Abstract
We report the degradation of quorum sensing N-acylhomoserine lactone molecules by a bacterium isolated from a Malaysian marine water sample. MALDI-TOF and phylogenetic analysis indicated this isolate BM1 clustered closely to Labrenzia sp. The quorum quenching activity of this isolate was confirmed by using a series of bioassays and rapid resolution liquid chromatography analysis. Labrenzia sp. degraded a wide range of N-acylhomoserine lactones namely N-(3-hexanoyl)-l-homoserine lactone (C6-HSL), N-(3-oxohexanoyl)-l-homoserine lactone (3-oxo-C6-HSL) and N-(3-hydroxyhexanoyl)-l-homoserine lactone (3-hydroxy-C6-HSL). Re-lactonisation bioassays confirmed Labrenzia sp. BM1 degraded these signalling molecules efficiently via lactonase activity. To the best of our knowledge, this is the first documentation of a Labrenzia sp. capable of degrading N-acylhomoserine lactones and confirmation of its lactonase-based mechanism of action.
Keywords: Labrenzia sp., quorum quenching, quorum sensing, marine, seawater, rapid resolution liquid chromatography (RRLC), N-acylhomoserine lactone, N-(3-oxohexanoyl)-l-homoserine lactone
1. Introduction
Quorum sensing (QS) is a form of cell-cell communication system that allows bacteria to communicate via the production and reception of small diffusible signal molecules, thereby regulating gene expression in response to bacterial cell density [1,2]. Once sufficient concentration of the signal has been reached, gene expression will be modulated either directly by interacting with a transcriptional regulator or indirectly by activating a signaling cascade [3,4]. QS was first discovered as a mechanism that regulated bioluminescence in Vibrio fischeri. Since then, QS has been documented in many different bacteria and various types of signal molecules have been identified [5–7].
Three types of QS molecules exist in bacteria, which are (1) LuxI/LuxR-type QS in proteobacteria which use N-acylhomoserine lactones (AHL) as signaling molecules; (2) the luxS-encoded autoinducer-2 (AI-2) system that exists in both Gram-positive and Gram-negative bacteria; (3) post-translationally modified oligopeptide-two-component-type QS in most Gram-positive bacteria [5]. However, the types that are most widely investigated are AHL and AI-2 [5,8]. QS control several phenotypes such as bioluminescence, biofilm, swarming, which has been shown to contribute to bacterial pathogenesis [9–11]. Therefore, it is not surprising that the study of QS systems has important implications in the control of microbial infections.
On the other hand, quorum quenching (QQ) refers to the disruption of the bacterial QS process. It was first reported by Dong et al. in a Gram-positive Bacillus species which inactivates AHLs via lactonase activity [12]. Since then, QQ mechanisms have been found in various organisms to countermeasure the benefit that QS brings to their competitors [13]. QQ can be achieved through enzymatic inactivation of AHL either by detachment of the N-acyl side chain from the lactone ring via acylase or the opening of the lactone ring moiety by AHL lactonase, and both ways will inactivate the AHLs [13–16].
In a polymicrobial environment such as that of the marine ecosystem, QS bacteria are likely to gain competitive advantage as they can react to stimuli and compete in population-density synchronized manner. Therefore, to overcome this, competitors to QS bacteria that are equipped with QQ ability will be able to countermeasure the benefits accorded by the QS mechanism. Moreover, QS controls several phenotypes, especially virulence factors, and QQ has been regarded as a promising novel anti-infective therapy [13,14]. In view of this, we therefore investigated the presence of QQ bacteria in Malaysian marine water samples with the hope of isolating novel QQ bacteria and exploring their QQ enzymes for downstream application such as attenuation of QS phenotypes [13].
2. Experimental Section
2.1. Collection of the Malaysian Seawater Sample
One seawater sample (50 mL) was collected using sterile polypropylene tubes from the shoreline of Batu Maung, Penang (N 05°18.6791′, E 100°17.971′), Malaysia. The pH of the collected marine water sample was recorded as pH 8. The seawater sample was immediately processed upon returning to the laboratory. To process the sample, the water sample was serially diluted and spread onto LB agar. Bacteria with observable different morphology were isolated after incubation for 24 h at 28 °C. Pure colonies were obtained with several passages on LB agar.
2.2. Bacterial Strains and Culture Conditions
Bacillus cereus and Escherichia coli TOP10 cells were used as positive and negative controls, respectively, for AHL degradation bioassays [17]. Chromobacterium violaceum CV026, an AHL biosensor, was also used in this study to detect the presence of short chain AHLs by the formation of a purple pigment when short chain AHLs are detected [18]. E. coli TOP10 cell was used as host for cloning. All bacteria were cultured at 28 °C in LB media, except for E. coli TOP10 which was cultured at 37 °C in LB media.
2.3. MALDI-TOF-Mass Spectrometry (MALDI-TOF-MS) Identification of Bacteria
Bacterial isolates were identified using a MALDI-TOF-MS (Bruker, Leipzig, Germany) extraction method with UV laser wavelength of 337 nm and acceleration voltage of 20 kV [19–21]. The spectra were then analyzed in the Bruker MALDI Biotyper Real Time Classification (RTC) Version 3.1 (Build 65) software. The dendrogram was generated using the standard MALDI Biotyper MSP creation method [19].
2.4. Phylogenetic Analysis of 16S rRNA Genes Sequences
Genomic DNA of isolate BM1 was extracted using QIAamp® DNA Mini Kit (QIAGEN GmBH, Hilden, Germany) and used for PCR as DNA template. 16S rRNA genes were PCR-amplified with the forward primer 27F (5′-AGAGTTTGATCMTGGCTCAG-3′), and the reverse primer 1525R (5′AAGGAGGTGWTCCARCC-3′), as described previously [22]. Briefly, The PCR cycles consisted of an initial denaturation at 94 °C for 5 min, followed by 30 cycles at 94 °C for 30 s, annealing at 63 °C for 30 s and extension at 72 °C for 1 min 30 s, and a final extension at 72 °C for 5 min. Gene sequences were compared with GenBank databases using the BLASTN program followed by sequence alignment and phylogenetic analyses using the Molecular Evolutionary Genetic Analysis (MEGA) version 5.1 with parameter Neighbour-Joining algorithm and bootstrap 1000 re-samplings [23]. Identification of bacterial isolate and their accession numbers acquired from GenBank.
2.5. AHL Inactivation Assay
An AHL inactivation assay was conducted to screen bacterial isolates capable of degrading AHLs [15,24]. Bacterial cells were harvested by centrifugation, cell pellets were washed twice and resuspended in PBS buffer (100 mM, pH 6.5). Selected synthetic AHLs (C6-HSL, 3-oxo-C6-HSL and 3-hydroxy-C6-HSL, Sigma-Aldrich, St. Louis, MO, USA) of various concentrations were dispensed into sterile micro-centrifuge tubes and dried by evaporation. Cells suspension (in PBS buffer) was added to rehydrate the AHL to the final concentration of 0.5 μM. Mixtures were then incubated at 28 °C with shaking at 220 rpm for 0 h and 24 h. Aliquots of AHLs were withdrawn at 0 h and 24 h and residual AHLs were detected using C. violaceum CV026. AHL aliquots were withdrawn at 0 h, 24 h (with and without HCl-treatment) after incubation with the QQ cells were analysed by using rapid resolution liquid chromatography (RRLC) and assayed with biosensor. For AHL inactivation assay, reaction mixtures were stopped by heat inactivation at 95 °C for 5 min and 10 μL of the reaction mixture was spotted onto sterile paper disc placed on C. violaceum CV026 lawn and incubated overnight at 28 °C. Reduction or abolishment of purple pigments after 24 h of incubation indicates AHL degradation. PBS and B. cereus served as negative and positive controls, respectively. To confirm whether Labrenzia sp. BM1 degraded AHLs via lactonase activity, we acidified the AHL degradation mixture to promote re-lactonisation of the opened lactone rings [25]. Formation of purple color pigmentation after the addition of 0.2 M HCl indicated lactonase production [15].
2.6. Rapid Resolution Liquid Chromatography (RRLC) Analysis
Sample preparation for RRLC analysis was performed similar to the whole-cell AHLs inactivation assay described. AHLs were extracted twice using ethyl acetate, followed by drying in the fume hood before resuspension in 100 μL of acetonitrile for RRLC analysis. AHLs degradation was analyzed using an Agilent Technologies (Agilent 1200 series, Waldbronn, Germany) 1200 series RRLC system, as described previously [26]. Briefly, AHLs samples were separated using an Agilent Poroshell120 EC-C18 column (4.6 mm × 100 mm, packed with 2.7 μm particle size) with the elution procedure consisting of an isocratic profile of acetonitrile/water (35:65, v/v). Flow rate of 0.7 mL/min was applied and AHL detection was carried out at 210 nm [6]. Known amounts of synthetic AHLs (C6-HSL), (3-oxo-C6-HSL) and (3-hydroxy-C6-HSL) were included as standards. AHL incubated with E. coli TOP10 cells and PBS served as a negative controls. Experiments were performed in duplicate.
3. Results and Discussion
3.1. Isolation and Identification of Strains
In this study, we have isolated four different bacteria colonies from our Malaysian shoreline marine water samples. MALDI-TOF-MS and 16S rRNA was performed to identify the isolates. Of these samples, BM1 showed high degree of degradation of various N-acylhomoserine lactones and hence was chosen for further work.
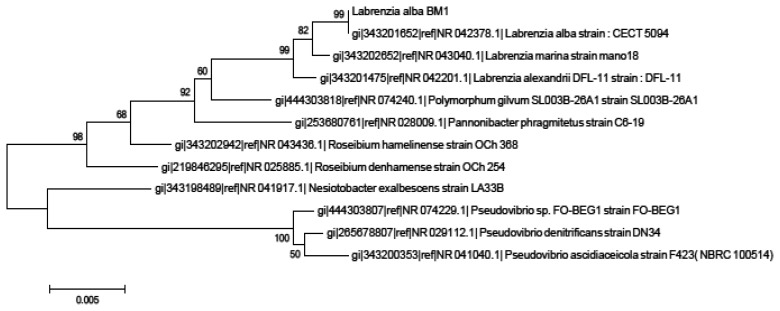
Isolate BM1 could not be identified due to the lack of reference spectra in the database that limits the identification of this sample to the genus or species level [27]. Hence, we proceeded to the molecular identification using phylogenetic trees generated on BM1 16S rRNA gene nucleotide sequences (accession number KJ 880043) which showed that our isolate BM1 is closely related to Labrenzia sp. with high bootstrap value (99%) (Figure 1).
Figure 1.
Phylogenetics analysis of isolate BM1. Tree was generated using MEGA 5.1. Isolate BM1 clustered closely to Labrenzia sp.
3.2. Degradation of AHLs and the Mechanism of QQ by Labrenzia sp. BM1
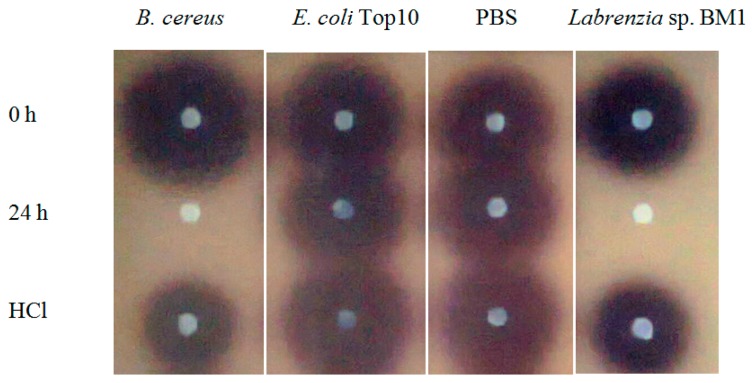
Labrenzia sp. BM1 showed significant degradation of C6-HSL which was depicted by the reduction or disappearance of the purple pigments after 24 h of incubation (Figure 2). Furthermore, Labrenzia sp. BM1 cells were also tested for their ability to degrade other types of AHLs including N-(3-oxo-hexanoyl)-l-homoserine lactone (3-oxo-C6-HSL) and N-(3-hydroxyhexanoyl)-l-homoserine lactone (3-hydroxy-C6-HSL), which represent AHLs with oxo and hydroxy groups, respectively (Table 1). Our data suggested that Labrenzia sp. BM1 could efficiently degrade both 3-oxo-C6-HSL and 3-hydroxy-C6-HSL.
Figure 2.
Degradation of C6-HSL by Labrenzia sp. BM1. C6-HSL was incubated with bacterial cell suspensions for 0 h, 24 h and HCl was added into aliquot incubated with QQ cells for 24 h. B. cereus served as positive control whereas E. coli Top10 and PBS were used as negative controls. The purple pigments after addition of HCl suggested re-lactonisation of the digested AHL, and this indicated that Labrenzia sp. BM1 produced lactonase which inactivated the tested AHLs.
Table 1.
Summary of AHLs inactivation assays detected using CV026 overlay.
| AHLs | B. cereus (Positive Control) | E. coli Top10 (Negative Control) | PBS Buffer (Negative Control) | Labrenzia sp. BM1 |
|---|---|---|---|---|
| C6-HSL | + | − | − | + |
| 3-oxo-C6-HSL | + | − | − | + |
| 3-hydroxy-C6-HSL | + | − | − | + |
+ denotes AHL was degraded, − denotes no AHL degradation was observed.
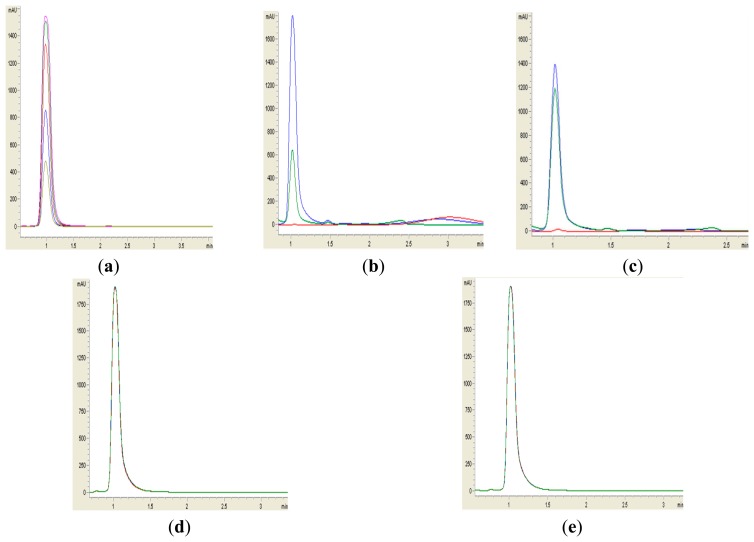
Formation of purple pigmentation after the addition of 0.2 M HCl indicated Labrenzia sp. BM1 inactivated AHL molecules via the enzyme lactonase [28]. When the opened ring AHL was acidified, this will promote re-lactonisation of the opened ring into intact AHLs [28]. RRLC was then conducted to further confirm the AHLs degradation activity via a lactonase mechanism (Figures 2 and 3). Using a similar approach, we used RRLC to analyse degradation of other AHLs and the data are summarised in Table 2. Figure 3 shows the representative chromatograms. Based on the whole-cell AHL inactivation assay and RRLC analyses on degradation of various AHL as lactonase substrate, strong QQ activity was observed in Labrenzia sp. BM1 (Figure 3, Table 2).
Figure 3.
RRLC analysis of 3-oxo-C6-HSL degradation. Residual 3-oxo-C6-HSL (with elution time of 1.00 ± 1.2 s), after degradation for 0 h (blue), 24 h (red) and relactonisation with HCl (green), was monitored at OD 210 nm. Degradation of 3-oxo-C6-HSL was depicted by the reduction of milliabsorbance unit (mAU) in the chromatogram. Samples containing standards 3-oxo-C6-HSL (0.2 μg/μL, 0.4μg/μL, 0.6 μg/μL, 0.8 μg/μL and 1.0 μg/μL) (corresponding to peaks with ascending height) (a) sample BM1 (b), B. cereus acted as positive control (c), E. coli Top10 (d), PBS buffer acted as the negative controls (e). Results show significant degradation and relactonisation of 3-oxo-C6-HSL by Labrenzia sp. BM1.
Table 2.
Summary of AHLs degradation analysed using Rapid Resolution Liquid Chromatography (RRLC).
| Time/Samples | AHLs | 0 h (mAU) | 24 h (mAU) | HCl (mAU) |
|---|---|---|---|---|
| Labrenzia sp. BM1 | 3-oxo-C6-HSL | 1700 | 0 | 650 |
| B. cereus (positive control) | 1400 | 0 | 1200 | |
| E. coli Top10 (negative control) | 1900 | 1900 | 1900 | |
| PBS buffer (negative control) | 1900 | 1900 | 1900 | |
|
| ||||
| Labrenzia sp. BM1 | 3-hydroxy-C6-HSL | 200 | 0 | 85 |
| B. cereus (positive control) | 200 | 0 | 145 | |
| E. coli Top10 (negative control) | 200 | 200 | 200 | |
| PBS buffer (negative control) | 200 | 200 | 200 | |
|
| ||||
| Labrenzia sp. BM1 | C6-HSL | 145 | 20 | 65 |
| B. cereus (positive control) | 165 | 0 | 40 | |
| E. coli Top10 (negative control) | 170 | 170 | 170 | |
| PBS buffer (negative control) | 200 | 200 | 200 | |
AHLs degradation was depicted as reduction of milli-absorbance unit (mAU).
Many QQ bacteria have been isolated from tropical marine environments [26]. For example, we have previously described a marine pseudomonad that shows both QS and QQ activities. In this work, we extended the search of marine QQ bacteria in another sampling spot with the aim to identify its QS interference ability and its QQ mechanism. Here, we described the isolation of Labrenzia sp. BM1 and its QQ properties were confirmed.
To date, many QQ genes have been well documented, for example AiiA lactonase homologs of Bacillus and PvdQ and QuiP of Psudomonas have been well characterized [29]. Various lactonase activities have been reported in many proteobacteria such as AiiA, AttM, AiiB [12,30] and AhlD [31]. There is also another class of AHL-degrading enzymes which is amidase/acylase exemplified by AiiD [13,29] that degrade AHLs by detaching the N-acyl side chain from the homoserine lactone. Under alkaline conditions AHLs are rapidly inactivated by pH-dependent lactonolysis in which the N-acyl- homoserine lactone ring is hydrolysed to the ring-opened form, a reaction that be reversed by acidification [8,9]. On the other hand, Acinetobacter sp. has shown a broad activity in degrading short chain AHLs via lactonase [15]. Interestingly, this is the first report that shows Labrenzia sp. BM1 exhibiting QQ activity. Our work presented here has added a new member of the Labrenzia sp. to the expanding list of QQ bacteria. This finding provides a basis to search for more QQ marine bacteria as most current work is focusing on QS mechanisms in marine bacteria. We hypothesise that QQ bacteria are important in the marine environment as they will be responsible for the turnover of AHLs thus preventing overaccumulation of AHLs in marine microbial niches such as biofilms.
Labrenzia sp. (formally classified as Stappia alba) has been studied for its general biochemical properties [32] but its QQ mechanism has not been documented before. Our data show for the first time, that Labrenzia sp. BM1 efficiently degraded AHLs. Since most Gram-negative bacteria use QS to modulate many phenotypes, including virulence determinants such biofilm formation, our work may path the way to explore Labrenzia sp. BM1 for biocontrol in aquaculture by destruction of QS signal molecules in marine QS pathogens and to attenuate them without the use of antibiotics [13,14], or prevention of biofouling. Labrenzia sp. BM1 may also serve as a model microorganism for microbial ecology studies of QS and QQ systems in the marine habitat.
4. Conclusions
We report here the QQ activity of Labrenzia sp. BM1 isolated from a Malaysian marine water sample. Our data demonstrated that Labrenzia sp. BM1 significantly degraded various N-acylhomoserine lactones (C6-HSL, 3-oxo-C6-HSL and 3-hydroxy-C6-HSL). Further investigations are being carried out to clone the QQ gene and to study the ecological role of QQ gene Labrenzia sp. BM1 in its marine environment.
Acknowledgments
Kok-Gan Chan thanks the University of Malaya for awarding the HIR Grant (UM-MOHE HIR Grant UM.C/625/1/HIR/MOHE/CHAN/01, Grant No. H-50001-A000001) that supported this work.
Author Contributions
NAG, SNMN, XYC, WFY did the sampling and performed the experiments. KGC secured and managed the funding, conceived the ideas, monitored and supervised the project. All authors prepared, proofread the draft and KGC approved the final submission.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Miller M.B., Bassler B.L. Quorum sensing in bacteria. Annu. Rev. Microbiol. 2001;55:165–199. doi: 10.1146/annurev.micro.55.1.165. [DOI] [PubMed] [Google Scholar]
- 2.Waters C.M., Bassler B.L. Quorum sensing: Cell-to-cell communication in bacteria. Annu. Rev. Cell Dev. Biol. 2005;21:319–346. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 3.Fuqua C., Parsek M.R., Greenberg E.P. Regulation of gene expression by cell-to-cell communication: Acyl-homoserine lactone quorum sensing. Annu. Rev. Genet. 2001;35:439–468. doi: 10.1146/annurev.genet.35.102401.090913. [DOI] [PubMed] [Google Scholar]
- 4.Williams P. Quorum sensing, communication and cross-kingdom signaling in the bacterial world. Microbiology. 2007;153:3923–3938. doi: 10.1099/mic.0.2007/012856-0. [DOI] [PubMed] [Google Scholar]
- 5.Norizan S.N.M., Yin W.F., Chan K.G. Caffeine as a potential quorum sensing inhibitor. Sensors. 2013;13:5117–5129. doi: 10.3390/s130405117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chong T.M., Koh C.L., Sam C.K., Choo Y.M., Yin W.F., Chan K.G. Characterization of quorum sensing and quorum quenching soil bacteria isolated from Malaysian tropical montane forest. Sensors. 2012;12:4846–4859. doi: 10.3390/s120404846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lau Y.Y., Sulaiman J., Chen J.W., Yin W.F., Chan K.G. Quorum sensing activity of Enterobacter asburiae isolated from lettuce leaves. Sensors. 2013;13:14189–14199. doi: 10.3390/s131014189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunny G.M., Leonard B.A. Cell-cell communication in gram-positive bacteria. Annu. Rev. Microbiol. 1997;51:527–564. doi: 10.1146/annurev.micro.51.1.527. [DOI] [PubMed] [Google Scholar]
- 9.Shih P.C., Huang C.T. Effect of quorum sensing deficiency on Pseudomonas aeruginosa biofilm formation and antibiotic resistance. J. Antimicrob. Chemother. 2002;49:309–314. doi: 10.1093/jac/49.2.309. [DOI] [PubMed] [Google Scholar]
- 10.Mellbye B., Schuster M. The sociomicrobiology of antivirulence drug resistance: A proof of concept. mBio. 2011;2 doi: 10.1128/mBio.00131-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shrout J.D., Chopp D.L., Just C.L., Hentzer M., Givskov M., Parsek M.R. The impact of quorum sensing and swarming motility on Pseudomonas aeruginosa biofilm formation is nutritionally conditional. Mol. Microbiol. 2006;62:1264–1277. doi: 10.1111/j.1365-2958.2006.05421.x. [DOI] [PubMed] [Google Scholar]
- 12.Dong Y.H., Xu J.L., Li X.Z., Zhang L.H. AiiA, an enzyme that inactivates the acylhomoserine lactone quorum sensing signal and attenuates the virulence of Erwinia carotovora. Proc. Natl. Acad. Sci. USA. 2000;97:3526–3531. doi: 10.1073/pnas.060023897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lin Y.H., Xu J.L., Hu J., Wang L.H., Ong S.L., Leadbetter J.R., Zhang L.H. Acyl-homoserine lactone acylase from Ralstonia strain XJ12B represents a novel and potent class of quorum-quenching enzymes. Mol. Microbiol. 2003;47:849–860. doi: 10.1046/j.1365-2958.2003.03351.x. [DOI] [PubMed] [Google Scholar]
- 14.Uroz S., Dessaux Y., Oger P. Quorum sensing and quorum quenching: The yin and yang of bacterial communication. ChemBioChem. 2009;10:205–216. doi: 10.1002/cbic.200800521. [DOI] [PubMed] [Google Scholar]
- 15.Chan K.G., Atkinson S., Mathee K., Sam C.K., Chhabra S.R., Koh C.L., Williams P. Characterization of N-acylhomoserine lactone-degrading bacteria associated with the Zingiber offinale (ginger) rhizosphere: Co-existence of quorum quenching and quorum sensing in Acinetobacter and Burkholderia. BMC Microbiol. 2011;11 doi: 10.1186/1471-2180-11-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ulrich R.L. Quorum quenching: Enzymatic disruption of N-acylhomoserine lactose-mediated bacterial communication in Burkholderia thailandensis. Appl. Environ. Microbiol. 2004;70:6173–6180. doi: 10.1128/AEM.70.10.6173-6180.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan K.G., Wong C.S., Yin W.F., Sam C.K., Koh C.L. Rapid degradation of N-3-oxoacylhomoserine lactones by a Bacillus cereus isolate from Malaysian rainforest soil. Antonie van Leeuwenhoek. 2010;98:299–305. doi: 10.1007/s10482-010-9438-0. [DOI] [PubMed] [Google Scholar]
- 18.McClean K.H., Winson M.K., Fish L., Taylor A., Chhabra S.R., Cámara M., Daykin M., Lamb J.H., Swift S., Brcroft B.W., et al. Quorum sensing and Chromobacterium violaceum: Exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology. 1997;143:3703–3711. doi: 10.1099/00221287-143-12-3703. [DOI] [PubMed] [Google Scholar]
- 19.Seng P., Drancourt M., Gouriet F., La Scola B., Fournier P.-E., Rolain J.M., Raoult D. Ongoing revolution in bacteriology: Routine identification of bacteria by matrix-assisted laser desorption ionization time-of-flight mass spectrometry. Clin. Infect. Dis. 2009;49:543–551. doi: 10.1086/600885. [DOI] [PubMed] [Google Scholar]
- 20.Chen J.W., Koh C.-L., Yin W.-F., Chan K.G. Short chain N-acyl homoserine lactone production by soil isolate Burkholderia sp. strain A9. Sensors. 2013;13:13217–13227. doi: 10.3390/s131013217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ngeow Y.F., Cheng H.J., Chen J.W., Yin W.-F., Chan K.G. Short chain N-acyl homoserine lactone production by clinical multidrug resistant Klebsiella pneumoniae strain CSG20. Sensors. 2013;13:15242–15251. doi: 10.3390/s131115242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan K.G., Yin W.F., Sam C.K., Koh C.L. A novel medium for the isolation of N-acylhomoserine lactone degrading bacteria. J. Ind. Microbiol. Biotechnol. 2009;36:247–251. doi: 10.1007/s10295-008-0491-x. [DOI] [PubMed] [Google Scholar]
- 23.Tamura K., Peterson D., Stacher G., Nei M., Kumar S. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 2011;28:2731–2739. doi: 10.1093/molbev/msr121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chan K.G. Expression of Klebsiella sp. lactonase ahlK gene is growth-phase, cell-population density and N-acylhomoserine lactone independent. Front. Life Sci. 2013;7:132–139. [Google Scholar]
- 25.Yates E.A., Philipp B., Buckley C., Atkinson S., Chhabra S.R., Sockett R.E., Goldner M., Dessaux Y., Cámara M., Smith H. N-acylhomoserine lactones undergo lactonolysis in a pH-, temperature-, and acyl chain length-dependent manner during growth of Yersinia pseudotuberculosis and Pseudomonas aeruginosa. Infect. Immun. 2002;70:5635–5646. doi: 10.1128/IAI.70.10.5635-5646.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong C.S., Yin W.F., Choo Y.M., Sam C.K., Koh C.L., Chan K.G. Coexistence of quorum-quenching and quorum-sensing in tropical marine Pseudomonas aeruginosa strain MW3A. World J. Microbiol. Biotechnol. 2011;28:453–461. doi: 10.1007/s11274-011-0836-x. [DOI] [PubMed] [Google Scholar]
- 27.Reich M., Bosshard P.P., Stark M., Beyser K., Borgmann S. Species identification of bacteria and fungi from solid and liquid culture media by MALDI-TOF mass spectrometry. J. Bacteriol. Parasitol. 2013;10:2155–9597. [Google Scholar]
- 28.Dong Y., Wang L., Xu J., Zhang H., Zhang X., Zhang L. Quenching quorum-sensing-dependent bacterial infection by an N-acyl homoserine lactonase. Nature. 2001;411:813–817. doi: 10.1038/35081101. [DOI] [PubMed] [Google Scholar]
- 29.Sio C.F., Otten L.G., Cool R.H., Diggle S.P., Braun P.G., Bos R., Daykin M., Cámara M., Williams P., Quax W.J. Quorum quenching by an N-acyl-homoserine lactone acylase from Pseudomonas aeruginosa PAO1. Infect. Immun. 2006;74:1673–1682. doi: 10.1128/IAI.74.3.1673-1682.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlier A., Uroz S., Smadja B., Fray R., Latour X., Dessaux Y., Faure D. The Ti plasmid of Agrobacterium tumefaciens harbors an attM- paralogous gene, aiiB, also encoding N-acylhomoserine lactonase activity. Appl. Environ. Microbiol. 2003;69:4989–4993. doi: 10.1128/AEM.69.8.4989-4993.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park S.Y., Lee S.J., Oh T.K., Oh J.W., Koo B.T., Yum D.Y., Lee J.K. AhlD, an N-acylhomoserine lactonase in Arthrobacter sp., and predicted homologues in other bacteria. Microbiology. 2003;149:1541–1550. doi: 10.1099/mic.0.26269-0. [DOI] [PubMed] [Google Scholar]
- 32.Biel H., Pukall R., Lunsdorf H., Schulz S., Allgaier M., Tindall B.J., Wagner D.I. Description of Labrenzia sp. alexandrii gen. nov., sp. nov., a novel alphaproteobacterium containing bacteriochlorophyll a, a proposal for reclassification of Stappia aggregate as Labrenzia sp. aggregate comb. nov., of Stappia alba as Labrenzia sp. comb. nov., and emended descriptions of the genera Pannonibacter, Stappia and Roseibium, and of the species Roseibium denhamense and Roseibium hamelinense. Int. J. Syst. Evol. Microbiol. 2007;57:1095–1107. doi: 10.1099/ijs.0.64821-0. [DOI] [PubMed] [Google Scholar]