Abstract
Background
Robotic training can help improve function of a paretic limb following a stroke, but individuals respond differently to the training. A predictor of functional gains might improve the ability to select those individuals more likely to benefit from robot based therapy. Studies evaluating predictors of functional improvement after a robotic training are scarce. One study has found that white matter tract integrity predicts functional gains following a robotic training of the hand and wrist.
Objective
Determine the predictive ability of behavioral and brain measures to improve selection of individuals for robotic training.
Methods
Twenty subjects with chronic stroke participated in an 8-week course of robotic exoskeletal training for the arm. Before training, a clinical evaluation, fMRI, diffusion tensor imaging, and transcranial magnetic stimulation (TMS) were each measured as predictors. Final functional gain was defined as change in the Box and Block Test (BBT). Measures significant in bivariate analysis were fed into a multivariate linear regression model.
Results
Training was associated with an average gain of 6±5 blocks on the BBT (p<0.0001). Bivariate analysis revealed that lower baseline motor evoked potential (MEP) amplitude on TMS, and lower laterality M1 index on fMRI each significantly correlated with greater BBT change. In the multivariate linear regression analysis, baseline MEP magnitude was the only measure that remained significant.
Conclusion
Subjects with lower baseline MEP magnitude benefited the most from robotic training of the affected arm. These subjects might have reserve remaining for the training to boost corticospinal excitability, translating into functional gains.
Keywords: predictor, robot, training, stroke, function
Introduction
Stroke is one of the leading causes of long term disability in the United States (1). In order to better understand the recovery process after stroke, studies have tried to find predictors of natural recovery of the affected limbs to minimize the impact of disability on function of stroke survivors. For the upper limb, both behavioral (eg. age, side of stroke lesion, degree of impairment) and neurophysiological (presence or absence of motor evoked potential) outcomes have been found to predict natural recovery of the affected upper limb after stroke (2, 3). Six months after stroke, 50% of the survivors are still left with some contralesional motor disability (1), greatly impacting performance of everyday activities (4).
Training the affected limb following a stroke is known to be an effective way to improve recovery and allow functional gains. One approach that is gaining popularity in rehabilitation settings is robotic training. Robotic devices have the capability to provide intense, repetitive, task-specific exercises in a reliable and controlled manner (5). Several studies evaluating the impact of robotic training have noted significant gains in function of the trained limb associated with improvement in activities of daily living (6-11). However, while group means show significant treatment gains, substantial inter subject variability in training responses is present, with some subjects presenting minimal to no gain (6-11). The reasons for this variability are as yet unclear. Determining which individuals are more likely to benefit from robotic training could help improve selection for this therapeutic approach, an important consideration given the need to use rehabilitation resources with optimal efficiency.
One approach to predicting response to specific therapies is to determine baseline measures capable of predicting functional gains following treatment (12-17). Descriptive and behavioral measures, such as age or motor impairment at the affected limb, have been found to predict final functional gains after stroke. For example, Lin et al (2009) found that baseline motor impairment of the affected hand and wrist was the best predictor of functional improvement after constraint-induced therapy (CIT) in chronic stroke survivors, such that individuals showing greater baseline motor ability benefited the most from therapy. Various brain measures have also been found to predict treatment effect. For example, at baseline, lower ipsilesional primary motor cortex activation by functional magnetic resonance imaging (fMRI) predicted arm motor gains from motor cortex stimulation and greater structural integrity of the ipsilesional corticospinal tract by diffusion tensor imaging (DTI) fractional anisotropy (FA) predicted arm motor gains from task-specific upper limb training (12, 18). Improved prediction of functional gain following therapy has also been noted when combining behavioral and brain status measures (12).
Despite the increasing evidence supporting the utility of robotic training to improve outcome after stroke, few studies have sought to identify predictors of functional gains. Riley et al (17) found that less white matter tract injury at baseline significantly predicted greater arm motor gains from a robotic intervention focused on the distal upper extremity. So far, no studies have attempted to determine predictors of functional gains following robotic training focused on the entire arm, i.e., both proximal and distal upper extremity. As behavioral and brain function measures can reflect the state of the motor cortex and corticospinal tract and their capacity to undergo plasticity with treatment, it is important to include both measures in determining the best predictors of functional gains from robotic therapy. The goal of the present study was to examine a range of measures, including behavioral, brain function via fMRI, DTI and neurophysiology via transcranial magnetic stimulation (TMS), to identify the best predictors of functional gains from robotic therapy. With our robotic therapy, it is thought that the assisted movement will prime the activity of the primary motor cortex circuits facilitating exercise-dependent plasticity (12). Knowing that functional gains following therapy rely on the integrity of the motor system (12, 17), we expected that, at baseline, greater MEP amplitude, greater ipsilesional M1 activation, lower FA asymmetry, and lesser motor impairments would be good predictors of larger gains following a robotic training of the entire affected arm.
Methods
Subjects
The study inclusion criteria were as follows: 1) between the age of 18-73 years old, 2) had a unilateral chronic stroke (three months or more), 3) be able to lift, move and drop at least two blocks on the Box and Block Test with the affected upper extremity, and 4) be able to undergo MRI, TMS, or both. Subjects were excluded if they met any of the following: 1) contracture at the upper extremity (modified Ashworth scale >4); 2) significant subluxation or pain (score < 1 on the pain section of the Fugl-Meyer) in affected shoulder; 3) inability to passively abduct or flex the affected shoulder to 90 degrees without pain; 4) severe neglect (19), apraxia (20), and severe sensation deficit (21) sufficient to preclude repetitive reaching to visual targets; 5) any substantial decrease in alertness, language reception, or attention (score ≥ 1 on question 1 or score of 3 on question 9 of the NIH Stroke scale); 6) concurrent severe medical problems (including neurological, cardiovascular, orthopedic or psychiatric problems); 7) antispasticity medication changes within six weeks of study entry; and 8) current participation in another trial of a rehabilitation therapy. Informed consent was obtained from each subject. The University of California, Irvine Institutional Review Board approved the study.
Clinical and brain assessments
A clinical assessment was performed by a trained physical therapist in the week before the beginning of the robotic training as well as at the end of the 8-week robotic training. The evaluator gathered demographic data as well as baseline clinical measures. The primary outcome measure was change in the Box and Block Test (BBT) (22) from baseline to week 8, a measure that assesses both proximal and distal upper extremity function. The BBT is scored as the number of blocks an individual can transfer from one BBT compartment to the other with the affected arm in 60 seconds. A higher number of blocks transferred indicates better manual dexterity. Additional clinical measures included the Fugl Meyer arm motor scale (23), the Wolf motor function test (24), the Motor Activity Log (25), grip and pinch strength, and the modified Ashworth scale (26).
Brain assessments consisted of an MRI scan and TMS evaluation, each obtained in the week preceding robotic training. MRI data were collected with a 3-T Philips scanner, using a T/R head coil. First, high-resolution T1-weighted anatomical images were acquired in axial orientation (TR 8.4 ms; TE 3.9 ms; flip angle 8°; number of slices 160; resolution 1×1×1 mm3). Second, fMRI data were obtained by use of gradient echo planar T2*-weighted imaging collected in axial orientation (TR 2000ms; TE 30ms; flip angle 70°; number of slices 31, gap between slices 1mm; slice thickness 4mm; in-plane voxel size 1.8.mm×1.8mm). Each fMRI run alternated 32 seconds blocks of rest with 0.25 Hz affected wrist flexion/extension. A total of 64 volumes were obtained for each run, and four such runs were obtained for a total of 256 brain volumes. During scanning, subject movements were guided by a moving figure representing the hand, which moved from wrist flexion to extension at a pace of 0.25 Hz. When the moving hand was red, subjects remained at rest, whereas when the hand turned green, subjects had to flex/extend their affected wrist following the 0.25 Hz pace of the figure. Overall, subjects performed 32 wrist movements (8 movements per direction, repeated 4 times). Before scanning, each subject practiced the task to make sure they properly understood the instructions. Third, DTI images were acquired using 32 non-colinear directions (TR 11 190ms, TE 69ms; number of slices 60; slice thickness 2mm; gap between slice 0mm; b values 800 s/mm2 and 0 s/mm2).
TMS was performed using each subject’s T1-weighted anatomical MRI imported into Brainsight stereotactic software (Rogue Research; Montreal, Quebec). A 10 × 10 grid with markers spaced 1 cm apart was superimposed on each subject’s T1 image, making sure to include premotor and peri Rolandic areas. The grid was intended to guide the consistency of brain stimulation at any given location and ensure adequate sampling of the motor cortex during determination of the hotspot. Single pulse TMS (Magstim 200-2 stimulator; MagStim Compagny, Dyfed, UK) was delivered to the ipsilesional motor cortex using a 70-mm figure-of-eight coil. Resting motor evoked potentials (MEP) were recorded from the affected extensor carpis radialis (ECR) with disposable pre-gelled silver-silver chloride surface EMG electrodes (gain= 10,000x, bandpass filters= 30-1,000Hz, sampling= 12,000). Electrodes were positioned 2 cm apart over the middle portion of the ECR belly and placed in parallel to the length of the muscle. A ground electrode was placed on the styloid process of the ulna. Scope (ADI; Colorado Springs, CO) was used to record EMG data. For each magnetic pulse, a 200 ms sample (40 ms pre and 160 ms post-TMS pulse) was displayed on a computer and stored for offline analysis. Background EMG was analyzed to ensure that the target muscle was at rest during testing. Thereby, mean corrected root mean square (RMS) EMG value of the 40 ms preceding each TMS pulse of the 110% I/O curve was calculated for each subject in whom an MEP could be elicited.
First, the site with the lowest resting motor threshold for a motor evoked response was determined (>100μV (27) in at least 4/7 trials). Once found, input/output curves were then recorded at 90%, 110%, 130%, and 150% of the motor threshold (10 pulses/stimulus intensity), with the order pseudorandomized to avoid cumulative effects of increasing the intensity of stimulation. If peak-to-peak MEP amplitude did not reach the chosen threshold of 100μV even at the maximum output of the stimulator (100%), the subject was considered as not having any MEP.
Brain data analysis
Analysis of fMRI data was done using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/). Images were realigned to the first image, coregistered, normalized to the MNI reference brain, and spatially smoothed (FWHM of 8mm). Images at rest were contrasted with images during the motor task. Any fMRI runs showing excess artifact due to head motion were removed from further analysis. Volume of activation, degree of activation (percent change in MRI signal between rest and motor task), and weighted laterality index (wLI), within specific regions of interest (ROI), were determined. Three 12-mm sphere ROI were drawn with Marsbar (http://marsbar.sourceforge.net/) based on the coordinates (x,y,z) of the meta-analysis of Mayka et al (2006) (28): ipsilesional primary motor cortex (M1 of the hand area) 37, −25, 62; ipsilesional dorsal premotor cortex (PMd) 30, −7, 63; and a single midline supplementary motor area (SMA) 0, −10, 59. The weighted laterality index was calculated according to Calautti et al (2007) (29) with values ranging from +1 (activation ipsilesionally, i.e., on the side contralateral to the wrist movement) to −1(activation contralesionally, i.e., on the side ipsilateral to the wrist movement).
DTI data were analyzed using FSL (http://www.fmrib.ox.ac.uk/fsl). Data preprocessing included eddy current correction as well as computation of diffusion parameters. Probabilistic tractography of the corticospinal tract was generated with the FDT toolbox of FSL using parameters for the seed, masks and targets described by Giorgio et al (2010) (30). Tract volumes were extracted for both sides. To determine values for FA and mean diffusivity (MD) within the corticospinal tract of the ipsilesional and contralesional hemisphere, tractography defined ROI were created separately for each subject. ROI were drawn at the peduncle, corresponding approximately to z=−22 in MNI space, and at the posterior limb of the internal capsule (PLIC), corresponding approximately to z=−8 in MNI space. Within the locations of interest, we defined a single voxel and centered a 4-mm box of fixed volume to make sure that the ROI would have the same volume between sides. FA asymmetry and MD asymmetry were computed following these formula: FAunaffected − FAaffected/FAunaffected + FAaffected, and MDunaffected − MDaffected/MDunaffected + MDaffected.
TMS data were analyzed offline using Scope (ADI; Colorado Springs, CO). Three groups of parameters were extracted. First, for each input stimulus intensity (90%, 110%, 130%, 150%), the peak-to-peak MEP amplitude was measured then averaged across the ten pulses. Second, latency, corresponding to the time (in ms) elapsed between the TMS pulse and the onset of the MEP signal, was extracted. Note that for the MEP amplitude and latency, values taken at 110% of motor threshold were retained. Third, recruitment curves were extracted by computing the slope of the averaged MEP amplitudes by the input stimulus intensity, following the procedure of Kleim et al. (31).
Robotic training program
The robotic training was performed using the Biomimetic Orthosis for the Neurorehabilitation of the Elbow and Shoulder (BONES). The features of BONES have been described in detail previously (32). Briefly, BONES assists in movement of the affected arm allowing it to move through normal wide workspace at the shoulder (flexion/extension, horizontal abduction/adduction, external/internal rotation), elbow (flexion/extension), forearm (pronation/supination), and wrist (flexion/extension), and also measures hand grasp and release. The high intensity, repetitive robotic training of the affected arm was held 3X/week for 8 weeks, for a total of 24 sessions. Each session lasted between 60 and 90 minutes and was supervised by a trained physical therapist. The computerized training comprised the tracking of a 3D phantom, one joint at a time, and functional games, where subjects had to use their entire affected arm to accomplish various computer simulated tasks such as driving or baseball (33). Subjects performed a total of 360 repetitions during each training session.
Statistical analysis
Change in the Box and Block Test following the robotic training was assessed by a paired t-test. The normality of data was assessed using the Shapiro-Wilk W test. For any data that were not normally distributed, transformation was attempted. Any data that could not be transformed were examined with non parametric statistical methods in the bivariate analysis. Bivariate analysis was performed to determine the relationship that change in BBT had with each baseline behavioral and brain assessment of interest, using Pearson’s product-moment correlation, for normally distributed data and Spearman’s rho correlation for not normally distributed data. All significant (p<0.05) baseline variables were then entered in a multivariate linear regression analysis using a forward stepwise model (probability to enter in the model p=0.25; probability to leave the model p=0.10) to determine which baseline variables retained a significant relationship with the change in BBT. Note that if a baseline variable that was not normally distributed showed a significant relationship with the change in BBT, it was either dichotomized or trichotomized based on visual inspection of the distribution and a hierarchical cluster analysis before being entered in the multivariate linear regression analysis. All statistical analysis were performed using SPSS® and JMP-5®, were two-tailed, and used level of statistical significance of p=0.05.
Results
Twenty subjects (12 male; 8 female) participated in the study. Fourteen subjects had right hemiparesis of the upper limb and six had left hemiparesis. Five subjects did not undergo TMS evaluation and one subject did not undergo MRI due to contraindications to the procedure (see Table 1 and Figure 1). For those subjects in whom an MEP could be detected, resting motor threshold ranged between 38 and 95% of stimulator output (mean 59 ± 20%), and RMS background EMG was (mean ± SD) 0.008 ± 0.005 mV [range: 0.005 0.019 mV]).
Table 1.
Distribution of all baseline potential predictive variables and correlation with the change in the Box and Block Test
| Baseline values (mean ± SD) |
Correlation with the change in the Box and Block Test |
p value | |
|---|---|---|---|
|
| |||
| Potential predictive variables | |||
| Age (years) [range] | 61 ± 6 [39;72] | −0.18 | 0.45 |
| Time since stroke (months) | 38 ± 38 | −0.40 | 0.079 |
| Box and Block Test (# blocks in 60 s) | 31 ± 13 | −0.26 | 0.27 |
| Fugl-Meyer arm motor scale (normal = 66) | 52 ± 8 | −0.35 | 0.13 |
| Wolf Motor Function Test : | |||
| Score (max=5) | 3.9 ± 0.6 | −0.37 | 0.11 |
| Time to completion (max = 1800 s) | 7.9 ± 11.6 | 0.11a | 0.63 |
| Weight (max = 20 lbs) | 13 ± 6 | −0.24a | 0.31 |
| Motor Activity Log: Amount of use (max = 5) | 2.8 ± 1.0 | 0.13 | 0.58 |
| Quality of movement (max = 5) | 2.4 ± 1.1 | 0.005 | 0.98 |
| Grip strength (kg) | 18.8 ± 14.0 | −0.39a | 0.089 |
| Pinch strength (kg) | 4.3± 2.5 | −0.35 | 0.13 |
| Modified Ashworth scale: Shoulder (normal = 0) | 0.3 ± 0.8 | 0.13a | 0.59 |
| Elbow (normal = 0) | 0.8 ± 1.0 | 0.15a | 0.54 |
| Baseline MEP amplitude (mV)b | 0.97 ± 1.13 | −0.61a | 0.016 |
| Presence (P) or absence (A) of MEPb | 8P/7A | −0.46 | 0.085 |
| Baseline recruitment slope (uV/% of stimulator output) | 0.03 ± 0.05 | −0.46a | 0.087 |
| Baseline latency (ms)b | 18.8 ± 1.9 | 0.20 | 0.63 |
| Volume of activation (mm3): M1 | 505 ± 252 | −0.43 | 0.063 |
| SMA | 528±216 | −0.25 | 0.31 |
| PMd | 381 ± 274 | −0.43 | 0.064 |
| Degree of activation (% change): M1 | 0.18 ± 0.71 | −0.21a | 0.38 |
| SMA | 0.53 ± 0.30 | −0.09a | 0.72 |
| PMd | 0.01 ± 0.58 | −0.29a | 0.22 |
| Weighted laterality indexc: M1 | 0.71 ± 0.52 | −0.53a | 0.022 |
| PMd | 0.37 ± 0.68 | −0.32a | 0.18 |
| FA asymmetryd: PLIC | 0.09 ± 0.11 | 0.21 | 0.31 |
| Peduncle | 0.12 ± 0.09 | −0.04 | 0.88 |
| MD asymmetry: PLIC | −0.06 ± 0.09 | −0.18a | 0.46 |
| Peduncle | −0.09 ± 0.14 | 0.13a | 0.60 |
| Corticospinal tract volume (mm3): Affected side | 7805 ± 3735 | 0.01 | 0.97 |
| Unaffected side | 11527±3190 | 0.02 | 0.97 |
| Lesion size (ml) | 8.9 ± 12.7 | 0.02 | 0.93 |
Spearman's rho correlation coefficient, otherwise Pearson's correlation coefficients are presented
For the MEP data (amplitude, presence/absence, and latency), values are presented for stimulation at 110% of motor threshold
For the weighted laterality index, no SMA value is shown because SMA was defined as a single midline ROI
Note that for the FA value for both the PLIC and peduncles, FA in the ipsilesional and contralesional corticospinal tracts each also showed no correlation
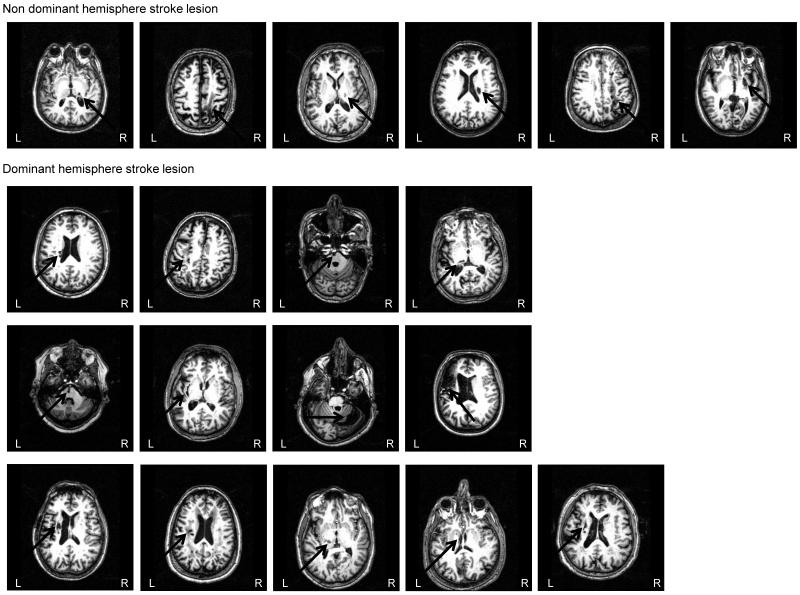
Figure 1.
Stroke location of each subject. Note that one subject did not undergo MRI scanning so data of 19 subjects are presented. L= left hemisphere; R = right hemisphere.
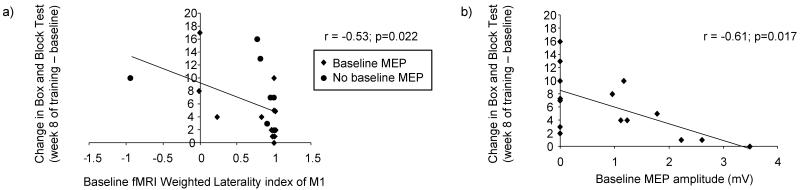
After 8 weeks of robotic training, a significant improvement in the Box and Block Test was noted with a mean gain of 6 ± 5 in the number of blocks taken in 60 s (mean value at baseline of 31 ± 13 vs. mean value at week 8 of 37 ± 13 blocks; p<0.0001). Further results of the impact of the robotic training on function of the affected upper limb are reported in greater detail in a separate report (33). Table 1 shows the baseline values of the predictive variables of interest. Two of these variables—baseline MEP amplitude at 110%, and the fMRI weighted laterality index of M1 -- showed a significant relationship with the change in the BBT and were retained for the stepwise forward multivariate linear regression model (Figure 2). Thus, at baseline, smaller MEP amplitude, and lower LI (ie interhemispheric activation balance more shifted contralesionally) each predicted larger gains from the subsequent 8-week course of robotic therapy. Before being entered in the multivariate linear regression analysis, baseline MEP amplitude was trichotomized (no MEP = 0; MEP between 0.96-1.23 = low; MEP >1.23 = high) and fMRI weighted laterality index of M1was dichotomized (<0.75 = low; >0.75 = high).
Figure 2.
Correlation between the change in the Box and Block Test and significant baseline potential predictive variables (a,b). Comparison of change in Box and Block Test and baseline MEP amplitude was done with stimulation at 110% of the motor threshold. Note that in Figure 2a and 2b any overlapping data points have been jittered slightly so that all points are visible.
The results of the stepwise analysis revealed that across all subjects, only baseline MEP magnitude remained as a significant variable (Table 2). In other words, subjects having less excitability of their corticospinal tract before training were the ones benefiting most from the robotic training, as assessed by the change in the BBT. When only the eight subjects who did have an MEP at baseline were examined in this model, results were identical, as baseline MEP magnitude remained the only predictor of improvement in BBT (r= −0.72; F(1,8); p=0.044). Interestingly, when this model was repeated using only the six subjects who had no baseline MEP (excluding one subject with an atypical fMRI weighted laterality index of −0.92), the fMRI weighted laterality index of M1 became the sole significant predictor of functional gains (r= −0.81; F(1,6); p=0.051).
Table 2.
Multivariate model summary
| Parameter | Estimate | t ratio | prob > |t| |
|---|---|---|---|
| Constant | 4.69 | 4.05 | 0.0014 |
| Baseline MEP magnitude* | −2.94 | −2.54 | 0.024 |
MEP was determined with stimulation at 110% of motor threshold The r2 of the overall model = 0.33
Discussion
This study aimed to identify predictors of functional gains from robotic training of the affected upper limb in subjects with chronic stroke. Results showed a significant improvement in the performance of the affected upper limb, as assessed by the change in score of the Box and Block Test, and that overall, this change in function was best predicted by a measure of excitability of the damaged corticospinal tract; in subjects whose motor cortex was unexcitable and thus did not have MEP, the fMRI LI measure was the best predictor of treatment gains. Together, these findings suggest that corticospinal tract integrity plays a crucial role in treatment gains post stroke and along with fMRI LI, it can reflect the state and capacity of the motor system to undergo plasticity following robotic therapy.
The results of the present study corroborate previous studies that found that TMS is useful for predicting functional changes derived from treatment (14, 18). Because magnitude of baseline corticospinal excitability best predicted the gains obtained, this means that an evaluation of neurophysiology before robotic training, in the form of MEP amplitude, could be useful to guide selection of subjects who are most likely to benefit from training. TMS is a non-invasive and rather inexpensive technique, and computation of MEP amplitude is readily performed. Thereby, TMS may be an effective tool for rapid stratification of patients at time of enrollment into a restorative stroke trial (34).
As opposed to what was hypothesized, lower baseline MEP magnitude was associated with larger gains in BBT performance. This result was surprising but could be explained by the study by Cramer et al (2007), where the authors noted that lower baseline cortical activity predicted greater gains from treatment. These authors described this fact by stating that if the brain is not already in a state of maximal capability, there is reserve remaining to enhance cortical activity and behavioral performance. It could be thought that in the current cohort of better-recovered subjects, those with lower baseline MEP magnitude had a remaining reserve for the robotic training to induce enhanced corticospinal excitability that paralleled improvement in function of the affected upper limb. Note that the same analogy can be made for fMRI LI as lower activation in the ipsilesional M1 hemisphere led to greater functional gains from the current robotic therapy, consistent with the concept that compensation using ipsilateral pathways was not fully developed before therapy in these subjects.
As noted in previous studies (17, 18), corticospinal tract integrity plays an important role in the recovery potential of stroke survivors, where greater damage to M1 tract has been associated with lesser functional gains following training (17). However, as opposed to the study by Stinear et al (2007), where FA asymmetry was the strongest predictor of improvement in motor function, FA asymmetry did not retain a predictive value in the current study. One reason for this discrepancy could be from the fact that different training regimens were provided to the subjects. Indeed, our high-intensity robotic training exercised each joint of the entire upper limb through a range of tasks as opposed to the training of Stinear, which consisted of picking up and moving small blocks. It could be thought that for training targeting specifically the affected hand, corticospinal tract integrity might play a more important role in predicting gains than for a training targeting the whole upper limb. Another reason could come from the fact that, as mentioned previously, our subjects were well-recovered from their stroke. This is reflected in the FA asymmetry of the PLIC (Stinear: 0.15 vs. ours: 0.09), where a value closer to 0 indicates symmetrical FA in the PLIC between sides. Because most of our subjects showed better motor recovery and a low degree of asymmetry in injury to corticospinal tract, reflected by the low FA asymmetry value in both PLIC and peduncle (see Table 1), this measure might not have been as useful as a treatment gain predictor in the current cohort of subjects as compared with a cohort with more severe motor system injury.
Across the entire group, laterality index of M1 did not survive the stepwise model, but this measure became the only variable predicting the final functional gains when examining only the subjects without baseline MEP values. Thus, lateralization gave further predictive power for this subgroup of subjects. Knowing that absence of MEP is related to poorer functional outcome (18), lateralization of M1 cortical activity towards the unaffected cortex might be a key element allowing these subjects to perform a functional task with their affected upper limb. Changes in cortical function are important for behavioral status among subjects with more severe motor system injury following stroke. Consistent with these findings, lateralization of M1 has previously been found to predict final functional gains after constraint induced therapy, in a subset of more impaired stroke survivors (13). Following the current results, it seems that, for subjects without MEP, a more sophisticated brain evaluation, by mean of the fMRI weighted laterality index, is needed to determine their potential to benefit from robotic therapy at the chronic stage of recovery.
The fact that no behavioral measures retained a predictive value along with our measure of neural function is not in line with a previous study by Cramer et al (2007), where both baseline Fugl-Meyer score and baseline degree of activation in primary motor cortex predicted higher gains following therapy (12). This discrepancy could come from the fact that since our subjects were better-recovered from their stroke, with a mean Fugl-Meyer score of 52/66 as opposed to 32/66 for the subjects of Cramer et al (2007), the psychometric properties of behavioral tools, such as their ceiling effect, may not be as useful a treatment gain predictor for subjects with milder motor impairments as opposed to brain measures.
Limitations of the study
Because of the inclusion criteria of this study, all subjects, to a different extent, were able to actively open/close the affected hand to pick up and drop blocks during the BBT and thus were on the milder end of the spectrum of post-stroke motor deficits. Further study is needed to ascertain if the current findings regarding MEP as a predictor of response to robotic therapy extend to stroke survivors who have more severe baseline motor impairments. Moreover, the association between the change in BBT and the baseline fMRI weighted laterality index of the subjects without MEP was computed based on data from 6 subjects only. Caution should be made when interpreting the results and a study using a greater sample size is needed to corroborate this finding. In addition, because an association between lower baseline MEP magnitude and better response to the robotic training was found, that could be explained by a reserve capacity of the brain to undergo plasticity, it could be thought that, after training, an increase in MEP magnitude would have occurred and be associated with the gains in BBT. Assessing post-training MEP amplitude would have allowed us to validate this idea. Additionally, 33% of the variance in the change in BBT was accounted by the multivariate model. Other variables not considered in the current study, such as prestroke experience or genetic factors (35) that play a role in post-stroke recovery (36), could also contribute to the variance observed. Finally, although this study allowed the determination of predictors of functional gains, providing a potential way to select subjects for robotic therapy and thereby optimize treatment benefit, it did not answer an important question: based on the predicting variables, what are the most promising alternative treatments that can be offered to the subjects who are not the best candidates for robotic exoskeletal training therapy? Future studies should thoroughly investigate the factors predicting the gains possible with other treatment options to ensure that all stroke survivors have the best chance of an optimal recovery.
Conclusion
High-intensity robotic exoskeletal training of the entire affected upper limb translated into significant improvement in functional performance, as assessed by the change in BBT. Several brain status measures showed an ability to predict the change in function of the trained limb. However, only one measure of brain status, baseline MEP magnitude, measured at 110% of motor threshold, survived the stepwise model. In other words, subjects presenting lower baseline MEP magnitude are those that benefited the most from a robotic training. Future studies should explore predictors of functional gains following robotic training of the entire upper limb in a subset of more impaired individuals to further improve selection of candidates for robot-based therapy.
Acknowledgments
The project was conducted at the Human Performance Laboratory at the Institute for Clinical and Translational Science University of California, Irvine, with funds provided by the National Center of Research Resources, 5M011 RR-00827-29, US Public Health Service. The project was financed by the National Institute of Health Contract N01-HD-3-3352 from NIBIB and NCMRR. Marie-Hélène Milot held a scholarship from the Canadian Institutes of Health Research and the Fonds de la Recherche en santé du Québec. David Reinkensmeyer has a financial interest in Hocoma, A.G. and Flint Rehabilitaton Devices, companies that make rehabilitation devices.
Footnotes
The terms of these arrangements have been reviewed and approved by the University of California, Irvine, in accordance with its conflict of interest policies.
References
- 1.Lloyd-Jones D, Adams RJ, Brown TM, et al. Heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 2.Coupar F, Pollock A, Rowe P, Weir C, Langhorne P. Predictors of upper limb recovery after stroke: a systematic review and meta-analysis. Clin Rehabil. 2012;26:291–313. doi: 10.1177/0269215511420305. [DOI] [PubMed] [Google Scholar]
- 3.Burke E, Cramer SC. Biomarkers and predictors of restorative therapy effects after stroke. Curr Neurol Neurosci Rep. 2013;13:329. doi: 10.1007/s11910-012-0329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartman-Maeir A, Soroker N, Ring H, Avni N, Katz N. Activities, participation and satisfaction one-year post stroke. Disabil Rehabil. 2007:559–66. doi: 10.1080/09638280600924996. [DOI] [PubMed] [Google Scholar]
- 5.Pignolo L. Robotics in neuro-rehabilitation. J Rehabil Med. 2009;41:955–60. doi: 10.2340/16501977-0434. [DOI] [PubMed] [Google Scholar]
- 6.Kahn LE, Zygman ML, Rymer WZ, Reinkensmeyer DJ. Robot-assisted reaching exercise promotes arm movement recovery in chronic hemiparetic stroke: a randomized controlled pilot study. J Neuroeng Rehabil. 2006;3:12. doi: 10.1186/1743-0003-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lo AC, Guarino PD, Richards LG, et al. Robot-assisted therapy for long-term upper-limb impairment after stroke. N Engl J Med. 2010;362:1772–83. doi: 10.1056/NEJMoa0911341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lum PS, Burgar CG, Shor PC, Majmundar M, Van der Loos M. Robot-assisted movement training compared with conventional therapy techniques for the rehabilitation of upper-limb motor function after stroke. Arch Phys Med Rehabil. 2002;83:952–9. doi: 10.1053/apmr.2001.33101. [DOI] [PubMed] [Google Scholar]
- 9.Reinkensmeyer DJ, Maier MA, Guigon E, et al. Do robotic and non-robotic arm movement training drive motor recovery after stroke by a common neural mechanism? Experimental evidence and a computational model. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:2439–41. doi: 10.1109/IEMBS.2009.5335353. [DOI] [PubMed] [Google Scholar]
- 10.Staubli P, Nef T, Klamroth-Marganska V, Riener R. Effects of intensive arm training with the rehabilitation robot ARMin II in chronic stroke patients: four single-cases. J Neuroeng Rehabil. 2009;6:46. doi: 10.1186/1743-0003-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahashi CD, Der-Yeghiaian L, Le V, Motiwala RR, Cramer SC. Robot-based hand motor therapy after stroke. Brain. 2008;131:425–37. doi: 10.1093/brain/awm311. [DOI] [PubMed] [Google Scholar]
- 12.Cramer SC, Parrish TB, Levy RM, et al. Predicting functional gains in a stroke trial. Stroke. 2007;38:2108–14. doi: 10.1161/STROKEAHA.107.485631. [DOI] [PubMed] [Google Scholar]
- 13.Dong Y, Dobkin BH, Cen SY, Wu AD, Winstein CJ. Motor cortex activation during treatment may predict therapeutic gains in paretic hand function after stroke. Stroke. 2006;37:1552–5. doi: 10.1161/01.STR.0000221281.69373.4e. [DOI] [PubMed] [Google Scholar]
- 14.Koski L, Mernar TJ, Dobkin BH. Immediate and long-term changes in corticomotor output in response to rehabilitation: correlation with functional improvements in chronic stroke. Neurorehabil Neural Repair. 2004;18:230–49. doi: 10.1177/1545968304269210. [DOI] [PubMed] [Google Scholar]
- 15.Lin KC, Huang YH, Hsieh YW, Wu CY. Potential predictors of motor and functional outcomes after distributed constraint-induced therapy for patients with stroke. Neurorehabil Neural Repair. 2009;23:336–42. doi: 10.1177/1545968308321773. [DOI] [PubMed] [Google Scholar]
- 16.Platz T, Kim IH, Engel U, Kieselbach A, Mauritz KH. Brain activation pattern as assessed with multi-modal EEG analysis predict motor recovery among stroke patients with mild arm paresis who receive the Arm Ability Training. Restor Neurol Neurosci. 2002;20:21–35. [PubMed] [Google Scholar]
- 17.Riley JD, Le V, Der-Yeghiaian L, et al. Anatomy of stroke injury predicts gains from therapy. Stroke. 2011;42:421–6. doi: 10.1161/STROKEAHA.110.599340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stinear CM, Barber PA, Smale PR, Coxon JP, Fleming MK, Byblow WD. Functional potential in chronic stroke patients depends on corticospinal tract integrity. Brain. 2007;130:170–80. doi: 10.1093/brain/awl333. [DOI] [PubMed] [Google Scholar]
- 19.Albert ML. A simple test of visual neglect. Neurology. 1973;23:658–64. doi: 10.1212/wnl.23.6.658. [DOI] [PubMed] [Google Scholar]
- 20.Alexander MP, Baker E, Naeser MA, Kaplan E, Palumbo C. Neuropsychological and neuroanatomical dimensions of ideomotor apraxia. Brain. 1992;115:87–107. doi: 10.1093/brain/115.1.87. [DOI] [PubMed] [Google Scholar]
- 21.Lincoln NB, Jackson JM, Adams SA. Reliability and Revision of the Nottingham Sensory Assessment for Stroke Patients. Physiotherapy. 1998;84:358–65. [Google Scholar]
- 22.Mathiowetz V, Volland G, Kashman N, Weber K. Adult norms for the Box and Block Test of manual dexterity. Am J Occup Ther. 1985;39:386–91. doi: 10.5014/ajot.39.6.386. [DOI] [PubMed] [Google Scholar]
- 23.Fugl-Meyer AR, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1 a method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 24.Wolf SL, Thompson PA, Morris DM, et al. The EXCITE trial: attributes of the Wolf Motor Function Test in patients with subacute stroke. Neurorehabil Neural Repair. 2005;19:194–205. doi: 10.1177/1545968305276663. [DOI] [PubMed] [Google Scholar]
- 25.Uswatte G, Taub E, Morris D, Light K, Thompson PA. The Motor Activity Log-28: assessing daily use of the hemiparetic arm after stroke. Neurology. 2006;67:1189–94. doi: 10.1212/01.wnl.0000238164.90657.c2. [DOI] [PubMed] [Google Scholar]
- 26.Katz RT, Rovai GP, Brait C, Rymer WZ. Objective quantification of spastic hypertonia: correlation with clinical findings. Arch Phys Med Rehabil. 1992;73:339–47. doi: 10.1016/0003-9993(92)90007-j. [DOI] [PubMed] [Google Scholar]
- 27.Rossini PM, Barker AT, Berardelli A, et al. Non-invasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol. 1994;91:79–92. doi: 10.1016/0013-4694(94)90029-9. [DOI] [PubMed] [Google Scholar]
- 28.Mayka MA, Corcos DM, Leurgans SE, Vaillancourt DE. Three-dimensional locations and boundaries of motor and premotor cortices as defined by functional brain imaging: a meta-analysis. Neuroimage. 2006;31:1453–74. doi: 10.1016/j.neuroimage.2006.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Calautti C, Naccarato M, Jones PS, et al. The relationship between motor deficit and hemisphere activation balance after stroke: A 3T fMRI study. Neuroimage. 2007;34:322–31. doi: 10.1016/j.neuroimage.2006.08.026. [DOI] [PubMed] [Google Scholar]
- 30.Giorgio A, Watkins KE, Chadwick M, et al. Longitudinal changes in grey and white matter during adolescence. Neuroimage. 2010;49:94–103. doi: 10.1016/j.neuroimage.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Kleim JA, Kleim ED, Cramer SC. Systematic assessment of training-induced changes in corticospinal output to hand using frameless stereotaxic transcranial magnetic stimulation. Nat Protoc. 2007;2:1675–84. doi: 10.1038/nprot.2007.206. [DOI] [PubMed] [Google Scholar]
- 32.Klein J, Spencer S, Allington J, Bobrow JE, Reinkensmeyer DJ. Optimization of a parallel shoulder mechanism to achieve a high-force, low-mass, robotic-arm exoskeleton. IEEE Trans Robot. 2010;26:710–5. [Google Scholar]
- 33.Milot MH, Spencer SJ, Chan V, et al. A crossover pilot study evaluating the functional outcomes of two different types of robotic movement training in chronic stroke survivors using the arm exoskeleton BONES. J Neuroeng Rehabil. 2013;10:112. doi: 10.1186/1743-0003-10-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cramer SC. Stratifying patients with stroke in trials that target brain repair. Stroke. 2010;41:S114–6. doi: 10.1161/STROKEAHA.110.595165. [DOI] [PubMed] [Google Scholar]
- 35.Pearson-Furhrop K, Cramer SC. Genetic influences on neural plasticity. PMR. 2010;2:S227–S40. doi: 10.1016/j.pmrj.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 36.Cramer SC. Repairing the human brain after stroke: I. Mechanisms of spontaneous recovery. Ann Neurol. 2008;63:272–87. doi: 10.1002/ana.21393. [DOI] [PubMed] [Google Scholar]