Abstract
Background
The therapeutic mechanisms of deep brain stimulations (DBS) are not fully understood. Axonal block induced by high frequency stimulation (HFS) has been suggested as one possible underlying mechanism of DBS.
Objective
To investigate the mechanism of the generation of HFS-induced axonal block.
Methods
High frequency pulse trains were applied to the fiber tracts of alveus and Schaffer collaterals in the hippocampal CA1 neurons in anaesthetized rats at 50, 100 and 200 Hz. The amplitude changes of antidromic-evoked population spikes (APS) were measured to determine the degree of axonal block. The amplitude ratio of paired-pulse evoked APS was used to assess the changes of refractory period.
Results
There were two distinct recovery stages of axonal block following the termination of HFS. One frequency-dependent faster phase followed by another frequency-independent slower phase. Experiments with specially designed temporal patterns of stimulation showed that HFS produced an extension of the duration of axonal refractory period thereby causing a fast recovery phase of the axonal block. Thus, prolonged gaps inserted within HFS trains could eliminate the axonal block and induced large population spikes and even epileptiform activity in the upstream or downstream regions.
Conclusions
Extension of refractory period plays an important role on HFS induced axonal block. Stimulation pattern with properly designed pauses could be beneficial for different requirements of excitation or inhibition in DBS therapies.
Keywords: deep brain stimulation, axon block, refractory period, hippocampus, temporal patterns of stimulation
1. Introduction
The application of deep brain stimulations (DBS) in the clinic has generated significant excitement as its therapeutic applications have been extended to various brains disorders such as Parkinson’s disease, epilepsy, pain, addiction, stroke and depression [1–5]. However, its therapeutic mechanisms are not fully understood. Two important questions remain unanswered: 1) why frequencies greater than 100 Hz are required to produce therapeutic efficacy [6,7]; and 2) which temporal pattern of DBS is more effective: continuous or intermittent stimulation [8,9]?
Recently, strong evidence has shown that the conduction of axons can be blocked by high frequency stimulation (HFS) in the hippocampus and subthalamus both in in-vivo and in-vitro preparation [10–15]. In particular, this axonal block can provide a reversible functional disconnection in both the afferent and efferent axons of hippocampal neurons by HFS with frequencies over 100 Hz [13]. Considering that axonal conduction is essential for the normal function of any complex neuronal networks [6], the effect of HFS on axons could have significant implication on brain function. The goal of the present study is to answer the two questions listed above by focusing on the axonal effects of HFS.
With the application of repeated stimuli of HFS, axons can undergo activity-dependent changes resulting in conduction failures. The changes involve the kinetics alteration of ion channel (e.g., channel inactivation) and the accumulation of ions in intracellular and extracellular areas [16–18] with different time constants. On one hand, the time constant of ion channel kinetics is very short, e.g., ~1 ms activated duration of sodium channels [19], suggesting a fast process. On the other hand, the changes of ionic concentrations, such as increase of extracellular potassium concentration ([K+]o) during intense firings of action potentials and diffusion of the accumulation [K+]o, suggest a slower process [20]. Therefore, we hypothesized that multiple mechanisms with different time constants could be responsible for both the development and the recovery of axonal failures.
In order to test this hypothesis, we applied HFS with biphasic pulses in the alveus fiber tract of hippocampal CA1 pyramidal cells to induce axonal block in anaesthetized rats. Previous studies indicate that the effective frequency of DBS is in the range of 100–200 Hz, especially 130 Hz. Therefore, we chose 50, 100 and 200 Hz in a wider frequency range to investigate the frequency-dependence of HFS effects. The time courses of the axon failure were examined respectively during and following HFS trains by evaluating the antidromically-evoked population spikes. Two distinct recovery stages of the axon failure with significantly different time constants were observed following HFS with different stimulation frequencies. Furthermore, we studied the mechanism underlying the effect of HFS on axonal block by measuring the changes of refractory period of axons during high frequency stimulation.
2. Methods and Materials
2.1 Surgical procedures
All procedures used in this study were carried out in accordance with the Guide for the Care and Use of Laboratory Animals (China Ministry of Health). Adult Sprague Dawley rats (250 – 350 g) were anesthetized with urethane (1.25 g/kg, i.p.) and placed in a stereotaxic apparatus (Stoelting Co.). Body temperature was maintained at ~37 °C by a wrap with a heating patch and towels. The skull was partially opened to allow the placement of electrodes. A 16 channel recording electrode array (NeuroNexus Technologies) was inserted into the hippocampal CA1 region (AP, −3.5; ML, 2.7; DV 2.5). Two stimulation electrodes, concentric bipolar stainless-steel electrodes (FHC, Bowdoin, ME 04287, USA), were positioned respectively in two axonal fiber tracts in CA1: the Schaffer collaterals (AP, −2.2; ML, 2.0; DV 2.8) for orthodromic stimulation and the alveus (AP, −4.8; ML, 2.7; DV 2.3) for antidromic stimulation. Two separate stainless steel screws were fixed in the nose bone and served as reference electrode and ground electrode. Both multiple unit activity (MUA) and patterns of the evoked potentials at each of the 16 channel recordings were judged to guide the final positions of the recording probe and the stimulation electrodes. Saline was placed over the exposed surface of the dura to keep it moist [13]. At the end of each experiment, the rat was immediately euthanized by an intracardiac injection of saturated potassium chloride.
2.2 Recording and Stimulation
Potential signals collected by recording probes were amplified by a 16-channel extracellular amplifier (Model 3600, A-M system Inc.) with a filtering frequency range of 0.3 Hz - 5 kHz. The amplified signals were then sampled by a ML880 Powerlab 16/30 data acquisition system (ADInstruments Inc.) at a sampling rate of 20 kHz/channel with 16-bit precision, and were stored into a hard disk for off-line analysis. The channel with the largest evoked population spikes (PS) and dense unit activity was taken as the location of the pyramidal layer of the CA1 region and the PS amplitudes were calculated.
Custom-made MATLAB codes were used to remove the stimulation artifacts during HFS by linearly interpolating ~1.0 ms signal at each stimulation point. The algorithm was able to remove the artifacts with minimal distortion of the neural signals [13].
Biphasic HFS pulses with constant currents were generated by the Model 2100 isolated pulse stimulator (A-M system Inc.) with a pulse duration of 0.1 ms per phase. According to input-output curves of the orthodromic- or antidromic-stimulations, a current intensity (0.3 – 0.5 mA) that evoked PSs with an amplitude approximately 3/4 of the maximal PS amplitude was used as HFS stimulation intensity. The inter-pulse intervals (IPI) within HFS trains were set as 20, 10 and 5 ms to obtain the stimulation frequencies of 50, 100 and 200 Hz, respectively. The length of HFS train was set at 1 min. Test stimuli with an identical intensity as HFS were applied at various time points following HFS to show the recovery course of PS activity. Within some of the HFS trains, one prolonged IPI gap of 20 or 100 ms was inserted every 2 s to reveal the change of refractory period of axons. The intervals between sequential HFS trains were >30 min to ensure recovery from previous HFS.
All statistical data were represented as mean ± standard deviation. One-way ANOVA with Post hoc Bonferroni test were used to judge the statistical significance of the differences among data groups.
3 Results
3.1 Two distinct stages in axonal recovery following HFS trains
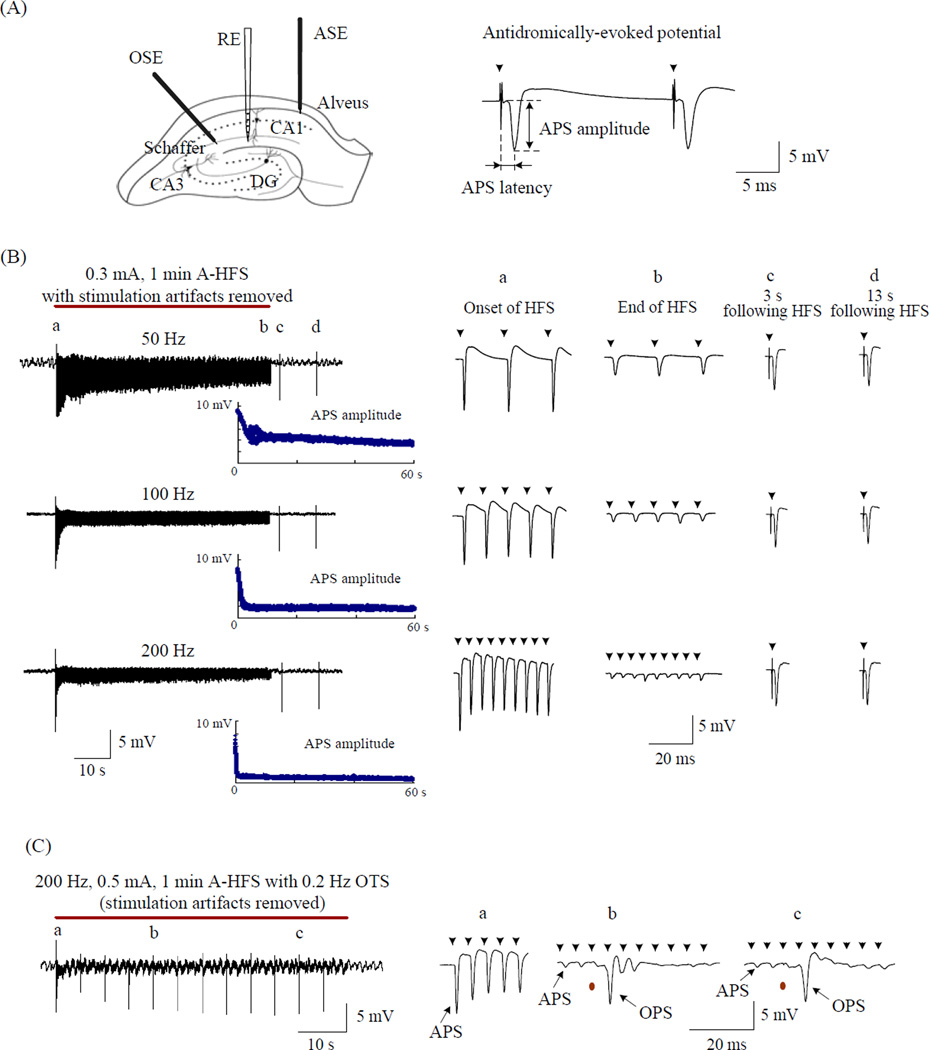
The axonal block induced by antidromic-HFS (A-HFS) and the time course of its recovery were investigated by stimulation of the alveus, a fiber tract consisting mainly of axons of the hippocampal CA1 pyramidal cells. Under normal condition, paired-pulse stimulation with a 20 ms IPI applied to the alveus induced two antidromically-evoked population spikes (APS) with similar large amplitudes (Fig. 1A). During 1 min long trains of A-HFS with frequencies of 50, 100 or 200 Hz, large APS waveforms were able to follow each stimulation pulse at the beginning of HFS. But, the APS amplitudes decreased rapidly towards the end of HFS trains. The suppression of the APS increased as the frequency of the HFS increased (Fig. 1B). Test pulses applied 3 s and 13 s following each HFS train evoked large and similar APS. Furthermore, the amplitudes of the partially recovered APS at the two time points were similar for all the HFS trains with three different frequencies, in spite of the fact that, at the end of HFS, the APS amplitudes were suppressed to a greater extent at higher frequencies.
Figure 1.
Rapid recovery of APS suppression following A-HFS. (A) Schematic diagram of the locations of recording electrode (RE) in hippocampal CA1 region, antidromic-stimulation electrodes (ASE) in the alveus, and orthdromic-stimulation electrodes (OSE) in the Schaffer collaterals, and an example of a paired-pulse evoked APS with inter-pulse interval of 20 ms. (B) Left: Examples of wide-band (0.3 Hz - 5 KHz) recorded signals in the CA1 pyramidal layer during 1 min long (denoted by the red bar) of 50, 100 and 200 Hz A-HFS with stimulation intensity of 0.3 mA. Amplitude scatter diagram of each evoked-APS is drawn below. Right: Expanded evoked APS waveforms at the time indicated by “a” (the onset of HFS), “b” (the end of HFS), “c” 3 s following HFS and “d” 13 s following HFS. Although the APS was suppressed greater at the end of 200 Hz HFS than 50 and 100 Hz, it recovered quickly to a large amplitude similar to the 50 and 100 Hz HFS at 3s following the HFS. (C) OPS evoked by 0.5 mA orthodromic test stimuli (OTS) during a 200 Hz A-HFS. Expanded waveforms on the right show large OPS maintained when the APS were suppressed markedly. In the expanded plots, arrows above the waveforms denote the removed artifacts of A-HFS, and the red dots below waveforms denote the removed artifacts of OTS.
As previously reported [13], the suppression of APS could be explained by axon failure since the large orthodromically-evoked PSs (OPS) could still be evoked by orthodromic test stimuli (OTS) during the A-HFS while the APS was markedly suppressed. Since action potentials in CA1 pyramidal cells are thought to be triggered in the axon initial segment, these results indicate that the neuronal cell bodies retained a high level of excitability while the axons were blocked (Fig. 1C). Therefore, in the present study, the amplitudes or areas of APS were used to evaluate the firing and conduction state of the alveus axons during and following HFS.
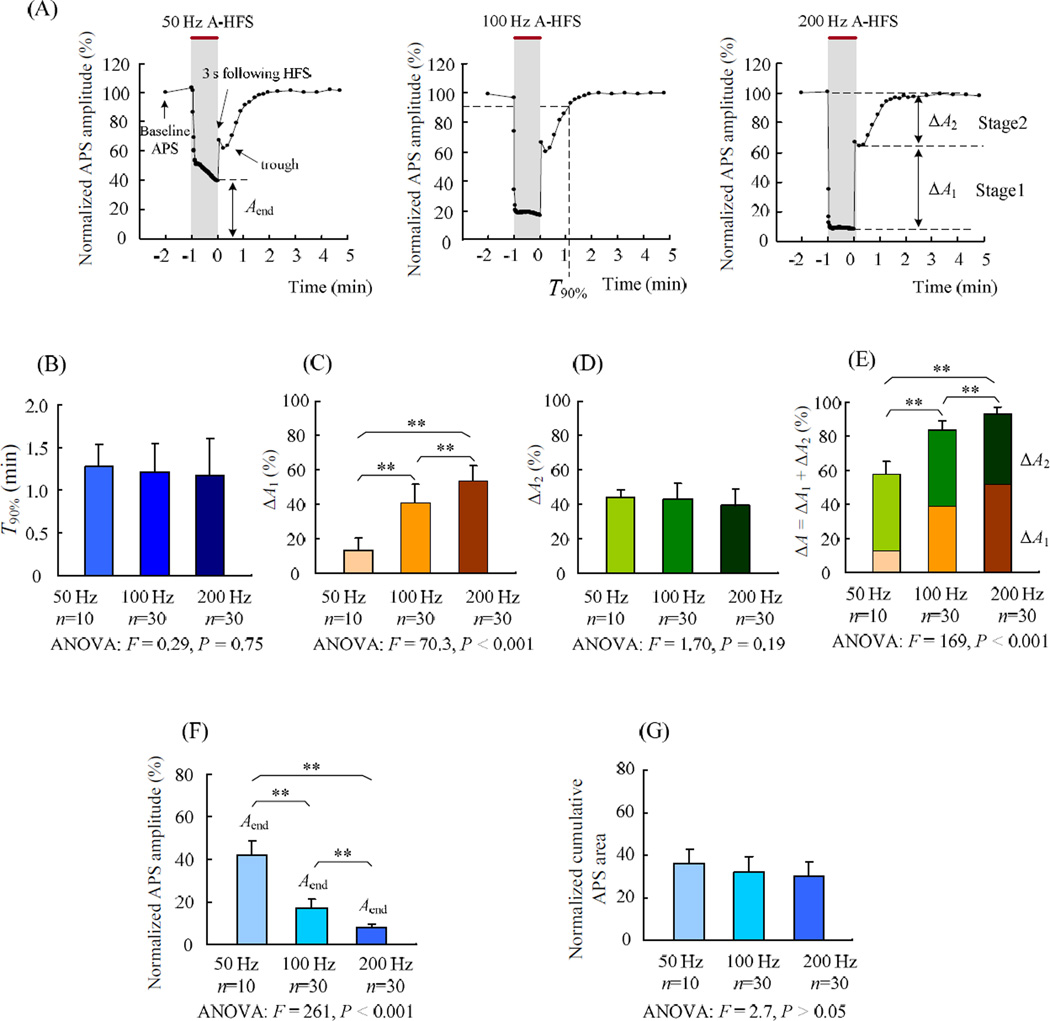
Although the recovery of the APS immediately following HFS train appeared to be rapid, it was not complete (Fig. 1B). In order to determine the full recovery time course of axon failure induced by A-HFS with different frequencies, we continued the application of test stimuli for 5 min following HFS trains (Fig. 2A). The interval between the test stimuli was set as 10 s for the first 2 min of the test (with the first test stimulus applied at 3 s following the HFS) and then changed to 30 s for the other 3 min. Interestingly, the recovery occurred in two distinct and consecutive phases (fast and slow phases). Within a few seconds following the HFS, the APS amplitude recovered quickly (fast phase). However, the complete recovery of the APS amplitude was much slower and took several minutes (slow phase)(Fig. 2A).
Figure 2.
Two recovery stages of APS following 1 min long A-HFS. The end of HFS was set as time 0. (A) Example curves of the APS amplitude normalized by baseline recording during and following 1 min long 50, 100 and 200 Hz A-HFS indicate that there were two distinct recovery stages of APS. Within 3 s following the HFS, the APS amplitude recovered rapidly (stage 1) Following stage 1, the APS dropped slightly before starting a slower recovery phase (stage 2). Shadings with red bars on top denote HFS durations. Each dot (except the 1st one) within the HFS period represents mean APS amplitude per second. The other dots following HFS represent each evoked APS. The two distinct stages in the recovery curves were separated by the lowest point after the 3 s point. (B) No significant difference in the recovery time T90% for 50, 100 and 200 Hz HFS was observed. (C) Recovery amounts of stage 1, ΔA1, significantly increased with the higher frequency. (D) There was no significant difference in the recovery amounts of stage 2, ΔA2, among the HFS groups. (E) Total amount of APS suppression, i.e., ΔA = ΔA1 + ΔA2, significantly increased with the higher frequency. (F) Mean APS amplitudes at the final 1 s of 1 min long HFS, Aend, significantly decreased with the higher frequency. (G) Cumulative APS areas within the final 1s of HFS normalized to the area of the 1st APS of HFS. The results of One-way ANOVA with Post hoc Bonferroni test are denoted in the plots of B – G: ** P < 0.001.
Based on these recovery curves, we defined two recovery stages (1 and 2) by setting the separation between them at the lowest point of the small “trough” after the 3 s point (see Fig. 2A). The mean time of the separation points was 15.9 ± 5.5 s for all of the total 70 trains of 50, 100 and 200 Hz HFS, and it was not significantly different among the three frequency groups. The time required for the APS to recover to 90% of baseline amplitude, T90%, was also not significantly different among the HFS groups with different frequencies (Fig. 2B). The mean value of T90% for all of the total 70 trains was 1.20 ± 0.37 min.
In stage 1, the amount of APS recovery amplitude normalized by the baseline APS amplitude, ΔA1, was frequency-dependent. It increased from ΔA1 = 13 ± 7.0 % (n = 10) of 50 Hz HFS to ΔA1 = 41 ± 11 % (n = 30) of 100 Hz HFS, and then to ΔA1 = 54 ± 9.0 % (n = 30) of 200 Hz HFS. The differences of ΔA1 among the three groups were significant and marked (Fig. 2C). However, in stage 2, the amount of APS recovery amplitude (ΔA2) was frequency-independent. There was no significant difference of ΔA2 among the three frequency groups (Fig. 2D). Taken together, there was a significant difference in the total recovery amount ΔA1 + ΔA2 (i.e., the suppression amount of APS amplitude by the HFS) for the three frequency groups (Fig. 2E).
The frequency-independent feature of the recovery stage 2 suggests a common mechanism underlying the effect of HFS applied at various frequencies. Presumably, this mechanism would be related to the intensity of neuronal firings induced by HFS. Considering that the intensity of firings depends on both the APS areas and the APS numbers for a given time period [21], we measured the sum of all the APS areas per one second window. Although the APS amplitude at the end (Aend) of 100 and 200 Hz HFS was much smaller than that of 50 Hz HFS (Fig. 1B and Fig. 2F), the number of APS induced by 100 and 200 Hz HFS were 2 and 4 times of 50 Hz HFS, respectively. At the final 1 s window of HFS, the cumulative areas of APS normalized by the 1st APS of HFS, Aarea, were 36 ± 6.6 (n = 10), 32 ± 7.7 (n = 30) and 30 ± 7.0 (n = 30) for the three frequency groups (Fig. 2G). The similar levels of APS areas generated at different frequencies support the hypothesis that the stage 2 recovery period is frequency-independent
3.2 Rapid recovery of axonal block induced by A-HFS
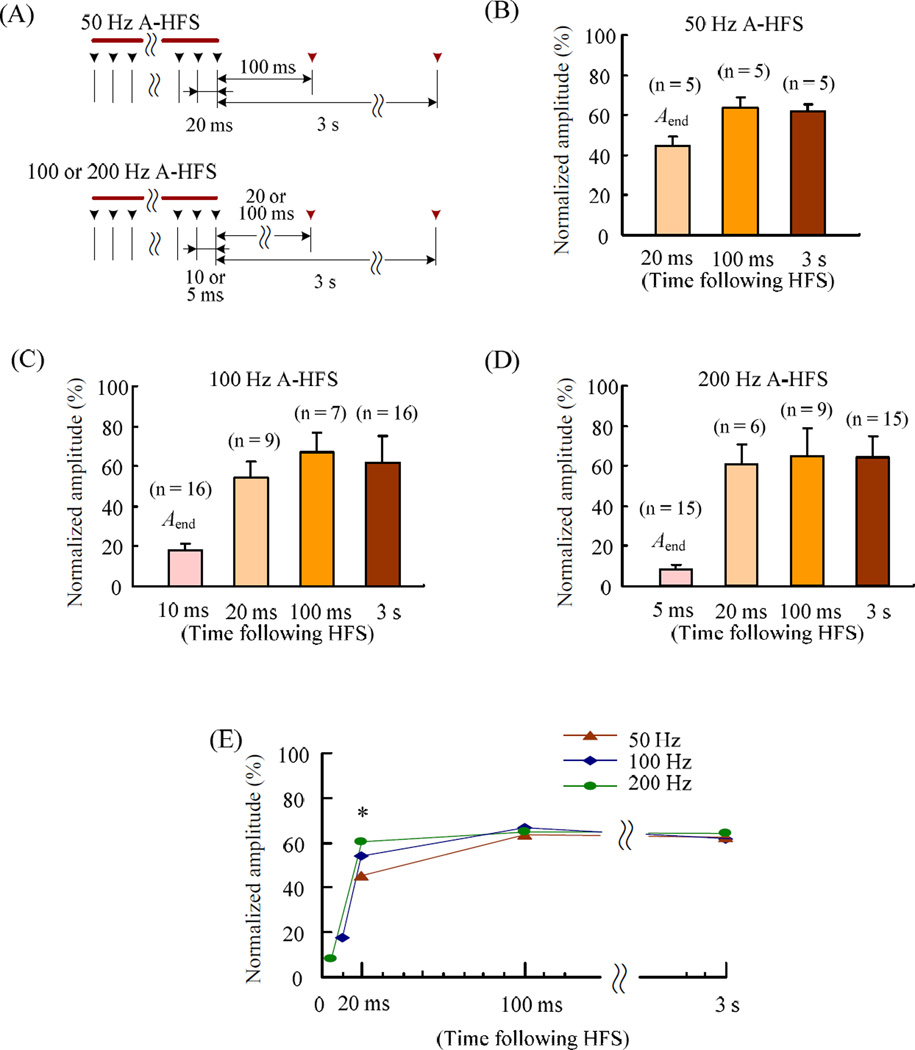
Although the slow recovery (stage 2) was frequency-independent, the fast recovery (stage 1) clearly was not. To study this recovery stage with higher time resolution, additional test stimuli were applied 20 and 100 ms immediately following A-HFS before the 3 s test. To avoid possible interactions among test pulses too close in time, the 20 and 100 ms tests were applied in separated HFS trials. For the 50 Hz HFS, because its IPI was just 20 ms, the final stimulus of HFS was treated as 20 ms test (Fig. 3A). Similarly, the final stimuli of the 100 and 200 Hz HFS trains were respectively treated as 10 and 5 ms test for comparison. The APS evoked by the additional test stimuli was compared with the Aend (see Fig. 2A and 2F) and together with APS evoked by the 3 s tests for the different frequencies of 50, 100 and 200 Hz (Fig. 3B – 3D). Even at 100 ms test, the APS already recovered to a level similar to the 3 s test for all of the frequencies. Interestingly, although the Aend of 200 Hz HFS was significantly smaller than those of 50 and 100 Hz HFS (Fig. 2F), at 20 ms test point, its APS jumped to a level higher than 50 and 100 Hz HFS (Fig. 3E).
Figure 3.
Recovery time courses of APS suppression immediately following A-HFS with different stimulation frequencies. (A) Sequence of test stimuli that were added at 20 or 100 ms and 3 s immediately following HFS trains. The black and the red arrows respectively denote the pulses within and following the HFS. The plots (B) – (D) show the statistical data of APS amplitudes normalized to baseline recordings for HFS trains of 50, 100 and 200 Hz with additional 20 or 100 ms test. The Aend bars were the mean APS amplitudes at the final 1 s of 1 min long HFS (see Fig 2), representing the final APS at intervals of 20, 10 and 5 ms in the respective frequency. To avoid possible interactions among test pulses too close in time, the 20 and 100 ms tests added in separated 100 and 200 Hz A-HFS trials, and the data were pooled in the plots. (G) Summary data collected from (B) – (D). The error bars are omitted for clarity since they are already showed in plots (B) – (D). * One-way ANOVA F = 5.27, P = 0.017; P < 0.02, Post hoc Bonferroni test, 50 Hz vs. 200 Hz.
Thus, a substantial portion of the amplitude suppression induced by higher frequency of 100 and 200 Hz stimulation could recover even within a short time duration less than 20 ms. This fast recovery suggests that the APS suppression could be related to the refractory period of axons. The HFS might extend the refractory period to a duration longer than 5 or 10 ms of the intervals of 200 and 100 Hz HFS thereby causing axonal block and suppressing the APS. Therefore, we next tested this hypothesis by evaluating the changes of axonal refractory period during HFS trains.
3.3 HFS could extend the refractory period of axons
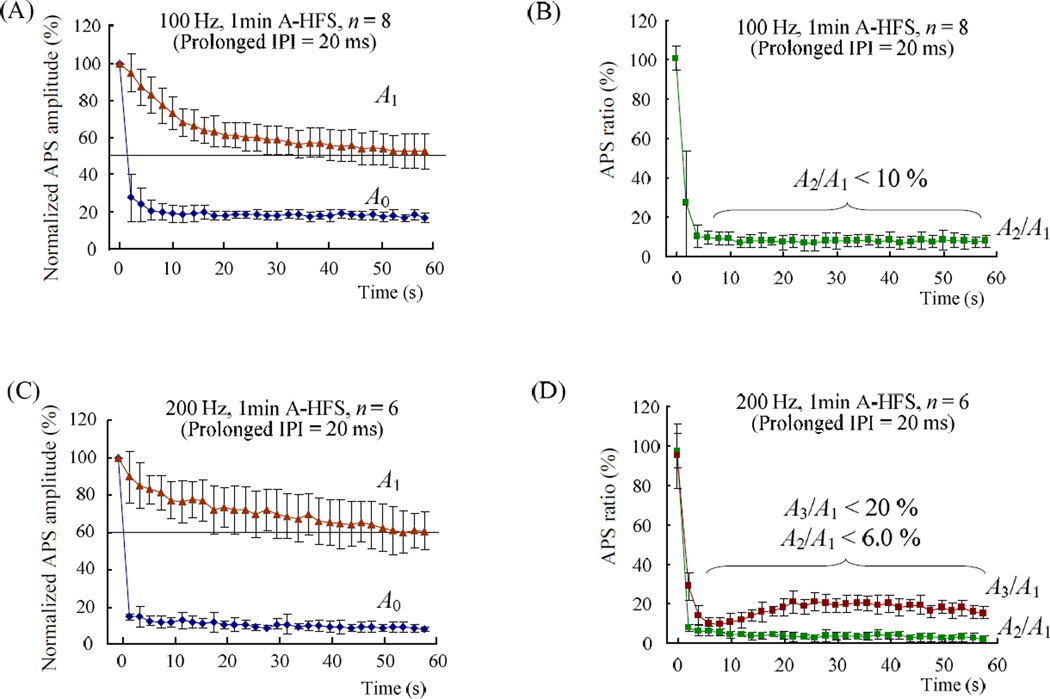
To determine the role of the refractory period (RP), the amplitude ratio of paired-pulse evoked APS was used to assess the changes of RP duration [11]. In addition, several prolonged intervals were inserted during HFS trains to obtain large enough APS for the detection of RP.
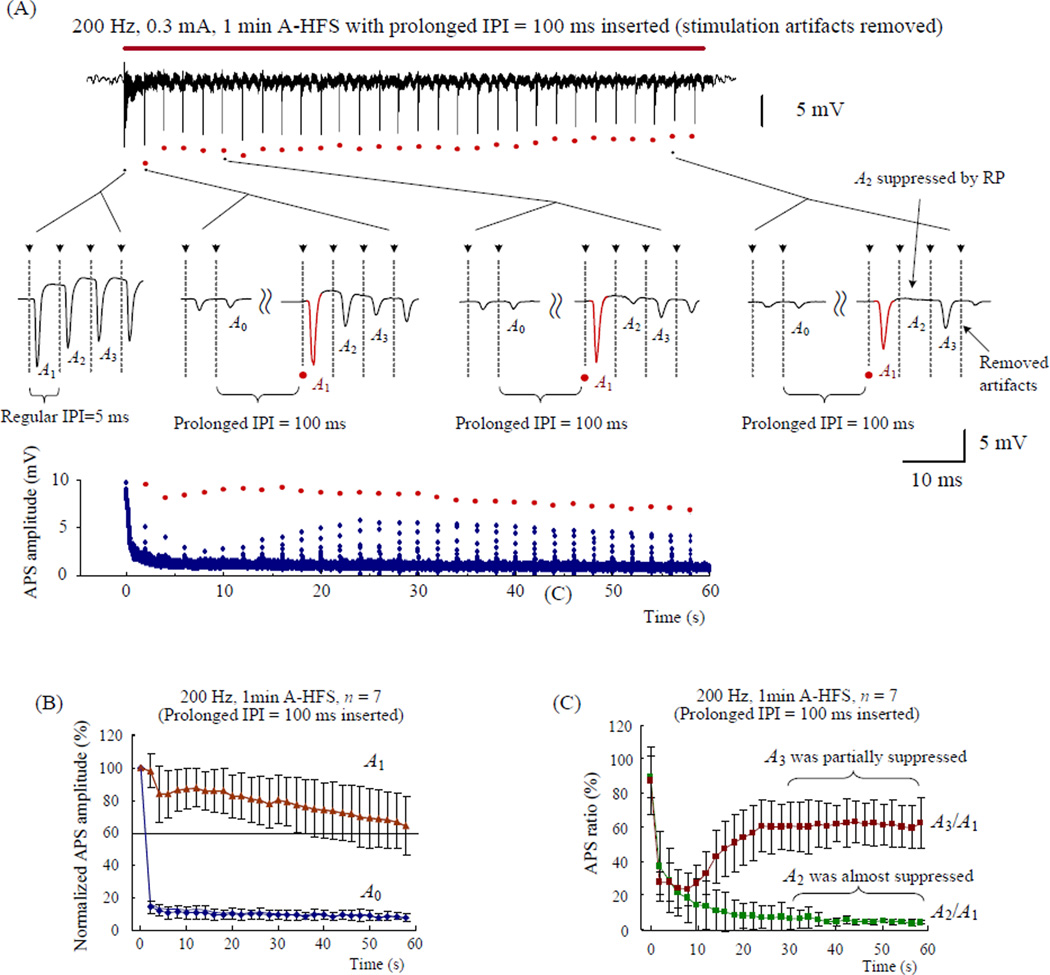
One prolonged IPI of 100 ms was inserted every 2 s to set a total of 29 gaps in 1 min long 200 Hz A-HFS trains (the IPI gaps are indicated by red dots in Fig. 4A). The APSs just following the gaps (the red waveforms termed A1 in Fig. 4A) were all significantly larger than the preceding response A0 that followed regular intervals (Fig. 4B). However, the second APS (termed A2) decreased and almost disappeared towards the end of HFS (Fig. 4A). The statistical data showed that (see Fig. 4C), at the very beginning of HFS, the amplitude ratio of the first paired-APS (i.e., A2/A1 for the 1th and 2th APS of HFS) was 90 ± 11 % (n = 7), indicating that A2 was not obviously suppressed. However, during the final 30 s period of HFS, A2/A1 < 7.0 % (n = 7), indicating that A2 at ~5 ms delay (the normal IPI of 200 Hz) was almost totally suppressed by the gradually extended RP of A1.
Figure 4.
The amplitude ratios of paired APS were used to assess the changes of refractory period during 200 Hz A-HFS trains. (A) Top: typical recording of 1 min long 0.3 mA 200 Hz A-HFS with prolonged IPI gaps inserted every 2 s. The red bar above the signal denotes the HFS durations. The red dots below the signal denote the evoked APSs subsequent to the gaps. Middle: expanded insets show the evoked APS waveforms at the onset of HFS (A2 ≈ A1) and around 3 examples of the total 29 gaps (A2 < A1). The APS waveforms subsequent to the prolonged IPI gaps (i.e., A1) are drawn by red color. The dashed lines with arrow heads in the expanded plots denote the location of the removed stimulation artifacts. Bottom: the scatter diagram of APS amplitude evoked by every stimulation pulse. The APS subsequent to the gaps (i.e., A1) are denoted by red dots. (B) Amplitudes of the preceding APS (A0) and subsequent APS (A1) to the gaps, normalized by the amplitude of the very first APS at the onset of HFS, indicate sufficient recovery of A1 while A0 was suppressed. (C) Ratios of A2/A1 and A3/A1 during HFS indicate that the refractory period extended to longer than 5 ms. Note: the regular interval of 200 Hz is 5 ms.
Next, we examined amplitude of A3 at ~10 ms from A1 by calculating the ratio of A3/A1 to determine whether the RP was long enough to suppress A3. The mean values of A3/A1 remained above 60 % during the final 30 s period of HFS while the amplitudes of A2 were small enough to be ignored (Fig. 4C). However, this result does not necessarily mean that the RP duration did not change to values longer than 10 ms. In order to overcome the effect of the gap duration on the detection of current RP, the 100 ms gap was next shortened to 20 ms.
The results of 20 ms gaps for both 100 and 200 Hz HFS are shown in Fig. 5. For 100 Hz HFS, the mean normalized amplitudes of A1 were all above 50% (n = 8, Fig. 5A), indicating sufficient recovery of APS subsequent to the 20 ms gaps. However, except ~ 4 s long period at the beginning of HFS, during the rest of HFS, A2 was suppressed markedly and A2/A1 < 10 % (n = 8, Fig. 5B), indicating that the RP of A1 was longer than 10 ms. For 200 Hz HFS with 20 ms gaps, the statistical data showed that A2/A1 < 6.0 % and A3/A1 < 20 % for most HFS period with enough large A1 > 60% (n = 6, Fig. 5C and 5D), indicating that the RP were also longer than 10 ms.
Figure 5.
Changes of the refractory period during 100 and 200 Hz A-HFS trains were assessed by inserting shorter gaps of 20 ms (refer Fig 4A for the definition of A0 ~ A3). (A) Normalized amplitudes of the preceding APS (A0) and subsequent APS (A1) to the 20 ms prolonged IPI gaps during 100 Hz A-HFS indicate sufficient recovery of A1 amplitudes. (B) Ratios of A2/A1 during 100 Hz A-HFS indicate that the refractory period produced by A1 extended to enough long to significantly suppress the A2 with ~10 ms delay. (C) Similar to (A), but for 200 Hz HFS. (D) Ratios of A2/A1 and A3/A1 during 200 Hz A-HFS also indicate that the refractory period extended to longer than 10 ms.
Taken together, the results suggest that during 100 and 200 Hz HFS trains, the duration of axonal RP was extended to values longer than 10 ms thereby suppressing the APS.
3.4 Persistant stimulation is required for continuous HFS effects
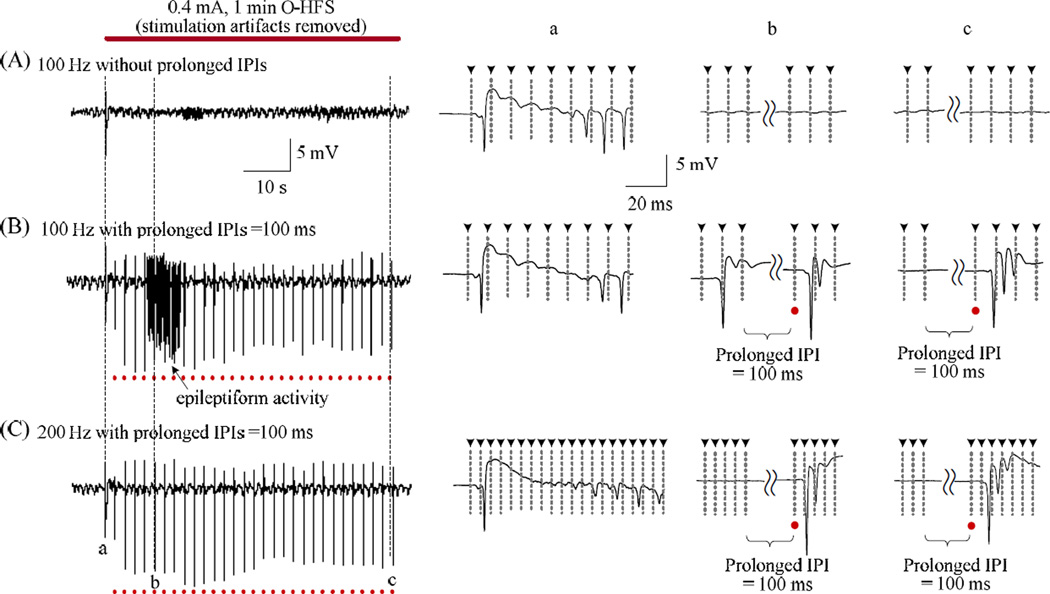
In the above experiments, HFS was applied at the alveus fiber of CA1 to study the conduction of axonal excitation and the responses of upstream neurons. However, since axons conduct bi-directionally, the effects of HFS could also propagate to the downstream neurons. Based on the rapid recovery feature of axonal block, we hypothesized that the stimulation of HFS should be persistent without intermission since prolonged gaps would allow axons to recover and propagate activation to downstream neurons, This hypothesis was tested by orthodromic-HFS (O-HFS), i.e., applying HFS on another fiber tract, the Schaffer collateral of CA1.
As showed in Figure 6, except for the orthodromically-evoked population spike (OPS) at the onset of HFS, the amplitude of OPS was quickly suppressed during a continuous 1 min long 100 Hz O-HFS train without prolonged gaps (Fig. 6A). The results are in agreement with a previous report [13]. However, during an O-HFS train with 100 ms prolonged IPI gaps inserted every 2 s, OPS with multiple spikes followed the gaps and even epileptiform activity with continuous spikes appeared (Fig. 6B). Similarly, within an O-HFS with a higher frequency of 200 Hz, large multiple-spike OPS activity could still be evoked by the stimuli immediately following the prolonged 100 ms IPI gaps while the regular stimuli of the HFS were not able to evoke any obvious PS activity (Fig. 6C). Similar results were observed repeatedly in 6 rat experiments.
Figure 6.
Comparison of continuous and intermittent orthodromic-HFS. (A) Typical regular 100 Hz O-HFS train without prolonged IPI gaps. The time expanded insets on the right show that except a few of the large OPS at the onset of HFS, there was no more obvious OPS appeared in the remained period of the HFS. (B) Epileptiform activity appeared during a 100 Hz O-HFS train with insertions of 100 ms prolonged IPI gaps. (C) In a 200 Hz O-HFS train, large OPS activity could still be evoked by the stimuli immediately following the prolonged 100 ms IPI gaps, while they were not able to be evoked by the regular stimuli of the HFS. The dashed lines with arrow heads denote the location of the removed stimulation artifacts. The red dots denote the location of stimuli immediately following the 100 ms prolonged IPI gaps.
These results indicate that short periods with absence of stimulation were able to interrupt the axonal block during HFS and could induce intensive firing activity in the downstream neurons. Therefore, persistent stimulation with high enough frequency is necessary for the maintenance of HFS-induced axon block.
4 Discussion
The major findings in this study include: 1) two distinct mechanisms are involved in the recovery from HFS-induced axonal block. One is frequency-dependent and rapid; the other is frequency-independent but much slower. 2) HFS produced an extension of the duration of axonal refractory period thereby causing axonal block with fast recovery. 3) Prolonged gaps inserted within HFS trains could eliminate the axonal block, induced large population spikes and even epileptiform activity in the upstream and downstream regions. Based on these findings, the underlying mechanisms of axonal block and their clinic implications are discussed below.
Plausible mechanisms underlying the extension of axon refractory periods
One of the unique findings of the study is that HFS could block the axonal conduction by extending the refractory period of axons. The axonal refractory period is normally less than 2 ms [11]. At the onset of A-HFS, the original refractory period generated by the very first APS of HFS was too short to suppress the second APS even with a 5 ms delay (in 200 Hz HFS). However, during HFS, the refractory periods increased and APSs at 10 ms delay were suppressed significantly within both 100 and 200 Hz HFS trains. In addition, the actual refractory periods must be longer than 10 ms within normal HFS trains without the test gaps providing recovery. This significant extension of refractory periods could be explained by changes of dynamics of ionic channels and changes of ion concentrations.
Previous simulations and experiments have shown that electrical currents generally produce a conduction block in nerves due to depolarization of the nerve membrane, resulting in an inactivation of the sodium channels [22,23]. Presumably, during the period of HFS, the continuous action of electrical pulses could keep the axons in a prolonged depolarization level that increases the inactivation duration of sodium channels following each event of axonal firing [16,17,24]. In addition, it has been shown that HFS can elevate [K+]o and suppress the firings of neurons and axons [25,26]. The intense activation of axons at the onset of HFS would raise [K+]o thereby facilitating the depolarization block. Therefore, the evoked-APS amplitudes would decrease rapidly at the beginning of HFS.
With continuous stimulation of HFS at high frequency, the incoming pulses would keep the refractory periods of axons at a prolonged level even when the elevated [K+]o concentrations began to drop [10]. From time to time, small subsets of axons that finally recovered from their refractory periods would be activated by next stimulation pulse, fire immediately and enter refractory periods again. Thus, each pulse of HFS could only generate small APS amplitude. In addition, although we did not test the effect of the frequency higher than 200Hz in these experiments, HFS with a higher frequency could presumably extend the refractory periods even longer and suppress the axon conduction further, but with higher power consumption. An appropriate frequency would be a compromise between the degree of axonal block needed and the electrical power consumption.
Plausible mechanisms underlying the slow recovery stage
Another interesting result of the study is that the overall recovery time (i.e., T90%) of HFS-induced APS suppression is similar for all of the 50, 100 and 200 Hz trials. It is frequency-independent and dominated by a “slow effect”.
The underlying mechanism of the “slow recovery” could be attributed to the after-hyperpolarization of the membrane that has been studied intensively [16]. This hyperpolarization following high frequency repeated axon firings is thought to be related to the hyper-activation of Na+/K+-pump. Normally, an action potential starts by a depolarization phase of Na+ influx and is then followed by repolarization phase of K+ efflux. The repolarization undershoots and causes hyperpolarization [19]. The hyperpolarization is due to the action of the sodium-dependent Na+/K+-pump that exchanges three intracellular Na+ ions for two extracellular K+ ions, resulting in a net outwards current [27–29].
Upon the termination of HFS, the repeated excitation of stimulation pulses ceased and the axonal membrane repolarized rapidly, forming the stage 1. Then, axons could undergo a phase of hyperpolarization generated by an increased activity of the Na+/K+-pump. The increase would in turn be generated by accumulation of intracellular Na+ ions during the long HFS period lasting several minutes and causing a prolonged after-hyperpolarization period [16]. This type of after-hyperpolarization could decrease the excitability of axons and be responsible for the recovery stage 2 reported in the present study. Although the inhibition mechanism of the Na+/K+-pump could also be activated during HFS since ion concentrations were changing in the intracellular and extracellular spaces. But the effect might be too weak and slow to overcome the strong and rapid depolarization effects of stimulation pulses [22, 27]. Therefore, only after the cease of HFS would the hyperpolarization effects of Na+/K+-pump dominate the membrane potentials of axons and form the slow recovery of stage 2.
The frequency-independent feature of the recovery stage 2 could be explained by the fact that the HFS-induced after-hyperpolarization is related to the cumulative effect of axonal firings. The intracellular and extracellular exchange amount of various ions generated by action potentials is reflected by both the area and the number of APS (i.e., the intensity of neuronal firings), but not by the amplitude of individual APS. Although the A-HFS with a higher frequency resulted in a much smaller APS amplitude at the end of HFS, the total number of action potentials generated during the same period of time was not greatly different for 50, 100 and 200 Hz HFS (Fig. 2G). Therefore, the accumulation of ion concentrations and the strength of the after-hyperpolarization should be similar for the HFS with different frequencies in the range. This could result in a frequency-independent recovery amount of APS amplitude and frequency-independent recovery time of the stage 2 (see Fig. 2B and 2D).
Clinical implication
The finding of a rapid recovery feature of HFS-induced axon failures suggests that the stimulation pulses of DBS should be applied with a high enough frequency and should avoid pauses with long duration to prevent over excitation in upstream and downstream neurons. In clinical applications, DBS usually involves the delivery of continuous electrical pulses with constant inter-pulse-interval (i.e., fixed-frequency) to specific brain regions. Recently, in order to search for optimal effects of DBS and to save battery power of the implanted pulse generator, various patterns of stimulation including pauses with tens to hundreds milliseconds have been tested in DBS for animals, patients and computer simulation models [8,9,30]. The results have shown that continuous stimulation without pauses is most effective therapeutics in suppressing tremor with thalamic DBS, while inconsistent intervals between DBS pulses could induce burst firings of neurons [31,32]. The present study sheds light on an important mechanism of extended refractory period induced by HFS to explain these phenomena. Long pauses might eliminate the inhibitory effect of axonal refractory period thereby decreased the effectiveness of DBS. However, a temporal pattern of stimulation with enough short pauses could improve the therapeutic effects of DBS as previously reported [9].
DBS is being applied to various brains disorders other than Parkinson’s disease and inhibition or excitation could be required to treat different diseases [33,34]. A stimulation pattern with properly designed pauses could be beneficial for a specific brain region that requires various amount of excitation/inhibition for optimum therapeutic outcome.
Limitations of the study
The study was performed on urethane anesthetized rats. Urethane is known to slightly decrease cell excitability and suppress neurotransmission in rat hippocampus[35]. However, large PS with amplitudes of 8 ~ 12 mV could be evoked by moderate current amplitudes of 0.3 – 0.5 mA at the onset of orthodromic- and antidromic-HFS, indicating that the effects of the anesthesia was not significant. Further studies with awake animals are needed to provide more accurate parameters of HFS (e.g., stimulation frequency and intensity) and more data of HFS recovery.
In addition, the present results were obtained from extracellular population recordings in in-vivo preparation of normal rats. Intracellular recordings of the changes of axon states during and following HFS would confirm the underlying mechanisms of axonal block. Also, further studies on animals with neurological disorders (e.g., epilepsy) would provide more information of actual therapeutic efficacy of DBS with different frequencies and different patterns. Finally, because HFS is required for effective DBS, the stage 2 process should be irrelevant to therapeutic efficacy, since it can be elicited at lower frequencies. Therefore, more efforts concentrated on ways to improve the stage 1 process may be needed to develop new DBS protocols. Based on the present results, stimulation paradigms that provide longer battery life and the optimum efficacy of HFS could be interesting to advance the development of DBS stimulators.
Conclusion
The results of present study clearly show the role of extended refractory period on HFS induced axonal block. They indicate that, at least in the hippocampus, continuous stimulation is required for sustained suppression of axons. Intermittent stimulation can produce excitation and even induce epileptiform activity as with a lower frequency or by short pauses within HFS trains. Therefore, the results could explain why a higher frequency over 100 Hz is necessary for a safe and effective DBS therapy, and provide guidelines for the designs of temporal pattern of DBS.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 30970753), by Major State Basic Research Development Program of China (No. 2011CB504400) and by NIH (NINDS grant #:_ 2R01NS060757-05A1).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Awan NR, Lozano A, Hamani C. Deep brain stimulation: current and future perspectives. Neurosurg. Focus. 2009;27:E2. doi: 10.3171/2009.4.FOCUS0982. [DOI] [PubMed] [Google Scholar]
- 2.Hamani C, Andrade D, Hodaie M, et al. Deep brain stimulation for the treatment of epilepsy. Int J Neural Syst. 2009;19:213–226. doi: 10.1142/S0129065709001975. [DOI] [PubMed] [Google Scholar]
- 3.Kuhn J, Möller M, Müller U, et al. Deep brain stimulation for the treatment of addiction. Addiction. 2011;106:1536–1537. doi: 10.1111/j.1360-0443.2011.03452.x. [DOI] [PubMed] [Google Scholar]
- 4.Datta A, Baker JM, Bikson M, et al. Individualized model predicts brain current flow during transcranial direct-current stimulation treatment in responsive stroke patient. Brain Stimul. 2011;4:169–174. doi: 10.1016/j.brs.2010.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mayberg HS, Lozano AM, Voon V, et al. Deep brain stimulation for treatment-resistant depression. Neuron. 2005;45:651–660. doi: 10.1016/j.neuron.2005.02.014. [DOI] [PubMed] [Google Scholar]
- 6.Hammond C, Ammari R, Bioulac B, et al. Latest view on the mechanism of action of deep brain stimulation. Mov Disord. 2008;23:2111–2121. doi: 10.1002/mds.22120. [DOI] [PubMed] [Google Scholar]
- 7.Lee HW, Webber WR, Crone N, et al. When is electrical cortical stimulation more likely to produce afterdischarges? Clin Neurophysiol. 2010;121:14–20. doi: 10.1016/j.clinph.2009.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Birdno MJ, Kuncel AM, Dorval AD, et al. Stimulus features underlying reduced tremor suppression with temporally patterned deep brain stimulation. J Neurophysiol. 2012;107:364–383. doi: 10.1152/jn.00906.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hess CW, Vaillancourt DE, Okun MS. The temporal pattern of stimulation may be important to the mechanism of deep brain stimulation. Exp Neurol. 2013;247:296–302. doi: 10.1016/j.expneurol.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jensen AL, Durand DM. Suppression of axonal conduction by sinusoidal stimulation in rat hippocampus in vitro. J Neural Eng. 2007;4:1–16. doi: 10.1088/1741-2560/4/2/001. [DOI] [PubMed] [Google Scholar]
- 11.Jensen AL, Durand DM. High frequency stimulation can block axonal conduction. Exp Neurol. 2009;220:57–70. doi: 10.1016/j.expneurol.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng F, Lammert K, Nixdorf-Bergweiler BE, et al. Axonal failure during high frequency stimulation of rat subthalamic nucleus. J Physiol. 2011;589(Pt 11):2781–2793. doi: 10.1113/jphysiol.2011.205807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng Z, Zheng X, Yu Y, et al. Functional disconnection of axonal fibers generated by high frequency stimulation in the hippocampal CA1 region in-vivo. Brain Res. 2013;1509:32–42. doi: 10.1016/j.brainres.2013.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim E, Owen B, Holmes WR, et al. Decreased afferent excitability contributes to synaptic depression during high-frequency stimulation in hippocampal area CA1. J Neurophysiol. 2012;108:1965–1976. doi: 10.1152/jn.00276.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosenbaum R, Zimnik A, Zheng F, et al. Axonal and synaptic failure suppress the transfer of firing rate oscillations, synchrony and information during high frequency deep brain stimulation. Neurobiol Dis. 2013;62C:86–99. doi: 10.1016/j.nbd.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bucher D, Goaillard JM. Beyond faithful conduction: short-term dynamics, neuromodulation, and long-term regulation of spike propagation in the axon. Prog Neurobiol. 2011;94:307–346. doi: 10.1016/j.pneurobio.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Debanne D, Campanac E, Bialowas A, et al. Axon physiology. Physiol Rev. 2011;91:555–602. doi: 10.1152/physrev.00048.2009. [DOI] [PubMed] [Google Scholar]
- 18.Chomiak T, Hu B. Axonal and somatic filtering of antidromically evoked cortical excitation by simulated deep brain stimulation in rat brain. J Physiol. 2007;579(Pt 2):403–412. doi: 10.1113/jphysiol.2006.124057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fletcher A. Action potential: generation and propagation. Anaesthesia & Intensive Care Medicine. 2008;9:251–255. [Google Scholar]
- 20.Kríz N, Syková E, Ujec E, et al. Changes of extracellular potassium concentration induced by neuronal activity in the sinal cord of the cat. J Physiol. 1974;238:1–15. doi: 10.1113/jphysiol.1974.sp010507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Theoret Y, Brown A, Fleming SP, et al. Hippocampal field potential: a microcomputer aided comparison of amplitude and integral. Brain Res Bull. 1984;12(5):589–595. doi: 10.1016/0361-9230(84)90178-3. [DOI] [PubMed] [Google Scholar]
- 22.Bhadra N, Kilgore KL. High*frequency electrical conduction block of mammalian peripheral motor nerve. Muscle Nerve. 2005;32:782–790. doi: 10.1002/mus.20428. [DOI] [PubMed] [Google Scholar]
- 23.Tai C, de Groat WC, Roppolo JR. Simulation of nerve block by high-frequency sinusoidal electrical current based on the Hodgkin-Huxley model. IEEE Trans Neural Syst Rehabil Eng. 2005;13:415–422. doi: 10.1109/TNSRE.2005.847356. [DOI] [PubMed] [Google Scholar]
- 24.Meeks JP, Mennerick S. Selective effects of potassium elevations on glutamate signaling and action potential conduction in hippocampus. J Neurosci. 2004;24:197–206. doi: 10.1523/JNEUROSCI.4845-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shin DS, Samoilova M, Cotic M, et al. High frequency stimulation or elevated K+ depresses neuronal activity in the rat entopeduncular nucleus. Neuroscience. 2007;149:68–86. doi: 10.1016/j.neuroscience.2007.06.055. [DOI] [PubMed] [Google Scholar]
- 26.Bellinger SC, Miyazawa G, Steinmetz PN. Submyelin potassium accumulation may functionally block subsets of local axons during deep brain stimulation: a modeling study. J Neural Eng. 2008;5:263–274. doi: 10.1088/1741-2560/5/3/001. [DOI] [PubMed] [Google Scholar]
- 27.Moldovan M, Krarup C. Evaluation of Na+/K+ pump function following repetitive activity in mouse peripheral nerve. J Neurosci Methods. 2006;155:161–171. doi: 10.1016/j.jneumeth.2005.12.015. [DOI] [PubMed] [Google Scholar]
- 28.Kiernan MC, Baker MD, Bostock H. Characteristics of late Na+ current in adult rat small sensory neurons. Neuroscience. 2003;119:653–660. doi: 10.1016/s0306-4522(03)00194-5. [DOI] [PubMed] [Google Scholar]
- 29.Kiernan MC, Lin CSY, Burke D. Differences in activity*dependent hyperpolarization in human sensory and motor axons. J Physiol. 2004;558:341–349. doi: 10.1113/jphysiol.2004.063966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker KB, Zhang J, Vitek JL. Pallidal stimulation: effect of pattern and rate on bradykinesia in the non-human primate model of Parkinson's disease. Exp Neurol. 2011;231:309–313. doi: 10.1016/j.expneurol.2011.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Birdno MJ, Cooper SE, Rezai AR, et al. Pulse-to-pulse changes in the frequency of deep brain stimulation affect tremor and modeled neuronal activity. J Neurophysiol. 2007;98:1675–1684. doi: 10.1152/jn.00547.2007. [DOI] [PubMed] [Google Scholar]
- 32.Kuncel AM, Birdno MJ, Swan BD, et al. Tremor reduction and modeled neural activity during cycling thalamic deep brain stimulation. Clin Neurophysiol. 2012;123:1044–1052. doi: 10.1016/j.clinph.2011.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deniau JM, Degos B, Bosch C, et al. Deep brain stimulation mechanisms: beyond the concept of local functional inhibition. Eur J Neurosci. 2010;32:1080–1091. doi: 10.1111/j.1460-9568.2010.07413.x. [DOI] [PubMed] [Google Scholar]
- 34.Yokoyama T, Sugiyama K, Nishizawa S, et al. The optimal stimulation site for chronic stimulation of the subthalamic nucleus in Parkinson's disease. Stereotact Funct Neurosurg. 2001;77:61–67. doi: 10.1159/000064598. [DOI] [PubMed] [Google Scholar]
- 35.Shirasaka Y, Wasterlain CG. The effect of urethane anesthesia on evoked potentials in dentate gyrus. Eur J Pharmacol. 1995;282:11–17. doi: 10.1016/0014-2999(95)00244-f. [DOI] [PubMed] [Google Scholar]