Abstract
This study was performed to investigate differences between children who did and did not experience peer rejection in psychological state through surveys and in emotion processing during an interpersonal stress challenge task to reflect naturalistic interpersonal face-to-face relationships. A total of 20 right-handed children, 10 to 12 yr of age, completed self-rating questionnaires inquiring about peer rejection in school, depression, and anxiety. They then underwent an interpersonal stress challenge task simulating conditions of emotional stress, in reaction to positive, negative and neutral facial expression stimuli, using interpersonal feedbacks, and functional magnetic resonance imaging (FMRI) for an analysis of neural correlates during the task. Ten were the peer-rejection group, whereas the remainder were the control group. Based on the behavioral results, the peer-rejection group exhibited elevated levels of depression, state anxiety, trait anxiety and social anxiety as compared to the control group. The FMRI results revealed that the peer-rejection group exhibited greater and remarkably more extensive activation of brain regions encompassing the amygdala, orbitofrontal cortex and ventrolateral prefrontal cortex in response to negative feedback stimuli of emotional faces. The different brain reactivities characterizing emotion processing during interpersonal relationships may be present between children who do and do not experience peer rejection.
Graphical Abstract
Keywords: Peer Rejection, Child, Magnetic Resonance Imaging, Emotional Face Processing
INTRODUCTION
Interpersonal relationships with peers are important for the mental and physical development of school-aged children. However, peer rejection, ostracism by peers or social exclusion may occur as a form of school violence that is emerging as one of the most serious problems in children (1, 2, 3).
Recently, several studies describing neural activity correlated with experimentally-induced peer rejection situations like a cyberball task in adults and adolescents have been performed. Associated brain regions including the amygdala, insula, dorsal anterior cingulate cortex, ventrolateral prefrontal cortex and ventral striatum have been found from these researches (4, 5). However, there is little research conducted on emotion processing during interpersonal relationships in children experiencing peer rejection.
We performed this study to investigate emotion processing of children experiencing peer rejection during interpersonal relationships and to find the effect of peer rejection in school on emotion processing of children during interpersonal relationships. The study was designed to compare processing of emotional facial expression, the neural responses to facial expressions of emotion, between children who did and did not experience peer rejection in school via functional magnetic resonance imaging (FMRI). As an experimental paradigm, the interpersonal stress challenge task to simulate conditions of emotional stress, in reaction to positive, negative and neutral facial expression stimuli, using interpersonal feedbacks was utilized, in order to simulate naturalistic interpersonal face-to-face relationships.
The experience of peer rejection can result in negative effects on mental and physical health evoked by psychological symptoms, including depression and anxiety (2, 6, 7, 8). Children experiencing peer rejection express more generalized negative emotion than their peers (9). Recently, the amygdala has been found to be associated with emotional face processing and the amygdala activity during adolescence has been suggested to be more reactive to emotional face than either childhood or adulthood (10, 11). Other brain regions involved in facial emotion recognition such as face-selective regions (the lateral fusiform gyrus and inferior occipital gyrus), the superior temporal sulcus, anterior temporal pole, and the limbic system (the orbitofrontal cortex and retrosplenial or posterior cingulated regions) have been reported as members of the distributed face network (12).
Therefore, on the basis of the findings above mentioned, we hypothesized that children who experience peer rejection would exhibit different neural responses to emotion during interpersonal relationships compared to children who do not experience peer rejection, especially in activities of brain regions involved in face perception, emotion processing and emotion regulation, including amygdala, and also in response to interpersonal feedback stimuli of negative emotion.
Herein, the differences between children who do and do not experience peer rejection in psychological state through surveys and in neural correlates to emotion processing during the FMRI task were assessed.
MATERIALS AND METHODS
Participants
Two-hundred-three children in the 5th and 6th grades from five elementary schools participated in the screening survey using the ostracism scale (2). Of the 203 children, 19 children rated highly on the ostracism scale (≥28 points). Among the 19 children, 14 children who and whose parents consented to the study participated in a neuroimaging study as a peer-rejection group. The children's parents and teachers noted their experiences of peer rejection in school and their experiences of peer rejection were confirmed by interviews of one single psychiatrist with the children, their parents and their teachers. Ten of the 14 children completed the study, and 4 children dropped out. The control group consisted of 10 children who scored less than 18 points on the ostracism scale. The participants were right-handed and presented with no medical or neurological disorders, no history of craniocerebral injury and no psychological disorders. All the children of the peer-rejection group were confirmed not to have depressive or anxiety disorders after the diagnostic evaluation by the single psychiatrist via structured clinical interviews using Kiddie-Schedule for Affective disorders and Schizophrenia-Present and Lifetime (K-SADS-PL).
Questionnaires
The ostracism scale (2) was developed as self-rating questionnaires to assess experiences of peer rejection, and composed of 12 items that were manifestations of peer rejection in school determined by surveying 2,972 middle school students (2). Each item was rated on five-point scale (1 to 5), from "none" to "almost everyday," according to the frequency over the past one year from the time of survey. In the study of developing the ostracism scale 157 of the 2,972 middle school students whose scores upon the ostracism scale were higher than the value that is twice the standard deviation above the average (mean + 2SD =27.25) were determined as a group experiencing peer rejection in school (2). We used 28 points as a cutoff value for the peer rejection group and 18 points (which is mean - 2SD in the study of developing the ostracism scale) for the control group. The Korean version of the Kovacs Children's Depression Inventory (KKCDI) (13, 14) was used to evaluate the severity of depressive symptoms; the Korean version of the Spielberger State-Trait Anxiety Inventory for Children (KSS-TAI-C) (15, 16) was used to examine trait and state anxiety. Social anxiety was measured using the Korean Social Anxiety Scale for Children and Adolescent (KSAS-CA) (17), which was developed based on the Social Anxiety Scale for Children-Revised by LaGreca and Stone (18), as well as the Social Phobia and Anxiety Inventory for Children by Beidel et al. (19).
FMRI paradigms and post-scan ratings
During the scanning the subjects solved geometric puzzles modified from Standard Raven's Progressive Matrices that were all unsolvable and equally difficult. After selecting answers to each puzzle, they were shown pre-recorded video clips of facial expressions of an intelligence assessment expert, depicting positive, negative and neutral interpersonal feedbacks about their performances in a pseudo-random order. Each puzzle was presented for 4 sec, followed by 4 sec of an interpersonal feedback response, and then a 4 sec period in which subjects rated their subjective emotional responses to the feedbacks they received. Each trial lasted a total of 28 sec including fixations, and the whole experiment included 60 trials. After completing the FMRI session, the participants were asked to rate their degree of attention (1=not attentive, 9=very attentive), the impact for the feedbacks (1=not impacted, 9=very impacted), the difficulty of the task (1=not difficult, 9=very difficult) and the self-relatedness to the task (1=related to the performance, 9=related to themselves).
Acquisition of imaging data
Magnetic resonance (MR) images were acquired using a 1.5T Avanto system (Siemens AG, Erlangen, Germany). Functional images were acquired as T2*-weighted echo-planar MR images across three consecutive sessions. At each session, a total of 307, 341 and 312 whole brain images were collected, respectively, with the blood-oxygen-level dependent (BOLD) contrast (repetition time=2,000 msec, echo time=24 msec, slice number=29, slice thickness=4 mm, matrix size=64×64, in-plane resolution=3.59 mm×3.59 mm). A structural T1-weighted MR image was acquired for coregistration to functional images (slice number=160, slice thickness=1 mm, matrix size=512×512, in-plane resolution=0.45 mm×0.45 mm).
Data analysis
For the analysis of demographic characteristics, behavioral results, the subjective emotional responses, and the validity of the FMRI paradigm, the paired t-test, the chi-square test and correlation analyses were used using SAS/PC version 9.2 (SAS Institute Inc., Cary, NC, USA).
FMRI data were preprocessed and analyzed using the statistical parametric mapping platform (SPM8; Wellcome Department of Cognitive Neurology, London, UK; http://www.fil.ion.ucl.ac.uk/spm/). The scans were slice-time corrected, motion corrected, warped (nonlinear normalisation), resliced to match a standardized template in Montreal Neurological Institute space, and smoothed with a 5×5×5-µL kernel in the first level analyses. The study design used a standard 2-level hierarchical general linear model. First-level linear contrasts were generated to assess BOLD activity changes during each of the three experimental feedback conditions compared to the baseline resting condition (e.g., crosshair fixation). Second-level random-effects group analyses were performed using participants' individual first-level contrast images. To compare the brain areas activated in response to the interpersonal feedbacks between the two groups, an analysis of covariance was performed with baseline depression and anxiety scores used as covariates. A significance threshold was set to P=0.001 (uncorrected) paired with a minimum cluster size threshold of 10 voxels.
Ethics statement
This study was approved by the institutional review board at the Uijeongbu St. Mary's Hospital, The Catholic University of Korea (IRB number: UC 10EASV0033). Informed consent was obtained from all participants and their parents.
RESULTS
Demographic characteristics and behavioral results
There were no significant group differences with respect to age and sex (Table 1). The scores of ostracism scale, KKCDI, KSSAI-C, KSTAI-C, KSAS-CA and three sub-factors of KSAS-CA (fear of negative evaluation, avoidance, and non-assertiveness) were significantly higher for the peer-rejection group, as compared to the control group (ostracism scale, P<0.001; KKCDI, P<0.001; KSSAI-C, P<0.05; KSTAI-C, P<0.05; KSAS-CA, P<0.05; the three sub-factors of KSAS-CA, P<0.05) (Table 1).
Table 1.
Demographic characteristics and behavioral results of the participants
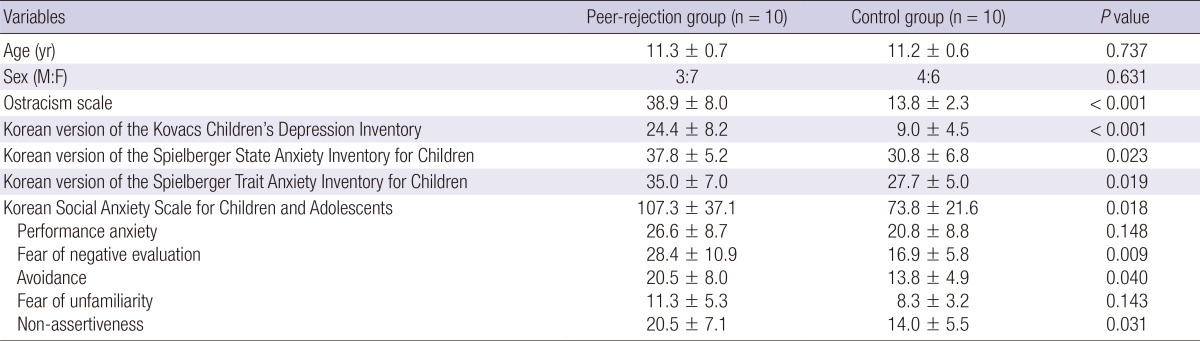
Continuous variables are given as mean±standard deviation.
Subjective emotional response and validity of FMRI paradigm
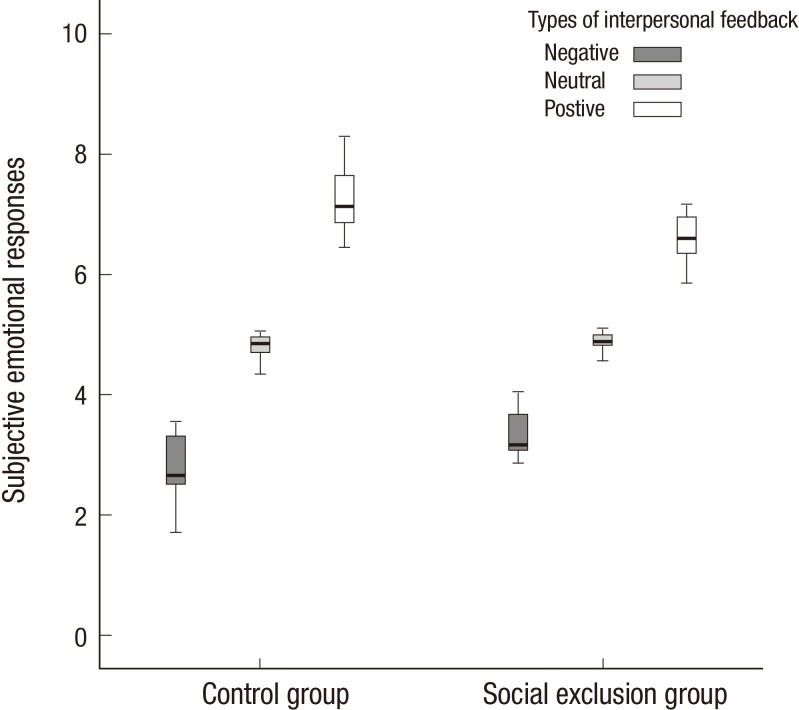
There were no significant group differences in the ratings of subjective emotional response to the positive, negative, and neutral interpersonal feedbacks (Fig. 1). The attention level was significantly lower in the peer-rejection group, as compared to the control group (P<0.05) (Table 2). There were no significant group differences with regard to the ratings of the impact for the interpersonal feedbacks during the task, the difficulty of the task, and the self-relatedness to the task. However, the subjective emotional response was not correlated with the measures of the degree of attention, the impact for the feedback, task difficulty or the self-relatedness to the task.
Fig. 1.
Subjective emotional response of the participants to the different types of interpersonal feedback.
Table 2.
Comparison of post-scan ratings between the two groups
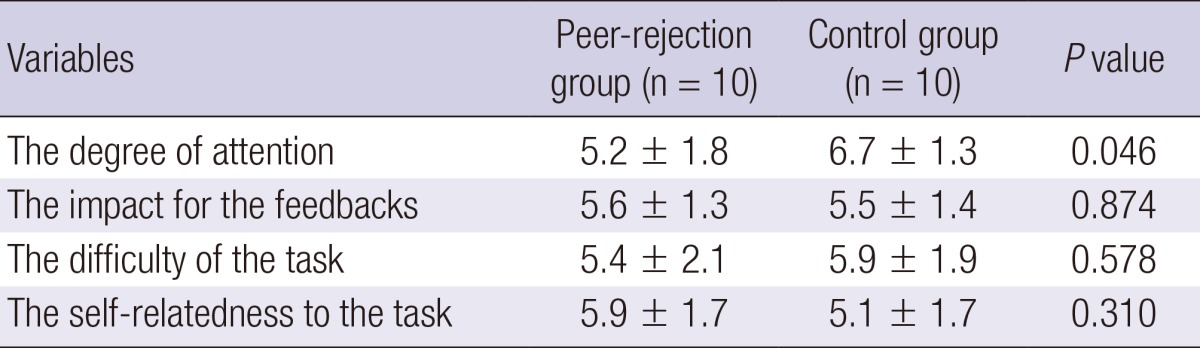
Continuous variables are given as mean±standard deviation.
Brain areas exhibiting altered activity in response to different types of interpersonal feedback by FMRI
In the peer-rejection group, negative interpersonal feedback was characterized by significant activation of the right middle occipital gyrus, left inferior occipital gyrus, left midbrain/hypothalamus, right amygdala/hippocampus, bilateral superior temporal gyrus, bilateral middle temporal gyrus, bilateral cerebellum, right precuneus/cuneus, bilateral inferior frontal gyrus/Brodmann area (BA)47, left calcarine/lingual lobe, left inferior parietal lobe, and right ventrolateral/orbitofrontal lobe. In contrast, responses to positive feedback were related to significant activity in the right middle occipital gyrus and right middle temporal gyrus. The neutral feedback was characterized by significant activation of the right middle frontal gyrus/BA46, right superior frontal gyrus/BA9, and bilateral occipital lobe/BA18 (Table 3).
Table 3.
The regions with altered brain activity in response to different types of interpersonal feedback in the peer-rejection group (height threshold T = 4.3, uncorrected P < 0.001, extended threshold k = 10)
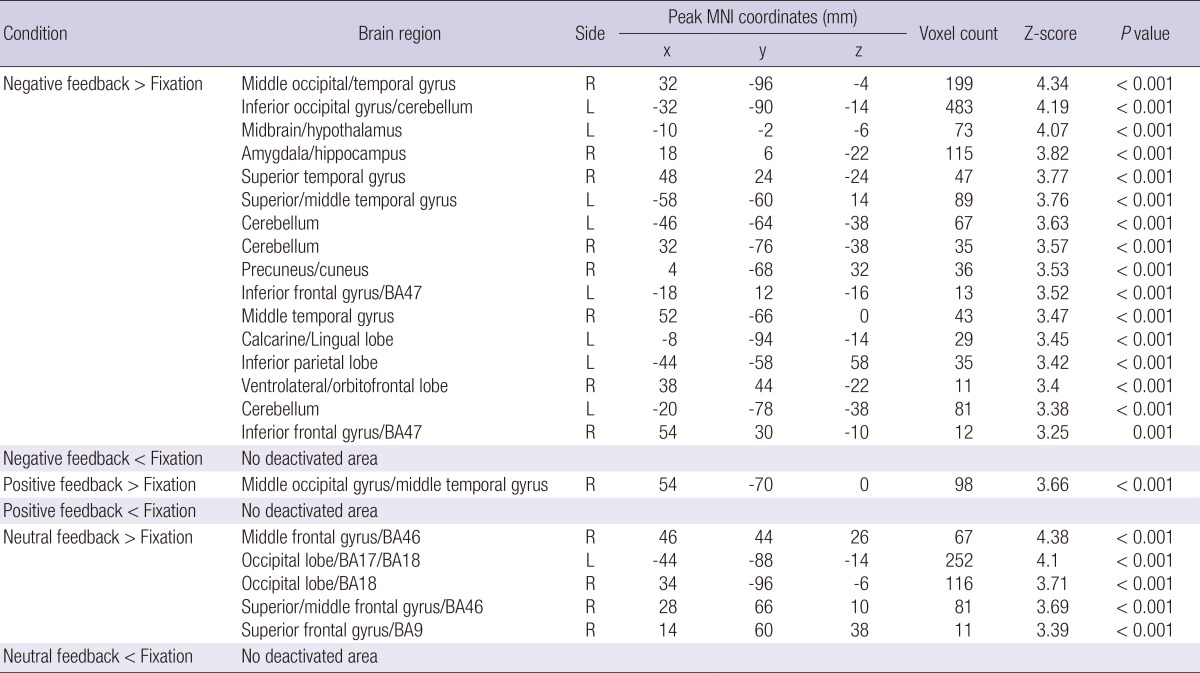
L, left hemisphere; R, right hemisphere; BA, Brodmann's Area. The x, y, and z coordinates are in the Montreal Neurological Institute (MNI) coordinates.
In the control group, there were no significantly activated areas in response to the negative interpersonal feedback, and only the right superior temporal gyrus was significantly activated during the positive feedback condition. In contrast, the right supplementary motor area/BA6, right middle temporal gyrus, right superior frontal gyrus/BA10/BA9, right ventrolateral prefrontal cortex/BA45/BA 46, right lateral orbitofrontal cortex/BA47, left inferior parietal lobe and left occipital lobe/BA17 were significantly activated in response to the neutral feedback (Table 4).
Table 4.
The regions with altered brain activity in response to different types of interpersonal feedback in the control group (height threshold T = 4.3, uncorrected P < 0.001, extended threshold k = 10)
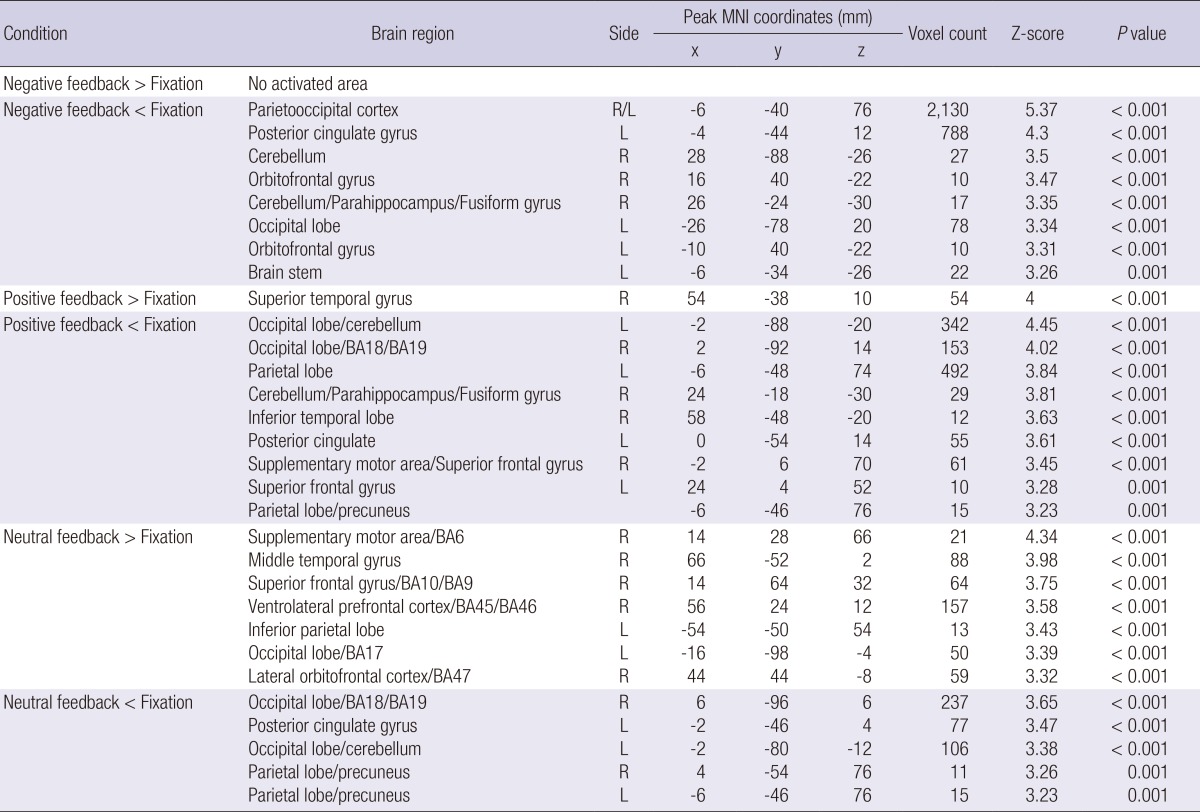
L, left hemisphere; R, right hemisphere; BA, Brodmann's Area. The x, y, and z coordinates are in the Montreal Neurological Institute (MNI) coordinates.
Comparison of brain activity between the two groups
In the peer-rejection group, the bilateral orbitofrontal gyrus/BA11/ventrolateral prefrontal cortex/BA47, left middle and inferior occipital gyrus/fusiform gyrus, left postcentral gyrus, bilateral posterior cingulate gyrus, bilateral cerebellum, right temporal pole, bilateral parahippocampus/amygdala, right cuneus, right supplementary motor area/cingulated gyrus/BA24, and left inferior temporal gyrus were significantly more activated than the control group during the negative interpersonal feedback condition (Table 5 and Fig. 2). Furthermore, the peer-rejection group showed significantly greater activation than the control group in the bilateral occipital lobe/BA17/BA18 and right inferior occipital gyrus in response to the positive interpersonal feedback, whereas greater activation of the right inferior occipital gyrus and right occipital lobe/cuneus was observed in the peer-rejection group in response to the neutral feedback (Table 5).
Table 5.
Comparison of activated brain areas in response to interpersonal feedbacks between the peer-rejection group and the control group controlled for baseline depression and anxiety scores (height threshold T = 3.69, uncorrected P < 0.001, extended threshold k = 10)
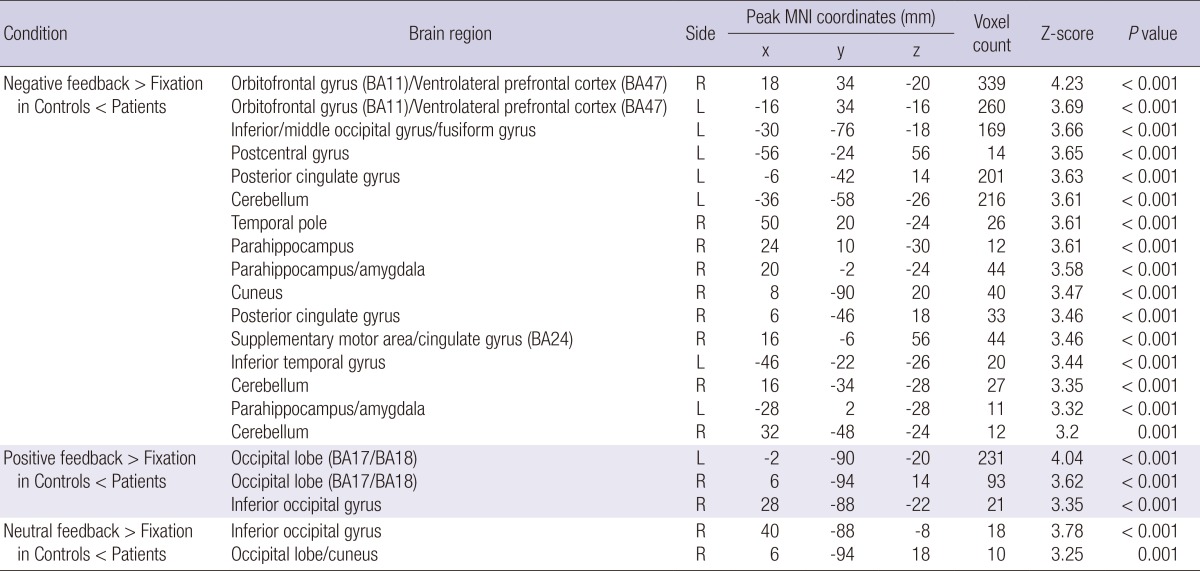
L, left hemisphere; R, right hemisphere; BA, Brodmann's Area. The x, y, and z coordinates are in the Montreal Neurological Institute (MNI) coordinates.
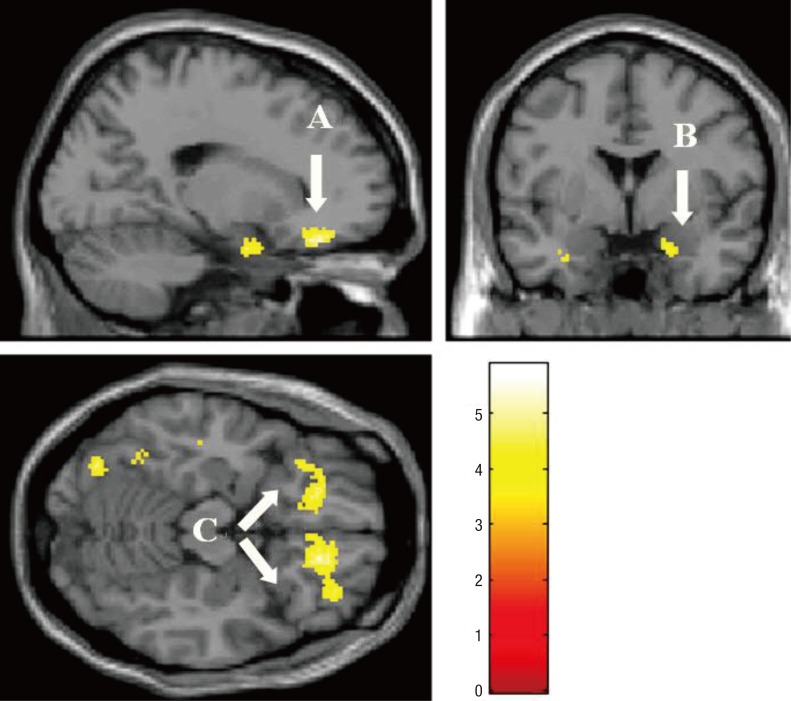
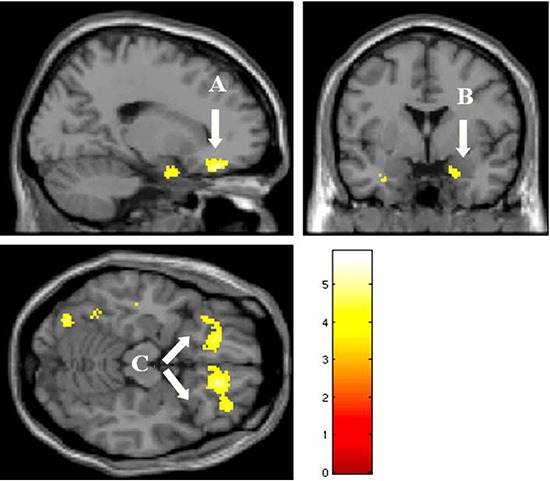
Fig. 2.
Activated brain regions in the negative interpersonal feedback condition relative to fixation condition in the peer-rejection group than the control group. (A, ventrolateral prefrontal cortex; B, amygdala; C, orbitofrontal gyrus) Baseline depression and anxiety scores were controlled for this comparison (height threshold T = 3.69, uncorrected P < 0.001, extended threshold k = 10).
DISCUSSION
In light of the behavioral results of our study, the children who reported higher peer rejection experiences exhibited elevated levels of depression, state anxiety, trait anxiety and social anxiety as compared to the control group. These results correspond well with the results from earlier works. Previous survey studies regarding peer rejection have shown that the peer-rejected students were overly sensitive to how they are perceived by others, had difficulties expressing their opinions, and showed some of symptoms of psychosis, such as depression, anxiety, obsessive behavior, sensitivity, and aggression (2, 6, 7, 8, 20, 21).
The FMRI results revealed that the peer-rejection group exhibited greater neural activity in response to facial expression stimuli with and without emotion across all three feedback conditions, primarily via activation of the visual areas and face perception areas, as compared to the control group. However, there were no differences in the subjective emotional responses to the three feedback conditions between the two groups. The more remarkable differences appeared during the negative feedback condition. Specifically, the peer-rejection group showed more extensive activation of brain regions encompassing the amygdala, orbitofrontal cortex and ventrolateral prefrontal cortex, which are implicated in emotion processing, as compared to the children in the control group. The FMRI findings that the peer-rejection group exhibited robust patterns of neural activation in response to negative feedback stimuli, despite their lower attention levels, as compared to the control children reinforced their survey results. Namely their high social anxiety due to a fear of negative evaluation by others, and these results may have strong implications in understanding their neural function. Social anxiety or social phobia individuals have been documented to have abnormal facial information processing characterized by a bias for negative emotions (22).
The amygdala, which is engaged in negative emotions such as fear (23), is thought to play an important role in stress-related processing in the peer-rejection group in response to the negative feedback facial stimuli. The negative feedback stimuli are believed to be recognized as fear-relevant stimuli to the peer-rejection children. It has been reported that the amygdala activity positively correlated with social anxiety such as peer rejection during fear perception in adolescents (24) and was heightened in response to fearful face in children with anxiety disorders (25).
The orbitofrontal cortex is involved in the cognitive processing of decision making, and it was recently reported to be associated with facial perception and the regulation of negative emotions (26, 27). The ventrolateral prefrontal cortex is implicated in the regulation of distress associated with both physical pain and negative emotional experiences (4, 28). Furthermore, it has been reported to have a reciprocal relationship with the amygdala in previous studies of social exclusion conditions and negative affect that related to the labeling of emotional face perception (4, 29, 30). In the current study, the orbitofrontal cortex and ventrolateral prefrontal cortex are believed to mediate cognitive function and negative emotion regulation.
The control children showed no amygdala activity for any feedback stimuli. Furthermore, they recruited the greatest extent of brain regional activation, including the right lateral orbitofrontal cortex and right ventrolateral prefrontal cortex, in response to neutral facial feedback, which can be interpreted as an obscure answer to the children.
Recently, other brain areas such as the ventral striatum, ventromedial prefrontal cortex and insula have been reported engaged in facial emotion processing in children (31, 32). However, these neural activities were not observed in the present study.
This study suggests that children who experience peer rejection may exhibit depressive and anxious mental states. Furthermore, these children may show neural activity that is more sensitive to negative emotional faces, which are perceived as salient or fear-relevant stimuli, as a means of recruiting the extensive brain regions, such as the amygdala and emotion regulation cortices. Consequently, distinct brain reactivity of emotional face processing may distinguish between children who do and do not experience peer rejection.
The study has limitations that require consideration, such as the small size of the trial group, which cautions against generalizing the results. For evaluation of attentiveness of the subjects studied during the task, we did not perform objective measurements such as for eye-tracking or a task for evaluating attentiveness. Recently a study for peer rejection in adolescence has been reported, suggesting time spent with friends in adolescence associated with less neural sensitivity to later peer rejection (33). The information of time spent with friends from the subjects studied was not obtained and was not integrated with the results. Finally, this study was not designed and conducted to identify whether the psychological symptoms associated with peer rejection were in relation to real psychosis, through follow-up of the subjects studied. Further and more elaborate investigations considering these numerous factors will be needed.
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Olweus D. Bullying at school: what we know and what we can do. Oxford: Blackwell Publishers; 1993. [Google Scholar]
- 2.Shin SW, Kwon SW, Shin MS, Cho SC. A survey of the psychosis among school violence victims. J Child Adolesc Psychiatry. 2000;11:124–143. [Google Scholar]
- 3.Kahng UH, Lee EH, Yim EJ. The bullying and psyhological traits. Korean J Couns Psychother. 2002;14:445–460. [Google Scholar]
- 4.Masten CL, Eisenberger NI, Borofsky LA, Pfeifer JH, McNealy K, Mazziotta JC, Dapretto M. Neural correlates of social exclusion during adolescence: understanding the distress of peer rejection. Soc Cogn Affect Neurosci. 2009;4:143–157. doi: 10.1093/scan/nsp007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisenberger NI, Lieberman MD, Williams KD. Does rejection hurt? an FMRI study of social exclusion. Science. 2003;302:290–292. doi: 10.1126/science.1089134. [DOI] [PubMed] [Google Scholar]
- 6.Rigby K. Consequences of bullying in schools. Can J Psychiatry. 2003;48:583–590. doi: 10.1177/070674370304800904. [DOI] [PubMed] [Google Scholar]
- 7.Williams KD. Ostracism. Annu Rev Psychol. 2007;58:425–452. doi: 10.1146/annurev.psych.58.110405.085641. [DOI] [PubMed] [Google Scholar]
- 8.Masten CL, Eisenberger NI. Exploring the experience of social rejection in adults and adolescents: a social cognitive neurosicence perspective. In: Harris MJ, editor. Bullying, rejection and peer victimization: a social cognitive neurosicence perspective. New York: Springer publishing company; 2009. pp. 53–78. [Google Scholar]
- 9.Hubbard JA. Emotion expression processes in children's peer interaction: the role of peer rejection, aggression, and gender. Child Dev. 2001;72:1426–1438. doi: 10.1111/1467-8624.00357. [DOI] [PubMed] [Google Scholar]
- 10.Killgore WD, Yurgelun-Todd DA. Unconscious processing of facial affect in children and adolescents. Soc Neurosci. 2007;2:28–47. doi: 10.1080/17470910701214186. [DOI] [PubMed] [Google Scholar]
- 11.Passarotti AM, Sweeney JA, Pavuluri MN. Neural correlates of incidental and directed facial emotion processing in adolescents and adults. Soc Cogn Affect Neurosci. 2009;4:387–398. doi: 10.1093/scan/nsp029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vuilleumier P, Pourtois G. Distributed and interactive brain mechanisms during emotion face perception: evidence from functional neuroimaging. Neuropsychologia. 2007;45:174–194. doi: 10.1016/j.neuropsychologia.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 13.Cho SC, Lee YS. Development of the Korean form of the Kovacs' children's depression inventory. J Korean Neuropsychiatr Assoc. 1990;29:943–956. [Google Scholar]
- 14.Kovacs M, Beck AT. An empirical-clinical approach toward a definition of childhood depression. In: Schulterbrandt JG, Raskin A, editors. Depression in childhood: diagnosis, treatment, and conceptual models. New York: Raven Press; 1977. pp. 1–25. [Google Scholar]
- 15.Cho SC, Choi JS. Development of the Korean form of the state-trait anxiety inventory for children. Seoul J Psychiatry. 1989;14:150–157. [Google Scholar]
- 16.Spielberger CD. Manual for State-Trait Anxiety Inventory for children. Palo Alto: Consulting Psychologist Press; 1972. [Google Scholar]
- 17.Moon HS, Oh KA. Validation study of the Korean Social Anxiety Scale for Children and Adolescents. Korean J Clin Psychol. 2002;21:429–443. [Google Scholar]
- 18.LaGreca AM, Stone WL. Social anxiety scale for children-revised: factor structure and concurrent validity. J Clin Child Psychol. 1993;22:17–27. [Google Scholar]
- 19.Beidel DC, Turner SM, Morris TL. A new inventory to assess childhood social anxiety and phobia: the social phobia and anxiety inventory for children. Psychol Assess. 1995;7:73–79. [Google Scholar]
- 20.Bhang SY, Yoo HK, Kim JH, Kim B, Lee YS, Ahn D, Suh DS, Cho SC, Hwang JW, Bahn GH. Victims of bullying among Korean adolescents: prevalence and association with psychopathology evaluated using the adolescent mental health and problem behavior screening questionnaire-II standardization study data. J Korean Acad Child Adolesc Psychiatry. 2012;23:23–30. [Google Scholar]
- 21.Kwon SJ, Park TW, Park SH, Yang JC, Chung YC, Chung SK. Prevalence of school bullying and related psychopathology in children and adolescents. J Korean Acad Child Adolesc Psychiatry. 2012;23:143–153. [Google Scholar]
- 22.Machado-de-Sousa JP, Arrais KC, Alves NT, Chagas MH, de Meneses-Gaya C, Crippa JA, Hallak JE. Facial affect processing in social anxiety: tasks and stimuli. J Neurosci Methods. 2010;193:1–6. doi: 10.1016/j.jneumeth.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 23.Furmark T, Fischer H, Wik G, Larsson M, Fredrikson M. The amygdala and individual differences in human fear conditioning. Neuroreport. 1997;8:3957–3960. doi: 10.1097/00001756-199712220-00021. [DOI] [PubMed] [Google Scholar]
- 24.Killgore WD, Yurgelun-Todd DA. Social anxiety predicts amygdala activation in adolescents viewing fearful faces. Neuroreport. 2005;16:1671–1675. doi: 10.1097/01.wnr.0000180143.99267.bd. [DOI] [PubMed] [Google Scholar]
- 25.Thomas KM, Drevets WC, Dahl RE, Ryan ND, Birmaher B, Eccard CH, Axelson D, Whalen PJ, Casey BJ. Amygdala response to fearful faces in anxious and depressed children. Arch Gen Psychiatry. 2001;58:1057–1063. doi: 10.1001/archpsyc.58.11.1057. [DOI] [PubMed] [Google Scholar]
- 26.Mak AK, Hu ZG, Zhang JX, Xiao ZW, Lee TM. Neural correlates of regulation of positive and negative emotions: an fmri study. Neurosci Lett. 2009;457:101–106. doi: 10.1016/j.neulet.2009.03.094. [DOI] [PubMed] [Google Scholar]
- 27.Goodkind MS, Sollberger M, Gyurak A, Rosen HJ, Rankin KP, Miller B, Levenson R. Tracking emotional valence: the role of the orbitofrontal cortex. Hum Brain Mapp. 2012;33:753–762. doi: 10.1002/hbm.21251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Petrovic P, Ingvar M. Imaging cognitive modulation of pain processing. Pain. 2002;95:1–5. doi: 10.1016/s0304-3959(01)00467-5. [DOI] [PubMed] [Google Scholar]
- 29.Hariri AR, Tessitore A, Mattay VS, Fera F, Weinberger DR. The amygdala response to emotional stimuli: a comparison of faces and scenes. Neuroimage. 2002;17:317–323. doi: 10.1006/nimg.2002.1179. [DOI] [PubMed] [Google Scholar]
- 30.Lieberman MD, Eisenberger NI, Crockett MJ, Tom SM, Pfeifer JH, Way BM. Putting feelings into words: affect labeling disrupts amygdala activity in response to affective stimuli. Psychol Sci. 2007;18:421–428. doi: 10.1111/j.1467-9280.2007.01916.x. [DOI] [PubMed] [Google Scholar]
- 31.Pfeifer JH, Masten CL, Moore WE, 3rd, Oswald TM, Mazziotta JC, Iacoboni M, Dapretto M. Entering adolescence: resistance to peer influence, risky behavior, and neural changes in emotion reactivity. Neuron. 2011;69:1029–1036. doi: 10.1016/j.neuron.2011.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pitskel NB, Bolling DZ, Kaiser MD, Crowley MJ, Pelphrey KA. How grossed out are you? the neural bases of emotion regulation from childhood to adolescence. Dev Cogn Neurosci. 2011;1:324–337. doi: 10.1016/j.dcn.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Masten CL, Telzer EH, Fuligni AJ, Lieberman MD, Eisenberger NI. Time spent with friends in adolescence relates to less neural sensitivity to later peer rejection. Soc Cogn Affect Neurosci. 2012;7:106–114. doi: 10.1093/scan/nsq098. [DOI] [PMC free article] [PubMed] [Google Scholar]