Abstract
Purpose: Experimental study of a new system for digital 2D and 3D full-field mammography (FFDM) using a high resolution detector based on two shifts of a-Se. Material and Methods: Images were acquired using the new FFDM system Amulet® (FujiFilm, Tokio, Japan), an a-Se detector (receptor 24 × 30 cm2, pixel size 50 µm, memory depth 12 bit, spatial resolution 10 lp/mm, DQE > 0.50). Integrated in the detector is a new method for data transfer, based on optical switch technology. The object of investigation was the Wisconsin Mammographic Random Phantom, Model 152A (Radiation Measurement Inc., Middleton, WI, USA) and the same parameters and exposure data (Tungsten, 100 mAs, 30 kV) were consistently used. We acquired 3 different pairs of images in the c-c and ml planes (2D) and in the c-c and c-c planes with an angle of 4 degrees (3D). Five radiologists experienced in mammography (experience ranging from 3 months to more than 5 years) analyzed the images (monitoring) which had been randomly encoded (random generator) with regard to the recognition of details such as specks of aluminum oxide (200–740 µm), nylon fibers (0.4–1.6 mm) and round lesions/masses (diameters 5–14 mm), using special linear glasses for 3D visualization, and compared the results. Results: A total of 225 correct positive decisions could be detected: we found 222 (98.7 %) correct positive results for 2D and 3D visualization in each case. Conclusion: The results of this phantom study showed the same detection rates for both 2D and 3D imaging using full field digital mammography. Our results must be confirmed in further clinical trials.
Key words: breast, breast tumor, mammographic density
Abstract
Zusammenfassung
Ziel: Experimentelle Studie mit einem System zur Erstellung digitaler 2-D- resp. 3-D-Vollfeld-Mammografien (FFDM) und einem neu entwickelten hochauflösenden Detektor auf der Basis von 2 Shifts a-Selens. Material und Methode: Die Untersuchungen wurden durchgeführt mit dem FFDM-System Amulet® (FujiFilm, Tokio, Japan): a-Se-Detektor (Rezeptor 24 × 30 cm2, Pixelgröße 50 µm, Speichertiefe 12 Bit, Auflösung 10 Lp/mm, DQE > 0,50). Integriert in den Detektor ist eine neue Methode des Datentransfers, basierend auf der Optical Switch Technology. Untersuchungsobjekt war das Wisconsin Mammographic Random Phantom, Model 152 A (Radiation Measurement Inc., Middleton, WI, USA). Es bestanden immer gleiche Untersuchungsparameter und Belichtungsdaten (Wolfram, 100 mAs, 30 kV). Wir fertigten 3 unterschiedliche Bilder (Aufnahmepaare) in c-c und ml Ebene (2-D) resp. in c-c und c-c Ebene mit einem Winkel von plus 4° (3-D) an. Fünf in der Mammografie erfahrene Radiologen (3 Monate bis mehr als 5 Jahre) werteten die zufallsverteilten Bilder (Monitoring) in Hinblick auf die Detektion von Aluminiumoxidpartikeln (200–740 µm), Nylonfäden (0,4–1,6 mm) und Rundherden (Durchmesser 5–14 mm), wobei zur 3-D-Visualisierung eine lineare Polfilterbrille (3-D) verwandt wurde, aus und verglichen die Ergebnisse. Ergebnisse: 225 richtig positive Entscheidungen waren möglich: Wir fanden für die 2-D- und 3-D-Visualisierung jeweils 222 (98,7 %) richtig positive Ergebnisse. Schlussfolgerung: Die Ergebnisse dieser Phantomstudie demonstrieren sowohl für die 2-D- als auch 3-D-Bildgebung in der digitalen Vollfeld-Mammografie gleiche Entdeckungsraten. Weitere klinische Studien sind hierzu notwendig.
Schlüsselwörter: Mamma, Mammakarzinom, mammografische Dichte
Introduction
The development of digital image receptor systems in mammography has progressed to such an extent over the past few years that, in addition to their conventional application, digital mammography systems can now also be used as a platform for further, new examination methods such as contrast mammography or tomosynthesis 1, 2, 3, 4, 5, 6.
Digital breast tomosynthesis (DBT) has the potential to remove undesirable masking as a result of superimposed layers and thus reduces false-positive or false-negative examination findings. Whether DBT can be regarded as an alternative to digital mammography or whether it is just an additional examination method in assessment diagnostics is as yet not clear 7, 8, 9, 10, 11, 12, 13, 14.
A novel method comprising 2 stereo mammography images (stereoscopy) (Fig. 3) using linearly polarised filter glasses produces a 3-dimensional (3D) overall image (visualisation) of the breast.
Fig. 3 a.

to c 3D digital full field mammography. Monitoring with mammographies in the c-c view and c-c view with an angle of plus 4° (stereoscopy), respectively (a and b) with the use of linearly polarised filter glasses in order to obtain a holistic 3D image of the breast (c).
The aim of the pilot study was to examine the detection of simulated mammographic lesions (micro-calcifications and tumour-like masses) with these novel mammography methods and to contrast these with the findings from another, already established mammography system.
Material and Method
For the examinations the Amulet digital mammography system (FujiFilm, Tokyo, Japan) was used. This system operates with a 24 × 30 cm2 detector which is firmly integrated into the system 15, 16. This totally novel detector is constructed from two superimposed layers of high-purity amorphous selenium, separated by a very thin layer of selenium that is just 1 µm thick and has been doped in a targeted manner with foreign atoms. This detector achieves a pixel size of 50 µm2.
The mammography system is fitted with a bimetal x-ray tube, which offers the options of molybdenum or tungsten as anode material in combination with molybdenum or rhodium filters. Within the scope of the present investigation all images were made with a manually set molybdenum/molybdenum combination and an x-ray current-time product of 100 mAs at a tube voltage of 30 kV (Table 1).
Table 1 Parameters of the mammography system during examination.
| Amulet | |
|---|---|
| Manufacturer | FujiFilm |
| Anode | Mo, W |
| Filter | Mo, Rh |
| Scattered radiation grid | linear |
| Conversion material | Semiconductora-Se |
| Sampling process | Optically induced sampling |
| Pixel size | 50 µm |
| Spatial resolution (Nyquist Frequency) | 10 lp/mm |
| Field size | 24 × 30 cm2 |
We initially took 3 different images (image pairs) in c-c and ml views (2D) and in the c-c view and c-c view with an angle of plus 4° (stereoscopy), respectively.
As in earlier studies 17 the Wisconsin Mammographic Random Phantom (Model 152 A, Gammex Inc.) was selected as test object (Fig. 1). This phantom contains a total of 5 elements with microcalcifications, 6 with thread-like structures and 4 with tumour-like masses. These 15 simulated lesions are located in 15 separate wax blocks in the phantom; in addition one empty wax block without lesions is present. The wax blocks are interchangeable, so that different distribution patterns in the phantom can be realised. A learning effect can thus be avoided for the evaluators. The complete phantom recreates a standard breast with a compression layer thickness of approx. 4.5 to 5 cm.
Fig. 1.
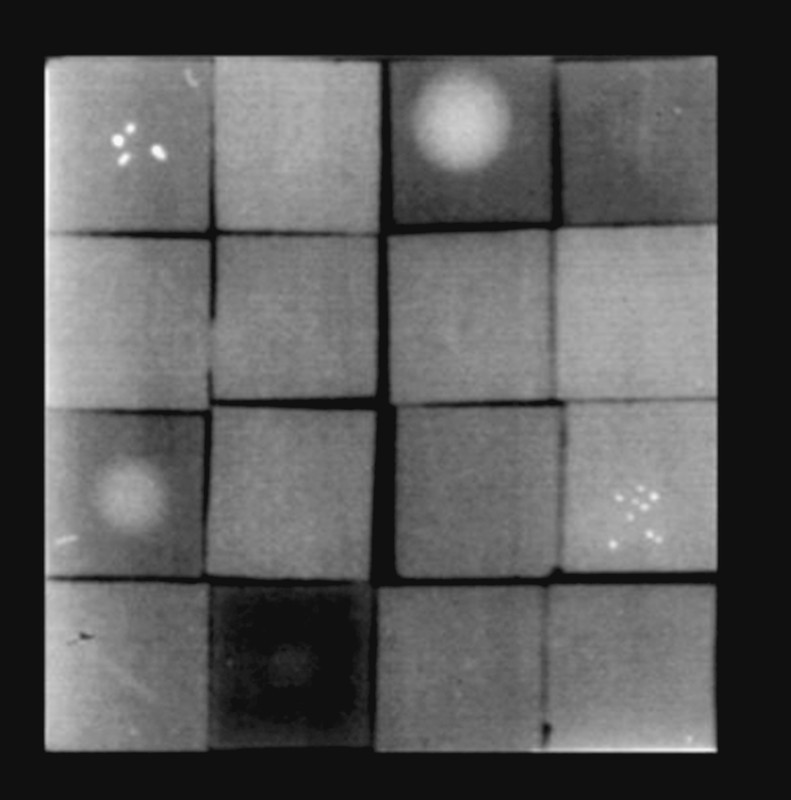
Wisconsin RMI Phantom, Model 152 A, Radiation Measurements, Middleton, WI, USA. 16 wax blocks with 4 round lumps, 5 calcifications, 6 threads, 1 empty, raised radiolucent position 4.5 cm, net density 1.5.
The evaluation of the phantom image was performed on a 2.5 × 2.5 k monitor by five radiologists with varying years of mammographic experience. Each radiologist was shown 3 image pairs (images in c-c and ml views) (2D) with different phantom compositions and in the c-c view and c-c view with an angle of plus 4°, respectively, using linearly polarised glasses (3D) for evaluation. In linearly polarised filter glasses the light is polarised linearly, i.e. the light oscillates within a plane determined by the filter. Here the filters must be positioned at a right angle to one another for the left and right viewing position in order to enable the separation of the two views and to achieve a 3D visualisation (Figs. 2 and 3). The viewing time per image was limited to a maximum of 5 minutes. As part of the study the detection sensitivity with the new mammography method was to be evaluated – for this reason the correct positive rate for each individual lesion type was determined.
Fig. 2 a.
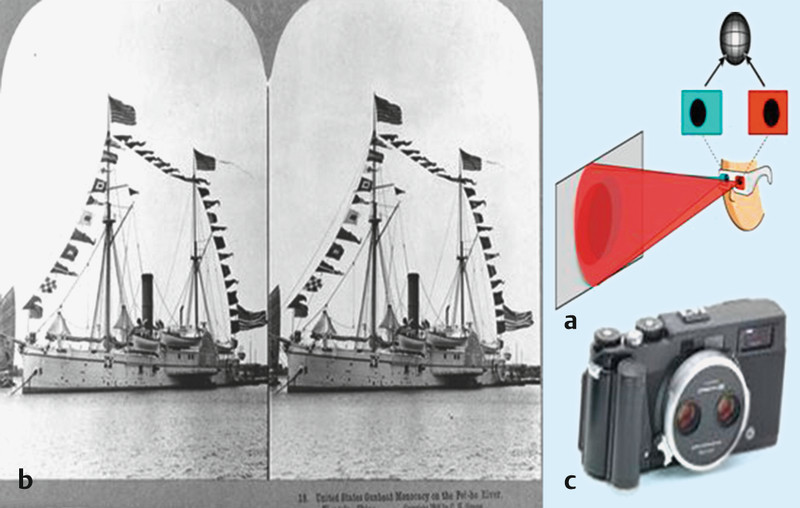
to c Stereoscopy. The distance between the human eyes is roughly 65 mm, i.e. each eye has a different view of the three-dimensional world. Through these 2 different viewpoints of a virtual system we are able to determine the relative depth of different objects in the object viewed, because the 2 independent images are combined within the brain to determine the depth (a and b). This process is used in photography by making 2 standard images (0° and 4° projection) in order to simulate natural sight (c).
Findings
Table 2 lists the findings of the study determined by the 5 evaluators, broken down according to the digital mammography system used, the different examination methods (2D and 3D respectively) and the lesion type. Additionally, the number of lesions actually present and the resulting detection rate, as well as the mean finding determined on the basis of the three lesion types, are given.
Table 2 Number of correctly detected simulated lesions using the digital mammography system and the detection rate in per cent averaged over the five evaluators, each three phantom configurations (c-c and ml plane [2D] and c-c and c-c plus 4° plane, respectively [3D]).
| Amuletc-c and oblique plane2D | Amuletc-c and c-c plus 4° plane3D | Really existing lesions | |
|---|---|---|---|
| Threads | 18.0 (100 %) | 18.0 (100 %) | 18 |
| Microcalcifications | 14.3 (95.6 %) | 14.3 (95.6 %) | 15 |
| Tumour-like masses | 12.0 (100 %) | 12.0 (100 %) | 12 |
| All lesions | 44.4 (98.7 %) | 44.4 (98.7 %) | 45 |
With the digital mammography system and the dual layer selenium detector a detection rate of 97.7 % was computed both for each of the 3 image pairs (images in the c-c and ml views) (2D) with differing phantom composition and the 3 image pairs in the c-c view and c-c plane with an angle of plus 4° (stereoscopy), respectively, using linearly polarised filter glasses for visualisation (3D).
Discussion
The most important image-producing method for the early diagnosis of breast cancer remains x-ray mammography. It is the only method with proven use as a quality-assured screening method to lower the breast cancer mortality rate. Full-field digital mammography, or FFDM, is today regarded as the standard mammographic method, both in curative mammography and in the preventive mammographic screening of women without any symptoms and, in particular, is more effective for the detection of pathological findings in women with dense breasts than standard film foil mammography 1, 2, 3, 4, 5, 6. Nonetheless, as with all radiological projection methods, digital mammography suffers from the fact that it depicts three-dimensional information as a two-dimensional image. Superimposed structures are projected onto one image plane, so that lesions of clinical relevance can easily be covered and their viewing obstructed by overlapping tissue. This increases the frequency of false-negative examination findings, i.e. existing carcinomas are overlooked. Such overlapping of normal breast tissue may also result in false-positive examination results, in that they mock malign lesions which then lead to the patient having to undergo an unnecessary repeat examination and may even result in an unnecessary biopsy.
The development of digital image receptor systems in mammography has progressed to such an extent over the past few years that, in addition to their conventional application, digital mammography systems can now also be used as a platform for further, new examination methods such as contrast mammography or tomosynthesis. Digital breast tomosynthesis, or DBT, is an imaging technology which can deliver layered images free from overlaps on the basis of a limited number of individual images taken at different projection angles. The exposure parameters for each individual layer are selected in such a way that the radiation exposure resulting from all images taken is preferably lower than the radiation dose from a 2-plane mammography. With the aid of different reconstruction algorithms the breast is subsequently visualised in the layers of interest at various depths parallel to the detector surface.
Digital breast tomosynthesis has the potential to remove undesirable masking as a result of superimposed layers and thus reduces false-positive or false-negative examination findings. Whether DBT can be regarded as an alternative to digital mammography or whether it is just an additional examination method in assessment diagnostics is as yet not clear 7, 8, 9, 10, 11, 12, 13, 14.
A novel method on the basis of 2 mammography images (c-c and c-c plus 4°) (stereoscopy) (Fig. 2) has been developed in order to obtain a three-dimensional overall image of the breast (3D) (Fig. 3) with the use of linearly polarised filter glasses.
The present study is the first phantom study based on a digital mammography system (Table 1) to compare the detection of simulated microcalcifications, thread-like structures and tumour-like masses in 2D and 3D visualisation (Fig. 1) 15, 16. The detected findings listed in Table 2 do not, however, show any differences. While the simulated microcalcifications could be detected with an efficiency of up to 95.6 % with both visualisation methods – 100 % of the thread-like structures and tumour-type masses were detected. This shows that the phantom used is at least sufficient for a rough orientation, but is not sufficient for a differentiated evaluation of powerful digital imaging systems. This is also confirmed by the fact that, in terms of the phantom examination, no great differences in the detection rates occurred despite the great discrepancy in experience between the 5 evaluators (from 3 months to 5 years).
Despite all limitations, the present findings with the Wisconsin Mammographic Random Phantom (Fig. 1) 17 must be evaluated as meeting all necessary minimum requirements. They cannot furnish an adequate basis for unlimited clinical application of the new system. Such conclusive findings could only be furnished by a comprehensive study on the contrast resolution capability with the currently valid national and international standard phantom CDMAM Phantom 18, 19. However, even with this test specimen, disregarding any anatomical noise only objects in front of a homogenous background can be detected. Furthermore, newly developed phantom systems exist 20, 21, the final findings of which are yet to be determined.
The new method for the three-dimensional representation of the breast in one holistic image with the use of linearly polarised filter glasses represents an important innovation, in particular in comparison with tomosynthesis 22. In addition, at the same time it is the deciding step towards a real, so to speak, online 3D visualisation of the breast in future made available at any desired workplace (PACS). To this end, besides the respective software, monitors with a resolution of at least 3.5 × 3.5 k are necessary, making additional aids (e.g. glasses) obsolete. Developments in this respect are still in the experimental stage. Whether an improvement in the detection rate of examination findings to be clarified (correct positive findings) while at the same time reducing false-negative findings – also by using computer-aided diagnostic systems (CAD) – can actually be achieved, remains to be seen. However, the prospect of real 3D-controlled intervention and planning oncology management, including plastic surgery, appears to be promising.
Footnotes
Conflict of Interest None.
Supporting Information
German supporting information for this article
References
- 1.Pisano E D, Gatsonis C, Hendrick E. et al. Diagnostic performance of digital versus film mammography for breast-cancer screening. N Engl J Med. 2006;353:1773–1783. doi: 10.1056/NEJMoa052911. [DOI] [PubMed] [Google Scholar]
- 2.Diekmann F, Bick U. Tomosynthesis and contrast-enhanced digital mammography: recent advances in digital mammography. Eur Radiol. 2007;17:3086–3092. doi: 10.1007/s00330-007-0715-x. [DOI] [PubMed] [Google Scholar]
- 3.Skaane P. Studies comparing screen-film mammography and full-field digital mammography in breast cancer screening: updated review. Acta Radiol. 2009;50:3–14. doi: 10.1080/02841850802563269. [DOI] [PubMed] [Google Scholar]
- 4.Schulz-Wendtland R, Fuchsjäger M, Wacker T. et al. Digital mammography: an update. Eur J Radiol. 2009;72:258–265. doi: 10.1016/j.ejrad.2009.05.052. [DOI] [PubMed] [Google Scholar]
- 5.Heywang-Köbrunner S H, Hacker A, Sedlacek S. Contrast-enhanced MRI for early detection and staging of breast cancer: do we need it? Geburtsh Frauenheilk. 2010;70:184–193. [Google Scholar]
- 6.Ruckhäberle E, Rody A, Kaufmann M. Report of the 32nd Annual San Antonio Breast Cancer Symposium, December 10–13, 2009. An international scientific symposium for interaction and exchange among basic scientists and clinicians in breast cancer. Geburtsh Frauenheilk. 2010;70:177–183. [Google Scholar]
- 7.Poplack S P, Tosteson T D, Kogel C A. et al. Digital breast tomosynthesis: initial experience in 98 women with abnormal digital screening mammography. AJR. 2007;189:616–623. doi: 10.2214/AJR.07.2231. [DOI] [PubMed] [Google Scholar]
- 8.Andersson I, Ikeda D M, Zackrisson S. et al. Breast tomosynthesis and digital mammography: a comparison of breast cancer visibility and BIRADS classification in a population of cancers with subtle mammographic findings. Eur Radiol. 2008;18:2817–2825. doi: 10.1007/s00330-008-1076-9. [DOI] [PubMed] [Google Scholar]
- 9.Good W F, Abrams G S, Catullo V J. et al. Digital breast tomosynthesis: a pilot observer study. AJR. 2008;190:865–869. doi: 10.2214/AJR.07.2841. [DOI] [PubMed] [Google Scholar]
- 10.Smith A P, Rafferty E A, Niklason L. Clinical performance of breast tomosynthesis as a function of radiologist experience level. LNCS. 2008;5116:61–66. [Google Scholar]
- 11.Gur D, Abrams G S, Chough D M. et al. Digital breast tomosynthesis: observer performance study. AJR. 2009;193:586–591. doi: 10.2214/AJR.08.2031. [DOI] [PubMed] [Google Scholar]
- 12.Kontos D, Bakic P R, Carton A K. et al. Parenchymal texture analysis in digital breast tomosynthesis for breast cancer risk estimation: a preliminary study. Acad Radiol. 2009;16:283–298. doi: 10.1016/j.acra.2008.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fömvik D, Zackrisson S, Ljunberg O. et al. Breast tomosynthesis: Accuracy of tumor measurement compared with digital mammography and ultrasonography. Acta Radiologica. 2010;51:240–247. doi: 10.3109/02841850903524447. [DOI] [PubMed] [Google Scholar]
- 14.Teertstra H J, Loo C E, van den Bosch M A. et al. Breast tomosynthesis in clinical practice: initial results. Eur Radiol. 2010;20:16–24. doi: 10.1007/s00330-009-1523-2. [DOI] [PubMed] [Google Scholar]
- 15.Schulz-Wendtland R, Hermann K P, Wenkel E. et al. First experiments for the detection of simulated mammographic lesions: Digital full field mammography with a new detector with a double plate of pure selenium. Radiologe. 2011;51:130–134. doi: 10.1007/s00117-010-2077-7. [DOI] [PubMed] [Google Scholar]
- 16.Schulz-Wendtland R, Hermann K P, Adamietz B. et al. Comparison of dignity determination of mammographic microcalcification with two systems for digital full-field mammography with different detector resolution: A retrospective clinical study (n = 50) Radiologe. 2011;51:126–129. doi: 10.1007/s00117-010-2078-6. [DOI] [PubMed] [Google Scholar]
- 17.Schulz-Wendtland R, Hermann K P, Lell M. et al. Phantomstudie zur Detektion simulierter Läsionen an fünf verschiedenen digitalen und einem konventionellen Mammographiesystem. Fortschr Röntgenstr. 2004;176:1127–1132. doi: 10.1055/s-2004-813261. [DOI] [PubMed] [Google Scholar]
- 18.Blendl C, Hermann K P, Mertelmeier T. Berlin: Beuth Verlag; 2005. PAS 1054: Anforderungen und Prüfverfahren für digitale Mammographie-Einrichtungen. [Google Scholar]
- 19.van Engen R, van Woudenberg S, Bosmans H, Luxembourg: European Communities; 2006. European Protocol for the Quality Control of the physical and technical Aspects of Mammography Screening; pp. 157–166. [Google Scholar]
- 20.Carton A K, Bakic P, Ullberg C. et al. Development of a physical 3D anthropomorphic breast phantom. Med Phys. 2011;38:891–896. doi: 10.1118/1.3533896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Engelken F J Sack I Klatt D et al. Evaluation of tomosynthesis elastography in a breast-mimicking phantom Eur J Radiol 2011. Jul 1 [epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 22.Webb L J, Samei E, Lo J Y. et al. Comparative performance of multiview stereoscopic and mammographic display modalities for breast lesion detection. Med Phys. 2011;38:1972–1980. doi: 10.1118/1.3562901. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
German supporting information for this article


