Abstract
The biosynthesis of soluble, properly folded recombinant proteins in large quantities from Escherichia coli is desirable for academic research and industrial protein production. The basal E. coli protein homeostasis (proteostasis) network capacity is often insufficient to efficiently fold overexpressed proteins. Herein we demonstrate that a transcriptionally reprogrammed E. coli proteostasis network is generally superior for producing soluble, folded, and functional recombinant proteins. Reprogramming is accomplished by overexpressing a negative feedback deficient heat-shock response transcription factor before and during overexpression of the protein-of-interest. The advantage of transcriptional reprogramming versus simply overexpressing select proteostasis network components (e.g., chaperones and co-chaperones, which has been explored previously) is that a large number of proteostasis network components are upregulated at their evolved stoichiometry, thus maintaining the system capabilities of the proteostasis network that are currently incompletely understood. Transcriptional proteostasis network reprogramming mediated by stress-responsive signaling in the absence of stress should also be useful for protein production in other cells.
Production of large quantities of soluble, properly folded, and functional recombinant proteins-of-interest remains a major challenge in both academic and industrial settings. Escherichia coli is an easily cultured organism often used for recombinant protein production; however, the quantity obtained of a soluble, folded, and functional protein-of-interest is often undesirably low, because many proteins-of-interest aggregate to form inclusion bodies in the cytoplasm when overexpressed.1 This happens because the innate E. coli proteostasis network capacity is insufficient to support the efficient folding of large quantities of a protein-of-interest as well as the endogenous proteome during bacterial cell growth. Therefore, one potentially general strategy to improve recombinant protein production is to enhance the E. coli’s proteostasis network capacity to facilitate the proper folding of recombinant proteins during their overexpression, without compromising the folding of the endogenous proteome.2,3
The proteostasis network capacity of E. coli is determined by the concentration and relative stoichiometry of the proteostasis network components, including chaperones, co-chaperones, chaperonins, folding enzymes, and proteases.4−7 Overexpression of select proteostasis network components, such as the molecular chaperones (DnaK), and/or co-chaperones (DnaJ and GrpE), and/or chaperonins (GroEL and GroES), either alone or in combination, effectively increases the biosynthetic yield of certain proteins-of-interest.8−11 However, this approach is limited—individual chaperones often handle specific protein substrates,12,13 making it difficult to predict a priori a suitable chaperone system for a specific protein. Thus, extensive experimentation is required to determine the chaperone pathway required to fold a specific protein.11−13 The E. coli cytosolic proteostasis network is transcriptionally regulated by the heat-shock response (HSR) stress-responsive signaling pathway.14 The advantage of transcriptional reprogramming versus overexpressing select proteostasis network components is that the system capabilities of the proteostasis network are maintained because the proteostasis network components are upregulated at their evolved stoichiometry.15−18
The HSR transcriptional program is controlled by the transcriptional factor σ32,19 whose induction through stress has been shown to elevate the mRNA and protein levels of the majority of proteostasis network components.20 Elevated temperature or the coexpression of σ32 has been used previously to improve recombinant protein production.21−23 However, these methods can be problematic: (i) an elevated temperature causes the endogenous proteome to misfold, consuming much of the HSR-enhanced folding capacity; (ii) the HSR or the coexpression of σ32 is transient, due to a negative feedback pathway and degradation of the σ32 transcriptional factor,20,24 thus presenting a timing challenge; and (iii) growing E. coli at high temperature for an extended period compromises cellular health and requires a lot of electricity for large scale production.
Herein, we transcriptionally reprogram E. coli’s cytosolic proteostasis capacity at a permissive growth temperature before and during production to maximize the quality and quantity of recombinant proteins-of-interest (Figure 1a). To accomplish this goal, we overexpressed the previously reported I54N mutant of σ32 (σ32-I54N)25 that is insensitive to negative feedback regulation, affording persistently high proteostasis network component concentrations at the stoichiometry optimized by evolution (Figure 1b). σ32-I54N was subcloned into a pBAD vector, which allows the expression of σ32-I54N to be regulated by l-arabinose. Addition of arabinose to the cell culture (final concentration of 0.02% (w/v)) increased σ32-I54N levels over a period of ∼24 h, even at 37 °C, a temperature that is known not to induce a HSR (Figure 2a, top panel). As expected, σ32-I54N expression substantially increased the levels of major chaperones (DnaK), co-chaperones (DnaJ and GrpE), and chaperonins (GroEL and GroES) over a period of ∼24 h (Figure 2a). In contrast, the concentration of trigger factor, a cotranslational chaperone not under σ32 regulation,20 was largely unchanged over this time period (Figure 2a). Importantly, proteostasis network capacity could also be enhanced when the cells were grown in minimal media (M9, Supplementary Figure S1), rendering this approach suitable for the production of metabolically labeled proteins.
Figure 1.
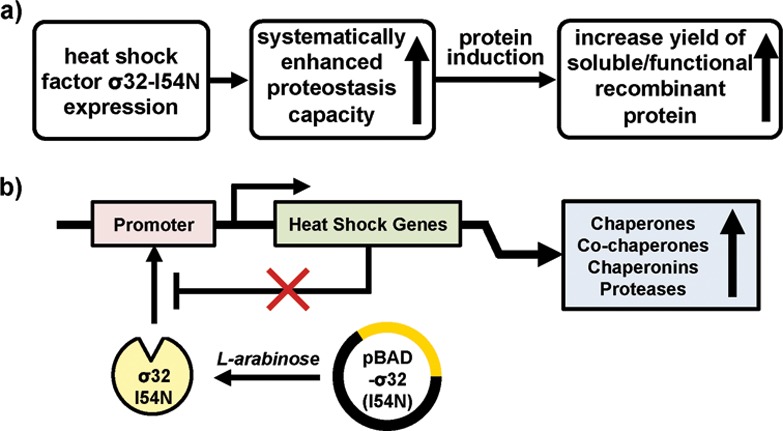
Enhancing cellular proteostasis capacity for high-yield, high-quality protein production in E. coli. (a) The proposed strategy to increase production of soluble, folded, and functional recombinant proteins by enhancing the proteostasis capacity of the E. coli through overexpression of a negative feedback deficient mutant of heat-shock factor σ32, σ32-I54N. (b) Induction of σ32-I54N expression by the addition of arabinose results in a persistent induction of cellular chaperones, co-chaperones, and chaperonins at ambient temperatures.
Figure 2.
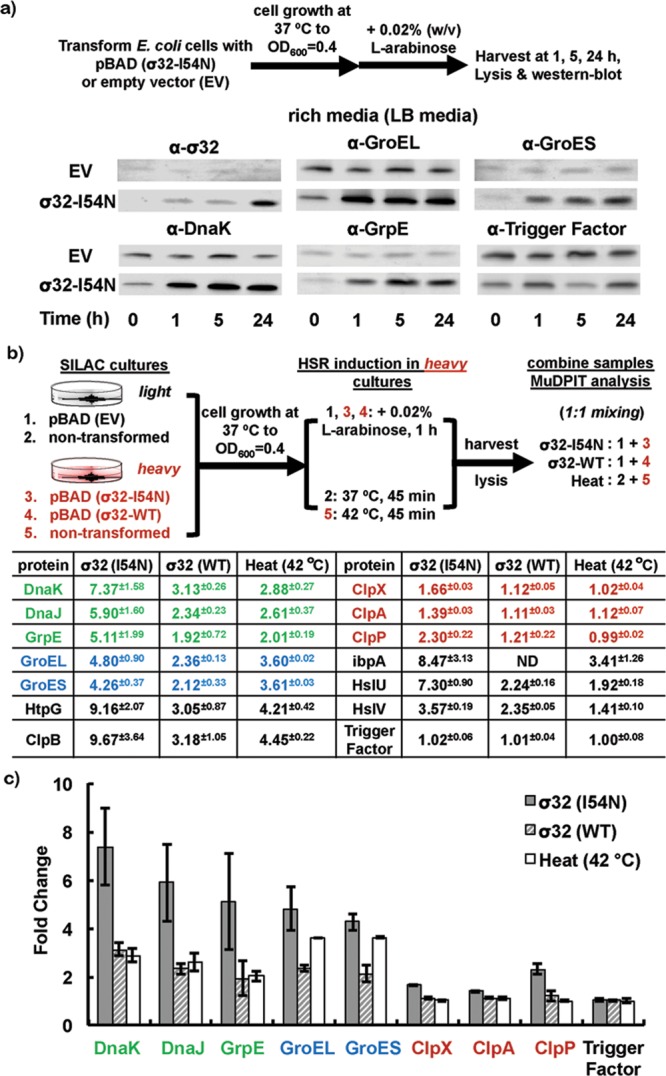
Overexpression of σ32-I54N increased the cellular concentration of major proteostasis network components in E. coli for durations that are suitable for recombinant protein expression. (a) In LB media, σ32-I54N expression increased the cellular concentration of σ32-I54N, chaperonins GroEL and GroES, chaperone DnaK, and co-chaperone GrpE, as determined by Western blotting analyses (experimental procedures outlined in the top panel). Trigger factor is not regulated by σ32 and serves as a loading control. EV: empty vector. (b,c) σ32-I54N expression for 1 h followed by cell lysis increased the concentration of major heat-shock proteins and maintained their naturally evolved stoichiometry, as quantified by whole cell SILAC MudPIT proteomic analyses (experimental procedures outlined in the top panel). Changes in heat-shock protein levels in response to wild-type (WT) σ32 expression or thermal (42 °C) stress are shown for comparison and more comprehensively in Supplementary Table S1. The HSR-regulated proteins that belong to specific folding or degradation pathways are color coded as green (DnaK/DnaJ/GrpE), blue (GroEL/GroES), and red (ClpX/P and ClpA/P AAA+ proteases). Trigger factor is not regulated by σ32 and serves as a control.
Using a quantitative whole cell proteomics approach (Figure 2b, top panel; stable isotopic labeling by amino acids in cell culture (SILAC) combined with multidimensional protein identification technology (MuDPIT) mass spectrometry), we found that σ32-I54N expression produced a HSR-like transcriptionally remodeled proteostasis network without perturbing the majority of the endogenous cellular proteome (Figures 2b,c and 3a). First, σ32-I54N expression for 1 h (Figure 2b, top panel) resulted in an elevated level of HSR regulated proteins within the σ32 regulon, including the major chaperones, chaperonins, and the AAA+ proteases (Figures 2b and c; proteins with >1.5-fold upregulation following σ32-I54N expression can be found in Supplementary Table S1, along with their fold changes following wild type σ32 expression or resulting from a thermal HSR). These results demonstrate that the σ32-I54N mutant faithfully recapitulates the transcriptional program of wild-type σ32 or a thermal-induced HSR; however, the fold change was higher with σ32-I54N than with wild-type σ32 or heat-shock due to the loss of feedback inhibition. Second, we found that the σ32-I54N HSR transcriptional program largely maintained the proper stoichiometry of proteostasis network components in comparison to the wild-type σ32 transcriptional program or a thermal HSR (Figure 2b,c and Supplementary Table S1). This is evidenced by the extent of the fold change of chaperones or chaperonins associated with individual pathways. For example, components comprising the Hsp70 (DnaK, DnaJ, and GrpE) and the Hsp60 (GroEL and GroES) pathways were each increased by a factor of ∼6 and ∼4, respectively (green and blue in Figure 2b,c). Similarly, the ClpX, ClpP and ClpA AAA+ proteases were increased by ∼2-fold (red in Figure 2b,c). Since productive folding of a protein-of-interest is the result of a collaboration between a number of folding pathways competing with proteolysis,5,16−18,26 it is crucial that balance between these pathways be maintained as well as possible. Third, σ32-I54N expression minimally perturbed the endogenous E. coli proteome, with the exception of those proteins in the σ32 regulon—89% of the proteins detected in the SILAC MudPIT proteomics study were changed by less than 50% in response to σ32-I54N expression (Figure 3a). Consistent with this data, σ32-I54N expression minimally perturbed E. coli growth (Figure 3b). Collectively, these results indicate that σ32-I54N expression results in healthy E. coli exhibiting an enhanced proteostasis network capacity, providing a pro-folding environment that also has the capacity to degrade misfolded proteins, envisioned to minimize inclusion body formation.
Figure 3.
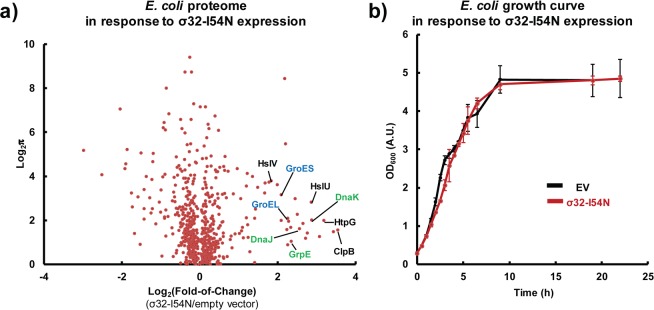
σ32-I54N expression minimally perturbs the E. coli proteome, with the exception of the heat-shock response genes, and thus does not affect cell growth. (a) Volcano plot relating the fold change (FC) of the proteome in response to σ32-I54N expression to the FC variability between duplicate SILAC MudPIT proteomics experiments. Variability is expressed as log2 π, where π = |FC – 1|/σFC, wherein σFC is the standard deviation of FC. Experimental procedures are outlined in Figure 2b, top panel. (b) Overexpression of σ32-I54N did not affect growth of E. coli during recombinant protein overexpression at 37 °C in LB media.
Next, we investigated whether the HSR transcriptional program was beneficial for recombinant protein production. Toward this end, the pBAD vector encoding σ32-I54N was cotransformed with a pET29b(+) vector harboring the protein-of-interest into the Bl21 (DE3) E. coli strain commonly used for protein overexpression, which is deficient in Lon and OmpT proteases (Figure 4a). σ32-I54N expression was initiated by the addition of l-arabinose (0.02%, w/v) after the culture reached an OD600 of 0.4 at 37 °C (red pathway in Figure 4a). Transcriptional reprogramming to enhance the E. coli proteostasis network capacity (Figure 4a) was started 1 h before IPTG induction of the protein-of-interest, which was expressed for 4 h. During the period of protein-of-interest expression, l-arabinose was present in the culture to continuously enhance E. coli proteostasis network capacity (red pathway in Figure 4a). For comparison, d-glucose (0.02%, w/v) was used to inhibit σ32-I54N expression, resulting in a basal E. coli proteostasis network capacity (black pathway in Figure 4a). The σ32-I54N transcriptional program moderately to substantially increased the soluble concentration of three aggregation-prone recombinant proteins exhibiting distinct structural scaffolds and organismal origins (Figure 4b). A de novo designed, mutation-destabilized retro-aldolase (RA) and the industrially important endoxylanase (XynA) enzyme mainly form inclusion bodies, with only a small soluble fraction, when produced in E. coli featuring a basal proteostasis network.27−29 When these proteins were overexpressed in E. coli featuring an enhanced proteostasis network capacity, the solubility of RA and XynA increased by 2- and 3-fold, respectively (Figure 4b), suggesting that the transcriptionally reprogrammed proteostasis network is able to rescue aggregation-prone proteins from inclusion body formation.
Figure 4.
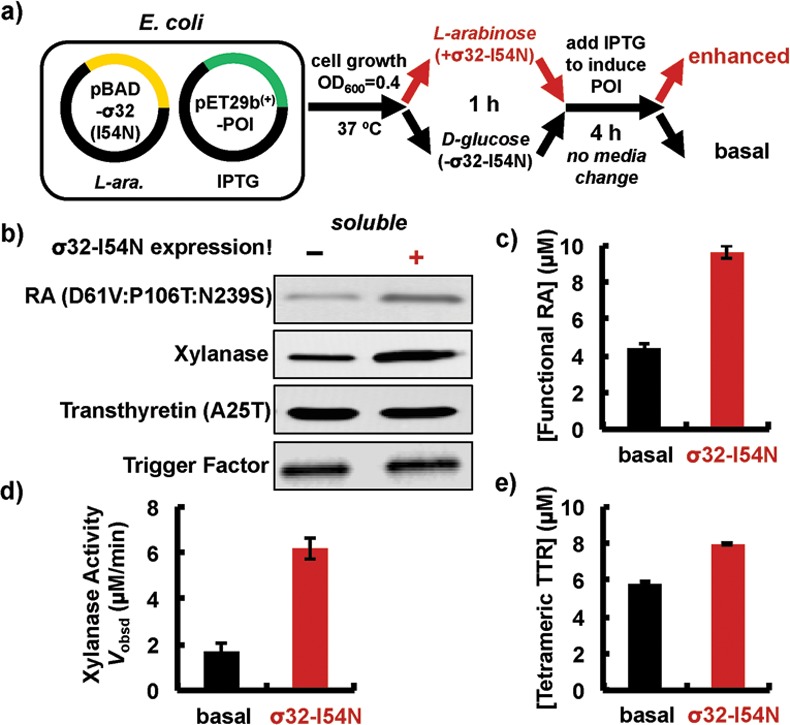
HSR transcriptional program increases the yield of soluble, folded, and functional recombinant proteins. (a) Schematic showing recombinant protein overexpression in a HSR transcriptionally enhanced E. coli proteostasis network. d-Glucose inhibits σ32-I54N expression, resulting in a basal proteostasis capacity. POI: protein-of-interest. IPTG: isopropyl β-d-1-thiogalactopyranoside. (b) Western blotting analysis of soluble recombinant proteins produced in the same number of E. coli cells featuring enhanced (+) or basal (−) proteostasis capacities. Trigger factor serves as a loading control. (c) Concentration of functional destabilized RA mutant in lysates measured using the previously described RA folding probe.28 (d) Xylanase activity in lysates measured using the EnzChekUltraxylanase assay kit. (e) Concentration of native, tetrameric A25T-TTR in lysates measured using the previously published TTR-tetramer folding probe.28
Using protein folding probes, we have recently shown that not all soluble proteins are functional when overexpressed in E. coli.28 Thus, the increase in soluble protein may not reflect an increase in the levels of functional protein. The functional concentration of a protein-of-interest can either be quantified using protein folding probes as previously reported28 or assessed directly using functional assays. Using the RA folding probe, we found that the enhanced proteostasis network capacity increased the functional RA concentration in cell lysates by 2-fold (9.80 ± 0.41 μM vs 4.12 ± 0.36 μM; Figure 4c and Supplementary Figure S2), comparable to the 2-fold increase in the soluble fraction. Similarly with xylanase, the HSR transcriptional program produced a ∼4-fold higher XynA activity, assessed using a fluorescence-based xylanase activity assay, relative to XynA produced within a basal proteostasis network (6.19 ± 0.45 μM/min vs 1.69 ± 0.34 μM/min; Figure 4d and Supplementary Figure S3). Thus, it appears that the enhanced proteostasis network capacity improves the yield of recombinant RA and XynA proteins as folded and functional proteins.
We also assessed the solubility and folding of the A25T mutant of human transthyretin (TTR). A25T TTR is a soluble protein when overexpressed in E. coli, and its solubility did not change when A25T TTR was expressed within an enhanced proteostasis network (Figure 4b). However, <50% of the soluble A25T TTR assumed its native tetrameric conformation when expressed within a basal proteostasis network (Figure 4e).28 Under a σ32-I54N-mediated enhanced proteostasis network, 39% more of the soluble A25T TTR forms native tetramers in comparison to TTR produced in a basal proteostasis network (7.98 ± 0.11 μM versus 5.75 ± 0.13 μM; Figure 4e and Supplementary Figure S4), suggesting that the enhanced proteostasis network capacity promotes the folding and assembly of A25T TTR into its functional quaternary structure.
Collectively, our data show that the σ32-I54N HSR-like reprogrammed proteostasis network promotes the production of soluble, folded, and functional recombinant proteins. This approach for recombinant protein production differs from previous approaches. First, by avoiding an environmental stress to induce the HSR, the cells maintain a healthy cellular physiology. Second, the HSR transcriptional program increases the cellular levels of chaperones, co-chaperones, chaperonins, and proteases (except Lon and ompT in the Bl21 DE3 strain) in their naturally evolved stoichiometry, which is important for maintaining the system attributes of the cytosolic proteostasis network. Such a global enhancement of E. coli cytosolic proteostasis capacity is able to mediate folding of a variety of client proteins, eliminating the necessity to know which chaperone or chaperonin pathway handles a particular protein-of-interest. Third, the σ32-I54N transcription factor is resistant to the feedback inhibition and degradation that limits the proteostasis network enhancement that can be achieved by wild-type σ32 overexpression. Therefore, higher concentrations of most proteostasis network components can be achieved with σ32-I54N reprogramming (Figure 2b), resulting in higher quantities of XynA (Supplementary Figure S5) relative to WT σ32 reprogramming. Lastly, although σ32-I54N transcriptional reprogramming also increases protease levels, it primarily affords a pro-folding environment, suited to folding recombinant proteins. The yield of XynA was minimally affected by lengthening the expression period from 4 to 6 h, suggesting that the proteolytic capacity did not override the pro-folding capacity (Supplementary Figure S6). Thus, we expect this approach to be effective for improving the yield of a variety of recombinant proteins without the need for extensive optimization. However, optimization of the temperature, the culture density at the time of HSR transcriptional program induction, and the timing of protein-of-interest induction could further enhance yield.
The principles that we have demonstrated for improved recombinant protein overexpression in bacteria should be readily applicable to other cellular expression systems, including eukaryotic cells.15−18 Transcriptional reprogramming retains the system attributes of the proteostasis network, enabling enhanced proteostasis network capacities to be used to improve the yield of soluble, folded, and functional recombinant proteins. Genetic strategies and chemical approaches for the activation of stress responsive signaling in the absence of stress are now becoming available for multiple organisms.30−34 Thus, it is now practical to transcriptionally reprogram proteostasis network capacity for improved production of difficult to fold recombinant proteins.
Methods
Recombinant Protein Overexpression in the Heat-Shock-Like Expression System
A pET29b(+) vector (kanamycin resistance) encoding the gene of a protein-of-interest was transformed into the Bl21 (DE3) strain harboring the pBAD-σ32-I54N vector (ampicillin resistance). When cultures of Bl21 (DE3) cells bearing both vectors reached an OD600 of 0.4, σ32-I54N expression was induced with 0.02% (w/v) l-arabinose. After incubation for 1 h, isopropyl β-d-1-thiogalactopyranoside (IPTG, final concentration of 1 mM) was added to induce overexpression of the protein-of-interest, which could be induced for as long as 24 h. During protein-of-interest expression, l-arabinose was kept in the cell culture to ensure that the E. coli proteostasis network capacity was constantly enhanced.
Acknowledgments
This work was supported by the National Institutes of Health grants NS050636 and DK046335 (J.W.K.) as well as the Skaggs Institute for Chemical Biology and the Lita Annenberg Hazen Foundation. X.Z. was a Howard Hughes Medical Institute Fellow of the Helen Hay Whitney Foundation and is currently supported by the Burroughs Wellcome Fund Career Award at the Scientific Interface. J.C.G. was supported by an NRSA from the NHLBI (F32-HL099245) and is currently supported by a postdoctoral fellowship from the American Heart Association. We thank M. Saure and M. T. Dendle for technical support and C. Fearns for carefully reading and editing the manuscript.
Supporting Information Available
Supplementary figures, methods, and references. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- Baneyx F.; Mujacic M. (2004) Recombinant protein folding and misfolding in Escherichia coli. Nat. Biotechnol. 22, 1399–1408. [DOI] [PubMed] [Google Scholar]
- Thomas J. G.; Ayling A.; Baneyx F. (1997) Molecular chaperones, folding catalysts, and the recovery of active recombinant proteins from E. coli - To fold or to refold. Appl. Biochem. Biotechnol. 66, 197–238. [DOI] [PubMed] [Google Scholar]
- de Marco A. (2011) Molecular and chemical chaperones for improving the yields of soluble recombinant proteins. Methods Mol. Biol. 705, 31–51. [DOI] [PubMed] [Google Scholar]
- Bukau B.; Deuerling E.; Pfund C.; Craig E. A. (2000) Getting newly synthesized proteins into shape. Cell 101, 119–122. [DOI] [PubMed] [Google Scholar]
- Hartl F. U.; Hayer-Hartl M. (2009) Converging concepts of protein folding in vitro and in vivo. Nat. Struct. Mol. Biol. 16, 574–581. [DOI] [PubMed] [Google Scholar]
- Horwich A. L.; Fenton W. A.; Chapman E.; Farr G. W. (2007) Two families of chaperonin: physiology and mechanism. Annu. Rev. Cell Dev. Biol. 23, 115–145. [DOI] [PubMed] [Google Scholar]
- Mamathambika B. S.; Bardwell J. C. (2008) Disulfide-linked protein folding pathways. Annu. Rev. Cell Dev. Biol. 24, 211–235. [DOI] [PubMed] [Google Scholar]
- Thomas J. G.; Baneyx F. (1996) Protein misfolding and inclusion body formation in recombinant Escherichia coli cells overexpressing Heat-shock proteins. J. Biol. Chem. 271, 11141–11147. [DOI] [PubMed] [Google Scholar]
- Baneyx F.; Palumbo J. L. (2003) Improving heterologous protein folding via molecular chaperone and foldase co-expression. Methods Mol. Biol. 205, 171–197. [DOI] [PubMed] [Google Scholar]
- de Marco A.; De Marco V. (2004) Bacteria co-transformed with recombinant proteins and chaperones cloned in independent plasmids are suitable for expression tuning. J. Biotechnol. 109, 45–52. [DOI] [PubMed] [Google Scholar]
- de Marco A. (2007) Protocol for preparing proteins with improved solubility by co-expressing with molecular chaperones in Escherichia coli. Nat. Protoc. 2, 2632–2639. [DOI] [PubMed] [Google Scholar]
- Rudiger S.; Germeroth L.; SchneiderMergener J.; Bukau B. (1997) Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries. EMBO J. 16, 1501–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houry W. A.; Frishman D.; Eckerskorn C.; Lottspeich F.; Hartl F. U. (1999) Identification of in vivo substrates of the chaperonin GroEL. Nature 402, 147–154. [DOI] [PubMed] [Google Scholar]
- Lindquist S. (1986) The heat-shock response. Annu. Rev. Biochem. 55, 1151–1191. [DOI] [PubMed] [Google Scholar]
- Balch W. E.; Morimoto R. I.; Dillin A.; Kelly J. W. (2008) Adapting proteostasis for disease intervention. Science 319, 916–919. [DOI] [PubMed] [Google Scholar]
- Powers E. T.; Morimoto R. I.; Dillin A.; Kelly J. W.; Balch W. E. (2009) Biological and chemical approaches to diseases of proteostasis deficiency. Annu. Rev. Biochem. 78, 959–991. [DOI] [PubMed] [Google Scholar]
- Hartl F. U.; Bracher A.; Hayer-Hartl M. (2011) Molecular chaperones in protein folding and proteostasis. Nature 475, 324–332. [DOI] [PubMed] [Google Scholar]
- Kim Y. E.; Hipp M. S.; Bracher A.; Hayer-Hartl M.; Hartl F. U. (2013) Molecular chaperone functions in protein folding and proteostasis. Annu. Rev. Biochem. 82, 323–355. [DOI] [PubMed] [Google Scholar]
- Straus D. B.; Walter W. A.; Gross C. A. (1987) The heat-shock response of Escherichia coli is regulated by changes in the concentration of σ-32. Nature 329, 348–351. [DOI] [PubMed] [Google Scholar]
- Zhao K.; Liu M. Z.; Burgess R. R. (2005) The global transcriptional response of Escherichia coli to induced σ(32) protein involves σ(32) regulon activation followed by inactivation and degradation of σ(32) in vivo. J. Biol. Chem. 280, 17758–17768. [DOI] [PubMed] [Google Scholar]
- Valdez-Cruz N. A.; Caspeta L.; Perez N. O.; Ramirez O. T.; Trujillo-Roldan M. A. (2010) Production of recombinant proteins in E. coli by the heat inducible expression system based on the phage lambda pL and/or pR promoters. Microb. Cell Fact. 9, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H.; Pei J.; Jiang Y.; Song X.; Shao W. (2010) pHsh vectors, a novel expression system of Escherichia coli for the large-scale production of recombinant enzymes. Biotechnol. Lett. 32, 795–801. [DOI] [PubMed] [Google Scholar]
- Hsu S. Y.; Lin Y. S.; Li S. J.; Lee W. C. (2014) Co-expression of a heat shock transcription factor to improve conformational quality of recombinant protein in Escherichia coli. J. Biosci. Bioeng. 10.1016/j.jbiosc.2014.1002.1012. [DOI] [PubMed] [Google Scholar]
- Guisbert E.; Herman C.; Lu C. Z.; Gross C. A. (2004) A chaperone network controls the heat shock response in E. coli. Genes Dev. 18, 2812–2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yura T.; Guisbert E.; Poritz M.; Lu C. Z.; Campbell E.; Gross C. A. (2007) Analysis of σ(32) mutants defective in chaperone-mediated feedback control reveals unexpected complexity of the heat shock response. Proc. Natl. Acad. Sci. U.S.A. 104, 17638–17643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukau B.; Weissman J.; Horwich A. (2006) Molecular chaperones and protein quality control. Cell 125, 443–451. [DOI] [PubMed] [Google Scholar]
- Bjelic S.; Kipnis Y.; Wang L.; Pianowski Z.; Vorobiev S.; Su M.; Seetharaman J.; Xiao R.; Kornhaber G.; Hunt J. F.; Tong L.; Hilvert D.; Baker D. (2014) Exploration of alternate catalytic mechanisms and optimization strategies for retroaldolase design. J. Mol. Biol. 426, 256–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y.; Tan Y. L.; Zhang X.; Bhabha G.; Ekiert D. C.; Genereux J. C.; Cho Y.; Kipnis Y.; Bjelic S.; Baker D.; Kelly J. W. (2014) Small molecule probes to quantify the functional fraction of a specific protein in a cell with minimal folding equilibrium shifts. Proc. Natl. Acad. Sci. U.S.A. 111, 4449–4454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y.; Wu A.; Zeng H.; Shao W. (2006) High-level expression of an α-l-arabinofuranosidase from Thermotoga maritima in Escherichia coli for the production of xylobiose from xylan. Biotechnol. Lett. 28, 351–356. [DOI] [PubMed] [Google Scholar]
- Calamini B.; Silva M. C.; Madoux F.; Hutt D. M.; Khanna S.; Chalfant M. A.; Saldanha S. A.; Hodder P.; Tait B. D.; Garza D.; Balch W. E.; Morimoto R. I. (2012) Small-molecule proteostasis regulators for protein conformational diseases. Nat. Chem. Biol. 8, 185–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoulders M. D.; Ryno L. M.; Cooley C. B.; Kelly J. W.; Wiseman R. L. (2013) Broadly applicable methodology for the rapid and dosable small molecule-mediated regulation of transcription factors in human cells. J. Am. Chem. Soc. 135, 8129–8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoulders M. D.; Ryno L. M.; Genereux J. C.; Moresco J. J.; Tu P. G.; Wu C.; Yates J. R. 3rd; Su A. I.; Kelly J. W.; Wiseman R. L. (2013) Stress-independent activation of XBP1s and/or ATF6 reveals three functionally diverse ER proteostasis environments. Cell Rep. 3, 1279–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. M.; Miyata Y.; Klinedinst S.; Peng H. M.; Chua J. P.; Komiyama T.; Li X.; Morishima Y.; Merry D. E.; Pratt W. B.; Osawa Y.; Collins C. A.; Gestwicki J. E.; Lieberman A. P. (2013) Activation of Hsp70 reduces neurotoxicity by promoting polyglutamine protein degradation. Nat. Chem. Biol. 9, 112–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryno L. M.; Genereux J. C.; Naito T.; Morimoto R. I.; Powers E. T.; Shoulders M. D.; Wiseman R. L. (2014) Characterizing the altered cellular proteome induced by the stress-independent activation of Heat Shock Factor 1. ACS Chem. Biol. 9, 1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


