Abstract
Despite compelling evidence for major genetic contributions to the etiology of obsessive-compulsive disorder (OCD), few genetic variants have been consistently associated with this debilitating illness. Molecular genetic studies in children and adolescents with OCD are of particular interest, since early onset of the disease has been observed to be associated with increased familiality. We replicate here for the first time in early-onset OCD patients, a previously reported association of OCD with the common gain-of-function LA allele at the serotonin transporter linked polymorphic region known as 5-HTTLPR in a collection of parent-offspring trios. The present meta-analysis of this recently refined serotonin transporter gene variant revealed further support for the LA allele conferring increased genetic susceptibility to OCD. We conclude that the 5-HTTLPR is currently the single best supported risk variant for OCD, in regards of early-onset OCD, albeit of modest effect size and the possibility that the conferred risk might not be specific to OCD.
Keywords: obsessive-compulsive disorder, serotonin transporter, 5 -HTTLPR, transmission disequilibrium test, meta -analysis
1. Introduction
Obsessive-compulsive disorder (OCD) is a debilitating neuropsychiatric disorder marked by recurring, anxiety-laden intrusive thoughts (obsessions) and repetitive behaviors (compulsions). OCD patients suffer from recurrent, persistent thoughts, ideas, impulses, and behavioural patterns imposing themselves on the patients against their will and perceived by them as distressing and excessive [2]. Obsessive–compulsive symptoms heritability has been estimated to be between 0.27 and 0.47 in adults and between 0.45 and 0.65 in children [22]. One of the most frequently investigated candidate genes for OCD is the serotonin transporter (SLC6A4) with its transcriptional control insertion/deletion polymorphism known as the 5-HTTLPR. Traditionally, 5-HTTLPR alleles have been divided into long (L) alleles with high expressing function and short (S) alleles with low expressing function. Recently the L alleles at the 5-HTTLPR were subdivided into truly high-expressing LA and low-expressing LG alleles, calling for a re-appreciation of previous research [11]. Hu et al. showed that as many as one in three L alleles (depending on ethnicity) are actually low-expressing (LG) and thus functionally equivalent to the short (S) variant [11]. This improved understanding of 5-HTTLPR functionality enabled them to identify a significant association of the gain-of-function LA allele with OCD in an unrelated case-control sample and a collection of trios. Although three subsequent replication attempts were statistically non-significant, all showed increased odds ratio for association of the LA allele with OCD [20,24,28].
While OCD onset occurs most often in adolescence and early adulthood (median age of onset 19 years reported in the National Comorbidity Survey Replication), symptoms can occur already in young children [12]. Early–onset OCD has been associated with higher prevalence of OCD in first-degree relatives, suggesting higher familiality in this group [reviewed in 18,26]. In addition, some differences in gender distribution, the nature of OCD symptoms and the illness course, as well as pattern of co-morbidity are present between early and late-onset OCD [13]. A stratified meta-analysis has pointed towards a possible particular association of the 5-HTTLPR long (L) allele with early-onset OCD and OCD in Caucasians [3]. This finding and the known especially high familiality in early-onset OCD prompted us to further evaluate the role of the refined 5-HTTLPR in a separate sample including only children and adolescents with OCD and both of their biological parents.
Here, we replicate for the first time the originally reported association of the LA allele with OCD [11] in a collection of early-onset OCD trios and further demonstrate a strong association of this allele with OCD in a meta-analysis for the LA allele.
2. Materials and Methods
2.1. Study group and genotyping
We collected a total of 103 trios from OCD-affected children and from both of their biological parents. The study sample included patients who had received treatment for OCD at the Departments of Child and Adolescent Psychiatry at the Universities of Würzburg, Marburg, Freiburg or Technical University of Aachen, according to protocols approved by the local institutional review boards and after obtaining informed written consent. All included patients with OCD were children and adolescents with an age at onset of less than 18 years, and all participants of the included trios were of European ancestry. All OCD probands fulfilled the diagnostic criteria for OCD according to DSM-IV [1]. Criteria for OCD were assessed by interviewing the children and parents with the respective versions of “Diagnostisches Interview bei psychischen Störungen im Kindes- und Jugendalter” (Kinder-DIPS) [21]. Severity and further characteristics of OCD were examined by interviewing the patients with the Children’s Yale-Brown Obsessive Compulsive Scale (CY-BOCS) (with a summary score above 16 points determined to be the cut-off for clinical impairment caused by OCD symptomatology) [16].
Criteria for co-morbid disorders were assessed with Kinder-DIPS, which screens for a wide range of psychiatric disorders in children and adolescents. These include affective-, anxiety-, eating- and tic disorders, attention-deficit/hyperactivity disorder (ADHD), conduct-, oppositional disorder, as well as a screening component for substance use, abuse, psychosis and somatic diseases. Screening for autistic spectrum disorders was carried out with CASCAP-D [4]. Diagnostic assessments of present and lifetime Tourette’s syndrome and tic disorders were performed with the adapted German version [10] of the Child and Adult Schedule for Tourette and Other Behavioral Syndromes (STOBS) [14]. Exclusion criteria were: lifetime history of psychotic disorder, Tourette’s syndrome, autistic disorder, alcohol dependence and mental retardation (IQ<70). Co-morbid disorders in the probands included ADHD (10.9%), mood (affective) disorders (5.8%), anorexia nervosa (2.5%), tics (10.9%, no Tourette’s syndrome), phobias (5.9%) and other anxiety disorders (1.7%). Probands with co-morbid disorders were included in the study only when OCD symptoms predominated. The mean age ± SD of the OCD-affected children was 12.84 ± 2.91 years. Mean age of disease onset ± SD was 11 ± 3.19 years.
Psychiatric disorders in parents of the OCD probands were assessed systematically with the lifetime version of the schedule for affective disorders and schizophrenia (SADS-L) [7]. On average 3.8% of the mothers and 2.1% of the fathers of the probands met the criteria for OCD. Other psychiatric disorders observed in the parents included most commonly anxiety (28.3% in mothers and 12.8% in fathers) and affective (17% in mothers and 19.1% in fathers) disorders. Less frequently diagnosed disorders in the parents were eating disorders, tics, alcohol abuse and ADHD. This collection is an extension and re-evaluation of a previously published, non-significant association analysis of the original (bi-allelic) 5-HTTLPR in OCD [25]. Genomic DNA was extracted from whole blood at the local sites and genotyped as described previously [27] under a protocol approved by the Institutional Review Board of the National Institute of Mental Health Division of Intramural Research Programs in Bethesda, MD (protocol number 96-M-0124). No-template controls and duplicate samples consistently yielded expected results; there were no Mendel errors; founder genotypes did not deviate from Hardy-Weinberg equilibrium (exact test, P = 0.57). The transmission disequilibrium test (TDT) was performed on child-parents trios [17]. The software package PLINK was used for the TDT analysis [15].
2.2. Meta-analysis
Relevant studies were identified by searching the database PUBMED (http://www.ncbi.nlm.nih.gov/pubmed/) for the terms (“obsessive-compulsive disorder” OR “OCD”) AND (“5-HTTLPR” OR “serotonin transporter”). From the search results all published original articles, which investigated the association of the refined 5-HTTLPR with OCD (both early- and late-onset) as of 30th April 2014 were included, and combined with the data from the current study. Since both case-control and family-based studies were included in the meta-analysis, and patient populations were not identical between studies, a random-effects model with the DerSimonian-Laird estimator of between-study variance (T2) was used. The results obtained were very similar using other estimators (e.g. restricted maximum likelihood or empirical Bayes). Variability due to between-study heterogeneity was estimated to be I2 = 29 % (95% CI = 0 to 90; Q(5) = 7.02, p = 0.219). The studies were also analyzed with fixed-effects meta-analyses. Funnel plot and a “trim and fill” analysis were used to assess whether there was any evidence of publication bias [5, 6]. The analysis was conducted with the metafor package in R (www.r-project.org) [23].
3. Results
We observed a significant over-transmission of the LA allele at the 5-HTTLPR to affected offspring in our OCD-affected child-parents trios (transmitted:non-transmitted, 68:39, χ2 = 7.86, df = 1, P = 0.0054, odds ratio 2.06) (Table 1). Our results thus support the notion of increased susceptibility to OCD being conferred by gain-of-function variation within the 5-HTTLPR of SLC6A4.
Table 1.
Transmission disequilibrium test for association of 5-HTTLPR with early-onset OCD.
| Allele | Transmitted | Non-transmitted | p-value |
|---|---|---|---|
| 5-HTTLPR LA allele | 68 | 39 | P=0.0054 |
As a next step, we included these data in a meta-analysis of all published studies of the refined 5-HTTLPR and OCD (Table 2). We conducted random-effects inverse-variance weighted meta-analysis of the effect estimates across all studies, and observed a significant meta-analysis P value of 0.003 for association of the LA allele with OCD (odds ratio 1.33, 95% confidence interval 1.10 – 1.61, Table 2). We used random-effects modeling under the assumption of a distribution of effects given that the studies originate from multiple centers that used different recruitment and ascertainment strategies. Fixed-effects meta-analyses yielded almost identical results (odds ratio 1.31, 95% confidence interval 1.14 – 1.52, P = 0.00021). No significant heterogeneity of effects was detected in the meta-analysis. There was some asymmetry in the funnel plot assessing publication bias (Supplementary Figure S1) and the trim and fill analysis indicated that one study was missing. If the missing study is included in an updated analysis the odds ratio decreases slightly from 1.33 to 1.27 (95% confidence interval 1.03 – 1.56, p = 0.0225), but the overall conclusions remain the same.
Table 2.
Summary and meta-analysis of all published association analyses of the serotonin transporter 5-HTTLPR gain-of-function LA allele and obsessive-compulsive disorder. Black bars in the forest plot represent 95% confidence intervals for odds ratio; the sample size is reflected in symbol size. The sample for the present report consisted of European-ancestry early-onset (< 18 years of age) probands; demographic and phenotypic characteristics for the remaining studies are as follows: Hu et al., 2006 [11] - case-control: European-ancestry individuals, early- and adult-onset probands; Hu et al., 2006 [11] - trios: predominantly European-ancestry families, early- and adult-onset probands; Wendland et al., 2007 [28] - European-ancestry individuals, early- and adult-onset probands; Tibrewal et al., 2010 [20] - Asian-ancestry individuals, early- and adult-onset probands; Voyiaziakis et al., 2011 [24] - primarily European-ancestry families, early-onset probands and at least two affected siblings per family.
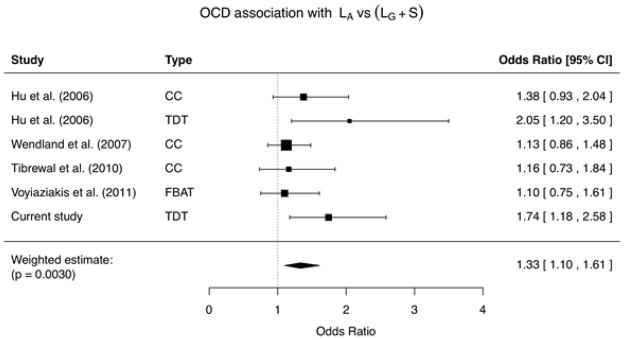
|
Abbreviations: CC, case-control; TDT, transmission disequilibrium test; FBAT, family-based association testing; CI, confidence interval.
4. Discussion
Molecular genetic studies in children and adolescents with OCD are of special interest, because formal genetic studies showed strong relationship between increased familiality and an early onset of OCD. Furthermore, early-onset OCD can be distinguished from later onset OCD by a different pattern of comorbidity and some differences in gender distribution, as well as differences in the nature of OCD symptoms and the illness course [13]. Our trio study in a children and adolescents OCD sample constitutes the first replication of the initial report on the refined 5-HTTLPR and OCD in adults [11].
The L-allele at the 5-HTTLPR has been repeatedly associated with OCD, even though not all studies have replicated these findings [13]. The report of Hu et al., 2006 showing that the 5-HTTLPR L-allele is subdivided into truly high-expressing LA and low-expressing LG alleles, and that the LA allele is associated with OCD, has pointed out the need for a reappraisal of previous association studies results [11]. Although three subsequent reports to the Hu et al. study [11] did not associate the LA allele with OCD at statistical significance [20,24,28], all three studies showed an increased odds ratio for this gain-of-function allele consistent with the original observation and with our results (Table 2). Differences in results between our study and previous replication attempts of the Hu et al. report [11] may be related to phenotypic and/or genotypic heterogeneity, polygenic contribution or additional functional variation within SLC6A4. It has been suggested that younger age of onset may represent a discrete, more aetiological-based OCD subgroup that can be used as a factor to reduce heterogeneity and provide more power for genetic investigation [13]. From the previous replication attempts of the association of the 5-HTTLPR LA allele with OCD, only the study of Voyiaziakis et al. [24] investigated retrospectively an early-onset OCD sample with at least two affected siblings in a large multicenter US family study pedigree design. In contrast, our sample consisted of OCD-affected child-parents trios, including a higher number of sporadic OCD cases. Some differences in symptoms presentation have been described between familial and sporadic early-onset OCD [9] and it is possible that they are related to differences in the gene variants conferring risk to the disease.
A recent meta-analysis, assessing published reports on the association of the refined 5-HTTLPR with OCD, found an association of the 5-HTTLPR LA allele with the disease (odds ratio 1.251, 99% confidence interval 1.048 – 1.492, P = 0.001) [19]. We conducted a meta-analysis combining the previously published data with the results of the present study on an early-onset OCD sample, and confirmed a strong association of the 5-HTTLPR LA allele with OCD. Together, these data suggest that in comparison to other genetic findings the gain-of-function LA allele currently represents the most strongly and consistently associated common susceptibility allele for OCD. The notion of serotonin transporter gain-of-function being a risk factor for OCD is further supported by the rare gain-of-function coding variant known as Ile425Val that is associated with a complex, predominantly OCD-like neuropsychiatric phenotype [29]. Several non-coding SLC6A4 variants, including rs25532 and rs16965628, have been found to modulate 5-HTTLPR functionality. When the presumably higher-expressing alleles at the 5-HTTLPR triallelic polymorphism, rs25532 and rs16965628 were studied together as a haplotype, it was significantly overrepresented in OCD probands, while no significant effect was observed when the three polymorphisms were studied as individual loci. These findings also corroborated the hypothesis of increased serotonin transporter functioning association with OCD [30]. These genetic observations are also in line with the well-documented therapeutic efficacy of selective serotonin reuptake inhibitors (SSRIs), which directly target the serotonin transporter protein encoded by SLC6A4 [8].
Due to the heterogeneity of OCD, studying genetic profiles of OCD in subtypes of the disorder and across different classes of obsessive-compulsive symptoms may provide valuable additional information for genetic predisposition to the disease [13]. Our study replicated for the first time the association of the LA allele with OCD using an early-onset OCD sample. Future studies of risk haplotypes, including the LA 5-HTTLPR, in OCD subtypes, including early-onset OCD, and their possible association with symptoms constellations, may provide additional insight into the genetic predisposition to OCD.
Supplementary Material
Highlights.
We studied triallelic 5-HTTLPR in early-onset obsessive-compulsive disorder (OCD)
103 trios of OCD-affected children and both their parents were investigated
Over-transmission of the LA allele to affected offspring was observed
Meta-analysis confirmed association of the LA allele with OCD
Our data support the 5-HTTLPR LA allele as a risk variant for early-onset OCD
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute of Mental Health (contract MH000336-30) and by the Swiss National Science Foundation (project number 130237). We would like to thank Prof. Steven Taylor for his help with the meta-analysis.
Footnotes
Declaration of interest
Dr. Jens R. Wendland is currently a full-time employee of Pfizer. Prof. Susanne Walitza has received research funding from Vifor Pharma, Switzerland and was on the speakers’ bureau of Eli Lilly, Janssen-Cilag and Astra Zeneca.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association and American Psychiatric Association Task Force on DSM-IV. Diagnostic and statistical manual of mental disorders: DSM-IV-TR. 2000 [Google Scholar]
- 2.American Psychiatric Association and American Psychiatric Association Task Force on DSM-5. Diagnostic and statistical manual of mental disorders: DSM-5. 2013 [Google Scholar]
- 3.Bloch MH, Landeros-Weisenberger A, Sen S, Dombrowski P, Kelmendi B, Coric V, Pittenger C, Leckman JF. Association of the serotonin transporter polymorphism and obsessive-compulsive disorder: systematic review. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:850–858. doi: 10.1002/ajmg.b.30699. [DOI] [PubMed] [Google Scholar]
- 4.Döpfner M, Berner W, Flechtner H, Lehmkuhl G, Steinhausen H. Psychopathologisches Befundsystem für Kinder und Jugendliche (CASCAP-D) Hogrefe Verlag; G̈öttingen: 1999. [Google Scholar]
- 5.Duval SJ, Tweedie RL. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–463. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 6.Duval SJ, Tweedie RL. A nonparametric “trim and fill” method of accounting for publication bias in meta-analysis. J Am Stat Assoc. 2000;95:89–98. [Google Scholar]
- 7.Endicott J, Spitzer RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch Gen Psychiatry. 1978;35:837–843. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- 8.Goddard AW, Shekhar A, Whiteman AF, McDougle CJ. Serotoninergic mechanisms in the treatment of obsessive-compulsive disorder. Drug Discov Today. 2008;13:325–332. doi: 10.1016/j.drudis.2007.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Hanna GL, Fischer DJ, Chadha KR, Himle JA, Van Etten M. Familial and sporadic subtypes of early-onset obsessive-compulsive disorder. Biol Psychiatry. 2005;57:895–900. doi: 10.1016/j.biopsych.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 10.Hebebrand J, Klug B, Fimmers R, Seuchter SA, Wettke-Schäfer R, Deget F, Camps A, Lisch S, Hebebrand K, von Gontard A, Lehmkuhl G, Poustka F, Schmidt M, Baur MP, Remschmidt H. Rates for tic disorders and obsessive compulsive symptomatology in families of children and adolescents with Gilles de la Tourette syndrome. J Psychiatr Res. 1997;31:519–530. doi: 10.1016/s0022-3956(97)00028-9. [DOI] [PubMed] [Google Scholar]
- 11.Hu XZ, Lipsky RH, Zhu G, Akhtar LA, Taubman J, Greenberg BD, Xu K, Arnold PD, Richter MA, Kennedy JL, Murphy DL, Goldman D. Serotonin transporter promoter gain-of-function genotypes are linked to obsessive-compulsive disorder. Am J Hum Genet. 2006;78:815–826. doi: 10.1086/503850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 13.Murphy DL, Moya PR, Fox MA, Rubenstein LM, Wendland JR, Timpano KR. Anxiety and affective disorder comorbidity related to serotonin and other neurotransmitter systems: obsessive-compulsive disorder as an example of overlapping clinical and genetic heterogeneity. Phil Trans R Soc B. 2013;368:20120435. doi: 10.1098/rstb.2012.0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pauls DL, Raymond CL, Stevenson JM, Leckman JF. A family study of Gilles de la Tourette syndrome. Am J Hum Genet. 1991;48:154–163. [PMC free article] [PubMed] [Google Scholar]
- 15.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scahill L, Riddle MA, McSwiggin-Hardin M, Ort SI, King RA, Goodman WK, Cicchetti D, Leckman JF. Children’s Yale-Brown Obsessive Compulsive Scale: reliability and validity. J Am Acad Child Adolesc Psychiatry. 1997;36:844–852. doi: 10.1097/00004583-199706000-00023. [DOI] [PubMed] [Google Scholar]
- 17.Spielmann RS, Mc Ginnies RE, Ewens WJ. Transmission test for linkage disequilibrium: the insulin gene region and insulin-dependent diabetes mellitus (IDDM) Am J Hum Genet. 1993;52:506–516. [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor S. Early versus late onset obsessive-compulsive disorder: evidence for distinct subtypes. Clin Psychol Rev. 2011;31:1083–1100. doi: 10.1016/j.cpr.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 19.Taylor S. Molecular genetics of obsessive-compulsive disorder: a comprehensive meta-analysis of genetic association studies. Mol Psychiatry. 2013;18:799–805. doi: 10.1038/mp.2012.76. [DOI] [PubMed] [Google Scholar]
- 20.Tibrewal P, Kumar HB, Shubha GN, Subhashree D, Purushottam M, Thennarasu K, Reddy YC, Jain S. Association of serotonin transporter gene polymorphisms with obsessive-compulsive disorder (OCD) in a south Indian population. Indian J Med Res. 2010;132:690–695. [PMC free article] [PubMed] [Google Scholar]
- 21.Unnewehr S, Schneider S, Margraf J. Diagnostisches Interview bei psychischen Störungen im Kindes- und Jugendalter. Springer; Berlin, Heidelberg, New York: 1995. Kinder-DIPS. [Google Scholar]
- 22.van Grootheest DS, Cath DC, Beekman AT, Boomsma DI. Twin studies on obsessive–compulsive disorder: a review. Twin Res Hum Genet. 2005;8:450–458. doi: 10.1375/183242705774310060. [DOI] [PubMed] [Google Scholar]
- 23.Viechtbauer W. Conducting meta-analyses in R with the metafor package. Journal of Statistical Software. 2010;36:1–48. [Google Scholar]
- 24.Voyiaziakis E, Evgrafov O, Li D, Yoon HJ, Tabares P, Samuels J, Wang J, Riddle MA, Grados MA, Bienvenu OJ, Shugart YY, Liang KY, Greenberg BD, Rasmussen SA, Murphy DL, Wendland JR, McCracken JT, Piacentini J, Rauch SL, Pauls DL, Nestadt G, Fyer AJ, Knowles JA. Association of SLC6A4 variants with obsessive-compulsive disorder in a large multicenter US family study. Mol Psychiatry. 2011;16:108–120. doi: 10.1038/mp.2009.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walitza S, Wewetzer C, Gerlach M, Klampfl K, Geller F, Barth N, Hahn F, Herpertz-Dahlmann B, Gössler M, Fleischhaker C, Schulz E, Hebebrand J, Warnke A, Hinney A. Transmission disequilibrium studies in children and adolescents with obsessive-compulsive disorders pertaining to polymorphisms of genes of the serotonergic pathway. J Neural Transm. 2004;111:817–825. doi: 10.1007/s00702-004-0134-y. [DOI] [PubMed] [Google Scholar]
- 26.Walitza S, Wendland JR, Gruenblatt E, Warnke A, Sontag TA, Tucha O, Lange KW. Genetics of early-onset obsessive-compulsive disorder. Eur Child Adolesc Psychiatry. 2010;19:227–235. doi: 10.1007/s00787-010-0087-7. [DOI] [PubMed] [Google Scholar]
- 27.Wendland JR, Martin BJ, Kruse MR, Lesch KP, Murphy DL. Simultaneous genotyping of four functional loci of human SLC6A4, with a reappraisal of 5-HTTLPR and rs25531. Mol Psychiatry. 2006;11:224–226. doi: 10.1038/sj.mp.4001789. [DOI] [PubMed] [Google Scholar]
- 28.Wendland JR, Kruse MR, Cromer KR, Murphy DL. A large case-control study of common functional SLC6A4 and BDNF variants in obsessive-compulsive disorder. Neuropsychopharmacology. 2007;32:2543–2551. doi: 10.1038/sj.npp.1301394. [DOI] [PubMed] [Google Scholar]
- 29.Wendland JR, DeGuzman TB, McMahon F, Rudnick G, Detera-Wadleigh SD, Murphy DL. SERT Ileu425Val in autism, Asperger syndrome and obsessive-compulsive disorder. Psychiatr Genet. 2008;18:31–39. doi: 10.1097/YPG.0b013e3282f08a06. [DOI] [PubMed] [Google Scholar]
- 30.Wendland JR, Moya PR, Kruse MR, Ren-Patterson RF, Jensen CL, Timpano KR, Murphy DL. A novel, putative gain-of-function haplotype at SLC6A4 associates with obsessive-compulsive disorder. Hum Mol Genet. 2008;17:717–723. doi: 10.1093/hmg/ddm343. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


