Abstract
Background
Hypoxic-ischemic injury (HI) to preterm brain results in white matter loss. The endogenous oligodendroglial response to perinatal HI is characterized by increased oligodendroglial progenitor cells (OPCs). MicroRNAs (miRs) are important post-transcriptional regulators of gene expression, and a few miRs have been shown to regulate differentiation of OPCs into mature oligodendroglia (OLs). We tested the hypothesis that microRNAs play a role in the increase in OPCs in response to perinatal HI.
Methods
We inducibly deleted the miR-processing enzyme Dicer in OPCs using a tamoxifen-inducible NG2CreERT2 transgene in Dicerfl/fl mice. After HI, mice were analyzed for OPC differentiation using immunofluorescence and for white matter formation by Luxol fast blue (LFB) staining. Functional recovery from injury was investigated using digital gait analysis. We also tested whether HI changed miRs known to regulate OPC differentiation using quantitative RT-PCR.
Results
Perinatal HI induced significant increases in miR-138 and miR-338, two microRNAs known to regulate OPC differentiation. Knockdown of Dicer increased myelin basic protein (MBP) and LFB staining within corpus callosum after HI. In addition, there was significant improvement in motor function 14 and 24 days post lesion.
Conclusion
Changes in specific mature miRs expressed in OPCs following HI may contribute to white matter injury.
Introduction
Preterm babies are at particular risk for white matter injury due to hypoxia-ischemia (HI). This is likely because oligodendrocyte progenitors cells (OPCs) that are abundant at the time of preterm birth are particularly vulnerable to this type of injury (1). Nonetheless, HI results in an increase in OPCs yet a decrease in mature oligodendrocytes (OLs) and myelin (2,3). This is thought to be caused by the inability of new OPCs to differentiate into mature OLs (3). Little is known about the mechanism leading to this arrest in OPC differentiation.
MicroRNAs (miRs) are small non-coding RNAs that are important post-transcriptional regulators of gene expression (4) that were first identified as regulators of developmental transitions, functioning to deplete mRNAs leftover from an earlier developmental stage (5). MiRs are particularly enriched in the brain where they function in neural stem/progenitor cell development (6,7). MiRs are sequentially processed, progressing from a primary form to a premature form with a stem-loop structure and finally to the mature form that suppresses protein translation by binding to the 3’UTR of mRNA to either inhibit translation or augment degradation. Progression from the premature form to the mature form requires the ribonuclease Dicer (4,7). Conditional loss of Dicer has demonstrated the critical role for miRs in many mouse tissues.
MiRs have been demonstrated to be important for normal central nervous system myelination as evidenced by delayed myelination in Olig2Cre;Dicerfl/fl and CNPase;Dicerfl/fl mice (8), decreased myelin in Olig1Cre;Dicerfl/fl mice (9) and dysmyelination in PLPCre;Dicerfl/fl mice (10). To test the hypothesis that miRs are involved in the oligodendroglial lineage response to perinatal HI, we established a mutant mouse strain in which Dicer can be inducibly excised from NG2 cells (NG2CreERT2;Dicerfl/fl mice), neural progenitors that are distributed widely throughout the brain and that differentiate predominantly into OPCs (11).
Results
Knockdown of Dicer in neural progenitors following perinatal hypoxia-ischemia increases mature oligodendrocytes and white matter within the corpus callosum
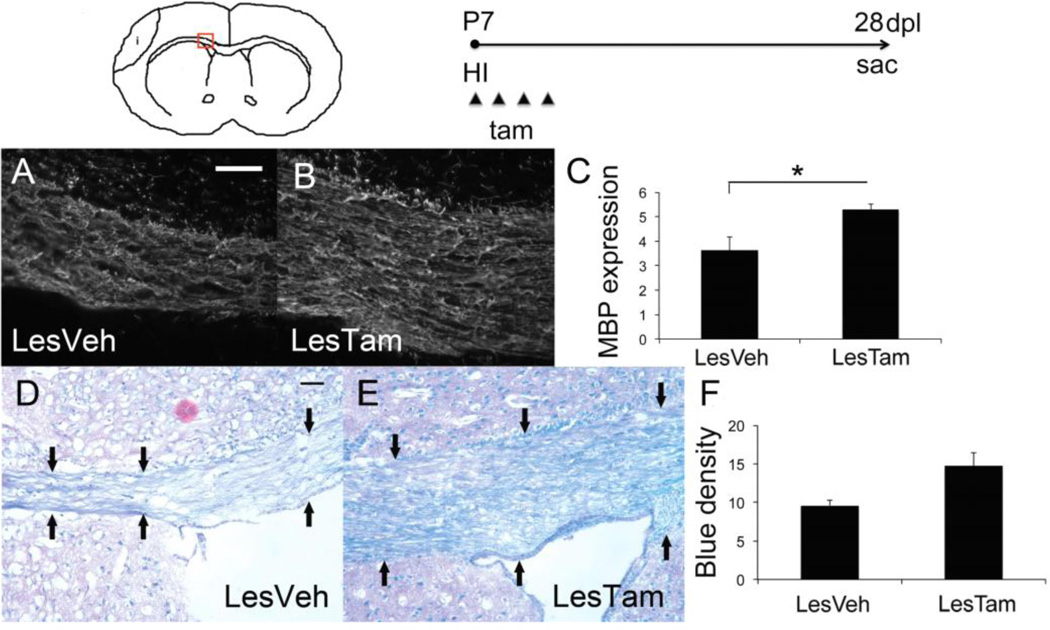
To inducibly excise the Dicer 1 intron within NG2+ progenitor cells, tamoxifen was administered to NG2CreERT2;Dicerflx/flx mice immediately after recovery from HI and daily thereafter for a total of 4 days (P7, P8, P9 and P10). Mice were sacrificed 28 days post lesion (dpl) when we used immunofluorescence to probe for markers of differentiated OLs. We compared the following 2 groups: HI/vehicle-treated (LesVeh) and HI/tamoxifen-treated (LesTam). We assayed for changes in expression of myelin basic protein (MBP), a marker of mature myelinating OL, within corpus callosum, again with knockdown of Dicer 0–4dpl. We compared protein expression by relative fluorescence and found a significant 1.46-fold increased expression of MBP in corpus callosum of the LesTam group compared to the LesVeh group (LesTam 5.30±0.2 average intensity v. LesVeh 3.63±0.5 average intensity, p=0.048; n=4 LesVeh and 4 LesTam mice) (Figure 1A, B and C). We also assayed for changes in expression of CNPase, a marker that is expressed by immature OL. However, we found only a 1.29-fold trend towards increased expression that did not reach significance. To further substantiate an increase in mature myelinating OLs we performed histologic stains for myelin using Luxol fast blue (LFB) on sections collected at 28dpl. We found qualitatively improved myelin-staining in the LesTam group compared to the LesVeh group (Figure 1D and E). Quantification of blue density demonstrated a 1.54-fold increase in the LesTam group compared to the LesVeh group that did not reach significance (Figure 1F). These findings support the hypothesis that miRs regulated by Dicer in neural progenitors antagonize the formation of OLs after perinatal HI.
Figure 1. Loss of Dicer in NG2 expressing cells following hypoxia-ischemia results in increased mature oligodendrocytes and improved myelin.
Schematic of injury area and timeline of experimental procedure (i=infarct area, P=postnatal day, dpl=days post lesion, HI=hypoxia-ischemia, sac=sacrifice, tam=tamoxifen). Red box denotes area of corpus callosum imaged on coronal sections. Representative micrographs of myelin basic protein (MBP) staining within the corpus callosum of A) a lesioned mouse treated with vehicle (LesVeh), 40x, scale bar=25 µm, and B) a lesioned mouse treated with treated with tamoxifen (LesTam). C) Quantification of MBP expression within corpus callosum, error bars represent SEM, *p=0.048; n=4 LesVeh and 4 LesTam. Representative micrographs of Luxol fast blue (LFB) staining within the corpus callosum of D) a lesioned mouse treated with vehicle (LesVeh), magnification=20x, scale bar=25 µm, black arrows indicate edge of corpus callosum, and E) a lesioned mouse treated with tamoxifen (LesTam). F) Quantification of LFB expression within corpus callosum, error bars represent SEM; n=5 LesVeh and 4 LesTam.
To explore whether the increase in mature myelinating OL results from an increased total number of oligodendroglia, we used immunofluorescence to probe for Olig1, a transcription factor expressed by cells along the continuum of the oligodendroglial lineage, from OPCs to mature OLs. Following the same induction protocol, mice were sacrificed 7dpl and we assayed for changes in numbers of Olig1+ cells within corpus callosum comparing lesioned mice after tamoxifen treatment to lesioned mice that received vehicle; we found no change in Olig1+ cells between treatment groups. Next, we probed for Olig2, a transcription factor that is expressed by neural progenitors and OPCs, comparing the same groups at the same time points. Interestingly, we observed a 0.38-fold trend towards decreased numbers of Olig2+ cells that did not reach significance due to variance (Supplemental Figure 1A (online)). Conversely, when we probed for PDGFRA, a growth factor receptor that marks OPCs, we observed a 1.90-fold trend towards increased numbers of PDGFRA+ cells that did not reach significance due to variance (Supplemental Figure 1B (online)). These findings indicate that excision of Dicer did not alter the total number of cells in the OL lineage and suggest that miRs may regulate the commitment of neural progenitor cells to OPCs.
Knockdown of Dicer in neural progenitors following perinatal hypoxia-ischemia improves ambulation
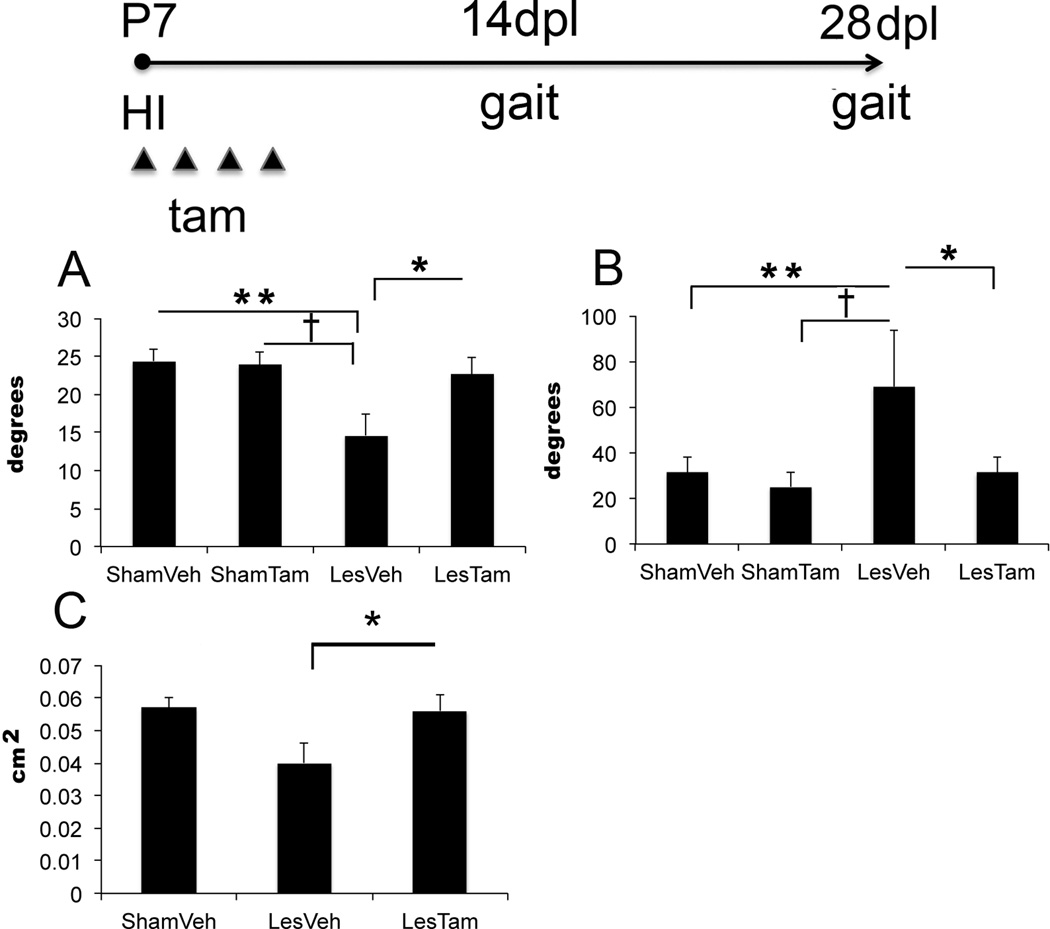
Since we observed an increase in myelinating OLs after excision of Dicer, we used digital gait analysis to assess ambulation, comparing sham-injury mice who received vehicle (ShamVeh) to sham-injury mice who received tamoxifen (ShamTam), LesTam mice and LesVeh mice. We assayed mice walking at a speed of 10cm/sec at both 14dpl and 28dpl. Because our lesions were unilateral, we expected to see the greatest differences between the left limbs of the LesVeh and the LesTam groups, and this is what we observed. At 14dpl, the LesTam group displayed significant differences in left hind limb absolute paw angle compared to the LesVeh group (LesTam 22.7±4.4 degrees v. LesVeh 14.6±4.1 degrees, p=0.034; n=3 ShamVeh, 3 ShamTam, 2 LesVeh and 4 LesTam mice). LesVeh mice also differed compared to ShamVeh and ShamTam mice for this parameter (LesVeh 14.6±4.1 degrees v. ShamVeh 24.4±2.8 degrees, p=0.019 and ShamTam 24.0±2.8 degrees, p=0.023, respectively) (Figure 2A). We were surprised to find that the LesTam group also displayed significant differences in right hind limb paw angle variability compared to the LesVeh group (LesTam 31.3±13 degrees v. LesVeh 69.1±35 degrees, p=0.033; n=3 ShamVeh, 3 ShamTam, 2 LesVeh and 4 LesTam mice). LesVeh mice also differed compared to ShamVeh and ShamTam mice for this parameter (LesVeh 69.1±35 degrees v. ShamVeh 31.1±12 degrees, p=0.040 and ShamTam 25.1±12 degrees, p=0.022, respectively) (Figure 2B). Right hind limb changes may be compensatory for changes in left hind limb function. Together, the digital gait analysis supports the hypothesis that miRs regulated by Dicer in neural progenitors worsen the outcomes of perinatal HI injured mice.
Figure 2. Loss of Dicer in NG2 expressing cells following hypoxia-ischemia results in improved parameters of ambulation at 14 and 28 days after injury.
Timeline of experimental procedure (P=postnatal day, dpl=days post lesion, HI=hypoxia-ischemia, gait=digital gait analysis, tam=tamoxifen). A) Absolute Paw Angle. Quantification of absolute paw angle at left hind limb at 14dpl, error bars = SD, *p=0.034, **p=0.0192, †p=0.023, n=3 ShamVeh, 3 ShamTam, 2 LesVeh and 4 LesTam mice. B) Paw Angle Variability. Quantification of paw angle variability at right hind limb at 14dpl, error bars = SD, *p=0.033, **p=0.040, †p=0.022, n=3 ShamVeh, 3 ShamTam, 2 LesVeh and 4 LesTam mice. C) Paw Area Variability. Quantification of paw area variability at left hind limb 28dpl, error bars = SD, *p=0.049, n=3 ShamVeh, 3 LesVeh and 5 LesTam mice.
When we assessed ambulation at 28dpl, again at a speed of 10cm/sec, the LesTam group displayed significant differences in left hind limb paw area variability compared to the LesVeh group (LesTam 0.056 cm2±0.011 cm2 v. LesVeh 0.04 cm2±0.010 cm2, p=0.049, n=3ShamVeh, 3LesVeh and 5LesTam) (Figure 2C). For each of these parameters, LesTam differed significantly from LesVeh animals and approximated ShamVeh animals, suggesting rescue by Dicer excision. All other limbs did not show any significant differences between LesTam and LesVeh groups at either time point.
Perinatal hypoxia-ischemia increases expression of microRNAs known to regulate OPC differentiation
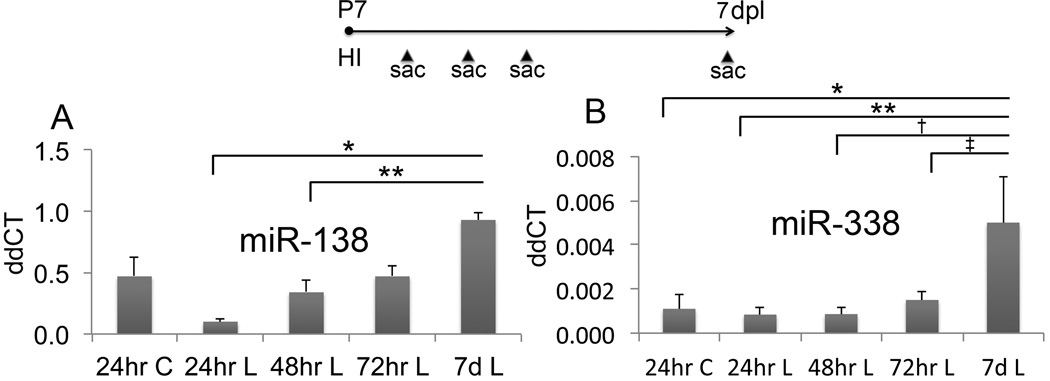
Since our experiments showed that knocking down mature miRs in OPCs in neural progenitors improved several parameters following HI, we wanted to test whether microRNAs known to regulate differentiation of OPCs were affected by HI. We used quantitative RT-PCR to measure four specific mature microRNAs: miR-9, miR-138, miR-219 and miR-338. We collected cortex and subcortical white matter at 24h, 48h and 72h as well as 7d after injury, isolated total RNA, produced cDNA and amplified the microRNAs. We found significant increases in miR-138 7d after injury (0.932±0.06) compared to 24h after injury (0.103±0.02, p=0.006) and compared to 48h after injury (0.343±0.10, p=0.04) (n=3 for each treatment groups and time point) (Figure 3A). We also found significant increases in miR-338 7d after injury (0.005±0.002) compared to 24h after sham surgery (0.001±0.001, p=0.008), and compared to 24h after injury (0.001±0.0003, p=0.004), 48h after injury (0.001±0.0003, p=0.004) and 72h after injury (0.001±0.0004, p=0.013) (n=3 for each treatment groups and time point) (Figure 3B). In contrast, we found no significant changes in miR-9 levels at 24h, 48h, 72h and 7d after injury. Moreover, we were unable to detect miR-219 at any of these time points. It is possible that miR-219 may not be expressed in mouse until later than 2 weeks postnatal age.
Figure 3. Hypoxia-ischemia in wild type mice increases expression of microRNAs known to regulate differentiation of oligodendrocyte progenitor cells.
Timeline of experimental procedure (P=postnatal day, dpl=days post lesion, HI=hypoxia-ischemia, sac=sacrifice). A) Quantification of miR-138 (ddCT=delta delta cycle time, C=control, L=lesion, miR=microRNA), error bars = SEM, *p=0.006, **p=0.04, n=3 for each treatment group and time point. B) Quantification of miR-338, *p=0.008, **p=0.004, †p=0.004 and ‡p=0.013, n=3 for each treatment group and time point.
Discussion
MicroRNAs are now recognized as important regulators of gene expression during cell differentiation, even in fully mature organisms. Indeed, the expression of certain genes is more dependent on levels of miRs than on mRNAs (12). Greater than one-third of all human genes are predicted to be regulated by miRs (13). Genes unique to the brain and oligodendroglial genes in particular are no exception.
Production of functional miRs is a complex multi-step process. The dsRNA nuclease Dicer is essential to this process. It cleaves premature miRs to form 10–25 nucleotide double-stranded mature miRs. Next, mature miRs complex with RNA Induced Silencing Complex/Argonaute and suppress protein translation by binding to the 3’UTR of mRNA, inhibiting translation or marking translated mRNA for degradation (4). Thus, since Dicer is involved in the initial step in the production of mature miRs, excision of Dicer is an effective strategy to prevent the function of mature miRs generally.
Because complete loss of function of Dicer is embryonic lethal, conditional knockdown is required (7). Telencephalon-specific Dicer mutants have implicated miRs as important in brain development (4). The Nestin-CreER;Dicerfl/fl mouse, in which Dicer is deleted in radial glia, displays embryonic-lethality whereas the Emx1-Cre;Dicerfl/fl mouse, in which Dicer is deleted at a later developmental stage, survives until postnatal day 30. Nestin-Cre;Dicerfl/fl mice have thinner cortices, smaller ventricular and subventricular zones and a thin cortical plate at E18.5 compared to wildtype, implicating Dicer in the regulation of neural stem cell proliferation. Emx-1Cre;Dicerfl/fl mice similarly have thinner cortices, smaller ventricular and intermediate zones, but in contrast have an enlarged cortical plate and no detectable hippocampus at E17.5, additionally implicating Dicer in the regulation of early neural stem cell differentiation (6).
MiRs in general continue to be important after commitment of neural progenitors to the oligodendroglial lineage, as evidenced by delayed myelination in Olig2Cre;Dicerfl/fl and CNPaseCre;Dicerfl/fl mice (8), decreased myelin in Olig1Cre;Dicerfl/fl mice (9) and dysmyelination in PLPCre;Dicer fl/fl mice (10). Furthermore, mature OL are decreased in Olig2Cre;Dicerfl/fl but not in CNPaseCre;Dicerfl/fl mice (8). Interestingly, the effects of lost Dicer in both Olig2Cre;Dicerfl/fl and CNPaseCre;Dicerfl/fl mice are cell autonomous (8). This is in contrast to the GFAPCre; Dicerfl/fl mouse in which non-cell autonomous effects result in death of neurons (14). In the peripheral nervous system, mature miRs are required for maturation of Schwann cells as evidenced by the lack of mature myelin in DhhCre;Dicerfl/fl mice (15,16) and POCre;Dicerfl/fl (17) mice. In vitro, Schwann cells transduced with lentiviral vector encoding shRNA to Dicer also fail to myelinate co-cultured dorsal root ganglion (18).
None of these studies tested the effect of knocking down Dicer in a disease or injury state. In particular, no studies to date address the role of Dicer within the context of perinatal HI. Ours is the first study to show that mature miRs in general suppress a regenerative oligodendroglial response to perinatal HI. We showed that if Dicer is knocked down specifically within OPCs following perinatal HI, NG2CreERT2;Dicerfl/fl mice fared better in terms of quantity of mature oligodendrocytes, quality of myelin and measurable parameters of ambulation. Because Dicer knockdown would prevent the production of all mature miRs, the effect we observed suggests that the net effect of all mature miRs induced by perinatal HI is to suppress the production of mature oligodendroglia. Our experiments suggest that mature miRs induced by perinatal HI may decrease the maturation rate of immature OLs into mature OLs; furthermore, they may decrease the differentiation rate of OPCs into OLs; alternatively, they may increase the proliferation rate of OPCs at the expense of differentiation.
Beyond Dicer knockouts, specific miRs have been identified as important regulators of neural stem cells. Following commitment of neural stem cells to the oligodendroglial lineage, specific miRs have been implicated in regulation of further differentiation. Lau et al., performed microarray analysis on fluorescence activated cell sorting (FACS) sorts of A2B5+GalC- cells from rat brain, compared them to FACS sorts of A2B5-GalC+ cells and showed, in combination with other published microarray studies, that at least 38 miRs are uniquely expressed in OLs but not neurons nor astrocytes. From their microarray analysis, they found that miR-9 was significantly downregulated during OPC differentiation. They examined miR-9 expression and the expression of its predicted target PMP22 mRNA and PMP22 protein and showed a negative target bias relationship in both OLs centrally and Schwann cells peripherally. In addition, their microarray analysis showed preferential expression of miR-338 in differentiating OLs (19). Zhao et al., in their microarray study of Olig1Cre;Dicerfl/fl mice and Olig1 null mice, found miR-338 and miR-219 to be significantly downregulated in their myelin-deficient mutants (9). Dugas et al. showed miR-219 and miR-138 were induced when OPC cultures were differentiated through microarray studies of rat OPCs cultured in PDGF+/T3- media compared to OPCs cultured in PDGF-/T3+ media (8). Thus, several miRs have been implicated in oligodendroglial differentiation, but only 4 specific miRs have been well-validated.
None of these studies evaluated the expression of specific oligodendroglia-regulating miRs in the context of a CNS disease state. One study that did identify specific miRs in the context of a CNS disease state modeled fetal ethanol exposure using human fetal neurospheres to explore miR-mediated effects on neural stem/progenitor cells. Ethanol at high levels suppressed miR-21 and miR-335, but ethanol at lower levels increased miR-335. Knockdown of miR-21 resulted in apoptosis whereas knockdown of miR-335 resulted in cell proliferation and blockade of the miR-21 knockdown-mediated apoptosis. Moreover, miR-335 was downregulated during OL differentiation whereas miR-21 was upregulated. These results suggest antagonistic actions of select miR on neural progenitor differentiation (20). Another study modeled perinatal HI using primary neuronal cultures and primary astrocyte cultures from fetal rats subjected to oxygen-glucose deprivation (OGD) to examine changes in specific miRs. This study found that in neurons, miR-29b was upregulated starting 6h after OGD, peaking at 24h after OGD, and miR-21 was upregulated 24h after OGD. Similarly in astrocytes, miR-29b and miR-21 were both upregulated 12h after OGD (21). Specific miRs have been studied in models of adult brain ischemia. Jeyaseelam et al. performed miR microarrays on blood and brain of adult rats after transient middle cerebral artery occlusion (MCAO) and found that miR-124a and miR-223 were highly expressed 24h and 48h after injury (22). Dharap et al., who also used microarray and the same injury model, identified miR-145 as highly expressed between 3h and 3 days after injury (23). Liu et al, used a permanent MCAO model as well as models of intracerebral hemorrhage and kainate-induced seizures and found that miR-298 is upregulated in blood and brain across all three injury models (24). None of these studies addressed miRs regulating the oligodendroglial lineage specifically.
Ours is the first study to show in an in vivo disease model of perinatal HI that specific miRs are altered, namely that miR-138 and miR-338 were specifically upregulated. We have also observed that miR-21 is significantly upregulated 72h post injury (data not shown). It would be interesting to explore whether miR-138, miR-21 or miR-338 were part of the mechanism leading to blocked maturation of OPCs seen after perinatal HI. Indeed, loss of Dicer in DhhCre;Dicerfl/fl mice led to the proliferation of immature Schwann cells (15). We speculate that a precise temporal knockdown of miRs specifically regulating OPC differentiation might prevent white matter loss due to perinatal HI. In a first in vivo attempt to manipulate miRs after oligodendroglial development, Zhao et al., electroporated miR-338 and miR-219 expression vectors in ovo and achieved ectopic formation of OLs and precocious OL differentiation in spinal cord; conversely, knockdown of these miRs inhibited OL maturation. Similarly, Dugas et al., used miR-219 and miR-138 mimetics to promote OL differentiation and partially rescued OL in Dicer negative OPC cultures (8). Although these studies used miR mimetics and miR anti-sense inhibitors to evaluate normal development, none used these strategies to rescue pathology in white matter disease. Dharap et al., did test whether knockdown of miR-145 effected protection following transient MCAO; they found that miR-145 antagonists resulted in increased superoxide dismutase 2 expression in peri-infarct neurons as well as decreased cortical infarct size (23).
It is our view that HI transiently inhibits Dicer, resulting in delayed and exuberant expression of mature miRs. In the case of perinatal HI, miR-138 and miR-338, which are normally expressed upon differentiation of OPCs to OLs, are delayed in their expression. This in turn delays their normal function of repressing promoters of OPC proliferation, namely PDGFRA, Sox6 and FoxJ3 (8). MiR-338 additionally targets ZNF238, another promoter of OPC proliferation (8,25). Such an effect is consistent with observed increases in OPCs following perinatal HI (2,3). A search of the bioinformatics database miRWalk, shows that miR-138, miR-338 and miR-9 are all implicated in the PDGF pathway (25). Other validated targets of miR-138 include EGF, EGFR, and CCND1 (25), which are all involved in cell proliferation; this makes sense given differentiation requires decreased proliferation. Interestingly, DICER1 is a validated target common to miR-138, miR-338 and miR-9, RNASEN is a validated target of miR-138 and miR-9, and AGO1 and AGO2 are validated targets of miR-9 (28). Thus, high levels of these miRs may regulate these essential components of miR processing in a negative feedback mechanism. In this way, excessively elevated levels of miR-138 and miR-338 may yield the paradoxic effect of fewer mature OLs rather than more mature OLs. Knocking down Dicer in OPCs in the days following perinatal HI may restore levels of oligodendroglia-regulating miRs closer to levels seen in normal development.
A major strength of our study is the use of a genetic model to tease out mechanisms leading to white matter injury and rescue. Moreover, by design, we induced loss of Dicer to temporally follow the injury, much the way interventions would be administered clinically. Our study was limited by the fact that we pooled Dicer heterozygous and homozygous mutants. Likely we would have observed more robust differences compared to wildtype mice in both cell counts and function by distinguishing between heterozygotes and homozygyotes. Nonetheless, we were able to show that mature miRs are required for the white matter loss that occurs following perinatal HI and that specific miRs are upregulated following perinatal HI. Stated otherwise, mature miRs play a role in the perturbation of oligodendroglial differentiation following perinatal HI; important candidates likely include miR-138 and miR-338. We anticipate future studies using the strategy of specific miR mimetics and specific miR inhibitors of these candidates and others to prevent white matter disease following premature birth.
Materials and Methods
Animals
CD-1 mice (Charles River, Chicago, IL) were used for quantitative real time RT-PCR experiments. NG2CreERT2;Dicerfl/fl mice were used for the remainder of the experiments; Dicer homozygous and heterozygous mutants were pooled and analyzed as Dicer floxed mutants. NG2CreERT2 mice were obtained from Dr. Akiko Nishiyama (University of Connecticut – Storrs, CT) and crossed with the Dicer floxed mouse that was obtained with the permission of Dr. Brian Harfe (University of Florida – Gainesville, FL). All mice were backcrossed to a C57Bl6 background for at least 6 generations. Mice were housed in a facility with a 12h light/dark cycle and allowed access to food and water ad libitum. Experiments were conducted according to protocols approved by the Institutional Animal Care and Use Committee and Northwestern Center for Comparative Medicine.
Neonatal Hypoxic-Ischemic Lesions
Postnatal day 7 (P7) mice were anesthetized with isoflurane, and the right common carotid artery was ligated and transected. After 2h recovery, pups were placed in humidified hypoxia chambers (8% oxygen/92% balanced air) kept at 37°C for 45min for CD-1 mice or 60min for NG2CreERT2;Dicer floxed mice, were allowed to recover for 1h, then returned to the dam as previously described (26,27). Non-lesioned controls underwent sham surgeries. Conditional inducible mice were treated with either tamoxifen (Sigma T5648) (Sigma, St. Louis, MO) 60mg/kg dissolved in sterile corn oil: 100% ethanol (9:1) or vehicle only IP once daily starting after HI on P7, P8, P9 and P10.
Immunofluorescence
Mice were anesthetized with carbon dioxide, transcardially perfused with 0.9% NaCl followed by 4% paraformaldehyde (PFA) in PBS, brains were removed, post-fixed overnight in ice cold 4% PFA, cryoprotected in 30% sucrose in PBS, embedded and frozen in O.C.T. Compound (Sakura Finetek 4583) (Sakura, Torrence, CA), cut into 10µm sections using a cryostat, and immunostained using the following antibodies: CNPase 1:200 (Sternberger SMI91) (Covance, Princeton, NJ), MBP 1:500 (Sternberger SMI99), Olig1 1:600 (Chemicon AB5540) (EMD Millipore, Billirica, MA), Olig2 1:400 (Chemicon AB9610), PDGFRA 1:200 (Fitzgerald CD140a) (Fitzgerald, North Acton, MA), AlexaFluor 488 Goat anti-Rabbit 1:500, AlexaFluor 594 Goat anti-Mouse IgG1 1:500, AlexaFluor 594 Goat anti-Mouse IgG2b 1:500, AlexaFluor 594 Goat anti-Mouse IgM 1:500 (Invitrogen) (Life Technologies, Grand Island, NY).
Histochemistry
Frozen sections were hydrated sequentially from 70% ethanol for 30min to 95% ethanol for 30min, then they were placed in 1% Luxol fast blue solution (Luxol fast blue 1g (S3382, Sigma-Aldrich, St. Louis, MO), 95% ethanol 100ml, 10% acetic acid 5ml) at 60° for 16h. Slides were rinsed with 95% ethanol, then ddH2O, then differentiated in 0.05% lithium carbonate solution (lithium carbonate 0.5g (L4283, Sigma-Aldrich), ddH2O 1000ml) for 5s followed by 70% ethanol for 5s twice, then rinsed with ddH2O. Differentiation steps were repeated until gray matter had cleared. Slides were counterstained in 0.25% cresyl echt violet solution (cresyl violet acetate (C1791, Sigma-Aldrich) 0.1gm, ddH2O 100ml, glacial acetic acid 10 drops) for 40s then rinsed in ddH2O and differentiated in 95% ethanol for 5min, then rinsed in 100% ethanol for 5min twice. Slides were dehydrated through from 95% ethanol through 100% ethanol, cleared with xylene and coverslips were mounted with Permount (SP15-500, Fisher Scientific, Pittsburgh, PA).
Quantitative RT-PCR
Mice were anesthetized with carbon dioxide, then were decapitated, brains were removed and chilled in ice-cold PBS for 5min, cut into 1mm sections using a stainless steel mouse brain slicer, then an approximately 15mg piece of intact peri-infarct cortex/subcortical white matter was dissected using tungsten needles at 24, 48, and 72h and 7 days after lesioning. Tissue was collected into PBS. Total RNA was obtained using the RNAqueous-4PCR kit (Ambion) (Life Technologies, Grand Island, NY). A total of 1µg of RNA was used for generating cDNA using the Thermoscript reverse transcriptase and oligo-dT primers (Invitrogen) (Life Technologies). cDNA was stored at −80°C until used. Quantitative real-time PCR for mature miR-9, miR-138 and miR-338 was performed for mature miR-21 was performed using the Taqman microRNA Assays for these mature miRs and snoRNA202 for internal controls (Taqman) (Life Technologies). A total of 1µl of cDNA was used per PCR. The following cycling parameters were used: 95°C x10min, for 40 cycles, 95°C x15s, 60°C x1min.
Digital Gait Analysis
Digital video images of the underside of mice walking at a speed of 10cm/sec were collected and analyzed using the DigiGait Imaging System and software (Mouse Specifics, Boston, MA) as previously described (28). Mouse paw area over time was used to calculate various parameters of gait. Results of lesioned animals that received tamoxifen were compared to results of lesioned animals that received vehicle.
Statistics and Analysis
Two to 5 mice were used per treatment group. Mid-striatal coronal sections were collected (between +0.98mm anterior to Bregma and +0.38 posterior to Bregma). For each animal, four 40x fields were captured from corpus callosum and the supracallosal radiations, using Axiovision 4.6 software (Carl Zeiss Vision, Thornwood, NY). Image files were encoded to mask the study group, areas were measured and cells were counted using Axiovision 4.6 and Image J software (NIH) by investigators blinded to group. After counts were completed, the data was decoded, cell counts per area were calculated for each field and averaged. Relative fluorescence was measured using Metamorph software (Molecular Devices, Sunnyvale, CA). Integrated density was measured using ImageJ (NIH, Bethesda, MD). Two group comparisons (cell counts and relative expression) were analyzed by the Student’s t-test and the significance level was set at p<0.05. Four group comparisons were analyzed by ANOVA, using Fisher’s PLSD for post-hoc comparisons with statistical significance set at p<0.05.
Supplementary Material
Acknowledgments
This work was supported by the Northwestern University Behavioral Phenotyping Core.
Statement of Financial Support: This work was supported by the National Institutes of Health (NIH) National Institute of Neurological Disorders and Stroke (NINDS), Bethesda, Maryland, USA, grants 5K08 NS053529, NS20778-28 and NS20013-29.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury. J Neurosci. 2001;21:1302–1312. doi: 10.1523/JNEUROSCI.21-04-01302.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dizon M, Szele F, Kessler JA. Hypoxia-ischemia induces an endogenous reparative response by local neural progenitors in the postnatal mouse telencephalon. Dev Neurosci. 2010;32:173–183. doi: 10.1159/000313468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Segovia KN, McClure M, Moravec M, et al. Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury. Ann Neurol. 2008;63:520–530. doi: 10.1002/ana.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stefani G, Slack FJ. Small non-coding RNAs in animal development. Nat Rev Mol Cell Biol. 2008;9:219–230. doi: 10.1038/nrm2347. [DOI] [PubMed] [Google Scholar]
- 5.Yi R, Fuchs E. A miR image of stem cells and their lineages. Current topics in developmental biology. 2012;99:175–199. doi: 10.1016/B978-0-12-387038-4.00007-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kawase-Koga Y, Otaegi G, Sun T. Different timings of Dicer deletion affect neurogenesis and gliogenesis in the developing mouse central nervous system. Developmental dynamics : an official publication of the American Association of Anatomists. 2009;238:2800–2812. doi: 10.1002/dvdy.22109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petri R, Malmevik J, Fasching L, Akerblom M, Jakobsson J. miRNAs in brain development. Experimental cell research. 2013 doi: 10.1016/j.yexcr.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 8.Dugas JC, Cuellar TL, Scholze A, et al. Dicer1 and miR-219 Are required for normal oligodendrocyte differentiation and myelination. Neuron. 2010;65:597–611. doi: 10.1016/j.neuron.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhao X, He X, Han X, et al. MicroRNA-mediated control of oligodendrocyte differentiation. Neuron. 2010;65:612–626. doi: 10.1016/j.neuron.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shin D, Shin JY, McManus MT, Ptacek LJ, Fu YH. Dicer ablation in oligodendrocytes provokes neuronal impairment in mice. Ann Neurol. 2009;66:843–857. doi: 10.1002/ana.21927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu X, Hill RA, Dietrich D, Komitova M, Suzuki R, Nishiyama A. Age-dependent fate and lineage restriction of single NG2 cells. Development. 2011;138:745–753. doi: 10.1242/dev.047951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Johnson CD, Esquela-Kerscher A, Stefani G, et al. The let-7 microRNA represses cell proliferation pathways in human cells. Cancer Res. 2007;67:7713–7722. doi: 10.1158/0008-5472.CAN-07-1083. [DOI] [PubMed] [Google Scholar]
- 13.Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 14.Tao J, Wu H, Lin Q, et al. Deletion of astroglial Dicer causes non-cell-autonomous neuronal dysfunction and degeneration. J Neurosci. 2011;31:8306–8319. doi: 10.1523/JNEUROSCI.0567-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bremer J, O'Connor T, Tiberi C, Rehrauer H, Weis J, Aguzzi A. Ablation of Dicer from murine Schwann cells increases their proliferation while blocking myelination. PLoS One. 2010;5:e12450. doi: 10.1371/journal.pone.0012450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pereira JA, Baumann R, Norrmen C, et al. Dicer in Schwann cells is required for myelination and axonal integrity. J Neurosci. 2010;30:6763–6775. doi: 10.1523/JNEUROSCI.0801-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yun B, Anderegg A, Menichella D, Wrabetz L, Feltri ML, Awatramani R. MicroRNA-deficient Schwann cells display congenital hypomyelination. J Neurosci. 2010;30:7722–7728. doi: 10.1523/JNEUROSCI.0876-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Verrier JD, Semple-Rowland S, Madorsky I, Papin JE, Notterpek L. Reduction of Dicer impairs Schwann cell differentiation and myelination. J Neurosci Res. 2010;88:2558–2568. doi: 10.1002/jnr.22418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lau P, Verrier JD, Nielsen JA, Johnson KR, Notterpek L, Hudson LD. Identification of dynamically regulated microRNA and mRNA networks in developing oligodendrocytes. J Neurosci. 2008;28:11720–11730. doi: 10.1523/JNEUROSCI.1932-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sathyan P, Golden HB, Miranda RC. Competing interactions between micro-RNAs determine neural progenitor survival and proliferation after ethanol exposure: evidence from an ex vivo model of the fetal cerebral cortical neuroepithelium. J Neurosci. 2007;27:8546–8557. doi: 10.1523/JNEUROSCI.1269-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ziu M, Fletcher L, Rana S, Jimenez DF, Digicaylioglu M. Temporal differences in microRNA expression patterns in astrocytes and neurons after ischemic injury. PLoS One. 2011;6:e14724. doi: 10.1371/journal.pone.0014724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jeyaseelan K, Lim KY, Armugam A. MicroRNA expression in the blood and brain of rats subjected to transient focal ischemia by middle cerebral artery occlusion. Stroke. 2008;39:959–966. doi: 10.1161/STROKEAHA.107.500736. [DOI] [PubMed] [Google Scholar]
- 23.Dharap A, Bowen K, Place R, Li LC, Vemuganti R. Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. J Cereb Blood Flow Metab. 2009;29:675–687. doi: 10.1038/jcbfm.2008.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu DZ, Tian Y, Ander BP, et al. Brain and blood microRNA expression profiling of ischemic stroke, intracerebral hemorrhage, and kainate seizures. J Cereb Blood Flow Metab. 2010;30:92–101. doi: 10.1038/jcbfm.2009.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dweep H, Sticht C, Pandey P, Gretz N. miRWalk - database: prediction of possible miRNA binding sites by "walking" the genes of 3 genomes. J Biomedical Informatics. 44:839–837. doi: 10.1016/j.jbi.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 26.Ditelberg JS, Sheldon RA, Epstein CJ, Ferriero DM. Brain injury after perinatal hypoxia-ischemia is exacerbated in copper/zinc superoxide dismutase transgenic mice. Pediatr Res. 1996;39:204–208. doi: 10.1203/00006450-199602000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Plane JM, Liu R, Wang TW, Silverstein FS, Parent JM. Neonatal hypoxic-ischemic injury increases forebrain subventricular zone neurogenesis in the mouse. Neurobiol Dis. 2004;16:585–595. doi: 10.1016/j.nbd.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 28.Hampton TG, Stasko MR, Kale A, Amende I, Costa AC. Gait dynamics in trisomic mice: quantitative neurological traits of Down syndrome. Physiol Behav. 2004;82:381–389. doi: 10.1016/j.physbeh.2004.04.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.