Abstract
During the last several decades, colorectal cancer surgery has experienced some major perioperative improvements. Preoperative risk-assessment of nutrition, frailty, and sarcopenia followed by interventions for patient optimization or an adapted surgical strategy, contributed to improved postoperative outcomes. Enhanced recovery programs or fast-track surgery also resulted in reduced length of hospital stay and overall complications without affecting patient safety. After an initially indecisive start due to uncertainty about oncological safety, the most significant improvement in intraoperative care was the introduction of laparoscopy. Laparoscopic surgery for colon and rectal cancer is associated with better short-term outcomes, whereas long-term outcomes regarding survival and recurrence rates are comparable. Nevertheless, long-term results in rectal surgery remain to be seen. Early recognition of anastomotic leakage remains a challenge, though multiple improvements have allowed better management of this complication.
Keywords: Laparoscopic surgery, Colorectal surgery, Anastomotic leakage, Frailty, Nutritional status, Sarcopenia, Enhanced recovery after surgery, Audits
Core tip: Laparoscopic surgery is a fundamental improvement in oncological colorectal surgery, associated with better short-term outcomes. However, anastomotic leakage still presents a major challenge in the postoperative course. Future research should therefore aim at the prevention, timely recognition and treatment of this complication. Correction of nutritional compromise, frailty and muscle loss, optimization of fluid and microcirculatory status, implementation of clinical and laboratory diagnostic markers, and the use of clinical audits may all contribute to a reduction of anastomotic leakage.
INTRODUCTION
Colorectal cancer is one of the predominant types of cancer and the fourth leading cause of cancer-associated deaths worldwide[1]. In numbers, 600000 patients died of colorectal cancer in 2008, and disability-adjusted life-years lost from colorectal cancer were 300 per 100000 patients, which was estimated to be 7% of the total cancer burden worldwide[2]. Although treatment of colorectal cancer is multidisciplinary nowadays, optimal surgery remains the cornerstone of improved survival[3,4]. Sixty-six percent of patients with colorectal cancer will undergo at least one major surgical resection[5]. The perioperative course of colorectal surgery for malignancy is crucial for the clinical outcome of treatment, in terms of mortality, tolerance, efficacy, and functional recovery, and has a considerable impact on health care resources[6,7]. In the past decades, perioperative care improved largely due to advances in anesthetic and analgesic approaches, minimally invasive operative techniques, and the introduction of fast-track protocols[8,9]. However, complications are still observed after oncological colorectal surgery, leading to prolonged hospital stay and high readmission rates with concurrent health care costs[10]. Early recognition and adequate intervention of complications will attenuate severity and may prevent mortality.
Anastomotic leakage is among the most prevalent and detrimental complications of colorectal surgery. Of 10017 registered resections for colorectal cancer in the Netherlands in 2012, 691 (6.9%) were complicated by anastomotic leakage requiring re-intervention (Dutch Surgical Colorectal Audit 2012)[11], making anastomotic leakage the primary complication requiring re-intervention. Anastomotic leakage is associated with high morbidity[12], mortality[11], reoperation[7], and duration of hospitalization[13]. In cancer, anastomotic leakage is related to diminished disease-specific survival and higher recurrence rates[7,14,15]. It is therefore imperative to keep searching for strategies to prevent, diagnose and treat anastomotic leakage.
This review article describes pre-, intra- en postoperative advancements in oncological colorectal surgery and highlights the opportunities for further improvement, particularly aiming at laparoscopy and the prevention and recognition of anastomotic leakage.
ADVANCES IN PREOPERATIVE CARE
Risk factors for anastomotic leakage are numerous and already widely described[16]. Therefore, describing risk factors is beyond the scope of this review article. The focus is on preoperative patient assessment and optimization.
Nutritional status
A powerful and easily obtainable tool to assess the patient’s physical and/or mental condition is the use of questionnaires. Various questionnaires have been developed to evaluate nutritional status. A poor nutritional condition correlates well with impaired quality of life and physical functioning[17]. The short nutritional assessment questionnaire (SNAQ) and malnutrition universal screening tool (MUST) (Table 1) scores are commonly used nutritional screening tools in surgical patients. These questionnaires accurately detect malnutrition, and the MUST score predicts postoperative complications in cardiac surgery[18]. Evidence for the value of nutritional screening tools to predict postoperative outcome in colorectal surgery is lacking. As one in five patients undergoing colorectal surgery is malnourished[19], the detection of nutritional depletion is of great importance, especially with neo-adjuvant therapies compromising the nutritional and metabolic status. A disbalance between energy expenditure and nutritional supplementation is the fundamental physiologic derangement leading to cancer-induced weight loss. Both malnourishment and weight loss are associated with poor clinical outcome after surgery[20,21].
Table 1.
Items of the short nutritional assessment questionnaire and malnutrition universal screening tool
| Tool | Item | Score |
| SNAQ | Weight loss | |
| > 6 kg in the last 6 mo | 3 | |
| > 3 kg in the last month | 2 | |
| The experience of a decreased appetite over the last month | 1 | |
| The use supplemental drinks or tube feeding over the last month | 1 | |
| MUST | BMI kg/m2 | |
| > 20 | 0 | |
| 18.5-20 | 1 | |
| < 18.5 | 2 | |
| Unplanned weight loss in past 3-6 mo | ||
| < 5% | 0 | |
| 5%-10% | 1 | |
| > 10% | 2 |
Adapted from Kruizenga et al[17] (interpretation: 2 = moderate malnutrition, 3 = severe malnutrition) and Lomivorotov et al[18] (interpretation: 0 = low risk of malnutrition, 1 = medium risk of malnutrition, ≥ 2 = high risk of malnutrition). SNAQ: Short nutritional assessment questionnaire; MUST: Malnutrition universal screening tool.
Although nutritional supplementation strategies in oncological colorectal surgery can improve handgrip strength, pulmonary function and insulin resistance[22], nutritional support has not been proven unequivocally effective to reduce length of hospital stay and anastomotic leakage rates[23,24]. It may be concluded that only severely malnourished patients benefit from nutritional support[25]. Nutritional status questionnaires may however not only be used to identify malnourished patients for nutritional support. As a tool for accurate prediction of postoperative complications, SNAQ and MUST scores could lead the way to other treatment options, for example surgery without a primary anastomosis or protection of the anastomosis using a diverting stoma. The predictive value of these scores for postoperative complications remains yet to be determined.
Contradictory to malnourishment, also obesity, particularly abdominal visceral obesity measured by computed tomography (CT)-based fat volumetry, is considered a predictor of postoperative complications, prolonged hospital stay and higher intraoperative conversion (from laparoscopic to conventional surgery) rates in colon surgery[26,27]. The effect on anastomotic leakage particularly is not known yet. Obviously, the presence of (visceral) obesity does not necessarily equal a sufficient nutritional status.
Frailty and sarcopenia
Advanced age is associated with an increased incidence of cancer[28]. The number of elderly cancer patients is concomitantly increasing. Fifty percent of patients with colorectal cancer is above the age of 70[11]. While survival of all cancer types is increasing, improvement of cancer outcome has been relatively limited in older patients[29]. Higher age is an independent predictor of disease-specific perioperative mortality in patients undergoing surgery for colorectal cancer[30,31]. Especially in older patients, weight loss, cachexia and nutritional compromise are associated with impaired response to chemotherapy and decreased survival[32,33].
Frail elderly undergoing colorectal surgery have a 4-fold increased risk of major postoperative complications[34]. Frailty is a state of increased vulnerability towards stressors in older individuals, leading to an increased risk of developing adverse health outcomes[35]. The definitions and biological characteristics of frailty are subject of debate. Weight loss, decreased muscle strength, reduced physical activity, exhaustion, and reduced walking speed are symptoms of a physical definition of frailty[34,36], whereas comorbidity, polypharmacy, decreased physical functioning, impaired nutritional and cognitive status, depression and social support are components of a more multidimensional description of frailty[37]. A simple screening instrument for frailty is the groningen frailty index, based on physical, cognitive, social and emotional items[38]. Skeletal muscle depletion or sarcopenia is an element of frailty in both definitions. Sarcopenia, mostly assessed by measurement of muscle area at the level of the third lumbar spine at CT-scan (Figure 1), is associated with prolonged hospital stay, infectious complications and decreased recurrence and survival rates following colorectal and liver surgery[39,40]. Moreover, sarcopenia frequently occurs in obese patients too and the combination of sarcopenia and obesity (sarcopenic obesity) may result in even a worse outcome in terms of physical ability and survival[41]. However, a specific effect of sarcopenia on the anastomotic leakage rate has not yet been established.
Figure 1.
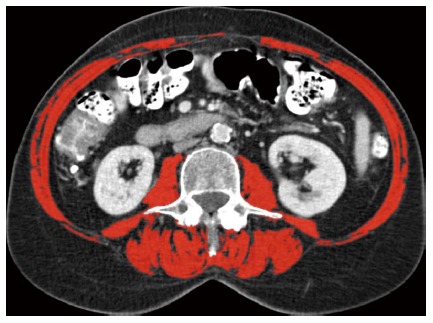
Computed tomography image at the third lumbar vertebral level. The following skeletal muscles are outlined in red: psoas, paraspinal, transverse abdominal, external oblique, internal oblique and rectus abdominis muscles. This female sarcopenia patient had an L3 (third lumbar spine) muscle index of 34.3 cm²/m².
Currently, with increasing age and incidence of colorectal malignancies, new research should aim at frailty and sarcopenia as risk factors for anastomotic leakage. Moreover, new options for preoperative treatment of frailty and sarcopenia should be investigated. Both nutritional and muscle exercise interventions have been proposed to counteract sarcopenia, with the best effects when both strategies are combined, which elicits the greatest anabolic response[42]. Effectiveness of such a dual approach on postoperative outcomes has not been investigated thus far.
Enhanced recovery after surgery
Several meta-analyses have shown that enhanced recovery after surgery (ERAS) programs result in reduced length of hospital stay and overall complications without affecting patient safety[9,43,44]. Although strong evidence exists for many recommendations, such as antibiotic prophylaxis and preoperative bowel preparation, controversies remain around perioperative fluid therapy, oxygen supplementation and use of non-steroidal anti-inflammatory drugs (NSAIDs)[45,46].
Furthermore, the effect of thoracic epidural anesthesia on splanchnic blood flow and anastomotic healing remains uncertain and demands future research[47,48]. After all, adequate tissue perfusion and oxygenation is imperative for anastomotic healing[49-52]. Major surgery accompanied by systemic hypotension and blood loss is thought to lead to redistribution of blood to preserve the vital organs (brain and heart) at the expense of the splanchnic circulation[53,54]. Indeed, intraoperative hypotension and blood loss are associated with an increased risk of anastomotic leakage in patients undergoing colorectal surgery[55]. Nevertheless, alterations in the microcirculation have been observed although systemic hemodynamic parameters, such as blood pressure, may be within an acceptable range[56]. Compromised visceral circulation, due to atherosclerosis of the visceral (celiac and superior/inferior mesenteric) or iliac arteries, is not associated with anastomotic leakage[57]. These findings imply that not the macroscopic circulation, but the microvascular flow at site of the anastomosis is of uppermost importance for anastomotic healing and that the microcirculation can be considered as a separate entity. Hence, the role in anastomotic healing and potential therapeutic targets for optimization of the gastrointestinal microcirculation remain to be clarified.
To reduce cardiopulmonary complications, restrictive fluid regimens seem superior to liberal fluid treatment[58]. Liberal and restrictive fluid therapies may induce hypoperfusion of the anastomosis by causing local edema or hypovolemia, which could be avoided by individualized, goal-directed fluid therapy. Individualized fluid therapy based on cardiac output measurement has been proposed as the ideal treatment strategy regarding complications, mortality and length of hospital stay[59]. However, a recent randomized controlled trial (RCT) using esophageal Doppler monitoring for cardiac output measurement could not prove a reduction in postoperative complications[60]. The effect of goal-directed fluid therapy on intestinal (microvascular) perfusion, damage and healing therefore needs further exploration.
Although an inspired oxygen concentration of over 80% during surgery and in the first two hours after surgery has been shown to reduce surgical-site infections[61], little is known about the effect on anastomotic oxygenation and healing. Only a small randomized trial describes a decrease in anastomotic leakage prevalence when administering 80% oxygen during surgery and in the first six hours following surgery[62]. Supplementation of high inspired oxygen concentration seems beneficial for anastomotic healing and should be evaluated in more patients and on the intestinal oxygenation level to draw definite conclusions.
Several studies have indicated that the use of NSAIDs is markedly correlated with anastomotic leakage following colorectal surgery[63-65]. The ERAS guidelines state that sufficient evidence is lacking to stop using NSAIDs as a component of multimodal analgesia[45]. The mechanisms by which NSAIDs exert their detrimental effects on colonic surgical wound healing are not known, which deserves further investigation.
ADVANCES IN INTRA-OPERATIVE CARE: LAPAROSCOPIC COLORECTAL SURGERY
Although strong evidence exists that a diverting stoma significantly reduces anastomotic leakage in rectal surgery[66,67], other measurements, such as omentoplasty, prophylactically leaving intra-abdominal drains, and application of a sealant (i.e., fibrin glue) around the anastomosis, seem obsolete in preventing anastomotic leakage[68-70]. Moreover, stapled anastomoses and hand-sewn techniques have comparable effects[71]. The most significant improvement in intraoperative care last decades, was the introduction of laparoscopy. Large incisions are avoided and surgical trauma is minimized.
Laparoscopic colon cancer surgery
Following the successful introduction of laparoscopic cholecystectomy and appendectomy, laparoscopic colon resection was first described by Jacobs and colleagues in 1991[72]. However, skepticism about its safety and feasibility rose, since early reports described high port or wound site cancer recurrence rates[73]. Consequently, laparoscopic colectomies were performed decreasingly and surgical societies summoned to only perform these under the auspices of randomized trials. Therefore, one single-center and three multi-center phase 3 randomized clinical trials were initialized to compare oncological outcomes between laparoscopic and conventional open colectomy for cancer: the Barcelona trial[74] and the clinical outcomes of surgical therapy (COST)[75], conventional vs laparoscopic-assisted surgery in colorectal cancer (CLASICC)[76] and colon cancer laparoscopic or open resection (COLOR)[77] trials, respectively.
Short-term benefits of laparoscopic colon cancer surgery
The early reports of these trials described similar[75-77] or even lower[74] postoperative complication rates for laparoscopic surgery compared with open surgery. Thirty-day mortality rates were not significantly different[74-77]. On the one hand, operative time was longer for laparoscopy in all studies. On the other hand, patients in the laparoscopic arm had significantly less blood loss[74,77], earlier return of bowel function[74,76,77], earlier resumption of fluid intake and regular diet[76,77], shorter use of oral and parenteral analgesics[75] and shorter hospital stay[74-77]. Radicality of resection, reflected by resection margins and the number of lymph nodes in the resected specimen, did not differ significantly[74-77]. Equivalence of the number of harvested lymph nodes was later confirmed in a meta-analysis[78].
The four exploring randomized trials were followed by multiple others. These trials also found short-term benefits in laparoscopic compared with open surgery[79-82] and confirmed similar postoperative complication and 30-d or in-hospital mortality rates for either operative modality[79-83]. Since the laparoscopic surgical technique is more similar to the conventional approach for right colectomies compared with other colorectal procedures, benefits seem less significant for this laparoscopic procedure[84]. Both long- and short-term health-related quality of life are higher in laparoscopic compared with open colon cancer surgery[85-87].
Hence, laparoscopic colon cancer surgery is associated with multiple short-term benefits compared with conventional surgery. Although these benefits might be clinically less important, laparoscopy seems more comfortable for the patient.
Laparoscopic colon cancer surgery and its oncological safety
Primary endpoints of the four pioneering trials mostly consisted of oncological parameters, since oncological safety was the main concern in early years. The Barcelona trial aimed to compare cancer-related survival after laparoscopic and open colon cancer resection. After a median follow-up of 43 mo, cancer-related survival was significantly higher after laparoscopic surgery[74]. Updated results with a median follow-up of 95 mo showed a tendency of higher cancer-related survival and overall survival for the laparoscopic group. Moreover, laparoscopic surgery was independently associated with a reduced risk of tumor recurrence[88]. This superiority of laparoscopy was mainly caused by the results in patients with stage III disease. This led to the hypothesis that the effect of surgery on the immune system is reduced in laparoscopy[89].
Time to tumor recurrence was the primary endpoint of the non-inferiority COST trial. Concerning this, it showed laparoscopic surgery to be non-inferior to open surgery. Moreover, no significant differences were found in cumulative incidence of recurrence, overall-survival, and disease-free survival. Tumor recurrence in surgical wounds was rare in both treatments groups (less than 1%) and did not differ between groups[75]. The 5-year results of the COST trial confirmed non-inferiority of laparoscopic surgery in terms of disease-free 5-year survival, overall 5-year survival, overall recurrence rates and recurrence distribution[90].
The CLASICC trial was initiated to investigate oncological safety regarding overall and disease-free survival and recurrence rates. No significant differences were found for either endpoint after three years[91]. Moreover, 5-year analysis of the data showed similar (local and distant) recurrence, and overall and disease-free survival rates for both study arms[92].
Primary outcome of the COLOR non-inferiority trial was 3-year disease-free survival, which differed 2% (95%CI: 3.2-7.2); 74.2% in the laparoscopic group and 76.2% in the open surgery group (P = 0.70), when all stages of disease combined were analyzed. Despite exceeding the predetermined non-inferiority boundary of 7% of the upper 95%CI, the authors concluded that this difference was clinically acceptable and that laparoscopy could safely be implemented into daily practice. After all, a per-protocol analysis showed laparoscopic surgery to be non-inferior to open surgery. The combined 3-year overall-survival did not significantly differ[93].
To enhance power, the transatlantic laparoscopically assisted vs open colectomy trials study group conducted a meta-analysis including the individual databases of the four mentioned trials (3-year results of the CLASICC and COLOR trial were not yet published at that moment). It showed similar 3-year disease-free and overall survival rates for all stages of disease in the two study arms. Furthermore, recurrence rates and patterns were comparable[94]. Later performed trials reproduced comparable long-term oncological results for both treatment groups[79,80,95]. Moreover, equivalence in long-term oncological outcomes was confirmed in multiple meta-analyses including high-quality RCTs[96-98].
In conclusion, early concerns regarding oncological safety have been invalidated nowadays. Particularly wound or port site recurrence rates are comparable in trials specifically reporting its incidence[74,80,90,93]. Laparoscopic surgery for curative colon malignancies is proven feasible, safe and non-inferior compared with conventional open surgery with multiple short-term advantages in patients with disease stages I-III on basis of solid level I evidence.
Laparoscopic rectal surgery
The CLASICC study was the first randomized trial also comparing oncological outcomes in laparoscopic and open surgery for rectal cancer. Laparoscopic rectal resection was considered technically more demanding than laparoscopic colon resection, as a high conversion rate (34%) and longer operation time were reported[76]. Feasibility and safety of laparoscopic rectal cancer surgery were questioned since. Nevertheless, successful total mesorectal excision was more frequently performed in laparoscopic procedures[76]. The suggested better practicability of this technique in laparoscopic surgery is essential, since complete resection of the mesorectum (with preservation of pelvic autonomic nerves) has shown to improve survival and recurrence rates[99]. Reacting on the CLASICC trial results, a research group from Hong Kong performed an updated subgroup analysis on rectal cancer[100] of their prospective randomized trial on laparoscopic resection of rectosigmoid carcinomas. This analysis showed faster recovery after and similar survival and disease-free survival rates in laparoscopic surgery compared with open surgery[101]. Due to these controversies, additional high-quality trials investigating oncological safety of laparoscopic rectal resection were required.
Short-term benefits of laparoscopic rectal cancer surgery
This need was fulfilled by the performance of multiple RCTs with both short- and long-term parameters as primary outcome. Several benefits of laparoscopic over open rectal surgery have been identified, which are almost identical to those in colon surgery. Laparoscopy is associated with significantly less blood loss or a trend towards significance[102-107]. This resulted in fewer blood transfusions, known as a risk factor for anastomotic leakage, in one trial reporting on this outcome[102]. Postoperative recovery was enhanced in laparoscopy, reflected by faster bowel recovery (shorter time to first postoperative peristalsis[76,105-108], flatus[103,106], stool[103,108], or resumption of normal diet[76,103,105,106]), and less analgesic use[103,105-107]. Moreover, length of hospital stay was shorter in the laparoscopic group, reaching significance in three single-center studies with short-term recovery as its primary outcome[102,106,107]. A wide range in postoperative complication rates is reported. Nonetheless, a meta-analysis reported significantly less postoperative complications after laparoscopic surgery[109]. On the other hand, like in colon cancer surgery, significantly longer operative time or a trend towards significance in laparoscopy is reported in most trials[76,102-108]. This issue would be of decreasing importance nowadays, since experience is growing.
In contrast to colon cancer surgery, higher health-related quality of life in laparoscopic compared with open rectal cancer surgery was only reported on the short-term (one week postoperatively) in a prospective study[110], whereas no difference was found on the long-term in the COLOR II cohort (one year postoperatively)[111].
Laparoscopic rectal cancer surgery and its oncological safety
No significant differences were found for both short- and long-term oncological outcomes in terms of proximal, distal and radial resection margins, number of lymph nodes harvested, three- or five-year overall-, cancer-related- or disease-free survival, and local recurrence rates in patients undergoing both sphincter-sparing (low anterior) resection for mid or high and abdominoperineal resection (APR) for low rectal carcinoma[102-104,106,108,112]. Since these studies incorporated relatively small patient numbers, consequently lacking power, and were mostly conducted in a single-center setting by one team or even one surgeon, a phase 3 non-inferiority multicenter trial including 1103 participants undergoing LAR or APR was conducted comparing oncological outcomes (COLOR II trial). Locoregional recurrence was its primary endpoint. Like the COLOR trial for colon cancer, the COLOR II trial showed that postoperative recovery was improved after laparoscopy, whereas radicality of resection, intra- and postoperative complications, and 30-d mortality were comparable in both groups. Moreover, laparoscopic rectal cancer surgery was considered feasible with a conversion rate of 16%. However, it should be noted that these findings are not applicable to all rectal cancer patients, since T3 tumors within 2 mm from the endopelvic fascia and T4 tumors were not included in the trial[113].
Meanwhile, long-term results of the CLASICC trial have been reported. Although higher positive resection margin rates were reported in the early results[76], 3-year results showed no differences in (local) recurrence and mortality rates. Moreover, similar results for APR and LAR were reported[91]. Multiple meta-analyses confirmed comparable short- and long-term oncological outcomes in laparoscopic and open surgery[114,115]. The Hong Kong study group reported equal overall survival, cancer-related survival, disease-free survival, and local and distant recurrence rates after ten years in patients with stage I-IV upper rectal cancer undergoing LAR[105]. Results for locoregional recurrence of the COLOR II trial are expected at the end of 2013[113].
In conclusion, rectal cancer surgery has short-term advantages over conventional open surgery and seems oncological safe. Nevertheless, recurrence and long-term survival rates of the COLOR II trial need to be awaited before laparoscopic resection of rectal cancer is indisputably proven to be oncological safe.
Surgical feasibility of laparoscopic colorectal surgery
Intraoperative conversion is considered an important measure of feasibility of a laparoscopic procedure. Due to the non-selective design of RCTs and the inability of researches to adequately choose patients eligible to be randomized to either surgical approach, high conversion rates were reported in early trials. In the CLASICC trial up to 25% of colon and 34% of rectal cancer patients underwent conversion[76]. However, no uniform definition of conversion was used in different trials. Conversion rates of the CLASICC trial improved each year, from 38% in the first year to 16% in the sixth year of the study[76]. Furthermore, low conversion rates of 2.8% to 14.6% were reported in later performed RCTs (allowing surgeons to be more experienced) and single-center studies with a specialized laparoscopic surgical team[74,79-81]. This demonstrates its function of the learning curve.
The COLOR case volume study demonstrated that high volume centers (> 10 cases per year) had fewer complications, greater lymph node harvest and shorter hospital stay compared with medium (5-10 cases per year) and low volume (< 5 cases per year) centers[116], again addressing the learning curve of laparoscopic surgery. Moreover, operative time was significantly shorter in high volume centers[116]. Hence, laparoscopic experience is a major factor in outcome. Higher costs for laparoscopy, despite the shorter mean hospital stay, may be reduced in high volume centers. After all, the main cause for higher costs are operative expenses[117].
Patient-tailored strategies
Although conversion rates were high in the firstly performed multicenter RCTs, laparoscopic colon surgery is considered standard of care in many countries nowadays. Intraoperative conversion was associated with higher complication rates and prolonged hospital stay[76,118]. Moreover, cases converted were associated with a worse overall[92] or 5-year disease-free[81] survival compared with laparoscopically completed or open surgery. The most common reasons for conversion in the early performed RCTs were tumor fixation and advanced disease, uncertainty of tumor clearance, and obesity[76]. As intraoperative conversion is nowadays mainly needed due to unfavorable tumor characteristics instead of inexperience of surgeons, these cases have a worse outcome. Moreover, laparoscopic surgery is currently regarded safe and suitable for obese patients also[119].
Besides surgical experience, a patient-tailored treatment strategy with optimal selection of patients eligible for laparoscopic surgery seems essential to optimize results. Adequate patient selection leads to lower conversion rates as shown in the LAPKON II trial[83]. Patients with rectal cancer were randomized after initial diagnostic laparoscopy to assess feasibility of laparoscopic resection. Hence, conversion should not be considered as surgical failure, but as a judicious decision. Nevertheless, appropriate selection should preferably be performed preoperatively. Feasibility and safety of laparoscopic surgery in elderly colorectal cancer patients for instance, have been underlined in a recent randomized study[120]. Another possible vulnerable population suitable for laparoscopy could be malnourished patients, since malnourishment and weight loss are associated with impaired clinical outcome[20,21].
Laparoscopic colorectal surgery in the light of enhanced recovery programs
As previously described, another major improvement in colorectal cancer surgery has been the introduction of fast-track or enhanced recovery programs. However, only one trial described in this review was performed within such a program. The LAFA trial, a multicenter randomized trial comparing laparoscopic and open surgery plus or minus a fast track program in segmental (right and left-sided) colectomy, concluded that the best perioperative treatment is laparoscopy combined with fast track surgery regarding total hospital stay. Secondary outcomes (postoperative hospital stay, morbidity, reoperation rate, readmission rate, in-hospital mortality, quality of life at two and four weeks, patient satisfaction and in-hospital costs) did not significantly differ between the four treatment groups[82]. Currently, a multicenter randomized trial is performed to investigate the hypothesis that laparoscopic surgery is superior compared with conventional surgery even when both treatments are optimized within the ERAS program[121].
ADVANCES IN POSTOPERATIVE CARE: DETECTION OF ANASTOMOTIC LEAKAGE
The clinical presentation of anastomotic leakage is heterogeneous and may be nonspecific. Anastomotic leakage is frequently diagnosed late due to a low index of suspicion based on clinical and conventional laboratory findings[122]. Moreover, abdominal CT-scan with intraluminal contrast undoubtedly has a role in timely recognition of anastomotic leakage, but yields low sensitivity (68%), which may delay the diagnosis and appropriate treatment[123]. Intervening weekends may further delay diagnosis and re-interventions[124]. Delay in recognizing and consequently treating anastomotic leakage after colorectal surgery is associated with increased mortality[125,126].
Clinical signs for accurate and early detection of anastomotic leakage have been widely investigated. den Dulk et al[127] standardized postoperative monitoring and developed a leakage-score, consisting of general, local physical examination, laboratory investigation and dietary items. The use of this score resulted in a significantly shorter delay in the diagnosis of anastomotic leakage.
Accurate diagnostic markers are needed to detect anastomotic leakage early after colorectal surgery. Various biomarkers have been investigated, although none has been validated clinically and studies are difficult to compare, mainly due to different definitions of anastomotic leakage[128]. C-reactive protein (CRP) has been widely proposed as an early indicator to diagnose anastomotic leakage on postoperative day 2-4[129]. However, the test characteristics are not convincingly robust, with approximately 70%-80% sensitivity and specificity[129]. Currently, the PRECIOUS trial investigates a step-up approach in major abdominal surgery combining CRP and CT imaging of the abdomen to diagnose severe complications, including anastomotic leakage[98]. Furthermore, specific plasma markers for intestinal cell damage and inflammation may provide better accuracy.
Finally, intraperitoneal microdialysis measuring intraperitoneal cytokines after rectal surgery has sometimes been used for early detection of anastomotic leakage. Lactate/ pyruvate ratio and interleukin (IL)-6, IL-10 and tumor necrosis factor-α were increased before clinical signs were detected in patients with anastomotic leakage[130,131]. Moreover, higher levels of intraperitoneal cytokines compared with systemic cytokines suggest the gastrointestinal tract to be the origin of the postoperative inflammatory response after colorectal surgery[132]. However, it should be noted that intraperitoneal microdialysis is an invasive method, with two catheters left behind intra-abdominally, that could involve complications. When a drain is left after surgery, measuring biomarkers in the peritoneal fluid, such as cytokines and metalloproteinases, could attribute to early detection of anastomotic leakage[133,134]. Also, a polymerase chain reaction for Enterococcus faecalis in drain fluids could be used as a screening test for anastomotic leakage[135]. Nevertheless, drains should not be used routinely, microdialysis is an invasive method, and the value of these markers in the absence of other clinical signs is limited.
ADVANCES IN ORGANIZATION OF CARE: AUDITS
Clinical auditing has been initiated in several countries and is considered an important tool for quality assessment and the identification of factors needing improvement. Furthermore, clinical audits provide a unique dataset for research as well. Starting in 2009, a nationwide audit for colorectal surgery has been initiated in the netherlands, the dutch surgical colorectal audit (DSCA)[136]. Later adopted as a quality indicator for the health care inspectorate, the DSCA has become a performance index for colorectal surgeons. Postoperative mortality and anastomotic leakage rates indeed decreased between 2010 and 2012[11,137]. This is in line with audits in other countries, including the United States, Belgium, Germany, United Kingdom, Spain, Italy, Denmark, Norway, and Sweden[138,139]. Whether these improvements are directly related to the introduction of the audit has to be determined. Yet, it can at least be stated that the DSCA has effectuated increased awareness of and insight in aspects of improvement.
Clinical audits have revealed several interesting findings with respect to postoperative complications. Hospitals with higher mortality rates had only slightly higher incidences of postoperative complications. However, the ability to let patients with a serious complication survive was significantly lower in high-mortality centers[136]. This phenomenon is addressed as failure to rescue and was previously described for other gastro-intestinal operations[140]. Data from the American College of Surgeons National Surgical Quality Improvement Program showed that although complication incidences did not vary between hospitals, mortality rates, largely contributed to death after major complications, significantly varied, indicating that timely recognition and treatment of complications deserves greater attention[141]. Future research should aim at identifying and improving the fundamental aspects causing failure to rescue. Another important finding was that anastomotic leakage rate variation between hospitals was mainly due to treatment-associated factors, such as blood loss or transfusion and operation time, than population characteristics, as was the case with postoperative mortality[142]. Therefore, anastomotic leakage is proposed as an accurate read-out for quality of care, underlining the importance of anastomotic leakage rate reduction in colorectal surgery. In conclusion, clinical audits provide unique insight in aspects associated with health care quality and more studies have to be done to find in-hospital factors correlated with anastomotic leakage for further improvement.
CONCLUSION
During the last several decades, colorectal cancer surgery has experienced some major perioperative improvements. Preoperative risk-assessment of nutrition, frailty, and sarcopenia followed by interventions for patient optimization or an adapted surgical strategy, contributed to improved postoperative outcomes. Enhanced recovery programs or fast-track surgery also resulted in reduced length of hospital stay and overall complications without affecting patient safety. After an initially indecisive start due to uncertainty about oncological safety, the most significant improvement in intraoperative care was the introduction of laparoscopy. Laparoscopic surgery for colon and rectal cancer is associated with better short-term outcomes, whereas long-term outcomes regarding survival and recurrence rates are comparable. Nevertheless, long-term results in rectal surgery remain to be seen. Early recognition of anastomotic leakage remains a challenge, though multiple improvements have allowed better management of this complication.
Footnotes
P- Reviewer: Aly EH, Samuel JC S- Editor: Gou SX L- Editor: A E- Editor: Liu XM
References
- 1.Cancer fact sheet N 297. World Health Organization, 2013. Available from: http://www.who.int/mediacentre/factsheets/fs297/en/
- 2.Soerjomataram I, Lortet-Tieulent J, Parkin DM, Ferlay J, Mathers C, Forman D, Bray F. Global burden of cancer in 2008: a systematic analysis of disability-adjusted life-years in 12 world regions. Lancet. 2012;380:1840–1850. doi: 10.1016/S0140-6736(12)60919-2. [DOI] [PubMed] [Google Scholar]
- 3.Pramateftakis MG. Optimizing colonic cancer surgery: high ligation and complete mesocolic excision during right hemicolectomy. Tech Coloproctol. 2010;14 Suppl 1:S49–S51. doi: 10.1007/s10151-010-0609-9. [DOI] [PubMed] [Google Scholar]
- 4.Pramateftakis MG, Kanellos D, Vrakas G, Tsachalis T, Raptis D, Makrantonakis A, Koukouritaki Z, Kanellos I. Progress in rectal cancer staging and treatment. Tech Coloproctol. 2010;14 Suppl 1:S29–S31. doi: 10.1007/s10151-010-0619-7. [DOI] [PubMed] [Google Scholar]
- 5.National Cancer Intelligence Networ. Major surgical resections report 2011 [Google Scholar]
- 6.Law WL, Choi HK, Lee YM, Ho JW. The impact of postoperative complications on long-term outcomes following curative resection for colorectal cancer. Ann Surg Oncol. 2007;14:2559–2566. doi: 10.1245/s10434-007-9434-4. [DOI] [PubMed] [Google Scholar]
- 7.Walker KG, Bell SW, Rickard MJ, Mehanna D, Dent OF, Chapuis PH, Bokey EL. Anastomotic leakage is predictive of diminished survival after potentially curative resection for colorectal cancer. Ann Surg. 2004;240:255–259. doi: 10.1097/01.sla.0000133186.81222.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kehlet H. Fast-track colorectal surgery. Lancet. 2008;371:791–793. doi: 10.1016/S0140-6736(08)60357-8. [DOI] [PubMed] [Google Scholar]
- 9.Zhuang CL, Ye XZ, Zhang XD, Chen BC, Yu Z. Enhanced recovery after surgery programs versus traditional care for colorectal surgery: a meta-analysis of randomized controlled trials. Dis Colon Rectum. 2013;56:667–678. doi: 10.1097/DCR.0b013e3182812842. [DOI] [PubMed] [Google Scholar]
- 10.Wick EC, Shore AD, Hirose K, Ibrahim AM, Gearhart SL, Efron J, Weiner JP, Makary MA. Readmission rates and cost following colorectal surgery. Dis Colon Rectum. 2011;54:1475–1479. doi: 10.1097/DCR.0b013e31822ff8f0. [DOI] [PubMed] [Google Scholar]
- 11.Snijders HS, Wouters MW, van Leersum NJ, Kolfschoten NE, Henneman D, de Vries AC, Tollenaar RA, Bonsing BA. Meta-analysis of the risk for anastomotic leakage, the postoperative mortality caused by leakage in relation to the overall postoperative mortality. Eur J Surg Oncol. 2012;38:1013–1019. doi: 10.1016/j.ejso.2012.07.111. [DOI] [PubMed] [Google Scholar]
- 12.Mäkelä JT, Kiviniemi H, Laitinen S. Risk factors for anastomotic leakage after left-sided colorectal resection with rectal anastomosis. Dis Colon Rectum. 2003;46:653–660. doi: 10.1007/s10350-004-6627-9. [DOI] [PubMed] [Google Scholar]
- 13.Golub R, Golub RW, Cantu R, Stein HD. A multivariate analysis of factors contributing to leakage of intestinal anastomoses. J Am Coll Surg. 1997;184:364–372. [PubMed] [Google Scholar]
- 14.Krarup PM, Nordholm-Carstensen A, Jorgensen LN, Harling H. Anastomotic leak increases distant recurrence and long-term mortality after curative resection for colonic cancer: a nationwide cohort study. Ann Surg. 2014;259:930–938. doi: 10.1097/SLA.0b013e3182a6f2fc. [DOI] [PubMed] [Google Scholar]
- 15.Mirnezami A, Mirnezami R, Chandrakumaran K, Sasapu K, Sagar P, Finan P. Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg. 2011;253:890–899. doi: 10.1097/SLA.0b013e3182128929. [DOI] [PubMed] [Google Scholar]
- 16.Daams F, Luyer M, Lange JF. Colorectal anastomotic leakage: aspects of prevention, detection and treatment. World J Gastroenterol. 2013;19:2293–2297. doi: 10.3748/wjg.v19.i15.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kruizenga HM, de Jonge P, Seidell JC, Neelemaat F, van Bodegraven AA, Wierdsma NJ, van Bokhorst-de van der Schueren MA. Are malnourished patients complex patients? Health status and care complexity of malnourished patients detected by the Short Nutritional Assessment Questionnaire (SNAQ) Eur J Intern Med. 2006;17:189–194. doi: 10.1016/j.ejim.2005.11.019. [DOI] [PubMed] [Google Scholar]
- 18.Lomivorotov VV, Efremov SM, Boboshko VA, Nikolaev DA, Vedernikov PE, Lomivorotov VN, Karaskov AM. Evaluation of nutritional screening tools for patients scheduled for cardiac surgery. Nutrition. 2013;29:436–442. doi: 10.1016/j.nut.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 19.Burden ST, Hill J, Shaffer JL, Todd C. Nutritional status of preoperative colorectal cancer patients. J Hum Nutr Diet. 2010;23:402–407. doi: 10.1111/j.1365-277X.2010.01070.x. [DOI] [PubMed] [Google Scholar]
- 20.Correia MI, Waitzberg DL. The impact of malnutrition on morbidity, mortality, length of hospital stay and costs evaluated through a multivariate model analysis. Clin Nutr. 2003;22:235–239. doi: 10.1016/s0261-5614(02)00215-7. [DOI] [PubMed] [Google Scholar]
- 21.Deslauriers J, Ginsberg RJ, Dubois P, Beaulieu M, Goldberg M, Piraux M. Current operative morbidity associated with elective surgical resection for lung cancer. Can J Surg. 1989;32:335–339. [PubMed] [Google Scholar]
- 22.Lidder P, Thomas S, Fleming S, Hosie K, Shaw S, Lewis S. A randomized placebo controlled trial of preoperative carbohydrate drinks and early postoperative nutritional supplement drinks in colorectal surgery. Colorectal Dis. 2013;15:737–745. doi: 10.1111/codi.12130. [DOI] [PubMed] [Google Scholar]
- 23.Oguz M, Kerem M, Bedirli A, Mentes BB, Sakrak O, Salman B, Bostanci H. L-alanin-L-glutamine supplementation improves the outcome after colorectal surgery for cancer. Colorectal Dis. 2007;9:515–520. doi: 10.1111/j.1463-1318.2006.01174.x. [DOI] [PubMed] [Google Scholar]
- 24.Von Meyenfeldt MF, Meijerink WJ, Rouflart MM, Builmaassen MT, Soeters PB. Perioperative nutritional support: a randomised clinical trial. Clin Nutr. 1992;11:180–186. doi: 10.1016/0261-5614(92)90026-m. [DOI] [PubMed] [Google Scholar]
- 25.Bozzetti F, Gavazzi C, Miceli R, Rossi N, Mariani L, Cozzaglio L, Bonfanti G, Piacenza S. Perioperative total parenteral nutrition in malnourished, gastrointestinal cancer patients: a randomized, clinical trial. JPEN J Parenter Enteral Nutr. 2000;24:7–14. doi: 10.1177/014860710002400107. [DOI] [PubMed] [Google Scholar]
- 26.Cecchini S, Cavazzini E, Marchesi F, Sarli L, Roncoroni L. Computed tomography volumetric fat parameters versus body mass index for predicting short-term outcomes of colon surgery. World J Surg. 2011;35:415–423. doi: 10.1007/s00268-010-0888-3. [DOI] [PubMed] [Google Scholar]
- 27.Tsujinaka S, Konishi F, Kawamura YJ, Saito M, Tajima N, Tanaka O, Lefor AT. Visceral obesity predicts surgical outcomes after laparoscopic colectomy for sigmoid colon cancer. Dis Colon Rectum. 2008;51:1757–1765; discussion 1765-1767. doi: 10.1007/s10350-008-9395-0. [DOI] [PubMed] [Google Scholar]
- 28.DePinho RA. The age of cancer. Nature. 2000;408:248–254. doi: 10.1038/35041694. [DOI] [PubMed] [Google Scholar]
- 29.Berrino F, Verdecchia A, Lutz JM, Lombardo C, Micheli A, Capocaccia R. Comparative cancer survival information in Europe. Eur J Cancer. 2009;45:901–908. doi: 10.1016/j.ejca.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 30.McMillan DC, McArdle CS, Morrison DS. A clinical risk score to predict 3-, 5- and 10-year survival in patients undergoing surgery for Dukes B colorectal cancer. Br J Cancer. 2010;103:970–974. doi: 10.1038/sj.bjc.6605864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matthiessen P, Hallböök O, Rutegård J, Sjödahl R. Population-based study of risk factors for postoperative death after anterior resection of the rectum. Br J Surg. 2006;93:498–503. doi: 10.1002/bjs.5282. [DOI] [PubMed] [Google Scholar]
- 32.Schiesser M, Kirchhoff P, Müller MK, Schäfer M, Clavien PA. The correlation of nutrition risk index, nutrition risk score, and bioimpedance analysis with postoperative complications in patients undergoing gastrointestinal surgery. Surgery. 2009;145:519–526. doi: 10.1016/j.surg.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 33.Persson C, Glimelius B. The relevance of weight loss for survival and quality of life in patients with advanced gastrointestinal cancer treated with palliative chemotherapy. Anticancer Res. 2002;22:3661–3668. [PubMed] [Google Scholar]
- 34.Tan KY, Kawamura YJ, Tokomitsu A, Tang T. Assessment for frailty is useful for predicting morbidity in elderly patients undergoing colorectal cancer resection whose comorbidities are already optimized. Am J Surg. 2012;204:139–143. doi: 10.1016/j.amjsurg.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 35.Fried LP, Hadley EC, Walston JD, Newman AB, Guralnik JM, Studenski S, Harris TB, Ershler WB, Ferrucci L. From bedside to bench: research agenda for frailty. Sci Aging Knowledge Environ. 2005;2005:pe24. doi: 10.1126/sageke.2005.31.pe24. [DOI] [PubMed] [Google Scholar]
- 36.Makary MA, Segev DL, Pronovost PJ, Syin D, Bandeen-Roche K, Patel P, Takenaga R, Devgan L, Holzmueller CG, Tian J, et al. Frailty as a predictor of surgical outcomes in older patients. J Am Coll Surg. 2010;210:901–908. doi: 10.1016/j.jamcollsurg.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 37.Saxton A, Velanovich V. Preoperative frailty and quality of life as predictors of postoperative complications. Ann Surg. 2011;253:1223–1229. doi: 10.1097/SLA.0b013e318214bce7. [DOI] [PubMed] [Google Scholar]
- 38.Schuurmans H, Steverink N, Lindenberg S, Frieswijk N, Slaets JP. Old or frail: what tells us more? J Gerontol A Biol Sci Med Sci. 2004;59:M962–M965. doi: 10.1093/gerona/59.9.m962. [DOI] [PubMed] [Google Scholar]
- 39.Lieffers JR, Bathe OF, Fassbender K, Winget M, Baracos VE. Sarcopenia is associated with postoperative infection and delayed recovery from colorectal cancer resection surgery. Br J Cancer. 2012;107:931–936. doi: 10.1038/bjc.2012.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.van Vledder MG, Levolger S, Ayez N, Verhoef C, Tran TC, Ijzermans JN. Body composition and outcome in patients undergoing resection of colorectal liver metastases. Br J Surg. 2012;99:550–557. doi: 10.1002/bjs.7823. [DOI] [PubMed] [Google Scholar]
- 41.Prado CM, Lieffers JR, McCargar LJ, Reiman T, Sawyer MB, Martin L, Baracos VE. Prevalence and clinical implications of sarcopenic obesity in patients with solid tumours of the respiratory and gastrointestinal tracts: a population-based study. Lancet Oncol. 2008;9:629–635. doi: 10.1016/S1470-2045(08)70153-0. [DOI] [PubMed] [Google Scholar]
- 42.Breen L, Phillips SM. Skeletal muscle protein metabolism in the elderly: Interventions to counteract the ‘anabolic resistance’ of ageing. Nutr Metab (Lond) 2011;8:68. doi: 10.1186/1743-7075-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Varadhan KK, Neal KR, Dejong CH, Fearon KC, Ljungqvist O, Lobo DN. The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: a meta-analysis of randomized controlled trials. Clin Nutr. 2010;29:434–440. doi: 10.1016/j.clnu.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 44.Spanjersberg WR, Reurings J, Keus F, van Laarhoven CJ. Fast track surgery versus conventional recovery strategies for colorectal surgery. Cochrane Database Syst Rev. 2011;(2):CD007635. doi: 10.1002/14651858.CD007635.pub2. [DOI] [PubMed] [Google Scholar]
- 45.Gustafsson UO, Scott MJ, Schwenk W, Demartines N, Roulin D, Francis N, McNaught CE, MacFie J, Liberman AS, Soop M, et al. Guidelines for perioperative care in elective colonic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr. 2012;31:783–800. doi: 10.1016/j.clnu.2012.08.013. [DOI] [PubMed] [Google Scholar]
- 46.Nygren J, Thacker J, Carli F, Fearon KC, Norderval S, Lobo DN, Ljungqvist O, Soop M, Ramirez J. Guidelines for perioperative care in elective rectal/pelvic surgery: Enhanced Recovery After Surgery (ERAS®) Society recommendations. Clin Nutr. 2012;31:801–816. doi: 10.1016/j.clnu.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 47.Freise H, Fischer LG. Intestinal effects of thoracic epidural anesthesia. Curr Opin Anaesthesiol. 2009;22:644–648. doi: 10.1097/ACO.0b013e32832eb7e8. [DOI] [PubMed] [Google Scholar]
- 48.Richards ER, Kabir SI, McNaught CE, MacFie J. Effect of thoracic epidural anaesthesia on splanchnic blood flow. Br J Surg. 2013;100:316–321. doi: 10.1002/bjs.8993. [DOI] [PubMed] [Google Scholar]
- 49.Hirano Y, Omura K, Tatsuzawa Y, Shimizu J, Kawaura Y, Watanabe G. Tissue oxygen saturation during colorectal surgery measured by near-infrared spectroscopy: pilot study to predict anastomotic complications. World J Surg. 2006;30:457–461. doi: 10.1007/s00268-005-0271-y. [DOI] [PubMed] [Google Scholar]
- 50.Karliczek A, Benaron DA, Baas PC, Zeebregts CJ, Wiggers T, van Dam GM. Intraoperative assessment of microperfusion with visible light spectroscopy for prediction of anastomotic leakage in colorectal anastomoses. Colorectal Dis. 2010;12:1018–1025. doi: 10.1111/j.1463-1318.2009.01944.x. [DOI] [PubMed] [Google Scholar]
- 51.Millan M, García-Granero E, Flor B, García-Botello S, Lledo S. Early prediction of anastomotic leak in colorectal cancer surgery by intramucosal pH. Dis Colon Rectum. 2006;49:595–601. doi: 10.1007/s10350-006-0504-7. [DOI] [PubMed] [Google Scholar]
- 52.Attard JA, Raval MJ, Martin GR, Kolb J, Afrouzian M, Buie WD, Sigalet DL. The effects of systemic hypoxia on colon anastomotic healing: an animal model. Dis Colon Rectum. 2005;48:1460–1470. doi: 10.1007/s10350-005-0047-3. [DOI] [PubMed] [Google Scholar]
- 53.Moore FA. The role of the gastrointestinal tract in postinjury multiple organ failure. Am J Surg. 1999;178:449–453. doi: 10.1016/s0002-9610(99)00231-7. [DOI] [PubMed] [Google Scholar]
- 54.Derikx JP, van Waardenburg DA, Thuijls G, Willigers HM, Koenraads M, van Bijnen AA, Heineman E, Poeze M, Ambergen T, van Ooij A, et al. New Insight in Loss of Gut Barrier during Major Non-Abdominal Surgery. PLoS One. 2008;3:e3954. doi: 10.1371/journal.pone.0003954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Post IL, Verheijen PM, Pronk A, Siccama I, Houweling PL. Intraoperative blood pressure changes as a risk factor for anastomotic leakage in colorectal surgery. Int J Colorectal Dis. 2012;27:765–772. doi: 10.1007/s00384-011-1381-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.De Backer D, Ortiz JA, Salgado D. Coupling microcirculation to systemic hemodynamics. Curr Opin Crit Care. 2010;16:250–254. doi: 10.1097/MCC.0b013e3283383621. [DOI] [PubMed] [Google Scholar]
- 57.Kornmann VN, van Werkum MH, Bollen TL, van Ramshorst B, Boerma D. Compromised visceral circulation does not affect the outcome of colorectal surgery. Surg Today. 2014;44:1220–1226. doi: 10.1007/s00595-013-0730-2. [DOI] [PubMed] [Google Scholar]
- 58.Rahbari NN, Zimmermann JB, Schmidt T, Koch M, Weigand MA, Weitz J. Meta-analysis of standard, restrictive and supplemental fluid administration in colorectal surgery. Br J Surg. 2009;96:331–341. doi: 10.1002/bjs.6552. [DOI] [PubMed] [Google Scholar]
- 59.Giglio MT, Marucci M, Testini M, Brienza N. Goal-directed haemodynamic therapy and gastrointestinal complications in major surgery: a meta-analysis of randomized controlled trials. Br J Anaesth. 2009;103:637–646. doi: 10.1093/bja/aep279. [DOI] [PubMed] [Google Scholar]
- 60.Brandstrup B, Svendsen PE, Rasmussen M, Belhage B, Rodt SÅ, Hansen B, Møller DR, Lundbech LB, Andersen N, Berg V, et al. Which goal for fluid therapy during colorectal surgery is followed by the best outcome: near-maximal stroke volume or zero fluid balance? Br J Anaesth. 2012;109:191–199. doi: 10.1093/bja/aes163. [DOI] [PubMed] [Google Scholar]
- 61.Greif R, Akça O, Horn EP, Kurz A, Sessler DI. Supplemental perioperative oxygen to reduce the incidence of surgical-wound infection. N Engl J Med. 2000;342:161–167. doi: 10.1056/NEJM200001203420303. [DOI] [PubMed] [Google Scholar]
- 62.Schietroma M, Carlei F, Cecilia EM, Piccione F, Bianchi Z, Amicucci G. Colorectal Infraperitoneal anastomosis: the effects of perioperative supplemental oxygen administration on the anastomotic dehiscence. J Gastrointest Surg. 2012;16:427–434. doi: 10.1007/s11605-011-1717-1. [DOI] [PubMed] [Google Scholar]
- 63.Gorissen KJ, Benning D, Berghmans T, Snoeijs MG, Sosef MN, Hulsewe KW, Luyer MD. Risk of anastomotic leakage with non-steroidal anti-inflammatory drugs in colorectal surgery. Br J Surg. 2012;99:721–727. doi: 10.1002/bjs.8691. [DOI] [PubMed] [Google Scholar]
- 64.Holte K, Andersen J, Jakobsen DH, Kehlet H. Cyclo-oxygenase 2 inhibitors and the risk of anastomotic leakage after fast-track colonic surgery. Br J Surg. 2009;96:650–654. doi: 10.1002/bjs.6598. [DOI] [PubMed] [Google Scholar]
- 65.Klein M, Gögenur I, Rosenberg J. Postoperative use of non-steroidal anti-inflammatory drugs in patients with anastomotic leakage requiring reoperation after colorectal resection: cohort study based on prospective data. BMJ. 2012;345:e6166. doi: 10.1136/bmj.e6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hüser N, Michalski CW, Erkan M, Schuster T, Rosenberg R, Kleeff J, Friess H. Systematic review and meta-analysis of the role of defunctioning stoma in low rectal cancer surgery. Ann Surg. 2008;248:52–60. doi: 10.1097/SLA.0b013e318176bf65. [DOI] [PubMed] [Google Scholar]
- 67.Tan WS, Tang CL, Shi L, Eu KW. Meta-analysis of defunctioning stomas in low anterior resection for rectal cancer. Br J Surg. 2009;96:462–472. doi: 10.1002/bjs.6594. [DOI] [PubMed] [Google Scholar]
- 68.Hao XY, Yang KH, Guo TK, Ma B, Tian JH, Li HL. Omentoplasty in the prevention of anastomotic leakage after colorectal resection: a meta-analysis. Int J Colorectal Dis. 2008;23:1159–1165. doi: 10.1007/s00384-008-0532-y. [DOI] [PubMed] [Google Scholar]
- 69.Huh JW, Kim HR, Kim YJ. Anastomotic leakage after laparoscopic resection of rectal cancer: the impact of fibrin glue. Am J Surg. 2010;199:435–441. doi: 10.1016/j.amjsurg.2009.01.018. [DOI] [PubMed] [Google Scholar]
- 70.Jesus EC, Karliczek A, Matos D, Castro AA, Atallah AN. Prophylactic anastomotic drainage for colorectal surgery. Cochrane Database Syst Rev. 2004;(4):CD002100. doi: 10.1002/14651858.CD002100.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Slieker JC, Daams F, Mulder IM, Jeekel J, Lange JF. Systematic review of the technique of colorectal anastomosis. JAMA Surg. 2013;148:190–201. doi: 10.1001/2013.jamasurg.33. [DOI] [PubMed] [Google Scholar]
- 72.Jacobs M, Verdeja JC, Goldstein HS. Minimally invasive colon resection (laparoscopic colectomy) Surg Laparosc Endosc. 1991;1:144–150. [PubMed] [Google Scholar]
- 73.Berends FJ, Kazemier G, Bonjer HJ, Lange JF. Subcutaneous metastases after laparoscopic colectomy. Lancet. 1994;344:58. doi: 10.1016/s0140-6736(94)91079-0. [DOI] [PubMed] [Google Scholar]
- 74.Lacy AM, García-Valdecasas JC, Delgado S, Castells A, Taurá P, Piqué JM, Visa J. Laparoscopy-assisted colectomy versus open colectomy for treatment of non-metastatic colon cancer: a randomised trial. Lancet. 2002;359:2224–2229. doi: 10.1016/S0140-6736(02)09290-5. [DOI] [PubMed] [Google Scholar]
- 75.Clinical Outcomes of Surgical Therapy Study Group. A comparison of laparoscopically assisted and open colectomy for colon cancer. N Engl J Med. 2004;350:2050–2059. doi: 10.1056/NEJMoa032651. [DOI] [PubMed] [Google Scholar]
- 76.Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): multicentre, randomised controlled trial. Lancet. 2005;365:1718–1726. doi: 10.1016/S0140-6736(05)66545-2. [DOI] [PubMed] [Google Scholar]
- 77.Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ, Haglind E, Påhlman L, Cuesta MA, Msika S, et al. Laparoscopic surgery versus open surgery for colon cancer: short-term outcomes of a randomised trial. Lancet Oncol. 2005;6:477–484. doi: 10.1016/S1470-2045(05)70221-7. [DOI] [PubMed] [Google Scholar]
- 78.Wu Z, Zhang S, Aung LH, Ouyang J, Wei L. Lymph node harvested in laparoscopic versus open colorectal cancer approaches: a meta-analysis. Surg Laparosc Endosc Percutan Tech. 2012;22:5–11. doi: 10.1097/SLE.0b013e3182432b49. [DOI] [PubMed] [Google Scholar]
- 79.Braga M, Frasson M, Zuliani W, Vignali A, Pecorelli N, Di Carlo V. Randomized clinical trial of laparoscopic versus open left colonic resection. Br J Surg. 2010;97:1180–1186. doi: 10.1002/bjs.7094. [DOI] [PubMed] [Google Scholar]
- 80.Liang JT, Huang KC, Lai HS, Lee PH, Jeng YM. Oncologic results of laparoscopic versus conventional open surgery for stage II or III left-sided colon cancers: a randomized controlled trial. Ann Surg Oncol. 2007;14:109–117. doi: 10.1245/s10434-006-9135-4. [DOI] [PubMed] [Google Scholar]
- 81.Hewett PJ, Allardyce RA, Bagshaw PF, Frampton CM, Frizelle FA, Rieger NA, Smith JS, Solomon MJ, Stephens JH, Stevenson AR. Short-term outcomes of the Australasian randomized clinical study comparing laparoscopic and conventional open surgical treatments for colon cancer: the ALCCaS trial. Ann Surg. 2008;248:728–738. doi: 10.1097/SLA.0b013e31818b7595. [DOI] [PubMed] [Google Scholar]
- 82.Vlug MS, Wind J, Hollmann MW, Ubbink DT, Cense HA, Engel AF, Gerhards MF, van Wagensveld BA, van der Zaag ES, van Geloven AA, et al. Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: a randomized clinical trial (LAFA-study) Ann Surg. 2011;254:868–875. doi: 10.1097/SLA.0b013e31821fd1ce. [DOI] [PubMed] [Google Scholar]
- 83.Neudecker J, Klein F, Bittner R, Carus T, Stroux A, Schwenk W. Short-term outcomes from a prospective randomized trial comparing laparoscopic and open surgery for colorectal cancer. Br J Surg. 2009;96:1458–1467. doi: 10.1002/bjs.6782. [DOI] [PubMed] [Google Scholar]
- 84.Braga M, Frasson M, Vignali A, Zuliani W, Di Carlo V. Open right colectomy is still effective compared with laparoscopy: results of a randomized trial. Ann Surg. 2007;246:1010–1014; discussion 1014-1015. doi: 10.1097/SLA.0b013e31815c4065. [DOI] [PubMed] [Google Scholar]
- 85.Stucky CC, Pockaj BA, Novotny PJ, Sloan JA, Sargent DJ, O’Connell MJ, Beart RW, Skibber JM, Nelson H, Weeks JC. Long-term follow-up and individual item analysis of quality of life assessments related to laparoscopic-assisted colectomy in the COST trial 93-46-53 (INT 0146) Ann Surg Oncol. 2011;18:2422–2431. doi: 10.1245/s10434-011-1650-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Weeks JC, Nelson H, Gelber S, Sargent D, Schroeder G. Short-term quality-of-life outcomes following laparoscopic-assisted colectomy vs open colectomy for colon cancer: a randomized trial. JAMA. 2002;287:321–328. doi: 10.1001/jama.287.3.321. [DOI] [PubMed] [Google Scholar]
- 87.Janson M, Lindholm E, Anderberg B, Haglind E. Randomized trial of health-related quality of life after open and laparoscopic surgery for colon cancer. Surg Endosc. 2007;21:747–753. doi: 10.1007/s00464-007-9217-9. [DOI] [PubMed] [Google Scholar]
- 88.Lacy AM, Delgado S, Castells A, Prins HA, Arroyo V, Ibarzabal A, Pique JM. The long-term results of a randomized clinical trial of laparoscopy-assisted versus open surgery for colon cancer. Ann Surg. 2008;248:1–7. doi: 10.1097/SLA.0b013e31816a9d65. [DOI] [PubMed] [Google Scholar]
- 89.Kuhry E, Jeekel J, Bonjer HJ. Effect of laparoscopy on the immune system. Semin Laparosc Surg. 2004;11:37–44. doi: 10.1177/107155170401100107. [DOI] [PubMed] [Google Scholar]
- 90.Fleshman J, Sargent DJ, Green E, Anvari M, Stryker SJ, Beart RW, Hellinger M, Flanagan R, Peters W, Nelson H. Laparoscopic colectomy for cancer is not inferior to open surgery based on 5-year data from the COST Study Group trial. Ann Surg. 2007;246:655–662; discussion 662-664. doi: 10.1097/SLA.0b013e318155a762. [DOI] [PubMed] [Google Scholar]
- 91.Jayne DG, Guillou PJ, Thorpe H, Quirke P, Copeland J, Smith AM, Heath RM, Brown JM. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J Clin Oncol. 2007;25:3061–3068. doi: 10.1200/JCO.2006.09.7758. [DOI] [PubMed] [Google Scholar]
- 92.Jayne DG, Thorpe HC, Copeland J, Quirke P, Brown JM, Guillou PJ. Five-year follow-up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg. 2010;97:1638–1645. doi: 10.1002/bjs.7160. [DOI] [PubMed] [Google Scholar]
- 93.Buunen M, Veldkamp R, Hop WC, Kuhry E, Jeekel J, Haglind E, Påhlman L, Cuesta MA, Msika S, Morino M, et al. Survival after laparoscopic surgery versus open surgery for colon cancer: long-term outcome of a randomised clinical trial. Lancet Oncol. 2009;10:44–52. doi: 10.1016/S1470-2045(08)70310-3. [DOI] [PubMed] [Google Scholar]
- 94.Bonjer HJ, Hop WC, Nelson H, Sargent DJ, Lacy AM, Castells A, Guillou PJ, Thorpe H, Brown J, Delgado S, et al. Laparoscopically assisted vs open colectomy for colon cancer: a meta-analysis. Arch Surg. 2007;142:298–303. doi: 10.1001/archsurg.142.3.298. [DOI] [PubMed] [Google Scholar]
- 95.Bagshaw PF, Allardyce RA, Frampton CM, Frizelle FA, Hewett PJ, McMurrick PJ, Rieger NA, Smith JS, Solomon MJ, Stevenson AR. Long-term outcomes of the australasian randomized clinical trial comparing laparoscopic and conventional open surgical treatments for colon cancer: the Australasian Laparoscopic Colon Cancer Study trial. Ann Surg. 2012;256:915–919. doi: 10.1097/SLA.0b013e3182765ff8. [DOI] [PubMed] [Google Scholar]
- 96.Di B, Li Y, Wei K, Xiao X, Shi J, Zhang Y, Yang X, Gao P, Zhang K, Yuan Y, et al. Laparoscopic versus open surgery for colon cancer: a meta-analysis of 5-year follow-up outcomes. Surg Oncol. 2013;22:e39–e43. doi: 10.1016/j.suronc.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 97.Kuhry E, Schwenk WF, Gaupset R, Romild U, Bonjer HJ. Long-term results of laparoscopic colorectal cancer resection. Cochrane Database Syst Rev. 2008;(2):CD003432. doi: 10.1002/14651858.CD003432.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Theophilus M, Platell C, Spilsbury K. Long-term survival following laparoscopic and open colectomy for colon cancer: a meta-analysis of randomized controlled trials. Colorectal Dis. 2014;16:O75–O81. doi: 10.1111/codi.12483. [DOI] [PubMed] [Google Scholar]
- 99.Kapiteijn E, Putter H, van de Velde CJ. Impact of the introduction and training of total mesorectal excision on recurrence and survival in rectal cancer in The Netherlands. Br J Surg. 2002;89:1142–1149. doi: 10.1046/j.1365-2168.2002.02196.x. [DOI] [PubMed] [Google Scholar]
- 100.Ng SS, Leung KL, Lee JF, Yiu RY, Li JC. MRC CLASICC trial. Lancet. 2005;366:713; author reply 713–714. doi: 10.1016/S0140-6736(05)67170-X. [DOI] [PubMed] [Google Scholar]
- 101.Leung KL, Kwok SP, Lam SC, Lee JF, Yiu RY, Ng SS, Lai PB, Lau WY. Laparoscopic resection of rectosigmoid carcinoma: prospective randomised trial. Lancet. 2004;363:1187–1192. doi: 10.1016/S0140-6736(04)15947-3. [DOI] [PubMed] [Google Scholar]
- 102.Braga M, Frasson M, Vignali A, Zuliani W, Capretti G, Di Carlo V. Laparoscopic resection in rectal cancer patients: outcome and cost-benefit analysis. Dis Colon Rectum. 2007;50:464–471. doi: 10.1007/s10350-006-0798-5. [DOI] [PubMed] [Google Scholar]
- 103.Kang SB, Park JW, Jeong SY, Nam BH, Choi HS, Kim DW, Lim SB, Lee TG, Kim DY, Kim JS, et al. Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short-term outcomes of an open-label randomised controlled trial. Lancet Oncol. 2010;11:637–645. doi: 10.1016/S1470-2045(10)70131-5. [DOI] [PubMed] [Google Scholar]
- 104.Lujan J, Valero G, Hernandez Q, Sanchez A, Frutos MD, Parrilla P. Randomized clinical trial comparing laparoscopic and open surgery in patients with rectal cancer. Br J Surg. 2009;96:982–989. doi: 10.1002/bjs.6662. [DOI] [PubMed] [Google Scholar]
- 105.Ng SS, Leung KL, Lee JF, Yiu RY, Li JC, Hon SS. Long-term morbidity and oncologic outcomes of laparoscopic-assisted anterior resection for upper rectal cancer: ten-year results of a prospective, randomized trial. Dis Colon Rectum. 2009;52:558–566. doi: 10.1007/DCR.0b013e31819ec20c. [DOI] [PubMed] [Google Scholar]
- 106.Ng SS, Leung KL, Lee JF, Yiu RY, Li JC, Teoh AY, Leung WW. Laparoscopic-assisted versus open abdominoperineal resection for low rectal cancer: a prospective randomized trial. Ann Surg Oncol. 2008;15:2418–2425. doi: 10.1245/s10434-008-9895-0. [DOI] [PubMed] [Google Scholar]
- 107.Zhou ZG, Hu M, Li Y, Lei WZ, Yu YY, Cheng Z, Li L, Shu Y, Wang TC. Laparoscopic versus open total mesorectal excision with anal sphincter preservation for low rectal cancer. Surg Endosc. 2004;18:1211–1215. doi: 10.1007/s00464-003-9170-1. [DOI] [PubMed] [Google Scholar]
- 108.Liang X, Hou S, Liu H, Li Y, Jiang B, Bai W, Li G, Wang W, Feng Y, Guo J. Effectiveness and safety of laparoscopic resection versus open surgery in patients with rectal cancer: a randomized, controlled trial from China. J Laparoendosc Adv Surg Tech A. 2011;21:381–385. doi: 10.1089/lap.2010.0059. [DOI] [PubMed] [Google Scholar]
- 109.Gao F, Cao YF, Chen LS. Meta-analysis of short-term outcomes after laparoscopic resection for rectal cancer. Int J Colorectal Dis. 2006;21:652–656. doi: 10.1007/s00384-005-0079-0. [DOI] [PubMed] [Google Scholar]
- 110.Li J, Chen R, Xu YQ, Wang XC, Zheng S, Zhang SZ, Ding KF. Impact of a laparoscopic resection on the quality of life in rectal cancer patients: results of 135 patients. Surg Today. 2010;40:917–922. doi: 10.1007/s00595-009-4156-9. [DOI] [PubMed] [Google Scholar]
- 111.Andersson J, Angenete E, Gellerstedt M, Angerås U, Jess P, Rosenberg J, Fürst A, Bonjer J, Haglind E. Health-related quality of life after laparoscopic and open surgery for rectal cancer in a randomized trial. Br J Surg. 2013;100:941–949. doi: 10.1002/bjs.9144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pechlivanides G, Gouvas N, Tsiaoussis J, Tzortzinis A, Tzardi M, Moutafidis M, Dervenis C, Xynos E. Lymph node clearance after total mesorectal excision for rectal cancer: laparoscopic versus open approach. Dig Dis. 2007;25:94–99. doi: 10.1159/000099176. [DOI] [PubMed] [Google Scholar]
- 113.van der Pas MH, Haglind E, Cuesta MA, Fürst A, Lacy AM, Hop WC, Bonjer HJ. Laparoscopic versus open surgery for rectal cancer (COLOR II): short-term outcomes of a randomised, phase 3 trial. Lancet Oncol. 2013;14:210–218. doi: 10.1016/S1470-2045(13)70016-0. [DOI] [PubMed] [Google Scholar]
- 114.Huang MJ, Liang JL, Wang H, Kang L, Deng YH, Wang JP. Laparoscopic-assisted versus open surgery for rectal cancer: a meta-analysis of randomized controlled trials on oncologic adequacy of resection and long-term oncologic outcomes. Int J Colorectal Dis. 2011;26:415–421. doi: 10.1007/s00384-010-1091-6. [DOI] [PubMed] [Google Scholar]
- 115.Ohtani H, Tamamori Y, Azuma T, Mori Y, Nishiguchi Y, Maeda K, Hirakawa K. A meta-analysis of the short- and long-term results of randomized controlled trials that compared laparoscopy-assisted and conventional open surgery for rectal cancer. J Gastrointest Surg. 2011;15:1375–1385. doi: 10.1007/s11605-011-1547-1. [DOI] [PubMed] [Google Scholar]
- 116.Kuhry E, Bonjer HJ, Haglind E, Hop WC, Veldkamp R, Cuesta MA, Jeekel J, Påhlman L, Morino M, Lacy A, et al. Impact of hospital case volume on short-term outcome after laparoscopic operation for colonic cancer. Surg Endosc. 2005;19:687–692. doi: 10.1007/s00464-004-8920-z. [DOI] [PubMed] [Google Scholar]
- 117.Janson M, Björholt I, Carlsson P, Haglind E, Henriksson M, Lindholm E, Anderberg B. Randomized clinical trial of the costs of open and laparoscopic surgery for colonic cancer. Br J Surg. 2004;91:409–417. doi: 10.1002/bjs.4469. [DOI] [PubMed] [Google Scholar]
- 118.Tjandra JJ, Chan MK. Systematic review on the short-term outcome of laparoscopic resection for colon and rectosigmoid cancer. Colorectal Dis. 2006;8:375–388. doi: 10.1111/j.1463-1318.2006.00974.x. [DOI] [PubMed] [Google Scholar]
- 119.Makino T, Shukla PJ, Rubino F, Milsom JW. The impact of obesity on perioperative outcomes after laparoscopic colorectal resection. Ann Surg. 2012;255:228–236. doi: 10.1097/SLA.0b013e31823dcbf7. [DOI] [PubMed] [Google Scholar]
- 120.Fujii S, Ishibe A, Ota M, Yamagishi S, Watanabe K, Watanabe J, Kanazawa A, Ichikawa Y, Oba M, Morita S, et al. Short-term results of a randomized study between laparoscopic and open surgery in elderly colorectal cancer patients. Surg Endosc. 2014;28:466–476. doi: 10.1007/s00464-013-3223-x. [DOI] [PubMed] [Google Scholar]
- 121.Kennedy RH, Francis A, Dutton S, Love S, Pearson S, Blazeby JM, Quirke P, Franks PJ, Kerr DJ. EnROL: a multicentre randomised trial of conventional versus laparoscopic surgery for colorectal cancer within an enhanced recovery programme. BMC Cancer. 2012;12:181. doi: 10.1186/1471-2407-12-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hyman N, Manchester TL, Osler T, Burns B, Cataldo PA. Anastomotic leaks after intestinal anastomosis: it’s later than you think. Ann Surg. 2007;245:254–258. doi: 10.1097/01.sla.0000225083.27182.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Kornmann VN, Treskes N, Hoonhout LH, Bollen TL, van Ramshorst B, Boerma D. Systematic review on the value of CT scanning in the diagnosis of anastomotic leakage after colorectal surgery. Int J Colorectal Dis. 2013;28:437–445. doi: 10.1007/s00384-012-1623-3. [DOI] [PubMed] [Google Scholar]
- 124.Doeksen A, Tanis PJ, Vrouenraets BC, Lanschot van JJ, Tets van WF. Factors determining delay in relaparotomy for anastomotic leakage after colorectal resection. World J Gastroenterol. 2007;13:3721–3725. doi: 10.3748/wjg.v13.i27.3721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Alves A, Panis Y, Trancart D, Regimbeau JM, Pocard M, Valleur P. Factors associated with clinically significant anastomotic leakage after large bowel resection: multivariate analysis of 707 patients. World J Surg. 2002;26:499–502. doi: 10.1007/s00268-001-0256-4. [DOI] [PubMed] [Google Scholar]
- 126.Macarthur DC, Nixon SJ, Aitken RJ. Avoidable deaths still occur after large bowel surgery. Scottish Audit of Surgical Mortality, Royal College of Surgeons of Edinburgh. Br J Surg. 1998;85:80–83. doi: 10.1046/j.1365-2168.1998.00554.x. [DOI] [PubMed] [Google Scholar]
- 127.den Dulk M, Noter SL, Hendriks ER, Brouwers MA, van der Vlies CH, Oostenbroek RJ, Menon AG, Steup WH, van de Velde CJ. Improved diagnosis and treatment of anastomotic leakage after colorectal surgery. Eur J Surg Oncol. 2009;35:420–426. doi: 10.1016/j.ejso.2008.04.009. [DOI] [PubMed] [Google Scholar]
- 128.Hirst NA, Tiernan JP, Millner PA, Jayne DG. Systematic review of methods to predict and detect anastomotic leakage in colorectal surgery. Colorectal Dis. 2014;16:95–109. doi: 10.1111/codi.12411. [DOI] [PubMed] [Google Scholar]
- 129.Platt JJ, Ramanathan ML, Crosbie RA, Anderson JH, McKee RF, Horgan PG, McMillan DC. C-reactive protein as a predictor of postoperative infective complications after curative resection in patients with colorectal cancer. Ann Surg Oncol. 2012;19:4168–4177. doi: 10.1245/s10434-012-2498-9. [DOI] [PubMed] [Google Scholar]
- 130.Matthiessen P, Strand I, Jansson K, Törnquist C, Andersson M, Rutegård J, Norgren L. Is early detection of anastomotic leakage possible by intraperitoneal microdialysis and intraperitoneal cytokines after anterior resection of the rectum for cancer? Dis Colon Rectum. 2007;50:1918–1927. doi: 10.1007/s10350-007-9023-4. [DOI] [PubMed] [Google Scholar]
- 131.Ellebaek Pedersen M, Qvist N, Bisgaard C, Kelly U, Bernhard A, Møller Pedersen S. Peritoneal microdialysis. Early diagnosis of anastomotic leakage after low anterior resection for rectosigmoid cancer. Scand J Surg. 2009;98:148–154. doi: 10.1177/145749690909800304. [DOI] [PubMed] [Google Scholar]
- 132.Jansson K, Redler B, Truedsson L, Magnuson A, Matthiessen P, Andersson M, Norgren L. Intraperitoneal cytokine response after major surgery: higher postoperative intraperitoneal versus systemic cytokine levels suggest the gastrointestinal tract as the major source of the postoperative inflammatory reaction. Am J Surg. 2004;187:372–377. doi: 10.1016/j.amjsurg.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 133.Komen N, de Bruin RW, Kleinrensink GJ, Jeekel J, Lange JF. Anastomotic leakage, the search for a reliable biomarker. A review of the literature. Colorectal Dis. 2008;10:109–115; discussion 115-117. doi: 10.1111/j.1463-1318.2007.01430.x. [DOI] [PubMed] [Google Scholar]
- 134.Cini C, Wolthuis A, D’Hoore A. Peritoneal fluid cytokines and matrix metalloproteinases as early markers of anastomotic leakage in colorectal anastomosis: a literature review and meta-analysis. Colorectal Dis. 2013;15:1070–1077. doi: 10.1111/codi.12192. [DOI] [PubMed] [Google Scholar]
- 135.Komen N, Slieker J, Willemsen P, Mannaerts G, Pattyn P, Karsten T, de Wilt H, van der Harst E, van Leeuwen W, Decaestecker C, et al. Polymerase chain reaction for Enterococcus faecalis in drain fluid: the first screening test for symptomatic colorectal anastomotic leakage. The Appeal-study: analysis of parameters predictive for evident anastomotic leakage. Int J Colorectal Dis. 2014;29:15–21. doi: 10.1007/s00384-013-1776-8. [DOI] [PubMed] [Google Scholar]
- 136.Henneman D, Snijders HS, Fiocco M, van Leersum NJ, Kolfschoten NE, Wiggers T, Wouters MW, Tollenaar RA. Hospital variation in failure to rescue after colorectal cancer surgery: results of the Dutch Surgical Colorectal Audit. Ann Surg Oncol. 2013;20:2117–2123. doi: 10.1245/s10434-013-2896-7. [DOI] [PubMed] [Google Scholar]
- 137.Jaarrapportage 2010: uitkomst van zorg registratie. Dutch Surgical Colorectal Audit, 2010. Available from: http://www.clinicalaudit.nl/jaarrapportage/archief/DSCA Jaarrapportage 2010.pdf.
- 138.van Gijn W, van de Velde CJ. 2010 SSO John Wayne clinical research lecture: rectal cancer outcome improvements in Europe: population-based outcome registrations will conquer the world. Ann Surg Oncol. 2011;18:691–696. doi: 10.1245/s10434-010-1326-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Wise J. Survival rates after bowel cancer surgery reach all time high, audit shows. BMJ. 2013;347:f4266. doi: 10.1136/bmj.f4266. [DOI] [PubMed] [Google Scholar]
- 140.Ghaferi AA, Birkmeyer JD, Dimick JB. Hospital volume and failure to rescue with high-risk surgery. Med Care. 2011;49:1076–1081. doi: 10.1097/MLR.0b013e3182329b97. [DOI] [PubMed] [Google Scholar]
- 141.Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. N Engl J Med. 2009;361:1368–1375. doi: 10.1056/NEJMsa0903048. [DOI] [PubMed] [Google Scholar]
- 142.Snijders HS, Henneman D, van Leersum NL, ten Berge M, Fiocco M, Karsten TM, Havenga K, Wiggers T, Dekker JW, Tollenaar RA, et al. Anastomotic leakage as an outcome measure for quality of colorectal cancer surgery. BMJ Qual Saf. 2013;22:759–767. doi: 10.1136/bmjqs-2012-001644. [DOI] [PubMed] [Google Scholar]


