Abstract
The nucleoprotein of negative strand RNA viruses forms a major component of the ribonucleoprotein complex that is responsible for viral transcription and replication. However, the precise role of nucleoprotein in viral RNA transcription and replication is not clear. Here we show that nucleoprotein of influenza A virus is entirely dispensable for replication and transcription of short viral RNA-like templates in vivo, suggesting that nucleoprotein represents an elongation factor for the viral RNA polymerase. We also find that the recruitment of nucleoprotein to nascent ribonucleoprotein complexes during replication of full length viral genes is mediated through nucleoprotein-nucleoprotein homo-oligomerisation in a “tail loop-first” orientation and is independent of RNA binding. This work demonstrates that nucleoprotein does not regulate the initiation and termination of transcription and replication by the viral polymerase in vivo and provides new mechanistic insights into the assembly and regulation of viral ribonucleoprotein complexes.
Keywords: assembly, influenza A virus, nucleoprotein, regulation, RNA-dependent RNA polymerase
Introduction
The genomes of all negative strand RNA viruses (NSVs) are coated by the viral nucleoprotein (NP). Together with the viral RNA-dependent RNA polymerase, these ribonucleoprotein (RNP) complexes form the minimal functional unit for all viral transcription and replication. Whereas a great deal is known about the functionality of the viral polymerase, the role of NP remains unclear. In addition, little is known about the mechanism of NP assembly into RNPs or how the polymerase is able to read through NP-bound RNA templates. The atomic structures of NP from different NSVs1 have revealed two prominent common features, namely a positively charged RNA binding cleft and the presence of extensive contacts between neighbouring NP molecules, suggesting at least some commonality in function and mechanism.
The genome of influenza A virus is comprised of 8 single-stranded negative sense RNA (vRNA) segments2. Each genome segment is associated with one heterotrimeric viral RNA-dependent RNA polymerase and multiple copies of NP, forming a viral genomic (v)RNP complex that serves as the template for both transcription and replication3. Transcription is initiated with a 10 – 13 nucleotide long capped primer that is endonucleolytically cleaved from host cellular mRNA4-6, and is prematurely terminated by polyadenylation at an oligo(U) sequence near the 5′ end of each vRNA through a stuttering mechanism7-9. In contrast, replication initiates de novo and generates a full length copy of the template RNA10. The distinct structures of the positive sense RNAs suggest that the initiation and termination mechanisms for vRNA-templated replication and transcription may be coupled i.e. initiation by cap-snatching is coupled to polyadenylation and de novo initiation is coupled to suppression of premature termination.
The role of NP during transcription and replication of the influenza virus genome has long remained an enigma. NP binds RNA with high affinity in a sequence-independent manner11,12, most likely through the positively charged cleft identified in the atomic structure between the head and body domains13,14. In addition, the structure revealed the presence of a tail loop by which NP molecules self-associate into oligomeric structures, which has been shown by mutagenesis to be required for RNP activity15. NP has also been shown biochemically to interact with polymerase subunits PB1 and PB216, but information on the interaction domains is limited17. It is generally thought that during replication the 5′ terminus of the nascent transcript is bound sequence-specifically and co-transcriptionally by “free” polymerase which then serves as a nucleation step for the sequence-independent sequential encapsidation of the transcript by NP3,13,18,19. Although the viral polymerase cannot replicate full-length genomic RNA in the absence of NP, it has previously been shown in vitro and more recently in vivo that replication of short RNA templates can occur in the absence of NP20-24. Binding of NP in vitro was shown to melt the secondary structure of an artificial mini vRNA of 81 nucleotides and it was suggested that one role of NP may be to facilitate RNA transcription11. Indeed, it has previously been proposed that NP may play a central role in genome replication by supporting the elongation of nascent transcripts20,25.
Besides this structural role in organising the RNP complex, NP has been implicated in the regulation of transcription and replication of influenza virus, as several temperature-sensitive NP mutations have been identified that result in defective replication at non-permissive temperatures26,27. Although several models have been proposed (reviewed in Portela and Digard19), the mechanism behind this role is unclear. More recently, the stabilisation model proposed that the synthesis of cRNA or mRNA from the virion-derived vRNPs is stochastic, but that the expression of both polymerase and NP are required for the stabilisation (and replication) of cRNA28. In support of this model, our laboratory has recently shown that the RNA binding activity of NP is crucial for its role in stabilising cRNA, whereas both RNA binding and NP oligomerisation are needed to support replication29.
Evidence which shows that the interaction of viral proteins with cellular factors is required for efficient viral transcription and replication has been accumulating30,31. NP has been shown to interact with numerous cellular factors; most notably, it has been suggested that NP is maintained as a monomer by binding to importin α5 while retaining its RNA binding activity, whereas UAP56 has been proposed to act as a chaperone to facilitate the binding of NP to RNA30,32,33. The minichromosome maintenance replicative helicase complex has been shown to be required for cRNA synthesis in vivo and to stimulate the elongation of nascent cRNA in vitro25,34.
Minireplicon systems, in which artificial, genome-like reporter RNAs are used as model templates that can be replicated and transcribed in vivo by co-expressed viral polymerase and NP, have been available for over two decades. The authenticity of this approach is demonstrated by the rescue of recombinant influenza virus through the simultaneous generation of eight recombinant RNPs in vivo35,36. Here, we have used the minireplicon system with short influenza A virus vRNA-like templates to show that NP plays no role in the regulation of in vivo RNP initiation or termination activity. Our data support the view that the template-associated NP is an essential cofactor required for full processivity of the viral RNA-dependent RNA polymerase during replication of the viral genome. We further find that the co-transcriptional addition of NP to nascent viral RNA is mediated unidirectionally by NP homo-oligomerisation independently of RNA binding.
Results
Micro vRNP-like complexes lacking NP are active in vivo
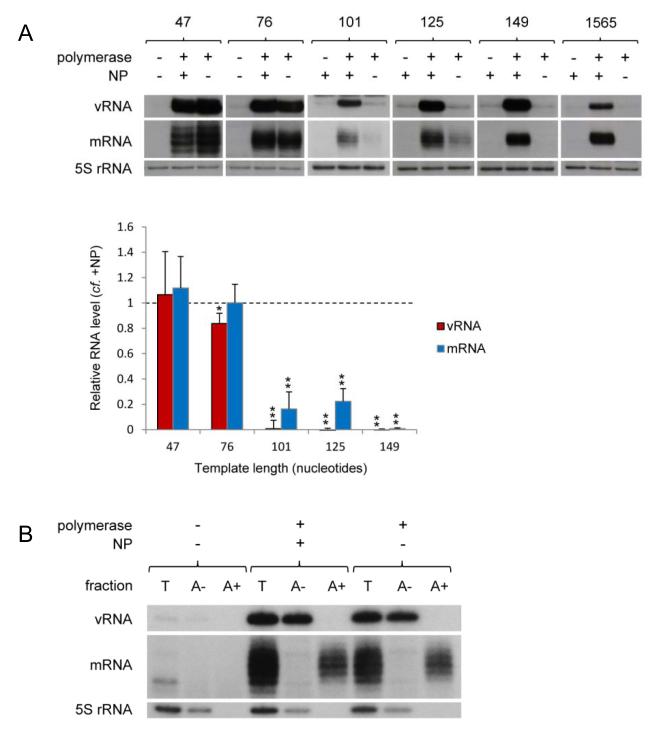
Recently, Resa-Infante et al.21 described the in vivo replication of a micro 46 nucleotide long influenza vRNA-like RNA in the absence of NP. In order to investigate what role, if any, NP plays in the regulation of transcription and replication, we constructed a series of short genome segments based on gene segment 5 by internal deletions, minimally retaining the conserved 3′ and 5′ termini and the oligo(U) stretch near to the 5′ terminus (Supplementary Fig. S1). Following in vivo RNP reconstitution, the accumulation of the negative and positive sense viral RNA was analysed by primer extension (Supplementary Table S1). We found that a microgenome segment of 47 nucleotides in length can be replicated efficiently by the viral RNA polymerase in the absence of NP (Fig. 1A), in agreement with Resa-Infante et al.21. In addition, RNA with a 5′ terminal extension, corresponding to mRNA, was also detected both in the presence and absence of NP. Using longer RNA templates, we determined that the viral polymerase is also able to efficiently replicate and transcribe a 76 nucleotide long template in the absence of NP. However, NP was found to be essential for the replication of templates of 101 nucleotides and longer (Fig. 1A). In order to further characterise the RNA species accumulating during replication and transcription of microgenome segments in the presence and absence of NP, total RNA samples were separated by oligo(dT) chromatography and the fractions were then analysed by primer extension (Fig. 1B). No differences were observed in the character of the RNA species accumulating in the presence or absence of NP. RNA that was bound to oligo(dT) and is thus poly-adenylated, was found to possess a 5′ terminal extension, as expected of viral mRNA. In contrast, vRNA, which is not poly-adenylated, did not bind to oligo(dT). In order to determine whether NP plays any role in replication and transcription of micro RNA templates, titrations of NP and polymerase were carried out. These data revealed that the presence of increasing concentrations of NP stimulates replication and transcription of a 76 nucleotide long template (Supplementary Fig. S2), especially at lower polymerase concentrations. Although we do not currently know the mechanism for this stimulation, significant levels of replication and transcription were detected in the absence of NP at all concentrations of polymerase tested. Overall, therefore, these data suggest that NP is not essential for the activity of reconstituted micro RNPs containing templates of up to 76 nucleotides in length, neither in the initiation or termination of replication or transcription or any possible coupling between these activities. However, NP is required for the transcription and replication of longer templates, suggesting that NP primarily plays a role as an elongation factor.
Fig. 1. The role of NP in the activity of vRNP-like complexes.
(A) The accumulation of vRNA and mRNA following in vivo reconstitution of vRNPs from trimeric polymerase and varying lengths of segment 5-based vRNA-like templates in the presence or absence of wild type NP was analysed by primer extension. PA was omitted as a negative control. The lengths of the vRNA-like gene segments (in nucleotides) are indicated above. The 1565 nucleotide long template represents full length segment 5 containing mutations to prevent expression of NP. Quantification was performed by phosphorimage analysis of at least three independent experiments. Analysis of the 5S rRNA levels served as an internal control. RNA levels detected in the negative control were set to 0. A graph depicting the vRNA and mRNA levels accumulating from each construct in the absence of NP relative to those accumulating in the presence of NP is shown. Error bars indicate standard error of the mean (SEM), and the asterisks indicate a significant difference from 1 (one sample t test; *P < 0.05; **P < 0.01). (B) Primer extension analysis of RNA samples separated by oligo(dT) chromatography from in vivo reconstituted RNPs with the 76 nucleotide long template in the presence or absence of wild type NP. Fractions: T = total RNA; A− = RNA not bound to oligo(dT); A+ = RNA bound to oligo(dT).
Monomeric NP supports activity of mini vRNP-like complexes
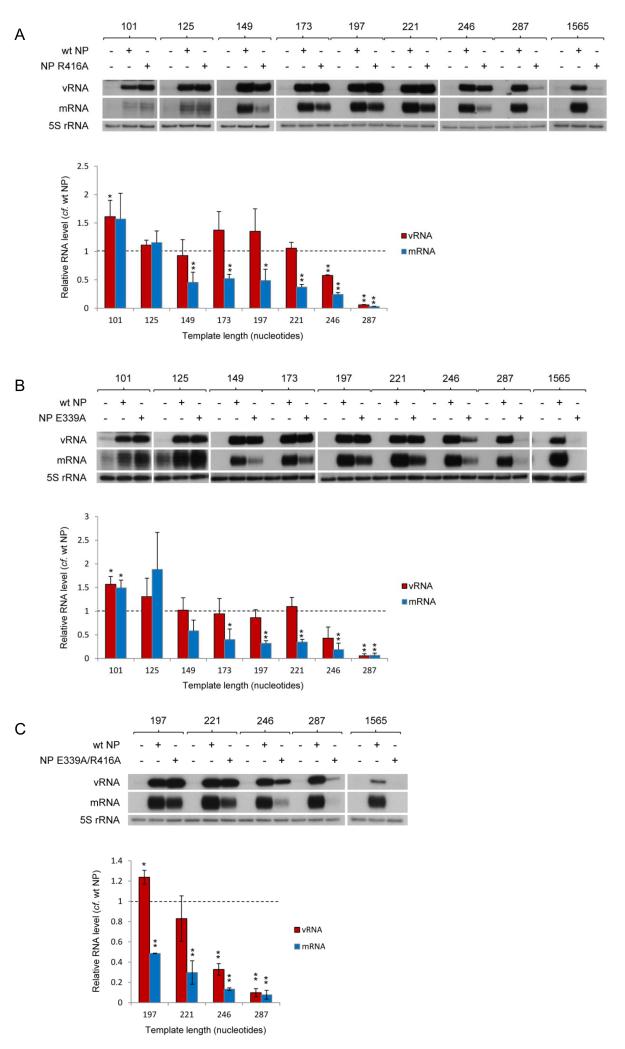
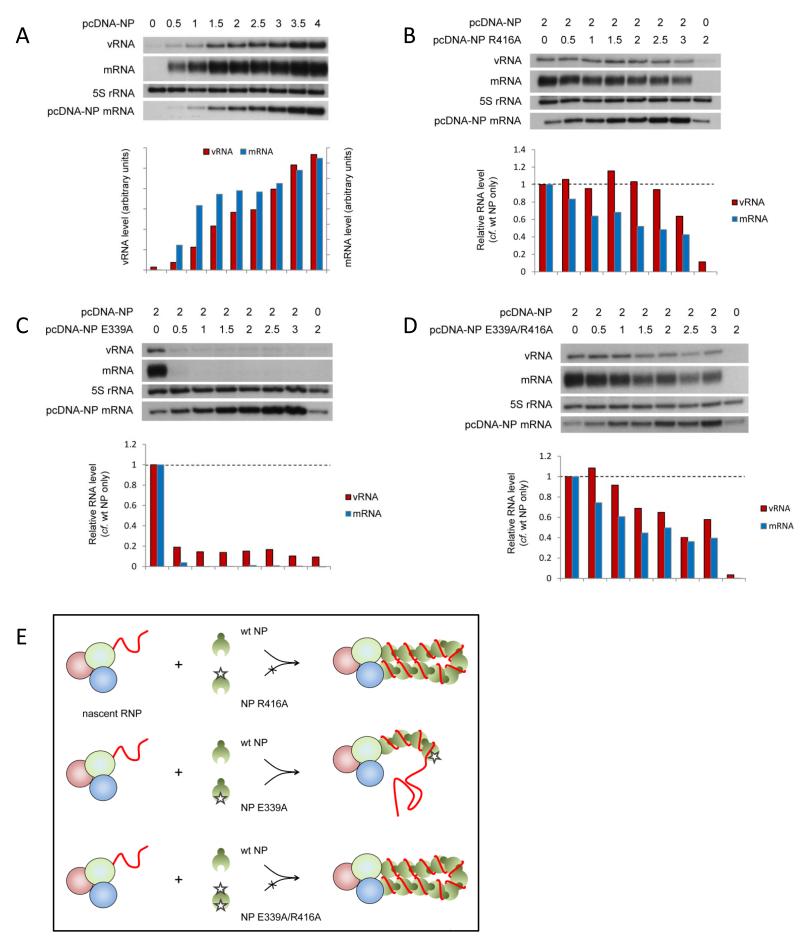
NP has been found to self-assemble into oligomers through a tail loop structure (residues 402–428) mediating the interaction of neighbouring molecules13-15. The identities of the essential molecular contacts between neighbouring NPs, notably R416 and E339, and the relevance of the interaction for viral RNP function, have been confirmed through mutagenesis studies13,15,37. As each NP in the RNP structure is believed to be associated with approximately 24 nucleotides38,39, we speculated that the addition of monomeric NP may suffice for the replication and transcription of a 101 nucleotide long template. Indeed, the addition of oligomerisation mutant NP R416A (Fig. 2A) or E339A (Fig. 2B) promoted replication and transcription of the 101 nucleotide long template as well as or better than wild type NP. To our surprise, templates up to 221 nucleotides long were also replicated as well in the presence of the oligomerisation mutant NP as in the presence of wild type NP. Intriguingly, despite wild type levels of replication, mRNA transcribed from the 149 to 221 nucleotide long templates in the presence of mutant NP accumulated to only ~50% compared with the level achieved in the presence of wild type NP. Replication and transcription of a 246 nucleotide long template was significantly reduced in the presence of mutant NP compared to replication and transcription in the presence of wild type NP and negligible for templates of 287 nucleotides and longer. Similar results were obtained with double mutant NP E339A/R416A (Fig. 2C). It should be pointed out that although the expression level (Supplementary Fig. S3) and sub-cellular localisation (Supplementary Fig. S4) of the oligomerisation mutant NPs were shown to be similar to wild type NP, we found that at lower concentrations oligomerisation mutant NP R416A was less efficient than wild type NP at supporting replication and transcription (Supplementary Fig. S5). Overall, we find that non-oligomerising mutant NP supports the replication and transcription of intermediate length templates.
Fig. 2. The role of NP oligomerisation in the activity of vRNP-like complexes.
The accumulation of vRNA and mRNA following in vivo reconstitution of vRNPs from trimeric polymerase and varying lengths of segment 5-based vRNA-like templates in the presence of wild type or oligomerisation mutant [R416A (A), E339A (B) or E339A/R416A (C)] NP was analysed by primer extension. NP was omitted as a negative control. The lengths of the vRNA-like gene segments (in nucleotides) are indicated above. The 1565 nucleotide long template represents full length segment 5 containing mutations to prevent expression of NP. Quantification was performed by phosphorimage analysis of at least three independent experiments. Analysis of the 5S rRNA levels served as an internal control. RNA levels detected in the negative control were set to 0. Graphs depicting the vRNA and mRNA levels accumulating from each construct in the presence of mutant NP relative to those accumulating in the presence of wild type NP are shown. Error bars indicate standard error of the mean (SEM), and the asterisks indicate a significant difference from 1 (one sample t test; *P < 0.05; **P < 0.01).
RNA-binding by NP is crucial for vRNP complex activity
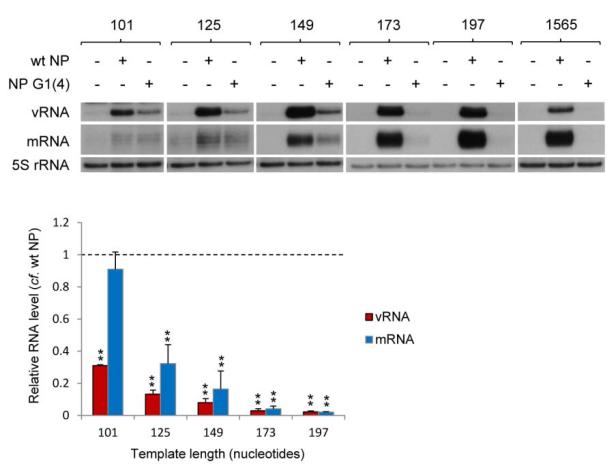
NP in the context of the RNP is known to bind the resident polymerase and template RNA12,16,37. In order to study whether the RNA binding activity of NP is required for its role in supporting RNP activity, we examined whether NP lacking RNA binding activity could promote replication of intermediate length templates. For this purpose, we used NP with mutations at four of the positively charged amino acids in the proposed RNA binding groove, NP G1(4). The RNA-binding affinity of NP G1(4) has previously been shown to be too low to be determined by surface plasmon resonance, yielding only a weak response even at a high concentration (6.25 μM) of NP G1(4), in contrast to the high RNA-binding affinity of wild type NP which was shown to have a dissociation constant (KD) of 16.5 nM29. In addition, NP G1(4) was shown to possess only residual activity in supporting full length viral gene replication29. As shown in Fig. 3, we found that NP G1(4) supported replication of the 101-149 nucleotide long templates very weakly (10-30% compared to wild type NP). Only barely detectable replication or transcription of templates longer than 149 nucleotides was observed in the presence of RNA binding mutant NP G1(4). Interestingly, we observed that RNA binding mutant NP G1(4) was able to support transcription of the 101 nucleotide template. In general, we conclude that the RNA binding activity of NP is important for its role in supporting replication, in agreement with previous observations27.
Fig. 3. The role of RNA-binding by NP in the activity of vRNP-like complexes.
The accumulation of vRNA and mRNA following in vivo reconstitution of vRNPs from trimeric polymerase and varying lengths of segment 5-based vRNA-like templates in the presence of wild type or RNA binding mutant [G1(4)] NP was analysed by primer extension. NP was omitted as a negative control. The lengths of the vRNA-like gene segments (in nucleotides) are indicated above. The 1565 nucleotide long template represents full length segment 5 containing mutations to prevent expression of NP. Quantification was performed by phosphorimage analysis of three independent experiments. Analysis of the 5S rRNA levels served as an internal control. RNA levels detected in the negative control were set to 0. A graph depicting the vRNA and mRNA levels accumulating from each construct in the presence of mutant NP relative to those accumulating in the presence of wild type NP is shown. Error bars indicate standard error of the mean (SEM), and the asterisks indicate a significant difference from 1 (one sample t test; **P < 0.01).
The role of NP is conserved for different vRNP templates
In order to confirm that the data presented above are not peculiar to the particular set of templates used, which are based on gene segment 5, representative mini-genome constructs based on gene segment 8 were also analysed. In vivo replication and transcription of segment 8-based vRNA templates 60, 172 or 890 nucleotides in length (Supplementary Fig. S1), the latter representing the full length genome segment, were compared in the presence or absence of wild type and mutant NP (Fig. 4). We found that the accumulation levels of vRNA and mRNA from these templates were not necessarily the same as those from the segment 5-based gene segments. For instance, replication and transcription of the segment 8-based 172 nucleotide long template in the presence of NP R416A was significantly reduced compared to that of segment 5-based 173 nucleotide long template. We propose that this may be due to the different templates having different secondary structures. However, overall, these data confirmed that (1) micro vRNA templates can be replicated and transcribed efficiently in the absence of NP, whereas replication and transcription of even the shortest full length genome segment requires wild type NP, (2) oligomerisation of NP is non-essential for replication of intermediate length vRNA templates but their transcription is partially inhibited by mutations in oligomerisation domains of NP, and (3) the RNA binding activity of NP is critical to the role of NP in RNP activity.
Fig. 4. The conservation of the role of NP in the activity of vRNP-like complexes.
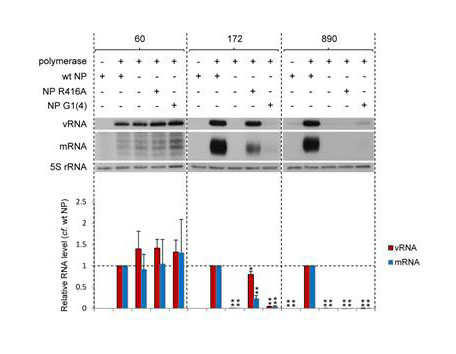
The accumulation of vRNA and mRNA following in vivo reconstitution of vRNPs from trimeric polymerase and varying lengths of segment 8-based vRNA-like templates in the presence or absence of wild type or mutant [oligomerisation mutant R416A or RNA binding mutant G1(4)] NP was analysed by primer extension. PA was omitted as a negative control. The lengths of the vRNA-like gene segments (in nucleotides) are indicated above. The 890 nucleotide long template represents full length segment 8. Quantification was performed by phosphorimage analysis of three independent experiments. Analysis of the 5S rRNA levels served as an internal control. RNA levels detected in the negative control were set to 0. A graph depicting the vRNA and mRNA levels accumulating from each construct in the absence of NP or in the presence of mutant NP relative to those accumulating in the presence of wild type NP is shown. Error bars indicate standard error of the mean (SEM), and the asterisks indicate a significant difference from 1 (one sample t test; *P < 0.05; **P < 0.01).
Monomeric NP is incorporated into mini-vRNP complexes
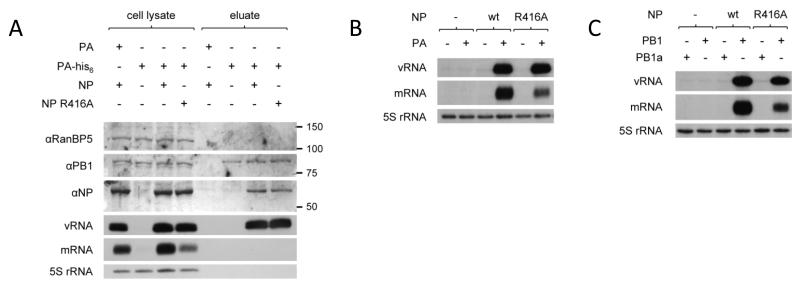
To address the question whether non-oligomerising mutant NP is incorporated into progeny RNP during replication of intermediate length templates, we carried out RNP reconstitution experiments with the 221 nucleotide template in the presence of a his-tagged PA and analysed the constituents of complexes co-purified during nickel affinity chromatography (Fig. 5A). Western blot and primer extension analyses clearly demonstrated the co-purification of wild type or non-oligomerising NP and vRNA, providing evidence for the assembly of RNP complexes. We also examined the requirement for active trimeric polymerase by RNP reconstitution experiments carried out in the presence or absence of PA (Fig. 5B), or in the presence of wild type PB1 or PB1 with a double mutation in the conserved SDD motif of the active site (Fig. 5C). As expected, wild type and non-oligomerising mutant NP were only able to support replication and transcription in the presence of active trimeric polymerase. Therefore, we conclude that replication and transcription of intermediate length templates is dependent on the assembly of active polymerase and wild type or non-oligomerising mutant NP into RNPs.
Fig. 5. Components assembled in active vRNP-like complexes.
(A) The proteins and RNA co-isolated during nickel affinity chromatography of vRNPs reconstituted in vivo from trimeric polymerase (his-tag on PA) and the 221 nucleotide long vRNA-like template in the presence of wild type or oligomerisation mutant (R416A) NP were analysed by western blot and primer extension respectively. The use of untagged PA or the omission of NP served as negative controls. The expression of comparable levels of NP, PB1, vRNA and mRNA was confirmed in the cell lysates; RanBP5 and 5S rRNA were detected as loading controls. vRNP components co-isolated with PA-his6 were detected in the eluates of nickel affinity chromatography. (B-C) Primer extension analysis of the accumulation of vRNA and mRNA derived from in vivo reconstituted vRNPs with the 221 nucleotide long template in the presence or absence of PA (B) or in the presence of wild type or inactive mutant (D445A/D446A) PB1 (C) and wild type or oligomerisation mutant (R416A) NP. NP was omitted as a negative control. Analysis of the 5S rRNA levels served as an internal control.
NP recruitment to RNP complexes is oligomerisation-mediated
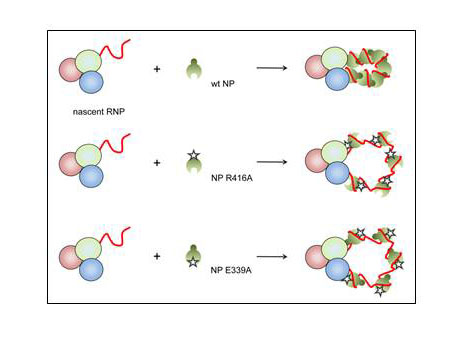
It has been proposed that specific binding of the viral polymerase to the 5′ terminal sequences of nascent viral RNAs may act as a nucleation signal for the assembly of progeny RNP by sequential association of NP, possibly mediated through oligomerisation3,13,18,19. We hypothesised that if this was the case, NP assembly on the progeny RNP would be unidirectional i.e. NP is assembled either tail loop- or insertion groove-first. In order to test this, we performed competition assays between wild type and mutant NP during RNP reconstitution. Initially, the effect of NP concentration on viral replication and transcription was determined by titration (Fig. 6A). Conditions of limited NP concentration that allow detection of increasing or decreasing replication and transcription (transfection of 2μg of wild type NP-expressing plasmid with 0.5μg of each plasmid expressing polymerase subunits and 1565 nucleotide long RNA template) were selected. Next, increasing amounts of plasmid expressing oligomerisation mutant NP, either R416A, E339A or E339A/R416A, were transfected with a constant amount of plasmid expressing wild type NP and the accumulation of viral RNA was analysed. As can be seen from Fig. 6B, the co-expression of NP with a mutation in the tail loop (NP R416A) had limited or no inhibitory effect on replication in the presence of wild type NP, even when expressed in excess of wild type NP. On the other hand, NP with a mutation in the insertion groove was highly dominant negative, inhibiting RNP activity even at a ratio of 1 in 4 relative to wild type NP (Fig. 6C). However, the inclusion of a tail loop mutation (R416A) abrogated this strong inhibitory effect of the mutation in the insertion groove (E339A), as seen in Fig. 6D with double mutant NP (E339A/R416A), resulting in only a limited inhibitory effect as observed with the R416A mutant alone. These data strongly suggest that NP joins the growing RNP unidirectionally in a tail loop-first orientation (Fig. 6E). NP R416A and NP E339A/R416A cannot be added to the growing RNP due to the mutation in the tail loop and are therefore non-competitive, although partial inhibition of RNP activity suggests limited competitive assembly of mutant NP into the RNP, possibly through non-oligomerisation mediated interaction with the RNP, e.g. with the polymerase or RNA. On the other hand, the wild type tail loop of NP E339A allows inclusion into the nascent RNP as efficiently as wild type NP, but the mutation in its insertion groove prevents the assembly of further NP, resulting in loss of RNP activity.
Fig. 6. Directionality of NP assembly onto RNP complexes.
(A-D) The accumulation of vRNA and mRNA following in vivo reconstitution of vRNPs from trimeric polymerase and full length segment 5 vRNA in the presence of varying concentrations of wild type NP (A) or wild type and oligomerisation mutant [R416A (B), E339A (C) or E339A/R416A (D)] NP was analysed by primer extension. 0.5μg of each of the polymerase subunit-expressing plasmids and the plasmid expressing the full length segment 5 RNA template (containing mutations to prevent expression of NP) were transfected together with the amount of NP-expressing plasmid shown in μg [in a total of 6μg (A) or 7μg (B-D)]. Analysis of the 5S rRNA levels served as an internal control. The level of mRNA derived from the NP-expressing plasmid is also shown. Quantification was performed by phosphorimage analysis. Graphs depicting the vRNA and mRNA levels accumulating in the presence of varying concentrations of wild type and mutant NP relative to those accumulating in the presence of wild type NP alone are shown. (E) Schematic depiction of competition assays demonstrating the directional assembly of NP onto nascent RNPs by homo-oligomerisation. NP recruitment to the nascent RNP is mediated through NP homo-oligomerisation, the tail loop of the incoming NP interacting with the insertion groove of the resident NP.
RNP complexes recruit NP independent of RNA binding
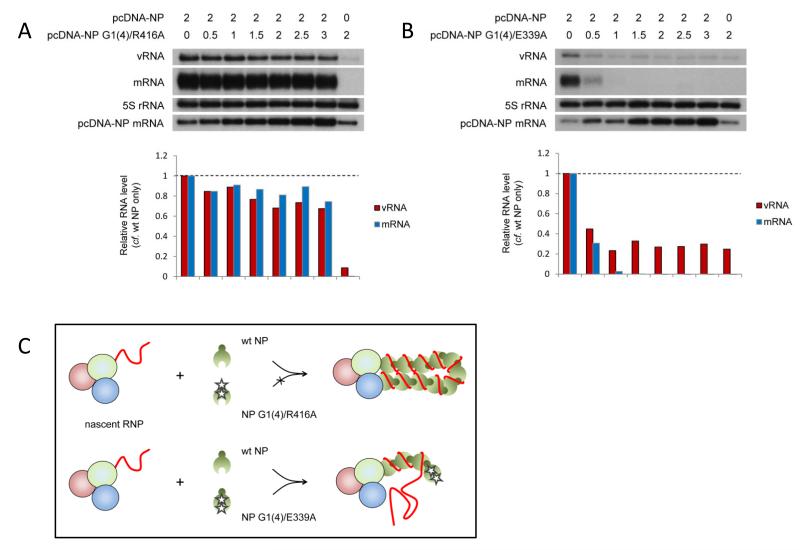
In order to address whether homo-oligomerisation between neighbouring NP molecules was mediated by RNA binding, or whether binding of NP to the RNA template follows homo-oligomerisation-mediated recruitment, we constructed NP G1(4)/E339A, adding mutations in the RNA binding groove to abolish RNA binding of the dominant negative oligomerisation mutant NP E339A. We hypothesised that a resultant loss of the dominant negative phenotype in competition assays would suggest that RNA binding was a prerequisite for assembly of NP on the nascent RNP and homo-oligomerisation. As a control, NP with mutations in the RNA binding groove and the tail loop domain (NP G1(4)/R416A) (Fig. 7A) showed only weak inhibition in competition assays with wild type NP. However, mutant NP G1(4)/E339A despite reduced expression levels compared to wild type NP (Supplementary Fig. S3), retained a similar dominant negative phenotype as NP E339A over wild type NP (Fig. 7B). This implies that the loss of RNA binding had little impact on the ability of the NP E339A to assemble on the nascent RNP and to prevent the recruitment of further NP. Therefore, it appears that NP may be recruited to the nascent RNP through homo-oligomerisation, independent of RNA binding, and that binding to the nascent RNA may occur subsequently (Fig. 7C).
Fig. 7. The role of RNA-binding in recruitment of NP to RNP complexes.
(A-B) The accumulation of vRNA and mRNA following in vivo reconstitution of vRNPs from trimeric polymerase and full length segment 5 vRNA in the presence of varying concentrations of wild type and dual RNA binding and oligomerisation mutants [G1(4)/R416A (A) or G1(4)/E339A (B)] NP was analysed by primer extension. 0.5μg of each of the polymerase subunit-expressing plasmids and the plasmid expressing the full length segment 5 RNA template (containing mutations to prevent expression of NP) were transfected together with the amount of NP-expressing plasmid shown in μg (in a total of 7μg). Analysis of the 5S rRNA levels served as an internal control. The level of mRNA derived from the NP-expressing plasmid is also shown. Quantification was performed by phosphorimage analysis. Graphs depicting the vRNA and mRNA levels accumulating in the presence of varying concentrations of wild type and mutant NP relative to those accumulating in the presence of wild type NP alone are shown. (C) Schematic depiction of competition assays demonstrating the proposed RNA binding-independent assembly of NP onto nascent full length RNPs by homo-oligomerisation. NP is recruited to the nascent RNP through docking of a wild type tail loop to the wild type insertion groove of the resident NP, independent of RNA binding. Binding to the nascent RNA may occur subsequently.
Discussion
Whereas it has previously been shown that the viral RNA polymerase is minimally sufficient for RNA synthesis from a short naked RNA template in vitro, NP has been found to be essential for replication and transcription of full-length genomic RNA in vitro and in vivo20,22-24,40,41. In the case of influenza A virus, short templates of 46 nucleotides have recently been found to be replicated in vivo in the absence of NP21. Here we have demonstrated firstly that the influenza A virus polymerase is sufficient to carry out authentic in vivo replication and transcription of short vRNA-like templates efficiently in the absence of NP (Fig. 1A and B). Therefore, while NP plays an important role in viral RNP activity, it is not essential for determining the mode of initiation or termination of replication or transcription (capped primer initiated versus primer-independent initiation and termination with or without polyadenylation) by the viral polymerase or for the regulation of these activities. Instead, our findings demonstrate that template-associated NP represents an elongation factor that plays a direct role in the stimulation of polymerase processivity, as has previously been proposed20. The latter model was based on in vitro data using virus-derived vRNPs stripped of NP. These authors suggested that the addition of NP facilitates escape of the viral polymerase from the promoter 12-19 nucleotides from the 3′ end. Similarly, NP of vesicular stomatitis virus, a non-segmented NSV, has recently been observed to be dispensable for in vitro synthesis of short RNA transcripts of approximately 21 nucleotides on naked RNA templates42. In contrast, we find that the influenza A virus polymerase is able to replicate vRNA templates of at least 76 nucleotides in the absence of NP in vivo, possibly implicating a cellular factor, such as minichromosome maintenance complex34, in facilitating promoter escape of the polymerase in the absence of NP. We also find that the presence of NP promotes replication and transcription of very short templates, especially at low concentrations of polymerase (Supplementary Fig. S2). Although we do not currently know the mechanism, it is possible that this reflects the multifunctional role of NP as a key adaptor molecule between virus and host cell processes19,43. For instance, NP may play a role through its interaction with the polymerase directly, either in the context of an RNP or free non-template associated polymerase, in nuclear and cytoplasmic trafficking or indeed in polymerase activity.
We also investigated the role of homo-oligomerisation and RNA binding in RNP assembly and NP function. NP of H1N1 and H5N1 NP were both crystallised in trimeric form13,14, revealing the likely mode of interaction between neighbouring NP molecules in the RNP through the flexible tail loop structure15. Although the role of homo-oligomerisation of NP is currently not known, it is generally thought to play a role in the assembly of RNPs. According to this model, NP is initially recruited to RNP through direct interaction with PB1 and PB2 of the polymerase bound to the nascent 5′ end of viral genomic or antigenomic RNA. Binding of subsequent NP to the RNA template may then be mediated by NP-NP homo-oligomerisation3,13,18,19. However, homo-oligomerisation has also been speculated to be RNA mediated15,44,45, and oligomerisation mutants of NP have previously been demonstrated to be impaired in RNA binding46. Indeed, surface plasmon resonance studies showed that although the RNA-binding affinities of oligomerisation mutants NP R416A and NP E339A remained at submicromolar levels, their dissociation constants (KD) were about 60 times higher compared to wild type NP (975 nM and 858 nM for NP R416A and NP E339A respectively compared to 15.8 nM for wild type NP)15. This is likely to be due to oligomeric wild type NP possessing more RNA binding sites, contributing to a higher association rate and higher RNA affinity15. We discovered that the mutation in the insertion groove of NP (NP E339A) which prevents NP homo-oligomerisation has a dominant negative phenotype in the assembly of a functional full length wild type RNP whereas the mutation of the corresponding interactive residue in the tail loop (NP R416A) was non-competitive (Fig. 6B and C). It is unlikely that these results are related to differences in RNA-binding affinities between mutant and wild type NPs as the two oligomerisation mutants have similar RNA-binding affinities 15 and similar phenotypes in supporting replication of intermediate length templates (Fig. 2B and C) and in stabilising full length cRNA29, but opposite effects in competition assays with wild type NP. In addition, the dominant negative phenotype of the insertion groove mutant NP E339A is abrogated by the introduction of the mutation (R416A) in the tail loop (Fig. 6D), whereas the addition of mutations in the RNA-binding groove of NP E339A had little effect on the dominant negative phenotype (Fig 7B). Therefore, we suggest that NP recruitment to the nascent RNP is mediated through NP homo-oligomerisation, the tail loop of the incoming NP interacting with the insertion groove of the resident NP. In similar experiments, Shen et al.47 found that mutant NP lacking the tail loop was uncompetitive in wild type NP-supported transcription of a luciferase reporter gene in agreement with our data whereas, surprisingly, both NP with the R416A tail loop mutation and E339A insertion groove mutation were dominant negative. Although the reason for this discrepancy is not clear, it may relate to these authors’ use of tagged NP. Nonetheless, these authors show the importance of the R416-E339 salt bridge for RNP assembly and activity during viral replication and the potential for antiviral drugs targeting this interaction. Our description of tail loop-first association of influenza A virus NP on nascent RNA is highly reminiscent of the directionality of NP of non-segmented NSVs revealed by the crystal structures of RNP complexes48,49 and allows deduction of the orientation of the RNA strand in the vRNP structures published while this manuscript was under review50,51.
We further determined that NP recruitment to the nascent RNP is not primarily mediated by RNA binding (Fig. 7A and B). Instead, we propose that NP is recruited by homo-oligomerisation as described above, hypothetically followed by RNA binding, thereby stabilising the nascent RNP and maintaining a continuously unfolded RNA template to facilitate replication. However, this raises the question of how homo-oligomerisation mutants are recruited to nascent full length RNA to form inactive but stable RNPs as shown previously29, or to form functional RNPs up to 221 nucleotides in length as shown here (Fig. 2A, B and C). As pointed out earlier, the efficiency of oligomerisation mutant NP assembly into RNPs is dependent on the concentration of NP (Supplementary Fig. S5). We propose that NP can be recruited to nascent RNP in an oligomerisation-independent manner, possibly through interaction with the polymerase and/or the RNA template, when present in sufficient concentration, thereby stabilising even full length RNPs29 (Fig. 8). However, we suggest that non-oligomerising NP recruited in this fashion only supports replication of intermediate length templates up to approximately 250 nucleotides in length, either due to insufficient assembly of monomeric NP onto longer transcripts or due to a role for NP homo-oligomerisation in modulating RNP template function beyond its role in RNP assembly. For instance, homo-oligomerisation may play an additional role to enable transient displacement and repositioning of the NP on the template during the passage of the polymerase.
Fig. 8. Model for the recruitment of monomeric NP to RNP-like complexes.
Schematic depiction of the proposed assembly of RNP-like complexes containing wild type or oligomerisation mutant NP. Recruitment of wild type NP to nascent RNP-like complexes is mediated through homo-oligomerisation whereas oligomerisation mutant NP is recruited through polymerase- and/or RNA-binding. The assembly of RNP-like complexes containing oligomerisation mutant NP is more sensitive to the concentration of NP than the assembly of RNP-like complexes containing wild type NP.
In summary, we have shown here that NP of influenza A virus does not regulate the initiation or termination of transcription and replication. Instead, it supports the progress of the polymerase during the elongation phase. We have also shown that NP is unidirectionally recruited to nascent RNPs through homo-oligomerisation and independently of RNA binding. The assays we have developed should allow more detailed investigation into the role of NP and the mechanisms of RNP replication and transcription. Furthermore, the approach described here to study the function and assembly mechanism of NP in influenza A virus RNPs should be readily adaptable to other NSVs. It will be interesting to determine the extent to which the polymerases of other NSVs are able to replicate and transcribe short vRNA-like templates in the absence of NP in vivo and the extent to which assembly mechanisms of NP onto nascent RNPs are conserved.
Methods
Cells and plasmids
Human embryonic kidney 293T cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% foetal calf serum (FCS). Plasmids expressing the PB2, PB1, PB1a (D445A/D446A), PA, PA-His6 and NP proteins of influenza A virus A/WSN/33 (pcDNA-PB2, pcDNA-PB1, pcDNA-PB1a, pcDNA-PA, pcDNA-PA-His6 and pcDNA-NP) have been described previously28,52, as have plasmids expressing NP G1(4) (R74A/R75A/R174A/R175A), NP R416A and NP E339A [pcDNA-NP G1(4), pcDNA-NP R416A and pcDNA-NP E339A}29 and the vRNA expressing plasmids pPOLI-NP-RT and pPOLI-NS-RT 35. Internal deletion mutants of pPOLI-NP-RT and pPOLI-NS-RT (Supplementary Fig. S1) were made by round-the-plasmid PCR amplification using two primers outwardly directed on the segment 5 or 8 gene sequence. PCR products were digested with SpeI and circularised by ligation. The NP open reading frame in the plasmid expressing full length (1565 nucleotides) segment 5 vRNA template was modified by site-directed mutagenesis, thereby eliminating the NP start codon (M1S) and introducing a stop codon (M238stop), to prevent expression of NP from transcribed mRNA. Plasmids expressing NP with mutations in both the oligomerisation and/or RNA binding domains [pcDNA-NP E339A/R416A, pcDNA-NP G1(4)/R416A and pcDNA-NP G1(4)/E339A] were constructed as chimeras from the individual mutants by restriction enzyme-mediated subcloning. Expression levels of mutant NP in transfected 293T cells were mostly comparable with wild type NP, with the exception of NP G1(4)/E339A which exhibited reduced accumulation (Supplementary Fig. S3). The sub-cellular localisation pattern of mutant NP compared to wild type NP in transfected 293T cells was determined by immunofluorescence microscopy (Supplementary Fig. S4).
In vivo vRNP reconstitution
1 ml suspensions of 293T cells (approximately 106 cells) in DMEM supplemented with 10% FCS were transfected in 35 mm dishes using 1 μl of Lipofectamine 2000 (Invitrogen) per μg of plasmid mixed with 150 μl OPTIMEM (Invitrogen). 1 μg of each of the pcDNA plasmids expressing the polymerase subunits and NP together with 1μg of the pPOLI plasmid expressing the template RNA were transfected (in a total of 5 μg), except where indicated otherwise. Transfected DNA quantities in each experiment were kept constant by supplementing with pcDNA3A empty vector where necessary. Cells were harvested 28 to 40 hours post-transfection.
RNA isolation and primer extension analysis
Total RNA was extracted from 106 transfected 293T cells with 1 ml Trizol (Invitrogen). 1/30th of each RNA sample was mixed with an excess of 32P-labelled DNA primers designed to detect negative- and positive sense RNA of the different templates (Supplementary Table S1) in 5 μl and denatured by heating at 95 °C for 5 min. The mixture was cooled on ice and transferred to 50 °C for 1 min prior to the addition of 5 μl 2x transcription mix [2x First Strand Buffer (Invitrogen), 20 mM DTT, 1 mM dNTP mix and 50 U SuperScript III RNase H− reverse transcriptase (Invitrogen)] also heated to 50 °C. The reaction was stopped after 1 hour by the addition of 8 μl 90% formamide, and heating at 95 °C for 5 min. Transcription products were separated on 12% polyacrylamide gels containing 7 M urea in TBE buffer and detected by autoradiography with intensifying screens. The presence of the 5′ terminal capped extension (which is of variable length) on mRNA allows clear separation of the mRNA reverse transcripts from those of cRNA. The level of vRNA template derived from RNA polymerase I-driven transcription of the input plasmid was determined by the omission of a single subunit of the viral polymerase and set to zero. The accumulation of vRNA was used as a measure of replication efficiency as the level of cRNA detected was considered to be too weak above background to quantitate. Quantitation was done using a Fuji phosphorimager and analysed using AIDA densitometry software.
Oligo(dT) chromatography
Total RNA from 3×106 transfected 293T cells harvested at 48 hours post-transfection was extracted with Trizol (Invitrogen) and denatured at 95°C for 5 minutes. RNA was mixed with 5 mg oligo(dT) cellulose (Sigma) in 200 μl binding buffer (0.5 M NaCl, 10 mM Tris-HCl, pH 7.5) and incubated with agitation at room temperature for 1 hour. Oligo(dT)-cellulose was pelleted in a microcentrifuge for 10 s and the supernatant (representing the unbound poly(A)- RNA fraction) was collected. The pellet was washed twice in binding buffer (1 ml) at room temperature, followed by incubation in 0.2 ml of low-salt buffer (0.25 M NaCl, 10 mM Tris-HCl, pH 7.5) at 37°C for 15 min and repelleting. After washing the pellet with an additional 0.2 ml of low-salt buffer, the bound poly(A)+ RNA was eluted with 200μl pre-warmed RNase-free water by incubating at 50°C for 15 minutes and repelleting. The poly(A)+ and poly(A)− RNA fractions were recovered by Trizol extraction.
Nickel-agarose affinity isolation of his-tagged RNP complexes
10 cm cell culture dishes of 293T cells were transfected as before using 9 μg of each plasmid (pcDNA-PB1, pcDNAPB2, pcDNA-PA or pcDNA-PA-His6, pcDNA-NP or pcDNA-NP R416A and pPOLINP221) and 67.5 μl Lipofectamine 2000 (Invitrogen). Cells were harvested 30 hours post-transfection, lysed in 1.5 ml of Tris-lysis buffer (50 mM Tris-HCl (pH 8.0), 200 mM NaCl, 20 mM imidazole, 33% glycerol, 0.5% Igepal CA-630, 1 mM DTT and 1x complete mini EDTA-free protease inhibitor cocktail tablet) at 4°C for 1 hour and centrifuged at 13000 rpm for 5 min. The cell lysate was then diluted 1 in 5 in Ni-agarose binding buffer (20 mM Tris-HCl (pH 8.0), 200 mM NaCl, 20 mM imidazole, 1 mM MgCl2 and 1x complete mini EDTA-free protease inhibitor cocktail tablet) and incubated with 50 μl Ni-NTA agarose (Qiagen) for 4 h at 4 °C. The Ni-NTA agarose was washed five times in wash buffer (20 mM Tris-HCl (pH 8.0), 200 mM NaCl, 20 mM imidazole, 1 mM MgCl, 10% glycerol, 0.1% Igepal CA-630). The Ni-NTA agarose was incubated at 4 °C for 2 hours in 800 μl elution buffer (Ni-agarose wash buffer with 250 mM imidazole) and pelleted at 13000 rpm for 5 min. The supernatant was analysed by western blotting.
Western blotting
Transfected 293T cells were washed in 1 ml PBS. 25% was resuspended in lysis buffer (250 mM Tris-HCl pH6.8, 2% SDS, 20% glycerol, 1.44 M β-mercaptoethanol) and the remaining 75% was used for RNA extraction. Cell lysates were analysed by SDS-PAGE followed by western blotting. Rabbit polyclonal antibody recognising NP (1:10000) was a kind gift from O. Haller. Rabbit polyclonal anti-PB1 (1:500), anti-RanBP5 (1:500) (Santa Cruz Biotechnology, Santa Cruz) or anti-beta actin (1:500) (Abcam, Cambridge) antibodies were used for loading controls.
Immunofluorescence microscopy
Vero cells grown on 13-mm coverslips in 24-well plates were transfected as approximately 70% confluent monolayers with 1 μg of expression plasmid and 1 μl of Lipofectamine 2000. The cells were incubated for 24 hours, then washed with PBS and fixed with 4% paraformaldehyde in 250 mM HEPES (pH 7.5) for 15 mins at room temperature. The cells were then permeabilized with 0.5% Triton X-100 in phosphate-buffered saline for 15 min at room temperature, blocked overnight at 4 °C in blocking solution (PBS containing 1% BSA and 0.2% gelatine pH8.0) and incubated with a rabbit polyclonal antibody recognising NP (1:20000) (a gift from O. Haller) in blocking solution for 1 hour at room temperature. Cy3-conjugated anti-rabbit (1:500) was used as the secondary antibody. Coverslips were mounted in Mowiol containing 0.001% 4′,6′-diamidino-2-phenylindole (DAPI; Sigma). Cells were viewed by using a Zeiss microscope with a Zeiss ×63/1.25 oil immersion objective, and the images were processed using ImageJ.
Supplementary Material
Acknowledgements
We thank Otto Haller for antibodies. This work was supported by the MRC (G1100138 to FTV and G0700848 to EF).
Footnotes
Competing Interests The authors declare no competing interests.
References
- 1.Ruigrok RW, Crepin T, Kolakofsky D. Nucleoproteins and nucleocapsids of negative-strand RNA viruses. Curr Opin Microbiol. 2011;14:504–10. doi: 10.1016/j.mib.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 2.Palese P, Shaw ML. Orthomyxoviridae: The viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. Vol. 2. Wolters Kluwer and Lippincott Williams & Wilkins; Philadelphia: 2007. pp. 1647–1689. [Google Scholar]
- 3.Resa-Infante P, Jorba N, Coloma R, Ortin J. The influenza virus RNA synthesis machine: advances in its structure and function. RNA Biol. 2011;8:207–15. doi: 10.4161/rna.8.2.14513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Plotch SJ, Bouloy M, Ulmanen I, Krug RM. A unique cap(m7GpppXm)-dependent influenza virion endonuclease cleaves capped RNAs to generate the primers that initiate viral RNA transcription. Cell. 1981;23:847–58. doi: 10.1016/0092-8674(81)90449-9. [DOI] [PubMed] [Google Scholar]
- 5.Dias A, et al. The cap-snatching endonuclease of influenza virus polymerase resides in the PA subunit. Nature. 2009;458:914–8. doi: 10.1038/nature07745. [DOI] [PubMed] [Google Scholar]
- 6.Yuan P, et al. Crystal structure of an avian influenza polymerase PA(N) reveals an endonuclease active site. Nature. 2009;458:909–13. doi: 10.1038/nature07720. [DOI] [PubMed] [Google Scholar]
- 7.Luo GX, Luytjes W, Enami M, Palese P. The polyadenylation signal of influenza virus RNA involves a stretch of uridines followed by the RNA duplex of the panhandle structure. J Virol. 1991;65:2861–7. doi: 10.1128/jvi.65.6.2861-2867.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robertson JS, Schubert M, Lazzarini RA. Polyadenylation sites for influenza virus mRNA. J Virol. 1981;38:157–63. doi: 10.1128/jvi.38.1.157-163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poon LL, Pritlove DC, Fodor E, Brownlee GG. Direct evidence that the poly(A) tail of influenza A virus mRNA is synthesized by reiterative copying of a U track in the virion RNA template. J Virol. 1999;73:3473–6. doi: 10.1128/jvi.73.4.3473-3476.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hay AJ, Skehel JJ, McCauley J. Characterization of influenza virus RNA complete transcripts. Virology. 1982;116:517–22. doi: 10.1016/0042-6822(82)90144-1. [DOI] [PubMed] [Google Scholar]
- 11.Baudin F, Bach C, Cusack S, Ruigrok RW. Structure of influenza virus RNP. I. Influenza virus nucleoprotein melts secondary structure in panhandle RNA and exposes the bases to the solvent. Embo J. 1994;13:3158–65. doi: 10.1002/j.1460-2075.1994.tb06614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamanaka K, Ishihama A, Nagata K. Reconstitution of influenza virus RNA-nucleoprotein complexes structurally resembling native viral ribonucleoprotein cores. J Biol Chem. 1990;265:11151–5. [PubMed] [Google Scholar]
- 13.Ye Q, Krug RM, Tao YJ. The mechanism by which influenza A virus nucleoprotein forms oligomers and binds RNA. Nature. 2006;444:1078–82. doi: 10.1038/nature05379. [DOI] [PubMed] [Google Scholar]
- 14.Ng AK, et al. Structure of the influenza virus A H5N1 nucleoprotein: implications for RNA binding, oligomerization, and vaccine design. FASEB J. 2008;22:3638–47. doi: 10.1096/fj.08-112110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan WH, et al. Functional analysis of the influenza virus H5N1 nucleoprotein tail loop reveals amino acids that are crucial for oligomerization and ribonucleoprotein activities. J Virol. 2010;84:7337–45. doi: 10.1128/JVI.02474-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Biswas SK, Boutz PL, Nayak DP. Influenza virus nucleoprotein interacts with influenza virus polymerase proteins. J Virol. 1998;72:5493–501. doi: 10.1128/jvi.72.7.5493-5501.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Poole E, Elton D, Medcalf L, Digard P. Functional domains of the influenza A virus PB2 protein: identification of NP- and PB1-binding sites. Virology. 2004;321:120–33. doi: 10.1016/j.virol.2003.12.022. [DOI] [PubMed] [Google Scholar]
- 18.Tao YJ, Ye Q. RNA virus replication complexes. PLoS Pathog. 2010;6:e1000943. doi: 10.1371/journal.ppat.1000943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Portela A, Digard P. The influenza virus nucleoprotein: a multifunctional RNA-binding protein pivotal to virus replication. J Gen Virol. 2002;83:723–34. doi: 10.1099/0022-1317-83-4-723. [DOI] [PubMed] [Google Scholar]
- 20.Honda A, Ueda K, Nagata K, Ishihama A. RNA polymerase of influenza virus: role of NP in RNA chain elongation. J Biochem. 1988;104:1021–6. doi: 10.1093/oxfordjournals.jbchem.a122569. [DOI] [PubMed] [Google Scholar]
- 21.Resa-Infante P, Recuero-Checa MA, Zamarreno N, Llorca O, Ortin J. Structural and functional characterization of an influenza virus RNA polymerase-genomic RNA complex. J Virol. 2010;84:10477–87. doi: 10.1128/JVI.01115-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee MT, et al. Definition of the minimal viral components required for the initiation of unprimed RNA synthesis by influenza virus RNA polymerase. Nucleic Acids Res. 2002;30:429–38. doi: 10.1093/nar/30.2.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newcomb LL, et al. Interaction of the influenza a virus nucleocapsid protein with the viral RNA polymerase potentiates unprimed viral RNA replication. J Virol. 2009;83:29–36. doi: 10.1128/JVI.02293-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang S, et al. Biochemical and kinetic analysis of the influenza virus RNA polymerase purified from insect cells. Biochem Biophys Res Commun. 2009 doi: 10.1016/j.bbrc.2009.11.100. [DOI] [PubMed] [Google Scholar]
- 25.Kawaguchi A, Momose F, Nagata K. Replication-coupled and host factor-mediated encapsidation of the influenza virus genome by viral nucleoprotein. J Virol. 2011;85:6197–204. doi: 10.1128/JVI.00277-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shapiro GI, Krug RM. Influenza virus RNA replication in vitro: synthesis of viral template RNAs and virion RNAs in the absence of an added primer. J Virol. 1988;62:2285–90. doi: 10.1128/jvi.62.7.2285-2290.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Medcalf L, Poole E, Elton D, Digard P. Temperature-sensitive lesions in two influenza A viruses defective for replicative transcription disrupt RNA binding by the nucleoprotein. J Virol. 1999;73:7349–56. doi: 10.1128/jvi.73.9.7349-7356.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vreede FT, Jung TE, Brownlee GG. Model suggesting that replication of influenza virus is regulated by stabilization of replicative intermediates. J Virol. 2004;78:9568–72. doi: 10.1128/JVI.78.17.9568-9572.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vreede FT, Ng AK, Shaw PC, Fodor E. Stabilization of influenza virus replication intermediates is dependent on the RNA-binding but not the homo-oligomerization activity of the viral nucleoprotein. J Virol. 2011;85:12073–8. doi: 10.1128/JVI.00695-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mayer D, et al. Identification of cellular interaction partners of the influenza virus ribonucleoprotein complex and polymerase complex using proteomic-based approaches. J Proteome Res. 2007;6:672–82. doi: 10.1021/pr060432u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rameix-Welti MA, Tomoiu A, Dos Santos Afonso E, van der Werf S, Naffakh N. Avian Influenza A virus polymerase association with nucleoprotein, but not polymerase assembly, is impaired in human cells during the course of infection. J Virol. 2009;83:1320–31. doi: 10.1128/JVI.00977-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boulo S, et al. Human importin alpha and RNA do not compete for binding to influenza A virus nucleoprotein. Virology. 2011;409:84–90. doi: 10.1016/j.virol.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Momose F, et al. Cellular splicing factor RAF-2p48/NPI-5/BAT1/UAP56 interacts with the influenza virus nucleoprotein and enhances viral RNA synthesis. J Virol. 2001;75:1899–908. doi: 10.1128/JVI.75.4.1899-1908.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kawaguchi A, Nagata K. De novo replication of the influenza virus RNA genome is regulated by DNA replicative helicase, MCM. Embo J. 2007;26:4566–75. doi: 10.1038/sj.emboj.7601881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fodor E, et al. Rescue of influenza A virus from recombinant DNA. J Virol. 1999;73:9679–82. doi: 10.1128/jvi.73.11.9679-9682.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Neumann G, et al. Generation of influenza A viruses entirely from cloned cDNAs. Proc Natl Acad Sci U S A. 1999;96:9345–50. doi: 10.1073/pnas.96.16.9345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coloma R, et al. The structure of a biologically active influenza virus ribonucleoprotein complex. PLoS Pathog. 2009;5:e1000491. doi: 10.1371/journal.ppat.1000491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Compans RW, Content J, Duesberg PH. Structure of the ribonucleoprotein of influenza virus. J Virol. 1972;10:795–800. doi: 10.1128/jvi.10.4.795-800.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ortega J, et al. Ultrastructural and functional analyses of recombinant influenza virus ribonucleoproteins suggest dimerization of nucleoprotein during virus amplification. J Virol. 2000;74:156–63. doi: 10.1128/jvi.74.1.156-163.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Huang TS, Palese P, Krystal M. Determination of influenza virus proteins required for genome replication. J Virol. 1990;64:5669–73. doi: 10.1128/jvi.64.11.5669-5673.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de la Luna S, Martin J, Portela A, Ortin J. Influenza virus naked RNA can be expressed upon transfection into cells co-expressing the three subunits of the polymerase and the nucleoprotein from simian virus 40 recombinant viruses. J Gen Virol. 1993;74(Pt 3):535–9. doi: 10.1099/0022-1317-74-3-535. [DOI] [PubMed] [Google Scholar]
- 42.Morin B, Rahmeh AA, Whelan SP. Mechanism of RNA synthesis initiation by the vesicular stomatitis virus polymerase. Embo J. 2012;31:1320–9. doi: 10.1038/emboj.2011.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cianci C, Gerritz SW, Deminie C, Krystal M. Influenza Nucleoprotein: Promising Target for Antiviral Chemotherapy. Antivir Chem Chemother. 2012 doi: 10.3851/IMP2235. [DOI] [PubMed] [Google Scholar]
- 44.Tarus B, et al. Molecular dynamics studies of the nucleoprotein of influenza a virus: role of the protein flexibility in RNA binding. PLoS One. 2012;7:e30038. doi: 10.1371/journal.pone.0030038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tarus B, et al. Oligomerization paths of the nucleoprotein of influenza A virus. Biochimie. 2012;94:776–85. doi: 10.1016/j.biochi.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 46.Elton D, Medcalf L, Bishop K, Harrison D, Digard P. Identification of amino acid residues of influenza virus nucleoprotein essential for RNA binding. J Virol. 1999;73:7357–67. doi: 10.1128/jvi.73.9.7357-7367.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen YF, et al. E339...R416 salt bridge of nucleoprotein as a feasible target for influenza virus inhibitors. Proc Natl Acad Sci U S A. 2011;108:16515–20. doi: 10.1073/pnas.1113107108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Green TJ, Zhang X, Wertz GW, Luo M. Structure of the vesicular stomatitis virus nucleoprotein-RNA complex. Science. 2006;313:357–60. doi: 10.1126/science.1126953. [DOI] [PubMed] [Google Scholar]
- 49.Albertini AA, et al. Crystal structure of the rabies virus nucleoprotein-RNA complex. Science. 2006;313:360–3. doi: 10.1126/science.1125280. [DOI] [PubMed] [Google Scholar]
- 50.Arranz R, et al. The Structure of Native Influenza Virion Ribonucleoproteins. Science. 2012 doi: 10.1126/science.1228172. [DOI] [PubMed] [Google Scholar]
- 51.Moeller A, Kirchdoerfer RN, Potter CS, Carragher B, Wilson IA. Organization of the Influenza Virus Replication Machinery. Science. 2012 doi: 10.1126/science.1227270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fodor E, et al. A single amino acid mutation in the PA subunit of the influenza virus RNA polymerase inhibits endonucleolytic cleavage of capped RNAs. J Virol. 2002;76:8989–9001. doi: 10.1128/JVI.76.18.8989-9001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.