Abstract
Aims
Calcineurin inhibitors (CNIs) taken after heart transplantation lead to excellent short-term outcomes, but long-term use may cause chronic nephrotoxicity. Our aim was to identify, appraise, select and analyse all high-quality research evidence relevant to the question of the clinical impact of CNI-sparing strategies in heart transplant patients.
Methods
We carried out a systematic review and meta-analysis of randomized controlled trials on CNI reduction in heart transplant recipients. Primary outcomes were kidney function and acute rejection after 1 year. Secondary outcomes included graft loss, all-cause mortality and adverse events.
Results
Eight open-label studies were included, with 723 patients (four tested de novo CNI reduction and four maintenance CNI reduction). Calcineurin inhibitor reduction did not improve creatinine clearance at 12 months 5.46 [−1.17, 12.03] P = 0.32 I2 = 65.4%. Acute rejection at 12 months (55/360 vs. 52/332), mortality (18/301 vs. 15/270) and adverse event rates (55/294 vs. 52/281) did not differ between the low-CNI and standard-CNI groups. There was significant benefit on creatinine clearance in patients with impaired renal function at 6 months [+12.23 (+5.26, +18.82) ml min−1, P = 0.0003] and at 12 months 4.63 [−4.55, 13.82] P = 0.32 I2 = 75%.
Conclusions
This meta-analysis did not demonstrate a favourable effect of CNI reduction on kidney function, but there was no increase in acute rejection. To provide a better analysis of the influence of CNI reduction patterns and associated treatments, a meta-analysis of individual patient data should be performed.
Keywords: calcineurin inhibitor, heart transplantation, meta-analysis, renal function
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Calcineurin inhibitors (CNIs) lead to excellent short-term outcomes in heart transplant patients.
Long-term use of CNIs may cause kidney damage.
WHAT THIS STUDY ADDS
The risk/benefit ratio of CNI reduction after heart transplantation has not been demonstrated.
There is a possible benefit on kidney function without raising the risk of acute rejection.
Introduction
Heart transplantation is a widely accepted treatment for patients with end-stage heart failure or other life-threatening heart diseases. Patient survival has increased mainly because of advances in immunosuppressive therapy [1]. Calcineurin inhibitors (CNIs), namely ciclosporin and tacrolimus, are the keystone of immunosuppressive therapy. Calcineurin inhibitors lead to excellent short-term outcomes [1]. However, the long-term use of CNIs may cause chronic CNI nephrotoxicity, a progressive decline in the glomerular filtration rate (GFR), progressive tubulo-interstitial damage and glomerulosclerosis [2].
The availability of mycophenolate mofetil (MMF) and proliferation signal inhibitors (PSIs) as more powerful immunosuppressive drugs than azathioprine (AZA) prompted the development of several approaches to attenuate renal impairment in solid organ transplant recipients, by reducing the CNI dose without any apparent increase in the incidence of acute rejection [2–5].
Even with the wide variety of study designs, drug regimens and outcomes evaluated, these studies show that overall CNI reduction or minimization is effective in improving renal function, without significant changes in clinical end-points (e.g. biopsy-proven rejection episodes and mortality). However, these trials were not sufficiently powered to assess clinical outcomes [4,6–10].
We performed a systematic review and meta-analysis of randomized controlled trials, which are currently regarded as the strongest form of medical evidence, to compare reduced and standard doses of CNIs in patients who underwent heart transplantation. Our aim was to identify, appraise, select and analyse all high-quality research evidence relevant to the question of the clinical impact of CNI-sparing strategies in these patients.
Methods
Inclusion criteria
We analysed published and unpublished randomized or quasi-randomized controlled trials that included heart transplant recipients treated with CNIs (ciclosporin or tacrolimus). We looked at studies evaluating a wide range of approaches, as follows: gradual CNI reduction, CNI discontinuation, and a combination of reduced dose CNIs with PSI inhibitors and CNI discontinuation, regardless of post-transplant timing, or a combination of immunosuppressive agents. Therapeutic drug monitoring was either based on the trough level (C0) or the CNI level 2 hours after administration of the morning dose (C2), using a specific high-performance liquid chromatography CNI dosage [11]. Based on the heterogeneity of the studies, we also analysed CNI dose reduction in subgroups.
Outcomes
The primary outcomes were kidney function and biopsy-proven acute rejection. Kidney function was measured according to creatinine clearance (CrCl), using the the Cockroft–Gault formula, and the estimated glomerular filtration rate (eGFR), according to the Modification of Diet in Renal Disease (MDRD) equation [12,13]. Secondary outcomes included the following: graft loss, all-cause mortality, left ventricular ejection fraction [measured by echocardiography at 6 months and at 1, 2 and 5 years (if available)], adverse events, infection episodes, major cardiac events (death, myocardial infarction, cardiac arrest, stent thrombosis or the need for revascularization).
Search strategy
We searched for studies on Medline and the Cochrane Library trial registry, and hand searched articles in reference lists up to January 2012. No language restrictions were applied. Keywords used were as follows: ‘Cyclosporin’, ‘Ciclosporine’, ‘Cyclosporine’, ‘Ciclosporin’, ‘Neoral’, or ‘Sandimmum’, ‘Tacrolimus’, ‘FK 506’, ‘Advagraf’, or ‘Prograf;’ ‘Calcineurin’, ‘Calcineurin Inhibitor;’ ‘Heart transplantation’, ‘Heart transplant;’ ‘Withdrawal’, ‘Tapering’, or ‘Minimizing;’ ‘Randomized clinical trials’ or ‘Quasi randomized clinical trials’. Unpublished trials were also identified by directly contacting the principal investigators.
Study selection
Two investigators (CC and CD) independently reviewed the identified abstracts or manuscripts to determine the eligibility of studies for inclusion in the meta-analysis. Studies that did not meet inclusion criteria were excluded. The investigators retrieved and evaluated the full-text versions of potentially relevant studies. The Jadad score was used to assess the methodological quality of selected articles and the Review Manager scale for primary outcomes [14,15].
Data collection
Data extraction was performed by two investigators (CC and CD) using a predefined data collection form. They wrote to the first author of published manuscripts to check data and collect missing data. These authors were also invited to participate in the present meta-analysis by providing additional information for the subgroup of patients with impaired kidney function at inclusion, defined as serum creatinine >120 μmol l−1 or eGFR <60 ml min−1.
Statistical analysis
The main outcome of this meta-analysis was kidney function and biopsy-proven acute rejection using CrCl. The level of significance was set at 5%. A test of association was performed at a level of significance of α = 5%. Binary criteria (incidence of acute rejection, graft loss and all-cause mortality) were analysed using a fixed-effect model or a random-effect model (Mantel–Haenszel) when heterogeneity was found. Results were expressed as relative risks (RRs) with 95% confidence intervals (CIs).
Assessment of study quality
The quality of studies was assessed using the Jadad score, a widely used procedure to assess independently the methodological quality of a clinical trial. It is based on whether the trial was randomized and the adequacy of the randomization method, whether the study was double blind and the adequacy of the blinding method, and the number of withdrawals and dropouts.
Assessment of heterogeneity
Statistical heterogeneity across trials was assessed with χ2 (P < 0.1) and I2 statistics. The I2 statistic is derived from Cochran's Q, i.e. χ2 statistic [(Q − d.f./Q) × 100], and measures the proportion of overall variation that is attributable to between-study heterogeneity. The statistical test of heterogeneity was significant if the P value was <0.1. Heterogeneity was considered high if the I2 was >50%. The value of τ2 was calculated in order to determine the size and clinical relevance of heterogeneity when detected using the previous method. A random-effect model was used when the statistical test of heterogeneity was significant. The following subgroup and heterogeneity analyses were performed: (i) de novo CNI reduction vs. maintenance CNI reduction; (ii) tacrolimus vs. ciclosporin; (iii) combined treatments (PSIs vs. no PSIs); (iv) good-quality vs. poor-quality trials (based on Jadad scale); and (v) a subgroup of patients with impaired kidney function (serum creatinine >120 μmol l−1 or eGFR <60 ml min−1) at the time of randomization vs. patients with serum creatinine <120 μmol l−1 or eGFR >60 ml min−1). All analyses were performed with Review Manager (RevMan) [15]. A funnel plot was performed to assess potential publication bias.
Results
We found 350 abstracts (339 from databases and 11 from other sources). The flow diagram of studies is shown in Figure 1. A total of 294 nonrandomized trials were excluded. Six studies did not evaluate the outcomes we selected. Nine were not randomized controlled trials. Five were not efficacy studies. Six studies were not on heart transplants and 21 did not match up with the treatment protocol we wanted to evaluate. Another study was excluded because it was published as an abstract only [16]. One had a 4 month follow-up [17].
Figure 1.
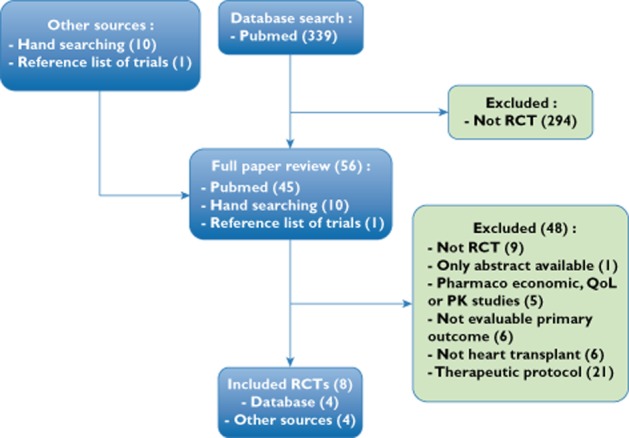
Flow diagram of studies
Eight randomized controlled trials were included in the meta-analysis, with a total of 723 patients [16,18–25]. All authors were contacted, and six provided supplementary data (Boissonnat, Gleissner, Groetzner, Gullestad, Lemkuhl and Potena). All were open-label studies. Included studies are described in Table 1.
Table 1.
Characteristics of included randomized studies (ciclosporin levels, C0 or C2 are shown in milligrams per millilitre)
| Boissonnat et al. (2011) [25] | Gullestad et al. (2010) [21] | Potena et al. (2010) [23] | Groetzner et al. (2009) [20] | Lehmkuhl et al. (2009) [22] | Wang et al. (2010) [24] | Cantarovich et al. (2008) [18] | Gleissner et al. (2006) [19] | |
|---|---|---|---|---|---|---|---|---|
| Participants (n) | 95 | 282 | 34 | 63 | 176 | 25 | 76 (cohort A) | 36 |
| Low dose/standard | 48/47 | 140/142 | 17/17 | 19/14 | 92/84 | 12/13 | 25/51 | 16/20 |
| Follow-up duration (months) | 12 | 12 | 12 | 12 | 12 | 6 | 12 | 6 |
| Intervention = low CNI | CsA | CNI | CsA | CNI-free + mTOR (Siro) + MMF | CsA C0 50–100 (7–12 months) + mTOR (Eve) | CsA | CsA 12 month C0 183 (A3) | CNI-free + mTOR (Siro) + MMF |
| C0 130–200 + MMF | C0 30–70% of baseline + mTOR (Eve) + MMF or AZA | C0 40–90 + mTOR (Eve) | C2 300–500 (day 150–180) + mTOR (Eve) | 197 (A2) + MMF | ||||
| Standard | CsA | CsA or Tacro + MMF or AZA | CsA | CNI | CsA C0 100–250 (7–12 months) + MMF | CsA C2 600–1000 (days 150–180) + Eve | C2 1600–1800 (A1 group) + MMF | CsA C0 < 80 + MMF |
| C0 200–300 + MMF | C0 100–150 + MMF | C0 reduced by 40% + MMF | ||||||
| Additional immunosuppressive treatment (control and experimental groups) | MMF + corticosteroids | MMF or AZA + corticosteroids | Eve (experimental group) | MMF + corticosteroids | Eve (experimental group) | CsA and Eve + corticosteroids | MMF + corticosteroids | MMF + corticosteroids |
| MMF (control group) + corticosteroids | MMF (control group) + corticosteroids | |||||||
| Renal function inclusion criteria | Creatinine ≤250 μmol l−1 | GFR 20–90 ml min−1 | GFR 20–60 ml min−1 | GFR <60 ml min−1 | GFR ≥ 50 ml min−1 | Creatinine ≤ 246.4 μmol l−1 | Creatinine ≤ 170 μmol l−1 | Patients with renal failure |
| Renal function calculation | Cockroft, MDRD | Measured glomerular filtration rate, MDRD | Cockroft | ? calculated creatinine clearance | Cockroft | Cockroft | Cockroft | Cockroft |
| Baseline mean age (years) | 49 (SD 11) | 58 (SD 8) | 63 (SD 6) | 60.9 | 51 (SD 11) | 44 | 49 (SD 12) | 64 |
| Percentage of men | 65 | 72 | 79 | 95.2 | 81 | 84 | 86 | 86 |
| Time post-transplant | <96 h | >1 year | 1–4 years | >6 months | ≤72 h | ≤72 h | 4–6 h | >6 months |
| Mean time from transplant (years) | De novo | 6 | 2.5 | De novo | De novo | De novo | De novo | 8.2 |
| Baseline renal function* | 126 mmol l−1 | 50 (SD 14) ml min−1 | 43.5 (SD 9.1) ml min−1 | 39 (SD 15) ml min−1 | 72.5 (SD 28) ml min−1 | 85 (SD 18) ml min−1 | 72.37 (SD 25.64) ml min−1 | 48.28 (SD 13) ml min−1 |
| CsA C0 level achieved in intervention group | 161 (SD 40.7) | 60 (SD 35) | 60 (SD 32) | CNI withdrawal | 110 (SD 50) | 505.5 (SD 187.8) | 190 (SD 190.5) | CNI withdrawal 6 months |
| C2 | ||||||||
| CsA C0 level in control group | 176.2 (SD 42) | 115 (SD 50) | 136 (SD 34) | 75 (SD 19) | 180 (SD 55) | 644.4 (SD 167.9) | 269 (SD 96) | 62.9 (SD 20.6) |
| C2 | 6 months | |||||||
| Percentage decrease in intervention group | −8.63 | −47.83 | −55.88 | −100.00 | −38.89 | −21.55 | −29.37 | −100.00 |
| Jadad score | 3 | 3 | 3 | 3 | 3 | 1 | 1 | 1 |
Abbreviations are as follows: AZA, azathiprine; CNI, calcineurin inhibitor; CsA, ciclosporin; C0, CsA dosage before CsA administration; C2, CsA concentration 2 h after administration; Eve, everolimus; GFR, glomerular filtration rate; MDRD, Modification of Diet in Renal Disease; MMF, mycofenolate mofetil; mTOR, mammalian target of rapamycin; Siro, sirolimus; and Tacro, tacrolimus.
Baseline renal function was measured using either serum creatinine or creatinine clearance.
The study by Cantarovich et al. [18] included two cohorts, A and B. As cohort B patients were randomized into two reduced doses without a standard dose, we considered only cohort A. Patients randomized into ‘high’ C2 range were the ‘standard-CNI’ group, and those randomized into the ‘intermediate’ and ‘low’ C2 range were included in the ‘low-CNI’ group [18].
The definition of acute rejection was based on the International Society of Heart and Lung Transplantation (ISHLT) classification. Acute rejection was defined by a score ≥3A in most studies, ≥2R in [19,23] and ≥1B in [20].
Patient data on impaired kidney function at randomization (serum creatinine >120 μmol l−1 or eGFR <60 ml min−1) was available for five studies [19–21,23,25]. Two studies specifically included patients with chronic kidney disease [19,20]. Three authors (Boissonnat, Gleissner and Potena) provided data for this subgroup [19,23,25]. Additional data were not available in one study [18].
Six studies were sponsored by pharmaceutical companies [18–22,25], one by a foundation [23], and the source of funding was not specified in one study [24].
Primary outcomes
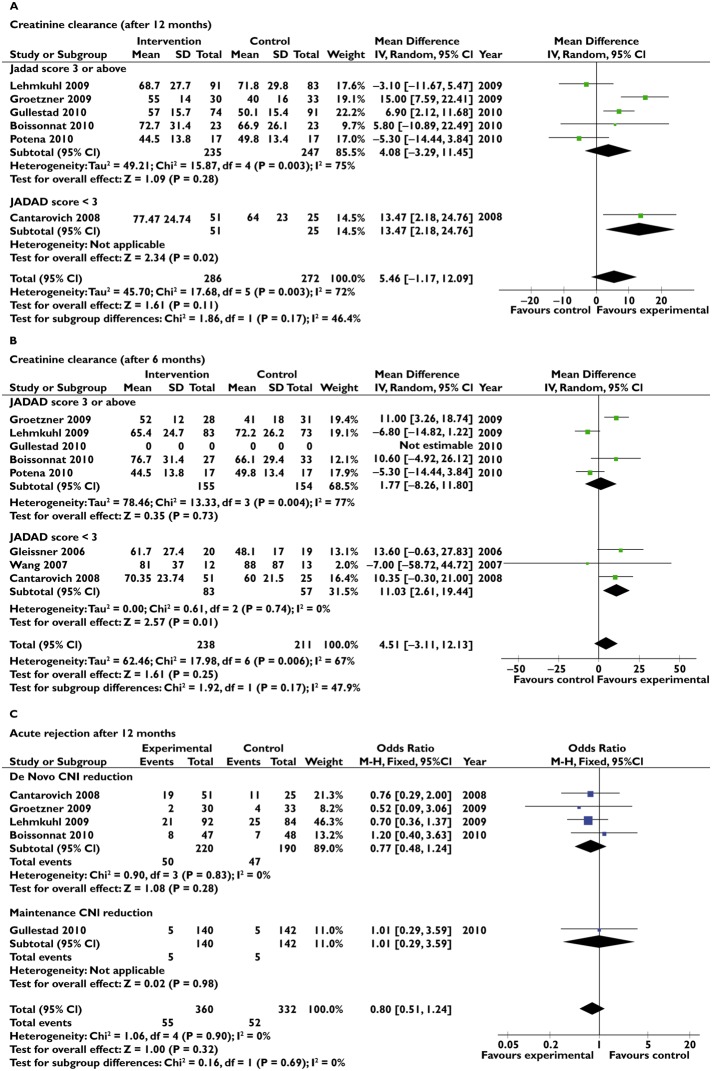
Using a fixed-effect model, CNI reduction did not improve CrCl at 6 months; it improved CrCl at 12 months, but there was a significant heterogeneity with I2 = 72% (Figure 2A,B). When using a random-effect model, the CrCl increase was not significant at 6 months [4.51 (−3.11, 12.13) ml min−1, P = 0.25] or at 1 year [5.46 (−1.17, 12.09), P = 0.11]. The incidence of acute rejection at 6 (43/218 vs. 40/187) and 12 months (55/360 vs. 52/332) did not differ between the low-CNI and standard-CNI groups, using a random-effect model (Figure 2C). Mortality (18/301 vs. 15/270) and severe adverse event rates (55/294 vs. 52/281) did not differ between low-dose CNI and standard-dose CNI groups.
Figure 2.
Forest plots for the primary outcome. (A) Creatinine clearance after 12 months. (B) Creatinine clearance after 6 months. (C) Acute rejection after 12 months
Subgroup analyses
Subgroup analyses were performed on the primary outcome based on the following criteria: (i) the study quality, there is a significant interaction between Jadad score, but the numbers of studies are low in each subgroup; (ii) the schedule of CNI reduction (de novo vs. maintenance); (iii) intensity of CNI reduction; and (iv) concomitant treatments (results not shown).
The subgroup analysis on patients with impaired renal function showed a significant benefit on CrCl at 12 months [4.23 (0.26, 8.20) ml min−1, P = 0.0003], with significant heterogeneity (Figure 3A), or 6 months [12.23 (5.65, 18.82) ml min−1, P = 0.0003; Figure 3B]. Data were not available for the complementary subgroup of patients with preserved kidney function; consequently, we were not able to analyse the interaction between kidney function at inclusion and outcome.
Figure 3.
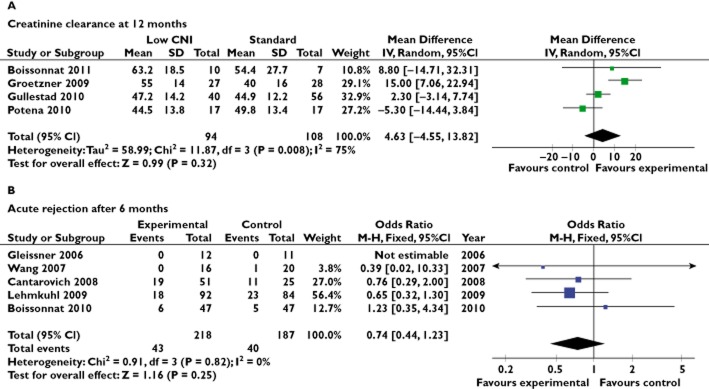
Subgroup analysis of patients with impaired renal function at inclusion. (A) Creatinine clearance at 12 months. (B) Acute rejection after 6 months.
Even though the funnel plot does not suggest a publication bias, its interpretation is limited because of the low number of studies.
Discussion
With this meta-analysis, it is not possible to conclude without a doubt that CNI reduction after a heart transplant leads to improved kidney function. Even though kidney function seems to be preserved, there is significant heterogeneity between trials and the number of patients is limited. The incidence of acute rejection did not increase in the CNI reduction group, but the power of the analysis is limited. As rejection rates fall with time after transplantation, it might be expected that the effects of CNI reduction may differ whether done de novo or many years after transplantation. However, there is no interaction between subgroups and treatment effect.
Other safety criteria, such as graft loss, mortality, adverse events and infections, did not differ between CNI-reduction and standard groups. However, the numbers of patients and events were low, as well as the statistical power. A detrimental effect could not be shown with this data set because of the heterogeneity of adverse event reporting. A detrimental effect on acute rejection could be anticipated with a CNI dose reduction. A total of 2600 subjects per group would be necessary to demonstrate non-inferiority compared with standard immunosuppression, with a relative risk of 1.30 (i.e. a 30% increase in the risk of acute rejection) and 80% power.
No kidney protection by CNI reduction was observed in several studies [17,22,25], whereas other studies found either a trend [24] or better kidney protection, with the greatest benefit immediately after transplantation [21]. In the study by Cantarovich et al., the lower C2 group was associated with a trend towards a lower incidence of acute rejection [18]. Potena et al. showed a possible increase in the risk of infection [23]. Gleissner et al. and Groetzner et al. found an increase in adverse effects with sirolimus/MMF [19,20]. This meta-analysis did not confirm unambiguously that early CNI reduction may be more beneficial than delayed CNI reduction.
Several studies suggested that patients with impaired renal function may benefit more from CNI reduction. This hypothesis was confirmed by the present meta-analysis. The combination of PSIs and MMF was suspected to induce higher mortality or more graft loss in renal transplant patients [26]. This could not be investigated in our meta-analysis. We also could not compare the impact of ciclosporin and tacrolimus on measured outcomes, because too few studies had such strategies.
The limitations of this meta-analysis relate to the fact that we included trials with CNI withdrawal, de novo CNI reduction and maintenance CNI reduction; therefore, the intensity of CNI reduction is very different between studies and may have introduced heterogeneity in the meta-analysis. Two studies appear different from other studies [22], [23]; these two studies used CNI reduction combined with PSIs, an association which has been recognized as having a potentially negative effect on renal function [27]. However, in the analysis without these two studies there is still heterogeneity (τ2 = 28.01, I2 = 64%). All the studies were open studies, with a biological end-point, which we can assume was measured blindly in treatment groups. Renal function was calculated using the Cockroft–Gault formula, except in two studies [20], [21]. The Cockroft–Gault equation can overestimate renal function in patients with impaired renal function. This is, however, unlikely to have altered the results, because the difference between groups was considered and not the absolute value. In addition, all studies were randomized, and the same calculation was applied to all groups. This is also true when considering the subgroup analysis of patients with impaired renal function. This subgroup might include patients with better renal function than expected, and the real effect might be greater than shown here. Heterogeneity could not be explained either by post-transplant delay or combined treatment (PSIs or not). No publication bias was identified using the funnel plot; however, the interpretation of the funnel asymmetry is difficult with a small number of studies.
Conclusion
In conclusion, the risk/benefit ratio of reducing CNI after heart transplantation has still not been demonstrated, even though there seems to be a favourable effect on kidney function without an increase in the risk of acute rejection. A meta-analysis on individual patient data using multivariate techniques should be performed on the effects of all aspects of immunosuppressive treatment (type of drug, dosage, kinetics of CNI reduction, etc.) on the risk/benefit ratio.
Acknowledgments
No specific funding was received for this systematic review. The authors would like to thank Kent Neal (supported by the French Cochrane Center) for proofreading the manuscript.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; CD works for Ipsen; MC received grants from Astellas, Hoffman LaRoche, Novartis and Pfizer, as well as consulting fees from Astellas; JG receives grants from Wyeth Pharma and Roche and consulting fees from Wyeth Pharma; LP is on the board of Teva, receives grants from Novartis and payment for lectures from Novartis; LS is on the board of Novartis and has received consulting fees from Astellas in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.John R, Rajasinghe HA, Chen JM, Weinberg AD, Sinha P, Mancini DM, Naka Y, Oz MC, Smith CR, Rose EA, Edwards NM. Long-term outcomes after cardiac transplantation: an experience based on different eras of immunosuppressive therapy. Ann Thorac Surg. 2001;72:440–449. doi: 10.1016/s0003-4975(01)02784-9. [DOI] [PubMed] [Google Scholar]
- 2.Naesens M, Kuypers DR, Sarwal M. Calcineurin inhibitor nephrotoxicity. Clin J Am Soc Nephrol. 2009;4:481–508. doi: 10.2215/CJN.04800908. [DOI] [PubMed] [Google Scholar]
- 3.Ekberg H, Bernasconi C, Tedesco-Silva H, Vitko S, Hugo C, Demirbas A, Acevedo RR, Grinyo J, Frei U, Vanrenterghem Y, Daloze P, Halloran P. Calcineurin inhibitor minimization in the Symphony study: observational results 3 years after transplantation. Am J Transplant. 2009;9:1876–1885. doi: 10.1111/j.1600-6143.2009.02726.x. [DOI] [PubMed] [Google Scholar]
- 4.Gaston RS, Kaplan B, Shah T, Cibrik D, Shaw LM, Angelis M, Mulgaonkar S, Meier-Kriesche HU, Patel D, Bloom RD. Fixed- or controlled-dose mycophenolate mofetil with standard- or reduced-dose calcineurin inhibitors: the Opticept trial. Am J Transplant. 2009;9:1607–1619. doi: 10.1111/j.1600-6143.2009.02668.x. [DOI] [PubMed] [Google Scholar]
- 5.Kobashigawa J, Miller L, Renlund D, Mentzer R, Alderman E, Bourge R, Costanzo M, Eisen H, Dureau G, Ratkovec R, Hummel M, Ipe D, Johnson J, Keogh A, Mamelok R, Mancini D, Smart F, Valantine H. A randomized active-controlled trial of mycophenolate mofetil in heart transplant recipients. Mycophenolate Mofetil Investigators. Transplantation. 1998;66:507–515. doi: 10.1097/00007890-199808270-00016. [DOI] [PubMed] [Google Scholar]
- 6.Cohen D, Vincenti F. A comparative open label study to evaluate graft function in de novo renal allograft recipients treated with reduced dose or standard dose cyclosporine in combination with sirolimus and corticosteroids. Am J Transplant. 2003;3(Suppl. 5):S465. [Google Scholar]
- 7.de Sevaux RG, Gregoor PJ, Hene RJ, Hoitsma AJ, Vos P, Weimar W, Van Gelder T, Hilbrands LB. A controlled trial comparing two doses of cyclosporine in conjunction with mycophenolate mofetil and corticosteroids. J Am Soc Nephrol. 2001;12:1750–1757. doi: 10.1681/ASN.V1281750. [DOI] [PubMed] [Google Scholar]
- 8.Gonwa TA, Hricik DE, Brinker K, Grinyo JM, Schena FP. Improved renal function in sirolimus-treated renal transplant patients after early cyclosporine elimination. Transplantation. 2002;74:1560–1567. doi: 10.1097/00007890-200212150-00013. [DOI] [PubMed] [Google Scholar]
- 9.Kahan BD, Julian BA, Pescovitz MD, Vanrenterghem Y, Neylan J. Sirolimus reduces the incidence of acute rejection episodes despite lower cyclosporine doses in caucasian recipients of mismatched primary renal allografts: a phase II trial. Rapamune Study Group. Transplantation. 1999;68:1526–1532. doi: 10.1097/00007890-199911270-00016. [DOI] [PubMed] [Google Scholar]
- 10.Muhlbacher F, Paczek L. An open-label study to evaluate the efficacy & safety of cyclosporine reduction in de novo renal allograft recipients receiving sirolimus: a dose comparative study. Am J Transplant. 2002;2(Suppl. 3):S238. doi: 10.1111/tri.12228. [DOI] [PubMed] [Google Scholar]
- 11.Bourge RC, Kirklin JK, White-Williams C, Naftel DC, George JF, Morrow R, Tarkka M, Welborn JM. Methotrexate pulse therapy in the treatment of recurrent acute heart rejection. J Heart Lung Transplant. 1992;11:1116–1124. [PubMed] [Google Scholar]
- 12.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 13.Stevens LA, Greene T, Levey AS. Surrogate end points for clinical trials of kidney disease progression. Clin J Am Soc Nephrol. 2006;1:874–884. doi: 10.2215/CJN.00600206. [DOI] [PubMed] [Google Scholar]
- 14.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, McQuay HJ. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 15.Review Manager (RevMan) Copenhagen: Nordic Cochrane Centre, Cochrane Collaboration; 2011. 5.1 for Windows [computer program] Edition. [Google Scholar]
- 16.Wang S, Zuckermann A, Keogh A, Ross H, Frigerio M, Eisen H, Bara C, Laufer G, Cotrufo M, Lena S. Cyclosporine Reduction in the Presence of Concentration-Controlled Everolimus in de Novo Cardiac Transplantation: 6-Month Study Results. Prague, Czech Republic: European Society for Organ Transplantation, ESOT; 2007. [Google Scholar]
- 17.Waser M, Maggiorini M, Binswanger U, Keusch G, Carrel T, von Segesser L, Gallino A, Turina M. Irreversibility of cyclosporine-induced renal function impairment in heart transplant recipients. J Heart Lung Transplant. 1993;12:846–850. [PubMed] [Google Scholar]
- 18.Cantarovich M, Ross H, Arizon JM, Gomez MA, Straatman L, Orus J, Alonso-Pulpon L, Molina BD, Wang S, Lage E, Crespo MG, Manito N, Howlett J, Haddad H. Benefit of Neoral C2 monitoring in de novo cardiac transplant recipients receiving basiliximab induction. Transplantation. 2008;85:992–999. doi: 10.1097/TP.0b013e318169bf43. [DOI] [PubMed] [Google Scholar]
- 19.Gleissner CA, Doesch A, Ehlermann P, Koch A, Sack FU, Katus HA, Dengler TJ. Cyclosporine withdrawal improves renal function in heart transplant patients on reduced-dose cyclosporine therapy. Am J Transplant. 2006;6:2750–2758. doi: 10.1111/j.1600-6143.2006.01527.x. [DOI] [PubMed] [Google Scholar]
- 20.Groetzner J, Kaczmarek I, Schulz U, Stegemann E, Kaiser K, Wittwer T, Schirmer J, Voss M, Strauch J, Wahlers T, Sohn HY, Wagner F, Tenderich G, Stempfle HU, Mueller-Ehmsen J, Schmid C, Vogeser M, Koch KC, Reichenspurner H, Daebritz S, Meiser B, Reichart B. Mycophenolate and sirolimus as calcineurin inhibitor-free immunosuppression improves renal function better than calcineurin inhibitor-reduction in late cardiac transplant recipients with chronic renal failure. Transplantation. 2009;87:726–733. doi: 10.1097/TP.0b013e3181963371. [DOI] [PubMed] [Google Scholar]
- 21.Gullestad L, Iversen M, Mortensen SA, Eiskjaer H, Riise GC, Mared L, Bjortuft O, Ekmehag B, Jansson K, Simonsen S, Gude E, Rundqvist B, Fagertun HE, Solbu D, Bergh CH. Everolimus with reduced calcineurin inhibitor in thoracic transplant recipients with renal dysfunction: a multicenter, randomized trial. Transplantation. 2010;89:864–872. doi: 10.1097/TP.0b013e3181cbac2d. [DOI] [PubMed] [Google Scholar]
- 22.Lehmkuhl HB, Arizon J, Vigano M, Almenar L, Gerosa G, Maccherini M, Varnous S, Musumeci F, Hexham JM, Mange KC, Livi U. Everolimus with reduced cyclosporine versus MMF with standard cyclosporine in de novo heart transplant recipients. Transplantation. 2009;88:115–122. doi: 10.1097/TP.0b013e3181aacd22. [DOI] [PubMed] [Google Scholar]
- 23.Potena L, Bianchi IG, Magnani G, Masetti M, Coccolo F, Fallani F, Russo A, Grigioni F, Branzi A, Ponticelli C. Cyclosporine lowering with everolimus or mycophenolate to preserve renal function in heart recipients: a randomized study. Transplantation. 2010;89:263–265. doi: 10.1097/TP.0b013e3181c42b95. [DOI] [PubMed] [Google Scholar]
- 24.Wang SS, Chou NK, Chi NH, Huang SC, Wu IH, Wang CH, Yu HY, Chen YS, Tsao CI, Ko WJ, Shun CT. Can cyclosporine blood level be reduced to half after heart transplantation? Transplant Proc. 2010;42:930–933. doi: 10.1016/j.transproceed.2010.03.014. [DOI] [PubMed] [Google Scholar]
- 25.Boissonnat P, Gaillard S, Redonnet M, Lelong B, Mattei M, Mouly-Bandini A, Treilhaud M, Pattier S, Sirinelli A, Epailly E, Varnous S, Billes M, Sebbag L, Ecochard R, Cornu C, Gueyffier F. 2012;13:231. doi: 10.1186/1745-6215-13-231. Impact of the early reduction of cyclosporine on renal function in heart transplant patients: a French randomised clinical trial. Trials. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sharif A, Shabir S, Chand S, Cockwell P, Ball S, Borrows R. Meta-analysis of calcineurin-inhibitor-sparing regimens in kidney transplantation. J Am Soc Nephrol. 2011;22:2107–2118. doi: 10.1681/ASN.2010111160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gurk-Turner C, Manitpisitkul W, Cooper M. A comprehensive review of everolimus clinical reports: a new mammalian target of rapamycin inhibitor. Transplantation. 2012;15:659–668. doi: 10.1097/TP.0b013e31825b411c. [DOI] [PubMed] [Google Scholar]