Abstract
AIM: To evaluate the diagnostic accuracy of serum Immunoglobulin A (IgA) for differentiating early stage nonalcoholic fatty liver disease (NAFLD) from nonalcoholic steatohepatitis (NASH).
METHODS: All cases had fatty liver change confirmed by ultrasound and aminotransferases of at least twice the normal level. Clinical and biochemical data, including serum IgA, were obtained from 50 histologically proven NAFLD cases and 54 healthy controls. Fasting whole blood samples were obtained from the study population. Immunoturbidimetric methods were used to measure the IgA levels. All NAFLD cases were hospitalized for liver biopsy. Liver specimens were examined for steatosis, steatohepatitis and fibrosis within hepatocytes. Patients were categorized into two groups: NASH and non-NASH. Variables were compared within cases (NASH vs non-NASH) and controls. Cut-off values of serum IgA were evaluated using analysis of receiver operating characteristic (ROC curves). Associations between the variables were tested using calculations of correlation coefficients. Statistical significances were assigned to P values < 0.05.
RESULTS: The extent of liver fibrosis correlated positively with IgA levels. Subjects with no fibrosis in their liver biopsies had a lower IgA level (301.5 ± 91.2 mg/dL) than subjects with any degree of fibrosis (388.8 ± 140.8 mg/dL), (P = 0.01). IgA levels were higher in NASH cases, and its value was significantly higher for higher degrees of fibrosis. Patients with perisinusoidal or pericellular fibrosis had significantly higher levels of IgA (403.5 ± 133.9 mg/dL, 418.2 ± 129.5 mg/dL) compared to those without it (301.8 ± 94.9 mg/dL, 297.7 ± 91.5 mg/dL), respectively. No significant correlation was found between steatosis grade and serum IgA levels. Based on ROC analysis, the best predictive IgA cutoff value for detecting liver fibrosis was 360 mg/dL (61% sensitivity, 81% specificity).
CONCLUSION: The serum IgA level is useful to evaluate the severity of liver fibrosis and can be used serially for evaluation and follow-up of NAFLD cases.
Keywords: Non-alcoholic Fatty Liver Disease, Biological Markers, Immunoglobulin A, Liver Cirrhosis, Fibrosis
Core tip: Nonalcoholic steatohepatitis (NASH) is a severe subtype of Nonalcoholic Fatty Liver Disease (NAFLD) that can progress to advanced liver disease and hepatocellular carcinoma. In this study, we investigated the diagnostic accuracy of serum Immunoglobulin A (IgA) as a biomarker for differentiating early stage NAFLD from NASH. We found that the extent of liver fibrosis correlated positively with IgA levels and these were higher in NASH cases than in non-NASH cases. Based on receiver operating characteristic analysis, the best predictive IgA cutoff value for detecting liver fibrosis was 360 mg/dL (61% sensitivity, 81% specificity).
INTRODUCTION
Nonalcoholic fatty liver disease (NAFLD) is strongly associated with metabolic syndrome[1] and represents a wide spectrum of histological changes in the liver, extending from simple steatosis, to nonalcoholic steatohepatitis (NASH), liver cirrhosis and hepatocellular carcinoma (HCC)[2,3]. The prevalence of NAFLD is rising rapidly worldwide[4-6]. In the United States, it varies considerably among different ethnic groups, ranging from 24% in African Americans, to 33% in Caucasians and 45% in Hispanics[7]. In Asia, the prevalence of NAFLD is reported as about 10% in the general population[8]. However, these reported figures may vary according to study populations and the modality used for the diagnosis. The increase in prevalence of NAFLD reflects a rise in risk factors of NAFLD: older age, type 2 diabetes, obesity and hypertriglyceridemia[4,9,10] and can be attributable to changes in life style, including western diet and sedentary life[11]. Nevertheless, the disease can be diagnosed in patients with normal Body Mass Index (BMI)[2].
The natural history of NAFLD is uncertain. Classically, NAFLD patients have minimally increased liver enzyme values, no history of excessive alcohol consumption, and no evidence of viral hepatitis, autoimmune liver disease or congenital chronic hepatitis. Frequently, NAFLD is discovered incidentally during routine workups[12]. In fact, despite its serious possible complications, the majority of the patients remain asymptomatic during the course of disease or they experience minimal symptoms, such as abdominal complaints or tender hepatomegaly in an abdominal exam. The discrepancy between the high prevalence of NAFLD in the population and the low prevalence of significant clinical symptoms has led to skepticism toward its clinical importance[13]. Simple hepatic steatosis is regarded as a mild hepatic injury. However, it cannot be considered a benign disease, because it can progress to steatohepatitis in many patients[12,14]. NASH is a potentially fatal stage of NAFLD. It is estimated that 20% of NAFLD cases have NASH, which has an inclination to develop into advanced liver disease, cirrhosis and ultimately to HCC[12,15]. Previous studies indicated that about 30%-40% of patients with NASH have advanced fibrosis and 10%-15% have established cirrhosis at the time of diagnosis[9,13]. Thus, it is important to differentiate NASH with and without fibrosis from simple steatosis for patient counseling and management[6]. Predictors of disease severity have not yet been clarified. At present, the gold standard technique for the diagnosis of NASH is liver biopsy, which is recognized as the only method to evaluate the presence and extent of necro-inflammatory changes and fibrosis in liver. However, it is an invasive procedure with possible serious complications and limitations[12,16-18]. A reliable non-invasive test as a replacement for liver biopsy would allow definite diagnosis in NAFLD patients and enables us to feasibly re-evaluate patients during a follow-up.
Other diagnostic procedures have their own limitations. For example, imaging tools like conventional ultrasound and CT scans can discover lipid accumulation within hepatocytes, but they fail to determine the stage of fibrosis[19,20]. Inflammation is considered to be a key component of NASH development. Consequently, systemic inflammatory biomarkers can increase during the process of the disease[21]. In the search for non-invasive indicators for the NAFLD severity, numerous potential biological parameters are being studied and their applicability for distinguishing the extent of NAFLD has been discussed[2,22,23]. However, there is still no definite biomarker validated as a predictor of the disease. Ferritin, CRP and α-TNF appear promising in the evaluation of NAFLD patients[22,24]. Apoptosis is the main phenomenon in liver fibrosis; therefore, serum biomarkers associated with apoptosis, such as CK-18, could help us to differentiate simple steatosis from NASH[6,25,26]. Furthermore, Immunoglobulin A (IgA), has been evaluated in previous studies[27-29] and discovered to be an independent predictor for evaluating the pre-cirrhotic progression of NASH[30]. In the search for a non-invasive approach to differentiate early stage NAFLD from NASH, we studied serum IgA concentrations, as a mediator of inflammation.
MATERIALS AND METHODS
This case-control study was based on data from a total of 104 Iranian patients (50 NAFLD cases and 54 healthy control subjects) at Mazandaran University of Medical Sciences, Imam Khomeini Hospital, Sari, Iran, 2011-2012. The Institutional Review Board approved the study protocol and all patients gave written informed consent for the use of their clinical data just for research purposes before participation. All cases had fatty liver changes confirmed by ultrasound and their aminotransferase levels were at least twice normal. Exclusion criteria included: serological evidence of viral hepatitis (positive HBsAg and/or HBcAb), chronic hepatitis C, primary biliary cirrhosis, primary sclerosing cholangitis, autoimmune hepatitis and hereditary metabolic disease (hemochromatosis, Wilson). Cases with a history of consuming hepatotoxic drugs were not enrolled in this study. Those with history of past or current ethanol consumption (men with more than 20 grams and women with more than 10 grams daily usage) were also excluded. Coagulation tests were done for all subjects who fulfilled the inclusion criteria and those with normal results were considered for liver biopsy.
Control subjects were selected from those who had normal BMI, liver ultrasound, aminotransferases and did not have diabetes or hyperlipidemia. Characteristics of patients and controls were blinded entirely for the investigators while performing validation assays and subsequent analysis.
Laboratory evaluation
Following at least 12 h fasting, 10 mL whole blood samples were obtained from the study population by vein puncture and collected in sterile tubes. Serum was separated from the cells within 20 min of the collection by centrifugation in 1500 × g for 10 min at room temperature. To preserve the serum components, the sera were quickly aliquoted in microtubes and flash frozen at -80 °C until further assay. Immunoturbidometric methods (DiaSys Diagnostics, Holzheim, Germany) were used to measure serum IgA, performed by the Pars-Azmon kit (Tehran, Iran).
Aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP) were measured enzymatically according to the International Federation of Clinical Chemistry with pyridoxal-5′-phosphate. In addition, a Cobas Integra 800® (Roche, Basel, Switzerland) performed enzymatic assays for serum triglycerides, total cholesterol (TC), low density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol and fasting plasma glucose. The bromocresol green method, using bovine serum albumin as standard protein on an LKB spectrophotometer (Biochrom Ltd, Cambridge, United Kingdom), measured serum albumin.
Liver histopathology
All NAFLD cases were hospitalized for liver biopsy, which was done by an experienced hepatologist under ultrasound guidance. Before performing the liver biopsy, coagulation tests were checked for each patient and normal subjects considered for the procedure. All liver biopsies were done using an automatic liver biopsy needle (Bard, gauge #16) under standardized conditions along with intensive 24 h follow up of patients for any possible complications. All specimens were fixed in formalin, processed and stained with hematoxylin-eosin and trichrome, and then examined by two experienced pathologists who were unaware of the clinical and biochemical findings of each patient. Histological samples were assessed according to these features within hepatic acini: steatosis, lobular inflammation, ballooning, and perisinusoidal/pericellular fibrosis. Diagnosis of steatosis was established based on presence of macrovesicular fat within at least 5% of hepatocytes and graded as follows: Grade 0: No evidence of steatosis, Grade 1: < 33%, Grade 2: 33%-66% and Grade 3: 66% < of hepatocytes contained macrovesicular fat. The minimum criteria for diagnosis of steatohepatitis included the presence of lobular inflammation and ballooning of cells or perisinusoidal/pericellular fibrosis in zone 3 of the hepatic acini and scored as follows: steatosis (0-3), lobular inflammation (0-3) and ballooning (0-2). NAFLD cases were categorized into three subgroups based on overall NAS activity score: definite NASH (score ≤ 5), borderline NASH (score 3-4) and non-NASH (score < 3). Histological staging was done according to the method of Kleiner et al[15] Fibrosis stages were expressed as the following four-point scale: stage 0: No fibrosis, stage 1: perivenular or perisinusoidal fibrosis in zone 3, stage 2: pericellular and periportal fibrosis, stage 3: septal/bridging fibrosis, stage 4: cirrhosis. On the basis of this classification, patients were categorized into two groups: NASH and non-NASH, according to Kleiner et al[15]. Data were recorded and analyzed for each patient in different subclasses based on the previously mentioned categories.
Statistical analysis
Descriptive statistics were expressed as means and standard deviations for continuous variables and the number (percent) for categorical variables. Statistical analysis was performed using SPSS, version 19 (SPSS, Inc., Chicago, IL, United States). The t-test or Pearson χ2 test was used for univariate comparisons between patient groups. The diagnostic values for sensitivity and specificity and cut-off values of the serum IgA were assessed using analysis of receiver operating characteristic (ROC curves). Associations between the variables were tested using calculations of correlation coefficients. Statistical significances were assigned to P values < 0.05.
RESULTS
From 2011-2012, 50 cases and 54 controls were referred for interdisciplinary evaluation and their data were included in the data set. The clinical and biochemical information on patients and controls is illustrated in Table 1. The clinical characteristics of patients with and without NASH are illustrated in Table 2.
Table 1.
Clinical and biochemical data of the study population (mean ± SD)
| Variable | Cases n = 50 | Controls n = 54 | P value |
| Age (yr) | 41.44 ± 13.04 | 32.28 ± 8.35 | 0.000 |
| Sex (n) | M:32 | M:29 | 0.287 |
| F:18 | F:25 | ||
| Weight (kg) | 79.14 ± 12.3 | 64.61 ± 10.96 | 0.000 |
| Height (cm) | 167.64 ± 9.48 | 166.34 ± 24.79 | 0.730 |
| BMI (kg/m²) | 0.000 | ||
| < 25 | 23.79 ± 0.8 | 22.7 ± 1.8 | |
| 25-29.9 | 27.8 ± 1.3 | 25.2 ± 0.3 | |
| ≥ 30 | 33.2 ± 3.6 | - | |
| Abdomen (cm) | 92.80 ± 13.49 | 78.55 ± 13.11 | 0.001 |
| Hip (cm) | 100.05 ± 10.06 | 87.18 ± 13.01 | 0.001 |
| ALT (U/mL) | 79.66 ± 35.93 | 20.07 ± 8.00 | 0.000 |
| AST (U/mL) | 47.62 ± 18.78 | 19.93 ± 5.19 | 0.000 |
| AST/ALT ratio | 0.64 ± 0.19 | 1.09 ± 0.35 | 0.000 |
| ALP (mg/dL) | 209.10 ± 75.21 | - | - |
| Albumin (mg/dL) | 4.45 ± 0.47 | - | - |
| FBS (mg/dL) | 102.74 ± 22.57 | - | - |
| TG (mg/dL) | 210.14 ± 104.81 | - | - |
| Cholesterol (g/dL) | 201.76 ± 37.01 | - | - |
| HDL (mg/dL) | 46.22 ± 10.49 | - | - |
| LDL (mg/dL) | 119.50 ± 39.42 | - | - |
| IgA (mg/dL) | 324.20 ± 111.63 | 318.111 ± 82.49 | 0.751 |
ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; ALP: Alkaline phosphatase; HDL: High-density lipoprotein; HDL: High-density lipoprotein; IgA: Immunoglobulin A.
Table 2.
Comparison of the clinical characteristics of patients with and without nonalcoholic steatohepatitis (mean ± SD)
| Variable | NASH n =28 | Non-NASH n = 22 | P value |
| Age (yr) | 41.8 ± 11.2 | 40.9 ± 15.3 | 0.80 |
| Weight (kg) | 77.0 ± 10.3 | 81.8 ± 14.1 | 0.17 |
| Height (cm) | 167.1 ± 9.2 | 168.2 ± 9.9 | 0.68 |
| BMI (kg/m²) | 0.153 | ||
| < 25 | 23.63 ± 0.7 | 23.97 ± 1.1 | |
| 25-29.9 | 28.09 ± 1.4 | 27.25 ± 1.3 | |
| ≥ 30 | 31.25 ± 1.5 | 34.25 ± 4.0 | |
| ALT (U/mL) | 89.7 ± 40.5 | 66.7 ± 24.3 | 0.02 |
| AST (U/mL) | 51.7 ± 20.4 | 42.4 ± 15.3 | 0.08 |
| AST/ALT ratio | 0.6 ± 0.1 | 0.6 ± 0.2 | 0.28 |
| ALP (mg/dL) | 217.3 ± 88.3 | 198.5 ± 54.3 | 0.38 |
| Albumin (mg/dL) | 4.5 ± 0.4 | 4.3 ± 0.5 | 0.18 |
| FBS (mg/dL) | 108.5 ± 27.3 | 95.3 ± 11 | 0.03 |
| TG (mg/dL) | 238.7 ± 110.8 | 173.6 ± 85.7 | 0.02 |
| Cholesterol (g/dL) | 206.7 ± 39.7 | 195.3 ± 32.9 | 0.28 |
| HDL (mg/dL) | 44.8 ± 10.3 | 47.9 ± 10.6 | 0.31 |
| LDL (mg/dL) | 117.7 ± 29.5 | 121.7 ± 49.9 | 0.72 |
| IgA (mg/dL) | 351.3 ± 125.9 | 289.6 ± 80.4 | 0.052 |
ALT: Alanine aminotransferase; AST: Aspartate aminotransferase; ALP: Alkaline phosphatase; HDL: High-density lipoprotein; HDL: High-density lipoprotein; IgA: Immunoglobulin A.
Clinical findings
Most of the NAFLD patients (78%) were overweight (mean BMI: 28.2 ± 3.8) and 24 % were obese (BMI > 30) (Table 1); however, our examinations for BMI with cutoff values adopted as 25 kg/m² (P = 0.47) and 30 kg/m² (P = 0.67) revealed no significant correlation between this variable with serum IgA levels. Moreover, considering age, sex, weight and height we found no statistically significant correlations between serum IgA level and these factors in our cases.
IgA level and biochemical variables
Statistical analysis was done for serum IgA concentrations and the following biochemical factors: AST, ALT, ALP, albumin, FBS, total cholesterol, HDL, LDL and TG.
We found that albumin with a cutoff point of 4 mg/dL was the only biochemical item that was statistically correlated (P = 0.03) with serum IgA concentration in this survey. We can concluded that serum IgA level can help distinguishing albumin levels more or less than 4 mg/dL in NAFLD patients.
In line with our inclusion criteria, aminotransferase levels were higher than normal among cases (Table 1). Mean serum IgA in those with AST and ALT more than 30 U/mL, was 329.1 ± 108.8 mg/dL and 322.6 ± 110.5 mg/dL, respectively. However, they were not correlated with IgA levels (AST P = 0.58 and ALT P = 0.62). Furthermore, the majority of our patients had an impaired lipid profile (Table 1); however, lipid levels did not correlate significantly with plasma IgA concentrations. 46% of patients had FBS more than 100 mg/dL and had higher amounts of IgA (331.3 ± 123.1 mg/dL), but it was not statistically significant (P = 0.67). None of the other studied biochemical factors correlated significantly with IgA levels in the case group.
IgA level and fibrosis stage
Of the 50 cases with NAFLD, 37 (74%) were histopathologically classified as stage 0. Fibrosis stages of 1 through 4 were found in six (12%), two (4%), four (8%) and one (2%) patients, respectively (Table 3). Based on Kleiner criteria (15), cases were classified as 28 (56%) NASH and 22 (44%) non-NASH. Not surprisingly, more severe forms of liver fibrosis were seen in NASH cases. Clinical characteristics were evaluated in each subgroup and compared for any significant correlation (Table 2).
Table 3.
Histopathological findings in patients with and without nonalcoholic steatohepatitis
| Variable | NASH (n) | Non-NASH (n) | P value |
| Lobular inflammation 25 | 18 | 7 | 0.04 |
| Ballooning 12 | 9 | 3 | 0.18 |
| Perisinusoidal fibrosis 11 | 8 | 3 | 0.30 |
| Pericellular fibrosis 11 | 9 | 2 | 0.08 |
| Steatosis Grade | |||
| 1 | 7 | 13 | 0.019 |
| 2 | 10 | 7 | |
| 3 | 11 | 2 | |
| Fibrosis Stage | 0.358 | ||
| 0 | 19 | 18 | |
| 1 | 3 | 3 | |
| 2 | 1 | 1 | |
| 3 | 4 | 0 | |
| 4 | 1 | 0 |
NASH: Non-alcoholic steatohepatitis.
Serum IgA levels in cases (324.2 ± 111.6 mg/dL) and controls (318.1 ± 82.5 mg/dL) had considerable overlap and no significant difference could be seen (P = 0.75). Considering the two major groups of cases, namely non-NASH (351.3 ± 125.9 mg/dL) and NASH (289.7 ± 80.4 mg/dL) subjects, although the IgA levels were higher in NASH cases, the difference did not reach statistical significance (P = 0.052).
There was a significant correlation of serum IgA level and fibrosis in the liver (Figure 1). Subjects with no fibrosis in their liver biopsies had a significantly lower level of IgA (301.5 ± 91.2 mg/dL) than subjects with any degree of fibrosis (388.8 ± 140.8 mg/dL) (P = 0.01). Even when comparing higher levels of fibrosis with lower levels, significant differences could be shown with the small numbers of cases in those groups (stage 0-1 vs stages 2-4 and stages 0-2 vs 3-4 had P values of 0.00).
Figure 1.
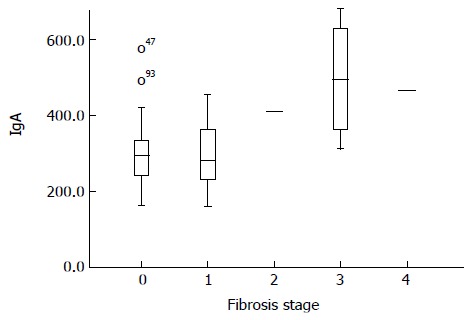
Serum Immunoglobulin A levels in different stages of fibrosis.
We also performed ROC curves analysis by plotting the sensitivity against the reverse specificity (1 minus the specificity) for each value (Figure 2). To detect any degree of fibrosis (F1-4) vs no fibrosis (F0), the area under the curve (AUC) was 0.690. Based on this analysis, the best cutoff point was defined at 360 mg/dL with a sensitivity of 61% and a specificity of 81%. A lower cutoff point of 269 mg/dL would have a sensitivity of about 85% and higher cutoff point of 414 mg/dL will be corresponding to a specificity of 90%.
Figure 2.
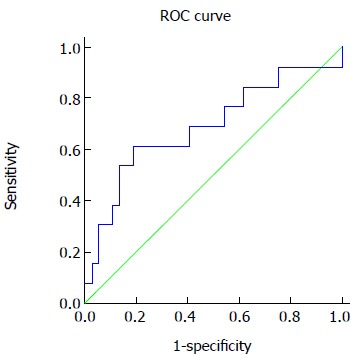
Receiver operating characteristic curves analysis of serum Immunoglobulin A and fibrosis (F0 vs F1-4). ROC: Receiver operating characteristic.
IgA levels and steatosis grade
Analysis of the serum IgA concentrations in relation to the histological grade of steatosis showed no relation between serum IgA levels and the steatosis grade in any combination [grade 1 vs grade 2-3 (P = 0.65), grade 1-2 vs grade 3 (P = 0.76), grade 1 vs 2 (P = 0.48), grade 1 vs 3 (P = 0.98), grade 2 vs grade 3 (P = 0.58). Similarly, we found no significant differences in IgA levels between ultrasound grade 1 vs 2-3 (P = 0.12)].
IgA levels and individual pathologic findings
At this stage, IgA levels were checked in different subgroups of individual components of the pathological examination, namely lobular inflammation, ballooning, perisinusoidal and pericellular fibrosis. No correlation was found between the former two items; but significant differences were observed in IgA levels for the latter two components. Eleven subjects had perisinusoidal fibrosis and their IgA levels were higher than in those without perisinusoidal fibrosis (403.5 ± 133.9 mg/dL vs 301.8 ± 94.9 mg/dL, respectively with a P = 0.06). A similar result was observed for the 11 patients that had pericellular fibrosis (418.2 ± 129.5 mg/dL vs 297.7 ± 91.5 mg/dL; P = 0.001).
Nonalcoholic Activity Score (NAS), a pathological score that was defined originally for the quantitative changes in histology of the liver in treatment trials, has been used in many studies as a tool to diagnose and stage NAFLD patients. Based on this score, we also classified our patients as the following: 16 (32%) simple steatosis, 15 (30%) borderline NASH and 19 (38%) patients had definite NASH. We found no statistically significant difference in IgA levels between the subjects based on NAS score in any combination.
DISCUSSION
Nonalcoholic fatty liver disease has become the world’s most common hepatic disease[9] and is becoming the top reason for liver transplantation[31,32]. Considering the growing incidence of obesity, hyperlipidemia, diabetes and other components of metabolic syndrome worldwide, one could expect that the prevalence of NAFLD will have an incremental pattern for the coming decades. Liver biopsy is the gold standard in the diagnosis and follow-up for the severity of the disease and its prognosis; however, considering the previously mentioned complications, it will be difficult to use this in the huge number potential cases.
In medicine, a biomarker is a measurable characteristic that reflects the severity or presence of some disease state. In other words, it is an indicator of a particular pathological condition or some other physiological state of an organism. Many serological biomarkers have been used in the diagnosis, evaluation and sub-classifying NAFLD cases into benign simple steatosis and more advanced forms of the continuum of the disease[33]. Yet, no single biomarker has been introduced to discriminate this continuum with an acceptable level of potency: many biomarkers with variable characteristics have been tested in this regard.
Immunoglobulin A, a circulating and secretory immunoglobulin, has been tested in NAFLD cases in one study[30]. It has also been studied in other immune-based chronic liver disease, such as autoimmune hepatitis, primary sclerosing cholangitis and primary biliary cirrhosis[34-36]. IgA could be a reliable biomarker for the establishment of early fibrosis in NAFLD subjects. In the current study, we tested serum IgA level in fifty biopsy proven NAFLD cases. This study is the second study to evaluate this biomarker in the context of NAFLD. A literature search identified many studies with conflicting results for the evaluation of various biomarkers. One biomarker should be tested in multiple centers, races and studies and after comparable results are achieved, one could claim the applicability of that particular test or biomarker.
We have focused on this biomarker and have evaluated its’ potency in various aspects of the NAFLD disease. As shown in the present study, comparing a reasonable population of cases and controls, no role could be found for the serum IgA level in the diagnosis of the fatty liver disease; however, this biomarker could be used to distinguish various grades of steatosis. However, when the disease progresses into steatohepatitis and fibrosis begins to be settled in the hepatic tissue, IgA shows its value in significantly dividing cases without fibrosis from those with fibrosis. As far as the liver itself is concerned, simple steatosis is a benign form of the disease and most cases with simple steatosis will stay at that stage and will not progress to the advanced forms of the disease. Some authorities believe that the subjects with advanced forms of the disease are originally different from the simple steatosis cases and these two categories are not the extremes of a continuum[33]. Whatever pathophysiology is accepted, the development of fibrosis is the first insult that happens in the long way to the development of cirrhosis and, eventually, HCC. Considering this issue, any biomarker focusing on this stage of disease should be considered seriously and if approved in parallel studies, could be recommended in daily practice of these patients. One can imagine considering those patients with higher levels of IgA for advanced forms of treatment, if the usual recommendations of diet and exercise do not change the test result.
After accepting IgA as a reliable biomarker in the context of NAFLD, we evaluated the best cutoff point. Tomita et al[30] suggested a cutoff of 315 mg/dL with an AUC of 0.756 as the best point. Our best cutoff point of 360 mg/dL is a little different from the original study. Lower levels of serum IgA will have higher sensitivity (e.g., 269 mg/dL correspond with a sensitivity of around 85%), thus it can be used in the screening protocols; whereas a higher level (e.g., 414 mg/dL with a specificity of 90%) can be used for ruling in of fibrosis in patient approach algorithms.
Analyzing serum IgA is a cheap and available test in most parts of the world. As a non-invasive test, it can be used serially in the evaluation and follow-up of NAFLD cases.
A major limitation of this study was the sample size. This study is the second to focus on the role of IgA in the field of NAFLD. Further studies with larger numbers of cases and among different ethnic groups are recommended to clarify the possible role of IgA for the detection of fibrosis in these patients. Further studies on the changes of IgA levels in NAFLD cases after weight changes and its correlation with blood sugar and lipid profile would be reasonable.
ACKNOWLEDGMENTS
We would like to thank M.A. Pourhoseingholi for his great review and comments on the statistical aspects.
COMMENTS
Background
Non-alcoholic steatohepatitis (NASH), the advanced stage of nonalcoholic fatty liver disease (NAFLD), has tendency to develop into liver cirrhosis. Differentiation of this subtype of the disease from the benign form (i.e., simple steatosis) relies on liver biopsy, which is invasive. Numerous biomarkers have been studied as replacements for liver biopsy in this regard. However, no single, reliable biomarker has been validated as a predictor for NAFLD progression. Immunoglobulin A (IgA) has been shown to be an available, simple, inexpensive biomarker that could indicate liver fibrosis in these patients. Ultimately, it appears that a combination of serum biomarkers and imaging studies could replace liver biopsy in the near future.
Research frontiers
IgA is a well-known subtype of immunoglobulin. Variation of serum IgA levels has been noted in some disorders. Recently, a study concerning the association between increased IgA in the setting of non-alcoholic fatty liver disease with liver fibrosis was performed. Liver fibrosis is a cornerstone in the natural history of fatty liver disease, and if present, denotes a more severe and advanced form of the disease, namely steatohepatitis, which can progress to liver cirrhosis and hepatocellular carcinoma.
Innovations and breakthroughs
The authors have rechecked the association between serum IgA levels and state of NAFLD on a different group of patients who were from a different genetic background and were completely non-alcoholic. They showed that serum IgA could not differentiate NAFLD cases from normal subjects; however, within NAFLD cases it has a well-documented role in separating different levels of liver fibrosis. They also found a different cutoff point for IgA in our cases, which is slightly higher than in the previous study.
Applications
As a substitute for liver biopsy (the gold standard in the evaluation of NAFLD patients), serum IgA can be used to find advanced forms of the disease along with other biomarkers in yet to be described panels.
Terminology
NAFLD is a form of chronic liver disease that is associated with obesity, diabetes and hyperlipidemia. NAFLD has been described as the hepatic manifestation of metabolic syndrome. It encompasses a continuum from simple steatosis to steatohepatitis. In the latter form, varying degrees of inflammation, apoptosis and liver fibrosis are found. Steatohepatitis can progress to liver cirrhosis and is a precursor for hepatocellular carcinoma.
Peer review
The study is well described, seems to be well performed and is supported by good techniques, methods and statistics.
Footnotes
Supported by Mazandaran University of Medical Sciences
P- Reviewer: Fuchs CD, Hatting M, Locatelli I, Wisse E S- Editor: Qi Y L- Editor: Stewart G E- Editor: Liu XM
References
- 1.Vizzutti F, Arena U, Nobili V, Tarquini R, Trappoliere M, Laffi G, Marra F, Pinzani M. Non-invasive assessment of fibrosis in non-alcoholic fatty liver disease. Ann Hepatol. 2009;8:89–94. [PubMed] [Google Scholar]
- 2.Fierbinteanu-Braticevici C, Dina I, Petrisor A, Tribus L, Negreanu L, Carstoiu C. Noninvasive investigations for non alcoholic fatty liver disease and liver fibrosis. World J Gastroenterol. 2010;16:4784–4791. doi: 10.3748/wjg.v16.i38.4784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torres DM, Harrison SA. Diagnosis and therapy of nonalcoholic steatohepatitis. Gastroenterology. 2008;134:1682–1698. doi: 10.1053/j.gastro.2008.02.077. [DOI] [PubMed] [Google Scholar]
- 4.Bedogni G, Miglioli L, Masutti F, Tiribelli C, Marchesini G, Bellentani S. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44–52. doi: 10.1002/hep.20734. [DOI] [PubMed] [Google Scholar]
- 5.Bellentani S, Bedogni G, Miglioli L, Tiribelli C. The epidemiology of fatty liver. Eur J Gastroenterol Hepatol. 2004;16:1087–1093. doi: 10.1097/00042737-200411000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Grandison GA, Angulo P. Can NASH be diagnosed, graded, and staged noninvasively? Clin Liver Dis. 2012;16:567–585. doi: 10.1016/j.cld.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, Grundy SM, Hobbs HH. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 8.Chitturi S, Wong VW, Farrell G. Nonalcoholic fatty liver in Asia: Firmly entrenched and rapidly gaining ground. J Gastroenterol Hepatol. 2011;26 Suppl 1:163–172. doi: 10.1111/j.1440-1746.2010.06548.x. [DOI] [PubMed] [Google Scholar]
- 9.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 10.Ratziu V, Giral P, Charlotte F, Bruckert E, Thibault V, Theodorou I, Khalil L, Turpin G, Opolon P, Poynard T. Liver fibrosis in overweight patients. Gastroenterology. 2000;118:1117–1123. doi: 10.1016/s0016-5085(00)70364-7. [DOI] [PubMed] [Google Scholar]
- 11.Sakugawa H, Nakayoshi T, Kobashigawa K, Yamashiro T, Maeshiro T, Miyagi S, Shiroma J, Toyama A, Nakayoshi T, Kinjo F, et al. Clinical usefulness of biochemical markers of liver fibrosis in patients with nonalcoholic fatty liver disease. World J Gastroenterol. 2005;11:255–259. doi: 10.3748/wjg.v11.i2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powell EE, Jonsson JR, Clouston AD. Dangerous liaisons: the metabolic syndrome and nonalcoholic fatty liver disease. Ann Intern Med. 2005;143:753–754. doi: 10.7326/0003-4819-143-10-200511150-00015. [DOI] [PubMed] [Google Scholar]
- 13.Serfaty L, Lemoine M. Definition and natural history of metabolic steatosis: clinical aspects of NAFLD, NASH and cirrhosis. Diabetes Metab. 2008;34:634–637. doi: 10.1016/S1262-3636(08)74597-X. [DOI] [PubMed] [Google Scholar]
- 14.Milić S, Stimac D. Nonalcoholic fatty liver disease/steatohepatitis: epidemiology, pathogenesis, clinical presentation and treatment. Dig Dis. 2012;30:158–162. doi: 10.1159/000336669. [DOI] [PubMed] [Google Scholar]
- 15.Kleiner DE, Brunt EM. Nonalcoholic fatty liver disease: pathologic patterns and biopsy evaluation in clinical research. Semin Liver Dis. 2012;32:3–13. doi: 10.1055/s-0032-1306421. [DOI] [PubMed] [Google Scholar]
- 16.Vuppalanchi R, Chalasani N. Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: Selected practical issues in their evaluation and management. Hepatology. 2009;49:306–317. doi: 10.1002/hep.22603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Younossi ZM, Jarrar M, Nugent C, Randhawa M, Afendy M, Stepanova M, Rafiq N, Goodman Z, Chandhoke V, Baranova A. A novel diagnostic biomarker panel for obesity-related nonalcoholic steatohepatitis (NASH) Obes Surg. 2008;18:1430–1437. doi: 10.1007/s11695-008-9506-y. [DOI] [PubMed] [Google Scholar]
- 18.Bell LN, Theodorakis JL, Vuppalanchi R, Saxena R, Bemis KG, Wang M, Chalasani N. Serum proteomics and biomarker discovery across the spectrum of nonalcoholic fatty liver disease. Hepatology. 2010;51:111–120. doi: 10.1002/hep.23271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Adams LA, Talwalkar JA. Diagnostic evaluation of nonalcoholic fatty liver disease. J Clin Gastroenterol. 2006;40 Suppl 1:S34–S38. doi: 10.1097/01.mcg.0000168642.38945.f1. [DOI] [PubMed] [Google Scholar]
- 20.Chave G, Milot L, Pilleul F. [Out of phase magnetic resonance imaging and liver applications] J Radiol. 2005;86:993–997. doi: 10.1016/s0221-0363(05)81482-9. [DOI] [PubMed] [Google Scholar]
- 21.Miller MH, Ferguson MA, Dillon JF. Systematic review of performance of non-invasive biomarkers in the evaluation of non-alcoholic fatty liver disease. Liver Int. 2011;31:461–473. doi: 10.1111/j.1478-3231.2011.02451.x. [DOI] [PubMed] [Google Scholar]
- 22.Yoneda M, Mawatari H, Fujita K, Iida H, Yonemitsu K, Kato S, Takahashi H, Kirikoshi H, Inamori M, Nozaki Y, et al. High-sensitivity C-reactive protein is an independent clinical feature of nonalcoholic steatohepatitis (NASH) and also of the severity of fibrosis in NASH. J Gastroenterol. 2007;42:573–582. doi: 10.1007/s00535-007-2060-x. [DOI] [PubMed] [Google Scholar]
- 23.Maleki I, Rastgar A, Hosseini V, Taghvaei T, Rafiei A, Barzin M, Torabizadeh Z, Naghshvar F, Khalilian A. High sensitive CRP and pentraxine 3 as noninvasive biomarkers of nonalcoholic fatty liver disease. Eur Rev for Med Pharmacol Sci. 2014;18:1583–1590. [PubMed] [Google Scholar]
- 24.Crespo J, Cayón A, Fernández-Gil P, Hernández-Guerra M, Mayorga M, Domínguez-Díez A, Fernández-Escalante JC, Pons-Romero F. Gene expression of tumor necrosis factor alpha and TNF-receptors, p55 and p75, in nonalcoholic steatohepatitis patients. Hepatology. 2001;34:1158–1163. doi: 10.1053/jhep.2001.29628. [DOI] [PubMed] [Google Scholar]
- 25.Wieckowska A, McCullough AJ, Feldstein AE. Noninvasive diagnosis and monitoring of nonalcoholic steatohepatitis: present and future. Hepatology. 2007;46:582–589. doi: 10.1002/hep.21768. [DOI] [PubMed] [Google Scholar]
- 26.Yilmaz Y, Dolar E, Ulukaya E, Akgoz S, Keskin M, Kiyici M, Aker S, Yilmaztepe A, Gurel S, Gulten M, et al. Soluble forms of extracellular cytokeratin 18 may differentiate simple steatosis from nonalcoholic steatohepatitis. World J Gastroenterol. 2007;13:837–844. doi: 10.3748/wjg.v13.i6.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Olas K, Butterweck H, Teschner W, Schwarz HP, Reipert B. Immunomodulatory properties of human serum immunoglobulin A: anti-inflammatory and pro-inflammatory activities in human monocytes and peripheral blood mononuclear cells. Clin Exp Immunol. 2005;140:478–490. doi: 10.1111/j.1365-2249.2005.02779.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gonzalez-Quintela A, Alende R, Gude F, Campos J, Rey J, Meijide LM, Fernandez-Merino C, Vidal C. Serum levels of immunoglobulins (IgG, IgA, IgM) in a general adult population and their relationship with alcohol consumption, smoking and common metabolic abnormalities. Clin Exp Immunol. 2008;151:42–50. doi: 10.1111/j.1365-2249.2007.03545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzàlez-Quintela A, Alende MR, Gamallo R, Gonzàlez-Gil P, López-Ben S, Tomé S, Otero E, Torre JA. Serum immunoglobulins (IgG, IgA, IgM) in chronic hepatitis C. A comparison with non-cirrhotic alcoholic liver disease. Hepatogastroenterology. 2003;50:2121–2126. [PubMed] [Google Scholar]
- 30.Tomita K, Teratani T, Yokoyama H, Suzuki T, Irie R, Ebinuma H, Saito H, Hokari R, Miura S, Hibi T. Serum immunoglobulin a concentration is an independent predictor of liver fibrosis in nonalcoholic steatohepatitis before the cirrhotic stage. Dig Dis Sci. 2011;56:3648–3654. doi: 10.1007/s10620-011-1771-2. [DOI] [PubMed] [Google Scholar]
- 31.Singal AK, Guturu P, Hmoud B, Kuo YF, Salameh H, Wiesner RH. Evolving frequency and outcomes of liver transplantation based on etiology of liver disease. Transplantation. 2013;95:755–760. doi: 10.1097/TP.0b013e31827afb3a. [DOI] [PubMed] [Google Scholar]
- 32.Wong RJ, Cheung R, Ahmed A. Nonalcoholic steatohepatitis is the most rapidly growing indication for liver transplantation in patients with hepatocellular carcinoma in the U.S. Hepatology. 2014;59:2188–2195. doi: 10.1002/hep.26986. [DOI] [PubMed] [Google Scholar]
- 33.Yilmaz Y. Review article: is non-alcoholic fatty liver disease a spectrum, or are steatosis and non-alcoholic steatohepatitis distinct conditions? Aliment Pharmacol Ther. 2012;36:815–823. doi: 10.1111/apt.12046. [DOI] [PubMed] [Google Scholar]
- 34.Gabeta S, Norman GL, Liaskos C, Papamichalis PA, Zografos T, Garagounis A, Rigopoulou EI, Dalekos GN. Diagnostic relevance and clinical significance of the new enhanced performance M2 (MIT3) ELISA for the detection of IgA and IgG antimitochondrial antibodies in primary biliary cirrhosis. J Clin Immunol. 2007;27:378–387. doi: 10.1007/s10875-007-9092-0. [DOI] [PubMed] [Google Scholar]
- 35.Gabeta S, Norman GL, Gatselis N, Liaskos C, Papamichalis PA, Garagounis A, Zachou K, Rigopoulou EI, Dalekos GN. IgA anti-b2GPI antibodies in patients with autoimmune liver diseases. J Clin Immunol. 2008;28:501–511. doi: 10.1007/s10875-008-9211-6. [DOI] [PubMed] [Google Scholar]
- 36.Berglin L, Björkström NK, Bergquist A. Primary sclerosing cholangitis is associated with autoreactive IgA antibodies against biliary epithelial cells. Scand J Gastroenterol. 2013;48:719–728. doi: 10.3109/00365521.2013.786131. [DOI] [PubMed] [Google Scholar]


