6 | 1291 | Mean Difference (IV, Random, 95% CI) | 22.84 [17.69, 27.98] |
| 28.2 change from baseline in ng/ml - of men only | 2 | 70 | Mean Difference (IV, Random, 95% CI) | 19.91 [13.64, 26.18] |
| 28.3 change from baseline in ng/ml - of women only | 1 | 71 | Mean Difference (IV, Random, 95% CI) | 41.4 [29.64, 53.16] |
| 29 Adverse effects: 8a. Metabolic - cholesterol - significant cholesterol increase | 1 | 266 | Risk Ratio (M-H, Random, 95% CI) | 0.78 [0.44, 1.38] |
| 30 Adverse effects: 8b. Metabolic - cholesterol - change from baseline in mg/dl | 7 | 1391 | Mean Difference (IV, Random, 95% CI) | −10.36 [−14.43, −6. 28] |
| 31 Adverse effects: 8c. Metabolic - glucose - abnormally high fasting glucose value | 3 | 670 | Risk Ratio (M-H, Random, 95% CI) | 0.50 [0.22, 1.16] |
| 32 Adverse effects: 8d. Metabolic -glucose - change from baseline in mg/dl | 7 | 1201 | Mean Difference (IV, Random, 95% CI) | −7.58 [−11.23, −3.93] |
| 33 Adverse effects: 8e. Metabolic -weight gain | 11 | 2594 | Risk Ratio (M-H, Random, 95% CI) | 0.55 [0.43, 0.72] |
| 33.1 significant weight gain (as defined by the original studies) | 8 | 1873 | Risk Ratio (M-H, Random, 95% CI) | 0.54 [0.39, 0.76] |
| 33.2 as “weight gain” reported adverse event | 3 | 721 | Risk Ratio (M-H, Random, 95% CI) | 0.60 [0.39, 0.90] |
| 34 Adverse effects: 8f. Metabolic - weight gain - change from baseline in kg | 13 | 2116 | Mean Difference (IV, Random, 95% CI) | −2.61 [−3.74, −1.48] |
Comparison 5. RISPERIDONE versus QUETIAPINE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1a. No clinically significant response (as def. by the original studies) | 4 | 1274 | Risk Ratio (M-H, Random, 95% CI) | 0.93 [0.82, 1.05] |
| 2 Global state: 1b. No clinically important change (as defined by the original studies) | 4 | 1274 | Risk Ratio (M-H, Random, 95% CI) | 0.90 [0.79, 1.02] |
| 2.1 short term | 3 | 1007 | Risk Ratio (M-H, Random, 95% CI) | 0.91 [0.78, 1.06] |
| 2.2 long term | 1 | 267 | Risk Ratio (M-H, Random, 95% CI) | 0.85 [0.62, 1.15] |
| 3 Leaving the study early | 10 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 due to any reason | 10 | 2278 | Risk Ratio (M-H, Random, 95% CI) | 0.94 [0.87, 1.02] |
| 3.2 due to adverse events | 7 | 1851 | Risk Ratio (M-H, Random, 95% CI) | 0.84 [0.56, 1.27] |
| 3.3 due to inefficacy | 7 | 1851 | Risk Ratio (M-H, Random, 95% CI) | 0.79 [0.62, 1.01] |
| 4 Mental state: 1a General - no clinically important change - short term (less than 30% PANSS total score reduction) | 2 | 982 | Risk Ratio (M-H, Random, 95% CI) | 0.90 [0.71, 1.15] |
| 5 Mental state: 1b. General - no clinicallly important change - short term (less than 20% BPRS total score reduction) | 1 | 25 | Risk Ratio (M-H, Random, 95% CI) | 1.03 [0.66, 1.60] |
| 6 Mental state: 1c. General - average endpoint score (PANSS total score, high = poor) | 9 | 1953 | Mean Difference (IV, Random, 95% CI) | −3.09 [−5.16, −1.01] |
| 6.1 short term | 5 | 1064 | Mean Difference (IV, Random, 95% CI) | −2.44 [−5.69, 0.81] |
| 6.2 medium term | 2 | 146 | Mean Difference (IV, Random, 95% CI) | −6.27 [−16.48, 3.94] |
| 6.3 long term | 2 | 743 | Mean Difference (IV, Random, 95% CI) | −3.11 [−5.82, −0.40] |
| 7 Mental state: 1d. General - average endpoint score - short term (BPRS total score, high = poor) | 1 | 25 | Mean Difference (IV, Random, 95% CI) | −1.68 [−11.69, 8.33] |
| 8 Mental state: 2a. Positive symptoms - no clinically important change - short term (less than 40% PANSS positive reduction) | 1 | 673 | Risk Ratio (M-H, Random, 95% CI) | 1.00 [0.90, 1.12] |
| 9 Mental state: 2b. Positive symptoms - average endpoint score - (PANSS positive subscore, high = poor) | 7 | 1264 | Mean Difference (IV, Random, 95% CI) | −1.82 [−2.48, −1.16] |
| 9.1 short term | 4 | 1037 | Mean Difference (IV, Random, 95% CI) | −2.10 [−3.19, −1.00] |
| 9.2 medium term | 2 | 146 | Mean Difference (IV, Random, 95% CI) | −2.15 [−4.31, 0.01] |
| 9.3 long term | 1 | 81 | Mean Difference (IV, Random, 95% CI) | −1.30 [−2.73, 0.13] |
| 10 Mental state: 2c. Positive symptoms - average endpoint score - short term (BPRS positive subscore, high = poor) | 1 | 25 | Mean Difference (IV, Random, 95% CI) | −1.1 [−2.02, −0.18] |
| 11 Mental state: 3a. Negative symptoms - no clinicallly important change - short term (less than 40% PANSS negative reduction) | 1 | 673 | Risk Ratio (M-H, Random, 95% CI) | 1.02 [0.96, 1.08] |
| 12 Mental state: 3b. Negative symptoms - average endpoint score - (PANSS negative subscore, high = poor) | 7 | 1183 | Mean Difference (IV, Random, 95% CI) | 0.34 [−1.26, 1.94] |
| 12.1 short term | 4 | 956 | Mean Difference (IV, Random, 95% CI) | 1.46 [−1.19, 4.11] |
| 12.2 medium term | 2 | 146 | Mean Difference (IV, Random, 95% CI) | −1.30 [−3.35, 0.75] |
| 12.3 long term | 1 | 81 | Mean Difference (IV, Random, 95% CI) | −0.83 [−2.27, 0.61] |
| 13 Mental state: 3c. Negative symptoms - average endpoint score - short term (BPRS negative subscore, high = poor) | 1 | 25 | Mean Difference (IV, Random, 95% CI) | −0.57 [−0.97, −0.17] |
| 14 Quality of life: General - average endpoint score - short term (QLS total score, high = poor) | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 0.5 [−12.87, 13.87] |
| 15 Service use: Number of participants rehospitalised | 2 | 877 | Risk Ratio (M-H, Random, 95% CI) | 0.75 [0.56, 1.00] |
| 15.1 medium term | 1 | 199 | Risk Ratio (M-H, Random, 95% CI) | 0.77 [0.42, 1.41] |
| 15.2 long term | 1 | 678 | Risk Ratio (M-H, Random, 95% CI) | 0.74 [0.53, 1.03] |
| 16 Adverse effects: 1. General - at least one adverse effect | 8 | 2226 | Risk Ratio (M-H, Random, 95% CI) | 0.96 [0.85, 1.08] |
| 17 Adverse effects: 2. Death | 5 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 17.1 natural causes | 2 | 982 | Risk Ratio (M-H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 17.2 suicide attempt | 2 | 945 | Risk Ratio (M-H, Random, 95% CI) | 2.30 [0.34, 15.65] |
| 17.3 suicide | 3 | 1139 | Risk Ratio (M-H, Random, 95% CI) | 0.71 [0.05, 9.16] |
| 18 Adverse effects: 3a. Cardiac effects - QTc prolongation | 2 | 1351 | Risk Ratio (M-H, Random, 95% CI) | 1.15 [0.39, 3.40] |
| 19 Adverse effects: 3b. Cardiac effects - QTc abnormalities - change from baseline in ms | 3 | 940 | Mean Difference (IV, Random, 95% CI) | −2.21 [−9.48, 5.05] |
| 20 Adverse effects: 4. Central nervous system - sedation | 8 | 2226 | Risk Ratio (M-H, Random, 95% CI) | 0.82 [0.69, 0.97] |
| 21 Adverse effects: 5a. Extrapyramidal effects | 8 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 21.1 akathisia | 6 | 2170 | Risk Ratio (M-H, Random, 95% CI) | 1.61 [0.89, 2.94] |
| 21.2 akinesia | 1 | 267 | Risk Ratio (M-H, Random, 95% CI) | 1.10 [0.73, 1.65] |
| 21.3 dystonia | 1 | 673 | Risk Ratio (M-H, Random, 95% CI) | 18.16 [2.44, 135.27] |
| 21.4 extrapyramidal symptoms | 2 | 872 | Risk Ratio (M-H, Random, 95% CI) | 1.69 [1.23, 2.34] |
| 21.5 parkinsonism | 2 | 717 | Risk Ratio (M-H, Random, 95% CI) | 17.0 [1.04, 277.61] |
| 21.6 rigor | 1 | 309 | Risk Ratio (M-H, Random, 95% CI) | 2.24 [0.80, 6.30] |
| 21.7 use of antiparkinson medication | 6 | 1715 | Risk Ratio (M-H, Random, 95% CI) | 1.98 [1.16, 3.39] |
| 22 Adverse effects: 5b. Extrapyramidal effects - scale measured | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 22.1 abnormal involuntary movement: AIMS (high = poor) | 2 | 958 | Mean Difference (IV, Random, 95% CI) | 0.34 [−0.08, 0.75] |
| 22.2 akathisia: Barnes Akathisia Scale (high = poor) | 2 | 700 | Mean Difference (IV, Random, 95% CI) | 0.73 [−0.54, 2.00] |
| 22.3 extrapyramidal symptoms: Simpson-Angus Scale (high = poor) | 5 | 1077 | Mean Difference (IV, Random, 95% CI) | 0.59 [0.02, 1.16] |
| 23 Adverse effects: 6. Haematological: Important decline in white blood cells | 1 | 673 | Risk Ratio (M-H, Random, 95% CI) | 0.34 [0.01, 8.23] |
| 24 Adverse effects: 7a. Prolactin associated side effects | 6 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 24.1 amenorrhea | 4 | 359 | Risk Ratio (M-H, Random, 95% CI) | 2.14 [1.26, 3.64] |
| 24.2 dysmenorrhea | 1 | 163 | Risk Ratio (M-H, Random, 95% CI) | 2.23 [0.42, 11.86] |
| 24.3 galactorrhea | 5 | 478 | Risk Ratio (M-H, Random, 95% CI) | 2.65 [1.19, 5.91] |
| 24.4 gynecomastia | 1 | 78 | Risk Ratio (M-H, Random, 95% CI) | 4.33 [1.34, 14.02] |
| 24.5 sexual dysfunction | 6 | 2157 | Risk Ratio (M-H, Random, 95% CI) | 1.24 [0.99, 1.54] |
| 25 Adverse effects: 7b. Prolactin - change from baseline in mg/dl | 6 | 1731 | Mean Difference (IV, Random, 95% CI) | 35.28 [26.19, 44.36] |
| 26 Adverse effects: 8a. Metabolic - cholesterol - significant cholesterol increase | 2 | 940 | Risk Ratio (M-H, Random, 95% CI) | 0.79 [0.45, 1.39] |
| 27 Adverse effects: 8b. Metabolic - cholesterol - change from baseline in mg/dl | 5 | 1433 | Mean Difference (IV, Random, 95% CI) | −8.49 [−12.23, −4.75] |
| 28 Adverse effects: 8c. Metabolic - glucose - abnormally high fasting glucose value | 2 | 940 | Risk Ratio (M-H, Random, 95% CI) | 0.72 [0.29, 1.79] |
| 29 Adverse effects: 8d. Metabolic -glucose - change from baseline in mg/dl | 5 | 1436 | Mean Difference (IV, Random, 95% CI) | 0.04 [−2.83, 2.92] |
| 30 Adverse effects: 8e. Metabolic -weight gain of 7% or more of total body weight | 7 | 1942 | Risk Ratio (M-H, Random, 95% CI) | 1.03 [0.88, 1.22] |
| 31 Adverse effects: 8f. Metabolic - weight gain - change from baseline in kg | 7 | 1446 | Mean Difference (IV, Random, 95% CI) | −0.71 [−2.47, 1.04] |
Comparison 6. RISPERIDONE versus SERTINDOLE - all data short term.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state - 1a. No clinically significant response (as defined by the original studies) | 1 | 187 | Risk Ratio (M-H, Random, 95% CI) | 1.14 [0.92, 1.40] |
| 2 Global state - 1b. No clinically important change (as defined by the original studies) | 1 | 187 | Risk Ratio (M-H, Random, 95% CI) | 1.24 [0.91, 1.68] |
| 3 Leaving the study early | 2 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 due to any reason | 2 | 508 | Risk Ratio (M-H, Random, 95% CI) | 0.81 [0.63, 1.06] |
| 3.2 due to adverse events | 2 | 508 | Risk Ratio (M-H, Random, 95% CI) | 0.73 [0.39, 1.35] |
| 3.3 due to inefficacy | 2 | 508 | Risk Ratio (M-H, Random, 95% CI) | 0.76 [0.46, 1.25] |
| 4 Mental state: 1a. General - no clinically important change (less than 50% PANSS total score reduction) | 1 | 187 | Risk Ratio (M-H, Random, 95% CI) | 1.14 [0.92, 1.40] |
| 5 Mental state: 1b. General - average endpoint score (PANSS total, high = poor) | 2 | 493 | Mean Difference (IV, Random, 95% CI) | −1.98 [−12.20, 8.24] |
| 6 Mental state: 2. Positive symptoms - average endpoint score (PANSS positive, high = poor) | 1 | 172 | Mean Difference (IV, Random, 95% CI) | 0.80 [−1.35, 2.95] |
| 7 Mental state: 3. Negative symptoms - average endpoint score (PANSS negative, high = poor) | 1 | 172 | Mean Difference (IV, Random, 95% CI) | 1.30 [−0.53, 3.13] |
| 8 General functioning: General - average endpoint score - (GAF total, high = poor) | 1 | 114 | Mean Difference (IV, Random, 95% CI) | 2.90 [−2.61, 8.41] |
| 9 Adverse effects: 1. General - at least one adverse effect | 2 | 508 | Risk Ratio (M-H, Random, 95% CI) | 0.97 [0.90, 1.05] |
| 10 Adverse effects: 2. Death - suicide | 1 | 187 | Risk Ratio (M-H, Random, 95% CI) | 3.3 [0.14, 79.98] |
| 11 Adverse effects: 3a. Cardiac effects - QTc prolongation | 2 | 508 | Risk Ratio (M-H, Random, 95% CI) | 0.21 [0.08, 0.51] |
| 12 Adverse effects: 3b. Cardiac effects - QTc abnormalities - change from baseline in ms | 2 | 495 | Mean Difference (IV, Random, 95% CI) | −18.60 [−22.37, −14. 83] |
| 13 Adverse effects: 4. Central nervous system - sedation | 2 | 508 | Risk Ratio (M-H, Random, 95% CI) | 1.15 [0.69, 1.92] |
| 14 Adverse effects: 5a. Extrapyramidal effects | 2 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 14.1 akathisia | 1 | 321 | Risk Ratio (M-H, Random, 95% CI) | 2.24 [1.02, 4.92] |
| 14.2 extrapyramidal symptoms | 1 | 187 | Risk Ratio (M-H, Random, 95% CI) | 1.53 [0.90, 2.61] |
| 14.3 parkinsonism | 1 | 321 | Risk Ratio (M-H, Random, 95% CI) | 4.11 [1.44, 11.73] |
| 14.4 tremor | 1 | 187 | Risk Ratio (M-H, Random, 95% CI) | 0.66 [0.16, 2.69] |
| 15 Adverse effects: 5b. Extrapyramidal symptom scales | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 15.1 abnormal involuntary movement: AIMS (high = poor) | 2 | 477 | Mean Difference (IV, Random, 95% CI) | 0.31 [−0.25, 0.86] |
| 15.2 akathisia: Barnes Akathisia Scale (high = poor) | 2 | 500 | Mean Difference (IV, Random, 95% CI) | 0.22 [0.03, 0.41] |
| 15.3 extrapyramidal symptoms: Simpson-Angus Scale (high = poor) | 2 | 500 | Mean Difference (IV, Random, 95% CI) | 0.37 [−0.61, 1.35] |
| 16 Adverse effects: 6. Prolactin associated side effects - sexual dysfunction | 2 | 437 | Risk Ratio (M-H, Random, 95% CI) | 0.34 [0.16, 0.76] |
| 16.1 abnormal ejaculation | 1 | 250 | Risk Ratio (M-H, Random, 95% CI) | 0.41 [0.16, 1.03] |
| 16.2 sexual dysfunction | 1 | 187 | Risk Ratio (M-H, Random, 95% CI) | 0.22 [0.05, 0.98] |
| 17 Adverse effects: 7a. Metabolic - cholesterol - change from baseline in mg/dl | 1 | 176 | Mean Difference (IV, Random, 95% CI) | 4.90 [−3.73, 13.53] |
| 18 Adverse effects: 7b. Metabolic - glucose - change from baseline in mg/dl | 1 | 176 | Mean Difference (IV, Random, 95% CI) | 2.00 [−5.85, 9.85] |
| 19 Adverse effects: 7c. Metabolic - weight gain | 1 | 187 | Risk Ratio (M-H, Random, 95% CI) | 0.77 [0.41, 1.43] |
| 20 Adverse effects: 7d. Metabolic - weight gain - change from baseline in kg | 2 | 328 | Mean Difference (IV, Random, 95% CI) | −0.99 [−1.86, −0.12] |
Comparison 7. RISPERIDONE versus ZIPRASIDONE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1a. No clinically significant response (as defined by the original studies) | 1 | 296 | Risk Ratio (M-H, Random, 95% CI) | 1.02 [0.93, 1.12] |
| 2 Global state: 1b. No clinically important change - short term (as defined by the original studies) | 1 | 296 | Risk Ratio (M-H, Random, 95% CI) | 0.81 [0.63, 1.04] |
| 3 Leaving the study early | 3 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 due to any reason | 3 | 1029 | Risk Ratio (M-H, Random, 95% CI) | 0.90 [0.83, 0.98] |
| 3.2 due to adverse events | 3 | 1029 | Risk Ratio (M-H, Random, 95% CI) | 0.82 [0.50, 1.32] |
| 3.3 due to inefficacy | 3 | 1029 | Risk Ratio (M-H, Random, 95% CI) | 0.88 [0.60, 1.27] |
| 4 Mental state: 1a. General - no clinically important change - short term (less than 50% PANSS total reduction) | 1 | 296 | Risk Ratio (M-H, Random, 95% CI) | 1.02 [0.93, 1.12] |
| 5 Mental state: 1b. General - average endpoint score (PANSS total, high = poor) | 3 | 1016 | Mean Difference (IV, Random, 95% CI) | −3.91 [−7.55, −0.27] |
| 5.1 short term | 1 | 296 | Mean Difference (IV, Random, 95% CI) | −1.5 [−3.16, 0.16] |
| 5.2 medium term | 1 | 204 | Mean Difference (IV, Random, 95% CI) | −6.30 [−12.77, 0.17] |
| 5.3 long term | 1 | 516 | Mean Difference (IV, Random, 95% CI) | −6.01 [−10.03, −1.99] |
| 6 Mental state: 1c. General - avergae endpoint score - short term (BPRS total, high = poor) | 1 | 296 | Mean Difference (IV, Random, 95% CI) | −0.70 [−4.33, 2.93] |
| 7 Mental state: 2a. Positive symptoms - average endpoint score - medium term (PANSS positive, high = poor) | 1 | 204 | Mean Difference (IV, Random, 95% CI) | −2.5 [−4.62, −0.38] |
| 8 Mental State: 2b. Positive symptoms - average endpoint score - short term (BPRS positive, high = poor) | 1 | 296 | Mean Difference (IV, Random, 95% CI) | −0.5 [−1.15, 0.15] |
| 9 Mental state: 3. Negative symptoms - average endpoint score (PANSS negative, high = poor) | 2 | 500 | Mean Difference (IV, Random, 95% CI) | −0.04 [−1.20, 1.12] |
| 9.1 short term | 1 | 296 | Mean Difference (IV, Random, 95% CI) | 0.0 [−1.48, 1.48] |
| 9.2 medium term | 1 | 204 | Mean Difference (IV, Random, 95% CI) | −0.10 [−1.98, 1.78] |
| 10 Service use: Number of patients rehospitalised | 2 | 767 | Risk Ratio (M-H, Random, 95% CI) | 0.87 [0.63, 1.22] |
| 10.1 medium term | 1 | 241 | Risk Ratio (M-H, Random, 95% CI) | 0.96 [0.53, 1.73] |
| 10.2 long term | 1 | 526 | Risk Ratio (M-H, Random, 95% CI) | 0.84 [0.56, 1.25] |
| 11 Adverse effects: 1. General - at least one adverse effect | 3 | 1063 | Risk Ratio (M-H, Random, 95% CI) | 1.07 [0.98, 1.17] |
| 12 Adverse effects: 2. Death | 2 | 767 | Risk Ratio (M-H, Random, 95% CI) | 0.84 [0.16, 4.58] |
| 12.1 suicide attempt | 1 | 526 | Risk Ratio (M-H, Random, 95% CI) | 1.09 [0.10, 11.89] |
| 12.2 suicide | 1 | 241 | Risk Ratio (M-H, Random, 95% CI) | 0.66 [0.06, 7.17] |
| 13 Adverse effects: 3a. Cardiac effects - QTc prolongation | 2 | 822 | Risk Ratio (M-H, Random, 95% CI) | 1.90 [0.40, 9.05] |
| 14 Adverse effects: 3b. Cardiac effects - QTc abnormalities - change from baseline in ms | 3 | 793 | Mean Difference (IV, Random, 95% CI) | −2.24 [−6.39, 1.92] |
| 15 Adverse effects: 4. Central nervous system - sedation | 3 | 1063 | Risk Ratio (M-H, Random, 95% CI) | 1.15 [0.83, 1.59] |
| 16 Adverse effects: 5a. Extrapyramidal effects | 3 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 16.1 akathisia | 3 | 1063 | Risk Ratio (M-H, Random, 95% CI) | 1.02 [0.55, 1.89] |
| 16.2 extrapyramidal symptoms | 1 | 241 | Risk Ratio (M-H, Random, 95% CI) | 3.16 [1.15, 8.69] |
| 16.3 tremor | 1 | 296 | Risk Ratio (M-H, Random, 95% CI) | 0.95 [0.47, 1.89] |
| 16.4 use of antiparkinson medication | 822 | Risk Ratio (M-H, Random, 95% CI) | 1.42 [1.03, 1.96] | |
| 17 Adverse effects: 5b. Extrapyramidal symptoms -scale measured | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 17.1 abnormal involuntary movement: AIMS (high = poor) | 1 | 296 | Mean Difference (IV, Random, 95% CI) | 0.21 [0.17, 0.25] |
| 17.2 akathisia: Barnes Akathisia Scale (high = poor) | 1 | 296 | Mean Difference (IV, Random, 95% CI) | 0.56 [0.51, 0.61] |
| 17.3 extrapyramidal symptoms: Simpson-Angus Scale (high = poor) | 1 | 296 | Mean Difference (IV, Random, 95% CI) | 0.34 [0.26, 0.42] |
| 18 Adverse effects: 6a. Prolactin associated side effects | 3 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 18.1 abnormal ejaculation | 1 | 215 | Risk Ratio (M-H, Random, 95% CI) | 2.10 [0.65, 6.75] |
| 18.2 amenorrhea | 2 | 225 | Risk Ratio (M-H, Random, 95% CI) | 1.19 [0.60, 2.39] |
| 18.3 decreased libido | 1 | 296 | Risk Ratio (M-H, Random, 95% CI) | 1.65 [0.70, 3.86] |
| 18.4 erectile dysfunction | 1 | 215 | Risk Ratio (M-H, Random, 95% CI) | 1.05 [0.38, 2.88] |
| 18.5 galactorrhea | 2 | 159 | Risk Ratio (M-H, Random, 95% CI) | 5.07 [0.61, 42.45] |
| 18.6 orgastic dysfunction | 2 | 822 | Risk Ratio (M-H, Random, 95% CI) | 1.44 [1.03, 2.02] |
| 19 Adverse effects: 6b. Prolactin - change from baseline in ng/ml | 2 | 767 | Mean Difference (IV, Random, 95% CI) | 21.97 [16.60, 27.34] |
| 20 Adverse effects: 7a. Metabolic - cholesterol - change from baseline in mg/dl | 2 | 767 | Mean Difference (IV, Random, 95% CI) | 8.58 [1.11, 16.04] |
| 21 Adverse effects: 7b. Metabolic -glucose - change from baseline in mg/dl | 2 | 767 | Mean Difference (IV, Random, 95% CI) | 4.94 [−1.91, 11.80] |
| 22 Adverse effects: 7c. Metabolic -weight gain of 7% or more of total body weight | 3 | 1063 | Risk Ratio (M-H, Random, 95% CI) | 2.03 [1.35, 3.06] |
| 23 Adverse effects: 7d. Metabolic - weight gain - change from baseline in kg | 1 | 461 | Mean Difference (IV, Random, 95% CI) | 1.1 [−0.15, 2.35] |
Comparison 8. RISPERIDONE versus CLOZAPINE - sensitivity analysis (skewed data included).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mental state: 1. General - average endpoint score (BPRS total, high = poor) | 3 | 368 | Mean Difference (IV, Random, 95% CI) | 2.84 [−1.36, 7.03] |
| 1.1 short term | 2 | 316 | Mean Difference (IV, Random, 95% CI) | 5.19 [2.12, 8.26] |
| 1.2 long term | 1 | 52 | Mean Difference (IV, Random, 95% CI) | −0.20 [−4.12, 3.72] |
| 2 Mental state: 2. Positive symptoms - average endpoint score (PANSS positive, high = poor) | 4 | 442 | Mean Difference (IV, Random, 95% CI) | 0.60 [−1.34, 2.53] |
| 2.1 short term | 3 | 361 | Mean Difference (IV, Random, 95% CI) | 0.24 [−2.72, 3.19] |
| 2.2 medium term | 1 | 81 | Mean Difference (IV, Random, 95% CI) | 0.40 [−2.78, 3.58] |
| 3 Mental state: 3. Negative symptoms - average endpoint score (PANSS negative, high = poor) | 4 | 442 | Mean Difference (IV, Random, 95% CI) | 0.14 [−2.02, 2.31] |
| 3.1 short term | 3 | 361 | Mean Difference (IV, Random, 95% CI) | −0.39 [−3.37, 2.59] |
| 3.2 medium term | 1 | 81 | Mean Difference (IV, Random, 95% CI) | 1.40 [−1.42, 4.22] |
Comparison 9. RISPERIDONE versus OLANZAPINE - sensitivity analysis (skewed data excluded).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mental state: 1a. General - average endpoint score (PANSS total, high = poor) | 14 | 2348 | Mean Difference (IV, Random, 95% CI) | 1.99 [0.60, 3.37] |
| 1.1 short term | 6 | 686 | Mean Difference (IV, Random, 95% CI) | 1.02 [−1.11, 3.15] |
| 1.2 medium term | 3 | 231 | Mean Difference (IV, Random, 95% CI) | 4.11 [−0.71, 8.93] |
| 1.3 long term | 5 | 1431 | Mean Difference (IV, Random, 95% CI) | 2.59 [0.20, 4.98] |
| 2 Mental state: 2. General - average endpoint score (BPRS total, high = poor) | 2 | 393 | Mean Difference (IV, Random, 95% CI) | 4.28 [−1.34, 9.91] |
| 2.1 Long term | 2 | 393 | Mean Difference (IV, Random, 95% CI) | 4.28 [−1.34, 9.91] |
| 3 Mental state: 3. Positive symptoms - average endpoint score (PANSS positive, high = poor) | 12 | 1663 | Mean Difference (IV, Random, 95% CI) | 0.62 [0.03, 1.21] |
| 3.1 short term | 4 | 622 | Mean Difference (IV, Random, 95% CI) | −0.28 [−1.62, 1.07] |
| 3.2 medium term | 3 | 231 | Mean Difference (IV, Random, 95% CI) | 1.58 [−0.03, 3.20] |
| 3.3 long term | 5 | 810 | Mean Difference (IV, Random, 95% CI) | 0.68 [−0.04, 1.40] |
| 4 Adverse effects: 1. Extrapyramidal effects - scale measured | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 dyskinesia: ESRS subscore for dyskinesia (high = poor) | 2 | 401 | Mean Difference (IV, Random, 95% CI) | 0.10 [−0.42, 0.62] |
| 4.2 extrapyramidal symptoms: ESRS total score (high = poor) | 2 | 530 | Mean Difference (IV, Random, 95% CI) | 0.16 [−0.58, 0.90] |
| 4.3 extrapyramidal symptoms: Simpson-Angus Scale (high = poor) | 4 | 487 | Mean Difference (IV, Random, 95% CI) | 0.75 [−0.10, 1.59] |
| 4.4 parkinsonism: ESRS subscore for parkinsonism (high = poor) | 2 | 401 | Mean Difference (IV, Random, 95% CI) | 1.06 [−1.31, 3.42] |
| 5 Adverse effects: 2. Prolactin associated side effects - change from baseline in ng/ml | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 change from baseline in ng/ml | 5 | 1256 | Mean Difference (IV, Random, 95% CI) | 24.70 [21.08, 28.33] |
Comparison 10. RISPERIDONE versus QUETIAPINE - sensitivity analysis (skewed data included).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mental state: 1. Positive symptoms - average endpoint score (PANSS positive, high = poor) | 6 | 1225 | Mean Difference (IV, Random, 95% CI) | −1.76 [−2.48, −1.04] |
| 1.1 short term | 3 | 998 | Mean Difference (IV, Random, 95% CI) | −2.08 [−3.56, −0.60] |
| 1.2 medium term | 2 | 146 | Mean Difference (IV, Random, 95% CI) | −2.15 [−4.31, 0.01] |
| 1.3 long term | 1 | 81 | Mean Difference (IV, Random, 95% CI) | −1.30 [−2.73, 0.13] |
| 2 Adverse effects: 1. Extrapyramidal effects - scale measured | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 extrapyramidal symptoms: Simpson-Angus Scale (high = poor) | 4 | 1033 | Mean Difference (IV, Random, 95% CI) | 0.82 [−0.31, 1.95] |
Analysis 1.1. Comparison 1 RISPERIDONE versus AMISULPRIDE, Outcome 1 Global state: 1a. No clinically significant response (as defined by the original studies).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 1 RISPERIDONE versus AMISULPRIDE
Outcome: 1 Global state: 1a. No clinically significant response (as defined by the original studies)
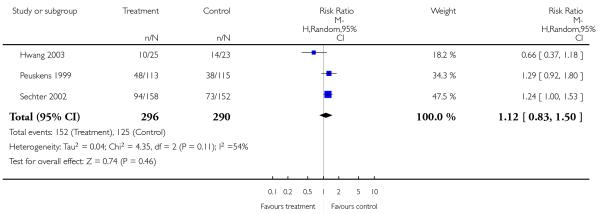
|
Analysis 1.2. Comparison 1 RISPERIDONE versus AMISULPRIDE, Outcome 2 Global state: 1b. No clinically important change (as defined by the original studies).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 1 RISPERIDONE versus AMISULPRIDE
Outcome: 2 Global state: 1b. No clinically important change (as defined by the original studies)
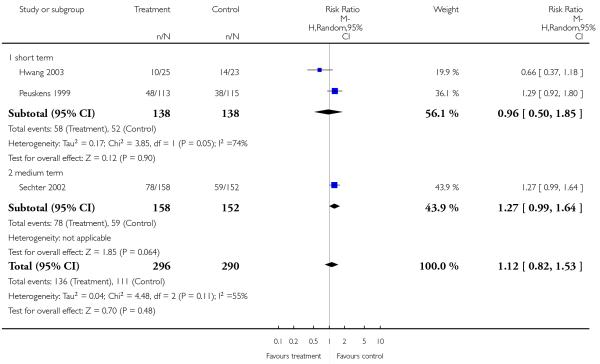
|
Analysis 1.3. Comparison 1 RISPERIDONE versus AMISULPRIDE, Outcome 3 Global state: 1c. Relapse - medium term (as defined by the original studies).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 1 RISPERIDONE versus AMISULPRIDE
Outcome: 3 Global state: 1c. Relapse - medium term (as defined by the original studies)
|
Analysis 1.4. Comparison 1 RISPERIDONE versus AMISULPRIDE, Outcome 4 Leaving the study early.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 1 RISPERIDONE versus AMISULPRIDE
Outcome: 4 Leaving the study early

|
Analysis 1.5. Comparison 1 RISPERIDONE versus AMISULPRIDE, Outcome 5 Mental state: 1a. General - no clinically important change - medium term (less than 50% PANSS total reduction).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 1 RISPERIDONE versus AMISULPRIDE
Outcome: 5 Mental state: 1a. General - no clinically important change - medium term (less than 50% PANSS total reduction)
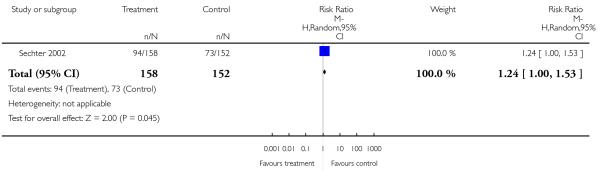
|
Analysis 1.6. Comparison 1 RISPERIDONE versus AMISULPRIDE, Outcome 6 Mental state: 1b. General - no clinically important change - short term (less than 20% PANSS total reduction).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 1 RISPERIDONE versus AMISULPRIDE
Outcome: 6 Mental state: 1b. General - no clinically important change - short term (less than 20% PANSS total reduction)

|
Analysis 1.7. Comparison 1 RISPERIDONE versus AMISULPRIDE, Outcome 7 Mental state: 1c. General - no clinically important change - medium term (less than 50% BPRS total reduction).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 1 RISPERIDONE versus AMISULPRIDE
Outcome: 7 Mental state: 1c. General - no clinically important change - medium term (less than 50% BPRS total reduction)
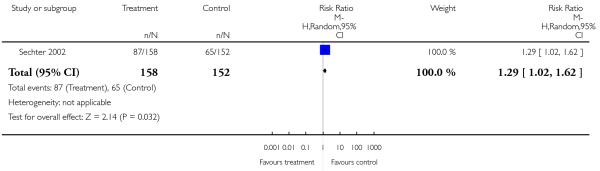
|
Analysis 1.8. Comparison 1 RISPERIDONE versus AMISULPRIDE, Outcome 8 Mental state: 1d. General - no clinically important change - short term (less than 40% BPRS total reduction).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 1 RISPERIDONE versus AMISULPRIDE
Outcome: 8 Mental state: 1d. General - no clinically important change - short term (less than 40% BPRS total reduction)
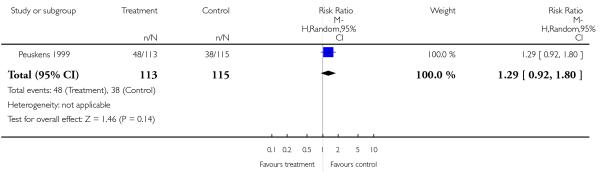
|
Analysis 1.9. Comparison 1 RISPERIDONE versus AMISULPRIDE, Outcome 9 Mental state: 1e. General - average endpoint score - (PANSS total, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 1 RISPERIDONE versus AMISULPRIDE
Outcome: 9 Mental state: 1e. General - average endpoint score - (PANSS total, high = poor)
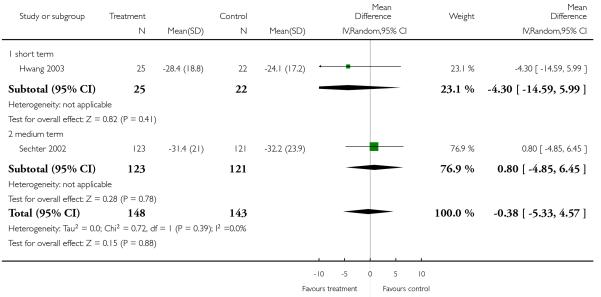
|
Analysis 1.10. Comparison 1 RISPERIDONE versus AMISULPRIDE, Outcome 10 Mental state: 1f. General - average endpoint score - BPRS total score (high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 1 RISPERIDONE versus AMISULPRIDE
Outcome: 10 Mental state: 1f. General - average endpoint score - BPRS total score (high = poor)
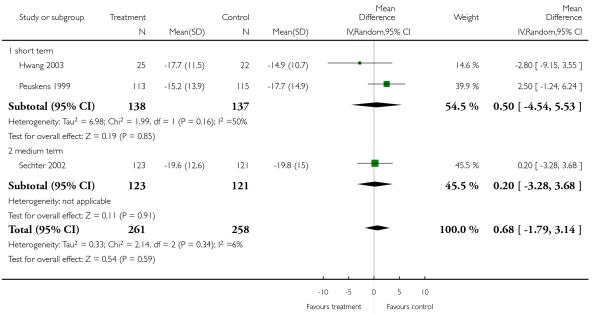
|
Analysis 1.11. Comparison 1 RISPERIDONE versus AMISULPRIDE, Outcome 11 Mental state: 2a. Positive symptoms - average endpoint score - (PANSS positive, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 1 RISPERIDONE versus AMISULPRIDE
Outcome: 11 Mental state: 2a. Positive symptoms - average endpoint score - (PANSS positive, high = poor)
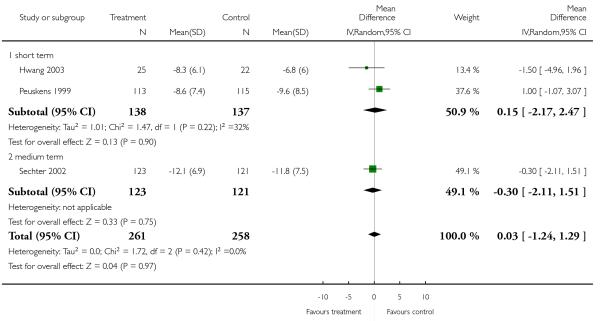
|
Analysis 1.12. Comparison 1 RISPERIDONE versus AMISULPRIDE, Outcome 12 Mental state: 2b. Positive symptoms - average endpoint score - short term (BPRS positive, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 1 RISPERIDONE versus AMISULPRIDE
Outcome: 12 Mental state: 2b. Positive symptoms - average endpoint score - short term (BPRS positive, high = poor)
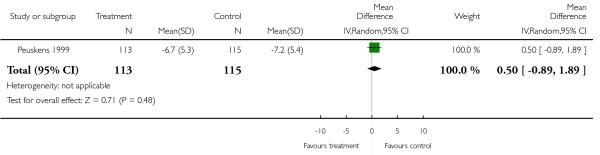
|
Analysis 1.13. Comparison 1 RISPERIDONE versus AMISULPRIDE, Outcome 13 Mental state: 3a. Negative symptoms - average endpoint score (PANSS negative, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 1 RISPERIDONE versus AMISULPRIDE
Outcome: 13 Mental state: 3a. Negative symptoms - average endpoint score (PANSS negative, high = poor)
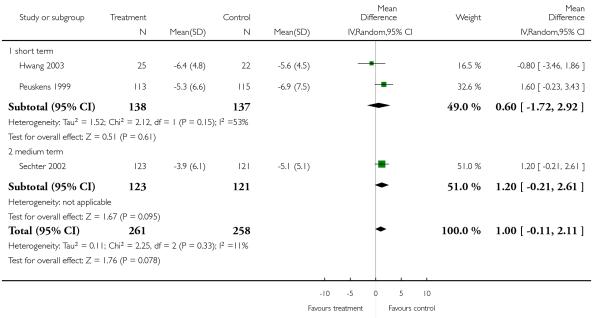
|
Analysis 1.14. Comparison 1 RISPERIDONE versus AMISULPRIDE, Outcome 14 Mental state: 3b. Negative symptoms - average endpoint scale (SANS total, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 1 RISPERIDONE versus AMISULPRIDE
Outcome: 14 Mental state: 3b. Negative symptoms - average endpoint scale (SANS total, high = poor)
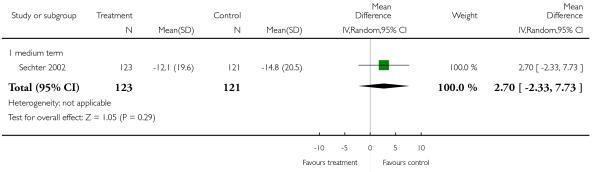
|
Analysis 1.15. Comparison 1 RISPERIDONE versus AMISULPRIDE, Outcome 15 General functioning: General 1a. No clinically important change - medium term (less than 50% SOFAS total score reduction).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 1 RISPERIDONE versus AMISULPRIDE
Outcome: 15 General functioning: General 1a. No clinically important change - medium term (less than 50% SOFAS total score reduction)
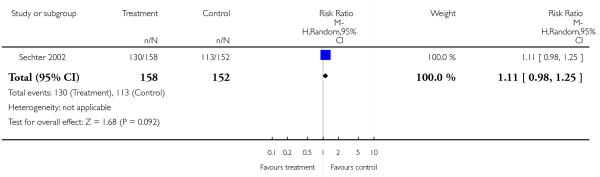
|
Analysis 1.16. Comparison 1 RISPERIDONE versus AMISULPRIDE, Outcome 16 General functioning: General 1b. average endpoint score - (SOFAS total score, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 1 RISPERIDONE versus AMISULPRIDE
Outcome: 16 General functioning: General 1b. average endpoint score - (SOFAS total score, high = poor)

|
Analysis 1.17. Comparison 1 RISPERIDONE versus AMISULPRIDE, Outcome 17 Adverse effects: 1. General - at least one adverse effect.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 1 RISPERIDONE versus AMISULPRIDE
Outcome: 17 Adverse effects: 1. General - at least one adverse effect
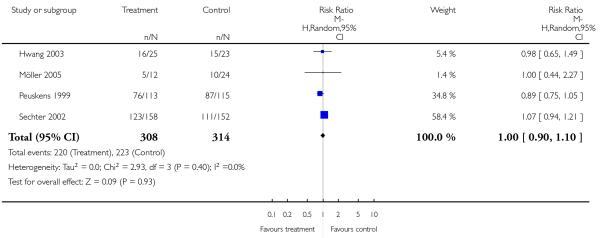
|
Analysis 1.18. Comparison 1 RISPERIDONE versus AMISULPRIDE, Outcome 18 Adverse effects: 2. Death.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 1 RISPERIDONE versus AMISULPRIDE
Outcome: 18 Adverse effects: 2. Death
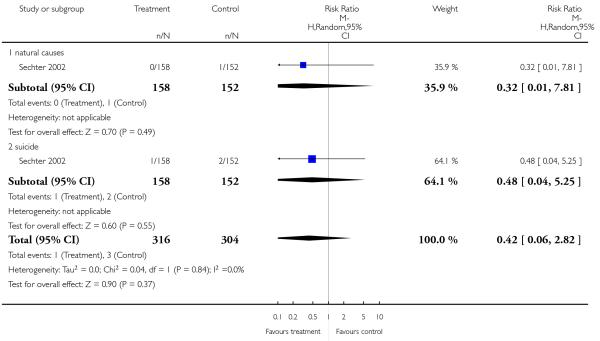
|
Analysis 1.19. Comparison 1 RISPERIDONE versus AMISULPRIDE, Outcome 19 Adverse effects: 3a. Cardiac effects - QTc prolongation.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 1 RISPERIDONE versus AMISULPRIDE
Outcome: 19 Adverse effects: 3a. Cardiac effects - QTc prolongation

|
Analysis 1.20. Comparison 1 RISPERIDONE versus AMISULPRIDE, Outcome 20 Adverse effects: 4a. Central nervous system - sedation.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 1 RISPERIDONE versus AMISULPRIDE
Outcome: 20 Adverse effects: 4a. Central nervous system - sedation
|
Analysis 1.21. Comparison 1 RISPERIDONE versus AMISULPRIDE, Outcome 21 Adverse effects: 4b. Central nervous system - seizures.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 1 RISPERIDONE versus AMISULPRIDE
Outcome: 21 Adverse effects: 4b. Central nervous system - seizures
|
Analysis 1.22. Comparison 1 RISPERIDONE versus AMISULPRIDE, Outcome 22 Adverse effects: 5a. Extrapyramidal effects.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 1 RISPERIDONE versus AMISULPRIDE
Outcome: 22 Adverse effects: 5a. Extrapyramidal effects
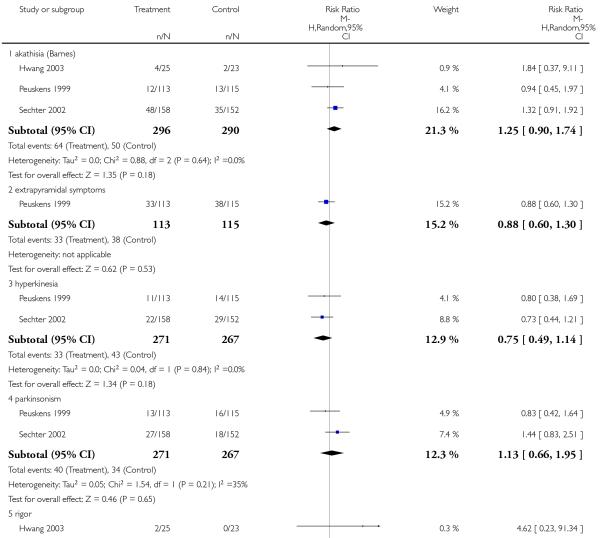
|

|
Analysis 1.23. Comparison 1 RISPERIDONE versus AMISULPRIDE, Outcome 23 Adverse effects: 5b Extrapyramidal effects - scale measured.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 1 RISPERIDONE versus AMISULPRIDE
Outcome: 23 Adverse effects: 5b Extrapyramidal effects - scale measured

|
Analysis 1.24. Comparison 1 RISPERIDONE versus AMISULPRIDE, Outcome 24 Adverse effects: 6. Prolactin associated side effects.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 1 RISPERIDONE versus AMISULPRIDE
Outcome: 24 Adverse effects: 6. Prolactin associated side effects
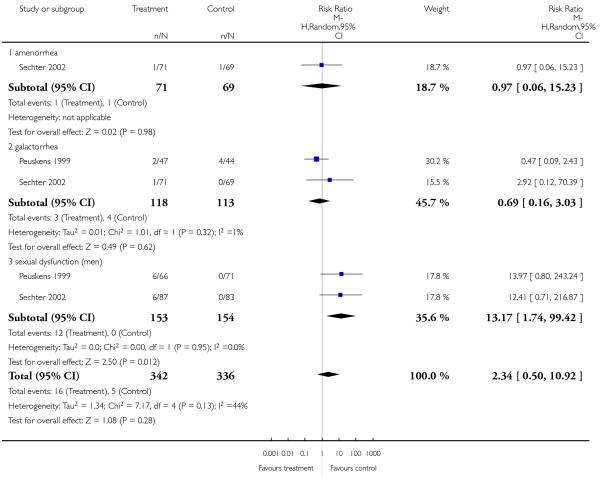
|
Analysis 1.25. Comparison 1 RISPERIDONE versus AMISULPRIDE, Outcome 25 Adverse effects: 8a. Metabolic effects - weight gain.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 1 RISPERIDONE versus AMISULPRIDE
Outcome: 25 Adverse effects: 8a. Metabolic effects - weight gain
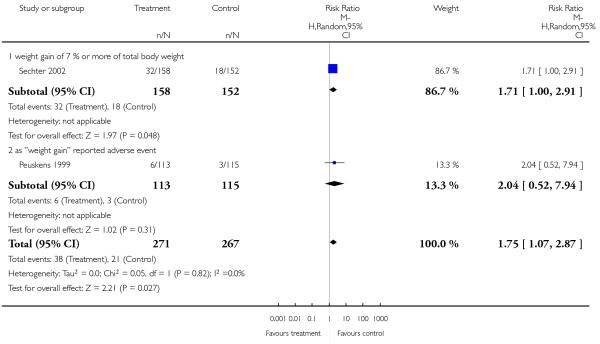
|
Analysis 1.26. Comparison 1 RISPERIDONE versus AMISULPRIDE, Outcome 26 Adverse effects: 8b Metabolic effects - weight gain - change from baseline in kg.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 1 RISPERIDONE versus AMISULPRIDE
Outcome: 26 Adverse effects: 8b Metabolic effects - weight gain - change from baseline in kg

|
Analysis 2.1. Comparison 2 RISPERIDONE versus ARIPIPRAZOLE - all data short term, Outcome 1 Global state: 1a. No clinically significant response (as defined by the original studies).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 2 RISPERIDONE versus ARIPIPRAZOLE - all data short term
Outcome: 1 Global state: 1a. No clinically significant response (as defined by the original studies)
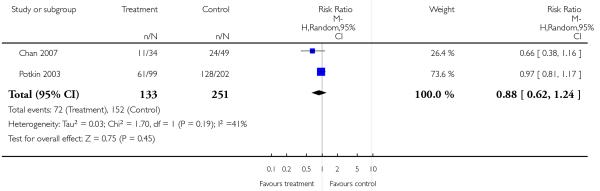
|
Analysis 2.2. Comparison 2 RISPERIDONE versus ARIPIPRAZOLE - all data short term, Outcome 2 Global state: 1b. No clinically important change (as defined by the original studies).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 2 RISPERIDONE versus ARIPIPRAZOLE - all data short term
Outcome: 2 Global state: 1b. No clinically important change (as defined by the original studies)
|
Analysis 2.3. Comparison 2 RISPERIDONE versus ARIPIPRAZOLE - all data short term, Outcome 3 Leaving the study early.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 2 RISPERIDONE versus ARIPIPRAZOLE - all data short term
Outcome: 3 Leaving the study early
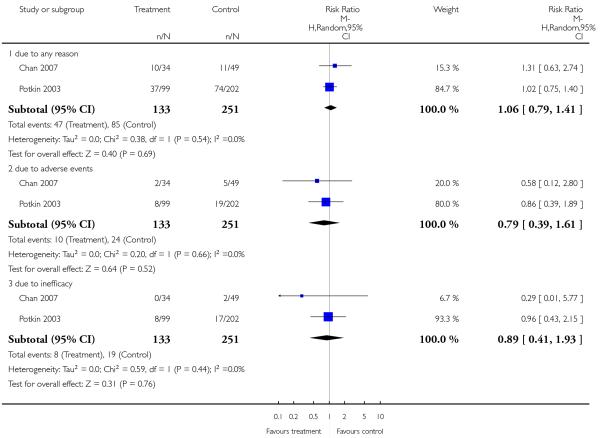
|
Analysis 2.4. Comparison 2 RISPERIDONE versus ARIPIPRAZOLE - all data short term, Outcome 4 Mental state: 1a. General - average endpoint score (PANSS total, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 2 RISPERIDONE versus ARIPIPRAZOLE - all data short term
Outcome: 4 Mental state: 1a. General - average endpoint score (PANSS total, high = poor)
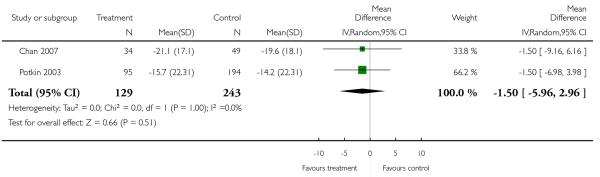
|
Analysis 2.5. Comparison 2 RISPERIDONE versus ARIPIPRAZOLE - all data short term, Outcome 5 Mental state: 2. Positive symptoms average endpoint score (PANSS positive, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 2 RISPERIDONE versus ARIPIPRAZOLE - all data short term
Outcome: 5 Mental state: 2. Positive symptoms average endpoint score (PANSS positive, high = poor)
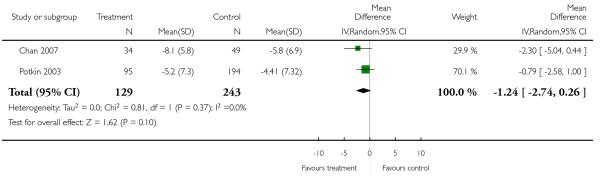
|
Analysis 2.6. Comparison 2 RISPERIDONE versus ARIPIPRAZOLE - all data short term, Outcome 6 Mental state: 3. Negative symptoms - average endpoint score - (PANSS negative, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 2 RISPERIDONE versus ARIPIPRAZOLE - all data short term
Outcome: 6 Mental state: 3. Negative symptoms - average endpoint score - (PANSS negative, high = poor)
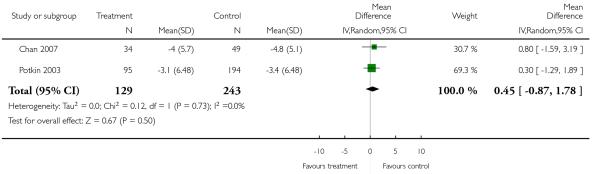
|
Analysis 2.7. Comparison 2 RISPERIDONE versus ARIPIPRAZOLE - all data short term, Outcome 7 Adverse effects: 1. General - at least one adverse effect.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 2 RISPERIDONE versus ARIPIPRAZOLE - all data short term
Outcome: 7 Adverse effects: 1. General - at least one adverse effect
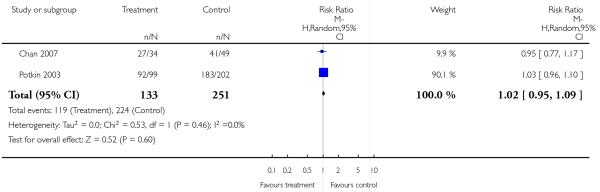
|
Analysis 2.8. Comparison 2 RISPERIDONE versus ARIPIPRAZOLE - all data short term, Outcome 8 Adverse effects: 2a. Cardiac effects - QTc prolongation.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 2 RISPERIDONE versus ARIPIPRAZOLE - all data short term
Outcome: 8 Adverse effects: 2a. Cardiac effects - QTc prolongation
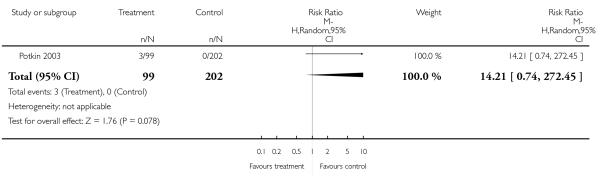
|
Analysis 2.9. Comparison 2 RISPERIDONE versus ARIPIPRAZOLE - all data short term, Outcome 9 Adverse effects: 2b. Cardiac effects - QTc abnormalities - change from baseline in ms.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 2 RISPERIDONE versus ARIPIPRAZOLE - all data short term
Outcome: 9 Adverse effects: 2b. Cardiac effects - QTc abnormalities - change from baseline in ms
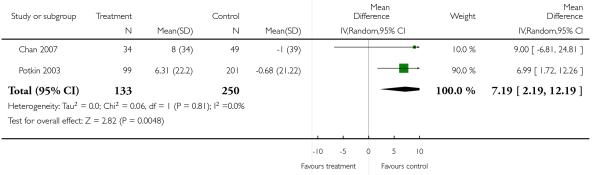
|
Analysis 2.10. Comparison 2 RISPERIDONE versus ARIPIPRAZOLE - all data short term, Outcome 10 Adverse effects: 3a. Extrapyramidal effects.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 2 RISPERIDONE versus ARIPIPRAZOLE - all data short term
Outcome: 10 Adverse effects: 3a. Extrapyramidal effects

|
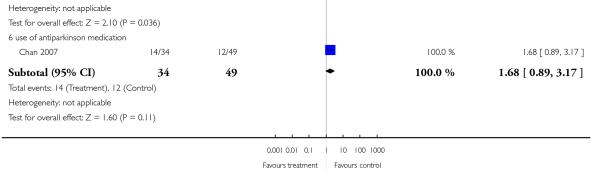
|
Analysis 2.11. Comparison 2 RISPERIDONE versus ARIPIPRAZOLE - all data short term, Outcome 11 Adverse effects: 3b. Extrapyramidal effects - scale measured.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 2 RISPERIDONE versus ARIPIPRAZOLE - all data short term
Outcome: 11 Adverse effects: 3b. Extrapyramidal effects - scale measured
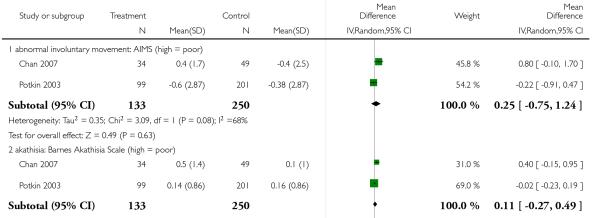
|
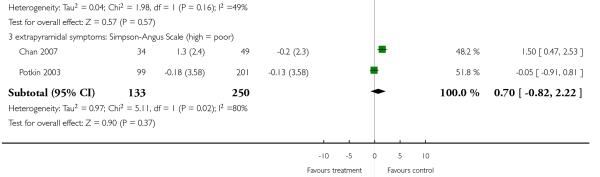
|
Analysis 2.12. Comparison 2 RISPERIDONE versus ARIPIPRAZOLE - all data short term, Outcome 12 Adverse effects: 4a. Prolactin associated side effects.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 2 RISPERIDONE versus ARIPIPRAZOLE - all data short term
Outcome: 12 Adverse effects: 4a. Prolactin associated side effects

|
Analysis 2.13. Comparison 2 RISPERIDONE versus ARIPIPRAZOLE - all data short term, Outcome 13 Adverse effects: 4b. Prolactin - change from baseline in ng/ml.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 2 RISPERIDONE versus ARIPIPRAZOLE - all data short term
Outcome: 13 Adverse effects: 4b. Prolactin - change from baseline in ng/ml
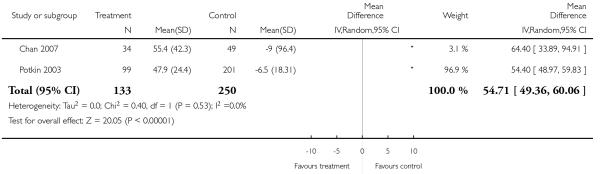
|
Analysis 2.14. Comparison 2 RISPERIDONE versus ARIPIPRAZOLE - all data short term, Outcome 14 Adverse effects: 5a. Metabolic effects - cholesterol - change from baseline in mg/dl.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 2 RISPERIDONE versus ARIPIPRAZOLE - all data short term
Outcome: 14 Adverse effects: 5a. Metabolic effects - cholesterol - change from baseline in mg/dl
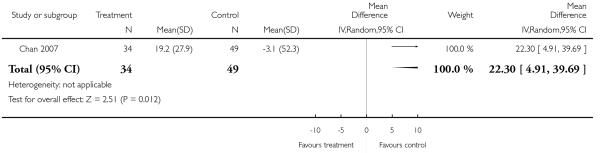
|
Analysis 2.15. Comparison 2 RISPERIDONE versus ARIPIPRAZOLE - all data short term, Outcome 15 Adverse effects: 5b. Metabolic effects - glucose - change from baseline in mg/dl.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 2 RISPERIDONE versus ARIPIPRAZOLE - all data short term
Outcome: 15 Adverse effects: 5b. Metabolic effects - glucose - change from baseline in mg/dl
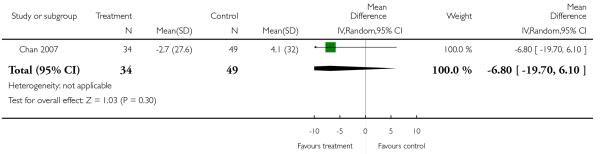
|
Analysis 2.16. Comparison 2 RISPERIDONE versus ARIPIPRAZOLE - all data short term, Outcome 16 Adverse effects: 5c. Metabolic effects - weight gain of 7% or more of total body weight.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 2 RISPERIDONE versus ARIPIPRAZOLE - all data short term
Outcome: 16 Adverse effects: 5c. Metabolic effects - weight gain of 7% or more of total body weight
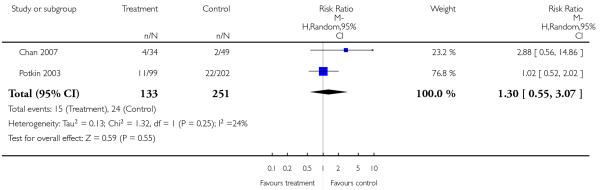
|
Analysis 2.17. Comparison 2 RISPERIDONE versus ARIPIPRAZOLE - all data short term, Outcome 17 Adverse effects: 5d. Metabolic effects - weight gain - change from baseline in kg.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 2 RISPERIDONE versus ARIPIPRAZOLE - all data short term
Outcome: 17 Adverse effects: 5d. Metabolic effects - weight gain - change from baseline in kg
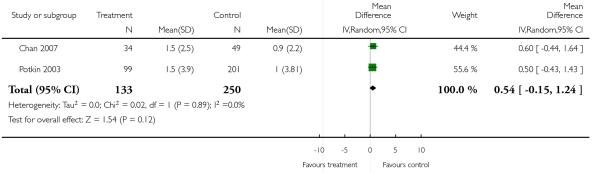
|
Analysis 3.1. Comparison 3 RISPERIDONE versus CLOZAPINE, Outcome 1 Global state: 1a. No clinically significant response (as defined by the original studies).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 3 RISPERIDONE versus CLOZAPINE
Outcome: 1 Global state: 1a. No clinically significant response (as defined by the original studies)
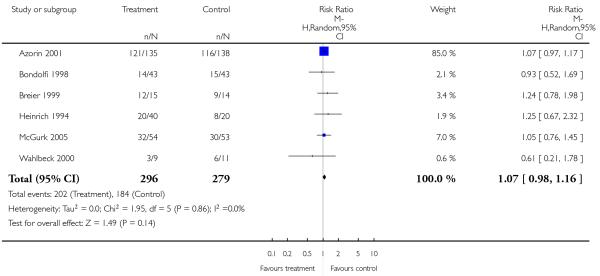
|
Analysis 3.2. Comparison 3 RISPERIDONE versus CLOZAPINE, Outcome 2 Global state: 1b. No clinically important change - short term (as defined by the original studies).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 3 RISPERIDONE versus CLOZAPINE
Outcome: 2 Global state: 1b. No clinically important change - short term (as defined by the original studies)
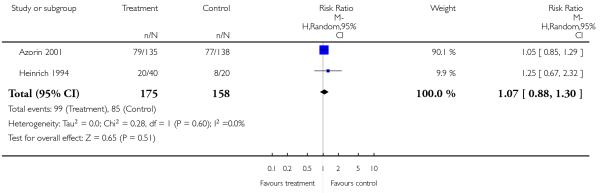
|
Analysis 3.3. Comparison 3 RISPERIDONE versus CLOZAPINE, Outcome 3 Leaving the study early.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 3 RISPERIDONE versus CLOZAPINE
Outcome: 3 Leaving the study early
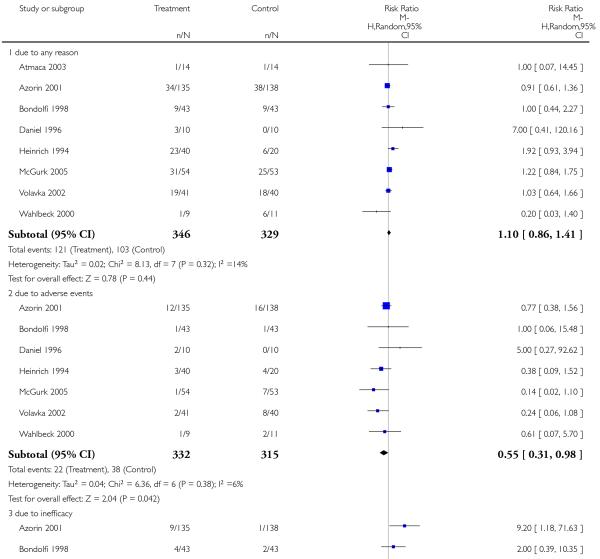
|
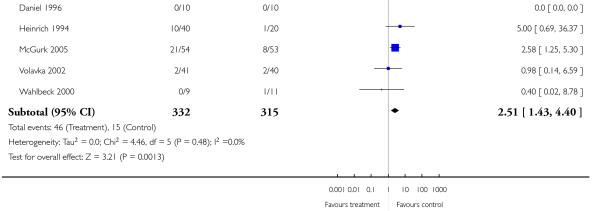
|
Analysis 3.4. Comparison 3 RISPERIDONE versus CLOZAPINE, Outcome 4 Mental state: 1a.General - no clinically important change - short term (less than 20 % PANSS total reduction).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 3 RISPERIDONE versus CLOZAPINE
Outcome: 4 Mental state: 1a.General - no clinically important change - short term (less than 20 % PANSS total reduction)
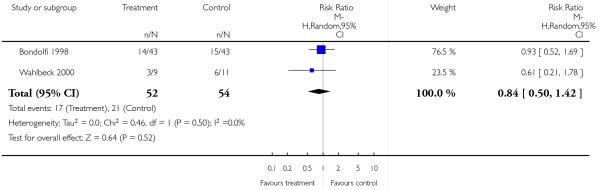
|
Analysis 3.5. Comparison 3 RISPERIDONE versus CLOZAPINE, Outcome 5 Mental state: 1b. General - no clinically important change - short term (as defined by the original studies).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 3 RISPERIDONE versus CLOZAPINE
Outcome: 5 Mental state: 1b. General - no clinically important change - short term (as defined by the original studies)
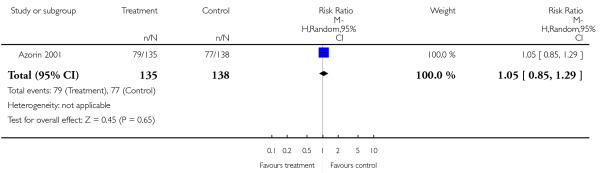
|
Analysis 3.6. Comparison 3 RISPERIDONE versus CLOZAPINE, Outcome 6 Mental State: 1c. General - no clinically important change - long term (less than 40% BPRS reduction).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 3 RISPERIDONE versus CLOZAPINE
Outcome: 6 Mental State: 1c. General - no clinically important change - long term (less than 40% BPRS reduction)

|
Analysis 3.7. Comparison 3 RISPERIDONE versus CLOZAPINE, Outcome 7 Mental State: 1d. General - no clinically important change - short term (less than 20% BPRS reduction).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 3 RISPERIDONE versus CLOZAPINE
Outcome: 7 Mental State: 1d. General - no clinically important change - short term (less than 20% BPRS reduction)

|
Analysis 3.8. Comparison 3 RISPERIDONE versus CLOZAPINE, Outcome 8 Mental state: 1e. General - average endpoint score (PANSS total, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 3 RISPERIDONE versus CLOZAPINE
Outcome: 8 Mental state: 1e. General - average endpoint score (PANSS total, high = poor)

|
Analysis 3.9. Comparison 3 RISPERIDONE versus CLOZAPINE, Outcome 9 Mental state: 1f. General - average endpoint score (BPRS total, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 3 RISPERIDONE versus CLOZAPINE
Outcome: 9 Mental state: 1f. General - average endpoint score (BPRS total, high = poor)

|
Analysis 3.10. Comparison 3 RISPERIDONE versus CLOZAPINE, Outcome 10 Mental state: 2a. Positive symptoms - average endpoint score (PANSS positive, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 3 RISPERIDONE versus CLOZAPINE
Outcome: 10 Mental state: 2a. Positive symptoms - average endpoint score (PANSS positive, high = poor)
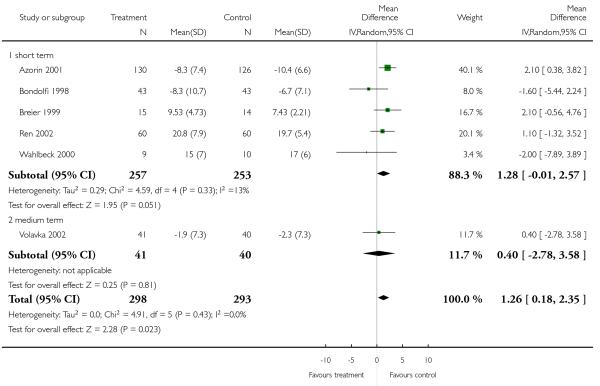
|
Analysis 3.11. Comparison 3 RISPERIDONE versus CLOZAPINE, Outcome 11 Mental state: 2b. Positive symptoms - average endpoint score - short term (BPRS positive, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 3 RISPERIDONE versus CLOZAPINE
Outcome: 11 Mental state: 2b. Positive symptoms - average endpoint score - short term (BPRS positive, high = poor)
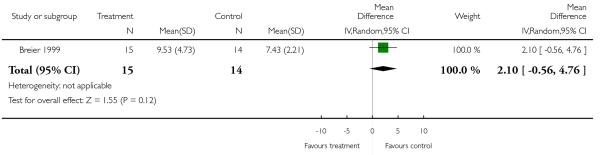
|
Analysis 3.12. Comparison 3 RISPERIDONE versus CLOZAPINE, Outcome 12 Mental state: 3a. Negative symptoms - average endpoint score (PANSS negative, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 3 RISPERIDONE versus CLOZAPINE
Outcome: 12 Mental state: 3a. Negative symptoms - average endpoint score (PANSS negative, high = poor)
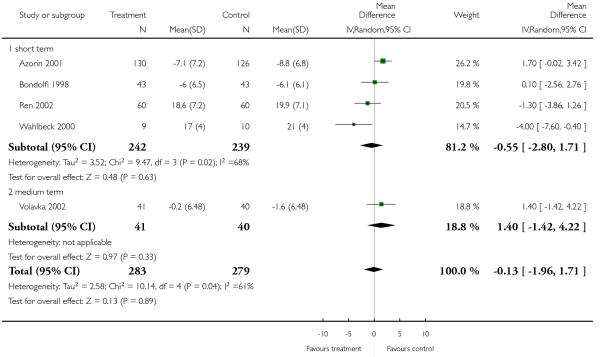
|
Analysis 3.13. Comparison 3 RISPERIDONE versus CLOZAPINE, Outcome 13 Mental state: 3b. Negative symptoms - average endpoint score - short term (SANS total, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 3 RISPERIDONE versus CLOZAPINE
Outcome: 13 Mental state: 3b. Negative symptoms - average endpoint score - short term (SANS total, high = poor)
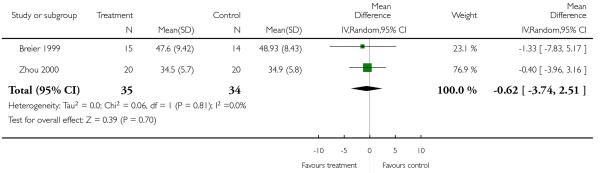
|
Analysis 3.14. Comparison 3 RISPERIDONE versus CLOZAPINE, Outcome 14 General functioning: 1a. General - average endpoint score - short term (GAF total, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 3 RISPERIDONE versus CLOZAPINE
Outcome: 14 General functioning: 1a. General - average endpoint score - short term (GAF total, high = poor)

|
Analysis 3.15. Comparison 3 RISPERIDONE versus CLOZAPINE, Outcome 15 General functioning: 1b. Social functioning - average endpoint score - short term (SFS, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 3 RISPERIDONE versus CLOZAPINE
Outcome: 15 General functioning: 1b. Social functioning - average endpoint score - short term (SFS, high = poor)
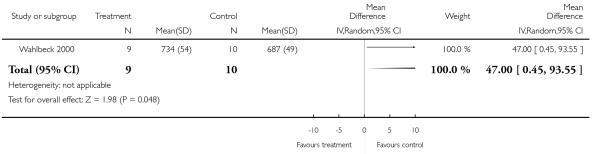
|
Analysis 3.16. Comparison 3 RISPERIDONE versus CLOZAPINE, Outcome 16 Cognitive functioning: 1a. Global - no clinically important change in global neurocognitive score - medium term (less than 1/2 SD).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 3 RISPERIDONE versus CLOZAPINE
Outcome: 16 Cognitive functioning: 1a. Global - no clinically important change in global neurocognitive score - medium term (less than 1/2 SD)
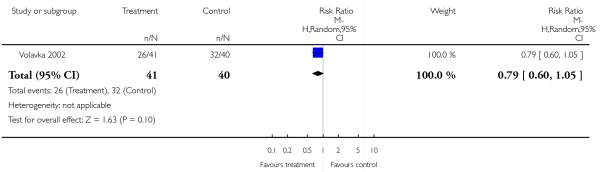
|
Analysis 3.17. Comparison 3 RISPERIDONE versus CLOZAPINE, Outcome 17 Cognitive functioning: 1b. Global - average endpoint score - medium term - (global neurocognitive score, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 3 RISPERIDONE versus CLOZAPINE
Outcome: 17 Cognitive functioning: 1b. Global - average endpoint score - medium term - (global neurocognitive score, high = poor)
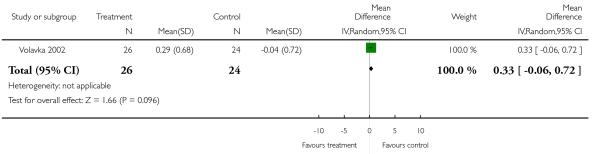
|
Analysis 3.18. Comparison 3 RISPERIDONE versus CLOZAPINE, Outcome 18 Adverse effects: 1. General - at least one adverse effect.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 3 RISPERIDONE versus CLOZAPINE
Outcome: 18 Adverse effects: 1. General - at least one adverse effect

|
Analysis 3.19. Comparison 3 RISPERIDONE versus CLOZAPINE, Outcome 19 Adverse effects: 2. Death.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 3 RISPERIDONE versus CLOZAPINE
Outcome: 19 Adverse effects: 2. Death

|
Analysis 3.20. Comparison 3 RISPERIDONE versus CLOZAPINE, Outcome 20 Adverse effects: 3. Cardiac effects.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 3 RISPERIDONE versus CLOZAPINE
Outcome: 20 Adverse effects: 3. Cardiac effects
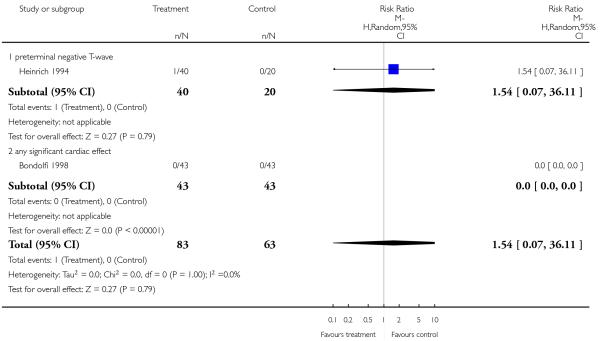
|
Analysis 3.21. Comparison 3 RISPERIDONE versus CLOZAPINE, Outcome 21 Adverse effects: 4a. Central nervous system - sedation.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 3 RISPERIDONE versus CLOZAPINE
Outcome: 21 Adverse effects: 4a. Central nervous system - sedation
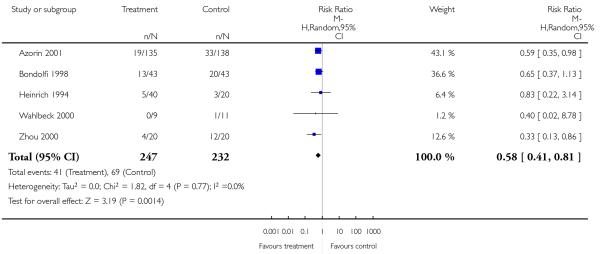
|
Analysis 3.22. Comparison 3 RISPERIDONE versus CLOZAPINE, Outcome 22 Adverse effects: 4b. Central nervous system - seizures.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 3 RISPERIDONE versus CLOZAPINE
Outcome: 22 Adverse effects: 4b. Central nervous system - seizures

|
Analysis 3.23. Comparison 3 RISPERIDONE versus CLOZAPINE, Outcome 23 Adverse effects: 5a. Extrapyramidal effects.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 3 RISPERIDONE versus CLOZAPINE
Outcome: 23 Adverse effects: 5a. Extrapyramidal effects

|
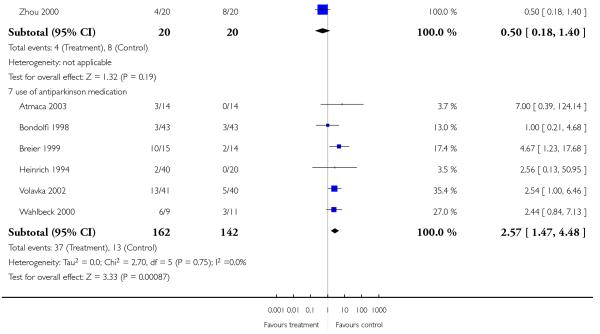
|
Analysis 3.24. Comparison 3 RISPERIDONE versus CLOZAPINE, Outcome 24 Adverse effects: 5b. Extrapyramidal symptoms - scale measured.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 3 RISPERIDONE versus CLOZAPINE
Outcome: 24 Adverse effects: 5b. Extrapyramidal symptoms - scale measured
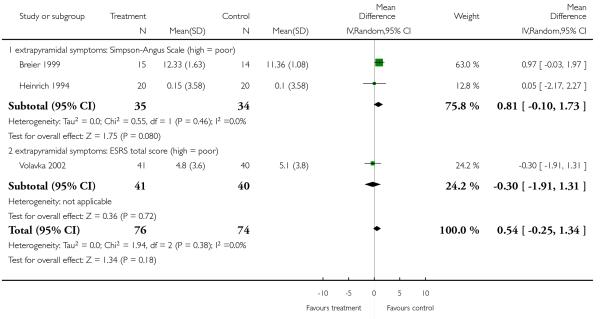
|
Analysis 3.25. Comparison 3 RISPERIDONE versus CLOZAPINE, Outcome 25 Adverse effects: 6. Haematological - white blood cells - significant low white blood cell count (as def. by the original studies).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 3 RISPERIDONE versus CLOZAPINE
Outcome: 25 Adverse effects: 6. Haematological - white blood cells - significant low white blood cell count (as def. by the original studies)
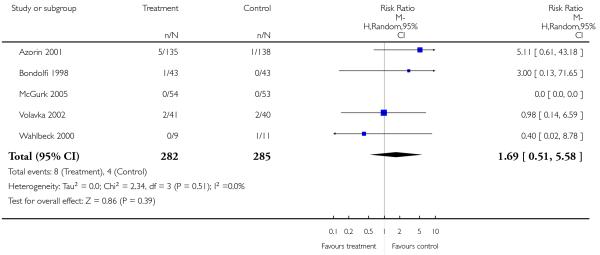
|
Analysis 3.26. Comparison 3 RISPERIDONE versus CLOZAPINE, Outcome 26 Adverse effects: 7a. Prolactin associated side effects.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 3 RISPERIDONE versus CLOZAPINE
Outcome: 26 Adverse effects: 7a. Prolactin associated side effects
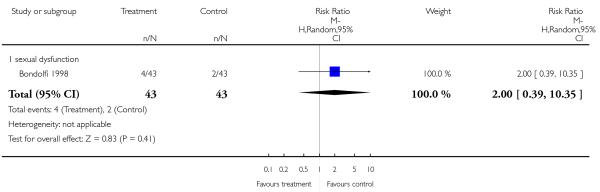
|
Analysis 3.27. Comparison 3 RISPERIDONE versus CLOZAPINE, Outcome 27 Adverse effects: 7b. Prolactin associated side effects - change from baseline in ng/ml.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 3 RISPERIDONE versus CLOZAPINE
Outcome: 27 Adverse effects: 7b. Prolactin associated side effects - change from baseline in ng/ml

|
Analysis 3.28. Comparison 3 RISPERIDONE versus CLOZAPINE, Outcome 28 Adverse effects: 3a. Metabolic - cholesterol - change from baseline in mg/dl.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 3 RISPERIDONE versus CLOZAPINE
Outcome: 28 Adverse effects: 3a. Metabolic - cholesterol - change from baseline in mg/dl

|
Analysis 3.29. Comparison 3 RISPERIDONE versus CLOZAPINE, Outcome 29 Adverse effects: 3b. Metabolic - glucose - change from baseline in mg/dl.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 3 RISPERIDONE versus CLOZAPINE
Outcome: 29 Adverse effects: 3b. Metabolic - glucose - change from baseline in mg/dl
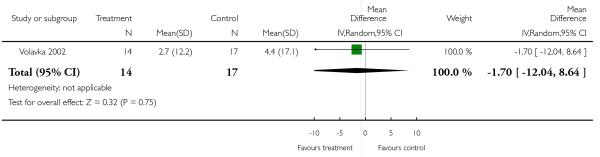
|
Analysis 3.30. Comparison 3 RISPERIDONE versus CLOZAPINE, Outcome 30 Adverse effects: 3c. Metabolic - weight gain.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 3 RISPERIDONE versus CLOZAPINE
Outcome: 30 Adverse effects: 3c. Metabolic - weight gain

|
Analysis 3.31. Comparison 3 RISPERIDONE versus CLOZAPINE, Outcome 31 Adverse effects: 3d. Metabolic - weight gain - change from baseline in kg.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 3 RISPERIDONE versus CLOZAPINE
Outcome: 31 Adverse effects: 3d. Metabolic - weight gain - change from baseline in kg
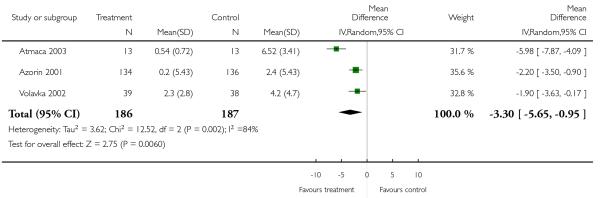
|
Analysis 4.1. Comparison 4 RISPERIDONE versus OLANZAPINE, Outcome 1 Global state: 1a. No clinically significant response (as defined by the original studies).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 4 RISPERIDONE versus OLANZAPINE
Outcome: 1 Global state: 1a. No clinically significant response (as defined by the original studies)

|
Analysis 4.2. Comparison 4 RISPERIDONE versus OLANZAPINE, Outcome 2 Global state: 1b. No clinically important change (as defined by the original studies).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 4 RISPERIDONE versus OLANZAPINE
Outcome: 2 Global state: 1b. No clinically important change (as defined by the original studies)
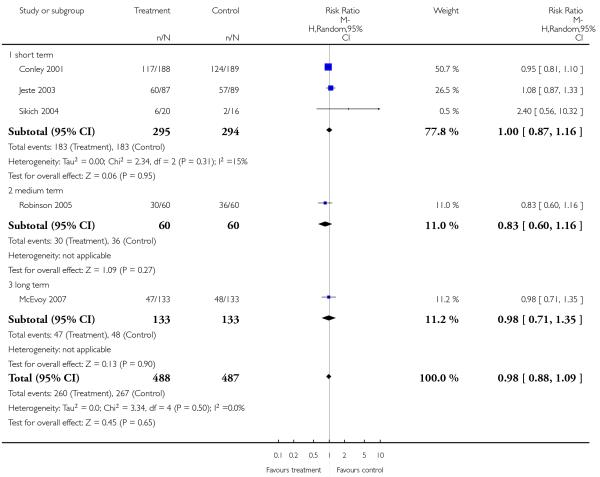
|
Analysis 4.3. Comparison 4 RISPERIDONE versus OLANZAPINE, Outcome 3 Global state: 1c. Relapse (as defined by the original studies).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 4 RISPERIDONE versus OLANZAPINE
Outcome: 3 Global state: 1c. Relapse (as defined by the original studies)
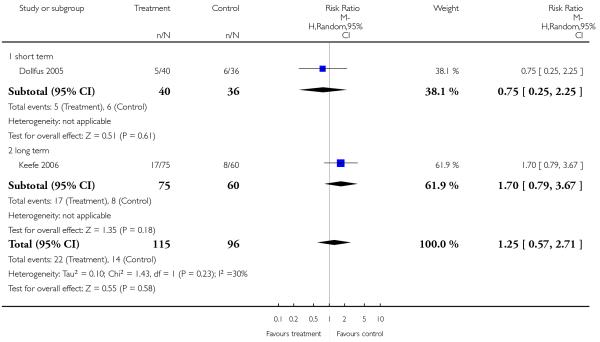
|
Analysis 4.4. Comparison 4 RISPERIDONE versus OLANZAPINE, Outcome 4 Leaving the study early.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 4 RISPERIDONE versus OLANZAPINE
Outcome: 4 Leaving the study early
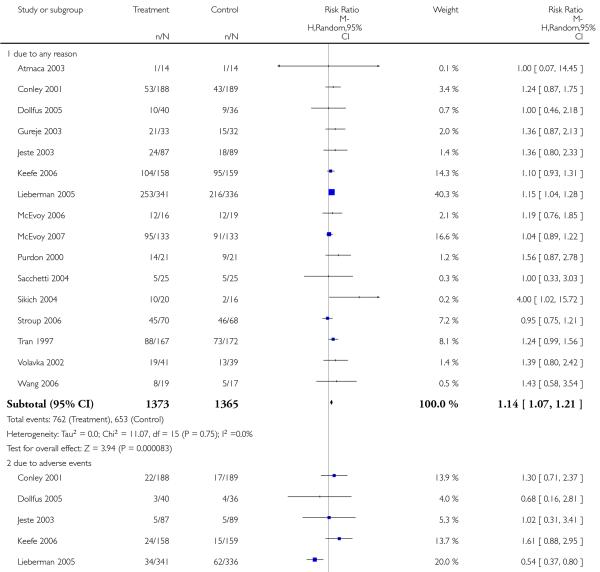
|
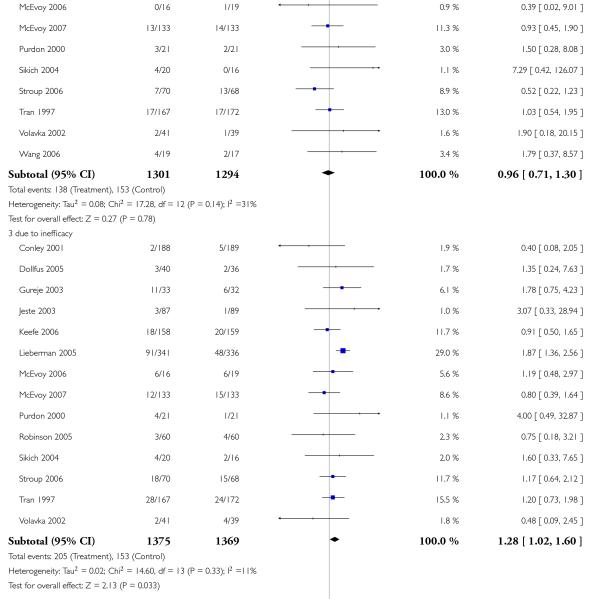
|
Analysis 4.5. Comparison 4 RISPERIDONE versus OLANZAPINE, Outcome 5 Mental state: 1a. General - no clinically important change (less than 50% PANSS total score reduction).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 4 RISPERIDONE versus OLANZAPINE
Outcome: 5 Mental state: 1a. General - no clinically important change (less than 50% PANSS total score reduction)

|
Analysis 4.6. Comparison 4 RISPERIDONE versus OLANZAPINE, Outcome 6 Mental state: 1b. General - no clinically important change - short term (less than 20% PANSS total score reduction).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 4 RISPERIDONE versus OLANZAPINE
Outcome: 6 Mental state: 1b. General - no clinically important change - short term (less than 20% PANSS total score reduction)
|
Analysis 4.7. Comparison 4 RISPERIDONE versus OLANZAPINE, Outcome 7 Mental state: 1c. General - average endpoint score (PANSS total, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 4 RISPERIDONE versus OLANZAPINE
Outcome: 7 Mental state: 1c. General - average endpoint score (PANSS total, high = poor)
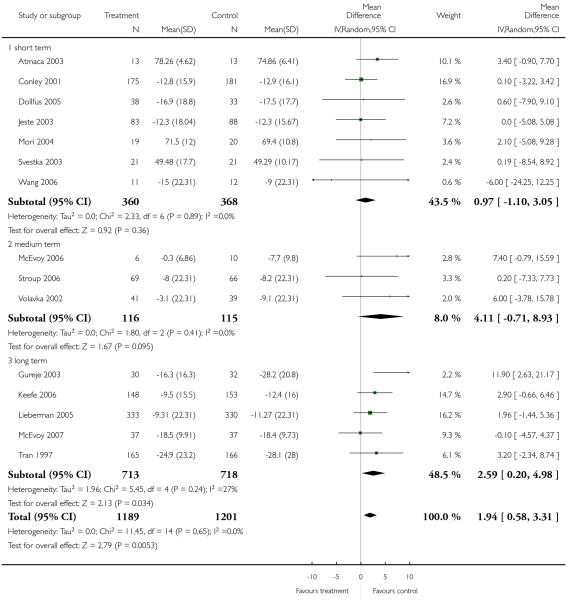
|
Analysis 4.8. Comparison 4 RISPERIDONE versus OLANZAPINE, Outcome 8 Mental state: 1d. General - average endpoint score (BPRS total score, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 4 RISPERIDONE versus OLANZAPINE
Outcome: 8 Mental state: 1d. General - average endpoint score (BPRS total score, high = poor)
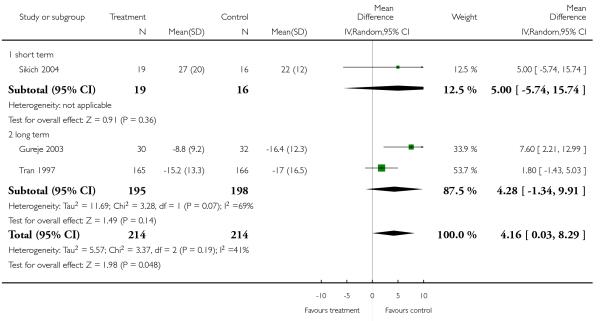
|
Analysis 4.9. Comparison 4 RISPERIDONE versus OLANZAPINE, Outcome 9 Mental state: 2a. Positive symptoms - no clinically important change - short term (less than 50% PANSS positive subscore reduction).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 4 RISPERIDONE versus OLANZAPINE
Outcome: 9 Mental state: 2a. Positive symptoms - no clinically important change - short term (less than 50% PANSS positive subscore reduction)
|
Analysis 4.10. Comparison 4 RISPERIDONE versus OLANZAPINE, Outcome 10 Mental state: 2b. Positive symptoms - average endpoint score (PANSS positive, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 4 RISPERIDONE versus OLANZAPINE
Outcome: 10 Mental state: 2b. Positive symptoms - average endpoint score (PANSS positive, high = poor)
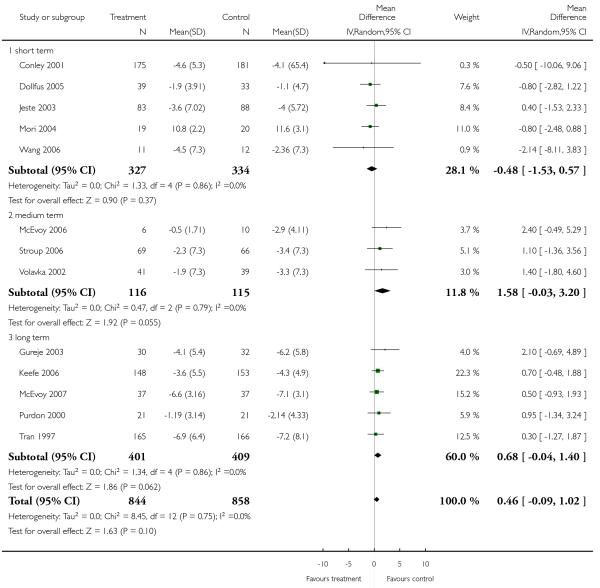
|
Analysis 4.11. Comparison 4 RISPERIDONE versus OLANZAPINE, Outcome 11 Mental state: 3a. Negative symptoms - average endpoint score (PANSS negative, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 4 RISPERIDONE versus OLANZAPINE
Outcome: 11 Mental state: 3a. Negative symptoms - average endpoint score (PANSS negative, high = poor)

|
Analysis 4.12. Comparison 4 RISPERIDONE versus OLANZAPINE, Outcome 12 Mental state: 3b. Negative symptoms - average endpoint score - long term (SANS total, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 4 RISPERIDONE versus OLANZAPINE
Outcome: 12 Mental state: 3b. Negative symptoms - average endpoint score - long term (SANS total, high = poor)
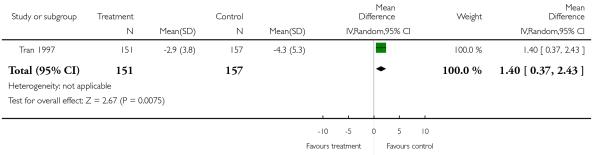
|
Analysis 4.13. Comparison 4 RISPERIDONE versus OLANZAPINE, Outcome 13 Quality of life: General - average endpoint score - long term (QLS total score, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 4 RISPERIDONE versus OLANZAPINE
Outcome: 13 Quality of life: General - average endpoint score - long term (QLS total score, high = poor)

|
Analysis 4.14. Comparison 4 RISPERIDONE versus OLANZAPINE, Outcome 14 Cognitive functioning: 1a.General - no clinically important change - medium term (less than ½ SD in Global Neurocognitive Score improved).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 4 RISPERIDONE versus OLANZAPINE
Outcome: 14 Cognitive functioning: 1a.General - no clinically important change - medium term (less than SD in Global Neurocognitive Score improved)
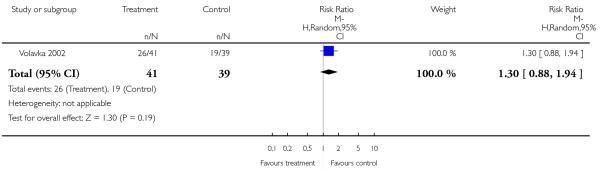
|
Analysis 4.15. Comparison 4 RISPERIDONE versus OLANZAPINE, Outcome 15 Cognitive functioning: 1b. General - average endpoint score - medium term (global neurocognitive score, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 4 RISPERIDONE versus OLANZAPINE
Outcome: 15 Cognitive functioning: 1b. General - average endpoint score - medium term (global neurocognitive score, high = poor)
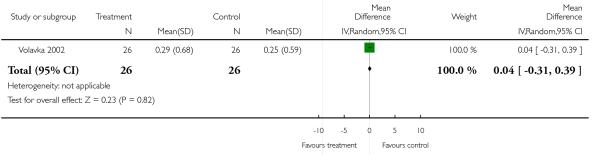
|
Analysis 4.16. Comparison 4 RISPERIDONE versus OLANZAPINE, Outcome 16 Cognitive functioning: 1c. General - average endpoint score - long term (neurocognitive composite score, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 4 RISPERIDONE versus OLANZAPINE
Outcome: 16 Cognitive functioning: 1c. General - average endpoint score - long term (neurocognitive composite score, high = poor)
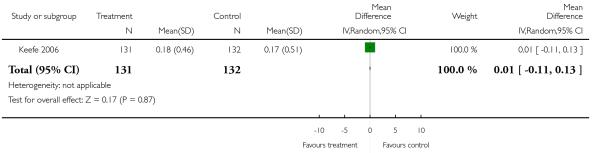
|
Analysis 4.17. Comparison 4 RISPERIDONE versus OLANZAPINE, Outcome 17 Service use - number of patients rehospitalised.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 4 RISPERIDONE versus OLANZAPINE
Outcome: 17 Service use - number of patients rehospitalised
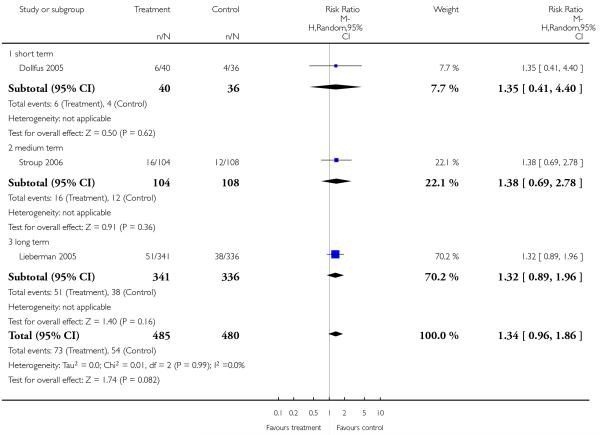
|
Analysis 4.18. Comparison 4 RISPERIDONE versus OLANZAPINE, Outcome 18 Adverse effects: 1. General - at least one adverse effect.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 4 RISPERIDONE versus OLANZAPINE
Outcome: 18 Adverse effects: 1. General - at least one adverse effect
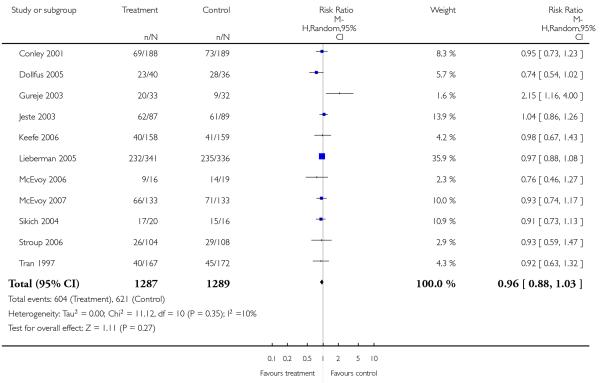
|
Analysis 4.19. Comparison 4 RISPERIDONE versus OLANZAPINE, Outcome 19 Adverse effects: 2. Death.
Review: Risperidone versus other atypical antipsychotics for schizophreni
Comparison: 4 RISPERIDONE versus OLANZAPINE
Outcome: 19 Adverse effects: 2. Death

|
Analysis 4.20. Comparison 4 RISPERIDONE versus OLANZAPINE, Outcome 20 Adverse effects: 3a. Cardiac effects.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 4 RISPERIDONE versus OLANZAPINE
Outcome: 20 Adverse effects: 3a. Cardiac effects

|
Analysis 4.21. Comparison 4 RISPERIDONE versus OLANZAPINE, Outcome 21 Adverse effects: 3b. Cardiac effects - QTc abnormalities - change from baseline in ms.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 4 RISPERIDONE versus OLANZAPINE
Outcome: 21 Adverse effects: 3b. Cardiac effects - QTc abnormalities - change from baseline in ms
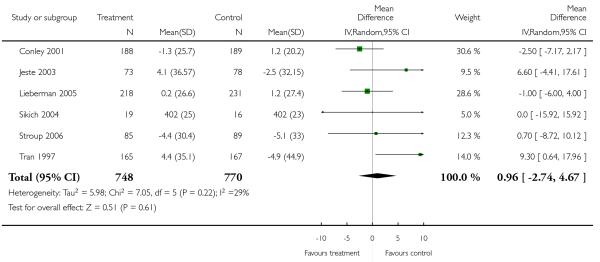
|
Analysis 4.22. Comparison 4 RISPERIDONE versus OLANZAPINE, Outcome 22 Adverse effects: 4a. Central nervous system - sedation.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 4 RISPERIDONE versus OLANZAPINE
Outcome: 22 Adverse effects: 4a. Central nervous system - sedation
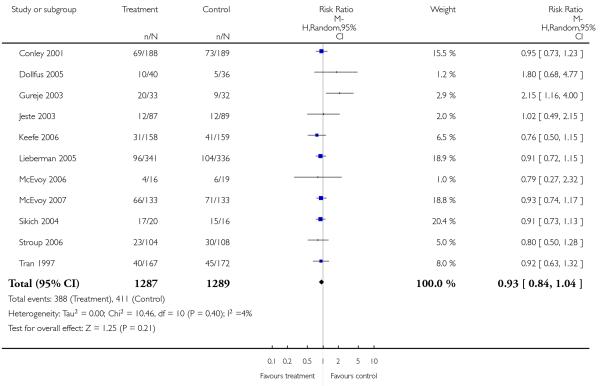
|
Analysis 4.23. Comparison 4 RISPERIDONE versus OLANZAPINE, Outcome 23 Adverse effects: 4b. Central nervous system - seizures.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 4 RISPERIDONE versus OLANZAPINE
Outcome: 23 Adverse effects: 4b. Central nervous system - seizures
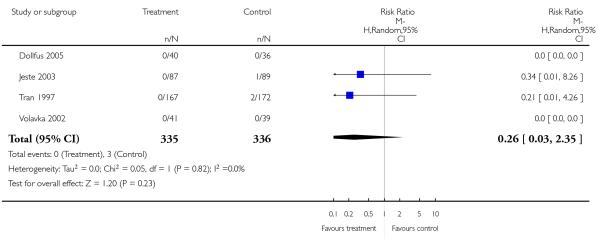
|
Analysis 4.24. Comparison 4 RISPERIDONE versus OLANZAPINE, Outcome 24 Adverse effects: 5a. Extrapyramidal effects.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 4 RISPERIDONE versus OLANZAPINE
Outcome: 24 Adverse effects: 5a. Extrapyramidal effects

|
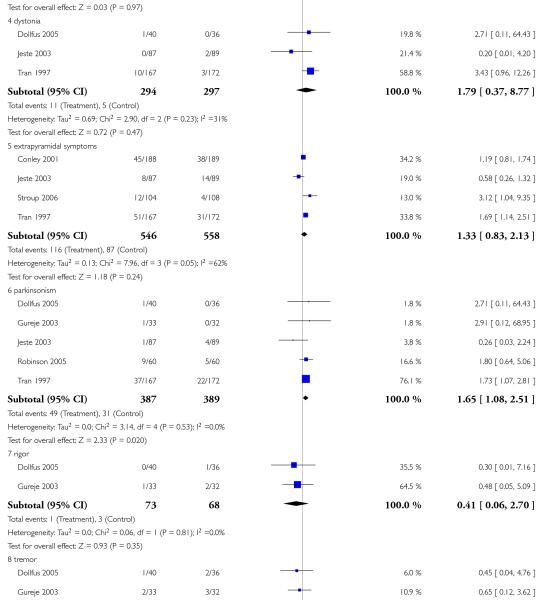
|

|
Analysis 4.25. Comparison 4 RISPERIDONE versus OLANZAPINE, Outcome 25 Adverse effects: 5b. Extrapyramidal effects - scale measured.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 4 RISPERIDONE versus OLANZAPINE
Outcome: 25 Adverse effects: 5b. Extrapyramidal effects - scale measured
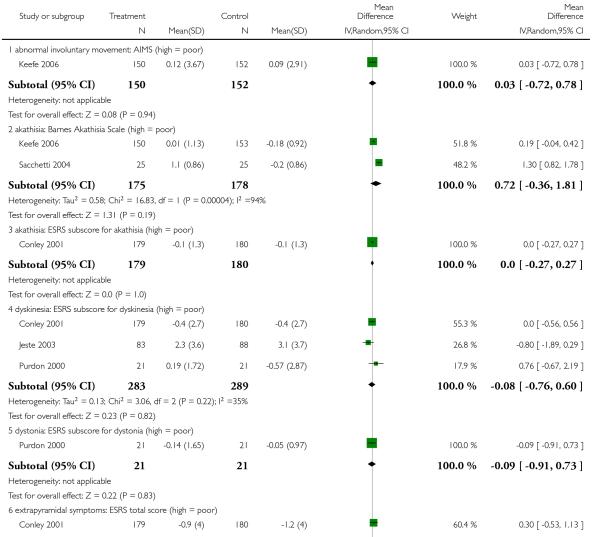
|

|
Analysis 4.26. Comparison 4 RISPERIDONE versus OLANZAPINE, Outcome 26 Adverse effects: 6. Haematological: white blood cells - significant low white blood cell count (as def. by the original studies).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 4 RISPERIDONE versus OLANZAPINE
Outcome: 26 Adverse effects: 6. Haematological: white blood cells - significant low white blood cell count (as def. by the original studies)
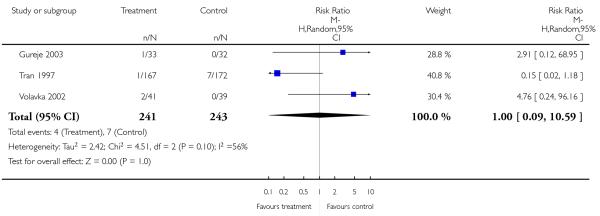
|
Analysis 4.27. Comparison 4 RISPERIDONE versus OLANZAPINE, Outcome 27 Adverse effects: 7a. Prolactin associated side effects.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 4 RISPERIDONE versus OLANZAPINE
Outcome: 27 Adverse effects: 7a. Prolactin associated side effects

|

|

|
Analysis 4.28. Comparison 4 RISPERIDONE versus OLANZAPINE, Outcome 28 Adverse effects: 7b. Prolactin - change from baseline in ng/ml.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 4 RISPERIDONE versus OLANZAPINE
Outcome: 28 Adverse effects: 7b. Prolactin - change from baseline in ng/ml
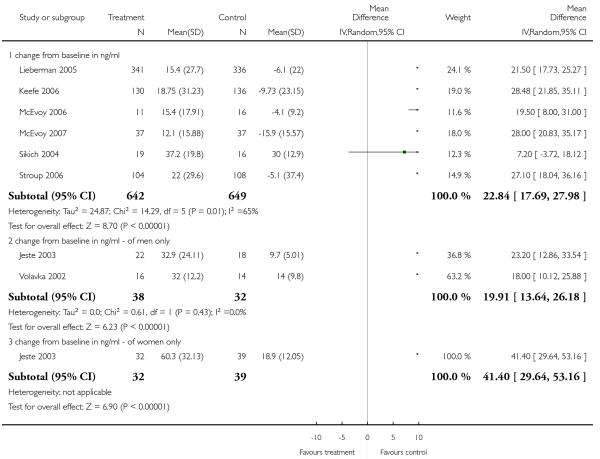
|
Analysis 4.29. Comparison 4 RISPERIDONE versus OLANZAPINE, Outcome 29 Adverse effects: 8a. Metabolic - cholesterol - significant cholesterol increase.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 4 RISPERIDONE versus OLANZAPINE
Outcome: 29 Adverse effects: 8a. Metabolic - cholesterol - significant cholesterol increase
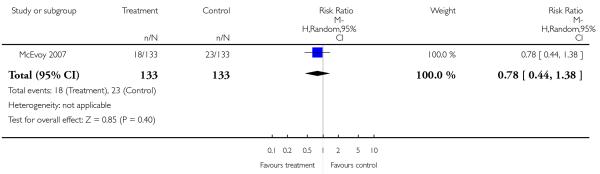
|
Analysis 4.30. Comparison 4 RISPERIDONE versus OLANZAPINE, Outcome 30 Adverse effects: 8b. Metabolic - cholesterol - change from baseline in mg/dl.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 4 RISPERIDONE versus OLANZAPINE
Outcome: 30 Adverse effects: 8b. Metabolic - cholesterol - change from baseline in mg/dl
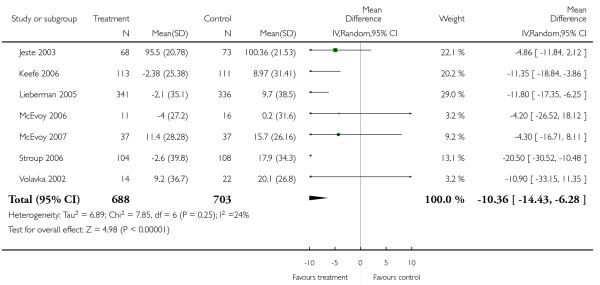
|
Analysis 4.31. Comparison 4 RISPERIDONE versus OLANZAPINE, Outcome 31 Adverse effects: 8c. Metabolic - glucose - abnormally high fasting glucose value.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 4 RISPERIDONE versus OLANZAPINE
Outcome: 31 Adverse effects: 8c. Metabolic - glucose - abnormally high fasting glucose value
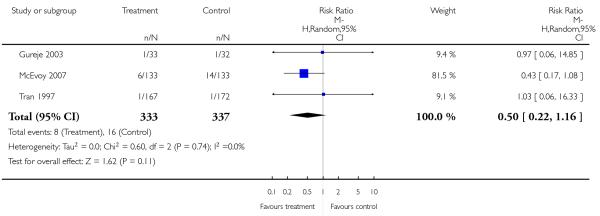
|
Analysis 4.32. Comparison 4 RISPERIDONE versus OLANZAPINE, Outcome 32 Adverse effects: 8d. Metabolic - glucose - change from baseline in mg/dl.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 4 RISPERIDONE versus OLANZAPINE
Outcome: 32 Adverse effects: 8d. Metabolic - glucose - change from baseline in mg/dl
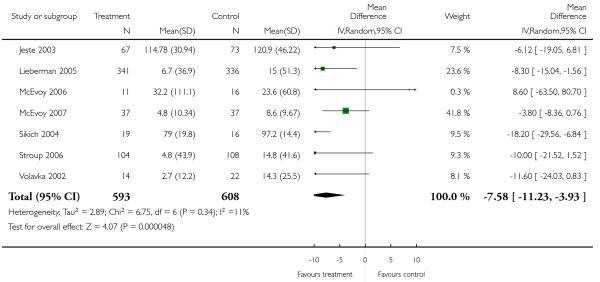
|
Analysis 4.33. Comparison 4 RISPERIDONE versus OLANZAPINE, Outcome 33 Adverse effects: 8e. Metabolic - weight gain.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 4 RISPERIDONE versus OLANZAPINE
Outcome: 33 Adverse effects: 8e. Metabolic - weight gain

|
Analysis 4.34. Comparison 4 RISPERIDONE versus OLANZAPINE, Outcome 34 Adverse effects: 8f. Metabolic - weight gain - change from baseline in kg.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 4 RISPERIDONE versus OLANZAPINE
Outcome: 34 Adverse effects: 8f. Metabolic - weight gain - change from baseline in kg
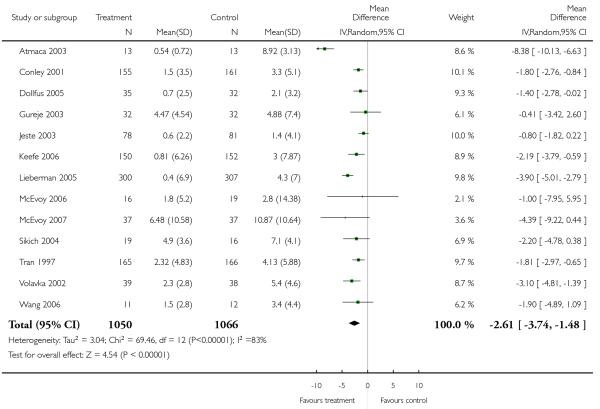
|
Analysis 5.1. Comparison 5 RISPERIDONE versus QUETIAPINE, Outcome 1 Global state: 1a. No clinically significant response (as def. by the original studies).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 5 RISPERIDONE versus QUETIAPINE
Outcome: 1 Global state: 1a. No clinically significant response (as def. by the original studies)
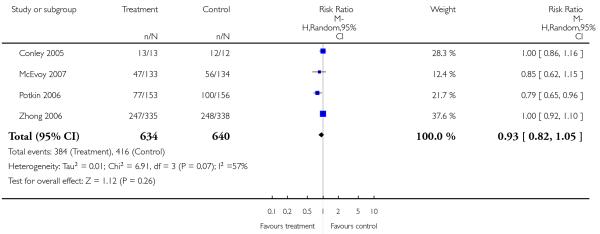
|
Analysis 5.2. Comparison 5 RISPERIDONE versus QUETIAPINE, Outcome 2 Global state: 1b. No clinically important change (as defined by the original studies).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 5 RISPERIDONE versus QUETIAPINE
Outcome: 2 Global state: 1b. No clinically important change (as defined by the original studies)
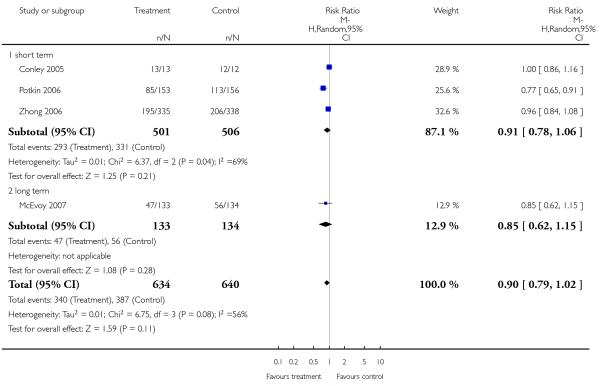
|
Analysis 5.3. Comparison 5 RISPERIDONE versus QUETIAPINE, Outcome 3 Leaving the study early.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 5 RISPERIDONE versus QUETIAPINE
Outcome: 3 Leaving the study early
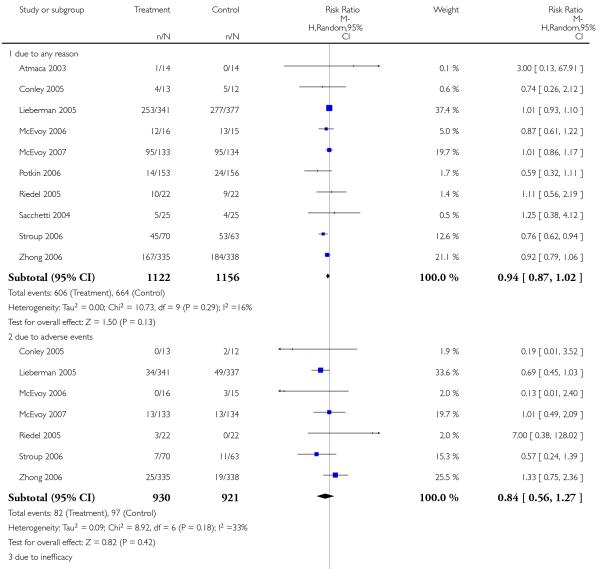
|
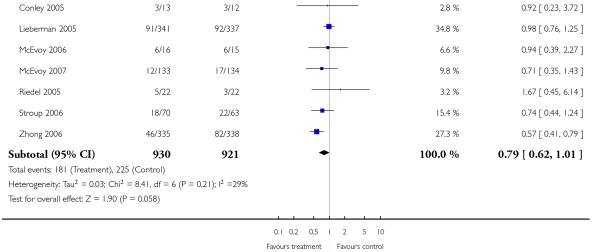
|
Analysis 5.4. Comparison 5 RISPERIDONE versus QUETIAPINE, Outcome 4 Mental state: 1a General - no clinically important change - short term (less than 30% PANSS total score reduction).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 5 RISPERIDONE versus QUETIAPINE
Outcome: 4 Mental state: 1a General - no clinically important change - short term (less than 30% PANSS total score reduction)
|
Analysis 5.5. Comparison 5 RISPERIDONE versus QUETIAPINE, Outcome 5 Mental state: 1b. General - no clinicallly important change - short term (less than 20% BPRS total score reduction).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 5 RISPERIDONE versus QUETIAPINE
Outcome: 5 Mental state: 1b. General - no clinicallly important change - short term (less than 20% BPRS total score reduction)
|
Analysis 5.6. Comparison 5 RISPERIDONE versus QUETIAPINE, Outcome 6 Mental state: 1c. General - average endpoint score (PANSS total score, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 5 RISPERIDONE versus QUETIAPINE
Outcome: 6 Mental state: 1c. General - average endpoint score (PANSS total score, high = poor)
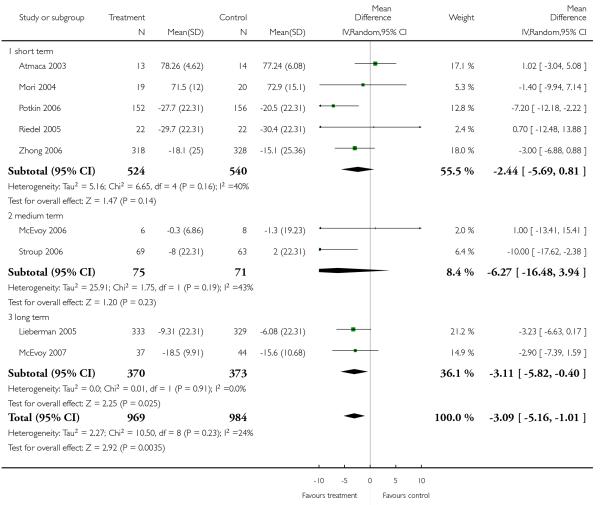
|
Analysis 5.7. Comparison 5 RISPERIDONE versus QUETIAPINE, Outcome 7 Mental state: 1d. General - average endpoint score - short term (BPRS total score, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 5 RISPERIDONE versus QUETIAPINE
Outcome: 7 Mental state: 1d. General - average endpoint score - short term (BPRS total score, high = poor)
|
Analysis 5.8. Comparison 5 RISPERIDONE versus QUETIAPINE, Outcome 8 Mental state: 2a. Positive symptoms - no clinically important change - short term (less than 40% PANSS positive reduction).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 5 RISPERIDONE versus QUETIAPINE
Outcome: 8 Mental state: 2a. Positive symptoms - no clinically important change - short term (less than 40% PANSS positive reduction)
|
Analysis 5.9. Comparison 5 RISPERIDONE versus QUETIAPINE, Outcome 9 Mental state: 2b. Positive symptoms - average endpoint score - (PANSS positive subscore, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 5 RISPERIDONE versus QUETIAPINE
Outcome: 9 Mental state: 2b. Positive symptoms - average endpoint score - (PANSS positive subscore, high = poor)

|
Analysis 5.10. Comparison 5 RISPERIDONE versus QUETIAPINE, Outcome 10 Mental state: 2c. Positive symptoms - average endpoint score - short term (BPRS positive subscore, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 5 RISPERIDONE versus QUETIAPINE
Outcome: 10 Mental state: 2c. Positive symptoms - average endpoint score - short term (BPRS positive subscore, high = poor)
|
Analysis 5.11. Comparison 5 RISPERIDONE versus QUETIAPINE, Outcome 11 Mental state: 3a. Negative symptoms - no clinicallly important change - short term (less than 40% PANSS negative reduction).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 5 RISPERIDONE versus QUETIAPINE
Outcome: 11 Mental state: 3a. Negative symptoms - no clinicallly important change - short term (less than 40% PANSS negative reduction)
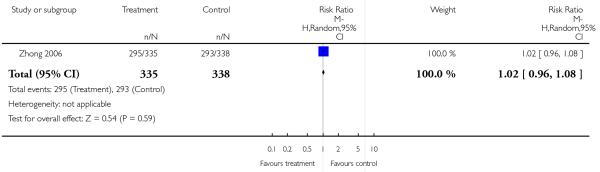
|
Analysis 5.12. Comparison 5 RISPERIDONE versus QUETIAPINE, Outcome 12 Mental state: 3b. Negative symptoms - average endpoint score - (PANSS negative subscore, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 5 RISPERIDONE versus QUETIAPINE
Outcome: 12 Mental state: 3b. Negative symptoms - average endpoint score - (PANSS negative subscore, high = poor)

|
Analysis 5.13. Comparison 5 RISPERIDONE versus QUETIAPINE, Outcome 13 Mental state: 3c. Negative symptoms - average endpoint score - short term (BPRS negative subscore, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 5 RISPERIDONE versus QUETIAPINE
Outcome: 13 Mental state: 3c. Negative symptoms - average endpoint score - short term (BPRS negative subscore, high = poor)
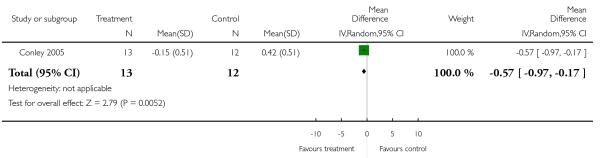
|
Analysis 5.14. Comparison 5 RISPERIDONE versus QUETIAPINE, Outcome 14 Quality of life: General - average endpoint score - short term (QLS total score, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 5 RISPERIDONE versus QUETIAPINE
Outcome: 14 Quality of life: General - average endpoint score - short term (QLS total score, high = poor)
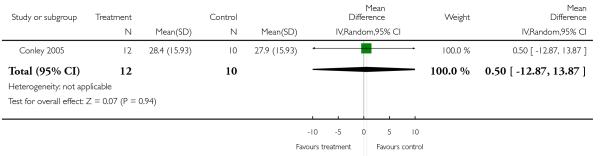
|
Analysis 5.15. Comparison 5 RISPERIDONE versus QUETIAPINE, Outcome 15 Service use: Number of participants rehospitalised.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 5 RISPERIDONE versus QUETIAPINE
Outcome: 15 Service use: Number of participants rehospitalised
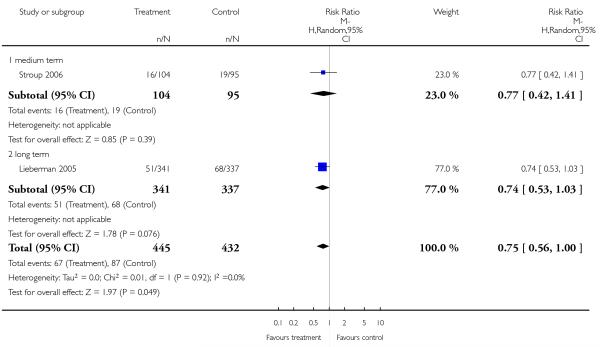
|
Analysis 5.16. Comparison 5 RISPERIDONE versus QUETIAPINE, Outcome 16 Adverse effects: 1. General - at least one adverse effect.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 5 RISPERIDONE versus QUETIAPINE
Outcome: 16 Adverse effects: 1. General - at least one adverse effect
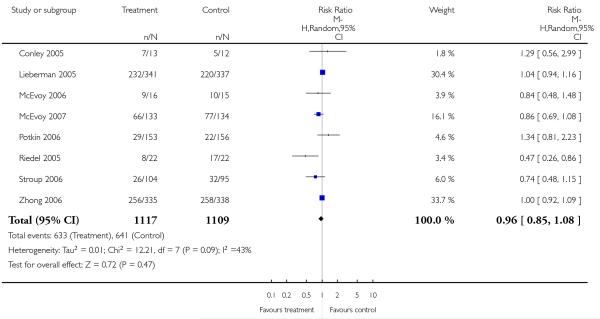
|
Analysis 5.17. Comparison 5 RISPERIDONE versus QUETIAPINE, Outcome 17 Adverse effects: 2. Death.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 5 RISPERIDONE versus QUETIAPINE
Outcome: 17 Adverse effects: 2. Death
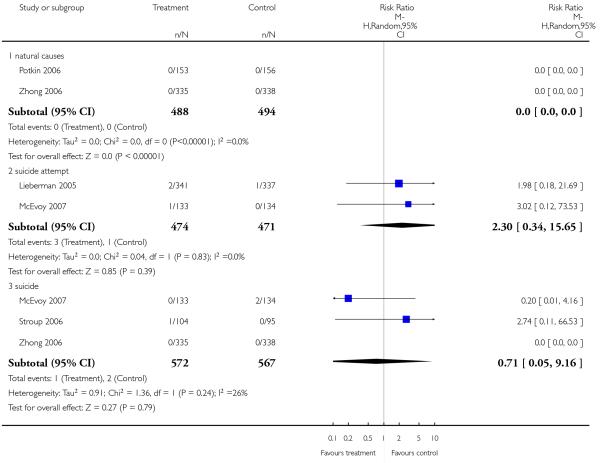
|
Analysis 5.18. Comparison 5 RISPERIDONE versus QUETIAPINE, Outcome 18 Adverse effects: 3a. Cardiac effects - QTc prolongation.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 5 RISPERIDONE versus QUETIAPINE
Outcome: 18 Adverse effects: 3a. Cardiac effects - QTc prolongation
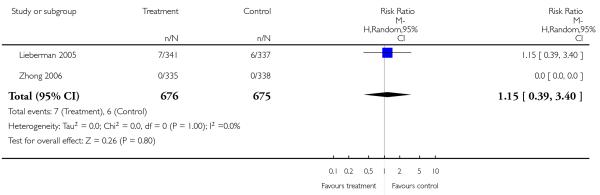
|
Analysis 5.19. Comparison 5 RISPERIDONE versus QUETIAPINE, Outcome 19 Adverse effects: 3b. Cardiac effects - QTc abnormalities - change from baseline in ms.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 5 RISPERIDONE versus QUETIAPINE
Outcome: 19 Adverse effects: 3b. Cardiac effects - QTc abnormalities - change from baseline in ms

|
Analysis 5.20. Comparison 5 RISPERIDONE versus QUETIAPINE, Outcome 20 Adverse effects: 4. Central nervous system - sedation.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 5 RISPERIDONE versus QUETIAPINE
Outcome: 20 Adverse effects: 4. Central nervous system - sedation
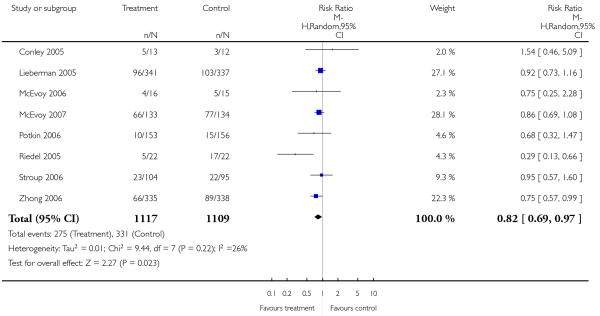
|
Analysis 5.21. Comparison 5 RISPERIDONE versus QUETIAPINE, Outcome 21 Adverse effects: 5a. Extrapyramidal effects.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 5 RISPERIDONE versus QUETIAPINE
Outcome: 21 Adverse effects: 5a. Extrapyramidal effects

|

|
Analysis 5.22. Comparison 5 RISPERIDONE versus QUETIAPINE, Outcome 22 Adverse effects: 5b. Extrapyramidal effects - scale measured.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 5 RISPERIDONE versus QUETIAPINE
Outcome: 22 Adverse effects: 5b. Extrapyramidal effects - scale measured
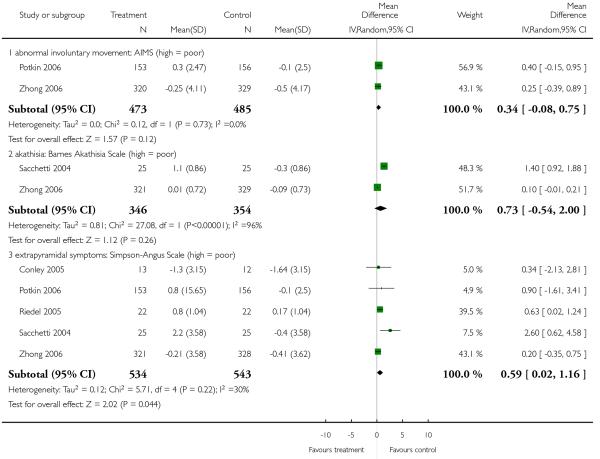
|
Analysis 5.23. Comparison 5 RISPERIDONE versus QUETIAPINE, Outcome 23 Adverse effects: 6. Haematological: Important decline in white blood cells.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 5 RISPERIDONE versus QUETIAPINE
Outcome: 23 Adverse effects: 6. Haematological: Important decline in white blood cells
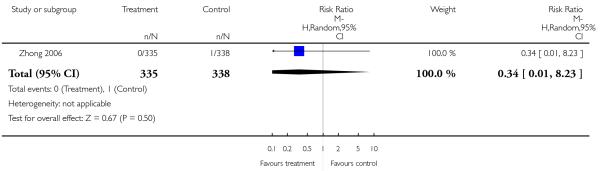
|
Analysis 5.24. Comparison 5 RISPERIDONE versus QUETIAPINE, Outcome 24 Adverse effects: 7a. Prolactin associated side effects.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 5 RISPERIDONE versus QUETIAPINE
Outcome: 24 Adverse effects: 7a. Prolactin associated side effects
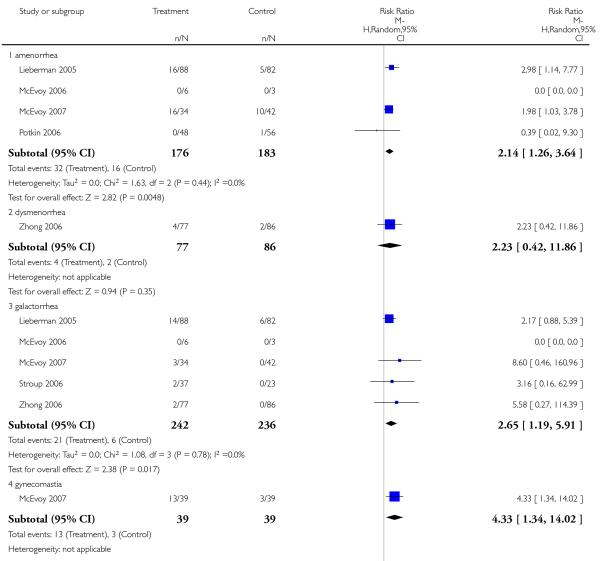
|

|
Analysis 5.25. Comparison 5 RISPERIDONE versus QUETIAPINE, Outcome 25 Adverse effects: 7b. Prolactin - change from baseline in mg/dl.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 5 RISPERIDONE versus QUETIAPINE
Outcome: 25 Adverse effects: 7b. Prolactin - change from baseline in mg/dl
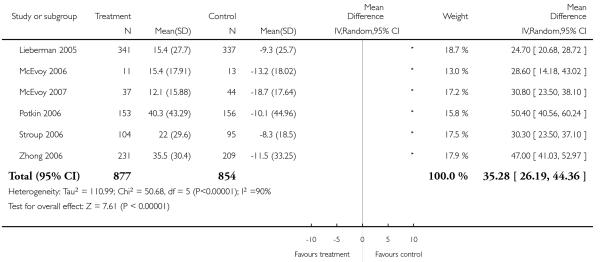
|
Analysis 5.26. Comparison 5 RISPERIDONE versus QUETIAPINE, Outcome 26 Adverse effects: 8a. Metabolic - cholesterol - significant cholesterol increase.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 5 RISPERIDONE versus QUETIAPINE
Outcome: 26 Adverse effects: 8a. Metabolic - cholesterol - significant cholesterol increase
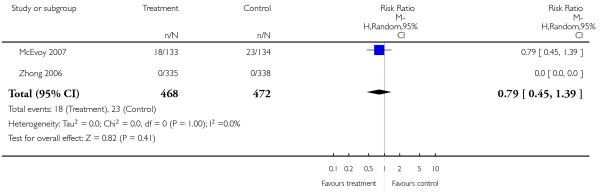
|
Analysis 5.27. Comparison 5 RISPERIDONE versus QUETIAPINE, Outcome 27 Adverse effects: 8b. Metabolic - cholesterol - change from baseline in mg/dl.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 5 RISPERIDONE versus QUETIAPINE
Outcome: 27 Adverse effects: 8b. Metabolic - cholesterol - change from baseline in mg/dl
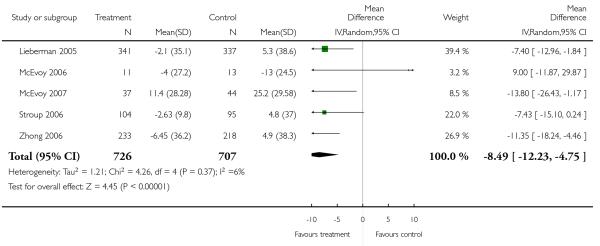
|
Analysis 5.28. Comparison 5 RISPERIDONE versus QUETIAPINE, Outcome 28 Adverse effects: 8c. Metabolic - glucose - abnormally high fasting glucose value.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 5 RISPERIDONE versus QUETIAPINE
Outcome: 28 Adverse effects: 8c. Metabolic - glucose - abnormally high fasting glucose value
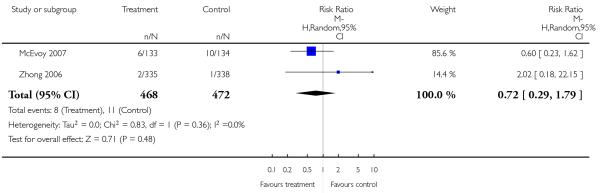
|
Analysis 5.29. Comparison 5 RISPERIDONE versus QUETIAPINE, Outcome 29 Adverse effects: 8d. Metabolic - glucose - change from baseline in mg/dl.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 5 RISPERIDONE versus QUETIAPINE
Outcome: 29 Adverse effects: 8d. Metabolic - glucose - change from baseline in mg/dl
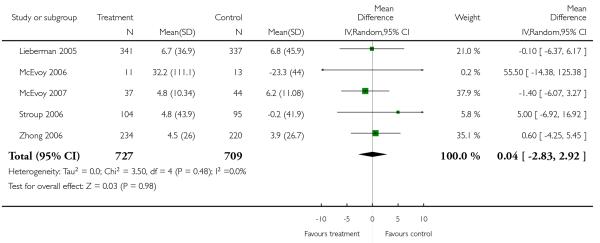
|
Analysis 5.30. Comparison 5 RISPERIDONE versus QUETIAPINE, Outcome 30 Adverse effects: 8e. Metabolic - weight gain of 7% or more of total body weight.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 5 RISPERIDONE versus QUETIAPINE
Outcome: 30 Adverse effects: 8e. Metabolic - weight gain of 7% or more of total body weight
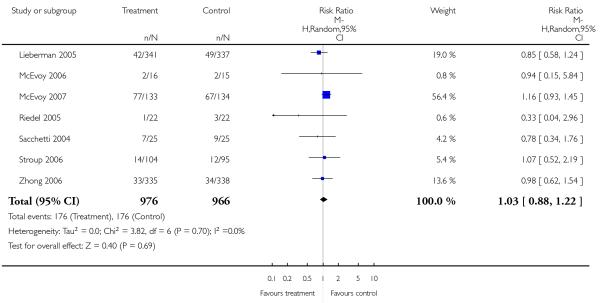
|
Analysis 5.31. Comparison 5 RISPERIDONE versus QUETIAPINE, Outcome 31 Adverse effects: 8f. Metabolic - weight gain - change from baseline in kg.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 5 RISPERIDONE versus QUETIAPINE
Outcome: 31 Adverse effects: 8f. Metabolic - weight gain - change from baseline in kg

|
Analysis 6.1. Comparison 6 RISPERIDONE versus SERTINDOLE - all data short term, Outcome 1 Global state - 1a. No clinically significant response (as defined by the original studies).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 6 RISPERIDONE versus SERTINDOLE - all data short term
Outcome: 1 Global state - 1a. No clinically significant response (as defined by the original studies)
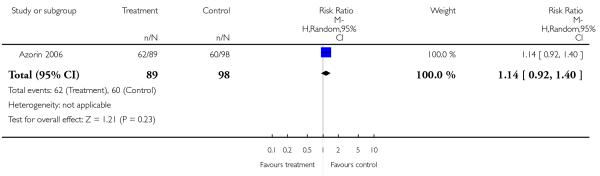
|
Analysis 6.2. Comparison 6 RISPERIDONE versus SERTINDOLE - all data short term, Outcome 2 Global state - 1b. No clinically important change (as defined by the original studies).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 6 RISPERIDONE versus SERTINDOLE - all data short term
Outcome: 2 Global state - 1b. No clinically important change (as defined by the original studies)
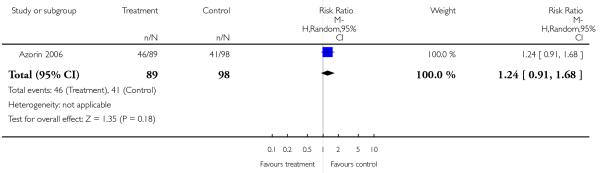
|
Analysis 6.3. Comparison 6 RISPERIDONE versus SERTINDOLE - all data short term, Outcome 3 Leaving the study early.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 6 RISPERIDONE versus SERTINDOLE - all data short term
Outcome: 3 Leaving the study early
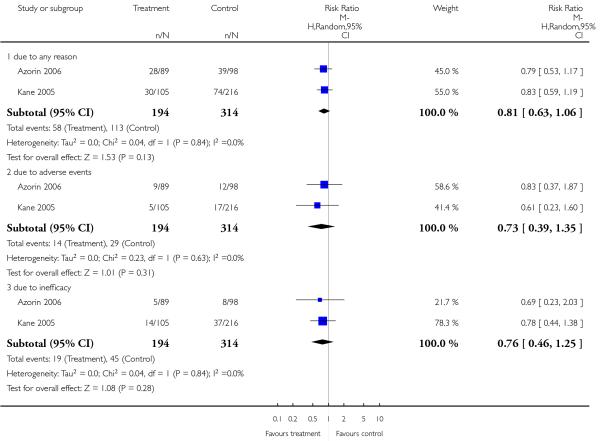
|
Analysis 6.4. Comparison 6 RISPERIDONE versus SERTINDOLE - all data short term, Outcome 4 Mental state: 1a. General - no clinically important change (less than 50% PANSS total score reduction).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 6 RISPERIDONE versus SERTINDOLE - all data short term
Outcome: 4 Mental state: 1a. General - no clinically important change (less than 50% PANSS total score reduction)
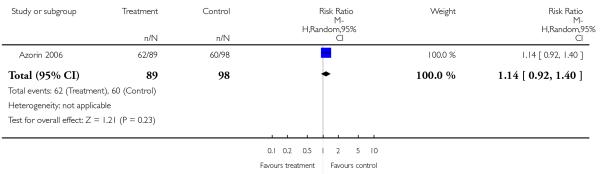
|
Analysis 6.5. Comparison 6 RISPERIDONE versus SERTINDOLE - all data short term, Outcome 5 Mental state: 1b. General - average endpoint score (PANSS total, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 6 RISPERIDONE versus SERTINDOLE - all data short term
Outcome: 5 Mental state: 1b. General - average endpoint score (PANSS total, high = poor)
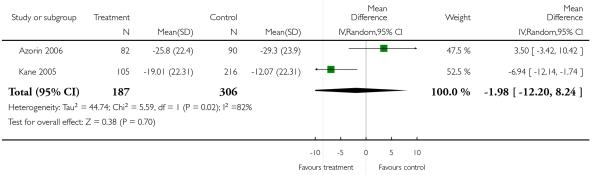
|
Analysis 6.6. Comparison 6 RISPERIDONE versus SERTINDOLE - all data short term, Outcome 6 Mental state: 2. Positive symptoms - average endpoint score (PANSS positive, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 6 RISPERIDONE versus SERTINDOLE - all data short term
Outcome: 6 Mental state: 2. Positive symptoms - average endpoint score (PANSS positive, high = poor)
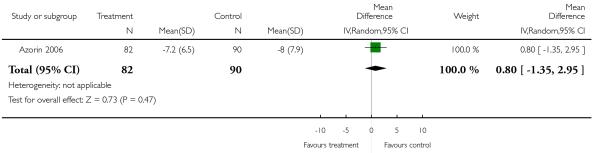
|
Analysis 6.7. Comparison 6 RISPERIDONE versus SERTINDOLE - all data short term, Outcome 7 Mental state: 3. Negative symptoms - average endpoint score (PANSS negative, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 6 RISPERIDONE versus SERTINDOLE - all data short term
Outcome: 7 Mental state: 3. Negative symptoms - average endpoint score (PANSS negative, high = poor)
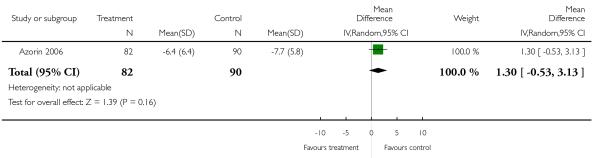
|
Analysis 6.8. Comparison 6 RISPERIDONE versus SERTINDOLE - all data short term, Outcome 8 General functioning: General - average endpoint score - (GAF total, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 6 RISPERIDONE versus SERTINDOLE - all data short term
Outcome: 8 General functioning: General - average endpoint score - (GAF total, high = poor)

|
Analysis 6.9. Comparison 6 RISPERIDONE versus SERTINDOLE - all data short term, Outcome 9 Adverse effects: 1. General - at least one adverse effect.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 6 RISPERIDONE versus SERTINDOLE - all data short term
Outcome: 9 Adverse effects: 1. General - at least one adverse effect

|
Analysis 6.10. Comparison 6 RISPERIDONE versus SERTINDOLE - all data short term, Outcome 10 Adverse effects: 2. Death - suicide.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 6 RISPERIDONE versus SERTINDOLE - all data short term
Outcome: 10 Adverse effects: 2. Death - suicide

|
Analysis 6.11. Comparison 6 RISPERIDONE versus SERTINDOLE - all data short term, Outcome 11 Adverse effects: 3a. Cardiac effects - QTc prolongation.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 6 RISPERIDONE versus SERTINDOLE - all data short term
Outcome: 11 Adverse effects: 3a. Cardiac effects - QTc prolongation
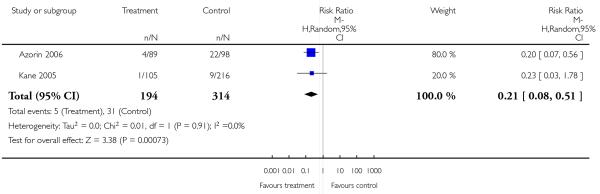
|
Analysis 6.12. Comparison 6 RISPERIDONE versus SERTINDOLE - all data short term, Outcome 12 Adverse effects: 3b. Cardiac effects - QTc abnormalities - change from baseline in ms.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 6 RISPERIDONE versus SERTINDOLE - all data short term
Outcome: 12 Adverse effects: 3b. Cardiac effects - QTc abnormalities - change from baseline in ms
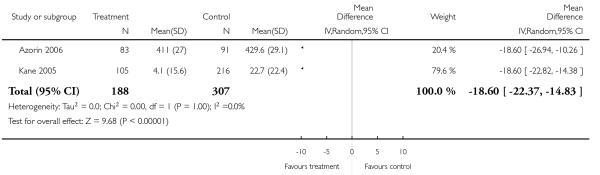
|
Analysis 6.13. Comparison 6 RISPERIDONE versus SERTINDOLE - all data short term, Outcome 13 Adverse effects: 4. Central nervous system - sedation.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 6 RISPERIDONE versus SERTINDOLE - all data short term
Outcome: 13 Adverse effects: 4. Central nervous system - sedation
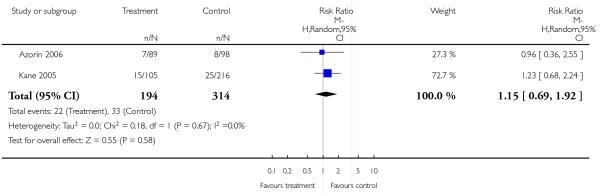
|
Analysis 6.14. Comparison 6 RISPERIDONE versus SERTINDOLE - all data short term, Outcome 14 Adverse effects: 5a. Extrapyramidal effects.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 6 RISPERIDONE versus SERTINDOLE - all data short term
Outcome: 14 Adverse effects: 5a. Extrapyramidal effects
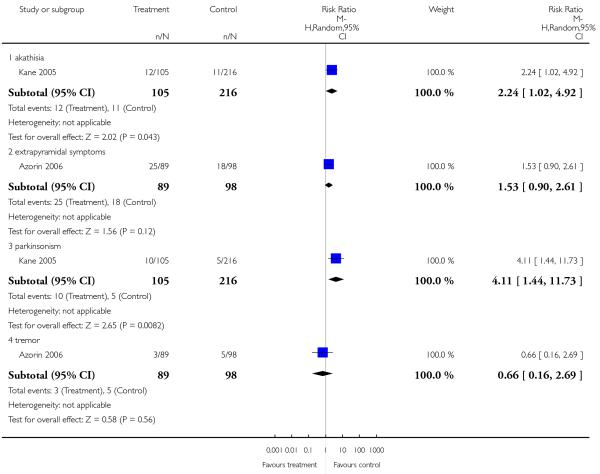
|
Analysis 6.15. Comparison 6 RISPERIDONE versus SERTINDOLE - all data short term, Outcome 15 Adverse effects: 5b. Extrapyramidal symptom scales.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 6 RISPERIDONE versus SERTINDOLE - all data short term
Outcome: 15 Adverse effects: 5b. Extrapyramidal symptom scales
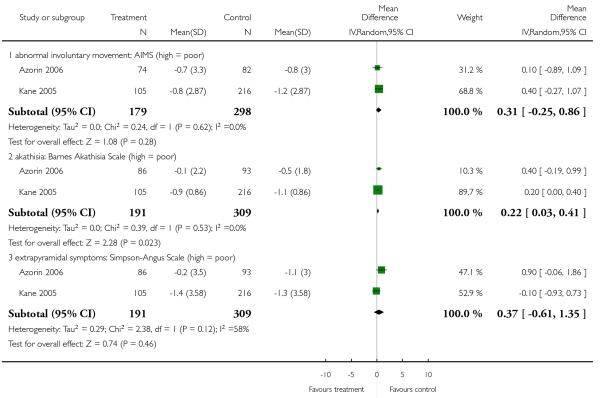
|
Analysis 6.16. Comparison 6 RISPERIDONE versus SERTINDOLE - all data short term, Outcome 16 Adverse effects: 6. Prolactin associated side effects - sexual dysfunction.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 6 RISPERIDONE versus SERTINDOLE - all data short term
Outcome: 16 Adverse effects: 6. Prolactin associated side effects - sexual dysfunction
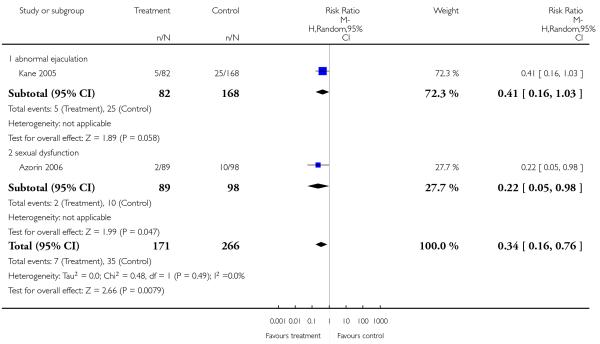
|
Analysis 6.17. Comparison 6 RISPERIDONE versus SERTINDOLE - all data short term, Outcome 17 Adverse effects: 7a. Metabolic - cholesterol - change from baseline in mg/dl.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 6 RISPERIDONE versus SERTINDOLE - all data short term
Outcome: 17 Adverse effects: 7a. Metabolic - cholesterol - change from baseline in mg/dl
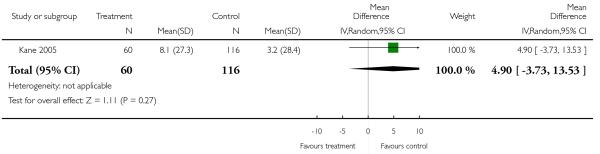
|
Analysis 6.18. Comparison 6 RISPERIDONE versus SERTINDOLE - all data short term, Outcome 18 Adverse effects: 7b. Metabolic - glucose - change from baseline in mg/dl.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 6 RISPERIDONE versus SERTINDOLE - all data short term
Outcome: 18 Adverse effects: 7b. Metabolic - glucose - change from baseline in mg/dl

|
Analysis 6.19. Comparison 6 RISPERIDONE versus SERTINDOLE - all data short term, Outcome 19 Adverse effects: 7c. Metabolic - weight gain.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 6 RISPERIDONE versus SERTINDOLE - all data short term
Outcome: 19 Adverse effects: 7c. Metabolic - weight gain
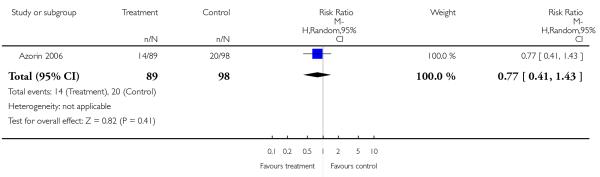
|
Analysis 6.20. Comparison 6 RISPERIDONE versus SERTINDOLE - all data short term, Outcome 20 Adverse effects: 7d. Metabolic - weight gain - change from baseline in kg.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 6 RISPERIDONE versus SERTINDOLE - all data short term
Outcome: 20 Adverse effects: 7d. Metabolic - weight gain - change from baseline in kg
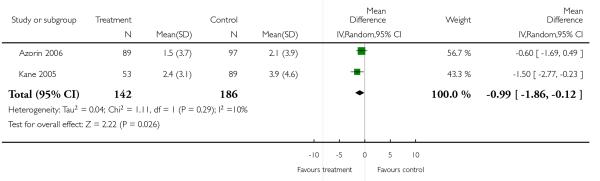
|
Analysis 7.1. Comparison 7 RISPERIDONE versus ZIPRASIDONE, Outcome 1 Global state: 1a. No clinically significant response (as defined by the original studies).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 7 RISPERIDONE versus ZIPRASIDONE
Outcome: 1 Global state: 1a. No clinically significant response (as defined by the original studies)
|
Analysis 7.2. Comparison 7 RISPERIDONE versus ZIPRASIDONE, Outcome 2 Global state: 1b. No clinically important change - short term (as defined by the original studies).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 7 RISPERIDONE versus ZIPRASIDONE
Outcome: 2 Global state: 1b. No clinically important change - short term (as defined by the original studies)
|
Analysis 7.3. Comparison 7 RISPERIDONE versus ZIPRASIDONE, Outcome 3 Leaving the study early.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 7 RISPERIDONE versus ZIPRASIDONE
Outcome: 3 Leaving the study early

|
Analysis 7.4. Comparison 7 RISPERIDONE versus ZIPRASIDONE, Outcome 4 Mental state: 1a. General - no clinically important change - short term (less than 50% PANSS total reduction).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 7 RISPERIDONE versus ZIPRASIDONE
Outcome: 4 Mental state: 1a. General - no clinically important change - short term (less than 50% PANSS total reduction)
|
Analysis 7.5. Comparison 7 RISPERIDONE versus ZIPRASIDONE, Outcome 5 Mental state: 1b. General - average endpoint score (PANSS total, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 7 RISPERIDONE versus ZIPRASIDONE
Outcome: 5 Mental state: 1b. General - average endpoint score (PANSS total, high = poor)
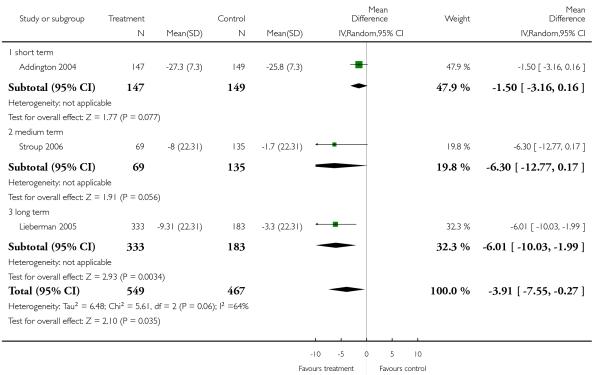
|
Analysis 7.6. Comparison 7 RISPERIDONE versus ZIPRASIDONE, Outcome 6 Mental state: 1c. General - avergae endpoint score - short term (BPRS total, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 7 RISPERIDONE versus ZIPRASIDONE
Outcome: 6 Mental state: 1c. General - avergae endpoint score - short term (BPRS total, high = poor)
|
Analysis 7.7. Comparison 7 RISPERIDONE versus ZIPRASIDONE, Outcome 7 Mental state: 2a. Positive symptoms - average endpoint score - medium term (PANSS positive, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 7 RISPERIDONE versus ZIPRASIDONE
Outcome: 7 Mental state: 2a. Positive symptoms - average endpoint score - medium term (PANSS positive, high = poor)
|
Analysis 7.8. Comparison 7 RISPERIDONE versus ZIPRASIDONE, Outcome 8 Mental State: 2b. Positive symptoms - average endpoint score - short term (BPRS positive, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 7 RISPERIDONE versus ZIPRASIDONE
Outcome: 8 Mental State: 2b. Positive symptoms - average endpoint score - short term (BPRS positive, high = poor)
|
Analysis 7.9. Comparison 7 RISPERIDONE versus ZIPRASIDONE, Outcome 9 Mental state: 3. Negative symptoms - average endpoint score (PANSS negative, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 7 RISPERIDONE versus ZIPRASIDONE
Outcome: 9 Mental state: 3. Negative symptoms - average endpoint score (PANSS negative, high = poor)
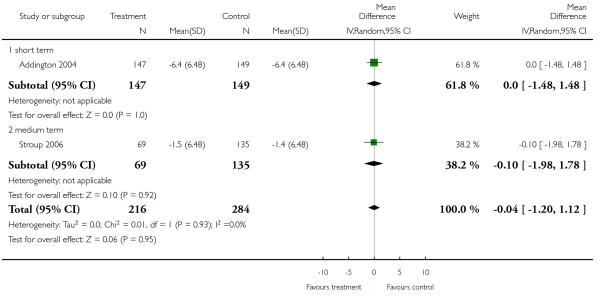
|
Analysis 7.10. Comparison 7 RISPERIDONE versus ZIPRASIDONE, Outcome 10 Service use: Number of patients rehospitalised.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 7 RISPERIDONE versus ZIPRASIDONE
Outcome: 10 Service use: Number of patients rehospitalised
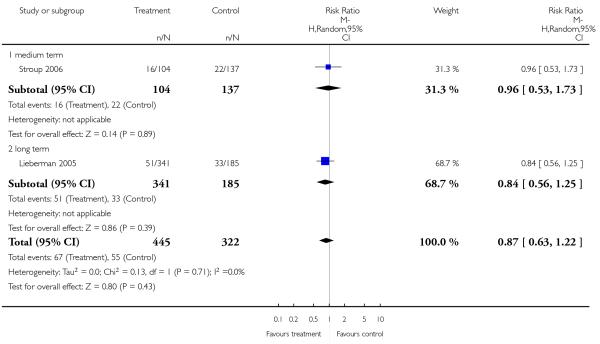
|
Analysis 7.11. Comparison 7 RISPERIDONE versus ZIPRASIDONE, Outcome 11 Adverse effects: 1. General - at least one adverse effect.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 7 RISPERIDONE versus ZIPRASIDONE
Outcome: 11 Adverse effects: 1. General - at least one adverse effect

|
Analysis 7.12. Comparison 7 RISPERIDONE versus ZIPRASIDONE, Outcome 12 Adverse effects: 2. Death.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 7 RISPERIDONE versus ZIPRASIDONE
Outcome: 12 Adverse effects: 2. Death
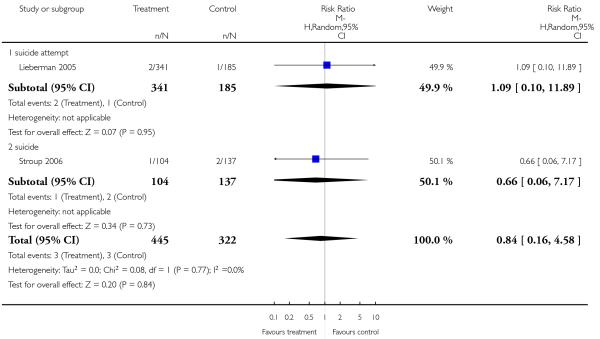
|
Analysis 7.13. Comparison 7 RISPERIDONE versus ZIPRASIDONE, Outcome 13 Adverse effects: 3a. Cardiac effects - QTc prolongation.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 7 RISPERIDONE versus ZIPRASIDONE
Outcome: 13 Adverse effects: 3a. Cardiac effects - QTc prolongation
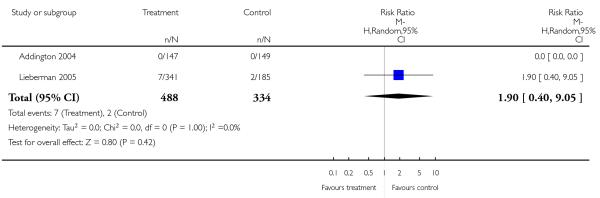
|
Analysis 7.14. Comparison 7 RISPERIDONE versus ZIPRASIDONE, Outcome 14 Adverse effects: 3b. Cardiac effects - QTc abnormalities - change from baseline in ms.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 7 RISPERIDONE versus ZIPRASIDONE
Outcome: 14 Adverse effects: 3b. Cardiac effects - QTc abnormalities - change from baseline in ms

|
Analysis 7.15. Comparison 7 RISPERIDONE versus ZIPRASIDONE, Outcome 15 Adverse effects: 4. Central nervous system - sedation.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 7 RISPERIDONE versus ZIPRASIDONE
Outcome: 15 Adverse effects: 4. Central nervous system - sedation
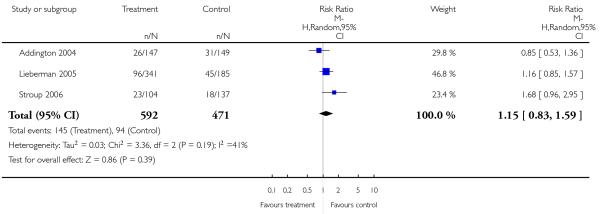
|
Analysis 7.16. Comparison 7 RISPERIDONE versus ZIPRASIDONE, Outcome 16 Adverse effects: 5a. Extrapyramidal effects.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 7 RISPERIDONE versus ZIPRASIDONE
Outcome: 16 Adverse effects: 5a. Extrapyramidal effects
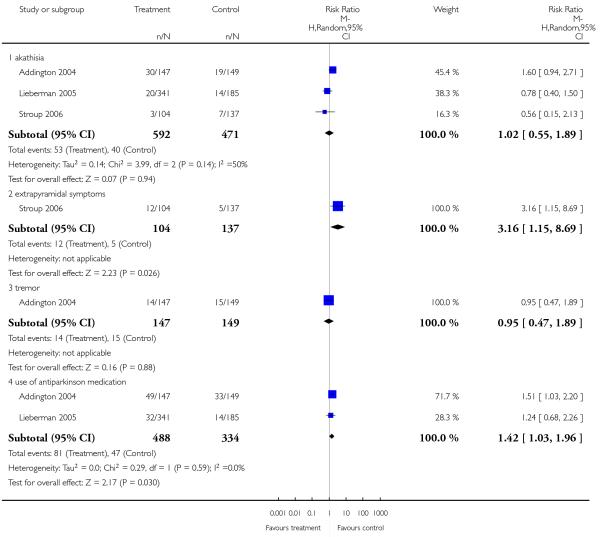
|
Analysis 7.17. Comparison 7 RISPERIDONE versus ZIPRASIDONE, Outcome 17 Adverse effects: 5b. Extrapyramidal symptoms - scale measured.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 7 RISPERIDONE versus ZIPRASIDONE
Outcome: 17 Adverse effects: 5b. Extrapyramidal symptoms - scale measured
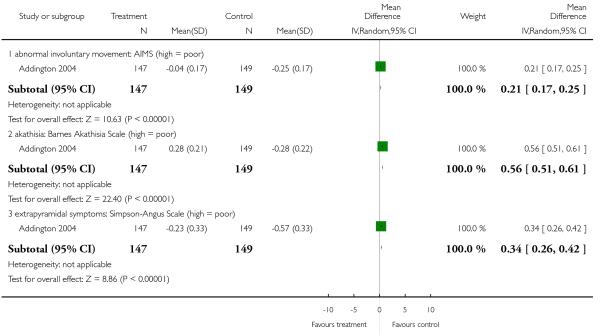
|
Analysis 7.18. Comparison 7 RISPERIDONE versus ZIPRASIDONE, Outcome 18 Adverse effects: 6a. Prolactin associated side effects.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 7 RISPERIDONE versus ZIPRASIDONE
Outcome: 18 Adverse effects: 6a. Prolactin associated side effects
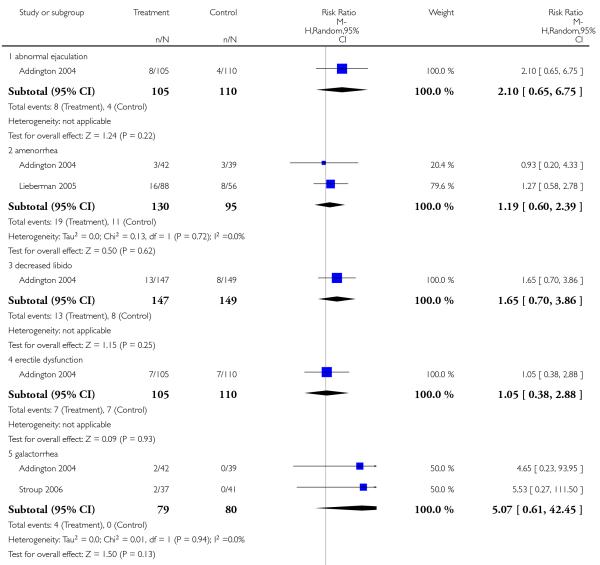
|
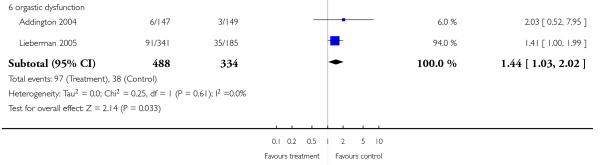
|
Analysis 7.19. Comparison 7 RISPERIDONE versus ZIPRASIDONE, Outcome 19 Adverse effects: 6b. Prolactin - change from baseline in ng/ml.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 7 RISPERIDONE versus ZIPRASIDONE
Outcome: 19 Adverse effects: 6b. Prolactin - change from baseline in ng/ml
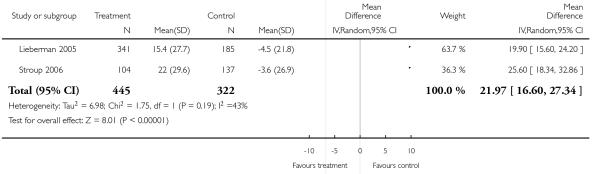
|
Analysis 7.20. Comparison 7 RISPERIDONE versus ZIPRASIDONE, Outcome 20 Adverse effects: 7a. Metabolic - cholesterol - change from baseline in mg/dl.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 7 RISPERIDONE versus ZIPRASIDONE
Outcome: 20 Adverse effects: 7a. Metabolic - cholesterol - change from baseline in mg/dl
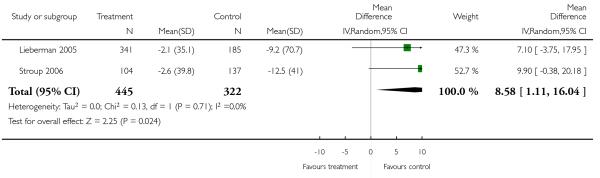
|
Analysis 7.21. Comparison 7 RISPERIDONE versus ZIPRASIDONE, Outcome 21 Adverse effects: 7b. Metabolic - glucose - change from baseline in mg/dl.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 7 RISPERIDONE versus ZIPRASIDONE
Outcome: 21 Adverse effects: 7b. Metabolic - glucose - change from baseline in mg/dl
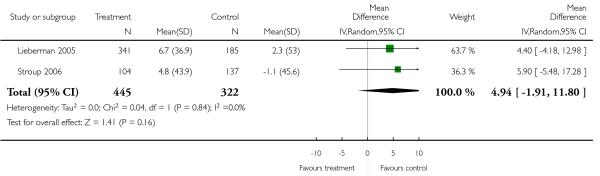
|
Analysis 7.22. Comparison 7 RISPERIDONE versus ZIPRASIDONE, Outcome 22 Adverse effects: 7c. Metabolic - weight gain of 7% or more of total body weight.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 7 RISPERIDONE versus ZIPRASIDONE
Outcome: 22 Adverse effects: 7c. Metabolic - weight gain of 7% or more of total body weight
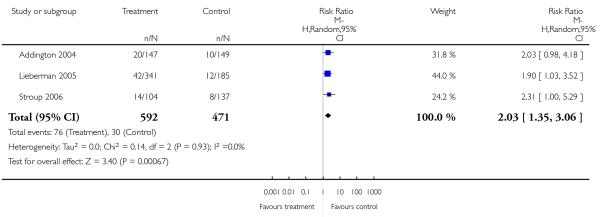
|
Analysis 7.23. Comparison 7 RISPERIDONE versus ZIPRASIDONE, Outcome 23 Adverse effects: 7d. Metabolic - weight gain - change from baseline in kg.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 7 RISPERIDONE versus ZIPRASIDONE
Outcome: 23 Adverse effects: 7d. Metabolic - weight gain - change from baseline in kg
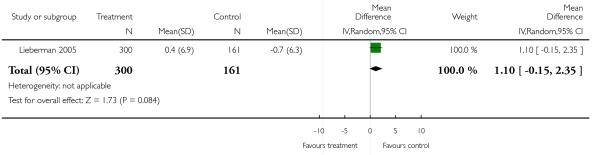
|
Analysis 8.1. Comparison 8 RISPERIDONE versus CLOZAPINE - sensitivity analysis (skewed data included), Outcome 1 Mental state: 1. General - average endpoint score (BPRS total, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 8 RISPERIDONE versus CLOZAPINE - sensitivity analysis (skewed data included)
Outcome: 1 Mental state: 1. General - average endpoint score (BPRS total, high = poor)
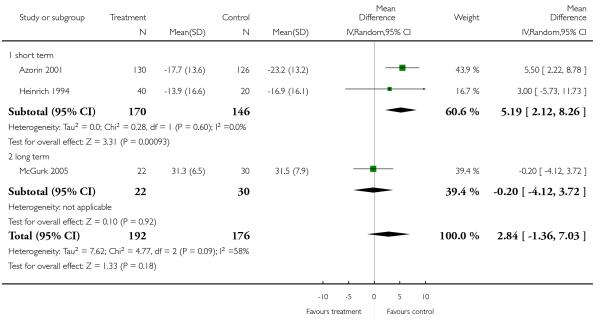
|
Analysis 8.2. Comparison 8 RISPERIDONE versus CLOZAPINE - sensitivity analysis (skewed data included), Outcome 2 Mental state: 2. Positive symptoms - average endpoint score (PANSS positive, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 8 RISPERIDONE versus CLOZAPINE - sensitivity analysis (skewed data included)
Outcome: 2 Mental state: 2. Positive symptoms - average endpoint score (PANSS positive, high = poor)
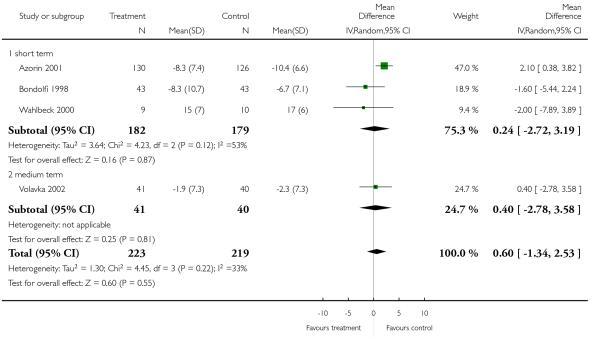
|
Analysis 8.3. Comparison 8 RISPERIDONE versus CLOZAPINE - sensitivity analysis (skewed data included), Outcome 3 Mental state: 3. Negative symptoms - average endpoint score (PANSS negative, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 8 RISPERIDONE versus CLOZAPINE - sensitivity analysis (skewed data included)
Outcome: 3 Mental state: 3. Negative symptoms - average endpoint score (PANSS negative, high = poor)
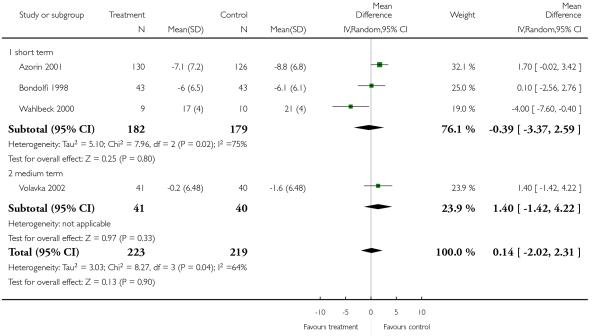
|
Analysis 9.1. Comparison 9 RISPERIDONE versus OLANZAPINE - sensitivity analysis (skewed data excluded), Outcome 1 Mental state: 1a. General - average endpoint score (PANSS total, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 9 RISPERIDONE versus OLANZAPINE - sensitivity analysis (skewed data excluded)
Outcome: 1 Mental state: 1a. General - average endpoint score (PANSS total, high = poor)
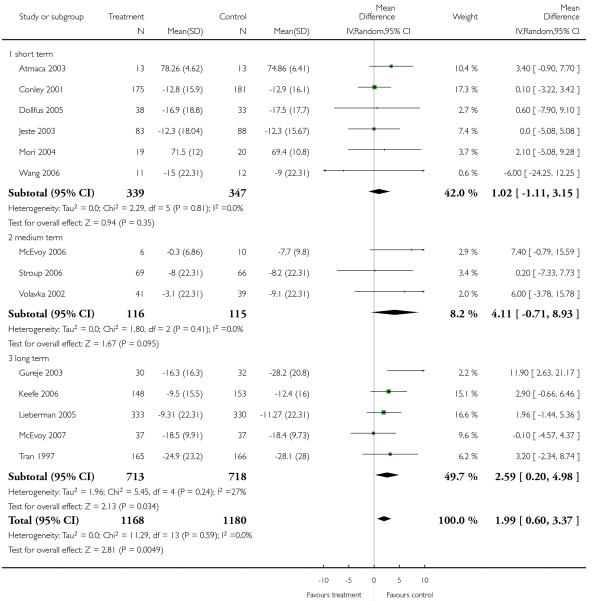
|
Analysis 9.2. Comparison 9 RISPERIDONE versus OLANZAPINE - sensitivity analysis (skewed data excluded), Outcome 2 Mental state: 2. General - average endpoint score (BPRS total, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 9 RISPERIDONE versus OLANZAPINE - sensitivity analysis (skewed data excluded)
Outcome: 2 Mental state: 2. General - average endpoint score (BPRS total, high = poor)
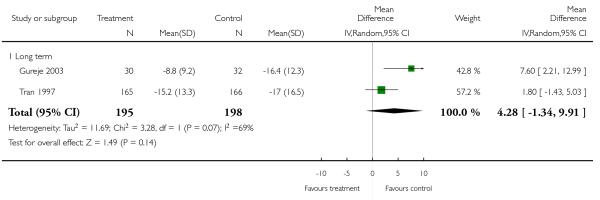
|
Analysis 9.3. Comparison 9 RISPERIDONE versus OLANZAPINE - sensitivity analysis (skewed data excluded), Outcome 3 Mental state: 3. Positive symptoms - average endpoint score (PANSS positive, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 9 RISPERIDONE versus OLANZAPINE - sensitivity analysis (skewed data excluded)
Outcome: 3 Mental state: 3. Positive symptoms - average endpoint score (PANSS positive, high = poor)
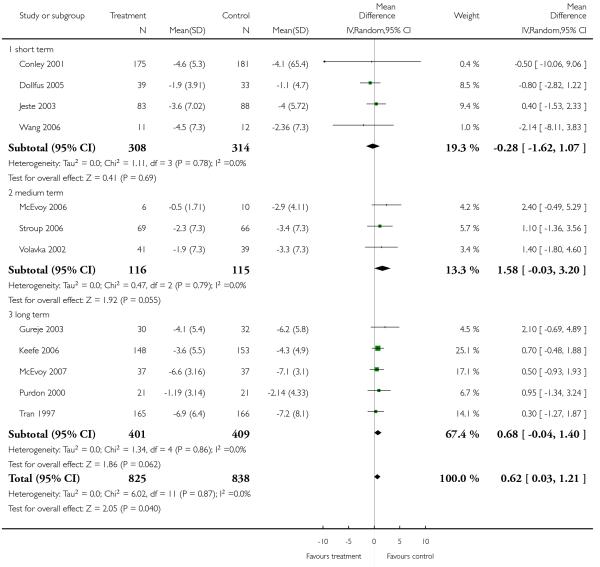
|
Analysis 9.4. Comparison 9 RISPERIDONE versus OLANZAPINE - sensitivity analysis (skewed data excluded), Outcome 4 Adverse effects: 1. Extrapyramidal effects - scale measured.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 9 RISPERIDONE versus OLANZAPINE - sensitivity analysis (skewed data excluded)
Outcome: 4 Adverse effects: 1. Extrapyramidal effects - scale measured
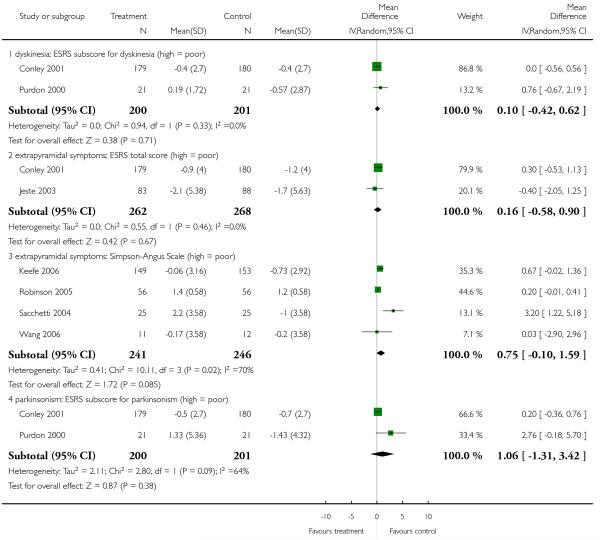
|
Analysis 9.5. Comparison 9 RISPERIDONE versus OLANZAPINE - sensitivity analysis (skewed data excluded), Outcome 5 Adverse effects: 2. Prolactin associated side effects - change from baseline in ng/ml.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 9 RISPERIDONE versus OLANZAPINE - sensitivity analysis (skewed data excluded)
Outcome: 5 Adverse effects: 2. Prolactin associated side effects - change from baseline in ng/ml

|
Analysis 10.1. Comparison 10 RISPERIDONE versus QUETIAPINE - sensitivity analysis (skewed data included), Outcome 1 Mental state: 1. Positive symptoms - average endpoint score (PANSS positive, high = poor).
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 10 RISPERIDONE versus QUETIAPINE - sensitivity analysis (skewed data included)
Outcome: 1 Mental state: 1. Positive symptoms - average endpoint score (PANSS positive, high = poor)

|
Analysis 10.2. Comparison 10 RISPERIDONE versus QUETIAPINE - sensitivity analysis (skewed data included), Outcome 2 Adverse effects: 1. Extrapyramidal effects - scale measured.
Review: Risperidone versus other atypical antipsychotics for schizophrenia
Comparison: 10 RISPERIDONE versus QUETIAPINE - sensitivity analysis (skewed data included)
Outcome: 2 Adverse effects: 1. Extrapyramidal effects - scale measured

|
WHAT’S NEW
Last assessed as up-to-date: 21 May 2008.
| Date | Event | Description |
|---|---|---|
| 12 December 2012 | Amended | Contact details updated. |
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
The review was slightly adapted to new functions available in Review Manager 5, namely the risk of bias table.
Footnotes
DECLARATIONS OF INTEREST
Katja Komossa: none known.
Stefan Leucht: has received speaker/consultancy/advisory board honoraria from SanofiAventis, BMS, EliLilly, Essex Pharma, Glaxo-SmithKline, Janssen/Johnson and Johnson, Lundbeck and Pfizer including the fees and travel expenses for attending these functions; and he has received funding for research projects from EliLilly and SanofiAventis.
Christine Rummel received lecture honoraria and travel grants to attend scientific meetings from AstraZeneca, Janssen-Cilag, Eli Lilly and Pfizer.
Werner Kissling: received speaker or consultancy honoraria from SanofiAventis, BMS, Lilly, Janssen, Lundbeck, Bayer and Pfizer.
Heike Hunger: none known.
Franziska Schmid: none known.
Sandra Schwarz: none known.
References to studies included in this review
- Addington 2004 {published data only} .Addington D, Pantelis C, Benattia I. Ziprasidone vs risperidone in schizophrenia: efficacy and safety in an 8-week trial. International Journal of Neuropsychopharmacology. 2002;5(Suppl 1):125. [Google Scholar]
- *; Addington DE, Pantelis C, Dineen M, Benattia I, Romano SJ. Efficacy and tolerability of ziprasidone versus risperidone in patients with acute exacerbation of schizophrenia or schizoaffective disorder: an 8-week, double-blind, multicenter trial. Journal of Clinical Psychiatry. 2004;65(12):1624–33. doi: 10.4088/jcp.v65n1207. [DOI] [PubMed] [Google Scholar]
- Atmaca 2003 {published data only} .Atmaca M, Kuloglu M, Tezcan E, Ustundag B. Serum leptin and triglyceride levels in patients on treatment with atypical antipsychotics. Journal of Clinical Psychiatry. 2003;64(5):598–604. doi: 10.4088/jcp.v64n0516. [DOI] [PubMed] [Google Scholar]
- Azorin 2001 {published data only} .*; Azorin JM, Spiegel R, Remington G, Vanelle JM, Pere JJ, Giguere M, Bourdeix I. A double-blind comparative study of clozapine and risperidone in the management of severe chronic schizophrenia. American Journal of Psychiatry. 2001;158(8):1305–13. doi: 10.1176/appi.ajp.158.8.1305. [DOI] [PubMed] [Google Scholar]
- Azorin 2006 {published data only} .Azorin J-M, Strub N, Loft H. A double-blind, controlled study of sertindole versus risperidone in the treatment of moderate-to-severe schizophrenia. International Clinical Psychopharmacology. 2006;21(1):49–56. doi: 10.1097/01.yic.0000177020.26311.a7. [DOI] [PubMed] [Google Scholar]
- Bondolfi 1998 {published data only} .Bondolfi G, Baumann P, Dufour H. Treatment-resistant schizophrenia - clinical experience with new antipsychotics. European Neuropsychopharmacology. 1996;6(Suppl 2):21–5. doi: 10.1016/0924-977x(96)00012-0. [DOI] [PubMed] [Google Scholar]
- *; Bondolfi G, Dufour H, Patris M, May JP, Billeter U, Eap CB, Baumann P. Risperidone versus clozapine in treatment-resistant chronic schizophrenia: a randomised double-blind study. The Risperidone Study Group. American Journal of Psychiatry. 1998;155(4):499–504. doi: 10.1176/ajp.155.4.499. [DOI] [PubMed] [Google Scholar]
- Breier 1999 {published data only} .*; Breier AF, Malhotra AK, Su TP, Pinals DA, Elman I, Adler CM, Lafargue RT, Clifton A, Pickar D. Clozapine and risperidone in chronic schizophrenia: effects on symptoms, parkinsonian side effects, and neuroendocrine response. American Journal of Psychiatry. 1999;156(2):294–8. doi: 10.1176/ajp.156.2.294. [DOI] [PubMed] [Google Scholar]
- Su T-P, Elman I, Malhotra AK, Adler CM, Pickar D, Breier AF. Comparison of weight gain during risperidone and clozapine treatment in chronic schizophrenia; Proceedings of the 149th Annual Meeting of the American Psychiatric Association; New York, New York, USA. 1996 Oct 2 - May 9.1996. [Google Scholar]
- Canive 2000 {published data only} .Canive JM, Edgar JC, LaNoue MD, Miller GA, Weisend MP, Tuason VB. A magnetoencephalographic examination on the effects of olanzapine and risperidone in patients with schizophrenia; Proceedings of the 40th Annual Meeting of the New Clinical Drug Evaluation Unit; Boca Raton, Florida, USA. USA. 2000 May 30 - Jun 2.2000. [Google Scholar]
- Canive JM, Miller GA, Irwin JG, Moses SN, Thoma RJ, Edgar JC, Sherwood A, Torres F, LaNoue M, Lewis S, Hanlos F, Weisend MP, Mead V, Tuason VB. Efficacy of Olanzapine and Risperidone in Schizophrenia: A Randomized Double-Blind Crossover Design. Psychopharmacology Bulletin. 2000;39(1):105–16. [PubMed] [Google Scholar]
- Chan 2007 {published data only} .*; Chan HY, Lin WW, Lin SK, Hwang TJ, Su TP, Chiang SC, Hwu HG. Efficacy and safety of aripiprazole in the acute treatment of schizophrenia in Chinese patients with risperidone as an active control: A randomized trial. Journal of Clinical Psychiatry. 2007;68(1):29–36. doi: 10.4088/jcp.v68n0104. [DOI] [PubMed] [Google Scholar]
- Hwang TJ, Chan HY, Lin WW, Lin SK, Hwu HG, Cheng MY, Forbes RA. Aripiprazole versus risperidone in the treatment of acutely relapsed patients with schizophrenia in Taiwan: a randomised controlled trial. Journal of the European College of Neuropsychopharmacology. 2005;14(Suppl 3):497. [Google Scholar]
- Conley 2001 {published data only} .*; Conley RR, Mahmoud R. A randomized double-blind study of risperidone and olanzapine in the treatment of schizophrenia or schizoaffective disorder. American Journal of Psychiatry. 2001;158(5):765–74. doi: 10.1176/appi.ajp.158.5.765. [DOI] [PubMed] [Google Scholar]
- Conley RR, Mahmoud R, Risperidone Study Group Risperidone versus olanzapine in patients with schizophrenia and schizoaffective psychosis [Risperidon versus olanzapin bei patienten mit schizophrenie und schizoaffektiven psychosen] Nervenheilkunde. 2000;19(5):110–2. [Google Scholar]
- Conley RR, Mahmoud RA. Risperidone versus olanzapine in patients with schizophrenia and schizoaffective disorder; Proceedings of the 155th Annual Meeting of the American Psychiatric Association; Philadelphia, Pennsylvania, USA. 2002 May 18-23.2002. [Google Scholar]
- Harvey PD, Green MF, McGurk SR, Meltzer HY. Changes in cognitive functioning with risperidone and olanzapine treatment: a large-scale, double-blind, randomised study. Psychopharmacology. 2003;169(3-4):404–11. doi: 10.1007/s00213-002-1342-5. [DOI] [PubMed] [Google Scholar]
- Conley 2005 {published data only} .Conley RR, Kelly DL, Nelson MW, Richardson CM, Feldman S, Benham R, Steiner P, Yu Y, Khan I, McMullen R, Gale E, Mackowick M, Love RC. Risperidone, quetiapine, and fluphenazine in the treatment of patients with therapy-refractory schizophrenia. Clinical Neuropharmacology. 2005;28(4):163–8. doi: 10.1097/01.wnf.0000172993.89879.0f. Pharm D, BCPP. [DOI] [PubMed] [Google Scholar]
- *; Kelly DL, Conley RR. A randomised double-blind 12-week study of quetiapine, risperidone or fluphenazine on sexual functioning in people with schizophrenia. Psychoneuroendocrinology. 2006;31(3):340–6. doi: 10.1016/j.psyneuen.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Kelly DL, Conley RR. Thyroid function in treatment-resistant schizophrenia patients treated with quetiapine, risperidone, or fluphenazine. Journal of Clinic Psychiatry. 2005;66:80–4. doi: 10.4088/jcp.v66n0111. [DOI] [PubMed] [Google Scholar]
- Richardson CM, Kelly DL, Gold JM, McMahon R, Yu Y, Conley RR. Risperidone vs quetiapine vs fluphenazine in treatment - resistant schizophrenia: neuropsychological outcome. Schizophrenia Bulletin. 2005;31:501–2. [Google Scholar]
- Daniel 1996 {published data only} .*; Daniel DG, Goldberg TE, Weinberger DR, Kleinman JE, Pickar D, Lubick LJ, Williams TS. Different side effect profiles of risperidone and clozapine in 20 outpatients with schizophrenia or schizoaffective disorder: a pilot study. American Journal of Psychiatry. 1996;153(3):417–9. doi: 10.1176/ajp.153.3.417. [DOI] [PubMed] [Google Scholar]
- Dollfus 2005 {published data only} .Dollfus S. The treatment of post-psychotic depression. Journal of the European College of Neuropsychopharmacology. 2006;16(Suppl 4):165. [Google Scholar]
- *; Dollfus S, Olivier V, Chabot B, Deal C, Perrin E. Olanzapine versus risperidone in the treatment of post-psychotic depression in schizophrenic patients. Schizophrenia Research. 2005;78(2-3):157–9. doi: 10.1016/j.schres.2005.06.001. [DOI] [PubMed] [Google Scholar]
- Dolnak 2001 {published data only} .Dolnak R, Rapaport MH. A prospective, randomised, double blind study examining functioning in schizophrenic patients treated with olanzapine and risperidone. Schizophrenia Research. 2001;49(1, 2):225–6. [Google Scholar]
- Gureje 2003 {published data only} .*; Gureje O, Miles W, Keks N, Grainger D, Lambert T, McGrath J, Tran P, Catts S, Fraser A, Hustig H, Andersen S, Crawford AM. Olanzapine versus risperidone in the management of schizophrenia: a randomized double-blind trial in Australia and New Zealand. Schizophrenia Research. 2003;61:303–14. doi: 10.1016/s0920-9964(02)00226-8. [DOI] [PubMed] [Google Scholar]
- Heinrich 1994 {published data only} .Heinrich K, Klieser E, Lehmann E, Kinzler E. Experimental comparison of the efficacy and compatibility of risperidone and clozapine in acute schizophrenia; Proceedings of the 17th Collegium Internationale Neuro-Psychopharmacologicum Congress; Kyoto, Japan. 1990 Sep 10-14.1991. pp. 37–9. [Google Scholar]
- *; Heinrich K, Klieser E, Lehmann E, Kinzler E, Hruschka H. Risperidone versus clozapine in the treatment of schizophrenic patients with acute symptoms: a double blind, randomized trial. Progress in Neuropsychopharmacology and Biological Psychiatry. 1994;18(1):129–37. doi: 10.1016/0278-5846(94)90029-9. [DOI] [PubMed] [Google Scholar]
- Klieser E, Lehmann E, Kinzler E, Wurthmann C, Heinrich K. Randomized, double-blind, controlled trial of risperidone versus clozapine in patients with chronic schizophrenia. Journal of Clinical Psychopharmacology. 1995;15(1 Suppl 1):45–51. doi: 10.1097/00004714-199502001-00008. [DOI] [PubMed] [Google Scholar]
- Hwang 2003 {published data only} .Hwang TJ, Lee S-M, Sun HJ, Lin H-N, Tsai S-J, Lee Y-C, Chen Y-S. Amisulpride versus risperidone in the treatment of schizophrenic patients: a double-blind pilot study in Taiwan. Journal of the Formosan Medical Association. 2003;102(1):30–6. [PubMed] [Google Scholar]
- Jeste 2003 {published data only} .Jeste D, Madhusoodanan S, Barak Y, Martinez RA, Mahmoud R, Kershaw P. Risperidone and olanzapine in elderly patients with schizophrenia and schizoaffective disorder. International Psychogeriatrics. 2001;13(2):295. [Google Scholar]
- *; Jeste DV, Barak Y, Madhusoodanan S, Gossman F, Gharabawi G. International multisite double-blind trial of the atypical antipsychotics risperidone and olanzapine in 175 elderly patients with chronic schizophrenia. American Journal of Geriatric Psychiatry. 2003;11(6):638–47. doi: 10.1176/appi.ajgp.11.6.638. [DOI] [PubMed] [Google Scholar]
- Jeste DV, Madhusoodanan S, Barak F, Martinez RA. Risperidone versus olanzapine in elderly patients with schizophrenia; 155th Annual Meeting of the American Psychiatric Association; Philadelphia, Pennsylvania, USA. 2002 May 18-23.2002. [Google Scholar]
- Jeste DV, Madhusoodanan S, Barak F, Martinez RA. Risperidone versus olanzapine in elderly patients with schizophrenia; Proceedings of the 154th Annual Meeting of the American Psychiatric Association; New Orleans, Louisiana, USA. 2001 May 5-10; 2001. Marathon Multimedia. [Google Scholar]
- Kane 2005 {published data only} .*; Kane JM, Potkin S, Buckley P, Daniel DG. Safety and efficacy of sertindole and risperidone in treatment resistant patients with schizophrenia. Schizophrenia Bulletin. 2005;31:490. [Google Scholar]
- Keefe 2006 {published data only} .Keefe R. Neurocognitive efficacy in patients with chronic schizophrenia. Journal of the European College of Neuropsychopharmacology. 2006;16(Suppl 4):577. [Google Scholar]
- *; Keefe RSE, Young CA, Rock SL, Purdon SE, Gold JM, Breier A. One-year double-blind study of the neurocognitive efficacy of olanzapine, risperidone, and haloperidol in schizophrenia. Schizophrenia Research. 2006;81(1):1–15. doi: 10.1016/j.schres.2005.07.038. [DOI] [PubMed] [Google Scholar]
- Namjoshi M, Young C, Huang L, Edgell ET, Breier A. Conical and quality-of-life outcomes associated with olanzapine, risperidone, and haloperidol treatment in patients with schizophrenia: results from a US random study. International Journal of Neuropsychopharmacology. 2002;5(Suppl 1):127. [Google Scholar]
- Namjoshi M, Young C, Huang L, Edgell ET, Breier A. Hospitalization rates associated with olanzapine, risperidone, and haloperidol treatment in patients with schizophrenia: results from a U.S. randomized controlled trial. Journal of the European College of Neuropsychopharmacology. 2002;12(Suppl 3):315. [Google Scholar]
- Namjoshi M, Young CA, Huang L, Edgell E, Breier A. Cost-effectiveness of olanzapine compared to risperidone and haloperidol in the treatment of patients with schizophrenia: results from a U.S. randomized controlled trial. Schizophrenia Research. 2003;60(1):296. [Google Scholar]
- Lieberman 2005 {published data only} .Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Keefe R, Perkins DO, Davis S, Davis CE, Lebowitz B, Hsiao J. CATIE trial results. Journal of the European College of Neuropsychopharmacology. 2006;16(Suppl 4):S184. [Google Scholar]
- McEvoy 2006 {published data only} .*; McEvoy JP, Lieberman JA, Stroup TS, Davis SM, Meltzer HY, Rosenheck RA, Swartz MS, Perkins DO, Keefe RS, Davis CE, Severe J, Hsiao JK. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. American Journal of Psychiatry. 2006;163(4):600–10. doi: 10.1176/ajp.2006.163.4.600. [DOI] [PubMed] [Google Scholar]
- McEvoy 2007 {published data only} .Keefe RSE, Gu H, Sweeney JA, Perkins DO, McEvoy JP, Hamer RM, Lieberman JA. The effects of olanzapine, quetiapine and risperidone on neurocognitive function in first-episode psychosis: a double-blind 52-week comparison; Proceedings of the 159th Annual Meeting of the American Psychiatric Association; Toronto, Canada. 2006 May 20-25.2006. [Google Scholar]
- Lieberman J, McEvoy JP, Perkins D, Hamer RH. Comparison of atypicals in first-episode psychosis: a randomized, 52-week comparison of olanzapine, quetiapine, and risperidone. Journal of the European College of Neuropsychopharmacology. 2005;15(Suppl 3):525. [Google Scholar]
- McEvoy JP. Comparison of clozapine versus other atypical drugs in prospectively defined, unresponsive patients; Proceedings of the 159th Annual Meeting of the American Psychiatric Association; Toronto, Canada. 2006 May 20-25.2006. [Google Scholar]
- *; McEvoy JP, Lieberman JA, Perkins DO, Hamer RM, Gu H, Lazarus A, Sweitzer D, Olexy C, Weiden P, Strakowski SD. Efficacy and tolerability of olanzapine, quetiapine, and risperidone in the treatment of early psychosis: A randomized, double-blind 52-week comparison. American Journal of Psychiatry. 2007;164:1050–60. doi: 10.1176/ajp.2007.164.7.1050. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Perkins DO, Gu H, Hamer RM, Lieberman JA. Clinical effectiveness and predictors of treatment non-adherence: comparison of olanzapine, quetiapine, and risperidone in first-episode psychosis. Schizophrenia Research. 2006;86(Suppl 1):130. [Google Scholar]
- McGurk 2005 {published data only} .Bellack AS, Schooler NR, Marder SR, Kane JM, Brown CH, Yang Y. Do clozapine and risperidone affect social competence and problem solving? American Journal of Psychiatry. 2004;161(2):364–7. doi: 10.1176/appi.ajp.161.2.364. [DOI] [PubMed] [Google Scholar]
- *; McGurk SR, Carter C, Goldman R, Green MF, Marder SR, Xie H, Schooler NR, Kane JM. The effects of clozapine and risperidone on spatial working memory in schizophrenia. American Journal of Psychiatry. 2005;162(5):1013–6. doi: 10.1176/appi.ajp.162.5.1013. [DOI] [PubMed] [Google Scholar]
- Mori 2004 {published data only} .*; Mori K, Nagao M, Yamashita H, Morinobu S, Yamawaki S. Effect of switching to atypical antipsychotics on memory in patients with chronic schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 2004;28(4):659–65. doi: 10.1016/j.pnpbp.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Mori K, Nagao M, Yamashita H, Yamawaki S. Effects of switching to atypical antipsychotics on memory in chronic schizophrenic patients. International Journal of Neuropsychopharmacology. 2002;5(Suppl 1):80. [Google Scholar]
- Möller 2005 {published data only} .Möller HJ, Riedel M, Eich FX. A randomised, double-blind clinical trial comparing treatment with amisulpride or risperidone for six weeks in elderly patients with schizophrenia. Journal of the European College of Neuropsychopharmacology. 2005;15(Suppl 3):511. [Google Scholar]
- Peuskens 1999 {published data only} .Peuskens J, Bech P, Möller HJ, Bale R, Fleurot O, Rein W. Amisulpride versus risperidone in the treatment of acute exacerbation of schizophrenia. Journal of Medical Economics. 2003;6:31–41. doi: 10.1016/s0165-1781(99)00075-x. [DOI] [PubMed] [Google Scholar]
- Potkin 2003 {published data only} .Potkin SG, Saha AR, Kujawa MJ, Carson WH, Ali M, Stock E, Stringfellow J, Ingenito G, Marder SR. Aripiprazole, an antipsychotic with a novel mechanism of action, and risperidone vs placebo in patients with schizophrenia and schizoaffective disorder. Archives of General Psychiatry. 2003;60(7):681–90. doi: 10.1001/archpsyc.60.7.681. [DOI] [PubMed] [Google Scholar]
- Potkin 2006 {published data only} .Gharabawi GM, Greenspan A, Rupnow MFT, Kosik-Gonzalez C, Bossie CA, Zhu Y, Kalali AH, Awad AG. Reduction in psychotic symptoms as a predictor of patient satisfaction with antipsychotic medication in schizophrenia: data from a randomized double-blind trial. BMC Psychiatry. 2006;6(45):1–7. doi: 10.1186/1471-244X-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan A, Kosik-Gonzalez C, Moreau-Mallet V, Bossie CA, Rupnow MFT, Zhu Y, Gharabawi GM. Risperidone vs quetiapine in inpatients with schizophrenia: a double-blind placebo-controlled study. Journal of the European College of Neuropsychopharmacology. 2005;15(Suppl 3):503. [Google Scholar]
- *; Potkin SG, Gharabawi GM, Greenspan AJ, Mahmoud R, Kosik-Gonzalez C, Rupnow MFT, Bossie CA, Davidson M, Burtea V, Zhu Y, Trivedi JK. A double-blind comparison of risperidone, quetiapine and placebo in patients with schizophrenia experiencing an acute exacerbation requiring hospitalization. Schizophrenia Research. 2006;85(1-3):254–65. doi: 10.1016/j.schres.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Potkin SG, Greenspan A, Kosik-Gonzalez C, Bossie C, Rupnow M, Zhu Y, Gharabawi G. A placebo - controlled study of risperidone vs quetiapine for symptom response and readiness for discharge among agitated inpatients with schizophrenia. Schizophrenia Bulletin. 2005;31:501. [Google Scholar]
- Purdon 2000 {published data only} .Purdon SE, Canadian Cognition and Outcome Study Group Neuropsychological change in early phase schizophrenia over twelve months of treatment with olanzapine, risperidone, or haloperidol. Schizophrenia Research. 1998;29(1, 2):132–3. doi: 10.1001/archpsyc.57.3.249. [DOI] [PubMed] [Google Scholar]
- *; Purdon SE, Jones BD, Stip E, Labelle A, Addington D, David SR, Breier A, Tollefson GD. Neuropsychological change in early phase schizophrenia during 12 months of treatment with olanzapine, risperidone, or haloperidol. The Canadian Collaborative Group for research in schizophrenia. Archives of General Psychiatry. 2000;57(3):249–58. doi: 10.1001/archpsyc.57.3.249. [DOI] [PubMed] [Google Scholar]
- Purdon SE, Woodward N, Lindborg SR, Stip E. Procedural learning in schizophrenia after 6 months of double-blind treatment with olanzapine, risperidone, and haloperidol. Psychopharmacology. 2003;169(3-4):390–7. doi: 10.1007/s00213-003-1505-z. [DOI] [PubMed] [Google Scholar]
- Ren 2002 {published data only} .Ren Q, Lu Y, Tian M. Comparison of quality of life of schizophrenic outpatients treated with risperdal or clozapine. Nervous Disease and Mental Hygiene. 2002;1(2):40. [Google Scholar]
- Riedel 2005 {published data only} .Riedel M, Möller H-J, Strassnig M, Spellmann I, Müller-Arends A, Dehning S, Sadowsky N, Müller N. Efficacy of quetiapine against negative symptoms in schizophrenia; Proceedings of the XIII World Congress of Psychiatry; Cairo, Egypt. 2005 10-15th Sept.2005. [Google Scholar]
- *; Riedel M, Muller N, Strassnig M, Spellmann I, Engel RR, Musil R, Dehning S, Douhet A, Schwarz MJ, Moller H-J. Quetiapine has equivalent efficacy and superior tolerability to risperidone in the treatment of schizophrenia with predominantly negative symptoms. European Archives of Psychiatry and Clinical Neuroscience. 2005;255(6):432–7. doi: 10.1007/s00406-005-0622-6. [DOI] [PubMed] [Google Scholar]
- Robinson 2005 {published data only} .*; Robinson DG, Woerner MG, Napolitano B, Patel RC, Sevy SM, Gunduz-Bruce H, Soto-Porello JM, Mendelowitz A, Khadivi A, Miller R, McCormack J, Lorell BS, Lesser ML, Schooler NR, Kane JM. Randomized comparison of olanzapine versus risperidone for the treatment of first-episode schizophrenia: 4-month outcomes. American Journal of Psychiatry. 2006;163(12):2096–102. doi: 10.1176/ajp.2006.163.12.2096. [DOI] [PubMed] [Google Scholar]
- Sacchetti 2004 {published data only} .Sacchetti E, Valsecchi P, Regini C, Galluzo a, Cacciani P, Agrimi E, Mencacci C. Comparison of quetiapine, olanzapine and risperidone in patients with schizophrenia: interim results of a randomised, rater-blinded study. Schizophrenia Research. 2004;67(1):150. [Google Scholar]
- *; Sacchetti E, Valsecchi P, Regini C, Galluzzo A, Cacciani P, Agrimi E, Mencacci C. Comparison of quetiapine, olanzapine, and risperidone in schizophrenia. Journal of the European College of Neuropsychopharmacology. 2004;14(Suppl 3):286. [Google Scholar]
- Sacchetti E, Valsecchi P, Regini C, Galluzzo A, Cacciani P, Agrimi E, Mencacci C. Comparison of quetiapine, olanzapine and risperidone in patients with schizophrenia: interim results of a randomised, rater-blinded study. Journal of the European College of Neuropsychopharmacology. 2003;13(4):350. [Google Scholar]
- Sechter 2002 {published data only} .*; Sechter D, Peuskens J, Fleurot O, Rein W, Lecrubier Y, Amisulpride Study Group Amisulpride vs. risperidone in chronic schizophrenia: results of a 6-month double-blind study. Neuropyschopharmacology. 2002;27(6):1071–81. doi: 10.1016/S0893-133X(02)00375-5. [DOI] [PubMed] [Google Scholar]
- Sikich 2004 {published data only} .Sikich L. Critical decisions in the treatment of adolescent and pediatric psychosis; Proceedings of the 154th Annual Meeting of the American Psychiatric Association; New Orleans, Louisiana, USA. 2001 May 5-10; 2001. Marathon Multimedia. [Google Scholar]
- *; Sikich L, Hamer RM, Bashford RA, Sheitman BB, Lieberman JA. A pilot study of risperidone, olanzapine, and haloperidol in psychotic youth: a double-blind, randomized, 8-week trial. Neuropyschopharmacology. 2004;29(1):133–45. doi: 10.1038/sj.npp.1300327. [DOI] [PubMed] [Google Scholar]
- Sikich L, Hooper SR, Malekpour AH, Sheitman BB, Lieberman JA. A double blind comparison of typical versus atypical antipsychotic agents on selected neurocognitive functions in children and adolescents with psychotic disorders. Schizophrenia Research. 2001;49(1, 2):245. [Google Scholar]
- Sikich L, Horrigan JP, Lieberman JA, Barnhill LJ, Sheitman BB, Courvoisie HE. Comparative use of olanzapine and risperidone in psychotic youth; Proceedings of the 154th Annual Meeting of the American Psychiatric Association; New Orleans, Louisiana, USA. 2001 May 5-10; 2001. Marathon Multimedia. [Google Scholar]
- Sikich L, Horrigan JP, Lieberman JA, Barnhill LJJr, Sheitman BB, Courvoisie HE. Comparative use of olanzapine and risperidone in psychotic youth; Proceedings of the 155th Annual Meeting of the American Psychiatric Association; Philadelphia, Pennsylvania, USA. 2002 May 18-23.2002. [Google Scholar]
- Sikich L, Williamson K, Malekpour A, Bashford RA, Hooper S, Sheitman B, Lieberman JA. Interim results of a randomized controlled trial of haloperidol, risperidone, and olanzapine in psychotic youth; Proceedings of the 38th Annual Meeting of the American College of Neuropsychopharmacology; Acapulco, Mexico. USA. 1999 Dec 12-16.1999. [Google Scholar]
- Stroup 2006 {published data only} .*; Stroup TS, Lieberman JA, McEvoy JP, Swartz MS, Davis SM, Rosenheck RA, Perkins DO, Keefe RS, Davis CE, Severe J, Hsiao JK. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. American Journal of Psychiatry. 2006;163(4):611–22. doi: 10.1176/ajp.2006.163.4.611. [DOI] [PubMed] [Google Scholar]
- Svestka 2003 {published data only} .*; Svestka J, Synek O, Zourkova A. Olanzapine versus risperidone in first-episode schizophrenic and schizoform disorders: a double-blind comparison. Journal of the European College of Neuropsychopharmacology. 2003;13(4):291. [Google Scholar]
- Tran 1997 {published data only} .Ahmed S, Zhang F, Walker D, Beglinger L, Earley WR, Tran PV. Olanzapine versus risperidone for treatment of negative symptoms in schizophrenia; Proceedings of the 156th Annual Meeting of the American Psychiatric Association; San Francisco, California, USA. 2003 May 17-22.2003. [Google Scholar]
- Edgell ET, Andersen SW, Johnstone BM, Dulisse B, Revicki D, Breier A. Olanzapine versus risperidone: a prospective comparison of clinical and economic outcomes in schizophrenia. International Journal of Neuropsychopharmacology. 2000;3(Suppl 1):92. doi: 10.2165/00019053-200018060-00004. [DOI] [PubMed] [Google Scholar]
- Feldman PD, Kaiser CJ, Kennedy JS, Sutton VK, Tran PV, Tollefson GD, Zhang F, Breier A. Comparison of risperidone and olanzapine in the control of negative symptoms of chronic schizophrenia and related psychotic disorders in patients aged 50 to 65 years. Journal of Clinical Psychiatry. 2003;64(9):998–1004. doi: 10.4088/jcp.v64n0904. [DOI] [PubMed] [Google Scholar]
- Glick ID, Berg PH. Time to study discontinuation, relapse, and compliance with atypical or conventional antipsychotics in schizophrenia and related disorders. International Clinical Psychopharmacology. 2002;17(2):65–8. doi: 10.1097/00004850-200203000-00004. [DOI] [PubMed] [Google Scholar]
- Tollefson GD, Tran PV, Hamilton S, Kuntz A. Olanzapine versus risperidone in the treatment of psychosis. Preliminary report. Schizophrenia Research. 1997;24(1, 2):191. [Google Scholar]
- *; Tran PV, Hamilton SH, Kuntz AJ, Potvin JH, Andersen SW, Beasley C, Tollefson GD. Double-blind comparison of olanzapine versus risperidone in the treatment of schizophrenia and other psychotic disorders. Journal of Clinical Psychopharmacology. 1997;17(5):407–18. doi: 10.1097/00004714-199710000-00010. [DOI] [PubMed] [Google Scholar]
- Tran PV, Tollefson GD, Andersen SW, Kuntz AJ, Hamilton SH. Olanzapine versus risperidone in the treatment of schizophrenia and other psychotic disorders; Proceedings of the 10th European College of Neuropsychopharmacology Congress; Vienna, Austria. 1997 Sep 13-17; 1997. [DOI] [PubMed] [Google Scholar]
- Van Nimwegen 2006 {published data only} .Van Nimwegen L, De Haan L, Van Beveren N, Laan W, Van de Brink W, Linszen D. Obsessive compulsive symptoms in a randomized double blind; Proceedings of the 13th Biennial Winter Workshop on Schizophrenia; Davos, Switzerland. 2006 Feb 6-10.2006. [Google Scholar]
- *; Van Nimwegen L, De Haan L, Van Beveren N, Laan W, Van de Brink W, Linszen D. Subjective well-being and craving for cannabis in first psychosis, a randomized double blind comparison of olanzapine versus risperidone; Proceedings of the 13th Biennial Winter Workshop on Schizophrenia; Davos, Switzerland. 2006 Feb 6-10.2006. [Google Scholar]
- Volavka 2002 {published data only} .Bilder RM, Goldman RS, Volavka J, Czobor P, Hoptman M, Sheitman B, Lindenmayer JP, Citrome L, McEvoy J, Kunz M, Chakos M, Cooper TB, Horowitz TL, Lieberman JA. Neurocognitive effects of clozapine, olanzapine, risperidone, and haloperidol in patients with chronic schizophrenia or schizoaffective disorder. American Journal of Psychiatry. 2002;159(6):1018–28. doi: 10.1176/appi.ajp.159.6.1018. [DOI] [PubMed] [Google Scholar]
- Bilder RM, Goldman RS, Volavka J, Czobor P, Hoptman M, Sheitman B, Lindenmayer JP, Citrome L, McEvoy J, Kunz M, Chakos M, Lieberman JA. Neurocognitive effects of clozapine, olanzapine, risperidone, and haloperidol on treatment-resistant patients with schizophrenia and schizoaffective disorder. European Neuropsychopharmacology. 2001;11(3):256. [Google Scholar]
- Bilder RM, Goldman RS, Volavka J, Czobor P, Hoptman M, Sheitman B, Lindenmayer J-P, Citrome L, McEvoy J, Kunz M, Chakos M, Lieberman JA. Neurocognitive effects of clozapine, olanzapine, risperidone, and haloperidol on treatment-resistant patients with schizophrenia and schizoaffective disorder. Schizophrenia Research. 2002;53(3 Suppl 1):194. doi: 10.1176/appi.ajp.159.6.1018. [DOI] [PubMed] [Google Scholar]
- Citrome L, Volavka J, Czobor P, Sheitman B, Lindenmayer J-P, McEvoy J, Cooper TB, Chakos M, Lieberman JA. Effects of clozapine, olanzapine, risperidone, and haloperidol on hostility among patients with schizophrenia. Psychiatric Services. 2001;52(11):1510–4. doi: 10.1176/appi.ps.52.11.1510. [DOI] [PubMed] [Google Scholar]
- Lindenmayer JP, Czobor P, Volavka J, Citrome L, Sheitman B, McEvoy JP, Cooper TB, Chakos M, Lieberman JA. Changes in glucose and cholesterol levels in patients with schizophrenia treated with typical or atypical antipsychotics. American Journal of Psychiatry. 2003;160(2):290–6. doi: 10.1176/appi.ajp.160.2.290. [DOI] [PubMed] [Google Scholar]
- Lindenmayer JP, Czobor P, Volavka J, Lieberman JA, Citrome L, Sheitman B, McEvoy JP, Cooper TB, Chakos M. Effects of atypical antipsychotics on the syndromal profile in treatment-resistant schizophrenia. Journal of Clinical Psychiatry. 2004;65(4):551–6. doi: 10.4088/jcp.v65n0416. [DOI] [PubMed] [Google Scholar]
- Volavka J, Czobor P, Cooper TB, Sheitman B, Lindenmayer JP, Citrome L, McEvoy JP, Lieberman JA. Prolactin levels in schizophrenia and schizoaffective disorder patients treated with clozapine, olanzapine, risperidone, or haloperidol. Journal of Clinical Psychiatry. 2004;65(1):57–61. doi: 10.4088/jcp.v65n0109. [DOI] [PubMed] [Google Scholar]
- Volavka J, Czobor P, Nolan K, Sheitman B, Lindenmayer JP, Citrome LMJP, Cooper TB, Lieberman JA. Overt aggression and psychotic symptoms in patients with schizophrenia treated with clozapine, olanzapine, risperidone, or haloperidol. Journal of Clinical Psychopharmacology. 2004;24(2):225–8. doi: 10.1097/01.jcp.0000117424.05703.29. [DOI] [PubMed] [Google Scholar]
- *; Volavka J, Czobor P, Sheitman B, Lindenmayer JP, Citrome L, McEvoy JP, Cooper TB, Chakos M, Lieberman JA. Clozapine, olanzapine, risperidone, and haloperidol in the treatment of patients with chronic schizophrenia and schizoaffective disorder. American Journal of Psychiatry. 2002;159(2):255–62. doi: 10.1176/appi.ajp.159.2.255. [DOI] [PubMed] [Google Scholar]
- Wahlbeck 2000 {published data only} .*; Wahlbeck K, Cheine M, Tuisku K, Ahokas A, Joffe G, Rimon R. Risperidone versus clozapine in treatment-resistant schizophrenia: a randomized pilot study. Progress in Neuropsychopharmacology and Biological Psychiatry. 2000;24(6):911–22. doi: 10.1016/s0278-5846(00)00118-4. [DOI] [PubMed] [Google Scholar]
- Wahlbeck K, Cheine M, Tuisku K, Ahokas A, Joffe G, Rimón R. Risperidone versus clozapine in treatment-resistant schizophrenia: a randomized pilot study. International Journal of Neuropsychopharmacology. 2000;3(Suppl 1):122. doi: 10.1016/s0278-5846(00)00118-4. [DOI] [PubMed] [Google Scholar]
- Wahlbeck K, Cheine M, Tuisku K, Joffe G. Risperidone versus clozapine in treatment resistant schizophrenia: a randomised pilot trial. Schizophrenia Research. 2000;41(1):197. doi: 10.1016/s0278-5846(00)00118-4. [DOI] [PubMed] [Google Scholar]
- Wang 2006 {published data only} .*; Wang X, Savage R, Borisov A, Rosenberg J, Woolwine B, Tucker M, May R, Feldman J, Nemeroff CB, Miller AH. Efficacy of risperidone versus olanzapine in patients with schizophrenia previously on chronic conventional antipsychotic therapy: a switch study. Journal of Psychiatric Research. 2006;40(7):669–76. doi: 10.1016/j.jpsychires.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Wynn 2007 {published data only} .Wynn JK, Green MF, Sprock J, Light GA, Widmark C, Reist C, Erhart S, Marder SR, Mintz J, Braff DL. Effects of olanzapine, risperidone and haloperidol on prepulse inhibition in schizophrenia patients: A double-blind, randomized controlled trial. Schizophrenia Research. 2007;5:1–9. doi: 10.1016/j.schres.2007.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong 2006 {published data only} .Zhong K, Harvey P, Brecher M, Sweitzer D. A randomized double - blind study of quetiapine and risperidone in the treatment of schizophrenia. Schizophrenia Bulletin. 2005;31:508. [Google Scholar]
- *; Zhong KX, Sweitzer DE, Hamer RM, Lieberman JA. Comparison of quetiapine and risperidone in the treatment of schizophrenia: a randomized, double-blind, flexibledose, 8-week study. Journal of Clinical Psychiatry. 2006;67(7):1093–103. doi: 10.4088/jcp.v67n0712. [DOI] [PubMed] [Google Scholar]
- Zhou 2000 {published data only} .Zhou Z, Shu Z, Yu Y. A control study of risperidone and clozapine in the treatment of the negative symptoms of schizophrenia. Aerospace Medicine. 2000;11:69–71. [Google Scholar]
References to studies excluded from this review
- Akdede 2006 {published data only} .Akdede BB, Anil Yaciolu AE, Alptekin K, Turgut TI, Tumuklu M, Yazici MK, Jayathilake K, Tunca Z, Gogus A, Meltzer HY. A double-blind study of combination of clozapine with risperidone in patients with schizophrenia: effects on cognition. Journal of Clinical Psychiatry. 2006;67(12):1912–9. [PubMed] [Google Scholar]
- Almond 1999 {published data only} .*; Almond S, O’Donnell O, McKendrick J. The cost-analysis of olanzapine compared with haloperidol and risperidone in the treatment of schizophrenia in the UK. Journal of the European College of Neuropsychopharmacology. 1999;9:289. [Google Scholar]
- Alvarez 2006 {published data only} .*; Alvarez E, Ciudad A, Olivares JM, Bousono M, Gomez JC. A randomized, 1-year follow-up study of olanzapine and risperidone in the treatment of negative symptoms in outpatients with schizophrenia. Journal of Clinical Psychopharmacology. 2006;26(3):238–49. doi: 10.1097/01.jcp.0000222513.63767.de. [DOI] [PubMed] [Google Scholar]
- Alvarez-Jimenez 2006 {published data only} .*; Alvarez-Jimenez M, Gonzalez-Blanch C, Vazquez-Barquero JL, Perez-Iglesias R, Martinez-Garcia O, Perez-Pardal T, Ramirez-Bonilla ML, Crespo-Facorro B. Attenuation of antipsychotic-induced weight gain with early behavioral intervention in drug-naive first-episode psychosis patients: a randomized controlled trial. Journal of Clinical Psychiatry. 2006;67(8):1253–60. doi: 10.4088/jcp.v67n0812. [DOI] [PubMed] [Google Scholar]
- Antonova 2005 {published data only} .*; Antonova E, Kumari V, Halari R, Zachariah E, Mehrotra R, Kumar A, Sharma T. Superior cognitive efficacy of atypical antipsychotics olanzapine, risperidone, and quetiapine, as a group, relative to low doses of conventional antipsychotics. Schizophrenia Bulletin. 2005;31:474. [Google Scholar]
- Apiquian 2003 {published data only} .*; Apiquian R, Fresan A, Herrera K, Ulloa RE, Loyzaga C, De LaFuente-Sandoval C, Gutierrez D, Nicolini H. Minimum effective doses of haloperidol for the treatment of first psychotic episode: a comparative study with risperidone and olanzapine. International Journal of Neuropsychopharmacology. 2003;6(4):403–8. doi: 10.1017/S1461145703003742. [DOI] [PubMed] [Google Scholar]
- Aquila 2000 {published data only} .*; Aquila R, Weiden PJ, Kinon BJ, Milton DR, Zygmunt A, Swindle RW, Stauffer VL. Effectiveness of olanzapine upon psychiatric and vocational rehabilitation outcomes; Proceedings of the 153rd Annual Meeting of the American Psychiatric Association; Chicago, Illinois, USA. 2000 May 13-18.2000. [Google Scholar]
- Ascher-Svanum 2006 {published data only} .*; Ascher-Svanum H, Zhu B, Faries D, Landbloom R, Swartz M, Swanson J. Time to discontinuation of atypical versus typical antipsychotics in the naturalistic treatment of schizophrenia. BMC Psychiatry. 2006;6(8):1–16. doi: 10.1186/1471-244X-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai 2003 {published data only} .Bai YM, Yu SC, Lin CC. Risperidone for severe tardive dyskinesia: a 12-week randomized, double-blind, placebo-controlled study. Journal of Clinical Psychiatry. 2003;64:1342–8. [PubMed] [Google Scholar]
- Baloescu 2006 {published data only} .Baloescu A, Vasile D, Gheorghe MD, Grigorescu G. Side effects of atypical antipsychotics - prediction factor for compliance. European College of Neuropsychopharmacology. 2006;16(Suppl 4):403. [Google Scholar]
- Basson 2001 {published data only} .*; Basson BR, Kinon BJ, Taylor CC, Szymanski KA, Gilmore JA, Tollefson GD. Factors influencing acute weight change in patients with schizophrenia treated with olanzapine, haloperidol, or risperidone. Journal of Clinical Psychiatry. 2001;62(4):231–8. doi: 10.4088/jcp.v62n0404. [DOI] [PubMed] [Google Scholar]
- Beasley 2001 {published data only} .*; Beasley CM, Berg PH, Dananberg J, Kwong KC, Taylor CCM, Breier A. Treatment-emergent potential impaired glucose tolerance and potential diabetes with olanzapine compared to other antipsychotic agents and placebo. Biological Psychiatry. 2001;49(8):121. [Google Scholar]
- Beasley 2003 {published data only} .*; Beasley CM, Sowell MO, Carlson C, Mukhopadhyay N, Dananberg J, Henry R, Breier A, Cavazzoni P. Prospective evaluation of insulin sensitivity by the hyperinsulinemic, euglycemic clamp in healthy volunteers treated with olanzapine, risperidone or placebo. Schizophrenia Research. 2003;60(1):309. doi: 10.1210/jc.2002-021884. [DOI] [PubMed] [Google Scholar]
- Bera 2001 {published data only} .*; Bera RB. A comparison of patient satisfaction between seroquel, olanzapine and risperidal. Schizophrenia Research. 2001;49(1, 2):220. [Google Scholar]
- Beuzen 2005 {published data only} .*; Beuzen J-N, Pans M, Modell S, Hagens P, McQuade R, Iwamoto T, Carson W. Naturalistic study of aripiprazole treatment; Proceedings of the XIII World Congress of Psychiatry; Cairo, Egypt. 2005 Sept 10-15.2005. [Google Scholar]
- Bitter 2004 {published data only} .*; Bitter I, Basson BR, Dossenbach MR. Antipsychotic treatment and sexual functioning in first-time neuroleptic treated schizophrenic patients. International Clinical Psychopharmacology. 2005;20:19–21. doi: 10.1097/00004850-200501000-00004. [DOI] [PubMed] [Google Scholar]
- Boylan 2004 {published data only} .*; Boylan LS, Labovitz DL. Unbalanced statistical analysis of combined divalproex and antipsychotic therapy for schizophrenia. Neuropyschopharmacology. 2004;29(3):636. doi: 10.1038/sj.npp.1300368. [DOI] [PubMed] [Google Scholar]
- Briken 2002 {published data only} .*; Briken P, Nika E, Moritz S, Haasen C, Perro C, Yagdiran O, Naber D, Krausz M. Effect of zotepine, olanzapine and risperidone on hostility in schizophrenic patients. Schizophrenia Research. 2002;57:311–3. doi: 10.1016/s0920-9964(01)00322-x. [DOI] [PubMed] [Google Scholar]
- Byerly 2006 {published data only} .*; Byerly MJ, Nakonezny P, Bugno R, Boles J. A randomized, double-blind pilot trial of switching to quetiapine vs. Risperidone continuation in outpatients with risperidone-associated sexual dysfunction; Proceedings of the 159th Annual Meeting of the American Psychiatric Association; Toronto, Canada. 2006 May 20-25.2006. [Google Scholar]
- Cai 2000 {published data only} .Cai C, Wan C, Cheng F. The controlled investigation of risperidone and clozapine in the treatment of schizophrenia. Health Psychology Journal. 2000;8(2):152–4. [Google Scholar]
- Canas 2006 {published data only} .*; Canas F, Perez V, Tafalla M. Quetiapine and risperidone in the treatment of schizophrenia: a short- and long-term, non-randomized study; Proceedings of the 159th Annual Meeting of the American Psychiatric Association; Toronto, Canada. 2006 May 20-25.2006. [Google Scholar]
- Cao 2001 {published data only} .*; Cao D, Xie S. Different effects of clozapine and risperidone on levels of blood glucose in patients with schizophrenia. Modern Rehabilitation. 2001;5(12):144–5. [Google Scholar]
- Cao 2003 {published data only} .Cao W, Liu X-L, Wei H-Y. A comparative study of the therapeutic effects of clozapine and risperidone in the treatment of schizophrenia. Herald of Medicine. 2003;22(10):679–80. [Google Scholar]
- Cao 2005 {published data only} .Cao D, Xie S, Chen Q, Yuan Y, Fan Q. Comparison of the effects of chlorpromazine, risperidone and quetiapine on hypothalamic-pituary -gonodal axis and sexual function in male patiens with schizophrenia. Chinese Journal of Clinical Rehabilitation. 2005;9:148–9. [Google Scholar]
- Cha 2002 {published data only} .Cha A, Xu Y, Zhu XH. The curative effect comparison of risperidone and clozapine to treat recurrent schizophrenia. Chinese Medical Journal of Metallurgical Industry. 2002;19(3):143–4. [Google Scholar]
- Chaudhry 2006 {published data only} .*; Chaudhry HR, Niaz S, Arshad N, Peracha F, Ayub A, Mufti KA. Comparison of risperidone, olanzapine and quetiapine in relation to body weight, serum blood glucose and prolactin levels. Journal of the European College of Neuropsychopharmacology. 2006;16(Suppl 4):241. [Google Scholar]
- Chen 1999 {published data only} .Chen ZA, Lan LM, Zhu PJ. Risperidone vs clozapine in treatment of schizophrenia. Chinese Journal of New Drugs And Clinical Remedies. 1999;18(5):277–80. [Google Scholar]
- Chen 2002 {published data only} .Chen L. A comparative stndy of EEg changes in patients treated by risperidone, chlorpromazine and clozpine. Journal of Qiqihar Medical. 2002;23(5):485–6. [Google Scholar]
- Chen 2003 {published data only} .Chen C-Q. A study of risperidone and clozapine in treatment of first episode schizophrenia. HuaXia medicine. 2003;27:578–9. [Google Scholar]
- Chen 2004 {published data only} .Chen Y-H, Li Y-M, Li S. A study of risperidone with haloperidol and clozapine in treatment of positive symptom schizophrenia. Suzhou University Journal of Medical Science. 2004;24:912–7. [Google Scholar]
- Chen 2004a {published data only} .Chen Q, Huang C-F. The influence of risperidone and clozapine in the quality of life of schizophrenia. Chinese Journal of Clinical Rehabilitation. 2004;8(18):3640–1. [Google Scholar]
- Chen 2005 {published data only} .Chen Q, Tang Y-L, Mao P-X, Cai Z-J. Effects of clozapine and risperidone on the glucose-regulating mechanism of patients with schizophrenia. Chinese Journal of Clinical Rehabilitation. 2005;9(4):116–8. [Google Scholar]
- Chen 2005a {published data only} .Chen B, Liu X, Zhang X. A study of risperidone combination with clozapine in the treatment of the schizophrenia. Heath Psychology Journal. 2005;13(2):116–7. [Google Scholar]
- Chen 2005b {published data only} .Chen Q, Yuan Y, Fang Q. Characteristics of the sexual disturbance caused by chlorpromazine, risperidone, quetiapine and olanzapine and their associations with the changes of blood glucose and blood lipids in male patients with schizophrenia. Chinese Clinical Rehabilitation. 2005;9:63–4. [Google Scholar]
- Cheng 2002 {published data only} .Cheng S-Z, Chi X-Z, Li Q-H, Jiang Y-Q. A study of risperidone and clozapine in treatment of resistant schizophrenia. Heath Psychology Journal. 2002;10(1):44–6. [Google Scholar]
- Chou 1999 {published data only} .Chou X, Chen Q, Li J. A clinical controlled study between risperidone and clozapine in the treatment of chronic schizophrenia. Sichuan Mental Health. 1999;12(4):244–6. [Google Scholar]
- Chowdhury 1999 {published data only} .*; Chowdhury AN, Mukherjee A, Ghosh K, Chowdhury S, Das Sen K. Horizon of a new hope: Recovery of schizophrenia in India. International Medical Journal. 1999;6(3):181–5. [Google Scholar]
- Citrome 2004 {published data only} .*; Citrome L, Casey DE, Daniel DG, Wozniak P, Kochan LD, Tracy KA. Adjunctive divalproex and hostility among patients with schizophrenia receiving olanzapine or risperidone. Psychiatric Services. 2004;55(3):290–4. doi: 10.1176/appi.ps.55.3.290. [DOI] [PubMed] [Google Scholar]
- Ciudad 2004 {published data only} .Ciudad A, Alvarez E, Bousono M, Cuesta M, Gomez JC, Olivares JM. Olanzapine versus risperidone: results of a one year randomized trial in outpatients with schizophrenia with prominent negative symptoms. Schizophrenia Research. 2004;67(1):161–2. [Google Scholar]
- Conley 1999 {published data only} .*; Conley J, Goldman RS, Bilder RM, Bates J, Reiter G, Pappadopulos E, Robinson D, Alvir JMA, Liebrman J, Schooler N. A comparison of the neurocognitive effects of treatment with typical and atypical neuroleptics in first-episode schizophrenia. Schizophrenia Research. 1999;36(1-3):128. [Google Scholar]
- Cornblatt 2002 {published data only} .*; Cornblatt B, Kern RS, Carson WH, Stock E, Ali M, Ingenito G, Green MF. Neurocognitive effects of aripiprazole versus olanzapine in patients with stable psychosis. Schizophrenia Research. 2002;53(3 Suppl 1):27. [Google Scholar]
- Crespo-Facorro 2006 {published data only} .*; Crespo-Facorro B, Perez-Iglesias R, Ramirez-Bonilla M, Martinez-Garcia O, Llorca J, Vazquez-Barquero JL. A practical clinical trial comparing haloperidol, risperidone, and olanzapine for the acute treatment of first-episode nonaffective psychosis. Journal of Clinical Psychiatry. 2006;67(10):1511–21. doi: 10.4088/jcp.v67n1004. [DOI] [PubMed] [Google Scholar]
- Cui 2002 {published data only} .Cui B, Shen J, Tian H, Wang Y, Guo X. A comparative study of the effects of risperidone, chlorpromazine and clozapine to patients with schizophrenia by the electrocardiograms. Tianjin Pharmacy. 2002;14(3):54–5. [Google Scholar]
- Czekalla 2001 {published data only} .*; Czekalla J, Beasley CM, Jr, Dellva MA, Berg PH, Grundy S. Analysis of the qtc interval during olanzapine treatment of patients with schizophrenia and related psychosis. Journal of Clinical Psychiatry. 2001;62(3):191–8. doi: 10.4088/jcp.v62n0310. [DOI] [PubMed] [Google Scholar]
- David 1999 {published data only} .*; David SR, Meehan KM, Sutton VK, Taylor CC. Treatment of negative symptoms with olanzapine in comparison with other novel antipsychotic agents. Journal of the European College of Neuropsychopharmacology. 1999;9:292. [Google Scholar]
- David 2000 {published data only} .*; David SR, Taylor CC, Kinon BJ, Breier A. The effects of olanzapine, risperidone, and haloperidol on plasma prolactin levels in patients with schizophrenia. Clinical Therapeutics. 2000;22(9):1085–96. doi: 10.1016/S0149-2918(00)80086-7. [DOI] [PubMed] [Google Scholar]
- De Haan 2002 {published data only} .*; De Haan L, Beuk N, Hoogenboom B, Dingemans P, Linszen D. Obsessive-compulsive symptoms during treatment with olanzapine and risperidone: a prospective study of 113 patients with recent-onset schizophrenia or related disorders. Journal of Clinical Psychiatry. 2002;63(2):104–7. doi: 10.4088/jcp.v63n0203. [DOI] [PubMed] [Google Scholar]
- Ding 2004 {published data only} .Ding Y, An B, Li G. A comparative study of risperidone and quetiapine in the treatment of schizophrenia and relationship between efficacy and the serum levels of IL-2, SIL-2R. Shangdong Arch Psychiatry. 2004;17:76–8. [Google Scholar]
- Ding 2005 {published data only} .Ding Z, Yu Z, Xu G. Control studies of increase of blood sugar induced by neo-antipsychotics. Journal of Clinical Psychosomatic Diseases. 2005;11(4):309–10. [Google Scholar]
- Dossenbach 2005 {published data only} .*; Dossenbach M, Arango-Davila C, Ibarra HS, Landa E, Aguilar J, Caro O, Leadbetter J, Assuncao S. Response and relapse in patients With schizophrenia treated with olanzapine, risperidone, quetiapine, or haloperidol: 12-month follow-up of the intercontinental schizophrenia outpatients health outcomes (IC-SOHO) study. Journal of Clinical Psychiatry. 2005;66(8):1021–30. doi: 10.4088/jcp.v66n0810. [DOI] [PubMed] [Google Scholar]
- Du 2003 {published data only} .Du W-J, Yu J-L, Zheng H-B, Zhong X-Q, Ma C. The influence of chlorpromazine, clozapine and risperidone on the cognitive function of schizophrenia. International Medicine. 2003;9:86–88. [Google Scholar]
- Du 2004 {published data only} .Du Z-H, Wu H-L. Comparison of chlorpromazine, clozapine and risperidone in the effect on indices of liver function. Journal of Practical Medical Techniques. 2004;11(6A):885–6. [Google Scholar]
- Du 2004a {published data only} .Du Z-H. A study on the change of serum thyroxin treated with risperdal or clozapine. Journal of Practical Medical Techniques. 2004;11(68):1020–1. [Google Scholar]
- Du 2005 {published data only} .Du Z, Wu H. Effects of clozapine and risperidone on the glucose metabolism in schizophrenic patients. Qilu Journal of Medicall Aboratory Sciences. 2005;16(1):8–9. [Google Scholar]
- Earnst 1999 {published data only} .*; Earnst KS, Taylor SF, Smet IC, Goldman RS, Tandon R, Berent S. The effects of typical antipsychotics, clozapine, and risperidone on neuropsychological test performance in schizophrenia. Schizophrenia Research. 1999;40(3):255–6. doi: 10.1016/s0920-9964(99)00064-x. [DOI] [PubMed] [Google Scholar]
- Ertugrul 2006 {published data only} .*; Ertugrul A, Anil Yagcioglu AE, Woodward ND, Jayathilake K, Meltzer HY. Genetic predictors of cognitive function and response to treatment in schizophrenia. Journal of the European College of Neuropsychopharmacology. 2006;16(Suppl 4):406. [Google Scholar]
- Estrella 1996 {published data only} .*; Estrella MH, Soria FL, Gonzalez CJC, Butron MAL, Torres JA, Escareño RR. Cost-effectiveness of clozapine vs respiridona for treatment-resistant schizophrenic patients; Proceedings of the 10th World Congress of Psychiatry; Madrid, Spain. 1996 Aug 23-28.1996. [Google Scholar]
- Fan 2003 {published data only} .Fan C, Leiying Wang K. The effect of clozapine and risperidone on blood sugar of patients with schizophrenia. Shandong Archives of Psychiatry. 2003;16(3):131–2. [Google Scholar]
- Feng 2004 {published data only} .Feng Y-P. The influence of clozapine and risperidone on the weight. Jounal of Clinical Psychiatry. 2004;14:101. [Google Scholar]
- Gan 1999 {published data only} .Gan JL. A control study of risperidone and clozapine in the freatment of first episode schizophrenia. Medical Journal of Chinese Civil Administration. 1999;11(1):61. [Google Scholar]
- García 2006 {published data only} .*; García MC, Vidal M, Ramos R. Sexual side effects of antipsychoticcs and treatment adherence. Journal of the European College of Neuropsychopharmacology. 2006;16(Suppl 4):378. [Google Scholar]
- Ge 2004 {published data only} .Ge Q, Xiong L, Liang X. A comparative study of risperidone, clozapine and haloperidol in the treatment of patients with schizophrenia. Sichuan Mental Health. 2004;17(2):79–81. [Google Scholar]
- Goldberg 2000 {published data only} .*; Goldberg TE, Dodge M, Aloia M, Egan MF, Weinberger DR. Effects of neuroleptic medications on speech disorganization in schizophrenia: biasing associative networks towards meaning. Psychological Medicine. 2000;30(5):1123–30. doi: 10.1017/s0033291799002639. [DOI] [PubMed] [Google Scholar]
- Guan 2005 {published data only} .Guan D-H, Song H-F. A control study of social function improvement in schizophrenia patients with risperdal and clozapine. Medical Journal of Chinese People Health. 2005;17(9):506–7. [Google Scholar]
- Guo 2001 {published data only} .Guo HR, Zhang SR, Sun FG. Clinical controlled study of risperidone and clozapine in treatment of schizophrenia. Journal of Xinxiang Medical College. 2001;18(3):174–6. [Google Scholar]
- Guo 2003 {published data only} .Guo S, Lu L, Cheng W. Effect of clozapine and risperidone on interleukin-2 on schizophrenia. Shanghai Archives of Psychiatry. 2003;15(1):35–8. [Google Scholar]
- Hagger 1997 {published data only} .*; Hagger C, Mitchell D, Wise AL, Schulz SC. Effects of oral ziprasidone and risperidone on cognitive functioning in patients with schizophrenia or schizoaffective disorder: Preliminary data; Proceedings of the 10th European College of Neuropsychopharmacology Congress; Vienna, Austria. 1997 Sep 13-17.1997. [Google Scholar]
- Han 2000 {published data only} .Han P. A study of risperidone and clozapine in treatment of resistant schizophrenia. Journal of Jining Medical College. 2000;23(3):75. [Google Scholar]
- Han 2005 {published data only} .Han Z-Y, Wang L-J, Wang J. Analysis of risperidone clonazepam in treatment of positive symptom schizophrenia. Ningxia Medical Journal. 2005;27:631–2. [Google Scholar]
- Harrigan 2004 {published data only} .*; Harrigan EP, Miceli JJ, Anziano R, Watsky E, Reeves KR, Cutler NR, Sramek J, Shiovitz T, Middle M. A randomized evaluation of the effects of six antipsychotic agents on QTC, in the absence and presence of metabolic inhibition. Journal of Clinical Psychopharmacology. 2004;24(1):62–9. doi: 10.1097/01.jcp.0000104913.75206.62. [DOI] [PubMed] [Google Scholar]
- He 2005 {published data only} .He Y-Q, Ma S-H, Du J. A controlled study on hostility and side effects in clozapine and risperidone for treatment of patients with schizophrenia. Journal of Xinxiang Medical College. 2005;22(2):118–20. [Google Scholar]
- Heresco-Levy 2005 {published data only} .*; Heresco-Levy U, Javitt DC, Ebstein R, Vass A, Lichtenberg P, Bar GCS, Ermilov M. D-serine efficacy as add-on pharmacotherapy to risperidone and olanzapine for treatment-refractory schizophrenia. Biological Psychiatry. 2005;57(6):577–85. doi: 10.1016/j.biopsych.2004.12.037. [DOI] [PubMed] [Google Scholar]
- Hou 2001 {published data only} .Hou J, Xu G, Ma C. The effects of clozapine and risperidone on serum prolactin levels of female schizophrenia. Journal of Clinical Psychological Medicine. 2001;11(1):1–3. [Google Scholar]
- Hrdlicka 2001 {published data only} .*; Hrdlicka M, Rosillon D, Duchesne I. Czech results of the RODOS study: comparison of risperidone and olanzapine from the point of view of efficacy, tolerability and treatment costs [Ceske vysledky studie RODOS: Porovnani risperidonu a olanzapinu z hlediska ucinnosti, snasenlivosti a nakladu lecby] Ceska A Slovenská Psychiatrie. 2001;97(7):343–9. [Google Scholar]
- Hu 2000 {published data only} .Hu X-C, Tao R-F. A study of risperidone and clozapine in treatment of schizophrenia. Journal of Handan Medical College. 2000;13(2):91. [Google Scholar]
- Hu 2005 {published data only} .Hu T-S. A study of risperidone in treatment of dominant positive symptoms in schizophrenia. Chinese Journal of Behavioral Medical Science. 2005;14(5):428. [Google Scholar]
- Huang 2000 {published data only} .Huang Y-H, Luo B-M. A study of risperidone and chlorpromazine clozapine. Health Psychology Journal. 2000;9:321–2. [Google Scholar]
- Huang 2001 {published data only} .Huang Q-M, Li J, Zhu X-W, Mel Q-Y. A study of risperidone and clozapine in treatment of the behaviors in schizophrenia. Shanghai Archives of Psychiatry. 2001;13(3):152–4. [Google Scholar]
- Huber 2004 {published data only} .*; Huber TJ, Borsutzky M, Schneider U, Emrich HM. Psychotic disorders and gonadal function: evidence supporting the oestrogen hypothesis. Acta Psychiatrica Scandinavica. 2004;109(4):269–74. doi: 10.1046/j.1600-0447.2003.00251.x. [DOI] [PubMed] [Google Scholar]
- Janssen-Ortho 2006 {published data only} .*; Janssen-Ortho Inc. Pragmatic randomised trial of RISPERDAL ® CONSTA ™ versus oral atypical antipsychotics in poorly adherent subjects with schizophrenia in a routine care setting: UK Revised Protocol V2. 2006 Unpublished report. [Google Scholar]
- Jones 2006 {published data only} .*; Jones PB, Barnes TRE, Davies L, Dunn G, Lloyd H, Hayhurst KP, Murray RM, Markwick A, Lewis SW. Randomized controlled trial of the effect on quality of life of second-vs first-generation antipsychotic drugs in schizophrenia: Cost utility of the latest antipsychotic drugs in schizophrenia study (CUtLASS 1) Archives of General Psychiatry. 2006;63(10):1079–87. doi: 10.1001/archpsyc.63.10.1079. [DOI] [PubMed] [Google Scholar]
- Karow 2002 {published data only} .*; Karow A, Naber D. Subjective well-being and quality of life under atypical antipsychotic treatment. Psychopharmacology. 2002;162:3–10. doi: 10.1007/s00213-002-1052-z. [DOI] [PubMed] [Google Scholar]
- Keks 2006 {published data only} .*; Keks NA, Tonso M, Tabone K, McHugh M, Thomas R, Tune P, Gelman M. Clinical experience with atypical antipsychotics in an acute inpatient unit: Focus on quetiapine. International Journal of Psychiatry in Clinical Practice. 2006;10(2):138–41. doi: 10.1080/13651500600579068. [DOI] [PubMed] [Google Scholar]
- Kelemen 2006 {published data only} .*; Kelemen O, Nagy O, Máttyássy A, Kiss I, Janka Z, Kéri S. Do second-generation antipsychotics disrupt decision-making abilities in schizophrenia? European College of Neuropsychopharmacology. 2006;16(Suppl 4):430. [Google Scholar]
- Kim 2004 {published data only} .*; Kim JG, Cho DH, Choi HK, Kim HJ, Cho JH, Kang SH, Lee SJ, Lee JG, Kim HT. The comparison of risperidone, olanzapine and quetiapine in the treatment of chronic schizophrenia and schizoaffective disorder. European College of Neuropsychopharmacology. 2004;14(Suppl 3):245. [Google Scholar]
- Knegtering 2004 {published data only} .*; Knegtering R, Castelein S, Bous H, Van Der Linde J, Bruggeman R, Kluiter H, Van Den Bosch RJ. A randomized open-label study of the impact of quetiapine versus risperidone on sexual functioning. Journal of Clinical Psychopharmacology. 2004;24(1):56–61. doi: 10.1097/01.jcp.0000106220.36344.04. [DOI] [PubMed] [Google Scholar]
- Koenigsberg 2000 {published data only} .*; Koenigsberg HW, Goodman M, Mitropoulou V, New AS, Siever LJ. Risperidone in the treatment of schizotypal personality; Proceedings of the 153rd Annual Meeting of the American Psychiatric Association; Chicago, Illinois, USA. 2000 May 13-18.2000. [Google Scholar]
- Kolff 2000 {published data only} .*; Kolff M, Coenen A, Van Dis H, Duigemans P. Differential effects of antipsychotic drugs on clinical symptoms and cognitive functions in the treatment of schizophrenia. European College of Neuropsychopharmacology. 2000;10(Suppl 2):S59. [Google Scholar]
- Kong 2001 {published data only} .Kong Q-R, Yang R-X, Li S-C, Lu Z-S, Li J-Y, Chen X. A study of clozapine with risperidone and clozapine with sulpiride in treatment of resistant schizophrenia. Archives of General Psychiatry. 2001;14:119–20. [Google Scholar]
- Kores 2003 {published data only} .*; Kores Plesnicar B, Zalar B, Tomori M, Krajnc I. Measurement of simple reaction time in antipsychotic treatment of patients with schizophrenia. Wiener Klinische Wochenschrift. 2003;115(1-2):58–62. doi: 10.1007/BF03040274. [DOI] [PubMed] [Google Scholar]
- Lee 2005 {published data only} .*; Lee JN, Yang BH, Nam CW. The influences of risperidone and clozapine on body weight and glucose level in patients with chronic schizophrenia - comparison with haloperidol. European College of Neuropsychopharmacology. 2005;15(Suppl 3):457. [Google Scholar]
- Lee 2006 {published data only} .*; Lee C, Wu K-H, Habil H, Dyachkova Y, Lee P. Treatment with olanzapine, risperidone or typical antipsychotic drugs in Asian patients with schizophrenia. Australian and New Zealand Journal of Psychiatry. 2006;40(5):437–45. doi: 10.1080/j.1440-1614.2006.01820.x. [DOI] [PubMed] [Google Scholar]
- Lei 2002 {published data only} .Lei X, Liang T, Zhang X. A controlled study on risperidone and clozapine in treatment of schizophrenia. China Pharmacist. 2002;5(3):169–71. [Google Scholar]
- Lewis 2004 {published data only} .*; Lewis SW, Davies L, Jones PB, Barnes TRE, Murray RM, Kerwin R, Taylor D, Hayhurst KP, Lloyd H, Markwick A. A randomised, controlled trial of new atypical drugs versus clozapine in treatment-resistant schizophrenia. Schizophrenia Research. 2004;67(1):6–7. doi: 10.3310/hta10170. [DOI] [PubMed] [Google Scholar]
- Li 2000 {published data only} .Li X-X. A study of risperidone and clozapine in treatment of chronic schizophrenia. He Nan Medical Information. 2000;8:52–3. [Google Scholar]
- Li 2001 {published data only} .Li J, Dai X. A comparative study of rispredone and sulpride in treating schizophrenia. Medical Journal of Chinese Civil Administration. 2001;13(3):136–8. [Google Scholar]
- Li 2003 {published data only} .Li T, Xue XM, Gong CF. A comparison study on the efficacy of risperidone vs dozapine in cognition of schizophrenia. Medical Journal of Chinese People Health. 2003;15(11):653–6. [Google Scholar]
- Li 2003a {published data only} .Li X, Lao G. Comparison of efficacy and safety of seroquel and risperidone in the treatment of schizophrenia. Chinese Journal of Behavioural Medical Science. 2003;12:45–6. [Google Scholar]
- Li 2004 {published data only} .Li Z. A comparative study of clozapine and risperdone lead to putting on weight. Heath Psychology Journal. 2004;12(5):396–7. [Google Scholar]
- Li 2005 {published data only} .Li G, Chen Q, Zhang Q. A comparison analysis on the efficacy of quetiapine and risperidone in cognitive function of schizophrenia. Chinese Journal of Behavioral Medical Science. 2005;14:1007–9. [Google Scholar]
- Liang 2005 {published data only} .Liang Z-Y, Huang X-S. A study of risperidone and clozapine in treatment of first episode schizophrenia. Medical Journal of Chinese People Health. 2005;17(9):509–10. [Google Scholar]
- Liao 2004 {published data only} .Liao C, Yang H, Gao H. Effects of clozapine and risperidone on blood routine examinations of patients with schizophrenia. Journal of Clinical Psychosomatic Diseases. 2004;10(3):156–7. [Google Scholar]
- Lin 2005 {published data only} .Lin Z, Li F, Yu Y, Zhong T. A controlled study of olanzapine and risperidone in the treatment of elderly patients with schizophrenia. Hainan Medical Journal. 2005;16(8):37–9. [Google Scholar]
- Lipkovich 2005 {published data only} .*; Lipkovich I, Baron D, Houston J, Ahl J, Rotelli M. Flexible-dose clinical trials: predictors and outcomes of antipsychotic dose adjustments. Journal of Clinical Psychopharmacology. 2005;25(4):381–6. doi: 10.1097/01.jcp.0000167791.70664.d4. [DOI] [PubMed] [Google Scholar]
- Littrell 1999 {published data only} .*; Littrell KH. Patients switched from depot antipsychotics to oral risperidone or olanzapine: an open-label randomized trial; Proceedings of the 152nd Annual Meeting of the American Psychiatric Association; Washington DC, USA. 1999 May 15-20.1999. [Google Scholar]
- Liu 1999 {published data only} .Liu Q, Man C, Li X. A control study of risperidone and colzapine to treating the positive symptoms of schizophrenia. Sichuan Mental Health. 1999;12(2):98–9. [Google Scholar]
- Liu 2001 {published data only} .Liu Q, Li X. A comparative study on the efficacy of combining risperidone and clozapine in the treatment of schizophrenia. Shandong Archives of Psychiatry. 2001;14(1):28–30. [Google Scholar]
- Liu 2003 {published data only} .Liu F, Dai M. The effects of risperidone and clozapine to patients with schizophrenia’s life quality. Sichuan Mental Health. 2003;16(4):203–4. [Google Scholar]
- Liu 2003a {published data only} .Liu Y, Li H, Wang H. Effect and related factors of clozapine and risperidone on glucose-insulin homeostasis in schizophrenic patients. Shanghai Archives of Psychiatry. 2003;15(4):257–59. [Google Scholar]
- Liu 2004 {published data only} .Liu TF, Yang JZ. Risperidone vs clozapine for treatment of schizophrenia in 30 patients each. Chinese Journal of New Drugs and Clinical Remedies. 2004;3(3):147–9. [Google Scholar]
- Liu 2004a {published data only} .Liu J-B, Wu J. The influence of clozapine, risperidone and chlorpromazine on the Glu.TG.TC and the weight in the first-episode schizophrenia. Chinese Journal of Nerval and Mental Diseases. 2004;30(4):293–4. [Google Scholar]
- Liu 2004b {published data only} .Liu N-H, Zhang J-Y. A study of risperidone with clonazepam in treatment of schizophrenia. Journal of Hai Nan Medical College. 2004;10:394–5. [Google Scholar]
- Liu 2004c {published data only} .Liu Y, Li H-F, Wang H-F, Wang K-X, Gao Z-S, Gu N-F. Influence of clozapine and risperidone on body weight, leptin level and lipid metabolism in schizophrenic patients. Chinese Journal of New Drugs and Clinical Remedies. 2004;9(23):579–82. [Google Scholar]
- Liu 2005 {published data only} .Liu K, Jiao Z-A. A study of the influence of clozapine and risperidone on the weight in schizophrenia. Medical Journal of Chinese People’s Health. 2005;17:513–4. [Google Scholar]
- Liu 2005a {published data only} .Liu G-F, Zhou J-S. The influence of risperidone and clozapine in the quality of life of schizophrenia. Medical Journal of Chinese People Health. 2005;17(6):289–90. [Google Scholar]
- Liu 2005c {published data only} .Liu Y. A comparative study of risperidone and clozapine in the treatment of refractory schizophrenia. Journal of Heze Medical College. 2005;17(1):13–4. [Google Scholar]
- Loza 2005 {published data only} .*; Loza B, Bartyzel M, Matysiewicz W, Mazurek I, Mosiolek A, Opielak G, Varghese S. Hyperprolactinemia during schizophrenia treatment with atypical antipsychotics: oral risperidone, depot risperidone, and oral olanzapine. European College of Neuropsychopharmacology. 2005;15(Suppl 3):522. [Google Scholar]
- Lu 2002 {published data only} .Lu Y, Ren Q, Tian M. A comparison of cognitive function in the first-onset schizophrenia treated with risperidone and clozapine. Shandong Archives of Psychiatry. 2002;15(4):206–7. [Google Scholar]
- Lu 2004 {published data only} .Lu L-X, Guo S-Q, Chen W, Li Q, Cheng J, Zhang H-Y, Shi T-Y, Shi Y. Effects of clozapine and risperidone on serum interleukin-2,-10,-18 in adolescent schizophrenia patients. Journal of Applied Clinical Pediatrics. 2004;19(11):983–6. [Google Scholar]
- Lu 2005 {published data only} .Lu S, Zhang S-A, Ren Y. Comparative study on the psychopathology and the quality of life of schizophrenia treated with risperidone, clozapine and quetiapine. Journal of Nursing Science. 2005;20(3):57–9. [Google Scholar]
- Ma 1999 {published data only} .Ma Q-M, Guan H-Y, Jiang L, Zhang Y-Y, Yi X-R, Guo J-M, Sun S-G, Liu K-H, Gao S-Q, Wu H-R, Xu S-W. A study of risperidone in treatment of chronic schizophrenia. Heath Psychology Journal. 1999;7(3):290–3. [Google Scholar]
- Mai 2005 {published data only} .Mai G, Cai GH, Huang JM. Comparative Study on the effect of aripiprazole and risperidone on schizophrenia. Chinese Journal of Rehabilitation. 2005;20(3):184–6. [Google Scholar]
- Malla 2004 {published data only} .*; Malla A, Norman R, Scholten D, Townsend L, Manchanda R, Takhar J, Haricharan R. A comparison of two novel antipsychotics in first episode non-affective psychosis: one-year outcome on symptoms, motor side effects and cognition. Psychiatry Research. 2004;129:159–69. doi: 10.1016/j.psychres.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Malyarov 1999 {published data only} .Malyarov S, Dzub G. Comparative assessment of the positive and negative symptom dynamics in schizophrenic patients treated with atypical antipsychotics or haloperidol. European College of Neuropsychopharmacology. 1999;9:296. [Google Scholar]
- Malykhin 2003 {published data only} .*; Malykhin N. Comparative efficacy of risperidone, clozapine and haloperidol in the treatment of schizoaffective disorders with manic symptoms. European College of Neuropsychopharmacology. 2003;13(4):304. [Google Scholar]
- Mao 2000 {published data only} .Mao Y, Da Z. Study of cost-effectiveness on risperidone vs clozapine in the treatment of inpatients with schizophrenia. Shanghai Archives of Psychiatry. 2000;12(4):211–3. [Google Scholar]
- Mazurek 2003 {published data only} .*; Mazurek I, Loza B, Lecyk A. Atypical versus typical antipsychotic treatment prognosis based on veps and wcst scores in paranoid schizophrenia. European College of Neuropsychopharmacology. 2003;13(4):347. [Google Scholar]
- Mei 2001 {published data only} .Mei Q, Zhu X, Shen J. A comparative study of social function in schizophrenic patients treated with risperidone or clozapine. Journal of Clinical Psychological Medicine. 2001;11(2):83–5. [Google Scholar]
- Meltzer 2002 {published data only} .*; Meltzer HY, Gilliam JH, Nasdahl C. Olanzapine causes greater increases in serum lipids than risperidone; Proceedings of the 155th Annual Meeting of the American Psychiatric Association; Philadelphia, Pennsylvania, USA. 2002 May 18-23.2002. [Google Scholar]
- Meltzer 2004 {published data only} .*; Meltzer HY, Arvanitis L, Bauer D, Rein W, Meta-Trial Study Group Placebo-controlled evaluation of four novel compounds for the treatment of schizophrenia and schizoaffective disorder. American Journal of Psychiatry. 2004;161(6):975–84. doi: 10.1176/appi.ajp.161.6.975. [DOI] [PubMed] [Google Scholar]
- Mintzer 2004 {published data only} .*; Mintzer JE, Mullen JA, Sweitzer DE. A comparison of extrapyramidal symptoms in older outpatients treated with quetiapine or risperidone. Current Medical Research and Opinion. 2004;20(9):1483–91. doi: 10.1185/030079904X2312. [DOI] [PubMed] [Google Scholar]
- Mohr 2000 {published data only} .*; Mohr E, Mendis T, Hildebrand K, De Deyn PP. Risperidone in the treatment of dopamine-induced psychosis in Parkinson’s disease: an open pilot study. Movement Disorder. 2000;15(6):1230–7. doi: 10.1002/1531-8257(200011)15:6<1230::aid-mds1026>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Mu 2002 {published data only} .Mu Z-Q, Zong Y-S, Luo Y. A study of risperidone instead of typical antipsychotics and clozapine in treatment of chronic schizophrenia. Health Psychology Journal. 2002;10:234–5. [Google Scholar]
- Mullen 2001 {published data only} .*; Mullen J, Jibson MD, Sweitzer D. A comparison of the relative safety, efficacy, and tolerability of quetiapine and risperidone in outpatients with schizophrenia and other psychotic disorders: the quetiapine experience with safety and tolerability (QUEST) study. Clinical Therapeutics. 2001;23(11):1839–54. doi: 10.1016/s0149-2918(00)89080-3. [DOI] [PubMed] [Google Scholar]
- Musil 2006 {published data only} .*; Musil RL, Spellmann I, Riedel M, Douhet A, Dehning S, Maino K, Zill P, Müller N, Möller HJ, Bondy B. SNAP-25 gene polymorphisms and weight gain in schizophrenic patients treated with atypical antipsychotics. European College of Neuropsychopharmacology. 2006;16(Suppl 4):415. [Google Scholar]
- Naber 2001 {published data only} .*; Naber D, Moritz S, Lambert M, Pajonk F, Holzbach R, Mass R, Andresen B. Improvement of schizophrenic patients’ subjective well-being under atypical antipsychotic drugs. Schizophrenia Research. 2001;50:79–88. doi: 10.1016/s0920-9964(00)00166-3. [DOI] [PubMed] [Google Scholar]
- Naber 2002 {published data only} .Naber D, Karow A, Lambert M. Psychosocial outcomes in patients with schizophrenia: quality of life and reintegration. Current Opinion in Psychiatry. 2002;15:31–6. [Google Scholar]
- Nan 2001 {published data only} .Nan Z, Wang J, Ji H. A controlled study of risperidone and clozapine both influences congnitive function of patients with schizophrenia. Sichuan Mental Health. 2001;14(4):198–200. [Google Scholar]
- Ni 2001 {published data only} .Ni J-L, Jiiang L-C, Hong X, Yang H, Zheng X-P. A study of clozapine and risperidone in treatment of schizophrenia. Heath Psychology Journal. 2001;9(3):181–2. [Google Scholar]
- Nicholls 2003 {published data only} .*; Nicholls CJ, Hale AS, Freemantle N. Cost-effectiveness of amisulpride compared with risperidone in patients with schizophrenia... first published as: Journal of Medical Economics 2003;6:31-41. Journal of Drug Assessment. 2003;6(2):79–89. [Google Scholar]
- Opjordsmoen 2000 {published data only} .*; Opjordsmoen S, Brunsvik S, Melle I, Dahl A, Friis S, Haahr U, Hustoft K, Johannessen JO, Larsen TK, McGlashan TH, Simonsen E, Vaglum P. A comparison between novel and traditional antipsychotics as first-line medication in early psychosis; Proceedings of the 2nd International Conference on Early Psychosis; New York, New York, USA. 2000 Mar 31 - Apr 2.2000. [Google Scholar]
- Ortega-Soto 1997 {published data only} .*; Ortega-Soto H, Apiquian R, Ulloa RE, Salas M, Loyzaga C, Mendizabal A, Brunner E. Olanzapine vs risperidone. A double blind trial in Mexican patients; Proceedings of the Collegium Internationale Neuro-Psychopharmacologicum Regional Meeting; Acapulco, Mexico. 1997 Aug 21-23.1997. [Google Scholar]
- Pan 2004 {published data only} .Pan M, Zhang S, Zhao Z. A comparative study of domestic quetiapine and risperidone in the treatment of schizophrenia. Medical Journal of Chinese People Health. 2004;16(10):597–8. [Google Scholar]
- Pan 2004a {published data only} .Pan M, Zhang S-Q, Zhao Z. Influence of three antipsychotic drugs on electrocardiogram in patients with schizophrenia. Journal of Xin Xiang Medical College. 2004;21(5):403–4. [Google Scholar]
- Peng 2001 {published data only} .Peng H, Kuang Y, Huang X. A control study of risperidone in combination with clozapine in treating refractory schizophrenia. Journal of Modern Clinical Medical Bioengineering. 2001;7(2):100–2. [Google Scholar]
- Peng 2004 {published data only} .Peng J-F, Xu Y-M, Wang Y-B. Effects of clozapine and risperidone on the serum prolactin levels and body weight regulation. Journal of Clinical Psychological Medicine. 2004;14(5):268–9. [Google Scholar]
- Perro 1999 {published data only} .*; Perro C, Naber D, Lambert M, Moritz S, Krause M. A prospective clinical comparative-study of four atypical antipsychotic agents in the treatment of schizophrenia; Proceedings of the 11th World Congress of Psychiatry; Hamburg, Germany. 1999 Aug 6-11.1999. p. 13. [Google Scholar]
- Peuskens 2004 {published data only} .*; Peuskens J, Deberdt W, Van Brunt D, Hill A, Liu-Seifert H, Csernansky J, Buckley P. Medication-specific correlates of relapse risk in schizophrenia. European College of Neuropsychopharmacology. 2004;14(Suppl 3):238. [Google Scholar]
- Qi 2004a {published data only} .Qi F, Wang L, Zhen Y. Comparison of efficacy and safety of quetiapine and risperidone in the treatment of schizophrenia. Shanghai Archives of Psychiatry. 2004;16:78–80. [Google Scholar]
- Qian 2004 {published data only} .Qian D, Pan B, Yang G. Cost-effectiveness analysis of 3 kinds of therapeutic schemes for schizophrenia. Evaluation and Analysis of Drug-use in Hospital of China. 2004;4(2):110–1. [Google Scholar]
- Qin 2005 {published data only} .Qin Y, Kang W, Song L. A comparative study of risperidone combining alprazolam and clozapine in the treatment of patients with schizophrenia. Heath Psychology Journal. 2005;13(6):444–5. [Google Scholar]
- Rabinowitz 2005 {published data only} .*; Rabinowitz J. Pattern mixture approach to comparing outcomes - of benefit in guideline development; Proceedings of the 18th European College of Neuropsychopharmacology Congress; Amsterdam, Netherlands. 2005 October 22-26.2005. [Google Scholar]
- Ren 2000 {published data only} .Ren J-J, Su J-L, Li B-Q. A study of risperidone and clozapine in treatment of resistant schizophrenia. Baotou Medical Journal. 2000;35(2):6–7. [Google Scholar]
- Ren 2004 {published data only} .Ren K, Zhao X, Jiang X. Effects of risperidone and clozapine on life quality of schizophrenics. Journal of Clinical Psychosomatic Diseases. 2004;10(1):3–4. [Google Scholar]
- Roerig 2004 {published data only} .*; Roerig JL, Mitchell JE, De Zwaan M, Crosby RD, Gosnell BA, Pederson K. A comparison of the effects of olanzapine and risperidone versus placebo on eating behaviors and ghrelin plasma levels in normal human subjects; Proceedings of the Thematic Conference of the World Psychiatric Association on “Treatments in Psychiatry: An Update”; Florence, Italy. 2004 Nov 10-13.2004. [Google Scholar]
- Ryu 2006 {published data only} .*; Ryu SH, Jang WS, Cho EY, Kim SK, Lee DS, Hong KS. Association of leptin gene polymorphism with antipsychotic drug-induced weight gain. European College of Neuropsychopharmacology. 2006;16(Suppl 4):419. [Google Scholar]
- Sajatovic 2002 {published data only} .*; Sajatovic M, Mullen JA, Sweitzer D. Efficacy of quetiapine and risperidone against depressive symptoms in outpatients with psychotic disorders; Proceedings of the 155th Annual Meeting of the American Psychiatric Association; Philadelphia, Pennsylvania, USA. 2002 May 18-23.2002. [Google Scholar]
- Shao 1999 {published data only} .Shao Y-Q. The extrapyramidal side effects of risperidone. Journal of Southeast China National Defense Medicinal Science. 1999;1(1):28–29. [Google Scholar]
- Sheng 2003 {published data only} .Sheng Y, Liu T, Li Y. A comparison study of the blood sugar in the treatment of schizophrenic patients with risperidone, clozapine and chlorpromazine. Qilu Journal of Medicall Aboratory Sciences. 2003;14(1):11–2. [Google Scholar]
- Sheng 2005 {published data only} .Sheng Y. Effects of clozapine and risperidone on serum interleukin-2 and interleukin-6 of patients with first episode schizophrenia. Qilu Journal of Medical Laboratory Sciences. 2005;16(5):14–5. [Google Scholar]
- Shi 2000 {published data only} .Shi F, Cheng X, Zhang Y. Controlled study of risperidone and clozapine in the treatment of schizophrenia. Shanghai Archives of Psychiatry. 2000;12(1):27–9. [Google Scholar]
- Shi 2001 {published data only} .Shi X-H. A study of risperidone and clozapine in treatment of schizophrenia. Medical Journal of Communications. 2001;15(6):652–3. [Google Scholar]
- Shi 2004 {published data only} .Shi T, Zhang R, Guo X. Influences of risperidone and clozapine on plasma levels of cytokine in first-episode schizophrenics. Chinese Journal of Nervous and Mental Diseases. 2004;30(5):339–41. [Google Scholar]
- Simpson 2004 {published data only} .*; Simpson GM, Fayek M, Weiden PJ, Murray S, Warrington L, Loebel A. Ziprasidone: long-term post-switch efficacy in schizophrenia. World Psychiatry. 2004;3(S1):220. [Google Scholar]
- Sowell 2002 {published data only} .*; Sowell M, Cavazzoni P, Roychowdhury SM, Breier A. Antipsychotics and diabetes: lack of evidence for a causal relationship. International Journal of Neuropsychopharmacology. 2002;5(Suppl 1):170. [Google Scholar]
- Su 2004 {published data only} .Sun D, Jiang Q, Su S. Effect of risperidone and clozapine on blood fat in patients with schizophrenia. Journal of Hebei Medical College for Continuing Education. 2004;21(2):8–9. [Google Scholar]
- Su 2005 {published data only} .*; Su K-P, Wu P-L, Pariante CM. A crossover study on lipid and weight changes associated with olanzapine and risperidone. Psychopharmacology. 2005;183(3):383–6. doi: 10.1007/s00213-005-0205-2. [DOI] [PubMed] [Google Scholar]
- Sun 2000 {published data only} .Sun T. A controlled study comparing risperidone and clozapine in the treatment of schizophrenia. Health Psychology Journal. 2000;8(3):290–1. [Google Scholar]
- Sun 2001 {published data only} .Sun Q-Z, Liang J-S, Yu G-H, Huang X. A study of risperidone and clozapine in treatment of negative symptoms of resistant schizophrenia. Journal of Qiqihar Medical. 2001;22(11):1247–8. [Google Scholar]
- Swanson 2006 {published data only} .*; Swanson JW, Swartz MS, Van Dorn RA. Effectiveness of atypical antipsychotics for substance abuse in schizophrenia patients; Proceedings of the 159th Annual Meeting of the American Psychiatric Association; Toronto, Canada. 2006 May 20-25.2006. [Google Scholar]
- Tandon 2006 {published data only} .*; Tandon R, Marcus RN, Stock EG, Riera LC, Kostic D, Pans M, McQuade RD, Nyilas M, Iwamoto T, Crandall DT. A prospective, multicenter, randomized, parallel-group, open-label study of aripiprazole in the management of patients with schizophrenia or schizoaffective disorder in general psychiatric practice: Broad Effectiveness Trial With Aripiprazole (BETA) Schizophrenia Research. 2006;84(1):77–89. doi: 10.1016/j.schres.2005.12.857. [DOI] [PubMed] [Google Scholar]
- Tang 2002 {published data only} .Tang M, Wang H, Huang J. Influences of treating schizophrenics with risperidone and clozapine on the quality of life of their spouses and parents. Nervous Diseases and Mental Hygiene. 2002;2(2):67–9. [Google Scholar]
- Tang 2002a {published data only} .Tang M, Zhao G, Wang H. Comparison of quality of life of family with schizophrenic outpatients. Nervous Disease and Mental Hygiene. 2002;2:67–9. [Google Scholar]
- Tian 2005 {published data only} .Tian R-F. A study of risperidone and clozapine in treatment of schizophrenia in children. Journal of Clinical Psychosomatic Diseases. 2005;11(3):261. [Google Scholar]
- Tong 2005 {published data only} .Tong S-N, Gan H-Q, Liu Y-Y. A study of clozapine and risperidone influencing on IQ and memory in schizophrenia. Chinese Journal of Behavioral Medical Science. 2005;14(7):634. [Google Scholar]
- Tunis 2006 {published data only} .*; Tunis SL, Faries DE, Nyhuis AW, Kinon BJ, Ascher-Svanum H, Aquila R. Cost-effectiveness of olanzapine as first-line treatment for schizophrenia: results from a randomized, open-label, 1-year trial. Value in Health. 2006;9(2):77–89. doi: 10.1111/j.1524-4733.2006.00083.x. [DOI] [PubMed] [Google Scholar]
- Van Bruggen 2003 {published data only} .Van Bruggen J, Tijssen J, Dingemans P, Gersons B, Linszen D. Symptom response and side-effects of olanzapine and risperidone in young adults with recent onset schizophrenia. International Clinical Psychopharmacology. 2003;18(6):341–6. doi: 10.1097/00004850-200311000-00005. [DOI] [PubMed] [Google Scholar]
- Vaughan 2000 {published data only} .*; Vaughan K, McConaghy N, Wolf C, Myhr C, Black T. Community treatment orders: Relationship to clinical care, medication compliance, behavioural disturbance and readmission. Australian and New Zealand Journal of Psychiatry. 2000;34(5):801–8. doi: 10.1080/j.1440-1614.2000.00813.x. [DOI] [PubMed] [Google Scholar]
- Wang 2000a {published data only} .Wang M, Liu L. A controlled study on seroquel and risperidone in the treatment of schizophrenic patients. Journal of Clinical Psychology in Medical Settings. 2000;10(4):200–1. [Google Scholar]
- Wang 2001 {published data only} .Wang J, Nan Z, Bai J. The relationship of schizophrenic cognitive function impairment with positive and negative symptoms. Journal of Clinical Psychology in Medical Settings. 2001;11:346–7. [Google Scholar]
- Wang 2002 {published data only} .Wang G, Chen Z, Wang H. Comparative study on quality of life of schizophrenics treated with risperidone or clozapine. Chinese Mental Health Journal. 2002;16(3):200–2. [Google Scholar]
- Wang 2002a {published data only} .Wang R, Geng Y, Pan D. A comparative trial on the efficacy of risperidone and clozapine in treatment-resistant schizophrenia. Shandong Archives of Psychiatry. 2002;15(4):221–2. [Google Scholar]
- Wang 2003 {published data only} .Wang J, Zhao J, Wang J. A comparative study of risperidone and clozapine on life quality of schizophrenia. Shandong Archires of Psychiatry. 2003;16(3):149–50. [Google Scholar]
- Wang 2003a {published data only} .Wang J-J. A study of risperidone and clozapine with sulpiride in treatment of schizophrenia. Shandong Archires of Psychiatry. 2003;16:234–5. [Google Scholar]
- Wang 2005 {published data only} .Wang C-H, Li Y, Ma J-D, Wang L-H, Pan M, Mu J-L. Comparison of cognitive function and p300 potentials in first-episode schizophrenia treated with risperidone and clozapine. Chinese Journal of Nervous and Mental Diseases. 2005;31(4):267–71. [Google Scholar]
- Wang 2005a {published data only} .Wang S-Y, Liu Z, Bian J, Xu J-G. The influence of risperidone in the quality of life in schizophrenia. Nervous Diseases and Mental Hygiene. 2005;5(2):153–4. [Google Scholar]
- Wang 2005b {published data only} .Wang X, Yang J, Guo H. Clinical study of quetiapine and risperidone in the treatment of schizophrenia. Journal of Clinical Psychosomatic Diseases. 2005;11(2):118–9. [Google Scholar]
- Weickert 2003 {published data only} .*; Weickert TW, Goldberg TE, Marenco S, Bigelow LB, Egan MF, Weinberger DR. Comparison of cognitive performances during a placebo period and an atypical antipsychotic treatment period in schizophrenia: critical examination of confounds. Neuropyschopharmacology. 2003;28(8):1491–500. doi: 10.1038/sj.npp.1300216. [DOI] [PubMed] [Google Scholar]
- Weng 1998 {published data only} .Weng Y. A controlled trial of risperidone versus clozapine in the treatment of schizophrenia. Journal of Clinical Psychological Medicine. 1998;8(2):83–5. [Google Scholar]
- Wolf 2002 {published data only} .*; Wolf K, Wolf K, Mass R, Kiefer F, Wiedemann K, Naber D. Improvement of facial expression of emotions (fee) of schizophrenic patients under olanzapine versus risperidone. A prospective facial-emg study; Proceedings of the 12th World Congress of Psychiatry; Yokohama, Japan. 2002 Aug 24-29.2002. [Google Scholar]
- Wolf 2005 {published data only} .*; Wolf K, Mass R, Kiefer F, Eckert K, Stritzky AV, Haasen C, Wiedemann K, Naber D. The influence of olanzapine versus risperidone on facial expression of emotions in schizophrenia-preliminary results of a facial electromyogram study. Journal of Clinical Psychopharmacology. 2005;25(3):278–81. doi: 10.1097/01.jcp.0000165739.55142.23. [DOI] [PubMed] [Google Scholar]
- Wu 2001 {published data only} .Wu T, Li Y, Li M. Efficacy of low dosage risperidone in the treatment of first episode schizophrenia. Journal of Clinical Psychology in Medical Settings. 2001;11:152–4. [Google Scholar]
- Wu 2002 {published data only} .Wu L. A control study of risperidone and clozapine combination for the treatment of refractory schizophrenia. Health Psychology Journal. 2002;10(2):135–7. [Google Scholar]
- Wu 2004 {published data only} .Wu J-F, Li Z-G. A study of risperidone and clozapine in treatment of schizophrenia. Medical Journal of Chinese People Health. 2004;16(4):213–4. [Google Scholar]
- Wu 2006 {published data only} .*; Wu R-R, Zhao J-P, Liu Z-N, Zhai J-G, Guo X-F, Guo W-B, Tang J-S. Effects of typical and atypical antipsychotics on glucose-insulin homeostasis and lipid metabolism in first-episode schizophrenia. Psychopharmacology. 2006;186(4):572–8. doi: 10.1007/s00213-006-0384-5. [DOI] [PubMed] [Google Scholar]
- Wyszogrodzka 2006 {published data only} .*; Wyszogrodzka-Kucharska A, Rabe-Jablonska J. Vitamin D deficiency and impaired parathormone metabolism as a risk factor of low bone mineral density in patients with diagnosis of schizophrenia treated with second generation antipsychotics. Journal of the European College of Neuropsychopharmacology. 2006;16(Suppl 4):373. [Google Scholar]
- Xiao 2003 {published data only} .Xiao X-M, Song X-C, Jiang H-Y. A study of risperidone and clozapine in treatment of schizophrenia. Journal of Clinical Psychology Medicine. 2003;21:33. [Google Scholar]
- Xie 2005 {published data only} .Xie B-Q, Wang Z-M, Han Q-L. A control study of risperidone and clozapine in the treatment the chronic schizophrenia. Shandong Archives of Psychiatry. 2005;18(2):76–7. [Google Scholar]
- Xin 2001 {published data only} .Xin X, Du B, Zeng Z. A controlled clinical study of risperidone and low dose of clozapine in treating schizophrenia. Herald of Medicine. 2001;20(8):501–2. [Google Scholar]
- Xu 2001 {published data only} .Xu C, Tang J, Zhang X. The effect of risperidone and clozapine treatment on serum interleukin 8, interleukin 15 in schizophrenis. Medical Journal of Chinese Civil Administration. 2001;13(3):132–5. [Google Scholar]
- Xu 2005 {published data only} .Xu X, Zhang X, Zhaao C, Dai X. Comparison of efficacy and safety of seroquel and risperidone in the treatment of schizophrenia. Journal of Clinical Medicine in Practice. 2005;9:20–1. [Google Scholar]
- Yagdiran 2000 {published data only} .*; Yagdiran O, Krausz M. Depressive symptoms under atypical neuroleptic treatment in schizophrenia. Nervenarzt. 2000;71(Suppl 1):135–6. [Google Scholar]
- Yamashita 2005 {published data only} .*; Yamashita H, Mori K, Nagao M, Okamoto Y, Morinobu S, Yamawaki S. Influence of aging on the improvement of subjective sleep quality by atypical antipsychotic drugs in patients with schizophrenia: comparison of middle-aged and older adults. American Journal of Geriatric Psychiatry. 2005;13(5):377–84. doi: 10.1176/appi.ajgp.13.5.377. [DOI] [PubMed] [Google Scholar]
- Yan 2002 {published data only} .Yan M, Wu B-Q. A study of risperidone and clozapine in treatment of chronic schizophrenia. Journal of Chinese Politic Medicine. 2002;14(6):350–1. [Google Scholar]
- Yang 1998 {published data only} .Yang Z, Xu H, Song Y. A controlled study of efficacy of risperidone and clozapine on treating schizophrenia whose predominant clinical features were negative symptoms. Sichuan Mental Health. 1998;11(3):151–2. [Google Scholar]
- Yang 2003 {published data only} .Yang X, Mei Q. A comparison of olanzapine and risperidone in the treatment of first episode schizophrenia. Shanghai Archives of Psychiatry. 2003;15:338–9. [Google Scholar]
- Yang 2004 {published data only} .Yang X-N, Mei Q-Y. A study of risperidone with clonazepam in treatment of schizophrenia. Journal of Clinical Psychiatry. 2004;25:274. [Google Scholar]
- Yang 2004a {published data only} .Yang B, Wang YD, Zhang L. Effects of clozapine, risperidone and haloperidol on plasma leptin in first-episode schizophrenic patients. Journal of The Fourth Military Medical University. 2004;25(14):1323–5. [Google Scholar]
- Ye 2005 {published data only} .Ye S-C, Zhang W-Y, Zhang Y-M. Observation of developments of the level of serum prolactin of first-episode schizophrenia patients during the treatment. Medical Journal of Chinese People Health. 2005;17(6):267–8. [Google Scholar]
- Ye 2005a {published data only} .Ye X, Xia X. A comparative study between aripiprazole and risperidone in treatment of first-onset schizophrenia. Medical Journal of National Defending Forces in Southwest China. 2005;15(6):616–7. [Google Scholar]
- Yin 2002 {published data only} .Yin Y, Son L. The impact of clozapine and risperidone on QTc. Shanghai Archives of Psychiatry. 2002;14(3):154–5. [Google Scholar]
- Yin 2004 {published data only} .Yin H-R, Fang Y-M, Huang R-H, Yang X-Q. A study of risperidone and clozapine in treatment of schizophrenia. China Medical Engineering. 2004;12(3):87–8. [Google Scholar]
- Yu 1999 {published data only} .Yu G-H, Huang X. A study of risperidone and clozapine in treatment of resistant patients of schizophrenia. Chinese Journal of Nerval and Mental Diseases. 1999;25:366–7. [Google Scholar]
- Yu 2002 {published data only} .Yu G, Ding G, Li X. An economic burden comparison of typical and atypical antipsychotic therapies for schizophrenia. Chinese Journal of Psychiatry. 2002;35(3):177–9. [Google Scholar]
- Yu 2005 {published data only} .Yu D-S, Xu J, Sun D-H, Zeng Y-Y, Gao X-N, Jiang X-J. A comparative study of clozapine vs risperidone on body posture balance. Chinese Journal of New Drugs and Clinical Remedies. 2005;24(2):125–8. [Google Scholar]
- Yue 2004 {published data only} .Yue Y, Song L, Xu Y. A comparative study on risperidone in the two-year treatment of schizophrenia. Shanghai Archives of Psychiatry. 2004;16(3):165–7. [Google Scholar]
- Zelaschi 2006 {published data only} .*; Zelaschi NM, Sr, Rodriguez JL, Sr, Gaitan S, Sr, Palacios Vallejos ME, Sr, Zieher LM., Sr The effects of the switch of conventional neuroleptics to atypical antipsychotics: a follow-up study of patients with chronic schizophrenia; Proceedings of the 159th Annual Meeting of the American Psychiatric Association; Toronto, Canada. 2006 May 20-25.2006. [Google Scholar]
- Zeng 2002 {published data only} .Zeng Y, Fang Q, Gao X. The 4th day serum prolactin level predicting the effective dose taking risperidone or clozapine. Journal of Clinical Psychological Medicine. 2002;12(5):277–9. [Google Scholar]
- Zhan 2002 {published data only} .Zhan L-Y. Analysis of risperidone and clozapine in behavioral change in schizophrenia. Modern Rehabilitation. 2002;6(19):2943. [Google Scholar]
- Zhang 1999 {published data only} .Zhang B-S, Liu B-Q, Shang F-Z, Song C-A, Huang C-B, Zhang R-F, Liu X-F. A double-blinded study of risperidone and clozapine in treatment of schizophrenia. Psychiatry Medicine of Shandong. 1999;12:7–9. [Google Scholar]
- Zhang 2000 {published data only} .Zhang C. A controlled study comparing risperidone and clozapine in the treatment of schizophrenia. Journal of Clinical Psychology. 1997;7:74–5. [Google Scholar]
- Zhang 2002 {published data only} .Zhang Q. A comparison of cognitive function in the first-onset schizophrenia treated with risperidone and clozapine. Journal of Qiqihar Medical. 2002;23(8):852–3. [Google Scholar]
- Zhang 2002a {published data only} .Zhang XZ, Yu J-L. Control studies of relapses between risperidone and clozapine in schizophrenia. Journal of Clinical Psychosomatic Diseases. 2002;8(3):145–7. [Google Scholar]
- Zhang 2003 {published data only} .Zhang L, Wu Y, Guo C. A comparative study between quetiapine and risperidone in the treatment of schizophrenia. Health Psychology Journal. 2003;11:262–4. [Google Scholar]
- Zhang 2005 {published data only} .Zhang J-X, Zhu F-Y, Shi X-M, Zhang X-M, Wei Li-H, Ji Z-F, Lin R-M. Controlled study of risperidone versus clozapine in treatment of refractory schizophrenia with depressive symptoms. Journal of Clinical Psychological Medicine. 2005;15(4):201–2. [Google Scholar]
- Zhao 2004 {published data only} .Zhao F, Zhang ZH, Li H. Control studies of quetiapine and risperidone in the treatment of schizophrenia. Journal of Clinical Psychosomatic Diseases. 2004;10:25–6. [Google Scholar]
- Zhao 2005 {published data only} .Zhao L, Li Y, Qi F. Control study of quetiapine and risperidone in treatment of schizophrenics. Chinese Journal of Health Psychology. 2005;13:79–80. [Google Scholar]
- Zheng 2001 {published data only} .Zheng X-X, Huang J-H, Huang Z-Z. A study of risperidone and clozapine in treatment of the side-effects in schizophrenia. Strait Pharmaceutical Journal. 2001;13:79–80. [Google Scholar]
- Zheng 2003 {published data only} .Zheng YJ, Wang GH, Cheng ZL. Effects of clozapine and risperidone on the glucose metabolism in first-episode schizophrenic patients. Chinese Journal of Psychiatry. 2003;36(4):207–10. [Google Scholar]
- Zhi 2005 {published data only} .Zhi S. Comparison study of aripiprazole and risperidone on schizophrenia. Modern Journal of Integrated Traditional Chinese and Western Medicine. 2005;14:2787–2. [Google Scholar]
- Zhong 2003 {published data only} .Zhong H, Wang M. The influence of risperidone, chlorpromazine and clozapine on ECG [2003] Journal of Gannan Medical College. 2003;23:644–5. [Google Scholar]
- Zhong 2006a {published data only} .Zhong Z-Y, Tao J, Wang X-L, Wu X-L, Zhang J-B. Effect of antipsychotic plus buflomedil hydrochlorde in ameliorating the negative symptoms of patients with schizophrenia. Zhongguo Linchuang Kangfu. 2006;10(2):30–2. [Google Scholar]
- Zhou 2005 {published data only} .Zhou B, Tang Y, Zhu L. Effects of risperidone vs clozapine on blood-lipid of first-episode schizophrenia. Journal of Clinical Psychosomatic Diseases. 2005;11(4):315–6. [Google Scholar]
- Zhu 1999 {published data only} .Zhu X, Mei Q. A controlled study comparing drug compliance with risperidone and clozapine in treatment of schizophrenia. Journal of Clinical Psychological Medicine. 1999;9(3):151–2. [Google Scholar]
- Zhu 2002 {published data only} .Zhu H, Ma C, Hou J, Yin Q, Hu H, Guo Y. Study of serum il-6 and a-ifn in the pre-and post-treatment of 68 patients with first episode schizophrenia. Chinese Journal of Nervous and Mental Diseases. 2002;28(5):324–6. [Google Scholar]
- Zhu 2003 {published data only} .Zhu F, Lin R, Zhang J. Risperidone versus clozapine in treatment-resistant schizophrenia: a randomized controlled study. Shanghai Archives of Psychiatry. 2003;15(3):168–71. [Google Scholar]
- Zhu 2003a {published data only} .Zhu Q-Y, Zhang S-W, Pi J-F. 15 cases of risperidone in treatment of resistant schizophrenia. Herald of Medicine. 2003;22:13–4. [Google Scholar]
- Zoccali 2003 {published data only} .*; Zoccali R, Muscatello MR, Torre DL, Malara G, Canale A, Crucitti DDC, Spina E. Lack of a pharmacokinetic interaction between mirtazapine and the newer antipsychotics clozapine, risperidone and olanzapine in patients with chronic schizophrenia. Pharmacological Research. 2003;48(4):411–4. doi: 10.1016/s1043-6618(03)00178-6. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
- Eli Lilly 2003 {published data only} .Eli Lilly, Company . Safety study of olanzapine and a comparator in patients with schizophrenia and schizoaffective disorder. Eli Lilly and Company Clinical Trial Registry; 2003. [Google Scholar]
- Eli Lilly 2006 {published data only} .Eli Lilly, Company . Efficacy study of early onset of antipsychotic drug action in schizophrenia. Eli Lilly and Company Clinical Trial Registry; 2006. [Google Scholar]
- Gafoor 2005 {published data only} .Gafoor R, Power P, Craig T, Kerwin R, McGuire P. A comparative study of quetiapine and risperidone in patients with first-episode psychosis. Journal of the European College of Neuropsychopharmacology. 2005;15(Suppl 3):509. [Google Scholar]
- Lieberman 2001 {published data only} .Lieberman JA. Risperidone and clozapine in chronic schizophrenia. 2001. CRISP database. [Google Scholar]
- Ratna 2003 {published data only} .Ratna L. A multi-centre, double-blind, randomised study to compare the safety tolerability and efficacy of quetiapine and risperidone in the treatment of schizophrenia in subjects with inadequate symptom control Improved Response In Schizophrenia - IRIS. Vol. 1. National Research Register; 2003. [Google Scholar]
- Reveley 2000 {published data only} .Reveley M. Ris-int-45 an international, multicentre, randomised double-blind parallel-group trial comparing the safety and efficacy of risperidone and olanzapine in the treatment of patients with schizophrenia. National Research Register; 2000. [Google Scholar]
- Sireling 2003 {published data only} .Sireling . Study a) Sertindole versus risperidone safety outcome study: a randomised, partially blinded, parallel-group, active-controlled, post marketing study. Study b) Sertindole post-marketing surveillance study. Vol. 2. National Research Register; 2003. 2003. [Google Scholar]
Additional references
- Altman 1996 .Altman DG, Bland JM. Detecing skewness from summary information. BMJ. 1996;313:1200. doi: 10.1136/bmj.313.7066.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen 1983 .Andreasen NC. Scale for the assessment of negative symptoms. University of Iowa; 1983. [Google Scholar]
- Andreasen 1984 .Andreasen NC. Scale for the assessment of positive symptoms. The University of Iowa; Iowa City, IA: 1984. [Google Scholar]
- APA 1994 .American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders (DSM-IV) 4th Edition American Psychiatric Association; 1994. [Google Scholar]
- APA 2004 .American Psychiatric Association . Practice guidelines for the treatment of patients with schizophrenia. 2nd Edition. American Psychiatric Publishing, Inc; 2004. pp. 1–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnt 1998 .Arnt J, Skarsfeldt T. Do novel antipsychotics have similar pharmacological characteristics? A review of the evidence. Neuropsychopharmacology. 1998;18:63–101. doi: 10.1016/S0893-133X(97)00112-7. [DOI] [PubMed] [Google Scholar]
- Barnes 1989 .Barnes TR. A rating scale for drug-induced akathisia. British Journal of Psychiatry. 1989;154:672–6. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- Bland 1997 .Bland JM, Kerry SM. Statistics notes. Trials randomised in clusters. BMJ. 1997;315:600. doi: 10.1136/bmj.315.7108.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissel 1999 .Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, Boutitie F, Nony P, Haugh M, Mignot G. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use. Therapie. 1999;54(4):405–11. [PubMed] [Google Scholar]
- Carpenter 1984 .Heinrichs DW, Hanlon ET, Carpenter WT., Jr. The quality of life scale: an instrument for rating the schizophrenic deficit syndrome. Schizophrenia Bulletin. 1984;10:388–96. doi: 10.1093/schbul/10.3.388. [DOI] [PubMed] [Google Scholar]
- Carpenter 1994 .Carpenter WT, Jr, Buchanan RW. Schizophrenia. New England Journal of Medicine. 1994;330:681–90. doi: 10.1056/NEJM199403103301006. [DOI] [PubMed] [Google Scholar]
- Chouinard 1980 .Chouinard G, Ross-Chouinard A, Annable L, Jones BD. The extrapyramidal symptom rating scale. Canadian Journal of Neurological Sciences. 1980;7:233. [Google Scholar]
- Curtis 1995 .Curtis V, Kerwin R. A risk benefit assessment of risperidone in schizophrenia. Drug Safety. 1995;12:139–45. doi: 10.2165/00002018-199512020-00006. [DOI] [PubMed] [Google Scholar]
- Deeks 2000 .Deeks J. Issues in the selection for meta-analyses of binary data; Proceedings of the 8th International Cochrane Colloquium; Cape Town, South Africa. 2000 Oct 25-28th.2000. [Google Scholar]
- Divine 1992 .Divine GW, Brown JT, Frazer LM. The unit of analysis error in studies about physicians’ patient care behavior. Journal of General Internal Medicine. 1992;7:623–9. doi: 10.1007/BF02599201. [DOI] [PubMed] [Google Scholar]
- Donner 2002 .Donner A, Klar N. Issues in the meta-analysis of cluster randomized trials. Statistics in Medicine. 2002;21:2971–80. doi: 10.1002/sim.1301. [DOI] [PubMed] [Google Scholar]
- Duggan 2005 .Duggan L, Fenton M, Rathbone J, Dardennes R, El-Dosoky A, Indran S. Olanzapine for schizophrenia. Cochrane Database of Systematic Reviews. 2005;(2) doi: 10.1002/14651858.CD001359.pub2. DOI: 10.1002/14651858.CD001359.pub2. [DOI] [PubMed] [Google Scholar]
- Egger 1997 .Egger M, Davey-Smith G, Schneider M, Minder CSO. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;13:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayeh 2006 .El-Sayeh HG, Morganti C. Aripiprazole for schizophrenia. Cochrane Database of Systematic Reviews. 2006;(2) doi: 10.1002/14651858.CD004578.pub3. DOI: 10.1002/14651858. CD004578.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbourne 2002 .Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving crossover trials: methodological issues. International Journal of Epidemiology. 2002;31(1):140–9. doi: 10.1093/ije/31.1.140. [DOI] [PubMed] [Google Scholar]
- Fleischhacker 1989 .Fleischhacker WW, Bergman KJ, Perovich R, Pestreich LK, Borenstein M, Lieberman JA, Kane JM. The Hillside Akathisia Scale: a new rating instrument for neuroleptic induced akathisia. Psychopharmacology Bulletin. 1989;25:222–6. [PubMed] [Google Scholar]
- Furukawa 2006 .Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. Journal of Clinical Epidemiology. 2006;59:7–10. doi: 10.1016/j.jclinepi.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Gaebel 2006 .Gaebel W, Falkai P, Weinmann S, Wobrock T. Treatment guidelines for schizophrenia [Behandlungsleitlinie Schizophrenie] Steinkopf; 2006. [Google Scholar]
- Gilbody 2000 .Gilbody SM, Bagnall AM, Duggan L, Tuunainen A. Risperidone versus other atypical antipsychotic medication for schizophrenia. Cochrane Database of Systematic Reviews. 2000;(3) doi: 10.1002/14651858.CD002306. DOI: 10.1002/14651858.CD002306. [DOI] [PubMed] [Google Scholar]
- Goldman 1992 .Goldman HH, Skodol AE, Lave TR. Revising axis V for DSM-IV: a review of measures of social functioning. American Journal of Psychiatry. 1992;149:1148–56. doi: 10.1176/ajp.149.9.1148. [DOI] [PubMed] [Google Scholar]
- Gulliford 1999 .Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community-based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology. 1999;149:876–83. doi: 10.1093/oxfordjournals.aje.a009904. [DOI] [PubMed] [Google Scholar]
- Gupta 1994 .Gupta S, Black D, Smith D. Risperidone: review of its pharmacology and therapeutic use in schizophrenia. Annals of Clinical Psychiatry. 1994;6:173–80. doi: 10.3109/10401239409149000. [DOI] [PubMed] [Google Scholar]
- Guy 1976 .Guy U. ECDEU assessment manual for psychopharmacology. National Institute of Mental Health; 1976. [Google Scholar]
- Heres 2006 .Heres S, Davis J, Maino K, Jetzinger E, Kissling W, Leucht S. Why olanzapine beats risperidone, risperidone beats quetiapine, and quetiapine beats olanzapine. American Journal of Psychiatry. 2006;163:185–94. doi: 10.1176/appi.ajp.163.2.185. [DOI] [PubMed] [Google Scholar]
- Higgins 2003 .Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins 2008 .Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1. The Cochrane Collaboration; 2008. updated September 2008. 2008. Available from www.cochrane-handbook.org. [Google Scholar]
- Janssen-Cilag 2005 .Janssen-Cilag . Risperdal. Janssen-Cilag Product Information; 2005. [Google Scholar]
- Jayaram 2006 .Jayaram MB, Hosalli P, Stroup S. Risperidone versus olanzapine for schizophrenia. Cochrane Database of Systematic Reviews. 2006;(2) doi: 10.1002/14651858.CD005237.pub2. DOI: 10.1002/14651858.CD005237.pub2. [DOI] [PubMed] [Google Scholar]
- Kane 1988 .Kane JM, Honigfeld G, Singer J, Meltzer H, Clozaril Collaborative Study Group Clozapine for the treatment of treatment-resistant schizophrenia: a double-blind comparison with chlorpromazine. Archives of General Psychiatry. 1988;45:789–96. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- Kane 1993 .Kane JM. Treatment programme and long term outcome in chronic schizophrenia. Acta Psychiatrica Scandinavica. 1993;46:585–93. doi: 10.1111/j.1600-0447.1990.tb05309.x. [DOI] [PubMed] [Google Scholar]
- Kay 1986 .Kay SR, Opler LA, Fiszbein A. Positive and negative syndrome scale (PANSS) manual. Multi-Health Systems; North Tonawanda (NY): 1986. [Google Scholar]
- Leucht 2005a .Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. What does the PANSS mean? Schizophrenia Research. 2005;79:231–8. doi: 10.1016/j.schres.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Leucht 2005b .Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of Brief Psychiatric Rating Scale Scores. British Journal of Psychiatry. 2005;187:366–71. doi: 10.1192/bjp.187.4.366. [DOI] [PubMed] [Google Scholar]
- Lewis 2006 .Lewis SW, Davies L, Jones P, Barnes TRE, Murray RM, Kerwin R, Taylor D, Hayhurst KP, Marwick A, Lloyd H, Dunn G. Randomised controlled trials of conventional antipsychotic versus new atypical drugs, and new atypical drugs versus clozapine, in people with schizophrenia responding poorly to, or intolerant of, current drug treatment. Health Technology Assessment. 2006;10(17):iii–iv. ix–xi, 1–165. doi: 10.3310/hta10170. [DOI] [PubMed] [Google Scholar]
- Marder 1994 .Marder SR, Meibach RC. Risperidone in the treatment of schizophrenia. American Journal of Psychiatry. 1994;151:825–35. doi: 10.1176/ajp.151.6.825. [DOI] [PubMed] [Google Scholar]
- Marshall 2000 .Marshall M, Lockwood A, Adams C, Bradley C, Joy C, Fenton M. Unpublished rating scales - a major source of bias in randomised controlled trials of treatments for schizophrenia? British Journal of Psychiatry. 2000;176:249–52. doi: 10.1192/bjp.176.3.249. [DOI] [PubMed] [Google Scholar]
- Marvaha 2004 .Marvaha S, Johnson S. Schizophrenia and employment - a review. Social Psychiatry and Psychiatric Epidemiology. 2004;39:337–49. doi: 10.1007/s00127-004-0762-4. [DOI] [PubMed] [Google Scholar]
- Moher 2001 .Moher D, Schulz KF, Altman D. The CONSORT statement: Revised recommendations for improving the quality of reports of parallel-group randomised trials. JAMA. 2001;285:1987–1. doi: 10.1001/jama.285.15.1987. [DOI] [PubMed] [Google Scholar]
- Möller 2000 .Möller HJ. New assessment of atypical antipsychotics [Aktuelle Bewertung neuer/atypischer Neuroleptika] Nervenarzt. 2000;71:329–44. doi: 10.1007/s001150050567. [DOI] [PubMed] [Google Scholar]
- Overall 1962 .Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- Rosenheck 1997 .Rosenheck R, Cramer J, Xu WC, Thomas J, Henderson W, Frisman L, Fye C, Charney D. A comparison of clozapine and haloperidol in hospitalized patients with refractory schizophrenia. New England Journal of Medicine. 1997;337:809–15. doi: 10.1056/NEJM199709183371202. [DOI] [PubMed] [Google Scholar]
- Rust 1989 .Rust J, Golombok S. Modern Psychometrics. Routledge; London: 1989. [Google Scholar]
- Simpson 1970 .Simpson EN, Angus JWF. A rating scale for extrapyrmidal side-effects. Acta Psychiatrica Scandinavica. 1970;212:11–9. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- Simpson 1999 .Simpson GM, Josiassen RC, Stanilla JK, De Leon J, Nair C, Abraham G, Odom WA, Turner RM. Double-blind study of clozapine dose response in chronic schizophrenia. American Journal of Psychiatry. 1999;156:1744–50. doi: 10.1176/ajp.156.11.1744. [DOI] [PubMed] [Google Scholar]
- Tandon 2008 .Tandon R, Keshavan MS, Nasralllah HA. Schizophrenia, “Just the Facts” What we know in 2008. 2. Epidemiology and etiology. Schizophrenia research. 2008;102:1–18. doi: 10.1016/j.schres.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Tarnow-Mordi 1999 .Tarnow-Mordi WO, Healy MJ. Distinguishing between “no evidence of effect” and “evidence of no effect” in randomised controlled trials and other comparisons. Archives of Diseases in Children. 1999;80:210–11. doi: 10.1136/adc.80.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuang 1978 .Tsuang MT. Suicide in schizophrenics, manics, depressives, and surgical controls: a comparison with general population suicide mortality. Archives of General Psychiatry. 1978;35:153–55. doi: 10.1001/archpsyc.1978.01770260031002. [DOI] [PubMed] [Google Scholar]
- Ukoumunne 1999 .Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area-wide and organisation-based interventions in health and health care: a systematic review. Health Technology Assessment. 1999;3(5):3–92. [PubMed] [Google Scholar]
- Van Os 2006 .Van Os J, Burns T, Cavallaro R, Leucht S, Peuskens J, Helldin L, Bernardo M, Arango C, Fleischhacker W, Lachaux B, Kane JM. Standardized remission criteria in schizophrenia. Acta Psychiatrica Scandinavica. 2006;113:91–5. doi: 10.1111/j.1600-0447.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- Wahlbeck 2001 .Wahlbeck K, Tuunainen A, Ahokas A, Leucht S. Drop-out rates in randomised antipsychotic drug trials. Psychopharmacology. 2001;155:230–3. doi: 10.1007/s002130100711. [DOI] [PubMed] [Google Scholar]
- WHO 2001 .WHO . The World Health Report 2001 - Mental Health: New Understanding, New Hope. World Health Organisation; Geneva: 2001. [Google Scholar]
- Xia 2007 .Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El-Sayeh H, Pinfold V, Takriti Y. The Leeds Outcomes Stakeholders Survey (LOSS) Study; Proceedings of the 15th Cochrane Colloquium; Sao Paulo. 2007 Oct 23-27.2007. [Google Scholar]
References to other published versions of this review
- Leucht 2008 .Leucht S, Komossa K, Rummel-Kluge C, Corves C, Hunger H, Schmid F, Asenjo Lobos C, Schwarz S, Davis JM. A meta-analysis of head-to-head comparisons of second generation antipsychotics in the treatment of schizophrenia. American Journal of Psychiatry. 2008;166(2):152–63. doi: 10.1176/appi.ajp.2008.08030368. [DOI] [PubMed] [Google Scholar]
- * Indicates the major publication for the study