Abstract
Epidemiological data of Bordetella pertussis infection among adolescents and adults are limited in Korea. Patients (≥ 11 yr of age) with a bothersome cough for less than 30 days were enrolled during a 1-yr period at 22 hospitals in Korea. Nasopharyngeal swabs were collected for polymerase chain reaction (PCR) and for bacteriologic culture. In total, 490 patients were finally enrolled, and 34 (6.9%) patients tested positive for B. pertussis; cough duration (14.0 days [7.0-21.0 days]) and age distribution were diverse. The incidence was the highest in secondary referral hospitals, compared to primary care clinics or tertiary referral hospitals (24/226 [10.6%] vs. 3/88 [3.4%] vs. 7/176 [4.0%], P = 0.012), and the peak incidence was observed in February and August (15.8% and 15.9%), with no confirmed cases between March and June. In the multivariate analysis, post-tussive vomiting was significantly associated with pertussis (odds ratio, 2.508; 95% confidence interval, 1.146-5.486) and secondary referral hospital showed a borderline significance. In conclusion, using a PCR-based method, 6.9% of adolescent and adult patients with an acute cough illness had pertussis infection in an outpatient setting. However, hospital levels and seasonal trends must be taken into account to develop a better strategy for controlling pertussis.
Graphical Abstract
Keywords: Pertussis, Adult, Hospitals, Incidence, Seasons
INTRODUCTION
Pertussis is a highly infectious disease and can cause significant morbidity in infants and the elderly (1, 2). However, the incidence of this vaccine-preventable disease has increased substantially in adolescents and adults since 1980, and pertussis has resurged in many countries (3, 4). A large-scaled European study demonstrated an increased incidence of pertussis infection in adults rather than children, and a large outbreak occurred in the United States in 2012 (3, 5).
Studies on the epidemiology of pertussis demonstrated that 7%-17% of cases of prolonged cough were attributable to Bordetella pertussis infection in adolescents and adults, and suggested that the disease is endemic in the present vaccine era (7,8,9). However, the diagnosis of pertussis is challenging in adult patients, and the waning immunity following vaccination can contribute to the persistence of pertussis; household contacts can be the main source of pertussis transmission (4, 6). Therefore, the early detection and prevention of adolescent and adult pertussis is of great importance (1). Although several Korean studies indicate a relatively low prevalence among adults (10, 11), recent outbreaks in other countries have alerted us to the dangers of pertussis (3, 5).
Up to date, many studies have been conducted about the epidemiological and clinical characteristics of adult pertussis. However, data are very limited among adult patients in Korea. In addition, we hypothesized that their detection rates would be different by hospital levels (i.e., levels of healthcare system) and season in ordinary non-epidemic settings. Serologic tests are important in epidemiological studies but they are not always readily available and are time-consuming in real-world clinical settings. Therefore, we conducted this multicenter study on patients with an acute cough illness (≤30 days) using a PCR-based test. We collaborated with a professional laboratory and the Korean Centers for Disease Control and Prevention (KCDC) to conduct the study.
MATERIALS AND METHODS
Sites and subjects
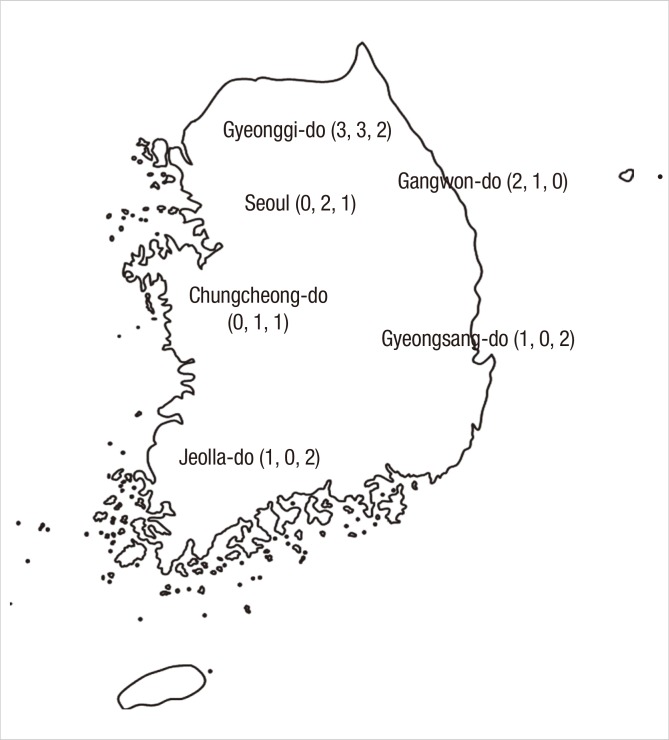
This study was conducted from July 2011 to June 2012 at 22 hospitals (7 primary care clinics, 7 secondary referral hospitals, and 8 tertiary referral hospitals) in six major provinces in Korea; among them, 11 hospitals (or clinics) were selected in Seoul and Gyeonggi-do because about a half of the nation's population reside around the Seoul metropolitan area (Fig. 1). No standardized definitions of the level of the healthcare system exist, and the range of services provided by each level of system depends on the available resources. In Korea, secondary referral hospitals provide specialized services at the regional level and usually have 30 to 500 beds (community or university hospitals). Tertiary referral hospitals are large-sized, university-affiliated, provide specialized services, train physicians and conduct research.
Fig. 1.
Six provinces and numbers of participating hospitals. In total, 22 hospitals (or clinics) participated in this study; numbers in parenthesis refer to those of participating primary, secondary, and tertiary hospitals, respectively.
Outpatients (adolescents or adults aged≥11 yr) who presented with a bothersome cough of a duration≤30 days were enrolled in this study. Exclusion criteria included a history of antibiotic treatment within 7 days, active lesion on the chest or paranasal sinus radiographs (when available), a fever of >38.0℃, immunocompromised status (e.g., AIDS, leukemia, aplastic anemia, organ transplant, autoimmune diseases, or chemotherapy), or a confirmed alternative cause for current illness (e.g., drugs [angiotensin-converting enzyme inhibitors], pneumonia, allergic rhinitis, sinusitis, or gastro-esophageal reflux).
Clinical data and specimen collection
Clinical information was collected by the participating investigators at outpatient departments. Data on age, sex, chronic respiratory diseases, comorbidities, tobacco usage, cough duration, classical pertussis symptoms, other respiratory symptoms and vaccination history (e.g., diphtheria, tetanus, and pertussis [DTP] or diphtheria, tetanus, and acellular pertussis [DTaP]) were collected for all enrolled patients. All specimens were collected via nasopharyngeal swab (NPS; liquid Amies medium on flocked swabs; Copan Diagnostic, Inc., Murrieta, CA, USA) and transferred at room temperature to the central laboratory (i.e., Seegene Medical Foundation) and plated on culture media within 24 hr. To ensure consistent specimen quality, independent personnel, usually trained registered nurses, performed the sampling procedure in each hospital; the KCDC educated all the participating hospitals, and all microbiological testing was performed in the central laboratory.
Case definition
To meet the clinical criteria for pertussis from the Centers for Disease Control and Prevention (CDC), patients must have a cough illness of ≥2 weeks duration and one of the following classical symptoms: 1) paroxysmal coughing, 2) inspiratory whooping, or 3) post-tussive vomiting (12). The laboratory criteria for diagnosis are isolation of B. pertussis or a positive polymerase-chain-reaction (PCR) assay. A 'confirmed case' is diagnosed when a patient satisfied both the clinical and laboratory criteria (16). In the present study, however, we define a confirmed case as a patient with a positive PCR or culture and a cough illness. We do not consider the duration of the cough illness in the diagnosis of pertussis due to the atypical characteristics of pertussis in adolescents and adults.
Specimens and microbiological tests
PCR and the bacteriologic culture were performed on all specimens. Regan and Lowe agar media (charcoal agar supplemented with 10% horse blood) with 40 mg/mL cephalexin were used for culture (13). After inoculation, the plates were incubated for at least 7 days under humid conditions (35-36℃). Identification was based on both biological characteristics and PCR (14).
For PCR, a portion of each specimen was boiled for 5 min. After centrifugation, 1-2 µL of the supernatant was used as a PCR template. Although no standardized PCR method exists for pertussis diagnosis, the "repeated-insertion sequence" and the "pertussis toxin (PT) promoter region" are used most commonly as target regions (15, 16). In the present study, we used the repeated-insertion sequence: primers BP1 (5'-GATTCAATAGGTTGTATGCATGGTT-3') and BP2 (5'-TTCAGGCACACAAACTTGATGGGCG-3'). PCR was performed using a commercially available pre-mixed Taq polymerase (AccuPower PRC PreMix; BIONEER, Daejeon, Korea).
PCR conditions were as follows: 95℃ for 5 min (pre-denaturation), followed by 40 cycles of 95℃ for 5 sec (denaturation) and 55℃ for 10 sec (annealing). PCR products were resolved by electrophoresis on 2% agarose gels, and identification of a 180-bp band indicated a positive result.
Data analyses
The primary outcomes were the incidence rate of B. pertussis infection among adolescents and adults and its relationships with hospital levels and season. Secondary outcomes were comparisons of incidence rates across age groups and six provinces. Clinical characteristics of confirmed cases were also compared to cases of non-confirmed cough illness. Data are expressed as means±standard deviations (or medians and interquartile ranges [IQRs]) for continuous variables and as percentages for categorical variables, unless otherwise indicated. Student's t-test was performed for continuous data, whereas chi-square or Fisher's exact tests were used for categorical data. A multivariate analysis by logistic regression (with a backward likelihood ratio method) was performed with covariates significant in univariate analysis to investigate risk factors for pertussis. All reported P values were two-sided, and P<0.05 indicated statistical significance. All analyses were conducted using the SPSS statistical software (IBM SPSS Statistics 21, Standard for Medical Network).
Ethics statement
All patients provided written informed consent, and the protocol of this study was approved by the institutional review board of Hallym University Sacred Heart Hospital (IRB No. 2011-I050) and each participating hospital. The authors assert that all procedures contributing to this work comply with the Helsinki Declaration of 1975 and its later amendments.
RESULTS
Demographics and clinical symptoms
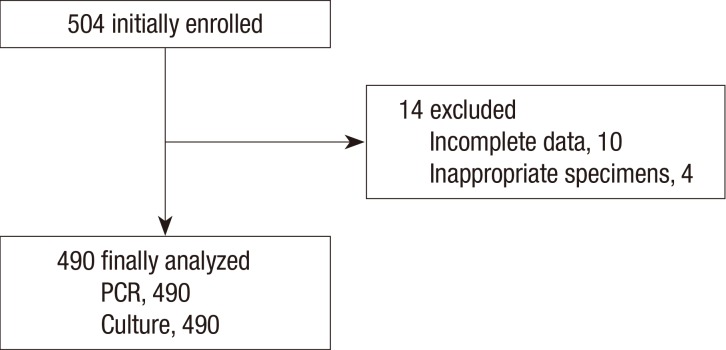
Among 504 patients initially enrolled, 14 were excluded due to their incomplete clinical data or inappropriate specimens (Fig. 2). Therefore, a total of 490 patients (88 from primary care clinics, 226 from secondary referral hospitals and 176 from tertiary referral hospitals) were finally included in the study. The mean age of the patients was 44.3±16.0 yr, and 72.7% were females (Table 1). Median cough duration was 14 days (7~21 days); and 72.4% of the patients reported a paroxysmal cough, while 13.1% and 17.8% reported whooping cough and post-tussive vomiting, respectively. Hypertension was the most common co-morbidity reported, and 5.7% had a chronic airway disease. Among clinical symptoms, sputum (65.1%) and rhinorrhea (37.8%) were the most frequent symptoms, when cough was excluded. Only 15.1% of patients could recall if they had received the DTP (or DTaP) vaccination.
Fig. 2.
Flow diagram of enrolled patients. PCR, polymerase chain reaction.
Table 1.
Baseline characteristics of the participants (n = 490)
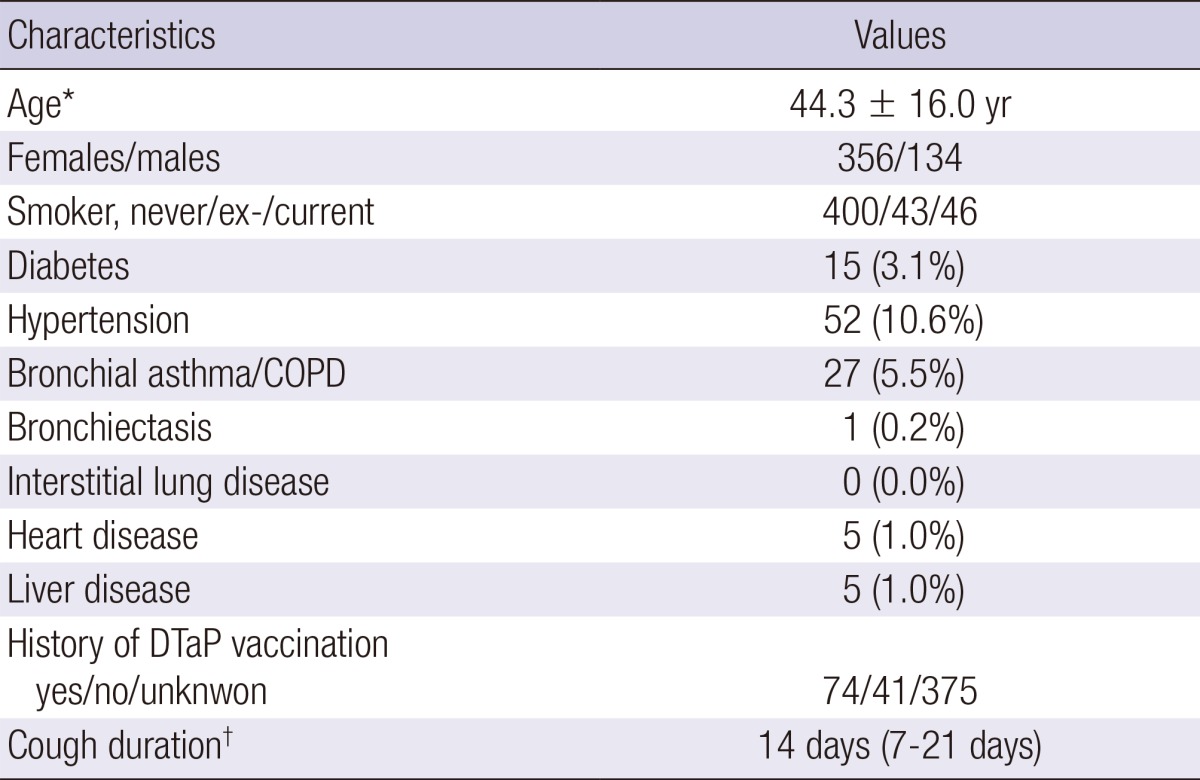
*Mean±SD; †Median (Interquartile range). COPD, chronic obstructive pulmonary disease; DTaP, diphtheria, tetanus, acellular pertussis.
Microbiologic data
We collected 490 nasopharyngeal swab samples from 22 hospitals during the 1-yr period. In total, 34 (6.9%) patients were PCR positive (i.e., confirmed case), and of these, 10 (2.0%) were culture positive (B. pertussis) concomitantly. We investigated further 20 close contacts including family members of the 11 confirmed cases. Of 20 contacted cases, five (25.0%) were PCR positive.
Incidence by hospital levels
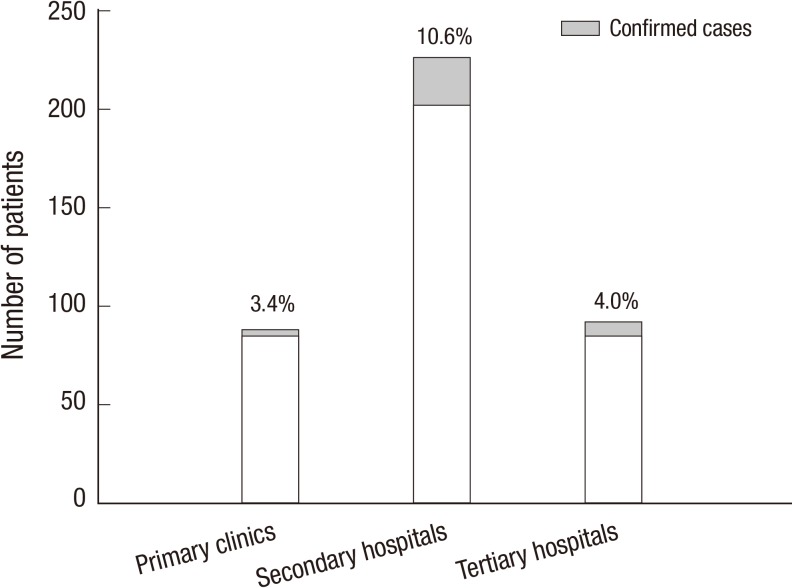
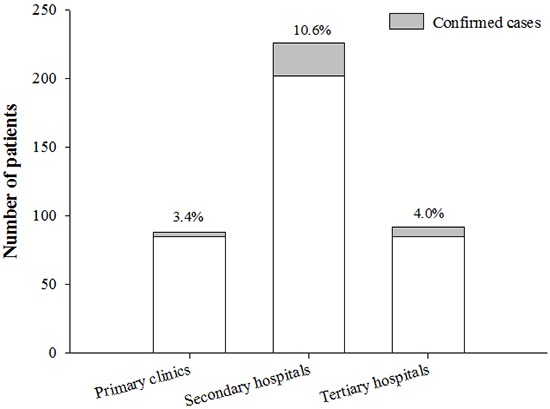
When stratified by the hospital levels, the highest incidence was in secondary referral hospitals (10.6% [24/226] vs. 3.4% [3/88] in primary care clinics vs. 4.0% [7/176] in tertiary referral hospitals; P=0.012; Fig. 3). In terms of age, sex, and cough duration, there were no significant differences among the three hospital groups (data not shown).
Fig. 3.
Incidence of pertussis by hospital levels. The incidence was the highest in secondary referral hospitals compared to tertiary or primary care hospitals (P = 0.012).
Incidence by season and province
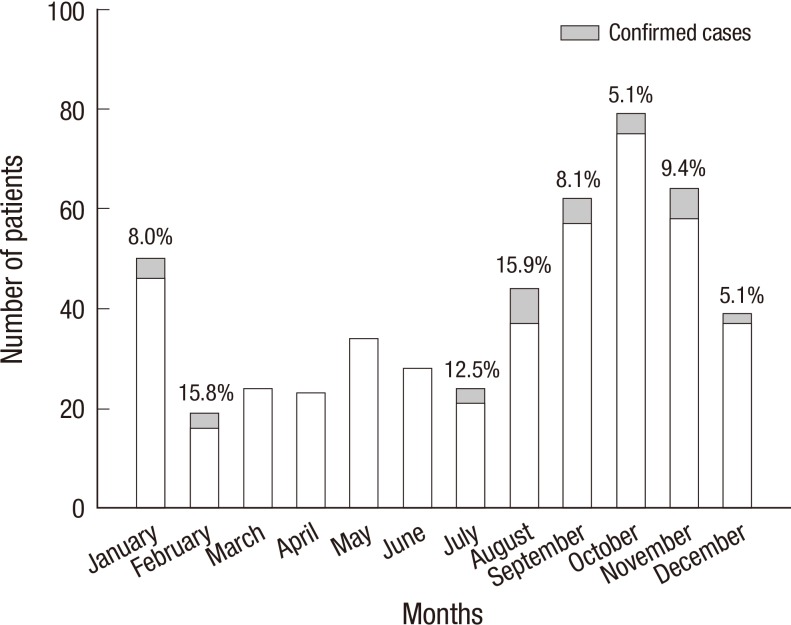
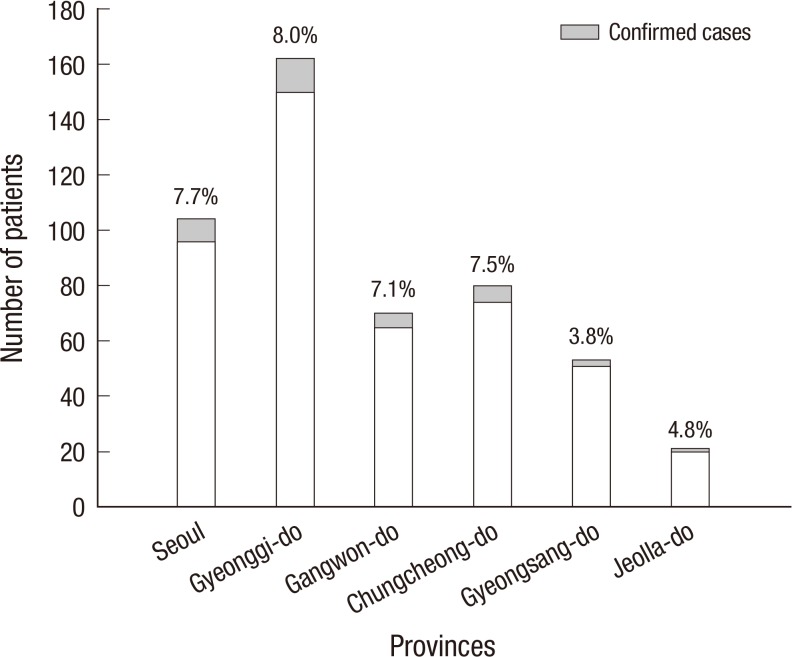
As shown in Fig. 4, peak incidence was in February and August (15.8% [3/19] and 15.9% [7/44], respectively). However, there were no pertussis cases between March and June. In terms of regional distribution (Fig. 5), we identified confirmed cases from all the six provinces, and the incidence rates ranged from 3.8% to 8.0%, with the lowest rates in the two southern provinces.
Fig. 4.
Seasonal trends of the incidence of pertussis. Peak incidences were observed in February and August; no pertussis cases were identified between March and June.
Fig. 5.
Incidence of pertussis by provinces. The incidence rates range from 3.8% to 8.0% among the six provinces and the two southern provinces (Gyeongsang-do and Jeolla-do) show the lowest rates, with no statistical significance.
Clinical characteristics and risk factors
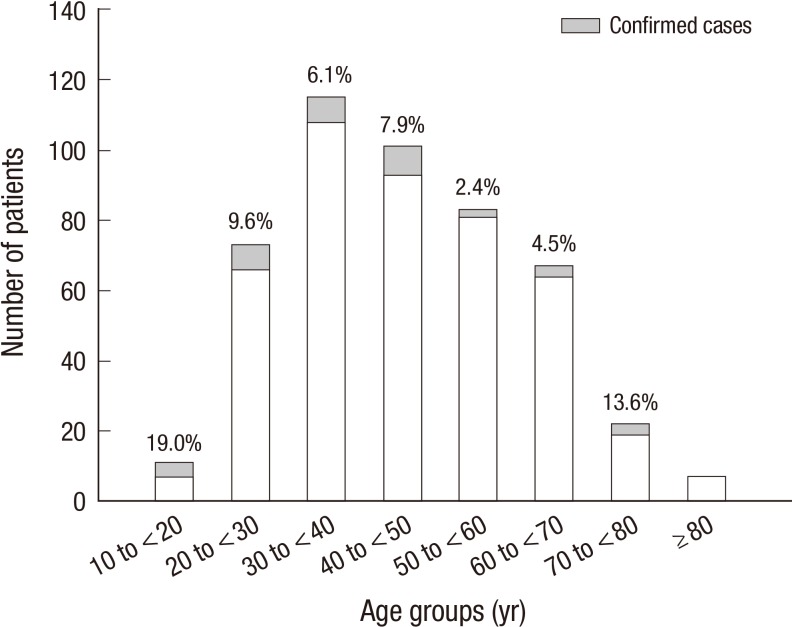
The age distribution of the confirmed cases was diverse, with the highest incidence rate among patients aged of 10 to <20 yr (Fig. 6). The median cough duration was 14.0 days (7.0-21.0 days); and 16 patients (45.7%) had a cough of duration<14 days. Among the confirmed cases, 27 patients (79.4%) complained of paroxysmal cough, but only 11.8% and 33.4% had inspiratory whooping and post-tussive vomiting, respectively. In particular, chills, crackles and wheezing were absent among the confirmed cases, and none of them had a history of chronic lung disease, either (Table 2).
Fig. 6.
Incidence of pertussis by age group. The age distribution of the confirmed cases was diverse (P > 0.05 by chi-square test).
Table 2.
Clinical characteristics between confirmed cases and other patients
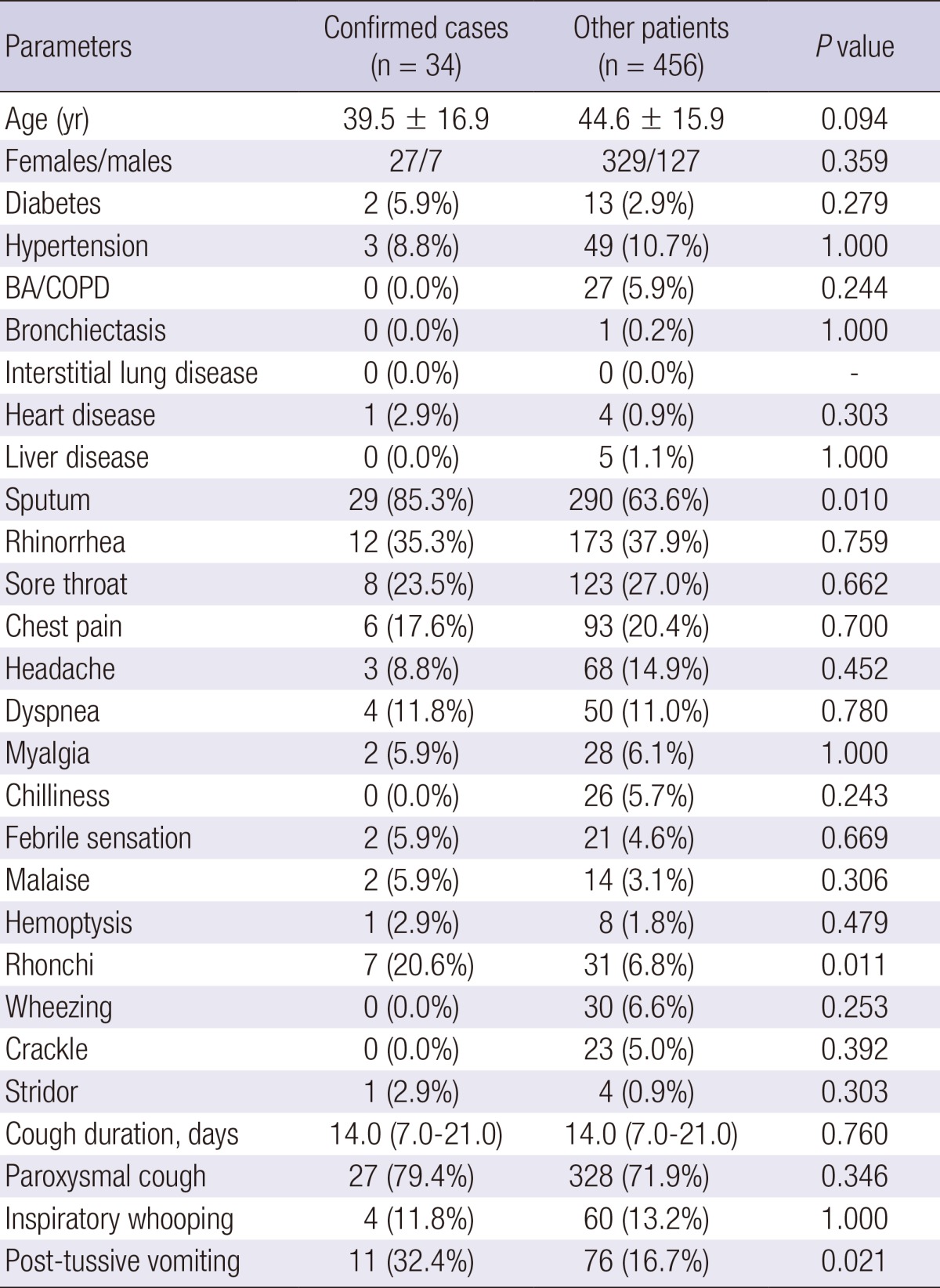
BA, bronchial asthma; COPD, chronic obstructive pulmonary disease.
The clinical characteristics of the confirmed cases are shown in Table 2. There were no significant differences in co-morbid illnesses between the two groups. However, sputum, rhonchi, and post-tussive vomiting were more frequent in the confirmed cases when compared to the other cases of cough illness (P=0.010, P=0.011, and P=0.021, respectively). In the multivariate analysis, where age, sputum, rhonchi, post-tussive vomiting, and hospital levels were included, post-tussive vomiting was significantly associated with pertussis (odds ratio [OR], 2.508; 95% confidence interval [CI], 1.146-5.486), and secondary referral hospitals showed a borderline significance (OR, 3.198; 95% CI, 0.921-11.105; Table 3).
Table 3.
Multivariate analysis for risk factors for predicting pertussis infection*
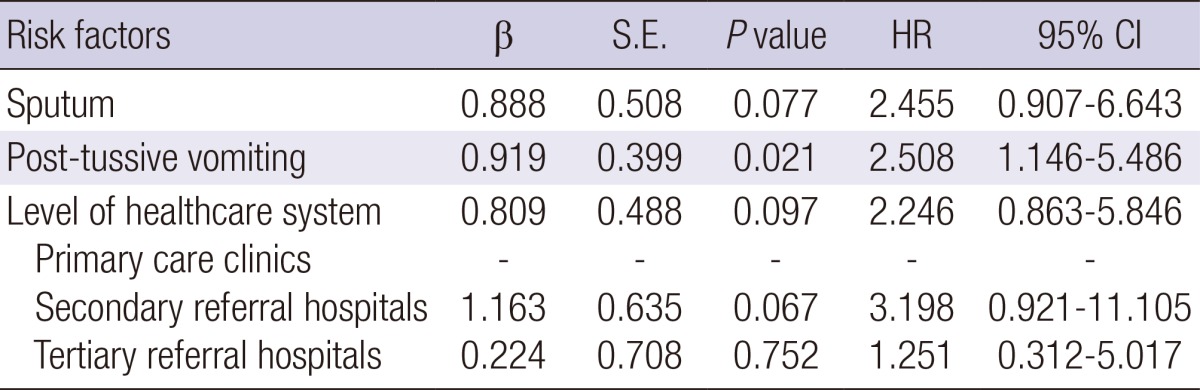
*Hosmer-Lemeshow test, chi-square=1.502 and P=0.959. CI, confidence interval; HR, hazard ratio; S.E., standard error.
DISCUSSION
In the present study, we found several interesting results. First, the incidence of pertussis infection was 6.9% in a non-epidemic outpatient setting. Second, more than two-thirds of confirmed cases (70.6%) were diagnosed at secondary referral hospitals. Third, the peak incidence of pertussis was in February and August, with no confirmed case during March-June. Fourth, post-tussive vomiting was significantly associated with PCR positivity for B. pertussis.
Despite the introduction of a vaccine, a worldwide resurgence of pertussis infection has been reported in recent years, and the incidence in adolescents and adults has increased dramatically (1, 3). In particular, the US experienced a large outbreak in 2012, in which some US states experienced an incidence similar to those in the 1940s and 1950s (5). This worldwide increase in the incidence of pertussis may be explained by multiple mechanisms: 1) development of more sensitive diagnostic methods, 2) changes in nationwide surveillance systems for communicable diseases, 3) waning immunity among adolescents and adults, and 4) vaccine failure (or genetic changes) (1, 17). Although pertussis are preventable by vaccination in childhood, the infection in adolescents and adults remains common and seems to be an endemic feature in the present vaccine era (8).
In Korea, the surveillance of pertussis relies mostly on clinical notification systems, and most reported cases are in children (18). Studies of adult pertussis are rare. Park et al. (10) reported that the incidence of pertussis was 2.9% among adult patients; in our previous study, it was 0.5% (11). These low positivity rates may be partly due to methodological problems such as the inclusion of patients with viral upper respiratory infection, variable specimen quality, and the absence of serological tests. In the present study, we enrolled only patients who visited clinics or hospitals on weekdays to insure specimen transfer within 24 hr, and one professional laboratory performed all microbiological tests. We did not perform serological tests, which have proven useful for diagnosis of the infection after 3-4 weeks (19,20,21), because these tests are not readily available in routine clinical practice. Therefore, we decided to use both PCR and culture for sample testing, and only enrolled patients with a cough duration of ≤30 days. However, the actual incidence of B. pertussis infection may have been underestimated. Additionally, PCR testing has also a risk of false positives (22, 23).
However, the incidence rate can vary by the length of cough illness used as an inclusion criterion. In several Western studies (7, 24), the incidence rate was reported to be 26%-31% among adults with subacute or chronic cough illnesses (a duration of ≥3 weeks), but a study from Israel reported a rate of 7% among adults with acute cough illnesses (25). According to the clinical definition of the CDC for B. pertussis infection, a cough illness of ≥2 weeks duration is required. In particular, previous studies showed that a mean duration of cough illnesses was 36-48 days among adult pertussis (24). However, we only focused on patients with acute cough illnesses in the present study; the median duration of cough illnesses was 14 days among the confirmed cases. Although the inclusion criteria were different from previous studies, we think that our results can provide an evidence of B. pertussis infection among adolescents and adults with acute cough illnesses.
One of the interesting findings in the present study was that confirmed cases were the most frequent in patients who visited secondary referral hospitals, which are usually community hospitals or medium-sized university hospitals. Only 3 of 34 confirmed cases were diagnosed at primary care clinics. Although the statistical analysis showed a borderline significance, this result can imply an important point that has been previously overlooked. That is, the patients with severe cough are less likely to attend primary care clinics. These patients can go directly to a secondary referral hospital in the Korean healthcare system. Otherwise, it is possible that B. pertussis infection is rarely suspected or diagnostic tests are unlikely to be performed by primary physicians. This can be due to the symptoms of B. pertussis infection that are not easily differentiated from those of the common cold in adults or adolescents. However, based on the results of our study, we can suggest that in countries such as Korea, we need to pay more attention to patients visiting secondary referral hospitals, and the different incidence by level of healthcare system should be taken into account in a future strategy for controlling pertussis. In addition, a continued education of healthcare providers will also be critical to detect adult (or adolescent) pertussis cases in the early period of their illness, when they usually visit primary care clinics.
With regard to the seasonal trend, experts reported no definite seasonal pattern of pertussis. However, they acknowledged that the incidence may increase in summer and fall (26). However, a Danish study reported that the annual peak incidence was in August (27). The authors also found that monthly trends in adults showed a high correlation with trends in children but there was no evidence of a relationship between the increase in incidence and the opening of school. However, in a study by Rendi-Wagner et al. (28), there was no seasonal occurrence. In Korea, there seems to be no definite seasonal pattern when data from the national clinical reporting systems are analyzed (18). However, we identified a peak incidence in February and August but no confirmed cases in March-June in the present study. Although we evaluated the incidence of infection during an only 1-yr period and our study was not population-based, these findings are interesting. Therefore, the role of a seasonal effect should be further examined in the future. In addition, a study of the safety of concurrent vaccination for seasonal influenza and pertussis has been reported (29). Considering the similarity of the symptoms of the two illnesses such as cough, studies of the epidemiological relationship and a vaccination strategy to cover both diseases in adults would be intriguing.
In the present study, 72.4% of all patients reported a paroxysmal cough, which was similar to our previous study. However, only 7.6% (n=27) of patients reporting a paroxysmal cough were PCR positive. Additionally, only eight patients (1.6%) had all three classical pertussis symptoms, only two of which were PCR positive. Although the diagnostic accuracy of the three classical symptoms remains questionable (11, 30, 31), we found that post-tussive vomiting was significantly associated with pertussis in the present study. Miyashita et al. (32) also demonstrated the importance of post-tussive vomiting in the clinical diagnosis of pertussis infection. Therefore, physicians should be alert to the presence of post-tussive vomiting among adolescent and adult patients with a cough illness. In addition, we plotted the receiver operating characteristic (ROC) curves for sputum, rhonchi, and the three classical pertussis symptoms for predicting B. pertussis infection. However, none of these symptoms alone (or in combination) showed a significant area under the curve value (data not shown).
This study had several limitations. First, as mentioned above, because no serologic tests were performed, we may have underestimated the true incidence of B. pertussis infection. Second, we only investigated 20 household contacts of confirmed cases. Therefore, we were unable to calculate the secondary attack rate. Third, B. pertussis PCR testing is not standardized; therefore, the possibility of false positives should be considered. Fourth, the numbers of patients and participating hospitals were not distributed evenly across the six provinces. Therefore, we acknowledge that our results can be limited by several biases. However, this was a multi-center, prospective study, which included primary care clinics as well as secondary and tertiary referral hospitals across the country. Our goal was to investigate several epidemiological characteristics of adult (and/or adolescent) pertussis in a non-epidemic outpatient setting. Therefore, we believe that our study has meaningful results for the future epidemiological strategy.
In conclusion, using a PCR-based method, 6.9% of adolescent and adult patients with a bothersome cough (≤30 days) were shown to have pertussis in a non-epidemic outpatient setting. The incidence was the highest in patients who visited secondary referral hospitals and there were no confirmed cases in spring. Therefore, the different incidence by hospital levels and season must be taken into account to develop a better strategy for controlling B. pertussis infection.
ACKNOWLEDGMENTS
The authors would like to thank Seung Hun Jang, Yong Il Hwang, Joo-Hee Kim (Hallym University Sacred Heart Hospital), Kyeong Min Shon (Inseong Clinic, Chuncheon), Yong Cheol Lee (Chonbuk University Hospital), Yee Hyeong Kim (Kyung Hee University Hospital) and A-Sook Shim (Seegen Medical Foundation) for their contribution to this multicenter study.
Footnotes
The authors have no conflicts of interest to declare.
This study was supported by the Korea Centers of Disease Control and Prevention (KCDC; 2011 Academic Research Fund, 2011-E46003-00).
References
- 1.Hewlett EL, Edwards KM. Clinical practice: pertussis - not just for kids. N Engl J Med. 2005;352:1215–1222. doi: 10.1056/NEJMcp041025. [DOI] [PubMed] [Google Scholar]
- 2.Stojanov S, Liese J, Belohradsky BH. Hospitalization and complications in children under 2 years of age with Bordetella pertussis infection. Infection. 2000;28:106–110. doi: 10.1007/s150100050056. [DOI] [PubMed] [Google Scholar]
- 3.Celentano LP, Massari M, Paramatti D, Salmaso S, Tozzi AE EUVAC-NET Group. Resurgence of pertussis in Europe. Pediatr Infect Dis J. 2005;24:761–765. doi: 10.1097/01.inf.0000177282.53500.77. [DOI] [PubMed] [Google Scholar]
- 4.Mooi FR, Van Der Maas NA, De Melker HE. Pertussis resurgence: waning immunity and pathogen adaptation - two sides of the same coin. Epidemiol Infect. 2014;142:685–694. doi: 10.1017/S0950268813000071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cherry JD. Epidemic pertussis in 2012: the resurgence of a vaccine-preventable disease. N Engl J Med. 2012;367:785–787. doi: 10.1056/NEJMp1209051. [DOI] [PubMed] [Google Scholar]
- 6.Kwon HJ, Yum SK, Choi UY, Lee SY, Kim JH, Kang JH. Infant pertussis and household transmission in Korea. J Korean Med Sci. 2012;27:1547–1551. doi: 10.3346/jkms.2012.27.12.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cherry JD. The epidemiology of pertussis: a comparison of the epidemiology of the disease pertussis with the epidemiology of Bordetella pertussis infection. Pediatrics. 2005;115:1422–1427. doi: 10.1542/peds.2004-2648. [DOI] [PubMed] [Google Scholar]
- 8.Cherry JD. Epidemiology of pertussis. Pediatr Infect Dis J. 2006;25:361–362. doi: 10.1097/01.inf.0000210478.60841.69. [DOI] [PubMed] [Google Scholar]
- 9.Jackson LA, Cherry JD, Wang SP, Grayston JT. Frequency of serological evidence of Bordetella infections and mixed infections with other respiratory pathogens in university students with cough illnesses. Clin Infect Dis. 2000;31:3–6. doi: 10.1086/313911. [DOI] [PubMed] [Google Scholar]
- 10.Park S, Lee MG, Lee KH, Park YB, Yoo KH, Park JW, Kim C, Lee YC, Park JS, Kwon YS, et al. A Multicenter Study of Pertussis Infection in Adults with Coughing in Korea: PCR-Based Study. Tuberc Respir Dis (Seoul) 2012;73:266–272. doi: 10.4046/trd.2012.73.5.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Park WB, Park SW, Kim HB, Kim EC, Oh M, Choe KW. Pertussis in adults with persistent cough in South Korea. Eur J Clin Microbiol Infect Dis. 2005;24:156–158. doi: 10.1007/s10096-005-1277-y. [DOI] [PubMed] [Google Scholar]
- 12.Broder KR, Cortese MM, Iskander JK, Kretsinger K, Slade BA, Brown KH, Mijalski CM, Tiwari T, Weston EJ, Cohn AC, et al. Preventing tetanus, diphtheria, and pertussis among adolescents: use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccines recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2006;55:1–34. [PubMed] [Google Scholar]
- 13.Regan J, Lowe F. Enrichment medium for the isolation of Bordetella. J Clin Microbiol. 1977;6:303–309. doi: 10.1128/jcm.6.3.303-309.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lautrop H. Laboratory diagnosis of whooping-cough or Bordetella infections. Bull World Health Organ. 1960;23:15–35. [PMC free article] [PubMed] [Google Scholar]
- 15.Glare EM, Paton JC, Premier RR, Lawrence AJ, Nisbet IT. Analysis of a repetitive DNA sequence from Bordetella pertussis and its application to the diagnosis of pertussis using the polymerase chain reaction. J Clin Microbiol. 1990;28:1982–1987. doi: 10.1128/jcm.28.9.1982-1987.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Houard S, Hackel C, Herzog A, Bollen A. Specific identification of Bordetella pertussis by the polymerase chain reaction. Res Microbiol. 1989;140:477–487. doi: 10.1016/0923-2508(89)90069-7. [DOI] [PubMed] [Google Scholar]
- 17.Cherry JD. The science and fiction of the "resurgence" of pertussis. Pediatrics. 2003;112:405–406. doi: 10.1542/peds.112.2.405. [DOI] [PubMed] [Google Scholar]
- 18.Korean Centers for Disease Control and Prevention. Sentinal surveillance systems for communicable infectious diseases. [accessed 30 December 2012]. Available at http://www.cdc.go.kr.
- 19.Birkebaek NH, Kristiansen M, Seefeldt T, Degn J, Moller A, Heron I, Andersen PL, Moller JK, Ostergård L. Bordetella pertussis and chronic cough in adults. Clin Infect Dis. 1999;29:1239–1242. doi: 10.1086/313448. [DOI] [PubMed] [Google Scholar]
- 20.Cattaneo LA, Reed GW, Haase DH, Wills MJ, Edwards KM. The seroepidemiology of Bordetella pertussis infections: a study of persons ages 1-65 years. J Infect Dis. 1996;173:1256–1259. doi: 10.1093/infdis/173.5.1256. [DOI] [PubMed] [Google Scholar]
- 21.Miller E, Fleming DM, Ashworth LA, Mabbett DA, Vurdien JE, Elliott TS. Serological evidence of pertussis in patients presenting with cough in general practice in Birmingham. Commun Dis Public Health. 2000;3:132–134. [PubMed] [Google Scholar]
- 22.Loeffelholz MJ, Thompson CJ, Long KS, Gilchrist MJ. Comparison of PCR, culture, and direct fluorescent-antibody testing for detection of Bordetella pertussis. J Clin Microbiol. 1999;37:2872–2876. doi: 10.1128/jcm.37.9.2872-2876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zouari A, Smaoui H, Kechrid A. The diagnosis of pertussis: which method to choose? Crit Rev Microbiol. 2012;38:111–121. doi: 10.3109/1040841X.2011.622715. [DOI] [PubMed] [Google Scholar]
- 24.Von König CH, Halperin S, Riffelmann M, Guiso N. Pertussis of adults and infants. Lancet Infect Dis. 2002;2:744–750. doi: 10.1016/s1473-3099(02)00452-8. [DOI] [PubMed] [Google Scholar]
- 25.Lieberman D, Shvartzman P, Lieberman D, Ben-Yaakov M, Lazarovich Z, Hoffman S, Mosckovitz R, Ohana B, Leinonen M, Luffy D, et al. Etiology of respiratory tract infection in adults in a general practice setting. Eur J Clin Microbiol Infect Dis. 1998;17:685–689. doi: 10.1007/s100960050161. [DOI] [PubMed] [Google Scholar]
- 26.Centers for Disease Control and Prevention. Epidemiology and Prevention of Vaccine-Preventable Diseases. 12th ed. Atlanta: Pinkbook; 2012. [Google Scholar]
- 27.De Greeff SC, Dekkers AL, Teunis P, Rahamat-Langendoen JC, Mooi FR, De Melker HE. Seasonal patterns in time series of pertussis. Epidemiol Infect. 2009;137:1388–1395. doi: 10.1017/S0950268809002489. [DOI] [PubMed] [Google Scholar]
- 28.Rendi-Wagner P, Paulke-Korinek M, Stanek G, Khanakah G, Kollaritsch H. Impact of a pertussis booster vaccination program in adolescents and adults on the epidemiology of pertussis in Austria. Pediatr Infect Dis J. 2007;26:806–810. doi: 10.1097/INF.0b013e318124a9dd. [DOI] [PubMed] [Google Scholar]
- 29.Weston WM, Friedland LR, Wu X, Howe B. Vaccination of adults 65 years of age and older with tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Boostrix®): results of two randomized trials. Vaccine. 2012;30:1721–1728. doi: 10.1016/j.vaccine.2011.12.055. [DOI] [PubMed] [Google Scholar]
- 30.Strebel P, Nordin J, Edwards K, Hunt J, Besser J, Burns S, Amundson G, Baughman A, Wattigney W. Population-based incidence of pertussis among adolescents and adults, Minnesota, 1995-1996. J Infect Dis. 2001;183:1353–1359. doi: 10.1086/319853. [DOI] [PubMed] [Google Scholar]
- 31.Yaari E, Yafe-Zimerman Y, Schwartz SB, Slater PE, Shvartzman P, Andoren N, Branski D, Kerem E. Clinical manifestations of Bordetella pertussis infection in immunized children and young adults. Chest. 1999;115:1254–1258. doi: 10.1378/chest.115.5.1254. [DOI] [PubMed] [Google Scholar]
- 32.Miyashita N, Akaike H, Teranishi H, Kawai Y, Ouchi K, Kato T, Hayashi T, Okimoto N. Diagnostic value of symptoms and laboratory data for pertussis in adolescent and adult patients. BMC Infect Dis. 2013;13:129. doi: 10.1186/1471-2334-13-129. [DOI] [PMC free article] [PubMed] [Google Scholar]