Abstract
Adenocarcinosarcoma, a neoplasm containing both carcinomatous and sarcomatous components, is a rare form of a cancer and the pathophysiology is currently poorly understood. Moreover, definitive treatment guidelines for this disease have not yet been established. Pancreatic adenocarcinosarcoma is even more rare and the prognosis is fatal. Here, we report a case of a 77-year-old male with pancreatic adenocarcinosarcoma and metastasis to the liver. The patient presented at our hospital with uncontrolled glucose levels and diabetes mellitus. The patient’s laboratory findings were unremarkable with the exception of elevated carbohydrate antigen 19-9 levels. Biopsies of the tumors in the pancreas and the liver revealed two types of tumors: pancreatic adenocarcinoma and a poorly differentiated sarcoma. To determine if KRAS mutations were present, we performed a peptide nucleic acid (PNA) clamp PCR-based assay. DNA sequencing by PNA clamp PCR identified a point mutation in codon 12 of exon 2 within KRAS from both tumor types. Because the KRAS mutation is observed in both tumor components, our findings support a monoclonal tumor origin followed by subsequent divergent differentiation into the sarcomatous and carcinomatous tumor populations. After we considered the patient’s status and the late stage of tumor detection, gemcitabine chemotherapy was administered.
Keywords: Pancreas, Adenocarcinosarcoma, Metastasis, KRAS, DNA sequencing
Core tip: Pancreatic adenocarcinosarcoma is a very rare form of cancer. Here, we present a case report of a 77 year-old male with pancreatic adenocarcinosarcoma and metastasis to the liver. Using peptide nucleic acid clamp PCR, we identified a mutation in KRAS in both tumors. These results indicate that both the sarcomatous and carcinomatous components of the tumor likely arose from a monoclonal origin.
INTRODUCTION
Adenocarcinosarcoma is a rare form of cancer that is composed of both carcinomatous and sarcomatous tumor tissues[1,2]. Adenocarcinosarcomas are most frequently observed in the uterus, though they have been observed in other locations such as the pancreas[3]. Metastasis of pancreatic adenocarcinosarcoma to the liver, however, is incredibly rare and has been infrequently reported in the literature.
Here, we report a case of pancreatic adenocarcinosarcoma with liver metastasis in a 77 year-old male patient. Using a peptide nucleic acid (PNA) clamp PCR-based approach, we tested whether the tumor had a mutation in the KRAS oncogene. PNA clamp PCR revealed a point mutation in codon 12 of exon 2 in KRAS in both tumor components. We also review the current literature regarding this rare form of cancer, including the immunohistopathologic characteristics, clinical features and diagnostic tools.
CASE REPORT
A 77 year-old male patient was admitted to our hospital for evaluation of a pancreatic mass after complaining of poorly controlled blood sugar that had developed more recently. Seven years ago, the patient was diagnosed with diabetes mellitus (DM). The patient received regular endocrinology evaluations, did not take medications and presented with normal glycated hemoglobin levels. Because his fasting glucose levels were measuring higher than usual over the past two months, the patient was prescribed DM medication and referred for a pancreatic sonogram. After an abdominal sonogram revealed a 2.2 cm hypoechoic mass in the pancreas, the patient was transferred to our hospital. Aside from the high blood sugar, the patient had no other symptoms such as abdominal pain, nausea or vomiting. The patient had no history of smoking, pancreatitis, significant weight loss or alcohol use. A physical examination did not reveal any signs of abdominal tenderness. The patient was hemodynamically stable and had no palpable abdominal masses. Laboratory results for aspartate transaminase, alanine transaminase, total bilirubin, alkaline phosphatase, r-glutamyl transpeptidase and amylase/lipase levels were within normal ranges while carbohydrate antigen 19-9 levels were elevated (160.57 U/mL).
An abdominal computed tomography (CT) scan performed by our hospital revealed a 2.2 cm mass in the pancreas with a dilated pancreatic duct (Figure 1A). The patient was diagnosed with pancreatic cancer. A 1.2 cm cyst in the uncinate process of the pancreatic head and body was identified and resembled an intraductal papillary mucinous neoplasm. The abdominal CT also revealed a 1.3 cm low attenuated hepatic nodule in S5, indicating that there was hepatic metastasis (Figure 1B). Abnormal signals from the body of the pancreas were observed through magnetic resonance imaging (MRI) and confirmed the presence of a tumor (Figure 2A). We did not observe invasion of the tumors into the superior mesenteric or portal veins. A positron emission tomography CT only revealed fluorodeoxyglucose uptake at the pancreatic and liver masses identified by the abdominal CT.
Figure 1.
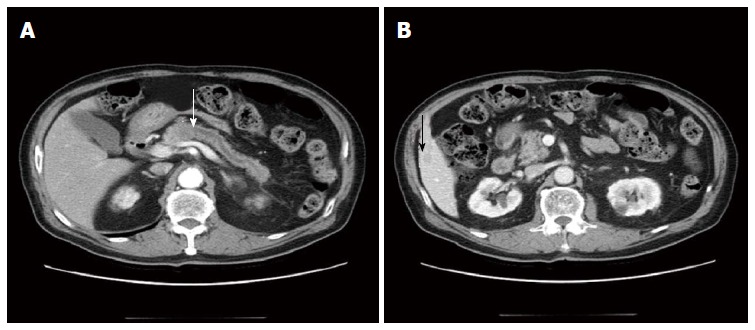
Axial pancreas-enhanced computed tomography findings. A: A 2.2 cm low attenuated mass in the pancreas body with diffuse pancreas duct dilatation (white arrow: pancreatic head mass); B: A 1.3 cm low attenuated hepatic nodule in S5 (black arrow: hepatic mass).
Figure 2.
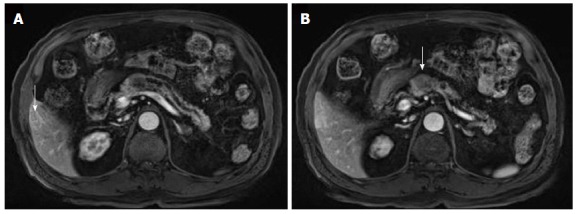
Magnetic resonance imaging findings. A: A T1 weighted magnetic resonance imaging (MRI) revealed a 2.2 cm mild low signal intensity pancreatic body mass with diffuse distal pancreatic duct dilatation (arrow: hepatic mass in S5); B: An MRI in the arterial phase revealed a 1.3 cm round, peripheral enhanced mass in S5 of the liver (arrow: pancreatic head mass).
Before beginning treatment, it was first necessary to distinguish between hepatic metastasis and double primary cancer from a benign neoplasm. To this end, we biopsied the liver and pancreatic masses and found pancreatic adenocarcinosarcoma and metastatic adenocarcinosarcoma. Microscopically, the tumor had dual characteristics (Figure 3A). The adenomatous portion of the tumor contained glands and the nuclei were dysmorphic. The sarcomatous portion contained pleomorphic tumor cells and high cellularity with vesicular and abnormal nuclei (Figure 3B). Because the sarcomatous portion of the tumor was very poorly differentiated, it was difficult to identify what type of tumor it was initially. The carcinomatous portion contained ductal adenocarcinoma cells (Figure 3C). Immunohistochemical staining results are listed in Table 1. The adenocarcinomatous part had strong immunoreactivity for pan-cytokeratin (CK), CK-7 and CK-19. The sarcomatous part was positive for pan-CK (Figure 4A, B) and vimentin (Figure 4C, D).
Figure 3.
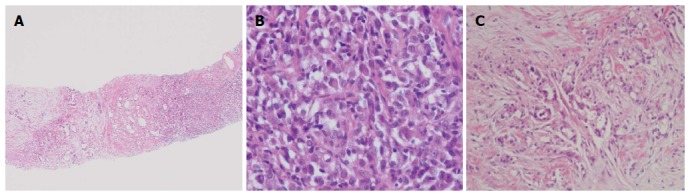
Pancreatic neoplasia biopsy. A: Dual disparate sarcomatous (right) and carcinomatous (left) components of the tumor [hematoxylin and eosin (HE) stain, × 100); B: Sarcomatous component (HE stain, × 400); C: Carcinomatous component (HE stain, × 200).
Table 1.
Immunohistochemistry markers used in this study
| Marker |
Tumor component |
|
| Adenocarcinoma | Sarcoma | |
| Pan-CK | Positive | Positive |
| CK-7 | Positive | Negative |
| CK-19 | Positive | Negative |
| Vimentin | Negative | Positive |
| Desmin | Negative | Negative |
| CD34 | Negative | Negative |
| CD117 | Negative | Negative |
| Actin | Negative | Negative |
| AFP | Negative | Negative |
CK: cytokeratin; AFP: alpha-fetoprotein.
Figure 4.
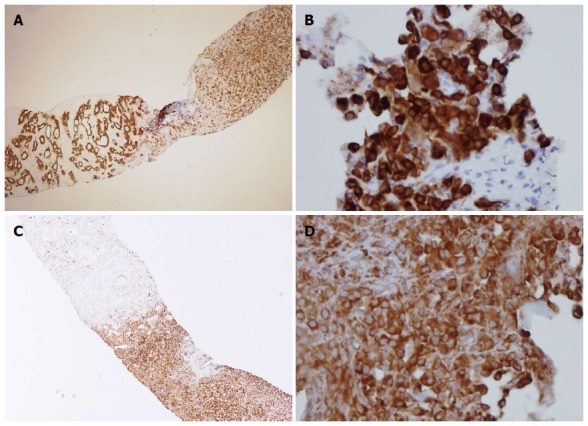
Immunohistopathology of the pancreatic tumor. A: A pancreatic biopsy revealed a sarcomatoid component (right) containing mesenchymal tumor cells and a carcinomatous component that gave rise to tumor glands (left) [pan-cytokeratin (CK) staining, × 40]; B: The sarcomatoid component from the liver was also positive for pan-CK (× 400); C: In the pancreatic biopsy, the sarcomatous component stained positive for vimentin (right) while the adenomatous component was negative for vimentin (left) (× 40); D: The sarcomatoid component from the liver stained positive for vimentin (× 400).
Using PNA clamp PCR, we analyzed KRAS for mutations from both the sarcomatous and carcinomatous portions of the pancreatic tumor. From both tumor components, we detected a point mutation in codon 12 of exon 2 in the KRAS oncogene (Table 2). The patient received chemotherapy with gemcitabine, but the tumor did not change in size in a follow-up abdominal CT.
Table 2.
Mutation in codon 12 of exon 2 in KRAS
|
Ct |
∆Ct-2 |
∆Ct-1 |
|||||
| Non-PNA | KRAS 12 | KRAS 13 | KRAS 12 | KRAS 13 | KRAS 12 | KRAS 13 | |
| The sarcomatous portions of the pancreatic tumor | |||||||
| T1 | 27.05 | 29.65 | 38.00 | 2.61 | 10.95 | 5.35 | -2.00 |
| Control | 25.22 | 37.59 | 39.25 | - | - | - | - |
| Standard Ct | - | 35.00 | 36.00 | ||||
| Result | KRAS codon 12 mutation | ||||||
| The carcinomatous portions of the pancreatic tumor | |||||||
| T2 | 24.96 | 27.50 | 38.00 | 2.54 | 13.04 | 7.50 | -2.00 |
| Control | 25.22 | 37.59 | 39.25 | - | - | - | - |
| Standard Ct | - | 35.00 | 36.00 | ||||
| Result | KRAS codon 12 mutation | ||||||
A mutation was identified if the ∆Ct-1 ≥ 2.0, meaning the mutated DNA sequences were amplified at least two cycles earlier than wildtype; If 0 ≤ ∆Ct-1 ≤ 2.0, ∆Ct-2 must be ≤ 6; The ∆Ct is the beginning value of sample amplification; The standard Ct designates the value where the wildtype sample began to amplify; ∆Ct-1 = Standard Ct – Sample Ct; and ∆Ct-2 = Sample Ct – Non-PNA Ct. Ct: threshold cycle; PNA: peptide nucleic acid.
DISCUSSION
Adenocarcinosarcoma is a rare form of cancer in which the tumor contains a complex of both carcinomatous and sarcomatous components. The origin of adenocarcinosarcomas is still controversial. Several histogenetic mechanisms have been attributed to the existence of carcinoma and sarcoma portions[3]. It may be that an initial carcinoma subsequently transforms into a sarcoma. Alternatively, a single stem cell may give rise to epithelial and mesenchymal progeny. It has also been hypothesized that two different tumors form and invade into each other. The most recent evidence, however, supports a model where the tumors arise from a monoclonal origin from a single stem cell[1]. In the case reported here, both the sarcomatous and carcinomatous tumor components had the same KRAS mutation in codon 12 of exon 2[4]. Thus, our data support the ”combination“ theory, where the sarcomatous and carcinomatous portions shared a monoclonal origin.
Biopsies of the sarcomatous component showed a heterogeneous mixture of cell types, with leiomyosarcoma, osteoclastic giant cells, primitive fibroblastic and mesenchymal characteristics[5,6]. In the current case, it was difficult to determine what components made up the sarcoma, in part because the sarcomatous portion was quite small. Moreover, the sarcomatous portion was too poorly differentiated to be defined by histopathology. Our immunohistochemical analysis demonstrated that the sarcomatous portion strongly expressed vimentin and pan-CK.
Because the incidence of pancreatic adenocarcinosarcoma is so rare, the pathology is still poorly understood. It has been hypothesized that the prognosis is influenced based on the sarcoma portion of the tumor; however, we did not have enough evidence from our case to support this idea.
In this case report, we present a patient with pancreatic adenocarcinosarcoma with metastasis to the liver. Because this form of cancer is rare, little information for diagnosis or treatment exists in the literature. In contrast to the cases previously reported, we obtained biopsies prior to pancreaticoduodenectomy and liver lobectomy surgical procedures. In another case report describing a patient with pancreatic adenocarcinosarcoma and liver metastasis, the patient underwent pancreaticoduodenectomy and hepatic lobectomy, but subsequently died two months after surgery due to multi-organ failure[7]. Thus, the recommendations for treatment or appropriate surgical approaches in this type of cancer are still lacking. Treatment should therefore be determined on a case-by-case basis. The biopsies in the present case were performed prior to surgery for several reasons. Primarily, it was to determine whether resectability would be feasible. A single liver mass could be a benign nodule, a double primary cancer or a hepatic cell carcinoma with pancreatic metastasis, although this option was less likely[8]. If the liver mass was benign or a double primary cancer, a surgical approach would be warranted. Because, however, the mass was a liver metastasis, the patient was given gemcitabine chemotherapy given the poor prognosis and late stage of diagnosis.
In other cases of adenocarcinosarcoma, patients complained of epigastric pain, a palpable abdominal mass, anemia, nausea with vomiting etc. In the present case, the patient only presented with poorly controlled blood sugar, a known feature of pancreatic cancer. This represents the first case where the earliest symptom of pancreatic adenocarcinosarcoma was poorly controlled blood sugar.
In conclusion, we present the first case, to the best of our knowledge, of pancreatic adenocarcinosarcoma reported in the gastrointestinal field. Analysis of the KRAS sequence identified the same mutation in both the sarcoma and carcinoma components of the tumor, supporting a monoclonal origin for the tumor.
COMMENTS
Case characteristics
A patient was admitted to our hospital with a recent bout of of poorly controlled blood sugar.
Clinical diagnosis
An abdominal sonogram detected a 2.2 cm mass in the pancreas.
Differential diagnosis
Pancreatic adenocarcinoma or a pancreatic benign neoplasm were considered in the differential diagnosis after the pancreatic mass was detected by sonogram.
Laboratory diagnosis
Analysis of blood chemistry was unremarkable with the exception of an elevation in carbohydrate antigen 19-9 (160.57 U/mL).
Imaging diagnosis
Abdominal computed tomography revealed a 2.2 cm mass in the body of the pancreas and a dilated pancreatic duct; a 1.3 cm mass in the S5 hepatic nodule was also detected and the patient was diagnosed with pancreatic cancer with hepatic metastasis.
Pathological diagnosis
Immunohistopathological analysis of the tumor revealed both sarcomatous and carcinomatous components and the patient was diagnosed with pancreatic adenocarcinosarcoma.
Treatment
The patient was treated with gemcitabine chemotherapy.
Related reports
It is currently hypothesized that these rare adenocarcinosarcomas arise from a single stem cell that gives rise to both epithelial and mesenchymal progeny.
Term explanation
An adenocarcinosarcoma is a rare type of cancer in which the tumor has both sarcomatous and carcinomatous components.
Experiences and lessons
Because both portions of the tumor had the same mutation in the KRAS oncogene, we hypothesize that the pancreatic adenocarcinosarcoma in this case report came from a monoclonal origin.
Peer review
Here, the authors report a rare case of pancreatic adenocarcinosarcoma with metastasis to the liver. Due to the poor prognosis and late stage of diagnosis, the patient was administered gemcitabine chemotherapy. This article is clinically relevant and offers new information on diagnosing and treating a rare form of pancreatic cancer.
Footnotes
P- Reviewer: Coskun A, Tan HJ S- Editor: Ma N L- Editor: A E- Editor: Du P
References
- 1.van den Berg W, Tascilar M, Offerhaus GJ, Albores-Saavedra J, Wenig BM, Hruban RH, Gabrielson E. Pancreatic mucinous cystic neoplasms with sarcomatous stroma: molecular evidence for monoclonal origin with subsequent divergence of the epithelial and sarcomatous components. Mod Pathol. 2000;13:86–91. doi: 10.1038/modpathol.3880013. [DOI] [PubMed] [Google Scholar]
- 2.Oymaci E, Argon A, Coşkun A, Uçar AD, Carti E, Erkan N, Yildirim M. Pancreatic carcinosarcoma: case report of a rare type of pancreatic neoplasia. JOP. 2013;14:212–215. doi: 10.6092/1590-8577/1309. [DOI] [PubMed] [Google Scholar]
- 3.Zhu WY, Liu TG, Zhu H. Long-term recurrence-free survival in a patient with pancreatic carcinosarcoma: a case report with a literature review. Med Oncol. 2012;29:140–143. doi: 10.1007/s12032-010-9804-9. [DOI] [PubMed] [Google Scholar]
- 4.Nakano T, Sonobe H, Usui T, Yamanaka K, Ishizuka T, Nishimura E, Hanazaki K. Immunohistochemistry and K-ras sequence of pancreatic carcinosarcoma. Pathol Int. 2008;58:672–677. doi: 10.1111/j.1440-1827.2008.02289.x. [DOI] [PubMed] [Google Scholar]
- 5.Watanabe M, Miura H, Inoue H, Uzuki M, Noda Y, Fujita N, Yamazaki T, Sawai T. Mixed osteoclastic/pleomorphic-type giant cell tumor of the pancreas with ductal adenocarcinoma: histochemical and immunohistochemical study with review of the literature. Pancreas. 1997;15:201–208. doi: 10.1097/00006676-199708000-00013. [DOI] [PubMed] [Google Scholar]
- 6.Wayne M, Gur D, Ascunce G, Abodessa B, Ghali V. Pancreatic mucinous cystic neoplasm with sarcomatous stroma metastasizing to liver: a case report and review of literature. World J Surg Oncol. 2013;11:100. doi: 10.1186/1477-7819-11-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shen ZL, Wang S, Ye YJ, Wang YL, Sun KK, Yang XD, Jiang KW. Carcinosarcoma of pancreas with liver metastasis combined with gastrointestinal stromal tumour of the stomach: is there a good prognosis with the complete resection? Eur J Cancer Care (Engl) 2010;19:118–123. doi: 10.1111/j.1365-2354.2008.00977.x. [DOI] [PubMed] [Google Scholar]
- 8.Wojcicki M, Lubikowski J, Post M, Chmurowicz T, Wiechowska-Kozlowska A, Krawczyk M. Aggressive surgical management of recurrent lymph node and pancreatic head metastases of resected fibrolamellar hepatocellular carcinoma: a case report. JOP. 2012;13:529–532. doi: 10.6092/1590-8577/839. [DOI] [PubMed] [Google Scholar]


