Abstract
Background
In many countries of the industrialised world second generation (’atypical’) antipsychotic drugs have become the first line drug treatment for people with schizophrenia. It is not clear how the effects of the various second generation antipsychotic drugs differ.
Objectives
To evaluate the effects of quetiapine compared with other second generation antipsychotic drugs for people with schizophrenia and schizophrenia-like psychosis.
Search methods
We searched the Cochrane Schizophrenia Group Trials Register (April 2007), inspected references of all identified studies, and contacted relevant pharmaceutical companies, drug approval agencies and authors of trials for additional information.
Selection criteria
We included all randomised control trials comparing oral quetiapine with oral forms of amisulpride, aripiprazole, clozapine, olanzapine, risperidone, sertindole, ziprasidone or zotepine in people with schizophrenia or schizophrenia-like psychosis.
Data collection and analysis
We extracted data independently. For dichotomous data we calculated relative risks (RR) and their 95% confidence intervals (CI) on an intention-to-treat basis based on a random-effects model. We calculated numbers needed to treat/harm (NNT/NNH) where appropriate. For continuous data, we calculated weighted mean differences (WMD) again based on a random-effects model.
Main results
The review currently includes 21 randomised control trials (RCTs) with 4101 participants. These trials provided data on four comparisons - quetiapine versus clozapine, olanzapine, risperidone or ziprasidone.
A major limitation to all findings is the high number of participants leaving studies prematurely (57.6%) and the substantial risk of biases in studies. Efficacy data favoured olanzapine and risperidone compared with quetiapine (PANSS total score versus olanzapine:10 RCTs, n=1449, WMD 3.66 CI 1.93 to 5.39; versus risperidone: 9 RCTs, n=1953, WMD 3.09 CI 1.01 to 5.16), but clinical meaning is unclear. There were no clear mental state differences when quetiapine was compared with clozapine or ziprasidone.
Compared with olanzapine, quetiapine produced slightly fewer movement disorders (6 RCTs, n=1090, RR use of antiparkinson medication 0.49 CI 0.3 to 0.79, NNH 25 CI 14 to 100) and less weight gain (7 RCTs, n=1173, WMD −2.81 CI −4.38 to −1.24) and glucose elevation, but more QTc prolongation (3 RCTs, n=643, WMD 4.81 CI 0.34 to 9.28). Compared with risperidone, quetiapine induced slightly fewer movement disorders (6 RCTs, n=1715, RR use of antiparkinson medication 0.5 CI 0.3 to 0.86, NNH 20 CI 10 to 100), less prolactin increase (6 RCTs, n=1731, WMD −35.28 CI −44.36 to −26.19) and some related adverse effects, but more cholesterol increase (5 RCTs, n=1433, WMD 8.61 CI 4.66 to 12.56). Compared with ziprasidone, quetiapine induced slightly fewer extrapyramidal adverse effects (1 RCT, n=522, RR use of antiparkinson medication 0.43 CI 0.2 to 0.93, NNH not estimable) and prolactin increase. On the other hand quetiapine was more sedating and led to more weight gain (2 RCTs, n=754, RR 2.22 CI 1.35 to 3.63, NNH 13 CI 8 to 33) and cholesterol increase than ziprasidone.
Authors’ conclusions
Best available evidence from trials suggests that most people who start quetiapine stop taking it within a few weeks. Comparisons with amisulpride, aripiprazole, sertindole and zotepine do not exist. Most data that has been reported within existing comparisons are of very limited value because of assumptions and biases within them. There is much scope for further research into the effects of this widely used drug.
Medical Subject Headings (MeSH): Antipsychotic Agents [adverse effects; * therapeutic use], Benzodiazepines [adverse effects; therapeutic use], Clozapine [adverse effects; therapeutic use], Dibenzothiazepines [adverse effects; * therapeutic use], Medication Adherence [statistics & numerical data], Piperazines [adverse effects; therapeutic use], Randomized Controlled Trials as Topic, Risperidone [adverse effects; therapeutic use], Schizophrenia [* drug therapy], Thiazoles [adverse effects; therapeutic use]
MeSH check words: Humans
BACKGROUND
Description of the condition
Schizophrenia can be a disabling psychiatric disorder which afflicts approximately one per cent of the population world-wide with little gender differences. The annual incidence of schizophrenia averages 15 per 100,000, the point prevalence averages approximately 4.5 per population of 1000, and the risk of developing the illness over one’s lifetime averages 0.7% (Tandon 2008). Its typical manifestations are positive symptoms such as fixed, false beliefs (delusions) and perceptions without cause (hallucinations), negative symptoms such as apathy and lack of drive, disorganisation of behaviour and thought, and catatonic symptoms such as mannerisms and bizarre posturing (Carpenter 1994). The degree of suffering and disability is considerable with 80% - 90% not working (Marvaha 2004) and up to 10% dying (Tsuang 1978). In the 15-44 years age group, schizophrenia is among the top ten leading causes of disease-related disability in the world (WHO 2001). Conventional antipsychotic drugs, such as chlorpromazine and haloperidol, have traditionally been used as first line antipsychotic drugs for people with schizophrenia (Kane 1993). The reintroduction of clozapine in the USA, and findings to indicate that clozapine seemed more effective than other drugs, as well as being associated with fewer movement disorders than chlorpromazine (Kane 1988), boosted development of new/second/atypical generation antipsychotic drugs (SGA).
Description of the intervention
There is no good definition of what an ’atypical’ antipsychotic is, but they were initially said to differ from typical antipsychotic drugs in that they do not cause movement disorders (catalepsy) in rats at clinically effective doses (Arnt 1998). The terms ’new’ or ’second generation’ antipsychotic drugs are not much better, because clozapine is now a very old drug. According to treatment guidelines (APA 2004, Gaebel 2006) second generation antipsychotic drugs include drugs such as amisulpride, aripiprazole, clozapine, olanzapine, quetiapine, risperidone, sertindole, ziprasidone and zotepine, although it is unclear whether some old and inexpensive compounds such as sulpiride or perazine have similar properties (Möller 2000). The second generation antipsychotic drugs raised major hopes of superior effects in a number of areas such as compliance, cognitive functioning, negative symptoms, movement disorders, quality of life, and the treatment of people whose illness had formerly been resistant to treatment.
How the intervention might work
Experimental laboratory studies have suggested that quetiapine is a clozapine-like atypical antipsychotic (Migler 1993, Goldstein 1993, Saller 1993). In contrast to olanzapine, risperidone, sertindole and ziprasidone have high affinities (<50 nM) to both D2 and 5-HT2A receptors, quetiapine is similar to clozapine in having only moderate affinities (<500 nM) to these sites (Goldstein 1995). Quetiapine has a high affinity for histamine receptors (<50 nM) (Srisurapanont 2004).
Why it is important to do this review
The debate as to how far the second generation antipsychotic drugs improve these outcomes compared with conventional antipsychotic drugs continues (Duggan 2005, El-Sayeh 2006) and the results from recent studies were sobering (Liebermann 2005, Jones 2006). Nevertheless, in some parts of the world, especially in the highly industrialised countries, second generation antipsychotic drugs have become the mainstay of treatment. They also differ in terms of their costs: while amisulpride and risperidone are already generic in many countries, quetiapine for example is still not. Therefore the question as to whether they differ from each other in their clinical effects becomes increasingly important. In this review we aim to summarise evidence from randomised controlled trials that compared quetiapine with other second generation antipsychotic drugs.
OBJECTIVES
To review the effects of quetiapine compared with other atypical antipsychotic drugs for people with schizophrenia and schizophrenia-like psychosis.
METHODS
Criteria for considering studies for this review
Types of studies
We included relevant randomised controlled trials which were at least single-blind (blind raters). Where a trial was described as double-blind, but it was only implied that the study was randomised, we included these trials in a sensitivity analysis. If there was no substantive difference within primary outcomes (see Types of outcome measures) when these implied randomisation studies were added, then we included these in the final analysis. If there was a substantive difference, we only used clearly randomised trials and described the results of the sensitivity analysis in the text. We excluded quasi-randomised studies, such as those allocating by using alternate days of the week.
We included randomised cross-over studies but only data up to the point of first cross-over because of the instability of the problem behaviours and the likely carry-over effects of all treatments.
Types of participants
We included people with schizophrenia and other types of schizophrenia-like psychosis (e.g. schizophreniform and schizoaffective disorders), irrespective of the diagnostic criteria used. There is no clear evidence that the schizophrenia-like psychoses are caused by fundamentally different disease processes or require different treatment approaches (Carpenter 1994).
Types of interventions
Quetiapine: any oral form of application, any dose.
Other ’atypical’ antipsychotic drugs: amisulpride, aripiprazole, clozapine, olanzapine, risperidone, sertindole, ziprasidone, zotepine: any oral form of application, any dose.
Types of outcome measures
We grouped outcomes into the short term (up to 12 weeks), medium term (13-26 weeks) and long term (over 26 weeks).
Primary outcomes
Global State: No clinically important response as defined by the individual studies (e.g. global impression less than much improved or less than 50% reduction on a rating scale)
Secondary outcomes
-
1
Leaving the studies early (any reason, adverse events, inefficacy of treatment)
-
2
Global state
-
2.1
No clinically important change in global state (as defined by individual studies)
-
2.2
Relapse (as defined by the individual studies)
-
3
Mental state (with particular reference to the positive and negative symptoms of schizophrenia)
-
3.1
No clinically important change in general mental state score
-
3.2
Average endpoint general mental state score
-
3.3
Average change in general mental state score
-
3.4
No clinically important change in specific symptoms (positive symptoms of schizophrenia, negative symptoms of schizophrenia)
-
3.5
Average endpoint specific symptom score
-
3.6
Average change in specific symptom score
-
4
General functioning
-
4.1
No clinically important change in general functioning
-
4.2
Average endpoint general functioning score
-
4.3
Average change in general functioning score
-
5
Quality of life/satisfaction with treatment
-
5.1
No clinically important change in general quality of life
-
5.2
Average endpoint general quality of life score
-
5.3
Average change in general quality of life score
-
6
Cognitive functioning
-
6.1
No clinically important change in overall cognitive functioning
-
6.2
Average endpoint of overall cognitive functioning score
-
6.3
Average change of overall cognitive functioning score
-
7
Service use
-
7.1
Admitted
-
8
Adverse effects
-
8.1
Number of people with at least one adverse effect
-
8.2
Clinically important specific adverse effects (cardiac effects, death, movement disorders, prolactin increase and associated effects, sedation, seizures, weight gain, effects on white blood cell count)
-
8.3
Average endpoint in specific adverse effects
-
8.4
Average change in specific adverse effects
Search methods for identification of studies
No language restriction was applied within the limitations of the search tools.
Electronic searches
We searched the Cochrane Schizophrenia Group’s Specialised Register (April 2007) using the phrase: [((quetiapin* AND (amisulprid* OR aripiprazol* OR clozapin* OR olanzapin* OR risperidon* OR sertindol* OR ziprasidon* OR zotepin*)) in title, abstract or index terms of REFERENCE) or ((quetiapin* AND (amisulprid* OR aripiprazol* OR clozapin* OR olanzapin* OR risperidon* OR sertindol* OR ziprasidon* OR zotepin*)) in interventions of STUDY)]
This register is compiled by systematic searches of major databases, hand searches and conference proceedings (see Group Module). The Cochrane Schizophrenia Group Trials Register is maintained on Meerkat 1.5. This version of Meerkat stores references as studies. When an individual reference is selected through a search, all references which have been identified as the same study are also selected.
Searching other resources
Reference searching We inspected the references of all identified studies for more trials.
Personal contact We contacted the first author of each included study for missing information.
Drug companies We contacted the manufacturers of all atypical antipsychotic drugs included for additional data.
Data collection and analysis
Selection of studies
KK, CRK and SL independently inspected all reports. We resolved any disagreement by discussion, and where there was still doubt, we acquired the full article for further inspection. Once the full articles were obtained, we independently decided whether the studies met the review criteria. If disagreement could not be resolved by discussion, we sought further information and added these trials to the list of those awaiting assessment.
Data extraction and management
1. Data extraction
KK, CRK and SL independently extracted data from selected trials. When disputes arose we attempted to resolve these by discussion. When this was not possible and further information was necessary to resolve the dilemma, we did not enter data and added the trial to the list of those awaiting assessment.
2. Management
KK, CRK, FS, HH, SS and SL extracted data onto standard simple forms. Where possible, we entered data in such a way that the area to the left of the line of no effect indicated a favourable outcome for quetiapine.
3. Rating scales
A wide range of instruments are available to measure outcomes in mental health studies. These instruments vary in quality and many are not validated, or are even ad hoc. It is accepted generally that measuring instruments should have the properties of reliability (the extent to which a test effectively measures anything at all) and validity (the extent to which a test measures that which it is supposed to measure) (Rust 1989). Unpublished scales are known to be subject to bias in trials of treatments for schizophrenia (Marshall 2000). Therefore continuous data from rating scales were included only if the measuring instrument had been described in a peer-reviewed journal.
Assessment of risk of bias in included studies
Again working independently, KK and SL assessed risk of bias using the tool described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). This tool encourages consideration of how the sequence was generated, how allocation was concealed, the integrity of blinding at outcome, the completeness of outcome data, selective reporting and other biases.
The risk of bias in each domain and overall were assessed and categorised into:
Low risk of bias: plausible bias unlikely to seriously alter the results (categorised as ’Yes’ in Risk of Bias table)
High risk of bias: plausible bias that seriously weakens confidence in the results (categorised as ’No’ in Risk of Bias table)
Unclear risk of bias: plausible bias that raises some doubt about the results (categorised as ’Unclear’ in Risk of Bias table)
Trials with high risk of bias (defined as at least four out of seven domains) were categorised as ’No’) or where allocation was clearly not concealed were not included in the review. If the raters disagreed, the final rating was made by consensus with the involvement of another member of the review group. Where inadequate details of randomisation and other characteristics of trials are provided, authors of the studies were contacted in order to obtain further information. Non-concurrence in quality assessment was reported.
Measures of treatment effect
1. Data types
We assessed outcomes using continuous (for example changes on a behaviour scale), categorical (for example, one of three categories on a behaviour scale, such as ’little change’, ’moderate change’ or ’much change’) or dichotomous (for example, either ’no important changes or ’important change’ in a person’s behaviour) measures. Currently RevMan does not support categorical data so we were unable to analyse this.
2. Dichotomous data
We carried out an intention to treat analysis. Everyone allocated to the intervention were counted, whether they completed the follow up or not. It was assumed that those who dropped out had no change in their outcome. This rule is conservative concerning response to treatment, because it assumes that those discontinuing the studies would not have responded. It is not conservative concerning adverse effects, but we felt that assuming that all those leaving early would have developed side effects would overestimate risk. Where possible, efforts were made to convert outcome measures to dichotomous data. This can be done by identifying cut off points on rating scales and dividing participants accordingly into ’clinically improved’ or ’not clinically improved’. It was generally assumed that if there had been a 50% reduction in a scale-derived score such as the Brief Psychiatric Rating Scale (BPRS, Overall 1962) or the Positive and Negative Syndrome Scale (PANSS, Kay 1986), this could be considered as a clinically significant response (Leucht 2005a, Leucht 2005b). If data based on these thresholds were not available, we used the primary cut-off presented by the original authors.
We calculated the relative risk (RR) and its 95% confidence interval (CI) based on the random effects model, as this takes into account any differences between studies even if there is no statistically significant heterogeneity. It has been shown that RR is more intuitive (Boissel 1999) than odds ratios and that odds ratios tend to be interpreted as RR by clinicians (Deeks 2000). This misinterpretation then leads to an overestimate of the impression of the effect. When the overall results were significant we calculated the number needed to treat (NNT) and the number-needed-to-harm (NNH) as the inverse of the risk difference.
3. Continuous data
3.1 Normal distribution of the data
The meta-analytic formulas applied by RevMan Analyses (the statistical programme included in RevMan) require a normal distribution of data. The software is robust towards some skew, but to which degree of skewness meta-analytic calculations can still be reliably carried out is unclear. On the other hand, excluding all studies on the basis of estimates of the normal distribution of the data also leads to a bias, because a considerable amount of data may be lost leading to a selection bias. Therefore, we included all studies in the primary analysis. In a sensitivity analysis we excluded potentially skewed data applying the following rules:
When a scale started from the finite number zero the standard deviation, when multiplied by two, was more than the mean (as otherwise the mean is unlikely to be an appropriate measure of the centre of the distribution, Altman 1996).
If a scale started from a positive value (such as PANSS which can have values from 30 to 210) the calculation described above was modified to take the scale starting point into account. In these cases skew is present if 2SD>(S-Smin), where S is the mean score and Smin is the minimum score.
In large studies (as a cut-off we used 200 participants) skewed data pose less of a problem. In these cases we entered the data in a synthesis.
The rules explained in a) and b) do not apply to change data.
The reasons is that when continuous data are presented on a scale which includes a possibility of negative values, it is difficult to tell whether data are non-normally distributed (skewed) or not. This is also the case for change data (endpoint minus baseline). In the absence of individual patient data it is impossible to know if data are skewed, though this is likely. After consulting the ALL-STAT electronic statistics mailing list, we presented change data in RevMan Analyses in order to summarise available information. In doing this, it was assumed either that data were not skewed or that the analysis could cope with the unknown degree of skew. Without individual patient data it is impossible to test this assumption. Change data were therefore included and a sensitivity analysis was not applied.
For continuous outcomes we estimated a weighted mean difference (WMD) between groups. WMDs were again based on the random effects model, as this takes into account any differences between studies even if there is no statistically significant heterogeneity. We combined both endpoint data and change data in the analysis, because there is no principal statistical reason why endpoint and change data should measure different effects (Higgins 2008). When standard errors instead of standard deviations (SD) were presented, we converted the former to standard deviations. If both were missing we estimated SDs from p-values or used the average SD of the other studies (Furukawa 2006).
Unit of analysis issues
1. Cluster trials
Studies increasingly employ ’cluster randomisation’ (such as randomisation by clinician or practice) but analysis and pooling of clustered data poses problems. Firstly, authors often fail to account for intraclass correlation in clustered studies, leading to a ’unit of analysis’ error (Divine 1992) whereby p values are spuriously low, confidence intervals unduly narrow and statistical significance overestimated. This causes type I errors (Bland 1997, Gulliford 1999).
Where clustering was not accounted for in primary studies, we presented the data in a table, with a (*) symbol to indicate the presence of a probable unit of analysis error. In subsequent versions of this review we will seek to contact first authors of studies to obtain intraclass correlation coefficients of their clustered data and to adjust for this using accepted methods (Gulliford 1999). Where clustering has been incorporated into the analysis of primary studies, we will also present these data as if from a non-cluster randomised study, but adjusted for the clustering effect.
We have sought statistical advice and have been advised that the binary data as presented in a report should be divided by a ’design effect’. This is calculated using the mean number of participants per cluster (m) and the intraclass correlation coefficient (ICC) [Design effect=1+(m-1)*ICC] (Donner 2002). If the ICC was not reported it was assumed to be 0.1 (Ukoumunne 1999).
If cluster studies had been appropriately analysed taking into account intraclass correlation coefficients and relevant data documented in the report, we synthesised these with other studies using the generic inverse variance technique.
2. Cross-over trials
A major concern of cross-over trials is the carry-over effect. It occurs if an effect (e.g. pharmacological, physiological or psychological) of the treatment in the first phase is carried over to the second phase. As a consequence on entry to the second phase the participants can differ systematically from their initial state despite a wash-out phase. For the same reason cross-over trials are not appropriate if the condition of interest is unstable (Elbourne 2002). As both effects are very likely in schizophrenia, we will only use data of the first phase of cross-over studies.
3. Studies with multiple treatment groups
Where a study involved more than two treatment groups, if relevant, the additional treatment groups were presented in additional relevant comparisons. Data were not double counted. Where the additional treatment groups were not relevant, these data were not reproduced.
Dealing with missing data
At some degree of loss of follow-up data must lose credibility (Xia 2007). Although high rates of premature discontinuation are a major problem in this field, we felt that it is unclear which degree of attrition leads to a high degree of bias. We, therefore, did not exclude trials on the basis of the percentage of participants completing them. However we addressed the attrition problem in all parts of the review, including the abstract. For this purpose we calculated, presented and commented on frequency statistics (overall rates of leaving the studies early in all studies and comparators pooled).
Assessment of heterogeneity
1. Clinical heterogeneity
We considered all the included studies within any comparison to judge for clinical heterogeneity.
2. Statistical
2.1 Visual inspection
We visually inspected graphs to investigate the possibility of statistical heterogeneity.
2.2 Employing the I2 statistic
Visual inspection was supplemented using, primarily, the I2statistic. This provides an estimate of the percentage of variability due to heterogeneity rather than chance alone. Where the I2 estimate was greater than or equal to 50% we interpreted this as indicating the presence of considerable levels of heterogeneity (Higgins 2003).
Assessment of reporting biases
Reporting biases arise when the dissemination of research findings is influenced by the nature and direction of results (Egger 1997). These are described in section 10.1 of the Cochrane Handbook (Higgins 2008). We are aware that funnel plots may be useful in investigating small study effects but are of limited power to detect such effects when there are few studies. We entered data from all identified and selected trials into a funnel graph (trial effect versus trial size) in an attempt to investigate the likelihood of overt publication bias. We did not undertake a formal test for funnel plot asymmetry.
Data synthesis
Where possible for both dichotomous and continuous data we used the random-effects model for data synthesis as this takes into account any differences between studies even if there is no statistically significant heterogeneity. We understand that there is no closed argument for preference for use of fixed or random-effects models. The random-effects method incorporates an assumption that the different studies are estimating different, yet related, intervention effects. This does seem true to us, however, random-effects does put added weight onto the smaller of the studies - those trials that are most vulnerable to bias.
Subgroup analysis and investigation of heterogeneity
If data are clearly heterogeneous we checked that data are correctly extracted and entered and that we had made no unit of analysis errors. If inconsistency was high and clear reasons explaining the heterogeneity were found, we presented the data separately. If not, we commented on the heterogeneity of the data.
Sensitivity analysis
We planned sensitivity analyses for examining the change in robustness of the sensitivity to including studies with potentially skewed data. A recent report showed that some of the comparisons of atypical antipsychotic drugs may have been biased by using inappropriate comparator doses (Heres 2006). We, therefore, also analysed whether the exclusion of studies with inappropriate comparator doses changed the results of the primary outcome and the general mental state.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies.
For substantive description of studies please see Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
The overall search strategy yielded 3620 reports of which 104 were closely inspected.
Included studies
Twenty-one studies with 4101 participants met the inclusion criteria. Six studies were sponsored by pharmaceutical companies producing quetiapine, three were sponsored by the manufacturer of the comparator antipsychotic, and eight had a neutral sponsor. For the remaining four studies the sponsor was unclear.
1. Length of trials
Fifteen studies were short term with a duration of 2-12 weeks. Three studies were medium term and two trials fell into the long term category.
2. Setting
Seven trials were conducted in an in- or outpatient setting, nine studies were conducted exclusively in an inpatient setting and one study was conducted exclusively in an outpatient setting. Four studies did not report the setting.
3. Participants
Seventeen studies included participants with diagnoses according to the Diagnostic and Statistical Manual Fourth revision (DSM-IV). Riedel 2005 additionally used the International Classification of Diseases Version 10 (ICD-10). Li 2002, Li 2005 and Liu 2004 diagnosed participants according to the Chinese Classification of Mental Disorders Version 3 (CCMD-3). Li 2003b used CCMD-2. Two studies included only acutely ill people (Riedel 2007, Svestka 2003b) and one included only people with a first episode of schizophrenia (McEvoy 2007). Two studies included people with chronic schizophrenia or people with more than one schizophrenic episode (Lieberman 2005, Stroup 2006). Only one study focused on treatment resistant participants (Conley 2005).
4. Study size
Lieberman 2005 was the largest study with 1453 participants, while Ozguven 2004 was the smallest, randomising only 22 people. Five studies had less than 50 participants but two randomised more than 400 people.
5. Interventions
5.1 Quetiapine: all included studies used flexible dosing
Overall, quetiapine was given in a dose range from 50 mg/day to 800 mg/day. Only Conley 2005 limited the upper dose range to 500 mg/day and Ozguven 2004 had a mean dose which was higher than the upper dose range of 800mg/day (827 mg/day).
5.2 Comparators: the comparator drugs were clozapine, olanzapine, risperidone and ziprasidone, again given in flexible doses
Some studies included treatment arms with fluphenazine, perphenazine and perospirone, as well, but as these are not second generation antipsychotic drugs we did not report the results.
6. Outcomes
6.1 Leaving the study early
The number of participants leaving the studies early were reported for the categories ‘any reason’, ‘adverse events’ and ‘lack of efficacy’.
6.2 No clinically significant response
We pre-specified at least 50% PANSS/BPRS reduction from baseline as a clinical relevant cut-off to define, but only Svestka 2003b reported this outcome. Instead, Liu 2004 indicated at least 50% SANS reduction from baseline, Potkin 2006 and Zhong 2006a at least 30 % PANSS total score reduction from baseline, Ozguven 2004 at least 20% SANS total score reduction from baseline, Conley 2005 a Clinical Global Impression (Guy 1976) of mild or better combined with at least 20% BPRS total reduction from baseline and McEvoy 2007 all PANSS items mild or better plus a Clinical Global Impression of mild or better.
6.3 Outcome scales
Details of scales that provided usable data are shown below. Reasons for exclusion of data from other instruments are given under ’Outcomes’ in the ’Included studies’ section.
6.3.1 Global state scales
6.3.1.1 Clinical Global Impression Scale - CGI (Guy 1976)
This is used to assess both severity of illness and clinical improvement, by comparing the conditions of the person standardised against other people with the same diagnosis. A seven point scoring system is usually used with low scores showing decreased severity and/or overall improvement.
6.3.2 Mental state scales
6.3.2.1 Positive and Negative Syndrome Scale - PANSS (Kay 1986)
This schizophrenia scale has 30 items, each of which can be defined on a seven-point scoring system varying from 1 (absent) to 7 (extreme). It can be divided into three sub-scales for measuring the severity of general psychopathology, positive symptoms (PANSS-P) and negative symptoms (PANSS-N). A low score indicates lesser severity.
6.3.2.2 Brief Psychiatric Rating Scale - BPRS (Overall 1962)
This is used to assess the severity of abnormal mental state. The original scale has 16 items, but a revised 18 item scale is commonly used. Each item is defined on a seven point scale varying from ’not present’ to ’extremely severe’, scoring from 0-6 or 1-7. Scores can range from 0-126 with high scores indicating more severe symptoms.
6.3.2.3 Scale for the Assessment of Negative Symptoms - SANS (Andreasen 1989)
This six point scale gives a global rating of the following negative symptoms: alogia, affective blunting, avolition-apathy, anhedonia-associality and attention impairment. Higher scores indicate more symptoms.
6.3.2.4 Scale for the Assessment of Positive Symptoms - SAPS (Andreasen 1984)
This four point scale gives a global rating of the following positive symptoms: hallucination, delusion, bizarre attitudes and positive formal thought disorder.
6.3.3 Global Assessment of Functioning - GAF (DSM IV 1994)
A rating scale for a patients’ overall capacity of psychosocial functioning, scoring from 1-100. Higher scores indicating a higher level of functioning.
6.3.4. Quality of Life Scale - QLS (Carpenter 1984)
This semi-structured interview is administered and rated by trained clinicians. It contains 21 items rated on a seven point scale based on the interviewers’ judgement of patient functioning. A total QLS and four sub-scale scores are calculated, with higher scores indicating less impairment.
6.3.5 Adverse effects scales
6.3.5.1 Abnormal Involuntary Movement Scale - AIMS (Guy 1976)
This has been used to assess tardive dyskinesia, a long-term, drug-induced movement disorder and short-term movement disorders such as tremor.
6.3.5.2 Barnes Akathisia Scale - BAS (Barnes 1989)
The scale comprises items rating the observable, restless movements that characterise akathisia, a subjective awareness of restlessness and any distress associated with the condition. These items are rated from 0 - normal to 3 - severe. In addition, there is an item for rating global severity (from 0 - absent to 5 - severe). A low score indicates low levels of akathisia.
6.3.5.3 Extrapyramidal Symptom Rating Scale - ESRS (Chouinard 1980)
This is a questionnaire relating to parkinsonian symptoms (nine items), a physician’s examination for parkinsonism and dyskinetic movements (eight items), and a clinical global impression of tardive dyskinesia. High scores indicate severe levels of movement disorder.
6.3.5.4 Simpson Angus Scale - SAS (Simpson 1970)
This is a ten item scale, with a scoring system of 0-4 for each item, measures drug-induced parkinsonism, a short-term drug-induced movement disorder. A low score indicates low levels of Parkinsonism.
6.4 Other adverse effects
Other adverse effects were reported as continuous variables for QTc prolongation (ms), cholesterol level (mg/dl), glucose level (mg/dl), prolactin level (ng/ml) and weight (kg). Other adverse events were reported in a dichotomous manner in terms of the number of people with a given effect.
6.5 Service use
Service use was described as the number of patients re-hospitalised during the trial.
Excluded studies
Eighty three studies had to be excluded for the following reasons: eleven were not randomised, 64 were open label, three employed inappropriate intervention, four reported no usable data and one was a pooled analysis rather than a trial.
Awaiting assessment
No studies are waiting assessment.
Ongoing studies
Four randomised trials comparing quetiapine with other antipsychotic drugs seem to be ongoing (Eli Lilly 2004b, Gafoor 2005, Ratna 2003, Reynolds 2001). For further details see ’Characteristics of ongoing studies’.
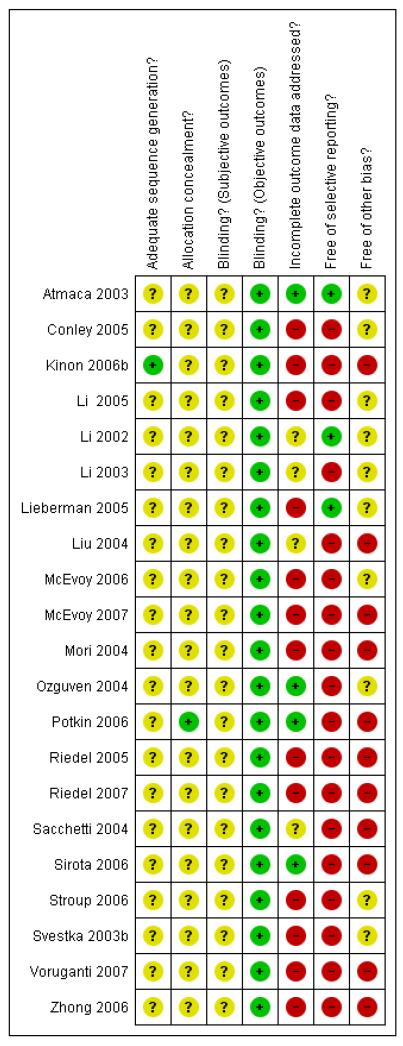
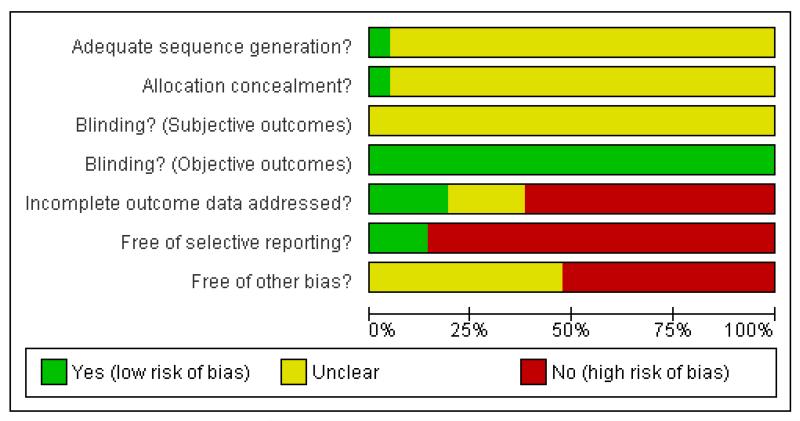
Risk of bias in included studies
For details please refer to risk of bias tables (Figure 1, Figure 2).
Figure 1. Methodological quality summary: review authors’ judgements about each methodological quality item for each included study.
Figure 2. Methodological quality graph: review authors’ judgements about each methodological quality item presented as percentages across all included studies.
Allocation
All of the included studies were described as randomised. Only two studies gave further information about the type of randomisation, Kinon 2006b described computer generated randomisation and Potkin 2006 described using an interactive voice response system for allocation concealment, for all other studies it was unclear whether the allocation strategies were appropriate.
Blinding
Seven of the included studies were ’single-blind’ (blind raters), all other included studies were ’double-blind’. Four studies used identical capsules for blinding (Lieberman 2005, McEvoy 2006, Potkin 2006, Riedel 2005). The other trials did not provide any information on the blinding procedure. No study examined whether blinding was effective. We found that the adverse effect profiles of some of the compounds are quite different and think that this may have made blinding difficult. We therefore conclude that the risk of bias for objective outcomes (e.g. death or laboratory values) was less than that for subjective outcomes, and for the latter there was a considerable risk as a result of poor blinding.
Incomplete outcome data
Fifteen studies indicated the number of participants leaving the studies early for any reason. In these fifteen studies the reasons for premature study discontinuation were usually well described. A major problem, however, was the very high attrition which in nine studies was higher than 30% (57.6% overall) (Conley 2005, Kinon 2006b, Lieberman 2005, McEvoy 2006, McEvoy 2007, Riedel 2005, Riedel 2007, Stroup 2006, Zhong 2006). In most studies the last-observation-carried-forward method was used to account for attrition. This is an imperfect method. It assumes that a participant’s outcome would not have changed if he/she had remained in the study which is often wrong. It is, however, questionable whether other methods (e.g. imputation strategies or mixed effect models) could have coped better with such dramatically high rates of attrition. The high loss to follow up is a clear threat to the validity of findings.
Selective reporting
Only two studies were judged to be free of selective reporting (Atmaca 2003, Li 2002). For most of the other trials there was a high risk of bias, mainly for the reason of incomplete reporting of predefined outcomes (Conley 2005, Kinon 2006b, Li 2005, Li 2003, Liu 2004, McEvoy 2006, Mori 2004, Ozguven 2004, Riedel 2005, Riedel 2007, Sacchetti 2004, Sirota 2006, Stroup 2006, Svestka 2003b, Voruganti 2007). In other studies only adverse events that occurred in at least 5% or 10% of participants, or which were moderately severe, have been reported (McEvoy 2007, Potkin 2006, Zhong 2006). The former method is problematic, because rare but important adverse effects may have been missed. In Lieberman 2005 all data from one site were excluded before analysis because of concerns about their integrity.
Other potential sources of bias
No study was clearly free of other potential sources of bias. In six the risk of ‘other bias’ was, however, unclear. Nine studies were industry sponsored (Kinon 2006b, McEvoy 2007, Potkin 2006, Riedel 2005, Riedel 2007, Sacchetti 2004, Sirota 2006, Voruganti 2007, Zhong 2006). There is evidence that pharmaceutical companies sometimes highlight the benefits of their compounds and tend to suppress their disadvantages (Heres 2006). Other reasons for potential bias were heterogeneity of pre-study treatment (Atmaca 2003, Stroup 2006), lack of or only short wash-out phases (Li 2005, Lieberman 2005, McEvoy 2006, Mori 2004, Voruganti 2007), baseline imbalance in terms of number of previous hospitalisations (Conley 2005), no information on the allowed dose range (Atmaca 2003, Li 2003, Voruganti 2007), or a too fast titration of clozapine which may be associated with more adverse events (Liu 2004).
Effects of interventions
1. Comparison 1. QUETIAPINE versus CLOZAPINE - all data short term
Five studies met the inclusion criteria for the comparison of quetiapine with clozapine.
1.1 Global state
1.1.1 No clinically significant response - as defined by the original studies
There was no significant difference (1 RCT, n=72, RR 0.94 CI 0.78 to 1.13).
1.1.2 No clinically important change - as defined by the original studies
There was no significant difference (1 RCT, n=72, RR 0.94 CI 0.74 to 1.18).
1.2 Leaving the study early
There was no significant difference in the number of participants leaving the studies early due to any reason (2 RCTs, n=95, RR 0.67 CI 0.18 to 2.43), due to adverse events (1 RCT, n=72, RR 0.14 CI 0.01 to 2.6) or due to inefficacy of treatment (1 RCT, n= 72, RR not estimable).
1.3 Mental state
1.3.1 General mental state: no clinically important change (less than 50% PANSS total score reduction from baseline)
There was no clear difference to be found (1 RCT, n=63, RR 1.07 CI 0.53 to 2.14).
1.3.2 General mental state: average score at endpoint - PANSS total
Four short term studies did not indicate a significant difference (4 RCTs, n=232, WMD −0.5 CI −2.85 to 1.86).
1.3.3 General mental state: average score at endpoint - BPRS total There was no significant difference (1 RCT, n=67, WMD −0.89 CI −3.20 to 1.42).
1.3.4 Positive symptoms: average score at endpoint - PANSS positive subscore
There was no significant difference (2 RCTs, n=142, WMD −0.7 CI −2.07 to 0.68).
1.3.5 Negative symptoms: average score at endpoint - PANSS negative subscore
Two small Chinese studies showed a significant superiority of quetiapine (2 RCTs, n=142, WMD −2.23 CI −3.48 to −0.99).
1.3.6 Negative symptoms: no clinically important change (less than 50% SANS total score reduction from baseline)
There was no significant difference (1 RCT, n=72, RR 0.94 CI 0.78 to 1.13).
1.3.7 Negative symptoms: average score at endpoint - SANS total There was no significant difference (1 RCT, n=67, WMD −1.64 CI −8.17 to 4.89).
1.4 Adverse effects
1.4.1 Numbers of participants with at least one adverse effect
There was a significant difference, based on data from Li 2002, favouring the treatment group (1 RCT, n=63, RR 0.42 CI 0.26 to 0.66, NNH 2 CI 1 to 3).
1.4.2 Cardiac effects - ECG abnormalities
There was a significant difference favouring quetiapine (1 RCT, n=72, RR 0.13 CI 0.02 to 0.95, NNH 5 CI 3 to 20).
1.4.3 Central nervous system - sedation
Fewer participants in the quetiapine group reported this outcome (2 RCTs, n=135, RR 0.22 CI 0.11 to 0.47, NNH 3 CI 2 to 8).
1.4.4 Extrapyramidal effects
There was no significant difference in akathisia (2 RCTs, n=135, RR 0.4 CI 0.08 to 1.99), rigor (1 RCT, n=63, RR 1.94 CI 0.18 to 20.3), tremor (2 RCTs, n=135, RR 0.99 CI 0.29 to 3.34) or use of antiparkinsonian medication (1 RCT, n=28, RR not estimable).
1.4.5 Haematological: important decline in white blood cells
There was no significant difference (1 RCT, n=33, RR 0.19 CI 0.01 to 3.88).
1.4.6 Metabolic - weight gain (number of participants with significant weight gain)
There was no significant difference (2 RCTs, n=135, RR 0.53 CI 0.25 to 1.11).
1.4.7 Metabolic - weight gain (change from baseline in kg)
One small study reported a trend in favour of quetiapine (1 RCT, n=27, WMD −2.11 CI −4.3 to 0.08).
1.5 Publication bias
We did not perform a funnel plot analysis because there were so few studies.
1.6 Investigation for heterogeneity and sensitivity analysis
The exclusion of Li 2002, Li 2003b, Li 2005 from the analysis of the PANSS total score due to possibly skewed data did not change the results to a marked extent.
2. Comparison 2. QUETIAPINE versus OLANZAPINE
Thirteen studies met the inclusion criteria for this comparison.
2.1 Global state
2.1.1 No clinically significant response - as defined by the original studies
There was no significant difference (3 RCTs, n=339, RR 1.11 CI 0.86 to 1.43).
2.1.2 No clinically important change - as defined by the original studies
There was no significant difference (2 RCTs, n=309, RR 1.18 CI 0.89 to 1.57).
2.2 Leaving the study early
Fewer participants in the olanzapine group (57%) compared with the quetiapine group (70%) left the studies early because of ‘any reason’ (10 RCTs, n=1651, RR 1.22 CI 1.13 to 1.32, NNH 10 CI 6 to 33) or ‘inefficacy’ (14% versus 25%, 8 RCTs, n=1563, RR 1.8 CI 1.42 to 2.27, NNH 11 CI 6 to 50), but not due to adverse events (12% versus 11%, 8 RCTs, n=1573, RR 0.90 CI 0.69 to 1.18).
2.3 Mental state
2.3.1 General mental state: no clinically important change - short term (less than 50% PANSS total score reduction)
There was no significant difference (1 RCT, n=42, RR 0.91 CI 0.54 to 1.53).
2.3.2 General mental state: average score at endpoint - PANSS total
There was a significant difference favouring olanzapine (10 RCTs, n=1449, WMD 3.66 CI 1.93 to 5.39) in the short term (4 RCTs, n=142, WMD 2.17 CI -1.51 to 5.85), medium term (3 RCTs, n= 483, WMD 5.57 CI 1.97 to 9.17) and long term (3 RCT, n=825, WMD 3.40 CI 0.91 to 5.88)
2.3.3 Positive symptoms: no clinically important change - short term (less than 20% SAPS total score reduction)
There was no difference identified with confidence (1 RCT, n=30, RR 15.0 CI 0.93 to 241.2).
2.3.4 Positive symptoms: average score at endpoint - PANSS positive subscore
There was a significant difference in favour of olanzapine (7 RCTs, n=679, WMD 1.8 CI 1.02 to 2.59), short term (3 RCTs, n=115, WMD 1.05 CI −0.75 to 2.85), medium term (3 RCTs, n=483, WMD 2.21 CI 0.90 to 3.52), long term (1 RCT, n=81, WMD 1.80 CI 0.39 to 3.21)
2.3.5 Positive symptoms: average score at endpoint - SAPS total score - short term (percentage change from baseline)
There was a significant difference favouring olanzapine (1 RCT, n=30, WMD 40.84 CI 23.97 to 57.71).
2.3.6 Negative symptoms: no clinically important change - short term (less than 20% SANS total score reduction from baseline)
There was no significant difference (1 RCT, n=30, RR 1.5 CI 0.53 to 4.26).
2.3.7 Negative symptoms: average score at endpoint - PANSS negative subscore
There was no significant difference (7 RCTs, n=679, WMD 0.41 CI −0.36 to 1.18), short term (3 RCTs, n=115, WMD 0.01 CI −1.72 to 1.73), medium term (3 RCTs, n=483, WMD 0.40 CI −0.67 to 1.47), long term (1 RCT, n=81, WMD 0.70 CI −0.73 to 2.13)
2.3.8 Negative symptoms: average score at endpoint - SANS total score
There was no significant difference (1 RCT, n=335, WMD 3.7 CI −0.48 to 7.88).
2.3.9 Negative symptoms: average score at endpoint - SANS total score (percent change from baseline)
There was no significant difference (1 RCT, n=30, WMD 2.46 CI −31.9 to 36.82).
2.4 General functioning: average endpoint total score -medium term GAF
There was a significant difference in favour of olanzapine (1 RCT, n=278, WMD 3.8 CI 0.77 to 6.83).
2.5 Quality of life: average endpoint total score -medium term QLS
There was no significant difference (1 RCT, n=286, WMD 1.8 CI −2.42 to 6.02).
2.6 Service use: number of participants re-hospitalised
There was a significant difference favouring olanzapine (2 RCTs, n=876, RR 1.79 CI 1.30 to 2.47, NNH 11 CI 7 to 25).
2.7 Adverse effects
2.7.1 Numbers of participants with at least one adverse effect
There was no significant difference (6 RCTs, n=1269, RR 0.97 CI 0.88 to 1.06).
2.7.2 Death
There was no significant difference (4 RCTs, n=1410, RR 0.74 CI 0.13 to 4.23).
2.7.3 Cardiac effects
2.7.3.1 Number of participants with QTc prolongation
There was no significant difference (1 RCT, n=673, RR 12.96 CI 0.73 to 229.17).
2.7.3.2 Mean change of QTc interval from baseline in ms
There was a significant difference favouring olanzapine (3 RCTs, n=643, WMD 4.81 CI 0.34 to 9.28).
2.7.4 Central nervous system
2.7.4.1 Sedation
There was no significant difference (7 RCTs, n=1615, RR 0.97 CI 0.78 to 1.2).
2.7.4.2 Seizures
There was no significant difference (1 RCT, n=40, RR 3.3 CI 0.14 to 76.46).
2.7.5 Extrapyramidal effects
2.7.5.1 Extrapyramidal effects
Fewer participants in the quetiapine group used antiparkinson medication at least once (6 RCTs, n=1090, RR 0.49 CI 0.3 to 0.79, NNH 25 CI 14 to 100). Apart from this, no significant differences in EPS were found for akathisia (6 RCTs, n=1277, RR 0.98 CI 0.68 to 1.4), akinesia (1 RCT, n=267, RR 1.02 CI 0.67 to 1.56), dystonia (1 RCT, n=42, RR 4.57 CI 0.23 to 89.72), any extrapyramidal symptom (2 RCTs, n=245, RR 1.62 CI 0.72 to 3.67), parkinsonism (1 RCT, n=40, RR 0.66 CI 0.18 to 2.41) and tremor (1 RCT, n=44, RR 0.39 CI 0.12 to 1.31).
2.7.5.2 Scale measured
Extrapyramidal adverse effects were evaluated with the Barnes Akathisia Scale, the Extrapyramidal Side Effects Rating Scale and the Simpson-Angus Scale. None of these indicated a significant difference between groups.
2.7.6 Prolactin associated side effects
Fewer participants in the quetiapine group suffered from sexual dysfunction (4 RCTs, n=1177, RR 0.8 CI 0.64 to 0.99, NNH 20 CI 10 to 100).
There was no significant difference in abnormally high prolactin (1 RCT, n=42, RR 0.10 CI 0.01 to 1.77), amenorrhoea (3 RCTs, n=252, RR 0.66 CI 0.36 to 1.21), galactorrhoea (4 RCTs, n=1025, RR 0.66 CI 0.25 to 1.73) and gynaecomastia (1 RCT, n=267, RR 0.33 CI 0.09 to 1.20).
2.7.7 Prolactin - change from baseline in ng/ml
Quetiapine was associated with less prolactin increase than olanzapine (5 RCTs, n=1021, RR −5.89 CI −11.62 to −0.16), but the data were heterogeneous. Nevertheless, the single-studies reported a consistent effect in favour of quetiapine (Svestka 2003b: n=35, WMD −40.07 CI −64.10 to −16.04, Lieberman 2005: n=673, WMD −3.20 CI − 6.81 to 0.41, McEvoy 2006: n=29, WMD −9.10 CI −19.88 to 1.68, Stroup 2006: n=203, WMD −3.20 CI −11.17 to 4.77, and McEvoy 2007: n=81, WMD −2.80 CI −10.03 to 4.43). Heterogeneity seems more due to differences in degree of prolactin increase rather than direction of effect.
2.7.8 Metabolic
2.7.8.1 Cholesterol - number of participants with abnormally high cholesterol increase
There was no significant difference (1 RCT, n=267, RR 0.99 CI 0.59 to 1.68).
2.7.8.2 Cholesterol - mean change from baseline in mg/dl
Overall data on cholesterol change from baseline did not show a statistically significant difference between groups (4 RCTs, n=986, WMD −4.69 CI −13.84 to 4.45). There was significant heterogeneity due to one outlier (McEvoy 2007) which was a first-episode study and showed a trend in favour of olanzapine. Excluding this study revealed a significant difference in favour of quetiapine (3 RCTs, n=643, WMD −7.84 CI −14.12 to −1.57).
2.7.8.3 Glucose - number of participants with abnormally high fasting glucose
There was no significant difference (1 RCT, n=267, RR 0.71 CI 0.33 to 1.54).
2.7.8.4 Glucose - change from baseline in mg/dl
The mean increase of glucose from baseline was lower in the quetiapine group than in the olanzapine group (4 RCTs, n=986, WMD −9.32 CI −17.82 to −0.82). The data remained heterogeneous even after an outlier study (McEvoy 2007) was excluded, but the superiority of quetiapine remained.
2.7.8.5 Weight gain
Fewer participants in the quetiapine group had a significant weight gain (8 RCTs, n=1667, RR 0.68 CI 0.51 to 0.92, NNH not estimable).
2.7.8.6 Weight gain - change from baseline in kg
Overall participants in the quetiapine group gained less weight than in the olanzapine group (7 RCT, n=1173, WMD −2.68 CI −4.26 to −1.10). Again, there was significant heterogeneity, but the results of the single studies consistently favoured quetiapine (Atmaca 2003: n=27, WMD −4.51 CI −6.57 to −2.45, Lieberman 2005: n=612, WMD −3.8 CI −4.91 to −2.69, Kinon 2006b: n=346, WMD −0.64 CI −1.76 to 0.48), McEvoy 2006: n=34, WMD −2.3 CI −10.18 to 5.58, Sirota 2006: n=40, WMD −3.2 CI −5.51 to −0.89, McEvoy 2007: n=81, WMD −5.18 CI −10.00 to −0.36, Riedel 2007: n=33, WMD −0.48 CI −2.52 to 1.56).
2.8 Publication bias
Funnel plots did not suggest a possible publication bias.
2.9 Investigation for heterogeneity and sensitivity analysis
When Mori 2004 was excluded from the evaluation of the PANSS positive score due to possibly skewed data olanzapine remained more effective.
3. Comparison 3. QUETIAPINE versus RISPERIDONE
Eleven studies met the inclusion criteria for the comparison of quetiapine with risperidone.
3.1 Global state
3.1.1 No clinically significant response - as defined by the original studies
Overall there was no significant difference. As the results were heterogeneous we present the single studies separately. Potkin 2006 reported a significant difference in favour of risperidone (n=177, RR 1.27 CI 1.05 to 1.55), while Conley 2005 (n=25, RR not estimable), Zhong 2006a (n=495, RR 1.0 CI 0.91 to 1.09) and McEvoy 2007 (n=103, RR 1.18 CI 0.87 to 1.6) found no significant difference between groups. The first three studies reported short term and only McEvoy 2007 reported long term data.
3.1.2 No clinically important change (as defined by the original studies)
There was a small superiority of risperidone which did not reach statistical significance (4 RCTs, n=1374, RR 1.16 CI 0.99 to 1.35).
3.2 Leaving the study early
There was no significant difference in the number of participants leaving the studies early due to any reason (quetiapine 57%, risperidone 54%, 10 RCTs, n=2278, RR 1.06 CI 0.98 to 1.15) or due to adverse events (11% versus 9%, 7 RCTs, n=1851, RR 1.19 CI 0.78 to 1.8). Leaving early due to inefficacy showed an almost significant superiority of risperidone (24% versus 19%, 7 RCTs, n=1851, RR 1.26 CI 0.99 to 1.61).
3.3 Mental state
3.3.1 General mental state: no clinically important change - short term (less than 30% PANSS total score reduction from baseline)
There was no significant difference (2 RCTs, n=984, RR 1.11 CI 0.87 to 1.42), but the results were heterogeneous. We therefore present the single studies separately: Potkin 2006 (n=177, RR 1.27 CI 1.05 to 1.55) and Zhong 2006a (n=495, RR 1.0 CI 0.91 to 1.09).
3.3.2 General mental state: no clinically important change - short term (less than 20% BPRS total score reduction) There was no significant difference (1 RCT, n=25, RR 0.98 CI 0.63 to 1.52).
3.3.3 General mental state: average score at endpoint - PANSS total
There was a significant difference in favour of risperidone: overall (9 RCTs, n=1953, WMD 3.09 CI 1.01 to 5.16), short term (5 RCTs, n=1064, WMD 2.44 CI −0.81 to 5.69), medium term (2 RCTs, n=146, WMD 6.27 CI −3.94 to 16.48), long term (2 RCTs, n=743, WMD 3.11 CI 0.40 to 5.82)
3.3.4 General mental state: average score at endpoint - short term - BPRS total
There was no significant difference (1 RCT, n=25, WMD 1.68 CI −8.33 to 11.69).
3.3.5 Positive symptoms - no clinically important change - short term (less than 40% PANSS positive score reduction from baseline)
There was no significant difference (1 RCT, n=673, RR 1.00 CI 0.9 to 1.12).
3.3.6 Positive symptoms: average score at endpoint - PANSS positive subscore
There was a significant difference favouring risperidone overall (7 RCTs, n=1264, WMD 1.82 CI 1.16 to 2.48), short term (4 RCTs, n=1037, WMD 2.10 CI 1.00 to 3.19), medium term (2 RCTs, n=146, WMD 2.15 CI −0.01 to 4.31), long term (1 RCT, n=81, WMD 1.30 CI −0.13 to 2.73)
3.3.7 Positive symptoms: average score at endpoint - short term-BPRS positive subscore
There was a significant difference favouring risperidone (1 RCT, n=25, WMD 1.1 CI 0.18 to 2.02).
3.3.8 Negative symptoms - no clinically important change - short term (less than 40% PANSS negative score reduction from baseline)
There was no significant difference (1 RCT, n=673, RR 0.98 CI 0.93 to 1.04).
3.3.9 Negative symptoms: average score at endpoint PANSS negative subscore
There was no significant difference (short term studies, 4 RCTs, n=956, WMD −1.46 CI −4.11 to 1.19; medium term studies, 2 RCTs, n=146, WMD 1.3 CI −0.75 to 3.35; long term stud, 1 RCT, n=81, WMD 0.8 CI −0.64 to 2.24). The short-term results were highly heterogeneous. Excluding a small outlier study (Riedel 2005) in a sensitivity analysis there was a significant superiority of risperidone (6 RCTs, n=1139, WMD 0.79 CI 0.04 to 1.54).
3.3.10 Negative symptoms: average score at endpoint - short term - BPRS negative subscore
There was a significant difference favouring risperidone (1 RCT, n=25, WMD 0.57 CI 0.17 to 0.97).
3.4 Quality of life: average endpoint score - short term - QLS total score
There was no significant difference (1 RCT, n=25, WMD −0.5 CI −13.87 to 12.87).
3.5 Service use: number of participants re-hospitalised
The difference almost reached statistical significance with a slight benefit for the risperidone group (2 RCTs, n=877, RR 1.34 CI 1.0 to 1.79).
3.6 Adverse effects
3.6.1 General: at least one adverse effect
There was no significant difference (8 RCTs, n=2226, RR 1.04 CI 0.93 to 1.17).
3.6.2 Death
There was no significant difference (7 RCTs, n=3066, RR 0.73 CI 0.17 to 3.09).
3.6.3 Cardiac effects
3.6.3.1. Number of participants with QTc prolongation
There was no significant difference (2 RCTs, n=1351, RR 0.87 CI 0.29 to 2.55).
3.6.3.2 Mean change of QTc interval from baseline in ms
Overall there was no significant difference (3 RCT, n= 940, WMD 2.21 CI −5.05 to 9.48). The data were heterogeneous. In the individual studies Lieberman 2005 found a significant difference in favour of risperidone (n=432, WMD 5.7 CI 0.57 to 10.83), while Stroup 2006 (n=166, WMD 6.3 CI −3.41 to 16.01) and Zhong 2006a (n=342, WMD −3.6 CI −7.55 to 0.35) found no significant difference between groups.
3.6.4 Central nervous system - sedation
There was a significant difference favouring risperidone (8 RCTs, n=2226, RR 1.21 CI 1.06 to 1.38, NNH 20 CI 11 to 50).
3.6.5 Extrapyramidal effects
3.6.5.1 Extrapyramidal effects
Quetiapine produced fewer movement disorders than risperidone in terms of ’extrapyramidal symptoms’ (2 RCTs, n=872, RR 0.59 CI 0.43 to 0.81, NNH 14 CI 8 to 33), dystonia (1 RCT, n= 673, RR 0.06 CI 0.01 to 0.41, NNH 20 CI 13 to 33) and use of antiparkinson medication at least once (6 RCTs, n=1715, RR 0.5 CI 0.3 to 0.86, NNH 20 CI 10 to 100). However, there was no significant difference in akathisia (6 RCTs, n=2170, RR 0.62 CI 0.34 to 1.13), akinesia (1 RCT, n=267, RR 0.91 CI 0.61 to 1.37) or parkinsonism (1 RCT, n=44, RR 0.06 CI 0.0 to 0.96).
3.6.5.2 As measured by scales
Quetiapine produced fewer extrapyramidal side effects than risperidone according to the Simpson-Angus Scale (5 RCTs, n= 1077, WMD −0.59 CI −1.16 to −0.02). There was no significant difference in dyskinesia (AIMS, 2 RCTs, n=958, WMD −0.34 CI-0.75 to 0.08) and akathisia (BAS, 2 RCTs, n=700, WMD −0.73 CI −2.0 to 0.54).
3.6.6 Haematological: important decline in white blood cells
There was no significant difference (1 RCT, n=673, RR 2.97 CI 0.12 to 72.73).
3.6.7 Prolactin
3.6.7.1 Prolactin associated adverse effects
Quetiapine produced significantly fewer cases of amenorrhoea (4 RCTs, n=359, RR 0.47 CI 0.28 to 0.79, NNT not estimable), galactorrhoea (5 RCTs, n=478, RR 0.38 CI 0.17 to 0.84, NNT 25 CI 13 to 100) and gynaecomastia (1 RCT, n=78, RR 0.23 CI 0.07 to 0.75, NNT 4 CI 2 to 11), but not dysmenorrhoea (1 RCT, n=163, RR 0.45 CI 0.08 to 2.38). Data on sexual dysfunction showed an almost significant superiority of quetiapine (6 RCTs, n=2157, RR 0.70 CI 0.48 to 1.01).
3.6.7.2 Change from baseline in ng/ml
There was a significant and consistent difference favouring quetiapine although the amount of the difference varied leading to statistical heterogeneity (6 RCTs, n=1731, WMD −35.28 CI −44.36 to −26.19; the results of the single studies were: Lieberman 2005, n=678, WMD −24.70 CI −28.72 to −20.68; McEvoy 2006, n=24, WMD −28.6 CI −43.02 to −14.18; Potkin 2006 n=309, WMD −50.4 CI −60.24 to −40.56; Stroup 2006, n=199, WMD −30.3 CI −37.1 to −23.5; Zhong 2006a, n=440, WMD −47.0 CI −52.97 to −41.03), McEvoy 2007, n=81, WMD −30.8 CI −38.1 to −23.5).
3.6.8 Metabolic
3.6.8.1 Cholesterol - number of participants with a significant cholesterol increase
There was no significant difference (2 RCTs, n=940, RR 1.27 CI 0.72 to 2.24).
3.6.8.2 Cholesterol - mean change from baseline in mg/dl
There was a significant difference favouring risperidone (5 RCTs, n=1433, WMD 8.61 CI 4.66 to 12.56).
3.6.8.3 Glucose - number of participants with abnormally high fasting glucose
There was no significant difference (2 RCTs, n=940, RR 1.39 CI 0.56 to 3.45).
3.6.8.4 Glucose - mean change from baseline in mg/dl
There was no significant difference (5 RCTs, n=1436, WMD −0.04 CI −2.92 to 2.83).
3.6.8.5 Weight gain - number of participants with 7% or more gain of total body weight
There was no significant difference (7 RCTs, n=1942, RR 0.97 CI 0.82 to 1.14).
3.6.8.6 Weight gain - mean change from baseline in kg
Overall there was no significant difference, but the data were highly heterogeneous presumably due to one small outlier study (Atmaca 2003) that showed a dramatic advantage of risperidone (7 RCTs, n=1446, WMD 0.71 CI −1.04 to 2.47). Nevertheless, excluding this study did not change the overall result.
3.7 Publication bias
A reasonable funnel plot analysis was only possible for the PANSS total score (>10 included studies). It did not suggest a possible publication bias.
3.8 Investigation for heterogeneity and sensitivity analysis
Excluding Mori 2004 from the evaluation of the PANSS positive subscore due to possibly skewed data did not reveal markedly different results. The data on akathisia (Barnes Akathisia Scale) indicated a considerable heterogeneity but clear reasons explaining this could not be found.
4. Comparison 4. QUETIAPINE versus ZIPRASIDONE
Two studies met the inclusion criteria for the comparison quetiapine versus ziprasidone.
4.1 Leaving the study early
There was no significant difference in the number of participants leaving the studies early due to any reason (2 RCTs, n=722, RR 1.05 CI 0.97 to 1.13), due to adverse events (2 RCTs, n=722, RR 1.04 CI 0.72 to 1.49) or due to inefficacy of treatment (2 RCTs, n=722, RR 1.14 CI 0.89 to 1.47).
4.2 Mental state
4.2.1 General mental State: average score at endpoint - PANSS total
There was no significant difference, but the data of two studies were heterogeneous and are therefore presented separately. Neither Stroup 2006 (medium term data) n=198, WMD 3.7 CI −2.97 to 10.37 nor Lieberman 2005 (long term data) n=512, WMD −2.78 CI −6.81 to 1.25 found a a significant difference between groups.
4.2.2 Positive Symptoms: average score at endpoint - medium term - PANSS positive subscore
There was no significant difference (1 RCT, n=198, WMD 0.0 CI −2.18 to 2.18).
4.2.3 Negative Symptoms: average score at endpoint - medium term - PANSS negative subscore
There was no significant difference (1 RCT, n=198, WMD 1.6 CI −0.34 to 3.54).
4.3 Service use: number of participants re-hospitalised
There was no significant difference neither in the overall analysis (2 RCTs, n=754, RR 1.17 CI 0.85 to 1.59) nor in the analysis of medium term data (1 RCT, n=232, RR 1.25 CI 0.71 to 2.17), or long term data (1 RCT, n=522, RR 1.13 CI 0.78 to 1.65)
4.4 Adverse effects
4.4.1 General - at least one adverse effect
There was no significant difference (2 RCTs, n=754, RR 1.03 CI 0.91 to 1.17).
4.4.2 Death
There was no significant difference (2 RCTs, n=754, RR 0.41 CI 0.05 to 3.15).
4.4.3 Cardiac effects
4.4.3.1 Number of participants with QTc prolongation
There was no significant difference (1 RCT, n=522, RR 1.65 CI 0.34 to 8.08).
4.4.3.2 mean change of QTc interval ms
There was no significant difference (2 RCTs, n=549, WMD 3.41 CI −1.37 to 8.18).
4.4.4 Central nervous system -sedation
Significantly fewer participants in the ziprasidone group than in the quetiapine group felt sedated (2 RCTs, n=754, RR 1.36 CI 1.04 to 1.77, NNH 14 CI 7 to 100).
4.4.5 Extrapyramidal effects
Significantly fewer people in the quetiapine group used antiparkinson medication at least once (1 RCT, n=522, RR 0.43 CI 0.2 to 0.93), but there were no clear differences in akathisia (2 RCTs, n=754, RR 0.78 CI 0.42 to 1.45) or ’any extrapyramidal symptoms’ (1 RCT, n=232, RR 2.02 CI 0.66 to 6.17).
4.4.6 Prolactin
4.4.6.1 Prolactin-associated adverse effects
There was no significant difference in amenorrhoea (1 RCT, n=138, RR 0.43 CI 0.15 to 1.24), galactorrhoea (2 RCTs, n=202, RR 0.68 CI 0.23 to 2.01) or sexual dysfunction (2 RCTs, n=754, RR 0.96 CI 0.64 to 1.42).
4.4.6.2 Mean change from baseline in ng/ml
There was a significant difference in favour of quetiapine (2 RCTs, n=754, WMD −4.77 CI −8.16 to −1.37).
4.4.7 Metabolic
4.4.7.1 Cholesterol - mean change from baseline in mg/dl
Ziprasidone was associated with significantly less cholesterol increase than quetiapine (2 RCTs, n=754, WMD 16.01 CI 8.57 to 23.46).
4.4.7.2 Glucose - mean change from baseline in mg/dl
There was no significant difference (2 RCTs, n=754, WMD 3.1 CI −3.99 to 10.19).
4.4.7.3 Weight gain - number of participants with 7% or more gain of total body weight
Significantly more participants in the quetiapine group than in the ziprasidone group gained weight (2 RCTs, n=754, RR 2.22 CI 1.35 to 3.63, NNH 13 CI 8 to 33).
4.4.7.4 Weight gain - change from baseline in kg
There was a superiority of ziprasidone which almost reached statistical significance (1 RCT, n= 466, WMD 1.2 CI −0.05 to 2.45).
4.5 Publication bias
Due to small number of included studies we did not perform a funnel plot analysis.
4.6 Investigation for heterogeneity and sensitivity analysis
The reasons for the preplanned sensitivity analysis did not apply and were therefore not performed.
DISCUSSION
Summary of main results
1. General
This analysis of the effects of quetiapine compared with other second generation antipsychotic drugs in the treatment of schizophrenia currently includes 21 studies reporting data on only four of eight possible comparisons. High discontinuation rates (overall 57.6 %) limit the value of findings. In addition, 15 of the 21 included studies randomised less than 100 people. The duration of the trials was usually short and we identified only two long term studies. Short term trials are not ideal to judge efficacy and tolerability of treatments for a chronic disease. Nine of the 21 studies were sponsored by a pharmaceutical industry with a clear pecuniary interest in the result. This is likely to be a further problem.
2. Comparison 1. QUETIAPINE versus CLOZAPINE
Five studies with a total of 334 participants fell into this comparison.
2.1 Leaving the studies early
The overall rate of participants leaving studies early was remarkably low (8.4%) and showed no clear difference between groups. Nevertheless, this finding was based on only two small (n=135), short term trials limiting any interpretation.
2.2 Efficacy outcomes (global state, overall and specific mental state)
There was no significant difference in global state, general mental state or positive symptoms. Quetiapine reduced negative symptoms more than clozapine, but this result must be interpreted with great caution as it was based on two small trials from China (Li 2003b, Li 2005).
2.3 Adverse effects
We found limited data on ‘at least one adverse effect’, cardiac effects, extrapyramidal symptoms, sedation, weight gain and white blood cell count. Results on ‘at least one adverse effect’, cardiac effects and sedation indicated an advantage for quetiapine. As these findings were based on only one or two studies they can not be considered to be robust.
3. Comparison 2. QUETIAPINE versus OLANZAPINE
Most of the studies included in the review contributed data to this comparison (N=13, n=1820).
3.1 Leaving the studies early
Less people in the olanzapine group compared with the quetiapine group left studies early because of ‘any reason’ or due to ‘inefficacy of treatment’. This finding suggests that olanzapine is a more acceptable treatment than quetiapine, at least in the confines of clinical trials. Nevertheless, the overall rate of premature study discontinuations was high (63.2%), limiting the validity of all other results.
3.2 Efficacy outcomes (global state, overall and specific mental state)
Quetiapine seems to be slightly less effective than olanzapine for the general mental state and for positive symptoms. There was no significant difference in the reduction of negative symptoms. The interpretation of the latter finding is, however, limited by the fact that most studies included participants with predominant positive symptoms. Such studies are not ideal for evaluating the effects of antipsychotic drugs on negative symptoms.
3.3 General functioning and quality of life
Very limited data on these important outcomes are available. Olanzapine may improve general functioning (GAF total score) more than quetiapine, but this result was based on a single study and needs to be replicated. There are no data indicating a difference in measures of quality of life.
3.4 Service use: number of participants re-hospitalised
The number of participants re-hospitalised was significantly higher in the quetiapine group. Again, this may reflect a certain efficacy advantage of olanzapine, but as this result was based on only two studies more data are needed.
3.5 Adverse effects
Adverse effects were reported as at least one adverse effect, cardiac effects, QTc abnormalities, increase of serum cholesterol, serum glucose, serum prolactin and associated side effects, death, extrapyramidal symptoms, the occurrence of sedation, seizures and weight gain. Among these adverse effects a benefit for quetiapine was found for the use of antiparkinson medication (a proxy measure for extrapyramidal adverse effects), weight gain, glucose elevation, prolactin increase, and some prolactin-associated adverse effects. On the other hand there was a certain superiority of olanzapine in terms of QTc prolongation. Overall, it seems that quetiapine may be more tolerable than olanzapine, but this is weighed against slightly less efficacy.
4. Comparison 3. QUETIAPINE versus RISPERIDONE
Eleven studies with 3770 participants met the inclusion criteria for this comparison.
4.1 Leaving the studies early
There was no clear difference in the number of participants leaving the studies early suggesting a similar overall acceptability of quetiapine and risperidone. Nevertheless, the overall discontinuation rate was high (56.7%) limiting the interpretation of all other results.
4.2 Efficacy outcomes (global state, overall and specific mental state)
The only differences in efficacy were found for the general mental state and positive symptoms. Quetiapine was less effective than risperidone in these aspects of psychopathology. Nevertheless, the differences were small (e.g. only three points on the PANSS total score).
4.3 Adverse effects
Adverse effects were available for at least one adverse effect, cardiac effects, cholesterol increase, changes in serum glucose, increase of prolactin level and associated side effects, death, extrapyramidal adverse effects, sedation, weight gain and white blood cell count. Among these, quetiapine was better than risperidone in various measures of extrapyramidal adverse effects and prolactin associated effects. On the other hand quetiapine was associated with more sedation and cholesterol increase than risperidone. These differences in the adverse effect profile and the slightly lower efficacy of quetiapine may be weighed in drug choice.
5. Comparison 4. QUETIAPINE versus ZIPRASIDONE
Only two studies with 722 participants provided data on this comparison.
5.1 Leaving the studies early
The overall number of participants leaving the studies early very high (80.7%), clearly limiting the interpretation of any findings beyond the outcome of ‘leaving the study early’. There was no significant difference between groups, but the acceptability of both compounds seems to be poor.
5.2 Efficacy outcomes (global state, overall and specific mental state)
Various efficacy outcomes revealed no difference between quetiapine and ziprasidone. There is currently no randomised data suggesting that either drug should be preferred due to better efficacy.
5.3 Adverse effects
Adverse effects were reported as at least one adverse effect, cardiac effects, death, extrapyramidal side effects, changes in cholesterol, glucose and prolactin, the occurrence of sedation and weight gain. Among those reported there was an advantage of quetiapine in use of antiparkinson medication and prolactin levels, while weight gain and sedation favoured ziprasidone. Treatment decisions should take these differences in the adverse effect profiles into account.
Overall completeness and applicability of evidence
We did not identify a single study for almost half of the possible comparisons of quetiapine with other second generation antipsychotic drugs. Evidence, therefore, is incomplete. Only two studies were long term, limiting applicability of the evidence as, after all, schizophrenia is often a chronic, often life-long, disorder. Furthermore, most of the included studies were efficacy studies, therefore external validity is limited and further effectiveness [pragmatic/real world] studies are needed.
Quality of the evidence
All studies were randomised and at least single-blind, but details were rarely presented. Therefore it is unclear in almost all studies whether randomisation and blinding were really appropriately done. Furthermore the high numbers of participants leaving the studies early (overall 57.6%) and the small number of long term studies (Lieberman 2005, McEvoy 2007, Voruganti 2007) call the validity of the findings into question. Selective reporting was evident in all but two studies and nine studies were industry sponsored. All these factors limit the quality of the evidence.
Potential biases in the review process
We are not aware of obvious flaws in our review process.
Agreements and disagreements with other studies or reviews
A previous Cochrane review compared the effects of quetiapine with placebo, first generation antipsychotic drugs and second generation antipsychotic drugs for schizophrenia (Srisurapanont 2004). A single study fell in the last category and compared quetiapine with risperidone. This update and reformatting of the review has identified many new studies, and data are far more comprehensive.
AUTHORS’ CONCLUSIONS
Implications for practice
1. For people with schizophrenia
For people with schizophrenia it may be important to know that most people who start the drug within short trials choose to stop taking it within a few weeks. Quetiapine may also be slightly less effective than risperidone and olanzapine. Quetiapine may have low risk for extrapyramidal adverse effects and prolactin increase and may lead to less weight gain and associated problems than olanzapine, but more so than risperidone and ziprasidone.
2. For clinicians
Clinicians should know that, for only four out of eight possible comparisons of quetiapine with other second generation antipsychotic drugs, relevant studies were identified and that the evidence is limited because very high rates of participants leave the studies early. Our most robust finding is that if a person is started on quetiapine most will be off this drug within a few weeks. Certainly, more studies comparing quetiapine with other second generation antipsychotic drugs are needed.
3. For managers/policy makers
Little information on service use (such as time in hospital) or functioning is available, but the limited data suggest that people on quetiapine may need to be hospitalised more frequently than those receiving risperidone or olanzapine. This may be accompanied by higher overall costs in some settings. Furthermore, a single study suggested better general functioning of participants treated with olanzapine. We do not feel that these findings are sufficiently robust to guide managers.
Implications for research
1. General
We stress how important it is that future studies strictly adhere to the CONSORT statement (Moher 2001). Following these recommendations would clearly improve the conduct and reporting of clinical trials.
2. Specific
Comparisons with amisulpride, aripiprazole, sertindole and zotepine do not exist. Most data that has been reported within existing comparisons are almost without value because of the assumptions and biases within them. There is, therefore, plenty of room for further research into the effects of this widely used drug. We realize that planning for such studies needs meticulous attention to detail but do suggest some pointers that have come from our reading and understanding of the existing trials (see Table 1).
Table 1.
Suggested design of future study
| Methods | Allocation: randomised - clearly described generation of sequence and concealment of allocation. Blindness: double - described and tested. Duration: 6 months minimum. |
| Participants | Diagnosis: schizophrenia (operational criteria). N=2700.* Age: any. Gender: both. History: any. |
| Interventions |
|
| Outcomes | Leaving study early (any reason, adverse events, inefficacy). Service outcomes: hospitalised, time in hospital, attending out patient clinics. Global impression: CGI**, relapse. Mental state: PANSS. Adverse events: UKU. Employment, family satisfaction, patient satisfaction. |
power calculation suggested 300/group would allow good chance of showing a 10% difference between groups for primary outcome.
Primary outcome
PLAIN LANGUAGE SUMMARY.
Quetiapine versus other atypical antipsychotic drugs for schizophrenia
This review compares the effects of quetiapine compared with other second generation antipsychotic drugs. There was a high number of participants leaving the studies early and we identified random controlled trials for only half of the possible drug comparisons. This limits the interpretation of the relative effects of quetiapine compared with other second generation antipsychotic drugs. Nevertheless, quetiapine may be slightly less effective than olanzapine and risperidone. It produced comparably few extrapyramidal symptoms, and prolactin increase. It produced less weight gain than olanzapine but more so than risperidone and ziprasidone.
ACKNOWLEDGEMENTS
We would like to thank the editorial base of the Cochrane Schizophrenia Group for its assistance. We would also like to thank the following authors for providing additional information on their studies: R Conley, Y Liu, H Ozguven, M Riedel, J Svestka and K Zhong.
SOURCES OF SUPPORT
Internal sources
Psychiatrische Klinik, Klinikum rechts der Isar, Tu München, Freistaat Bayern, Germany.
External sources
Bundesministerium für Bildung und Forschung, Nr FKZ: 01 KG 0606, GZ:GF-GFKG01100506, Germany.
CHARACTERISTICS OF STUDIES
Characteristics of included studies [ordered by study ID]
| Methods | Allocation: random, no further details. Blindness: single, rater-blinded. Duration: 6 weeks. Design: parallel. Location: single centre. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia. N=56. Gender: 24 M, 29 F. Age: 19-46 years (mean clozapine=31.3 years, mean olanzapine=29.6 years, mean quetiapine=30.1 years, mean risperidone=27.9 years, mean control group=32.1 years). History: duration ill mean clozapine=6.6 years, mean olanzapine=6.3 years, mean quetiapine=5.9 years, mean risperidone=5.6, age at onset: not reported |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason. Mental state: PANSS total score. Adverse effects: EPS (use of antiparkinson medication), weight gain (BMI), laboratory (serum leptin, triglycerid levels) |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Random, no further details. |
| Allocation concealment? | Unclear | No further details. |
| Blinding? Subjective outcomes |
Unclear | Single, rater-blind. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? Objective outcomes |
Yes | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
Yes | The overall attrition was low (5.4%). Reason for leaving early were not assessed, only completer data were presented. But due to the very low rate we do not think that there was a risk of bias |
| Free of selective reporting? | Yes | Probably free of bias. The study focused on serum leptin and triglyceride levels which were adequately described |
| Free of other bias? | Unclear | Data on the allowed dose range have not been presented. Furthermore, the pre-study treatment was quite heterogeneous as 19 participants had never taken any psychotropic drugs while most other participants had a long history of previous treatment |
| Methods | Allocation: random, no further details. Blindness: double, no further details. Duration: 12 weeks. Design: parallel. Location: not reported. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia, treatment resistance, persistant positive psychotic symptoms, BPRS total score of 35 or more plus CGI score of 4 or more. N=38. Gender: 30 M, 8 F. Age: 18-65 years (mean fluphenazine=44.2 years, mean quetiapine=43.7 years, mean risperidone=46.3 years). History: duration ill not reported, age at onset not reported. Setting: inpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Global State: CGI. Mental State: BPRS total score, BPRS positive subscore, BPRS negative subscore. Cognitive functioning: Neuropsychological testing. Quality of life: QLS. Adverse effects: open interviews, EPS (use of antiparkinson medication, SAS), prolactin increase, sexual dysfunction, sedation, weight gain, laboratory (thyroidal hormones) Unable to use- Prolactin increase: no useable data. Sexual dysfunction: no useable data. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Random, no further details. |
| Allocation concealment? | Unclear | No further details. |
| Blinding? Subjective outcomes |
Unclear | Double, no further details. Whether blinding was successful has not been examined, but both compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? Objective outcomes |
Yes | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
No | Overall attrition was moderate 36%. The analysis was based on mixed effect models. It is unclear whether this statistical method can account for such a relatively high attrition rate |
| Free of selective reporting? | No | Not all of the predefined adverse effects were reported. |
| Free of other bias? | Unclear | There was a slight baseline imbalance in terms of mean age and the mean number of previous hospitalisations (14 in the risperidone and 9.7 in the quetiapine group) |
| Methods | Allocation: random, computer-generated randomisation. Blindness: double, identical capsules. Duration: 26 weeks. Design: parallel. Location: multicentre. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia (n=230), schizoaffective disorder (n=116), prominent negative symptoms. N=346. Gender: 228 M, 118 F. Age: mean olanzapine=41.67 years, mean quetiapine=40.45 years. History: duration ill mean olanzapine=17.57 years, quetiapine=17.78 years, age at onset mean olanzapine=24.16 years, quetiapine=22.59 years. Setting: outpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Mental State: PANSS total score, PANSS positive subscore, PANSS negative subscore, SANS total score, depression (Calgary Depression Scale). General functioning: GAF, Case Manager Rating Scale, Patient Functioning Rating Scale. Quality of life: QLS total score. Adverse effects: Sedation, weight gain, laboratory (hematology, uric acid) Unable to use- Leukopenia: no useable data. Use of antiparkinson medication: no data. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Yes | Random, computer-generated randomisation. |
| Allocation concealment? | Unclear | No further details. |
| Blinding? Subjective outcomes |
Unclear | Double, identical capsules. Whether blinding was successful has not been examined, but both compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? Objective outcomes |
Yes | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
No | The overall attrition was very high (54.9%). The last-observation-carried-forward method was used to account for people leaving the study early. It assumes that a participant who discontinued the study would not have had a change of his condition if he had remained in the study. This assumption can obviously be wrong |
| Free of selective reporting? | No | The numbers of participants who received antiparkinson medication or who had leukopenia were not indicated |
| Free of other bias? | No | The study was sponsored by the manufacturer of olanzapine. |
| Methods | Allocation: random, no further details. Blindness: double, no further details. Duration: 12 weeks. Design: parallel. Location: single centre. |
|
| Participants | Diagnosis: (CCMD-3) schizophrenia. N=67. Gender: not reported. Age: mean=26.18 years. History: duration ill mean clozapine=0.49 years, mean quetiapine=0.5 years, age at onset not reported. Setting: inpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason. Mental State: PANSS total score, PANSS positive subscore, PANSS negative subscore. Adverse effects: EPS, sedation. Unable to use- Extrapyramidal symptoms: no data. Sedation: no data. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Random, no further details. |
| Allocation concealment? | Unclear | No further details. |
| Blinding? Subjective outcomes |
Unclear | Double, no further details. Whether blinding was successful has not been examined, but both compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? Objective outcomes |
Yes | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
No | The overall attrition was 9.1%. Numbers leaving early were only reported due to any reason. Only completer data were assessed |
| Free of selective reporting? | No | There was no reporting on adverse effects. |
| Free of other bias? | Unclear | Baseline characteristics have not been presented for both groups separately. Therefore, baseline imbalance can not be excluded. Furthermore, there was no washout period |
| Methods | Allocation: random, no further details. Blindness: double, no further details. Duration: 8 weeks. Design: parallel. Location: single centre. |
|
| Participants | Diagnosis: (CCMD-3) schizophrenia. N=63. Gender: not reported. Age: mean clozapine=30 years, mean quetiapine=28 years. History: duration ill mean clozapine=0.63 years, mean quetiapine=0.65 years, age at onset not reported. Setting: in- and outpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason. Global State: CGI. Mental State: PANSS total score. Adverse effects: open interviews, cardiac effects (palpitation), EPS (akathisia, rigor, tremor), sedation, weight gain, laboratory (white blood cell count) Unable to use - Leaving the study early: due to adverse events (not fully reported) |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Random, no further details. |
| Allocation concealment? | Unclear | No further details |
| Blinding? Subjective outcomes |
Unclear | Double, probably identical capsules. Whether blinding was successful has not been examined, but both compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? Objective outcomes |
Yes | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
Unclear | Two participants left the study early due to adverse events in the clozapine group. There is some doubt whether all data on leaving the study early have been presented |
| Free of selective reporting? | Yes | We did not find evidence for selective reporting. |
| Free of other bias? | Unclear | There were no data on pre study medication, therefore baseline imbalance can not be excluded |
| Methods | Allocation: random, no further details. Blindness: single, rater-blinded. Duration: 8 weeks. Design: parallel. Location: single centre. |
|
| Participants | Diagnosis: (CCMD-2) schizophrenia. N=76. Gender: not reported. Age: mean clozapine=36.2 years, mean quetiapine=34.7 years. History: duration ill mean clozapine=6.12 years, mean quetiapine=5.71 years, age at onset not reported. Setting: inpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early. Global State. General Mental State: PANSS total score, PANSS positive subscore, PANSS negative subscore. Adverse effects: treatment emergent symptom scale. Unable to use - Leaving the study early: not fully reported. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Random, no further details. |
| Allocation concealment? | Unclear | No further details. |
| Blinding? Subjective outcomes |
Unclear | Single, rater-blind. Whether blinding was successful has not been examined, but both compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? Objective outcomes |
Yes | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
Unclear | One participant in the quetiapine group left the study early due to inefficacy. This participant was not included in the analysis. There is some doubt whether all data on leaving the study early have been presented |
| Free of selective reporting? | No | The study duration was eight weeks, but outcomes only at four weeks were available |
| Free of other bias? | Unclear | The allowed dose range was not indicated. |
| Methods | Allocation: random, no further details. Blindness: double, identical capsules. Duration: 78 weeks. Design: parallel. Location: multicentre. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia, previously more than one schizophrenic episode, responder. N=1493. Gender: 1080 M, 380 F. Age: 18-65 years (mean=40.6 years). History: duration ill, age at onset: not reported. Setting: in- and outpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Global State: CGI-S. Mental State: PANSS total score. Service use: number of patients re-hospitalised. Adverse effects: open interviews, Death (suicide attempt), EPS (use of antiparkinson medication, akathisia), cardiac effects (ECG), prolactin-associated side-effects, sedation, weight gain, laboratory (prolactin, lipids, glucose) Unable to use - Leaving the study early: due to extrapyramidal effects (no usable data) |
|
| Notes | 33 participants were excluded from the analysis. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Random, no further details. |
| Allocation concealment? | Unclear | No further details. |
| Blinding? Subjective outcomes |
Unclear | Double, identical capsules. Whether blinding was successful has not been examined, but the examined compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? Objective outcomes |
Yes | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
No | The attrition rate was very high (75%). Continuous outcomes were evaluated based on a mixed effects model. It is unclear whether any statistical method can account for such a high attrition rate |
| Free of selective reporting? | Yes | There was no evidence for selective reporting. |
| Free of other bias? | Unclear | Dose ranges were quite different, the upper dose range of olanzapine was 30 mg whereas risperidone could only be titrated up to 6mg/day. There was no wash-out period. An overlap in the administration of formerly given antipsychotics was permitted for the first four weeks after randomisation. Allocation to ziprasidone treatment was not possible from the start of the study due to later availability of ziprasidone |
| Methods | Allocation: random, no further details. Blindness: single, rater-blinded. Duration: 12 weeks. Design: parallel. Location: single centre. |
|
| Participants | Diagnosis: (CCMD-3) schizophrenia. N=72. Gender: not reported. Age: mean clozapine=37.44 years, mean quetiapine=36.86 years. History: duration ill mean clozapine=9.36 years, mean quetiapine=8.64 years, age at onset: not reported. Setting: inpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Global State. Mental State: BPRS total, SANS total. Adverse effects: open interviews, cardiac effects (ECG) EPS (akathisia, tremor), sedation, dry mouth, hypersalivation, hypotonia, liver function, dizziness, hyperemesis, maldigestion, weight gain Unable to use - Leaving the study early: due to any reason (not fully reported) |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Random, no further details. |
| Allocation concealment? | Unclear | No further details. |
| Blinding? Subjective outcomes |
Unclear | Single, rater-blind. Whether blinding was successful has not been examined, but both compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? Objective outcomes |
Yes | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
Unclear | Five participants left the study early, three due to adverse events in the clozapine group and two due to unclear reasons in the quetiapine group. These five participants were not included in the analysis. There is some doubt whether all data on leaving the study early have been presented |
| Free of selective reporting? | No | The mean doses of the medications used were not indicated. |
| Free of other bias? | No | Clozapine was titrated to 400mg/day within 10 days. Such a fast dose increase can be accompanied by a higher rate of adverse effects |
| Methods | Allocation: random, no further details. Blindness: double, identical capsules. Duration: 52 weeks (26 weeks observed, because of small group sizes). Design: parallel. Location: multicentre. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia, inadequate efficacy in previous study, clozapine treatment (n=49) was open-label. N=99 (observed N=50). Gender: 80 M, 19 F. Age: 18-65 years (mean=39.7 years). History: duration ill, age at onset: not reported. Setting: in- and outpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Global state: CGI. Mental State: PANSS total score, PANSS positive subscore, PANSS negative subscore. Adverse effects: open interviews, amenorrhoea, galactorrhoea, sexual dysfunction, sedation, laboratory (lipids, glucose, prolactin, haemoglobin A1C level), weight gain Unable to use - Global state: CGI (no data). |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Random, no further details. |
| Allocation concealment? | Unclear | No further details. |
| Blinding? Subjective outcomes |
Unclear | Double, identical capsules. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side-effects. This can be a problem for blinding |
| Blinding? Objective outcomes |
Yes | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
No | The overall attrition was extremely high (74%). We doubt that the validity of the findings was not affected by this high number |
| Free of selective reporting? | No | Due to small numbers and the very high attrition only data on 26 weeks treatment (rather than the full duration of 52 weeks) were presented |
| Free of other bias? | Unclear | The dose ranges were quite different, the upper dose range of olanzapine was 30 mg/day whereas risperidone could only be given in a maximum dose of 6 mg/day. Patients had a history of former inefficacy to one of the medications. It was excluded that the same medication could be given again but still this might implicate a risk of bias due to baseline imbalance in terms of former treatment. There was no wash-out phase |
| Methods | Allocation: random, no further details. Blindness: double, no further details. Duration: 52 weeks. Design: parallel. Location: multicentre. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia (n=231), schizophreniform disorder (n=115) or schizoaffective disorder (n=54), first episode, psychotic symptoms for 1 month to 5 years, PANSS psychosis and CGI-S score of 4 or more. N=400. Gender: 292 M, 108 F. Age: 16-40 years (mean=24.5 years). History: duration ill mean=1.08 years, age at onset 23.5 years. Setting: in- and outpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Global State: CGI. Mental State: PANSS total, PANSS positive subscore, PANSS negative subscore, depression Calgary depression scale. Adverse effects: open interviews, death (suicide attempt, suicide, EPS (akathisia, akinesia, use of antiparkinson medication, laboratory (cholesterol, fasting glucose, prolactin), prolactin associated side effects (amenorrhoea, galactorrhoea, gynaecomastia, sexual dysfunction), sedation, insomnia, dry mouth, orthostatic faintness, constipation, sialorrhoea, skin rash, gynaecomastia, urinary hesitancy, incontinence, weight gain (BMI, waist circumference) |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Random, no further details. |
| Allocation concealment? | Unclear | No further details. |
| Blinding? Subjective outcomes |
Unclear | Double, no further details. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? Objective outcomes |
Yes | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
No | The overall attrition was high (70.3.%). The primary analysis was based on a mixed effect model, secondary outcomes used the last-observation-carried forward approach and included only study completers. Nevertheless, it is unclear whether any statistical method can account for such a high attrition |
| Free of selective reporting? | No | Adverse events were presented only in case of moderate or worse severity |
| Free of other bias? | No | The study was sponsored by the manufacturer of quetiapine. |
| Methods | Allocation: random, no further details. Blindness: double, no further details. Duration: 8 weeks (last 4 weeks observed). Design: parallel. Location: single centre. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia disorganised (n=23), paranoid (n=10), undifferentiated (n=34). N=77. Gender: 39 M, 38 F. Age: 28-84 years (mean=59.9 years). History: duration ill mean=34.51 years, age at onset: not reported. Setting: inpatient. |
|
| Interventions |
|
|
| Outcomes | Mental State: PANSS total score, PANSS positive subscore, PANSS negative subscore. Cognitive functioning: digit span distractibility test. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Random, no further details. |
| Allocation concealment? | Unclear | No further details. |
| Blinding? Subjective outcomes |
Unclear | Double, no further details. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? Objective outcomes |
Yes | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
No | Data on leaving the study early have not been presented. |
| Free of selective reporting? | No | Adverse events were not reported. Numbers on use of antiparkinson medication have not been presented |
| Free of other bias? | No | There was no wash-out period. The previous antipsychotic treatment was gradually tapered over four weeks. Thus, during a period of 4 weeks the participants were on two drugs |
| Methods | Allocation: random, no further details. Blindness: single, no further details. Duration: 6 weeks. Design: parallel. Location: not reported. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia. N=30. Gender: 8M, 22 F. Age: mean=35.3 years. History: duration ill, age at onset: not reported. Setting: not reported. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Global state: CGI. Mental state: SAPS total score, SANS total score. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Random, no further details. |
| Allocation concealment? | Unclear | No further details. |
| Blinding? Subjective outcomes |
Unclear | Single, rater blind. Whether blinding was successful has not been examined, but both compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? Objective outcomes |
Yes | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
Yes | The overall attrition rate was 13%. The method used to address incomplete outcomes has not been presented. Nevertheless, due to the low rate we consider the risk to be low |
| Free of selective reporting? | No | The study has only been published as an abstract. Efficacy data have only been presented as percentage change from baseline |
| Free of other bias? | Unclear | Only female participants could be included, so that a possible gender bias can not be excluded |
| Methods | Allocation: random, no further details. Blindness: double, identical capsules. Duration: 6 weeks (2 weeks observed). Design: parallel. Location: multicentre. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia (n=341) disorganised, paranoid or undifferentiated or schizoaffective disorder (n=30) plus (n=11), CGI-S of 5 or more, recent exacerbation. N=382. Gender: 251 M, 131 F. Age: 18-65 years (mean=34.8 years). History: duration ill, age at onset: not reported. Setting: inpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason. Global State: CGI. Mental State: PANSS total score, PANSS positive subscore, PANSS negative subscore, Depression Hamilton Rating Scale for Depression, Readiness for Discharge Questionnaire. Satisfaction of treatment: Study Medication Satisfaction. Adverse effects: open interviews, death (natural cause), cardiac effects (ECG), EPS (akathisia, rigor, AIMS, BAS, SAS), prolactin associated side effects (amenorrhoea, decreased libido), sedation, headache , insomnia, constipation, laboratory (prolactin) Unable to use - BAS: no data. Cardiac effects-QTc-prolongation: no data. |
|
| Notes | * data not analysed from placebo group. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Random, no further details. |
| Allocation concealment? | Yes | The participants were assigned using a centralized interactive voice response system. Probably a correct method |
| Blinding? Subjective outcomes |
Unclear | Double, identical capsules. Whether blinding was successful has not been examined, but both compounds differ quite substantially in side-effects. This can be a problem for blinding |
| Blinding? Objective outcomes |
Yes | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
Yes | The overall attrition was 12%. The last-observation-carried-forward method was used to account for people leaving the study early. It assumes that a participant who discontinued the study would not have had a change of his condition if he had remained in the study. This assumption can obviously be wrong. Nevertheless, due to the overall low attrition it is unlikely that the results have been affected |
| Free of selective reporting? | No | Data on some adverse effects were not available. Side effects had to occur in at least 10% to be reported. Important side effects may have been missed by this procedure |
| Free of other bias? | No | The study was sponsored by the manufacturer of risperidone. |
| Methods | Allocation: random, no further details. Blindness: double, identical capsules. Duration: 12 weeks. Design: parallel. Location: not reported. |
|
| Participants | Diagnosis: (DSM-IV or ICD-10) schizophrenia, predominant negative symptoms, CGI of 4 or more, PANSS negative subscore of 21 or more. N=44. Gender: 27 M, 17 F. Age: mean quetiapine=30.6 years, mean risperidone=39.3 years. History: duration ill mean quetiapine=5.4 years, mean risperidone=2.5 years, age at onset mean quetiapine=25.3 years, mean risperidone=36.9 years. Setting: partially in- and outpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Mental State: PANSS total score, PANSS positive subscore, PANSS negative subscore, SANS total score. Cognitive functioning: Auditory verbal memory test, Trail Making Test, Wechsler visual memory scale. Adverse effects: open interviews, cardiac effects (ECG), EPS (akathisia, parkinsonism, use of antiparkinson medication, SAS), sedation, headache, nausea, insomnia, dizziness, weight gain, laboratory (prolactin) Unable to use - SANS total score: no data. Prolactin change from baseline in ng/ml: no data. Cardiac effects: no data. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Random, no further details. |
| Allocation concealment? | Unclear | No further details. |
| Blinding? Subjective outcomes |
Unclear | Double, identical capsules. Whether blinding was successful has not been examined, but both compounds differ quite substantially in side-effects. This can be a problem for blinding |
| Blinding? Objective outcomes |
Yes | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
No | The overall attrition was considerable (45.2%). The data were analysed using a last-observation-carried-forward and a completer analysis. Nevertheless, it is questionable whether any statistical method can account for such a high attrition |
| Free of selective reporting? | No | Data on negative symptoms (SANS) and some adverse effects were not available |
| Free of other bias? | No | The study was sponsored by the manufacturer of quetiapine. |
| Methods | Allocation: random, no further details. Blindness: double, no further details. Duration: 8 weeks. Design: parallel. Location: single centre. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia, acute episode, CGI of more than 4, PANSS total score of more than 60. N=52. Gender: 21 M, 12 F (of completers, here defined as those who completed cognitive assessments at two or more time points out of three (baseline, week 4, weeks 8)). Age: 18-65 years (mean olanzapine=34.47 years, mean quetiapine=36.69 years) (of completers). History: duration ill mean olanzapine=4.71 years, mean quetiapine=8.44 years (of completers), age at onset mean olanzapine=29.76 years, mean quetiapine=28.25 years (of completers). Setting: inpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events. Global state: CGI. Mental State: PANSS total score, PANSS positive subscore, PANSS negative subscore. Adverse effects: open interviews, UKU EPS (akathisia, use of antiparkinson medication, BAS, ESRS), sedation, headache, dizziness, obstipation, weight gain Unable to use - Global state: no data. BAS: no data. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Random, no further details. |
| Allocation concealment? | Unclear | No further details. |
| Blinding? Subjective outcomes |
Unclear | Double, no further details. Whether blinding was successful has not been examined, but both compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? Objective outcomes |
Yes | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
No | The overall attrition was very high 61.5%.The last-observation-carried-forward method was used to account for people leaving the study early. It assumes that a participant who discontinued the study would not have had a change of his condition if he had remained in the study. This assumption can obviously be wrong |
| Free of selective reporting? | No | Data on global state have not been presented. |
| Free of other bias? | No | The study was sponsored by the manufacturer of olanzapine. |
| Methods | Allocation: random, no further details. Blindness: single (rater-blinded). Duration: 16 weeks (8 weeks observed). Design: parallel. Location: multicentre. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia, PANSS total score of 70 or more, PANSS positive subscore of 4 or more on at least 2 items. N=75. Gender: not reported. Age: 18-65 years. History: duration ill not reported., age at onset not reported. Setting: inpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason. Mental State: PANSS total score, BPRS hostility cluster score, PANSS positive subscore, PANSS negative subscore). Adverse effects: EPS (BAS, SAS), weight gain. Unable to use - Mental State: PANSS total score, PANSS positive subscore, PANSS negative subscore (no usable data) |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Random, no further details. |
| Allocation concealment? | Unclear | No further details. |
| Blinding? Subjective outcomes |
Unclear | Single, rater-blind. Whether blinding was successful has not been examined, but the compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? Objective outcomes |
Yes | Single, rater-blind. Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
Unclear | The attrition rate was 18.6%. The last-observation-carried-forward method was used to account for people leaving the study early. It assumes that a participant who discontinued the study would not have had a change of his condition if he had remained in the study. This assumption can obviously be wrong |
| Free of selective reporting? | No | Efficacy data (PANSS) were only presented as percentage change, without indications of standard deviations, standard errors, p-values or ranges. Only interim data after half of the patients had been recruited have been presented |
| Free of other bias? | No | The study was sponsored by the manufacturer of quetiapine. |
| Methods | Allocation: random, no further details. Blindness: single, rater-blinded. Duration: 12 weeks. Design: parallel. Location: single centre. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia, PANSS negative subscore of more than 15, SANS total score more than 60. N=40. Gender: 32 M, 8 F. Age: 21-64 years (mean olanzapine=36.2 years, mean quetiapine=38.3 years). History: duration ill mean olanzapine=13.3 years, mean quetiapine=15.9 years, age at onset not reported. Setting: inpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Mental State: PANSS total score, SANS. Adverse effects: open interviews, cardiac effects (ECG), EPS (akathisia, parkinsonism, use of antiparkinson medication, SAS, AIMS, BAS), sedation, insomnia, abdominal pain, fever, rhinitis, conjunctivitis, seizures, weight gain Unable to use - Mental State: PANSS total score, negative symptoms SANS (median change). EPS scales: no data. Cardiac effects: no data. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Random, no further details. |
| Allocation concealment? | Unclear | No further details. |
| Blinding? Subjective outcomes |
Unclear | Single, rater-blind. Whether blinding was successful has not been examined, but both compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? Objective outcomes |
Yes | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
Yes | The overall attrition was quite low (12%). The last-observation-carried-forward method was used to account for people leaving the study early. It assumes that a participant who discontinued the study would not have had a change of his condition if he had remained in the study. This assumption can obviously be wrong. Nevertheless, due to the low attrition we do not think that this led to bias |
| Free of selective reporting? | No | Efficacy data (PANSS, SANS) have only been presented as median change. There were no data on extrapyramidal side-effects and cardiac effects |
| Free of other bias? | No | The study was sponsored by the manufacturer of quetiapine. |
| Methods | Allocation: random, 2 steps of randomisation before and after availability of ziprasidone, subjects received other medication than in previous phase 1 treatment. Re-randomised. Blindness: double, identical capsules. Duration: 26 weeks. Design: parallel. Location: not reported. |
|
| Participants | Diagnosis: (DSM-IV) chronic schizophrenia. N=444. Gender: 308 M, 136 F. Age: 18-65 years (mean olanzapine=40.0 years, mean quetiapine=40.1 years, mean risperidone=41.8 years, mean ziprasidone=41.3 years). History: duration ill not reported, age at onset not reported. Setting: in- and outpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Global State: CGI. Mental State: PANSS total score. Adverse effects: open interviews, death (suicide), EPS (akathisia), cardiac effects (ECG), prolactin-associated side-effects, weight gain, laboratory (prolactin, glucose, cholesterol) |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Random, 2 steps of randomisation before and after availability of ziprasidone, subjects received other medication than in previous phase 1 treatment. Re-randomised |
| Allocation concealment? | Unclear | No further details. |
| Blinding? Subjective outcomes |
Unclear | Double, identical capsules. Whether blinding was successful has not been examined, but both compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? Objective outcomes |
Yes | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
No | The attrition rate was very high (72.5%). Continuous data were analysed based on mixed effect models. It is unclear whether any statistical method can account for such high rates of leaving the study early |
| Free of selective reporting? | No | Use of antiparkinson medication was permitted but data on this outcome have not been presented |
| Free of other bias? | Unclear | Patients had a history of former intolerance to atypical antipsychotic treatment but baseline data on this were not provided |
| Methods | Allocation: random, no further details. Blindness: double, no further details. Duration: 6 weeks. Design: parallel. Location: not reported. |
|
| Participants | Diagnosis: (ICD-10) acute schizophrenia (n=32), schizoaffective disorder (n=10). N=42. Gender: 42 F. Age: mean=35.78 years. History: duration ill mean=7.05 years, age at onset not reported. Setting: inpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: inefficacy. Global State: CGI. Mental State: PANSS total score, PANSS positive subscore, PANSS negative subscore. Adverse effects: Cardiac effects (QTc), EPS (akathisia, dystonia, extrapyramidal symptoms, tremor), weight gain, laboratory (cholesterol, glucose, prolactin) Unable to use - Cholesterol: no data. Glucose: no data. |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Random, no further details. |
| Allocation concealment? | Unclear | No further details. |
| Blinding? Subjective outcomes |
Unclear | Double, no further details. Whether blinding was successful has not been examined, but both compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? Objective outcomes |
Yes | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
No | Data on the overall attrition rate were not available. |
| Free of selective reporting? | No | For some metabolic parameters there were no data available. |
| Free of other bias? | Unclear | There was a slight baseline imbalance in terms of mean age, which was described being non significant |
| Methods | Allocation: random, no further details. Blindness: single, rater-blinded. Duration: 52 weeks. Design: parallel. Location: not reported. |
|
| Participants | Diagnosis: schizophrenia. N=86. Gender: not reported. Age: not reported. History: duration ill, age at onset: not reported. Setting: not reported. |
|
| Interventions |
|
|
| Outcomes | Mental State: PANSS total score, PANSS positive subscore, PANSS negative subscore. General functioning: GAF. Cognitive functioning: PANSS cognitive cluster, Wisconsin card sorting test. Adverse effects: UKU, EPS (SAS, AIMS, BAS), weight gain, number of dysglycaemics Unable to use - At the time the publication was available the update search was finished, therefore most of the data except for PANSS total, could not be considered |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Random, no further details. |
| Allocation concealment? | Unclear | No further details. |
| Blinding? Subjective outcomes |
Unclear | Single, rater-blind. Whether blinding was successful has not been examined, but both compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? Objective outcomes |
Yes | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
No | There is a discrepancy between the abstract in the text. While according to the abstract there were fewer participants leaving the study early, this finding was no longer mentioned in the text according to which the overall attrition was only 1.2% |
| Free of selective reporting? | No | Use of antiparkinson medication was permitted but numbers have not been presented |
| Free of other bias? | No | The study was sponsored by the manufacturer of quetiapine. There was no wash-out period |
| Methods | Allocation: random, no further details. Blindness: double, no further details. Duration: 8 weeks. Design: parallel. Location: multicentre. |
|
| Participants | Diagnosis: (DSM-IV) schizophrenia, PANSS of 60 or more, CGI-S of 4 or more. N=673. Gender: 510 M, 163 F. Age: 18-65 years (mean quetiapine=40.2 years, mean risperidone=39.6 years). History: duration ill, age at onset: not reported. Setting: in- and outpatient, initially inpatient. |
|
| Interventions |
|
|
| Outcomes | Leaving the study early: any reason, adverse events, inefficacy. Global State: CGI. Mental State: PANSS total score, PANSS positive subscore, PANSS negative subscore. Adverse effects: open interviews, cardiac effects (QTc), death (natural causes, suicide), EPS (akathisia, dystonia, parkinsonism, use of antiparkinson medication, AIMS, BAS, SAS), sedation, prolactin associated side effects (dysmenorrhea, galactorrhea, sexual dysfunction) weight gain, laboratory (cholesterol, glucose, prolactin, white blood cell count) |
|
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Adequate sequence generation? | Unclear | Random, no further details. |
| Allocation concealment? | Unclear | No further details. |
| Blinding? Subjective outcomes |
Unclear | Double, no further details. Whether blinding was successful has not been examined, but both compounds differ quite substantially in side effects. This can be a problem for blinding |
| Blinding? Objective outcomes |
Yes | Objective outcomes such as laboratory measures or death are unlikely to have been much affected by problems of blinding |
| Incomplete outcome data addressed? All outcomes |
No | The overall attrition was high (52.1%). The last-observation-carried-forward method was used to account for people leaving the study early. It assumes that a participant who discontinued the study would not have had a change of his condition if he had remained in the study. This assumption can obviously be wrong. Data on study completers were also available. Nevertheless, it is unclear whether any statistical method can account for such a degree of attrition |
| Free of selective reporting? | No | Adverse events were only presented with an incidence of at least 5% among the participants, therefore important side effects may have been missed by this procedure |
| Free of other bias? | No | The study was sponsored by the manufacturer of quetiapine. |
Diagnostic tool
DSM III-R and DSM-IV - Diagnostic Statistical Manual version 3 Revised and version 4.
ICD 10 - The International Statistical Classification of Diseases and Related Health Problems.
BMI - Body Mass Index.
Rating Scales:
Global rating scales:
CGI - Clinical Global Impressions.
CGI-S - Clinical Global Impression-Severity.
CGI-I - Clinical Global Impression-Improvement.
Mental state:
BPRS - Brief Psychiatric Rating Scale.
MADRS - Montgomery-Asberg Depression Rating Scale.
MMSE - Wiing Mini Mental State Examination.
PANSS - Positive and Negative Syndrome Scale.
SANS - Scale for the Assessment of Negative Symptoms.
Side effects:
AIMS - Abnormal Involuntary Movement Scale.
BAS - Barnes Akathisia Scale.
BMI - Body mass index.
EPS- Extrapyramidal symptoms.
ESRS - Extrapyramidal Syndrome Rating Scale.
HAS - Hillside Akathisia Scale.
SAS - Simpson-Angus Index - for neurological side effects.
UKU - Udvalg for kliniske ndersogelser Side Effect Rating Scale -side effect rating scale.
Quality of Life:
QoL - Quality of Life Scale.
SWN -Subjective Well-being List.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| An 2003 | Allocation: randomised. Blindness: open-label. |
| Antonova 2005 | Allocation: randomised. Blindness: single-blind (rater-blind). Participants: people with schizophrenia. Interventions: olanzapine, risperidone and quetiapine versus conventional antipsychotics. Outcomes: no usable data. |
| Ascher-Svanum 2006 | Allocation: not randomised, cohort study. |
| Baloescu 2006 | Allocation: not randomised, controlled clinical trial. |
| Beuzen 2005 | Allocation: randomised Blindness: open-label. |
| Byerly 1999 | Allocation: not reported. Blindness: not reported. Participants: people with schizophrenia. Interventions: clozapine versus quetiapine. Outcomes: no usable data. |
| Byerly 2006 | Allocation: randomised. Blindness: double-blind. Participants: people with schizophrenia. Interventions: quetiapine versus risperidone. Outcomes: no usable data. |
| Canas 2006 | Allocation: not randomised, controlled clinical trial. |
| Cao 2005 | Allocation: randomised. Blindness: open-label. |
| Cao 2005a | Allocation: randomised. Blindness: open-label. |
| Chaudhry 2006 | Allocation: randomised. Blindness: open-label. |
| Dai 2004 | Allocation: randomised. Blindness: open-label. |
| Dai 2005 | Allocation: randomised. Blindness: open-label. |
| Ding 2004 | Allocation: randomised. Blindness: open-label. |
| Dossenbach 2005 | Allocation: not randomised, cohort study. |
| Du 2003 | Allocation: randomised. Blindness: open-label. |
| Emsley 2005 | Allocation: randomised. Blindness: investigator-blind. Participants: people with schizophrenia. Interventions: inappropriate intervention. |
| Fan 2005 | Allocation: randomised. Blindness: open-label. |
| Fleischhacker 2005 | Allocation: randomised. Blindness: open-label. |
| Fu 2005 | Allocation: randomised. Blindness: open-label. |
| Gao 2003 | Allocation: randomised. Blindness: open-label. |
| Garcia 2006 | Allocation: not randomised, case series. |
| Harrigan 2004 | Allocation: randomised. Blindness: open-label. |
| He 2003 | Allocation: randomised. Blindness: open-label. |
| Huang 2003 | Allocation: randomised. Blindness: open-label. |
| Huber 2004 | Allocation: unclear. Blindness: unclear. Intervention: other aims. |
| Karow 2002 | Allocation: not randomised, review. |
| Keks 2006 | Allocation: randomised. Blindness: open-label. |
| Kelemen 2006 | Allocation: not randomised, controlled clinical trial. |
| Kim 2004 | Allocation: not randomised, controlled clinical trial. |
| Knegtering 2004 | Allocation: randomised. Blindness: open-label. |
| Li 2001 | Allocation: randomised. Blindness: open-label. |
| Li 2002a | Allocation: not randomised. |
| Li 2003a | Allocation: randomised. Blindness: open-label. |
| Li 2003b | Allocation: randomised. Blindness: open-label. |
| Li 2005 | Allocation: randomised. Blindness: open-label. |
| Liu 2004a | Allocation: randomised. Blindness: open-label. |
| Liu 2005 | Allocation: randomised. Blindness: open-label. |
| Lu 2005 | Allocation: randomised. Blindness: open-label. |
| Luo 2005 | Allocation: randomised. Blindness: open-label. |
| Mintzer 2004 | Allocation: randomised. Blindness: open-label. |
| Mullen 2001 | Allocation: randomised. Blindness: open-label |
| Musil 2006 | Allocation: not randomised, cohort study. |
| Pan 2004 | Allocation: randomised. Blindness: open-label. |
| Pan 2004a | Allocation: randomised. Blindness: open-label. |
| Pan 2004b | Allocation: randomised. Blindness: open-label. |
| Pang 2002 | Allocation: randomised. Blindness: open-label. |
| Peng 2004 | Allocation: randomised. Blindness: not mentioned. Participants: people with schizophrenia. Interventions: inappropriate intervention. |
| Qi 2004 | Allocation: randomised. Blindness: open-label. |
| Qian 2004 | Allocation: randomised. Blindness: open-label. |
| Reznik 2004 | Allocation: randomised. Blindness: open-label. |
| Ryu 2006 | Allocation: not randomised, controlled clinical trial. |
| Sajatovic 2002 | Allocation: randomised. Blindness: open-label. |
| Swanson 2006 | Allocation: randomised. Blindness: open-label. |
| Tang 2003 | Allocation: randomised. Blindness: open-label. |
| Tang 2005 | Allocation: randomised. Blindness: open-label. |
| Wang 2000 | Allocation: randomised. Blindness: open-label. |
| Wang 2004 | Allocation: randomised. Blindness: open-label. |
| Wang 2004a | Allocation: not randomised. |
| Wang 2005 | Allocation: randomised. Blindness: open-label. |
| Wang 2005a | Allocation: randomised. Blindness: open-label. |
| Wang 2005b | Allocation: randomised. Blindness: open-label. |
| Wang 2005c | Allocation: randomised. Blindness: open-label. |
| Wang 2005d | Allocation: randomised. Blindness: open-label. |
| Weickert 2003 | Allocation: randomised. Participants: people with schizophrenia. Interventions: inappropriate intervention. |
| Xiang 2005 | Allocation: randomised. Blindness: open-label. |
| Xu 2002 | Allocation: randomised. Blindness: open-label. |
| Xu 2003 | Allocation: randomised. Blindness: open-label. |
| Xu 2005 | Allocation: randomised. Blindness: open-label. |
| Yamashita 2005 | Allocation: not randomised, case series. |
| Yang 2004 | Allocation: randomised. Blindness: open-label. |
| Yang 2005 | Allocation: randomised. Blindness: open-label. |
| Yu 2003 | Allocation: randomised. Blindness: open-label. |
| Yuan 2005 | Allocation: randomised. Blindness: open-label. |
| Zhang 2003 | Allocation: randomised. Blindness: open-label. |
| Zhang 2005 | Allocation: randomised. Blindness: open-label. |
| Zhang 2005a | Allocation: randomised. Blindness: open-label. |
| Zhang 2005b | Allocation: randomised. Blindness: open-label. |
| Zhang 2005c | Allocation: randomised. Blindness: open-label. |
| Zhao 2004 | Allocation: randomised. Blindness: open-label. |
| Zhao 2005 | Allocation: randomised. Blindness: open-label. |
| Zhao 2005a | Allocation: randomised. Blindness: open-label. |
| Zhong 2006a | Allocation: randomised. Blindness: open-label. |
| Zhou 2003 | Allocation: randomised. Blindness: open-label. |
| Zhou 2003a | Allocation: randomised. Blindness: open-label. |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Trial 8894 F1D-US-HGLR. |
| Methods | Allocation: random, no further details. Blindness: double, no further details. Duration: 26 weeks. Design: parallel. Location: not reported. |
| Participants | Diagnosis: schizophrenia or schizoaffective disorder. N=not reported. Gender: not reported. Age: 18-75 years. History: duration ill not reported., age at onset not reported. Setting: not reported. |
| Interventions |
|
| Outcomes | Response to treatment. Leaving the study early: any reason, lack of efficacy or worsening of psychiatric syndromes. Global state: CGI, PG-I. Mental State: PANSS, depression MADRS General functioning: DAI-10, GAF. Quality of life: SF-36. Adverse effects: EPS (SAS, BAS, AIMS), vital signs, weight (waist circumference, BMI, appetite, metabolic syndrome), laboratory (fasting glucose, haemoglobin A1c, lipids, insulin) |
| Starting date | July 2004. |
| Contact information | Eli Lilly and company. |
| Notes |
| Trial name or title | A comparative study of quetiapine and risperidone in patients with first episode psychosis |
| Methods | Allocation: random, no further details. Blindness: rater-blinded. |
| Participants | Diagnosis: first episode of schizophreniform psychosis (ICD-10 criteria) |
| Interventions |
|
| Outcomes | Global state: CGI. Mental state: PANSS positive subscale, PANSS negative subscale, Calgary Depression Scale for Schizophrenia, Calgary Anxiety Scale Schizophrenia. General functioning: GAF. |
| Starting date | Not known. |
| Contact information | |
| Notes |
| Trial name or title | Improved response in Schizophrenia -IRIS. |
| Methods | Allocation: random, no further details. Blindness: double, no further details. |
| Participants | Diagnosis: schizophrenia. |
| Interventions |
|
| Outcomes | Global state: CGI-S. Mental state: PANSS, GAS, HAM-D scores. Quality of life - SQLS and care giving inventory scores. Health Economics. Adverse effects: EPS (AIMS, SAS, BAS). |
| Starting date | 1 October 2002. |
| Contact information | Dr Lawrence Ratna Barnet Hospital Wellhouse Lane Barnet EN5 3DJ UK Telephone: 020 8216 4617 Fax: 020 8216 4595 |
| Notes |
| Trial name or title | A six month, rater blind comparison of quetiapine and risperidone in the treatment of tardive dyskinesia in people with schizophrenia |
| Methods | Allocation: random, no further details. Blindness: single, rater-blinded. |
| Participants | Diagnosis: schizophrenia. N=30. |
| Interventions |
|
| Outcomes | Not known. |
| Starting date | Not known. |
| Contact information | Not known. |
| Notes |
DATA AND ANALYSES
Comparison 1. QUETIAPINE versus CLOZAPINE - all data short term.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1a. No clinically significant response (as defined by original studies) | 1 | 72 | Risk Ratio (M-H, Random, 95% CI) | 0.94 [0.78, 1.13] |
| 2 Global state: 1b. No clinically important change - short term (as defined by the original studies) | 1 | 76 | Risk Ratio (M-H, Random, 95% CI) | 0.94 [0.74, 1.18] |
| 3 Leaving the study early | 3 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 any reason | 2 | 95 | Risk Ratio (M-H, Random, 95% CI) | 0.67 [0.18, 2.43] |
| 3.2 due to adverse events | 1 | 72 | Risk Ratio (M-H, Random, 95% CI) | 0.14 [0.01, 2.67] |
| 3.3 due to inefficacy | 1 | 72 | Risk Ratio (M-H, Random, 95% CI) | Not estimable |
| 4 Mental state: 1a. General - no clinically important change - short term (less than 50% PANSS total score reduction) | 1 | 63 | Risk Ratio (M-H, Random, 95% CI) | 1.07 [0.53, 2.14] |
| 5 Mental state: 1b. General -average endpoint score - short term (PANSS total, high=poor) | 4 | 232 | Mean Difference (IV, Random, 95% CI) | −0.50 [−2.85, 1.86] |
| 6 Mental state: 1c. General -average endpoint score - short term (BPRS total, high=poor) | 1 | 67 | Mean Difference (IV, Random, 95% CI) | −0.89 [−3.20, 1.42] |
| 7 Mental state: 2. Positive symptoms - average endpoint score (PANSS positive subscore, high=poor) | 2 | 142 | Mean Difference (IV, Random, 95% CI) | −0.70 [−2.07, 0.68] |
| 8 Mental state: 3a. Negative symptoms - no clinically important change - short term (less than 50% SANS total score reduction) | 1 | 72 | Risk Ratio (M-H, Random, 95% CI) | 0.94 [0.78, 1.13] |
| 9 Mental state: 3b. Negative symptoms - average endpoint score - short term (PANSS negative subscore, high=poor) | 2 | 142 | Mean Difference (IV, Random, 95% CI) | −2.23 [−3.48, −0.99] |
| 10 Mental state: 3c. Negative symptoms - average endpoint score - short term (SANS total, high=poor) | 1 | 67 | Mean Difference (IV, Random, 95% CI) | −1.64 [−8.17, 4.89] |
| 11 Adverse effects: 1. General - at least one adverse effect | 1 | 63 | Risk Ratio (M-H, Random, 95% CI) | 0.42 [0.26, 0.66] |
| 12 Adverse effects: 2. Cardiac effects: ECG abnormalities | 1 | 72 | Risk Ratio (M-H, Random, 95% CI) | 0.13 [0.02, 0.95] |
| 13 Adverse effects: 3. Central nervous system - sedation | 2 | 135 | Risk Ratio (M-H, Random, 95% CI) | 0.22 [0.11, 0.47] |
| 14 Adverse effects: 4. Extrapyramidal effects | 3 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 14.1 akathisia | 2 | 135 | Risk Ratio (M-H, Random, 95% CI) | 0.40 [0.08, 1.99] |
| 14.2 rigor | 1 | 63 | Risk Ratio (M-H, Random, 95% CI) | 1.94 [0.18, 20.30] |
| 14.3 tremor | 2 | 135 | Risk Ratio (M-H, Random, 95% CI) | 0.99 [0.29, 3.34] |
| 14.4 use of antiparkinson medication | 1 | 28 | Risk Ratio (M-H, Random, 95% CI) | Not estimable |
| 15 Adverse effects: 5. Haematological: Important decline in white blood cells | 1 | 63 | Risk Ratio (M-H, Random, 95% CI) | 0.19 [0.01, 3.88] |
| 16 Adverse effects: 6a. Metabolic -weight - gain | 2 | 135 | Risk Ratio (M-H, Random, 95% CI) | 0.53 [0.25, 1.11] |
| 17 Adverse effects: 6b. Metabolic -weight - change from baseline (kg) | 1 | 27 | Mean Difference (IV, Random, 95% CI) | −2.11 [−4.30, 0.08] |
Comparison 2. QUETIAPINE versus OLANZAPINE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1a. No clinically significant response (as defined by the original studies) | 3 | 339 | Risk Ratio (M-H, Random, 95% CI) | 1.11 [0.86, 1.43] |
| 2 Global state: 1b. No clinically important change (as defined by the original studies) | 2 | 309 | Risk Ratio (M-H, Random, 95% CI) | 1.18 [0.89, 1.57] |
| 2.1 short term | 1 | 42 | Risk Ratio (M-H, Random, 95% CI) | 1.36 [0.59, 3.15] |
| 2.2 long term | 1 | 267 | Risk Ratio (M-H, Random, 95% CI) | 1.16 [0.86, 1.57] |
| 3 Leaving the study early | 11 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 any reason | 10 | 1651 | Risk Ratio (M-H, Random, 95% CI) | 1.22 [1.13, 1.32] |
| 3.2 due to adverse events | 8 | 1573 | Risk Ratio (M-H, Random, 95% CI) | 0.90 [0.69, 1.18] |
| 3.3 due to inefficacy | 8 | 1563 | Risk Ratio (M-H, Random, 95% CI) | 1.80 [1.42, 2.27] |
| 4 Mental state: 1a. General - no clinically important change-short term (less than 50% PANSS total score reduction) | 1 | 42 | Risk Ratio (M-H, Random, 95% CI) | 0.91 [0.54, 1.53] |
| 5 Mental state: 1b. General -average endpoint score (PANSS total, high=poor) | 10 | 1449 | Mean Difference (IV, Random, 95% CI) | 3.66 [1.93, 5.39] |
| 5.1 short term | 4 | 142 | Mean Difference (IV, Random, 95% CI) | 2.17 [−1.51, 5.85] |
| 5.2 medium term | 3 | 482 | Mean Difference (IV, Random, 95% CI) | 5.57 [1.97, 9.17] |
| 5.3 long term | 3 | 825 | Mean Difference (IV, Random, 95% CI) | 3.40 [0.91, 5.88] |
| 6 Mental state: 2a. Positive symptoms - no clinically important change-short term (less than 20% SAPS total score reduction) | 1 | 30 | Risk Ratio (M-H, Random, 95% CI) | 15.0 [0.93, 241.20] |
| 7 Mental state: 2b. Positive symptoms - average endpoint score (PANSS positive subscore, high=poor) | 7 | 679 | Mean Difference (IV, Random, 95% CI) | 1.80 [1.02, 2.59] |
| 7.1 short term | 3 | 115 | Mean Difference (IV, Random, 95% CI) | 1.05 [−0.75, 2.85] |
| 7.2 medium term | 3 | 483 | Mean Difference (IV, Random, 95% CI) | 2.21 [0.90, 3.52] |
| 7.3 long term | 1 | 81 | Mean Difference (IV, Random, 95% CI) | 1.80 [0.39, 3.21] |
| 8 Mental state: 2c. Positive symptoms - SAPS total score - percent change-short term (high=poor) | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 40.84 [23.97, 57.71] |
| 9 Mental state: 3a. Negative symptoms - no clinically important change-short term (less than 20% SANS total score reduction) | 1 | 30 | Risk Ratio (M-H, Random, 95% CI) | 1.5 [0.53, 4.26] |
| 10 Mental state: 3b. Negative symptoms - average endpoint score (PANSS negative subscore, high=poor) | 7 | 679 | Mean Difference (IV, Random, 95% CI) | 0.41 [−0.36, 1.18] |
| 10.1 short term | 3 | 115 | Mean Difference (IV, Random, 95% CI) | 0.01 [−1.72, 1.73] |
| 10.2 medium term | 3 | 483 | Mean Difference (IV, Random, 95% CI) | 0.40 [−0.67, 1.47] |
| 10.3 long term | 1 | 81 | Mean Difference (IV, Random, 95% CI) | 0.70 [−0.73, 2.13] |
| 11 Mental state: 3c. Negative symptoms - average endpoint score-medium term (SANS total score, high=poor) | 1 | 335 | Mean Difference (IV, Random, 95% CI) | 3.70 [−0.48, 7.88] |
| 12 Mental state: 3d. Negative symptoms - average endpoint score-short term (SANS total score- percent change, high= poor) | 1 | 30 | Mean Difference (IV, Random, 95% CI) | 2.46 [−31.90, 36.82] |
| 13 General functioning: General - average endpoint score-medium term (GAF total score, high=poor) | 1 | 278 | Mean Difference (IV, Random, 95% CI) | 3.80 [0.77, 6.83] |
| 14 Quality of life: General -average endpoint score-medium term (QLS total score, high=poor) | 1 | 286 | Mean Difference (IV, Random, 95% CI) | 1.80 [−2.42, 6.02] |
| 15 Service use: number of participants re-hospitalised | 2 | 876 | Risk Ratio (M-H, Random, 95% CI) | 1.79 [1.30, 2.47] |
| 15.1 medium term | 1 | 203 | Risk Ratio (M-H, Random, 95% CI) | 1.8 [0.92, 3.51] |
| 15.2 long term | 1 | 673 | Risk Ratio (M-H, Random, 95% CI) | 1.78 [1.24, 2.58] |
| 16 Adverse effects: 1. General - at least one adverse effect | 6 | 1269 | Risk Ratio (M-H, Random, 95% CI) | 0.97 [0.88, 1.06] |
| 17 Adverse effects: 2. Death | 3 | 1410 | Risk Ratio (M-H, Random, 95% CI) | 0.74 [0.13, 4.23] |
| 17.1 suicide attempt | 2 | 940 | Risk Ratio (M-H, Random, 95% CI) | 0.35 [0.05, 2.29] |
| 17.2 suicide | 2 | 470 | Risk Ratio (M-H, Random, 95% CI) | 4.96 [0.24, 102.41] |
| 18 Adverse effects: 3a. Cardiac effects - QTc prolongation | 1 | 673 | Risk Ratio (M-H, Random, 95% CI) | 12.96 [0.73, 229.17] |
| 19 Adverse effects: 3b. Cardiac effects - QTc abnormalities -change from baseline in ms | 3 | 643 | Mean Difference (IV, Random, 95% CI) | 4.81 [0.34, 9.28] |
| 20 Adverse effects: 4a. Central nervous system - sedation | 7 | 1615 | Odds Ratio (M-H, Fixed, 95% CI) | 0.97 [0.78, 1.20] |
| 21 Adverse effects: 4b. Central nervous system - seizures | 1 | 40 | Risk Ratio (M-H, Random, 95% CI) | 3.3 [0.14, 76.46] |
| 22 Adverse effects: 5a. Extrapyramidal effects | 8 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 22.1 akathisia | 6 | 1277 | Risk Ratio (M-H, Random, 95% CI) | 0.98 [0.68, 1.40] |
| 22.2 akinesia | 1 | 267 | Risk Ratio (M-H, Random, 95% CI) | 1.02 [0.67, 1.56] |
| 22.3 dystonia | 1 | 42 | Risk Ratio (M-H, Random, 95% CI) | 4.57 [0.23, 89.72] |
| 22.4 extrapyramidal symptoms | 2 | 245 | Risk Ratio (M-H, Random, 95% CI) | 1.62 [0.72, 3.67] |
| 22.5 parkinsonism | 1 | 40 | Risk Ratio (M-H, Random, 95% CI) | 0.66 [0.18, 2.41] |
| 22.6 tremor | 1 | 42 | Risk Ratio (M-H, Random, 95% CI) | 0.39 [0.12, 1.31] |
| 22.7 use of antiparkinson medication | 6 | 1090 | Risk Ratio (M-H, Random, 95% CI) | 0.49 [0.30, 0.79] |
| 23 Adverse effects: 5b. Extrapyramidal effects - scale measured | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 23.1 akathisia: Barnes Akathisia Scale (high=poor) | 1 | 50 | Mean Difference (IV, Random, 95% CI) | −0.10 [−0.58, 0.38] |
| 23.2 extrapyramidal symptoms: ESRS total score (high=poor) | 1 | 33 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 23.3 extrapyramidal symptoms: Simpson-Angus Scale (high=poor) | 1 | 50 | Mean Difference (IV, Random, 95% CI) | 0.6 [−1.38, 2.58] |
| 24 Adverse effects: 6a. Prolactin associated side effects | 5 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 24.1 abnormally high prolactin value | 1 | 42 | Risk Ratio (M-H, Random, 95% CI) | 0.10 [0.01, 1.77] |
| 24.2 amenorrhea | 3 | 252 | Risk Ratio (M-H, Random, 95% CI) | 0.66 [0.36, 1.21] |
| 24.3 galactorrhea | 4 | 1025 | Risk Ratio (M-H, Random, 95% CI) | 0.66 [0.25, 1.73] |
| 24.4 gynecomastia | 1 | 267 | Risk Ratio (M-H, Random, 95% CI) | 0.33 [0.09, 1.20] |
| 24.5 sexual dysfunction | 4 | 1177 | Risk Ratio (M-H, Random, 95% CI) | 0.80 [0.64, 0.99] |
| 25 Adverse effects: 6b. Prolactin -change from baseline in ng/ml | 5 | 1021 | Mean Difference (IV, Random, 95% CI) | −5.89 [−11.62, −0.16] |
| 26 Adverse effects: 7a. Metabolic - cholesterol - significant cholesterol increase | 1 | 267 | Risk Ratio (M-H, Random, 95% CI) | 0.99 [0.59, 1.68] |
| 27 Adverse effects: 7b. Metabolic - cholesterol - change from baseline in mg/dl | 4 | 986 | Mean Difference (IV, Random, 95% CI) | −4.69 [−13.84, 4.45] |
| 28 Adverse effects: 7c. Metabolic - glucose - abnormally high fasting glucose value | 1 | 267 | Risk Ratio (M-H, Random, 95% CI) | 0.71 [0.33, 1.54] |
| 29 Adverse effects: 7d. Metabolic -glucose - change from baseline in mg/dl | 4 | 986 | Mean Difference (IV, Random, 95% CI) | −9.32 [−17.82, −0.82] |
| 30 Adverse effects: 7e. Metabolic -weight - gain | 8 | 1667 | Risk Ratio (M-H, Random, 95% CI) | 0.68 [0.51, 0.92] |
| 30.1 significant weight gain (as defined by the original studies) | 7 | 1321 | Risk Ratio (M-H, Random, 95% CI) | 0.69 [0.51, 0.95] |
| 30.2 as “weight gain” reported adverse events | 1 | 346 | Risk Ratio (M-H, Random, 95% CI) | 0.49 [0.04, 5.34] |
| 31 Adverse effects: 7f. Metabolic -weight - change from baseline in kg | 7 | 1173 | Mean Difference (IV, Random, 95% CI) | −2.68 [−4.26, −1.10] |
Comparison 3. QUETIAPINE versus RISPERIDONE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Global state: 1a. No clinically significant response (as defined by the original studies) | 4 | 1274 | Risk Ratio (M-H, Random, 95% CI) | 1.12 [0.93, 1.35] |
| 2 Global state: 1b. No clinically important change (as defined by the original studies) | 4 | 1274 | Risk Ratio (M-H, Random, 95% CI) | 1.16 [0.99, 1.35] |
| 2.1 short term | 3 | 1007 | Risk Ratio (M-H, Random, 95% CI) | 1.16 [0.94, 1.44] |
| 2.2 long term | 1 | 267 | Risk Ratio (M-H, Random, 95% CI) | 1.18 [0.87, 1.60] |
| 3 Leaving the study early | 10 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 3.1 any reason | 10 | 2278 | Risk Ratio (M-H, Random, 95% CI) | 1.06 [0.98, 1.15] |
| 3.2 due to adverse events | 7 | 1851 | Risk Ratio (M-H, Random, 95% CI) | 1.19 [0.78, 1.80] |
| 3.3 due to inefficacy | 7 | 1851 | Risk Ratio (M-H, Random, 95% CI) | 1.26 [0.99, 1.61] |
| 4 Mental state: 1a General - no clinically important change - short term (less than 30% PANSS total score reduction) | 2 | 982 | Risk Ratio (M-H, Random, 95% CI) | 1.11 [0.87, 1.42] |
| 5 Mental state: 1b. General - no clinicallly important change - short term (less than 20% BPRS total score reduction) | 1 | 25 | Risk Ratio (M-H, Random, 95% CI) | 0.98 [0.63, 1.52] |
| 6 Mental state: 1c. General - average endpoint score (PANSS total score, high=poor) | 9 | 1953 | Mean Difference (IV, Random, 95% CI) | 3.09 [1.01, 5.16] |
| 6.1 short term | 5 | 1064 | Mean Difference (IV, Random, 95% CI) | 2.44 [−0.81, 5.69] |
| 6.2 medium term | 2 | 146 | Mean Difference (IV, Random, 95% CI) | 6.27 [−3.94, 16.48] |
| 6.3 long term | 2 | 743 | Mean Difference (IV, Random, 95% CI) | 3.11 [0.40, 5.82] |
| 7 Mental state: 1d. General -average endpoint score - short term (BPRS total score, high= poor) | 1 | 25 | Mean Difference (IV, Random, 95% CI) | 1.68 [−8.33, 11.69] |
| 8 Mental state: 2a. Positive symptoms - no clinically important change - short term (less than 40% PANSS positive reduction) | 1 | 673 | Risk Ratio (M-H, Random, 95% CI) | 1.00 [0.90, 1.12] |
| 9 Mental state: 2b. Positive symptoms - average endpoint score - (PANSS positive subscore, high=poor) | 7 | 1264 | Mean Difference (IV, Random, 95% CI) | 1.82 [1.16, 2.48] |
| 9.1 short term | 4 | 1037 | Mean Difference (IV, Random, 95% CI) | 2.10 [1.00, 3.19] |
| 9.2 medium term | 2 | 146 | Mean Difference (IV, Random, 95% CI) | 2.15 [−0.01, 4.31] |
| 9.3 long term | 1 | 81 | Mean Difference (IV, Random, 95% CI) | 1.30 [−0.13, 2.73] |
| 10 Mental state: 2c. Positive symptoms - average endpoint score - short term (BPRS positive subscore, high=poor) | 1 | 25 | Mean Difference (IV, Random, 95% CI) | 1.1 [0.18, 2.02] |
| 11 Mental state: 3a. Negative symptoms - no clinicallly important change - short term (less than 40% PANSS negative reduction) | 1 | 673 | Risk Ratio (M-H, Random, 95% CI) | 0.98 [0.93, 1.04] |
| 12 Mental state: 3b. Negative symptoms - average endpoint score - (PANSS negative subscore, high=poor) | 7 | 1183 | Mean Difference (IV, Random, 95% CI) | −0.35 [−1.95, 1.26] |
| 12.1 short term | 4 | 956 | Mean Difference (IV, Random, 95% CI) | −1.46 [−4.11, 1.19] |
| 12.2 medium term | 2 | 146 | Mean Difference (IV, Random, 95% CI) | 1.30 [−0.75, 3.35] |
| 12.3 long term | 1 | 81 | Mean Difference (IV, Random, 95% CI) | 0.80 [−0.64, 2.24] |
| 13 Mental state: 3c. Negative symptoms - average endpoint score - (BPRS negative subscore, high=poor) | 1 | 25 | Mean Difference (IV, Random, 95% CI) | 0.57 [0.17, 0.97] |
| 14 Quality of life: General- average endpoint score - short term (QLS total score, high=poor) | 1 | 22 | Mean Difference (IV, Random, 95% CI) | −0.5 [−13.87, 12.87] |
| 15 Service use: number of participants re-hospitalised | 2 | 877 | Risk Ratio (M-H, Random, 95% CI) | 1.34 [1.00, 1.79] |
| 15.1 medium term | 1 | 199 | Risk Ratio (M-H, Random, 95% CI) | 1.3 [0.71, 2.38] |
| 15.2 long term | 1 | 678 | Risk Ratio (M-H, Random, 95% CI) | 1.35 [0.97, 1.88] |
| 16 Adverse effects: 1. General - at least one adverse effect | 8 | 2226 | Risk Ratio (M-H, Random, 95% CI) | 1.04 [0.93, 1.17] |
| 17 Adverse effects: 2. Death | 5 | 3066 | Risk Ratio (M-H, Random, 95% CI) | 0.73 [0.17, 3.09] |
| 17.1 natural causes | 2 | 982 | Risk Ratio (M-H, Random, 95% CI) | Not estimable |
| 17.2 suicide attempt | 2 | 945 | Risk Ratio (M-H, Random, 95% CI) | 0.43 [0.06, 2.95] |
| 17.3 suicide | 3 | 1139 | Risk Ratio (M-H, Random, 95% CI) | 1.41 [0.11, 18.32] |
| 18 Adverse effects: 3a. Cardiac effects - QTc prolongation | 2 | 1351 | Risk Ratio (M-H, Random, 95% CI) | 0.87 [0.29, 2.55] |
| 19 Adverse effects: 3b. Cardiac effects - QTc abnormalities -change from baseline in ms | 3 | 940 | Mean Difference (IV, Random, 95% CI) | 2.21 [−5.05, 9.48] |
| 20 Adverse effects: 4. Central nervous system - sedation | 8 | 2226 | Risk Ratio (M-H, Fixed, 95% CI) | 1.21 [1.06, 1.38] |
| 21 Adverse effects: 5a. Extrapyramidal effects | 8 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 21.1 akathisia | 6 | 2170 | Risk Ratio (M-H, Random, 95% CI) | 0.62 [0.34, 1.13] |
| 21.2 akinesia | 1 | 267 | Risk Ratio (M-H, Random, 95% CI) | 0.91 [0.61, 1.37] |
| 21.3 dystonia | 1 | 673 | Risk Ratio (M-H, Random, 95% CI) | 0.06 [0.01, 0.41] |
| 21.4 extrapyramidal symptoms | 2 | 872 | Risk Ratio (M-H, Random, 95% CI) | 0.59 [0.43, 0.81] |
| 21.5 parkinsonism | 2 | 717 | Risk Ratio (M-H, Random, 95% CI) | 0.06 [0.00, 0.96] |
| 21.6 rigor | 1 | 309 | Risk Ratio (M-H, Random, 95% CI) | 0.45 [0.16, 1.25] |
| 21.7 use of antiparkinson medication | 6 | 1715 | Risk Ratio (M-H, Random, 95% CI) | 0.50 [0.30, 0.86] |
| 22 Adverse effects: 5b. Extrapyramidal effects - scale measured | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 22.1 abnormal involuntary movement: AIMS (high=poor) | 2 | 958 | Mean Difference (IV, Random, 95% CI) | −0.34 [−0.76, 0.08] |
| 22.2 akathisia: Barnes Akathisia Scale (high=poor) | 2 | 700 | Mean Difference (IV, Random, 95% CI) | −0.73 [−2.00, 0.54] |
| 22.3 extrapyramidal symptoms: Simpson-Angus Scale (high=poor) | 5 | 1077 | Mean Difference (IV, Random, 95% CI) | −0.59 [−1.16, −0.02] |
| 23 Adverse effects: 6. Haematological: important decline in white blood cells | 1 | 673 | Risk Ratio (M-H, Random, 95% CI) | 2.97 [0.12, 72.73] |
| 24 Adverse effects: 7a. Prolactin associated side effects | 6 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 24.1 amenorrhea | 4 | 359 | Risk Ratio (M-H, Random, 95% CI) | 0.47 [0.28, 0.79] |
| 24.2 dysmenorrhea | 1 | 163 | Risk Ratio (M-H, Random, 95% CI) | 0.45 [0.08, 2.38] |
| 24.3 galactorrhea | 5 | 1188 | Risk Ratio (M-H, Random, 95% CI) | 0.37 [0.16, 0.85] |
| 24.4 gynecomastia | 1 | 267 | Risk Ratio (M-H, Random, 95% CI) | 0.23 [0.07, 0.79] |
| 24.5 sexual dysfunction | 6 | 2157 | Risk Ratio (M-H, Random, 95% CI) | 0.70 [0.48, 1.01] |
| 25 Adverse effects: 7b. Prolactin -change from baseline in mg/dl | 6 | 1731 | Mean Difference (IV, Random, 95% CI) | −35.28 [−44.36, −26. 19] |
| 26 Adverse effects: 8a. Metabolic - cholesterol - significant cholesterol increase | 2 | 940 | Risk Ratio (M-H, Random, 95% CI) | 1.27 [0.72, 2.24] |
| 27 Adverse effects: 8b. Metabolic - cholesterol - change from baseline in mg/dl | 5 | 1433 | Mean Difference (IV, Random, 95% CI) | 8.61 [4.66, 12.56] |
| 28 Adverse effects: 8c. Metabolic - glucose - abnormally high fasting glucose value | 2 | 940 | Risk Ratio (M-H, Random, 95% CI) | 1.39 [0.56, 3.45] |
| 29 Adverse effects: 8d. Metabolic -glucose - change from baseline in mg/dl | 5 | 1436 | Mean Difference (IV, Random, 95% CI) | −0.04 [−2.92, 2.83] |
| 30 Adverse effects: 8e. Metabolic -weight gain of 7% or more of total body weight | 7 | 1942 | Risk Ratio (M-H, Random, 95% CI) | 0.97 [0.82, 1.14] |
| 31 Adverse effects: 8f. Metabolic - weight gain - change from baseline in kg | 7 | 1446 | Mean Difference (IV, Random, 95% CI) | 0.71 [−1.04, 2.47] |
Comparison 4. QUETIAPINE versus ZIPRASIDONE.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Leaving the study early | 2 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 1. 1 any reason | 2 | 722 | Risk Ratio (M-H, Random, 95% CI) | 1.05 [0.97, 1.13] |
| 1.2 adverse events | 2 | 722 | Risk Ratio (M-H, Random, 95% CI) | 1.04 [0.72, 1.49] |
| 1.3 inefficacy | 2 | 722 | Risk Ratio (M-H, Random, 95% CI) | 1.14 [0.89, 1.47] |
| 2 Mental state: 1. General - average endpoint score (PANSS total score, high=poor) | 2 | 710 | Mean Difference (IV, Random, 95% CI) | −0.11 [−6.36, 6.14] |
| 2.1 medium term | 1 | 198 | Mean Difference (IV, Random, 95% CI) | 3.70 [−2.97, 10.37] |
| 2.2 long term | 1 | 512 | Mean Difference (IV, Random, 95% CI) | −2.78 [−6.81, 1.25] |
| 3 Mental state: 2. Positive symptoms - average endpoint score - medium term (PANSS positive subscore, high=poor) | 1 | 198 | Mean Difference (IV, Random, 95% CI) | Not estimable |
| 4 Mental state: 3. Negative symptoms - average endpoint score - medium term (PANSS negative subscore, high=poor) | 1 | 198 | Mean Difference (IV, Random, 95% CI) | 1.60 [−0.34, 3.54] |
| 5 Service use: number of participants re-hospitalised | 2 | 754 | Risk Ratio (M-H, Random, 95% CI) | 1.17 [0.85, 1.59] |
| 5.1 medium term | 1 | 232 | Risk Ratio (M-H, Random, 95% CI) | 1.25 [0.71, 2.17] |
| 5.2 long term | 1 | 522 | Risk Ratio (M-H, Random, 95% CI) | 1.13 [0.78, 1.65] |
| 6 Adverse effects: 1. General - at least one adverse effect | 2 | 754 | Risk Ratio (M-H, Random, 95% CI) | 1.03 [0.91, 1.17] |
| 7 Adverse effects: 2. Death | 2 | 754 | Risk Ratio (M-H, Random, 95% CI) | 0.41 [0.05, 3.15] |
| 7.1 suicide attempt | 1 | 522 | Risk Ratio (M-H, Random, 95% CI) | 0.55 [0.03, 8.73] |
| 7.2 suicide | 1 | 232 | Risk Ratio (M-H, Random, 95% CI) | 0.29 [0.01, 5.92] |
| 8 Adverse effects: 3a. Cardiac effects - QTc prolongation | 1 | 522 | Risk Ratio (M-H, Random, 95% CI) | 1.65 [0.34, 8.08] |
| 9 Adverse effects: 3b. Cardiac effects - QTc abnormalities -change from baseline in ms | 2 | 549 | Mean Difference (IV, Random, 95% CI) | 3.41 [−1.37, 8.18] |
| 10 Adverse effects: 4. Central nervous system - sedation | 2 | 754 | Risk Ratio (M-H, Fixed, 95% CI) | 1.36 [1.04, 1.77] |
| 11 Adverse effects: 5. Extrapyramidal effects | 2 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 11.1 akathisia | 2 | 754 | Risk Ratio (M-H, Random, 95% CI) | 0.78 [0.42, 1.45] |
| 11.2 extrapyramidal symptoms | 1 | 232 | Risk Ratio (M-H, Random, 95% CI) | 2.02 [0.66, 6.17] |
| 11.3 use of antiparkinson medication | 1 | 522 | Risk Ratio (M-H, Random, 95% CI) | 0.43 [0.20, 0.93] |
| 12 Adverse effects: 6a. Prolactin associated effects | 2 | Risk Ratio (M-H, Random, 95% CI) | Subtotals only | |
| 12.1 amenorrhea | 1 | 138 | Risk Ratio (M-H, Random, 95% CI) | 0.43 [0.15, 1.24] |
| 12.2 galactorrhea | 2 | 572 | Risk Ratio (M-H, Random, 95% CI) | 0.55 [0.18, 1.68] |
| 12.3 sexual dysfunction | 2 | 754 | Risk Ratio (M-H, Random, 95% CI) | 0.96 [0.64, 1.42] |
| 13 Adverse effects: 6b. Prolactin -change from baseline in ng/ml | 2 | 754 | Mean Difference (IV, Random, 95% CI) | −4.77 [−8.16,−1.37] |
| 14 Adverse effects: 7a. Metabolic - cholesterol - change from baseline in mg/dl | 2 | 754 | Mean Difference (IV, Random, 95% CI) | 16.01 [8.57, 23.46] |
| 15 Adverse effects: 7b. Metabolic -glucose- change from baseline in mg/dl | 2 | 754 | Mean Difference (IV, Random, 95% CI) | 3.10 [−3.99, 10.19] |
| 16 Adverse effects: 7c. Metabolic -weight gain of 7% or more of total body weight | 2 | 754 | Risk Ratio (M-H, Random, 95% CI) | 2.22 [1.35, 3.63] |
| 17 Adverse effects: 7d. Metabolic - weight gain - change from baseline in kg | 1 | 466 | Mean Difference (IV, Random, 95% CI) | 1.2 [−0.05, 2.45] |
Comparison 5. QUETIAPINE versus CLOZAPINE- sensitivity analysis (skewed data excluded).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mental state: 1. General - average endpoint score - short term (PANSS total, high=poor) | 1 | 27 | Mean Difference (IV, Random, 95% CI) | 0.18 [−4.11, 4.47] |
| 1.1 short term | 1 | 27 | Mean Difference (IV, Random, 95% CI) | 0.18 [−4.11, 4.47] |
Comparison 6. QUETIAPINE versus OLANZAPINE- sensitivity analysis (skewed data excluded).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mental state: 1. Positive symptoms - average endpoint score (PANSS positive subscore, high=poor) | 6 | 639 | Mean Difference (IV, Random, 95% CI) | 1.82 [0.98, 2.65] |
| 1.1 short term | 2 | 75 | Mean Difference (IV, Random, 95% CI) | 0.09 [−2.76, 2.93] |
| 1.2 medium term | 3 | 483 | Mean Difference (IV, Random, 95% CI) | 2.21 [0.90, 3.52] |
| 1.3 long term | 1 | 81 | Mean Difference (IV, Random, 95% CI) | 1.80 [0.39, 3.21] |
Comparison 7. QUETIAPINE versus RISPERIDONE- sensitivity analysis (skewed data excluded).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mental state: 6. Positive symptoms - average endpoint score - (PANSS positive subscore, high=poor) | 6 | 1225 | Mean Difference (IV, Random, 95% CI) | 1.76 [1.04, 2.48] |
| 1.1 short term | 3 | 998 | Mean Difference (IV, Random, 95% CI) | 2.08 [0.60, 3.56] |
| 1.2 medium term | 2 | 146 | Mean Difference (IV, Random, 95% CI) | 2.15 [−0.01, 4.31] |
| 1.3 long term | 1 | 81 | Mean Difference (IV, Random, 95% CI) | 1.30 [−0.13, 2.73] |
| 2 Adverse effects: 1. Extrapyramidal effects -Simpson-Angus Scale (high= poor) | 4 | 1033 | Mean Difference (IV, Random, 95% CI) | −0.82 [−1.95, 0.31] |
Analysis 1.1. Comparison 1 QUETIAPINE versus CLOZAPINE - all data short term, Outcome 1 Global state: 1a. No clinically significant response (as defined by original studies).
Review: Quetiapine versus other atypical antipsychotics: for schizophrenia
Comparison: 1QUETIAPINE versus CLOZAPINE - all data short term
Outcome: 1 Global state: 1 a. No clinically significant response (as defined by original studies)
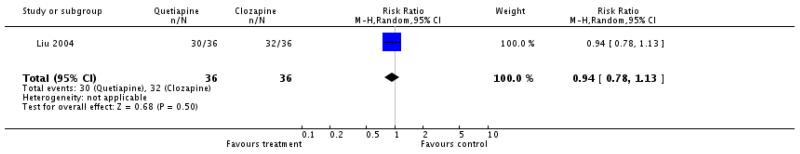
|
Analysis 1.2. Comparison 1 QUETIAPINE versus CLOZAPINE - all data short term, Outcome 2 Global state: 1b. No clinically important change - short term (as defined by the original studies).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 QUETIAPINE versus CLOZAPINE - all data short term
Outcome: 2 Global state: lb. No clinically important change - short term (as defined by the original studies)
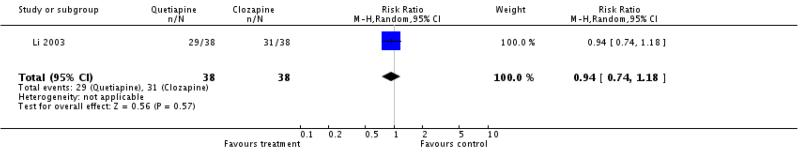
|
Analysis 1.3. Comparison 1 QUETIAPINE versus CLOZAPINE - all data short term, Outcome 3 Leaving the study early.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 QUETIAPINE versus CLOZAPINE - all data short term
Outcome: 3 Leaving the study early
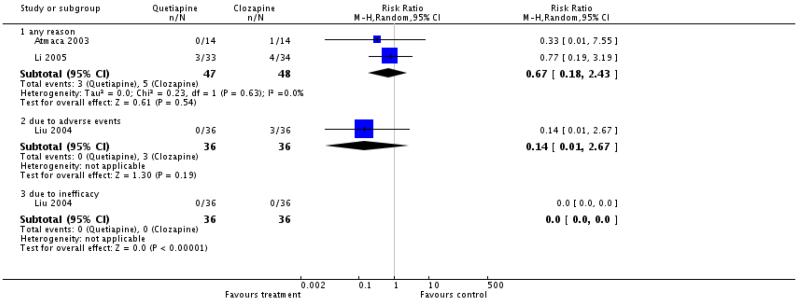
|
Analysis 1.4. Comparison 1 QUETIAPINE versus CLOZAPINE - all data short term, Outcome 4 Mental state: 1a. General - no clinically important change - short term (less than 50% PANSS total score reduction).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 QUETIAPINE versus CLOZAPINE - all data short term
Outcome: 4 Mental state: la. General - no clinically im portant change - short term (less than 50% PANSS total score reduction)

|
Analysis 1.5. Comparison 1 QUETIAPINE versus CLOZAPINE - all data short term, Outcome 5 Mental state: 1b. General - average endpoint score - short term (PANSS total, high=poor).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 1QUETIAPINE versus CLOZAPINE - all data short term
Outcome: 5 Mental state: lb. General - average endpoint score - short term (PANSS total, high = poor)

|
Analysis 1.6. Comparison 1 QUETIAPINE versus CLOZAPINE - all data short term, Outcome 6 Mental state: 1c. General - average endpoint score - short term (BPRS total, high=poor).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 QUETIAPINE versus CLOZAPINE - all data short term
Outcome: 6 Mental state: lc. General - average endpoint score - short term (BPRS total, high = poor)
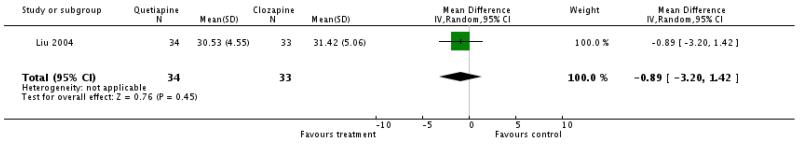
|
Analysis 1.7. Comparison 1 QUETIAPINE versus CLOZAPINE - all data short term, Outcome 7 Mental state: 2. Positive symptoms - average endpoint score (PANSS positive subscore, high=poor).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 QUETIAPINE versus CLOZAPINE - all data short term
Outcome: 7 Mental state: 2. Positive symptoms - average endpoint score (PANSS positive subscore, high=poor)
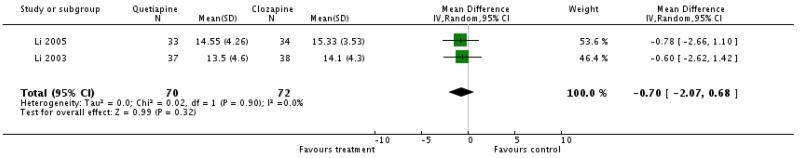
|
Analysis 1.8. Comparison 1 QUETIAPINE versus CLOZAPINE - all data short term, Outcome 8 Mental state: 3a. Negative symptoms - no clinically important change - short term (less than 50% SANS total score reduction).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 QUETIAPINE versus CLOZAPINE - all data short term
Outcome: 9 Mental state: 3a. Negative symptoms - no clinically important change - short term (less than 50% SANS total score reduction)
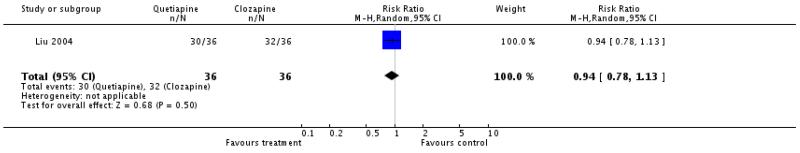
|
Analysis 1.9. Comparison 1 QUETIAPINE versus CLOZAPINE - all data short term, Outcome 9 Mental state: 3b. Negative symptoms - average endpoint score - short term (PANSS negative subscore, high=poor).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 QUETIAPINE versus CLOZAPINE - all data short term
Outcome: 9 Mental state: 3b. Negative symptoms - average endpoint score - short term (PANSS negative subscore, high=poor)
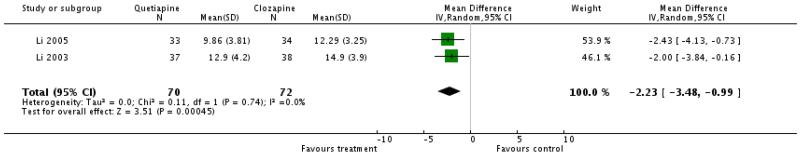
|
Analysis 1.10. Comparison 1 QUETIAPINE versus CLOZAPINE - all data short term, Outcome 10 Mental state: 3c. Negative symptoms - average endpoint score - short term (SANS total, high=poor).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 QUETIAPINE versus CLOZAPINE - all data short term
Outcome: 10 Mental state: 3c. Negative symptoms - average endpoint score - short term (SANS total, high = poor)
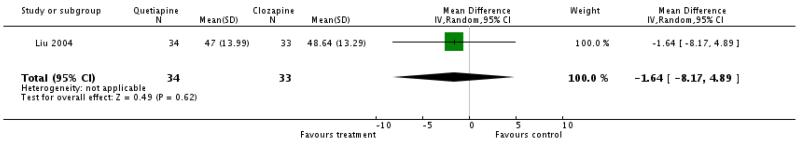
|
Analysis 1.11. Comparison 1 QUETIAPINE versus CLOZAPINE - all data short term, Outcome 11 Adverse effects: 1. General - at least one adverse effect.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 QUETIAPINE versus CLOZAPINE - all data short term
Outcome: 11 Adverse effects: 1. General - at least one adverse effect
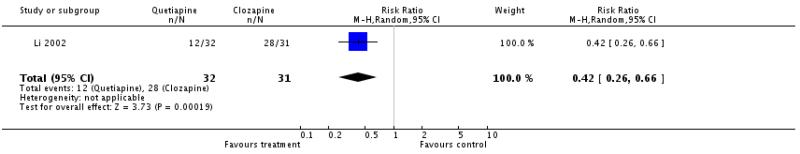
|
Analysis 1.12. Comparison 1 QUETIAPINE versus CLOZAPINE - all data short term, Outcome 12 Adverse effects: 2. Cardiac effects: ECG abnormalities.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 QUETIAPINE versus CLOZAPINE - all data short term
Outcome: 12 Adverse effects: Z. Cardiac effects: ECG abnormalities

|
Analysis 1.13. Comparison 1 QUETIAPINE versus CLOZAPINE - all data short term, Outcome 13 Adverse effects: 3. Central nervous system - sedation.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 QUETIAPINE versus CLOZAPINE - all data short term
Outcome: 13 Adverse effects: 3. Central nervous system - sedation
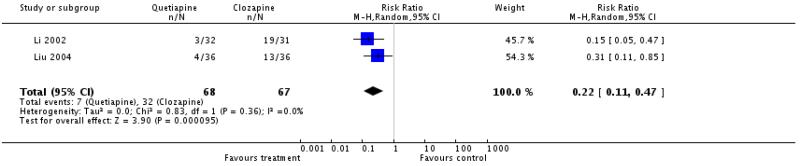
|
Analysis 1.14. Comparison 1 QUETIAPINE versus CLOZAPINE - all data short term, Outcome 14 Adverse effects: 4. Extrapyramidal effects.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 QUETIAPINE versus CLOZAPINE - all data short term
Outcome: 14 Adverse effects: 4. Extrapyramidal effects
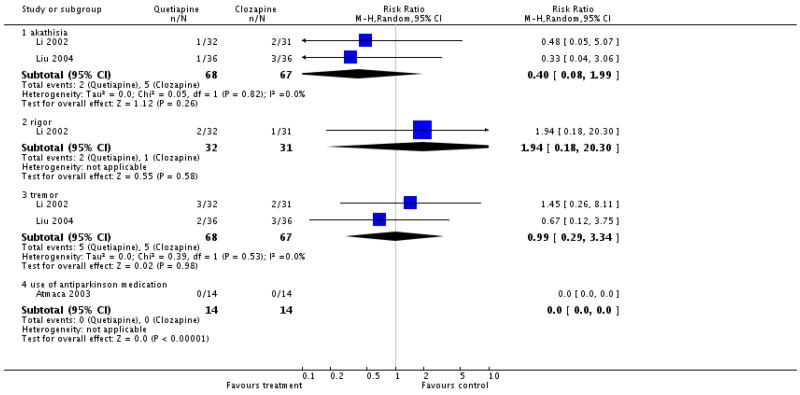
|
Analysis 1.15. Comparison 1 QUETIAPINE versus CLOZAPINE - all data short term, Outcome 15 Adverse effects: 5. Haematological: Important decline in white blood cells.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 QUETIAPINE versus CLOZAPINE - all data short term
Outcome: 15 Adverse effects: 5. Haematological: Important decline in white blood cells
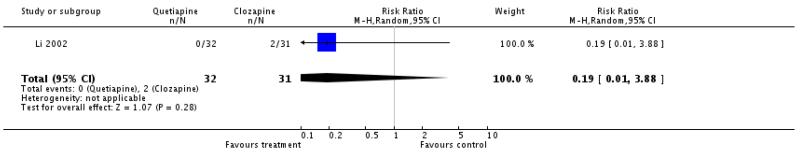
|
Analysis 1.16. Comparison 1 QUETIAPINE versus CLOZAPINE - all data short term, Outcome 16 Adverse effects: 6a. Metabolic - weight - gain.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 QUETIAPINE versus CLOZAPINE - all data short term
Outcome: 16 Adverse effects: 6a. Metabolic - weight - gain
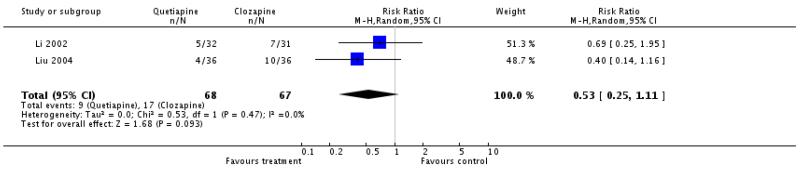
|
Analysis 1.17. Comparison 1 QUETIAPINE versus CLOZAPINE - all data short term, Outcome 17 Adverse effects: 6b. Metabolic - weight - change from baseline (kg).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 1 QUETIAPINE versus CLOZAPINE - all data short term
Outcome: 11 Adverse effects: 6b. Metabolic - weight - change from baseline (kg)
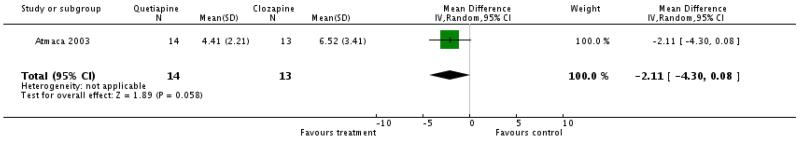
|
Analysis 2.1. Comparison 2 QUETIAPINE versus OLANZAPINE, Outcome 1 Global state: 1a. No clinically significant response (as defined by the original studies).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 2 QUETIAPINE versus OLANZAPINE
Outcome: 1 Global state: 1a. No clinically significant response (as defined by the original studies)
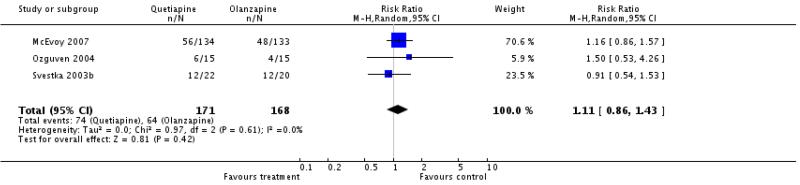
|
Analysis 2.2. Comparison 2 QUETIAPINE versus OLANZAPINE, Outcome 2 Global state: 1b. No clinically important change (as defined by the original studies).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 2 QUETIAPINE versus OLANZAPINE
Outcome: 2 Global state: lb. No clinically im portant change (as defined by the original studies)
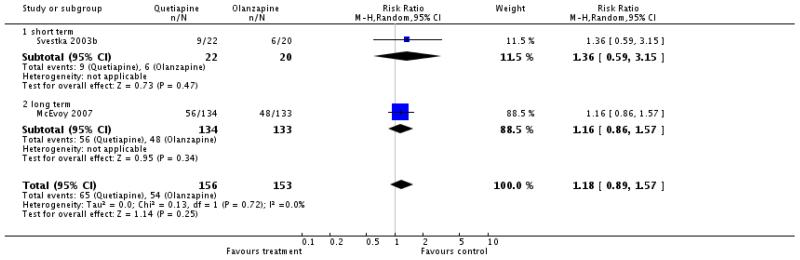
|
Analysis 2.3. Comparison 2 QUETIAPINE versus OLANZAPINE, Outcome 3 Leaving the study early.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 2 QUETIAPINE versus OLANZAPINE
Outcome: 3 Leaving the study early
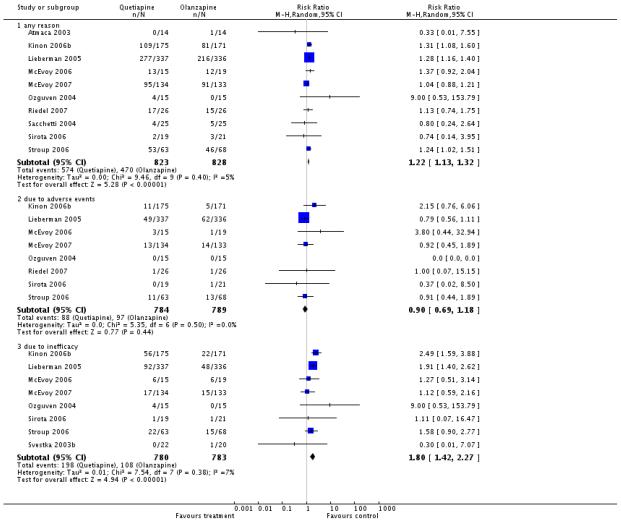
|
Analysis 2.4. Comparison 2 QUETIAPINE versus OLANZAPINE, Outcome 4 Mental state: 1a. General - no clinically important change-short term (less than 50% PANSS total score reduction).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 2 QUETIAPINE versus OLANZAPINE
Outcome: 4 Mental state: 1a. General - no clinically important change-short term (less than 50% PANSS total score reduction)
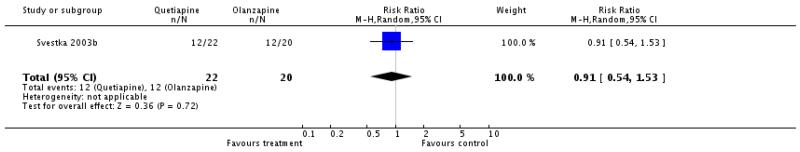
|
Analysis 2.5. Comparison 2 QUETIAPINE versus OLANZAPINE, Outcome 5 Mental state: 1b. General - average endpoint score (PANSS total, high=poor).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 2 QUETIAPINE versus OLANZAPINE
Outcome: 5 Mental state: lb. General - average endpoint score (PANSS total, high = poor)
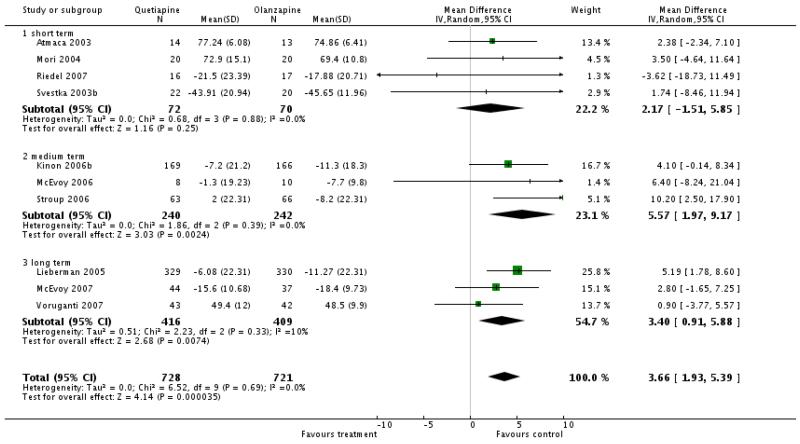
|
Analysis 2.6. Comparison 2 QUETIAPINE versus OLANZAPINE, Outcome 6 Mental state: 2a. Positive symptoms - no clinically important change-short term (less than 20% SAPS total score reduction).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 2 QUETIAPINE versus OLANZAPINE
Outcome: 6 Mental state: 2a. Positive symptoms - no clinically important change-short term (less than 20% SAPS total score reduction)

|
Analysis 2.7. Comparison 2 QUETIAPINE versus OLANZAPINE, Outcome 7 Mental state: 2b. Positive symptoms - average endpoint score (PANSS positive subscore, high=poor).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 2 QUETIAPINE versus OLANZAPINE
Outcome: 7 Mental state: 2b. Positive symptoms - average endpoint score (PANSS positive subscore, high = poor)

|
Analysis 2.8. Comparison 2 QUETIAPINE versus OLANZAPINE, Outcome 8 Mental state: 2c. Positive symptoms - SAPS total score - percent change-short term (high=poor).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 2 QUETIAPINE versus OLANZAPINE
Outcome: 8 Mental state: 2c. Positive symptoms - SAPS total score - percent change-short term (high = poor)

|
Analysis 2.9. Comparison 2 QUETIAPINE versus OLANZAPINE, Outcome 9 Mental state: 3a. Negative symptoms - no clinically important change-short term (less than 20% SANS total score reduction).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 2 QUETIAPINE versus OLANZAPINE
Outcome: 9 Mental state: 3a. Negative symptoms - no clinically im portant change-short term (less than 2 OS SANS total score reduction)
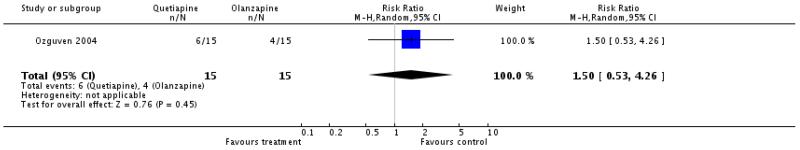
|
Analysis 2.10. Comparison 2 QUETIAPINE versus OLANZAPINE, Outcome 10 Mental state: 3b. Negative symptoms - average endpoint score (PANSS negative subscore, high=poor).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 2 QUETIAPINE versus OLANZAPINE
Outcome: 10 Mental state: 3b. Negative symptoms - average endpoint score (PANSS negative subscore, high = poor)
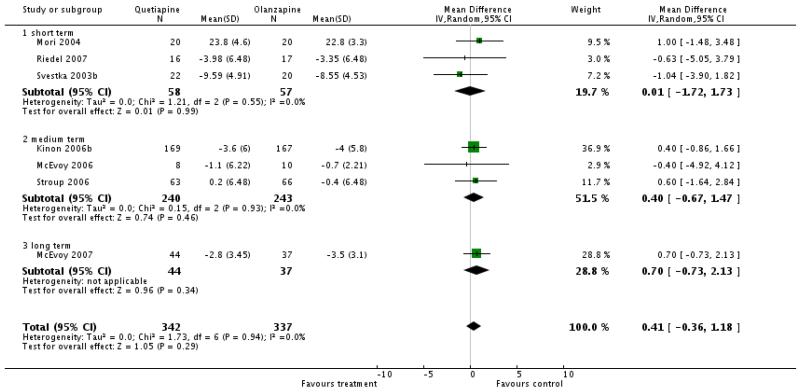
|
Analysis 2.11. Comparison 2 QUETIAPINE versus OLANZAPINE, Outcome 11 Mental state: 3c. Negative symptoms - average endpoint score-medium term (SANS total score, high=poor).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 2 QUETIAPINE versus OLANZAPINE
Outcome: 11 Mental state: 3c. Negative symptoms - average endpoint score-medium term (SANS total score, high = poor)
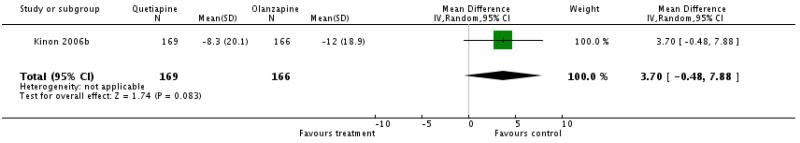
|
Analysis 2.12. Comparison 2 QUETIAPINE versus OLANZAPINE, Outcome 12 Mental state: 3d. Negative symptoms - average endpoint score-short term (SANS total score-percent change, high=poor).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 2 QUETIAPINE versus OLANZAPINE
Outcome: 12 Mental state: 3d. Negative symptoms - average endpoint score-short term (SANS total score-percent change, high = poor)
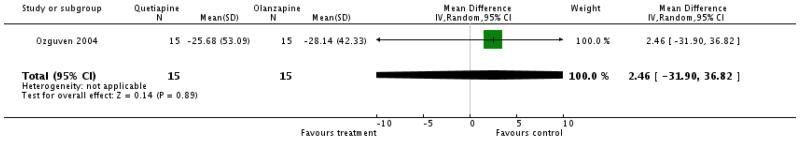
|
Analysis 2.13. Comparison 2 QUETIAPINE versus OLANZAPINE, Outcome 13 General functioning: General - average endpoint score-medium term (GAF total score, high=poor).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 2 QUETIAPINE versus OLANZAPINE
Outcome: 13 General functioning: General - average endpoint score-medium term (GAF total score, high = poor)

|
Analysis 2.14. Comparison 2 QUETIAPINE versus OLANZAPINE, Outcome 14 Quality of life: General - average endpoint score-medium term (QLS total score, high=poor).
Review: 2 Quetiapine versus OLANZAPINE
Outcome 14 Quality of versus other atypical antipsychotics for schizophrenia
Comparison: 2 QUETIAPINE versus OLANZAPINE
Outcome: 14 Quality of life: General - average endpoint score-m edium term (QLS total score, high = poor)

|
Analysis 2.15. Comparison 2 QUETIAPINE versus OLANZAPINE, Outcome eric use nm fatin osale.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 2 QUETIAPINE versus OLANZAPINE
Outcome: 15 Service use: num ber of paiticipants re-hospitalised
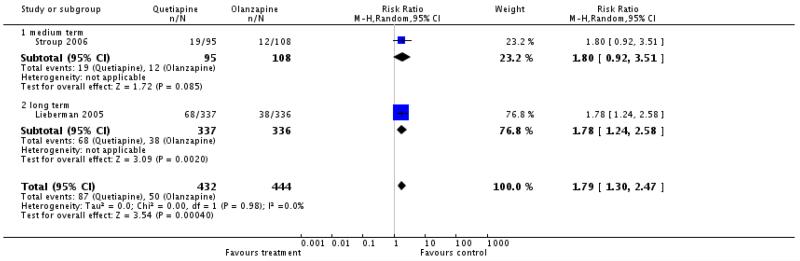
|
Analysis 2.16. Comparison 2 QUETIAPINE versus OLANZAPINE, Outcome 16 Adverse effects: 1. General - at least one adverse effect.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 2 QUETIAPINE versus OLANZAPINE
Outcome: 16 Adverse effects: 1. General - at least one adverse effect
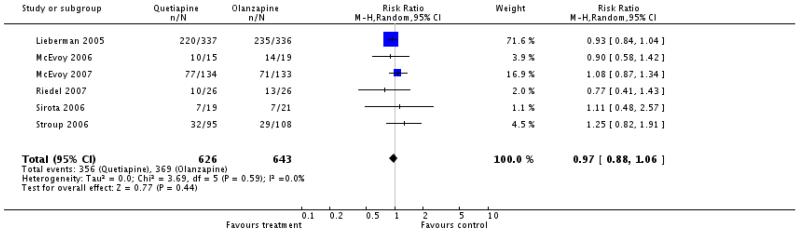
|
Analysis 2.17. Comparison 2 QUETIAPINE versus OLANZAPINE, Outcome 17 Adverse effects: 2. Death.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 2 QUETIAPINE versus OLANZAPINE
Outcome: 17 Adverse effects: 2. Death

|
Analysis 2.18. Comparison 2 QUETIAPINE versus OLANZAPINE, Outcome 18 Adverse effects: 3a. Cardiac effects - QTc prolongation.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 2 QUETIAPINE versus OLANZAPINE
Outcome: 18 Adverse effects: 3a. Cardiac effects - QTc prolongation
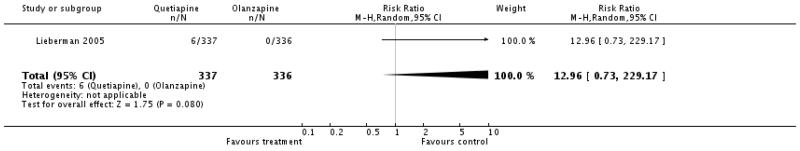
|
Analysis 2.19. Comparison 2 QUETIAPINE versus OLANZAPINE, Outcome 19 Adverse effects: 3b. Cardiac effects - QTc abnormalities - change from baseline in ms.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 2 QUETIAPINE versus OLANZAPINE
Outcome: 19 Adverse effects: 3b. Cardiac effects - CLTc abnormalities - change from baseline in ms

|
Analysis 2.20. Comparison 2 QUETIAPINE versus OLANZAPINE, Outcome 20 Adverse effects: 4a. Central nervous system - sedation.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 2 QUETIAPINE versus OLANZAPINE
Outcome: 20 Adverse effects: 4a. Central nervous system - sedation

|
Analysis 2.21. Comparison 2 QUETIAPINE versus OLANZAPINE, Outcome 21 Adverse effects: 4b. Central nervous system - seizures.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 2 QUETIAPINE versus OLANZAPINE
Outcome: 21 Adverse effects: 4b. Central nervous system - seizures
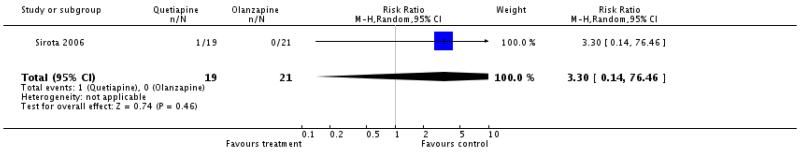
|
Analysis 2.22. Comparison 2 QUETIAPINE versus OLANZAPINE, Outcome 22 Adverse effects: 5a. Extrapyramidal effects.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 2 QUETIAPINE versus OLANZAPINE
Outcome: 22 Adverse effects: 5a. Extrapyramidal effects
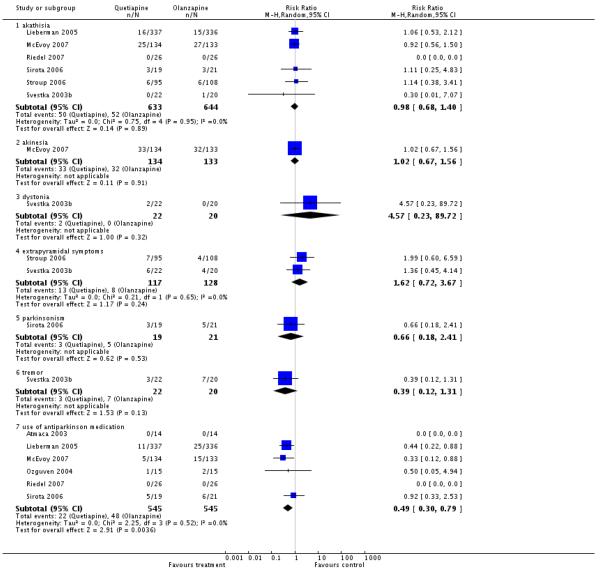
|
Analysis 2.23. Comparison 2 QUETIAPINE versu OLANZAPINE, Outcome 23 Adverse effects: 5b. Extrapyramidal effects - scale measured.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 2 QUETIAPINE versus OLANZAPINE
Outcome: 23 Adverse effects: 5b. Extrapyramidal effects - scale measured
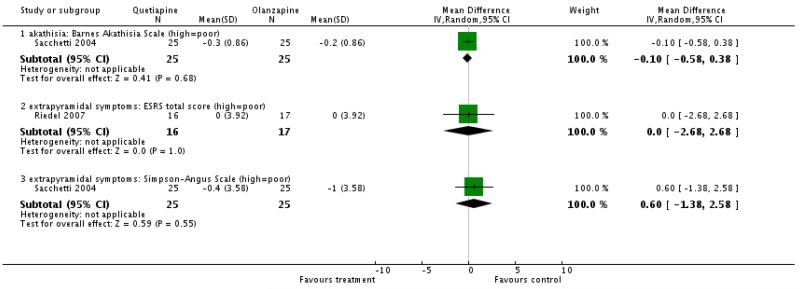
|
Analysis 2.24. Comparison 2 QUETIAPINE versus OLANZAPINE, Outcome 24 Adverse effects: 6a. Prolactin associated side effects.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 2 QUETIAPINE versus OLANZAPINE
Outcome: 24 Adverse effects: 6a. Prolactin associated side effects
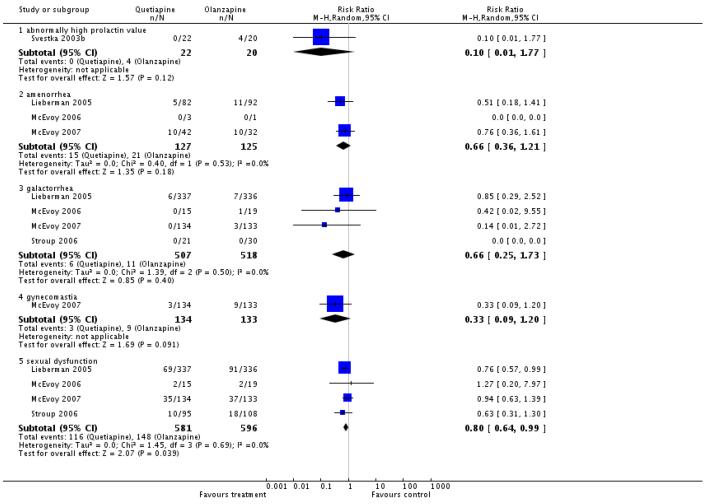
|
Analysis 2.25. Comparison 2 QUETIAPINE versus OLANZAPINE, Outcome 25 Adverse effects: 6b. Prolactin - change from baseline in ng/ml.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 2 QUETIAPINE versus OLANZAPINE
Outcome: 25 Adverse effects: 6b. Prolactin - change from baseline in ng/ml

|
Analysis 2.26. Comparison 2 QUETIAPINE versus OLANZAPINE, Outcome 26 Adverse effects: 7a. Metabolic - cholesterol - significant cholesterol increase.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 2 QUETIAPINE versus OLANZAPINE
Outcome: 26 Adverse effects: 7a. Metabolic - cholesterol - significant cholesterol increase
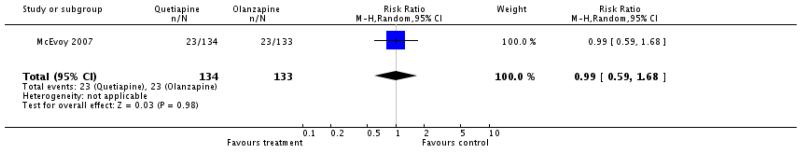
|
Analysis 2.27. Comparison 2 QUETIAPINE versus OLANZAPINE, Outcome 27 Adverse effects: 7b. Metabolic - cholesterol - change from baseline in mg/dl.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 2 QUETIAPINE versus OLANZAPINE
Outcome: 7.1 Adverse effects: 7b. Metabolic - cholesterol - change from baseline in mg/dl
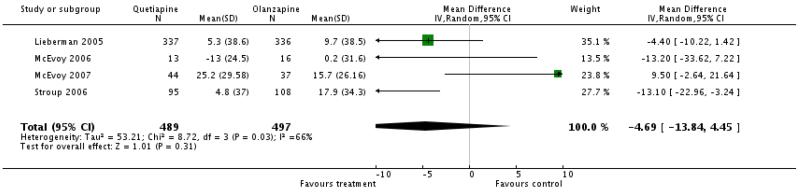
|
Analysis 2.28. Comparison 2 QUETIAPINE versus OLANZAPINE, Outcome 28 Adverse effects: 7c. Metabolic - glucose - abnormally high fasting glucose value.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 2 QUETIAPINE versus OLANZAPINE
Outcome: 2B Adverse effects: 7c. Metabolic - glucose - abnorm ally high fasting glucose value
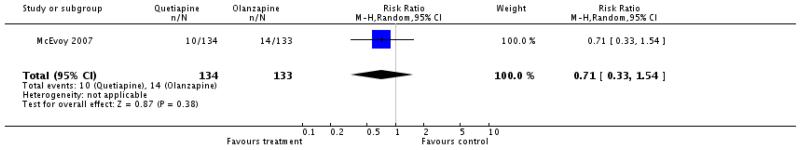
|
Analysis 2.29. Comparison 2 QUETIAPINE versus OLANZAPINE, Outcome 29 Adverse effects: 7d. Metabolic - glucose - change from baseline in mg/dl.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 2 QUETIAPINE versus OLANZAPINE
Outcome: 29 Adverse effects: 7d. Metabolic - glucose - change from baseline in mg/dl

|
Analysis 2.30. Comparison 2 QUETIAPINE versus OLANZAPINE, Outcome 30 Adverse effects: 7e. Metabolic - weight - gain.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 2 QUETIAPINE versus OLANZAPINE
Outcome: 30 Adverse effects: 7e. Metabolic - weight - gain
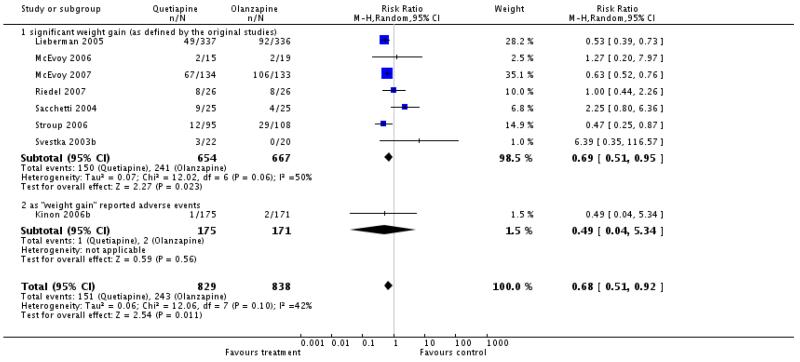
|
Analysis 2.31. Comparison 2 QUETIAPINE versus OLANZAPINE, Outcome 31 Adverse effects: 7f. Metabolic - weight - change from baseline in kg.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 2 QUETIAPINE versus OLANZAPINE
Outcome: 31 Adverse effects: 7f. Metabolic - weight - change from baseline in kg
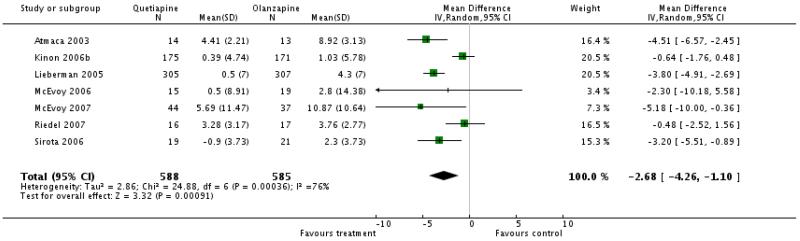
|
Analysis 3.1. Comparison 3 QUETIAPINE versus RISPERIDONE, Outcome 1 Global state: 1a. No clinically significant response (as defined by the original studies).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 QUETIAPINE versus RISPERIDONE
Outcome: 1 Global state: 1a. No clinically significant response (as defined by the original studies)

|
Analysis 3.2. Comparison 3 QUETIAPINE versus RISPERIDONE, Outcome 2 Global state: 1b. No clinically important change (as defined by the original studies).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 QUETIAPINE versus RISPERIDONE
Outcome: 2 Global state: lb. No clinically im portant change (as defined by the original studies)

|
Analysis 3.3. Comparison 3 QUETIAPINE versus RISPERIDONE, Outcome 3 Leaving the study early.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 QUETIAPINE versus RISPERIDONE
Outcome: 3 Leaving the study early
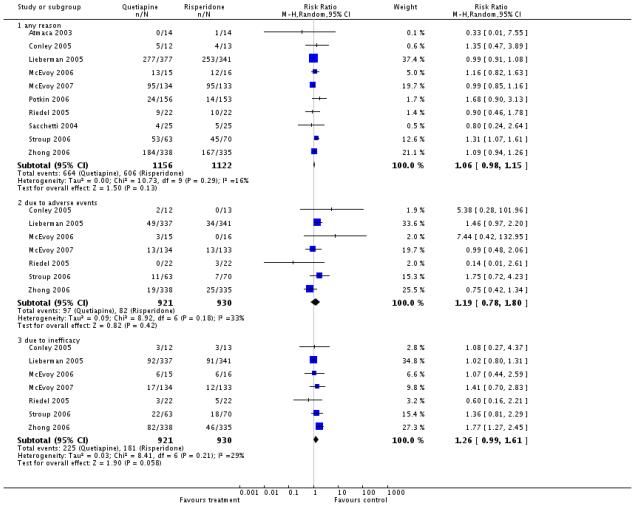
|
Analysis 3.4. Comparison 3 QUETIAPINE versus RISPERIDONE, Outcome 4 Mental state: 1a General - no clinically important change - short term (less than 30% PANSS total score reduction).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 QUETIAPINE versus RISPERIDONE
Outcome: 4 Mental state: 1a General - no clinically im poitant change - shoit term (less than 30% PANSS total score reduction)
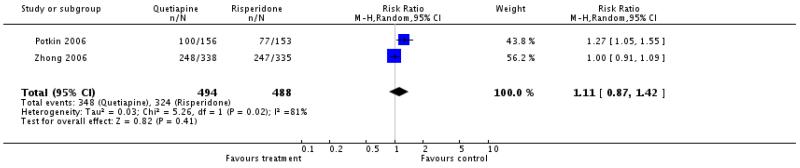
|
Analysis 3.5. Comparison 3 QUETIAPINE versus RISPERIDONE, Outcome 5 Mental state: 1b. General - no clinicallly important change - short term (less than 20% BPRS total score reduction).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 QUETIAPINE versus RISPERIDONE
Outcome: 5 Mental state: lb. General - no clinicallly im portant change - short term (less than 2 OS BPRS total score reduction)
|
Analysis 3.6. Comparison 3 QUETIAPINE versus RISPERIDONE, Outcome 6 Mental state: 1c. General - average endpoint score (PANSS total score, high=poor).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 QUETIAPINE versus RISPERIDONE
Outcome: 6 Mental state: lc. General - average endpoint score (PANSS total score, high = poor)
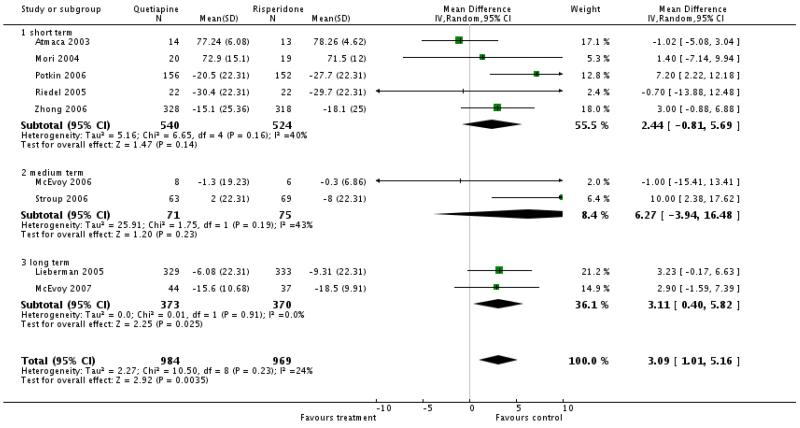
|
Analysis 3.7. Comparison 3 QUETIAPINE versus RISPERIDONE, Outcome 7 Mental state: 1d. General - average endpoint score - short term (BPRS total score, high=poor).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 QUETIAPINE versus RISPERIDONE
Outcome: 7 Mental state: 1d. General - average endpoint score - short term (BPRS total score, high = poor)
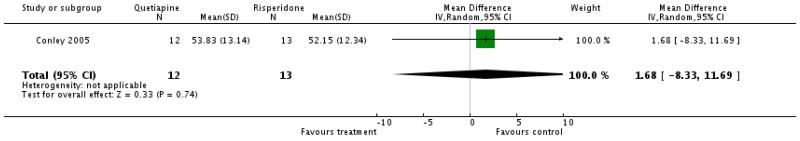
|
Analysis 3.8. Comparison 3 QUETIAPINE versus RISPERIDONE, Outcome 8 Mental state: 2a. Positive symptoms - no clinically important change - short term (less than 40% PANSS positive reduction).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 QUETIAPINE versus RISPERIDONE
Outcome: 8 Mental state: 2a. Positive symptoms - no clinically important change - short term (less than 40% PANSS positive reduction)
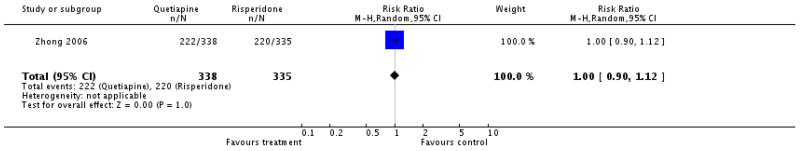
|
Analysis 3.9. Comparison 3 QUETIAPINE versus RISPERIDONE, Outcome 9 Mental state: 2b. Positive symptoms - average endpoint score - (PANSS positive subscore, high=poor).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 QUETIAPINE versus RISPERIDONE
Outcome: 9 Mental state: 2b. Positive symptoms - average endpoint score - (PANSS positive subscore, high = poor)
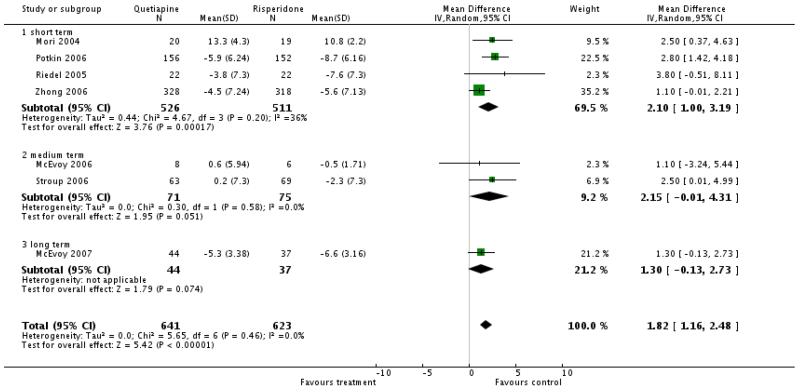
|
Analysis 3.10. Comparison 3 QUETIAPINE versus RISPERIDONE, Outcome 10 Mental state: 2c. Positive symptoms - average endpoint score - short term (BPRS positive subscore, high=poor).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 QUETIAPINE versus RISPERIDONE
Outcome: IB Mental state: 2c. Positive symptoms - average endpoint score - short term (BPRS positive subscore, high = poor)

|
Analysis 3.11. Comparison 3 QUETIAPINE versus RISPERIDONE, Outcome 11 Mental state: 3a. Negative symptoms - no clinicallly important change - short term (less than 40% PANSS negative reduction).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 QUETIAPINE versus RISPERIDONE
Outcome: 11 Mental state: 3a. Negative symptoms - no clinicallly im portant change - short term (less than 40% PANSS negative reduction)

|
Analysis 3.12. Comparison 3 QUETIAPINE versus RISPERIDONE, Outcome 12 Mental state: 3b. Negative symptoms - average endpoint score - (PANSS negative subscore, high=poor).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 QUETIAPINE versus RISPERIDONE
Outcome: 12 Mental state: 3b. Negative symptoms - average endpoint score - (PANSS negative subscore, high = poor)
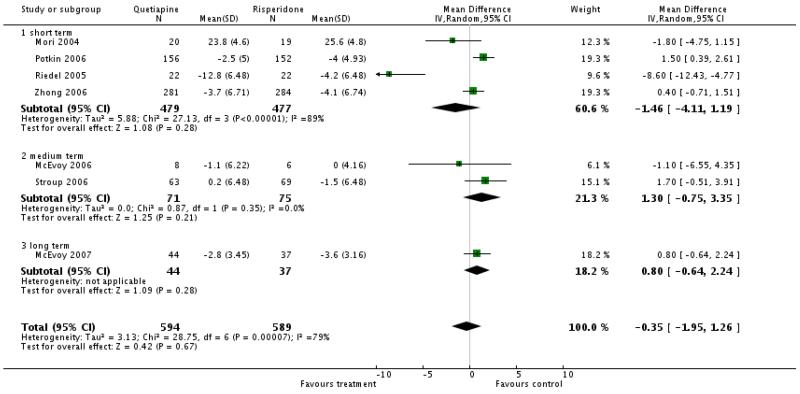
|
Analysis 3.13. Comparison 3 QUETIAPINE versus RISPERIDONE, Outcome 13 Mental state: 3c. Negative symptoms - average endpoint score - (BPRS negative subscore, high=poor).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 QUETIAPINE versus RISPERIDONE
Outcome: 13 Mental state: 3c. Negative symptoms - average endpoint score - (BPRS negative subscore, high = poor)
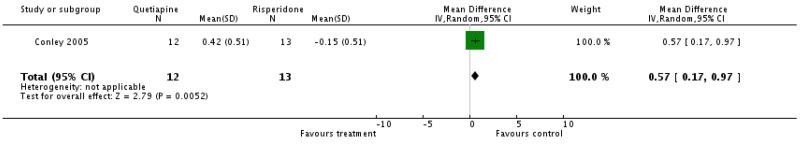
|
Analysis 3.14. Comparison 3 QUETIAPINE versus RISPERIDONE, Outcome 14 Quality of life: General- average endpoint score - short term (QLS total score, high=poor).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 QUETIAPINE versus RISPERIDONE
Outcome: 14 Quality of life: General- average endpoint score - short term (QLS total score, high = poor)

|
Analysis 3.15. Comparison 3 QUETIAPINE versus RISPERIDONE, Outcome 15 Service use: number of participants re-hospitalised.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 QUETIAPINE versus RISPERIDONE
Outcome: 15 Service use: num ber of participants re-hospitalised
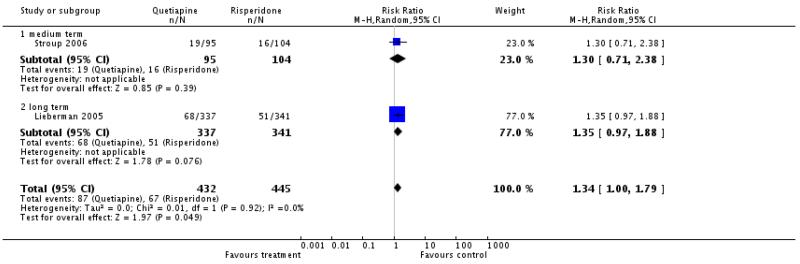
|
Analysis 3.16. Comparison 3 QUETIAPINE versus RISPERIDONE, Outcome 16 Adverse effects: 1. General - at least one adverse effect.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 QUETIAPINE versus RISPERIDONE
Outcome: 16 Adverse effects: 1. General - at least one adverse effect

|
Analysis 3.17. Comparison 3 QUETIAPINE versus RISPERIDONE, Outcome 17 Adverse effects: 2. Death.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 QUETIAPINE versus RISPERIDONE
Outcome: 17 Adverse effects: 2. Death

|
Analysis 3.18. Comparison 3 QUETIAPINE versus RISPERIDONE, Outcome 18 Adverse effects: 3a. Cardiac effects - QTc prolongation.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 QUETIAPINE versus RISPERIDONE
Outcome: 18 Adverse effects: 3a. Cardiac effects - QTc prolongation

|
Analysis 3.19. Comparison 3 QUETIAPINE versus RISPERIDONE, Outcome 19 Adverse effects: 3b. Cardiac effects - QTc abnormalities - change from baseline in ms.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 QUETIAPINE versus RISPERIDONE
Outcome: 19 Adverse effects: 3b. Cardiac effects - QTc abnormalities - change from baseline in ms

|
Analysis 3.20. Comparison 3 QUETIAPINE versus RISPERIDONE, Outcome 20 Adverse effects: 4. Central nervous system - sedation.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 QUETIAPINE versus RISPERIDONE
Outcome: 20 Adverse effects: 4. Central nervous system - sedation

|
Analysis 3.21. Comparison 3 QUETIAPINE versus RISPERIDONE, Outcome 21 Adverse effects: 5a. Extrapyramidal effects.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 QUETIAPINE versus RISPERIDONE
Outcome: 21 Adverse effects: 5a. Extrapyramidal effects

|
Analysis 3.22. Comparison 3 QUETIAPINE versus RISPERIDONE, Outcome 22 Adverse effects: 5b. Extrapyramidal effects - scale measured.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 QUETIAPINE versus RISPERIDONE
Outcome: 22 Adverse effects: 5b. Extrapyramidal effects - scale measured

|
Analysis 3.23. Comparison 3 QUETIAPINE versus RISPERIDONE, Outcome 23 Adverse effects: 6. Haematological: important decline in white blood cells.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 QUETIAPINE versus RISPERIDONE
Outcome: 23 Adverse effects: S. Haematological: important decline in white blood cells
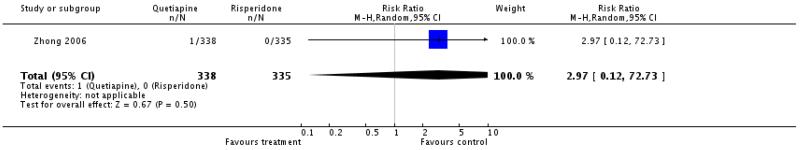
|
Analysis 3.24. Comparison 3 QUETIAPINE versus RISPERIDONE, Outcome 24 Adverse effects: 7a. Prolactin associated side effects.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 QUETIAPINE versus RISPERIDONE
Outcome: 24 Adverse effects: 1a. Prolactin associated side effects

|
Analysis 3.25. Comparison 3 QUETIAPINE versus RISPERIDONE, Outcome 25 Adverse effects: 7b. Prolactin - change from baseline in mg/dl.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 QUETIAPINE versus RISPERIDONE
Outcome: 25 Adverse effects: ?b. Prolactin - change from baseline in mg/dl
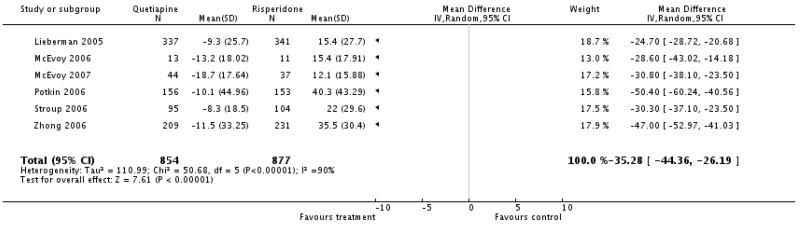
|
Analysis 3.26. Comparison 3 QUETIAPINE versus RISPERIDONE, Outcome 26 Adverse effects: 8a. Metabolic - cholesterol - significant cholesterol increase.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 QUETIAPINE versus RISPERIDONE
Outcome: 26 Adverse effects: 8a. Metabolic - cholesterol - significant cholesterol increase
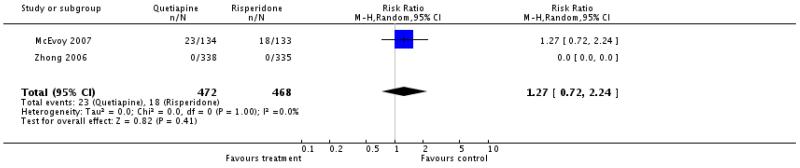
|
Analysis 3.27. Comparison 3 QUETIAPINE versus RISPERIDONE, Outcome 27 Adverse effects: 8b. Metabolic - cholesterol - change from baseline in mg/dl.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 QUETIAPINE versus RISPERIDONE
Outcome: 21 Adverse effects: 8b. Metabolic - cholesterol - change from baseline in mg/dl
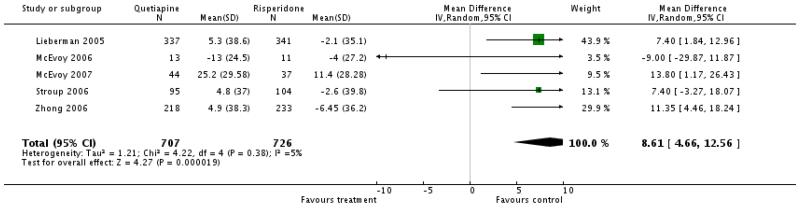
|
Analysis 3.28. Comparison 3 QUETIAPINE versus RISPERIDONE, Outcome 28 Adverse effects: 8c. Metabolic - glucose - abnormally high fasting glucose value.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 QUETIAPINE versus RISPERIDONE
Outcome: 2B Adverse effects: 8c. Metabolic - glucose - abnorm ally high fasting glucose value
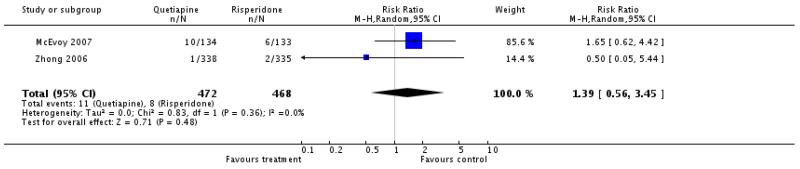
|
Analysis 3.29. Comparison 3 QUETIAPINE versus RISPERIDONE, Outcome 29 Adverse effects: 8d. Metabolic - glucose - change from baseline in mg/dl.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 QUETIAPINE versus RISPERIDONE
Outcome: 29 Adverse effects: 8d. Metabolic - glucose - change from baseline in mg/dl
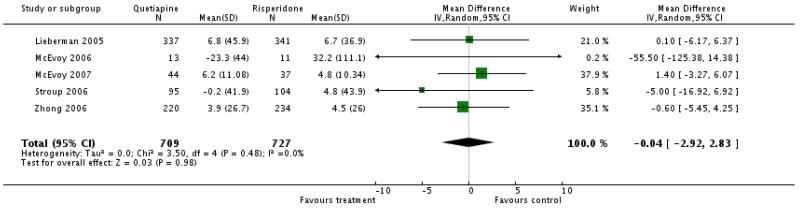
|
Analysis 3.30. Comparison 3 QUETIAPINE versus RISPERIDONE, Outcome 30 Adverse effects: 8e. Metabolic - weight gain of 7% or more of total body weight.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 QUETIAPINE versus RISPERIDONE
Outcome: 30 Adverse effects: 8e. Metabolic - weight gain of 7% or m ore of total body weight

|
Analysis 3.31. Comparison 3 QUETIAPINE versus RISPERIDONE, Outcome 31 Adverse effects: 8f. Metabolic - weight gain - change from baseline in kg.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 3 QUETIAPINE versus RISPERIDONE
Outcome: 31 Adverse effects: 8f. Metabolic - weight gain - change from baseline in kg
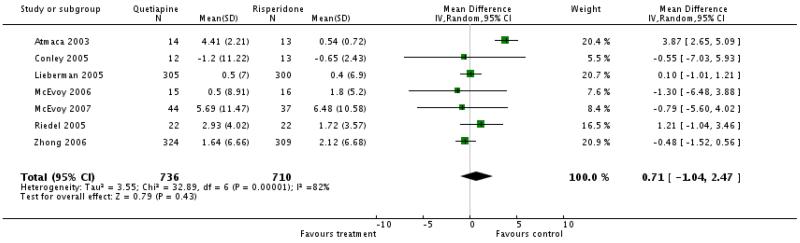
|
Analysis 4.1. Comparison 4 QUETIAPINE versus ZIPRASIDONE, Outcome 1 Leaving the study early.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 4 QUETIAPINE versus ZIPRASIDONE
Outcome: 1 Leaving the study early
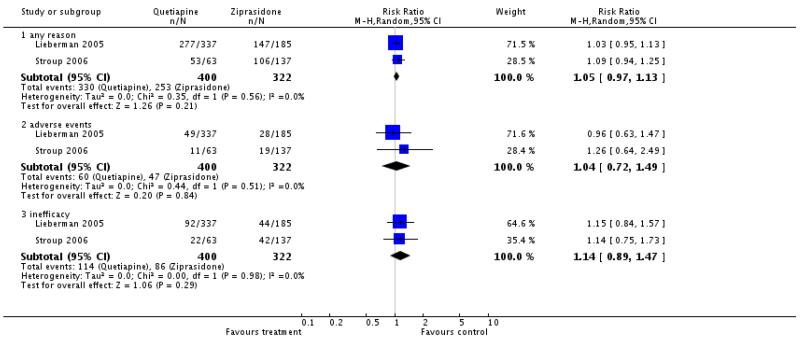
|
Analysis 4.2. Comparison 4 QUETIAPINE versus ZIPRASIDONE, Outcome 2 Mental state: 1. General - average endpoint score (PANSS total score, high=poor).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 4 QUETIAPINE versus ZIPRASIDONE
Outcome: 2 Mental state: 1. General - average endpoint score (PANSS total score, high = pnor)

|
Analysis 4.3. Comparison 4 QUETIAPINE versus ZIPRASIDONE, Outcome 3 Mental state: 2. Positive symptoms - average endpoint score - medium term (PANSS positive subscore, high=poor).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 4 QUETIAPINE versus ZIPRASIDONE
Outcome: 3 Mental state: 2. Positive symptoms - average endpoint score - medium term (PANSS positive subscore, high = poor)
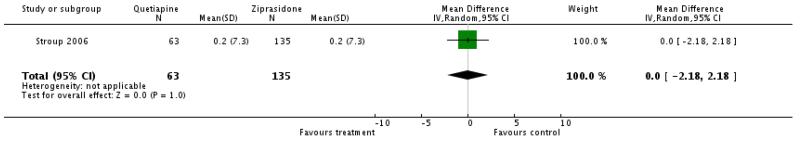
|
Analysis 4.4. Comparison 4 QUETIAPINE versus ZIPRASIDONE, Outcome 4 Mental state: 3. Negative symptoms - average endpoint score - medium term (PANSS negative subscore, high=poor).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 4 QUETIAPINE versus ZIPRASIDONE
Outcome: 4 Mental state: 3. Negative symptoms - average endpoint score - medium term (PANSS negative subscore, high = poor)
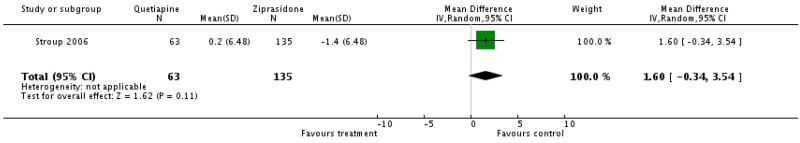
|
Analysis 4.5. Comparison 4 QUETIAPINE versus ZIPRASIDONE, Outcome 5 Service use: number of participants re-hospitalised.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 4 QUETIAPINE versus ZIPRASIDONE
Outcome: 5 Service use: number of participants re-hospitalised
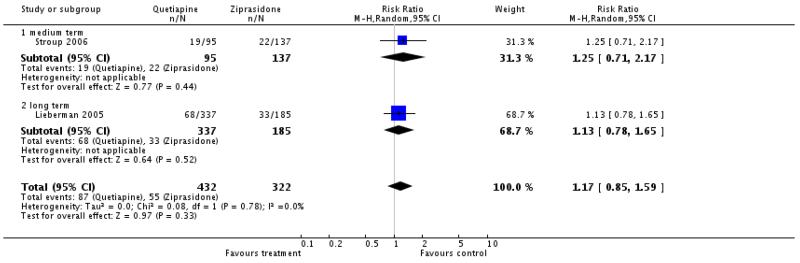
|
Analysis 4.6. Comparison 4 QUETIAPINE versus ZIPRASIDONE, Outcome 6 Adverse effects: 1. General - at least one adverse effect.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 4 QUETIAPINE versus ZIPRASIDONE
Outcome: 6 Adverse effects: 1. General - at least one adverse effect
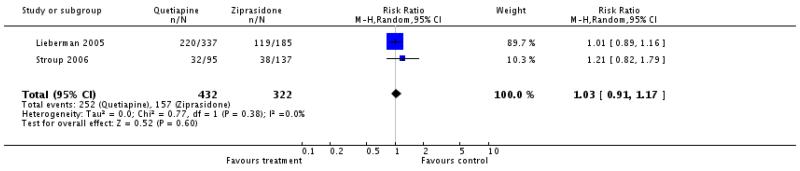
|
Analysis 4.7. Comparison 4 QUETIAPINE versus ZIPRASIDONE, Outcome 7 Adverse effects: 2. Death.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 4 QUETIAPINE versus ZIPRASIDONE
Outcome: 7 Adverse effects: 2. Death
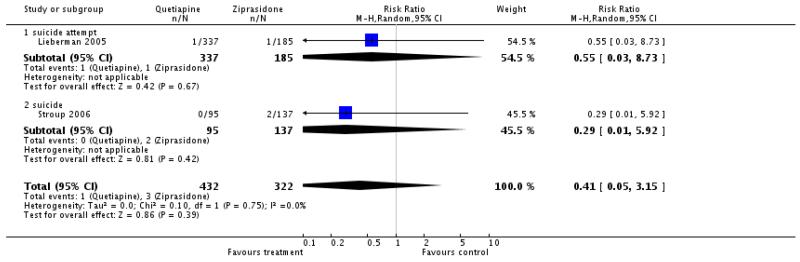
|
Analysis 4.8. Comparison 4 QUETIAPINE versus ZIPRASIDONE, Outcome 8 Adverse effects: 3a. Cardiac effects - QTc prolongation.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 4 QUETIAPINE versus ZIPRASIDONE
Outcome: 8 Adverse effects: 3a. Cardiac effects - QTc prolongation
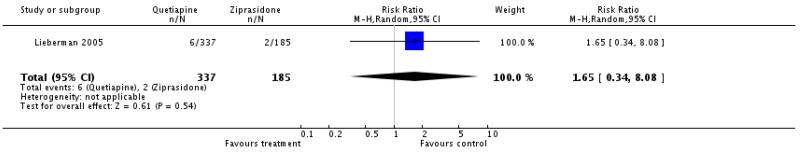
|
Analysis 4.9. Comparison 4 QUETIAPINE versus ZIPRASIDONE, Outcome 9 Adverse effects: 3b. Cardiac effects - QTc abnormalities - change from baseline in ms.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 4 QUETIAPINE versus ZIPRASIDONE
Outcome: 9 Adverse effects: 3b. Cardiac effects - Q.Tc abnormalities - change from baseline in ms
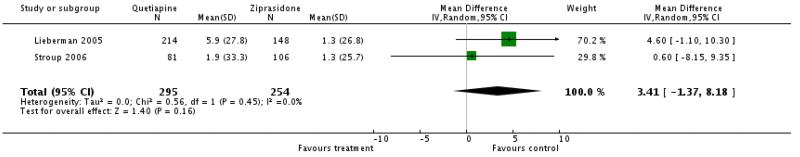
|
Analysis 4.10. Comparison 4 QUETIAPINE versus ZIPRASIDONE, Outcome 10 Adverse effects: 4. Central nervous system - sedation.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 4 QUETIAPINE versus ZIPRASIDONE
Outcome: 10 Adverse effects: 4. Central nervous system - sedation
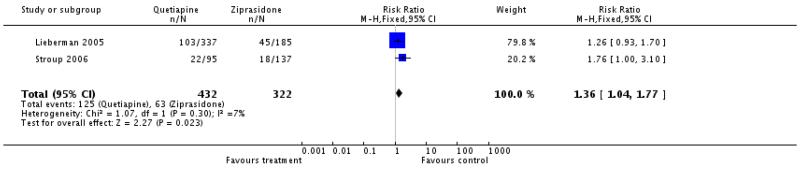
|
Analysis 4.11. Comparison 4 QUETIAPINE versus ZIPRASIDONE, Outcome 11 Adverse effects: 5. Extrapyramidal effects.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 4 QUETIAPINE versus ZIPRASIDONE
Outcome: 11 Adverse effects: 5. Extrapyramidal effects
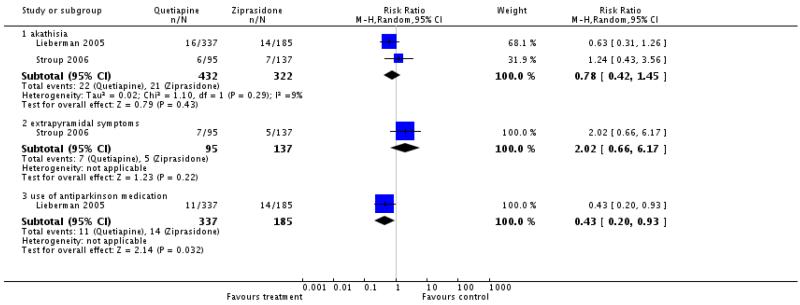
|
Analysis 4.12. Comparison 4 QUETIAPINE versus ZIPRASIDONE, Outcome 12 Adverse effects: 6a. Prolactin associated effects.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 4 QUETIAPINE versus ZIPRASIDONE
Outcome: 12 Adverse effects: Sa. Prolactin associated effects
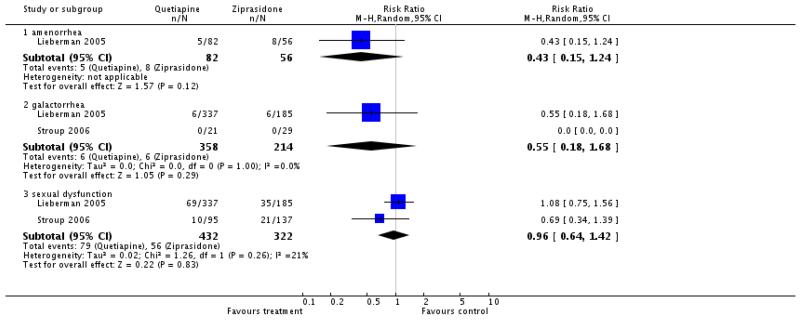
|
Analysis 4.13. Comparison 4 QUETIAPINE versus ZIPRASIDONE, Outcome 13 Adverse effects: 6b. Prolactin - change from baseline in ng/ml.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 4 QUETIAPINE versus ZIPRASIDONE
Outcome: 13 Adverse effects: 6b. Prolactin - change from baseline in ng/m I

|
Analysis 4.14. Comparison 4 QUETIAPINE versus ZIPRASIDONE, Outcome 14 Adverse effects: 7a. Metabolic - cholesterol - change from baseline in mg/dl.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 4 QUETIAPINE versus ZIPRASIDONE
Outcome: 14 Adverse effects: 7a. Metabolic - cholesterol - change from baseline in mg/dl
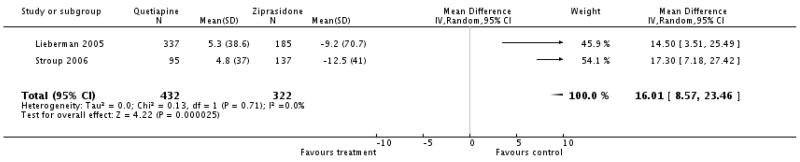
|
Analysis 4.15. Comparison 4 QUETIAPINE versus ZIPRASIDONE, Outcome 15 Adverse effects: 7b. Metabolic - glucose- change from baseline in mg/dl.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 4 QUETIAPINE versus ZIPRASIDONE
Outcome: 15 Adverse effects: 7b. Metabolic - glucose- change from baseline in mg/dl

|
Analysis 4.16. Comparison 4 QUETIAPINE versus ZIPRASIDONE, Outcome 16 Adverse effects: 7c. Metabolic - weight gain of 7% or more of total body weight.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 4 QUETIAPINE versus ZIPRASIDONE
Outcome: 1 6 Adverse effects: 7c. M etabolic - weight gain of 7% or m ore of total body weight
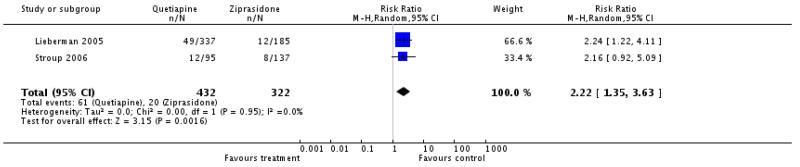
|
Analysis 4.17. Comparison 4 QUETIAPINE versus ZIPRASIDONE, Outcome 17 Adverse effects: 7d. Metabolic - weight gain - change from baseline in kg.
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 4 QUETIAPINE versus ZIPRASIDONE
Outcome: 17 Adverse effects: 7d. Metabolic - weight gain - change from baseline in kg
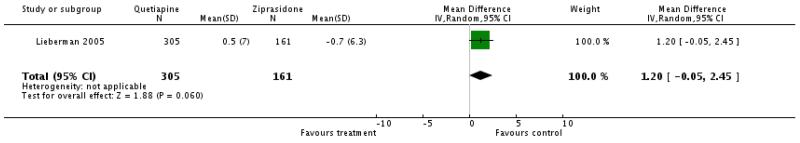
|
Analysis 5.1. Comparison 5 QUETIAPINE versus CLOZAPINE- sensitivity analysis (skewed data excluded), Outcome 1 Mental state: 1. General - average endpoint score -short term (PANSS total, high=poor).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 5 QUETIAPINE vers us CLOZAPINE- sensitivity analysis (skewed data excluded)
Outcome: 1 Mental state: 1. General - average endpoint score - short term (PANSS total, high = poor)

|
Analysis 6.1. Comparison 6 QUETIAPINE versus OLANZAPINE- sensitivity analysis (skewed data excluded), Outcome 1 Mental state: 1. Positive symptoms - average endpoint score (PANSS positive subscore, high=poor).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 6 QUETIAPINE versus OLANZAPINE- sensitivity analysis (skewed data excluded)
Outcome: 1 Mental state: 1. Positive symptoms - average endpoint score (PANSS positive subscore, high = poor)
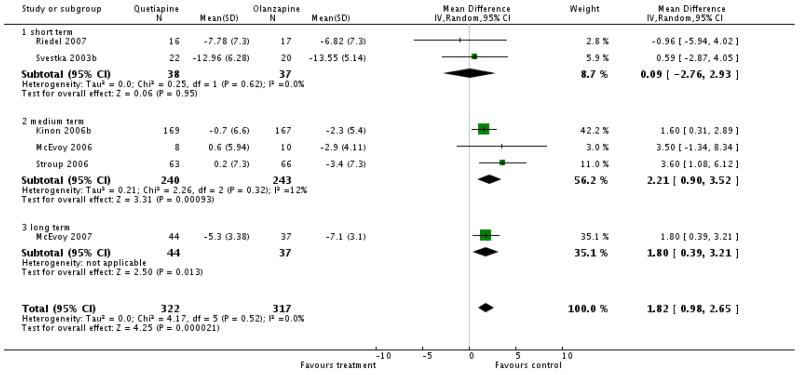
|
Analysis 7.1. Comparison 7 QUETIAPINE versus RISPERIDONE- sensitivity analysis (skewed data excluded), Outcome 1 Mental state: 6. Positive symptoms - average endpoint score - (PANSS positive subscore, high=poor).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 7 QUETIAPINE versus RISPERIDONE- sensitivity analysis (skewed data excluded)
Outcome: 1 Mental state: 6. Positive symptoms - average endpoint score - (PANSS positive subscore, high = poor)

|
Analysis 7.2. Comparison 7 QUETIAPINE versus RISPERIDONE- sensitivity analysis (skewed data excluded), Outcome 2 Adverse effects: 1. Extrapyramidal effects -Simpson-Angus Scale (high=poor).
Review: Quetiapine versus other atypical antipsychotics for schizophrenia
Comparison: 7 QUETIAPINE vers us RISPERIDONE- sensitivity analysis (skewed data excluded)
Outcome: 2 Adverse effects: 1. Extrapyramidal effects - Simpson-Angus Scale (high = poor)
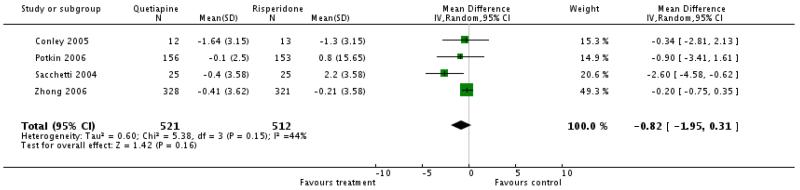
|
HISTORY
Protocol first published: Issue 3, 2007
Review first published: Issue 1, 2010
| Date | Event | Description |
|---|---|---|
| 15 October 2008 | Amended | Converted to new review format. |
DIFFERENCES BETWEEN PROTOCOL AND REVIEW
The review was adapted to new formatting and functions available in Review Manager 5, notably the inclusion of risk of bias tables.
Footnotes
DECLARATIONS OF INTEREST
Katja Komossa: none known.
Stefan Leucht: has received speaker/consultancy honoria from Sanofi-Aventis, BMS, Eli Lilly, Janssen, Lundbeck and Pfizer. He received research support from Sanofi-Aventis and Eli Lilly.
Christine Rummel: has received lecture honoraria and travel grants to attend scientific meetings from AstraZeneca, Janssen-Cilag, Eli Lilly and Pfizer.
Werner Kissling: has received speaker or consultancy honoraria from SanofiAventis, BMS, Lilly, Janssen, Lundbeck, Bayer and Pfizer
Heike Hunger: none known.
Franziska Schmid: none known.
Sandra Schwarz: none known.
Manit Srisurapanont: has received honoraria and support for attending national and international scientific meetings from AstraZeneca (Thailand), Eli Lilly Asia, Inc. (Thailand), GlaxoSmithKline (Thailand), Janssen-Cilag (Thailand), Servier (Thailand) and Solvay Pharmaceuticals (Thailand).
References to studies included in this review
- Atmaca 2003 {published data only} .*; Atmaca M, Kuloglu M, Tezcan E, Ustundag B. Serum leptin and triglyceride levels in patients on treatment with atypical antipsychotics. Journal of Clinical Psychiatry. 2003;64(5):598–604. doi: 10.4088/jcp.v64n0516. [DOI] [PubMed] [Google Scholar]
- Conley 2005 {published data only} .*; Conley RR, Kelly DL, Nelson MW, Richardson CM, Feldman S, Benham R, Steiner P, Yu Y, Khan I, McMullen R, Gale E, Mackowick M, Love RC. Risperidone, quetiapine, and fluphenazine in the treatment of patients with therapiy-refractory schizophrenia. Clinical Neuropharmacology. 2005;28(4):163–68. doi: 10.1097/01.wnf.0000172993.89879.0f. Pharm D, BCPP. [DOI] [PubMed] [Google Scholar]
- Kelly DL, Conley RR. A randomized double-blind 12-week study of quetiapine, risperidone or fluphenazine on sexual functioning in people with schizophrenia. Psychoneuroendocrinology. 2006;31(3):340–6. doi: 10.1016/j.psyneuen.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Kelly DL, Conley RR. Thyroid function in treatment-resistant schizophrenia patients treated with quetiapine, risperidone, or fluphenazine. Journal of Clinical Psychiatry. 2005;66:80–84. doi: 10.4088/jcp.v66n0111. [DOI] [PubMed] [Google Scholar]
- Richardson CM, Kelly DL, Gold JM, McMahon R, Yu Y, Conley RR. Risperidone vs quetiapine vs fluphenazine in treatment - resistant schizophrenia: neuropsychological outcome. Schizophrenia Bulletin. 2005;31:501–2. [Google Scholar]
- Kinon 2006b {published data only} .*; Kinon BJ, Lipkovich I, Edwards SB, Adams DH, Ascher-Svanum H, Siris SG. A 24-week randomized study of olanzapine versus ziprasidone in the treatment of schizophrenia or schizoaffective disorder in patients with prominent depressive symptoms. Journal of Clinical Psychopharmacology. 2006;26(2):157–62. doi: 10.1097/01.jcp.0000204137.82298.06. [DOI] [PubMed] [Google Scholar]
- Li 2005 {published data only} .*; Li Y, Feng YG. A double blind comparing study between the effects of quetiapine and clozapine on the life quality of the patients with schizophrenia. Medical Journal of Chinese Peoples Health. 2005;17:262–64. [Google Scholar]
- Li 2002 {published data only} .*; Li Y, Wang CH, Zhang DH, Wang LH, Zhao Z. A study of quetiapine and clozapine in treatment of first-episode schizophrenia. Chinese Journal of Nerv-and Mental Disease. 2002;28:219–20. [Google Scholar]
- Li 2003 {published data only} .*; Li CH. A study of quetiapine and clozapine in treatment of schizophrenia. Chinese Journal of Nerv-and Mental Disease. 2003;29:306–7. [Google Scholar]
- Lieberman 2005 {published data only} .*; Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RSE, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New England Journal of Medicine. 2005;353(12):1209–23. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- Liu 2004 {published data only} .*; Liu Y, Xu M, Chen X. A controlled study of quetiapine and clozapine in the treatment of schizophrenia with predominantly negative symptoms. Shandong Archires of Psychiatry. 2004;17(1):6–8. [Google Scholar]
- McEvoy 2006 {published data only} .*; McEvoy JP, Lieberman JA, Stroup TS, Davis SM, Meltzer HY, Rosenheck RA, Swartz MS, Perkins DO, Keefe RS, Davis CE, Severe J, Hsiao JK. Effectiveness of clozapine versus olanzapine, quetiapine, and risperidone in patients with chronic schizophrenia who did not respond to prior atypical antipsychotic treatment. American Journal of Psychiatry. 2006;163(4):600–10. doi: 10.1176/ajp.2006.163.4.600. [DOI] [PubMed] [Google Scholar]
- McEvoy 2007 {published data only} .Keefe RSE, Gu H, Sweeney JA, Perkins DO, McEvoy JP, Hamer RM, Lieberman JA. The effects of olanzapine, quetiapine and risperidone on neurocognitive function in first-episode psychosis: a double-blind 52-week comparison; Proceedings of the 159th Annual Meeting of the American Psychiatric Association; Toronto, Canada. 2006 May 20-25.2006. [Google Scholar]
- Lieberman J, McEvoy JP, Perkins D, Hamer RH. Comparison of atypicals in first-episode psychosis: a randomized, 52-week comparison of olanzapine, quetiapine, and risperidone. Journal of the European College of Neuropsychopharmacology. 2005;15(Suppl 3):S525. [Google Scholar]
- McEvoy JP. Comparison of clozapine versus other atypical drugs in prospectively defined, unresponsive patients; Proceedings of the 159th Annual Meeting of the American Psychiatric Association; Toronto, Canada. 2006 May 20-25.2006. [Google Scholar]
- *; McEvoy JP, Lieberman JA, Perkins DO, Hamer RM, Gu H, Lazarus A, Sweitzer D, Olexy C, Weiden P, Strakowski SD. Efficacy and tolerability of olanzapine, quetiapine, and risperidone in the treatment of early psychosis: A randomized, double-blind 52-week comparison. American Journal of Psychiatry. 2007;164:1050–1060. doi: 10.1176/ajp.2007.164.7.1050. [DOI] [PubMed] [Google Scholar]
- McEvoy JP, Perkins DO, Gu H, Hamer RM, Lieberman JA. Clinical effectiveness and predictors of treatment non-adherence: comparison of olanzapine, quetiapine, and risperidone in first-episode psychosis. Schizophrenia Research. 2006;86(Suppl 1):S130. doi: 10.1016/j.schres.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Mori 2004 {published data only} .*; Mori K, Nagao M, Yamashita H, Morinobu S, Yamawaki S. Effect of switching to atypical antipsychotics on memory in patients with chronic schizophrenia. Progress in NeuroPsychopharmacology and Biological Psychiatry. 2004;28(4):659–65. doi: 10.1016/j.pnpbp.2004.01.019. [DOI] [PubMed] [Google Scholar]
- Ozguven 2004 {published data only} .*; Ozguven Hd, Oner O, Baskak B, Oner P, Atbasoglu Ec. The metabolic and clinical effects of olanzapine and quetiapine: preliminary findings from a randomized single-blind trial in patients with schizophrenia. Schizophrenia Research. 2004;67(1):190–1. [Google Scholar]
- Potkin 2006 {published data only} .Gharabawi GM, Greenspan A, Rupnow MFT, Kosik-Gonzalez C, Bossie CA, Zhu Y, Kalali AH, Awad AG. Reduction in psychotic symptoms as a predictor of patient satisfaction with antipsychotic medication in schizophrenia: data from a randomized double-blind trial. BMC Psychiatry. 2006;6(45):1–7. doi: 10.1186/1471-244X-6-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenspan A, Kosik-Gonzalez C, Moreau-Mallet V, Bossie CA, Rupnow MFT, Zhu Y, Gharabawi GM. Risperidone vs quetiapine in inpatients with schizophrenia: a double-blind placebo-controlled study. Journal of the European College of Neuropsychopharmacology. 2005;15(Suppl 3):S503. [Google Scholar]
- *; Potkin SG, Gharabawi GM, Greenspan AJ, Mahmoud R, Kosik-Gonzalez C, Rupnow MFT, Bossie CA, Davidson M, Burtea V, Zhu Y, Trivedi JK. A double-blind comparison of risperidone, quetiapine and placebo in patients with schizophrenia experiencing an acute exacerbation requiring hospitalization. Schizophrenia Research. 2006;85(1-3):254–65. doi: 10.1016/j.schres.2006.03.027. [DOI] [PubMed] [Google Scholar]
- Potkin SG, Greenspan A, Kosik-Gonzalez C, Bossie C, Rupnow M, Zhu Y, Gharabawi G. A placebo - controlled study of risperidone vs quetiapine for symptom response and readiness for discharge among agitated inpatients with schizophrenia. Schizophrenia Bulletin. 2005;31:501. [Google Scholar]
- Riedel 2005 {published data only} .Riedel M, Muller N, Strassnig M, Spellmann I, Engel RR, Musil R, Dehning S, Douhet A, Schwarz MJ, Moller HJ. Quetiapine has equivalent efficacy and superior tolerability to risperidone in the treatment of schizophrenia with predominantly negative symptoms. European Archives of Psychiatry and Clinical Neuroscience. 2005;255(6):432–7. doi: 10.1007/s00406-005-0622-6. [DOI] [PubMed] [Google Scholar]
- Riedel 2007 {published data only} .*; Riedel M, Müller N, Spellmann I, Engel RR, Musil R, Valdevit R, Dehning S, Douhet A, Cerovecki A, Strassnig M, Möller HJ. Efficacy of olanzapine versus quetiapine on cognitive dysfunctions with an acute episode of schizophrenia. European Archieves of Psychiatry and Clinical Neuroscience. 2007;748:360–70. doi: 10.1007/s00406-007-0748-9. [DOI] [PubMed] [Google Scholar]
- Riedel M, Muller N, Strassnig M, Spellmann I, Engel RR, Musil R, Dehning S, Douhet A, Schwarz MJ, Moller HJ. Quetiapine has equivalent efficacy and superior tolerability to risperidone in the treatment of schizophrenia with predominantly negative symptoms. European Archives of Psychiatry and Clinical Neuroscience. 2005;255(6):432–7. doi: 10.1007/s00406-005-0622-6. [DOI] [PubMed] [Google Scholar]
- Sacchetti 2004 {published data only} .Sacchetti E, Valsecchi P, Regini C, Galluzo a, Cacciani P, Agrimi E, Mencacci C. Comparison of quetiapine, olanzapine and risperidone in patients with schizophrenia: interim results of a randomised, rater-blinded study. Schizophrenia Research. 2004;67(1):150. [Google Scholar]
- Sacchetti E, Valsecchi P, Regini C, Galluzzo A, Cacciani P. Comparison of quetiapine, olanzapine and risperidone in a randomized study in patients with schizophrenia; Proceedings of the Thematic Conference of the World Psychiatric Association on “Treatments in Psychiatry: An Update”; Florence, Italy. 2004 Nov 10-13.2004. [Google Scholar]
- *; Sacchetti E, Valsecchi P, Regini C, Galluzzo A, Cacciani P, Agrimi E, Mencacci C. Comparison of quetiapine, olanzapine, and risperidone in schizophrenia. Journal of the European College of Neuropsychopharmacology. 2004;14(Suppl 3):S286. [Google Scholar]
- Sacchetti E, Valsecchi P, Regini C, Galluzzo A, Cacciani P, Agrimi E, Mencacci C. Comparison of quetiapine, olanzapine and risperidone in patients with schizophrenia: interim results of a randomised, rater-blinded study. Journal of the European College of Neuropsychopharmacology. 2003;13(4):S350. [Google Scholar]
- Sacchetti E, Valsecchi P, Regini C, Galluzzo A, Cacciani P, Agrimi E, Mencacci C. Comparison of quetiapine, olanzapine and risperidone in patients with schizophrenia: interim results of a randomised, rater-blinded study; Proceedings of the 16th European College of Neuropsychopharmacology Congress; Prague, Czech Republic. 2003 Sep 20-24.2003. [Google Scholar]
- Sirota 2006 {published data only} .*; Sirota P, Pannet I, Koren A, Tchernichovsky E. Quetiapine versus olanzapine for the treatment of negative symptoms in patients with schizophrenia. Human Psychopharmacology. 2006;21(4):227–34. doi: 10.1002/hup.763. [DOI] [PubMed] [Google Scholar]
- Sirota P, Tchernichowsky E, Panet I, Koren A. The effectiveness of quetiapine versus olanzapine in improving negative symptoms of patients with schizophrenia. Schizophrenia Research. 2004;67(1):170. [Google Scholar]
- Stroup 2006 {published data only} .*; Stroup TS, Lieberman JA, McEvoy JP, Swartz MS, Davis SM, Rosenheck RA, Perkins DO, Keefe RS, Davis CE, Severe J, Hsiao JK. Effectiveness of olanzapine, quetiapine, risperidone, and ziprasidone in patients with chronic schizophrenia following discontinuation of a previous atypical antipsychotic. American Journal of Psychiatry. 2006;163(4):611–22. doi: 10.1176/ajp.2006.163.4.611. [DOI] [PubMed] [Google Scholar]
- Svestka 2003b {published data only} .Svestka J, Synek O, Zourkova A. A double-blind comparison of olanzapine and quetiapine in treatment of acute exacerbations of schizophrenic or schizoaffective disorders. Journal of the European College of Neuropsychopharmacology. 2003;13(4):S291. [Google Scholar]
- Voruganti 2007 {published data only} .*; Voruganti LP, Awad AG, Parker G, Forrest C, Usmani Y, Fernando MLD, Senthilal S. Cognition, functioning and quality of life in schizophrenia treatment: Results of a one-year randomized controlled trial of olanzapine and quetiapine. Schizophrenia Research. 2007;96(1):146–55. doi: 10.1016/j.schres.2007.08.002. [DOI] [PubMed] [Google Scholar]
- Zhong 2006 {published data only} .Harvey PD, Patterson TL, Potter LS, Zhong K, Brecher M. Improvement in social competence with short-term atypical antipsychotic treatment: a randomized, double-blind comparison of quetiapine versus risperidone for social competence, social cognition, and neuropsychological functioning. American Journal of Psychiatry. 2006;163(11):1918–25. doi: 10.1176/ajp.2006.163.11.1918. [DOI] [PubMed] [Google Scholar]
- *; Zhong KX, Sweitzer DE, Hamer RM, Lieberman JA. Comparison of quetiapine and risperidone in the treatment of schizophrenia: a randomized, double-blind, flexible-dose, 8-week study. Journal of Clinical Psychiatry. 2006;67(7):1093–103. doi: 10.4088/jcp.v67n0712. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- An 2003 {published data only} .An BF, Liu XQ, Chen JX. A comparative study between quetiapine and clozapine in the treatment of schizophrenia and the relation with the plasman levels of IL-2, SIL-2R. Schuan Mental Health. 2003;16(3):152–4. [Google Scholar]
- Antonova 2005 {published data only} .Antonova E, Kumari V, Halari R, Zachariah E, Mehrotra R, Kumar A, Sharma T. Superior cognitive efficacy of atypical antipsychotics olanzapine, risperidone, and quetiapine, as a group, relative to low doses of conventional antipsychotics. Schizophrenia Bulletin. 2005;31:474. [Google Scholar]
- Ascher-Svanum 2006 {published data only} .Ascher-Svanum H, Zhu B, Faries D, Landbloom R, Swartz M, Swanson J. Time to discontinuation of atypical versus typical antipsychotics in the naturalistic treatment of schizophrenia. BMC Psychiatry. 2006;6(8):1–16. doi: 10.1186/1471-244X-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baloescu 2006 {published data only} .Baloescu A, Vasile D, Gheorghe MD, Grigorescu G. Side effects of atypical antipsychotics - prediction factor for compliance. Journal of the European College of Neuropsychopharmacology. 2006;16(Suppl 4):S403. [Google Scholar]
- Beuzen 2005 {published data only} .Beuzen J-N, Pans M, Modell S, Hagens P, McQuade R, Iwamoto T, Carson W. Naturalistic study of aripiprazole treatment; Proceedings of the XIII World Congress of Psychiatry; Cairo, Egypt. 2005 Sept 10-15th.2005. [Google Scholar]
- Byerly 1999 {published data only} .Byerly M, Weber M. Clozapine versus quetiapine for schizophrenia. Stanley Foundation Research Programs. 1999 [Google Scholar]
- Byerly 2006 {published data only} .Byerly MJ, Nakonezny P, Bugno R, Boles J. A randomized, double-blind pilot trial of switching to quetiapine vs. risperidone continuation in outpatients with risperidone-associated sexual dysfunction; Proceedings of the 159th Annual Meeting of the American Psychiatric Association; Toronto, Canada. 2006 May 20-25.2006. [Google Scholar]
- Canas 2006 {published data only} .Canas F, Perez V, Tafalla M. Quetiapine and risperidone in the treatment of schizophrenia: a short- and long-term, non-randomized study; Proceedings of the 159th Annual Meeting of the American Psychiatric Association; Toronto, Canada. 2006 May 20-25.2006. [Google Scholar]
- Cao 2005 {published data only} .Cao D, Xie S-P, Chen Q-B, Yuan Y-G, Fang Q. Characteristics of the sexual disturbance caused by chlorpromazine, risperidone, quetiapine and olanzapine and their associations with the changes of blood glucose and blood lipids in male patients with schizophrenia. Chinese Journal of Clinical Rehabilitation. 2005;9(36):63–8. [Google Scholar]
- Cao 2005a {published data only} .Cao D, Xie S-P, Chen Q-B, Yuan Y-G, Fang Q. Comparison of the effects of chlorpromazine, risperidone and quetiapine on hypothalamic-pituitary-gonadal axis and sexual function in male patients with schizophrenia. Chinese Journal of Clinical Rehabilitation. 2005;9(20):148–51. [Google Scholar]
- Chaudhry 2006 {published data only} .Chaudhry HR, Niaz S, Arshad N, Peracha F, Ayub A, Mufti KA. Comparison of risperidone, olanzapine and quetiapine in relation to body weight, serum blood glucose and prolactin levels. Journal of the European College of Neuropsychopharmacology. 2006;16(Suppl 4):S241. [Google Scholar]
- Dai 2004 {published data only} .Dai J-P, Zhao Z-H, Mai G-Y. Comparative study on the effect of olanzapine and seroquel of schizophrenia. Chinese Journal of Behavioral Medical Science. 2004;13(3):291–93. [Google Scholar]
- Dai 2005 {published data only} .Dai J-P, Zhao Z-H, Liu G-X. Comparison of efficacy and safety of aripiprazole and quetiapine in the treatment of schizophrenia. Chinese Journal of Behavioral Medical Sience. 2005;14(8):712–14. [Google Scholar]
- Ding 2004 {published data only} .Ding Y, An B, Li G, Li Y. A comparative study of risperidone and quetiapine in the treatment of schizophrenia and relationship between efficacy and the serum levels of il-2?sil-2r. Shandong Archives of Psychiatry. 2004;17(2):76–78. [Google Scholar]
- Dossenbach 2005 {published data only} .Dossenbach M, Arango-Davila C, Silva Ibarra H, Landa E, Aguilar J, Caro O, Leadbetter J, Assuncao S. Response and relapse in patients with schizophrenia treated with olanzapine, risperidone, quetiapine, or haloperidol: 12-month follow-up of theiIntercontinental schizophrenia outpatient health outcomes (IC-SOHO) study. Journal of Clinical Psychiatry. 2005;66:1021–30. doi: 10.4088/jcp.v66n0810. [DOI] [PubMed] [Google Scholar]
- Du 2003 {published data only} .Feng D-J, Zhou T, Xiong L-H, Thian D-F. A study of quetiapine and clozapine in treatment of chronic schizophrenia. Journal of Luzhou Medical College. 2003;26(6):511–12. [Google Scholar]
- Emsley 2005 {published data only} .Emsley R, Turner HJ, Schronen J, Botha K, Smit R, Oosthuizen PP. Effects of quetiapine and haloperidol on body mass index and glycaemic control: a long-term, randomized, controlled trial. International Journal of Neuropsychopharmacology. 2005;1:1–8. doi: 10.1017/S1461145705005067. [DOI] [PubMed] [Google Scholar]
- Fan 2005 {published data only} .Fan J-H, Li W-J. A study of quetiapine in treatment of negative symptoms dominant schizophrenia. Sichuai Psychiatry. 2005;18:178. [Google Scholar]
- Fleischhacker 2005 {published data only} .Fleischhacker WW, Keet IPM, Kahn RS. The European First Episode Schizophrenia Trial (EUFEST): rationale and design of the trial. Schizophrenia Research. 2005;78(2-3):147–56. doi: 10.1016/j.schres.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Fu 2005 {published data only} .Fu H, Yu H, Huo J. A comparative study of quetiapine vs clozapine in the treatment of schizophrenia. Journal of Clinical Psychosomatic Diseases. 2005;11(4):313–4. [Google Scholar]
- Gao 2003 {published data only} .Gao CZ, Gao Z. A study of quetiapine in the treatment of first-onset schizophrenia. Journal of Clinical Psychological Medicine. 2003;13(4):221–2. [Google Scholar]
- García 2006 {published data only} .García MC, Vidal M, Ramos R. Sexual side effects of antipsychoticcs and treatment adherence. Journal of the European College of Neuropsychopharmacology. 2006;16(Suppl 4):S378. [Google Scholar]
- Harrigan 2004 {published data only} .Harrigan EP, Miceli JJ, Anziano R, Watsky E, Reeves KR, Cutler NR, Sramek J, Shiovitz T, Middle M. A randomized evaluation of the effects of six antipsychotic agents on QTC, in the absence and presence of metabolic inhibition. Journal of Clinical Psychopharmacology. 2004;24(1):62–9. doi: 10.1097/01.jcp.0000104913.75206.62. [DOI] [PubMed] [Google Scholar]
- He 2003 {published data only} .He J, Chen YX. A controlled study on quetiapine and clozapine in the treatment of schizophrenic patients. Medical Journal of Chinese Civil Administration. 2003;15(6):335–6. [Google Scholar]
- Huang 2003 {published data only} .Huang S-P, Ma Z-W, Guo B-Y. Effectiveness of quetiatine vs clozapine in the treatment of schizophrenia. Journal of Clinical Psychosomatic Diseases. 2003;9(4):206–7. [Google Scholar]
- Huber 2004 {published data only} .Huber TJ, Borsutzky M, Schneider U, Emrich HM. Psychotic disorders and gonadal function: Evidence supporting the oestrogen hypothesis. Acta Psychiatrica Scandinavica. 2004;109(4):269–74. doi: 10.1046/j.1600-0447.2003.00251.x. [DOI] [PubMed] [Google Scholar]
- Karow 2002 {published data only} .Karow A, Naber D. Subjective well-being and quality of life under atypical antipsychotic treatment. Psychopharmacology. 2002;162:3–10. doi: 10.1007/s00213-002-1052-z. [DOI] [PubMed] [Google Scholar]
- Keks 2006 {published data only} .Keks NA, Tonso M, Tabone K, McHugh M, Thomas R, Tune P, Gelman M. Clinical experience with atypical antipsychotics in acute inpatient unit: Focus on quetiapine. International Journal of Psychiatry in Clinical Practice. 2006;10(2):1–4. doi: 10.1080/13651500600579068. [DOI] [PubMed] [Google Scholar]
- Kelemen 2006 {published data only} .Kelemen O, Nagy O, Máttyássy A, Kiss I, Janka Z, Kéri S. Do second-generation antipsychotics disrupt decision-making abilities in schizophrenia? Journal of the European College of Neuropsychopharmacology. 2006;16(Suppl 4):S430. [Google Scholar]
- Kim 2004 {published data only} .Kim JG, Cho DH, Choi HK, Kim HJ, Cho JH, Kang SH, Lee SJ, Lee JG, Kim HT. The comparison of risperidone, olanzapine and quetiapine in the treatment of chronic schizophrenia and schizoaffective disorder. Journal of the European College of Neuropsychopharmacology. 2004;14(Suppl 3):S245. [Google Scholar]
- Knegtering 2004 {published data only} .Knegtering R, Castelein S, Bous H, Van Der Linde J, Bruggeman R, Kluiter H, Van Den Bosch RJ. A randomized open-label study of the impact of quetiapine versus risperidone on sexual functioning. Journal of Clinical Psychopharmacology. 2004;24(1):56–61. doi: 10.1097/01.jcp.0000106220.36344.04. [DOI] [PubMed] [Google Scholar]
- Li 2001 {published data only} .Li HF, Gu NF, Xie B, Li M, Zhang L, Cheng Y, Wang MJ. Quetiapine in treatment of schizophrenia: a randomized, controlled, multicentre study. Chinese Journal of New Drugs and Clinical Remedies. 2001;20(4):260–3. [Google Scholar]
- Li 2002a {published data only} .Li D-G, Zheng M-X, Zheng J-J. A comparative study between quetiapine and clozapine in the treatment of schizophrenia. Jiuheyuan Hospital of Shishou. 2002;12(5):267–8. [Google Scholar]
- Li 2003a {published data only} .Li C-M. A comparison of cognitive function in the first-onset schizophrenia treated with quetiapine and clozapine. Medical Journal of Chinese People Health. 2003;15(12):718–721. [Google Scholar]
- Li 2003b {published data only} .Li X, Lao G, Cao L. Comparison of efficacy and safety of seroquel and resperidone in the treatment of schizophrenia. Chinese Journal of Behavioral Medical Science. 2003;12(1):45–6. [Google Scholar]
- Li 2005 {published data only} .Li G, Chen Q-G, Zhang Q-H. A comparison analysis on the efficacy of quetiapine and risperidone in cognitive function of schizophrenia. Chinese Journal of Behavioral Medical Science. 2005;14(11):1007–8. 36. [Google Scholar]
- Liu 2004a {published data only} .Liu Y, Xu M, Chen X. A controlled study of quetiapine and clozapine in the treatment of schizophrenia with predominantly negative symptoms. Shandong Archires of Psychiatry. 2004;17(1):6–8. [Google Scholar]
- Liu 2005 {published data only} .Liu C-X, Liu S-H, Tu Z-M, Liu B. A study of quetiapine with small doze of clozapine in treatment of resistant schizophrenia. Medical Journal of Chinese Peoplé s Health. 2005;17:748–49. [Google Scholar]
- Lu 2005 {published data only} .Lu S, Zhang S-A, Ren Y. Comparative study on the psychopathology and the quality of life of schizophrenia treated with risperidone, clozapine and quetiapine. Journal of Nursing Science. 2005;20(3):57–9. [Google Scholar]
- Luo 2005 {published data only} .Luo Y, Zhang S-W, Wang A-Q. The comparative study of quetiapine and clozapine in the treatment of female schizophrene. Medical Journal of Chinese People Health. 2005;17(6):269–70. [Google Scholar]
- Mintzer 2004 {published data only} .Mintzer JE, Mullen JA, Sweitzer DE. A comparison of extrapyramidal symptoms in older outpatients treated with quetiapine or risperidone. Current Medical Research and Opinion. 2004;20(9):1483–91. doi: 10.1185/030079904X2312. [DOI] [PubMed] [Google Scholar]
- Mullen 2001 {published data only} .Mullen J, Jibson MD, Sweitzer D. A comparison of the relative safety, efficacy, and tolerability of quetiapine and risperidone in outpatients with schizophrenia and other psychotic disorders: the quetiapine experience with safety and tolerability (QUEST) study. Clinical Therapeutics. 2001;23(11):1839–54. doi: 10.1016/s0149-2918(00)89080-3. [DOI] [PubMed] [Google Scholar]
- Musil 2006 {published data only} .Musil RL, Spellmann I, Riedel M, Douhet A, Dehning S, Maino K, Zill P, Müller N, Möller HJ, Bondy B. SNAP-25 gene polymorphisms and weight gain in schizophrenic patients treated with atypical antipsychotics. Journal of the European College of Neuropsychopharmacology. 2006;16(Suppl 4):S415. [Google Scholar]
- Pan 2004 {published data only} .Pan M, Wang H, Zhang S. A controlled study of domestic quetiapine and risperidone in the treatment of first-episode schizophrenia. Journal of Clinical Psychosomatic Diseases. 2004;10(4):244–46. [Google Scholar]
- Pan 2004a {published data only} .Pan M, Zhang S-Q, Zhao Z. A comparative study of domestic quetiapine and risperidone in the treatment of schizophrenia. Medical Journal of Chinese Peoples Health. 2004;16(10):597–598. 637. [Google Scholar]
- Pan 2004b {published data only} .Pan M, Zhang S, Zhao Z. Influence of three antipsychotic drugs on electrocardiogram in patients with schizophrenia. Journal of XinXiang Medical College. 2004;21:403–404. [Google Scholar]
- Pang 2002 {published data only} .Pang D, Wang C, Cui A. Effect of quetiapine fumarate and clozapine on clinical rehabilitation of schizophrenia:a controlled study. Chinese Journal of Clinical Rehabilitation. 2002;6(7):1007. [Google Scholar]
- Peng 2004 {published data only} .Peng X-X, Huang X. Control study of quetiapine on schizophrenia mainly with negative symptoms. Modern Journal of Integrated Traditional Chinese and Western Medicine. 2004;13(12):1557–58. [Google Scholar]
- Qi 2004 {published data only} .Qi F, Wang L, Zhen Y. Comparison of efficacy and safety of quetiapine and risperidone in the treatment of schizophrenia. Shanghai Archives of Psychiatry. 2004;16(2):78–79. 119. [Google Scholar]
- Qian 2004 {published data only} .Qian D, Pan B, Yang G. Cost-effectiveness analysis of 3 kinds of therapeutic schemes for schizophrenia. Evaluation and Analysis of Drug-use in Hospital of China. 2004;4(2):110–111. [Google Scholar]
- Reznik 2004 {published data only} .Reznik I, Slavkin L, Shabash E, Shaked G, Kertzman S, Spivak B, Weizman A, Kotler M. Quetiapine (“Seroquel”) and olanzapine for acute treatment of patients with schizophrenia: an open-label, comparative study; Proceedings of the XXIV Collegium Internationale NeuroPsychopharmacologicum; Paris, France. 2004 June 20-24.2004. pp. 1–4. [Google Scholar]
- Ryu 2006 {published data only} .Ryu SH, Jang WS, Cho EY, Kim SK, Lee DS, Hong KS. Association of leptin gene polymorphism with antipsychotic drug-induced weight gain. Journal of the European College of Neuropsychopharmacology. 2006;16(Suppl 4):S419. [Google Scholar]
- Sajatovic 2002 {published data only} .Sajatovic M, Mullen JA, Sweitzer D. Efficacy of quetiapine and risperidone against depressive symptoms in outpatients with psychotic disorders; Proceedings of the 155th Annual Meeting of the American Psychiatric Association; Philadelphia, Pennsylvania, USA. 2002 May 18-23.2002. [Google Scholar]
- Swanson 2006 {published data only} .Swanson JW, Swartz MS, Van Dorn RA. Effectiveness of atypical antipsychotics for substance abuse in schizophrenia patients; Proceedings of the 159th Annual Meeting of the American Psychiatric Association; Toronto, Canada. 2006 May 20-25.2006. [Google Scholar]
- Tang 2003 {published data only} .Tang Y. A controlled study of schizophrenia treated with quetiapine and clozapine. Shanghai Archives of Psychiatry. 2003;15(1):27–9. [Google Scholar]
- Tang 2005 {published data only} .Tang Z-K, Xu C-M, Chen D. A comparative study of quetiapine and clozapine in the treatment of recurrent schizophrenia. Shandong Archives of Psychiatry. 2005;18(3):167–8. [Google Scholar]
- Wang 2000 {published data only} .Wang M, Liu M, Lu L. A controlled study on seroquel and risperidone in the treatment of schizophrenic patients. Journal of Clinical Psychological Medicine. 2000;10(4):200–1. [Google Scholar]
- Wang 2004 {published data only} .Wang X. Comparative study of effects of quetiapine and clozapine on quality of life of schizophrenics. Journal of Clinical Psychosomatic Diseases. 2004;10(3):167–68. [Google Scholar]
- Wang 2004a {published data only} .Wang M, Han F, Ma H. Comparation of quetiapine and clozapine in treatment of patients with first-onset schizophrenia. Chinese Journal of Clinical Pharmacology and Therapeutics. 2004;9(5):551–54. [Google Scholar]
- Wang 2005 {published data only} .Wang C-H, Li Y, Liu X. Cognitive function and p300 potentials in first-episode schizophrenia treated with quetiapine and risperidone. Chinese Mental Health Journal. 2005;19(5):333–6. [Google Scholar]
- Wang 2005a {published data only} .Wang C-H, Li Y, Wang L-H, Pan M, ZHao Z, Mu J-L. Comparison of cognitive function and event related potentials in first episode schizophrenic treated with antipsychotic drugs. Journal of Clinical Psychological Medicine. 2005;15(3):168–70. [Google Scholar]
- Wang 2005b {published data only} .Wang X, Yang J, Guo H. Clinical study of quetiapine and risperidone in the treatment of schizophrenia. Journal of Clinical Psychosomatic Diseases. 2005;11(2):118–9. [Google Scholar]
- Wang 2005c {published data only} .Wang X-F, Wang W-H, Wang J-C. Analysis of quetiapine in treatment of first-episode schizophrenia. Sichuai Psychiatry Medicine. 2005;18:235–36. [Google Scholar]
- Wang 2005d {published data only} .Wang Y, Sun M, Shao X. A comparative study between quetiapine and clozapine in the treatment of schizophrenia. Heath Psychology Journal. 2005;13(4):271–2. [Google Scholar]
- Weickert 2003 {published data only} .Weickert TW, Goldberg TE, Marenco S, Bigelow LB, Egan MF, Weinberger DR. Comparison of cognitive performances during a placebo period and an atypical antipsychotic treatment period in schizophrenia: critical examination of confounds. Neuropyschopharmacology. 2003;28(8):1491–500. doi: 10.1038/sj.npp.1300216. [DOI] [PubMed] [Google Scholar]
- Xiang 2005 {published data only} .Xiang D-F, Liu X-L. Treatment of 31 cases of schizophrenia with quetiapine. Herald of Medicine. 2005;24(1):40–42. [Google Scholar]
- Xu 2002 {published data only} .Xu M-Q, Peng M-Y. A study of Quetiapine in treatment of schizophrenia. Journal of Clinical Psychology Medicine. 2002;12:227–28. [Google Scholar]
- Xu 2003 {published data only} .Xu LZ, Ouyang JL, Gao SZ. Effects of domestic quetiapine vs clozapine in treatment of schizophrenia. Chinese Journal of New Drugs and Clinical Remedies. 2003;7477(9):542–5. [Google Scholar]
- Xu 2005 {published data only} .Xu X-F, Zhang X-B, Zhou C-Y, Dai X-G. Comparison of efficacy and safety of seroquel and risperidone in the treatment of schizophrenia. Journal of Jiangsu Clinical Medicine. 2005;9(1):20–22. [Google Scholar]
- Yamashita 2005 {published data only} .Yamashita H, Mori K, Nagao M, Okamoto Y, Morinobu S, Yamawaki S. Influence of aging on the improvement of subjective sleep quality by atypical antipsychotic drugs in patients with schizophrenia: comparison of middle-aged and older adults. American Journal of Geriatric Psychiatry. 2005;13(5):377–84. doi: 10.1176/appi.ajgp.13.5.377. [DOI] [PubMed] [Google Scholar]
- Yang 2004 {published data only} .Yang F-S, Yang Y-L, Zhang Z-H. Control study on quetiapine and clozapine in treatment of refractory schizophrenia. Medical Journal of Chinese People Health. 2004;16(1):12–13. [Google Scholar]
- Yang 2005 {published data only} .Yang Q-P, Huang Y-P, Yang B-X. The effect of quetiapine and risperidone on the EEG of first-episode schizophrenic patients. Shandong Archives of Psychiatry. 2005;18(3):159–60. [Google Scholar]
- Yu 2003 {published data only} .Yu G, Liang S, He Z-G. Comparison of Quetiapine and Clozapine in treatment of schizophrenia. Journal of Clinical Psychiatry Medicine. 2003;25:4–5. [Google Scholar]
- Yuan 2005 {published data only} .Yuan F-J, Gao M-Z, Liu X. A contrast observation of quetiapine and clozapine in affecting electroencephalogram. Journal of Practical Medical Techniques. 2005;12(2B):482–4. [Google Scholar]
- Zhang 2003 {published data only} .Zhang L, Wu Y, Guo C. A comparative study between quetiapine and risperidone in the treatment of schizophrenia. Health Psychology Journal. 2003;11(4):262–3. [Google Scholar]
- Zhang 2005 {published data only} .Zhang M, Zhang H-F, Gu W. A comparative study of quetiapine and clozapine in the treatment of schizophrenia. Shandong Archives of Psychiatry. 2005;18(2):86–8. [Google Scholar]
- Zhang 2005a {published data only} .Zhang H, Zhang M, Gu W. A comparative study of social function in schizophrenia patients treated with seroquel (quetiapine) or clozapine. Sichuan Mental Health. 2005;18(2):91–3. [Google Scholar]
- Zhang 2005b {published data only} .Zhang R-X, Cao Y-F. A comparative study of EKG changed by quetiapine and clozapine in the treatment of schizophrenia. Medical Journal of Chinese People Health. 2005;17(6):290–1. [Google Scholar]
- Zhang 2005c {published data only} .Zhang Y, Jiang Y, Zhao S. A comparative study of quetiapine combined with clomipramine in treatment of schizophrenia with obsessive-compulsive symptoms. Chinese Journal of Health Psychology. 2005;13(4):260–2. [Google Scholar]
- Zhao 2004 {published data only} .Zhao F, Zhang Z, Li H. Control studies of quetiapine and risperidone in the treatment of schizophrenia. Journal of Clinical Psychosomatic Diseases. 2004;10(1):25–26. [Google Scholar]
- Zhao 2005 {published data only} .Zhao L, Li Y, Qi F. Control study of quetiapine and risperidone in treatment of schizophrenics. Heath Psychology Journal. 2005;13(1):56, 79–80. [Google Scholar]
- Zhao 2005a {published data only} .Zhao Y-H, Fang J-H, Wang S-H, An C-G, Ma X, Li H-X. A study of quetiapine and clozapine in treatment of schizophrenia. Journal of Clinical Psychology Medicine. 2005;15:231–32. [Google Scholar]
- Zhong 2006a {published data only} .Zhong Z-Y, Tao J, Wang X-L, Wu X-L, Zhang J-B. Effect of antipsychotic plus buflomedil hydrochlorde in ameliorating the negative symptoms of patients with schizophrenia. Zhongguo Linchuang Kangfu. 2006;10(2):30–2. [Google Scholar]
- Zhou 2003 {published data only} .Zhou P, Gong F, Fan C. A control of treating chronic schizophrenia with seroquel or clozapine. Jiangxi Medical Journal. 2003;38(6):395–6. [Google Scholar]
- Zhou 2003a {published data only} .Zhou R, Dai X. A comparative study of quetiapine and clozapine in the treatment of patients with schizophrenia. Shanghai Archives of Psychiatry. 2003;15(4):215–7. [Google Scholar]
References to ongoing studies
- Eli Lilly 2004b {published data only} .Eli Lilly, Company Comparison of continuing olanzapine to switching to quetiapine in overweight or obese patients with schizophrenia and schizoaffective disorder. Eli Lilly and Company Clinical Trial Registry. 2004:8894. [Google Scholar]
- Gafoor 2005 {published data only} .Gafoor R, Power P, Craig T, Kerwin R, McGuire P. A comparative study of quetiapine and risperidone in patients with first-episode psychosis. Journal of the European College of Neuropsychopharmacology. 2005;15(Suppl 3):S509. [Google Scholar]
- Ratna 2003 {published data only} .Ratna L. A multi-centre, double-blind, randomised study to compare the safety tolerability and efficacy of quetiapine and risperidone in the treatment of schizophrenia in subjects with inadequate symptom control mproved Response In Schizophrenia - IRIS. National Research Register. 2003;1 [Google Scholar]
- Reynolds 2001 {published data only} .Reynolds C. A six month, rater blind comparison of quetiapine and risperidone in the treatment of tardive dyskinesia in patients with schizophrenia. National Research Register. 2001;3 [Google Scholar]
Additional references
- Altman 1996 .Altman DG, Bland JM. Detecing skewness from summary information. BMJ. 1996;313:1200. doi: 10.1136/bmj.313.7066.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreasen 1984 .Andreasen NC. Scale for the assessment of positive symptoms (SAPS) The University of Iowa; 1984. [Google Scholar]
- Andreasen 1989 .Andreasen NC. Scale for the assessment of negative symptoms (SANS) British Journal of Psychiatry. 1989;7:53–8. [PubMed] [Google Scholar]
- APA 2004 .American Psychiatric Association . American Journal of Psychiatry. 2nd Edition. American Journal of Psychiatry; 2004. Practice guidelines for the treatment of patients with schizophrenia; pp. 1–114. [Google Scholar]
- Arnt 1998 .Arnt J, Skarsfeldt T. Do novel antipsychotics have similar pharmacological characteristics? A review of the evidence. Neuropsychopharmacology. 1998;18:63–101. doi: 10.1016/S0893-133X(97)00112-7. [DOI] [PubMed] [Google Scholar]
- Barnes 1989 .Barnes TR. A rating scale for drug induced akathisia. British Journal of Psychiatry. 1989;154:672–6. doi: 10.1192/bjp.154.5.672. [DOI] [PubMed] [Google Scholar]
- Bland 1997 .Bland JM, Kerry SM. Statistics notes. Trials randomised in clusters. BMJ. 1997;315:600. doi: 10.1136/bmj.315.7108.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissel 1999 .Boissel JP, Cucherat M, Li W, Chatellier G, Gueyffier F, Buyse M, Boutitie F, Nony P, Haugh M, Mignot G. The problem of therapeutic efficacy indices. 3. Comparison of the indices and their use. Therapie. 1999;54(4):405–11. [PubMed] [Google Scholar]
- Carpenter 1984 .Heinrichs DW, Hanlon ET, Carpenter WT., Jr. The quality of life scale: an instrument for rating the schizophrenic deficit syndrom. Schizophrenia Bulletin. 1984;10:388–96. doi: 10.1093/schbul/10.3.388. [DOI] [PubMed] [Google Scholar]
- Carpenter 1994 .Carpenter WT, Jr, Buchanan RW. Schizophrenia. New England Journal of Medicine. 1994;330:681–90. doi: 10.1056/NEJM199403103301006. [DOI] [PubMed] [Google Scholar]
- Chouinard 1980 .Chouinard G, Ross-Chouinard A, Annable L, Jones BD. The extrapyramidal symptom rating scale. Canadian Journal of Neurological Sciences. 1980;7:233. [Google Scholar]
- Deeks 2000 .Deeks J. Issues in the selection for meta-analyses of binary data; Proceedings of the 8th International Cochrane Colloquium; Cape Town, South Africa. 2000 Oct 25-28th.2000. [Google Scholar]
- Divine 1992 .Divine GW, Brown JT, Frazer LM. The unit of analysis error in studies about physicians’ patient care behavior. Journal of General Internal Medicine. 1992;7:623–9. doi: 10.1007/BF02599201. [DOI] [PubMed] [Google Scholar]
- Donner 2002 .Donner A, Klar N. Issues in the meta-analysis of cluster randomized trials. Statistics in Medicine. 2002;21:2971–80. doi: 10.1002/sim.1301. [DOI] [PubMed] [Google Scholar]
- DSM IV 1994 .American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 4th Edition APA; Washington DC: 1994. [Google Scholar]
- Duggan 2005 .Duggan L, Fenton M, Rathbone J, Dardennes R, El-Dosoky A, Indran S. Olanzapine for schizophrenia. Cochrane Database of Systematic Reviews. 2005;(2) doi: 10.1002/14651858.CD001359.pub2. DOI: 10.1002/14651858.CD001359.pub2. [DOI] [PubMed] [Google Scholar]
- Egger 1997 .Egger M, Davey-Smith G, Schneider M, Minder CSO. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;13:629–34. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayeh 2006 .El-Sayeh HG, Morganti C. Aripiprazole for schizophrenia. Cochrane Database of Systematic Reviews. 2006;(2) doi: 10.1002/14651858.CD004578.pub3. DOI: DOI: 10.1002/14651858. CD004578.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbourne 2002 .Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving crossover trials: methodological issues. International Journal of Epidemiology. 2002;31(1):140–9. doi: 10.1093/ije/31.1.140. [DOI] [PubMed] [Google Scholar]
- Furukawa 2006 .Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta-analyses can provide accurate results. Journal of Clinical Epidemiology. 2006;59:7–10. doi: 10.1016/j.jclinepi.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Gaebel 2006 .Gaebel W, Falkai P, Weinmann S, Wobrock T. Treatment guidelines for schizophrenia [Behandlungsleitlinie Schizophrenie] Steinkopf. 2006 [Google Scholar]
- Goldstein 1993 .Goldstein JM, Litwin LC, Sutton EM, Malick JB. Seroquel: electrophysiological profile of a potential atypical antipsychotic. Psychopharmacology. 1993;112:293–8. doi: 10.1007/BF02244924. [DOI] [PubMed] [Google Scholar]
- Goldstein 1995 .Goldstein JM. Preclinical tests that predict clozapine-like atypical antipsychotic actions. Critical issues in the treatment of schizophrenia. International Academy of biochemical Drug Research. 1995;10:95–101. [Google Scholar]
- Gulliford 1999 .Gulliford MC, Ukoumunne OC, Chinn S. Components of variance and intraclass correlations for the design of community-based surveys and intervention studies: data from the Health Survey for England 1994. American Journal of Epidemiology. 1999;149:876–83. doi: 10.1093/oxfordjournals.aje.a009904. [DOI] [PubMed] [Google Scholar]
- Guy 1976 .Guy U. ECDEU assessment manual for psychopharmacology. National Institute of Mental Health; 1976. [Google Scholar]
- Heres 2006 .Heres S, Davis J, Maino K, Jetzinger E, Kissling W, Leucht S. Why olanzapine beats risperidone, risperidone beats quetiapine, and quetiapine beats olanzapine. American Journal of Psychiatry. 2006;163:185–94. doi: 10.1176/appi.ajp.163.2.185. [DOI] [PubMed] [Google Scholar]
- Higgins 2003 .Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins 2008 .Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions 5.0.1 [updated September 2008] The Cochrane Collaboration; 2008. Available from www.cochrane-handbook.org. [Google Scholar]
- Jones 2006 .Jones PB, Barnes TRE, Davies L, Dunn G, Lloyd H, Hayhurst KP, Murray RM, Markwick A, Lewis SW. Randomized controlled trial of the effect on quality of life. Archives of General Psychiatry. 2006;63:1079–6. doi: 10.1001/archpsyc.63.10.1079. [DOI] [PubMed] [Google Scholar]
- Kane 1988 .Kane JM, Honigfeld G, Singer J, Meltzer H. Clozaril Collaborative Study Group. Clozapine for the treatment of treatment-resistant schizophrenia: a double-blind comparison with chlorpromazine. Archives of General Psychiatry. 1988;45:789–96. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- Kane 1993 .Kane JM. Treatment programme and long term outcome in chronic schizophrenia. Acta Psychiatrica Scandinavica. 1993;46:585–93. doi: 10.1111/j.1600-0447.1990.tb05309.x. [DOI] [PubMed] [Google Scholar]
- Kay 1986 .Kay SR, Opler LA, Fiszbein A. Positive and negative syndrome scale (PANSS) manual. Multi-Health Systems; North Tonawanda (NY): 1986. [Google Scholar]
- Leucht 2005a .Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. What does the PANSS mean? Schizophrenia Research. 2005;79:231–8. doi: 10.1016/j.schres.2005.04.008. [DOI] [PubMed] [Google Scholar]
- Leucht 2005b .Leucht S, Kane JM, Kissling W, Hamann J, Etschel E, Engel R. Clinical implications of Brief Psychiatric Rating Scale Scores (BPRSS) British Journal of Psychiatry. 2005;187:366–71. doi: 10.1192/bjp.187.4.366. [DOI] [PubMed] [Google Scholar]
- Liebermann 2005 .Liebermann JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RSE, Davis SM. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. New England Journal of Medicine. 2005;353:1209–23. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- Marshall 2000 .Marshall M, Lockwood A, Adams C, Bradley C, Joy C, Fenton M. Unpublished rating scales - a major source of bias in randomised controlled trials of treatments for schizophrenia? British Journal of Psychiatry. 2000;176:249–52. doi: 10.1192/bjp.176.3.249. [DOI] [PubMed] [Google Scholar]
- Marvaha 2004 .Marvaha S, Johnson S. Schizophrenia and employment - a review. Social Psychiatry and Psychiatric Epidemiology. 2004;39:337–49. doi: 10.1007/s00127-004-0762-4. [DOI] [PubMed] [Google Scholar]
- Migler 1993 .Migler BM, Warawa EJ, Malick JB. Seroquel: behavioral profile of a potential atypical antipsychotic. Psychopharmacology. 1993;112:299–307. doi: 10.1007/BF02244925. [DOI] [PubMed] [Google Scholar]
- Moher 2001 .Moher D, Schulz KF, Altman D. The CONSORT statement: Revised recommendations for improving the quality of reports of parallel group randomized trials. JAMA. 2001;285:1987–1. doi: 10.1001/jama.285.15.1987. [DOI] [PubMed] [Google Scholar]
- Möller 2000 .Möller HJ. New assessment of atypical antipsychotics [Aktuelle Bewertung neuer/atypischer Neuroleptika] Nervenarzt. 2000;71:329–44. doi: 10.1007/s001150050567. [DOI] [PubMed] [Google Scholar]
- Overall 1962 .Overall JE, Gorham DR. The Brief Psychiatric Rating Scale. Psychological Reports. 1962;10:799–812. [Google Scholar]
- Rust 1989 .Rust J, Golombok S. Modern Psychometrics. Routledge; London: 1989. [Google Scholar]
- Saller 1993 .Saller CF, Salama AI. Seroquel: biochemical profile of a potential atypical antipsychotic. Psychopharmacology. 1993;112:285–92. doi: 10.1007/BF02244923. [DOI] [PubMed] [Google Scholar]
- Simpson 1970 .Simpson EN, Angus JWF. A rating scale for extrapyramidal side-effects. Acta Psychiatrica Scandinavica Supplementum. 1970;212:11–9. doi: 10.1111/j.1600-0447.1970.tb02066.x. [DOI] [PubMed] [Google Scholar]
- Srisurapanont 2004 .Srisurapanont M, Maneeton B, Maneeton N. Quetiapine for schizophrenia. Cochrane Database of Systematic Reviews. 2004;(2) doi: 10.1002/14651858.CD000967.pub2. DOI: 10.1002/14651858.CD000967.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tandon 2008 .Tandon R, Keshavan MS, Nasralllah HA. Schizophrenia, “Just the Facts” What we know in 2008. 2. Epidemiology and etiology. Schizophrenia research. 2008;102:1–18. doi: 10.1016/j.schres.2008.04.011. [DOI] [PubMed] [Google Scholar]
- Tsuang 1978 .Tsuang MT. Suicide in schizophrenics, manics, depressives, and surgical controls: a comparison with general population suicide mortality. Archives of General Psychiatry. 1978;35:153–55. doi: 10.1001/archpsyc.1978.01770260031002. [DOI] [PubMed] [Google Scholar]
- Ukoumunne 1999 .Ukoumunne OC, Gulliford MC, Chinn S, Sterne JAC, Burney PGJ. Methods for evaluating area-wide and organisation-based interventions in health and health care: a systematic review. Health Technology Assessment. 1999;3(5):3–92. [PubMed] [Google Scholar]
- WHO 2001 .World Health Organisation . The World Health Report 2001 - Mental health: New understanding, new hope. WHO; Geneva: 2001. [Google Scholar]
- Xia 2007 .Xia J, Adams CE, Bhagat N, Bhagat V, Bhoopathi P, El-Sayeh H. The Leeds Outcomes Stakeholders Survey (LOSS) Study; Proceedings of the 15th Cochrane Colloquium; Sao Paulo. 2007 Oct 23-27.2007. [Google Scholar]
References to other published versions of this review
- Leucht 2008 .Leucht S, Komossa K, Rummel-Kluge C, Corves C, Hunger H, Schmid F, Asenjo Lobos C, Schwarz S, Davis JM. A meta-analysis of head-to-head comparisons of second-generation antipsychotics in the treatment of schizophrenia. American Journal of Psychiatry. 2009;166:152–63. doi: 10.1176/appi.ajp.2008.08030368. DOI: 10.1176/appi.ajp.2008.08030368. [DOI] [PubMed] [Google Scholar]
- * Indicates the major publication for the study