Abstract
Background. We report the first-in-human safety and immunogenicity assessment of a prototype hexon chimeric adenovirus (Ad) serotype 5 (Ad5) vector containing the hexon hypervariable regions of Ad serotype 48 (Ad48) and expressing human immunodeficiency virus (HIV) type 1 EnvA.
Methods. Forty-eight Ad5 and Ad48 seronegative, HIV-uninfected subjects were enrolled in a randomized, double-blind, placebo-controlled, dose escalation phase 1 study. Four groups of 12 subjects received 109 to 1011 viral particles (vp) of the Ad5HVR48.EnvA.01 vaccine (n = 10 per group) or placebo (n = 2 per group) at week 0 or weeks 0, 4, and 24. Safety and immunogenicity were assessed.
Results. Self-limited reactogenicity was observed after the initial immunization in the highest (1011 vp) dose group. Responses in vaccinees included Ad48 neutralizing antibody (nAb) titers higher than Ad5 nAb titers, EnvA-specific enzyme-linked immunosorbent assay titers, and EnvA-specific enzyme-linked immunospot assay responses, and these responses generally persisted at week 52. At week 28 in the 109, 1010, and 1011 vp 3-dose groups, geometric mean EnvA enzyme-linked immunosorbent assay titers were 5721, 10 929, and 3420, respectively, and Ad48 nAb titers were a median of 1.7-fold higher than for Ad5.
Conclusions. Ad5HVR48.ENVA.01 was safe, well tolerated, and immunogenic at all doses tested. Vector-elicited nAb responses were greater for Ad48 than Ad5, confirming that Ad-specific nAbs in humans are primarily, but not exclusively, directed against the hexon hypervariable regions.
Clinical Trials Registration. NCT00695877.
Keywords: HIV vaccine, adenovirus 5 HVR48, safety, immunogenicity, dose escalation
Development of a preventive human immunodeficiency virus 1 (HIV-1) vaccine is a global health priority, but to our knowledge only 4 vaccine concepts have been evaluated in field trials to date [1–7]. Adenoviruses are potent vectors [8], and although recombinant adenovirus (Ad) serotype 5 (rAd5) vectors have been studied as preventative HIV-1 vaccines both alone and in conjunction with DNA priming, they have not been efficacious [1, 7]. Potential limitations of Ad serotype 5 (Ad5) vectors include the high level of preexisting antivector immunity, particularly in the developing world [9, 10], as well as qualitative features of the immune responses elicited [11].
To overcome these potential limitations, adenoviruses with lower seroprevalence have been proposed as vectors [12]. Alternative serotype Ad vectors have notable biologic differences from rAd5 in terms of baseline seroepidemiologic findings, receptor usage, tropism, innate immune profile, adaptive immune phenotype, and protective efficacy in the simian immunodeficiency virus (SIV)/macaque model [13–16]. Several alternative serotype Ad vectors, including Ad26 and Ad35, have advanced into clinical trials as potential vaccine vectors [17–20].
An alternative approach to retain favorable immunologic features of Ad5 vectors while reducing the potential impact of preexisting Ad5 antibodies is to develop chimeric recombinant Ad vectors in which the key Ad5 neutralization epitopes have been removed. Because Ad5-specific neutralizing antibodies (nAbs) are directed primarily against the Ad5 hexon major capsid protein [21], specifically against the 7 hexon hypervariable regions on the hexon surface, we developed chimeric rAd5 vectors in which the hexon hypervariable regions were swapped with the corresponding regions from the rare Ad serotype 48 (Ad48) [22]. These chimeric vectors use the same cellular receptor as Ad5 and effectively circumvented dominant Ad5-specific nAbs directed against the hexon hypervariable regions [22]. Furthermore, a single injection of an Ad5HVR48 chimeric vaccine expressing SIV Env/Gag/Pol/Nef was immunogenic and led to improved peak and plateau viral loads after SIV challenge in macaques [23]. In this study, we report the first-in-human safety and immunogenicity evaluation of an Ad5HVR48 vectored HIV-1 vaccine, and we evaluated the capacity of this chimeric vector to induce nAbs against both Ad5 and Ad48.
METHODS
Vaccine
The Ad5HVR48.ENVA.01 vaccine was produced in complementing HER96 cells by Crucell Holland BV, Leiden, the Netherlands. A replication deficient (deletion in the early region 1/early region 3[Δ E1/E3]) rAd5 with hexon hypervariable regions from Ad48 was constructed with an HIV-1 clade A Env gene encoding a modified envelope gp140 protein [24, 25] (see Supplementary Material for details). The placebo was final formulation buffer.
Participants and Study Design
This study was a single center, randomized, double-blind, placebo-controlled, dose escalation trial to evaluate the safety and immunogenicity of a 3-dose regimen (weeks 0, 4, and 24) of Ad5HVR48.ENVA.01 at 109, 1010, or 1011 viral particles (vp) and a single-dose (week 0) regimen at 1010 vp in healthy HIV-uninfected volunteers. The 2 groups receiving 1010 vp allowed a comparison of 1 versus 3 vaccinations. Study subjects were Ad5 and Ad48 seronegative and at low risk for acquiring HIV according to standard criteria [26]. The protocol was approved by the institutional review board and biosafety committee and written informed consent was obtained from each subject. The study was registered at ClinicalTrials.gov (NCT00695877).
Groups 1, 2, and 3 received 3 inoculations of 109, 1010, 1011 vp, respectively, and group 4 received a single inoculation of 1010 vp. Each dose group had 12 subjects, 10 vaccinees and 2 placebo recipients, for a total sample size of 48. The placebo recipients in each group were pooled into an 8-subject placebo group for analysis (2 placebo recipients received 1 injection rather than 3). All vaccines were given by needle and syringe in the deltoid muscle.
Immunogenicity Studies
Immunogenicity assessments were performed on samples collected at weeks 0, 4, 8, 24, 28, and 52. Week 28 samples were not analyzed in group 4. All immunogenicity assays were performed in a blinded fashion under good clinical laboratory practices conditions. Luciferase-based Ad neutralization assays were performed to assess Ad5- and Ad48-specific nAbs, as described elsewhere [14, 16]. Direct enzyme-linked immunosorbent assay (ELISA) was performed with serum samples to assess EnvA-specific binding antibodies against the vaccine immunogen [13]. Interferon γ enzyme-linked immunospot assay (ELISPOT) was performed to assess EnvA-specific cellular immune responses using a pool of overlapping EnvA peptides [13]. Criteria for positive Ad5 and Ad48 nAb responses were titer >16 and for Env ELISA responses, titer ≥100. Criteria for ELISPOT assay positivity were ≥55 spot-forming cells (SFCs)/106 peripheral blood mononuclear cells (PBMCs) and ≥3 times background; ELISPOT responses that were <10 SFCs/106 PBMCs after subtraction of medium-only responses were considered baseline and imputed to 10. The EnvA protein and peptides were provided by the National Institutes of Health (NIH) Vaccine Research Center, Bethesda, Maryland.
Statistical Methods
All analyses are based on the intent-to-treat principle including all subjects in the group to which they were randomized. Summaries of responses are presented as geometric mean titers (GMTs) for the antibody data and medians for the HIV-1 ELISPOT data. Differences in proportions were tested with 2-sided Fisher exact tests. The Kruskal–Wallis nonparametric analysis of variance was used to test for differences among the groups. When a significant overall difference in the Kruskal–Wallis test was identified at a given time point, pair-wise tests of all possible treatment pairs were performed using the Mann–Whitney–Wilcoxon nonparametric test. Spearman correlations were used to assess the correlation between Ad5 or Ad48 nAb titers and the magnitude of EnvA-specific T-cell responses or ELISA titers for vaccinees for the 2 assays being compared. Tests with a 2-sided P value < .05 were considered significant. No adjustments were made for multiple comparisons.
RESULTS
Subject Characteristics and Demographics
Of the 585 subjects screened for Ad5 and Ad48 seropositivity between February 2009 through November 2011, 278 (47.5%) were positive for Ad5 nAb and 49 (8.4%) were positive for Ad48 nAb. Of the 48 subjects enrolled, 31 (65%) were female, 35 (73%) were <31 years old (median, 24 years; range, 18–50 years), 15 (31%) were nonwhite, and 4 (8%) were Hispanic. The mean body mass index was 23.8 kg/m2 (range, 16.9–34.9 kg/m2). The overall retention rate was 98%, and 119 (99%) of the 120 planned vaccinations were given (99 of 100 for active vaccine and all 20 for placebo injections).
Safety and Tolerability
The Ad5HVR48.ENVA.01 vaccine was safe and generally well tolerated at all doses studied (Supplementary Figure 1A and 1B). Minimal reactogenicity was observed in the 109 or 1010 vp dose groups. Some systemic reactogenicity was observed after the initial immunization with the 1011 vp dose and consisted of a constellation of moderate to severe symptoms including malaise, myalgia, headache, and/or chills in 9 subjects, which typically began on the evening of vaccination and spontaneously resolved within a day. The reactogenicity pattern with the initial injection of the 1011 vp dose is significantly different from that in the other dose groups (P = .01), but there were no significant differences among dose groups with the booster inoculations.
Local reactogenicity was relatively common in all dose groups with all 3 inoculations and consisted of mild to moderate erythema, induration, pain, tenderness, or itchiness at the inoculation site, which generally resolved within 4–7 days, either with no treatment or with over-the-counter analgesics. Local reactogenicity symptoms did not differ among the groups after the initial or second inoculation but were greater than in the placebo group after the 6-month booster injection (P = .01). Additional safety information and comparisons between antivector and anti-insert immune responses are presented in the Supplementary Material.
Ad5 nAb Responses
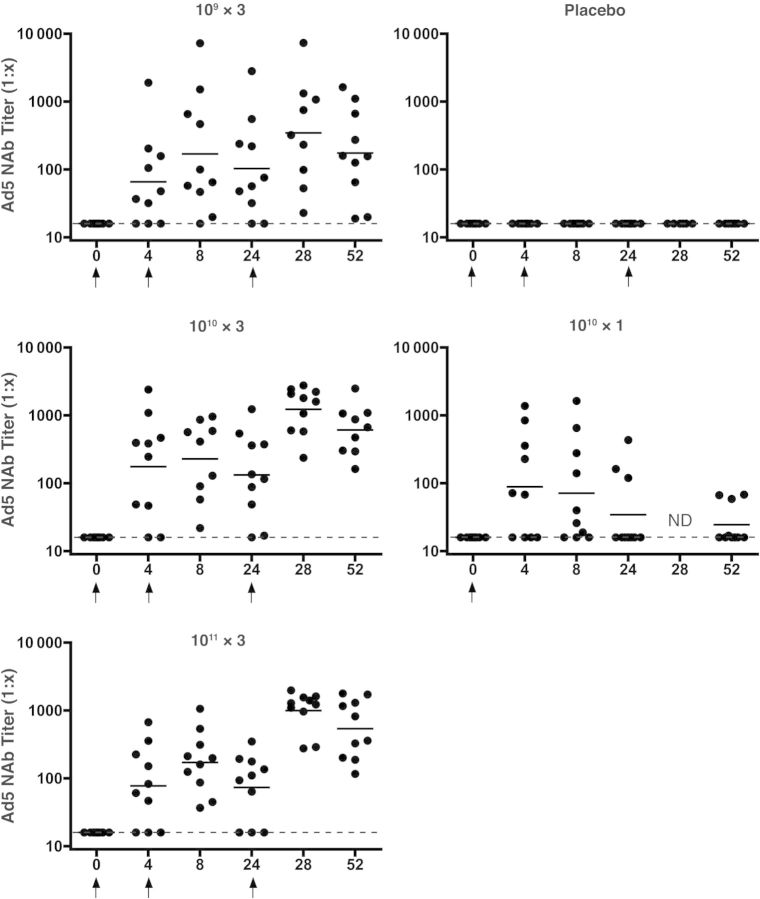
All subjects were seronegative for Ad5 at baseline. All placebo recipients had negative Ad5 nAb titers throughout the course of the study. Figure 1 shows the kinetics of the Ad5 nAb responses by dose group. Twenty-eight subjects (70%) had detectable Ad5 nAb titers by day 28 after first vaccination and all subjects who received a second injection seroconverted (titer >16) by 8 weeks except 1 subject in the lowest dose group. At week 52, the Ad5 nAb titers remained positive in all subjects who had received ≥1 booster vaccination, whereas only 40% of subjects (4 of 10) who received a single 1010 dose still had detectable Ad5 nAb at 1 year.
Figure 1.
Adenovirus serotype 5 (Ad5) neutralizing antibody (nAb) responses by group. Individual subject responses are shown by week and vaccine group. Dots indicate individual responses at a given time point; horizontal lines, geometric mean titers at a given time point for the group; dashed lines, lower limit of the assay; arrows, times when vaccine or placebo was administered. Abbreviation: ND, not done.
No clear dose-response trend was observed in the Ad5 nAb titers at week 4 after the initial immunization; GMTs were 66, 176, 78, and 89, respectively, in the groups that received 3 doses at 109, 1010, or 1011 or 1 dose at 1010 (hereafter 109 × 3, 1010 × 3, 1011 × 3, and 1010 × 1; P < .02 for all groups compared with placebo). At week 8 there was an increase in Ad5 nAb GMTs in groups 1–3 (4 weeks after second vaccination) but, as expected, not in group 4: 169 (109 × 3 group), 229 (1010 × 3 group), 172 (1011 × 3 group), and 42 (1010 × 1 group). A 32% decay in Ad5 nAb titers was noted at week 24 (before the third vaccination), but significant boosting was observed after the third vaccination (week 28) to 346 (109 × 3 group), 1234 (1010 × 3 group), and 996 (1011 × 3 group). At week 52, Ad5 nAb titers persisted with GMTs of 175, 612, and 538 in the 109 × 3, 1010 × 3, and 1011 × 3 groups, respectively, with a trend (P = .08) toward higher Ad5 nAb titers in the 2 higher-dose groups.
Ad48 nAb Responses
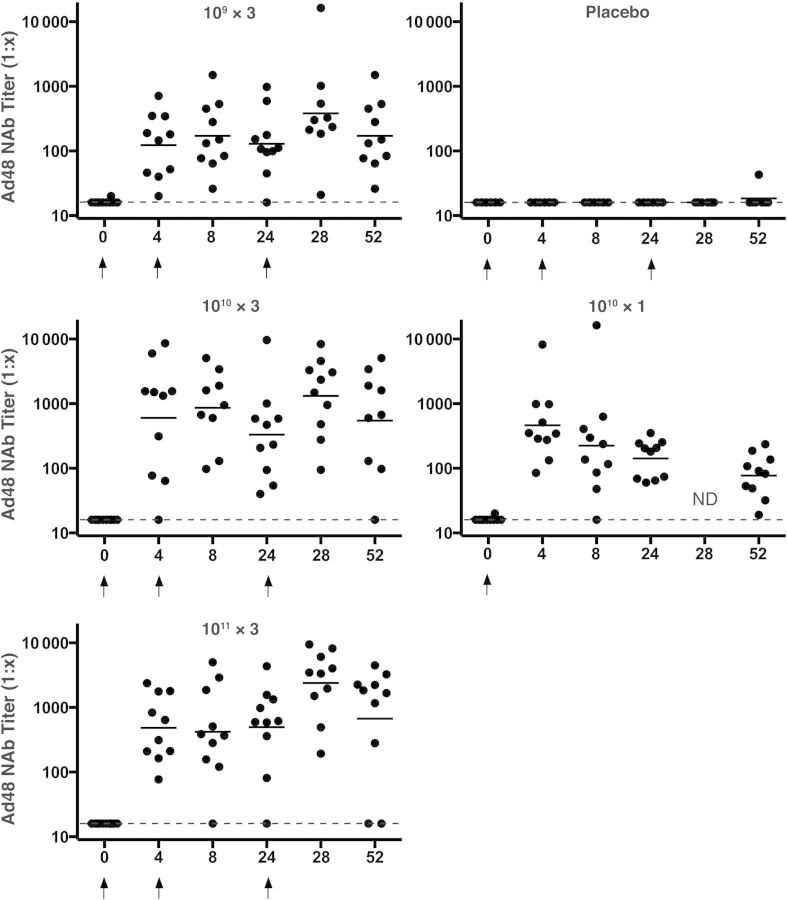
All subjects were seronegative for Ad48 at baseline. All placebo subjects were Ad48 nAb negative throughout the study except 1 subject who had a low titer response at week 52. Figure 2 shows the kinetics of the Ad48 nAb responses by dose group. Ninety-eight percent of the subjects (39 of 40) had a detectable Ad48 nAb titer by day 28 after first vaccination, and the single vaccinee (in the 1010 × 3 group) who did not respond to the first dose had a detectable titer by week 24. At week 52, the Ad48 nAb titer remained positive in most subjects who had received ≥1 booster vaccination (26 of 29; 90%) and in all 10 subjects who received a single vaccination.
Figure 2.
Adenovirus serotype 48 (Ad48) neutralizing antibody (nAb) responses by group. Individual subjects responses are shown by week and vaccine group. Dots indicate individual responses at a given time point; horizontal lines, geometric mean titers at a given time point for the group; dashed lines, lower limit of the assay; arrows, times when vaccine or placebo was administered. Abbreviation: ND, not done.
An increase in Ad48 nAb titer was observed as the dose was increased from 109 to 1010 or 1011 with GMTs at week 4 of 123, 601, 484, and 462 in the 109 × 3, 1010 × 3, 1011 × 3, and 1010 × 1 groups, respectively, at week 4 after the initial immunization (P < .02 for all groups compared with placebo). These responses were not significantly increased after the second vaccination, at week 8, in the 109 × 3, 1010 × 3, and 1011 × 3 groups but declined by about 32% by week 24. A significant increase in Ad48 nAb titer was observed after the third vaccination compared with the preboost GMTs, with increases from 129 to 382 (109 × 3 group), 330 to 1321 (1010 × 3 group), and 496 to 2395 (1011 × 3 group). At week 52, Ad48 nAb titers persisted in all groups: 171, 547, 673, and 77 in the 109 × 3, 1010 × 3, 1011 × 3, and 1010 × 1 groups, respectively.
Taken together, these data show that Ad5HVR48.ENVA.01 consistently elicited vector-specific nAbs that increased with a subsequent boost immunization at 6 months (but not with the 1-month boost) and could be detected for ≥1 year after a single inoculation. Higher titer and more durable nAb immune responses were elicited against Ad48 than against Ad5 and with 3 doses compared with a single dose. An analysis of all the vaccinees at peak immunogenicity time points (week 28 for the 109 × 3, 1010 × 3, 1011 × 3 groups and week 4 for the 1010 × 1 group) showed that they had a median 2.2-fold higher Ad48 nAb titer compared with Ad5 nAb titer (P = .02). Furthermore, 69% of vaccinees (27 of 39) had higher Ad48 than Ad5 nAb titers (P = .01). This is a striking finding, because the chimeric Ad5HVR48 vector contained >99% Ad5 sequences with <1% Ad48-derived sequences.
HIV-1 Env-Specific Antibody Responses
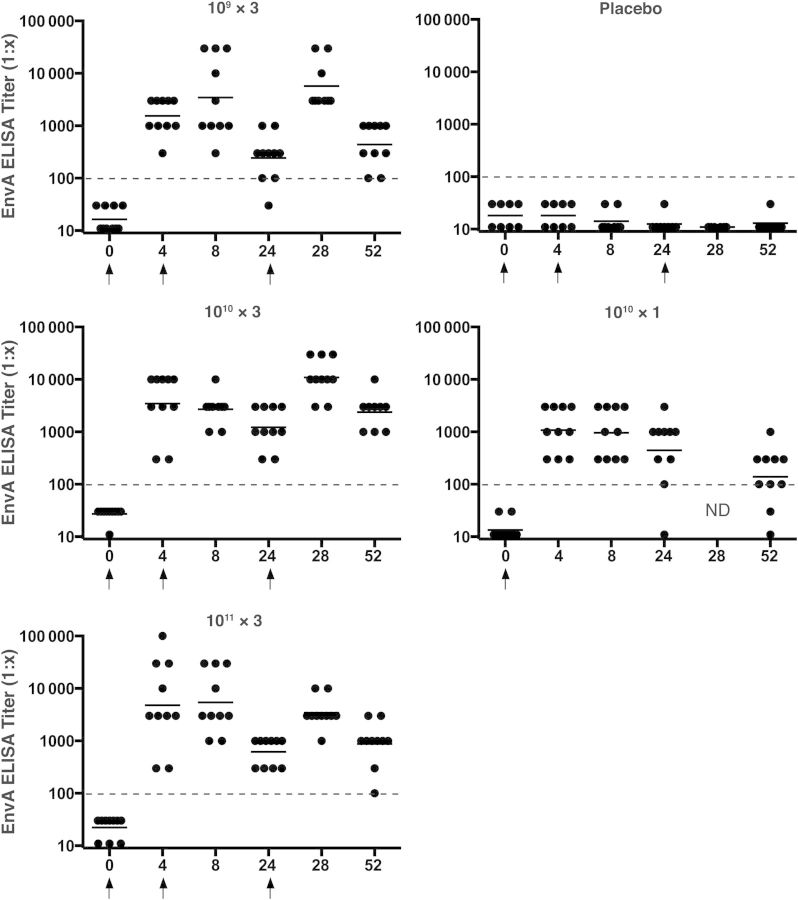
All subjects had negative EnvA-specific ELISA binding antibody titers at baseline, and all subjects in the placebo group remained negative throughout the study. Figure 3 shows the kinetics of the binding antibody responses by dose group. At week 4, after the initial vaccination, 100% (all 40) of the vaccinees had an ELISA response to the homologous EnvA antigen. At week 52 responses remained detectable in all subjects (100%) in the 109 × 3, 1010 × 3, and 1011 × 3 groups and in 80% (8 of 10) in the 1010 × 1 group. ELISA responses to a heterologous EnvA, UG37, were also detected but somewhat lower than responses to the homologous EnvA; these are presented in the Supplement and Supplementary Figure 2.
Figure 3.
EnvA enzyme-linked immunosorbent assay (ELISA) responses by group. Individual subject responses are shown by week and vaccine group. Dots indicate individual responses at a given time point; horizontal lines, geometric mean titers at a given time point for the group; dashed lines, cutoff for the assay; arrows, times when vaccine or placebo was administered. Abbreviation: ND, not done.
At week 4 a trend with an increase in EnvA ELISA GMT was observed, consistent with a dose response: 1536, 3456, 4805, and 1081 in the 109 × 3, 1010 × 3, 1011 × 3, and 1010 × 1 groups, respectively (P = .05). These titers were not significantly increased after the second vaccination and decreased by about 74% by week 24 to 243, 1220, and 618 in the 109 × 3, 1010 × 3, and 1011 × 3 groups, respectively. These titers then increased significantly after the third vaccination to 5721, 10 929, and 3420 (P < .0001) but declined by week 52 to 440, 2378, 877, and 139 in the 109 × 3, 1010 × 3, 1011 × 3, and 1010 × 1 groups, respectively.
The impact of vaccination schedule was assessed by comparing the 1010 × 3 and 1010 × 1 groups. As expected, similar ELISA GMTs were observed at week 4 after the first vaccination and were not significantly different at week 8, despite the second vaccination in the 1010 × 3 group. A decrease in GMT was observed in both groups by week 24 (1220 for 1010 × 3 and 444 for 1010 × 1), followed by an increase to 10 929 in the 1010 × 3 group after the third vaccination. At week 52, 17-fold higher ELISA responses were observed in the 1010 × 3 group than in the 1010 × 1 group (2378 vs 139; P < .0001). A similar pattern was observed with the heterologous UG37 antigen (Supplementary Figure 2).
These data show that Ad5HVR48.ENVA.01 consistently induced EnvA-specific binding antibody responses, and that these responses were substantially augmented by homologous boosting, despite the induction of robust vector-specific nAbs. No significant HIV-specific nAb responses were detected by TZM-bl assays against a panel of tier 1 viruses (DJ283.8, SF162.LS, MW965.26, and MS208.A1; data not shown).
HIV-1 EnvA-Specific Cellular Immune Responses
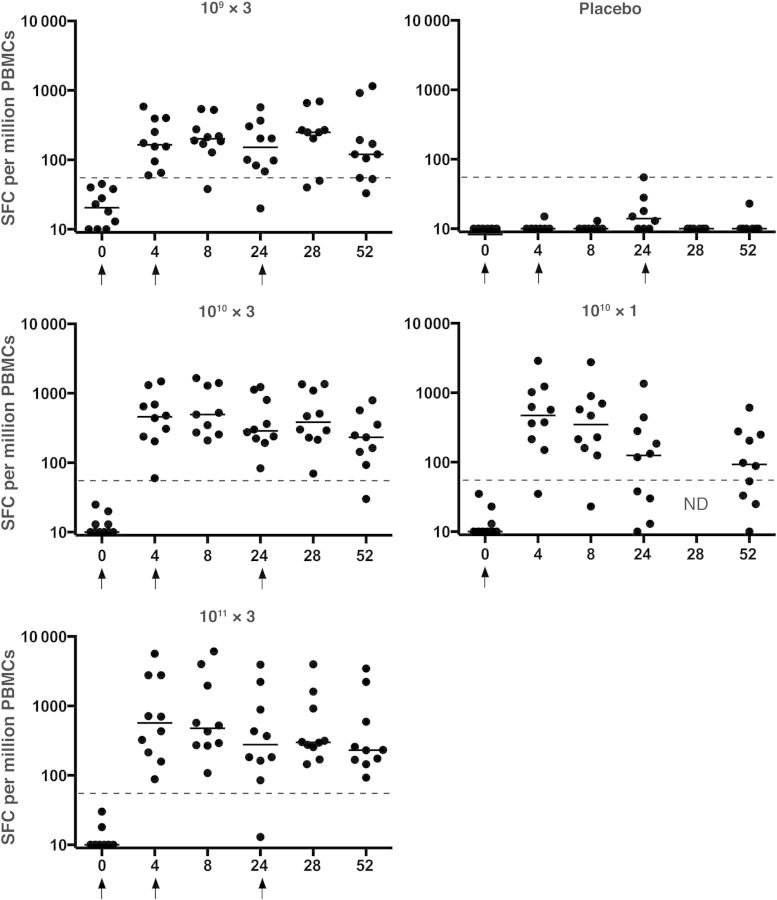
No EnvA-specific cellular immune responses were detected with interferon γ ELISPOT at baseline, and none of the subjects in the placebo group had EnvA-specific ELISPOT responses. Figure 4 shows the kinetics of the response by dose group. At week 4, 39 of 40 vaccinees (98%) had detectable ELISPOT responses. One subject in the 1010 × 1 group never had a detectable ELISPOT response despite a modest but transient ELISA response to EnvA. At week 52, persistent responses were detected in 32 of 39 subjects (82%): 8, 8, 10, and 6 subjects, respectively, in the 109 × 3, 1010 × 3, 1011 × 3, and 1010 × 1 groups.
Figure 4.
EnvA enzyme-linked immunospot assay responses by group. Individual subject responses are shown by week and vaccine group. Dots indicate individual responses at a given time point; horizontal lines, median values at a given time point for the group; dashed lines, cutoff for the assay; arrows, times when vaccine or placebo was administered. Abbreviations: ND, not done; PBMCs, peripheral blood mononuclear cells; SFCs, spot-forming cells.
The median ELISPOT responses at week 4 were 165 (109 × 3 group), 459 (1010 × 3 group), 568 (1011 × 3 group), and 473 SFCs/106 PBMCs (1010 × 1 group; all P < .0001 for comparison with placebo). These responses were similar in all groups at weeks 8, 24, and 28 despite the booster doses (at weeks 4 and 24) that the first 3 groups received, although there was a trend toward lower responses in the 109 × 3 group. At week 52, ELISPOT responses were slightly higher in the groups that had received booster vaccinations (120, 233, and 232 SFCs/106 PBMCs in the 109 × 3, 1010 × 3, and 1011 × 3 groups, respectively) compared with only a single vaccination (1010 × 1: 93). These data show that Ad5HVR48.ENVA.01 consistently induced EnvA-specific cellular immune responses and that these responses could be detected for ≥1 year after a single vaccination.
DISCUSSION
The novel recombinant Ad5HVR48.ENVA.01 HIV-1 vaccine candidate was generally safe, well tolerated, and immunogenic in this first-in-human evaluation of this chimeric Ad vector. Responses were induced after a single immunization in nearly all subjects, including those who received the lowest dose. All subjects who received the vaccine had both Ad5 and Ad48 vector-specific and EnvA insert-specific humoral immune responses that generally persisted for 1 year. These findings are consistent with the data in the nonhuman primate model where Ad5HVR48 vectors elicited consistent humoral and cellular immune responses and provided partial protection against SIV challenges [22, 23, 27]. Because Ad5HVR48 has many biologic properties that differ from those of Ad5, including a distinct innate immune cytokine profile [28], its potential as a vaccine platform warrants further study.
Interestingly, although our chimeric vector expressed only 104 amino acids (<1% of the Ad proteome) derived from 7 Ad48 hexon hypervariable domains, the anti-Ad48 nAb titers elicited were higher in magnitude and persisted longer than the anti-Ad5 nAb titers elicited by the remainder of the vector. This finding confirms the chimeric nature of the vector capsid and demonstrates that the 7 short hexon hypervariable regions represent a primary determinant of vector-specific nAbs. Although we have reported this in the context of preclinical studies [22], this has not previously been shown in a vaccine trial in humans. Our data also demonstrate that although dominant vector-specific nAbs are directed against the hexon hypervariable regions (as measured by the Ad48-specific nAbs), other nAb targets also exist (as measured by the Ad5-specific nAbs), potentially against other hexon and fiber epitopes [29, 30].
The impact of preexisting anti-Ad5 or anti-Ad48 nAbs elicited by natural infection could not be assessed in our study because our subjects were all screened to be seronegative for both. In 2 models of Ad5-vectored vaccines, preexisting Ad5-specific nAbs were found to have no impact on immune responses elicited by the insert [31, 32].
Env-specific T-cell responses were elicited at all 3 doses, including the lowest dose (109 vp). Because lower doses were not studied, the threshold for the induction of T-cell responses in humans with this vector is unknown. T-cell responses were only minimally boosted by dose (over a 100-fold range) or increased number of immunizations (3 vs 1). Although direct comparisons are not possible owing to differences in assay methods, the insert-specific responses induced by this Ad5HVR48 vector seem comparable to those elicited by Ad5, Ad26, and Ad35 vectors at similar doses and intervals [17, 24, 33].
Env-specific binding antibody responses detected with ELISA were elicited in nearly all subjects after the first immunization, including those who received the lowest dose. A dose-response trend was suggested, with higher titers in the 1011 vp group than in the other groups after the initial inoculation, but this effect was mitigated by the booster vaccinations. In the 1010 vp dose group, a direct comparison of the effect of the number of immunizations can be made. Here the 3-dose regimen elicited higher ELISA titers compared with the 1-dose regimen, and this difference was maintained at 1 year.
Despite the induction of robust vector-specific nAb responses, EnvA insert-specific antibody responses were increased after boosts with the homologous vectors, and we found no evidence that vector-specific nAbs inhibited insert-specific cellular or humoral immune responses. On the contrary, the few significant interactions we observed suggest that there is a modest, but positive, association between induction of antivector neutralization activity and immune responses elicited by the insert.
A phase IIB study of the Merck Ad5-gag/pol/nef vaccine showed no protective efficacy and possible enhanced HIV-1 acquisition in certain subgroups [1]. Our vaccine prototype is different then this prior vaccine in several important ways. First, we use chimeric Ad5 with hexon hypervariable regions derived from Ad48 as a vaccine vector, which has been shown in animal models to have major biologic differences, including lack of hepatic tropism and different innate immune profiles [11, 13, 15, 16, 28, 34]. Second, our vector expresses HIV-1 Env as a test antigen, which is potentially relevant because Env-specific antibodies seem to play a role in blocking acquisition of infection in both human [35] and nonhuman primate studies [36–38]. However a DNA-prime/Ad5-boost vaccine study was recently halted for lack of efficacy despite including HIV-1 Env immunogens in the regimen [7].
These data demonstrate the safety and immunogenicity of the novel recombinant Ad5HVR48.ENVA.01 vaccine in humans for the first time. Importantly, Env-specific humoral and cellular immune responses were consistently elicited over a 100-fold dose range with only minimal reactogenicity at the highest dose studied. Moreover, vector-elicited nAb responses were higher for Ad48 than Ad5, confirming that human Ad-specific nAbs are primarily, but not exclusively, directed against the hexon hypervariable regions. Further clinical evaluation is needed to determine whether preexisting Ad5 and/or Ad48 immunity will alter the safety or immunogenicity profile observed. The chimeric Ad5HVR48 vector is a biologically distinct vaccine vector compared with the parental Ad5 vector and therefore warrants additional investigation as a vaccine vector for both HIV and other pathogens.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the safety monitoring committee (Peter Wright [chair], Michael Keefer, and Paul Goepfert); the NIH Vaccine Research Center for the EnvA vaccine antigen, protein, and peptides; the Investigational Drug Service at Brigham and Women's Hospital (Alka Patel, Kinara Yang, Kevin Falchuk, and Jon Silverman); the clinical research staff at Brigham and Women's Hospital (Kathleen H. Krause, Rozalia Kocjan, Patrick Falahee, Caesar Angel-Lopez, Christine Matera, Trevon Mayers, Samuel Cohen, Daniel Worrall, Marissa Wilck, Elisa Choi, Yehuda Cohen, Nicolas Issa, and Francisco Marty); the research staff at Beth Israel Deaconess Medical Center (Mark J. Iampietro, Ann Cheung, Kara Brandariz, Annalena LaPorte, Anna G. McNally, Jennifer Shields, Kelly A. Stanley, Rebecca Dilan, Faye Stephens, Robyn Hamel, Giannina Santos, Elizabeth Christian, Alexis Burbank, Caroline Miller, Justin Dalrymple, Katherine E. Yanosick, James Perry, Elise Zablowsky, Alexander Robles, Anjali Chand, David J. Dominguez, Jetta Garrity, and Lauren E. Grandpre); Crucell staff for advice during the initial phases of the project (Jaap Goudsmit, Sandra Kik, Jerold Sadoff, Jort Vellinga, and Jerôme Custers); EMMES (Hayley Loblein); and the National Institute of Allergy and Infectious Diseases/NIH staff (Mike Pensiero, Dale Lawrence, Alan Fix, Elizabeth Adams, and Mary Anne Luzar). We also thank our volunteers for their generous participation in this study.
Disclaimer. This article was written by E. M. S. in her capacity as an employee of the NIH, but the views expressed herein do not necessarily represent those of the NIH.
Financial support. This work was supported by the NIH (grants AI066305, AI078526, AI096040, RR025758, and TR000170) and the Ragon Institute of MGH, MIT, and Harvard.
Potential conflicts of interest. M. G. P. and M. W. are employees of Crucell Holland. Patents for the Ad5HVR48.ENVA.01 construct are held in part by Crucell and Beth Israel Deaconess Medical Center, and no licensing agreements, royalties or income are associated with these patents.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Buchbinder SP, Mehrotra DV, Duerr A, et al. Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet. 2008;372:1881–93. doi: 10.1016/S0140-6736(08)61591-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Flynn NM, Forthal DN, Harro CD, et al. Placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis. 2005;191:654–65. doi: 10.1086/428404. [DOI] [PubMed] [Google Scholar]
- 3.McElrath MJ, De Rosa SC, Moodie Z, et al. HIV-1 vaccine-induced immunity in the test-of-concept Step Study: a case-cohort analysis. Lancet. 2008;372:1894–905. doi: 10.1016/S0140-6736(08)61592-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med. 2009;361:2209–20. doi: 10.1056/NEJMoa0908492. [DOI] [PubMed] [Google Scholar]
- 5.Gray GE, Allen M, Moodie Z, et al. Safety and efficacy of the HVTN 503/Phambili study of a clade-B-based HIV-1 vaccine in South Africa: a double-blind, randomised, placebo-controlled test-of-concept phase 2b study. Lancet Infect Dis. 2011;11:507–15. doi: 10.1016/S1473-3099(11)70098-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pitisuttithum P, Gilbert P, Gurwith M, et al. Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis. 2006;194:1661–71. doi: 10.1086/508748. [DOI] [PubMed] [Google Scholar]
- 7.Hammer SM, Sobieszczyk ME, Janes H, et al. Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N Engl J Med. 2013;369:2083–92. doi: 10.1056/NEJMoa1310566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnson JA, Barouch DH, Baden LR. Nonreplicating vectors in HIV vaccines. Curr Opin HIV AIDS. 2013;8:411–9. doi: 10.1097/COH.0b013e328363d3b7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barouch DH, Kik SV, Weverling GJ, et al. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine. 2011;29:5203–9. doi: 10.1016/j.vaccine.2011.05.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mast TC, Kierstead L, Gupta SB, et al. International epidemiology of human pre-existing adenovirus (Ad) type-5, type-6, type-26 and type-36 neutralizing antibodies: correlates of high Ad5 titers and implications for potential HIV vaccine trials. Vaccine. 2010;28:950–7. doi: 10.1016/j.vaccine.2009.10.145. [DOI] [PubMed] [Google Scholar]
- 11.Barouch DH. Novel adenovirus vector-based vaccines for HIV-1. Curr Opin HIV AIDS. 2010;5:386–90. doi: 10.1097/COH.0b013e32833cfe4c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Michael NL. Rare serotype adenoviral vectors for HIV vaccine development. J Clin Invest. 2012;122:25–7. doi: 10.1172/JCI60988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barouch DH, Liu J, Li H, et al. Vaccine protection against acquisition of neutralization-resistant SIV challenges in rhesus monkeys. Nature. 2012;482:89–93. doi: 10.1038/nature10766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Rhee EG, Masek-Hammerman K, Teigler JE, Abbink P, Barouch DH. Adenovirus serotype 26 utilizes CD46 as a primary cellular receptor and only transiently activates T lymphocytes following vaccination of rhesus monkeys. J Virol. 2012;86:10862–5. doi: 10.1128/JVI.00928-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu J, Ewald BA, Lynch DM, et al. Magnitude and phenotype of cellular immune responses elicited by recombinant adenovirus vectors and heterologous prime-boost regimens in rhesus monkeys. J Virol. 2008;82:4844–52. doi: 10.1128/JVI.02616-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lore K, Adams WC, Havenga MJ, et al. Myeloid and plasmacytoid dendritic cells are susceptible to recombinant adenovirus vectors and stimulate polyfunctional memory T cell responses. J Immunol. 2007;179:1721–9. doi: 10.4049/jimmunol.179.3.1721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baden LR, Walsh SR, Seaman MS, et al. First-in-human evaluation of the safety and immunogenicity of a recombinant adenovirus serotype 26 HIV-1 Env vaccine (IPCAVD 001) J Infect Dis. 2013;207:240–7. doi: 10.1093/infdis/jis670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keefer MC, Gilmour J, Hayes P, et al. A phase I double blind, placebo-controlled, randomized study of a multigenic HIV-1 adenovirus subtype 35 vector vaccine in healthy uninfected adults. PLoS One. 2012;7:e41936. doi: 10.1371/journal.pone.0041936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geisbert TW, Bailey M, Hensley L, et al. Recombinant adenovirus serotype 26 (Ad26) and Ad35 vaccine vectors bypass immunity to Ad5 and protect nonhuman primates against ebolavirus challenge. J Virol. 2011;85:4222–33. doi: 10.1128/JVI.02407-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radosevic K, Rodriguez A, Lemckert AA, et al. The Th1 immune response to Plasmodium falciparum circumsporozoite protein is boosted by adenovirus vectors 35 and 26 with a homologous insert. Clin Vaccine Immunol. 2010;17:1687–94. doi: 10.1128/CVI.00311-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sumida SM, Truitt DM, Lemckert AA, et al. Neutralizing antibodies to adenovirus serotype 5 vaccine vectors are directed primarily against the adenovirus hexon protein. J Immunol. 2005;174:7179–85. doi: 10.4049/jimmunol.174.11.7179. [DOI] [PubMed] [Google Scholar]
- 22.Roberts DM, Nanda A, Havenga MJ, et al. Hexon-chimaeric adenovirus serotype 5 vectors circumvent pre-existing anti-vector immunity. Nature. 2006;441:239–43. doi: 10.1038/nature04721. [DOI] [PubMed] [Google Scholar]
- 23.Barouch DH, Liu J, Lynch DM, et al. Protective efficacy of a single immunization of a chimeric adenovirus vector-based vaccine against simian immunodeficiency virus challenge in rhesus monkeys. J Virol. 2009;83:9584–90. doi: 10.1128/JVI.00821-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Catanzaro AT, Koup RA, Roederer M, et al. Phase 1 safety and immunogenicity evaluation of a multiclade HIV-1 candidate vaccine delivered by a replication-defective recombinant adenovirus vector. J Infect Dis. 2006;194:1638–49. doi: 10.1086/509258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kong WP, Huang Y, Yang ZY, Chakrabarti BK, Moodie Z, Nabel GJ. Immunogenicity of multiple gene and clade human immunodeficiency virus type 1 DNA vaccines. J Virol. 2003;77:12764–72. doi: 10.1128/JVI.77.23.12764-12772.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Andrasik MP, Karuna ST, Nebergall M, Koblin BA, Kublin JG. Behavioral risk assessment in HIV Vaccine Trials Network (HVTN) clinical trials: a qualitative study exploring HVTN staff perspectives. Vaccine. 2013;31:4398–405. doi: 10.1016/j.vaccine.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barouch DH, Klasse PJ, Dufour J, Veazey RS, Moore JP. Macaque studies of vaccine and microbicide combinations for preventing HIV-1 sexual transmission. Proc Natl Acad Sci U S A. 2012;109:8694–8. doi: 10.1073/pnas.1203183109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Teigler JE, Iampietro MJ, Barouch DH. Vaccination with adenovirus serotypes 35, 26, and 48 elicits higher levels of innate cytokine responses than adenovirus serotype 5 in rhesus monkeys. J Virol. 2012;86:9590–8. doi: 10.1128/JVI.00740-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bradley RR, Lynch DM, Iampietro MJ, Borducchi EN, Barouch DH. Adenovirus serotype 5 neutralizing antibodies target both hexon and fiber following vaccination and natural infection. J Virol. 2012;86:625–9. doi: 10.1128/JVI.06254-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bradley RR, Maxfield LF, Lynch DM, Iampietro MJ, Borducchi EN, Barouch DH. Adenovirus serotype 5-specific neutralizing antibodies target multiple hexon hypervariable regions. J Virol. 2012;86:1267–72. doi: 10.1128/JVI.06165-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morse MA, Chaudhry A, Gabitzsch ES, et al. Novel adenoviral vector induces T-cell responses despite anti-adenoviral neutralizing antibodies in colorectal cancer patients. Cancer Immunol Immunother. 2013;62:1293–301. doi: 10.1007/s00262-013-1400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smaill F, Jeyanathan M, Smieja M, et al. A human type 5 adenovirus-based tuberculosis vaccine induces robust T cell responses in humans despite preexisting anti-adenovirus immunity. Sci Transl Med. 2013;5:205ra134. doi: 10.1126/scitranslmed.3006843. [DOI] [PubMed] [Google Scholar]
- 33.Jaoko W, Karita E, Kayitenkore K, et al. Safety and immunogenicity study of multiclade HIV-1 adenoviral vector vaccine alone or as boost following a multiclade HIV-1 DNA vaccine in Africa. PLoS One. 2010;5:e12873. doi: 10.1371/journal.pone.0012873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Coughlan L, Bradshaw AC, Parker AL, et al. Ad5:Ad48 hexon hypervariable region substitutions lead to toxicity and increased inflammatory responses following intravenous delivery. Mol Ther. 2012;20:2268–81. doi: 10.1038/mt.2012.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haynes BF, Gilbert PB, McElrath MJ, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med. 2012;366:1275–86. doi: 10.1056/NEJMoa1113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Letvin NL, Rao SS, Montefiori DC, et al. Immune and genetic correlates of vaccine protection against mucosal infection by SIV in monkeys. Sci Transl Med. 2011;3:81ra36. doi: 10.1126/scitranslmed.3002351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barouch DH, Stephenson KE, Borducchi EN, et al. Protective efficacy of a global HIV-1 mosaic vaccine against heterologous SHIV challenges in rhesus monkeys. Cell. 2013;155:531–9. doi: 10.1016/j.cell.2013.09.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roederer M, Keele BF, Schmidt SD, et al. Immunological and virological mechanisms of vaccine-mediated protection against SIV and HIV. Nature. 2014;505:502–8. doi: 10.1038/nature12893. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.