SUMMARY
During development, vertebrates form a progression of up to three different kidneys that are comprised of functional units termed nephrons. Nephron composition is highly conserved across species, and an increasing appreciation of the similarities between zebrafish and mammalian nephron cell types has positioned the zebrafish as a relevant genetic system for nephrogenesis studies. A key component of the nephron blood filter is a specialized epithelial cell known as the podocyte. Podocyte research is of the utmost importance as a vast majority of renal diseases initiate with the dysfunction or loss of podocytes, resulting in a condition known as proteinuria that causes nephron degeneration and eventually leads to kidney failure. Understanding how podocytes develop during organogenesis may elucidate new ways to promote nephron health by stimulating podocyte replacement in kidney disease patients. In this review, we discuss how the zebrafish model can be used to study kidney development, and how zebrafish research has provided new insights into podocyte lineage specification and differentiation. Further, we discuss the recent discovery of podocyte regeneration in adult zebrafish, and explore how continued basic research using zebrafish can provide important knowledge about podocyte genesis in embryonic and adult environments.
Keywords: kidney, podocyte, glomerulus, renal corpuscle, zebrafish, development, regeneration
INTRODUCTION
Overview of Vertebrate Kidney Composition and Development
The kidney is an important excretory and regulatory organ that is essential for animal life. The vertebrate kidney, in particular, is quite complex in its anatomical composition and contains a diverse cellular population. In humans, for example, the kidney is comprised of more than 20 different epithelial and mesenchymal cell types (Reilly, et al., 2007). During embryogenesis, the renal lineages derive from the intermediate mesoderm (IM) and can be divided broadly into parenchymal (functional) and stromal (supporting) cells (McCampbell and Wingert, 2012). Cooperation between these cell types enables the kidney to perform sophisticated physiological functions that include blood filtration, nitrogenous waste excretion, reabsorption of metabolites, regulation of acid-base levels, maintenance of osmotic balance, and the secretion of hormones (Reilly, et al., 2007).
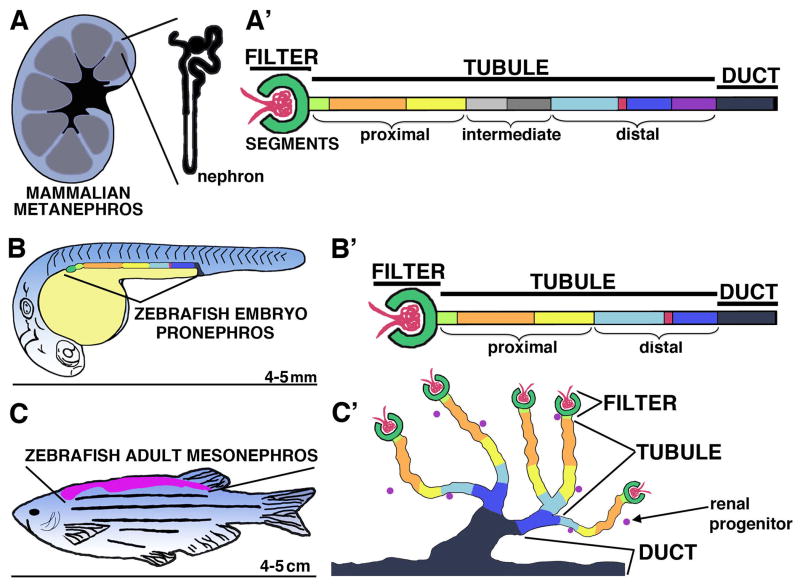
Vertebrates sequentially make as many as three different renal structures during development: the pronephros, mesonephros and metanephros (Saxen, 1987). Each kidney is subsequently more intricate, and once formed the previous structure is degraded or in some cases partially remodeled to help fashion other genitourinary structures (Saxen, 1987). Despite the architectural variations among kidneys, they are composed of excretory units termed nephrons (Saxen, 1987; Dressler, 2006; Wingert and Davidson, 2008). Nephrons cleanse the blood by gathering metabolic waste, and modify the solutions they collect to regulate water and electrolyte balance. Nephrons are tissue pipes constructed from epithelial cells, and typically have three major parts: a blood filter, a tubule, and a duct (Figure 1). During nephrogenesis, renal progenitors are patterned to form these parts, which are further organized into segments with cells that have unique ultrastructures, gene expression profiles and functional roles (Reilly et al., 2007; Wingert and Davidson, 2008). When plasma is gathered from the bloodstream by the filter, it enters the proximal end of the tubule and flows distally, during which time this filtrate is modified via active reabsorption and secretion of solutes by the tubular epithelium, then passes through a collecting duct where final refinements of electrolytes occurs. Nephrons reabsorb salts and other nutrients such as amino acids needed by the organism to maintain life, as the filter does not discriminate between essential materials and unwanted metabolic by-products. Nephron segment composition and organization have been conserved among vertebrates, likely due to the importance of these functions (Figure 1) (Reimschuessel, 2001). The molecular basis for segment patterning is not well understood (Constantini and Kopan, 2010; Little and McMahon, 2012), but ongoing research using animal models continues to provide new insights into nephrogenesis. Here, we address the utility of the zebrafish model to study the formation of a critical cell type, the podocyte, which is located at the blood filter.
FIGURE 1. Nephron segmental anatomy is conserved among vertebrates.
(A) Schematic of a mammalian metanephros with (A′) a linear diagram of nephron segments. (B) Schematic of a zebrafish embryo with lateral location of the pronephros indicated, and with (B′) a linear diagram of the nephron segments. The zebrafish embryo is approximately 4 mm long (tip to tail) by 5 dpf, and continues to utilize the pronephros as the fish grows. (C) Schematic of a zebrafish adult with dorsal location of the mesonephros, and with (C′) a diagram depicting the arborized arrangements of nephrons with common duct exitways. The adult zebrafish is typically 4–5 cm in length (tip to tail). Analogous nephron segments are color-coded, with the vasculature (red ball), podocytes (dark green), neck (light green), proximal tubule segments (orange, yellow), intermediate tubule segments (gray), distal tubule segments (light blue, dark blue, purple) with intervening macula densa (mammals) or corpuscle of Stannius (fish) (red), and finally the duct (black). [Reprinted from Transl Res, 163(2), McCampbell K, Wingert RA, New Tides: using zebrafish to study renal regeneration, Pages No. 109–122, Copyright 2014, with permission from Elsevier.]
Anatomy of the Nephron Blood Filter and the Role of Podocytes in Kidney Disease
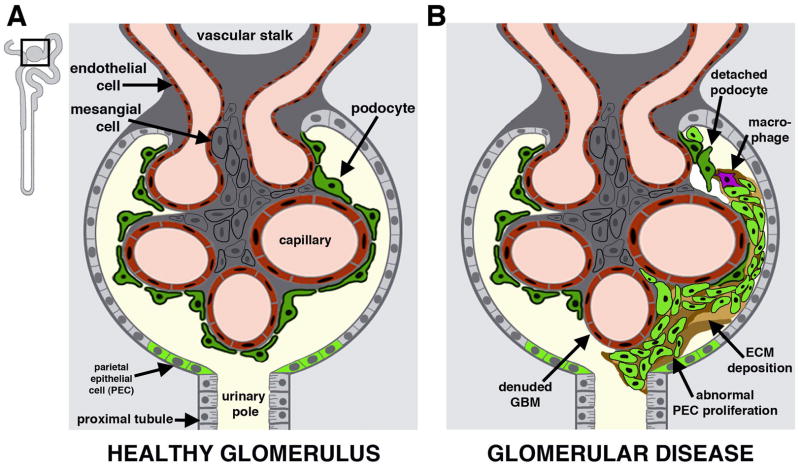
The nephron blood filter, also known as the renal corpuscle, connects the vascular and excretory systems (Figure 2A). Each blood filter is comprised of a capsule (Bowman’s capsule or capsula glomeruli) made up of parietal epithelial cells that surround a structure known as the glomerulus. The glomerulus is composed of vascular endothelial cells, mesangial cells, and podocytes (Figure 2A) (Mundel and Kriz, 1995; Kriz and Kaissling, 2007). At the glomerulus, the filtration sieve for the blood has three layers: the fenestrated endothelium, an intervening trilaminar glomerular basement membrane (GBM), and the podocytes (Reilly et al., 2007). Fluid from the circulation passes through the fenestrated cell membrane of the endothelial cells, across the GBM and then through the podocyte network by moving through the spaces between neighboring cells. Podocytes have extensive cell extensions known as foot processes which adhere to the GBM and interdigitate with the foot processes of adjacent podocytes. Foot processes are connected via junctional complexes known as slit diaphragms. Slit diaphrams are comprised of elaborate assemblies of adhesion proteins (e.g. Nephrin, Fat1) located at the cell surface that connect with underlying adaptor proteins (e.g. Podocin, CD2AP) and cytoskeletal associated proteins (e.g. Synaptopodin, α-Actinin 4), which link to the cytoskeleton (Deen, 2004; Patrakka and Tryggvason, 2010). The slit diaphragm has a filtration space that is 25–60 nm wide, which creates a barrier that impedes the passage of circulating macromolecules the size of albumin or larger (e.g. larger than approximately 70 kDa), but permits the passage of molecules including amino acids, sugars, and electrolytes that are recovered by the activities of the downstream tubular cells (Rodewald and Karnowsky, 1974; Ryan, 1981; Wartiovaara et al., 2004; Ha, 2013). Thus, the sieve is a selective mesh that keeps circulating cells and large protein complexes in the bloodstream while channeling a liquid filtrate with small molecules into the nephron.
FIGURE 2. Composition of the nephron blood filter.
(A) Healthy blood filter with intact glomerular components. Inset depicts nephron outline and boxed area enlarged in panel. (B) Maladaptive response to podocyte attrition, in which activated parietal epithelial cells proliferate and fill the capsule, impeding flow to the nephron tubule and leading to nephron atrophy. [Figure adapted from Clin Transl Med, 2(1), Li Y, Wingert RA, Regenerative medicine for the kidney: stem cell prospects and challenges, Pages 1–16, doi: 10.1186/2001-1326-2-11, Copyright 2013, permission through the creative commons license.]
Both the podocytes and the GBM are essential for the proper function and integrity of the sieve. For example, perturbations that lead to foot process effacement from the GBM can cause the loss of podocytes. If rare podocytes are dislodged and excreted in healthy individuals, residual podocytes hypertrophy to fill the unoccupied GBM (Kriz 2012). Reestablishment of the glomerular barrier is essential to keep high molecular weight compounds and cells from entering the nephron and causing damage. Genetic or environmental defects that more broadly disrupt podocytes or the production of GBM components lead to chronic renal problems due to subsequent alterations and/or damage to the renal corpuscle and tubule cells (Chiang and Inagi, 2010; Chen and Miner, 2012; Leeuwis, et al., 2010). Clinically, this is evidenced by proteinuria, or the presence of proteins in the urine. Such glomerular disruptions can lead to maladaptive responses, in which local epithelia proliferate and form cellular lesions within the capsule (Smeets and Moeller, 2012). Glomerular scars can block filtrate entry into the tubule, trigger inflammation, and lead to nephron degeneration (Figure 2B) (Kriz and LeHir, 2005). Unfortunately, there are no clinical methods to heal podocyte damage, and the progressive loss of nephrons leads to end stage renal disease (ESRD), where the majority of kidney function fails. Typically, ESRD is diagnosed when patients have lost 85–90% of their renal function. At present, the treatments for kidney failure are dialysis or organ transplant. These renal replacement therapy (RRT) interventions are life saving but still associated with high mortality rates. Further, they necessitate long-term medical care and thus have high socioeconomic burdens for patients and their families. Additionally, the availability of RRT is limited across the world, being initiated in less than 25% of patients with renal failure in developing countries (Ortiz, et al., 2014). Therefore, the identification of effective and cost-effective ways to implement treatments for the underlying causes of glomerular disorders, such as to promote podocyte regeneration, has been the subject of intense research focus.
While knowledge about podocyte formation has significant implications, the molecular and genetic pathways that orchestrate this process are only somewhat understood. In recent years there have been marked advancements in knowledge about podocyte development, some emerging from zebrafish studies, though many mechanistic aspects have yet to be uncovered (Quaggin and Kreidberg, 2008). For example, how podocytes are specified during renal development has remained largely a black box. Interestingly, some findings have suggested that podocyte stem cells may exist in mammals (Li and Wingert, 2013). The hypothesis of podocyte stem cells provided an attractive explanation to account for podocyte loss over the long-term (rather than compensation by hypertrophy alone), and was suggested to explain the origin of glomerular scars—with the idea being that scars emerge when podocyte stem/progenitor cells exhibit uncontrolled behavior (Lasagni and Romagnani, 2010). However, data obtained from murine genetic fate mapping studies now reputes the notion of podocyte regeneration from a stem cell pool in the adult mouse (Berger, et al., 2014). Interestingly, other vertebrates like zebrafish can regenerate podocytes during adulthood (Zhou and Hildebrandt, 2012; Huang, et al., 2013a). In this review, we provide an overview of the approaches that enable nephrology research with zebrafish, discuss how zebrafish genetic studies have provided insights into podocyte development during embryogenesis, and explore how zebrafish are being used to study podocyte regeneration.
THE ZEBRAFISH MODEL AND APPLICATIONS FOR KIDNEY RESEARCH
Zebrafish are a genetically tractable system to study vertebrate development and regeneration
Zebrafish are small freshwater teleosts first used for toxicology studies (Laale, 1977) that have since become a widespread biomedical research model (Lieschke and Currie, 2007) due to both their genetic tractability (Driever, et al., 1996; Haffter, et al., 1996; Haffter and Nüsslein-Volhard, 1996) and high degree of genetic conservation with humans (Howe, et al., 2013). Zebrafish have many characteristics that have promoted their popular scientific use (Lieschke and Currie, 2007; Santoriello and Zon, 2012; Pickart and Klee, 2014). Zebrafish can be kept in minimal space, which decreases the cost of maintenance of these animals. Additionally, they have large clutch sizes and can reproduce frequently, increasing the rate of experimentation. The external fertilization of their embryos allows for easy visualization of development, which is further enhanced because the embryos and larvae are largely transparent. This translucency is ideal for analysis of gene and protein expression by methods such as in situ hybridization and immunofluorescence, as well as the direct visualization of tissues in transgenic strains. Another key feature of zebrafish is that the majority of organs are formed by 24–72 hours post fertilization (hpf) (Kimmel et al., 1995). This rapid embryogenesis expedites research pursuits, increasing the rate at which data may be obtained as compared to other vertebrates.
To date, numerous genetic and molecular tools have been adapted from other model organisms or devised specifically for zebrafish experimentation. Organized community efforts have centralized information and many reagents are available through the Zebrafish Model Organism Database (ZFIN) and the Zebrafish International Resource Center (ZIRC) (Bradford, et al., 2011). Some of the most predominant methods used to gain information are through the use of forward and reverse genetic approaches (Lawson and Wolfe, 2011), as well as chemical genetics (Lessman, 2011). Large-scale forward diploid genetic screens have led to the identification of many mutants that recapitulate human congenital disorders (Driever, et al., 1996; Haffter et al., 1996; Amsterdam and Hopkins, 2006), and alternate strategies such as those using haploids enable moderate genome coverage in small-scale screening efforts (Kroeger, et al., 2014). While the identification of chemically-induced genetic lesions by positional cloning with meiotic mapping has historically been laborious (Zhou and Zon, 2011), the advent of next-generation sequencing technologies have enabled whole-genome sequencing (WGS) and whole-exome sequencing (WES) (Gupta et al., 2010; Bowen et al., 2012; Leshchiner et al., 2012; Obholzer, et al., 2012; Voz et al., 2012; Kettleborough, et al., 2013; Ryan et al., 2013). Mutants identified through forward or reverse screens have been further studied by implementing chemical screens to rescue or exacerbate the phenotype (Peterson, et al., 2010; Peterson and Fishman, 2011).
Genes of interest can also be further studied using gene knockdown technologies, of which several have been developed in zebrafish. Of these, morpholino technology has been the most widely used and is relatively successful, though still hindered by off-target and other secondary effects. Morpholinos are antisense oligonucleotides that inhibit protein production by blocking translation or inhibiting splicing of RNA targets, and can also be used to block other RNA targets such as microRNAs (Nasevicius and Ekker, 2000; Lan, et al., 2011). They are typically microinjected into the 1–2 cell stage embryos and can continue to knockdown the target for several days. Additionally, morpholino experiments have also been performed in adults using electroporation to facilitate cell uptake (Thummel et al., 2006; Thummel et al., 2008) and there are modified in vivo morpholinos that can circumvent the need for electroporation into the target tissue (Moulton and Jiang, 2009; Kim et al., 2010; Chablais, et al., 2010).
Recent advances in genome editing have provided a bevy of new avenues for reverse genetics in zebrafish, whereby one can selectively introduce mutations into a chromosomal target of interest and generate stable mutant lines for study. These tools include zinc finger nucleases, transcription activator-like effect nucleases (TALENs), and CRISPR-Cas based RNA-guided endonucleases (Gaj, et al., 2013; Auer and Del Bene, 2014; Cheng and Wingert, 2014). Each method involves the microinjection of engineered materials into the 1-cell stage embryo to introduce heritable genetic changes during embryogenesis. Zinc finger nuclease technology induces DNA cleavage in a sequence-specific fashion, but creation of the reagents can be laborious (Meng, et al., 2008; Urnov et al., 2011). In contrast, TALENs and CRISPR-Cas techniques enable relatively straightforward creation of genome editing tools (Li et al., 2011).
Conversely to gene knockdown, overexpression of genes can also be implemented in the zebrafish model system by injecting mRNAs into early embryos. Among other experiments, this technique is of extreme importance as it can accomplish a rescue in mutant or knockdown lines. Rescues are integral in validating positional cloning studies and confirming the specificity of knockdown reagents like morpholinos. Alternatively, global or localized overexpression can be performed using transgenics with the desired combinations of ubiquitous or tissue specific promoters, or controlled temporally with heat shock inducible promoters (Scheer and Campos-Ortega, 1999). Other inducible systems include the Cre-lox, Mifepristone-LexPR and the Gal4-UAS systems, which can control genes spatially and/or temporally (Suster et al., 2009a; Emelyanov and Parinov, 2008; Mosimann and Zon, 2011; Scott et al., 2007).
The ability to create transgenic lines has also been critically important in zebrafish for lineage analysis, cell labeling and other manipulations. Transgenic animals that have genetically incorporated fluorescent markers allows for visual tracking of the labeled tissue(s). In fact, the optical clarity of the embryo and larvae increase the visibility of low levels of fluorescence compared to other vertebrates. Transgenesis in zebrafish was revolutionized with the tol2 transposon system (Suster et al., 2009a) and BAC transgenesis using tol2, the latter of which has circumvented the challenges of working with lengthy promoter sequences (Suster et al., 2009b). Additionally, transgenics are now used to perform targeted cell, tissue, or organ ablation by creating strains that express bacterial nitroreductase (NTR), which in the presence of the prodrug metrodinazole (Mtz) produces a cytotoxic compound that triggers cell death (Curado, et al., 2007, 2008). Taken together, these techniques have created a diverse molecular toolkit for zebrafish developmental and regenerative studies, and been increasingly employed for nephrology studies.
Kidney research using the zebrafish: a prime model for podocyte biology
The zebrafish model can be used to study nephrogenesis both during development and adult life (Gerlach and Wingert, 2013; Cheng and Wingert, 2014; Cheng, et al., 2014; Li, et al., 2014; Marra and Wingert, 2014), as well as to study the regeneration of renal cell types (Johnson, et al., 2011; McCampbell and Wingert, 2014). Zebrafish utilize a pronephros for renal activity during embryonic and early larval stages, followed by a mesonephros that functions during late larval and adult stages (Drummond, et al., 1998; Drummond, 2003; Drummond, 2005). Unlike higher vertebrates, zebrafish do not develop the third metanephric kidney. Nevertheless, nephrons in both zebrafish kidney forms possess a similar segmental organization as other vertebrates (Figure 1) (Wingert et al., 2007; Diep, et al., 2011; McCampbell, et al., 2014). These broad similarities include a blood filter comprised of podocytes, a tubule with a series of proximal and distal segment domains, and a terminal duct, as further discussed in the following paragraphs (Figure 1).
Zebrafish form a simple pronephros composed of a pair of nephrons by 24 hpf, and the nephrons begin to function by 48 hpf (Figure 1B) (Kimmel et al., 1995; Drummond, et al., 1998). At the rostral end of the zebrafish is a single glomerulus, containing podocytes, where these nephrons meet. Following the common glomerulus, each nephron contains the following segments: a neck (N), proximal convoluted tubule (PCT), proximal straight tubule (PST), distal early (DE), corpuscle of Stannius (CS), distal late (DL), pronephric duct (PD), and terminates at the cloaca (Wingert et al., 2007). The pronephric nephrons mirror the archetypal segment composition present in mammals, though some differences have been appreciated. One difference is the absence of a thin limb segment in zebrafish (Wingert et al., 2007). The thin limb is involved in water reabsorption in mammals (Reilly, et al., 2007) and is held to be unnecessary in zebrafish as they are fresh water organisms with no need to conserve water or concentrate urine (Wingert and Davidson, 2008). Further, a neck segment is not found in nephrons of all mammals (e.g. humans) but it is present in some species (e.g. rabbits) (Reilly, et al., 2007). Finally, the CS is unique to bony fishes and regulates calcium and phosphate levels (Krishnamurthy, 1976; Bonga and Pang, 1992; Butler et al., 2003; Schein, et al., 2012), though it has been speculated that there may be an evolutionary link between the CS and the mammalian macula densa (Wingert and Davidson, 2008).
The zebrafish pronephros is functional for the first several weeks of larval life, and over this time a mesonephros is constructed. The mesonephros assembles gradually, as clusters of renal progenitors located near the pronephros undergo proliferation and morphogenesis to make more nephrons (Zhou et al., 2010; Diep et al., 2011). During the early stages of mesonephros development, the new nephrons emerge in close proximity to the pronephros and form connections to one of these original nephrons (Zhou et al., 2010; Diep et al., 2011). Over time, the nephron networks become increasingly elaborate as more units are added. Ultimately, the mesonephric kidney will contain several hundred nephrons, and persists as the adult kidney (Zhou et al., 2010; Diep et al., 2011). Mesonephric nephrons have a similar segmental organization as the pronephros, with glomeruli composed of podocytes and tubules that contain proximal and distal segments (Figure 1C) (Zhou et al., 2010; Diep et al., 2011; Gerlach, et al., 2011; McCampbell, et al., 2014). Generation of the mesonephric nephrons activate genes that are used in pronephros ontogeny (Zhou et al., 2010; Diep et al., 2011), though overall this is an under-scrutinized topic.
Interestingly, nephrogenesis occurs throughout the life of the zebrafish, likely due to the continued growth of the organism and the subsequent necessity to meet a higher demand for waste excretion (Zhou et al., 2010; Diep et al., 2011; Davidson, 2011; McCampbell and Wingert, 2014). Further, zebrafish undergo nephron epithelial regeneration and de novo nephron formation, termed neonephrogenesis, after experiencing kidney damage (Zhou et al., 2010; Diep et al., 2011). Numerous fish species, including goldfish, dogfish, skate, and medaka, likewise can regenerate damaged nephrons and undergo neonephrogenesis (Hentschel, 1988; Reimschuessel, et al., 1990; Reimschuessel and Williams, 1995; Augusto, et al., 1996; Salice, et al., 2001; Reimschuessel 2001; Elger, et al., 2003; Watanabe, et al., 2009). These capabilities are not present in higher vertebrates, whose response to acute kidney damage is limited to tubule epithelial regeneration, and upon chronic damage undergo scar formation that can lead to advancing nephron loss and end stage renal disease (Li and Wingert, 2013). Several damage paradigms are used in model organisms to better understand kidney tissue changes (McCampbell and Wingert, 2014). A common method used in fish is intraperitoneal injection of the aminoglycoside antibiotic gentamicin sulfate, which is a nephrotoxicant that causes proximal tubule cell death (Reimschuessel 2001; McCampbell and Wingert, 2014). More recently, transgenic injury models have been developed to selectively ablate podocytes using the NTR-Mtz system to study regeneration of this cell type throughout the zebrafish lifespan (Zhou and Hildebrandt, 2012; Huang et al., 2013a).
Given the consequences of podocyte health for overall renal function, understanding podocyte genesis in development and regeneration has vital importance. One significant feature common to the zebrafish pronephric and mesonephric kidney is the conservation of podocyte ultrastructure and gene expression characteristics with higher vertebrates including humans (Drummond, 2003; Kramer-Zucker, et al., 2005a; Ebarasi, et al., 2011). For example, zebrafish podocytes show extended foot processes with interdigitating foot processes in embryonic and adult nephrons based on transmission electron micrograph studies (Drummond, et al., 1998; Zhou and Hildenbrandt, 2012; Huang, et al., 2013a). Creation of the podocyte-specific reporter zebrafish line Tg(podocin:GFP) enabled 3-dimensional ultrastructural analysis based on scanning electron micrograph visualization of pronephric podocytes, which further emphasized their similarities with mammals (He, et al., 2011). Further, the podocytes sit on a trilaminar GBM with opposing capillary endothelial cells (Drummond, et al., 1998; Zhou and Hildenbrandt, 2012; Huang, et al., 2013a). Gene expression is conserved with other vertebrates during podocyte lineage development, with the expression of similar transcription factors in progenitors (e.g. wt1a, pax2a) (Drummond, et al., 1998), and later the expression of genes that encode slit diaphragm components as podocytes differentiate (e.g. nephrin, podocin) (Kramer-Zucker, et al., 2005a; Zhou and Hildenbrandt, 2012; Huang, et al., 2013a).
The simplicity of the zebrafish pronephros and its rapid development over the first two days of embryonic life have facilitated research using this kidney to delineate mechanisms of podocyte formation (Gerlach and Wingert, 2013) and study the genetic regulation of the glomerular filtration barrier (Hentschel, et al., 2007; Hanke, et al., 2013). As mentioned, transgenic models are now available to study podocyte regeneration in both embryonic and adult zebrafish. In the subsequent sections, we narrow the focus of this review to discuss what is known about podocyte formation during zebrafish pronephros development, and then the new models of inducible podocyte damage and regeneration.
DEVELOPMENT OF THE PODOCYTE LINEAGE IN THE ZEBRAFISH PRONEPHROS
Podocyte progenitor specification and early differentiation
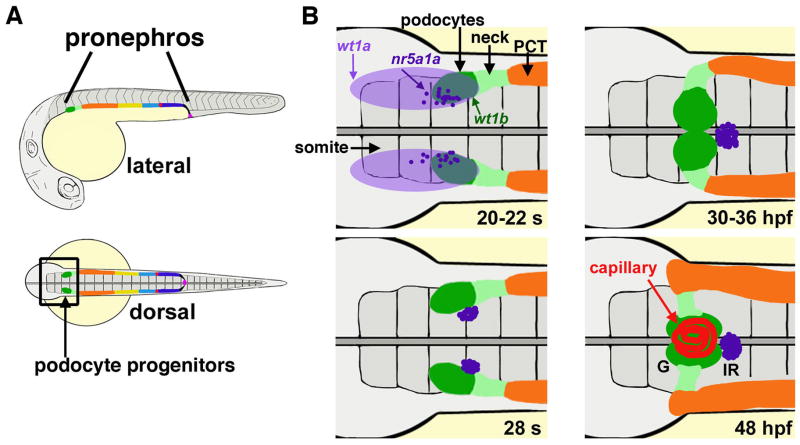
The pronephros, including the podocyte lineage, forms over the first day of development from bilateral stripes of renal progenitors that emerge from the intermediate mesoderm (Drummond 2003; Gerlach and Wingert, 2013). By the conclusion of embryonic gastrulation and the onset of somitogenesis (at ~10 hpf), the renal progenitors are distinguished by the expression of several transcription factors, among them pax2a, pax8, and lhx1a (Krauss, et al., 1991; Toyama and Dawid, 1997; Drummond, et al., 1998; Picker, et al., 2002; Swanhart, et al., 2010). A rostral region of the renal progenitor field expresses the Wilms tumor-1 gene paralog wt1a, though wt1a transcripts are not specific to renal progenitors and instead are found in a broad domain of the anterior trunk (Figure 3) (Drummond, et al., 1998; Serluca and Fishman, 2001; Bollig et al., 2006; Wingert, et al., 2007). A subset of the wt1a-expressing cells located next to somite 3 are fated to become the podocyte progenitors (Bollig et al., 2006; O’Brien, et al., 2011). By the 15 somite stage, the podocyte progenitors are specifically demarcated by expression of a second Wilms paralog, wt1b (Bollig et al., 2006), and also express pax2a, lhx1a, and the Notch effector hey1 (O’Brien, et al., 2011). By 24 hpf, the podocytes begin to express genes that encode components necessary to build the slit diaphragm, such as nephrin (Kramer-Zucker, et al., 2005a; O’Brien, et al., 2011). The podocytes mature and undergo morphogenesis over the next day of development to make the glomerulus, which begins blood filtration at ~48–50 hpf (discussed in a subsequent section).
FIGURE 3. Zebrafish podocyte lineage specification and glomerular development.
(A) The zebrafish pronephros contains podocytes (dark green) at the rostral-most position. (B) Developmental timecourse of the cell populations that develop in proximity to podocytes. Gene expression of wt1a (light purple) is broad, while wt1b transcripts (dark green) are restricted next to somite (s) three, and interrenal precursors marked by nr5a1a transcripts (dark purple) are interspersed in this region. The neck (light green) is located caudal to the podocytes, followed by the proximal convoluted tubule (PCT, orange). Cell movements between the 20 somite stage to 48 hours post fertilization (hpf) lead to formation of a single glomerulus (G) with central capillary nexus (red). The interrenal gland (IR) (dark purple) is situated just caudal to the glomerulus. [Figure adapted from Wiley Interdiscip Rev Dev Biol, 2, Gerlach G, Wingert RA, Kidney organogenesis in the zebrafish: Insights into vertebrate nephrogenesis and regeneration, Pages 559–585, Copyright 2013, with author permission].
wt1a and wt1b
Based on morpholino knockdown studies, wt1a is essential for normal podocyte specification in the zebrafish pronephros, but the roles of wt1b are somewhat less clear. Knockdown of wt1a leads to defective glomerulus development (Hsu, et al., 2003; Perner et al., 2007; O’Brien, et al., 2011). In the absence of wt1a activity, the number of podocytes that form is reduced. Expression of wt1a is necessary for the expression of numerous podocyte genes. For example, the podocyte progenitors in wt1a morphant embryos show weak expression of wt1b transcripts, but they do not proceed in differentiation, failing to express slit diaphragm components like nephrin, podocin, and podocalyxin (Hsu, et al., 2003; Perner et al., 2007; O’Brien, et al., 2011).
Thus far, the molecular changes that result from wt1b knockdown have been sparsely characterized. In contrast to wt1a, wt1b function is dispensable for the expression of podocyte genes surveyed to date although this list is rather short (Perner et al., 2007; O’Brien, et al., 2011). wt1b knockdown has been associated with pronephric cyst formation and renal failure in approximately 70% of morphants (Perner et al., 2007). As this was not linked with overt changes in podocyte specification or the expression of podocin or nephrin transcripts, alterations in subsequent maturation events might underlie the knockdown phenotype (Perner et al., 2007). However, dual wt1a/wt1b knockdown has been reported to result in similar outcomes as wt1a knockdown alone, providing contradictory data that suggest the role(s) of wt1b are redundant with wt1a (O’Brien, et al., 2011). Given the limitations of morpholino-based investigations, further studies are needed to clarify the activities of wt1b during pronephric development and this analysis may be best served by utilizing genome-editing based approaches.
Regulation of wt1a expression by retinoic acid (RA)
The emergence of podocyte progenitors that express wt1a and wt1b is reliant on normal RA signaling in the embryo. RA is a secreted morphogen that is well known to exert dose dependent effects on the patterning of many developing tissues and organs, where RA levels are controlled by the sources of RA biosynthesis and degradation (Duester, 2008). During zebrafish pronephros development, RA is required broadly to define proximal segment identities along the renal progenitor field (Wingert et al, 2007; Wingert and Davidson, 2011). Chemical genetics experiments in which RA biosynthesis was blocked by treating embryos with an inhibitor of aldehyde dehydrogenase (aldh) enzymes, known as diethylaminobenzaldehyde (DEAB), led to the formation of pronephros tubules that lacked podocytes (Wingert et al, 2007; Wingert and Davidson, 2011). Further, defects in aldh1a2 expression, either via morpholino knockdown or in aldh1a2 genetic mutants, is associated with a reduction in podocytes that can be rescued by exogenous RA treatment (Wingert et al, 2007; Wingert and Davidson, 2011).
The major source of RA that patterns the renal progenitors is the adjacent paraxial mesoderm that gives rise to the embryonic somites. Knockdown of tbx16, a T-box transcription factor required for normal paraxial mesoderm formation (Amacher, et al., 2002; Morley, et al., 2009), dramatically reduces aldh1a2 expression in the somites and is associated with reduced podocytes (Wingert and Davidson, 2011). While this tbx16 knockdown analysis links pronephros patterning to normal paraxial mesoderm development, other sources of RA could still be involved in renal progenitor specification and patterning (Wingert and Davidson, 2011). Importantly, the effects of RA occur early in development, similar to the developmental period when tbx16 is active, and the effects are dose-sensitive (Wingert et al, 2007; Wingert and Davidson, 2011).
Further research has provided strong evidence that RA is directly responsible for the level of wt1a expression in the pronephros (Bollig et al., 2009). RA signaling occurs when RA binds to heterodimers of the retinoic acid receptor (RAR) and retinoic X receptor (RXR) transcription factors (Duester, 2008). Analysis of the upstream region of wt1a in zebrafish revealed the presence of an enhancer that contains a predicted RXR heterodimer binding site (Bollig et al., 2009). In addition, this enhancer is conserved at the human Wt1 locus (Bollig et al., 2009). Through in vitro electrophoretic mobility shift assays, both the zebrafish wt1a and human Wt1 enhancers could bind RAR/RXR proteins (Bollig et al., 2009). Finally, this enhancer was found to control zebrafish pronephric wt1a expression in vivo based on experiments in which transgenic wt1a:GFP reporter fish were treated with exogenous RA (Bollig et al., 2009). Taken together, these data show the importance of RA in the specification of podocytes, and strongly suggest that RA promotes podocyte formation by activation of wt1a expression in the zebrafish pronephros. The conservation of the retinoid binding sites in the human Wt1 enhancer suggests that RA could regulate Wt1, though further studies are needed to address if and when such a mechanism operates during mammalian renal development.
Interactions between wt1a, foxc1a and Notch signaling
There have been some molecular insights into the mechanism by which wt1a, in turn, regulates podocyte formation and the expression of target genes like podocin and nephrin in the zebrafish pronephros (Miceli, et al., 2014). Researchers uncovered physical interactions in vitro between zebrafish wt1a, foxc1a, the NICD intracellular domain of Notch1, and the Notch effector rbpj, suggesting that these proteins form a transcriptional complex that controls a podocyte-specific gene regulatory network (O’Brien et al., 2011). In support of this, single knockdown of wt1a, foxc1a or rbpj only reduced the number of wt1b-expressing podocytes, but dual knockdown combinations were sufficient to entirely abrogate podocyte formation (O’Brien et al., 2011). These data suggest that combinatorial interactions between these factors direct podocyte development in zebrafish (O’Brien et al., 2011).
Additionally, the researchers used biochemical assays to address whether these findings represented conserved podocyte regulatory networks, as developmental studies of the Xenopus pronephros and the mouse metanephros have provided genetic evidence that podocyte formation is reliant on the homologs of Wt1, FoxC1/2, and Notch signaling (Cheng and Kopan, 2005; Takemoto et al., 2006; White, et al., 2010). The ability of different in vitro combinations and dosages of mammalian Wt1, FoxC2, and NICD1 to induce podocyte genes was evaluated by transfecting NIH3T3 cells with a number of luciferase reporter constructs and plasmids containing the various factors (O’Brien et al., 2011). They found that the combined activity of Wt1/FoxC2/NICD caused high Hey1 transcription, while Wt1/Foxc1a activity triggered Podocalyxin (O’Brien et al., 2011). Taken together, these findings suggest that there is broad conservation of the gene regulatory networks that control podocyte development. This is an intriguing area for future study, as it may lend insight into molecular disruptions that cause podocyte deficiency.
Downstream of wt1a: the role of osr1
In addition to the factors discussed above, the zinc-finger transcription factor odd skipped related 1 (osr1) is also expressed in the intermediate mesoderm and is required for normal development of both the podocytes and proximal tubule in the zebrafish pronephros, with no apparent role in distal pronephros development (Tena et al., 2007; Mudumana et al., 2008). Knockdown of osr1 is associated with the formation of podocytes that express wt1a transcripts, but fail to express nephrin by 24 hpf (Mudumana et al., 2008). Further, osr1 knockdown leads to a proximal reduction of lhx1a at the 4 somite stage and proximal reduction of pax2a at 24 hpf (Tena et al., 2007). These changes suggest that osr1 might regulate podocyte and/or proximal tubule development by controlling expression of lhx1a and pax2a in renal progenitors. Concomitant overexpression of pax2a is capable of rescuing proximal tubule formation in osr1 morphants, suggesting that osr1 normally acts to induce or maintain pax2a expression in renal progenitors (Mudumana et al., 2008). In a recent study, overexpression of lhx1a in osr1 morphants was found to rescue nephrin expression in podocytes, demonstrating that osr1 plays a key role in mediating podocyte differentiation downstream of wt1a (Tomar, et al., 2014).
Delineation of the podocyte lineage: relationships to the neck segment and the interrenal gland
As previously mentioned, podocytes express the paralogs wt1a and wt1b. It is thought that overlap of these markers is indicative of a podocyte progenitor cell (Bollig et al., 2006; O’Brien, et al., 2011). Within the renal progenitor field, pax2a expression initially overlaps with wt1a/wt1b expressing cells then becomes restricted to the neck segment (O’Brien, et al., 2011). Furthermore, as the fish develops to between 20 and 22 hpf, expression of some of the wt1a field overlaps with expression of the nuclear receptor marker nr5a1a (Figure 3) (Hsu et al., 2003). This population of cells will give rise to the interrenal gland, forming a single group of cells at the midline positioned posterior to the podocytes between 22 and 30 hpf (Figure 3) (Hsu et al., 2003; Liu, 2007). The zebrafish interrenal gland is an endocrine organ that is responsible for synthesis and secretion of steroids and is akin to the mammalian adrenal gland (Hsu et al., 2003; Liu, 2007). It is noteworthy that the interrenal and adrenal glands not only have similarities in function, but are also both tightly associated with kidney tissues. The molecular circuitry that regulates the spatiotemporal dynamics of wt1a, pax2a and nr5a1a expression is crucial for the precise emergence of the respective podocyte, neck and interrenal gland lineages, as further described below.
Role of pax2a in suppressing podocyte formation in the pronephros, and pax2a regulation by ponzr1
There is genetic evidence that the transcription factor pax2a is essential for restricting podocyte formation in the renal progenitor field. In no isthmus (noi) zebrafish that have loss of function mutations in pax2a, the absence of normal pax2a expression is associated with ectopic wt1a transcripts in the neck region (Majumdar et al., 2000). In addition, later in development these mutant cells lose epithelial markers and show expression of vegfaa, indicative of maturing podocytes (Majumdar et al., 2000). These findings suggest that pax2a inhibits expression of podocyte factors in the renal progenitors that will adopt the neck segment identity (Majumdar, et al., 2000).
The precise control of pax2a expression in podocyte progenitors is also vital, as further experimentation has shown that pax2a downregulation is required for normal glomerulus formation (Bedell et al., 2012). This regulation, at least in part, is accomplished by the gene product encoded by plac8 onzin related protein 1 (ponzr1), which belongs to a chordate-specific gene family (Bedell et al., 2012). ponzr1 can function in vivo as a transcription factor or cofactor based on activator/repressor overexpression tests in zebrafish embryos (Bedell et al., 2012). Knockdown of ponzr1 during zebrafish embryogenesis leads to expanded pax2a expression in podocyte progenitors and disrupted glomerular development, in which the central glomerulus fails to form (Bedell et al., 2012). This suggests that one role of ponzr1 is to mediate the domain of pax2a expression in renal progenitors to promote normal podocyte formation.
The intriguing relationship between the pronephros podocyte lineage and the interrenal organ
As mentioned, the interrenal progenitor field was shown as overlapping regions of wt1a and nr5a1a (Hsu et al., 2003). Interestingly, data also suggests that podocyte and interrenal progenitors are regulated in such a way that cells of one fate come at the expense of the cells from the other. Research has shown an increase in interrenal progenitors in both wt1a and rbpja/b morphants, with an exacerbated increase in double morphants (O’Brien et al., 2011). In addition, double-deficiency of wt1a and foxc1a led to an abrogation of interrenal progenitors (O’Brien et al., 2011). These data indicate there is an extremely complex interplay between all these proteins correlating with the balance of podocyte and interrenal progenitors (O’Brien et al., 2011). Fate mapping studies are needed to resolve the relationship between these lineages during normal development and in the context of genetic disruptions that alter the formation of these cell types.
Podocyte midline migration and vascular recruitment to assemble the glomerulus: precise coordination of blood flow and nephron fluid flow
The zebrafish pronephric glomerulus is formed after the bilateral podocyte clusters migrate to the midline, recruit vasculature, and form one composite glomerulus with these capillary endothelial cells (Drummond, 2003; Gerlach and Wingert, 2014). This process is relatively rapid, occurring over the second day of development such that by approximately 48–50 hpf, the pronephros begins to filter the blood (Figure 3) (Drummond, et al., 1998).
Initially, during development of the body plan, the renal progenitors undergo convergence related movements that situate them bilaterally (Lam, et al., 2009). Recently termed the pronephric glomerular primordia (PGP), the podocyte progenitors move toward the midline between 10 and 24 hpf (Huang, et al., 2013), which is reliant on midline signals (Liu, et al., 2000; Majumdar and Drummond, 2000). Subsequent to convergence-based migratory movements, the podocytes complete the journey to the midline between 24 hpf and 36 hpf (Huang, et al., 2013b). By histology, they appear as epithelial vesicles that undergo progressive morphogenesis between 40 hpf and 48 hpf to intermingle with endothelial cells (Drummond, et al., 1998; Ichimura, et al., 2012a). Interestingly, the embryonic heartbeat becomes synchronous at approximately 24 hpf, and impaired cardiovascular function leads to bilateral glomeruli, demonstrating that blood flow is required for glomerular development (Serluca, et al., 2002; Ichimura, et al., 2012b). Maturing podocytes secrete vegfaa, which recruits vascular endothelial cells (Majumdar and Drummond, 2000; Serluca et al., 2002). While podocyte maturation is coordinated with vascular recruitment, this maturation is not reliant on the presence of endothelial cells, as cloche mutants that are unable to develop endothelia form podocytes that are able to form foot processes (Majumdar and Drummond, 1999).
Further, the proper establishment of fluid flow within the nephron tubule, accomplished by the formation and function of motile cilia on tubular epithelial cells, is essential for maintaining normal fluid homeostasis and the rate of fluid movement across the glomerulus. Disruption of cilia-driven fluid flow leads to pronephric cyst formation, in which fluid accumulates adjacent to the glomerulus, thus affecting the ability of the nephrons to excrete fluid (Kramer-Zucker, et al., 2005b).
Podocyte terminal differentiation/maturation: establishment of foot processes & the slit diaphragm
Podocytes undergo terminal differentiation subsequent to their midline congregation and assembly of the glomerulus (Kramer-Zucker, et al., 2005a). As previously mentioned, pronephric podocyte progenitors begin to express transcripts that encode slit diaphragm components beginning as early as 24 hpf, such as nephrin and podocalyxin (Kramer-Zucker, et al., 2005a; O’Brien et al., 2011; Ichimura, et al., 2013a). Over the next day of development, podocyte gene expression profile is further altered, with the expression of additional slit diaphragm components, such as podocin and integrinα3 at 36 hpf (O’Brien et al., 2011). These observations suggest that the production of slit-diaphragm components is initiated prior to the start of blood filtration.
However, although blood filtration can be detected by 48–50 hpf, the slit diaphragm becomes increasingly refined over the next several days of development, and there is ongoing expression of slit diaphragm genes (e.g. nephrin) (Ichimura, et al., 2013b). Analysis of podocyte morphology using transmission electron microscopy (TEM) has revealed that spreading and elaboration of foot processes between 72–96 hpf, with fine interdigitations present by 96 hpf (Kramer-Zucker, et al., 2005a). Renal clearance, or the ability of nephrons to excrete fluid, can be assessed by vascular injection of visible molecules, such as fluorescent FITC-inulin (Hentschel, et al., 2005; Rider, et al., 2012). The actual size-selectivity, or barrier function, of the slit diaphragm can be actually measured based on clearance of high molecular weight fluorescently labeled molecules (Drummond, et al., 2003; Kramer-Zucker, et al., 2005; Hentschel, et al., 2007; Hanke, et al., 2013). At 72 hpf, the zebrafish glomerulus is relatively leaky, consistent with the rare appearance of slit-diaphragms by TEM: 70 kilodalton (kDa) rhodamine-dextran or 68 kDa Alexa-BSA can pass through the glomerular filter and enter the tubule, where it is endocytosed by proximal tubule cells (Kramer-Zucker, et al., 2005a). After 72 hpf, this leakiness is diminished. The use of larger molecules has been utilized to discern glomerular leakage: by 84 hpf, 500 kDA FITC-dextran is retained in the vascular system and only rarely can pass into the tubule, and leakiness of this molecule into the tubule has been implemented to measure glomerular integrity (Kramer-Zucker, et al., 2005a).
Functional comparisons between slit diaphragm components have provided compelling evidence that protein activities are highly conserved between the zebrafish pronephros and mammalian metanephros (Fukuyo, et al., 2014). Due to the conservation of podocyte structure and function between zebrafish and mammals, there have been many studies using the zebrafish pronephros to test whether particular genes encode essential components of the filtration barrier (Hentschel, et al., 2007; Kirsch, et al., 2013; Hanke, et al., 2013), such as the Neph/nephrin immunoglobulin domain-containing family members, e.g. nephrin and Neph1-3 (Neumann-Haefelin, et al., 2010; Wang, et al., 2012; Arif, et al., 2014). Through such studies, the zebrafish has become a useful model for several podocyte-specific diseases which arise from defects in proteins needed to maintain a selective slit diaphragm in mature podocytes (also see Swanhart et al., 2011 for an extensive listing of zebrafish renal disease genetic models).
Further, the zebrafish pronephros has been useful for the characterization of proteins that are present in podocytes but have unknown roles in their physiology. The function of such genes expressed in podocytes has been interrogated extensively using zebrafish morpholino studies. This work has identified essential roles for a bevy of factors needed to establish or maintain foot processes based on the observations of foot process effacement subsequent to gene knockdown. For example, cytoskeletal components that alter actin dynamics have dramatic influences on foot process attachment to the GBM, as shown by loss-of-function studies of LAT3 (a sodium-independent neutral L-amino acid transporter) (Sekine, et al., 2009), cofilin-1 (an actin depolymerizing factor) (Ashworth, et al., 2010, or most recently anillin (an F-actin binding protein) (Gbadegesin, et al., 2014). Disruptions of several myosin genes have also been studied with morpholino knockdown strategies using the zebrafish pronephros, and identified critical roles for several in podocyte morphogenesis, including MYH9 (non-muscle myosin heavy chain IIA) (Müller et al., 2011), and the unconventional class I myosins Myo1c (Arif, et al., 2013) and Myo1e (Mao, et al., 2013). Additionally, the gene Glcci1 (glucocorticoid-induced transcript 1), which encodes a protein that localizes to the cytoplasm of mammalian podocytes in mature glomeruli, was studied with knockdowns in developing zebrafish to ascertain its potential role(s). The researchers found that Glcci1 activity is needed to maintain the filtration barrier and prevent foot effacement (Nishibori, et al., 2011).
A systematic screen using zebrafish morpholino injections has also been performed to knockdown candidate glomerular factors annotated in GlomBase, a transcript bioinformatics database generated from the sequencing of cDNAs isolated from newborn and adult mouse glomeruli (Ebarasi et al., 2009). In this approach, the researchers used zebrafish for an in vivo functional screen in which they performed glomerular filtration assays with 500 kDa FITC-dextran on morphant embryos to assess filtration barrier integrity (Ebarasi et al., 2009). They discovered several relevant genes and through their analysis of the gene crb2b, a member of the Crumbs family of polarity factors, demonstrating that podocyte differentiation is reliant on proper cell polarity (Ebarasi et al., 2009). In an independent study, the vertebrate tight junction protein and immunoglobulin superfamily member CAR (coxsackie and adenovirus receptor) was shown to be essential for elaboration of foot process architecture during podocyte differentiation (Raschperger, et al., 2008). Furthermore, the interaction of podocytes with the basement membrane has been examined through genetic knockdown of P4H-TM (prolyl 4-hydroxylase transmembrane), which can catalyze the hydroxylation of collagens (Hyvärinen, et al., 2010). Elimination of P4H-TM expression in zebrafish embryos was associated with a fragmented GBM, podocytes with abnormally shaped foot processes, and proteinuria (Hyvärinen, et al., 2010).
In addition to these studies, a growing list of reports have implemented the zebrafish model for knockdown studies to evaluate the roles of genes mutated in human patients with renal dysfunctions associated with proteinuria or the nephrotic syndrome (the constellation of proteinuria, hypoalbuminemia, and edema). To date, these include studies of human PLCE1 (phospholipase C epsilon) (Hinkes, et al., 2006), COQ6 (coenzyme Q10 biosynthesis monooxygenase 6) (Heerings, et al., 2011), ADCK4 (aarF domain containing kinase 4) (Ashraf, et al., 2013), and ARHGDIA (Gee, et al., 2013). In each case, positional cloning or homozygosity mapping with affected human patient samples was combined with in vivo functional assessment of the identified gene candidate by performing knockdown of the zebrafish orthologue.
In sum, these studies highlight the utility of zebrafish to efficiently and rapidly explore the functional roles of podocyte-expressed genes in an in vivo setting that does not require the time or expense of mammalian genetics. Continued implementation of in vivo functional studies with the zebrafish pronephros is poised to make valuable ongoing contributions to the understanding of slit barrier establishment and maintenance, and the pathologies associated with glomerular defects.
PODOCYTE DEVELOPMENT DURING MESONEPHROS FORMATION
There have been limited research studies to date characterizing the process of zebrafish mesonephros development. Further, the signals that induce clusters of renal progenitors located near the pronephros to begin nephrogenesis are not known. However, the limited studies that have been published used the transgenic reporters Tg(wt1b:EGFP) or Tg(lhx1a:EGFP) to visualize renal progenitors, and have documented a number of similarities during nephrogenesis of the larval mesonephros and neonephrogenesis after injury in the adult mesonephros (Zhou et al., 2010; Diep et al., 2011).
In larval zebrafish, both of these reporters mark clusters of renal progenitors that appear in close proximity to the pronephros by approximately 12–14 days post fertilization (dpf). When each cluster of renal progenitors proliferates during mesonephros development, it undergoes morphogenesis to form an elongated nephron tubule (Zhou et al., 2010; Diep et al., 2011). Interestingly, the transgenic reporter Tg(wt1b:EGFP) initially marks the entire renal progenitor cluster, but during elongation becomes restricted in expression to the site of the future glomerulus (Zhou et al., 2010; Diep et al., 2011). The detection of the Tg(wt1b:EGFP) reporter in putative podocyte precursors during mesonephric nephron formation is consistent with a recapitulation of embryonic nephron formation. While this suggests that nephrogenesis involves similar pathways in embryonic and adult zebrafish kidney development, further work is needed to determine if the molecular mechanisms are in fact recapitulated later in life.
Advances in imaging technologies proffer a new opportunity to investigate mesonephros processes. In a recent report from Endlich, et al., (2014) two-photon microscopy was applied to the study of podocyte dynamics in the zebrafish embryo. The researchers explored whether podocytes in the zebrafish pronephros were motile by imaging Tg(wt1a:EGFP) fish between 5–7 dpf (Endlich, et al., 2014). Within the nephrology field, podocyte motility has been controversial, and one challenge has been the accurate visualization of foot processes in living samples. In this case, the authors were able to combine in vivo analysis and increase their resolving power for foot processes with their two-photon approach. While the authors demonstrate that pronephric podocytes were not migratory and had stable arrangements of foot processes over the duration of imaging (for example, over ~ 1 day) (Endlich, et al., 2014), the applications of this technique are not limited to the pronephros. Future use of these and other next generation imaging approaches provides exciting new opportunities to explore renal progenitor development in the mesonephros.
REGENERATION OF PODOCYTES IN THE MESONEPHROS
Renal progenitors in the zebrafish mesonephros can produce new nephrons that include podocytes
Zebrafish have the incredible ability to generate new functional nephrons throughout their lifetime, a process often referred to as neonephrogenesis. Neonephrogenesis involves production of both a blood filter and tubule (Zhou et al., 2010; Diep et al., 2011), though the extent of cell types that are made is still under scrutiny by many labs. The ability to undergo neonephrogenesis after embryonic development has been lost in higher vertebrates, therefore an interesting area of research is determining how the zebrafish have maintained this ability later in life. It is especially compelling that the zebrafish can generate new nephrons after almost all nephrons are destroyed (Zhou et al., 2010; Diep et al., 2011).
Not surprisingly, the renal progenitor cells that produce nephrons during the regeneration response express transcription factor genes such as pax2a, wt1b, and lhx1a that are expressed in pronephros renal progenitors (Zhou et al., 2010; Diep et al., 2011). Further, the aforementioned polarized domain of the Tg(wt1b:EGFP) reporter in elongating nephrons (discussed above) is also seen in adult zebrafish during neonephrogenesis, where the proximal area of new nephrons show fluorescence, consistent with the location of the newly formed glomerulus (Zhou et al., 2010; Diep et al., 2011). This data implies that regeneration-triggered neonephrogenesis responses in the adult mesonephros use similar transcription factors and signaling pathways as the initial generation of the pronephros and mesonephros, suggesting that regeneration recapitulates development in this case.
Additional research into the cells that express these developmental genes after adult kidney injury has shown that clusters of lhx1a+ cells can be transplanted into adult recipients (Diep et al., 2011). Further, the cells successfully produce new nephrons at sites away from the transplantation procedure, suggesting the possibility that the renal progenitors have features of (or are in fact) migratory mesenchymal stem cells. As the new nephrons elongate, they eventually integrate normally into the pre-existing nephrons of recipient kidneys (Diep et al., 2011). This revelation suggests that within these lhx1a+ clusters, there are renal progenitor cells that enable the regeneration of the entire nephron, including glomerular and tubular cell types, in the zebrafish kidney (Diep et al., 2011). What is more intriguing is that transplantation of lhx1a+/wt1b+ cells does not elicit this regeneration response, as nephrons are not formed. This suggests activation of wt1b may change the potential of these renal progenitor cells, possibly causing a differentiation event precluding these cells from becoming new nephrons (Diep et al., 2011). In addition, it has been shown that transplantation of single lhx1a+ cell cannot generate new nephrons, suggesting there is interplay between groups of lhx1a+ cells, allowing them to direct neonephrogenesis in the regenerating adult kidney (Diep et al., 2011).
Podocyte-specific regeneration models in zebrafish
While neonephrogenesis occurs in nephrons of zebrafish that have sustained damage, to tease apart if and how the organizational units of the nephron can individually regenerate, researchers have begun to employ transgenic models that enable inducible, targeted cell ablation in the kidney. Such systems can be engineered by placing the bacterial NTR gene under the control of a tissue-specific promoter, and then using this construct to establish a stable transgenic zebrafish line (Curado, et al., 2007; Curado et al., 2008). At the desired time point, the prodrug, Mtz, is added to the fish water (Curado, et al., 2007; Curado et al., 2008). This prodrug is then converted into a cytotoxin by the bacterial NTR, crosslinking DNA and inducing cell death (Curado, et al., 2007; Curado et al., 2008, Pisharath et al., 2007).
Two independent studies have recently used this system to ablate podocytes in adult and larval zebrafish (Zhou and Hildebrandt, 2012; Huang et al., 2013a). In these experiments, the podocyte-specific enhancer of the gene podocin was cloned upstream of the bacterial NTR gene. In each case, the researches cloned a fluorescent reporter downstream of NTR, so as to visualize the cells with NTR gene activity. In addition, the fluorescent reporter was used to visualize cell number in Mtz treated fish. The treated fish showed a decrease in the number of fluorescent cells indicating the NTR-Mtz system had ablated these podocin expressing podocytes. This cell death was verified by caspase-3 or TUNEL staining in these experiments, which was identified as a dose-dependent effect. Additionally, in both studies fish with compromised podocytes incur severe pericardial edema, as well as fluid buildup in other tissues (Zhou and Hildebrandt, 2012; Huang et al., 2013a).
Zhou and Hildebrandt experimented further by cloning the Vitamin-D binding protein (VDBP) and GFP downstream of a liver-specific promoter, which is similar to the mammalian albumin protein, less GFP, and the site of its generation (Zhou and Hildebrandt, 2012). Albumin is a large protein inhibited from entering the tubule by functional podocytes, however in kidneys with damaged podocytes this protein recapitulates proteinuria by entering the proximal convoluted tubule. The results showed that in Mtz treated zebrafish, VDBP was in the proximal tubule, indicating severe podocyte damage. In addition, they developed an ELISA assay to identify the relative quantity of GFP in the water, indicating the VDBP had been excreted as a waste product from the kidney. The researchers detected significantly more GFP in the water of Mtz treated fish, compared to control fish, and showed that this system worked in both zebrafish embryos and adult fish (Zhou and Hildebrandt, 2012). Overall, these experiments indicate that podocytes are being ablated, causing proteinuria. Thus, this system is a valuable new tool to study the factors that regulate proteinuria, which is relevant to many kidney diseases.
Huang and colleagues (2013a) reported similar success with their NTR-Mtz system in zebrafish larvae, validating that podocytes are ablated by decreased fluorescence, as well as an absence of nephrin transcripts as assayed by in situ hybridization. In addition, they performed electron microscopy to evaluate the disruption of foot processes, which are a strong indicator of podocyte dysfunction. After washout of Mtz, podocyte integrity and function were eventually restored, signifying a regenerative response. Their results demonstrate that four days after washout, the podocytes have regained a significant number of foot processes with slit diaphragm re-establishment. Seven days after washout, foot processes were repaired and slit diaphragms were formed, indicating returned functionality. In addition to electron microscopy, expression of the transgenic line incorporating NTR-GFP under the podocin reporter returns, as well as podocin and nephrin transcripts as analyzed by in situ hybridization. A small group of cells also incorporated BrdU, and re-expressed GFP, indicating podocyte proliferation after Mtz washout (Huang et al., 2013a). These data indicate that podocyte replenishment is possible in zebrafish, though whether podocytes are emerging from podocyte stem/progenitors or through the proliferation of pre-existing podocytes has yet to be determined.
These two manuscripts use an important genetic tool for research in the adult zebrafish kidney, as the system can be temporally and spatially regulated, in addition to being washed out after a certain period. The techniques allow for ablation of specific cells during a time course, thus researchers can follow the events of regeneration in real-time. In addition, through these experiments, it is clearly evident that podocytes can repopulate the glomerulus after injury, however the mechanism is currently unknown. This line of investigation is of extreme importance, as loss of podocyte function is a hallmark of many kidney diseases.
CONCLUSIONS
The zebrafish is a very useful model system to study developmental regenerative biology. Zebrafish have high genetic conservation with other vertebrates, which predicts the high likelihood that discoveries will be applicable to higher vertebrates, including humans. As explored in this review, zebrafish are an excellent model for studies of the molecular basis of nephrogenesis in embryonic and adult contexts. Here we have discussed how zebrafish have emerged as a useful model system for podocyte studies and have high potential for further developmental and regenerative research on this renal cell type. While numerous major questions still remain, the zebrafish is poised to address many of these and thus uncover some valuable answers.
Our knowledge about the genetic pathways that lead to glomerular dysfunction remains incomplete. Podocytes are essential cells for normal glomerular function (Wiggins, 2007; Patrakka and Tryggvason, 2009). There are many human diseases caused by dysfunctional podocytes such as minimal change disease, membranous nephropathy, classic focal segmental glomerulosclerosis (FSGS), cellular/collapsing FSGS, and diabetic nephropathy to name a few (Shankland, 2006). These diseases and others can lead to podocyte effacement, which effects slit diaphragm function, a change in podocyte number (usually a loss), or both, ultimately ending in proteinuria (Shankland, 2006). Ongoing systems biology work continues to identify relevant genetic loci in human disease patients (e.g. Pattaro, et al., 2012) and to identify podocyte-expressed genes (e.g. He, et al., 2007; Hartwig, et al., 2010; Lindenmeyer, et al. 2010). Using techniques described in this review, it is possible to investigate the functions of newly identified genes using the simple zebrafish pronephros for gain and loss of function interrogations. Such studies may reveal crucial insights applicable to the in vitro production of podocytes, such as by directed differentiation of induced pluripotent stem cells (Song, et al., 2012). In addition, the zebrafish provides a rather unique opportunity to perform in vivo small molecule screens—in this case, to identify podocyte-specific drugs for such disease models (Poureetezadi and Wingert, 2013; Poureetezadi, et al., 2014). These approaches can provide a powerful complement to ongoing investigations with cultured mammalian podocytes (Reiser, et al., 2010).
There is also a wide horizon of research to explore regarding the kidney regeneration response elicited by the zebrafish after damage. For example, scientists are still trying to identify bona fide stem cells in the kidney, specifically in the glomerulus. If these cells are identified, it would be of vast interest to find their location within the kidney. Proposed stem cells have included parietal epithelial cells (PECs), as these cells have some characteristics of stem cells (Lasagni and Romagnani, 2010; Sagrinati et al., 2006; Lazzeri et al., 2007). In mice, these cells express CD24 and CD133, but do not express podocalyxin, however podocyte precursors express all three. This is suggestive that the PEC has not yet differentiated enough to become a podocyte precursor, and may exhibit more stem-like qualities (Sagrinati et al., 2006). Further experiments showed that a subset of purified PECs can be grown in culture, self-renew, and generate clones with characteristics of developing nephrons (Sagrinati et al., 2006). It has also been shown that PECs line the Bowman’s capsule and migrate into the glomerular tuft to differentiate into podocytes during mouse embryonic kidney development (Appel et al., 2009). Finally, PECs divide at low frequency, express some stem cell markers, and can differentiate into several cell types (Pabst and Sterzel, 1983; Vogetseder et al., 2005; Sagrinati et al., 2006; Lazzeri et al., 2007). However, despite these studies, recent genetic fate mapping work in the mouse has revealed that PECs produce podocytes in juvenile mice, but not in adult mice (Berger, et al., 2014). This recent finding suggests that podocyte regeneration does not normally occur by proliferation of PEC progenitors in mammals. It remains to be seen whether signals that trigger podocyte replacement in other species, like zebrafish, could elicit podocyte regeneration in mammals. The first step will be the elucidation of signaling pathways that are necessary and sufficient to drive this process in zebrafish, as this will provide a place to begin testing whether similar behaviors might be elicited in the complex kidney of higher vertebrates.
Looking forward, zebrafish research can provide valuable insights into the molecular mechanisms that regulate podocyte regeneration. In addition to the points already discussed, determining the stem cell niche could lead identification of other genes that, when mutated, cause kidney defects. Understanding these topics may reveal targets that are sufficient to activating these regeneration pathways in humans with renal disorders, so as to attenuate their symptoms or possibly even cure their disease. The zebrafish provides a great model system for these advancements, along with future experiments to aid in our understanding of renal function and nephron regeneration.
Acknowledgments
Grant Support: This work was supported by funding from the following sources: National Institutes of Health grants K01DK083512, DP2OD008470, and R01DK100237; March of Dimes Basil O’Connor Starter Scholar Award #5-FY12-75; start up funds from the University of Notre Dame College of Science and Department of Biological Sciences; and a generous gift to the University of Notre Dame from Elizabeth and Michael Gallagher on behalf of the Gallagher Family to foster stem cell research.
We thank the staffs of the Department of Biological Sciences for their support, and the Center for Zebrafish Research at Notre Dame for their care of our zebrafish colony. We thank the members of our research lab for their support and discussions of the ideas presented in this review.
LITERATURE CITED
- Amacher SL, Draper BW, Summers BR, Kimmel CB. The zebrafish T-box genes no tail and spadetail are required for development of trunk and tail mesoderm and medial floor plate. Development. 2002;129:3311–3323. doi: 10.1242/dev.129.14.3311. [DOI] [PubMed] [Google Scholar]
- Amsterdam A, Hopkins N. Mutagenesis strategies in zebrafish for identifying genes involved in development and disease. Trends Genet. 2006;22:473–478. doi: 10.1016/j.tig.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Appel D, Kershaw DB, Smeets B, Yuan G, Fuss A, Frye B, Elger M, Kriz W, Floege J, Moeller MJ. Recruitment of podocytes from glomerular parietal epithelial cells. J Am Soc Nephrol. 2009;20:333–343. doi: 10.1681/ASN.2008070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arif E, Kumari B, Wagner MC, Zhou W, Holzman LB, Nihalani D. Myo1c is an unconventional myosin required for zebrafish glomerular development. Kid Int. 2013;84:1154–1165. doi: 10.1038/ki.2013.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arif E, Rathore YS, Kumari B, Ashish F, Wong HN, Holzman LB, Nihalani D. Slit diaphragm protein Neph1 and its signaling: a novel therapeutic target for protection of podocytes against glomerular injury. J Biol Chem. 2014;289:9502–9518. doi: 10.1074/jbc.M113.505743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashraf S, Gee HY, Woerner S, Xie LX, Vega-Warner V, Lovric S, Fang H, Song X, Cattran DC, Avila-Casado C, Paterson AD, Nitschké P, Bole-Feysot C, Cochat P, Esteve-Rudd J, Haberberger B, Allen SJ, Zhou W, Airik R, Otto EA, Barua M, Al-Hamed MH, Kari JA, Evans J, Bierzynska A, Saleem MA, Böckenhauer D, Kleta R, El Desoky S, Hacihamdioglu DO, Gok F, Washburn J, Wiggins RC, Choi M, Lifton RP, Levy S, Han Z, Salviati L, Prokisch H, Williams DS, Pollak M, Clarke CF, Pei Y, Antignac C, Hildebrandt F. ADCK4 mutations promote steroid-resistant nephrotic syndrome through CoQ10 biosynthesis disruption. J Clin Invest. 2013;123:5179–5189. doi: 10.1172/JCI69000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashworth S, Teng B, Kaufeld J, Miller E, Tossidou I, Englert C, Bollig F, Staggs L, Roberts IS, Park JK, Haller H, Schiffer M. Cofilin-1 inactivation leads to proteinuria-studies in zebrafish, mice and humans. PLoS One. 2010;5:e12626. doi: 10.1371/journal.pone.0012626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auer TO, Del Bene F. CRISPR/Cas9 and TALEN-mediated knock-in approaches in zebrafish. Methods. 2014 doi: 10.1016/j.ymeth.2014.03.027. S1046-2023(14)00129-7. [DOI] [PubMed] [Google Scholar]
- Augusto J, Smith B, Smith S, Robertson J, Reimschuessel R. Gentamicin-induced nephrotoxicity and nephroneogenesis in Oreochromis nilotica, a tilapian fish. Dis Aquatic Org. 1996;26:49–58. [Google Scholar]
- Bedell VM, Person AD, Larson JD, McLoon A, Balciunas D, Clark KJ, Neff KI, Nelson KE, Bill BR, Schimmenti LA, Beiraghi S, Ekker SC. The lineage-specific gene ponzr1 is essential for zebrafish pronephric and pharyngeal arch development. Development. 2012;139:793–804. doi: 10.1242/dev.071720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger K, Schulte K, Boor P, Kuppe C, van Kuppevelt TH, Floege J, Smeets B, Moeller MJ. The regenerative potential of parietal epithelial cells in adult mice. J Am Soc Nephrol. 2014;25:693–705. doi: 10.1681/ASN.2013050481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollig F, Mehringer R, Perner B, Hartung C, Schafer M, Schartl M, Volff JN, Winkler C, Englert C. Identification and comparative expression analysis of a second wt1 gene in zebrafish. Dev Dyn. 2006;235:554–561. doi: 10.1002/dvdy.20645. [DOI] [PubMed] [Google Scholar]
- Bollig F, Perner B, Besenbeck B, Kothe S, Ebert C, Taudien S, Englert C. A highly conserved retinoic acid responsive element controls wt1a expression in the zebrafish pronephros. Development. 2009;136:2883–2892. doi: 10.1242/dev.031773. [DOI] [PubMed] [Google Scholar]
- Bonga SEW, Pang PKT. Control of calcium regulating hormones in the vertebrates: parathyroid hormone, calcitonin, prolactin, and stanniocalcin. Int Rev Cytol. 1991;128:139–213. doi: 10.1016/s0074-7696(08)60499-4. [DOI] [PubMed] [Google Scholar]
- Bowen ME, Henke K, Siegfried KR, Warman ML, Harris MP. Efficient mapping and cloning of mutations in zebrafish by low-coverage whole-genome sequencing. Genetics. 2012;190:1017–1024. doi: 10.1534/genetics.111.136069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford Y, Conlin T, Dunn N, Fashena D, Frazer K, Howe DG, Knight J, Mani P, Martin R, Moxon SA, Paddock H, Pich C, Ramachandran S, Ruef BJ, Ruzicka L, Bauer Schaper H, Schaper K, Shao X, Singer A, Sprague J, Sprunger B, Van Slyke C, Westerfield M. ZFIN: enhancements and updates to the Zebrafish Model Organism Database. Nucleic Acids Res. 2011;39:D822–D829. doi: 10.1093/nar/gkq1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler DG, Zhang DH, Villadiego R, Oudit GY, Youson JH, Cadinouche MZ. Response by the corpuscles of stannius to hypotensive stimuli in three divergent ray-finned fishes (amia calva, anguilla rostrata, and catastomus commersoni): cardiovascular and morphological changes. Gen Comp Endocrinol. 2003;132:198–208. doi: 10.1016/s0016-6480(03)00080-7. [DOI] [PubMed] [Google Scholar]
- Chablais F, Jazwinska A. IGF signaling between blastema and wound epidermis is required for fin regeneration. Development. 2010;137:871–9. doi: 10.1242/dev.043885. [DOI] [PubMed] [Google Scholar]
- Chen YM, Miner JH. Glomerular basement membrane and related glomerular disease. Trans Res. 2012;160:291–297. doi: 10.1016/j.trsl.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng HT, Kopan R. The role of notch signaling in specification of podocyte and proximal tubules within the developing mouse kidney. Kidney Int. 2005;68:1951–1952. doi: 10.1111/j.1523-1755.2005.00627.x. [DOI] [PubMed] [Google Scholar]
- Cheng CN, Wingert RA. Chapter 9: Renal system development in the zebrafish: a basic model of the human kidney. In: Carver E, Lessman C, editors. Zebrafish: Topics in Reproduction & Development. Nova Scientific Publishers; 2014. [Google Scholar]
- Cheng CN, Li Y, Marra A, Verdun V, Wingert RA. Flat mount preparation for observation and analysis of fixed zebrafish embryo specimens. J Vis Exp. 2014 doi: 10.3791/51604. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang, Inagi Glomerular diseases: genetic causes and future therapeutics. Nat Rev Nephrol. 2010;6:539–554. doi: 10.1038/nrneph.2010.103. [DOI] [PubMed] [Google Scholar]
- Costantini F, Kopan R. Patterning a complex organ: Branching morphogenesis and nephron segmentation in kidney development. Dev Cell. 2010;18:698–712. doi: 10.1016/j.devcel.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curado S, Anderson RM, Jungblut B, Mumm J, Schroeter E, Stainier DYR. Conditional targeted cell ablation in zebrafish: a new tool for regeneration studies. Dev Dyn. 2007;236:1025–1035. doi: 10.1002/dvdy.21100. [DOI] [PubMed] [Google Scholar]
- Curado S, Stainier DY, Anderson RM. Nitroreductase-mediated cell/tissue ablation in zebrafish: A spatially and temporally controlled ablation method with applications in developmental and regeneration studies. Nat Protoc. 2008;3:948–954. doi: 10.1038/nprot.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AJ. Uncharted waters: Nephrogenesis and renal regeneration in fish and mammals. Pediatr Nephrol. 2011;26:1435–1443. doi: 10.1007/s00467-011-1795-z. [DOI] [PubMed] [Google Scholar]
- Deen WM. What determines glomerular capillary permeability? J Clin Invest. 2004;114:1412–1414. doi: 10.1172/JCI23577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diep CQ, Ma D, Deo RC, Holm TM, Naylor RW, Arora N, Wingert RA, Bollig F, Djordjevic G, Lichman B, Zhu H, Ikenaga T, Ono F, Englert C, Cowan CA, Hukriede NA, Handin RI, Davidson AJ. Identification of adult nephron progenitors capable of kidney regeneration in zebrafish. Nature. 2011;470:95–100. doi: 10.1038/nature09669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driever W, Solnica-Krezel L, Schier AF, Neuhauss SC, Malicki J, Stemple DL, Stainier DY, Zwartkruis F, Abdelilah S, Rangini Z, Belak J, Boggs C. A genetic screen for mutations affecting embryogenesis in zebrafish. Development. 1996;123:37–46. doi: 10.1242/dev.123.1.37. [DOI] [PubMed] [Google Scholar]
- Dressler GR. The cellular basis of kidney development. Annu Rev Cell Dev Biol. 2006;22:509–529. doi: 10.1146/annurev.cellbio.22.010305.104340. [DOI] [PubMed] [Google Scholar]
- Drummond IA, Majumdar A, Hentschel H, Elger M, Solnica-Krezel L, Schier AF, Neuhauss SC, Stemple DL, Zwartkruis F, Rangini Z, Driever W, Fishman MC. Early development of the zebrafish pronephros and analysis of mutations affecting pronephric function. Development. 1998;125:4655–4667. doi: 10.1242/dev.125.23.4655. [DOI] [PubMed] [Google Scholar]
- Drummond IA. Making a zebrafish kidney: a tale of two tubes. Trends Cell Biol. 2003;13:357–365. doi: 10.1016/s0962-8924(03)00124-7. [DOI] [PubMed] [Google Scholar]
- Drummond IA. Kidney development and disease in the zebrafish. J Am Soc Nephrol. 2005;16:299–304. doi: 10.1681/ASN.2004090754. [DOI] [PubMed] [Google Scholar]
- Duester G. Retinoic acid synthesis and signaling during early organogenesis. Cell. 2008;134:921–931. doi: 10.1016/j.cell.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebarasi L, He L, Hultenby K, Takemoto M, Betsholtz C, Tryggvason K, Majumdar A. A reverse genetic screen in the zebrafish identifies crb2b as a regulator of the glomerular filtration barrier. Dev Biol. 2009;334:1–9. doi: 10.1016/j.ydbio.2009.04.017. [DOI] [PubMed] [Google Scholar]
- Ebarasi L, Oddsson A, Hultenby K, Betsholtz C, Tryggvason K. Zebrafish: a model system for the study of vertebrate renal development, function, and pathophysiology. Curr Opin Nephrol Hypertens. 2011;20:416–424. doi: 10.1097/MNH.0b013e3283477797. [DOI] [PubMed] [Google Scholar]
- Elger M, Hentschel H, Litteral J, Wellner M, Kirsch T, Luft FC, Haller H. Nephrogenesis is induced by partial nephrectomy in the elasmobranch Leucoraja erinacea. J Am Soc Nephrol. 2003;14:1506–1518. doi: 10.1097/01.asn.0000067645.49562.09. [DOI] [PubMed] [Google Scholar]
- Emelyanov A, Parinov S. Mifepristone-inducible LexPR system to drive and control gene expression in transgenic zebrafish. Dev Biol. 2008;320:113–121. doi: 10.1016/j.ydbio.2008.04.042. [DOI] [PubMed] [Google Scholar]
- Endlich N, Simon O, Göpferich A, Wegner H, Moeller MJ, Rumpel E, Kotb AM, Endlich K. Two-photon microscopy reveals stationary podocytes in living zebrafish larvae. J Am Soc Nephrol. 2014;25:681–686. doi: 10.1681/ASN.2013020178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuyo Y, Nakamura T, Bubenshchikova E, Powell R, Tsuji T, Janknecht R, Obara T. Nephrin and Podocin functions are highly conserved between the zebrafish and mammalian metanephros. Mol Med Rep. 2014;9:457–465. doi: 10.3892/mmr.2013.1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaj T, Gersbach CA, Barbas CF., III ZFN, TALEN, and CRISPR/Cas-based methods for genome editing. Trends Biotechnol. 2013;31:397–405. doi: 10.1016/j.tibtech.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gbadegesin RA, Hall G, Adeyemo A, Hanke N, Tossidou I, Burchette J, Wu G, Homstad A, Sparks MA, Gomez J, Jiang R, Alonso A, Lavin P, Conlon P, Korstanje R, Stander MC, Shamsan G, Barua M, Spurney R, Singhal PC, Kopp JB, Haller H, Howell D, Pollak MR, Shaw AS, Schiffer M, Winn MP. Mutations in the gene that encodes F-actin binding protein anillin cause FSGS. J Am Soc Nephrol. 2014;25 doi: 10.1681/ASN.2013090976. epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee HY, Saisawat P, Ashraf S, Hurd TW, Vega-Warner V, Fang H, Beck BB, Gribouval O, Zhou W, Diaz KA, Natarajan S, Wiggins RC, Lovric S, Chernin G, Schoeb DS, Ovunc B, Frishberg Y, Soliman NA, Fathy HM, Goebel H, Hoefele J, Weber LT, Innis JW, Faul C, Han Z, Washburn J, Antignac C, Levy S, Otto EA, Hildebrandt F. ARHGDIA mutations cause nephrotic syndrome via defective RHO GTPase signaling. J Clin Invest. 2013;123:3243–3253. doi: 10.1172/JCI69134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach GF, Schrader LN, Wingert RA. Dissection of the adult zebrafish kidney. J Vis Exp. 2011;54:e2839. doi: 10.3791/2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlach GF, Wingert RA. Kidney organogenesis in the zebrafish: Insights into vertebrate nephrogenesis and regeneration. Wiley Interdiscip Rev Dev Biol. 2013;2:559–585. doi: 10.1002/wdev.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta T, Marlow FL, Ferriola D, Mackiewicz K, Dapprich J, Monos D, Mullins MC. Microtubule actin crosslinking factor 1 regulates the Balbiani body and animal-vegetal polarity of the zebrafish oocyte. PLoS Genet. 2010;6:e1001073. doi: 10.1371/journal.pgen.1001073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha TS. Roles of adaptor proteins in podocyte biology. World J Nephrol. 2013;2:1–10. doi: 10.5527/wjn.v2.i1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffter P, Granato M, Brand M, Mullins MC, Hammerschmidt M, Kane DA, Odenthal J, van Eeden FJ, Jiang YJ, Heisenberg CP, Kelsh RN, Furutani-Seiki M, Vogelsang E, Beuchle D, Schach U, Fabian C, Nüsslein-Volhard C. The identification of genes with unique and essential functions in the development of the zebrafish, Danio rerio. Development. 1996;123:1–36. doi: 10.1242/dev.123.1.1. [DOI] [PubMed] [Google Scholar]
- Haffter P, Nüsslein-Volhard C. Large scale genetics in a small vertebrate, the zebrafish. In J Dev Biol. 1996;40:221–227. [PubMed] [Google Scholar]
- Hanke N, Staggs L, Schroder P, Litteral J, Fleig S, Kaufeld J, Pauli C, Haller H, Schiffer M. “Zebrafishing” for novel genes relevant to the glomerular filtration barrier. Biomed Res Int. 2013;2013:658270. doi: 10.1155/2013/658270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwig S, Ho J, Pandey P, Macisaac K, Taglienti M, Xiang M, Alterovitz G, Ramoni M, Fraenkel E, Kreidberg JA. Genomic characterization of Wilms’ tumor suppressor 1 targets in nephron progenitor cells during kidney development. Development. 2010;137:1189–1203. doi: 10.1242/dev.045732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B, Ebarasi L, Hultenby K, Tryggvason K, Betsholtz C. Podocin-green fluorescence protein allows visualization and functional analysis of podocytes. J Am Soc Nephrol. 2011;22:1019–1023. doi: 10.1681/ASN.2010121291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L, Sun Y, Patrakka J, Mostad P, Norlin J, Xiao Z, Andrae J, Tryggvason K, Samuelsson T, Betsholtz C, Takemoto M. Glomerulus-specific mRNA transcripts and proteins identified through kidney expressed sequence tag database analysis. Kid Int. 2007;71:889–900. doi: 10.1038/sj.ki.5002158. [DOI] [PubMed] [Google Scholar]
- Heerings SF, Chernin G, Chaki M, Zhou W, Sloan AJ, Ji Z, Xie LX, Salviati L, Hurd TW, Vega-Warner V, Killen PD, Raphael Y, Ashraf S, Ovunc B, Schoeb DS, McLaughlin HM, Airik R, Vlangos CN, Gbadegesin R, Hinkes B, Saisawat P, Trevisson E, Doimo M, Casarin A, Pertegato V, Giorgi G, Prokisch H, Rötig A, Nürnberg G, Becker C, Wang S, Ozaltin F, Topaloglu R, Bakkaloglu A, Bakkaloglu SA, Müller D, Beissert A, Mir S, Berdeli A, Varpizen S, Zenker M, Matejas V, Santos-Ocaña C, Navas P, Kusakabe T, Kispert A, Akman S, Soliman NA, Krick S, Mundel P, Reiser J, Nürnberg P, Clarke CF, Wiggins RC, Faul C, Hildebrandt F. COQ6 mutations in human patients produce nephrotic syndrome with sensorineural deafness. J Clin Invest. 2011;121:2013–2024. doi: 10.1172/JCI45693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentschel H. Renal blood vascular system in the Elasmobranch, Raja erinacia Mitcheill, in relation to kidney zones. Am J Anat. 1988;183:130–147. doi: 10.1002/aja.1001830204. [DOI] [PubMed] [Google Scholar]
- Hentschel DM, Park KM, Cilenti L, Zervos AS, Drummond I, Bonventre JV. Acute renal failure in zebrafish: a novel system to study a complex disease. Am J Physiol Renal Physiol. 2005;288:F923–F929. doi: 10.1152/ajprenal.00386.2004. [DOI] [PubMed] [Google Scholar]
- Hentschel DM, Mengel M, Boehme L, Liebsch F, Albertin C, Bonventre JV, Haller H, Schiffer M. Rapid screening of glomerular slit diaphragm integrity in larval zebrafish. Am J Physiol Renal Physiol. 2007;293:F1746–F1750. doi: 10.1152/ajprenal.00009.2007. [DOI] [PubMed] [Google Scholar]
- Hinkes B1, Wiggins RC, Gbadegesin R, Vlangos CN, Seelow D, Nürnberg G, Garg P, Verma R, Chaib H, Hoskins BE, Ashraf S, Becker C, Hennies HC, Goyal M, Wharram BL, Schachter AD, Mudumana S, Drummond I, Kerjaschki D, Waldherr R, Dietrich A, Ozaltin F, Bakkaloglu A, Cleper R, Basel-Vanagaite L, Pohl M, Griebel M, Tsygin AN, Soylu A, Müller D, Sorli CS, Bunney TD, Katan M, Liu J, Attanasio M, O’toole JF, Hasselbacher K, Mucha B, Otto EA, Airik R, Kispert A, Kelley GG, Smrcka AV, Gudermann T, Holzman LB, Nürnberg P, Hildebrandt F. Positional cloning uncovers mutations in PLCE1 responsible for nephrotic syndrome variant that may be reversible. Nat Genet. 2006;38:1397–1405. doi: 10.1038/ng1918. [DOI] [PubMed] [Google Scholar]
- Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, Collins JE, Humphray S, McLaren K, Matthews L, McLaren S, Sealy I, Caccamo M, Churcher C, Scott C, Barrett JC, Koch R, Rauch GJ, White S, Chow W, Kilian B, Quintais LT, Guerra-Assunção JA, Zhou Y, Gu Y, Yen J, Vogel JH, Eyre T, Redmond S, Banerjee R, Chi J, Fu B, Langley E, Maguire SF, Laird GK, Lloyd D, Kenyon E, Donaldson S, Sehra H, Almeida-King J, Loveland J, Trevanion S, Jones M, Quail M, Willey D, Hunt A, Burton J, Sims S, McLay K, Plumb B, Davis J, Clee C, Oliver K, Clark R, Riddle C, Elliot D, Threadgold G, Harden G, Ware D, Begum S, Mortimore B, Kerry G, Heath P, Phillimore B, Tracey A, Corby N, Dunn M, Johnson C, Wood J, Clark S, Pelan S, Griffiths G, Smith M, Glithero R, Howden P, Barker N, Lloyd C, Stevens C, Harley J, Holt K, Panagiotidis G, Lovell J, Beasley H, Henderson C, Gordon D, Auger K, Wright D, Collins J, Raisen C, Dyer L, Leung K, Robertson L, Ambridge K, Leongamornlert D, McGuire S, Gilderthorp R, Griffiths C, Manthravadi D, Nichol S, Barker G, Whitehead S, Kay M, Brown J, Murnane C, Gray E, Humphries M, Sycamore N, Barker D, Saunders D, Wallis J, Babbage A, Hammond S, Mashreghi-Mohammadi M, Barr L, Martin S, Wray P, Ellington A, Matthews N, Ellwood M, Woodmansey R, Clark G, Cooper J, Tromans A, Grafham D, Skuce C, Pandian R, Andrews R, Harrison E, Kimberley A, Garnett J, Fosker N, Hall R, Garner P, Kelly D, Bird C, Palmer S, Gehring I, Berger A, Dooley CM, Ersan-Ürün Z, Eser C, Geiger H, Geisler M, Karotki L, Kirn A, Konantz J, Konantz M, Oberländer M, Rudolph-Geiger S, Teucke M, Lanz C, Raddatz G, Osoegawa K, Zhu B, Rapp A, Widaa S, Langford C, Yang F, Schuster SC, Carter NP, Harrow J, Ning Z, Herrero J, Searle SM, Enright A, Geisler R, Plasterk RH, Lee C, Westerfield M, de Jong PJ, Zon LI, Postlethwait JH, Nüsslein-Volhard C, Hubbard TJ, Roest Crollius H, Rogers J, Stemple DL. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503. doi: 10.1038/nature12111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu HJ, Lin G, Chung BC. Parallel early development of zebrafish interrenal glands and pronephros: Differential control by wt1 and ff1b. Development. 2003;130:2107–2116. doi: 10.1242/dev.00427. [DOI] [PubMed] [Google Scholar]
- Huang J, McKee M, Huang HD, Xiang A, Davidson AJ, Lu HA. A zebrafish model of conditional targeted podocyte ablation and regeneration. Kidney Int. 2013a;83:1193–1200. doi: 10.1038/ki.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang CJ, Wilson V, Pennings S, MacRae CA, Mullins J. Sequential effects of spadetail, one-eyed pinhead and no tail on midline convergence of nephric primordia during zebrafish embryogenesis. Dev Biol. 2013b;384:290–300. doi: 10.1016/j.ydbio.2013.07.002. [DOI] [PubMed] [Google Scholar]
- Hyvärinen J, Parikka M, Sormunen R, Rämet M, Tryggvason K, Kivirikko KI, Myllyharju J, Koivunen P. Deficiency of a transmembrane prolyl 4-hydroxylase in the zebrafish leads to basement membrane defects and compromised kidney function. J Biol Chem. 2010;285:42023–42032. doi: 10.1074/jbc.M110.145904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura K, Bubenshchikova E, Powell R, Fukuyo Y, Nakamura T, Tran U, Oda S, Tanaka M, Wessely O, Kurihara H, Sakai T, Obara T. A comparative analysis of glomerulus development in the pronephros of Medaka and zebrafish. PLoS One. 2012a;7:e45286. doi: 10.1371/journal.pone.0045286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura K, Fukuyo Y, Nakamura T, Powell R, Sakai T, Obara T. Structural disorganization of pronephric glomerulus in zebrafish mpp5a/nagi oko mutant. Dev Dyn. 2012b;241:1922–1932. doi: 10.1002/dvdy.23877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura K, Powell R, Nakamura T, Kurihara H, Sakai T, Obara T. Podocalyxin regulates pronephric glomerular development in zebrafish. Physiol Rep. 2013a;1:e00074. doi: 10.1002/phy2.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichimura K, Fukuyo Y, Nakamura T, Powell R, Sakai T, Janknecht R, Obara T. Developmental localization of nephrin in zebrafish and medaka pronephric glomerulus. J Histochem Cytochem. 2013b;61:313–324. doi: 10.1369/0022155413477115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson CS, Holzemer NF, Wingert RA. Laser ablation of the zebrafish pronephros to study renal epithelial regeneration. J Vis Exp. 2011;54:e2839. doi: 10.3791/2845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kettleborough RN, Busch-Nentwich EM, Harvey SA, Dooley CM, de Bruijn E, van Eeden F, Sealy I, White RJ, Herd C, Nijman IJ, Fényes F, Mehroke S, Scahill C, Gibbons R, Wali N, Carruthers S, Hall A, Yen J, Cuppen E, Stemple DL. A systematic genome-wide analysis of zebrafish protein-coding gene function. Nature. 2013;496:494–497. doi: 10.1038/nature11992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Radhakrishnan UP, Rajpurohit SK, Kulkarni V, Jagadeeswaran P. Vivo-Morpholino knockdown of alphaIIb: A novel approach to inhibit thrombocyte function in adult zebrafish. Blood Cells Mol Dis. 2010;44:169–74. doi: 10.1016/j.bcmd.2009.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Kirsch T, Kaufeld J, Korstanje R, Hentschel DM, Staggs L, Bollig F, Beese M, Schroder P, Boehme L, Haller H, Schiffer M. Knockdown of the hypertension-associated gene NOSTRIN alters glomerular barrier function in zebrafish (Danio rerio) Hypertension. 2013;62:726–730. doi: 10.1161/HYPERTENSIONAHA.113.01882. [DOI] [PubMed] [Google Scholar]
- Kramer-Zucker AG, Wiessner S, Jensen AM, Drummond IA. Organization of the pronephric filtration apparatus in zebrafish requires Nephrin, Podocin and the FERM domain protein Mosaic eyes. Dev Biol. 2005a;285:316–329. doi: 10.1016/j.ydbio.2005.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer-Zucker AG, Olale F, Haycraft CJ, Yoder BK, Schier AF, Drummond IA. Cilia-driven fluid flow in the zebrafish pronephros, brain and kupffer’s vesicle is required for normal organogenesis. Development. 2005b;132:1907–1921. doi: 10.1242/dev.01772. [DOI] [PubMed] [Google Scholar]
- Krauss S, Johansen T, Korzh V, Fjose A. Expression of the zebrafish paired box gene pax[zf-b] during early neurogenesis. Development. 1991;113:1193–1206. doi: 10.1242/dev.113.4.1193. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy VJ. Cytophysiology of corpuscles of Stannius. Int Rev Cytol. 1976;46:77–249. doi: 10.1016/s0074-7696(08)60992-4. [DOI] [PubMed] [Google Scholar]
- Kriz W, Kaissling B. Structural organization of the mammalian kidney. In: Alpern R, Herbert S, editors. Seldin and Giebish’s The Kidney - Physiology and Pathophysiology. Academic Press; 2007. pp. 479–563. [Google Scholar]
- Kriz W. Podocyte hypertrophy mismatch and glomerular disease. Nat Rev Nephrol. 2012;8:618–619. doi: 10.1038/nrneph.2012.198. [DOI] [PubMed] [Google Scholar]
- Kriz W, LeHir M. Pathways to nephron loss starting from glomerular diseases-insights from animal models. Kidney Int. 2005;67:404–419. doi: 10.1111/j.1523-1755.2005.67097.x. [DOI] [PubMed] [Google Scholar]
- Kroeger PT, Jr, Poureetezadi SJ, McKee R, Jou J, Miceli R, Wingert RA. Production of haploid zebrafish embryos by in vitro fertilization. J Vis Exp. 2014 doi: 10.3791/51708. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laale HW. The biology and use of zebrafish, Brachydanio rerio in fisheries research. A literature review. J Fish Biol. 1977;10:121–173. [Google Scholar]
- Lam PY, Webb SE, Leclerc C, Moreau M, Miller AL. Inhibition of stored Ca2+ release disrupts convergence-related cell movements in the lateral intermediate mesoderm resulting in abnormal positioning and morphology of the pronephric anlagen in intact zebrafish embryos. Develop Growth Differ. 2009;51:429–442. doi: 10.1111/j.1440-169X.2009.01106.x. [DOI] [PubMed] [Google Scholar]
- Lan CC, Leong IU, Lai D, Love DR. Disease modeling by gene targeting using microRNAs. Methods Cell Biol. 2011;105:419–436. doi: 10.1016/B978-0-12-381320-6.00018-7. [DOI] [PubMed] [Google Scholar]
- Lasagni L, Romagnani P. Glomerular epithelial stem cells: The good, the bad, and the ugly. J Am Soc Nephrol. 2010;21:1612–1619. doi: 10.1681/ASN.2010010048. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Wolfe SA. Forward and reverse genetic approaches for the analysis of vertebrate development in the zebrafish. Dev Cell. 2011;21:48–64. doi: 10.1016/j.devcel.2011.06.007. [DOI] [PubMed] [Google Scholar]
- Lazzeri E, Crescioli C, Ronconi E, Mazzinghi B, Sagrinati C, Netti GS, Angelotti ML, Parente E, Ballerini L, Cosmi L, Maggi L, Gesualdo L, Rotondi M, Annunziato F, Maggi E, Lasagni L, Serio M, Romagnani S, Vannelli GB, Romagnani P. Regenerative potential of embryonic renal multipotent progenitors in acute renal failure. J Am Soc Nephrol. 2007;18:3128–3138. doi: 10.1681/ASN.2007020210. [DOI] [PubMed] [Google Scholar]
- Leeuwis JW, Nguyen TQ, Dendooven A, Kok RJ, Goldschmeding R. Targeting podocyte-associated diseases. Adv Drug Deliv Rev. 2010;62:1325–1336. doi: 10.1016/j.addr.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Leshchiner I, Alexa K, Kelsey P, Adzhubei I, Austin-Tse CA, Cooney JD, Anderson H, King MJ, Stottmann RW, Garnaas MK, Ha S, Drummond IA, Paw BH, North TE, Beier DR, Goessling W, Sunyaev SR. Mutation mapping and identification by whole-genome sequencing. Genome Res. 2012;22:1541–1548. doi: 10.1101/gr.135541.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lessman CA. The developing zebrafish (Danio rerio): A vertebrate model for high-throughput screening of chemical libraries. Birth Defects Res C Embryo Today. 2011;93:268–280. doi: 10.1002/bdrc.20212. [DOI] [PubMed] [Google Scholar]
- Li T, Huang S, Zhao X, Wright DA, Carpenter S, Spalding MH, Weeks DP, Yang B. Modularly assembled designer TAL effector nucleases for targeted gene knockout and gene replacement in eukaryotes. Nucleic Acids Res. 2011;39:6315–6325. doi: 10.1093/nar/gkr188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Wingert RA. Regenerative medicine for the kidney: stem cell prospects and challenges. Clin Transl Med. 2013;2:11. doi: 10.1186/2001-1326-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Cheng CN, Verdun V, Wingert RA. Zebrafish nephrogenesis is regulated by interactions between retinoic acid, mecom, and Notch signaling. Dev Biol. 2014;386(1):111–122. doi: 10.1016/j.ydbio.2013.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieschke GJ, Currie PD. Animal models of human disease: zebrafish swim into view. Nat Rev Genet. 2007;8:353–367. doi: 10.1038/nrg2091. [DOI] [PubMed] [Google Scholar]
- Lindenmeyer MT, Eichinger F, Sen K, Anders HJ, Edenhofer I, Mattinzoli D, Kretzler M, Rastaldi MP, Cohen CD. Systematic analysis of a novel human renal glomerulus-enriched gene expression dataset. PLoS One. 2010;7:e11545. doi: 10.1371/journal.pone.0011545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little M, McMahon AP. Mammalian kidney development: principles, progress, and projections. Cold Spring Harb Perspect Biol. 2012;4:a008300. doi: 10.1101/cshperspect.a008300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu A, Majumdar A, Schauerte HE, Haffter P, Drummond IA. Zebrafish wnt4b expression in the floor plate is altered in sonic hedgehog and gli-2 mutants. Mech Dev. 2000;91:409–413. doi: 10.1016/s0925-4773(99)00308-1. [DOI] [PubMed] [Google Scholar]
- Liu YW. Interrenal organogenesis in the zebrafish model. Organogenesis. 2007;3:44–48. doi: 10.4161/org.3.1.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar A, Drummond IA. Podocyte differentiation in the absence of endothelial cells as revealed in the zebrafish avascular mutant, cloche. Dev Genet. 1999;24:220–229. doi: 10.1002/(SICI)1520-6408(1999)24:3/4<220::AID-DVG5>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Majumdar A, Drummond IA. The zebrafish floating head mutant demonstrates podocytes play an important role in directing glomerular differentiation. Dev Biol. 2000;222:147–157. doi: 10.1006/dbio.2000.9642. [DOI] [PubMed] [Google Scholar]
- Majumdar A, Lun K, Brand M, Drummond IA. Zebrafish no isthmus reveals a role for pax2.1 in tubule differentiation and patterning events in the pronephric primordia. Development. 2000;127:2089–2098. doi: 10.1242/dev.127.10.2089. [DOI] [PubMed] [Google Scholar]
- Mao J, Wang D, Mataleena P, He B, Niu D, Katayama K, Xu X, Ojala JR, Wang W, Shu Q, Du L, Liu A, Pikkarainen T, Patrakka J, Tryggvason K. Myo1e impairment results in actin reorganization, podocyte dysfunction, and proteinuria in zebrafish and cultured podocytes. PLoS One. 2013;8:e72750. doi: 10.1371/journal.pone.0072750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marra A, Wingert RA. Roles of Iroquois transcription factors in kidney development. Cell Dev Biol. 2014;3:131. doi: 10.4172/2168-9296.1000131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCampbell KK, Wingert RA. Renal stem cells: fact or science fiction? Biochem J. 2012;444:153–168. doi: 10.1042/BJ20120176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCampbell KK, Wingert RA. New tides: using zebrafish to study renal regeneration. Transl Res. 2014;163:109–122. doi: 10.1016/j.trsl.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCampbell KK, Springer K, Wingert RA. Analysis of nephron composition and function in the adult zebrafish kidney. J Vis Exp. 2014 doi: 10.3791/51644. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng X, Noyes MB, Zhu LJ, Lawson ND, Wolfe SA. Targeted gene inactivation in zebrafish using engineered zinc-finger nucleases. Nat Biotechnol. 2008;26:695–701. doi: 10.1038/nbt1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miceli R, Kroeger PT, Jr, Wingert RA. Molecular mechanisms of podocyte development revealed by zebrafish kidney research. Cell Dev Biol. 2014 doi: 10.4172/2168-9296.1000138. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley RH, Lachani K, Keefe D, Gilchrist MJ, Flicek P, Smith JC, Wardle FC. A gene regulatory network directed by zebrafish No tail accounts for its roles in mesoderm formation. Proc Natl Acad Sci USA. 2009;106:3829–3834. doi: 10.1073/pnas.0808382106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosimann C, Zon LI. Advanced zebrafish transgenesis with Tol2 and application for Cre/lox recombination experiments. Methods Cell Biol. 2011;104:173–194. doi: 10.1016/B978-0-12-374814-0.00010-0. [DOI] [PubMed] [Google Scholar]
- Moulton JD, Jiang S. Gene Knockdowns in Adult Animals: PPMOs and Vivo-Morpholinos. Molecules 2009. 2009;14:1304–1323. doi: 10.3390/molecules14031304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mudumana SP, Hentschel D, Liu Y, Vasilyev A, Drummond IA. Odd skipped related1 reveals a novel role for endoderm in regulating kidney versus vascular cell fate. Development. 2008;135:3355–3367. doi: 10.1242/dev.022830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller T, Rumpel E, Hradetzky S, Bollig F, Wegner H, Blumenthal A, Greinacher A, Endlich K, Endlich N. Non-muscle myosin IIA is required for the development of the zebrafish glomerulus. Kid Int. 2011;80:1055–1063. doi: 10.1038/ki.2011.256. [DOI] [PubMed] [Google Scholar]
- Mundel P, Kriz W. Structure and function of podocytes: an update. Anat Embryol. 1995;192:385–397. doi: 10.1007/BF00240371. [DOI] [PubMed] [Google Scholar]
- Nasevicius A, Ekker SC. Effective targeted gene ‘knockdown’ in zebrafish. Nat Genet. 2000;26:216–220. doi: 10.1038/79951. [DOI] [PubMed] [Google Scholar]
- Neumann-Haefelin E, Kramer-Zucker A, Slanchev K, Hartleben B, Noutsou F, Martin K, Wanner N, Ritter A, Godel M, Pagel P, Fu X, Muller A, Baumeister R, Walz G, Huber TB. A model organism approach: defining the role of Neph proteins as regulators of neuron and kidney morphogenesis. Hum Mol Genet. 2010;19:2347–2359. doi: 10.1093/hmg/ddq108. [DOI] [PubMed] [Google Scholar]
- Nishibori Y, Katayama K, Parikka M, Oddsson A, Nukui M, Hultenby K, Wernerson A, He B, Ebarasi L, Raschperger E, Norlin J, Uhlén M, Patrakka J, Betsholtz C, Tryggvason K. Glcci1 deficiency leads to proteinuria. J Am Soc Nephrol. 2011;22:2037–2046. doi: 10.1681/ASN.2010111147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obholzer N, Swinburne IA, Schwab E, Nechiporuk AV, Nicolson T, Megason SG. Rapid positional cloning of zebrafish mutations by linkage and homozygosity mapping using whole-genome sequencing. Development. 2012;139:4280–4290. doi: 10.1242/dev.083931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Brien LL, Grimaldi M, Kostun Z, Wingert RA, Selleck R, Davidson AJ. Wt1a, Foxc1a, and the notch mediator rbpj physically interact and regulate the formation of podocytes in zebrafish. Dev Biol. 2011;358:318–330. doi: 10.1016/j.ydbio.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz A, Covic A, Fliser D, Fouque D, Goldsmith D, Kanbay M, Mallamaci F, Massy ZA, Rossignol P, Vanholder R, Wiecek A, Zoccali C, London GM Board of the EURECA-m Working Group of ERA-EDTA. Epidemiology, contributors to, and clinical trials of mortality risk in chronic kidney failure. Lancet. 2014;383:1831–1843. doi: 10.1016/S0140-6736(14)60384-6. [DOI] [PubMed] [Google Scholar]
- Pabst R, Sterzel RB. Cell renewal of glomerular cell types in normal rats. an autoradiographic analysis. Kidney Int. 1983;24:626–631. doi: 10.1038/ki.1983.203. [DOI] [PubMed] [Google Scholar]
- Patrakka J, Tryggvason K. New insights into the role of podocytes in proteinuria. Nat Rev Nephrol. 2009;5:463–468. doi: 10.1038/nrneph.2009.108. [DOI] [PubMed] [Google Scholar]
- Patrakka J, Tryggvason K. Molecular make-up of the glomerular filtration barrier. Biochem Biophys Res Commun 2010. 2010;396:164–9. doi: 10.1016/j.bbrc.2010.04.069. [DOI] [PubMed] [Google Scholar]
- Pattaro C, Köttgen A, Teumer A, Garnaas M, Böger CA, Fuchsberger C, Olden M, Chen MH, Tin A, Taliun D, Li M, Gao X, Gorski M, Yang Q, Hundertmark C, Foster MC, O’Seaghdha CM, Glazer N, Isaacs A, Liu CT, Smith AV, O’Connell JR, Struchalin M, Tanaka T, Li G, Johnson AD, Gierman HJ, Feitosa M, Hwang SJ, Atkinson EJ, Lohman K, Cornelis MC, Johansson Å, Tönjes A, Dehghan A, Chouraki V, Holliday EG, Sorice R, Kutalik Z, Lehtimäki T, Esko T, Deshmukh H, Ulivi S, Chu AY, Murgia F, Trompet S, Imboden M, Kollerits B, Pistis G, Harris TB, Launer LJ, Aspelund T, Eiriksdottir G, Mitchell BD, Boerwinkle E, Schmidt H, Cavalieri M, Rao M, Hu FB, Demirkan A, Oostra BA, de Andrade M, Turner ST, Ding J, Andrews JS, Freedman BI, Koenig W, Illig T, Döring A, Wichmann HE, Kolcic I, Zemunik T, Boban M, Minelli C, Wheeler HE, Igl W, Zaboli G, Wild SH, Wright AF, Campbell H, Ellinghaus D, Nöthlings U, Jacobs G, Biffar R, Endlich K, Ernst F, Homuth G, Kroemer HK, Nauck M, Stracke S, Völker U, Völzke H, Kovacs P, Stumvoll M, Mägi R, Hofman A, Uitterlinden AG, Rivadeneira F, Aulchenko YS, Polasek O, Hastie N, Vitart V, Helmer C, Wang JJ, Ruggiero D, Bergmann S, Kähönen M, Viikari J, Nikopensius T, Province M, Ketkar S, Colhoun H, Doney A, Robino A, Giulianini F, Krämer BK, Portas L, Ford I, Buckley BM, Adam M, Thun GA, Paulweber B, Haun M, Sala C, Metzger M, Mitchell P, Ciullo M, Kim SK, Vollenweider P, Raitakari O, Metspalu A, Palmer C, Gasparini P, Pirastu M, Jukema JW, Probst-Hensch NM, Kronenberg F, Toniolo D, Gudnason V, Shuldiner AR, Coresh J, Schmidt R, Ferrucci L, Siscovick DS, van Duijn CM, Borecki I, Kardia SL, Liu Y, Curhan GC, Rudan I, Gyllensten U, Wilson JF, Franke A, Pramstaller PP, Rettig R, Prokopenko I, Witteman JC, Hayward C, Ridker P, Parsa A, Bochud M, Heid IM, Goessling W, Chasman DI, Kao WH, Fox CS CARDIoGRAM Consortium; ICBP Consortium; CARe Consortium; Wellcome Trust Case Control Consortium 2 (WTCCC2) Genome-wide association and functional follow-up reveals new loci for kidney function. PLoS Genet. 2012;8:e1002584. doi: 10.1371/journal.pgen.1002584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perner B, Englert C, Bollig F. The wilms tumor genes wt1a and wt1b control different steps during formation of the zebrafish pronephros. Dev Biol. 2007;309:87–96. doi: 10.1016/j.ydbio.2007.06.022. [DOI] [PubMed] [Google Scholar]
- Peterson RT, Link BA, Dowling JE, Schreiber SL. Small molecule developmental screens reveal the logic and timing of vertebrate development. Proc Natl Acad Sci USA. 2000;97:12965–12969. doi: 10.1073/pnas.97.24.12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson RT, Fishman MC. Designing zebrafish chemical screens. Methods Cell Biol. 2011;105:525–541. doi: 10.1016/B978-0-12-381320-6.00023-0. [DOI] [PubMed] [Google Scholar]
- Pickart MA, Klee EW. Zebrafish approaches enhance the translational research tackle box. Transl Res. 2014;163:65–78. doi: 10.1016/j.trsl.2013.10.007. [DOI] [PubMed] [Google Scholar]
- Picker A, Scholpp S, Böhli H, Takeda H, Brand M. A novel positive transcriptional feedback loop in midbrain-hindbrain boundary development is revealed through analysis of the zebrafish pax2.1 promoter in transgenic lines. Development. 2002;129:3227–3239. doi: 10.1242/dev.129.13.3227. [DOI] [PubMed] [Google Scholar]
- Poureetezadi SJ, Wingert RA. Congenital and acute kidney disease: translational research insights from zebrafish chemical genetics. General Med. 2013;1:112. doi: 10.4172/2327-5146.1000112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poureetezadi SJ, Donahue E, Wingert RA. High-throughput manual small molecule screening in zebrafish embryos. J Vis Exp. 2014 doi: 10.3791/52063. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaggin SE, Kreidberg JA. Development of the renal glomerulus: good neighbors and good fences. Development. 2008;135:609–620. doi: 10.1242/dev.001081. [DOI] [PubMed] [Google Scholar]
- Raschperger E, Neve EPA, Wernerson A, Hultenby K, Pettersson RF, Majumdar A. The coxsackie and adenovirus receptor (CAR) is required for renal epithelial differentiation within the zebrafish pronephros. Dev Biol. 2008;313:455–464. doi: 10.1016/j.ydbio.2007.10.049. [DOI] [PubMed] [Google Scholar]
- Reilly R, Bulger R, Kriz W. Diseases of the Kidney and Urinary Tract. Philadelphia: Lippincott Williams & Wilkins; 2007. Structural-functional relationships in the kidney; pp. 2–53. [Google Scholar]
- Reimschuessel R, Bennett RO, May EB, Lipsky MM. Renal tubular cell regeneration, cell proliferation and chronic nephrotoxicity in the goldfish Carassius auratus following exposure to a single sublethal dose of hexachlorobutadiene. Diseas Aquat Organ. 1990;8:211–224. [Google Scholar]
- Reimschuessel R, Williams D. Development of new nephrons in adult kidneys following gentamicin-induced nephrotoxicity. Ren Fail. 1995;17:101–106. doi: 10.3109/08860229509026246. [DOI] [PubMed] [Google Scholar]
- Reimschuessel R. A fish model of renal regeneration and development. Ilar j. 2001;42:285–291. doi: 10.1093/ilar.42.4.285. [DOI] [PubMed] [Google Scholar]
- Reiser J, Gupta V, Kistler AD. Toward the development of podocyte-specific drugs. Kid Int. 2010;77:662–668. doi: 10.1038/ki.2009.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rider SA, Tucker CS, del-Pozo J, Rose KN, MacRae CA, Bailey MA, Mullins JJ. Techniques for the in vivo assessment of cardio-renal function in zebrafish (Danio rerio) larvae. J Physiol. 2012;590:1803–1809. doi: 10.1113/jphysiol.2011.224352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodewald R, Karnovsky MJ. Porous substructure of the glomerular slit diaphragm in the rat and mouse. J Cell Biol. 1974;60:423–433. doi: 10.1083/jcb.60.2.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan GB. The glomerular sieve and the mechanisms of proteinuria. Aust N Z J Med. 1981;11:197–206. doi: 10.1111/j.1445-5994.1981.tb04233.x. [DOI] [PubMed] [Google Scholar]
- Ryan S, Willer J, Marjoram L, Bagwell J, Mankiewicz J, Leshchiner I, Goessling W, Bagnat M, Katsanis N. Rapid identification of kidney cyst mutations by whole exome sequencing in zebrafish. Development. 2013;140:4445–4451. doi: 10.1242/dev.101170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagrinati C, Netti GS, Mazzinghi B, Lazzeri E, Liotta F, Frosali F, Ronconi E, Meini C, Gacci M, Squecco R, Carini M, Gesualdo L, Francini F, Maggi E, Annunziato F, Lasagni L, Serio M, Romagnani S, Romagnani P. Isolation and characterization of multipotent progenitor cells from the bowman’s capsule of adult human kidneys. J Am Soc Nephrol. 2006;17:2443–2456. doi: 10.1681/ASN.2006010089. [DOI] [PubMed] [Google Scholar]
- Salice CJ, Rokous JS, Kane AS, Reimschuessel R. New nephron development in goldfish (Carassius auratus) kidneys following repeated gentamicin-induced nephrotoxicosis. Comp Med. 2001;51:56–59. [PubMed] [Google Scholar]
- Santoriello C, Zon LI. Hooked! modeling human disease in zebrafish. J Clin Invest. 2012;122:2337–2343. doi: 10.1172/JCI60434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxen L. Organogenesis of the kidney. Cambridge: Cambridge University Press; 1987. pp. 1–173. [Google Scholar]
- Scheer N, Campos-Ortega JA. Use of the Gal4-UAS technique for targeted gene expression in zebrafish. Mech Dev. 1999;80:153–158. doi: 10.1016/s0925-4773(98)00209-3. [DOI] [PubMed] [Google Scholar]
- Schein V, Cardoso JCR, Pinto PIS, Anjos L, Silva N, Power DM, Canario AVM. Four stanniocalcin genes in teleost fish: structure, phylogenetic analysis, tissue distribution and expression during hypercalcemic challenge. Gene Comp Endocrinol. 2012;175:344–356. doi: 10.1016/j.ygcen.2011.11.033. [DOI] [PubMed] [Google Scholar]
- Scott EK, Mason L, Arrenberg AB, Ziv L, Gosse NJ, Xiao T, Chi NC, Asakawa K, Kawakami K, Baier H. Targeting neural circuitry in zebrafish using GAL4 enhancer trapping. Nat Methods. 2007;4:323–326. doi: 10.1038/nmeth1033. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Nishibori Y, Akimoto Y, Kudo A, Ito N, Fukuhara D, Kurayama R, Higashihara E, Babu E, Kanai Y, Asanuma K, Nagata M, Majumdar A, Tryggvason K, Yan K. Amino acid transporter LAT3 is required for podocyte development and function. J Am Soc Nephrol. 2009;20:1586–1596. doi: 10.1681/ASN.2008070809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serluca FC, Fishman MC. Pre-pattern in the pronephric kidney field of zebrafish. Development. 2001;128:2233–2241. doi: 10.1242/dev.128.12.2233. [DOI] [PubMed] [Google Scholar]
- Serluca FC, Drummond IA, Fishman MC. Endothelial signaling in kidney morphogenesis: A role for hemodynamic forces. Curr Biol. 2002;12:492–497. doi: 10.1016/s0960-9822(02)00694-2. [DOI] [PubMed] [Google Scholar]
- Shankland SJ. The podocyte’s response to injury: Role in proteinuria and glomerulosclerosis. Kidney Int. 2006;69:2131–2147. doi: 10.1038/sj.ki.5000410. [DOI] [PubMed] [Google Scholar]
- Smeets B, Moeller MJ. Parietal epithelial cells and podocytes in glomerular diseases. Semin Nephrol. 2012;32:357–367. doi: 10.1016/j.semnephrol.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Song B, Smink AM, Jones CV, Callaghan JM, Firth SD, Bernard CA, Laslett AL, Kerr PG, Ricardo SD. The directed differentiation of human iPS cells into kidney podocytes. PLoS One. 2012;7:e46453. doi: 10.1371/journal.pone.0046453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suster ML, Kikuta H, Urasaki A, Asakawa K, Kawakami K. Transgenesis in zebrafish with the tol2 transposon system. Methods Mol Biol. 2009a;561:41–63. doi: 10.1007/978-1-60327-019-9_3. [DOI] [PubMed] [Google Scholar]
- Suster ML, Sumiyama K, Kawakami K. Transposon-mediated BAC transgenesis in zebrafish and mice. BMC Genomics. 2009b;10:477-2164-10-477. doi: 10.1186/1471-2164-10-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanhart LM, Takahashi N, Jackson RL, Gibson GA, Watkins SC, Dawid IB, Hukriede NA. Characterization of an lhx1a transgenic reporter in zebrafish. Int J Dev Biol. 2010;54:731–736. doi: 10.1387/ijdb.092969ls. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanhart LM, Cosentino CC, Diep CQ, Davidson AJ, de Caestecker M, Hukriede NA. Zebrafish kidney development: Basic science to translational research. Birth Defects Res C Embryo Today. 2011;93:141–156. doi: 10.1002/bdrc.20209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemoto M, He L, Norlin J, Patrakka J, Xiao Z, Petrova T, Bondjers C, Asp J, Wallgard E, Sun Y, Samuelsson T, Mostad P, Lundin S, Miura N, Sado Y, Alitalo K, Quaggin SE, Tryggvason K, Betsholtz C. Large-scale identification of genes implicated in kidney glomerulus development and function. EMBO J. 2006;25:1160–1174. doi: 10.1038/sj.emboj.7601014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tena JJ, Neto A, de la Calle-Mustienes E, Bras-Pereira C, Casares F, Gomez-Skarmeta JL. Odd-skipped genes encode repressors that control kidney development. Dev Biol. 2007;301:518–531. doi: 10.1016/j.ydbio.2006.08.063. [DOI] [PubMed] [Google Scholar]
- Thummel R, Bai S, Sarras MP, Jr, Song P, McDermott J, Brewer J, Perry M, Zhang X, Hyde DR, Godwin AR. Inhibition of zebrafish fin regeneration using in vivo electroporation of morpholinos against fgfr1 and msxb. Dev Dyn. 2006;235:336–346. doi: 10.1002/dvdy.20630. [DOI] [PubMed] [Google Scholar]
- Thummel R, Kassen SC, Montgomery JE, Enright JM, Hyde DR. Inhibition of muller glial cell division blocks regeneration of the light-damaged zebrafish retina. Dev Neurobiol. 2008;68:392–408. doi: 10.1002/dneu.20596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomar R, Mudumana SP, Pathak N, Hukreide NA, Drummond IA. osr1 is required for podocyte development downstream of wt1a. J Am Soc Nephrol. 2014 doi: 10.1681/ASN.2013121327. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topczewska JM, Topczewski J, Solnica-Krezel L, Hogan BL. Sequence and expression of zebrafish foxc1a and foxc1b, encoding conserved forkhead/winged helix transcription factors. Mech Dev. 2001;100:343–347. doi: 10.1016/s0925-4773(00)00534-7. [DOI] [PubMed] [Google Scholar]
- Toyama R, Dawid IB. lim6, a novel LIM homeobox gene in the zebrafish: comparison of its expression pattern with lim1. Dev Dyn. 1997;209:406–417. doi: 10.1002/(SICI)1097-0177(199708)209:4<406::AID-AJA8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Urnov FD, Rebar EJ, Holmes MC, Zhang HS, Gregory PD. Genome editing with engineered zinc finger nucleases. Nat Rev Genet. 2010;11:636–646. doi: 10.1038/nrg2842. [DOI] [PubMed] [Google Scholar]
- Vogetseder A, Karadeniz A, Kaissling B, Le Hir M. Tubular cell proliferation in the healthy rat kidney. Histochem Cell Biol. 2005;124:97–104. doi: 10.1007/s00418-005-0023-y. [DOI] [PubMed] [Google Scholar]
- Voz ML, Coppieters W, Manfroid I, Baudhuin A, Von Berg V, Charlier C, Meyer D, Driever W, Martial JA, Peers B. Fast homozygosity mapping and identification of a zebrafish ENU-induced mutation by whole-genome sequencing. PLoS ONE. 2012;7:e34671. doi: 10.1371/journal.pone.0034671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Lehtonen S, Chen YC, Heikkilä E, Panula P, Holthöfer H. Neph3 associates with regulation of glomerular and neural development in zebrafish. Differentiation. 2012;83:38–46. doi: 10.1016/j.diff.2011.08.007. [DOI] [PubMed] [Google Scholar]
- Watanabe N1, Kato M, Suzuki N, Inoue C, Fedorova S, Hashimoto H, Maruyama S, Matsuo S, Wakamatsu Y. Kidney regeneration through nephron neogenesis in medaka. Develop Growth Differ. 2009;51:135–143. doi: 10.1111/j.1440-169X.2009.01090.x. [DOI] [PubMed] [Google Scholar]
- White JT, Zhang B, Cerqueira DM, Tran U, Wessely O. Notch signaling, wt1 and foxc2 are key regulators of the podocyte gene regulatory network in Xenopus. Development. 2010;137:1863–1873. doi: 10.1242/dev.042887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggins RC. The spectrum of podocytopathies: A unifying view of glomerular diseases. Kidney Int. 2007;71:1205–1214. doi: 10.1038/sj.ki.5002222. [DOI] [PubMed] [Google Scholar]
- Wingert RA, Selleck R, Yu J, Song HD, Chen Z, Song A, Zhou Y, Thisse B, Thisse C, McMahon AP, Davidson AJ. The cdx genes and retinoic acid control the positioning and segmentation of the zebrafish pronephros. PLoS Genet. 2007;3:1922–1938. doi: 10.1371/journal.pgen.0030189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingert RA, Davidson AJ. The zebrafish pronephros: A model to study nephron segmentation. Kidney Int. 2008;73:1120–1127. doi: 10.1038/ki.2008.37. [DOI] [PubMed] [Google Scholar]
- Wingert RA, Davidson AJ. Zebrafish nephrogenesis involves dynamic spatiotemporal expression changes in renal progenitors and essential signals from retinoic acid and irx3b. Dev Dyn. 2011;240:2011–2027. doi: 10.1002/dvdy.22691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Boucher RC, Bollig F, Englert C, Hildebrandt F. Characterization of mesonephric development and regeneration using transgenic zebrafish. Am J Physiol Renal Physiol. 2010;299:F1040–F1047. doi: 10.1152/ajprenal.00394.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou W, Hildebrandt F. Inducible podocyte injury and proteinuria in transgenic zebrafish. J Am Soc Nephrol. 2012;23:1039–1047. doi: 10.1681/ASN.2011080776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Zon LI. The Zon laboratory guide to positional cloning in zebrafish. Methods Cell Biol. 2011;104:287–309. doi: 10.1016/B978-0-12-374814-0.00016-1. [DOI] [PubMed] [Google Scholar]