Abstract
Background
Clinical trials are essential to establish the effectiveness of new cancer therapies, but less than 5% of adults with cancer enroll in trials. In addition to ineligibility or lack of available trials, barriers to enrollment may include limited patient awareness about the option of participation.
Methods
We surveyed a multiregional cohort of patients with lung or colorectal cancer (or their surrogates) three to six months after diagnosis. We assessed whether respondents reported learning that clinical trial participation might be an option, and, if so, with whom they discussed trials. We used logistic regression to assess the association of patient characteristics with discussing trial participation and enrolling in trials. All statistical tests were two-sided.
Results
Of 7887 respondents, 1114 (14.1%) reported discussing the possibility of clinical trial participation; most learned about trials from their physicians, and 287 patients (3.6% of all patients, 25.8% of trial discussants) enrolled. Among 2173 patients who received chemotherapy for advanced (stage III/IV lung or stage IV colorectal) cancer, 25.7% discussed trials, and 7.6% (29.5% of trial discussants) enrolled. Discussions were less frequent among older patients, African American or Asian vs white patients, and those with lower incomes and more comorbidity. Enrollment was higher among patients reporting shared vs physician-driven decisions (all P < .05).
Conclusions
In this population-based cohort, only 14% of patients discussed participation in clinical trials. Discussions were more frequent among advanced cancer patients but were still reported by a minority of patients. Strategies to expand access to trials and facilitate patient-provider communication about participation may accelerate development of better cancer therapeutics.
Clinical trials in oncology are essential to establish the effectiveness of new therapeutic strategies. However, a recent Institute of Medicine report described an impending crisis in cancer clinical trials, raising concerns about the complexity of requirements for conducting trials, appropriate prioritization of trial proposals, cost, and low accrual rates (1). Up to 40% of National Cancer Institute (NCI) sponsored trials close without meeting accrual goals (1), and nearly one-third of phase III trials close because of poor accrual (2). Clinical trial enrollment rates in adult cancer populations have historically been 5% or less (1,3,4), with lower rates among minorities and older patients (4–10).
An important factor in determining whether patients participate in clinical trials is whether their health care providers discuss the option of participation (1). However, there is limited information regarding rates of discussions about participation, sources of patient information about trials, and the association of patient characteristics with these factors. One study of 235 patients and their physicians found that only 20% of patients potentially eligible for phase II/III trials were offered enrollment, yet most who were offered enrollment participated. Shared decision-making between patient and physician about trial enrollment was associated with the decision to enroll (11). However, this was a small study of patients at two NCI-designated comprehensive cancer centers, which may limit its generalizability.
A previous study from the large, population- and health-system-based, multiregional Cancer Care Outcomes Research and Surveillance (CanCORS) (12) Consortium reported that 5.3% of patients with lung or colorectal cancer participated in clinical trials within 14 months of diagnosis (3). This study found that younger age and stage III /IV disease, but few other patient factors, were associated with clinical trial participation (3). That analysis, however, focused primarily on associations between physician characteristics and trial enrollment. It did not address discussions with physicians about the possibility of enrollment or the patient decision-making process around trials.
In this study, we used additional data from the CanCORS study to better understand how patients with newly diagnosed lung or colorectal cancer decided whether to participate in clinical trials. We first assessed the proportion of patients who discussed clinical trials as a potential treatment option and examined demographic characteristics, beliefs, and clinical factors associated with these discussions. Second, among patients who discussed clinical trials as a treatment option, we further assessed participation rates and demographic characteristics, beliefs, and clinical or decision-making process factors associated with patients’ decisions to participate. We also assessed discussion and participation rates among patients who saw oncologists, received chemotherapy, and were treated with chemotherapy for advanced disease, for whom more trials may have been available. Next, we examined the sources from which clinical trial participants first learned about the trials in which they participated, and from which nonparticipants learned that a clinical trial was a possibility. Finally, we evaluated the main reasons for declining to participate among nonparticipants.
Methods
Study Design and Participants
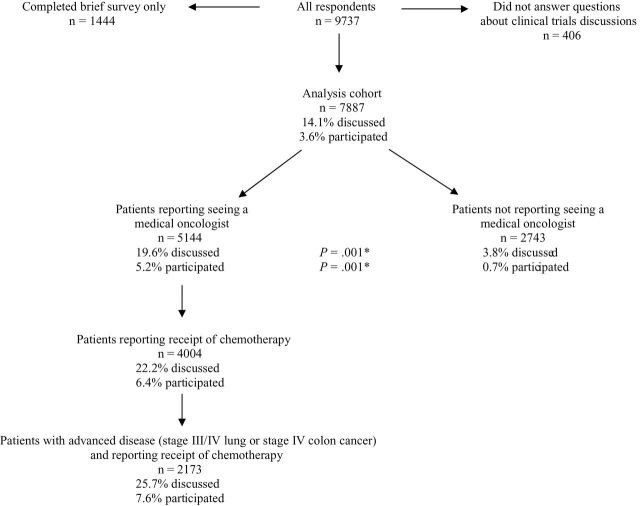
CanCORS was an observational study designed to investigate cancer care processes and outcomes (12). It included patients with lung or colorectal cancer diagnosed between 2003 and 2005 who lived in one of five geographic regions (Northern California, Los Angeles County, North Carolina, Iowa, or Alabama) or who received care in one of five health maintenance organizations or 15 Veterans Affairs sites (12,13). Patients were surveyed three to six months after diagnosis. If patients were deceased or too ill to participate, their surrogates were surveyed. American Association for Public Opinion Research (14) response rates were 49% for lung cancer and 53% for colorectal cancer patients; cooperation rates (participation among subjects successfully contacted) were 59% and 61%, respectively (13). We excluded 1444 of 9737 respondents who completed only a brief version of the survey and were not asked about clinical trial participation and 406 patients/surrogates who did not answer the question about clinical trials discussions or answered “don’t know,” yielding a final cohort of 7887 cases (Figure 1). Results were similar in a sensitivity analysis grouping those respondents with those answering “no.” The study was approved by the human subjects committees of all participating institutions.
Figure 1.
Rates of reported discussions about clinical trials and participation in trials according to management by oncologists.*Two-sided P value for comparison of discussion and participation rates among patients reporting vs not reporting a medical oncologist, based on the chi-square test.
Study Outcomes
The primary outcomes of interest were patient/surrogate-reported discussion of possible enrollment in clinical trials and reported participation in a treatment trial. We also examined sources of information about trials and reasons for declining participation among nonparticipants. Patients or surrogates were asked whether “anyone mentioned that enrollment in clinical trials” might be an option. Those answering affirmatively (“discussants”) were asked whether patients had “participated in a clinical trial or research study” since diagnosis. Trial participants were asked whether that trial involved surgery, radiation therapy, chemotherapy, other drugs, or other treatments, with multiple responses allowed. Participants were also asked from whom they first learned about the clinical trial in which they participated. Nonparticipants who had heard about trials were asked from whom they had learned that clinical trial participation was a possibility and why they had not participated in a trial.
Independent Variables
Independent variables included patient demographics, clinical characteristics, and beliefs. Demographics included age at diagnosis, sex, race/ethnicity, marital status, educational attainment, region of the country, income, insurance status, enrollment in an integrated health system (patients enrolled through the Veteran’s Affairs and health maintenance organization sites or Kaiser Permanente of Northern or Southern California), and survey type (patient, surrogate of living patient, or surrogate of deceased patient). Clinical characteristics included number of self-reported comorbid conditions (15–17), health status before diagnosis (measured by a subset of five questions from the Short-Form 12) (18), cancer type (colorectal, non-small cell lung cancer, small cell lung cancer), and stage at diagnosis (19). We included survey items that assessed attitudes about cancer and its treatment, because we hypothesized that these factors might affect motivation to consider clinical trials. These factors included fatalism based on a four-item Fatalism scale (20) and preferences regarding tradeoffs between length of life and quality of life (“If you had to make a choice now, would you prefer treatment that extends life as much as possible, even if it means having more pain and discomfort, or would you want treatment that focuses on relieving pain and discomfort as much as possible, even if it means not living as long?”) or cost savings (“If you had to make a choice now, would you prefer treatment that extends life as much as possible, even if it means using up all of your financial resources, or would you want treatment that costs you less, even if it means not living as long?”). Finally, we hypothesized that patient involvement in decision-making about trials may have been associated with participation rates. Respondents who reported trial discussions were asked to report patients’ roles in deciding whether to participate in a trial. Response options, based on the Degner five-point scale, included “you made the decision with little or no input from your doctors,” “you made the decision after considering your doctors’ opinions,” “you and your doctors made the decision together,” “your doctors made the decision after considering your opinion,” and “your doctors made the decision with little or no input from you” (21–23). We categorized the first two responses as patient-controlled decisions, the third as shared decisions, and the last two as physician-controlled decisions. Variables were categorized as shown in Table 1.
Table 1.
Characteristics of study cohort and association with clinical trial discussion and participation
| Characteristics | Cohort n (%) | Percentage of cohort with characteristic reporting discussion of a trial (n = 1114)* |
P† | Percentage of discussants with characteristic reporting participation in a trial (n = 287) | P† |
|---|---|---|---|---|---|
| Total | 7887 (100) | 14.1 | 25.8 | ||
| Age at diagnosis (quintiles) | |||||
| <57 y | 1653 (21) | 21.8 | <.001 | 26.7 | .14 |
| 57–64 y | 1500 (19) | 19.0 | 24.6 | ||
| 65–71 y | 1634 (21) | 13.0 | 21.7 | ||
| 72–78 y | 1610 (20) | 11.4 | 32.2 | ||
| >78 y | 1490 (19) | 5.0 | 21.6 | ||
| Sex | |||||
| Male | 4428 (56) | 14.5 | .31 | 27.3 | .17 |
| Female | 3459 (44) | 13.7 | 23.7 | ||
| Race | |||||
| White | 5483 (70) | 14.9 | .01 | 25.8 | .49 |
| Hispanic | 570 (7) | 11.8 | 17.9 | ||
| African American | 1031 (13) | 11.3 | 30.2 | ||
| Asian | 397 (5) | 13.1 | 26.9 | ||
| Other | 406 (5) | 15.0 | 24.6 | ||
| Marital status | |||||
| Married/partnered | 4826 (61) | 15.8 | <.001 | 27.0 | .16 |
| Unmarried | 3054 (39) | 11.5 | 23.1 | ||
| Not ascertained | 7 (0.1) | ||||
| Education attained | |||||
| Less than high school | 1701 (22) | 8.8 | <.001 | 22.2 | .46 |
| High school graduate | 4464 (57) | 14.0 | 27.0 | ||
| College graduate | 1663 (21) | 20.3 | 25.2 | ||
| Not ascertained | 59 (0.8) | ||||
| Region | |||||
| West | 4156 (53) | 14.2 | <.001 | 20.1 | <.001 |
| Midwest | 1119 (14) | 18.2 | 27.5 | ||
| South | 2543 (32) | 12.2 | 35.2 | ||
| Northeast | 69 (0.9) | 17.4 | 33.3 | ||
| Household income | |||||
| <$20,000/y | 2388 (30) | 9.7 | <.001 | 23.8 | .22 |
| $20,000–$40,000/y | 2131 (27) | 13.1 | 30.6 | ||
| $40,000–$60,000/y | 1103 (14) | 17.0 | 24.1 | ||
| > $60,000/y | 1478 (19) | 22.7 | 24.5 | ||
| Not ascertained | 787 (10) | ||||
| Insurance status | |||||
| Insured | 7629 (97) | 14.1 | .58 | 26.0 | .16 |
| Not insured | 181 (2) | 12.7 | 13.0 | ||
| Not ascertained | 77 (1) | ||||
| Integrated system | |||||
| No | 5341 (68) | 13.9 | .47 | 26.8 | .29 |
| Yes | 2546 (32) | 14.5 | 23.8 | ||
| Self-reported comorbid conditions | |||||
| 0 | 3168 (40) | 16.2 | <.001 | 26.1 | .12 |
| 1 | 2612 (33) | 12.9 | 27.7 | ||
| 2 | 1273 (16) | 13.0 | 26.5 | ||
| 3+ | 779 (10) | 11.7 | 15.4 | ||
| Not ascertained | 55 (1) | ||||
| Cancer type | |||||
| Colorectal | 3798 (48) | 11.0 | <.001 | 25.8 | .91 |
| Non-small cell lung | 3594 (46) | 17.2 | 26.0 | ||
| Small cell lung | 495 (6) | 15.4 | 23.7 | ||
| Stage at diagnosis | |||||
| I | 1716 (22) | 5.9 | <.001 | 24.8 | .57 |
| II | 1352 (17) | 10.0 | 20.7 | ||
| III | 2148 (27) | 16.5 | 26.3 | ||
| IV | 2220 (28) | 20.5 | 26.6 | ||
| Unknown | 451 (6) | ||||
| Fatalism, tertile | |||||
| 1 (Least fatalistic) | 1887 (24) | 12.9 | .002 | 34.0 | .008 |
| 2 | 1304 (17) | 16.7 | 22.0 | ||
| 3 (Most fatalistic) | 1437 (18) | 16.8 | 24.5 | ||
| Not ascertained | 3259 (41) | ||||
| Prefers life extension over symptom control | |||||
| Yes | 2613 (33) | 17.0 | <.001 | 26.5 | .93 |
| No | 2786 (35) | 13.5 | 26.8 | ||
| Not ascertained | 2488 (32) | ||||
| Prefers life extension over lower cost | |||||
| Yes | 3090 (39) | 16.1 | .02 | 27.3 | .92 |
| No | 2033 (26) | 13.7 | 27.0 | ||
| Not ascertained | 2764 (35) | ||||
| Prediagnosis health status (quartile) | |||||
| 1 | 1577 (20) | 14.7 | .56 | 21.6 | .15 |
| 2 | 1479 (19) | 14.1 | 28.9 | ||
| 3 | 1553 (20) | 15.5 | 30.3 | ||
| 4 | 1501 (19) | 15.7 | 25.9 | ||
| Not ascertained | 1777 (23) | ||||
| Survey type | |||||
| Patient | 5354 (68) | 14.9 | <.001 | 26.9 | .19 |
| Surrogate: living patient | 946 (12) | 14.6 | 26.1 | ||
| Surrogate: deceased patient | 1587 (20) | 11.2 | 20.3 | ||
| Among patients who discussed trials (n = 1114) | |||||
| Decision role | |||||
| Patient-controlled | 603 (54) | 29.2 | <.001 | ||
| Shared control | 260 (23) | 35.0 | |||
| Physician-controlled | 134 (12) | 13.4 | |||
| Not ascertained | 117 (11) | ||||
| Among trial participants (n = 287); n (%) of participants only; multiple responses allowed | |||||
| Surgery | 20 (7) | ||||
| Radiation therapy | 41 (14) | ||||
| Chemotherapy | 176 (61) | ||||
| Other drugs | 123 (43) | ||||
| Other treatment | 16 (6) | ||||
The numbers in Table 1 reflect unimputed data.
* For example, of 7887 patients in the overall cohort, 1653 (21%) were in age quintile 1. Of those 1653, 360 (21.8%) reported discussing the possibility of enrollment in a trial. Of those 360, 96 patients (26.7%) reported enrollment in a trial.
† Two-sided P value for the chi-square test, comparing the proportion of patients reporting clinical trial discussion or participation across the categories for each independent variable. All statistical tests were two-sided.
Statistical Analysis
We assessed trial discussion and participation rates among all patients and stratified among patients reporting seeing an oncologist, receiving chemotherapy, and having advanced disease (stage III/IV lung or stage IV colorectal cancer).
Among all patients, we used chi-square tests to analyze unadjusted associations between patient characteristics and dependent variables. Missing data were infrequent for most variables other than responses to survey questions not included in all versions of the baseline survey (patient, surrogate of living patient, and surrogate of deceased patient), including fatalism, baseline health status and tradeoffs between length of life and quality of life or cost; income data were also missing for 10% of patients (Table 1). Among patients who learned of the possibility of enrolling in a clinical trial, data for patient role in the enrollment decision were missing in 11% of cases. For unadjusted analyses, we excluded patients with missing values.
We used multivariable logistic regression to predict discussion of clinical trials and participation in a trial (among the patients who discussed one), adjusting for all independent variables described above and using multiple imputation to impute missing responses for independent variables (no data were missing for dependent variables based on cohort definition) (24). We did not impute values for patients with missing information on stage at diagnosis or for questions not included in the version of the baseline survey completed (for example, surrogates of patients who were deceased were not asked about preferences for life extension vs symptom control or cost), and we included a “missing” category for such variables. Values were not imputed for 48 patients who partially completed their survey version; these patients were excluded from statistical models.
Of 1114 respondents who reported a discussion of clinical trials, 12 responded “don’t know” to the question about participation in a trial; these cases were grouped with the 815 answering “no.” Analyses were performed using SAS, version 9.2, and Stata, version 13. Analyses treated all variables as categorical. Two-sided P values less than .05 were considered statistically significant.
Results
Characteristics of the 7887 patients and unadjusted associations between patient characteristics and clinical trials discussion and participation are listed in Table 1. Overall, 1114 (14.1%) of patients/surrogates reported discussing clinical trials as a potential option. In 287 cases (3.6% of the overall cohort, 25.8% of those who discussed trials as an option), patients participated in trials. Among trial participants, 7.0% reported participating in a trial involving surgery; 14.3% in a radiation trial; 61.3% in a chemotherapy trial; 42.9% in a trial involving other drugs; and 5.6% in a trial of another treatment. Trial discussion and participation rates were higher among patients who saw medical oncologists than those who did not (Figure 1). Among 2173 patients treated with chemotherapy for advanced disease (stage III/IV lung or stage IV colorectal cancer), 25.7% discussed a trial, and 7.6% (29.5% of discussants) enrolled. Among all respondents reporting clinical trial discussions, enrollment was less likely among those who described physician-controlled decisions about participation (13.4% enrolled) than among those describing shared decisions (35.0% enrolled) or patient-controlled decisions (29.2% enrolled; P < .001).
In adjusted analyses (Table 2), factors associated with clinical trial discussions included younger age, increasing educational attainment, higher income, lung (vs colorectal) cancer, and more advanced cancer stage. African American and Asian patients were less likely than white patients to report trial discussions. Among patients who discussed trials, those with ≥3 comorbidities were less likely than those without comorbidities to enroll (OR = 0.4, 95% CI = 0.2 to 0.9) (Table 2), as were those with somewhat higher levels of fatalism (OR = 0.6, 95% CI = 0.4 to 0.9 for middle vs lowest tertile of fatalism scores, with a statistically nonsignificant effect for the highest vs lowest tertile of fatalism scores, OR = 0.7, 95% CI = 0.5 to 1.1, P = .12). Compared with respondents from the West, patients from the South were more likely to participate following discussions (OR = 2.1, 95% CI = 1.5 to 3.1). Those reporting physician-controlled decisions regarding trial enrollment were less likely than those reporting shared decisions to participate (OR = 0.3, 95% CI = 0.2 to 0.6). This association persisted in a sensitivity analysis restricted to patients who first learned about trials from health care providers (OR = 0.3, 95% CI = 0.2 to 0.6).
Table 2.
Logistic regression analyses of clinical trial discussion and participation, adjusted
| Characteristic | Discussion of trials (n = 7839)* | Participation in trials among discussants (n = 1107) | ||
|---|---|---|---|---|
| OR (95% CI) | P† | OR (95% CI) | P† | |
| Age at diagnosis (quintiles) | ||||
| <57 y | Ref | <.001 | Ref | .12 |
| 57–64 y | 0.9 (0.7 to 1.0) | 0.9 (0.6 to 1.3) | ||
| 65–71 y | 0.6 (0.5 to 0.7) | 0.8 (0.5 to 1.2) | ||
| 72–78 y | 0.5 (0.4 to 0.7) | 1.4 (0.9 to 2.2) | ||
| >78 y | 0.2 (0.2 to 0.3) | 0.8 (0.4 to 1.6) | ||
| Sex | ||||
| Male | Ref | .48 | Ref | .37 |
| Female | 1.1 (0.9 to 1.2) | 0.9 (0.6 to 1.2) | ||
| Race | ||||
| White | Ref | .01 | Ref | .93 |
| Hispanic | 0.8 (0.6 to 1.1) | 0.9 (0.4 to 1.8) | ||
| African American | 0.7 (0.6 to 0.9) | 1.2 (0.7 to 1.9) | ||
| Asian | 0.7 (0.5 to 0.9) | 1.1 (0.5 to 2.3) | ||
| Other | 0.9 (0.7 to 1.2) | 1.1 (0.6 to 2.2) | ||
| Marital status | ||||
| Unmarried/unknown | Ref | .69 | Ref | .75 |
| Married/partnered | 1.0 (0.9 to 1.2) | 1.1 (0.7 to 1.5) | ||
| Education attained | ||||
| Less than high school | Ref | <.001 | Ref | .63 |
| High school | 1.3 (1.1 to 1.6) | 1.2 (0.8 to 2.0) | ||
| College | 1.9 (1.5 to 2.5) | 1.1 (0.6 to 2.0) | ||
| Region | ||||
| West | Ref | .08 | Ref | <.001 |
| South | 1.0 (0.8 to 1.2) | 2.1 (1.5 to 3.1) | ||
| Midwest | 1.3 (1.0 to 1.6) | 1.5 (1.0 to 2.3) | ||
| Northeast | 0.9 (0.4 to 1.7) | 2.6 (0.7 to 9.4) | ||
| Household income | ||||
| < $20,000/y | Ref | <.001 | Ref | .45 |
| $20,000–$40,000/y | 1.2 (1.0 to 1.5) | 1.4 (0.9 to 2.2) | ||
| $40,000–$60,000/y | 1.4 (1.1 to 1.8) | 1.1 (0.7 to 1.9) | ||
| >$60,000/y | 1.8 (1.4 to 2.3) | 1.2 (0.7 to 2.1) | ||
| Insured | ||||
| No | Ref | .46 | Ref | .22 |
| Yes | 1.2 (0.7 to 1.9) | 2.3 (0.6 to 8.5) | ||
| Integrated health system | ||||
| No | Ref | .54 | Ref | .43 |
| Yes | 1.0 (09 to 1.2) | 0.9 (0.6 to 1.2) | ||
| Self-reported comorbid conditions | ||||
| 0 | Ref | .22 | Ref | .09 |
| 1 | 0.8 (0.7 to 1.0) | 1.0 (0.7 to 1.4) | ||
| 2 | 0.9 (0.8 to 1.2) | 1.0 (0.6 to 1.6) | ||
| 3+ | 0.9 (0.7 to 1.2) | 0.4 (0.2 to 0.9) | ||
| Prediagnosis health status (quartile) | ||||
| 1 | Ref | .73 | Ref | .51 |
| 2 | 1.0 (0.8 to 1.2) | 1.4 (0.9 to 2.1) | ||
| 3 | 1.1 (0.9 to 1.4) | 1.3 (0.8 to 2.1) | ||
| 4 | 1.1 (0.9 to 1.4) | 1.1 (0.7 to 1.8) | ||
| Not ascertained ‡ | ||||
| Cancer type | ||||
| Colorectal | Ref | <.001 | Ref | .60 |
| Non-small cell lung | 1.9 (1.6 to 2.2) | 1.2 (0.8 to 1.6) | ||
| Small cell lung | 1.3 (1.0 to 1.8) | 0.9 (0.5 to 1.8) | ||
| Stage at diagnosis | ||||
| I | Ref | <.001 | Ref | .38 |
| II | 2.3 (1.7 to 3.0) | 0.9 (0.5 to 1.7) | ||
| III | 3.5 (2.8 to 4.5) | 1.2 (0.7 to 2.0) | ||
| IV | 5.0 (3.9 to 6.3) | 1.4 (0.8 to 2.4) | ||
| Unknown | 3.9 (2.8 to 5.5) | 1.5 (0.7 to 3.1) | ||
| Fatalism, tertile | ||||
| 1 (Least fatalistic) | Ref | .74 | Ref | .05 |
| 2 | 1.1 (0.9 to 1.3) | 0.6 (0.4 to 0.9) | ||
| 3 (Most fatalistic) | 1.1 (0.9 to 1.3) | 0.7 (0.5 to 1.1) | ||
| Not ascertained ‡ | ||||
| Chooses longer life over quality of life | ||||
| No | Ref | .34 | Ref | .44 |
| Yes | 1.1 (0.9 to 1.3) | 0.9 (0.6 to 1.2) | ||
| Not ascertained‡ | ||||
| Chooses longer life over cost | ||||
| No | Ref | .40 | Ref | .68 |
| Yes | 1.1 (0.9 to 1.3) | 1.1 (0.7 to 1.7) | ||
| Not ascertained ‡ | ||||
| Patient role in decision to participate | ||||
| Patient-controlled | N/A | 0.9 (0.6 to 1.2) | <.001 | |
| Shared control | Ref | |||
| Physician-controlled | 0.3 (0.2 to 0.6) | |||
| Survey type | ||||
| Patient | Ref | <.001 | Ref | .27 |
| Surrogate: living patient | 1.6 (1.2 to 2.0) | 0.8 (0.5 to 1.3) | ||
| Surrogate: deceased patient | 0.6 (0.5 to 0.9) | 0.6 (0.3 to 1.1) | ||
* Adjusted for all variables in the table. For the total 7887 cases in the cohort, multiple imputation was performed to address item nonresponse (see Table 1) and allow inclusion of patients with missing data in multivariable models. An additional 48 participants who completed only partial surveys were omitted from the multiple imputation and therefore excluded from models. These 48 patients were missing marital status (n = 7), income (n = 25), health insurance (n = 24), comorbidity (n = 25), prediagnosis health status (n = 15), fatalism (n = 11), trade-off between length of life and quality of life (n = 20), trade-off between length of life and cost of treatment (n = 20). OR = odds ratio; CI = confidence interval; Ref = reference group.
† Two-sided P value for test of combined significance (partial F test) of the possible values of each categorical variable in the model.
‡ After imputation, all ‘not ascertained’ responses for fatalism in the model were within the surrogate surveys for living and deceased patients, which did not ask the question. Similarly, all such responses for prediagnosis health status and trade-offs between length of life and quality of life or cost were within the deceased patient surrogate surveys. The survey type variable therefore controlled for these responses.
Patients’ sources of information about clinical trials are shown in Table 3. In 92.7% of cases, those who participated in a trial learned about that trial from a health care provider. Among nonparticipants, 75.8% first learned about the possibility of enrollment from a health care provider.
Table 3.
Source of information about clinical trials*
| Source | Nonparticipants (n = 827) | Participants (n = 287) |
|---|---|---|
| n (%) | n (%) | |
| Doctor or other health care professional | 627 (75.8) | 266 (92.7) |
| Family member | 56 (6.8) | 3 (1.0) |
| Internet | 33 (4.0) | 3 (1.0) |
| Read in newspaper, magazine, other | 23 (2.8) | 2 (0.7) |
| Friend or acquaintance | 21 (2.5) | 4 (1.4) |
| Heard on radio or saw on television | 7 (0.9) | 1 (0.3) |
| Patient support or advocacy group | 3 (0.4) | 1 (0.3) |
| Don’t know | 11 (1.3) | 3 (1.0) |
| Other | 46 (5.6) | 4 (1.4) |
* Respondents who endorsed clinical trials participation were asked from whom they first learned about the specific clinical trial in which participation occurred. Respondents who reported learning that a clinical trial was a possibility, but who denied participation, were asked from whom they first learned that enrolling in a trial was a possibility.
Among 293 respondents who discussed clinical trials but did not participate and indicated at least one reason for this decision (Table 4), the most common was that a trial was not an option or doctors did not think it would help (25.9%); other reasons included patient doubt that a trial would help (20.8%), being too sick to have a trial treatment (15.4%), and the possibility of receiving placebo (12.0%).
Table 4.
Reasons for nonparticipation in trials among patients who heard about a trial but did not participate (n = 293 providing at least one reason)
| Reason (multiple responses allowed) | n (%) |
|---|---|
| Trial not an option/doctors did not think it would help | 76 (25.9) |
| You did not think a trial would help | 61 (20.8) |
| You were too sick to have trial treatment | 45 (15.4) |
| You might get placebo rather than actual treatment | 35 (12.0) |
| You were worried about side effects of trial treatment | 30 (10.2) |
| You might be treated like a guinea pig | 27 (9.2) |
| You might receive treatment that had not been sufficiently tested | 21 (7.2) |
| Insurance coverage or payment was a problem | 11 (3.8) |
| You were worried you would have to switch doctors to participate | 7 (2.4) |
| ‘Other’ reasons (one response allowed) | |
| Undergoing other cancer treatment | 43 (14.7) |
| Other medical problems | 9 (3.1) |
| Problems scheduling trial treatment | 9 (3.1) |
| Difficulty with transportation | 5 (1.7) |
| Distance was too great | 4 (1.4) |
| Competing life needs (work, childcare, family responsibilities) | 3 (1.0) |
Discussion
Within a large, population- and health-system based cohort of patients with recently diagnosed lung or colorectal cancer, we found that only 14.1% of patients discussed the possibility of clinical trial enrollment and 3.6% participated in trials, consistent with prior reports (1,3,25). Rates were higher (25.7% and 7.6%) among patients receiving chemotherapy for advanced disease, for whom trials were more likely available. Nevertheless, as in the overall cohort, a minority of patients who discussed trials in this group participated (29.5%). Health care providers were the most frequently reported source of information about trials, illustrating the central role of discussions with physicians in decisions about participation.
Our observed rates of clinical trial discussion and enrollment were lower than rates of 40% and 9% recently reported from another large survey (5). However, that survey was limited by a low response rate of 8% and its focus on patients seeking online resources about cancer treatments, which is likely itself a predictor for trial participation. As others have found (5, 25–27), we identified racial and socioeconomic disparities in rates of clinical trials discussions. These associations were evident despite adjustment for a rich set of demographic and clinical factors. However, neither race/ethnicity nor income was associated with trial participation among those who discussed trials. This may have related to more limited statistical power in the smaller cohort of discussants, but these results may also suggest that expanding trial availability and targeting underrepresented groups for discussions about trials could help to address their lower rates of enrollment. We identified regional differences in participation among trial discussants, which may merit further investigation; several explanations are possible, including differences in the nature of available trials and care delivery structure across the United States.
Information about trial availability and eligibility was not available in this analysis, and these factors may especially contribute to lower rates of clinical trials participation among older patients (7–9). One prior study showed that older cancer patients were less likely to be eligible for clinical trials, but that among patients who were eligible for trials that were available to them, older patients were not statistically significantly less likely to participate (28). In our analysis, age was associated with lower rates of discussions about trials despite adjustment for comorbidity and health status before diagnosis, but among patients who learned that a clinical trial might be an option, there was no association between age and enrollment.
Shared decision-making in health care is considered desirable because of its potential to facilitate patient involvement in care and standardize and promote the use of beneficial interventions (29). Evidence that shared decision-making affects care process or outcome measures, however, has varied with the intervention under study (30–32). In our cohort, patients who reported physician-controlled decisions about trial enrollment were less likely to enroll than those reporting shared or patient-controlled decisions. This suggests that patient involvement in the decision-making process might optimize clinical trial participation rates. It is also possible that patients whose decisions about trial enrollment were shared or patient-controlled were more likely to enroll because they were good candidates for available trials. Patients whose providers recommended against clinical trials might instead have reported physician-controlled discussions. However, in a sensitivity analysis restricted to respondents reporting a health care provider as the primary source of information about trials, the association between a shared decision-making process and clinical trial participation persisted. This may indicate an intrinsic effect of shared decision-making among those patients whose providers believed them to be promising enough candidates to broach the topic of enrollment in a trial. We also observed that among trial discussants, more fatalistic patients were slightly less likely to enroll, possibly reflecting more doubt regarding the potential benefit of medical treatments, particularly experimental therapies.
Strengths of our study included its large, population- and health-system based, multiregional cohort with rigorous data collection and follow-up. One limitation is the possibility of recall bias; we may have underestimated discussion rates if patients and surrogates of patients who did not participate in trials were less likely to remember clinical trial discussions. However, we excluded respondents who reported they did not know whether they discussed trials, and patients were surveyed soon after diagnosis, likely minimizing this effect. Additional research is needed to validate patient self-report of clinical trial discussions. Further, some patients may have discussed and participated in trials after the survey, particularly as some developed recurrent or progressive disease. Nonetheless, a prior CanCORS analysis focusing on physician factors associated with trial participation (3) found an overall participation rate of 5.3% within approximately 14 months of diagnosis, which is only slightly higher than our estimate of 3.6% within three to six months of diagnosis. Our survey was also subject to nonresponse bias, although the cohort of patients enrolled in CanCORS has been demonstrated to be representative of US patients with lung and colorectal cancer (13). Rates of clinical trial discussion in our cohort may represent an upper limit within this population, since patients were included regardless of their initial sources of information about trials, not only if they learned about trials from physicians. Finally, we did not have information about trial availability and eligibility, which also play essential roles in clinical trial participation (33,34).
In conclusion, we observed relatively low rates of discussions about clinical trials among patients with recently diagnosed lung or colorectal cancer and even lower overall rates of participation in trials, consistent with prior studies. Even among patients treated with chemotherapy for advanced cancer, for whom investigational approaches should arguably be integrated into all initial considerations about treatment options, given a low chance of cure with standard therapy, the discussion rate was only 25.7%, with a participation rate of 7.6%. We also found that patients were less likely to learn that clinical trial participation was an option if they were older, minorities, or had lower income or educational attainment. Patients who reported a shared or patient-controlled decision-making process were more likely to participate once they heard about trials. These findings indicate that improving trial accrual and participation rates may require a two-pronged approach. First, trial availability and access must be expanded and patients educated about the option of enrollment. Second, enhanced efforts to address patient concerns about trials and to optimize communication between providers and eligible patients may further increase participation.
Funding
This work of the Cancer Care Outcomes Research and Surveillance (CanCORS) Consortium was supported by grants from the National Cancer Institute (NCI) to the Statistical Coordinating Center (Dana Farber Cancer Institute U01 CA093344) and the NCI-supported Primary Data Collection and Research Centers (Dana Farber Cancer Institute/Cancer Research Network U01 CA093332, Harvard Medical School/Northern California Cancer Center U01 CA093324, RAND/UCLA U01 CA093348, University of Alabama at Birmingham U01 CA093329, University of Iowa U01 CA093339, University of North Carolina U01 CA093326) and by a Department of Veterans Affairs grant to the Durham VA Medical Center CRS 02-164. Drs. Keating and Schrag were also supported by 1R01CA164021-01A1.
This analysis was performed using CanCORS core dataset version 1.17 and baseline survey dataset version 1.12; the authors would like to thank the staff of the CanCORS Statistical Coordinating Center (SCC) for assistance with the data. We also thank David Harrington, PhD for helpful comments on an earlier version of the manuscript. We are also grateful to Jane C. Weeks, MD, MS, for her feedback on plans for this analysis. The authors have no conflicts of interest to report.
References
- 1. Nass SJ, Moses HL, Mendelsohn J. A national cancer clinical trials system for the 21st century: reinvigorating the NCI Cooperative Group Program. Committee on Cancer Clinical Trials and the NCI Cooperative Group Program; Institute of Medicine, National Academies Press; 2010:1371. [PubMed] [Google Scholar]
- 2. Schroen AT, Petroni GR, Wang H, et al. Achieving sufficient accrual to address the primary endpoint in phase III clinical trials from U.S. Cooperative Oncology Groups. Clin Cancer Res. 2012;18(1):256–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Fouad MN, Lee JY, Catalano PJ, et al. Enrollment of patients with lung and colorectal cancers onto clinical trials. J Oncol Pract. 2013;9(2):e40–e47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Stewart JH, Bertoni AG, Staten JL, Levine EA, Gross CP. Participation in surgical oncology clinical trials: gender-, race/ethnicity-, and age-based disparities. Ann Surg Oncol. 2007;14(12):3328–3334 [DOI] [PubMed] [Google Scholar]
- 5. Unger JM, Hershman DL, Albain KS, et al. Patient income level and cancer clinical trial participation. J Clin Oncol. 2013;31(5):536–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jimenez R, Zhang B, Joffe S, et al. Clinical trial participation among ethnic/racial minority and majority patients with advanced cancer: what factors most influence enrollment? J Palliat Med. 2013;16(3):256–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kennedy BJ. Needed: clinical trials for older patients. J Clin Oncol. 1991;9(5):718–720 [DOI] [PubMed] [Google Scholar]
- 8. Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341(27):2061–2067 [DOI] [PubMed] [Google Scholar]
- 9. Talarico L, Chen G, Pazdur R. Enrollment of elderly patients in clinical trials for cancer drug registration: a 7-year experience by the US Food and Drug Administration. J Clin Oncol. 2004;22(22):4626–4631 [DOI] [PubMed] [Google Scholar]
- 10. Du W, Gadgeel SM, Simon MS. Predictors of enrollment in lung cancer clinical trials. Cancer. 2006;106(2):420–425 [DOI] [PubMed] [Google Scholar]
- 11. Albrecht TL, Eggly SS, Gleason MEJ, et al. Influence of clinical communication on patients’ decision making on participation in clinical trials. J Clin Oncol. 2008;26(16):2666–2673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ayanian JZ, Chrischilles EA, Fletcher RH, et al. Understanding cancer treatment and outcomes: the Cancer Care Outcomes Research and Surveillance Consortium. J Clin Oncol. 2004;22(15):2992–2996 [DOI] [PubMed] [Google Scholar]
- 13. Catalano PJ, Ayanian JZ, Weeks JC, et al. Representativeness of participants in the cancer care outcomes research and surveillance consortium relative to the surveillance, epidemiology, and end results program. Med Care. 2013;51(2):e9–e15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. The American Association for Public Opinion Research (AAPOR). Standard Definitions: Final Dispositions of Case Codes and Outcome Rates for Surveys. 5thed. Lenexa, Kansas; 2008 [Google Scholar]
- 15. Malin JL, Ko C, Ayanian JZ, et al. Understanding cancer patients’ experience and outcomes: development and pilot study of the Cancer Care Outcomes Research and Surveillance patient survey. Support Care Cancer. 2006;14(8):837–848 [DOI] [PubMed] [Google Scholar]
- 16. Katz JN, Chang LC, Sangha O, Fossel AH, Bates DW. Can comorbidity be measured by questionnaire rather than medical record review? Med Care. 1996;34(1):73–84 [DOI] [PubMed] [Google Scholar]
- 17. Klabunde CN, Reeve BB, Harlan LC, Davis WW, Potosky AL. Do patients consistently report comorbid conditions over time?: results from the prostate cancer outcomes study. Med Care. 2005;43(4):391–400 [DOI] [PubMed] [Google Scholar]
- 18. Ware J, Kosinski M, Keller SD. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220–233 [DOI] [PubMed] [Google Scholar]
- 19. Greene FL, Page DL, Fleming ID, eds. AJCC Cancer Staging Manual. 6thed. New York: Springer; 2002 [Google Scholar]
- 20. Jacobson CK. Denominational and Racial and Ethnic Differences in Fatalism. Rev Relig Res. 1999;41(1):9–20 [Google Scholar]
- 21. Degner LF, Sloan JA, Venkatesh P. The Control Preferences Scale. Can J Nurs Res. 1997;29(3):21–43 [PubMed] [Google Scholar]
- 22. Degner LF, Kristjanson LJ, Bowman D, et al. Information needs and decisional preferences in women with breast cancer. JAMA. 1997;277(18):1485–1492 [PubMed] [Google Scholar]
- 23. Keating NL, Landrum MB, Arora NK, et al. Cancer patients’ roles in treatment decisions: do characteristics of the decision influence roles? J Clin Oncol. 2010;28(28):4364–4370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. He Y, Zaslavsky AM, Landrum MB, Harrington DP, Catalano P. Multiple imputation in a large-scale complex survey: a practical guide. Stat Methods Med Res. 2010;19(6):653–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Murthy VH, Krumholz HM, Gross CP. Participation in cancer clinical trials: race-, sex-, and age-based disparities. JAMA. 2004;291(22):2720–2726 [DOI] [PubMed] [Google Scholar]
- 26. Kanarek NF, Tsai H-L, Metzger-Gaud S, et al. Geographic proximity and racial disparities in cancer clinical trial participation. J Natl Compr Canc Netw. 2010;8(12):1343–1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eggly S, Barton E, Winckles A, Penner LA, Albrecht TL. A disparity of words: racial differences in oncologist-patient communication about clinical trials. Health Expect. 2013. 10.1111/hex.12108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Javid SH, Unger JM, Gralow JR, et al. A prospective analysis of the influence of older age on physician and patient decision-making when considering enrollment in breast cancer clinical trials (SWOG S0316). Oncologist. 2012;17(9):1180–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Légaré F, Ratté S, Stacey D, et al. Interventions for improving the adoption of shared decision making by healthcare professionals. Cochrane Database Syst Rev. 2010;(5):CD006732. [DOI] [PubMed] [Google Scholar]
- 30. Stacey D, Bennett CL, Barry MJ, et al. Decision aids for people facing health treatment or screening decisions. Cochrane database Syst Rev. 2011;(10):CD001431. [DOI] [PubMed] [Google Scholar]
- 31. Edwards A, Elwyn G, Hood K, et al. Patient-based outcome results from a cluster randomized trial of shared decision making skill development and use of risk communication aids in general practice. Fam Pract. 2004;21(4):347–354 [DOI] [PubMed] [Google Scholar]
- 32. Tak HJ, Ruhnke GW, Meltzer DO. Association of patient preferences for participation in decision making with length of stay and costs among hospitalized patients. JAMA Intern Med. 2013;173(13):1195–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lara PN, Higdon R, Lim N, et al. Prospective evaluation of cancer clinical trial accrual patterns: identifying potential barriers to enrollment. J Clin Oncol. 2001;19(6):1728–1733 [DOI] [PubMed] [Google Scholar]
- 34. Go RS, Frisby KA, Lee JA, et al. Clinical trial accrual among new cancer patients at a community-based cancer center. Cancer. 2006;106(2):426–433 [DOI] [PubMed] [Google Scholar]