Abstract
An antiangiogenic homoisoflavanone, cremastranone, was synthesized for the first time. This scalable synthesis, which includes selective demethylation, could be used to develop lead molecules to treat angiogenesis-induced eye diseases. Synthetic cremastranone inhibited the proliferation, migration and tube formation ability of human retinal microvascular endothelial cells, important steps in pathological angiogenesis.
Retinopathy of prematurity (ROP), proliferative diabetic retinopathy (DR) and neovascular (“wet”) age-related macular degeneration (wet AMD) are three major ocular diseases, affecting children, adults and elderly people.1 These diseases are characterized by abnormal formation of blood vessels in the eye through the process of pathological angiogenesis. The blood vessels formed during pathological angiogenesis in the eye are fragile, porous and not completely differentiated. As a result haemorrhage, retinal detachment, fibrotic scarring and rapid photoreceptor degeneration occurs in the eye leading to vision loss.
Nearly 2 million people are affected by wet AMD in the United States, while about 75% of diabetic patients show clinical symptoms of DR.1 Blocking pathological angiogenesis in the eyes is the current treatment strategy for these diseases. The current mainstay of therapy is the use of anti-VEGF biologics. However, these treatments are associated with vision damaging side effects, unfavourable cost to benefit ratio, and incomplete patient response.2–5 Hence there is a critical requirement for new therapies. Towards this goal, we are pursuing development of small molecules as drug leads for these diseases, based on natural products.
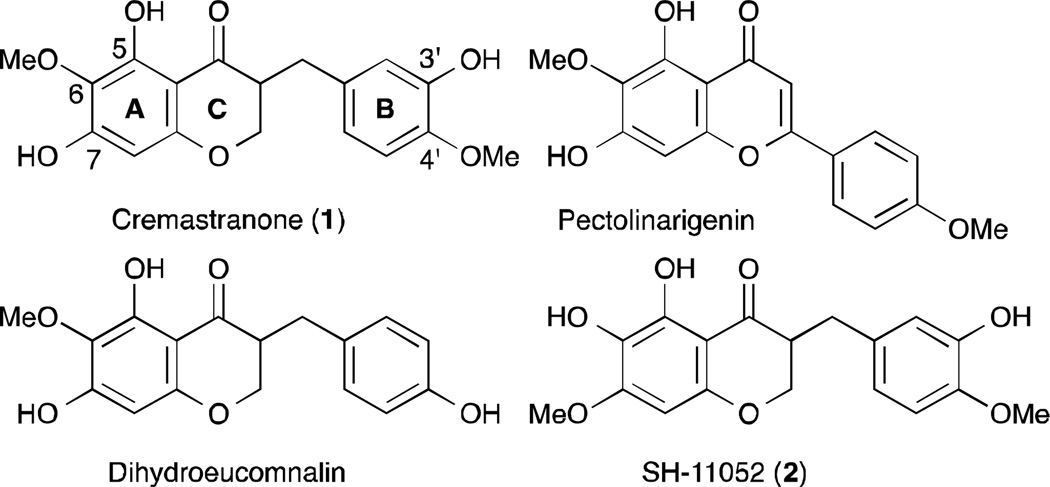
Homoisoflavanones are a small class of naturally occurring oxygen heterocycles that have been isolated from many geographically diverse plant genera. These compounds are categorized into four types: 3-benzyl-4-chromanones, 3-benzylidene-4-chromanones, 3-benzyl-3-hydroxy-4-chromanones and scillascillins.6 In general, these compounds are reported to show anti-inflammatory, anti-oxidative, anti-bacterial, anti-cancer, anti-histaminic, anti-viral, and anti-phosphodiesterase activities. Cremastranone (1), 5,7-dihydroxy-3-(3-hydroxy-4-methoxybenzyl)-6-methoxychroman-4-one, is a 3-benzyl-4-chromanone that has been isolated from the plants Muscari armeniacum,7 Chionodoxa luciliae,8 Scilla natalensis,9 Merwilla plumbea,10 and Cremastra appendiculata (D. Don)11 (1; Fig. 1). The bulb of the orchid C. appendiculata is used in East Asia as a traditional medicine, taken internally to treat several cancers, and applied externally for skin lesions.
Fig. 1.
The structures of cremastranone (1) and its congeners.
Cremastranone (1) was previously reported to possess antiangiogenic activity both in vitro and in vivo and was identified as a potent inhibitor of the proliferation of human umbilical vein endothelial cells (HUVECs).11 Also 1 inhibited vascular tube formation and new vessel growth induced by basic fibroblast growth factor. Its anti-angiogenic properties were further confirmed in vivo in the laser-induced choroidal neovascularization and oxygen induced retinopathy mouse models,12,13 used for testing novel therapies for wet AMD and ROP, respectively. Moreover, injection of compound 1 into the vitreous of normal adult mice showed no short-term cytotoxic or inflammatory effects on the retina, nor did it induce apoptosis of retinal cells.13 These results suggest that proliferative ocular vascular diseases such as ROP, DR, and AMD may be targeted using compound 1 or its derivatives. Other recent work has suggested that 1 has potent anti-inflammatory activity in the context of UV-induced skin inflammation14 and allergy.15
So far, the syntheses of 5,7-dihydroxy-6-methoxyflavones and 5,7-dihydroxy-6-methoxyhomoisoflavanones have been reported (Fig. 1).16, 17 But in spite of cremastranone’s interesting biological activities and the synthesis of these congeners, to the best of our knowledge, synthesis of 1 has not yet been reported. To allow future profiling and development of this homoisoflavanone and its analogs, we sought to develop a scalable synthesis suitable for quickly securing multi-gram quantities. During our previous synthetic approach toward cremastranone, the regioisomer (SH-11052, 2) was generated due to the challenge of selective deprotection of methyl phenyl ethers.18 Herein, we describe a short and efficient synthesis of cremastranone (1) featuring a solution to the issue of selective deprotection of the methyl phenyl ether. Moreover, we show for the first time that synthetic cremastranone has biological properties consistent with the natural-source compound, in a disease relevant cell model.
Our plan for the preparation of cremastranone entailed 4-chromanone formation and deprotection of a methyl aryl ether.19 This strategy would permit significant flexibility in the synthesis of 1 and thereby establish a platform that leads to a variety of derivatives for further structure-activity relationship studies. In addition, we envisioned our synthesis would become very useful for the syntheses of similar interesting homoisoflavanones.
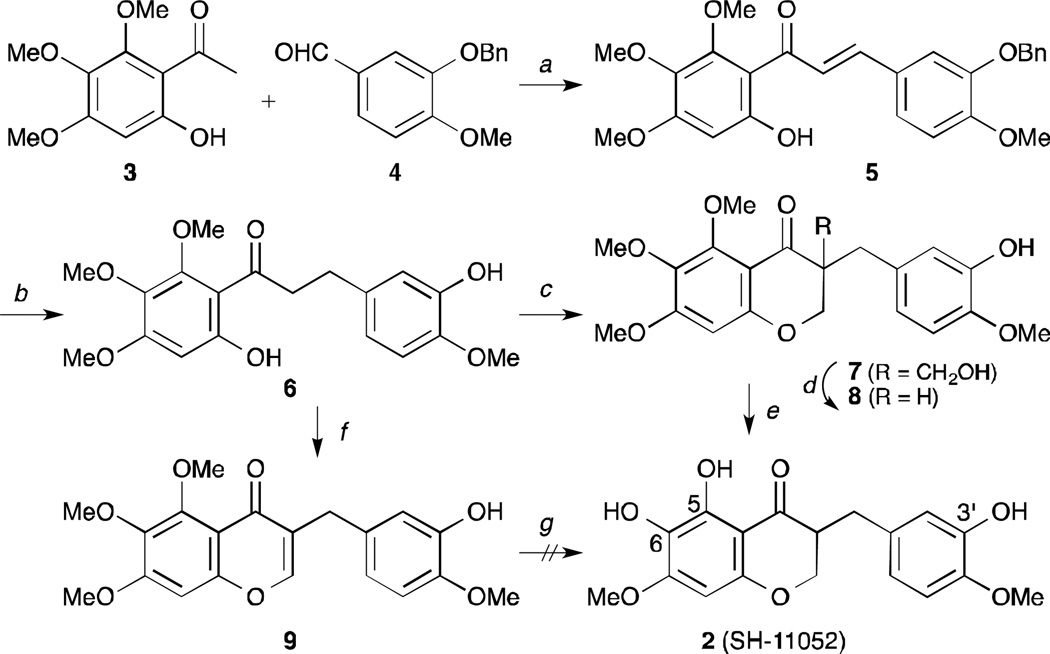
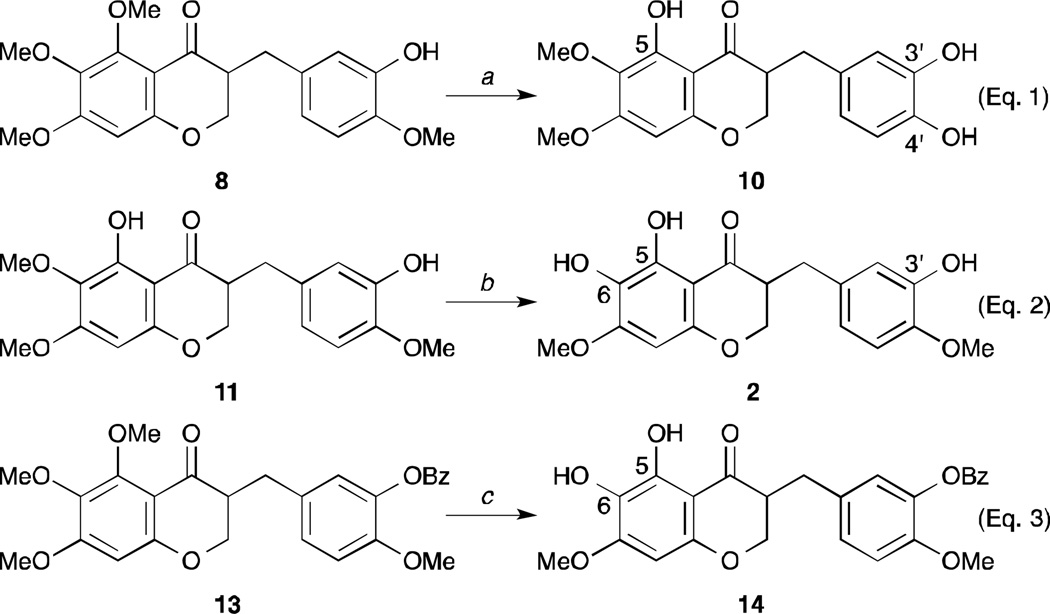
As shown in Scheme 1 and described previously,18 our initial approach toward the synthesis of cremastranone (1) began with an aldol condensation of the 6′-hydroxy-2′,3′,4′-trimethoxy-acetophenone 3 with 3-benzyloxy-4-methoxybenzaldehyde 4. Catalytic hydrogenation of the chalcone 5 and the cyclization of the dihydrochalcone 6 with formaldehyde led to the desired 4-chromanone 8 after byproducts (e.g. 7) were treated with K2CO3. Finally, in order to remove two methyl groups selectively, the 4-chromanone 8 would be demethylated with a variety of reagents. However, the selective removal of two methyl groups under most conditions was not satisfactory. Among them, the demethylation using an excess of TMSI in CHCl3 gave the best conversion,20 but its product was not identical to cremastranone but rather its regioisomer, called SH-11052 (2). Its structure was elucidated by 2D-NMR spectroscopies such as NOESY and HMBC.18 To overcome the unwanted result, the alternative 4-chromone 9 cyclized by DMF, PCl5 was demethylated and hydrogenated.21 Similarly, the desired product was not afforded from 9. Surprisingly, treatment of 8 with BBr3 led to the other isomer demethylated on the C4′ position (10).22 (Eq. 1, Scheme 2) The next trial was to remove the intermediates which were demethylated only on the C5 position or protected with a benzoyl group on the C3′ position. (Eq. 2 and 3, Scheme 2) Unfortunately, the free OH group at the C7 position could not be generated from any intermediates bearing 5,6,7-trimethoxy-4-chromanone.
Scheme 1.
The initial synthetic approach to cremastranone (1). Reagents and conditions: a) KOH, MeOH, 0°C, 54%; b) Pd/C, HCO2Na, HCO2H, 60°C, 79%; c) formalin, NaOH, 60°C; d) K2CO3, EtOH, 47% for 2 steps; e) TMSI (4 eq.), CHCl3, 50°C, 49%; f) PCl5, BF3-OEt2, DMF, 62%; g) HBr, AcOH then H2, Pd/C. MeOH.
Scheme 2.
The study of the demethylation of 5,6,7-trimethoxy-4-chromanone. Reagents and conditions: a) BBr3(or BCl3) (2.5 eq.), CH2Cl2, −78°C, 65%; b) TMSI, CHCl3, reflux, 38%; c) TMSI (4 eq.), CHCl3, reflux, 52%.
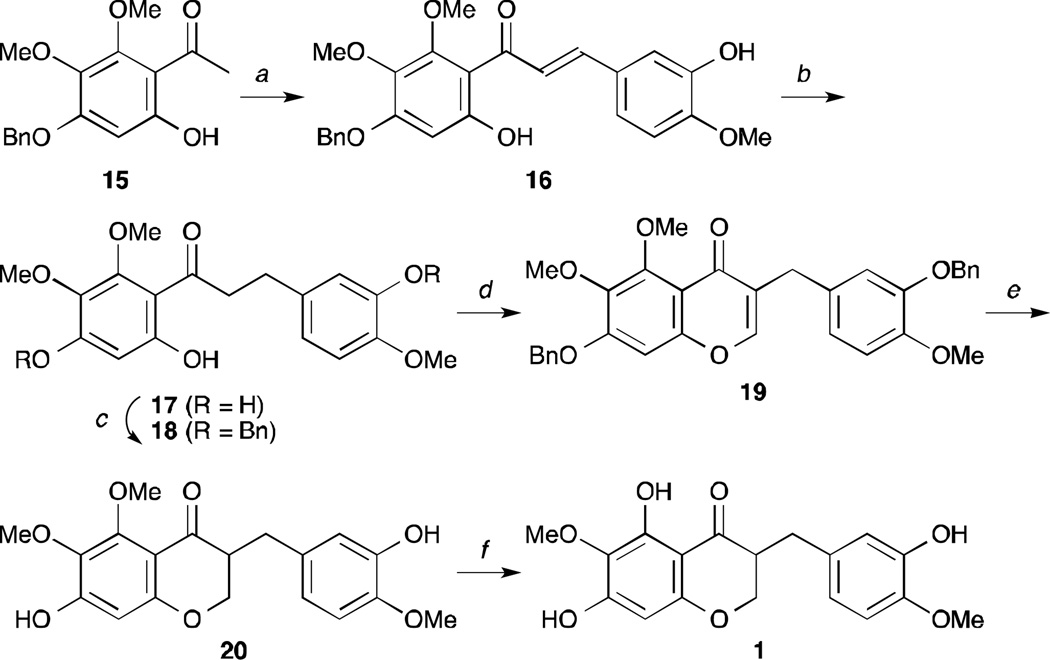
Finally, our key solution for an alternative route to cremastranone (1) was the generation of the phenol group on the C7 position prior to the C6 and C4′ positions. Thus 4′-benzyloxy-6′-hydroxy-2′,3′-dimethoxyacetophenone 15 was chosen as the starting material instead of 3 (Scheme 3). As reported before, 15 was prepared from 2′,4′,6′-trihydroxyacetophenone with several steps.23 Aldol condensation of 15 with isovanillin, followed by catalytic hydrogenation of the chalcone 16 under H2 and Pd/C afforded the dihydrochalcone 17. With the dihydrochalcone 17 in hand, the formation of the 4-chromone was directly attempted using N,N-dimethylformamide dimethyl acetal. However, the methylation of a phenol group accompanied this reaction. To overcome the side reaction, the dihydrochalcone 17 was carefully treated with benzyl bromide to afford the bis(benzyl ether) 18 which was converted to the corresponding 4-chromone 19. To this end, catalytic hydrogenation of 19 led to the desired 4-chromanone 20. Finally, methyl groups were removed by 2 equivalents of TMSI to give cremastranone (1). Thus the antiangiogenic homoisoflavanone cremastranone is available in 6 steps and 26.8% overall yield from the acetophenone 15. Although our synthetic cremastranone is a racemate, its chemical shifts in 1H-and13C-NMR spectra match those of the reported natural product (Supplementary Table 1).7 To date, the configuration at C3 for natural-source compound 1 has not been reported. Further experiments are necessary to determine the absolute configuration of the C3 position in compound 1.
Scheme 3.
First synthesis of (±)-cremastranone (1). Reagents and conditions: a) isovanillin, KOH, EtOH, rt, 53%; b) H2, Pd/C, MeOH, 94%; c) K2CO3, benzylbromide, acetone, reflux, 89%; d) (CH3)2NCH(OCH3)2, toluene, reflux, 80%; e) H2, Pd/C, MeOH, 87%; f) TMSI (2 eq.), CHCl3, 60°C, 87%.
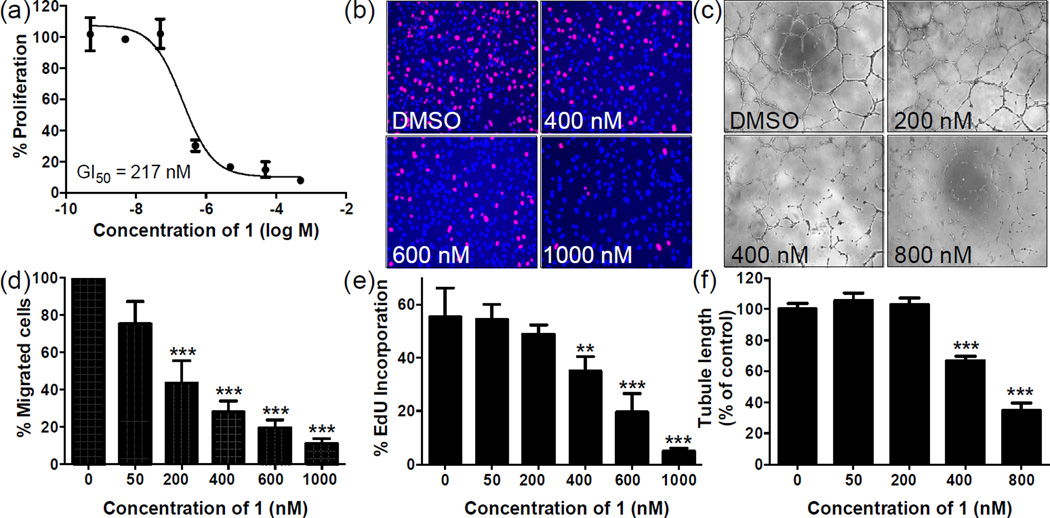
Previously, cremastranone isolated from C. appendiculata was tested for its anti-proliferative activity against an endothelial cell model, human umbilical vein endothelial cells (HUVECs), and the 50% growth inhibitory concentration (GI50) value was reported to be 1.5 µM.11 Hence, we tested the anti-proliferative activity of synthetic cremastranone 1 on HUVECs in an alamarBlue fluorescence cell proliferation assay and the GI50 was observed to be 377 nM. The slightly higher potency of synthetic 1 might be attributed to a difference in the proliferation assays used or compound purity. Further, we tested the anti-proliferative activity of 1 against a more ocular disease relevant endothelial cell model, human retinal microvascular endothelial cells (HRECs). We observed clear dose dependent inhibition of HREC proliferation by 1 in complete medium with a GI50 of 217 nM (Fig. 2a). In addition we also tested the proliferation of HRECs activated by vascular endothelial growth factor, a potent inducer of angiogenesis, in the presence of 1 and found the GI50 to be comparable (276 nM). Previously we reported a GI50 of 43 µM for regioisomer SH-11052 (2),18 which differed only in the positions of hydroxyl and methoxy groups at C6 and C7 as compared to 1. The importance of positions of the groups on the ‘A’ ring suggest that a structure-activity relationship can be established.
Fig. 2.
Synthetic cremastranone (1) blocks in vitro angiogenesis of human retinal microvascular endothelial cells (HRECs) in a dose-dependent manner. (a) The effect of 1 on proliferation of HRECs was tested using an alamarBlue fluorescence assay. (b, e) In an EdU incorporation assay, 1 blocked DNA synthesis as evidenced by a decrease in incorporation of EdU (pink) into the nuclei of HRECs (nuclei, blue). (c, f) Tube formation by HRECs on Matrigel was blocked by 1; the extent of tube formation was measured as tubule length. (d) Migration of HRECs in a scratch-wound assay was inhibited by 1. Original magnifications: 40×. The data points in all graphs indicate mean ± SEM; ** represents P < 0.01; *** represents P < 0.001 (ANOVA with Dunnett’s post hoc tests). Each of the panels is representative of three independent experiments.
We then measured the endothelial cell specificity of 1 by measuring its effects on the proliferation of non-endothelial ocular cell lines, Y79 (retinoblastoma cell line), 92-1 (uveal melanoma cell line) and ARPE-19 (retinal pigmented epithelial cells). The anti-proliferative potency of 1 on these non-endothelial cell lines was significantly lower than that on endothelial cells with GI50 values of 9.8 µM, 47 µM, and >250 µM on Y79, 92-1, and ARPE-19 cells respectively, indicating that compound 1 has highly specific antiproliferative activity toward endothelial cells. We further confirmed the inhibition of HREC proliferation by 1 in a secondary assay by monitoring the incorporation of thymidine analogue 5-ethynyl-2′-deoxyuridine (EdU) into DNA of endothelial cells in the presence of different concentrations of 1. In this EdU incorporation assay, compound 1 inhibited the DNA synthesis of HRECs in a dose dependent manner (Fig. 2b, e).
After establishing the inhibition of HREC proliferation by 1, we tested its anti-angiogenic activity in vitro. We tested the ability of HRECs to form tubes in vitro in a Matrigel tube formation assay, which recapitulates in vitro major events of physiological angiogenesis. Cremastranone 1 prevented the formation of closed tube structures in a dose dependent manner as measured by both tube length (Fig. 2c, f) and number of polygons formed (data not shown). This is consistent with previous findings with the natural-source cremastranone in HUVECs.11 Migration is also an important step in the angiogenesis process, wherein endothelial cells move from pre-existing capillaries to the site of blood vessel formation. We measured this ability of HRECs to migrate using the standard scratch-wound assay, in which HRECs were allowed to grow to confluency, then a scratch was introduced and movement of endothelial cells into the scratched area from the surrounding population was measured in the presence of various concentrations of 1 (Fig. 2d). The migration of HRECs was inhibited by 1 in a dose dependent manner.
In summary, a novel scalable strategy to synthesize the biologically active homoisoflavanone cremastranone was developed, and the resulting compound showed potent activity in cell models. Together, these results confirm the anti-angiogenic activity of synthetic cremastranone 1 in a disease-relevant cell type, and pave the way for development of analogues with higher potency and better pharmacological properties to treat blinding ocular diseases caused by pathological angiogenesis.
Supplementary Material
Acknowledgements
This work was supported by grants from the International Retinal Research Foundation, Ralph W. and Grace M. Showalter Research Trust, Retina Research Foundation, and NIH NCATS KL2TR001106 to TWC, a grant from the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (NRF-2013R1A1A2007151) to S-YS, and the Ausich Graduate Scholarship from Kemin Health to HDB. This publication was also supported in part by an unrestricted grant from Research to Prevent Blindness, Inc.
Footnotes
Electronic Supplementary Information (ESI) available: synthetic methodology, compound characterization, and biological methods. See DOI: 10.1039/c000000x/
References
- 1.Penn JS, Madan A, Caldwell RB, Bartoli M, Caldwell RW, Hartnett ME. Prog Retin Eye Res. 2008;27:331–371. doi: 10.1016/j.preteyeres.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Folk JC, Stone EM. N Engl J Med. 2010;363:1648–1655. doi: 10.1056/NEJMct1000495. [DOI] [PubMed] [Google Scholar]
- 3.Mitchell P, Annemans L, White R, Gallagher M, Thomas S. Pharmacoeconomics. 2011;29:107–131. doi: 10.2165/11585520-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 4.Hodge W, Brown A, Kymes S, Cruess A, Blackhouse G, Hopkins R, McGahan L, Sharma S, Pan I, Blair J, Vollman D, Morrison A. Can. J. Ophthalmol. 2010;45:223–230. doi: 10.3129/i10-047. [DOI] [PubMed] [Google Scholar]
- 5.Lux A, Llacer H, Heussen FM, Joussen AM. Br. J. Ophthalmol. 2007;91:1318–1322. doi: 10.1136/bjo.2006.113902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.du Toit K, Drewes SE, Bodenstein J. Nat Prod Res. 2010;24:457–490. doi: 10.1080/14786410903335174. [DOI] [PubMed] [Google Scholar]
- 7.Adinolfi M, Corsaro MM, Lanzetta R, Laonigro G, Mangoni L, Parrilli M. Phytochemistry. 1987;26:285–290. [Google Scholar]
- 8.Corsaro MM, Lanzetta R, Mancino A, Parrilli M. Phytochemistry. 1992;31:1395–1397. [Google Scholar]
- 9.Crouch NR, Bangani V, Mulholland DA. Phytochemistry. 1999;51:943–946. [Google Scholar]
- 10.du Toit K, Elgorashi EE, Malan SF, Drewes SE, van Staden J, Crouch NR, Mulholland DA. Bioorg. Med. Chem. 2005;13:2561–2568. doi: 10.1016/j.bmc.2005.01.036. [DOI] [PubMed] [Google Scholar]
- 11.Shim JS, Kim JH, Lee J, Kim SN, Kwon HJ. Planta Med. 2004;70:171–173. doi: 10.1055/s-2004-815496. [DOI] [PubMed] [Google Scholar]
- 12.Kim JH, Kim KH, Yu YS, Kim YM, Kim KW, Kwon HJ. Biochem. Biophys. Res. Commun. 2007;362:848–852. doi: 10.1016/j.bbrc.2007.08.100. [DOI] [PubMed] [Google Scholar]
- 13.Kim JH, Yu YS, Jun HO, Kwon HJ, Park KH, Kim KW. Mol. Vis. 2008;14:556–561. [PMC free article] [PubMed] [Google Scholar]
- 14.Hur S, Lee YS, Yoo H, Yang JH, Kim TY. J. Dermatol. Sci. 2010;59:163–169. doi: 10.1016/j.jdermsci.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Lee YS, Hur S, Kim TY. Allergy. 2014;69:453–462. doi: 10.1111/all.12356. [DOI] [PubMed] [Google Scholar]
- 16.Farkas L, Strelisky J. Tetrahedron Lett. 1970;11:187–190. [Google Scholar]
- 17.Farkas L, Gottsegen Á, Nógrádi M, Strelisky J. Tetrahedron. 1971;27:5049–5054. [Google Scholar]
- 18.Basavarajappa HD, Lee B, Fei X, Lim D, Callaghan B, Mund JA, Case J, Rajashekhar G, Seo SY, Corson TW. PLoS One. 2014;9:e95694. doi: 10.1371/journal.pone.0095694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.For deprotection of methyl aryl ethers Saadati F, Meftah-Booshehri H. Synlett. 2013;24:1702–1706. and references therein
- 20.Jung ME, Lyster MA. J. Org. Chem. 1977;42:3761–3764. [Google Scholar]
- 21.For the synthesis of 5,7-dihydroxy-6-methoxyflavone from 5,6,7-trimethoxyflavone, 47% HBr/AcOH was used. Huang W-H, Chien P-Y, Yang C-H, Lee A-R. Chem. Pharm. Bull. 2003;51:339–340. doi: 10.1248/cpb.51.339.
- 22.To confirm the structure of 10 given by BBr3, the compound synthesized through aldol condensation of 5,6,7-trimethoxy-4-chromanone with 3,4-dihydroxybenzaldehyde, olefin reduction, and mono-demethylation on the C5 is identical to the compound 10. For detailed scheme and experimental procedures, see the Electronic Supplementary Information
- 23.Kavvadias D, Sand P, Youdim KA, Qaiser MZ, Rice-Evans C, Baur R, Sigel E, Rausch WD, Riederer P, Schreier P. Br. J. Pharmacol. 2004;142:811–820. doi: 10.1038/sj.bjp.0705828. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.