Abstract
AIM: To detect high risk patients with a progressive disease course of ulcerative colitis (UC) requiring immunosuppressive therapy (IT).
METHODS: A retrospective, multicenter analysis of 262 UC patients from eight German tertiary inflammatory bowel disease centres was performed. Patients were divided into two groups depending on the patients need to initiate immunosuppressive therapy in the disease course. A comparison between the two groups was made with regard to demographics, clinical and laboratory parameters obtained within three months after UC diagnosis and the response to first medical therapy. Using this data, a prognostic model was established to predict the individual patients probability of requiring an immunosuppressive therapy.
RESULTS: In 104 (39.7%) out of 262 patients, UC therapy required an immunosuppressive treatment. Patients in this group were significantly younger at time of diagnosis (HR = 0.981 ± 0.014 per year, P = 0.009), and required significantly more often a hospitalisation (HR = 2.5 ± 1.0, P < 0.001) and a systemic corticosteroid therapy at disease onset (HR = 2.4 ± 0.8, P < 0.001), respectively. Response to steroid treatment was significantly different between the two groups of patients (HR = 5.2 ± 3.9 to 50.8 ± 35.6 compared to no steroids, P = 0.016 to P < 0.001). Furthermore, in the IT group an extended disease (HR = 3.5 ± 2.4 to 6.1 ± 4.0 compared to proctitis, P = 0.007 to P = 0.001), anemia (HR = 2.2 ± 0.8, P < 0.001), thrombocytosis (HR = 1.9 ± 1.8, P = 0.009), elevated C-reactive protein (CRP) (HR = 2.1 ± 0.9, P < 0.001), and extraintestinal manifestations in the course of disease (HR = 2.6 ± 1.1, P = 0.004) were observed. Six simple clinical items were used to establish a prognostic model to predict the individual risk requiring an IT. This probability ranges from less than 2% up to 100% after 5 years. Using this, the necessity of an immunosuppressive therapy can be predicted in 60% of patients. Our model can determine the need for an immunosuppressive drug therapy or if a “watch and wait” approach is reasonable already early in the treatment course of UC.
CONCLUSION: Using six simple clinical parameters, we can estimate the patients individual risk of developing a progressive disease course.
Keywords: Clinical practice, Parameter, Prediction model, Ulcerative colitis, Inflammatory bowel disease
Core tip: We performed a retrospective study to identify patients at risk for a progressive disease course of ulcerative colitis, characterized by the necessity of immunosuppressive treatment. Personal data, clinical and laboratory parameters during the first 3 mo after ulcerative colitis diagnosis and effects of initial medical therapy were evaluated. Six simple clinical items were used to develop a prognostic model predicting such a progressive disease course. Thereby, our model can help in deciding if patients will need immunosuppressive drugs early in the disease course or if a careful watch and wait strategy is justified.
INTRODUCTION
The clinical course of ulcerative colitis (UC) has a wide spectrum of severity. While some patients suffer from a single episode others may experience a chronic or potentially life-threatening disease course. The Inflammatory Bowel South-Eastern Norway study group published a population based prospective study over a period of 5 years. Four different disease courses have been characterized: decreasing symptoms after the first acute onset of disease (44%), increase of inflammation (3%), chronic continuous symptoms (24%) and chronic intermittent symptoms (29%), respectively[1]. The early introduction of immunosuppressive agents (i.e., thiopurines or calcineurin inhibitors) or anti-tumor necrosis factor (TNF)-α antibodies such as infliximab or adalimumab may have a positive impact on the occurrence of acute severe inflammation including complications such as a toxic megacolon or colorectal cancer in the long term. Substantially important may be the effect on the patients quality of life[2,3]. But, patients presenting with a less severe disease course who are treated early on with immunosuppressive agents could be posed with overtreatment. Continuing debates discuss the potential risk of side-effects of immunosuppressants as well as anti-TNF-α antibodies[4]. A population-based study from Olmsted, Minnesota, United States, concluded that 66% of UC patients did not need corticosteroid treatment at all. Furthermore, 49% of UC patients receiving initial corticosteroids after their one year follow up, were corticosteroid free and without the need of surgery[3]. Consequently, identifying those patients exhibiting a progressive disease course with the need for an immunosuppressive treatment as early as possible becomes critical, since therapeutic approaches could alter the course of UC and subsequently the occurrence of complications.
Prediction of a disabling clinical courses was studied in several population-based studies[5]. Young age at diagnosis and female gender were associated with a trend towards more frequent relapses[6]. Further, extensive colitis (defined as upper limit of macroscopic lesions proximal to the splenic flexure)[1,6,7] and parameters reflecting a systemic involvement at initial presentation (fever, weight loss)[7] resulted in a higher risk of colectomy within 10 years after diagnosis. Thus far, non-invasive, easily available and reliable methods, early on, have not been established nor introduced into a clinical algorithm in order to identify patients at a higher probability for a severe disease course.
Therefore, our goal was to discover and implement simple clinical parameters early after diagnosis of UC into an algorithm, predicting a severe disease course and the individual patients need for immunosuppressive therapy.
MATERIALS AND METHODS
Patients
We performed a retrospective, multicenter analysis in UC patients from eight German inflammatory bowel disease (IBD) centers. Besides gastroenterological outpatient clinics from four university clinics (University Clinic Jena, Charité Universitätsmedizin Berlin: Campus Virchow-Klinikum and Campus Benjamin Franklin, respectively, and Christian Albrechts-University, Kiel), one community-based hospital (Evangelisches Krankenhaus Kalk, Köln) and three specialized IBD private practices (Gastroenterologische Gemeinschaftspraxis, Herne, Gastroenterologische Gemeinschaftspraxis Minden and Internistische Gemeinschaftspraxis für Verdauungs- und Stoffwechselkrankheiten, Leipzig) were involved in the study. Patients diagnosed with UC between January 2003 and February 2008 with a follow-up period of at least 6 mo after diagnosis or with initiation of immunosuppressive therapy after at least three months after diagnosis of the disease were included in the study.
The need for immunosuppressive therapy served as a surrogate parameter of a progressive disease course in UC patients. Our patients were classified into two groups depending on requiring immunosuppressive therapy or not. We choose this parameter to differentiate two groups of patients (simple vs progressive disease course), as IT summarizes different unfavourable disease courses in only one parameter that is consistently documented in a patients’ health record. Immunosuppressive therapy contains thiopurines, methotrexate, anti-TNF-α antibodies, as well as cyclosporine A, and tacrolimus. Of note, topical (budesonide) and systemic corticosteroids were not regarded as “immunosuppressive therapy”. Further details on the frequency of the specific medication prescribed are provided in the section “immunosuppressive therapy” in the results section. Immunosuppressive therapy was initiated by physicians highly experienced in IBD treatment if UC patients underwent two or more flares within a time period of 12 mo. The definitions of active disease and remission followed the guidelines from the German Society Digestive Diseases and European Crohn’s and Colitis Organization[8,9]. Patients were excluded from the study in case of missing informed consent, or in case of loss of follow-up.
Medical records were investigated in order to identify patients who subsequently exhibited a severe course of the disease requiring immunosuppressive therapy. Personal data such as date of first diagnosis, date of first symptoms, gender, smoking habits and family history of IBD as well as clinical parameters available during an early phase after the initial diagnosis of UC were recorded. Moreover, extension of the disease, extraintestinal manifestations (articular, ocular and cutaneous manifestations, respectively), fever at the first flare of UC, abdominal tenderness, and laboratory parameters (hemoglobin, thrombocytes and CRP level) were investigated, too. Furthermore, we evaluated necessity, time of initiation, kind and effect of oral steroid therapy.
Ethical statement
The study was approved by the ethics committee of the University Hospital Jena (2104-08/07) and was performed in agreement with the principles of the Declaration of Helsinki.
Statistical analysis
Data was analyzed using SPSS 19.0 to identify significant differences between patients in need of immunosuppressive agents and those without. Each variable was analyzed using univariate Cox regression with a level of significance set at α ≤ 0.05 (2-sided). Welch’s t-test was used to determine temporal differences.
The multivariate Cox model was used to discriminate the influence of several concurrent risk factors indicating the need for immunosuppressive therapy. The event in this survival analysis was the requirement of immunosuppressive treatment. The Cox regression is based on the assumption that the effects of variables on survival (necessity for immunosuppressants) are constant over time (proportional hazards assumption). From the estimated regression parameters β1, ¡, β6 for the identified six risk factors x1, ¡, x6 the survival probability at time point t, S0(t) ist the survival function S(t, x, β) = S0(t)eβ1*x1 + ... + β6*x6 of the baseline population (x1 = 0, ¡, x6 = 0), which is also estimated in the model[10].
H0(t) is the baseline hazard at time point t, which is also estimated in the model.
RESULTS
In this study a total of 262 UC patients from gastroenterological University hospitals and community outpatient clinics as well as private practices were investigated.
Immunosuppressive therapy
In 104 patients (39.7%) the progress of UC disease required an immunosuppressive therapy. Ninety-eight (94.2%) of these patients received azathioprine, 23 (22.1%) 6-mercaptopurine, 36 (34.6%) infliximab, 3 (2.9%) adalimumab, and 1 (1.0%) received golimumab. The total count exceeds 100%, since several immunosuppressive drugs were combined (Table 1).
Table 1.
Patient characteristics
| Patient characteristics | n (%) |
| Age at diagnosis (yr) | 262 (34) |
| Gender | |
| Male | 127 (48.5) |
| Female | 135 (51.5) |
| Extent of the disease | |
| Proctitis | 43 (16.4) |
| Proctosigmoiditis | 47 (17.9) |
| Left-sided colitis | 50 (19.1) |
| Pancolitis | 114 (43.5) |
| Unknown | 8 (3.0) |
| Extraintestinal manifestations | |
| Eyes | 2 (0.8) |
| Skin | 6 (2.3) |
| Joints | 50 (19.1) |
| Immunosuppressive therapy | |
| Azathioprine | 98 (94.2) |
| 6-Mercaptopurine | 23 (22.1) |
| Infliximab | 36 (34.6) |
| Adalimumab | 3 (2.9) |
| Golimumab | 1 (1.0) |
| Methotrexate | 3 (2.9) |
| Miscellaneous | 12 (11.5) |
| Reason for IT | |
| Steroid-refractory disease course | 40 (38.4) |
| Chronic-active disease course | 31 (29.8) |
| Steroid-dependent disease course | 27 (26.0) |
| Miscellaneous | 6 (5.8) |
IT: Immunosuppressive treatment.
The amount of immunosuppressive agents an individual patient is shown in Figure 1. The initiation of an immunosuppressive therapy was necessary due to different factors: 38.4% of the patients received an immunosuppressive therapy due to steroid resistance, 29.8% of the patients experienced a chronic active disease course while 26.0% had a steroid dependent disease.
Figure 1.
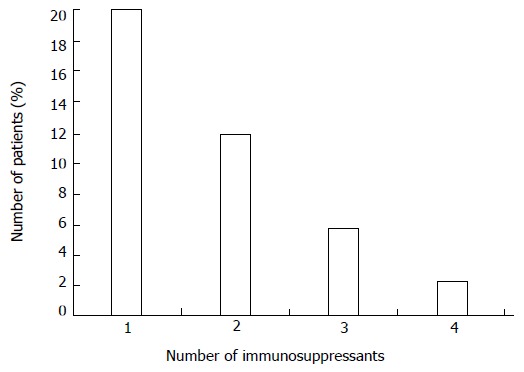
Representation of the number of immunosuppressive drugs per patient during follow-up.
Demographic parameters
At time of diagnosis patients with a progressive disease course were significantly younger at diagnosis (33.7 years vs 38.5 years, P = 0.011) compared with the patients without need for an immunosuppressive therapy. More precisely, if a patient was 10 years older at time of diagnosis, the need of initiating an immunosuppressive therapy was decreased by 19%. The study involved 48.5% male and 51.5% female patients with UC. Interestingly, gender was not associated with a significant higher risk probability for immunosuppressive treatment (male gender HR = 1, female gender HR = 1.33, P = 0.151). 10.7% of patients in our study group were smokers, while 41.2% were non-smokers and 10.3% were former smokers. Smoking status was unknown in 37.8% of patients. Notably, non-smoking patients did not have a higher probability for a progressive disease course requiring immunosuppressive drugs (P = 0.5).
Family history of inflammatory bowel disease was not associated with an increased risk for an immunosuppressive therapy (P = 0.973). In this cohort 8.0% of the patients had a first degree-relative suffering from IBD.
Disease specific parameters
We investigated the possible correlation between the extent of inflamed intestinal areas and the need for immunosuppressive treatment. Most of the patients presented with pancolitis (43.5%) followed by left-sided colitis (19.1%), proctosigmoiditis (17.9%) and proctitis (16.4%). Compared to patients with proctitis each of the other subgroups showed a significantly increased risk of requiring immunosuppressive therapy (P < 0.001). Presence of backwash-ileitis (only 4.2% of patients) showed a trend towards increased necessity of immunosuppressants in the course of the disease (P = 0.054).
Notably, patients with a high disease activity requiring hospitalisation at diagnosis (30.9%) significantly more often received immunosuppressive medication subsequently (HR = 2.47, P ≤ 0.001).
Only individual patients were suffering from extraintestinal manifestations (EIM) at diagnosis. For these patients, there was no significant difference in requiring immunosuppressive agents consecutively (P = 0.482). However, the occurence of extraintestinal manifestations increased during the course of UC. Articular manifestations (19.1%) were common, whereas cutaneous (1.5%) or ocular (0.8%) manifestations were described quite rarely. Patients suffering from extraintestinal manifestations in the course of UC significantly more often required immunosuppressive treatment than patients without EIM (P = 0.004). Accordingly, the number of EIM correlated with the necessity of immunosuppressive therapy (P < 0.001).
Steroid therapy
In total 174 patients (66.4%) required corticosteroids throughout the course of UC. Of those, 76 patients (29% of the entire study population) necessitated corticosteroids at the first flare of the disease.
We identified multiple differences with regard to steroid medication in patients with or without a following need for immunosuppressive treatment. Patients requiring a treatment with immunosuppressants in the course of UC significantly more often necessitated corticosteroids at the time of diagnosis (HR = 2.4, P < 0.001) and throughout the disease course (HR = 19, P < 0.001). Response to steroid therapy was also significantly associated with subsequent initiation of immunosupressive treatment (P < 0.001; Figure 2). Patients with no response to steroid therapy had a significantly higher need for immunosuppressive treatment (HR = 2.139, P = 0.002). Conversely, remission under steroid therapy was correlated with a reduced necessity of immunosuppressive therapy (HR = 0.23, P = 0.014).
Figure 2.
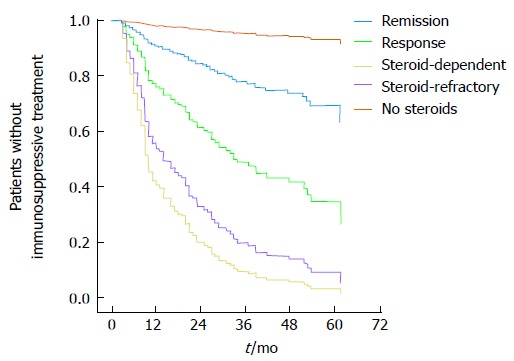
Statistically different necessity of immunosuppressive treatment in the course of the disease depending on response to steroid therapy.
Notably, patients necessitating steroid therapy within 1 year after onset of symptoms significantly more often required immunosuppressive treatment than patients being treated with steroids more than 12 mo after onset of symptoms (HR = 14.1, P ≤ 0.001).
In patients with a consecutive need for immunosuppressive agents, the time between steroid treated diseases episodes was significantly shorter (29 wk vs 55 wk, P = 0.007). The clinical response (remission, response or no effect, respectively) to steroid therapy at diagnosis showed a significant correlation with future need of immunosuppressive agents in univariate (P < 0.001) as well as in multivariate Cox regression analysis (P < 0.001).
Clinical and laboratory parameters
Patients with a severe or a mild UC disease course did not show significant differences with regard to fever at onset (2.3%) or at diagnosis (4.2%) and abdominal tenderness at first physical examination (0.8%) (P > 0.05).
Anemia (HR = 2.2, P ≤ 0.001), elevated CRP (HR = 2.11, P ≤ 0.001) and thrombocytosis (HR = 1.93, P ≤ 0.01) in the course of the disease were shown more often in patients with UC requiring immunosuppressive therapy. However, at time of diagnosis there were no significant differences concerning anemia, elevated CRP-levels and thrombocytosis comparing patients with or without future need for immunosuppressive treatment.
Multivariate Cox regression analysis
Using multivariate Cox regression analysis we detected six parameters available in the early course of UC, which allow us to predict the risk for the individual patient′s need for immunosuppressive treatment: (1) age at diagnosis; (2) gender; (3) extent of UC at initial presentation; (4) requirement to hospitalize a patient at diagnosis; (5) use of steroids at diagnosis; and (6) response to steroid therapy. A detailed representation of these parameters is shown in Table 2. Using these six parameters we established a prognostic model enabling us to assess the individual patient′s probability requiring immunosuppressants in the course of the disease. According to the established model, we illustrated three examples showing the probability to require immunosuppressive therapy (Figure 3). Most importantly, based on this retrospectively developed model we would be able to draw clinically meaningful conclusions in more than 60% of our patients. 20.2% of patients experience a mild disease course with a cumulative probability to require immunosuppressive drugs during the following 5 years of less than 20%. However, we would be able to identify 39.9% of patients with a probability of more than 80% to require immunosuppressants during the subsequent five years.
Table 2.
Independent parameters associated with a severe course of ulcerative colitis, predicting the risk for subsequent necessity of immunosuppressive therapy (multivariate analysis)
| Parameter | OR | 95%CI | P value |
| Age at diagnosis | 0.981 (per year) | 0.967-0.995 | 0.009 |
| Gender (female) | 1.3 | 0.9-2.0 | 0.156 |
| Steroid therapy at diagnosis | 2.4 | 1.6-3.7 | < 0.001 |
| Hospitalisation at diagnosis | 2.5 | 1.5-4 | < 0.001 |
| Extent of the disease | < 0.001 | ||
| Proctitis | 1 | ||
| Procto-sigmoiditis | 3.5 | 1.4-8.6 | 0.007 |
| Left-sided colitis | 5.3 | 1.6-15.3 | 0.002 |
| Pancolitis | 6.1 | 2.1-17.5 | 0.001 |
| Result of steroid therapy | < 0.001 | ||
| No steroids | 1 | ||
| Remission | 5.2 | 1.4-20.3 | 0.016 |
| Response | 15.3 | 4.7-50 | < 0.001 |
| Steroid-dependent | 50.8 | 15.2-170 | < 0.001 |
| Steroid-refractory | 34.8 | 10.5-114.8 | < 0.001 |
Figure 3.
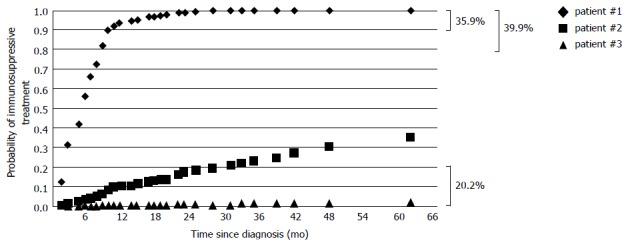
Results of our model to calculate the individual probability of immunosuppressive treatment depending on the identified risk factors; representation of three hypothetical patients. Right handside: Percentage of patients with a probability to require immunosuppressive treatment (IT) no more than 20%, at least 80% and at least 90%, respectively (Patient #1: female; 19 years at diagnosis; pancolitis; hospitalisation and steroids at diagnosis, steroid-refractory; Patient #2: male; 28 years at diagnosis, left-sided colitis; hospitalisation and steroids at diagnosis; remission; Patient #3: male; 47 years at diagnosis; proctitis; no hospitalisation and no steroids at diagnosis).
DISCUSSION
The objective of this study was to identify clinical parameters which allow to predict a severe disease characterized by the necessity for an immunosuppressive treatment early in the course of UC. By investigating patients from specialized IBD centres only, we could include a substantial number of patients receiving immunosuppressants, and we thus feel confident to have identified effective clinical parameters predicting a severe course of UC.
As a first important result our study showed that UC patients requiring hospitalization at diagnosis more often necessitated immunosuppressive therapy in the course of the disease (OR = 2.469; 95%CI: 1.513-4.032). In line with these findings, in a retrospective case-control study of 246 patients the requirement to hospitalize a patient to control disease activity was an independent risk factor of future colectomy (OR = 5.37; 95%CI: 2.00-14.46)[11]. Patients in our cohort requiring immunosuppressive agents were treated significantly more often with systemic corticosteroids at the first flare of the disease, had a lower probability of remission upon steroid therapy and did not respond to steroid treatment more frequently compared to patients without consecutive immunosuppressive therapy.
Secondly, we identified that disease extent by endoscopic assessment was an independent predictor of a complicated disease during follow-up. Using isolated rectal involvement as a comparator, the risk of requiring IT increased with disease extent from procto-sigmoiditis (OR = 3.84), through left-sided colitis (OR = 5.3) to pancolitis (OR = 6.1). It is well accepted, that endoscopic assessment is of central importance in diagnostic algorithms of UC, and that it can be used to predict disease behaviour[12]. Several groups described that an extensive disease in UC is correlated with a severe disease course, as well as with failure of medical therapy and with a higher rate of colectomy[13,14]. Even if the extent of disease at diagnosis predicts the consecutive need of immunosuppressive agents and the probability of requiring a colectomy, it may not influence the risk of relapse[15]. Farmer and co-workers described that patients with left-sided colitis or pancolitis experienced significantly higher frequencies of complications (severe or fulminant colitis, toxic dilatation or surgery) compared with those with proctitis only[16].
Third, in our cohort younger age at diagnosis has been identified as an independent parameter correlated with a higher risk for immunosuppressive treatment. Younger age at diagnosis has been previously shown to serve as a predictor of a more complicated disease course associated with more severe diarrhea, pancolitis and use of corticosteroids[17,18]. Complementary, patients who are diagnosed with UC at the age of 45 years or older experience fewer relapses[19]. An explanation could be that patients with a strong genetic background and multiple environmental risk factors will present earlier in their course of disease.
Several parameters such as family history of UC, cigarette smoking, presence of backwash ileitis, fever at diagnosis, extraintestinal manifestations at diagnosis, anemia, elevated CrP or thrombocytosis at diagnosis that we expected to predict a severe disease course according to other studies[5,20,21] we could not confirm in our study. Two reasons for these apparent differences may be the low frequency of some events in our study population (e.g., presence of EIM at diagnosis) and a lack of data in several patients due to the retrospective design (e.g., smoking status).
Based on the identification of several risk factors for subsequent IT, we developed a prognostic model consisting of six simple clinical parameters allowing to identify patients requiring immunosuppressive therapy in the future. The identified criteria were: (1) requirement of hospitalization at diagnosis; (2) use of steroids at diagnosis; (3) response to steroid therapy; (4) initial extent of UC; (5) age at diagnosis; and (6) gender. These parameters combined in a prognostic model allow us to predict a progressive disease course of UC characterized by the necessity of an immunosuppressive treatment. These parameters are easily accessible early in a patient’s medical history and therefore allow us to predict the further course of disease in clinical practice on an individual basis.
Prognostic scores should be reliable and applicable to the majority of patients. Using our predictive model it was possible to predict the disease course within the subsequent five years in a clinically useful manner in 60% of patients. Especially, for 40% of patients with an unfavourable prognosis, our model provides the basis for an accelerated therapeutic approach using immunosuppressants and anti-TNF antibodies to potentially prevent progression of UC. In another 20% of patients our model can exclude the necessity of IT with 80% probability, preventing these patients from treatment with potentially harmful substances.
Our study design has method-inherent weaknesses and strengths. The development of a simple model, using easily accessible clinical parameters early in the disease course, is a major strength. This allows the physician to predict the individual patient’s risk of developing a progressive disease course. Subsequently, this assessment can potentially influence clinical decision making. Moreover, the patient cohort includes a well balanced mixture of patients from tertiary referral centers and also highly experienced private practices, therefore not predisposed to selection bias. The involvement of dedicated IBD physicians ensured a high data quality. Since the study is designed retrospectivly, this could be considered as one potential weakness.
In conclusion, extent of disease, young age, hospitalization and steroid therapy at diagnosis and insufficient response to corticosteroid therapy are independently correlated with a more severe disease course in UC. The model we have developed in this study is based on easily accessible clinical parameters and enables the quantification of an individual UC patient′s probability to develop a progressive course of the disease. Therefore our model can aid in clinical decision making. If all six risk factors were present, the risk of developing a progressive disease course requiring immunosuppressive therapy was almost 100% during the subsequent period of five years. Concurrently, in the absence of any risk factor the probability was nearly 0%. Therefore, our model does not only provide statistically significant differences but it provides the opportunity to estimate the future need for immunosuppressive treatment in daily clinical practice.
COMMENTS
Background
Ulcerative colitis (UC) is a chronic inflammatory disorder of the gastrointestinal tract presenting with diarrhea, bloody stools and abdominal discomfort. The clinical course of the disease is variable ranging from a single episode of the disease to a chronic relapsing or chronic continuous disease activity. Finally, in a significant amount of patients with a severe disease course proctocolectomy may become necessary as a last therapeutic option.
Research frontiers
Patients with predictors of a disabling disease course of UC should be treated with immunosuppressants. But, in order to identify patients at a higher probability for a severe disease course at an early stage non-invasive, easily available and reliable parameters have not been established nor introduced into a clinical algorithm thus far.
Innovations and breakthroughs
The authors performed a retrospective, multicenter analysis of 262 UC patients from eight German tertiary inflammatory bowel disease centres. Personal data, clinical and laboratory parameters obtained during the first 3 mo after UC diagnosis and effects of initial medical treatment were evaluated. From this analysis, the authors identified 6 independent clinical parameters (age at diagnosis, gender, necessity of steroids or hospitalisation at diagnosis, extent of the disease and result of an initial steroid therapy) that can be identified early in an individual disease course. Using these parameters, the authors established a prognostic model to calculate the individuals probability of requiring immunosuppressive treatment (as a parameter of severe disease activity).
Applications
The model the authos developed uses six simple clinical parameters allowing an individualized estimation of a patient’s risk for experiencing a severe disease course. Using our model the treating physician can estimate the need for a immunosuppressive drug therapy early in the course of UC.
Terminology
Immunosuppressant: A drug that suppresses the immune system in order to reduce disease activity and clinical symptoms of the disease. Different immunosuppressants have been associated with side-effects like infections or an elevated risk of tumour development.
Peer review
The data does not only show statistically significant results, but the model provides the opportunity to estimate the future need for immunosuppressants in daily clinical practice. This paper reads well and the data is convincing.
Footnotes
P- Reviewer: M’Koma A S- Editor: Ding Y L- Editor: A E- Editor: Du P
References
- 1.Solberg IC, Lygren I, Jahnsen J, Aadland E, Høie O, Cvancarova M, Bernklev T, Henriksen M, Sauar J, Vatn MH, et al. Clinical course during the first 10 years of ulcerative colitis: results from a population-based inception cohort (IBSEN Study) Scand J Gastroenterol. 2009;44:431–440. doi: 10.1080/00365520802600961. [DOI] [PubMed] [Google Scholar]
- 2.Colombel JF, Rutgeerts P, Reinisch W, Esser D, Wang Y, Lang Y, Marano CW, Strauss R, Oddens BJ, Feagan BG, et al. Early mucosal healing with infliximab is associated with improved long-term clinical outcomes in ulcerative colitis. Gastroenterology. 2011;141:1194–1201. doi: 10.1053/j.gastro.2011.06.054. [DOI] [PubMed] [Google Scholar]
- 3.Cosnes J, Gower-Rousseau C, Seksik P, Cortot A. Epidemiology and natural history of inflammatory bowel diseases. Gastroenterology. 2011;140:1785–1794. doi: 10.1053/j.gastro.2011.01.055. [DOI] [PubMed] [Google Scholar]
- 4.Stallmach A, Hagel S, Bruns T. Adverse effects of biologics used for treating IBD. Best Pract Res Clin Gastroenterol. 2010;24:167–182. doi: 10.1016/j.bpg.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Beaugerie L, Sokol H. Clinical, serological and genetic predictors of inflammatory bowel disease course. World J Gastroenterol. 2012;18:3806–3813. doi: 10.3748/wjg.v18.i29.3806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henriksen M, Jahnsen J, Lygren I, Vatn MH, Moum B. Are there any differences in phenotype or disease course between familial and sporadic cases of inflammatory bowel disease? Results of a population-based follow-up study. Am J Gastroenterol. 2007;102:1955–1963. doi: 10.1111/j.1572-0241.2007.01368.x. [DOI] [PubMed] [Google Scholar]
- 7.Langholz E, Munkholm P, Davidsen M, Binder V. Course of ulcerative colitis: analysis of changes in disease activity over years. Gastroenterology. 1994;107:3–11. doi: 10.1016/0016-5085(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 8.Dignass A, Preiss JC, Aust DE, Autschbach F, Ballauff A, Barretton G, Bokemeyer B, Fichtner-Feigl S, Hagel S, Herrlinger KR, et al. Updated German guideline on diagnosis and treatment of ulcerative colitis, 2011. Z Gastroenterol. 2011;49:1276–1341. doi: 10.1055/s-0031-1281666. [DOI] [PubMed] [Google Scholar]
- 9.Dignass A, Van Assche G, Lindsay JO, Lémann M, Söderholm J, Colombel JF, Danese S, D’Hoore A, Gassull M, Gomollón F, et al. The second European evidence-based Consensus on the diagnosis and management of Crohn’s disease: Current management. J Crohns Colitis. 2010;4:28–62. doi: 10.1016/j.crohns.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Hosmer DW, Lemeshow S. Applied Survival Analysis. Regression Modeling of Time to Event Data. New York: Wiley; 1999. pp. 92–93. [Google Scholar]
- 11.Ananthakrishnan AN, Issa M, Beaulieu DB, Skaros S, Knox JF, Lemke K, Emmons J, Lundeen SH, Otterson MF, Binion DG. History of medical hospitalization predicts future need for colectomy in patients with ulcerative colitis. Inflamm Bowel Dis. 2009;15:176–181. doi: 10.1002/ibd.20639. [DOI] [PubMed] [Google Scholar]
- 12.Allez M, Lémann M. Role of endoscopy in predicting the disease course in inflammatory bowel disease. World J Gastroenterol. 2010;16:2626–2632. doi: 10.3748/wjg.v16.i21.2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carbonnel F, Lavergne A, Lémann M, Bitoun A, Valleur P, Hautefeuille P, Galian A, Modigliani R, Rambaud JC. Colonoscopy of acute colitis. A safe and reliable tool for assessment of severity. Dig Dis Sci. 1994;39:1550–1557. doi: 10.1007/BF02088063. [DOI] [PubMed] [Google Scholar]
- 14.Ho GT, Mowat C, Goddard CJ, Fennell JM, Shah NB, Prescott RJ, Satsangi J. Predicting the outcome of severe ulcerative colitis: development of a novel risk score to aid early selection of patients for second-line medical therapy or surgery. Aliment Pharmacol Ther. 2004;19:1079–1087. doi: 10.1111/j.1365-2036.2004.01945.x. [DOI] [PubMed] [Google Scholar]
- 15.Lau A, Chande N, Ponich T, Gregor JC. Predictive factors associated with immunosuppressive agent use in ulcerative colitis: a case-control study. Aliment Pharmacol Ther. 2008;28:606–613. doi: 10.1111/j.1365-2036.2008.03772.x. [DOI] [PubMed] [Google Scholar]
- 16.Farmer RG, Easley KA, Rankin GB. Clinical patterns, natural history, and progression of ulcerative colitis. A long-term follow-up of 1116 patients. Dig Dis Sci. 1993;38:1137–1146. doi: 10.1007/BF01295733. [DOI] [PubMed] [Google Scholar]
- 17.Etchevers MJ, Aceituno M, García-Bosch O, Ordás I, Sans M, Ricart E, Panés J. Risk factors and characteristics of extent progression in ulcerative colitis. Inflamm Bowel Dis. 2009;15:1320–1325. doi: 10.1002/ibd.20897. [DOI] [PubMed] [Google Scholar]
- 18.Lee JH, Cheon JH, Moon CM, Park JJ, Hong SP, Kim TI, Kim WH. Do patients with ulcerative colitis diagnosed at a young age have more severe disease activity than patients diagnosed when older? Digestion. 2010;81:237–243. doi: 10.1159/000253850. [DOI] [PubMed] [Google Scholar]
- 19.Ardizzone S, Cassinotti A, Duca P, Mazzali C, Penati C, Manes G, Marmo R, Massari A, Molteni P, Maconi G, et al. Mucosal healing predicts late outcomes after the first course of corticosteroids for newly diagnosed ulcerative colitis. Clin Gastroenterol Hepatol. 2011;9:483–489.e3. doi: 10.1016/j.cgh.2010.12.028. [DOI] [PubMed] [Google Scholar]
- 20.Yarur AJ, Strobel SG, Deshpande AR, Abreu MT. Predictors of aggressive inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2011;7:652–659. [PMC free article] [PubMed] [Google Scholar]
- 21.Blonski W, Buchner AM, Lichtenstein GR. Clinical predictors of aggressive/disabling disease: ulcerative colitis and crohn disease. Gastroenterol Clin North Am. 2012;41:443–462. doi: 10.1016/j.gtc.2012.01.008. [DOI] [PubMed] [Google Scholar]


