Abstract
Background :
Numerous studies have shown reduction of periprosthetic bone mineral density (BMD) after hip replacement. The effect on the whole limb, however, is still unexplored. This study’s objective was to analyse the postoperative development of BMD and muscle strength of the limb after total hip replacement (THR) and to determine links between these parameters.
Methods :
55 patients, who underwent THR, were included. Depending on therapeutic indication, either an uncemented stem (Group A, n=30) or a cemented stem (Group B, n=25) has been implanted. In the limbs, the measurement of BMD using DEXA and the maximum isometric muscle strength, detected by a leg press, were undertaken preoperatively and after 3, 6 and 12 months.
Results :
A total of 12 patients (Group A: n = 6, Group B: n = 6) were excluded due to reasons which were not relevant to the study. So, the results refer to the data of 43 patients. In Group A (uncemented, n = 24), a significant decrease of BMD on the operated extremity was seen after 3, 6 and 12 months compared with preoperative values. Isometric muscle strength on the affected extremity increased significantly after 6 and 12 months. In Group B (cemented, n = 19), with a lower baseline compared to group A, an increase in BMD of the affected limb was seen postoperatively. This rise was significant after 12 months. With regard to the isometric muscle strength, a significant increase could be observed in this group after 6 and 12 months.
Conclusion :
Analogous to postoperative reduction of periprosthetic bone density, a decrease of the entire limb BMD on the operated leg occurred after implantation of uncemented hip stems. In contrast, an increase in BMD was recorded for cemented stems. Regardless of the type of anchoring, a substantial increase in muscular strength could be observed postoperatively in both groups.
Keywords: Bone mineral density, hip replacement, limb, muscle strength, outcome.
BACKGROUND
Total hip replacement (THR) is currently one of the most successful procedures in orthopaedic surgery [1]. World-wide, more than 1 million THR are implanted annually, about 150,000 of them in Germany alone. While the uncemented joint replacement is intended particularly for younger and more active patients, cemented replacements are preferred with higher age and worse bone structure.
A durable endoprosthetic care requires a stable anchoring of the implant in the bone. For cemented replacements, this is achieved by creating an even cavity-free cement coat and by a constant cement penetration into the spongiosa. For cementless replacements, primary stability must first be achieved intraoperatively by use of "press-fit" fixation.
Through new bone formation around the implant, it subsequently leads to long-term secondary stability [2]. However, osseous anchoring of the implant can be affected by various factors.
The postoperative reduction of the periprosthetic bone density after implantation of uncemented [3-6] and cemented [6-8] stems might be a major problem in orthopaedic surgery. In general missing implant to bone contact or osteolysis around the stem might lead to failure of the prosthesis after a few years [9, 10]. However, diffuse bone loss around the implant is observed immediately after THR. It is possible that these bone resorptions might work against a sufficient osseous integration of the implant in the first postoperative months.
The early loss of periprosthetic bone density results primarily from the operational irritation, the reduced postoperative loading and also from the modified power flow caused by the prosthesis [11, 12]. In addition, the preoperative bone density is of great relevance [7, 12, 13]. Regardless of the type of prosthesis, the result is a significant loss of bone density around the implant within the first postoperative year [3, 7, 10, 14]. From the second postoperative year this loss slows down [3, 7, 8].
The musculature on the other hand is known to have a significant influence on the entire bone density. Physical activity and mechanical stress have a positive effect on the bone mass and the bone strength [15, 16]. However, immobilisation and inactivity atrophy affect the bone mass negatively.
In osteoarthritis, muscular atrophies and muscular contractures can occur as a result of pain-related protective postures and joint deformations. This may worsen the function of the hip joint, but also the general condition of the patient [17]. THR provides a solution leading to pain-free mobility and allows the patient to compensate existing conditional deficits. The postoperative outcome is dependent on fast recovery of joint function and hence also on the regeneration of the muscular system. Any injury of the muscles is immediately associated with reduction of the power and disorders of proprioception, which complicates the functional regeneration. Postoperatively, the result is a significant improvement in the function of periarticular muscles. Nevertheless, large deficits remain in the comparison with healthy age groups. This applies not only to the maximum force, but also to the muscular endurance [18]. The maximum force is considered an important instrument representing the muscular performance.
In addition to the successful use in osteoporosis diagnostics, dual-energy X-ray absorptiometry (DEXA) could be successfully used for the determination of periprosthetic bone density [3, 5, 7]. It is characterised by a high precision, a high sensitivity as well as by a high reproducibility of the results [19, 20]. Further advantages are a low radiation exposure [19] and short examination times. By using the “metal-removal” tool of the software, it is possible to eliminate the influences of metal implants. Thus, precise measurement of periprosthetic bone density can be obtained. After differentiation of body tissue composition, DEXA also allows determination of the entire body bone mineral density (BMD).
Numerous studies have shown a reduction of periprosthetic bone density after THR by means of DEXA. However, there are very few comparisons of these changes with bone density in other regions of the body [6, 8]. Currently there exist no studies regarding the entire lower limb bone density after hip prostheses implantation. So the question arises whether the development of bone density on the lower limb is similar or stands in contrast to the development of the periprosthetic bone density.
The aim of this study was to analyse whether possible correlations between the parameters of bone density and muscle strength on the operated lower limb can be proved. Here, the results of cementless and cemented hip stems were evaluated in terms of early clinical outcome and compared with the unoperated leg.
MATERIALS AND METHODS
Sample size calculation indicated that the number of participants required was 26 per group in order to detect a clinically relevant difference in BMD of 0.1 g/cm² with a two-sided 5% alpha-level and a power of 80%. A 12-month recruitment period was set to enrol this number of participants.
A total of 55 patients, who underwent THR, were recruited during the period from March 2009 to March 2010 and consented to participate in the study. The study protocol was evaluated by the ethical committee of the University Medicine Rostock (A 2009 02).
Inclusion criteria were, in case of osteoarthritis of the hip, the indication for primary implantation of a THR with an uncemented Hipstar® stem or a cemented Exeter® stem as well as an age between 50 and 85 years. Further criteria were the understanding in the scope of the prospective study and also the willingness to participate at the required follow-up.
Exclusion criteria were, in addition to osteoporosis (T-score <-2.5) and a body mass index >35 kg/m², the contralateral THR within the last or planned for the following year, as well as an ipsilateral prosthetic joint replacement within the follow-up period. Furthermore, neurosensory or motor deficits, tumour diseases, infections, diabetes mellitus type I, dialysis-dependent renal failure and the lack of compliance or unavailability for follow-up were considered as exclusion criteria. The patients confirmed their consent in the study in written form.
Depending on the patient´s age, the prosthetic care occurred either with an uncemented Hipstar® or a cemented Exeter® stem in combination with the Trident® TC cup system with X3® PE-insert (Polyethylene) and a ceramic head. According to our institutional rules that all THR patients over the age of 70 years are provided with cemented implants the patients in Group B are all over the age of 70. The implantation of the THR was carried out by one of the three main surgeons at our department of orthopaedics. According to the anchoring of the stem, patients were assigned to two different groups.
Group A contained of 30 patients, who had received an uncemented stem (Hipstar®). The mean age of the patients in this group was 62.9±7.0 years, 17 men and 13 women were included.
Group B was composed of 25 patients, who had been supplied with a cemented stem (Exeter®). Here the mean age was 75.0±5.0 years. This group consisted of 10 male and 15 female patients.
The postoperative treatment was standardised. All patients were mobilised by physical therapy with crutches from the 1st postoperative day on. After completing wound healing and mobilisation under full weight-bearing on ward level and stairs, the patients could be released for a follow-up treatment to a rehabilitation facility normally on the 8th-12th postoperative day.
In the process of 3 (3.0±0.75), 6 (6.0±0.75) and 12 (12.0±0.75) months postoperatively, the patients were summoned to the follow-up examination.
DEXA
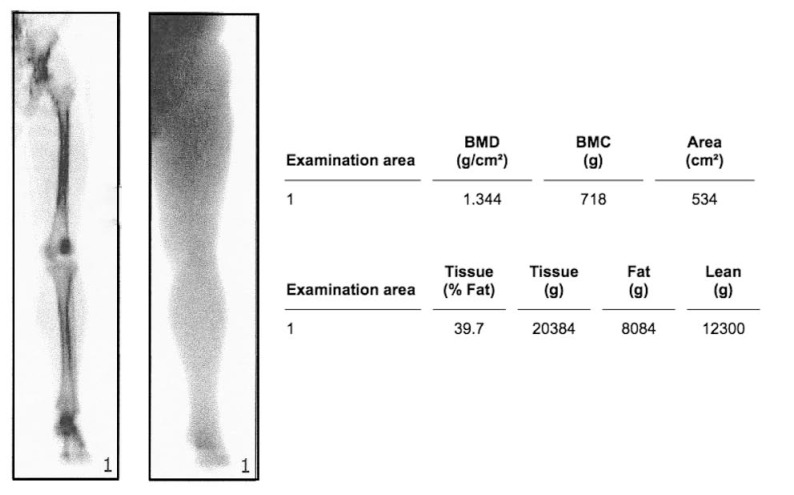
To exclude osteoporosis, we preoperatively carried out measurements of BMD at the lumbar spine (a.p.) or the proximal femur by using DEXA. Therefore, we used a Lunar Prodigy™ DEXA (General Electric (GE) Healthcare, Munich, Germany) and the associated software enCORE™ 2007 (version 11.40.004, General Electric (GE) Medical Systems, Madison, WI, USA). The patient was positioned centrally on the scanner table and the full-body scan feature was traversed from the iliac crest down to the foot. The BMD was obtained by setting a region of interest (ROI) at the affected and unaffected lower limb (Fig. 1). Within the frame of the follow-up after 3, 6 and 12 months BMD of the lower limbs were controlled. The metallic implant was captured and eliminated automatically using the software´s "metal-removal” function. For determining age-related changes in bone density, BMD at the lumbar spine (L1-L4) was measured preoperatively as well as 12 months postoperatively. For quality assurance we performed coefficient of variation (CV) measurments with a calibration phantom every day of DEXA evaluation (BMD CV = 0.3 % for DEXA Phantom). Additionaly we calculated the least significant change (LSC) for our specific whole limb ROI dexa assessment of 6 %.
Fig. (1).
Evaluation of a DEXA scan to measure bone mineral density on the lower limb of a contralateral side.
Muscular Strength
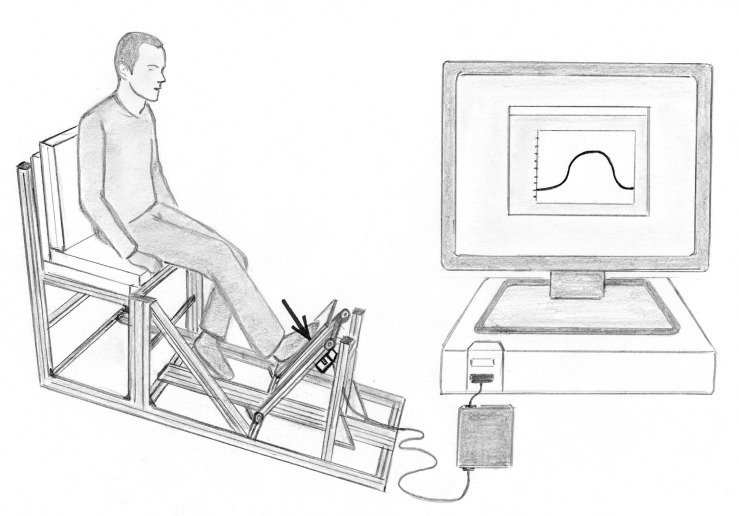
The maximum isometric muscle strength in the entire power chain of the affected and unaffected lower limb was monitored preoperatively as well as 3, 6 and 12 months postoperatively. For this purpose, a special leg press test bench was constructed, consisting of a seat for the patient and an associated base plate (Fig. 2). Under this plate a compression force sensor (force sensor KM40, ME measuring systems GmbH, Hennigsdorf) was attached. Before the leg press dynamometer was used on patient evaluation, the intra- and inter-session reliability for the isometric maximum voluntary strength was calculated. The maximum isometric muscle strength demonstrated high intra- and inter-session reliability, with intraclass correlation coefficient (ICC) higher than 0.95. The ICC was slightly higher for intra-session compared to inter-session analysis. The CV for muscle strength were small, but slightly above 5 %, indicating moderate reliability. The percentage change in the mean between the trials within the same session was -2.3 % (95%-CI: -6.0-1.4 %) and between the sessions 2.8 % (95%-CI: -3.5-9.4 %). No significant intra- and inter-session differences were observed.
Fig. (2).
Demonstration of the measurement of the maximum isometric muscle strength in the entire power chain of the lower limb.
For each measurement, the patient was positioned on the test bench with reproducible seat position and lever ratios using a mechanical goniometer. In doing so, the angle between torso and upper leg had to be 90°, the angle between upper and lower leg set to 130°±5° and the angle between lower leg and foot 90°±5°. Subsequently, the patients were asked to press with greatest possible force against the base plate of the test bench and to hold this condition for approximately 3 seconds. Afterwards they had to take the foot off the plate again. Per each evaluation date, 3 measurements were made on both legs, after a preliminary assessment.
The maximum isometric muscle strength of the lower limb, recorded by the force sensor, was redirected via a USB measuring amplifier (GSV3USB, ME measuring systems, Hennigsdorf) to a measuring PC. By using the software ME GSV control (ME measuring systems, Hennigsdorf) the data could be recorded. The measured values were stored as a text file and later processed in an Excel spreadsheets and SPSS. From the maximum values of each measurement mean and standard deviation were calculated, based on which the statistical analysis of the data was carried out.
Scores
Clinical evaluation was undertaken preoperatively as well as 3, 6 and 12 months postoperatively. At this the clinical and functional outcome was rated by Harris Hip Score (HHS) [21]. The analysis of patient-related outcome was carried out by use of Western Ontario and McMaster University Osteoarthritis Index (WOMAC) [22].
Statistics
All data were statistically analysed using the SPSS statistical software package 15.0 (SPSS Inc. Chicago, Illinois, United States). Power calculations and Cohen’s d effect size were estimated with the statistical software package G*Power 3 (version 3.1.5.) [23].
In the first step, a descriptive analysis was carried out. All variables (BMD, muscle strength, HHS, WOMAC) have been described by mean and standard deviation, minimum, maximum and number of available observations.
Using Friedman-test (FR) or Wilcoxon-test (WI) for pair differences, comparisons were made within the groups between the different times of evaluation (preoperative, 3 months postoperative, 6 months postoperative and 12 months postoperative). At this, values of p<0.05 were determined as statistically significant.
Depending on the distribution, it was examined whether there are significant differences between operated and contralateral leg using the u-test of Mann-Whitney for independent samples.
RESULTS
At the beginning of the study the median age over all patients was 68.4±8.6 years. 27 men and 28 women were included.
After the exclusion of a total of 12 patients (Group A, n = 6; Group B, n = 6) as a result of postoperative partial weight-bearing (n = 3), contralateral THR within the follow-up period (n = 2), Re-OP after trochanter fracture (n = 1) or sintering of the stem (n = 1), death (n = 1) or private purposes (n = 3) as well as lack of availability (n = 1), the data from 43 patients were evaluable (mean age 68.2±8.9 years; f/m = 20/23).
Comparing the two groups in matters of age, patients in Group A were younger (mean±SD 62.7±6.9 years, p = 0.001) than patients in Group B (75.1±5.6 years). The patients of Group A had a higher preoperative bone mineral density (1.337±0.143 g/cm²) on the affected limb than patients of Group B (1.239±0.127 g/cm²). No differences between the two patient groups arose with regard to the other specific parameters.
Hipstar®
In Group A (Hipstar®), which was composed of a total of 30 patients, in two cases it came to intraoperative complications that required a partial weight-bearing postoperatively. As a result, these patients were excluded from the study.
Within the framework of the follow-up examination, in one case a trochanteric fracture was detected, in another case a sintering of the stem was assured. These circumstances required a renewed operational supply. One patient was unable to take part in the follow-up examination due to health reasons and one patient was postoperatively no longer available. Therefore, Group A comprised 24 complete records and consisted of 14 male and 10 female patients.
In Group A there was a significant decrease of BMD on the operated extremity in the postoperative stages compared to baseline. With a basic value of 1.337±0.143 g/cm², the BMD after 3 months postoperative was 1.313±0.147 g/cm² (WI-test p=0.045), after 6 months 1.294±0.150 g/cm² (WI-test p=0.001) and after 12 months 1.256±0.153 g/cm² (WI-test p=0.005) (Fig. 3, Table 1). There were no significant changes in BMD between the separate postoperative examination dates. The BMD at the lumbar spine decreased from 1.305±0.212 g/cm² preoperatively to 1.282±0.196 g/cm² after 12 months (WI-test p=0.028). BMD of the contralateral leg remained constant at a mean of 1.325±0.13 g/cm².
Fig. (3).
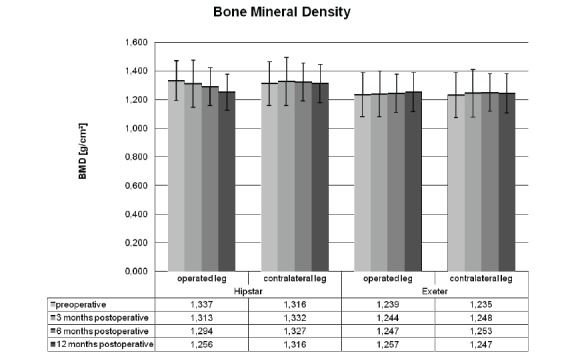
Postoperative development of the bone mineral density on the affected and unaffected limb after total hip replacement.
Table 1.
Development of BMD and isometric muscle strength of operated and contralateral leg.
| BMD (g/cm²) | Strength (N) | ||||||
|---|---|---|---|---|---|---|---|
| Operated Leg | Contralateral leg | p of Mann- Whitney-Test |
Operated Leg |
Contralateral Leg |
p of Mann-Whitney-Test |
||
| Group A (uncemented Hipstar®) | preoperative (baseline) |
1.337±0.143 | 1.316±0.160 | 0.540 | 918±635 | 1089±114 | 0.580 |
| 3 months postoperative |
1.313±0.147 p=0.045 * | 1.332±0.163 p=0.153 | 0.940 | 926±397 p=0.199 | 1211±110 p=0.300 | 0.171 | |
| 6 months postoperative |
1.294±0.150 p=0.001 * | 1.327±0.155 p=0.616 | 0.754 | 1162±501 p=0.002 * | 1500±104 p=0.016 * | 0.128 | |
| 12 months postoperative |
1.256±0.153 p=0.005 * | 1.316±0.161 p=0.449 | 0.560 | 1363±682 p<0.001 * | 1549±90 p=0.016 * | 0.501 | |
| Group B (cemented Exeter®) | preoperative (baseline) |
1.239±0.127 | 1.235±0.120 | 0.706 | 691±356 | 715±70 | 0.815 |
| 3 months postoperative |
1.244±0.128 p=0.643 | 1.248±0.130 p=0.36* | 0.706 | 825±498 p=0.212 | 931±84 p=0.074 | 0.602 | |
| 6 months postoperative |
1.247±0.132 p=0.896 | 1.253±0.131 p=0.163 | 0.667 | 942±535 p=0.007 * | 1021±62 p=0.002 * | 0.760 | |
| 12 months postoperative |
1.257±0.126 p=0.049 * | 1.247±0.133 p=0.147 | 0.733 | 1190±450 p<0.001 * | 1094±95 p<0.001 * | 0.577 | |
Mean±SD, * p < 0.05 Wilcoxon-test compared with baseline.
Isometric muscle strength of the operated lower extremity was 918±635 N preoperatively and remained nearly constant 3 months postoperatively (926±397 N). A significant increase in muscle strength, compared to baseline, came 6 months postoperatively on 1162±501 N (WI-test p=0.002) and 12 months postoperatively on 1363±682 N (WI-test p<0.001). There was also a significant development after 6 (WI-test p<0.001) and 12 months (WI-test p<0.001) compared to the muscle strength 3 months postoperatively. The isometric muscle strength of contralateral leg was 1089±114 N at preoperative evaluation point, 3 months postoperatively 1211±110 N, 6 months postoperatively 1500±104 N and 12 months postoperatively 1549±90 N. The difference between operated and unaffected leg was not significant.
The clinical and functional outcome improved significantly within the follow-up period. The preoperative HHS was 50.69±13.67 points. After 3 months 72.47±18.18 points (WI-test p<0.001), after 6 months 81.46±11.79 points (WI-test p<0.001) and after 12 months 80.91±17.00 points (WI-test p<0.001) were reached. Significant changes occurred after 6 (WI-test p=0.016) and 12 months (WI-test p=0.004) also in comparison with the values 3 months postoperatively.
In terms of patient-related outcomes, a significant improvement was also seen 3, 6 and 12 months postoperatively. The WOMAC rose from preoperative 46.53±16.82 points to 66.24±18.29 points after 3 months (WI-test p<0.001), rose again to 73.35±15.57 after 6 months (WI-test p<0.001) and reached a value of 75.01±16.92 points (WI-test p<0.001) 12 months postoperatively. Also, on the basis of the score achieved 3 months postoperative, a significant improvement was seen after 6 (WI-test p=0.003) and 12 months (WI-test p=0.002).
Exeter®
In Group B (Exeter®), which consisted of 25 patients at the beginning of the study, in two cases patients had been excluded due to a contralateral THR within the follow-up period. One patient received partial weight-bearing postoperatively. Two patients dropped out of the study due to health or private reasons and one patient died within the follow-up period.
Thus at the end of the study, Group B consisted of a total of 19 patients (6 male/13 female). The median age was 75.1±5.6 years.
In Group B, based on a preoperative BMD of the lower limb of 1.239±0.127 g/cm², neither 3 months (1.244±0.128 g/cm²) nor 6 months (1.247±0.132 g/cm²) postoperatively showed significant changes. As recently as 12 months postoperatively a significant increase in BMD on the operated limb to 1.257±0.126 g/cm² (WI-test p=0.049) was detected. Between the separate clinical review dates no significant changes could be observed. BMD of the contralateral leg was 1.235±0.120 g/cm2 preoperatively, increased up to 1.248±0.130 g/cm2 after 3 months and persisted nearly constantly at this value for 6- and 12-month postoperative evaluation point (Fig. 3, Table 1).
From a preoperative value of 691±356 N, the isometric strength of the operated limb did not change significantly after 3 months (825±498 N). However, 6 months postoperatively there was an increase in muscle strength to 942±535 N. This development was significant when compared to the preoperative value (WI-test p=0.007), as well as to the value 3 months postoperatively (WI-test p<0.027). Further increases occurred 12 months postoperatively on 1190±450 N. This development was significant when compared with the examination dates (preoperatively: WI-test p<0.001; 6 months postoperatively: WI-test p=0.007) (Table 1). Muscle strength of contralateral side increased from 715±70 N preoperatively to 931±84 N (3 months), 1021±62 N (6 months) and 1094±95 N 12 months postoperatively (Table 1).
Also in this group the clinical and functional outcome improved significantly. The preoperative HHS increased from 51.63±11.21 points to 77.44±13.92 (WI-test p<0.001) after 3 months, grew further to 81.06±11.89 points after 6 months (WI-test p<0.001) and reached 89.0±10.86 (WI-test p<0.001) after 12 months. The increase of HHS 12 months postoperatively was also significant when compared to the results 3 (WI-test p=0.008) and 6 (WI-test p=0.076) months postoperatively.
There was also a significant improvement in terms of patient-related outcome. The WOMAC Score increased from 48.90±11.17 points preoperatively to 71.93±15.07 points (WI-test p<0.001) after 3 months. After 6 months 79.17±12.23 points (WI-test p<0.001) and after 12 months 78.72±12.09 points (WI-test p<0.001) were reached. Between 3 and 6 months (WI-test p=0.01), as well as between 3 and 12 months (WI-test p=0.026), significant changes could be detected.
DISCUSSION
In the present study, BMD and muscle strength of the affected and unaffected limb were analysed over a period of one year (preoperative, 3, 6 and 12 months postoperatively) after implantation of total hip arthroplasty. In addition, clinical and patient-relevant outcome were evaluated.
Regardless of the type of anchoring, an improvement in the clinical and patient-related outcome as well as an increase in muscle strength on the operated and contralateral extremity was seen in the postoperative stages. After implantation of cementless stems, a decrease of BMD on the operated extremity after 3, 6 and 12 months was recorded. After implantation of cemented stems, BMD tends to result in an increase after 3 and 6 months. 12 months postoperatively this increase was significant.
Outcome
In terms of clinical-functional and patient-related outcomes, good results could be achieved, which are comparable to those of other studies [24, 25]. At any time during the course of observation, group differences on the HHS or WOMAC were detectable. The early postoperative results confirm the fact that THR is one of the most successful procedures in orthopaedics.
Muscular Strength
The aim of an artificial joint replacement is to achieve absence from pain and to improve mobility so that independence in everyday life is ensured. With increasing biomechanical strain the regeneration of muscles and postoperative increase of muscular strength can occur. However, in terms of maximum strength and muscular endurance, remarkable gaps remain when compared to healthy age groups [26, 27]. In the present study, in both groups an increase in the maximum isometric muscle strength on the affected and unaffected lower limb was seen postoperatively, according to the expectations. Improved clinical and functional outcomes reflect that this development is not only a result of the muscular regeneration alone, but that is also a result of postoperative reduced pain, increased physical activity and improved joint function.
Physical activity and biomechanical stress result in an increase in muscle strength. Torsional and bending stress of the bone triggered by muscle contractions, as well as axial load, result in an increase in bone mass. Each qualitative and quantitative change of load will result in a restructuring of the bone. According to Wolffs law, changing biomechanical conditions leads to bone remodelling [15]. Also, other authors postulate a change in bone mineral density as a result of mechanical stress above threshold [28, 29].
With this in mind and because of a good clinical outcome and probably enhanced physical activity, we expected a postoperative increase of BMD on the lower limb in the cemented and uncemented group.
Bone Mineral Density
Actually, there was a slight increase in BMD on the operated extremity 12 months after implantation of cemented stems. However, this was not the case after cementless THR where the BMD on the lower limb declined during the postoperative period (Table 1).
Currently, no comparable studies of the development of BMD on the entire affected extremity are available after THR. However, extensive investigations exist with regard to the change of the periprosthetic bone mineral density after THR.
Whether the findings from these studies can be applied to the entire lower limb was evaluated in the present study.
Analogous to various studies, which describe a periprosthetic bone loss after cementless THR [3-6, 30], we found a decrease in BMD at the entire operated limb 3, 6 and 12 months after implantation of cementless stems.
In contrast, after implantation of cemented hip stems we found a slight increase in BMD on the operated limb 3, 6 and 12 months postoperatively.
Most of the current studies on periprosthetic bone density also demonstrate a decreasing BMD after implantation of cemented hip stems [6-8, 31], however some studies found an increase in periprosthetic bone density postoperatively [30].
Reasons for the early decrease of periprosthetic bone density after THR are the operational irritation, such as reaming and rasping the medullary cavity or the thermal trauma of curing bone cement [30], the reduced load on the operated leg and the "stress shielding" as a result of altered power caused by the prosthesis [11, 14].
In addition, the development of the periprosthetic bone density is affected by the preoperative BMD. So various studies found a greater periprosthetic bone loss after THR in patients with a preoperative lower bone density [7, 10, 12].
The effect of the periprosthetic changes in BMD caused by operative trauma or “stress shielding”, for example, are relatively small in respect to the BMD of the whole leg. Rather than the preoperative bone density, the activity level of the patient and the loading of the operated extremity seem to have a greater influence on the bone remodelling after THR.
In the follow-up, patients from Group A mentioned pain in the operated limb after implantation of cementless stems more often. 12 Months postoperatively, a total of 12 patients of this group reported residual complaints in the operated extremity. 9 patients gave an account of an occasional stress or motion pain in the operated hip or in the thigh (n = 6). Others reported a feeling of a foreign object (n = 2) or stiffness (n = 1) in the hip joint. In addition, some patients´ complaints (n = 3) were due to other diseases of the operated extremity (spinal stenosis and thrombophlebitis). One can assume that these complaints resulted in a reduced load of the operated extremity, which could explain the postoperative decrease of BMD. The decline of the BMD in the lumbal spine 12 months postoperatively could speak for a reduced mobility of patients from Group A as well. On the other hand, patients from Group B rarely complained about pain in the operated extremity. Only 4 patients still indicated occasional complaints under load (n = 3) or a foreign body sensation (n = 1) 12 months after implantation of THR with cemented stem. With this in mind, it is to be presumed that patients from Group B have a higher benefit from the implantation of an artificial hip joint after 12 months. A corresponding load of the operated limb may explain the increase in BMD 12 months postoperatively. The BMD at the lumbar spine remained stable in the postoperative stages.
METHODS
DEXA is a method with high precision, sensitivity and reproducibility of results [19, 20]. Thus, the evaluation of the smallest changes in bone mineral density is possible. For measurement of bone density after joint arthroplasty, implants can be eliminated via the "metal-removal" function, so reliable BMD values can be delivered. However, with regard to the complete elimination of the cement mantle after implantation of cemented hip stems, different views exist. It is possible that the cement causes artifacts and so positively distorts the bone density values [7, 30]. Some authors have tried to eliminate the cement mantle around the prosthesis [6, 31], while others saw no possibility for doing so [8].
In the present study it must be pointed out that the BMD of the entire lower limb was analysed, so the cement surrounding stem is likely to have negligible effect on the result. Also, an influence of the BMD values caused by the cement would only explain the trend increase in BMD 3 months postoperatively, but not its further increase after 6 and 12 months. So it must be assumed by a real change of bone density.
Muscular Strength
The maximum strength is considered as a decisive criterion for assessing the overall performance of the muscles. In the present study the maximum isometric muscle strength was determined. Confounding factors, such as the speed of the joint movement or the change in the muscle length could be eliminated. The procedure of isometric muscle strength measurement is used in numerous studies and considered to be appropriate. The method is characterised by controlled contractions with constant muscle length and a high reproducibility. The high standard deviation values which occurred during muscle strength measurement of the affected limb are likely due to pre- and postoperative pain, changed proprioceptive perception and the cautious behaviour of the patients during the bench press test after THR.
Criticism
Critiques of the present study are the small number of patients and the difference of mean age between the two groups due to our institutional rules, the fact that the preoperative BMD and muscle strength were used as baseline values, as well as the relatively late first investigation of postperative BMD and the short follow-up observation period of one year. However, since the present study is observational and no comparison between cemented and uncemented has been done due to the large age differences between group A and B, the results we obtained are valid and show interesting tendencies. Therefore, the aim of further studies should be to examine the correlation between bone density and muscle strength in the lower limb over a longer period using larger samples.
In view of the fact that physical activity has a relevant impact on BMD, activity must be registered more precisely by means of appropriate questionnaires or small wearable activity monitores in subsequent studies.
Also, the question arises whether the decrease of BMD goes hand in hand with an increased fracture rate after implantation of cement-free THR and, therefore, a drug treatment is useful. More detailed studies are required to verify this.
CONCLUSION
The question to be answered in this study was whether objectified, easy to determine parameters, such as strength or BMD measurement of the limb after primary hip replacement show a characteristic profile and to find out to what extent the previously described periprosthetic bone loss applies to the entire limb, so that by an objective assessment the possible functionality of a hip prosthesis can be determind.
After implantation of uncemented hip stems a decrease of the entire BMD on the operated limb occurred similar to the known postoperative reduction of periprosthetic bone density. In contrast, a slight increase in limb BMD was recorded for cemented stems. Regardless of the type of anchoring, a substantial increase in muscular strength could be observed postoperatively. The present study revealed no link between postoperative BMD and muscle strength in whole limb. As a result of this investigation we conclude that the measurement of strength after surgery increases significantly. The bone density changes, however, varies between the implantation technique and clinical outcome, which in itself is quite an interesting observation, and in the overall context could permit the conclusion that the development of bone density is rather delayed.
ACKNOWLEDGEMENTS
We like to thank Anett Mau-Möller and Robert Jacksteit for conducting validation studies of the leg press dynamometer. This work has been funded by the Department of Orthopaedics, University Medicine Rostock. All authors except WS are employees of the Department of Orthopaedics at University Medicine Rostock. WS is employee at Orthopädisch-Neurochirugisches Zentrum, Datteln and prosecutes his research interests at the Department of Orthopaedics at the University Medicine Rostock.
ABBREVIATIONS
- BMC
= Bone Mineral Content
- BMD
= Bone Mineral Density
- CI
= Confidence Interval
- CV
= Coefficient of Variation
- DEXA
= Dual Energy X-Ray Absorptiometrie
- FR
= Friedman-test
- HHS
= Harris Hip Score
- ICC
= Intraclass Correlation Coefficient
- LSC
= Least Significant Change
- PE
= Polyethylene
- ROI
= Region of Interest
- SD
= Standard deviation
- THR
= Total Hip Replacement
- WI
= Wilcoxon-test
- WOMAC
= Western Ontario and McMaster University Osteoarthritis Index.
AUTHORS’ CONTRIBUTIONS
CKr and CKa collected the data, performed DEXA and Strength measurements, analysed data and wrote parts of the manuscript. TL developed the leg press, analysed data, did statistics, wrote parts of the manuscript and drafted the manuscript. SF did statistics. RS designed the study, supervised the measurements and the study and wrote parts of the manuscript. WS and WM revised the manuscript critically for important intellectual content made contributions to conception and design of the study. All authors listed made substantial contributions to the final revision of the manuscript.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Malchau H, Herberts P, Eisler T, Garellick G, Soderman P. The Swedish Total Hip Replacement Register. J Bone Joint Surg Am. 2002;84-A:2–20. doi: 10.2106/00004623-200200002-00002. [DOI] [PubMed] [Google Scholar]
- 2.Orlik J, Zhurov A, Middleton J. On the secondary stability of coated cementless hip replacement parameters that affected interface strength. Med Eng Phys. 2003;25: 825–31. doi: 10.1016/s1350-4533(03)00099-7. [DOI] [PubMed] [Google Scholar]
- 3.Venesmaa PK, Kröger HP, Miettinen HJ, Jurvelin JS, Suomalainen OT, Alhava EM. Monitoring of periprosthetic BMD after uncemented total hip arthroplasty with dual-energy X-ray absorptiometry - a 3-year follow-up study. J Bone Miner Res. 2001;16: 1056–61. doi: 10.1359/jbmr.2001.16.6.1056. [DOI] [PubMed] [Google Scholar]
- 4.Rahmy AIA, Gosens T, Blake GM, Tonino A, Fogelman I. Periprosthetic bone remodelling of two types of uncemented femoral implant with proximal hydroxyapatite coating a 3-year follow-up study addressing the influence of prosthesis design and preoperative bone density on periprosthetic bone loss. Osteoporos Int. 2004;15: 281–9. doi: 10.1007/s00198-003-1546-5. [DOI] [PubMed] [Google Scholar]
- 5.Nysted M, Benum P, Klaksvik J, Foss O, Aamodt A. Periprosthetic bone loss after insertion of an uncemented, customized femoral stem and an uncemented anatomical stem.A randomized DXA study with 5-year follow-up. Acta Orthop. 2011;82: 410–6. doi: 10.3109/17453674.2011.588860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dan D, Germann D, Burki H , et al. Bone loss after total hip arthroplasty. Rheumatol Int. 2006;26: 792–8. doi: 10.1007/s00296-005-0077-0. [DOI] [PubMed] [Google Scholar]
- 7.Venesmaa PK, Kröger HPJ, Jurvelin JS , et al. Periprosthetic bone loss after cemented total hip arthroplasty a prospective 5-year dual energy radiographic absorptiometry study of 15 patients. Acta Orthop Scand. 2003;74: 31–6. doi: 10.1080/00016470310013617. [DOI] [PubMed] [Google Scholar]
- 8.Damborg F, Nissen N, Jørgensen HRI, Abrahamsen B, Brixen K. Changes in bone mineral density (BMD) around the cemented Exeter stem a prospective study in 18 women with 5 years follow-up. Acta Orthop. 2008;79: 494–8. doi: 10.1080/17453670710015481. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi S, Saito N, Horiuchi H, Iorio R, Takaoka K. Poor bone quality or hip structure as risk factors affecting survival of total-hip arthroplasty. Lancet. 2000;355: 1499–504. doi: 10.1016/S0140-6736(00)02164-4. [DOI] [PubMed] [Google Scholar]
- 10.Nishii T, Sugano N, Masuhara K, Shibuya T, Ochi T, Tamura S. Longitudinal evaluation of time related bone remodeling after cementless total hip arthroplasty. Clin Orthop Relat Res. 1997;339: 121–31. doi: 10.1097/00003086-199706000-00017. [DOI] [PubMed] [Google Scholar]
- 11.Bryan JM, Sumner DR, Hurwitz DE, Tompkins GS, Andriacchi TP, Galante JO. Altered load history affects periprosthetic bone loss following cementless total hip arthroplasty. J Orthop Res. 1996;14: 762–8. doi: 10.1002/jor.1100140513. [DOI] [PubMed] [Google Scholar]
- 12.Huiskes R, Weinans H, van Rietbergen B. The relationship between stress shielding and bone resorption around total hip stems and the effects of flexible materials. Clin Orthop Relat Res. 1992;274: 124–34. [PubMed] [Google Scholar]
- 13.Engh CA, McGovern TF, Bobyn JD, Harris WH. A quantitative evaluation of periprosthetic bone-remodeling after cementless total hip arthroplasty. J Bone Joint Surg Am. 1992;74: 1009–20. [PubMed] [Google Scholar]
- 14.Van der Wal BCH, Rahmy A, Grimm B, Heyligers I, Tonino A. Preoperative bone quality as a factor in dual-energy X-ray absorptiometry analysis comparing bone remodelling between two implant types. Int Orthop. 2008;32: 39–45. doi: 10.1007/s00264-006-0279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolf HJ. Julis Wolff and his "law of bone remodeling. Orthopade. 1995;24: 378–86. [PubMed] [Google Scholar]
- 16.Chow R, Harrison JE, Notarius C. Effect of two randomised exercise programmes on bone mass of healthy postmenopausal women. BMJ. 1987;295: 1441–4. doi: 10.1136/bmj.295.6611.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garbuz DS, Xu M, Duncan CP, Masri BA, Sobolev B. Delays worsen quality of life outcome of primary total hip arthroplasty. Clin Orthop Relat Res. 2006;447: 79–84. doi: 10.1097/01.blo.0000203477.19421.ed. [DOI] [PubMed] [Google Scholar]
- 18.Horstmann T, Jörger G, Heitkamp HC, Mayer F, Winter E, Dickhuth HH. Auswirkungen von Hüftsport auf Gangbild, Kraftverhalten und Lebensqualität von Koxarthrotikern. Aktuelle Rheumatol. 2001;26: 162–8. [Google Scholar]
- 19.Kiratli BJ, Heiner JP, McBeath AA, Wilson MA. Determination of bone mineral density by dual x-ray absorptiometry in patients with uncemented total hip arthroplasty. J Orthop Res. 1992;10: 836–44. doi: 10.1002/jor.1100100613. [DOI] [PubMed] [Google Scholar]
- 20.artini FM, Lebherz C, Mayer F, Leichtle U, Kremling E, Sell S. Precision of the measurements of periprosthetic bone mineral density in hips with a custom-made femoral stem. J Bone Joint Surg Br. 2000;82: 1065–71. doi: 10.1302/0301-620x.82b7.9791. [DOI] [PubMed] [Google Scholar]
- 21.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures treatment by mold arthroplasty.An end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51: 737–55. [PubMed] [Google Scholar]
- 22.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15: 1833–40. [PubMed] [Google Scholar]
- 23.Faul F, Erdfelder E, Lang AG, Buchner A G. *Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39: 175–91. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- 24.Bergschmidt P, Bader R, Finze S, Gankovych A, Kundt G, Mittelmeier W. Cementless total hip replacement a prospective clinical study of the early functional and radiological outcomes of three different hip stems. Arch Orthop Trauma Surg. 2010;130: 125–33. doi: 10.1007/s00402-009-0907-8. [DOI] [PubMed] [Google Scholar]
- 25.Aarons H, Hall G, Hughes S, Salmon S. Short-term recovery from hip and knee arthroplasty. J Bone Joint Surg Am. 1996;78: 555–8. [PubMed] [Google Scholar]
- 26.Horstmann T, Heitkamp HC, Haupt G, Merk J, Mayer F, Dickhuth HH. Possibilities and limitations of sports therapy in patients with osteoarthrosis and prothesis of the hip. Dtsch Z Sportmed. 2001;52: 274–8. [Google Scholar]
- 27.Jandric SD. Effects of rehabilitation and arthroplasty on the hip isometric muscle strength in patients with osteoarthritis of the hip. Med Pregled. 2009;62: 236–40. doi: 10.2298/mpns0906236j. [DOI] [PubMed] [Google Scholar]
- 28.Frost HM. From Wolff’s law to the mechanostat a new face of physiology. J Orthop Sci. 1998;3: 282–6. doi: 10.1007/s007760050054. [DOI] [PubMed] [Google Scholar]
- 29.Chilibeck PD, Sale DG, Webber CE. Exercise and bone mineral density. Sports Med. 1995;19: 103–22. doi: 10.2165/00007256-199519020-00003. [DOI] [PubMed] [Google Scholar]
- 30.Kröger H, Miettinen H, Arnala I, Koski E, Rushton N, Suomalainen O. Evaluation of periprosthetic bone using dual-energy x-ray absorptiometry precision of the method and effect of operation on bone mineral density. J Bone Miner Res. 1996;11: 1526–30. doi: 10.1002/jbmr.5650111020. [DOI] [PubMed] [Google Scholar]
- 31.Marchetti ME, Steinberg CG, Greene JM, Jenis LG, Baran DT. A prospective study of proximal femur bone mass following cemented and uncemented hip arthroplasty. J Bone Miner Res. 1996;1: 1033–9. doi: 10.1002/jbmr.5650110722. [DOI] [PubMed] [Google Scholar]