Vaccines currently recommended during pregnancy include inactivated influenza, acellular pertussis, and tetanus toxoid vaccines. Maternal immunization provides protection to the pregnant woman and fetus, and protects the infant through transplacental maternal antibody transfer. New maternal vaccine candidates are under development.
Keywords: maternal immunization, influenza, tetanus, diphtheria, pertussis
Abstract
Maternal immunization has the potential to protect the pregnant woman, fetus, and infant from vaccine-preventable diseases. Maternal immunoglobulin G is actively transported across the placenta, providing passive immunity to the neonate and infant prior to the infant's ability to respond to vaccines. Currently inactivated influenza, tetanus toxoid, and acellular pertussis vaccines are recommended during pregnancy. Several other vaccines have been studied in pregnancy and found to be safe and immunogenic and to provide antibody to infants. These include pneumococcus, group B Streptococcus, Haemophilus influenzae type b, and meningococcus vaccines. Other vaccines in development for potential maternal immunization include respiratory syncytial virus, herpes simplex virus, and cytomegalovirus vaccines.
Many vaccine-preventable diseases cause substantial morbidity and mortality in pregnant women, neonates, and infants. However, vaccination of very young infants by direct immunization is limited by poor immunogenicity and interference from maternal antibody [1]. Immunization during pregnancy has the potential to provide protection to the mother, fetus, and infant through transplacental transfer of vaccine-specific maternal immunoglobulin G (IgG). Maternal IgG provides passive immunity during the first 6 months of an infant's life prior to the infant's ability to fully respond to vaccination. Maternal immunization can also prevent disease in both the pregnant woman and fetus during a high-risk period in their lives. This has been most recently emphasized with the increased mortality from pandemic H1N1 influenza in pregnant women and fetuses compared with the general population [2, 3]. Finally, pregnant women are an otherwise healthy population who respond well to vaccines and are easily accessed through routine prenatal care.
Several factors influence the transfer of maternal IgG during pregnancy, including placental integrity, total maternal IgG concentration, IgG subtype, and timing of vaccination. Maternal infection with HIV or malaria can reduce the ability of the placenta to transport IgG through impairment of Fc receptor function [4]. Higher levels of total maternal IgG may also reduce transfer of antigen-specific IgG by competitive binding to placental Fc receptors [5]. IgG transfer also varies by subtype. IgG1, which is induced primary by protein antigens such as tetanus toxoid, is more efficiently transferred than IgG2, which is induced by polysaccharide antigens such as pneumococcus [6]. Active transport of maternal IgG occurs primarily after 32 weeks’ gestation; infants born prematurely have low levels of maternal antibody. By the time of delivery of a full-term infant, the level of IgG may be higher in the infant than the mother due to active transport [7]. Infant antibody titers rise approximately 2 weeks after maternal vaccination. In a study of Haemophilus influenzae type b (Hib) conjugate vaccine, transmission of antibodies was greatest in mothers vaccinated >4 weeks before delivery [8]. Vaccination between 28 and 32 weeks may optimize the amount of disease-specific IgG present at time of delivery and ensure the greatest period of protection for neonates. For influenza, where there is substantial risk to the pregnant woman and fetus as well as the infant, the Advisory Committee on Immunization Practices (ACIP) recommends vaccination at the beginning of the seasonal epidemic [9].
The safety of maternal immunization has been shown for many vaccines. In the United States, vaccination of pregnant women against influenza and poliomyelitis was recommended and widely implemented in the 1950s and 1960s. Longitudinal surveillance studies following mothers and infants until 7 years of age showed no increased risk for development of learning disabilities, malignancy, or congenital malformations [10]. Maternal tetanus toxoid (TT) has been administered to millions of women worldwide with no known risks to mother or fetus. Vaccination during pregnancy with live vaccines, such as varicella or yellow fever, has been relatively contraindicated due to a concern for potential transmission of infection across the placenta to the fetus, although this is evaluated on a case-by-case basis [9]. Pregnancy is an exclusion criterion for enrollment into many vaccine trials, limiting the availability of data regarding safety of routine vaccines in pregnancy. Policy regarding use of vaccines in pregnancy is often guided by postmarketing vaccine surveillance systems, such as the Vaccine Adverse Event Reporting System in the United States, as well as data from the small numbers of pregnant women inadvertently vaccinated in clinical trials. Limitations of use of vaccine surveillance systems include lack of long-term follow-up data as well as a limited ability to detect rare pregnancy-associated adverse events.
RECOMMENDED AND AVAILABLE VACCINES
Tetanus
Neonatal tetanus mortality has been reduced by 92% with the advent of universal TT administration during pregnancy in combination with improved hygienic birthing practices (Figure 1) [11]. TT is a protein-based subunit vaccine that elicits an IgG1 immune response, with antibody actively transported across the placenta with >100% efficiency. The World Health Organization (WHO) recommends the administration of 2 doses of TT in the first pregnancy and one in each subsequent pregnancy for a maximum of 5 doses. Implementation of TT is widely used in resource-limited settings with 80% coverage of pregnancies worldwide (Table 1). In the United States, TT is administered as part of the tetanus toxoid, diphtheria toxoid, and acellular pertussis vaccine (Tdap) during pregnancy, given primarily for protection against neonatal pertussis. Administration of TT alone during pregnancy in the United States is not indicated for protection against tetanus in women who have completed the recommended immunization series prior to conception. The infrastructure for delivery of TT in resource-limited settings can potentially be used for scale-up of universal maternal immunization programs for other vaccines, such as influenza [23].
Figure 1.
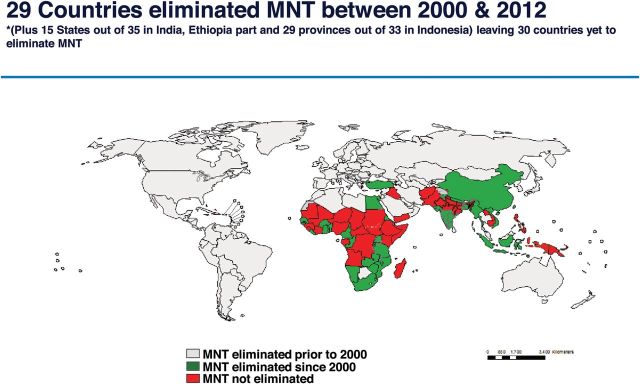
Elimination of neonatal tetanus by 2012. Reprinted with permission from the World Health Organization. Abbreviation: MNT, maternal and neonatal tetanus.
Table 1.
Vaccines Recommended Routinely and in Special Circumstances, and Vaccines Currently in Development
| Vaccine | Timing | Immunogenicity in Pregnancy | Safety in Pregnancy Documented | Placental Transfer Efficiency | Antibody Duration in Infant |
|---|---|---|---|---|---|
| Routinely recommended | |||||
| Inactivated influenza virus | Annually during influenza season | Yes [13] | Yes | 95% | 2–3 mo |
| Tetanus, diphtheria, and acellular pertussis | 27–36 wk gestation of every pregnancya | Yes [14] | Yes | >100% for tetanus >100% for pertussis |
2 mo for pertussis |
| In special circumstances (travel to endemic areas, exposure, and during outbreaks) | |||||
| PPSV23 or conjugate PCV13 | Studies performed in third trimester | Yes [15, 16] | Yes | 30%–44% for polysaccharide | 5 mo |
| Haemophilus influenzae type b conjugate and polysaccharide | Studies performed in third trimester | Yes [8] | Yes | 82%–92%; IgG1 > IgG2 | 2 mo |
| Meningococcus conjugate and polysaccharide | Studies performed in third trimester | Yes for polysaccharide [17] | Yes for polysaccharide, ND for conjugate | 30%–44% for polysaccharide [18] | 2–4 mo |
| Inactivated poliovirus | Prior to travel, during outbreaks | Yes [10] | Yes | ND | ND |
| Typhoid | Prior to travel | ND | ND | ND | ND |
| Cholera | Prior to travel | ND | ND | ND | ND |
| Hepatitis A | Prior to travel | Yes | Yesb | ND | ND |
| Hepatitis B | Prior to travel | Yes | Yesb | ND | ND |
| Rabies | Prior to travel and after exposure | Yes | Yesb | ND | ND |
| Japanese encephalitis | Prior to travel | ND | ND | ND | ND |
| Yellow fever | Prior to travel | Yes [19] | Unclearc | ND | ND |
| Oral poliovirus | During outbreaks | Yes [20] | Yesb | Yes | ND |
| Contraindicated in pregnancy [9] | |||||
| Measles, mumps | … | No | Yesd | >100% for measles [21] | 9–12 mo for measles |
| Rubella | … | Yes | Yesb | ND | ND |
| Varicella | … | No | Yesd | ND | ND |
| Zoster | … | No | Yes | ND | ND |
| LAIV | … | No | Yesd | ND | ND |
| Vaccines under development | |||||
| Herpes simplex virus | ND | ND | ND | ND | ND |
| Cytomegaloviruse | ND | ND | ND | ND | ND |
| Respiratory syncytial virus | Third trimester | ND | ND | ND | ND |
| Group B Streptococcus | Third trimester | Yes [22] | Yes | 77% | 2 mo |
Adapted from Munoz and Englund [12].
Abbreviations: IgG, immunoglobulin G; LAIV, live attenuated influenza vaccine; ND, no data; PCV13, 13-valent pneumococcal conjugate vaccine; PPSV23, 23-valent pneumococcal polysaccharide vaccine.
a Recommendations by the Centers for Disease Control for United States populations.
b No adverse events have been identified in registry programs of infants born to women inadvertently vaccinated during pregnancy.
c One of 81 infants of mothers vaccinated with yellow fever vaccine had evidence of yellow fever immunoglobulin M antibody in cord blood.
d Contraindicated due to live vaccine. However, no adverse events have been identified in registry programs of infants born to women inadvertently vaccinated during pregnancy.
e Target population for cytomegalovirus (CMV) vaccine would be adolescent or adult females prior to pregnancy to prevent primary CMV infection during pregnancy.
Influenza
Pregnant women are at higher risk for severe complications from influenza than the general population, particularly in the third trimester. Influenza infection during pregnancy in the 2009 H1N1 pandemic was associated with an increased risk of maternal and fetal death, whereas administration of the H1N1 vaccine has been shown to be safe and efficacious in pregnancy [2, 3, 24]. Influenza vaccination during pregnancy generates a protective antibody response and decreases clinical illness in both mothers and infants [13]. Vaccination with 1 dose of inactivated monovalent 2009 H1N1 vaccine during pregnancy produces a protective antibody response in 93% of pregnant women, with efficient transplacental antibody transfer and generation of protective antibody responses in 87% of infants [25]. Influenza vaccination during pregnancy also reduces the risk of an influenza diagnosis in the mother [2, 3]. A randomized clinical trial of trivalent inactivated influenza vaccination (TIV) during pregnancy in Bangladesh had a vaccine efficacy of 63%, with reduction of influenza-like illness in infants and mothers by 29% and 36%, respectively [13]. The WHO has made pregnant women the top priority group for influenza vaccination. Use of the live-attenuated influenza vaccine, however, is not recommended during pregnancy.
The effect of influenza vaccination on birth outcomes, including a potential effect on decreased incidence of small for gestational age (SGA), preterm birth, and low-birthweight infants in pregnant women, have been analyzed in many retrospective cohort studies and 1 prospective randomized controlled trial (Table 2) [28, 31]. In the prospective study in Bangladesh, receipt of TIV during pregnancy was associated with an increased birthweight of 200 g and decreased incidence of SGA by 34%. Retrospective studies have also shown a decreased risk of preterm birth with both H1N1 vaccine and TIV administration during pregnancy [30, 32]. Several prospective clinical trials are under way in South Africa, Mali, and Nepal and will add further data regarding the effect of vaccination on birth outcomes [33]. Preliminary results from the South African study demonstrate efficacy in both pregnant women and their infants [34, 35].
Table 2.
Studies Reporting Birth Outcomes With Trivalent Inactivated or H1N1 Influenza Vaccine Administered During Pregnancy
| First Author | Site | Trial Design | Intervention | Control | Risk of Preterm Birth (95% CI) | Risk of Low Birth Weight (95% CI) | Risk of Small for Gestational Age (95% CI) |
|---|---|---|---|---|---|---|---|
| Omer, 2011 [27] | Georgia, USA | Retrospective cohort analysis | TIV N = 578 |
No vaccine N = 3748 |
OR: 0.28 (.11–.74) | … | OR: 0.31 (.13–.75) |
| Steinhoff, 2012 [28] | Dhaka, Bangladesh | Randomized controlled clinical trial | TIV N = 172 |
Pneumococcal polysaccharide vaccine N = 168 |
OR: 0.48 (.08–2.74) | OR: 0.19 (.02–1.64) | OR: 0.43 (.20–.94) |
| Kallen, 2012 [29] | Sweden | Retrospective population-based cohort analysis | H1N1 vaccine N = 18 612 |
No vaccine N = 136 914 |
OR: 0.86 (.77–.96) | OR: 0.86 (.77–.96) | OR: 1.04 (.92–1.17) |
| Fell, 2012 [30] | Ontario, Canada | Retrospective population-based cohort analysis | H1N1 vaccine N = 23 340 |
No vaccine N = 32 230 |
RR: 0.73 (.58–.91) | … | RR 0.90 (.85–.96) |
| Pasternak, 2012 [24] | Denmark | Retrospective population-based cohort study | H1N1 vaccine N = 6989 infants |
No vaccine N = 46 443 infants |
1st trimester: OR: 1.32 (.76–2.31) 2nd/3rd trimester: OR: 1.00 (.84–1.17) |
1st trimester: OR: 0.83 (.41–1.67) 2nd/3rd trimester: OR: 1.14 (.94–1.38) |
1st trimester: OR: 0.79 (.46–1.37); 2nd/3rd trimester: OR: 0.97 (.87–1.09) |
| Richards, 2013 [31] | Georgia, USA | Retrospective cohort analysis | H1N1 vaccine N = 1125 |
No vaccine N = 1581 |
OR: 0.63 (.47–.84) | OR: 0.79 (.56–1.10) | OR: 1.26 (.94–1.69) |
Adapted from Steinhoff and Omer [26].
Abbreviations: CI, confidence interval; OR, odds ratio; RR, relative risk; TIV, trivalent inactivated influenza vaccine.
Pertussis
In the United States, the majority of hospitalizations and deaths from pertussis have occurred in infants <2 months of age. Administration of Tdap to postpartum mothers and family members has been recommended by the ACIP since 2005, but was logistically difficult to implement and did not provide protection during the first few weeks of the infants' life prior to generation of an antibody response [36, 37]. Efficacy of programs to immunize family members prior to birth has not been formally evaluated. Studies evaluating infant cord blood pertussis antibody levels showed significantly higher antibody titers in infants born to mothers who were vaccinated with Tdap during pregnancy [14]. Additional studies of pertussis booster administration to healthy adolescents and adults showed that levels of antibody remained sustained for several months after vaccination [38]. Based on these studies, the ACIP modified their recommendations in 2011 to include administration of Tdap to all unvaccinated pregnant women, and then subsequently updated these recommendations in 2012 to include vaccination of all pregnant women, regardless of previous immunization status, during the third trimester of pregnancy [39]. This modification was in response to concerns of potentially inadequate vaccination histories, as well as additional data showing that mothers who were vaccinated before the third trimester of the current pregnancy did not transfer sufficient protective antibody titers to their infants [40].
Routine Tdap immunization of pregnant women between 28 and 38 weeks of gestation was implemented in the United Kingdom in 2012. Evidence to date indicates good safety in a cohort of 18 000 immunized pregnant women, and more than half a million pregnant women in the United Kingdom have been immunized with Tdap (Repevax, Sanofi Pasteur, Lyon, France), a vaccine against diphtheria, polio, pertusiss, and tetanus, to date [41]. The number of fatal infant pertussis cases in the United Kingdom decreased from 12 deaths in 2012 to 2 deaths in 2013; both fatalities in 2013 were in infants born to mothers who were not immunized. A recent small controlled clinical trial in the United States demonstrated that maternal Tdap administration does not appear to impact subsequent infant immune responses to infant immunization with diphtheria, tetanus, and acellular pertussis vaccines, particularly following the fourth booster dose of vaccine. Evaluation of infant antibody responses in other clinical trials is ongoing [42]. Potential adverse events associated with repeated administration of Tdap during closely spaced pregnancies is also not yet evaluated.
WELL-STUDIED AND POTENTIALLY RECOMMENDED VACCINES
Pneumococcus
Pneumococcal pneumonia, meningitis, and bacteremia, as well as suppurative otitis media, are the most common causes of invasive bacterial infections worldwide. Infections in infants are associated with high mortality and long-term sequelae. Use of pneumococcal conjugate vaccines in infants has prevented disease through generation of a serum antibody response, reduction of nasopharyngeal carriage, and induction of herd immunity. The 13-valent pneumococcal polysaccharide-protein conjugate vaccine (PCV13) is recommended for immunization of infants in the United States starting at age 2 months for a 3-dose primary series in the first year of life [43]. However, invasive disease in infants can occur before 2 months of age.
In adults, administration of the 23-valent pneumococcal polysaccharide vaccine (PPSV23) has been recommended for >20 years. Studies of PPSV administered in the third trimester of pregnancy showed the vaccine to be safe and immunogenic, with efficient transplacental antibody transfer [15, 16]. Pneumococcal-specific antibody half-life in infants was approximately 35 days, with antibody levels in serum and breast milk sustained at 5 months of age [15]. However, a recent review found insufficient evidence that vaccination with PPSV during pregnancy has any effect on reduction of neonatal infection or nasopharyngeal colonization at 2–3 months of age [16, 44, 45]. In contrast to conjugate vaccines, polysaccharide vaccines do not induce a robust T-cell and memory B-cell response. Clinical trials comparing conjugate vaccine to PPSV23 in adults have shown a superior T-cell dependent memory B-cell antibody response with the conjugate vaccine [46, 47]. The conjugate vaccine is now recommended by the ACIP for adults with certain high-risk conditions [9]. Studies of the conjugate vaccine in pregnant women have not been reported.
Haemophilus influenzae Type b
Hib was the most common cause of bacterial meningitis in children aged <5 years in the United States before the availability of conjugate vaccines in 1988. More than 95% of the disease burden in the United States has been eliminated with vaccination, which also eliminates nasopharyngeal carriage and provides herd immunity [48]. In resource-limited settings, Hib continues to be a serious cause of bacterial meningitis and sepsis in children <5 years, and vaccine implementation has been slow due to lack of infrastructure, cost, and concerns about sustainability [49]. Type b polysaccharide conjugate vaccines against polyribosylribitol phosphate antigen are currently used in the United States in infants, but require 2–3 doses in infancy, followed by a booster dose at 12–18 months, to achieve sustained protective levels of antibodies [50, 51]. Because a large proportion of disease can occur in infants before 18 months of age, maternal immunization can be considered as a strategy to protect young infants in regions of the world without high levels of herd immunity. In studies of Hib vaccine conducted in the United States and developing countries, maternal vaccination with Hib conjugate and polysaccharide vaccines in the third trimester has been shown to be safe and immunogenic, and increases postvaccination serum and cord blood antibody titers [52, 53]. Conjugate vaccine was found to be superior to polysaccharide vaccine in increasing infant antibody titers at birth and 2 months [8, 54]. In addition, maternal vaccination did not blunt the infant response to conjugate vaccine when compared with infants of unvaccinated mothers [54].
Meningococcus
Invasive meningococcal disease is caused primarily by serogroups A, B, C, Y, or W135, and remains a significant cause of mortality worldwide, with yearly epidemics of primarily serogroup A in sub-Saharan Africa. Polysaccharide (MPSV4) and conjugate (MenACWY) vaccines with protection against A, C, Y, and W135 are available in the United States. The quadrivalent vaccine is currently available and licensed for infants as young as 2 months of age, but only recommended for those at high risk of infection. Prior studies have shown rise in antibody concentrations following meningococcal polysaccharide vaccine in maternal and infant sera and breast milk, although efficiency of transplacental transmission was variable, ranging from 30% to 44% for A and C, respectively [17, 18]. A serogroup A meningitis polysaccharide-tetanus toxoid conjugate vaccine (PsA-TT, MenAfriVac) was administered to 1.8 million persons in Chad in 2011, including pregnant women, with a 94% reduction in meningitis incidence between vaccinated and unvaccinated regions [55]. Further analysis of this vaccine in pregnant women is not yet available. No major safety concerns have been identified in pregnant women or newborns of women vaccinated during pregnancy with either MPSV4 or MenACWY in vaccine registries [56, 57]. Serogroup B disease disproportionately affects infants. A vaccine against serogroup B became available in Europe in 2013, although this has not been studied in pregnant women and is not licensed in the United States [58].
Polio
Inactivated poliovirus vaccine (IPV) is currently not recommended for pregnant women, although it has been well studied in the 1950s in the United States during a time of routine maternal vaccination. IPV has been shown to be safe and immunogenic to the mother and infant [59]. Antibody levels in mothers are high in response to both IPV and oral poliovirus vaccine (OPV), and there is evidence of efficient transplacental antibody transfer [60]. OPV, a live vaccine, could theoretically infect or cause sequelae to the fetus, but use of OPV during pregnancy has been studied as part of massive vaccination campaigns. No association has been found with congenital malformations or other adverse fetal outcomes [61].
VACCINES CURRENTLY UNDER DEVELOPMENT
Group B Streptococcus
Group B Streptococcus (GBS) is the most common cause of invasive disease in infants <3 months of age in the United States, and also causes bacteremia, urinary tract infections, chorioamnionitis, and endometritis in pregnant women. Rectal or vaginal GBS carriage is a prerequisite to invasive infection. Carriage rates during pregnancy at 23–26 weeks’ gestation are estimated at 20% [62]. Early disease with onset before 7 days is prevented with intrapartum antibiotics in women with rectal carriage of GBS, although this does not prevent late disease in infants or disease in pregnant women. Early studies of polysaccharide vaccines showed variable immunogenicity [63]. A monovalent polysaccharide conjugate vaccine has been studied in pregnant women, with results showing safety and immunogenicity, efficient transplacental antibody transfer to the fetus, and persistence of antibody until 2 months of age [22]. Currently, a trivalent polysaccharide conjugate vaccine composed of capsular serotypes Ia, Ib, and III is in phase 2 clinical trials in several countries in Europe and Africa [64]. The combination of these 3 serotypes causes the majority of early onset GBS disease. Studies are ongoing to define a serologic correlate to protection from disease in infants.
Respiratory Syncytial Virus
Respiratory syncytial virus (RSV) causes severe bronchiolitis and pneumonia, disproportionately affecting infants <6 months of age worldwide [65]. No vaccine is yet available to prevent RSV, and treatment is mainly supportive. A monoclonal antibody, palivizumab, is administered to high-risk infants to protect against infection during RSV season in many developed countries, but this is expensive and needs to be given by monthly injection [66]. Higher levels of maternal IgG are associated with less severe disease in infants, and prophylaxis with palivizumab is effective in reduction of hospitalizations due to RSV [67]. A formalin-inactivated vaccine was tested in infants in the 1960s, and caused augmentation of disease after subsequent wild-type infection in vaccinees, putting a halt to vaccine studies for many years [68]. Vaccine studies in infants <6 months of age have been difficult due to poor immunogenicity of vaccine candidates in this age group, with live attenuated RSV vaccines the most likely candidate vaccine for young infants [69]. Maternal vaccination would be an ideal strategy to prevent disease in neonates and young infants. The extensive experience with the use of palivizumab indicates the potential safety and benefit of an RSV fusion protein vaccine during pregnancy. A purified fusion protein (PFP-2) vaccine for RSV has been shown to be safe in pregnancy, although it did not significantly increase neutralizing antibody titers to RSV [70]. Currently, several new vaccines are in development, including one in phase 2 trials in women of childbearing age [68]. New studies have clarified the conformational structure of the RSV fusion protein, and identified novel antigenic sites that could be used for vaccine development [68].
Herpes Simplex Virus
Neonatal herpes simplex virus (HSV) is associated with a 60% mortality rate if untreated. The route of acquisition is most commonly through exposure in the genital tract during vaginal delivery. The highest risk of neonatal herpes occurs in women who acquire primary disease during pregnancy, rather than those who reactivate their disease [71]. Most women with genital herpes are asymptomatic or have subclinical infection. An appropriate vaccine candidate would prevent acquisition of HSV-1 and HSV-2 in pregnant women. HSV vaccine candidates in the past have not been successful in preventing primary infection from both HSV-1 and HSV-2, although several candidate vaccines are in the pipeline [72, 73].
Cytomegalovirus
Congenital cytomegalovirus (CMV) infection may cause hearing loss and neurodevelopmental sequelae in children [74]. Seronegative pregnant women are at highest risk to acquire CMV infection, frequently from young children. Women with primary infection during pregnancy are at highest risk to transmit CMV to the fetus. A vaccine administered to young children or adolescent females prior to pregnancy would prevent primary infection in pregnant women. Several candidate vaccines are in phase 1 and 2 trials, including a subunit vaccine, gB/MF59, that targets a glycoprotein complex involved in fusion of the virus with the host cell membrane. In a phase 2 randomized clinical trial conducted in seronegative postpartum women, this vaccine was found to have an efficacy of 50% in preventing acquisition of primary CMV infection. Congenital CMV infection was detected in 1% of infants born to mothers in the vaccine group, and 4% in the placebo group [75]. Currently, additional phase 2 trials are ongoing in healthy, adolescent female volunteers.
Hepatitis E Virus
A discussion of hepatitis E vaccination is included in the Supplementary Appendix.
CONCLUSIONS
Maternal immunization is a safe, efficacious, and logistically feasible strategy to provide protection against vaccine-preventable diseases in pregnant women, fetuses, and neonates. Pregnant women are able to generate robust immune responses to vaccines and to transmit antibody to infants through the placenta. The success of the neonatal tetanus elimination program has demonstrated the acceptability of providing vaccines as an integrated part of prenatal care. Maternal immunization with influenza vaccine is gaining worldwide acceptance following the 2009 influenza A/H1N1 pandemic. Novel vaccine candidates against RSV, CMV, HSV, and group B Streptococcus are under development. Maternal immunization has the potential to prevent maternal and neonatal disease in a vulnerable population.
Notes
Financial support. This work was supported by the National Institutes of Health (grant number K23-AI103105 to H. Y. C.).
Potential conflicts of interest. J. A. E. has received research support from the Bill & Melinda Gates Foundation, PATH, Gilead, GlaxoSmithKline, and Roche; serves as a consultant for GlaxoSmithKline; has received payment for lectures from Abbvie; and has had travel expenses paid for by the Global Pertussis Initiative, ESPID, International RSV Society, and GlaxoSmithKline. H. Y. C. has received research support from the Bill & Melinda Gates Foundation and PATH.
Both authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Englund J, Glezen WP, Piedra PA. Maternal immunization against viral disease. Vaccine. 1998;16:1456–63. doi: 10.1016/s0264-410x(98)00108-x. [DOI] [PubMed] [Google Scholar]
- 2.Haberg SE, Trogstad L, Gunnes N, et al. Risk of fetal death after pandemic influenza virus infection or vaccination. N Engl J Med. 2013;368:333–40. doi: 10.1056/NEJMoa1207210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Siston AM, Rasmussen SA, Honein MA, et al. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA. 2010;303:1517–25. doi: 10.1001/jama.2010.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.de Moraes-Pinto MI, Verhoeff F, Chimsuku L, et al. Placental antibody transfer: influence of maternal HIV infection and placental malaria. Arch Dis Child Fetal Neonatal Ed. 1998;79:F202–5. doi: 10.1136/fn.79.3.f202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartter HK, Oyedele OI, Dietz K, Kreis S, Hoffman JP, Muller CP. Placental transfer and decay of maternally acquired antimeasles antibodies in Nigerian children. Pediatr Infect Dis J. 2000;19:635–41. doi: 10.1097/00006454-200007000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Glezen WP, Alpers M. Maternal immunization. Clin Infect Dis. 1999;28:219–24. doi: 10.1086/515122. [DOI] [PubMed] [Google Scholar]
- 7.Linder N, Ohel G. In utero vaccination. Clin Perinatol. 1994;21:663–74. [PubMed] [Google Scholar]
- 8.Englund JA, Glezen WP, Turner C, Harvey J, Thompson C, Siber GR. Transplacental antibody transfer following maternal immunization with polysaccharide and conjugate Haemophilus influenzae type b vaccines. J Infect Dis. 1995;171:99–105. doi: 10.1093/infdis/171.1.99. [DOI] [PubMed] [Google Scholar]
- 9.Bridges CB, Woods L, Coyne-Beasley T. Advisory Committee on Immunization Practices (ACIP) recommended immunization schedule for adults aged 19 years and older—United States, 2013. MMWR Surveill Summ. 2013;62(suppl 1):9–19. [PubMed] [Google Scholar]
- 10.Heinonen OP, Shapiro S, Monson RR, Hartz SC, Rosenberg L, Slone D. Immunization during pregnancy against poliomyelitis and influenza in relation to childhood malignancy. Int J Epidemiol. 1973;2:229–35. doi: 10.1093/ije/2.3.229. [DOI] [PubMed] [Google Scholar]
- 11.Healy CM. Vaccines in pregnant women and research initiatives. Clin Obstet Gynecol. 2012;55:474–86. doi: 10.1097/GRF.0b013e31824f3acb. [DOI] [PubMed] [Google Scholar]
- 12.Munoz FM, Englund JA. Vaccines in pregnancy. Infect Dis Clin North Am. 2001;15:253–71. doi: 10.1016/s0891-5520(05)70278-6. [DOI] [PubMed] [Google Scholar]
- 13.Zaman K, Roy E, Arifeen SE, et al. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med. 2008;359:1555–64. doi: 10.1056/NEJMoa0708630. [DOI] [PubMed] [Google Scholar]
- 14.Gall SA, Myers J, Pichichero M. Maternal immunization with tetanus-diphtheria-pertussis vaccine: effect on maternal and neonatal serum antibody levels. Am J Obstet Gynecol. 2011;204:334.e1–5. doi: 10.1016/j.ajog.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 15.Shahid NS, Steinhoff MC, Hoque SS, Begum T, Thompson C, Siber GR. Serum, breast milk, and infant antibody after maternal immunisation with pneumococcal vaccine. Lancet. 1995;346:1252–7. doi: 10.1016/s0140-6736(95)91861-2. [DOI] [PubMed] [Google Scholar]
- 16.Munoz FM, Englund JA, Cheesman CC, et al. Maternal immunization with pneumococcal polysaccharide vaccine in the third trimester of gestation. Vaccine. 2001;20:826–37. doi: 10.1016/s0264-410x(01)00397-8. [DOI] [PubMed] [Google Scholar]
- 17.Shahid NS, Steinhoff MC, Roy E, Begum T, Thompson CM, Siber GR. Placental and breast transfer of antibodies after maternal immunization with polysaccharide meningococcal vaccine: a randomized, controlled evaluation. Vaccine. 2002;20:2404–9. doi: 10.1016/s0264-410x(02)00061-0. [DOI] [PubMed] [Google Scholar]
- 18.O'Dempsey TJ, McArdle T, Ceesay SJ, et al. Meningococcal antibody titres in infants of women immunised with meningococcal polysaccharide vaccine during pregnancy. Arch Dis Child Educ Pract Ed. 1996;74:F43–6. doi: 10.1136/fn.74.1.f43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Staples JE, Gershman M, Fischer M. Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2010;59:1–27. [PubMed] [Google Scholar]
- 20.Linder N, Handsher R, Fruman O, et al. Effect of maternal immunization with oral poliovirus vaccine on neonatal immunity. Pediatr Infect Dis J. 1994;13:959–62. doi: 10.1097/00006454-199411000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Leuridan E, Van Damme P. Passive transmission and persistence of naturally acquired or vaccine-induced maternal antibodies against measles in newborns. Vaccine. 2007;25:6296–304. doi: 10.1016/j.vaccine.2007.06.020. [DOI] [PubMed] [Google Scholar]
- 22.Baker CJ, Rench MA, McInnes P. Immunization of pregnant women with group B streptococcal type III capsular polysaccharide-tetanus toxoid conjugate vaccine. Vaccine. 2003;21:3468–72. doi: 10.1016/s0264-410x(03)00353-0. [DOI] [PubMed] [Google Scholar]
- 23.Ortiz JR, Neuzil KM, Ahonkhai VI, et al. Translating vaccine policy into action: a report from the Bill & Melinda Gates Foundation Consultation on the prevention of maternal and early infant influenza in resource-limited settings. Vaccine. 2012;30:7134–40. doi: 10.1016/j.vaccine.2012.09.034. [DOI] [PubMed] [Google Scholar]
- 24.Pasternak B, Svanstrom H, Molgaard-Nielsen D, et al. Risk of adverse fetal outcomes following administration of a pandemic influenza A(H1N1) vaccine during pregnancy. JAMA. 2012;308:165–74. doi: 10.1001/jama.2012.6131. [DOI] [PubMed] [Google Scholar]
- 25.Jackson LA, Patel SM, Swamy GK, et al. Immunogenicity of an inactivated monovalent 2009 H1N1 influenza vaccine in pregnant women. J Infect Dis. 2011;204:854–63. doi: 10.1093/infdis/jir440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steinhoff MC, Omer SB. A review of fetal and infant protection associated with antenatal influenza immunization. Am J Obstetr Gynecol. 2012;207(3 suppl):S21–7. doi: 10.1016/j.ajog.2012.06.071. [DOI] [PubMed] [Google Scholar]
- 27.Omer SB, Goodman D, Steinhoff MC, et al. Maternal influenza immunization and reduced likelihood of prematurity and small for gestational age births: a retrospective cohort study. PLoS Med. 2011;8:e1000441. doi: 10.1371/journal.pmed.1000441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Steinhoff MC, Omer SB, Roy E, et al. Neonatal outcomes after influenza immunization during pregnancy: a randomized controlled trial. CMAJ. 2012;184:645–53. doi: 10.1503/cmaj.110754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kallen B, Olausson PO. Vaccination against H1N1 influenza with Pandemrix((R)) during pregnancy and delivery outcome: a Swedish register study. BJOG. 2012;119:1583–90. doi: 10.1111/j.1471-0528.2012.03470.x. [DOI] [PubMed] [Google Scholar]
- 30.Fell DB, Sprague AE, Liu N, et al. H1N1 influenza vaccination during pregnancy and fetal and neonatal outcomes. Am J Public Health. 2012;102:e33–40. doi: 10.2105/AJPH.2011.300606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Richards JL, Hansen C, Bredfeldt C, et al. Neonatal outcomes after antenatal influenza immunization during the 2009 H1N1 influenza pandemic: impact on preterm birth, birth weight, and small for gestational age birth. Clin Infect Dis. 2013;56:1216–22. doi: 10.1093/cid/cit045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Legge A, Dodds L, Macdonald NE, Scott J, McNeil S. Rates and determinants of seasonal influenza vaccination in pregnancy and association with neonatal outcomes. CMAJ. 2014;186:E157–64. doi: 10.1503/cmaj.130499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adegbola R, Nesin M, Wairagkar N. Immunogenicity and efficacy of influenza immunization during pregnancy: recent and ongoing studies. Am J Obstet Gynecol. 2012;207(3 suppl):S28–32. doi: 10.1016/j.ajog.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Madhi S, Cutland C, Hugo A, et al. Efficacy and immunogenicity of inactivated influenza vaccine in pregnant women: a randomized, double-blind, placebo controlled trial [Abstract 63003]. 16th International Congress on Infectious Diseases; 4 April; Cape Town, South Africa. 2014. Available at: http://www.xcdsystem.com/icid2014/63.003.html . Accessed 14 April 2014. [Google Scholar]
- 35.Madhi S, Cutland C, Jones S, et al. Randomized, placebo-controlled trial on safety and efficacy of inactivated influenza vaccination of pregnant women in preventing illness in their infants [Abstract 1401].. 16th International Congress on Infectious Diseases; 4 April; Cape Town, South Africa. 2014. Available at: http://www.xcdsystem.com/icid2014/14.001.html. Accessed 14 April 2014. [Google Scholar]
- 36.Centers for Disease Control and Prevention Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) in pregnant women and persons who have or anticipate having close contact with an infant aged <12 months—Advisory Committee on Immunization Practices (ACIP), 2011. MMWR Morb Mortal Wkly Rep. 2011;60:1424–6. [PubMed] [Google Scholar]
- 37.Healy CM, Rench MA, Baker CJ. Implementation of cocooning against pertussis in a high-risk population. Clin Infect Dis. 2011;52:157–62. doi: 10.1093/cid/ciq001. [DOI] [PubMed] [Google Scholar]
- 38.Le T, Cherry JD, Chang SJ, et al. Immune responses and antibody decay after immunization of adolescents and adults with an acellular pertussis vaccine: the APERT Study. J Infect Dis. 2004;190:535–44. doi: 10.1086/422035. [DOI] [PubMed] [Google Scholar]
- 39.Centers for Disease Control and Prevention Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women—Advisory Committee on Immunization Practices (ACIP), 2012. MMWR Morb Mortal Wkly Rep. 2013;62:131–5. [PMC free article] [PubMed] [Google Scholar]
- 40.Healy CM, Rench MA, Baker CJ. Importance of timing of maternal combined tetanus, diphtheria, and acellular pertussis (Tdap) immunization and protection of young infants. Clin Infect Dis. 2013;56:539–44. doi: 10.1093/cid/cis923. [DOI] [PubMed] [Google Scholar]
- 41.National Health Service. Whooping cough vaccination in pregnancy. Available at: http://www.nhs.uk/conditions/pregnancy-and-baby/Pages/Whooping-cough-vaccination-pregnant.aspx . Accessed 9 June 2014.
- 42.Munoz FM, Bond NH, Maccatto M, et al. Safety and immunogenicity of Tdap immunization of pregnant women: A randomized clinical trial. JAMA. 2014;311:1760–9. doi: 10.1001/jama.2014.3633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nuorti JP, Whitney CG. Prevention of pneumococcal disease among infants and children - use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine—recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2010;59:1–18. [PubMed] [Google Scholar]
- 44.Chaithongwongwatthana S, Yamasmit W, Limpongsanurak S, et al. Pneumococcal vaccination during pregnancy for preventing infant infection. Cochrane Database Syst Rev. 2012;7:CD004903. doi: 10.1002/14651858.CD004903.pub2. [DOI] [PubMed] [Google Scholar]
- 45.Quiambao BP, Nohynek HM, Kayhty H, et al. Immunogenicity and reactogenicity of 23-valent pneumococcal polysaccharide vaccine among pregnant Filipino women and placental transfer of antibodies. Vaccine. 2007;25:4470–7. doi: 10.1016/j.vaccine.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 46.Paradiso PR. Pneumococcal conjugate vaccine for adults: a new paradigm. Clin Infect Dis. 2012;55:259–64. doi: 10.1093/cid/cis359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clutterbuck EA, Lazarus R, Yu LM, et al. Pneumococcal conjugate and plain polysaccharide vaccines have divergent effects on antigen-specific B cells. J Infect Dis. 2012;205:1408–16. doi: 10.1093/infdis/jis212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Progress toward eliminating Haemophilus influenzae type b disease among infants and children—United States, 1987–1997. MMWR Morb Mortal Wkly Rep. 1998;47:993–8. [PubMed] [Google Scholar]
- 49.Watt JP, Wolfson LJ, O'Brien KL, et al. Burden of disease caused by Haemophilus influenzae type b in children younger than 5 years: global estimates. Lancet. 2009;374:903–11. doi: 10.1016/S0140-6736(09)61203-4. [DOI] [PubMed] [Google Scholar]
- 50.Berrington JE, Cant AJ, Matthews JN, O'Keeffe M, Spickett GP, Fenton AC. Haemophilus influenzae type b immunization in infants in the United Kingdom: effects of diphtheria/tetanus/acellular pertussis/Hib combination vaccine, significant prematurity, and a fourth dose. Pediatrics. 2006;117:e717–24. doi: 10.1542/peds.2005-0348. [DOI] [PubMed] [Google Scholar]
- 51.Perrett KP, John TM, Jin C, et al. Long-term persistence of immunity and B-cell memory following Haemophilus influenzae type b conjugate vaccination in early childhood and response to booster. Clin Infect Dis. 2014;58:949–59. doi: 10.1093/cid/ciu001. [DOI] [PubMed] [Google Scholar]
- 52.Englund JA, Glezen WP. Maternal immunization with Haemophilus influenzae type b vaccines in different populations. Vaccine. 2003;21:3455–9. doi: 10.1016/s0264-410x(03)00350-5. [DOI] [PubMed] [Google Scholar]
- 53.Mulholland K, Suara RO, Siber G, et al. Maternal immunization with Haemophilus influenzae type b polysaccharide-tetanus protein conjugate vaccine in The Gambia. JAMA. 1996;275:1182–8. [PubMed] [Google Scholar]
- 54.Englund JA, Glezen WP, Thompson C, Anwaruddin R, Turner CS, Siber GR. Haemophilus influenzae type b-specific antibody in infants after maternal immunization. Pediatr Infect Dis J. 1997;16:1122–30. doi: 10.1097/00006454-199712000-00005. [DOI] [PubMed] [Google Scholar]
- 55.Daugla D, Gami J, Gamougam K, et al. Effect of a serogroup A meningococcal conjugate vaccine (PsA-TT) on serogroup A meningococcal meningitis and carriage in Chad: a community study. Lancet. 2014;383:40–7. doi: 10.1016/S0140-6736(13)61612-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ball R, Braun MM, Mootrey GT. Safety data on meningococcal polysaccharide vaccine from the Vaccine Adverse Event Reporting System. Clin Infect Dis. 2001;32:1273–80. doi: 10.1086/319982. [DOI] [PubMed] [Google Scholar]
- 57.Zheteyeva Y, Moro PL, Yue X, Broder K. Safety of meningococcal polysaccharide-protein conjugate vaccine in pregnancy: a review of the Vaccine Adverse Event Reporting System. Am J Obstet Gynecol. 2013;208:478.e1–6. doi: 10.1016/j.ajog.2013.02.027. [DOI] [PubMed] [Google Scholar]
- 58.Moxon R, Snape MD. The price of prevention: what now for immunisation against meningococcus B? Lancet. 2013;382:369–70. doi: 10.1016/S0140-6736(13)61572-X. [DOI] [PubMed] [Google Scholar]
- 59.Prevots DR, Burr RK, Sutter RW, Murphy TV. Poliomyelitis prevention in the United States. Updated recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2000;49:1–22. quiz CE1–7. [PubMed] [Google Scholar]
- 60.Brown GC, Carroll CJ. Antibody response of pregnant women to poliomyelitis vaccine and passive transfer to infants. J Immunol. 1958;81:389–95. [PubMed] [Google Scholar]
- 61.Harjulehto T, Aro T, Hovi T, Saxen L. Congenital malformations and oral poliovirus vaccination during pregnancy. Lancet. 1989;1:771–2. doi: 10.1016/s0140-6736(89)92584-1. [DOI] [PubMed] [Google Scholar]
- 62.Regan JA, Klebanoff MA, Nugent RP. The epidemiology of group B streptococcal colonization in pregnancy. Vaginal infections and prematurity study group. Obstet Gynecol. 1991;77:604–10. [PubMed] [Google Scholar]
- 63.Munoz FM, Ferrieri P. Group B Streptococcus vaccination in pregnancy: moving toward a global maternal immunization program. Vaccine. 2013;31(suppl 4):D46–51. doi: 10.1016/j.vaccine.2012.11.026. [DOI] [PubMed] [Google Scholar]
- 64.Madhi SA, Dangor Z, Heath PT, et al. Considerations for a phase-III trial to evaluate a group B Streptococcus polysaccharide-protein conjugate vaccine in pregnant women for the prevention of early- and late-onset invasive disease in young-infants. Vaccine. 2013;31(suppl 4):D52–7. doi: 10.1016/j.vaccine.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 65.Hall CB, Weinberg GA, Iwane MK, et al. The burden of respiratory syncytial virus infection in young children. N Engl J Med. 2009;360:588–98. doi: 10.1056/NEJMoa0804877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.The IMpact-RSV Study Group. Palivizumab, a humanized respiratory syncytial virus monoclonal antibody, reduces hospitalization from respiratory syncytial virus infection in high-risk infants. Pediatrics. 1998;102:531–7. [PubMed] [Google Scholar]
- 67.Glezen WP, Paredes A, Allison JE, Taber LH, Frank AL. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J Pediatr. 1981;98:708–15. doi: 10.1016/s0022-3476(81)80829-3. [DOI] [PubMed] [Google Scholar]
- 68.Anderson LJ, Dormitzer PR, Nokes DJ, Rappuoli R, Roca A, Graham BS. Strategic priorities for respiratory syncytial virus (RSV) vaccine development. Vaccine. 2013;31(suppl 2):B209–15. doi: 10.1016/j.vaccine.2012.11.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Karron RA, Buchholz UJ, Collins PL. Live-attenuated respiratory syncytial virus vaccines. Curr Top Microbiol Immunol. 2013;372:259–84. doi: 10.1007/978-3-642-38919-1_13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Munoz FM, Piedra PA, Glezen WP. Safety and immunogenicity of respiratory syncytial virus purified fusion protein-2 vaccine in pregnant women. Vaccine. 2003;21:3465–7. doi: 10.1016/s0264-410x(03)00352-9. [DOI] [PubMed] [Google Scholar]
- 71.Corey L, Wald A. Maternal and neonatal herpes simplex virus infections. N Engl J Med. 2009;361:1376–85. doi: 10.1056/NEJMra0807633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Roth K, Ferreira VH, Kaushic C. HSV-2 vaccine: current state and insights into development of a vaccine that targets genital mucosal protection. Microb Pathog. 2013;58:45–54. doi: 10.1016/j.micpath.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 73.Belshe RB, Leone PA, Bernstein DI, et al. Efficacy results of a trial of a herpes simplex vaccine. N Engl J Med. 2012;366:34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Adler SP, Nigro G. Prevention of maternal-fetal transmission of cytomegalovirus. Clin Infect Dis. 2013;57(suppl 4):S189–92. doi: 10.1093/cid/cit585. [DOI] [PubMed] [Google Scholar]
- 75.Pass RF, Zhang C, Evans A, et al. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med. 2009;360:1191–9. doi: 10.1056/NEJMoa0804749. [DOI] [PMC free article] [PubMed] [Google Scholar]


