Abstract
AIM: To investigate the role of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase in colon epithelial cells in the pathogenesis of acute and chronic colon inflammation in a mouse model of dextran sulphate sodium (DSS)-induced colitis.
METHODS: Balb/c mice were divided into three groups: 8 mice with acute DSS-induced colitis (3.5% DSS solution; 7 d), 8 mice with chronic DSS-induced colitis (3.5% DSS solution for 5 d + water for 6 d; 4 cycles; total: 44 d) and 12 mice without DSS supplementation as a control group. Primary colonic epithelial cells were isolated using chelation method. The cells were cultivated in the presence of mediators (lipopolysaccharide (LPS), apocynin or diphenyleneiodonium). Viability of cells was assessed by fluorescent microscopy. Production of reactive oxygen species (ROS) by the cells was measured fluorometrically using Amplex Red. Production of tumour necrosis factor-alpha (TNF-α) by the colonic epithelial cells was analysed by ELISA. Nox1 gene expression was assessed by real-time PCR.
RESULTS: Our study showed that TNF-α level was increased in unstimulated primary colonic cells both in the acute and chronic colitis groups, whereas decreased viability, increased ROS production, and expression of Nox1 was characteristic only for chronic DSS colitis mice when compared to the controls. The stimulation by LPS increased ROS generation via NADPH oxidase and decreased cell viability in mice with acute colitis. Treatment with NADPH oxidase inhibitors increased cell viability and decreased the levels of ROS and TNF-α in the LPS-treated cells isolated from mice of both acute and chronic colitis groups.
CONCLUSION: Our study revealed the importance of NADPH oxidase in the pathogenesis of both acute and chronic inflammation of the colon.
Keywords: Apocynin; Diphenyleneiodonium; Dextran sulphate sodium-induced colitis; Inflammatory bowel disease, Nicotinamide adenine dinucleotide phosphate oxidase; Reactive oxygen species; Tumour necrosis factor-α
Core tip: Reactive oxygen species (ROS)-induced oxidative stress is one of the most important etiological factors involved in the inflammation. The key producers of ROS in cells are nicotinamide adenine dinucleotide phosphate (NADPH) oxidase enzymes. Several studies have shown that epithelial NADPH oxidase might be responsible for normal immune response to antigens in the gut. On the other hand, little is known about the molecular pathways controlling ROS production via NADPH oxidase in primary intestinal epithelial cells during inflammation. The aim of this study was to investigate the role of NADPH oxidase in colon epithelial cells in the pathogenesis of acute and chronic colon inflammation using a mouse model of dextran sulphate sodium-induced colitis. The results of our study revealed the importance of NADPH oxidase in the pathogenesis of colon inflammation.
INTRODUCTION
Ulcerative colitis (UC) is a chronic inflammatory bowel disease (IBD) that affects the intestinal mucosa[1]. The pathogenesis of UC seems to involve primary defects in one or more elements responsible for the recognition of bacteria and normal immune response to antigens in the gut[2,3]. Previous studies indicated the importance of reactive oxygen species (ROS)-induced oxidative stress in the development of IBD. The key producers of ROS in phagocytic and non-phagocytic cells are NADPH oxidase enzymes[4,5]. NADPH oxidase-derived ROS act as intracellular messengers for a variety of cellular receptor signal transduction pathways, and play pivotal roles in various biological activities, including host defence, cell growth and differentiation, stimulation of pro-inflammatory genes, and cell death[4,6-8]. The epithelial NADPH oxidase homologs (Nox1 and DUOX2) generate a higher level of ROS in the colon compared to phagocytic NADPH oxidase (Nox2)[4]. Nox1, the so-called ‘‘colon NADPH oxidase’’, is highly expressed in the colon, particularly in colon epithelial cells[5]. This enzyme comes into close contact with normal and pathogenic bacteria and may play an important role in local innate immune and inflammatory responses in the gut[5,9,10]. Several studies have shown that bacterial products and pro-inflammatory cytokines such as interleukin-18 (IL-18), interferon gamma and tumour necrosis factor-alpha (TNF-α) can stimulate the NADPH oxidase expression and ROS production in intestinal epithelial cell cultures in vitro[10,11]. Anti-inflammatory cytokines interleukin-10 (IL-10) and transforming growth factor-beta effectively block the stimulatory actions of pro-inflammatory cytokines and decrease NADPH oxidase activity in colon cancer cell lines[10,11]. However, molecular pathways controlling ROS production via NOX enzymes in primary intestinal epithelial cells during acute and chronic inflammation are poorly understood.
The aim of this study was to investigate the role of NADPH oxidase in colon epithelial cells in the acute and chronic colon inflammation using mice with dextran sulphate sodium (DSS)-induced colitis.
MATERIALS AND METHODS
Animals
Male Balb/c mice were used for the experiments (Lithuanian University of Health Sciences, Veterinary Academy, vivarium, Lithuania). All mice were 6-8-wk-old and had an approximate weight of 16-20 g at the beginning of the experiment. Mice were housed in individual plastic cages (1 mouse per cage) in a 12-h light/dark cycle at 22 °C room temperature and were provided with food and water ad libitum. Procedures involving animal care were conducted conforming to national and international laws and policies. The experimental design was approved by the Lithuanian Animal Ethics Committee (Protocol No. 0201).
Induction of colitis with DSS
Colon inflammation in Balb/c mice was induced by oral administration of 3.5% DSS dissolved in the distilled drinking water and supplied ad libitum (molecular mass 40 kDa, TdB Consultancy, Uppsala, Sweden). We used a protocol that was established by Wirtz et al[12], which was slightly modified as follows: animals were divided into three study groups: 8 mice with acute DSS-induced colitis (mice were given 3.5% DSS in the drinking water over 7 d; 1 cycle; total number of days: 7), 8 mice with chronic DSS-induced colitis (mice were given 3.5% DSS in the drinking water over 5 d and water for 6 d; this cycle was repeated 4 times; total number of days: 44), and 12 mice as a control group without DSS supplementation.
Evaluation of colonic inflammation
Assessment of clinical parameters: Clinical parameters recorded in the experiments with Balb/c mice were colon length (cm), colon weight (mg, without faeces), spleen weight (mg), body weight (g), mortality, diarrhoea (assessment of faeces was performed using the Bristol scale) and rectal bleeding (seen by ocular inspection)[13]. Bristol scale is designed to classify faeces into seven groups according to faeces consistency in points from 1 (very hard) to 7 (entirely liquid)[14]. The clinical and morphological parameters of the animals were expressed as a mean of the group.
Assessment of histological score in mice: All chemicals were obtained from Sigma-Aldrich (Steinheim, Germany) unless otherwise stated. Each colonic segment of mice was washed with phosphate-buffered solution without Mg2+ or Ca2+ (PBS) (Invitrogen, Carlsbad, CA, United States) and immediately fixed with 10% neutral formalin for 4 h at room temperature for paraffin embedding. Serial 3-μm sections were cut for each tract and stained with haematoxylin and eosin for histological examination. Images of tissue were analysed using an OLYMPUS IX71 microscope with Q IMAGING EXI aqua camera (Tokyo, Japan). Histological examination was performed using the method suggested by Hausmann et al[15].
Isolation and cultivation of primary mouse colonic epithelial cells
Large intestine was removed and placed in PBS for washing procedure. Subsequently, the intestine was cut into 1-mm fragments and transferred in PBS solution containing 50 IU/mL penicillin, 50 μg/mL streptomycin, and 0.5 mg/mL gentamycin. The primary mouse colonic epithelial cells were isolated using chelation method as described by Meijssen et al[16], but slightly modified as follows. The intestine fragments were incubated in 2.5 mmol/L EDTA dissolved in PBS for 40-45 min at room temperature to liberate the single epithelial cells from the lamina propria. The isolated epithelial cells were suspended in DMEM supplemented with 15% fetal calf serum, 10 mmol/L HEPES buffer, and antibiotics. Approximately 107 cells per well were seeded and cultured with mediators (20 μg/mL of lipopolysaccharide (LPS), 1 mmol/L of apocynin, 20 μg/mL LPS + 1 mmol/L apocynin) in 24-well plates coated with bovine dermal collagen for 24 h at 37 °C in an atmosphere of 5% CO2 and at 90% relative humidity. After 24 h, supernatants were collected and stored at -20 °C[1]. In order to assess the purity of isolated epithelial cell culture, i.e., the content of inflammatory cells, the expression of specific cell type markers (Ptprc - a hematopoietic cell marker, Cd3e - a T cell marker, Cd68 - a monocyte/macrophage marker, Vill - an epithelial cell marker) was detected using real-time PCR method (described in detail below).
Measurement of hydrogen peroxide production
NADPH oxidase generates superoxide, which is rapidly converted to hydrogen peroxide[17]. Hydrogen peroxide production by cells was measured fluorometrically using 1 μmol/L Amplex Red and 10 units/mL horseradish peroxidase. Mouse colonic epithelial cells (approximately 105 cells/mL) were resuspended in PBS and incubated with either 5 units/mL of catalase (endogenous control), or 10 μg/mL of LPS, or 20 μmol/L of diphenyleneiodonium (DPI), or 10 μg/mL LPS + 20 μmol/L DPI for 30 min at 37 °C. Biopsy samples were accurately shredded, suspended in PBS solution and incubated without stimulation for 30 min. Then the Amplex Red and peroxidase were added and the rate of hydrogen peroxide production was measured by following the increase in fluorescence (excitation at 544 nm, emission at 590 nm) with a Fluoroskan Ascent microplate fluorometer (The Thermo Scientific, Waltham, MA)[1].
Apocynin interferes with detection of ROS in assay systems selective for hydrogen peroxide or hydroxyl radicals. This inhibitor acts as a radical scavenger and inhibits Amplex Red oxidation. Thus ROS are not measured accurately and cannot reflect the effect of apocynin on the NADPH oxidase activity. Therefore, for the assessment of NADPH oxidase activity we applied another large-spectrum inhibitor DPI[1,18]. Estimation of effect of DPI on cell viability and TNF-α has not been performed, because DPI is a less specific inhibitor of Nox than apocynin and can inhibit other oxidases, e.g., mitochondrial complex I[4].
Assessment of cell viability
The viability of cultured mouse colonic epithelial cells was assessed by propidium iodide (PI, 7 μmol/L) and Hoechst 33342 (4 μg/mL) staining using an OLYMPUS IX71S1F-3 fluorescence microscope (Tokyo, Japan). PI-negative cells with weak Hoechst-staining were considered to be viable, whereas cells showing nuclear shrinkage or fragmentation and intensive Hoechst staining but still lacking PI staining were classified as chromatin condensed/fragmented (apoptotic). PI-positive cells were classified as necrotic. Mouse colonic epithelial cells were counted in at least 5 microscopic fields per well (three wells per treatment). Data are expressed as percentage of viable, necrotic or apoptotic cells of the total number of cells per field[1,19].
Assessment of TNF-α concentration
Concentration of TNF-α was assessed in the supernatants of primary mouse epithelial cell cultures using a commercially available two-site ELISA kit (Invitrogen, Carlsbad, CA). The lowest limit of sensitivity of the test system for TNF-α was 3 pg/mL. The optical densities at 450 nm and at a correction wavelength of 490 nm were measured on an ELISA microplate reader (MRX microplate reader, Dynex Technologies, Denkendorf, Germany)[1].
Assessment of gene expression in colonic epithelial cells by real-time PCR
Isolated mouse epithelial cells were stabilised with RNAlater® solution and kept in -80 °C until further analysis. Total RNA was isolated using the GeneJET RNA Purification Kit (Thermo Fisher Scientific, Vilnius, Lithuania). Purified RNA was re-suspended in RNase-free water and stored at -80 °C. RNA purity and integrity were assessed using a spectrophotometer (Nanodrop Technologies, Wilmington, DE, United States). cDNA was synthesised from total RNA (200 ng) using First Strand cDNA Synthesis Kit (Thermo Fisher Scientific, Vilnius, Lithuania). Reaction for the real-time PCR was performed using predesigned TaqMan® Gene Expression Assays (Gene expression assays IDs: Nox1 - Mm00549170_m1, Actb - Mm00607939_s1, Ptprc - Mm01293577_m1; Cd3e - Mm00599684_g1; Cd68 - Mm03047340_m1; Vill - Mm00457074_m1) and TaqMan® Universal PCR Master Mix, according to the manufacturer’s instructions (Life Technologies, Carlsbad, United States). Actb was used as the endogenous control gene. The experiments for each sample were performed in triplicate. Expression in the mouse epithelial cells was determined using ABI Fast 7500 System (Life Technologies, Carlsbad, CA, United States). The PCR conditions were 1 cycle of 95 °C for 10 min followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. The changes of gene expression was analysed using the 2ΔCT method.
Statistical analysis
Statistical analyses were performed using the SPSS statistical package (version 16.0; Chicago, IL). Data in text and figures are presented as mean ± standard error. Statistical analyses were performed using one-way ANOVA. The least significant difference test was used as a post hoc test. A P-value < 0.05 was considered statistically significant[1].
RESULTS
Assessment of the clinical symptoms of colitis mice
Oral administration of 3.5% DSS solution for 7 d induced severe acute colitis in mice with a significant reduction in the body weight, diarrhoea, rectal bleeding, decreased colon length, increased spleen weight when compared to control mice (Table 1). Administration of 3.5% DSS for 44 d induced less severe damage, however, a significant body weight loss and liquefaction of faeces were observed compared to the control mice (Table 1). The animals with acute colitis had more severe symptoms in comparison to chronic colitis mice, including pronounced weight loss, rectal bleeding, decrease in colon length, and increase in spleen weight. The mice in the control group did not show any clinical symptoms of the disease.
Table 1.
Assessment of clinical symptoms and morphological alterations after dextran sulphate sodium administration in Balb/c mice
| Group | n | Length of colon (cm) | Colon weight (mg) | Weight of colon/ length of colon | Spleen weight (mg) | Body weight (g) | Assessment of faeces | Rectal bleeding | Mortality |
| Control | 12 | 7.8 ± 0.78 | 337.6 ± 69.04 | 44.26 ± 7.11 | 97.1 ± 31.35 | 21.9 ± 3.89 | 2.5 ± 0.52 | 0/12 | 0/12 |
| (0%/100%) | (0%/100%) | ||||||||
| Acute colitis | 8 | 5.9 ± 0.97a | 321.9 ± 55.29 | 55.03 ± 6.48a | 197.9 ± 93.58a | 17.0 ± 2.30a | 5.0 ± 0.85a | 5/8 | 0/8 |
| (62%/100%) | (0%/100%) | ||||||||
| Chronic colitis | 8 | 7.2 ± 0.27e | 354.2 ± 69.29 | 49.39 ± 6.28 | 119.0 ± 20.74e | 18.8 ± 1.06ce | 4.2 ± 0.45ce | 0/8 | 0/8 |
| (0%/100%) | (0%/100%) |
Colon inflammation was induced by the administration of 3.5% DSS solution in Balb/c mice. Assessment of faeces was performed using the Bristol scale (points 1 to 7). Rectal bleeding - number of mice with rectal bleeding (percentages)/total number of mice (percentages). Mortality - number of dead mice (percentages)/total number of mice (percentages).
P < 0.05, acute DSS-induced colitis groups vs control;
P < 0.05, chronic DSS-induced colitis groups vs control;
P < 0.05, acute DSS-induced colitis vs chronic DSS-induced colitis groups. Results are presented as mean ± SE.
Histological assessment of colon inflammation in mice
In mice with acute colitis we observed major epithelium damage with loss of crypts in large areas (3.8 ± 0.42, P = 0.01) and inflammatory cell infiltration of the lamina submucosa (3.9 ± 0.35, P = 0.01) (Figure 1B). In mice with chronic colitis, loss of goblet cells in large areas (2.9 ± 0.19, P = 0.02) and inflammatory cells infiltration of the lamina muscularis mucosae (2.2 ± 0.30, P = 0.02) were observed (Figure 1C). Control mice had no histological alterations in the colon tissue (Figure 1A).
Figure 1.
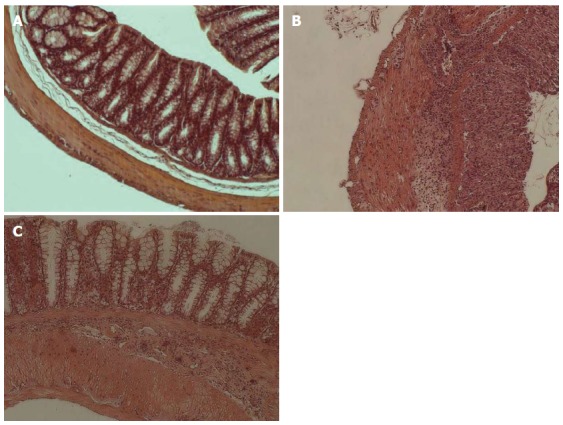
Effect of dextran sulphate sodium treatment on histological parameters in Balb/c mice. Analysis of histological parameters was performed as described in Materials and Methods (original magnification × 10). A: Untreated mice (n = 12); B: Acute dextran sulphate sodium (DSS)-induced colitis group (oral administration of 3.5% DSS solution for 7 d; n = 8); C: Chronic DSS-induced colitis group (oral administration of 3.5% DSS for 44 d; n = 8).
Assessment of purity of isolated epithelial cell culture
The purity of the primary colonic epithelial cell culture was assessed by detecting the expression of gene markers (Ptprc - a hematopoietic cell marker; Cd3e - a T cell marker; Cd68 - a monocyte/macrophage marker; Vill - an epithelial cell marker). As shown in Figure 2, the expression levels of Ptprc, Cd3e, and Cd68 gene markers, specific for inflammatory cells, were minor compared to the expression of epithelial cell marker Vill in primary colonic epithelial cells. Moreover, expression levels of these genes were identical in cell cultures obtained from all three experimental groups. These findings indicate that inflammatory cells did not affect ROS, TNF-α production and Nox1 expression in our cell culture.
Figure 2.
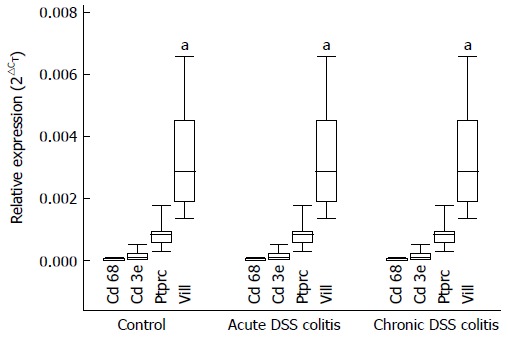
Expression of Cd68, Cd3e, Ptprc, and Vill in the colonic epithelial cells of male Balb/c mice. Cd68, Cd3e, Ptprc, and Vill were specific cell type markers (Ptprc - a hematopoietic cell marker, Cd3e - a T cell marker, Cd68 - a monocyte/macrophage marker, Vill - an epithelial cells marker). The expression level of genes in the cells was determined by real-time PCR. aStatistically significant differences in expression levels of inflammatory cell markers (Cd68, Cd3e, Ptprc) and epithelial cell specific marker Vill in all the experimental groups. Values are presented as mean ± SE. Control group: n = 12. Both DSS-induced colitis groups: n = 8. aP < 0.05, both DSS-induced colitis groups vs control. DSS: Dextran sulphate sodium.
Assessment of viability of mouse colonic epithelial cells
The viability of cells isolated from chronic colitis mice after 24 h was significantly lower and number of necrotic cells was higher than those in the control group, whereas no significant difference between acute colitis and control groups was observed (Figure 3A, B and C). Treatment of cells with NADPH oxidase inhibitor apocynin substantially increased the viability of cells (to 68%) and reduced necrosis (to 30%) in mice with chronic colitis, whereas apocynin had no significant effect on cell survival in the control and acute colitis groups (Figure 3C). Stimulation of colonic epithelial cells with LPS significantly reduced the viability of the cells in the control (to 50%) and both acute and chronic colitis groups (to 47% and to 38%, respectively) by increasing the number of necrotic cells (Figure 3A, B and C). However, treatment with apocynin prevented the inhibitory effect of LPS on colonic epithelial cell viability in all groups of mice. Cell viability was significantly increased to 60% in the control group, to 64% in the acute colitis and to 55% in chronic colitis groups and number of necrotic cells was significantly decreased (36%, 32% and 40%, respectively, Figure 3A, B and C). The percentage of apoptotic cells was minor (3%-8%) in all study groups.
Figure 3.
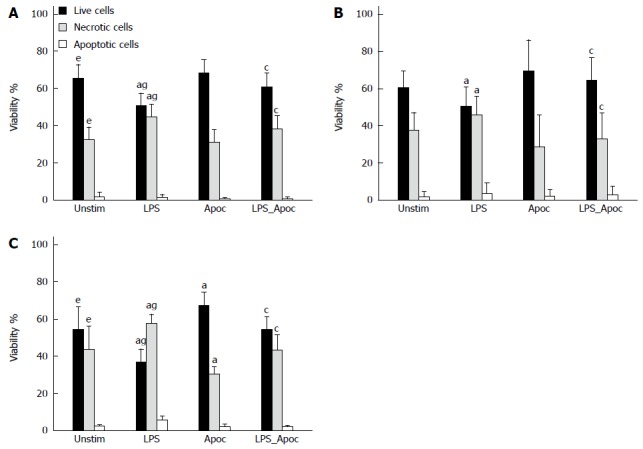
Assessment of viability of mouse colonic epithelial cells. Cells were incubated with 20 μg/mL LPS, 1 mmol/L apocynin (Apoc), 20 μg/mL LPS + 1 mmol/L Apoc, and without stimulation for 24 h. A: Untreated mice; B: Acute DSS-induced colitis group; C: Chronic DSS-induced colitis group. aP < 0.05, DSS-induced colitis groups untreated vs one of the stimulated subgroups in the control; c P < 0.05, DSS-induced colitis groups vs LPS and LPS + Apoc treated subgroups in the control; eP < 0.05, chronic DSS-induced colitis groups in the untreated subgroup vs control; gP < 0.05, chronic DSS-induced colitis groups in the LPS subgroup vs control; Values are presented as mean ± SE. Untreated mouse group: n = 12. Both DSS-induced colitis groups: n = 8. LPS: Lipopolysaccharide.
Assessment of hydrogen peroxide production in cells
The level of extracellular hydrogen peroxide production was significantly higher in chronic colitis mouse cells than in the control group (Figure 4A). Similar results were obtained when hydrogen peroxide production was measured directly in the colonic biopsies, i.e., hydrogen peroxide production was higher in the biopsies of both colitis groups when compared to controls (Figure 4B).
Figure 4.
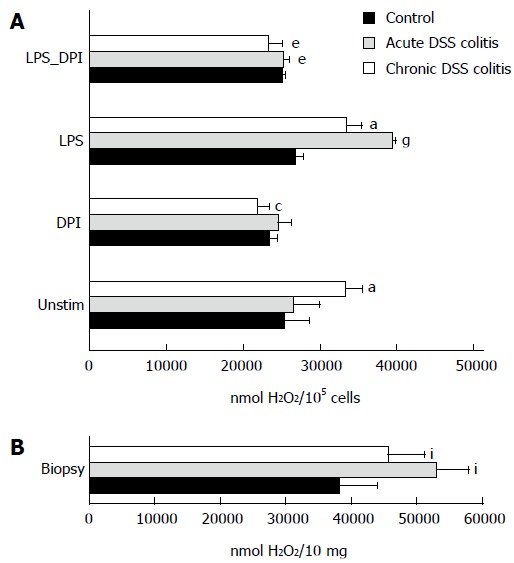
Assessment of hydrogen peroxide production. A: Mouse colonic epithelial cells were incubated for 30 min with 10 μg/mL LPS, 10 μmol/L DPI, 10 μg/mL LPS + 10 μmol/L DPI or without stimulation; B: Colonic biopsies (10 mg) of DSS-induced colitis mice and control subjects were incubated without stimulation for 30 min. aP < 0.05, DSS-induced colitis groups vs stimulated and un-stimulated cell subgroups within control. cP < 0.05, untreated vs DPI treated subgroups in the chronic DSS-induced colitis group. eP < 0.05, LPS vs LPS + DPI treated cell subgroups in both DSS-induced colitis groups. gP < 0.05, untreated vs LPS treated cell subgroups in the acute DSS-induced colitis group. iP < 0.05, DSS-induced colitis groups in the mouse colonic biopsies vs control. Values are mean ± SE. Untreated mouse group: n = 12. Both DSS-induced colitis groups: n = 8.
NADPH oxidase inhibitor DPI decreased the level of ROS production in the chronic colitis group when compared to the control by 1.5 times (Figure 4A). Treatment of cells with bacterial endotoxin LPS significantly increased the production of hydrogen peroxide in both colitis groups (1.5-fold higher in acute colitis group and 1.3-fold higher in chronic colitis group). The addition of DPI to LPS-treated colonic epithelial cells significantly decreased the level of ROS production in all experimental colitis groups. In the acute colitis group, ROS level was approximately 1.6-fold lower compared to the LPS-treated cells. Similar results were obtained in the chronic colitis group, where hydrogen peroxide production decreased approximately 1.5-fold (Figure 4A).
Assessment of TNF-α concentration in colonic epithelial cells
We investigated the influence of NADPH oxidase on the production of pro-inflammatory cytokine TNF-α. As shown in Figure 5, the level of TNF-α was significantly increased in both acute and chronic colitis groups compared to the control (4.5 and 3.6-fold, respectively). In chronic colitis mice treatment with LPS nearly doubled the levels of TNF-α production. Treatment with NADPH oxidase inhibitor apocynin prevented the stimulating effect of LPS on TNF-α production in primary colonic cells in both acute and chronic colitis groups (1.5 and 1.6-fold, respectively, Figure 5).
Figure 5.
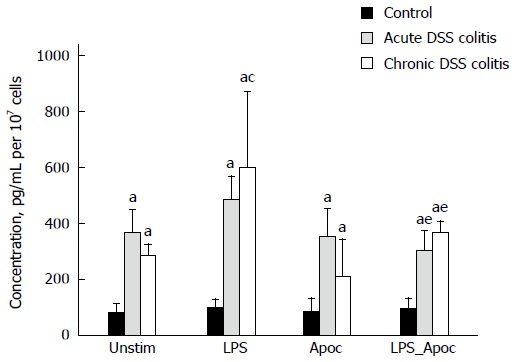
Assessment of TNF-α production by mouse colonic epithelial cells. TNF-α concentration was measured in cell media after 24 h incubation with 20 μg/mL of LPS, 1 mmol/L of apocynin (Apoc), 20 μg/mL LPS + 1 mmol/L Apoc or without stimulation; aP < 0.05, DSS-induced colitis groups in all subgroups vs control; cP < 0.05, untreated vs LPS treated subgroup in the chronic DSS-induced colitis group; eP < 0.05, LPS vs LPS + apocynin treated subgroup in both DSS-induced colitis groups. Values are presented as mean ± SE. Untreated mouse group: n = 12. Both DSS-induced colitis groups: n = 8.
Expression of Nox1 in colonic epithelial cells
We investigated the expression levels of Nox1 in the colonic epithelial cells of mice using real-time PCR analysis. As demonstrated in Figure 6, the expression level of Nox1 in the epithelial cells during chronic colitis was approximately 6.4-fold higher when compared to controls. Expression level of Nox1 did not differ significantly in the epithelial cells isolated from mice with acute colitis and the control group.
Figure 6.
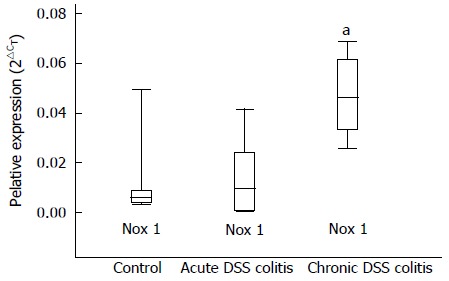
Expression of Nox1 in the colonic epithelial cells of male Balb/c mice. The expression levels of genes in these cells were determined by real-time PCR. aP < 0.05 chronic DSS-induced colitis group vs both other groups. Values are presented as mean ± SE. Control group: n = 12. Both DSS-induced colitis groups: n = 8. DSS: Dextran sulphate sodium.
DISCUSSION
Several studies have shown that epithelial NADPH oxidase mediated formation of ROS is involved in inflammatory responses and host defence system at mucosal surfaces[9,20,21]. Increased production of ROS via NADPH oxidase has been implicated in tissue damage observed in chronic inflammatory disorders, such as IBD[1,22,23]. In this study, using an experimental colitis mouse model, we examined the role of NADPH oxidase in colon epithelial cells in the acute and chronic colon inflammation.
The results of this study revealed that expression level of Nox1 is increased in the colon epithelium of mice with chronic colitis. Similar findings were observed in the human colonic epithelium of patients with ulcerative colitis[21]. Unstimulated cells of chronic colitis mice had decreased viability and increased ROS production via NADPH oxidase and TNF-α concentration when compared to the control group. This observation suggests that NADPH oxidase is an active player in chronic inflammatory response in the primary colonic epithelial cells. Increased activity of NADPH oxidase triggers unbalanced inflammation cascade and non-phagocytic cell damage, and can act as an oncogenic factor which increases the risk of intestinal cancer[3,9]. Furthermore, overproduction of ROS may induce chronic inflammation[5,6,19,22] and damage of the colon tissue[23,24].
Interestingly, we did not find significant differences in Nox1 expression, cell viability or ROS synthesis in the colonic epithelium of mice with acute colitis when compared to the control mice. Nevertheless, TNF-α level was increased in acute colitis mice, indicating active inflammatory process. Our results suggest that TNF-α level did not affect Nox1 expression and ROS generation in the acute colitis model. This observation could be explained by the action of anti-inflammatory cytokines such as IL-10 which may suppress TNF-α-stimulated activation of Nox1[22,25]. Furthermore, the results of our study revealed that bacterial products were involved in the activation of NADPH oxidase in acute colon inflammation. Stimulation of epithelial cells with LPS dramatically diminished cell viability and increased ROS production via NADPH oxidase. Our results suggest that NADPH oxidase might contribute to the development of acute inflammation in the colon epithelium. It is well known that this enzyme is rapidly activated during infection or after stimulation by bacterial endotoxins, when innate immune system activates protective cascades against microbial invaders in phagocytic and non-phagocytic cells[26-29].
In order to eliminate the potential bias for our results, the purity of colonic epithelial cell culture was confirmed by specific cell markers Ptprc, Cd3e, Cd68 and Will. The expression analyses of these cell markers revealed that colonic epithelial cells produced inflammation mediators themselves and generated inflammatory responses to antigens.
The inhibition of NADPH oxidase represents an attractive therapeutic strategy for the treatment of many chronic diseases[1]. Apocynin has been widely used as a selective inhibitor of the complex NADPH oxidase in the experimental models of inflammation[24,29,30]. Apocynin possesses very low toxicity and anti-inflammatory activity, i.e., reducing the level of inflammatory cytokines including TNF-α, protecting cells from damage induced by bacterial products and decreasing damage in the colon tissue[1,24,30-34]. In our study in both colitis groups, apocynin decreased necrosis and TNF-α production and increased cell viability in LPS-treated colonic epithelial cells. Similar findings were determined in the chronic colitis group, where apocynin substantially increased the viability of cells and reduced necrosis. We hypothesize that the protective mechanism of apocynin might be associated not only with anti-inflammatory action of this inhibitor but also with the decreased ROS generation via NADPH oxidase and reduced oxidative stress in cells. Recently, this effect of apocynin was observed in the primary colonic epithelial cells of patients with ulcerative colitis[1]. However, the effect of apocynin on colon epithelial regeneration needs to be investigated in the future.
The results of our study showed that epithelial NADPH oxidase is directly involved in chronic colon inflammation; however, stimulation by bacterial products is required for NADPH oxidase activation during acute colitis. In both cases, the molecular mechanism for activation of NADPH oxidase is similar. The signalling cascade for activation of NADPH oxidase in colonic epithelial cells might be associated with the toll-like receptor (TLR) pathway, where LPS strains potently stimulate ROS production by Nox1 through TLR4[1,10,35,36]. Further studies should be designed to investigate the role and exact mechanism of LPS/TLR4/TNF-α/NOX signalling in the intestine epithelium in acute and chronic colitis[1]. The detection of expression changes of other NADPH oxidase homologs and evaluation of superoxide in primary epithelial cells during colon inflammation should be conducted in novel studies.
In conclusion, our study revealed the importance of NADPH oxidase in pathogenesis of acute and chronic colon inflammation. Moreover, treatment with NADPH oxidase inhibitors had a protective impact against pro-inflammatory action of bacterial endotoxins in mouse colon epithelial cells during acute and chronic colitis.
COMMENTS
Background
Reactive oxygen species (ROS)-induced oxidative stress is one of the most important etiological factors involved in inflammation. The key producers of ROS in the epithelial cells are nicotinamide adenine dinucleotide phosphate (NADPH) oxidase enzymes. Several studies have shown that epithelial NADPH oxidase might be responsible for normal immune response to antigens in the gut. Increased production of ROS via NADPH oxidase has been implicated in tissue damage observed in chronic inflammatory disorders, such as inflammatory bowel disease (IBD).
Research frontiers
The aim of this study was to investigate the role of NADPH oxidase in colon epithelial cells in the acute and chronic colon inflammation using a mouse colitis model. In this study, the authors revealed the importance of NADPH oxidase in the pathogenesis of both acute and chronic colon inflammation.
Innovations and breakthroughs
Currently, the genes encoding NADPH oxidase enzymes and their localization have been investigated extensively; however, knowledge about the expression of NADPH oxidase in tissues, their activation mechanisms and possible biological functions is still lacking. Most research investigations of biological functions of NADPH oxidase have been performed using the cancer cell lines, but not in primary cell cultures. The results of this study revealed the importance of NADPH oxidase in the pathogenesis of colon inflammation. Moreover, the treatment with NADPH oxidase inhibitors had a protective impact against pro-inflammatory action of bacterial endotoxins in mouse colon epithelial cells during inflammation.
Applications
Obtained knowledge will expand the existing theoretical knowledge about the molecular mechanisms of inflammation and contribute to the development of new therapeutic targets for ulcerative colitis.
Terminology
NADPH oxidases are a family of integral membrane proteins that catalyse the transfer of electrons from NADPH to molecular oxygen to produce ROS. Epithelial NADPH oxidase comes into close contact with normal and pathogenic microorganisms and may play an important role in local innate immunity and inflammatory responses in the gut. They believe that this enzyme is involved in the molecular mechanism of colon inflammation.
Peer review
The authors examined the role of NADPH oxidase in colon epithelial cells in the pathogenesis of acute and chronic colon inflammation. They showed that TNF-α level was increased in unstimulated primary colonic cells both in the acute and chronic colitis groups, whereas decreased viability, increased ROS generation via NADPH oxidase, and expression of Nox1 were characteristic only for chronic DSS-induced colitis mice when compared to the controls. Treatment with NADPH oxidase inhibitors increased cell viability, decreased the levels of ROS and TNF-α in the LPS-treated cells isolated from mice of both acute and chronic DSS-induced colitis groups. The results are interesting and may expand the existing theoretical knowledge about the molecular mechanisms of colon inflammation.
Footnotes
P- Reviewer: Huerta-Franco MR, Marie JC, Yeligar SM S- Editor: Qi Y L- Editor: Wang TQ E- Editor: Liu XM
References
- 1.Ramonaite R, Skieceviciene J, Kiudelis G, Jonaitis L, Tamelis A, Cizas P, Borutaite V, Kupcinskas L. Influence of NADPH oxidase on inflammatory response in primary intestinal epithelial cells in patients with ulcerative colitis. BMC Gastroenterol. 2013;13:159. doi: 10.1186/1471-230X-13-159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roda G, Sartini A, Zambon E, Calafiore A, Marocchi M, Caponi A, Belluzzi A, Roda E. Intestinal epithelial cells in inflammatory bowel diseases. World J Gastroenterol. 2010;16:4264–4271. doi: 10.3748/wjg.v16.i34.4264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ismail AS, Hooper LV. Epithelial cells and their neighbors. IV. Bacterial contributions to intestinal epithelial barrier integrity. Am J Physiol Gastrointest Liver Physiol. 2005;289:G779–G784. doi: 10.1152/ajpgi.00203.2005. [DOI] [PubMed] [Google Scholar]
- 4.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 5.Bokoch GM, Knaus UG. NADPH oxidases: not just for leukocytes anymore. Trends Biochem Sci. 2003;28:502–508. doi: 10.1016/S0968-0004(03)00194-4. [DOI] [PubMed] [Google Scholar]
- 6.Debnath T, Kim da H, Lim BO. Natural products as a source of anti-inflammatory agents associated with inflammatory bowel disease. Molecules. 2013;18:7253–7270. doi: 10.3390/molecules18067253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim KA, Kim JY, Lee YA, Min A, Bahk YY, Shin MH. Entamoeba histolytica induces cell death of HT29 colonic epithelial cells via NOX1-derived ROS. Korean J Parasitol. 2013;51:61–68. doi: 10.3347/kjp.2013.51.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M, Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Kawahara T, Kuwano Y, Teshima-Kondo S, Takeya R, Sumimoto H, Kishi K, Tsunawaki S, Hirayama T, Rokutan K. Role of nicotinamide adenine dinucleotide phosphate oxidase 1 in oxidative burst response to Toll-like receptor 5 signaling in large intestinal epithelial cells. J Immunol. 2004;172:3051–3058. doi: 10.4049/jimmunol.172.5.3051. [DOI] [PubMed] [Google Scholar]
- 10.Rokutan K, Kawahara T, Kuwano Y, Tominaga K, Nishida K, Teshima-Kondo S. Nox enzymes and oxidative stress in the immunopathology of the gastrointestinal tract. Semin Immunopathol. 2008;30:315–327. doi: 10.1007/s00281-008-0124-5. [DOI] [PubMed] [Google Scholar]
- 11.O'Leary DP, Bhatt L, Woolley JF, Gough DR, Wang JH, Cotter TG, Redmond HP. TLR-4 signalling accelerates colon cancer cell adhesion via NF-κB mediated transcriptional up-regulation of Nox-1. PLoS One. 2012;7:e44176. doi: 10.1371/journal.pone.0044176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wirtz S, Neufert C, Weigmann B, Neurath MF. Chemically induced mouse models of intestinal inflammation. Nat Protoc. 2007;2:541–546. doi: 10.1038/nprot.2007.41. [DOI] [PubMed] [Google Scholar]
- 13.Axelsson LG, Landström E, Bylund-Fellenius AC. Experimental colitis induced by dextran sulphate sodium in mice: beneficial effects of sulphasalazine and olsalazine. Aliment Pharmacol Ther. 1998;12:925–934. doi: 10.1046/j.1365-2036.1998.00357.x. [DOI] [PubMed] [Google Scholar]
- 14.Lewis SJ, Heaton KW. Stool form scale as a useful guide to intestinal transit time. Scand J Gastroenterol. 1997;32:920–924. doi: 10.3109/00365529709011203. [DOI] [PubMed] [Google Scholar]
- 15.Hausmann M, Obermeier F, Paper DH, Balan K, Dunger N, Menzel K, Falk W, Schoelmerich J, Herfarth H, Rogler G. In vivo treatment with the herbal phenylethanoid acteoside ameliorates intestinal inflammation in dextran sulphate sodium-induced colitis. Clin Exp Immunol. 2007;148:373–381. doi: 10.1111/j.1365-2249.2007.03350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Meijssen MA, Brandwein SL, Reinecker HC, Bhan AK, Podolsky DK. Alteration of gene expression by intestinal epithelial cells precedes colitis in interleukin-2-deficient mice. Am J Physiol. 1998;274:G472–G479. doi: 10.1152/ajpgi.1998.274.3.G472. [DOI] [PubMed] [Google Scholar]
- 17.Babior BM. NADPH oxidase. Curr Opin Immunol. 2004;16:42–47. doi: 10.1016/j.coi.2003.12.001. [DOI] [PubMed] [Google Scholar]
- 18.Heumüller S, Wind S, Barbosa-Sicard E, Schmidt HH, Busse R, Schröder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- 19.Cizas P, Budvytyte R, Morkuniene R, Moldovan R, Broccio M, Lösche M, Niaura G, Valincius G, Borutaite V. Size-dependent neurotoxicity of beta-amyloid oligomers. Arch Biochem Biophys. 2010;496:84–92. doi: 10.1016/j.abb.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. J Immunol. 2004;173:3589–3593. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 21.Szanto I, Rubbia-Brandt L, Kiss P, Steger K, Banfi B, Kovari E, Herrmann F, Hadengue A, Krause KH. Expression of NOX1, a superoxide-generating NADPH oxidase, in colon cancer and inflammatory bowel disease. J Pathol. 2005;207:164–176. doi: 10.1002/path.1824. [DOI] [PubMed] [Google Scholar]
- 22.Davies GR, Simmonds NJ, Stevens TR, Grandison A, Blake DR, Rampton DS. Mucosal reactive oxygen metabolite production in duodenal ulcer disease. Gut. 1992;33:1467–1472. doi: 10.1136/gut.33.11.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McKenzie SJ, Baker MS, Buffinton GD, Doe WF. Evidence of oxidant-induced injury to epithelial cells during inflammatory bowel disease. J Clin Invest. 1996;98:136–141. doi: 10.1172/JCI118757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marín M, Giner RM, Ríos JL, Recio Mdel C. Protective effect of apocynin in a mouse model of chemically-induced colitis. Planta Med. 2013;79:1392–1400. doi: 10.1055/s-0033-1350710. [DOI] [PubMed] [Google Scholar]
- 25.Kamizato M, Nishida K, Masuda K, Takeo K, Yamamoto Y, Kawai T, Teshima-Kondo S, Tanahashi T, Rokutan K. Interleukin 10 inhibits interferon gamma- and tumor necrosis factor alpha-stimulated activation of NADPH oxidase 1 in human colonic epithelial cells and the mouse colon. J Gastroenterol. 2009;44:1172–1184. doi: 10.1007/s00535-009-0119-6. [DOI] [PubMed] [Google Scholar]
- 26.Bogdan C. Oxidative burst without phagocytes: the role of respiratory proteins. Nat Immunol. 2007;8:1029–1031. doi: 10.1038/ni1007-1029. [DOI] [PubMed] [Google Scholar]
- 27.Hultqvist M, Olsson LM, Gelderman KA, Holmdahl R. The protective role of ROS in autoimmune disease. Trends Immunol. 2009;30:201–208. doi: 10.1016/j.it.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Ogier-Denis E, Mkaddem SB, Vandewalle A. NOX enzymes and Toll-like receptor signaling. Semin Immunopathol. 2008;30:291–300. doi: 10.1007/s00281-008-0120-9. [DOI] [PubMed] [Google Scholar]
- 29.Geiszt M. NADPH oxidases: new kids on the block. Cardiovasc Res. 2006;71:289–299. doi: 10.1016/j.cardiores.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 30.Petrônio MS, Zeraik ML, Fonseca LM, Ximenes VF. Apocynin: chemical and biophysical properties of a NADPH oxidase inhibitor. Molecules. 2013;18:2821–2839. doi: 10.3390/molecules18032821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stefanska J, Pawliczak R. Apocynin: molecular aptitudes. Mediators Inflamm. 2008;2008:106507. doi: 10.1155/2008/106507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi EM, Lee YS. Protective effect of apocynin on antimycin A-induced cell damage in osteoblastic MC3T3-E1 cells. J Appl Toxicol. 2012;32:714–721. doi: 10.1002/jat.1689. [DOI] [PubMed] [Google Scholar]
- 33.Impellizzeri D, Esposito E, Mazzon E, Paterniti I, Di Paola R, Bramanti P, Cuzzocrea S. Effect of apocynin, a NADPH oxidase inhibitor, on acute lung inflammation. Biochem Pharmacol. 2011;81:636–648. doi: 10.1016/j.bcp.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 34.Van den Worm E, Beukelman CJ, Van den Berg AJ, Kroes BH, Labadie RP, Van Dijk H. Effects of methoxylation of apocynin and analogs on the inhibition of reactive oxygen species production by stimulated human neutrophils. Eur J Pharmacol. 2001;433:225–230. doi: 10.1016/s0014-2999(01)01516-3. [DOI] [PubMed] [Google Scholar]
- 35.Geiszt M, Leto TL. The Nox family of NAD(P)H oxidases: host defense and beyond. J Biol Chem. 2004;279:51715–51718. doi: 10.1074/jbc.R400024200. [DOI] [PubMed] [Google Scholar]
- 36.Rada B, Leto TL. Oxidative innate immune defenses by Nox/Duox family NADPH oxidases. Contrib Microbiol. 2008;15:164–187. doi: 10.1159/000136357. [DOI] [PMC free article] [PubMed] [Google Scholar]


