Abstract
A case is presented of a 36-year-old male with primary sclerosing cholangitis-associated inflammatory bowel disease (PSC-IBD) and two synchronous stage 1 adenocarcinomata of the colon, who was initially treated with a subtotal colectomy with ileostomy. One year later, the patient presented with extensive intra-abdominal lymphadenopathy and peritoneal carcinomatosis, as well as a markedly elevated serum level of alpha-fetoprotein (AFP). Fine needle aspiration biopsy of a porta hepatis lymph node revealed a metastatic hepatoid adenocarcinoma. Subsequent review of the previous colectomy specimen showed that one of the previously identified adenocarcinomata had features suggestive of a hepatoid colonic adenocarcinoma. The patient was subsequently treated with a cytotoxic regimen of FOLFOX (oxaliplatin, leucovorin, 5-fluorouracil) and bevacizumab, with stable results being achieved after six months. This case presents the first known report of PSC-IBD associated with synchronous typical and hepatoid adenocarcinomata of the colon and highlights the importance of considering hepatoid adenocarcinoma as a differential diagnosis in patients with an increasing serum AFP level.
Keywords: Hepatoid adenocarcinoma, Inflammatory bowel disease, Colon cancer, Alpha-fetoprotein, Ulcerative colitis, Primary sclerosing cholangitis, Recurrence, Metastasis
Core tip: This is the first reported case of a synchronous presentation of typical and hepatoid adenocarcinomata of the colon in a patient with long-standing ulcerative colitis and primary sclerosing cholangitis. This report addresses the clinical importance of probing for hepatoid adenocarcinomata in patients with increased serum alpha-fetoprotein levels in the absence of hepatocellular carcinoma.
INTRODUCTION
Hepatoid adenocarcinoma (HA) is a rare neoplasm, which was first described in the stomach by Bourreille et al[1] and termed by Ishikura et al[2] after reporting a similar case in 1985. The frequent occurrence of HA in the stomach has been attributed to the common embryologic origin of the stomach and liver from the foregut, though it can occur in other organs, including the colon, genitourinary tract, thymus, biliary system, esophagus, pancreas, peritoneum, kidney, ovary or lung[3]. The histologic features and biochemical profile of hepatoid tumors are reminiscent of hepatocellular carcinoma (HCC)[4,5]. HA are composed of polygonal tumor cells arranged in both trabecular and glandular structures[6], and can be identified with alpha-fetoprotein (AFP), hepatocyte specific antigen (HSA), and MOC31 antibodies, which usually do not react with hepatocytes[3]. This case report describes the discovery of a metastatic HA in the colon of a patient with primary sclerosing cholangitis (PSC)-associated inflammatory bowel disease (IBD).
CASE REPORT
A 36-year-old Caucasian male was first diagnosed with ulcerative pancolitis at the age of 24 and treated with mesalamine. Eight years after the initial diagnosis, the patient became jaundiced and imaging of his bile duct showed changes typical of PSC. A subsequent dysplasia surveillance colonoscopy showed a polypoid lesion within the proximal transverse colon, along with chronic inflammatory mucosal changes. Biopsies of the lesion revealed an infiltrating, moderately differentiated colonic adenocarcinoma. The patient then underwent a subtotal colectomy and ileostomy with a Roux-en-Y hepaticojejunostomy.
Although only one lesion was documented during colonoscopy, histopathologic examination of the resection specimen showed two completely excised synchronous colonic tumors, 9 cm apart, within the transverse colon. The first tumor measured 2 cm in size and showed a high-grade (poorly differentiated) colonic adenocarcinoma, characterized by solid sheets of neoplastic cells arising from dysplastic surface epithelium. The second larger tumor (4.7 cm) had a low-grade histologic appearance, composed of moderately differentiated colonic glands invading into the superficial portions of the submucosa. Both lesions invaded the submucosa but did not penetrate the muscularis propria, and thus were staged as pT1 lesions. Lymphatic or venous invasion was not observed (via D2-40 and CD31 staining) and 45 regional colonic lymph nodes were negative for metastatic disease. The background colonic mucosa showed chronic active ulcerative pancolitis with minimal severity.
The histologic staging and review by a multidisciplinary team did not indicate adjuvant chemotherapy. However, four months after the initial colectomy, the patient developed ascites and was found to be cirrhotic. An enlarging left renal perihilar lymph node identified during a pre-liver transplant work-up was monitored for six months by serial image scanning. A positron emission tomography (PET) scan revealed multiple PET-positive paraaortic lymph nodes and one left inguinal lymph node, which was suspicious for malignancy. However, no neoplastic changes were observed in an excisional biopsy of the left inguinal lymph node. The only abnormal biomedical value coinciding with the PET-positive result was an abnormal AFP value of 4896 μg/L that was encountered during the standard HCC screening protocol. Moreover, the patient’s AFP levels had slowly but steadily increased over the previous twelve months. A biphasic abdominal computed tomography (CT) scan and hepatic magnetic resonance imaging both failed to show any hepatic mass lesions.
Sixteen months after the initial colectomy, the patient underwent an endoscopic ultrasound and a fine needle aspiration biopsy of the largest porta hepatis lymph node. Histologic examination showed the lymph node to be positive for metastatic carcinoma, as indicated by intermediate-sized dysplastic cells with vacuolated cytoplasm and occasional markedly enlarged malignant nuclei-forming lumina (Figure 1A). Immunohistochemistry revealed that the tumor cells were positive for AFP (Figure 1B) and strongly positive for caudal-related homeobox transcription factor 2 (CDX-2) (Figure 1C). In addition, staining for HSA was unequivocal (Figure 1D). These histopathologies were consistent with a metastasis of the known primary colonic tumor, and the AFP positivity suggested a hepatoid variant. A subsequent review of the original colonic specimens revealed that the smaller, poorly differentiated tumor was in fact composed of polygonal-shaped cells with granular eosinophilic cytoplasm, centrally located nuclei, and prominent nucleoli (Figure 2A, B) that were immune-positive for AFP (inset of Figure 2B), HSA, and CDX-2. In contrast, the second tumor identified from the original specimen was negative for AFP and HSA immunostaining, and showed a more typical colonic morphologic differentiation (Figure 2C, D). This indicated that the tumor in the porta hepatis node represented a metastasis of the colonic HA.
Figure 1.
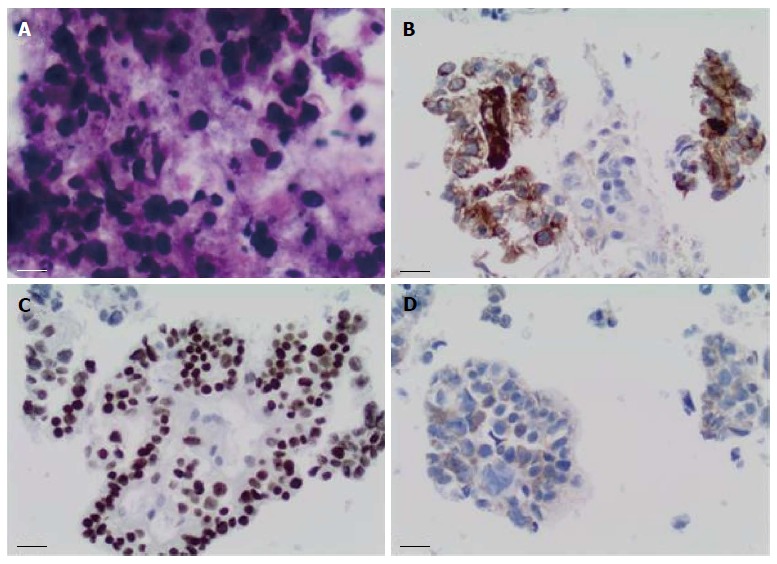
Fine needle aspiration biopsy of enlarged porta hepatis node. A: Papanicolaou staining showed multiple intermediate-sized dysplastic cells with vacuolated cytoplasm and occasional markedly-enlarged malignant nuclei-forming lumina (× 40 magnification); B: Immunohistochemistry showed that tumor cells stained positive for alpha-fetoprotein (× 20 magnification); C: Tumor cells showed strong nuclear immunostaining for caudal-related homeobox transcription factor 2 (× 20 magnification); D: Staining for hepatocyte-specific antigen was unequivocal (× 20 magnification).
Figure 2.
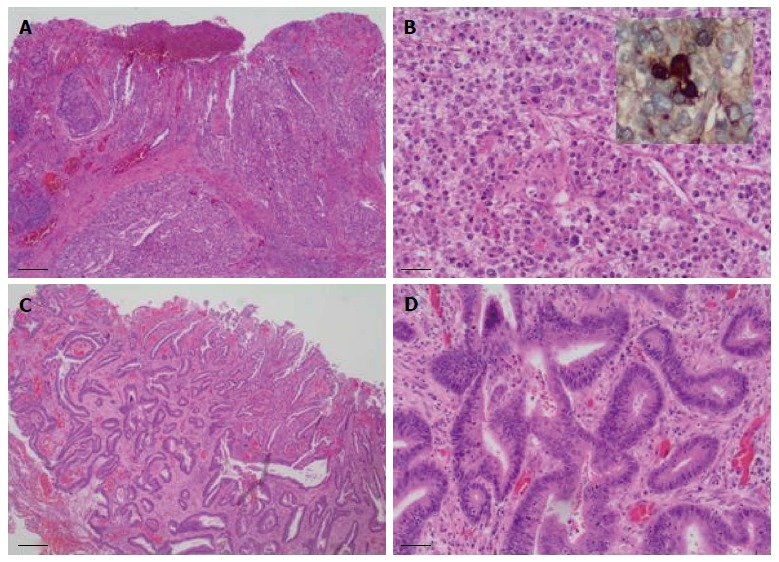
Colectomy specimen showed two synchronous tumors with divergent histologic differentiation. A: The larger tumor was poorly differentiated and composed of sheets of polygonal-shaped cells with granular eosinophilic cytoplasm, centrally located nuclei and prominent nucleoli (hematoxylin and eosin staining, × 40 magnification); B: Larger tumor at × 200 magnification, inset shows immunostaining for alpha-fetoprotein; C: The second tumor demonstrated a more typical colonic morphologic differentiation, characterized by infiltrating submucosal glands (hematoxylin and eosin staining, × 40 magnification); D: Second tumor at × 200 magnification.
Following this diagnosis, a repeat CT scan of the abdomen was performed, which showed prominent paracolic peritoneal enhancement that was suggestive of peritoneal carcinomatosis. The patient was thus started on palliative chemotherapy with FOLFOX (oxaliplatin, leucovorin, 5-fluorouracil) and bevacizumab. Serum AFP levels decreased to 260 μg/L after three cycles of chemotherapy, and the patient remains stable six months after the diagnosis of metastatic HA of the colon.
DISCUSSION
This case describes a patient with long-standing ulcerative colitis and primary sclerosing cholangitis who was diagnosed with metastatic colonic HA that was denoted by markedly elevated AFP levels. To the best of our knowledge, this is the first reported case of two synchronous colonic cancers with divergent histologic subtypes arising in a background of PSC-IBD. HA of the colon or rectum is distinctly rare, representing only 2% of all reported cases[7]. Four of the seven previously reported cases of colonic HA were associated with IBD[8-11]. This strong correlation with IBD may be related to the ongoing chronic inflammation in these patients, but to date, no causal link has been established. A review of 271 patients showed that HA occurs predominantly in males (male:female = 2.3:1), with a median age of 63.0 ± 12.8 years (range: 21 to 100 years)[6]. The majority of the patients (84.8%) had elevated serum AFP levels, ranging from < 1.0 to 475000 μg/L. AFP, a glycoprotein produced in large amounts in the fetus, is found at a concentration of < 10 μg/L in healthy adult individuals and is mildly elevated in benign liver diseases, such as hepatitis and cirrhosis. AFP levels are also mildly elevated in HCC and other tumors, including non-seminomatous germ cell tumors, pancreatic ductal and gallbladder adenocarcinomata, and gastrointestinal tract carcinomata. Although a small percentage of HA do not produce AFP (15.2%)[6], histologic examination of AFP immunoreactivity remains the gold standard for diagnosis.
The treatment of HAs involves surgical resection of the tumor, followed by organ specific adjuvant chemotherapy. FOLFOX or FOLFIRI (leucovorin, 5-fluorouracil, irinotecan) regimens are the treatment of choice for patients with colorectal HA, though outcomes vary significantly. For example, Cappetta et al[11] reported a 75-year-old female with colonic HA who had a poor response to surgical resection and standard chemotherapy for colorectal cancer (FOLFOX-4 and later FOLFIRI plus bevacizumab), whereas Slotta et al[7] reported a 59-year-old female patient with a 27-mo recurrence-free survival after surgical resection and adjuvant chemotherapy (FOLFOX-4). Despite combined surgical resection and adjuvant chemotherapy, the overall prognosis for HA patients remains poor in comparison to “typical” solid organ tumors. A 55% one-year survival rate for HA has been reported, along with an 11-mo overall median survival that encompasses a wide range depending on the primary tumor site[3]. The median survival for colorectal HA is 19.4 mo[7].
Although the pathogenesis of HA is not clearly understood, it is thought that hepatoid cells originate more proximal to the totipotent cell. This may allow early intravascular proliferation of these tumors, which may explain their poor prognosis[8,11]. This case study exemplifies the clinical importance of serum AFP levels, which, in the absence of a hepatic lesion, can be an indicator of an unsuspected HA. Future studies are needed to understand the mechanism that triggers differentiation to a hepatoid morphology and phenotype, as well as to identify an effective chemotherapy regimen.
COMMENTS
Case characteristics
A 36-year-old male with a history of ulcerative colitis, primary sclerosing cholangitis, and two colonic carcinomata that was treated with a subtotal colectomy and ileostomy and later presented with increased abdominal girth.
Clinical diagnosis
Ascites.
Differential diagnosis
Liver cirrhosis, peritoneal carcinomatosis.
Laboratory diagnosis
Marked elevation of alpha-fetoprotein (AFP; 4896 μg/L) that had slowly increased over a period of 12 mo.
Imaging diagnosis
Positron emission tomography (PET) scan showed multiple PET-positive paraaortic lymph nodes and one left inguinal lymph node.
Pathological diagnosis
Fine needle aspiration biopsy of the porta hepatis lymph node showed metastatic carcinoma and positive AFP and caudal-related homeobox transcription factor 2 (CDX-2) immunostaining. One of the original colonic tumor specimens demonstrated histologic features suggestive of hepatoid adenocarcinoma (HA) and stained positive for AFP, CDX-2, and hepatocyte specific antigen.
Treatment
The patient was treated with chemotherapy FOLFOX (oxaliplatin, leucovorin, 5-fluorouracil) in combination with bevacizumab.
Related reports
The tumor biology of HA is not very well understood and its recurrence cannot be reliably predicted.
Term explanation
HA is a rare and aggressive extra-hepatic tumor that displays biochemical and histologic features reminiscent of hepatocellular carcinoma and is often associated with a high serum level of AFP.
Experiences and lessons
This case not only represents the first reported case of two synchronous colonic cancers with divergent histologic subtypes arising in a background of primary sclerosing cholangitis-associated inflammatory bowel disease, but also addresses the importance of considering HA as a differential diagnosis in patients with an increasing serum AFP level in the absence of a hepatic lesion.
Peer review
A rare occurrence of colonic HA is reported that was identified by steadily increasing serum AFP levels.
Footnotes
P- Reviewer: Amarapurkar DN, Ciaccio E, Kishimoto T, Tran TA S- Editor: Gou SX L- Editor: A E- Editor: Zhang DN
References
- 1.Bourreille J, Metayer P, Sauger F, Matray F, Fondimare A. [Existence of alpha feto protein during gastric-origin secondary cancer of the liver] Presse Med. 1970;78:1277–1278. [PubMed] [Google Scholar]
- 2.Ishikura H, Kirimoto K, Shamoto M, Miyamoto Y, Yamagiwa H, Itoh T, Aizawa M. Hepatoid adenocarcinomas of the stomach. An analysis of seven cases. Cancer. 1986;58:119–126. doi: 10.1002/1097-0142(19860701)58:1<119::aid-cncr2820580121>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 3.Metzgeroth G, Ströbel P, Baumbusch T, Reiter A, Hastka J. Hepatoid adenocarcinoma - review of the literature illustrated by a rare case originating in the peritoneal cavity. Onkologie. 2010;33:263–269. doi: 10.1159/000305717. [DOI] [PubMed] [Google Scholar]
- 4.Ishikura H, Kishimoto T, Andachi H, Kakuta Y, Yoshiki T. Gastrointestinal hepatoid adenocarcinoma: venous permeation and mimicry of hepatocellular carcinoma, a report of four cases. Histopathology. 1997;31:47–54. doi: 10.1046/j.1365-2559.1997.5740812.x. [DOI] [PubMed] [Google Scholar]
- 5.Terracciano LM, Glatz K, Mhawech P, Vasei M, Lehmann FS, Vecchione R, Tornillo L. Hepatoid adenocarcinoma with liver metastasis mimicking hepatocellular carcinoma: an immunohistochemical and molecular study of eight cases. Am J Surg Pathol. 2003;27:1302–1312. doi: 10.1097/00000478-200310000-00002. [DOI] [PubMed] [Google Scholar]
- 6.Su JS, Chen YT, Wang RC, Wu CY, Lee SW, Lee TY. Clinicopathological characteristics in the differential diagnosis of hepatoid adenocarcinoma: a literature review. World J Gastroenterol. 2013;19:321–327. doi: 10.3748/wjg.v19.i3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Slotta JE, Jüngling B, Kim YJ, Wagner M, Igna D, Schilling MK. Hepatoid adenocarcinoma of the transverse colon. Int J Colorectal Dis. 2012;27:989–991. doi: 10.1007/s00384-011-1305-6. [DOI] [PubMed] [Google Scholar]
- 8.Borgonovo G, Razzetta F, Assalino M, Varaldo E, Puglisi M, Ceppa P. Rectal hepatoid carcinoma with liver metastases in a patient affected by ulcerative colitis. Hepatobiliary Pancreat Dis Int. 2008;7:539–543. [PubMed] [Google Scholar]
- 9.Liu Q, Bannan M, Melamed J, Witkin GB, Nonaka D. Two cases of hepatoid adenocarcinoma of the intestine in association with inflammatory bowel disease. Histopathology. 2007;51:123–125. doi: 10.1111/j.1365-2559.2007.02715.x. [DOI] [PubMed] [Google Scholar]
- 10.Lattes C, Carella R, Faggioli S, Gabusi E, Grigioni WF. Hepatoid adenocarcinoma of the rectum arising in ulcerative colitis: report of a case. Dis Colon Rectum. 2000;43:105–108. doi: 10.1007/BF02237252. [DOI] [PubMed] [Google Scholar]
- 11.Cappetta A, Bergamo F, Mescoli C, Lonardi S, Rugge M, Zagonel V. Hepatoid adenocarcinoma of the colon: what should we target? Pathol Oncol Res. 2012;18:93–96. doi: 10.1007/s12253-011-9424-5. [DOI] [PubMed] [Google Scholar]


