Abstract
Peritoneal carcinomatosis (PC), the dissemination of cancer cells throughout the lining of the abdominal cavity, is the second most common presentation of colon cancer distant metastasis. Despite remarkable advances in cytotoxic chemotherapy and targeted therapy for colon cancer over the last 15 years, it has been repeatedly shown that these therapies remain ineffective for colon cancer PC. Recently, there has been a rapid accumulation of reports that cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy (CRS-HIPEC) prolongs the life of colon cancer PC patients. Here, we will review the clinical presentation, the mechanisms of disease progression, and current treatment options for colon cancer PC, with a focus on the benefits and limitations of CRS-HIPEC.
Keywords: Cancer, Colorectal, Carcinomatosis, Peritoneal, Hyperthermic intraperitoneal chemotherapy, Intraperitoneal chemotherapy, Cytoreductive surgery, Mechanism
Core tip: This paper aims to review the clinical presentation, the mechanisms of disease progression, and current treatment options for colon cancer peritoneal carcinomatosis, with a focus on the benefits and limitations of cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy.
INTRODUCTION
The most critical and ultimately life threatening feature of malignant disease is its ability to metastasize to other organs[1-6]. The peritoneum is second only to the liver as a site for colon cancer metastasis. Approximately 4%-7% of patients with colon cancer are noted to have peritoneal carcinomatosis (PC) at the time of diagnosis, despite recent advances in early detection of the disease[7-9]. Although the tumors are primarily confined to the peritoneum with no discernable metastasis elsewhere in approximately 20%-25% of patients with colon cancer PC[10-12], the prognosis of these patients is poor with a median survival of 6-9 mo after diagnosis[7,9,13,14]. As a result, until recent years, colon cancer PC had been regarded as a form of systemic metastasis portending a terminal state of colon cancer for which only palliative surgery (such as diverting colostomy) and/or systemic chemotherapy was recommended[15-18]. Here, we will review the presentation, mechanisms of disease progression, and current treatment options of colon cancer PC, which have been reported to prolong life of those patients.
CLINICAL PRESENTATION
The symptoms of colon cancer PC can vary and are nonspecific; many patients, however, experience extreme fatigue, abdominal discomfort, and abdominal pain because of ascites and associated bowel obstruction[19,20]. If ascites is present, cytology from aspirated fluid can help to establish the diagnosis in half of these patients. Approximately 40% of the patients can be diagnosed by imaging modalities including computed tomography (CT), magnetic resonance imaging, positron emission tomography (PET) and ultrasound, but in 8% of the patients, colon cancer PC is found at the time of laparotomy[21]. In many cases diagnostic laparoscopy can be helpful to gain more detailed information on the intra-peritoneal dissemination of the tumor or histological confirmation of the suspicion of PC[22]. PC rates are highest among women, patients with primary mucinous adenocarcinomas, tumor stage T4, lymph node stage N2, and among patients with a positive resection margin[23]. Time to recurrent disease has been reported with a median time of 14-16 mo[8,24]. In terms of PC, median time to diagnosis of metachronous disease was 18 mo[23].
MECHANISM OF ESTABLISHMENT AND PROGRESSION OF COLON CANCER PC
The multistep process through which PC is established has been conceptualized to include the five following steps (Figure 1)[25,26].
Figure 1.
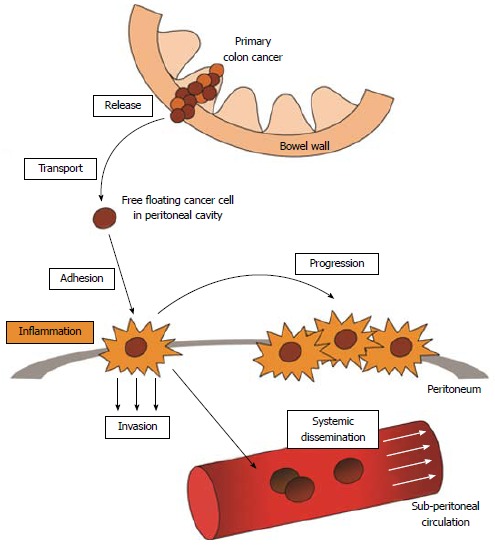
A multistep process is involved in establishment and progression of colon cancer peritoneal carcinomatosis. First, loose cancer cells are released from the primary tumor that has penetrated the colon wall. The free floating cancer cells enter the peritoneal cavity and are transported to the peritoneal surface, where with inflammation playing a major role, the cells become adhesed to the peritoneum. The cancer cells then invade into the submesothelial layers and ultimately are able to access to the systemic circulation. Note that the progression of peritoneal carcinomatosis is not only at the single adhesion site, but its spill over to the neighboring peritoneum.
Release of loose cancer cells from the primary tumor into the abdominal cavity
In order for the cancer cell to disseminate in the abdominal cavity, it needs to be released from the primary colon cancer mass. This process can occur through several mechanisms including spontaneous and/or iatrogenic exfoliation of cancer cells from the primary tumor. In colon cancer PC, the expressions and/or the functions of cell-cell adhesion molecules, such as E-cadherin, can be down-regulated thereby promoting cancer cell detachment[27-29]. Iatrogenic causes include incomplete resection, inadvertent breach of the tumor’s integrity, or transection of blood or lymph vessels with subsequent leakage[30].
Transport in abdominal cavity
Once the cancer cells are released into the abdominal cavity, they are transported to different anatomical regions of the abdomen governed by gravity, peristaltic gastrointestinal movement, and the mechanics of the negative pressure generated by movement of the diaphragm[31]. The most common sites of disease localization include the right lower quadrant, the right diaphragm, the hepatoduodenal ligament, the omentum, the pelvic viscera and the parietal peritoneum[32].
Adhesion to the peritoneum
In order for the floating cancer cells to establish PC, the cells need to adhere to the peritoneum. Evidence suggests that inflammation plays a major role in this process by enhancing expression of adhesion molecules. Adhesion of cancer cells to the mesothelial layer is mediated by adhesion molecules, such as vascular cell adhesion molecule 1 [VCAM-1 (CD106)], intercellular adhesion molecule 1 [ICAM-1 (CD54)], and platelet endothelial cell adhesion molecule [PECAM-1 (CD31)], many of which are also expressed by endothelial cells[33,34]. Pro-inflammatory mediators, IL-1β, IL-6 and the epidermal growth factor, have been shown to increase peritoneal adhesion of cancer cells[35-37], and TNF-α has been shown to enhance the expression of ICAM-1[38]. Adhesive interactions have also been reported between the mesothelial hyaluronan coat and the transmembrane glycoprotein CD44, a molecule expressed by many cancer types[39]. The mechanisms responsible for this site specificity remain incompletely understood, but may relate to the pro-angiogenic environment elicited by the dense capillary network surrounding these immune aggregates[40].
Invasion into the submesothelial layers
Loose cancer cells gain access to submesothelial tissue at areas of peritoneal discontinuity. The cells themselves may precipitate these areas of weakness in the peritoneum, as cancer cells have been shown to induce apoptosis of mesothelial cells by a FAS dependent mechanism[41]. Alternatively, the extracellular matrix might become exposed by contraction of mesothelial cells and disruption of intercellular junctions in response to inflammatory mediators[42]. Invasion of the submesothelial tissue is accompanied by adhesion to and degradation of the existing extracellular matrix through various integrins and proteases respectively[43].
Access to the systemic circulation
Once the submesothelial stroma is invaded, cancer cells can then gain access to the blood and lymphatic microcirculation. Particulate matter, including free peritoneal cancer cells, can enter the systemic circulation through subperitoneal lymphatic lacunae located between the muscle fibers of the diaphragm[44]. The peritoneum layering the pouch of Douglas, for example, is rich in subperitoneal lymphatic vessels and milky spots. Intraperitoneal fluid containing free cancer cells, once reaching the pelvic subperitoneal lymphatics, then travels toward the rectum, and finally flows into the lymph nodes around the iliac artery. In contrast, the peritoneum covering the liver, as well as the serosal surface of small bowel and spleen, are devoid of lymphatic stomata and milky spots and thus are involved in systemic dissemination of cancer cells only in the late stage of PC[45].
TREATMENT OPTIONS AND OUTCOMES
Systemic chemotherapy
PC has been considered to be a form of systemic distant metastasis and a terminal state in colon cancer, thus historically palliative systemic chemotherapy was utilized. The few retrospective studies examining this palliative therapy invariably show disappointing responses to systemic chemotherapy by 5-fluorouracil (5-FU) and leucovorin with a poor prognosis compared to other metastatic sites[13,46-49]. Recently, more effective cytotoxic chemotherapies and biologic targeted therapies using oxaliplatin, irinotecan, bevacizumab, cetuximab, or panitumumab have been reported to improve the survival of patients with metastatic colon cancer. However, since the majority of patients involved in these studies are those with liver or lung metastases, the effects of these agents on PC remain unclear[50,51]. Only a few papers have described the outcome of systemic chemotherapy for colon cancer PC. A pooled analysis of north central cancer treatment group phase III trials N9741 and N9841 demonstrates that current systemic chemotherapy, such as 5-FU based combination therapy, FOLFOX, is not effective for PC and fails to significantly improve survival[52].
Intraperitoneal chemotherapy
Intraperitoneal delivery of anti-neoplastic agents for cancer into the abdominal cavity has been attempted since antiquity. In the mid-18th century, English surgeon Christopher Warrik injected a mixture of “Bristol water” and “Claret” (a Bordeaux wine) into the peritoneal cavity of a woman suffering from intractable ascites[19]. Following the discovery of the anti-neoplastic potential of nitrogen mustard, its effect in the abdominal cavity was studied within the mid-20th century[53].
Approximately one in five patients with colon cancer harbors minimal residual disease in the peritoneum after surgical resection, while PC develops in approximately one in seven patients[25]. This observation led to a hypothesis that intraperitoneal chemotherapy following complete resection of a T3 or T4 stage colon cancer may decrease peritoneal recurrence. In animal models of colon cancer, intraperitoneal administration of chemotherapeutic drugs successfully prevented tumor development following colon cancer cell implantation into abdominal cavity[54]. Clinical studies, however, have thus far not shown any benefit from adjuvant intraperitoneal chemotherapy for colon cancer. Vaillant et al[55] randomized 267 stage II and III colon cancer patients to either surgery alone or surgery combined with intraoperative intravenous 5-FU and postoperative intraperitoneal 5-FU administration. Overall, adjuvant 5-FU failed to improve disease free survival (DFS), overall survival, or the frequency of peritoneal recurrence. An unplanned subgroup analysis indicated a DFS benefit in patients with stage II disease. Similarly, a randomized trial by Nordlinger et al[56] failed to show any benefit from immediate postoperative regional chemotherapy (intraperitoneal or intraportal administration by the choice of participating center) followed by intravenous chemotherapy compared with intravenous chemotherapy alone in patients with resected stage II or III colorectal cancer. Taken together, neither of these two trials were able to show any decrease in peritoneal recurrence from adjuvant intraperitoneal chemotherapy in patients with colorectal cancer.
Early postoperative intraperitoneal chemotherapy
Early postoperative intraperitoneal chemotherapy (EPIC) is infusion of chemotherapy directly into the abdominal cavity through ports, allowing circulation and/or drainage of the drugs for one to five days after cytoreductive surgery (CRS) to kill remaining microscopic cancerous tumors and free floating cells. EPIC may be given in multiple cycles for several months after surgery.
In 1995, a French group attempted to conduct a randomized controlled clinical trial comparing CRS plus EPIC to systemic chemotherapy, but it was rapidly abandoned because of patients’ dissatisfaction with the treatment in the control arm. Subsequently, a modified clinical trial comparing CRS plus EPIC versus CRS without EPIC was initiated in 1996. This study by Elias et al[57] closed prematurely after 4 years in 2000 with only 35 of the required 90 patients enrolled. In this incomplete trial, EPIC failed to demonstrate any survival benefit; however, analysis of these 35 patients showed that complete CRS (CCR-0) resulted in a 2-year survival rate of 60%, an impressive improvement from the historic 10% survival rate among patients treated with systemic chemotherapy and palliative surgery[57].
Mahteme et al[58] compared 18 colon cancer PC patients who underwent CRS, EPIC (5-FU, cisplatin, or irinotecan) and systemic 5-FU based chemotherapy with 18 matched control patients who underwent systemic chemotherapy alone. Patients in the CRS group had a median survival of 32 mo, with 2-year and 5-year survival of 60% and 28%, respectively, whereas patients in the control group had a median survival of 14 mo, with 2-year and 5-year survival of 10% and 5%, respectively (P = 0.01, vs CRS group). Glehen et al[59] analyzed a multi-institutional registry (28 international institutions) involving 506 colorectal cancer PC patients who underwent CRS and EPIC with a median follow-up of 53 mo, finding a median survival of 19 mo, and 1-, 3- and 5-year survival to be 72%, 39% and 19%, respectively. Two hundred seventy-one patients had CCR-0 and their median survival was 32 mo, with 1-, 3- and 5- year survival of 87%, 47% and 31%, respectively. The details of CRS and chemotherapy regimens differed among the institutions, limiting the conclusions that can be drawn from this study[59]. A systematic review by Yan et al[60] confirmed the importance of achieving CCR-0, as it was determined that those procedures that succeeded in this fashion resulted in improved survival to 60 from 28 mo when compared with procedures leaving residual disease. CCR-0 resulted in prolonged overall survival from 22% to 49% at 5 years. The overall quality of the available evidence is, however, rather low, and a potentially serious postoperative complication rate should be taken into account. A consistent finding is that optimal results can be expected in patients with a limited disease burden in whom a complete resection can be achieved.
Cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy
Recently Cytoreductive Surgery combined with Hyperthermic Intraperitoneal Chemotherapy (CRS-HIPEC) has been introduced for colorectal cancer PC, which appears to be the most effective approach for prolonging survival in selected patients[49,59,61-63]. CRS-HIPEC is a type of hyperthermic therapy used in combination with direct application of chemotherapy inside the abdominal cavity after surgical removal of as much cancer as possible. HIPEC is also known as intraperitoneal hyperthermic chemoperfusion, intra-abdominal hyperthermic chemoperfusion, or intraoperative chemo hyperthermia peritoneal perfusion. In this procedure, warmed anti-cancer drugs are infused and circulated in the abdominal cavity in direct contact with the peritoneum. These procedures last as long as 8-10 h and carry a significant rate of complications. According to the National Cancer Institute database, currently there are eight ongoing clinical trials worldwide that include HIPEC as a keyword; 2 phase III, 4 phase II, and 2 phase I trials (from www.cancer.gov, consulted 26th of June 2013)[64].
Verwaal et al[49,65] reported 105 patients with colon cancer PC without evidence of hematogenous metastases enrolled between 1998 and 2001, and randomly allocated to receive CRS-HIPEC followed by IV 5-FU and leucovorin vs 5-FU and leucovorin with or without palliative surgery. The median disease specific survival was 22.2 mo in the CRS-HIPEC arm and 12.6 mo in the control arm (P = 0.028). The survival was significantly better in patients with no more than five of seven abdominal PC regions and in patients in whom a macroscopic complete resection (CCR-0) was achieved. Unfortunately, the value of this randomized clinical trial is limited by the fact that the systemic chemotherapy regimen was not the standard of care (which includes Irinotecan and/or Oxaliplatin), appendiceal (n = 18) and rectal (n = 12) tumors were not balanced in the two groups, and the HIPEC protocol was based only on mitomycin C in the perfusate. In order to assess the effect of CRS-HIPEC with modern systemic chemotherapies, Elias et al[66] conducted a comparison of the survival of patients with isolated and resectable PC treated with systemic chemotherapy containing oxaliplatin or irinotecan or treated with CRS- HIPEC. In this study, the patients treated with modern systemic chemotherapy achieved a median survival of 24 mo, while median survival was 63 mo with a 5-year survival rate of 51% after CRS-HIPEC.
Franko et al[67] compared 67 patients treated with mitomycin C-based HIPEC to 38 controls, with all 105 patients receiving 5-FU, irinotecan, oxaliplatin and bevacizumab or cetuximab. Unfortunately, in this study the number of patients with liver metastases and the number receiving oxaliplatin and targeted therapies were both greater in the HIPEC group (78% vs 18% and 59% vs 18% respectively).
Among non-randomized, retrospective multi-institutional studies, the largest published series comes from the French registry and includes 523 patients with colon cancer PC from 1990 to 2007 treated with CRS-HIPEC[68]. Despite the fact that 16% of patients had incomplete CRS with macroscopic residuals (CCR-1), making it difficult to extrapolate data about survival, and that the large number of participating centers adds variability (relating to learning curve and surgical standardization), the reported 30-d mortality was only 3%, which was remarkably lower than that reported in the Dutch trial (8%)[49]. Since PC without systemic spread is found in 3% of patients with colon cancer, this subgroup might benefit from CRS-HIPEC[9]. In regards to long-term survival, complete cytoreduction (CCR-0) appears to be critical given the results from a multinational retrospective analysis of 506 patients with CRC treated with CRS-HIPEC showing an overall median survival of 32.4 mo with CCR-0 scores compared to 19 mo with suboptimal resection[59]. Multivariate analysis revealed that other variables associated with survival include treatment by a second procedure, limited disease extent, age less than 65 years, and use of adjuvant chemotherapy.
One randomized trial and several prospective cohort studies have shown improvements in disease-free and overall survival associated with CRS-HIPEC with/without EPIC when compared to palliative-intent surgery and/or systemic chemotherapy[65-67,69]. Chua et al[69] enrolled 294 patients, comparing supportive care and palliation with postoperative systemic chemotherapy based on 5-FU, irinotecan, oxaliplatin, Capecitabine and monoclonal antibodies, with or without HIPEC (low-dose mitomycine C) and EPIC (high dose 5-FU). The type of perioperative intraperitoneal chemotherapy did not have an effect on survival (median survival HIPEC = 36 mo, EPIC =38 mo, HIPEC and EPIC = 43 mo; P = 0.715). On the other hand, the rate of grade III/IV complications was reported to be significantly higher when HIPEC was combined with EPIC, especially in patients with a high total tumor burden. Thus, the use of EPIC in combination with CRS-HIPEC is associated with an increased rate of complications[70].
Since the total tumor burden of PC is critical to predicting the outcome of CRS-HIPEC, several scoring methods have been proposed in an effort to quantify both the extent of peritoneal disease and the completeness of resection. The peritoneal cancer index (PCI, Washington Cancer Institute), first described by Sugarbaker in 1996, is commonly used for quantification. The scores range from 0 to 39 and can be determined prior to surgery using CT images[71,72]. The PCI is semi-quantitative, aiming to define and measure the peritoneal involvement. It is easily reproducible and has been validated by several studies and by expert consensus[59,73-76]. Other scores have been reported that attempt to preoperatively differentiate between patients that might benefit from curative or palliative therapy such as the Peritoneal Surface Disease Severity Score[69]. It is extremely important to select appropriate patients with colorectal PC for CRS-HIPEC, though the perioperative mortality is reduced at high-volume centers with extensive experience in the procedure, morbidity remains high regardless[77]. Also Segelman et al[78] reported predicting the individual risk of metachronous PC after surgery for non-metastatic CC. The models may help in the planning of treatment and follow-up of patients.
Although currently the most common chemotherapeutic agents infused during HIPEC are mitomycin-C and cisplatin, the drug regimen differs considerably between institutions. The intraperitoneal application of catumaxomab is a new treatment option. This tri-functional antibody which is active against the EpCAM-antigen of the peritoneal tumor cells has been proven to be effective in the treatment of malignant ascites and is licensed for this indication[79,80]. It is possible that morbidity and effectiveness of CRS-HIPEC may be improved as knowledge and technology continue to develop leading to new drugs, more effective patient selection, and improved perioperative management.
FUTURE DIRECTIONS
Peritoneal carcinomatosis historically has been regarded as an untreatable disease and despite advances, remains a significant challenge for oncologists and surgeons. For many years, patients with PC have been considered to be beyond the realm of curative thearpy, but in recent years promising results have been reported in a variety of tumor types using CRS-HIPEC. A randomized trial has demonstrated a significant survival gain compared with palliative therapy alone in colorectal cancer PC. However, CRS-HIPEC is only available in specialized institutions and is not indicated for the majority of the colorectal PC patients due to its high morbidity and mortality. Furthermore, many clinicians continue to have reservations about recommending patients to undergo such a highly morbid procedure with a goal of prolonging life for median of 10 mo, especially given that any expectation of curing PC remains unrealistic. Likewise, CRS-HIPEC has no effect on alleviating systemic symptoms such as cachexia, weight loss, and malnutrition that significantly hinder the remaining quality of life. Further elucidating the biological behavior of colon PC and developing molecularly targeted therapy may serve as an avenue of future research.
Footnotes
Supported by NIH grants, No. R01CA160688 (to Takabe K) and No. T32CA085159-10 (to Terracina KP)
P- Reviewer: Roncucci L S- Editor: Ma YJ L- Editor: A E- Editor: Liu XM
References
- 1.House MG, Ito H, Gönen M, Fong Y, Allen PJ, DeMatteo RP, Brennan MF, Blumgart LH, Jarnagin WR, D’Angelica MI. Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg. 2010;210:744–752, 752-755. doi: 10.1016/j.jamcollsurg.2009.12.040. [DOI] [PubMed] [Google Scholar]
- 2.Kanas GP, Taylor A, Primrose JN, Langeberg WJ, Kelsh MA, Mowat FS, Alexander DD, Choti MA, Poston G. Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors. Clin Epidemiol. 2012;4:283–301. doi: 10.2147/CLEP.S34285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Spolverato G, Ejaz A, Azad N, Pawlik TM. Surgery for colorectal liver metastases: The evolution of determining prognosis. World J Gastrointest Oncol. 2013;5:207–221. doi: 10.4251/wjgo.v5.i12.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Go PH, Klaassen Z, Meadows MC, Chamberlain RS. Gastrointestinal cancer and brain metastasis: a rare and ominous sign. Cancer. 2011;117:3630–3640. doi: 10.1002/cncr.25940. [DOI] [PubMed] [Google Scholar]
- 5.Villeneuve PJ, Sundaresan RS. Surgical management of colorectal lung metastasis. Clin Colon Rectal Surg. 2009;22:233–241. doi: 10.1055/s-0029-1242463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li WH, Peng JJ, Xiang JQ, Chen W, Cai SJ, Zhang W. Oncological outcome of unresectable lung metastases without extrapulmonary metastases in colorectal cancer. World J Gastroenterol. 2010;16:3318–3324. doi: 10.3748/wjg.v16.i26.3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemmens VE, Klaver YL, Verwaal VJ, Rutten HJ, Coebergh JW, de Hingh IH. Predictors and survival of synchronous peritoneal carcinomatosis of colorectal origin: a population-based study. Int J Cancer. 2011;128:2717–2725. doi: 10.1002/ijc.25596. [DOI] [PubMed] [Google Scholar]
- 8.Segelman J, Granath F, Holm T, Machado M, Mahteme H, Martling A. Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2012;99:699–705. doi: 10.1002/bjs.8679. [DOI] [PubMed] [Google Scholar]
- 9.Jayne DG, Fook S, Loi C, Seow-Choen F. Peritoneal carcinomatosis from colorectal cancer. Br J Surg. 2002;89:1545–1550. doi: 10.1046/j.1365-2168.2002.02274.x. [DOI] [PubMed] [Google Scholar]
- 10.Minsky BD, Mies C, Rich TA, Recht A, Chaffey JT. Potentially curative surgery of colon cancer: patterns of failure and survival. J Clin Oncol. 1988;6:106–118. doi: 10.1200/JCO.1988.6.1.106. [DOI] [PubMed] [Google Scholar]
- 11.Russell AH, Tong D, Dawson LE, Wisbeck WM, Griffin TW, Laramore GE, Luk KH. Adenocarcinoma of the retroperitoneal ascending and descending colon: sites of initial dissemination and clinical patterns of recurrence following surgery alone. Int J Radiat Oncol Biol Phys. 1983;9:361–365. doi: 10.1016/0360-3016(83)90297-3. [DOI] [PubMed] [Google Scholar]
- 12.Tong D, Russell AH, Dawson LE, Wisbeck WM, Griffin TW, Laramore GE, Luk KH. Adenocarcinoma of the cecum: natural history and clinical patterns of recurrence following radical surgery. Int J Radiat Oncol Biol Phys. 1983;9:357–360. doi: 10.1016/0360-3016(83)90296-1. [DOI] [PubMed] [Google Scholar]
- 13.Sadeghi B, Arvieux C, Glehen O, Beaujard AC, Rivoire M, Baulieux J, Fontaumard E, Brachet A, Caillot JL, Faure JL, et al. Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer. 2000;88:358–363. doi: 10.1002/(sici)1097-0142(20000115)88:2<358::aid-cncr16>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 14.Elias D, Blot F, El Otmany A, Antoun S, Lasser P, Boige V, Rougier P, Ducreux M. Curative treatment of peritoneal carcinomatosis arising from colorectal cancer by complete resection and intraperitoneal chemotherapy. Cancer. 2001;92:71–76. doi: 10.1002/1097-0142(20010701)92:1<71::aid-cncr1293>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 15.Sugarbaker PH. Surgical management of carcinomatosis from colorectal cancer. Clin Colon Rectal Surg. 2005;18:190–203. doi: 10.1055/s-2005-916280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fiori E, Lamazza A, De Cesare A, Bononi M, Volpino P, Schillaci A, Cavallaro A, Cangemi V. Palliative management of malignant rectosigmoidal obstruction. Colostomy vs. endoscopic stenting. A randomized prospective trial. Anticancer Res. 2004;24:265–268. [PubMed] [Google Scholar]
- 17.Sugarbaker PH. Review of a personal experience in the management of carcinomatosis and sarcomatosis. Jpn J Clin Oncol. 2001;31:573–583. doi: 10.1093/jjco/hye088. [DOI] [PubMed] [Google Scholar]
- 18.Blair SL, Chu DZ, Schwarz RE. Outcome of palliative operations for malignant bowel obstruction in patients with peritoneal carcinomatosis from nongynecological cancer. Ann Surg Oncol. 2001;8:632–637. doi: 10.1007/s10434-001-0632-1. [DOI] [PubMed] [Google Scholar]
- 19.Ceelen WP, Flessner MF. Intraperitoneal therapy for peritoneal tumors: biophysics and clinical evidence. Nat Rev Clin Oncol. 2010;7:108–115. doi: 10.1038/nrclinonc.2009.217. [DOI] [PubMed] [Google Scholar]
- 20.Piso P, Arnold D. Multimodal treatment approaches for peritoneal carcinosis in colorectal cancer. Dtsch Arztebl Int. 2011;108:802–808. doi: 10.3238/arztebl.2011.0802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berthelot C, Morel O, Girault S, Verrièle V, Poirier AL, Moroch J, Boucher Y, Le Jeune JJ, Lorimier G. Use of FDG-PET/CT for peritoneal carcinomatosis before hyperthermic intraperitoneal chemotherapy. Nucl Med Commun. 2011;32:23–29. doi: 10.1097/MNM.0b013e328340e730. [DOI] [PubMed] [Google Scholar]
- 22.Yan TD, Morris DL, Shigeki K, Dario B, Marcello D. Preoperative investigations in the management of peritoneal surface malignancy with cytoreductive surgery and perioperative intraperitoneal chemotherapy: Expert consensus statement. J Surg Oncol. 2008;98:224–227. doi: 10.1002/jso.21069. [DOI] [PubMed] [Google Scholar]
- 23.van Gestel YR, Thomassen I, Lemmens VE, Pruijt JF, van Herk-Sukel MP, Rutten HJ, Creemers GJ, de Hingh IH. Metachronous peritoneal carcinomatosis after curative treatment of colorectal cancer. Eur J Surg Oncol. 2014;40:963–969. doi: 10.1016/j.ejso.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 24.Galandiuk S, Wieand HS, Moertel CG, Cha SS, Fitzgibbons RJ, Pemberton JH, Wolff BG. Patterns of recurrence after curative resection of carcinoma of the colon and rectum. Surg Gynecol Obstet. 1992;174:27–32. [PubMed] [Google Scholar]
- 25.Ceelen WP, Bracke ME. Peritoneal minimal residual disease in colorectal cancer: mechanisms, prevention, and treatment. Lancet Oncol. 2009;10:72–79. doi: 10.1016/S1470-2045(08)70335-8. [DOI] [PubMed] [Google Scholar]
- 26.Ceelen W, Van Nieuwenhove Y, Pattyn P. Surgery and intracavitary chemotherapy for peritoneal carcinomatosis from colorectal origin. Acta Gastroenterol Belg. 2008;71:373–378. [PubMed] [Google Scholar]
- 27.Terauchi M, Kajiyama H, Yamashita M, Kato M, Tsukamoto H, Umezu T, Hosono S, Yamamoto E, Shibata K, Ino K, et al. Possible involvement of TWIST in enhanced peritoneal metastasis of epithelial ovarian carcinoma. Clin Exp Metastasis. 2007;24:329–339. doi: 10.1007/s10585-007-9070-1. [DOI] [PubMed] [Google Scholar]
- 28.Kokenyesi R, Murray KP, Benshushan A, Huntley ED, Kao MS. Invasion of interstitial matrix by a novel cell line from primary peritoneal carcinosarcoma, and by established ovarian carcinoma cell lines: role of cell-matrix adhesion molecules, proteinases, and E-cadherin expression. Gynecol Oncol. 2003;89:60–72. doi: 10.1016/s0090-8258(02)00152-x. [DOI] [PubMed] [Google Scholar]
- 29.Pocard M, Debruyne P, Bras-Gonçalves R, Mareel M, Dutrillaux B, Poupon MF. Single alteration of p53 or E-cadherin genes can alter the surgical resection benefit in an experimental model of colon cancer. Dis Colon Rectum. 2001;44:1106–1112. doi: 10.1007/BF02234630. [DOI] [PubMed] [Google Scholar]
- 30.Hansen E, Wolff N, Knuechel R, Ruschoff J, Hofstaedter F, Taeger K. Tumor cells in blood shed from the surgical field. Arch Surg. 1995;130:387–393. doi: 10.1001/archsurg.1995.01430040049007. [DOI] [PubMed] [Google Scholar]
- 31.Meyers MA. Distribution of intra-abdominal malignant seeding: dependency on dynamics of flow of ascitic fluid. Am J Roentgenol Radium Ther Nucl Med. 1973;119:198–206. doi: 10.2214/ajr.119.1.198. [DOI] [PubMed] [Google Scholar]
- 32.Sugarbaker PH. Observations concerning cancer spread within the peritoneal cavity and concepts supporting an ordered pathophysiology. Cancer Treat Res. 1996;82:79–100. doi: 10.1007/978-1-4613-1247-5_6. [DOI] [PubMed] [Google Scholar]
- 33.Jonjić N, Peri G, Bernasconi S, Sciacca FL, Colotta F, Pelicci P, Lanfrancone L, Mantovani A. Expression of adhesion molecules and chemotactic cytokines in cultured human mesothelial cells. J Exp Med. 1992;176:1165–1174. doi: 10.1084/jem.176.4.1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bittinger F, Klein CL, Skarke C, Brochhausen C, Walgenbach S, Röhrig O, Köhler H, Kirkpatrick CJ. PECAM-1 expression in human mesothelial cells: an in vitro study. Pathobiology. 1996;64:320–327. doi: 10.1159/000164067. [DOI] [PubMed] [Google Scholar]
- 35.van Grevenstein WM, Hofland LJ, van Rossen ME, van Koetsveld PM, Jeekel J, van Eijck CH. Inflammatory cytokines stimulate the adhesion of colon carcinoma cells to mesothelial monolayers. Dig Dis Sci. 2007;52:2775–2783. doi: 10.1007/s10620-007-9778-4. [DOI] [PubMed] [Google Scholar]
- 36.Ziprin P, Ridgway PF, Pfistermüller KL, Peck DH, Darzi AW. ICAM-1 mediated tumor-mesothelial cell adhesion is modulated by IL-6 and TNF-alpha: a potential mechanism by which surgical trauma increases peritoneal metastases. Cell Commun Adhes. 2003;10:141–154. [PubMed] [Google Scholar]
- 37.van Rossen ME, Hofland LJ, van den Tol MP, van Koetsveld PM, Jeekel J, Marquet RL, van Eijck CH. Effect of inflammatory cytokines and growth factors on tumour cell adhesion to the peritoneum. J Pathol. 2001;193:530–537. doi: 10.1002/1096-9896(2000)9999:9999<::AID-PATH805>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 38.Klein CL, Bittinger F, Skarke CC, Wagner M, Köhler H, Walgenbach S, Kirkpatrick CJ. Effects of cytokines on the expression of cell adhesion molecules by cultured human omental mesothelial cells. Pathobiology. 1995;63:204–212. doi: 10.1159/000163953. [DOI] [PubMed] [Google Scholar]
- 39.Cannistra SA, Kansas GS, Niloff J, DeFranzo B, Kim Y, Ottensmeier C. Binding of ovarian cancer cells to peritoneal mesothelium in vitro is partly mediated by CD44H. Cancer Res. 1993;53:3830–3838. [PubMed] [Google Scholar]
- 40.Gerber SA, Rybalko VY, Bigelow CE, Lugade AA, Foster TH, Frelinger JG, Lord EM. Preferential attachment of peritoneal tumor metastases to omental immune aggregates and possible role of a unique vascular microenvironment in metastatic survival and growth. Am J Pathol. 2006;169:1739–1752. doi: 10.2353/ajpath.2006.051222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Heath RM, Jayne DG, O’Leary R, Morrison EE, Guillou PJ. Tumour-induced apoptosis in human mesothelial cells: a mechanism of peritoneal invasion by Fas Ligand/Fas interaction. Br J Cancer. 2004;90:1437–1442. doi: 10.1038/sj.bjc.6601635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Warn R, Harvey P, Warn A, Foley-Comer A, Heldin P, Versnel M, Arakaki N, Daikuhara Y, Laurent GJ, Herrick SE, et al. HGF/SF induces mesothelial cell migration and proliferation by autocrine and paracrine pathways. Exp Cell Res. 2001;267:258–266. doi: 10.1006/excr.2001.5240. [DOI] [PubMed] [Google Scholar]
- 43.Carreiras F, Rigot V, Cruet S, Andre F, Gauduchon P, Marvaldi J. Migration properties of the human ovarian adenocarcinoma cell line IGROV1: importance of alpha(v)beta3 integrins and vitronectin. Int J Cancer. 1999;80:285–294. doi: 10.1002/(sici)1097-0215(19990118)80:2<285::aid-ijc19>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 44.Abu-Hijleh MF, Habbal OA, Moqattash ST. The role of the diaphragm in lymphatic absorption from the peritoneal cavity. J Anat. 1995;186(Pt 3):453–467. [PMC free article] [PubMed] [Google Scholar]
- 45.Kusamura S, Baratti D, Zaffaroni N, Villa R, Laterza B, Balestra MR, Deraco M. Pathophysiology and biology of peritoneal carcinomatosis. World J Gastrointest Oncol. 2010;2:12–18. doi: 10.4251/wjgo.v2.i1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chu DZ, Lang NP, Thompson C, Osteen PK, Westbrook KC. Peritoneal carcinomatosis in nongynecologic malignancy. A prospective study of prognostic factors. Cancer. 1989;63:364–367. doi: 10.1002/1097-0142(19890115)63:2<364::aid-cncr2820630228>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 47.Köhne CH, Cunningham D, Di Costanzo F, Glimelius B, Blijham G, Aranda E, Scheithauer W, Rougier P, Palmer M, Wils J, et al. Clinical determinants of survival in patients with 5-fluorouracil-based treatment for metastatic colorectal cancer: results of a multivariate analysis of 3825 patients. Ann Oncol. 2002;13:308–317. doi: 10.1093/annonc/mdf034. [DOI] [PubMed] [Google Scholar]
- 48.Bloemendaal AL, Verwaal VJ, van Ruth S, Boot H, Zoetmulder FA. Conventional surgery and systemic chemotherapy for peritoneal carcinomatosis of colorectal origin: a prospective study. Eur J Surg Oncol. 2005;31:1145–1151. doi: 10.1016/j.ejso.2005.06.002. [DOI] [PubMed] [Google Scholar]
- 49.Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, Zoetmulder FA. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21:3737–3743. doi: 10.1200/JCO.2003.04.187. [DOI] [PubMed] [Google Scholar]
- 50.Goldberg RM, Sargent DJ, Morton RF, Fuchs CS, Ramanathan RK, Williamson SK, Findlay BP, Pitot HC, Alberts S. Randomized controlled trial of reduced-dose bolus fluorouracil plus leucovorin and irinotecan or infused fluorouracil plus leucovorin and oxaliplatin in patients with previously untreated metastatic colorectal cancer: a North American Intergroup Trial. J Clin Oncol. 2006;24:3347–3353. doi: 10.1200/JCO.2006.06.1317. [DOI] [PubMed] [Google Scholar]
- 51.Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350:2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- 52.Franko J, Shi Q, Goldman CD, Pockaj BA, Nelson GD, Goldberg RM, Pitot HC, Grothey A, Alberts SR, Sargent DJ. Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J Clin Oncol. 2012;30:263–267. doi: 10.1200/JCO.2011.37.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weisberger AS, Levine B, Storaasli JP. Use of nitrogen mustard in treatment of serous effusions of neoplastic origin. J Am Med Assoc. 1955;159:1704–1707. doi: 10.1001/jama.1955.02960350004002. [DOI] [PubMed] [Google Scholar]
- 54.Hribaschek A, Meyer F, Schneider-Stock R, Pross M, Ridwelski K, Lippert H. Comparison of intraperitoneal with intravenous administration of taxol in experimental peritoneal carcinomatosis. Chemotherapy. 2007;53:410–417. doi: 10.1159/000110005. [DOI] [PubMed] [Google Scholar]
- 55.Vaillant JC, Nordlinger B, Deuffic S, Arnaud JP, Pelissier E, Favre JP, Jaeck D, Fourtanier G, Grandjean JP, Marre P, et al. Adjuvant intraperitoneal 5-fluorouracil in high-risk colon cancer: A multicenter phase III trial. Ann Surg. 2000;231:449–456. doi: 10.1097/00000658-200004000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nordlinger B, Rougier P, Arnaud JP, Debois M, Wils J, Ollier JC, Grobost O, Lasser P, Wals J, Lacourt J, et al. Adjuvant regional chemotherapy and systemic chemotherapy versus systemic chemotherapy alone in patients with stage II-III colorectal cancer: a multicentre randomised controlled phase III trial. Lancet Oncol. 2005;6:459–468. doi: 10.1016/S1470-2045(05)70222-9. [DOI] [PubMed] [Google Scholar]
- 57.Elias D, Delperro JR, Sideris L, Benhamou E, Pocard M, Baton O, Giovannini M, Lasser P. Treatment of peritoneal carcinomatosis from colorectal cancer: impact of complete cytoreductive surgery and difficulties in conducting randomized trials. Ann Surg Oncol. 2004;11:518–521. doi: 10.1245/ASO.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 58.Mahteme H, Hansson J, Berglund A, Påhlman L, Glimelius B, Nygren P, Graf W. Improved survival in patients with peritoneal metastases from colorectal cancer: a preliminary study. Br J Cancer. 2004;90:403–407. doi: 10.1038/sj.bjc.6601586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Glehen O, Kwiatkowski F, Sugarbaker PH, Elias D, Levine EA, De Simone M, Barone R, Yonemura Y, Cavaliere F, Quenet F, et al. Cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for the management of peritoneal carcinomatosis from colorectal cancer: a multi-institutional study. J Clin Oncol. 2004;22:3284–3292. doi: 10.1200/JCO.2004.10.012. [DOI] [PubMed] [Google Scholar]
- 60.Yan TD, Black D, Savady R, Sugarbaker PH. Systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for peritoneal carcinomatosis from colorectal carcinoma. J Clin Oncol. 2006;24:4011–4019. doi: 10.1200/JCO.2006.07.1142. [DOI] [PubMed] [Google Scholar]
- 61.Verwaal VJ, van Ruth S, Witkamp A, Boot H, van Slooten G, Zoetmulder FA. Long-term survival of peritoneal carcinomatosis of colorectal origin. Ann Surg Oncol. 2005;12:65–71. doi: 10.1007/s10434-004-1167-z. [DOI] [PubMed] [Google Scholar]
- 62.Königsrainer I, Beckert S. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: where are we? World J Gastroenterol. 2012;18:5317–5320. doi: 10.3748/wjg.v18.i38.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Al-Shammaa HA, Li Y, Yonemura Y. Current status and future strategies of cytoreductive surgery plus intraperitoneal hyperthermic chemotherapy for peritoneal carcinomatosis. World J Gastroenterol. 2008;14:1159–1166. doi: 10.3748/wjg.14.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coccolini F, Gheza F, Lotti M, Virzì S, Iusco D, Ghermandi C, Melotti R, Baiocchi G, Giulini SM, Ansaloni L, et al. Peritoneal carcinomatosis. World J Gastroenterol. 2013;19:6979–6994. doi: 10.3748/wjg.v19.i41.6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Verwaal VJ, Bruin S, Boot H, van Slooten G, van Tinteren H. 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol. 2008;15:2426–2432. doi: 10.1245/s10434-008-9966-2. [DOI] [PubMed] [Google Scholar]
- 66.Elias D, Lefevre JH, Chevalier J, Brouquet A, Marchal F, Classe JM, Ferron G, Guilloit JM, Meeus P, Goéré D, et al. Complete cytoreductive surgery plus intraperitoneal chemohyperthermia with oxaliplatin for peritoneal carcinomatosis of colorectal origin. J Clin Oncol. 2009;27:681–685. doi: 10.1200/JCO.2008.19.7160. [DOI] [PubMed] [Google Scholar]
- 67.Franko J, Ibrahim Z, Gusani NJ, Holtzman MP, Bartlett DL, Zeh HJ. Cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion versus systemic chemotherapy alone for colorectal peritoneal carcinomatosis. Cancer. 2010;116:3756–3762. doi: 10.1002/cncr.25116. [DOI] [PubMed] [Google Scholar]
- 68.Elias D, Gilly F, Boutitie F, Quenet F, Bereder JM, Mansvelt B, Lorimier G, Dubè P, Glehen O. Peritoneal colorectal carcinomatosis treated with surgery and perioperative intraperitoneal chemotherapy: retrospective analysis of 523 patients from a multicentric French study. J Clin Oncol. 2010;28:63–68. doi: 10.1200/JCO.2009.23.9285. [DOI] [PubMed] [Google Scholar]
- 69.Chua TC, Morris DL, Saxena A, Esquivel J, Liauw W, Doerfer J, Germer CT, Kerscher AG, Pelz JO. Influence of modern systemic therapies as adjunct to cytoreduction and perioperative intraperitoneal chemotherapy for patients with colorectal peritoneal carcinomatosis: a multicenter study. Ann Surg Oncol. 2011;18:1560–1567. doi: 10.1245/s10434-010-1522-1. [DOI] [PubMed] [Google Scholar]
- 70.McConnell YJ, Mack LA, Francis WP, Ho T, Temple WJ. HIPEC + EPIC versus HIPEC-alone: differences in major complications following cytoreduction surgery for peritoneal malignancy. J Surg Oncol. 2013;107:591–596. doi: 10.1002/jso.23276. [DOI] [PubMed] [Google Scholar]
- 71.Sugarbaker PH, Jablonski KA. Prognostic features of 51 colorectal and 130 appendiceal cancer patients with peritoneal carcinomatosis treated by cytoreductive surgery and intraperitoneal chemotherapy. Ann Surg. 1995;221:124–132. doi: 10.1097/00000658-199502000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359–374. doi: 10.1007/978-1-4613-1247-5_23. [DOI] [PubMed] [Google Scholar]
- 73.Kianmanesh R, Scaringi S, Sabate JM, Castel B, Pons-Kerjean N, Coffin B, Hay JM, Flamant Y, Msika S. Iterative cytoreductive surgery associated with hyperthermic intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis of colorectal origin with or without liver metastases. Ann Surg. 2007;245:597–603. doi: 10.1097/01.sla.0000255561.87771.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Glehen O, Cotte E, Schreiber V, Sayag-Beaujard AC, Vignal J, Gilly FN. Intraperitoneal chemohyperthermia and attempted cytoreductive surgery in patients with peritoneal carcinomatosis of colorectal origin. Br J Surg. 2004;91:747–754. doi: 10.1002/bjs.4473. [DOI] [PubMed] [Google Scholar]
- 75.Portilla AG, Shigeki K, Dario B, Marcello D. The intraoperative staging systems in the management of peritoneal surface malignancy. J Surg Oncol. 2008;98:228–231. doi: 10.1002/jso.21068. [DOI] [PubMed] [Google Scholar]
- 76.Rivard JD, McConnell YJ, Temple WJ, Mack LA. Cytoreduction and heated intraperitoneal chemotherapy for colorectal cancer: are we excluding patients who may benefit? J Surg Oncol. 2014;109:104–109. doi: 10.1002/jso.23446. [DOI] [PubMed] [Google Scholar]
- 77.Gusani NJ, Cho SW, Colovos C, Seo S, Franko J, Richard SD, Edwards RP, Brown CK, Holtzman MP, Zeh HJ, et al. Aggressive surgical management of peritoneal carcinomatosis with low mortality in a high-volume tertiary cancer center. Ann Surg Oncol. 2008;15:754–763. doi: 10.1245/s10434-007-9701-4. [DOI] [PubMed] [Google Scholar]
- 78.Segelman J, Akre O, Gustafsson UO, Bottai M, Martling A. Individualized prediction of risk of metachronous peritoneal carcinomatosis from colorectal cancer. Colorectal Dis. 2014;16:359–367. doi: 10.1111/codi.12552. [DOI] [PubMed] [Google Scholar]
- 79.Heiss MM, Murawa P, Koralewski P, Kutarska E, Kolesnik OO, Ivanchenko VV, Dudnichenko AS, Aleknaviciene B, Razbadauskas A, Gore M, et al. The trifunctional antibody catumaxomab for the treatment of malignant ascites due to epithelial cancer: Results of a prospective randomized phase II/III trial. Int J Cancer. 2010;127:2209–2221. doi: 10.1002/ijc.25423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ströhlein MA, Lordick F, Rüttinger D, Grützner KU, Schemanski OC, Jäger M, Lindhofer H, Hennig M, Jauch KW, Peschel C, et al. Immunotherapy of peritoneal carcinomatosis with the antibody catumaxomab in colon, gastric, or pancreatic cancer: an open-label, multicenter, phase I/II trial. Onkologie. 2011;34:101–108. doi: 10.1159/000324667. [DOI] [PubMed] [Google Scholar]


