Abstract
High-throughput DNA sequencing has revolutionized cancer genomics with numerous discoveries relevant to cancer diagnosis and treatment. The latest sequencing and analysis methods have successfully identified somatic alterations including single nucleotide variants (SNVs), insertions and deletions (indels), structural aberrations, and gene fusions. Additional computational techniques have proved useful to define those mutations, genes, and molecular networks that drive diverse cancer phenotypes as well as determine clonal architectures in tumour samples. Collectively, these tools have advanced the study of genomic, transcriptomic, epigenomic alterations and their association to clinical properties. Here, we review cancer genomics software and the insights that have been gained from their application.
Introduction
Fred Sanger and colleagues jump-started the nascent field of genomics in 1977 with their development of chain-termination DNA sequencing1,2. It founded a series of commercial instruments that helped produce numerous early milestones, including the sequence of the first human genome3. Work was slow and expensive (the Human Genome Project rang-up about 1 billion dollars) and enormous gains in economy and speed would be needed before the approach could be applied widely. Enter ‘next generation sequencing’, the generic name for a raft of advanced techniques, including pyrosequencing[G], sequencing-by-ligation[G] and sequencing-by-synthesis[G]. State-of-the-art instruments now process a whole genome in less than a week and for nominally less than ten-thousand dollars. Many thousands of genomes and exomes have since been sequenced and their data have had an enormous impact on cancer research. Cancer genomics is a now-recognized sub-specialty that grew out of adapting sequencing for cancer research. It broadly seeks to characterize germline variants and somatic mutations in the individual, to use such data from cohorts to identify driver mutations[G], germline predispositions and environmental factors related to cancer and, ultimately, to synthesize such information into mechanistic theories and to develop information systems to assist clinicians with diagnosis and treatment decisions.
Aside from instrument advancements, cancer genomics owes a considerable debt to computing hardware and software. Biology has been steadily absorbing the knowledge, techniques and analytical culture of computer science and mathematics, and this has enabled the development of workhorse algorithms for sequence alignment, detection of somatic events and identification of significantly mutated genes[G] (SMGs). However, expansion in computing power is no longer pacing increases in instrument throughput, meaning the bottleneck is quickly shifting from data generation to data analysis. Taken with newer high-throughput streams, like RNA and protein sequences, as well as incorporation of data-intensive diagnostics like imaging, and the scope of the problem is clear; As the gap between the investigator’s abilities to generate and analyze data grows, genomics will increasingly experience the kinds of “Big Data” pains already familiar to other data-centric disciplines like particle physics. One of the foremost issues will be integrating the grand corpus of these many data types to open new frontiers in research.
The field has advanced substantially since the first cancer genome was sequenced, a mere 5 years ago4. Whole-genome, exome and RNA-sequencing are now routinely used in cancer studies and tools continue to be deployed for even more sophisticated analysis, for example combining genome and RNA-seq data for detecting fusion genes and interpreting cancer genomes across multiple patients to discover driver mutations and pathways. Such analyses have led to discovery of new cancer genes and cancer-causing mutations and have demonstrated how environmental exposure leads to characteristic mutational spectra. In this review, we discuss state-of-the-art data generation in cancer genomics, as well as current methods for pre-processing the raw data to detect signals and higher-level analysis of individuals (Level I) and cohorts (Level II) for research questions and clinical application (Figure 1). Moreover, we remark on some important open problems, and speculate on where the field is moving in the next several years.
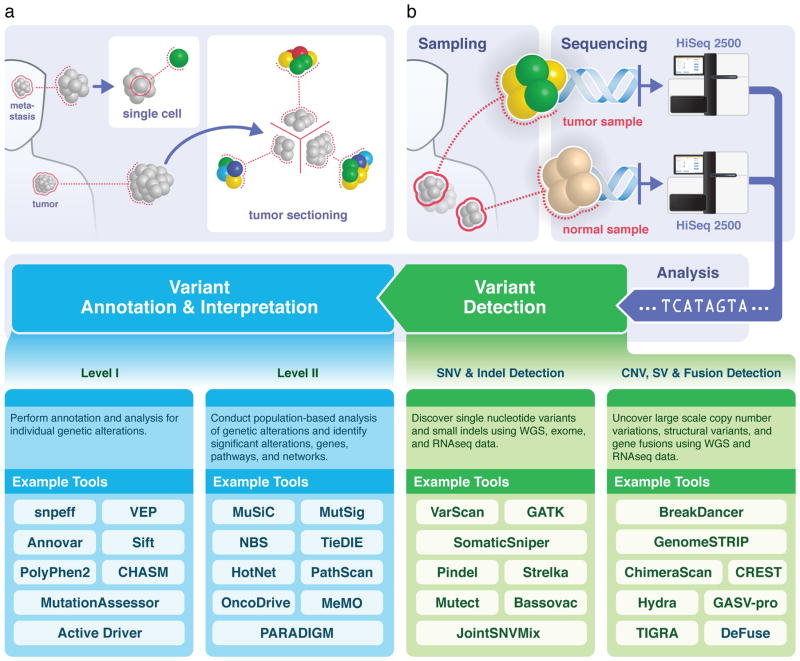
Figure 1. Sample procurement, sequencing, and analysis roadmap.
(A) Sequencing strategy: Most cancer genomics investigations sequence the genome of a tumour sample from primary or metastatic lesion, starting with a non-specific ‘global’ sample pooled from biopsy or resection. Because the spatial distribution of any resident subclones is not known a priori, it will become increasingly common to sequence specific regions from a tumor section separately. In the limit, single-cell sequencing can also be performed on flow-sorted nuclei to assess cellular diversity (B) Overview of the sequencing and analysis process: tumour and adjacent healthy tissue samples are sequenced using high-throughput instruments to obtain genome, exome, RNA and other types of data. After alignment, a battery of detection tools identifies both small (SNV, indel) and large (copy number, structural variation, gene fusions) alterations, which are then annotated and analyzed individually (Level I) —for example, for likely functional implications — and collectively (Level II) —for example, to identify relevant gene pathways and networks
Sequencing strategies
“Sequencing” is a broad term for interrogating a variety of molecular entities, including an entire static genome (whole genome sequencing)5, strictly the coding genomic regions (exome sequencing)6, the transcriptome7 as a snapshot of mRNA presence at a given time and tissue location, genomic methylation patterns8, and peptides (protein sequence). Because coding genomic sequences comprise only 1–2% of the genome, the cost for exome sequencing is still appreciably lower than for whole genome sequencing. However, differences are gradually becoming less important, as technology improvements continue to decrease overall sequencing costs. Despite its higher cost, whole-genome sequencing might be preferable, as it provides information on structural and non-coding variants, which cannot be captured from exome-only data. Whole genome data are therefore considered to be the unbiased “gold standard”9 and the field is likely to shift increasingly towards this form of data.
Traditional sequencing analysis
For the individual cancer patient, the immediate goal of any sequencing procedure is to identify germline and somatic variants linked to the cancer phenotype. Typically, tumour and normal tissue samples are collected, sequenced, aligned to the reference genome, and compared against each other to identify genomic differences (Figure 1). Many of the reported differences represent bona fide somatic aberrations, but such findings are ideally validated by more comprehensive data from an independent platform. There are many different kinds and sizes of mutational events, for example single nucleotide variations (SNVs), copy number aberrations (CNAs) and small insertions and deletions (collectively referred to as indels), each of which is detected through specific algorithmic methods. In actual practice, detection of all germline and somatic aberrations is a formidable challenge, due to limitations in current analysis algorithms (discussed below) and the quantity/quality of sequence data. Important events may be missed when sequence coverage is too low, or when repetitive or complex genomic regions complicate the alignment and assembly of sequence variants. Sequence coverage theory [G] has co-evolved with sequencing technologies and predicts that data must increase as we seek to identify increasingly subtle genomic signals (see Box 1).
Box 1. Coverage Considerations.
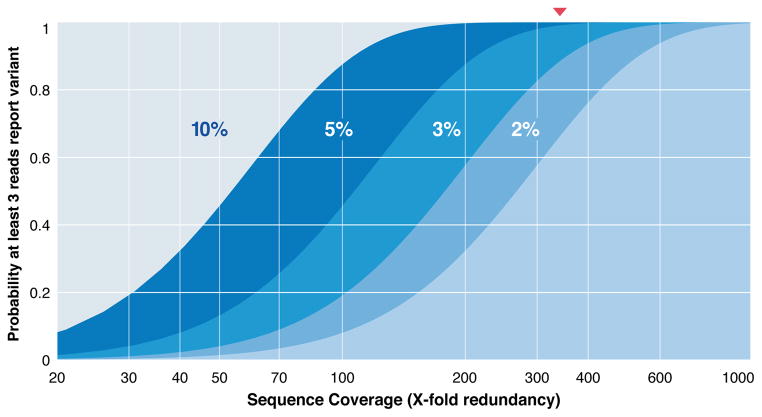
Early sequencing projects were based on Lander-Waterman theory155 for haploid coverage, which recommended a redundancy factor, ρ, of around 10X. Absent biases, this implies that loci are spanned by an average of 10 sequence reads and that >99.99% of the genome is represented by the data155. However, for medical applications, both alleles must be reliably identified. If the minimum condition is ≥3 spanning reads per allele, a figure of ρ ≈ 30X is then required for attaining roughly the same 99.99% standard156. This has been the de facto redundancy for cancer sequencing projects with respect to detecting SNVs, although it does not speak to other types of events. For example, presence of an indel is suggested whenever the reference-aligned average length of spanning fragments is significantly different from the average fragment length of the originating library.52 Application of this principle49,157 is tricky, because a genuine event has to be distinguished from cases in which predominantly shorter or longer fragments were sampled merely by chance. At 30X, the size range of insertions for which indels can be detected with low Type I error is rather narrow, suggesting more data are needed158. Data requirements elevate further if somatic events within subclones of a tumor are to be identified. For a subclone whose mass is a fraction μ of the total tumor, the probability of at least 3 reads reporting a heterozygous variant is
| (1) |
where R=μρ/2. In a 5% mass subclone, the remarkable figure of 340X data is required if 99% of subclonal variants should meet the detection conditions (Box 1 Figure). Smaller subclones require even more data, readily topping 500X in certain instances. The biomedical relevance of subclones, coupled with the growing throughput of instruments means that amounts of data generated for cancer genomics projects will continue to increase.
Box 1 Figure. Data requirements for capturing heterozygous variants.
Identifying a single-nucleotide variant (SNV) requires its observation in multiple reads, usually at least 3, but accrual of these reads is governed by the random dynamics of sampling and coverage, quantified in the ideal case (pure samples, perfect data, and no sequence bias) by Eq. (1) for various tumour mass fractions. Data requirements are pushed appreciably higher by subclones that comprise smaller fractions of the entire tumour mass. Red triangle indicates redundancy of 340X for 99% probability of observing ≥3 reads in a 5% subclone.
Subclonal analysis
As sequencing costs continue to decline, researchers are able to sequence tumor samples more deeply, enabling new analyses. For example, cancer progression has long been known to be a fundamentally clonal process10 and sequence coverages are now becoming sufficiently large to permit detection of the low prevalence events routinely associated with tumour subclones11. In recent years, multi-site and/or stage sequencing and tumour sectioning experiments have begun to identify founding clones and subclones contributing to cancer progression11–14 (Figure 1A). Mutations in subclones are typically mapped at low variant allele fraction (VAF) and often occur against a background of impure tumour and/or normal sample collection. Their identification is extremely difficult, an observation that is partially quantified by coverage theory (Box 1).
Single cell sequencing
Pioneering work on assessing CNAs in multiple tumor subpopulations13 was followed by single-cell sequencing15 using whole genome amplification (WGA) of DNA extracted from flow sorted nuclei (Figure 1A). Single-cell DNA and RNA sequencing are now routinely used for revealing cellular diversities within a tumour. However, there are still important challenges, such as amplification biases from degenerate oligonucleotide-primed WGA15 and multiple displacement amplification techniques16,17. Biases lead to uneven coverage and consequent difficulties for identifying somatic alterations, including SNVs, CNAs and structural aberrations. Sensitivity is most affected by allele dropout, owing to the preferential amplification of one of two alleles, with rates of 8 to 40%16,18 reported. Large CNAs can still be examined with low genome coverage (e.g., 5–6%) by computing read counts in variable-sized bins19, whereas unequal coverage renders analysis of smaller copy number and structural variants extremely difficult. Despite these challenges, recent advances, such as assembly algorithms that handle uneven sequence coverage20, point to widespread application of single-cell sequencing in the future.
Dissecting genomic changes in cancer
Somatic aberrations contain crucial information about the mechanisms of tumour development, progression, and metastasis/relapse. In addition, a subset that are “clinically actionable” have important implications in inferring prognosis and guiding decisions about treatment. The need for accurately identifying these events has spawned a wide collection of algorithms and software (Figure 1B). Most tools use either a composite statistical “score” or a formal probability test, though simple heuristic thresholds persist too. Table 1 lists some of the more widely used algorithms for detection of SNVs, indels, structural variants, fusions, as well as for additional analyses, including driver gene identification.
Table 1.
Computational tools for detecting and interpreting cancer genome alterations.
| SV / Indel Detection
| ||
|---|---|---|
| Program | Analysis | Synopsis |
| Bassovac (unpublished, Wendl, C et al.) | SNV/indel detection | Bayesian with tumor/normal impurity and clonality |
| GATK23 | SNV/indel detection | analysis framework using MapReduce |
| JointSNVMix31 | SNV detection | binomial/multinomial probability with pre-filtering |
| Mutect28 | SNV/indel detection | Bayesian probability with pre- and post-filtering |
| Pindel38 | Indel detection | pattern growth learning method |
| SomaticSniper27 | SNV/indel detection | Bayesian probability with posterior filtering |
| Strelka29 | SNV/indel detection | Bayesian probability with posterior filtering |
| SNVMix30 | SNV detection | Binomial mixture model |
| VarScan24,25 | SNV/indel detection | Fisher exact test, fitering, and FDR correction |
| Copy Number / SV / Fusion Detection
| ||
|---|---|---|
| Program | Analysis | Synopsis |
| BreakDancer54 | SV/indel detection | Kolmogorov-Smirnov test on discordant reads |
| BreakFusion68 | fusion detection | alignment-based pipeline for transcriptome data |
| BreakTrans73 | fusion mapping | integrate fusion discovery and breakpoint tools |
| ChimeraScan67 | chimeric transcription | discordant read pairs with posterior filtering |
| CREST55 | SV detection | heuristics/binomial test on soft-clipped reads |
| DeFuse65 | fusion detection | dynamic programming split and discordant reads |
| Delly40 | SV detection | integrated method of discordant and split reads |
| GASV-pro57 | SV detection | plane sweep for segment intersection |
| GenomeStrip59 | SV detection | depth and split/discordant reads on populations |
| Hydra139 | SV detection | discordant reads with assembly validation |
| Lumpy (unpublished, Layer, RM et al.) | SV detection | integrated method of discordant and split reads |
| TIGRA42 | SV detection | DeBruijn graph-based assembly |
| Level I Annotation & Interpretation
| ||
|---|---|---|
| Program | Analysis | Synopsis |
| Absolute148 | purity/ploidy/clonality | optimization of log scores |
| Annovar74 | functional prediction | annotation-based prediction |
| ASCAT166 | purity/ploidy/clonality | goodness of fit ranking of candidate solutions |
| TUSON Explorer100 | Gene classification | Oncogene or suppressor using mutation signatures |
| Classy (unpublished, Bharadwaj, M et al.) | gene classification | oncogene or suppressor using nearest neighbor |
| CHASM84,85 | functional preduction | random forest classifier |
| MutationAssessor83 | functional prediction | conservation-based prediction (entropy score) |
| PolyPhen281,167 | functional prediction | structure and alignment based probability model |
| SciClone (unpublished, Miller, C et al.) | tumor clonality | Bayesian mixture model |
| Sift82 | functional prediction | conservation-based prediction |
| Snpeff75 | functional prediction | annotation and coding effect prediction |
| THetA151 | purity/ploidy/clonality | maximum likelihood of mixture composition |
| VEP168 | functional prediction | annotation-based prediction |
| Level II Annotation & Interpretation
| ||
|---|---|---|
| Program | Analysis | Synopsis |
| Dendrix128 | mutation analysis | de novo discovery of mutual exclusive mutations |
| HotNet119 | network analysis | diffusion model for significant networks |
| MeMO122 | network analysis | network modules with mutual exclusivity |
| MuSiC92 | mutation analysis | framework for significance analysis of mutations |
| Multi-Dendrix129 | mutation analysis | de novo discovery of multiple sets of exclusive mutations |
| MutSigCV93 | mutation analysis | gene significance with variable background rate |
| NBS121 | network analysis | clustering using non-negative matrix factorization |
| OncoDrive-CIS169/-CLUST170 | mutation analysis | z-statistics for copy numbers of driver genes |
| Paradigm126 | gene expression | network analysis of gene expression |
| PathScan109 | pathway analysis | probability model for mutation-enriched pathways |
| TieDIE125 | network analysis | network diffusion model linking mutations to gene expression |
Despite impressive progress, the variant calling problem remains unresolved and we believe there is yet appreciable room for improvement in algorithm sophistication and accuracy. Numerous ad-hoc procedures have been developed to wring more performance out of existing tools. For example, it is known empirically that a candidate event called by several independent algorithms is significantly less likely to be a false positive than if it were called by any single one alone. Consequently, multi-caller strategies have now become more common, where several detectors21 are used under a “majority rules” aegis. Of course, sensitivity can suffer, as the overall discovery power now depends jointly on the individual tools’ powers. Although preliminary work22 has been reported, there are no conclusive studies that recommend specific tool combinations for optimally balancing Type I [G] versus Type II errors [G], perhaps because such studies require enormous computation. As there are more than two dozen published SNV-specific detectors alone, a 3-caller approach would demand evaluating more than 2000 possible combinations. Knowing the best algorithm combinations for various types of somatic events would be tremendously valuable to the community.
Single nucleotide variant detection
SNVs are the most frequent alterations in cancer genomes. Numerous SNV detection algorithms have been developed, including GATK23 [This is a broad and widely-used toolkit for variant discovery and data processing.], VarScan24,25 [VarScan is one of the early programs for single-nucleotide somatic detection and has since added additional capability for germline, copy number, and indel events.], SAMtools26 [SAMtools is a broad set of utilities for processing sequence data in the standardized SAM/BAM format, including variant calling.], SomaticSniper27, Mutect28 [MuTect is a widely used program for identifying single nucleotide somatic events in tumor-normal pair sequencing data.], Strelka29, and JointSNVMix/SNVMix30,31 (Figure 1B and Table 1). The first three handle both germline and somatic variants, whereas the others were designed for calling somatic mutations using tumour and matched normal genomic sequences. Although heterozygous variant allele frequencies of 50% are expected in germline samples, this number often does not hold for somatic sites in tumours, mainly owing to normal contamination and/or tumour heterogeneity. Algorithm development is now focusing on handling somatic mutations over a wide range of variant allele fractions. One example is the Bassovac algorithm that considers dependence upon bi-directional impurities and tumour subclonal structures (heterogeneity) at the read level, a necessary condition for avoiding ad hoc modeling and heuristics (Wendl et al., unpublished observations). Preliminary findings show improved performance, especially for events having low allelic fractions.
Indel detection
Indel detection is still challenging, largely owing to both their lower frequencies compared with those of SNVs32,33 and to mapping difficulties34. Although existing alignment tools are adequate for mapping reads containing SNVs, they lack the necessary accuracy and sensitivity for reads that overlap with indels or structural variants. Most tools by default allow for only two mismatches and no gaps in ‘seeded’ regions (that is in the first 28 bp in a read), which prohibits indel-containing reads from aligning to the reference. Paired-end mapping [G] is tremendously helpful in identifying larger indels when the ends occur in flanking regions, enabling inference of altered intervening sequences (Box 1). Gapped alignment [G], split read [G], and de novo assembly [G] are common approaches for detecting indels. VarScan25 and GATK Unified Genotyper23 are based on heuristics for indel calling using raw statistics, such as coverage, numbers of indel-supporting reads, read mapping qualities and mismatch counts.
Many existing tools23,25,26 work well for detecting short indels (<5–8 bp), but suffer from lack of precision [G] (Figure 1B and Table 1). Further, they often cannot detect medium-size indels, including some known druggable and/or prognostic events, using short read data. For example, internal tandem duplications of FLT3 (FLT3 ITD), present in 20% of patients with acute myeloid leukaemia (AML) and associated with poor prognosis35, are often overlooked because of mapping difficulties36. Finally, detection around low-complexity regions (such as homopolymers) is particularly challenging. SAMtools26 finds short indels by correcting for the effect of flanking tandem repeats, usually producing a large number of indel calls in the process. Dindel37 applies a Bayesian approach for calling small (<50 nucleotides) indels by realigning previously-mapped reads to generate candidate haplotypes and computes a posterior probability for each haplotype for downstream analysis. Conversely, Pindel38 [Pindel is focused on identifying breakpoints at single base resolution of indels, inversions, and tandem duplications.] takes a pattern growth approach borrowed from protein data analysis39 to detect indel breakpoints using both split reads and paired-end reads. A similar approach is employed in Delly40. Pindel achieves high precision and its sensitivity has been improved by allowing mismatches during the pattern matching process. The recent application of BWA-MEM41 alignment allows better mapping around long indels and structural variants. Moreover, local de novo assembly or multiple alignments around candidate indel sites (for example, using GATK haplotype caller and TIGRA local assembly42) reduce the number of false-positive indels. This process is utilized in many pipelines for indel detection.
Copy number aberration and structural variant detection
Unlike SNVs or small indels, CNAs typically affect more than one gene. Traditionally, single nucleotide polymorphism (SNP) genotyping data have been utilized for studying CNAs in cancer, and the CNA landscape across multiple cancer types has been reported43,44. Accurate inference of copy number from sequence data requires normalization procedures that consider certain biases inherent to short read sequencing methods (such as GC content and library biases). Approaches have been implemented for both GC-based coverage normalization and mapping bias45,46. GISTIC47 [GISTIC is one of the standard tools for finding genes affected by copy number changes that have a bearing on cancer initiation or progression.] and CMDS48 have been developed for the identification of recurrent CNVs.
Structural changes in chromosomes, such as chromosome deletions, insertions, inversions and translocations represent another major source of somatic variation in cancer genomes. The majority of known cancer genes are affected in varying degrees by rearrangements that result in either a fusion transcript or transcriptional dysregulation. Cytogenetics, spectral karyotyping, and fluorescent in situ hybridization have previously identified large chromosomal events in multiple cancer types (such as the BCR–ABL translocation in chronic myelogeneous leukaemia (CML)). Early end-sequencing profiling of bacterial artificial chromosome (BAC) or Fosmid libraries revealed complex chromosomal architectures in several human cancers49–53. In recent years, high-throughput whole-genome sequencing of tumour samples has further improved the ability to detect somatic rearrangements and to characterize their breakpoints with base pair resolution. The identification and analysis of read pairs that do not align as anticipated enable the detection of a wide range of structural alterations, including deletions, tandem or inverted duplications, inversions, insertions and translocations in many cancer genomes. BreakDancer54 [BreakDancer is a general tool for identifying structural variations, including insertions, deletions, inversions, and translocations using the concept of discordant read pairs.], CREST55, VariationHunter56, GASV-Pro57,58 and GenomeSTRIP59 are among the pioneering and most popular algorithms for such analysis (Figure 1B and Table 1). BreakDancer performs de novo prediction of deletions, insertions, inversions and translocations based on a Poisson model for the number of supporting reads, size of anchoring regions and overall genome coverage. CREST utilizes the soft-clipping performed by the software package Burrows Wheeler aligner (BWA) and similar aligners to predict diverse structural events. GASV analyzes structural variants, improving breakpoint identification using a geometric bounding algorithm; GASV-Pro extends this approach incorporating read depth to further improve variant calls. GenomeSTRIP characterizes genome deletion polymorphism using population-level concepts to reinterpret the technical features of sequence data that often reflect structural variation. Although these approaches are quite sensitive, the paired-end strategy tends to yield many false positives owing to sequencing errors or read mis-alignments, especially within repetitive sequences. As for indel detection, local assembly is also widely considered to be a reliable supplement for reducing false positives and improving breakpoint resolution of structural variants.
Fusion detection
The expression of gene fusions that arise through genomic structural rearrangements is a major mechanism for tumour initiation and progression. BCR–ABL1 in CML60, PML–RARa in acute promyelocytic leukemia61,62 and TMPRSS2–ERG in prostate cancer63 are among the most recurrent, functional gene fusions identified to date. Recently, algorithms such as Tophat-fusion64, deFuse65, MapSplice66, ChimeraScan67 and BreakFusion68 have been developed to detect fusions from RNA sequencing data (Figure 1B and Table 1). These tools are algorithmically similar to their genomic counterparts, although they focus primarily on mapping and ascertaining novel sequence junctions produced by mRNA-splicing and depend more on genome annotations. It is increasingly clear that fusions can arise from both simple translocations involving only two distal genomic loci60 and complex rearrangements consisting of multiple distal loci69,70. Therefore, concurrent detection of gene fusions and the originating rearrangements using systematic approaches can improve the accuracy of predictions, as well as help to delineate the underlying mechanistic aspects of gene fusion products. Two tools, Comrad71 and nFuse72, were developed to address this challenge. Both align raw whole-genome and RNA sequencing reads, while simultaneously corroborating fusions and genomic breakpoint discovery. Comrad, which was the first to be developed, only maps a single fusion breakpoint to a single genomic breakpoint through the application of a set of ad hoc rules. An extension, nFuse maps fusion breakpoints to complex structural rearrangements using a graph-theoretic approach. Their advantage is that they account for ambiguous read alignment and therefore minimize errors caused by misalignments. We have recently developed BreakTrans73, which jointly analyzes whole-genome and transcriptome sequencing data to test hypotheses produced by other tools, such as Tophat-fusion, MapSplice, BreakDancer and CREST, for further delineating the mechanistic components of gene fusions. Variants of various types and sizes described above require sophisticated tools for annotating and interpreting their effects and significance.
Variant Annotation and Prediction of Driver Mutations and Pathways
Following the identification of somatic alterations, the next challenge is to distinguish driver mutations from passenger mutations [G]. Because of the ease of assessing the recurrence and frequency of somatic mutations relative to the efforts necessary to validate their function, many computational and statistical techniques have been introduced to predict driver mutations and genes. These techniques can be divided into three general categories based on their underlying strategies: variant effect prediction; recurrence/frequency assessment; and pathway/network analysis.
Annotations and functional predictions
Recent years have seen consolidation of various genome annotation databases into centralized sources, with great improvement in quality and comprehensiveness. Ensembl and UCSC have emerged as leading repositories of genes and transcripts from GENCODE and Refseq; regulatory elements identified by ENCODE, TransFac and RegulomeDB; noncoding RNAs from Noncode, BodyMap and MiRBase; and protein annotations from Pfam and Interpro (see online links). There has been a concurrent emergence of software that leverages these resources to perform genome-wide annotation of variants in coding and non-coding regions. Annovar74 [Annovar is a versatile and widely-used tool for functional annotation of variants. It is often accessed through its web interface wAnnovar] and SNPeff75 provide annotation of transcript variants, SKIPPY76 predicts cryptic splice effectors, and Ensembl VEP, FunSeq77 and SNPnexus78 all extend support to include annotation of noncoding elements and regulatory features (Figure 1B and Table 1). Further, VAAST79 and GEMINI80 allow for comprehensive analysis and integration of coding variants, noncoding variants, regulatory elements and phenotype. In cancer studies, PolyPhen81 [A concatenation of “polymorphism phenotyping”, PolyPhen predicts the impact of amino acid changes on proteins and is often used in conjunction with SIFT.], SIFT82 [SIFT (sorting intolerant from tolerant) infers whether amino acid substitution has an effect on subsequent functioning of protein and is often used in conjunction with PolyPhen], MutationAssessor83 and Condel84 are commonly used to predict deleterious mutations. In addition, CHASM85,86 [CHASM is a popular tool for assessing functional impact of somatic missense mutations based on whether they furnish selective advantage to cancerous cells.], TransFIC87 and OncodriveFM88 use features learned from known cancer mutations for highlighting potential driver mutations. Finally, tools such as ActiveDriver89 have been developed to predict effects related to protein aggregation, protein stability and alterations of residues targeted by post-translational modification.
Significantly mutated genes
The most widely used approach to distinguish driver mutations from passenger mutations is to identify those mutations that occur more often than expected by chance. This approach is generally applicable across cancer types and is especially well suited for mutagenic phenomena associated with specific kinds of cancers, for example viral disruption in ovarian and cervical cancers, smoking and tobacco-induced mutations in lung and oral cancers, and ultraviolet (UVA, UVB or UVC) radiation-induced mutations in melanoma (Figure 2 and Box 2). In the simplest case, one assumes that the background mutation rate [G] (BMR) of a gene is known and evaluates the probability of passenger mutations in a given number of samples using a statistical test90,91.
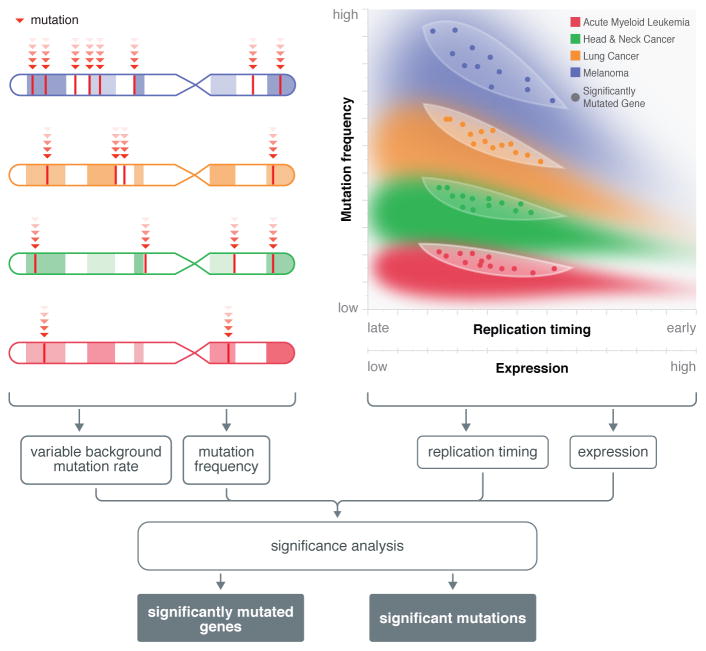
Figure 2. Biological factors relevant to assessing significant genes in cancer.
Genomic analysis establishes mutation frequencies of genes and helps characterize background mutation rates. Specific mutation hot spots have been found in the various cancer types. Other factors have also been shown to affect the background mutation rate of a gene, including gene length, expression level, and replication timing. State-of-the-art tools, such as MuSiC and MutSig give proper consideration to these and many other factors, for example transition versus transversion frequency, in determining the significantly mutated genes that contribute substantively to cancer initiation and progression.
Box 2. Detection of environmental impact on cancer genomes.
Healthy cells are subjected to various external insults that promote mutagenesis, well-known examples being cigarette smoke, asbestos, and ultraviolet radiation159 (Box 2 Figure). Viral infection and age also play roles160. These factors leave their marks on the cancer genome. For example, comparison of the mutation profiles across 12 common cancer types reveals that lung tumours contain higher proportions of C→A transversions131, which are classical signatures of exposure to cigarette smoke. Mutation dynamics are compliant with circumstances133, such as by ultraviolet exposure in melanomas, mismatch repair defects in colon cancers, or viral infections in head and neck tumors133,161,162. There is also growing appreciation that viral sequences, both episomal and those integrated into a genome, are more important in cancer than previously thought. Several oncoviruses have already been confirmed, including human papilloma virus (HPV), hepatitis B virus (HBV), hepatitis C virus (HCV), Epstein-Barr virus (EBV), human T-lymphotropic virus and Merkel cell polyomavirus, but there are undoubtedly more and they affect 15% to 20% of all human cancers163. Efforts to systematically characterize viruses in cancer are forthcoming and screening cancer genomes for viral sequences will likely be routine in the future. Despite their propensity for rapid evolution, it is likely that viral sequences will be reasonably detectable owing to their size, for example using homology-based read alignment and comparison with viral and bacterial databases. PathSeq164 and RINS165 investigate microbial sequences using the traditional subtraction and intersection approaches, respectively and research is now underway for developing additional tools for viral discovery.
Box 2 Figure. Environmental factor contributing to cancer risk.
Smoking, viruses, and radiation can strongly affect mutation rates across the cancer genome and mutation profiles across cancer types and human populations. Signatures of these effects can often be detected in tumour genome sequences.
The primary difficulty is to obtain good BMR estimates, as inaccuracies can lead to incorrect association of a gene with cancer. Many factors are known to affect the BMR of a gene (Figure 2), including covariates, variation among samples, and errors in upstream analysis. Covariates include differences in gene length, expression level, and replication timing. Mutation frequencies can differ not only across patients within a cancer type, but also because of diverse mutation spectra across cancer types that are possibly associated with environmental factors and viral signatures. Finally, incorrect or biased annotation of mutations can contribute markedly to potential false positives in cancer gene analysis. For example, multiple open reading frames in genes like TTN or incomplete description of pseudogenes in olfactory receptors can lead to incorrect assignment and annotation of mutations resulting in false predictions. Inadequate sequence coverage of a gene exacerbates these problems. Software that accounts for these contingencies includes MuSiC92 and MutSig93, which have been extensively used in many large-scale cancer studies94–98. Both tools integrate heterogeneities using convolution to obtain probability tails. There are additional covariates not accounted for and it is likely that frequency methods will continue to be developed.
Another method that has been used to distinguish between driver and passenger mutations is to examine whether mutations cluster in specific residues of the protein sequence. The so-called ‘20/20 Rule’99 advises that a gene be classified as an oncogene if at least 20% of its missense mutations (or identical in-frame indels) are located at a particular residue. Conversely, a gene is classified as a tumour suppressor if at least 20% of the mutations are inactivating (nonsense, frame-shift, splice site, or stop codon read-through). This heuristic is applicable to many well-known cancer genes, but is also somewhat arbitrary in the use of a fixed 20% threshold. It is now being supplemented by algorithms that assess patterns of mutational signatures100 and clustering of mutations in protein sequence101 or 3D protein structure102 using more rigorous statistical scores. Recent methods have shown that combining different signals of positive selection holds great potential for finding reliable lists of driver genes103.
Pathway and network analysis
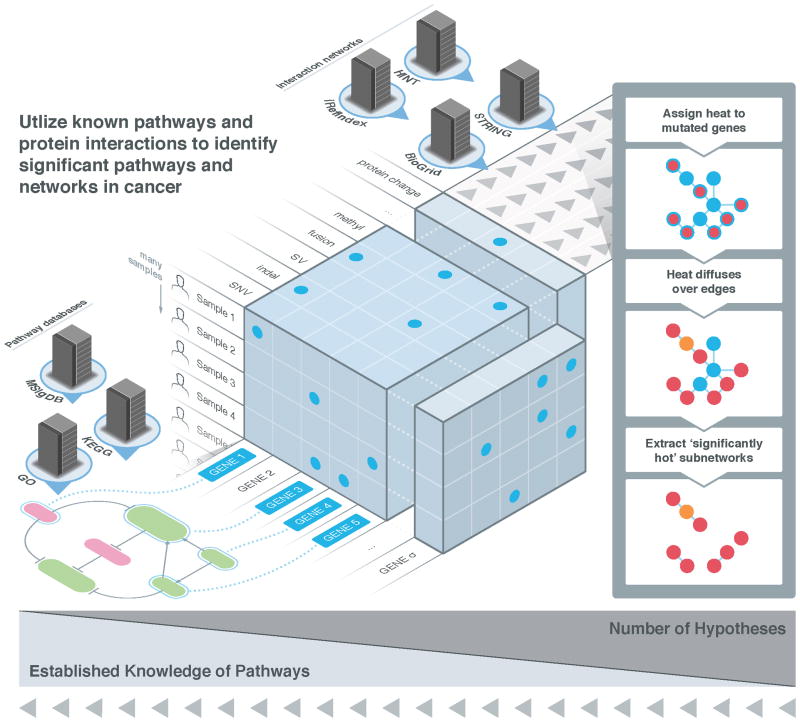
Enhanced understanding of somatic mutations can be gained by examining collections of mutations in signaling, regulatory, or metabolic pathways (Figure 3). It is well established that functional somatic mutations deregulate these pathways, and researchers have used a variety of approaches to assess the clustering of mutations in known pathways and interaction networks. These approaches can be divided into two classes: those that analyze known (curated) pathways, represented as gene sets, and those that analyze interaction networks to implicitly build pathways de novo.
Figure 3. Significantly mutated genes, pathways and networks.
Given the mutational status of genes across multiple patients, one can distinguish driver from passenger mutations using several strategies. Single-gene tests determine whether the observed number of samples having a mutation in the gene is significantly greater than what is expected under an appropriate null model. Pathway or gene set approaches examine whether multiple genes in pre-defined sets, as obtained for example from a curated database like KEGG, GO, or MSigDB, have more mutations than expected. These tests are biased to the prior knowledge of gene cascades residing in these databases, but the numbers of tests are relatively small, so the risks associated with Type I error [G] tend to be manageable. Conversely, network approaches rely only on knowledge of known protein-protein or protein-DNA interactions in examining combinations of mutations on whole-genome interaction networks, for example using the analog of heat diffusion. Because these approaches are unbiased, they furnish the possibility of inferring novel combinations of genes relevant to cancer, but larger numbers of hypothesis tests imply that greater care must be taken for multiple testing correction.
A straightforward approach to evaluate combinations of mutated genes is to examine the overlap between lists of mutated genes and pre-defined gene sets having known biological function. This technique has been used for over a decade in gene expression analysis to evaluate lists of differentially-expressed genes. Databases that record functional annotations of human genes include KEGG104, GO105, MSigDB106 and others. For example, suppose we have a list M of mutated genes, and we aim to see whether this list contains genes involved in regulation of cell cycle. Using the KEGG database, we find the list L of over two dozen cell cycle genes. There are two statistical tests that can be used to test whether M and L have significant overlap. First, if the list M of mutated genes is ranked (for example, using one of the mutation significance scores described above), gene-set enrichment analysis (GSEA)106 can be used to determine whether the genes in L are near the top of the ranked list M107. Second, if the list M is unranked, then the overlap between the lists M and L can be assessed using a hypergeometric test108. More recently, specialized tests for SMG sets have been introduced. The most direct approach is to adapt one of the SMG tests (e.g., MuSiC and MutSig) described above. More sophisticated approaches such as PathScan109 and the method of Boca, et al.110 allow for varying BMR across annotated genes.
Examination of gene sets overcomes some of the limitations of single-gene tests of recurrence; in particular, these tests can assign significance to rarely mutated genes, when these genes appear in the same pathway. However, these tests also have some limitations. Human gene annotations and pathway databases remain incomplete and there is extensive crosstalk between pathways, meaning that decisions regarding which genes form the boundary of a pathway are somewhat arbitrary. The crosstalk is represented in gene set and pathway databases by the presence of multiple, overlapping gene sets, thus complicating the interpretation of reported enrichments. Finally, signaling and regulatory pathways have a rich topology of activating and inhibitory interactions, and this information is not represented in the list of genes genes/proteins that are members of the pathway.
To overcome these limitations, a second approach to analyzing combinations of mutations is to utilize biological interaction networks. A variety of genome-scale protein–protein interaction networks have been constructed in the past few years. For example, HPRD111, KEGG104 and Reactome112 summarize experimentally validated protein–protein interactions, whereas other databases, such as BioGrid113, STRING114, HINT115 and iRefIndex116 integrate interaction information from multiple data sources including protein–protein interactions derived from high-throughput experiments. The resulting protein–protein interaction networks contain over 10,000 proteins and 50,000 interactions. More recently, protein–DNA interactions from the ENCODE project117 have been integrated into these networks118.
Interaction networks have been used in place of gene sets to determine combinations of mutations that should be further evaluated. However, most biological networks have a non-uniform topology that is characterized by the presence of hubs or nodes. This topology must be taken into account when defining mutated subnetworks. HotNet119 is a method to find subnetworks of a large interaction network that are mutated in more samples than expected by chance. HotNet employs a heat diffusion model to simultaneously encode both the topology of the network and the significance of the observed frequencies of each mutated gene. Genes (or their corresponding proteins) are assigned an initial heat according to their mutation frequency or significance. This heat then diffuses over the edges of a network. Thus, significantly mutated subnetworks correspond to hotspots on the network. The number and size of such subnetworks is then tested for statistical significance. HotNet has been used to determine subnetworks in multiple cancer types analyzed in the context of TCGA 95,97,120, and has, for example, implicated mutations in the Notch signaling pathway in ovarian carcinoma95.
Recently, network-based stratification (NBS)121 used a similar heat diffusion model to define subtypes of tumour samples by clustering smoothed mutation profiles. MeMo122 is another approach to find mutated subnetworks, using the observation that driver mutations in interacting proteins are often mutually exclusive across patients123,124(see also below). MeMo first defines modules of highly-connected nodes in the network, and then assesses whether these network modules exhibit mutually exclusive mutations. MeMo has been used in several cancer types reported in the TCGA 96,120. Another approach used in TCGA studies120 is TieDIE125, which employs a network diffusion approach to connect genetic abnormalities (e.g. somatic mutations) to transcriptional changes. Many other methods have been introduced to examine networks using gene expression126, which are not discussed in detail here.
The third approach that has been used to analyze combinations of mutations is the identification of mutually-exclusive sets of mutations. For example, PIK3CA mutations and PTEN deletion are mutually exclusive in breast cancer127. Inverting this idea, one might find combinations of driver mutations by identifying mutually exclusive sets of mutations. MeMo122 uses this idea to examine genes with known interactions, as noted above. Alternatively, one may attempt to discover sets of mutually exclusive genes de novo, with no prior restrictions on the sets of genes. This idea is the basis of the De Novo Driver Exclusivity (Dendrix) algorithm128, as well as the Multi-Dendrix129 and RME130 algorithms. The Dendrix algorithm was used in the TCGA acute myeloid leukemia project97 and in Pan-Cancer TCGA analysis of 12 cancer types131.
Today, a substantial number of significant genes and pathways have been identified in individual cancer types as well as across cancer types. The next challenge is to better understand how these genes and pathways interact and function in concert in individual cancer patient.
Genome integrity and clonal architectures
Accumulation of somatic mutations in a population of tumor cells is the foundation of the clonal theory of cancer, as described by Peter Nowell in 197610. High-throughput sequencing has led to new insights into this process, including the discovery of novel mutational processes and the quantification of the clonal architecture of tumors.
Kataegis, chromothripsis and chromoplexy
One of the more fascinating observations from cancer-genome sequencing studies are genomes with extreme numbers and types on mutations. Kataegis [G] is the occurrence of an unusually large number of single nucleotide mutations clustered in a single locus, and was first reported in breast tumors132 and other cancer types133. Kataegisis identified from “rainfall plots” that illustrate the frequency of single nucleotide mutations across the genome.
The analogous phenomenon of many genome rearrangement breakpoints clustered at a single locus has long been observed from lower resolution microarray and cytogenetic studies134. However, genome sequencing has revealed a different phenomenon of chromosome shattering, or chromothripsis [G], where one or more loci undergo a catastrophic event of simultaneous breakage and aberrant repair at multiple breakpoints in a single cell division70. Chromothripsis was originally reported in ~2–3% of all cancers, but was shown to be particularly common in bone cancers (~25%). It was later reported in pediatric medulloblastomas135, and associated with TP53 mutations, suggesting a possible mechanism for its appearance136,137. A related process called chromoplexy [G] has now been observed in prostate cancers138.
Distinguishing chromothripsis/chromplexy from sequential accumulation of chromosomal rearrangements over multiple cellular generations is a challenge. Secondary rearrangements often obscure the signatures of chromothripsis and chromoplexy. The distinction between simultaneous and sequential rearrangement is typically made via simulations70,135,139, although there have been criticisms of these approaches140. In lieu of simulation, putative signatures of chromothripsis have been proposed141. Tools, such as PREGO142, nFuse72 and extensions of Hydra139 that simultaneously analyze multiple rearrangement breakpoints facilitate the evaluation of these signatures. However, more work is needed to find quantitative measures that distinguish chromothripsis and chromoplexy from sequential accumulation of rearrangements.
Defining clonal architecture in heterogeneous tumours
All genomic alterations discussed above have a role in clonal evolution [G]. Tumour clones are subject to changing selective pressures and continually accumulating mutations (Figure 4A). Genomic alterations collectively reflect this evolutionary history and can be used to reconstruct the subclonal architectures and progression processes that might have led to relapse or metastasis. Such information is enormously important, as clonality has already been implicated in numerous aspects of cancer, including clinical outcome143, increased progression and malignancy144 and drug resistance145.
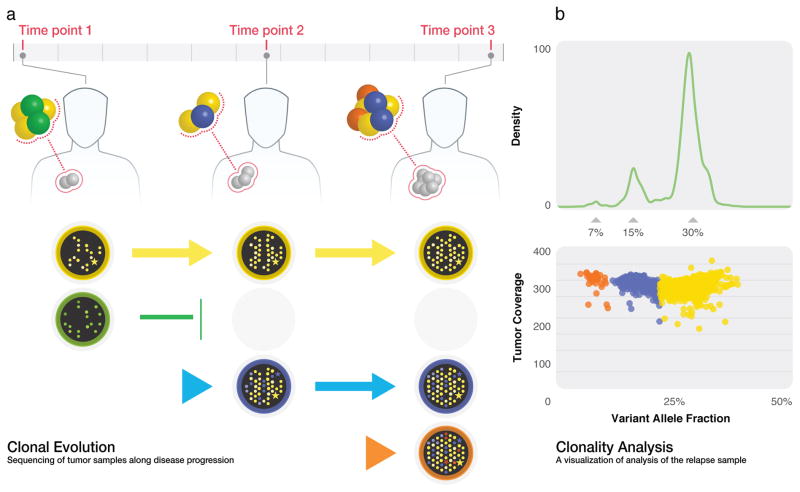
Figure 4. Conceptual example of clonal evolution model and clonality analysis.
(A) The founding clone (yellow) persists during the course of the disease. Another clone (green) present at time point 1 faces extinction before time point 2, but new subclones (blue/time point 2 and orange/time point 3) emerge during disease progression. (B) SciClone algorithm detects the three mutation clusters present at time point 3.
Clonal inference can be challenging. The number and positioning of clones within a tumour is often unknown, so uniform sampling is routinely presumed. Dot-plots are often used to obtain visual estimates of clones. For example; each heterozygous SNV event can be represented by a dot positioned on orthogonal axes of VAF versus frequency or total reads representing the event. Because the process is stochastic, such plots cluster into “dot clouds” (Figure 4) that are suggestive of clones. If the collective distribution is non-Gaussian (as determined by tests like Shapiro-Wilk or D’Agostino K-squared), multiple clones are presumed to be present. The process of discerning clones individually then encounters more confounders from both experimental contingencies (such as mutual impurities of the tumor and normal samples owing to suffusion or insufficient margin) and biological complications (such as copy number variations within the tumor genome). There are also subtle statistical factors, including differences in variances of clonal VAF distributions. Specifically, mutations that exist in all tumour cells, namely those present in the most recent common ancestor, have a variance (σ2) proportional to unity (σ2 ∝ 1). Conversely, mutations present in a minor subclone whose mass is a fraction μ of the total tumor have σ2 ∝ μ, meaning its distribution is “flattened” in this dimension.
Some clonal discovery methods center around the mathematical concept of density estimation, a process through which a probability density function (PDF) that best describes the observed data is constructed, for example using the Parzen-Rosenblatt146,147 “window” method. If clusters are sufficiently separated by VAF, the PDF readily identifies the tumour clones. There are a variety of more recent and sophisticated methods. For example ABSOLUTE148 adds an optimally-fitted copy number alteration model and karyotype likelihood model. Conversely, PyClone149 and the methods of Nik-Zainal et al. identify clones using hierarchical Bayesian clustering11,150. We have developed a method called SciClone (Miller et al. unpublished observations) (Figure 4B) that uses Bayesian mixture modeling to examine multiple samples from a patient over time (initial and relapsing tumour samples) or space (multiple biopsies). The THetA algorithm151 accounts for the presence of copy number aberrations, which can confound analysis of VAFs. Like the variant calling problem, progress has been significant, but substantial improvement is still needed. Not only will better variant detection improve clonal analysis, but also additional classes of information, including cancer-specific and pan-cancer population data, as well as information from other affected family members, will help to better define tumour architecture. Finally, direct integration with phylogenetic analysis algorithms may help to arbitrate among certain kinds of multiple alternatives that are currently undecidable148.
Conclusion: basic and clinical applications
In the short time since cancer genomics burst onto the biomedical scene it has made numerous fundamental contributions: 1) cancer-associated genes and pathways have been identified; 2) germline predispositions have been established; 3) technologies and algorithms have been improved; 4) vast datasets have been organized and recorded; and 5) knowledge has been classified into new databases. These accomplishments can be attributed to many individual research lab driven projects as well as large scale collaborative projects conducted by The Cancer Genome Atlas (TCGA) and International Cancer Genome Consortium (ICGC) using cutting edge computational approaches152,153. TCGA has completed nearly 10,000 cancer cases across 20 cancer types and ICGC will be sequencing approximately 25,000 additional genomes across 50 cancer types over the next several years. Further, efforts by the Cancer Cell Line Encyclopedia154 and Genomics of Drugs sensitivity in Cancer (http://www.cancerrxgene.org/) will help establish genomic determinants of resistance or sensitivity to drugs. The information and knowledge that will pour out of such projects are expected to have enormous implications for understanding cancer broadly, as well as for diagnosing and treating tumours at the individual patient level. This will be a tangible step towards personalized medicine.
Widespread clinical application of cancer genome and transcriptome sequencing is a certainty, although the timing remains unclear because of several outstanding issues related to both cost and reliability. First, the “data spectrum” and associated analysis tools are not yet complete. A significant portion of driver events in cancer are DNA or RNA alterations that affect protein expression, but proteomics has not yet ramped to the same high-throughput rates and sample census that genomic sequencing has. In our view, proteomic data are increasingly important in ascertaining driver genes and pathways, especially in terms of winnowing false positives from the large lists of hypotheses generated by pathway, network and significant gene mutation algorithms. However, it is clear that the proteomic gap is starting to close. For example, the Clinical Proteomic Tumor Analysis Consortium (CPTAC) launched by the National Cancer Institute (NCI) will further many goals, including characterizing tumour protein inventories, integrating genomics with proteomics and developing biomarker assays for high-priority proteins. Associated bioinformatics tools will be further developed, as well. This will be an increasingly fertile area of research. The second factor is the reality of cost. The sequence of an individual genome has dropped about 5 orders of magnitude, from about $1B for the first human genome to around $10K today. Technology development continues apace, but the overall cost for an entire “package” (DNA, RNA, and proteomic sequencing and companion systematic analysis) will likely have to drop yet another order of magnitude before sequencing can become anything like a routine clinical test. There will probably be some form of certification process for analysis software, as well.
There have even been a few early clinical victories, like the amazing case of Dr. Lukas Wartman, where comprehensive genome, exome and RNA analysis implicated FLT3 over-expression in his particular form of leukaemia (In Treatment for Leukemia, Glimpses of the Future, The New York Times, July 7th, 2012). This analysis led to the decision to administer Sutent, an FDA-approved tyrosine kinase inhibitor targeting FLT3 expression that quickly put Dr. Wartman’s disease into remission, which continues today. The next chapter of cancer research will undoubtedly see further pushes toward clinical application, as well as increased involvement of big pharmain developing new therapeutic agents. The cancer landscape will look vastly different from today in a decade and we will be at the threshold, if not well into the era of finding cures (or means of conferring long-term remission) for some cancers. Stay tuned.
Online summary.
High-throughput sequencing of cancer genomes, exomes, and transcriptomes has enabled the identification of many novelsomatic aberrations, providing new insights into cancer biology and new therapeutic targets.
Computational and statistical tools are necessary to interpret the large and complex datasets that result from high-throughput sequencing approaches.
Mature software for detecting single-nucleotide variants, indels, copy number aberrations, structural aberrations, and gene fusions in cancer genomes are now available. Additional challenges remain in increasing the sensitivity and specificity of these algorithms.
Computational techniques are essential to prioritize somatic aberrations that are likely to be functional for further experimental validation. Two common approaches are to predict functional impact of individual mutations using prior biological knowledge, and to identify recurrently mutated genes, pathways, and networks across many samples.
Algorithms to infer the clonal structure and evolutionary history of a tumor from ultra-deep sequencing data have recently been introduced. Applications of these techniques have shown that minority mutations in primary tumors may rise to majority in relapse/metastasis.
Sequencing of cancer genomes has shown wide range of specialized mutational processes including features like kategis, chromothripsis, and chromoplexy that result in rapid genomic change and punctuated tumor evolution.
Acknowledgments
This work was supported by the National Human Genome Research Institute grants U01HG006517 to L.D. and R01HG005690 and R01HG007069 to B.J.R. and the National Cancer Institute grant R01CA180006 to L.D. We would like to thank Kai Ye and Michael D. McLellan for helpful comments.
Glossary
- Background mutation rate
Rate at which spontaneous mutations occur due to uncorrected copying errors
- Chromoplexy
A mutational event that results in significant, complex rearrangements involving multiple loci, though not as dramatic as chromothripsis and involving less clustering of rearrangement breakpoints
- Chromothripsis
A catastrophic mutational event that “shatters” one or more chromosomes, with simultaneous loss and rearrangement of multiple chromosomal segments
- Clonal evolution
the emergence of novel clones having improved survival or propagational fitness according to the particular sets of somatic mutations they have accumulated
- Driver mutation
A somatic mutation that plays a causal role in initiation, progression, metastasis, or recurrence of cancer
- De novo assembly
Reconstruction of a genomic target by assessing consensus sequence from alignments of overlapping reads and clones
- Gapped alignment
Alignment process where small gaps are allowed if they support a better fit
- Kataegis
The appearance of regions of local hyper-mutation in a tumor genome
- Paired-end mapping
Coordinated mapping of both sequenced ends of a fragment to a reference genome, where their approximately known separation furnishes extra information against misalignments
- Passenger mutations
Somatic mutations that arise incidentally and play no mechanistic role in cancer initiation or progression
- Precision
The fraction of the total number of called events that are true, sometimes called the positive predictive value
- Pyrosequencing
Specific sequencing-by-synthesis method where detection is based on chemiluminescent signals from luciferin conversion
- Sequence coverage theory
Characterization of sequencing processes mathematically in order to support development of detection methods and analysis and design of sequencing projects
- Sequencing-by-ligation
Sequencing based on using the mismatch sensitivity of DNA ligase to detect nucleotides
- Sequencing-by-synthesis
Sequential polymerization of nucleotides to a template with each incorporation inferred by an imaging process, usually from a fluorescent dye attached to the added nucleotide
- Significantly mutated gene
A gene having a rate of somatic mutation that is higher than what can be attributed to a random background rate, suggesting a role in tumor initiation or progression
- Split read
The phenomenon in which a read spans a deleted site, whereby it appears to be split in its alignment to a reference
- Type I error
Declaring an effect where none actually exists, which leads to a “false positive”
- Type II error
Overlooking an actual effect, which leads to a “false negative”
Biographies
Li Ding has concentrated her research on understanding somatic/germline genetic changes relevant to cancer initiation and progression as well as drug response. Her recent efforts include the discovery of 127 cancer genes across over 3,000 tumors from 12 major cancer types. She is the principle investigator for the NHGRI sponsored genome sequencing informatics (GS-IT) center at Washington University, an Assistant Director at the Genome Institute and an Assistant Professor of Medicine and Genetics at Washington University in St. Louis.
Michael Wendl focuses on applying mathematics and computational methods to pressing problems in the biomedical sciences. He developed much of DNA sequencing theory and co-wrote the PHRED trace analyzer used for processing Sanger sequencing data, including in the Human Genome Project. He now concentrates on problems in cancer genomics, including somatic detection, pathway analysis, and clonal evolution modeling.
Joshua McMichael creates user interfaces and data visualizations for bioinformatics, specializing in cancer genomics. He worked on the Genome Modeling System for high throughput sequencing data analysis and has produced many of the visualizations for cancer genomics discoveries including clonal evolution in acute myeloid leukemia. He currently works as a software developer at the Genome Institute at Washington University in St. Louis.
Ben Raphael develops novel combinatorial and statistical algorithms for the interpretation of genomes. Recent work focuses on structural variation in human and cancer genomes and on network/pathway analysis of somatic mutations in cancer. He is an Associate Professor in the Department of Computer Science and Director of the Center for Computational Molecular Biology at Brown University.
Footnotes
Online links
Ensembl - http://www.ensembl.org/Homo_sapiens/Info/Index
UCSC - http://genome.ucsc.edu/cgi-bin/hgGateway
GENCODE - http://www.gencodegenes.org/data.html
RefSeq - http://www.ncbi.nlm.nih.gov/refseq/
ENCODE - http://www.genome.gov/Encode/
TransFac - http://www.gene-regulation.com/pub/databases.html
RegulomeDB - http://www.regulomedb.org/
Noncode - http://www.noncode.org/
BodyMap - http://www.illumina.com/science/data_library.ilmn
http://www.ebi.ac.uk/arrayexpress/experiments/E-MTAB-513/
miRBase - http://www.mirbase.org/
Pfam - http://pfam.sanger.ac.uk/
Interpro - https://www.ebi.ac.uk/interpro/
References
- 1.Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. 1977. Biotechnology. 1992;24:104–108. [PubMed] [Google Scholar]
- 3.Lander ES, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 4.Ley TJ, et al. DNA sequencing of a cytogenetically normal acute myeloid leukaemia genome. Nature. 2008;456:66–72. doi: 10.1038/nature07485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shendure J, Lieberman Aiden E. The expanding scope of DNA sequencing. Nat Biotechnol. 2012;30:1084–1094. doi: 10.1038/nbt.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Majewski J, Schwartzentruber J, Lalonde E, Montpetit A, Jabado N. What can exome sequencing do for you? J Med Genet. 2011;48:580–589. doi: 10.1136/jmedgenet-2011-100223. [DOI] [PubMed] [Google Scholar]
- 7.Ozsolak F, Milos PM. RNA sequencing: advances, challenges and opportunities. Nat Rev Genet. 2011;12:87–98. doi: 10.1038/nrg2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Krueger F, Kreck B, Franke A, Andrews SR. DNA methylome analysis using short bisulfite sequencing data. Nat Methods. 2012;9:145–151. doi: 10.1038/nmeth.1828. [DOI] [PubMed] [Google Scholar]
- 9.Ding L, et al. Genome remodelling in a basal-like breast cancer metastasis and xenograft. Nature. 2010;464:999–1005. doi: 10.1038/nature08989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 11.Ding L, et al. Clonal evolution in relapsed acute myeloid leukaemia revealed by whole-genome sequencing. Nature. 2012;481:506–510. doi: 10.1038/nature10738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gerlinger M, et al. Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med. 2012;366:883–892. doi: 10.1056/NEJMoa1113205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Navin N, et al. Inferring tumor progression from genomic heterogeneity. Genome Res. 2010;20:68–80. doi: 10.1101/gr.099622.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Navin NE, Hicks J. Tracing the tumor lineage. Mol Oncol. 2010;4:267–283. doi: 10.1016/j.molonc.2010.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navin N, et al. Tumour evolution inferred by single-cell sequencing. Nature. 2011 doi: 10.1038/nature09807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hou Y, et al. Single-cell exome sequencing and monoclonal evolution of a JAK2-negative myeloproliferative neoplasm. Cell. 2012;148:873–885. doi: 10.1016/j.cell.2012.02.028. [DOI] [PubMed] [Google Scholar]
- 17.Xu X, et al. Single-cell exome sequencing reveals single-nucleotide mutation characteristics of a kidney tumor. Cell. 2012;148:886–895. doi: 10.1016/j.cell.2012.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gundry M, Li W, Maqbool SB, Vijg J. Direct, genome-wide assessment of DNA mutations in single cells. Nucleic Acids Res. 2012;40:2032–2040. doi: 10.1093/nar/gkr949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baslan T, et al. Genome-wide copy number analysis of single cells. Nat Protoc. 2012;7:1024–1041. doi: 10.1038/nprot.2012.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bankevich A, et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J Comput Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SY, Speed TP. Comparing somatic mutation-callers: beyond Venn diagrams. BMC Bioinformatics. 2013;14:189. doi: 10.1186/1471-2105-14-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goode DL, et al. A simple consensus approach improves somatic mutation prediction accuracy. Genome Med. 2013;5:90. doi: 10.1186/gm494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKenna A, et al. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res. 2010;20:1297–1303. doi: 10.1101/gr.107524.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koboldt DC, et al. VarScan: variant detection in massively parallel sequencing of individual and pooled samples. Bioinformatics. 2009;25:2283–2285. doi: 10.1093/bioinformatics/btp373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koboldt DC, et al. VarScan 2: somatic mutation and copy number alteration discovery in cancer by exome sequencing. Genome Res. 2012;22:568–576. doi: 10.1101/gr.129684.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, et al. The Sequence Alignment/Map format and SAMtools. Bioinformatics. 2009;25:2078–2079. doi: 10.1093/bioinformatics/btp352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larson DE, et al. SomaticSniper: identification of somatic point mutations in whole genome sequencing data. Bioinformatics. 2012;28:311–317. doi: 10.1093/bioinformatics/btr665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cibulskis K, et al. Sensitive detection of somatic point mutations in impure and heterogeneous cancer samples. Nat Biotechnol. 2013;31:213–219. doi: 10.1038/nbt.2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saunders CT, et al. Strelka: accurate somatic small-variant calling from sequenced tumor-normal sample pairs. Bioinformatics. 2012;28:1811–1817. doi: 10.1093/bioinformatics/bts271. [DOI] [PubMed] [Google Scholar]
- 30.Goya R, et al. SNVMix: predicting single nucleotide variants from next-generation sequencing of tumors. Bioinformatics. 2010;26:730–736. doi: 10.1093/bioinformatics/btq040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Roth A, et al. JointSNVMix: a probabilistic model for accurate detection of somatic mutations in normal/tumour paired next-generation sequencing data. Bioinformatics. 2012;28:907–913. doi: 10.1093/bioinformatics/bts053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lunter G. Probabilistic whole-genome alignments reveal high indel rates in the human and mouse genomes. Bioinformatics. 2007;23:i289–296. doi: 10.1093/bioinformatics/btm185. [DOI] [PubMed] [Google Scholar]
- 33.Cartwright RA. Problems and solutions for estimating indel rates and length distributions. Molecular biology and evolution. 2009;26:473–480. doi: 10.1093/molbev/msn275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li H, Ruan J, Durbin R. Mapping short DNA sequencing reads and calling variants using mapping quality scores. Genome Res. 2008;18:1851–1858. doi: 10.1101/gr.078212.108. gr.078212.108 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith CC, et al. Validation of ITD mutations in FLT3 as a therapeutic target in human acute myeloid leukaemia. Nature. 2012;485:260–263. doi: 10.1038/nature11016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spencer DH, et al. Detection of FLT3 internal tandem duplication in targeted, short-read-length, next-generation sequencing data. J Mol Diagn. 2013;15:81–93. doi: 10.1016/j.jmoldx.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 37.Albers CA, et al. Dindel: accurate indel calls from short-read data. Genome Res. 2011;21:961–973. doi: 10.1101/gr.112326.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ye K, Schulz MH, Long Q, Apweiler R, Ning Z. Pindel: a pattern growth approach to detect break points of large deletions and medium sized insertions from paired-end short reads. Bioinformatics. 2009;25:2865–2871. doi: 10.1093/bioinformatics/btp394. btp394 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ye K, Kosters WA, Ijzerman AP. An efficient, versatile and scalable pattern growth approach to mine frequent patterns in unaligned protein sequences. Bioinformatics. 2007;23:687–693. doi: 10.1093/bioinformatics/btl665. btl665 [pii] [DOI] [PubMed] [Google Scholar]
- 40.Rausch T, et al. DELLY: structural variant discovery by integrated paired-end and split-read analysis. Bioinformatics. 2012;28:i333–i339. doi: 10.1093/bioinformatics/bts378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H. Aligning sequence reads, clone sequences and assembly contigs with BWA-MEM. 2013. arXiv:1303.3997 [q-bio.GN] [Google Scholar]
- 42.Chen K, et al. TIGRA: A Targeted Iterative Graph Routing Assembler for breakpoint assembly. Genome Res. 2013 doi: 10.1101/gr.162883.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bignell GR, et al. Signatures of mutation and selection in the cancer genome. Nature. 2010;463:893–898. doi: 10.1038/nature08768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Beroukhim R, et al. The landscape of somatic copy-number alteration across human cancers. Nature. 2010;463:899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoon S, Xuan Z, Makarov V, Ye K, Sebat J. Sensitive and accurate detection of copy number variants using read depth of coverage. Genome Res. 2009;19:1586–1592. doi: 10.1101/gr.092981.109. gr.092981.109 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Campbell PJ, et al. Identification of somatically acquired rearrangements in cancer using genome-wide massively parallel paired-end sequencing. Nat Genet. 2008;40:722–729. doi: 10.1038/ng.128. ng.128 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Beroukhim R, et al. Assessing the significance of chromosomal aberrations in cancer: methodology and application to glioma. Proc Natl Acad Sci U S A. 2007;104:20007–20012. doi: 10.1073/pnas.0710052104. 0710052104 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Q, et al. CMDS: a population-based method for identifying recurrent DNA copy number aberrations in cancer from high-resolution data. Bioinformatics. 2010;26:464–469. doi: 10.1093/bioinformatics/btp708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Raphael BJ, Volik S, Collins C, Pevzner PA. Reconstructing tumor genome architectures. Bioinformatics. 2003;19(Suppl 2):ii162–171. doi: 10.1093/bioinformatics/btg1074. [DOI] [PubMed] [Google Scholar]
- 50.Raphael BJ, et al. A sequence-based survey of the complex structural organization of tumor genomes. Genome Biol. 2008;9:R59. doi: 10.1186/gb-2008-9-3-r59. gb-2008-9-3-r59 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Volik S, et al. Decoding the fine-scale structure of a breast cancer genome and transcriptome. Genome Res. 2006;16:394–404. doi: 10.1101/gr.4247306. gr.4247306 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Volik S, et al. End-sequence profiling: sequence-based analysis of aberrant genomes. Proc Natl Acad Sci U S A. 2003;100:7696–7701. doi: 10.1073/pnas.1232418100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bignell GR, et al. Architectures of somatic genomic rearrangement in human cancer amplicons at sequence-level resolution. Genome Res. 2007;17:1296–1303. doi: 10.1101/gr.6522707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chen K, et al. BreakDancer: an algorithm for high-resolution mapping of genomic structural variation. Nat Methods. 2009;6:677–681. doi: 10.1038/nmeth.1363. nmeth.1363 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J, et al. CREST maps somatic structural variation in cancer genomes with base-pair resolution. Nat Methods. 2011;8:652–654. doi: 10.1038/nmeth.1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hormozdiari F, Alkan C, Eichler EE, Sahinalp SC. Combinatorial algorithms for structural variation detection in high-throughput sequenced genomes. Genome Res. 2009;19:1270–1278. doi: 10.1101/gr.088633.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sindi S, Helman E, Bashir A, Raphael BJ. A geometric approach for classification and comparison of structural variants. Bioinformatics. 2009;25:i222–230. doi: 10.1093/bioinformatics/btp208. btp208 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sindi SS, Onal S, Peng LC, Wu HT, Raphael BJ. An integrative probabilistic model for identification of structural variation in sequencing data. Genome Biol. 2012;13:R22. doi: 10.1186/gb-2012-13-3-r22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Handsaker RE, Korn JM, Nemesh J, McCarroll SA. Discovery and genotyping of genome structural polymorphism by sequencing on a population scale. Nat Genet. 2011;43:269–276. doi: 10.1038/ng.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rowley JD. Letter: A new consistent chromosomal abnormality in chronic myelogenous leukaemia identified by quinacrine fluorescence and Giemsa staining. Nature. 1973;243:290–293. doi: 10.1038/243290a0. [DOI] [PubMed] [Google Scholar]
- 61.Huang ME, et al. Use of all-trans retinoic acid in the treatment of acute promyelocytic leukemia. Blood. 1988;72:567–572. [PubMed] [Google Scholar]
- 62.Huang ME. Treatment of acute promyelocytic leukemia with all-trans retinoic acid. Zhonghua yi xue za zhi. 1988;68:131–133. 110. [PubMed] [Google Scholar]
- 63.Tomlins SA, et al. Integrative molecular concept modeling of prostate cancer progression. Nat Genet. 2007;39:41–51. doi: 10.1038/ng1935. [DOI] [PubMed] [Google Scholar]
- 64.Kim YK, et al. Cooperation of H2O2-mediated ERK activation with Smad pathway in TGF-beta1 induction of p21WAF1/Cip1. Cellular signalling. 2006;18:236–243. doi: 10.1016/j.cellsig.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 65.McPherson A, et al. deFuse: an algorithm for gene fusion discovery in tumor RNA-Seq data. PLoS Comput Biol. 2011;7:e1001138. doi: 10.1371/journal.pcbi.1001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang K, et al. MapSplice: accurate mapping of RNA-seq reads for splice junction discovery. Nucleic Acids Res. 2010;38:e178. doi: 10.1093/nar/gkq622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Iyer MK, Chinnaiyan AM, Maher CA. ChimeraScan: a tool for identifying chimeric transcription in sequencing data. Bioinformatics. 2011;27:2903–2904. doi: 10.1093/bioinformatics/btr467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen K, et al. BreakFusion: targeted assembly-based identification of gene fusions in whole transcriptome paired-end sequencing data. Bioinformatics. 2012;28:1923–1924. doi: 10.1093/bioinformatics/bts272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Berger MF, et al. The genomic complexity of primary human prostate cancer. Nature. 2011;470:214–220. doi: 10.1038/nature09744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stephens PJ, et al. Massive genomic rearrangement acquired in a single catastrophic event during cancer development. Cell. 2011;144:27–40. doi: 10.1016/j.cell.2010.11.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.McPherson A, et al. Comrad: detection of expressed rearrangements by integrated analysis of RNA-Seq and low coverage genome sequence data. Bioinformatics. 2011;27:1481–1488. doi: 10.1093/bioinformatics/btr184. [DOI] [PubMed] [Google Scholar]
- 72.McPherson A, et al. nFuse: discovery of complex genomic rearrangements in cancer using high-throughput sequencing. Genome Res. 2012;22:2250–2261. doi: 10.1101/gr.136572.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen K, et al. BreakTrans: uncovering the genomic architecture of gene fusions. Genome Biol. 2013;14:R87. doi: 10.1186/gb-2013-14-8-r87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wang K, Li M, Hakonarson H. ANNOVAR: functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 2010;38:e164. doi: 10.1093/nar/gkq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cingolani P, et al. A program for annotating and predicting the effects of single nucleotide polymorphisms, SnpEff: SNPs in the genome of Drosophila melanogaster strain w1118; iso-2; iso-3. Fly. 2012;6:80–92. doi: 10.4161/fly.19695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Woolfe A, Mullikin JC, Elnitski L. Genomic features defining exonic variants that modulate splicing. Genome Biol. 2010;11:R20. doi: 10.1186/gb-2010-11-2-r20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Khurana E, et al. Integrative annotation of variants from 1092 humans: application to cancer genomics. Science. 2013;342:1235587. doi: 10.1126/science.1235587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chelala C, Khan A, Lemoine NR. SNPnexus: a web database for functional annotation of newly discovered and public domain single nucleotide polymorphisms. Bioinformatics. 2009;25:655–661. doi: 10.1093/bioinformatics/btn653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yandell M, et al. A probabilistic disease-gene finder for personal genomes. Genome Res. 2011;21:1529–1542. doi: 10.1101/gr.123158.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Paila U, Chapman BA, Kirchner R, Quinlan AR. GEMINI: integrative exploration of genetic variation and genome annotations. PLoS Comput Biol. 2013;9:e1003153. doi: 10.1371/journal.pcbi.1003153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Nakken S, Alseth I, Rognes T. Computational prediction of the effects of non-synonymous single nucleotide polymorphisms in human DNA repair genes. Neuroscience. 2007;145:1273–1279. doi: 10.1016/j.neuroscience.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 82.Ng PC, Henikoff S. SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res. 2003;31:3812–3814. doi: 10.1093/nar/gkg509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Reva B, Antipin Y, Sander C. Predicting the functional impact of protein mutations: application to cancer genomics. Nucleic Acids Res. 2011;39:e118. doi: 10.1093/nar/gkr407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gonzalez-Perez A, Lopez-Bigas N. Improving the assessment of the outcome of nonsynonymous SNVs with a consensus deleteriousness score, Condel. Am J Hum Genet. 2011;88:440–449. doi: 10.1016/j.ajhg.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wong WC, et al. CHASM and SNVBox: toolkit for detecting biologically important single nucleotide mutations in cancer. Bioinformatics. 2011;27:2147–2148. doi: 10.1093/bioinformatics/btr357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Carter H, et al. Cancer-specific high-throughput annotation of somatic mutations: computational prediction of driver missense mutations. Cancer Res. 2009;69:6660–6667. doi: 10.1158/0008-5472.CAN-09-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gonzalez-Perez A, Deu-Pons J, Lopez-Bigas N. Improving the prediction of the functional impact of cancer mutations by baseline tolerance transformation. Genome Med. 2012;4:89. doi: 10.1186/gm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gonzalez-Perez A, Lopez-Bigas N. Functional impact bias reveals cancer drivers. Nucleic Acids Res. 2012;40:e169. doi: 10.1093/nar/gks743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Reimand J, Bader GD. Systematic analysis of somatic mutations in phosphorylation signaling predicts novel cancer drivers. Molecular systems biology. 2013;9:637. doi: 10.1038/msb.2012.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Greenman C, Wooster R, Futreal PA, Stratton MR, Easton DF. Statistical analysis of pathogenicity of somatic mutations in cancer. Genetics. 2006;173:2187–2198. doi: 10.1534/genetics.105.044677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Getz G, et al. Comment on “The consensus coding sequences of human breast and colorectal cancers”. Science. 2007;317:1500. doi: 10.1126/science.1138764. [DOI] [PubMed] [Google Scholar]
- 92.Dees ND, et al. MuSiC: Identifying mutational significance in cancer genomes. Genome Res. 2012;22:1589–1598. doi: 10.1101/gr.134635.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lawrence MS, et al. Mutational heterogeneity in cancer and the search for new cancer-associated genes. Nature. 2013;499:214–218. doi: 10.1038/nature12213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 2008;455:1061–1068. doi: 10.1038/nature07385. nature07385 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Integrated genomic analyses of ovarian carcinoma. Nature. 2011;474:609–615. doi: 10.1038/nature10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Comprehensive molecular portraits of human breast tumours. Nature. 2012;490:61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Genomic and Epigenomic Landscapes of Adult De Novo Acute Myeloid Leukemia. N Engl J Med. 2013 doi: 10.1056/NEJMoa1301689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ding L, et al. Somatic mutations affect key pathways in lung adenocarcinoma. Nature. 2008;455:1069–1075. doi: 10.1038/nature07423. nature07423 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vogelstein B, et al. Cancer genome landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Davoli T, et al. Cumulative haploinsufficiency and triplosensitivity drive aneuploidy patterns and shape the cancer genome. Cell. 2013;155:948–962. doi: 10.1016/j.cell.2013.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ye J, Pavlicek A, Lunney EA, Rejto PA, Teng CH. Statistical method on nonrandom clustering with application to somatic mutations in cancer. BMC Bioinformatics. 2010;11:11. doi: 10.1186/1471-2105-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ryslik GA, Cheng Y, Cheung KH, Modis Y, Zhao H. Utilizing protein structure to identify non-random somatic mutations. BMC Bioinformatics. 2013;14:190. doi: 10.1186/1471-2105-14-190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lawrence MS, et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. 2014;505:495–501. doi: 10.1038/nature12912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kanehisa M, Goto S, Furumichi M, Tanabe M, Hirakawa M. KEGG for representation and analysis of molecular networks involving diseases and drugs. Nucleic Acids Res. 2010;38:D355–360. doi: 10.1093/nar/gkp896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ashburner M, et al. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Subramanian A, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Lin J, et al. A multidimensional analysis of genes mutated in breast and colorectal cancers. Genome Res. 2007;17:1304–1318. doi: 10.1101/gr.6431107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wendl MC, et al. PathScan: a tool for discerning mutational significance in groups of putative cancer genes. Bioinformatics. 2011;27:1595–1602. doi: 10.1093/bioinformatics/btr193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Boca SM, Kinzler KW, Velculescu VE, Vogelstein B, Parmigiani G. Patient-oriented gene set analysis for cancer mutation data. Genome Biol. 2010;11:R112. doi: 10.1186/gb-2010-11-11-r112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Peri S, et al. Development of human protein reference database as an initial platform for approaching systems biology in humans. Genome Res. 2003;13:2363–2371. doi: 10.1101/gr.1680803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Croft D, et al. Reactome: a database of reactions, pathways and biological processes. Nucleic Acids Res. 2011;39:D691–697. doi: 10.1093/nar/gkq1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chatr-Aryamontri A, et al. The BioGRID interaction database: 2013 update. Nucleic Acids Res. 2013;41:D816–823. doi: 10.1093/nar/gks1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Franceschini A, et al. STRING v9.1: protein-protein interaction networks, with increased coverage and integration. Nucleic Acids Res. 2013;41:D808–815. doi: 10.1093/nar/gks1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Das J, Yu H. HINT: High-quality protein interactomes and their applications in understanding human disease. BMC systems biology. 2012;6:92. doi: 10.1186/1752-0509-6-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Razick S, Magklaras G, Donaldson IM. iRefIndex: a consolidated protein interaction database with provenance. BMC Bioinformatics. 2008;9:405. doi: 10.1186/1471-2105-9-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Bernstein BE, et al. An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489:57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Khurana E, Fu Y, Chen J, Gerstein M. Interpretation of genomic variants using a unified biological network approach. PLoS Comput Biol. 2013;9:e1002886. doi: 10.1371/journal.pcbi.1002886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vandin F, Upfal E, Raphael BJ. Algorithms for detecting significantly mutated pathways in cancer. J Comput Biol. 2011;18:507–522. doi: 10.1089/cmb.2010.0265. [DOI] [PubMed] [Google Scholar]
- 120.Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature. 2013;499:43–49. doi: 10.1038/nature12222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hofree M, Shen JP, Carter H, Gross A, Ideker T. Network-based stratification of tumor mutations. Nat Methods. 2013;10:1108–1115. doi: 10.1038/nmeth.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ciriello G, Cerami E, Sander C, Schultz N. Mutual exclusivity analysis identifies oncogenic network modules. Genome Res. 2012;22:398–406. doi: 10.1101/gr.125567.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vogelstein B, Kinzler KW. Cancer genes and the pathways they control. Nat Med. 2004;10:789–799. doi: 10.1038/nm1087. [DOI] [PubMed] [Google Scholar]
- 124.Yeang CH, McCormick F, Levine A. Combinatorial patterns of somatic gene mutations in cancer. Faseb J. 2008;22:2605–2622. doi: 10.1096/fj.08-108985. [DOI] [PubMed] [Google Scholar]
- 125.Paull EO, et al. Discovering causal pathways linking genomic events to transcriptional states using Tied Diffusion Through Interacting Events (TieDIE) Bioinformatics. 2013;29:2757–2764. doi: 10.1093/bioinformatics/btt471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Vaske CJ, et al. Inference of patient-specific pathway activities from multi-dimensional cancer genomics data using PARADIGM. Bioinformatics. 2010;26:i237–245. doi: 10.1093/bioinformatics/btq182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Saal LH, et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 2005;65:2554–2559. doi: 10.1158/0008-5472-CAN-04-3913. [DOI] [PubMed] [Google Scholar]
- 128.Vandin F, Upfal E, Raphael BJ. De novo discovery of mutated driver pathways in cancer. Genome Res. 2012;22:375–385. doi: 10.1101/gr.120477.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Leiserson MD, Blokh D, Sharan R, Raphael BJ. Simultaneous identification of multiple driver pathways in cancer. PLoS Comput Biol. 2013;9:e1003054. doi: 10.1371/journal.pcbi.1003054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Miller CA, Settle SH, Sulman EP, Aldape KD, Milosavljevic A. Discovering functional modules by identifying recurrent and mutually exclusive mutational patterns in tumors. BMC Med Genomics. 2011;4:34. doi: 10.1186/1755-8794-4-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kandoth C, et al. Mutational landscape and significance across 12 major cancer types. Nature. 2013;502:333–339. doi: 10.1038/nature12634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Nik-Zainal S, et al. Mutational processes molding the genomes of 21 breast cancers. Cell. 2012;149:979–993. doi: 10.1016/j.cell.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Alexandrov LB, et al. Signatures of mutational processes in human cancer. Nature. 2013;500:415–421. doi: 10.1038/nature12477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Albertson DG, Collins C, McCormick F, Gray JW. Chromosome aberrations in solid tumors. Nat Genet. 2003;34:369–376. doi: 10.1038/ng1215. [DOI] [PubMed] [Google Scholar]
- 135.Rausch T, et al. Genome sequencing of pediatric medulloblastoma links catastrophic DNA rearrangements with TP53 mutations. Cell. 2012;148:59–71. doi: 10.1016/j.cell.2011.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Maher CA, Wilson RK. Chromothripsis and human disease: piecing together the shattering process. Cell. 2012;148:29–32. doi: 10.1016/j.cell.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Forment JV, Kaidi A, Jackson SP. Chromothripsis and cancer: causes and consequences of chromosome shattering. Nat Rev Cancer. 2012;12:663–670. doi: 10.1038/nrc3352. [DOI] [PubMed] [Google Scholar]
- 138.Baca SC, et al. Punctuated evolution of prostate cancer genomes. Cell. 2013;153:666–677. doi: 10.1016/j.cell.2013.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Malhotra A, et al. Breakpoint profiling of 64 cancer genomes reveals numerous complex rearrangements spawned by homology-independent mechanisms. Genome Res. 2013;23:762–776. doi: 10.1101/gr.143677.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sorzano CO, Pascual-Montano A, Sanchez de Diego A, Martinez AC, van Wely KH. Chromothripsis: breakage-fusion-bridge over and over again. Cell Cycle. 2013;12:2016–2023. doi: 10.4161/cc.25266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Korbel JO, Campbell PJ. Criteria for inference of chromothripsis in cancer genomes. Cell. 2013;152:1226–1236. doi: 10.1016/j.cell.2013.02.023. [DOI] [PubMed] [Google Scholar]
- 142.Oesper L, Ritz A, Aerni SJ, Drebin R, Raphael BJ. Reconstructing cancer genomes from paired-end sequencing data. BMC Bioinformatics. 2012;13(Suppl 6):S10. doi: 10.1186/1471-2105-13-S6-S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Landau DA, et al. Evolution and impact of subclonal mutations in chronic lymphocytic leukemia. Cell. 2013;152:714–726. doi: 10.1016/j.cell.2013.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Keats JJ, et al. Clonal competition with alternating dominance in multiple myeloma. Blood. 2012;120:1067–1076. doi: 10.1182/blood-2012-01-405985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Turke AB, et al. Preexistence and clonal selection of MET amplification in EGFR mutant NSCLC. Cancer Cell. 2010;17:77–88. doi: 10.1016/j.ccr.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Parzen E. On estimation of a probability density function and mode. Annals of Mathematical Statistics. 1962;33:1065–1076. [Google Scholar]
- 147.Rosenblatt M. Remarks on some non-parametric estimates of a density function. Annals of Mathematical Statistics. 1956;27:832–837. [Google Scholar]
- 148.Carter SL, et al. Absolute quantification of somatic DNA alterations in human cancer. Nat Biotechnol. 2012;30:413–421. doi: 10.1038/nbt.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shah SP, et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012;486:395–399. doi: 10.1038/nature10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nik-Zainal S, et al. The life history of 21 breast cancers. Cell. 2012;149:994–1007. doi: 10.1016/j.cell.2012.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Oesper L, Mahmoody A, Raphael BJ. THetA: inferring intra-tumor heterogeneity from high-throughput DNA sequencing data. Genome Biol. 2013;14:R80. doi: 10.1186/gb-2013-14-7-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Gonzalez-Perez A, et al. Computational approaches to identify functional genetic variants in cancer genomes. Nat Methods. 2013;10:723–729. doi: 10.1038/nmeth.2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Raphael BJ, Dobson JR, Oesper L, Vandin F. Identifying driver mutations in sequenced cancer genomes: computational approaches to enable precision medicine. Genome Med. 2014;6:5. doi: 10.1186/gm524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Barretina J, et al. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature. 2012;483:603–607. doi: 10.1038/nature11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Lander ES, Waterman MS. Genomic mapping by fingerprinting random clones: a mathematical analysis. Genomics. 1988;2:231–239. doi: 10.1016/0888-7543(88)90007-9. [DOI] [PubMed] [Google Scholar]
- 156.Wendl MC, Wilson RK. Aspects of coverage in medical DNA sequencing. BMC Bioinformatics. 2008;9:239. doi: 10.1186/1471-2105-9-239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bashir A, Volik S, Collins C, Bafna V, Raphael BJ. Evaluation of paired-end sequencing strategies for detection of genome rearrangements in cancer. PLoS Comput Biol. 2008;4:e1000051. doi: 10.1371/journal.pcbi.1000051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wendl MC, Wilson RK. Statistical aspects of discerning indel-type structural variation via DNA sequence alignment. BMC Genomics. 2009;10:359. doi: 10.1186/1471-2164-10-359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Boffetta P, Nyberg F. Contribution of environmental factors to cancer risk. British medical bulletin. 2003;68:71–94. doi: 10.1093/bmp/ldg023. [DOI] [PubMed] [Google Scholar]
- 160.Cerwenka A, Lanier LL. Natural killer cells, viruses and cancer. Nature reviews. Immunology. 2001;1:41–49. doi: 10.1038/35095564. [DOI] [PubMed] [Google Scholar]
- 161.Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Stransky N, et al. The mutational landscape of head and neck squamous cell carcinoma. Science. 2011;333:1157–1160. doi: 10.1126/science.1208130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Parkin DM. The global health burden of infection-associated cancers in the year 2002. Int J Cancer. 2006;118:3030–3044. doi: 10.1002/ijc.21731. [DOI] [PubMed] [Google Scholar]
- 164.Kostic AD, et al. PathSeq: software to identify or discover microbes by deep sequencing of human tissue. Nat Biotechnol. 2011;29:393–396. doi: 10.1038/nbt.1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bhaduri A, Qu K, Lee CS, Ungewickell A, Khavari PA. Rapid identification of non-human sequences in high-throughput sequencing datasets. Bioinformatics. 2012;28:1174–1175. doi: 10.1093/bioinformatics/bts100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Van Loo P, et al. Allele-specific copy number analysis of tumors. Proc Natl Acad Sci U S A. 2010;107:16910–16915. doi: 10.1073/pnas.1009843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Adzhubei IA, et al. A method and server for predicting damaging missense mutations. Nat Methods. 2010;7:248–249. doi: 10.1038/nmeth0410-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.McLaren W, et al. Deriving the consequences of genomic variants with the Ensembl API and SNP Effect Predictor. Bioinformatics. 2010;26:2069–2070. doi: 10.1093/bioinformatics/btq330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Tamborero D, Lopez-Bigas N, Gonzalez-Perez A. Oncodrive-CIS: a method to reveal likely driver genes based on the impact of their copy number changes on expression. PLoS One. 2013;8:e55489. doi: 10.1371/journal.pone.0055489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tamborero D, Gonzalez-Perez A, Lopez-Bigas N. OncodriveCLUST: exploiting the positional clustering of somatic mutations to identify cancer genes. Bioinformatics. 2013;29:2238–2244. doi: 10.1093/bioinformatics/btt395. [DOI] [PubMed] [Google Scholar]