Abstract
Background
Migraine is a highly disabling condition for the individual and also has wide‐reaching implications for society, healthcare services, and the economy. Sumatriptan is an abortive medication for migraine attacks, belonging to the triptan family.
Objectives
To determine the efficacy and tolerability of oral sumatriptan compared to placebo and other active interventions in the treatment of acute migraine attacks in adults.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, online databases, and reference lists for studies through 13 October 2011.
Selection criteria
We included randomised, double‐blind, placebo‐ and/or active‐controlled studies using oral sumatriptan to treat a migraine headache episode, with at least 10 participants per treatment arm.
Data collection and analysis
Two review authors independently assessed trial quality and extracted data. We used numbers of participants achieving each outcome to calculate relative risk (or 'risk ratio') and numbers needed to treat to benefit (NNT) or harm (NNH) compared to placebo or a different active treatment.
Main results
Sixty‐one studies (37,250 participants) compared oral sumatriptan with placebo or an active comparator. Most of the data were for the 50 mg and 100 mg doses. Sumatriptan surpassed placebo for all efficacy outcomes. For sumatriptan 50 mg versus placebo the NNTs were 6.1, 7.5, and 4.0 for pain‐free at two hours and headache relief at one and two hours, respectively. NNTs for sustained pain‐free and sustained headache relief during the 24 hours postdose were 9.5 and 6.0, respectively. For sumatriptan 100 mg versus placebo the NNTs were 4.7, 6.8, 3.5, 6.5, and 5.2, respectively, for the same outcomes. Results for the 25 mg dose were similar to the 50 mg dose, while sumatriptan 100 mg was significantly better than 50 mg for pain‐free and headache relief at two hours, and for sustained pain‐free during 24 hours. Treating early, during the mild pain phase, gave significantly better NNTs for pain‐free at two hours and sustained pain‐free during 24 hours than did treating established attacks with moderate or severe pain intensity.
Relief of associated symptoms, including nausea, photophobia, and phonophobia, was greater with sumatriptan than with placebo, and use of rescue medication was lower with sumatriptan than with placebo. For the most part, adverse events were transient and mild and were more common with the sumatriptan than with placebo, with a clear dose response relationship (25 mg to 100 mg).
Sumatriptan was compared directly with a number of active treatments, including other triptans, paracetamol (acetaminophen), acetylsalicylic acid, non‐steroidal anti‐inflammatory drugs (NSAIDs), and ergotamine combinations.
Authors' conclusions
Oral sumatriptan is effective as an abortive treatment for migraine attacks, relieving pain, nausea, photophobia, phonophobia, and functional disability, but is associated with increased adverse events.
Plain language summary
Sumatriptan (oral route of administration) for acute migraine attacks in adults
Sumatriptan is one of the triptan family of drugs used to treat migraine attacks. It is widely available as an oral tablet. This review found that a single dose was effective in relieving migraine headache pain and associated symptoms of nausea, sensitivity to light, and sensitivity to sound. Pain was reduced from moderate or severe to no pain by two hours in about 3 in 10 people (32%) taking sumatriptan 100 mg, compared with about 1 in 10 (11%) taking placebo. Pain was reduced from moderate or severe to no worse than mild pain by two hours in 6 in 10 people (61%) taking sumatriptan 100 mg, compared with about 3 in 10 (32%) taking placebo. Almost a quarter (24%) of people taking sumatriptan 100 mg had freedom from pain at two hours which was sustained during 24 hours without the use of rescue medication, compared with fewer than 1 in 10 (8%) taking placebo. In addition to relieving headache pain, sumatriptan also relieved symptoms of nausea and sensitivity to light and sound by two hours in about half of those who took it, compared with about one‐third of those taking placebo. Adverse events were mostly of short duration and mild or moderate in severity, and were experienced by about 4 in 10 (43%) of people taking sumatriptan 100 mg, and by 2 in 10 (23%) taking placebo. The 50 mg dose had slightly lower efficacy, but was associated with fewer adverse events. Treating attacks while pain was still mild was more effective than treating established attacks with moderate or severe pain intensity.
Background
Description of the condition
Migraine is a common, disabling headache disorder, with considerable social and economic impact (Hazard 2009). Recent reviews found a one‐year prevalence of 15% for adults in European countries (Stovner 2010) and 13% for all ages in the US (Victor 2010). Migraine is more prevalent in women than in men (by a factor of two to three), and in the age range 30 to 50 years.
The International Headache Society (IHS) classifies two major subtypes. Migraine without aura is the most common subtype. It is characterised by attacks lasting 4 to 72 hours that are typically of moderate to severe pain intensity, unilateral, pulsating, aggravated by normal physical activity, and associated with nausea and/or photophobia and phonophobia. Migraine with aura is characterised by reversible focal neurological symptoms that develop over a period of 5 to 20 minutes and last for less than 60 minutes, followed by headache with the features of migraine without aura. In some cases the headache may lack migrainous features or be absent altogether (IHS 2004).
A recent large prevalence study in the US found that over half of migraineurs had severe impairment or required bed rest during attacks. Despite this high level of disability and a strong desire for successful treatment, only a proportion of migraine sufferers seek professional advice for the treatment of attacks. The majority were not taking any preventive medication, although one‐third met guideline criteria for offering or considering it. Nearly all (98%) migraineurs used acute treatments for attacks, with 49% using over‐the‐counter (OTC) medication only, 20% using prescription medication, and 29% using both. OTC medication included aspirin, other non‐steroidal anti‐inflammatory drugs (NSAIDs), paracetamol (acetaminophen), and paracetamol with caffeine (Bigal 2008; Diamond 2007; Lipton 2007). Similar findings have been reported from other large studies in France and Germany (Lucas 2006; Radtke 2009).
The significant impact of migraine with regard to pain, disability, social functioning, quality of relationships, emotional well‐being, and general health (Edmeads 1993; Osterhaus 1994; Solomon 1997) results in a huge burden for the individual, health services, and society (Clarke 1996; Ferrari 1998; Hazard 2009; Hu 1999; Solomon 1997). The annual US economic burden relating to migraine, including missed days of work and lost productivity, is USD 14 billion (Hu 1999). Thus successful treatment of acute migraine attacks not only benefits patients by reducing their disability and improving health‐related quality of life, but also reduces the need for healthcare resources and increases economic productivity (Jhingran 1996; Lofland 1999).
Description of the intervention
The symptomatic treatment of migraine advanced significantly with the development of the triptan class of drugs, of which sumatriptan was the first, in 1991. It is available as a standard oral tablet, nasal spray, subcutaneous injection, and rectal suppository. It is available only by prescription in most countries, but in the UK packs of 2 x 50 mg oral tablets are available OTC as Imigran Recovery for individuals with previously diagnosed migraine. Generic (non‐proprietary) formulations are also available for the standard tablets in many countries. The nasal spray, subcutaneous, and rectal formulations may be particularly useful for individuals who experience severe nausea or vomiting with their attacks. This review will investigate only oral sumatriptan. In the UK in 2010 there were over 910,000 prescriptions for sumatriptan, of which about 782,000 were for the oral formulation, with about two‐thirds for the 50 mg tablet and one‐third for the 100 mg tablet (PCA 2011); the majority of prescribing (96%) was for generic sumatriptan.
In order to establish whether sumatriptan is an effective treatment for migraine at a specified dose in acute migraine attacks, it is necessary to study its effects in circumstances that permit detection of pain relief. Such studies are carried out in individuals with established pain of moderate to severe intensity, using single doses of the interventions. Participants who experience an inadequate response with either placebo or active treatment are permitted to use rescue medication, and the intervention is considered to have failed in those individuals. In clinical practice, however, individuals would not normally wait until pain is of at least moderate severity, and may take a second dose of medication if the first dose does not provide adequate relief. Once efficacy is established in studies using single doses in established pain, further studies may investigate different treatment strategies and patient preferences. These are likely to include treating the migraine attack early while pain is mild, and using a low dose initially, with a second dose if response is inadequate.
How the intervention might work
Sumatriptan is a 5‐HT1 agonist, selectively targeting the 5‐HT (serotonin) 1B and 1D receptors. It has three putative mechanisms of therapeutic action (Ferrari 2002; Goadsby 2007):
vasoconstriction of dilated meningeal blood vessels;
inhibition of the release of vasoactive neuropeptides from perivascular trigeminal sensory neurons;
reduction of pain signal transmission in the trigeminal dorsal horn.
It is used for acute treatment, having no efficacy in preventing future attacks.
Why it is important to do this review
Sumatriptan was the first marketed triptan and is by far the most used triptan worldwide. Since it came off patent, generic formulations have greatly increased its availability, and sumatriptan has become the standard against which new acute migraine treatments are compared. An earlier Cochrane review of oral sumatriptan for acute migraine headaches searched for studies to the end of 2001 (McCrory 2003) and included comparisons with placebo, no intervention, other drug treatments, and behavioural or physical therapies. More studies have been published since that time and an update is needed to include and evaluate the data from these. We decided to include all routes of administration in the update, and to limit comparators to placebo and other pharmacological interventions. Owing to the very large amount of information now available, particularly for the oral formulation, we carried out separate reviews for each route of administration (Derry 2012a; Derry 2012b; Derry 2012c; Derry 2012d), together with an overview of all routes of administration (Derry (forthcoming)). These sumatriptan reviews form part of a larger series of reviews planned for acute treatments for migraine attacks.
Objectives
The objective of this review is to determine the efficacy and tolerability of oral sumatriptan compared to placebo and other active interventions in the treatment of acute migraine attacks in adults.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised, double‐blind, placebo‐ and/or active‐controlled studies using oral sumatriptan to treat a migraine headache episode. Studies had to have a minimum of 10 participants per treatment arm and report dichotomous data for at least one of the outcomes specified below. We accepted studies reporting treatment of consecutive headache episodes if outcomes for the first, or each, episode were reported separately. Cross‐over studies were accepted if there was adequate washout (≥ 48 hours) between treatments.
Types of participants
Studies enrolled adults (at least 18 years of age) with migraine. We used the definition of migraine specified by the International Headache Society (IHS 1988; IHS 2004), although we accepted diagnostic criteria equivalent to those of IHS 1988 where a specific reference was not provided. There were no restrictions on migraine frequency, duration, or type (with or without aura). Participants taking stable prophylactic therapy to reduce migraine frequency were accepted; where reported, details on the prophylactic therapy prescribed or allowed are provided in the Characteristics of included studies table.
Types of interventions
We included studies in which self administered oral sumatriptan was used to treat a migraine headache episode. There were no restrictions on dose, dosing regimen (e.g. single dose versus optional second dose) or timing of the first dose in relation to headache intensity (e.g. taking the first dose when pain was of moderate or severe intensity versus when pain was only mild).
A placebo comparator is essential to demonstrate that sumatriptan is effective in this condition. Active‐controlled trials without a placebo were considered as secondary evidence. We excluded studies designed to demonstrate prophylactic efficacy in reducing the number or frequency of migraine headaches.
Types of outcome measures
Primary outcomes
In selecting the main outcome measures for this review, we considered scientific rigour, availability of data, and patient preferences (Lipton 1999). Patients with acute migraine headaches have rated complete pain relief, no headache recurrence, rapid onset of pain relief, and no side effects as the four most important outcomes (Lipton 1999).
In view of these patient preferences and in line with the guidelines for controlled trials of drugs in migraine issued by the IHS (IHS 2000), we considered the following primary outcomes:
pain‐free at two hours, without the use of rescue medication;
reduction in headache pain ('headache relief') at one and two hours (pain reduced from moderate or severe to none or mild without the use of rescue medication);
sustained pain‐free during the 24 hours postdose (pain‐free within two hours, with no use of rescue medication or recurrence of moderate to severe pain within 24 hours);
sustained headache relief during the 24 hours postdose (headache relief at two hours, sustained for 24 hours, with no use of rescue medication or a second dose of study medication).
Pain intensity or pain relief had to be measured by the patient (not the investigator or carer). Pain measures accepted for the primary outcomes were:
pain intensity: four‐point categorical scale, with wording equivalent to none, mild, moderate, and severe; or 100 mm visual analogue scale (VAS);
pain relief: five‐point categorical scale, with wording equivalent to none, a little, some, a lot, complete; or 100 mm VAS.
All included studies used one or more of these standard scales and reported outcomes as defined above.
We considered only data obtained directly from the patient.
Secondary outcomes
Secondary outcomes considered were:
use of rescue medication;
participants with any adverse event during the 24 hours postdose;
participants with particular adverse events during the 24 hours postdose;
withdrawals due to adverse events;
headache‐associated symptoms: relief and/or presence at two hours;
functional disability: relief and/or presence at two hours.
Although recurrence of headache is perceived to be a problem with triptan medication, we chose not to analyse this outcome because of variation in the definition of 'recurrence' and poor reporting, such that it is often unclear whether the result is reported as a proportion of the whole treatment group or only of those who experienced headache relief at two hours. Furthermore, because recurrence is dependent upon first experiencing headache relief at two hours ‐ an outcome that varies across different treatment groups ‐ interpretation of the result is difficult. We believe that the outcome of sustained headache relief at 24 hours qualitatively provides the same information to patients, but in a more rigorous and intuitive way.
Definitions of important terms, including all measured outcomes, are provided in Appendix 1.
Search methods for identification of studies
Electronic searches
We searched the following databases:
the Cochrane Central Register of Controlled Trials (CENTRAL) (2011, Issue 10);
MEDLINE (via OVID) (to 13 October 2011);
EMBASE (via OVID) (to 13 October 2011);
Oxford Pain Relief Database (Jadad 1996a).
See Appendix 2, Appendix 3, and Appendix 4 for the search strategies for MEDLINE, EMBASE, and CENTRAL, respectively. There were no language restrictions.
Searching other resources
We searched reference lists of retrieved studies and review articles for additional studies. We also searched online databases of clinical trials (www.gsk‐clinicalstudyregister.com and www.clinicaltrials.gov). We made a written request for information about both published and unpublished data from the manufacturer of sumatriptan (GlaxoSmithKline), and asked specifically for further details on a number of studies published only on their clinical trial database. We did not search grey literature and short abstracts.
Data collection and analysis
Selection of studies
Two review authors independently carried out the searches and selected studies for inclusion. We viewed titles and abstracts of all studies identified by electronic searches on screen and excluded any that clearly did not satisfy the inclusion criteria. We read full copies of the remaining studies to identify those suitable for inclusion. Disagreements were settled by discussion with a third review author.
Data extraction and management
Two review authors independently extracted data from included studies using a standard data extraction form. Disagreements were settled by discussion with a third review author. One author entered data into RevMan 5.1 (RevMan 2011).
Assessment of risk of bias in included studies
We assessed methodological quality using the Oxford Quality Score (Jadad 1996b).
The scale is used as follows:
Is the study randomised? If yes, give one point.
Is the randomisation procedure reported and is it appropriate? If yes, add one point; if no, deduct one point.
Is the study double‐blind? If yes, add one point.
Is the double‐blind method reported and is it appropriate? If yes, add one point; if no, deduct one point.
Are the reasons for patient withdrawals and dropouts described? If yes, add one point.
The scores for each study are reported in the Characteristics of included studies table.
We also completed a 'Risk of bias' table for each study, using assessments of random sequence generation, allocation concealment, blinding, and study size.
Measures of treatment effect
We used relative risk (or 'risk ratio', RR) to establish statistical difference. Numbers needed to treat (NNT) and pooled percentages were used as absolute measures of benefit or harm.
We used the following terms to describe adverse outcomes in terms of harm or prevention of harm:
When significantly fewer adverse outcomes occurred with sumatriptan than with control (placebo or active) we used the term the number needed to treat to prevent one event (NNTp).
When significantly more adverse outcomes occurred with sumatriptan compared with control (placebo or active) we used the term the number needed to harm or cause one event (NNH).
Unit of analysis issues
We accepted randomisation at the individual patient level only.
Dealing with missing data
The most likely source of missing data was in cross‐over studies. Where this might be problematic (e.g. where data were missing for > 10% of participants), we used only first‐period data where available. In all cases (cross‐over or parallel‐group) we proposed to comment if there were substantial missing data and perform sensitivity analysis if possible.
Assessment of heterogeneity
We assessed heterogeneity of response rates using L'Abbé plots, a visual method for assessing differences in results of individual studies (L'Abbé 1987).
Assessment of reporting biases
We assessed publication bias by examining the number of participants in trials with zero effect (relative risk of 1.0) needed for the point estimate of the NNT to increase beyond a clinically useful level (Moore 2008). In this case, we specified a clinically useful level as a NNT ≥ 8 for pain‐free at two hours, and NNT ≥ 6 for headache relief at two hours.
Data synthesis
We analysed studies using a single dose of sumatriptan in established pain of at least moderate intensity separately from studies in which medication was taken before pain became well established or in which a second dose of medication was permitted.
We calculated effect sizes and combined data for analysis only for comparisons and outcomes where there were at least two studies and 200 participants (Moore 1998). We calculated relative risk of benefit or harm with 95% confidence intervals (CIs) using a fixed‐effect model (Morris 1995). We calculated NNT, NNTp, and NNH with 95% CIs using the pooled number of events by the method of Cook and Sackett (Cook 1995). A statistically significant difference from control was assumed when the 95% CI of the relative risk of benefit or harm did not include the number one.
We determined significant differences between NNT, NNTp, and NNH for different doses of active treatment, or between groups in the sensitivity analyses, using the z test (Tramer 1997).
We describe data from comparisons and outcomes with only one study or fewer than 200 participants in the summary tables and text where appropriate for information and comparison, but we did not analyse these data quantitatively.
Subgroup analysis and investigation of heterogeneity
We analysed different doses separately. We performed subgroup analysis for different formulations of the oral treatment.
Sensitivity analysis
We planned sensitivity analysis for study quality (Oxford Quality Score of 2 versus 3 or more) and for migraine type (with aura versus without aura). A minimum of two studies and 200 participants were required for any sensitivity analysis. Where studies allowed a second dose of study medication but did not report incidence of adverse events separately for participants taking a single dose only, we carried out sensitivity analysis, removing these data to determine any effect of multiple dosing.
Results
Description of studies
Included studies
Sixty‐one studies (58 publications) fulfilled the inclusion criteria for this review; 55 were published in full peer‐reviewed journals (Banerjee 1992; Brandes 2007 Study 1 and Study 2; Bussone 2000; Carpay 2004; Cutler 1995; Dahlof 1991; Dahlof 2009; Diener 2004a; Diener 2004b; DKSMSG 1999; Dodick 2002; Dowson 2002; Ensink 1991; Freitag 2001; Gallagher 2000; Geraud 2000; Goadsby 1991; Goadsby 2000; Goldstein 1998; Goldstein 2005; Gruffyd‐Jones 2001; Havanka 2000; Ishkanian 2007; Jelinski 2006; Kaniecki 2006; Kolodny 2004; Kudrow 2005; Latere 1991; Lines 2001; Lipton 2000; Mathew 2003; Myllyla 1998; Nappi 1994; Nett 2003; Patten 1991; Pfaffenrath 1998; Pini 1995; Pini 1999; Sandrini 2002; Sandrini 2007; Sargent 1995; Savani 1999; Schulman 2003; Sheftell 2005 Study 1 and Study 2; Smith 2005; Spierings 2001; Tfelt‐Hansen 1995; Tfelt‐Hansen 1998; Tfelt‐Hansen 2006; Thomson 1992; Visser 1996; Winner 2003 Study 1 and Study 2), five were available as Results Summaries on the manufacturer's website (GL/MIG/001/92; GL/MIG/001A/92; GL/MIG/002; GL/MIG/002A; GL/MIG/009), and one was a clinical trial report provided by the manufacturer (160‐104). These studies provided data on 37,250 participants.
All of the included studies recruited adult participants only, with the majority (46/61) recruiting participants between 18 and 65 years of age (mean ages ranged from 33 to 43 years), and the remainder ranging from a 55 year maximum age to no upper limit on age. The majority of participants were female (70% to 100%) and suffering from migraine without aura (14% to 93%). All studies required participants to have had at least a 6‐ or 12‐month history of migraine attacks (except one (160‐104) which made no specific requirement for migraine history) meeting IHS (or equivalent) diagnostic criteria (IHS 1988; IHS 2004) before screening. Twenty‐four studies required participants to discontinue any prophylactic medication at least two weeks before receiving study medication, while 13 studies allowed stable prophylactic medications (often excluding monoamine oxidase inhibitors, methysergide, and ergotamine or ergotamine‐containing medications), and the remaining 24 studies did not report on prophylaxis. Twenty‐two studies restricted participants from taking study medication within a defined time period of other acute migraine medications. This was most often 24 hours for any opiate, ergotamine, or triptan use, and six hours for any simple analgesics or antiemetics. The majority of the studies did not report on restricted acute migraine medications.
Participants were generally excluded for: pregnancy or breast‐feeding; inadequate contraception; confirmed or suspected cardiovascular or cerebrovascular disease (particularly history of ischaemic heart disease); uncontrolled hypertension (diastolic ≥ 95 mmHg or systolic ≥ 160 mmHg); current or past drug abuse; psychiatric illness; epilepsy; hepatic disease; Raynaud's syndrome; and/or opthalmoplegic, basilar, or hemiplegic migraine. In addition, nine studies excluded participants if they had previously taken sumatriptan, while four studies required participants to have experience of sumatriptan to be eligible.
The baseline headache intensity at which study medication was administered varied amongst the included studies. The majority (35/61) administered the study drug when migraine headache pain was of moderate or severe intensity, but 14 studies required that medication should be taken at the first recognised signs of migraine attack, and six studies explicitly required the migraine to still be in the mild pain phase when treated. Six studies did not report the baseline headache intensity at which study medication was administered. Those studies requiring that medication be taken at the first recognised signs of migraine attack but not explicitly requiring pain to still be mild were dominated by participants treating moderate or severe attacks and provided data based specifically on this population. Similarly, in those studies not reporting the baseline headache intensity required for treatment, the vast majority of participants had moderate or severe migraine attacks at the time of dosing, and data were provided specifically on those participants.
Most of the included studies used a parallel‐group design (53/61), treating a single migraine attack (34/61). Of those studies treating multiple attacks, most treated either two or three separate attacks (7 and 15 studies, respectively). The response of headaches to study treatment was measured using a standard four‐point pain intensity scale in all 61 studies. The majority of the studies (58/61) reported at least one IHS‐preferred outcome (IHS 2000); three studies reported only secondary outcomes. Just over half of the studies (31/61) offered participants the option of a second dose of study medication if either the initial response had been inadequate, or the participant experienced recurrence (defined as a relapse of moderate or severe intensity headache after an initial response) (14 studies); or to treat recurrence alone (17 studies). All studies but one reported allowing rescue medication if the response to study treatment was insufficient after a defined time period. Forty‐two studies allowed some form of rescue medication after two hours, and 18 studies allowed it after four hours (one study reported allowing rescue medication but did not report at what time). In some cases rescue medication was available to treat recurrence as well as inadequate response, but most studies did not address this question specifically.
Twenty‐four studies used only a placebo comparator, 13 studies used only active comparators, and 24 used both active and placebo comparators. The 61 studies reported on 57 different treatment comparisons.
Sumatriptan 25 mg versus placebo (160‐104; Cutler 1995; Goldstein 1998; Kolodny 2004; Pfaffenrath 1998; Sargent 1995).
Sumatriptan 25 mg versus isometheptene mucate + dichloralphenazone + acetaminophen (Freitag 2001).
Sumatriptan 25 mg versus zolmitriptan 2.5 mg (Gallagher 2000).
Sumatriptan 25 mg versus zolmitriptan 5 mg (Gallagher 2000).
Sumatriptan 25 mg versus rizatriptan 5 mg (Goldstein 1998; Kolodny 2004).
Sumatriptan 25 mg versus rizatriptan 10 mg (Goldstein 1998; Kolodny 2004).
Sumatriptan 25 mg versus eletriptan 40 mg (160‐104).
Sumatriptan 25 mg versus eletriptan 80 mg (160‐104).
Sumatriptan 50 mg versus placebo (160‐104; Bussone 2000; Carpay 2004; Cutler 1995; Dahlof 2009; Diener 2004a; Diener 2004b; Goldstein 1998; Goldstein 2005; Ishkanian 2007; Jelinski 2006; Kolodny 2004; Kudrow 2005; Lines 2001; Lipton 2000; Nett 2003; Pfaffenrath 1998; Pini 1999; Sandrini 2002; Sargent 1995; Savani 1999; Sheftell 2005 Study 1 and Study 2; Smith 2005; Tfelt‐Hansen 2006; Winner 2003 Study 1 and Study 2).
Sumatriptan 50 mg versus tonabersat 20 mg (Dahlof 2009).
Sumatriptan 50 mg versus tonabersat 40 mg (Dahlof 2009).
Sumatriptan 50 mg versus effervescent acetylsalicylic acid (ASA) 1000 mg (Diener 2004a; Diener 2004b).
Sumatriptan 50 mg versus ibuprofen 400 mg (Diener 2004b).
Sumatriptan 50 mg versus zolmitriptan 2.5 mg (Gallagher 2000; Gruffyd‐Jones 2001).
Sumatriptan 50 mg versus zolmitriptan 5 mg (Gallagher 2000; Gruffyd‐Jones 2001).
Sumatriptan 50 mg versus rizatriptan 5 mg (Goldstein 1998; Kolodny 2004; Lines 2001).
Sumatriptan 50 mg versus rizatriptan 10 mg (Goldstein 1998; Kolodny 2004).
Sumatriptan 50 mg versus paracetamol (acetaminophen) 1000 mg + aspirin 1000 mg + caffeine 260 mg (Goldstein 2005).
Sumatriptan 50 mg versus valdecoxib 20 mg (Kudrow 2005).
Sumatriptan 50 mg versus valdecoxib 40 mg (Kudrow 2005).
Sumatriptan 50 mg versus eletriptan 40 mg (160‐104; Sandrini 2002).
Sumatriptan 50 mg versus eletriptan 80 mg (160‐104; Sandrini 2002).
Sumatriptan 50 mg versus indomethacin 25 mg + prochlorperazine 2 mg + caffeine 75 mg (Indoprocaf) (Sandrini 2007).
Sumatriptan 50 mg versus sumatriptan 50 mg + metoclopramide 10 mg (Schulman 2003).
Sumatriptan 50 mg versus sumatriptan 50 mg + naproxen 500 mg (Smith 2005).
Sumatriptan 50 mg versus naproxen 500 mg (Smith 2005).
Sumatriptan 50 mg versus almotriptan 12.5 mg (Spierings 2001).
Sumatriptan 85 mg versus placebo (Brandes 2007 Study 1 and Study 2).
Sumatriptan 85 mg versus sumatriptan 85 mg + naproxen 500 mg (Brandes 2007 Study 1 and Study 2).
Sumatriptan 85 mg versus naproxen 500 mg (Brandes 2007 Study 1 and Study 2).
Sumatriptan 100 mg versus placebo (Carpay 2004; Cutler 1995; Dahlof 1991; DKSMSG 1999; Dodick 2002; Dowson 2002; Ensink 1991; Geraud 2000; Goadsby 1991; Goadsby 2000; Havanka 2000; Jelinski 2006; Kaniecki 2006; Mathew 2003; Myllyla 1998; Nappi 1994; Nett 2003; Patten 1991; Pfaffenrath 1998; Pini 1995; Sandrini 2002; Sargent 1995; Sheftell 2005 Study 1 and Study 2; Tfelt‐Hansen 1995; Tfelt‐Hansen 1998; Visser 1996; Winner 2003 Study 1 and Study 2).
Sumatriptan 100 mg versus diclofenac potassium 50 mg (DKSMSG 1999).
Sumatriptan 100 mg versus diclofenac potassium 100 mg (DKSMSG 1999).
Sumatriptan 100 mg versus almotriptan 12.5 mg (Dodick 2002; Dowson 2002).
Sumatriptan 100 mg versus almotriptan 25 mg (Dowson 2002).
Sumatriptan 100 mg versus zolmitriptan 5 mg (Geraud 2000).
Sumatriptan 100 mg versus paracetamol 1000 mg + metoclopramide (MCP) 10 mg (GL/MIG/001/92; GL/MIG/001A/92).
Sumatriptan 100 mg versus buclizine hydrochloride 12.5 mg + paracetamol 1000 mg + codeine phosphate 16 mg (Migraleve) (GL/MIG/002; GL/MIG/002A).
Sumatriptan 100 mg versus ergotamine tartrate 2 mg + cyclizine hydrochloride 50 mg + caffeine hydrate 100 mg (Migril) (GL/MIG/009).
Sumatriptan 100 mg versus eletriptan 20 mg (Goadsby 2000).
Sumatriptan 100 mg versus eletriptan 40 mg (Goadsby 2000; Mathew 2003; Sandrini 2002).
Sumatriptan 100 mg versus eletriptan 80 mg (Goadsby 2000; Sandrini 2002).
Sumatriptan 100 mg versus naratriptan 1 mg (Havanka 2000).
Sumatriptan 100 mg versus naratriptan 2.5 mg (Havanka 2000).
Sumatriptan 100 mg versus naratriptan 5 mg (Havanka 2000).
Sumatriptan 100 mg versus naratriptan 7.5 mg (Havanka 2000).
Sumatriptan 100 mg versus naratriptan 10 mg (Havanka 2000).
Sumatriptan 100 mg versus ergotamine tartrate 2 mg + caffeine 200 mg (Cafergot) (Latere 1991).
Sumatriptan 100 mg versus tolfenamic acid 200 mg (Myllyla 1998).
Sumatriptan 100 mg versus rizatriptan 5 mg (Tfelt‐Hansen 1998).
Sumatriptan 100 mg versus rizatriptan 10 mg (Tfelt‐Hansen 1998; Visser 1996).
Sumatriptan 100 mg versus rizatriptan 20 mg (Visser 1996).
Sumatriptan 100 mg versus rizatriptan 40 mg (Visser 1996).
Sumatriptan 100 mg versus acetylsalicylic acid (ASA) 900 mg + metoclopramide (MCP) 10 mg (Tfelt‐Hansen 1995; Thomson 1992).
Sumatriptan 200 mg versus placebo (Banerjee 1992; Dahlof 1991; Patten 1991).
Sumatriptan 300 mg versus placebo (Dahlof 1991; Patten 1991).
In total, 2111 participants were treated with sumatriptan 25 mg, 8081 with sumatriptan 50 mg, 849 with sumatriptan 85 mg, 8094 with sumatriptan 100 mg, 460 with sumatriptan 200 mg, 454 with sumatriptan 300 mg, 7016 with placebo, 1103 with naproxen, 855 with sumatriptan 85 mg + naproxen 500 mg, 251 with sumatriptan 50 mg + naproxen 500 mg, 131 with diclofenac potassium 50 mg, 122 with diclofenac potassium 100 mg, 134 with tonabersat 20 mg, 137 with tonabersat 40 mg, 369 with effervescent ASA 1000 mg, 212 with ibuprofen 400 mg, 958 with almotriptan 12.5 mg, 191 with almotriptan 25 mg, 65 with isometheptene mucate + dichloralphenazone + acetaminophen, 878 with zolmitriptan 2.5 mg, 1395 with zolmitriptan 5 mg, 675 with paracetamol 100 mg + MCP 10 mg, 710 with buclizine hydrochloride 12.5 mg + paracetamol 1000 mg + codeine phosphate 16 mg, 258 with ergotamine tartrate 2 mg + cyclizine hydrochloride 50 mg + caffeine hydrate 100 mg, 144 with eletriptan 20 mg, 1317 with eletriptan 40 mg, 485 with eletriptan 80 mg, 1606 with rizatriptan 5 mg, 1590 with rizatriptan 10 mg, 82 with rizatriptan 20 mg, 121 with rizatriptan 40 mg, 69 with acetaminophen 1000 mg + aspirin 1000 mg + caffeine 260 mg, 85 with naratriptan 1 mg, 87 with naratriptan 2.5 mg, 93 with naratriptan 5 mg, 93 with naratriptan 7.5 mg, 96 with naratriptan 10 mg, 137 with valdecoxib 20 mg, 152 with valdecoxib 40 mg, 289 with ergotamine tartrate 2 mg + caffeine 200 mg, 47 with tolfenamic acid 200 mg, 142 with indomethacin 25 mg + prochlorperazine 2 mg + caffeine 75 mg, 16 with sumatriptan 50 mg + MCP 10 mg, and 320 with ASA 900 mg + MCP 10 mg.
Some studies were inconsistent in the treatment group denominators reported, so that the population varied slightly in size for different outcomes or at different time points. Where this variability was not explained in the text, the denominators were changed to match the treated efficacy population if this gave a more conservative estimate of the efficacy of the drug.
Of the 61 included studies, 38 were either directly supported by the manufacturers of sumatriptan (GlaxoSmithKline, Glaxo Wellcome, or Glaxo) and are therefore very likely to have used branded sumatriptan (Imigran or Imitrex), or specifically reported using branded sumatriptan. Only three of the included studies did not report involvement of any pharmaceutical company, and the remaining 20 studies were supported by a different pharmaceutical company. For these 23 studies it is unknown whether branded sumatriptan or the generic equivalent was used; many of them may have used encapsulated branded sumatriptan, which, it has been suggested, is subject to delayed bioavailability and, possibly, reduced efficacy. The effect of this, in this analysis, would be conservative.
Full details of included studies are provided in the Characteristics of included studies table.
Excluded studies
We excluded 23 studies after reading the full report (Cady 1994; Cady 2000; Centonze 1995; Colman 2001; Dowson 2000; Dowson 2005; Ferrari 1994; Gobel 2000; Landy 2004 (Study 1); Midelfart 1994; Padma 1998; Pradel 2005; Rapoport 1995; Rederich 1995; Salonen 1999; Savani 2001; Scott 1996; Sramek 1999; SUMA4014; Tepper 2006; Tfelt‐Hansen 2000; Wells 2001; Wells 2003). The reasons for these exclusions are provided in the Characteristics of excluded studies table.
Risk of bias in included studies
Included studies were all randomised and double‐blind. The majority of the studies provided information about withdrawals and dropouts, although 18 studies either made no statement about withdrawals or did not give an adequate explantation for differing treatment group denominators. The reliability of the trials was determined using the Oxford Quality Scale. Thirteen studies scored 5 of 5 on the scale, 19 studies scored 4 of 5, 15 studies scored 3 of 5, and 11 studies scored 2 of 5. Points were lost due to inadequate description of the methods of randomisation or double‐blinding, and also lack of information about withdrawals and dropouts. Details are provided in the Characteristics of included studies table.
In addition, we created a 'Risk of bias' table which considered random sequence generation, allocation concealment, blinding, and study size (Figure 1). No studies were considered to be at high risk of bias from random sequence generation, allocation concealment, or blinding. Six studies (Banerjee 1992; Goldstein 2005; Myllyla 1998; Sargent 1995; Schulman 2003; Tfelt‐Hansen 2006) did not include 50 or more participants in each treatment arm and were therefore considered to be at high risk of bias from their size.
1.
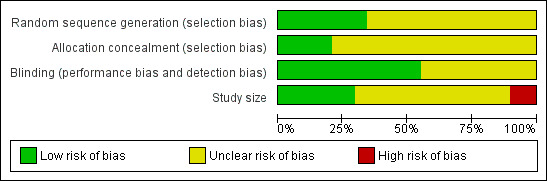
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Effects of interventions
Details of results for efficacy in individual studies are provided in Appendix 5.
Although two very similar studies (Brandes 2007 Study 1 and Study 2) reported on the efficacy and safety of sumatriptan 85 mg verus placebo and active comparators, we chose not to analyse the data for this dose of sumatriptan. Sumatriptan at a dose of 85 mg is not available outside of a combination treatment (sumatriptan plus naproxen) which is reviewed elsewhere (Law 2010), and falls between the two commonly used doses of sumatriptan, so does not contribute to our understanding of any potential dose response relationship.
Pain‐free at two hours
Sumatriptan 25 mg versus placebo
Three studies (1108 participants) in participants with moderate or severe baseline pain provided data (160‐104; Cutler 1995; Goldstein 1998).
The proportion of participants pain‐free at two hours with sumatriptan 25 mg was 25% (201/809; range 16% to 28%).
The proportion of participants pain‐free at two hours with placebo was 9% (26/299; range 8% to 9%).
The relative benefit of treatment compared with placebo was 2.7 (1.8 to 4.0; Analysis 1.1); the NNT was 6.2 (4.9 to 8.5).
1.1. Analysis.
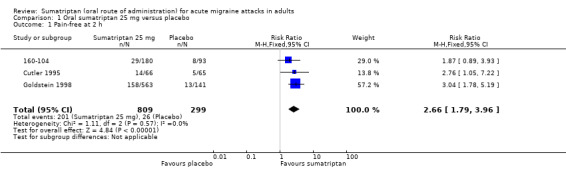
Comparison 1 Oral sumatriptan 25 mg versus placebo, Outcome 1 Pain‐free at 2 h.
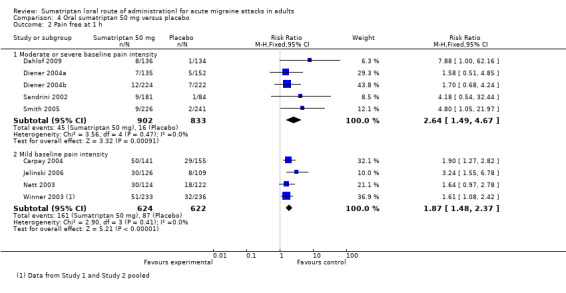
Sumatriptan 50 mg versus placebo
Thirteen studies (6447 participants) in participants with moderate or severe baseline pain intensity provided data (160‐104; Cutler 1995; Dahlof 2009; Diener 2004a; Diener 2004b; Goldstein 1998; Ishkanian 2007; Lipton 2000; Sandrini 2002; Savani 1999; Sheftell 2005 Study 1 and Study 2; Smith 2005).
The proportion of participants pain‐free at two hours with sumatriptan 50 mg was 28% (1080/3922; range 16% to 40%).
The proportion of participants pain‐free at two hours with placebo was 11% (282/2525; range 3% to 21%).
The relative benefit of treatment compared with placebo was 2.7 (2.4 to 3.1; Analysis 4.1; Figure 2); the NNT was 6.1 (5.5 to 6.9).
4.1. Analysis.
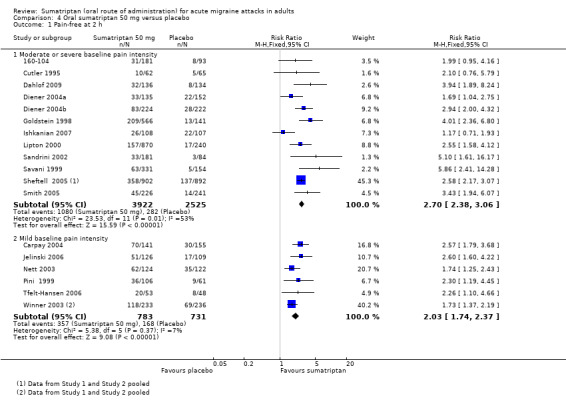
Comparison 4 Oral sumatriptan 50 mg versus placebo, Outcome 1 Pain‐free at 2 h.
2.
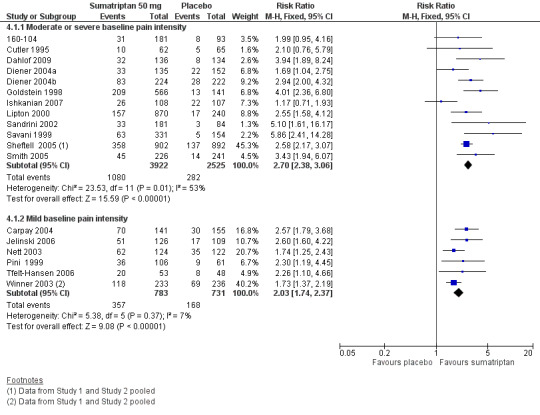
Forest plot of comparison: 4 Oral sumatriptan 50 mg versus placebo, outcome: 4.1 Pain‐free at 2 h.
Seven studies (1514 participants) in participants with mild baseline pain intensity provided data (Carpay 2004; Jelinski 2006; Nett 2003; Pini 1999; Tfelt‐Hansen 2006; Winner 2003 Study 1 and Study 2).
The proportion of participants pain‐free at two hours with sumatriptan 50 mg was 46% (357/783; range 34% to 51%).
The proportion of participants pain‐free at two hours with placebo was 23% (168/731; range 15% to 29%).
The relative benefit of treatment compared with placebo was 2.0 (1.7 to 2.4; Analysis 4.1); the NNT was 4.4 (3.8 to 5.7).
Treating early, while headache was still in the mild pain phase was significantly more effective than treating established moderate or severe headache pain (z = 2.283; P = 0.023; see Summary of results B).
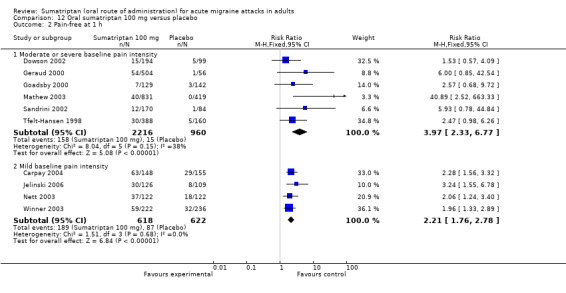
Sumatriptan 100 mg versus placebo
Sixteen studies (6571 participants) in participants with moderate or severe baseline pain intensity provided data (Cutler 1995; Dodick 2002; Dowson 2002; Ensink 1991; Geraud 2000; Goadsby 2000; Kaniecki 2006; Mathew 2003; Myllyla 1998; Nappi 1994; Sandrini 2002; Sheftell 2005 Study 1 and Study 2; Tfelt‐Hansen 1995; Tfelt‐Hansen 1998; Visser 1996).
The proportion of participants pain‐free at two hours with sumatriptan 100 mg was 32% (1291/4017; range 17% to 50%).
The proportion of participants pain‐free at two hours with placebo was 11% (272/2554; range 4% to 16%).
The relative benefit of treatment compared with placebo was 3.2 (2.8 to 3.6; Analysis 12.1); the NNT was 4.7 (4.3 to 5.1).
12.1. Analysis.
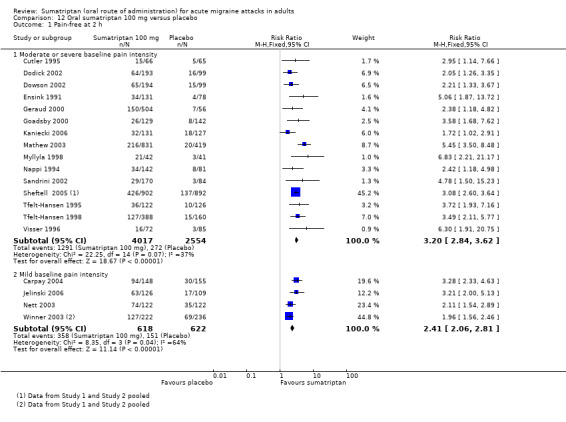
Comparison 12 Oral sumatriptan 100 mg versus placebo, Outcome 1 Pain‐free at 2 h.
Sumatriptan 100 mg was significantly more effective than sumatriptan 50 mg in participants with moderate or severe baseline pain intensity (z = 3.451; P = 0.0007; see Summary of results B).
Five studies (1240 participants) in participants with mild baseline pain intensity provided data (Carpay 2004; Jelinski 2006; Nett 2003; Winner 2003 Study 1 and Study 2).
The proportion of participants pain‐free at two hours with sumatriptan 100 mg was 58% (358/618; range 50% to 64%).
The proportion of participants pain‐free at two hours with placebo was 24% (151/622; range 16% to 29%).
The relative benefit of treatment compared with placebo was 2.4 (2.1 to 2.8; Analysis 12.1); the NNT was 3.0 (2.6 to 3.5).
Treating early, while headache was still in the mild pain phase was significantly more effective than treating established moderate or severe headache pain (z = 4.351; P < 0.00006).
Sumatriptan 100 mg was significantly more effective than sumatriptan 50 mg in participants with mild baseline pain intensity (z = 3.124; P = 0.002; see Summary of results B).
Sumatriptan 25 mg versus rizatriptan 5 mg
Two studies (2210 participants) in participants with moderate or severe baseline pain provided data (Goldstein 1998; Kolodny 2004).
The proportion of participants pain‐free at two hours with sumatriptan 25 mg was 28% (310/1117; range 27% to 28%).
The proportion of participants pain‐free at two hours with rizatriptan 5 mg was 33% (363/1093; range 33% to 33%).
The relative benefit of sumatriptan compared with rizatriptan was 0.84 (0.74 to 0.95; Analysis 2.1); the NNT was 18 (11 to 62) in favour of rizatriptan.
2.1. Analysis.
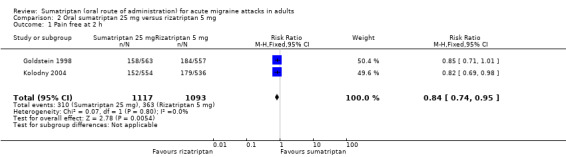
Comparison 2 Oral sumatriptan 25 mg versus rizatriptan 5 mg, Outcome 1 Pain free at 2 h.
Sumatriptan 25 mg versus rizatriptan 10 mg
Two studies (2231 participants) in participants with moderate or severe baseline pain provided data (Goldstein 1998; Kolodny 2004).
The proportion of participants pain‐free at two hours with sumatriptan 25 mg was 28% (310/1117; range 27% to 28%).
The proportion of participants pain‐free at two hours with rizatriptan 10 mg was 39% (440/1114; range 38% to 41%).
The relative benefit of sumatriptan compared with rizatriptan was 0.70 (0.62 to 0.79; Analysis 3.1); the NNT was 8.5 (6.4 to 13) in favour of rizatriptan.
3.1. Analysis.
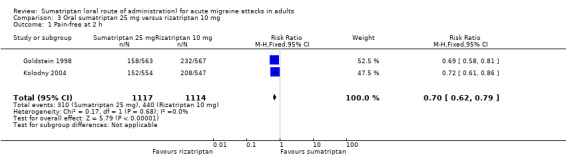
Comparison 3 Oral sumatriptan 25 mg versus rizatriptan 10 mg, Outcome 1 Pain‐free at 2 h.
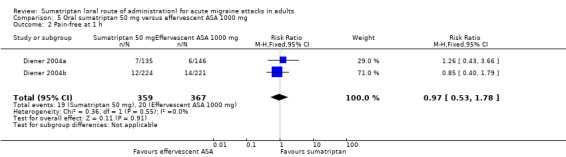
Sumatriptan 50 mg versus effervescent acetylsalicylic acid 1000 mg
Two studies (726 participants) in participants with moderate or severe baseline pain provided data (Diener 2004a; Diener 2004b).
The proportion of participants pain‐free at two hours with sumatriptan 50 mg was 32% (116/359; range 24% to 37%).
The proportion of participants pain‐free at two hours with effervescent ASA 1000 mg was 26% (97/367; range 25% to 27%).
The relative benefit of sumatriptan compared with effervescent ASA was 1.2 (0.97 to 1.5; Analysis 5.1); there was no significant difference between treatments.
5.1. Analysis.
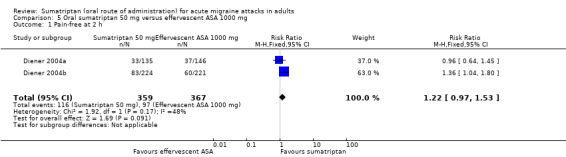
Comparison 5 Oral sumatriptan 50 mg versus effervescent ASA 1000 mg, Outcome 1 Pain‐free at 2 h.
Sumatriptan 50 mg versus rizatriptan 5 mg
Two studies (2209 participants) provided data in participants with moderate or severe baseline pain (Goldstein 1998; Kolodny 2004).
The proportion of participants pain‐free at two hours with sumatriptan 50 mg was 35% (394/1116; range 34% to 37%).
The proportion of participants pain‐free at two hours with rizatriptan 5 mg was 33% (363/1093; range 33% to 33%).
The relative benefit of sumatriptan compared with rizatriptan was 1.1 (0.95 to 1.2; Analysis 8.1); there was no significant difference between treatments.
8.1. Analysis.
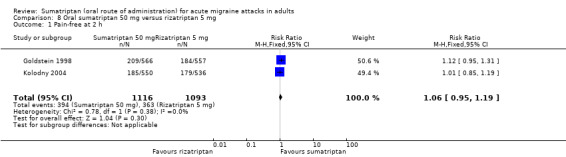
Comparison 8 Oral sumatriptan 50 mg versus rizatriptan 5 mg, Outcome 1 Pain‐free at 2 h.
Sumatriptan 50 mg versus rizatriptan 10 mg
Two studies (2230 participants) in participants with moderate or severe baseline pain provided data (Goldstein 1998; Kolodny 2004).
The proportion of participants pain‐free at two hours with sumatriptan 50 mg was 35% (394/1116; range 34% to 37%).
The proportion of participants pain‐free at two hours with rizatriptan 10 mg was 39% (440/1114; range 38% to 41%).
The relative benefit of sumatriptan compared with rizatriptan was 0.89 (0.80 to 1.0; Analysis 9.1); there was no significant difference between treatments.
9.1. Analysis.
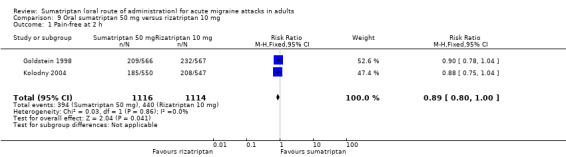
Comparison 9 Oral sumatriptan 50 mg versus rizatriptan 10 mg, Outcome 1 Pain‐free at 2 h.
Sumatriptan 50 mg versus eletriptan 40 mg
Two studies (721 participants) in participants with moderate or severe baseline pain provided data (160‐104; Sandrini 2002).
The proportion of participants pain‐free at two hours with sumatriptan 50 mg was 18% (64/362; range 17% to 18%).
The proportion of participants pain‐free at two hours with eletriptan 40 mg was 24% (86/359; range 18% to 30%).
The relative benefit of sumatriptan compared with eletriptan was 0.74 (0.55 to 0.98; Analysis 10.1); the NNT was 16 (8.2 to 270) in favour of eletriptan.
10.1. Analysis.
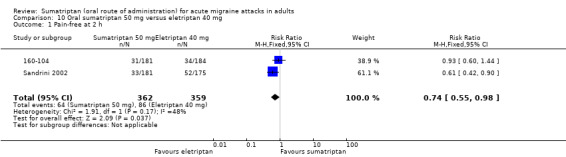
Comparison 10 Oral sumatriptan 50 mg versus eletriptan 40 mg, Outcome 1 Pain‐free at 2 h.
Sumatriptan 50 mg versus eletriptan 80 mg
Two studies (706 participants) in participants with moderate or severe baseline pain provided data (160‐104; Sandrini 2002).
The proportion of participants pain‐free at two hours with sumatriptan 50 mg was 18% (64/362; range 17% to 18%).
The proportion of participants pain‐free at two hours with eletriptan 40 mg was 30% (104/344; range 25% to 36%).
The relative benefit of sumatriptan compared with eletriptan was 0.58 (0.44 to 0.76; Analysis 11.1); the NNT was 8.0 (5.3 to 16) in favour of eletriptan.
11.1. Analysis.
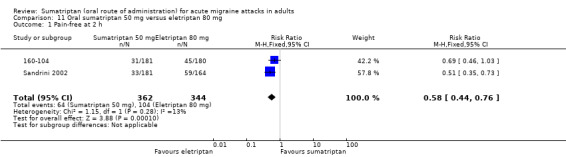
Comparison 11 Oral sumatriptan 50 mg versus eletriptan 80 mg, Outcome 1 Pain‐free at 2 h.
Sumatriptan 100 mg versus eletriptan 40 mg
Three studies (2263 participants) in participants with moderate or severe baseline pain provided data (Goadsby 2000; Mathew 2003; Sandrini 2002).
The proportion of participants pain‐free at two hours with sumatriptan 100 mg was 24% (271/1130; range 17% to 26%).
The proportion of participants pain‐free at two hours with eletriptan 40 mg was 32% (366/1133; range 25% to 34%).
The relative benefit of sumatriptan compared with eletriptan was 0.74 (0.65 to 0.85; Analysis 13.1); the NNT was 12 (8.3 to 22) in favour of eletriptan.
13.1. Analysis.
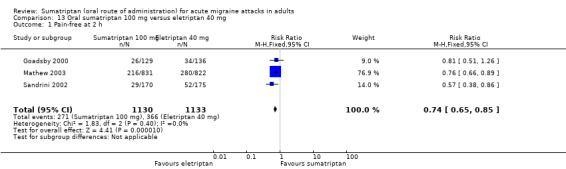
Comparison 13 Oral sumatriptan 100 mg versus eletriptan 40 mg, Outcome 1 Pain‐free at 2 h.
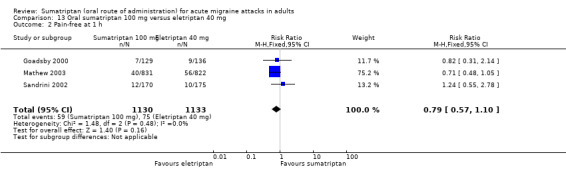
Sumatriptan 100 mg versus eletriptan 80 mg
Two studies (604 participants) in participants with moderate or severe baseline pain provided data (Goadsby 2000; Sandrini 2002).
The proportion of participants pain‐free at two hours with sumatriptan 100 mg was 18% (55/299; range 17% to 20%).
The proportion of participants pain‐free at two hours with eletriptan 80 mg was 34% (103/305; range 31% to 36%).
The relative benefit of sumatriptan compared with eletriptan was 0.54 (0.41 to 0.72; Analysis 14.1); the NNT was 6.5 (4.5 to 12) in favour of eletriptan.
14.1. Analysis.
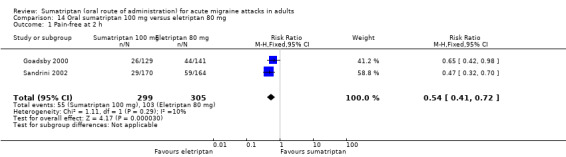
Comparison 14 Oral sumatriptan 100 mg versus eletriptan 80 mg, Outcome 1 Pain‐free at 2 h.
Sumatriptan 100 mg versus rizatriptan 10 mg
Two studies (936 participants) in participants with moderate or severe baseline pain provided data (Tfelt‐Hansen 1998; Visser 1996).
The proportion of participants pain‐free at two hours with sumatriptan 100 mg was 31% (143/460; range 22% to 33%).
The proportion of participants pain‐free at two hours with rizatriptan 10 mg was 37% (178/476; range 26% to 40%).
The relative benefit of sumatriptan compared with rizatriptan was 0.82 (0.69 to 0.98; Analysis 15.1); the NNT was 16 (8.1 to 410) in favour of rizatriptan.
15.1. Analysis.
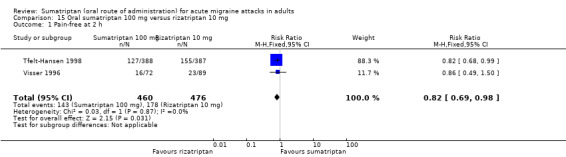
Comparison 15 Oral sumatriptan 100 mg versus rizatriptan 10 mg, Outcome 1 Pain‐free at 2 h.
Sumatriptan 100 mg versus almotriptan 12.5 mg
Two studies (754 participants) in participants with moderate or severe baseline pain provided data (Dodick 2002; Dowson 2002).
The proportion of participants pain‐free at two hours with sumatriptan 100 mg was 33% (129/387; range 33% to 34%).
The proportion of participants pain‐free at two hours with almotriptan 12.5 mg was 28% (102/367; range 28% to 28%).
The relative benefit of sumatriptan compared with almotriptan was 1.2 (0.97 to 1.5; Analysis 16.1); there was no significant difference between treatments.
16.1. Analysis.
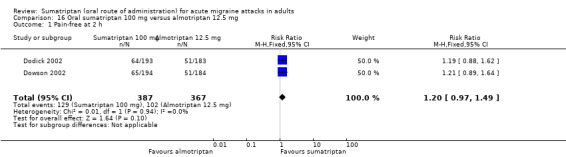
Comparison 16 Oral sumatriptan 100 mg versus almotriptan 12.5 mg, Outcome 1 Pain‐free at 2 h.
Sumatriptan 100 mg versus acetylsalicylic acid 900 mg + metoclopramide 10 mg
Two studies (575 participants) in participants with moderate or severe baseline pain provided data (Tfelt‐Hansen 1995; Thomson 1992).
The proportion of participants pain‐free at two hours with sumatriptan 100 mg was 26% (71/275; range 23% to 30%).
The proportion of participants pain‐free at two hours with ASA + MCP was 16% (48/300; range 12% to 21%).
The relative benefit of sumatriptan compared with ASA + MCP was 1.6 (1.2 to 2.3; Analysis 18.1); the NNT was 10 (6.1 to 31).
18.1. Analysis.
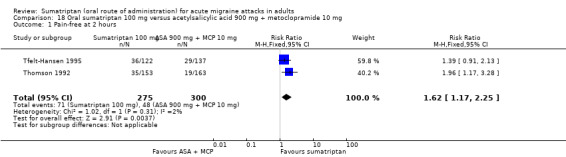
Comparison 18 Oral sumatriptan 100 mg versus acetylsalicylic acid 900 mg + metoclopramide 10 mg, Outcome 1 Pain‐free at 2 hours.
Pain‐free at one hour
Sumatriptan 50 mg versus placebo
Five studies (1735 participants) in participants with moderate or severe baseline pain intensity provided data (Dahlof 2009; Diener 2004a; Diener 2004b; Sandrini 2002; Smith 2005).
The proportion of participants pain‐free at one hour with sumatriptan 50 mg was 5% (45/902; range 4% to 6%).
The proportion of participants pain‐free at one hour with placebo was 2% (16/833; range 1% to 3%).
The relative benefit of treatment compared with placebo was 2.6 (1.5 to 4.6; Analysis 4.2); the NNT was 33 (21 to 73).
4.2. Analysis.
Comparison 4 Oral sumatriptan 50 mg versus placebo, Outcome 2 Pain free at 1 h.
Five studies (1246 participants) in participants with mild baseline pain intensity provided data (Carpay 2004; Jelinski 2006; Nett 2003; Winner 2003 Study 1 and Study 2).
The proportion of participants pain‐free at one hour with sumatriptan 50 mg was 26% (161/624; range 22% to 35%).
The proportion of participants pain‐free at one hour with placebo was 14% (87/622; range 7% to 19%).
The relative benefit of treatment compared with placebo was 1.9 (1.5 to 2.4; Analysis 4.2); the NNT was 8.5 (6.2 to 13).
Sumatriptan 100 mg versus placebo
Six studies (3176 participants) in participants with moderate or severe baseline pain intensity provided data (Dowson 2002; Geraud 2000; Goadsby 2000; Mathew 2003; Sandrini 2002; Tfelt‐Hansen 1998).
The proportion of participants pain‐free at one hour with sumatriptan 100 mg was 7% (158/2216; range 5% to 11%).
The proportion of participants pain‐free at one hour with placebo was 2% (15/960; range 0% to 2%).
The relative benefit of treatment compared with placebo was 4.0 (2.3 to 6.8; Analysis 12.2); the NNT was 18 (15 to 24).
12.2. Analysis.
Comparison 12 Oral sumatriptan 100 mg versus placebo, Outcome 2 Pain‐free at 1 h.
Five studies (1240 participants) in participants with mild baseline pain intensity provided data (Carpay 2004; Jelinski 2006; Nett 2003; Winner 2003 Study 1 and Study 2).
The proportion of participants pain‐free at one hour with sumatriptan 100 mg was 31% (189/618; range 24% to 43%).
The proportion of participants pain‐free at one hour with placebo was 14% (87/622; range 7% to 19%).
The relative benefit of treatment compared with placebo was 2.2 (1.8 to 2.8; Analysis 12.2); the NNT was 6.0 (4.7 to 8.3).
Sumatriptan 50 mg versus effervescent acetylsalicylic acid 1000 mg
Two studies (726 participants) in participants with moderate or severe baseline pain intensity provided data (Diener 2004a; Diener 2004b).
The proportion of participants pain‐free at one hour with sumatriptan 50 mg was 5% (19/359).
The proportion of participants pain‐free at one hour with effervescent ASA 1000 mg was 5% (20/367; range 4% to 6%).
The relative benefit of sumatriptan compared with effervescent ASA was 0.97 (0.53 to 1.8; Analysis 5.2); there was no significant difference between treatments.
5.2. Analysis.
Comparison 5 Oral sumatriptan 50 mg versus effervescent ASA 1000 mg, Outcome 2 Pain‐free at 1 h.
Sumatriptan 100 mg versus eletriptan 40 mg
Three studies (2263 participants) in participants with moderate or severe baseline pain intensity provided data (Goadsby 2000; Mathew 2003; Sandrini 2002).
The proportion of participants pain‐free at one hour with sumatriptan 100 mg was 5% (59/1130; range 5% to 7%).
The proportion of participants pain‐free at one hour with eletriptan 40 mg was 7% (75/1133; range 6% to 7%).
The relative benefit of sumatriptan compared with eletriptan was 0.79 (0.57 to 1.1; Analysis 13.2); there was no significant difference between treatments.
13.2. Analysis.
Comparison 13 Oral sumatriptan 100 mg versus eletriptan 40 mg, Outcome 2 Pain‐free at 1 h.
Sumatriptan 100 mg versus eletriptan 80 mg
Two studies (604 participants) in participants with moderate or severe baseline pain intensity provided data (Goadsby 2000; Sandrini 2002).
The proportion of participants pain‐free at one hour with sumatriptan 100 mg was 6% (19/299; range 5% to 7%).
The proportion of participants pain‐free at one hour with eletriptan 80 mg was 13% (40/305; range 12% to 14%).
The relative benefit of sumatriptan compared with eletriptan was 0.48 (0.29 to 0.82; Analysis 14.2); the NNT was 15 (8.7 to 48) in favour of eletriptan.
14.2. Analysis.
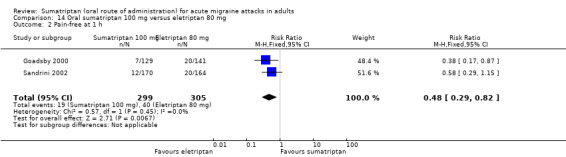
Comparison 14 Oral sumatriptan 100 mg versus eletriptan 80 mg, Outcome 2 Pain‐free at 1 h.
Headache relief at one hour
All participants experiencing outcomes of headache relief must, by definition, have had moderate to severe pain at baseline.
Sumatriptan 25 mg versus placebo
Three studies (745 participants) provided data (160‐104; Pfaffenrath 1998; Sargent 1995).
The proportion of participants with headache relief at one hour with sumatriptan 25 mg was 27% (137/514; range 23% to 29%).
The proportion of participants with headache relief at one hour with placebo was 16% (36/231; range 6% to 22%).
The relative benefit of treatment compared with placebo was 1.6 (1.2 to 2.3; Analysis 1.2); the NNT was 9.0 (5.8 to 20).
1.2. Analysis.
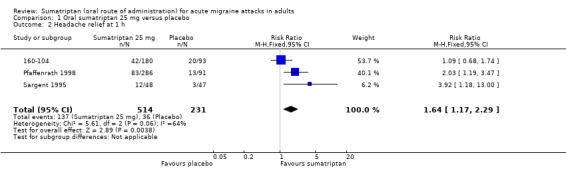
Comparison 1 Oral sumatriptan 25 mg versus placebo, Outcome 2 Headache relief at 1 h.
Sumatriptan 50 mg versus placebo
Nine studies (2766 participants) provided data (160‐104; Diener 2004a; Diener 2004b; Goldstein 2005; Pfaffenrath 1998; Sandrini 2002; Sargent 1995; Savani 1999; Smith 2005).
The proportion of participants with headache relief at one hour with sumatriptan 50 mg was 27% (454/1655; range 13% to 50%).
The proportion of participants with headache relief at one hour with placebo was 14% (157/1111; range 6% to 30%).
The relative benefit of treatment compared with placebo was 1.8 (1.5 to 2.1; Analysis 4.3); the NNT was 7.5 (6.2 to 9.7).
4.3. Analysis.
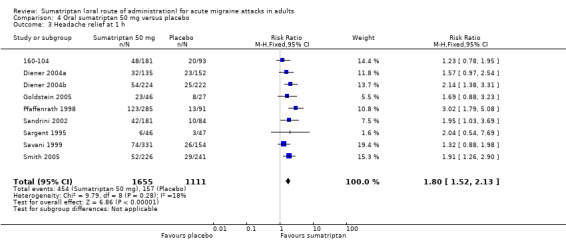
Comparison 4 Oral sumatriptan 50 mg versus placebo, Outcome 3 Headache relief at 1 h.
Sumatriptan 100 mg versus placebo
Ten studies (3983 participants) provided data (Dowson 2002; Geraud 2000; Goadsby 2000; Havanka 2000; Mathew 2003; Pfaffenrath 1998; Sandrini 2002; Sargent 1995; Tfelt‐Hansen 1998; Visser 1996).
The proportion of participants with headache relief at one hour with sumatriptan 100 mg was 29% (795/2709; range 18% to 38%).
The proportion of participants with headache relief at one hour with placebo was 15% (187/1274; range 6% to 29%).
The relative benefit of treatment compared with placebo was 1.9 (1.6 to 2.2; Analysis 12.3); the NNT was 6.8 (5.8 to 8.3).
12.3. Analysis.
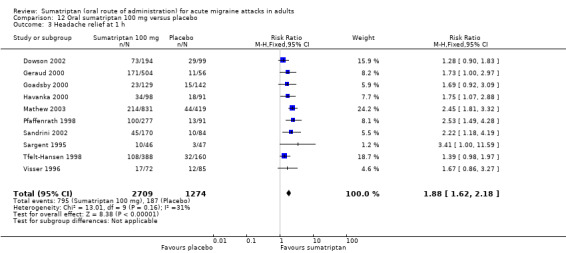
Comparison 12 Oral sumatriptan 100 mg versus placebo, Outcome 3 Headache relief at 1 h.
Sumatriptan 25 mg versus rizatriptan 5 mg
Two studies (2210 participants) provided data (Goldstein 1998; Kolodny 2004).
The proportion of participants with headache relief at one hour with sumatriptan 25 mg was 34% (375/1117; range 33% to 34%).
The proportion of participants with headache relief at one hour with rizatriptan 5 mg was 37% (404/1093; range 36% to 38%).
The relative benefit of sumatriptan compared with rizatriptan was 0.91 (0.81 to 1.0; Analysis 2.2); the NNT was 29 (14 to 170) in favour of rizatriptan.
2.2. Analysis.
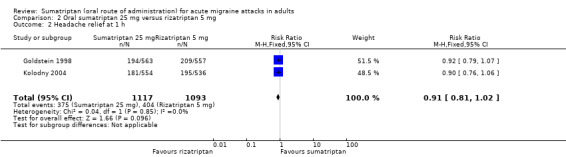
Comparison 2 Oral sumatriptan 25 mg versus rizatriptan 5 mg, Outcome 2 Headache relief at 1 h.
Sumatriptan 25 mg versus rizatriptan 10 mg
Two studies (2231 participants) provided data (Goldstein 1998; Kolodny 2004).
The proportion of participants with headache relief at one hour with sumatriptan 25 mg was 34% (375/1117; range 33% to 34%).
The proportion of participants with headache relief at one hour with rizatriptan 10 mg was 41% (456/1114; range 40% to 42%).
The relative benefit of sumatriptan compared with rizatriptan was 0.82 (0.74 to 0.91; Analysis 3.2); the NNT was 14 (8.8 to 30) in favour of rizatriptan.
3.2. Analysis.
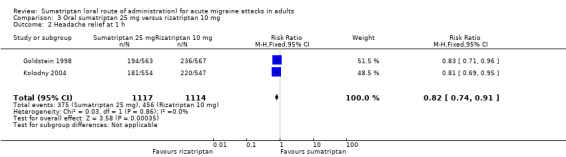
Comparison 3 Oral sumatriptan 25 mg versus rizatriptan 10 mg, Outcome 2 Headache relief at 1 h.
Sumatriptan 50 mg versus effervescent acetylsalicylic acid 1000 mg
Two studies (726 participants) provided data (Diener 2004a; Diener 2004b).
The proportion of participants with headache relief at one hour with sumatriptan 50 mg was 24% (86/359).
The proportion of participants with headache relief at one hour with effervescent ASA 1000 mg was 31% (113/367; range 25% to 34%).
The relative benefit of sumatriptan compared with effervescent ASA was 0.78 (0.61 to 0.98; Analysis 5.3); the NNT was 15 (7.5 to 270) in favour of effervescent ASA.
5.3. Analysis.
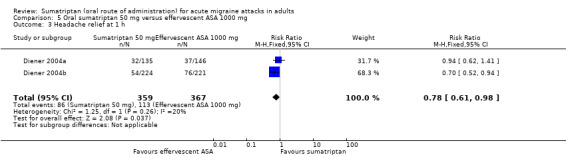
Comparison 5 Oral sumatriptan 50 mg versus effervescent ASA 1000 mg, Outcome 3 Headache relief at 1 h.
Sumatriptan 50 mg versus zolmitriptan 2.5 mg
Two studies (1609 participants) provided data (Gallagher 2000; Gruffyd‐Jones 2001).
The proportion of participants with headache relief at one hour with sumatriptan 50 mg was 41% (330/814; range 35% to 44%).
The proportion of participants with headache relief at one hour with zolmitriptan 2.5 mg was 40% (318/795; range 35% to 43%).
The relative benefit of sumatriptan compared with zolmitriptan was 1.0 (0.90 to 1.1; Analysis 6.1); there was no significant difference between treatments.
6.1. Analysis.
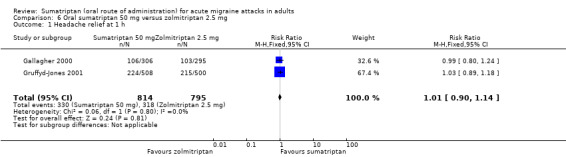
Comparison 6 Oral sumatriptan 50 mg versus zolmitriptan 2.5 mg, Outcome 1 Headache relief at 1 h.
Sumatriptan 50 mg versus zolmitriptan 5 mg
Two studies (1633 participants) provided data (Gallagher 2000; Gruffyd‐Jones 2001).
The proportion of participants with headache relief at one hour with sumatriptan 50 mg was 41% (330/814; range 35% to 44%).
The proportion of participants with headache relief at one hour with zolmitriptan 5 mg was 39% (320/819; range 37% to 40%).
The relative benefit of sumatriptan compared with zolmitriptan was 1.0 (0.90 to 1.2; Analysis 7.1); there was no significant difference between treatments.
7.1. Analysis.
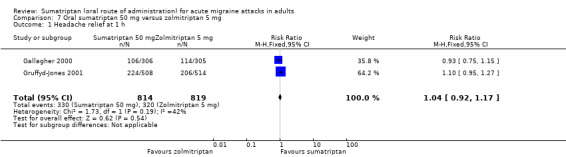
Comparison 7 Oral sumatriptan 50 mg versus zolmitriptan 5 mg, Outcome 1 Headache relief at 1 h.
Sumatriptan 50 mg versus rizatriptan 5 mg
Two studies (2209 participants) provided data (Goldstein 1998; Kolodny 2004).
The proportion of participants with headache relief at one hour with sumatriptan 50 mg was 37% (409/1116; range 35% to 39%).
The proportion of participants with headache relief at one hour with rizatriptan 5 mg was 37% (404/1093; range 36% to 38%).
The relative benefit of sumatriptan compared with rizatriptan was 0.99 (0.89 to 1.1; Analysis 8.2); there was no significant difference between treatments.
8.2. Analysis.
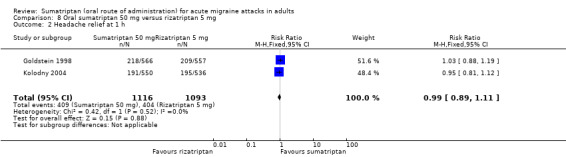
Comparison 8 Oral sumatriptan 50 mg versus rizatriptan 5 mg, Outcome 2 Headache relief at 1 h.
Sumatriptan 50 mg versus rizatriptan 10 mg
Two studies (2230 participants) provided data (Goldstein 1998; Kolodny 2004).
The proportion of participants with headache relief at one hour with sumatriptan 50 mg was 37% (409/1116; range 35% to 39%).
The proportion of participants with headache relief at one hour with rizatriptan 10 mg was 41% (456/1114; range 40% to 42%).
The relative benefit of sumatriptan compared with rizatriptan was 0.90 (0.81 to 1.0; Analysis 9.2); there was no significant difference between treatments.
9.2. Analysis.
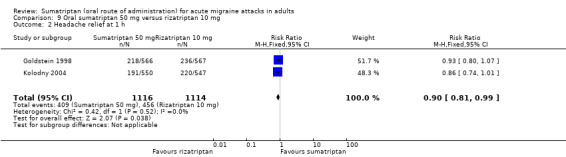
Comparison 9 Oral sumatriptan 50 mg versus rizatriptan 10 mg, Outcome 2 Headache relief at 1 h.
Sumatriptan 50 mg versus eletriptan 40 mg
Two studies (721 participants) provided data (160‐104; Sandrini 2002).
The proportion of participants with headache relief at one hour with sumatriptan 50 mg was 25% (90/362; range 23% to 27%).
The proportion of participants with headache relief at one hour with eletriptan 40 mg was 25% (90/359; range 21% to 30%).
The relative benefit of sumatriptan compared with eletriptan was 0.99 (0.77 to 1.3; Analysis 10.2); there was no significant difference between treatments.
10.2. Analysis.
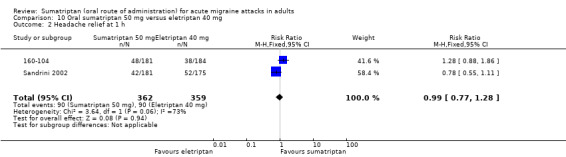
Comparison 10 Oral sumatriptan 50 mg versus eletriptan 40 mg, Outcome 2 Headache relief at 1 h.
Sumatriptan 50 mg versus eletriptan 80 mg
Two studies (706 participants) provided data (160‐104; Sandrini 2002).
The proportion of participants with headache relief at one hour with sumatriptan 50 mg was 25% (90/362; range 23% to 27%).
The proportion of participants with headache relief at one hour with eletriptan 80 mg was 35% (119/344; range 34% to 35%).
The relative benefit of sumatriptan compared with eletriptan was 0.72 (0.57 to 0.91; Analysis 11.2); the NNT was 10 (6.1 to 33) in favour of eletriptan.
11.2. Analysis.
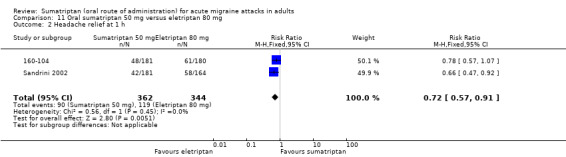
Comparison 11 Oral sumatriptan 50 mg versus eletriptan 80 mg, Outcome 2 Headache relief at 1 h.
Sumatriptan 100 mg versus eletriptan 40 mg
Three studies (2263 participants) provided data (Goadsby 2000; Mathew 2003; Sandrini 2002).
The proportion of participants with headache relief at one hour with sumatriptan 100 mg was 25% (282/1130; range 18% to 26%).
The proportion of participants with headache relief at one hour with eletriptan 40 mg was 32% (368/1133; range 30% to 33%).
The relative benefit of sumatriptan compared with eletriptan was 0.77 (0.67 to 0.88; Analysis 13.3); the NNT was 13 (8.9 to 26) in favour of eletriptan.
13.3. Analysis.
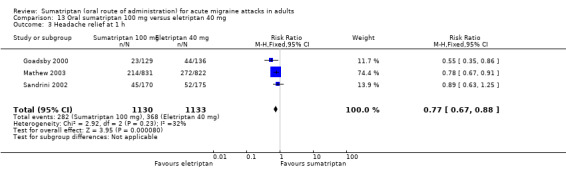
Comparison 13 Oral sumatriptan 100 mg versus eletriptan 40 mg, Outcome 3 Headache relief at 1 h.
Sumatriptan 100 mg versus eletriptan 80 mg
Two studies (604 participants) provided data (Goadsby 2000; Sandrini 2002).
The proportion of participants with headache relief at one hour with sumatriptan 100 mg was 23% (68/299; range 18% to 26%).
The proportion of participants with headache relief at one hour with eletriptan 80 mg was 35% (106/305; range 34% to 35%).
The relative benefit of sumatriptan compared with eletriptan was 0.65 (0.50 to 0.84; Analysis 14.3); the NNT was 8.3 (5.2 to 21) in favour of eletriptan.
14.3. Analysis.
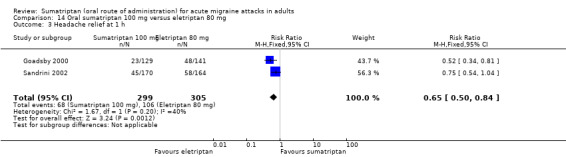
Comparison 14 Oral sumatriptan 100 mg versus eletriptan 80 mg, Outcome 3 Headache relief at 1 h.
Sumatriptan 100 mg versus rizatriptan 10 mg
Two studies (936 participants) provided data (Tfelt‐Hansen 1998; Visser 1996).
The proportion of participants with headache relief at one hour with sumatriptan 100 mg was 26% (120/460; range 24% to 27%).
The proportion of participants with headache relief at one hour with rizatriptan 10 mg was 34% (163/476; range 25% to 36%).
The relative benefit of sumatriptan compared with rizatriptan was 0.76 (0.62 to 0.92; Analysis 15.2); the NNT was 12 (7.1 to 43) in favour of rizatriptan.
15.2. Analysis.
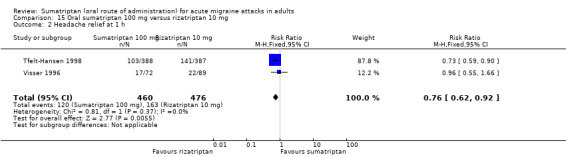
Comparison 15 Oral sumatriptan 100 mg versus rizatriptan 10 mg, Outcome 2 Headache relief at 1 h.
Headache relief at two hours
All participants experiencing outcomes of headache relief must, by definition, have had moderate to severe pain at baseline.
Sumatriptan 25 mg versus placebo
Five studies (1580 participants) provided data (160‐104; Cutler 1995; Goldstein 1998; Pfaffenrath 1998; Sargent 1995).
The proportion of participants with headache relief at two hours with sumatriptan 25 mg was 56% (638/1143; range 49% to 62%).
The proportion of participants with headache relief at two hours with placebo was 32% (140/437; range 17% to 38%).
The relative benefit of treatment compared with placebo was 1.7 (1.4 to 1.9; Analysis 1.3); the NNT was 4.2 (3.5 to 5.4).
1.3. Analysis.
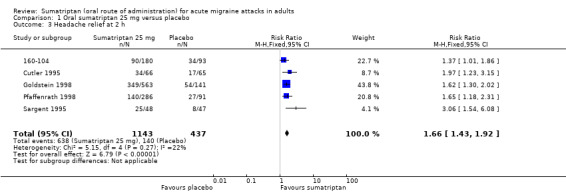
Comparison 1 Oral sumatriptan 25 mg versus placebo, Outcome 3 Headache relief at 2 h.
Sumatriptan 50 mg versus placebo
Nineteen studies (8102 participants) provided data (160‐104; Bussone 2000; Cutler 1995; Dahlof 2009; Diener 2004a; Diener 2004b; Goldstein 1998; Goldstein 2005; Ishkanian 2007; Kudrow 2005; Lines 2001; Lipton 2000; Pfaffenrath 1998; Sandrini 2002; Sargent 1995; Savani 1999; Sheftell 2005 Study 1 and Study 2; Smith 2005).
The proportion of participants with headache relief at two hours with sumatriptan 50 mg was 57% (2822/4955; range 42% to 69%).
The proportion of participants with headache relief at two hours with placebo was 32% (1007/3147; range 17% to 52%).
The relative benefit of treatment compared with placebo was 1.8 (1.7 to 1.9; Analysis 4.4; Figure 3); the NNT was 4.0 (3.7 to 4.4).
4.4. Analysis.
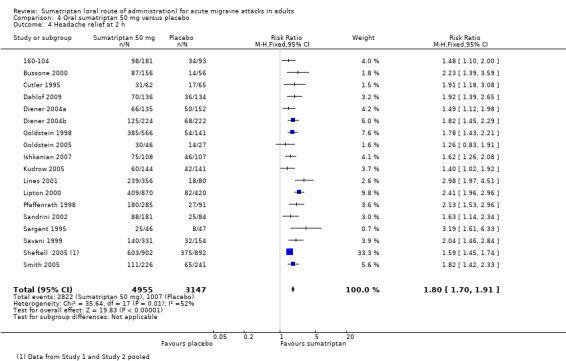
Comparison 4 Oral sumatriptan 50 mg versus placebo, Outcome 4 Headache relief at 2 h.
3.
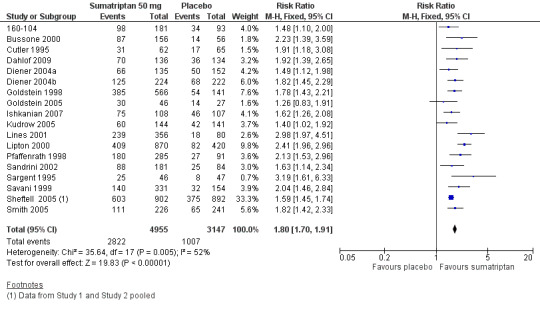
Forest plot of comparison: 4 Oral sumatriptan 50 mg versus placebo, outcome: 4.4 Headache relief at 2 h.
Sumatriptan 100 mg versus placebo
Twenty‐one studies (7811 participants) provided data (Cutler 1995; Dahlof 1991; Dowson 2002; Ensink 1991; Geraud 2000; Goadsby 1991; Goadsby 2000; Havanka 2000; Kaniecki 2006; Mathew 2003; Myllyla 1998; Nappi 1994; Patten 1991; Pfaffenrath 1998; Sandrini 2002; Sargent 1995; Sheftell 2005 Study 1 and Study 2; Tfelt‐Hansen 1995; Tfelt‐Hansen 1998; Visser 1996).
The proportion of participants with headache relief at two hours with sumatriptan 100 mg was 61% (2877/4751; range 46% to 79%).
The proportion of participants with headache relief at two hours with placebo was 32% (967/3060; range 10% to 43%).
The relative benefit of treatment compared with placebo was 1.9 (1.8 to 2.0; Analysis 12.4); the NNT was 3.5 (3.2 to 3.7).
12.4. Analysis.
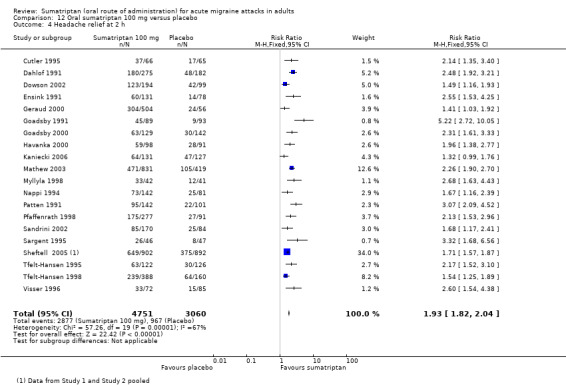
Comparison 12 Oral sumatriptan 100 mg versus placebo, Outcome 4 Headache relief at 2 h.
Sumatriptan 100 mg was significantly more effective than sumatriptan 50 mg (z = 2.407; P = 0.016; see Summary of results B).
Sumatriptan 200 mg versus placebo
Three studies (749 participants) provided data (Banerjee 1992; Dahlof 1991; Patten 1991).
The proportion of participants with headache relief at two hours with sumatriptan 200 mg was 72% (311/429; range 62% to 75%).
The proportion of participants with headache relief at two hours with placebo was 26% (82/320; range 22% to 32%).
The relative benefit of treatment compared with placebo was 2.8 (2.3 to 3.5; Analysis 19.1); the NNT was 2.1 (1.9 to 2.5).
19.1. Analysis.
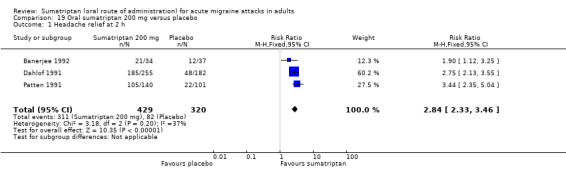
Comparison 19 Oral sumatriptan 200 mg versus placebo, Outcome 1 Headache relief at 2 h.
Sumatriptan 200 mg was significantly more effective than sumatriptan 100 mg (z = 5.212; P < 0.00006).
Sumatriptan 300 mg versus placebo
Two studies (709 participants) provided data (Dahlof 1991; Patten 1991).
The proportion of participants with headache relief at two hours with sumatriptan 300 mg was 67% (286/426; range 66% to 69%).
The proportion of participants with headache relief at two hours with placebo was 25% (70/283; range 22% to 26%).
The relative benefit of treatment compared with placebo was 2.7 (2.2 to 3.4; Analysis 20.1); the NNT was 2.4 (2.0 to 2.8).
20.1. Analysis.
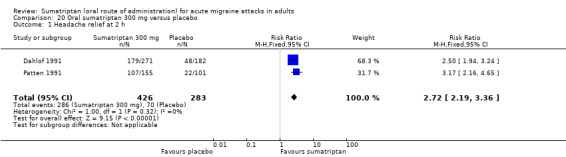
Comparison 20 Oral sumatriptan 300 mg versus placebo, Outcome 1 Headache relief at 2 h.
Sumatriptan 25 mg versus rizatriptan 5 mg
Two studies (2210 participants) provided data (Goldstein 1998; Kolodny 2004).
The proportion of participants with headache relief at two hours with sumatriptan 25 mg was 35% (386/1117; range 12% to 58%).
The proportion of participants with headache relief at two hours with rizatriptan 5 mg was 67% (731/1093; range 66% to 68%).
The relative benefit of sumatriptan compared with rizatriptan was 0.90 (0.84 to 0.95; Analysis 2.3); the NNT was 14 (9.1 to 34) in favour of rizatriptan.
2.3. Analysis.
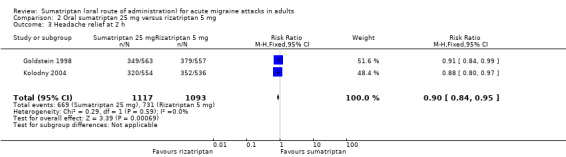
Comparison 2 Oral sumatriptan 25 mg versus rizatriptan 5 mg, Outcome 3 Headache relief at 2 h.
Sumatriptan 25 mg versus rizatriptan 10 mg
Two studies (2231 participants) provided data (Goldstein 1998; Kolodny 2004).
The proportion of participants with headache relief at two hours with sumatriptan 25 mg was 35% (386/1117; range 12% to 58%).
The proportion of participants with headache relief at two hours with rizatriptan 10 mg was 70% (780/1114; range 68% to 72%).
The relative benefit of sumatriptan compared with rizatriptan was 0.86 (0.80 to 0.91; Analysis 3.3); the NNT was 9.9 (7.1 to 16) in favour of rizatriptan.
3.3. Analysis.
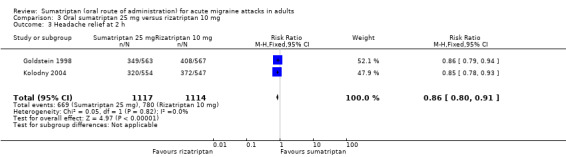
Comparison 3 Oral sumatriptan 25 mg versus rizatriptan 10 mg, Outcome 3 Headache relief at 2 h.
Sumatriptan 50 mg versus effervescent acetylsalicylic acid 1000 mg
Two studies (726 participants) provided data (Diener 2004a; Diener 2004b).
The proportion of participants with headache relief at two hours with sumatriptan 50 mg was 53% (191/359; range 49% to 56%).
The proportion of participants with headache relief at two hours with effervescent ASA 1000 mg was 42% (153/367; range 25% to 52%).
The relative benefit of sumatriptan compared with effervescent ASA was 1.3 (1.1 to 1.5; Analysis 5.4); the NNT was 8.7 (5.3 to 23).
5.4. Analysis.
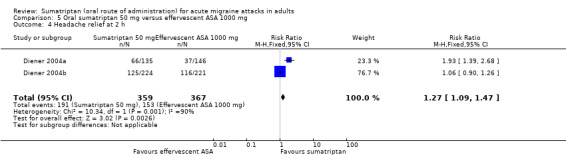
Comparison 5 Oral sumatriptan 50 mg versus effervescent ASA 1000 mg, Outcome 4 Headache relief at 2 h.
Sumatriptan 50 mg versus zolmitriptan 2.5 mg
Two studies (1609 participants) provided data (Gallagher 2000; Gruffyd‐Jones 2001).
The proportion of participants with headache relief at two hours with sumatriptan 50 mg was 67% (543/814; range 59% to 71%).
The proportion of participants with headache relief at two hours with zolmitriptan 2.5 mg was 66% (523/795; range 65% to 67%).
The relative benefit of sumatriptan compared with zolmitriptan was 1.0 (0.95 to 1.1; Analysis 6.2); there was no significant difference between treatments.
6.2. Analysis.
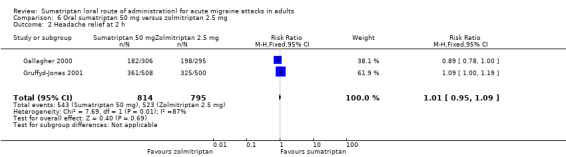
Comparison 6 Oral sumatriptan 50 mg versus zolmitriptan 2.5 mg, Outcome 2 Headache relief at 2 h.
Sumatriptan 50 mg versus zolmitriptan 5 mg
Two studies (1633 participants) provided data (Gallagher 2000; Gruffyd‐Jones 2001).
The proportion of participants with headache relief at two hours with sumatriptan 50 mg was 67% (543/814; range 59% to 71%).
The proportion of participants with headache relief at two hours with zolmitriptan 5 mg was 66% (537/819; range 65% to 66%).
The relative benefit of sumatriptan compared with zolmitriptan was 1.0 (0.95 to 1.1; Analysis 7.2); there was no significant difference between treatments.
7.2. Analysis.
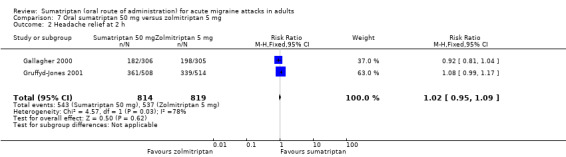
Comparison 7 Oral sumatriptan 50 mg versus zolmitriptan 5 mg, Outcome 2 Headache relief at 2 h.
Sumatriptan 50 mg versus rizatriptan 5 mg
Three studies (2911 participants) provided data (Goldstein 1998; Kolodny 2004; Lines 2001).
The proportion of participants with headache relief at two hours with sumatriptan 50 mg was 65% (949/1469; range 62% to 67%).
The proportion of participants with headache relief at two hours with rizatriptan 5 mg was 66% (951/1442; range 63% to 68%).
The relative benefit of sumatriptan compared with rizatriptan was 0.98 (0.93 to 1.0; Analysis 8.3); there was no significant difference between treatments.
8.3. Analysis.
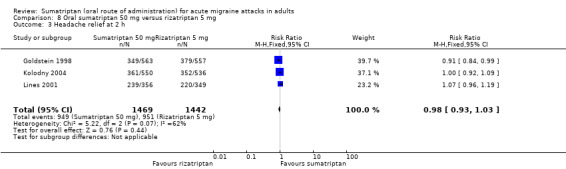
Comparison 8 Oral sumatriptan 50 mg versus rizatriptan 5 mg, Outcome 3 Headache relief at 2 h.
Sumatriptan 50 mg versus rizatriptan 10 mg
Two studies (2227 participants) provided data (Goldstein 1998; Kolodny 2004).
The proportion of participants with headache relief at two hours with sumatriptan 50 mg was 64% (710/1113; range 62% to 66%).
The proportion of participants with headache relief at two hours with rizatriptan 10 mg was 70% (780/1114; range 68% to 72%).
The relative benefit of sumatriptan compared with rizatriptan was 0.91 (0.86 to 0.97; Analysis 9.3); the NNT was 16 (9.9 to 43) in favour of rizatriptan.
9.3. Analysis.
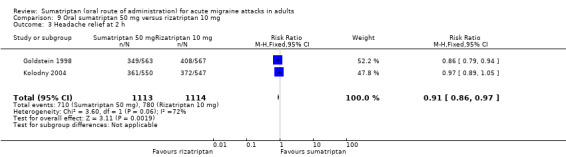
Comparison 9 Oral sumatriptan 50 mg versus rizatriptan 10 mg, Outcome 3 Headache relief at 2 h.
Sumatriptan 50 mg versus eletriptan 40 mg
Two studies (721 participants) provided data (160‐104; Sandrini 2002).
The proportion of participants with headache relief at two hours with sumatriptan 50 mg was 51% (186/362; range 49% to 54%).
The proportion of participants with headache relief at two hours with eletriptan 40 mg was 60% (217/359; range 59% to 62%).
The relative benefit of sumatriptan compared with eletriptan was 0.85 (0.75 to 0.97; Analysis 10.3); the NNT was 11 (6.1 to 54) in favour of eletriptan.
10.3. Analysis.
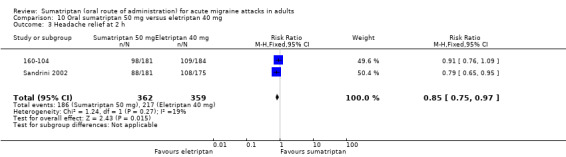
Comparison 10 Oral sumatriptan 50 mg versus eletriptan 40 mg, Outcome 3 Headache relief at 2 h.
Sumatriptan 50 mg versus eletriptan 80 mg
Two studies (706 participants) provided data (160‐104; Sandrini 2002).
The proportion of participants with headache relief at two hours with sumatriptan 50 mg was 51% (186/362; range 49% to 54%).
The proportion of participants with headache relief at two hours with eletriptan 80 mg was 66% (226/344; range 65% to 66%).
The relative benefit of sumatriptan compared with eletriptan was 0.78 (0.69 to 0.89; Analysis 11.3); the NNT was 7.0 (4.7 to 14) in favour of eletriptan.
11.3. Analysis.
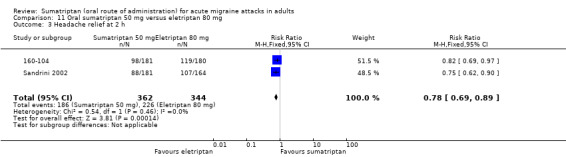
Comparison 11 Oral sumatriptan 50 mg versus eletriptan 80 mg, Outcome 3 Headache relief at 2 h.
Sumatriptan 100 mg versus eletriptan 40 mg
Three studies (2263 participants) provided data (Goadsby 2000; Mathew 2003; Sandrini 2002).
The proportion of participants with headache relief at two hours with sumatriptan 100 mg was 55% (622/1130; range 50% to 57%).
The proportion of participants with headache relief at two hours with eletriptan 40 mg was 62% (706/1133; range 56% to 64%).
The relative benefit of sumatriptan compared with eletriptan was 0.88 (0.82 to 0.95; Analysis 13.4); the NNT was 14 (8.9 to 31) in favour of eletriptan.
13.4. Analysis.
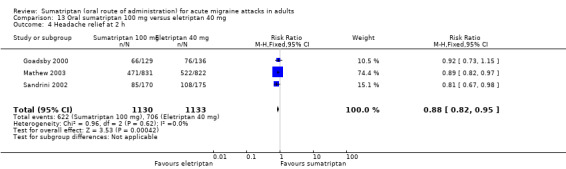
Comparison 13 Oral sumatriptan 100 mg versus eletriptan 40 mg, Outcome 4 Headache relief at 2 h.
Sumatriptan 100 mg versus eletriptan 80 mg
Two studies (604 participants) provided data (Goadsby 2000; Sandrini 2002).
The proportion of participants with headache relief at two hours with sumatriptan 100 mg was 51% (151/299; range 50% to 51%).
The proportion of participants with headache relief at two hours with eletriptan 80 mg was 65% (198/305; range 65% to 65%).
The relative benefit of sumatriptan compared with eletriptan was 0.78 (0.68 to 0.89; Analysis 14.4); the NNT was 6.9 (4.5 to 15) in favour of eletriptan.
14.4. Analysis.
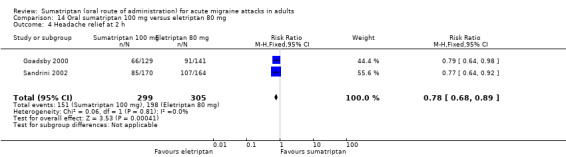
Comparison 14 Oral sumatriptan 100 mg versus eletriptan 80 mg, Outcome 4 Headache relief at 2 h.
Sumatriptan 100 mg versus paracetamol 1000 mg + metoclopramide 10 mg
Two studies (1035 participants) provided data (GL/MIG/001/92; GL/MIG/001A/92).
The proportion of participants with headache relief at two hours with sumatriptan 100 mg was 45% (233/514; range 42% to 49%).
The proportion of participants with headache relief at two hours with paracetamol 1000 mg + MCP 10 mg was 43% (225/521; range 41% to 45%).
The relative benefit of sumatriptan compared with paracetamol + MCP was 1.1 (0.91 to 1.2; Analysis 17.1); there was no significant difference between the treatments.
17.1. Analysis.
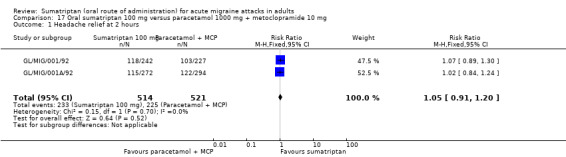
Comparison 17 Oral sumatriptan 100 mg versus paracetamol 1000 mg + metoclopramide 10 mg, Outcome 1 Headache relief at 2 hours.
Sumatriptan 100 mg versus acetylsalicylic acid 900 mg + metoclopramide 10 mg
Two studies (575 participants) provided data (Tfelt‐Hansen 1995; Thomson 1992).
The proportion of participants with headache relief at two hours with sumatriptan 100 mg was 50% (137/275; range 48% to 52%).
The proportion of participants with headache relief at two hours with ASA 900 mg + MCP 10 mg was 46% (138/300; range 38% to 55%).
The relative benefit of sumatriptan compared with ASA + MCP was 1.1 (0.92 to 1.3; Analysis 18.2); there was no significant difference between the treatments.
18.2. Analysis.
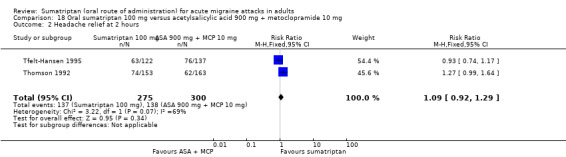
Comparison 18 Oral sumatriptan 100 mg versus acetylsalicylic acid 900 mg + metoclopramide 10 mg, Outcome 2 Headache relief at 2 hours.
Sustained pain‐free during the 24 hours postdose
Sumatriptan 50 mg versus placebo
Four studies (2526 participants) in participants with moderate or severe baseline pain intensity provided data (Sandrini 2002; Sheftell 2005 Study 1 and Study 2; Smith 2005).
The proportion of participants with a 24‐hour sustained pain‐free response with sumatriptan 50 mg was 17% (226/1309; range 11% to 20%).
The proportion of participants with a 24‐hour sustained pain‐free response with placebo was 7% (82/1217; range 4% to 8%).
The relative benefit of treatment compared with placebo was 2.6 (2.1 to 3.4; Analysis 4.5; Figure 4); the NNT was 9.5 (7.7 to 12).
4.5. Analysis.
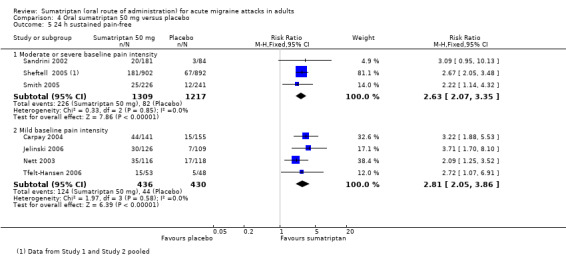
Comparison 4 Oral sumatriptan 50 mg versus placebo, Outcome 5 24 h sustained pain‐free.
4.
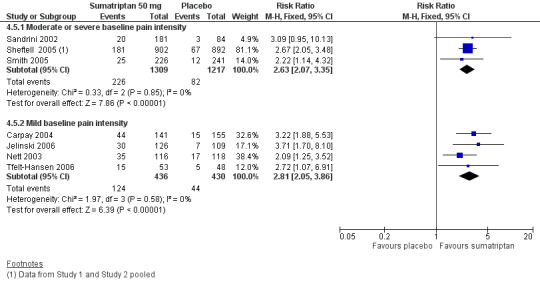
Forest plot of comparison: 4 Oral sumatriptan 50 mg versus placebo, outcome: 4.5 24 h sustained pain‐free.
Four studies (866 participants) in participants with mild baseline pain intensity provided data (Carpay 2004; Jelinski 2006; Nett 2003; Tfelt‐Hansen 2006).
The proportion of participants with a 24‐hour sustained pain‐free response with sumatriptan 50 mg was 28% (124/436; range 24% to 31%).
The proportion of participants with a 24‐hour sustained pain‐free response with placebo was 10% (44/430; range 6% to 14%).
The relative benefit of treatment compared with placebo was 2.8 (2.1 to 3.9; Analysis 4.5); the NNT was 5.5 (4.3 to 7.6).
Treating early, while headache was still in the mild pain phase was significantly more effective than treating established moderate or severe headache pain (z = 2.648; P = 0.008; see Summary of results B).
Sumatriptan 100 mg versus placebo
Six studies (2891 participants) in participants with moderate or severe baseline pain intensity provided data (Dodick 2002; Dowson 2002; Kaniecki 2006; Sandrini 2002; Sheftell 2005 Study 1 and Study 2).
The proportion of participants with a 24‐hour sustained pain‐free response with sumatriptan 100 mg was 24% (374/1590; range 14% to 29%).
The proportion of participants with a 24‐hour sustained pain‐free response with placebo was 8% (106/1301; range 4% to 17%).
The relative benefit of treatment compared with placebo was 2.8 (2.4 to 3.5; Analysis 12.5); the NNT was 6.5 (5.6 to 7.8).
12.5. Analysis.
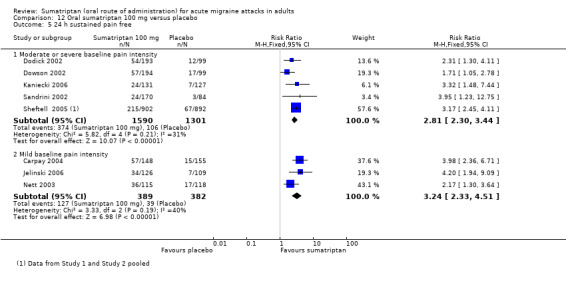
Comparison 12 Oral sumatriptan 100 mg versus placebo, Outcome 5 24 h sustained pain free.
Sumatriptan 100 mg was significantly more effective than sumatriptan 50 mg in participants with moderate or severe baseline pain intensity (z = 2.663; P = 0.008; see Summary of results B).
Three studies (771 participants) in participants with mild baseline pain intensity provided data (Carpay 2004; Jelinski 2006; Nett 2003).
The proportion of participants with a 24‐hour sustained pain‐free response with sumatriptan 100 mg was 33% (127/389; range 27% to 39%).
The proportion of participants with a 24‐hour sustained pain‐free response with placebo was 10% (39/382; range 6% to 14%).
The relative benefit of treatment compared with placebo was 3.2 (2.3 to 4.5; Analysis 12.5); the NNT was 4.5 (3.6 to 5.9).
Treating early, while headache was still in the mild pain phase was significantly more effective than treating established moderate or severe headache pain (z = 2.261; P = 0.024; see Summary of results B).
Sumatriptan 100 mg versus almotriptan 12.5 mg
Two studies (754 participants) in participants with moderate or severe baseline pain intensity provided data (Dodick 2002; Dowson 2002).
The proportion of participants with a 24‐hour sustained pain‐free response with sumatriptan 100 mg was 29% (111/387; range 28% to 29%).
The proportion of participants with a 24‐hour sustained pain‐free response with almotriptan 12.5 mg was 30% (110/367; range 25% to 35%).
The relative benefit of sumatriptan compared with almotriptan was 0.96 (0.77 to 1.2; Analysis 16.2); there was no significant difference between treatments.
16.2. Analysis.
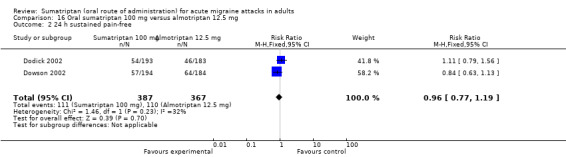
Comparison 16 Oral sumatriptan 100 mg versus almotriptan 12.5 mg, Outcome 2 24 h sustained pain‐free.
Sustained headache relief during the 24 hours postdose
All participants experiencing outcomes of headache relief must, by definition, have had moderate to severe pain at baseline.
Sumatriptan 50 mg versus placebo
Four studies (2526 participants) provided data (Sandrini 2002; Sheftell 2005 Study 1 and Study 2; Smith 2005).
The proportion of participants with 24‐hour sustained headache relief with sumatriptan 50 mg was 35% (454/1309; range 29% to 36%).
The proportion of participants with 24‐hour sustained headache relief with placebo was 18% (220/1217; range 17% to 21%).
The relative benefit of treatment compared with placebo was 1.9 (1.7 to 2.2; Analysis 4.6); the NNT was 6.0 (5.0 to 7.6).
4.6. Analysis.
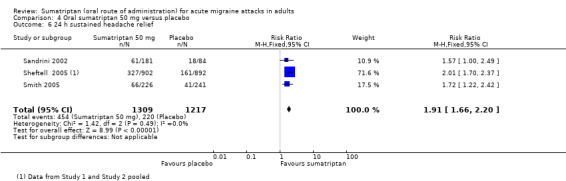
Comparison 4 Oral sumatriptan 50 mg versus placebo, Outcome 6 24 h sustained headache relief.
Sumatriptan 100 mg versus placebo
Six studies (4116 participants) provided data (Geraud 2000; Kaniecki 2006; Mathew 2003; Sandrini 2002; Sheftell 2005 Study 1 and Study 2).
The proportion of participants with 24‐hour sustained headache relief with sumatriptan 100 mg was 36% (922/2538; range 33% to 39%).
The proportion of participants with 24‐hour sustained headache relief with placebo was 17% (270/1578; range 14% to 25%).
The relative benefit of treatment compared with placebo was 2.1 (1.9 to 2.4; Analysis 12.6); the NNT was 5.2 (4.6 to 6.0).
12.6. Analysis.
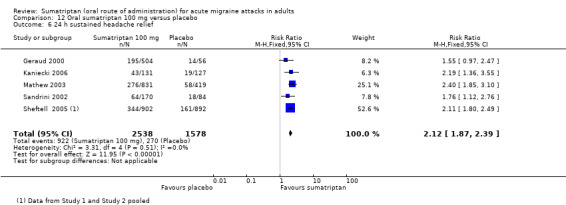
Comparison 12 Oral sumatriptan 100 mg versus placebo, Outcome 6 24 h sustained headache relief.
Sumatriptan 100 mg versus eletriptan 40 mg
Two studies (1998 participants) provided data (Mathew 2003; Sandrini 2002).
The proportion of participants with 24‐hour sustained headache relief with sumatriptan 100 mg was 34% (340/1001; range 33% to 38%).
The proportion of participants with 24‐hour sustained headache relief with eletriptan 40 mg was 43% (430/997; range 42% to 50%).
The relative benefit of sumatriptan compared with eletriptan was 0.79 (0.70 to 0.88; Analysis 13.5); the NNT was 11 (7.5 to 20) in favour of eletriptan.
13.5. Analysis.
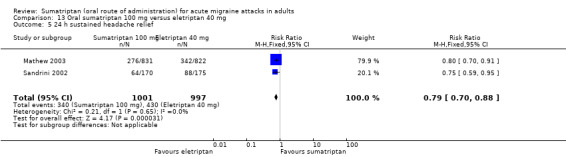
Comparison 13 Oral sumatriptan 100 mg versus eletriptan 40 mg, Outcome 5 24 h sustained headache relief.
| Summary of results A: Pain‐free and headache relief | ||||||
| Studies |
Attacks treated |
Treatment (%) |
Placebo or comparator (%) |
Relative risk (95% CI) |
NNT (95% CI) |
|
| Pain‐free at 2 hours | ||||||
| Sumatriptan 25 mg versus placebo (moderate or severe baseline pain intensity) | 3 | 1108 | 25 | 9 | 2.7 (1.8 to 4.0) | 6.2 (4.9 to 8.5) |
| Sumatriptan 50 mg versus placebo (moderate or severe baseline pain intensity) | 13 | 6447 | 28 | 11 | 2.7 (2.4 to 3.1) | 6.1 (5.5 to 6.9) |
| Sumatriptan 50 mg versus placebo (mild baseline pain intensity) | 7 | 1514 | 46 | 23 | 2.0 (1.7 to 2.4) | 4.4 (3.7 to 5.6) |
| Sumatriptan 100 mg versus placebo (moderate or severe baseline pain intensity) | 16 | 6571 | 32 | 11 | 3.2 (2.8 to 3.6) | 4.7 (4.3 to 5.1) |
| Sumatriptan 100 mg versus placebo (mild baseline pain intensity) | 5 | 1240 | 58 | 24 | 2.4 (2.1 to 2.8) | 3.0 (2.6 to 3.5) |
| Sumatriptan 25 mg versus rizatriptan 5 mg (moderate or severe baseline pain intensity) | 2 | 2210 | 28 | 33 | 0.84 (0.74 to 0.95) | ‐18 (‐11 to ‐62) |
| Sumatriptan 25 mg versus rizatriptan 10 mg (moderate or severe baseline pain intensity) | 2 | 2231 | 28 | 39 | 0.70 (0.62 to 0.79) | ‐8.5 (‐6.4 to ‐13) |
| Sumatriptan 50 mg versus effervescent ASA 1000 mg (moderate or severe baseline pain intensity) | 2 | 726 | 32 | 26 | 1.2 (0.97 to 1.5) | Not calculated |
| Sumatriptan 50 mg versus rizatriptan 5 mg (moderate or severe baseline pain intensity) | 2 | 2209 | 35 | 33 | 1.1 (0.95 to 1.2) | Not calculated |
| Sumatriptan 50 mg versus rizatriptan 10 mg (moderate or severe baseline pain intensity) | 2 | 2230 | 35 | 39 | 0.89 (0.80 to 0.99) | ‐24 (‐12 to ‐560) |
| Sumatriptan 50 mg versus eletriptan 40 mg (moderate or severe baseline pain intensity) | 2 | 721 | 18 | 24 | 0.74 (0.55 to 0.98) | ‐16 (‐8.2 to ‐270) |
| Sumatriptan 50 mg versus eletriptan 80 mg (moderate or severe baseline pain intensity) | 2 | 706 | 18 | 30 | 0.58 (0.44 to 0.76) | ‐8.0 (‐5.3 to ‐16) |
| Sumatriptan 100 mg versus eletriptan 40 mg (moderate or severe baseline pain intensity) | 3 | 2263 | 24 | 32 | 0.74 (0.65 to 0.85) | ‐12 (‐8.3 to ‐22) |
| Sumatriptan 100 mg versus eletriptan 80 mg (moderate or severe baseline pain intensity) | 2 | 604 | 18 | 34 | 0.54 (0.41 to 0.72) | ‐6.5 (‐4.5 to ‐12) |
| Sumatriptan 100 mg versus rizatriptan 10 mg (moderate or severe baseline pain intensity) | 2 | 936 | 31 | 37 | 0.82 (0.69 to 0.98) | ‐16 (‐8.1 to ‐410) |
| Sumatriptan 100 mg versus almotriptan 12.5 mg (moderate or severe baseline pain intensity) | 2 | 754 | 33 | 28 | 1.2 (0.97 to 1.5) | Not calculated |
| Sumatriptan 100 mg versus ASA 900 mg + MCP 10 mg (moderate or severe baseline pain intensity) | 2 | 575 | 26 | 16 | 1.6 (1.2 to 2.3) | 10 (6.1 to 31) |
| Pain‐free at 1 hour | ||||||
| Sumatriptan 50 mg versus placebo (moderate or severe baseline pain intensity) | 5 | 1735 | 5 | 2 | 2.6 (1.5 to 4.7) | 33 (21 to 73) |
| Sumatriptan 50 mg versus placebo (mild baseline pain intensity) | 5 | 1246 | 26 | 14 | 1.9 (1.5 to 2.4) | 8.5 (6.2 to 13) |
| Sumatriptan 100 mg versus placebo (moderate or severe baseline pain intensity) | 6 | 3176 | 7 | 2 | 4.0 (2.3 to 6.8) | 18 (15 to 24) |
| Sumatriptan 100 mg versus placebo (mild baseline pain intensity) | 5 | 1240 | 31 | 14 | 2.2 (1.8 to 2.8) | 6.0 (4.7 to 8.3) |
| Sumatriptan 50 mg versus effervescent ASA 1000 mg (moderate or severe baseline pain intensity) | 2 | 726 | 5 | 5 | 0.97 (0.53 to 1.8) | Not calculated |
| Sumatriptan 100 mg versus eletriptan 40 mg (moderate or severe baseline pain intensity) | 3 | 2263 | 5 | 7 | 0.79 (0.57 to 1.1) | Not calculated |
| Sumatriptan 100 mg versus eletriptan 80 mg (moderate or severe baseline pain intensity) | 2 | 604 | 6 | 13 | 0.48 (0.29 to 0.82) | ‐15 (‐8.7 to ‐48) |
| Headache relief at 1 hour | ||||||
| Sumatriptan 25 mg versus placebo | 3 | 745 | 27 | 16 | 1.6 (1.2 to 2.3) | 9.0 (5.8 to 20) |
| Sumatriptan 50 mg versus placebo | 9 | 2766 | 27 | 14 | 1.8 (1.5 to 2.1) | 7.5 (6.2 to 9.7) |
| Sumatriptan 100 mg versus placebo | 10 | 3983 | 29 | 15 | 1.9 (1.6 to 2.2) | 6.8 (5.8 to 8.3) |
| Sumatriptan 25 mg versus rizatriptan 5 mg | 2 | 2210 | 34 | 37 | 0.91 (0.81 to 1.0) | Not calculated |
| Sumatriptan 25 mg versus rizatriptan 10 mg | 2 | 2231 | 34 | 41 | 0.82 (0.74 to 0.91) | ‐14 (‐8.8 to ‐30) |
| Sumatriptan 50 mg versus effervescent ASA 1000 mg | 2 | 726 | 24 | 31 | 0.78 (0.61 to 0.98) | ‐15 (‐7.5 to ‐270) |
| Sumatriptan 50 mg versus zolmitriptan 2.5 mg | 2 | 1609 | 41 | 40 | 1.0 (0.90 to 1.1) | Not calculated |
| Sumatriptan 50 mg versus zolmitriptan 5 mg | 2 | 1633 | 41 | 39 | 1.0 (0.92 to 1.2) | Not calculated |
| Sumatriptan 50 mg versus rizatriptan 5 mg | 2 | 2209 | 37 | 37 | 0.99 (0.89 to 1.1) | Not calculated |
| Sumatriptan 50 mg versus rizatriptan 10 mg | 2 | 2230 | 37 | 41 | 0.90 (0.81 to 1.0) | ‐23 (‐12 to ‐410) |
| Sumatriptan 50 mg versus eletriptan 40 mg | 2 | 721 | 25 | 25 | 0.99 (0.77 to 1.3) | Not calculated |
| Sumatriptan 50 mg versus eletriptan 80 mg | 2 | 706 | 25 | 35 | 0.72 (0.57 to 0.91) | ‐10 (‐6.1 to ‐33) |
| Sumatriptan 100 mg versus eletriptan 40 mg | 3 | 2263 | 25 | 32 | 0.77 (0.67 to 0.88) | ‐13 (‐8.9 to ‐26) |
| Sumatriptan 100 mg versus eletriptan 80 mg | 2 | 604 | 23 | 35 | 0.65 (0.50 to 0.84) | ‐8.3 (‐5.2 to ‐21) |
| Sumatriptan 100 mg versus rizatriptan 10 mg | 2 | 936 | 26 | 34 | 0.76 (0.62 to 0.92) | ‐12 (‐7.1 to ‐43) |
| Headache relief at 2 hours | ||||||
| Sumatriptan 25 mg versus placebo | 5 | 1580 | 56 | 32 | 1.7 (1.4 to 1.9) | 4.2 (3.5 to 5.4) |
| Sumatriptan 50 mg versus placebo | 19 | 8102 | 57 | 32 | 1.8 (1.7 to 1.9) | 4.0 (3.7 to 4.4) |
| Sumatriptan 100 mg versus placebo | 21 | 7811 | 61 | 32 | 1.9 (1.8 to 2.0) | 3.5 (3.2 to 3.7) |
| Sumatriptan 200 mg versus placebo | 3 | 749 | 72 | 26 | 2.8 (2.3 to 3.5) | 2.1 (1.9 to 2.5) |
| Sumatriptan 300 mg versus placebo | 2 | 709 | 67 | 25 | 2.7 (2.2 to 3.4) | 2.4 (2.0 to 2.8) |
| Sumatriptan 25 mg versus rizatriptan 5 mg | 2 | 2210 | 60 | 67 | 0.90 (0.84 to 0.95) | ‐14 (‐9.1 to ‐34) |
| Sumatriptan 25 mg versus rizatriptan 10 mg | 2 | 2231 | 60 | 70 | 0.86 (0.80 to 0.91) | ‐9.9 (‐7.1 to ‐16) |
| Sumatriptan 50 mg versus effervescent ASA 1000 mg | 2 | 726 | 53 | 42 | 1.3 (1.1 to 1.5) | 8.7 (5.3 to 23) |
| Sumatriptan 50 mg versus zolmitriptan 2.5 mg | 2 | 1609 | 67 | 66 | 1.0 (0.95 to 1.1) | Not calculated |
| Sumatriptan 50 mg versus zolmitriptan 5 mg | 2 | 1633 | 67 | 66 | 1.0 (0.95 to 1.1) | Not calculated |
| Sumatriptan 50 mg versus rizatriptan 5 mg | 3 | 2911 | 65 | 66 | 0.98 (0.93 to 1.0) | Not calculated |
| Sumatriptan 50 mg versus rizatriptan 10 mg | 2 | 2227 | 64 | 70 | 0.91 (0.86 to 0.97) | ‐16 (‐9.9 to ‐43) |
| Sumatriptan 50 mg versus eletriptan 40 mg | 2 | 721 | 51 | 60 | 0.85 (0.75 to 0.97) | ‐11 (‐6.1 to ‐54) |
| Sumatriptan 50 mg versus eletriptan 80 mg | 2 | 706 | 51 | 66 | 0.78 (0.69 to 0.89) | ‐7.0 (‐4.7 to ‐14) |
| Sumatriptan 100 mg versus eletriptan 40 mg | 3 | 2263 | 55 | 62 | 0.88 (0.82 to 0.95) | ‐14 (‐8.8 to ‐31) |
| Sumatriptan 100 mg versus eletriptan 80 mg | 2 | 604 | 51 | 65 | 0.78 (0.68 to 0.89) | ‐6.9 (‐4.5 to ‐15) |
| Sumatriptan 100 mg versus paracetamol 1000 mg + MCP 10 mg | 2 | 1035 | 45 | 43 | 1.1 (0.92 to 1.2) | Not calculated |
| Sumatriptan 100 mg versus ASA 900 mg + MCP 10 mg | 2 | 575 | 50 | 46 | 1.1 (0.92 to 1.3) | Not calculated |
| Sustained pain‐free during the 24 hours postdose | ||||||
| Sumatriptan 50 mg versus placebo (moderate or severe baseline pain intensity) | 4 | 2526 | 17 | 7 | 2.6 (2.1 to 3.4) | 9.5 (7.7 to 12) |
| Sumatriptan 50 mg versus placebo (mild baseline pain intensity) | 4 | 866 | 28 | 10 | 2.8 (2.1 to 3.9) | 5.5 (4.3 to 7.6) |
| Sumatriptan 100 mg versus placebo (moderate or severe baseline pain intensity) | 6 | 2891 | 24 | 8 | 2.8 (2.4 to 3.5) | 6.5 (5.6 to 7.8) |
| Sumatriptan 100 mg versus placebo (mild baseline pain intensity) | 3 | 771 | 33 | 10 | 3.2 (2.3 to 4.5) | 4.5 (3.6 to 5.9) |
| Sumatriptan 100 mg versus almotriptan 12.5 mg (moderate or severe baseline pain intensity) | 2 | 754 | 29 | 30 | 0.96 (0.77 to 1.2) | Not calculated |
| Sustained headache relief during the 24 hours postdose | ||||||
| Sumatriptan 50 mg versus placebo | 4 | 2526 | 35 | 18 | 1.9 (1.7 to 2.2) | 6.0 (5.0 to 7.6) |
| Sumatriptan 100 mg versus placebo | 6 | 4116 | 36 | 17 | 2.1 (1.9 to 2.4) | 5.2 (4.6 to 6.0) |
| Sumatriptan 100 mg versus eletriptan 40 mg | 2 | 1998 | 34 | 43 | 0.79 (0.70 to 0.88) | ‐11 (‐7.5 to ‐20) |
| Summary of results B: Statistical tests for the effect of dose and baseline pain intensity | ||
| z | P | |
| Pain‐free at 2 hours | ||
| Sumatriptan 50 mg, mild versus moderate/severe baseline pain | 2.450 | 0.014 |
| Sumatriptan 50 mg versus 100 mg (moderate/severe baseline pain) | 3.798 | 0.0001 |
| Sumatriptan 50 mg versus 100 mg (mild baseline pain) | 3.124 | 0.002 |
| Sumatriptan 100 mg, mild versus moderate/severe baseline pain | 4.351 | < 0.00006 |
| Headache relief at 2 hours | ||
| Sumatriptan 50 mg versus 100 mg | 2.585 | 0.010 |
| Sumatriptan 100 mg versus 200 mg | 5.212 | < 0.00006 |
| Sustained pain‐free during the 24 hours postdose | ||
| Sumatriptan 50 mg, mild versus moderate/severe baseline pain | 2.648 | 0.008 |
| Sumatriptan 50 mg versus 100 mg (moderate/severe baseline pain) | 2.663 | 0.008 |
| Sumatriptan 100 mg, mild versus moderate/severe baseline pain | 2.261 | 0.024 |
Subgroup analyses
Formulation: dispersible/rapid‐release tablet versus standard tablet
For headache relief at two hours (Analysis 12.14) with sumatriptan 100 mg versus placebo, there was no difference between dispersible (i.e. disintegrating)/rapid‐release formulations and standard tablets (P = 0.59). There were insufficient data to investigate effect of formulation for other doses or outcomes.
12.14. Analysis.
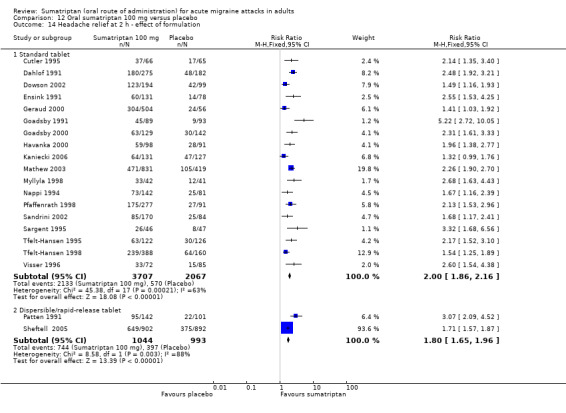
Comparison 12 Oral sumatriptan 100 mg versus placebo, Outcome 14 Headache relief at 2 h ‐ effect of formulation.
Sensitivity analyses
We carried out sensitivity analyses to take into consideration and assess the effect of variation in methodological quality of the included studies. We considered studies with an Oxford Quality Score of 2 of 5 to be at greater risk of bias and therefore analysed them separately for each outcome. Where there were insufficient data to provide a meaningful comparison of these lower‐quality trials with the higher quality trials (scoring 3 or more of 5) for a particular outcome, we performed sensitivity analyses simply to remove the lower‐quality trials from the original all‐trials analyses.
There were insufficient data to carry out any sensitivity analyses for participants with and without aura.
Pain‐free at two hours
Sumatriptan 50 mg versus placebo
Of the 12 studies originally analysed comparing sumatriptan 50 mg with placebo in participants with moderate or severe baseline pain intensity, two had a quality score of 2 of 5 (Cutler 1995; Savani 1999). There was no significant difference (P = 0.30) between the two groups of studies (Analysis 4.14).
4.14. Analysis.
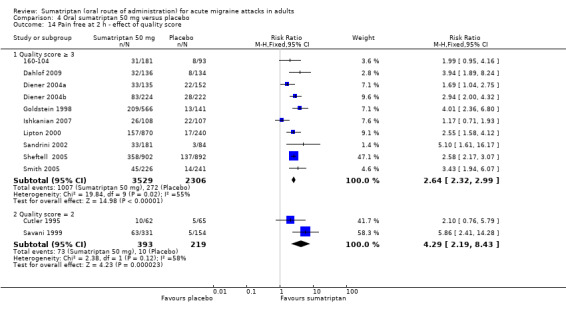
Comparison 4 Oral sumatriptan 50 mg versus placebo, Outcome 14 Pain free at 2 h ‐ effect of quality score.
Of the six studies originally analysed comparing sumatriptan 50 mg with placebo in participants with mild baseline pain intensity, two had a quality score of 2 of 5 (Carpay 2004; Pini 1999). There was no significant difference between the two groups of studies (analysis not shown).
Sumatriptan 100 mg versus placebo
Of the 16 studies originally analysed comparing sumatriptan 100 mg with placebo in participants with moderate or severe baseline pain intensity, three had a quality score of 2 of 5 (Cutler 1995; Dodick 2002; Ensink 1991). There was no significant difference (P = 0.32) between the two groups of studies (Analysis 12.15).
12.15. Analysis.
Comparison 12 Oral sumatriptan 100 mg versus placebo, Outcome 15 Pain‐free at 2 h ‐ effect of quality score.
Of the four studies originally analysed comparing sumatriptan 100 mg with placebo in participants with mild baseline pain intensity, one had a quality score of 2 of 5 (Carpay 2004). Removing this study from the analysis made no significant difference to the calculated relative benefit of treatment versus placebo (analysis not shown).
Pain‐free at one hour
None of the 10 studies providing data on pain‐free at one hour in participants with moderate or severe baseline pain intensity had a quality score of less than 3 of 5.
Of the four studies originally analysed comparing sumatriptan (either 50 mg or 100 mg) with placebo in participants with mild baseline pain intensity, one had a quality score of 2 of 5 (Carpay 2004). Removing this study from the analysis of either dose made no significant difference to the calculated relative benefit of treatment versus placebo (analysis not shown).
Headache relief at one hour
Sumatriptan 50 mg versus placebo
Of the nine studies originally analysed comparing sumatriptan 50 mg with placebo, only one had a quality score of 2 of 5 (Savani 1999). Removing this study from the analysis made no significant difference to the calculated relative benefit of treatment versus placebo (Analysis 4.15).
4.15. Analysis.
Comparison 4 Oral sumatriptan 50 mg versus placebo, Outcome 15 Headache relief at 1 h ‐ effect of quality score.
Headache relief at two hours
Sumatriptan 25 mg versus placebo
Of the five studies originally analysed comparing sumatriptan 50 mg with placebo, only one had a quality score of 2 of 5 (Cutler 1995). Removing this study from the analysis made no significant difference to the calculated relative benefit of treatment versus placebo (Analysis 1.10).
1.10. Analysis.
Comparison 1 Oral sumatriptan 25 mg versus placebo, Outcome 10 Headache relief at 2 h ‐ effect of quality score.
Sumatriptan 50 mg versus placebo
Of the 18 studies originally analysed comparing sumatriptan 50 mg with placebo, four had a quality score of 2 of 5 (Bussone 2000; Cutler 1995; Lines 2001; Savani 1999). There was a significant difference between the studies with a quality score of 2 and those with a score of 3 or more (z = 2.61, P = 0.009) (Analysis 4.16). Removing low‐quality studies did not substantially change the calculated relative benefit and NNT.
4.16. Analysis.
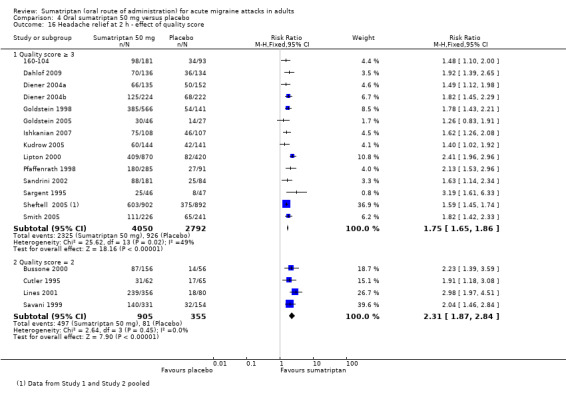
Comparison 4 Oral sumatriptan 50 mg versus placebo, Outcome 16 Headache relief at 2 h ‐ effect of quality score.
Sumatriptan 100 mg versus placebo
Of the 20 studies originally analysed comparing sumatriptan 50 mg with placebo, four had a quality score of 2 of 5 (Cutler 1995; Dahlof 1991; Ensink 1991; Patten 1991). There was a significant difference between the studies with a quality score of 2 and those with a score of 3 or more (z = 2.98, P = 0.003) (Analysis 12.16). Removing low‐quality studies did not substantially change the calculated relative benefit and NNT.
12.16. Analysis.
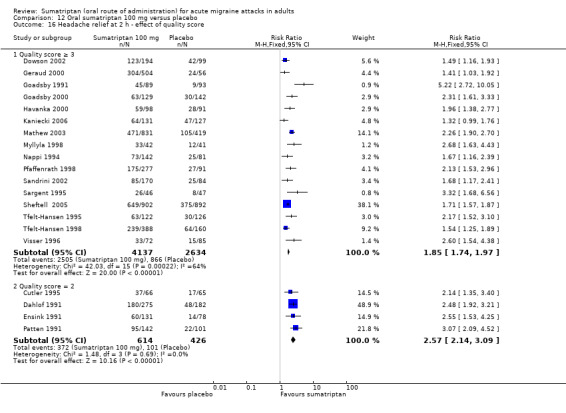
Comparison 12 Oral sumatriptan 100 mg versus placebo, Outcome 16 Headache relief at 2 h ‐ effect of quality score.
Sustained pain‐free during the 24 hours postdose
None of the studies providing data for 24‐hour sustained freedom from pain in participants with moderate or severe baseline pain scored a quality score of less than 3, so no sensitivity analyses were possible.
Of the four and three studies originally analysed for this outcome comparing sumatriptan 50 mg and 100 mg, respectively, with placebo in participants with mild baseline pain, one study had a quality score of 2 of 5 (Carpay 2004). Removing this study from the analysis of either dose made no significant difference to the calculated relative benefit of treatment versus placebo (analysis not shown).
Use of rescue medication
All studies but one (Schulman 2003) allowed participants whose symptoms were not adequately controlled to take additional rescue or 'escape' medication (usually a different analgesic, or in some studies a second dose of test medication). Participants were asked to wait, usually for two hours, before taking any additional medication in order to give the test medication enough time to have an effect. Use of rescue medication at or after a defined time point was reported in most studies and is a measure of treatment failure (lack of efficacy). The time over which use of rescue medication was measured varied between studies. Some reported use of rescue medication up to four hours after initial dosing, while the others reported use of rescue medication up to 24 hours after initial dosing.
Sumatriptan 25 mg versus placebo
None of the included studies provided data for this comparison up to 24 hours after initial dosing.
Two studies (1282 participants) provided data for the use of rescue medication up to four hours after initial dosing, in participants with moderate or severe baseline pain intensity (Goldstein 1998; Kolodny 2004).
The proportion of participants requiring rescue medication with sumatriptan 25 mg was 24% (207/853; range 23% to 25%).
The proportion of participants requiring rescue medication with placebo was 41% (175/429; range 39% to 45%).
The relative benefit of treatment compared with placebo was 0.57 (0.48 to 0.68; Analysis 1.4); the NNTp was 6.1 (4.6 to 9.0).
1.4. Analysis.
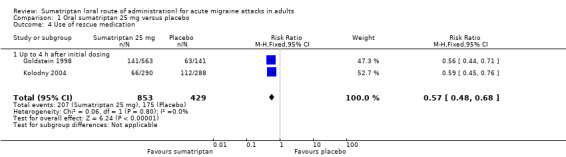
Comparison 1 Oral sumatriptan 25 mg versus placebo, Outcome 4 Use of rescue medication.
Sumatriptan 50 mg verus placebo
Four studies (2079 participants) provided data for the use of rescue medication up to 24 hours after initial dosing, in participants with moderate or severe baseline pain intensity (Diener 2004a; Ishkanian 2007; Lipton 2000; Smith 2005).
The proportion of participants requiring rescue medication with sumatriptan 50 mg was 20% (266/1339; range 7% to 51%).
The proportion of participants requiring rescue medication with placebo was 42% (309/740; range 8% to 63%).
The relative benefit of treatment compared with placebo was 0.77 (0.68 to 0.87; Analysis 4.7); the NNTp was 4.6 (3.8 to 5.6).
4.7. Analysis.
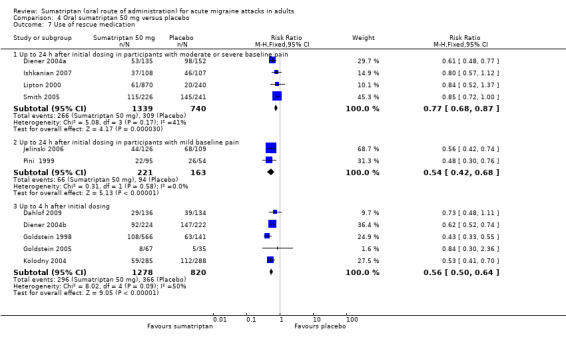
Comparison 4 Oral sumatriptan 50 mg versus placebo, Outcome 7 Use of rescue medication.
Two studies (384 participants) provided data for the use of rescue medication up to 24 hours after initial dosing, in participants with mild baseline pain intensity (Jelinski 2006; Pini 1999).
The proportion of participants requiring rescue medication with sumatriptan 50 mg was 30% (66/221; range 23% to 35%).
The proportion of participants requiring rescue medication with placebo was 58% (94/163; range 48% to 62%).
The relative benefit of treatment compared with placebo was 0.54 (0.42 to 0.68; Analysis 4.7); the NNTp was 3.6 (2.7 to 5.5).
Treating early, while headache was still in the mild pain phase did not significantly affect the use of rescue medication.
Five studies (2098 participants) provided data for the use of rescue medication up to four hours after initial dosing, in participants with moderate or severe baseline pain intensity (Dahlof 2009; Diener 2004b; Goldstein 1998; Goldstein 2005; Kolodny 2004).
The proportion of participants requiring rescue medication with sumatriptan 50 mg was 23% (296/1278; range 12% to 41%).
The proportion of participants requiring rescue medication with placebo was 45% (366/820; range 14% to 66%).
The relative benefit of treatment compared with placebo was 0.56 (0.49 to 0.63; Analysis 4.7); the NNTp was 4.7 (3.9 to 5.8).
Sumatriptan 100 mg versus placebo
Six studies (2810 participants) provided data for the use of rescue medication up to 24 hours after initial dosing, in participants with moderate or severe baseline pain intensity (Dodick 2002; Geraud 2000; Goadsby 2000; Havanka 2000; Mathew 2003; Tfelt‐Hansen 1995).
The proportion of participants requiring rescue medication with sumatriptan 100 mg was 33% (621/1877; range 26% to 63%).
The proportion of participants requiring rescue medication with placebo was 58% (543/933; range 53% to 81%).
The relative benefit of treatment compared with placebo was 0.57 (0.52 to 0.62; Analysis 12.7); the NNTp was 4.0 (3.5 to 4.7).
12.7. Analysis.
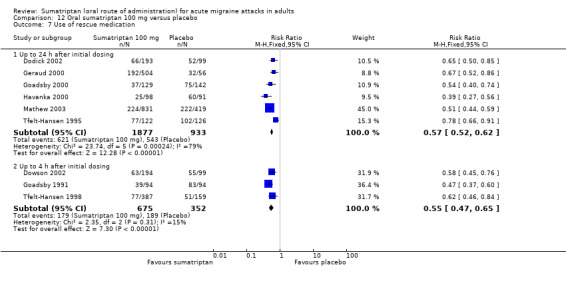
Comparison 12 Oral sumatriptan 100 mg versus placebo, Outcome 7 Use of rescue medication.
Three studies (1027 participants) provided data for the use of rescue medication up to four hours after initial dosing, in participants with moderate or severe baseline pain intensity (Dowson 2002; Goadsby 1991; Tfelt‐Hansen 1998).
The proportion of participants requiring rescue medication with sumatriptan 100 mg was 27% (179/675; range 20% to 41%).
The proportion of participants requiring rescue medication with placebo was 54% (189/352; range 32% to 88%).
The relative benefit of treatment compared with placebo was 0.55 (0.47 to 0.65; Analysis 12.7); the NNTp was 3.7 (3.0 to 4.8).
Sumatriptan 25 mg versus rizatriptan 5 mg
Two studies (1698 participants) provided data for the use of rescue medication up to four hours after initial dosing, in participants with moderate or severe baseline pain intensity (Goldstein 1998; Kolodny 2004).
The proportion of participants requiring rescue medication with sumatriptan 25 mg was 24% (207/853; range 23% to 25%).
The proportion of participants requiring rescue medication with rizatriptan 5 mg was 25% (213/845; range 23% to 30%).
The relative benefit of sumatriptan compared with rizatriptan was 0.96 (0.82 to 1.1; Analysis 2.4); there was no significant difference between treatments.
2.4. Analysis.
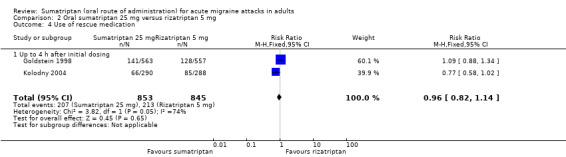
Comparison 2 Oral sumatriptan 25 mg versus rizatriptan 5 mg, Outcome 4 Use of rescue medication.
Sumatriptan 25 mg versus rizatriptan 10 mg
Two studies (1716 participants) provided data for the use of rescue medication up to four hours after initial dosing, in participants with moderate or severe baseline pain intensity (Goldstein 1998; Kolodny 2004).
The proportion of participants requiring rescue medication with sumatriptan 25 mg was 24% (207/853; range 23% to 25%).
The proportion of participants requiring rescue medication with rizatriptan 10 mg was 20% (175/863; range 19% to 23%).
The relative benefit of sumatriptan compared with rizatriptan was 1.2 (1.0 to 1.4; Analysis 3.4); there was no significant difference between treatments.
3.4. Analysis.
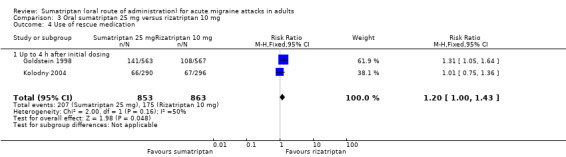
Comparison 3 Oral sumatriptan 25 mg versus rizatriptan 10 mg, Outcome 4 Use of rescue medication.
Sumatriptan 50 mg versus rizatriptan 5 mg
Two studies (1696 participants) provided data for the use of rescue medication up to four hours after initial dosing, in participants with moderate or severe baseline pain intensity (Goldstein 1998; Kolodny 2004).
The proportion of participants requiring rescue medication with sumatriptan 50 mg was 20% (167/851; range 19% to 21%).
The proportion of participants requiring rescue medication with rizatriptan 5 mg was 25% (213/845; range 23% to 30%).
The relative benefit of sumatriptan compared with rizatriptan was 0.78 (0.65 to 0.93; Analysis 8.4); the NNTp was 18 (10 to 62).
8.4. Analysis.
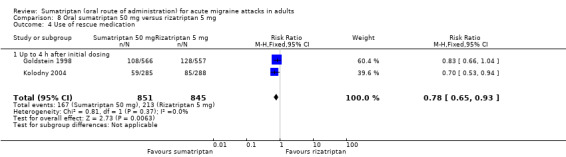
Comparison 8 Oral sumatriptan 50 mg versus rizatriptan 5 mg, Outcome 4 Use of rescue medication.
Sumatriptan 50 mg versus rizatriptan 10 mg
Two studies (1714 participants) provided data for the use of rescue medication up to four hours after initial dosing, in participants with moderate or severe baseline pain intensity (Goldstein 1998; Kolodny 2004).
The proportion of participants requiring rescue medication with sumatriptan 50 mg was 20% (167/851; range 19% to 21%).
The proportion of participants requiring rescue medication with rizatriptan 10 mg was 20% (175/863; range 19% to 23%).
The relative benefit of sumatriptan compared with rizatriptan was 0.97 (0.80 to 1.2; Analysis 9.4); there was no significant difference between treatments.
9.4. Analysis.
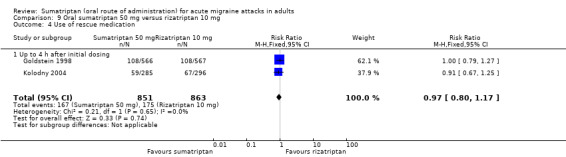
Comparison 9 Oral sumatriptan 50 mg versus rizatriptan 10 mg, Outcome 4 Use of rescue medication.
Sumatriptan 100 mg versus eletriptan 40 mg
Two studies (1918 participants) provided data for the use of rescue medication up to 24 hours after initial dosing, in participants with moderate or severe baseline pain intensity (Goadsby 2000; Mathew 2003).
The proportion of participants requiring rescue medication with sumatriptan 100 mg was 27% (261/960; range 27% to 29%).
The proportion of participants requiring rescue medication with eletriptan 40 mg was 21% (203/958; range 20% to 29%).
The relative benefit of sumatriptan compared with rizatriptan was 1.3 (1.1 to 1.5; Analysis 13.6); the NNTp was 17 (10 to 46) in favour of eletriptan.
13.6. Analysis.
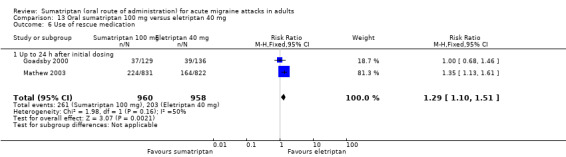
Comparison 13 Oral sumatriptan 100 mg versus eletriptan 40 mg, Outcome 6 Use of rescue medication.
Sumatriptan 100 mg versus paracetamol 1000 mg + metoclopramide 10 mg
Two studies (1243 participants) provided data for the use of rescue medication up to 24 hours after initial dosing, in participants with moderate or severe baseline pain intensity (GL/MIG/001/92; GL/MIG/001A/92).
The proportion of participants requiring rescue medication with sumatriptan 100 mg was 33% (198/606; range 27% to 37%).
The proportion of participants requiring rescue medication with paracetamol 1000 mg + MCP 10 mg was 38% (245/637; range 32% to 44%).
The relative benefit of sumatriptan compared with paracetamol + MCP was 0.86 (0.74 to 0.99; Analysis 17.3); the NNTp was 17 (9.0 to 210).
17.3. Analysis.
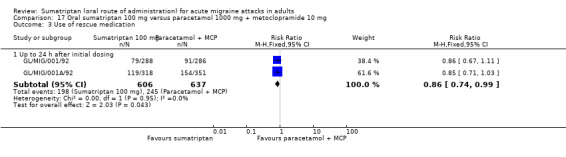
Comparison 17 Oral sumatriptan 100 mg versus paracetamol 1000 mg + metoclopramide 10 mg, Outcome 3 Use of rescue medication.
| Summary of results C: Use of rescue medication | ||||||
| Studies | Attacks treated | Treatment (%) |
Placebo or comparator (%) |
Relative risk (95% CI) | NNTp (95% CI) | |
| Use of rescue medication up to 24 hours after initial dosing | ||||||
| Sumatriptan 50 mg versus placebo (moderate or severe baseline pain intensity) | 4 | 2079 | 20 | 42 | 0.77 (0.68 to 0.87) | 4.6 (3.8 to 5.6) |
| Sumatriptan 50 mg versus placebo (mild baseline pain intensity) | 2 | 384 | 30 | 58 | 0.54 (0.42 to 0.68) | 3.6 (2.7 to 5.5) |
| Sumatriptan 100 mg versus placebo | 6 | 2810 | 33 | 58 | 0.57 (0.52 to 0.62) | 4.0 (3.5 to 4.7) |
| Sumatriptan 100 mg versus eletriptan 40 mg | 2 | 1918 | 27 | 21 | 1.3 (1.1 to 1.5) | ‐17 (‐10 to ‐46) |
| Sumatriptan 100 mg versus paracetamol 1000 mg + metoclopramide 10 mg | 2 | 1243 | 33 | 38 | 0.86 (0.74 to 0.99) | 17 (9.0 to 210) |
| Use of rescue medication up to 4 hours after initial dosing | ||||||
| Sumatriptan 25 mg versus placebo | 2 | 1282 | 24 | 41 | 0.57 (0.48 to 0.68) | 6.1 (4.6 to 9.0) |
| Sumatriptan 50 mg versus placebo | 5 | 2098 | 23 | 45 | 0.56 (0.50 to 0.64) | 4.7 (3.9 to 5.8) |
| Sumatriptan 100 mg versus placebo | 3 | 1027 | 27 | 54 | 0.55 (0.47 to 0.65) | 3.7 (3.0 to 4.8) |
| Sumatriptan 25 mg versus rizatriptan 5 mg | 2 | 1698 | 24 | 25 | 0.96 (0.82 to 1.1) | Not calculated |
| Sumatriptan 25 mg versus rizatriptan 10 mg | 2 | 1716 | 24 | 20 | 1.2 (1.0 to 1.4) | Not calculated |
| Sumatriptan 50 mg versus rizatriptan 5 mg | 2 | 1696 | 20 | 25 | 0.78 (0.65 to 0.93) | 18 (10 to 62) |
| Sumatriptan 50 mg versus rizatriptan 10 mg | 2 | 1714 | 20 | 20 | 0.97 (0.80 to 1.2) | Not calculated |
Relief of headache‐associated symptoms
In general, relief of headache‐associated symptoms (defined as a symptom reduction from any intensity at baseline to none at two hours) was inconsistently reported. Of the 28 studies that reported any data for symptom relief, only seven reported on relief of all four major symptoms of interest. Not all studies reported baseline incidence of associated symptoms from which relief could be calculated, however the majority reported presence of symptoms two hours after treatment. The incidence of vomiting was very low in all studies and where reported did not permit analysis.
Fourteen of the studies providing data on relief of associated symptoms included a small number (< 10%) of participants with mild baseline pain intensity. It is possible that these participants had fewer or less severe associated symptoms, but the number was considered small enough that even if this were so, there would not be a major effect on the overall result; these studies were therefore included in any pooled analyses to which they were relevant.
Effects of treatment on relieving headache‐associated symptoms are presented in Summary of results D. Sumatriptan at doses of 25 mg, 50 mg, and 100 mg showed efficacy in relief of the associated symptoms of nausea, photophobia, and phonophobia, compared to placebo. There was no obvious dose response relationship over 25 mg to 100 mg. The majority of the data were for participants with moderate or severe baseline pain intensity, for whom about 45% to 50% of symptoms present at baseline were relieved within two hours of sumatriptan treatment, while about 30% to 35% were relieved with placebo (Analysis 1.5; Analysis 4.8; Analysis 12.8), giving NNTs of 4 to 8.
1.5. Analysis.
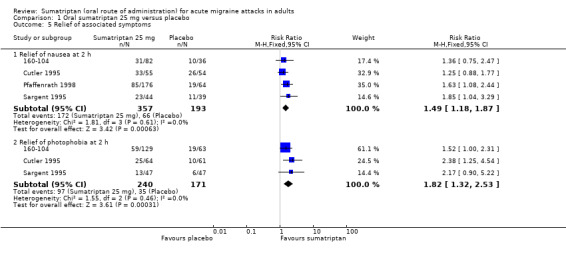
Comparison 1 Oral sumatriptan 25 mg versus placebo, Outcome 5 Relief of associated symptoms.
4.8. Analysis.
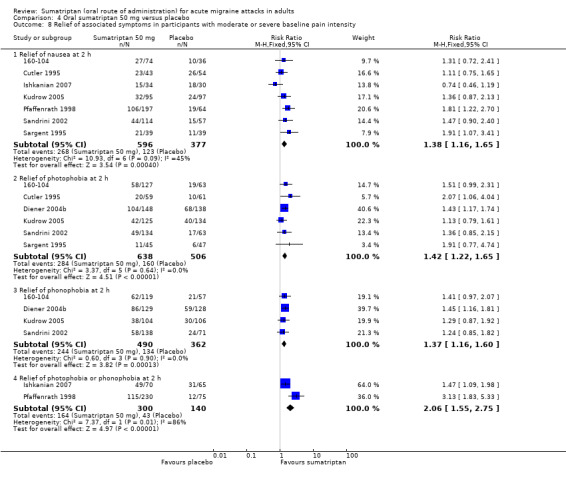
Comparison 4 Oral sumatriptan 50 mg versus placebo, Outcome 8 Relief of associated symptoms in participants with moderate or severe baseline pain intensity.
12.8. Analysis.
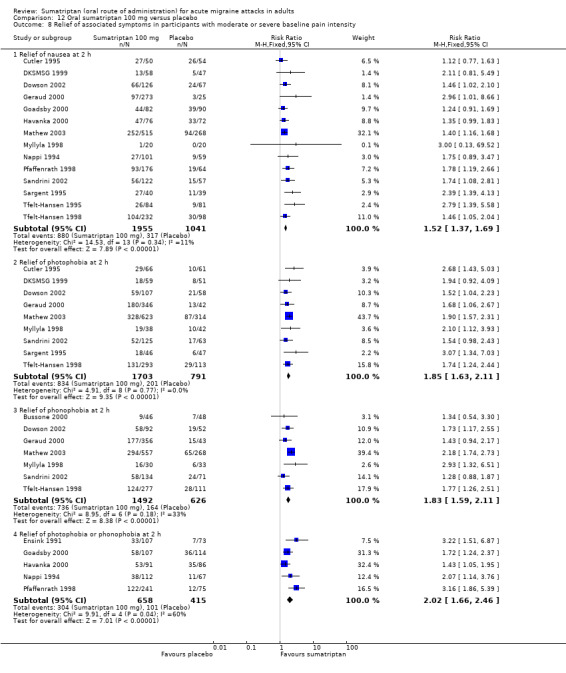
Comparison 12 Oral sumatriptan 100 mg versus placebo, Outcome 8 Relief of associated symptoms in participants with moderate or severe baseline pain intensity.
Three studies in participants with mild baseline pain intensity provided data on relief of headache‐associated symptoms. About 50% to 60% of symptoms present at baseline were relieved within two hours of sumatriptan treatment, while only 10% to 20% were relieved with placebo (Analysis 4.9; Analysis 12.9), giving NNTs 2 to 3. These results must be interpreted cautiously due to the fact that only small numbers of participants were involved in the same three studies providing data for relief of each associated symptom. In addition the studies made no comment on the severity of the symptoms, and it may be that the symptoms present at baseline in participants with moderate or severe headache are not comparable with those present at baseline in participants with mild headache.
4.9. Analysis.
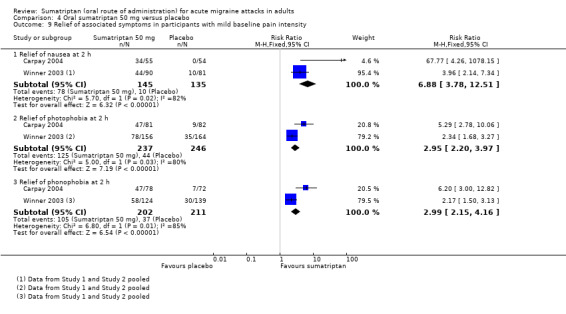
Comparison 4 Oral sumatriptan 50 mg versus placebo, Outcome 9 Relief of associated symptoms in participants with mild baseline pain intensity.
12.9. Analysis.
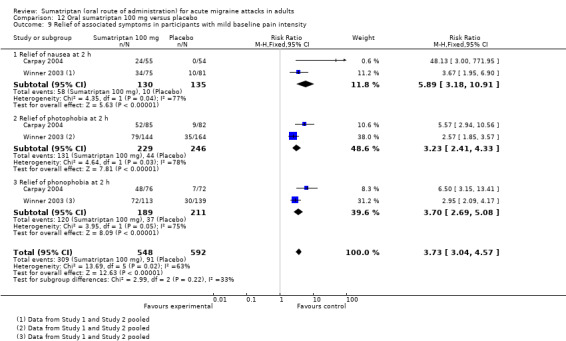
Comparison 12 Oral sumatriptan 100 mg versus placebo, Outcome 9 Relief of associated symptoms in participants with mild baseline pain intensity.
Sumatriptan 50 mg was inferior to eletriptan 40 mg for the relief of nausea (NNT of 8.2 in favour of eletriptan), but no significant difference was found between the two for relief of photophobia or phonophobia (Analysis 10.4). Eletriptan 80 mg, on the other hand, was found to be superior to sumatriptan 50 mg for the relief of photophobia and phonophobia (NNTs of 6.1 and 9.0 respectively), but there was no significant difference between the two for relief of nausea (Analysis 11.4).
10.4. Analysis.
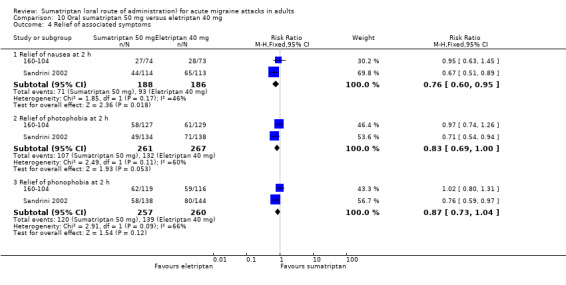
Comparison 10 Oral sumatriptan 50 mg versus eletriptan 40 mg, Outcome 4 Relief of associated symptoms.
11.4. Analysis.
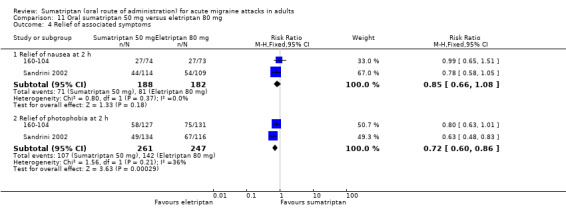
Comparison 11 Oral sumatriptan 50 mg versus eletriptan 80 mg, Outcome 4 Relief of associated symptoms.
Sumatriptan 100 mg was inferior to eletriptan 40 mg for the relief of nausea, photophobia, and phonophobia, with NNTs of 11 to 16 in favour of eletriptan (Analysis 13.7). Similarly sumatriptan 100 mg was inferior to eletriptan 80 mg for the relief of nausea and photophobia, with NNTs of 8.9 and 6.4, respectively, in favour of eletriptan (Analysis 14.5). There was no clear difference between sumatriptan 100 mg and ASA 900 mg + MCP 10 mg for the relief of nausea (Analysis 18.3), or paracetamol 1000 mg + MCP 10 mg for the relief of either photophobia or phonophobia (Analysis 17.2).
13.7. Analysis.
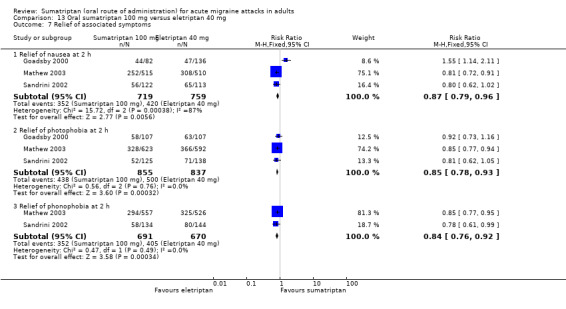
Comparison 13 Oral sumatriptan 100 mg versus eletriptan 40 mg, Outcome 7 Relief of associated symptoms.
14.5. Analysis.
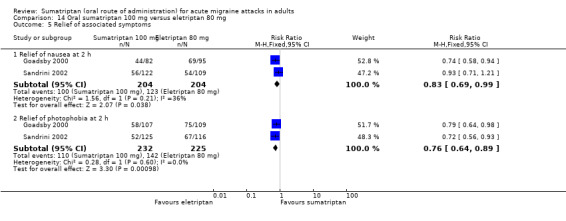
Comparison 14 Oral sumatriptan 100 mg versus eletriptan 80 mg, Outcome 5 Relief of associated symptoms.
18.3. Analysis.
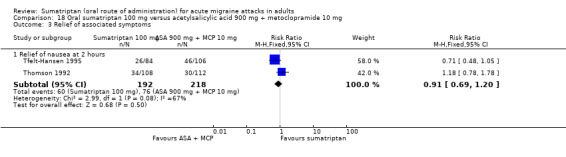
Comparison 18 Oral sumatriptan 100 mg versus acetylsalicylic acid 900 mg + metoclopramide 10 mg, Outcome 3 Relief of associated symptoms.
17.2. Analysis.
Comparison 17 Oral sumatriptan 100 mg versus paracetamol 1000 mg + metoclopramide 10 mg, Outcome 2 Relief of associated symptoms.
| Summary of results D: relief of associated symptoms at 2 hours | ||||||
| Intervention | Studies | Attacks with symptom present | Treatment (%) |
Placebo or comparator (%) |
Relative risk (95% CI) | NNT |
| Nausea | ||||||
| Sumatriptan 25 mg versus placebo | 4 | 550 | 48 | 34 | 1.5 (1.2 to 1.9) | 7.2 (4.5 to 18) |
| Sumatriptan 50 mg versus placebo (moderate or severe baseline pain intensity) | 7 | 973 | 45 | 33 | 1.4 (1.2 to 1.7) | 8.1 (5.4 to 16) |
| Sumatriptan 50 mg versus placebo (mild baseline pain intensity) | 3 | 280 | 54 | 7 | 6.9 (3.8 to 13) | 2.2 (1.8 to 2.7) |
| Sumatriptan 100 mg versus placebo (moderate or severe baseline pain intensity) | 14 | 2996 | 45 | 30 | 1.5 (1.3 to 1.7) | 6.9 (5.5 to 9.1) |
| Sumatriptan 100 mg versus placebo (mild baseline pain intensity) | 3 | 265 | 45 | 7 | 5.9 (3.2 to 11) | 2.7 (2.1 to 3.6) |
| Sumatriptan 50 mg versus eletriptan 40 mg | 2 | 374 | 38 | 50 | 0.76 (0.60 to 0.95) | ‐8.2 (‐4.5 to 44) |
| Sumatriptan 50 mg versus eletriptan 80 mg | 2 | 370 | 38 | 45 | 0.85 (0.67 to 1.1) | Not calculated |
| Sumatriptan 100 mg versus eletriptan 40 mg | 3 | 1478 | 49 | 55 | 0.87 (0.79 to 0.96) | ‐16 (‐8.7 to ‐77) |
| Sumatriptan 100 mg versus eletriptan 80 mg | 2 | 408 | 49 | 60 | 0.83 (0.69 to 0.99) | ‐8.9 (‐4.8 to ‐60) |
| Sumatriptan 100 mg versus ASA 900 mg + MCP 10 mg | 2 | 410 | 31 | 35 | 0.91 (0.69 to 1.2) | Not calculated |
| Photophobia | ||||||
| Sumatriptan 25 mg versus placebo | 3 | 411 | 40 | 20 | 1.8 (1.3 to 2.5) | 5.0 (3.5 to 8.9) |
| Sumatriptan 50 mg versus placebo (moderate or severe baseline pain intensity) | 6 | 1144 | 45 | 32 | 1.4 (1.2 to 1.7) | 7.8 (5.4 to 14) |
| Sumatriptan 50 mg versus placebo (mild baseline pain intensity) | 3 | 483 | 53 | 18 | 3.0 (2.2 to 4.0) | 2.9 (2.3 to 3.7) |
| Sumatriptan 100 mg versus placebo (moderate or severe baseline pain intensity) | 9 | 2494 | 49 | 25 | 1.9 (1.6 to 2.1) | 4.2 (3.7 to 5.1) |
| Sumatriptan 100 mg versus placebo (mild baseline pain intensity) | 3 | 475 | 57 | 18 | 3.2 (2.4 to 4.3) | 2.5 (2.1 to 3.2) |
| Sumatriptan 50 mg versus eletriptan 40 mg | 2 | 528 | 41 | 49 | 0.83 (0.69 to 1.0) | Not calculated |
| Sumatriptan 50 mg versus eletriptan 80 mg | 2 | 508 | 41 | 57 | 0.72 (0.60 to 0.86) | ‐6.1 (‐4.0 to ‐13) |
| Sumatriptan 100 mg versus eletriptan 40 mg | 3 | 1692 | 51 | 60 | 0.85 (0.78 to 0.93) | ‐12 (‐7.6 to ‐26) |
| Sumatriptan 100 mg versus eletriptan 80 mg | 2 | 457 | 47 | 63 | 0.76 (0.64 to 0.89) | ‐6.4 (‐4.1 to ‐15) |
| Phonophobia | ||||||
| Sumatriptan 50 mg versus placebo (moderate or severe baseline pain intensity) | 4 | 852 | 50 | 37 | 1.4 (1.2 to 1.6) | 7.8 (5.1 to 16) |
| Sumatriptan 50 mg versus placebo (mild baseline pain intensity) | 3 | 413 | 52 | 18 | 3.0 (2.2 to 4.2) | 2.9 (2.3 to 3.9) |
| Sumatriptan 100 mg versus placebo (moderate or severe baseline pain intensity) | 7 | 2118 | 49 | 26 | 1.8 (1.6 to 2.1) | 4.3 (3.7 to 5.3) |
| Sumatriptan 100 mg versus placebo (mild baseline pain intensity) | 3 | 400 | 63 | 18 | 3.7 (2.7 to 5.1) | 2.2 (1.8 to 2.7) |
| Sumatriptan 50 mg versus eletriptan 40 mg | 2 | 517 | 47 | 53 | 0.87 (0.73 to 1.0) | Not calculated |
| Sumatriptan 50 mg versus eletriptan 80 mg | 2 | 508 | 47 | 58 | 0.81 (0.69 to 0.96) | ‐9.0 (‐5.1 to ‐41) |
| Sumatriptan 100 mg versus eletriptan 40 mg | 2 | 1361 | 51 | 60 | 0.84 (0.76 to 0.92) | ‐11 (‐6.8 to ‐24) |
| Photophobia or phonophobia | ||||||
| Sumatriptan 50 mg versus placebo | 2 | 440 | 55 | 31 | 2.1 (1.6 to 2.8) | 4.2 (3.0 to 6.9) |
| Sumatriptan 100 mg versus placebo | 5 | 1073 | 46 | 24 | 2.0 (1.7 to 2.5) | 4.6 (3.6 to 6.2) |
| Sumatriptan 100 mg versus paracetamol 1000 mg + MCP 10 mg | 2 | 1001 | 32 | 32 | 0.99 (0.83 to 1.2) | Not calculated |
We also analysed studies according to the presence of associated symptoms two hours after treatment, irrespective of whether they were present at baseline, and calculated NNTps (Appendix 6). Significantly fewer participants reported symptoms of nausea, photophobia, or phonophobia with sumatriptan 25 mg, 50 mg, and 100 mg than with placebo, with no obvious dose response relationship. Again, the majority of the data were for participants treating established moderate or severe migraine attacks. Significantly fewer participants reported photophobia at two hours with sumatriptan than with any comparator, with NNTps ranging from 5.7 to 7.1. Similarly, fewer participants reported nausea and phonophobia with sumatriptan than with any comparator, although the difference was less pronounced, with NNTps ranging from 7.3 to 15. There was no significant difference between sumatriptan of any dose and placebo for incidence of vomiting at two hours. The small amount of data available for participants treating mild migraine attacks indicated that sumatriptan 50 mg and 100 mg were also effective against headache‐associated symptoms at two hours compared to placebo. There was no obvious dose response relationship between the 50 mg and 100 mg doses. Calculated NNTps were not significantly different to those for participants treating moderate or severe headache, with the exception of phonophobia, for which sumatriptan 100 mg administered during the mild pain phase was significantly more effective (P = 0.002) than the same dose administered when the headache had reached moderate or severe intensity.
Significantly fewer participants reported nausea with rizatriptan 5 mg and 10 mg than with sumatriptan 25 mg or 50 mg (NNTps ranged from 15 to 23 in favour of rizatriptan). Rizatriptan 10 mg was also more effective against photophobia and phonophobia after two hours than sumatriptan 25 mg. Eletriptan 40 mg and 80 mg were significantly superior to sumatriptan 100 mg against nausea, photophobia, and phonophobia at two hours (NNTps ranged from 7 to 14 in favour of eletriptan). However, only eletriptan 80 mg was found to be significantly better than sumatriptan 50 mg against photophobia and phonophobia at two hours (NNTps of 8.0 and 14 in favour of eletriptan). Otherwise there were no significant differences in the presence of headache‐associated symptoms at two hours between sumatriptan and the other active migraine treatments for which data were analysed.
Relief of functional disability
Few studies reported relief of functional disability (defined as improvement from moderate or severe disability at baseline to mild or none at two hours on a four‐point scale). The following analyses involve studies in which participants had moderate or severe (or predominantly moderate or severe) baseline pain. Only one study (Carpay 2004) assessing participants with mild baseline pain intensity reported relief of functional disability as defined in this way, and therefore no separate pooled analyses could be performed.
Sumatriptan 25 mg versus placebo
Three studies (381 participants) provided data (160‐104; Cutler 1995; Sargent 1995).
The proportion of participants with relief of functional disability at two hours with sumatriptan 25 mg was 49% (107/220; range 33% to 52%).
The proportion of participants with relief of functional disability at two hours with placebo was 32% (51/161; range 15% to 50%).
The relative benefit of treatment compared with placebo was 1.4 (1.1 to 1.8; Analysis 1.6); the NNT was 5.9 (3.7 to 14).
1.6. Analysis.
Comparison 1 Oral sumatriptan 25 mg versus placebo, Outcome 6 Relief of functional disability at 2 h.
Sumatriptan 50 mg versus placebo
Four studies (607 participants) provided data (160‐104; Cutler 1995; Sandrini 2002; Sargent 1995).
The proportion of participants with relief of functional disability at two hours with sumatriptan 50 mg was 49% (186/378; range 29% to 58%).
The proportion of participants with relief of functional disability at two hours with placebo was 31% (72/229; range 15% to 50%).
The relative benefit of treatment compared with placebo was 1.5 (1.2 to 1.8; Analysis 4.10); the NNT was 5.6 (3.9 to 10).
4.10. Analysis.
Comparison 4 Oral sumatriptan 50 mg versus placebo, Outcome 10 Relief of functional disability at 2 h.
Sumatriptan 100 mg versus placebo
Six studies (1827 participants) provided data (Cutler 1995; Goadsby 2000; Havanka 2000; Mathew 2003; Sandrini 2002; Sargent 1995).
The proportion of participants with relief of functional disability at two hours with sumatriptan 100 mg was 58% (651/1113; range 46% to 62%).
The proportion of participants with relief of functional disability at two hours with placebo was 31% (220/714; range 15% to 34%).
The relative benefit of treatment compared with placebo was 1.9 (1.7 to 2.1; Analysis 12.10); the NNT was 3.6 (3.1 to 4.3).
12.10. Analysis.
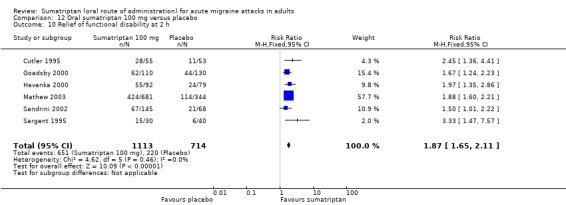
Comparison 12 Oral sumatriptan 100 mg versus placebo, Outcome 10 Relief of functional disability at 2 h.
Sumatriptan 50 mg versus eletriptan 40 mg
Two studies (590 participants) provided data (160‐104; Sandrini 2002).
The proportion of participants with relief of functional disability at two hours with sumatriptan 50 mg was 51% (153/298; range 46% to 58%).
The proportion of participants with relief of functional disability at two hours with eletriptan 40 mg was 62% (180/292; range 60% to 63%).
The relative benefit of sumatriptan compared with eletriptan was 0.83 (0.72 to 0.96; Analysis 10.5); the NNT was 9.7 (5.5 to 43) in favour of eletriptan.
10.5. Analysis.
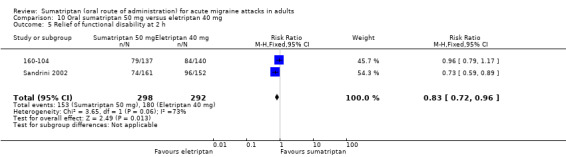
Comparison 10 Oral sumatriptan 50 mg versus eletriptan 40 mg, Outcome 5 Relief of functional disability at 2 h.
Sumatriptan 50 mg versus eletriptan 80 mg
Two studies (570 participants) provided data (160‐104; Sandrini 2002).
The proportion of participants with relief of functional disability at two hours with sumatriptan 50 mg was 51% (153.298; range 46% to 58%).
The proportion of participants with relief of functional disability at two hours with eletriptan 80 mg was 62% (168/272; range 55% to 68%).
The relative benefit of sumatriptan compared with eletriptan was 0.84 (0.73 to 0.97; Analysis 11.5); the NNT was 9.6 (5.4 to 43) in favour of eletriptan.
11.5. Analysis.
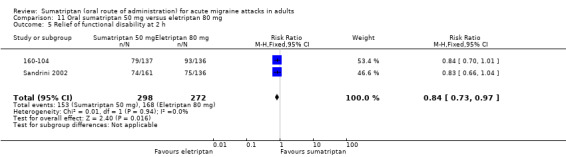
Comparison 11 Oral sumatriptan 50 mg versus eletriptan 80 mg, Outcome 5 Relief of functional disability at 2 h.
Sumatriptan 100 mg versus eletriptan 40 mg
Three studies (1880 participants) provided data (Goadsby 2000; Mathew 2003; Sandrini 2002).
The proportion of participants with relief of functional disability at two hours with sumatriptan 100 mg was 59% (553/936; range 46% to 62%).
The proportion of participants with relief of functional disability at two hours with eletriptan 40 mg was 68% (645/944; range 63% to 70%).
The relative benefit of sumatriptan compared with eletriptan was 0.86 (0.81 to 0.92; Analysis 13.8); the NNT was 11 (7.4 to 20) in favour of eletriptan.
13.8. Analysis.
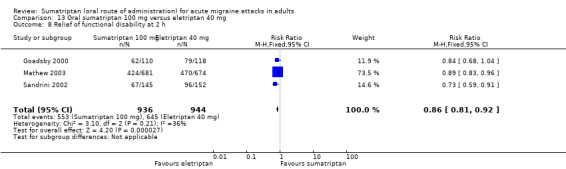
Comparison 13 Oral sumatriptan 100 mg versus eletriptan 40 mg, Outcome 8 Relief of functional disability at 2 h.
Sumatriptan 100 mg versus eletriptan 80 mg
Two studies (516 participants) provided data (Goadsby 2000; Sandrini 2002).
The proportion of participants with relief of functional disability at two hours with sumatriptan 100 mg was 51% (129/255; range 46% to 56%).
The proportion of participants with relief of functional disability at two hours with eletriptan 80 mg was 66% (173/261; range 55% to 78%).
The relative benefit of sumatriptan compared with eletriptan was 0.77 (0.67 to 0.90; Analysis 14.6); the NNT was 6.4 (4.2 to 14) in favour of eletriptan.
14.6. Analysis.
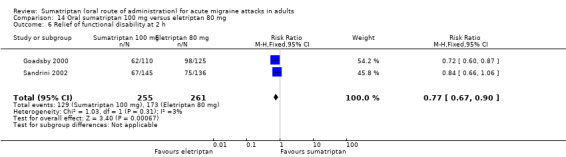
Comparison 14 Oral sumatriptan 100 mg versus eletriptan 80 mg, Outcome 6 Relief of functional disability at 2 h.
We also analysed studies according to the presence of functional disability (of moderate or severe intensity on a four‐point scale) two hours after treatment, and irrespective of whether it was present at baseline, and calculated NNTps (Appendix 6). Significantly fewer participants reported functional disability two hours after treatment with sumatriptan 25 mg, 50 mg, and 100 mg than with placebo, with the 100 mg dose having a lower (better) NNTp of 3.8 than the two lower doses (NNTps of 6.4 and 6.7, respectively). Eletriptan 40 mg and 80 mg were significantly more effective than sumatriptan 50 mg and 100 mg against functional disability at two hours (NNTps ranged from 6.6 to 13 in favour of eletriptan).
Adverse events
Details of results for adverse events and withdrawals in individual studies are provided in Appendix 7.
All except five studies (Dodick 2002; Lines 2001; Goadsby 1991; Goldstein 2005; Sandrini 2002) reported on the total number of participants experiencing any adverse event after treatment, although there was significant variability in many details of adverse event reporting in those studies providing data. Most studies appeared to collect data using spontaneous reports in diary cards and at follow‐up review after the end of treatment. The duration over which data were collected was not always specific, and where it was, there were differences across studies. Most studies probably collected data during the 24 hours postdose, but some specified different time periods: Sargent 1995 specified 72 hours; Pini 1995 "during the study"; Dahlof 2009 six days; 160‐104, Mathew 2003, and Sheftell 2005 seven days; and GL/MIG/001/92, GL/MIG/001A/92, GL/MIG/002, and GL/MIG/002A collected data over several weeks. The majority of studies reported adverse events regardless of their causal relationship to the study drug, but eight studies (Carpay 2004; Dahlof 1991; Freitag 2001; Jelinski 2006; Kaniecki 2006; Lipton 2000; Pini 1995; Sheftell 2005) reported only events considered to be related to the study medication.
In some studies a second, and sometimes third, dose of study medication was taken, and in all but one study rescue medication was allowed if there was an inadequate response after a given period of time. It is likely that in all cases adverse event data continued to be collected after such additional medication. Furthermore, a number of studies treated more than one attack. In Gallagher 2000, Goldstein 1998, and Kolodny 2004 first attack data were reported for adverse events, while Lipton 2000 reported events in all attacks combined. Banerjee 1992, Bussone 2000, Dahlof 1991, Diener 2004b, DKSMSG 1999, Gruffyd‐Jones 2001, Latere 1991, Myllyla 1998, Patten 1991, Pfaffenrath 1998, Sandrini 2007, Savani 1999, Schulman 2003, Tfelt‐Hansen 1995, and Thomson 1992 reported events per participant, but it is unclear how multiple attacks were combined.
Despite these inconsistencies, we have included as much data as possible in the adverse event analyses in order to be more inclusive and conservative, but analyses of pooled data on adverse events should be interpreted cautiously.
Treatments were generally described as well tolerated, with most adverse events being of mild or moderate severity and self limiting.
Participants experiencing any adverse event during the 24 hours postdose
Sumatriptan 25 mg versus placebo
Four studies (1550 participants) in participants with moderate or severe baseline pain intensity provided data (Cutler 1995; Goldstein 1998; Kolodny 2004; Pfaffenrath 1998).
The proportion of participants experiencing adverse events within 24 hours with sumatriptan 25 mg was 39% (371/956; range 24% to 71%).
The proportion of participants experiencing adverse events within 24 hours with placebo was 37% (220/594; range 20% to 74%).
The relative harm of treatment compared with placebo was 1.1 (1.0 to 1.3; Analysis 1.7); there was no significant difference between the two.
1.7. Analysis.
Comparison 1 Oral sumatriptan 25 mg versus placebo, Outcome 7 Any adverse event within 24 h.
Sumatriptan 50 mg versus placebo
Ten studies (3728 participants) in participants with moderate or severe baseline pain intensity provided data (Cutler 1995; Diener 2004a; Diener 2004b; Goldstein 1998; Ishkanian 2007; Kolodny 2004; Kudrow 2005; Pfaffenrath 1998; Savani 1999; Smith 2005).
The proportion of participants experiencing adverse events within 24 hours with sumatriptan 50 mg was 32% (667/2114; range 14% to 68%).
The proportion of participants experiencing adverse events within 24 hours with placebo was 24% (389/1614; range 10% to 74%).
The relative harm of treatment compared with placebo was 1.3 (1.2 to 1.4; Analysis 4.11; Figure 5); the NNH was 13 (9.7 to 22).
4.11. Analysis.
Comparison 4 Oral sumatriptan 50 mg versus placebo, Outcome 11 Any adverse event within 24 h.
5.
Forest plot of comparison: 4 Oral sumatriptan 50 mg versus placebo, outcome: 4.11 Any adverse event within 24 h.
Sumatriptan 50 mg caused significantly more adverse events than sumatriptan 25 mg (z = 1.941; P = 0.052).
Six studies (1242 participants) in participants with mild baseline pain intensity provided data (Jelinski 2006; Nett 2003; Pini 1999; Tfelt‐Hansen 2006; Winner 2003 Study 1 and Study 2).
The proportion of participants experiencing adverse events within 24 hours with sumatriptan 50 mg was 16% (104/642; range 8% to 51%).
The proportion of participants experiencing adverse events within 24 hours with placebo was 7% (43/600; range 5% to 15%).
The relative harm of treatment compared with placebo was 2.3 (1.6 to 3.2; Analysis 4.11); the NNH was 11 (8.0 to 18).
Sumatriptan 100 mg versus placebo
Twelve studies (3257 participants) in participants with moderate or severe baseline pain intensity provided data (Cutler 1995; DKSMSG 1999; Dowson 2002; Ensink 1991; Geraud 2000; Goadsby 2000; Havanka 2000; Nappi 1994; Pfaffenrath 1998; Tfelt‐Hansen 1995; Tfelt‐Hansen 1998; Visser 1996).
The proportion of participants experiencing adverse events within 24 hours with sumatriptan 100 mg was 43% (931/2171; range 22% to 64%).
The proportion of participants experiencing adverse events within 24 hours with placebo was 23% (255/1086; range 6% to 74%).
The relative harm of treatment compared with placebo was 1.7 (1.5 to 1.9; Analysis 12.11); the NNH was 5.2 (4.4 to 6.2).
12.11. Analysis.
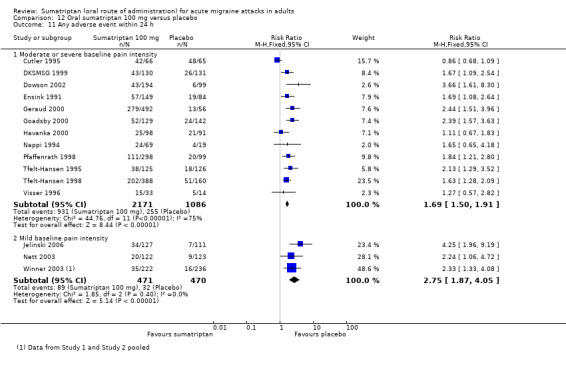
Comparison 12 Oral sumatriptan 100 mg versus placebo, Outcome 11 Any adverse event within 24 h.
Sumatriptan 100 mg caused significantly more adverse events than sumatriptan 50 mg (z = 5.379; P < 0.00006).
Four studies (941 participants) in participants with mild baseline pain intensity provided data (Jelinski 2006; Nett 2003; Winner 2003 Study 1 and Study 2).
The proportion of participants experiencing adverse events within 24 hours with sumatriptan 100 mg was 19% (89/471; range 12% to 27%).
The proportion of participants experiencing adverse events within 24 hours with placebo was 7% (32/470; range 6% to 8%).
The relative harm of treatment compared with placebo was 2.8 (1.9 to 4.1; Analysis 12.11); the NNH was 8.3 (6.1 to 13).
Sumatriptan 25 mg versus rizatriptan 5 mg
Two studies (1169 participants) in participants with moderate or severe baseline pain intensity provided data (Goldstein 1998; Kolodny 2004).
The proportion of participants experiencing adverse events within 24 hours with sumatriptan 25 mg was 43% (250/587; range 39% to 46%).
The proportion of participants experiencing adverse events within 24 hours with rizatriptan 5 mg was 41% (238/582; range 38% to 44%).
The relative harm of sumatriptan compared with rizatriptan was 1.0 (0.91 to 1.2; Analysis 2.5); there was no significant difference between the two treatments.
2.5. Analysis.
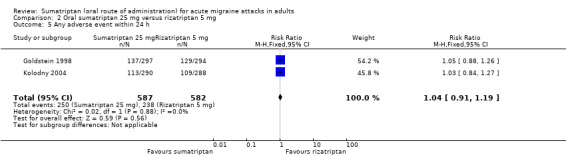
Comparison 2 Oral sumatriptan 25 mg versus rizatriptan 5 mg, Outcome 5 Any adverse event within 24 h.
Sumatriptan 25 mg versus rizatriptan 10 mg
Two studies (1186 participants) in participants with moderate or severe baseline pain intensity provided data (Goldstein 1998; Kolodny 2004).
The proportion of participants experiencing adverse events within 24 hours with sumatriptan 25 mg was 43% (250/587; range 39% to 46%).
The proportion of participants experiencing adverse events within 24 hours with rizatriptan 10 mg was 46% (276/599; range 45% to 47%).
The relative harm of sumatriptan compared with rizatriptan was 0.92 (0.81 to 1.1; Analysis 3.5); there was no significant difference between the two treatments.
3.5. Analysis.
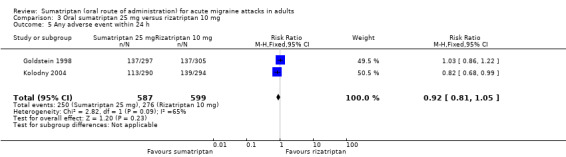
Comparison 3 Oral sumatriptan 25 mg versus rizatriptan 10 mg, Outcome 5 Any adverse event within 24 h.
Sumatriptan 50 mg versus effervescent acetylsalicylic acid 1000 mg
Two studies (730 participants) in participants with moderate or severe baseline pain intensity provided data (Diener 2004a; Diener 2004b).
The proportion of participants experiencing adverse events within 24 hours with sumatriptan 50 mg was 18% (64/361; range 14% to 20%).
The proportion of participants experiencing adverse events within 24 hours with effervescent ASA 1000 mg was 15% (55/369; range 13% to 16%).
The relative harm of sumatriptan compared with effervescent ASA was 1.2 (0.85 to 1.6; Analysis 5.5); there was no significant difference between the two treatments.
5.5. Analysis.
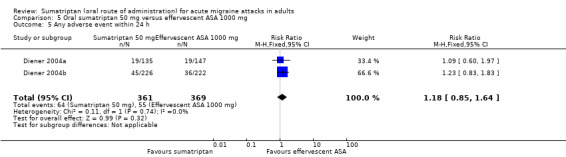
Comparison 5 Oral sumatriptan 50 mg versus effervescent ASA 1000 mg, Outcome 5 Any adverse event within 24 h.
Sumatriptan 50 mg versus zolmitriptan 2.5 mg
Two studies (1771 participants) in participants with moderate or severe baseline pain intensity provided data (Gallagher 2000; Gruffyd‐Jones 2001).
The proportion of participants experiencing adverse events within 24 hours with sumatriptan 50 mg was 32% (290/893; range 29% to 34%).
The proportion of participants experiencing adverse events within 24 hours with zolmitriptan 2.5 mg was 32% (283/878; range 28% to 35%).
The relative harm of sumatriptan compared with zolmitriptan was 1.0 (0.88 to 1.2; Analysis 6.3); there was no significant difference between the two treatments.
6.3. Analysis.
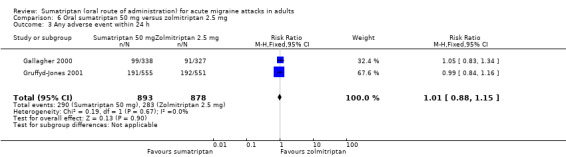
Comparison 6 Oral sumatriptan 50 mg versus zolmitriptan 2.5 mg, Outcome 3 Any adverse event within 24 h.
Sumatriptan 50 mg versus zolmitriptan 5 mg
Two studies (1790 participants) in participants with moderate or severe baseline pain intensity provided data (Gallagher 2000; Gruffyd‐Jones 2001).
The proportion of participants experiencing adverse events within 24 hours with sumatriptan 50 mg was 32% (290/893; range 29% to 34%).
The proportion of participants experiencing adverse events within 24 hours with zolmitriptan 5 mg was 36% (322/897; range 33% to 38%).
The relative harm of sumatriptan compared with zolmitriptan was 0.91 (0.80 to 1.0; Analysis 7.3); there was no significant difference between the two treatments.
7.3. Analysis.
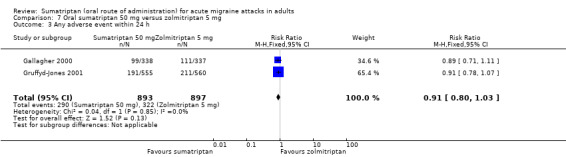
Comparison 7 Oral sumatriptan 50 mg versus zolmitriptan 5 mg, Outcome 3 Any adverse event within 24 h.
Sumatriptan 50 mg versus rizatriptan 5 mg
Two studies (1160 participants) in participants with moderate or severe baseline pain intensity provided data (Goldstein 1998; Kolodny 2004).
The proportion of participants experiencing adverse events within 24 hours with sumatriptan 50 mg was 48% (276/578; range 46% to 49%).
The proportion of participants experiencing adverse events within 24 hours with rizatriptan 5 mg was 41% (238/582; range 38% to 44%).
The relative harm of sumatriptan compared with rizatriptan was 1.2 (1.0 to 1.3; Analysis 8.5); there was no significant difference between the two treatments.
8.5. Analysis.
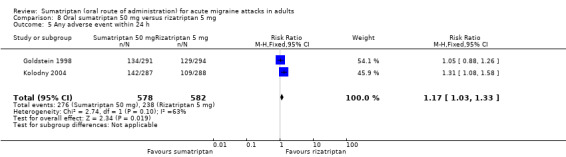
Comparison 8 Oral sumatriptan 50 mg versus rizatriptan 5 mg, Outcome 5 Any adverse event within 24 h.
Sumatriptan 50 mg versus rizatriptan 10 mg
Two studies (1177 participants) in participants with moderate or severe baseline pain intensity provided data (Goldstein 1998; Kolodny 2004).
The proportion of participants experiencing adverse events within 24 hours with sumatriptan 50 mg was 48% (276/578; range 46% to 49%).
The proportion of participants experiencing adverse events within 24 hours with rizatriptan 10 mg was 46% (276/599; range 45% to 47%).
The relative harm of sumatriptan compared with rizatriptan was 1.0 (0.92 to 1.2; Analysis 9.5); there was no significant difference between the two treatments.
9.5. Analysis.
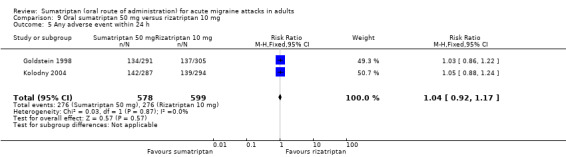
Comparison 9 Oral sumatriptan 50 mg versus rizatriptan 10 mg, Outcome 5 Any adverse event within 24 h.
Sumatriptan 100 mg versus rizatriptan 10 mg
Two studies (856 participants) in participants with moderate or severe baseline pain intensity provided data (Tfelt‐Hansen 1998; Visser 1996).
The proportion of participants experiencing adverse events within 24 hours with sumatriptan 100 mg was 52% (217/421; range 45% to 52%).
The proportion of participants experiencing adverse events within 24 hours with rizatriptan 10 mg was 47% (203/435; range 47% to 48%).
The relative harm of sumatriptan compared with rizatriptan was 1.1 (0.96 to 1.3; Analysis 15.3); there was no significant difference between the two treatments.
15.3. Analysis.
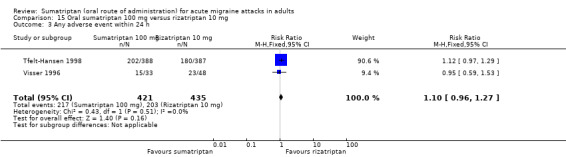
Comparison 15 Oral sumatriptan 100 mg versus rizatriptan 10 mg, Outcome 3 Any adverse event within 24 h.
Sumatriptan 100 mg versus acetylsalicylic acid 900 mg + metoclopramide 10 mg
Two studies (621 participants) in participants with moderate or severe baseline pain intensity provided data (Tfelt‐Hansen 1995; Thomson 1992).
The proportion of participants experiencing adverse events within 24 hours with sumatriptan 100 mg was 37% (112/300; range 30% to 42%).
The proportion of participants experiencing adverse events within 24 hours with ASA 900 mg + MCP 10 mg was 24% (78/321; range 18% to 29%).
The relative harm of sumatriptan compared with ASA + MCP was 1.5 (1.2 to 1.9; Analysis 18.4); the NNH was 7.7 (4.9 to 17).
18.4. Analysis.
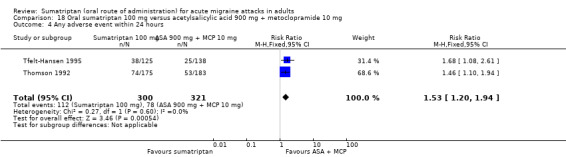
Comparison 18 Oral sumatriptan 100 mg versus acetylsalicylic acid 900 mg + metoclopramide 10 mg, Outcome 4 Any adverse event within 24 hours.
| Summary of results E: Number of participants experiencing any adverse event within 24 hours of study treatment | ||||||
| Comparison | Studies | Participants | Treatment (%) | Comparator (%) | Relative risk (95% CI) | NNH (95% CI) |
| Sumatriptan 25 mg versus placebo | 4 | 1550 | 39 | 37 | 1.1 (1.0 to 1.3) | Not calculated |
| Sumatriptan 50 mg versus placebo (in participants with moderate or severe baseline pain intensity) | 10 | 3728 | 32 | 24 | 1.3 (1.2 to 1.4) | 13 (9.7 to 22) |
| Sumatriptan 50 mg versus placebo (in participants with mild baseline pain intensity) | 6 | 1242 | 16 | 7 | 2.3 (1.6 to 3.2) | 11 (8.0 to 18) |
| Sumatriptan 100 mg versus placebo (in participants with moderate or severe baseline pain intensity) | 12 | 3257 | 43 | 23 | 1.7 (1.5 to 1.9) | 5.2 (4.4 to 6.2) |
| Sumatriptan 100 mg versus placebo (in participants with mild baseline pain intensity) | 4 | 941 | 19 | 7 | 2.8 (1.9 to 4.1) | 8.3 (6.1 to 13) |
| Sumatriptan 25 mg versus rizatriptan 5 mg | 2 | 1169 | 43 | 41 | 1.0 (0.91 to 1.2) | Not calculated |
| Sumatriptan 25 mg versus rizatriptan 10 mg | 2 | 1186 | 43 | 46 | 0.92 (0.81 to 1.1) | Not calculated |
| Sumatriptan 50 mg versus effervescent ASA 1000 mg | 2 | 730 | 18 | 15 | 1.2 (0.85 to 1.6) | Not calculated |
| Sumatriptan 50 mg versus zolmitriptan 2.5 mg | 2 | 1771 | 32 | 32 | 1.0 (0.88 to 1.2) | Not calculated |
| Sumatriptan 50 mg versus zolmitriptan 5 mg | 2 | 1790 | 32 | 36 | 0.91 (0.80 to 1.0) | Not calculated |
| Sumatriptan 50 mg versus rizatriptan 5 mg | 2 | 1160 | 48 | 41 | 1.2 (1.0 to 1.3) | Not calculated |
| Sumatriptan 50 mg versus rizatriptan 10 mg | 2 | 1177 | 48 | 46 | 1.0 (0.92 to 1.2) | Not calculated |
| Sumatriptan 100 mg versus rizatriptan 10 mg | 2 | 856 | 52 | 47 | 1.1 (0.96 to 1.3) | Not calculated |
| Sumatriptan 100 mg versus ASA 900 mg + MCP 10 mg | 2 | 621 | 37 | 24 | 1.5 (1.2 to 1.9) | 7.7 (4.9 to 17) |
| Summary of results F: Statistical tests for the effect of dose and baseline pain intensity | ||
| z | P | |
| Sumatriptan 25 mg versus 50 mg | 1.941 | 0.052 |
| Sumatriptan 50 mg versus 100 mg | 5.379 | <0.00006 |
There was a clear dose response relationship for sumatriptan in comparisons with placebo, with significantly more participants experiencing adverse events with each dose increment (NNHs of 5.2, 13, and 'not statistically significant' for sumatriptan 100 mg, 50 mg, and 25 mg, respectively). There was no significant difference between sumatriptan 25 mg, 50 mg, or 100 mg and rizatriptan at either 5 mg or 10 mg. Likewise there was no significant difference in incidence of adverse events within 24 hours between sumatriptan 50 mg and effervescent ASA 1000 mg, or zolmitriptan 2.5 mg and 5 mg. Only in the comparison between sumatriptan 100 mg and ASA 900 mg + MCP 10 mg was sumatriptan significantly worse than its comparator (NNH of 7.7).
Sensitivity analyses
We carried out sensitivity analyses to take into consideration and assess the effect of including adverse event data collected from participants who may have had more than one dose of study medication. Where there were sufficient data based on only a single dose to provide a meaningful analysis, we performed sensitivity analyses simply to remove the potentially contaminated data.
Sumatriptan 25 mg versus placebo
Of the four studies originally analysed comparing sumatriptan 25 mg with placebo for incidence of any adverse event within 24 hours, one offered participants a second dose of study medication (Pfaffenrath 1998). Removing this study from the analysis made no significant difference to the calculated relative risk of treatment versus placebo (analysis not shown).
Sumatriptan 50 mg versus placebo
Of the 10 studies originally analysed comparing sumatriptan 50 mg with placebo for incidence of any adverse event within 24 hours, three offered participants a second dose of study medication (Kudrow 2005; Pfaffenrath 1998; Savani 1999). Removing these studies from the analysis made no significant difference to the calculated relative risk of treatment versus placebo (analysis not shown).
Sumatriptan 100 mg versus placebo
Of the 12 studies originally analysed comparing sumatriptan 50 mg with placebo for incidence of any adverse event within 24 hours, four offered participants a second dose of study medication and did not report adverse events according to the number of doses received (Dowson 2002; Ensink 1991; Goadsby 2000; Pfaffenrath 1998). Removing these studies from the analysis made no significant difference to the calculated relative risk of treatment versus placebo (analysis not shown).
Participants experiencing specific adverse events
Six studies did not report on the incidence of individual adverse events (Diener 2004b; Dodick 2002; Goadsby 1991; Lines 2001; Lipton 2000; Sandrini 2007), and a further four studies reported only the incidence of events by body system affected (Bussone 2000; Diener 2004a; Goldstein 2005; Pini 1999). The remaining 51 studies reported the incidence of at least one specific adverse event, although there was significant variability in the manner of reporting that further limited the number of studies providing data for pooled analyses. Two studies (Latere 1991; Myllyla 1998) reported the number of events, rather than the number of participants experiencing an event in each treatment arm, and therefore did not provide data for analysis. One study (Tfelt‐Hansen 2006) reported only the total number of participants experiencing a specific adverse event, and did not break this down by treatment arm. As discussed previously, the duration over which adverse event data were collected varied between studies and, as with the total incidence of adverse events, 11 studies (160‐104; Dahlof 2009; GL/MIG/001/92; GL/MIG/001A/92; GL/MIG/002; GL/MIG/002A; Mathew 2003; Pini 1995; Sargent 1995; Sheftell 2005 Study 1 and Study 2) were not included in pooled analyses due to inappropriate collection periods.
Individual adverse events were reported inconsistently between studies. The majority of studies reported only the most commonly occurring adverse events, for example those occurring in more than 3% of participants in any of the treatment arms, while others used different terms to describe the same or similar events. In order to be as inclusive as possible we have pooled related adverse events into groups (described in detail in Appendix 8). Where one study provided data on more than one event in a particular group, for example reporting both malaise/fatigue and asthenia, we have counted the event with the higher incidence and ignored the other in order not to double count participants, because it is possible that any one participant may have experienced both events. This will lead to an underestimation of incidence if all those with the less frequent event did not also have the more frequent one. Since there were no significant differences in the overall incidence of adverse events between participants treating attacks of mild baseline pain intensity and participants treating attacks of moderate or severe intensity, we performed analysis of individual adverse events on all participants, regardless of pain intensity at baseline.
One final limitation of these analyses was that a significant proportion of studies reported only events judged to be related to the study medication. In these cases it was not clear whether it was the event, in general terms, that was judged to be related, or whether it was a specific event in a specific patient. The latter may provide an underestimate of the incidence of this event in the study population as a whole, and therefore the results should be interpreted cautiously.
Where two or more placebo‐controlled studies reported data for at least 200 participants investigating the incidence of a specific adverse event within 24 hours of study treatment, we carried out pooled analysis to calculate the relative risk, and where appropriate the NNH (Summary of results G; Analysis 1.8; Analysis 4.12; Analysis 12.12; Analysis 19.3; Analysis 20.2).
1.8. Analysis.
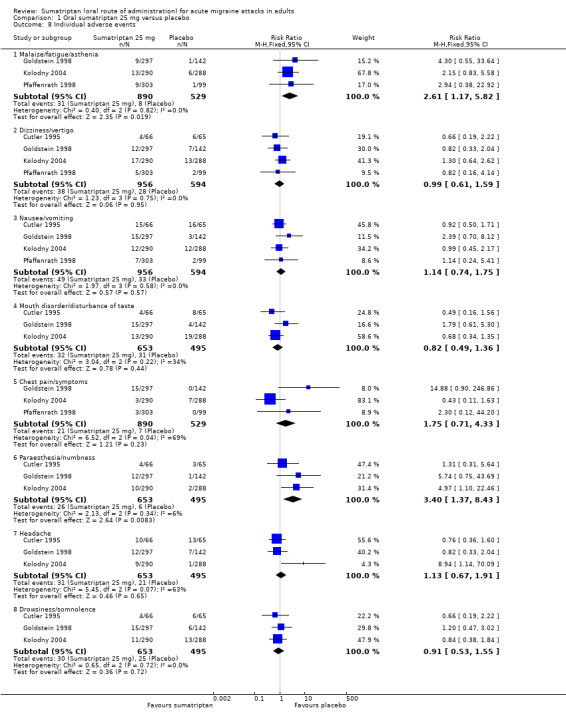
Comparison 1 Oral sumatriptan 25 mg versus placebo, Outcome 8 Individual adverse events.
4.12. Analysis.
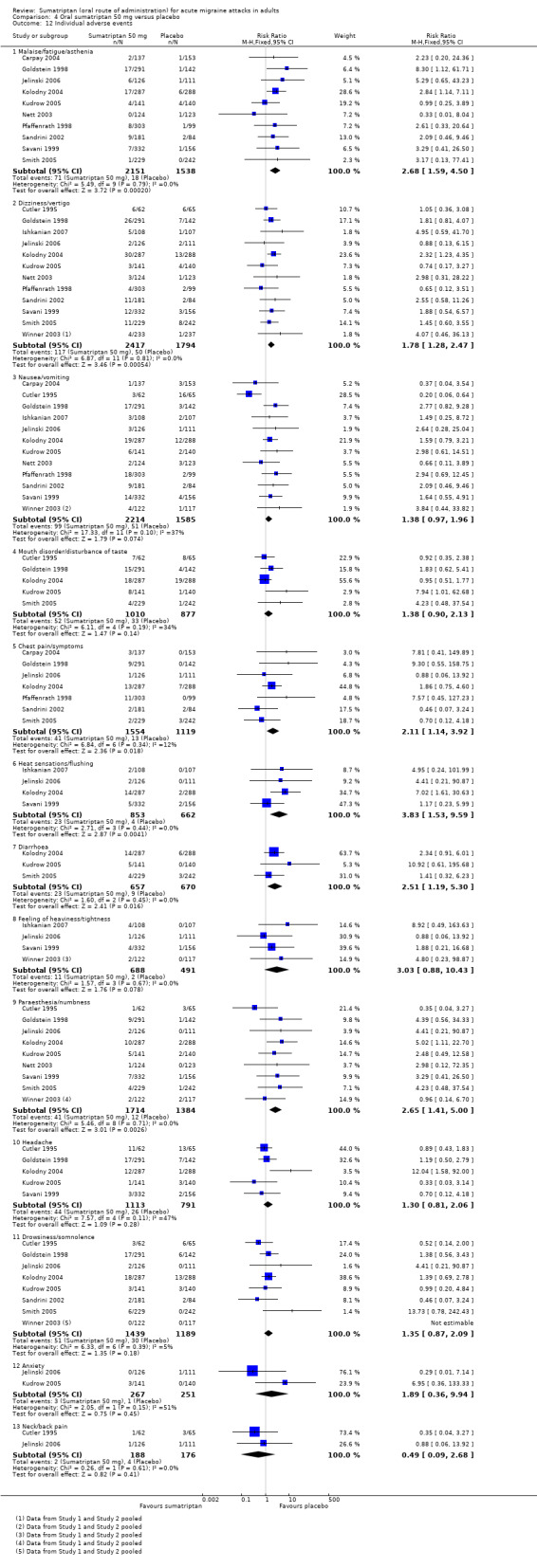
Comparison 4 Oral sumatriptan 50 mg versus placebo, Outcome 12 Individual adverse events.
12.12. Analysis.
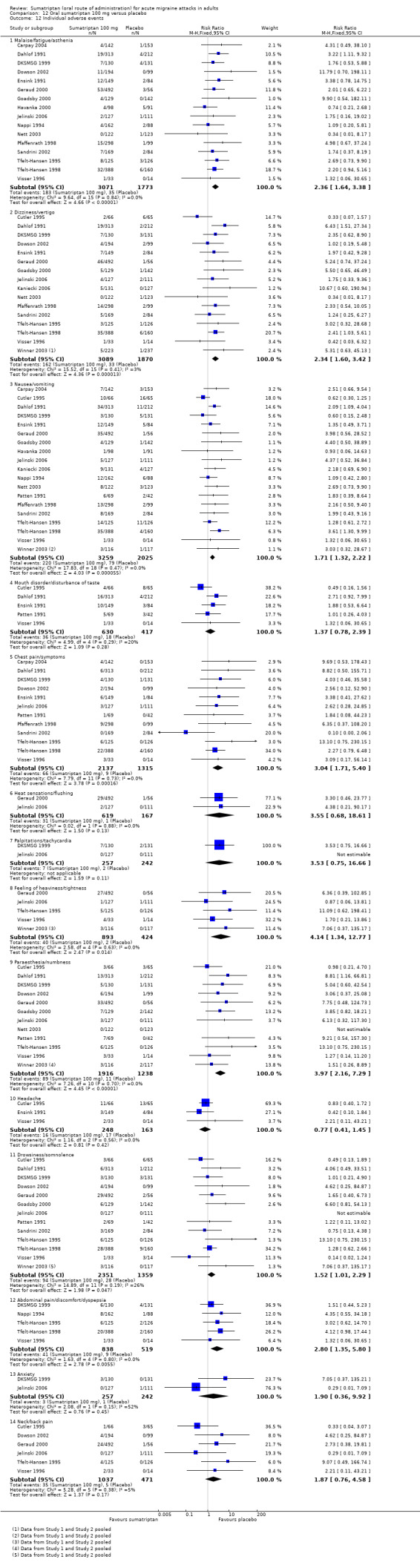
Comparison 12 Oral sumatriptan 100 mg versus placebo, Outcome 12 Individual adverse events.
19.3. Analysis.
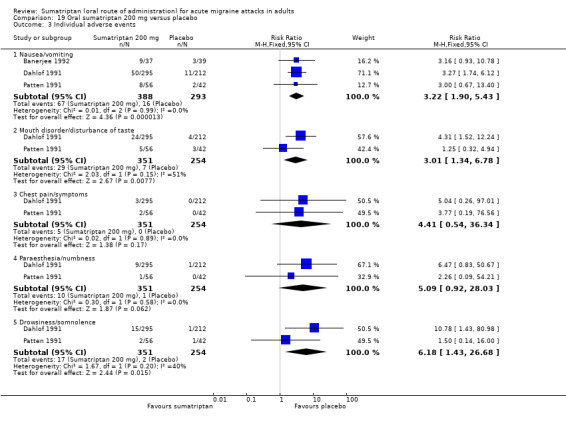
Comparison 19 Oral sumatriptan 200 mg versus placebo, Outcome 3 Individual adverse events.
20.2. Analysis.
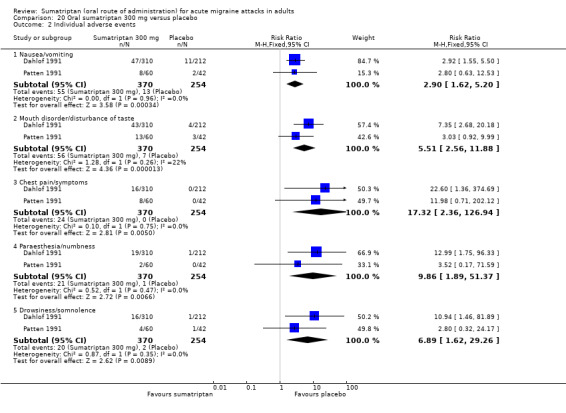
Comparison 20 Oral sumatriptan 300 mg versus placebo, Outcome 2 Individual adverse events.
| Summary of results G: Number of participants experiencing specific adverse events within 24 hours of study treatment in placebo‐controlled studies | ||||||
| Studies |
Participants treated |
Treatment (%) | Placebo (%) | Relative risk (95% CI) | NNH (95% CI) | |
| Malaise/fatigue/asthenia | ||||||
| Sumatriptan 25 mg | 3 | 1419 | 3 | 2 | 2.6 (1.2 to 5.8) | 51 (28 to 260) |
| Sumatriptan 50 mg | 10 | 3689 | 3 | 1 | 2.7 (1.6 to 4.5) | 47 (33 to 85) |
| Sumatriptan 100 mg | 16 | 4844 | 6 | 2 | 2.4 (1.6 to 3.4) | 25 (20 to 35) |
| Dizziness/vertigo | ||||||
| Sumatriptan 25 mg | 4 | 1550 | 4 | 5 | 0.99 (0.61 to 1.6) | Not calculated |
| Sumatriptan 50 mg | 13 | 4211 | 5 | 3 | 1.8 (1.3 to 2.5) | 49 (31 to 110) |
| Sumatriptan 100 mg | 17 | 4959 | 5 | 2 | 2.3 (1.6 to 3.4) | 29 (22 to 41) |
| Nausea/vomiting | ||||||
| Sumatriptan 25 mg | 4 | 1550 | 5 | 6 | 1.1 (0.74 to 1.8) | Not calculated |
| Sumatriptan 50 mg | 13 | 3799 | 4 | 3 | 1.4 (0.97 to 2.0) | Not calculated |
| Sumatriptan 100 mg | 20 | 5284 | 7 | 4 | 1.7 (1.3 to 2.2) | 35 (25 to 61) |
| Sumatriptan 200 mg | 3 | 681 | 17 | 5 | 3.2 (1.9 to 5.4) | 8.5 (6.1 to 14) |
| Sumatriptan 300 mg | 2 | 624 | 15 | 5 | 2.9 (1.6 to 5.2) | 10 (7.0 to 19) |
| Mouth disorder/disturbance of taste | ||||||
| Sumatriptan 25 mg | 3 | 1148 | 5 | 6 | 0.82 (0.49 to 1.4) | Not calculated |
| Sumatriptan 50 mg | 5 | 1887 | 5 | 4 | 1.4 (0.90 to 2.1) | Not calculated |
| Sumatriptan 100 mg | 5 | 1047 | 6 | 4 | 1.4 (0.78 to 2.4) | Not calculated |
| Sumatriptan 200 mg | 2 | 605 | 8 | 3 | 3.0 (1.3 to 6.7) | 18 (11 to 50) |
| Sumatriptan 300 mg | 2 | 624 | 15 | 3 | 5.5 (2.6 to 12) | 8.1 (6.0 to 12) |
| Chest pain/symptoms | ||||||
| Sumatriptan 25 mg | 3 | 1419 | 2 | 1 | 1.8 (0.70 to 4.3) | Not calculated |
| Sumatriptan 50 mg | 7 | 2673 | 3 | 1 | 2.1 (1.1 to 3.9) | 69 (40 to 260) |
| Sumatriptan 100 mg | 12 | 3452 | 3 | 1 | 3.0 (1.7 to 5.4) | 44 (31 to 73) |
| Sumatriptan 200 mg | 2 | 605 | 1 | 0 | 4.4 (0.54 to 36) | Not calculated |
| Sumatriptan 300 mg | 2 | 624 | 6 | 0 | 17 (2.4 to 127) | 16 (11 to 27) |
| Heat sensations/flushing | ||||||
| Sumatriptan 50 mg | 4 | 1515 | 3 | 1 | 3.8 (1.5 to 9.6) | 49 (30 to 130) |
| Sumatriptan 100 mg | 2 | 786 | 5 | 1 | 3.6 (0.68 to 19) | Not calculated |
| Palpitations/tachycardia | ||||||
| Sumatriptan 100 mg | 2 | 499 | 3 | 1 | 3.5 (0.75 to 17) | Not calculated |
| Diarrhoea | ||||||
| Sumatriptan 50 mg | 3 | 1327 | 4 | 1 | 2.5 (1.2 to 5.3) | 46 (26 to 210) |
| Feeling of heaviness/tightness | ||||||
| Sumatriptan 50 mg | 5 | 1179 | 2 | 0 | 3.0 (0.88 to 10) | Not calculated |
| Sumatriptan 100 mg | 6 | 1317 | 4 | 0 | 4.1 (1.3 to 13) | 26 (18 to 46) |
| Paraesthesia/numbness | ||||||
| Sumatriptan 25 mg | 3 | 1148 | 4 | 1 | 3.4 (1.4 to 8.4) | 36 (22 to 100) |
| Sumatriptan 50 mg | 10 | 3089 | 2 | 1 | 2.7 (1.4 to 5.0) | 66 (42 to 160) |
| Sumatriptan 100 mg | 13 | 3154 | 5 | 1 | 27 (21 to 37) | |
| Sumatriptan 200 mg | 2 | 605 | 3 | 0 | 5.1 (0.92 to 28) | Not calculated |
| Sumatriptan 300 mg | 2 | 624 | 6 | 0 | 9.9 (1.9 to 51) | 19 (13 to 38) |
| Headache | ||||||
| Sumatriptan 25 mg | 3 | 1148 | 5 | 4 | 1.1 (0.67 to 1.9) | Not calculated |
| Sumatriptan 50 mg | 5 | 1904 | 4 | 3 | 1.3 (0.82 to 2.1) | Not calculated |
| Sumatriptan 100 mg | 3 | 411 | 6 | 10 | 0.77 (0.41 to 1.4) | Not calculated |
| Drowsiness/somnolence | ||||||
| Sumatriptan 25 mg | 3 | 1148 | 5 | 5 | 0.91 (0.53 to 1.6) | Not calculated |
| Sumatriptan 50 mg | 9 | 2628 | 4 | 3 | 1.4 (0.87 to 2.1) | Not calculated |
| Sumatriptan 100 mg | 14 | 3710 | 4 | 2 | 1.5 (1.0 to 2.3) | 53 (33 to 130) |
| Sumatriptan 200 mg | 2 | 605 | 5 | 1 | 6.2 (1.4 to 27) | 25 (15 to 64) |
| Sumatriptan 300 mg | 2 | 624 | 5 | 1 | 6.9 (1.6 to 29) | 22 (14 to 48) |
| Abdominal pain/discomfort/dyspepsia | ||||||
| Sumatriptan 100 mg | 5 | 1357 | 5 | 2 | 3.0 (1.4 to 6.3) | 32 (20 to 76) |
| Anxiety | ||||||
| Sumatriptan 50 mg | 2 | 518 | 1 | 0 | 1.9 (0.36 to 9.9) | Not calculated |
| Sumatriptan 100 mg | 2 | 499 | 1 | 0 | 1.9 (0.36 to 9.9) | Not calculated |
| Neck/back pain | ||||||
| Sumatriptan 50 mg | 2 | 364 | 1 | 2 | 0.49 (0.09 to 2.7) | Not calculated |
| Sumatriptan 100 mg | 6 | 1508 | 3 | 1 | 1.9 (0.73 to 4.7) | Not calculated |
We also analysed studies for the incidence of specific adverse events in sumatriptan versus active comparators and, where appropriate, calculated NNHs (Appendix 9). For the majority of adverse events there was no significant difference between sumatriptan and the comparator. Sumatriptan 100 mg was found to cause significantly fewer occurrences of malaise/fatigue/asthenia and nausea/vomiting than eletriptan 80 mg (NNHs of ‐17 and ‐24 respectively). Sumatriptan 25 mg caused fewer occurrences of dizziness/vertigo and drowsiness/somnolence than rizatriptan 10 mg (NNHs of ‐20 and ‐34 respectively). Conversely, sumatriptan 50 mg caused significantly more headache than rizatriptan of either 5 or 10 mg (NNHs of 38 and 34 respectively), while sumatriptan 100 mg caused significantly more nausea/vomiting, chest pain/symptoms, feeling of heaviness/tightness, and paraesthesia/numbness than ASA 900 mg + MCP 10 mg (NNHs of 19, 33, 33, and 37 respectively).
Participants experiencing serious adverse events
Twenty‐five studies did not specifically comment on serious adverse events (Cutler 1995; Diener 2004a; Dodick 2002; Dowson 2002; Ensink 1991; Freitag 2001; GL/MIG/001/92; GL/MIG/001A/92; GL/MIG/002; GL/MIG/002A; Goadsby 1991; Goadsby 2000; Havanka 2000; Ishkanian 2007; Kolodny 2004; Kudrow 2005; Lines 2001; Myllyla 1998; Nappi 1994; Patten 1991; Pfaffenrath 1998; Pini 1995; Sargent 1995; Tfelt‐Hansen 2006; Thomson 1992), 15 studies reported that there were none during the study (Bussone 2000; Carpay 2004; DKSMSG 1999; Goldstein 2005; Gruffyd‐Jones 2001; Jelinski 2006; Kaniecki 2006; Nett 2003; Pini 1999; Schulman 2003; Smith 2005; Spierings 2001; Tfelt‐Hansen 1998; Winner 2003 Study 1 and Study 2), and six reported that there were no drug‐related serious adverse events (Geraud 2000; Goldstein 1998; Mathew 2003; Sandrini 2002; Sheftell 2005 Study 1 and Study 2). The remaining 15 studies all reported at least one serious adverse event, although most were judged to be unrelated to any study medication.
In studies reporting occurrence of serious adverse events separately for sumatriptan and comparator treatment arms (160‐104; Banerjee 1992; Brandes 2007; Dahlof 1991; Diener 2004b; GL/MIG/009; Latere 1991; Sandrini 2007; Visser 1996), or the absence of such events (Bussone 2000; Carpay 2004; DKSMSG 1999; Goldstein 2005; Gruffyd‐Jones 2001; Jelinski 2006; Kaniecki 2006; Nett 2003; Pini 1999; Schulman 2003; Smith 2005; Spierings 2001; Tfelt‐Hansen 1998; Winner 2003 Study 1 and Study 2), the incidence was less than 1% in any treatment arm, except three (160‐104 placebo group, 2/93 treated participants; Banerjee 1992 placebo group, 1/39 treated participants; Visser 1996 rizatriptan 20 mg group, 1/82 treated participants).
Sumatriptan versus placebo
Eighteen studies (7687 participants) provided data on sumatriptan of any dose versus placebo (160‐104; Banerjee 1992; Brandes 2007 Study 1 and Study 2; Bussone 2000; Carpay 2004; Dahlof 1991; Diener 2004b; DKSMSG 1999; Goldstein 2005; Jelinski 2006; Kaniecki 2006; Nett 2003; Pini 1999; Smith 2005; Tfelt‐Hansen 1998; Visser 1996; Winner 2003 Study 1 and Study 2).
The overall incidence of serious adverse events was 0.12% (6/4829) for all doses of sumatriptan (including second doses and rescue medication), and 0.10% (3/2858) for placebo. There were too few events to calculate relative risk or NNH. Further details of individual studies are in Appendix 7.
Sumatriptan versus active comparators
Sixteen studies (11,599 participants/attacks) provided data on sumatriptan of any dose versus active comparators (160‐104; Brandes 2007 Study 1 and Study 2; Diener 2004b; DKSMSG 1999; Gallagher 2000; GL/MIG/009; Goldstein 2005; Gruffyd‐Jones 2001; Latere 1991; Sandrini 2007; Schulman 2003; Smith 2005; Spierings 2001; Tfelt‐Hansen 1998; Visser 1996). In all cases there were too few events to calculate relative risk or NNH.
Three studies (1946 participants) comparing sumatriptan with naproxen provided data (Brandes 2007 Study 1 and Study 2; Smith 2005). The overall incidence was 0.10% (1/964) for all doses of sumatriptan (50 to 85 mg), and 0% (0/982) for naproxen (500 mg).
Three studies (1952 participants) comparing sumatriptan with sumatriptan + naproxen provided data (Brandes 2007 Study 1 and Study 2; Smith 2005). The overall incidence was 0.10% (1/964) for all doses of sumatriptan (50 to 85 mg), and 0% (0/988) for sumatriptan + naproxen (50‐85/500 mg).
Two studies (2878 participants/attacks) comparing sumatriptan with zolmitriptan provided data (Gallagher 2000; Gruffyd‐Jones 2001). The overall incidence was 0.51% (6/1167) for all doses of sumatriptan (25 to 50 mg), and 0.23% (4/1711) for all doses of zolmitriptan (2.5 to 5 mg).
Two studies (1303 participants) comparing sumatriptan with rizatriptan provided data (Tfelt‐Hansen 1998; Visser 1996). The overall incidence was 0% (0/460) for sumatriptan (100 mg), and 0.12% (1/843) for all doses of rizatriptan (5 to 40 mg).
Withdrawals due to adverse events
Thirty studies did not specifically report on adverse event withdrawals or did not report data for each treatment arm separately. The remaining 31 studies reported the number of withdrawals due to adverse events per treatment group (160‐104; Banerjee 1992; Dahlof 1991; Dahlof 2009; DKSMSG 1999; Dowson 2002; Gallagher 2000; GL/MIG/001/92; GL/MIG/001A/92; GL/MIG/002; GL/MIG/002A; GL/MIG/009; Goadsby 2000; Goldstein 1998; Gruffyd‐Jones 2001; Havanka 2000; Latere 1991; Nappi 1994; Nett 2003; Patten 1991; Pfaffenrath 1998; Sandrini 2002; Sandrini 2007; Sargent 1995; Sheftell 2005 Study 1 and Study 2; Spierings 2001; Tfelt‐Hansen 1995; Tfelt‐Hansen 1998; Thomson 1992; Visser 1996). Some of these studies either did not report how multiple attacks were combined or reported only drug‐related adverse event withdrawals, but were included anyway in order to be more inclusive and conservative.
In studies reporting the occurrence of adverse event withdrawals, nine reported none (Dahlof 2009; Dowson 2002; Havanka 2000; Nett 2003; Sargent 1995; Sheftell 2005 Study 1 and Study 2; Spierings 2001; Visser 1996), four reported an incidence in any treatment arm of less than 2% (DKSMSG 1999; Goadsby 2000; Goldstein 1998; Tfelt‐Hansen 1998), 12 reported an incidence in any treatment arm of less than 5% (Gallagher 2000; GL/MIG/001A/92; GL/MIG/002; GL/MIG/002A; Gruffyd‐Jones 2001; Latere 1991; Nappi 1994; Pfaffenrath 1998; Sandrini 2002; Sandrini 2007; Tfelt‐Hansen 1995; Thomson 1992), and six studies reported an incidence of greater than 5% in at least one treatment arm (160‐104; Banerjee 1992; Dahlof 1991; GL/MIG/001/92; GL/MIG/009; Patten 1991).
Sumatriptan versus placebo
Nineteen studies (10,059 participants) provided data on sumatriptan of any dose versus placebo (160‐104; Banerjee 1992; Dahlof 1991; Dahlof 2009; DKSMSG 1999; Dowson 2002; Goadsby 2000; Goldstein 1998; Havanka 2000; Nappi 1994; Nett 2003; Patten 1991; Pfaffenrath 1998; Sandrini 2002; Sargent 1995; Sheftell 2005; Tfelt‐Hansen 1995; Tfelt‐Hansen 1998; Visser 1996).
The overall incidence of adverse event withdrawal was 1.6% (113/7133) for all doses of sumatriptan (including second doses and rescue medication), and 0.65% (19/2926) for placebo. If studies using initial doses of sumatriptan of greater than 100 mg were removed from this analysis, the overall incidence of adverse event withdrawal was 0.71% (45/6349) for all doses of sumatriptan ≤ 100 mg. A total of 1041 participants provided data comparing sumatriptan > 100 mg with placebo, giving an NNH of 14 (10 to 23) for treatment with sumatriptan. Other than for doses > 100 mg there was no evidence of a dose response relationship, and there were too few events to calculate relative risk or NNH. Further details of individual studies are in Appendix 7.
Sumatriptan versus active comparators
Twenty‐two studies (15,099 participants) provided data on sumatriptan of any dose versus active comparators (160‐104; Dahlof 2009; DKSMSG 1999; Dowson 2002; Gallagher 2000; GL/MIG/001/92; GL/MIG/001A/92; GL/MIG/002; GL/MIG/002A; GL/MIG/009; Goadsby 2000; Goldstein 1998; Gruffyd‐Jones 2001; Havanka 2000; Latere 1991; Sandrini 2002; Sandrini 2007; Spierings 2001; Tfelt‐Hansen 1995; Tfelt‐Hansen 1998; Thomson 1992; Visser 1996). In all cases there were too few events to calculate relative risk or NNH.
Two studies (1742 participants) comparing sumatriptan 50 mg or 100 mg with almotriptan 12.5 mg or 25 mg provided data (Dowson 2002; Spierings 2001). Neither trial reported any withdrawals due to adverse events.
Two studies (3004 participants/attacks) comparing sumatriptan 25 mg or 50 mg with zolmitriptan 2.5 mg or 5 mg provided data (Gallagher 2000; Gruffyd‐Jones 2001). The overall incidence was 2.5% (31/1229) for all doses of sumatriptan, and 2.9% (52/1775) for all doses of zolmitriptan.
Two studies (1328 participants) comparing sumatriptan 100 mg with paracetamol 100 mg + MCP 10 mg provided data (GL/MIG/001/92; GL/MIG/001A/92). The overall incidence was 4.7% (31/653) for sumatriptan, and 1.9% (13/675) for paracetamol + MCP.
Two studies (1426 participants) comparing sumatriptan 100 mg with buclizine hydrochloride 12.5 mg + paracetamol 1000 mg + codeine phosphate 16 mg (Migraleve) provided data (GL/MIG/002; GL/MIG/002A). The overall incidence was 4.6% (33/716) for sumatriptan, and 1.7% (12/710) for Migraleve.
Three studies (1779 participants) comparing sumatriptan 25 mg, 50 mg, or 100 mg with eletriptan 20 mg, 40 mg, or 80 mg provided data (160‐104; Goadsby 2000; Sandrini 2002). The overall incidence was 1.2% (10/841) for sumatriptan, and 1.3% (12/938) for eletriptan.
Three studies (2482 participants) comparing sumatriptan 25 mg, 50 mg, or 100 mg with rizatriptan 5 mg or 10 mg provided data (Goldstein 1998; Tfelt‐Hansen 1998; Visser 1996). The overall incidence was 0.38% (4/1048) for sumatriptan, and 0.28% (4/1434) for rizatriptan.
Two studies (621 participants) comparing sumatriptan 100 mg with ASA 900 mg + MCP 10 mg provided data (Tfelt‐Hansen 1995; Thomson 1992). The overall incidence was 3.0% (9/300) for sumatriptan, and 0.31% (1/321) for ASA + MCP.
Consistency of response
Of the 27 included studies that involved participants treating more than one migraine attack, 13 provided some data on consistency of response to treatment across successive attacks. Consistency was addressed in two distinct ways in these 13 studies. Twelve studies (Bussone 2000; Gallagher 2000; GL/MIG/001/92; GL/MIG/001A/92; GL/MIG/002; GL/MIG/002A; GL/MIG/009; Gruffyd‐Jones 2001; Myllyla 1998; Pfaffenrath 1998; Tfelt‐Hansen 1995; Thomson 1992) reported the proportion of participants achieving a particular outcome (responding) for each successive attack separately, thereby providing a measure of the consistency of the population's response to treatment over successive attacks. Four studies (Gallagher 2000; Gruffyd‐Jones 2001; Pfaffenrath 1998; Sandrini 2002) reported on the proportion of participants achieving a particular outcome in a defined fraction (for example in two‐thirds) of their treated attacks. This provides a measure of the consistency of an individual's response to treatment over successive attacks.
Eight studies (GL/MIG/001/92; GL/MIG/001A/92; GL/MIG/002; GL/MIG/002A; Myllyla 1998; Pfaffenrath 1998; Tfelt‐Hansen 1995; Thomson 1992) provided separate data for the proportion of participants achieving headache relief at two hours with sumatriptan 100 mg in up to three successive attacks. Response rates ranged from 39% to 79% for an individual attack. There were no clear trends over successive attacks in the same study (Figure 6), suggesting that the proportion of participants achieving headache relief at two hours was consistent. Results for headache relief at two hours in participants with other doses of sumatriptan were similar and, in fact, no efficacy outcome for which data over successive attacks were reported separately showed any evidence of an inconsistent response. One study (Gallagher 2000) provided data on the incidence of adverse events over the course of six treated attacks. In this case, for both the 25 mg and 50 mg doses of sumatriptan, the proportion of participants experiencing an adverse event within 24 hours appeared to decrease as successive attacks were treated (Figure 7), such that the incidence of adverse events was much lower in participants treating their sixth attack than those treating their first.
6.
Consistency of response: proportion of participants treated with sumatriptan 100 mg achieving headache relief at 2 hours over successive attacks
7.
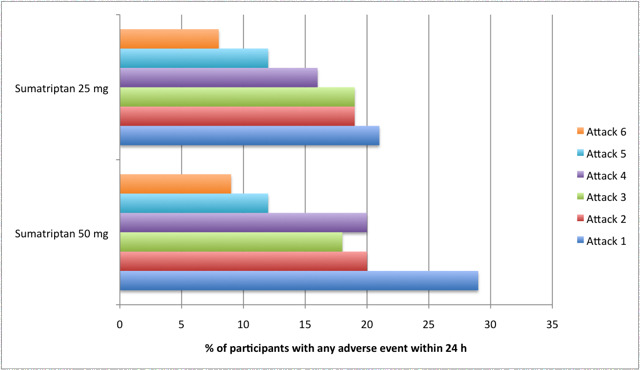
Consistency of response: proportion of participants treated with sumatriptan 25 mg or 50 mg experiencing any adverse events within 24 hours over successive attacks (data from Gallagher 2000)
Two studies (Pfaffenrath 1998; Sandrini 2002) reported the percentage of participants achieving headache relief at two hours in two‐thirds or more of their treated attacks. Approximately 50% of participants treated with sumatriptan 25 mg responded to treatment in at least 2/3 attacks, while about 55% did so when treated with sumatriptan 50 mg, and about 60% when treated with sumatriptan 100 mg. Two studies (Gallagher 2000; Gruffyd‐Jones 2001) reported the percentage of participants achieving headache relief at two hours in 80% or more of their treated attacks. Again, the lower dose of 25 mg resulted in a smaller fraction of participants achieving this level of consistency than the 50 mg dose (approximately 30% with sumatriptan 25 mg and 40% with sumatriptan 50 mg). Finally one study (Sandrini 2002) reported the proportion of participants experiencing headache relief at two hours in 3/3 of their treated attacks (i.e. 100%). Twenty‐two percent of participants treated with sumatriptan 50 mg achieved headache relief at two hours in every attack treated, while 24% of those treated with sumatriptan 100 mg did so.
Discussion
Summary of main results
This review included 61 randomised, double‐blind, controlled studies with 37,250 participants. Twenty‐four studies had only a placebo control, 13 had only active comparators, and 24 had both placebo and active comparators. Active comparators were isometheptene mucate + dichloralphenazone + acetaminophen, zolmitriptan, rizatriptan, tonabersat, effervescent acetylsalicylic acid, ibuprofen, paracetamol (acetaminophen) + aspirin + caffeine, valdecoxib, eletriptan, indomethacin + prochlorperazine + caffeine (Indoprocaf), sumatriptan + metoclopramide, sumatriptan + naproxen, naproxen, almotriptan, diclofenac potassium, naratriptan, ergotamine tartrate + caffeine (Cafergot), tolfenamic acid, acetylsalicylic acid + metoclopramide, buclizine hydrochloride + paracetamol + codeine phosphate (Migraleve), and ergotamine tartrate + cyclizine hydrochloride + caffeine hydrate (Migril). Sumatriptan was studied in doses of 25, 50, 85, 100, 200, and 300 mg in an oral tablet (either standard or disintegrating) formulation. Most of the data were for the 50 mg and 100 mg doses. In most studies participants treated established attacks of moderate to severe intensity, but some treated when pain was still mild. Separate analyses were carried out for these different levels of baseline pain.
For nearly all efficacy outcomes, sumatriptan of any dose was superior to placebo and gave clinically useful numbers needed to treat (NNTs), the one exception to this being pain‐free at one hour. The remarkably consistent response between studies for the primary outcomes, as illustrated by L'Abbé plots (Appendix 10), was not unexpected given the inclusion criteria for the studies and the well‐defined outcomes. There was a trend for lower (better) NNTs at higher doses, with significant differences between 50 mg and 100 mg for pain‐free and headache relief at two hours, and sustained pain‐free during the 24 hours postdose; and between 100 mg and 200 mg for headache relief at two hours. Where treatment of participants with mild baseline pain was compared with treatment at moderate or severe baseline pain, treatment while pain was still mild was found to be significantly more effective for outcomes of pain‐free at two hours and sustained pain‐free during 24 hours.
For the International Headache Society (IHS)‐preferred outcome of pain‐free at two hours, sumatriptan 25 mg, 50 mg, and 100 mg compared with placebo gave NNTs of 6.2, 6.1, and 4.7, respectively, in participants treating established attacks with moderate or severe pain. In participants treating attacks while pain was still mild, sumatriptan 50 mg and 100 mg compared with placebo gave NNTs of 4.4 and 3.0, respectively. About 30% of participants with moderate or severe baseline pain were responders with sumatriptan compared to 10% with placebo, while response rates were higher for participants with mild baseline pain (about 45% to 60% responders with sumatriptan compared to 24% with placebo). For pain‐free at one hour in participants with moderate or severe baseline pain, the NNTs were 33 and 18 for sumatriptan 50 mg and 100 mg, respectively (5% to 7% responders with sumatriptan, 2% with placebo). In participants with mild baseline pain, the NNTs were 8.5 and 6.0 for the 50 mg and 100 mg doses, respectively (about 25% to 30% responders with sumatriptan, 14% with placebo). For headache relief at one hour, sumatriptan 25 mg, 50 mg, and 100 mg compared with placebo gave NNTs of 9.0, 7.5, and 6.8, respectively (about 28% responders with sumatriptan, 13% with placebo), and for headache relief at two hours sumatriptan 25, 50, 100, 200, and 300 mg gave NNTs of 4.2, 4.0, 3.5, 2.1, and 2.4, respectively, when compared with placebo (about 60% to 70% responders with sumatriptan, 30% with placebo). For sustained pain‐free during the 24 hours postdose the NNTs for sumatriptan 50 mg and 100 mg in participants with moderate or severe baseline pain were 9.5 and 6.5, respectively (about 20% responders with sumatriptan, 8% with placebo), while in participants with mild baseline pain they were 5.5 and 4.5, respectively (about 30% responders with sumatriptan, 10% with placebo). The NNTs for sustained headache relief during 24 hours, treating with sumatriptan 50 mg and 100 mg were 6.0 and 5.2, respectively (about 35% responders with sumatriptan, 17% with placebo).
Data were available for the use of rescue medication, the relief of headache‐associated symptoms, and the relief of functional disability. The number of participants requiring rescue medication after treating with placebo (about 40% to 60%) was approximately double that after treating with sumatriptan 25 mg, 50 mg, or 100 mg (about 20% to 30%). This relationship appeared to hold regardless of whether the headache was treated early during the mild pain phase or when more established. Reported headache‐associated symptoms included nausea, vomiting, photophobia, and phonophobia; vomiting occurred too infrequently for reliable analysis. Sumatriptan 25 mg, 50 mg, and 100 mg compared with placebo gave NNTs of between 7 and 8 for relief of nausea at two hours, and between 4 and 8 for relief of photophobia and phonophobia. Approximately half of participants treated with sumatriptan achieved relief of these symptoms, compared with approximately one‐third of those treated with placebo. Functional disability was relieved (i.e. reduced from moderate or severe at baseline to mild or none at two hours) in approximately 50% of participants treated with sumatriptan 25 mg and 50 mg, and 60% of participants treated with sumatriptan 100 mg, compared with 25% to 30% of participants treated with placebo. This gave NNTs for relief of functional disability of 5.9, 5.6, and 3.6 for sumatriptan 25 mg, 50 mg, and 100 mg, respectively, when compared with placebo.
Analysis of adverse events was compromised by the fact that some studies did not specify the time period over which data were collected, and some specified time periods different from the 24‐hour period specified in our review protocol. Furthermore, studies allowed use of rescue medication for inadequate response (usually after two hours), and many allowed a second dose of study medication for headache recurrence (or sometimes lack of efficacy), without specifying whether adverse event data continued to be collected from participants who had taken additional medication. With these caveats, we chose to pool as much data as possible. More participants experienced adverse events with sumatriptan than with placebo, and a dose response relationship was seen over the range 25 mg to 100 mg, with NNHs ranging from 'not statistically significant' to 5.2. For the most part adverse events were described as mild to moderate in intensity and self limiting. It was noteworthy that adverse events with placebo were reported by 7% of participants with mild baseline pain, but by 23% with moderate or severe baseline pain, and correspondingly more with active treatment.
Serious adverse events were uncommon, and only four were reported as related to the study drug: one after treating with sumatriptan 85 mg (heart palpitations), one after treating with sumatriptan 300 mg (chest tightness and pressure), one after treating with ibuprofen 400 mg (perforation of duodenal ulcer), and one after treating with two doses of rizatriptan 20 mg (micturition‐associated syncope). Withdrawals due to adverse events were uncommon. In placebo‐controlled studies, excluding those using doses of sumatriptan greater than 100 mg, the rate of adverse event withdrawal after treating with sumatriptan was equivalent to that after placebo. Doses of sumatriptan greater than 100 mg showed slightly increased rates of withdrawal due to adverse events. For the most part individual adverse events occurred significantly more often with sumatriptan ≥ 100 mg than with placebo. Sumatriptan at doses of 50 mg or less was significantly different from placebo only for malaise/fatigue/asthenia and paraesthesia/numbness.
Of the active comparators used in the included studies, only rizatriptan 5 mg and 10 mg, effervescent ASA 1000 mg, zolmitriptan 2.5 mg and 5 mg, eletriptan 40 mg and 80 mg, almotriptan 12.5 mg, paracetamol 1000 mg + MCP 10 mg, and ASA 900 mg + MCP 10 mg provided sufficient data to be analysed for a particular outcome. Rizatriptan 5 mg was superior to sumatriptan 25 mg for pain‐free at two hours and headache relief at two hours, but there was no significant difference between the treatments for headache relief at one hour; neither of these doses is commonly used. There was no difference between rizatriptan 5 mg and sumatriptan 50 mg for any outcomes reported. Rizatriptan 10 mg was superior to sumatriptan 25 mg, 50 mg, and 100 mg for all reported outcomes, including pain‐free at two hours and headache relief at one and two hours. Effervescent ASA 1000 mg was more effective than sumatriptan 50 mg for headache relief at one hour, but there was no difference between the treatments for pain‐free at one or two hours, and sumatriptan 50 mg was significantly superior for headache relief at two hours. For zolmitriptan 2.5 mg and 5 mg compared with sumatriptan 50 mg, there was no significant difference for headache relief at either one or two hours, and for almotriptan 12.5 mg compared with sumatriptan 100 mg, there was no significant difference for pain‐free at two hours or sustained pain‐free during the 24 hours postdose. Eletriptan 40 mg and 80 mg were superior to sumatriptan 50 mg and 100 mg for most reported outcomes, including pain‐free at two hours, and headache relief at one and two hours. However, there was no significant difference between sumatriptan 50 mg and eletriptan 40 mg for headache relief at one hour, or sumatriptan 100 mg and eletriptan 40 mg for pain‐free at one hour. There was no significant difference between sumatriptan 100 mg and either paracetamol + MCP, or ASA + MCP for headache relief at two hours. Sumatriptan 100 mg was, however, significantly superior to ASA + MCP for pain‐free at two hours. For the majority of adverse events there was no significant difference between sumatriptan and any active comparator.
It should be noted that there were a very large number of analyses, with no correction for multiple comparisons, as is common with Cochrane reviews. The standard of statistical significance was a probability of a result occurring by chance of less than 5%, or 1 in 20. It is likely that many of the significant results obtained would not survive correction for multiple comparisons.
Overall completeness and applicability of evidence
Included participants suffered from migraine in accordance with IHS criteria (even if not specifically referenced in a few cases), with the majority suffering around one to six attacks per month and with a history of attacks for at least six months, and usually one year. In the majority of studies treated attacks had to be established, with moderate or severe pain intensity, before medication could be taken. The use of prophylactic medication during the study period was variable, with some studies requiring participants to discontinue any prophylactic medication at least two weeks before receiving study medication, while others allowed stable prophylactic medications, and others failed to comment at all. Nine studies excluded participants if they had previously taken sumatriptan, while four studies required participants to have experience of sumatriptan to be eligible for inclusion. Overall there did not appear to be a particular bias towards a certain type of migraine patient, but many studies recruited participants through headache clinics, which may have selected for those with more severe or hard‐to‐treat pain. Individuals were carefully screened before study entry, and those with certain conditions, particularly cardio‐ or cerebrovascular disease, were excluded from the studies. Other exclusions included pregnant or lactating women, individuals with hepatic disease or who regularly experience vomiting, and individuals who suffer from frequent non‐migraine headaches or basilar, ophthalmic, or hemiplegic migraine. This may mean that the study population is not a reflection of a less carefully screened general population who may use sumatriptan.
While most studies reported IHS‐preferred outcomes, they did not all report all the outcomes of interest for this review so that numbers of participants in any comparison were usually smaller than numbers treated.
Single‐dose studies provide only limited information about adverse events, and individual studies are generally underpowered to assess harm, but pooling adverse event data from similar studies may allow more robust estimates for short‐term use. In these studies the number of participants who experienced any adverse event was slightly increased with sumatriptan compared to placebo. However it is important to remember that in many studies rescue medication, or a second dose of study medication, was permitted if study medication failed to provide adequate relief, or in the event of recurrence, and this may disproportionately increase rates of adverse events in the placebo group. Some studies in this review reported data for individual adverse events only if they occurred at a specified rate, which differed across studies (> 1% to ≥ 5%), and inevitably means that some events occurring at lower frequencies were not reported in some studies.
Twenty studies allowed participants to treat a migraine attack during the mild pain phase. Despite this option to treat early, the majority of participants reported moderate or severe baseline pain intensity, and only six provided efficacy data for sumatriptan versus any comparator in participants with mild baseline pain intensity. In clinical practice many people treat their headache during this mild pain phase, and there is also some evidence that treating attacks in the early stages is beneficial (Gendolla 2008; Pascual 2002), which is supported by the data presented here. More studies reporting consistently on early treatment and different dosing strategies are needed to inform the best clinical use of oral sumatriptan.
The vast majority of included studies were industry‐sponsored and are likely to have used GlaxoSmithKline‐branded formulations of sumatriptan. Increasingly, migraine sufferers use generic formulations of sumatriptan (91% in the UK), and no clinical trials specifically using generic sumatriptan were found for inclusion in this review.
Quality of the evidence
The majority of included studies were of good methodological quality, with only 11/61 deemed to be of low quality (scoring 2 of 5 using the Oxford Quality Scale). However, 40 studies did not adequately describe random sequence generation, 47 studies did not provide information about allocation concealment, and 28 studies did not provide details on the method of blinding. In a number of studies withdrawals and dropouts were not reported adequately by treatment group, and for some outcomes reported denominators differed from the intention‐to‐treat (ITT) population, presumably because some participants failed to record data at that point. Wherever an adequate explanation was not given we have used the ITT denominator if it gave a more conservative estimate; in general, the number of missing participants was not sufficient to significantly alter the results. Nineteen studies had at least 200 participants in each treatment arm, a further 36 had between 50 and 200 in one or more treatment arms (the placebo arm was often smallest with other treatment arms having over 200 participants), and six had fewer than 50 participants in all treatment arms. Overall methodological quality of the included studies was good, and treatment group sizes were sufficiently big to avoid major bias in the results for efficacy.
While most studies used patient diaries and reported some information about adverse events, the outcomes were not always our preferred ones, and the time over which data were collected was frequently not explicit. It is likely that data continued to be collected after intake of rescue medication or a second dose of study medication, so that total dose over the period assessed is uncertain.
Potential biases in the review process
We identified a large amount of data in comparisons with placebo, particularly for the 50 mg and 100 mg doses. Over 4000 additional participants would have to have been involved in unpublished trials with zero treatment effect for the NNT for headache relief at two hours to increase above 6 (which we considered the limit of clinical utility in this situation) for the 50 mg dose (Moore 2008). This equates to 10 studies with over 400 participants in sumatriptan 50 mg and placebo treatment arms. Similarly, over 2000 additional participants would have to have been involved in unpublished trials with zero treatment effects for the NNT for pain‐free at two hours to increase above 8 (considered to be the limit of clinical utility in this situation), equivalent to 10 trials with over 200 sumatriptan 50 mg and placebo‐treated participants in each. It is unlikely that such a large amount of unidentified data exists, so publication bias is not a concern.
The methods of review were such as to minimise bias due to the review process itself, but use of data from both phases of cross‐over studies and from studies reporting combined data from several attacks may introduce unknown biases. For cross‐over studies a 48‐hour period between qualifying attacks should limit potential for carryover effects, and for multiple attacks there is some evidence of consistency of response (in terms of proportion of participants achieving the outcome) for aspirin in migraine (Kirthi 2010) and within some studies in this review (Bussone 2000; Gallagher 2000; GL/MIG/001/92; GL/MIG/001A/92; GL/MIG/002; GL/MIG/002A; GL/MIG/009; Gruffyd‐Jones 2001; Myllyla 1998; Pfaffenrath 1998; Sandrini 2002; Tfelt‐Hansen 1995; Thomson 1992).
We specified that a minimum of 200 participants in at least two studies were required before carrying out any pooled analysis, but ideally we would need at least 200 participants in each treatment arm where there is an event rate of 50% to be reasonably confident in the size of an effect (Moore 2010). The magnitude of effect for outcomes with fewer participants and/or lower event rates should be interpreted with caution.
Agreements and disagreements with other studies or reviews
The earlier Cochrane review of sumatriptan for acute migraine (McCrory 2003) reported results for sumatriptan 100 mg, 50 mg, and 25 mg versus placebo. These results were broadly consistent with those reported here, with the additional data included in this review resulting in much tighter confidence intervals. The new data did, however, change the estimated effects of sumatriptan 50 mg versus placebo for pain‐free at two hours (which appears to have been overestimated previously) and sumatriptan 100 mg versus placebo for incidence of adverse events (which appears to have been underestimated previously).
Similarly, the results presented here were also largely consistent with those presented in a previous review of triptan use in acute migraine (Gawel 2001) which included data from 13 studies comparing oral sumatriptan with placebo, of which all but two (Centonze 1995; Rederich 1995) were included in our review. Again additional data included in our review resulted in a slightly reduced estimate of efficacy for both the 50 mg and 100 mg doses and tighter confidence intervals.
Ferrari and colleagues (Ferrari 2001) reviewed all triptan studies available up to 2001. They used the approach of presenting the absolute percentage benefiting, and the absolute benefit increase (active minus placebo). Results in that analysis are in line with those presented here.
Oldman 2002 reviewed all pharmacological treatments for acute migraine, including 15 studies involving oral sumatriptan, all of which are included here (two unpublished studies included have subsequently been published as Goadsby 2000 and Sandrini 2002 and are included in this review). Again, the results are in good agreement with those presented in our review: NNTs for headache relief at two hours with both sumatriptan 50 mg and 100 mg were very similar, while NNTs for headache relief at one hour with the two doses of sumatriptan were slightly decreased with the additional data included in this review.
Authors' conclusions
Implications for practice.
Oral sumatriptan is an effective treatment for the relief of headache pain, other symptoms associated with migraine, and functional disability, with single doses of 25 mg or more providing clinically useful levels of relief in some people. Higher doses are effective in more individuals, but at the expense of greater numbers of adverse events. Most events were described as mild and of short duration. The number of participants experiencing headache relief by one hour after administration is low, and the number pain‐free by one hour is not clinically useful. Treating attacks early, during the mild pain phase, results in significantly greater efficacy, but does not significantly change the incidence of adverse events.
These data support the general guideline advice to use 50 mg as the starting dose, with increases to 100 mg if necessary and tolerated. Some experienced patients may find that a 25 mg dose is sufficient.
Implications for research.
A useful line of research would be to investigate whether sumatriptan is a useful second‐line treatment for individuals who fail to get an adequate response with simple analgesics, such as ibuprofen or aspirin.
There is an abundance of data on the efficacy of sumatriptan in terms of pain relief, but in general, reporting of long‐term (sustained to 24 hours or 48 hours) and secondary outcomes such as relief of headache‐associated symptoms, functional disability, and adverse events is less good. Future studies should address sustained outcomes and consistently report relief of associated symptoms, functional disability and adverse events using standard definitions.
More studies are needed to establish whether treating pain early, while still mild, gives better short‐term (two‐hour) and long‐term (sustained to 24 hours or 48 hours) outcomes, and better patient satisfaction.
What's new
| Date | Event | Description |
|---|---|---|
| 29 May 2019 | Amended | Contact details updated. |
| 1 May 2015 | Review declared as stable | A search for studies is likely to identify potentially relevant studies, but the studies are unlikely to change conclusions. |
Notes
This review is one of a series of reviews on sumatriptan for acute migraine attacks in adults which replaces an earlier Cochrane review of oral sumatriptan (McCrory 2003).
Acknowledgements
GlaxoSmithKline provided additional information relating to methods in five of the unpublished studies included in this review. Pfizer provided a clinical trial report for eletriptan protocol 160‐104, the sixth unpublished study included in this review.
We would like to acknowledge the helpful comments and editorial expertise of Timothy Steiner, Douglas McCory, and Rebecca Gray, and those who contributed to the various stages of peer review.
Appendices
Appendix 1. Definitions
All terms relating to primary efficacy outcomes are defined according to the effect of the treatment on headache pain, measured using a four‐point pain intensity scale (ranging from 0 to 3 or none, mild, moderate, and severe).
Baseline pain intensity ‐ level of pain participant must be experiencing in order to receive study medication, either 1 (mild pain) or 2/3 (moderate or severe pain).
Pain‐free at two hours ‐ number of participants with a pain intensity of 0 (none) at 2 hours after administration of study medication, expressed as a fraction of the treated participants with the appropriate baseline pain.
Headache relief at two hours ‐ number of participants with a reduction in pain intensity from 2/3 (moderate/severe) to 0/1 (none/mild) at two hours after administration of study medication, expressed as a fraction of the treated participants with grade 2/3 baseline pain.
24‐hour sustained headache relief ‐ number of participants with a reduction in pain intensity from 2/3 (moderate/severe) to 0/1 (none/mild) at two hours after administration of study medication which is then sustained between 2 and 24 hours without recurrence of headache or use of rescue medication, expressed as a fraction of the treated participants with grade 2/3 baseline pain.
24‐hour sustained pain‐free ‐ number of participants with a pain intensity of 0 (none) at two hours after administration of study medication which is then sustained between 2 and 24 hours without recurrence of headache or use of rescue medication expressed as a fraction of the treated participants with the appropriate baseline pain.
Use of rescue medication ‐ number of participants requiring the use of additional medication to treat either recurrence of headache or an inadequate response to study medication, provided that the additional medication is not, or does not include, the study drug.
Relief of associated symptoms ‐ number of participants with an absence of a headache‐associated symptom (nausea, vomiting, photophobia, or phonophobia) at two hours after administration of study medication, expressed as a fraction of the treated participants for whom the symptom was present at baseline.
Presence of associated symptoms ‐ presence of a headache‐associated symptom (nausea, vomiting, photophobia, or phonophobia) at two hours after administration of study medication, expressed as a fraction of all treated participants.
Relief of functional disability ‐ reduction in the level of functional disability, measured using a four‐point scale, from moderate or severe disability (grade 2/3) at baseline to mild or none (grade 1/0) at two hours after administration of study medication, expressed as a fraction of the treated participants with moderate or severe functional disability at baseline.
Presence of functional disability ‐ presence of functional disability (of moderate or severe intensity) at two hours after administration of study medication, expressed as a fraction of all treated participants.
Appendix 2. Search strategy for MEDLINE (via OVID)
Serotonin Agonists/ OR Tryptamines/
(sumatriptan OR Imitrex OR Imigran).mp.
1 OR 2
Headache/ OR exp Headache Disorders/ OR exp Migraine Disorders/
(headach* OR migrain* OR cephalgi* OR cephalalgi*).mp.
4 OR 5
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab.
drug therapy.fs.
randomly.ab.
trial.ab.
groups.ab.
OR/7‐14
3 AND 6 AND 15
Appendix 3. Search strategy for EMBASE (via OVID)
Serotonin Agonists/ OR Tryptamines/
(sumatriptan OR Imitrex OR Imigran).mp.
1 OR 2
exp Headache and facial pain
exp Migraine
(headach* OR migrain* OR cephalgi* OR cephalalgi*).mp.
4 OR 5 OR 6
clinical trials.sh.
controlled clinical trials.sh.
randomized controlled trial.sh.
double‐blind procedure.sh.
(clin* adj25 trial*).ab.
((doubl* or trebl* or tripl*) adj25 (blind* or mask*)).ab.
placebo*.ab.
random*.ab.
OR/8‐15
3 AND 7 AND 16
Appendix 4. Search strategy for CENTRAL
MeSH descriptor Serotonin Agonists OR MeSH descriptor Tryptamines
(sumatriptan OR Imitrex OR Imigran):ti,ab,kw
1 OR 2
MeSH descriptor Headache/ OR MeSH descriptor Headache Disorders explode all trees
MeSH descriptor Migraine Disorders explode all trees
(headach* OR migrain* OR cephalgi* OR cephalalgi*):ti,ab,kw
4 OR 5 OR 6
3 AND 7
Limit 8 to Clinical Trials (CENTRAL)
Appendix 5. Summary of outcomes: efficacy
| Study ID | Treatment | Headache relief 1 h | Headache relief 2 h | Pain‐free 1 h | Pain‐free 2 h | Sustained headache relief 24 h | Sustained pain‐free 24 h | Use of rescue medication |
| 160‐104 | Numbers of participants treating first attack: (1) Sumatriptan 25 mg, n = 180 (2) Sumatriptan 50 mg, n = 181 (3) Eletriptan 40 mg, n = 184 (4) Eletriptan 80 mg, n = 180 (5) Placebo, n = 93 |
1st attack: (1) 42/180 (2) 48/181 (3) 38/184 (4) 61/180 (5) 20/93 | 1st attack: (1) 90/180 (2) 98/181 (3) 109/184 (4) 119/180 (5) 34/93 | No data | 1st attack: (1) 29/180 (2) 31/181 (3) 34/184 (4) 45/180 (5) 8/93 | No data | No data | No useable data |
| Banerjee 1992 | (1) Sumatriptan (dispersible) 200 mg, n = 37 (34 for efficacy) (2) Placebo, n = 39 (37 for efficacy) |
No data | 1st attack: (1) 21/34 (2) 12/37 |
No data | 1st attack: (1) 5/34 (2) 1/37 |
No data | No data | At 2 h: (1) 9/37 (2) 22/39 |
| Brandes 2007 | Study 1 (1) Sumatriptan 85 mg, n = 415 (365 for efficacy) (2) Naproxen 500 mg, n = 419 (361 for efficacy) (3) Sumatriptan 85 mg + naproxen 500 mg, n = 422 (370 for efficacy) (4) Placebo, n = 421 (365 for efficacy) Study 2 (1) Sumatriptan 85 mg, n = 434 (370 for efficacy) (2) Naproxen 500 mg, n = 434 (371 for efficacy) (3) Sumatriptan 85 mg + naproxen 500 mg, n = 433 (367 for efficacy) (4) Placebo, n = 435 (387 for efficacy) |
No data | Study 1 (1) 200/365 (2) 157/361 (3) 237/370 (4) 102/365 Study 2 (1) 182/370 (2) 158/371 (3) 207/367 (4) 109/387 |
No data | Study 1 (1) 90/365 (2) 53/361 (3) 125/370 (4) 33/365 Study 2 (1) 82/370 (2) 57/371 (3) 107/367 (4) 37/387 |
Study 1 (1) 127/365 (2) 107/361 (3) 174/370 (4) 64/365 Study 2 (1) 121/370 (2) 102/371 (3) 158/367 (4) 64/387 |
Study 1 (1) 59/365 (2) 37/361 (3) 90/370 (4) 30/365 Study 2 (1) 51/370 (2) 37/371 (3) 83/367 (4) 25/387 |
At 24 h: Study 1 (1) 115/361 (2) 135/356 (3) 81/364 (4) 192/360 Study 2 (1) 137/362 (2) 143/364 (3) 83/362 (4) 223/382 |
| Bussone 2000 | (1) Sumatriptan 50 mg, n = 156 (2) Placebo, n = 56 |
No data | (1) 87/156 (2) 14/56 | No data | No data | No data | No data | No useable data |
| Carpay 2004 | (1) Sumatriptan (fast disintegrating) 50 mg, n = 141 (2) Sumatriptan (fast disintegrating) 100 mg, n = 148 (3) Placebo, n = 155 |
No data | No data | (1) 50/141 (2) 63/148 (3) 29/155 |
(1) 70/141 (2) 94/148 (3) 30/155 |
No data | (1) 44/141 (2) 57/148 (3) 15/155 |
No data |
| Cutler 1995 | (1) Sumatriptan 25 mg, n = 66 (2) Sumatriptan 50 mg, n = 62 (3) Sumatriptan 100 mg, n = 66 (4) Placebo, n = 65 |
No data | (1) 34/66 (2) 31/62 (3) 37/66 (4) 17/65 |
No data | (1) 14/66 (2) 10/62 (3) 15/66 (4) 5/65 |
No data | No data | No data |
| Dahlof 1991 | (1) Sumatriptan 100 mg, n = 305 (275 with moderate or severe baseline pain intensity) (2) Sumatriptan 200 mg, n = 283 (255 with moderate or severe baseline pain intensity) (3) Sumatriptan 300 mg, n = 299 (271 with moderate or severe baseline pain intensity) (4) Placebo, n = 205 (182 with moderate or severe baseline pain intensity) |
No data | (1) 180/275 (2) 185/255 (3) 179/271 (4) 48/182 |
No data | No data | No data | No data | No useable data |
| Dahlof 2009 | (1) Sumatriptan 50 mg, n = 136 (2) Tonabersat 20 mg, n = 134 (3) Tonabersat 40 mg, n = 137 (4) Placebo, n = 134 |
(1) 41/136 (2) 28/134 (3) 24/137 (4) 29/134 |
(1) 70/136 (2) 39/134 (3) 43/137 (4) 36/134 |
(1) 8/136 (2) 2/134 (3) 4/137 (4) 1/134 |
(1) 32/136 (2) 7/134 (3) 6/137 (4) 8/134 |
No data | No data | At 3 h: (1) 29/136 (2) 40/134 (3) 41/137 (4) 39/134 |
| Diener 2004a | (1) Sumatriptan 50 mg, n = 135 (2) Effervescent acetylsalicylic acid 1000 mg, n = 147 (146 for efficacy) (3) Placebo, n = 153 (152 for efficacy) |
(1) 32/135 (2) 37/146 (3) 23/152 |
(1) 66/135 (2) 72/146 (3) 50/152 |
(1) 7/135 (2) 6/146 (3) 5/152 |
(1) 33/135 (2) 37/146 (3) 22/152 |
No data | No data | At 24 h: (1) 53/135 (2) 62/146 (3) 98/152 |
| Diener 2004b | (1) Sumatriptan 50 mg, n = 226 (2) Ibuprofen 400 mg, n = 212 (3) Effervescent acetylsalicylic acid 1000 mg, n = 222 (4) Placebo, n = 222 |
(1) 54/224 (2) 65/211 (3) 76/221 (4) 25/222 |
(1) 125/224 (2) 127/211 (3) 116/221 (4) 68/222 |
(1) 12/224 (2) 21/211 (3) 14/221 (4) 7/222 |
(1) 83/224 (2) 70/211 (3) 60/221 (4) 28/222 |
No useable data | No data | At 2 h: (1) 92/224 (2) 87/211 (3) 99/221 (4) 147/222 |
| DKSMSG 1999 | (1) Sumatriptan 100 mg, n = 130 (2) Diclofenac‐potassium 50 mg, n = 131 (3) Diclofenac‐potassium 100 mg, n = 122 (4) Placebo, n = 131 |
No useable data | No useable data | No data | No useable data | No data | No data | At 24 h: (1) 47/115 (2) 41/115 (3) 41/115 (4) 69/115 |
| Dodick 2002 | Protocol CL13 (1) Sumatriptan 100 mg, n = 193 (2) Almotriptan 12.5 mg, n = 183 (3) Placebo, n = 99 |
No data | No data | No data | (1) 64/193 (2) 51/183 (3) 16/99 |
No data | (1) 54/193 (2) 46/183 (3) 12/99 |
At 24 h: (1) 66/193 (2) 70/183 (3) 52/99 |
| Dowson 2002 | (1) Sumatriptan 100 mg, n = 194 (2) Almotriptan 12.5 mg, n = 184 (3) Almotriptan 25 mg, n = 191 (4) Placebo, n = 99 |
(1) 73/194 (2) 65/184 (3) 59/191 (4) 29/99 |
(1) 123/194 (2) 104/184 (3) 108/191 (4) 42/99 |
(1) 15/194 (2) 9/184 (3) 21/191 (4) 5/99 | (1) 65/194 (2) 51/184 (3) 66/191 (4) 15/99 |
No data | No data | At 2 h: (1) 63/194 (2) 71/184 (3) 73/191 (4) 55/99 |
| Ensink 1991 | (1) Sumatriptan 100 mg, n = 148 (131 with moderate or severe baseline pain intensity) (2) Placebo, n = 84 (78 with moderate or severe baseline pain intensity) |
No data | (1) 60/131 (2) 14/78 |
No data | (1) 34/131 (2) 4/78 |
No data | No data | No useable data |
| Freitag 2001 | (1) Sumatriptan 25 mg (+ additional dose of 25 mg at 2 h), n = 61 (2) Isometheptene combination 2 doses (+ additional single doses at 1, 2 and 3 h), n = 65 |
(1) 18/40 (2) 19/42 |
No data | No data | No data | No data | No data | No data |
| Gallagher 2000 | (1) Sumatriptan 25 mg, n = 336 (306 for efficacy) (2) Sumatriptan 50 mg, n = 338 (306 for efficacy) (3) Zolmitriptan 2.5 mg, n = 327 (295 for efficacy) (4) Zolmitriptan 5 mg, n = 337 (305 for efficacy) |
(1) 101/306 (2) 106/306 (3) 103/295 (4) 114/305 |
(1) 182/306 (2) 195/306 (3) 198/295 (4) 198/305 |
No data | No data | (1) 101/306 (2) 117/306 (3) 120/295 (4) 130/305 |
No data | No data |
| Geraud 2000 | (1) Sumatriptan 100 mg, n = 504 (2) Zolmitriptan 5 mg, n = 498 (3) Placebo, n = 56 |
(1) 171/504 (2) 163/498 (3) 11/56 |
(1) 304/504 (2) 288/498 (3) 24/56 |
(1) 54/504 (2) 37/498 (3) 1/56 | (1) 150/504 (2) 144/498 (3) 7/56 |
(1) 195/504 (2) 180/498 (3) 14/56 |
No data | At 24 h: (1) 192/504 (2) 189/498 (3) 32/56 |
| GL/MIG/001/92 | (1) Sumatriptan 100 mg, n = 305 (242 with moderate or severe baseline pain intensity) (2) Paracetamol 1000 mg + metoclopramide 10 mg, n = 302 (227 with moderate or severe baseline pain intensity) |
No data | 1st attack: (1) 118/242 (2) 103/227 |
No data | No data | No data | No data | 1st attack, at 24 h: (1) 79/288 (2) 91/286 |
| GL/MIG/001A/92 | (1) Sumatriptan 100 mg, n = 348 (272 with moderate or severe baseline pain intensity) (2) Paracetamol 1000 mg + metoclopramide 10 mg, n = 373 (294 with moderate or severe baseline pain intensity) |
No data | 1st attack: (1) 115/272 (2) 122/294 |
No data | No data | No data | No data | 1st attack, at 24 h: (1) 119/318 (2) 154/351 |
| GL/MIG/002 | (1) Sumatriptan 100 mg, n = 374 (262 with moderate or severe baseline pain intensity) (2) Migraleve (buclizine hydrochloride 12.5 mg + paracetamol 1000 mg + codeine phosphate 16 mg), n = 378 (275 with moderate or severe baseline pain intensity) |
No data | 1st attack: (1) 131/262 (2) 111/275 |
No data | No data | No data | No data | 1st attack, at 24 h: (1) 71/351 (2) 106/358 |
| GL/MIG/002A | (1) Sumatriptan 100 mg, n = 342 (261 with moderate or severe baseline pain intensity) (2) Migraleve (buclizine hydrochloride 12.5 mg + paracetamol 1000 mg + codeine phosphate 16 mg), n = 332 (257 with moderate or severe baseline pain intensity) |
No data | No useable data | No data | No data | No data | No data | 1st attack, at 24 h: (1) 60/305 (2) 106/308 |
| GL/MIG/009 | (1) Sumatriptan 100 mg, n = 255 (203 with moderate or severe baseline pain intensity) (2) Migril (ergotamine tartrate 2 mg + cyclizine hydrochloride 50 mg + caffeine hydrate 100 mg), n = 258 (204 with moderate or severe baseline pain intensity) |
1st attack: (1) 69/203 (2) 56/204 |
1st attack: (1) 95/203 (2) 76/204 |
1st attack: (1) 24/203 (2) 11/204 |
1st attack: (1) 56/203 (2) 39/204 |
No data | No data | 1st attack, at 24 h: (1) 27/234 (2) 27/234 |
| Goadsby 1991 | Number of attacks in efficacy population: (1) Sumatriptan 100 mg, n = 94 (89 of moderate or severe intensity) (2) Placebo, n = 94 (93 of moderate or severe intensity) |
No data | (1) 45/89 (2) 9/93 |
No data | No data | No data | No data | At 2 h: (1) 39/94 (2) 83/94 |
| Goadsby 2000 | (1) Sumatriptan 100 mg, n = 129 (2) Eletriptan 20 mg, n = 144 (3) Eletriptan 40 mg, n = 136 (4) Eletriptan 80 mg, n = 141 (5) Placebo, n = 142 |
(1) 23/129 (2) 31/144 (3) 44/136 (4) 48/141 (5) 15/142 |
(1) 63/129 (2) 70/144 (3) 76/136 (4) 91/141 (5) 30/142 |
(1) 7/129 (2) 3/144 (3) 9/136 (4) 20/141 (5) 3/142 |
(1) 26/129 (2) 25/144 (3) 34/136 (4) 44/141 (5) 8/142 |
No data | No data | At 24 h: (1) 37/129 (2) 43/144 (3) 39/136 (4) 37/141 (5) 75/142 |
| Goldstein 1998 | (1) Sumatriptan 25 mg, n = 563 (2) Sumatriptan 50 mg, n = 566 (3) Rizatriptan 5 mg, n = 557 (4) Rizatriptan 10 mg, n = 567 (5) Placebo, n = 141 |
(1) 194/563 (2) 218/566 (3) 209/557 (4) 236/567 (5) no data |
(1) 349/563 (2) 385/566 (3) 379/557 (4) 408/567 (5) 54/141 |
(1) 36/563 (2) 43/566 (3) 61/557 (4) 63/567 (5) no data |
(1) 158/563 (2) 209/566 (3) 184/557 (4) 232/567 (5) 13/141 |
No data | No data | 1st attack, at 4 h: (1) 141/563 (2) 108/566 (3) 128/557 (4) 108/567 (5) 63/141 |
| Goldstein 2005 | (1) Sumatriptan 50 mg, n = 67 (2) Acetaminophen 1000 mg + aspirin 1000 mg + caffeine 260 mg combination, n = 69 (3) Placebo, n = 35 |
(1) 23/46 (2) 25/50 (3) 8/27 |
(1) 30/46 (2) 42/50 (3) 14/27 |
No data | No data | No data | No data | At 4 h: (1) 8/67 (2) 1/69 (3) 5/35 |
| Gruffyd‐Jones 2001 | (1) Sumatriptan 50 mg, n = 555 (508 for efficacy) (2) Zolmitriptan 2.5 mg, n = 551 (500 for efficacy) (3) Zolmitriptan 5 mg, n = 560 (514 for efficacy) |
1st attack: (1) 224/508 (2) 215/500 (3) 206/514 |
1st attack: (1) 361/508 (2) 325/500 (3) 339/514 |
1st attack: (1) 66/508 (2) 50/500 (3) 62/514 |
1st attack: (1) 183/508 (2) 160/500 (3) 185/514 |
No useable data | 1st attack: (1) 137/508 (2) 125/500 (3) 123/514 |
All attacks, at 24 h: (1) 620/2693 (2) 631/2671 (3) 608/2744 |
| Havanka 2000 | (1) Sumatriptan 100 mg, n = 98 (2) Naratriptan 1 mg, n = 85 (3) Naratriptan 2.5 mg, n = 87 (4) Naratriptan 5 mg, n = 93 (5) Naratriptan 7.5 mg, n = 93 (6) Naratriptan 10 mg, n = 96 (95 with moderate or severe baseline pain intensity) (7) Placebo, n = 91 |
(1) 34/98 (2) 21/85 (3) 26/87 (4) 32/93 (5) 40/93 (6) 38/95 (7) 18/91 |
(1) 59/98 (2) 49/85 (3) 45/87 (4) 50/93 (5) 63/93 (6) 66/95 (7) 28/91 |
No data | No data | No useable data | No data | At 24 h: (1) 25/98 (2) 40/85 (3) 30/87 (4) 36/93 (5) 23/93 (6) 21/96 (7) 60/91 |
| Ishkanian 2007 | (1) Sumatriptan 50 mg, n = 108 (2) Placebo, n = 108 (107 for efficacy) |
No data | (1) 75/108 (2) 46/107 |
No data | (1) 26/108 (2) 22/107 |
No data | No data | At 24 h: (1) 37/108 (2) 46/107 |
| Jelinski 2006 | (1) Sumatriptan 50 mg, n = 126 (2) Sumatriptan 100 mg, n = 126 (3) Placebo, n = 109 |
No data | No data | (1) 30/126 (2) 30/126 (3) 8/109 |
(1) 51/126 (2) 63/126 (3) 17/109 |
No data | (1) 30/126 (2) 34/126 (3) 7/109 |
No data |
| Kaniecki 2006 | (1) Sumatriptan 100 mg, n = 131 (2) Placebo, n = 127 |
No data | (1) 64/131 (2) 47/127 |
No data | (1) 32/131 (2) 18/127 |
(1) 43/131 (2) 19/127 |
(1) 24/131 (2) 7/127 |
At 24 h: (1) 55/131 (2) 79/127 |
| Kolodny 2004 | (1) Sumatriptan 25 mg, n = 554 (290 1st attack only) (2) Sumatriptan 50 mg, n = 550 (285 1st attack only) (3) Rizatriptan 5 mg, n = 536 (288 1st attack only) (4) Rizatriptan 10 mg, n = 547 (296 1st attack only) (5) Placebo, n = 288 |
(1) 181/554 (2) 191/550 (3) 195/536 (4) 220/547 (5) no data |
(1) 320/554 (2) 361/550 (3) 352/536 (4) 372/547 (5) no data |
No data | (1) 152/554 (2) 185/550 (3) 179/536 (4) 208/547 (5) no data |
No data | No data | 1st attack, at 4 h: (1) 66/290 (2) 59/285 (3) 85/288 (4) 67/296 (5) 112/288 |
| Kudrow 2005 | (1) Sumatriptan 50 mg, n = 144 (2) Valdecoxib 20 mg, n = 137 (3) Valdecoxib 40 mg, n = 152 (4) Placebo, n = 141 |
No data | (1) 60/144 (2) 61/137 (3) 72/152 (4) 42/141 |
No data | No data | No data | No data | No useable data |
| Latere 1991 | (1) Sumatriptan (dispersible) 100 mg, n = 288 (220 with moderate or severe baseline pain intensity) (2) Cafergot, n = 289 (246 with moderate or severe baseline pain intensity) |
No data | 1st attack: (1) 145/220 (2) 118/246 |
No data | 1st attack: (1) 77/220 (2) 32/246 |
No data | No data | At 2 h: (1) 69/288 (2) 127/289 |
| Lines 2001 | (1) Sumatriptan 50 mg, n = 356 (2) Rizatriptan 5 mg, n = 349 (3) Placebo, n = 80 |
No data | (1) 239/356 (2) 220/349 (3) 18/80 |
No data | No data | No data | No data | No data |
| Lipton 2000 | Treated attacks: (1) Sumatriptan 50 mg, n = 870 (2) Placebo, n = 240 |
No data | All attacks: (1) 409/870 (2) 82/240 |
No data | All attacks (1) 157/870 (2) 17/240 |
No data | No data | All attacks, at 24 h: (1) 61/870 (2) 20/240 |
| Matthew 2003 | (1) Sumatriptan 100 mg, n = 831 (2) Eletriptan 40 mg, n = 822 (3) Placebo, n = 419 |
(1) 214/831 (2) 272/822 (3) 44/419 |
(1) 471/831 (2) 522/822 (3) 105/419 |
(1) 40/831 (2) 56/822 (3) 0/419 |
(1) 216/831 (2) 280/822 (3) 20/419 |
(1) 276/831 (2) 342/822 (3) 58/419 |
No data | At 24 h: (1) 224/831 (2) 164/822 (3) 222/419 |
| Myllyla 1998 | (1) Sumatriptan 100 mg (+ optional dose of placebo after 1 h), n = 46 (42 for efficacy) (2) Tolfenamic acid 200 mg (+ optional 2nd dose after 1 h), n = 47 (43 for efficacy) (3) Placebo (+ optional dose of placebo after 1 h), n = 46 (41 for efficacy) |
No data | 1st attack: (1) 33/42 (2) no useable data (3) 12/41 |
No data | 1st attack: (1) 21/42 (2) no useable data (3) 3/41 |
No data | No data | No useable data |
| Nappi 1994 | (1) Sumatriptan 100 mg, n = 158 (148 with moderate or severe baseline pain intensity) (2) Placebo, n = 86 (81 with moderate or severe baseline pain intensity) |
No data | (1) 73/142 (2) 25/81 |
No data | (1) 34/142 (2) 8/81 |
No data | No data | No useable data |
| Nett 2003 | (1) Sumatriptan 50 mg, n = 124 (124 for efficacy, 116 for per‐protocol efficacy) (2) Sumatriptan 100 mg, n = 122 (122 for efficacy, 115 for per‐protocol efficacy) (3) Placebo, n = 123 (122 for efficacy, 118 for per‐protocol efficacy) |
No data | No data | (1) 30/124 (2) 37/122 (3) 18/122 |
(1) 62/124 (2) 74/122 (3) 35/122 |
No data | (1) 35/116 (2) 36/115 (3) 17/118 |
No data |
| Patten 1991 | (1) Sumatriptan (dispersible) 100 mg, n = 142 (2) Sumatriptan (dispersible) 200 mg, n = 140 (3) Sumatriptan (dispersible) 300 mg, n = 155 (4) Placebo, n = 101 |
No data | 1st attack: (1) 95/142 (2) 105/140 (3) 107/155 (4) 22/101 |
No data | No data | No data | No data | No data |
| Pfaffenrath 1998 | (1) Sumatriptan 25 mg, n = 303 (286 with moderate or severe baseline pain intensity) (2) Sumatriptan 50 mg, n = 303 (285 with moderate or severe baseline pain intensity) (3) Sumatriptan 100 mg, n = 298 (277 with moderate or severe baseline pain intensity) (4) Placebo, n = 99 (91 with moderate or severe baseline pain intensity) |
1st attack: (1) 83/286 (2) 123/285 (3) 100/277 (4) 13/91 |
1st attack: (1) 140/286 (2) 180/285 (3) 175/277 (4) 27/91 |
No data | No useable data | No data | No data | No data |
| Pini 1999 | (1) Sumatriptan 50 mg, n = 137 (106 for efficacy) (2) Placebo, n = 82 (61 for efficacy) |
No data | No data | No data | (1) 36/106 (2) 9/61 |
No data | No data | At 4 h: (1) 22/95 (2) 26/54 |
| Pini 1995 | (1) Sumatriptan 100 mg, n = 151 (2) Placebo, n = 87 |
No data | No data | No data | No data | No data | No data | No useable data |
| Sandrini 2002 | (1) Sumatriptan 50 mg, n = 181 (2) Sumatriptan 100 mg, n = 170 (3) Eletriptan 40 mg, n = 175 (4) Eletriptan 80 mg, n = 164 (5) Placebo, n = 84 |
(1) 42/181 (2) 45/170 (3) 52/175 (4) 58/164 (5) 10/84 |
(1) 88/181 (2) 85/170 (3) 108/175 (4) 107/164 (5) 25/84 |
(1) 9/181 (2) 12/170 (3) 10/175 (4) 20/164 (5) 1/84 |
(1) 33/181 (2) 29/170 (3) 52/175 (4) 59/164 (5) 3/84 |
(1) 61/181 (2) 64/170 (3) 88/175 (4) 87/164 (5) 18/84 |
(1) 20/181 (2) 24/170 (3) 42/175 (4) 46/164 (5) 3/84 |
No useable data |
| Sandrini 2007 | (1) Sumatriptan 50 mg, n = 139 (138 for efficacy) (2) Indoprocaf, n = 143 |
No data | 1st attack: (1) 79/138 (2) 82/143 |
All attacks: (1) 18/264 (2) 14/276 |
1st attack: (1) 49/138 (2) 45/143 |
1st attack: (1) 60/138 (2) 64/143 |
1st attack: (1) 29/138 (2) 23/143 |
No useable data |
| Sargent 1995 | (1) Sumatriptan 25 mg, n = 48 (2) Sumatriptan 50 mg, n = 46 (3) Sumatriptan 100 mg, n = 46 (4) Placebo, n = 47 |
(1) 12/48 (2) 6/46 (3) 10/46 (4) 3/47 |
(1) 25/48 (2) 25/46 (3) 26/46 (4) 8/47 |
No data | No data | No data | No data | No data |
| Savani 1999 | (1) Sumatriptan 50 mg, n = 331 (2) Placebo, n = 154 |
1st attack: (1) 74/331 (2) 26/154 |
1st attack: (1) 140/331 (2) 32/154 |
No data | 1st attack: (1) 63/331 (2) 5/154 |
No useable data | No data | No useable data |
| Schulman 2003 | (1) Sumatriptan 50 mg, n = 16 (2) Sumatriptan 50 mg + metoclopramide 10 mg, n = 16 |
No data | (1) 5/16 (2) 7/16 |
No data | No data | No data | No data | No data |
| Sheftell 2005 | Study 1: (1) Sumatriptan (rapid‐release) 50 mg, n = 494 (448 for efficacy) (2) Sumatriptan (rapid‐release) 100 mg, n = 488 (462 for efficacy) (3) Placebo, n = 495 (456 for efficacy) Study 2: (1) Sumatriptan (rapid‐release) 50 mg, n = 496 (454 for efficacy) (2) Sumatriptan (rapid‐release) 100 mg, n = 485 (440 for efficacy) (3) Placebo, n = 494 (436 for efficacy) |
No data | Study 1 (1) 310/448 (2) 331/462 (3) 208/456 Study 2 (1) 293/454 (2) 318/440 (3) 167/436 |
No data | Study 1 (1) 180/448 (2) 219/462 (3) 84/456 Study 2 (1) 178/454 (2) 207/440 (3) 53/436 |
Study 1 (1) 154/448 (2) 163/462 (3) 92/456 Study 2 (1) 173/454 (2) 181/440 (3) 69/436 |
Study 1 (1) 85/448 (2) 107/462 (3) 46/456 Study 2 (1) 96/454 (2) 108/440 (3) 21/436 |
No useable data |
| Smith 2005 | (1) Sumatriptan 50 mg, n = 229 (226 for efficacy) (2) Sumatriptan 50 mg, + naproxen 500 mg, n = 251 (250 for efficacy) (3) Naproxen 500 mg, n = 250 (248 for efficacy) (4) Placebo, n = 241 |
(1) 52/226 (2) 73/250 (3) 67/248 (4) 29/241 |
(1) 111/226 (2) 163/250 (3) 114/248 (4) 65/241 |
(1) 9/226 (2) 20/250 (3) 7/248 (4) 2/241 |
(1) 45/226 (2) 85/250 (3) 45/248 (4) 14/241 |
(1) 66/226 (2) 115/250 (3) 62/248 (4) 41/241 |
(1) 25/226 (2) 63/250 (3) 30/248 (4) 12/241 |
At 24 h: (1) 115/226 (2) 88/250 (3) 129/248 (4) 154/241 |
| Spierings 2001 | (1) Sumatriptan 50 mg, n = 582 (2) Almotriptan 12.5 mg, n = 591 |
(1) 206/582 (2) 202/591 |
(1) 333/582 (2) 343/591 |
(1) 41/582 (2) 32/591 |
(1) 143/582 (2) 106/591 |
No data | No data | At 24 h: (1) 193/582 (2) 217/591 |
| Tfelt‐Hansen 1995 | (1) Sumatriptan 100 mg, n = 122 (2) Lysine acetylsalicylate 1620 mg + metoclopramide 10 mg, n = 137 (3) Placebo, n = 126 |
No data | 1st attack: (1) 63/122 (2) 76/137 (3) 30/126 |
No data | 1st attack: (1) 36/122 (2) 29/137 (3) 10/126 |
No data | No data | 1st attack, at 24 h: (1) 77/122 (2) 74/137 (3) 102/126 |
| Tfelt‐Hansen 1998 | (1) Sumatriptan 100 mg, n = 388 (2) Rizatriptan 5 mg, n = 164 (3) Rizatriptan 10 mg, n = 387 (4) Placebo, n = 160 |
(1) 108/388 (2) 49/164 (3) 141/387 (4) 32/160 |
(1) 239/388 (2) 99/164 (3) 258/387 (4) 64/160 |
(1) 30/388 (2) 11/164 (3) 40/387 (4) 5/160 | (1) 127/388 (2) 41/164 (3) 155/387 (4) 15/160 |
No data | No data | At 2 h: (1) 77/387 (2) no data (3) 69/385 (4) 51/159 |
| Tfelt‐Hansen 2006 | (1) Sumatriptan 50 mg, n = 53 (2) Placebo, n = 48 |
No data | No data | No data | (1) 20/53 (2) 8/48 |
No data | (1) 15/53 (2) 5/48 |
No data |
| Thomson 1992 | (1) Sumatriptan 100 mg, n = 175 (153 with moderate or severe baseline pain intensity) (2) Aspirin 900 mg + metoclopramide 10 mg, n = 183 (163 with moderate or severe baseline pain intensity) |
No data | 1st attack: (1) 74/153 (2) 62/163 |
No data | 1st attack: (1) 35/153 (2) 19/163 |
No data | No data | 1st attack, at 48 h: (1) 57/168 (2) 101/181 |
| Visser 1996 | (1) Sumatriptan 100 mg, n = 72 (2) Rizatriptan 10 mg, n = 89 (3) Rizatriptan 20 mg, n = 82 (4) Rizatriptan 40 mg, n = 121 (5) Placebo, n = 85 |
(1) 17/72 (2) 22/89 (3) 24/82 (4) 47/121 (5) 12/85 |
(1) 33/72 (2) 46/89 (3) 46/82 (4) 80/121 (5) 15/85 |
No data | (1) 16/72 (2) 23/89 (3) 29/82 (4) 59/121 (5) 3/85 |
No data | No data | No data |
| Winner 2003 | Study 1: (1) Sumatriptan 50 mg, n = 122 (2) Sumatriptan 100 mg, n = 115 (3) Placebo, n = 117 Study 2: (1) Sumatriptan 50 mg, n = 111 (2) Sumatriptan 100 mg, n = 107 (3) Placebo, n = 119 |
No data | No data | Study 1 (1) 27/122 (2) 29/115 (3) 15/117 Study 2 (1) 24/111 (2) 30/107 (3) 17/119 |
Study 1 (1) 59/122 (2) 61/115 (3) 34/117 Study 2 (1) 59/111 (2) 66/107 (3) 35/119 |
No data | No data | No useable data |
Appendix 6. Associated symptoms: presence two hours after treatment
| Associated symptoms: symptom present 2 hours after taking study medication | ||||||
| Intervention | Studies | Attacks treated | Treatment (%) | Placebo (%) | Relative risk (95% CI) | NNTp (95% CI) |
| Nausea | ||||||
| Sumatriptan 25 mg versus placebo | 5 | 1587 | 30 | 42 | 0.76 (0.66 to 0.88) | 8.9 (6.0 to 17) |
| Sumatriptan 50 mg versus placebo (moderate or severe baseline pain intensity) | 10 | 3098 | 31 | 42 | 0.79 (0.72 to 0.87) | 9.3 (7.0 to 14) |
| Sumatriptan 50 mg versus placebo (mild baseline pain intensity) | 5 | 1223 | 21 | 33 | 0.63 (0.52 to 0.76) | 8.3 (5.9 to 14) |
| Sumatriptan 100 mg versus placebo (moderate or severe baseline pain intensity) | 15 | 4927 | 35 | 46 | 0.77 (0.71 to 0.81) | 9.0 (7.2 to 12) |
| Sumatriptan 100 mg versus placebo (mild baseline pain intensity) | 5 | 1218 | 21 | 33 | 0.63 (0.52 to 0.76) | 8.2 (5.8 to 14) |
| Sumatriptan 25 mg versus rizatriptan 5 mg | 2 | 2210 | 29 | 22 | 1.3 (1.1 to 1.5) | ‐15 (‐9.6 to ‐32) |
| Sumatriptan 25 mg versus rizatriptan 10 mg | 2 | 2231 | 29 | 23 | 1.3 (1.1 to 1.5) | ‐17 (‐10 to ‐44) |
| Sumatriptan 50 mg versus rizatriptan 5 mg | 2 | 2209 | 28 | 22 | 1.2 (1.1 to 1.4) | ‐19 (‐11 to ‐63) |
| Sumatriptan 50 mg versus rizatriptan 10 mg | 2 | 2230 | 28 | 23 | 1.2 (1.0 to 1.4) | Not calculated |
| Sumatriptan 50 mg versus eletriptan 40 mg | 2 | 686 | 34 | 27 | 1.2 (0.98 to 1.5) | Not calculated |
| Sumatriptan 50 mg versus eletriptan 80 mg | 2 | 675 | 34 | 31 | 1.1 (0.88 to 1.4) | Not calculated |
| Sumatriptan 100 mg versus eletriptan 40 mg | 3 | 2132 | 34 | 27 | 1.3 (1.1 to 1.5) | ‐13 (‐8.8 to ‐28) |
| Sumatriptan 100 mg versus eletriptan 80 mg | 2 | 548 | 38 | 29 | 1.3 (1.0 to 1.7) | ‐11 (‐5.9 to ‐80) |
| Sumatriptan 100 mg versus ASA 900 mg + MCP 10 mg | 2 | 612 | 45 | 45 | 1.0 (0.85 to 1.2) | Not calculated |
| Vomiting | ||||||
| Sumatriptan 50 mg versus placebo | 3 | 1438 | 4 | 4 | 1.0 (0.59 to 1.7) | Not calculated |
| Sumatriptan 100 mg versus placebo | 6 | 1335 | 9 | 10 | 0.93 (0.67 to 1.3) | Not calculated |
| Sumatriptan 25 mg versus rizatriptan 5 mg | 2 | 2210 | 3 | 2 | 1.6 (0.90 to 2.8) | Not calculated |
| Sumatriptan 25 mg versus rizatriptan 10 mg | 2 | 2231 | 3 | 2 | 1.4 (0.82 to 2.4) | Not calculated |
| Sumatriptan 50 mg versus rizatriptan 5 mg | 2 | 2209 | 3 | 2 | 2.0 (1.1 to 3.4) | ‐60 (‐34 to ‐290) |
| Sumatriptan 50 mg versus rizatriptan 10 mg | 2 | 2230 | 3 | 2 | 1.7 (1.0 to 2.9) | Not calculated |
| Photophobia | ||||||
| Sumatriptan 25 mg versus placebo | 4 | 1185 | 50 | 67 | 0.77 (0.69 to 0.85) | 5.7 (4.3 to 8.7) |
| Sumatriptan 50 mg versus placebo (moderate or severe baseline pain intensity) | 9 | 2928 | 45 | 59 | 0.76 (0.71 to 0.82) | 7.1 (5.6 to 9.5) |
| Sumatriptan 50 mg versus placebo (mild baseline pain intensity) | 4 | 988 | 35 | 52 | 0.66 (0.57 to 0.77) | 5.7 (4.2 to 8.7) |
| Sumatriptan 100 mg versus placebo (moderate or severe baseline pain intensity) | 9 | 3381 | 38 | 55 | 0.71 (0.66 to 0.77) | 5.8 (4.8 to 7.3) |
| Sumatriptan 100 mg versus placebo (mild baseline pain intensity) | 4 | 983 | 28 | 52 | 0.54 (0.46 to 0.64) | 4.2 (3.3 to 5.5) |
| Sumatriptan 25 mg versus rizatriptan 5 mg | 2 | 2210 | 51 | 46 | 1.1 (1.0 to 1.2) | Not calculated |
| Sumatriptan 25 mg versus rizatriptan 10 mg | 2 | 2231 | 51 | 42 | 1.2 (1.1 to 1.3) | ‐11 (‐7.7 to ‐21) |
| Sumatriptan 50 mg versus effervescent ASA 1000 mg | 2 | 727 | 27 | 30 | 0.90 (0.72 to 1.1) | Not calculated |
| Sumatriptan 50 mg versus rizatriptan 5 mg | 2 | 2209 | 45 | 46 | 0.98 (0.89 to 1.1) | Not calculated |
| Sumatriptan 50 mg versus rizatriptan 10 mg | 2 | 2230 | 45 | 42 | 1.1 (0.96 to 1.2) | Not calculated |
| Sumatriptan 50 mg versus eletriptan 40 mg | 2 | 687 | 44 | 40 | 1.1 (0.93 to 1.3) | Not calculated |
| Sumatriptan 50 mg versus eletriptan 80 mg | 2 | 678 | 44 | 32 | 1.4 (1.1 to 1.7) | ‐8.0 (‐5.1 to ‐19) |
| Sumatriptan 100 mg versus eletriptan 40 mg | 3 | 2134 | 39 | 32 | 1.2 (1.1 to 1.4) | ‐14 (‐8.8 to ‐30) |
| Sumatriptan 100 mg versus eletriptan 80 mg | 2 | 551 | 45 | 30 | 1.5 (1.2 to 1.9) | ‐6.7 (‐4.4 to ‐15) |
| Phonophobia | ||||||
| Sumatriptan 25 mg versus placebo | 2 | 958 | 40 | 47 | 0.83 (0.70 to 0.98) | 15 (7.2 to 120) |
| Sumatriptan 50 mg versus placebo (moderate or severe baseline pain intensity) | 7 | 2709 | 38 | 50 | 0.75 (0.69 to 0.82) | 8.3 (6.3 to 12) |
| Sumatriptan 50 mg versus placebo (mild baseline pain intensity) | 4 | 989 | 30 | 45 | 0.66 (0.56 to 0.78) | 6.6 (4.7 to 11) |
| Sumatriptan 100 mg versus placebo (moderate or severe baseline pain intensity) | 7 | 3158 | 34 | 48 | 0.70 (0.64 to 0.77) | 7.3 (5.8 to 10) |
| Sumatriptan 100 mg versus placebo (mild baseline pain intensity) | 4 | 981 | 20 | 45 | 0.45 (0.37 to 0.55) | 4.1 (3.3 to 5.4) |
| Sumatriptan 25 mg versus rizatriptan 5 mg | 2 | 2210 | 42 | 37 | 1.2 (1.0 to 1.3) | Not calculated |
| Sumatriptan 25 mg versus rizatriptan 10 mg | 2 | 2231 | 42 | 34 | 1.2 (1.1 to 1.4) | ‐12 (‐8.3 to ‐25) |
| Sumatriptan 50 mg versus effervescent ASA 1000 mg | 2 | 727 | 26 | 27 | 0.96 (0.76 to 1.2) | Not calculated |
| Sumatriptan 50 mg versus rizatriptan 5 mg | 2 | 2209 | 37 | 37 | 0.99 (0.89 to 1.1) | Not calculated |
| Sumatriptan 50 mg versus rizatriptan 10 mg | 2 | 2230 | 37 | 34 | 1.1 (0.96 to 1.2) | Not calculated |
| Sumatriptan 50 mg versus eletriptan 40 mg | 2 | 689 | 39 | 36 | 1.1 (0.91 to 1.3) | Not calculated |
| Sumatriptan 50 mg versus eletriptan 80 mg | 2 | 679 | 39 | 32 | 1.2 (0.99 to 1.5) | ‐14 (‐6.9 to 1900) |
| Sumatriptan 100 mg versus eletriptan 40 mg | 2 | 1898 | 35 | 28 | 1.3 (1.1 to 1.5) | ‐14 (‐8.7 to ‐32) |
| Functional disability | ||||||
| Sumatriptan 25 mg versus placebo | 3 | 486 | 42 | 58 | 0.75 (0.62 to 0.91) | 6.4 (4.1 to 15) |
| Sumatriptan 50 mg versus placebo | 4 | 747 | 44 | 59 | 0.76 (0.66 to 0.88) | 6.7 (4.5 to 13) |
| Sumatriptan 100 mg versus placebo | 6 | 1897 | 41 | 67 | 0.61 (0.56 to 0.67) | 3.8 (3.3 to 4.6) |
| Sumatriptan 50 mg versus eletriptan 40 mg | 2 | 705 | 44 | 35 | 1.3 (1.1 to 1.5) | ‐11 (‐6.0 to ‐46) |
| Sumatriptan 50 mg versus eletriptan 80 mg | 2 | 690 | 44 | 33 | 1.3 (1.1 to 1.6) | ‐9.5 (‐5.6 to ‐30) |
| Sumatriptan 100 mg versus eletriptan 40 mg | 3 | 2231 | 35 | 27 | 1.3 (1.2 to 1.5) | ‐13 (‐8.5 to ‐24) |
| Sumatriptan 100 mg versus eletriptan 80 mg | 2 | 563 | 48 | 32 | 1.5 (1.2 to 1.8) | ‐6.6 (‐4.3 to ‐14) |
Appendix 7. Summary of outcomes: adverse events and withdrawals
| Study ID | Treatment | > 1 dose of study medication available | Any AE | Specific AEs | Serious AEs | AE withdrawal | Other withdrawals/exclusions |
| 160‐104 | Numbers of participants treating first attack: (1) Sumatriptan 25 mg, n = 180 (2) Sumatriptan 50 mg, n = 181 (3) Eletriptan 40 mg, n = 184 (4) Eletriptan 80 mg, n = 180 (5) Placebo, n = 93 |
Yes | Within 7 days: Attack 1 1st dose only: (1) 32/99 (2) 39/113 (3) 54/124 (4) 76/138 (5) 17/42 |
Occurring in > 5% of subjects in any treatment arm Attack 1 1st dose only: Asthenia: (1) 1/99; (2) 0/113; (3) 5/124; (4) 7/138; (5) 0/42 Dry mouth: (1) 3/99; (2) 2/113; (3) 4/124; (4) 9/138; (5) 2/42 Nausea: (1) 5/99; (2) 10/113; (3) 9/124; (4) 14/138; (5) 3/42 Dizziness: (1) 4/99; (2) 9/113; (3) 11/124; (4) 7/138; (5) 1/42 Paraesthesia: (1) 2/99; (2) 1/113; (3) 6/124; (4) 7/138; (5) 1/42 Somnolence: (1) 4/99; (2) 3/113; (3) 4/124; (4) 16/138; (5) 1/42 | (1) 1/180 (ovarian cyst) (2) 0/181 (3) 1/184 (acute cerebral infarction ‐ not related) (4) 1/180 (uterine haemorrhage) (5) 2/93 (urinary tract infection and ectopic pregnancy) | Attack 1: (1) 2/180 (2) 5/181 (3) 3/90 (4) 5/88 (5) 3/93 |
Other withdrawals (no further details): Attack 1 (1) 55/180 (2) 54/181 (3) 21/90 (4) 10/88 (5) 22/93 |
| Banerjee 1992 | (1) Sumatriptan (dispersible) 200 mg, n = 37 (2) Placebo, n = 39 |
No | Within 24 hours: (1) 13/37 (2) 6/39 |
Nausea/vomiting: (1) 9/37; (2) 3/39 |
(1) 0/37 (2) 1/39 |
(1) 4/37 (2) 2/39 | No data |
| Brandes 2007 | Study 1 (1) Sumatriptan 85 mg, n = 415 (365 for efficacy) (2) Naproxen 500 mg, n = 419 (361 for efficacy) (3) Sumatriptan 85 mg + naproxen 500 mg, n = 422 (370 for efficacy) (4) Placebo, n = 421 (365 for efficacy) Study 2 (1) Sumatriptan 85 mg, n = 434 (370 for efficacy) (2) Naproxen 500 mg, n = 434 (371 for efficacy) (3) Sumatriptan 85 mg + naproxen 500 mg, n = 433 (367 for efficacy) (4) Placebo, n = 435 (387 for efficacy) |
No | Within 24 hours: Study 1 (1) 89/365 (2) 48/361 (3) 100/370 (4) 45/365 Study 2 (1) 105/370 (2) 52/371 (3) 97/367 (4) 39/387 |
Reported in 2% or more of participants in any treatment arm: Study 1 Dizziness: (1) 8/365; (2) 6/361; (3) 17/370; (4) 9/365 Paraesthesia: (1) 9/365; (2) 2/361; (3) 11/370; (4) 1/365 Somnolence: (1) 7/365; (2) 6/361; (3) 11/370; (4) 8/365 Nausea: (1) 9/365; (2) 3/361; (3) 11/370; (4) 6/365 Dry mouth: (1) 5/365; (2) 1/361; (3) 8/370; (4) 3/365 Chest discomfort: (1) 5/365; (2) 1/361; (3) 8/370; (4) 0/365 Study 2 Somnolence: (1) 10/370; (2) 6/371; (3) 13/367; (4) 7/387 Dizziness: (1) 8/370; (2) 5/371; (3) 11/367; (4) 7/387 Paraesthesia: (1) 8/370; (2) 0/371; (3) 7/367; (4) 2/387 Nausea: (1) 12/370; (2) 2/371; (3) 13/367; (4) 4/387 Dyspepsia: (1) 11/370; (2) 4/371; (3) 9/367; (4) 2/387 Dry mouth: (1) 10/370; (2) 0/371; (3) 7/367; (4) 5/387 |
Study 1 (1) 1/365 (2) 0/361 (3) 0/370 (4) 0/365 Study 2 (1) 0/370 (2) 0/371 (3) 0/367 (4) 0/387 |
No data | No data |
| Bussone 2000 | (1) Sumatriptan 50 mg, n = 156 (2) Placebo, n = 56 |
Yes | No useable data | No useable data ‐ adverse events reported by body system rather than individually | (1) 0/156 (2) 0/56 |
5 (groups not reported) | 60 other withdrawals: 33 failed to return, 18 lack of efficacy, 3 investigator's decision, 6 other reasons |
| Carpay 2004 | (1) Sumatriptan (fast disintegrating) 50 mg, n = 141 (2) Sumatriptan (fast disintegrating) 100 mg, n = 148 (3) Placebo, n = 155 |
Yes | Within 24 hours: Drug‐related (1) 14/137 (2) 24/142 (3) 8/153 |
Reported in ≥ 3% of participants in any treatment group: Nausea and vomiting: (1) 1/137; (2) 7/142; (3) 3/153 Chest symptoms: (1) 3/137; (2) 4/142; (3) 0/153 Malaise and fatigue: (1) 2/137; (2) 4/142; (3) 1/153 |
(1) 0/137 (2) 0/142 (3) 0/153 |
(1) 1/137 (2) 0/142 (3) 0/153 |
36 other withdrawals: 1 consent withdrawn, 9 lost to follow‐up, 24 no occasion to treat migraine, 1 other and 1 missing data |
| Cutler 1995 | (1) Sumatriptan 25 mg, n = 66 (2) Sumatriptan 50 mg, n = 62 (3) Sumatriptan 100 mg, n = 66 (4) Placebo, n = 65 |
No | Within 24 hours: (1) 47/66 (2) 42/62 (3) 42/66 (4) 48/65 |
Experienced by ≥ 3 participants in any treatment group: Nausea/vomiting: (1) 23/66; (2) 5/62; (3) 15/66; (4) 25/65 Migraine: (1) 17/66; (2) 23/62; (3) 20/66; (4) 26/65 Headache: (1) 15/66; (2) 18/62; (3) 17/66; (4) 20/65 Mouth disorder: (1) 6/66; (2) 11/62; (3) 5/66; (4) 12/65 Dizziness/vertigo: (1) 6/66; (2) 10/62; (3) 3/66; (4) 9/65 Drowsiness/sedation: (1) 6/66; (2) 5/62; (3) 5/66; (4) 9/65 Tingling: (1) 6/66; (2) 2/62; (3) 5/66; (4) 5/65 Sleep disturbance: (1) 0/66; (2) 2/62; (3) 2/66; (4) 5/65 Taste disturbance: (1) 5/66; (2) 2/62; (3) 6/66; (4) 5/65 Neck pain/stiffness: (1) 2/66; (2) 2/62; (3) 2/66; (4) 5/65 |
No data | 1 (group not reported) | No data |
| Dahlof 1991 | (1) Sumatriptan 100 mg, n = 305 (2) Sumatriptan 200 mg, n = 283 (3) Sumatriptan 300 mg, n = 299 (4) Placebo, n = 205 |
No | Within 24 hours: Drug‐related (1) 113/313 (2) 139/295 (3) 164/310 (4) 36/212 |
Bad taste: (1) 16/313; (2) 24/295; (3) 43/310; (4) 4/212 Malaise/fatigue: (1) 19/313; (2) 27/295; (3) 31/310; (4) 4/212 Dizziness/vertigo: (1) 19/313; (2) 15/295; (3) 19/310; (4) 2/212 Drowsiness/sedation: (1) 6/313; (2) 15/295; (3) 16/310; (4) 1/212 Nausea and/or vomiting: (1) 34/313; (2) 50/295; (3) 47/310; (4) 11/212 Chest symptoms: (1) 6/313; (2) 3/295; (3) 16/310; (4) 0/212 Numbness/paraesthesia/ tingling: (1) 13/313; (2) 9/295; (3) 19/310; (4) 1/212 Feeling of heaviness: (1) 6/313; (2) 15/295; (3) 9/310; (4) 1/212 Weakness: (1) 13/313; (2) 12/295; (3) 6/310; (4) 1/212 |
(1) 2/313 (2) 1/295 (3) 1/310 (4) 0/212 |
(1) 10/313 (2) 20/295 (3) 26/310 (4) 3/212 |
38 excluded from efficacy analysis due to protocol violations |
| Dahlof 2009 | (1) Sumatriptan 50 mg, n = 136 (2) Tonabersat 20 mg, n = 134 (3) Tonabersat 40 mg, n = 137 (4) Placebo, n = 134 |
No | Within 6 days: (1) 44/136 (2) 43/134 (3) 56/137 (4) 34/134 |
Occurring in ≥ 3% of participants: Dizziness: (1) 3/136; (2) 8/134; (3) 13/137; (4) 2/134 Paraesthesia: (1) 4/136; (2) 0/134; (3) 5/137; (4) 2/134 Vertigo: (1) 1/136; (2) 4/134; (3) 7/137; (4) 1/134 Nausea: (1) 4/136; (2) 6/134; (3) 11/137; (4) 3/134 Somnolence: (1) 3/136; (2) 2/134; (3) 5/137; (4) 1/134 |
2 (groups not reported) | (1) 0/136 (2) 0/134 (3) 0/137 (4) 0/134 |
147 other exclusions: 88 did not have an attack meeting IHS criteria, 33 for protocol violations, 25 for vomiting within 1 h of treatment, 1 for treating a mild headache |
| Diener 2004a | (1) Sumatriptan 50 mg, n = 135 (2) Effervescent acetylsalicylic acid 1000 mg, n = 147 (146 for efficacy) (3) Placebo, n = 153 (152 for efficacy) |
No | Within 24 hours: (1) 19/135 (2) 19/147 (3) 16/153 |
No useable data ‐ only adverse events for single body system reported | No data | No data | 2 excluded from efficacy analysis due to failure to return patient diaries |
| Diener 2004b | (1) Sumatriptan 50 mg, n = 226 (2) Ibuprofen 400 mg, n = 212 (3) Effervescent acetylsalicylic acid 1000 mg, n = 222 (4) Placebo, n = 222 |
No | Within 24 hours: (1) 45/226 (2) 26/212 (3) 36/222 (4) 32/222 |
No data | (1) 0/226 (2) 1/212 (3) 1/222 (4) 0/222 |
(1) 0/226 (2) 1/212 (3) 1/222 (4) 0/222 |
1 excluded from efficacy analysis due to failure to return patient diary |
| DKSMSG 1999 | (1) Sumatriptan 100 mg, n = 130 (2) Diclofenac‐potassium 50 mg, n = 131 (3) Diclofenac‐potassium 100 mg, n = 122 (4) Placebo, n = 131 |
No | Within 24 hours: (1) 43/130 (2) 25/131 (3) 18/122 (4) 26/131 |
Occurring in ≥ 2% participants for at least 1 treatment: Asthenia: (1) 4/130; (2) 1/131; (3) 1/122; (4) 2/131 Fatigue: (1) 7/130; (2) 5/131; (3) 1/122; (4) 4/131 Chest pain: (1) 4/130; (2) 0/131; (3) 0/122; (4) 1/131 Dizziness: (1) 7/130; (2) 1/131; (3) 0/122; (4) 3/131 Paraesthesia: (1) 5/130; (2) 2/131; (3) 0/122; (4) 1/131 Somnolence: (1) 3/130; (2) 8/131; (3) 1/122; (4) 3/131 Dyspepsia: (1) 1/130; (2) 3/131; (3) 3/122; (4) 1/131 Nausea: (1) 3/130; (2) 3/131; (3) 1/122; (4) 5/131 Abdominal pain: (1) 6/130; (2) 1/131; (3) 6/122; (4) 4/131 Vomiting: (1) 0/130; (2) 0/131; (3) 0/122; (4) 4/131 Tachycardia: (1) 7/130; (2) 2/131; (3) 1/122; (4) 2/131 Anxiety: (1) 3/130; (2) 1/131; (3) 0/122; (4) 0/131 |
(1) 0/130 (2) 0/131 (3) 0/122 (4) 0/131 |
(1) 0/130 (2) 0/131 (3) 2/122 (4) 2/131 |
25 other withdrawals: 5 withdrew consent, 1 no longer required treatment, 2 lost to follow‐up, 17 did not report sufficient attacks |
| Dodick 2002 | Protocol CL13 (1) Sumatriptan 100 mg, n = 193 (2) Almotriptan 12.5 mg, n = 183 (3) Placebo, n = 99 |
Yes | No data | No data | No data | No data | No data |
| Dowson 2002 | (1) Sumatriptan 100 mg, n = 194 (2) Almotriptan 12.5 mg, n = 184 (3) Almotriptan 25 mg, n = 191 (4) Placebo, n = 99 |
Yes | Within 24 hours: (1) 43/194 (2) 16/184 (3) 35/191 (4) 6/99 |
Occurring in more than 2% of participants in any treatment group: Back pain: (1) 4/194; (2) 2/184; (3) 1/191; (4) 0/99 Fatigue: (1) 11/194; (2) 1/184; (3) 2/191; (4) 0/99 Paraesthesia: (1) 6/194; (2) 1/184; (3) 2/191; (4) 1/99 Dizziness: (1) 4/194; (2) 0/184; (3) 4/191; (4) 2/99 Somnolence: (1) 4/194; (2) 1/184; (3) 3/191; (4) 0/99 |
No data | (1) 0/194 (2) 0/184 (3) 0/191 (4) 0/99 |
(1) 1/194 (protocol violation) (2) 1/184 (protocol violation) (3) 6/191 (use of prohibited medication and protocol violation) (4) 0/99 |
| Ensink 1991 | (1) Sumatriptan 100 mg, n = 148 (2) Placebo, n = 84) |
Yes | Within 24 hours: (1) 57/149 (2) 19/84 |
Most commonly reported adverse events: Nausea and/or vomiting: (1) 12/149; (2) 5/84 Malaise/fatigue: (1) 12/149; (2) 2/84 Disturbance of taste: (1) 10/149; (2) 3/84 Dizziness/vertigo: (1) 7/149; (2) 2/84 Headache: (1) 3/149; (2) 4/84 Chest symptoms: (1) 6/149; (2) 1/84 Weakness: (1) 4/149; (2) 1/84 |
No data | (1) 2/149 (2) 1/84 |
1 excluded from efficacy analysis due to protocol violation |
| Freitag 2001 | (1) Sumatriptan 25 mg (+ additional dose of 25 mg at 2 h), n = 61 (2) Isometheptene combination 2 doses (+ additional single doses at 1, 2 and 3 h), n = 65 |
No | Within 24 hours: Drug‐related (1) 11/61 (2) 13/65 |
Abdominal pain or cramps: (1) 1/61; (2) 2/65 Nausea: (1) 1/61; (2) 2/65 Diarrhoea: (1) 1/61; (2) 0/65 Lightheadedness: (1) 3/61; (2) 4/65 Sleepiness: (1) 3/61; (2) 2/65 Dry mouth: (1) 3/61; (2) 1/65 Heat flashes: (1) 0/61; (2) 1/65 Head pressure: (1) 2/61; (2) 0/65 Tremor: (1) 1/61; (2) 0/65 Sweating: (1) 1/61; (2) 0/65 Palpitations: (1) 0/61; (2) 1/65 Chest pain: (1) 2/61; (2) 0/65 Enlarged thyroid: (1) 0/61; (2) 1/65 Sore throat: (1) 2/61; (2) 0/65 Laryngitis: (1) 1/61; (2) 0/65 Bruises: (1) 1/61; (2) 0/65 Stiff neck: (1) 1/61; (2) 0/65 Drug taste: (1) 1/61; (2) 0/65 Confusion: (1) 0/61; (2) 1/65 |
No data | No data | 11 other withdrawals: 7 due to failure to treat within allotted time frame, 2 lost to follow‐up, 1 for protocol violation and 1 due to vomiting immediately after taking drug |
| Gallagher 2000 | (1) Sumatriptan 25 mg, n = 336 (306 for efficacy) (2) Sumatriptan 50 mg, n = 338 (306 for efficacy) (3) Zolmitriptan 2.5 mg, n = 327 (295 for efficacy) (4) Zolmitriptan 5 mg, n = 337 (305 for efficacy) |
Yes | Within 24 hours: Attack 1 (1) 69/336 (2) 99/338 (3) 91/327 (4) 111/337 |
Infection: (1) 6/336; (2) 19/338; (3) 19/327; (4) 14/337 Tightness: (1) 3/336; (2) 9/338; (3) 7/327; (4) 22/337 Nausea: (1) 14/336; (2) 25/338; (3) 23/327; (4) 38/337 Vomiting: (1) 13/336; (2) 20/338; (3) 12/327; (4) 14/337 Dizziness: (1) 15/336; (2) 17/338; (3) 20/327; (4) 27/337 Paraesthesia: (1) 12/336; (2) 15/338; (3) 16/327; (4) 27/337 Somnolence: (1) 12/336; (2) 13/338; (3) 14/327; (4) 26/337 Pharyngitis: (1) 17/336; (2) 14/338; (3) 23/327; (4) 26/337 |
10 (groups not reported) | (1) 9/336 (2) 7/338 (3) 6/327 (4) 12/337 |
No data |
| Geraud 2000 | (1) Sumatriptan 100 mg, n = 504 (2) Zolmitriptan 5 mg, n = 498 (3) Placebo, n = 56 |
No | Probably within 24 hours: (1) 279/492 (2) 287/491 (3) 13/56 |
With an incidence of at least 5%: Asthenia: (1) 53/492; (2) 53/491; (3) 3/56 Dizziness/vertigo: (1) 46/492; (2) 44/491; (3) 1/56 Somnolence: (1) 29/492; (2) 37/491; (3) 2/56 Paraesthesia: (1) 33/492; (2) 29/491; (3) 0/56 Heaviness other than chest or neck: (1) 27/492; (2) 29/491; (3) 0/56 Nausea: (1) 35/492; (2) 30/491; (3) 1/56 Warm sensation: (1) 29/492; (2) 24/491; (3) 1/56 Neck pain: (1) 24/492; (2) 17/491; (3) 1/56 |
No drug related serious AEs | No data | No data |
| GL/MIG/001/92 | (1) Sumatriptan 100 mg, n = 305 (2) Paracetamol 1000 mg + metoclopramide 10 mg, n = 302 |
Yes | Collected over > 24 hours (1) 162/305 (2) 98/302 |
Dizziness: (1) 22/305; (2) 13/302 Nausea: (1) 44/305; (2) 5/302 Numbness of extremities: (1) 2/305 (2) 0/302 Pins and needles through whole body: (1) 2/305; (2) 0/302 Anxiety: (1) 1/305; (2) 0/302 Backache: (1) 1/305; (2) 0/302 Chest pain: (1) 1/305; (2) 0/302 Feeling of heaviness in head: (1) 1/305; (2) 0/302 Hot sweats: (1) 1/305; (2) 0/302 Sweaty: (1) 8/305; (2) 0/302 Vomiting: (1) 7/305; (2) 6/302 Warm sensation: (1) 1/305; (2) 0/302 Weakness: (1) 6/305; (2) 1/302 Diarrhoea: (1) 3/305; (2) 5/302 Headache: (1) 5/305; (2) 6/302 Drowsiness: (1) 7/305; (2) 5/302 Dyspepsia: (1) 5/305; (2) 0/302 Tiredness: (1) 4/305; (2) 9/302 |
No useable data | (1) 18/305 (2) 5/302 |
Other withdrawals: (1) 35/305 (2) 42/302 Withdrawal due to lack of efficacy: (1) 0/305 (2) 8/302 |
| GL/MIG/001A/92 | (1) Sumatriptan 100 mg, n = 348 (2) Paracetamol 1000 mg + metoclopramide 10 mg, n = 373 |
No | Collected over > 24 hours (1) 142/348 (2) 93/373 |
Chest pain: (1) 4/348; (2) 0/373 Nausea: (1) 30/348; (2) 6/373 Cervicalgia: (1) 3/348; (2) 0/373 Headache: (1) 2/348; (2) 2/373 Dizziness: (1) 24/348; (2) 9/373 Sweaty: (1) 2/348; (2) 0/373 Diarrhoea: (1) 5/348; (2) 4/373 Vomiting: (1) 11/348; (2) 3/373 Cold sensation: (1) 1/348; (2) 0/373 Feeling of heaviness: (1) 7/348; (2) 0/373 Pins and needles in arms: (1) 1/348; (2) 0/373 Tightness in chest: (1) 1/348; (2) 0/373 Abdominal pain: (1) 0/348; (2) 1/373 Anxiety states: (1) 0/348; (2) 1/373 |
No useable data | (1) 13/348 (2) 8/373 |
Other withdrawals: (1) 95/348 (2) 92/373 Withdrawal due to lack of efficacy: (1) 11/347 (2) 11/373 |
| GL/MIG/002 | (1) Sumatriptan 100 mg, n = 374 (2) Migraleve (buclizine hydrochloride 12.5 mg + paracetamol 1000 mg + codeine phosphate 16 mg), n = 378 |
Yes | Collected over > 24 hours (1) 183/374 (2) 127/378 |
Dizziness: (1) 31/374; (2) 16/378 Nausea: (1) 46/374; (2) 22/378 Headache: (1) 31/374; (2) 55/378 Tiredness: (1) 8/374; (2) 11/378 Dyspepsia: (1) 7/374; (2) 5/378 Cervicalgia: (1) 5/374; (2) 7/378 Drowsiness: (1) 3/374; (2) 6/378 Sweaty: (1) 2/374; (2) 6/278 Vomiting: (1) 2/374; (2) 7/378 Weakness: (1) 2/374; (2) 0/378 Abdominal ache: (1) 1/374; (2) 0/378 Feeling of heaviness: (1) 1/374; (2) 0/378 Numbness in body: (1) 1/374; (2) 0/378 Chest pain: (1) 0/374; (2) 1/378 |
No useable data | (1) 18/374 (2) 5/378 |
Other withdrawals: (1) 27/374 (2) 27/378 Withdrawal due to lack of efficacy: (1) 6/374 (2) 7/378 |
| GL/MIG/002A | (1) Sumatriptan 100 mg, n = 342 (2) Migraleve (buclizine hydrochloride 12.5 mg + paracetamol 1000 mg + codeine phosphate 16 mg), n = 332 |
No | Collected over > 24 hours (1) 146/342 (2) 95/332 |
Dizziness: (1) 24/342; (2) 19/332 Nausea: (1) 20/342; (2) 11/332 Headache: (1) 15/342; (2) 9/332 Tiredness: (1) 13/342; (2) 3/332 Drowsiness: (1) 6/342; (2) 2/332 Vomiting: (1) 5/342; (2) 4/332 Hot: (1) 4/342; (2) 4/332 Backache: (1) 3/342; (2) 3/332 Sweaty: (1) 4/342; (2) 5/332 Chest pain: (1) 1/342; (2) 1/332 Abdominal pain: (1) 1/342; (2) 0/332 Flushing of face: (1) 1/342; (2) 0/332 Heaviness in arm/leg: (1) 1/342; (2) 0/332 Palpitations: (1) 0/342; (2) 1/332 |
No useable data | (1) 15/342 (2) 7/332 |
Other withdrawals: (1) 58/342 (2) 52/332 Withdrawals due to lack of efficacy: (1) 4/342 (2) 8/332 |
| GL/MIG/009 | (1) Sumatriptan 100 mg, n = 255 (2) Migril (ergotamine tartrate 2 mg + cyclizine hydrochloride 50 mg + caffeine hydrate 100 mg), n = 258 |
Yes | Within 24 hours: (1) 75/255 (2) 68/258 |
Most frequent adverse events: Neck stiffness: (1) 17/255; (2) 0/258 Nausea: (1) 11/255; (2) 22/258 Dizziness: (1) 10/255; (2) 14/258 Diarrhoea: (1) 9/255; (2) 3/258 Vomiting: (1) 4/255; (2) 12/258 Light‐headedness: (1) 7/255; (2) 6/258 Tiredness: (1) 7/255; (2) 4/258 Drowsiness: (1) 5/255; (2) 15/258 Sore throat: (1) 5/255; (2) 2/258 Vertigo: (1) 5/255; (2) 1/258 |
(1) 1/255 (2) 0/258 |
(1) 14/255 (2) 13/258 |
Other withdrawals: (1) 26/255 (2) 29/258 Withdrawals due to lack of efficacy: (1) 1/255 (2) 5/258 |
| Goadsby 1991 | Number of attacks in efficacy population: (1) Sumatriptan 100 mg, n = 94 (2) Placebo, n = 94 |
No | No data | No data | No data | No data | No data |
| Goadsby 2000 | (1) Sumatriptan 100 mg, n = 129 (2) Eletriptan 20 mg, n = 144 (3) Eletriptan 40 mg, n = 136 (4) Eletriptan 80 mg, n = 141 (5) Placebo, n = 142 |
Yes | Within 24 hours: (1) 52/129 (2) 49/144 (3) 48/136 (4) 72/141 (5) 24/142 |
Events with ≥ 4% incidence: Asthenia: (1) 4/129; (2) 3/144; (3) 4/136; (4) 14/141; (5) 0/142 Drowsiness: (1) 6/129; (2) 3/144; (3) 1/136; (4) 6/141; (5) 2/142 Nausea: (1) 4/129; (2) 4/144; (3) 2/136; (4) 10/141; (5) 1/142 Dizziness: (1) 5/129; (2) 3/144; (3) 5/136; (4) 6/141; (5) 1/142 Paraesthesia: (1) 7/129; (2) 6/144; (3) 3/136; (4) 11/141; (5) 2/142 |
None after eletriptan treatment, no other details reported | (1) 1/129 (2) 2/144 (3) 0/136 (4) 0/141 (5) 1/142 |
Other withdrawals: (1) 3/129 (2) 1/144 (3) 2/136 (4) 5/141 (5) 2/142 |
| Goldstein 1998 | (1) Sumatriptan 25 mg, n = 563 (2) Sumatriptan 50 mg, n = 566 (3) Rizatriptan 5 mg, n = 557 (4) Rizatriptan 10 mg, n = 567 (5) Placebo, n = 141 |
No | Within 24 hours: Attack 1 (1) 137/297 (2) 134/291 (3) 129/294 (4) 137/305 (5) 50/142 |
Incidence ≥ 5% in any 1 treatment group: Asthenia/fatigue: (1) 9/297; (2) 17/291; (3) 12/294; (4) 9/305; (5) 1/142 Chest pain: (1) 15/297; (2) 9/291; (3) 9/294; (4) 6/305; (5) 0/142 Dizziness: (1) 12/297; (2) 26/291; (3) 26/294; (4) 34/305; (5) 7/142 Dry mouth: (1) 15/297; (2) 15/291; (3) 21/294; (4) 15/305; (5) 4/142 Headache: (1) 12/297; (2) 17/291; (3) 6/294; (4) 6/305; (5) 7/142 Nausea: (1) 15/297; (2) 17/291; (3) 12/294; (4) 15/305; (5) 3/142 Paraesthesia: (1) 12/297; (2) 9/291; (3) 9/294; (4) 15/305; (5) 1/142 Somnolence: (1) 15/297; (2) 17/291; (3) 12/294; (4) 21/305; (5) 6/142 |
No drug‐related serious AEs | Attack 1 (1) 2/297 (2) 1/291 (3) 4/294 (4) 0/297 (5) 0/142 |
No data |
| Goldstein 2005 | (1) Sumatriptan 50 mg, n = 67 (2) Acetaminophen 1000 mg + aspirin 1000 mg + caffeine 260 mg combination, n = 69 (3) Placebo, n = 35 |
No | No data | No useable data ‐ adverse events reported by body system rather than individually | (1) 0/67 (2) 0/69 (3) 0/35 |
No data | No data |
| Gruffyd‐Jones 2001 | (1) Sumatriptan 50 mg, n = 555 (2) Zolmitriptan 2.5 mg, n = 551 (3) Zolmitriptan 5 mg, n = 560 |
Yes | Within 24 hours: (1) 191/555 (2) 192/551 (3) 211/560 |
Occurring in ≥ 4% of participants in any treatment group: Asthenia: (1) 25/555; (2) 29/551; (3) 37/560 Paraesthesia: (1) 30/555; (2) 29/551; (3) 29/560 Tightness: (1) 17/555; (2) 19/551; (3) 28/560 Dizziness: (1) 28/555; (2) 19/551; (3) 32/560 Somnolence: (1) 25/555; (2) 17/551; (3) 28/560 |
(1) 0/555 (2) 0/551 (3) 0/560 |
(1) 15/555 (2) 15/551 (3) 19/560 |
Other withdrawals: (1) 192/597 (2) 192/597 (3) 187/593 |
| Havanka 2000 | (1) Sumatriptan 100 mg, n = 98 (2) Naratriptan 1 mg, n = 85 (3) Naratriptan 2.5 mg, n = 87 (4) Naratriptan 5 mg, n = 93 (5) Naratriptan 7.5 mg, n = 93 (6) Naratriptan 10 mg, n = 96 (7) Placebo, n = 91 |
No | Within 24 hours: (1) 25/98 (2) 17/85 (3) 18/87 (4) 30/93 (5) 34/93 (6) 34/96 (7) 21/91 |
Most common adverse events: Malaise/fatigue: (1) 4/98; (2) 2/85; (3) 1/87; (4) 3/93; (5) 10/93; (6) 11/96; (7) 5/91 Nausea: (1) 1/98; (2) 0/85; (3) 3/87; (4) 4/93; (5) 4/93; (6) 4/96; (7) 1/91 |
No data | (1) 0/98 (2) 0/85 (3) 0/87 (4) 0/93 (5) 0/93 (6) 0/96 (7) 0/91 |
No data |
| Ishkanian 2007 | (1) Sumatriptan 50 mg, n = 108 (2) Placebo, n = 108 |
No | Probably within 24 hours: (1) 21/108 (2) 12/108 |
Most common study drug‐related AEs: Dizziness: (1) 5/108; (2) 1/107 Nausea: (1) 3/108; (2) 2/107 Other pressure/tightness: (1) 4/108; (2) 0/107 Temperature sensations: (1) 2/108; (2) 0/107 |
(1) 0/108 (2) 0/108 |
No data | 1 excluded from efficacy analysis due to failure to return patient diary |
| Jelinski 2006 | (1) Sumatriptan 50 mg, n = 126 (2) Sumatriptan 100 mg, n = 126 (3) Placebo, n = 109 |
Yes | Within 24 hours: Drug‐related (1) 21/126 (2) 28/126 (3) 3/109 |
Most frequent adverse events: Nausea: (1) 3/126; (2) 5/127; (3) 1/111 Dizziness: (1) 2/126; (2) 4/127; (3) 2/111 Tingling: (1) 2/126; (2) 3/127; (3) 0/111 Chest tightness: (1) 0/126; (2) 3/127; (3) 1/111 Fatigue: (1) 6/126; (2) 2/127; (3) 1/111 Hot flushes: (1) 2/126; (2) 2/127; (3) 0/111 Tightness of throat: (1) 1/126; (2) 2/127; (3) 0/111 Weakness: (1) 1/126; (2) 2/127; (3) 0/111 Palpitations: (1) 2/126; (2) 0/127; (3) 0/111 Somnolence: (1) 2/126; (2) 0/127; (3) 0/111 Neck pain: (1) 1/126; (2) 0/127; (3) 1/111 Chest pain: (1) 1/126; (2) 0/127; (3) 0/111 Drowsiness: (1) 1/126; (2) 0/127; (3) 0/111 Numbness: (1) 1/126; (2) 0/127; (3) 0/111 Anxiety: (1) 0/126; (2) 0/127; (3) 1/111 |
(1) 0/126 (2) 0/126 (3) no data |
No data | 3 excluded due to loss at follow‐up |
| Kaniecki 2006 | (1) Sumatriptan 100 mg, n = 131 (2) Placebo, n = 127 |
Yes | Probably within 24 hours: Drug‐related (1) 17/131 (2) 6/127 |
Reported in > 2% of participants: Nausea: (1) 9/131; (2) 4/127 Dizziness: (1) 5/131; (2) 0/127 |
(1) 0/131 (2) 0/127 |
No data | 5 excluded from efficacy analysis as failed to provide post‐baseline efficacy assessments 3 excluded from placebo treatment arm (no details reported) |
| Kolodny 2004 | (1) Sumatriptan 25 mg, n = 554 (290 1st attack only) (2) Sumatriptan 50 mg, n = 550 (287 1st attack only) (3) Rizatriptan 5 mg, n = 536 (288 1st attack only) (4) Rizatriptan 10 mg, n = 547 (294 1st attack only) (5) Placebo, n = 288 |
No | Within 24 hours: Attack 1 (1) 113/290 (2) 142/287 (3) 109/288 (4) 139/294 (5) 102/288 |
With common AEs: Asthenia/fatigue: (1) 13/290; (2) 17/287; (3) 15/288; (4) 11/294; (5) 6/288 Chest pain: (1) 3/290; (2) 13/287; (3) 5/288; (4) 10/294; (5) 7/288 Diarrhoea: (1) 3/290; (2) 14/287; (3) 4/288; (4) 3/294; (5) 6/288 Dry mouth: (1) 13/290; (2) 18/287; (3) 11/288; (4) 18/294; (5) 19/288 Flushing: (1) 12/290; (2) 14/287; (3) 3/288; (4) 9/294; (5) 2/288 Nausea: (1) 12/290; (2) 19/287; (3) 13/288; (4) 17/294; (5) 12/288 Dizziness: (1) 17/290; (2) 30/287; (3) 19/288; (4) 25/294; (5) 13/288 Headache: (1) 9/290; (2) 12/287; (3) 8/288; (4) 6/294; (5) 1/288 Paraesthesia: (1) 10/290; (2) 10/287; (3) 6/288; (4) 13/294; (5) 2/288 Somnolence: (1) 11/290; (2) 18/287; (3) 17/288; (4) 23/294; (5) 13/288 |
No data | 3 (groups not reported) | 157 other discontinuations: 94 did not have 2nd headache, 18 lost to follow‐up, 13 withdrew from study, 14 were unco‐operative, 18 discontinued due to lack of therapeutic response, need for concomitant medication, or inclusion/exclusion criteria not met |
| Kudrow 2005 | (1) Sumatriptan 50 mg, n = 144 (2) Valdecoxib 20 mg, n = 137 (3) Valdecoxib 40 mg, n = 152 (4) Placebo, n = 141 |
Yes | Within 24 hours: (1) 45/141 (2) 32/133 (3) 42/149 (4) 41/140 |
Occurring in > 2% of dosed participants: Dry mouth: (1) 8/141; (2) 3/133; (3) 2/149; (4) 1/140 Nausea: (1) 6/141; (2) 3/133; (3) 3/149; (4) 2/140 Diarrhoea: (1) 5/141; (2) 2/133; (3) 3/149; (4) 0/140 Paraesthesia: (1) 5/141; (2) 4/133; (3) no data; (4) 2/140 Fatigue: (1) 4/141; (2) 2/133; (3) 4/149; (4) 4/140 Somnolence: (1) 3/141; (2) 4/133; (3) 4/149; (4) 3/140 Dizziness (excl. vertigo): (1) 3/141; (2) 3/133; (3) 7/149; (4) 4/140 Anxiety: (1) 3/141; (2) 0/133; (3) 1/149; (4) 0/140 Headache: (1) 1/141; (2) 2/133; (3) 2/149; (4) 3/140 Hypoaesthesia: (1) 1/141; (2) 2/133; (3) 1/149; (4) 3/140 |
No data | No data | Total withdrawals (possibly including AE withdrawals, but not specified): (1) 3/144 (2) 4/137 (3) 5/152 (4) 2/141 |
| Latere 1991 | (1) Sumatriptan (dispersible) 100 mg, n = 288 (2) Cafergot, n = 289 |
No | Within 24 hours: (1) 130/290 (2) 113/290 |
No useable data ‐ no. of events rather than participants with event reported | (1) 2/290 (2) 0/290 |
(1) 6/290 (2) 9/290 |
No data |
| Lines 2001 | (1) Sumatriptan 50 mg, n = 356 (2) Rizatriptan 5 mg, n = 349 (3) Placebo, n = 80 |
No | No data | No data | No data | No data | No data |
| Lipton 2000 | Treated attacks: (1) Sumatriptan 50 mg, n = 870 (2) Placebo, n = 240 |
No | Within 24 hours: Attacks treated with drug‐related AE (1) 83/1358 (2) 19/356 |
No data | 1 (group not reported) | 7 drug‐related AE withdrawals (groups not reported) | 62 excluded from efficacy analysis due to failure to treat (53), incomplete data (2) or noncompliance (7) |
| Matthew 2003 | (1) Sumatriptan 100 mg, n = 831 (2) Eletriptan 40 mg, n = 822 (3) Placebo, n = 419 |
Yes | Within 7 days: (1) 314/849 (2) 259/835 (3) 146/429 |
Incidence ≥ 2%: Nausea: (1) 125/849; (2) 99/835; (3) 54/429 Vomiting: (1) 49/849; (2) 49/835; (3) 46/429 Photophobia: (1) 39/849; (2) 34/835; (3) 24/429 Asthenia: (1) 20/849; (2) 13/835; (3) 4/429 Chest symptoms: (1) 17/849; (2) 13/835; (3) 2/429 Paraesthesia: (1) 20/849; (2) 9/835; (3) 0/429 |
No drug‐related serious AEs | No data | Total withdrawals (possibly including AE withdrawals, but not specified): (1) 1/849 (2) 3/835 (3) 0/429 |
| Myllyla 1998 | (1) Sumatriptan 100 mg (+ optional dose of placebo after 1 h), n = 46 (2) Tolfenamic acid 200 mg (+ optional 2nd dose after 1 h), n = 47 (3) Placebo (+ optional dose of placebo after 1 h), n = 46 |
Yes | No useable data | No useable data | No data | 2 (groups not reported) | 8 withdrawn due to lack of attacks (groups not reported) 3 lost to follow‐up |
| Nappi 1994 | (1) Sumatriptan 100 mg, n = 158 (2) Placebo, n = 86 |
Yes | Within 24 hours: Participants receiving 1 dose only (1) 24/69 (2) 4/19 |
Reported by at least 4 sumatriptan‐treated participants: Nausea and/or vomiting: (1) 12/162; (2) 6/88 Gastric symptoms: (1) 8/162; (2) 1/88 Malaise/fatigue: (1) 4/162; (2) 2/88 Abdominal discomfort: (1) 4/162; (2) 1/88 |
No data | (1) 8/162 (2) 2/88 |
6 excluded from efficacy analysis due to protocol violations |
| Nett 2003 | (1) Sumatriptan 50 mg, n = 124 (2) Sumatriptan 100 mg, n = 122 (3) Placebo, n = 123 |
No | Probably within 24 hours: (1) 10/124 (2) 20/122 (3) 9/123 |
Reported by more than 2% of participants in any treatment group: Nausea: (1) 2/124; (2) 8/122; (3) 3/123 Paraesthesia: (1) 1/124; (2) 0/122; (3) 0/123 Dizziness: (1) 3/124; (2) 0/122; (3) 1/123 Malaise/fatigue: (1) 0/124; (2) 0/122; (3) 1/123 |
(1) 0/124 (2) 0/122 (3) 0/123 |
(1) 0/124 (2) 0/122 (3) 0/123 |
48 excluded: 18 lost to follow‐up, 22 did not treat, 2 protocol violations, 5 withdrew consent, 3 other |
| Patten 1991 | (1) Sumatriptan (dispersible) 100 mg, n = 142 (2) Sumatriptan (dispersible) 200 mg, n = 140 (3) Sumatriptan (dispersible) 300 mg, n = 155 (4) Placebo, n = 101 |
No | No useable data | Chest symptoms: (1) 1/69; (2) 2/56; (3) 8/60; (4) 0/42 Heaviness/pressure/ warmth: (1) 3/69; (2) 9/56; (3) 6/60; (4) 2/42 Tingling/prickling: (1) 7/69; (2) 1/56; (3) 2/60; (4) 0/42 Drowsiness/sedation: (1) 2/69; (2) 2/56; (3) 4/60; (4) 1/42 Nausea/vomiting: (1) 6/69; (2) 8/56; (3) 8/60; (4) 2/42 Bitter taste: (1) 5/69; (2) 5/56; (3) 13/60; (4) 3/42 |
No data | (1) 2/69 (2) 7/46 (3) 11/60 (4) 1/42 |
No data |
| Pfaffenrath 1998 | (1) Sumatriptan 25 mg, n = 303 (2) Sumatriptan 50 mg, n = 303 (3) Sumatriptan 100 mg, n = 298 (4) Placebo, n = 99 |
Yes | Within 24 hours: (1) 74/303 (2) 82/303 (3) 111/298 (4) 20/99 |
Incidence of ≥ 4% in any treatment group, after single dose only: Malaise/fatigue: (1) 9/303; (2) 8/303; (3) 15/298; (4) 1/99 Nausea/vomiting: (1) 7/303; (2) 18/303; (3) 13/298; (4) 2/99 Dizziness: (1) 5/303; (2) 4/303; (3) 14/298; (4) 2/99 Chest pressure/heaviness: (1) 3/303; (2) 11/303; (3) 9/298; (4) 0/99 Muscle pain: (1) 3/303; (2) 1/303; (3) 3/298; (4) 0/99 |
No data | (1) 2/303 (2) 3/303 (3) 8/298 (4) 1/99 |
88 other withdrawals: 21 due to lack of efficacy, 6 lost to follow‐up, 20 due to protocol violation, 41 other |
| Pini 1999 | (1) Sumatriptan 50 mg, n = 137 (106 for efficacy) (2) Placebo, n = 82 (61 for efficacy) |
Yes | Within 24 hours: (1) 10/106 (2) 4/82 |
No useable data ‐ adverse events reported by body system rather than individually | (1) 0/106 (2) 0/82 |
No data | Total withdrawals (possibly including AE withdrawals, but not specified): 12 (groups not given) |
| Pini 1995 | (1) Sumatriptan 100 mg, n = 151 (2) Placebo, n = 87 |
No | Within 48 hours: Drug‐related (1) 18/151 (2) 6/87 |
Occurring in over 1% of participants: Malaise/fatigue: (1) 7/151; (2) 0/87 Nausea and/or vomiting: (1) 5/151; (2) 1/87 Paraesthesia: (1) 3/151; (2) 1/87 Numbness: (1) 3/151; (2) 0/87 Throat symptoms/neck stiffness: (1) 3/151; (2) 0/87 Chest symptoms: (1) 2/151; (2) 8/87 Heaviness/pressure sensation: (1) 2/151; (2) 0/87 |
No data | No data | No data |
| Sandrini 2002 | (1) Sumatriptan 50 mg, n = 181 (2) Sumatriptan 100 mg, n = 170 (3) Eletriptan 40 mg, n = 175 (4) Eletriptan 80 mg, n = 164 (5) Placebo, n = 84 |
Yes | No data | Occurring in ≥ 5% in any treatment group: Asthenia: (1) 9/181; (2) 7/169; (3) 11/175; (4) 15/164; (5) 2/84 Nausea: (1) 9/181; (2) 8/169; (3) 5/175; (4) 15/164; (5) 2/84 Dizziness: (1) 11/181; (2) 5/169; (3) 11/175; (4) 15/164; (5) 2/84 Somnolence: (1) 2/181; (2) 3/169; (3) 12/175; (4) 6/164; (5) 2/84 Chest symptoms: (1) 2/181; (2) 0/169; (3) 1/175; (4) 7/164; (5) 2/84 Sweating: (1) 0/181; (2) 3/169; (3) 8/175; (4) 3/164; (5) 2/84 |
No drug‐related serious AEs | (1) 1/181 (after 2 doses) (2) 2/170 (after 2 doses) (3) 2/175 (1 after 2 doses and the other after single dose) (4) 1/164 (after single dose) (5) 2/84 |
No data |
| Sandrini 2007 | (1) Sumatriptan 50 mg, n = 139 (138 for efficacy) (2) Indoprocaf, n = 143 |
Yes | Within 24 hours: (1) 25/139 (2) 31/143 |
No data | (1) 1/139 (2) 0/143 |
(1) 1/139 (2) 3/143 |
1 excluded from efficacy analysis due to failure to return patient diary |
| Sargent 1995 | (1) Sumatriptan 25 mg, n = 48 (2) Sumatriptan 50 mg, n = 46 (3) Sumatriptan 100 mg, n = 46 (4) Placebo, n = 47 |
No | Probably within 72 hours: (1) 15/48 (2) 17/46 (3) 15/46 (4) 14/47 |
Experienced by ≥ 3 participants in any treatment group: Nausea/vomiting: (1) 1/48; (2) 5/46; (3) 6/46; (4) 8/47 Mouth disorder: (1) 2/48; (2) 1/46; (3) 0/46; (4) 0/47 Dizziness/vertigo: (1) 1/48; (2) 3/46; (3) 2/46; (4) 2/47 Drowsiness/sedation: (1) 3/48; (2) 0/46; (3) 2/46; (4) 0/47 Tingling: (1) 3/48; (2) 2/46; (3) 3/46; (4) 2/47 Chills: (1) 3/48; (2) 0/46; (3) 1/46; (4) 2/47 |
No data | (1) 0/48 (2) 0/46 (3) 0/46 (4) 0/47 |
No data |
| Savani 1999 | (1) Sumatriptan 50 mg, n = 331 (2) Placebo, n = 154 |
Yes | Within 24 hours: (1) 82/332 (2) 32/156 |
Experienced by 1% or more of participants in any treatment group: Paraesthesia: (1) 7/332; (2) 1/156 Numbness: (1) 5/332; (2) 1/156 Tingling: (1) 5/332; (2) 1/156 Warm or hot sensation: (1) 5/332; (2) 2/156 Feeling of tightness: (1) 4/332; (2) 1/156 Nausea or vomiting: (1) 14/332; (2) 4/156 Dizziness: (1) 12/332; (2) 3/156 Headache: (1) 3/332; (2) 2/156 Malaise and fatigue: (1) 7/332; (2) 1/156 |
1 (group not reported) | 12 (groups not reported) | 52 withdrawals: 5 due to lack of efficacy, 15 lost to follow‐up, 32 for other reasons |
| Schulman 2003 | (1) Sumatriptan 50 mg, n = 16 (2) Sumatriptan 50 mg + metoclopramide 10 mg, n = 16 |
No | Within 24 hours: (1) 2/16 (2) 4/16 |
No data | (1) 0/16 (2) 0/16 |
No data | No data |
| Sheftell 2005 | Study 1: (1) Sumatriptan (rapid‐release) 50 mg, n = 494 (2) Sumatriptan (rapid‐release) 100 mg, n = 488 (3) Placebo, n = 495 Study 2: (1) Sumatriptan (rapid‐release) 50 mg, n = 496 (2) Sumatriptan (rapid‐release) 100 mg, n = 485 (3) Placebo, n = 494 |
Yes | Within 7 days: Drug‐related Study 1 (1) 40/494 (2) 57/488 (3) 17/495 Study 2 (1) 58/496 (2) 94/485 (3) 25/494 |
Reported in > 2% of participants in any treatment group: Nausea: Study 1 (1) 11/494; (2) 13/488; (3) 5/495 Study 2 (1) 10/496; (2) 16/485; (3) 5/494 Paraesthesia Study 1 (1) 4/494; (2) 3/488; (3) 0/495 Study 2 (1) 5/496; (2) 14/485; (3) 1/494 |
No drug related serious AEs | Study 1 (1) 0/494 (2) 0/488 (3) 0/495 Study 2 (1) 0/496 (2) 0/485 (3) 0/494 |
"Premature withdrawals": Study 1 (1) 72/556 (2) 67/551 (3) 75/558 Study 2 (1) 66/561 (2) 65/550 (3) 61/555 |
| Smith 2005 | (1) Sumatriptan 50 mg, n = 229 (2) Sumatriptan 50 mg, + naproxen 500 mg, n = 251 (3) Naproxen 500 mg, n = 250 (4) Placebo, n = 241 |
No | Within 24 hours: (1) 55/229 (2) 58/251 (3) 43/250 (4) 36/241 |
Reported in > 2% of participants in any treatment group: Chest tightness: (1) 2/229; (2) 4/250; (3) 5/251; (4) 3/242 Diarrhoea: (1) 4/229; (2) 6/250; (3) 0/251; (4) 3/242 Dizziness (not vertigo): (1) 11/229; (2) 4/250; (3) 9/251; (4) 8/242 Dry mouth: (1) 4/229; (2) 3/250; (3) 4/251; (4) 1/242 Fatigue: (1) 1/229; (2) 0/250; (3) 5/251; (4) 0/242 Nausea aggravated: (1) 3/229; (2) 2/250; (3) 1/251; (4) 4/242 Paraesthesia: (1) 4/229; (2) 1/250; (3) 2/251; (4) 1/242 Somnolence: (1) 6/229; (2) 2/250; (3) 3/251; (4) 0/242 Tinnitus: (1) 4/229; (2) 4/250; (3) 6/251; (4) 2/242 |
(1) 0/229 (2) 0/251 (3) 0/250 (4) 0/241 |
No data | 7 excluded from efficacy analysis for protocol violations |
| Spierings 2001 | (1) Sumatriptan 50 mg, n = 582 (2) Almotriptan 12.5 mg, n = 591 |
Yes | Within 24 hours: (1) 113/582 (2) 90/591 |
Occurring in at least 1% of subjects: Chest pain: (1) 13/582; (2) 2/591 Headache: (1) 9/582; (2) 8/591 Vasodilation: (1) 8/582; (2) 6/591 Diarrhoea: (1) 3/582; (2) 6/591 Nausea: (1) 20/582; (2) 13/591 Dizziness: (1) 10/582; (2) 12/591 Paraesthesia: (1) 5/582; (2) 7/591 Somnolence: (1) 11/582; (2) 8/591 Palpitations: (1) 0/582; (2) 2/591 |
(1) 0/582 (2) 0/591 |
(1) 0/582 (2) 0/591 |
Other withdrawals: 8 withdrawn (4 from each group), no further details reported |
| Tfelt‐Hansen 1995 | (1) Sumatriptan 100 mg, n = 122 (2) Lysine acetylsalicylate 1620 mg + metoclopramide 10 mg, n = 137 (3) Placebo, n = 126 |
No | Within 24 hours: (1) 38/125 (2) 25/138 (3) 18/126 |
Nausea/vomiting: (1) 14/125; (2) 3/138; (3) 11/126 Somnolence: (1) 6/125; (2) 12/138; (3) 0/126 Fatigue/weakness: (1) 8/125; (2) 3/138; (3) 3/126 Abdominal pain: (1) 6/125; (2) 7/138; (3) 2/126 Constriction of throat/chest pain: (1) 6/125; (2) 0/138; (3) 0/126 Paraesthesia: (1) 6/125; (2) 0/138; (3) 0/126 Heaviness in lower limbs: (1) 5/125; (2) 0/138; (3) 0/126 Back or neck pain: (1) 4/125; (2) 0/138; (3) 0/126 Syncope: (1) 3/125; (2) 0/138; (3) 0/126 Vertigo/dizziness: (1) 3/125; (2) 1/138; (3) 1/126 |
1 (group not reported) | (1) 4/125 (2) 1/138 (3) 2/126 |
4 excluded from the efficacy analysis due to incomplete patient diary data |
| Tfelt‐Hansen 1998 | (1) Sumatriptan 100 mg, n = 388 (2) Rizatriptan 5 mg, n = 164 (3) Rizatriptan 10 mg, n = 387 (4) Placebo, n = 160 |
No | Within 24 hours: (1) 202/388 (2) 64/164 (3) 180/387 (4) 51/160 |
Reported in > 5% of participants: Somnolence: (1) 28/388; (2) 12/164; (3) 33/387; (4) 9/160 Dizziness: (1) 35/388; (2) 9/164; (3) 30/387; (4) 6/160 Asthenia/fatigue: (1) 32/388; (2) 4/164; (3) 30/387; (4) 6/160 Nausea: (1) 35/388; (2) 8/164; (3) 22/387; (4) 4/160 Vomiting: (1) 10/388; (2) 5/164; (3) 12/387; (4) 8/160 Abdominal pain: (1) 20/388; (2) 7/164; (3) 12/387; (4) 2/160 Chest pain: (1) 22/388; (2) 2/164; (3) 13/387; (4) 4/160 |
(1) 0/388 (2) 0/164 (3) 0/387 (4) 0/160 |
Drug‐related (1) 1/388 (2) 0/164 (3) 0/387 (4) 0/160 |
7 other discontinuations: 2 lost to follow‐up, 1 withdrew from study, 4 for protocol violations |
| Tfelt‐Hansen 2006 | (1) Sumatriptan 50 mg, n = 53 (2) Placebo, n = 48 |
Yes | Within 24 hours: (1) 27/53 (2) 7/48 |
No useable data ‐ no. of individual adverse events not reported separately by treatment arm | No data | No data | 2 withdrawals (one from each group) for protocol violations |
| Thomson 1992 | (1) Sumatriptan 100 mg, n = 175 (2) Aspirin 900 mg + metoclopramide 10 mg, n = 183 |
No | Within 24 hours: (1) 74/175 (2) 53/183 |
Incidence ≥ 2%: Nausea and/or vomiting: (1) 18/175; (2) 14/183 Malaise/fatigue: (1) 11/175; (2) 6/183 Dizziness/vertigo: (1) 9/175; (2) 4/183 Disturbance of taste: (1) 8/175; (2) 4/183 Sweating: (1) 7/175; (2) 1/183 Abdominal discomfort: (1) 5/175; (2) 3/183 Throat symptoms: (1) 6/175; (2) 2/183 Headache: (1) 6/175; (2) 1/183 Chest symptoms: (1) 4/175; (2) 1/183 Feeling of heaviness: (1) 4/175; (2) 0/183 Neck pain/stiffness: (1) 3/175; (2) 3/183 Paraesthesia: (1) 3/175; (2) 1/183 Diarrhoea: (1) 2/175; (2) 8/183 Tachycardia: (1) 1/175; (2) 3/183 Gastroesophageal reflux: (1) 1/175; (2) 3/183 Mouth/tongue disorder: (1) 1/175; (2) 4/183 Drowsiness/sedation: (1) 1/175; (2) 3/183 |
No data | Drug‐related (1) 5/175 (2) 0/183 |
3 excluded from efficacy analysis due to failure to return patient diary |
| Visser 1996 | (1) Sumatriptan 100 mg, n = 72 (2) Rizatriptan 10 mg, n = 89 (3) Rizatriptan 20 mg, n = 82 (4) Rizatriptan 40 mg, n = 121 (5) Placebo, n = 85 |
Yes | Within 24 hours: In participants taking only single dose (1) 15/33 (2) 23/48 (3) 31/46 (4) 66/80 (5) 5/14 |
Incidence ≥ 5% in any 1 treatment group after single dose only: Dizziness: (1) 1/33; (2) 4/48; (3) 14/46; (4) 29/80; (5) 1/14 Drowsiness: (1) 1/33; (2) 5/48; (3) 11/46; (4) 16/80; (5) 3/14 Asthenia/fatigue: (1) 1/33; (2) 2/48; (3) 6/46; (4) 14/80; (5) 0/14 Nausea: (1) 1/33; (2) 1/48; (3) 2/46; (4) 9/80; (5) 0/14 Paraesthesia: (1) 3/33; (2) 2/48; (3) 3/46; (4) 8/80; (5) 1/14 Dry mouth: (1) 1/33; (2) 1/48; (3) 5/46; (4) 7/80; (5) 0/14 Heaviness, regional: (1) 4/33; (2) 2/48; (3) 1/46; (4) 6/80; (5) 1/14 Chest pain: (1) 3/33; (2) 2/48; (3) 2/46; (4) 6/80; (5) 0/14 Mental acuity decreased: (1) 0/33; (2) 1/48; (3) 1/46; (4) 4/80; (5) 1/14 Abdominal pain: (1) 1/33; (2) 1/48; (3) 0/46; (4) 4/80; (5) 0/14 Stiffness: (1) 2/33; (2) 0/48; (3) 0/46; (4) 2/80; (5) 0/14 Headache: (1) 2/33; (2) 0/48; (3) 6/46; (4) 2/80; (5) 0/14 Neck pain: (1) 2/33; (2) 0/48; (3) 1/46; (4) 0/80; (5) 0/14 |
(1) 0/72 (2) 0/89 (3) 1/82 (after 2 doses) (4) 0/121 (5) 0/85 |
(1) 0/72 (2) 0/89 (3) 0/82 (4) 0/121 (5) 0/85 |
No other withdrawals after taking study medication |
| Winner 2003 | Study 1: (1) Sumatriptan 50 mg, n = 122 (2) Sumatriptan 100 mg, n = 115 (3) Placebo, n = 117 Study 2: (1) Sumatriptan 50 mg, n = 111 (2) Sumatriptan 100 mg, n = 107 (3) Placebo, n = 119 |
Yes | Within 24 hours: Study 1 (1) 22/122 (2) 22/115 (3) 9/117 Study 2 (1) 10/111 (2) 13/107 (3) 7/119 |
Occurring at a rate of 3% or greater: Study 1 Nausea: (1) 4/122; (2) 3/116; (3) 1/117 Dizziness: (1) 4/122; (2) 2/116; (3) 0/117 Somnolence: (1) 0/122; (2) 3/116; (3) 0/117 Paraesthesia: (1) 2/122; (2) 3/116; (3) 2/117 Other pressure/tightness: (1) 2/122; (2) 3/116; (3) 0/117 Study 2 Dizziness: (1) 0/111; (2) 3/107; (3) 1/120 |
Study 1 (1) 0/122 (2) 0/115 (3) 0/117 Study 2 (1) 0/111 (2) 0/107 (3) 0/119 |
No data | Total withdrawals (possibly including AE withdrawals): Study 1 (1) 16/138 (2) 23/138 (3) 24/141 Study 2 (1) 11/122 (2) 21/127 (3) 14/133 |
Appendix 8. Breakdown of individual adverse event groups
We used the following groupings of individual adverse events in all four reviews of sumatriptan whenever it was possible to combine studies for analysis (all routes of administration except rectal).
Malaise/fatigue/asthenia:
Malaise/fatigue
Fatigue
Malaise and fatigue
Asthenia/fatigue
Fatigue/weakness
Asthenia
Weakness
Dizziness/vertigo:
Dizziness/vertigo
Dizziness
Dizziness (excl. vertigo)
Dizziness (not vertigo)
Nausea/vomiting:
Nausea/vomiting
Nausea
Vomiting
Nausea and vomiting
Disorder of mouth/disturbance of taste:
Disorder of mouth/tongue
Mouth disorder
Dry mouth
Disturbance of taste
Bad taste
Drug taste
Chest pain/symptoms:
Chest pressure/heaviness
Chest tightness
Chest discomfort
Chest pain
Chest symptoms
Constriction of throat/chest pain
Tightness of throat
Heat sensations/flushing:
Warm/hot sensation
Flushing
Vasodilation
Heat flashes
Warm sensation
Temperature sensations
Hot flush
Burning sensation
Palpitations/tachycardia:
Palpitations
Tachycardia
Diarrhoea:
Diarrhoea
Feeling of tightness/heaviness:
Feeling of heaviness
Heaviness other than chest or neck
Feeling of heaviness in head
Heaviness/pressure sensation
Heaviness in lower limbs
Heaviness, regional
Head pressure
Tightness
Other pressure/tightness
Sweating:
Sweating
Abdominal pain/discomfort/dyspepsia:
Abdominal discomfort
Abdominal pain
Abdominal pain or cramps
Dyspepsia
Gastric symptoms
Gastroesophageal reflux
Paraesthesia/numbness:
Paraesthesia
Tingling
Numbness/paraesthesia/tingling
Numbness
Headache:
Headache
Drowsiness/somnolence:
Drowsiness/sedation
Somnolence
Sleepiness
Drowsiness
Anxiety:
Anxiety
Neck/back pain:
Neck pain/stiffness
Neck pain
Back or neck pain
Back pain
Disorder of nasal cavity/sinuses:
Disorder of nasal cavity/sinuses
Nasal discomfort
Nasal stuffiness
Wet nostrils
Throat symptoms
Throat symptoms
Throat discomfort
Injection‐site reaction:
Injection‐site reaction
Application site reaction
Appendix 9. Specific adverse events: sumatriptan versus active comparators
| Summary of results E: Number of participants experiencing specific adverse events within 24 hours of study treatment | ||||||
| Studies |
Participants treated |
Treatment (%) | Comparator (%) | Relative risk (95% CI) | NNH (95% CI) | |
| Malaise/fatigue/asthenia | ||||||
| Sumatriptan 25 mg versus rizatriptan 5 mg | 2 | 1169 | 4 | 5 | 0.81 (0.47 to 1.4) | Not calculated |
| Sumatriptan 25 mg versus rizatriptan 10 mg | 2 | 1186 | 4 | 3 | 1.1 (0.62 to 2.0) | Not calculated |
| Sumatriptan 50 mg versus rizatriptan 5 mg | 2 | 1160 | 6 | 5 | 1.3 (0.78 to 2.1) | Not calculated |
| Sumatriptan 50 mg verus rizatriptan 10 mg | 2 | 1177 | 6 | 3 | 1.8 (1.0 to 3.0) | Not calculated |
| Sumatriptan 100 mg versus eletriptan 40 mg | 2 | 609 | 4 | 5 | 0.76 (0.36 to 1.6) | Not calculated |
| Sumatriptan 100 mg versus eletriptan 80 mg | 2 | 603 | 4 | 10 | 0.39 (0.20 to 0.76) | ‐17 (‐10 to ‐53) |
| Sumatriptan 100 mg versus ASA 900 mg + MCP 10 mg | 2 | 621 | 6 | 3 | 2.3 (1.0 to 4.9) | Not calculated |
| Sumatriptan 100 mg versus rizatriptan 10 mg | 2 | 856 | 8 | 7 | 1.1 (0.66 to 1.7) | Not calculated |
| Dizziness/vertigo | ||||||
| Sumatriptan 25 mg versus rizatriptan 5 mg | 2 | 1169 | 5 | 8 | 0.64 (0.41 to 1.0) | Not calculated |
| Sumatriptan 25 mg versus rizatriptan 10 mg | 2 | 1186 | 5 | 10 | 0.50 (0.33 to 0.77) | ‐20 (‐13 to ‐51) |
| Sumatriptan 50 mg versus rizatriptan 5 mg | 2 | 1160 | 10 | 8 | 1.3 (0.86 to 1.8) | Not calculated |
| Sumatriptan 50 mg versus rizatriptan 10 mg | 2 | 1177 | 10 | 10 | 0.98 (0.69 to 1.4) | Not calculated |
| Sumatriptan 100 mg versus eletriptan 40 mg | 2 | 609 | 3 | 5 | 0.65 (0.30 to 1.4) | Not calculated |
| Sumatriptan 100 mg versus eletriptan 80 mg | 2 | 603 | 3 | 7 | 0.48 (0.23 to 1.0) | Not calculated |
| Sumatriptan 100 mg versus ASA 900 mg + MCP 10 mg | 2 | 621 | 4 | 2 | 2.5 (0.91 to 7.1) | Not calculated |
| Sumatriptan 100 mg versus rizatriptan 10 mg | 2 | 856 | 9 | 8 | 1.1 (0.69 to 1.7) | Not calculated |
| Nausea/vomiting | ||||||
| Sumatriptan 25 mg versus rizatriptan 5 mg | 2 | 1169 | 5 | 4 | 1.1 (0.63 to 1.8) | Not calculated |
| Sumatriptan 25 mg versus rizatriptan 10 mg | 2 | 1186 | 5 | 5 | 0.86 (0.52 to 1.4) | Not calculated |
| Sumatriptan 50 mg versus rizatriptan 5 mg | 2 | 1160 | 6 | 4 | 1.5 (0.88 to 2.4) | Not calculated |
| Sumatriptan 50 mg versus rizatriptan 10 mg | 2 | 1177 | 6 | 5 | 1.2 (0.73 to 1.9) | Not calculated |
| Sumatriptan 100 mg versus eletriptan 40 mg | 2 | 609 | 4 | 2 | 1.8 (0.71 to 4.5) | Not calculated |
| Sumatriptan 100 mg versus eletriptan 80 mg | 2 | 603 | 4 | 8 | 0.49 (0.25 to 0.95) | ‐24 (‐13 to ‐270) |
| Sumatriptan 100 mg versus ASA 900 mg + MCP 10 mg | 2 | 621 | 11 | 5 | 2.0 (1.1 to 3.5) | 19 (10 to 91) |
| Sumatriptan 100 mg versus rizatriptan 10 mg | 2 | 856 | 9 | 5 | 1.6 (0.95 to 2.6) | Not calculated |
| Mouth disorder/disturbance of taste | ||||||
| Sumatriptan 25 mg versus rizatriptan 5 mg | 2 | 1169 | 5 | 5 | 0.87 (0.53 to 1.4) | Not calculated |
| Sumatriptan 25 mg versus rizatriptan 10 mg | 2 | 1186 | 5 | 6 | 0.87 (0.53 to 1.4) | Not calculated |
| Sumatriptan 50 mg versus rizatriptan 5 mg | 2 | 1160 | 6 | 5 | 1.0 (0.65 to 1.7) | Not calculated |
| Sumatriptan 50 mg versus rizatriptan 10 mg | 2 | 1177 | 6 | 6 | 1.0 (0.65 to 1.7) | Not calculated |
| Chest pain/symptoms | ||||||
| Sumatriptan 25 mg versus rizatriptan 5 mg | 2 | 1169 | 3 | 2 | 1.3 (0.64 to 2.5) | Not calculated |
| Sumatriptan 25 mg versus rizatriptan 10 mg | 2 | 1186 | 3 | 3 | 1.2 (0.59 to 2.2) | Not calculated |
| Sumatriptan 50 mg versus rizatriptan 5 mg | 2 | 1170 | 4 | 2 | 1.6 (0.83 to 3.1) | Not calculated |
| Sumatriptan 50 mg versus rizatriptan 10 mg | 2 | 1177 | 4 | 3 | 1.4 (0.75 to 2.7) | Not calculated |
| Sumatriptan 100 mg versus ASA 900 mg + MCP 10 mg | 2 | 621 | 3 | 0 | 7.5 (1.4 to 41) | 33 (19 to 120) |
| Sumatriptan 100 mg versus rizatriptan 10 mg | 2 | 856 | 6 | 3 | 1.7 (0.93 to 3.3) | Not calculated |
| Feeling of heaviness/tightness | ||||||
| Sumatriptan 100 mg versus ASA 900 mg + MCP 10 mg | 2 | 621 | 3 | 0 | 11 (1.4 to 83) | 33 (19 to 110) |
| Paraesthesia/numbness | ||||||
| Sumatriptan 25 mg versus rizatriptan 5 mg | 2 | 1169 | 4 | 3 | 1.5 (0.76 to 2.8) | Not calculated |
| Sumatriptan 25 mg versus rizatriptan 10 mg | 2 | 1186 | 4 | 5 | 0.80 (0.46 to 1.4) | Not calculated |
| Sumatriptan 50 mg versus rizatriptan 5 mg | 2 | 1160 | 3 | 3 | 1.3 (0.65 to 2.5) | Not calculated |
| Sumatriptan 50 mg versus rizatriptan 10 mg | 2 | 1177 | 3 | 5 | 0.70 (0.40 to 1.3) | Not calculated |
| Sumatriptan 100 mg versus ASA 900 mg + MCP 10 mg | 2 | 621 | 3 | 0 | 6.8 (1.2 to 38) | 37 (21 to 170) |
| Headache | ||||||
| Sumatriptan 25 mg versus rizatriptan 5 mg | 2 | 1169 | 4 | 2 | 1.5 (0.76 to 2.9) | Not calculated |
| Sumatriptan 25 mg versus rizatriptan 10 mg | 2 | 1186 | 4 | 2 | 1.8 (0.89 to 3.6) | Not calculated |
| Sumatriptan 50 mg versus rizatriptan 5 mg | 2 | 1160 | 5 | 2 | 2.1 (1.1 to 3.9) | 38 (21 to 230) |
| Sumatriptan 50 mg versus rizatriptan 10 mg | 2 | 1183 | 5 | 2 | 2.5 (1.3 to 4.8) | 34 (20 to 114) |
| Drowsiness/somnolence | ||||||
| Sumatriptan 25 mg versus rizatriptan 5 mg | 2 | 1169 | 4 | 5 | 0.89 (0.53 to 1.5) | Not calculated |
| Sumatriptan 25 mg versus rizatriptan 10 mg | 2 | 1186 | 4 | 7 | 0.60 (0.38 to 0.97) | ‐34 (‐18 to ‐410) |
| Sumatriptan 50 mg versus rizatriptan 5 mg | 2 | 1160 | 6 | 5 | 1.2 (0.75 to 2.0) | Not calculated |
| Sumatriptan 50 mg versus rizatriptan 10 mg | 2 | 1177 | 6 | 7 | 0.82 (0.54 to 1.3) | Not calculated |
| Sumatriptan 100 mg versus eletriptan 40 mg | 2 | 609 | 3 | 4 | 0.72 (0.31 to 1.7) | Not calculated |
| Sumatriptan 100 mg versus eletriptan 80 mg | 2 | 603 | 3 | 4 | 0.78 (0.34 to 1.8) | Not calculated |
| Sumatriptan 100 mg versus ASA 900 mg + MCP 10 mg | 2 | 621 | 2 | 5 | 0.51 (0.21 to 1.2) | Not calculated |
| Sumatriptan 100 mg versus rizatriptan 10 mg | 2 | 856 | 7 | 9 | 0.79 (0.49 to 1.3) | Not calculated |
| Abdominal pain/discomfort/dyspepsia | ||||||
| Sumatriptan 100 mg versus ASA 900 mg + MCP 10 mg | 2 | 621 | 4 | 3 | 1.2 (0.51 to 2.8) | Not calculated |
| Sumatriptan 100 mg versus rizatriptan 10 mg | 2 | 856 | 5 | 3 | 1.7 (0.84 to 3.3) | Not calculated |
| Neck/back pain | ||||||
| Sumatriptan 100 mg versus ASA 900 mg + MCP 10 mg | 2 | 621 | 2 | 1 | 2.3 (0.65 to 8.0) | Not calculated |
Appendix 10. L'Abbé plots for sumatriptan 50 mg versus placebo
L'Abbé plots for sumatriptan 50 mg versus placebo for the outcomes headache relief at two hours (Figure 125), pain‐free at two hours (Figure 126), and sustained pain‐free at 24 hours (Figure 127) show consistency in response across studies for these outcomes.
8.
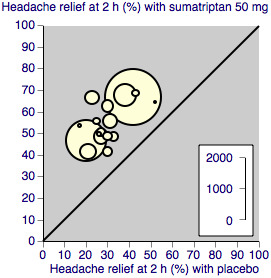
L'Abbé plot showing results for sumatriptan 50 mg versus placebo for headache relief at 2 hours. Each circle represents a different study; size of circle is proportional to size of study; diagonal is line of equivalence
9.
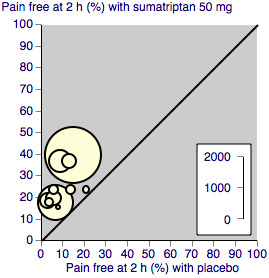
L'Abbé plot showing results for sumatriptan 50 mg versus placebo for pain‐free at 2 hours. Each circle represents a different study; size of circle is proportional to size of study; diagonal is line of equivalence
10.
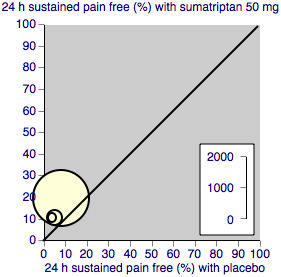
L'Abbé plot showing results for sumatriptan 50 mg versus placebo for sustained pain‐free during 24 hours. Each circle represents a different study; size of circle is proportional to size of study; diagonal is line of equivalence
Data and analyses
Comparison 1. Oral sumatriptan 25 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain‐free at 2 h | 3 | 1108 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.66 [1.79, 3.96] |
| 2 Headache relief at 1 h | 3 | 745 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.17, 2.29] |
| 3 Headache relief at 2 h | 5 | 1580 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.43, 1.92] |
| 4 Use of rescue medication | 2 | 1282 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.48, 0.68] |
| 4.1 Up to 4 h after initial dosing | 2 | 1282 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.48, 0.68] |
| 5 Relief of associated symptoms | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Relief of nausea at 2 h | 4 | 550 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [1.18, 1.87] |
| 5.2 Relief of photophobia at 2 h | 3 | 411 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.82 [1.32, 2.53] |
| 6 Relief of functional disability at 2 h | 3 | 381 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.07, 1.77] |
| 7 Any adverse event within 24 h | 4 | 1550 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [1.00, 1.30] |
| 8 Individual adverse events | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Malaise/fatigue/asthenia | 3 | 1419 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.61 [1.17, 5.82] |
| 8.2 Dizziness/vertigo | 4 | 1550 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.61, 1.59] |
| 8.3 Nausea/vomiting | 4 | 1550 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.74, 1.75] |
| 8.4 Mouth disorder/disturbance of taste | 3 | 1148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.49, 1.36] |
| 8.5 Chest pain/symptoms | 3 | 1419 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.71, 4.33] |
| 8.6 Paraesthesia/numbness | 3 | 1148 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.40 [1.37, 8.43] |
| 8.7 Headache | 3 | 1148 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.67, 1.91] |
| 8.8 Drowsiness/somnolence | 3 | 1148 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.53, 1.55] |
| 9 Any adverse event withdrawal | 2 | 841 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.20, 7.26] |
| 10 Headache relief at 2 h ‐ effect of quality score | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 All studies | 5 | 1580 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.66 [1.43, 1.92] |
| 10.2 Only quality score ≥ 3 | 4 | 1449 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [1.40, 1.90] |
1.9. Analysis.
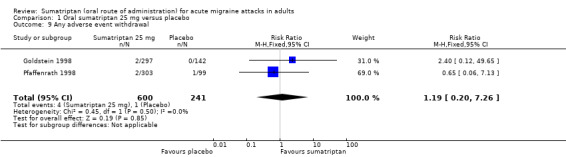
Comparison 1 Oral sumatriptan 25 mg versus placebo, Outcome 9 Any adverse event withdrawal.
Comparison 2. Oral sumatriptan 25 mg versus rizatriptan 5 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain free at 2 h | 2 | 2210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.74, 0.95] |
| 2 Headache relief at 1 h | 2 | 2210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.81, 1.02] |
| 3 Headache relief at 2 h | 2 | 2210 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.84, 0.95] |
| 4 Use of rescue medication | 2 | 1698 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.82, 1.14] |
| 4.1 Up to 4 h after initial dosing | 2 | 1698 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.82, 1.14] |
| 5 Any adverse event within 24 h | 2 | 1169 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.19] |
| 6 Individual adverse events | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Paraesthesia | 2 | 1169 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [0.76, 2.77] |
| 6.2 Chest symptoms | 2 | 1169 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.64, 2.53] |
| 6.3 Dizziness/vertigo | 2 | 1169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.41, 1.00] |
| 6.4 Somnolence | 2 | 1169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.53, 1.49] |
| 6.5 Nausea/vomiting | 2 | 1169 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.63, 1.82] |
| 6.6 Dry mouth/mouth disorder | 2 | 1169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.53, 1.42] |
| 6.7 Malaise/fatigue/asthenia | 2 | 1169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.47, 1.40] |
2.6. Analysis.
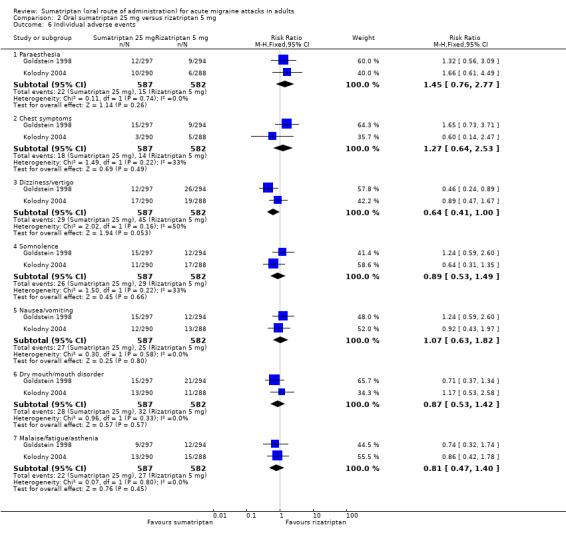
Comparison 2 Oral sumatriptan 25 mg versus rizatriptan 5 mg, Outcome 6 Individual adverse events.
Comparison 3. Oral sumatriptan 25 mg versus rizatriptan 10 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain‐free at 2 h | 2 | 2231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.62, 0.79] |
| 2 Headache relief at 1 h | 2 | 2231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.74, 0.91] |
| 3 Headache relief at 2 h | 2 | 2231 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.80, 0.91] |
| 4 Use of rescue medication | 2 | 1716 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.00, 1.43] |
| 4.1 Up to 4 h after initial dosing | 2 | 1716 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.00, 1.43] |
| 5 Any adverse event within 24 h | 2 | 1186 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.81, 1.05] |
| 6 Individual adverse events | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Paraesthesia | 2 | 1186 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.46, 1.39] |
| 6.2 Chest symptoms | 2 | 1186 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.59, 2.23] |
| 6.3 Dizziness/vertigo | 2 | 1186 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.33, 0.77] |
| 6.4 Somnolence | 2 | 1186 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.38, 0.97] |
| 6.5 Nausea/vomiting | 2 | 1186 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.52, 1.42] |
| 6.6 Dry mouth/mouth disorder | 2 | 1186 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.53, 1.41] |
| 6.7 Malaise/fatigue/asthenia | 2 | 1186 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.62, 2.03] |
3.6. Analysis.
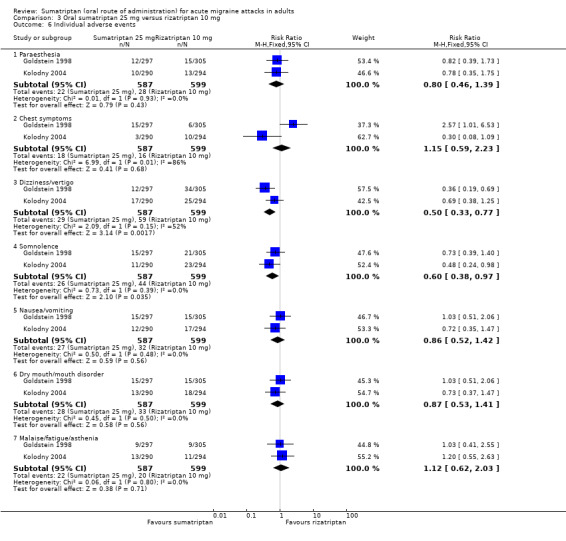
Comparison 3 Oral sumatriptan 25 mg versus rizatriptan 10 mg, Outcome 6 Individual adverse events.
Comparison 4. Oral sumatriptan 50 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain‐free at 2 h | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Moderate or severe baseline pain intensity | 12 | 6447 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.70 [2.38, 3.06] |
| 1.2 Mild baseline pain intensity | 6 | 1514 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.03 [1.74, 2.37] |
| 2 Pain free at 1 h | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Moderate or severe baseline pain intensity | 5 | 1735 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.64 [1.49, 4.67] |
| 2.2 Mild baseline pain intensity | 4 | 1246 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.48, 2.37] |
| 3 Headache relief at 1 h | 9 | 2766 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.52, 2.13] |
| 4 Headache relief at 2 h | 18 | 8102 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.70, 1.91] |
| 5 24 h sustained pain‐free | 7 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Moderate or severe baseline pain intensity | 3 | 2526 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.63 [2.07, 3.35] |
| 5.2 Mild baseline pain intensity | 4 | 866 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.81 [2.05, 3.86] |
| 6 24 h sustained headache relief | 3 | 2526 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.91 [1.66, 2.20] |
| 7 Use of rescue medication | 11 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Up to 24 h after initial dosing in participants with moderate or severe baseline pain | 4 | 2079 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.68, 0.87] |
| 7.2 Up to 24 h after initial dosing in participants with mild baseline pain | 2 | 384 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.42, 0.68] |
| 7.3 Up to 4 h after initial dosing | 5 | 2098 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.50, 0.64] |
| 8 Relief of associated symptoms in participants with moderate or severe baseline pain intensity | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Relief of nausea at 2 h | 7 | 973 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [1.16, 1.65] |
| 8.2 Relief of photophobia at 2 h | 6 | 1144 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [1.22, 1.65] |
| 8.3 Relief of phonophobia at 2 h | 4 | 852 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [1.16, 1.60] |
| 8.4 Relief of photophobia or phonophobia at 2 h | 2 | 440 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [1.55, 2.75] |
| 9 Relief of associated symptoms in participants with mild baseline pain intensity | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Relief of nausea at 2 h | 2 | 280 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.88 [3.78, 12.51] |
| 9.2 Relief of photophobia at 2 h | 2 | 483 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.95 [2.20, 3.97] |
| 9.3 Relief of phonophobia at 2 h | 2 | 413 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.99 [2.15, 4.16] |
| 10 Relief of functional disability at 2 h | 4 | 607 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.45 [1.17, 1.79] |
| 11 Any adverse event within 24 h | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Moderate or severe baseline pain intensity | 10 | 3728 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [1.17, 1.44] |
| 11.2 Mild baseline pain intensity | 5 | 1242 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.26 [1.62, 3.16] |
| 12 Individual adverse events | 13 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Malaise/fatigue/asthenia | 10 | 3689 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.68 [1.59, 4.50] |
| 12.2 Dizziness/vertigo | 12 | 4211 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [1.28, 2.47] |
| 12.3 Nausea/vomiting | 12 | 3799 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.97, 1.96] |
| 12.4 Mouth disorder/disturbance of taste | 5 | 1887 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.38 [0.90, 2.13] |
| 12.5 Chest pain/symptoms | 7 | 2673 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.11 [1.14, 3.92] |
| 12.6 Heat sensations/flushing | 4 | 1515 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.83 [1.53, 9.59] |
| 12.7 Diarrhoea | 3 | 1327 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.51 [1.19, 5.30] |
| 12.8 Feeling of heaviness/tightness | 4 | 1179 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.88, 10.43] |
| 12.9 Paraesthesia/numbness | 9 | 3098 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.65 [1.41, 5.00] |
| 12.10 Headache | 5 | 1904 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.30 [0.81, 2.06] |
| 12.11 Drowsiness/somnolence | 8 | 2628 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.87, 2.09] |
| 12.12 Anxiety | 2 | 518 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.36, 9.94] |
| 12.13 Neck/back pain | 2 | 364 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.09, 2.68] |
| 13 Any adverse event withdrawal | 4 | 1553 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.18, 7.08] |
| 14 Pain free at 2 h ‐ effect of quality score | 12 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 Quality score ≥ 3 | 10 | 5835 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.64 [2.32, 2.99] |
| 14.2 Quality score = 2 | 2 | 612 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.29 [2.19, 8.43] |
| 15 Headache relief at 1 h ‐ effect of quality score | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 All studies | 9 | 2766 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.52, 2.13] |
| 15.2 Only quality score ≥ 3 | 8 | 2281 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.92 [1.59, 2.31] |
| 16 Headache relief at 2 h ‐ effect of quality score | 18 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 Quality score ≥ 3 | 14 | 6842 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [1.65, 1.86] |
| 16.2 Quality score = 2 | 4 | 1260 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.31 [1.87, 2.84] |
4.13. Analysis.
Comparison 4 Oral sumatriptan 50 mg versus placebo, Outcome 13 Any adverse event withdrawal.
Comparison 5. Oral sumatriptan 50 mg versus effervescent ASA 1000 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain‐free at 2 h | 2 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.97, 1.53] |
| 2 Pain‐free at 1 h | 2 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.53, 1.78] |
| 3 Headache relief at 1 h | 2 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.61, 0.98] |
| 4 Headache relief at 2 h | 2 | 726 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [1.09, 1.47] |
| 5 Any adverse event within 24 h | 2 | 730 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.85, 1.64] |
Comparison 6. Oral sumatriptan 50 mg versus zolmitriptan 2.5 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Headache relief at 1 h | 2 | 1609 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.90, 1.14] |
| 2 Headache relief at 2 h | 2 | 1609 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.09] |
| 3 Any adverse event within 24 h | 2 | 1771 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.88, 1.15] |
Comparison 7. Oral sumatriptan 50 mg versus zolmitriptan 5 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Headache relief at 1 h | 2 | 1633 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.92, 1.17] |
| 2 Headache relief at 2 h | 2 | 1633 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.95, 1.09] |
| 3 Any adverse event within 24 h | 2 | 1790 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.80, 1.03] |
Comparison 8. Oral sumatriptan 50 mg versus rizatriptan 5 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain‐free at 2 h | 2 | 2209 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.95, 1.19] |
| 2 Headache relief at 1 h | 2 | 2209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.89, 1.11] |
| 3 Headache relief at 2 h | 3 | 2911 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.93, 1.03] |
| 4 Use of rescue medication | 2 | 1696 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.65, 0.93] |
| 4.1 Up to 4 h after initial dosing | 2 | 1696 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.65, 0.93] |
| 5 Any adverse event within 24 h | 2 | 1160 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.03, 1.33] |
Comparison 9. Oral sumatriptan 50 mg versus rizatriptan 10 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain‐free at 2 h | 2 | 2230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.80, 1.00] |
| 2 Headache relief at 1 h | 2 | 2230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.81, 0.99] |
| 3 Headache relief at 2 h | 2 | 2227 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.86, 0.97] |
| 4 Use of rescue medication | 2 | 1714 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.80, 1.17] |
| 4.1 Up to 4 h after initial dosing | 2 | 1714 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.80, 1.17] |
| 5 Any adverse event within 24 h | 2 | 1177 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.92, 1.17] |
Comparison 10. Oral sumatriptan 50 mg versus eletriptan 40 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain‐free at 2 h | 2 | 721 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.55, 0.98] |
| 2 Headache relief at 1 h | 2 | 721 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.77, 1.28] |
| 3 Headache relief at 2 h | 2 | 721 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.75, 0.97] |
| 4 Relief of associated symptoms | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Relief of nausea at 2 h | 2 | 374 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.60, 0.95] |
| 4.2 Relief of photophobia at 2 h | 2 | 528 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.69, 1.00] |
| 4.3 Relief of phonophobia at 2 h | 2 | 517 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.73, 1.04] |
| 5 Relief of functional disability at 2 h | 2 | 590 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.72, 0.96] |
Comparison 11. Oral sumatriptan 50 mg versus eletriptan 80 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain‐free at 2 h | 2 | 706 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.44, 0.76] |
| 2 Headache relief at 1 h | 2 | 706 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.57, 0.91] |
| 3 Headache relief at 2 h | 2 | 706 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.69, 0.89] |
| 4 Relief of associated symptoms | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Relief of nausea at 2 h | 2 | 370 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.66, 1.08] |
| 4.2 Relief of photophobia at 2 h | 2 | 508 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.60, 0.86] |
| 5 Relief of functional disability at 2 h | 2 | 570 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.73, 0.97] |
Comparison 12. Oral sumatriptan 100 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain‐free at 2 h | 19 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Moderate or severe baseline pain intensity | 15 | 6571 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.20 [2.84, 3.62] |
| 1.2 Mild baseline pain intensity | 4 | 1240 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.41 [2.06, 2.81] |
| 2 Pain‐free at 1 h | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Moderate or severe baseline pain intensity | 6 | 3176 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.97 [2.33, 6.77] |
| 2.2 Mild baseline pain intensity | 4 | 1240 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.21 [1.76, 2.78] |
| 3 Headache relief at 1 h | 10 | 3983 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [1.62, 2.18] |
| 4 Headache relief at 2 h | 20 | 7811 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.93 [1.82, 2.04] |
| 5 24 h sustained pain free | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Moderate or severe baseline pain intensity | 5 | 2891 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.81 [2.30, 3.44] |
| 5.2 Mild baseline pain intensity | 3 | 771 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.24 [2.33, 4.51] |
| 6 24 h sustained headache relief | 5 | 4116 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.12 [1.87, 2.39] |
| 7 Use of rescue medication | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Up to 24 h after initial dosing | 6 | 2810 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.52, 0.62] |
| 7.2 Up to 4 h after initial dosing | 3 | 1027 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.47, 0.65] |
| 8 Relief of associated symptoms in participants with moderate or severe baseline pain intensity | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Relief of nausea at 2 h | 14 | 2996 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.37, 1.69] |
| 8.2 Relief of photophobia at 2 h | 9 | 2494 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [1.63, 2.11] |
| 8.3 Relief of phonophobia at 2 h | 7 | 2118 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.83 [1.59, 2.11] |
| 8.4 Relief of photophobia or phonophobia at 2 h | 5 | 1073 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.02 [1.66, 2.46] |
| 9 Relief of associated symptoms in participants with mild baseline pain intensity | 2 | 1140 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.73 [3.04, 4.57] |
| 9.1 Relief of nausea at 2 h | 2 | 265 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.89 [3.18, 10.91] |
| 9.2 Relief of photophobia at 2 h | 2 | 475 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.23 [2.41, 4.33] |
| 9.3 Relief of phonophobia at 2 h | 2 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.70 [2.69, 5.08] |
| 10 Relief of functional disability at 2 h | 6 | 1827 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [1.65, 2.11] |
| 11 Any adverse event within 24 h | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 Moderate or severe baseline pain intensity | 12 | 3257 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.50, 1.91] |
| 11.2 Mild baseline pain intensity | 3 | 941 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.75 [1.87, 4.05] |
| 12 Individual adverse events | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 12.1 Malaise/fatigue/asthenia | 16 | 4844 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.36 [1.64, 3.38] |
| 12.2 Dizziness/vertigo | 16 | 4959 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.34 [1.60, 3.42] |
| 12.3 Nausea/vomiting | 19 | 5284 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [1.32, 2.22] |
| 12.4 Mouth disorder/disturbance of taste | 5 | 1047 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.78, 2.39] |
| 12.5 Chest pain/symptoms | 12 | 3452 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.04 [1.71, 5.40] |
| 12.6 Heat sensations/flushing | 2 | 786 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.55 [0.68, 18.61] |
| 12.7 Palpitations/tachycardia | 2 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.53 [0.75, 16.66] |
| 12.8 Feeling of heaviness/tightness | 5 | 1317 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.14 [1.34, 12.77] |
| 12.9 Paraesthesia/numbness | 12 | 3154 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.97 [2.16, 7.29] |
| 12.10 Headache | 3 | 411 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.41, 1.45] |
| 12.11 Drowsiness/somnolence | 13 | 3710 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [1.01, 2.29] |
| 12.12 Abdominal pain/discomfort/dyspepsia | 5 | 1357 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.80 [1.35, 5.80] |
| 12.13 Anxiety | 2 | 499 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [0.36, 9.92] |
| 12.14 Neck/back pain | 6 | 1508 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.87 [0.76, 4.58] |
| 13 Any adverse event withdrawal | 9 | 2744 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.77, 3.47] |
| 14 Headache relief at 2 h ‐ effect of formulation | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 14.1 Standard tablet | 18 | 5774 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [1.86, 2.16] |
| 14.2 Dispersible/rapid‐release tablet | 2 | 2037 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.80 [1.65, 1.96] |
| 15 Pain‐free at 2 h ‐ effect of quality score | 15 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 15.1 Quality score ≥ 3 | 12 | 5939 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.26 [2.87, 3.71] |
| 15.2 Quality score = 2 | 3 | 632 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.68 [1.80, 4.00] |
| 16 Headache relief at 2 h ‐ effect of quality score | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 Quality score ≥ 3 | 16 | 6771 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.85 [1.74, 1.97] |
| 16.2 Quality score = 2 | 4 | 1040 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.57 [2.14, 3.09] |
12.13. Analysis.
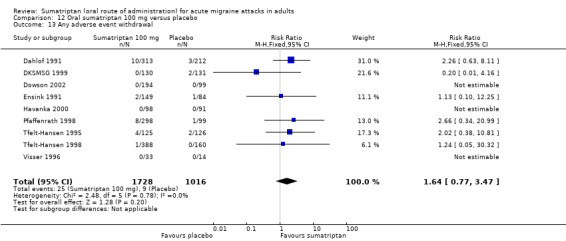
Comparison 12 Oral sumatriptan 100 mg versus placebo, Outcome 13 Any adverse event withdrawal.
Comparison 13. Oral sumatriptan 100 mg versus eletriptan 40 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain‐free at 2 h | 3 | 2263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.65, 0.85] |
| 2 Pain‐free at 1 h | 3 | 2263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.57, 1.10] |
| 3 Headache relief at 1 h | 3 | 2263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.67, 0.88] |
| 4 Headache relief at 2 h | 3 | 2263 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.82, 0.95] |
| 5 24 h sustained headache relief | 2 | 1998 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.70, 0.88] |
| 6 Use of rescue medication | 2 | 1918 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.10, 1.51] |
| 6.1 Up to 24 h after initial dosing | 2 | 1918 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [1.10, 1.51] |
| 7 Relief of associated symptoms | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 Relief of nausea at 2 h | 3 | 1478 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.79, 0.96] |
| 7.2 Relief of photophobia at 2 h | 3 | 1692 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.78, 0.93] |
| 7.3 Relief of phonophobia at 2 h | 2 | 1361 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.76, 0.92] |
| 8 Relief of functional disability at 2 h | 3 | 1880 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.81, 0.92] |
Comparison 14. Oral sumatriptan 100 mg versus eletriptan 80 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain‐free at 2 h | 2 | 604 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.41, 0.72] |
| 2 Pain‐free at 1 h | 2 | 604 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.29, 0.82] |
| 3 Headache relief at 1 h | 2 | 604 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.50, 0.84] |
| 4 Headache relief at 2 h | 2 | 604 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.68, 0.89] |
| 5 Relief of associated symptoms | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Relief of nausea at 2 h | 2 | 408 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.69, 0.99] |
| 5.2 Relief of photophobia at 2 h | 2 | 457 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.64, 0.89] |
| 6 Relief of functional disability at 2 h | 2 | 516 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.67, 0.90] |
Comparison 15. Oral sumatriptan 100 mg versus rizatriptan 10 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain‐free at 2 h | 2 | 936 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.69, 0.98] |
| 2 Headache relief at 1 h | 2 | 936 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.62, 0.92] |
| 3 Any adverse event within 24 h | 2 | 856 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.96, 1.27] |
Comparison 16. Oral sumatriptan 100 mg versus almotriptan 12.5 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain‐free at 2 h | 2 | 754 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [0.97, 1.49] |
| 2 24 h sustained pain‐free | 2 | 754 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.77, 1.19] |
Comparison 17. Oral sumatriptan 100 mg versus paracetamol 1000 mg + metoclopramide 10 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Headache relief at 2 hours | 2 | 1035 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.91, 1.20] |
| 2 Relief of associated symptoms | 2 | 1001 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.83, 1.18] |
| 2.1 Relief of photophobia or phonophobia | 2 | 1001 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.83, 1.18] |
| 3 Use of rescue medication | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Up to 24 h after initial dosing | 2 | 1243 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.74, 0.99] |
| 4 Any adverse event within 24 h | 2 | 1328 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.64 [1.42, 1.89] |
17.4. Analysis.
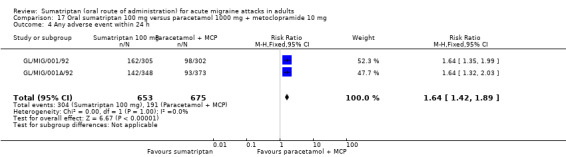
Comparison 17 Oral sumatriptan 100 mg versus paracetamol 1000 mg + metoclopramide 10 mg, Outcome 4 Any adverse event within 24 h.
Comparison 18. Oral sumatriptan 100 mg versus acetylsalicylic acid 900 mg + metoclopramide 10 mg.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain‐free at 2 hours | 2 | 575 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.62 [1.17, 2.25] |
| 2 Headache relief at 2 hours | 2 | 575 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.92, 1.29] |
| 3 Relief of associated symptoms | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Relief of nausea at 2 hours | 2 | 410 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.69, 1.20] |
| 4 Any adverse event within 24 hours | 2 | 621 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.20, 1.94] |
Comparison 19. Oral sumatriptan 200 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Headache relief at 2 h | 3 | 749 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.84 [2.33, 3.46] |
| 2 Any adverse event withdrawal | 2 | 583 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.83 [1.46, 10.06] |
| 3 Individual adverse events | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Nausea/vomiting | 3 | 681 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.22 [1.90, 5.43] |
| 3.2 Mouth disorder/disturbance of taste | 2 | 605 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.01 [1.34, 6.78] |
| 3.3 Chest pain/symptoms | 2 | 605 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.41 [0.54, 36.34] |
| 3.4 Paraesthesia/numbness | 2 | 605 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.09 [0.92, 28.03] |
| 3.5 Drowsiness/somnolence | 2 | 605 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.18 [1.43, 26.68] |
19.2. Analysis.
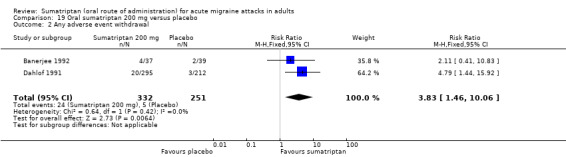
Comparison 19 Oral sumatriptan 200 mg versus placebo, Outcome 2 Any adverse event withdrawal.
Comparison 20. Oral sumatriptan 300 mg versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Headache relief at 2 h | 2 | 709 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.72 [2.19, 3.36] |
| 2 Individual adverse events | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Nausea/vomiting | 2 | 624 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.90 [1.62, 5.20] |
| 2.2 Mouth disorder/disturbance of taste | 2 | 624 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.51 [2.56, 11.88] |
| 2.3 Chest pain/symptoms | 2 | 624 | Risk Ratio (M‐H, Fixed, 95% CI) | 17.32 [2.36, 126.94] |
| 2.4 Paraesthesia/numbness | 2 | 624 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.86 [1.89, 51.37] |
| 2.5 Drowsiness/somnolence | 2 | 624 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.89 [1.62, 29.26] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
160‐104.
| Methods | Multicentre, randomised, double‐blind, double‐dummy, placebo‐controlled, parallel‐group. Single dose to treat each of up to 3 separate attacks. Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0.5, 1, 2, and 4 hours after dosing Second dose (either same as first dose of study medication or a double‐blind placebo) available after 2 hours for inadequate response, or for recurrence of headache within 24 hours of initial dosing Alternative rescue medication available 2 hours after second dose if appropriate |
|
| Participants | Aged 18 years or over and suffering at least 1 acute attack of migraine, with or without aura (IHS 1988), every 6 weeks Participants excluded if ever taken sumatriptan before (any formulation) or oral eletriptan No prescription analgesic or antiemetic within 6 hours prior to study treatment. No sumatriptan, ergotamine, or ergotamine‐like agent within previous 48 hours. N = 818 (treated first attack) M 150, F 668 (82%) Mean age 35 years Without aura 86% |
|
| Interventions | Numbers of participants treating first attack Sumatriptan 25 mg, n = 180 Sumatriptan 50 mg, n = 181 Eletriptan 40 mg, n = 184 Eletriptan 80 mg, n = 180 Placebo, n = 93 |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 2 h) Relief of nausea, photophobia, and phonophobia at 2 hours Relief of functional disability at 2 hours Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5. Pharmaceutical industry support: Pfizer |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated pseudo‐random code using the method of random permuted blocks |
| Allocation concealment (selection bias) | Low risk | Next consecutive number corresponding to study drug in blister card |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
Banerjee 1992.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat each of up to 3 separate attacks. Medication administered as soon as migraine attack with aura recognised Assessments at 2 and 6 h after dosing Rescue medication available after 2 h for inadequate symptom relief At least a 48‐h interval between treated attacks |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with aura. At least 1‐year history of migraine (untreated severity ≥ moderate) with an average of 1 to 6 attacks per month. Migraine prophylaxis discontinued at least 2 weeks prior to entering the study N = 94 (71 for efficacy) M 14, F 80 (85%) Mean age 35 years Proportion with/without aura not reported |
|
| Interventions | Sumatriptan (dispersible) 200 mg, n = 37 (34 for efficacy) Placebo, n = 39 (37 for efficacy) |
|
| Outcomes | Headache relief (at 2 h) Pain‐free (at 2 h) Persistence of nausea, vomiting, and photophobia at 2 h Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3. Pharmaceutical industry support: Glaxo Group Research Ltd. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | High risk | Treatment groups < 50 participants |
Brandes 2007.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered when migraine headache pain was of moderate or severe intensity Assessment at 0.5, 1, 1.5, and 2 h and then hourly from 2 to 24 h after dosing Rescue medication available after 2 h, excepting ergot‐containing medications, serotonin agonists, or NSAID‐containing products 2 replicate studies: Study 1 and 2 |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (2004) with or without aura. At least 6‐month history of migraine (untreated severity ≥ moderate) with an average of 2 to 6 attacks per month. Participants excluded if experienced more than 6 migraine attacks per month in the 2 months before screening, or had chronic daily headache (≥ 15 days per month of non‐migraine headaches) during the 3 months before screening No ergotamine use within 3 months, no monoamine oxidase inhibitor use within 2 weeks, and no St. John's wort use within 4 weeks of taking study medication. No regular NSAID use. No NSAID, opiate, or ergotamine use within 24 h, and no other analgesic or antiemetic use within 6 h of taking study medication. Study 1 N = 1677 (1461 for efficacy) M 187, F 1254 (87%) Mean age 40 years Without aura 74% Study 2 N = 1736 (1495 for efficacy) M 153, F 1317 (90%) Mean age 40 years Without aura 77% |
|
| Interventions | Study 1 Sumatriptan 85 mg, n = 415 (365 for efficacy) Naproxen 500 mg, n = 419 (361 for efficacy) Sumatriptan 85 mg + naproxen 500 mg, n = 422 (370 for efficacy) Placebo, n = 421 (365 for efficacy) Study 2 Sumatriptan 85 mg, n = 434 (370 for efficacy) Naproxen 500 mg, n = 434 (371 for efficacy) Sumatriptan 85 mg + naproxen 500 mg, n = 433 (367 for efficacy) Placebo, n = 435 (387 for efficacy) |
|
| Outcomes | Headache relief (at 2 h) Pain‐free (at 2 h) 24 h sustained headache relief 24 h sustained pain‐free Improvement in nausea, photophobia, and phonophobia at 2 h Improvement in functional disability at 2 h (from Landy 2007 (secondary reference for this study)) Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3. Pharmaceutical industry support: GlaxoSmithKline and Pozen Inc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | Low risk | Treatment groups > 200 participants |
Bussone 2000.
| Methods | Multicentre, randomised, double‐blind, cross‐over. Single dose to treat each of up to 12 consecutive attacks. Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 2 and 4 h after dosing Rescue medication available after 4 h for inadequate relief Second dose of study medication available for recurrence between 4 and 24 h At least 24 h between separate attacks, otherwise defined as recurrence |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) with an average of 1 to 6 attacks per month. Ergotamine and migraine prophylaxis discontinued before taking study medication N = 233 M 49, F 184 (79%) Mean age 37 years Proportion with/without aura not reported |
|
| Interventions | Sumatriptan 50 mg, n = 156 Placebo, n = 56 |
|
| Outcomes | Headache relief (at 2 h) Persistence of functional disability at 2 h Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W0. Total = 2. Pharmaceutical industry support: Glaxo Wellcome |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
Carpay 2004.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered within 1 h of the onset of mild pain while pain was still mild Assessments at 0.5, 0.75, 1, 2, and 24 h after dosing Second dose of study medication available to treat recurrence in individuals experiencing pain‐free results at 2 h Rescue medication (excluding ergot‐containing medication or triptans) available after 2 h for inadequate relief or recurrence (in individuals not wanting a second dose of study medication) |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate), typically preceded by a mild‐pain phase, and with an average of 1 to 6 attacks per month. Participants excluded if they had more than 6 migraines per month during either of the 2 months before screening. Migraine prophylactic medication containing ergotamine, ergotamine‐derivatives, or methysergide, and use of monoamine oxidase inhibitors was discontinued 2 weeks before the study. N = 481 (444 for efficacy) M 74, F 358 (83%) Mean age 41 years Without aura 71% |
|
| Interventions | Sumatriptan (fast disintegrating) 50 mg, n = 141 Sumatriptan (fast disintegrating) 100 mg, n = 148 Placebo, n = 155 |
|
| Outcomes | Pain‐free (at 1 and 2 h) 24 h sustained pain‐free Improvement in nausea, photophobia, and phonophobia at 2 h Improvement in functional disability at 2 h (from Barbanti 2004 (secondary reference for this study)) Adverse events |
|
| Notes | Oxford Quality Score: R1, DB1, W0. Total = 2. Pharmaceutical industry support: GlaxoSmithKline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
Cutler 1995.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered when migraine headache pain was of moderate or severe intensity. Assessment at 0.5, 1, 1.5, 2, 2.5, 3, 3.5, and 4 h after dosing. Rescue medication (acetaminophen) was available after 2 h if pain had not improved relative to predose levels. After 4 h, rescue medication other than acetaminophen was allowed if pain had still not improved. |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) with an average of 1 to 6 attacks per month. Migraine prophylaxis not allowed during 2‐week period preceding treatment. No opioid‐containing agents or ergotamine within 24 h, or simple analgesics within 6 h of taking study medication. N = 259 M 22, F 237 (92%) Mean age 39 years Proportion with/without aura not reported |
|
| Interventions | Sumatriptan 25 mg, n = 66 Sumatriptan 50 mg, n = 62 Sumatriptan 100 mg, n = 66 Placebo, n = 65 |
|
| Outcomes | Headache relief (at 2 h) Pain‐free (at 2 h) Improvement in nausea and photophobia at 2 h Improvement in functional disability at 2 h Adverse events |
|
| Notes | Oxford Quality Score: R1, DB1, W0. Total = 2. Pharmaceutical industry support: Glaxo Research Institute |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
Dahlof 1991.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat each of 3 consecutive attacks. Medication was administered at the earliest sign of an attack Assessment at 2 h after dosing Rescue medication (provided it did not contain ergotamine) was available after 2 h for inadequate symptom relief Minimum of 48 h between treated attacks |
|
| Participants | Aged 18 to 60 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) with an average of 1 to 6 attacks per month. Use of migraine prophylactic therapy was stopped at least 2 weeks before receipt of study medication N = 1130 (984 with moderate or severe baseline pain intensity) M 187, F 943 (83%) Mean age 40 years Without aura 33% |
|
| Interventions | Sumatriptan 100 mg, n = 305 (275 with moderate or severe baseline pain intensity) Sumatriptan 200 mg, n = 283 (255 with moderate or severe baseline pain intensity) Sumatriptan 300 mg, n = 299 (271 with moderate or severe baseline pain intensity) Placebo, n = 205 (182 with moderate or severe baseline pain intensity) |
|
| Outcomes | Headache relief (at 2 h) Improvement in nausea, vomiting, and photophobia at 2 h Patients' opinion of treatment Use of rescue medication Consistency of response Adverse events Withdrawals due to adverse events |
|
| Notes | Oxford Quality Score: R1, DB1, W0. Total = 2. Pharmaceutical industry support: Glaxo Group Research Ltd. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | Low risk | Treatment groups > 200 participants |
Dahlof 2009.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0.5, 1, 2, 4, and 24 h after dosing Rescue medication available after 2 h |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) with an average of 1 to 6 attacks per month. Participants excluded if they treated non‐migrainous headaches with analgesia for more than 10 days per month over the 6 months before screening No ergotamine, ergot‐derivatives, or triptans within 24 h, or any analgesics within 6 h of taking study medication N = 667 (541 for efficacy) M 85, F 456 (84%) Mean age 40 years Without aura 74% |
|
| Interventions | Sumatriptan 50 mg, n = 136 Tonabersat 20 mg, n = 134 Tonabersat 40 mg, n = 137 Placebo, n = 134 |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 1 and 2 h) Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB1, W1. Total = 4. Pharmaceutical industry support: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Low risk | Remote allocation, sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
Diener 2004a.
| Methods | Multicentre, randomised, double‐blind, double‐dummy, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0.5, 1, 1.5, 2, and 24 h after dosing Participants were encouraged to wait until 2 h after dosing before taking rescue medication if they experienced inadequate symptomatic relief, although it was available at any time during the study |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 6‐month history of migraine (untreated severity ≥ moderate) with an average of 1 to 6 attacks per month. At the time of treatment participants had to be without aura with each of the following associated symptoms was present: nausea, photophobia, and phonophobia Participants must have been free from any previous migraine for at least 24 h N = 435 (433 for efficacy) M 66, F 367 (85%) Mean age 43 years Without aura 79% |
|
| Interventions | Sumatriptan 50 mg, n = 135 Effervescent acetylsalicylic acid 1000 mg, n = 147 (146 for efficacy) Placebo, n = 153 (152 for efficacy) |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 1 and 2 h) Improvement in nausea, photophobia, and phonophobia at 2 h Patients' opinion of treatment Use of rescue medication Adverse events |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5. Pharmaceutical industry support: Bayer AG |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy technique |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
Diener 2004b.
| Methods | Multicentre, randomised, double‐blind, double‐dummy, placebo‐controlled, cross‐over. Single dose to treat each of 3 successive attacks. Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0.5, 1, 1.5, and 2 h after dosing Participants were encouraged to wait until 2 h after dosing before taking rescue medication if they experienced inadequate symptomatic relief, although it was available at any time during the study Minimum of 48 h between consecutive study treatments |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) with an average of 1 to 6 attacks per month. Participants were excluded if they experienced any other type of headache, including tension‐type headache N = 313 (312 for efficacy) M 59, F 253 (81%) Mean age 38 years Without aura 79% |
|
| Interventions | Sumatriptan 50 mg, n = 226 Ibuprofen 400 mg, n = 212 Effervescent acetylsalicylic acid 1000 mg, n = 222 Placebo, n = 222 |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 1 and 2 h) Improvement in nausea, vomiting, photophobia, and phonophobia at 2 h Patients' opinion of treatment Use of rescue medication Adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4. Pharmaceutical industry support: Bayer AG |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy technique |
| Study size | Low risk | Treatment groups > 200 participants |
DKSMSG 1999.
| Methods | Multicentre, randomised, double‐blind, double‐dummy, within‐patient cross‐over. Single dose to treat each of 4 consecutive attacks. Medication administered at the first sign of migraine pain Assessments at 0.3, 0.7, 1, 1.5, 2, 3, 4, 6, and 8 h after dosing Paracetamol available as rescue medication after 2 h for inadequate symptom relief Each treated attack separated by at least a 48‐h period free of acute headache medication and migraine symptoms |
|
| Participants | Aged 18 years or over, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) with an average of 2 to 6 attacks per month. N = 156 (144 received at least 1 treatment, 115 completed treatment for all 4 attacks) M 37, F 119 (76%) Mean age 33 years Proportion with/without aura not reported |
|
| Interventions | Sumatriptan 100 mg, n = 130 Diclofenac‐potassium 50 mg, n = 131 Diclofenac‐potassium 100 mg, n = 122 Placebo, n = 131 |
|
| Outcomes | Improvement in nausea, vomiting, photophobia, and phonophobia at 2 h Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4. Pharmaceutical industry support: Novartis Pharma |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy technique |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
Dodick 2002.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered when migraine headache pain was of moderate or severe intensity Assessments over 24 h following dosing and specifically at 2 h postdose Second dose of study medication available to treat recurrence within 24 h Rescue medication (excluding ergot alkaloids and 5‐HT1B/1D agonists) was available if moderate‐to‐severe migraine pain persisted 2 h after initial dosing Of the 3 studies reported, only protocol CL13 is relevant |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) with an average of 1 to 6 attacks per month, each separated by at least a 24‐h headache‐free period. Participants were excluded if they had a history of migraine with prolonged aura or if they experienced more than 6 headaches per month. No migraine medications (e.g. analgesics, NSAIDS, 5‐HT1B/1D receptor agonists, or dopamine agonists) for 2 days prior to intake of study medication. No antipsychotic or antidepressant medication within the 3 months preceding study enrolment, or any investigational drug within 1 month of study enrolment. Protocol CL13 N = 475 M 69, F 406 (85%) Mean age 43 years Without aura 79% |
|
| Interventions | Protocol CL13 Sumatriptan 100 mg, n = 193 Almotriptan 12.5 mg, n = 183 Placebo, n = 99 |
|
| Outcomes | Pain‐free (at 2 h) 24‐h sustained pain‐free Use of rescue medication |
|
| Notes | Oxford Quality Score: R1, DB1, W0. Total = 2. Pharmaceutical industry support: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
Dowson 2002.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 1, 2, and 24 h after dosing Second dose of study medication available to treat recurrence within 24 h Rescue medication (excluding ergot‐derivatives) available if migraine pain did not disappear or become mild within 2 h of treatment |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) with an average of 1 to 6 attacks per month, each separated by at least a 24‐h headache‐free period. Participants were excluded if they had a history of migraine with prolonged aura or if they needed symptomatic medication for migraine in the 2 days before taking study medication. No investigational drug within 1 month of study treatment. No monoamine oxidase inhibitors, lithium, selective serotonin reuptake inhibitors, ergots or derivatives, or methysergide in the 2 weeks prior to study medication. N = 668 M 101, F 567 (85%) Mean age 42 years Without aura 78% |
|
| Interventions | Sumatriptan 100 mg, n = 194 Almotriptan 12.5 mg, n = 184 Almotriptan 25 mg, n = 191 Placebo, n = 99 |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 1 and 2 h) 24‐h sustained pain‐free (from Dowson 2004 (secondary reference for this study)) Improvement in nausea, vomiting, photophobia, and phonophobia at 2 h Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3. Pharmaceutical industry support: Almirall SA |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
Ensink 1991.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered as soon as possible after onset of headache Assessments at 2, 4, and 24 h after dosing Second dose of study medication available after 2 h if headache persisted. Alternative rescue medication available 2 h after the second dose of study medication if their headache had not resolved. Third dose of study medication available to treat headache recurrence within 24 h |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) with an average of 1 to 6 attacks per month. No prophylactic medication within 2 weeks of the start of the study N = 233 (232 for efficacy, 209 with moderate or severe baseline pain intensity) M 34, F 198 (85%) Mean age 41 years Without aura 67% |
|
| Interventions | Sumatriptan 100 mg, n = 148 (131 with moderate or severe baseline pain intensity) Placebo, n = 84 (78 with moderate or severe baseline pain intensity) |
|
| Outcomes | Headache relief (at 2 h) Pain‐free (at 2 h) Persistence of nausea and vomiting at 2 h Improvement in photo/phonophobia at 2 h Improvement in any headache associated symptoms at 2 h Patients' opinion of treatment Use of rescue medication Adverse events |
|
| Notes | Oxford Quality Score: R1, DB1, W0. Total = 2. Pharmaceutical industry support: Glaxo Group Research Ltd. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
Freitag 2001.
| Methods | Multicentre, randomised, double‐blind, double‐dummy, parallel‐group. Multiple doses to treat single attack, after initial dose participants were allowed additional doses on a hourly basis for the next 3 h (see interventions for precise dosing schedule). Medication administered at the first signs or symptoms of acute migraine attack Assessments at 0.5, 1, 2, 3, 4, and 24 h after initial dosing Participants asked to refrain from taking rescue medications until at least 2 h after initial dosing |
|
| Participants | Aged 18 or over, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) with an average of 2 to 8 attacks per month. Participants were excluded if their migraines were accompanied by vomiting more than 20% of the time or required bed rest for at least half of their attacks Prophylactic migraine medications were continued if the dose had been stable prior to study enrolment No monoamine oxidase inhibitors or methysergide within 2 weeks of study enrolment N = 128 (126 for efficacy) M 14, F 112 (89%) Mean age 42 years Without aura 90% |
|
| Interventions | Sumatriptan 25 mg (+ additional dose of 25 mg at 2 h), n = 61 Isometheptene combination (isometheptene mucate + dichloralphenazone + acetaminophen) 2 doses (+ additional single doses at 1, 2 and 3 h), n = 65 |
|
| Outcomes | Headache relief (at 1 and 2 h) Improvement in nausea, photophobia, and phonophobia at 1 h Improvement in functional disability at 1 h Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4. Pharmaceutical industry support: Carnrick Laboratories |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy technique |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
Gallagher 2000.
| Methods | Multicentre, randomised, double‐blind, parallel‐group. Single dose to treat each of at least 2 separate attacks (up to 6). Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 1, 2, 4, and 24 h after dosing Second dose of study medication available to treat recurrence occurring 4 to 24 h after the initial dose Rescue medication (excluding acute antimigraine treatments such as sumatriptan, ergotamine, dihydroergotamine, and isometheptene) was available 2 h after the last dose of study medication to treat persistent headache |
|
| Participants | Aged 18 to 65, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate). Participants were excluded if they had experienced non‐migraine headache for 10 or more days per month over the previous 6 months No monoamine oxidase inhibitors, methysergide, methylergonovine, fenfluramine, or dexfenfluramine use during the study period. N = 1338 (1212 for efficacy) M 150, F 1062 (88%) Mean age 40 years Without aura 57% |
|
| Interventions | Sumatriptan 25 mg, n = 336 (306 for efficacy) Sumatriptan 50 mg, n = 338 (306 for efficacy) Zolmitriptan 2.5 mg, n = 327 (295 for efficacy) Zolmitriptan 5 mg, n = 337 (305 for efficacy) |
|
| Outcomes | Headache relief (at 1 and 2 h) 24‐h sustained headache relief Improvement in nausea and photophobia Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3. Pharmaceutical industry support: Zeneca Inc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | Low risk | Treatment groups > 200 participants |
Geraud 2000.
| Methods | Multicentre, randomised, double‐blind, double‐dummy, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 1, 2, 4, and 24 h after dosing Rescue medication was available after 2 h if migraine symptoms persisted. However ergot derivatives were not permitted until 12 h after study medication, and sumatriptan could not be used as a rescue medication. |
|
| Participants | Aged 18 to 65, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) with an average of 1 to 6 attacks per month. Participants were excluded if they had taken sumatriptan or zolmitriptan previously Participants were permitted to use medications such as β‐blockers, calcium channel blockers (excluding flunarizine), clonidine, and valproic acid for migraine prophylaxis. However, they were excluded if they had received regular treatment during the month preceding the study with psychoactive drugs or drugs with a clinically important action at a 5‐HT receptor. N = 1058 M 174, F 884 (84%) Mean age 38 years Without aura 73% |
|
| Interventions | Sumatriptan 100 mg, n = 504 Zolmitriptan 5 mg, n = 498 Placebo, n = 56 |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 1 and 2 h) 24‐h sustained headache relief Improvement in nausea, photophobia, and phonophobia at 2 h Improvement in functional disability at 2 h Use of rescue medication Adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4. Pharmaceutical industry support: Glaxo Wellcome |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy technique |
| Study size | Unclear risk | Active treatment groups > 200 participants, placebo treatment group 56 participants |
GL/MIG/001/92.
| Methods | Multicentre, randomised, double‐blind, double‐dummy, parallel‐group. Single dose to treat each of up to 3 attacks. Assessments at 2 and 4 h after dosing Second dose of either sumatriptan (sumatriptan‐treated group) or placebo (paracetamol/metoclopramide group) was available after 2 h if necessary Rescue medication (usual non‐ergotamine containing migraine treatments) was available after 4 h if study medication had not provided adequate relief |
|
| Participants | Aged 20 to 65, at least 1‐year history of migraine (diagnostic criteria equivalent to IHS 1988) with or without aura, and a frequency of at least 1 attack every 8 weeks Participants were excluded if they had taken sumatriptan previously No migraine prophylactic therapy or ergotamine‐containing medications within 2 weeks before study treatment N = 607 (469 with moderate or severe baseline pain intensity) M 98, F 509 (84%) Mean age 39 years Proportion with/without aura not reported |
|
| Interventions | Sumatriptan 100 mg, n = 305 (242 with moderate or severe baseline pain intensity) Paracetamol 1000 mg + metoclopramide 10 mg, n = 302 (227 with moderate or severe baseline pain intensity) |
|
| Outcomes | Headache relief (at 2 h) Improvement in nausea, vomiting, and photo/phonophobia at 2 h Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5. Pharmaceutical industry support: Glaxo Group Research Ltd. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation code |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy technique |
| Study size | Low risk | Treatment groups > 200 participants |
GL/MIG/001A/92.
| Methods | Multicentre, randomised, double‐blind, double‐dummy, parallel‐group. Single dose to treat each of up to 3 attacks. Assessments at 2 and 4 h after dosing Rescue medication (usual non‐ergotamine containing migraine treatments) was available after 4 h if study medication had not provided adequate relief |
|
| Participants | Aged 20 to 65, at least 1‐year history of migraine (diagnostic criteria equivalent to IHS 1988) with or without aura, and a frequency of at least 1 attack every 8 weeks Participants were excluded if they had taken sumatriptan previously No migraine prophylactic therapy or ergotamine‐containing medications within 2 weeks before study treatment N = 721 (566 with moderate or severe baseline pain intensity) M 124, F 597 (83%) Mean age 40 years Proportion with/without aura not reported |
|
| Interventions | Sumatriptan 100 mg, n = 348 (272 with moderate or severe baseline pain intensity) Paracetamol 1000 mg + metoclopramide 10 mg, n = 373 (294 with moderate or severe baseline pain intensity) |
|
| Outcomes | Headache relief (at 2 h) Improvement in photo/phonophobia at 2 h Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5. Pharmaceutical industry support: Glaxo Group Research Ltd. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation code |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy technique |
| Study size | Low risk | Treatment groups > 200 participants |
GL/MIG/002.
| Methods | Multicentre, randomised, double‐blind, parallel‐group. Single dose to treat each of up to 3 attacks. Medication administered at the onset of migraine Assessments at 2 and 4 h after dosing Second dose of either sumatriptan (sumatriptan‐treated group) or placebo (Migraleve group) was available for inadequate relief after 2 h Rescue medication was available after 4 h if study medication had not provided adequate relief |
|
| Participants | Aged 18 to 65, at least 1‐year history of migraine (diagnostic criteria equivalent to IHS 1988) with or without aura (untreated severity ≥ moderate), and a frequency of at least 1 attack every 8 weeks Participants were excluded if they had taken sumatriptan previously, or were receiving prophylactic therapy for migraine, constant analgesic therapy for other diseases, or antiemetics (regularly or irregularly) No ergotamine‐containing medications within 2 weeks before study treatment N = 752 (of which 709 treated attack 1, and 532 had ≥ moderate baseline pain intensity) M 112, F 640 (85%) Mean age 41 years Proportion with/without aura not reported |
|
| Interventions | Sumatriptan 100 mg, n = 374 (262 with moderate or severe baseline pain intensity) Migraleve (buclizine hydrochloride 12.5 mg + paracetamol 1000 mg + codeine phosphate 16 mg), n = 378 (275 with moderate or severe baseline pain intensity) |
|
| Outcomes | Headache relief (at 2 h) Improvement in nausea, vomiting, and photo/phonophobia at 2 h Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3. Pharmaceutical industry support: Glaxo Group Research Ltd. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | Low risk | Treatment groups > 200 participants |
GL/MIG/002A.
| Methods | Multicentre, randomised, double‐blind, parallel‐group. Single dose to treat each of up to 3 attacks. Medication administered at the onset of migraine Assessments at 2 and 4 h after dosing Rescue medication was available after 4 h if study medication had not provided adequate relief |
|
| Participants | Aged 18 to 65, at least 1 year history of migraine (diagnostic criteria equivalent to IHS 1988) with or without aura (untreated severity ≥ moderate), and a frequency of at least 1 attack every 8 weeks Participants were excluded if they had taken sumatriptan previously, or were receiving prophylactic therapy for migraine, constant analgesic therapy for other diseases, or antiemetics (regularly or irregularly) No ergotamine‐containing medications within 2 weeks before study treatment N = 674 (of which 617 treated attack 1, and 518 had ≥ moderate baseline pain intensity) M 112, F 562 (83%) Mean age 41 years Proportion with/without aura not reported |
|
| Interventions | Sumatriptan 100 mg, n = 342 (261 with moderate or severe baseline pain intensity) Migraleve (buclizine hydrochloride 12.5 mg + paracetamol 1000 mg + codeine phosphate 16 mg), n = 332 (257 with moderate or severe baseline pain intensity) |
|
| Outcomes | Improvement in photo/phonophobia at 2 h Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3. Pharmaceutical industry support: Glaxo Group Research Ltd. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | Low risk | Treatment groups > 200 participants |
GL/MIG/009.
| Methods | Multicentre, randomised, double‐blind, parallel‐group. Single dose to treat each of up to 3 attacks. Medication administered at the onset of migraine Assessments at 1, 2, and 4 h after dosing Second dose of study medication was available for inadequate relief after 2 h Alternative rescue medication was available after 4 h if study medication had not provided adequate relief |
|
| Participants | Aged 18 to 65, at least 1‐year history of migraine (diagnostic criteria equivalent to IHS 1988) with or without aura (untreated severity ≥ moderate), and a frequency of at least 1 attack every 4 weeks with at least 24 hours of freedom from headache between attacks No migraine prophylactic therapy, or ergotamine‐containing medications within 2 weeks before study treatment N = 513 (of which 468 treated attack 1, and 407 had ≥ moderate baseline pain intensity) M 83, F 430 (84%) Mean age 40 years Proportion with/without aura not reported |
|
| Interventions | Sumatriptan 100 mg, n = 255 (203 with moderate or severe baseline pain intensity) Migril (ergotamine tartrate 2 mg + cyclizine hydrochloride 50 mg + caffeine hydrate 100 mg), n = 258 (204 with moderate or severe baseline pain intensity) |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 1 and 2 h) Improvement in nausea, vomiting, and photo/phonophobia at 2 h Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3. Pharmaceutical industry support: Glaxo Group Research Ltd. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | Low risk | Treatment groups > 200 participants |
Goadsby 1991.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, cross‐over. Single dose to treat each of 4 successive attacks. Medication was administered as soon as participants were confident that they were having a migraine headache Assessment at 2 h after dosing Rescue medication available after 2 h |
|
| Participants | Aged 18 to 60, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) with an average of 1 to 6 attacks per month. Current prophylaxis was continued during the trial N = 61 (47 for efficacy) Proportion of male/female participants not reported Mean age 39 years Proportion with/without aura not reported |
|
| Interventions | Number of attacks in efficacy population Sumatriptan 100 mg, n = 94 (89 of moderate or severe intensity) Placebo, n = 94 (93 of moderate or severe intensity) |
|
| Outcomes | Headache relief (at 2 h) Use of rescue medication |
|
| Notes | Oxford Quality Score: R1, DB2, W0. Total = 3. Pharmaceutical industry support: Glaxo Group Research Ltd. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Matching placebo |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
Goadsby 2000.
| Methods | Multicentre, randomised, double‐blind, double‐dummy, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered when migraine headache pain was of moderate or severe intensity, and only if the aura phase had ended. Assessments at 0.5, 1, 1.5, and 2 h after dosing. Second blinded dose of study medication was available to treat recurrence within 24 h Rescue medication (analgesics, NSAIDs, or antiemetics) available as needed beginning 2 h after initial dosing |
|
| Participants | Aged 18 or over, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) with frequency of at least one attack every 6 weeks. Participants were excluded if they had more than 6 attacks per month No sumatriptan or any ergotamine‐like compound within 48 h of taking study medication N = 692 M 124, F 568 (82%) Mean age 40 years Without aura 68% |
|
| Interventions | Sumatriptan 100 mg, n = 129 Eletriptan 20 mg, n = 144 Eletriptan 40 mg, n = 136 Eletriptan 80 mg, n = 141 Placebo, n = 142 |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 1 and 2 h) Improvement in nausea and photo/phonophobia at 2 h Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5. Pharmaceutical industry support: Pfizer Inc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated pseudorandom code using method of random permuted blocks |
| Allocation concealment (selection bias) | Low risk | Study medication supplied pre‐packed, dispensed as next consecutive number |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy technique |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
Goldstein 1998.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, cross‐over. Single dose to treat each of 2 successive attacks. Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0.5, 1, 2, 3, and 4 h after dosing Rescue medication available after 2 h for inadequate headache response Each treated attack was separated by a minimum of 5 days |
|
| Participants | Aged 18 to 91, meeting IHS criteria for migraine (1988) with or without aura. At least 6‐month history of migraine (untreated severity ≥ moderate) with an average of 1 to 8 attacks per month. No monoamine oxidase inhibitors, propranolol, or lithium within 2 weeks; no sumatriptan, ergot derivatives, or opiates within 24 h; and no other form of analgesia or antiemetic within 6 h of taking study medication Standard migraine prophylaxis was permitted with the exception of NSAIDs and propranolol N = 1329 (1205 for efficacy) M 162, F 1167 (88%) Mean age 40 years Without aura 89% |
|
| Interventions | Sumatriptan 25 mg, n = 563 Sumatriptan 50 mg, n = 566 Rizatriptan 5 mg, n = 557 Rizatriptan 10 mg, n = 567 Placebo, n = 141 |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 1 and 2 h) Persistence of nausea, vomiting, photophobia, and phonophobia at 2 h Persistence of functional disability at 2 h Patients' opinion of treatment Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3. Pharmaceutical industry support: Merck Research Laboratories (supplies of sumatriptan provided by Glaxo Wellcome) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | Unclear risk | Active treatment groups > 200 participants, placebo treatment group 141 participants |
Goldstein 2005.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered when the first symptoms usually recognised as the beginning of a migraine attack occurred Assessment at 0.25, 0.5, 0.75, 1, 1.5, 2, 3, and 4 h after dosing Rescue medication permitted, but no further details reported |
|
| Participants | Meeting IHS criteria for migraine (1988) with or without aura. At least 6‐month history of migraine (untreated severity ≥ moderate) with an average of 1 to 8 attacks per month. Participants were excluded if their migraines were accompanied by vomiting more than 20% of the time or required bed rest for at least half of their attacks N = 171 (123 with moderate or severe baseline pain intensity) M 32, F 139 (81%) Mean age 38 years Without aura 14% |
|
| Interventions | Sumatriptan 50 mg, n = 67 Acetaminophen 1000 mg + aspirin 1000 mg + caffeine 260 mg combination, n = 69 Placebo, n = 35 |
|
| Outcomes | Headache relief (at 1 and 2 h) Improvement in photophobia at 1.5 h Improvement in phonophobia at 2 h Improvement in functional disability at 4 h Patients' opinion of treatment Use of rescue medication Serious and specific adverse events |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5. Pharmaceutical industry support: Bristol‐Myers Squibb |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Study medications were individually encapsulated to preserve study blinding |
| Study size | High risk | Active treatment groups 50 to 200 participants, placebo treatment group 35 participants |
Gruffyd‐Jones 2001.
| Methods | Multicentre, randomised double‐blind, double‐dummy, parallel‐group. Single dose to treat each of up to 6 consecutive attacks. Medication administered when migraine headache pain was of moderate or severe intensity, provided a migraine‐free period of at least 24 h had elapsed since the previous treated attack Assessments at 1, 2, 4, and 24 h after dosing Second dose of study medication available to treat recurrence between 2 and 24 h after the initial dosing Rescue medication (analgesics, NSAIDs, antiemetics, or sedatives) available after 2 h to treat persistent migraine headache. However, ergotamine derivatives not permitted until at least 6 h after initial dosing. |
|
| Participants | Aged 18 to 65, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) with an average of 1 to 6 attacks per month. Participants were excluded if they had suffered non‐migraine headaches on more than 10 days per month over the preceding 6 months No monoamine oxidase inhibitors, methysergide, or methylergonovine within 2 weeks of randomisation N = 1666 (1522 for efficacy) M 223, F 1299 (85%) Mean age 42 years Without aura 57% |
|
| Interventions | Sumatriptan 50 mg, n = 555 (508 for efficacy) Zolmitriptan 2.5 mg, n = 551 (500 for efficacy) Zolmitriptan 5 mg, n = 560 (514 for efficacy) |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 1 and 2 h) 24‐h sustained pain‐free Improvement in nausea, photophobia, and phonophobia at 2 h Patients' opinion of treatment Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5. Pharmaceutical industry support: AstraZeneca Pharmaceuticals |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers scheme |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy technique |
| Study size | Low risk | Treatment groups > 200 participants |
Havanka 2000.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 10, 20, 30, 60, 90, 120, 180, and 240 minutes after dosing Rescue medication available 4 h after dosing for persistent headache |
|
| Participants | Aged 18 to 55, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) with an average of 1 to 6 attacks per month. No use of monoamine oxidase inhibitors, serotonin reuptake inhibitors, lithium, of flunarizine during the study period No sumatriptan or ergot‐containing medications within 24 h before or after study drug administration, and no antiemetics or analgesics within 6 h of study drug administration Migraine prophylactic medication stopped at least 2 weeks before administration of study medication N = 643 (642 for efficacy) M 77, F 566 (88%) Mean age not reported Without aura 75% |
|
| Interventions | Sumatriptan 100 mg, n = 98 Naratriptan 1 mg, n = 85 Naratriptan 2.5 mg, n = 87 Naratriptan 5 mg, n = 93 Naratriptan 7.5 mg, n = 93 Naratriptan 10 mg, n = 96 (95 with moderate or severe baseline pain intensity) Placebo, n = 91 |
|
| Outcomes | Headache relief (at 1 and 2 h) 24‐h sustained headache relief Improvement in nausea and photo/phonophobia at 2 h Improvement in functional disability at 2 h Patients' opinion of treatment Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB1, W1. Total = 4. Pharmaceutical industry support: Glaxo Wellcome |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation numbers |
| Allocation concealment (selection bias) | Low risk | Numbers assigned in consecutive order, starting with the lowest available |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
Ishkanian 2007.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0.5, 1, 2, and 4 h after dosing Rescue medication available after 2 h |
|
| Participants | Aged 18 to 65, suffering at least 6 self described or physician‐diagnosed "sinus" headaches in the 6 months prior to screening which, upon careful review at screening, were determined to satisfy IHS diagnostic criteria for migraine (1988) with or without aura. Participants must have had no previous diagnosis of migraine and have had no previous use of migraine‐specific medications, such as 5‐HT1B/1D agonists, ergotamine, or ergot‐like medications. Participants with evidence of other types of headache, such as chronic daily headache (more than 15 headache days per month), were excluded No monoamine oxidase inhibitors or sumatriptan within 2 weeks of trial screening. No analgesics, antiemetics, or other acute migraine medications, or sinus/nasal medications (e.g. antihistamines, nasal sprays and decongestants) within 24 h of taking study medication. N = 216 (215 for efficacy) M 64, F 151 (70%) Mean age 40 years Without aura 90% |
|
| Interventions | Sumatriptan 50 mg, n = 108 Placebo, n = 108 (107 for efficacy) |
|
| Outcomes | Headache relief (at 2 h) Pain‐free (at 2 h) Improvement in nausea and photo/phonophobia at 2 h Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5. Pharmaceutical industry support: GlaxoSmithKline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedules |
| Allocation concealment (selection bias) | Low risk | Remote allocation, assignments sealed and remained intact |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Matching placebo |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
Jelinski 2006.
| Methods | Multicentre, randomised, double‐blind, double‐dummy, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered within 2 h of the first sign of migraine pain, while the pain was still considered to be mild. Assessments at 0.5, 1, 2, 4, and 24 h after dosing Second dose of study medication available to treat recurrence 2 to 24 h after initial dosing Rescue medication (analgesics, antiemetics, or other acute migraine medications) were available after 2 h for inadequate symptom relief |
|
| Participants | Aged 18 to 65, meeting IHS criteria for migraine (1988) with or without aura. Had 1 to 6 migraine attacks per month in the 2 months prior to screening, and typically experienced moderate to severe migraine pain preceded by a mild pain phase. No use of monoamine oxidase inhibitors during the study period No analgesics, antiemetics, or other acute migraine medications within 6 h of taking study medication. No ergotamine, ergot‐type medications, or other 5HT1 agonists within 24 h of study medication use. Participants permitted to continue their use of prophylactic medications (excluding methysergide) during the study, provided the dose was stable for at least 1 month before study entry N = 361 M 52, F 309 (86%) Mean age 40 years Without aura 67% |
|
| Interventions | Sumatriptan 50 mg, n = 126 Sumatriptan 100 mg, n = 126 Placebo, n = 109 |
|
| Outcomes | Pain‐free (at 1 and 2 h) 24 h sustained pain‐free Persistence of nausea, vomiting, photophobia, and phonophobia at 2 h (from SUM40291) Use of rescue medication (from SUM40291) Adverse events (with additional data from SUM40291) Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5. Pharmaceutical industry support: GlaxoSmithKline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation code |
| Allocation concealment (selection bias) | Low risk | Treatment group assignment was unknown to patients and investigators |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy technique |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
Kaniecki 2006.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0.5, 1, 2, 4, and 24 h after dosing Second dose of study medication available after 2 h to treat recurrence or for pain if participant had at least a partial response to the first dose Alternative rescue medication (excluding ergotamine‐containing medications and monoamine oxidase inhibitors) available after 2 h for persistent pain |
|
| Participants | Aged 18 to 65, self reporting tension/stress‐type headache, who were given a diagnosis of migraine with or without aura according to IHS criteria (1988) at a screening visit. At least 1‐year history of headache (untreated severity ≥ moderate) with an average of 1 to 6 attacks per month. Participants excluded if they had ever used a triptan, ergotamine, or an ergot derivative, or had persistent head or neck pain outside of migraine attacks (more than 15 days per month during the 2 months before screening) No monoamine oxidase inhibitors within 2 weeks of study entry N = 258 M 69, F 184 (73%) Mean age 37 years Proportion with/without aura not reported |
|
| Interventions | Sumatriptan 100 mg, n = 131 Placebo, n = 127 |
|
| Outcomes | Headache relief (at 2 h) Pain‐free (at 2 h) 24‐h sustained headache relief 24‐h sustained pain‐free Patients' opinion of treatment Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3. Pharmaceutical industry support: GlaxoSmithKline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
Kolodny 2004.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, cross‐over. Single dose to treat each of 2 consecutive attacks. Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0.5, 1, 1.5, 2, 3, and 4 h after dosing Rescue medication (analgesics or antiemetics) was permitted from 2 h onwards in case of treatment failure or headache recurrence |
|
| Participants | Aged 18 years or older, meeting IHS criteria for migraine (1988) with or without aura. At least 6‐month history of migraine (untreated severity ≥ moderate). No monoamine oxidase inhibitors, methysergide, or propranolol during the study period Standard antimigraine prophylactic medications (with the exception of NSAIDs, daily analgesics, or propanolol) were permitted N = 1447 (1287 for efficacy) M 203, F 1244 (86%) Mean age 40 years Proportion with/without aura not reported |
|
| Interventions | Sumatriptan 25 mg, n = 554 (290 1st attack only) Sumatriptan 50 mg, n = 550 (285 1st attack only) Rizatriptan 5 mg, n = 536 (288 1st attack only) Rizatriptan 10 mg, n = 547 (296 1st attack only) Placebo, n = 288 |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 2 h) Persistence of nausea, vomiting, photophobia, and phonophobia at 2 h Persistence of functional disability at 2 h Use of rescue medication Adverse events |
|
| Notes | Oxford Quality Score: R2, DB2, W0. Total = 4. Pharmaceutical industry support: Merck & Co. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated allocation schedule |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Matched placebos |
| Study size | Low risk | Treatment groups > 200 participants |
Kudrow 2005.
| Methods | Multicentre, randomised, double‐blind, double‐dummy, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0.5, 1, 1.5, 2, 3, 4, 8, 12, and 24 h after dosing Second dose of study medication available if headache worsened, failed to improve or recurred within 24 h Rescue medication available 2 h after initial dosing (encouraged wait, not enforced) |
|
| Participants | Aged 18 to 65, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) with an average of 2 to 8 attacks per month, at least 2 of which were of moderate or severe intensity. Participants were only eligible for entry if they had previously used sumatriptan Changes to (or initiation of) migraine prophylactic medication less than 2 weeks before study screening visit were prohibited Chronic use (more than 3 days per week) of analgesics, COX‐2 inhibitors, or non‐specific NSAIDs not permitted No ergotamine‐containing or ergot‐type medication, 5‐HT1D or 5‐HT1B/1D medication, or COX‐2 inhibitors within 48 h of receiving study medication N = 574 M 48, F 526 (92%) Mean age 41 years Without aura 64% |
|
| Interventions | Sumatriptan 50 mg, n = 144 Valdecoxib 20 mg, n = 137 Valdecoxib 40 mg, n = 152 Placebo, n = 141 |
|
| Outcomes | Headache relief (at 2 h) Improvement in nausea, vomiting, photophobia, and phonophobia at 2 h Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4. Pharmaceutical industry support: Pfizer Inc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy technique |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
Latere 1991.
| Methods | Multicentre, randomised, double‐blind, double‐dummy, parallel‐group. Single dose to treat each of 3 successive attacks. Assessment at 2 h Rescue medication available after 2 h Minimum of 48 h between treatments with trial medication |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) with an average of 1 to 6 attacks per month. All prophylactic therapy stopped at the initial screening visit N = 577 M 98, F 479 (83%) Mean age 40 years Without aura 70% |
|
| Interventions | Sumatriptan (dispersible) 100 mg, n = 288 (220 with moderate or severe baseline pain intensity) Cafergot, n = 289 (246 with moderate or severe baseline pain intensity) |
|
| Outcomes | Headache relief (at 2 h) Pain‐free (at 2 h) Improvement in nausea, vomiting, and photo/phonophobia at 2 h Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5. Pharmaceutical industry support: Glaxo Group Research Ltd. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation code |
| Allocation concealment (selection bias) | Low risk | Participants entered in ascending sequential order of patient number at each centre |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy technique |
| Study size | Low risk | Treatment groups > 200 participants |
Lines 2001.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0.5, 1, 1.5, 2, 3, and 4 h after dosing Rescue medications, consisting of standard analgesics or antiemetics, were allowed from 2 h onwards |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 6‐month history of migraine (untreated severity ≥ moderate) with an average of 1 to 8 attacks per month. No details of prohibited medications reported N = 792 (785 for efficacy) M 158, F 634 (80%) Mean age 40 years Proportion with/without aura not reported |
|
| Interventions | Sumatriptan 50 mg, n = 356 Rizatriptan 5 mg, n = 349 Placebo, n = 80 |
|
| Outcomes | Headache relief (at 2 h) | |
| Notes | Oxford Quality Score: R1, DB1, W0. Total = 2. Pharmaceutical industry support: Merck & Co. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | Unclear risk | Active treatment groups > 200 participants, placebo treatment group 80 participants |
Lipton 2000.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, cross‐over. Single dose to treat each of up to 10 attacks. Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 2, 3, and 24 h after dosing Rescue medication available after 4 h 24 h headache‐free interval was required between treated headaches |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 6‐month history of migraine (untreated severity ≥ moderate) with an average of 1 to 10 attacks per month. Participants with clinical diagnosis of migrainous headache and episodic tension‐type headache were also included in the study, although only those with IHS‐diagnosed migraine were used for efficacy analysis Participants were required to have an HIQ score of 250 or greater at screening No monoamine oxidase inhibitor use during the study period N = 311 (249 with migraine diagnosis for efficacy) M 35, F 214 (86%) Mean age 38 years Proportion with/without aura not reported Total number of treated attacks = 1110 |
|
| Interventions | Treated attacks: Sumatriptan 50 mg, n = 870 Placebo, n = 240 |
|
| Outcomes | Headache relief (at 2 h) Pain‐free (at 2 h) Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W0. Total = 4. Pharmaceutical industry support: Glaxo Wellcome |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical appearing placebo |
| Study size | Low risk | Treatment groups > 200 participants |
Mathew 2003.
| Methods | Multicentre, randomised, double‐blind, double‐dummy, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0.5, 1, 1.5, 2, 4, and 24 h after dosing Second dose of study medication available to treat recurrence after 2 h Rescue medication available after 2 h for inadequate headache relief, although participants not permitted to take any other triptan, ergotamine, or ergotamine‐like substance for 24 h after initial dosing |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura and a monthly frequency of 1 to 6 attacks. No use of potent CYP3A4 inhibitors or monoamine oxidase inhibitors within 2 weeks prior to study entry. No analgesic or antiemetic within 6 h, or triptan, ergotamine‐containing or ergot‐type medication within 48 h of taking study medication N = 2113 (2072 for efficacy) M 277, F 1795 (87%) Mean age 42 years Without aura 65% |
|
| Interventions | Sumatriptan 100 mg, n = 831 Eletriptan 40 mg, n = 822 Placebo, n = 419 |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 1 and 2 h) 24‐h sustained headache relief Improvement in nausea, photophobia, and phonophobia at 2 h Improvement in functional disability at 2 h Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4. Pharmaceutical industry support: Pfizer Ltd. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy technique |
| Study size | Low risk | Treatment groups > 200 participants |
Myllyla 1998.
| Methods | Multicentre, randomised, double‐blind, double‐dummy, placebo‐controlled, parallel‐group. Up to 2 doses to treat each of 2 successive attacks. Medication administered at the first symptoms of a migraine attack Assessment at 2 h Second dose of study medication if headache not improved after 1 h Alternative rescue medication (paracetamol, acetylsalicylic acid, naproxen, ketoprofen, prochlorperazine, or diazepam) available after 2 h if headache relief still insufficient At least 48 h required between the treatment of 2 successive attacks |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) with an average of 1 to 4 attacks per month. N = 154 (126 for efficacy) M 15, F 126 (89%) Mean age 39 years Without aura 72% |
|
| Interventions | Sumatriptan 100 mg (+ optional dose of placebo after 1 h), n = 46 (42 for efficacy) Tolfenamic acid 200 mg (+ optional 2nd dose after 1 h), n = 47 (43 for efficacy) Placebo (+ optional dose of placebo after 1 h), n = 46 (41 for efficacy) |
|
| Outcomes | Headache relief (at 2 h) Pain‐free (at 2 h) Improvement in nausea, vomiting, photophobia, and phonophobia at 2 h Use of rescue medication Adverse events |
|
| Notes | Oxford Quality Score: R2, DB2, W0. Total = 4. Pharmaceutical industry support: A/S GEA Farmaceutisk Fabrik (medication used was Imigran) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation code |
| Allocation concealment (selection bias) | Low risk | Complete randomisation blocks assigned to centres, participants entered in ascending sequential order of patient number |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy technique |
| Study size | High risk | Treatment groups < 50 participants |
Nappi 1994.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered at the first sign of migraine Assessments at 2 and 4 h Second dose of study medication available if symptom relief was inadequate at 2 h Alternative rescue medication (not ergotamine) was available if the response after 4 h was still inadequate Headache recurrence after either the first or second dose could be treated by a third dose of study medication, providing it was more than 2 h after the most recent dose and less than 24 h after the first dose |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate). Participants were excluded if they were taking migraine prophylaxis N = 250 (244 for efficacy) M 56, F 188 (77%) Mean age 38 years Without aura 87% |
|
| Interventions | Sumatriptan 100 mg, n = 158 (148 with moderate or severe baseline pain intensity) Placebo, n = 86 (81 with moderate or severe baseline pain intensity) |
|
| Outcomes | Headache relief (at 2 h) Pain‐free (at 2 h) Improvement in nausea, vomiting, and photo/phonophobia at 2 h Patients' opinion of treatment Use of second dose of study medication Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3. Pharmaceutical industry support: Glaxo Group Research Ltd. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
Nett 2003.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single menstrually associated migraine attack. Medication administered within 1 h of the onset of pain, but only if the pain was mild at onset and only if the pain was still mild at the time of treatment Assessments at 0.5, 1, and 2 h after dosing Rescue medication or a second double‐blind dose of study medication were available to treat either inadequate response after 2 h or recurrence between 2 and 24 h |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine with a minimum of 6 months of regularly occurring menstrually associated migraines (defined as occurring between day ‐2 to day 4 relative to the first day of flow). Participants had to have had menstrually associated migraine in at least 2 of their last 3 perimenstrual periods before screening that were typically associated with moderate to severe pain preceded by a mild pain phase Participants were excluded if they had tension‐type headache for more than 15 days per month or more than 6 migraine attacks per month in either of the 2 months before screening No monoamine oxidase inhibitors or ergotamine‐containing or ergotamine‐type migraine prophylactic medication during the study period. Other migraine prophylactic medications were permitted, provided they had been on a constant regimen for at least 1 month before screening and the regimen remained constant throughout the study. No analgesics, antiemetics, or non‐serotonin‐agonist acute migraine medications within 6 h of taking study medication N = 369 (368 for efficacy, 349 for per‐protocol efficacy) All F Mean age 36 years Without aura 75% |
|
| Interventions | Sumatriptan 50 mg, n = 124 (124 for efficacy, 116 for per‐protocol efficacy) Sumatriptan 100 mg, n = 122 (122 for efficacy, 115 for per‐protocol efficacy) Placebo, n = 123 (122 for efficacy, 118 for per‐protocol efficacy) |
|
| Outcomes | Pain‐free (at 1 and 2 h) 24‐h sustained pain‐free Persistence of nausea and photo/phonophobia at 2 h Improvement in functional disability at 2 h (from Landy 2004 (secondary reference for this study) Study 2) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5. Pharmaceutical industry support: GlaxoSmithKline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Remote allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All tablets were visually indistinguishable |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
Patten 1991.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat each of up to 3 successive attacks. Medication administered at the earliest sign of a migraine attack, provided at least 48 h had elapsed since the previous study treatment. Assessment at 2 h after dosing Rescue medication (excluding ergotamine‐containing medication) was available after 2 h if symptoms were not adequately relieved |
|
| Participants | Aged 18 to 60 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) with an average of 1 to 6 attacks per month. All use of prophylactic migraine therapy was stopped at least 2 weeks before starting on the study medication N = 624 (538 with moderate or severe baseline pain intensity) No demographic data given |
|
| Interventions | Sumatriptan (dispersible) 100 mg, n = 142 Sumatriptan (dispersible) 200 mg, n = 140 Sumatriptan (dispersible) 300 mg, n = 155 Placebo, n = 101 |
|
| Outcomes | Headache relief (at 2 h) Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W0. Total = 2. Pharmaceutical industry support: Glaxo Group Research Ltd. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
Pfaffenrath 1998.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat each of 3 separate attacks. Assessment at 0.5, 1, 2, 3, and 4 h after dosing Second randomised dose of study medication available to treat headache recurrence from 2 to 24 h after initial dosing Rescue medication (excluding ergotamine‐containing preparations or sumatriptan) was permitted if headache relief was inadequate 4 h after initial dosing |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) with an average of 1 to 6 attacks per month. No use of lithium, monoamine oxidase inhibitors, serotonin reuptake inhibitors, or ergotamine‐containing migraine prophylactic medications during the study period No analgesics or antiemetics within 6 h and no ergotamine‐containing medications within 24 h of taking study medication N = 1003 (939 with moderate or severe baseline pain intensity) M 157, F 846 (84%) Mean age 40 years Without aura 66% |
|
| Interventions | Sumatriptan 25 mg, n = 303 (286 with moderate or severe baseline pain intensity) Sumatriptan 50 mg, n = 303 (285 with moderate or severe baseline pain intensity) Sumatriptan 100 mg, n = 298 (277 with moderate or severe baseline pain intensity) Placebo, n = 99 (91 with moderate or severe baseline pain intensity) |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 2 h) Improvement in nausea and photo/phonophobia at 2 h Persistence of functional disability at 2 h Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4. Pharmaceutical industry support: Glaxo Wellcome |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Matching placebo |
| Study size | Unclear risk | Active treatment groups > 200 participants, placebo treatment group 99 participants |
Pini 1999.
| Methods | Multicentre, 2 phase study Phase 1: Randomised, open‐label treatment of a single attack with 1 of 3 standard over‐the‐counter migraine medications when migraine headache pain was of mild or moderate intensity. Participants who failed to respond in phase 1 then went on to phase 2. Phase 2: Randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication was administered when migraine headache pain was of mild or moderate intensity. Assessments at 2 and 4 h after dosing Second dose of study medication was available to treat recurrence between 4 and 24 h Rescue medication was available for insufficient relief of symptoms 4 h after initial dosing |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity mild or moderate) with an average of 1 to 8 attacks per month. No migraine prophylaxis containing ergotamine during the study period No sumatriptan or ergotamine‐containing drugs within 24 h, or other analgesics or antiemetics within 6 h of taking study medication Phase 2: N = 219 (167 for efficacy) M 44, F 175 (80%) Mean age 37 years Proportion with/without aura not reported |
|
| Interventions | Sumatriptan 50 mg, n = 137 (106 for efficacy) Placebo, n = 82 (61 for efficacy) |
|
| Outcomes | Pain‐free (at 2 h) Use of rescue medication Adverse events |
|
| Notes | Oxford Quality Score: R1, DB1, W0. Total = 2. Pharmaceutical industry support: Glaxo Wellcome (medication used was Imigran) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
Pini 1995.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered at the earliest sign of migraine attack Assessments every 30 minutes up to 4 h, and then every 6 h up to 48 h after dosing Rescue medication (ergotamine‐free) was available after 4 h if the headache was not controlled |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 6‐month history of migraine (untreated severity ≥ moderate). No details of prohibited medications reported. N = 238 (222 for efficacy) M 52, F 186 (78%) Mean age 37 years Without aura 61% |
|
| Interventions | Sumatriptan 100 mg, n = 151 Placebo, n = 87 |
|
| Outcomes | All efficacy data at 4 h Adverse events |
|
| Notes | Oxford Quality Score: R1, DB1, W0. Total = 2. Pharmaceutical industry support: Glaxo |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
Sandrini 2002.
| Methods | Multicentre, randomised, double‐blind, double‐dummy, placebo‐controlled, parallel‐group. Single dose to treat each of up to 3 successive attacks. Medication administered within 6 h of onset of a migraine attack, when the headache pain was of moderate or severe intensity, and if any aura phase had ended Assessments at 0.5, 1, 2, 4, and 24 h after dosing Second, blinded and randomised dose of study medication was available if there was no response to treatment after 2 h, or if there was a recurrence of headache within 24 h Rescue medication was available 2 h after the second dose if there was still no improvement in headache |
|
| Participants | Aged 18 years or older, meeting IHS criteria for migraine (1988) with or without aura, and suffering at least 1 attack every 6 weeks. Participants were excluded if they had previously taken oral eletriptan or any formulation of sumatriptan. No ergotamine or any ergotamine‐like agent within 48 h before, or 24 h after, taking study medication. No proprietary analgesic or antiemetic within 6 h of taking study medication. N = 774 M 93, F 681 (88%) Mean age 38 years Without aura 65% |
|
| Interventions | Sumatriptan 50 mg, n = 181 Sumatriptan 100 mg, n = 170 Eletriptan 40 mg, n = 175 Eletriptan 80 mg, n = 164 Placebo, n = 84 |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 1 and 2 h) 24‐h sustained headache relief 24‐h sustained pain‐free Improvement in nausea, photophobia, and phonophobia at 2 h Improvement in functional disability at 2 h Use of rescue medication Serious and specific adverse events Withdrawals due to adverse events |
|
| Notes | Oxford Quality Score: R1, DB2, W0. Total = 3. Pharmaceutical industry support: Pfizer Ltd. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy technique |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
Sandrini 2007.
| Methods | Multicentre, randomised, double‐blind, double‐dummy, placebo‐controlled, parallel‐group. Single dose to treat each of 2 consecutive attacks separated by at least 48 h. Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0.5, 1, 1.5, 2, 3, 4, and 5 h after dosing Second dose of study medication available as rescue medication after 2 h if headache relief was inadequate, or to treat recurrence within 48 h of initial dosing Alternative rescue medication was available 2 h after the second dose if headache relief remained inadequate. Ergot derivatives and opiates could not be used as a rescue medication. |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) and suffering an average of 1 to 6 attacks per month. Use of migraine prophylaxis or ergot derivatives during the month of screening and the study period was prohibited Participants were not permitted to take coffee or beverages containing caffeine during the migraine attack N = 282 (281 for efficacy) M 61, F 220 (78%) Mean age 35 years Without aura 93% |
|
| Interventions | Sumatriptan 50 mg, n = 139 (138 for efficacy) Indoprocaf (indomethacin 25 mg + prochlorperazine 2 mg + caffeine 75 mg), n = 143 |
|
| Outcomes | Headache relief (at 2 h) Pain‐free (at 1 and 2 h) 24‐h sustained headache relief 24‐h sustained pain‐free Improvement in nausea, vomiting, photophobia, and phonophobia at 2 h Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5. Pharmaceutical industry support: Solvay Pharma |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated code list |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy technique |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
Sargent 1995.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered when migraine headache pain was of moderate or severe intensity Assessments every 30 mins up to 4 h after dosing Rescue medication (acetaminophen) available after 2 h if pain had not improved relative to predose levels. Rescue medication other than acetaminophen was allowed beginning 4 h after initial dosing. |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) and suffering an average of 1 to 6 attacks per month. Migraine prophylaxis was not allowed during the 2‐week period preceding treatment No simple analgesics during 6 h preceding treatment, and no opioid‐containing agents or ergotamine during the 24 h preceding treatment N = 187 M 16, F 171 (91%) Mean age 40 years Without aura 80% |
|
| Interventions | Sumatriptan 25 mg, n = 48 Sumatriptan 50 mg, n = 46 Sumatriptan 100 mg, n = 46 Placebo, n = 47 |
|
| Outcomes | Headache relief (at 1 and 2 h) Improvement in nausea and photophobia at 2 h Improvement in functional disability at 2 h Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3. Pharmaceutical industry support: Glaxo Research Institute |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | High risk | Treatment groups < 50 participants |
Savani 1999.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat each of up to 3 separate attacks. Assessments at 0.5, 1, 2, 3, and 4 h after dosing. Second dose of study medication available to treat recurrence from 4 to 24 h after initial dosing Rescue medication (excluding ergotamine‐containing preparations or sumatriptan) was permitted if headache relief was inadequate 4 h after taking study medication |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) and suffering an average of 1 to 6 attacks per month. Participants were excluded if they had ever taken sumatriptan previously or were currently using a monoamine oxidase inhibitor, a serotonin reuptake inhibitor, or lithium No analgesics or antiemetics within 6 h, or ergotamine or ergotamine‐containing medication within 24 h of taking study medication. Normal prophylactic medication for migraine was permitted (unchanged throughout the study, if possible) N = 485 (less than 1% of which had mild pain at baseline) M 68, F 417 (86%) Mean age 36 to 40 years Without aura 67% to 87% |
|
| Interventions | Sumatriptan 50 mg, n = 331 Placebo, n = 154 |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 2 h) Adverse events |
|
| Notes | Oxford Quality Score: R1, DB1, W0. Total = 2. Pharmaceutical industry support: Glaxo Wellcome |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | Unclear risk | Active treatment group > 200 participants, placebo treatment group 154 participants |
Schulman 2003.
| Methods | Single centre, randomised, double‐blind, cross‐over. Each participant treated 2 successive attacks with a single dose, one with each study treatment. Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 30, 60, 90, and 120 minutes after dosing |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura, and suffered an average of 1 to 8 attacks per month Participants were only eligible for entry if they had failed to receive adequate self defined relief from triptans previously, had at least 15 headache‐free days per month, and did not experience vomiting in greater than 25% of their migraine attacks Migraine prophylaxis was permitted, provided the dose of the medications had been stable for at least 30 days before initiating the trial N = 18 (16 for efficacy) M 3, F 13 (81%) Mean age 40 years Without aura 81% |
|
| Interventions | Sumatriptan 50 mg, n = 16 Sumatriptan 50 mg + metoclopramide 10 mg, n = 16 |
|
| Outcomes | Headache relief (at 2 h) Improvement in nausea, photophobia, and phonophobia at 2 h Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4. Pharmaceutical industry support: GlaxoSmithKline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical pills |
| Study size | High risk | Treatment groups < 50 participants |
Sheftell 2005.
| Methods | Two identically designed studies, multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered when migraine headache pain was of moderate or severe intensity Assessments every 10 minutes for the first 2 h, and then at 4 and 24 h after dosing Second dose of study medication or non‐prohibited acute migraine medication available after 2 h to treat recurrence Rescue medication available after 2 h if pain not reduced to mild or none within 2 h after initial dosing |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 6‐month history of migraine (untreated severity ≥ moderate) and suffering an average of 1 to 6 attacks per month. Participants were excluded if they experienced headache on more than 15 days per month in any of the 3 months before screening. No migraine prophylactic medication containing ergotamine, an ergot derivative, or methysergide, or use of monoamine oxidase inhibitor within 2 weeks before screening. Study 1: N = 1477 (1366 for efficacy) M 196, F 1170 (86%) Mean age 41 years Without aura 70% Study 2: N = 1475 (1330 for efficacy) M 204, F 1126 (85%) Mean age 40 years Without aura 67% |
|
| Interventions | Study 1: Sumatriptan (rapid‐release) 50 mg, n = 494 (448 for efficacy) Sumatriptan (rapid‐release) 100 mg, n = 488 (462 for efficacy) Placebo, n = 495 (456 for efficacy) Study 2: Sumatriptan (rapid‐release) 50 mg, n = 496 (454 for efficacy) Sumatriptan (rapid‐release) 100 mg, n = 485 (440 for efficacy) Placebo, n = 494 (436 for efficacy) |
|
| Outcomes | Headache relief (at 2 h) Pain‐free (at 2 h) 24‐h sustained headache relief 24‐h sustained pain‐free Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3. Pharmaceutical industry support: GlaxoSmithKline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | Remote allocation generated by the study sponsor and not available to the investigators |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | Low risk | Treatment groups > 200 participants |
Smith 2005.
| Methods | Multicentre, randomised, double‐blind, double‐dummy, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered when migraine headache pain was of moderate or severe intensity Assessments every 15 minutes up to 2 h, every 30 minutes between 2 and 4 h and hourly between 4 and 24 h after dosing Rescue medication available after 2 h |
|
| Participants | Aged 18 years or older, meeting IHS criteria for migraine (1988 and 2004) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) and suffering an average of 2 to 6 attacks per month. Participants had a history of tolerating oral treatment with a 5‐HT agonist for migraine N = 972 (965 for efficacy) M 92, F 880 (91%) Mean age 42 years Without aura 75% |
|
| Interventions | Sumatriptan 50 mg, n = 229 (226 for efficacy) Sumatriptan 50 mg, + naproxen 500 mg, n = 251 (250 for efficacy) Naproxen 500 mg, n = 250 (248 for efficacy) Placebo, n = 241 |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 1 and 2 h) 24‐h sustained headache relief 24‐h sustained pain‐free Persistence of nausea, photophobia, and phonophobia at 2 h Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4. Pharmaceutical industry support: Pozen Inc. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy technique |
| Study size | Low risk | Treatment groups > 200 participants |
Spierings 2001.
| Methods | Multicentre, randomised, double‐blind, parallel‐group. Single dose to treat single attack. Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0.5, 1, 2, 4, 24, and 48 h after dosing Second dose of study medication available to treat recurrence between 2 and 24 h Rescue medication (excluding triptans or ergotamine) available 2 h after taking study medication if migraine pain had not decreased to mild or none |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 6‐month history of migraine (untreated severity ≥ moderate) and suffering at least 2 attacks per month, with a minimum interval of 24 h between consecutive attacks. Preventative migraine treatment was allowed, with the exception of monoamine oxidase inhibitors, lithium carbonate, cyproheptadine hydrochloride, methysergide maleate, ergotamine tartrate, and dihydroergotamine mesylate which had to be discontinued at least 2 weeks before enrolment. Participants were excluded if they had ever taken almotriptan before, but could not be triptan naive N = 1173 M 129, F 1044 (89%) Mean age 41 years Proportion with/without aura not reported |
|
| Interventions | Sumatriptan 50 mg, n = 582 Almotriptan 12.5 mg, n = 591 |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 1 and 2 h) Improvement in nausea, vomiting, photophobia, and phonophobia at 2 h Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4. Pharmaceutical industry support: Pharmacia |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Identical‐looking capsules |
| Study size | Low risk | Treatment groups > 200 participants |
Tfelt‐Hansen 1995.
| Methods | Multicentre, randomised, double‐blind, double‐dummy, placebo‐controlled, parallel‐group. Single dose to treat each of 2 consecutive attacks. Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 2 and 24 h after dosing Rescue medication (except for ergot alkaloids or morphinomimetic drugs) was allowed if the headache was inadequately controlled after 2 h |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) and suffering an average of 2 to 6 attacks per month. N = 389 (385 for efficacy) M 94, F 327 (78%) Mean age 39 years Without aura 85% |
|
| Interventions | Sumatriptan 100 mg, n = 122 Lysine acetylsalicylate 1620 mg + metoclopramide 10 mg, n = 137 Placebo, n = 126 |
|
| Outcomes | Headache relief (at 2 h) Improvement in nausea and vomiting at 2 h Patients' opinion of treatment Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4. Pharmaceutical industry support: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy technique |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
Tfelt‐Hansen 1998.
| Methods | Multicentre, randomised, double‐blind, triple‐dummy, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0.5, 1, 1.5, 2, 3, and 4 h after dosing Rescue medication was available to treat non‐response at 2 h, or recurrence within 24 of initial dosing. Sumatriptan, Midrin, and ergot derivatives were prohibited as rescue medications until 24 after initial dosing. |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 6‐month history of migraine (untreated severity ≥ moderate) and suffering an average of 1 to 8 attacks per month. Participants were excluded if they had ever been exposed to rizatriptan before No monoamine oxidase inhibitors, methysergide, or lithium within 2 weeks; sumatriptan, Midrin, or ergot derivatives within 48 h; any opiate within 24 h; or any other form of analgesia or antiemetic within 6 h of taking study medication Standard migraine prophylaxis was permitted with the exception of NSAIDs N = 1099 M 201, F 898 (82%) Mean age 38 years Without aura 84% |
|
| Interventions | Sumatriptan 100 mg, n = 388 Rizatriptan 5 mg, n = 164 Rizatriptan 10 mg, n = 387 Placebo, n = 160 |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 1 and 2 h) Improvement in nausea, photophobia, and phonophobia at 2 h Improvement in functional disability at 2 h Use of rescue medication Adverse events Withdrawals due to adverse events |
|
| Notes | Oxford Quality Score: R2, DB2, W0. Total = 4. Pharmaceutical industry support: Merck & Co. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated schedule |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Triple‐dummy technique |
| Study size | Unclear risk | Some active treatment groups > 200 participants, others and placebo treatment group 50 to 200 participants |
Tfelt‐Hansen 2006.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered within 1 h after the start of an attack, but only if the attack was still in the mild headache phase Assessments at 0.5, 1, and 2 h after dosing Second dose available to treat recurrence between 2 and 24 h Rescue medication available after 2 h if pain relief was incomplete. However, triptans or ergotamine could not be used as rescue medication within 24 of taking study medication. |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine, in which attacks became moderate or severe following an initial mild pain phase, and suffered a total of 6 to 12 attacks per year. Participants were excluded if they had treated a migraine with a triptan within the last 6 months. N = 101 M 22, F 79 (78%) Mean age 38 years Without aura 80% |
|
| Interventions | Sumatriptan 50 mg, n = 53 Placebo, n = 48 |
|
| Outcomes | Pain‐free (at 2 h) 24‐h sustained pain‐free Patients' opinion of treatment Adverse events |
|
| Notes | Oxford Quality Score: R1, DB1, W1. Total = 3. Pharmaceutical industry support: GlaxoSmithKline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Study size | High risk | Active treatment group 50 to 200 participants, placebo treatment group < 50 participants |
Thomson 1992.
| Methods | Multicentre, randomised, double‐blind, double‐dummy, parallel‐group. Single dose to treat each of up to 3 separate attacks. Assessment at 2 h after dosing. Rescue medication (not containing ergotamine, aspirin or metoclopramide) available after 2 h for inadequate headache response Minimum interval of 48 h between consecutive study treatments |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine (untreated severity ≥ moderate) and suffering an average of 1 to 6 attacks per month. All migraine prophylaxis was discontinued at least 2 weeks prior to the use of study medication No ergotamine‐containing medication within 24 h of taking study medication N = 358 (316 for efficacy) M 72, F 283 (80%) Mean age 41 years Without aura 64% |
|
| Interventions | Sumatriptan 100 mg, n = 175 (153 with moderate or severe baseline pain intensity) Aspirin 900 mg + metoclopramide 10 mg, n = 183 (163 with moderate or severe baseline pain intensity) |
|
| Outcomes | Headache relief (at 2 h) Pain‐free (at 2 h) Improvement in nausea, vomiting, and photo/phonophobia at 2 h Patients' opinion of treatment Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R2, DB2, W1. Total = 5. Pharmaceutical industry support: Glaxo Group Research Ltd. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation code |
| Allocation concealment (selection bias) | Low risk | Complete randomisation blocks allocated to centres and participants assigned in order of registration for study |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy technique |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
Visser 1996.
| Methods | Multicentre, randomised, double‐blind, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered when migraine headache pain was of moderate or severe intensity Assessments at 0.5, 1, 1.5, and 2 h after dosing Second, blinded dose of study medication available after 2 h for inadequate headache response Rescue medication (opiates, acetaminophen, or NSAIDs) available after 4 h, and sumatriptan or ergotamine‐derivatives available after 24 h |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 6‐month history of migraine (untreated severity ≥ moderate) and suffering 8 or fewer migraine attacks per month. No fluoxetine hydrochloride within 6 weeks, prophylactic antimigraine treatment within 2 weeks, ergot derivatives or sumatriptan within 48 h, opiate within 24 h, or any other form of analgesia within 6 h of taking study medication N = 449 M 47, F 402 (90%) Mean age 40 years Proportion with/without aura not reported |
|
| Interventions | Sumatriptan 100 mg, n = 72 Rizatriptan 10 mg, n = 89 Rizatriptan 20 mg, n = 82 Rizatriptan 40 mg, n = 121 Placebo, n = 85 |
|
| Outcomes | Headache relief (at 1 and 2 h) Pain‐free (at 2 h) Persistence of functional disability at 2 h Use of rescue medication Adverse events Withdrawals |
|
| Notes | Oxford Quality Score: R1, DB2, W1. Total = 4. Pharmaceutical industry support: Merck Research Laboratories |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Matching capsules |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
Winner 2003.
| Methods | Two identical studies, multicentre, randomised, double‐blind, double‐dummy, placebo‐controlled, parallel‐group. Single dose to treat single attack. Medication administered at the first sign of pain, while the pain was mild Assessments at 0.5, 1, 2, 4, and 24 h after dosing Second dose of study medication available to treat recurrence between 2 and 24 h after initial dosing Rescue medication (analgesics, antiemetics, or other acute migraine medications) available 4 h after initial dosing |
|
| Participants | Aged 18 to 65 years, meeting IHS criteria for migraine (1988) with or without aura. At least 1‐year history of migraine with an average of 1 to 6 attacks per month. All participants were required to experience moderate or severe migraine pain preceded by a mild pain phase. No use of monoamine oxidase inhibitors for a minimum of 2 weeks before screening or throughout the course of the study. Otherwise allowed to continue migraine prophylactic medications. No analgesics, antiemetics, or other migraine medication within the 6 h before taking study medication, and no ergotamine, ergot‐type medications, or other serotonin1B/1D agonists within 24 h of study medication use Study 1: N = 362 (354 for efficacy, of which 3% did not have mild pain at baseline) M 43, F 311 (88%) Mean age 41 years Without aura 73% Study 2: N = 354 (337 for efficacy, of which 4 % did not have mild pain at baseline) M 59, F 298 (88%) Mean age 43 years Without aura 79% |
|
| Interventions | Study 1: Sumatriptan 50 mg, n = 122 Sumatriptan 100 mg, n = 115 Placebo, n = 117 Study 2: Sumatriptan 50 mg, n = 111 Sumatriptan 100 mg, n = 107 Placebo, n = 119 |
|
| Outcomes | Pain‐free (at 1 and 2 h) Improvement in nausea, photophobia, and phonophobia at 2 h Use of rescue medication Adverse events |
|
| Notes | Oxford Quality Score: R2, DB2, W0. Total = 4. Pharmaceutical industry support: GlaxoSmithKline |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation schedule |
| Allocation concealment (selection bias) | Low risk | Treatment assignment sealed and remained intact throughout the study |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐dummy technique |
| Study size | Unclear risk | Treatment groups 50 to 200 participants |
DB: double‐blinding; F: female; h: hour; HIQ: Headache Impact Questionnaire; IHS: International Headache Society; M: male; NSAID: non‐steroidal anti‐inflammatory drug; R: randomisation; W: withdrawals
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cady 1994 | First dose of subcutaneous sumatriptan not randomised, only for subsequent doses of oral sumatriptan for recurrence (from 2 to 24 h after initial dosing) were patients randomised to either sumatriptan or placebo |
| Cady 2000 | Insufficient number of participants in placebo group (≤ 10) |
| Centonze 1995 | No IHS diagnosis |
| Colman 2001 | No useable outcomes reported |
| Dowson 2000 | No IHS diagnosis of migraine ‐ attacks were GP diagnosed with no subsequent re‐analysis according to IHS criteria |
| Dowson 2005 | No useable data ‐ only 4 h data reported and number of patients analysed for particular outcome uninterpretable |
| Ferrari 1994 | First dose of sumatriptan not randomised, only for a second dose (after 2 h) and potentially a third dose for subsequent recurrence were patients randomised to either sumatriptan or placebo |
| Gobel 2000 | No useable data ‐ only 4 h efficacy data reported |
| Landy 2004 (Study 1) | High proportion of patients treated moderate or severe headache when protocol required treatment of headache when mild, therefore baseline pain intensity too heterogeneous to interpret results. Also discrepancies in reporting make number of patients analysed for a particular outcome un‐interpretable. |
| Midelfart 1994 | No useable data ‐ only 4 h data reported and number of patients analysed for particular outcome un‐interpretable |
| Padma 1998 | Inadequate randomisation ‐ alternating allocation |
| Pradel 2005 | No useable data ‐ no outcomes reported for individual study treatments |
| Rapoport 1995 | First dose of subcutaneous sumatriptan not randomised, only for a second dose (after 4 h) and potentially a third dose for subsequent recurrence were patients randomised to either oral sumatriptan or placebo |
| Rederich 1995 | No useable data ‐ outcomes reported as % of patients experiencing outcome, but number of patients analysed for particular outcomes not reported |
| Salonen 1999 | Only compares different doses of sumatriptan, no placebo or alternative active comparator |
| Savani 2001 | One dose of sumatriptan compared against another with no placebo control, and baseline population enriched/selected for tolerance of adverse events and non‐response to 50 mg sumatriptan |
| Scott 1996 | First dose of sumatriptan not randomised, only for a second dose (after 4 h) and potentially a third dose for subsequent recurrence were patients randomised to either sumatriptan or placebo |
| Sramek 1999 | No useable data ‐ only pooled data for 3 different doses of sumatriptan reported |
| SUMA4014 | First dose of sumatriptan is non‐randomised and only single‐blinded; only participants who fail to respond to the initial dose are given a subsequent randomised, double‐blinded dose of sumatriptan (25 mg or 50 mg) or placebo |
| Tepper 2006 | Only attacks meeting IHS criteria for probably migraine (2004 IHS) or migrainous disorder (1988 IHS) eligible |
| Tfelt‐Hansen 2000 | Data reported in Tfelt‐Hansen 1995 |
| Wells 2001 | No useable data ‐ efficacy data reported in Sandrini 2002 |
| Wells 2003 | No useable data ‐ efficacy data reported in Sandrini 2002 |
GP: general practitioner; h: hour; IHS: International Headache Society
Differences between protocol and review
We have considered data for three outcomes not specified in the protocol.
Use of rescue medication was reported by the majority of studies, and provides a measure of efficacy from the point of view of the patient. In taking rescue medication the patient is saying that the efficacy of the medication is not adequate and that they need alternative analgesia. They are effectively withdrawing due to lack of efficacy, where efficacy is defined by their preparedness to carry on without additional analgesia, rather than a predefined outcome such as headache relief at two hours. We believe this is useful additional information relevant to clinical practice.
Pain‐free at one hour provides, along with headache relief at one hour, a measure of the speed of onset of the medication. This is an important feature of some anti‐migraine treatments and can vary significantly between different routes of administration of the same drug. We chose to analyse pain‐free at one hour to provide a stringent measure of the early efficacy of oral sumatriptan, which we believe to be important information for clinical practice.
Headache relief over multiple (two or three) attacks was reported in five studies. We chose to analyse this because it provides useful information about whether initial response to medication is maintained in subsequent attacks.
Several studies allowed participants the option of a second dose of study medication under certain conditions and apparently continued to collect adverse event data after this. We performed sensitivity analyses to investigate the possible effect on the incidence of adverse events of including these studies.
We have included data for withdrawals due to adverse events over reporting periods longer than the 24 hours stated in the protocol. Many studies collected adverse event data for longer than 24 hours after treatment, and it is likely that in these cases data on withdrawals due to adverse events were also collected over longer time periods. Adverse event withdrawals were infrequent in all of the trials reporting, regardless of the time period over which they were collected, but are an important measure of drug safety and tolerability. We therefore decided to be as inclusive as possible with data on adverse event withdrawals, in the hope of providing the most comprehensive picture possible of sumatriptan tolerability.
For calculations of susceptibility to publication bias we have used a NNT of ≥ 8 as the limit of clinical utility for pain‐free at two hours and ≥ 6 for headache relief at two hours. In the protocol we said we would use a NNT of ≥ 8 for headache relief at two hours, but made the change following a discussion with the field editor.
Contributions of authors
SD and RAM wrote the protocol. CD and SD carried out searches, data extraction, and analyses. RAM acted as arbitrator. All authors were involved with writing the final review.
Sources of support
Internal sources
Oxford Pain Relief Trust, UK.
External sources
Cochrane Review Incentive Scheme 2010, UK.
-
Lifting The Burden: the Global Campaign against Headache, UK.
Funding for administrative costs associated with editorial and peer review
Declarations of interest
RAM and SD have received research support from charities, government, and industry sources at various times. RAM has consulted for various pharmaceutical companies, including GlaxoSmithKline, the manufacturers of sumatriptan. RAM has received lecture fees from pharmaceutical companies related to analgesics and other healthcare interventions. CD has no interests to declare. GlaxoSmithKline were not in any way involved in conducting this review.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
160‐104 {unpublished data only}
- A multicentre, double‐blind, placebo controlled, parallel group study of two dose levels of oral eletriptan and two dose levels of oral sumatriptan given for the acute treatment of migraine. Pfizer clinical trial report 1998.
Banerjee 1992 {published data only}
- Banerjee M, Findley LJ. Sumatriptan in the treatment of acute migraine with aura. Cephalalgia 1992;12(1):39‐44. [DOI] [PubMed] [Google Scholar]
Brandes 2007 {published data only}
- Brandes JL, Kudrow D, Stark SR, O'Carroll CP, Adelman JU, O'Donnell FJ, et al. Sumatriptan‐naproxen for acute treatment of migraine: a randomized trial. JAMA 2007;297(13):1443‐54. [DOI] [PubMed] [Google Scholar]
- Landy S, DeRossett SE, Rapoport A, Rothrock J, Ames MH, McDonald SA, et al. Two double‐blind, multicenter, randomized, placebo‐controlled, single‐dose studies of sumatriptan/naproxen sodium in the acute treatment of migraine: function, productivity, and satisfaction outcomes. MedGenMed 2007;9(2):53. [PMC free article] [PubMed] [Google Scholar]
Bussone 2000 {published data only}
- Bussone G, Manzoni GC, Cortelli P, Roncolato M, Fabbri L, Benassuti C. Efficacy and tolerability of sumatriptan in the treatment of multiple migraine attacks. Journal of the Neurological Sciences 2000;21(5):272‐8. [DOI: 10.1007/%24100720070064] [DOI] [PubMed] [Google Scholar]
Carpay 2004 {published data only}
- Barbanti P, Carpay JA, Kwong WJ, Ahmad F, Boswell D. Effects of a fast disintegrating/rapid release oral formulation of sumatriptan on functional ability in patients with migraine. Current Medical Research and Opinion 2004;20(12):2021‐9. [DOI] [PubMed] [Google Scholar]
- Carpay J, Schoenen J, Ahmad F, Kinrade F, Boswell D. Efficacy and tolerability of sumatriptan tablets in a fast‐disintegrating, rapid‐release formulation for the acute treatment of migraine: results of a multicenter, randomized, placebo‐controlled study. Clinical Therapeutics 2004;26(2):214‐23. [DOI: ] [DOI] [PubMed] [Google Scholar]
Cutler 1995 {published data only}
- Cutler N, Mushet GR, Davis R, Clements B, Whitcher L. Oral sumatriptan for the acute treatment of migraine: evaluation of three dosage strengths. Neurology 1995;45(8 Suppl 7):S5‐9. [PubMed] [Google Scholar]
Dahlof 1991 {published data only}
- The Oral Sumatriptan Dose‐Defining Study Group. Sumatriptan‐‐an oral dose‐defining study. European Neurology 1991;31(5):300‐5. [DOI] [PubMed] [Google Scholar]
Dahlof 2009 {published data only}
- Dahlof CG, Hauge AW, Olesen J. Efficacy and safety of tonabersat, a gap‐junction modulator, in the acute treatment of migraine: a double‐blind, parallel‐group, randomized study. Cephalalgia 2009;29 Suppl 2:7‐16. [PUBMED: 19723121] [DOI] [PubMed] [Google Scholar]
Diener 2004a {published data only}
- Diener HC, Eikermann A, Gessner U, Gobel H, Haag G, Lange R, et al. Efficacy of 1,000 mg effervescent acetylsalicylic acid and sumatriptan in treating associated migraine symptoms. European Neurology 2004;52(1):50‐6. [DOI: 10.1159/000079544] [DOI] [PubMed] [Google Scholar]
Diener 2004b {published data only}
- Diener HC, Bussone G, Liano H, Eikermann A, Englert R, Floeter T, et al. Placebo‐controlled comparison of effervescent acetylsalicylic acid, sumatriptan and ibuprofen in the treatment of migraine attacks. Cephalalgia 2004;24(11):947‐54. [PUBMED: 15482357] [DOI] [PubMed] [Google Scholar]
DKSMSG 1999 {published data only}
- The Diclofenac‐K/Sumatriptan Migraine Study Group. Acute treatment of migraine attacks: efficacy and safety of a nonsteroidal anti‐inflammatory drug, diclofenac‐potassium, in comparison to oral sumatriptan and placebo. Cephalalgia 1999;19(4):232‐40. [DOI] [PubMed] [Google Scholar]
Dodick 2002 {published data only}
- Dodick DW. Almotriptan increases sustained pain‐free outcomes in acute migraine: results from three controlled clinical trials. Headache 2002;42(1):21‐7. [DOI: 10.1046/j.1526-4610.2002.02009.x] [DOI] [PubMed] [Google Scholar]
Dowson 2002 {published data only}
- Dowson AJ, Massiou H, Lainez JM, Cabarrocas X. Almotriptan improves response rates when treatment is within 1 hour of migraine onset. Headache 2004;44(4):318‐22. [DOI] [PubMed] [Google Scholar]
- Dowson AJ, Massiou H, LaÍnez JM, Cabarrocas X. Almotriptan is an effective and well‐tolerated treatment for migraine pain: results of a randomized, double‐blind, placebo‐controlled clinical trial. Cephalalgia 2002;22(6):453‐61. [PUBMED: 12133045] [DOI] [PubMed] [Google Scholar]
Ensink 1991 {published data only}
- Ensink FB, Sumatriptan International Study Group. Subcutaneous sumatriptan in the acute treatment of migraine. Journal of Neurology 1991;238 Suppl 1:S66‐9. [PUBMED: 1646291] [DOI] [PubMed] [Google Scholar]
Freitag 2001 {published data only}
- Freitag FG, Cady R, DiSerio F, Elkind A, Gallagher RM, Goldstein J, et al. Comparative study of a combination of isometheptene mucate, dichloralphenazone with acetaminophen and sumatriptan succinate in the treatment of migraine. Headache 2001;41(4):391‐8. [DOI: 10.1046/j.1526-4610.2001.111006391.x] [DOI] [PubMed] [Google Scholar]
Gallagher 2000 {published data only}
- Gallagher RM, Dennish G, Spierings EL, Chitra R. A comparative trial of zolmitriptan and sumatriptan for the acute oral treatment of migraine. Headache 2000;40(2):119‐28. [DOI: 10.1046/j.1526-4610.2000.00017.x] [DOI] [PubMed] [Google Scholar]
Geraud 2000 {published data only}
- Geraud G, Olesen J, Pfaffenrath V, Tfelt‐Hansen P, Zupping R, Diener HC, et al. Comparison of the efficacy of zolmitriptan and sumatriptan: issues in migraine trial design. Cephalalgia 2000;20(1):30‐8. [PUBMED: 10817444] [DOI] [PubMed] [Google Scholar]
GL/MIG/001/92 {unpublished data only}
- A double‐blind, general practice study to compare GR43175 with paracetamol and metoclopramide in the acute treatment of migraine (Original protocol). www.gsk‐clinicalstudyregister.com/ 1992.
GL/MIG/001A/92 {unpublished data only}
- A double‐blind, general practice study to compare GR43175 with paracetamol and metoclopramide in the acute treatment of migraine (Amended protocol). www.gsk‐clinicalstudyregister.com/ 1992.
GL/MIG/002 {unpublished data only}
- A double‐blind general practice study to compare sumatriptan with Migraleve in the acute treatment of migraine (Original protocol). www.gsk‐clinicalstudyregister.com/ 1990.
GL/MIG/002A {unpublished data only}
- A double‐blind general practice study to compare sumatriptan with Migraleve in the acute treatment of migraine (Amended protocol). www.gsk‐clinicalstudyregister.com/ 1991.
GL/MIG/009 {unpublished data only}
- A double blind study to compare acute sumatriptan with Migril in the treatment of migraine. www.gsk‐clinicalstudyregister.com/ 1991.
Goadsby 1991 {published data only}
- Goadsby PJ, Zagami AS, Donnan GA, Symington G, Anthony M, Bladin PF, et al. Oral sumatriptan in acute migraine. Lancet 1991;338(8770):782‐3. [DOI: 10.1016/0140-6736(91)90666-D] [DOI] [PubMed] [Google Scholar]
Goadsby 2000 {published data only}
- Goadsby PJ, Ferrari MD, Olesen J, Stovner LJ, Senard JM, Jackson NC, et al. Eletriptan Steering Committee. Eletriptan in acute migraine: a double‐blind, placebo‐controlled comparison to sumatriptan. Neurology 2000;54(1):156‐63. [DOI] [PubMed] [Google Scholar]
Goldstein 1998 {published data only}
- Goldstein J, Ryan R, Jiang K, Getson A, Norman B, Block GA, et al. Rizatriptan Protocol 046 Study Group. Crossover comparison of rizatriptan 5 mg and 10 mg versus sumatriptan 25 mg and 50 mg in migraine. Headache 1998;38(10):737‐47. [DOI: 10.1046/j.1526-4610.1998.3810737.x] [DOI] [PubMed] [Google Scholar]
Goldstein 2005 {published data only}
- Goldstein J, Silberstein SD, Saper JR, Elkind AH, Smith TR, Gallagher RM, et al. Acetaminophen, aspirin, and caffeine versus sumatriptan succinate in the early treatment of migraine: results from the ASSET trial. Headache 2005;45(8):973‐82. [DOI: 10.1111/j.1526-4610.2005.05177.x] [DOI] [PubMed] [Google Scholar]
Gruffyd‐Jones 2001 {published data only}
- Gruffyd‐Jones K, Kies B, Middleton A, Mulder LJ, Røsjø Ø, Millson DS. Zolmitriptan versus sumatriptan for the acute oral treatment of migraine: a randomized, double‐blind, international study. European Journal of Neurology 2001;8(3):237‐45. [DOI] [PubMed] [Google Scholar]
Havanka 2000 {published data only}
- Havanka H, Dahlöf C, Pop PH, Diener HC, Winter P, Whitehouse H, et al. Naratriptan S2WB2004 Study Group. Efficacy of naratriptan tablets in the acute treatment of migraine: a dose‐ranging study. Clinical Therapeutics 2000;22(8):970‐80. [DOI: 10.1016/S0149-2918(00)80068-5] [DOI] [PubMed] [Google Scholar]
Ishkanian 2007 {published data only}
- Ishkanian G, Blumenthal H, Webster CJ, Richardson MS, Ames M. Efficacy of sumatriptan tablets in migraineurs self‐described or physician‐diagnosed as having sinus headache: a randomized, double‐blind, placebo‐controlled study. Clinical Therapeutics 2007;29(1):99‐109. [DOI: 10.1016/j.clinthera.2007.01.012] [DOI] [PubMed] [Google Scholar]
Jelinski 2006 {published data only}
- Jelinski SE, Becker WJ, Christie SN, Ahmad FE, Pryse‐Phillips W, Simpson SD. Pain free efficacy of sumatriptan in the early treatment of migraine. Canadian Journal of Neurological Sciences 2006;33(1):73‐9. [http://cjns.metapress.com/openurl.asp?genre=article&issn=0317‐1671&volume=33&issue=2&spage=228] [DOI] [PubMed] [Google Scholar]
- SUM40291. A randomized, double‐blind, placebo‐controlled, parallel‐group, single‐attack evaluation of sumatriptan 50 mg and 100 mg versus placebo during a migraine headache at the first sign of pain. www.gsk‐clinicalstudyregister.com/ 2001.
Kaniecki 2006 {published data only}
- Kaniecki R, Ruoff G, Smith T, Barrett PS, Ames MH, Byrd S, et al. Prevalence of migraine and response to sumatriptan in patients self‐reporting tension/stress headache. Current Medical Research and Opinion 2006;22(8):1535‐44. [DOI: 10.1185/030079906X118685] [DOI] [PubMed] [Google Scholar]
Kolodny 2004 {published data only}
- Kolodny A, Polis A, Battisti WP, Johnson‐Pratt L, Skobieranda F, Rizatriptan Protocol 052 Study Group. Comparison of rizatriptan 5 mg and 10 mg tablets and sumatriptan 25 mg and 50 mg tablets. Cephalalgia 2004;24(7):540‐6. [DOI] [PubMed] [Google Scholar]
Kudrow 2005 {published data only}
- Kudrow D, Thomas HM, Ruoff G, Ishkanian G, Sands G, Le VH, et al. Valdecoxib for treatment of a single, acute, moderate to severe migraine headache. Headache 2005;45(9):1151‐62. [DOI: 10.1111/j.1526-4610.2005.00238.x] [DOI] [PubMed] [Google Scholar]
Latere 1991 {published data only}
- The Multinational Oral Sumatriptan and Cafergot Comparative Study Group. A randomized, double‐blind comparison of sumatriptan and Cafergot in the acute treatment of migraine. European Neurology 1991;31(5):314‐22. [DOI] [PubMed] [Google Scholar]
Lines 2001 {published data only}
- Lines CR, Vandormael K, Malbecq W. A comparison of visual analog scale and categorical ratings of headache pain in a randomized controlled clinical trial with migraine patients. Pain 2001;93(2):185‐90. [DOI: 10.1016/S0304-3959(01)00315-3] [DOI] [PubMed] [Google Scholar]
Lipton 2000 {published data only}
- Lipton RB, Stewart WF, Cady R, Hall C, O'Quinn S, Kuhn T, et al. 2000 Wolfe Award. Sumatriptan for the range of headaches in migraine sufferers: results of the Spectrum Study. Headache 2000;40(10):783‐91. [DOI: 10.1111/j.1526-4610.2000.00143.x] [DOI] [PubMed] [Google Scholar]
Mathew 2003 {published data only}
- Mathew NT, Schoenen J, Winner P, Muirhead N, Sikes CR. Comparative efficacy of eletriptan 40 mg versus sumatriptan 100 mg. Headache 2003;43(3):214‐22. [DOI: 10.1046/j.1526-4610.2003.03044.x] [DOI] [PubMed] [Google Scholar]
Myllyla 1998 {published data only}
- Myllylä VV, Havanka H, Herrala L, Kangasniemi P, Rautakorpi I, Turkka J, et al. Tolfenamic acid rapid release versus sumatriptan in the acute treatment of migraine: comparable effect in a double‐blind, randomized, controlled, parallel‐group study. Headache 1998;38(3):201‐7. [DOI: 10.1046/j.1526-4610.1998.3803201.x] [DOI] [PubMed] [Google Scholar]
Nappi 1994 {published data only}
- Nappi G, Sicuteri F, Byrne M, Roncolato M, Zerbini O. Oral sumatriptan compared with placebo in the acute treatment of migraine. Journal of Neurology 1994;241(3):138‐44. [DOI] [PubMed] [Google Scholar]
Nett 2003 {published data only}
- Landy S, Savani N, Shackelford S, Loftus J, Jones M. Efficacy and tolerability of sumatriptan tablets administered during the mild‐pain phase of menstrually associated migraine. International Journal of Clinical Practice 2004;58(10):913‐9. [DOI: 10.1111/j.1368-5031.2004.00295.x] [DOI] [PubMed] [Google Scholar]
- Nett R, Landy S, Shackelford S, Richardson MS, Ames M, Lener M. Pain‐free efficacy after treatment with sumatriptan in the mild pain phase of menstrually associated migraine. Journal of Obstetrics and Gynecology 2003;102(4):835‐42. [DOI] [PubMed] [Google Scholar]
Patten 1991 {published data only}
- Patten JP. Clinical experience with oral sumatriptan: a placebo‐controlled, dose‐ranging study. Oral Sumatriptan Dose‐defining Study Group. Journal of Neurology 1991;238 Suppl 1:S62‐5. [DOI] [PubMed] [Google Scholar]
Pfaffenrath 1998 {published data only}
- Pfaffenrath V, Cunin G, Sjonell G, Prendergast S. Efficacy and safety of sumatriptan tablets (25 mg, 50 mg, and 100 mg) in the acute treatment of migraine: defining the optimum doses of oral sumatriptan. Headache 1998;38(3):184‐90. [DOI: 10.1046/j.1526-4610.1998.3803184.x] [DOI] [PubMed] [Google Scholar]
Pini 1995 {published data only}
- Pini LA, Sternieri E, Fabbri L, Zerbini O, Bamfi F, The Oral Sumatriptan Italian Study Group. High efficacy and low frequency of headache recurrence after oral sumatriptan. Journal of International Medical Research 1995;23(2):96‐105. [DOI] [PubMed] [Google Scholar]
Pini 1999 {published data only}
- Pini LA, Fabbri L, Cavazzuti L, The Sumatriptan 50 mg Italian Study Group. Efficacy and safety of sumatriptan 50 mg in patients not responding to standard care, in the treatment of mild to moderate migraine. International Journal of Clinical Pharmacology Research 1999;19(2):57‐64. [PubMed] [Google Scholar]
Sandrini 2002 {published data only}
- Sandrini G, Färkkilä M, Burgess G, Forster E, Haughie S, Eletriptan Steering Committee. Eletriptan vs sumatriptan: a double‐blind, placebo‐controlled, multiple migraine attack study. Neurology 2002;59(8):1210‐7. [DOI] [PubMed] [Google Scholar]
Sandrini 2007 {published data only}
- Sandrini G, Cerbo R, Bene E, Ferrari A, Genco S, Grazioli I, et al. Efficacy of dosing and re‐dosing of two oral fixed combinations of indomethacin, prochlorperazine and caffeine compared with oral sumatriptan in the acute treatment of multiple migraine attacks: a double‐blind, double‐dummy, randomised, parallel group, multicentre study. International Journal of Clinical Practice 2007;61(8):1256‐69. [DOI: 10.1111/j.1742-1241.2007.01458.x] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sargent 1995 {published data only}
- Sargent J, Kirchner JR, Davis R, Kirkhart B. Oral sumatriptan is effective and well tolerated for the acute treatment of migraine: results of a multicenter study. Neurology 1995;45(8 Suppl 7):S10‐4. [PubMed] [Google Scholar]
Savani 1999 {published data only}
- Savani N, Brautaset NJ, Reunanen M, Szirmai I, Ashford EA, Hassani H, et al. Sumatriptan Tablets S2CM07 Study Group. A double‐blind placebo‐controlled study assessing the efficacy and tolerability of 50 mg sumatriptan tablets in the acute treatment of migraine. International Journal of Clinical Practice. Supplement 1999;105:7‐15. [PubMed] [Google Scholar]
Schulman 2003 {published data only}
- Schulman EA, Dermott KF. Sumatriptan plus metoclopramide in triptan‐nonresponsive migraineurs. Headache 2003;43(7):729‐33. [DOI: 10.1046/j.1526-4610.2003.03130.x] [DOI] [PubMed] [Google Scholar]
Sheftell 2005 {published data only}
- Sheftell FD, Dahlöf CG, Brandes JL, Agosti R, Jones MW, Barrett PS. Two replicate randomized, double‐blind, placebo‐controlled trials of the time to onset of pain relief in the acute treatment of migraine with a fast‐disintegrating/rapid‐release formulation of sumatriptan tablets. Clinical Therapeutics 2005;27(4):407‐17. [DOI] [PubMed] [Google Scholar]
Smith 2005 {published data only}
- Smith TR, Sunshine A, Stark SR, Littlefield DE, Spruill SE, Alexander WJ. Sumatriptan and naproxen sodium for the acute treatment of migraine. Headache 2005;45(8):983‐91. [DOI: 10.1111/j.1526-4610.2005.05178.x] [DOI] [PubMed] [Google Scholar]
Spierings 2001 {published data only}
- Spierings EL, Gomez‐Mancilla B, Grosz DE, Rowland CR, Whaley FS, Jirgens KJ. Oral almotriptan vs. oral sumatriptan in the abortive treatment of migraine: a double‐blind, randomized, parallel‐group, optimum‐dose comparison. Archives of Neurology 2001;58(6):944‐50. [DOI] [PubMed] [Google Scholar]
Tfelt‐Hansen 1995 {published data only}
- Tfelt‐Hansen P, Henry P, Mulder LJ, Scheldewaert RG, Schoenen J, Chazot G. The combination of oral lysine‐acetylsalicylate and metoclopramide compared with oral sumatriptan in the treatment of migraine attacks. A randomized, double‐blind, placebo‐controlled clinical trial. Ugeskrift for Laeger 1996;158(45):6435‐9. [PubMed] [Google Scholar]
- Tfelt‐Hansen P, Henry P, Mulder LJ, Scheldewaert RG, Schoenen J, Chazot G. The effectiveness of combined oral lysine acetylsalicylate and metoclopramide compared with oral sumatriptan for migraine. Lancet 1995;346(8980):923‐6. [DOI] [PubMed] [Google Scholar]
Tfelt‐Hansen 1998 {published data only}
- Tfelt‐Hansen P, Teall J, Rodriguez F, Giacovazzo M, Paz J, Malbecq W, et al. Rizatriptan 030 Study Group. Oral rizatriptan versus oral sumatriptan: a direct comparative study in the acute treatment of migraine. Headache 1998;38(10):748‐55. [DOI: 10.1046/j.1526-4610.1998.3810748.x] [DOI] [PubMed] [Google Scholar]
Tfelt‐Hansen 2006 {published data only}
- Tfelt‐Hansen P, Bach FW, Daugaard D, Tsiropoulos I, Riddersholm B. Treatment with sumatriptan 50 mg in the mild phase of migraine attacks in patients with infrequent attacks: a randomised, double‐blind, placebo‐controlled study. Journal of Headache and Pain 2006;7(6):389‐94. [DOI: 10.1007/s10194-006-0333-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomson 1992 {published data only}
- The Oral Sumatriptan and Aspirin plus Metoclopramide Comparative Study Group. A study to compare oral sumatriptan with oral aspirin plus oral metoclopramide in the acute treatment of migraine. European Neurology 1992;32(3):177‐84. [DOI] [PubMed] [Google Scholar]
Visser 1996 {published data only}
- Visser WH, Terwindt GM, Reines SA, Jiang K, Lines CR, Ferrari MD. Rizatriptan vs sumatriptan in the acute treatment of migraine. A placebo‐controlled, dose‐ranging study. Dutch/US Rizatriptan Study Group. Archives of Neurology 1996;53(11):1132‐7. [DOI] [PubMed] [Google Scholar]
Winner 2003 {published data only}
- Winner P, Mannix LK, Putnam DG, McNeal S, Kwong J, O'Quinn S, et al. Pain‐free results with sumatriptan taken at the first sign of migraine pain: 2 randomized, double‐blind, placebo‐controlled studies. Mayo Clinic Proceedings 2003;78(10):1214‐22. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Cady 1994 {published data only}
- Cady RK, Rubino J, Crummett D, Littlejohn TW 3rd. Oral sumatriptan in the treatment of recurrent headache. Archives of Family Medicine 1994;3(9):766‐72. [DOI] [PubMed] [Google Scholar]
Cady 2000 {published data only}
- Cady RK, Lipton RB, Hall C, Stewart WF, O'Quinn S, Gutterman D. Treatment of mild headache in disabled migraine sufferers: results of the Spectrum Study. Headache 2000;40(10):792‐7. [DOI] [PubMed] [Google Scholar]
Centonze 1995 {published data only}
- Centonze V, Polito MB, Bari M, Fabbri L, Cassiano MA, Bassi A, et al. Evaluation of the efficacy of oral sumatriptan in the management of migraine attacks. Clinical results [Italian]. La Clinica Terapeutica 1995;146(11):721‐8. [PubMed] [Google Scholar]
Colman 2001 {published data only}
- Colman SS, Brod MI, Krishnamurthy A, Rowland CR, Jirgens KJ, Gomez‐Mancilla B. Treatment satisfaction, functional status, and health‐related quality of life of migraine patients treated with almotriptan or sumatriptan. Clinical Therapeutics 2001;23(1):127‐45. [DOI] [PubMed] [Google Scholar]
Dowson 2000 {published data only}
- Dowson A, Ball K, Haworth D. Comparison of a fixed combination of domperidone and paracetamol (Domperamol) with sumatriptan 50 mg in moderate to severe migraine: a randomised UK primary care study. Current Medical Research and Opinion 2000;16(3):190‐7. [DOI: ] [PubMed] [Google Scholar]
Dowson 2005 {published data only}
- Dowson AJ, Massiou H, Aurora SK. Managing migraine headaches experienced by patients who self‐report with menstrually related migraine: a prospective, placebo‐controlled study with oral sumatriptan. Journal of Headache and Pain 2005;6(2):81‐7. [DOI: 10.1007/s10194-005-0156-3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferrari 1994 {published data only}
- Ferrari MD, James MH, Bates D, Pilgrim A, Ashford E, Anderson BA, et al. Oral sumatriptan: effect of a second dose, and incidence and treatment of headache recurrences. Cephalalgia 1994;14(5):330‐8. [DOI] [PubMed] [Google Scholar]
Gobel 2000 {published data only}
- Göbel H, Winter P, Boswell D, Crisp A, Becker W, Hauge T, et al. Naratriptan International Recurrence Study Group. Comparison of naratriptan and sumatriptan in recurrence‐prone migraine patients. Clinical Therapeutics 2000;22(8):981‐9. [DOI: 10.1016/S0149-2918(00)80069-7] [DOI] [PubMed] [Google Scholar]
Landy 2004 (Study 1) {published data only}
- Landy S, Savani N, Shackelford S, Loftus J, Jones M. Efficacy and tolerability of sumatriptan tablets administered during the mild‐pain phase of menstrually associated migraine. International Journal of Clinical Practice 2004;58(10):913‐9. [DOI: 10.1111/j.1368-5031.2004.00295.x] [DOI] [PubMed] [Google Scholar]
Midelfart 1994 {published data only}
- Midelfart E, Winnem M. Sumatriptan in the treatment of migraine in general practice [Norwegian]. Tidsskr Nor Laegeforen 1994;114(29):3430‐2. [PubMed] [Google Scholar]
Padma 1998 {published data only}
- Padma MV, Jain S, Maheshwari MC, Misra S, Karak B, Singh AK, et al. Efficacy and tolerability of oral sumatriptan in Indian patients with acute migraine; a multicentre study. Neurology India 1998;46(2):105‐8. [PubMed] [Google Scholar]
Pradel 2005 {published data only}
- Pradel FG, Subedi P, Varghese AA, Mullins CD, Weis KA. Does earlier headache response equate to earlier return to functioning in patients suffering from migraine?. Cephalalgia 2005;26(4):428‐35. [DOI] [PubMed] [Google Scholar]
Rapoport 1995 {published data only}
- Rapoport AM, Visser WH, Cutler NR, Alderton CJ, Paulsgrove LA, Davis RL, et al. Oral sumatriptan in preventing headache recurrence after treatment of migraine attacks with subcutaneous sumatriptan. Neurology 1995;45(8):1505‐9. [DOI] [PubMed] [Google Scholar]
Rederich 1995 {published data only}
- Rederich G, Rapoport A, Cutler N, Hazelrigg R, Jamerson B. Sumatriptan for the long‐term treatment of migraine: clinical findings. Neurology 1995;45(8 Suppl 7):S15‐20. [PubMed] [Google Scholar]
Salonen 1999 {published data only}
- Salonen R, Ashford EA, Gibbs M, Hassani H. Patient preference for oral sumatriptan 25 mg, 50 mg, or 100 mg in the acute treatment of migraine: a double‐blind, randomized, crossover study. Sumatriptan Tablets S2CM11 Study Group. International Journal of Clinical Practice. Supplement 1999;105:16‐24. [PubMed] [Google Scholar]
Savani 2001 {published data only}
- Savani N, Pfaffenrath V, Rice L, Boswell D, Black L, Jones M. Sumatriptan SUMB4007 Study Group. Efficacy, tolerability, and patient satisfaction with 50‐ and 100‐mg sumatriptan tablets in those initially dissatisfied with the efficacy of 50‐mg sumatriptan tablets. Clinical Therapeutics 2001;23(2):260‐71. [DOI: 10.1016/S0149-2918(01)80008-4] [DOI] [PubMed] [Google Scholar]
Scott 1996 {published data only}
- Scott RJ, Aitchison WR, Barker PR, McLaren GI. Oral sumatriptan in the acute treatment of migraine and migraine recurrence in general practice. QJM 1996;89(8):613‐22. [DOI] [PubMed] [Google Scholar]
Sramek 1999 {published data only}
- Sramek JJ, Hussey EK, Clements B, Cutler NR. Oral sumatriptan pharmacokinetics in the migraine state. Clinical Drug Investigation 1999;17(2):137‐44. [Google Scholar]
SUMA4014 {unpublished data only}
- A double‐blind, placebo‐controlled, parallel group study to evaluate the efficacy of a second sumatriptan succinate tablet (25 or 50 mg) in the acute treatment of migraine. www.gsk‐clinicalstudyregister.com/ 1998.
Tepper 2006 {published data only}
- Tepper SJ, Cady R, Dodick D, Freitag FG, Hutchinson SL, Twomey C, et al. Oral sumatriptan for the acute treatment of probable migraine: first randomized, controlled study. Headache 2006;46(1):115‐24. [DOI: 10.1111/j.1526-4610.2006.00300.x] [DOI] [PubMed] [Google Scholar]
Tfelt‐Hansen 2000 {published data only}
- Tfelt‐Hansen P. The effectiveness of combined oral lysine acetylsalicylate and metoclopramide (Migpriv) in the treatment of migraine attacks. Comparison with placebo and oral sumatriptan. Functional Neurology 2000;15 Suppl 3:196‐201. [PubMed] [Google Scholar]
Wells 2001 {published data only}
- Wells NE. Comparison of the effectiveness of eletriptan, sumatriptan and Cafergot in reducing the time loss associated with migraine attacks. Journal of Medical Economics 2001;4:157‐66. [Google Scholar]
Wells 2003 {published data only}
- Wells N, Hettiarachchi J, Drummond M, Carter D, Parpia T, Pang F. A cost‐effectiveness analysis of eletriptan 40 and 80 mg versus sumatriptan 50 and 100 mg in the acute treatment of migraine. Value Health 2003;6(4):438‐47. [DOI] [PubMed] [Google Scholar]
Additional references
Bigal 2008
- Bigal ME, Serrano D, Reed M, Lipton RB. Chronic migraine in the population: burden, diagnosis, and satisfaction with treatment. Neurology 2008;71(8):559‐66. [DOI: 10.1212/01.wn1.0000323925.29520.e7] [DOI] [PubMed] [Google Scholar]
Clarke 1996
- Clarke CE, MacMillan L, Sondhi S, Wells NEJ. Economic and social impact of migraine. Quarterly Journal of Medicine 1996;89(1):77‐84. [DOI] [PubMed] [Google Scholar]
Cook 1995
- Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ 1995;310(6977):452‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry (forthcoming)
- Derry CJ, Derry S, Moore RA. Sumatriptan (all routes of administration) for acute migraine attacks in adults: an overview of Cochrane reviews. Cochrane Database of Systematic Reviews (forthcoming). [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2012a
- Derry CJ, Derry S, Moore RA. Sumatriptan (oral route of administration) for acute migraine attacks in adults. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD008615] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2012b
- Derry CJ, Derry S, Moore RA. Sumatriptan (subcutaneous route of administration) for acute migraine attacks in adults. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD009665] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2012c
- Derry CJ, Derry S, Moore RA. Sumatriptan (intranasal route of administration) for acute migraine attacks in adults. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD009663] [DOI] [PMC free article] [PubMed] [Google Scholar]
Derry 2012d
- Derry CJ, Derry S, Moore RA. Sumatriptan (rectal route of administration) for acute migraine attacks in adults. Cochrane Database of Systematic Reviews 2012, Issue 2. [DOI: 10.1002/14651858.CD009664] [DOI] [PMC free article] [PubMed] [Google Scholar]
Diamond 2007
- Diamond S, Bigal ME, Silberstein S, Loder E, Reed M, Lipton RB. Patterns of diagnosis and acute and preventive treatment for migraine in the United States: results from the American Migraine Prevalence and Prevention study. Headache 2007;47(3):355‐63. [DOI: 10.1111/j.1526-4610.2006.00631.x] [DOI] [PubMed] [Google Scholar]
Edmeads 1993
- Edmeads J, Findlay H, Tugwell P, Pryse‐Phillips W, Nelson RF, Murray TJ. Impact of migraine and tension‐type headache on life‐style, consulting behaviour, and medication use: a Canadian population survey. Canadian Journal of Neurological Sciences 1993;20(2):131‐7. [DOI] [PubMed] [Google Scholar]
Ferrari 1998
- Ferrari MD. The economic burden of migraine to society. Pharmacoeconomics 1998;13(6):667‐76. [DOI] [PubMed] [Google Scholar]
Ferrari 2001
- Ferrari MD, Roon KI, Lipton RB, Goadsby PJ. Oral triptans (serotonin 5‐HT(1B/1D) agonists) in acute migraine treatment: a meta‐analysis of 53 trials. Lancet 2001;358:1668‐75. [DOI] [PubMed] [Google Scholar]
Ferrari 2002
- Ferrari MD, Goadsby PJ, Roon KI, Lipton RB. Triptans (serotonin, 5‐HT1B/1D agonists) in migraine: detailed results and methods of a meta‐analysis of 53 trials. Cephalalgia 2002;22(8):633‐58. [DOI: 10.1046/j.1468-2982.2002.00404.x] [DOI] [PubMed] [Google Scholar]
Gawel 2001
- Gawel MJ, Worthington I, Maggisano A. A systematic review of the use of triptans in acute migraine. Canadian Journal of Neurological Sciences 2001;28(1):30‐41. [DOI] [PubMed] [Google Scholar]
Gendolla 2008
- Gendolla A. Early treatment in migraine: how strong is the current evidence?. Cephalalgia 2008;28 Suppl 2:28‐35. [DOI] [PubMed] [Google Scholar]
Goadsby 2007
- Goadsby PJ. Recent advances in understanding migraine mechanisms, molecules and therapeutics. Trends in Molecular Medicine 2007;13(1):39‐44. [DOI: 10.1016/j.molmed.2006.11.005] [DOI] [PubMed] [Google Scholar]
Hazard 2009
- Hazard E, Munakata J, Bigal ME, Rupnow MF, Lipton RB. The burden of migraine in the United States: current and emerging perspectives on disease management and economic analysis. Value in Health 2009;12(1):55‐64. [DOI: 10.1111/j.1524-4733.2008.00404.x] [DOI] [PubMed] [Google Scholar]
Hu 1999
- Hu XH, Markson LE, Lipton RB, Stewart WF, Berger ML. Burden of migraine in the United States: disability and economic costs. Archives of Internal Medicine 1999;159(8):813‐8. [DOI] [PubMed] [Google Scholar]
IHS 1988
- Headache Classification Committee of the International Headache Society. Classification and diagnostic criteria for headache disorders, cranial neuralgias and facial pain. Cephalalgia 1988;8 Suppl 7:1‐96. [PubMed] [Google Scholar]
IHS 2000
- International Headache Society Clinical Trials Subcommittee. Guidelines for controlled trials of drugs in migraine: second edition. Cephalalgia 2000;20(9):765‐86. [DOI] [PubMed] [Google Scholar]
IHS 2004
- Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004;24 Suppl 1:1‐160. [DOI] [PubMed] [Google Scholar]
Jadad 1996a
- Jadad AR, Carroll D, Moore A, McQuay H. Developing a database of published reports of randomised clinical trials in pain research. 1996 1996;66(2‐3):239‐46. [DOI] [PubMed] [Google Scholar]
Jadad 1996b
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJM, Gavaghan DJ, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary?. Controlled Clinical Trials 1996;17(1):1‐12. [DOI] [PubMed] [Google Scholar]
Jhingran 1996
- Jhingran P, Cady RK, Rubino J, Miller D, Grice RB, Gutterman DL. Improvements in health‐related quality of life with sumatriptan treatment for migraine. Journal of Family Practice 1996;42(1):36‐42. [PubMed] [Google Scholar]
Kirthi 2010
- Kirthi V, Derry S, Moore RA, McQuay HJ. Aspirin with or without an antiemetic for acute migraine headaches in adults. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD008041.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
L'Abbé 1987
- L'Abbé KA, Detsky AS, O'Rourke K. Meta‐analysis in clinical research. Annals of Internal Medicine 1987;107(2):224‐33. [DOI] [PubMed] [Google Scholar]
Law 2010
- Law S, Derry S, Moore RA. Sumatriptan plus naproxen for acute migraine headaches in adults. Cochrane Database of Systematic Reviews 2010, Issue 6. [DOI: 10.1002/14651858.CD008541] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lipton 1999
- Lipton RB, Stewart WF. Acute migraine therapy: do doctors understand what patients with migraine want from therapy?. Headache 1999;39 Suppl 2:S20‐6. [Google Scholar]
Lipton 2007
- Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, AMPP Advisory Group, et al. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 2007;68(5):343‐9. [DOI] [PubMed] [Google Scholar]
Lofland 1999
- Lofland JH, Johnson NE, Batenhorst AS, Nash DB. Changes in resource use and outcomes for patients with migraine treated with sumatriptan: a managed care perspective. Archives of Internal Medicine 1999;159(8):857‐63. [DOI] [PubMed] [Google Scholar]
Lucas 2006
- Lucas C, Géraud G, Valade D, Chautard MH, Lantéri‐Minet M. Recognition and therapeutic management of migraine in 2004, in France: results of FRAMIG 3, a French nationwide population‐based survey. Headache 2006;46(5):715‐25. [DOI: 10.1111/j.1526-4610.2006.00430.x] [DOI] [PubMed] [Google Scholar]
McCrory 2003
- McCrory DC, Gray RN. Oral sumatriptan for acute migraine. Cochrane Database of Systematic Reviews 2003, Issue 3. [DOI: 10.1002/14651858.CD002915] [DOI] [PubMed] [Google Scholar]
Moore 1998
- Moore RA, Gavaghan D, Tramèr MR, Collins SL, McQuay HJ. Size is everything ‐ large amounts of information are needed to overcome random effects in estimating direction and magnitude of treatment effects. Pain 1998;78(3):209‐16. [DOI] [PubMed] [Google Scholar]
Moore 2008
- Moore RA, Barden J, Derry S, McQuay HJ. Managing potential publication bias. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:15‐24. [Google Scholar]
Moore 2010
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. ‘‘Evidence” in chronic pain – establishing best practice in the reporting of systematic reviews. Pain 2010;150:386–9. [DOI] [PubMed] [Google Scholar]
Morris 1995
- Morris JA, Gardner MJ. Calculating confidence intervals for relative risk, odds ratios and standardised ratios and rates. In: Gardner MJ, Altman DG editor(s). Statistics with Confidence ‐ Confidence Intervals and Statistical Guidelines. London: British Medical Journal, 1995:50‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oldman 2002
- Oldman AD, Smith LA, McQuay HJ, Moore RA. Pharmacological treatments for acute migraine: quantitative systematic review. Pain 2002;97(3):247‐57. [DOI] [PubMed] [Google Scholar]
Osterhaus 1994
- Osterhaus JT, Townsend RJ, Gandek B, Ware JE Jr. Measuring the functional status and well‐being of patients with migraine headache. Headache 1994;34(6):337‐43. [DOI] [PubMed] [Google Scholar]
Pascual 2002
- Pascual J. Clinical benefits of early triptan therapy for migraine. Headache 2002;42 Suppl 1:10‐7. [DOI: 10.1046/j.1526-4610.2002.0420s1010.x] [DOI] [PubMed] [Google Scholar]
PCA 2011
- Anonymous. Prescription cost analysis, England 2010. The NHS Information Centre, Prescribing Support Unit 2011. [ISBN: 978‐1‐84636‐542‐3]
Radtke 2009
- Radtke A, Neuhauser H. Prevalence and burden of headache and migraine in Germany. Headache 2009;49(1):79‐89. [DOI: 10.1111/j.1526-4610.2008.01263.x] [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Solomon 1997
- Solomon GD, Price KL. Burden of migraine. A review of its socioeconomic impact. Pharmacoeconomics 1997;11 Suppl 1:1‐10. [DOI] [PubMed] [Google Scholar]
Stovner 2010
- Stovner LJ, Andree C. Prevalence of headache in Europe: a review for the Eurolight project. Journal of Headache and Pain 2010;11(4):289‐99. [DOI: 10.1007/s10194-010-0217-0] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tramer 1997
- Tramèr MR, Reynolds DJM, Moore RA, McQuay HJ. Impact of covert duplicate results on meta‐analysis: a case study. BMJ 1997;315(7109):635‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Victor 2010
- Victor TW, Hu X, Campbell JC, Buse DC, Lipton RB. Migraine prevalence by age and sex in the United States: a life‐span study. Cephalalgia 2010;30(9):1065‐72. [DOI: 10.1177/0333102409355601] [DOI] [PubMed] [Google Scholar]


