Abstract
Background
Chronic kidney disease (CKD) is associated with an increased risk of heart failure (HF). We aimed to evaluate the role of large artery stiffness, brachial and central blood pressure as predictors of incident hospitalized HF in the Chronic Renal Insufficiency Cohort (CRIC), a multi-ethnic multi-center prospective observational study of patients with CKD.
Methods and Results
We studied 2602 participants who were free of HF at baseline. Carotid-femoral pulse wave velocity (CF-PWV, the gold-standard index of large artery stiffness), brachial and central pressures (estimated via radial tonometry and a generalized transfer function) were assessed at baseline. Participants were prospectively followed to assess the development of new-onset hospitalized HF. During 3.5 years of follow-up, 154 participants had a first hospital admission for HF. CF-PWV was a significant independent predictor of incident hospitalized HF. Compared to the lowest tertile, the HR among subjects in the middle and top CF-PWV tertiles were 2.33 (95%CI=1.37-3.97; P=0.002) and 5.24 (95%CI=3.22-8.53; P<0.0001), respectively. After adjustment for multiple confounders, the HR for the middle and top CF-PWV tertiles were 1.95 (95%CI=0.92-4.13; P=0.079) and 3.01 (95%CI=1.45-6.26; P=0.003), respectively. Brachial systolic and pulse pressure were also independently associated with incident hospitalized HF, whereas central pressures were less consistently associated with this endpoint. The association between CF-PWV and incident HF persisted after adjustment for systolic blood pressure.
Conclusions
Large artery stiffness is an independent predictor of incident HF in CKD, an association with strong biologic plausibility given the known effects of large artery stiffening of left ventricular pulsatile load.
Keywords: chronic kidney disease, heart failure, pulse wave velocity, arterial stiffness, central pressures
Chronic kidney disease (CKD) is independently associated with an increased risk of cardiovascular disease1;2. Patients with CKD are also at an increased risk of heart failure (HF), which is a major cause of morbidity and mortality in this population 3-6. Whereas several studies have been performed regarding predictors of overall cardiovascular risk in CKD (assessed using composite cardiovascular endpoints), the predictors of HF as a specific endpoint have not been adequately characterized in subjects with CKD. Of note, composite cardiovascular endpoints usually include several atherosclerotic and non-atherosclerotic events, for which risk factors may differ. HF in CKD has been proposed to be largely independent of atherosclerotic occlusive disease and more closely related to structural myocardial disease 3.
Elevated blood pressure is a well-known risk factor for HF in the general population and a candidate mechanism for increased HF risk in CKD 7. However, a recent study reported that moderate CKD increases the risk of HF even in the absence of hypertension (defined from brachial pressure measurements) or diabetes mellitus at baseline 4. Central pressure profiles have been investigated in the prediction of cardiovascular risk in patients with end-stage kidney disease 8, but the relationship between central pressures and incident HF has never been examined in earlier stages of CKD. Similarly, increased large artery stiffness has been proposed as a major contributor to HF risk in CKD 3 due to its well-known effects on left ventricular pulsatile afterload 9, which promote left ventricular hypertrophy and myocardial dysfunction. Despite these important physiologic considerations, the relationship between large artery stiffness, central pressures and incident HF in CKD has not been investigated.
In this study, we aimed to evaluate the role of large artery stiffness, brachial and central blood pressure as predictors of incident hospitalized HF in the Chronic Renal Insufficiency Cohort (CRIC), a multi-ethnic multi-center prospective observational study of patients with CKD10.
Methods
Study Overview
The CRIC Study is a prospective cohort study of 3939 participants enrolled from June 2003 through August 2008 through 7 clinical centers across the USA10. Participants constitute a racially diverse group of men and women aged 21-74 years who were identified as having CKD, approximately half of whom were diabetic, and who were enrolled using age-stratified criteria for kidney function by estimated glomerular filtration rate (eGFR) 11. The level of eGFR used to define eligibility was based on the four variable Modification of Diet in Renal Disease (MDRD) estimating equation12, using a serum creatinine measured at each enrolling site and then calibrated to the Cleveland Clinic laboratory 13. Demographic characteristics of the entire CRIC cohort have been published 11 .
Exclusion criteria included disorders that would be likely to compromise life expectancy during follow-up (e.g. NYHA class 3 and 4 heart failure, cancer, and immunosuppressive therapies within 6 months of enrollment). We note that some subjects were enrolled in the parent CRIC study who had a diagnosis of HF with NYHA functional class 1 and 2 at baseline (n=214). These subjects were excluded from this particular study. The study was approved by the Institutional Review Boards of all participating centers. All subjects provided informed consent.
Brachial and Central pressure measurements
Study personnel in the CRIC Study were trained to take standard brachial blood pressure in the dominant arm using the American Heart Association standard protocol using a Tyco aneroid sphygmomanometer 14. Central aortic systolic and pulse pressures were incorporated into the CRIC protocol at the second year follow up visit, and measured supine after 5 minutes of rest using the SphygmoCorPVx System (AtCor Medical, West Ryde, Australia) via the right radial artery at all of the CRIC sites, as previously described 15. Briefly, the coordinator captured 10 seconds of a stable right radial artery waveform signal using a high-fidelity Millar applanation tonometer, from which the central aortic pressure profile was estimated using the generalized transfer function of the Sphygmocor device 16;17.
Carotid-Femoral Pulse Wave Velocity Measurement
Carotid-femoral PWV measurements were incorporated in the CRIC protocol beginning with the 2-year follow-up visit at all study sites. Carotid-femoral PWV measurements were performed in the supine position, after at least 5 minutes of rest using the right carotid and right femoral arteries as previously described 18. Briefly, the SphygmoCorPVx (AtCor Medical, West Ryde, Australia) device was used by trained, certified study personnel. Carotid-femoral distance was computed as the distance from the sternal notch to the umbilicus plus the distance from the umbilicus to the point of palpable femoral pulse minus the distance from the sternal notch to the point of the palpable carotid pulse. This distance was further adjusted, as we reported previously 18, by a correction factor for the exaggeration of the sternal notch to femoral measurement produced by large waist circumference 18. A Millar tonometer captured ten seconds of acceptable carotid followed immediately by 10 seconds of femoral pulse waveforms (in series). The QRS complex timing was used as a fiducial point to compute the delay in pulse arrival between the carotid and femoral sites.
Prospective Follow-up and Heart Failure Event Adjudication
After enrollment, participants were followed until death or withdrawal of informed consent. The primary goals of the CRIC Study are to investigate standard as well as novel risk factors for CKD progression, as well as the incidence and progression of cardiovascular disease (CVD). This is accomplished by yearly in-person follow-up visits at which time a standard protocol assessing physical findings, interval medical history, EKG and laboratory testing were performed. The interval medical history specifically queries hospitalized CVD events at both the yearly in-person visits as well as by an interval 6-month telephone contact. Anytime an event is reported by the participant, records are sought and the de-identified medical records are sent to two CRIC investigator-physicians for adjudication (with discordances solved by consensus or an appeal to a third physician adjudicator when needed).
A HF event is adjudicated when there is hospitalization for clinical symptoms (dyspnea on exertion or rest, paroxysmal nocturnal dyspnea, and/or orthopnea) with at least one of the following objective findings: (1) Radiographic evidence of pulmonary edema or pulmonary congestion; (2) Physical Exam findings consistent with CHF to include at least two of the following: Inspiratory crackles (“rales”) involving at least 1/3 of the lower lung fields, S3 gallop on auscultation, elevated jugular venous pressure or peripheral edema; (3) Invasive hemodynamic or echocardiographic evidence of HF, including any of the following: pulmonary capillary wedge pressure >18 mmHg, cardiac index <2.0 L/min per m2 of body surface area or left ventricular ejection fraction ≤35%. This analysis includes adjudicated, hospitalized incident HF events occurring through March 2011.
Since we aimed to assess the risk factors for incident HF in this study, this analysis excluded participants who were enrolled in the CRIC Study and who had a pre-existing diagnosis of HF (n=214), those who had an incident HF event between enrollment and the first available measurement of carotid-femoral pulse wave velocity (Cf-PWV; n=3).
Statistical Analysis
Baseline characteristics were described using means with standard deviations for continuous variables, and number (%) for categorical data. Participant characteristics were analyzed in the entire sample and compared among tertiles of Cf-PWV values. Survival across Cf-PWV tertiles and various parameters of interest was examined with the Kaplan-Meier estimator. Cox proportional hazards models were used to analyze the relationship between various parameters of interest, including brachial and central systolic blood pressure (SBP), brachial and central pulse pressure (PP) and Cf-PWV. Hazard ratios (HR) and 95% confidence intervals (CIs) were computed across tertiles of these variables, using the lowest tertile as the referent group, after testing the proportional hazards assumption. We constructed 3 types of models for each analysis: an unadjusted model (Model 1), a model adjusted for age, race, sex, and enrollment site (Model 2) and a model further adjusted for baseline values of various other potential confounders, including diabetes mellitus, proteinuria, the presence of chronic obstructive pulmonary disease, mean arterial pressure, heart rate, HDL-cholesterol, LDL-cholesterol, body mass index, triglycerides, history of MI/revascularization and current smoking. Harrell's C values, an index of model discrimination, were computed for each Cox model. Analyses were executed in SAS 9.3 (SAS Institute, Cary, NC).
Results
Baseline characteristics of the study sample are shown in Table 1. The mean (SD) age of the study population was 59.9 years. Women constituted 43% of participants. Among the 2,602 study participants included in this analysis, 46% were non-Hispanic white, 38% were non-Hispanic black and 12% were Hispanic. Mean GFR was 45.3 mL/min/1.73 m2. The mean values of brachial systolic and diastolic pressures were 126.7 and 70.1 mmHg, respectively. Mean carotid-femoral PWV was 9.69 m/s. Diabetes mellitus was present in 31% of the sample, whereas a history of prior revascularization or myocardial infarction was present in 19% of the sample.
Table 1. Comparison of Chronic Renal Insufficiency Cohort (CRIC) Study participants without HF at study entry by tertile of carotid-femoral PWV.
| All n=2602 |
PWV ≤7.9 m/s n=888 |
PWV 7.9 to 10.3 m/s n=889 |
PWV >10.3 m/s n=825 |
P value |
|
|---|---|---|---|---|---|
| Age | 59.89 (11.07) | 54.47 (11.99) | 60.96 (9.91) | 64.57 (8.45) | <0.001 |
| Female gender, % | 1124 (43%) | 427 (48.1%) | 373 (42%) | 324 (39.3%) | <0.001 |
| Race-Ethnicity | <0.001 | ||||
| Hispanic | 321 (12%) | 102 (11.5%) | 113 (12.7%) | 106 (12.8%) | <0.001 |
| Non-Hispanic Black | 982 (38%) | 265 (29.8%) | 343 (38.6%) | 374 (45.3%) | . |
| Non-Hispanic White | 1190 (46%) | 482 (54.3%) | 391 (44%) | 317 (38.4%) | . |
| Other | 109 (4%) | 39 (4.4%) | 42 (4.7%) | 28 (3.4%) | . |
| Systolic blood pressure, mmHg | 126.71 (21.75) | 117.58 (17.15) | 125.67 (19.72) | 137.73 (23.37) | <0.001 |
| Diastolic blood pressure, mmHg | 70.14 (12.47) | 70.69 (11.39) | 70.37 (12.26) | 69.28 (13.73) | 0.053 |
| Mean arterial pressure, mmHg | 89.00 (13.38) | 86.32 (11.76) | 88.80 (12.85) | 92.12 (14.87) | <0.001 |
| Brachial Pulse pressure, mmHg | 56.58 (19.28) | 46.89 (14.40) | 55.31 (17.08) | 68.51 (19.79) | <0.001 |
| Central systolic pressure, mmHg | 116.59 (21.86) | 108.74 (19.11) | 115.77 (20.18) | 125.91 (22.86) | <0.001 |
| Central pulse pressure, mmHg | 45.56 (18.95) | 37.24 (16.03) | 44.66 (17.47) | 55.49 (18.80) | <0.001 |
| Resting heart rate, beats/minute | 67.79 (11.31) | 67.20 (11.13) | 67.28 (11.03) | 68.97 (11.72) | 0.001 |
| Aortic Augmentation Index | 27.04 (12.40) | 24.77 (13.20) | 27.79 (12.40) | 28.68 (11.09) | <0.001 |
| Pulse wave velocity, m/s | 9.46 (2.97) | 6.66 (0.89) | 9.04 (0.70) | 12.93 (2.41) | ----- |
| eGFR, mL/[min 1.73 m2]* | 45.26 (18.25) | 50.71 (20.00) | 45.35 (16.96) | 39.20 (15.52) | <0.001 |
| Prevalence of Hypertension, % | 2301 (88.4%) | 686 (29.8%) | 819 (35.6%) | 796 (34.6%) | <0.001 |
| Body mass index, kg/m2 | 31.05 (6.65) | 30.79 (6.83) | 30.98 (6.61) | 31.42 (6.50) | 0.131 |
| Diabetes, % | 1181 (45%) | 236 (26.6%) | 396 (44.5%) | 549 (66.5%) | <0.001 |
| Urine protein, g | |||||
| <0.10 | 859 (40%) | 367 (49.1%) | 299 (40.7%) | 193 (29.2%) | <0.001 |
| 0.10 -<0.50 | 644 (30%) | 209 (28%) | 222 (30.2%) | 213 (32.2%) | . |
| 0.50 -<1.50 | 313 (15%) | 99 (13.3%) | 103 (14%) | 111 (16.8%) | . |
| 1.50+ | 327 (15%) | 72 (9.6%) | 110 (15%) | 145 (21.9%) | . |
| COPD,% | 116 (4%) | 24 (2.7%) | 42 (4.8%) | 50 (6.1%) | 0.003 |
| Prior MI or revascularization,% | 498 (19%) | 105 (11.8%) | 176 (19.8%) | 217 (26.3%) | <0.001 |
| Current smoker, % | 288 (11%) | 77 (8.7%) | 99 (11.1%) | 112 (13.6%) | 0.005 |
| HDL-cholesterol, mg/dL | 48.28 (15.77) | 49.29 (15.20) | 48.55 (16.64) | 46.84 (15.38) | 0.011 |
| LDL-cholesterol, mg/dL | 100.97 (33.74) | 103.15 (33.56) | 101.92 (34.14) | 97.48 (33.31) | 0.004 |
| Triglycerides, md/dL | 149.09 (114.74) | 140.19 (98.53) | 156.34 (138.81) | 151.49 (102.40) | 0.020 |
Number in parentheses represent the standard deviation for continuous variables and proportions for categorical variables.
During a mean of 3.5 years of follow-up, 154 participants (5.9%) developed HF. Table 2 shows the association of various baseline characteristics and incident HF in unadjusted analyses. Factors associated with incident HF included age, blood pressure, the presence of chronic obstructive pulmonary disease (COPD), LDL-cholesterol, eGFR, urinary protein excretion, body mass index, diabetes mellitus, a history of myocardial infarction or coronary revascularization, aortic augmentation index corrected for a heart rate and Cf-PWV (Table 2).
Table 2. Unadjusted hazard ratios for incident HF associated with hemodynamic factors in the Chronic Renal Insufficiency Cohort (CRIC) Study.
| Hazard Ratios (95% CI) | P value | |
|---|---|---|
| Age | 1.05 (1.03, 1.06) | <0.001 |
| Female gender, % | 0.77 (0.55, 1.07) | 0.12 |
| Race * | ||
| Non-Hispanic Black | ||
| Hispanic | ||
| Other | ||
| Systolic blood pressure, mmHg | 1.02 (1.01, 1.03) | <0.001 |
| Diastolic blood pressure, mmHg | 0.98 (0.97, 1.00) | 0.016 |
| Mean arterial pressure, mmHg | 1.01 (1.00, 1.02) | 0.037 |
| Brachial Pulse pressure, mmHg | 1.03 (1.02, 1.03) | <0.001 |
| Central systolic pressure, mmHg | 1.02 (1.01, 1.02) | <0.001 |
| Central pulse pressure, mmHg | 1.02 (1.02, 1.03) | <0.001 |
| Resting heart rate, beats/minute | 1.01 (1.00, 1.03) | 0.10 |
| Aortic Augmentation Index | 1.01 (1.00, 1.02) | 0.11 |
| Aortic Augmentation index (@HR75) | 1.02 (1.01, 1.04) | 0.003 |
| Pulse wave velocity, m/s | 1.18 (1.13, 1.22) | <0.001 |
| eGFR, mL/[min 1.73 m2]* | 0.96 (0.95, 0.97) | <0.001 |
| Body mass index, kg/m2 | 1.04 (1.01, 1.06) | 0.002 |
| 24-hour urine protein excretion † | ||
| 0.10 to<0.50 g/24h | 2.86 (1.60, 5.13) | <0.001 |
| 0.50 to<1.50 g/24h | 5.86 (3.25, 10.55) | <0.001 |
| ≥1.50 g/24h | 6.79 (3.81, 12.10) | <0.001 |
| Diabetes, % | 3.05 (2.16, 4.32) | <0.001 |
| COPD, % | 3.62 (2.21, 5.93) | <0.001 |
| Prior revascularization or myocardial infarction,% | 2.87 (2.08, 3.97) | <0.001 |
| Current smoker, % | 1.27 (0.80, 2.04) | 0.31 |
| HDL-cholesterol, mg/dL | 0.99 (0.98, 1.00) | 0.15 |
| LDL-cholesterol, mg/dL | 0.99 (0.98, 0.99) | <0.001 |
| Triglycerides, md/dL | 1.00 (1.00, 1.00) | 0.018 |
Reference category is urine protein <0.10 g/24h
Carotid-femoral PWV, brachial pressures and central pressures as predictors of incident HF
Table 3 shows the HR (95% CI) associated with tertiles of either: (1) CF-PWV; (2) Brachial systolic pressure; (3) Central systolic pressure; (4) Brachial pulse pressure; (5) Central pulse pressure. For each of these hemodynamic variables, Table 3 presents unadjusted analyses, analyses adjusted for demographic factors (age, race, sex and enrollment site) and analyses further adjusted for diabetes mellitus, proteinuria, the presence of chronic obstructive pulmonary disease, mean arterial pressure, heart rate, HDL-cholesterol, LDL-cholesterol, body mass index, triglycerides, history of MI/revascularization and current smoking. Harrell's C values are also presented for each model.
Table 3.
Carotid-femoral PWV, central and brachial pressure tertiles and risk of incident HF (events=154; censored participants with no events=2448).
| Model 1 (unadjusted) * | Model 2 † | Model 3 ‡ | ||||
|---|---|---|---|---|---|---|
| Hazard Ratios (95% CI) |
P value | Hazard Ratios (95% CI) |
P value | Hazard Ratios (95% CI) |
P value | |
| Carotid-Femoral PWV (m/s) | Harrell's C=0.67 | Harrell's C=0.729 | Harrell's C=0.843 | |||
| ≤7.8 | Referent | Referent | ||||
| 7.8 -≤10.3 | 2.33 (1.37, 3.97) | 0.002 | 1.80 (1.05, 3.10) | 0.03 | 1.95 (0.92, 4.13) | 0.079 |
| >10.3 | 5.24 (3.22, 8.53) | <0.001 | 3.59 (2.14, 6.03) | <0.001 | 3.01 (1.45, 6.26) | 0.003 |
| Brachial systolic blood pressure (mmHg) | Harrell's C=0.642 | Harrell's C=0.721 | Harrell's C=0.84 | |||
| ≤116 | Referent | Referent | Referent | |||
| 116 -≤133.3 | 1.36 (0.84, 2.20) | 0.21 | 1.22 (0.75, 1.98) | 0.42 | 1.91 (0.88, 4.12) | 0.10 |
| >133.3 | 3.21 (2.09, 4.93) | <0.001 | 2.39 (1.53, 3.75) | <0.001 | 2.92 (1.18, 7.22) | 0.021 |
| Central systolic blood pressure (mmHg) | Harrell's C=0.615 | Harrell's C=0.708 | Harrell's C=0.839 | |||
| ≤106 | Referent | Referent | Referent | |||
| 106 -≤122.5 | 1.32 (0.83, 2.09) | 0.24 | 1.19 (0.74, 1.89) | 0.47 | 1.50 (0.76, 2.96) | 0.25 |
| >122.5 | 2.50 (1.64, 3.80) | <0.001 | 1.86 (1.20, 2.89) | 0.005 | 1.65 (0.74, 3.69) | 0.23 |
| Brachial pulse pressure (mmHg) | Harrell's C=0.680 | Harrell's C=0.74 | Harrell's C=0.848 | |||
| ≤46.7 | Referent | Referent | Referent | |||
| 46.7 -≤62.7 | 1.64 (0.97, 2.76) | 0.063 | 1.25 (0.73, 2.14) | 0.41 | 1.87 (0.79, 4.44) | 0.16 |
| >62.7 | 4.89 (3.09, 7.74) | <0.001 | 3.35 (2.03, 5.52) | <0.001 | 3.53 (1.47, 8.46) | 0.005 |
| Central pulse pressure (mmHg) | Harrell's C=0.669 | Harrell's C=0.733 | Harrell's C=0.847 | |||
| ≤35.4 | Referent | Referent | Referent | |||
| 35.4 -≤50.8 | 1.68 (1.01, 2.78) | 0.045 | 1.33 (0.79, 2.24) | 0.28 | 1.24 (0.58, 2.65) | 0.58 |
| >50.8 | 4.32 (2.75, 6.80) | <0.001 | 3.00 (1.84, 4.91) | <0.001 | 2.45 (1.14, 5.27) | 0.02 |
Model 1 is unadjusted
Model 2 is adjusted for age, race, sex, and enrollment site
Model 3 is additionally adjusted for diabetes mellitus, proteinuria, the presence of chronic obstructive pulmonary disease, mean arterial pressure, heart rate, HDL-cholesterol, LDL-cholesterol, body mass index, triglycerides, history of MI/revascularization, current smoking, hemoglobin, human recombinant erythropoietin use, angiotensin-converting enzyme inhibitor use, beta blocker use, calcium channel blocker use, history of hypertension, fasting glucose and serum albumin.
Carotid-femoral PWV was significantly associated with the development of HF in unadjusted analyses (Table 2 and Model 1 in Table 3). Event rates and Kaplan-Meier curves for the 3 tertiles of CF-PWV are shown in Figure 1A and 1B, respectively. Compared to the lowest tertile, the HR for HF among subjects in the middle and top tertiles were 2.33 (95%CI=1.37-3.97; P=0.002) and 5.24 (95%CI=3.22-8.53; P<0.0001), respectively. This association persisted after adjustment for age, race, sex and enrollment site. Upon further adjustment for other covariates, the hazard ratios for HF among subjects in the middle and top tertiles were 1.95 (95%CI=0.92-4.13; P=0.079) and 3.01 (95%CI=1.45-6.26; P=0.003), respectively.
Figure 1A.
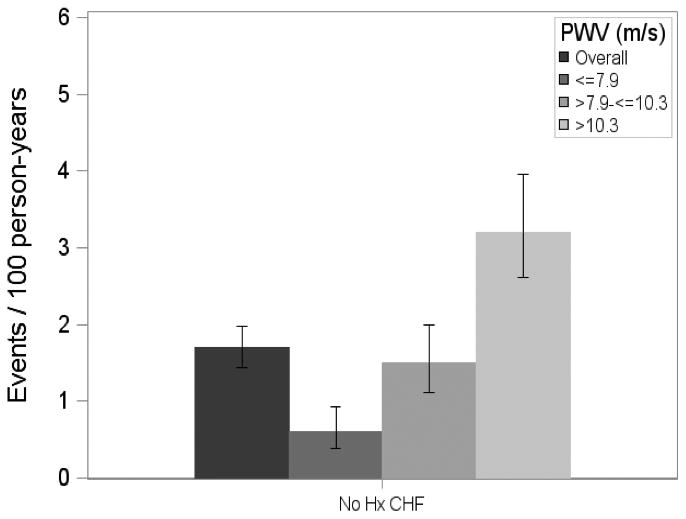
Incident HF event rates among Chronic Renal Insufficiency Cohort (CRIC) Study participants without HF at study entry overall and by tertile of carotid-femoral PWV.
Figure 1B.

Kaplan-Meier curves for incident HF by tertile of carotid-femoral PWV.
Compared to subjects in the lowest tertile of brachial SBP, those in the highest tertile (but not those in the middle tertile) demonstrated a significantly increased risk of HF (HR=3.21; 95%CI=2.09-4.93; P<0.001). This association persisted in fully adjusted analyses (HR=2.92; 95%CI=1.18-7.22; P=0.02). In contrast to brachial SBP, central SBP was not associated with incident HF in a fully adjusted models (Model 3 in Table 3, P>0.05).
Compared to subjects in the lowest tertile of brachial PP, those in the highest tertile (but not those in the middle tertile) demonstrated a significantly increased risk of HF (HR=4.89; 95%CI=3.09-7.74; P<0.001). This association persisted in fully adjusted analyses (HR=3.53; 95%CI=1.47-8.46; P=0.005). Subjects in the highest tertile of central PP also demonstrated a greater risk of incident HF in unadjusted analyses, as well as in fully adjusted analyses (HR=2.45; 95%CI=1.14-5.27; P=0.02).
Association between carotid-femoral PWV after adjustment for blood pressure
Carotid-femoral PWV remained independently associated with incident HF after adjustment for SBP. In these analyses, compared to the lowest tertile, the HR for HF among subjects in the middle and highest tertiles of CF-PWV were 1.79 (95%CI=0.88-3.66; P=0.022) and 2.58 (95%CI=1.29-5.16; P=0.022), respectively.
Discussion
In this study, we assessed the relationship between large artery stiffness (assessed as Cf-PWV), brachial pressures, central pressures and the risk of incident HF in a large cohort of subjects with CKD. We demonstrate, for the first time, that Cf-PWV is associated with a pronounced increase in the risk of incident HF in this population, an association that persists after adjustment for blood pressure and other confounders. In addition, we demonstrate that brachial systolic and pulse pressure are both predictive of incident HF, whereas the association between central systolic and pulse pressure and incident HF was weaker and/or less consistent with increasing adjustment for covariates.
Further characterization of risk factors for incident cardiovascular events in CKD is an important goal. In particular, prospective studies characterizing the factors related to incident HF in early CKD are lacking. HF is a major cause of morbidity and mortality in CKD 6. Our study is the first to investigate the associations between various factors present at baseline and incident HF in a large cohort of subjects with CKD. We demonstrate that HF is a common event in this cohort (Figure 1) and that large artery stiffening is an important independent risk factor for incident HF. Carotid-femoral PWV is considered the “gold-standard” index of large artery stiffness. Whereas we included both SBP and Cf-PWV to assess whether a measurement of the latter can be prognostic independently of the former, it should be recognized that SBP (and PP) are also importantly influenced by large artery stiffness. Our study thus clearly demonstrates an independent association between large artery stiffening and incident HF in CKD, which has strong biologic plausibility. Various mechanisms can explain the association between Cf-PWV, SBP, PP and incident HF. Large artery stiffness occurs secondary to degeneration, fibrosis and calcification of the medial layer of the aorta (arteriosclerosis) which is a process distinct from atherosclerosis, an intimal process that leads to occlusive vascular events (such as myocardial infarction) 19. HF in CKD has been proposed to be largely independent of atherosclerotic occlusive disease and more closely related to structural myocardial disease 3. Large artery stiffness has an important effect on left ventricular pulsatile afterload, through its effects on the early aortic systolic pressure rise, the total compliance of the arterial system and the velocity at which the pulse waves travel forward in the arteries and reflected waves travel backward toward the heart 20. In early systole, the forward-traveling energy pulse from ventricular contraction favors an increase in pressure and forward flow in the proximal aorta 21. Proximal aortic stiffening favors a greater characteristic impedance, which in turn results in a greater amount of pressure increase for any given early systolic flow 20. Similarly, large artery stiffening has an important effect on the time of return of reflected waves to the heart. Stiffer aortas conduct the forward and backward traveling waves at greater velocity, therefore promoting an earlier arrival of the reflected wave for any given distance to reflection sites 20. Large artery stiffening also results in a lower arterial compliance, since large arteries normally provide most of the total compliance of the arterial tree. Finally, the lower diastolic pressure associated with large artery stiffening contributes to a decreased coronary perfusion pressure.
The abnormalities in pulsatile afterload that occurs as a consequence of large artery stiffness result in increased systolic wall stress 22, diastolic and systolic dysfunction and left ventricular hypertrophy 23-27. An association between increased large artery stiffness and increased extracellular matrix turnover (as measured by higher amino-terminal pro-peptide of type III procollagen) has also been demonstrated 28. Hundley et al 29 have shown that stiffening of the aorta is closely associated with a reduced peak aerobic exercise capacity (measured as peak VO2) in patients with HF and preserved ejection fraction. Similarly, proximal aortic stiffening has been demonstrated in patients with established systolic HF 21.
Although increased left ventricular pulsatile afterload is likely to be the mechanism linking aortic stiffness with the risk of incident HF, additional mechanisms may be at play. Arterial stiffness, although not directly related to atherosclerosis, shares risk factors with atherosclerosis and thus tends to be associated with coronary atherosclerosis 30. Large artery stiffness may also lead to a faster deterioration of renal function over time, which in turn may favor hypervolemia, hypertension, and further large artery stiffening and calcification, thus leading to a vicious circle that culminates in clinically-evident HF. Future studies will be required to assess the link between progressive large artery stiffness, progressive renal dysfunction and new-onset HF in patients with CKD.
Although we found that brachial SBP and PP were associated with incident HF in this population, central SBP and PP were less consistently associated with this outcome and therefore did not provide any additional value over brachial pressures. These results confirm recent findings from the Multi-Ethnic Study of Atherosclerosis (MESA), which demonstrated a strong association between brachial pressures and incident HF, without a significant incremental value of central SBP and PP once brachial pressures are known 31. Of note, the latter study did demonstrate a strong relationship between wave reflections and incident HF. This approach remains to be tested in future studies in CKD populations.
Our study also found that older age, diabetes mellitus, a history of myocardial infarction, coronary revascularization, known arrhythmia, greater body mass index, lower eGFR and urinary protein excretion were associated with incident HF. Older age is a well-known risk factor for HF, an observation that our study extends to the CKD population. Diabetes mellitus is associated with a variety of myocardial metabolic, structural and functional abnormalities 32, arterial stiffening 18;33 with increased pulsatile left ventricular afterload and coronary artery disease, all of which may contribute to its associated with HF in CKD. Similarly, the association between kidney function and incident HF has been reported in previous studies that included individuals with and without established CKD (e.g., older individuals) and has been shown to be independent of the presence of diabetes or hypertension at baseline4;5;34;35. Renal dysfunction may lead to HF through several mechanisms, including plasma volume expansion, overactivity of the sympathetic nervous system, and the renin-angiotensin-aldosterone axis, hyperphosphatemia and increased fibroblast growth factor-23 levels 36. The higher risk of HF among black participants is also consistent with previous data demonstrating that blacks are more likely to develop CKD 37;38, HF 39 and to progress from sub-clinical systolic and diastolic dysfunction and/or LV remodeling to frank HF 40-42 than their white counterparts. Similarly, the Health, Aging and Body Compositions Study demonstrated an association between CKD and incident HF among older individuals, which was stronger for black, relative to white individuals, with a population-attributable risk for HF associated with moderate or high cystatin C levels of 47% for blacks vs. 5% for whites, despite a similar prevalence of this abnormality at baseline 5. Finally, the observed association between BMI and incident HF in our study is consistent with the well-known association between obesity and the risk of HF, previously reported in the general population 43.
Our study findings need to be interpreted in the context of its strengths and limitations. Our large, well-characterized, multiethnic sample, with an assessment of large artery stiffness using the gold standard measurement and the careful, systematic adjudication of HF events, are important strengths of our study. Additionally, our study population of mild to moderate diabetic and non-diabetic kidney disease represents one that has not been adequately represented in prior similar investigations. Our study also has limitations. We did not assess pulsatile left ventricular afterload or wave reflection magnitude, which will need to be optimally assessed using central pressure-flow analyses, to better determine the pulsatile hemodynamic abnormalities that are associated with incident HF in this population. Further studies will also be required to assess the relationship between large artery stiffness, left ventricular remodeling at baseline and incident HF. Although we demonstrate an independent relationship between baseline large artery stiffness and future new-onset HF, our study cannot establish causality. Finally, our adjudication did not discriminate between heart failure with reduced ejection fraction and heart failure with preserved ejection fraction, nor did we have enough HF events to assess how the baseline predictors of HF relate to one type vs. the other. This should be the focus of future studies in this cohort. We note that our study was not designed to address the potential enhancement in individual risk prediction provided by the use of cf-PWV in conjuction with, or addition to, existing clinical heart failure risk scores, nor it intended to develop a CKD-specific risk score. This should be addressed in focus of future studies In conclusion, large artery stiffness independently predicts the onset of incident HF in subjects with CKD. These findings are important because they identify an important link between non-atherosclerotic vascular dysfunction and HF in this population. Our findings contribute to a better understanding of the determinants of HF risk in CKD and may be useful for the design of future intervention studies to reduce the risk of HF in this population.
Supplementary Material
Acknowledgments
Sources of Funding: Funding for the CRIC Study was obtained under a cooperative agreement from National Institute of Diabetes and Digestive and Kidney Diseases (U01DK060990, U01DK060984, U01DK061022, U01DK061021, U01DK061028, U01DK060980, U01DK060963, and U01DK060902). In addition, this work was supported in part by: the Perelman School of Medicine at the University of Pennsylvania Clinical and Translational Science Award NIH/NCATS UL1TR000003, Johns Hopkins University UL1 TR-000424, University of Maryland GCRC M01 RR-16500, Clinical and Translational Science Collaborative of Cleveland, UL1TR000439 from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research, Michigan Institute for Clinical and Health Research (MICHR) UL1TR000433, University of Illinois at Chicago Clinical and Translational Science Award UL1RR029879, Tulane University Translational Research in Hypertension and Renal Biology P30GM103337, Kaiser Permanente National Institutes of Health/National Center for Research Resourced University of California, San Francisco/Clinical and Translational Science Institute UL1 RR-024131.
Disclosures: Dr. Chirinos has received research grants from the National Institutes of Health and the Department of Veterans Affairs for research related to arterial stiffness, has received consulting fees (modest) from Fukuda-Denshi and High Point Pharmaceuticals and received free equipment loans from Atcor Medical, IEM, Fukuda-Denshi and Health-Stats for research in arterial stiffness.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY. Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Van BW, De BD, Verbeke F, Delanghe J, Lameire N, Vanholder R. The glomerular filtration rate in an apparently healthy population and its relation with cardiovascular mortality during 10 years. Eur Heart J. 2007;28:478–483. doi: 10.1093/eurheartj/ehl455. [DOI] [PubMed] [Google Scholar]
- 3.Chue CD, Townend JN, Steeds RP, Ferro CJ. Arterial stiffness in chronic kidney disease: causes and consequences. Heart. 2010;96:817–823. doi: 10.1136/hrt.2009.184879. [DOI] [PubMed] [Google Scholar]
- 4.Dhingra R, Gaziano JM, Djousse L. Chronic kidney disease and the risk of heart failure in men. Circ Heart Fail. 2011;4:138–144. doi: 10.1161/CIRCHEARTFAILURE.109.899070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bibbins-Domingo K, Chertow GM, Fried LF, Odden MC, Newman AB, Krtichevsky SB, Harris TB, Satterfield S, Cummings SR, Shlipal MG. Renal function and heart failure risk in older black and white individuals: the Health, Aging, and Body Composition Study. Arch Intern Med. 2006;166:1396–1402. doi: 10.1001/archinte.166.13.1396. [DOI] [PubMed] [Google Scholar]
- 6.Foley RN, Murray AM, Li S, Herzog CA, McBean AM, Eggers PW, Collins AJ. Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol. 2005;16:489–495. doi: 10.1681/ASN.2004030203. [DOI] [PubMed] [Google Scholar]
- 7.Taddei S, Nami R, Bruno RM, Quatrini I, Nuti R. Hypertension, left ventricular hypertrophy and chronic kidney disease. Heart Fail Rev. 2011;16:615–620. doi: 10.1007/s10741-010-9197-z. [DOI] [PubMed] [Google Scholar]
- 8.Safar ME, Blacher J, Pannier B, Guerin AP, Marchais SJ, Guyonvarch PM, London GM. Central pulse pressure and mortality in end-stage renal disease. Hypertension. 2002;39:735–738. doi: 10.1161/hy0202.098325. [DOI] [PubMed] [Google Scholar]
- 9.Chirinos JA. Arterial stiffness: basic concepts and measurement techniques. J Cardiovasc Transl Res. 2012;5:243–255. doi: 10.1007/s12265-012-9359-6. [DOI] [PubMed] [Google Scholar]
- 10.Feldman HI, Appel LJ, Chertow GM, Cifelli D, Cizman B, Daugirdas J, Fink JC, Franklin-Becker ED, Go AS, Hamm LL, He J, Hostetter T, Hsu CY, Jamerson K, Joffe M, Kusek JW, Landis JR, Lash JP, Miller ER, Mohler ER, 3rd, Muntner P, Ojo AO, Rahman M, Townsend RR, Wright JT. The Chronic Renal Insufficiency Cohort (CRIC) Study: Design and Methods. J Am Soc Nephrol. 2003;14:S148–S153. doi: 10.1097/01.asn.0000070149.78399.ce. [DOI] [PubMed] [Google Scholar]
- 11.Lash JP, Go AS, Appel LJ, He J, Ojo A, Rahman M, Townsend RR, Xie D, Cifelli D, Cohan J, Fink JC, Fischer MJ, Gadegbeku C, Hamm LL, Kusek JW, Landis JR, Narva A, Robinson N, Teal V, Feldman HI. Chronic Renal Insufficiency Cohort (CRIC) Study: baseline characteristics and associations with kidney function. Clin J Am Soc Nephrol. 2009;4:1302–1311. doi: 10.2215/CJN.00070109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 13.Coresh J, Astor BC, McQuillan G, Kusek J, Greene T, Van Lente F, Levey AS. Calibration and random variation of the serum creatinine assay as critical elements of using equations to estimate glomerular filtration rate. Am J Kidney Dis. 2002;39:920–929. doi: 10.1053/ajkd.2002.32765. [DOI] [PubMed] [Google Scholar]
- 14.Perloff D, Grim C, Flack J, Frohlich ED, Hill M, McDonald M, Morgenstern BZ. Human blood pressure determination by sphygmomanometry. Circulation. 1993;88:2460–2470. doi: 10.1161/01.cir.88.5.2460. [DOI] [PubMed] [Google Scholar]
- 15.Townsend RR, Chirinos JA, Parsa A, Weir MA, Sozio SM, Lash JP, Chen J, Steigerwalt SP, Go AS, Hsu CY, Rafey M, Wright JT, Jr, Duckworth MJ, Gadegbeku CA, Joffe MP. Central Pulse Pressure in Chronic Kidney Disease. A Chronic Renal Insufficiency Cohort Ancillary Study. Hypertension. 2010;56:518–524. doi: 10.1161/HYPERTENSIONAHA.110.153924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pauca AL, O'Rourke MF, Kon ND. Prospective evaluation of a method for estimating ascending aortic pressure from the radial artery pressure waveform. Hypertension. 2001;38:932–937. doi: 10.1161/hy1001.096106. [DOI] [PubMed] [Google Scholar]
- 17.Chen CH, Nevo E, Fetics B, Pak PH, Yin FC, Maughan WL, Kass DA. Estimation of central aortic pressure waveform by mathematical transformation of radial tonometry pressure. Validation of generalized transfer function. Circulation. 1997;95:1827–1836. doi: 10.1161/01.cir.95.7.1827. [DOI] [PubMed] [Google Scholar]
- 18.Townsend RR, Chirinos JA, Parsa A, Weir MA, Sozio SM, Lash JP, Chen J, Steigerwalt SP, Go AS, Hsu CY, Rafey M, Wright JT, Jr, Duckworth MJ, Gadegbeku CA, Joffe MP. Aortic PWV in chronic kidney disease: a CRIC ancillary study. Am J Hypertens. 2010;23:282–289. doi: 10.1038/ajh.2009.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aronow WS, Lee NH, Sales FF, Etienne F. Prevalence of postural hypotension in elderly patients in a long-term health care facility. Am J Cardiol. 1988;62:336. doi: 10.1016/0002-9149(88)90243-3. [DOI] [PubMed] [Google Scholar]
- 20.Nichols WW, O'Rourke MF, Vlachopoulos C. McDonald's blood flow in arteries. 6th. London: Hodder Arnold; 2011. [Google Scholar]
- 21.Mitchell GF, Tardif JC, Arnold JM, Marchiori G, O'Brien TX, Dunlap ME, Pfeffer MA. Pulsatile hemodynamics in congestive heart failure. Hypertension. 2001;38:1433–1439. doi: 10.1161/hy1201.098298. [DOI] [PubMed] [Google Scholar]
- 22.Chirinos JA, Segers P, Gillebert TC, Gupta AK, De Buyzere ML, De Bacquer D, St John-Sutton M, Rietzschel ER. Arterial properties as determinants of time-varying myocardial stress in humans. Hypertension. 2012;60:64–70. doi: 10.1161/HYPERTENSIONAHA.112.190710. [DOI] [PubMed] [Google Scholar]
- 23.Westerhof N, O'Rourke MF. Haemodynamic basis for the development of left ventricular failure in systolic hypertension and for its logical therapy. J Hypertens. 1995;13:943–952. doi: 10.1097/00004872-199509000-00002. [DOI] [PubMed] [Google Scholar]
- 24.Denardo SJ, Nandyala R, Freeman GL, Pierce GL, Nichols WW. Pulse wave analysis of the aortic pressure waveform in severe left ventricular systolic dysfunction. Circ Heart Fail. 2010;3:149–156. doi: 10.1161/CIRCHEARTFAILURE.109.862383. [DOI] [PubMed] [Google Scholar]
- 25.Russo C, Jin Z, Palmieri V, Homma S, Rundek T, Elkind MS, Sacco RL, Di Tullio MR. Arterial stiffness and wave reflection: sex differences and relationship with left ventricular diastolic function. Hypertension. 2012;60:362–368. doi: 10.1161/HYPERTENSIONAHA.112.191148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chirinos JA, Segers P, Rietzschel ER, De Buyzere ML, Raja MW, Claessens T, De Bacquer D, St John Sutton M, Gillebert TC. Early and late systolic wall stress differentially relate to myocardial contraction and relaxation in middle-aged adults: The asklepios study. Hypertension. 2013;61:296–303. doi: 10.1161/HYPERTENSIONAHA.111.00530. [DOI] [PubMed] [Google Scholar]
- 27.Mottram PM, Haluska BA, Leano R, Carlier S, Case C, Marwick TH. Relation of arterial stiffness to diastolic dysfunction in hypertensive heart disease. Heart. 2005;91:1551–1556. doi: 10.1136/hrt.2004.046805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonapace S, Rossi A, Cicoira M, Golia G, Zanolla L, Franceschini L, Conte L, Marino P, Zardini P, Vassanelli C. Aortic stiffness correlates with an increased extracellular matrix turnover in patients with dilated cardiomyopathy. Am Heart J. 2006;152:93–96. doi: 10.1016/j.ahj.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 29.Hundley WG, Kitzman DW, Morgan TM, Hamilton CA, Darty SN, Stewart KP, Herrington DM, Link KM, Little WC. Cardiac cycle-dependent changes in aortic area and distensibility are reduced in older patients with isolated diastolic heart failure and correlate with exercise intolerance. J Am Coll Cardiol. 2001;38:796–802. doi: 10.1016/s0735-1097(01)01447-4. [DOI] [PubMed] [Google Scholar]
- 30.van Popele NM, Mattace-Raso FU, Vliegenthart R, Grobbee DE, Asmar R, van der Kuip DA, Hofman A, de Feijter PJ, Oudkerk M, Witteman JC. Aortic stiffness is associated with atherosclerosis of the coronary arteries in older adults: the Rotterdam Study. J Hypertens. 2006;24:2371–2376. doi: 10.1097/01.hjh.0000251896.62873.c4. [DOI] [PubMed] [Google Scholar]
- 31.Chirinos JA, Kips JG, Jacobs DR, Jr, Brumback L, Duprez DA, Kronmal R, Bluemke DA, Townsend RR, Vermeersch S, Segers P. Arterial Wave Reflections and Incident Cardiovascular Events and Heart Failure: MESA (Multiethnic Study of Atherosclerosis) J Am Coll Cardiol. 2012;60:2170–2177. doi: 10.1016/j.jacc.2012.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cas AD, Spigoni V, Ridolfi V, Metra M. Diabetes and chronic heart failure: from diabetic cardiomyopathy to therapeutic approach. Endocr Metab Immune Disord Drug Targets. 2013;13:38–50. doi: 10.2174/1871530311313010006. [DOI] [PubMed] [Google Scholar]
- 33.Cruickshank K, Riste L, Anderson SG, Wright JS, Dunn G, Gosling RG. Aortic pulse-wave velocity and its relationship to mortality in diabetes and glucose intolerance: an integrated index of vascular function? Circulation. 2002;106:2085–2090. doi: 10.1161/01.cir.0000033824.02722.f7. [DOI] [PubMed] [Google Scholar]
- 34.Fried LF, Shlipak MG, Crump C, Bleyer AJ, Gottdiener JS, Kronmal RA, Kuller LH, Newman AB. Renal insufficiency as a predictor of cardiovascular outcomes and mortality in elderly individuals. J Am Coll Cardiol. 2003;41:1364–1372. doi: 10.1016/s0735-1097(03)00163-3. [DOI] [PubMed] [Google Scholar]
- 35.Gottdiener JS, Arnold AM, Aurigemma GP, Polak JF, Tracy RP, Kitzman DW, Gardin JM, Rutledge JE, Boineau RC. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J Am Coll Cardiol. 2000;35:1628–1637. doi: 10.1016/s0735-1097(00)00582-9. [DOI] [PubMed] [Google Scholar]
- 36.Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro OM, Kusek JW, Keane MG, Wolf M. FGF23 induces left ventricular hypertrophy. J Clin Invest. 2011;121:4393–4408. doi: 10.1172/JCI46122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stehman-Breen CO, Gillen D, Steffes M, Jacobs DR, Jr, Lewis CE, Kiefe CI, Siscovick D. Racial differences in early-onset renal disease among young adults: the coronary artery risk development in young adults (CARDIA) study. J Am Soc Nephrol. 2003;14:2352–2357. doi: 10.1097/01.asn.0000083392.11042.14. [DOI] [PubMed] [Google Scholar]
- 38.Krop JS, Coresh J, Chambless LE, Shahar E, Watson RL, Szklo M, Brancati FL. A community-based study of explanatory factors for the excess risk for early renal function decline in blacks vs whites with diabetes: the Atherosclerosis Risk in Communities study. Arch Intern Med. 1999;159:1777–1783. doi: 10.1001/archinte.159.15.1777. [DOI] [PubMed] [Google Scholar]
- 39.Coughlin SS, Labenberg JR, Tefft MC. Black-white differences in idiopathic dilated cardiomyopathy: the Washington DC dilated Cardiomyopathy Study. Epidemiology. 1993;4:165–172. doi: 10.1097/00001648-199303000-00013. [DOI] [PubMed] [Google Scholar]
- 40.Laurent S, Briet M, Boutouyrie P. Arterial stiffness as surrogate end point: needed clinical trials. Hypertension. 2012;60:518–522. doi: 10.1161/HYPERTENSIONAHA.112.194456. [DOI] [PubMed] [Google Scholar]
- 41.Regnault V, Thomas F, Safar ME, Osborne-Pellegrin M, Khalil RA, Pannier B, Lacolley P. Sex difference in cardiovascular risk: role of pulse pressure amplification. J Am Coll Cardiol. 2012;59:1771–1777. doi: 10.1016/j.jacc.2012.01.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilkinson IB, McEniery CM, Schillaci G, Boutouyrie P, Segers P, Donald A, Chowienczyk PJ. ARTERY Society guidelines for validation of non-invasive haemodynamic measurement devices: Part 1, arterial pulse wave velocity. Artery Research. 2010;4:34–40. [Google Scholar]
- 43.Kenchaiah S, Evans JC, Levy D, Wilson PW, Benjamin EJ, Larson MG, Kannel WB, Vasan RS. Obesity and the risk of heart failure. N Engl J Med. 2002;347:305–313. doi: 10.1056/NEJMoa020245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


