Abstract
We describe here the X-ray crystal structure of NF-κB p50/RelB heterodimer bound to a κB DNA. Although the global modes of subunit association and κB DNA recognition are similar to other NF-κB/DNA complexes, this complex reveals distinctive features not observed for non-RelB complexes. For example, Lys274 of RelB is removed from the protein–DNA interface whereas the corresponding residues in all other subunits make base-specific contacts. This mode of binding suggests that RelB may allow the recognition of more diverse κB sequences. Complementary surfaces on RelB and p50, as revealed by the crystal contacts, are highly suggestive of assembly of multiple p50/RelB heterodimers on tandem κB sites in solution. Consistent with this model our in vitro binding experiments reveal optimal assembly of two wild-type p50/RelB heterodimers on tandem HIV κB DNA with 2 bp spacing but not by a mutant heterodimer where one of the RelB packing surface is altered. We suggest that multiple NF-κB dimers assemble at diverse κB promoters through direct interactions utilizing unique protein–protein interaction surfaces.
Keywords: NF-κB, RelB, p50, transcription, X-ray crystallography
Introduction
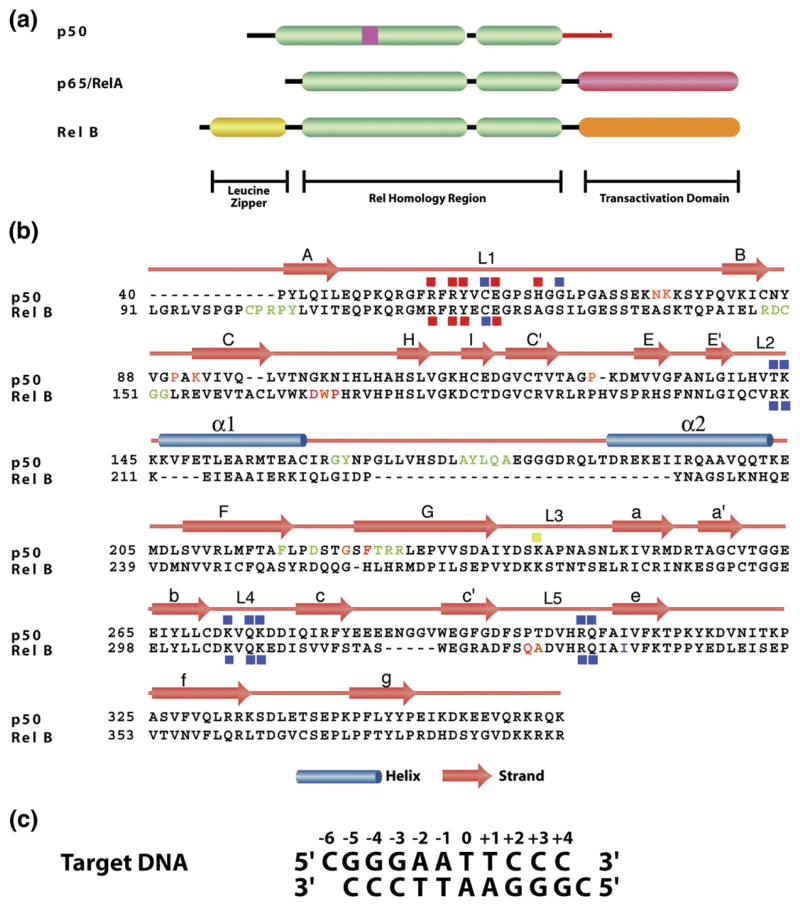
NF-κBs constitute a family of transcription activators that modulate the expression of a large number of genes that are responsible for key cellular functions including development, proliferation, survival and inflammation.1,2 This family consists of five members, p50, p52, RelA (p65), c-Rel and RelB which share a ∼300 residues segment of high sequence homology at or near their N terminus (Figure 1). This homologous segment, known as the Rel homology region (RHR) is critical for nearly all the functions, including DNA binding, dimerization, inhibitor binding and nuclear localization.1 p50 and p52 are processed products of p105 and p100, respectively. Unlike the other NF-κB proteins, p50 and p52 lack a transactivation domain but contain inserts within the RHR. These two subunits associate with RelA, c-Rel and RelB to form the predominant NF-κB dimers responsible for gene activation.2,3 In resting cells, NF-κB activators are retained in an inactive state by binding to inhibitor IκB proteins. On stimulation, IκB kinase (IKK) phosphorylates IκB of IκB/NF-κB complexes, which leads to its ubiquitination and subsequent proteasomal degradation.4 Free NF-κB then activates transcription. The NF-κB specific target sites are collectively referred to as κB DNA which is roughly 10 bp in length. Extensive studies have been carried out to elucidate the rules that govern the binding of different NF-κB dimers to different target DNA sites. Physiological gene promoters have been shown to contain innumerable variations of this consensus. Compilation of the promoter sequences of all known target genes has revealed that a great number of these promoters contain multiple κB sites‡. The precise role of each κB site within a promoter containing multiple sites is mostly unknown. However, cases that have been tested so far have demonstrated cross-talk between these κB sites.5
Figure 1.
Primary and secondary structures of NF-κB and κB DNA. (a) A schematic representation of the domain architectures of three of the NF-κB family members. (b) Primary sequences alignment of the RHR. The secondary structures are mapped onto the primary sequence. Subunit and DNA contacting amino acids are denoted by red (base-specific) and blue (backbone) squares. The crystal contact residues are colored. Similar color denotes interaction pairs from two dimers. (c) κB sequence used for crystallization.
RelB does not stringently follow the “NF-κB family rules”. First identified as an immediate-early gene in serum stimulated fibroblasts, RelB is known to be a critical factor for secondary lymphoid organ development.6,7 It is the only member that is not known to form a homodimer and has restricted ability to heterodimerize. RelB preferentially heterodimerizes with p50 and p52 in vivo. RelB has an extended N terminus known as the leucine zipper (LZ) domain (Figure 1). However, this domain does not participate in homo-and heterodimerization and its function is not known.6–8 Moreover, RelB is inhibited by the atypical IκB protein p100, and not by the prototypical IκB proteins such as IκBα.9 The p52/RelB heterodimer is formed in response to non-classical NF-κB activation pathways.3,10–12 Sustained activation of NF-κB target genes requires RelB. The current model suggests that immediately after induction, expression of target genes is regulated by rapidly activated RelA and c-Rel dimers, and the RelB dimers take over in the later stages.13,14 This takeover appears to be the result of slow activation of RelB dimers and post-induced repression of RelA and c-Rel. These results also suggest that the RelB dimers must be able to bind different κB sequences on these target genes. However, there are at least some target genes that exclusively require RelB such as the chemokines SDF and BLC.15 The consensus κB sequences found in the promoters of these genes, 5′-GGGRN W TTTC-3′, are somewhat different from the consensus κB sequence bound by the p50/RelB heterodimer 5′ GGGRN W YYCC-3′ (where R denotes a purine, N denotes any base, W denotes an adenine or thymine and Y denotes a pyrimidine base).16
Barring RelB, X-ray structures of all NF-κB subunits bound to DNA have been solved.17–22 Since RelB seems to have several unique features, which is not common to the other members, we attempted to crystallize and solve the structure of the p50/RelB heterodimer bound to DNA. We report here the X-ray structure of p50/RelB heterodimer bound to a consensus κB site. Though this structure has resemblance to other NF-κB dimers complexed to κB DNA, it shows some unique binding interactions. Our structure reveals hydrophobic interactions between two crystallographic complexes. In vitro binding studies demonstrate that the hydrophobic interactions between the two NF-κB p50/RelB dimers bound to tandem κB sites occur in solution as well. This interaction may play a critical role in the multi-protein complex formation at the promoter and in the transcription activation of target genes.
Results
Overall structure of the p50/RelB heterodimer bound to DNA
Recombinant heterodimer containing the RHR of murine p50 (amino acid residues (aa) 39–363) and murine RelB (aa 67–400) were co-crystallized with a target 10-mer κB DNA containing a one base overhang at the 5′ end, 5′ CGGGAATTCCC 3′ (Figure 1). The structure was solved using molecular replacement and refined to a resolution of 3.04 Å. In the crystal, complex molecules are arranged along the DNA long axis where the free 5′-cytosine bases are stacked forming a nearly continuous DNA helix. The packing is further facilitated by the protein–protein interactions along the same axis. The possible significance of this molecular arrangement will be discussed later.
The overall structure of the DNA bound p50/RelB heterodimer complex is similar to the other known structures of NF-κB/DNA complexes (Figure 2(a)). The complex buries 3160 Å2 solvent accessible surface area, which compares favorably to other NF-κB DNA complexes. Although, RelB contained 34 residues N-terminal to its RHR, only three residues immediately N-terminal to the RHR showed electron density. Each RHR folds into two Ig domains, an N-terminal DNA binding domain and a C-terminal dimerization domain. No significant bending and deformation of DNA in the complex are observed. Like all known DNA bound to NF-κB, the DNA minor groove in this complex is also compressed.
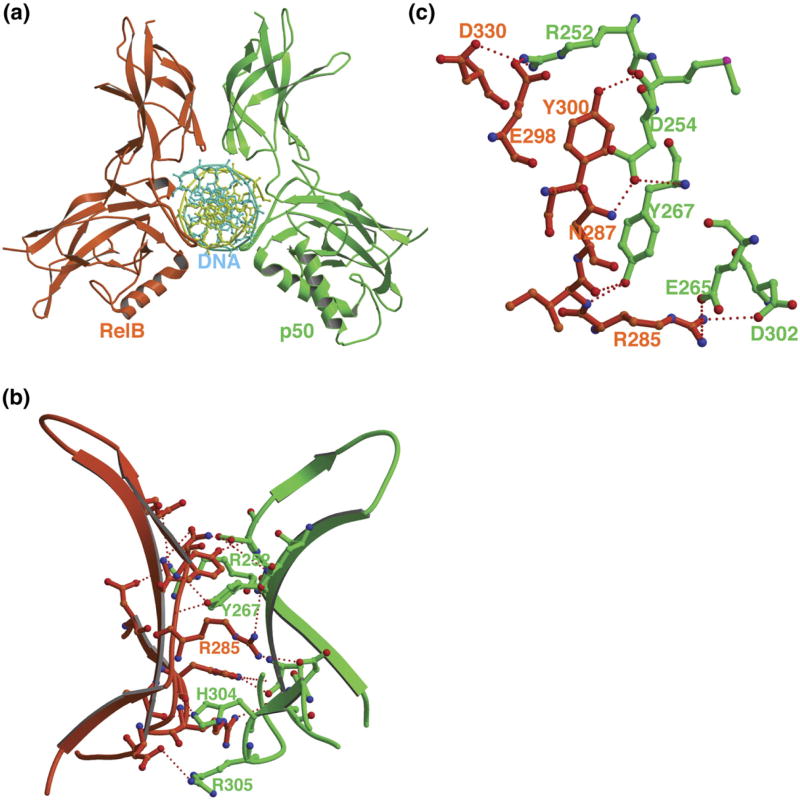
Figure 2.
Overall structure and the subunit interface of the p50/RelB. (a) The ribbon representation of the complex viewed along the long DNA axis. Throughout the paper the same color code will be maintained for the p50 (green) and RelB (red) subunits. (b) Hydrogen bonding network at the subunit interface. (c) Detailed view of the core of the subunit interface.
The dimer interface
The overall feature of the p50/RelB dimer interface is similar to all other dimers known to date except for the RelB homodimer. The interface is characterized by a central hydrophobic core, consisting of pseudo-symmetrical residues from both subunits and is surrounded by a network of hydrogen bonds (Figure 2(b)).23,24 Two equivalent tyrosines (Tyr267 of p50 and Tyr300 of RelB; hereinafter all RelB residues will be shown in italics) located at the center of the interface dominate dimerization (Figure 2(c)). In addition to several van der Waals contacts, these tyrosine residues also make several hydrogen bonding contacts. Mutational experiments have revealed that Tyr267 in p50 is the most critical for homodimer formation. The hydroxyl of Tyr267 in p50 makes hydrogen bonds with the backbone carbonyl of Val251 and side-chain of Arg252 of the opposing subunits in both p50 homodimer.23 However, in the p50/RelB heterodimer both tyrosine residues contact only the backbone. This altered pattern is a direct result of the shift of these two side-chains towards each other but away from the side-chain functional group of Arg252 of p50 or equivalent Arg333 of RelB. The unique hydrogen bond between Asp254 of p50 and Asn200 of RelA,17 which is thought to contribute to the enhanced stability of the p50/RelA heterodimer versus the homodimers recurs between Asp254 of p50 and the homologous Asn284 of RelB. However, its contribution to the overall stability of this heterodimer is not known.
Protein–DNA interactions
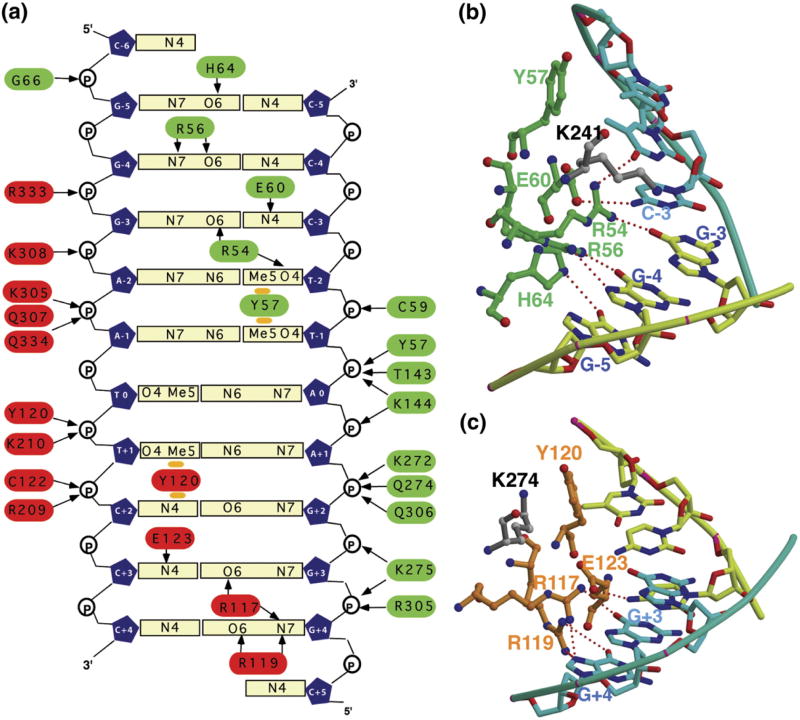
The base-specific and non-specific contacts observed in the complex are shown schematically in Figure 3(a). As expected, the heterodimer binds the symmetrical target DNA asymmetrically. The p50 subunit recognizes the half-site comprising of 5 bp whereas the RelB subunit recognizes the 4 bp half site. These two subunit-contacting sub-sites are separated by a single AT base-pair. His64 in p50 contacts the first G:C base-pair (G:C-5) in the duplex (Figure 3(b)). The lack of a histidine in RelB, at an equivalent position similar to RelA and c-Rel, is responsible for the asymmetric binding (Figure 3(b)). Two features that are strikingly different in this complex compared to the previous complexes are the following; first, less than expected number of base-pairs at each half site make specific hydrogen bonding contacts with the protein sub-units and second, patterns of base-specific hydrogen bonds are different as compared to the other NF-κB complexes. In all previously known p50/DNA sub-complexes, all 5 bp were observed to contact the p50 subunit directly by hydrogen bonds.17,21 In this case only 4 bp make direct hydrogen bonds with the protein. The fifth one, the methyl group of T-1, makes van der Waals contacts with Tyr57. The same is also true for the RelB/DNA sub-complex; only 3 bp form hydrogen bonds with RelB whereas the last base-pair (A:T at position +1) contacts Tyr120 through van der Waals interactions. This difference in hydrogen bonding pattern is observed due to the altered positions of two homologous lysine residues; Lys241 of p50 and Lys274 of RelB. The ammonium group of Lys241 is positioned over 4 Å away from nearest DNA atoms (Figure 3(b)). However, it still can influence DNA binding through electrostatic interactions. Lys274 of RelB is located 7 Å away from the protein DNA interface and this lysine is not expected to directly influence DNA binding by the heterodimer as discussed below (Figure 3(c)).
Figure 3.
Protein–DNA contacts. (a) Schematic representation of base-specific and non-specific contacts between the heterodimer and κB DNA. (b) Detailed sequence specific interactions between amino acids from p50 and RelB (in (c)). The DNA non-contacting residues in this complex are highlighted in grey color. The homologous residues make contact in other complexes.
Conformation of loop 3 (linker) is different in RelB compared to other NF-κB subunits
Loop 3 is apparently a flexible linker that connects the N-terminal DNA binding domain and C-terminal dimerization domain (DD) (Figure 4(a)). In structures of all NF-κB/DNA complexes known to date, this linker forms a loop structure with helical propensity that makes direct contact with DNA and is stabilized upon forming the protein/DNA complex. Lys241 of p50 or Arg187 of RelA that makes direct DNA contact are located within this loop.16 In addition, a conserved proline also makes van der Waals contact with the sugar moiety of the DNA backbone. In RelB this proline is substituted with a threonine. The helical architecture of this segment is more prominent in RelB than the corresponding segment in p50 due to the proline to threonine substitution (Figure 4(b) and (c)). The backbone hydrogen bond between the carbonyl of Lys274 and amide of Thr276 allows the rotation of the side-chain such that it can make a salt bridge with Asp272 which further stabilizes the helix (Figure 4(d)). The stacking interaction between Lys274 and Tyr120 as described earlier also contributes to adopt the helical geometry by further stabilizing the conformation of Lys274. Consequent to these intramoleuclar interactions the linker helix of RelB moves away from DNA making fewer number of direct DNA contacts. Although the p50 linker segment lies closer to DNA than that of RelB, in this complex it adopts a slightly different conformation than in other p50/DNA complexes. The ammonium group of Lys241 is located 4.3 Å from the carbonyl group of T(−1) making the direct contact difficult. However, a paucity of direct protein–DNA contacts around the center of the DNA in this complex does not imply a low affinity. In vitro binding experiments show that the p50/RelB heterodimer binds κB sequences with similar affinities as compared to other NF-κB dimers (data not shown). There is a significant number of positively charged residues located within 5 Å from the DNA which may contribute to the affinity through long-range electrostatic interactions.
Figure 4.
Comparison of the loop 3 conformations. (a) Overlay of the dimerization domains of RelB and p50 reveals difference in their loop 3 conformations. The RelB loop 3 is more helical, which allows more intra-subunit hydrogen bonds between side-chains. (b) A proline at the center of loop 3 breaks the helical structure in p50. (c) Helical conformation in RelB is stabilized by main-chain hydrogen bonds.
Crystallographic contacts between RelB and p50 at sites away from DNA contact sites
The packing arrangement of the complex in the crystal reveals several notable contacts between two p50/RelB:DNA complexes. We discuss here contacts along the b and c axes because of their possible significance in protein–protein interaction in vivo. Crystal contact between the two p50/RelB heterodimers along the b-axis involves the N terminus of RelB (Figure 5(a)). In this case, the penta peptide N-terminal to the RelB RHR and a tetra peptide encompassing residues Asp149 to Gly152 form a surface that interacts with the complementary surface formed by the insert of p50 burying a surface area of 1390 Å2. The interaction site in p50 is formed largely by the loop connecting the two helices. A part of the region interacts with the tetra peptide of RelB and the other part of this segment (Gly162, Tyr163, Gln177 and Ala178) forms a pocket by positioning close to βF-βG loop and βG, which involves residues Phe217, Phe225, Thr226, Arg227 and Arg228. The penta peptide from RelB fits snugly into this hydrophobic pocket of p50 mostly by making van der Waals contacts. Additional hydrogen bonding interactions between RelB (Arg102 and Tyr104) and p50 (Thr226 and Arg228) might also be stabilizing crystal packing (Figure 5(a), bottom). The insert is largest in p50 which folds into a two-helix bundle where the helices are connected by a long loop. In p52, the insert is slightly shorter resulting in the shortening of the loop connecting the helices. In general, the cavity can only be formed by these two members in the NF-κB family (Figure 1(a) and (b)).
Figure 5.
Crystal packing contacts between multiple complexes. (a) Overall view of the packing between RelB and p50 subunits from two different complexes translated one unit cell along the b-axis. (b) Interaction between the two complexes related by a 2-fold axis. A close up view of the same contact surface is shown in two parts. (c) Overall view of the packing interactions along the 2-fold axis. The dimerization domains are shown in grey. (d) Close up view of the same contact surface.
The most dominant packing interaction appears to occur by the stacking of the two unpaired cytosines related by the 2-fold axis forming a pseudo-continuous double helix. Two protein–protein contact sites between p50 from one dimer to RelB of another on the same face as the DNA contact face appear to further stabilize the packing interaction along this side (Figure 5(b)). One of these contact surfaces is predominantly hydrophobic in nature involving the two N-terminal domains. The second contact area is predominantly polar, which involves the dimerization domains of RelB. The hydrophobic interaction is centered at residue Trp167 of RelB and lies in a groove formed by multiple hydrophobic residues of p50, namely, Pro90, Pro119, Pro127, Gly223 and Phe225. In addition to Trp167, Asp166 and Pro168 of RelB are also in close proximity to the hydrophobic surface formed by p50. RelB residues are unique and the p50 residues are shared only by p52 in the NF-κB family. The second contact surface is composed of Gln328 and Asp329 of RelB and Asn75 and Lys76 of p50 (Figure 5(c)). Again these contact residues in both the subunits are unique to the p50/RelB heterodimer. In this packing arrangement, two p50/RelB heterodimers appear to stably interact with each other by binding to two κB sites separated by 1 to 2 bp.
Crystal contacts mimics multi-protein promoter assembly in solution
The packing arrangements along the long c axis prompted us to examine if the p50/RelB heterodimer can indeed cooperate with itself by binding to tandem κB sites, and if such cooperation is RelB-dimer specific. We used HIV-κB sites as a model system where the κB sites are arranged in tandem with 4 bp spacing between them. However, the κB sites are arranged in a direct repeat as opposed to the dyad symmetric arrangement as seen in our structure. Therefore, contrary to the interactions between the p50 and RelB N-terminal domains (NTDs) seen in our structure, two identical NTDs would interact with each other in the direct repeat arrangement. Nevertheless, the same hydrophobic surfaces of RelB and p50 would be involved in the homomeric interactions: the tripeptide (Trp167, Asp166 and Pro168) surface of RelB from one dimer would interact with a RelB surface (similar to the p50 surface seen in our structure) from another dimer. The reciprocal surfaces in p50 NTDs would also be in close proximity and may mediate interactions.
We tested how the p50/RelB heterodimer binding to tandem κB sites varied when spacings between the two κB sites change from 0, 1, 2, 3 and the natural 4 bp. For the electrophoretic mobility shift assay (EMSA) we used pure p50/RelB RHR heterodimer, which also included the entire LZ domain of RelB. We roughly estimated efficiency by visualization of the slower migrating species that corresponds to the ternary complex where two dimers are bound to the tandem κB sites present in the probe (Figure 6). Our EMSA results reveal that ternary complex formation is more efficient when the spacing is 2 bp, which suggests that when the spacing is optimum the hydrophobic patches are involved in the assembly of the two heterodimers on the tandem κB sites. The ternary complex formation is significantly reduced when the spacing is 0 and noticeably reduced when the spacing is 1, 3 and 4 bp.
Figure 6.
EMSA analysis using human immunodeficiency virus-long terminal repeat (HIV-LTR) as probes. (a) HIV-LTR DNA probes sequences. (b) EMSA analysis of recombinant protein using HIV-LTR probes with different spacing as indicated. Single and double arrowheads denote binary and ternary complexes, respectively. (c)–(e) a EMSA analysis of cell lysates using HIV2HIV, HIV4HIV, and HIVMut probes, respectively. For supershift analysis, RelB antibody from Snata Cruz Biotechnology (sc-226) against the RelB C terminus peptide was used. (f) Protein expression check visualized by Western blotting with Flag antibody.
To test the role of the RelB tripeptide (Trp167, Asp166 and Pro168) in the cooperative assembly between two p50/RelB heterodimers, we deleted these three residues and compared the assembly activity of wild-type and mutant heterodimers onto both HIV2HIVand HIV4HIV DNA sequences. Here, we used full length heterodimers with either wt RelB or mutant RelB with the three amino acid deletion expressed in mammalian cells. HEK293T cells were co-transfected with p50 and wild-type RelB or mutant RelB, and relative amounts of the dimers were estimated by Western blot. EMSA does not show any difference in binding of wild-type and mutant heterodimer to a probe containing only a single HIV κB site, suggesting that the deletion does not affect the binary complex formation. Consistent with previous reports, we do not observe DNA binding by RelB alone, whereas p50 alone does bind DNA. The binary and ternary complexes with DNA are supershifted with RelB antibody, which demonstrate that these complexes contain both RelB and p50.
Consistent with in vitro binding experiments, we observe that the assembly of ternary complex with wild-type p50/RelB heterodimers onto HIV2HIV is significantly more efficient as compared to the native HIV4HIV DNA. The mutant p50/RelB heterodimer is significantly defective in the formation of ternary complex on both tandem DNAs. These observations suggest that protein–protein interaction at a site away from the protein–DNA interface has a role in the assembly of multiple dimers onto promoters containing multiple κB sites arranged in tandem.
Discussion
Dimerization and DNA binding by RelB
RelB forms highly specialized dimers with p50 and p52 subunits. The p50/RelB heterodimer is more stable than the corresponding homodimers in vitro, with little or no homodimer of either type observed. Although the broad mechanism of dimerization within the NF-κB family is similar (with the exception of RelB homodimer), the actual nature of interactions differ significantly among the various dimers. p50/RelB and p52/RelB heterodimers can make contacts across the subunit that are not possible in other dimers. However, in the absence of detailed biochemical analysis of various mutants it is difficult to assign the exact contribution of each residue at the interface in the stabilization of the dimer. It is possible that the same residue can have a different effect on the stabilization depending on the nature of the partner subunit. Also, it is important to consider the role played by the residues not present at the interface. Crystallographic analysis showed that RelB forms a domain swapped homodimer, presumably due to sequence changes outside the dimer interface.24 This altered amino acid probably resulted in a destabilized monomer and consequently two monomers could not associate in a normal manner. In contrast, when RelB associates with p50 it forms a regular NF-κB dimer. In this case, strong contacts at the heterodimer interface gained sufficient energy, which is compensated for the lack of RelB domain fold stability. In all, a large number of residues in the dimerization domain play a role in the fine-tuning of NF-κB dimer formation.
The general strategy in DNA recognition is similar for all NF-κB subunits.25,26 Four critical specificity-determining residues (Arg117, Arg119, Tyr120 and Glu123 in RelB) are conserved in all members and make similar contacts in all known complexes. However, the presence of an acidic residue at position 121 instead of a hydrophobic residue as in other cases may influence the binding interaction. In addition, Arg125 is positioned close to DNA and could potentially interact with a longer DNA. The structure of the linker that connects the two domains is significantly different between RelB and other members. A lysine (Lys274 in RelB and Lys241 in p50) from this linker region can make base-specific contacts. In addition to the pivotal proline that positions the entire linker in the complex, the DNA sequence and conformation play a role in determining the position of this lysine, thereby dictating the base it contacts.25 In RelB, this lysine is not located near the DNA. However, it is unclear if the lack of contact by this residue is responsible for dictating the strength of the protein/DNA complex. These observations suggest that all NF-κB dimers are able to recognize target DNA with certain degree of variability in the binding modes and this becomes more pronounced with the variability of target κB sequences.
Assembly of multiple NF-κB dimers onto the tandem κB sites
A large number of NF-κB target genes contain multiple κB sites in their promoters. Requirement of multiple κB sites for transcription have been tested for several cases using both transfection-based and genetic experiments. In all of these studies multiple κB sites have been found to be essential for target gene transcription by NF-κB. Genetic studies so far have provided direct evidence that in the case of IP-10 and MCP-1, the two κB sites are functionally linked and are involved in cross-regulation.5,27 How multiple κB sites are utilized by the NF-κB dimers for target gene transcription is presently unclear. The question of assembly becomes more complex when one considers that in addition to the differences in sequences, the spacing between the κB sites and their orientations are also different. In the case of IP-10, κB sequences direct the assembly of NF-κB dimers in a co-activator-specific manner.5,28 We show that direct interactions between two of the p50/RelB heterodimers are important for the promoter assembly. Complementary surfaces exist in p50 and RelB and these surfaces can be involved in protein–protein interactions. The same molecule can also have surfaces that can complement each other and induce protein–protein interaction. These interactions are presumably weak and are facilitated only when bound to DNA. Our results suggest that depending on the spacing and phasing between the two κB sites, different surfaces could participate in the protein–protein interactions. In case of the HIV promoter, the hydrophobic surface centered at residue Trp167 of RelB is engaged in the interaction with another hydrophobic surface from a second RelB molecule when the spacing between the two κB sites is 2 bp. If two sites are arranged in a symmetric manner then RelB and p50 can mediate similar interactions so long as the spacing between the two sites is 2 bp. These interactions would be less than optimal when inter-site spacing is longer as in the case of the wild-type HIV promoter. When the spacing is less than 2 bp, efficiency of assembly is reduced as the two dimers would have difficulty in occupying both sites comfortably without steric clash.
Our structure also identifies a new protein–protein interaction surface, which involve the LZ domain of RelB and the insert of p50. This surface is highly specific for the p50/RelB heterodimer. However, the DNA must bend nearly 180° in order for the assembly of two p50/RelB heterodimers using these complementary surfaces. Such a severe bending is possible only when two sites are separated by hundreds of base-pairs in which case large DNA looping is possible without costing too much energy. In all, these results allow us to propose a model of how p50/RelB heterodimers can assemble onto promoters containing two κB sites and how the assembly could occur through direct interactions between distinct surfaces. This also explains how the same dimer can be assembled on different promoters where the spacing and orientations between them could be different.
However, it is unclear if thermodynamically stable assembly of transcription activators at the promoter is required for optimal transcription. Transcription might be regulated at the kinetic level where faster on and/or off rates of promoter binding by NF-κB is more suitable for transient assembly and in that case neighboring sites may help that process and in such cases κB orientation and separation between them may not be an essential factor for transcriptional output.29 Further studies are required to show how thermodynamically and kinetically stable activator/promoter complexes regulate transcription.
Materials and Methods
Purification of the p50/RelB heterodimer
Untagged p50 (37–363) was expressed in Escherichia coli and purified in two steps as described. His-tagged RelB(1–400) expressed in E. coli and the protein was partially purified by Nickel affinity chromatography in the presence of denaturant. p50 and RelB were then mixed in the molar ratio of 1:1.1 in denaturing buffer and refolded. The refolded proteins were further purified by cation exchange chromatography (HiTrap S column) where the p50/RelB heterodimer eluted as a single, distinct peak. The peak fractions were pooled, concentrated and stored at −80C.
Crystallization
Our initial attempt was to crystallize the p50 RHR bound to RelB N terminus containing both the N-terminal leucine zipper domain and RHR. However, thrombin cleavage of the RelB histidine tag lead to the loss of the leucine zipper domain due to the presence of a near perfect cleavage site at residue 67 within the domain. Crystals of the complex were grown by hanging drop vapor diffusion method at room temperature where 10 mg/ml complex (in 25 mM Tris–HCl (pH 7.5), 100 mM NaCl and 1 mM DTT) was mixed with 1:1 ratio of reservoir solution which contained 5% (w/v) PEG3350, 100 mM sodium citrate, 0.5% (v/v) β-octyl glucoside, 1 mM spermine and 5 mM DTT.
Data collection and structure solution
Before data collection, crystals were soaked for about 1 min in a cryo-protectant buffer containing the original reservoir solution plus 28% (v/v) glycerol and flash cooled under liquid nitrogen. X-ray diffraction data ware collected at APS ID19 synchrotron source. The diffraction pattern revealed that the crystal belongs to the hexagonal space group P6122 or p6522 with unit cell: a=b=91.44 Å, c=419.97 Å. There is one RelB/p50/DNA complex in the asymmetric unit, with a solvent fraction of 65%. X-ray diffraction data were integrated and scaled using HKL2000.30 The data processing statistics are included in Table 1.
Table 1. Summary of crystallographic analysis.
| Data collection | |
| Space group | P6122 |
| Unit cell (Å) | |
| a | 91.44 |
| b | 91.44 |
| c | 419.97 |
| Resolution (Å) | 3.04(3.14–3.04) |
| I/σ | 11.6(2.51) |
| Completeness (%) | 92(91) |
| Rsymm (%)a | 6.4(51.7) |
| Refinement | |
| No. of reflections | 19,087 |
| No. of protein atoms | 4658 |
| No. of DNA atoms | 442 |
| No. of water molecules | 34 |
| Rcrystal (%)b | 23.8 |
| Rfree (%)c | 27.6 |
| rms deviations | |
| Bond length (Å) | 0.007 |
| Bond angle (°) | 1.38 |
Rsymm=Σ|Iobs−Iavg|ΣIavg.
Rcryst=Σ ‖Fobs|−|Fcalc‖/ΣFobs.
Rfree was calculated with 10% of the data.
The X-ray crystal structure was determined by molecular replacement using AMoRe31 with the p50/p65/DNA(Ig/HIV-1) complex as the search model. The solution was obtained in space group P6122. The molecular replacement solution was top peak with 4.8σ in the rotation function and 19.0σ in the translation function. Rigid body fitting of the solution in AMoRe gave an R factor of 49.1% in the 10.0 Å–3.9 Å resolution range. The orientation and position of this initial model were refined by rigid body refinement in CNS.32 The structure was further refined using minimization and simulated annealing with a maximum likelihood target function and a flat bulk-solvent correction using the CNS system. The residues of p65 subunit were replaced by RelB sequences based on 2FO–FC maps using Xtalview.33 Thirtyfour water molecules were deduced from FO–FC difference electron-density maps and accepted on the basis of hydrogen bond geometry and temperature factor criteria. The progress of the model rebuilding and refinement was monitored using Rfree, which was calculated from a randomly chosen test set comprising 5% of the data. After the group temperature factors were included in the refinement, the R factor was 23.8% and free R factor was 27.6% for the final model. The detailed results of the refinement are included in Table 1.
Protein Data Bank accession code
The coordinates have been deposited into the RCSB Protein Data Base as entry code 2V2t.
Mammalian cell culture and transient protein expression
HEK293T cells were maintained in DMEM (CellGro) supplemented with 10% (v/v) fetal bovine serum (Omega) at 37 °C. Flag-tagged mammalian expression vector was generated by cloning Flag sequence (DYKDDDDK) into pEYFP-C1 vector (Clontech). Mouse p50 and RelB were generated by PCR and cloned into pEYFP-C1-YFP+ NTFlag vector to express N-terminal Flag fusion protein in mammalian cells. HEK293T cells were transfected using Lipofectectamin 2000 (Invitrogen). Cell lysates were prepared 24 h after transfection. Protein expressions were checked by Western blot, and cell lysates were used for EMSA analysis.
Electrophoretic mobility shift assay
The oligonucleotides containing κB sites from different genes used for EMSA were PAGE purified, end radiolabeled with 32P using T4-polynucleotide kinase and [γ-32P]ATP, and annealed to the complementary strands. Binding reaction mixtures contained recombinant p50/RelB heterodimer at different concentrations, binding buffer (10 mM Tris (pH7.5), 50 mM NaCl, 10% (v/v) glycerol, 1% (v/v) NP-40, 1 mM EDTA, 0.1 mg/ml of poly (dI-dC)), and 10000 cpm of 32P-labeled DNA. For EMSA with crude cellular extracts, amounts of wild-type and mutant heterodimers were estimated by WB. We used untrasfected 293T cell extracts to normalization and dilution of the transfected extracts. Reaction mixtures were incubated at room temperature for 20 min and analyzed by electrophoresis on a 5% (w/v) non-denatured polyacrylamide gel at 200 V for 1 h 40 min in 25 mM Tris, 190 mM glycine, and 1 mM EDTA. The gels were then dried, exposed to a phosphorimager, and scanned by Typhoon scanner 9400 from Amershan Bioscience. Gels were quantified by using ImageQuant version5.2 from Molecular Dynamics.
Acknowledgments
We thank Amanda Fusco and Sulakshana Mukherjee for critically reading the manuscript. This work is supported by an NIH grant (CA71871).
Abbreviations used
- RHR
Rel homology region
- aa
amino acid residue(s)
- NTD
N-terminal domain
- EMSA
electrophoretic mobility shift assay
- HIV-LTR
human immunodeficiency virus-long terminal repeat
Footnotes
References
- 1.Baldwin AS., Jr The NF-kappa B and I kappa B proteins: new discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 2.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 3.Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109:S81–S96. doi: 10.1016/s0092-8674(02)00703-1. [DOI] [PubMed] [Google Scholar]
- 4.Karin M, Ben-Neriah Y. Phosphorylation meets ubiquitination: the control of NF-[kappa]B activity. Annu Rev Immunol. 2000;18:621–663. doi: 10.1146/annurev.immunol.18.1.621. [DOI] [PubMed] [Google Scholar]
- 5.Leung TH, Hoffmann A, Baltimore D. One nucleotide in a kappaB site can determine cofactor specificity for NF-kappaB dimers. Cell. 2004;118:453–464. doi: 10.1016/j.cell.2004.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Ruben SM, Klement JF, Coleman TA, Maher M, Chen CH, Rosen CA. I-Rel: a novel rel-related protein that inhibits NF-kappa B transcriptional activity. Genes Dev. 1992;6:745–760. doi: 10.1101/gad.6.5.745. [DOI] [PubMed] [Google Scholar]
- 7.Ryseck RP, Bull P, Takamiya M, Bours V, Siebenlist U, Dobrzanski P, Bravo R. RelB, a new Rel family transcription activator that can interact with p50-NF-kappa B. Mol Cell Biol. 1992;12:674–684. doi: 10.1128/mcb.12.2.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dobrzanski P, Ryseck RP, Bravo R. Both N-and C-terminal domains of RelB are required for full transactivation: role of the N-terminal leucine zipper-like motif. Mol Cell Biol. 1993;13:1572–1582. doi: 10.1128/mcb.13.3.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dobrzanski P, Ryseck RP, Bravo R. Specific inhibition of RelB/p52 transcriptional activity by the C-terminal domain of p100. Oncogene. 1995;10:1003–1007. [PubMed] [Google Scholar]
- 10.Claudio E, Brown K, Park S, Wang H, Siebenlist U. BAFF-induced NEMO-independent processing of NF-kappa B2 in maturing B cells. Nature Immunol. 2002;3:958–965. doi: 10.1038/ni842. [DOI] [PubMed] [Google Scholar]
- 11.Coope HJ, Atkinson PG, Huhse B, Belich M, Janzen J, Holman MJ, et al. CD40 regulates the processing of NF-kappaB2 p100 to p52. EMBO J. 2002;21:5375–5385. doi: 10.1093/emboj/cdf542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Senftleben U, Cao Y, Xiao G, Greten FR, Krahn G, Bonizzi G, et al. Activation by IKKalpha of a second, evolutionary conserved, NF-kappa B signaling pathway. Science. 2001;293:1495–1499. doi: 10.1126/science.1062677. [DOI] [PubMed] [Google Scholar]
- 13.Saccani S, Pantano S, Natoli G. Two waves of nuclear factor kappaB recruitment to target promoters. J Exp Med. 2001;193:1351–1359. doi: 10.1084/jem.193.12.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saccani S, Pantano S, Natoli G. Modulation of NF-kappaB activity by exchange of dimers. Mol Cell. 2003;11:1563–1574. doi: 10.1016/s1097-2765(03)00227-2. [DOI] [PubMed] [Google Scholar]
- 15.Bonizzi G, Bebien M, Otero DC, Johnson-Vroom KE, Cao Y, Vu D, et al. Activation of IKKalpha target genes depends on recognition of specific kappaB binding sites by RelB:p52 dimers. EMBO J. 2004;23:4202–4210. doi: 10.1038/sj.emboj.7600391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huxford T, Malek S, Ghosh G. Structure and mechanism in NF-kappa B/I kappa B signaling. Cold Spring Harbor Symp Quant Biol. 1999;64:533–540. doi: 10.1101/sqb.1999.64.533. [DOI] [PubMed] [Google Scholar]
- 17.Chen FE, Huang DB, Chen YQ, Ghosh G. Crystal structure of p50/p65 heterodimer of transcription factor NF-kappaB bound to DNA. Nature. 1998;391:410–413. doi: 10.1038/34956. [DOI] [PubMed] [Google Scholar]
- 18.Chen YQ, Sengchanthalangsy LL, Hackett A, Ghosh G. NF-kappaB p65 (RelA) homodimer uses distinct mechanisms to recognize DNA targets. Struct Fold Des. 2000;8:419–428. doi: 10.1016/s0969-2126(00)00123-4. [DOI] [PubMed] [Google Scholar]
- 19.Ghosh G, van Duyne G, Ghosh S, Sigler PB. Structure of NF-kappa B p50 homodimer bound to a kappa B site. Nature. 1995;373:303–310. doi: 10.1038/373303a0. [DOI] [PubMed] [Google Scholar]
- 20.Huang DB, Chen YQ, Ruetsche M, Phelps CB, Ghosh G. X-ray crystal structure of proto-oncogene product c-Rel bound to the CD28 response element of IL-2. Structure (Camb) 2001;9:669–678. doi: 10.1016/s0969-2126(01)00635-9. [DOI] [PubMed] [Google Scholar]
- 21.Muller CW, Rey FA, Sodeoka M, Verdine GL, Harrison SC. Structure of the NF-kappa B p50 homodimer bound to DNA. Nature. 1995;373:311–317. doi: 10.1038/373311a0. [DOI] [PubMed] [Google Scholar]
- 22.Cramer P, Larson CJ, Verdine GL, Muller CW. Structure of the human NF-kappaB p52 homodimer-DNA complex at 2.1 Å resolution. EMBO J. 1997;16:7078–7090. doi: 10.1093/emboj/16.23.7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang DB, Huxford T, Chen YQ, Ghosh G. The role of DNA in the mechanism of NFkappaB dimer formation: crystal structures of the dimerization domains of the p50 and p65 subunits. Structure. 1997;5:1427–1436. doi: 10.1016/s0969-2126(97)00293-1. [DOI] [PubMed] [Google Scholar]
- 24.Huang DB, Vu D, Ghosh G. NF-kappaB RelB forms an intertwined homodimer. Structure (Camb) 2005;13:1365–1373. doi: 10.1016/j.str.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 25.Chen FE, Ghosh G. Regulation of DNA binding by Rel/NF-kappaB transcription factors: structural views. Oncogene. 1999;18:6845–6852. doi: 10.1038/sj.onc.1203224. [DOI] [PubMed] [Google Scholar]
- 26.Hoffmann A, Natoli G, Ghosh G. Transcriptional regulation via the NF-kappaB signaling module. Oncogene. 2006;25:6706–6716. doi: 10.1038/sj.onc.1209933. [DOI] [PubMed] [Google Scholar]
- 27.Ping D, Boekhoudt GH, Rogers EM, Boss JM. Nuclear factor-kappa B p65 mediates the assembly and activation of the TNF-responsive element of the murine monocyte chemoattractant-1 gene. J Immunol. 1999;162:727–734. [PubMed] [Google Scholar]
- 28.Ogawa S, Lozach J, Benner C, Pascual G, Tangirala RK, Westin S, et al. Molecular determinants of crosstalk between nuclear receptors and toll-like receptors. Cell. 2005;122:707–721. doi: 10.1016/j.cell.2005.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bosisio D, Marazzi I, Agresti A, Shimizu N, Bianchi ME, Natoli G. A hyper-dynamic equilibrium between promoter-bound and nucleoplasmic dimers controls NF-kappaB-dependent gene activity. EMBO J. 2006;25:798–810. doi: 10.1038/sj.emboj.7600977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Otwinowski Z, Minor W. Processing of x-ray diffraction data collected in oscillation mode. In: Sweet RM, Carter CW, editors. Methods in Enzymology. Academic Press; New York: 1997. pp. 307–326. [DOI] [PubMed] [Google Scholar]
- 31.Navaza J. Implementation of molecular replacement in AMoRe. Acta Crystallog sect D. 2001;57:1367–1372. doi: 10.1107/s0907444901012422. [DOI] [PubMed] [Google Scholar]
- 32.Brunger AT, Adams PD, Clore GM, DeLano WL, Gros P, Grosse-Kunstleve RW, et al. Crystallography and NMR system: a new software suite for macromolecular structure determination. Acta Crystallog sect D. 1998;54:905–921. doi: 10.1107/s0907444998003254. [DOI] [PubMed] [Google Scholar]
- 33.McRee DE. XtalView/Xfit-A versatile program for manipulating atomic coordinates and electron density. J Struct Biol. 1999;125:156–165. doi: 10.1006/jsbi.1999.4094. [DOI] [PubMed] [Google Scholar]