Abstract
Background
Deep brain stimulation of the subthalamic nucleus (STN DBS) reduces Parkinson disease (PD) motor symptoms but has unexplained, variable effects on mood.
Objective
The study tested the hypothesis that pre-existing mood and/or anxiety disorders or increased symptom severity negatively affects mood response to STN DBS.
Methods
Thirty-eight PD participants with bilateral STN DBS and on PD medications were interviewed with Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID) and completed Beck Depression Inventory (BDI) and Spielberger State Anxiety Inventory (SSAI) self-reports. Subsequently, during OFF and optimal ON (clinical settings) STN DBS conditions and while off PD medications, motor function was assessed with the United Parkinson Disease Rating Scale (UPDRS, part III), and participants rated their mood with Visual Analogue Scales (VAS), and again completed SSAI. VAS mood variables included anxiety, apathy, valence and emotional arousal.
Results
STN DBS improved UPDRS scores and mood. Unexpectedly, PD participants diagnosed with current anxiety or mood disorders experienced greater STN DBS-induced improvement in mood than those diagnosed with remitted disorders or who were deemed as having never met threshold criteria for diagnosis. BDI and SSAI scores did not modulate mood response to STN DBS, indicating that clinical categorical diagnosis better differentiates mood response to STN DBS than self-rated symptom severity. SCID diagnosis, BDI and SSAI scores did not modulate motor response to STN DBS.
Conclusions
PD participants diagnosed with current mood or anxiety disorders are more sensitive to STN DBS-induced effects on mood, possibly indicating altered basal ganglia circuitry in this group.
Keywords: Parkison disease, subthalamic nucleus, deep brain stimulation, mood, mood disorder, anxiety disorder
Introduction
Twenty-five to 40% of individuals with Parkinson disease (PD) suffer from mood and anxiety disorders that substantially impair quality of life [1–2]. While impairments in motor behavior in PD arise primarily from basal ganglia dysfunction [3], the neurobiological underpinnings of comorbid psychiatric disorders in PD remain less clear. PD patients in the advanced stages of the disease are particularly susceptible to psychiatric symptoms [1]. Since patients treated with subthalamic nucleus deep brain stimulation (STN DBS) typically have advanced motor symptoms, they may fall within this vulnerable population. Although PD patients are frequently screened for current psychiatric disorders prior to STN DBS surgery [4], they may have recovered at the time of screening from past illness, or may develop new psychiatric symptoms after surgery as the disease progresses and treatment changes.
PD patients with STN DBS provide a unique opportunity to investigate the neural underpinnings of mood and anxiety disorders in PD. The STN may have substantial functional heterogeneity, given its convergent inputs from and projections to motor, limbic and associative cortical regions [5–8]. Growing evidence demonstrates that STN DBS, a therapy aimed at decreasing motor impairment and dopaminergic medication use in PD, also can alter mood [9–10]. Some studies have found reduced depression, apathy and psychiatric symptoms with stimulators turned ON relative to OFF [11–13]. By contrast, case studies demonstrate that some patients experience adverse changes in mood-related behavior with STN DBS, including fits of laughter [14], hypomania [15], and severe transient depression [16–17]. Case reports [17] and other studies [18–19], although not designed to experimentally test whether past psychiatric disorders affect acute alterations in mood induced by STN DBS, highlight the importance of considering the effects of past and current psychiatric disorders on the mood response to STN DBS, which can be quite variable across PD patients.
Here, we test whether past and present psychiatric history modulate the acute effects of STN DBS on mood using a double-blind OFF/clinically optimal ON STN DBS experimental design and well-validated measures of acute mood and behavioral change. In addition, PD participants refrained from dopaminergic medication overnight to reduce confounding the effects of STN DBS on mood [12–14]. Based on past findings from our laboratory [11], we predicted that STN DBS would induce beneficial acute effects on mood in PD participants without past or current mood or anxiety symptomatology. By contrast, we hypothesized that STN DBS would acutely cause adverse alterations in mood in participants with remitted or current mood and anxiety symptoms based on evidence that preexisting psychiatric conditions may render PD patients more susceptible to adverse mood alterations induced by STN DBS [17–19].
Materials and methods
Participants
Thirty-eight participants with PD and bilateral STN-DBS were recruited from the Washington University in St. Louis Movement Disorders Center. Six of these participants previously participated in a different study that measured mood response to STN DBS [11]. Participants were informed of all relevant risks and provided signed consent forms in accordance with the Declaration of Helsinki; the study was approved by the Washington University in St. Louis Human Research Protection Office. Subjects were included based on clinically definite diagnosis of PD [20–22], previously implanted bilateral STN-DBS electrodes and an absence of neurological deficits including dementia, head injury or stroke. Details regarding the specific surgical technique used to implant DBS electrodes and the programming paradigm can be found elsewhere [23]. Soletra or Activa (Medtronic Inc.) pulse generators were used in all participants. DBS implants were previously optimized for motor benefit using monopolar stimulation prior to recruitment into the study.
Localization of STN DBS electrode contacts
Pre-operative clinical MRIs were obtained with a Siemens Vision 1.5T scanner. MRIs were aligned to post-operative computed tomography (CT) images and atlas registration was performed using a validated method [24]. The atlas location of each electrode contact was visualized by overlaying the fused MRI-CT image (resliced to match the Mai atlas [25]) on Mai atlas slices where contact coordinates were plotted [24].
Behavioral protocol
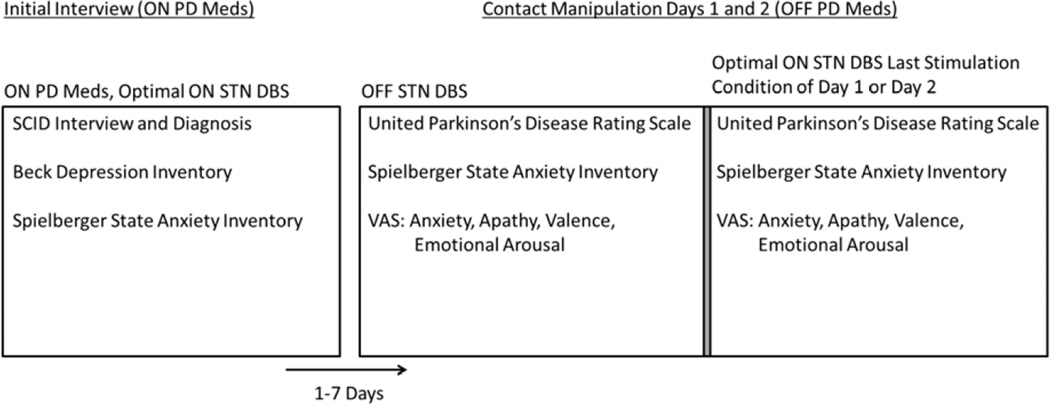
The experimental procedure is diagrammed in Figure 1 and described below.
Figure 1.
Experimental procedure detailing interviews, self-report questionnaires, motor assessment, and computer tasks (Visual Analogue Scales self-ratings) from which dependent variables were obtained. Participants underwent contact manipulation conditions 1–7 days after the Initial Interview. In the case of participants who underwent 2 days of stimulation conditions, OFF STN DBS dependent measure scores were obtained by averaging across both OFF conditions.
Initial Interview
Prior to contact manipulation days, subjects were evaluated with their clinically-determined optimal STN DBS stimulation settings while on anti-parkinsonian medications (optimal ON DBS, on medications) (see Figure 1). Presence of current or remitted mood or anxiety disorders was determined by administration of the Structural Clinical Interview for DSM-IV-TR Axis I Disorders (SCID-I/NP [26]) by a movement disorders-trained neuropsychiatrist (KJB), except that the DSM-IV-TR causation criteria were ignored as suggested by a consensus panel [27], e.g. Major Depressive Disorder was diagnosed rather than Mood Disorder Due to Parkinson Disease. Current depressive and anxiety symptoms were further assessed by 2 self-report questionnaires: the Beck Depression Inventory-II (BDI-II [28]) and the Spielberger State-Trait Anxiety Inventory (SSAI [29]).
For some analyses (described below), the SCID was used to separate groups of participants based on the presence of a threshold-level (as defined by the SCID and as determined by the interviewing psychiatrist) current (threshold criteria met during the last month) or remitted mood or anxiety disorder. The union of these two groups includes all subjects who were diagnosed with past and/or current mood and/or anxiety disorders during the Initial Interview. Due to low numbers of participants who were diagnosed with current mood disorders, we did not analyze these disorders separately. Diagnoses of participants with other Axis I disorders (psychosis, substance abuse or dependence, somatoform or eating disorders) did not occur frequently enough in this sample for reliable data analysis.
Contact Manipulation Days
One to 7 days after the Initial Interview, participants underwent electrode contact manipulation days, during which they underwent a series of stimulation conditions including OFF DBS and off PD medications, and clinically optimal settings ON DBS and off PD medications. Participants abstained from PD medications overnight prior to contact manipulation days and were in the ‘practical defined off state’ [30]. Participants continued to take other medications, including psychiatric medications, and received optimal ON DBS until the first contact manipulation of the dayOptimal ON DBS, off PD medications was always the last stimulation condition of the day. The order of other stimulation conditions was randomized over 1–2 days (see Figure 1). In studies lasting 2 days, an OFF condition occurred on each day and data collected from these conditions were averaged to obtain average OFF scores. Motor and mood outcomes were obtained 30–60 min following each contact manipulation (STN DBS turned OFF or ON) [30].
During each stimulation condition, motor signs were evaluated by a trained clinician blind to stimulation condition using the motor subscale (part III) of the Unified Parkinson’s Disease Rating Scale (UPDRS [31]). Self-rated mood was measured by visual analogue scales (VAS [32]) and the SSAI (“state” only). VAS ratings were linked to the Circumplex Model of emotion [33]. The following summary measures were used as dependent variables: valence and emotional arousal (calculated as described in [34]), anxiety (average of responses to VAS items with anchors calm/nervous, relaxed/distressed, and calm/tense), and apathy (response to a VAS with anchors motivated/apathetic). For clarity in graphic representation, anxiety and apathy scores were reversed by subtracting the raw score from 50 so that scores are centered at zero and lower scores indicate lower anxiety or apathy.
Data analyses
For analyses described below, dependent variables included UPDRS scores, VAS anxiety, apathy, valence and emotional arousal scores, and SSAI anxiety scores, all obtained on contact manipulation days, which included OFF DBS, off PD medication and optimal ON DBS, off PD medication sessions. Due to technical difficulties, one participant did not have SSAI scores and another did not have VAS scores on contact manipulation days. Both of these participants were diagnosed with remitted mood disorders.
Acute effects of STN DBS on mood and motor behavior
Since UPDRS scores consist of ranks, a paired Wilcoxon signed ranks test was used to test for differences in UPDRS scores between OFF DBS, off PD medication and optimal ON STN DBS, off PD medication conditions; paired t-tests were used for VAS valence, anxiety, apathy and arousal and SSAI anxiety variables.
Modulation of STN DBS-induced changes in mood and motor behavior by psychiatric diagnosis
General linear model (GLM) univariate and non-parametric Kruskal-Wallis analyses of variance (ANOVA) determined if age, disease duration, time between DBS surgery and Initial Interview, proportion of participants currently taking psychiatric medications, SSAI scores (during Initial Interview), BDI scores and race and gender distributions differed across 3 groups of participants, including 1) participants diagnosed with a current mood and/or anxiety disorder (n = 15; these participants may also have remitted mood and/or anxiety disorders); 2) participants diagnosed with a remitted mood or anxiety disorder (n = 11, no current diagnosis); and 3) participants deemed to have never met threshold criteria for a mood or anxiety disorder diagnosis (n = 12). UPDRS , SSAI, VAS valence, VAS arousal, VAS anxiety and VAS apathy scores obtained during the OFF DBS, off PD medications condition were also compared across groups with Kruskal-Wallis or univariate ANOVA.
Difference scores for all dependent variables were calculated by subtracting scores obtained during OFF DBS, off PD medication from those obtained during optimal ON DBS, off PD medication conditions. To avoid Type I error due to multiple comparisons and because VAS measures can be highly correlated with each other although they represent different aspects of mood, two separate GLM multivariate ANOVA (MANOVA) were performed to determine whether diagnosis group, as described above, modulated STN DBS-induced VAS difference scores. Since valence and arousal are the main constructs that represent emotional state in the circumplex model of emotion [33] and are scored on the same scale, valence and arousal difference scores were included as dependent variables in the first MANOVA. The second MANOVA included VAS anxiety and apathy difference scores as dependent variables. Significant main effects of diagnosis group by MANOVA and subsequent univariate ANOVA were followed up with post hoc least square difference comparisons. STN DBS-induced differences in SSAI and UPDRS scores were compared across the three diagnosis groups with a univariate ANOVA and a non-parametric Kruskal-Wallis ANOVA, respectively.
Modulation of STN DBS-induced changes in mood and motor behavior by psychiatric symptom severity
The influence of psychiatric symptom severity (measured by the BDI and SSAI during the Initial Interview) on STN DBS induced changes in VAS mood scores were tested in a manner similar to the MANOVAs described in the paragraph above except that BDI or SSAI was treated as a covariate and all participants were included in the analyses instead of partitioned into groups based on SCID diagnoses. Pearson’s r or Spearman’s ρ tested for relationships between Initial Interview BDI or SSAI (from Initial Interview) scores and SSAI (from contact manipulation days) and UPDRS difference scores, respectively.
Relationships between STN DBS-induced changes in mood variables and motor behavior
To determine if STN DBS-induced changes in mood were related to changes in motor function, correlations between mood and UPDRS difference scores were performed with Spearman’s ρ across all participants as well as within diagnostic groups.
The threshold for significance for all analyses was set at p ≤ 0.05, followed by Bonferroni multiple comparisons correction when appropriate.
Results
Participants
Participant characteristics are summarized in Table 1.
Table 1.
Participant characteristics.
| Sex | 18 M, 20 F |
| Age (years) | 63.0 (8.0) |
| Race | 34 White, 1 Black, 3 Native American |
| Duration of PD (years) | 13.6 (5.0) |
| Months from surgery to participation | 15.5 (8.4) |
| UPDRS Motor Score (OFF stimulation, off PD medication) | 33.9 (11.4) |
| BDI-II Score | 10.0 (5.5); range = 0–21 |
| SSAI Score | 30.4 (7.3); range = 20–45 |
| Psychiatric Medication Type α * | None, n = 15; SSRI, n = 8; SNRI, n = 1; TeCA, n = 3; TCA n = 2; nTCA n = 3; BZD/BZD-like n = 11; Other n = 2 |
Mean (S.D.) shown.
Participants may take more than 1 type of psychiatric medication.
data not obtained from 2 participants.
BDI-II, Beck Depression Inventory II; SSAI, Spielberger State Anxiety Inventory; SSRI, selective serotonin reuptake inhibitor; SNRI, serotonin and norepinephrine inhibitor; TeCA, tetracyclic antidepressant; TCA, tricyclic antidepressant; nTCA, non-tricyclic antidepressant (bupropion); BZD, benzodiazepine; Other, neudexta and lamotrigine
Stimulation Parameters and Clinical Contact Locations
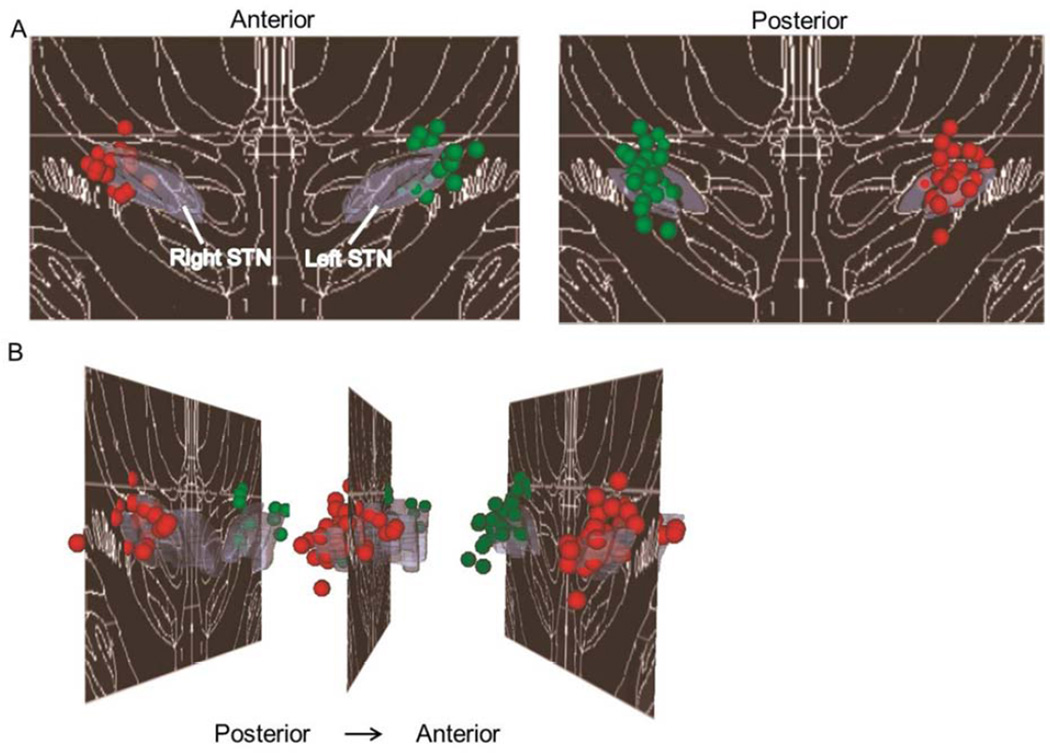
All participants had bilateral STN DBS with a monopolar configuration, with 185 Hz frequency, 1.3 – 3.6 V amplitude, and 60 or 90 µs pulse width. STN DBS contact locations were mostly localized to the posterior STN and adjacent regions (see Figure 2).
Figure 2.
Three-dimensional distribution of clinically optimized STN DBS electrode contacts for the sample studied (N = 38). They are presented (A) coronally and (B) sagittally, overlaid on the Mai atlas [25], 17.2 mm posterior to the anterior commissure. For display purposes, a 0.75 mm radius sphere was centered on each contact location. Violet = STN; red spheres = right electrode contact locations; green spheres = left electrode contact locations.
Acute Effects of STN DBS
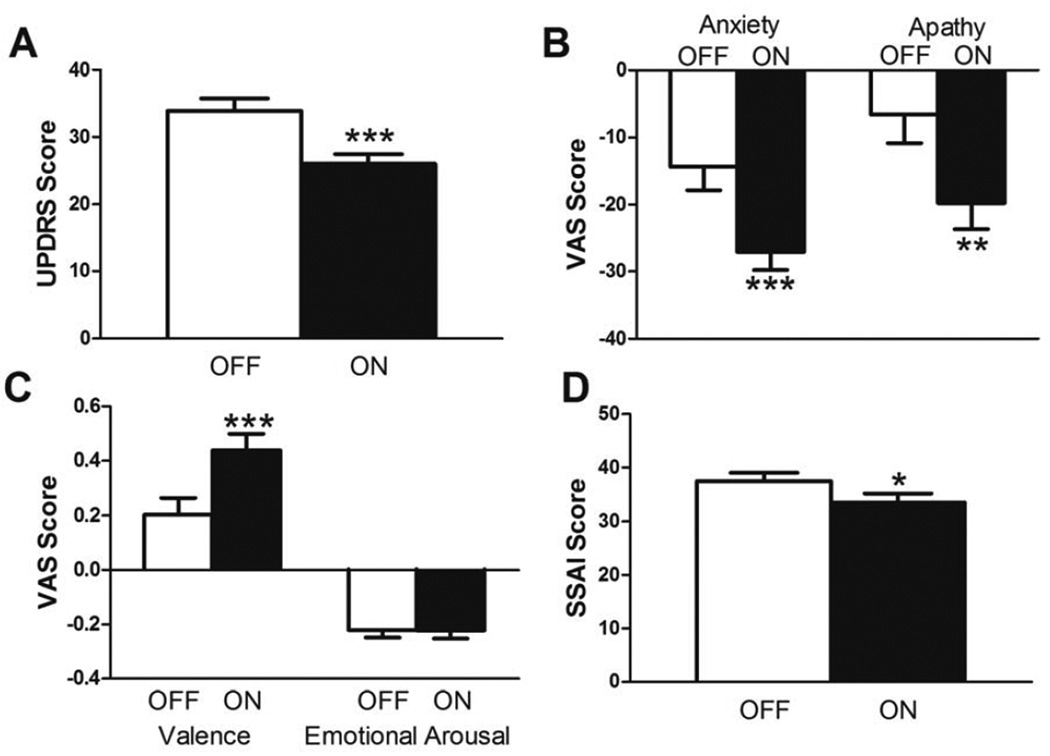
Relative to OFF DBS (off PD medications), optimal ON DBS (off PD medications) improved motor symptoms (UPDRS: Z37 = −4.64, p < 0.001), self-rated anxiety (VAS: t36 = 4.45, p < 0.001; SSAI: t36 = 2.56, p < 0.05), apathy (VAS: t36 = 3.37, p < 0.01) and affective valence (VAS: t36 = −4.72, p < 0.001), but did not affect affective arousal (VAS: t36 = 0.10, p = 0.93) (see Figure 3). Multiple comparisons correction was not applied here because we predicted that STN DBS would improve mood and motor function based on previous results from our laboratory [11].
Figure 3.
Acute effects of STN DBS on mood and motor behavior. Relative to OFF DBS (off PD medications), clinically optimal STN DBS (off PD medications) (A) improved motor symptoms, (B, D) decreased anxiety and apathy, and (C) increased valence (improved mood), but had no effect on emotional arousal. Mean + SEM shown. *, p < 0.05, **, p < 0.01, ***, p < 0.001 relative to OFF. VAS, visual analogue scale; SSAI, Spielberger State Anxiety Inventory; UPDRS, United Parkinson’s Disease Rating Scale.
Clinician Diagnoses and Symptom Severity
Tables 2 and 3 detail the distribution of subjects according to SCID diagnosis at the Initial Interview and psychiatric medication use.
Table 2.
Distribution of diagnosed disorders among PD participants in sample.
| SCID-I/NP Diagnoses α | Number of participants | Number of participants taking psychiatric medications* |
|---|---|---|
| Current mood disorder | 3 | 3 |
| Remitted mood disorder | 15 | 13 |
| No mood disorder ever | 21 | 6 |
| Current anxiety disorder | 15 | 9 |
| Remitted anxiety disorder | 5 | 3 |
| No anxiety disorder ever | 19 | 9 |
Participants may belong to more than 1 diagnostic category.
data not obtained from 2 participants.
Table 3.
Number of subjects diagnosed with various mood and anxiety disorders with the SCID-I/NP.α
| Remitted | Current | |
|---|---|---|
| Mood Disorders | ||
| Major Depressive Disorder | 10 | 2 |
| Depressive Disorder NOS | 3 | 1 |
| Substance Induced | 1 | 0 |
| Anxiety Disorders | ||
| Social Phobia | 3 | 6 |
| Specific Phobia | 0 | 4 |
| Anxiety Disorder NOS | 0 | 4 |
| Panic Disorder without Agoraphobia | 1 | 1 |
| Obsessive Compulsive Disorder | 1 | 0 |
Participants may be diagnosed with more than one disorder. SCID, Structural Clinical Interview for DSM-IV-TR Axis I Disorders; NOS, not otherwise specified.
Modulation of STN DBS-induced changes in mood and motor behavior by psychiatric diagnosis
Participants with a current mood or anxiety disorder diagnosis (n = 15) did not differ from those with remitted diagnoses (n = 11) or from participants deemed to have never met threshold for diagnoses (n = 12) in age (p = 0.14), disease duration (p = 0.92; data missing for 1 participant in the group of participants in the non-diagnosed group), the number of months between STN DBS surgery and the Initial Interview (p = 0.55), gender (p = 0.64), proportion of participants currently taking psychiatric medications (p = 0.20), BDI scores (p = 0.20), or SSAI scores (p = 0.32). Racial distribution did differ among diagnosis groups (p < 0.05) (Table 4). SSAI (p = 0.69), VAS valence (p = 0.39), VAS arousal (p = 0.45), VAS anxiety (p = 0.34), VAS apathy (p = 0.21) and UPDRS (p = 0.12; see Table 4) scores obtained during the OFF DBS, off PD medication condition did not differ across groups.
Table 4.
Participant characteristics by SCID diagnosis group.
| Current Mood or Anxiety Disorder Diagnosis (n = 15) |
Remitted Mood or Anxiety Disorder Diagnosis (n = 11) |
No Mood or Anxiety Disorder Diagnosis Ever (n = 12) |
|
|---|---|---|---|
| Sex | 6 M, 9 F | 5 M, 6 F | 7 M, 5 F |
| Age (years) | 60.9 (7.7) | 61.6 (9.3) | 66.8 (6.4) |
| Race | 11 White, 1 Black, 3 Native American |
11 White | 12 White |
| Duration of PD (years) | 13.8 (5.4) | 13.1 (4.9) | 13.9α(5.0) |
| Months from surgery to participation |
16.5 (7.1) | 16.5 (10.3) | 13.2 (8.2) |
| UPDRS Motor Score (OFF DBS, off PD medications) |
37.9 (13.9) | 33.5 (9.6) | 29.4 (7.9) |
| BDI-II Score | 11.9 (5.8) | 8.1 (3.8) | 9.4 (6.1) |
| SSAI Score | 31.6 (6.9) | 27.5 (5.3) | 31.4 (9.0) |
Mean (S.D.) shown. Data missing for
1 participant.
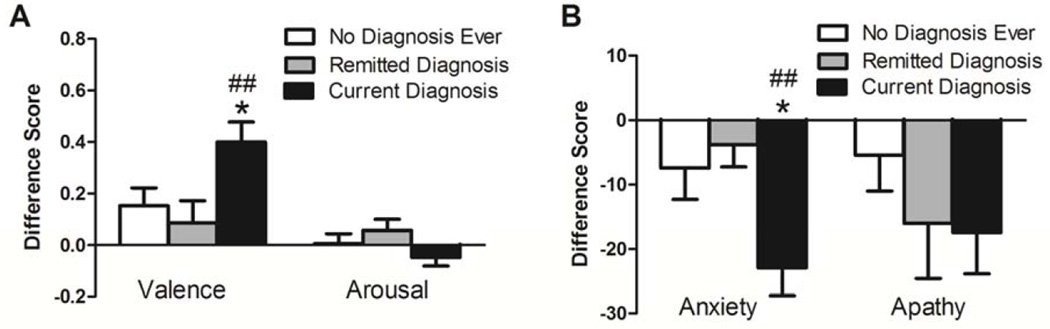
Participants with current mood or anxiety disorder diagnoses experienced increased STN DBS-induced improvement in valence and anxiety (as measured by VAS) but not arousal or apathy compared to participants who were remitted or deemed to never have met threshold for diagnosis. (Figure 4A–B, Table 5). STN DBS-induced changes in SSAI (F2,34 = 67.36, p = 0.47) and UPDRS (X236 = 1.03, p = 0.60) scores did not differ across the three diagnosis groups (data not shown).
Figure 4.
STN DBS-induced improvements in mood are greater in participants with current anxiety or mood disorder diagnoses relative to participants with remitted diagnoses or deemed never to have met threshold criteria for diagnosis. Optimal ON, off medication STN DBS-induced improvements in self-rated (A) valence but not arousal and (B) anxiety but not apathy were significantly elevated in currently diagnosed participants relative to remitted and never diagnosed groups. Mean + SEM shown. *, p < 0.05 relative to no diagnosis ever; ##p < 0.01 relative to remitted diagnosis.
Table 5.
Results for MANOVAs: Diagnostic group modulation of STN DBS-induced changes in VAS measures. Current diagnosis n = 15; remitted diagnoses n = 10; no diagnosis ever n = 12. Also, see Figure 4
| Dependent Measure | Main effect of Diagnostic Group |
Main effect of Diagnostic Group by VAS measure |
post hoc LSD results |
|---|---|---|---|
| VAS Valence and Arousal Difference Scores |
F4,68 = 2.56, p = 0.046 | Valence: F2,34 = 4.65, p= 0.016 | Valence: current vs. remitted, p= 0.009; current vs. never, p= 0.027; remitted vs. never, p = 0.575 |
| Arousal: F2,34 = 1.92, p = 0.162 | Arousal: N/A (no main effect) | ||
| VAS Anxiety and Apathy Difference Scores |
F4,68 = 3.27, p = 0.016 | Anxiety: F2,34 = 5.61, p= 0.008 | Anxiety: current vs. remitted, p= 0.005; current vs. never, p= 0.014; remitted vs. never, p = 0.308 |
| Apathy: F2,34 = 0.94, p = 0.401 | Apathy: N/A (no main effect) |
Modulation of STN DBS-induced changes in mood and motor behavior by psychiatric symptom severity
BDI-II scores obtained during the Initial Interview (optimal ON, on medications) did not significantly modulate STN DBS-induced changes in any VAS measure (valence and arousal MANCOVA: F2,34 = 0.90, p = 0.42; anxiety and apathy MANCOVA: F2,34 = 0.53, p = 0.59), SSAI (r37 = 0.22, p = 0.19) or UPDRS (ρ38 = 0.21, p = 0.21) scores (data not shown).
SSAI scores obtained during the Initial Interview (optimal ON, on medications) did not significantly modulate STN DBS-induced changes in any VAS measure (valence and arousal MANCOVA: F2,34 = 1.51, p = 0.24; anxiety and apathy MANCOVA: F2,34 = 0.16, p = 0.85), SSAI (r37 = 0.09, p = 0.62) or UPDRS (ρ38 = 0.13, p = 0.44) scores (data not shown).
Relationships Between Motor and Mood Responses to STN DBS
Across all participants (n = 37 excluding 1 participant each for VAS and SSAI analyses due to missing scores), DBS-induced change in UPDRS scores did not significantly correlate with DBS-induced change in anxiety (VAS: ρ37 = 0.04, p = 0.80; SSAI: ρ37 = 0.22, p = 0.18), apathy (ρ37 = 0.11, p = 0.51), valence (ρ37 = −0.01, p = 0.97) or arousal (ρ37 = −0.06, p = 0.71) (data not shown). STN DBS-induced changes in self-rated VAS and SSAI scores also were not significantly related to DBS-induced change in UPDRS scores within any diagnostic group: 1) participants who had current mood or anxiety diagnoses (n = 15) (ρ15 ≤ 0.51, p ≥ 0.05 for all correlations, data not shown); 2) participants diagnosed with remitted mood or anxiety disorders (n = 10) (ρ10 ≤ 0.58, p ≥ 0.08 for all correlations, data not shown); 3) participants deemed to never have had a threshold-level current or remitted mood or anxiety disorder (n = 12) (ρ12 ≤ 0.18, p ≥ 0.57 for all correlations, data not shown). None of the correlational p-values survived Bonferroni multiple comparisons corrections.
Discussion
The current study is the first analysis of the influence of mood or anxiety disorder diagnoses and self-reported psychiatric symptom severity on acute mood response to STN DBS with a rigorous OFF vs. ON DBS experimental design while off PD medications. As expected, STN DBS exerted acute positive effects on mood and motor behavior. Unexpectedly, PD participants diagnosed with current mood or anxiety disorders were more sensitive to the beneficial mood effects of STN DBS than those that did not meet threshold criteria for current diagnosis. Although STN DBS acutely improved motor manifestations, change in motor function did not correlate with change in self-rated mood, suggesting that these effects occurred independently of each other on an individual level. Taken together, these findings suggest that current mood or anxiety disorders in PD alter the response of mood-related circuitry to STN DBS and provide further evidence that the STN is a functionally heterogeneous brain region embedded in both sensorimotor and limbic circuitry [5–6,8].
Bilateral STN DBS acutely decreased self-reported anxiety and apathy while increasing affective valence, but did not affect emotional arousal. These results support previous studies with similar experimental designs (ON vs. OFF stimulation, off PD medications) [11–13]. Replication of these findings both at a different clinical site from those in previous studies [12–13] and within our own laboratory [11] provides strong evidence for their validity. However, our results do contrast with those of other studies [17–19] in which STN DBS induced adverse effects on mood. However, these psychiatric adverse effects appear to occur in some but not all PD patients in one study [19], and the other two are retrospective case reports [17–18]. Importantly, none of these studies [17–19] employed planned experiments designed to test for acute changes in mood induced by STN DBS with an OFF control condition. Furthermore, as described and as shown in Figure 2, the optimal STN DBS contact locations for participants in our study were in and around the caudal dorsolateral STN region, which is the surgical target for STN DBS. Perhaps DBS at more ventral STN sites induces more profound and/or adverse effects on mood. Indeed, DBS of dorsal and ventral/ventromedial regions of the STN is widely hypothesized to be disproportionately associated with alterations in motor behavior and mood, respectively [35–38].
Contrary to our hypothesis, PD participants diagnosed with current mood or anxiety disorders were more sensitive to STN DBS-induced improvements in valence and anxiety, as measured by VAS, than those that were deemed to be remitted or to not have ever met threshold criteria for diagnosis. It seems unlikely that the currently diagnosed group showed increased STN DBS-induced benefit in mood solely due to worse mood state at baseline since, relative to the remitted group and participants that were deemed to have never met criteria for diagnosis, they did not differ in BDI or SSAI scores during the Initial Interview or VAS or SSAI scores during the OFF DBS, off medication condition. Given that the study was designed to carefully control for confounding effects such as placebo, lesion, and PD medications, it seems reasonable to infer that PD participants diagnosed with current mood or anxiety disorders likely have disturbed brain circuitry that is acutely more responsive to STN DBS compared to PD participants not currently diagnosed. In PD participants remitted for mood or anxiety disorders, this system may not be more responsive to STN DBS compared to participants never meeting criteria for a mood or anxiety disorder diagnosis because the disorder occurred prior to the onset of PD and/or disturbed circuitry may be recovered due to medication, other therapy, spontaneous remission, possibly ongoing STN DBS or any combination of these factors. STN DBS may acutely improve mood and anxiety by affecting neurotransmission and/or other signaling features of the motor, associative and limbic circuitry that project to and/or receive input from the STN. The exact mechanism by which high-frequency DBS exerts its effects remains unknown but it likely reduces disturbances in basal ganglia thalamocortical network activity by increasing both excitatory and inhibitory signaling in the STN and adjacent fiber tracts [39]. In our study, DBS-induced improvements in self-rated mood and anxiety did not correlate with improved motor function. Our findings suggest that optimized STN DBS can impact mood-related neural circuitry in addition to and/or separately from its effects on motor symptoms.
Self-reported depressive and anxiety symptoms as measured by BDI and SSAI, respectively, did not modulate mood response to STN DBS. BDI and SSAI scores also did not differ between PD participants diagnosed with current mood or anxiety disorders and those without; nor did they differ between PD participants remitted for these disorders and those deemed to have never met criteria for diagnosis. These results are surprising because study participants completed these questionnaires at a maximum of one week prior to contact manipulation days, suggesting that relatively recent self-reported symptom severity is not necessarily an accurate indicator of current or past threshold-level clinical symptoms of mood and anxiety disorders in PD. Furthermore, our findings indicate that self-reported symptom severity does not predict mood or anxiety response to STN DBS while categorical clinical diagnosis does. Interestingly, unlike VAS anxiety scores, psychiatric diagnosis did not modulate STN DBS-induced changes in SSAI scores. The causes and implications of these findings deserve further study.
There are some limitations to this study. First, the majority of current SCID-diagnosed psychiatric disorders in this study were anxiety disorders whereas past SCID-diagnosed psychiatric disorders were primarily mood disorders. The current study was not designed to test for differential modulation of acute mood response by mood vs. anxiety disorders. The small sample size and overlap of these symptoms in the same participants limits our ability to disentangle the influence of these two types of disorders. Indeed, all 3 participants diagnosed with current mood disorders were also diagnosed with current anxiety disorders.
Second, although participants were blinded to stimulation condition, fatigue and relief to be nearly done with the study could contribute to improved mood and/or motor behavior since the optimal ON condition was the last stimulation condition of the day. However, similar results from our laboratory [11] and others [12–13] in which OFF and ON DBS conditions were randomized indicate that observed acute effects of STN DBS on mood and anxiety in our study are not an artifact of anticipated relief. Furthermore, visual inspection of the time course over the study day shows that, with the exception of arousal, mood and motor function do not appear to improve over time (Figure 1, Appendix). It is also possible that DBS effects did not completely dissipate during the OFF DBS, off medication condition, which may account for the lack of difference among diagnostic groups on the SSAI and VAS measures. Indeed, reversible neuropsychiatric symptom rebounds are associated with gradualDBS (in regions other than STN) battery depletion over time for treatment-resistant depression [40] and obsessive compulsive disorder [41]. Since we investigated acute rather than long-term DBS effects , long-term wearing-off of DBS most likely does not account for diagnostic group differences in mood responsivity.
Finally, we controlled for PD medication but not psychiatric medication use. However, as detailed in Table 2, a substantial number of PD participants who were diagnosed with remitted or deemed to never have had a mood or anxiety disorder were taking psychiatric medications during the study and the proportion of participants with current diagnoses that were taking psychiatric medications did not differ between these groups. Therefore, although future studies should include investigation of psychiatric medication effects on behavior, it is unlikely that psychiatric medication is responsible for the positive modulation of mood response to STN DBS by current mood or anxiety disorder diagnoses.
Conclusions
PD participants diagnosed with current mood or anxiety disorders are more sensitive to STN DBS-induced improvements in mood, a possible indication that basal ganglia circuitry may be further altered in this group relative to those with remitted disorders or those deemed to have never met threshold criteria for diagnosis. Importantly, our findings support the notion that the STN plays a role in both motor and psychiatric manifestations in PD and may serve as an integration site for motor and limbic information as suggested by Haynes and Haber [5]. Future studies should investigate the effect of prolonged STN DBS on mood with longitudinal studies as well as the impact of baseline psychiatric symptomatology on the relationship between STN DBS location and alterations in mood in PD.
Supplementary Material
Highlights.
“Acute changes in mood induced by subthalamic deep brain stimulation in Parkinson disease are modulated by psychiatric diagnosis” (original Ms. Ref. No.: BRS-D-14-00109) by SA Eisenstein, WB Dewispelaere, MC Campbell, HM Lugar, JS Perlmutter, KJ Black, T Hershey.
Motor and mood responses to acute STN DBS were studied in 38 PD participants.
STN DBS improved motor and mood outcomes relative to OFF DBS.
Current psychiatric diagnosis was related to increased DBS-induced mood benefit.
Brain circuitry may be altered in PD participants with psychiatric diagnoses.
Acknowledgments
The authors would like to thank Deboragh Moore, Colleen Considine, Tasha D. Doty, Mary Creech, Emily C. Bihun, Samantha A. Ranck, Dawn Lintzenich, Johanna Hartlein and Angela Wernle for data collection. The authors also thank the following for support: the National Institutes of Health (R01NS058797 [TH, SAE], R01NS41509 [JSP], K24MH087913 [KJB], UL1TR000448 [WBD, CTSA]); Brain & Behavior Research Foundation [MCC]; American Parkinson Disease Association (APDA) Greater St. Louis chapter; APDA Advanced Research Center at Washington University; and Barnes Jewish Hospital Foundation. The R01NS058797 supported collection, analysis, and interpretation of data and writing this report. Other funding sources did not have specific roles in study design.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure of Financial Interests and Potential Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Black KJ, Pandya A. Depression in Parkinson disease. In: Gilliam F, Sheline YI, editors. Depression and Brain Dysfunction. Oxon, UK: Parthenon Publishing; 2005. pp. 199–237. [Google Scholar]
- 2.Cummings JL. Depression and Parkinson’s disease: a review. Am J Psychiatry. 1992;149:443–454. doi: 10.1176/ajp.149.4.443. [DOI] [PubMed] [Google Scholar]
- 3.Lee RG. Physiology of the basal ganglia and pathophysiology of Parkinson’s disease. Can J Neurol Sci. 1987;14:373–380. doi: 10.1017/s0317167100037768. [DOI] [PubMed] [Google Scholar]
- 4.Siddiqui MS, Ellis T, Tatter SS, Foote KD, Okun MS. Deep Brain Stimulation: Patient Selection in Parkinson’s Disease, Other Movement Disorders, and Neuropsychiatric Disorders. In: Tarsy D, Vitek JL, Starr PA, Okun MS, editors. Deep Brain Stimulation in Neurological and Psychiatric Disorders. Totowa, NJ: Humana Press; 2008. pp. 83–98. [Google Scholar]
- 5.Haynes WIA, Haber SN. The organization of prefrontal-subthalamic inputs in primates provides an anatomical substrate for both functional specificity and integration: Implications for basal ganglia models and deep brain stimulation. J Neurosci. 2013;33:4804–4814. doi: 10.1523/JNEUROSCI.4674-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambert C, Zrinzo L, Nagy Z, Lutti A, Hariz M, Foltynie T, et al. Confirmation of functional zones within the human subthalamic nucleus: patterns of connectivity and subparcellation using diffusion weighted imaging. Neuroimage. 2012;60:83–94. doi: 10.1016/j.neuroimage.2011.11.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- 8.Parent A, Hazrati LN. Functional anatomy of the basal ganglia. II. The place of subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res Brain Res Rev. 1995;20:128–154. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
- 9.Burn DJ, Tröster AI. Neuropsychiatric complications of medical and surgical therapies for Parkinson’s disease. J Geriatr Psychiatry Neurol. 2004;17:172–180. doi: 10.1177/0891988704267466. [DOI] [PubMed] [Google Scholar]
- 10.Takeshita S, Kurisu K, Trop L, Arita K, Akimitsu T, Verhoeff NP. Effect of subthalamic stimulation on mood state in Parkinson’s disease: evaluation of previous facts and problems. Neurosurg Rev. 2005;28:179–186. doi: 10.1007/s10143-005-0387-4. [DOI] [PubMed] [Google Scholar]
- 11.Campbell MC, Black KJ, Weaver PM, Lugar HM, Videen TO, Tabbal SD, et al. Mood response to deep brain stimulation of the subthalamic nucleus in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 2012;24:28–36. doi: 10.1176/appi.neuropsych.11030060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Czernecki V, Pillon B, Houeto JL, Welter ML, Mesnage V, Agid Y, et al. Does bilateral stimulation of the subthalamic nucleus aggravate apathy in Parkinson’s disease? J Neurol Neurosurg Psychiatry. 2005;76:775–779. doi: 10.1136/jnnp.2003.033258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schneider F, Habel U, Volkmann J, Regel S, Kornischka J, Sturm V, et al. Deep brain stimulation of the subthalamic nucleus enhances emotional processing in Parkinson disease. Arch Gen Psychiatry. 2003;60:296–302. doi: 10.1001/archpsyc.60.3.296. [DOI] [PubMed] [Google Scholar]
- 14.Krack P, Kumar R, Ardouin C, Dowsey PL, McVicker JM, Benabid AL, et al. Mirthful laughter induced by subthalamic nucleus stimulation. Mov Disord. 2001;16:867–875. doi: 10.1002/mds.1174. [DOI] [PubMed] [Google Scholar]
- 15.Mandat TS, Hurwitz T, Honey CR. Hypomania as an adverse effect of subthalamic nucleus stimulation: report of two cases. Acta Neurochir (Wien) 2006;148:895–897. doi: 10.1007/s00701-006-0795-4. [DOI] [PubMed] [Google Scholar]
- 16.Bejjani BP, Damier P, Arnulf I, Thivard L, Bonnet AM, Dormont D, et al. Transient acute depression induced by high-frequency deep-brain stimulation. N Engl.J Med. 1999;340:1476–1480. doi: 10.1056/NEJM199905133401905. [DOI] [PubMed] [Google Scholar]
- 17.Doshi PK, Chhaya N, Bhatt MH. Depression leading to attempted suicide after bilateral subthalamic nucleus stimulation for Parkinson’s disease. Mov Disord. 2002;17:1084–1085. doi: 10.1002/mds.10198. [DOI] [PubMed] [Google Scholar]
- 18.Houeto JL, Mesnage V, Mallet L, Pillon B, Gargiulo M, du Moncel ST, et al. Behavioural disorders, Parkinson’s disease and subthalamic stimulation. J Neurol Neurosurg Psychiatry. 2002;72:701–707. doi: 10.1136/jnnp.72.6.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thobois S, Mertens P, Guenot M, Hermier M, Mollion H, Bouvard M, et al. Subthalamic nucleus stimulation in Parkinson’s disease: clinical evaluation of 18 patients. J Neurol. 2002;249:529–534. doi: 10.1007/s004150200059. [DOI] [PubMed] [Google Scholar]
- 20.Calne DB, Snow BJ, Lee C. Criteria for diagnosing Parkinson’s disease. Ann Neurol. 1992;32:S125–S127. doi: 10.1002/ana.410320721. [DOI] [PubMed] [Google Scholar]
- 21.Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: A clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–184. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Racette BA, Rundle M, Parsian A, Perlmutter JS. Evaluation of a screening questionnaire for genetic studies of Parkinson’s disease. Am J Med Genet. 1999;88:539–543. [PubMed] [Google Scholar]
- 23.Tabbal SD, Revilla FJ, Mink JW, Schneider-Gibson P, Wernle AR, de Erausquin GA, et al. Safety and efficacy of subthalamic nucleus deep brain stimulation performed with limited intraoperative mapping for treatment of Parkinson’s disease. Neurosurgery. 2007;61:119–127. doi: 10.1227/01.neu.0000289725.97211.51. [DOI] [PubMed] [Google Scholar]
- 24.Videen TO, Campbell MC, Tabbal SD, Karimi M, Hershey T, Perlmutter JS. Validation of a fiducial-based atlas localization method for deep brain stimulation contacts in the area of the subthalamic nucleus. J Neurosci Methods. 2008;168:275–281. doi: 10.1016/j.jneumeth.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mai JK, Assheuer J, Paxinos G. Atlas of the Human Brain. San Diego: Elsevier Academic Press; 2004. [Google Scholar]
- 26.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV-TR Axis I Disorders – Non-patient Edition (SCID-I/NP), 4/2005 revision) New York: Biometrics Research, New York State Psychiatric Institute; 2005. [Google Scholar]
- 27.Marsh L, McDonald WM, Cummings JL, Ravina B. NINDS/NIMH Work Group on Depression and Parkinson’s Disease. Provisional Diagnostic Criteria for Depression in Parkinson’s Disease: Report of an NINDS/NIMH Work Group. Mov Disord. 2006;21:148–158. doi: 10.1002/mds.20723. [DOI] [PubMed] [Google Scholar]
- 28.Beck AT, Steer RA, Brown G. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 29.Spielberger CD, Gorsuch R, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 30.Moro E, Scerrati M, Romito LM, Roselli R, Tonali P, Albanese A. Chronic subthalamic nucleus stimulation reduces medication requirements in Parkinson’s disease. Neurology. 1999;53:85–90. doi: 10.1212/wnl.53.1.85. [DOI] [PubMed] [Google Scholar]
- 31.Lang AE, Fahn S. Assessment of Parkinson’s disease. In: Munsat TL, editor. Quantification of Neurologic Deficit. Boston: Butterworths; 1989. pp. 285–309. [Google Scholar]
- 32.Folstein MF, Luria R. Reliability, validity, and clinical application of the Visual Analogue Mood Scale. Psychol Med. 1973;3:479–486. doi: 10.1017/s0033291700054283. [DOI] [PubMed] [Google Scholar]
- 33.Larsen RJ, Diener E. Promises and problems with the circumplex model of emotion. In: Clarck MS, editor. Emotion: Review of personality and social psychology. Thousand Oaks, CA: Sage Publications, INC; 1992. pp. 25–59. [Google Scholar]
- 34.Limsoontarakul S, Campbell MC, Black KJ. A perfusion MRI study of emotional valence and arousal in Parkinson’s disease. Parkinson’s Disease. 2011;2011:742907. doi: 10.4061/2011/742907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chopra A, Tye SJ, Lee KH, Sampson S, Matsumoto J, Adams A, et al. Underlying neurobiology and clinical correlates of mania status after subthalamic nucleus deep brain stimulation in Parkinson’s disease: a review of the literature. J Neuropsychiatry Clin Neurosci. 2012;24:102–110. doi: 10.1176/appi.neuropsych.10070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greenhouse I, Gould S, Houser M, Hicks G, Gross J, Aron AR. Stimulation at dorsal and ventral electrode contacts targeted at the subthalamic nucleus has different effects on motor and emotion functions in Parkinson’s disease. Neuropsychologia. 2011;49:528–534. doi: 10.1016/j.neuropsychologia.2010.12.030. [DOI] [PubMed] [Google Scholar]
- 37.Raucher-Chene D, Charrel CL, de Maindreville AD, Limosin F. Manic episode with psychotic symptoms in a patient with Parkinson’s disease treated by subthalamic nucleus stimulation: improvement on switching the target. J Neurol Sci. 2008;273:116–117. doi: 10.1016/j.jns.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 38.Schilbach L, Weiss PH, Kuhn J, Timmermann L, Klosterkotter J, Huff W. Pharmacological treatment of deep brain stimulation-induced hypomania leads to clinical remission while preserving motor benefits. Neurocase. 2012;18:152–159. doi: 10.1080/13554794.2011.568502. [DOI] [PubMed] [Google Scholar]
- 39.Miocinovic S, Somayajula S, Chitnis S, Vitek JL. History, applications, and mechanisms of deep brain stimulation. JAMA Neurol. 2013;70:163–171. doi: 10.1001/2013.jamaneurol.45. [DOI] [PubMed] [Google Scholar]
- 40.Kennedy SH, Giacobbe P, Rizvi SJ, Placenza FM, Nishikawa Y, Mayberg HS, et al. Deep brain stimulation for treatment-resistant depression: follow-up after 3 to 6 years. Am J Psychiatry. 2011;168:502–510. doi: 10.1176/appi.ajp.2010.10081187. [DOI] [PubMed] [Google Scholar]
- 41.Vora AK, Ward H, Foote KD, Goodman WK, Okun MS. Rebound symptoms following battery depletion in the NIH OCD DBS cohort: clincial and reimbursement issues. Brain Stimul. 2012;5:599–604. doi: 10.1016/j.brs.2011.10.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.