Abstract
Purpose
To clarify sotalol’s classification in the BCS versus BDDCS systems through cellular, rat everted sac and PAMPA permeability studies.
Methods
Studies were carried out in Madin Darby canine kidney (MDCK) and MDR1-transfected MDCK (MDCK-MDR1) cell lines, rat everted gut sacs and the Parallel Artificial Membrane Permeability Assay (PAMPA) system. Three-hour transport studies were conducted in MDCK cell lines (with apical pH changes) and MDCK-MDR1 cells (with and without the P-glycoprotein inhibitor GG918); male Sprague-Dawley rats (300 ~ 350 g) were used to prepare everted sacs. In the PAMPA studies, drug solutions at different pH’s were dosed in each well and incubated for 5 hours. Samples were measured by LC-MS/MS, or liquid scintillation counting and apparent permeability (Papp) was calculated.
Results
Sotalol showed low permeability in all of the cultured-cell lines, everted sacs and PAMPA systems. It might be a border line P-glycoprotein substrate. The PAMPA study showed that sotalol’s permeability increased with a higher apical pH, while much less change was found in MDCK cells.
Conclusion
The low permeability rate for sotalol correlates with its Class 3 BDDCS assignment and lack of in vivo metabolism.
Keywords: Permeability, Biopharmaceutics drug disposition classification system (BDDCS), P-glycoprotein, MDCK cells, Solute transporters
INTRODUCTION
In 1995, Amidon et al. developed the Biopharmaceutics Classification System (BCS) (1), which categorizes drugs into four classes based on their solubility and permeability. Later in 2000, FDA adopted the BCS system as an underlying principle for the <Guidance for Industry: Waiver of In Vivo Bioavailability and Bioequivalence Studies for Immediate - Release Solid Oral Dosage Forms Based on a Biopharmaceutical Classification System> (2). According to BCS, sponsors can apply for a waiver of in vivo bioequivalence studies for immediate - release formulations if the drug exhibits high water solubility and high permeability. When the highest dose strength of a drug is soluble in 250 mL or less in aqueous media over the pH range of 1 ~ 7.5 at 37°C, the drug substance is considered to be highly soluble. Although the original work of Amidon and coworker (1) measured intestinal permeability rates the preferred criterion in the BCS Guidance (2) is the extent of absorption of a drug substance in humans, where ≥ 90% absorption is considered high permeability. However, the BCS Guidance (2) also indicates that in vivo or in situ animal studies and excised intestinal tissues, or monolayers of suitable epithelial cells may be used to demonstrate high permeability rate, which may serve as a measure of high permeability. In contrast the EMA only allows biowaivers based on the extent of absorption in humans (3).
In general, it is agreed (4) that drugs exhibiting high intestinal permeability rates will also exhibit a high extent of absorption/permeability, but certain drugs showing low cellular permeability rates are still completely absorbed and thus qualify for a waiver of in vivo bioequivalence studies.
In 2005, Wu and Benet developed the Biopharmaceutics Drug Disposition Classification System (BDDCS) (5). They and others suggested that metabolism maybe used as a surrogate predictor for permeability rate, as well as an alternate method in defining ≥ 90% absorbed Class 1 drugs suitable for a waiver of in vivo studies of bioequivalence (6).
Although BCS and BDDCS are based on different in vivo processes, there is usually a reasonably good correlation between the extent of absorption and the extent of metabolism. However, discrepancies between BCS and BDDCS have been observed(5, 7). In 2008, Chen and Yu studied 51 drugs, and found that only 73% (37/51) of the “highly permeable” drugs exhibit extensive metabolism (8). Thus, they concluded that highly permeable drugs may be or may not be metabolized extensively. Of the 14 drugs identified by Chen and Yu as not having extensive metabolism (which would be classified as low permeability rate drugs in BDDCS), in vitro permeability studies (9–13) were available for three drugs (sotalol, levofloxacin and ofloxacin), all of which exhibited lower permeability rates than found for metoprolol. Thus, where in vitro permeability rates were available, the low values correlate with the drugs’ poor extent of metabolism.
Sotalol is an antiarrhythmic agent, with combined class II and III properties. Following oral administration, the absolute bioavailability can reach 100% (14), with no first-pass gut and hepatic metabolism. FDA classifies sotalol as a Class 1 drug based on its extent of absorption, despite cultured-cell permeability studies showing that sotalol is a low permeability rate drug (4,8).
In order to study the disparity between permeability rate and extent of absorption, Dahan et al. carried out rat intestinal perfusion studies with different intestinal segments (15). In their studies, although sotalol’s permeability rate was shown to be lower than that of metoprolol’s for all small intestinal segments investigated, they reported that sotalol’s permeability rate at pH 7.5 exceeded that of metoprolol’s at pH 6.5, and matches metoprolol’s permeability rate at pH 7.0. By this comparison, Dahan et al. (15) concluded that there was no discrepancy between permeability rate and extent of absorption. Sotalol is highly absorbed, and exhibits a high permeability rate, not necessarily in the jejunum, but somewhere along relevant intestinal regions.
Here, we carried out cultured-cell, rat everted sac, and PAMPA system studies to investigate sotalol’s permeability and the effect of pH, as well as to investigate potential transporters that may be involved in sotalol’s transmembrane transport, in order to further characterize sotalol’s permeability in different systems and pH environments.
MATERIALS AND METHODS
Chemicals
Sotalol (racemic), metoprolol, labetalol, verapamil, mannitol, and propranolol were purchased from Sigma-Aldrich Inc. (St. Louis, MO). [3H]-Verapamil was purchased from American Radiolabeled Chemicals (Saint Louis, MO). [14C]-Mannitol was obtained from Perkin-Elmer (Boston, MA). GG918 was from Sequoia Research (Oxford, U.K.). All other reagents (acetonitrile, formic acid) and solvents used in drug analysis were commercial products and of HPLC grade.
Cell Culture Media and Reagents
The MDCK and MDCK-MDR1 cell lines were kind gifts from Dr. Ira Pastan from the National Institute of Health (Bethesda, MD). Cell culture media and supplement as well as drug transport study media were purchased from the Cell Culture Facility in the University of California San Francisco (UCSF, San Francisco, CA). Six-well tissue culture treated polystyrene plates were obtained from Corning Life Science (Acton, MA). Falcon polyethylene cell culture inserts (pore size 0.4 μm, growth area 4.2 cm2) were obtained from BD Biosciences (Bedford, MA). The integrity of the monolayers was ensured by measuring the transepithelial electrical resistance (TEER) values using the Millicell electrical resistance system (Millipore Corporation, Bedford, MA).
Bidirectional Transport Studies
To determine whether sotalol is a substrate of P-glycoprotein, bidirectional transport studies were performed in MDCK and MDCK-MDR1 cells. MDCK and MDCK–MDR1 cells were grown in Dulbecco’s modified Eagle media including fetal bovine serum (FBS, 10%), penicillin (100 μunit/ml), streptomycin (100 μg/ml) and colchicine (80 ng/ml, only for MDCK-MDR1). All cells were fed fresh media (but no colchicine) the day before the transport studies. On the day of the experiments, the cells were washed once and pre-incubated for ~15 min at 37°C in 5% CO2 with Hank’s Balanced Salt Solution (HBSS) containing 25 mM HEPES and 1% FBS. Four 10 μM sotalol dosing solutions were prepared in the same buffer adjusted to different pH (6.5, 7.0, 7.4), and with the P-gp inhibitor GG918 (0.5 μM) at pH 7.4. Three-hour transport studies were conducted in MDCK cells with apical pH at 6.5, 7.0, and 7.4, as well as MDCK-MDR1 cells with and without GG918 (pH 7.4). Each experiment was started by the addition of dosing solution into the donor compartment in two directions, 2.5 ml on the basal side for basolateral to apical (B to A) studies, and 1.5 ml on the apical side for apical to basolateral (A to B) studies, and blank HBSS into the receiver compartments. Samples were collected from the receiver sides at 30, 60, 90 and 180 min, and replaced with blank HBSS. All experiments were performed in triplicate (3 wells) and placed in a shaking incubator at 37°C. At the end of the experiment, solutions from both A- and B- sides were sampled, and each insert with cells was washed 3 times with an ice-cold phosphate buffered saline followed by sonicated 15 minutes with 25% acetonitrile (ACN) to measure intracellular concentrations and calculate mass balances.
Rat Everted Sac Studies
Male Sprague-Dawley rats (300 ~ 350 g) from Charles River Laboratories (Wilmington, MA) were housed in the UCSF Laboratory Animal Resource Center with a 12 h light/dark cycle and allowed free access to water and food. All physiological buffers were ordered from the UCSF Cell Culture Facility. Study protocols were approved by the Institutional Animal Care and Use Committee, UCSF. Rats were anesthetized using a mixture of 80 mg/ml ketamine (Butler Schein™ Animal Health, Dublin, OH) and 12 mg/ml xylazine (Lloyd Laboratories, Shenandoah, IA) at a dose of 1 ml/kg by intraperitoneal injection. Small intestine was removed and placed in ice cold phosphate buffered saline. Everted sacs were prepared as previously described (16) from 3 cm jejunal segments after stripping of the serosal layer using fine tweezers. The sacs, filled with blank HBSS (pH 7.4), were incubated at 37°C and oxygenated in 25 ml scintillation vials containing 20 ml blank HBSS. Stock solutions (50 mM) of sotalol and metoprolol were prepared in dimethyl sulfoxide, and 20 μl were added into the vials to start the experiment (1:1000 dilution). The vials were kept in a 37°C water bath with shaking racks and gently oxygenated (95% O2) to avoid disruption of the enterocytes. Samples (150 μl) were taken at 15, 30, 45 and 60 min from inside the sacs, and replaced with blank HBSS at pH 7.4. Samples were measure by LC-MS/MS after a one-step protein precipitation by acetonitrile.
PAMPA System Studies
A BD Gentest pre-coated PAMPA system was obtained from BD Biosciences (Bedford, MA). The plate was brought to room temperature 30 min before use. Sotalol, metoprolol, labetalol, verapamil, and mannitol 50 μM dosing solutions were prepared in HBSS at the different pHs to be tested. Trace [3H]-verapamil and [14C]-mannitol were added into the 50 μM dosing solutions. Drug solutions were added in either the bottom or top wells, and taken from the receiver wells after 5 hours shaking in a 37°C incubator. Samples were prepared by either acetonitrile precipitation for LC-MS/MS analysis or by adding 5 ml cocktail to 100 μl samples for liquid scintillation counting.
Stock Solutions and Sample Preparation
All stock solutions and working solutions were prepared at 50 mM in dimethyl sulfoxide and stored at −20°C. Both standard working solution and samples were diluted 10 times by acetonitrile, and then 1:1 dilution with acetonitrile containing (200 nM) of propranolol as internal standard. Following thorough vortex mixing and centrifugation, 80 μl was transferred into HPLC vials.
Analytical Methods
A 4000 triple quadrupole tandem-mass spectrometer (AB Sciex Pte. Ltd., Foster City, CA) co-operated with the Schimadzu (Carlsbad, CA) HPLC binary pump system (LC-MS/MS) was used for the drug analysis. The TurboIonSpray voltage was set at 5500 V and was operated at 550 °C in positive ESI mode. Multiple reactions monitoring (MRM) was used for a better selectivity. Transition (m/z) and collision energies were as follows, sotalol 273.050 → 133.200 (37 V), metoprolol 268.070 → 116.100 (25 V), labetalol 329.223 → 90.873 (37 V), and propranolol 260.107 → 183.071 (27 V).
Separation was carried out on a Waters Xterra MS C-18 (4.6 × 50mm, 5 μm, Milford, MA) analytical column. The mobile phase A consisted of water with 0.1% formic acid; mobile phase B consisted of acetonitrile with 0.1% formic acid. A gradient elution was carried out from 0% B to 100% B in 7 min, decreased to 0% B in 0.01 min and equilibrated until 9.5 min. The flow rate was set at 0.5 ml/min; 5 μl aliquots were injected into the system.
[3H]-Verapamil and [14C]-mannitol were measured in Econo-Safe scintillation cocktail (RPI Corp., Mount Prospect, IL) with a liquid scintillation counter (LS 6000TA, Beckman Coulter).
Data Analysis
Quantification of samples measured by LC-MS/MS was performed with Analyst version 1.5 Workstation (Applied Biosystems, Foster City, CA). For cellular transport and rat everted sac studies, permeability (Papp) was calculated using the following equation:
where dC/dt represents the change of concentration over time, VR represents the volume in the receiver chamber, A is the surface area of the cell monolayer/intestinal tissue, and C0 is the initial concentration.
For the PAMPA study, the apparent permeability (Pe) was calculated using the following equation:
where Cequilibrium represents the average concentration in both wells, i.e., Cequilibrium = [CD (t) * VD + CA (t) * VA]/(VD + VA). CD (t) and CA (t) are the compound concentrations in donor and acceptor (receiver) wells, respectively, at time t; VD and VA are the volumes in donor and acceptor wells, respectively; A is the PAMPA filter area, and t is the incubation time.
RESULTS
MDCK Transport Studies
TEER values and microscopy were used to confirm the formation of confluent MDCK monolayers. The average TEER value was 466 ± 5 Ω for MDCK cells (all pHs), and 1644 ± 53 Ω in MDCH-MDR1 cells. As shown in Table I, sotalol showed changes in permeability with pH variation (p < 0.05) in MDCK cells, with efflux ratios below 2.0. In contrast, in MDCK-MDR1 cells, the B to A flux was 7-fold greater than A to B flux, and this directionality was markedly diminished when P-glycoprotein was inhibited by GG918 (Table I). Intracellular concentrations were also unchanged as a function of pH in MDCK cells, while P-glycoprotein inhibition increased intracellular concentrations in MDCK-MDR1 cells (Table II). Mass balance was between 99.7 ~ 111.7% in all of these cellular studies.
Table I.
Sotalol’s Papp in MDCK and MDCK-MDR1 Cells
| Apical pH | MDCK cells
|
MDCK-MDR1 cells
|
|||
|---|---|---|---|---|---|
| 7.4 | 7.0 | 6.5 | Control | W/GG918 | |
|
|
|
||||
| Papp (A→B) (× 10−6 cm/s) | 0.26 ± 0.03 | 0.21 ± 0.02 | 0.17 ± 0.02 | 0.15 ± 0.01 | 0.19 ± 0.04 |
| Papp (B→A) (× 10−6 cm/s) | 0.31 ± 0.01 | 0.33 ± 0.01 | 0.31 ± 0.03 | 1.05 ± 0.06 | 0.20 ± 0.01 |
| Efflux Ratio Papp (B→A)/Papp (A→B) | 1.2 | 1.6 | 1.8 | 7.0 | 1.1 |
Table II.
Sotalol’s Intracellular Accumulation in MDCK and MDCK-MDR1 Cells
| Apical pH | MDCK cells
|
MDCK-MDR1 cells
|
|||
|---|---|---|---|---|---|
| 7.4 | 7.0 | 6.5 | Control | W/GG918 | |
|
|
|
||||
| A to C (nmole/L) | 22.9 ± 4.9 | 19.3 ± 3.2 | 20.0 ± 9.4 | 16.7 ± 2.5 | 28.0 ± 6.0 |
| B to C (nmole/L) | 103 ± 4 | 97.6 ± 5.5 | 100 ± 11 | 68.6 ± 6.6 | 206 ± 32 |
Rat Gut Everted Sac Studies
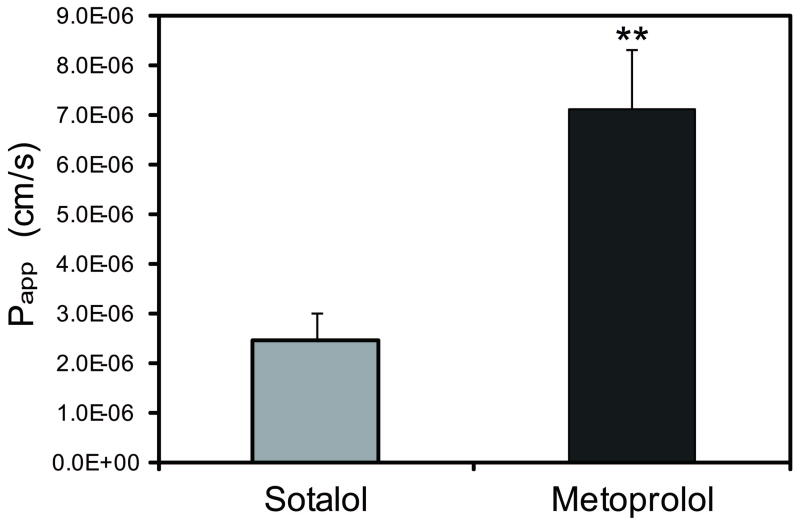
The tightness of the everted sacs was confirmed by carefully checking for any leaking after filling blank buffer into the sacs, and the integrity was further evaluated by adding 50 μM phenol red into the dosing solution, as a color marker of the integrity of the sacs. The study was done in duplicate and repeated on two different days. Sotalol’s Papp was shown to be significantly lower than metoprolol (p<0.05; Figure 1). The recovery for sotalol was 90~110%, and 78~106% for metoprolol.
Figure 1.
Transepithelial transport of 50 μM sotalol and metoprolol in rat jejunum everted sac studies. N = 4. ** P < 0.01.
PAMPA Studies
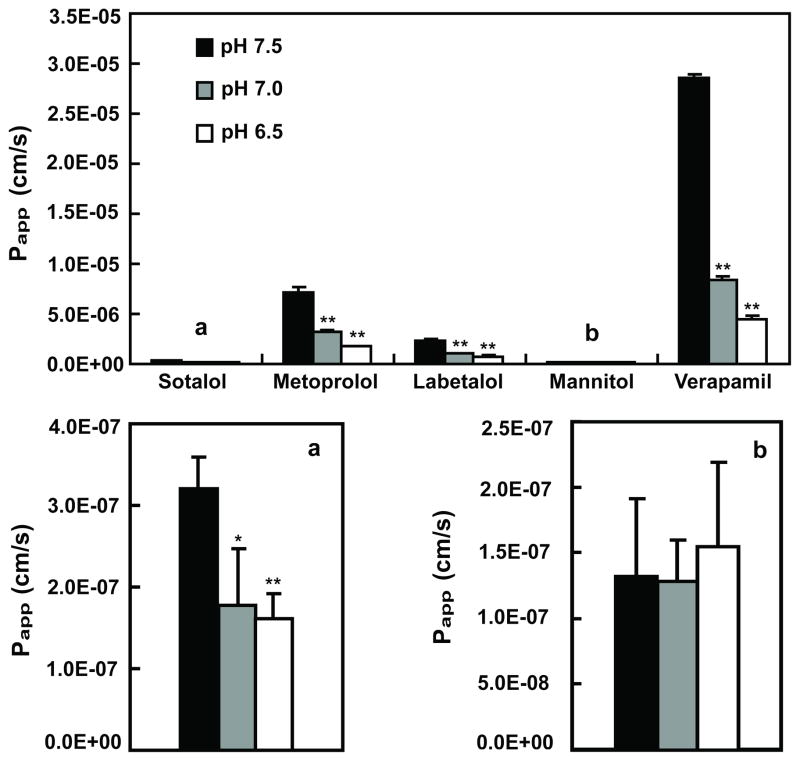
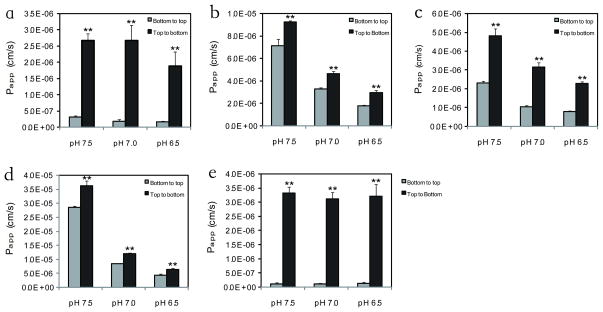
PAMPA studies were conducted on three different days, and each drug was dosed in at least 3 wells in parallel on each day. Mannitol and verapamil were used as low permeability and high permeability controls, respectively. In Figure 2 it can be seen that sotalol’s Papp was about 30 and 100 times lower than metoprolol and verapamil, respectively, and equivalent to about 3 times higher than mannitol over the pH range 6.5 to 7.5. Labetalol was also evaluated since FDA has accepted this compound as a reference drug, in addition to metoprolol (11). All drugs except mannitol exhibited pH-dependent permeability, with higher Papp values at higher pH. When dosed from the top compartment of the PAMPA (opposite to the recommended direction), sotalol and mannitol’s permeability increased by 10-fold with little pH dependence (Figure 3). In contrast, the directionality effect was much less with metoprolol, labetalol, and verapamil with a similar pH effect seen as that observed in the bottom compartment dosing (Figure 3).
Figure 2.
Papp for sotalol, metoprolol, labetalol, verapamil and mannitol from PAMPA system in pH 7.5 (solid bars), 7.0 (grey bars) and 6.5 (open bars) at 50 μM (N=3). aEnlargement of sotalol data y-axis. bEnlargement of mannitol data y-axis. * P < 0.05, ** P < 0.01.
Figure 3.
Directionality and pH effects in the PAMPA system (a. sotalol; b. metoprolol; c. labetalol; d. verapamil; e. mannitol). Dosing solutions were added from bottom compartments (grey bars) and top compartments (solid bars) of the PAMPA system. N=3. ** P < 0.01.
DISCUSSION
Sotalol is a BDDCS Class 3 drug since it is poorly metabolized after oral administration, but exhibits high solubility. From BDDCS this poor metabolism would be expected to correspond to a low permeability rate. Based on BDDCS, both uptake and efflux transporters may be important contributors for sotalol’s disposition. The high solubility enables a high drug concentration in the gut lumen, but an uptake transporter will be needed to overcome the poor permeability to cross the cell membranes. However, once the drug gets into the enterocytes, it could also be a substrate for efflux transporters.
Previous studies exhibited differing results. In the studies of Alt et al. (10) and Yang et al. (13), sotalol did not exhibit P-glycoprotein mediated transport in Caco-2 cells. Bachmakov et al. (12) observed polarized transport (efflux ratio less than 2.0) that was diminished by P-glycoprotein inhibition, also in Caco-2 cells. Thus, they concluded that other factors (e.g. kidney function) were far more important for sotalol disposition than P-glycoprotein function.
Being one of the most hydrophilic β-blockers, sotalol is proposed to pass through the intestinal membranes through the paracellular pathway, as well as the carrier-mediated transcellular pathway, since such hydrophilic compounds don’t exhibit the sufficient lipophilicity needed to penetrate the cell membrane. Yang et al. (13) studied the role of tight junction in the permeability of sotalol, and observed an increase of sotalol Papp with the decrease of the TEER values compared to metoprolol, suggesting that the hydrophilic sotalol is able to pass through the leaky intercellular junction via the paracellular pathway. It was also reported by Yamashita et al. (17) that certain bile salts could decrease the TEER values and result in a higher permeability.
Kato et al. (18) confirmed that sotalol is a substrate of Organic Anion Transporting Polypeptide 1A2 (OATP1A2). They reported that the uptake of sotalol by oocytes transfected with OATP1A2 increased in a time-dependent manner and was much higher than that observed in control oocytes. Since OATP1A2 is localized to the apical brush border membrane together with P-glycoprotein in human enterocytes (19), we suspect it would be involved in sotalol’s absorption.
It is well known that many transporter proteins are expressed in Caco-2 cells, including uptake transporters such as OATPs. Therefore we might expect a P-glycoprotein effect to be diminished in Caco-2 cells. In contrast, MDCK cells express low levels of transporters, and thus this cell line serves as an ideal negative control for the identification of human P-glycoprotein substrates. Caco-2 and MDCK cells yield similar permeability values, and a good correlation (r2 = 0.79) for drugs absorbed by passive diffusion (9), but many transporters can affect drug permeability results for actively transported drugs. Thus, Caco-2 cells are not an ideal cell line for the characterization of transporter substrates of specific transport proteins.
Therefore, we conducted sotalol transport studies in MDCK and MDR1 transfected MDCK (MDCK-MDR1) cell lines. The efflux ratio reached 7.0 in MDCK-MDR1 cells, and decreased to 1.1 when P-glycoprotein was inhibited (Table I). Intracellular accumulation studies also showed that MDCK-MDR1 cells have lower accumulation from the B direction compared with MDCK cells, because P-glycoprotein helped to transport sotalol into the apical compartment. When P-glycoprotein was inhibited by GG918, the accumulation was increased by 3-fold (Table II).
Very recently, our laboratory reviewed P-glycoprotein data for 53 drugs that had been studied in both the Borst and NIH cell lines, finding that the NIH efflux ratio (ER) values were on average 4.26 fold higher (20). Thus, we now suggest that when using a 2.0 ER cut off for the Borst cell line to define P-gp substrates, as proposed by Polli et al. (21), that an 8.5 ER cut off may be more appropriate for the NIH cell line. Therefore, from our study in the NIH cell line we have shown that sotalol is a P-glycoprotein substrate, but that the relevance is border-line (i.e. ER < 8.5).
The PAMPA system is a 96-well phospholipid-coated filter separating two compartments, which serves an in vitro model for passive, transcellular permeation. It can be used to evaluate permeability over a large pH range, therefore is valuable for the estimation of the drug absorption across the entire gastrointestinal tract. Studies have demonstrated that the lipid/oil/lipid tri-layer PAMPA system (Gentest precoated PAMPA system) yields good correlations with Caco-2 cell line data as well as human absorption data (22), although the differences in absolute permeability values between studies can vary 100 fold.
In our PAMPA studies, sotalol’s Papp was 30 and 100 times lower than metoprolol and verapamil, at all three pH values tested (Figure 2). All drugs except mannitol showed higher Papp at pH 7.5 than pH 6.5, which correlates with the general pH partition theory. For these weakly basic drugs with pKa ~9.0, more nonionized molecules will be present at pH 7.5 compared to pH 6.5, facilitating passage through the hydrophobic phospholipid membranes. Since P-glycoprotein activity is increased in an acidic environment (23), this could modulate the differences at different pHs. However, the pH effect on permeability appears to be more significant than on the p-glycoprotein as a pH effect was observed in the MDCK cell studies (Table I). Interestingly, we noticed directionality differences in the PAMPA system for the BDDCS Class 3 compounds, sotalol and mannitol, which was much less than seen with the BDDCS Class 1 compounds, metoprolol, labetalol and verapamil (Figure 3). When dosed from the top compartment, the Papp values for sotalol and mannitol were 10 times higher than when dosed from the bottom compartment (Figure 3a and 3e). As far as we can determine no previous reverse direction studies with PAMPA have been reported. However, it may be that the very large differences in permeability for sotalol and mannitol, with much less of a pH effect, may be characteristic of paracellular absorption.
Our present results correlate well with the results from the study of Dahan et al.(15), where sotalol exhibited a higher Papp in the ileum at the higher physiological pH, as well as at each pH studied, where sotalol’s Papp is lower than that for the reference drug metoprolol.
From above studies, we could conclude that sotalol is a BDDCS Class 3 drug, with high solubility and low permeability rate, which correlates with its lack of in vivo metabolism. Furthermore sotalol appears to be a substrate for both an uptake transporter and an efflux transporter in the intestine as predicted by BDDCS for Class 3 drugs, although the clinical relevance of these transporters may be low due to potential paracellular absorption and a border-line efflux ratio.
Acknowledgments
We thank the Chinese Scholarship Council for providing financial support for Wei Liu to study and carry out a portion of these studies in Dr. Benet’s laboratory at the University of California, San Francisco. The studies in Dr. Benet’s lab were funded in part by NIH grant RR031474.
ABBREVIATIONS
- BCS
Biopharmaceutics Classification System
- BDDCS
Biopharmaceutics Drug Disposition Classification System
- FBS
Fetal bovine serum
- HBSS
Hank’s balanced salt solution
- MDCK
Madin Darby Canine Kidney
- MDR-1 (P-gp)
Multidrug Resistance -1 (P-glycoprotein)
- PAMPA
Parallel Artificial Membrane Permeability Assay
- TEER
Transepithelial electrical resistance
References
- 1.Amidon GL, Lennernäs H, Shah VP, Crison JR. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm Res. 1995;12:413–20. doi: 10.1023/a:1016212804288. [DOI] [PubMed] [Google Scholar]
- 2.Research CfDEa, U.S. Food and Drug Administration. Guidance for industry: Waiver of in vivo bioavailability and bioequivalence studies for immediate-release solid oral dosage forms based on a biopharmaceutics classification system. 2000 Aug; http://www.fda.gov/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm064964.htm (Available from)
- 3.Use CfMPfH, European Medicines Agency. Guideline on the investigation of bioequivalence. 2010 Jan 20; doi: 10.1111/j.1742-7843.2009.00518.x. http://www.ema.europa.eu/ema/pages/includes/document/open_document.jsp?webContentId=WC500070039 (Available from) [DOI] [PubMed]
- 4.Chen M-L, Amidon GL, Benet LZ, Lennernäs H, Yu LX. The bcs, bddcs, and regulatory guidances. Pharm Res. 2011;28:1774–8. doi: 10.1007/s11095-011-0438-1. [DOI] [PubMed] [Google Scholar]
- 5.Wu C-Y, Benet LZ. Predicting drug disposition via application of bcs: Transport/absorption/elimination interplay and development of a biopharmaceutics drug disposition classification system. Pharm Res. 2005;22:11–23. doi: 10.1007/s11095-004-9004-4. [DOI] [PubMed] [Google Scholar]
- 6.Benet LZ, Amidon GL, Barends D, Lennernäs H, Polli J, Shah V, Stavchansky S, Yu LX. The use of bddcs in classifying the permeability of marketed drugs. Pharm Res. 2008;25:483–8. doi: 10.1007/s11095-007-9523-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Takagi T, Ramachandran C, Bermejo M, Yamashita S, Yu LX, Amidon GL. A provisional biopharmaceutical classification of the top 200 oral drug products in the united states, great britain, spain, and japan. Mol Pharm. 2006;3:631–43. doi: 10.1021/mp0600182. [DOI] [PubMed] [Google Scholar]
- 8.Chen M-L, Yu LX. The use of drug metabolism for prediction of intestinal permeability. Mol Pharm. 2009;6:74–81. doi: 10.1021/mp8001864. [DOI] [PubMed] [Google Scholar]
- 9.Irvine JD, Takahashi L, Lockhart K, Cheong J, Tolan JW, Selick HE, Grove JR. Mdck (madin-darby canine kidney) cells: A tool for membrane permeability screening. J Pharm Sci. 1999;88:28–33. doi: 10.1021/js9803205. [DOI] [PubMed] [Google Scholar]
- 10.Alt A, Potthast H, Moessinger J, Sickmüller B, Oeser H. Biopharmaceutical characterization of sotalol-containing oral immediate release drug products. Eur J Pharm Biopharm. 2004;58:145–50. doi: 10.1016/j.ejpb.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 11.Volpe DA. Permeability classification of representative fluoroquinolones by a cell culture method. AAPS Pharm Sci. 2004:6. doi: 10.1208/ps060213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bachmakov I, Werner U, Endress B, Auge D, Fromm MF. Characterization of β-adrenoceptor antagonists as substrates and inhibitors of the drug transporter p-glycoprotein. Fund Clin Pharmacol. 2006;20:273–82. doi: 10.1111/j.1472-8206.2006.00408.x. [DOI] [PubMed] [Google Scholar]
- 13.Yang Y, Faustino PJ, Volpe DA, Ellison CD, Lyon RC, Yu LX. Biopharmaceutics classification of selected β-blockers: Solubility and permeability class membership. Mol Pharm. 2007;4:608–14. doi: 10.1021/mp070028i. [DOI] [PubMed] [Google Scholar]
- 14.Anttila M, Arstila M, Pfeffer M, Tikkanen R, Vallinkoski V, Sundquist H. Human pharmacokinetics of sotalol. Acta Pharmacol Toxicol (Copenh) 1976;39:118–28. doi: 10.1111/j.1600-0773.1976.tb03162.x. [DOI] [PubMed] [Google Scholar]
- 15.Dahan A, Miller JM, Hilfinger JM, Yamashita S, Yu LX, Lennernäs H, Amidon GL. High-permeability criterion for bcs classification: Segmental/ph dependent permeability considerations. Mol Pharm. 2010;7:1827–34. doi: 10.1021/mp100175a. [DOI] [PubMed] [Google Scholar]
- 16.Nayak RK, Benet LZ. Drug transfer across rat intestinal musculature after edetic acid treatment. J Pharm Sci. 1971;60:1508–11. doi: 10.1002/jps.2600601014. [DOI] [PubMed] [Google Scholar]
- 17.Yamashita S, Masada M, Nadai T, TK Effect of adjuvants on charge-selective permeability and electrical resistance of rat jejunal membrane. J Pharm Sci. 1990;79:579–83. doi: 10.1002/jps.2600790706. [DOI] [PubMed] [Google Scholar]
- 18.Kato Y, Miyazaki T, Kano T, Sugiura T, Kubo Y, Tsuji A. Involvement of influx and efflux transport systems in gastrointestinal absorption of celiprolol. J Pharm Sci. 2009;98:2529–39. doi: 10.1002/jps.21618. [DOI] [PubMed] [Google Scholar]
- 19.Glaeser H, Bailey DG, Dresser GK, Gregor JC, Schwarz UI, McGrath JS, Jolicoeur E, Lee W, Leake BF, Tirona RG, Kim RB. Intestinal drug transporter expression and the impact of grapefruit juice in humans. Clin Pharmacol Ther. 2007;81:362–70. doi: 10.1038/sj.clpt.6100056. [DOI] [PubMed] [Google Scholar]
- 20.Broccatelli F, Larregieu CA, Cruciani G, Oprea TI, Benet LZ. Improving the prediction of the brain disposition for orally administered drugs using BDDCS. Adv Drug Deliv Rev. 2012 doi: 10.1016/j.addr.2011.12.008. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polli JW, Wring SA, Humphreys JE, Huang L, Morgan JB, Webster LO, Serabjit-Singh CS. Rational use of in vitro p-glycoprotein assays in drug discovery. Journal of Pharmacology and Experimental Therapeutics. 2001;299:620–8. [PubMed] [Google Scholar]
- 22.Chen X, Murawski A, Patel K, Crespi C, Balimane P. A novel design of artificial membrane for improving the pampa model. Pharm Res. 2008;25:1511–20. doi: 10.1007/s11095-007-9517-8. [DOI] [PubMed] [Google Scholar]
- 23.Thews O, Gassner B, Kelleher DK, Schwerdt G, Gekle M. Impact of extracellular acidity on the activity of p-glycoprotein and the cytotoxicity of chemotherapeutic drugs. Neoplasia. 2006;8:143–52. doi: 10.1593/neo.05697. [DOI] [PMC free article] [PubMed] [Google Scholar]