Abstract
Objective
To determine the association between generalized evoked pressure pain sensitivity with distal pressure-pain threshold (PPT) and the presence, severity, or number of involved knee/hip joints with radiographic osteoarthritis (rOA) or related symptoms.
Methods
Data for these cross-sectional analyses come from the second follow-up (2008–11) of the Johnston County Osteoarthritis Project (n=1,602). Pressure-pain threshold measurements were averaged over two trials from both the left and right trapezius. Outcomes of radiographic knee and hip OA were both defined by a Kellgren-Lawrence score of 2–4 and site-specific symptoms were ascertained at clinical interview. Associations were determine with multiple logistic regression models, and two-way interactions were tested at p<0.05.
Results
The sample was 67.2% female and 31.0% African American. Participants’ mean age was 67.9 (SD 9.0); mean body mass index was 31.5 (SD 7.1); mean Center for Epidemiologic Studies Depression Scale score was 6.5 (SD 7.4); and mean total PPT was 3.6kg (SD 0.7). Significant associations were found between PPT and self-reported knee/hip symptoms. No significant associations were found between PPT and presence, severity, or number of joints with knee and hip rOA without accompanying symptoms. No significant interactions were found with demographic or clinical characteristics.
Conclusion
Pressure-pain threshold was significantly associated with self-reported single and multi-joint symptoms. In contrast, after adjustment, PPT measured at the trapezius was not associated with asymptomatic knee or hip rOA. As such, PPT may prove to be a useful indicator of rOA pain processing and of why individuals respond favorably and others do not to treatments targeting rOA.
Introduction
Osteoarthritis (OA) is the most common form of arthritis, affecting an estimated 27 million adults in the United States.1 Osteoarthritis is also one of the leading causes of disability and a significant cause of decreased quality of life.2,3 There are several structural sources of knee or hip pain including, but not limited to, synovium5, bone marrow lesions, and soft tissues6. Conservative treatments for OA typically consist of symptom management as well as preservation of, or increase in, physical function.4 Plain film radiographs are commonly used in clinical practice to evaluate OA. However, the relationship between the presence of radiographic OA (rOA) and self-reported symptoms is variable, with only modest associations found between radiologic structural findings and pain.7 This mismatch between radiographic structural findings and symptoms has led to the study of other factors, such as pain processes that can be evaluated by pressure-pain threshold (PPT) measurement. This measurement may improve the understanding of an individual’s pain perception by identifying augmented pain processing.8 An improved understanding of the association between pain perception and knee or hip symptoms and rOA could be useful towards understanding why some patients require increased pain control for similar rOA and why some patients experience an ongoing refractory pain process following interventions, such as total joint arthroplasty, and others do not.13
An individual’s perception of pain is modulated by peripheral, spinal and supraspinal processes, which includes increased excitability of dorsal horn neurons, producing hypersensitivity in a segmental distribution.9 The study of altered pain processing is not new in the area of musculoskeletal pain disorders10 such as low back pain8, fibromyalgia11, and atypical facial pain12. Findings from these studies suggest augmented pain processing mechanisms in which pain results from altered pain processing rather than damaged or inflamed peripheral structures.8
The influence of altered pain processing in OA has been shown by studies of hand rOA14 and, more recently, with knee rOA15–17. A recent systematic review and meta-analysis found a significant relationship between PPT and symptomatic radiographic knee OA13, indicating that patients with symptomatic radiographic knee OA have a lower threshold for pain. Studies included in this review, however, were primarily clinic-based case-control studies with a limited and select sample from which to compare different subgroups of symptomatic and asymptomatic rOA. The lack of asymptomatic rOA participants among these studies limits the evidence that can be gained for the link between pain processing and rOA. In addition, this review did not address hip rOA. Morphometric differences between rOA in the knee versus hip suggests different pathophysiological processes.18,19 Similarities in vibratory perception threshold have been found to exist between knee and hip OA.20 However, there is limited evidence that abnormal pain processing measured with PPT is present with hip rOA and is similar to knee rOA. The purpose of the present analyses is to determine the association between PPT, knee/hip rOA, and self-reported knee/hip symptoms, as well as whether knee and hip rOA share similar pain processing or differ by joint site. We hypothesized that a significant association would be observed between PPT measures and self-reported symptoms, but no significant association would be found with rOA measures of disease without knowledge of symptoms regardless of joint site examined (i.e., knee and/or hip joint).
Methods
Study Participants
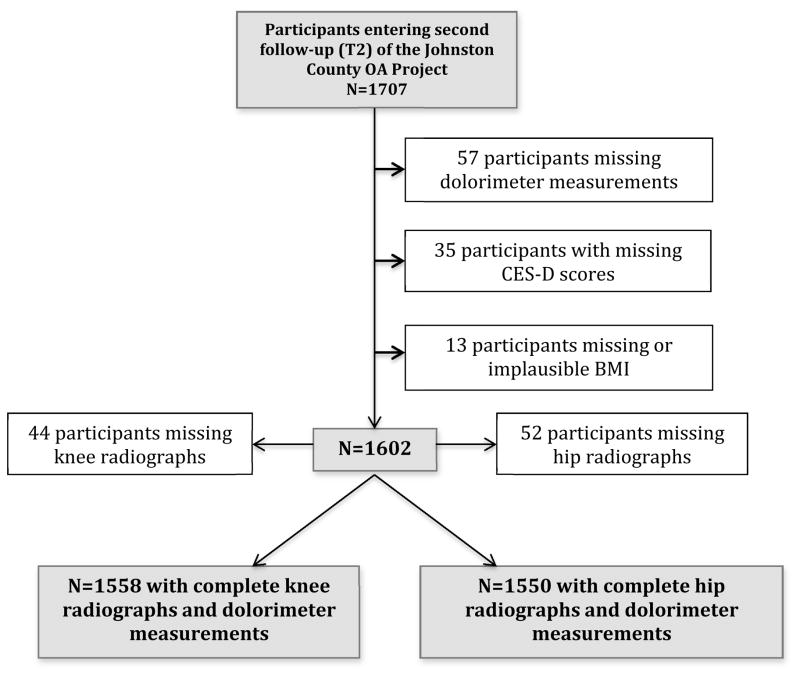
Data for these cross-sectional analyses were from the Johnston County (JoCo) OA Project, an ongoing, population-based study in six rural townships of JoCo, North Carolina. The purpose of the JoCo OA Project is to determine the incidence, prevalence and progression of knee, hip, hand, and spine rOA among African Americans (AAs) and Caucasians. Details of the sampling strategy and recruitment methods have been described in detail elsewhere.18 Briefly, baseline (T0) enrollment (1991–98) included civilian, noninstitutionalized adults aged 45 years and older recruited by probability sampling, with over-sampling of African Americans. Follow-up for each enrollment occurs approximately every 5 years where the majority of participants complete clinical interview and examination including radiographic assessment on the same day. The current analyses used data from the 1,707 participants who completed second follow-up (T2) from 2008–11. Of these participants, there were 57 with missing PPT measures, 13 with missing or implausible BMI measurements, and another 35 subjects missing Centers for Epidemiologic Studies-Depression Scale (CES-D) scores. Some participants were missing knee radiographs (n=52) and some hip radiographs (n=44), resulting in n= 1,558 for knee rOA analyses and n = 1,550 for hip rOA analyses (Figure 1).
Figure 1.
Description of Participants included in the Analyses.
Pressure-pain threshold measurements
Pain-pressure thresholds are inversely related to pressure pain sensitivity, i.e., a decreased PPT indicates increased pain sensitivity. PPT measurements, using a standard mechanical pressure-based dolorimeter, were used to assess each participant’s threshold (measured in kilograms of pressure) for distal pressure-pain. A single trained research assistant conducted all PPT clinical measurement following an a-priori measurement protocol. This type of training method has been shown to have good reliability.21 The measurement begins with a “practice trial” where a demonstration of the device is conducted with the participant. Measurements were then collected from both the left and right upper trapezius muscle in a systematic fashion consisting of two total measurements. Beginning with the left side, pressure was applied to the trapezius at a rate of 1 kg per second until selfreported pain. If a participant did not report pain at 4 kg of pressure, the value of pressure pain was recorded as “>4.0 kg”. Trials were continued until two consecutive readings were within +/−0.4 kg for a maximum of four trials. The same procedure was repeated for the right side. Values from the left and right trapezius were averaged to provide a single PPT score.
Knee and Hip Symptoms
Participants were asked at clinical interview about symptoms in left and right knees and left and right hips, both separately: “On most days do you have symptoms of pain, aching or stiffness in your [left/right] [knee/hip]?” Participants were considered to have chronic knee symptoms if they answered affirmatively to the knee symptoms question and to have chronic hip symptoms if they answered affirmatively to the hip symptoms question.
Radiographic Assessment
Bilateral posterior-anterior knee radiography was obtained for both knees with a Synaflexer (CCBR Synarc, San Francisco, CA) positioning device, and bilateral hip radiography with supine anterior-posterior pelvis radiographs. The presence of knee or hip prosthesis due to arthroplasty was the primary reason for missing radiographic data. All hip and knee radiographs were read for Kellgren- Lawrence (K-L) score by a single bone and joint radiologist (JBR). Presence of hip and knee rOA was defined as a K-L score of 2–4. Severity of hip and knee rOA was quantified as none with a K-L score=0 or 1, mild with a KL score=2 and moderate/severe with a K-L score=3 or 4. Inter-rater reliability (comparison of radiograph readings between JBR and another radiologist) and intra-rater reliability (comparison of radiograph readings completed by JBR at two separate times) have been reported previously with a weighted kappa for inter-rater reliability of 0.9 and kappa for intra-rater reliability of 0.9.22
Demographic and Clinical Characteristics
At the time of clinical interview, the following participant characteristics were collected: self-reported race (African American or Caucasian), age (in years), body mass index (BMI) at baseline (calculated as weight measured with a balance beam scale in kilograms/height measured without shoes in meters squared), and depressive symptoms (using the CESD23).
Statistical Analysis
Descriptive statistics were calculated for the entire sample and for subgroups of interest. Cochran–Mantel–Haenszel statistics for categorical variables and one-way ANOVA testing of linear trend for continuous variables compared all demographic and clinical characteristics (gender, race, age, BMI, knee rOA, hip rOA, knee symptoms, and hip symptoms, and CES-D Scale) across the combined (right and left) average distal PPT.
Multiple logistic regression models, with common referent coding, were used to examine the associations between PPT and both self-reported knee/hip symptoms (both presence (“yes” or “no”) and severity (none, mild, moderate or severe) and knee and/or hip rOA. All models were adjusted for age, race, sex, BMI, and CES-D Scale scores while models with the outcome of symptoms were also adjusted for the presence of rOA (to check for independence of associations). Because of the small number of both “severe” rOA and symptom responses, these values were collapsed with their respective “moderate” category. Two-way interactions were tested between demographic and clinical characteristics with each outcome at a significance of p<0.05. Odds ratios (ORs) and their 95% confidence intervals were the measure of association for all analyses. In multi-joint and multisite models, linear tests for trend were conducted with the natural log of the odds ratio. All statistical computations were performed using SAS Version 9.2 software (SAS Institute, Cary, NC).
Results
Table 1 provides descriptive statistics for the demographic and clinical characteristics of the entire study sample. The overall average of the summed left and right dolorimeter measurements was 3.6 kg of pressure (SD 0.7) with a median of 4.0 kg and range of 0–8 kg. Mean total PPT did not differ significantly by right or left measurements (mean difference − 0.02, p=0.28). Mean age was 67.9 years (SD 9.0) and mean BMI was 31.5 kg/m2 (SD 7.1). The majority were women (67.2%), and 31.0% of the sample consisted of AAs. Radiographic knee OA was present in nearly half the sample (45.7%) while hip OA was slightly less prevalent (40.3%). The presence of knee and hip symptoms was also common, with 38.4% reporting knee symptoms and 29.3% reporting hip symptoms.
Table 1.
Characteristics of the 1,602 participants with pain-pressure threshold measurements
| Characteristic | Mean (SD) or N (%) |
|---|---|
| Dolorimeter Average (kg)* | 3.6 (0.7) |
| Age (years) | 67.9 (9.0) |
| BMI (kg/m2) | 31.5 (7.1) |
| CES-D | 6.5 (7.4) |
| African American | 497 (31.0) |
| Women | 1,077 (67.2) |
| Knee rOA | |
| Present | 732 (45.7) |
| Mild | 245 (15.3) |
| Moderate/severe | 487 (30.4) |
| Knee symptoms | |
| Present | 615 (38.4) |
| Mild | 202 (12.6) |
| Moderate/severe | 276 (17.2) |
| Hip rOA | |
| Present | 646 (40.3) |
| Mild | 580 (36.2) |
| Moderate/severe | 66 (4.1) |
| Hip symptoms | |
| Present | 469 (29.3) |
| Mild | 155 (9.7) |
| Moderate/severe | 298 (18.6) |
median 4.0kg range (0–4kg)
Several significant differences in PPT averages were found across subgroups. Women had a significantly lower average PPT (3.52 kg [SD=0.80] vs. 3.89 kg [SD=0.41], p<0.001) than men, indicating elevated pain sensitivity among this subgroup. Participants with hip rOA had significantly lower PPTs than those without hip rOA (3.69 kg [SD=0.67] vs. 3.58 kg [SD=0.77], p=0.002); no significant difference was found between those with and without knee rOA. A lower average PPT was found among participants with knee symptoms compared to participants without knee symptoms (3.47 kg [SD=0.84] vs, 3.74 kg [SD=0.61], p=0.001) as well as with hip symptoms when compared to participants without hip symptoms (3.43 kg [SD=0.86] vs, 3.73 kg [SD=0.63], p<0.0001).
Table 2 presents the adjusted odds ratios (aOR) and 95% confidence intervals (95% CI) for associations between a one-unit increase in pressure-pain measurements and the presence and severity of knee/hip rOA or symptoms. As a participant’s threshold for pain increased, indicating decreased pain sensitivity, there was a 39% (aOR=0.61 ((95% CI 0.50, 0.74)) decreased odds of having moderate/severe self-reported hip symptoms. A similar association (aOR=0.59 ((95% CI 0.50, 0.70)) was found between PPT and self-reported moderate/severe knee symptoms. Radiographic OA status did not modify the relationship between PPT and self-reported symptoms for either the knee (interaction p-value=0.96) or hip (p=0.80). No significant associations were found between PPT and the presence of either knee or hip rOA.
Table 2.
Adjusted odds ratios (aOR) and 95% confidence intervals (95% CI) for associations between a 1-unit increase in pressure-pain threshold measurements and presence and severity of knee/hip rOA or symptoms.
| Outcomes* | rOA | Symptoms | |||
|---|---|---|---|---|---|
| aOR** | 95% CI | aOR*** | 95% CI | ||
| Knee | |||||
| Presence | Yes vs no | 1.05 | 0.89–1.23 | 0.64 | 0.54–0.76 |
| Severity | Mild vs no | 1.09 | 0.86–1.36 | 0.84 | 0.66–1.06 |
| Moderate/severe vs no | 1.03 | 0.86–1.24 | 0.61 | 0.50–0.74 | |
|
| |||||
| Hip | |||||
| Presence | Yes vs no | 0.91 | 0.78–1.06 | 0.67 | 0.57–0.79 |
| Severity | Mild vs no | 0.90 | 0.77–1.05 | 0.82 | 0.64–1.06 |
| Moderate/severe vs no | 1.01 | 0.70–1.45 | 0.59 | 0.50–0.70 | |
Referent for all outcomes is none (no rOA for rOA outcomes, no symptoms for symptoms outcomes)
Adjusted for age, sex, race/ethnicity, BMI, and CES-D.
Adjusted for age, sex, race/ethnicity, BMI, CES-D, and rOA.
Table 3 presents the adjusted associations between a one-unit increase in PPT among symptomatic and asymptomatic knee or hip rOA. A one-unit increase in PPT was significantly associated with the presence of knee symptoms with (aOR=0.77 ((95% CI 0.63–0.95)) and without (aOR=0.61 ((95% CI 0.49–0.77)) the presence of rOA in the knee. A similar association was found between the presence of hip symptoms with (aOR=0.63 ((95% CI 0.50–0.78)) and without (aOR=0.69 ((95% CI 0.56–0.86)) the presence of hip rOA. No significant associations were observed between PPT and knee or hip rOA without knowledge of symptoms.
Table 3.
Adjusted odds ratios (aOR) and 95% confidence intervals (95% CI) for associations between a 1-unit increase in pressure-pain threshold and knee or hip radiographic osteoarthritis (rOA) with and without symptoms.
| Outcomes | Categories | aOR* | 95% CI |
|---|---|---|---|
| Knee | |||
| rOA and Symptoms | without rOA and symptoms | referent | |
| without rOA, with symptoms | 0.61 | 0.49–0.77 | |
| with rOA, without symptoms | 1.14 | 0.89–1.47 | |
| with rOA and symptoms | 0.77 | 0.63–0.95 | |
|
| |||
| Hip | |||
| rOA and Symptoms | without rOA and symptoms | referent | |
| without rOA, with symptoms | 0.69 | 0.56–0.86 | |
| with rOA, without symptoms | 0.97 | 0.78–1.19 | |
| with rOA and symptoms | 0.63 | 0.50–0.78 | |
Adjusted for age, sex, race/ethnicity, BMI, and CES-D.
Table 4 presents the adjusted associations between a one-unit increase in PPT and the single and combined presence of knee and/or hip rOA or the presence of knee and/or hip symptoms. Significant associations were found among participants with hip symptoms (aOR=0.68 ((95% CI 0.53–0.88)) and a similar association among participants with knee symptoms (aOR=0.64 ((95% CI 0.51–0.80)). A stronger association was found among participants with both knee and hip symptoms. Increased threshold for pain (decreased pain sensitivity) was associated with a 50% decreased odds of self-reported knee and hip symptoms (aOR=0.50 ((95% CI 0.40–0.62)). No significant associations were found between PPT and either knee and/or hip rOA.
Table 4.
Adjusted odds ratios (aOR) and 95% confidence intervals (95% CI) for associations between a 1-unit increase in pressure-pain threshold and knee or hip radiographic osteoarthritis (rOA).
| Outcomes | Categories | aOR | 95% CI |
|---|---|---|---|
| rOA* | |||
| Knee and Hip | without knee or hip OA | referent | |
| without knee OA, with hip OA | 0.82 | 0.66–1.01 | |
| with knee OA, without hip OA | 0.98 | 0.79–1.21 | |
| both knee and hip OA | 0.96 | 0.76–1.20 | |
|
| |||
| Symptoms (Sx)** | |||
| Knee and Hip | without knee or hip Sx | referent | |
| without knee Sx, with hip Sx | 0.68 | 0.53–0.88 | |
| with knee Sx, without hip Sx | 0.64 | 0.51–0.80 | |
| both knee and hip Sx | 0.50 | 0.40–0.62 | |
Adjusted for age, sex, race/ethnicity, BMI, and CES-D.
Adjusted for age, sex, race/ethnicity, BMI, CES-D and rOA.
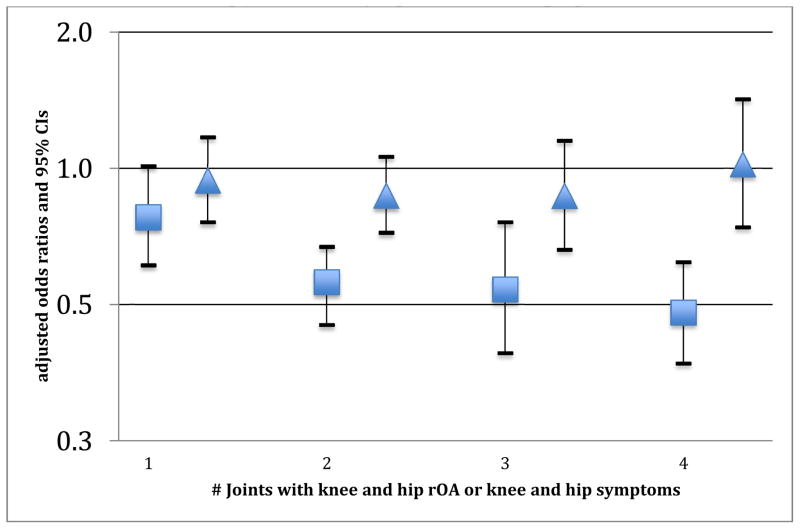
A significant trend (p-value for trend=0.001) was found between PPT-derived pain sensitivity and number of symptomatic knee or hip joints. Participants with lower pain sensitivity (i.e., greater PPT thresholds) were significantly less likely to have a greater total number of symptomatic knee and/or hip joints (1 joint=aOR 0.78 ((95% CI 0.61–1.01)), 2 joints=aOR 0.56 ((95% CI 0.45–0.67)), 3 joints=aOR 0.54 ((95% CI 0.39–0.76)), and 4 joints=aOR 0.48 ((95% CI 0.37–0.62)). In contrast, this relationship was not observed with knee and hip rOA involved joints (p-value for trend=0.78). (Figure 2) No significant two-way interaction effects were found with demographic or clinical characteristics in any of the models.
Figure 2. Adjusted associations between increased pressure-pain threshold and number of knee and hip joints with symptoms or radiographic osteoarthritis*.
*referent = those with no knees or hips with symptoms or rOA. Triangles = knee and hip rOA. Squares= knee and hip symptoms.
Discussion
Our study sought to determine the association between distal PPT, as a measure of augmented pain processing, and rOA and/or self-reported knee/hip symptoms. We hypothesized that PPT would be associated with self-reported symptoms but not with rOA. Indeed, as a participant’s sensitivity for pressure-pain decreased, there were several significant associations with presence, severity and number of joints with symptoms, regardless of the knee or hip joint. In contrast, across all rOA outcomes modeled, no significant associations were found between PPT and presence, severity or number of joints with knee or hip rOA. When comparing associations between PPT and self-reported symptoms, both the knee and hip joints had a similar strength and direction of association estimates. We also found that the relationship between a participant’s threshold for pain and self-reported symptoms was not influenced by the presence of either knee or hip rOA alone. These findings are similar to the weak and inconsistent relationships reported between structural changes in rOA and self-reported symptoms.7
Our results are consistent with previous case-control studies evaluating remote or distal PPT, finding modest correlations with patients who have symptomatic knee rOA awaiting knee replacement surgery15,24 or among clinic based participants25,26. PPT from a distal measurement such as the upper trapezius muscle evaluated in our study was not routinely evaluated in these studies. However, Immura and colleagues15 demonstrated consistent significant correlations between several different dermatomal, myotomal and sclerotomal sites and knee OA, thus indicating a constant differential PPT measurement across these structures. A significantly lower threshold for pain among distal and remote PPT measurements in symptomatic OA when compared to healthy controls has been reported in a recent meta-analysis.13 Many of these studies used for this meta-analysis did not examine the relationship between PPT and non-symptomatic knee rOA, limiting inferences about the role of localized symptoms. Our analyses included participants recruited independently of rOA and knee symptom status, which allowed several comparisons including presence of rOA with and without knee symptoms. Thus, we found that the presence of knee symptoms, and not the presence of knee rOA, was the important factor in understanding an individual’s pain sensitivity as measured by the threshold for pressure pain.
To our knowledge, ours is the first community-based cohort study to determine the association between distal pressure-pain and symptom status with hip rOA. Similar to one previous study of vibratory perception threshold, we determined if differences in PPT measurements would be present among those with hip rOA when compared to knee OA. We found a significant difference in distal PPT mean values across presence of hip rOA but not knee rOA. However, this difference may reflect known gender differences in presence and severity of hip rOA27,28 as this association was not found after adjustment in multivariable models. Our findings are consistent with two previous case-control studies finding that patients with symptomatic hip rOA have lower PPTs using remote pressurepain measurements when compared to those without hip rOA.29,30 However, as identical to knee rOA, our findings indicate that self-reported symptoms rather than the combination of rOA and symptoms are the major influence on the association with pressure-pain sensitivity.
The majority of previous studies using pain perception thresholds have focused on knee OA alone. This study compared PPT measurements for combinations of knee or hip rOA and knee or hip symptoms. Our findings indicate that the associations were similar in strength and followed the same directional trend. When both knee and hip symptoms were combined, the number of symptomatic joints had a significant increasing trend that was not observed with both knee and hip joint with rOA. These findings suggest that the presence of augmented pain processing in the knee and hip are similar and additive, regardless of the morphometric abnormalities cited as common etiologies for hip OA that may differ from knee OA.18,19, 31–33
A deeper understanding of the basic science of pain pathways mediating symptoms may lead to improved clinical management of pain in OA.6 Our results improve the understanding of the role of pain processing on the magnitude of OA symptoms, which, with future research, may have a large impact on clinical care and decision-making. For example, PPT measurement may be a useful predictor of refractory symptoms following joint replacement, as patients with lower thresholds for pain may be more susceptible to ongoing symptoms.13 These patients with lower thresholds for pain or refractory pain symptoms may benefit from pharmaceutical therapies that target pain-processing pathways.6 Another potential use of PPT measurement may be to identify patients who might respond differently for clinical trials of pain processing agents, or to identify patients that transition from acute to chronic pain13 or facilitate early identification of multiple joint OA or generalized symptoms. Early identification may in turn lead to early management and treatment tools that may be more effective than those in current practice.34 Our study also suggests that those with more severe knee or hip symptoms, those with combined knee and hip symptoms, or those with more numbers of knees and hips with symptoms might be more likely to be candidates for pharmaceutical or other interventions that address pain processing. Such concepts would require clinical trials to test if this supposition could be used clinically to select patients for specific pain-processing interventions.
Our cross-sectional analyses were derived from the largest and only community-based cohort study examining both knee/hip symptoms and knee/hip rOA with PPT. Additionally, our sample included African Americans and Caucasians of both genders, with similar findings across these demographic groups. Our large sample size, therefore, allowed for several comparison groups and assessment of trends to support our findings of association between symptoms and PPT. Our study, however, is not without limitations as we were not able to examine a regional pain process as all of our PPT measurements were conducted at sites distal (i.e., trapezius) from the areas of symptoms and rOA. Our results therefore may be more representative of a central pain process. Measurement bias is certainly possible with the PPT scores. However, we would assume that this bias is nondifferential and our estimates would underestimate the true effect. Furthermore, we utilized one individual to conduct PPT measurements following an a-priori protocol and the test-retest reliability of PPT in this fashion has been found to be reliable.21 Since our results come from a cross-sectional design, we could not infer a temporal relationship between pressure pain and rOA or symptoms. Furthermore, our questions related to knee/hip symptoms (i.e., on most days) may not provide a complete understanding of the chronicity of symptoms experienced by participants as it lacks a well-defined duration. Lastly, we adjusted for factors that may influence the relationship between PPT and rOA; however, we did not adjust for every known factor that may influence PPT measures such as fibromyalgia, low back pain or other musculoskeletal disorders.
Despite these limitations, our study provides a new understanding of an augmented pain processing in both the knee and hip joints as they relate to both symptoms and rOA. Based upon these results, self-reported symptoms are driven primarily by mechanisms of pain perception versus radiographic structural changes. This suggests, that at least in some individual’s, PPT evaluates an altered pain perception which may be a systemic state independent of structure. Continued work in the area of pain processing and response to interventions for OA-related pain is needed.
Significance and Innovations.
Sensitivity to painful pressure evaluated by the pressure-pain threshold (PPT) is significantly associated with both the presence and number of self-reported symptoms in the knee and/or hip joints.
No significant associations were found with PPT and radiographic measures of osteoarthritis (rOA) in knee and/or hip joints.
These findings improve our understanding of augmented pain processing in both the knee and hip joints as they relate to both symptoms and rOA
Acknowledgments
Funding Sources
NIH Loan Repayment Program, National Institute of Arthritis Musculoskeletal and Skin Diseases (1-L30-AR057661-02).
Supported by Agency for Health Care Research and Quality (AHRQ) K-12 Comparative Effectiveness Career Development Award grant number HS19479-01.
The Johnston County Osteoarthritis Project is supported in part by cooperative agreements S043, S1734, and S3486 from the Centers for Disease Control and Prevention/Association of Schools of Public Health; the NIAMS Multipurpose Arthritis and Musculoskeletal Disease Center grant 5-P60-AR30701; and the NIAMS Multidisciplinary Clinical Research Center grant 5 P60 AR49465-03.
We would like to acknowledge Holly R. Thompson, BA for her assistance with manuscript preparation and editing.
Footnotes
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of ARHQ, Centers for Disease Control and Prevention or NIAMS.
Contributor Information
Adam P. Goode, Assistant Professor, Department of Community and Family Medicine, Duke University.
Xiaoyan A. Shi, SAS Institute, Cary, NC.
Richard H. Gracely, Professor, Director Clinical Research, Center for Pain and Innovation, University of North Carolina at Chapel Hill.
Jordan B. Renner, Professor, Department of Radiology, University of North Carolina at Chapel Hill.
Joanne M. Jordan, Professor, Department of Rheumatology, Medicine and Epidemiology, Director Thurston Arthritis Center, University of North Carolina at Chapel Hill.
References
- 1.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58(1):26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dillon CF, Rasch EK, Gu Q, Hirsch R. Prevalence of knee osteoarthritis in the United States: arthritis data from the Third National Health and Nutrition Examination Survey 1991–94. The Journal of rheumatology. 2006;33(11):2271–2279. [PubMed] [Google Scholar]
- 3.Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54(1):226–229. doi: 10.1002/art.21562. [DOI] [PubMed] [Google Scholar]
- 4.Hochberg MC, Altman RD, April KT, Benkhalti M, Guyatt G, McGowan J, et al. American College of Rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis care & research. 2012;64(4):465–474. doi: 10.1002/acr.21596. [DOI] [PubMed] [Google Scholar]
- 5.Baker K, Grainger A, Niu J, Clancy M, Guermazi A, Crema M, et al. Relation of synovitis to knee pain using contrast-enhanced MRIs. Annals of the rheumatic diseases. 2010;69(10):1779–1783. doi: 10.1136/ard.2009.121426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sofat N, Ejindu V, Kiely P. What makes osteoarthritis painful? The evidence for local and pain processing. Rheumatology (Oxford) 2011;50(12):2157–2165. doi: 10.1093/rheumatology/ker283. [DOI] [PubMed] [Google Scholar]
- 7.Claessens AA, Schouten JS, van den Ouweland FA, Valkenburg HA. Do clinical findings associate with radiographic osteoarthritis of the knee? Annals of the rheumatic diseases. 1990;49(10):771–774. doi: 10.1136/ard.49.10.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Giesecke T, Gracely RH, Grant MA, Nachemson A, Petzke F, Williams DA, et al. Evidence of augmented pain processing in idiopathic chronic low back pain. Arthritis and rheumatism. 2004;50(2):613–623. doi: 10.1002/art.20063. [DOI] [PubMed] [Google Scholar]
- 9.Schadrack J, Neto FL, Ableitner A, Castro-Lopes JM, Willoch F, Bartenstein P, et al. Metabolic activity changes in the rat spinal cord during adjuvant monoarthritis. Neuroscience. 1999;94(2):595–605. doi: 10.1016/s0306-4522(99)00186-4. [DOI] [PubMed] [Google Scholar]
- 10.Geisser ME, Glass JM, Rajcevska LD, Clauw DJ, Williams DA, Kileny PR, et al. A psychophysical study of auditory and pressure sensitivity in patients with fibromyalgia and healthy controls. J Pain. 2008;9(5):417–422. doi: 10.1016/j.jpain.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 11.Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis and Rheumatism. 2002;46(5):1333–1343. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 12.Derbyshire SW, Jones AK, Devani P, Friston KJ, Feinmann C, Harris M, et al. Cerebral responses to pain in patients with atypical facial pain measured by positron emission tomography. J Neurol Neurosurg Psychiatry. 1994;57(10):1166–1172. doi: 10.1136/jnnp.57.10.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Suokas AK, Walsh DA, McWilliams DF, Condon L, Moreton B, Wylde V, et al. Quantitative sensory testing in painful osteoarthritis: a systematic review and meta-analysis. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2012;20(10):1075–1085. doi: 10.1016/j.joca.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Farrell M, Gibson S, McMeeken J, Helme R. Pain and hyperalgesia in osteoarthritis of the hands. The Journal of rheumatology. 2000;27(2):441–447. [PubMed] [Google Scholar]
- 15.Imamura M, Imamura ST, Kaziyama HH, Targino RA, Hsing WT, de Souza LP, et al. Impact of nervous system hyperalgesia on pain, disability, and quality of life in patients with knee osteoarthritis: a controlled analysis. Arthritis and rheumatism. 2008;59(10):1424–1431. doi: 10.1002/art.24120. [DOI] [PubMed] [Google Scholar]
- 16.Geisser ME, Gracely RH, Giesecke T, Petzke FW, Williams DA, Clauw DJ. The association between experimental and clinical pain measures among persons with fibromyalgia and chronic fatigue syndrome. Eur J Pain. 2007;11(2):202–207. doi: 10.1016/j.ejpain.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 17.Harris RE, Williams DA, McLean SA, Sen A, Hufford M, Gendreau RM, et al. Characterization and consequences of pain variability in individuals with fibromyalgia. Arthritis and Rheumatism. 2005;52(11):3670–3674. doi: 10.1002/art.21407. [DOI] [PubMed] [Google Scholar]
- 18.Jordan JM, Helmick CG, Renner JB, Luta G, Dragomir AD, Woodard J, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2007;34(1):172–180. [PubMed] [Google Scholar]
- 19.Jordan JM, Helmick CG, Renner JB, Luta G, Dragomir AD, Woodard J, et al. Prevalence of hip symptoms and radiographic and symptomatic hip osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. The Journal of rheumatology. 2009;36(4):809–815. doi: 10.3899/jrheum.080677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shakoor N, Lee KJ, Fogg LF, Block JA. Generalized vibratory deficits in osteoarthritis of the hip. Arthritis and rheumatism. 2008;59(9):1237–1240. doi: 10.1002/art.24004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wylde V, Palmer S, Learmonth ID, Dieppe P. Test-retest reliability of Quantitative Sensory Testing in knee osteoarthritis and healthy participants. Osteoarthritis and cartilage/OARS, Osteoarthritis Research Society. 2011;19(6):655–658. doi: 10.1016/j.joca.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Feldman C, Nothstein G, Somaiya CK, Obeidallah H, Silverthorne E, Wunderlich S, et al. An exploratory investigation of the risk of pathogenic contamination at selected New Jersey skilled nursing and assisted living residences. Perspect Public Health. 2011;131(2):85–88. doi: 10.1177/1757913910391042. [DOI] [PubMed] [Google Scholar]
- 23.Burton AK, Balague F, Cardon G, Eriksen HR, Henrotin Y, Lahad A, et al. Chapter 2. European guidelines for prevention in low back pain : November 2004. European spine journal : official publication of the European Spine Society, the European Spinal Deformity Society, and the European Section of the Cervical Spine Research Society. 2006;15 (Suppl 2):S136–168. doi: 10.1007/s00586-006-1070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wylde V, Hewlett S, Learmonth ID, Dieppe P. Persistent pain after joint replacement: prevalence, sensory qualities, and postoperative determinants. Pain. 2011;152(3):566–572. doi: 10.1016/j.pain.2010.11.023. [DOI] [PubMed] [Google Scholar]
- 25.Arendt-Nielsen L, Nie H, Laursen MB, Laursen BS, Madeleine P, Simonsen OH, et al. Sensitization in patients with painful knee osteoarthritis. Pain. 2010;149(3):573–581. doi: 10.1016/j.pain.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Lee YC, Lu B, Bathon JM, Haythornthwaite JA, Smith MT, Page GG, et al. Pain sensitivity and pain reactivity in osteoarthritis. Arthritis care & research. 2011;63(3):320–327. doi: 10.1002/acr.20373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maillefert JF, Gueguen A, Monreal M, Nguyen M, Berdah L, Lequesne M, et al. Sex differences in hip osteoarthritis: results of a longitudinal study in 508 patients. Annals of the rheumatic diseases. 2003;62(10):931–934. doi: 10.1136/ard.62.10.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zouboulis CC, Bechara FG, Fritz K, Kurzen H, Liakou AI, Marsch WC, et al. S1 guideline for the treatment of hidradenitis suppurativa/acne inversa * (number ICD-10 L73.2) J Dtsch Dermatol Ges. 2012;10 (Suppl 5):S1–31. doi: 10.1111/j.1610-0387.2012.08006.x. [DOI] [PubMed] [Google Scholar]
- 29.Kosek E, Ordeberg G. Abnormalities of somatosensory perception in patients with painful osteoarthritis normalize following successful treatment. Eur J Pain. 2000;4(3):229–238. doi: 10.1053/eujp.2000.0175. [DOI] [PubMed] [Google Scholar]
- 30.O’Driscoll SL, Jayson MI. Proceedings: Pain threshold (PT) analysis in patients with osteoarthritis of the hip. Annals of the rheumatic diseases. 1975;34(2):195–196. doi: 10.1136/ard.34.2.195-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gregory JS, Waarsing JH, Day J, Pols HA, Reijman M, Weinans H, et al. Early identification of radiographic osteoarthritis of the hip using an active shape model to quantify changes in bone morphometric features: can hip shape tell us anything about the progression of osteoarthritis? Arthritis and Rheumatism. 2007;56(11):3634–3643. doi: 10.1002/art.22982. [DOI] [PubMed] [Google Scholar]
- 32.Gosvig KK, Jacobsen S, Sonne-Holm S, Palm H, Troelsen A. Prevalence of malformations of the hip joint and their relationship to sex, groin pain, and risk of osteoarthritis: a population-based survey. The Journal of bone and joint surgery. 2010;92(5):1162–1169. doi: 10.2106/JBJS.H.01674. American volume. [DOI] [PubMed] [Google Scholar]
- 33.Dudda M, Kim YJ, Zhang Y, Nevitt MC, Xu L, Niu J, et al. Morphologic differences between the hips of Chinese women and white women: could they account for the ethnic difference in the prevalence of hip osteoarthritis? Arthritis and Rheumatism. 2011;63(10):2992–2999. doi: 10.1002/art.30472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.A call to action from the Chronic Osteoarthritis Management Initiative (COAMI) September 2012. Orthop Nurs. 2012;31(6):359–361. doi: 10.1097/NOR.0b013e318278767a. [DOI] [PubMed] [Google Scholar]