Abstract
Our study aimed to investigate whether serum leucine-rich alpha-2-glycoprotein (LRG) levels are elevated in patients with rheumatoid arthritis (RA). In addition, we assessed their correlation with disease activity parameters and pro-inflammatory cytokine, tumor necrosis factor-α (TNF-α). Our study included 69 patients with RA and 48 age- and sex-matched healthy controls. Serum concentrations of TNF-α and LRG were determined by enzyme-linked immunosorbent assay. Serum LRG concentrations were significantly elevated in patients with RA compared with those in healthy controls (30.8±14.4 vs. 22.2±6.1 ng/mL; P<0.001). In patients with RA, serum LRG levels were found to be correlated with disease activity score 28 (DAS28), erythrocyte sedimentation rate, and C-reactive protein levels (γ=0.671; γ=0.612; and γ=0.601, P<0.001, respectively), but not with serum TNF-α levels. Serum LRG levels in patients with an active disease status (DAS28≥2.6) were significantly higher than those in remission (DAS28<2.6) (36.45±14.36 vs. 24.63±8.81 ng/mL; P<0.001). Our findings suggest that serum LRG could contribute to the inflammatory process independent of TNF-α and it may be a novel biomarker for assessing inflammatory activity in patients with RA.
Graphical Abstract
Keywords: Leucine-Rich Alpha-2-Glycoprotein; Arthritis, Rheumatoid; Disease Activity; Tumor Necrosis Factor-alpha
INTRODUCTION
Rheumatoid arthritis (RA) is a chronic autoimmune disease that primarily affects the synovial lining of the diarthrodial joints (1). The primary features of RA are synovial hyperplasia, inflammatory cell infiltration of the synovium, neoangiogenesis, imbalance between pro- and anti-inflammatory cytokines, and progressive destruction of articular cartilage and bone (1). Recent treatment guidelines emphasize early and aggressive treatment and aim for remission as a therapeutic target (2, 3). To improve outcomes, patients must be closely monitored for disease activity at each clinician visit, and disease-modifying anti-rheumatic drugs (DMARDs) must be appropriately administered. Although erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) levels are routinely measured in clinical practice to assess the disease activity of RA, their usage has been limited, as they are often discordant with each other and their values are even in the normal range in approximately 50% of active patients with RA (4, 5). For this reason, many investigators aimed to identify reliable biomarkers to monitor disease activity.
Leucine-rich alpha-2 glycoprotein (LRG) is an approximately 50 kDa glycoprotein that contains a leucine-rich motif (6, 7). The function of LRG is not fully understood; however, its expression in various cells has been reported (8, 9, 10, 11). In a recent study that involved proteome analysis of the serum of patients with chronic inflammatory diseases (inflammatory bowel disease, rheumatoid arthritis, and Behçet disease), a significant elevation of serum LRG concentrations was noted, suggesting that LRG may be a useful biomarker for inflammatory conditions (12). Recent research demonstrated that LRG expression in colon cells was induced by interleukin (IL)-6, tumor-necrosis factor (TNF)-α, and IL-22 (8), which also play pivotal roles in the pathophysiology of RA (13, 14).
The aims of our study were to compare the serum LRG levels in patients with RA with those of healthy controls and to compare the usefulness of serum LRG as a marker of disease activity in patients with RA with that of other disease activity parameters and pro-inflammatory cytokine, TNF-α.
MATERIALS AND METHODS
Study design and subjects
This cross-sectional study included 69 patients with RA and 48 age- and sex- matched healthy controls. All patients were diagnosed with RA between June 2012 and May 2013 at an outpatient rheumatology clinic (Chung-Ang University College of Medicine, Seoul, Korea). All patients fulfilled the 1987 revised American College of Rheumatology (15) or the 2010 revised American College of Rheumatology/European League Against Rheumatism classification criteria for RA (16). Patients with acute infection, malignancies, or combined autoimmune diseases were excluded to remove possible biases. Forty-eight age- and sex- matched healthy individuals who visited the Health Examination Center for medical check-ups were included as the control group.
Clinical and laboratory assessments
Patient age, sex, medication history, height, body weight, disease duration, and extra-articular manifestations of RA were recorded. Clinical and laboratory data were collected during blood sampling. The number of tender and swollen joints and visual analogue scores judged globally by the physician and subjects were assessed. All joint examinations were conducted by the same rheumatologist. Complete blood count, biochemical analysis, IgM type of rheumatoid factor, anti-cyclic citrullinated peptide antibody, and anti-nuclear antibody were assessed. ESR was determined from whole blood using Westergren method (Afifax, Padova, Italy), and CRP concentration was assessed in serum via a nephelometric method (Beckman Coulter, Inc., Fullerton, CA, USA). Disease Activity Score 28 (DAS28) was calculated to determine RA disease activity (17). Patients were divided into two subgroups according to their disease activity as follows: inactive group, DAS28≤2.6; active group, DAS28>2.6 (17).
Measurement of serum LRG and cytokines
Venous blood samples were obtained from patients with RA and healthy controls. The collected serum samples were centrifuged at 1,500 rpm for 15 min, divided into aliquots, and stored frozen at -80℃ until used. Serum LRG (IBL Co., Ltd., Gunma, Japan) and TNF-α (R&D Systems, Inc., Minneapolis, MN, USA) levels were measured via enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's protocol. Intra- and inter-assay coefficients of variation of LRG were 3.0%-4.9% and 4.2%-5.1%, respectively, and those of TNF-α were 4.6%-5.2% and 5.4%-7.4%, respectively. The coefficients of determination (R2) were 0.9973 and 0.9943 for LRG and TNF-α, respectively. All samples were analyzed in duplicate.
Statistical analysis
All statistical analyses were conducted using the IBM SPSS Statistics software version 20.0 (IBM Corp., Armonk, NY, USA). Continuous variables were expressed as mean±standard deviations. Mean differences between continuous variables were evaluated using Student's t-test and Mann-Whitney U-test. Categorical variables were analyzed via the chi-square test. The ability of LRG level to distinguish RA from healthy controls was evaluated using a receiver-operating characteristic (ROC) analysis. Correlations between parameter studies were calculated with the Pearson's correlation coefficient. In all analyses, a P value<0.05 was considered statistically significant.
Ethics statement
This study was approved by the institutional review board of Chung-Ang University Hospital (C2013169-1129). Informed consent was confirmed by the board, and we obtained informed consent from all patients and controls in the study.
RESULTS
Serum concentrations of LRG in healthy controls and patients with RA
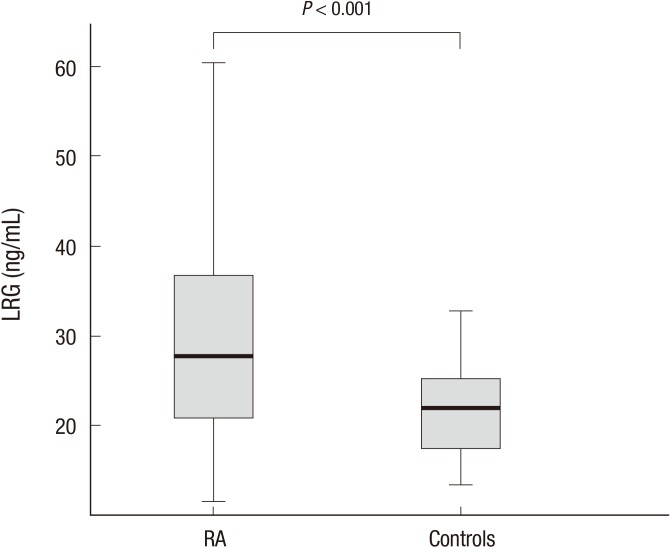
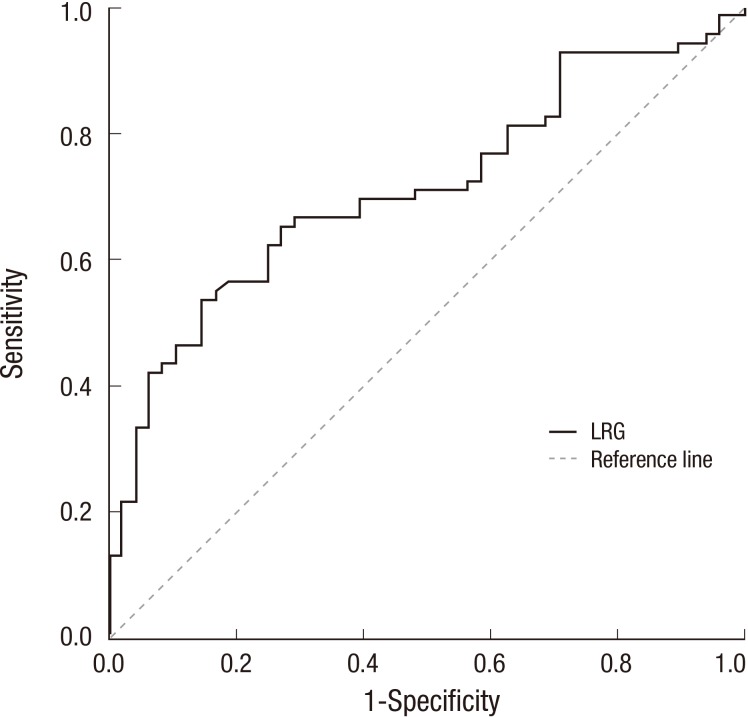
Our study included 69 patients with RA and 48 healthy controls. There was no statistically significant differences in the mean age or the sex ratio between the two groups (age, 56.0±13.5 vs. 55.1±3.3 yr; P=0.635; female ratio 53/69 [76.8%] vs. 29/48 [60.4%)]; P=0.057). The CRP levels in patients with RA were significantly higher than those in the healthy controls (1.07±2.30 vs. 0.15±0.22 mg/dL; P=0.001). However, there were no significant differences in CRP levels between the inactive RA group and the healthy control group (0.24±0.21 vs. 0.15±0.22 mL; P=1.000). Serum LRG levels were significantly elevated in patients with RA compared with those in the healthy controls (30.8±14.4 vs. 22.2±6.1 ng/mL; P<0.001; Fig. 1). ROC analysis for LRG to discriminate between RA and healthy control resulted in an area under the curve (AUC) of 0.712 (95% CI, 0.619-0.804) and an optimal cut-point at 27 ng/mL. With that cut-off level, the sensitivity was 53.6% and the specificity was 85.4% (Fig. 2).
Fig. 1.
Serum leucine-rich alpha-2 glycoprotein (LRG) levels in rheumatoid arthritis (RA) patients and controls. Mean LRG concentrations were significantly elevated in the serum of patients with RA compared with those in healthy controls (P < 0.001).
Fig. 2.
Receiver-operating characteristic (ROC) analysis for leucine-rich alpha-2 glycoprotein (LRG) to discriminate between rheumatoid arthritis (RA) and healthy control. It resulted in an area under curve (AUC) of 0.712 (95% CI, 0.619-0.804) and an optimal cut-point at 27 ng/mL. With that cut-off level, the sensitivity was 53.6% and the specificity was 85.4%.
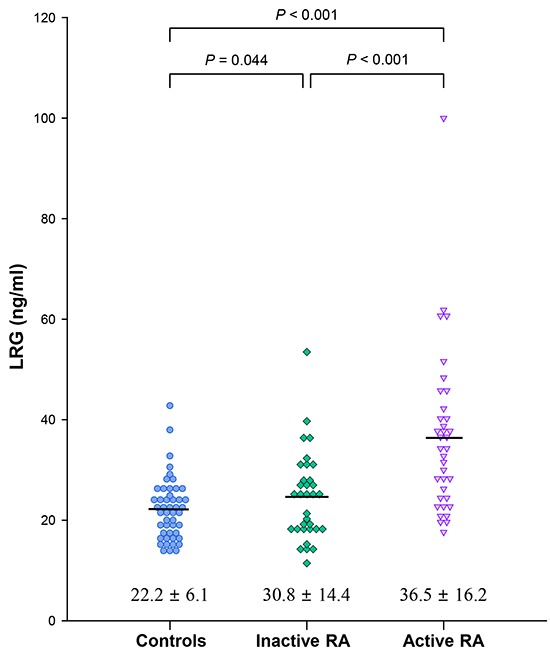
Characteristics of patients with RA and comparison according to disease activity status
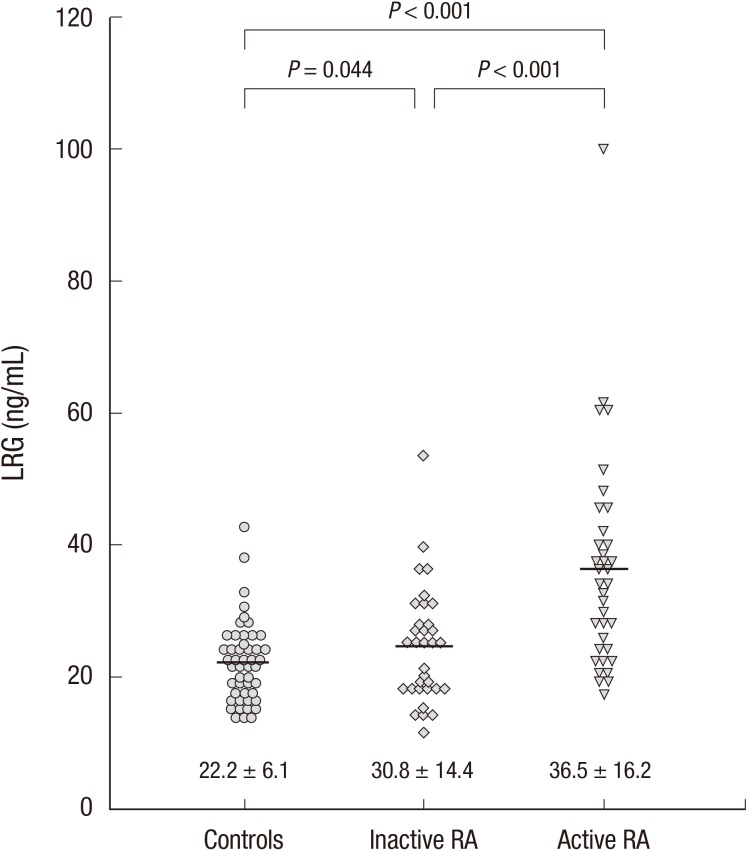
The clinical features and laboratory values of patients with RA are described in Table 1. All patients with RA were divided into an active group (n=36) or an inactive group (n=33) according to their DAS28 scores. Patients in the active group had higher levels of ESR, CRP, and DAS28 than those in the inactive group. Mean serum LRG levels were higher in the active group than in the inactive group (36.5±16.2 vs. 30.8±14.4 ng/mL; P<0.001; Fig. 3). Mean LRG levels in both the active and inactive groups significantly differed from those in the healthy controls (P<0.001 and P=0.044, respectively; Fig. 3). However, mean serum TNF-α levels were not significantly different between these two groups.
Table 1.
Demographics and clinical characteristics of patients with rheumatoid arthritis according to disease activity
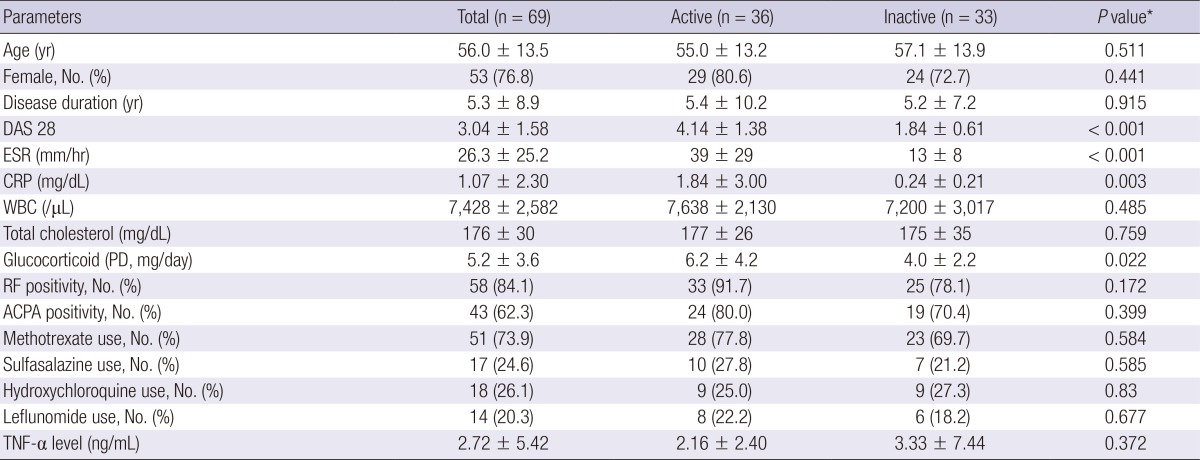
*P values were compared between active group and inactive group. DAS28, 28-joint disease activity score; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; WBC, white blood cell; PD, prednisolone; RF, rheumatoid factor; APCA, anti-citrullinated protein antibody; TNF, tumor necrosis factor.
Fig. 3.
Differences in serum concentrations of leucine-rich alpha-2 glycoprotein (LRG) between patients with active and inactive rheumatoid arthritis (RA) and healthy controls. Serum concentrations of LRG in the active RA group are higher than those in the inactive RA group and controls (P < 0.001). Patients with inactive RA show higher LRG levels than the controls (P = 0.044).
Correlations between serum LRG and TNF-α levels and disease activity parameters in patients with RA
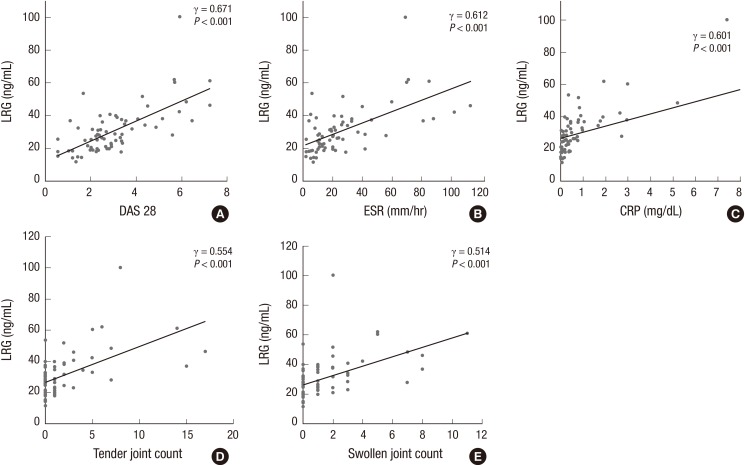
We analyzed the relationship between serum concentrations of LRG and other disease activity parameters in patients with RA. The serum LRG levels were significantly correlated with DAS28 (γ=0.671; P<0.001; Fig. 4A), ESR (γ=0.612; P<0.001; Fig. 4B), CRP (γ=0.671; P<0.001; Fig. 4C), and the number of tender joints (γ=0.554; P<0.001; Fig. 4D) and swollen joints (γ=0.514; P<0.001; Fig 4E). However, serum LRG levels were not correlated with serum TNF-α levels (γ=0.007; P=0.954) and neutrophil count (γ=0.099; P=0.420).
Fig. 4.
Correlations between disease activity parameters and serum concentrations of leucine-rich alpha-2 glycoprotein (LRG) in patients with rheumatoid arthritis (RA). Serum levels of LRG are positively correlated with disease activity score 28 (DAS28) (γ = 0.671, P < 0.001), (A) erythrocyte sedimentation rate (ESR) (γ = 0.612, P < 0.001), (B) C-reactive protein (CRP) (γ = 0.601, P < 0.001), (C) number of tender joints (γ = 0.554, P < 0.001), (D) and number of swollen joints (γ = 0.514, P < 0.001), (E).
DISCUSSION
In the present study, we demonstrated that the serum concentration of LRG was significantly elevated in patients with RA compared with that in the healthy controls. Serum LRG levels were found to be correlated with DAS28, suggesting that LRG could be a useful marker for assessing disease activity in patients with RA.
LRG is a serum protein, which was the first isolated from human serum in 1977 (7). The amino acid sequence of LRG was identified in 1985, and showed 66 leucine residues among 312 amino acid residues. LRG contains a leucine-rich repeat that consists of periodic eight-unit repeats of a 3 amino acid sequence (leucine-proline-asparagine) (6). Although its function and regulation are unclear, LRG has been detected in the sera of healthy individuals. Studies have reported that LRG is expressed in brain astrocyte, hepatocyte, and neutrophil (9, 10, 11). In a mouse study published in 2002, LRG expression was found to be a possible indication of inflammation and granulocyte differentiation (11). Shirai et al. (10) demonstrated that LRG expression in HepG2 cells was up-regulated by IL-6, and its secretory pattern was similar to that of acute-phase proteins such as CRP.
Cytokines play a critical role in the pathogenesis of RA. Pro-inflammatory cytokines such as TNF-α, IL-1, IL-6, IL-17, and IL-33, are activated in the synovium by various cell populations (14, 18). In our study, serum TNF-α levels were not correlated with DAS28 or serum LRG levels. Previous studies showed discrepancy about whether serum TNF-α levels were correlated with clinical disease activity (19, 20, 21). A recent study showed that LRG expression in colon cells was induced by IL-6, TNF-α, and IL-22 (8). However, in a previous proteomic analysis of serum from patients with RA, Behçet disease, and Crohn disease, serum LRG levels were not found to correlate with serum IL-6 levels (12). When considered together, serum LRG levels might be regulated in part cytokines other than TNF-α or IL-6 in patients with RA. Future studies that involve additional cytokine analysis will be needed to clarify the regulatory mechanisms of circulating LRG levels in RA.
Previous studies suggested that LRG may be a protein secreted by the inflammatory process and that reflects the inflammatory burden (10). However, LRG has been shown to bind cytochrome C and has been hypothesized to play a role in cell survival (22). A recent study demonstrated that LRG promotes pathological angiogenesis via the transforming growth factor-β signaling pathway (23). To date, there have been no reports regarding the expression of LRG in the synovium of RA patients. Regarding the contribution of neoangiogenesis and defective apoptosis to perpetuation of chronic synovial inflammation in RA (24, 25), LRG may play an important role in the pathogenesis of RA. Further studies are warranted to elucidate the role of LRG in the pathophysiology of RA.
LRG may be a useful biomarker in various conditions such as infection, malignancies, and chronic inflammatory diseases. Kentsis et al. (26) suggested urine LRG as a useful biomarker to differentiate patients with appendicitis from those with acute abdominal pain. Elevated serum LRG levels have been observed in ovarian, biliary tract, colorectal, and non-small cell lung cancers (27, 28, 29, 30). These findings indicate that serum LRG levels could increase in various inflammatory and pathologic conditions. Hence, elevated serum LRG levels may not be used as a specific diagnostic marker of certain diseases. However, serum LRG concentrations were significantly elevated in patients with ulcerative colitis compared with those in patients in remission. Similarly, patients with a DAS28 score>2.6 were found to have higher serum LRG levels than those with a DAS28 score≤2.6 (Fig. 2). Moreover, the present study demonstrated that serum LRG levels are correlated with disease activity indicators such as DAS28, ESR, CRP, and the number of tender and swollen joints, which indicates that measuring serum LRG levels could be helpful for assessing disease activity in patients with RA.
In our study, ROC analyses showed that LRG has an ability to distinguish between RA and normal controls. While CRP levels in patients with inactive RA were not significantly different from those in the healthy controls, the LRG level in inactive RA was significantly higher than that in healthy control. Therefore we think that serum LRG levels more than 27 ng/mL could be an indication for RA exacerbation before clinical manifestation. However, considering the cost of measuring LRG levels by ELISA, future study to investigate whether LRG is superior to cheap acute phase reactants (ESR and CRP) should be performed to apply LRG in the clinical practice.
We expected that the serum LRG levels in patients with RA were correlated with neutrophil count because LRG expression was up-regulated during neutrophilic differentiation (11). However, no correlation was found between serum LRG level and neutrophil count in patients with RA. The reason is unclear, yet we may consider that LRG had little connection with neutrophils in RA pathogenesis because LRG can be expressed in other cells (8, 9, 10). The use of prednisolone would be another confounding factor, in that it induces neutrophilia independent to the pathophysiology of RA (31).
Our study has several limitations. First, the study population was not large enough to achieve statistical significance. However, our study population was >2-folds the size of the previous study (12). Second, due to the cross-sectional design, the causal relationship and serial change in serum LRG level according to the change in the disease activity of RA could not be defined. Also, considering the percentage of RA patients in remission, disease activity (DAS28) of our study population was not so high. Further large-sized, longitudinal study can clarify the LRG concentration at different disease activity status (remission, mild, moderate and high disease activity) as well as serial change of LRG levels according to the change of disease activity. Third, the levels of other cytokines, including IL-1, IL-6, and IL-17, in the pathophysiology of RA were not measured, and their relations to LRG were not analyzed.
Our study demonstrated that serum LRG levels were elevated in patients with RA compared with those in the healthy controls, and that serum LRG level is correlated with disease activity parameters of RA independent of TNF-α. These findings suggest that LRG reflects the burden of systemic inflammation in RA and may play a role in the inflammatory process. Serum LRG could be a useful disease activity marker in patients with RA.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376:1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 2.Smolen JS, Aletaha D, Bijlsma JW, Breedveld FC, Boumpas D, Burmester G, Combe B, Cutolo M, de Wit M, Dougados M, et al. Treating rheumatoid arthritis to target: recommendations of an international task force. Ann Rheum Dis. 2010;69:631–637. doi: 10.1136/ard.2009.123919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smolen JS, Landewé R, Breedveld FC, Dougados M, Emery P, Gaujoux-Viala C, Gorter S, Knevel R, Nam J, Schoels M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 2010;69:964–975. doi: 10.1136/ard.2009.126532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Graf J, Scherzer R, Grunfeld C, Imboden J. Levels of C-reactive protein associated with high and very high cardiovascular risk are prevalent in patients with rheumatoid arthritis. PLoS One. 2009;4:e6242. doi: 10.1371/journal.pone.0006242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wolfe F. The many myths of erythrocyte sedimentation rate and C-reactive protein. J Rheumatol. 2009;36:1568–1569. doi: 10.3899/jrheum.090386. [DOI] [PubMed] [Google Scholar]
- 6.Takahashi N, Takahashi Y, Putnam FW. Periodicity of leucine and tandem repetition of a 24-amino acid segment in the primary structure of leucine-rich alpha 2-glycoprotein of human serum. Proc Natl Acad Sci U S A. 1985;82:1906–1910. doi: 10.1073/pnas.82.7.1906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haupt H, Baudner S. Isolation and characterization of an unknown, leucine-rich 3.1-S-alpha2-glycoprotein from human serum (author's transl) Hoppe Seylers Z Physiol Chem. 1977;358:639–646. [PubMed] [Google Scholar]
- 8.Serada S, Fujimoto M, Terabe F, Iijima H, Shinzaki S, Matsuzaki S, Ohkawara T, Nezu R, Nakajima S, Kobayashi T, et al. Serum leucine-rich alpha-2 glycoprotein is a disease activity biomarker in ulcerative colitis. Inflamm Bowel Dis. 2012;18:2169–2179. doi: 10.1002/ibd.22936. [DOI] [PubMed] [Google Scholar]
- 9.Nakajima M, Miyajima M, Ogino I, Watanabe M, Hagiwara Y, Segawa T, Kobayashi K, Arai H. Brain localization of leucine-rich α2-glycoprotein and its role. Acta Neurochir Suppl. 2012;113:97–101. doi: 10.1007/978-3-7091-0923-6_20. [DOI] [PubMed] [Google Scholar]
- 10.Shirai R, Hirano F, Ohkura N, Ikeda K, Inoue S. Up-regulation of the expression of leucine-rich alpha(2)-glycoprotein in hepatocytes by the mediators of acute-phase response. Biochem Biophys Res Commun. 2009;382:776–779. doi: 10.1016/j.bbrc.2009.03.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Donnell LC, Druhan LJ, Avalos BR. Molecular characterization and expression analysis of leucine-rich alpha2-glycoprotein, a novel marker of granulocytic differentiation. J Leukoc Biol. 2002;72:478–485. [PubMed] [Google Scholar]
- 12.Serada S, Fujimoto M, Ogata A, Terabe F, Hirano T, Iijima H, Shinzaki S, Nishikawa T, Ohkawara T, Iwahori K, et al. iTRAQ-based proteomic identification of leucine-rich alpha-2 glycoprotein as a novel inflammatory biomarker in autoimmune diseases. Ann Rheum Dis. 2010;69:770–774. doi: 10.1136/ard.2009.118919. [DOI] [PubMed] [Google Scholar]
- 13.Xie Q, Wang SC, Li J. Interleukin 22, a potential therapeutic target for rheumatoid arthritis. J Rheumatol. 2012;39:2220. doi: 10.3899/jrheum.120757. [DOI] [PubMed] [Google Scholar]
- 14.Klareskog L, Catrina AI, Paget S. Rheumatoid arthritis. Lancet. 2009;373:659–672. doi: 10.1016/S0140-6736(09)60008-8. [DOI] [PubMed] [Google Scholar]
- 15.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 16.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, Birnbaum NS, Burmester GR, Bykerk VP, Cohen MD, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 17.Prevoo ML, van't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight-joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 18.Hong YS, Moon SJ, Joo YB, Jeon CH, Cho ML, Ju JH, Oh HJ, Heo YJ, Park SH, Kim HY, et al. Measurement of interleukin-33 (IL-33) and IL-33 receptors (sST2 and ST2L) in patients with rheumatoid arthritis. J Korean Med Sci. 2011;26:1132–1139. doi: 10.3346/jkms.2011.26.9.1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Altomonte L, Zoli A, Mirone L, Scolieri P, Magaró M. Serum levels of interleukin-1b, tumour necrosis factor-a and interleukin-2 in rheumatoid arthritis: correlation with disease activity. Clin Rheumatol. 1992;11:202–205. doi: 10.1007/BF02207957. [DOI] [PubMed] [Google Scholar]
- 20.Yen JH, Chen JR, Tsai WJ, Liu HW. Correlation of tumor necrosis factor alpha levels with disease activity of rheumatoid arthritis. Zhonghua Min Guo Wei Sheng Wu Ji Mian Yi Xue Za Zhi. 1992;25:232–243. [PubMed] [Google Scholar]
- 21.Wascher TC, Hermann J, Brezinschek R, Brezinschek HP, Wilders-Truschnig M, Rainer F, Krejs GJ. Serum levels of interleukin-6 and tumour-necrosis-factor-alpha are not correlated to disease activity in patients with rheumatoid arthritis after treatment with low-dose methotrexate. Eur J Clin Invest. 1994;24:73–75. doi: 10.1111/j.1365-2362.1994.tb02063.x. [DOI] [PubMed] [Google Scholar]
- 22.Cummings C, Walder J, Treeful A, Jemmerson R. Serum leucine-rich alpha-2-glycoprotein-1 binds cytochrome c and inhibits antibody detection of this apoptotic marker in enzyme-linked immunosorbent assay. Apoptosis. 2006;11:1121–1129. doi: 10.1007/s10495-006-8159-3. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Abraham S, McKenzie JA, Jeffs N, Swire M, Tripathi VB, Luhmann UF, Lange CA, Zhai Z, Arthur HM, et al. LRG1 promotes angiogenesis by modulating endothelial TGF-β signalling. Nature. 2013;499:306–311. doi: 10.1038/nature12345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marrelli A, Cipriani P, Liakouli V, Carubbi F, Perricone C, Perricone R, Giacomelli R. Angiogenesis in rheumatoid arthritis: a disease specific process or a common response to chronic inflammation? Autoimmun Rev. 2011;10:595–598. doi: 10.1016/j.autrev.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 25.Liu H, Pope RM. The role of apoptosis in rheumatoid arthritis. Curr Opin Pharmacol. 2003;3:317–322. doi: 10.1016/s1471-4892(03)00037-7. [DOI] [PubMed] [Google Scholar]
- 26.Kentsis A, Lin YY, Kurek K, Calicchio M, Wang YY, Monigatti F, Campagne F, Lee R, Horwitz B, Steen H, et al. Discovery and validation of urine markers of acute pediatric appendicitis using high-accuracy mass spectrometry. Ann Emerg Med. 2010;55:62–70.e4. doi: 10.1016/j.annemergmed.2009.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Choi JW, Liu H, Shin DH, Yu GI, Hwang JS, Kim ES, Yun JW. Proteomic and cytokine plasma biomarkers for predicting progression from colorectal adenoma to carcinoma in human patients. Proteomics. 2013;13:2361–2374. doi: 10.1002/pmic.201200550. [DOI] [PubMed] [Google Scholar]
- 28.Liu Y, Luo X, Hu H, Wang R, Sun Y, Zeng R, Chen H. Integrative proteomics and tissue microarray profiling indicate the association between overexpressed serum proteins and non-small cell lung cancer. PLoS One. 2012;7:e51748. doi: 10.1371/journal.pone.0051748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sandanayake NS, Sinclair J, Andreola F, Chapman MH, Xue A, Webster GJ, Clarkson A, Gill A, Norton ID, Smith RC, et al. A combination of serum leucine-rich α-2-glycoprotein 1, CA19-9 and interleukin-6 differentiate biliary tract cancer from benign biliary strictures. Br J Cancer. 2011;105:1370–1378. doi: 10.1038/bjc.2011.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Andersen JD, Boylan KL, Jemmerson R, Geller MA, Misemer B, Harrington KM, Weivoda S, Witthuhn BA, Argenta P, Vogel RI, et al. Leucine-rich alpha-2-glycoprotein-1 is upregulated in sera and tumors of ovarian cancer patients. J Ovarian Res. 2010;3:21. doi: 10.1186/1757-2215-3-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dale DC, Fauci AS, Guerry DI, Wolff SM. Comparison of agents producing a neutrophilic leukocytosis in man: hydrocortisone, prednisone, endotoxin, and etiocholanolone. J Clin Invest. 1975;56:808–813. doi: 10.1172/JCI108159. [DOI] [PMC free article] [PubMed] [Google Scholar]