Abstract
Orphanin FQ/nociceptin (OFQ/N) inhibits the activity of proopiomelanocortin (POMC) neurones located in the hypothalamic arcuate nucleus (ARH) that regulate female sexual behaviour and energy balance. We tested the hypothesis that gonadal steroids differentially modulate the ability of OFQ/N to inhibit these cells via presynaptic inhibition of transmitter release and postsynaptic activation of G protein-gated, inwardly-rectifying K+ (GIRK)-1 channels. Whole-cell patch clamp recordings were performed in hypothalamic slices prepared from ovariectomised rats. OFQ/N (1 μM) decreased the frequency of miniature excitatory postsynaptic currents (mEPSCs) and inhibitory postsynaptic currents (mIPSCs), and also caused a robust outward current in the presence of tetrodotoxin, in ARH neurones from vehicle- treated animals. A priming dose of oestradiol benzoate (EB; 2 μg) increased basal mEPSC frequency, markedly diminished both the OFQ/N-induced decrease in mEPSC frequency and the activation of GIRK-1 currents, and potentiated the OFQ/N-induced decrease in mIPSC frequency. Steroid treatment regimens that facilitate sexual receptivity reinstate the basal mEPSC frequency, the OFQ/N-induced decrease in mEPSC frequency and the activation of GIRK-1 currents to levels observed in vehicle-treated controls, and largely abolish the ability of OFQ/N to decrease mIPSC frequency. These effects were observed in an appreciable population of identified POMC neurones, nearly one-half of which projected to the medial preoptic nucleus. Taken together, these data reveal that gonadal steroids influence the pleiotropic actions of OFQ/N on ARH neurones, including POMC neurones, in a disparate manner. These temporal changes in OFQ/N responsiveness further implicate this neuropeptide system as a critical mediator of the gonadal steroid regulation of reproductive behaviour.
Keywords: estradiol, progesterone, POMC, orphanin FQ, lordosis
Introduction
The neuropeptide orphanin FQ (a.k.a., nociceptin (OFQ/N) is widely distributed in the brain (1-3). It activates its cognate opioid receptor like (ORL)-1 receptor, which is a metabotropic, Gi/o-coupled receptor that highly expressed in hypothalamic nuclei such as the medial preoptic nucleus (MPN), paraventricular nucleus, arcuate nucleus (ARH) and ventromedial nucleus (VMN; (3-6)). The OFQ/N-ORL-1 system has been shown to regulate reproduction and energy balance in the female (see (7,8) for review). Within the ARH, OFQ/N stimulates ORL-1 receptors expressed in POMC neurones that are important in both sexual behaviour and feeding to activate G protein-gated, inwardly rectifying K+ (GIRK)-1 channels (9-12). The efflux of K+ through these GIRK channels causes an outward current and associated membrane hyperpolarization that lowers the membrane potential below the threshold for activation of voltage-gated Na+ channels, thereby resulting in a cessation of neuronal firing (13,14). In addition, OFQ/N activates upstream ORL-1 receptors that are poised to presynaptically inhibit glutamatergic input impinging upon POMC neurones (10,12). This reduces the extent of stimulation of postsynaptic ionotropic glutamate receptors that would otherwise depolarise the cell membrane to increase the probability of activating voltage-gated Na+ channels and thus generating action potentials (15,16). On the other hand, OFQ/N can also inhibit GABA release from brain regions such as the cortex (17), amygdala (18) and the hypothalamic suprachiasmatic nucleus (SCN; (19)). Given that glutamatergic and GABAergic inputs converge on POMC neurones (20-22), and that activation of GABAA receptors culminates in the predominate form of fast inhibitory neurotransmission in the hypothalamus (21,23-26), it is likely that the OFQ/N-induced presynaptic inhibition of GABA release also plays a role in the ORL-1 receptor-mediated regulation of ARH neuronal activity.
A subpopulation of ARH POMC neurones project to the MPN and are important for steroid regulation of sexual receptivity (11,27). Oestradiol increases the expression and release of β-endorphin (a post- translational product of the POMC gene) in rodents and primates (28-30). In the female rat, a priming dose of oestradiol (2 μg) acts through a multisynaptic circuit to rapidly induce and maintain the release of β-endorphin that activates MPN μ-opioid receptors to inhibit sexual receptivity for 48 hr (30-32). Oestradiol accomplishes this by decoupling Gi/o-coupled metabotropic receptors from their ability to presynaptically decrease excitatory glutamate transmission to the POMC neuron, and to activate postsynaptic GIRK-1 currents in, and the associated hyperpolarization of, these cells (12,33). To facilitate lordosis the oestradiol-induced activation of MPN-projecting POMC neurones needs to be reversed (11,31,34). Indeed, subsequent progesterone treatment of oestrogen-primed female rats facilitates lordosis and deactivates MPN μ-opioid receptors, which indicates that excitation of POMC neurones has been reduced (34). Alternatively, if a single bolus of a high dose of oestradiol (5-50 μg) is given, then the steroid exerts biphasic actions. Initially, POMC neurones are stimulated for at least 24 hr. By 48 hr after treatment the increase in POMC neuronal excitability and MPN μ-opioid receptor activation have subsided, which then facilitates lordosis behaviour. These results indicate that the activity of POMC neurones that project to the MPN is regulated by steroids in a dose- and time-dependent manner. The OFQ/N-ORL-1 system facilitates lordosis through the deactivation of MPN μ-opioid receptors (11). It follows, therefore, that steroid treatment regimens that ultimately facilitate lordosis do so by enhancing pleiotropic ORL-1 receptor-mediated signalling mechanisms that inhibit the output of ARH POMC neurones.
The purpose of the present study was to test the hypothesis that gonadal steroids differentially modulate presynaptic and postsynaptic indices of ORL-1 receptor-mediated signalling in ARHneurones. To this end, the ability of OFQ/N to reduce excitatory and inhibitory input onto ARH neurones, as well as to activate GIRK-1 channels in ARH neurones, was evaluated in hypothalamic slices taken from OVX animals that were treated cyclically either with vehicle or a priming dose of EB, and subsequently with either with vehicle or progesterone on the morning of experimentation. In some experiments we monitored ORL-1 receptor-mediated responses in slices from animals treated with a high dose of EB 48 hr prior to experimentation that also produces sexual receptivity (11,31,34). We expect that steroid priming will diminish both the ORL-1 receptor-mediated presynaptic inhibition of excitatory neurotransmission and the activation of postsynaptic GIRK-1 channels, and enhance the ORL-1 receptor- mediated presynaptic inhibition of inhibitory neurotransmission, which would functionally translate into an increase in the excitability of POMC neurones. Further, we anticipate that steroid priming paradigms facilitating sexual receptivity will augment both the ORL-1 receptor-mediated signalling via presynaptic inhibition of excitatory neurotransmission and the activation of postsynaptic GIRK-1 channels, as well as dampen the ORL-1 receptor-mediated presynaptic inhibition of inhibitory neurotransmission, which would culminate in a reduction in POMC neuronal activity.
Materials and Methods
Animals
Adult Long-Evans female rats (200-225 g) were purchased from Charles River Laboratory Inc., (Wilmington, MA). Bilateral ovariectomies (OVX) were performed by the supplier. Animals were housed in a climate and light controlled room (12/12 L/D cycle, lights on 0700 h) with food and water available ad libitum. All procedures were approved by the Western University of Health Sciences IACUC in accordance with institutional guidelines based on NIH standards. Animals were treated once every four days for three cycles with either oestradiol benzoate (EB; 2 μg; s.c.) or its sesame oil vehicle (0.1 ml total volume; s.c.). Twenty-six hr after the final EB treatment, animals were given either progesterone (500 μg; s.c.) or sesame oil, and brains were collected 4 hr thereafter. In some experiments, animals were administered a 50 μg dose of EB 48 hr prior to experimentation. These treatment paradigms have long been known to elicit sexual receptivity in female rodents, due at least in part via modulation of an inhibitory ARH-MPN reproductive behaviour circuit (3,11,27,32,34-37).
Drugs
Unless otherwise indicated all drugs were purchased through Tocris Cookson, Inc. (Ellisville, MO, USA). EB and progesterone (Steraloids, Newport, R.I., USA) was initially prepared as 1 mg/ml stock solutions in punctilious ethanol. A known quantity of this stock solution was added to a volume of sesame oil sufficient to produce final concentrations of 20 μg/ml and 500 μg/ml EB, as well as 5 mg/ml progesterone. OFQ/N, the voltage-gated Na+ channel blocker tetrodotoxin (TTX) with citrate (Alomone Labs, Jerusalem, Israel), the GABAA receptor antagonist 6-imino-3-(4-methoxyphenyl)-1(6H)- pyridazinebutanoic acid hydrobromide (SR 95531) and the α-amino-3-hydroxyl-5-methyl-4-isoxazole- propionate (AMPA) receptor antagonist 2,3-dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7- sulfonamide (NBQX) were dissolved in UltraPure H20 to stock concentrations of 1 mM, 1 mM, 10 mM and 10 mM, respectively. The N-methyl-D-aspartate receptor antagonist cis-4-[Phosphomethyl]-2- piperidinecarboxylic acid (CGS 19755) was dissolved in 0.1 N NaOH and diluted to the appropriate volume with UltraPure H2O (final concentration 10 mM). Aliquots of these stock solutions were stored at −20°C.
Stereotaxic Surgery
Some animals were focally injected with the retrograde tracer Fluorogold (Fluorochrome, LLC, Denver, CO.) into the MPN 6-8 days prior to experimentation. They were fitted in a stereotaxic apparatus (Digital Lab Standard; Stoelting Co., Wood Dale, IL, USA) while under 3% isoflurane anaesthesia. The scalp was opened with a 2-2.5 cm incision made down the midline of the skull beginning at the front of the orbits towards the occipital lobe with a scalpel blade. The periosteum was rubbed from the scalp by sterile cotton swabs. A single hole was drilled so that an injection needle could be slowly lowered into the MPN (coordinates from bregma, anterior, −0.1 mm; lateral, −0.8 mm; and ventral, −6.0 mm from dura; tooth bar, −3.3 mm). The injection needle was held at these coordinates for one minute prior to the start of infusion. The retrograde tract tracer, Fluorogold (5% dissolved in sterile saline; 0.5 μL total volume), was slowly injected into the MPN using a Stoelting manual injector system. The injection needle remained in place for 10 minutes after infusion to allow for diffusion from the tip and then slowly removed from the brain to reduce potential spread of Fluorogold. Sterile bone wax was placed in the hole to seal the cavity and help promote clotting. After surgery, the rats were given oral antibiotics in drinking water (0.5 mg/ml of sulfamethoxazole and 0.1 mg/ml of trimethoprim; Hi-Tech Pharmacal, Amityvill, NY) as well as Carprofen (5 mg/kg; s.c.; Sigma Aldrich Corp., St. Louis, MO, USA) to help control post-operative pain.
Electrophysiology
On the day of experimentation, the OVX rat was anaesthetised with 32% isoflurane, decapitated, brain removed from the skull and the hypothalamus dissected. In experiments where Fluorogold was focally injected into the MPN, we routinely saved the rostral block containing the MPN and prepared sections at 20 μm thickness so that we could precisely determine the site of injection. Only those animals in which FG was administered directly into the MPN were included in the present study. The hypothalamic block was mounted on a cutting platform, secured in a vibratome well filled with an ice-cold, oxygenated (95% O2, 5% CO2) artificial cerebrospinal fluid (aCSF) in which the majority of sodium was replaced by sucrose (sucrose, 208; NaHCO3, 26; KCl, 2; NaH2PO4, 1.25; dextrose, 10; HEPES, 10; MgSO4, 2; MgCl2 1; CaCl2, 1; in mM). Three to four coronal slices (300 μM) through the rostro-caudal extent of the ARH, were cut at 1 °C. The slices were then transferred to an auxiliary chamber and incubated at room temperature in oxygenated aCSF containing (in mM): NaCl, 124; NaHCO3 26; dextrose, 10; HEPES, 10; KCl, 5; NaH2PO4, 2.6; MgSO4, 2; CaCl2, 1. They were kept under these conditions until electrophysiological recording.
During whole-cell patch recording from ARH neurones, slices were maintained in a chamber perfused with a warmed (35 °C), oxygenated aCSF in which the CaCl2 concentration was raised to 2 mM. Artificial CSF and all drugs (diluted with aCSF) were perfused via a peristaltic pump at a rate of 1.5 ml/min. Patch electrodes are assembled from borosilicate glass (World Precision Instruments; Sarasota, FL, USA; 1.5 mm O.D.) pulled on a P-97 Flaming Brown puller (Sutter Instrument Co., Novato, CA, USA), and filled with the following (in mM): potassium gluconate, 128; NaCl, 10; MgCl2, 1; EGTA, 11; HEPES, 10; ATP, 1; GTP, 0.25; 0.5% biocytin; adjusted to a pH of 7.3 with KOH. Electrode resistances vary from 3-8 MΩ. A Multiclamp 700A preamplifier (Axon Instruments, Foster City, CA, USA) amplified potentials and passed current through the electrode. Membrane currents were recorded in voltage clamp with access resistances that typically range from 8-22 MΩ, and underwent analog-digital conversion via a Digidata 1322A interface coupled to pClamp 8.2 software (Axon Instruments). The access resistance, as well as the resting membrane potential (RMP) and the input resistance (Rin), were monitored throughout the course of the recording. If the access resistance deviated greater than 10% of its original value, the recording was ended. Low-pass filtering of the currents was conducted at a frequency of two KHz. The liquid junction potential was calculated to be −10 mV, and corrected for during data analysis using pClamp software.
To evaluate if OFQ/N presynaptically inhibits excitatory and inhibitory neurotransmission at ARH synapses, we recorded miniature excitatory postsynaptic currents (mEPSCs) and inhibitory postsynaptic currents (mIPSCs) in the presence of either the GABAA receptor antagonist SR 95531 (10 μM) and TTX (500 nM), or the ionotropic glutamate receptor antagonists NBQX and CGS 19755 as well as TTX, from respective holding potentials of −75 mV or −30 mV using an internal solution in which Cs+ was substituted for K+. After collecting a 3-4 min segment of baseline data, we perfused OFQ/N (30 nM-1 μM) for four min, and then recorded mEPSCs or mIPSCs in the presence of the peptide. The threshold for event detection was set at least three pA below (for EPSCs) or above (for IPSCs) the baseline holding current as assessed from the headstage output, and continuously monitored throughout each 3-4 min recording period. This was done to ensure that the smaller amplitude events were not inadvertently omitted from the analysis. Synaptic events were detected and analyzed using Clampfit 8.2 (Axon Instruments) in combination with the SigmaPlot (IBM/SPSS, New York, NY, USA) and StatGraphics (StatPoint, Inc., Warrenton, VA, USA) programs. When we analyzed the data to determine mE/IPSC frequency and amplitude for the ≥100 contiguous synaptic events per condition, we poured over each 250-msec sweep in the entire range to ensure that each event that we included in the analysis bore the classic kinetic profile of a fast E/IPSC. We used this information to evaluate OFQ/N-induced alterations in mEPSC and mIPSC frequency and amplitude as assessed from cumulative probability plots.
To determine whether OFQ/N activates postsynaptic K+ currents that are highly prevalent in ARH neurones, recordings using the whole-cell configuration from holding potentials of −60 mV were performed. OFQ/N was perfused along with 1 μM TTX until a new steady-state holding current was established (3-5 min). Current-voltage relationships were generated before and immediately following peptide application over a range centering on the equilibrium potential for K+. This was accomplished with step command potentials ranging from −50 to −130 mV (1 sec duration; 10 mV increments).
Immunohistochemistry
Following electrophysiological recording, slices were fixed with 4% paraformaldehyde in Sorensen’s phosphate buffer (pH 7.4) for 90-180 min (38). They then were immersed overnight in 20% sucrose dissolved in Sorensen’s buffer, and frozen in Tissue-Tek embedding medium (Miles, Inc., Elkhart, IN, USA) the next day. Coronal sections (20 μm) were cut on a cryostat, and mounted on slides. These sections were washed with 0.1 M sodium phosphate buffer (pH 7.4), and then processed with streptavidin-Alexa Fluor (AF) 488 (Molecular Probes, Inc., Eugene, OR, USA) at a 1:300 dilution. After localizing the biocytin-filled neuron via fluorescence microscopy, the slides containing the appropriate sections were processed with a polyclonal antibodies directed against either β- endorphin (Immunostar, Inc., Hudson, WI, USA; 1:400 dilution), α-melanocyte-stimulating hormone (α-MSH, Immunostar; 1:200 dilution) or cocaine-amphetamine-regulated transcript (CART; Phoenix Pharmaceuticals, Inc., Burlingame, CA, USA; 1:2000 dilution) using fluorescence immunohistochemistry (38).
Statistical Analyses
Comparisons between groups were made with either the one-way, multifactorial or rank-transformed multifactorial analysis of variance (ANOVA) followed by the Least Significant Difference (LSD) test. Comparisons of the mEPSC and mIPSC interval and amplitude distributions observed under basal and OFQ/N-treated conditions were evaluated via the Kolmogorov-Smirnov test. Sample sizes for the treatment groups undergoing statistical analyses were defined as the number of cells/treatment group. Differences were considered statistically significant if the probability of error was <5%.
Results
We made recordings from a total of 141 ARH neurones in slices obtained from 54 animals treated with either EB (2 μg; n = 21) or its sesame oil vehicle 30 hr (0.1 mL; n = 25). EB-primed animals were subsequently treated with either progesterone (500 μg; n = 10) or sesame oil (n = 11) four hr prior to experimentation, whereas vehicle-treated animals were given sesame oil. Eight additional animals were treated with a high dose (50 μg) of EB. Neither EB nor EB/progesterone had any effect on either the RMP (vehicle: −53.5 ± 1.3 mV; EB: −52.6 ± 1.9 mV; EB/progesterone: −55.7 ± 1.6 mV) or Rin (vehicle: 527.3 ± 61.6 MΩ; EB: 498.1 ± 49.3 MΩ; EB/progesterone: 439.4 ± 43.5 MΩ; n = 26-53).
Experiment #1: Gonadal Steroid Modulation of Basal mEPSC and mIPSC frequency in ARH Neurones
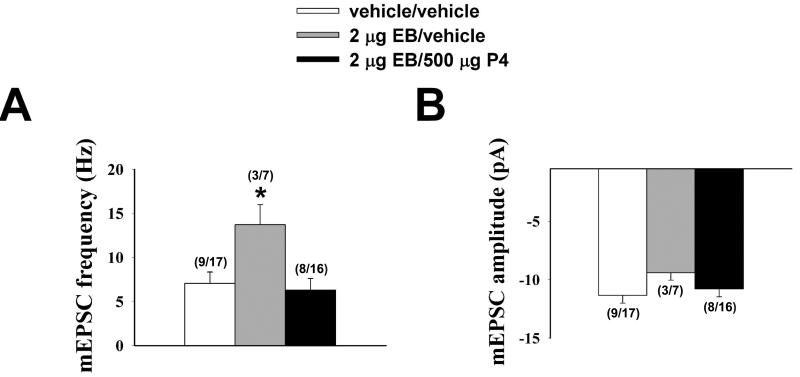
As shown in the bar graph in Figure 1A and the membrane current traces in Figure 2, EB nearly doubled the basal mEPSC frequency compared to vehicle-treated controls. Progesterone administered to EB- primed animals 4 hr prior to experimentation reversed the EB-induced increase in mEPSC frequency (one-way ANOVA/LSD, Fsteroid = 4.19, P < 0.03, df = 2). This effect was specific to mEPSC frequency, as both EB and EB/progesterone were without effect on mEPSC amplitude (Figure 1B; one-way ANOVA/LSD, Fsteroid = 1.04, P < 0.37, df = 2). On the other hand, neither EB nor EB/progesterone influenced mIPSC frequency (vehicle: 5.7 ± 1.0 Hz; EB: 4.3 ± 1.4 Hz; EB/progesterone: 3.8 ± 0.8 Hz; one-way ANOVA/LSD, Fsteroid = 0.82, P < 0.47, df = 2) or amplitude (vehicle: 11.9 ± 1.3 pA; EB: 14.5 ± 3.2 pA; EB/progesterone: 13.2 ± 2.2 pA; one-way ANOVA/LSD, Fsteroid = 0.46, P < 0.65, df = 2; n = 6-17).
Figure 1.
Baseline mEPSC frequency but not amplitude is increased in ARH neurones from OVX rats 30 hr after EB priming, which was negated four hr after subsequent in vivo progesterone (P4) treatment. Bars represent means and vertical lines 1 S.E.M. of the basal mEPSC frequency and amplitude measured from recordings in slices from vehicle- (open columns), EB- (grey columns) and EB/P4-treated (black columns). The numbers in parenthesis refer to the number of animals (on the left) and the number of cells (on the right). * = P < 0.05, one-way ANOVA/LSD.
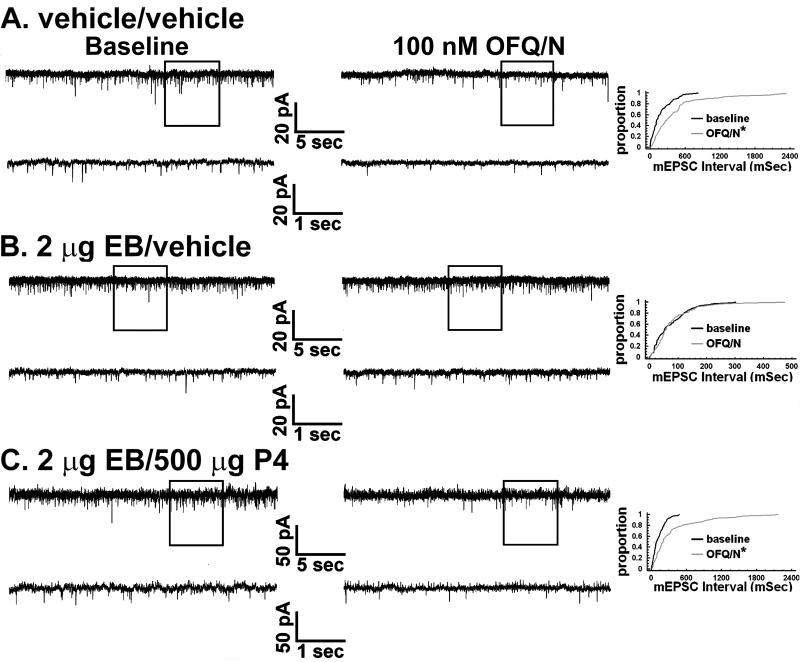
Figure 2.
Gonadal steroids modulate the OFQ/N-induced presynaptic inhibition of excitatory input onto ARH neurones in a disparate fashion. In the left-hand column of panels A-C are baseline traces of membrane current from identified POMC neurones in slices collected from OVX rats 30 hr after vehicle or EB priming, and four hr after a subsequent second vehicle or progesterone (P4) treatment. In the middle column are traces from the cells in A, B and C recorded in the presence of 100 nM OFQ/N. In the right column are the quantile plots derived from these same cells that represent the distributions of mEPSC interval measured under baseline conditions and in the presence of OFQ/N. * = P < 0.05, Kolmogorov-Smirnov.
Experiment #2: Gonadal Steroid Modulation of the OFQ/N-induced Presynaptic Inhibition of Excitatory and Inhibitory Input onto ARH Neurones
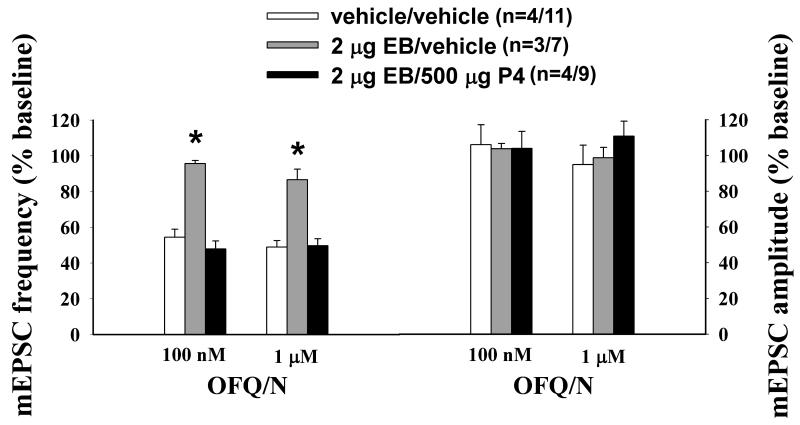
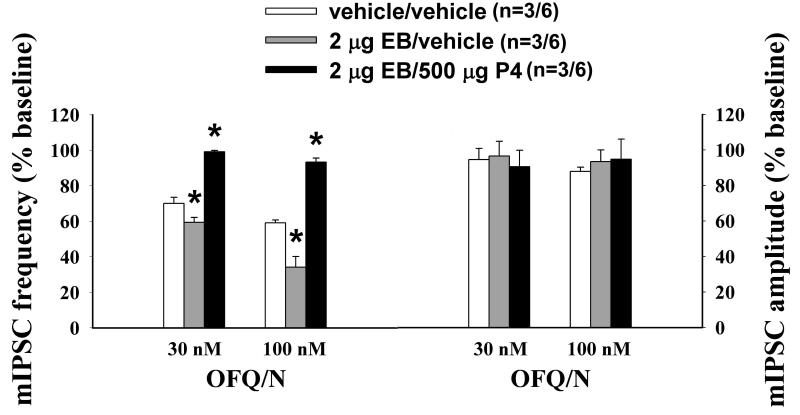
Next we endeavored to ascertain whether gonadal steroids influence OFQ/N-induced changes in synaptic input impinging upon ARH neurones. As shown in Figures 2 and 3, OFQ/N evoked a sizable decrease in mEPSC frequency in recordings of ARH neurones from vehicle-treated animals (Kolmogorov-Smirnov, K-S statistic = 2.17771, P < 0.0002). EB per se appreciably diminished his effect (Kolmogorov-Smirnov, K-S statistic = 1.01558, P < 0.26). However, progesterone administered 26 hr after EB treatment, and 4 hr prior to experimentation, restored the ability of OFQ/N to decrease mEPSC frequency (Kolmogorov- Smirnov, K-S statistic = 2.19203, P < 0.0002; Rank-transformed multifactorial ANOVA/LSD, Fsteroid = 20.15, P < 0.0001, df = 2). OFQ/N also dose-dependently decreased mIPSC frequency during recordings of ARH neurones in slices from vehicle-treated animals (Figures 4 and 5; Kolmogorov-Smirnov, K-S statistic = 1.27279, P < 0.05). Interestingly, EB alone significantly potentiated the OFQ/N-induced reduction in mIPSC frequency (Kolmogorov-Smirnov, K-S statistic = 2.40416, P < 0.0001), whereas in ARH neurones from EB-primed, progesterone-treated animals it was markedly attenuated (Kolmogorov- Smirnov, K-S statistic = 0.707107, P < 0.70; Rank-transformed multifactorial ANOVA/LSD, Fsteroid = 28.59, P < 0.0001, df = 2, Fdose OFQ/N = 13.44, p < 0.006, df = 1; n = 6-11). Gonadal steroids did not affect the inability of OFQ/N to alter mEPSC or mIPSC amplitude (Figure 3 (mEPSC): Rank-transformed multifactorial ANOVA/LSD, Fsteroid = 0.28, P < 0.77, df = 2, Fdose OFQ/N = 0.09, p < 0.77, df = 1; Figure 5 (mIPSC): Rank-transformed multifactorial ANOVA/LSD, Fsteroid = 0.10, P < 0.91, df = 2, Fdose OFQ/N = 0.13, p < 0.73, df = 1; n = 6-11)
Figure 3.
Composite bar graph that illustrates the differential gonadal steroid modulation of the OFQ/N- induced decrease in mEPSC frequency. Estradiol priming (EB/vehicle) blocks the OFQ/N-induced decrease in mEPSC frequency and amplitude observed in ARH neurones from OVX vehicle-treated (vehicle/vehicle) rats, which was reinstated by subsequent progesterone (EB/P4) treatment. Bars represent means and vertical lines 1 S.E.M. of the mEPSC frequency that was normalized to values observed under baseline conditions within their respective treatment groups. The numbers in parenthesis refer to the number of animals (on the left) and the number of cells (on the right). * = P < 0.05, rank- transformed multifactorial ANOVA/LSD.
Figure 4.
Gonadal steroids differentially modulate the OFQ/N-induced presynaptic inhibition of inhibitory input impinging upon ARH neurones. Thirty hours after estradiol treatment, the decrease in mIPSC frequency caused by OFQ/N is augmented, and this is blocked four hours after subsequent progesterone (P4) treatment. In the left-hand column are baseline traces of membrane current in identified POMC neurones from A) vehicle-treated, B) EB-treated and C) EB/P4-treated animals. In the middle column are traces from the cells in A, B and C recorded in the presence of 100 nM OFQ/N. In the right column are the quantile plots derived from these same cells that represent the distributions of mIPSC interval measured under baseline conditions and in the presence of OFQ/N. When comparing A and B it is worthy to note that in the one-second excerpts enclosed by the rectangle there were 31 synaptic events under baseline conditions in the example from the vehicle-treated animal, and OFQ/N reduced this number to 16 (51.6 percent of baseline). Conversely, in the expanded traces from the representative, oestrogen-primed example there were 19 synaptic events under baseline conditions, which were reduced to four in the presence of OFQ/N (21.1 percent of baseline). * = P < 0.05, Kolmogorov-Smirnov; **, P < 0.006, Kolmogorov-Smirnov.
Figure 5.
Composite bar graph depicting the gonadal steroid modulation of the OFQ/N-induced decrease in mIPSC frequency. Bars represent means and vertical lines 1 S.E.M. of the mIPSC frequency and amplitude that were normalized to values observed under baseline conditions within their designated treatment groups. The numbers in parenthesis refer to the number of animals (on the left) and the number of cells (on the right). * = P < 0.05, rank-transformed multifactorial ANOVA/LSD.
Experiment #3: Gonadal Steroid Modulation of the OFQ/N-induced Activation of Postsynaptic K+ Currents in ARH Neurones
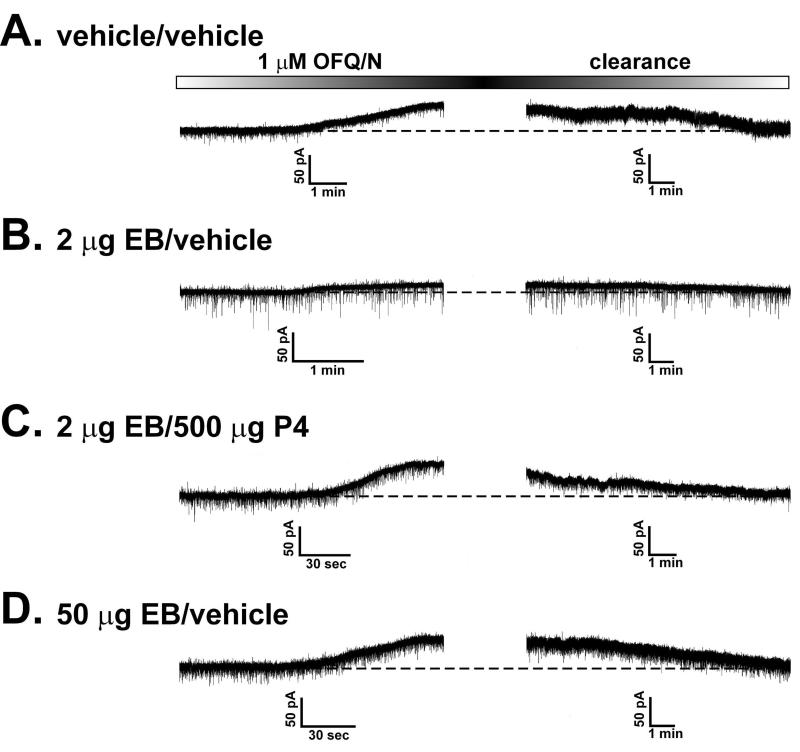
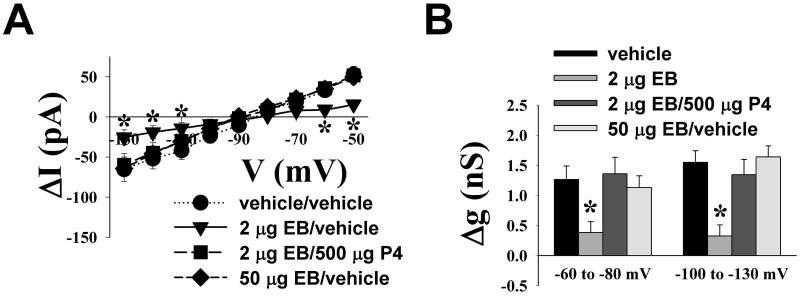
Given the disparate gonadal steroid influences that we observed for the OFQ/N-induced presynaptic inhibition of excitatory and inhibitory input onto ARH neurones, we next set out to determine if they could similarly elicit a differential modulation of the activation of postsynaptic K+ currents caused by the peptide. As shown in Figure 6, OFQ/N produced a sizable outward current in ARH neurones from vehicle-treated animals that reversed upon clearance of the peptide from the slice. This effect was associated with an increase in conductance, and reversed polarity near the Nernst equilibrium potential for K+ (Figure 7). EB treatment appreciably reduced the ability of OFQ/N to activate this postsynaptic K+ current. By contrast, progesterone treatment following EB priming abrogated the negative oestrogenic modulation of the magnitude of the OFQ/N-induced outward current. Importantly, we saw a similarly robust OFQ/N-induced outward current in ARH neurones from animals treated 48 hr prior with a high dose of EB (50 ug) that increases sexual receptivity like that found in EB-primed, progesterone-treated subjects. These observations were corroborated upon examination of the I/V plots and the conductance changes caused by OFQ/N (Figure 7; ΔI: multifactorial ANOVA/LSD, Fsteroid = 2.28, P < 0.08, df = 3; Fvoltage = 34.07, P < 0.0001, df = 8; Finteraction = 2.35, P < 0.0004, df = 24; Δg: multifactorial ANOVA/LSD, Fsteroid = 11.26, P < 0.0001, df = 3; Fvoltage = 0.78, P < 0.38, df = 1; Finteraction = 0.51, P < 0.68, df = 3; n = 11-32).
Figure 6.
Membrane current traces that illustrate the OFQ/N-induced outward currents recorded in identified POMC neurones from OVX rats either 30 hr after the initial vehicle or EB priming and four hr after the second vehicle or progesterone (P4) treatment (A-C), or 48 hr after a high dose of EB (50 μg) that elicits sexual receptivity much like that seen in EB-primed, P4-treated animals (D). These recordings were performed in the presence of 1 μM TTX to ensure a direct postsynaptic effect. For each example, OFQ/N application commenced as signified by the left-hand side of the OFQ/N application timeline box located above each set of traces. I/V relationships were routinely generated prior to, and in the presence of, OFQ/N. This necessitated switching between gap-free and episodic protocols, which precluded the acquisition of a single trace showing both the outward current and its wash-out. The time necessary to conduct the second I/V and then switch back to the gap-free recording of washout took, at most, 90 sec. The two panels, both of equal dimensions, were aligned along their respective X-axes. The time required for complete OFQ/N washout was variable, and in some instances, it took as long as 20 min to be achieved. This is why the time scales for the two halves of the recording are different.
Figure 7.
Gonadal steroids regulate the OFQ/N-induced activation of postsynaptic K+ currents in a hormonally dependent, behaviorally relevant manner. A) This I/V plot illustrates the OFQ/N-induced currents measured at different voltages that were altered to varying extents by either 2 μg EB, 2 μg EB/500 μg progesterone (P4) or 50 μg EB. B) Composite bar graph illustrating the disparate influences on the OFQ/N-induced increase in slope conductance (Δg) caused by these different steroid treatment paradigms. * = P < 0.05, multi-factorial ANOVA/LSD, n = 14 animals/32 cells from vehicle/vehicle- treated group; eight animals/19 cells from 2 μg EB/vehicle-treated group; four animals/11 cells from EB/500 μg P4-treated group; eight animals/12 cells from the50 μg EB-treated group.
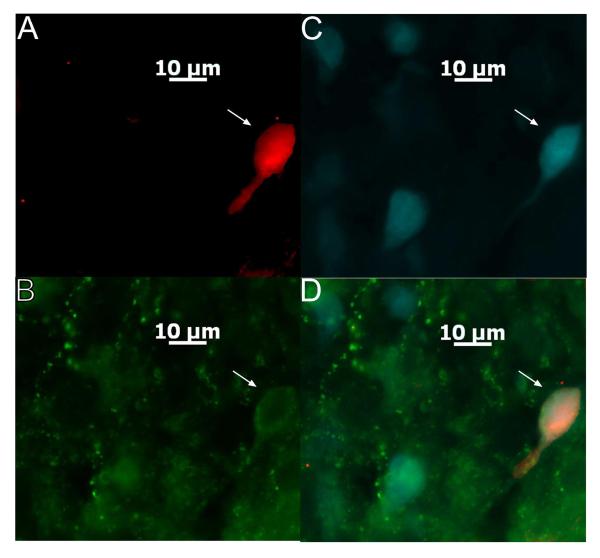
After electrophysiological recording, we identified whether 54 of the ARH neurones evaluated in the present study that exhibited electrophysiological characteristics of POMC neurones (e.g., hyperpolarization-activated cationic current, A-type K+ current (39-41)) were indeed POMC neurones. Of these, 45 cells were immunocytochemically identified using phenotypic markers of POMC neurones, including 19 that also were labelled with Fluorogold (Figure 8). Of the nine POMC-negative cells, only one projected to the MPN. All of the representative electrophysiological traces shown in the figures that follow were derived from identified POMC neurones. We separated the data collected in identified POMC neurones from that collected in POMC negative/identified cells, and summarized the resultant findings in Table 1. Of particular note is that OFQ/N elicited a comparatively larger inhibitory outward current (and increase in slope conductance) in POMC neurones. A priming dose of oestradiol antagonized the outward current and increase in slope conductance caused by OFQ/N to similar degrees in POMC neurones and POMC-negative/unidentified cells. Subsequent progesterone treatment abrogated the oestrogenic attenuation of the OFQ/N-induced outward current, but did not significantly affect the change in conductance, in both POMC and POMC negative/unidentified cells. On the other hand, the high (50 μg) dose of oestradiol, which also promotes female sexual receptivity, fully restores all of these effects of OFQ/N in both POMC neurones and POMC negative/unidentified cells. Of the 10 POMC neurones that exhibited this significantly more robust postsynaptic response to OFQ/N than the POMC- negative/unidentified cells, four were Fluorogold-positive. The RMP, Rin, ΔI and Δg of Fluorogold- positive POMC neurones were no different than those of FG-negative POMC neurones. By contrast, OFQ/N decreased mEPSC and mIPSC frequency to a similar degree in POMC vs. non-POMC/unidentified cells. The ability of oestrogen priming to diminish, and subsequent progesterone treatment to replenish, the OFQ/N-induced decrease in mEPSC frequency was indistinguishable between POMC neurones and POMC negative/unidentified cells. Likewise, there was no difference in the ability of oestrogen priming to accentuate, or subsequent progesterone treatment to attenuate, the OFQ/N- induced decrease mIPSC frequency in POMC vs. POMC negative/unidentified neurones.
Figure 8.
A colour photomicrograph of a MPN-projecting ARC POMC neuron from which an electrophysiological recording was taken. A) Biocytin labeling of the neuron filled throughout the recording and visualized with AF546. B) Neuronal somata and fibers that are immunopositive for CART as visualized by AF488. C) Retrogradely labelled ARH neurones filled with Fluorogold that was injected into the MPN. D) Merged photomicrographs from A-C illustrating the ARH neuron (denoted by the arrows) that was triple-labelled with Fluorogold, biocytin and CART immunoreactivity.
Table 1.
Summary of the postsynaptic and presynaptic actions of OFQ/N on POMC neurones and unidentified ARH neurones in slices from vehicle- and steroid-treated animals.
| Postsynaptic | Presynaptic | ||||
|---|---|---|---|---|---|
| ΔI (−60 mV; pA) |
Δg (−60 to −80 mV; nS) |
Δg (−100 to −130 mV; nS) |
mEPSC frequency (% baseline) |
mIPSC frequency (% baseline) |
|
| POMC cells, vehicle |
76.0 ± 11.2* (10) |
2.37 ± 0.41* (10) |
2.20 ± 0.28* (10) |
49.1 ± 3.0 (5) | 56.7 ± 1.1 (3) |
| non-POMC cells, vehicle |
40.1 ± 7.2 (21) |
0.90 ± 0.21 (21) |
1.40 ± 0.25 (21) |
52.4 ± 5.1 (6) | 62.7 ± 1.8 (3) |
| POMC cells, 2 μg EB |
18.9 ± 9.7*,# (4) |
0.29 ± 0.25# (4) |
0.71 ± 0.30*,# (4) |
94.8 ± 9.7# (3) | 32.8 ± 5.5# (3) |
| non-POMC cells, 2 μg EB |
10.5 ± 5.5# (15) |
0.46 ± 0.20 (15) |
0.21 ± 0.19# (15) |
96.2 ± 1.5# (4) | 42.7 ± 0.3# (3) |
| POMC cells, 2 μg EB/500 μg P4 |
51.0 ± 14.9* (7) |
1.26 ± 0.35# (7) |
1.43 ± 0.32*,# (7) |
45.7 ± 8.0 (4) | 92.8 ± 6.3# (3) |
| non-POMC cells, 2 μg EB/500 μg P4 |
31.8 ± 12.4 (4) |
1.05 ± 0.38 (4) |
0.83 ± 0.16# (4) |
50.5 ± 3.8 (5) | 94.2 ± 1.6# (3) |
| POMC cells, 50 μg EB |
60.2* ± 13.7 (3) |
2.11 ± 0.42* (3) |
2.27 ± 0.34* (3) |
-- | -- |
| non-POMC cells, 50 μg EB |
35.3 ± 5.3 (9) |
0.75 ± 0.12 (9) |
1.27 ± 0.28 (9) |
-- | -- |
The postsynaptic activation of GIRK-1 channels was assessed by measuring the change in the membrane holding current (ΔI) at −60 mV, and the change in slope conductance (Δg) measured between −60 to −80 mV and −100 to −130 mV, in the presence of 1 μM OFQ/N. The presynaptic inhibition of glutamate and GABA release was evaluated by examining the change in mEPSC frequency and mIPSC frequency, respectively, in the presence of 100 nM OFQ/N.
Values from POMC neurones that are significantly different (P < 0.05, two-way ANOVA/LSD) than those from unidentified ARH neurones.
Values in neurones from steroid-treated animals that are significantly different (P < 0.05, two-way ANOVA/LSD) than those from their vehicle-treated counterparts.
Discussion
The results of the present study demonstrate that gonadal steroids differentially modulate the pleiotropic actions of OFQ/N in a critical region of the limbic-hypothalamic reproductive circuitry in a manner that is coincident with changes the sexual receptive state of the female rodent. These conclusions are based on the following observations: 1) oestradiol priming that does not induce lordosis per se increases basal excitatory input onto ARH neurones, attenuates both the OFQ/N-induced presynaptic inhibition of excitatory input onto ARH neurones and the activation of postsynaptic GIRK-1 channels in ARH neurones, and facilitates the OFQ/N-induced presynaptic inhibition of inhibitory input onto ARH neurones, 2) when the steroidal milieu favors sexual receptivity (i.e., in EB-primed, progesterone-treated animals, or in animals treated 48 hr prior with a high dose of EB), the basal level of excitatory input onto ARH neurones is restored, as is the ability of OFQ/N to presynaptically inhibit excitatory input onto ARH neurones and postsynaptically activate GIRK-1 channels in ARH neurones, 3) under the latter conditions the ability of OFQ/N to also presynaptically inhibit inhibitory input onto ARH neurones is virtually abolished, and 4) all of these effects are seen in a sizable population of POMC neurones, many of which also project to the MPN.
The OFQ/N-induced presynaptic inhibition of excitatory and inhibitory input converging upon POMC and other ARH neurones that we observed presently is consistent with that found in the cortex (17), amygdala (18) and SCN (19). We have shown previously that this excitatory input is blocked by ionotropic glutamate receptor antagonists (10,12,21,38), whereas the inhibitory input is abolished by GABAA receptor antagonists (21). In addition, the OFQ/N-induced presynaptic inhibition of this excitatory input is blocked by the ORL-1 receptor antagonist UFP-101 (12), and altogether absent in ORL-1 receptor knockout mice (10).
Much of the work in recent decades on the oestrogenic regulation of neurotransmission has been associated with discovering and understanding the rapid effects of oestradiol. These types of studies, mostly performed in vitro, have been important for moving the field forward in demonstrating that steroids can rapidly affect presynaptic and postsynaptic elements of neurotransmission by activating particular receptor subtypes of receptors and signal transduction pathways (8). However, we now know that these oestrogenic effects can be sustained for at least 24 hr after systemic administration, and are beginning to be placed into physiological contexts (see 8,12,21,33 for review). Presently, we found that oestrogen priming resulted in an increase in glutamatergic input onto POMC and other ARH neurones, which is in agreement with increases in asymmetric synapse formation reported in the hippocampus (42) and the ARH (43), as well as with upregulated GluR1-3 subunit expression in the hypothalamus (44). It also diminished the ORL-1 receptor-mediated presynaptic inhibition of glutamate release at POMC synapses, which is congruent with the oestrogenic reduction of the CB1 receptor-mediated presynaptic inhibition of glutamate release at POMC synapses (21). On the other hand, oestrogen priming enhanced the OFQ/N-induced presynaptic inhibition of GABAergic input onto POMC and other ARH neurones. This is consistent with the oestrogenic reduction of GABAergic input onto hippocampal neurones (45), with the oestrogenic diminution of GABAergic axosomatic contacts in the ARH (46), and with theoestrogenic accentuation of the CB1 receptor-mediated presynaptic inhibition of GABA release at POMC synapses (21). Thus, a priming dose of oestradiol that induces the activation of μ-opioid receptors in the MPN differentially modulates the ability of OFQ/N to control the output of the ARH neurones via presynaptic inhibition. Most interestingly, progesterone treatment that reduces POMC neuronal activity as evidenced by a decrease in the oestradiol-induced μ-opioid receptor activation in the MPN diminished excitatory glutamate release onto POMC and other ARH neurones to levels observed in OVX vehicle- treated controls, and restored the ability of OFQ/N to presynaptically inhibit this glutamatergic input. This is consistent with the rapid decline in VMN glutamate levels seen following progesterone administration to oestrogen-primed females (47). Arguably the most seminal finding from this study is that oestradiol and progesterone treatment markedly attenuated the ability of OFQ/N to presynaptically inhibit GABAergic input onto POMC and other ARH neurones, which would have the effect of enhancing the inhibition. It is known that the progesterone derivative allopregnanolone enhances GABAA receptor-mediated currents in hypothalamic gonadotropin-releasing hormone (GnRH) neurones (48,49). However, progesterone reduces GABAergic input impinging on these cells when administered to oestrogen-primed animals (50). Our results indicate that the progesterone-induced uncoupling of presynaptically localised ORL-1 receptors from the mechanisms that inhibit GABA release represents another important way in which the steroid positively modulates GABAergic neurotransmission in the hypothalamus. In addition, they suggest that progesterone may regulate GABAergic neurotransmission in a site specific manner.
The OFQ/N-induced outward current that we presently encountered in POMC neurones is associated with membrane hyperpolarization and a reduction in neuronal firing (9,12). It is greatly diminished by UFP-101 and the GIRK channel blockers Ba2+ and tertiapin (9,12), and completely ablated in ORL-1 receptor knockout mice (10). This ORL-1 receptor-mediated activation of GIRK-1 channels also inhibits other ARH neurones such as the A12 dopamine and GnRH neurones (9), which accounts, at least in part, for the effects of the peptide on the secretion of the anterior pituitary hormones prolactin and luteinizing hormone (51,52). However, when we split the data up between POMC neurones and non- POMC/unidentified cells, we found that the former responded in a significantly more robust fashion to the postsynaptic actions of OFQ/N than the latter. The same did not hold true for the presynaptic actions of OFQ/N, in that the peptide inhibited mE/IPSC frequency to similar degrees in both POMC and POMC- negative/unidentified neurones. It follows that the ORL1 receptor-mediated activation of postsynaptic GIRK-1 channels in POMC neurones is more physiologically relevant than it is for other ARH cell types. Given what is known about how POMC neurones impact homeostatic function, it stands to reason that the gonadal steroid modulation of the hyperpolarizing response in these cells imparts a critically important mechanism though which estradiol and progesterone exert changes in female sexual behavior and energy homeostasis (see (8) for review). Just like with the presynaptic inhibition of glutamatergic input, oestrogen priming attenuated the ORL-1 receptor-mediated activation of postsynaptic GIRK-1 channels in POMC and other ARH neurones. This is similar to the oestrogenic disruption of the μ-opioid and GABAB receptor-mediated activation of GIRK-1 channels observed in these cells (33), and suggests that the oestrogenic uncoupling of metabotropic Gi/o-coupled receptors from their postsynaptic effect or systems represents a fundamental mechanism through which oestrogen priming controls POMC neuronal activity. The oestrogenic regulation of other ARH neurones like A12 dopamine neurones that tonically inhibit prolactin secretion and GnRH neurones that drive the reproductive axis is well documented. Oestrogen-primed, progesterone-treated animals, as well as those treated 48 hr prior with a 50 μg dose of EB, exhibit sexual receptivity concomitant with decreased μ-opioid receptor activation in the MPN. In oestrogen-primed, progesterone-treated animals, the ability of OFQ/N to elicit the activation of GIRK-1 channels in POMC neurones was increased but only partially restored to levels observed in OVX vehicle- treated controls, whereas animals treated 48 hr prior with 50 μg EB displayed full restoration of the responsiveness of POMC neurones to the OFQ/N-induced activation of GIRK-1 channels. This is in agreement with the fact that ORL-1 receptor antagonism and immunoneutralization blocks the increase in sexual receptivity caused by 50 μg EB but not by progesterone in EB-primed animals (11). Nevertheless, the partial progesterone-induced restoration following oestrogen priming is in line with what we observed for basal glutamatergic release, and for the ORL-1 receptor-mediated presynaptic inhibition of glutamatergic input onto ARH neurones. Progesterone has been shown to directly inhibit voltage-gated K+ channels in striatal neurones (53), and to decrease K+ flux through both large conductance, Ca2+- activated K+ channels and GIRK channels in Xenopus oocytes (54,55). By contrast, Kir6.2 expression is upregulated in the preoptic area of oestrogen-primed, progesterone-treated rats (56), and this is in agreement with our present finding that progesterone can facilitate the coupling of Gi/o-coupled receptors to GIRK-1 channels in ARH neurones. Our results also show a dose-dependent oestrogenic regulation of the ORL-1 receptor-mediated postsynaptic actions at a critical neuroanatomical component of a physiologically relevant circuit controlling reproductive behaviour. This is in keeping with our recent finding that the 50 μg dose, but not the priming dose, of oestradiol reduces levels of plasma membrane oestrogen receptor (ER)α, as well as ERα complexing with and signaling through metabotropic glutamate receptor 1 in the ARH, 48 hr after administration (54), which would render POMC neurones refractory to further oestrogenic stimulation.
These gonadal steroid-induced changes in ORL-1 receptor-mediated signalling at POMC synapses temporally coincide with alterations in sexual receptivity. Oestrogen priming per se that activates MPN μ-opioid receptors to inhibit sexual receptivity (11,34) can be attributed, at least in part, to the ability of oestradiol to increase basal glutamatergic input onto POMC neurones, to attenuate the ORL-1 receptor- mediated presynaptic inhibition of glutamate release onto POMC neurones and the postsynaptic activation of GIRK-1 channels in POMC neurones, and to accentuate the OFQ/N-induced presynaptic inhibition of GABAergic input onto these cells. These actions culminate in an increase in the excitability of POMC neurones, and the increased release of β-endorphin then stimulates μ-opioid receptors in MPN neurones (11,30-32,34). This is critical for the subsequent development of sexual receptivity, as evidenced by the fact that pretreatment with the opioid receptor antagonists blocks the oestradiol-induced internalization of μ-opioid receptors in the MPN (30,34) and significantly reduces the lordosis quotient oestrogen-primed, progesterone-treated animals (57). Lordosis quotient and score also are decreased in oestrogen-primed, progesterone-treated μ-opioid receptor knockout mice relative to their wildtype controls (58). Moreover, β-endorphin administered into the third ventricle concomitantly with oestrogen priming potentiates lordosis behaviour when tested five hr after subsequent progesterone treatment (59). OFQ/N could also be facilitating lordosis through activation of ORL-1 receptors located in other hypothalamic areas like the VMN to regulate descending lordosis motor output pathways (60). However, infusions of OFQ/N in the VMN region that facilitated lordosis also deactivated the MPN μ-opioid receptors; indicating that a reduction in the activity of POMC neurones projecting to the MPN was still required for the behavioral expression of sexual receptivity (11). Conversely, under steroidal conditions that favor sexual receptivity, the basal glutamatergic input impinging upon POMC neurones, as well as the ability of OFQ/N to presynaptically inhibit glutamate release onto POMC neurones and to postsynaptically activate GIRK-1channels in POMC neurones, are restored, whereas the OFQ/N-induced decrease in GABA release at POMC synapses is markedly diminished. Collectively, this results in a decrease in POMC neuronal excitability that is associated with a reduction in MPN μ-opioid receptor activation (34). When μ-opioid receptors are activated by exogenous agonists administered into the MPN of oestrogen-primed, progesterone-treated rats, lordosis behaviour is appreciably attenuated (34). Thus, our present data indicate that OFQ/N-induced presynaptic inhibition of neurotransmission and levels of postsynaptic ORL- 1 signalling are modulated by oestradiol and progesterone over time in a behaviourally relevant fashion.
In conclusion, our results demonstrate that gonadal steroids differentially modulate pleiotropic ORL-1 receptor-mediated signalling at POMC synapses in a dose- and time-specific manner. As such, they provide a newfound appreciation for how this neuropeptide system is involved in the temporal dynamics of the gonadal steroid regulation of female reproductive behaviour. Given that OFQ/N stimulates appetite (10,61,62), and that oestradiol and progesterone respectively decrease and increase appetite (8,63,64), our findings also have important implications for how gonadal steroids influence energy homeostasis.
Acknowledgements
The authors thank Cecilia Meza for her excellent technical assistance. This study was supported by PHS Grants DA024314 and HD058638, and by the Instituto de Salud Carlos III, Cooperative Research Thematic Networks in Health Grant RD2012/0028/0021.
References
- 1.Reinscheid RK, Nothacker H-P, Bourson A, Ardati A, Henningsen RA, Bunzow JR, Grandy DK, Langen H, Monsma FJ, Civelli O, Orphanin FQ. A neuropeptide that activates an opioidlike G protein-coupled receptor. Science. 1995;270:792–794. doi: 10.1126/science.270.5237.792. [DOI] [PubMed] [Google Scholar]
- 2.Darland T, Heinricher MM, Grandy DK. Orphanin FQ/nociceptin: a role in pain and analgesia, but so much more. Trends Neurosci. 1998;21:215–221. doi: 10.1016/s0166-2236(97)01204-6. [DOI] [PubMed] [Google Scholar]
- 3.Sinchak K, Romeo HE, Micevych PE. Site-specific estrogen and progestin regulation of orphanin FQ/nociceptin and nociceptin opioid receptor mRNA expression in the female rat limbic hypothalamic system. J Comp Neurol. 2006;496:252–268. doi: 10.1002/cne.20949. [DOI] [PubMed] [Google Scholar]
- 4.Bunzow JR, Saez C, Mortrud M, Bouvier C, Williams JT, Low M, Grandy DK. Molecular cloning and tissue distribution of a putative member of the rat opioid receptor gene family that is not a μ, Δ or kappa opioid receptor type. FEBS Lett. 1994;347:284–288. doi: 10.1016/0014-5793(94)00561-3. [DOI] [PubMed] [Google Scholar]
- 5.Mollereau C, Parmentier M, Mailleux P, Butour J-L, Moisand C, Chalon P, Caput D, Vassart G, Meunier J-C. ORL1, a novel member of the opioid receptor family. FEBS Lett. 1994;341:33–38. doi: 10.1016/0014-5793(94)80235-1. [DOI] [PubMed] [Google Scholar]
- 6.Sim LJ, Xiao RY, Childers SR. Identification of opioid receptor-like (ORL1) peptide-stimulated [35S]GTPgammaS binding in rat brain. Neuroreport. 1996;7:729–733. doi: 10.1097/00001756-199602290-00012. [DOI] [PubMed] [Google Scholar]
- 7.Olszewski PK, Levine AS. Minireview: characterization of influence of central nociceptin/orphanin FQ on consummatory behavior. Endocrinology. 2004;145:2627–2632. doi: 10.1210/en.2004-0016. [DOI] [PubMed] [Google Scholar]
- 8.Sinchak K, Wagner EJ. Estradiol signaling in the regulation of reproduction and energy balance. Frontiers Neuroendocrinol. 2012;33:342–363. doi: 10.1016/j.yfrne.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wagner EJ, Rønnekleiv OK, Grandy DK, Kelly MJ. The peptide orphanin FQ inhibits β- endorphin neurons and neurosecretory cells in the hypothalamic arcuate nucleus by activating an inwardly-rectifying K+ conductance. Neuroendocrinology. 1998;67:73–82. doi: 10.1159/000054301. [DOI] [PubMed] [Google Scholar]
- 10.Farhang B, Pietruszewski L, Lutfy K, Wagner EJ. The role of the NOP receptor in regulating food intake, meal pattern, and the excitability of proopiomelanocortin neurons. Neuropharmacology. 2010;59:190–200. doi: 10.1016/j.neuropharm.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanathara NM, Moraes J, Kanjiya S, Sinchak K. Orphanin FQ in the mediobasal hypothalamus facilitates sexual receptivity through the deactivation of medial preoptic mu-opioid receptors. Horm Behav. 2011;60:540–548. doi: 10.1016/j.yhbeh.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Borgquist A, Kachani M, Tavitian N, Sinchak K, Wagner EJ. Estradiol negatively modulates the pleiotropic actions of orphanin FQ/nociceptin at proopiomelanocortin synapses. Neuroendocrinology. 2013;98:60–72. doi: 10.1159/000351868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rudy B. Diversity and ubiquity of K channels. Neuroscience. 1988;25:729–749. doi: 10.1016/0306-4522(88)90033-4. [DOI] [PubMed] [Google Scholar]
- 14.Hille B. Potassium Channels and Chloride Channels. In: Hille B, editor. Ionic Channels of Excitable Membranes. Sinauer Associates, Inc.; Sunderland, Mass: 1992. pp. 115–139. [Google Scholar]
- 15.Curtis DR, Phillis JW, Watkins JC. Actions of amino-acids on the isolated hemisected spinal cord of the toad. Br J Pharmacol. 1961;16:262–283. doi: 10.1111/j.1476-5381.1997.tb06801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Curtis DR, Watkins JC. Acidic amino acids with strong excitatory actions on mammalian neurones. J Physiol (Lond) 1963;166:1–14. doi: 10.1113/jphysiol.1963.sp007087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bianchi C, Marani L, Barbieri M, Marino S, Beani L, Siniscalchi A. Effects of nociceptin/orphanin FQ and endomorphin-1 on glutamate and GABA release, intracellular [Ca2+] and cell excitability in primary cultures of rat cortical neurons. Neuropharmacology. 2004;47:873–883. doi: 10.1016/j.neuropharm.2004.06.017. [DOI] [PubMed] [Google Scholar]
- 18.Meis S, Pape H-C. Control of glutamate and GABA release by nociceptin/orphanin FQ in the rat lateral amygdala. J Physiol (Lond) 2001;532:701–712. doi: 10.1111/j.1469-7793.2001.0701e.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gompf HS, Moldavan MG, Irwin RP, Allen CN. Nociceptin/orphanin FQ (N/OFQ) inhibits excitatory and inhibitory synaptic signaling in the suprachiasmatic nucleus (SCN) Neuroscience. 2005;132:955–965. doi: 10.1016/j.neuroscience.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 20.Hentges ST, Low MJ, Williams JT. Differential regulation of synaptic inputs by constitutively released endocannabinoids and exogenous cannabinoids. J Neurosci. 2005;25:9746–9751. doi: 10.1523/JNEUROSCI.2769-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nguyen QH, Wagner EJ. Estrogen differentially modulates the cannabinoid-induced presynaptic inhibition of amino acid neurotransmission in proopiomelanocortin neurons of the arcuate nucleus. Neuroendocrinology. 2006;84:123–137. doi: 10.1159/000096996. [DOI] [PubMed] [Google Scholar]
- 22.Diaz S, Farhang B, Hoien J, Stahlman M, Adatia N, Cox JM, Wagner EJ. Sex differences in the cannabinoid modulation of appetite, body temperature and neurotransmission at POMC synapses. Neuroendocrinology. 2009;89:424–440. doi: 10.1159/000191646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Decavel C, van den Pol AN. GABA: A dominant neurotransmitter in the hypothalamus. J Comp Neurol. 1990;302:1019–1037. doi: 10.1002/cne.903020423. [DOI] [PubMed] [Google Scholar]
- 24.Obrietan K, van den Pol AN. GABA neurotransmission in the hypothalamus: Developmental reversal from Ca2+ elevating to depressing. J Neurosci. 1995;15:5065–5077. doi: 10.1523/JNEUROSCI.15-07-05065.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glaum SR, Hara M, Bindokas VP, Lee CC, Polonsky KS, Bell GI, Miller RJ. Leptin, the obese gene product, rapidly modulates synaptic transmission in the hypothalamus. Mol Pharmacol. 1996;50:230–235. [PubMed] [Google Scholar]
- 26.Jo Y-H, Role LW. Coordinate release of ATP and GABA at in vitro synapses of lateral hypothalamic neurons. J Neurosci. 2002;22:4794–4804. doi: 10.1523/JNEUROSCI.22-12-04794.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mills RH, Sohn RK, Micevych PE. Estrogen-induced μ-opioid receptor internalization in the medial preoptic nucleus is mediated via neuropeptide Y-Y1 receptor activation in the arcuate nucleus of female rats. J Neurosci. 2004;24:947–955. doi: 10.1523/JNEUROSCI.1366-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thornton JE, Loose MD, Kelly MJ, Rönnekleiv OK. Effects of estrogen on the number of neurons expressing β-endorphin in the medial basal hypothalamus of the female guinea pig. J Comp Neurol. 1994;341:68–77. doi: 10.1002/cne.903410107. [DOI] [PubMed] [Google Scholar]
- 29.Ferin M, Van Vugt D, Wardlaw S. The hypothalamic control of the menstrual cycle and the role of endogenous opioid peptides. Recent Prog Horm Res. 1984;40:441–485. doi: 10.1016/b978-0-12-571140-1.50015-3. [DOI] [PubMed] [Google Scholar]
- 30.Eckersell CB, Popper P, Micevych PE. Estrogen-induced alteration of μ-opioid receptor immunoreactivity in the medial preoptic nucleus and medial amygdala. J Neurosci. 1998;18:3967–3976. doi: 10.1523/JNEUROSCI.18-10-03967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych PE. Membrane estrogen receptor-α interactions with metabotropic glutamate receptor 1a modulate female receptivity in rats. J Neurosci. 2007;27:9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dewing P, Christensen A, Bondar G, Micevych PE. Protein kinase C signaling in the hypothalamic arcuate nucleus regulates sexual receptivity in female rats. Endocrinology. 2008;149:5934–5942. doi: 10.1210/en.2008-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelly MJ, Loose MD, Ronnekleiv OK. Estrogen suppresses μ-opioid- and GABAB-mediated hyperpolarization of hypothalamic arcuate neurons. J Neurosci. 1992;12:2745–2750. doi: 10.1523/JNEUROSCI.12-07-02745.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sinchak K, Micevych PE. Progesterone blockade of estrogen activation of μ-opioid receptors regulates reproductive behavior. J Neurosci. 2001;21:5723–5729. doi: 10.1523/JNEUROSCI.21-15-05723.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Priest CA, Vink KL, Micevych PE. Temporal regulation by estrogen of β-preprotachykinin mRNA expression in the rat ventromedial nucleus of the hypothalamus. Mol Brain Res. 1995;28:61–71. doi: 10.1016/0169-328x(94)00184-g. [DOI] [PubMed] [Google Scholar]
- 36.Geary N, Asarian L. Cyclic estradiol treatment normalizes body weight and test meal size in ovariectomized rats. Physiol Behav. 1999;67:141–147. doi: 10.1016/s0031-9384(99)00060-8. [DOI] [PubMed] [Google Scholar]
- 37.Asarian L, Geary N. Cyclic estradiol treatment normalizes body weight and restores physiologic patterns of spontaneous feeding and sexual receptivity in ovariectomized rats. Horm Behav. 2002;42:461–471. doi: 10.1006/hbeh.2002.1835. [DOI] [PubMed] [Google Scholar]
- 38.Ho J, Cox JM, Wagner EJ. Cannabinoid-induced hyperphagia: Correlation with inhibition of proopiomelanocortin neurons? Physiol Behav. 2007;92:507–519. doi: 10.1016/j.physbeh.2007.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ibrahim N, Bosch MA, Smart JL, Qiu J, Rubinstein M, Rønnekleiv OK, Low MJ, Kelly MJ. Hypothalamic proopiomelanocortin neurons are glucose responsive and express KATP channels. Endocrinology. 2003;144:1331–1340. doi: 10.1210/en.2002-221033. [DOI] [PubMed] [Google Scholar]
- 40.Tang SL, Tran V, Wagner EJ. Sex differences in the cannabinoid modulation of an A-type K+ current in neurons of the mammalian hypothalamus. J Neurophysiol. 2005;94:2983–2986. doi: 10.1152/jn.01187.2004. [DOI] [PubMed] [Google Scholar]
- 41.Kellert BA, Nguyen MC, Nguyen C, Nguyen QH, Wagner EJ. Estrogen rapidly attenuates cannabinoid-induced changes in energy homeostasis. Eur J Pharmacol. 2009;622:15–24. doi: 10.1016/j.ejphar.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jelks KB, Wylie R, Floyd CL, McAllister AK, Wise P. Estradiol targets synaptic proteins to induce glutamatergic synapse formation in cultured hippocampal neurons: critical role of estrogen receptor-α. J Neurosci. 2007;27:6903–6913. doi: 10.1523/JNEUROSCI.0909-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gao Q, Mezei G, Nie Y, Rao Y, Choi CS, Bechmann I, Leranth C, Toran-Allerand D, Priest CA, Roberts JL, Gao X-B, Mobbs C, Shulman GI, Diano S, Horvath TL. Anorectic estrogen mimics leptin’s effect on the rewiring of melanocortin cells and Stat3 signaling in obese animals. Nature Medicine. 2007;13:89–94. doi: 10.1038/nm1525. [DOI] [PubMed] [Google Scholar]
- 44.Diano S, Naftolin F, Horvath TL. Gonadal steroids target AMPA glutamate receptor-containing neurons in the rat hypothalamus, septum and amygdala: A morphological and biochemical study. Endocrinology. 1997;138:778–789. doi: 10.1210/endo.138.2.4937. [DOI] [PubMed] [Google Scholar]
- 45.Murphy DD, Cole NB, Greenberger V, Segal M. Estradiol increases dendritic spine density by reducing GABA neurotransmission in hippocampal neurons. J Neurosci. 1998;18:2550–2559. doi: 10.1523/JNEUROSCI.18-07-02550.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Párducz A, Perez J, Garcia-Segura LM. Estradiol induces plasticity of GABAergic synapses in the hypothalamus. Neuroscience. 1993;53:395–401. doi: 10.1016/0306-4522(93)90203-r. [DOI] [PubMed] [Google Scholar]
- 47.Luine VN, Grattan DR, Selmanoff M. Gonadal hormones alter hypothalamic GABA and glutamate levels. Brain Res. 1997;747:165–168. doi: 10.1016/s0006-8993(96)01255-3. [DOI] [PubMed] [Google Scholar]
- 48.Sim JA, Skynner MJ, Herbison AE. Direct regulation of postnatal GnRH neurons by the progesterone derivative allopregnanolone in the mouse. Endocrinology. 2001;142:4448–4453. doi: 10.1210/endo.142.10.8451. [DOI] [PubMed] [Google Scholar]
- 49.Sullivan SD, Moenter SM. Neurosteroids alter γ-aminobutyric acid postsynaptic currents in gonadotropin-releasing hormone neurons: a possible mechanism for direct steroidal control. Endocrinology. 2003;144:4366–4375. doi: 10.1210/en.2003-0634. [DOI] [PubMed] [Google Scholar]
- 50.Sullivan SD, Moenter SM. GABAergic integration of progesterone and androgen feedback to gonadotropin-releasing hormone neurons. Biol Reprod. 2005;72:33–41. doi: 10.1095/biolreprod.104.033126. [DOI] [PubMed] [Google Scholar]
- 51.Shieh K-R, Pan J-T. Effects of orphanin FQ on central dopaminergic neuronal activities and prolactin secretion. Am J Physiol Regul Integr Comp Physiol. 2001;280:R705–R712. doi: 10.1152/ajpregu.2001.280.3.R705. [DOI] [PubMed] [Google Scholar]
- 52.An X-F, Yu J-Y, Chen B-Y, Zhang S-L. Role of hypothalamus nociceptin/orphanin FQ in pre- ovulatory luteinizing hormone surge of estrogen and progesterone-primed, ovariectomized rats. Acta Pharmacol Sin. 2007;28:1189–1197. doi: 10.1111/j.1745-7254.2007.00587.x. [DOI] [PubMed] [Google Scholar]
- 53.Kelley BG, Mermelstein PG. Progesterone blocks multiple routes of ion flux. Mol Cell Neurosci. 2011;48:137–141. doi: 10.1016/j.mcn.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Evaul K, Jamnongjit M, Bhagavath B, Hammes SR. Testosterone and progesterone rapidly attenuate plasma membrane Gβγ-mediated signaling in Xenopus laevis oocytes by signaling through classical steroid receptors. Mol Endocrinol. 2007;21:186–196. doi: 10.1210/me.2006-0301. [DOI] [PubMed] [Google Scholar]
- 55.Wong C-M, Tsang S-Y, Yao X, Chan FL, Huang Y. Differential effects of estrogen and progesterone on potassium channels expressed in Xenopus oocytes. Steroids. 2008;73:272–279. doi: 10.1016/j.steroids.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 56.Huang W, Acosta-Martínez M, Levine JE. Ovarian steroids stimulate adenosine triphosphate- sensitive potassium (KATP) channel subunit gene expression and confer responsiveness of the gonadotropin-releasing hormone pulse generator to KATP channel modulation. Endocrinology. 2008;149:2423–2432. doi: 10.1210/en.2007-0830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Torii M, Kubo K, Sasaki T. Naloxone and initial estrogen action to induce lordosis in ovariectomized rats: The effect of a cut between the septum and preoptic area. Neurosci Lett. 1995;195:167–170. doi: 10.1016/0304-3940(95)11809-b. [DOI] [PubMed] [Google Scholar]
- 58.Sinchak K, Shahedi K, Dewing P, Micevych PE. Sexual receptivity is reduced in the female mu- opioid receptor knockout mouse. Neuroreport. 2005;16:1697–1700. doi: 10.1097/01.wnr.0000181585.49130.93. [DOI] [PubMed] [Google Scholar]
- 59.Torii M, Kubo K, Sasaki T. Influence of opioid peptides on the priming action of estrogen on lordosis in ovariectomized rats. Neurosci Lett. 1996;212:68–70. doi: 10.1016/0304-3940(96)12763-4. [DOI] [PubMed] [Google Scholar]
- 60.Calizo LH, Flanagan-Cato LM. Hormonal-neural integration in the female rat ventromedial hypothalamus: triple labeling for estrogen receptor-alpha, retrograde tract tracing from the periaqueductal gray, and mating-induced Fos expression. Endocrinology. 2003;144:5430–40. doi: 10.1210/en.2003-0331. [DOI] [PubMed] [Google Scholar]
- 61.Pomonis JD, Billington CJ, Levine AS. Orphanin FQ, agonist of the orphan opioid receptor, stimulates feeding in rats. Neuroreport. 1996;8:369–371. doi: 10.1097/00001756-199612200-00072. [DOI] [PubMed] [Google Scholar]
- 62.Bomberg EM, Grace MK, Levine AS, Olszewski PK. Functional interaction between nociceptin/orphanin FQ and α-melanocyte-stimulating hormone in the regulation of feeding. Peptides. 2006;27:1827–1834. doi: 10.1016/j.peptides.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 63.Johnson WG, Corrigan SA, Lemmon CR, Bergeron KB, Crusco AH. Energy regulation over the menstrual cycle. Physiol Behav. 1994;56:523–527. doi: 10.1016/0031-9384(94)90296-8. [DOI] [PubMed] [Google Scholar]
- 64.Corcoran C, Grinspoon S. Treatments for wasting in patients with the acquired immunodeficiency syndrome. New England J Med. 1999;340:1740–1750. doi: 10.1056/NEJM199906033402207. [DOI] [PubMed] [Google Scholar]