Abstract
Purpose
To determine whether shock wave lithotripsy (SWL) treatment of the kidney of metabolic syndrome (MetS) pigs worsens glucose tolerance or increases the risk of developing diabetes mellitus.
Materials and Methods
Nine-month-old female Ossabaw miniature pigs were fed a hypercaloric atherogenic diet to induce MetS. At 15 months of age, pigs were treated with 2000 SWs or 4000 SWs (24 kV at 120 SWs/min) using the unmodified Dornier HM3 lithotripter. SWs were targeted to the upper pole calyx of the left kidney so as to model treatment that would also expose the tail of the pancreas to SWs. Intravenous glucose tolerance tests (IVGTTs) were performed on conscious, fasting pigs before SWL and at 1 month and 2 months post-SWL with blood samples taken for glucose and insulin measurement.
Results
Pigs fed the hypercaloric atherogenic diet were obese, dyslipidemic, insulin resistant and glucose intolerant—consistent with the development of MetS. Assessment of insulin resistance, glucose tolerance and pancreatic beta cell function from fasting plasma glucose and insulin levels, and the glucose and insulin response profile to IVGTTs, were similar before and after SWL.
Conclusions
The MetS status of SWL treated pigs was unchanged 2 months following treatment of the kidney with 2000 high-amplitude SWs or overtreatment with 4000 high-amplitude SWs. These findings do not support a single SWL treatment of the kidney as a risk factor for the onset of diabetes mellitus.
Keywords: Swine, shock wave lithotripsy, metabolic syndrome, kidney
Introduction
Shock wave lithotripsy (SWL) is a common first-line modality for the treatment of urolithiasis.1,2 However, this technology is not without unwanted side effects in that renal SWL can injure the kidney and surrounding organs.3
The pancreas has been implicated as an organ at risk of injury following renal SWL, as evidenced by elevated plasma and urinary levels of pancreatic exocrine enzymes (e.g. amylase and lipase),4–9 the occurrence of pancreatic hematomas,4,6 and case reports of acute pancreatitis.6–9 However, a relationship between SWL and long-term pancreatic dysfunction had not been established until Krambeck and colleagues at the Mayo Clinic reported the results of a retrospective 19-year follow up study on stone patients treated with SWL, finding that such patients had a higher incidence of developing type 2 diabetes (T2D) compared to stone forming patients treated conservatively.10 Shortly after the publication of the Mayo Clinic report, there were 2 additional retrospective follow up studies of 17 years or less of stone patients treated with SWL that did not show a higher incidence of T2D.11,12 With this background of clinical information at hand, we embarked on an animal study to address the issue of whether SWL treatment could increase the risk of developing T2D. We used the Ossabaw miniature swine model that develops similar features of human metabolic syndrome (MetS)13,14—a cluster of factors that includes obesity, insulin resistance, impaired glucose tolerance, dyslipidemia and hypertension—and examined whether SWL of the kidney exacerbates the severity of MetS, i.e. increases the risk for diabetes mellitus.
Materials and Methods
Studies were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committees of Indiana University School of Medicine and Methodist Hospital. Seven to nine-month-old female Ossabaw pigs were fed a hypercaloric atherogenic diet of 1 kg/day to induce obesity and other features of MetS.13,14 After 6 months on the diet high in fat, cholesterol, and fructose, the MetS pigs underwent SWL treatment using the unmodified Dornier HM3 lithotripter. Anesthesia and survival surgery preparation have been described previously.15 SWs were targeted to an upper-pole calyx of the left kidney (2000 SWs or 4000 SWs, 24 kV, 120 SWs/min). X-ray verification of targeting was done every 500 SWs, and electrodes were replaced after every 1000 SWs. Intravenous glucose tolerance tests (IVGTTs) were performed on conscious, fasted pigs a few days before SWL and at 1 and 2 months post-SWL with intravenous blood samples taken for glucose and insulin measurement.
IVGTT
Pigs were anesthetized and central venous access gained using a non-surgical procedure to catheterize the jugular vein.16 Pigs were conditioned to a low-stress restraint sling for 4 days before the IVGTT. After an overnight fast, conscious pigs were placed in the sling and blood samples taken from the jugular vein catheter immediately before and at 5, 10, 20, 30, 40, 50 and 60 min after an intravenous bolus of sterile glucose solution (dextrose, 1 g/kg). Blood samples were collected in sodium heparinized tubes and kept on ice until blood glucose measurement (YSI 2300 Stat Plus analyzer) and then centrifuged to collect plasma, which was frozen at −80°C until assayed for insulin (Millipore Corporation, St. Charles, MO). Fasting blood samples were also collected for serum chemistries (Antech Diagnostics, Fishers, IN).
IVGTT in the presence of isoproterenol
Three pigs from the 4000 SWs treatment group underwent an additional IVGTT that was performed 1 to 3 days after the completion of the 2 months post-SWL IVGTT. This final IVGTT was done in the presence of isoproterenol, an insulin secretagogue, 17 to assess whether the pigs had additional pancreatic reserve to release insulin above the levels reached with the glucose challenge alone. Pigs were restrained in slings and sterile (±) isoproterenol infused into the jugular vein catheter at a rate of 50 ng/kg/min for 90 min. A bolus injection of sterile glucose solution (1 g/kg) was given at 30 min into the isoproterenol infusion. Blood samples were taken before and at 20 min and 30 min into the isoproterenol infusion and at 5, 10, 20, 30, 40, 50 and 60 min after glucose administration.
Pancreas exocrine function
Blood was withdrawn from indwelling jugular vein catheters (see above) in conscious fasting pigs 1 day before SWL and at days 1, 7 and 28 after treatment. Blood was added to tubes containing appropriate preservatives and then assayed for pancreatic lipase (clinical laboratory services at Methodist Hospital, Indianapolis, IN) and pancreatic amylase (ARUP Laboratories, Salt Lake City, UT).
Pancreas histology and insulin staining
The pancreas was perfusion fixed in situ with 10% phosphate buffered formalin and then kept in the same fixative for ~2 weeks before being transferred to a 70% ethanol solution. Pancreatic tail segments were paraffin embedded, serially sectioned at 6 μm intervals, then immunostained for insulin (rabbit anti-human insulin [Santa Cruz], [1:500]) and counterstained with hematoxylin as previously described.18 The beta cell area of 24–56 sections from at least six animals in each group was calculated with investigators blinded to the treatment groups. Additional sections underwent hematoxylin and eosin staining for histologic assessment. Control pancreatic tail tissue was obtained from Ossabaw pigs of similar sex, age and MetS phenotype as the SWL-treated pigs fed the same atherogenic diet, but were not treated with SWL.
Calculations
Insulin sensitivity was evaluated from fasting plasma values of glucose and insulin measured on the day of the IVGTT using the homeostasis model assessment for insulin resistance HOMA-IR = (glucose0 min x insulin0 min)/405, and the quantitative insulin sensitivity check index QUICKI = 1/(log glucose0 min + log insulin0 min).19 From the IVGTT the following parameters were calculated: modified homeostatic model assessment of insulin resistance (HOMA-IRIVGTT = product of plasma glucose and insulin concentration); area under the curve (AUC) for glucose and insulin; acute insulin response to glucose (AIRG = insulin5 min − insulin0 min); glucose tolerance (KG = −slope of ln(glucose)5–20 min x 100); beta cell function (BCFIVGTT = AIRG/(glucose5 min − glucose0 min); IVGTT insulin sensitivity index (S2 = KG/((AUCinsulin(0 – 20 min)/20 min) x Vd) where Vd = injected glucose dose/(glucose peak x body weight); disposition index (DIIVGTT = AIRG x S2).20,21
Statistical analysis
Continuous variables were summarized by means and standard errors of the means (SEMs). Student’s t-tests were used for comparisons between two groups. Repeated measures ANOVA were used to compare pre- and post-SWL values. In comparing the pancreatic beta cell areas, repeated measures ANOVA was used on the original measures, and rank-based nonparametric ANOVA was used on the minimal values of each segment block. Bootstrap was used to generate the p-value for the latter approach.22
Results
Blood Chemistry
Table 1 shows the serum chemistry profile of fasting pigs that were fed the hypercaloric atherogenic diet for 6 months and similar aged Ossabaw pigs fed a normal diet. Pigs fed the hypercaloric atherogenic diet were obese, hyperglycemic (i.e. impaired glucose tolerance), hyperinsulinemic (i.e. insulin resistant) and dyslipidemic (elevated levels of cholesterol and triglycerides)—hallmarks of the MetS phenotype.13,14 Serum liver enzymes were also elevated indicative of liver abnormalities.13
Table 1.
Fasting serum chemistry profile of lean and MetS Ossabaw pigs.
| Lean (n = 8–9) | MetS (n = 12–14) | P value | |
|---|---|---|---|
| Gender (Male/Female) | Female | Female | |
| Age (months) | ~ 15 | ~ 15 | |
| Atherogenic diet duration (months) | Not applicable | ~ 6 | |
| Body Wt. (kg) | 48 ± 2 | 86 ± 2 | <0.0001 |
| Glycemic measures: | |||
| Glucose (mg/dL) | 66 ± 2 | 81 ± 3 | 0.0003 |
| Insulin (μU/ml) | 10 ± 2 | 22 ± 4 | 0.0128 |
| Plasma lipids: | |||
| Cholesterol (mg/dL) | 62 ± 2 | 503 ± 58 | <0.0001 |
| Trigylcerides (mg/dL) | 17 ± 1 | 52 ± 8 | 0.0016 |
| Liver biochemistries: | |||
| AST (IU/L) | 27 ± 3 | 54 ± 7 | 0.0039 |
| ALT (IU/L) | 28 ± 3 | 69 ± 8 | 0.0003 |
| ALP (IU/L) | 79 ± 13 | 165 ± 24 | 0.0064 |
| GGT (IU/L) | 27 ± 1 | 71 ± 7 | 0.0001 |
| Muscle biochemistries: | |||
| CPK (IU/L) | 479 ± 54 | 568 ± 149 | 0.5846 |
Values are mean ± SEM. Normal and atherogenic diet compositions provided an average energy intake of ~2500 and ~6000 kcal/day with 10.5% and 46% of calories from fat sources, respectively.13 AST = aspartate aminotransferase, ALT = alanine aminotransferase, ALP = alkaline phosphatase, GGT = gamma-glutamyl transpeptidase, CPK = creatinine phosphokinase
Abdominal anatomy of the pig
Figure 1 shows a computed tomography image of the anatomical orientation of the pancreas and left kidney in an 18-month-old obese female MetS Ossabaw pig. The tail of the pancreas lies in close proximity to the anterior surface of the upper pole of the left kidney—both regions being within the blast path of the SW generated from the HM3 lithotripter. Consequently, SWs targeted to this region of the left kidney had the potential to also injure the pancreas.
Figure 1.
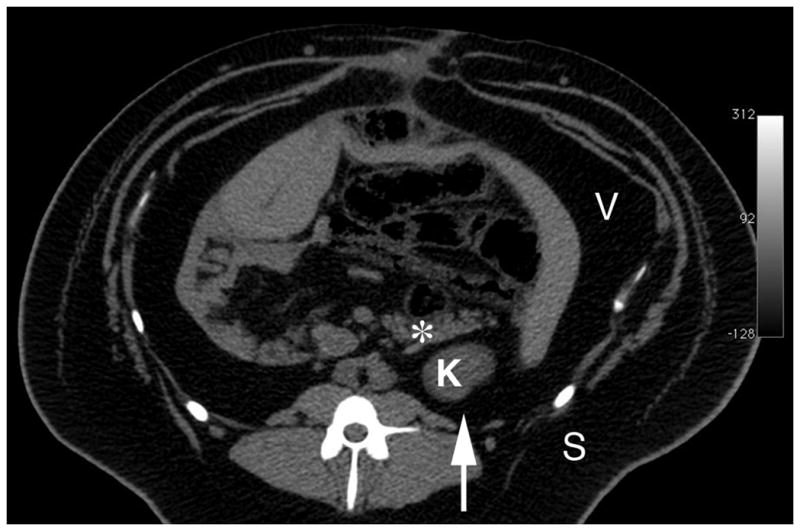
Abdominal computed tomography image of an 18-month-old obese female MetS Ossabaw pig on hypercaloric atherogenic diet for 12 months. Image was acquired with a published scanning protocol.16 The extreme obesity of the pig is evident from the several cm of subcutaneous and visceral fat. * = tail of pancreas, K = upper pole of left kidney, S = subcutaneous fat, V = visceral (intra-abdominal) fat. Arrow indicates the direction of SW propagation.
IVGTT
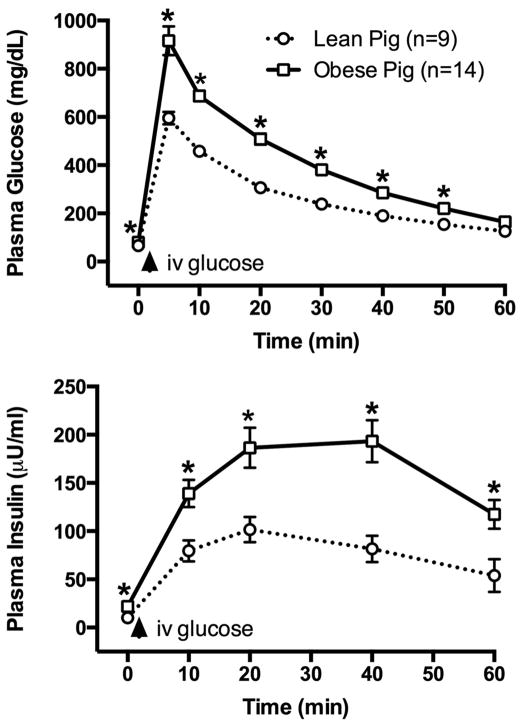
Figure 2 illustrates the mean glucose and insulin response profile to an IVGTT in the MetS pigs prior to SWL treatment. Shown for comparison is the corresponding responses in Ossabaw lean pigs of similar age fed a normal diet. The MetS pigs demonstrated higher peak glucose and insulin responses to the intravenous glucose challenge, indicative of robust impaired glucose tolerance and insulin resistance.
Figure 2.
IVGTT in lean and obese (MetS) Ossabaw pigs. Intravenous glucose bolus was given at 1 g/kg body weight. * = P<0.05 from corresponding value in control (lean pig) group. Data shown are mean ± SEM.
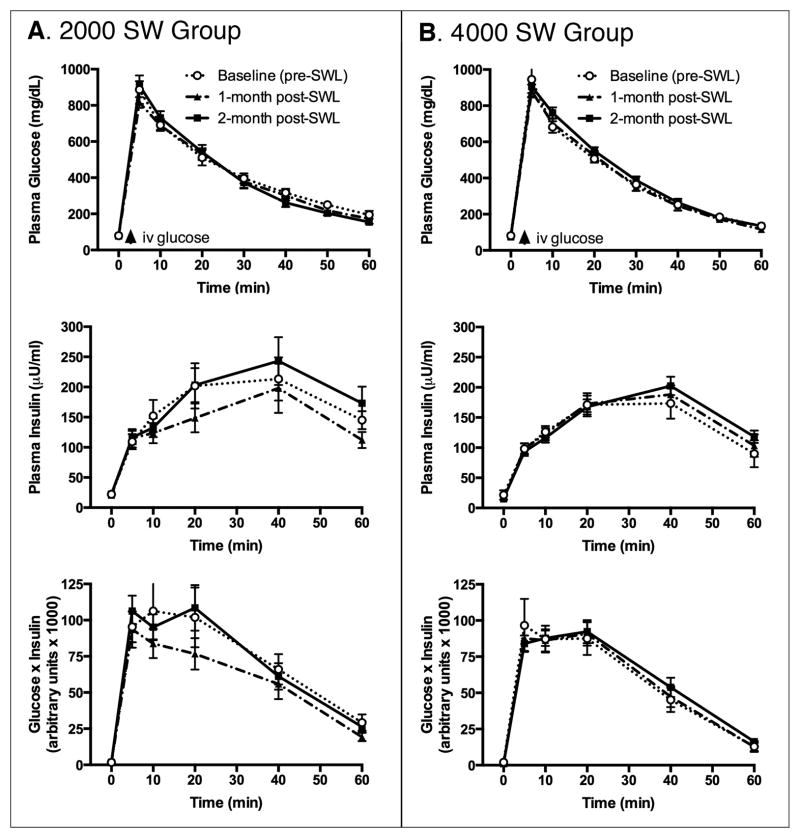
Fasting levels of plasma glucose and insulin of MetS pigs, as well as their response profile to an IVGTT, were similar before and at 1 month and 2 months after SWL treatment with 2000 SWs or 4000 SWs (Table 2, Figure 3). Calculation of indexes of insulin sensitivity, pancreatic beta cell function and glucose tolerance from fasting plasma glucose and insulin values, as well as IVGTT glucose and insulin kinetics, demonstrated that SWL treatment did not alter the severity of MetS, regardless of the delivered SW dose (Table 3).
Table 2.
Body weight, food intake, and fasting glycemia of SWL-treated MetS pigs.
| Pre-SWL | Post-SWL (1 month) | Post-SWL (2 month) | |
|---|---|---|---|
| Body wt. (kg) | |||
| SWL at 2000 SWs (n=7) | 89 ± 2 | 94 ± 3* | 100 ± 4*† |
| SWL at 4000 SWs (n=7) | 84 ± 3 | 87 ± 2* | 94 ± 2*† |
| SWL (2000 & 4000 SW data combined, n=14) | 86 ± 2 | 91 ± 2* | 97 ± 2*† |
| Estimated cumulative food intake (kg) | |||
| SWL at 2000 SWs | 213 ± 3 | 243 ± 3* | 268 ± 4*† |
| SWL at 4000 SWs | 178 ± 2 | 206 ± 2* | 234 ± 2*† |
| SWL (2000 & 4000 SW data combined) | 196 ± 5 | 225 ± 5* | 251 ± 5*† |
| Fasting plasma glucose (mg/dL) | |||
| SWL at 2000 SWs | 80 ± 2 | 79 ± 4 | 78 ± 5 |
| SWL at 4000 SWs | 81 ± 6 | 75 ± 3 | 79 ± 3 |
| SWL (2000 & 4000 SW data combined) | 80 ± 3 | 77 ± 3 | 78 ± 3 |
| Fasting plasma insulin (μU/ml) | |||
| SWL at 2000 SWs | 22 ± 3 | 21 ± 4 | 22 ± 4 |
| SWL at 4000 SWs | 20 ± 7 | 15 ± 3 | 16 ± 3 |
| SWL (2000 & 4000 SW data combined) | 21 ± 4 | 18 ± 2 | 19 ± 2 |
Data shown are mean ± SEM.
P < 0.05 from pre-SWL value;
P <0.05 from 1 month post-SWL value.
Figure 3.
IVGTT in MetS pigs before and after SWL. Shown are the plasma glucose and insulin responses and the calculated modified homeostatic model assessment of insulin resistance (HOMA-IRIVGTT = product of plasma glucose and insulin concentration). Intravenous glucose bolus was given at 1 g/kg body weight. Data shown are mean ± SEM.
Table 3.
Glycemia of SWL-treated MetS pigs.
| Pre-SWL | Post-SWL (1 month) | Post-SWL (2 month) | |
|---|---|---|---|
| Fasting: | |||
| HOMA-IR | |||
| SWL at 2000 SWs (n=7) | 4.35 ± 0.64 | 4.05 ± 0.76 | 4.51 ± 1.08 |
| SWL at 4000 SWs (n=7) | 4.65 ± 2.10 | 2.76 ± 0.41 | 3.19 ± 0.69 |
| SWL (2000 & 4000 SW data combined, n=14) | 4.51 ± 1.12 | 3.40 ± 0.45 | 3.85 ± 0.64 |
| QUICKI | |||
| SWL at 2000 SWs | 0.316 ± 0.006 | 0.319 ± 0.006 | 0.316 ± 0.007 |
| SWL at 4000 SWs | 0.324 ± 0.010 | 0.332 ± 0.008 | 0.327 ± 0.007 |
| SWL (2000 & 4000 SW data combined) | 0.320 ± 0.006 | 0.326 ± 0.005 | 0.322 ± 0.005 |
| IVGTT: | |||
| AUCglucose/AUCinsulin | |||
| SWL at 2000 SWs | 2.58 ± 0.34 | 3.42 ± 0.94 | 2.48 ± 0.57 |
| SWL at 4000 SWs | 2.90 ± 0.34 | 2.46 ± 0.17 | 2.58 ± 0.27 |
| SWL (2000 & 4000 SW data combined) | 2.74 ± 0.23 | 2.94 ± 0.48 | 2.53 ± 0.30 |
| KG (min−1) | |||
| SWL at 2000 SWs | 3.61 ± 0.54 | 2.91 ± 0.38 | 3.50 ± 0.44 |
| SWL at 4000 SWs | 3.89 ± 0.39 | 3.39 ± 0.35 | 3.32 ± 0.28 |
| SWL (2000 & 4000 SW data combined) | 3.76 ± 0.31 | 3.15 ± 0.26 | 3.41 ± 0.25 |
| AIRG (pmol/L) | |||
| SWL at 2000 SWs | 525 ± 80 | 566 ± 78 | 564 ± 60 |
| SWL at 4000 SWs | 446 ± 34 | 501 ± 42 | 465 ± 38 |
| SWL (2000 & 4000 SW data combined) | 492 ± 43 | 534 ± 43 | 514 ± 37 |
| BCF (pmol/mmol) | |||
| SWL at 2000 SWs | 12.43 ± 2.15 | 13.96 ± 2.03 | 12.22 ± 1.31 |
| SWL at 4000 SWs | 9.74 ± 0.95 | 11.18 ± 0.86 | 10.33 ± 1.19 |
| SWL (2000 & 4000 SW data combined) | 11.24 ± 1.19 | 12.57 ± 1.13 | 11.28 ± 0.89 |
| S2 (ml·min−1·pM−1·kg−1) | |||
| SWL at 2000 SWs | 0.64 ± 0.09 | 0.72 ± 0.14 | 0.63 ± 0.10 |
| SWL at 4000 SWs | 0.79 ± 0.08 | 0.66 ± 0.09 | 0.67 ± 0.07 |
| SWL (2000 & 4000 SW data combined) | 0.72 ± 0.06 | 0.69 ± 0.08 | 0.65 ± 0.06 |
| DI | |||
| SWL at 2000 SWs | 330 ± 56 | 372 ± 55 | 337 ± 47 |
| SWL at 4000 SWs | 337 ± 17 | 319 ± 35 | 300 ± 26 |
| SWL (2000 & 4000 SW data combined) | 330 ± 28 | 346 ± 32 | 318 ± 26 |
HOMA-IR = homeostasis model assessment for insulin resistance; QUICKI = quantitative insulin sensitivity check index; AUC = area under the curve for glucose and insulin; KG = glucose tolerance; AIRG = acute insulin response to glucose; BCF = beta cell function; S2 = insulin sensitivity index; DI = disposition index. Equations for each glycemic measure are described in the Calculations section in Methods and Materials. Data shown are mean ± SEM.
IVGTT ± isoproterenol
Figure 4 depicts the individual insulin responses to IVGTTs performed in 3 pigs at 2 months post-treatment with 4000 SWs and repeated in the presence of isoproterenol (an insulin secretagogue). Fasting plasma insulin levels were 23, 13 and 13 μU/ml and rose during the initial 30-min of isoproterenol infusion to 77, 74 and 27 μU/ml, respectively. The IVGTT in the presence of isoproterenol resulted in the peak insulin response being nearly doubled in 2 pigs and marginally elevated in 1 pig. Overall, the data suggest that the SWL-treated MetS pigs have pancreatic insulin reserves.
Figure 4.
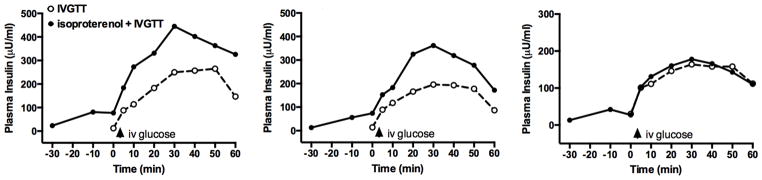
IVGTT in 2 month post-SWL treated MetS pigs and then repeated ~1 day later during the intravenous infusion of isoproterenol (insulin secretagogue).
Assessment of pancreatic injury
Pancreas exocrine function
As shown in Table 4, kidneys exposed to 2000 SWs were associated with a transient rise in serum amylase and lipase of ~20% within 1 day after SWL, with amylase remaining elevated at 7 days post-treatment. Renal SWL with 4000 SWs was also associated with ~25% elevation in serum lipase at post-treatment day 1, whereas serum amylase rose only ~8% at post-treatment day 28.
Table 4.
Effect of renal SWL on serum pancreatic enzyme levels of SWL-treated MetS pigs.
| Serum Amylase (μU/ml) | Serum Lipase (μU/ml) | |
|---|---|---|
| Treatment with 2000 SW (n=8) | ||
| Day −1 (Pre-SWL) | 2176 ± 254 | 9.9 ± 0.0 |
| Day +1 | 2504 ± 300* | 12.2 ± 0.7* |
| Day +7 | 2367 ± 224* | 10.1 ± 0.2 |
| Day +28 | 2143 ± 309 | 10.1 ± 0.2 |
| Treatment with 4000 SW (n=8) | ||
| Day −1 (Pre-SWL) | 1513 ± 241 | 12.7 ± 0.9 |
| Day +1 | 1559 ± 224 | 15.9 ± 1.3* |
| Day +7 | 1543 ± 235 | 13.0 ± 1.0 |
| Day +28 | 1634 ± 307* | 12.6 ± 1.0 |
Mean ± SEM values are shown. A value of 9.9 was assigned to all lipase measurements below the threshold of detection of 10 μU/ml.
P<0.05 from pre-SWL value.
Pancreas histopathology
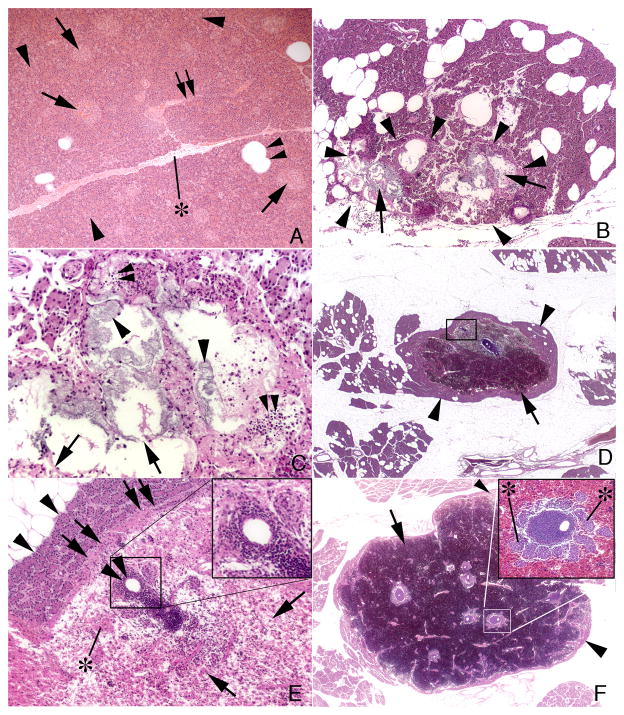
Micrographs of pancreatic tail tissue are shown in Figure 5. No pathological changes were noted in any pancreatic cell type of control MetS (no SWL) pigs (panel A). Two different types of injury were found in both SW-treated MetS groups. The first type of tissue injury was highly focal in location and was characterized by cellular necrosis of centroacinar cells within a grouping of pancreatic acini, injured acini surrounded and infiltrated with many inflammatory cells, and complete cell loss in some acini (panels B and C). This first type of injury was detected in the 2000 SW (2 of 7 pigs) and 4000 SW (1 of 7 pigs) groups. The second type of injury was characterized by a focal intra-parenchymal hematoma (panels D–F) and detected in the 2000 SW (1 of 7 pigs, hematoma measuring 6 mm in width is shown in panel D) and 4000 SW (3 of 7 pigs; hematomas measuring 10 to 18 mm in width with an example shown in panel F) groups. All hematomas occupied the central region of the pancreatic lobule and were walled off from a peripheral rim of normal appearing acinar cells by a fibrotic band or capsule (panel E). Within the boundaries of the hematoma, all acinar cells appear destroyed leaving islands of islets of Langerhans and various sized ductal and vascular profiles surrounded by chronic inflammatory cells (inserts in panels E and F).
Figure 5.
Pancreatic tail histology. Panel A is from a control MetS pig and shows islets of Langerhans (arrows), pancreatic acinar cells (arrowhead), interlobular ducts (double arrow), fat cells (double arrowhead) and interlobular septum (asterisk). Panel B shows the first type of injury, which is focal (outlined by arrowheads) and characterized by cellular necrosis of acini (arrows). Panel C is at higher magnification and shows necrotic centroacinar cells (arrowheads), complete loss of acinar cells (arrows) and infiltrating inflammatory cells (double arrowheads). Panels D–F show the second type of injury, which is characterized by a focal intra-parenchymal hematoma (arrows) that is separated from surrounding normal appearing tissue (arrowheads) by a fibrotic band or capsule (double arrows in panel E which is an enlargement of the box shown in panel D). Islands of islets of Langerhans (asterisk), and various sized ductal and vascular profiles (double arrowheads) surrounded by chronic inflammatory cells are seen within the boundaries of the hematoma (Panels D–F). Inserts in panels E and F show chronic inflammatory cells around an artery. Insert in panel F also shows several islets of Langerhans (asterisk) near the vascular cuffs.
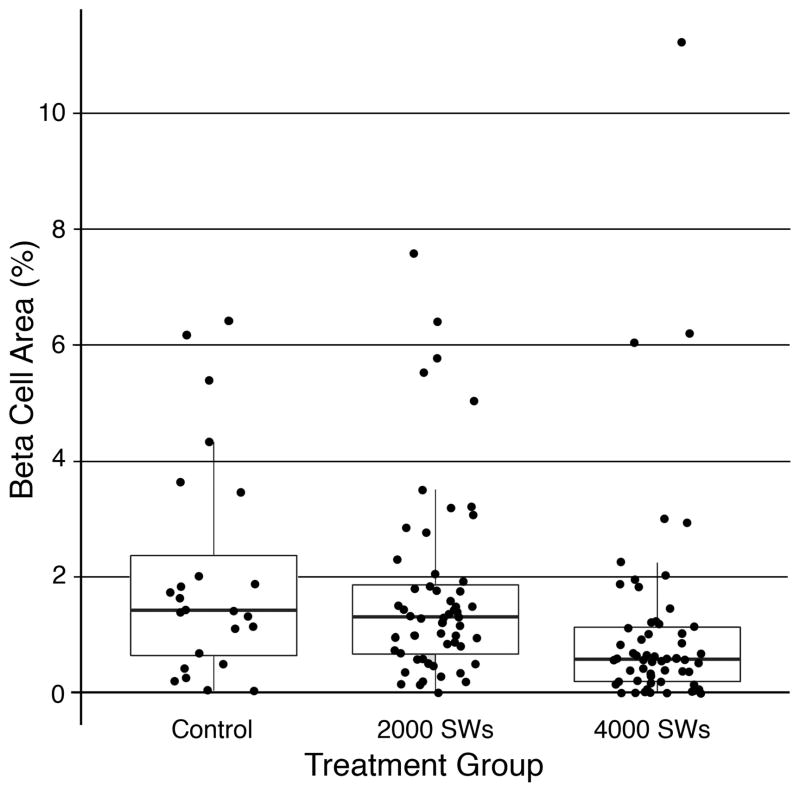
Insulin-immunoreactive beta cell area
A box plot of % beta cell areas calculated from insulin stained pancreatic tail tissue sections is shown in Figure 6. The majority of data points in the 4000 SW-treated pigs were clustered at lower beta cell area values than the control and 2000 SW-treated pigs. No differences were found on analyzing all three groups (P = 0.1320), whereas comparing the control versus 4000 SW-treated groups gave a P-value that approached statistical significance (P = 0.0752). There was a clear trend for a loss of insulin-containing beta cells within the pancreatic tail after renal SWL at the highest SW dose.
Figure 6.
A box plot of % beta cell area measured in insulin immunostained pancreatic tail tissue sections from the MetS control (24 data points from 6 pigs), 2000 SW-treated (56 data points from 7 pigs) and 4000 SW-treated (56 data points from 7 pigs) groups. Median value represented by midline within the box; interquartile range (25%–75% of data values) represented by the height of box; lowest and largest non-outlier values represented by end of whiskers.
Discussion
Ossabaw pigs were fed a hypercaloric atherogenic diet for over 6 months and developed robust features of MetS similar to those found in patients—that is, obesity, dyslipidemia, hyperinsulinemia and impaired glucose tolerance, with such atherogenic diet fed pigs previously shown to develop hypertension.13,14 We used this well-characterized porcine model of MetS 14 to assess whether SWL treatment of the kidney exacerbates the severity of MetS.
The spatial orientation of the kidneys and pancreas of the pig is similar to that of the human—the head of the pancreas lies adjacent to the hilum of the right kidney with the pancreatic body crossing the anterior surface of the left kidney just above its hilum, over which it tapers into the pancreatic tail.23 Because of this anatomical arrangement, SWs delivered by the HM3 lithotripter to an upper pole calyx of the pig’s left kidney has the potential to injure the pancreatic tail as a side effect of SW treatment. The tail of the pancreas is rich in insulin containing beta cells with estimates of insulin content being up to 2-fold greater than in the pancreatic head, neck or body.24,25 Therefore, destruction and failure of pancreatic insulin containing beta cells by SWL treatment, and by the subsequent inflammatory response, could potentially reduce pancreatic insulin reserves to such an extent that it would lead to further progression of the MetS phenotype, thereby accelerating the onset of diabetes mellitus.
Pancreatic lipase—and to a lesser extent pancreatic amylase—was consistently elevated immediately after renal SWL and then decreased and normalized promptly. Others have also shown similar temporal profiles for serum lipase and amylase after renal SWL.5,7,8 The rise in serum markers of pancreatic exocrine function observed in the present study likely reflects pancreatic trauma as collateral damage of renal SWL, but they remained within or close to normal limits. Regardless, we were able to confirm injury to the pancreatic tail histologically—sites of hemorrhage, chronic inflammation, loss of normal cell integrity, fibrotic regions and loss of insulin-immunoreactive beta cells. However, the degree of tissue injury was such that pigs were asymptomatic, which is in keeping with acute pancreatic complications being a rare event following renal SWL.9
SWL treatments did not influence fasting levels of plasma glucose and insulin as well as their kinetic response to an intravenous glucose challenge in MetS pigs—that is, glucose tolerance and insulin sensitivity were unchanged during the 2 month follow up period. Of particular note is that SWL did not significantly alter the disposition index, which is an integrated measure of the relationship between beta cell function and insulin sensitivity—i.e. insulin secretion and insulin action. A fall in the disposition index would reflect progression towards further glucose intolerance and diabetes,26 which clearly did not occur after SWL at either SW dose.
The motivation for our experimental animal study was the clinical report that SWL of renal and proximal ureteral stones was associated with the development of T2D.10 In a 19-year follow up study of a referral-based cohort of 288 adult patients treated with a median number of 1100 SWs at 20 kV using the HM3 lithotripter, Krambeck and colleagues from the Mayo Clinic reported a three-fold higher incidence of developing T2D compared to stone formers treated conservatively—T2D risk being related to the number of SWs administered and the total intensity of SWL treatment in a single session.10 These findings raised the concern that SWL may have long-term harmful effects. More recently, the same group of investigators failed to demonstrate an association between SWL and the onset of T2D in a population-based cohort of 423 adult patients during a mean follow up of ~9 years.27 Others have also reported no change in the incidence of T2D in adult patients whose kidney stones were treated with SWs using the HM3 or Medstone STS lithotripter, with follow ups that were of a 6 year,12 17 year 11 or 20 year duration.28 A group of 70 pediatric patients (mean age of 6.5 years old) with renal stones were treated with single or multiple SWL sessions using the Doli S or MFL 5000 lithotripters, and had normal blood sugar values over a mean follow up period of ~5 years—that is, no patient developed T2D after SWL.29 All these clinical studies were retrospective reviews using patient completed questionnaires, medical records and diagnostic codes; an assortment of control groups including kidney stone patients treated non-surgically, ureteral stone patients treated with SWL, and information gathered from background general population surveys; and as such have inherent weaknesses and limitations as discussed in each article. Despite the fact that we treated the kidneys of MetS pigs with SW doses that were higher than the clinical norm, and at SW doses that consistently damaged the kidney (not shown), our findings are in keeping with the growing clinical consensus that the long-term risk of T2D from renal SWL is likely negligible.
Our study is not without limitations in that the follow-up period after SWL was only 2 months. This short timeframe did not allow us to fully address whether damage to the pancreas by SWL accelerated the progression to T2D, only that SWL did not cause further impairment of glucose tolerance, beta-cell function or insulin sensitivity. In fact, the isoproterenol plus IVGTT experiments demonstrated that substantial pancreatic insulin reserves still remained in the SWL-treated MetS pigs, and together with the relatively small focal areas of pancreatic injury after SWL likely accounts for the unaltered MetS phenotype. No doubt, if pancreatic insulin reserves were severely compromised then any significant SWL-induced pancreatic injury—whether from single or multiple SWL sessions—could have functional consequences. Combining the 2000 SW and 4000 SW data sets to increase the sample size did not uncover any SWL-mediated changes in metrics of insulin secretion and action. Euglycemic-hyperinsulinemic and hyperglycemic-hyperinsulinemic clamp methods are regarded as the “gold standard” for measures of insulin sensitivity and beta cell function, respectively 20 —nevertheless, the less complicated and easily repeatable IVGTT was employed in the present study to provide acceptable estimations of these measures.20,21,30 Finally, there is a lack of information on the degree of pancreatic injury that is necessary to induce further glucose intolerance and progression to T2D in the MetS pig model.
In summary, we provide biochemical and histologic evidence that SWL treatment of the upper pole of the left kidney can injure the pancreatic tail and mildly reduce insulin containing beta cells in MetS pigs. However, this degree of injury was insufficient to alter measures of glucose tolerance and insulin sensitivity during the 2 months of follow up. Therefore, renal SWL does not exacerbate features of MetS—this was the case even after overtreatment of the kidney with 4000 high-amplitude SWs. These findings do not support a single session of SWL as a risk factor for worsening of glucose tolerance or the onset of diabetes mellitus.
Acknowledgments
The authors are grateful to Jennifer Stashevsky for sectioning and staining tissue, Dan Moss and Gary Considine for performing the insulin staining and quantifying beta cell area, and Philip Blomgren for figure and micrograph composition. This work was supported by PHS grant P01-DK43881, R01 DK093954 and VA Merit Award 1I01BX001733.
Abbreviations and Acronyms
- SWL
shock wave lithotripsy
- SW
shock wave
- MetS
metabolic syndrome
- T2D
type 2 diabetes
- IVGTT
intravenous glucose tolerance test
References
- 1.Preminger G, Tielius H, Assimos D, et al. EAU/AUA Nephrolithiasis Guideline Panel. 2007 Guideline for the management of ureteral calculi. J Urol. 2007;178:2418. doi: 10.1016/j.juro.2007.09.107. [DOI] [PubMed] [Google Scholar]
- 2.Türk C, Knoll T, Petrik A, et al. Guidelines on urolithiasis. European Association of Urology; 2013. http://www.uroweb.org/gls/pdf/21_Urolithiasis_LR.pdf. [Google Scholar]
- 3.Evan AP, Willis LR. Extracorporeal shock wave lithotripsy: complications. In: Smith AD, editor. Smith’s textbook on endourology. BC Decker Inc; Hamilton: 2007. pp. 353–365. [Google Scholar]
- 4.Mullen KD, Hoofnagle JH, Jones EA. Shock wave-induced pancreatic trauma. Am J Gastroenterol. 1991;86:630. [PubMed] [Google Scholar]
- 5.Kirkali Z, Kirkali G, Tanci S, et al. The effect of extracorporeal shock wave lithotripsy on pancreatic enzymes. Int Urol Nephrol. 1994;26:405. doi: 10.1007/BF02768009. [DOI] [PubMed] [Google Scholar]
- 6.Abe H, Nisimura T, Osawa S, et al. Acute pancreatitis caused by extracorporeal shock wave lithotripsy for bilateral renal pelvic calculi. Int J Urol. 2000;7:65. doi: 10.1046/j.1442-2042.2000.00139.x. [DOI] [PubMed] [Google Scholar]
- 7.Hassan I, Zietlow SP. Acute pancreatitis after extracorporeal shock wave lithotripsy for a renal calculus. Urology. 2002;60:1111iii. doi: 10.1016/s0090-4295(02)01984-2. [DOI] [PubMed] [Google Scholar]
- 8.Karakayali F, Sevmis S, Ayvaz I, et al. Acute necrotizing pancreatitis as a rare complication of extracorporeal shock wave lithotripsy. Int J Urol. 2006;13:613. doi: 10.1111/j.1442-2042.2006.01366.x. [DOI] [PubMed] [Google Scholar]
- 9.Weng CH, Ho PY, Tsai CC, et al. Severe acute pancreatitis with abscess after extracorporeal shock wave lithotripsy: a rare complication. Urolithiasis. 2013;41:133. doi: 10.1007/s00240-012-0535-6. [DOI] [PubMed] [Google Scholar]
- 10.Krambeck AE, Gettman MT, Rohlinger AL, et al. Diabetes Mellitus and Hypertension associated with shock wave lithotripsy of renal and proximal ureteral stones at 19 years of followup. J Urol. 2006;175:1742. doi: 10.1016/S0022-5347(05)00989-4. [DOI] [PubMed] [Google Scholar]
- 11.Sato Y, Tanda H, Kato S, et al. Shock wave lithotripsy for renal stones is not associated with hypertension and diabetes mellitus. Urol. 2008;71:586. doi: 10.1016/j.urology.2007.10.072. [DOI] [PubMed] [Google Scholar]
- 12.Makhlouf AA, Thorner D, Ugarte R, et al. Shock wave lithotripsy not associated with development of diabetes mellitus at 6 years of follow-up. Urol. 2009;73:4. doi: 10.1016/j.urology.2008.07.066. [DOI] [PubMed] [Google Scholar]
- 13.Lee L, Alloosh M, Saxena R, et al. Nutritional model of steatohepatitis and metabolic syndrome in the Ossabaw miniature swine. Hepatology. 2009;50:56. doi: 10.1002/hep.22904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sturek M. Ca2+ regulatory mechanisms of exercise protection against coronary artery disease in metabolic syndrome and diabetes. J Appl Physiol. 2011;111:573. doi: 10.1152/japplphysiol.00373.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willis LR, Evan AP, Connors BA, et al. Shockwave lithotripsy: Dose-related effects on renal structure, hemodynamics, and tubular function. J Endourol. 2005;19:90. doi: 10.1089/end.2005.19.90. [DOI] [PubMed] [Google Scholar]
- 16.Sturek M, Alloosh M, Wenzel J, et al. Ossabaw Island miniature swine: cardiometabolic syndrome assessment. In: Swindle MM, editor. Swine in the Laboratory: Surgery, Anesthesia, Imaging, and Experimental Techniques. Boca Raton: CRC Press; 2007. [Google Scholar]
- 17.Spangler RS, Phillips RW. Portal vein insulin response of glucose intolerant Yucatan miniature swine to common secretogogues. Horm Metabol Res. 1982;14:448. doi: 10.1055/s-2007-1019045. [DOI] [PubMed] [Google Scholar]
- 18.Sims EK, Hatanaka M, Morris DL, et al. Divergent compensatory responses to high-fat diet between C57BL6/J and C57BLKS/J inbred mouse strains. Am J Physiol Endocrinol Metab. 2013;305:E1495. doi: 10.1152/ajpendo.00366.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mather KJ, Hunt AE, Steinburg HO, et al. Repeatability characteristics of simple indices of insulin resistance: implications for research applications. J Clin Endocrinol Metab. 2001;86:5457. doi: 10.1210/jcem.86.11.7880. [DOI] [PubMed] [Google Scholar]
- 20.Pacini G, Mari A. Methods for clinical assessment of insulin sensitivity and β-cell function. Best Practice & Research Clinical Endocrinology & Metabolism. 2003;17:305. doi: 10.1016/s1521-690x(03)00042-3. [DOI] [PubMed] [Google Scholar]
- 21.Christoffersen B, Ribel U, Raun K, et al. Evaluation of different methods of assessment of insulin sensitivity in Göttingen minipigs: introduction to a new, simpler method. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1195. doi: 10.1152/ajpregu.90851.2008. [DOI] [PubMed] [Google Scholar]
- 22.Efron B, Tibshirani R. An Introduction to the Bootstrap. Boca Raton, FL: Chapman & Hall/CRC; 1993. [Google Scholar]
- 23.Rosse C, Gaddum-Rosse P. The Pancreas. 5. Lippincott-Raven Publishers; Philadelphia, PA: 1997. Hollinshead’s Textbook of Anatomy; pp. 562–568. [Google Scholar]
- 24.Wittingen J, Frey CF. Islet concentration in the head, body, tail and uncinate process of the pancreas. Ann Surg. 1974;179:412. doi: 10.1097/00000658-197404000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ulrichs K, Bosse M, Heiser A, et al. Histomorphological characteristics of the porcine pancreas as a basis for the isolation of islets of langerhaus. Xenotransplantation. 1995;2:176. [Google Scholar]
- 26.Weyer C, Bogardus C, Mott DM, et al. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J Clin Invest. 1999;104:787. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Cógáin M, Krambeck AE, Rule AD, et al. Shock wave lithotripsy and diabetes mellitus: a population-based cohort study. Urol. 2012;79:298. doi: 10.1016/j.urology.2011.07.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chew BH, Zavaglia B, Sutton C, et al. Twenty-year prevalence of diabetes mellitus and hypertension in patients receiving shock-wave lithotripsy for urolithiasis. BJU Int. 2012;109:444. doi: 10.1111/j.1464-410X.2011.10291.x. [DOI] [PubMed] [Google Scholar]
- 29.El-Nahas RA, Awad BA, El-Assmy AM, et al. Are there long-term effects of extracorporeal shockwave lithotripsy in paediatric patients? BJUI. 2013;111:666. doi: 10.1111/j.1464-410X.2012.11420.x. [DOI] [PubMed] [Google Scholar]
- 30.Bergman RN, Prager R, Volund A, et al. Equivalence of the insulin sensitivity index in man derived by the minimal model method and the euglycemic glucose clamp. J Clin Invest. 1987;79:790. doi: 10.1172/JCI112886. [DOI] [PMC free article] [PubMed] [Google Scholar]