Abstract
Tetanus toxin elicits spastic paralysis by cleaving VAMP-2 to inhibit neurotransmitter release in inhibitory neurons of the central nervous system. While the retrograde transport of TeNT from endosomes has been described, the initial steps that define how TeNT initiates trafficking to the retrograde system are undefined. The current study examines TeNT entry into primary cultured cortical neurons by TIRF microscopy. The initial association of TeNT with the plasma membrane was dependent upon ganglioside binding, but segregated from synaptophysin1 (Syp1), a synaptic vesicle (SV) protein. TeNT entry was unaffected by membrane depolarization and independent of SV cycling, while entry of the receptor-binding domain of TeNT (HCR/T) was stimulated by membrane depolarization and inhibited by blocking SV cycling. Measurement of the incidence of colocalization showed that TeNT segregated from Syp1, while HCR/T colocalized with Syp1. These studies show that while the HCR defines the initial association of TeNT with the cell membrane, regions outside the HCR define how TeNT enters neurons independent of SV cycling. This provides a basis for the unique entry of botulinum toxin and tetanus toxin into neurons.
Keywords: tetanus toxin, synaptic vesicles, endosomes
Introduction
The clostridial neurotoxins, botulinum neurotoxin (BoNT) and tetanus neurotoxin (TeNT) are the most toxic proteins for humans (1). TeNT is responsible for the paralytic disease tetanus, and BoNT is responsible for botulism. TeNT and BoNT share ~35% identity and ~65% similarity and overall structure-function properties (2). There are differences between TeNT and BoNT, notably clinical pathology and sequence diversity among isolated toxins. Intoxication with BoNT results in flaccid paralysis due to the inhibition of acetylcholine release of motor neurons, while TeNT intoxication yields a spastic paralysis due to inhibition of glycine release of inhibitory neurons (3). There is considerable genetic diversity amongst BoNT sequences, resulting the several serotypes (named A-H in order of discovery), while the genetic diversity of TeNT appears more limited (4).
Clostridial neurotoxins (CNT) are 150-kDa proteins expressed as a single chain and subsequently cleaved by bacterial or host proteases to form a di-chain protein linked by a disulfide bond (2). The 50-kDa light chain (LC) is a zinc-dependent protease, which cleaves neuron-specific SNARE proteins (5). SNARE proteins facilitate fusion of synaptic vesicles (SVs) to the plasma membrane. SNARE cleavage inhibits the fusion event is inhibited, resulting in a loss of neuronal signaling. The 100-kDa heavy chain (HC) contains two structurally distinct domains with separate functions. The translocation domain (HCT) undergoes a pH-induced insertion into the vesicle membrane that facilitates LC translocation from the lumen of the SV into the cell cytosol (6). The receptor-binding domain (HCR) contains binding sites for ganglioside and SV protein in BoNT: SV2 for BoNT serotypes A, D, E and F (7–11), or synaptotagmin for serotypes B and G (12, 13). The HCR of TeNT (HCR/T) contains binding sites for two gangliosides (14). Proposed protein receptors for TeNT include a GDI-anchored protein (15) and SV2 (16).
Differences in BoNT and TeNT pathology are not due to the specific substrate protein cleaved by the LC, but due to the unique trafficking of the toxins within neurons. BoNTs are endocytosed by motor neurons during recycling of SVs (17). Acidification of the SV lumen facilitates neurotransmitter uptake and the pH change results in translocation of the LC across the membrane and into the cytosol. Intoxication of the motor neuron results in loss of stimulatory signaling between neuron and muscle, yielding flaccid paralysis. In contrast, TeNT is endocytosed by motor neurons via a clathrin- and Rab5- dependent mechanism, and subsequently retrograde traffics to the soma in Rab7 enriched vesicles that have a neutral pH (18, 19). Neurotrophin receptors, cholera toxin and canine adenovirus-2 also use this retrograde pathway (19–21). Upon transcytosis from motor neuron into an inhibitory interneuron, the LC is delivered into the cytosol and cleaves VAMP2, which inhibits SV cycling. The resulting loss of this inhibitory signaling to the motor neuron leads to the spastic paralysis characteristic of tetanus.
Intoxication of an animal with higher concentrations of CNT results in aberrant paralysis, with TeNT causing a flaccid paralysis (22), and BoNT serotype A (BoNT/A) acting on the central nervous system (23, 24). Therefore, the trafficking events of BoNTs and TeNT that lead to flaccid and spastic paralysis are not exclusive. The pathologies associated with tetanus and botulism have been attributed to receptor binding and intracellular trafficking of the HCR domains to the respective toxins (17). As a single molecule, HCR/T is capable of retrograde trafficking within the motor neuron (19, 25). However, recent work indicates that in mice the HCR domain of TeNT (HCR/T) is not sufficient to cause retrograde trafficking of a BoNT-TeNT fusion protein (26). While there are several caveats to assigning the cellular basis for the observed trafficking patterns of the fusion proteins, these observations bring into question if the HCR domain of TeNT is sufficient to traffic the toxin to physiological targets beyond the motor neuron.
Endocytosis of SV proteins from the plasma membrane involves multiple pathways (27), including clathrin-mediated endocytosis from the plasma membrane, kiss-and-run from the incomplete collapse of a SV, and bulk endocytosis. These pathways regenerate SVs within seconds of exocytosis. Whether BoNTs utilize all or some of these pathways is unclear. We recently described an atoxic TeNT, TeNT(RY), in the Neuro-2a cell model (28) and reported that TeNT(RY) and HCR/T shared similar ganglioside-binding profiles, but only partially colocalized within intracellular vesicles. The current study examines the entry of TeNT(RY) into cultured cortical neurons in response to membrane depolarization and synaptic vesicle cycling. We report that TeNT(RY) trafficking was primarily via a membrane depolarization-independent and SV-cycling independent mechanism, which differed from HCR/T, which responded to membrane depolarization and SV cycling. This provides direct evidence for the contribution of region(s) outside the HCR in defining how TeNT enters polarized neurons.
Results
Unique entry of HCR/T and TeNT(RY) into cortical neurons
In this study, the intensities of cell-associated proteins were normalized to synaptophysin1, a SV marker protein. Pearson’s coefficients (29) established the incidence of colocalization (IOC) between two proteins and have an experimental range of ~0.1 (segregated) to 0.8 (colocalized), as previously determined (30). In the current study, Pearson’s coefficients describe colocalization between two proteins: 0.1 to 0.3 (segregated); 0.3 to 0.6 (partial); and > 0.6 (colocalized). The binding and entry of TeNT(RY) and HCR/T were measured in primary cortical neurons, which are enriched for complex gangliosides, exhibit Ca2+-dependent SV cycling, and can be imaged by total internal reflection fluorescence (TIRF) microscopy.
At 4°C, TeNT(RY) and HCR/T binding to cortical neurons was proportional to dose (data not shown). Assessment of binding (Figure 1) showed that ~15% more TeNT(RY) was cell associated than HCR/T. At 4°C, there were similar amounts of partial colocalization of HCR/T or TeNT(RY) with Syp1. Thus, in the absence of membrane fluidity, HCR/T and TeNT(RY) have similar associations with the plasma membrane. At 37°C, TeNT(RY) and HCR/T entry into cortical neurons was measured after 5 min in depolarizing (High K buffer) or non-depolarizing (Low K buffer) conditions. Cell association of TeNT(RY) and HCR/T increased with concentration (Figure 2A), and the relative binding of TeNT(RY) and HCR/T were similar, within 10%. The absolute amount of cell associated TeNT(RY) and HCR/T changed only fractionally with membrane depolarization.
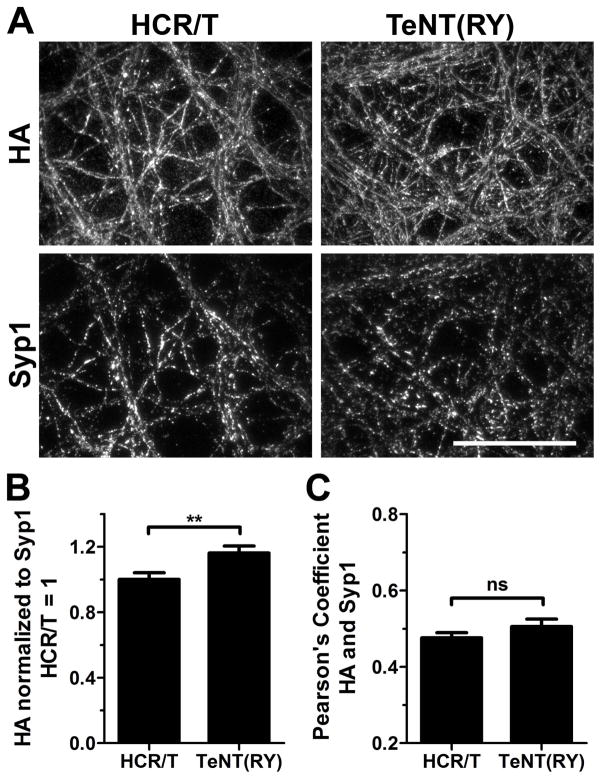
Figure 1. HCR/T and TeNT(RY) bind cortical neurons with similar efficiency.
Cortical neurons were cooled with ice-cold DPBS and then incubated with 40 nM HA-HCR/T or TeNT(RY) for 30 min in Low K buffer on ice. Neurons were washed, fixed and processed for immunofluorescence. Neurons were stained for synaptophysin1 (Syp1) and for the HA epitope to detect HCR/T and TeNT(RY). (A) Representative images are shown. (B) Intensity of HA staining was quantified and normalized to the Syp1 intensity for each field. HCR/T signal was set to 1. (C) The colocalization of each protein (HA) to Syp1 was quantified using Pearson’s coefficients. Statistics were performed using Student’s t-Test. Scale bar is 40 μm.
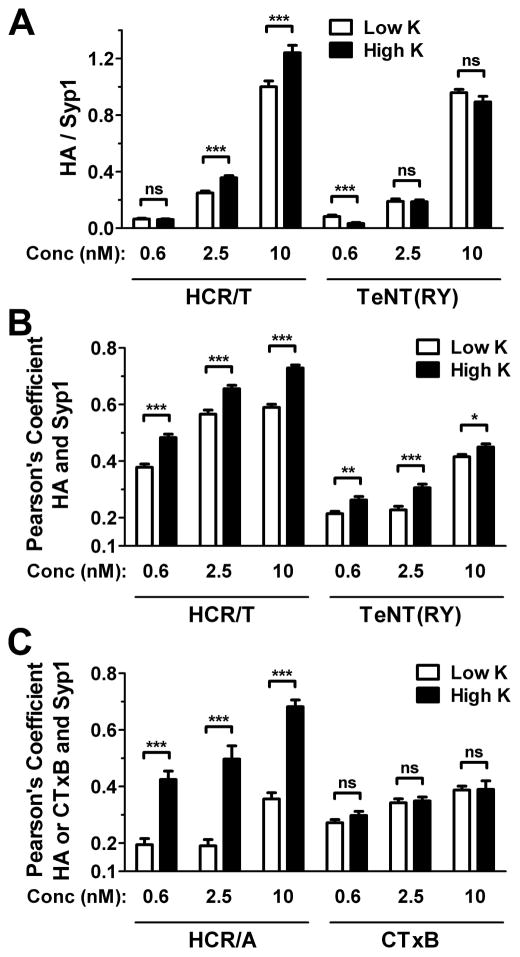
Figure 2. Differential entry of HCR/T and TeNT(RY) into primary cortical neurons.
Cortical neurons were incubated with 0.6, 2.5 or 10 nM HA-HCR/T and TeNT(RY) (A and B), or HA-HCR/A and Alexa647-CTxB (C), for 5 min at 37°C in either High K (filled bars) or Low K (open bars). Neurons were processed for immunofluorescence and probed for synaptophysin (Syp1) and the HA epitope to detect HCR/T and TeNT(RY) (A and B), and HCR/A (C). CTxB was detected by Alexa fluorescence (C). Intensity (A) and colocalization of HA to Syp1 (B and C) were quantified. HA intensity was normalized to Syp1, with HCR/T signal at 10 nM in Low K was set to 1. Data are from 12 random fields from at least three independent replicates. Statistics were performed using Student’s t-Test.
Association with SVs was established by measuring the IOC of TeNT(RY) or HCR/T with Syp1, a resident synaptic vesicle protein (Figure 2B). The receptor binding domain of BoNT/A (HCR/A), which utilizes SVs to enter neurons, served as a SV-dependent control. The B subunit of cholera toxin (CTxB), which utilizes GM1 as a receptor, served as a SV-independent control. As expected, upon incubation the IOC of HCR/A with Syp1 increased upon membrane depolarization, while CTxB did not show a significant increase in IOC with Syp1 upon membrane depolarization (Figure 2C). The overall association of HCR/T with SVs was greater than TeNT(RY) upon incubation in Low K buffer and upon membrane depolarization with High K buffer. At lower concentrations, TeNT(RY) segregated from SVs even upon membrane depolarization, though at all concentrations there was an increase in IOC in High K buffer. In contrast, HCR/T partially colocalized with SVs in low K buffer, and colocalized with SVs upon membrane depolarization. These findings showed that TeNT(RY) entry was primarily segregated from SVs, while HCR/T entry was associated with SVs.
Limited colocalization of HCR/T and TeNT(RY) in cortical neurons
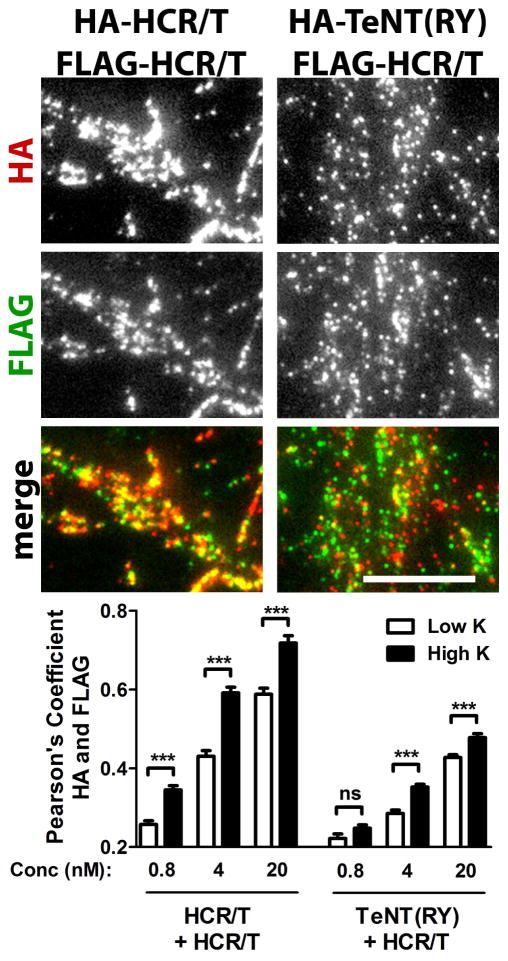
The difference in colocalization with Syp1 suggested that HCR/T and TeNT(RY) utilize different entry pathways, which was tested. Cortical neurons were incubated with FLAG-HCR/T and HA-HCR/T or FLAG-HCR/T and HA-TeNT(RY) (Figure 3). HCR/T molecules with different epitopes serve as controls for maximum colocalization expected from separate molecules with the same binding properties. Overall, the IOCs were greater for the different epitope-tagged forms of HCR/T than for TeNT(RY) and HCR/T. At the lowest concentration, the molecules segregated from each other, due to binding randomly to an excess of ganglioside receptor. At the highest concentration, the different epitope-tagged forms of HCR/T were colocalized upon incubation in both Low K buffer and High K buffer, while TeNT(RY) and HCR/T showed only partial colocalization. This is consistent with differential trafficking of TeNT(RY) and HCR/T in neurons.
Figure 3. Limited colocalization of HCR/T and TeNT(RY) in cortical neurons.
Cortical neurons were incubated with HA-HCR/T and FLAG-HCR/T or HA-TeNT(RY) and FLAG-HCR/T, for 2 min in High or Low K buffer. Neurons were probed for HA (red) and FLAG (green). Representative images are shown for High K conditions at 4 nM (above). Colocalization of HA to FLAG was quantified (below). Statistics were performed using Student’s t-Test. Scale bar is 10 μm.
Unique internalization of TeNT(RY) and HCR/T in cortical neurons
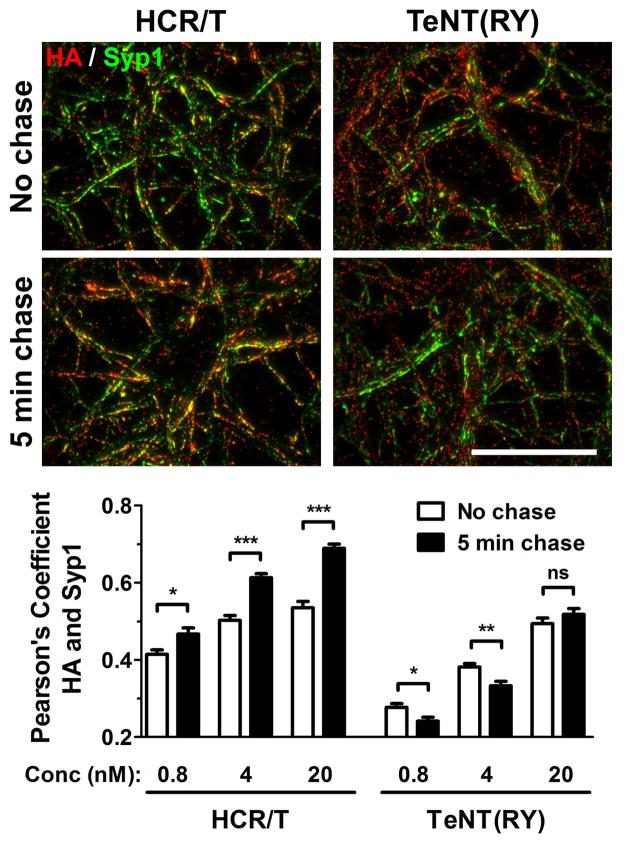
To assess movement on the cell membrane, the IOC of TeNT(RY) and HCR/T with Syp1 was examined in a pulse-chase experiment. Prior to and after the chase, the overall IOC of TeNT(RY) with Syp1 was lower than the IOC of HCR/T with Syp1 (Figure 4). Following the chase, the IOC of TeNT(RY) with Syp1 decreased at the lower concentrations, while the IOC of HCR/T with Syp1 increased. The decrease in the IOC with Syp1 during the chase showed that TeNT(RY) entry segregated from SVs and was more restricted than HCR/T, which showed an increased association with SVs over the chase.
Figure 4. Unique entry of TeNT(RY) and HCR/T in cortical neurons.
Cortical neurons were incubated with 0.8, 4 or 20 nM HA-HCR/T or TeNT(RY) for 2 min at 37°C in Low K. Neurons were washed in DPBS and either immediately fixed (no chase, open bars) or incubated for 5 min in Low K and then fixed (5 min chase, filled bars). Neurons were probed for synaptophysin1 (Syp1, green) and for HA epitope (red). Representative images of the 4 nM HCR/T or TeNT(RY) are shown (above). Colocalization of HA to Syp1 was quantified (below). Scale bar is 40 μm.
TeNT(RY) enters neurons independent of synaptic vesicle cycling
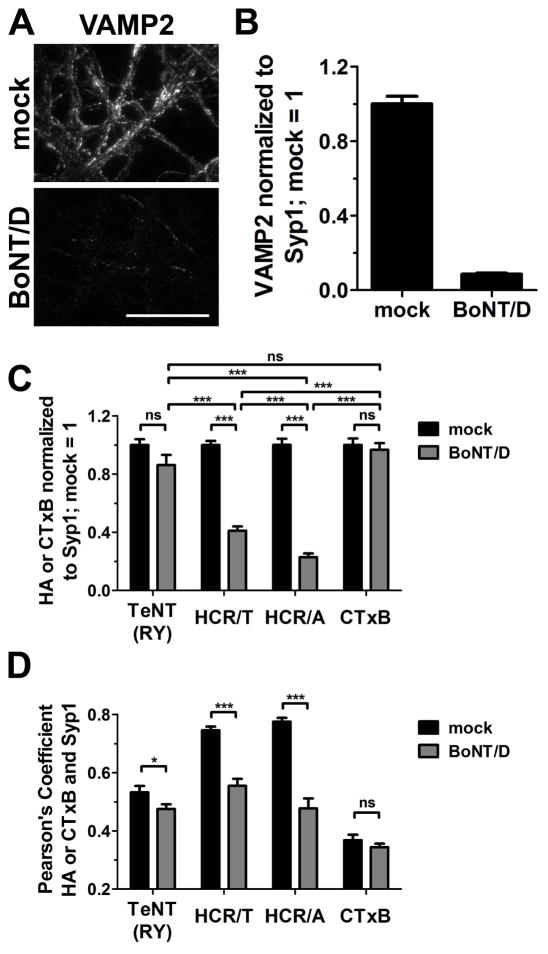
A direct assessment of SV-dependent entry was made by measuring the entry of TeNT(RY) into neurons that were inhibited for SV cycling. Cortical neurons were incubated overnight with BoNT/D, which reduced the steady state level of VAMP2 by ~ 90% (Figure 5). Controls showed that HCR/A had a reduced capacity to enter BoNT/D-treated neurons, while CTxB entry was independent of BoNT/D treatment. TeNT(RY) entered cortical neurons independent of BoNT/D treatment, while HCR/T entry was partially inhibited in BoNT/D-treated neurons. In addition, the change of IOC of TeNT(RY) with Syp1, with and without BoNT/D treatment, appeared more similar to the IOC of CTxB with Syp1 than of either HCR/A or HCR/T with Syp1. The data indicate that TeNT(RY) entry is largely independent of SV cycling.
Figure 5. TeNT(RY) enters neurons independent of SV cycling.
Cortical neurons were mock treated or intoxicated with BoNT/D for 20 hr. Neurons were incubated with 4 nM TeNT(RY), HCR/T, HCR/A or Alexa-labeled Cholera toxin B (CTxB) for 5 min at 37°C in High K. Cells were washed, fixed and processed for immunofluorescence. Representative images of VAMP2 staining in mock and BoNT/D intoxicated neurons are shown (A) and the intensity was quantified (B), with mock-treated neurons set to 1. Intensity (C) and colocalization to Syp1 (D) of TeNT(RY), HCR/T, HCR/A or CTxB were quantified. The intensity of each protein in mock-treated neurons was set to 1. Data was compiled from 12 fields from three independent replicates. Statistical comparisons were performed using a one-way ANOVA with Bonferroni post-test in (C) and Student’s t-test in (D). Scale bar is 40 μm.
Discussion
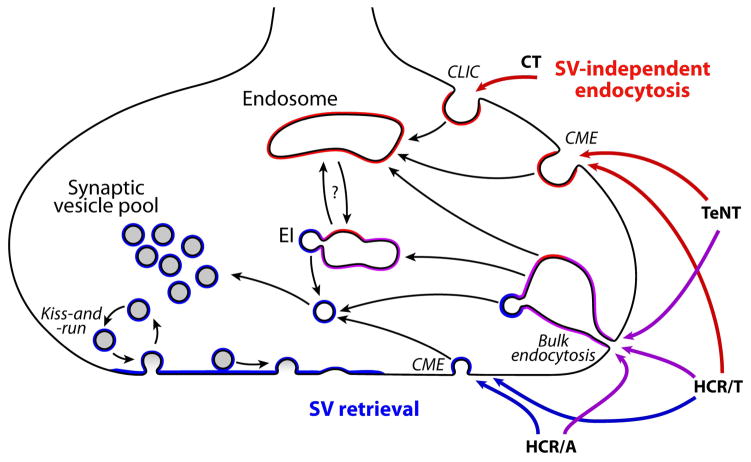
From the data presented, a model for the entry of TeNT into neurons is proposed (Figure 6). Initial affinities of TeNT and HCR/T for primary cortical neurons are similar and are independent of Syp1, a SV marker, consistent with the association to dual gangliosides (14). At 37°C, TeNT associates with neurons independent of SVs, while HCR/T binding is heterologous, either entering with SVs or entering independent of SVs. The data are consistent with TeNT entering neurons primarily via clathrin-mediated endocytosis (CME). Since the IOC between TeNT(RY) and Syp1 increased fractionally upon membrane depolarization, TeNT may also enter neurons via bulk phase endocytosis during non-specific uptake. This is consistent with higher concentrations of TeNT having flaccid-like paralytic symptoms (22). The differential entry of TeNT and HCR/T implicate a domain outside the HCR, either the HCT or LC, as contributing to the restricted trafficking of TeNT to CME via protein or lipid interactions. HCR/A and CTxB were functional controls establishing the parameters for entry via SV-cycling and SV-independent endocytosis, respectively.
Figure 6. Model for the entry of BoNT and TeNT into cortical neurons.
Synaptic vesicles are recycled by multiple mechanisms, including kiss-and-run, clathrin-mediated endocytosis (CME), and bulk endocytosis. Bulk endocytosis of SV protein-enriched membrane forms endocytic intermediates (EI) which subsequently leads to formation of SVs. Cholera toxin (CT) enters via clathrin-independent carriers (CLIC) into endosomes which will later retrograde traffic to the endoplasmic reticulum. HCR/A enters SVs during retrieval of SV proteins from the membrane. HCR/T enters SV-positive vesicles with and without depolarization via SV retrieval mechanisms, and enters by endocytosis. TeNT enters primarily via CME, and by bulk endocytosis during depolarization, largely segregated away from SVs. Vesicles and membranes positive for SV proteins are denoted in blue, endocytic vesicles are denoted in red, and vesicles containing both SV and endocytic proteins are denoted in purple.
Endocytosis of SV proteins from the plasma membrane involves multiple pathways, and is not completely defined (27). SV cycling is a specialized series of events capable of regenerating SVs within seconds of exocytosis. Clathrin-mediated endocytosis from the plasma membrane is a major contributor to the recycling of SV proteins, and retrieves complete SVs ready to undergo acidification and neurotransmitter uptake. The most controversial mechanism of recycling, kiss-and-run, involves incomplete collapse of the SV into the plasma membrane, and therefore permits rapid recycling of an intact SV. The kiss-and-run fusion pore is small enough to keep a 15 nm quantum dot trapped (31), and has been measured as 2.3 nm (32). The combined size limitation and selective nature of the fusion pore (33) make kiss-and-run an unlikely mechanism to allow CNT entry. The least specific mechanism of recycling occurs under conditions of high activity, such as under membrane depolarization, in which bulk endocytosis retrieves large amounts of SV protein-enriched plasma membrane into cisternae, which then from SVs. TeNT may utilize bulk endocytosis to enter the SV cycling pathway.
Entry of TeNT via SV cycling has been observed in several neuronal cell types under different conditions. Matteoli et al. observed uptake of 20 nM labeled TeNT into hippocampal neurons only upon depolarization using a 5 minute assay (34). Under these conditions, gold-labeled TeNT were observed within vesicles consistent with the size of SVs. Yeh et al. similarly observed uptake of 50 nM HCR/T by hippocampal and spinal cord neurons under depolarizing conditions, with a ~4-fold decrease in signal under non-depolarizing conditions (16). In cultured cortical neurons, 40 nM HCR/T entered by a SV-dependent pathway, which colocalized with HCR/A, and by a SV-independent pathway (30). The data presented here support HCR/T entry via SVs, but found that TeNT(RY) associated only minimally with SVs, and only with membrane depolarization. Unexpectedly, HCR/T colocalized with SV markers in non-depolarized conditions, indicating that HCR/T entered SVs via an endocytic route independent of active SV cycling.
The molecular basis of dual ganglioside binding by TeNT has been described (35). Similar to other clostridial neurotoxins, TeNT contains a conserved SxWY motif for binding to the Gal-GalNAc within the ganglioside backbone. On the same face that BoNT/B binds synaptotagmin (36), an arginine in TeNT mediates binding to the disialic acid in b-series gangliosides. Since the ganglioside binding profiles of TeNT(RY) and HCR/T are similar (28), an interaction with the neuron mediated solely by gangliosides would have resulted in similar entry properties, which was not observed. Yeh et al. (16) proposed SV2 as the protein receptor for TeNT, which remains debated within the field (28). The existence and identity of a protein receptor for TeNT remains hypothetical. However, the data presented here implicates differential entry of TeNT and HCR/T. This supports the existence of either an additional interaction mediated by the HCT or LC domains, or an interaction within the HCR stabilized by the HCT or LC domains. Whether this interaction is with a protein, sugar or lipid remains to be determined.
TeNT must enter into neurons via more than one pathway, which allows passage through peripheral motor neurons into the CNS and subsequently into inhibitory interneurons to deliver LC into the cytosol (37). TeNT entry and retrograde trafficking into motor neurons has been characterized using HCR/T, and is dependent on clathrin, Rab5 and Rab7 (19). vATPase is excluded from the retrograde vesicles (18), which provides a pathway through the motor neuron without experiencing an acidified environment and translocation of the LC. Subsequent TeNT intoxication of inhibitory interneurons requires entry into a vesicle, which will undergo acidification. A further complication to the trafficking of TeNT is the existence of two of more cellular fates for TeNT in a single neuron supported by toxicity experiments. In mice, a high amount of TeNT caused a flaccid paralysis similar to botulism (22). The high concentration of TeNT required to cause flaccid paralysis suggests the pathway leading to a BoNT-like paralysis is a low-affinity pathway. Conversely, recent studies have demonstrated the retrograde trafficking of BoNT/A via the optic nerve in rats (24, 38), and the retrograde trafficking of BoNT serotypes A and E in cultured rat motor neurons (38). HCR/T, HCR/A and CTxB retrograde traffic in cultured motor neurons (19, 20, 38). Rather than utilizing exclusive pathways, the trafficking of BoNT and TeNT appears based upon high- and low- affinity interactions, also observed in the current study. CTxB, Trk receptors, and p75NTR can retrograde traffic with HCR/T in the neurotrophin signaling endosome, having merged from clathrin-dependent and -independent pathways that merge in early endosomes, and subsequently formed fast transport carriers (20). In the current study, the multiple entry capabilities of HCR/T provides support for HCR/T, like TeNT, to enter independent from SVs, and sort into endosomes which form then retrograde carriers, though less efficiently.
A recent study found that efficient retrograde transport of BoNT-TeNT chimeras occurred only in toxins containing the entire TeNT molecule (26). Early experiments in the retrograde trafficking of TeNT established that Fragments BIIb and C (the HCR domain) are 50–100 times less efficient in ascending from the motor neuron (39). These observations support the existence of trafficking information contained outside the HCR domain of TeNT. This is consistent with previous studies in Neuro-2a cells and the present study in cortical neurons, showing the differential trafficking of TeNT(RY) and HCR/T (28).
Thus, TeNT and BoNT are both capable of retrograde trafficking and intoxicating motor neurons, though each via different mechanisms and with different affinities (38, 40, 41). This implicates a scaling model for the preferred entry of CNTs based upon binding preference and receptor affinity: BoNTs show more restricted trafficking into SVs, whereas TeNT shows preferred trafficking via SV-independent pathways. The restriction of TeNT from SVs appears based on regions outside the HCR domain, as HCR/T readily enters SVs. Understanding how TeNT identifies unique entry pathways into the central nervous system may provide a basis to develop new therapeutics against neurological diseases.
Methods
Production and purification of recombinant proteins
Hemagglutinin (HA)-tagged and FLAG-tagged proteins were produced and purified as previously described (28). Proteins produced include heavy chain receptor (HCR) binding domains of tetanus (HCR/T, residues 865–1315) and botulinum neurotoxin serotype A2 (C. botulinum str. A2 Kyoto F, HCR/A, residues 870–1296), His-FLAG-HA-Strep tagged nontoxic TeNT(RY) and His-HA-Strep tagged nontoxic TeNT(RY).
Primary rat neurons
Glass-bottomed 24-well cell culture plates (MatTek) were coated overnight at RT with 0.5 mL of 50 μg/mL poly-D-lysine (Sigma) and 3 μg/mL of mouse laminin (Sigma). Wells were washed twice with cell-culture grade water and incubated with Neurobasal media (Life Technologies) for 1 h at 37°C before neurons were plated. Rat E18 cortical neurons (BrainBits, LLC) were dissociated and plated as instructed by the company. Neurons were cultured in Neurobasal media supplemented with B27 (Life Technologies), 0.56 mM Glutamax (Life Technologies) and 0.1 mg/mL Primocin (Invivogen). Neurons were fed on day 7 in culture by replacing half the media with fresh media. Experiments with neurons were performed between days 10 and 14 in culture.
Neuron experiments
For binding studies, cortical neurons were washed (2X) with ice-cold Dulbecco’s phosphate-buffered saline (DPBS) and cooled for 5 min on ice. Neurons were incubated with 40 nM HCR/T or TeNT(RY) in ice-cold Low K buffer for 30 min. Neurons were washed twice in ice-cold DPBS and processed for immunofluorescence. For entry studies, neurons were washed twice in warm DPBS and incubated with 0.16–40 nM HCR/T, TeNT(RY), HCR/A, or Alexa647-labeled CTxB in Low K buffer (15 mM HEPES, 145 mM NaCl, 5.6 mM KCl, 2.2 mM CaCl2, 0.5 mM MgCl2, pH 7.4) or High K buffer (15 mM HEPES, 95 mM NaCl, 56 mM KCl, 2.2 mM CaCl2, 0.5 mM MgCl2, pH 7.4) at 37°C for 2 or 5 min. Neurons were washed twice in warm DPBS and processed for immunofluorescence. Where indicated, 1 nM BoNT/D was added to the neurons overnight prior to the entry experiment. BoNT/D was provided by Eric A. Johnson from University of Wisconsin-Madison (Madison, WI).
Immunofluorescence
Cells were fixed in 4% (w/v) paraformaldehyde in DPBS for 15 min at RT, washed in DPBS, permeabilized with 0.1% (v/v) TritonX-100 in 4% (v/v) formaldehyde in DPBS for 15 min at RT, washed in DPBS, incubated with 150 mM glycine in DPBS for 15 min at RT, and washed in DPBS. Cells were blocked in a blocking solution (10% FBS, 25 mg/mL gelatin from cold water fish skin, 0.1% TritonX-100 and 0.05% Tween-20) for 1 h at RT and incubated with primary antibodies in an antibody solution (5% FBS, 10 mg/mL gelatin from cold water fish skin, 0.1% TritonX-100 and 0.05% Tween-20) overnight at 4°C. Recombinant proteins were probed with rat anti-HA antibody (1:2000, Roche) or mouse anti-FLAG antibody (1:10,000, Sigma). Cellular proteins were probed with guinea pig anti-synaptophysin antibody (1:2000, SySy) and mouse anti-VAMP2 (1:2000, SySy). Cells were washed three times for 5 min in DPBS and incubated with Alexa-labeled secondary antibodies (1:1000 dilution, Molecular Probes) in antibody solution for 1 h at RT. Cells were washed four times for 5 min in DPBS. Cells were fixed with 4% paraformaldehyde in DPBS for 10 min at RT and washed in DPBS. Mounting solution Citifluor AF3 (Citifluor, Ltd) was added to neuron samples in glass-bottomed plates for storage and TIRF imaging. Images were captured using a Nikon TE2000 total internal reflection fluorescence (TIRF) microscope equipped with a Nikon Apo TIRF 100X/1.49 NA oil objective, using a Photometrics CoolSnap HQ2 camera and Nikon NIS Elements AR software.
Botulinum neurotoxin production
Botulinum neurotoxin type D was isolated from C. botulinum type D strain 1873. The 150 kDa protein was purified using biochemical methods similar to those previously described for isolation of other BoNT serotypes with minor modifications (42, 43). The specific activity was determined in mice as previously described in Malizio et al (40). Specific activity in mice was 1.1 x 108 LD50 Units/mg.
Data analysis
Image analyses of intensity and Pearson’s coefficients were performed using ImageJ 1.46r. Graphs were created using GraphPad Prism 5 and figures were compiled using Adobe Photoshop CS3.
Synopsis.
Total internal reflection fluorescence microscopy examined tetanus neurotoxin (TeNT) and receptor binding domain of TeNT (HCR/T) entry into rat primary cortical neurons. TeNT association was independent of membrane depolarization, unaffected by blocking synaptic vesicle (SV) cycling, and segregated from SVs. In contrast, HCR/T association was increased with membrane depolarization, inhibited by blocking SV cycling, and colocalized with SVs. Thus, TeNT enters neurons independent of synaptic vesicles and TeNT trafficking is directed by regions outside the HCR domain.
Acknowledgments
This study was supported by NIH R01 AI-031062 and NIH U54-AI-057153. JTB and EAJ are members of the Great Lakes Regional Center of Excellence (GLRCE) and acknowledge partial support by the GLRCE for these studies.
References
- 1.Gill DM. Bacterial toxins: a table of lethal amounts. Microbiol Mol Biol Rev. 1982;46(1):86–94. doi: 10.1128/mr.46.1.86-94.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lacy DB, Stevens RC. Sequence homology and structural analysis of the clostridial neurotoxins. J Mol Biol. 1999;291(5):1091–1104. doi: 10.1006/jmbi.1999.2945. [DOI] [PubMed] [Google Scholar]
- 3.Hatheway CL. Toxigenic clostridia. Clin Microbiol Rev. 1990;3(1):66–98. doi: 10.1128/cmr.3.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hill KK, Smith TJ, Helma CH, Ticknor LO, Foley BT, Svensson RT, Brown JL, Johnson EA, Smith LA, Okinaka RT, Jackson PJ, Marks JD. Genetic Diversity among Botulinum Neurotoxin-Producing Clostridial Strains. J Bacteriol. 2007;189(3):818–832. doi: 10.1128/JB.01180-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schiavo G, Rossetto O, Benfenati F, Poulain B, Montecucco C. Tetanus and botulinum neurotoxins are zinc proteases specific for components of the neuroexocytosis apparatus. Ann N Y Acad Sci. 1994;710:65–75. doi: 10.1111/j.1749-6632.1994.tb26614.x. [DOI] [PubMed] [Google Scholar]
- 6.Montal M. Botulinum neurotoxin: a marvel of protein design. Annu Rev Biochem. 2010;79:591–617. doi: 10.1146/annurev.biochem.051908.125345. [DOI] [PubMed] [Google Scholar]
- 7.Dong M, Yeh F, Tepp WH, Dean C, Johnson EA, Janz R, Chapman ER. SV2 Is the Protein Receptor for Botulinum Neurotoxin A. Science. 2006;312(5773):592–596. doi: 10.1126/science.1123654. [DOI] [PubMed] [Google Scholar]
- 8.Peng L, Tepp WH, Johnson EA, Dong M. Botulinum neurotoxin D uses synaptic vesicle protein SV2 and gangliosides as receptors. PLoS Pathog. 2011;7(3):e1002008. doi: 10.1371/journal.ppat.1002008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dong M, Liu H, Tepp WH, Johnson EA, Janz R, Chapman ER. Glycosylated SV2A and SV2B mediate the entry of botulinum neurotoxin E into neurons. Mol Biol Cell. 2008;19(12):5226–5237. doi: 10.1091/mbc.E08-07-0765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rummel A, Hafner K, Mahrhold S, Darashchonak N, Holt M, Jahn R, Beermann S, Karnath T, Bigalke H, Binz T. Botulinum neurotoxins C, E and F bind gangliosides via a conserved binding site prior to stimulation-dependent uptake with botulinum neurotoxin F utilising the three isoforms of SV2 as second receptor. J Neurochem. 2009;110(6):1942–1954. doi: 10.1111/j.1471-4159.2009.06298.x. [DOI] [PubMed] [Google Scholar]
- 11.Fu Z, Chen C, Barbieri JT, Kim JJ, Baldwin MR. Glycosylated SV2 and gangliosides as dual receptors for botulinum neurotoxin serotype F. Biochemistry. 2009;48(24):5631–5641. doi: 10.1021/bi9002138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishiki T, Kamata Y, Nemoto Y, Omori A, Ito T, Takahashi M, Kozaki S. Identification of protein receptor for Clostridium botulinum type B neurotoxin in rat brain synaptosomes. J Biol Chem. 1994;269(14):10498–10503. [PubMed] [Google Scholar]
- 13.Dong M, Richards DA, Goodnough MC, Tepp WH, Johnson EA, Chapman ER. Synaptotagmins I and II mediate entry of botulinum neurotoxin B into cells. J Cell Biol. 2003;162(7):1293–1303. doi: 10.1083/jcb.200305098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen C, Fu Z, Kim J-JP, Barbieri JT, Baldwin MR. Gangliosides as High Affinity Receptors for Tetanus Neurotoxin. J Biol Chem. 2009;284(39):26569–26577. doi: 10.1074/jbc.M109.027391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Munro P, Kojima H, Dupont JL, Bossu JL, Poulain B, Boquet P. High sensitivity of mouse neuronal cells to tetanus toxin requires a GPI-anchored protein. Biochem Biophys Res Commun. 2001;289(2):623–629. doi: 10.1006/bbrc.2001.6031. [DOI] [PubMed] [Google Scholar]
- 16.Yeh FL, Dong M, Yao J, Tepp WH, Lin G, Johnson EA, Chapman ER. SV2 Mediates Entry of Tetanus Neurotoxin into Central Neurons. PLoS Pathog. 2010;6(11):e1001207. doi: 10.1371/journal.ppat.1001207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bercsenyi K, Giribaldi F, Schiavo G. The elusive compass of clostridial neurotoxins: deciding when and where to go? Curr Top Microbiol Immunol. 2013;364:91–113. doi: 10.1007/978-3-642-33570-9_5. [DOI] [PubMed] [Google Scholar]
- 18.Bohnert S, Schiavo G. Tetanus Toxin Is Transported in a Novel Neuronal Compartment Characterized by a Specialized pH Regulation. J Biol Chem. 2005;280(51):42336–42344. doi: 10.1074/jbc.M506750200. [DOI] [PubMed] [Google Scholar]
- 19.Deinhardt K, Salinas S, Verastegui C, Watson R, Worth D, Hanrahan S, Bucci C, Schiavo G. Rab5 and Rab7 Control Endocytic Sorting along the Axonal Retrograde Transport Pathway. Neuron. 2006;52(2):293–305. doi: 10.1016/j.neuron.2006.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Schmieg N, Menendez G, Schiavo G, Terenzio M. Signalling endosomes in axonal transport: Travel updates on the molecular highway. Semin Cell Dev Biol. 2013 doi: 10.1016/j.semcdb.2013.10.004. [DOI] [PubMed] [Google Scholar]
- 21.Salinas S, Bilsland LG, Henaff D, Weston AE, Keriel A, Schiavo G, Kremer EJ. CAR-associated vesicular transport of an adenovirus in motor neuron axons. PLoS Pathog. 2009;5(5):e1000442. doi: 10.1371/journal.ppat.1000442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuda M, Sugimoto N, Ozutsumi K, Hirai T. Acute botulinum-like intoxication by tetanus neurotoxin in mice. Biochem Biophys Res Commun. 1982;104(2):799–805. doi: 10.1016/0006-291x(82)90708-2. [DOI] [PubMed] [Google Scholar]
- 23.Caleo M, Antonucci F, Restani L, Mazzocchio R. A reappraisal of the central effects of botulinum neurotoxin type A: by what mechanism? J Neurochem. 2009;109(1):15–24. doi: 10.1111/j.1471-4159.2009.05887.x. [DOI] [PubMed] [Google Scholar]
- 24.Restani L, Antonucci F, Gianfranceschi L, Rossi C, Rossetto O, Caleo M. Evidence for anterograde transport and transcytosis of botulinum neurotoxin A (BoNT/A) J Neurosci. 2011;31(44):15650–15659. doi: 10.1523/JNEUROSCI.2618-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris NP, Consiglio E, Kohn LD, Habig WH, Hardegree MC, Helting TB. Interaction of fragments B and C of tetanus toxin with neural and thyroid membranes and with gangliosides. J Biol Chem. 1980;255(13):6071–6076. [PubMed] [Google Scholar]
- 26.Wang J, Zurawski TH, Meng J, Lawrence GW, Aoki KR, Wheeler L, Dolly JO. Novel chimeras of botulinum and tetanus neurotoxins yield insights into their distinct sites of neuroparalysis. FASEB J. 2012;26(12):5035–5048. doi: 10.1096/fj.12-210112. [DOI] [PubMed] [Google Scholar]
- 27.Saheki Y, De Camilli P. Synaptic vesicle endocytosis. Cold Spring Harb Perspect Biol. 2012;4(9):a005645. doi: 10.1101/cshperspect.a005645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blum FC, Przedpelski A, Tepp WH, Johnson EA, Barbieri JT. Entry of a recombinant, full-length, atoxic tetanus neurotoxin into Neuro-2a cells. Infect Immun. 2014;82(2):873–881. doi: 10.1128/IAI.01539-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manders EM, Stap J, Brakenhoff GJ, van Driel R, Aten JA. Dynamics of three-dimensional replication patterns during the S-phase, analysed by double labelling of DNA and confocal microscopy. J Cell Sci. 1992;103 ( Pt 3):857–862. doi: 10.1242/jcs.103.3.857. [DOI] [PubMed] [Google Scholar]
- 30.Blum FC, Chen C, Kroken AR, Barbieri JT. Tetanus toxin and botulinum toxin a utilize unique mechanisms to enter neurons of the central nervous system. Infect Immun. 2012;80(5):1662–1669. doi: 10.1128/IAI.00057-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Q, Li Y, Tsien RW. The dynamic control of kiss-and-run and vesicular reuse probed with single nanoparticles. Science. 2009;323(5920):1448–1453. doi: 10.1126/science.1167373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He L, Wu XS, Mohan R, Wu LG. Two modes of fusion pore opening revealed by cell-attached recordings at a synapse. Nature. 2006;444(7115):102–105. doi: 10.1038/nature05250. [DOI] [PubMed] [Google Scholar]
- 33.Gandhi SP, Stevens CF. Three modes of synaptic vesicular recycling revealed by single-vesicle imaging. Nature. 2003;423(6940):607–613. doi: 10.1038/nature01677. [DOI] [PubMed] [Google Scholar]
- 34.Matteoli M, Verderio C, Rossetto O, Iezzi N, Coco S, Schiavo G, Montecucco C. Synaptic vesicle endocytosis mediates the entry of tetanus neurotoxin into hippocampal neurons. P Natl Acad Sci USA. 1996;93(23):13310–13315. doi: 10.1073/pnas.93.23.13310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen C, Baldwin MR, Barbieri JT. Molecular Basis for Tetanus Toxin Coreceptor Interactions. Biochemistry. 2008;47(27):7179–7186. doi: 10.1021/bi800640y. [DOI] [PubMed] [Google Scholar]
- 36.Jin R, Rummel A, Binz T, Brunger AT. Botulinum neurotoxin B recognizes its protein receptor with high affinity and specificity. Nature. 2006;444(7122):1092–1095. doi: 10.1038/nature05387. [DOI] [PubMed] [Google Scholar]
- 37.Rossetto O, Scorzeto M, Megighian A, Montecucco C. Tetanus neurotoxin. Toxicon. 2013;66:59–63. doi: 10.1016/j.toxicon.2012.12.027. [DOI] [PubMed] [Google Scholar]
- 38.Restani L, Giribaldi F, Manich M, Bercsenyi K, Menendez G, Rossetto O, Caleo M, Schiavo G. Botulinum neurotoxins A and E undergo retrograde axonal transport in primary motor neurons. PLoS Pathog. 2012;8(12):e1003087. doi: 10.1371/journal.ppat.1003087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Weller U, Taylor CF, Habermann E. Quantitative comparison between tetanus toxin, some fragments and toxoid for binding and axonal transport in the rat. Toxicon. 1986;24(11–12):1055–1063. doi: 10.1016/0041-0101(86)90132-7. [DOI] [PubMed] [Google Scholar]
- 40.Akaike N, Shin MC, Wakita M, Torii Y, Harakawa T, Ginnaga A, Kato K, Kaji R, Kozaki S. Transsynaptic inhibition of spinal transmission by A2 botulinum toxin. J Physiol. 2013;591(Pt 4):1031–1043. doi: 10.1113/jphysiol.2012.242131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mazzocchio R, Caleo M. More than at the Neuromuscular Synapse: Actions of Botulinum Neurotoxin A in the Central Nervous System. Neuroscientist. 2014 doi: 10.1177/1073858414524633. [DOI] [PubMed] [Google Scholar]
- 42.Malizio CJ, Goodnough MC, Johnson EA. Purification of Clostridium botulinum type A neurotoxin. Methods Mol Biol. 2000;145:27–39. doi: 10.1385/1-59259-052-7:27. [DOI] [PubMed] [Google Scholar]
- 43.Prabakaran S, Tepp W, DasGupta BR. Botulinum neurotoxin types B and E: purification, limited proteolysis by endoproteinase Glu-C and pepsin, and comparison of their identified cleaved sites relative to the three-dimensional structure of type A neurotoxin. Toxicon : official journal of the International Society on Toxinology. 2001;39(10):1515–1531. doi: 10.1016/s0041-0101(01)00124-6. [DOI] [PubMed] [Google Scholar]