Abstract
STUDY QUESTION
Is consumption of sugar-sweetened beverages (SSB) associated with semen quality?
SUMMARY ANSWER
Higher consumption of SSB was associated with lower sperm motility among healthy, young men.
WHAT IS KNOWN ALREADY
The existing literature on the potential role of SSBs on male reproductive function is scarce and primarily focused on the relation between caffeinated beverages and semen quality. However, a rodent model suggests that SSBs may hamper male fertility.
STUDY DESIGN, SIZE, DURATION
The Rochester Young Men's Study; a cross-sectional study of 189 healthy young men carried out at the University of Rochester during 2009–2010.
PARTICIPANTS/MATERIALS, SETTING, METHODS
Men aged 18–22 years provided semen and blood samples, underwent a physical examination and completed a previously validated food frequency questionnaire (FFQ). Linear regression was used to analyze the association of SSBs with sperm parameters and reproductive hormone levels while adjusting for potential confounders.
MAIN RESULTS AND THE ROLE OF CHANCE
SSB intake was inversely related to progressive sperm motility. Men in the highest quartile of SSB intake (≥1.3 serving/day) had 9.8 (95% CI: 1.9,17.8) percentage units lower progressive sperm motility than men in the lowest quartile of intake (<0.2 serving/day) (P, trend = 0.03). This association was stronger among lean men (P, trend = 0.005) but absent among overweight or obese men (P, trend = 0.98). SSB intake was unrelated to other semen quality parameters or reproductive hormones levels.
LIMITATIONS, REASONS FOR CAUTION
As in all cross-sectional studies, causal inference is limited. An additional problem is that only single semen sample was obtained from each subject.
WIDER IMPLICATIONS OF THE FINDINGS
To our knowledge, this is the first report on the relation between SSB intake and low semen quality beyond the contribution of caffeinated beverages. While our findings are in agreement with recent experimental data in rodents, more studies are required to draw conclusions on the relation of SSB with semen quality or male infertility.
STUDY FUNDING/COMPETING INTEREST(S)
Supported by the European Union Seventh Framework Program (Environment), ‘Developmental Effects of Environment on Reproductive Health’ (DEER) grant 212844. Grant P30 DK046200 and Ruth L. Kirschstein National Research Service Award T32 DK007703-16 and T32HD060454 from the National Institutes of Health. None of the authors has any conflicts of interest to declare.
Keywords: diet, sugar-sweetened beverage, semen quality
Introduction
Overweight and obesity have been consistently related to low sperm counts (Sermondade et al., 2013) and to decreased fertility in natural (Sallmen et al., 2006; Nguyen et al., 2007) and assisted conception (Bakos et al., 2011; Colaci et al., 2012). It has been suggested that the effects of excess body weight on sperm production could be explained by alterations in hypothalamic–pituitary–gonadal axis, increased scrotal temperature resulting from abdominal and scrotal fat deposition, or the accumulation of liposoluble endocrine disruptors in adipose tissue (Sermondade et al., 2013). However, it is possible that other metabolic consequences of obesity, such as dysregulated adipokine secretion, insulin resistance and increased systemic inflammation (Hammoud et al., 2008a,b; Palmer et al., 2012), could also be responsible for this relationship. In addition, dietary factors have been related to some of the metabolic consequences of obesity, suggesting that specific aspects of diet may affect sperm production through similar mechanisms.
Multiple studies (Malik et al., 2010; Mozaffarian et al., 2011; Pan et al., 2013), including two randomized trials (de Ruyter et al., 2012; Ebbeling et al., 2012), have shown that sugar-sweetened beverages (SSBs) cause weight gain and obesity. It is also well known that SSBs can elicit some of the metabolic consequences of obesity (Stanhope et al., 2009). For example, SSBs increase insulin resistance (Stanhope et al., 2009) which could negatively influence semen quality via increased oxidative stress (Park et al., 2009). However, very few studies have examined the relation of SSB intake with semen quality, reproductive hormone levels or male fertility. While a recent study in rodents found that sugary drinks negatively impact male fertility (Ruff et al., 2013), the existing literature in humans is scarce.
The purpose of this study was to evaluate the relation between SSB intake and semen quality among healthy young men. We hypothesized that higher consumption of SSBs would be associated with lower semen quality.
Methods
Study population
The Rochester Young Men's Study (RYMS) is a cross-sectional study of healthy young men conducted between 2009 and 2010. Men, aged 18–22 years, were recruited through flyers and newspapers at college campuses in the Rochester, NY, area. Subjects were eligible if they were born in the USA after 31 December 1987, were able to read and speak English, and were able to have their mothers complete a questionnaire. Of the 389 men who contacted the study, 305 met all eligibility criteria. Eighty-three eligible men did not join the study due to lack of interest after learning the details of the study or failure to arrange a study visit. The remaining 222 (73%) men participated in the study. Diet assessment was introduced after enrollment began. Of the 194 men who completed the dietary assessment, three men were excluded due to missing data on sperm morphology and two due to implausible caloric intake (>10 000 or <600 kcals/day), leaving 189 men for the final analysis. Upon entry, all subjects completed questionnaires on lifestyle, demographics, as well as medical and reproductive history. The study was approved by the University of Rochester Research Subjects Review Board and informed consent was obtained from all subjects.
Semen analyses
Men underwent a physical examination and provided semen and blood samples on the same day. The physical examination included measurement of weight and height, assessment of testes location while participants were in the standing position, and presence of varicocele.
Men were instructed to abstain from ejaculation for at least 48 h before sample collection; men who did not follow this instruction were identified but not excluded (n = 26). Semen samples were collected on site by masturbation. Participants were asked to report abstinence period at the time of sample collection. Abstinence times reported to be >240 h (n = 7) were truncated at 240 h. Ejaculate volume was estimated by specimen weight, assuming a semen density of 1.0 g/ml. Sperm concentration was evaluated by hemocytometer (Improved Neubauer; Hauser Scientific, Inc., Horsham, PA, USA). For that, samples were diluted in a solution of 0.6 M NaHCO3 and 0.4% (v/v) formaldehyde in distilled water. Sperm motility was classified as progressive (WHO class A+B) and total (WHO class A+B+C) (World Health Organization, 1999). Briefly, a 10 μl of well-mixed semen was placed on a clean glass slide that had been kept at 37°C and covered with a 22 × 22 mm coverslip. The preparation was placed on the heating stage of a microscope at 37°C and immediately examined at ×400 magnification. Morphology was assessed using strict criteria (Menkveld et al., 1990). Total sperm count (sperm concentration × volume), total motile count (sperm concentration × volume × percent motile) and total normal count (sperm concentration × volume × percent normal morphology) were calculated. As a quality control measure, six sets of duplicate semen samples were sent during the study from the University of Copenhagen's Department of Growth and Reproduction to the Andrology Laboratory (University of Rochester).
Reproductive hormones measurement
Blood serum was frozen at –80°C, and then shipped to Copenhagen, Denmark on dry ice and stored at –20°C until hormone analysis was performed at University Department of Growth and Reproduction at Rigshospitalet. Serum levels of FSH (Lifshitz et al.), LH (Mendiola et al.) and sex hormone-binding globulin (SHBG) were assessed using time-resolved immunoflourometric assays (DELFIA; PerkinElmer, Skovlunde, Denmark). The intra-assay variations were all <5.0% for the FSH, LH and SHBG assay. Serum testosterone (T) levels were determined by a time-resolved fluoroimmunoassay (DELFIA; PerkinElmer) with intra- and inter-assay variation <8%. Estradiol (E2) was measured by radioimmunoassay (Pantex, Santa Monica, CA, USA) with intra-assay variation of <8% and the inter-assay variation of <13%. Inhibin B levels were determined by a specific two-sided enzyme immunometric assay (Oxford Bio-Innovation Ltd, Bicester, UK) with intra- and inter-assay variation of 13 and 18%, respectively. Free testosterone (cFT) concentration was calculated using the equation of Vermeulen et al., assuming a fixed albumin concentration of 43 g/l (Vermeulen et al., 1999).
Dietary assessment
Diet was assessed using a previously validated 131-item FFQ (Rimm et al., 1992). Men were asked to report how often, on average, they had consumed specified amounts of each food, beverage and supplement over the past year. Food frequency options ranged from never to six or more times per day. For beverages, a serving was defined as one glass, bottle or can; size was assumed to be 12 oz (0.35 l) for nutrient estimation. Total SSB consumption was derived by summing intakes of carbonated SSBs with caffeine (e.g. Coke, Pepsi), carbonated SSBs without caffeine (e.g. Ginger Ale, 7-UP) and non-carbonated SSBs (e.g. sports drinks, sugared iced tea). In a validation study, the de-attenuated correlation (i.e. observed correlation corrected for random within-person variability) (Rosner and Willett, 1988) between two 1-week prospectively collected diet records collected 6 months apart and FFQ reports collected at the end of the year were 0.84 for caffeinated carbonated beverages and 0.55 for all other SSBs (Feskanich et al., 1993). Previously described dietary patterns were calculated to characterize overall food choices (Gaskins et al., 2012). Nutrient intakes were estimated using a nutrient database derived from the US Department of Agriculture (USDA) with additional information obtained from manufacturers (United States Department of Agriculture and Agriculural Research Service, 2008).
Statistical analyses
Men were classified into quartiles of SSB intake. Linear regression models were used to estimate the adjusted difference and 95% confidence interval (CI) in semen quality parameters and reproductive hormone levels in increasing quartiles of SSB intake using men in the lowest quartile as reference, while adjusting for potential confounders. Sperm concentration, total sperm count and FSH were log-transformed to meet normality assumptions of linear regression. Results for these parameters were back transformed to improve interpretability. Population marginal means were utilized to present marginal population averages adjusted for the covariates in the model (Searle et al., 1980). Tests for linear trend were performed across quartiles of SSB intake using median intake in each quartile as a continuous variable in the linear regression models. To examine the possibility of non-linear relationships, we evaluated potential threshold effects by dichotomizing SSB intake in increments of 0.25 servings/day. Non-linearity was also examined by fitting models with linear and quadratic terms.
Participant characteristics previously related to semen quality parameters in this population (Gaskins et al., 2012, 2013) or other studies (Li et al., 2011; Sermondade et al., 2013) were considered as potential confounders if they were also related to SSB intake at P < 0.20. Based on these criteria, models were adjusted for age (Vine et al.), smoking status (current/former or never), abstinence time (h), physical activity (h/week), TV viewing hour (h/week), total fat intake (% energy), total protein intake (% energy), total energy intake (kcal/day), total caffeine intake (g/d), total alcohol intake (g/d) and for the Prudent and Western dietary pattern summary scores. Analyses for sperm motility were additionally adjusted for time between ejaculation and start of semen analysis (Chavarro et al.). Models for reproductive hormones were adjusted for time of blood draw to account for circadian variation in hormone levels and the same set of covariates as semen parameters with the exception of abstinence time. Additional adjustment for BMI was planned regardless of statistical significance as it was hypothesized to mediate the relation between SSB and semen quality. In addition, effect modification by BMI (<25 and ≥25 kg/m2), smoking status (current and never/former smokers), physical activity (moderate-vigorous activity <10.5 and ≥10.5 h/week) and caffeine (<103.1 and ≥103.1 g/d) was tested using cross product-terms in the final model. Statistical analyses were performed with SAS v9.2 (SAS Institute, Cary, NC, USA).
Results
The median (range) age of participants was 19.6 (18–22) years. Most men were Caucasian (83%) and non-smokers (77%); 42% were overweight or obese (BMI ≥ 25 kg/m2) and no men were underweight (BMI ≤18.5 kg/m2). Participants were highly active, with a median (25th, 75th percentile) of 8.0 (5.0, 14.0) h/week spent on moderate to vigorous physical activities. The median [25th, 75th percentile] values of sperm parameters were 45.2 × 106/ml [20.5, 95.6] for concentration, 62.8% [55.5, 73.5] for motility, 8.6% [5%, 12%] for normal morphology and 3.5 ml [2.2, 4.4] for ejaculate volume. The median [25th, 75th percentile] SSB intake was 0.71 servings/day [0.22, 1.29], 45% of which was intake of non-carbonated SSBs. Most men did not consume diet beverages (63%) and 95% of men had an intake of diet beverage below 1 serving/day. On average, SSB intake amounted to 6.2% of total energy intake and 25.8% of total sugar intake. Men who consumed more SSBs had higher Western pattern scores, total caloric intake and carbohydrates intake but lower Prudent pattern scores and protein intakes (Table I).
Table I.
Characteristics of the Rochester Young Men's Study population according to quartiles of sugar-sweetened beverage (SSB) intake.
| Sugar-sweetened beverage |
|||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | P-valuea | |
| N | 48 | 45 | 48 | 48 | |
| Median, serving/day | 0.11 | 0.42 | 0.95 | 2.72 | |
| Range | 0–0.2 | 0.2–0.7 | 0.7–1.3 | 1.3–7.5 | |
| Median (IQR) or n (%) | |||||
| Demographics | |||||
| Age, years | 19.6 (19.1, 20.8) | 19.7 (19.0, 20.7) | 19.5 (18.8, 20.5) | 19.1 (18.7, 19.9) | 0.08 |
| BMI, kg/m2 | 24.8 (22.9, 26.6) | 25.3 (22.8, 27.6) | 24.0 (22.6, 25.9) | 24.5 (22.6, 25.3) | 0.26 |
| Current smoker, n (%) | 6 (13) | 14 (31) | 15 (31) | 8 (17) | 0.05 |
| TV viewing (hour/week) | 12 (4, 14) | 4 (4, 14) | 14 (4, 20) | 14 (4, 20) | 0.06 |
| Moderate-vigorous exercise, h/w | 8.5 (6.0, 14.5) | 9.0 (5.0, 11.0) | 8.0 (3.8, 13.0) | 8.0 (4.0, 15.0) | 0.29 |
| Total exercise, h/w | 14.0 (8.5, 20.0) | 12.0 (8.0, 18.0) | 13.0 (7.0, 19.5) | 15.0 (8.0, 26.0) | 0.43 |
| Race, n (%) | |||||
| White | 39 (81) | 39 (87) | 37 (77) | 41 (85) | 0.63 |
| Non-White | 9 (19) | 6 (13) | 11 (23) | 7 (15) | |
| Abstinence time, hours | 72.4 (61.6, 134.1) | 65.8 (53.8, 84.5) | 70.5 (52.0, 101.0) | 69.1 (54.9, 98.3) | 0.19 |
| Diet | |||||
| Alcohol, g/d | 8.0 (2.0, 22.2) | 14.4 (5.9, 21.4) | 12.7 (2.6, 28.4) | 14.4 (3.9, 27.1) | 0.38 |
| Caffeine, g/d | 20.6 (7.4, 57.6) | 50.9 (27.4, 135.8) | 70.2 (34.9, 111.3) | 84.9 (58.4, 183.9) | 0.10 |
| Total sugar intake, g/d | 149 (105, 179) | 139 (110, 187) | 143 (118, 193) | 225 (174, 307) | <0.0001 |
| Total carbohydrate, % energy | 48.9 (45.0, 55.8) | 47.9 (44.8, 54.3) | 48.8 (46.2, 52.3) | 52.3 (47.7, 57.2) | 0.02 |
| Total protein, % energy | 18.0 (15.7, 19.7) | 16.3 (14.8, 18.1) | 15.6 (14.4, 17.2) | 14.5 (12.8, 16.7) | <0.0001 |
| Total fat, % energy | 29.3 (25.4, 32.9) | 29.8 (27.9, 32.9) | 31.9 (29.4, 35.4) | 29.5 (27.6, 32.6) | 0.07 |
| Total energy intake, kcal/day | 2536 (2011, 3125) | 2629 (2275, 3246) | 2819 (2057, 3668) | 3460 (2841, 4441) | <0.0001 |
| Prudent pattern scoreb | 0.1 (−0.3, 1.0) | −0.0 (−0.6, 0.3) | −0.5 (−0.8, 0.3) | −0.4 (−0.7, −0.1) | 0.0006 |
| Western pattern scoreb | −0.8 (−1.2, −0.3) | −0.4 (−0.7, 0.3) | −0.0 (−0.5, 0.3) | 0.7 (0.1, 1.4) | <0.0001 |
| Multivitamin users, n (%) | 21 (44) | 10 (22) | 12 (25) | 10 (21) | 0.06 |
| Reproductive history | |||||
| Self-reported history of cryptorchidism, n (%) | 0 (0) | 3 (7) | 1 (2) | 1 (2) | 0.22 |
| Testis low in scrotum, n (%) | 41 (85) | 41 (91) | 45 (94) | 46 (96) | 0.32 |
| Genital diseasec, n (%) | 0 (0) | 4 (9) | 3 (6) | 4 (8) | 0.17 |
| Varicocele, n (%) | 1 (2) | 1 (2) | 0 (0) | 3 (6) | 0.34 |
| Hydrocele, n (%) | 0 (0) | 1 (3) | 0 (0) | 1 (2) | 0.60 |
IQR, interquartile range.
aFrom Kruskal–Wallis test for continuous variables and Fisher's exact test for categorical variables.
bDietary patterns were constructed by factor analysis as described in Gaskins et al. (2012). A higher score indicates higher adherence to the Prudent or Western dietary pattern.
cIncluding epididymitis, orchitis, prostatitis, urinary tract infection, gonorrhea, genital warts or herpes, chlamydia, torsion of the testes, hypospadia or other diseases of the penis, testicles, urinary tract or scrotum.
SSB intake was inversely related to sperm motility after adjustment for potential confounders (Table II). Further adjustment for BMI and overall dietary patterns did not have a major impact on the results. In this model, men in the top category of SSB intake had 6.3 (95% CI 1.0, 11.6) percentage units lower sperm motility than men in the lowest 3 quartiles of intake. Adjustment for intake of total sugars slightly strengthened the association. In the multivariate model with additional terms for total sugar intake, the adjusted percentage (95% CI) of total motile sperm in increasing quartiles of SSB intake were 64.2 (59.9, 68.5), 65.8 (61.8, 69.7), 63.9 (60.1, 67.7) and 57.2 (52.5, 61.9) (P, trend = 0.02).
Table II.
Directly measured semen quality parameters [mean (95% CI)] according to the intake of sugar-sweetened beverages (SSB).
| Sugar-sweetened beverage |
|||||
|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Ptrenda | |
| N | 48 | 45 | 48 | 48 | |
| SSB intake median, serving/day | 0.11 | 0.42 | 0.95 | 2.72 | |
| Range, serving/day | 0–0.2 | 0.2–0.7 | 0.7–1.3 | 1.3–7.5 | |
| Sperm concentration (millions/ml) | |||||
| Crude | 44.1 (33.3, 58.4) | 43.5 (32.5, 58.1) | 45.5 (34.4, 60.2) | 47.3 (35.7, 62.6) | 0.67 |
| Model 1b | 44.4 (33.7, 58.6) | 46.2 (34.8, 61.3) | 47.8 (36.3, 62.8) | 42.3 (31.7, 56.3) | 0.71 |
| Model 2c | 41.1 (31.0, 54.6) | 43.8 (33.3, 57.5) | 47.7 (36.6, 62.2) | 48.1 (35.9, 64.4) | 0.54 |
| Model 3d | 40.6 (30.0, 54.8) | 44.1 (33.4, 58.1) | 47.4 (36.3, 61.9) | 48.7 (35.5, 66.9) | 0.53 |
| Sperm motility (% motile A+B+C)e | |||||
| Crude | 63.0 (59.1, 66.8) | 66.3 (62.4, 70.3) | 63.6 (59.8, 67.4) | 58.2 (54.4, 62.1) | 0.01 |
| Model 1b | 63.9 (60.1, 67.8) | 66.3 (62.3, 70.2) | 63.8 (60.0, 67.6) | 57.1 (53.1, 61.1)* | 0.002 |
| Model 2c | 63.5 (59.5, 67.6) | 65.3 (61.4, 69.2) | 63.7 (59.9, 67.5) | 58.5 (54.4, 62.7) | 0.03 |
| Model 3d | 63.9 (59.7, 68.1) | 65.4 (61.5, 69.3) | 63.7 (59.9, 67.4) | 58.0 (53.6, 62.5) | 0.03 |
| Progressive motility (% motile A+B)e | |||||
| Crude | 58.5 (54.4, 62.5) | 61.8 (57.7, 66.0) | 59.8 (55.8, 63.8) | 53.6 (49.6, 57.7) | 0.01 |
| Model 1b | 59.6 (55.5, 63.6) | 61.8 (57.7, 65.9) | 60.0 (56.1, 64.0) | 52.3 (48.2, 56.5)* | 0.002 |
| Model 2c | 58.9 (54.7, 63.2) | 60.8 (56.7, 64.9) | 60.0 (56.0, 64.0) | 54.0 (49.6, 58.4) | 0.04 |
| Model 3d | 59.3 (54.8, 63.8) | 60.9 (56.8, 65.0) | 60.0 (56.0, 64.0) | 53.5 (48.8, 58.2) | 0.03 |
| Sperm morphology (% normal) | |||||
| Crude | 8.2 (6.9, 9.5) | 8.8 (7.5, 10.1) | 8.5 (7.2, 9.8) | 9.0 (7.8, 10.3) | 0.46 |
| Model 1b | 8.2 (6.9, 9.5) | 8.9 (7.5, 10.2) | 8.5 (7.2, 9.8) | 8.9 (7.6, 10.3) | 0.61 |
| Model 2c | 8.2 (6.8, 9.6) | 8.8 (7.5, 10.1) | 8.6 (7.3, 9.9) | 9.0 (7.5, 10.4) | 0.59 |
| Model 3d | 8.4 (6.9, 9.9) | 8.9 (7.5, 10.2) | 8.6 (7.3, 9.9) | 8.6 (7.1, 10.2) | 0.98 |
| Semen volume (ml) | |||||
| Crude | 3.6 (3.2, 4.1) | 3.2 (2.8, 3.7) | 3.6 (3.1, 4.0) | 3.3 (2.9, 3.8) | 0.58 |
| Model 1b | 3.5 (3.1, 3.9) | 3.3 (2.8, 3.7) | 3.6 (3.2, 4.0) | 3.4 (3.0, 3.9) | 0.98 |
| Model 2c | 3.5 (3.0, 3.9) | 3.3 (2.9, 3.7) | 3.7 (3.3, 4.1) | 3.3 (2.9, 3.8) | 0.77 |
| Model 3d | 3.5 (3.1, 4.0) | 3.3 (2.9, 3.8) | 3.7 (3.3, 4.1) | 3.2 (2.8, 3.7) | 0.51 |
aEstimated using median intake in each quartile as a continuous variable.
bAdjusted for total energy intake and abstinence time.
cAdjusted for total energy intake, abstinence time, age, smoking status, alcohol, caffeine, total protein intake, total fat intake, TV viewing hours and physical activity.
dAdjusted for total energy intake, abstinence time, age, smoking status, alcohol, caffeine, total protein intake, total fat intake, TV viewing hours, physical activity, BMI, and the Prudent and Western dietary patterns.
eAdditionally adjusted for time from current ejaculation to start of semen analysis.
*P-value for trend <0.05 compared with men in the lowest quartile of SSB intake.
Results for progressive motility closely paralleled the results for total motility (Table II). Men in the top quartile of SSB intake had 9.8 (95% CI 1.9,17.8) percentage units lower progressive sperm motility than men in the lowest quartile of intake. SSB intake was unrelated to sperm concentration, morphology and ejaculate volume (Table II). It was also unrelated to derived semen quality parameters (Supplementary Table SI). In addition, there were no differences between specific SSBs (carbonated SSBs with caffeine, carbonated SSBs without caffeine and non-carbonated SSBs) in their association with sperm motility (P = 0.17). Intake of fruit juices was not related to semen quality parameters.
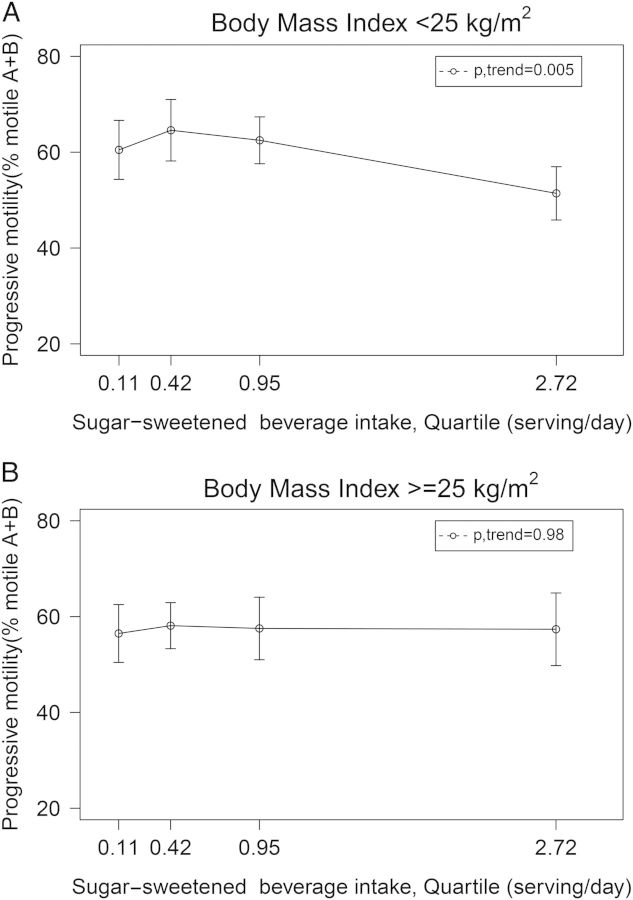
BMI modified the association between SSB consumption and progressive sperm motility (P, interaction = 0.002). SSBs were inversely related to progressive motility among lean men but not among overweight or obese men (Fig. 1). There was no evidence of significant heterogeneity on the relation between SSBs and motility by levels of physical activity (P, interaction = 0.88), smoking status (P, interaction = 0.95) or caffeine intake (P, interaction = 0.60).
Figure 1.
Adjusted progressive motility according to sugar-sweetened beverage (SSB) intake by BMI level. Values are adjusted progressive motility (95% CI) across quartiles of SSB intake among (A) men with BMI<25 kg/m2 (P-trend = 0.005) and (B) men with BMI ≥25 kg/m2 (P-trend = 0.98). X-axis labels median intake in each quartile. Results are adjusted for total energy intake, abstinence time, age, smoking status, alcohol, caffeine, total protein intake, total fat intake, TV viewing hours, physical activity, Prudent and Western dietary patterns, and time from current ejaculation to start of semen analysis. Tests for trend were conducted across quartiles using the median value of a variable in each quartile.
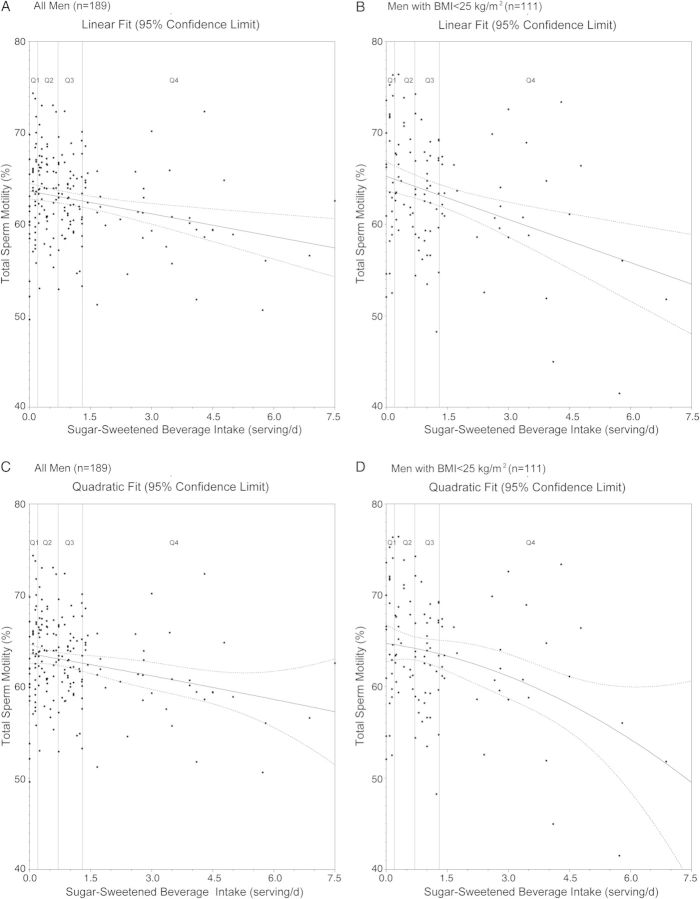
We assessed the possibility of a threshold or non-linear relation between SSB intake and motility. Analyses where SSB intake was dichotomized in increasing cutoffs showed that the inverse relation between SSB intake and progressive motility became statistically significant at intakes above 1 serving/day (Supplementary Table SII), roughly corresponding to the median intake in the third quartile of SSB intake. Non-linear models did not improve the model fit compared with a linear model (Fig. 2).
Figure 2.
Sugar-sweetened beverage (SSB) intake in relation to total sperm motility. Solid lines indicate estimate of linear regression model; dashed lines indicate 95% confidence limits of the estimate. (A) SSBs modeled as a linear predictor among all men. (B) SSBs modeled as a linear predictor among lean men. (C) SSBs modeled with linear and quadratic terms among all men. (D) SSBs modeled with linear and quadratic terms among lean men. All models are adjusted for total energy intake, abstinence time, age, BMI, smoking status, alcohol, caffeine, total protein intake, total fat intake, TV viewing hours, physical activity, Prudent and Western dietary patterns, and time from current ejaculation to start of semen analysis.
Lastly, we investigated the relation between SSB intake and reproductive hormone levels (Table III). There was an inverse association between SSB intake and FSH levels of borderline statistical significance (P, trend = 0.07). FSH levels, however, were not related to sperm motility (rSpearman = –0.08, P = 0.21) and further adjustment for FSH in the multivariate models of the relation between SSBs and sperm motility did not affect the results. SSBs were unrelated to levels of the remaining reproductive hormones.
Table III.
Reproductive hormone levels [mean (95% CI)] according to the intake of sugar-sweetened beverages (SSB).
| Sugar-sweetened beverages |
|||||
|---|---|---|---|---|---|
| SSB intake, quartiles | Q1 | Q2 | Q3 | Q4 | P trenda |
| Range, serving/day | 0–0.2 | 0.2–0.7 | 0.7–1.3 | 1.3–7.5 | |
| FSH (IU/l) | |||||
| Crude | 2.6 (2.3, 3.0) | 2.6 (2.3, 3.0) | 2.6 (2.3, 3.0) | 2.3 (2.0, 2.6) | 0.08 |
| Adjustedb | 2.7 (2.3, 3.2) | 2.6 (2.3, 3.1) | 2.6 (2.3, 3.0) | 2.2 (1.9, 2.6) | 0.07 |
| LH (IU/l) | |||||
| Crude | 3.6 (3.2, 4.0) | 3.6 (3.2, 4.1) | 3.8 (3.4, 4.2) | 3.8 (3.4, 4.2) | 0.54 |
| Adjustedb | 3.6 (3.2, 4.1) | 3.6 (3.2, 4.0) | 3.9 (3.5, 4.3) | 3.6 (3.2, 4.1) | 0.97 |
| Testosterone (nmol/l) | |||||
| Crude | 19.9 (18.0, 21.9) | 21.8 (19.8, 23.8) | 19.9 (17.9, 21.8) | 20.0 (18.1, 22.0) | 0.64 |
| Adjustedb | 20.5 (18.4, 22.7) | 21.8 (19.7, 23.8) | 20.0 (17.7, 21.6) | 19.7 (17.4, 21.9) | 0.37 |
| Free testosterone (nmol/l) | |||||
| Crude | 0.44 (0.40, 0.48) | 0.52 (0.48, 0.56)* | 0.47 (0.43, 0.52) | 0.48 (0.43, 0.52) | 0.79 |
| Adjustedb | 0.44 (0.39, 0.49) | 0.51 (0.46, 0.56)* | 0.48 (0.44, 0.52) | 0.48 (0.43, 0.53) | 0.95 |
| SHBG (nmol/l) | |||||
| Crude | 33.1 (29.8, 36.4) | 29.5 (26.0, 32.9) | 29.5 (26.1, 32.8) | 29.4 (26.1, 32.7) | 0.31 |
| Adjustedb | 34.5 (30.9, 38.0) | 30.5 (27.1, 33.8) | 28.4 (25.2, 31.5)* | 28.2 (24.5, 31.9)* | 0.12 |
| Estradiol (pmol/l) | |||||
| Crude | 88.6 (81.5, 95.7) | 95.9 (88.5, 103.2) | 90.7 (83.6, 97.8) | 90.2 (83.1, 97.3) | 0.79 |
| Adjustedb | 90.8 (82.7, 98.8) | 95.3 (87.8, 102.8) | 89.8 (82.6, 97.0) | 89.5 (81.0, 97.9) | 0.57 |
| Inhibin B (pg/ml) | |||||
| Crude | 190.5 (173.2, 207.9) | 187.9 (169.9, 205.8) | 197.2 (179.8, 214.6) | 203.7 (186.3, 221.1) | 0.20 |
| Adjustedb | 192.3 (172.8, 211.7) | 188.4 (170.3, 206.5) | 196.9 (179.6, 214.2) | 201.9 (181.5, 222.3) | 0.41 |
| Inhibin B/FSHb | 86.4 (62.4, 110.4) | 84.2 (61.9, 106.5) | 93.5 (72.2, 114.8) | 114.2 (89.1, 139.2) | 0.09 |
| Testosterone/LHb | 5.9 (4.9, 6.9) | 6.9 (6.0, 7.8) | 5.7 (4.8, 6.6) | 6.5 (5.5, 7.6) | 0.78 |
| cFT/LHb | 143.2 (110.7, 175.7) | 155.0 (124.8, 185.2) | 152.1 (123.2, 180.9) | 173.2 (139.2, 207.2) | 0.28 |
| E2/testosteroneb | 4.8 (4.4, 5.3) | 4.6 (4.2, 5.3) | 4.9 (4.5, 5.3) | 4.7 (4.3, 5.2) | 0.97 |
CI, confidence interval; SHBG, sex hormone-binding globulin; testosterone/LH, ratio of testosterone (nmol/l) to LH (IU); FT/LH, ratio of calculated free testosterone (pmol/l) to LH (IU); E2/testosterone, ratio of E2 (pmol/l) to testosterone (nmol/l); inhibin B/FSH, ratio of inhibin B (pg/ml) to FSH (IU).
aEstimated using median intake in each quartile as a continuous variable.
bAdjusted for total energy intake, age, BMI, smoking status, alcohol, caffeine, total protein intake, total fat intake, TV viewing hours, physical activity, the Prudent and Western dietary patterns, and hour of blood sampling.
*P-value for trend <0.05 compared with men in the lowest quartile of intake.
Discussion
Intake of SSBs was related to lower sperm motility (total and progressive) among young healthy men. This relation was independent of a large number of potential confounders, but was confined to lean men. There was also a suggestion of an inverse relation between SSB intake and FSH levels. SSB intake was not related to other semen quality parameters or reproductive hormone levels.
Our results are consistent with recent animal experimental data (Ruff et al., 2013). Male mice fed 25% of their total energy intake as a fructose/glucose solution designed to resemble SSBs had a 25% fewer offspring than control male mice (Ruff et al., 2013). SSBs accounted for a smaller proportion of calories in our study, 9.2% of total caloric intake among men in the highest quartile for SSB intake, than in the rodent experiment. It is, therefore, possible that the observed relations may be larger in populations with higher SSB intake. It is also important to point out that it is not possible to determine from our findings to what extent the observed relations with sperm motility might translate into fertility. Therefore, further evaluation of SSBs’ role in male reproductive function is needed.
SSB intake is known to have multiple metabolic effects that could explain the observed associations. Consumption of SSBs has been found to increase insulin resistance in adolescents (Kondaki et al., 2013) and adults (Stanhope et al., 2009). Insulin resistance is known to increase oxidative stress (Park et al., 2009), which in turn can negatively influence sperm motility (Benedetti et al., 2012; Chen et al., 2013). In addition, conditions characterized by insulin resistance, such as type 2 diabetes, have also been related to lower sperm motility (Echavarria Sanchez et al., 2007; Rama Raju et al., 2012). On the other hand, insulin resistance decreases hepatic production of SHBG (Pugeat et al., 1991, 2010). Lower circulating levels of SHBG initially increase the bioavailability of testosterone which then via a negative feedback loop decreases central production of gonadotrophins via GnRH to keep free testosterone unchanged. This could partly explain our finding of slightly lower FSH with higher SSB intake. This adaptation, however, would be expected also to result in lower inhibin B levels (secondary to lower FSH) but we did not observe any significant relation between SSB intake and inhibin B. While it is possible that this mismatch reflects that the feed-forward arm of the loop (FSH-inhibin B) is less robust than feedback loop (inhibin B-FSH) (Ramaswamy et al., 2000), it could also be a chance finding. Further evaluation of the effect of SSB on reproductive hormone homeostasis is warranted.
Given the strong relation between SSB intake and obesity and the well-characterized association between obesity and semen quality, we had hypothesized that an association between SSB intake and semen would be mediated through BMI. However, contrary to our hypothesis, we did not observe evidence of mediation by BMI. Instead, BMI modified this relation whereby the association between SSBs and sperm motility was observed among lean men but not among overweight or obese men. Total sperm count and sperm concentration are the parameters more strongly related to obesity (Sermondade et al., 2013) but excess weight has also been related to lower sperm motility in humans (Hammoud et al., 2008a,b; Sekhavat and Moein, 2010; Hammiche et al., 2012) and in animal models (Fernandez et al., 2011). The observed interaction may represent a true biological interaction where the strong deleterious effect of excess body weight on motility outweighs the modest association between SSB and this outcome and, therefore, the relation between SSB and poor semen quality can only be observed among men with higher baseline semen quality. Alternatively, contaminants such as bisphenol A (BPA) and phthalates leaching from plastic containers could also explain the observed relations (Hauser, 2008; Meeker et al., 2010; Mendiola et al., 2011; Jurewicz et al., 2013).
While SSBs have not been examined specifically in their relation to semen quality before, some have evaluated the relation between caffeinated sodas and semen quality in studies evaluating the relation between caffeine and semen quality (Curtis et al., 1997; Vine et al., 1997; Ramlau-Hansen et al., 2008; Jensen et al., 2010). Of closest relevance to our findings, Jensen et al. found that men who drank >1 l/day of cola had significantly lower total sperm counts and sperm concentration than men who did not consume these beverages; this relation was not explained by caffeine intake (Jensen et al., 2010). The divergent findings between Jensen's and the current study could result from the differences between the studies. These include the fact that most SSBs in this population were non-carbonated SSBs, such as sport drinks, which were not assessed by Jensen et al.; a difference in physical activity levels between the studies; and the lack of adjustment for other dietary factors that have been subsequently associated with semen quality in Jensen's study (Jensen et al., 2013). Other studies that have considered intake of SSBs as part of their assessment of sources of caffeine have not found associations with semen quality (Vine et al., 1997; Ramlau-Hansen et al., 2008), although the association between caffeinated soft drinks and semen quality was not specifically reported in these studies. Clearly, further examination of the relation between SSB intake and semen quality parameters deserves further consideration.
Strengths of our study include, first, a relatively homogenous population of young healthy men without knowledge of their fertility potential or the results of their semen analysis, which makes it unlikely that the results are explained by changes in diet made in response to fertility issues. On the other hand, the homogeneity hinders generalizability to other groups of men. Second, we used a previously validated dietary instrument, which not only gives confidence to the validity of self-reported SSB intake, but also allows us to assess and adjust for overall food choices thereby reducing the likelihood of residual confounding by other dietary behaviors. In addition, we had detailed information on a variety of lifestyle risk factors, reproductive history and results of a physical examination, which allowed for adjustment of potential confounders. Third, the range of SSB intake observed in this population is comparable to that of adult men in the USA (Kit et al., 2013).
The most important limitation of the study is its cross-sectional design, which severely hampers our ability to assess causality. However, as mentioned above, men were blinded to the study outcomes thereby limiting the possibility of reverse causation; one of the most serious sources of bias in cross-sectional studies. Second, the sensitive window for spermatogenesis is 3 months prior to the ejaculate (with a shorter time window for effects on sperm maturation in the epididymis manifested by an association with motility but not concentration, as observed here), while the FFQ asked men's typical daily intake during the previous year. Nevertheless, people generally tend to ‘telescope’ their reports. As a result, it may reflect more recent intake thus minimizing this limitation (Willett, 2012). An additional problem is that only single semen sample was obtained from each subject. However, previous work suggests that there is limited gain in information from using multiple samples per man in research settings (Carlsen et al., 2005; Stokes-Riner et al., 2007).
In conclusion, we found that SSB intake was inversely related to sperm motility among young healthy men. This association was confined to lean men. Our findings are consistent with recent experimental data in rodents (Smith et al.). To our knowledge, this is the first report on the relation between SSB intake and low semen quality beyond the contribution of specific SSBs as sources of caffeine. Therefore, it is important that this association should be further evaluated.
Supplementary data
Supplementary data are available at http://humrep.oxfordjournals.org/.
Authors' roles
S.H.S. was involved in study concept and design. J.M., J.E.C., S.H.S. and N.J. contributed to the acquisition of data. Y.H.C. analyzed data, wrote the manuscript and had a primary responsibility for final content; J.E.C. supervised analysis and edited the manuscript. M.A., A.J.G., P.L.W., J.M., N.J., S.H.S. and J.E.C. were involved in the critical revision of the manuscript. All authors read and approved the final manuscript.
Funding
Supported by the European Union Seventh Framework Program (Environment), ‘Developmental Effects of Environment on Reproductive Health’ (DEER) grant 212844. Grant P30 DK046200 and Ruth L. Kirschstein National Research Service Award T32 DK007703-16 and T32HD060454 from the National Institutes of Health.
Conflict of interest
None declared.
Supplementary Material
References
- Bakos HW, Henshaw RC, Mitchell M, Lane M. Paternal body mass index is associated with decreased blastocyst development and reduced live birth rates following assisted reproductive technology. Fertil Steril. 2011;95:1700–1704. doi: 10.1016/j.fertnstert.2010.11.044. [DOI] [PubMed] [Google Scholar]
- Benedetti S, Tagliamonte MC, Catalani S, Primiterra M, Canestrari F, De Stefani S, Palini S, Bulletti C. Differences in blood and semen oxidative status in fertile and infertile men, and their relationship with sperm quality. Reprod Biomed Online. 2012;25:300–306. doi: 10.1016/j.rbmo.2012.05.011. [DOI] [PubMed] [Google Scholar]
- Carlsen E, Swan SH, Petersen JH, Skakkebaek NE. Longitudinal changes in semen parameters in young Danish men from the Copenhagen area. Hum Reprod. 2005;20:942–949. doi: 10.1093/humrep/deh704. [DOI] [PubMed] [Google Scholar]
- Chavarro JE, Minguez-Alarcon L, Mendiola J, Cutillas-Tolin A, Lopez-Espin JJ, Torres-Cantero AM. Trans fatty acid intake is inversely related to total sperm count in young healthy men. Hum Reprod. 2014;29:429–440. doi: 10.1093/humrep/det464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SJ, Allam JP, Duan YG, Haidl G. Influence of reactive oxygen species on human sperm functions and fertilizing capacity including therapeutical approaches. Arch Gynecol Obstet. 2013;288:191–199. doi: 10.1007/s00404-013-2801-4. [DOI] [PubMed] [Google Scholar]
- Colaci DS, Afeiche M, Gaskins AJ, Wright DL, Toth TL, Tanrikut C, Hauser R, Chavarro JE. Men's body mass index in relation to embryo quality and clinical outcomes in couples undergoing in vitro fertilization. Fertil Steril. 2012;98:1193–1199 e1191. doi: 10.1016/j.fertnstert.2012.07.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis KM, Savitz DA, Arbuckle TE. Effects of cigarette smoking, caffeine consumption, and alcohol intake on fecundability. Am J Epidemiol. 1997;146:32–41. doi: 10.1093/oxfordjournals.aje.a009189. [DOI] [PubMed] [Google Scholar]
- de Ruyter JC, Olthof MR, Seidell JC, Katan MB. A trial of sugar-free or sugar-sweetened beverages and body weight in children. N Engl J Med. 2012;367:1397–1406. doi: 10.1056/NEJMoa1203034. [DOI] [PubMed] [Google Scholar]
- Ebbeling CB, Feldman HA, Chomitz VR, Antonelli TA, Gortmaker SL, Osganian SK, Ludwig DS. A randomized trial of sugar-sweetened beverages and adolescent body weight. N Engl J Med. 2012;367:1407–1416. doi: 10.1056/NEJMoa1203388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echavarria Sanchez MG, Franco Laguna E, Juarez Bengoa A, Villanueva Diaz CA. Seminal quality and hormones in patients with diabetes mellitus type 2. Ginecol Obstet Mex. 2007;75:241–246. [PubMed] [Google Scholar]
- Fernandez CD, Bellentani FF, Fernandes GS, Perobelli JE, Favareto AP, Nascimento AF, Cicogna AC, Kempinas WD. Diet-induced obesity in rats leads to a decrease in sperm motility. Reprod Biol Endocrinol. 2011;9:32. doi: 10.1186/1477-7827-9-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feskanich D, Rimm EB, Giovannucci EL, Colditz GA, Stampfer MJ, Litin LB, Willett WC. Reproducibility and validity of food intake measurements from a semiquantitative food frequency questionnaire. J Am Diet Assoc. 1993;93:790–796. doi: 10.1016/0002-8223(93)91754-e. [DOI] [PubMed] [Google Scholar]
- Gaskins AJ, Colaci DS, Mendiola J, Swan SH, Chavarro JE. Dietary patterns and semen quality in young men. Hum Reprod. 2012;27:2899–2907. doi: 10.1093/humrep/des298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskins AJ, Mendiola J, Afeiche M, Jorgensen N, Swan SH, Chavarro JE. Physical activity and television watching in relation to semen quality in young men. Br J Sports Med. 2013 doi: 10.1136/bjsports-2012-091644. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammiche F, Laven JS, Twigt JM, Boellaard WP, Steegers EA, Steegers-Theunissen RP. Body mass index and central adiposity are associated with sperm quality in men of subfertile couples. Hum Reprod. 2012;27:2365–2372. doi: 10.1093/humrep/des177. [DOI] [PubMed] [Google Scholar]
- Hammoud AO, Gibson M, Peterson CM, Meikle AW, Carrell DT. Impact of male obesity on infertility: a critical review of the current literature. Fertil Steril. 2008a;90:897–904. doi: 10.1016/j.fertnstert.2008.08.026. [DOI] [PubMed] [Google Scholar]
- Hammoud AO, Wilde N, Gibson M, Parks A, Carrell DT, Meikle AW. Male obesity and alteration in sperm parameters. Fertil Steril. 2008b;90:2222–2225. doi: 10.1016/j.fertnstert.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Hauser R. Urinary phthalate metabolites and semen quality: a review of a potential biomarker of susceptibility. Int J Androl. 2008;31:112–117. doi: 10.1111/j.1365-2605.2007.00844.x. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Swan SH, Skakkebaek NE, Rasmussen S, Jorgensen N. Caffeine intake and semen quality in a population of 2,554 young Danish men. Am J Epidemiol. 2010;171:883–891. doi: 10.1093/aje/kwq007. [DOI] [PubMed] [Google Scholar]
- Jensen TK, Heitmann BL, Jensen MB, Halldorsson TI, Andersson AM, Skakkebaek NE, Joensen UN, Lauritsen MP, Christiansen P, Dalgard C, et al. High dietary intake of saturated fat is associated with reduced semen quality among 701 young Danish men from the general population. Am J Clin Nutr. 2013;97:411–418. doi: 10.3945/ajcn.112.042432. [DOI] [PubMed] [Google Scholar]
- Jurewicz J, Radwan M, Sobala W, Ligocka D, Radwan P, Bochenek M, Hawula W, Jakubowski L, Hanke W. Human urinary phthalate metabolites level and main semen parameters, sperm chromatin structure, sperm aneuploidy and reproductive hormones. Reprod Toxicol. 2013;42:232–241. doi: 10.1016/j.reprotox.2013.10.001. [DOI] [PubMed] [Google Scholar]
- Kit BK, Fakhouri TH, Park S, Nielsen SJ, Ogden CL. Trends in sugar-sweetened beverage consumption among youth and adults in the United States: 1999–2010. Am J Clin Nutr. 2013;98:180–188. doi: 10.3945/ajcn.112.057943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondaki K, Grammatikaki E, Jimenez-Pavon D, De Henauw S, Gonzalez-Gross M, Sjostrom M, Gottrand F, Molnar D, Moreno LA, Kafatos A, et al. Daily sugar-sweetened beverage consumption and insulin resistance in European adolescents: the HELENA (Healthy Lifestyle in Europe by Nutrition in Adolescence) Study. Public Health Nutr. 2013;16:479–486. doi: 10.1017/S1368980012002613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Lin H, Li Y, Cao J. Association between socio-psycho-behavioral factors and male semen quality: systematic review and meta-analyses. Fertil Steril. 2011;95:116–123. doi: 10.1016/j.fertnstert.2010.06.031. [DOI] [PubMed] [Google Scholar]
- Lifshitz SJ, Trye S, Sapra K, Normand N, Bongiovanni AM, Witkin S, Gelber S. Neutrophil gelatinase-associated lipocalin as a diagnostic marker for preeclampsia. Am J Obstet Gynecol. 2013;208:S256. [Google Scholar]
- Malik VS, Popkin BM, Bray GA, Despres JP, Willett WC, Hu FB. Sugar-sweetened beverages and risk of metabolic syndrome and type 2 diabetes: a meta-analysis. Diabetes Care. 2010;33:2477–2483. doi: 10.2337/dc10-1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeker JD, Ehrlich S, Toth TL, Wright DL, Calafat AM, Trisini AT, Ye X, Hauser R. Semen quality and sperm DNA damage in relation to urinary bisphenol A among men from an infertility clinic. Reprod Toxicol. 2010;30:532–539. doi: 10.1016/j.reprotox.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendiola J, Stahlhut RW, Jorgensen N, Liu F, Swan SH. Shorter anogenital distance predicts poorer semen quality in young men in Rochester, New York. Environ Health Perspect. 2011;119:958–963. doi: 10.1289/ehp.1103421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menkveld R, Stander FS, Kotze TJ, Kruger TF, van Zyl JA. The evaluation of morphological characteristics of human spermatozoa according to stricter criteria. Hum Reprod. 1990;5:586–592. doi: 10.1093/oxfordjournals.humrep.a137150. [DOI] [PubMed] [Google Scholar]
- Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364:2392–2404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen RH, Wilcox AJ, Skjaerven R, Baird DD. Men's body mass index and infertility. Hum Reprod. 2007;22:2488–2493. doi: 10.1093/humrep/dem139. [DOI] [PubMed] [Google Scholar]
- Palmer NO, Bakos HW, Fullston T, Lane M. Impact of obesity on male fertility, sperm function and molecular composition. Spermatogenesis. 2012;2:253–263. doi: 10.4161/spmg.21362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan A, Malik VS, Hao T, Willett WC, Mozaffarian D, Hu FB. Changes in water and beverage intake and long-term weight changes: results from three prospective cohort studies. Int J Obes (Lond) 2013;37:1378–1385. doi: 10.1038/ijo.2012.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K, Gross M, Lee DH, Holvoet P, Himes JH, Shikany JM, Jacobs DR., Jr Oxidative stress and insulin resistance: the coronary artery risk development in young adults study. Diabetes Care. 2009;32:1302–1307. doi: 10.2337/dc09-0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pugeat M, Crave JC, Elmidani M, Nicolas MH, Garoscio-Cholet M, Lejeune H, Dechaud H, Tourniaire J. Pathophysiology of sex hormone binding globulin (SHBG): relation to insulin. J Steroid Biochem Mol Biol. 1991;40:841–849. doi: 10.1016/0960-0760(91)90310-2. [DOI] [PubMed] [Google Scholar]
- Pugeat M, Nader N, Hogeveen K, Raverot G, Dechaud H, Grenot C. Sex hormone-binding globulin gene expression in the liver: drugs and the metabolic syndrome. Mol Cell Endocrinol. 2010;316:53–59. doi: 10.1016/j.mce.2009.09.020. [DOI] [PubMed] [Google Scholar]
- Rama Raju GA, Jaya Prakash G, Murali Krishna K, Madan K, Siva Narayana T, Ravi Krishna CH. Noninsulin-dependent diabetes mellitus: effects on sperm morphological and functional characteristics, nuclear DNA integrity and outcome of assisted reproductive technique. Andrologia. 2012;44(Suppl 1):490–498. doi: 10.1111/j.1439-0272.2011.01213.x. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Marshall GR, McNeilly AS, Plant TM. Dynamics of the follicle-stimulating hormone (FSH)-inhibin B feedback loop and its role in regulating spermatogenesis in the adult male rhesus monkey (Macaca mulatta) as revealed by unilateral orchidectomy. Endocrinology. 2000;141:18–27. doi: 10.1210/endo.141.1.7276. [DOI] [PubMed] [Google Scholar]
- Ramlau-Hansen CH, Thulstrup AM, Bonde JP, Olsen J, Bech BH. Semen quality according to prenatal coffee and present caffeine exposure: two decades of follow-up of a pregnancy cohort. Hum Reprod. 2008;23:2799–2805. doi: 10.1093/humrep/den331. [DOI] [PubMed] [Google Scholar]
- Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- Rosner B, Willett WC. Interval estimates for correlation coefficients corrected for within-person variation: implications for study design and hypothesis testing. Am J Epidemiol. 1988;127:377–386. doi: 10.1093/oxfordjournals.aje.a114811. [DOI] [PubMed] [Google Scholar]
- Ruff JS, Suchy AK, Hugentobler SA, Sosa MM, Schwartz BL, Morrison LC, Gieng SH, Shigenaga MK, Potts WK. Human-relevant levels of added sugar consumption increase female mortality and lower male fitness in mice. Nat Commun. 2013;4:2245. doi: 10.1038/ncomms3245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallmen M, Sandler DP, Hoppin JA, Blair A, Baird DD. Reduced fertility among overweight and obese men. Epidemiology. 2006;17:520–523. doi: 10.1097/01.ede.0000229953.76862.e5. [DOI] [PubMed] [Google Scholar]
- Searle SR, Speed FM, Milliken GA. Population marginal means in the linear model: an alternative to least square means. Am Stat. 1980;34:216–221. [Google Scholar]
- Sekhavat L, Moein MR. The effect of male body mass index on sperm parameters. Aging Male. 2010;13:155–158. doi: 10.3109/13685530903536643. [DOI] [PubMed] [Google Scholar]
- Sermondade N, Faure C, Fezeu L, Shayeb AG, Bonde JP, Jensen TK, Van Wely M, Cao J, Martini AC, Eskandar M, et al. BMI in relation to sperm count: an updated systematic review and collaborative meta-analysis. Hum Reprod Update. 2013;19:221–231. doi: 10.1093/humupd/dms050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AD, Kim YI, Refsum H. Is folic acid good for everyone? Am J Clin Nutr. 2008;87:517–533. doi: 10.1093/ajcn/87.3.517. [DOI] [PubMed] [Google Scholar]
- Stanhope KL, Schwarz JM, Keim NL, Griffen SC, Bremer AA, Graham JL, Hatcher B, Cox CL, Dyachenko A, Zhang W, et al. Consuming fructose-sweetened, not glucose-sweetened, beverages increases visceral adiposity and lipids and decreases insulin sensitivity in overweight/obese humans. J Clin Invest. 2009;119:1322–1334. doi: 10.1172/JCI37385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stokes-Riner A, Thurston SW, Brazil C, Guzick D, Liu F, Overstreet JW, Wang C, Sparks A, Redmon JB, Swan SH. One semen sample or 2? Insights from a study of fertile men. J Androl. 2007;28:638–643. doi: 10.2164/jandrol.107.002741. [DOI] [PubMed] [Google Scholar]
- United States Department of Agriculture, Agriculural Research Service. 2008. USDA National Nutrient Database for Standard Reference, Release 21 URL http://www.ars.usda.gov/ba/bhnrc/ndl .
- Vermeulen A, Verdonck L, Kaufman JM. A critical evaluation of simple methods for the estimation of free testosterone in serum. J Clin Endocrinol Metab. 1999;84:3666–3672. doi: 10.1210/jcem.84.10.6079. [DOI] [PubMed] [Google Scholar]
- Vine MF, Setzer RW, Jr, Everson RB, Wyrobek AJ. Human sperm morphometry and smoking, caffeine, and alcohol consumption. Reprod Toxicol. 1997;11:179–184. doi: 10.1016/s0890-6238(97)00004-x. [DOI] [PubMed] [Google Scholar]
- Willett W. Nutritional Epidemiology. New York: Oxford University Press; 2012. [Google Scholar]
- World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction. New York: Cambridge university press; 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.