Abstract
Aims
Several epidemiological studies have evaluated the association between nonsteroidal anti-inflammatory drugs (NSAIDs) and brain tumour risk. However, results from these studies have been inconsistent. The aim of this detailed meta-analysis is to review and summarize the evidence on this association.
Methods
A comprehensive search for articles published up to September 2013 was performed. Studies evaluating the association between exposure to NSAIDs and risk of brain tumours were included. Random-effects meta-analytical models were used to calculate the relative risk (RR) and corresponding 95% confidence intervals (CIs). Sensitivity analyses, Galbraith plots and subgroup analyses were also performed.
Results
Ten studies (six case–control studies, three cohort studies and one randomized controlled trial), published between 2003 and 2013, were included in this analysis. Compared with non-use, overall use of NSAIDs was not statistically significantly associated with brain tumour risk based on the random-effects models (RR = 1.01; 95% CI = 0.89, 1.15). No differences were observed when analyses were stratified by gender and brain tumour subtype. Specific analysis for aspirin and non-aspirin NSAIDs yielded similar results. However, a slightly increased risk of brain tumour in NSAID users was observed in cohort studies (RR = 1.32; 95% CI = 1.06, 1.64; P = 0.014). Furthermore, our analysis did not show a significant association between frequency and dose of aspirin use and brain tumour risk.
Conclusions
Use of NSAIDs (aspirin and non-aspirin NSAIDs) does not appear to be associated with brain tumour risk, but larger studies are needed to substantiate this relationship.
Keywords: anti-inflammatory agents, brain neoplasms, glioma, meta-analysis, nonsteroidal, risk factor
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Several epidemiological studies have evaluated the association between nonsteroidal anti-inflammatory drugs (NSAIDs) and brain tumour risk.
However, the results obtained from these studies have been inconsistent; a protective association, a null association and an increased risk association have all been reported.
WHAT THIS STUDY ADDS
This is the first meta-analysis to evaluate the association between use of NSAIDs and brain tumour risk based on all the published literature.
Our results suggest that NSAID (aspirin and non-aspirin NSAID) use does not appear to be associated with brain tumours in any way.
Introduction
Brain and other nervous system tumours are the second leading cause of death from neurological diseases. Gliomas are the most common primary brain tumour in adults [1], and of these, glioblastoma is the most frequent and malignant histologic type [2]. The prognosis of glioma patients remains poor, and fewer than 3% of glioblastoma patients are still alive 5 years after diagnosis [2]. The aetiology of primary brain tumour is largely unknown, and despite extensive studies into their aetiology, there are few established risk factors for developing brain tumours [2,3]. Thus, prevention efforts, beyond reduction in X-ray radiation exposure (given that it is the primary risk factor), merit additional consideration.
Cyclo-oxygenase-2 (COX-2), an inducible enzyme, plays a key role in the inflammatory response and is overexpressed in both meningiomas [4,5] and gliomas [6,7]. It is well established that COX-2 is linked to inflammation and is related to tumour growth through its effect on apoptosis, cell migration, platelet aggregation and angiogenesis [8,9]. In vitro and in vivo studies have shown that nonsteroidal anti-inflammatory drugs (NSAIDs) inhibit glioma cell growth through COX-2-dependent and -independent mechanisms [7,9,10], and may be important chemopreventive agents [11].
To date, however, epidemiological studies and clinical trials on NSAID use and brain tumour risk have been inconsistent. Some previous case–control studies have reported a protective association between use of NSAIDs and risk of glioma [12,13] or glioblastoma multiforme [14]. One prospective cohort study [15], three further large case–control studies [16–18] and one randomized trial [19] have all reported null results for the association between non-aspirin NSAID or aspirin use and brain tumour risk. In contrast, two previous cohort studies [20,21] have reported an increased risk of brain tumours in users of aspirin or non-aspirin NSAIDs. Therefore, it is still unclear whether NSAID use can reduce the risk of brain tumours, and clarification of the potentially chemopreventive effect of NSAIDs is of great significance given their widespread and long-term use.
The overall aim of this meta-analysis was to evaluate the association between use of NSAIDs and brain tumour risk based on all the published literature. We also evaluated whether the association varies by type of NSAID (aspirin and non-aspirin NSAID), study design, gender, frequency of use and aspirin dose.
Methods
Systematic search
We carried out the meta-analysis following the guidelines from the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) statement [22]. In September 2013, two authors (SL and YL) independently performed a systematic literature search in the PubMed, Embase, Web of Science and Cochrane library databases. The search strategy was as follows: (‘brain tumour’ or ‘intracranial tumour’ or ‘brain neoplasms’ or ‘intracranial neoplasms’ or ‘brain cancer’ or ‘intracranial cancer’ or ‘glioma’ or ‘glioblastoma’ or ‘meningioma’) and (‘nonsteroidal anti-inflammatory drug’ or ‘NSAID’ or ‘aspirin’ or ‘cyclo-oxygenase-2 inhibitors’). Only human studies were included, and no language restrictions were imposed. The same authors retrieved and independently assessed potentially relevant articles reporting information on the association between NSAID use and brain tumour incidence. The titles and abstracts of the studies identified in the computerized search were scanned to exclude any studies that were clearly irrelevant. The full texts of the remaining articles were read to determine whether they contained information on the topic of interest. The reference lists of all papers of interest were manually checked to retrieve other pertinent publications.
Eligibility criteria
Studies were included if they met all the following inclusion criteria: (i) original cohort study, case–control study or randomized controlled trial (RCT) that evaluated exposure to NSAIDs and incidence of brain tumours; (ii) provided a relative risk (RR) estimate (risk ratio, rate ratio, hazard ratio or odds ratios) with the corresponding 95% confidence intervals (CIs) or sufficient data to calculate them; and (iii) when multiple reports were published on the same study population, we included in the meta-analysis only the most informative one, unless the reported outcomes were mutually exclusive. Inclusion was not restricted by study size. Disagreements in the study selection were discussed among the co-authors until consensus was reached.
Data extraction
Data were extracted by two independent authors using the same standardized form. Conflicts were resolved by consensus, referring back to the original article. We reviewed all the included studies and abstracted the following information: first author's name, year of publication, country, study design, number of subjects, type of NSAID, the definition of exposure, period of enrolment, tumour subtypes, participant characteristics, covariates adjusted for in the analysis, and adjusted risk estimates and the corresponding 95% CIs for individual drugs and tumours. If the required data for the meta-analysis were not readily available in the published article, the corresponding authors were contacted at least once.
Methodological quality assessment
The quality of each eligible study was assessed by two authors (YL and SL). Disagreements were discussed with a third author (XQ) and resolved by consensus. Methodological quality was assessed using the Cochrane Risk of Bias tool [23] for RCTs; the instruments are described in detail elsewhere [23]. For nonrandomized studies, the Newcastle–Ottawa Scale [24] was used, where observational studies were scored across three categories: selection (four questions, one star each), comparability (one question, up to two stars) and the exposure/outcome of interest (three questions, one star each). A ‘star’ represents a ‘high-quality’ choice of individual study. A high-quality study should achieve at least seven stars, a medium-quality study four to six stars and a poor-quality study less than four stars.
Statistical analysis
The pooled RR estimates were generated under a random-effects model (the DerSimonian and Laird method) [25] or a fixed-effects model (the Mantel–Haenszel method) [26]. When heterogeneity was found, data were analysed using a random-effects model [27]. Heterogeneity between studies was assessed using Cochran's Q test, defined as a P < 0.10, and inconsistency was measured using the I2 statistic [28,29]. Values of I2 of 25, 50 and 75% indicate a low, moderate and high degree of heterogeneity, respectively [29]. Sensitivity analyses were also conducted to assess the consistency of results and to investigate the influence of one study on the overall meta-analysis by sequential omission of individual studies [30]. Furthermore, the Galbraith plot was used to spot the outliers as the possible major sources of heterogeneity [31]. Publication bias was assessed graphically using a funnel plot and quantitatively using Begg's rank correlation test [32] and Egger's regression asymmetry test [33].
We included in this meta-analysis studies reporting different measures of relative risk (RR), as follows: the odds ratio in case–control studies; and rate ratio or risk ratio in cohort studies. In practice, these measures of effect mathematically yield very similar RR estimates, given that the absolute risk of brain tumours is low [34]. The use of NSAIDs is defined as ‘overall use’, i.e. all the reported intake levels of use of NSAIDs. Studies were grouped by the type of medicine (overall NSAIDs, aspirin or non-aspirin NSAIDs). In order to detect potential interactions, studies were stratified by study design, type of medicine, gender and tumour subtype. All values of P < 0.05 (two-sided) were considered as significant unless otherwise specified. All analyses were performed using STATA version 12.0 (StataCorp, College Station, TX, USA).
Results
Search results
A total of 2091 articles were identified during the initial search (Figure 1). After removing the duplicates and reviewing the titles and abstracts, 2071 articles were found to be ineligible. We retrieved the full copies of the remaining 20 articles, and 10 met the inclusion criteria [12–21]. The rejected studies did not include the outcome or the medicine of interest [35], reported estimates on mortality [36], reported pooled data analysis [37] or reported estimates on the brain and other nervous systems combined [38]. The characteristics of studies included in this meta-analysis are presented in Table 1. There were six case–control studies, three cohort studies and only one RCT investigating the association between NSAID use and risk of brain tumours (glioma, glioblastoma or meningioma); there was only one study conducted for meningioma [18]. The publication dates of the studies included in the meta-analysis ranged between 2003 and 2013. The majority of the studies were conducted in the USA [12–16,19,21], and three were carried out in the UK [18] and Denmark [13,17]. All studies adjusted for age and sex, and some studies controlled for additional variables, including race, alcohol use and smoking status, which are potential confounders. Each study had medium-high to high quality and low risk, so we included all studies for analysis.
Figure 1.
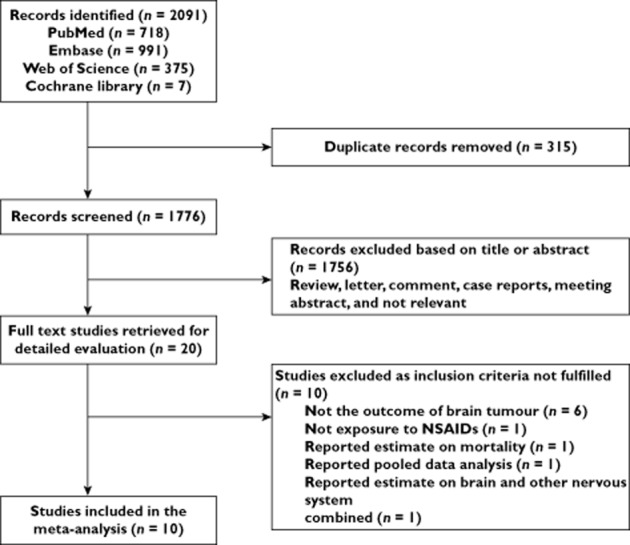
Flow chart depicting the selection of eligible studies
Table 1.
Studies on nonsteroidal anti-inflammatory drug use and brain tumour risk included in the meta-analysis
| Study | Country | No. of cases | All subjects | Exposure | Exposure definition | Types of brain cancer analysed | Exposure period (mean no. of years of follow-up) | Population details | Adjustment factors | Quality score |
|---|---|---|---|---|---|---|---|---|---|---|
| Case–control studies | ||||||||||
| Sivak-Sears et al. (2004) [14] | USA | 236 | 637 | NSAIDs | At least one pill per week for 10 years | Glioblastoma | 1997–2000 | Men and women; mean age 62 years | 1–6 | 7 |
| Scheurer et al. (2008) [12] | USA | 325 | 925 | NSAIDs | Any use | Glioma | 2001–2006 | Men and women; mean age 50 years | 1–4, 8–12 | 6 |
| Scheurer et al. (2011) [16] | USA | 1339 | 2873 | NSAIDs | At least one pill per week for at least 6 months | Glioma | 1997–2008 | Men and women; mean age 53.5 years | 1–4, 8–12 | 7 |
| Ferris et al. (2012) [13] | USA | 517 | 917 | NSAIDs | At least twice a week for at least 6 months | Glioma | 2007–2010 | Men and women; mean age (SD) 57.4 (13.8) years | 1–3, 14–15 | 7 |
| Bannon et al. (2013) [18] | UK | 5052 | 47 730 | Aspirin Non-aspirin NSAIDs | Aspirin: low dose <75 mg; high dose >75 mg | Glioma Meningioma | 1987–2009 (7) | Men and women; mean age (SD) 58 (15) years | 1, 2, 16–19 | 7 |
| Gaist et al. (2013) [17] | Denmark | 2688 | 21 536 | Aspirin Non-aspirin NSAIDs | At least prescription | Glioma | 2000–2009 | Men and women; aged 20–85 years | 1–4, 9, 15, 18, 20–22 | 7 |
| Cohort studies | ||||||||||
| Friis et al. (2003) [20] | Denmark | 70 | 29 470 | Aspirin | 75–150 mg daily | Brain | 1989–1995 (4.1) | Men and women; mean age at entry 70 years | 1, 2 | 7 |
| Sorensen et al. (2003) [21] | USA | 170 | 172 057 | Non-aspirin NSAIDs | At least one prescription | Brain | 1989–1995 (5.4) | Men and women; mean age (SD) 47.2 (18.6) years | 1, 2 | 8 |
| Daugherty et al. (2011) [15] | USA | 341 | 302 767 | Aspirin Non-aspirin NSAIDs | Regular use: more than twice a week | Glioblastoma Glioma | 1995–1997 (5.2) | Men and women; mean age 63.4 years | 1–3, 13 | 7 |
| RCT | ||||||||||
| Cook et al. (2005) [19] | USA | 31 | 39 876 | Aspirin | 100 mg every other day | Brain | 1992–2004 (10.1) | Women; mean (SD) 54.6 (7.0) years | 1, 6, 7 | Low risk |
Abbreviations are as follows: NSAIDs, nonsteroidal anti-inflammatory drugs; RCT, randomized controlled trial; SD, standard deviation. Adjustment factors: 1, age; 2, sex; 3, race; 4, education; 5, income; 6, vitamin E; 7, β-carotene assignments; 8, alcohol use; 9, smoking status; 10, family history of cancer; 11, family history of brain tumour; 12, inflammation-related events; 13, history of heart disease; 14, acetaminophen use; 15, use of statins; 16, history of osteoarthritis/arthralgia; 17, history of rheumatoid arthritis; 18, history of allergy; 19, history of hormone replacement therapy use; 20, asthma; 21, antihistamines; 22, anti-asthma medications.
Association between overall NSAID use and brain tumour risk
Of the 10 studies included, three [15,17,18] provided separate effect estimates on aspirin and non-aspirin NSAID use. To avoid double counting of the subjects exposed to both aspirin and non-aspirin NSAIDs, we included only the use of aspirin for these three studies in our analysis. Six case–control studies [12–14,16–18], three cohort studies [15,20,21] and one RCT [19], including 618 788 subjects and 10 555 cases overall, evaluated exposure to NSAIDs and brain tumour risk.
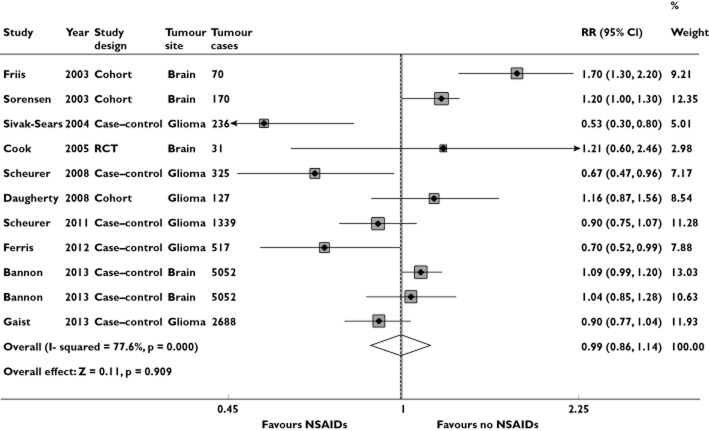
Figure 2 shows the study-specific and pooled RRs and 95% CIs of brain tumours for NSAID users vs. NSAID non-users. Two estimates from Bannon et al. [18] are shown because the study provided separate analysis for low-dose and high-dose aspirin, which have also been kept separate in this figure. Meta-analysis of all 10 studies showed no statistical significance for an association between NSAID use and risk of brain tumours (RR = 0.99; 95% CI = 0.86, 1.14). This time, Cochran's Q test had a P value of <0.001 and the corresponding quantity I2 was 77.6%, both indicating a statistically significant heterogeneity among the studies (Table 2). In contrast, the P values for Begg's and Egger's tests were P = 0.213 and P = 0.333, respectively, both suggesting a low probability of publication bias (Figure 3A).
Figure 2.
Forest plot of the association between NSAID use and risk of brain tumour
Table 2.
Meta-analysis results
| Exposure | No. of studies | Incident cases | RR (95% CI) | P value | Effect model | Heterogeneity | |
|---|---|---|---|---|---|---|---|
| P value | I2 (%) | ||||||
| NSAID use | |||||||
| Overall | 10 | 10 555 | 0.99 (0.86, 1.14) | 0.909 | R | <0.001 | 77.6 |
| Study design | |||||||
| Case–control studies | 6 | 10 157 | 0.87 (0.75, 1.02) | 0.077 | R | 0.001 | 72.3 |
| Cohort studies | 3 | 367 | 1.32 (1.06, 1.64) | 0.014 | R | 0.055 | 65.6 |
| RCT | 1 | 31 | 1.21 (0.60, 2.45) | 0.596 | – | – | – |
| Gender | |||||||
| Male | 4 | 547 | 0.98 (0.70, 1.37) | 0.920 | R | 0.054 | 60.9 |
| Female | 5 | 382 | 0.90 (0.52, 1.56) | 0.717 | R | <0.001 | 85.4 |
| Tumour subtype | |||||||
| Glioma | 5 | 6017 | 0.93 (0.81,1.07) | 0.292 | R | 0.038 | 60.5 |
| Meningioma | 1 | 861 | 1.07 (0.87, 1.32) | 0.525 | R | – | – |
| Glioblastoma | 2 | 366 | 0.74 (0.40, 1.37) | 0.340 | R | 0.041 | 76.0 |
| Aspirin use | |||||||
| Overall | 7 | 8704 | 1.01 (0.84, 1.21) | 0.946 | R | <0.001 | 78.8 |
| Study design | |||||||
| Case–control studies | 4 | 8476 | 0.89 (0.74, 1.07) | 0.214 | R | 0.007 | 77.0 |
| Cohort studies | 2 | 197 | 1.41 (0.97, 2.05) | 0.071 | R | 0.057 | 72.5 |
| RCT | 1 | 31 | 1.21 (0.60, 2.45) | 0.596 | – | – | – |
| Dose of aspirin | |||||||
| Regular | 4 | 894 | 0.83 (0.55, 1.24) | 0.353 | R | 0.011 | 73.1 |
| Low-dose aspirin | 3 | 7810 | 1.16 (0.89, 1.51) | 0.284 | R | <0.001 | 88.3 |
| High-dose aspirin | 1 | 5052 | 1.04 (0.85, 1.28) | 0.694 | – | – | – |
| Non-aspirin NSAID use | |||||||
| Overall | 3 | 7787 | 1.09 (0.99, 1.18) | 0.055 | R | 0.156 | 46.1 |
Abbreviations are as follows: CI, confidence interval; Effect model, R random-effects model; NSAIDs, nonsteroidal anti-inflammatory drugs; RCT, randomized controlled trial; RR, relative risk.
Figure 3.
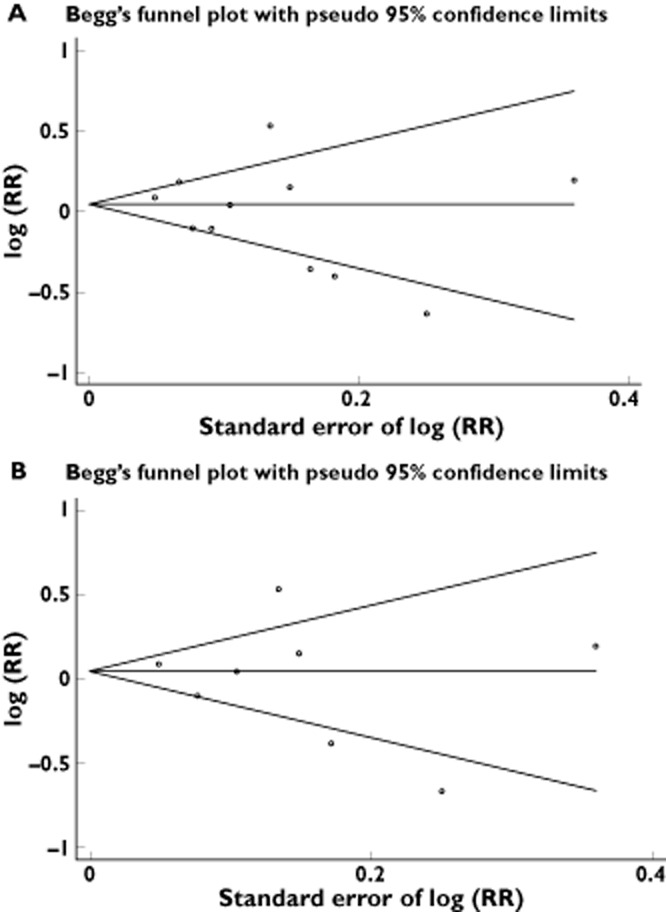
(A) Funnel plot for NSAIDs use and brain tumour risk in overall analysis. (B) Funnel plot for aspirin use and brain tumour risk
Sensitivity analysis was performed by sequential omission of individual studies using the random-effects model and demonstrated that no study appeared to influence the overall results (data not shown). When the Galbraith plot was analysed, five studies [12–14,20,21] were identified as the main contributors to heterogeneity (Figure 4A). By excluding these studies from the analysis, similar pooled RR and significance were obtained (RR = 1.01; 95% CI = 0.92, 1.11), and heterogeneity was low (I2 = 32.5 %; P = 0.192; data not shown).
Figure 4.

(A) Galbraith plots analysis of NSAIDs and brain tumour risk. (B) Galbraith plots analysis of aspirin and brain tumour risk.  , fitted values
, fitted values
After stratifying the data into subgroups based on study design, gender and specific brain tumour subtypes, we found no association between NSAID use and brain tumours in case–control studies (RR = 0.87; 95% CI = 0.75, 1.02), RCT (RR = 1.21; 95% CI = 0.60, 2.45), males (RR = 0.98; 95% CI = 0.70, 1.37), females (RR = 0.90; 95% CI = 0.52, 1.56), glioma (RR = 0.93; 95% CI = 0.81, 1.07), meningioma (RR = 1.07; 95% CI = 0.87, 1.32) and glioblastoma (RR = 0.74; 95% CI = 0.40, 1.37). However, a slight increase in the risk of brain tumours by taking NSAIDs was observed in cohort studies (RR = 1.32; 95% CI = 1.06, 1.64; Table 2). There was only one publication constructed by RCT [19] and one for meningioma [18]; therefore, the respective results relate entirely to the particular study.
Association between aspirin use and brain tumour risk
Four case–control studies [13,14,17,18], two cohort studies [15,20] and one RCT [19], encompassing 8704 incident cases of brain tumours, evaluated exposure to aspirin and brain tumour risk. Overall, ever use of aspirin appeared not to be significantly associated with brain tumour risk compared with no aspirin use (RR = 1.01; 95% CI = 0.84, 1.21; I2 = 78.8%; P < 0.001; Table 2 and Figure 5). The P values for Begg's and Egger's tests were P = 0.386 and P = 0.644, respectively, both suggesting a low probability of publication bias (Figure 3B). Two estimates from Bannon et al. [18] are shown because the study provided separate analysis for low-dose and high-dose aspirin, which have also been kept separate in these figures.
Figure 5.
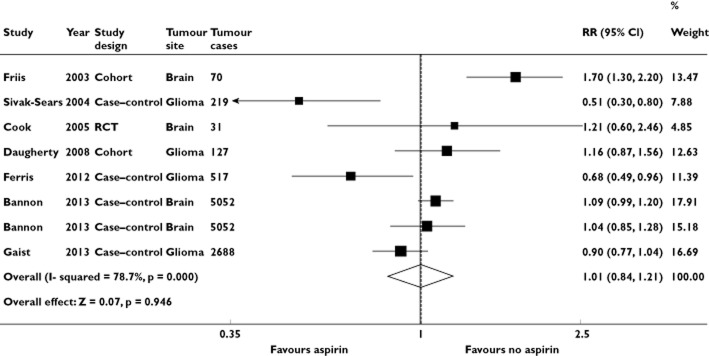
Forest plot of the association between aspirin use and risk of brain tumour
Sensitivity analysis demonstrated that no study apparently influenced the overall results (data not shown). When the Galbraith plot was analysed, three studies [13,14,20] were identified as the main contributors to heterogeneity (Figure 4B). We checked whether these studies influenced our results by repeating the analysis without these studies and obtained similar results (data not shown). To evaluate the consistency across varying study designs with different potential biases, we stratified data into subgroups based on study design. The association was not statistically significant among case–control studies (RR = 0.89; 95% CI = 0.74, 1.07) or among cohort studies (RR = 1.41; 95% CI = 0.97, 2.05; Table 2).
To analyse the association between the dose of aspirin and the risk of brain tumours, drug exposure was categorized as ‘regular’, ‘low-dose aspirin’ and ‘high-dose aspirin’, all as reported in the individual studies. There were three studies for the ‘low-dose aspirin’ analysis, where the definitions for ‘low dose’ were 75 mg day−1 [20], ≤75 mg day−1 [18] and ≤100 mg day−1 [17], and only one study for ‘high dose’ (≥75 mg day−1) aspirin analysis. There was no statistically significant difference between the calculated pooled RR estimates for the ‘regular’, ‘low dose’ and ‘high dose’ of aspirin (Table 2), providing no evidence of a dose-dependent relationship between the frequency of aspirin use and risk of brain tumours. The duration of drug use varied in each trial; hence, statistical analysis of significance between these groups was not valid.
Association between use of non-aspirin NSAIDs and brain tumour risk
Only two case–control studies [17,18] and one cohort study [15], encompassing 7787 incident cases of brain tumours, evaluated exposure to non-aspirin NSAIDs and brain tumour risk. The association between non-aspirin-NSAID use and brain tumour risk was not statistically significant based on a random-effects model (RR = 1.09; 95% CI = 0.99, 1.18), with evidence of low heterogeneity (I2 = 46.1%; P = 0.156; Table 2); therefore, Begg's and Egger's tests were used to provide statistical evidence of funnel plot symmetry. The results still did not suggest any evidence of publication bias (P = 1.00 for Begg's test and P = 0.410 for Egger's test). Subgroup analysis was not performed for studies with a small number of patients.
Discussion
There is a long-standing debate concerning the association between use of NSAIDs and cancer. Recent meta-analyses have discussed the potential chemoprevention of NSAID use against cancer at various sites, such as breast [39], lung [40], oesophageal [41], colorectal [42] and ovarian cancer [43]. In recent years, several epidemiological and clinical studies have been conducted to investigate the association of NSAID use and brain tumour risk [12–21]. Experimental studies indicated that COX-2 is overexpressed in both meningiomas [4,5] and gliomas [6,7]. Moreover, it is well established that COX-2 is related to tumour growth [8,9]. Nonsteroidal anti-inflammatory drugs may inhibit glioma cell growth through COX-2-dependent and -independent mechanisms [7,9,10], which may be the proposed mechanisms behind the hypotheses of the NSAID–brain tumour relationship.
In this systematic review of epidemiological data, NSAID use does not appear to be associated with brain tumours when compared with non-use. This finding is based on a total of 10 studies, including over 10 555 tumour cases, that provided risk estimates for overall NSAIDs or specific subtypes. Major analyses were performed by stratifying the data into subgroups based on study design, gender and tumour subtype. We also performed a pooled analysis on the effect of aspirin dosage. To the best of our knowledge, this is the first comprehensive meta-analysis to investigate the overall risk of brain tumours associated with NSAID use.
Despite NSAID use not appearing to be associated with brain tumours, a slightly increased risk of brain tumours following NSAID use was observed in cohort studies (RR = 1.32; 95% CI = 1.06, 1.64), with evidence of moderated heterogeneity (I2 = 65.6%; P = 0.055). There were three cohort studies included in this analysis [15,20,21]; however, the primary outcome of two studies was not brain tumours but colorectal cancer [20,21]. In addition, these two cohort studies estimated RR by adopting a standardized incidence ratio, which is the ratio of observed to expected cases, based on reference incidence rates for the general population [20,21]. All these factors may be the sources of heterogeneity observed in our results. As expected, when the Galbraith plot was analysed, these two studies were identified as the main contributors to heterogeneity [20,21]; by excluding these from the overall analysis, similar pooled RR and significance were obtained, providing evidence that our result of null association between NSAID use and brain tumours was robust and reliable to a certain extent.
The consideration of study bias is critical when a meta-analysis of published literature is performed. The existence of a bias in favour of publication of statistically significant results is well documented [44]. However, the likelihood of important selection or publication bias in our meta-analysis results is small. During the identification and selection process, we carried out a broad search covering multiple databases with a manual review, and no article was excluded because of methodological characteristics. In addition, the Begg's and the Egger's tests both suggested a low probability of publication bias. The results above indicate that publication bias was not evident in this meta-analysis.
Heterogeneity is a potential problem when interpreting the results of all meta-analyses, and finding the sources of heterogeneity is one of the most important goals of any meta-analysis [45]. In the present case, significant between-study heterogeneity in the pooled analyses of total eligible studies was observed (I2 = 77.6%; P < 0.001). Despite stratifying the data into subgroups based on study design, gender, tumour subtype and dosage of aspirin used, some heterogeneity was still detected. Aside from the factors analysed above, other factors, such as the difference of each study in ascertainment of drug exposure (for example, self-reported or medical records), definition of exposure, study population, sample size, family history, race and education, may also be responsible for heterogeneity. A pivotal unresolved problem is the definition of the duration and frequency of NSAID use, which could have chemoprophylactic effects.
Apart from the potential sources of heterogeneity mentioned above, five studies were identified as the main contributors to heterogeneity [12–14,20,21]; two of these were cohort studies, which have been discussed above [20,21]. The rest were case–control studies, all of which reported an inverse association between use of NSAIDs and risk of glioma or glioblastoma [12–14]. As is well known, case–control studies, in particular, could have potential for recall bias. For example, early and undiagnosed symptoms of the disease affect the use of aspirin. Moreover, this type of bias is of particular concern when studying brain tumours, because these can cause cognitive impairment and memory loss and, therefore, result in an underestimation of exposure among cases.
The results of this meta-analysis must be interpreted cautiously in light of the strengths and limitations of the included trials. A major strength of this study is that, with a large sample size (more than 618 788 subjects and 10 555 cases), we have enough statistical power to provide precise and reliable effect estimates. Secondly, all studies included were original studies with medium-high quality, which provided reliable sources of evidence. In addition, this investigation allowed the examination of the correlation of NSAID use separately for the main brain cancer subtypes and gender. Nevertheless, several limitations of this study must be considered. Firstly, two-thirds of the studies that assess the association between NSAID use and brain tumours are observational studies. Observational studies included in our meta-analysis may be affected by various sources of bias that, at least in part, may explain the lower pooled RR observed in case–control than in cohort studies. Secondly, the studies included did not all adjust for the same confounders. They generally failed to account for one or more of the following risk factors for brain tumours: therapeutic X-ray radiation, alcohol use, smoking state, family history of brain tumours, chemical exposure, diet and allergy. Thirdly, publication bias might occur, as we did not include unpublished data, although we did not find evidence of publication bias by using Begg's and Egger's tests. However, the number of studies included is so small that it may limit the statistical power. Fourthly, the limited available data restricted the scope for duration and dose–risk analyses. Our results are based on ‘regular’, ‘low-dose’ and ‘high-dose’ aspirin intake, which have not been precisely defined, and there are some similarities in the categorizations of ‘low dose’ and ‘high dose’ between the compared studies; this may result in a lack of reliability. Therefore, our results should be interpreted with caution.
In conclusion, despite the limitations of our meta-analysis, the results show that there was no strong association between use of NSAIDs (aspirin and non-aspirin-NSAIDs) and brain tumour risk. Considering the presence of heterogeneity in our analysis, more epidemiological studies are required to evaluate the relationship between NSAID and brain tumour risk further, and this should be explored in depth to clarify the optimal dose, frequency and duration of NSAID use.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.McKinney PA. Brain tumours: incidence, survival, and aetiology. J Neurol Neurosurg Psychiatry. 2004;75(Suppl. 2):ii12–17. doi: 10.1136/jnnp.2004.040741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ohgaki H. Epidemiology of brain tumors. Methods Mol Biol. 2009;472:323–342. doi: 10.1007/978-1-60327-492-0_14. [DOI] [PubMed] [Google Scholar]
- 3.Bondy ML, Scheurer ME, Malmer B, Barnholtz-Sloan JS, Davis FG, Il'yasova D, Kruchko C, McCarthy BJ, Rajaraman P, Schwartzbaum JA, Sadetzki S, Schlehofer B, Tihan T, Wiemels JL, Wrensch M, Buffler PA, Brain Tumor Epidemiology C. Brain tumor epidemiology: consensus from the Brain Tumor Epidemiology Consortium. Cancer. 2008;113:1953–1968. doi: 10.1002/cncr.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kato Y, Nishihara H, Mohri H, Kanno H, Kobayashi H, Kimura T, Tanino M, Terasaka S, Tanaka S. Clinicopathological evaluation of cyclooxygenase-2 expression in meningioma: immunohistochemical analysis of 76 cases of low and high-grade meningioma. Brain Tumor Pathol. 2012 doi: 10.1007/s10014-012-0127-8. doi: 10.1007/s10014-012-0127-8. [DOI] [PubMed] [Google Scholar]
- 5.Pistolesi S, Boldrini L, Gisfredi S, Ursino S, Ali G, Nuti S, De Ieso K, Pieracci N, Parenti G, Fontanini G. Expression of cyclooxygenase-2 and its correlation with vasogenic brain edema in human intracranial meningiomas. Cancer Invest. 2007;25:555–562. doi: 10.1080/07357900701508280. [DOI] [PubMed] [Google Scholar]
- 6.Temel SG, Kahveci Z. Cyclooxygenase-2 expression in astrocytes and microglia in human oligodendroglioma and astrocytoma. J Mol Histol. 2009;40:369–377. doi: 10.1007/s10735-009-9250-1. [DOI] [PubMed] [Google Scholar]
- 7.Joki T, Heese O, Nikas DC, Bello L, Zhang J, Kraeft SK, Seyfried NT, Abe T, Chen LB, Carroll RS, Black PM. Expression of cyclooxygenase 2 (COX-2) in human glioma and in vitro inhibition by a specific COX-2 inhibitor, NS-398. Cancer Res. 2000;60:4926–4931. [PubMed] [Google Scholar]
- 8.Taketo MM. Cyclooxygenase-2 inhibitors in tumorigenesis (part I) J Natl Cancer Inst. 1998;90:1529–1536. doi: 10.1093/jnci/90.20.1529. [DOI] [PubMed] [Google Scholar]
- 9.Fujita M, Kohanbash G, Fellows-Mayle W, Hamilton RL, Komohara Y, Decker SA, Ohlfest JR, Okada H. COX-2 blockade suppresses gliomagenesis by inhibiting myeloid-derived suppressor cells. Cancer Res. 2011;71:2664–2674. doi: 10.1158/0008-5472.CAN-10-3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wakimoto N, Wolf T, Yin D, O'Kelly J, Akagi T, Abramovitz L, Black KL, Tai HH, Koeffler HP. Nonsteroidal anti-inflammatory drugs suppress glioma via 15-hydroxyprostaglandin dehydrogenase. Cancer Res. 2008;68:6978–6986. doi: 10.1158/0008-5472.CAN-07-5675. [DOI] [PubMed] [Google Scholar]
- 11.Ulrich CM, Bigler J, Potter JD. Non-steroidal anti-inflammatory drugs for cancer prevention: promise, perils and pharmacogenetics. Nat Rev Cancer. 2006;6:130–140. doi: 10.1038/nrc1801. [DOI] [PubMed] [Google Scholar]
- 12.Scheurer ME, El-Zein R, Thompson PA, Aldape KD, Levin VA, Gilbert MR, Weinberg JS, Bondy ML. Long-term anti-inflammatory and antihistamine medication use and adult glioma risk. Cancer Epidemiol Biomarkers Prev. 2008;17:1277–1281. doi: 10.1158/1055-9965.EPI-07-2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferris JS, McCoy L, Neugut AI, Wrensch M, Lai R. HMG CoA reductase inhibitors, NSAIDs and risk of glioma. Int J Cancer. 2012;131:E1031–1037. doi: 10.1002/ijc.27536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sivak-Sears NR, Schwartzbaum JA, Miike R, Moghadassi M, Wrensch M. Case-control study of use of nonsteroidal antiinflammatory drugs and glioblastoma multiforme. Am J Epidemiol. 2004;159:1131–1139. doi: 10.1093/aje/kwh153. [DOI] [PubMed] [Google Scholar]
- 15.Daugherty SE, Moore SC, Pfeiffer RM, Inskip PD, Park Y, Hollenbeck A, Rajaraman P. Nonsteroidal anti-inflammatory drugs and glioma in the NIH-AARP Diet and Health Study cohort. Cancer Prev Res (Phila) 2011;4:2027–2034. doi: 10.1158/1940-6207.CAPR-11-0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scheurer ME, Amirian ES, Davlin SL, Rice T, Wrensch M, Bondy ML. Effects of antihistamine and anti-inflammatory medication use on risk of specific glioma histologies. Int J Cancer. 2011;129:2290–2296. doi: 10.1002/ijc.25883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gaist D, Garcia-Rodriguez LA, Sorensen HT, Hallas J, Friis S. Use of low-dose aspirin and non-aspirin nonsteroidal anti-inflammatory drugs and risk of glioma: a case-control study. Br J Cancer. 2013;108:1189–1194. doi: 10.1038/bjc.2013.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bannon FJ, O'Rorke MA, Murray LJ, Hughes CM, Gavin AT, Fleming SJ, Cardwell CR. Non-steroidal anti-inflammatory drug use and brain tumour risk: a case-control study within the Clinical Practice Research Datalink. Cancer Causes Control. 2013;24:2027–2034. doi: 10.1007/s10552-013-0279-9. [DOI] [PubMed] [Google Scholar]
- 19.Cook NR, Lee IM, Gaziano JM, Gordon D, Ridker PM, Manson JE, Hennekens CH, Buring JE. Low-dose aspirin in the primary prevention of cancer: the Women's Health Study: a randomized controlled trial. JAMA. 2005;294:47–55. doi: 10.1001/jama.294.1.47. [DOI] [PubMed] [Google Scholar]
- 20.Friis S, Sorensen HT, McLaughlin JK, Johnsen SP, Blot WJ, Olsen JH. A population-based cohort study of the risk of colorectal and other cancers among users of low-dose aspirin. Br J Cancer. 2003;88:684–688. doi: 10.1038/sj.bjc.6600760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorensen HT, Friis S, Norgard B, Mellemkjaer L, Blot WJ, McLaughlin JK, Ekbom A, Baron JA. Risk of cancer in a large cohort of nonaspirin NSAID users: a population-based study. Br J Cancer. 2003;88:1687–1692. doi: 10.1038/sj.bjc.6600945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA, Cochrane Bias Methods G, Cochrane Statistical Methods G. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa: Ottawa Hospital Research Institute; 2009. [Google Scholar]
- 25.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 26.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22:719–748. [PubMed] [Google Scholar]
- 27.Midgette AS, Wong JB, Beshansky JR, Porath A, Fleming C, Pauker SG. Cost-effectiveness of streptokinase for acute myocardial infarction: a combined meta-analysis and decision analysis of the effects of infarct location and of likelihood of infarction. Med Decis Making. 1994;14:108–117. doi: 10.1177/0272989X9401400203. [DOI] [PubMed] [Google Scholar]
- 28.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Copas J, Shi JQ. Meta-analysis, funnel plots and sensitivity analysis. Biostatistics. 2000;1:247–262. doi: 10.1093/biostatistics/1.3.247. [DOI] [PubMed] [Google Scholar]
- 31.Galbraith RF. A note on graphical presentation of estimated odds ratios from several clinical trials. Stat Med. 1988;7:889–894. doi: 10.1002/sim.4780070807. [DOI] [PubMed] [Google Scholar]
- 32.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50:1088–1101. [PubMed] [Google Scholar]
- 33.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greenland S. Quantitative methods in the review of epidemiologic literature. Epidemiol Rev. 1987;9:1–30. doi: 10.1093/oxfordjournals.epirev.a036298. [DOI] [PubMed] [Google Scholar]
- 35.Schlehofer B, Blettner M, Becker N, Martinsohn C, Wahrendorf J. Medical risk factors and the development of brain tumors. Cancer. 1992;69:2541–2547. doi: 10.1002/1097-0142(19920515)69:10<2541::aid-cncr2820691025>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 36.Ratnasinghe LD, Graubard BI, Kahle L, Tangrea JA, Taylor PR, Hawk E. Aspirin use and mortality from cancer in a prospective cohort study. Anticancer Res. 2004;24:3177–3184. [PubMed] [Google Scholar]
- 37.Rothwell PM, Fowkes FGR, Belch JFF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: analysis of individual patient data from randomised trials. Lancet. 2011;377:31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- 38.Thun MJ, Namboodiri MM, Calle EE, Flanders WD, Heath CW., Jr Aspirin use and risk of fatal cancer. Cancer Res. 1993;53:1322–1327. [PubMed] [Google Scholar]
- 39.Zhao YS, Zhu S, Li XW, Wang F, Hu FL, Li DD, Zhang WC, Li X. Association between NSAIDs use and breast cancer risk: a systematic review and meta-analysis. Breast Cancer Res Treat. 2009;117:141–150. doi: 10.1007/s10549-008-0228-6. [DOI] [PubMed] [Google Scholar]
- 40.Xu JL, Yin ZQ, Gao W, Liu LX, Wang RS, Huang PW, Yin YM, Liu P, Yu RB, Shu YQ. Meta-analysis on the association between nonsteroidal anti-inflammatory drug use and lung cancer risk. Clin Lung Cancer. 2012;13:44–51. doi: 10.1016/j.cllc.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 41.Tian WJ, Zhao YS, Liu SY, Li X. Meta-analysis on the relationship between nonsteroidal anti-inflammatory drug use and gastric cancer. Eur J Cancer Prev. 2010;19:288–298. doi: 10.1097/CEJ.0b013e328339648c. [DOI] [PubMed] [Google Scholar]
- 42.Din FVN, Theodoratou E, Farrington SM, Tenesa A, Barnetson RA, Cetnarskyj R, Stark L, Porteous ME, Campbell H, Dunlop MG. Effect of aspirin and NSAIDs on risk and survival from colorectal cancer. Gut. 2010;59:1670–1679. doi: 10.1136/gut.2009.203000. [DOI] [PubMed] [Google Scholar]
- 43.Ni XJ, Ma JJ, Zhao YC, Wang Y, Wang S. Meta-analysis on the association between non-steroidal anti-inflammatory drug use and ovarian cancer. Br J Clin Pharmacol. 2013;75:26–35. doi: 10.1111/j.1365-2125.2012.04290.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Easterbrook PJ, Berlin JA, Gopalan R, Matthews DR. Publication bias in clinical research. Lancet. 1991;337:867–872. doi: 10.1016/0140-6736(91)90201-y. [DOI] [PubMed] [Google Scholar]
- 45.Ioannidis JP, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ. 2007;335:914–916. doi: 10.1136/bmj.39343.408449.80. [DOI] [PMC free article] [PubMed] [Google Scholar]