Abstract
IMPORTANCE
Low-risk elective surgical procedures are common, but there are no clear guidelines for when preoperative consultations are required. Such consultations may therefore represent a substantial discretionary service.
OBJECTIVE
To assess temporal trends, explanatory factors, and geographic variation for preoperative consultation in Medicare beneficiaries undergoing cataract surgery, a common low-risk elective procedure.
DESIGN, SETTING, AND PARTICIPANTS
Cohort study using a 5% national random sample of Medicare part B claims data including a cohort of 556 637 patients 66 years or older who underwent cataract surgery from 1995 to 2006. Temporal trends in consultations were evaluated within this entire cohort, whereas explanatory factors and geographic variation were evaluated within the 89 817 individuals who underwent surgery from 2005 to 2006.
MAIN OUTCOMES AND MEASURES
Separately billed preoperative consultations (performed by family practitioners, general internists, pulmonologists, endocrinologists, cardiologists, nurse practitioners, or anesthesiologists) within 42 days before index surgery.
RESULTS
The frequency of preoperative consultations increased from 11.3% in 1998 to 18.4% in 2006. Among individuals who underwent surgery in 2005 to 2006, hierarchical logistic regression modeling found several factors to be associated with preoperative consultation, including increased age (75–84 years vs 66–74 years: adjusted odds ratio [AOR], 1.09 [95% CI, 1.04–1.13]), race (African American race vs other: AOR, 0.71 [95% CI, 0.65–0.78]), urban residence (urban residence vs isolated rural town: AOR, 1.64 [95% CI, 1.49–1.81]), facility type (outpatient hospital vs ambulatory surgical facility: AOR, 1.10 [95% CI, 1.05–1.15]), anesthesia provider (anesthesiologist vs non–medically directed nurse anesthetist: AOR, 1.16 [95% CI, 1.10–1.24), and geographic region (Northeast vs South: AOR, 3.09 [95% CI, 2.33–4.10]). The burden of comorbidity was associated with consultation, but the effect size was small (<10%). Variation in frequency of consultation across hospital referral regions was substantial (median [range], 12% [0–69%]), even after accounting for differences in patient-level, anesthesia provider–level, and facility-level characteristics.
CONCLUSIONS AND RELEVANCE
Between 1995 and 2006, the frequency of preoperative consultation for cataract surgery increased substantially. Referrals for consultation seem to be primarily driven by nonmedical factors, with substantial geographic variation.
Patients undergoing elective surgery with anesthesia have preoperative and preanesthesia evaluations performed by their operating surgeon and anesthesia provider, respectively. The routine provision of these services is included in Medicare’s global fees to surgeons and anesthesia providers. A subset of patients also receives a formal preoperative medical consultation that is distinct from the routine preoperative evaluations by surgeons and anesthesia providers. Preoperative medical consultation, which may be billed separately to Medicare, is a common health care service.1,2 However, for most surgical patients, there is no national guideline specifying criteria for referral for such consultations. Although it is recommended that patients with active cardiac conditions undergo further evaluation,3 it is not clear which considerations should trigger the referral of the much larger number of patients who are at low cardiac risk or scheduled to undergo low-risk surgical procedures. In fact, there is little information on how often preoperative consultation is performed among the vast number of patients who undergo elective low-risk procedures in the United States and how referral for consultation is influenced by characteristics at the level of the individual patient, the institution, and the geographic region.
To address this important gap in the literature, we undertook a retrospective cohort study to determine the proportion of US Medicare patients who undergo preoperative consultation before cataract surgery, beyond the routine preoperative evaluations by surgeons and anesthesiologists, and to identify factors that explain referral for such consultations. Cataract surgery is a particularly relevant scenario to study because it is associated with a low risk of perioperative medical complications and is the most common surgical procedure in the Medicare population, with more than 2 million beneficiaries undergoing cataract surgery annually.4,5 We also aimed to assess how the frequency of preoperative consultation for cataract surgery changed over time and whether there was substantial geographic variation in its frequency. We tested the hypotheses that referral for preoperative consultations (1) has increased during recent years, (2) is largely driven by nonmedical factors, and (3) shows substantial geographic variation.
Methods
Design, Population, and Data Sources
We conducted a retrospective cohort study of US residents 66 years or older using a 5% national random sample of Medicare part B files for the years 1994 to 2006 that were available to researchers at the University of Washington. These files include adjudicated claims for physician services to Medicare beneficiaries enrolled in traditional fee-for-service Medicare. Medicare denominator files provided information on patients’ demographic characteristics and date of enrollment in the Medicare program. All eligible cases of a first cataract surgical procedure were included. We identified patients undergoing a first cataract surgery by the occurrence of Current Procedural Terminology (CPT) codes 66982, 66983, or 66984 during the period 1995 through 2006. Records starting in 1994 were used to ascertain comorbidities. The entire cohort with dates of surgery in the period 1995 through 2006 was used to examine temporal trends in preoperative consultation, whereas we used only the 2 most recent years (2005 and 2006) for all other analyses. The University of Washington Human Subjects Division reviewed this study and determined that it did not involve research with human subjects. As a result of this determination, institutional review board approval and informed consent were not required.
Primary End Point: Preoperative Consultation
The primary end point was the occurrence of preoperative consultation, as identified by any codes for outpatient consultation (CPT codes 99242–99245) or inpatient consultation (CPT codes 99252–99255) within 42 days before the index surgery. Consultations are separately billed to Medicare using codes that reflect 5 levels of clinical complexity. The last digit of the CPT code reflects the respective level, and level 5 represents the highest level of complexity. We defined preoperative consultations as those that were provided by family physicians, general internists, pulmonologists, endocrinologists, cardiologists, nurse practitioners, or anesthesiologists (Centers for Medicare & Medicaid Services specialty codes 08, 11, 29, 46, 06, 50, and 05, respectively).
Predictors of Preoperative Consultation
We considered a priori several predictors of preoperative consultation, including patient demographic characteristics, co-morbidities, rural/urban residence status, surgical facility, anesthesia provider type, and geographic region.
Using diagnostic codes present within 365 days before surgery, we ascertained the presence of specific comorbidities, including ischemic heart disease, cerebrovascular disease, chronic renal insufficiency, heart failure, diabetes mellitus, peripheral vascular disease, dementia, chronic pulmonary disease, rheumatic disease, peptic ulcer disease, liver disease, paraplegia or hemiplegia, primary malignancy, and metastatic disease. We also calculated the Revised Cardiac Risk Index (RCRI),6 using the method described by Lindenauer et al,7 as well as the Quan et al8 modification of the Charlson Comorbidity Index. The RCRI is a predictive index for postoperative cardiac complications consisting of 6 equally weighted components, namely, ischemic heart disease, cerebrovascular disease, chronic renal insufficiency, heart failure, diabetes mellitus, and high-risk surgery.7
Place-of-surgery categories included ambulatory surgical centers, hospital outpatient departments, inpatient hospitals, and private offices, as indicated by Medicare’s place-of-service codes.
The type of anesthesia provider is indicated in Medicare claims by modifiers. We grouped these codes into 4 categories: (1) non–medically directed certified registered nurse anesthetists (CRNAs) (modifier QZ), (2) anesthesiologists personally providing anesthesia care (modifier AA), (3) both CRNAs and anesthesiologists involved with provision of anesthesia care in various medical direction or supervision ratios (all other modifier codes), and (4) a category of “unknown” (no modifier was reported).
Rural status was determined by linking the patient’s residential zip code to its Rural-Urban Commuting Area code9; these were aggregated into 4 categories: urban, large rural city, small rural town, and isolated rural town. The patient’s zip code was also used to assign patients to 1 of the 306 hospital referral regions (HRRs)10(pp19–35) and to 1 of the 4 major US regions as designated by the US Census Bureau.
Analysis
To examine time trends in preoperative consultations from 1995 to 2006 while accounting for any simultaneous temporal changes in patient-level characteristics, we fit a multivariable logistic regression model based on the entire 1995 to 2006 sample. The model adjusted for age, sex, race, comorbidities (Charlson comorbidity index), and rural/urban residence status. Calendar year was modeled as a categorical variable. Generalized estimating equations with robust sandwich variance estimates were used to account for correlation within HRRs. From the model, we estimated marginal predicted probabilities of preoperative consultation in the Medicare population by calendar year.
Using the subgroup of individuals who underwent surgery from 2005 to 2006, we conducted bivariate analyses to compare characteristics of patients who did or did not undergo preoperative consultation, calculating absolute standardized differences in addition to 2-sample Student t tests and χ2 tests. An absolute standardized difference greater than 10% is considered to represent meaningful imbalance.11 We also characterized the timing of consultations over the period preceding surgery, using frequency distributions by day, starting from 42 days prior to cataract surgery.
Hierarchical random intercept multivariable logistic regression models were used to determine the adjusted association of potential explanatory factors with preoperative consultation. In these models, HRR was treated as a random effect. Fixed-effect variables in the model included patient characteristics (age, sex, race, rural/urban residence status, Charlson comorbidity index), surgical setting, anesthesia provider type, and geographic region (Northeast, Midwest, South, West). Age was categorized into 3 groups (66–74, 75–84, or ≥85 years), whereas race was classified as African American vs other. In the primary analysis, we excluded the few observations with missing values for rural/urban residence status, surgical setting, and geographic region. Because the frequency of missing values for anesthesia provider type was not negligible (11%), a category for missingness (unknown) was created for this variable. We examined the influence of all variables with missing values (ie, rural/urban residence status, surgical setting, anesthesia provider, geographic region) using multiple imputation.
The hierarchical regression model allowed us to compare the relative importance of individual HRRs in predicting referral for preoperative consultation. We characterized variability between HRRs using the median odds ratio (OR).12 The OR is interpreted as the median value obtained when comparing the adjusted odds of undergoing consultation if 2 individuals with the same fixed effects had cataract surgery in 2 randomly chosen HRRs. Because it always involves comparisons of higher-ranked vs lower-ranked HRRs, the median OR always has a value of 1 or greater. It characterizes heterogeneity across HRRs, is adjusted for patient-level covariates, and may be directly compared against ORs of fixed-effect patient-level characteristics. For example, a value of 1.50 suggests 50% higher odds of receiving preoperative consultation if the same patient had surgery at one randomly selected HRR as opposed to another.
For all models, a 2-sided a level of .05 was required for statistical significance. The statistical software Stata, version 13 (StataCorp), was used for all analyses.
Results
Overall Study Population and Temporal Trends in Frequency of Consultation
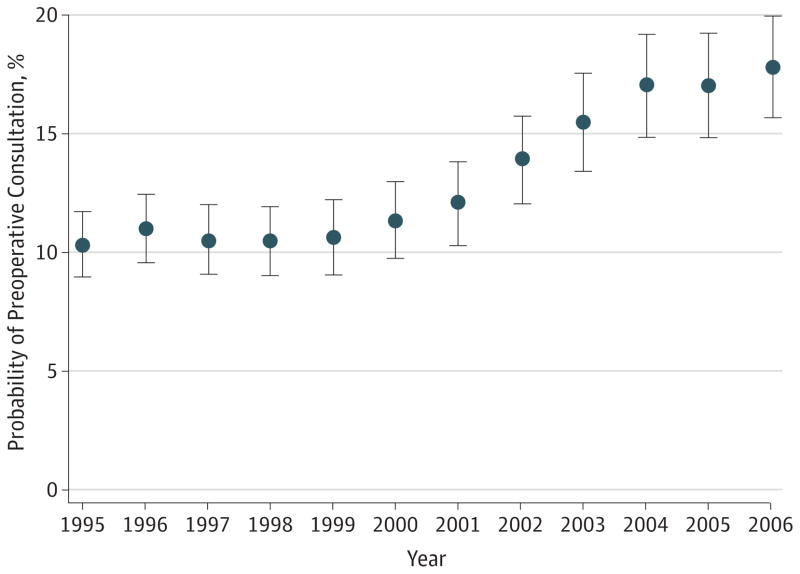
We identified 556 637 patients in our Medicare sample as having undergone a first cataract surgery between 1995 and 2006. The characteristics of the entire 1995 to 2006 cohort are presented in the online supplementary material (see eTable 1 in Supplement). The overall proportion of patients, in the entire cohort, that was provided a preoperative consultation was 14%. The frequency of preoperative consultation increased over the 12-year period from 1995 to 2006. The unadjusted probabilities of consultation in 1995, 1998, 2001, 2004, and 2006 were 11.0%, 11.3%, 12.8%, 17.8%, and 18.4%, respectively. The adjusted probabilities, with associated 95% CIs, of consultation for each year are presented in Figure 1.
Figure 1. Adjusted Probability of Preoperative Consultation by Calendar Year.
Circles represent the adjusted probability of preoperative consultation by calendar year, and vertical bars denote 95% CIs computed using robust variance estimation.
The overall increase in frequency of consultation was statistically significant (P < .001, test of linear trend) and was especially pronounced over the period 1999 to 2006, during which the unadjusted relative increase in probability of consultation was 61%.
Characteristics and Explanatory Variables of Preoperative Consultation
Of the entire cohort, 89 817 individuals underwent surgery in the 2-year period from 2005 to 2006. The remainder of analyses presented in the Results pertains to this subgroup from this 2-year period. Their mean age was 76 years, and 61% were female. Table 1 shows the characteristics of the 2005 to 2006 cohort and the bivariate associations of these characteristics with preoperative consultation. Eighteen percent of patients (n = 16 167) underwent preoperative consultation. Among the patients with consultations, 97% had only 1 consultation. With regard to sex, race, and comorbidities, patients who underwent consultation in the 42 days preceding the surgical procedure did not show a meaningful imbalance (>10%) compared with patients who did not. Patients who did or did not undergo preoperative consultation had meaningful covariate imbalance with respect to age, rural/urban status, type of surgical facility, type of anesthesia provider, and geographic region. Specifically, individuals undergoing consultation were less likely to be in the youngest age category and were more likely to undergo cataract surgery in an outpatient or inpatient hospital facility, have an anesthesiologist involved with their anesthesia care, reside in an urban area, and reside in the Northeast census region (Table 1).
Table 1.
Characteristics of the Subgroup of Patients Who Underwent Surgery in 2005 and 2006
| Characteristic | No. (%) | Absolute Standardized Difference, % | P Value | |
|---|---|---|---|---|
| Consultation (n = 16 167) | No Consultation (n = 73 650) | |||
| Age | ||||
| Mean (SD), y | 76.7 (6.2) | 76.1 (6.2) | 10.6 | <.001 |
| No. (%) | ||||
| 66–74 y | 6169 (38.2) | 31 816 (43.2) | 10.3 | <.001 |
| 75–84 y | 8164 (50.5) | 34 513 (46.9) | 7.3 | |
| ≥85 y | 1834 (11.3) | 7321 (9.9) | 4.6 | |
| Female sex | 10 049 (62.2) | 45 032 (61.1) | 2.1 | .02 |
| African American race | 718 (4.4) | 4484 (6.1) | 7.4 | <.001 |
| Rural-urban status | ||||
| Isolated rural town | 725 (4.5) | 5332 (7.2) | 11.7 | <.001 |
| Small rural town | 691 (4.3) | 6436 (8.7) | 18.2 | |
| Large rural city | 1634 (10.1) | 10 401 (14.2) | 12.3 | |
| Urban | 12 821 (79.3) | 51 108 (69.4) | 22.8 | |
| Unknown | 296 (1.8) | 373 (0.5) | 12.3 | |
| Comorbid disease | ||||
| Ischemic heart disease | 4690 (29.0) | 20 175 (27.4) | 3.6 | <.001 |
| Cerebrovascular disease | 2056 (12.7) | 8637 (11.7) | 3.0 | <.001 |
| Chronic renal insufficiency | 744 (4.6) | 3160 (4.3) | 1.5 | .08 |
| Congestive heart failure | 1864 (11.5) | 7874 (10.7) | 2.7 | .002 |
| Diabetes mellitus | 4505 (27.9) | 19 600 (26.6) | 2.8 | .001 |
| Peripheral vascular disease | 2591 (16) | 10 241 (13.9) | 6.0 | <.001 |
| Dementia | 354 (2.2) | 1633 (2.2) | 0.2 | .82 |
| Chronic pulmonary disease | 3447 (21.3) | 15 093 (20.5) | 2.0 | .02 |
| Rheumatic disease | 823 (5.1) | 3073 (4.2) | 4.4 | <.001 |
| Peptic ulcer disease | 263 (1.6) | 1250 (1.7) | 0.6 | .52 |
| Liver disease | 503 (3.1) | 2075 (2.8) | 1.7 | .04 |
| Paraplegia or hemiplegia | 100 (0.6) | 528 (0.7) | 1.2 | .17 |
| Cancer | ||||
| Primary malignancy | 2363 (14.6) | 9680 (13.1) | 4.3 | <.001 |
| Metastatic disease | 225 (1.4) | 815 (1.1) | 2.6 | .002 |
| Charlson Comorbidity Indexa | ||||
| 0 | 5426 (33.6) | 26 728 (36.3) | 5.7 | <.001 |
| 1 | 3828 (23.7) | 17 621 (23.9) | 0.6 | |
| 2 | 2594 (16.0) | 11 667 (15.8) | 0.6 | |
| 3 | 1801 (11.1) | 7930 (10.8) | 1.2 | |
| 4 | 1010 (6.2) | 4035 (5.5) | 3.3 | |
| ≥5 | 1508 (9.3) | 5669 (7.7) | 5.8 | |
| Revised Cardiac Risk Index | ||||
| 0 | 7629 (47.2) | 36 168 (49.1) | 3.8 | <.001 |
| 1 | 4860 (30.1) | 22 056 (29.9) | 0.2 | |
| 2 | 2394 (14.8) | 10 353 (14.1) | 2.1 | |
| ≥3 | 1284 (7.9) | 5073 (6.9) | 4.0 | |
| Surgical facility | ||||
| Ambulatory surgical center | 8277 (51.2) | 40 077 (54.4) | 6.5 | <.001 |
| Office | 1044 (6.5) | 7205 (9.8) | 12.2 | |
| Inpatient hospital | 57 (0.4) | 100 (0.1) | 4.4 | |
| Outpatient hospital | 6764 (41.8) | 26 203 (35.6) | 12.9 | |
| Unknown | 25 (0.2) | 65 (0.1) | 1.9 | |
| Anesthesia provider | ||||
| Non–medically directed CRNA | 3192 (19.7) | 23 460 (31.9) | 27.9 | <.001 |
| Medically directed CRNA | 5365 (33.2) | 18 525 (25.2) | 17.7 | |
| Anesthesiologist | 6368 (39.4) | 22 690 (30.8) | 18.1 | |
| Unknown | 1242 (7.7) | 8975 (12.2) | 15.1 | |
| Geographic regionb | ||||
| South | 4292 (26.5) | 31 605 (42.9) | 34.9 | <.001 |
| West | 1605 (9.9) | 12 364 (16.8) | 20.3 | |
| Midwest | 4536 (28.1) | 18 796 (25.5) | 5.7 | |
| Northeast | 5395 (33.4) | 10 274 (13.9) | 46.9 | |
| Unknown | 339 (2.1) | 611 (0.8) | 10.6 | |
Abbreviation: CRNA, certified registered nurse anesthetist.
Charlson Comorbidity Index is calculated using the Quan et al8 modification of this index.
Northeast includes Connecticut, Maine, Massachusetts, New Hampshire, New Jersey, New York, Pennsylvania, Rhode Island, and Vermont; Midwest includes Illinois, Indiana, Iowa, Kansas, Michigan, Minnesota, Missouri, Nebraska, North Dakota, Ohio, South Dakota, and Wisconsin; South includes Alabama, Arkansas, Delaware, Florida, Georgia, Kentucky, Louisiana, Maryland, Mississippi, North Carolina, Oklahoma, South Carolina, Tennessee, Texas, Virginia, Washington DC, and West Virginia; West includes Alaska, Arizona, California, Colorado, Hawaii, Idaho, Montana, New Mexico, Nevada, Oregon, Utah, Washington, and Wyoming.
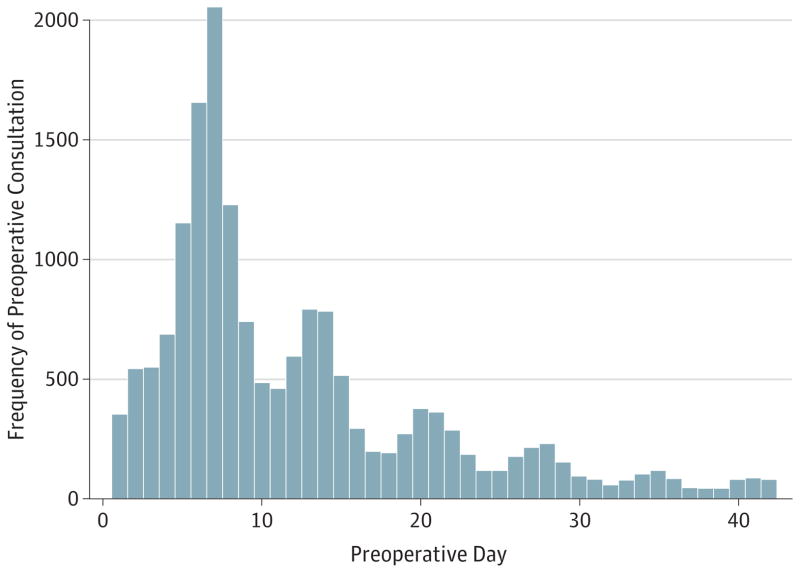
The proportion of consultations provided by family practitioners increased during the study period. In 2005 to 2006, internal medicine specialists provided the majority of consultations (53%), whereas family practitioners, cardiologists, nurse practitioners, and pulmonologists provided 32%, 9%, 2%, and 2%, respectively (see eFigure in Supplement). Endocrinologists and anesthesiologists each provided 1% of consultations. Level 3 (40%) and 4 (35%) preoperative consultations were the most common, whereas level 5 visits accounted for 11% and level 2 visits for 14% of all consultations. The distribution of the timing of preoperative consultation during the days preceding surgery showed peaks on weekly intervals (days 7, 14) (see Figure 2). The median (interquartile range) interval from consult to surgery was 8 (6–15) days (ie, 75% of consultations were provided within 15 days of surgery).
Figure 2. Distribution of Preoperative Consultations in the 42 Days Before Surgery.
Frequency distribution of consultations during the 42-day preoperative period for cataract surgery procedures in 2005 to 2006. A multimodal distribution is observed, with peaks at weekly intervals. The highest frequency occurred on preoperative day 7.
Table 2 shows unadjusted and adjusted associations between potential predictor variables and preoperative consultation in the 2005 to 2006 cohort. In adjusted analyses, patients aged 75 to 84 years were more likely to be seen in consultation than those aged 66 to 74 years. African American race and rural location of residence were associated with lower odds of a preoperative consultation. Only the highest category of the Charlson Comorbidity Index (≥5) was associated with higher adjusted odds for preoperative consultation. Patients who had the cataract surgery performed in an office setting had lower adjusted odds of having a preoperative consultation, and patients who had surgery in an inpatient or outpatient hospital had higher adjusted odds of having a consultation. Patients who had an anesthesiologist involved with their anesthesia care (either personally administering care or medically directing or supervising CRNAs) had higher adjusted odds of having a preoperative consultation. Geographic region was strongly associated with preoperative consultation, with patients in the Northeast having the greatest and those in the South and West the lowest adjusted odds of consultations (adjusted OR, 3.09 comparing Northeast with South).
Table 2.
Association of Consultation With Characteristics at the Level of the Patient, Provider, Facility, and Geographic Region
| Demographic Characteristic | Unadjusted Odds Ratio (95% CI) | P Value | Adjusted Odds Ratio (95% CI) | P Value |
|---|---|---|---|---|
| Age, y | ||||
| 66–74 | 1 [Reference] | <.001a | 1 [Reference] | <.001a |
| 75–84 | 1.22 (1.18–1.27) | 1.09 (1.04–1.13) | ||
| ≥85 | 1.29 (1.22–1.37) | 1.05 (0.98–1.12) | ||
| Female sex | 1.04 (1.01–1.08) | .02 | 1.02 (0.98–1.06) | .44 |
| African American race | 0.72 (0.66–0.78) | <.001 | 0.71 (0.65–0.78) | <.001 |
| Charlson Comorbidity Indexb | ||||
| 0 | 1 [Reference] | <.001a | 1 [Reference] | <.001a |
| 1 | 1.07 (1.02–1.12) | 1.01 (0.96–1.07) | ||
| 2 | 1.10 (1.04–1.15) | 1.00 (0.94–1.05) | ||
| 3 | 1.12 (1.05–1.19) | 1.01 (0.95–1.08) | ||
| 4 | 1.23 (1.14–1.33) | 1.08 (0.99–1.17) | ||
| ≥5 | 1.31 (1.23–1.40) | 1.09 (1.02–1.18) | ||
| Rural–urban status | ||||
| Isolated rural town | 1 [Reference] | <.001c | 1 [Reference] | <.001c |
| Small rural town | 0.79 (0.71–0.88) | 0.94 (0.83–1.06) | ||
| Large rural city | 1.16 (1.05–1.27) | 1.45 (1.30–1.61) | ||
| Urban | 1.84 (1.70–2.00) | 1.64 (1.49–1.81) | ||
| Surgical facility | ||||
| Ambulatory surgical center | 1 [Reference] | <.001c | 1 [Reference] | <.001c |
| Office | 0.70 (0.65–0.75) | 0.86 (0.79–0.93) | ||
| Inpatient hospital | 2.76 (1.99–3.82) | 1.22 (0.81–1.83) | ||
| Outpatient hospital | 1.25 (1.21–1.30) | 1.10 (1.05–1.15) | ||
| Anesthesia provider | ||||
| Non–medically directed CRNA | 1 [Reference] | <.001c | 1 [Reference] | <.001c |
| Medically directed CRNA | 2.13 (2.03–2.23) | 1.27 (1.19–1.35) | ||
| Anesthesiologist | 2.06 (1.97–2.16) | 1.16 (1.10–1.24) | ||
| Unknown | 1.02 (0.95–1.09) | 0.79 (0.73–0.86) | ||
| Geographic regiond | ||||
| South | 1 [Reference] | <.001c | 1 [Reference] | <.001c |
| West | 0.96 (0.90–1.02) | 0.97 (0.71–1.31) | ||
| Midwest | 1.78 (1.70–1.86) | 2.22 (1.71–2.89) | ||
| Northeast | 3.87 (3.69–4.05) | 3.09 (2.33–4.10) | ||
| Revised Cardiac Risk Indexe | ||||
| 0 | 1 [Reference] | <.001a | … | |
| 1 | 1.04 (1.00–1.09) | … | ||
| 2 | 1.10 (1.04–1.15) | … | ||
| ≥3 | 1.20 (1.12–1.28) | … | ||
Abbreviations: CRNA, certified registered nurse anesthetist.
Test of linear trend of ordered categories within variable.
Charlson Comorbidity Index is calculated using the Quan et al8 modification of this index.
Group test of all categories within variable.
Northeast includes Connecticut, Maine, Massachusetts, New Hampshire, New Jersey, New York, Pennsylvania, Rhode Island, and Vermont; Midwest includes Illinois, Indiana, Iowa, Kansas, Michigan, Minnesota, Missouri, Nebraska, North Dakota, Ohio, South Dakota, and Wisconsin; South includes Alabama, Arkansas, Delaware, Florida, Georgia, Kentucky, Louisiana, Maryland, Mississippi, North Carolina, Oklahoma, South Carolina, Tennessee, Texas, Virginia, Washington DC, and West Virginia; West includes Alaska, Arizona, California, Colorado, Hawaii, Idaho, Montana, New Mexico, Nevada, Oregon, Utah, Washington, and Wyoming.
Not incorporated into multivariable analysis.
Variation Across HRRs
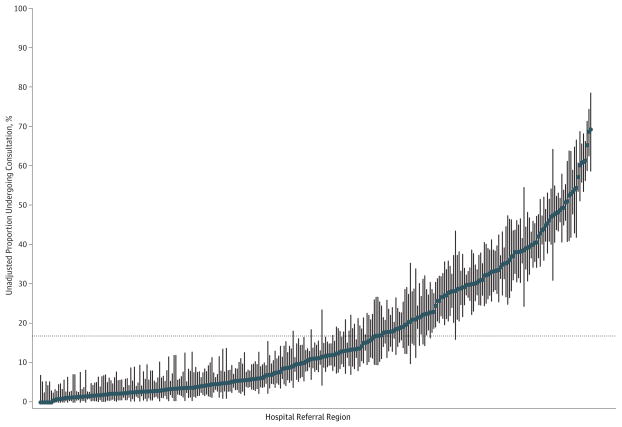
When the 306 HRRs were ranked with respect to frequency of preoperative consultation, there was considerable variation (Figure 3). The median (range) HRR-specific frequency of consultation was 12% (0–69%). The median OR across HRRs was 3.01, meaning that the median odds of receiving preoperative consultation was 3 times greater if patients with the same fixed-effect characteristics had surgery in 1 randomly selected HRR as opposed to another.
Figure 3. Variation in Frequency of Consultation Across Hospital Referral Regions.
Points represent unadjusted proportions of individuals undergoing consultation before cataract surgery across 306 hospital referral regions (HRRs) during 2005 to 2006. Vertical lines represent exact binomial 95% CIs. The dotted horizontal line denotes the overall proportion undergoing consultation (17.8%). The HRRs are ordered along the x-axis from lowest to highest frequency of consultations.
Discussion
In this cohort study of Medicare beneficiaries undergoing cataract surgery, we found a substantial increase in the frequency of referral for preoperative consultation from 1995 to 2006. Techniques for cataract surgery have progressed during the interval that we studied, with reduced surgical time, smaller wounds, and quicker recovery.4 These improvements have been associated with an increasing proportion of procedures performed under local and topical anesthesia.13,14 Despite these improvements in surgical techniques, referrals for preoperative consultation increased during this period, even after changes in patient characteristics were accounted for. It is not clear why referral for preoperative consultations has increased over time; there are no clear medical explanations such as increased co-morbidity, surgical complexity, or anesthetic risks. It is unclear why preoperative consultations increased more after 1999, and we believe that there are several possible explanations. First, Schein et al5 published a landmark study in the New England Journal of Medicine in 2000. This trial randomized almost 20 000 patients scheduled to undergo cataract surgery either to receive or not receive routine preoperative testing. It is possible that this report and accompanying editorial, which recommended more emphasis on preoperative physician assessments rather than routine testing, influenced the preoperative management of cataract surgery.15 Second, it is possible that the increase in referrals for preoperative consultations was not specific to cataract surgery. For example, a previous study reported an increase in overall physician referrals over a similar period.16 The trend is also consistent with the more rapid increase in inflation-adjusted overall Medicare spending after 1999.17 Third, Sharma et al18 reported that the proportion of Medicare patients experiencing comanagement was relatively unchanged from 1996 to 2000 and then increased sharply. They found that “the increase was entirely attributable to a surge in comanagement by generalist physicians.”18(p363) Although cataract surgery is performed on an outpatient basis, it is possible that generalists’ increased involvement with perioperative care extends to outpatient surgery. Finally, in 1997 Congress passed the Balanced Budget Act. This included reductions in reimbursements to some health care providers, and it is possible that they responded by maximizing income under existing incentives. Whereas a preoperative assessment is required by both the surgeon and anesthesia provider, and payment for this is included in the respective providers’ typical procedure reimbursement, they may have perceived the use of a separate pre-operative consultant to provide a full history and physical examination as a means to improve operating room efficiency.
We found substantial geographic variation in the use of pre-operative consultations. The median OR of 3.01 across HRRs may be interpreted as meaning that the odds of an individual patient having preoperative consultation is increased 3-fold if surgery was performed in 1 randomly selected HRR vs another. When compared with adjusted ORs associated with patient demographic characteristics, comorbidities, surgical facility type, and anesthesia provider type, the large value of this median OR suggests a strong geographic influence on whether patients are referred for consultation. The median OR of 3.01 is also consistent with a previous study19 on geographic variation in the use of preoperative consultations for major surgery in Ontario, Canada. In the absence of guidelines for the use of this intervention, this variation may represent uncertainty about when it is indicated. The geographic variation was also substantial when major US census regions were compared. The 3-fold higher odds of having a preoperative consultation in the Northeast relative to the South seems to be independent of differences in comorbidities or other patient characteristics, suggesting that provision of this service is discretionary and driven by regional factors. Notably, a previous study of a different periprocedural intervention, namely, sedation for endoscopy, also found that the Northeast region had the highest frequency of this service.20
Consultations do provide the opportunity to improve documentation of comorbid disease, perform risk stratification, optimize factors associated with preexisting medical conditions, and initiate interventions intended to decrease perioperative risk (such as use of β-blockers).19,21–24 Two previous studies have focused on preoperative medical evaluations for patients undergoing ophthalmic surgery.25,26 Both of these studies have emphasized the opportunity to improve patient care by identifying problems incidental to the surgical condition. These studies have not demonstrated improvements in specific perioperative or long-term outcomes, and the cost-effectiveness of such preoperative consultations has been questioned.27,28 Previous research has established that routine preoperative medical testing is not indicated for patients undergoing cataract surgery,5,29,30 and it has been argued that appropriate selective testing requires a preoperative physician assessment.15 It remains unclear when such physician assessment can be adequately provided by the operating surgeon or anesthesia provider (both of whom receive global fees that include reimbursement for a preoperative assessment), as opposed to a separate preoperative medical consultant. The weak association of traditional risk factors with consultation in the present study suggests 2 potential problems. Specifically, high-risk patients may not be receiving consultations, whereas low-risk patients who likely do not require consultations still receive them, thereby adding costs to the health care system.
Our results suggest several important areas for additional research. First, additional research is needed to better understand the cost and consequences of preoperative consultations in this population, as well as those undergoing other elective low-risk operations. Relative to no consultations, our data preliminarily suggest increased odds of performing electrocardiograms (unadjusted OR, 5.4 [95% CI, 5.2–5.6]) and blood draws (unadjusted OR, 2.3 [95% CI, 2.3–2.4]) in association with consultation. The inclusion of low surgical risk procedures is important because they are common, and it is likely that a high proportion of preoperative consultations are for patients undergoing low-risk and intermediate-risk procedures. Second, the specific types of patients who most need and benefit from consultation must be better defined, so that referral processes can be more standardized. Third, qualitative research is needed to better delineate the reasons for the substantial geographic variation in frequency of consultation. An understanding of such underlying reasons is a prerequisite to the development of any effective strategy to help reduce this variation.
Our study has several limitations. This study is based on administrative data, which may be affected by coding errors or misclassification of preoperative consultation. Nonetheless, the observation of peak visits on days 7 and 14 preoperatively, and low frequency of consultations occurring more than 28 days pre-operatively, suggests that the majority of these visits were associated with a planned surgery. Preoperative consultations may have resulted in delays in surgery beyond 42 days or cancellation of surgery, and we would not have captured these meaningful preoperative consultation visits. Therefore, misclassification could potentially bias toward under representing effective and important preoperative consultations. This bias is likely small because we included along preoperative window (42 days) to help allow time for delays. We investigated patterns of missingness and found that, overall, the frequency of missing data was low. With the use of multiple imputation for missing values, our adjusted estimates were substantially unchanged (eTable 2 in Supplement). In addition, Medicare data lack clinical details such as disease severity and laboratory test results. Some of the variation may be explained by unmeasured differences in disease severity, although this is unlikely to entirely explain the substantial variation that we observed. We used a random sample of Medicare files that we believe is representative of the entire Medicare population. It is unknown whether our findings can be extrapolated to other insurance coverage settings or outside the United States.
Conclusions
This large retrospective cohort study suggests that there was substantial use of preoperative medical consultation for cataract surgery and that referrals for consultation had increased during the study period. With the exception of age, referral for preoperative consultation seems driven primarily by nonmedical factors including practice setting, type of anesthesia provider, and geographical region. We observed substantial geographic variation, for which additional research is needed to better understand the underlying reasons. These data highlight an area of opportunity for interventions aimed at reducing unwanted practice variability in a process that has the potential to consume vast amounts of health care resources.
Supplementary Material
Acknowledgments
Funding/Support: This study was partially supported by funds from the Department of Anesthesiology and Pain Medicine, University of Washington, Seattle. Dr Thilen is supported by grant T32GM086270 from the National Institutes of Health, and by the Department of Anesthesiology and Pain Medicine, University of Washington, Seattle. Dr Wijeysundera is supported by a Clinician Scientist Award from the Canadian Institutes of Health Research and a Merit Award from the Department of Anesthesia at the University of Toronto, Toronto, Ontario, Canada. Drs Weaver and Lowy are partially supported by resources from the Veterans Affairs Puget Sound Health Care System, Seattle, Washington.
Role of the Sponsors: The funders had no role in the design and conduct of the study; collection, management, analysis, or interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Conflict of Interest Disclosures: None reported.
Previous Presentation: Preliminary data were presented at the annual meeting of the International Anesthesia Research Society; May 4, 2013; San Diego, California.
Additional Contributions: William Kreuter, MPA, Department of Health Services, School of Public Health, University of Washington, Seattle, assisted with computer programming and served as custodian of the original Medicare data files. He received financial compensation for his contributions to this study.
Author Contributions: Dr Thilen had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Thilen, Treggiari, Weaver, Wijeysundera.
Acquisition of data: Thilen.
Analysis and interpretation of data: All authors.
Drafting of the manuscript: Thilen, Lange, Wijeysundera.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Thilen, Lange, Lowy, Wijeysundera.
Obtained funding: Thilen.
Study supervision: Treggiari, Weaver, Wijeysundera.
References
- 1.Katz RI, Cimino L, Vitkun SA. Preoperative medical consultations: impact on perioperative management and surgical outcome. Can J Anaesth. 2005;52(7):697–702. doi: 10.1007/BF03016556. [DOI] [PubMed] [Google Scholar]
- 2.Thilen SR, Bryson CL, Reid RJ, Wijeysundera DN, Weaver EM, Treggiari MM. Patterns of preoperative consultation and surgical specialty in an integrated healthcare system. Anesthesiology. 2013;118(5):1028–1037. doi: 10.1097/ALN.0b013e31828ea68a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fleisher LA, Beckman JA, Brown KA, et al. 2009 ACCF/AHA focused update on perioperative beta blockade incorporated into the ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. J Am Coll Cardiol. 2009;54(22):e13–e118. doi: 10.1016/j.jacc.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 4.Schein OD, Cassard SD, Tielsch JM, Gower EW. Cataract surgery among Medicare beneficiaries. Ophthalmic Epidemiol. 2012;19(5):257–264. doi: 10.3109/09286586.2012.698692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schein OD, Katz J, Bass EB, et al. Study of Medical Testing for Cataract Surgery. The value of routine preoperative medical testing before cataract surgery. N Engl J Med. 2000;342(3):168–175. doi: 10.1056/NEJM200001203420304. [DOI] [PubMed] [Google Scholar]
- 6.Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation. 1999;100(10):1043–1049. doi: 10.1161/01.cir.100.10.1043. [DOI] [PubMed] [Google Scholar]
- 7.Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353(4):349–361. doi: 10.1056/NEJMoa041895. [DOI] [PubMed] [Google Scholar]
- 8.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 9.Washington, Wyoming, Alaska, Montana, Idaho (WWAMI) Rural Health Research Center. [Accessed December 19, 2012];Rural-Urban Commuting Area Codes (RUCAs) http://depts.washington.edu/uwruca/
- 10.Center for the Evaluative Clinical Sciences, Dartmouth Medical School. . The Dartmouth Atlas of Health Care. Chicago, IL: American Hospital Publishing; 1996. [PubMed] [Google Scholar]
- 11.Austin PC. Propensity-score matching in the cardiovascular surgery literature from 2004 to 2006: a systematic review and suggestions for improvement. J Thorac Cardiovasc Surg. 2007;134(5):1128–1135. doi: 10.1016/j.jtcvs.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 12.Larsen K, Merlo J. Appropriate assessment of neighborhood effects on individual health. Am J Epidemiol. 2005;161(1):81–88. doi: 10.1093/aje/kwi017. [DOI] [PubMed] [Google Scholar]
- 13.Leaming DV American Society of Cataract and Refractive Surgery. Practice styles and preferences of ASCRS members—2000 survey. J Cataract Refract Surg. 2001;27(6):948–955. doi: 10.1016/s0886-3350(01)00905-1. [DOI] [PubMed] [Google Scholar]
- 14.Leaming DV. Practice styles and preferences of ASCRS members—2003 survey. J Cataract Refract Surg. 2004;30(4):892–900. doi: 10.1016/j.jcrs.2004.02.064. [DOI] [PubMed] [Google Scholar]
- 15.Roizen MF. More preoperative assessment by physicians and less by laboratory tests. N Engl J Med. 2000;342(3):204–205. doi: 10.1056/NEJM200001203420311. [DOI] [PubMed] [Google Scholar]
- 16.Barnett ML, Song Z, Landon BE. Trends in physician referrals in the United States, 1999–2009. Arch Intern Med. 2012;172(2):163–170. doi: 10.1001/archinternmed.2011.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Himmelstein DU, Woolhandler S. Cost control in a parallel universe: Medicare spending in the United States and Canada. Arch Intern Med. 2012;172(22):1764–1766. doi: 10.1001/2013.jamainternmed.272. [DOI] [PubMed] [Google Scholar]
- 18.Sharma G, Kuo YF, Freeman J, Zhang DD, Goodwin JS. Comanagement of hospitalized surgical patients by medicine physicians in the United States. Arch Intern Med. 2010;170(4):363–368. doi: 10.1001/archinternmed.2009.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wijeysundera DN, Austin PC, Beattie WS, Hux JE, Laupacis A. Variation in the practice of preoperative medical consultation for major elective noncardiac surgery: a population-based study. Anesthesiology. 2012;116(1):25–34. doi: 10.1097/ALN.0b013e31823cfc03. [DOI] [PubMed] [Google Scholar]
- 20.Liu H, Waxman DA, Main R, Mattke S. Utilization of anesthesia services during outpatient endoscopies and colonoscopies and associated spending in 2003–2009. JAMA. 2012;307(11):1178–1184. doi: 10.1001/jama.2012.270. [DOI] [PubMed] [Google Scholar]
- 21.Wijeysundera DN, Austin PC, Beattie WS, Hux JE, Laupacis A. Outcomes and processes of care related to preoperative medical consultation. Arch Intern Med. 2010;170(15):1365–1374. doi: 10.1001/archinternmed.2010.204. [DOI] [PubMed] [Google Scholar]
- 22.Devereaux PJ, Ghali WA, Gibson NE, et al. Physicians’ recommendations for patients who undergo noncardiac surgery. Clin Invest Med. 2000;23(2):116–123. [PubMed] [Google Scholar]
- 23.Pausjenssen L, Ward HA, Card SE. An internist’s role in perioperative medicine: a survey of surgeons’ opinions. BMC Fam Pract. 2008;9:4. doi: 10.1186/1471-2296-9-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Katz RI, Barnhart JM, Ho G, Hersch D, Dayan SS, Keehn L. A survey on the intended purposes and perceived utility of preoperative cardiology consultations. Anesth Analg. 1998;87(4):830–836. doi: 10.1097/00000539-199810000-00016. [DOI] [PubMed] [Google Scholar]
- 25.Levinson W. Preoperative evaluations by an internist—are they worthwhile? West J Med. 1984;141(3):395–398. [PMC free article] [PubMed] [Google Scholar]
- 26.Phillips MB, Bendel RE, Crook JE, Diehl NN. Global health implications of preanesthesia medical examination for ophthalmic surgery. Anesthesiology. 2013;118(5):1038–1045. doi: 10.1097/ALN.0b013e31828ea5b2. [DOI] [PubMed] [Google Scholar]
- 27.Abrams J. Medical workups before eye operations. West J Med. 1984;141(3):373–374. [PMC free article] [PubMed] [Google Scholar]
- 28.Newman MF, Mathew JP, Aronson S. The evolution of anesthesiology and perioperative medicine. Anesthesiology. 2013;118(5):1005–1007. doi: 10.1097/ALN.0b013e31828ea5cb. [DOI] [PubMed] [Google Scholar]
- 29.Cavallini GM, Saccarola P, D’Amico R, Gasparin A, Campi L. Impact of preoperative testing on ophthalmologic and systemic outcomes in cataract surgery. Eur J Ophthalmol. 2004;14(5):369–374. [PubMed] [Google Scholar]
- 30.Keay L, Lindsley K, Tielsch J, Katz J, Schein O. Routine preoperative medical testing for cataract surgery. Cochrane Database Syst Rev. 2009;(2):CD007293. doi: 10.1002/14651858.CD007293.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.