Abstract
Background. Tools that estimate recent and long-term malaria transmission in a population would be highly useful for malaria elimination programs.
Methods. The prevalence of antibodies to 11 Plasmodium falciparum antigens was assessed by cytometric bead assay or enzyme-linked immunosorbent assay in 1000 people in a highland area of Kenya over 14 months, during a period of interrupted malaria transmission.
Results. Antibodies differed by antigen in acquisition with age: rapid (>80% antibody positive by age 20 years, 5 antigens), moderate (>40% positive by age 20 years, 3 antigens), or slow (<40% positive by age 20 years, 3 antigens). Antibody seroreversion rates in the 14 months between samples decreased with age rapidly (7 antigens), slowly (3 antigens), or remained high at all ages (schizont extract). Estimated antibody half-lives in individuals >10 years of age were long (40 to >80 years) for 5 antigens, moderate (5–20 years) for 3 antigens, and short (<1 year) for 3 antigens.
Conclusions. Antibodies to P. falciparum antigens in malaria-endemic areas vary by age, antigen, and time since last exposure to P. falciparum. Multiplex P. falciparum antibody testing could provide estimates of long-term and recent malaria transmission and potentially of a population's susceptibility to future clinical malaria.
Keywords: antibody, elimination, half-life, malaria, Plasmodium falciparum
Malaria remains a major cause of morbidity and death, affecting more than 200 million people and causing more than 660 000 deaths annually [1]. Malaria elimination is now a goal for many countries, but several roadblocks exist for elimination campaigns, including the lack of a rapid test to determine levels of recent and long-term malaria transmission in a population. A reliable test would allow for initial planning of interventions and for a simple way to determine the success of those interventions over time.
A recent study assessed immunoglobulin G (IgG) antibodies to merozoite surface protein-119 (MSP-119) in populations across a gradient of malaria transmission intensity [2]. The study estimated a very long half-life for antibodies to MSP-119, and models using antibodies to MSP-119 were able to predict prior malaria transmission in the area. These findings demonstrated the promise of antibody testing to create a seroprofile that allowed estimation of malaria transmission in the area. Antibodies to apical membrane antigen-1 (AMA-1) were also tested in this study and subsequent cross-sectional studies in other populations [3, 4] and were found to saturate in a population earlier than antibodies to MSP-119. Estimates in these studies were obtained from a single collection, however, so the estimates could not be refined by assessing actual changes over time in antibody prevalence and level, and antibody half-life was estimated by assuming that it was the same for all ages.
The half-life of antibodies to a given Plasmodium falciparum antigen may differ by age, and antibodies to different P. falciparum antigens may also differ in longevity [5]. Testing for antibodies to multiple antigens could therefore be a viable method for estimating recent and past malaria transmission, providing data that would be highly useful for malaria elimination campaigns. Antibodies to several P. falciparum antigens have also been correlated with protection from clinical malaria [6–11], so a seroprofile of antibodies to multiple P. falciparum antigens could potentially allow estimation of risk of clinical malaria in a population if malaria transmission recurred.
A 1-year period of interrupted transmission in a highland study site of Kenya provided an ideal opportunity to assess the half-life of IgG antibodies to multiple P. falciparum antigens in the absence of sustained transmission, using a newly developed cytometric bead assay.
MATERIALS AND METHODS
Study Site, Surveillance for Clinical Malaria, and Cohort Enrollment
The study site was the highland areas of Kapsisiywa and Kipsamoite, North Nandi County, Kenya, areas with highly seasonal P. falciparum malaria transmission. All individuals in the study site (population approximately 8000) were surveyed by demography and were requested to go the health center if they had any symptoms consistent with malaria (fever, chills, headache, or severe malaise). Clinical malaria was defined as microscopy testing positive for any human Plasmodium species in the presence of symptoms consistent with malaria.
Blood samples were collected after informed consent from a cohort of 1697 randomly selected individuals at the study site in May 2007 and July 2008 (an average of 14.3 months between sample collections). One thousand of these individuals were randomly selected for antibody testing.
Ethical approval for the study was obtained from the Kenya Medical Research Institute National Ethical Review Committee and the Institutional Review Board for Human Studies at the University of Minnesota. Informed consent was obtained from study individuals or, in the case of minors, from their parent or guardian.
P. falciparum and Epstein-Barr Virus Recombinant and Peptide Antigens
Recombinant proteins of the P. falciparum antigens apical membrane antigen-1 (AMA-1, full-length ectodomain, 3D7 and FVO strains), erythrocyte-binding antigen-175 (EBA-175, nonglycosylated region II), glutamate-rich protein (GLURP-R0, conserved nonrepeat N-terminal region, amino acids 25–514; R2, repeat C-terminal region, amino acids 705–1178, both 3D7 strain), merozoite surface protein-1 (MSP-119, E-KNG variant; MSP-142, 3D7, FUP and FVO strains), merozoite surface protein-3 (MSP-3, C-terminus, FVO strain), and liver-stage antigen-1 (LSA-1, C-terminal region, amino acids 1628 to 1909, 3D7 strain) were used for testing. Recombinant AMA-1 and LSA-1 were expressed in Escherichia coli and provided by Sheetij Dutta and David Lanar, respectively, Walter Reed Army Institute for Research. Recombinant MSP-142 and MSP-3 were expressed in E. coli, and recombinant EBA-175 expressed in Pichia pastoris, provided by David Narum, National Institutes of Health. Recombinant GLURP was expressed in E. coli and provided by Michael Theisen, Statens Seruminstitut, Copenhagen, Denmark. Recombinant MSP-119 was expressed in Saccharomyces cerevisiae and provided by the Malaria Research and Reference Reagent Resource Center (Manassas, VA). For circumsporozoite protein (CSP), the (NANP)5 repeat peptide was used. P. falciparum parasites from the 3D7 parasite clone were cultured in the preparation of schizont extract (SE) crude antigen used in enzyme-linked immunosorbent assays (ELISAs) [12]. Epstein–Barr virus (EBV) viral capsid antigen (VCA-p18) was provided by Jaap M. Middeldorp, Vrije Universiteit Medical Center, Amsterdam, The Netherlands. Recombinant antigens were chosen based on their association with prior malaria exposure or protection against clinical malaria in prior studies. Antibodies to the FVO variant for AMA-1 and MSP-142 are presented because antibodies to the 3D7 and FVO variants of AMA-1 were strongly correlated (r > 0.96, P < .0001), and antibodies to the 3D7, FUP, and FVO variants of MSP-142 were likewise strongly correlated (all r > .94, all P < .0001).
Microscopy and Polymerase Chain Reaction Testing for Plasmodium Species Infection
Microscopy testing for Plasmodium species was performed by Giemsa-stained thick and thin peripheral blood smears. Smears were examined independently by 2 microscopists, with a third reading performed for slides with discordant results [13]. Nested polymerase chain reaction (PCR) testing for P. falciparum infection was performed on filter paper blood spot samples as previously described [14, 15].
Testing for IgG Antibodies to P. falciparum Antigens or EBV Viral Capsid Antigen
Serologic responses to all P. falciparum antigens except CSP, schizont extract, and VCA-p18 were determined using a multiplex cytometric bead assay (CBA). Development and validation of this assay was previously described in detail [16]. Briefly, microspheres were coupled to P. falciparum antigens. Recombinant antigens were dissolved in 0.01 M phosphate-buffered saline (PBS) to the following concentrations, found optimal in previous studies [16]: 0.1 µg/mL (AMA-1, EBA-175, and GLURP-R2), 0.2 µg/mL (MSP-142), and 0.5 µg/mL (GLURP-R0, MSP-119 and MSP-3). Coated beads were added to microtiter plates (MABVN 1250, Millipore Corporation, Billerica, MA), incubated with plasma, and then washed. This reaction was incubated with goat antihuman IgG (gamma-chain specific) F(ab`)2 fragment-R-phycoerythrin (Sigma P8047, St. Louis, MO). The beads were analyzed on a Bioplex200 machine, and the results expressed as median fluorescence intensity (MFI). Antibodies to CSP, schizont extract, and VCA-p18 were tested by ELISA as previously described [17]. For CBA and ELISA testing, each testing plate contained plasma samples from both time points, and MFI or optical density (OD) values of wells with PBS alone were run on each plate and subtracted from sample values. Duplicate testing was performed on 10% of samples. Duplicate samples for all antigens correlated highly (Spearman's ρ > 0.87 for all, P < .0001), with a <10% coefficient of variation for all.
Statistical Analysis
Antibody levels were expressed in arbitrary units (AUs), which were calculated by dividing the MFI (for CBA) or OD (for ELISA) from the test plasma sample by the mean MFI or OD plus 3 SD from plasma samples from North American individuals never exposed to malaria. Values from nonexposed individuals were run on each plate. An AU > 1.0 was considered seropositive [18].
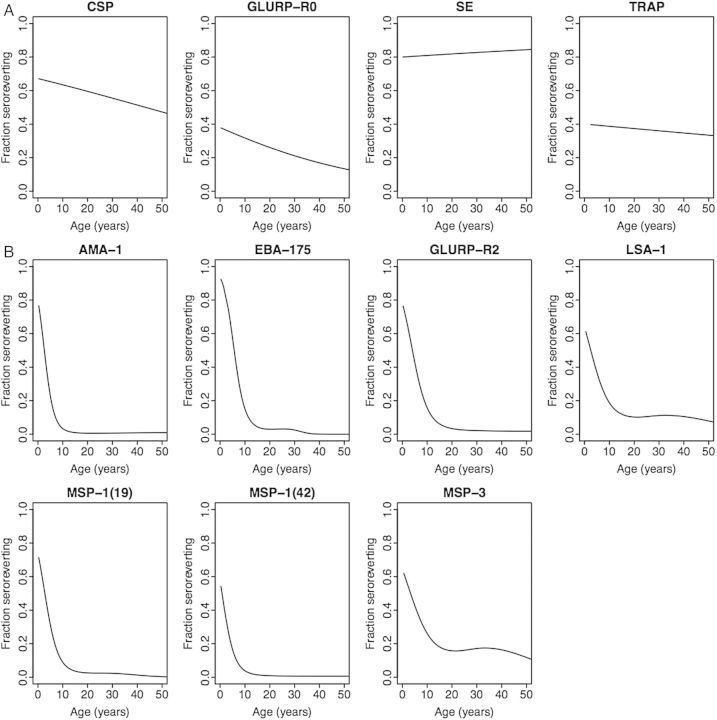
To assess the pattern of antibody acquisition according to antigen (Figure 1), subjects were sorted in order of age, divided into 8 groups of 125 persons each, and the fraction seropositive in each age group for each antigen was computed. The probability of seroreversion between the 2 blood samples (Figure 2) was estimated as a function of age for a given antigen by selecting all persons seropositive at the first blood sample and applying logistic regression, with dependent variable seroreversion at the second blood sample (yes/no) and predictor age (continuous) at the first blood draw. We considered 2 logistic regressions, one in which age entered linearly and one in which it entered as a 3-degree-of-freedom natural spline. For each antigen, we tested the spline fit versus the linear fit using the likelihood ratio test; if P >.05, we report the fit with age entered linearly, while if P ≤ .05, we report the natural-spline fit.
Figure 1.
Patterns of antibody acquisition to P. falciparum antigens in relation to age, in May 2007 (circles and solid lines) and July 2008 (triangles and dashed lines) in a highland area of western Kenya. The 3 typical patterns of antibody acquisition are rapid (A), moderate (B), and slow (C). Abbreviations: AMA-1, apical membrane antigen-1; CSP, circumsporozoite protein; EBA-175, erythrocyte-binding antigen-175; GLURP-R0, glutamate-rich protein-R0; GLURP-R2, glutamate-rich protein-R2; LSA-1, liver-stage antigen-1; MSP-119, merozoite surface protein-119; MSP-142, merozoite surface protein-142; MSP-3, merozoite surface protein-3; SE, schizont extract; TRAP, thrombospondin-related adhesive protein.
Figure 2.
Fraction seroreverting between the 2 blood samples (average 14.3 months between samples), by antigen and by age for each antigen. With respect to age, 2 patterns are typical: not strongly differentiated by age (A), and strongly differentiated by age (B), with very low fractions seroreverting after age 10–20 years and much higher fractions seroreverting in young children. For all 4 antigens following pattern (A), age entered the logistic regression linearly; for all 7 antigens following pattern (B), age entered the logistic regression as a natural spline (see Methods). Abbreviations: AMA-1, apical membrane antigen-1; CSP, circumsporozoite protein; EBA-175, erythrocyte-binding antigen-175; GLURP-R0, glutamate-rich protein-R0; GLURP-R2, glutamate-rich protein-R2; LSA-1, liver-stage antigen-1; MSP-119, merozoite surface protein-119; MSP-142, merozoite surface protein-142; MSP-3, merozoite surface protein-3; SE, schizont extract; TRAP, thrombospondin-related adhesive protein.
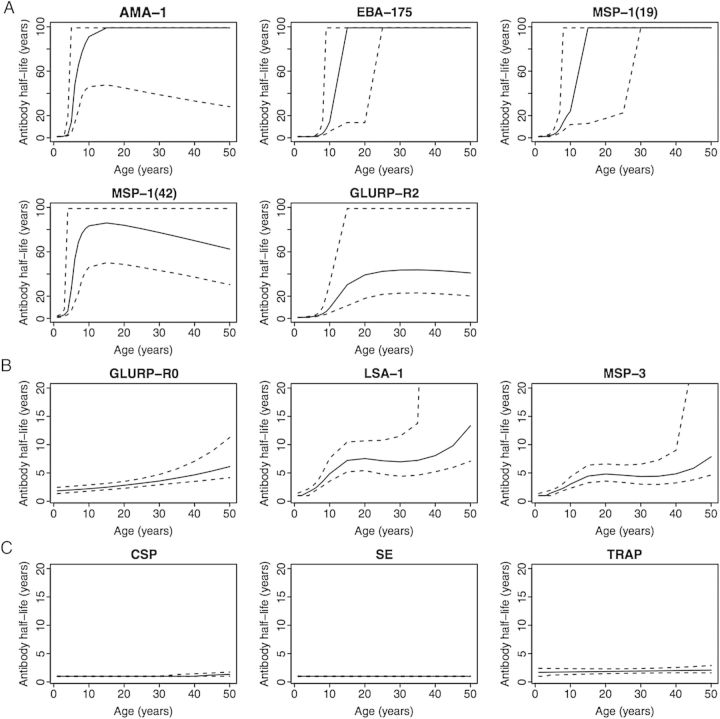
Serum half-lives were estimated for each antigen and age using the estimated probability of seroreversion at each age (described above). To estimate seroreversion half-life of an antigen for age a years, we computed the probability of seroreverting in the interval from age a to age a + 14.3 months, the interval age a + 14.3 months to age a + (2 × 14.3) months, and so forth, and summed until reaching the earliest age at which the probability of seroreversion exceeded 0.5. We then used linear interpolation between the last 2 ages considered, with probabilities of seroreversion bracketing 0.5, to estimate the age at which the probability of seroreversion equals 0.5. If the probability of seroreversion never exceeded 0.5, the half-life was set to 99 years. The confidence interval for an antigen was computed by drawing 1000 bootstrap samples with replacement from the subset of subjects who were seropositive at the first draw. For each bootstrap sample, the logistic regression was refit and the half-life estimated; the 95% confidence interval is the 2.5th and 97.5th percentiles of the 1000 bootstrapped estimates.
RESULTS
Study Cohort Characteristics and Incidence of Clinical Malaria
The study cohort had median age of 12.4 years, with ages ranging from 0.3 years to 96.7 years. Forty-eight percent of individuals were males. The typical seasonal pattern of malaria incidence was interrupted from March 2007 to April 2008, a period when no cases of clinical malaria were detected in the entire study site, likely due to spraying of >75% of households from March–July 2007 with the indoor residual insecticide lambda-cyhalothrin [19]. Between May 2007 and July 2008, the times of the 2 sample collections, no episodes of clinical malaria were recorded in any of the 1000 study participants tested for antibodies to P. falciparum.
In May 2007, 3 persons (0.3%) had asymptomatic parasitemia by microscopy testing, and in July 2008, 3 different persons (0.3%) had asymptomatic parasitemia by microscopy testing. PCR testing of 400 randomly selected persons from the site documented 1 (0.2%), 1 (0.2%), 0, 0, and 4 (1%) infected persons in May, August and November 2007 and April and July 2008, respectively.
Antibodies Are Acquired Rapidly, Moderately, or Slowly With Age, Depending on Antigen
In the study cohort as a whole, IgG antibodies to different antigens varied in prevalence according to antigen in 2007 and 2008 (Figure 1A). The prevalence of IgG antibodies at different ages differed according to antigen. Three general patterns of antibody acquisition occurred with age: rapid (>80% seropositive by 20 years of age: AMA-1, erythrocyte-binding antigen-175 [EBA-175], glutamate-rich protein [GLURP]-R2, MSP-119 and MSP-142, Figure 1A), moderate (>40% seropositive by 20 years of age, with further increases in seropositivity with greater age: liver-stage antigen-1 [LSA-1], GLURP-R0, and merozoite surface protein-3 [MSP-3], Figure 1B), and slow (<40% seropositive by 20 years of age, with modest increases in seropositivity with greater age: circumsporozoite protein [CSP], schizont extract [SE], and thrombospondin-related adhesive protein [TRAP], Figure 1C). For antibodies acquired rapidly, differences in seroprevalence between 2007 and 2008 were small (Figure 1A). For antibodies acquired at a moderate or slow rate, seroprevalence was lower in 2008 (Figure 1B and 1C). Decreases in seroprevalence in 2008 were most pronounced at older ages and for the antibodies acquired most slowly (Figure 1C).
Seroreversion Rate Decreases With Age, but the Decrease Varies by Antigen
In the cohort as a whole, 3 distinct patterns across ages were noted in the proportion of individuals seroreverting (changing from antibody positive to antibody negative) over the 14-month period between 2007 and 2008. Antibodies to 3 antigens (CSP, GLURP-R0, and TRAP) showed a slow, linear decrease in seroreversion rate with age (Figure 2A). Antibodies to SE showed no relationship to age, with high rates of seroreversion (>80%) in all ages (Figure 2A). In contrast, for antibodies to 7 antigens (AMA-1, EBA-175, GLURP-R2, LSA-1, MSP-119, MSP-142, and MSP-3), a high proportion of the youngest individuals seroreverted over the 14-month period, but seroreversion diminished to nearly zero by age 10 years (AMA-1, EBA-175, MSP-119, MSP-142), 20 years (GLURP-R2, LSA-1), or older (MSP-3) (Figure 2B).
Antibody Half-life May Be Short, Intermediate, or Long, Depending on Age and Antigen
Antibody half-life for each antigen was estimated for different ages as described in the Methods section. Estimated antibody half-life was low for young children for all antigens, but increased to essentially a lifetime (>80 years) by age 10–15 years for AMA-1, EBA-175, and MSP-119, to age 60 + years for MSP-142 by age 10–15 years; and to 40 + years by age 25 years for GLURP-R2 (Figure 3A). Antibody half-life increased with age to intermediate length for LSA-1 (>10 years by age 50 years), GLURP-R0, and MSP-3 (>5 years by age 50 years) (Figure 3B). Antibody half-life did not increase with age for CSP, SE, or TRAP, remaining <2 years even for adults ≥50 years of age (Figure 3C).
Figure 3.
Antibody half-lives to P. falciparum antigens according to age. The solid line is the estimated half-life for each age; dashed lines are upper and lower 95% confidence limits. The vertical axis is 0–100 years for Panel A and 0–20 years for Panels B and C. The half-lives show 3 typical patterns: short half-life for young children and long or very long half-life after age 10–20 years (A), short half-life for young children and half-life of 5–10 years for older children and adults (B), and very short half-life for all ages (C). Abbreviations: AMA-1, apical membrane antigen-1; CSP, circumsporozoite protein; EBA-175, erythrocyte-binding antigen-175; GLURP-R0, glutamate-rich protein-R0; GLURP-R2, glutamate-rich protein-R2; LSA-1, liver-stage antigen-1; MSP-119, merozoite surface protein-119; MSP-142, merozoite surface protein-142; MSP-3, merozoite surface protein-3; SE, schizont extract; TRAP, thrombospondin-related adhesive protein.
Antibody Levels to all P. falciparum Antigens But not Epstein-Barr Virus Viral Capsid Antigen Decrease in the Absence of Transmission
Antibody prevalence to many P. falciparum antigens was unchanged after 14 months with no episodes of clinical malaria, but antibody levels to all P. falciparum antigens decreased during this period (Table 1). In contrast, antibody levels to an unrelated antigen, the EBV viral capsid antigen (VCA-p18), were stable in all age groups over time (Table 1 and Supplementary Tables 1–4). In children <5 years of age, antibody levels to all P. falciparum antigens except EBA-175 and LSA-1 decreased over time, but at this age, the median AUs were <1.0 for all antigens, indicating that >50% children were seronegative at the time of first testing (Supplementary Table 1). For age groups 5–14 and 15–40, antibodies to all P. falciparum antigens decreased significantly (Supplementary Tables 2 and 3), while in individuals >40 years, levels decreased for all P. falciparum antigens except AMA-1, EBA-175, and MSP-142 (Supplementary Table 4).
Table 1.
Antibody Levels to P. falciparum Antigens (n = 1000) and Epstein Barr Virus VCA-p18 Antigen (n = 527) in a Highland Kenya Study Cohort in 2007 and 2008
| Antigen | Antibody Level Median (25th, 75th Percentile)a |
P Valueb | |
|---|---|---|---|
| May 2007 | July 2008 | ||
| AMA-1 | 19.9 (1.4, 45.2) | 15.4 (1.0, 43.2) | <.0001 |
| CSP | 0.7 (0.5, 1.3) | 0.5 (0.3, 1.0) | <.0001 |
| EBA-175 | 2.5 (0.4, 35.6) | 1.5 (0.3, 35.2) | <.0001 |
| GLURP-R0 | 0.6 (0.2, 3.2) | 0.5 (0.2, 1.9) | <.0001 |
| GLURP-R2 | 3.4 (0.4, 21.7) | 2.7 (0.4, 14.1) | <.0001 |
| LSA-1 | 2.2 (0.7, 6.5) | 1.7 (0.6, 4.9) | <.0001 |
| MSP-119 | 9.5 (0.5, 62.1) | 6.3 (0.4, 50.6) | <.0001 |
| MSP-142 | 51.8 (2.7, 147.1) | 39.6 (1.9, 136.7) | <.0001 |
| MSP-3 | 1.3 (0.4, 5.7) | 0.9 (0.3, 3.7) | <.0001 |
| SE | 0.7 (0.5, 1.3) | 0.4 (0.2, 0.7) | <.0001 |
| TRAP | 0.3 (0.2, 0.9) | 0.2 (0.1, 0.6) | <.0001 |
| EBV VCA-p18 | 1.4 (0.7, 2.0) | 1.4 (0.8, 2.1) | .70 |
Abbreviations: AMA-1, apical membrane antigen-1; CSP, circumsporozoite protein; EBA-175, erythrocyte-binding antigen-175; EBV VCA-p18, Epstein–Barr virus viral capsid antigen; GLURP-R0, glutamate-rich protein-R0; GLURP-R2, glutamate-rich protein-R2; LSA-1, liver-stage antigen-1; MSP-119, merozoite surface protein-119; MSP-142, merozoite surface protein-142; MSP-3, merozoite surface protein-3; SE, schizont extract; TRAP, thrombospondin-related adhesive protein.
a Antibody levels are in arbitrary units (see Methods section) for P. falciparum antigens and in optical density units for EBV VCA-p18.
b Wilcoxon paired signed–rank test.
DISCUSSION
Markers that indicate cumulative and recent malaria exposure and potential risk of future clinical malaria in a population would be valuable tools for malaria elimination programs. The present study demonstrates that antibody half-lives to P. falciparum antigens vary substantially by age and antigen in the absence of exposure. Multiplex antibody testing may therefore be a simple method to estimate recent and cumulative P. falciparum exposure in a population, and could potentially be used to predict risk of future clinical malaria in a population.
Figure 4 illustrates the potential of multiplex testing for assessing malaria exposure using the example of antibodies to AMA-1, CSP, and schizont extract in individuals of different ages in a population. A population profile like that of Figure 4A, in which only a small proportion of older people are seropositive to AMA-1, and none at any age are positive to CSP or SE, suggests exposure to malaria in the distant past. The profile in Figure 4B (most individuals >40 years old AMA-1 seropositive, a smaller proportion of individuals 10–20 years old AMA-1 positive, a very small proportion of older individuals CSP positive, and none SE positive) suggests exposure to malaria in the past 5–10 years. The profile in Figure 4C (high proportion of individuals >10 years old AMA-1 positive, small proportion CSP positive, very few SE positive), suggests a large decrease in or possible interruption of transmission over the prior year (Figure 4C). Finally, the profile in Figure 4D (high proportion of individuals >10 years old AMA-1 positive, moderate proportion of young children AMA-1 positive, moderate proportion of all ages CSP and SE positive) suggests recent transmission (Figure 4D). Exposure estimates could be refined by including antibodies to antigens with similar half-life patterns, reducing the “noise” arising from sampling and measurement error, and by assessing changes in antibody levels (rather than simply presence or absence of antibodies). Specifying such a method—including choice of antigens to include, sample size, and age distribution—would require significant additional analysis but is straightforward in principle.
Figure 4.
Examples of potential population seroprofiles of antibodies to the antigens AMA-1, CSP, and schizont extract. A, Seroprofile suggesting distant exposure in the past. B, Seroprofile suggesting exposure in the past 5–10 years. C, Seroprofile suggesting a large decrease in or interruption of transmission approximately 1 year before sampling. D, Seroprofile suggesting ongoing active transmission. Abbreviations: AMA-1, apical membrane antigen-1; CSP, circumsporozoite protein; SE, schizont extract.
Prior population-based seroepidemiology studies have suggested that antibodies to P. falciparum can act as serological markers of malaria [2, 3, 20]. Drakeley et al [2] provided the first population-based estimates of antibody half-lives, estimating that antibodies to MSP-119 have a long half-life (estimated at >40 years) once acquired. This finding contrasted with studies in Kenyan and Gambian children, which showed a short duration of persistence for IgG antibodies to MSP-119 [21] or multiple merozoite antigens [5]. The present study demonstrates that the study differences in antibody half-life estimates for MSP-119 reflect the effects of age and/or cumulative exposure on antibody half-life. Studies from the 1970s in central America, assessing antibodies to the whole parasite, showed similarly that antibodies to P. falciparum varied with age and that antibodies in young children were often short-lived (<6 months), while antibodies in adults were longer-lasting [22, 23]. Testing antibody levels to multiple P. falciparum antigens across ages, as opposed to antibodies to the whole parasite, provides a way to increase the precision and accuracy of malaria exposure estimates, and using a multiplex method makes such testing feasible for a large number of plasma or filter paper samples [24].
Memory B cells require antigenic stimulation to proliferate and differentiate into antibody-secreting cells [25, 26]. For this reason, the antibodies seen in this population in 2008, after a 1-year period of malaria interruption, likely reflect the activity of long-lived plasma cells [27–30]. A number of mechanisms may contribute to the differences seen in this study in the strength and longevity of antibody responses to specific P. falciparum antigens. These include differences in the type, number, and activity of long-lived plasma cells or memory B cells [31–35], and differences in antigen inoculum, immunogenicity of antigen, antigen antibody affinity, location in which the antigen encounters immune cells, and antigen interaction with T-helper cells [34]. The present study provides new information on variation in antibody half-lives, but a better understanding of the mechanisms that lead to differences in antibody levels and longevity will be important in designing malaria vaccines that generate long-lived, high-quality antibodies to P. falciparum antigens.
Numerous studies document the association between protection against clinical malaria and antibodies to specific P. falciparum antigens, including several antigens included in the present study [6, 7, 9, 11, 36]. Several studies also document that antibodies to multiple antigens correlate better with protection from clinical malaria than antibodies to a single antigen, and that the degree of protection may relate to antibody level rather than presence or absence of antibody [8, 17, 37, 38]. A multiplex assay incorporating the present study antigens and antigens newly associated with protection [36, 39] therefore has the potential to provide crude estimates of age-based risk of clinical malaria in a population if exposed to malaria, allowing for targeted interventions and assessment of the risk of a malaria epidemic in that population.
For broader applicability of such an assay, testing of multiple antigenic variants is needed to see if specific antibody responses differs between sites with differing prevalence of antigenic variants, and testing for optimal antigen concentration across sites with varying transmission intensities may also be required. A study across areas of differing transmission intensity would provide better information about the extent to which age versus exposure plays a role in acquisition of specific antibody responses. The utility of seroprevalence testing for the evaluation of malaria exposure and prediction of risk of clinical malaria will be best measured in cohorts in which malaria exposure has been carefully measured over several years, including time before and after the seroprevalence testing, and in cohorts that have a gradient of transmission intensity.
Testing many samples at 2 time points separated by malaria interruption allowed estimation of antibody half-lives in a context-simulating elimination, which makes this study's findings particularly applicable to programs targeting elimination. These findings may not be fully applicable to older children and adults in areas of high transmission who experience interruption of transmission after years of prior exposure, as their exposure can be >100-fold higher than that of our study participants, and this may alter many factors that affect antibody half-life, so additional studies across cohorts of differing transmission intensity are required. Identifying antigens that produce a seroprofile similar to schizont extract will be important in moving forward with a single multiplex cytometric bead assay, as it is unlikely that schizont extract can be successfully conjugated for a cytometric bead assay, yet its unique seroprofile strongly informed the study results.
In conclusion, the findings of the present study that antibody half-lives to P. falciparum antigens vary substantially by age and antigen in the absence of exposure provide a basis for multiplex antibody testing as a simple method to estimate recent and cumulative P. falciparum exposure in a population, and as a potential method to estimate future risk of clinical malaria in a population. Refining the assay with additional antigens and developing a standardized method of estimating exposure and risk are the next steps that will allow this approach to be validated in cohorts with known malaria exposure and incidence.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank the study participants and their families and the study team, including clinical officers, nurses, laboratory technologists, and data entry personnel. We thank Michael Thiessen and the Malaria Research and Reference Reagent Resource Center for the provision of antigens. This manuscript was approved for publication by the director of the Kenya Medical Research Institute.
Financial support. This work was supported by grants to C. C. J. from the National Institute of Allergy and Infectious Diseases (NIAID, U01 AI056270) and the Fogarty International Center (D43 TW0080085 and R25 TW009345). This research was supported, in part, by the Intramural Research Program of the NIAID, National Institutes of Health (NIH).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.WHO. Geneva: 2012. WHO World Malaria Report. [Google Scholar]
- 2.Drakeley CJ, Corran PH, Coleman PG, et al. Estimating medium- and long-term trends in malaria transmission by using serological markers of malaria exposure. Proc Natl Acad Sci USA. 2005;102:5108–13. doi: 10.1073/pnas.0408725102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bousema T, Youssef RM, Cook J, et al. Serologic markers for detecting malaria in areas of low endemicity, Somalia, 2008. Emerg Infect Dis. 2010;16:392–9. doi: 10.3201/eid1603.090732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cook J, Reid H, Iavro J, et al. Using serological measures to monitor changes in malaria transmission in Vanuatu. Malar J. 2010;9:169. doi: 10.1186/1475-2875-9-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akpogheneta OJ, Duah NO, Tetteh KK, et al. Duration of naturally acquired antibody responses to blood-stage Plasmodium falciparum is age dependent and antigen specific. Infect Immun. 2008;76:1748–55. doi: 10.1128/IAI.01333-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Reddy SB, Anders RF, Beeson JG, et al. High affinity antibodies to Plasmodium falciparum merozoite antigens are associated with protection from malaria. PLOS One. 2012;7:e32242. doi: 10.1371/journal.pone.0032242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McCarra MB, Ayodo G, Sumba PO, et al. Antibodies to Plasmodium falciparum erythrocyte-binding antigen-175 are associated with protection from clinical malaria. Pediatr Infect Dis J. 2011;30:1037–42. doi: 10.1097/INF.0b013e31822d1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dodoo D, Atuguba F, Bosomprah S, et al. Antibody levels to multiple malaria vaccine candidate antigens in relation to clinical malaria episodes in children in the Kasena-Nankana district of Northern Ghana. Malar J. 2011;10:108. doi: 10.1186/1475-2875-10-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Courtin D, Oesterholt M, Huismans H, et al. The quantity and quality of African children's IgG responses to merozoite surface antigens reflect protection against Plasmodium falciparum malaria. PLOS One. 2009;4:e7590. doi: 10.1371/journal.pone.0007590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stanisic DI, Richards JS, McCallum FJ, et al. Immunoglobulin G subclass-specific responses against Plasmodium falciparum merozoite antigens are associated with control of parasitemia and protection from symptomatic illness. Infect Immun. 2009;77:1165–74. doi: 10.1128/IAI.01129-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Giha HA, Staalsoe T, Dodoo D, et al. Antibodies to variable Plasmodium falciparum–infected erythrocyte surface antigens are associated with protection from novel malaria infections. Immunol Lett. 2000;71:117–26. doi: 10.1016/s0165-2478(99)00173-x. [DOI] [PubMed] [Google Scholar]
- 12.Sarr JB, Remoue F, Samb B, et al. Evaluation of antibody response to Plasmodium falciparum in children according to exposure of Anopheles gambiae s.l or Anopheles funestus vectors. Malar J. 2007;6:117. doi: 10.1186/1475-2875-6-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.John CC, McHugh MM, Moormann AM, Sumba PO, Ofulla AV. Low prevalence of Plasmodium falciparum infection among asymptomatic individuals in a highland area of Kenya. Trans R Soc Trop Med Hyg. 2005;99:780–6. doi: 10.1016/j.trstmh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 14.Menge DM, Ernst KC, Vulule JM, Zimmerman PA, Guo H, John CC. Microscopy underestimates the frequency of Plasmodium falciparum infection in symptomatic individuals in a low transmission highland area. Am J Trop Med Hyg. 2008;79:173–7. [PMC free article] [PubMed] [Google Scholar]
- 15.Snounou G, Viriyakosol S, Zhu XP, et al. High sensitivity of detection of human malaria parasites by the use of nested polymerase chain reaction. Mol Biochem Parasitol. 1993;61:315–20. doi: 10.1016/0166-6851(93)90077-b. [DOI] [PubMed] [Google Scholar]
- 16.Ondigo BN, Park GS, Gose SO, et al. Standardization and validation of a cytometric bead assay to assess antibodies to multiple Plasmodium falciparum recombinant antigens. Malar J. 2012;11:427. doi: 10.1186/1475-2875-11-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.John CC, Moormann AM, Pregibon DC. Correlation of high levels of antibodies to multiple pre-erythrocytic Plasmodium falciparum antigens and protection from infection. Am J Trop Med Hyg. 2005;73:222–8. [PubMed] [Google Scholar]
- 18.Noland GS, Hendel-Paterson B, Min XM, et al. Low prevalence of antibodies to preerythrocytic but not blood-stage Plasmodium falciparum antigens in an area of unstable malaria transmission compared to prevalence in an area of stable malaria transmission. Infect Immun. 2008;76:5721–8. doi: 10.1128/IAI.00591-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.John CC, Riedesel MA, Magak NG, et al. Possible interruption of malaria transmission, highland Kenya, 2007–2008. Emerg Infect Dis. 2009;15:1917–24. doi: 10.3201/eid1512.090627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corran P, Coleman P, Riley E, Drakeley C. Serology: a robust indicator of malaria transmission intensity? Trends Parasitol. 2007;23:575–82. doi: 10.1016/j.pt.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 21.Kinyanjui SM, Conway DJ, Lanar DE, Marsh K. IgG antibody responses to Plasmodium falciparum merozoite antigens in Kenyan children have a short half-life. Malar J. 2007;6:82. doi: 10.1186/1475-2875-6-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warren M, Collins WE, Jeffery GM, Skinner JC. The seroepidemiology of malaria in Middle America. II. Studies on the Pacific coast of Costa Rica . Am J Trop Med Hyg. 1975;24:749–54. doi: 10.4269/ajtmh.1975.24.749. [DOI] [PubMed] [Google Scholar]
- 23.Warren M, Collins WE, Cedillos R, Jeffery GM. The seroepidemiology of malaria in Middle America. III. Serologic assessment of localized Plasmodium falciparum epidemics. Am J Trop Med Hyg. 1976;25:20–5. doi: 10.4269/ajtmh.1976.25.20. [DOI] [PubMed] [Google Scholar]
- 24.Corran PH, Cook J, Lynch C, et al. Dried blood spots as a source of anti-malarial antibodies for epidemiological studies. Malar J. 2008;7:195. doi: 10.1186/1475-2875-7-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schittek B, Rajewsky K. Maintenance of B-cell memory by long-lived cells generated from proliferating precursors. Nature. 1990;346:749–51. doi: 10.1038/346749a0. [DOI] [PubMed] [Google Scholar]
- 26.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171:4969–73. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 27.Slifka MK, Antia R, Whitmire JK, Ahmed R. Humoral immunity due to long-lived plasma cells. Immunity. 1998;8:363–72. doi: 10.1016/s1074-7613(00)80541-5. [DOI] [PubMed] [Google Scholar]
- 28.Manz RA, Hauser AE, Hiepe F, Radbruch A. Maintenance of serum antibody levels. Annu Rev Immunol. 2005;23:367–86. doi: 10.1146/annurev.immunol.23.021704.115723. [DOI] [PubMed] [Google Scholar]
- 29.Wipasa J, Suphavilai C, Okell LC, et al. Long-lived antibody and B Cell memory responses to the human malaria parasites, Plasmodium falciparum and Plasmodium vivax. PLOS Pathog. 2010;6:e1000770. doi: 10.1371/journal.ppat.1000770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Traggiai E, Puzone R, Lanzavecchia A. Antigen dependent and independent mechanisms that sustain serum antibody levels. Vaccine. 2003;21(Suppl 2):S35–7. doi: 10.1016/s0264-410x(03)00198-1. [DOI] [PubMed] [Google Scholar]
- 31.Terstappen LW, Johnsen S, Segers-Nolten IM, Loken MR. Identification and characterization of plasma cells in normal human bone marrow by high-resolution flow cytometry. Blood. 1990;76:1739–47. [PubMed] [Google Scholar]
- 32.Slifka MK, Ahmed R. Long-lived plasma cells: a mechanism for maintaining persistent antibody production. Curr Opin Immunol. 1998;10:252–8. doi: 10.1016/s0952-7915(98)80162-3. [DOI] [PubMed] [Google Scholar]
- 33.Pauli NT, Henry Dunand CJ, Wilson PC. Exploiting human memory B cell heterogeneity for improved vaccine efficacy. Front Immunol. 2011;2:77. doi: 10.3389/fimmu.2011.00077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oracki SA, Walker JA, Hibbs ML, Corcoran LM, Tarlinton DM. Plasma cell development and survival. Immunol Rev. 2010;237:140–59. doi: 10.1111/j.1600-065X.2010.00940.x. [DOI] [PubMed] [Google Scholar]
- 35.Amanna IJ, Slifka MK. Mechanisms that determine plasma cell lifespan and the duration of humoral immunity. Immunol Rev. 2010;236:125–38. doi: 10.1111/j.1600-065X.2010.00912.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Richards JS, Stanisic DI, Fowkes FJ, et al. Association between naturally acquired antibodies to erythrocyte-binding antigens of Plasmodium falciparum and protection from malaria and high-density parasitemia. Clin Infect Dis. 2010;51:e50–60. doi: 10.1086/656413. [DOI] [PubMed] [Google Scholar]
- 37.Dodoo D, Hollingdale MR, Anum D, et al. Measuring naturally acquired immune responses to candidate malaria vaccine antigens in Ghanaian adults. Malar J. 2011;10:168. doi: 10.1186/1475-2875-10-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.John CC, Tande AJ, Moormann AM, et al. Antibodies to pre-erythrocytic Plasmodium falciparum antigens and risk of clinical malaria in Kenyan children. J Infect Dis. 2008;197:519–26. doi: 10.1086/526787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tran TM, Ongoiba A, Coursen J, et al. Naturally acquired antibodies specific for Plasmodium falciparum reticulocyte-binding protein homologue 5 inhibit parasite growth and predict protection from malaria. J Infect Dis. 2014;209:789–98. doi: 10.1093/infdis/jit553. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.