Abstract
The primary cilium (PC) is a very dynamic hair-like membrane structure that assembles/disassembles in a cell-cycle dependent manner and is present in almost every cell type. Despite being continuous with the plasma membrane, a diffusion barrier located at the ciliary base confers the PC properties of a separate organelle with very specific characteristics and membrane composition. Therefore, vesicle trafficking is the major process by which components are acquired for cilium formation and maintenance. In fact, a system of specific sorting signals controls the right of cargo admission into the cilia.
Disruption to the ciliary structure or its function leads to multi-organ diseases known as ciliopathies. These illnesses arise from a spectrum of mutations in any of the more than 50 loci linked to these conditions. Therefore, it is not surprising that symptom variability (specific manifestations and severity) among and within ciliopathies seems to be an emerging characteristic. Nevertheless, one can speculate that mutations occurring in genes whose products contribute to the overall vesicle trafficking to the PC (i.e., affecting cilia assembly) will lead to more severe symptoms, while those involved in the transport of specific cargoes will result in milder phenotypes. In this review, we summarize the trafficking mechanisms to the cilia and also provide a description of the trafficking defects observed in some ciliopathies which can be correlated to the severity of the pathology.
Keywords: Ciliopathies, vesicle trafficking, primary cilia, endocytic pathway, secretory pathway
Introduction
Non-motile cilia were first observed by Zimmerman (1) more than a century ago, and named ‘Primary cilium’ (PC) by Sergei Sorokin in 1968 (2). Initially, this plasma membrane-derived structure was thought to represent a vestigial swimming apparatus, and interest in it eventually faded away. Indeed, it was not until about a decade ago that the PC resurfaced as its presence was observed in virtually every cell type and its relevance for intracellular signaling and developmental diseases became clear (3).
The PC hosts a series of signaling pathways of critical importance for normal vertebrate development (Hedgehog (4), Wnt signaling pathways (5)), growth and differentiation (TGF-β (6), PDGF signaling (7)), sensory perception (Rhodopsin (8) and odorant receptors localization in the cilia (9)), hormonal regulation (Mchr1 and 5-HTr6 (10), Sstr3 (11)) and mechanical transduction (Polycystin-1 and Polycystin-2 (12)). These crucial sensory functions of the PC rely on its high concentration of membrane receptors that interpret a plethora of chemical, mechanical and other extracellular cues. In consequence, deficiencies in the assembly or function of this specialized area of the plasma membrane have severe consequences on the development and overall physiology of the affected organism (13). Therefore, abnormal cilia have been linked to a heterogeneous group of diseases, attributed to single gene mutations in more than 50 loci, known as ciliopathies (14). These illnesses can be lethal and are characterized by overlapping phenotypes that may include retinal degeneration, kidney dysfunction, infertility, cognitive impairment, polydactyly, situs inversus, obesity, diabetes and other manifestations. In fact, it is in great part due to its medical relevance that in recent times we have witnessed an immense surge of research aimed to better understand the mechanisms of assembly, maintenance and function of the PC.
Assembly and Architecture of the PC
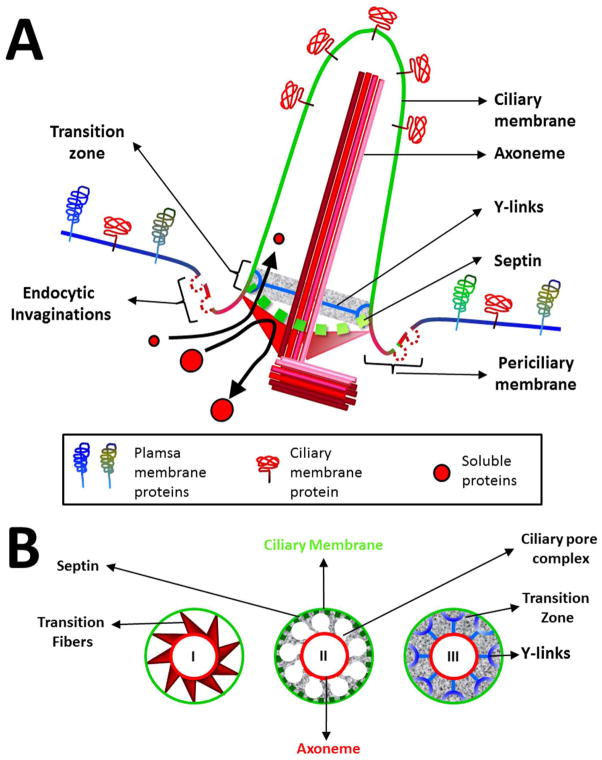
The PC is a sensory, cell surface-structure with a microtubule-based core (a non-motile 9+0 axoneme) originating from a basal body derived from the mother centriole, and ensheathed by the so-called ciliary membrane (Fig. 1A). For comprehensive descriptions of the PC organization, the reader is referred to excellent reviews available in the literature (3, 15, 16). Ciliogenesis in most cells is initiated by anchoring the basal body to the plasma membrane directly by the distal appendage protein C2cd3 that in turn leads other components namely Sclt1, Ccdc41, Cep89, Fbf1, and Cep164 to mark the position for assembly of the PC (17–19). Vesicles dock to the basal body and supply material for growth of the cilia. An alternative ciliary pathway begins in the cytosol, where vesicles dock onto the mother centriole, elongate the nascent cilium in the cytosol which is later inserted in the plasma membrane. In this case, the cilium remains partially buried under the plasma membrane, enclosed in a curved invagination of the plasma membrane called the ciliary pocket that continues with the ciliary membrane (20). Cilia elongation is mediated by a specialized bi-directional intraflagellar transport system (IFT) (see below under ‘Trafficking within the PC’).
Figure 1. The PC is an isolated domain.
(A) The barrier at the ciliary base gives the ciliary membrane its unique identity in terms of lipid and protein composition (see text for details). (B) Cross-sections bottom up of the ciliary base depicting the proximal transition fibres (I), Septin ring and the ciliary pore complex (II) and the distal Y-links (III) (See text for details).
Although the ciliary membrane presents continuity with the plasmalemma (Fig. 1A), the two have a very different composition when compared to each other (21, 22). The establishment of the primary cilia boundary is made possible by the presence of a barrier at the ciliary base whose components include the transition fibres (TFs), ciliary necklace and the ciliary pocket (Fig. 1A,B) that highly restricts protein diffusion to and from the ciliary membrane and the intraciliary space. Therefore, this barrier gives the cilia characteristics of a compartmentalized organelle and since there is no protein synthesis within the PC, it requires specialized trafficking machinery for the delivery and retrieval of components. Indeed, vesicle trafficking is essential for ciliogenesis (23).
This review will discuss the basics of ciliary transport to the base of the cilia, across the barrier and within the cilia. It will primarily highlight the role of genes affected in ciliopathies and involved in trafficking to and from the PC.
Vesicle trafficking in PC assembly, maintenance and function
Traffic Control: The Barrier
The most proximal line of defense against indiscriminate diffusion into the ciliary compartment is the periciliary membrane where distal appendages arising from the basal body, converted into the TFs, are attached. Transmission electron microscopy (TEM) has revealed a pin-wheel like structure for the TFs with inter-fiber space between the transition fibers too small for vesicles to pass through (24)(Fig. 1B). Instead, vesicles dock and fuse with the ciliary pocket (if present) and the periciliary membrane (20). The higher occurrence of endocytic events at the ciliary pocket sets it apart from the continuing plasma membrane (6) (Fig. 1A). Examination of the periciliary membrane by Laurdan Microscopy exhibited higher condensation of the membrane when compared to the ciliary membrane and the ciliary tip (22).
TEM also revealed, distal to the TFs, the presence of particles known as the ‘ciliary necklace’ circumferentially decorating the ciliary membrane (25). These membrane decorations were connected to the axoneme by champagne glass-shaped structures called “Y-links” (Fig. 1B). Many TZ (Transition Zone) proteins have been identified, namely the Nephronophthisis-Meckel Syndrome-Joubert Syndrome (NPHP-MKS-JBTS) complex and nucleoporins (26–31), and they have been proposed to be held in place by a septin barrier present between the periciliary and the ciliary compartments (30). In addition, isolation of the basal bodies from Tetrahymena pyriformis helped to identify a so-called ‘terminal plate’ which could serve as a ciliary partitioning system (32). Cryo-EM revealed that this ciliary pore complex has an outer ring, speculated to be the septin ring, with nine inner rings which are similar in size to the nuclear pore (32)(Fig. 1B). Indeed, similarities between the nuclear pore complex and the ciliary base have been proposed; however, whether nucleoporins form a pore complex at the ciliary base is still under debate (21, 31).
Membrane proteins
The septin ring was shown to prevent the lateral diffusion of membrane proteins to and from the PC, emerging as a major player in maintaining the ciliary membrane composition (33). The TZ seems to function as a ‘smart gate’ that validates the entry of cargo pre-loaded at the TFs on ‘IFT trains’ headed for the ciliary tip (34). Evidence points towards the existence of putative TZ resident proteins acting as ‘validating officers’ for each cargo to allow access to the ciliary membrane. Zhao et al (35) showed that the depletion of the components of the TZ zone B9d2 or Nephrocystin-5 (NPHP-5) affected the transport of the protein Opsin, but not Peripherin. Another example is the dramatic effect of depletion of the TZ proteins Tctn1, 2 and Cc2d2a on Smoothened (Smo), Polycystin-2 and Arl13 ciliary localization, but without consequences on Sstr3 trafficking to the cilia (27). In addition, depletion of more than one of its components led to ultrastructural defects in the TZ in Caenorhabditis elegans (29, 36).
Soluble proteins
The TZ also functions as a molecular sieve to restrict soluble proteins from moving across the barrier (Fig. 1A); although reports on the size-exclusion range vary, perhaps reflecting a cell-specific property (21, 31, 37). An extreme case of ciliary specialization is the photoreceptor, where the outer segment corresponds to the PC and is connected to the inner segment by a region analogous to the TZ. Nafaji et al (38) showed that the ability of soluble proteins to translocate into the photoreceptor cilia depended on their intrinsic size. However, the investigators proposed that the traffic of these proteins to the PC was controlled by steric hindrance exerted by layers of flattened membranous stacks present in the outer segment rather than by a diffusive barrier at the ciliary base (38).
Trafficking to the base of the PC
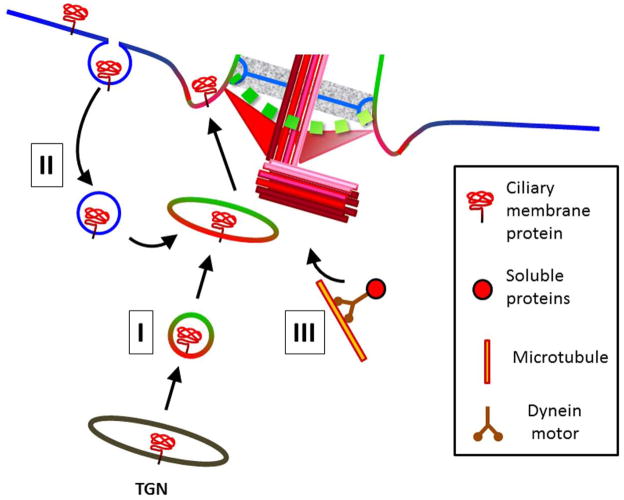
As mentioned above, vesicle trafficking is the major pathway by which cells deliver materials to the base of the cilia. Therefore, vesicle trafficking is required for the assembly, maintenance and functionality of the PC. This section summarizes different ciliary targeting mechanisms of ciliary lipids and proteins (Fig. 2).
Figure 2. Pathways for transport of material to the base of the cilia.
Secretory pathway (I) Endocytic-recycling pathway (II) Dynein-Dynactin complexes (III). (See text for details)
Sorting of PC cargo at the Golgi apparatus
Among the earliest evidence that Golgi-derived vesicles carry structural components of the cilia was the demonstration that the Golgi-disrupting drug Brefeldin A was capable of impairing ciliogenesis (39, 40). Since the lipid composition of the ciliary compartment is distinct from the plasma membrane, ciliary specific lipids are believed to be pre-sorted at the TGN and packaged into vesicles for delivery to the site of cilia assembly (41). PC-destined proteins containing ciliary targeting signals (CTS) get incorporated into these vesicles. Further, a growing body of evidence suggests that proper localization to the PC requires the interplay between several CTS (see Table I) present in the protein cargo, and that different CTS can be recognized by different elements of the sorting machinery at different stations enroute to the PC.
Table I.
Ciliary targeting consensus sequences present in cargos
| Cargo | Protein type | Organism | Signal1 (Consensus)a/binding partner | Signal2 (Consensus)a/binding partner |
|---|---|---|---|---|
| ODR-10 | GPCR | C.elegans | FR (ØB)/NDb(170) | |
| STR-1 | GPCR | C.elegans | YR (ØB)/ND (170) | |
| SSTR3 | GPCR | Mammalian | FK (ØB)/BBSome (4) | APSCQ (AX[S,A]XQ)/ND (171) |
| HTR6 | GPCR | Mammalian | FK (ØB)/ND (4) | ATAGQ (AX[S,A]XQ)/ND (171) |
| Rhodopsin | GPCR | Mammalian | FR (ØB)/ASAP1 (4, 46, 170) | QVSPA ([K,R,Q]VXPX)/Arf4 (42, 44) |
| Smoothened | GPCR | Mammalian | WR (ØB)/ND (4) | |
| MCHR1 | GPCR | Mammalian | APASQ (AX[S,A]XQ)/ND (171, 172) | |
| GPR161 | GPCR | Mammalian | [I,V]KARK/ND (113) | |
| Polycystin-1 | GPCR | Mammalian | KVHPSST ([K,R,Q]VXPX)/ND (173) | |
| PGR15L | GPCR | Mammalian | [R,K][I,L]W/ND (142) | |
| GPR83 | GPCR | Mammalian | [R,K][I,L]W/ND (142) | |
| NPY2R | GPCR | Mammalian | [R,K][I,L]W/ND (142) | |
| Polycystin-2 | 6 TM domains | Mammalian | RVQPQ ([K,R,Q]VXPX)/ND (49) | |
| Fibrocystin | Single-pass TM | Mammalian | CLVCCWFKKSKTRKIKP/Rab8 (174) | |
| RP2 | Membrane-associated protein | Mammalian | Consensus M9 sequence/Importin β2 (99) | |
| CNGB1b | Ion channel | Mammalian | RVSPG ([K,R,Q]VXPX)/ND (175) | |
| KIF17 | Soluble | Mammalian | KRKK (Similar to NLS)/Importinβ2 (98) |
Amino acids are indicated using the 1-letter code (e.g., Y=Tyrosine; N=Asparagine). “X” represents a position occupied by any amino acid. Ø= amino acid with a bulky-hydrophobic side chain (L, I, M, V, F). Amino acids within brackets indicate that one or the other can be found in that position within the consensus.
Not determined
Membrane proteins
The photoreceptor-enriched protein Rhodopsin has been a popular cargo to study trafficking to the PC (42–44), and a spatiotemporal model for the sorting of this protein has been proposed (45). Mazelova et al (42) identified a ternary complex composed of Arf4, ASAP1 and Rab11- FIP3 which functions at the TGN for the packaging of Rhodopsin into vesicles targeted to the cilia. Specifically, while a VXPX motif in Rhodopsin is recognized by the small GTPase Arf4, a FR signal is responsible for engaging the ARFGAP ASAP1. Disruption of either CTS prevented Rhodopsin localization to the PC (46). The BAR domain of ASAP1 is believed to confer the complex with the ability to deform membranes contributing to vesicle budding (47). FIP3, probably the last member of the complex to be recruited, stimulates the Arf4GAP activity of ASAP1 supporting the complex release from the TGN (48)(Fig. 2,I).
Although Polycystin-2 also contains a VXPX CTS (49), the membrane carriers containing this cargo pinch off from the cis-Golgi to reach the cilia without traversing the TGN (50). Interestingly, it has been demonstrated that full length Polycystin-2 and constitutively active Smo are routed towards the cilia at the cis-Golgi, whereas truncated Polycystin-2 and WT Smo found in the bulk of the plasma membrane are sorted at the trans-Golgi (50).
The tetrameric clathrin-associated adaptor complex AP1, clathrin and the Rab GTPase Rab8 contribute to the sorting of cargo to the PC at the exit of the TGN (9) (Fig. 2,I). Single AP1, clathrin or Rab8 mutants led to similar defects of ODR-10 trafficking, ciliary length and shape defects in C.elegans (9). However, AP1 and Rab8 seem to function at distinct steps during sorting because no co-localization was observed between them. Cargo seemed to move quickly through the AP1 compartment, but resided longer in the Rab8 compartment (9).
Rab8 was also found to interact with the inositol polyphosphate 5-phosphatase Ocrl1 (51). Ocrl1 is a protein capable of binding to several Rabs along the endocytic and secretory routes (51–53) and is required for membrane trafficking in both pathways (54). The loss of Ocrl1 was recently linked to deficient cilia assembly (43, 55). Coon et al (43) showed that Ocrl1 participates in the delivery of cargo to the PC via two routes: directly from the TGN involving Rab8 and an indirect route by internalizing cargo from plasma membrane and redirecting it from the endosomal compartment to the PC (Fig. 2,I and II). This latter mechanism involved Rab5 and the endosomal proteins IPIP27A/Ses. Some evidence suggested that both pathways coalesce at the base of the PC (43).
Additional evidence further supports the existence of an indirect, endocytosis- and recycling-dependent protein trafficking route to the PC (Fig. 2,II). For example knock-down of the tyrosine phosphatase PTPN2 in ht-RPE cells resulted in an accumulation of the typical PC-enriched protein Smo-EGFP in early endosomes (23). In addition, mice expressing a dominant negative version of the endocytic recycling protein Arl13b, displayed evidence of defective regulation of Smo in the PC and abnormal Sonic hedgehog (Shh) signaling (56, 57). Further, Arl13b was also shown to play a role in the ciliary targeting of the lipid phophatase Inpp5E (58). Similar to Arl13b’s function at the ciliary base, another GTPase, Arl3, co-ordinates with its GAP RP2 the traffic of proteins to the ciliary base (59–62).
Soluble Proteins
Soluble cytoplasmic proteins reach the ciliary base by diffusion; however, evidence suggest that the dynein-mediated, microtubule (MT)-dependent transport is a parallel, faster route than diffusion, to reach the centrioles (63) (Fig. 2,III). Specifically, several centriolar components namely pericentrin, γ-tubulin, ninein, centrin and PCM-1 are localized by dynein-dynactin mediated and microtubule (MT) dependent transport (63, 64). These components were mislocalized following MT depolymerizing or by disrupting dynein (63, 64).
BBS4 interaction with PCM-1 and the p150 glued subunit of dynactin suggests that BBS4 is an adaptor linking cargo to the dynein-dynactin motor for MT mediated transport (65). Another adaptor protein that plays a role in maintenance of the centriolar structure and function is Hook2 which also exists in a complex with PCM-1 (66, 67). The proposed idea is that PCM-1 shuttles between the cytosol and the centriole delivering cargo to the ciliary base, whose dispersion away from the centriole is regulated by Cep290 (68)(Fig. 2,III).
As a whole, these results emphasize the existence of multiple mechanisms and routes participating in the sorting of proteins to the PC.
Vesicle docking and fusion at the base of the PC
Since vesicles are prevented from entering the cilia by the barrier located at the base of the PC, carriers must dock and fuse at the ciliary base.
Rab8 is one of the major players in ciliogenesis and the only Rab that localizes to the PC. Indeed, this Rab GTPase is required for the docking and fusion of vesicles at the base of the PC (69) and for maintaining ciliary membrane identity (70). Multiple proteins such as RPGR (71), Cep290 (72), Ahi1 (73) and PCM1 (68) and the Rab11-Rabin8 complex contribute to Rab8 recruitment to the base of cilia.
Also of outstanding importance for cilia assembly is Rab11, which is involved in the recruitment of the Rab8 activator Rabin8 on pericentrosomal vesicles, activity that requires the ASAP1 scaffolding function (46, 74, 75). The activation of Rab8 and localization to the PC by the Rab11-Rabin8 complex is coupled to ciliary growth. Once the required cilia length is achieved, Rabin8 is removed from the centrosomes which also stops the trafficking of Rab8 into the cilium (75).
The TRAPPII (transport protein particle II) complex co-localizes with Rabin8-Rab8 in vesicles and in the centriole (75). However, it seems to facilitate the tethering of the ciliary vesicles to the centriole rather than to the periciliary membrane (75). In addition, it was recently shown that distal appendage components can act as an anchor for ciliary vesicles (18, 19, 76, 77) and interact with the vesicle trafficking machinery.
The role for the exocyst in vesicle fusion at the periciliary membrane is supported by several observations. Members of the exocyst complex (Sec6 and Sec8) have been found at the base of the cilia (78, 79). Overexpression of the exocyst component Sec10 led to the formation of longer PC in MDCK cells likely due to the excess delivery of ciliary components (80). The observation that the Sec15 subunit of the exocyst interacts with Rab11 and Rabin8 (81), suggested that the exocyst is linked to the Rab11-binding ternary complex (Arf4-ASAP1-FIP3) and facilitates membrane fusion (45). In fact, Rab8 regulation was coupled to the exocyst-mediated ‘kiss and run’ discharge of the contractile vacuole in Dictyostelium discoideum (82), supporting their role in vesicle tethering and fusion. Exocyst components also were found in a complex with IFT components and cargo (Polycystin-2); and knock-down of Sec10 resulted in a mislocalization of the cargo suggesting that the exocyst directs the IFT particle and its associated cargo to the cilia (83).
Trafficking in and out of the PC
Membrane proteins
The octameric complex referred to as the BBSome, constitutes the first canonical coat complex known to be involved in specialized protein trafficking into the cilium (84, 85). Although the BBSome may also play a role in trafficking to the ciliary base (86, 87), it localizes to the plasma membrane via Arl6 (BBS3) and mediates cargo translocation through the ciliary barrier (85, 88)(Fig. 3A). Specifically, the BBSome interacts with the C-terminus of ciliary cargoes such as Smo and Sstr3 (85, 89) and somehow drags them across the barrier (90). The BBSome complex binds Rabin8 at the ciliary base and it has been suggested that this interaction is the key for BBSome ciliary entry along with its cargo (84). Regulators of BBSome ciliary trafficking have also been reported, namely LZTFL1 and AZI1 which sequester the BBSome in the cytoplasm and the centriolar satellites respectively (91, 92).
Figure 3. Transport of Material across and within the cilia.
Movement across the cilia mediated by the BBSome and activated Ran gradient system. Co-ordination of IFT inside the cilia by the BBSome for anterograde and retrograde trafficking (See text for details).
There is also evidence that the BBSome is involved in the exit of proteins from the cilia. Loss of BBS proteins resulted in the accumulation of Smo and its negative regulator Patched (Ptch) in the cilia, leading to disruption of the essential development Shh signaling pathway (89, 91). These results suggested that BBS components are required for the regulated segregation of Smo from Ptch in the cilia, perhaps by facilitating the exit of the latter. Other studies indicated that the BBSome is required for the exit of Dopamine GPCRs from the primary cilia which could account for enhanced anxiety seen in BBS children (93, 94). It has also been suggested that the BBSome proteins controls cilia exit and entry by co-ordinating the IFT machinery (see below under ‘Trafficking within the cilium’). In order to achieve the right ciliary volume, BBS and Rab8 deliver material to the ciliary compartment while the endocytic players help to remove material from the periciliary compartment (95).
Soluble proteins
Nucleoporins also have been found to localize at the base of the cilia (31) and the identification of a ciliary pore complex (32) emphasizes the similarities between the nuclear and ciliary transport mechanisms. The nuclear pore is a highly selective port of entry to the nucleus which only allows proteins with nuclear localization signals recognized by importins (soluble carriers), to be transported into the nucleus following a GTP-bound Ran gradient (96). Similar mechanisms have been proposed for the ciliary transport of soluble protein KIF17 and membrane proteins Crumbs and RP2 (97–99)(Fig. 3). Presence of a nuclear localization signal in these cargoes has been confirmed (see Table I). However, how these proteins are selectively localized in the cilia and not the nucleus is yet to be established. Other studies have showed that soluble proteins move into the cilia depending on their size (21, 37) by diffusion across a molecular sieve at the ciliary base. Therefore, it is possible that larger molecules require active transport to move across the cilia.
Trafficking within the PC
Once inside the cilia, movement is made possible by the IFT complexes A and B, responsible for retrograde and anterograde transport, respectively (12, 34, 100–103) (Fig. 3). Both complexes have been found to be in association as a single string of particles which are known as ‘IFT trains’, allowing constant bi-directional movement or helping each other to successfully complete a cycle of transport to and fro in the cilia (104). The IFT trains move in the space between the axoneme and the membrane and associate with both, as seen in the electron-tomographs of Chlamydomonas reinhardtii flagella (105). The IFT particles are carried in the anterograde direction by kinesin-2 motors and in the retrograde direction by cytoplasmic dynein-2 motors (105–107)(Fig. 3).
In C.reinhardtii and C.elegans, cargo gets loaded on IFT particles at the ciliary base (34) and again at the ciliary tip (108). A recent study in C.reinhardtii showed that cargo loading at the ciliary base is regulated by the size of the growing cilia (109). Up to date, no direct IFT-cargo interaction has been reported (110), however cargo has been observed hopping on and off the ‘IFT trains’ (111). Interestingly, Mukhopadhyay et al (112, 113) showed evidence supporting the presence of an adaptor protein (Tulp-3) linking cargo and the IFT particle.
Investigations in C.elegans also suggested a role of the BBS proteins as co-ordinators of IFT particles and motors for efficient anterograde transport (114, 115)(Fig. 3). Specifically, C.elegans mutants of BBS7/8 displayed varying velocities of anterograde transport due to lack of co-ordination between the motors (114, 115). Similar roles have also been described for the ARL proteins Arl-3 and Arl-13 (116). How and whether these 2 families co-ordinate along the same pathway has not been determined. Further, loss of BBS proteins in C.elegans and C.reinhardtii led to the accumulation of IFT B particles and signaling molecules at the cilia tip (108, 117). Turnaround of IFT complexes and cargo loading at the ciliary tip occurred in a DYF-2 and BBS-1 dependent manner (108). This abnormality was also apparent in mouse models of BBS (BBS 1/2/4/6 null mice) showing bulged ciliary tip which points towards problems in the retrograde IFT transport (94, 118, 119).
Ciliopathies
Ciliopathies are a broad and heterogeneous group of diseases due to compromised cilium function or structure. Given that almost every cell type in our body assembles a PC at a certain moment, it is not surprising that most ciliopathies are multi-organ disorders.
Ciliopathies arise from different mutations in more than 50 genes, and mutations in some genes can lead to more than one ciliopathy with pleiotropic phenotypes that can vary from mild to severe. While Tables II–V summarizes the characteristics of different ciliopathies and their link to vesicle trafficking, the following section discusses the steps of ciliary trafficking specifically affected in each ciliopathy and assesses its correlation with the phenotypes/symptoms observed.
Table II.
Ciliary defects and phenotypes characteristic of Renal/Retinal-renal ciliopathies
| Genes | Other names | Ciliopathy | Cilia presence | Ciliary signaling functions | Other functions/defects | Animal model phenotypes |
|---|---|---|---|---|---|---|
| INV | NPHP2 | NPHP | Inv/inv mutant mice: Renal cilia not affected (176), structure of the nodal cilia not affected (177). | Negative regulator of Wnt/β-catenin pathway (178), required for cilia disassembly (179). | inv/inv mutant mice: Sustained cell proliferation in the kidney(180). | KOa mice: Situs inversus (181), renal fibrosis (182). |
| NPHP7 | GLIS2 | NPHP | KDb in zebrafish: Required for ciliary motilify (183) | Negative regulator of Wnt/β-catenin pathway (126) | Required for kidney architecture (127, 184) | KO mice: Tubular atrophy and fibrosis leading to kidney failure (127, 184) |
| NPHP9 | NEK8 | NPHP | KO mice: Not required for ciliogenesis (185). | Suggestive of Polycystin-2 dependent signaling from the cilia (185, 186). | Required for the kidney tubule maintenance (187), regulator of Hippo signaling (188). | KO mice: Die at birth due to situs inversus, cardiac anomalies, glomerular cysts (185). KD in zebrafish: pronephric cysts (189). |
| NPHP4 | Nephroretinin | NPHP, SLSN | Nphp4 mutant mice: No overall ciliogenesis defect, photoreceptor degeneration reported (190) | Negatively regulate Wnt/β-catenin pathway (191) | Negative regulator of Hippo pathways (188) | |
| NPHP5 | IQCB1 | NPHP, SLSN | Nphp5 KD: Decreased number of renal cilia (192) | Rhodopsin trafficking (192) | KO mice: Apoptosis, fibrosis and cysts (192) |
Knockout;
Knockdown
Renal ciliopathies
Among the ciliopathies, Polycystic kidney disease (PKD) and Nephronophthisis (NPHP) majorly involve renal malfunction.
PKD is caused by mutations in the renal-cilia specific signaling receptors Polycystin-1, Polycystin-2 or Fibrocystin (120). Abnormal function or PC targeting of these protein cargoes leads to mechanosensation defects which in turn conduces to cyst formation or tubulopathy (121).
NPHP is the leading cause of end stage renal disorder, the last stage of chronic kidney disease, a condition requiring immediate dialysis or kidney transplant. The products of the genes affected in NPHP are called nephrocystins, and are localized at the ciliary base. In addition to renal ciliopathies, some of these genes are also involved in multi-organ diseases (see below). NPHPs form distinct complexes with each other namely NPHP1-4-8, NPHP5-6, NPHP 2-3-9-16 found in the TZ (122). Although their specific functions are not known it is apparent that genes that cause the renal form of NPHP do not affect ciliogenesis (Table II). Some of the members of this protein family seem to play a role in signal transduction (123–125), for example Glis-2 is a negative regulator of the Wnt β-catenin pathway by functioning as its negative regulator (126). In fact, suppression of the canonical Wnt signaling is required for normal renal development and maintenance (125, 127) which when disrupted in PKD and NPHP leads to cystogenesis or fibrosis.
Multi-organ ciliopathies
Senior-Løken Syndrome (SLSN)
Affected NPHP genes are also often found associated with retinal abnormalities, in which case the corresponding pathology is known as SLSN. NPHP-5 is the classical SLSN causal gene as 100% of the cases are associated with retinal-renal pathology. The link between nephrocystin genes and retinal degeneration is possibly due to the interaction of nephrocystins with RPGR which is required for the maintenance of the photoreceptor by trafficking of Opsins to the outer segment (128–131). RPGR acts as a GEF for Rab8 and is also required for Rab8 localization (71). The adaptor protein RPGRIP1L seems to mediate the interaction between the RPGR and different NPHP complexes namely NPHP1-2-5 and NPHP 4-6-8 (71, 132). A role of the nephrocystins in sorting and trafficking of Opsins by correct loading of IFT particles on trains has also been described (133).
Oculo-Cerebro-Renal syndrome of Lowe (OCRL) or Lowe Syndrome (LS)
LS is characterized by cataracts, mental retardation and renal abnormalities such as LMW proteinuria. This disease is caused by mutations in the OCRL1 gene (see ‘Trafficking to the base of the PC’). However, OCRL1 mutations can also lead to a renal tubulopathy called Dent disease. It has been proposed that these different pathologies might be due to mutations resulting in different truncated forms of the protein (134).
It has been shown that LS patient and Ocrl1 knock-down cells show abnormal cilia (43, 55, 135) with reduced Rhodopsin trafficking to the PC (43). A zebrafish model of LS showed symptoms typical of ciliary dysfunction with curved bodies, underdeveloped eyes, pronephro cilia abnormalities and neurological lesions (43, 55, 136). Importantly, Ocrl1 interacts with several Rabs (underscoring its role in vesicle trafficking); and with highest affinity for Rab8, highlighting its role in ciliary function (53). In fact, simultaneous overexpression of Ocrl1 and Rab8 led to abnormal traffic to the cilia and to the formation of bulged PC likely due to cargo accumulation (43). In addition, Ocrl1 lack of function leads to RhoGTPase activation abnormalities which could be a cause or consequence of ciliary dysfunction (137, 138). Although the specific mechanism is still unknown, these abnormalities led to defects in cell spreading, cell migration, and fluid phase uptake (139) which in turn may be responsible for some of the developmental defects observed in LS patients.
Bardet-Biedl Syndrome (BBS)
BBS is characterized by obesity, rod-cone dystrophy, renal abnormalities, polydactyly, male hypogonadism and learning disabilities; animal models of BBS faithfully reproduced these phenotypes (See Table III). Most of the BBS causative genes are part of the BBSome or are required for regulation, assembly or functioning of the BBSome. As discussed previously, the BBSome plays a role in trafficking of cargoes across the cilia (See Section on ‘Trafficking In and Out of the Cilia’). However, it does not seem to be playing a critical role in cilia assembly (87, 89, 117, 140)(Table III). This is probably the reason why BBS is not characterized by severe phenotypes such as perinatal lethality. Indeed, BBS results in male infertility due to defective spermatogenesis caused by motile ciliary defect, not PC defect (140, 141).
Table III.
Ciliary defects and phenotypes characteristic of Bardet-Biedl Syndrome (BBS)
| Genes | Other names | Ciliopathy | Cilia presence | Ciliary signaling and trafficking functions | Phenotypes in animal models |
|---|---|---|---|---|---|
| BBS1 | BBS | KOa mice: Defective olfactory epithelium (193). Bbs1 M390R knockin mice: abnormally swollen distal end in their ependymal cilia, airway epithelial cilia, photoreceptor degeneration (118, 119) | Component of BBSome (85), leptin signaling (86), Hh signaling (89), required for turnaround of IFT particles (108). | Bbs1 M390R knock-in mice: ventriculomegaly, obesity,retinopathy, higher heart rate (118). KO mice: Embryonic lethal, anosmia (193). | |
| BBS2 | BBS | KO mice: Global cilia assembly not affected, photoreceptor degeneration, defective sperm flagella (140), bulged ciliary tips in airway epithelia (119), not required for ciliogenesis in the brain(87). MOb injected zebrafish: progressively defective KVc cilia (194). | Component of BBSome (85), Leptin signaling (86), Sstr3 and Mchr1 neuronal cilia localization (87), Dopamine receptor ciliary exit (93), Rhodopsin photoreceptor localization (140) | KO mice: obesity (86, 140), ventriculomegaly (118), retinopathy, male infertility, neurosensory phenotypes (140). | |
| BBS3 | ARL6 | BBS | MO injected zebrafish: Defective KV (195, 196). Mutant Arl6 overexpression in hTERT-RPE cells: increased ciliary length (88) | Required for BBSome targeting to the cilia (85), Hh signaling (91), Wnt/β-catenin signaling (88). | KO mice: Retinopathy (196). |
| BBS4 | BBS | KO mice: Global cilia assembly not affected, defective olfactory epithelium, photoreceptor degeneration, defective sperm development (141, 193, 197), not required for ciliogenesis in the brain (87), bulged ciliary tips in airway epithelia(119), longer renal cilia (198). MO injected zebrafish: Progressive defective KV cilia (194). Full length flagella assembled in the absence of BBSome components (117). | Component of BBSome (85), Leptin signaling (86), Rhodopsin, Arrestin and Transducin photoreceptor localization (197). Sstr3 and Mchr1 neuronal cilia localization (87). Dopamine receptor ciliary exit (93). Required for transport of PCM1 and its associated cargo to the ciliary base (65). Aberrant Rho A signaling (199). | KO mice: Neurosensory phentoypes (140), anosmia (193), male infertility (141). | |
| BBS5 | BBS | MO injected zebrafish: Progressive defective KV cilia (194, 200). | Component of BBSome (85), Hh signaling (91), interacts with the Dopamine receptor (93). | MO injected zebrafish: Altered heart looping, defective melanosome transport (200). | |
| BBS6 | MKKS | BBS | KO mice: retinal degeneration (201, 202), Olfactory epithilium cilia affected, bulged ciliary tips in airway epithelia (119). MO injected zebrafish: Progressive defective KV cilia (194). | Required for BBSome assembly (203), Leptin signaling (86). | KO mice: obesity (86), ventriculomegaly (118), retinal degeneration (201, 202). |
| BBS7 | BBS | KO mice: ependymal cilia abnormally bulged at the tips, photoreceptor degeneration (94). MO injected zebrafish: Progressive defective KV cilia (194). BBS7 null mice MEFsd: Cilia not affected (89) | Component of BBSome (85), Dopamine receptor ciliary exit (93). Co-ordination of IFT (114, 115). | KO mice: retinal degeneration, obesity, ventriculomegaly and male infertility (94). | |
| BBS8 | TCT8 | BBS | KO mice: Olfactory cilia affected (204). MO injected zebrafish: Progressive defective KV cilia (194). | Component of BBSome (85). Required for OSM-9 localization in C.elegans (205), ACIII and SLP3 localization in olfactory sensory neuronal cilia (204). Co-ordination of IFT (114, 115). | KO mice: Olfactory cilia affected (204) |
| BBS9 | PTHB1 | BBS | MO injected zebrafish: defective KV. KDe in IMCD3 cells: absence of cilia (206). | Component of BBSome (85) | |
| BBS10 | BBS | Required for BBSome assembly (203) | |||
| BBS11 | TRIM32 | BBS | MO injected zebrafish: progressive defective KV cilia (207) | KO mice: Myopathy and neurodegeneration (208) | |
| BBS12 | BBS | Required for BBSome assembly (203) | |||
| BBS17 | LZTFL1 | BBS | Negative regulator of BBSome, Hh signaling (91) | ||
| BB1P10 | BBS18 | BBS | NPY2R ciliary localization (142) | KO mice: Obesity, hyperphagia, retinal degeneration, male sterility (142). | |
| BBS15 | WDPCP, Fritz | BBS | Mutant mice: Kidney collecting duct, neuroepithelium and MEFs have defective ciliogenesis (209). KD in Xenopus: fewer and shorter cilia (210). | Mutant mice: anophthalmia, central polydactyly, kidney cysts, complex congenital heart defects, cloacal septal defects (209). | |
| SDCCAG8 | BBS16, NPHP10 | BBS, NPHP, SLSN | KO mice: No global ciliogenesis defect, photoreceptor degeneration (211). | Rhodopsin trafficking, DNA Damage signaling response (211). | MO injected zebrafish: body axis curvature, kidney cysts, hydrocephalus (212). |
Knockout;
Morpholino;
Kupffer’s vesicle;
Mouse Embryonic Fibroblasts;
Knockdown
Abnormal delivery of ciliary components for proper ciliary functioning can also lead to ciliary dysfunction, as it is apparent in the case of retinal degeneration due to mistrafficking of Rhodopsin. BBS proteins are required for maintenance of the photoreceptor by functioning at the connecting cilium; therefore, mutations in BBS components results in a degeneration of the photoreceptor. BBS proteins are also required for trafficking of neuronal cilia cargoes namely Sstr3, Mchr1 and D1 (87, 93, 94)(Table III). Defective Leptin receptor signaling and mistrafficking of the NeuropeptideY in the absence of the BBSome has been linked to obesity in these patients (86, 142)(Table III).
Lack of BBS protein functions led to structural ciliary defects with bulged ciliary tips due to accumulation of cargo along with IFT components (94, 118, 119). This is probably due to the role played by BBS in IFT co-ordination and turn-around in the cilia (108, 114, 115). Overall, these findings are suggestive of BBSome functioning in the delivery of cargo to the cilia in a cell-specific manner resulting in a degenerative disease.
Joubert Syndrome (JBTS)
JBTS is a multi-organ disorder with a characteristic hindbrain malformation (‘Molar tooth Sign’), along with ataxia and cognitive dysfunction. It is also frequently accompanied by renal and/or retinal symptoms. The animal models of JBTS display symptoms characteristic of ciliopathies: photoreceptor degeneration, brain malformations, laterality defects and cystic kidneys (133, 143–150)(Table IV).
Table IV.
Ciliary defects and phenotypes characteristic of Joubert Syndrome (JBTS)
| Genes | Other names | Ciliopathy | Cilia presence | Ciliary signaling and trafficking functions | Phenotypes in animal models |
|---|---|---|---|---|---|
| Inpp5E | JBTS1 | JBTS | Mutant mice MEFsa: Prematurely destabilized cilia in response to growth factor stimulation (155). MO injected zebrafish: decreased cilia in KVc and pronephric ducts (153) | INPP5E mutant mice: Embryonic lethal, skeletal abnormalities, kidney cysts, cerebral defects, hexadactyly (155). MOb injected zebrafish: microphthalmia, pronephros cysts, pericardial effusion, and left-right body axis asymmetry (153). | |
| AHI1 | JBTS3 | JBTS | Mutant mice: Photoreceptor degeneration (145, 146), normal cilia in kidneys and MEFs (213, 214). Ahi 1 KDd in IMCD3 cells and mutant mice MEF: affected for ciliogenesis (73, 147). Ahi1 morphant zebrafish: Defective ciliation in KV and in the pronephros cilia (147). | Wnt signaling (214), Shh signaling (56), Rab8 localization, vesicle docking and fusion (73), Opsin (145), Transducin and Rom1 trafficking (146). | KO mice: fibrosis, cystic kidney, midline fusion defect (213, 214), photoreceptor degeneration (145, 146). MO injected zebrafish: ear, hindbrain and eye abnormalities, cardiac-looping, cloacal dilation (147). |
| ARL13B | JBTS8 | JBTS | Mutant mice: Ciliary axoneme structure affected (56) | Hh signaling (56), Inpp5E, Polycystin-2 and ODR-10 ciliary localization (58, 116). Co-ordination of IFT (215). | KO mice: Embryonic lethal, open neural tube, randomized heart looping, polydactyly, abnormal eyes (56). Mutant zebrafish: curved bodies, pronephric cysts (144). |
| OFD1 | JBTS10 | JBTS | Mutant mice: nodal cilia and kidney cilia affected (143). Ofd1 mutant ESe cell lines: affected for ciliogenesis (216). | Required for the recruitment of Ift88 to the centrosome (216). | KO mice: embryonic lethal, left-right axis asymmetry, cystic kidneys (143). |
| KIF7 | JBTS12, COS2 | JBTS | KD in hTERT-RPE cells: reduced number of cilia (217). | Hh signaling (157, 218) | KO mice: skeletal abnormalities, Preaxial polydactyl (218), expanded motor neuron domain, embryonic lethal (157). |
| TCTN1 | JBTS13 | JBTS | KO mice: nodal and neural tube cilia affected (27). | Hh signaling, Ciliary localization of AC3, Polycystin-2 and Arl13b (27, 219) | KO mice: Embryonic lethal, defective limb bud patterning, neural tube patterning defects, cerebral abnormalities, holoprosencephaly (219) |
| TMEM237 | JBTS14 | JBTS | Patient fibroblasts failed to produce cilium (36). | Wnt signaling disrupted (36). | |
| CEP41 | JBTS15 | JBTS | Mutant zebrafish: kindey cilia motility was affected. Patient fibroblasts had a fewer percentage of cilia (149). | Mutant mice: embryonic lethal, brain malformation, laterality defects; MO injected zebrafish: laterality defects (149). | |
| PDE6D | JBTS | Inpp5E ciliary localization (58). | |||
| CSPP1 | JBTS | Patient fibroblasts affected for ciliogenesis (150). | Ciliary localization of ACIII and Arl13b (150) | MO injected zebrafish: curved bodies, pronephric cysts, cerebellar abnormalities (150). | |
| Cep164 | NPHP15 | NPHP, JBTS | KD in hTERT-RPE blocked docking of mother centrioles, docking of vesicle to the mother centriole and ciliogenesis (76, 148, 152) | Interacts with Rab8 (76), Inpp5E ciliary localization (58). Defective DNA damage response (148). | |
| NPHP1 | JBTS4 | NPHP, JBTS | Mutant mice: sperm development affected (220), photoreceptor degeneration (133), respiratory cilium motility defects (151). | Trafficking of Ift88, Rhodopsin and Transducin ciliary localization (133). | Nphp1 mutant mice: male sterility, retinal degeneration (133, 220). |
Mutant Embryonic Fibroblats,
Morpholino,
Kupffer’s vesicle,
Knockdown,
Embryonic stemcells
Genes affected in JBTS are required for ciliogenesis (Table IV). Absence of PC or decreased PC length is observed upon mutations in CSPP1, CEP41, TMEM237, TCTN1, KIF7, OFD1 and AHI1. Ciliary motility was also affected in animal models of JBTS: Nphp1 (151) and Cep41 (149). Ciliary defects were observed in several organs such as nodal cilia, connecting cilium of photoreceptors, neural tube cilia, limb bud mesenchyme and renal cilia (27, 143, 145–147) which accounts for the multi-organ disruptions associated with JBTS.
In addition, JBTS involves mutations in genes whose products are required in key vesicle trafficking processes during cilia assembly (Table IV). For example, the JBTS protein Cep164 is essential for ciliogenesis by docking the mother centriole to the apical membrane (148, 152) and vesicles to this structure (76). Ahi1 is required for vesicle delivery and fusion to the PC via its interaction with Rab8 (73). Arl13b is involved in trafficking material to the cilia (see ‘Trafficking to the base of the PC’) and its knockdown also resulted in a defective axoneme structure (56). Further, Arl13b, Cep164 and Pde6d form a complex that co-ordinates the localization of ciliary cargo (58), such as the lipid phosphatase Inpp5E. In turn, mutations in Inpp5E also lead to JBTS. Recently, the role of Inpp5E in ciliary development was demonstrated in morphant zebrafish (153). Although the exact mechanism of action is unknown; given its similarities to Ocrl1 in terms of substrate specificity (154, 155), Inpp5E may be also involved in vesicle transport to the PC. It is possible that this complex serves to transport more components to the cilia for its proper functioning (156). JBTS components also play a role in the regulation of ciliary signaling pathways, such as the essential Shh pathway (56, 157).
JBTS proteins are also required for proper functional co-ordination of IFTs, as is apparent from the disruption of IFT-A and B subcomplexes in ARL13b mutant C.elegans (116). Lack of function of other JBTS proteins have also been linked to IFT transport, namely Ofd1 defective cells were affected for Ift-88 ciliary base localization (4) and Nphp1 mutant photoreceptors showed defective trafficking of Ift-88 and Wdr-19 (133). It can be speculated that each JBTS protein might be functioning with certain IFT particles and co-ordinating the movement of the IFT particle associated cargo into the PC; further, IFT particles might carry different cargoes depending on the cell type leading to a complex, multi-organ disease. Collectively, the evidence indicates that JBTS is caused by disrupted ciliogenesis or by trafficking of components essential for defining and maintaining the ciliary compartment characteristics thus leading to more severe phenotypes than BBS.
Meckel-Gruber Syndrome (MKS)
This is the most severe of the multi-organ ciliopathies, characterized by occipital encephalocele, perinatal lethality, renal cysts and hepatic ductal malformations. Animal models of MKS faithfully reproduce these defects (30, 123, 124, 158, 159)(Table V). Similar to JBTS, MKS mutants display overall ciliary disruption in multiple tissues namely, the nodal cilia, neural cilia, limb-bud cilia and retinal degeneration (27, 30, 124, 129, 158, 160) (Table V).
Table V.
Ciliary defects and phenotypes characteristic of Meckel-Gruber Syndrome (MKS)
| Genes | Other names | Ciliopathy | Cilia presence | Ciliary signaling and trafficking functions | Phenotypes in animal models |
|---|---|---|---|---|---|
| B9D1 | MKS9, MKSR-1 | MKS | KOa mice: reduced cilia in kidney tubules (221), ciliogenesis affected in rapidly dividing tissues (30). KDb in IMCD3 cells: disrupted ciliogenesis (222). | Hh signaling, Ciliary localization of Arl13b, ACIII (221). MKS-1/MKSR-1/MKSR-2/MKS-3/MKS-6 along with NPHP1 and 4 are required for the formation of an intact barrier in C.elegans cilia (29). | KO mice: Embryonic lethal, polydactyly, kidney cysts, ductal plate malformation, abnormal neural tube patterning (221), microphthalmia, polydactyly, severe vascular defects (30). |
| B9D2 | MKS10, MKSR-2,Stumpy | MKS | KO mice: Ependymal cilia affected (159). KD in IMCD3 cells: disrupted ciliogenesis (222) | MKS-1/MKSR-1/MKSR-2/MKS-3/MKS-6 along with NPHP1 and 4 are required for the formation of an intact barrier in C.elegans cilia (29). | KO mice: hydrocephalus, cystic kidneys (159). |
| MKS1 | BBS13 | BBS, MKS | Mutant mice: Cilia numbers were affected in most epithelial and mesenchymal tissues (160), MEFsc and kidney (223). KD in IMCD3: ciliogenesis disrupted, by blocking centriole migration to the apical membrane (28, 161). | Hh signaling (28, 160). MKS-1/MKSR-1/MKSR-2/MKS-3/MKS-6 along with NPHP1 and 4 are required for the formation of an intact barrier in C.elegans cilia (29). | Mks1 mutant mice: embryonic lethal, biliary duct malformation, kidney cysts, polydactyly, occipital encephalocoele, hydrocephaly, skeletal defects (160); craniofacial defects, polydactyly, congenital heart defects, polycystic kidneys and randomized left-right patterning (223). |
| CEP290 | BBS14, MKS4, JBTS5, NPHP6 | BBS, MKS, JBTS, NPHP, SLSN | Mutant mice: Retinal degeneration (129). KD in IMCD3 and hTERT-RPE cells: Critical for ciliogenesis (28, 68) | Component of Y-links (26). Required for Rab8 localization (68), Rhodopsin and Arrestin ciliary localization (129). | Cep 290 null mice: Midline fusion defect (214). Cep290 mutant mice: retinal degeneration (129). |
| TMEM216 | JBTS2, MKS2 | JBTS, MKS | KD in IMCD3: ciliogenesis defects, centrosomal docking was prevented (224) | MKS-1/MKSR-1/MKSR-2/MKS-3/MKS-6 along with NPHP1 and 4 are required for the formation of an intact barrier in C.elegans cilia (29). | |
| TMEM67 | JBTS6, MKS3, NPHP11, Meckelin | JBTS, MKS, NPHP | Mutant mice: kidney tubule cilia affected (27), photoreceptor degeneration (225). KD in IMCD3 cells: ciliogenesis affected by blocking centriole migration to the apical membrane (161). | Ciliary localization of ACIII, Arl13b, Rhodopsin, Rom1, Transducin and Arrestin (27, 225). MKS-1/MKSR-1/MKSR-2/MKS-3/MKS-6 along with NPHP1 and 4 are required for the formation of an intact barrier in C.elegans cilia (29). | KO mice: kidney ciliary defects, kidney cysts (27), photoreceptor degeneration (225). |
| RPGRIP1l | JBTS7, MKS5, NPHP8, FTM | NPHP, JBTS, MKS, | Mutant mice: Fewer cilia in the neural tube and limb buds (158). Mutant C.elegans: ciliogenesis affected (29). | Hh signaling (158). | KO mice: death, craniofacial dysmorphology, microphthalmis, polydactyly, heterotaxia (158) |
| CC2D2A | JBTS9, MKS6 | NPHP, JBTS, MKS, | KO mice: nodal and neural tube cilia formation affected (27). | Required for ciliary localization of ACIII, Polycystin-2, Arl13b (27), opsin (162). MKS-1/MKSR-1/MKSR-2/MKS-3/MKS-6 along with NPHP1 and 4 are required for the formation of an intact barrier in C.elegans cilia (29). | KO mice: embryonic lethal, neural tube ciliary defects, laterality defects, holoprosencephaly, microphthalmia (27). Mutant zebrafish: photoreceptor degeneration (162). |
| TMEM138 | JBTS16 | JBTS, MKS | Marks vesicles which are ciliary bound (226). | ||
| TMEM231 | JBTS20 | JBTS, MKS | KO mice ciliogenesis defects in rapidly dividing tissues (30). | Hh signaling, required for diffusion barrier integrity (30). | KO mice: Microphthalmia, polydactyly, lethal due to severe vascular defects (30). |
| TCTN2 | MKS8 | JBTS, MKS | KO mice: nodal and neural tube cilia formation affected (27). | Hh signaling, required for ciliary localization of ACIII, Polycystin-2, Arl13b (27). | KO mice: Embryonic lethal, affected nodal cilia, neuronal cilia and limb bud mesenchyme cilia (27). |
| NPHP3 | MKS7 | NPHP, MKS, SLSN | MOd injected zebrafish: KVe cilia affected (124). | Wnt signaling (123, 124) | KO mice: Embryonic lethal, Situs inversus, renal-hepatic, pancreatic dysplasia, heart defects, tapetoretinal degeneration (123). MO in zebrafish: hydrocephalus, situs inversus (124). |
Knockout,
Knockdown,
Mouse embryonic fibroblasts,
Morpholino,
Kupffer’s vesicle
MKS proteins are involved in early ciliogenic events which involves establishment of the barrier at the base which controls protein trafficking to and from the PC. Most of these proteins are localized in the TZ and co-operate to form an intact TZ (29, 30)(Table V). Mutations in the corresponding TZ genes resulted in stunted cilia with abnormal membrane composition, due to loss of the ability to maintain the membrane diffusional barrier between the plasma and the ciliary membrane (29, 30). Other functions described for these MKS proteins involve basal body docking by Mks1 and Mks3 (161). Cep290 and Cc2d2a are required for Rab8 localization, which is the ciliary compartment identifier (68, 162). Taken together, mutations in these genes disrupt global ciliogenesis leading to severe phenotypes.
Overlap among ciliopathies
Lack of or abnormal function of certain genes can lead to the onset of several ciliopathies (See Fig. 4). For example, mutations in some MKS-causing genes (CEP290) also can lead to milder phenotypes resulting in NPHP, BBS or JBTS (See Table V). This spectrum in phenotype has been attributed to specific mutations and mutational load. Hypomorphic mutations in MKS1 and CC2D2A have shown to cause milder diseases categorized as BBS and JBTS respectively (163, 164). In the case of TMEM67, the position and nature of the mutation determines the severity of the disease (165). Interestingly, Cep290 is a protein that when mutated can manifest a varied spectrum of disorders (130), possibly due to the number and type of interactors that it has, namely, BBS proteins, Cc2d2a, Nphp5 and Rab8 (68, 166–168).
Figure 4.
A Graphical Representation of the overlap of genes involved in ciliopathies.
In addition, there are also reports that even the same mutation may lead to scenarios of different severity. For example, Ocrl1 mutations can cause LS, but also Dent’s disease (lacking mental retardation and ocular abnormalities) (169). The mechanisms responsible for this variability are not fully established yet, but the existence of critical genetic modifiers and the differential impact of specific mutations have been suggested.
Conclusions
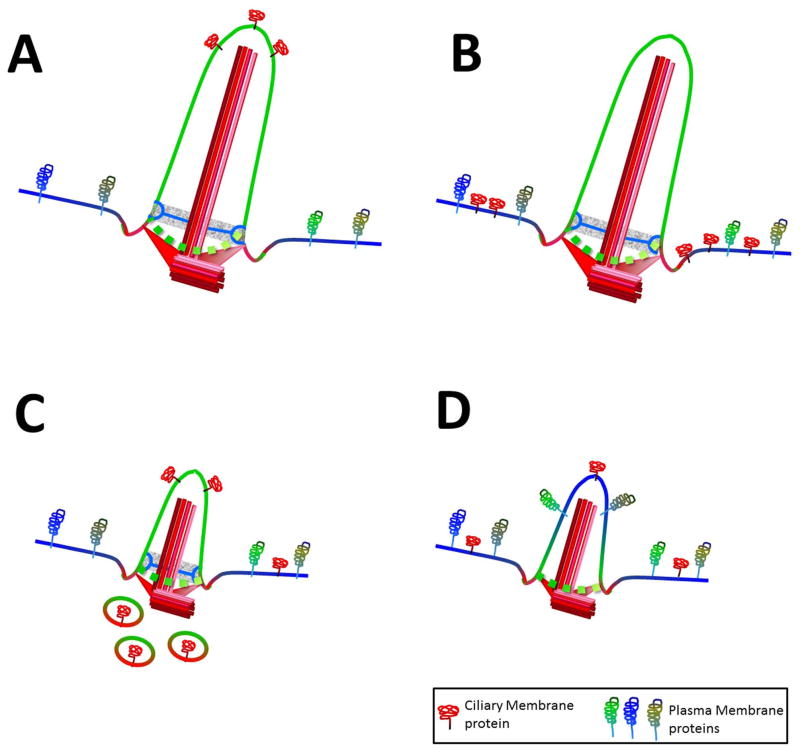
Broadly, ciliopathies can be divided in isolated (PKD, NPHP) versus multi-organ disorders (SLSN, BBS, JBTS, LS and MKS). Whereas specific ciliary signaling pathways are disrupted in NPHP and PKD, multi-organ ciliopathies display a more global effect on PC function. Importantly, the severity of the disease seems to depend on whether ciliogenesis is affected or not (Fig. 5). In the case of the milder BBS, while the cilia are intact, specific functions are disrupted due to mistrafficking of ciliary receptors (Fig. 5B). On the more severe end of the disease spectrum are LS, JBTS and MKS in which ciliogenesis is disrupted or affected (Fig. 5C and D).
Figure 5. Ciliary trafficking defects in ciliopathies.
(A) Normal Cilia. (B) NPHP and BBS: no ciliogenesis defect, specific ciliary cargo trafficking defect. (C) LS and JBTS: ciliogenesis affected due to defective material delivery for ciliary construction. (D) MKS: TZ components missing resulting in the loss of ciliary boundary
In summary, this review emphasized the importance of vesicle trafficking and protein sorting for the assembly and function of the primary cilia. As a whole, the evidence discussed also highlights the correlation between the impact of the mutation (depending on factors such as gene redundancy and the presence of genetic modifiers) on the PC (e.g., altering traffic of specific cargo vs. PC assembly deficiencies) and the severity of the corresponding ciliopathy. We speculate that in the near future, these emerging trends will contribute to our ability to make predictions in terms of gene product function or ciliopathy mechanism.
Synopsis.
The receptor-rich, signaling organelle known as Primary cilium (PC) has been implicated in a multitude of diseases collectively denominated as ciliopathies. The presence of a defined barrier at its base makes the PC an isolated compartment that needs vesicle trafficking for maintenance and function. This review discusses the major players involved in trafficking to the PC and highlights evidence correlating the severity of the ciliopathy to the trafficking defect involved. Specifically, mild and severe symptoms arise from specific ciliary receptor mislocalization and generalized ciliogenesis defect, respectively.
Acknowledgments
We thank members of the Aguilar for critical reading of the manuscript. We apologize to all authors whose original contributions we could not cite due to space limitations. For more complete listings please refer to the cited reviews and references therein. The Aguilar lab is supported by grants from the National Institutes of Health (5 R21 CA151961), the Lowe Syndrome Trust (PU/ICH/1010) and by the Center for Science of Information (CSoI), an NSF Science and Technology Center, under grant agreement CCF-0939370.
References
- 1.Zimmermann KW. zur Kenntniss einiger Drusen and Epithelien. Archiv fur Mikroskopische Anatomie. 1898;52:552–706. [Google Scholar]
- 2.Sorokin SP. RECONSTRUCTIONS OF CENTRIOLE FORMATION AND CILIOGENESIS IN MAMMALIAN LUNGS. Journal of Cell Science. 1968;3(2):207. doi: 10.1242/jcs.3.2.207. [DOI] [PubMed] [Google Scholar]
- 3.Satir P, Pedersen LB, Christensen ST. The primary cilium at a glance. Journal of Cell Science. 2010;123(4):499–503. doi: 10.1242/jcs.050377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DYR, Reiter JF. Vertebrate Smoothened functions at the primary cilium. Nature. 2005;437(7061):1018–1021. doi: 10.1038/nature04117. [DOI] [PubMed] [Google Scholar]
- 5.Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, Reiter JF. Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nature Cell Biology. 2008;10(1):70–U54. doi: 10.1038/ncb1670. [DOI] [PubMed] [Google Scholar]
- 6.Clement CA, Ajbro KD, Koefoed K, Vestergaard ML, Veland IR, de Jesus MPRH, Pedersen LB, Benmerah A, Andersen CY, Larsen LA, Christensen ST. TGF-beta Signaling Is Associated with Endocytosis at the Pocket Region of the Primary Cilium. Cell Reports. 2013;3(6):1806–1814. doi: 10.1016/j.celrep.2013.05.020. [DOI] [PubMed] [Google Scholar]
- 7.Schneider L, Clement CA, Teilmann SC, Pazour GJ, Hoffmann EK, Satir P, Christensen ST. PDGFR alpha alpha signaling is regulated through the primary cilium in fibroblasts. Current Biology. 2005;15(20):1861–1866. doi: 10.1016/j.cub.2005.09.012. [DOI] [PubMed] [Google Scholar]
- 8.Moritz OL, Tam BM, Papermaster DS, Nakayama T. A functional rhodopsin-green fluorescent protein fusion protein localizes correctly in transgenic Xenopus laevis retinal rods and is expressed in a time-dependent pattern. Journal of Biological Chemistry. 2001;276(30):28242–28251. doi: 10.1074/jbc.M101476200. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan OI, Molla-Herman A, Cevik S, Ghossoub R, Kida K, Kimura Y, Jenkins P, Martens JR, Setou M, Benmerah A, Blacque OE. The AP-1 clathrin adaptor facilitates cilium formation and functions with RAB-8 in C. elegans ciliary membrane transport. Journal of Cell Science. 2010;123(22):3966–3977. doi: 10.1242/jcs.073908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brailov I, Bancila M, Brisorgueil MJ, Miquel MC, Hamon M, Verge D. Localization of 5-HT6 receptors at the plasma membrane of neuronal cilia in the rat brain. Brain Research. 2000;872(1–2):271–275. doi: 10.1016/s0006-8993(00)02519-1. [DOI] [PubMed] [Google Scholar]
- 11.Handel M, Schulz S, Stanarius A, Schreff M, Erdtmann-Vourliotis M, Schmidt H, Wolf G, Hollt V. Selective targeting of somatostatin receptor 3 to neuronal cilia. Neuroscience. 1999;89(3):909–926. doi: 10.1016/s0306-4522(98)00354-6. [DOI] [PubMed] [Google Scholar]
- 12.Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB. Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Current Biology. 2002;12(11):R378–R380. doi: 10.1016/s0960-9822(02)00877-1. [DOI] [PubMed] [Google Scholar]
- 13.Goetz SC, Anderson KV. The primary cilium: a signalling centre during vertebrate development. Nature Reviews Genetics. 2010;11(5):331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker K, Beales PL. Making Sense of Cilia in Disease: The Human Cilloplathies. American Journal of Medical Genetics Part C-Seminars in Medical Genetics. 2009;151C(4):281–295. doi: 10.1002/ajmg.c.30231. [DOI] [PubMed] [Google Scholar]
- 15.Hoey DA, Downs ME, Jacobs CR. The mechanics of the primary cilium: An intricate structure with complex function. Journal of Biomechanics. 2012;45(1):17–26. doi: 10.1016/j.jbiomech.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilliam JC, Chang JT, Sandoval IM, Zhang Y, Li T, Pittler SJ, Chiu W, Wensel TG. Three-Dimensional Architecture of the Rod Sensory Cilium and Its Disruption in Retinal Neurodegeneration. Cell. 2012;151(5):1029–1041. doi: 10.1016/j.cell.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ye X, Zeng H, Ning G, Reiter JF, Liu A. C2cd3 is critical for centriolar distal appendage assembly and ciliary vesicle docking in mammals. Proceedings of the National Academy of Sciences of the United States of America. 2014;111(6):2164–2169. doi: 10.1073/pnas.1318737111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tanos BE, Yang H-J, Soni R, Wang W-J, Macaluso FP, Asara JM, Tsou M-FB. Centriole distal appendages promote membrane docking, leading to cilia initiation. Genes & Development. 2013;27(2):163–168. doi: 10.1101/gad.207043.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Joo K, Kim CG, Lee M-S, Moon H-Y, Lee S-H, Kim MJ, Kweon H-S, Park W-Y, Kim C-H, Gleeson JG, Kim J. CCDC41 is required for ciliary vesicle docking to the mother centriole. Proceedings of the National Academy of Sciences of the United States of America. 2013;110(15):5987–5992. doi: 10.1073/pnas.1220927110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Benmerah A. The ciliary pocket. Current Opinion in Cell Biology. 2013;25(1):78–84. doi: 10.1016/j.ceb.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Breslow DK, Koslover EF, Seydel F, Spakowitz AJ, Nachury MV. An in vitro assay for entry into cilia reveals unique properties of the soluble diffusion barrier. Journal of Cell Biology. 2013;203(1):129–147. doi: 10.1083/jcb.201212024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vieira OV, Gaus K, Verkade P, Fullekrug J, Vaz WLC, Simons K. FAPP2, cilium formation, and compartmentalization of the apical membrane in polarized Madin-Darby canine kidney (MDCK) cells. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(49):18556–18561. doi: 10.1073/pnas.0608291103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim J, Lee JE, Heynen-Genel S, Suyama E, Ono K, Lee K, Ideker T, Aza-Blanc P, Gleeson JG. Functional genomic screen for modulators of ciliogenesis and cilium length. Nature. 2010;464(7291):1048–U1114. doi: 10.1038/nature08895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson RG. 3-DIMENSIONAL STRUCTURE OF BASAL BODY FROM RHESUS-MONKEY OVIDUCT. Journal of Cell Biology. 1972;54(2):246. doi: 10.1083/jcb.54.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gilula NB, Satir P. CILIARY NECKLACE - CILIARY MEMBRANE SPECIALIZATION. Journal of Cell Biology. 1972;53(2):494. doi: 10.1083/jcb.53.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Craige B, Tsao C-C, Diener DR, Hou Y, Lechtreck K-F, Rosenbaum JL, Witman GB. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. Journal of Cell Biology. 2010;190(5):927–940. doi: 10.1083/jcb.201006105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garcia-Gonzalo FR, Corbit KC, Salome Sirerol-Piquer M, Ramaswami G, Otto EA, Noriega TR, Seol AD, Robinson JF, Bennett CL, Josifova DJ, Manuel Garcia-Verdugo J, Katsanis N, Hildebrandt F, Reiter JF. A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nature Genetics. 2011;43(8):776–U788. doi: 10.1038/ng.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sang L, Miller JJ, Corbit KC, Giles RH, Brauer MJ, Otto EA, Baye LM, Wen X, Scales SJ, Kwong M, Huntzicker EG, Sfakianos MK, Sandoval W, Bazan JF, Kulkarni P, et al. Mapping the NPHP-JBTS-MKS Protein Network Reveals Ciliopathy Disease Genes and Pathways. Cell. 2011;145(4):513–528. doi: 10.1016/j.cell.2011.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams CL, Li C, Kida K, Inglis PN, Mohan S, Semenec L, Bialas NJ, Stupay RM, Chen N, Blacque OE, Yoder BK, Leroux MR. MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. Journal of Cell Biology. 2011;192(6):1023–1041. doi: 10.1083/jcb.201012116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chih B, Liu P, Chinn Y, Chalouni C, Komuves LG, Hass PE, Sandoval W, Peterson AS. A ciliopathy complex at the transition zone protects the cilia as a privileged membrane domain. Nature Cell Biology. 2012;14(1):61–U97. doi: 10.1038/ncb2410. [DOI] [PubMed] [Google Scholar]
- 31.Kee HL, Dishinger JF, Blasius TL, Liu C-J, Margolis B, Verhey KJ. A size-exclusion permeability barrier and nucleoporins characterize a ciliary pore complex that regulates transport into cilia. Nature Cell Biology. 2012;14(4):431. doi: 10.1038/ncb2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ounjai P, Kim KD, Liu H, Dong M, Tauscher AN, Witkowska HE, Downing KH. Architectural Insights into a Ciliary Partition. Current Biology. 2013;23(4):339–344. doi: 10.1016/j.cub.2013.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hu Q, Milenkovic L, Jin H, Scott MP, Nachury MV, Spiliotis ET, Nelson WJ. A Septin Diffusion Barrier at the Base of the Primary Cilium Maintains Ciliary Membrane Protein Distribution. Science. 2010;329(5990):436–439. doi: 10.1126/science.1191054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deane JA, Cole DG, Seeley ES, Diener DR, Rosenbaum JL. Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Current Biology. 2001;11(20):1586–1590. doi: 10.1016/s0960-9822(01)00484-5. [DOI] [PubMed] [Google Scholar]
- 35.Zhao C, Malicki J. Nephrocystins and MKS proteins interact with IFT particle and facilitate transport of selected ciliary cargos. Embo Journal. 2011;30(13):2532–2544. doi: 10.1038/emboj.2011.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang L, Szymanska K, Jensen VL, Janecke AR, Innes AM, Davis EE, Frosk P, Li C, Willer JR, Chodirker BN, Greenberg CR, McLeod DR, Bernier FP, Chudley AE, Mueller T, et al. TMEM237 Is Mutated in Individuals with a Joubert Syndrome Related Disorder and Expands the Role of the TMEM Family at the Ciliary Transition Zone. American Journal of Human Genetics. 2011;89(6):713–730. doi: 10.1016/j.ajhg.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin Y-C, Niewiadomski P, Lin B, Nakamura H, Phua SC, Jiao J, Levchenko A, Inoue T, Rohatgi R, Inoue T. Chemically inducible diffusion trap at cilia reveals molecular sieve-like barrier. Nature Chemical Biology. 2013;9(7):437. doi: 10.1038/nchembio.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Najafi M, Maza NA, Calvert PD. Steric volume exclusion sets soluble protein concentrations in photoreceptor sensory cilia. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(1):203–208. doi: 10.1073/pnas.1115109109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Deretic D, Schmerl S, Hargrave PA, Arendt A, McDowell JH. Regulation of sorting and post-Golgi trafficking of rhodopsin by its C-terminal sequence QVS(A)PA. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(18):10620–10625. doi: 10.1073/pnas.95.18.10620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haller K, Fabry S. Brefeldin A affects synthesis and integrity of a eukaryotic flagellum. Biochemical and Biophysical Research Communications. 1998;242(3):597–601. doi: 10.1006/bbrc.1997.8015. [DOI] [PubMed] [Google Scholar]
- 41.Klemm RW, Ejsing CS, Surma MA, Kaiser H-J, Gerl MJ, Sampaio JL, de Robillard Q, Ferguson C, Proszynski TJ, Shevchenko A, Simons K. Segregation of sphingolipids and sterols during formation of secretory vesicles at the trans-Golgi network. Journal of Cell Biology. 2009;185(4):601–612. doi: 10.1083/jcb.200901145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mazelova J, Astuto-Gribble L, Inoue H, Tam BM, Schonteich E, Prekeris R, Moritz OL, Randazzo PA, Deretic D. Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. Embo Journal. 2009;28(3):183–192. doi: 10.1038/emboj.2008.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coon BG, Hernandez V, Madhivanan K, Mukherjee D, Hanna CB, Barinaga-Rementeria Ramirez I, Lowe M, Beales PL, Aguilar RC. The Lowe syndrome protein OCRL1 is involved in primary cilia assembly. Human molecular genetics. 2012;21(8):1835–1847. doi: 10.1093/hmg/ddr615. [DOI] [PubMed] [Google Scholar]
- 44.Lodowski KH, Lee R, Ropelewski P, Nemet I, Tian G, Imanishi Y. Signals Governing the Trafficking and Mistrafficking of a Ciliary GPCR, Rhodopsin. Journal of Neuroscience. 2013;33(34):13621–13638. doi: 10.1523/JNEUROSCI.1520-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J, Deretic D. Molecular complexes that direct rhodopsin transport to primary cilia. Progress in Retinal and Eye Research. 2014;38:1–19. doi: 10.1016/j.preteyeres.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J, Morita Y, Mazelova J, Deretic D. The Arf GAP ASAP1 provides a platform to regulate Arf4-and Rab11-Rab8-mediated ciliary receptor targeting. Embo Journal. 2012;31(20):4057–4071. doi: 10.1038/emboj.2012.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nie ZZ, Hirsch DS, Luo RB, Jian XY, Stauffer S, Cremesti A, Andrade J, Lebowitz J, Marino M, Ahvazi B, Hinshaw JE, Randazzo PA. A BAR domain in the N terminus of the Arf GAP ASAP1 affects membrane structure and trafficking of epidermal growth factor receptor. Current Biology. 2006;16(2):130–139. doi: 10.1016/j.cub.2005.11.069. [DOI] [PubMed] [Google Scholar]
- 48.Inoue H, Ha VL, Prekeris R, Randazzo PA. Arf GTPase-activating Protein ASAP1 Interacts with Rab11 Effector FIP3 and Regulates Pericentrosomal Localization of Transferrin Receptor-positive Recycling Endosome. Molecular Biology of the Cell. 2008;19(10):4224–4237. doi: 10.1091/mbc.E08-03-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Geng L, Okuhara D, Yu ZH, Tian X, Cai YQ, Shibazaki S, Somlo S. Polycystin-2 traffics to cilia independently of polycystin-1 by using an N-terminal RVxP motif. Journal of Cell Science. 2006;119(7):1383–1395. doi: 10.1242/jcs.02818. [DOI] [PubMed] [Google Scholar]
- 50.Hoffmeister H, Babinger K, Guerster S, Cedzich A, Meese C, Schadendorf K, Osten L, de Vries U, Rascle A, Witzgall R. Polycystin-2 takes different routes to the somatic and ciliary plasma membrane. Journal of Cell Biology. 2011;192(4):631–645. doi: 10.1083/jcb.201007050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hyvola N, Diao A, McKenzie E, Skippen A, Cockcroft S, Lowe M. Membrane targeting and activation of the Lowe syndrome protein OCRL1 by rab GTPases. Embo Journal. 2006;25(16):3750–3761. doi: 10.1038/sj.emboj.7601274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fukuda M, Kanno E, Ishibashi K, Itoh T. Large scale screening for novel Rab effectors reveals unexpected broad Rab binding specificity. Molecular & Cellular Proteomics. 2008;7(6):1031–1042. doi: 10.1074/mcp.M700569-MCP200. [DOI] [PubMed] [Google Scholar]
- 53.Hagemann N, Hou X, Goody RS, Itzen A, Erdmann KS. Crystal structure of the Rab binding domain of OCRL1 in complex with Rab8 and functional implications of the OCRL1/Rab8 module for Lowe syndrome. Small GTPases. 2012;3(2):107–110. doi: 10.4161/sgtp.19380. [DOI] [PubMed] [Google Scholar]
- 54.Mehta ZB, Pietka G, Lowe M. The Cellular and Physiological Functions of the Lowe Syndrome Protein OCRL1. Traffic. 2014;15(5):471–487. doi: 10.1111/tra.12160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Luo N, West CC, Murga-Zamalloa CA, Sun L, Anderson RM, Wells CD, Weinreb RN, Travers JB, Khanna H, Sun Y. OCRL localizes to the primary cilium: a new role for cilia in Lowe syndrome. Human Molecular Genetics. 2012;21(15) doi: 10.1093/hmg/dds163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Caspary T, Larkins CE, Anderson KV. The graded response to sonic hedgehog depends on cilia architecture. Developmental Cell. 2007;12(5):767–778. doi: 10.1016/j.devcel.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 57.Larkins CE, Aviles GDG, East MP, Kahn RA, Caspary T. Arl13b regulates ciliogenesis and the dynamic localization of Shh signaling proteins. Molecular Biology of the Cell. 2011;22(23):4694–4703. doi: 10.1091/mbc.E10-12-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Humbert MC, Weihbrecht K, Searby CC, Li Y, Pope RM, Sheffield VC, Seo S. ARL13B, PDE6D, and CEP164 form a functional network for INPP5E ciliary targeting. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(48):19691–19696. doi: 10.1073/pnas.1210916109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Veltel S, Kravchenko A, Ismail S, Wittinghofer A. Specificity of Arl2/Arl3 signaling is mediated by a ternary Arl3-effector-GAP complex. Febs Letters. 2008;582(17):2501–2507. doi: 10.1016/j.febslet.2008.05.053. [DOI] [PubMed] [Google Scholar]
- 60.Wright KJ, Baye LM, Olivier-Mason A, Mukhopadhyay S, Sang L, Kwong M, Wang W, Pretorius PR, Sheffield VC, Sengupta P, Slusarski DC, Jackson PK. An ARL3-UNC119-RP2 GTPase cycle targets myristoylated NPHP3 to the primary cilium. Genes & Development. 2011;25(22):2347–2360. doi: 10.1101/gad.173443.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schwarz N, Hardcastle AJ, Cheetham ME. Arl3 and RP2 mediated assembly and traffic of membrane associated cilia proteins. Vision Research. 2012;75:2–4. doi: 10.1016/j.visres.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 62.Schwarz N, Novoselova TV, Wait R, Hardcastle AJ, Cheetham ME. The X-linked retinitis pigmentosa protein RP2 facilitates G protein traffic. Human Molecular Genetics. 2012;21(4):863–873. doi: 10.1093/hmg/ddr520. [DOI] [PubMed] [Google Scholar]
- 63.Young A, Dictenberg JB, Purohit A, Tuft R, Doxsey SJ. Cytoplasmic dynein-mediated assembly of pericentrin and gamma tubulin onto centrosomes. Molecular Biology of the Cell. 2000;11(6):2047. doi: 10.1091/mbc.11.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dammermann A, Merdes A. Assembly of centrosomal proteins and microtubule organization depends on PCM-1. Journal of Cell Biology. 2002;159(2):255–266. doi: 10.1083/jcb.200204023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kim JC, Badano JL, Sibold S, Esmail MA, Hill J, Hoskins BE, Leitch CC, Venner K, Ansley SJ, Ross AJ, Leroux MR, Katsanis N, Beales PL. The Bardet-Biedl protein BBS4 targets cargo to the pericentriolar region and is required for microtubule anchoring and cell cycle progression. Nature Genetics. 2004;36(5):462–470. doi: 10.1038/ng1352. [DOI] [PubMed] [Google Scholar]
- 66.Szebenyi G, Hall B, Yu R, Hashim AI, Kraemer H. Hook2 localizes to the centrosome, binds directly to centriolin/CEP110 and contributes to centrosomal function. Traffic. 2007;8(1):32–46. doi: 10.1111/j.1600-0854.2006.00511.x. [DOI] [PubMed] [Google Scholar]
- 67.Baron Gaillard CL, Pallesi-Pocachard E, Massey-Harroche D, Richard F, Arsanto J-P, Chauvin J-P, Lecine P, Kramer H, Borg J-P, Le Bivic A. Hook2 is involved in the morphogenesis of the primary cilium. Molecular biology of the cell. 2011;22(23):4549–4562. doi: 10.1091/mbc.E11-05-0405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kim J, Krishnaswami SR, Gleeson JG. CEP290 interacts with the centriolar satellite component PCM-1 and is required for Rab8 localization to the primary cilium. Human Molecular Genetics. 2008;17(23):3796–3805. doi: 10.1093/hmg/ddn277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moritz OL, Tam BM, Hurd LL, Peranen J, Deretic D, Papermaster DS. Mutant rab8 impairs docking and fusion of rhodopsin-bearing post-Golgi membranes and causes cell death of transgenic Xenopus rods. Molecular Biology of the Cell. 2001;12(8):2341–2351. doi: 10.1091/mbc.12.8.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yoshimura S-i, Egerer J, Fuchs E, Haas AK, Barr FA. Functional dissection of Rab GTPases involved in primary cilium formation. Journal of Cell Biology. 2007;178(3):363–369. doi: 10.1083/jcb.200703047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Murga-Zamalloa CA, Atkins SJ, Peranen J, Swaroop A, Khanna H. Interaction of retinitis pigmentosa GTPase regulator (RPGR) with RAB8A GTPase: implications for cilia dysfunction and photoreceptor degeneration. Human Molecular Genetics. 2010;19(18):3591–3598. doi: 10.1093/hmg/ddq275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tsang WY, Bossard C, Khanna H, Peraenen J, Swaroop A, Malhotra V, Dynlacht BD. CP110 suppresses primary cilia formation through its interaction with CEP290, a protein deficient in human ciliary disease. Developmental Cell. 2008;15(2):187–197. doi: 10.1016/j.devcel.2008.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hsiao Y-C, Tong ZJ, Westfall JE, Ault JG, Page-McCaw PS, Ferland RJ. Ahi1, whose human ortholog is mutated in Joubert syndrome, is required for Rab8a localization, ciliogenesis and vesicle trafficking. Human Molecular Genetics. 2009;18(20):3926–3941. doi: 10.1093/hmg/ddp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Knoedler A, Feng S, Zhang J, Zhang X, Das A, Peranen J, Guo W. Coordination of Rab8 and Rab11 in primary ciliogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(14):6346–6351. doi: 10.1073/pnas.1002401107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Westlake CJ, Baye LM, Nachury MV, Wright KJ, Ervin KE, Phu L, Chalouni C, Beck JS, Kirkpatrick DS, Slusarski DC, Sheffield VC, Scheller RH, Jackson PK. Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(7):2759–2764. doi: 10.1073/pnas.1018823108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schmidt KN, Kuhns S, Neuner A, Hub B, Zentgraf H, Pereira G. Cep164 mediates vesicular docking to the mother centriole during early steps of ciliogenesis. Journal of Cell Biology. 2012;199(7):1083–1101. doi: 10.1083/jcb.201202126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sillibourne JE, Hurbain I, Grand-Perret T, Goud B, Tran P, Bornens M. Primary ciliogenesis requires the distal appendage component Cep123. Biology open. 2013;2(6):535–545. doi: 10.1242/bio.20134457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rogers KK, Wilson PD, Snyder RW, Zhang XY, Guo W, Burrow CR, Lipschutz JH. The exocyst localizes to the primary cilium in MDCK cells. Biochemical and Biophysical Research Communications. 2004;319(1):138–143. doi: 10.1016/j.bbrc.2004.04.165. [DOI] [PubMed] [Google Scholar]
- 79.Mazelova J, Ransom N, Astuto-Gribble L, Wilson MC, Deretic D. Syntaxin 3 and SNAP-25 pairing, regulated by omega-3 docosahexaenoic acid, controls the delivery of rhodopsin for the biogenesis of cilia-derived sensory organelles, the rod outer segments. Journal of Cell Science. 2009;122(12):2003–2013. doi: 10.1242/jcs.039982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zuo X, Guo W, Lipschutz JH. The Exocyst Protein Sec10 Is Necessary for Primary Ciliogenesis and Cystogenesis In Vitro. Molecular Biology of the Cell. 2009;20(10):2522–2529. doi: 10.1091/mbc.E08-07-0772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Feng S, Knoedler A, Ren J, Zhang J, Zhang X, Hong Y, Huang S, Peranen J, Guo W. A Rab8 Guanine Nucleotide Exchange Factor-Effector Interaction Network Regulates Primary Ciliogenesis. Journal of Biological Chemistry. 2012;287(19):15602–15609. doi: 10.1074/jbc.M111.333245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Essid M, Gopaldass N, Yoshida K, Merrifield C, Soldati T. Rab8a regulates the exocyst-mediated kiss-and-run discharge of the Dictyostelium contractile vacuole. Molecular Biology of the Cell. 2012;23(7):1267–1282. doi: 10.1091/mbc.E11-06-0576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fogelgren B, Lin S-Y, Zuo X, Jaffe KM, Park KM, Reichert RJ, Bell PD, Burdine RD, Lipschutz JH. The Exocyst Protein Sec10 Interacts with Polycystin-2 and Knockdown Causes PKD-Phenotypes. Plos Genetics. 2011;7(4) doi: 10.1371/journal.pgen.1001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC, Jackson PK. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129(6):1201–1213. doi: 10.1016/j.cell.2007.03.053. [DOI] [PubMed] [Google Scholar]
- 85.Jin H, White SR, Shida T, Schulz S, Aguiar M, Gygi SP, Bazan JF, Nachury MV. The Conserved Bardet-Biedl Syndrome Proteins Assemble a Coat that Traffics Membrane Proteins to Cilia. Cell. 2010;141(7):1208–U1198. doi: 10.1016/j.cell.2010.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Seo S, Guo D-F, Bugge K, Morgan DA, Rahmouni K, Sheffield VC. Requirement of Bardet-Biedl syndrome proteins for leptin receptor signaling. Human Molecular Genetics. 2009;18(7):1323–1331. doi: 10.1093/hmg/ddp031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Berbari NF, Lewis JS, Bishop GA, Askwith CC, Mykytyn K. Bardet-Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(11):4242–4246. doi: 10.1073/pnas.0711027105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wiens CJ, Tong Y, Esmail MA, Oh E, Gerdes JM, Wang J, Tempel W, Rattner JB, Katsanis N, Park H-W, Leroux MR. Bardet-Biedl Syndrome-associated Small GTPase ARL6 (BBS3) Functions at or near the Ciliary Gate and Modulates Wnt Signaling. Journal of Biological Chemistry. 2010;285(21):16218–16230. doi: 10.1074/jbc.M109.070953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang Q, Seo S, Bugge K, Stone EM, Sheffield VC. BBS proteins interact genetically with the IFT pathway to influence SHH-related phenotypes. Human Molecular Genetics. 2012;21(9):1945–1953. doi: 10.1093/hmg/dds004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Milenkovic L, Scott MP, Rohatgi R. Lateral transport of Smoothened from the plasma membrane to the membrane of the cilium. Journal of Cell Biology. 2009;187(3):365–374. doi: 10.1083/jcb.200907126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Seo S, Zhang Q, Bugge K, Breslow DK, Searby CC, Nachury MV, Sheffield VC. A Novel Protein LZTFL1 Regulates Ciliary Trafficking of the BBSome and Smoothened. Plos Genetics. 2011;7(11) doi: 10.1371/journal.pgen.1002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chamling X, Seo S, Searby CC, Kim G, Slusarski DC, Sheffield VC. The centriolar satellite protein AZI1 interacts with BBS4 and regulates ciliary trafficking of the BBSome. PLoS genetics. 2014;10(2):e1004083–e1004083. doi: 10.1371/journal.pgen.1004083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Domire JS, Green JA, Lee KG, Johnson AD, Askwith CC, Mykytyn K. Dopamine receptor 1 localizes to neuronal cilia in a dynamic process that requires the Bardet-Biedl syndrome proteins. Cellular and Molecular Life Sciences. 2011;68(17):2951–2960. doi: 10.1007/s00018-010-0603-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang Q, Nishimura D, Vogel T, Shao J, Swiderski R, Yin T, Searby C, Carter CS, Kim G, Bugge K, Stone EM, Sheffield VC. BBS7 is required for BBSome formation and its absence in mice results in Bardet-Biedl syndrome phenotypes and selective abnormalities in membrane protein trafficking. Journal of Cell Science. 2013;126(11):2372–2380. doi: 10.1242/jcs.111740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kaplan OI, Doroquez DB, Cevik S, Bowie RV, Clarke L, Sanders AAWM, Kida K, Rappoport JZ, Sengupta P, Blacque OE. Endocytosis Genes Facilitate Protein and Membrane Transport in C. elegans Sensory Cilia. Current Biology. 2012;22(6):451–460. doi: 10.1016/j.cub.2012.01.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wente SR, Rout MP. Cold Spring Harbor Perspectives in Biology. The Nuclear Pore Complex and Nuclear Transport. 2010;2(10) doi: 10.1101/cshperspect.a000562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fan S, Fogg V, Wang Q, Chen X-W, Liu C-J, Margolis B. A novel Crumbs3 isoform regulates cell division and ciliogenesis via importin beta interactions. Journal of Cell Biology. 2007;178(3):387–398. doi: 10.1083/jcb.200609096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Dishinger JF, Kee HL, Jenkins PM, Fan S, Hurd TW, Hammond JW, Truong YN-T, Margolis B, Martens JR, Verhey KJ. Ciliary entry of the kinesin-2 motor KIF17 is regulated by importin-beta 2 and RanGTP. Nature Cell Biology. 2010;12(7):703–U164. doi: 10.1038/ncb2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hurd TW, Fan S, Margolis BL. Localization of retinitis pigmentosa 2 to cilia is regulated by Importin beta 2. Journal of Cell Science. 2011;124(5):718–726. doi: 10.1242/jcs.070839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Follit JA, Tuft RA, Fogarty KE, Pazour GJ. The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Molecular Biology of the Cell. 2006;17(9):3781–3792. doi: 10.1091/mbc.E06-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Tsao C-C, Gorovsky MA. Different effects of Tetrahymena IFT172 domains on anterograde and retrograde intraflagellar transport. Molecular Biology of the Cell. 2008;19(4):1450–1461. doi: 10.1091/mbc.E07-05-0403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Iomini C, Babaev-Khaimov V, Sassaroli M, Piperno G. Protein particles in Chlamydomonas flagella undergo a transport cycle consisting of four phases. Journal of Cell Biology. 2001;153(1):13–24. doi: 10.1083/jcb.153.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Iomini C, Li L, Esparza JM, Dutcher SK. Retrograde Intraflagellar Transport Mutants Identify Complex A Proteins With Multiple Genetic Interactions in Chlamydomonas reinhardtii. Genetics. 2009;183(3):885–896. doi: 10.1534/genetics.109.101915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mencarelli C, Mitchell A, Leoncini R, Rosenbaum J, Lupetti P. Isolation of intraflagellar transport trains. Cytoskeleton. 2013;70(8):439–452. doi: 10.1002/cm.21121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Pigino G, Geimer S, Lanzavecchia S, Paccagnini E, Cantele F, Diener DR, Rosenbaum JL, Lupetti P. Electron-tomographic analysis of intraflagellar transport particle trains in situ. Journal of Cell Biology. 2009;187(1):135–148. doi: 10.1083/jcb.200905103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Pedersen LB, Rosenbaum JL. INTRAFLAGELLAR TRANSPORT (IFT): ROLE IN CILIARY ASSEMBLY, RESORPTION AND SIGNALLING. Ciliary Function in Mammalian Development. 2008;85:23–61. doi: 10.1016/S0070-2153(08)00802-8. [DOI] [PubMed] [Google Scholar]
- 107.Taschner M, Bhogaraju S, Lorentzen E. Architecture and function of IFT complex proteins in ciliogenesis. Differentiation. 2012;83(2):S12–S22. doi: 10.1016/j.diff.2011.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wei Q, Zhang Y, Li Y, Zhang Q, Ling K, Hu J. The BBSome controls IFT assembly and turnaround in cilia. Nature Cell Biology. 2012;14(9):950. doi: 10.1038/ncb2560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wren KN, Craft JM, Tritschler D, Schauer A, Patel DK, Smith EF, Porter ME, Kner P, Lechtreck KF. A Differential Cargo-Loading Model of Ciliary Length Regulation by IFT. Current Biology. 2013;23(24):2463–2471. doi: 10.1016/j.cub.2013.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bhogaraju S, Engel BD, Lorentzen E. Intraflagellar transport complex structure and cargo interactions. Cilia. 2013;2(1):10–10. doi: 10.1186/2046-2530-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ye F, Breslow DK, Koslover EF, Spakowitz AJ, Nelson WJ, Nachury MV. Single molecule imaging reveals a major role for diffusion in the exploration of ciliary space by signaling receptors. Elife. 2013:2. doi: 10.7554/eLife.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mukhopadhyay S, Wen X, Chih B, Nelson CD, Lane WS, Scales SJ, Jackson PK. TULP3 bridges the IFT-A complex and membrane phosphoinositides to promote trafficking of G protein-coupled receptors into primary cilia. Genes & Development. 2010;24(19):2180–2193. doi: 10.1101/gad.1966210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mukhopadhyay S, Wen X, Ratti N, Loktev A, Rangell L, Scales SJ, Jackson PK. The Ciliary G-Protein-Coupled Receptor Gpr161 Negatively Regulates the Sonic Hedgehog Pathway via cAMP Signaling. Cell. 2013;152(1–2):210–223. doi: 10.1016/j.cell.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 114.Blacque OE, Reardon MJ, Li CM, McCarthy J, Mahjoub MR, Ansley SJ, Badano LL, Mah AK, Beales PL, Davidson WS, Johnsen RC, Audeh M, Plasterk RHA, Baillie DL, Katsanis N, et al. Loss of C-elegans BBS-7 and BBS-8 protein function results in cilia defects and compromised intraflagellar transport. Genes & Development. 2004;18(13):1630–1642. doi: 10.1101/gad.1194004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ou GS, Blacque OE, Snow JJ, Leroux MR, Scholey JM. Functional coordination of intraflagellar transport motors. Nature. 2005;436(7050):583–587. doi: 10.1038/nature03818. [DOI] [PubMed] [Google Scholar]
- 116.Li Y, Wei Q, Zhang Y, Ling K, Hu J. The small GTPases ARL-13 and ARL-3 coordinate intraflagellar transport and ciliogenesis. Journal of Cell Biology. 2010;189(6):1039–1051. doi: 10.1083/jcb.200912001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Lechtreck K-F, Johnson EC, Sakai T, Cochran D, Ballif BA, Rush J, Pazour GJ, Ikebe M, Witman GB. The Chlamydomonas reinhardtii BBSome is an IFT cargo required for export of specific signaling proteins from flagella. Journal of Cell Biology. 2009;187(7):1117–1132. doi: 10.1083/jcb.200909183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Davis RE, Swiderski RE, Rahmouni K, Nishimura DY, Mullins RF, Agassandian K, Philp AR, Searby CC, Andrews MP, Thompson S, Berry CJ, Thedens DR, Yang B, Weiss RM, Cassell MD, et al. A knockin mouse model of the Bardet-Biedl syndrome 1 M390R mutation has cilia defects, ventriculomegaly, retinopathy, and obesity. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(49):19422–19427. doi: 10.1073/pnas.0708571104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Shah AS, Farmen SL, Moninger TO, Businga TR, Andrews MP, Bugger K, Searby CC, Nishimura D, Brogden KA, Kline JN, Sheffield VC, Welsh MJ. Loss of Bardet-Biedl syndrome proteins alters the morphology and function of motile cilia in airway epithelia. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(9):3380–3385. doi: 10.1073/pnas.0712327105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Lil XG, Elia AEH, Lu WN, Brown EM, Quinn SJ, Ingber DE, Zhou J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nature Genetics. 2003;33(2):129–137. doi: 10.1038/ng1076. [DOI] [PubMed] [Google Scholar]
- 121.Kotsis F, Boehlke C, Kuehn EW. The ciliary flow sensor and polycystic kidney disease. Nephrology Dialysis Transplantation. 2013;28(3):518–526. doi: 10.1093/ndt/gfs524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shiba D, Yokoyama T. The ciliary transitional zone and nephrocystins. Differentiation. 2012;83(2):S91–S96. doi: 10.1016/j.diff.2011.11.006. [DOI] [PubMed] [Google Scholar]
- 123.Bergmann C, Fliegauf M, Bruechle NO, Frank V, Olbrich H, Kirschner J, Schermer B, Schmedding I, Kispert A, Kraenzlin B, Nuernberg G, Becker C, Grimm T, Girschick G, Lynch SA, et al. Loss of nephrocystin-3 function can cause embryonic lethality, meckel-gruber-like syndrome, situs inversus, and renal-hepatic-pancreatic dysplasia. American Journal of Human Genetics. 2008;82(4):959–970. doi: 10.1016/j.ajhg.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhou W, Dai J, Attanasio M, Hildebrandt F. Nephrocystin-3 is required for ciliary function in zebrafish embryos. American Journal of Physiology-Renal Physiology. 2010;299(1):F55–F62. doi: 10.1152/ajprenal.00043.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A, Mlodzik M, Polok B, Driever W, Obara T, Walz G. Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nature Genetics. 2005;37(5):537–543. doi: 10.1038/ng1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kim Y-S, Kang HS, Jetten AM. The Kruppel-like zinc finger protein Glis2 functions as a negative modulator of the Wnt/beta-catenin signaling pathway. Febs Letters. 2007;581(5):858–864. doi: 10.1016/j.febslet.2007.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kim Y-S, Kang HS, Herbert R, Beak JY, Collins JB, Grissom SF, Jetten AM. Kruppel-like zinc finger protein Glis2 is essential for the maintenance of normal renal functions. Molecular and Cellular Biology. 2008;28(7):2358–2367. doi: 10.1128/MCB.01722-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Otto EA, Loeys B, Khanna H, Hellemans J, Sudbrak R, Fan SL, Muerb U, O’Toole JF, Helou J, Attanasio M, Utsch B, Sayer JA, Lillo C, Jimeno D, Coucke P, et al. Nephrocystin-5, a ciliary IQ domain protein, is mutated in Senior-Loken syndrome and interacts with RPGR and calmodulin. Nature Genetics. 2005;37(3):282–288. doi: 10.1038/ng1520. [DOI] [PubMed] [Google Scholar]
- 129.Chang B, Khanna H, Hawes N, Jimeno D, He S, Lillo C, Parapuram SK, Cheng H, Scott A, Hurd RE, Sayer JA, Otto EA, Attanasio M, O’Toole JF, Jin G, et al. In-frame deletion in a novel centrosomal/ciliary protein CEP290/NPHP6 perturbs its interaction with RPGR and results in early-onset retinal degeneration in the rd16 mouse. Human Molecular Genetics. 2006;15(11):1847–1857. doi: 10.1093/hmg/ddl107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Baala L, Romano S, Khaddour R, Saunier S, Smith UM, Audollent S, Ozilou C, Faivre L, Laurent N, Foliguet B, Munnich A, Lyonnet S, Salomon R, Encha-Razavi F, Gubler M-C, et al. The Meckel-Gruber syndrome gene, MKS3, is mutated in Joubert syndrome. American Journal of Human Genetics. 2007;80(1):186–194. doi: 10.1086/510499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brancati F, Travaglini L, Zablocka D, Boltshauser E, Accorsi P, Montagna G, Silhavy JL, Barrano G, Bertini E, Emma F, Rigoli L, Dallapiccola B, Gleeson JG, Valente EM, Int JSG. RPGRIP1L mutations are mainly associated with the cerebello-renal phenotype of Joubert syndrome-related disorders. Clinical Genetics. 2008;74(2):164–170. doi: 10.1111/j.1399-0004.2008.01047.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Gerner M, Haribaskar R, Puetz M, Czerwitzki J, Walz G, Schaefer T. The retinitis pigmentosa GTPase regulator interacting protein 1 (RPGRIP1) links RPGR to the nephronophthisis protein network. Kidney International. 2010;77(10):891–896. doi: 10.1038/ki.2010.27. [DOI] [PubMed] [Google Scholar]
- 133.Jiang S-T, Chiou Y-Y, Wang E, Chien Y-L, Ho H-H, Tsai F-J, Lin C-Y, Tsai S-P, Li H. Essential role of nephrocystin in photoreceptor intraflagellar transport in mouse. Human Molecular Genetics. 2009;18(9):1566–1577. doi: 10.1093/hmg/ddp068. [DOI] [PubMed] [Google Scholar]
- 134.Hichri H, Rendu J, Monnier N, Coutton C, Dorseuil O, Poussou RV, Baujat G, Blanchard A, Nobili F, Ranchin B, Remesy M, Salomon R, Satre V, Lunardi J. From Lowe Syndrome to Dent Disease: Correlations between Mutations of the OCRL1 Gene and Clinical and Biochemical Phenotypes. Human Mutation. 2011;32(4):379–388. doi: 10.1002/humu.21391. [DOI] [PubMed] [Google Scholar]
- 135.Rbaibi Y, Cui S, Mo D, Carattino M, Rohatgi R, Satlin LM, Szalinski CM, Swanhart LM, Folsch H, Hukriede NA, Weisz OA. OCRL1 Modulates Cilia Length in Renal Epithelial Cells. Traffic (Copenhagen, Denmark) 2012;13(9) doi: 10.1111/j.1600-0854.2012.01387.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Barinaga-Rementeria Ramirez I, Pietka GR, Jones D, Divecha N, Alia AC, Baraban SFL, Hurlstone A, Lowe M. Impaired Neural Development in a Zebrafish Model for Lowe Syndrome. doi: 10.1093/hmg/ddr608. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Madhivanan K, Mukherjee D, Aguilar RC. Lowe syndrome: Between primary cilia assembly and Rac1-mediated membrane remodeling. Communicative & integrative biology. 2012;5(6) doi: 10.4161/cib.21952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.van Rahden VA, Brand K, Najm J, Heeren J, Pfeffer SR, Braulke T, Kutsche K. The 5-phosphatase OCRL mediates retrograde transport of the mannose 6-phosphate receptor by regulating a Rac1-cofilin signalling module. Human Molecular Genetics. 2012;21(23):5019–5038. doi: 10.1093/hmg/dds343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Coon BG, Mukherjee D, Hanna CB, Riese DJ, II, Lowe M, Aguilar RC. Lowe syndrome patient fibroblasts display Ocrl1-specific cell migration defects that cannot be rescued by the homologous Inpp5b phosphatase. Human Molecular Genetics. 2009;18(23):4478–4491. doi: 10.1093/hmg/ddp407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Nishimura DY, Fath M, Mullins RF, Searby C, Andrews M, Davis R, Andorf JL, Mykytyn K, Swiderski RE, Yang BL, Carmi R, Stone EM, Sheffield VC. Bbs2-null mice have neurosensory deficits, a defect in social dominance, and retinopathy associated with mislocalization of rhodopsin. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(47):16588–16593. doi: 10.1073/pnas.0405496101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Mykytyn K, Mullins RF, Andrews M, Chiang AP, Swiderski RE, Yang BL, Braun T, Casavant T, Stone EM, Sheffield VC. Bardet-Biedl syndrome type 4 (BBS4)-null mice implicate Bbs4 in flagella formation but not global cilia assembly. Proceedings of the National Academy of Sciences of the United States of America. 2004;101(23):8664–8669. doi: 10.1073/pnas.0402354101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Loktev AV, Jackson PK. Neuropeptide Y Family Receptors Traffic via the Bardet-Biedl Syndrome Pathway to Signal in Neuronal Primary Cilia. Cell Reports. 2013;5(5):1316–1329. doi: 10.1016/j.celrep.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 143.Ferrante MI, Zullo A, Barra A, Bimonte S, Messaddeq N, Studer M, Dolle P, Franco B. Oral-facial-digital type I protein is required for primary cilia formation and left-right axis specification. Nature Genetics. 2006;38(1):112–117. doi: 10.1038/ng1684. [DOI] [PubMed] [Google Scholar]
- 144.Cantagrel V, Silhavy JL, Bielas SL, Swistun D, Marsh SE, Bertrand JY, Audollent S, Attie-Bitach T, Holden KR, Dobyns WB, Traver D, Al-Gazali L, Ali BR, Lindner TH, Caspary T, et al. Mutations in the cilia gene ARL13B lead to the classical form of Joubert syndrome. American Journal of Human Genetics. 2008;83(2):170–179. doi: 10.1016/j.ajhg.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Louie CM, Caridi G, Lopes VS, Brancati F, Kispert A, Lancaster MA, Schlossman AM, Otto EA, Leitges M, Groene H-J, Lopez I, Gudiseva HV, O’Toole JF, Vallespin E, Ayyagari R, et al. AHI1 is required for photoreceptor outer segment development and is a modifier for retinal degeneration in nephronophthisis. Nature Genetics. 2010;42(2):175–U117. doi: 10.1038/ng.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Westfall JE, Hoyt C, Liu Q, Hsiao Y-C, Pierce EA, Page-McCaw PS, Ferland RJ. Retinal Degeneration and Failure of Photoreceptor Outer Segment Formation in Mice with Targeted Deletion of the Joubert Syndrome Gene, Ahi1. Journal of Neuroscience. 2010;30(26):8759–8768. doi: 10.1523/JNEUROSCI.5229-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Simms RJ, Hynes AM, Eley L, Inglis D, Chaudhry B, Dawe HR, Sayer JA. Modelling a ciliopathy: Ahi1 knockdown in model systems reveals an essential role in brain, retinal, and renal development. Cellular and Molecular Life Sciences. 2012;69(6):993–1009. doi: 10.1007/s00018-011-0826-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Chaki M, Airik R, Ghosh AK, Giles RH, Chen R, Slaats GG, Wang H, Hurd TW, Zhou W, Cluckey A, Gee HY, Ramaswami G, Hong C-J, Hamilton BA, Cervenka I, et al. Exome Capture Reveals ZNF423 and CEP164 Mutations, Linking Renal Ciliopathies to DNA Damage Response Signaling. Cell. 2012;150(3):533–548. doi: 10.1016/j.cell.2012.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Lee JE, Silhavy JL, Zaki MS, Schroth J, Bielas SL, Marsh SE, Olvera J, Brancati F, Iannicelli M, Ikegami K, Schlossman AM, Merriman B, Attie-Bitach T, Logan CV, Glass IA, et al. CEP41 is mutated in Joubert syndrome and is required for tubulin glutamylation at the cilium. Nature Genetics. 2012;44(2):193–199. doi: 10.1038/ng.1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Tuz K, Bachmann-Gagescu R, O’Day DR, Hua K, Isabella CR, Phelps IG, Stolarski AE, O’Roak BJ, Dempsey JC, Lourenco C, Alswaid A, Boennemann CG, Medne L, Nampoothiri S, Stark Z, et al. Mutations in CSPP1 Cause Primary Cilia Abnormalities and Joubert Syndrome with or without Jeune Asphyxiating Thoracic Dystrophy. American Journal of Human Genetics. 2014;94(1):62–72. doi: 10.1016/j.ajhg.2013.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Fliegauf M, Horvath J, von Schnakenburg C, Olbrich H, Mueller D, Thumfart J, Schermer B, Pazour GJ, Neumann HPH, Zentgraf H, Benzing T, Omran H. Nephrocystin specifically localizes to the transition zone of renal and respiratory cilia and photoreceptor connecting cilia. Journal of the American Society of Nephrology. 2006;17(9):2424–2433. doi: 10.1681/ASN.2005121351. [DOI] [PubMed] [Google Scholar]
- 152.Graser S, Stierhof Y-D, Lavoie SB, Gassner OS, Lamla S, Le Clech M, Nigg EA. Cep164, a novel centriole appendage protein required for primary cilium formation. Journal of Cell Biology. 2007;179(2):321–330. doi: 10.1083/jcb.200707181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Luo N, Lu J, Sun Y. Evidence of a role of inositol polyphosphate 5-phosphatase INPP5E in cilia formation in zebrafish. Vision Research. 2012;75:98–107. doi: 10.1016/j.visres.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Bielas SL, Silhavy JL, Brancati F, Kisseleva MV, Al-Gazali L, Laszlo S, Bayoumi RA, Zaki MS, Abdel-Aleem A, Rosti RO, Kayserili H, Swistun D, Scott LC, Bertini E, Boltshauser E, et al. Mutations in INPP5E, encoding inositol polyphosphate-5-phosphatase E, link phosphatidyl inositol signaling to the ciliopathies. Nature Genetics. 2009;41(9):1032–U1108. doi: 10.1038/ng.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Jacoby M, Cox JJ, Gayral S, Hampshire DJ, Ayub M, Blockmans M, Pernot E, Kisseleva MV, Compere P, Schiffmann SN, Gergely F, Riley JH, Perez-Morga D, Woods CG, Schurmans S. INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nature Genetics. 2009;41(9):1027–U1102. doi: 10.1038/ng.427. [DOI] [PubMed] [Google Scholar]
- 156.Li Y, Zhang Q, Wei Q, Zhang Y, Ling K, Hu J. SUMOylation of the small GTPase ARL-13 promotes ciliary targeting of sensory receptors. Journal of Cell Biology. 2012;199(4):589–598. doi: 10.1083/jcb.201203150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liem KF, Jr, He M, Ocbina PJR, Anderson KV. Mouse Kif7/Costal2 is a cilia-associated protein that regulates Sonic hedgehog signaling. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(32):13377–13382. doi: 10.1073/pnas.0906944106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Vierkotten J, Dildrop R, Peters T, Wang B, Ruether U. Ftm is a novel basal body protein of cilia involved in Shh signalling. Development. 2007;134(14):2569–2577. doi: 10.1242/dev.003715. [DOI] [PubMed] [Google Scholar]
- 159.Town T, Breunig JJ, Sarkisian MR, Spilianakis C, Ayoub AE, Liu X, Ferrandino AF, Gallagher AR, Li MO, Rakic P, Flavell RA. The stumpy gene is required for mammalian ciliogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(8):2853–2858. doi: 10.1073/pnas.0712385105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Weatherbee SD, Niswander LA, Anderson KV. A mouse model for Meckel syndrome reveals Mks1 is required for ciliogenesis and Hedgehog signaling. Human Molecular Genetics. 2009;18(23):4565–4575. doi: 10.1093/hmg/ddp422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Dawe HR, Smith UM, Cullinane AR, Gerrelli D, Cox P, Badano JL, Blair-Reid S, Sriram N, Katsanis N, Attie-Bitach T, Afford SC, Copp AJ, Kelly DA, Gull K, Johnson CA. The Meckel-Gruber Syndrome proteins MKS1 and meckelin interact and are required for primary cilium formation. Human Molecular Genetics. 2007;16(2):173–186. doi: 10.1093/hmg/ddl459. [DOI] [PubMed] [Google Scholar]
- 162.Bachmann-Gagescu R, Phelps IG, Stearns G, Link BA, Brockerhoff SE, Moens CB, Doherty D. The ciliopathy gene cc2d2a controls zebrafish photoreceptor outer segment development through a role in Rab8-dependent vesicle trafficking. Human Molecular Genetics. 2011;20(20):4041–4055. doi: 10.1093/hmg/ddr332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Leitch CC, Zaghloul NA, Davis EE, Stoetzel C, Diaz-Font A, Rix S, Al-Fadhel M, Lewis RA, Eyaid W, Banin E, Dollfus H, Beales PL, Badano JL, Katsanis N. Hypomorphic mutations in syndromic encephalocele genes are associated with Bardet-Biedl syndrome. Nature Genetics. 2008;40(4):443–448. doi: 10.1038/ng.97. [DOI] [PubMed] [Google Scholar]
- 164.Mougou-Zerelli S, Thomas S, Szenker E, Audollent S, Elkhartoufi N, Babarit C, Romano S, Salomon R, Amiel J, Esculpavit C, Gonzales M, Escudier E, Leheup B, Loget P, Odent S, et al. CC2D2A Mutations in Meckel and Joubert Syndromes Indicate a Genotype-Phenotype Correlation. Human Mutation. 2009;30(11):1574–1582. doi: 10.1002/humu.21116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Iannicelli M, Brancati F, Mougou-Zerelli S, Mazzotta A, Thomas S, Elkhartoufi N, Travaglini L, Gomes C, Ardissino GL, Bertini E, Boltshauser E, Castorina P, D’Arrigo S, Fischetto R, Leroy B, et al. Novel TMEM67 Mutations and Genotype-phenotype Correlates in Meckelin-related Ciliopathies. Human Mutation. 2010;31(5):E1319–E1331. doi: 10.1002/humu.21239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Gorden NT, Arts HH, Parisi MA, Coene KLM, Letteboer SJF, van Beersum SEC, Mans DA, Hikida A, Eckert M, Knutzen D, Alswaid AF, Ozyurek H, Dibooglu S, Otto EA, Liu Y, et al. CC2D2A Is Mutated in Joubert Syndrome and Interacts with the Ciliopathy-Associated Basal Body Protein CEP290. American Journal of Human Genetics. 2008;83(5):559–571. doi: 10.1016/j.ajhg.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Stowe TR, Wilkinson CJ, Iqbal A, Stearns T. The centriolar satellite proteins Cep72 and Cep290 interact and are required for recruitment of BBS proteins to the cilium. Molecular Biology of the Cell. 2012;23(17):3322–3335. doi: 10.1091/mbc.E12-02-0134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Barbelanne M, Song J, Ahmadzai M, Tsang WY. Pathogenic NPHP5 mutations impair protein interaction with Cep290, a prerequisite for ciliogenesis. Human Molecular Genetics. 2013;22(12):2482–2494. doi: 10.1093/hmg/ddt100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Conduit SE, Dyson JM, Mitchell CA. Inositol polyphosphate 5-phosphatases; new players in the regulation of cilia and ciliopathies. FEBS letters. 2012;586(18) doi: 10.1016/j.febslet.2012.07.037. [DOI] [PubMed] [Google Scholar]
- 170.Dwyer ND, Adler CE, Crump JG, L’Etoile ND, Bargmann CI. Polarized dendritic transport and the AP-1 mu 1 clathrin adaptor UNC-101 localize odorant receptors to olfactory cilia. Neuron. 2001;31(2):277–287. doi: 10.1016/s0896-6273(01)00361-0. [DOI] [PubMed] [Google Scholar]
- 171.Berbari NF, Johnson AD, Lewis JS, Askwith CC, Mykytyn K. Identification of ciliary localization sequences within the third intracellular loop of G protein-coupled receptors. Molecular Biology of the Cell. 2008;19(4):1540–1547. doi: 10.1091/mbc.E07-09-0942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nagata A, Hamamoto A, Horikawa M, Yoshimura K, Takeda S, Saito Y. Characterization of ciliary targeting sequence of rat melanin-concentrating hormone receptor 1. General and Comparative Endocrinology. 2013;188:159–165. doi: 10.1016/j.ygcen.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 173.Ward HH, Brown-Glaberman U, Wang J, Morita Y, Alper SL, Bedrick EJ, Gattone VH, II, Deretic D, Wandinger-Ness A. A conserved signal and GTPase complex are required for the ciliary transport of polycystin-1. Molecular Biology of the Cell. 2011;22(18):3289–3305. doi: 10.1091/mbc.E11-01-0082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Follit JA, Li L, Vucica Y, Pazour GJ. The cytoplasmic tail of fibrocystin contains a ciliary targeting sequence. Journal of Cell Biology. 2010;188(1):21–28. doi: 10.1083/jcb.200910096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jenkins PM, Hurd TW, Zhang L, McEwen DP, Brown RL, Margolis B, Verhey KJ, Martens JR. Ciliary targeting of olfactory CNG channels requires the CNGB1b subunit and the kinesin-2 motor protein, KIF17. Current Biology. 2006;16(12):1211–1216. doi: 10.1016/j.cub.2006.04.034. [DOI] [PubMed] [Google Scholar]
- 176.Shiba D, Takamatsu T, Yokoyama T. Primary cilia of inv/inv mouse renal epithelial cells sense physiological fluid flow: Bending of primary cilia and Ca2+ influx. Cell Structure and Function. 2005;30(1–2):93–100. doi: 10.1247/csf.30.93. [DOI] [PubMed] [Google Scholar]
- 177.Watanabe D, Saijoh Y, Nonaka S, Sasaki G, Ikawa Y, Yokoyama T, Hamada H. The left-right determinant Inversin is a component of node monocilia and other 9+0 cilia. Development. 2003;130(9):1725–1734. doi: 10.1242/dev.00407. [DOI] [PubMed] [Google Scholar]
- 178.Bellavia S, Dahan K, Terryn S, Cosyns J-P, Devuyst O, Pirson Y. A homozygous mutation in INVS causing juvenile nephronophthisis with abnormal reactivity of the Wnt/beta-catenin pathway. Nephrology Dialysis Transplantation. 2010;25(12):4097–4102. doi: 10.1093/ndt/gfq519. [DOI] [PubMed] [Google Scholar]
- 179.Mergen M, Engel C, Mueller B, Follo M, Schaefer T, Jung M, Walz G. The nephronophthisis gene product NPHP2/Inversin interacts with Aurora A and interferes with HDAC6-mediated cilia disassembly. Nephrology Dialysis Transplantation. 2013;28(11):2744–2753. doi: 10.1093/ndt/gft316. [DOI] [PubMed] [Google Scholar]
- 180.Sugiyama N, Yokoyama T. Sustained cell proliferation of renal epithelial cells in mice with inv mutation. Genes to Cells. 2006;11(10):1213–1224. doi: 10.1111/j.1365-2443.2006.01011.x. [DOI] [PubMed] [Google Scholar]
- 181.Yokoyama T, Copeland NG, Jenkins NA, Montgomery CA, Elder FFB, Overbeek PA. REVERSAL OF LEFT-RIGHT ASYMMETRY - A SITUS-INVERSUS MUTATION. Science. 1993;260(5108):679–682. doi: 10.1126/science.8480178. [DOI] [PubMed] [Google Scholar]
- 182.Sugiyama N, Kohno M, Yokoyama T. Inhibition of the p38 MAPK pathway ameliorates renal fibrosis in an NPHP2 mouse model. Nephrology Dialysis Transplantation. 2012;27(4):1351–1358. doi: 10.1093/ndt/gfr550. [DOI] [PubMed] [Google Scholar]
- 183.Kim YH, Epting D, Slanchev K, Engel C, Walz G, Kramer-Zucker A. A Complex of BBS1 and NPHP7 Is Required for Cilia Motility in Zebrafish. Plos One. 2013;8(9) doi: 10.1371/journal.pone.0072549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Attanasio M, Uhlenhaut NH, Sousa VH, O’Toole JF, Otto E, Anlag K, Klugmann C, Treier A-C, Helou J, Sayer JA, Seelow D, Nurnberg G, Becker C, Chudley AE, Nurnberg P, et al. Loss of GLIS2 causes nephronophthisis in humans and mice by increased apoptosis and fibrosis. Nature Genetics. 2007;39(8):1018–1024. doi: 10.1038/ng2072. [DOI] [PubMed] [Google Scholar]
- 185.Manning DK, Sergeev M, van Heesbeen RG, Wong MD, Oh J-H, Liu Y, Henkelman RM, Drummond I, Shah JV, Beier DR. Loss of the Ciliary Kinase Nek8 Causes Left-Right Asymmetry Defects. Journal of the American Society of Nephrology. 2013;24(1):100–112. doi: 10.1681/ASN.2012050490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Sohara E, Luo Y, Zhang J, Manning DK, Beier DR, Zhou J. Nek8 regulates the expression and localization of polycystin-1 and polycystin-2. Journal of the American Society of Nephrology. 2008;19(3):469–476. doi: 10.1681/ASN.2006090985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Trapp ML, Galtseva A, Manning DK, Beier DR, Rosenblum ND, Quarmby LM. Defects in ciliary localization of Nek8 is associated with cystogenesis. Pediatric Nephrology. 2008;23(3):377–387. doi: 10.1007/s00467-007-0692-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Habbig S, Bartram MP, Saegmueller JG, Griessmann A, Franke M, Mueller R-U, Schwarz R, Hoehne M, Bergmann C, Tessmer C, Reinhardt HC, Burst V, Benzing T, Schermer B. The ciliopathy disease protein NPHP9 promotes nuclear delivery and activation of the oncogenic transcriptional regulator TAZ. Human Molecular Genetics. 2012;21(26):5528–5538. doi: 10.1093/hmg/dds408. [DOI] [PubMed] [Google Scholar]
- 189.Otto EA, Trapp ML, Schultheiss UT, Helou J, Quarmby LM, Hildebrandt F. NEK8 mutations affect ciliary and centrosomal localization and may cause nephronophthisis. Journal of the American Society of Nephrology. 2008;19(3):587–592. doi: 10.1681/ASN.2007040490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Won J, de Evsikova CM, Smith RS, Hicks WL, Edwards MM, Longo-Guess C, Li T, Naggert JK, Nishina PM. NPHP4 is necessary for normal photoreceptor ribbon synapse maintenance and outer segment formation, and for sperm development. Human Molecular Genetics. 2011;20(3):482–496. doi: 10.1093/hmg/ddq494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Borgal L, Habbig S, Hatzold J, Liebau MC, Dafinger C, Sacarea I, Hammerschmidt M, Benzing T, Schermer B. The Ciliary Protein Nephrocystin-4 Translocates the Canonical Wnt Regulator Jade-1 to the Nucleus to Negatively Regulate beta-Catenin Signaling. Journal of Biological Chemistry. 2012;287(30):25370–25380. doi: 10.1074/jbc.M112.385658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Ronquillo C, Frederick J, Baehr W. Nephrocystin-5 knockout mice recapitulate retina and kidney pathologies of Senior-Loken Syndrome. Investigative ophthalmology and Visual Science. 2013 [Google Scholar]
- 193.Kulaga HM, Leitch CC, Eichers ER, Badano JL, Lesemann A, Hoskins BE, Lupski JR, Beales PL, Reed RR, Katsanis N. Loss of BBS proteins causes anosmia in humans and defects in olfactory cilia structure and function in the mouse. Nature Genetics. 2004;36(9):994–998. doi: 10.1038/ng1418. [DOI] [PubMed] [Google Scholar]
- 194.Yen HJ, Tayeh MK, Stone EM, Sheffield VC, Slusarski DC. Bardet-Biedl syndrome genes are important in retrograde intracellular trafficking and Kupffer’s vesicle cilia function. Human Molecular Genetics. 2006;15(5):667–677. doi: 10.1093/hmg/ddi468. [DOI] [PubMed] [Google Scholar]
- 195.Tayeh MK, Yen H-J, Beck JS, Searby CC, Westfall TA, Griesbach H, Sheffield VC, Slusarski DC. Genetic interaction between Bardet-Biedl syndrome genes and implications for limb patterning. Human Molecular Genetics. 2008;17(13):1956–1967. doi: 10.1093/hmg/ddn093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Pretorius PR, Baye LM, Nishimura DY, Searby CC, Bugge K, Yang B, Mullins RF, Stone EM, Sheffield VC, Slusarski DC. Identification and Functional Analysis of the Vision-Specific BBS3 (ARL6) Long Isoform. Plos Genetics. 2010;6(3) doi: 10.1371/journal.pgen.1000884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Abd-El-Barr MM, Sykoudis K, Andrabi S, Eichers ER, Pennesi ME, Tan PL, Wilson JH, Katsanis N, Lupski JR, Wu SM. Impaired photoreceptor protein transport and synaptic transmission in a mouse model of Bardet-Biedl syndrome. Vision Research. 2007;47(27):3394–3407. doi: 10.1016/j.visres.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Mokrzan EM, Lewis JS, Mykytyn K. Differences in renal tubule primary cilia length in a mouse model of Bardet-Biedl syndrome. Nephron Experimental nephrology. 2007;106(3):e88–96. doi: 10.1159/000103021. [DOI] [PubMed] [Google Scholar]
- 199.Hernandez-Hernandez V, Pravincumar P, Diaz-Font A, May-Simera H, Jenkins D, Knight M, Beales PL. Bardet-Biedl syndrome proteins control the cilia length through regulation of actin polymerization. Human Molecular Genetics. 2013;22(19):3858–3868. doi: 10.1093/hmg/ddt241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Al-Hamed MH, van Lennep C, Hynes AM, Chrystal P, Eley L, Al-Fadhly F, El Sayed R, Simms RJ, Meyer B, Sayer JA. Functional modelling of a novel mutation in BBS5. Cilia. 2014;3(1):3–3. doi: 10.1186/2046-2530-3-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Fath MA, Mullins RF, Searby C, Nishimura DY, Wei J, Rahmouni K, Davis RE, Tayeh MK, Andrews M, Yang BL, Sigmund CD, Stone EM, Sheffield VC. Mkks-null mice have a phenotype resembling Bardet-Biedl syndrome. Human Molecular Genetics. 2005;14(9):1109–1118. doi: 10.1093/hmg/ddi123. [DOI] [PubMed] [Google Scholar]
- 202.Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S, Tan PL, Phillips HM, Leroux MR, Henderson DJ, Murdoch JN, et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nature Genetics. 2005;37(10):1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- 203.Seo S, Baye LM, Schulz NP, Beck JS, Zhang Q, Slusarski DC, Sheffield VC. BBS6, BBS10, and BBS12 form a complex with CCT/TRiC family chaperonins and mediate BBSome assembly. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(4):1488–1493. doi: 10.1073/pnas.0910268107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tadenev ALD, Kulaga HM, May-Simera HL, Kelley MW, Katsanis N, Reed RR. Loss of Bardet-Biedl syndrome protein-8 (BBS8) perturbs olfactory function, protein localization, and axon targeting. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(25):10320–10325. doi: 10.1073/pnas.1016531108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Tan PL, Barr T, Inglis PN, Mitsuma N, Huang SM, Garcia-Gonzalez MA, Bradley BA, Coforio S, Albrecht PJ, Watnick T, Germino GG, Beales PL, Caterina MJ, Leroux MR, Rice FL, et al. Loss of Bardet-Biedl syndrome proteins causes defects in peripheral sensory innervation and function. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(44):17524–17529. doi: 10.1073/pnas.0706618104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Veleri S, Bishop K, Nogare DED, English MA, Foskett TJ, Chitnis A, Sood R, Liu P, Swaroop A. Knockdown of Bardet-Biedl Syndrome Gene BBS9/PTHB1 Leads to Cilia Defects. Plos One. 2012;7(3) doi: 10.1371/journal.pone.0034389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Chiang AP, Beck JS, Yen HJ, Tayeh MK, Scheetz TE, Swiderski RE, Nishimura DY, Braun TA, Kim KYA, Huang J, Elbedour K, Carmi R, Slusarski DC, Casavant TL, Stone EM, et al. Homozygosity mapping with SNP arrays identifies TRIM32 an E3 ubiquitin ligase, as a Bardet-Biedl syndrome gene (BBS11) Proceedings of the National Academy of Sciences of the United States of America. 2006;103(16):6287–6292. doi: 10.1073/pnas.0600158103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Kudryashova E, Wu J, Havton LA, Spencer MJ. Deficiency of the E3 ubiquitin ligase TRIM32 in mice leads to a myopathy with a neurogenic component. Human Molecular Genetics. 2009;18(7):1353–1367. doi: 10.1093/hmg/ddp036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Cui C, Chatterjee B, Lozito TP, Zhang Z, Francis RJ, Yagi H, Swanhart LM, Sanker S, Francis D, Yu Q, San Agustin JT, Puligilla C, Chatterjee T, Tansey T, Liu X, et al. Wdpcp, a PCP Protein Required for Ciliogenesis, Regulates Directional Cell Migration and Cell Polarity by Direct Modulation of the Actin Cytoskeleton. Plos Biology. 2013;11(11) doi: 10.1371/journal.pbio.1001720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kim SK, Shindo A, Park TJ, Oh EC, Ghosh S, Gray RS, Lewis RA, Johnson CA, Attie-Bittach T, Katsanis N, Wallingford JB. Planar Cell Polarity Acts Through Septins to Control Collective Cell Movement and Ciliogenesis. Science. 2010;329(5997):1337–1340. doi: 10.1126/science.1191184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Airik R, Slaats G, Guo Z, Weiss A, Khan N, Ghosh A, Hurd T, Bekker-Jensen S, Schroder J, Elledge S, Andersen J, Kispert A, Castelli M, Boletta A, Giles R, et al. Renal-Retinal Ciliopathy Gene Sdccag8 Regulates DNA Damage Response Signaling. Journal of the American Society of Nephrology. 2014 doi: 10.1681/ASN.2013050565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Otto EA, Hurd TW, Airik R, Chaki M, Zhou W, Stoetzel C, Patil SB, Levy S, Ghosh AK, Murga-Zamalloa CA, van Reeuwijk J, Letteboer SJF, Sang L, Giles RH, Liu Q, et al. Candidate exome capture identifies mutation of SDCCAG8 as the cause of a retinal-renal ciliopathy. Nature Genetics. 2010;42(10):840. doi: 10.1038/ng.662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Lancaster MA, Louie CM, Silhavy JL, Sintasath L, DeCambre M, Nigam SK, Willert K, Gleeson JG. Impaired Wnt-beta-catenin signaling disrupts adult renal homeostasis and leads to cystic kidney ciliopathy. Nature Medicine. 2009;15(9):1046–U1101. doi: 10.1038/nm.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Lancaster MA, Gopal DJ, Kim J, Saleem SN, Silhavy JL, Louie CM, Thacker BE, Williams Y, Zaki MS, Gleeson JG. Defective Wnt-dependent cerebellar midline fusion in a mouse model of Joubert syndrome. Nature Medicine. 2011;17(6):726–U119. doi: 10.1038/nm.2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Cevik S, Hori Y, Kaplan OI, Kida K, Toivenon T, Foley-Fisher C, Cottell D, Katada T, Kontani K, Blacque OE. Joubert syndrome Arl13b functions at ciliary membranes and stabilizes protein transport in Caenorhabditis elegans. Journal of Cell Biology. 2010;188(6):953–969. doi: 10.1083/jcb.200908133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Singla V, Romaguera-Ros M, Manuel Garcia-Verdugo J, Reiter JF. Ofd1, a Human Disease Gene, Regulates the Length and Distal Structure of Centrioles. Developmental Cell. 2010;18(3):410–424. doi: 10.1016/j.devcel.2009.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Dafinger C, Liebau MC, Elsayed SM, Hellenbroich Y, Boltshauser E, Korenke GC, Fabretti F, Janecke AR, Ebermann I, Nurnberg G, Nurnberg P, Zentgraf H, Koerber F, Addicks K, Elsobky E, et al. Mutations in KIF7 link Joubert syndrome with Sonic Hedgehog signaling and microtubule dynamics. Journal of Clinical Investigation. 2011;121(7):2662–2667. doi: 10.1172/JCI43639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Cheung HO-L, Zhang X, Ribeiro A, Mo R, Makino S, Puviindran V, Lo Law KK, Briscoe J, Hui C-c. The Kinesin Protein Kif7 Is a Critical Regulator of Gli Transcription Factors in Mammalian Hedgehog Signaling. Science Signaling. 2009;2(76) doi: 10.1126/scisignal.2000405. [DOI] [PubMed] [Google Scholar]
- 219.Reiter JF, Skarnes WC. Tectonic, a novel regulator of the Hedgehog pathway required for both activation and inhibition. Genes & Development. 2006;20(1):22–27. doi: 10.1101/gad.1363606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Jiang S-T, Chiou Y-Y, Wang E, Lin H-K, Lee S-P, Lu H-Y, Wang C-KL, Tang M-J, Li H. Targeted disruption of Nphp1 causes male infertility due to defects in the later steps of sperm morphogenesis in mice. Human Molecular Genetics. 2008;17(21):3368–3379. doi: 10.1093/hmg/ddn231. [DOI] [PubMed] [Google Scholar]
- 221.Dowdle WE, Robinson JF, Kneist A, Salome Sirerol-Piquer M, Frints SGM, Corbit KC, Zaghloul NA, van Lijnschoten G, Mulders L, Verver DE, Zerres K, Reed RR, Attie-Bitach T, Johnson CA, Manuel Garcia-Verdugo J, et al. Disruption of a Ciliary B9 Protein Complex Causes Meckel Syndrome. American Journal of Human Genetics. 2011;89(1):94–110. doi: 10.1016/j.ajhg.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Bialas NJ, Inglis PN, Li C, Robinson JF, Parker JDK, Healey MP, Davis EE, Inglis CD, Toivonen T, Cottell DC, Blacque OE, Quarmby LM, Katsanis N, Leroux MR. Functional interactions between the ciliopathy-associated Meckel syndrome 1 (MKS1) protein and two novel MKS1-related (MKSR) proteins. Journal of Cell Science. 2009;122(5):611–624. doi: 10.1242/jcs.028621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Cui C, Chatterjee B, Francis D, Yu Q, SanAgustin JT, Francis R, Tansey T, Henry C, Wang B, Lemley B, Pazour GJ, Lo CW. Disruption of Mks1 localization to the mother centriole causes cilia defects and developmental malformations in Meckel-Gruber syndrome. Disease Models & Mechanisms. 2011;4(1):43–56. doi: 10.1242/dmm.006262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Valente EM, Logan CV, Mougou-Zerelli S, Lee JH, Silhavy JL, Brancati F, Iannicelli M, Travaglini L, Romani S, Illi B, Adams M, Szymanska K, Mazzotta A, Lee JE, Tolentino JC, et al. Mutations in TMEM216 perturb ciliogenesis and cause Joubert, Meckel and related syndromes. Nature Genetics. 2010;42(7) doi: 10.1038/ng.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Collin GB, Won J, Hicks WL, Cook SA, Nishina PM, Naggert JK. Meckelin Is Necessary for Photoreceptor Intraciliary Transport and Outer Segment Morphogenesis. Investigative Ophthalmology & Visual Science. 2012;53(2):967–974. doi: 10.1167/iovs.11-8766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lee JH, Silhavy JL, Lee JE, Al-Gazali L, Thomas S, Davis EE, Bielas SL, Hill KJ, Iannicelli M, Brancati F, Gabriel SB, Russ C, Logan CV, Sharif SM, Bennett CP, et al. Evolutionarily Assembled cis-Regulatory Module at a Human Ciliopathy Locus. Science. 2012;335(6071):966–969. doi: 10.1126/science.1213506. [DOI] [PMC free article] [PubMed] [Google Scholar]