Abstract
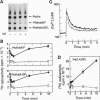
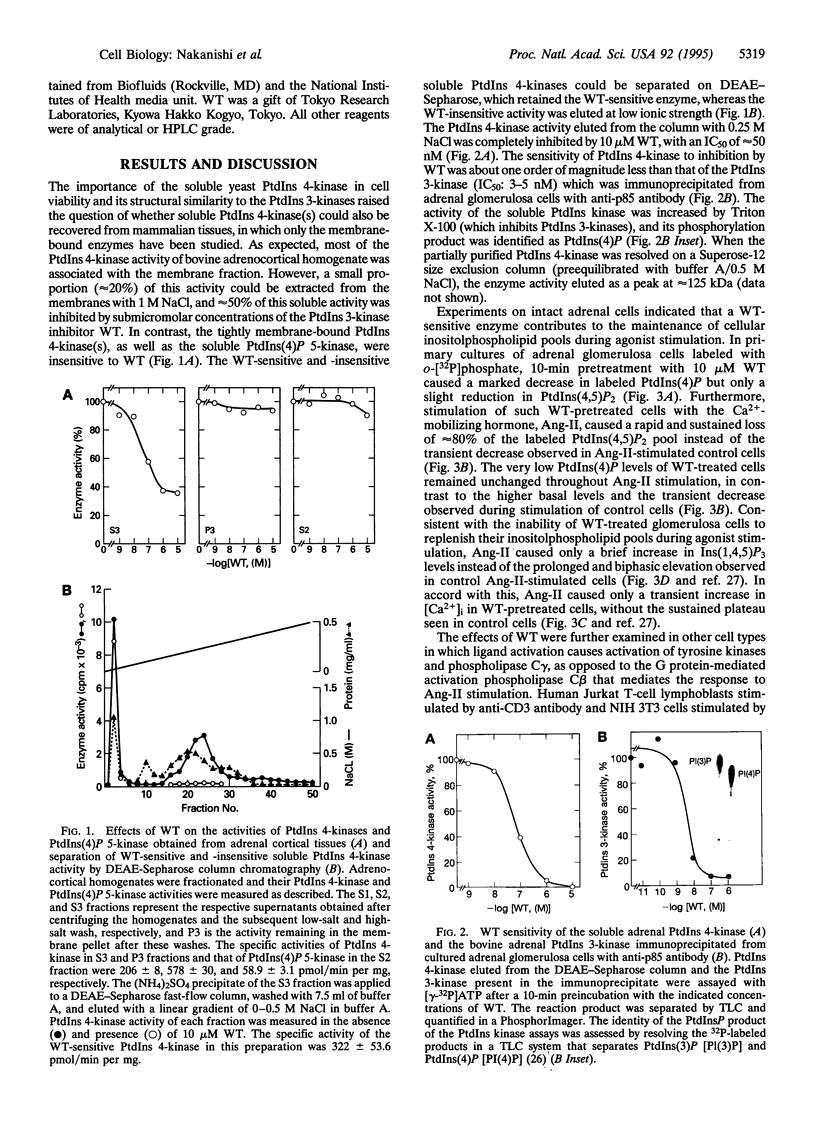
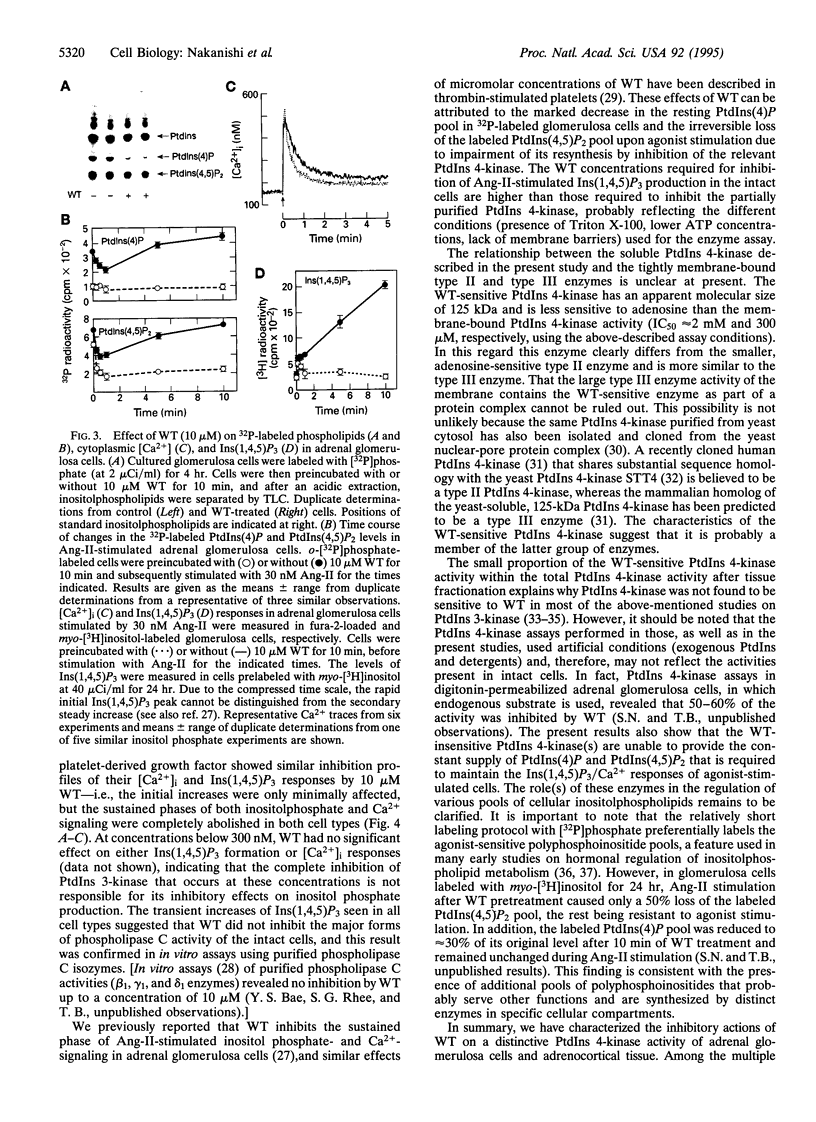
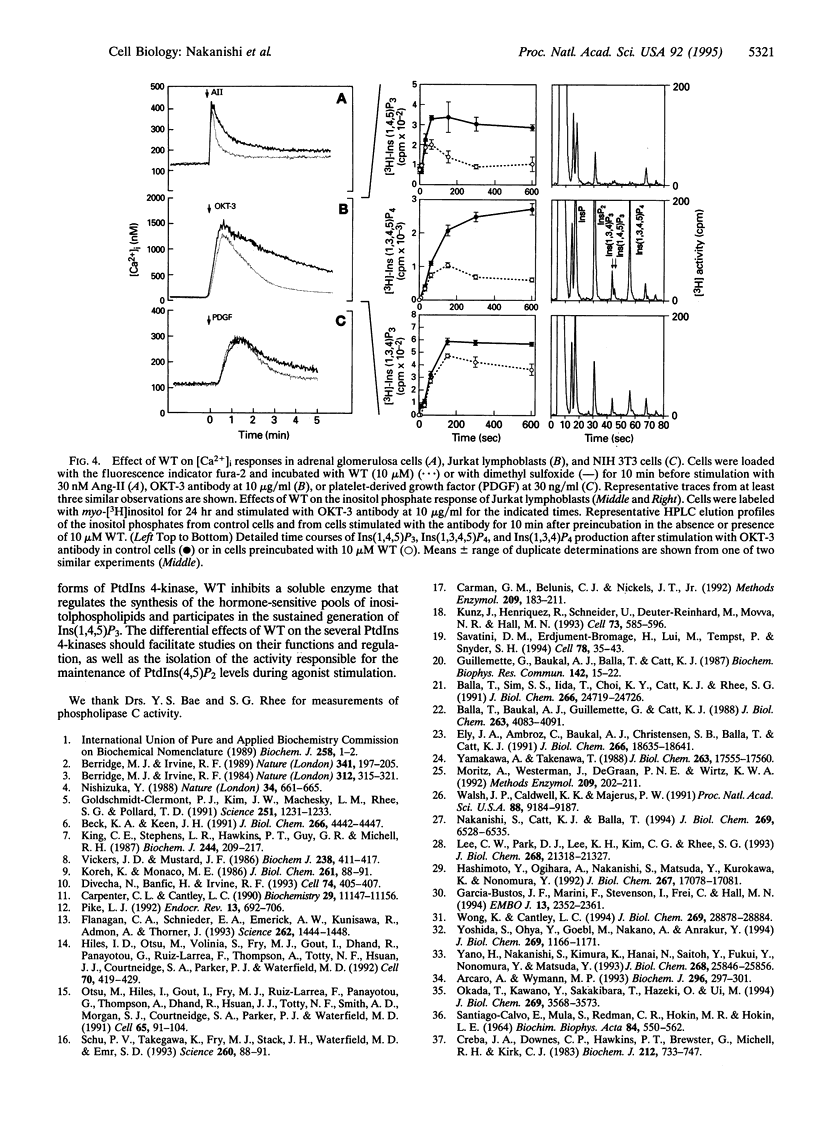
The synthesis of phosphatidylinositol 4,5-bisphosphate [PtdIns(4,5)P2], the immediate precursor of intracellular signals generated by calcium-mobilizing hormones and growth factors, is initiated by the conversion of phosphatidylinositol to phosphatidylinositol 4-phosphate [PtdIns(4)P] by phosphatidylinositol 4-kinase (PtdIns 4-kinase). Although cells contain several PtdIns 4-kinases, the enzyme responsible for regulating the synthesis of hormone-sensitive PtdIns(4,5)P2 pools has not been identified. In this report we describe the inhibitory effect of micromolar concentrations of wortmannin (WT) on the synthesis of hormone-sensitive PtdIns(4)P and PtdIns(4,5)P2 pools in intact adrenal glomerulosa cells, and the presence of a WT-sensitive PtdIns 4-kinase in adrenocortical extracts. In addition to its sensitivity to the PtdIns 3-kinase inhibitor WT, this enzyme is distinguished from the recognized membrane-bound PtdIns 4-kinases by its molecular size and weak membrane association. Inhibition of this PtdIns 4-kinase by WT results in rapid loss of the hormone-sensitive PtdIns(4,5)P2 pool in angiotensin II-stimulated glomerulosa cells. Consequently, WT treatment inhibits the sustained but not the initial increases in inositol 1,4,5-trisphosphate and cytoplasmic [Ca2+] in a variety of agonist-stimulated cells, including adrenal glomerulosa cells, NIH 3T3 fibroblasts, and Jurkat lymphoblasts. These results indicate that a specific WT-sensitive PtdIns 4-kinase is critical for the maintenance of the agonist-sensitive polyphosphoinositide pool in several cell types.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balla T., Baukal A. J., Guillemette G., Catt K. J. Multiple pathways of inositol polyphosphate metabolism in angiotensin-stimulated adrenal glomerulosa cells. J Biol Chem. 1988 Mar 25;263(9):4083–4091. [PubMed] [Google Scholar]
- Balla T., Sim S. S., Iida T., Choi K. Y., Catt K. J., Rhee S. G. Agonist-induced calcium signaling is impaired in fibroblasts overproducing inositol 1,3,4,5-tetrakisphosphate. J Biol Chem. 1991 Dec 25;266(36):24719–24726. [PubMed] [Google Scholar]
- Beck K. A., Keen J. H. Interaction of phosphoinositide cycle intermediates with the plasma membrane-associated clathrin assembly protein AP-2. J Biol Chem. 1991 Mar 5;266(7):4442–4447. [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Carman G. M., Belunis C. J., Nickels J. T., Jr Phosphatidylinositol 4-kinase from yeast. Methods Enzymol. 1992;209:183–189. doi: 10.1016/0076-6879(92)09022-u. [DOI] [PubMed] [Google Scholar]
- Carpenter C. L., Cantley L. C. Phosphoinositide kinases. Biochemistry. 1990 Dec 25;29(51):11147–11156. doi: 10.1021/bi00503a001. [DOI] [PubMed] [Google Scholar]
- Creba J. A., Downes C. P., Hawkins P. T., Brewster G., Michell R. H., Kirk C. J. Rapid breakdown of phosphatidylinositol 4-phosphate and phosphatidylinositol 4,5-bisphosphate in rat hepatocytes stimulated by vasopressin and other Ca2+-mobilizing hormones. Biochem J. 1983 Jun 15;212(3):733–747. doi: 10.1042/bj2120733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Divecha N., Banfić H., Irvine R. F. Inositides and the nucleus and inositides in the nucleus. Cell. 1993 Aug 13;74(3):405–407. doi: 10.1016/0092-8674(93)80041-c. [DOI] [PubMed] [Google Scholar]
- Ely J. A., Ambroz C., Baukal A. J., Christensen S. B., Balla T., Catt K. J. Relationship between agonist- and thapsigargin-sensitive calcium pools in adrenal glomerulosa cells. Thapsigargin-induced Ca2+ mobilization and entry. J Biol Chem. 1991 Oct 5;266(28):18635–18641. [PubMed] [Google Scholar]
- Flanagan C. A., Schnieders E. A., Emerick A. W., Kunisawa R., Admon A., Thorner J. Phosphatidylinositol 4-kinase: gene structure and requirement for yeast cell viability. Science. 1993 Nov 26;262(5138):1444–1448. doi: 10.1126/science.8248783. [DOI] [PubMed] [Google Scholar]
- Garcia-Bustos J. F., Marini F., Stevenson I., Frei C., Hall M. N. PIK1, an essential phosphatidylinositol 4-kinase associated with the yeast nucleus. EMBO J. 1994 May 15;13(10):2352–2361. doi: 10.1002/j.1460-2075.1994.tb06519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt-Clermont P. J., Kim J. W., Machesky L. M., Rhee S. G., Pollard T. D. Regulation of phospholipase C-gamma 1 by profilin and tyrosine phosphorylation. Science. 1991 Mar 8;251(4998):1231–1233. doi: 10.1126/science.1848725. [DOI] [PubMed] [Google Scholar]
- Guillemette G., Baukal A. J., Balla T., Catt K. J. Angiotensin-induced formation and metabolism of inositol polyphosphates in bovine adrenal glomerulosa cells. Biochem Biophys Res Commun. 1987 Jan 15;142(1):15–22. doi: 10.1016/0006-291x(87)90445-1. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Ogihara A., Nakanishi S., Matsuda Y., Kurokawa K., Nonomura Y. Two thrombin-activated Ca2+ channels in human platelets. J Biol Chem. 1992 Aug 25;267(24):17078–17081. [PubMed] [Google Scholar]
- Hiles I. D., Otsu M., Volinia S., Fry M. J., Gout I., Dhand R., Panayotou G., Ruiz-Larrea F., Thompson A., Totty N. F. Phosphatidylinositol 3-kinase: structure and expression of the 110 kd catalytic subunit. Cell. 1992 Aug 7;70(3):419–429. doi: 10.1016/0092-8674(92)90166-a. [DOI] [PubMed] [Google Scholar]
- King C. E., Stephens L. R., Hawkins P. T., Guy G. R., Michell R. H. Multiple metabolic pools of phosphoinositides and phosphatidate in human erythrocytes incubated in a medium that permits rapid transmembrane exchange of phosphate. Biochem J. 1987 May 15;244(1):209–217. doi: 10.1042/bj2440209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koréh K., Monaco M. E. The relationship of hormone-sensitive and hormone-insensitive phosphatidylinositol to phosphatidylinositol 4,5-bisphosphate in the WRK-1 cell. J Biol Chem. 1986 Jan 5;261(1):88–91. [PubMed] [Google Scholar]
- Kunz J., Henriquez R., Schneider U., Deuter-Reinhard M., Movva N. R., Hall M. N. Target of rapamycin in yeast, TOR2, is an essential phosphatidylinositol kinase homolog required for G1 progression. Cell. 1993 May 7;73(3):585–596. doi: 10.1016/0092-8674(93)90144-f. [DOI] [PubMed] [Google Scholar]
- Lee C. W., Park D. J., Lee K. H., Kim C. G., Rhee S. G. Purification, molecular cloning, and sequencing of phospholipase C-beta 4. J Biol Chem. 1993 Oct 5;268(28):21318–21327. [PubMed] [Google Scholar]
- Moritz A., Westerman J., de Graan P. N., Wirtz K. W. Phosphatidylinositol 4-kinase and phosphatidylinositol-4-phosphate 5-kinase from bovine brain membranes. Methods Enzymol. 1992;209:202–211. doi: 10.1016/0076-6879(92)09024-w. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Catt K. J., Balla T. Inhibition of agonist-stimulated inositol 1,4,5-trisphosphate production and calcium signaling by the myosin light chain kinase inhibitor, wortmannin. J Biol Chem. 1994 Mar 4;269(9):6528–6535. [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Okada T., Kawano Y., Sakakibara T., Hazeki O., Ui M. Essential role of phosphatidylinositol 3-kinase in insulin-induced glucose transport and antilipolysis in rat adipocytes. Studies with a selective inhibitor wortmannin. J Biol Chem. 1994 Feb 4;269(5):3568–3573. [PubMed] [Google Scholar]
- Otsu M., Hiles I., Gout I., Fry M. J., Ruiz-Larrea F., Panayotou G., Thompson A., Dhand R., Hsuan J., Totty N. Characterization of two 85 kd proteins that associate with receptor tyrosine kinases, middle-T/pp60c-src complexes, and PI3-kinase. Cell. 1991 Apr 5;65(1):91–104. doi: 10.1016/0092-8674(91)90411-q. [DOI] [PubMed] [Google Scholar]
- Pike L. J. Phosphatidylinositol 4-kinases and the role of polyphosphoinositides in cellular regulation. Endocr Rev. 1992 Nov;13(4):692–706. doi: 10.1210/edrv-13-4-692. [DOI] [PubMed] [Google Scholar]
- SANTIAGO-CALVO E., MULE S., REDMAN C. M., HOKIN M. R., HOKIN L. E. THE CHROMATOGRAPHIC SEPARATION OF POLYPHOSPHOINOSITIDES AND STUDIES ON THEIR TURNOVER IN VARIOUS TISSUES. Biochim Biophys Acta. 1964 Oct 2;84:550–562. doi: 10.1016/0926-6542(64)90125-8. [DOI] [PubMed] [Google Scholar]
- Sabatini D. M., Erdjument-Bromage H., Lui M., Tempst P., Snyder S. H. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell. 1994 Jul 15;78(1):35–43. doi: 10.1016/0092-8674(94)90570-3. [DOI] [PubMed] [Google Scholar]
- Schu P. V., Takegawa K., Fry M. J., Stack J. H., Waterfield M. D., Emr S. D. Phosphatidylinositol 3-kinase encoded by yeast VPS34 gene essential for protein sorting. Science. 1993 Apr 2;260(5104):88–91. doi: 10.1126/science.8385367. [DOI] [PubMed] [Google Scholar]
- Vickers J. D., Mustard J. F. The phosphoinositides exist in multiple metabolic pools in rabbit platelets. Biochem J. 1986 Sep 1;238(2):411–417. doi: 10.1042/bj2380411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh J. P., Caldwell K. K., Majerus P. W. Formation of phosphatidylinositol 3-phosphate by isomerization from phosphatidylinositol 4-phosphate. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):9184–9187. doi: 10.1073/pnas.88.20.9184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakawa A., Takenawa T. Purification and characterization of membrane-bound phosphatidylinositol kinase from rat brain. J Biol Chem. 1988 Nov 25;263(33):17555–17560. [PubMed] [Google Scholar]
- Yano H., Nakanishi S., Kimura K., Hanai N., Saitoh Y., Fukui Y., Nonomura Y., Matsuda Y. Inhibition of histamine secretion by wortmannin through the blockade of phosphatidylinositol 3-kinase in RBL-2H3 cells. J Biol Chem. 1993 Dec 5;268(34):25846–25856. [PubMed] [Google Scholar]
- Yoshida S., Ohya Y., Goebl M., Nakano A., Anraku Y. A novel gene, STT4, encodes a phosphatidylinositol 4-kinase in the PKC1 protein kinase pathway of Saccharomyces cerevisiae. J Biol Chem. 1994 Jan 14;269(2):1166–1172. [PubMed] [Google Scholar]